User login
Readmission to non-index hospital following acute stroke linked to worse outcomes
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
REPORTING FROM ANA 2018
Key clinical point:
Major finding: The adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
Study details: A review of 24,545 acute stroke patients 2013 from the Nationwide Readmissions Database.
Disclosures: The researchers reported having no financial disclosures.
Source: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
Older adults who self-harm face increased suicide risk
Adults aged 65 years and older with a self-harm history are more likely to die from unnatural causes – specifically suicide – than are those who do not self-harm, according to what researchers called the first study of self-harm that exclusively focused on older adults from the perspective of primary care.
“This work should alert policy makers and primary health care professionals to progress towards implementing preventive measures among older adults who consult with a GP,” lead author Catharine Morgan, PhD, and her coauthors, wrote in the Lancet Psychiatry.
The study, which reviewed the primary care records of 4,124 older adults in the United Kingdom with incidents of self-harm, found that , said Dr. Morgan, of the National Institute for Health Research (NIHR) Greater Manchester (England) Patient Safety Translational Research Centre at the University of Manchester, and her coauthors. They also noted that, “compared with their peers who had not harmed themselves, adults in the self-harm cohort were an estimated 20 times more likely to die unnaturally during the first year after a self-harm episode and three or four times more likely to die unnaturally in subsequent years.”
The coauthors also found that, compared with a comparison cohort, the prevalence of a previous mental illness was twice as high among older adults who had engaged in self-harm (hazard ratio, 2.10; 95% confidence interval, 2.03-2.17). Older adults with a self-harm history also had a 20% higher prevalence of a physical illness (HR, 1.20; 95% CI, 1.17-1.23), compared with those without such a history.
Dr. Morgan and her coauthors also uncovered differing likelihoods of referral to specialists, depending on socioeconomic status of the surrounding area. Older patients in “more socially deprived localities” were less likely to be referred to mental health services. Women also were more likely than men were to be referred, highlighting “an important target for improvement across the health care system.” They also recommended avoiding tricyclics for older patients and encouraged maintaining “frequent medication reviews after self-harm.”
The coauthors noted potential limitations in their study, including reliance on clinicians who entered the primary care records and reluctance of coroners to report suicide as the cause of death in certain scenarios. However, they strongly encouraged general practitioners to intervene early and consider alternative medications when treating older patients who exhibit risk factors.
“Health care professionals should take the opportunity to consider the risk of self-harm when an older person consults with other health problems, especially when major physical illnesses and psychopathology are both present, to reduce the risk of an escalation in self-harming behaviour and associated mortality,” they wrote.
The NIHR Greater Manchester Patient Safety Translational Research Centre funded the study. Dr. Morgan and three of her coauthors declared no conflicts of interest. Two authors reported grants from the NIHR, and one author reported grants from the Department of Health and Social Care and the Healthcare Quality Improvement Partnership.
SOURCE: Morgan C et al. Lancet Psychiatry. 2018 Oct 15. doi: 10.1016/S2215-0366(18)30348-1.
The study by Morgan et al. and her colleagues reinforced both the risks of self-harm among older adults and the absence of follow-up, but more research needs to be done, according to Rebecca Mitchell, PhD, an associate professor at the Australian Institute of Health Innovation at Macquarie University in Sydney.
Just 11.7% of older adults who self-harmed were referred to a mental health specialist, even though the authors found that the older adult cohort had twice the prevalence of a previous mental illness, compared with a matched comparison cohort. Though we may not always know the factors that contributed to these incidents of self-harm, “Morgan and colleagues have provided evidence that the clinical management of older adults who self-harm needs to improve,” Dr. Mitchell wrote.
Next steps could include “qualitative studies that focus on life experiences, social connectedness, resilience, and experience of health care use,” she wrote, painting a fuller picture of the intentions behind those self-harm choices.
“Further research still needs to be done on self-harm among older adults, including the replication of Morgan and colleagues’ research in other countries, to increase our understanding of how primary care could present an early window of opportunity to prevent repeated self-harm attempts and unnatural deaths,” Dr. Mitchell added.
These comments are adapted from an accompanying editorial (Lancet Psychiatry. 2018 Oct 15. doi: 10.1016/S2215-0366[18]30358-4). Dr. Mitchell declared no conflicts of interest.
The study by Morgan et al. and her colleagues reinforced both the risks of self-harm among older adults and the absence of follow-up, but more research needs to be done, according to Rebecca Mitchell, PhD, an associate professor at the Australian Institute of Health Innovation at Macquarie University in Sydney.
Just 11.7% of older adults who self-harmed were referred to a mental health specialist, even though the authors found that the older adult cohort had twice the prevalence of a previous mental illness, compared with a matched comparison cohort. Though we may not always know the factors that contributed to these incidents of self-harm, “Morgan and colleagues have provided evidence that the clinical management of older adults who self-harm needs to improve,” Dr. Mitchell wrote.
Next steps could include “qualitative studies that focus on life experiences, social connectedness, resilience, and experience of health care use,” she wrote, painting a fuller picture of the intentions behind those self-harm choices.
“Further research still needs to be done on self-harm among older adults, including the replication of Morgan and colleagues’ research in other countries, to increase our understanding of how primary care could present an early window of opportunity to prevent repeated self-harm attempts and unnatural deaths,” Dr. Mitchell added.
These comments are adapted from an accompanying editorial (Lancet Psychiatry. 2018 Oct 15. doi: 10.1016/S2215-0366[18]30358-4). Dr. Mitchell declared no conflicts of interest.
The study by Morgan et al. and her colleagues reinforced both the risks of self-harm among older adults and the absence of follow-up, but more research needs to be done, according to Rebecca Mitchell, PhD, an associate professor at the Australian Institute of Health Innovation at Macquarie University in Sydney.
Just 11.7% of older adults who self-harmed were referred to a mental health specialist, even though the authors found that the older adult cohort had twice the prevalence of a previous mental illness, compared with a matched comparison cohort. Though we may not always know the factors that contributed to these incidents of self-harm, “Morgan and colleagues have provided evidence that the clinical management of older adults who self-harm needs to improve,” Dr. Mitchell wrote.
Next steps could include “qualitative studies that focus on life experiences, social connectedness, resilience, and experience of health care use,” she wrote, painting a fuller picture of the intentions behind those self-harm choices.
“Further research still needs to be done on self-harm among older adults, including the replication of Morgan and colleagues’ research in other countries, to increase our understanding of how primary care could present an early window of opportunity to prevent repeated self-harm attempts and unnatural deaths,” Dr. Mitchell added.
These comments are adapted from an accompanying editorial (Lancet Psychiatry. 2018 Oct 15. doi: 10.1016/S2215-0366[18]30358-4). Dr. Mitchell declared no conflicts of interest.
Adults aged 65 years and older with a self-harm history are more likely to die from unnatural causes – specifically suicide – than are those who do not self-harm, according to what researchers called the first study of self-harm that exclusively focused on older adults from the perspective of primary care.
“This work should alert policy makers and primary health care professionals to progress towards implementing preventive measures among older adults who consult with a GP,” lead author Catharine Morgan, PhD, and her coauthors, wrote in the Lancet Psychiatry.
The study, which reviewed the primary care records of 4,124 older adults in the United Kingdom with incidents of self-harm, found that , said Dr. Morgan, of the National Institute for Health Research (NIHR) Greater Manchester (England) Patient Safety Translational Research Centre at the University of Manchester, and her coauthors. They also noted that, “compared with their peers who had not harmed themselves, adults in the self-harm cohort were an estimated 20 times more likely to die unnaturally during the first year after a self-harm episode and three or four times more likely to die unnaturally in subsequent years.”
The coauthors also found that, compared with a comparison cohort, the prevalence of a previous mental illness was twice as high among older adults who had engaged in self-harm (hazard ratio, 2.10; 95% confidence interval, 2.03-2.17). Older adults with a self-harm history also had a 20% higher prevalence of a physical illness (HR, 1.20; 95% CI, 1.17-1.23), compared with those without such a history.
Dr. Morgan and her coauthors also uncovered differing likelihoods of referral to specialists, depending on socioeconomic status of the surrounding area. Older patients in “more socially deprived localities” were less likely to be referred to mental health services. Women also were more likely than men were to be referred, highlighting “an important target for improvement across the health care system.” They also recommended avoiding tricyclics for older patients and encouraged maintaining “frequent medication reviews after self-harm.”
The coauthors noted potential limitations in their study, including reliance on clinicians who entered the primary care records and reluctance of coroners to report suicide as the cause of death in certain scenarios. However, they strongly encouraged general practitioners to intervene early and consider alternative medications when treating older patients who exhibit risk factors.
“Health care professionals should take the opportunity to consider the risk of self-harm when an older person consults with other health problems, especially when major physical illnesses and psychopathology are both present, to reduce the risk of an escalation in self-harming behaviour and associated mortality,” they wrote.
The NIHR Greater Manchester Patient Safety Translational Research Centre funded the study. Dr. Morgan and three of her coauthors declared no conflicts of interest. Two authors reported grants from the NIHR, and one author reported grants from the Department of Health and Social Care and the Healthcare Quality Improvement Partnership.
SOURCE: Morgan C et al. Lancet Psychiatry. 2018 Oct 15. doi: 10.1016/S2215-0366(18)30348-1.
Adults aged 65 years and older with a self-harm history are more likely to die from unnatural causes – specifically suicide – than are those who do not self-harm, according to what researchers called the first study of self-harm that exclusively focused on older adults from the perspective of primary care.
“This work should alert policy makers and primary health care professionals to progress towards implementing preventive measures among older adults who consult with a GP,” lead author Catharine Morgan, PhD, and her coauthors, wrote in the Lancet Psychiatry.
The study, which reviewed the primary care records of 4,124 older adults in the United Kingdom with incidents of self-harm, found that , said Dr. Morgan, of the National Institute for Health Research (NIHR) Greater Manchester (England) Patient Safety Translational Research Centre at the University of Manchester, and her coauthors. They also noted that, “compared with their peers who had not harmed themselves, adults in the self-harm cohort were an estimated 20 times more likely to die unnaturally during the first year after a self-harm episode and three or four times more likely to die unnaturally in subsequent years.”
The coauthors also found that, compared with a comparison cohort, the prevalence of a previous mental illness was twice as high among older adults who had engaged in self-harm (hazard ratio, 2.10; 95% confidence interval, 2.03-2.17). Older adults with a self-harm history also had a 20% higher prevalence of a physical illness (HR, 1.20; 95% CI, 1.17-1.23), compared with those without such a history.
Dr. Morgan and her coauthors also uncovered differing likelihoods of referral to specialists, depending on socioeconomic status of the surrounding area. Older patients in “more socially deprived localities” were less likely to be referred to mental health services. Women also were more likely than men were to be referred, highlighting “an important target for improvement across the health care system.” They also recommended avoiding tricyclics for older patients and encouraged maintaining “frequent medication reviews after self-harm.”
The coauthors noted potential limitations in their study, including reliance on clinicians who entered the primary care records and reluctance of coroners to report suicide as the cause of death in certain scenarios. However, they strongly encouraged general practitioners to intervene early and consider alternative medications when treating older patients who exhibit risk factors.
“Health care professionals should take the opportunity to consider the risk of self-harm when an older person consults with other health problems, especially when major physical illnesses and psychopathology are both present, to reduce the risk of an escalation in self-harming behaviour and associated mortality,” they wrote.
The NIHR Greater Manchester Patient Safety Translational Research Centre funded the study. Dr. Morgan and three of her coauthors declared no conflicts of interest. Two authors reported grants from the NIHR, and one author reported grants from the Department of Health and Social Care and the Healthcare Quality Improvement Partnership.
SOURCE: Morgan C et al. Lancet Psychiatry. 2018 Oct 15. doi: 10.1016/S2215-0366(18)30348-1.
FROM THE LANCET PSYCHIATRY
Key clinical point: Consider medications other than tricyclics and frequent medication reviews for older adults who self-harm.
Major finding: “Adults in the self-harm cohort were an estimated 20 times more likely to die unnaturally during the first year after a self-harm episode and three or four times more likely to die unnaturally in subsequent years.”
Study details: A multiphase cohort study involving 4,124 adults in the United Kingdom, aged 65 years and older, with a self-harm episode recorded during 2001-2014.
Disclosures: The National Institute for Health Research (NIHR) Greater Manchester Patient Safety Translational Research Centre funded the study. Dr. Morgan and three of her coauthors declared no conflicts of interest. Two authors reported grants from the NIHR, and one reported grants from the Department of Health and Social Care and the Healthcare Quality Improvement Partnership.
Source: Morgan C et al. Lancet Psychiatry. 2018 Oct 15. doi: 10.1016/S2215-0366(18)30348-1.
Adjuvanted flu vaccine reduces hospitalizations in oldest old
SAN FRANCISCO – presented at an annual scientific meeting on infectious diseases.
“It’s one thing to say you have a more immunogenic vaccine, it’s another thing to be able to say it offers clinical benefit, especially in the oldest old and the frailest frail,” says Stefan Gravenstein, MD, professor of medicine and health services, policy and practice at the Brown University School of Public Health, Providence, R.I. Dr. Gravenstein presented a poster outlying a randomized, clinical trial of the Fluad vaccine in nursing homes.
The study randomized the nursing homes so that some facilities would offer Fluad as part of their standard of care. The design helped address the problem of consent. Any clinical trial that requires individual consent would likely exclude many of the frailest patients, leading to an unrepresentative sample. “So if you want to have a generalizable result, you’d like to have it applied to the population the way you would in the real world, so randomizing the nursing homes rather than the people makes a lot of sense,” said Dr. Gravenstein.
Dr. Gravenstein chose to test the vaccine in nursing home residents, hoping to see a signal in a population in which flu complications are more common. “If you can get a difference in a nursing home population, that’s clinically important, that gives you hope that you can see it in all the other populations, too,” he said.
SOURCE: Gravenstein S et al. IDWeek 2018, Abstract 996.
SAN FRANCISCO – presented at an annual scientific meeting on infectious diseases.
“It’s one thing to say you have a more immunogenic vaccine, it’s another thing to be able to say it offers clinical benefit, especially in the oldest old and the frailest frail,” says Stefan Gravenstein, MD, professor of medicine and health services, policy and practice at the Brown University School of Public Health, Providence, R.I. Dr. Gravenstein presented a poster outlying a randomized, clinical trial of the Fluad vaccine in nursing homes.
The study randomized the nursing homes so that some facilities would offer Fluad as part of their standard of care. The design helped address the problem of consent. Any clinical trial that requires individual consent would likely exclude many of the frailest patients, leading to an unrepresentative sample. “So if you want to have a generalizable result, you’d like to have it applied to the population the way you would in the real world, so randomizing the nursing homes rather than the people makes a lot of sense,” said Dr. Gravenstein.
Dr. Gravenstein chose to test the vaccine in nursing home residents, hoping to see a signal in a population in which flu complications are more common. “If you can get a difference in a nursing home population, that’s clinically important, that gives you hope that you can see it in all the other populations, too,” he said.
SOURCE: Gravenstein S et al. IDWeek 2018, Abstract 996.
SAN FRANCISCO – presented at an annual scientific meeting on infectious diseases.
“It’s one thing to say you have a more immunogenic vaccine, it’s another thing to be able to say it offers clinical benefit, especially in the oldest old and the frailest frail,” says Stefan Gravenstein, MD, professor of medicine and health services, policy and practice at the Brown University School of Public Health, Providence, R.I. Dr. Gravenstein presented a poster outlying a randomized, clinical trial of the Fluad vaccine in nursing homes.
The study randomized the nursing homes so that some facilities would offer Fluad as part of their standard of care. The design helped address the problem of consent. Any clinical trial that requires individual consent would likely exclude many of the frailest patients, leading to an unrepresentative sample. “So if you want to have a generalizable result, you’d like to have it applied to the population the way you would in the real world, so randomizing the nursing homes rather than the people makes a lot of sense,” said Dr. Gravenstein.
Dr. Gravenstein chose to test the vaccine in nursing home residents, hoping to see a signal in a population in which flu complications are more common. “If you can get a difference in a nursing home population, that’s clinically important, that gives you hope that you can see it in all the other populations, too,” he said.
SOURCE: Gravenstein S et al. IDWeek 2018, Abstract 996.
REPORTING FROM ID WEEK 2018
CDC: Trivalent adjuvanted influenza vaccine aIIV3 safe in elderly adults
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
sworcester@frontlinemedcom.com
SOURCE: Haber P et al. ICEID 2018, Board 320.
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
sworcester@frontlinemedcom.com
SOURCE: Haber P et al. ICEID 2018, Board 320.
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
sworcester@frontlinemedcom.com
SOURCE: Haber P et al. ICEID 2018, Board 320.
REPORTING FROM ICEID 2018
Key clinical point: No new or unexpected adverse events were reported among the 630 reports related to the vaccine during the study period, of which 521 involved adults aged 65 years and older.
Major finding: Of 521 reports, 18 were serious, and there were two deaths.
Study details: A review of 521 reports to the Vaccine Adverse Event Reporting System in 2017-2018.
Disclosures: Ms. Haber reported having no disclosures.
Source: Haber P et al. ICEID 2018, Board 320.
PCV13 moderately effective in older adults
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
REPORTING FROM ICEID 2018
ED visits related to psychiatric complaints are up 20% among elderly
SAN DIEGO – Between 2011 and 2015, the proportion of elderly patients presenting to the emergency department with psychiatric complaints increased by 20%, according to a retrospective analysis of national hospital data.
In addition, 10-year increases in age, male sex, nursing home status, and Medicare insurance were associated with an increased likelihood of hospital admission.
“The growing geriatric patient population is a well-known phenomenon across every developed country,” lead researcher Derrick Huang said in an interview in advance of the annual meeting of the American College of Emergency Physicians. “A potent mix of increasing life expectancy, greater disease severity and comorbidities in the elderly, and large-scale demographic shifts has placed a significant strain on both our financial and health care resources. This study corroborates these trends in the emergency department and is a preliminary exploration of potential, newly evolving clinical challenges that the ED team will increasingly face into the future.”
For the study, Mr. Huang, a fourth-year medical student at Oakland University William Beaumont School of Medicine in Rochester, Mich., and his colleagues examined National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2015. They limited the analysis to emergency department visits with patients in the age groups of 65-74, 75-84, and 85 or older. For the primary outcome of interest, the researchers evaluated demographic variables of age group, sex, residential status, race and ethnicity, and insurance for association with hospital admission. For the secondary outcome of interest, they evaluated presenting ED complaints related to the clinical domains of cardiopulmonary disease, psychiatric disease, and fractures and dislocations for potential trends in the ED geriatric age group between 2011 and 2015.
Mr. Huang and his associates found that, as a percentage of total ED visits, those among patients aged 65 or older rose from 14.9% in 2011 to 15.6% in 2015, an increase of 4.7%. By age group, the proportion of visits during the study period was highest for those aged 65-74 years (43.8%), followed by those aged 75-84 years (34.7%) and those aged 85 and older (21.5%). On multivariate analysis, the 75-84 and age-85-and-older groups were 1.30 and 1.71 times more likely to be admitted to the hospital, compared with the 65-74 group, respectively (P less than .000 for both).
Men were 1.19 times more likely than were women to be admitted (P less than .000). In addition, elderly patients who reside in nursing homes were 1.70 times more likely to be admitted to the hospital, compared with those who lived in private homes (P less than .000), while those with Medicare insurance were 1.57 more likely to be admitted, compared with those who did not have insurance (P = .004).
On trend analysis, ED psychiatric complaints rose incrementally during the study period, from 3.9% in 2011 to 4.7% in 2015, a relative increase of 20.5%. The researchers identified no consistent trend with visit complaints related to cardiopulmonary disease, and fractures and dislocations.
“This was not too surprising, because these difficulties with older patients are not new and many investigators have sought out solutions,” Mr. Huang said. “For example, there have been many interventions both in the ED as well as in the primary care setting designed to identify risk factors and facilitate postdischarge care to prevent falls. These approaches are constantly evolving and will be of increasing importance.”
“For example, we may be seeing a larger of proportion of patients with acute mental health complaints,” Mr. Huang said. “We will need to continue developing our multidisciplinary approach to care by improving coordination with different specialties – and especially outpatient and community health care providers.”
The study’s senior author was Jason Wasserman, PhD of Oakland University William Beaumont School of Medicine. The researchers reported having no financial disclosures.
SOURCE: Huang D et al. Ann Emerg Med. 2018 Oct. doi: 10.1016/j.annemergmed.2018.08.212.
SAN DIEGO – Between 2011 and 2015, the proportion of elderly patients presenting to the emergency department with psychiatric complaints increased by 20%, according to a retrospective analysis of national hospital data.
In addition, 10-year increases in age, male sex, nursing home status, and Medicare insurance were associated with an increased likelihood of hospital admission.
“The growing geriatric patient population is a well-known phenomenon across every developed country,” lead researcher Derrick Huang said in an interview in advance of the annual meeting of the American College of Emergency Physicians. “A potent mix of increasing life expectancy, greater disease severity and comorbidities in the elderly, and large-scale demographic shifts has placed a significant strain on both our financial and health care resources. This study corroborates these trends in the emergency department and is a preliminary exploration of potential, newly evolving clinical challenges that the ED team will increasingly face into the future.”
For the study, Mr. Huang, a fourth-year medical student at Oakland University William Beaumont School of Medicine in Rochester, Mich., and his colleagues examined National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2015. They limited the analysis to emergency department visits with patients in the age groups of 65-74, 75-84, and 85 or older. For the primary outcome of interest, the researchers evaluated demographic variables of age group, sex, residential status, race and ethnicity, and insurance for association with hospital admission. For the secondary outcome of interest, they evaluated presenting ED complaints related to the clinical domains of cardiopulmonary disease, psychiatric disease, and fractures and dislocations for potential trends in the ED geriatric age group between 2011 and 2015.
Mr. Huang and his associates found that, as a percentage of total ED visits, those among patients aged 65 or older rose from 14.9% in 2011 to 15.6% in 2015, an increase of 4.7%. By age group, the proportion of visits during the study period was highest for those aged 65-74 years (43.8%), followed by those aged 75-84 years (34.7%) and those aged 85 and older (21.5%). On multivariate analysis, the 75-84 and age-85-and-older groups were 1.30 and 1.71 times more likely to be admitted to the hospital, compared with the 65-74 group, respectively (P less than .000 for both).
Men were 1.19 times more likely than were women to be admitted (P less than .000). In addition, elderly patients who reside in nursing homes were 1.70 times more likely to be admitted to the hospital, compared with those who lived in private homes (P less than .000), while those with Medicare insurance were 1.57 more likely to be admitted, compared with those who did not have insurance (P = .004).
On trend analysis, ED psychiatric complaints rose incrementally during the study period, from 3.9% in 2011 to 4.7% in 2015, a relative increase of 20.5%. The researchers identified no consistent trend with visit complaints related to cardiopulmonary disease, and fractures and dislocations.
“This was not too surprising, because these difficulties with older patients are not new and many investigators have sought out solutions,” Mr. Huang said. “For example, there have been many interventions both in the ED as well as in the primary care setting designed to identify risk factors and facilitate postdischarge care to prevent falls. These approaches are constantly evolving and will be of increasing importance.”
“For example, we may be seeing a larger of proportion of patients with acute mental health complaints,” Mr. Huang said. “We will need to continue developing our multidisciplinary approach to care by improving coordination with different specialties – and especially outpatient and community health care providers.”
The study’s senior author was Jason Wasserman, PhD of Oakland University William Beaumont School of Medicine. The researchers reported having no financial disclosures.
SOURCE: Huang D et al. Ann Emerg Med. 2018 Oct. doi: 10.1016/j.annemergmed.2018.08.212.
SAN DIEGO – Between 2011 and 2015, the proportion of elderly patients presenting to the emergency department with psychiatric complaints increased by 20%, according to a retrospective analysis of national hospital data.
In addition, 10-year increases in age, male sex, nursing home status, and Medicare insurance were associated with an increased likelihood of hospital admission.
“The growing geriatric patient population is a well-known phenomenon across every developed country,” lead researcher Derrick Huang said in an interview in advance of the annual meeting of the American College of Emergency Physicians. “A potent mix of increasing life expectancy, greater disease severity and comorbidities in the elderly, and large-scale demographic shifts has placed a significant strain on both our financial and health care resources. This study corroborates these trends in the emergency department and is a preliminary exploration of potential, newly evolving clinical challenges that the ED team will increasingly face into the future.”
For the study, Mr. Huang, a fourth-year medical student at Oakland University William Beaumont School of Medicine in Rochester, Mich., and his colleagues examined National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2015. They limited the analysis to emergency department visits with patients in the age groups of 65-74, 75-84, and 85 or older. For the primary outcome of interest, the researchers evaluated demographic variables of age group, sex, residential status, race and ethnicity, and insurance for association with hospital admission. For the secondary outcome of interest, they evaluated presenting ED complaints related to the clinical domains of cardiopulmonary disease, psychiatric disease, and fractures and dislocations for potential trends in the ED geriatric age group between 2011 and 2015.
Mr. Huang and his associates found that, as a percentage of total ED visits, those among patients aged 65 or older rose from 14.9% in 2011 to 15.6% in 2015, an increase of 4.7%. By age group, the proportion of visits during the study period was highest for those aged 65-74 years (43.8%), followed by those aged 75-84 years (34.7%) and those aged 85 and older (21.5%). On multivariate analysis, the 75-84 and age-85-and-older groups were 1.30 and 1.71 times more likely to be admitted to the hospital, compared with the 65-74 group, respectively (P less than .000 for both).
Men were 1.19 times more likely than were women to be admitted (P less than .000). In addition, elderly patients who reside in nursing homes were 1.70 times more likely to be admitted to the hospital, compared with those who lived in private homes (P less than .000), while those with Medicare insurance were 1.57 more likely to be admitted, compared with those who did not have insurance (P = .004).
On trend analysis, ED psychiatric complaints rose incrementally during the study period, from 3.9% in 2011 to 4.7% in 2015, a relative increase of 20.5%. The researchers identified no consistent trend with visit complaints related to cardiopulmonary disease, and fractures and dislocations.
“This was not too surprising, because these difficulties with older patients are not new and many investigators have sought out solutions,” Mr. Huang said. “For example, there have been many interventions both in the ED as well as in the primary care setting designed to identify risk factors and facilitate postdischarge care to prevent falls. These approaches are constantly evolving and will be of increasing importance.”
“For example, we may be seeing a larger of proportion of patients with acute mental health complaints,” Mr. Huang said. “We will need to continue developing our multidisciplinary approach to care by improving coordination with different specialties – and especially outpatient and community health care providers.”
The study’s senior author was Jason Wasserman, PhD of Oakland University William Beaumont School of Medicine. The researchers reported having no financial disclosures.
SOURCE: Huang D et al. Ann Emerg Med. 2018 Oct. doi: 10.1016/j.annemergmed.2018.08.212.
REPORTING FROM ACEP18
Key clinical point: An increasing proportion of elderly patients are presenting to the emergency department with mental health complaints.
Major finding: Emergency department psychiatric complaints among elderly patients rose from 3.9% in 2011, to 4.7% in 2015, a relative increase of 20.5%.
Study details: A retrospective analysis of National Ambulatory Medical Care Survey data between 2011 and 2015.
Disclosures: The researchers reported having no financial disclosures.
Source: Huang D et al. Ann Emerg Med. 2018 Oct. doi: 10.1016/j.annemergmed.2018.08.212.
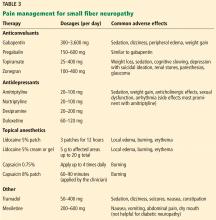
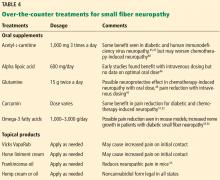
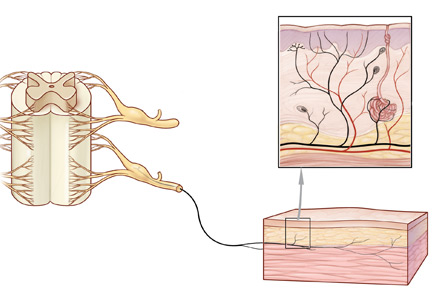
Office approach to small fiber neuropathy
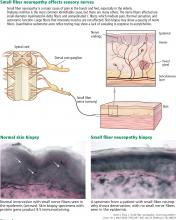
Peripheral neuropathy is the most common reason for an outpatient neurology visit in the United States and accounts for over $10 billion in healthcare spending each year.1,2 When the disorder affects only small, thinly myelinated or unmyelinated nerve fibers, it is referred to as small fiber neuropathy, which commonly presents as numbness and burning pain in the feet.
This article details the manifestations and evaluation of small fiber neuropathy, with an eye toward diagnosing an underlying cause amenable to treatment.
OLDER PATIENTS MOST AFFECTED
The epidemiology of small fiber neuropathy is not well established. It occurs more commonly in older patients, but data are mixed on prevalence by sex.3–6 In a Dutch study,3 the overall prevalence was at least 53 cases per 100,000, with the highest rate in men over age 65.
CHARACTERISTIC SENSORY DISTURBANCES
Sensations vary in quality and time
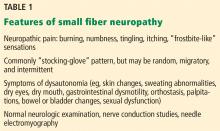
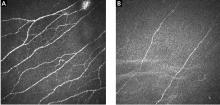
Patients with small fiber neuropathy typically present with a symmetric length-dependent (“stocking-glove”) distribution of sensory changes, starting in the feet and gradually ascending up the legs and then to the hands.
Commonly reported neuropathic symptoms include various combinations of burning, numbness, tingling, itching, sunburn-like, and frostbite-like sensations. Nonneuropathic symptoms may include tightness, a vise-like squeezing of the feet, and the sensation of a sock rolled up at the end of the shoe. Cramps or spasms may also be reported but rarely occur in isolation.7
Symptoms are typically worse at the end of the day and while sitting or lying down at night. They can arise spontaneously but may also be triggered by something as minor as the touch of clothing or cool air against the skin. Bedsheet sensitivity of the feet is reported so often that it is used as an outcome measure in clinical trials. Symptoms can also be exacerbated by extremes in ambient temperature and are especially worse in cold weather.
Random patterns suggest an immune cause
Symptoms may also have a non–length-dependent distribution that is asymmetric, patchy, intermittent, and migratory, and can involve the face, proximal limbs, and trunk. Symptoms may vary throughout the day, eg, starting with electric-shock sensations on one side of the face, followed by perineal numbness and then tingling in the arms lasting for a few minutes to several hours. While such patterns may be seen with diabetes and other common etiologies, they often suggest an underlying immune-mediated disorder such as Sjögren syndrome or sarcoidosis.8–10 Although large fiber polyneuropathy may also be non–length-dependent, the deficits are usually fixed, with no migratory component.
Autonomic features may be prominent
Autonomic symptoms occur in nearly half of patients and can be as troublesome as neuropathic pain.3 Small nerve fibers mediate somatic and autonomic functions, an evolutionary link that may reflect visceral defense mechanisms responding to pain as a signal of danger.11 This may help explain the multisystemic nature of symptoms, which can include sweating abnormalities, bowel and bladder disturbances, dry eyes, dry mouth, gastrointestinal dysmotility, skin changes (eg, discoloration, loss of hair, shiny skin), sexual dysfunction, orthostatic hypotension, and palpitations. In some cases, isolated dysautonomia may be seen.
TARGETED EXAMINATION
History: Medications, alcohol, infections
When a patient presents with neuropathic pain in the feet, a detailed history should be obtained, including alcohol use, family history of neuropathy, and use of neurotoxic medications such as metronidazole, colchicine, and chemotherapeutic agents.
Human immunodeficiency virus (HIV) and hepatitis C infection are well known to be associated with small fiber neuropathy, so relevant risk factors (eg, blood transfusions, sexual history, intravenous drug use) should be asked about. Recent illnesses and vaccinations are another important line of questioning, as a small-fiber variant of Guillain-Barré syndrome has been described.12
Assess reflexes, strength, sensation
On physical examination, particular attention should be focused on searching for abnormalities indicating large nerve fiber involvement (eg, absent deep tendon reflexes, weakness of the toes). However, absent ankle deep tendon reflexes and reduced vibratory sense may also occur in healthy elderly people.
Similarly, proprioception, motor strength, balance, and vibratory sensation are functions of large myelinated nerve fibers, and thus remain unaffected in patients with only small fiber neuropathy.
Evidence of a systemic disorder should also be sought, as it may indicate an underlying etiology.
DIAGNOSTIC TESTING
Although patients with either large or small fiber neuropathy may have subjective hyperesthesia or numbness of the distal lower extremities, the absence of significant abnormalities on neurologic examination should prompt consideration of small fiber neuropathy.
Electromyography worthwhile
Nerve conduction studies and needle electrode examination evaluate only large nerve fiber conditions. While electromyographic results are normal in patients with isolated small fiber neuropathy, the test can help evaluate subclinical large nerve fiber involvement and alternative diagnoses such as bilateral S1 radiculopathy. Nerve conduction studies may be less useful in patients over age 75, as they may lack sural sensory responses because of aging changes.13
Skin biopsy easy to do
Skin biopsy for evaluating intraepidermal nerve fiber density is one of the most widely used tests for small fiber neuropathy. This minimally invasive procedure can now be performed in a primary care office using readily available tools or prepackaged kits and analyzed by several commercial laboratories.
Reduced intraepidermal nerve fiber density on skin biopsy has been described in various other conditions such as fibromyalgia and chronic pain syndromes.16,17 The clinical significance of these findings remains uncertain.
Quantitative sudomotor axon reflex testing
Quantitative sudomotor axon reflex testing (QSART) is a noninvasive autonomic study that assesses the volume of sweat produced by the limbs in response to acetylcholine. A measure of postganglionic sympathetic sudomotor nerve function, QSART has a sensitivity of up to 80% and can be used to diagnose small fiber neuropathy.18 In a series of 115 patients with sarcoidosis small fiber neuropathy,9 the QSART and skin biopsy findings were concordant in 17 cases and complementary in 29, allowing for confirmation of small fiber neuropathy in patients whose condition would have remained undiagnosed had only one test been performed. QSART can also be considered in cases where skin biopsy may be contraindicated (eg, patient use of anticoagulation). Of note, the study may be affected by a number of external factors, including caffeine, tobacco, antihistamines, and tricyclic antidepressants; these should be held before testing.
Other diagnostic studies
Other tests may be helpful, as follows:
Tilt-table and cardiovagal testing may be useful for patients with orthostasis and palpitations.
Thermoregulatory sweat testing can be used to evaluate patients with abnormal patterns of sweating, eg, hyperhidrosis of the face and head.
INITIAL TESTING FOR AN UNDERLYING CAUSE
Glucose tolerance test for diabetes
Diabetes is the most common identifiable cause of small fiber neuropathy and accounts for about a third of all cases.5 Impaired glucose tolerance is also thought to be a risk factor and has been found in up to 50% of idiopathic cases, but the association is still being debated.21
While testing for hemoglobin A1c is more convenient for the patient, especially because it does not require fasting, a 2-hour oral glucose tolerance test is more sensitive for detecting glucose dysmetabolism.22
Lipid panel for metabolic syndrome
Small fiber neuropathy is associated with individual components of the metabolic syndrome, which include obesity, hyperglycemia, and dyslipidemia. Of these, dyslipidemia has emerged as the primary factor involved in the development of small fiber neuropathy, via an inflammatory pathway or oxidative stress mechanism.23,24
Vitamin B12 deficiency testing
Vitamin B12 deficiency, a potentially correctable cause of small fiber neuropathy, may be underdiagnosed, especially as values obtained by blood testing may not reflect tissue uptake. Causes of vitamin B12 deficiency include reduced intake, pernicious anemia, and medications that can affect absorption of vitamin B12 (eg, proton pump inhibitors, histamine 2 receptor antagonists, metformin).
Testing should include:
- Complete blood cell count to evaluate for vitamin B12-related macrocytic anemia and other hematologic abnormalities
- Serum vitamin B12 level
- Methylmalonic acid or homocysteine level in patients with subclinical or mild vitamin B12 deficiency, manifested as low to normal vitamin B12 levels (< 400 pg/mL); methylmalonic acid and homocysteine require vitamin B12 as a cofactor for enzymatic conversion, and either or both may be elevated in early vitamin B12 deficiency.
Celiac antibody panel
Celiac disease, a T-cell mediated enteropathy characterized by gluten intolerance and a herpetiform-like rash, can be associated with small fiber neuropathy.25 In some cases, neuropathy symptoms are preceded by the onset of gastrointestinal symptoms, or they may occur in isolation.25
Inflammatory disease testing
Sjögren syndrome accounts for nearly 10% of cases of small fiber neuropathy. Associated neuropathic symptoms are often non–length-dependent, can precede sicca symptoms for up to 6 years, and in some cases are the sole manifestation of the disease.10 Small fiber neuropathy may also be associated with vasculitis, systemic lupus erythematosus, and other connective tissue disorders.
Testing should include:
- Erythrocyte sedimentation rate, C-reactive protein, and antinuclear antibodies: though these are nonspecific markers of inflammation, they may support an immune-mediated etiology if positive
- Extractable nuclear antigen panel: Sjögren syndrome A and B autoantibodies are the most important components in this setting5,11
- The Schirmer test or salivary gland biopsy should be considered for seronegative patients with sicca or a suspected immune-mediated etiology, as the sensitivity of antibody testing ranges from only 10% to 55%.10
Thyroid function testing
Hypothyroidism, and less commonly hyperthyroidism, are associated with small fiber neuropathy.
Metabolic tests for liver and kidney disease
Renal insufficiency and liver impairment are well-known causes of small nerve fiber dysfunction. Testing should include:
- Comprehensive metabolic panel
- Gamma-glutamyltransferase if alcohol abuse is suspected, since heavy alcohol use is one of the most common causes of both large and small fiber neuropathy.
HIV and hepatitis C testing
For patients with relevant risk factors, HIV and hepatitis C testing should be part of the initial workup (and as second-tier testing for others). Patients who test positive for hepatitis C should undergo further testing for cryoglobulinemia, which can present with painful small fiber neuropathy.26
Serum and urine immunoelectrophoresis
Paraproteinemia, with causes ranging from monoclonal gammopathy of uncertain significance to multiple myeloma, has been associated with small fiber neuropathy. An abnormal serum or urine immunoelectrophoresis test warrants further investigation and possibly referral to a hematology-oncology specialist.
SECOND-TIER TESTING
Less common treatable causes of small fiber neuropathy may also be evaluated.
Copper, vitamin B1 (thiamine), or vitamin B6 (pyridoxine) deficiency testing. Although vitamin B6 toxicity may also result in neuropathy due to its toxic effect on the dorsal root ganglia, the mildly elevated vitamin B6 levels often found in patients being evaluated for neuropathy are unlikely to be the primary cause of symptoms. Many laboratories require fasting samples for accurate vitamin B6 levels.
Angiotensin-converting enzyme levels for sarcoidosis. Small fiber neuropathy is common in sarcoidosis, occurring in more than 30% of patients with systemic disease.27 However, screening for sarcoidosis by measuring serum levels is often falsely positive and is not cost-effective. In a study of 195 patients with idiopathic small fiber neuropathy,11 44% had an elevated serum level, but no evidence of sarcoidosis was seen on further testing, which included computed tomography of the chest in 29 patients.12 Thus, this test is best used for patients with evidence of systemic disease.
Amyloid testing for amyloidosis. Fat pad or bone marrow biopsy should be considered in the appropriate clinical setting.
Paraneoplastic autoantibody panel for occult cancer. Such testing may also be considered if clinically warranted. However, if a patient is found to have low positive titers of paraneoplastic antibodies and suspicion is low for an occult cancer (eg, no weight loss or early satiety), repeat confirmatory testing at another laboratory should be done before embarking on an extensive search for malignancy.
Ganglionic acetylcholine receptor antibody testing for autoimmune autonomic ganglionopathy. This should be ordered for patients with prominent autonomic dysfunction. The antibody test can be ordered separately or as part of an autoantibody panel. The antibody may indicate a primary immune-mediated process or a paraneoplastic disease.28
Genetic mutation testing. Recent discoveries of gene mutations leading to peripheral nerve hyperexcitability of voltage-gated sodium channels have elucidated a hereditary cause of small fiber neuropathy in nearly 30% of cases that were once thought to be idiopathic.29,30 Genetic testing for mutations in SCN9A and SCN10 (which code for the Nav1.7 and Nav1.8 sodium channels, respectively) is commercially available and may be considered for those with a family history of neuropathic pain in the feet or for young, otherwise healthy patients.
Fabry disease is an X-linked lysosomal disorder characterized by angiokeratomas, cardiac and renal impairment, and small fiber neuropathy. Treatment is now available, but screening is not cost-efficient and should only be pursued in patients with other symptoms of the disease.31,32
OTHER POSSIBLE CAUSES
Guillain-Barré syndrome
A Guillain-Barré syndrome variant has been reported that is characterized by ascending limb paresthesias and cerebrospinal fluid albuminocytologic dissociation in the setting of preserved deep tendon reflexes and normal findings on EMG.12 The clinical course is similar to that of typical Guillain-Barré syndrome, in that symptoms follow an upper respiratory or gastrointestinal tract infection, reach their nadir at 4 weeks, and then gradually improve. Some patients respond to intravenous immune globulin.
Vaccine-associated
Postvaccination small fiber neuropathy has also been reported. The nature of the association is unclear.33
Parkinson disease
Small fiber neuropathy is associated with Parkinson disease. It is attributed to a number of proposed factors, including neurodegeneration that occurs parallel to central nervous system decline, as well as intestinal malabsorption with resultant vitamin deficiency.34,35
Rapid glycemic lowering
Aggressive treatment of diabetes, defined as at least a 2-point reduction of serum hemoglobin A1c level over 3 months, may result in acute small fiber neuropathy. It manifests as severe distal extremity pain and dysautonomia.
In a retrospective study,36 104 (10.9%) of 954 patients presenting to a tertiary diabetic clinic developed treatment-induced diabetic neuropathy with symptoms occurring within 8 weeks of rapid glycemic control. The severity of neuropathy correlated with the degree and rate of glycemic lowering. The condition was reversible in some cases.
TREATING SPECIFIC DISORDERS
For patients with an identified cause of neuropathy, targeted treatment offers the best chance of halting progression and possibly improving symptoms. Below are recommendations for addressing neuropathy associated with the common diagnoses.
Diabetes, impaired glucose tolerance, and metabolic syndrome. In addition to glycemic- and lipid-lowering therapies, lifestyle modifications with a specific focus on exercise and nutrition are integral to treating diabetes and related disorders.
In the Look AHEAD (Action for Health in Diabetes) study,37 which evaluated the effects of intensive lifestyle intervention on neuropathy in 5,145 overweight patients with type 2 diabetes, patients in the intervention group had lower pain scores and better touch sensation in the toes compared with controls at 1 year. Differences correlated with the degree of weight loss and reduction of hemoglobin A1c and lipid levels.
As running and walking may not be feasible for many patients owing to pain, stationary cycling, aqua therapy, and swimming are other options. A stationary recumbent bike may be useful for older patients with balance issues.
Vitamin B12 deficiency. As reduced absorption rather than low dietary intake is the primary cause of vitamin B12 deficiency for many patients, parenteral rather than oral supplementation may be best. A suggested regimen is subcutaneous or intramuscular methylcobalamin injection of 1,000 µg given daily for 1 week, then once weekly for 1 month, followed by a maintenance dose once a month for at least 6 to 12 months. Alternatively, a daily dose of vitamin B12 1,000 µg can be taken sublingually.
Sjögren syndrome. According to anecdotal case reports, intravenous immune globulin, corticosteroids, and other immunosuppressants help painful small fiber neuropathy and dysautonomia associated with Sjögren syndrome.10
Sarcoidosis. Sarcoidosis-associated small fiber neuropathy may also respond to intravenous immune globulin, as well as infliximab and combination therapy.9 Culver et al38 found that cibinetide, an experimental erythropoetin agonist, resulted in improved corneal nerve fiber measures in patients with small fiber neuropathy associated with sarcoidosis.
Celiac disease. A gluten-free diet is the treatment for celiac disease and can help some patients.
GENERAL MANAGEMENT
For all patients, regardless of whether the cause of small fiber neuropathy has been identified, managing symptoms remains key, as pain and autonomic dysfunction can markedly impair quality of life. A multidisciplinary approach that incorporates pain medications, physical therapy, and lifestyle modifications is ideal. Integrative holistic treatments such as natural supplements, yoga, and other mind-body therapies may also help.
Pain control
Mexiletine, a voltage-gated sodium channel blocker used as an antiarrhythmic, may help refractory pain or hereditary small fiber neuropathy related to sodium channel dysfunction. However, it is not recommended for diabetic neuropathy.39
Combination regimens that use drugs with different mechanisms of action can be effective. In one study, combined gabapentin and nortriptyline were more effective than either drug alone for neuropathic pain.40
Inhaled cannabis reduced pain in patients with HIV and diabetic neuropathy in a number of studies. Side effects included euphoria, somnolence, and cognitive impairment.41,42 The use of medical marijuana is not yet legal nationwide and may affect employability even in states in which it has been legalized.
Owing to the opioid epidemic and high addiction potential, opioids are no longer a preferred recommendation for chronic treatment of noncancer-related neuropathy. A population-based study of 2,892 patients with neuropathy found that those on chronic opioid therapy (≥ 90 days) had worse functional outcomes and higher rates of addiction and overdose than those on short-term therapy.43 However, the opioid agonist tramadol was found to be effective in reducing neuropathic pain and may be a safer option for patients with chronic small fiber neuropathy.44
Integrative, holistic therapies
PROGNOSIS
For many patients, small fiber neuropathy is a slowly progressive disorder that reaches a clinical plateau lasting for years, with progression to large fiber involvement reported in 13% to 36% of cases; over half of patients in one series either improved or remained stable over a period of 2 years.5,57 Long-term studies are needed to fully understand the natural disease course. In the meantime, treating underlying disease and managing symptoms are imperative to patient care.
- Burke JF, Skolarus LE, Callaghan BC, Kerber KA. Choosing Wisely: highest-cost tests in outpatient neurology. Ann Neurol 2013; 73(5):679–683. doi:10.1002/ana.23865
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26(6):1790–1795. pmid:12766111
- Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: a survey in the Netherlands. Neurology 2013; 81(15):1356–1360. doi:10.1212/WNL.0b013e3182a8236e
- Periquet MI, Novak V, Collins MP, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology 1999; 53(8):1641–1647. pmid:10563606
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131(pt 7):1912–1925. doi:10.1093/brain/awn093
- Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002; 26(2):173–188. doi:10.1002/mus.10181
- Lopate G, Streif E, Harms M, Weihl C, Pestronk A. Cramps and small-fiber neuropathy. Muscle Nerve 2013; 48(2):252–255. doi:10.1002/mus.23757
- Khan S, Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve 2012; 45(1):86–91. doi:10.1002/mus.22255
- Tavee JO, Karwa K, Ahmed Z, Thompson N, Parambil J, Culver DA. Sarcoidosis-associated small fiber neuropathy in a large cohort: clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir Med 2017; 126:135–138. doi:10.1016/j.rmed.2017.03.011
- Berkowitz AL, Samuels MA. The neurology of Sjogren’s syndrome and the rheumatology of peripheral neuropathy and myelitis. Pract Neurol 2014; 14(1):14–22. doi:10.1136/practneurol-2013-000651
- Lang M, Treister R, Oaklander AL. Diagnostic value of blood tests for occult causes of initially idiopathic small-fiber polyneuropathy. J Neurol 2016; 263(12):2515–2527. doi:10.1007/s00415-016-8270-5
- Seneviratne U, Gunasekera S. Acute small fibre sensory neuropathy: another variant of Guillain-Barré syndrome? J Neurol Neurosurg Psychiatry 2002; 72(4):540–542. pmid:11909922
- Tavee JO, Polston D, Zhou L, Shields RW, Butler RS, Levin KH. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve 2014; 49(4):564–569. doi:10.1002/mus.23971
- Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med 2009; 76(5):297–305. doi:10.3949/ccjm.76a.08070
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999; 53(8):1634–1640. pmid:10563605
- Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013; 154(11):2310–2316. doi:10.1016/j.pain.2013.06.001
- Üçeyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136(pt 6):1857–1867. doi:10.1093/brain/awt053
- Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992; 15(6):661–665. doi:10.1002/mus.880150605
- Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003; 46(5):683–688. doi:10.1007/s00125-003-1086-8
- de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy—a large cohort study and review of the literature. Eur J Neurol 2018; 25(2):348–355. doi:10.1111/ene.13508
- Smith AG. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst 2012; 17(suppl 2):15–21. doi:10.1111/j.1529-8027.2012.00390.x
- Hoffman-Snyder C, Smith BE, Ross MA, Hernandez J, Bosch EP. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006; 63(8):1075–1079. doi:10.1001/archneur.63.8.noc50336
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 2009; 14(4):257–267. doi:10.1111/j.1529-8027.2009.00237.x
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009; 58(7):1634–1640. doi:10.2337/db08-1771
- Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology 2003; 60(10):1581–1585. pmid:12771245
- Gemignani F, Brindani F, Alfieri S, et al. Clinical spectrum of cryoglobulinaemic neuropathy. J Neurol Neurosurg Psychiatry 2005; 76(10):1410–1414. doi:10.1136/jnnp.2004.057620
- Bakkers M, Merkies IS, Lauria G, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology 2009; 73(14):1142–1148. doi:10.1212/WNL.0b013e3181bacf05
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012; 71(1):26–39. doi:10.1002/ana.22485
- Brouwer BA, Merkies IS, Gerrits MM, Waxman SG, Hoeijmakers JG, Faber CG. Painful neuropathies: the emerging role of sodium channelopathies. J Peripher Nerv Syst 2014; 19(2):53–65. doi:10.1111/jns5.12071
- Samuelsson K, Kostulas K, Vrethem M, Rolfs A, Press R. Idiopathic small fiber neuropathy: phenotype, etiologies, and the search for Fabry disease. J Clin Neurol 2014; 10(2):108–118. doi:10.3988/jcn.2014.10.2.108
- de Greef BT, Hoeijmakers JG, Wolters EE, et al. No Fabry disease in patients presenting with isolated small fiber neuropathy. PLoS One 2016; 11(2):e0148316. doi:10.1371/journal.pone.0148316
- Souayah N, Ajroud-Driss S, Sander HW, Brannagan TH, Hays AP, Chin RL. Small fiber neuropathy following vaccination for rabies, varicella or Lyme disease. Vaccine 2009; 27(52):7322–7325. doi:10.1016/j.vaccine.2009.09.077
- Nolano M, Provitera V, Manganelli F, et al. Loss of cutaneous large and small fibers in naive and l-dopa–treated PD patients. Neurology 2017; 89(8):776–784. doi:10.1212/WNL.0000000000004274
- Zis P, Grünewald RA, Chaudhuri RK, Hadjivassiliou M. Peripheral neuropathy in idiopathic Parkinson’s disease: a systematic review. J Neurol Sci 2017; 378:204–209. doi:10.1016/j.jns.2017.05.023
- Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015; 138(pt 1):43–52. doi:10.1093/brain/awu307
- Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia 2017; 60(6):980–988. doi:10.1007/s00125-017-4253-z
- Culver DA, Dahan A, Bajorunas D, et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci 2017; 58(6):BIO52–BIO60. doi:10.1167/iovs.16-21291
- Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2011; 3(4):345–352.e21. doi:10.1016/j.pmrj.2011.03.008
- Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009; 374(9697):1252–1261. doi:10.1016/S0140-6736(09)61081-3
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ. Association of long-term opioid therapy with functional status, adverse outcomes, and mortality among patients with polyneuropathy. JAMA Neurol 2017; 74(7):773–779. doi:10.1001/jamaneurol.2017.0486
- Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998; 50(6):1842–1846. pmid:9633738
- Sima AA, Calvani M, Mehra M, Amato A; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care 2005; 28(1):89–94. pmid:15616239
- Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995; 38(12):1425–1433. pmid:8786016
- Scarpini E, Sacilotto G, Baron P, Cusini M, Scarlato G. Effect of acetyl-L-carnitine in the treatment of painful peripheral neuropathies in HIV+ patients. J Peripher Nerv Syst 1997; 2(3):250-252. pmid: 10975731
- Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 2013; 31(20):2627-2633. doi:10.1200/JCO.2012.44.8738
- Amara S. Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann Pharmacother 2008; 42(10):1481-1485. doi:10.1345/aph.1L179
- Huang JS, Wu CL, Fan CW, Chen WH, Yeh KY, Chang PH. Intravenous glutamine appears to reduce the severity of symptomatic platinum-induced neuropathy: a prospective randomized study. J Chemother 2015; 27(4):235-240. doi:10.1179/1973947815Y.0000000011
- Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 2014; 723:202-206. doi:10.1016/j.ejphar.2013.11.033
- Mendonça LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi MD, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology 2013; 34:205-211. doi:10.1016/j.neuro.2012.09.011
- Wagner K, Lee KS, Yang J, Hammock BD. Epoxy fatty acids mediate analgesia in murine diabetic neuropathy. Eur J Pain 2017; 21(3):456-465. doi:10.1002/ejp.939
- Lewis EJ, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: a 12-month pilot trial. Neurology 2017; 88(24):2294–2301. doi:10.1212/WNL.0000000000004033
- Hu D, Wang C, Li F, et al. A combined water extract of frankincense and myrrh alleviates neuropathic pain in mice via modulation of TRPV1. Neural Plast 2017; 2017:3710821. doi:10.1155/2017/3710821
- Tavee J, Rensel M, Planchon SM, Butler RS, Stone L. Effects of meditation on pain and quality of life in multiple sclerosis and peripheral neuropathy: a pilot study. Int J MS Care 2011; 13(4):163–168. doi:10.7224/1537-2073-13.4.163
- Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol 2016; 73(6):684–690. doi:10.1001/jamaneurol.2016.0057
Peripheral neuropathy is the most common reason for an outpatient neurology visit in the United States and accounts for over $10 billion in healthcare spending each year.1,2 When the disorder affects only small, thinly myelinated or unmyelinated nerve fibers, it is referred to as small fiber neuropathy, which commonly presents as numbness and burning pain in the feet.
This article details the manifestations and evaluation of small fiber neuropathy, with an eye toward diagnosing an underlying cause amenable to treatment.
OLDER PATIENTS MOST AFFECTED
The epidemiology of small fiber neuropathy is not well established. It occurs more commonly in older patients, but data are mixed on prevalence by sex.3–6 In a Dutch study,3 the overall prevalence was at least 53 cases per 100,000, with the highest rate in men over age 65.
CHARACTERISTIC SENSORY DISTURBANCES
Sensations vary in quality and time
Patients with small fiber neuropathy typically present with a symmetric length-dependent (“stocking-glove”) distribution of sensory changes, starting in the feet and gradually ascending up the legs and then to the hands.
Commonly reported neuropathic symptoms include various combinations of burning, numbness, tingling, itching, sunburn-like, and frostbite-like sensations. Nonneuropathic symptoms may include tightness, a vise-like squeezing of the feet, and the sensation of a sock rolled up at the end of the shoe. Cramps or spasms may also be reported but rarely occur in isolation.7
Symptoms are typically worse at the end of the day and while sitting or lying down at night. They can arise spontaneously but may also be triggered by something as minor as the touch of clothing or cool air against the skin. Bedsheet sensitivity of the feet is reported so often that it is used as an outcome measure in clinical trials. Symptoms can also be exacerbated by extremes in ambient temperature and are especially worse in cold weather.
Random patterns suggest an immune cause
Symptoms may also have a non–length-dependent distribution that is asymmetric, patchy, intermittent, and migratory, and can involve the face, proximal limbs, and trunk. Symptoms may vary throughout the day, eg, starting with electric-shock sensations on one side of the face, followed by perineal numbness and then tingling in the arms lasting for a few minutes to several hours. While such patterns may be seen with diabetes and other common etiologies, they often suggest an underlying immune-mediated disorder such as Sjögren syndrome or sarcoidosis.8–10 Although large fiber polyneuropathy may also be non–length-dependent, the deficits are usually fixed, with no migratory component.
Autonomic features may be prominent
Autonomic symptoms occur in nearly half of patients and can be as troublesome as neuropathic pain.3 Small nerve fibers mediate somatic and autonomic functions, an evolutionary link that may reflect visceral defense mechanisms responding to pain as a signal of danger.11 This may help explain the multisystemic nature of symptoms, which can include sweating abnormalities, bowel and bladder disturbances, dry eyes, dry mouth, gastrointestinal dysmotility, skin changes (eg, discoloration, loss of hair, shiny skin), sexual dysfunction, orthostatic hypotension, and palpitations. In some cases, isolated dysautonomia may be seen.
TARGETED EXAMINATION
History: Medications, alcohol, infections
When a patient presents with neuropathic pain in the feet, a detailed history should be obtained, including alcohol use, family history of neuropathy, and use of neurotoxic medications such as metronidazole, colchicine, and chemotherapeutic agents.
Human immunodeficiency virus (HIV) and hepatitis C infection are well known to be associated with small fiber neuropathy, so relevant risk factors (eg, blood transfusions, sexual history, intravenous drug use) should be asked about. Recent illnesses and vaccinations are another important line of questioning, as a small-fiber variant of Guillain-Barré syndrome has been described.12
Assess reflexes, strength, sensation
On physical examination, particular attention should be focused on searching for abnormalities indicating large nerve fiber involvement (eg, absent deep tendon reflexes, weakness of the toes). However, absent ankle deep tendon reflexes and reduced vibratory sense may also occur in healthy elderly people.
Similarly, proprioception, motor strength, balance, and vibratory sensation are functions of large myelinated nerve fibers, and thus remain unaffected in patients with only small fiber neuropathy.
Evidence of a systemic disorder should also be sought, as it may indicate an underlying etiology.
DIAGNOSTIC TESTING
Although patients with either large or small fiber neuropathy may have subjective hyperesthesia or numbness of the distal lower extremities, the absence of significant abnormalities on neurologic examination should prompt consideration of small fiber neuropathy.
Electromyography worthwhile
Nerve conduction studies and needle electrode examination evaluate only large nerve fiber conditions. While electromyographic results are normal in patients with isolated small fiber neuropathy, the test can help evaluate subclinical large nerve fiber involvement and alternative diagnoses such as bilateral S1 radiculopathy. Nerve conduction studies may be less useful in patients over age 75, as they may lack sural sensory responses because of aging changes.13
Skin biopsy easy to do
Skin biopsy for evaluating intraepidermal nerve fiber density is one of the most widely used tests for small fiber neuropathy. This minimally invasive procedure can now be performed in a primary care office using readily available tools or prepackaged kits and analyzed by several commercial laboratories.
Reduced intraepidermal nerve fiber density on skin biopsy has been described in various other conditions such as fibromyalgia and chronic pain syndromes.16,17 The clinical significance of these findings remains uncertain.
Quantitative sudomotor axon reflex testing
Quantitative sudomotor axon reflex testing (QSART) is a noninvasive autonomic study that assesses the volume of sweat produced by the limbs in response to acetylcholine. A measure of postganglionic sympathetic sudomotor nerve function, QSART has a sensitivity of up to 80% and can be used to diagnose small fiber neuropathy.18 In a series of 115 patients with sarcoidosis small fiber neuropathy,9 the QSART and skin biopsy findings were concordant in 17 cases and complementary in 29, allowing for confirmation of small fiber neuropathy in patients whose condition would have remained undiagnosed had only one test been performed. QSART can also be considered in cases where skin biopsy may be contraindicated (eg, patient use of anticoagulation). Of note, the study may be affected by a number of external factors, including caffeine, tobacco, antihistamines, and tricyclic antidepressants; these should be held before testing.
Other diagnostic studies
Other tests may be helpful, as follows:
Tilt-table and cardiovagal testing may be useful for patients with orthostasis and palpitations.
Thermoregulatory sweat testing can be used to evaluate patients with abnormal patterns of sweating, eg, hyperhidrosis of the face and head.
INITIAL TESTING FOR AN UNDERLYING CAUSE
Glucose tolerance test for diabetes
Diabetes is the most common identifiable cause of small fiber neuropathy and accounts for about a third of all cases.5 Impaired glucose tolerance is also thought to be a risk factor and has been found in up to 50% of idiopathic cases, but the association is still being debated.21
While testing for hemoglobin A1c is more convenient for the patient, especially because it does not require fasting, a 2-hour oral glucose tolerance test is more sensitive for detecting glucose dysmetabolism.22
Lipid panel for metabolic syndrome
Small fiber neuropathy is associated with individual components of the metabolic syndrome, which include obesity, hyperglycemia, and dyslipidemia. Of these, dyslipidemia has emerged as the primary factor involved in the development of small fiber neuropathy, via an inflammatory pathway or oxidative stress mechanism.23,24
Vitamin B12 deficiency testing
Vitamin B12 deficiency, a potentially correctable cause of small fiber neuropathy, may be underdiagnosed, especially as values obtained by blood testing may not reflect tissue uptake. Causes of vitamin B12 deficiency include reduced intake, pernicious anemia, and medications that can affect absorption of vitamin B12 (eg, proton pump inhibitors, histamine 2 receptor antagonists, metformin).
Testing should include:
- Complete blood cell count to evaluate for vitamin B12-related macrocytic anemia and other hematologic abnormalities
- Serum vitamin B12 level
- Methylmalonic acid or homocysteine level in patients with subclinical or mild vitamin B12 deficiency, manifested as low to normal vitamin B12 levels (< 400 pg/mL); methylmalonic acid and homocysteine require vitamin B12 as a cofactor for enzymatic conversion, and either or both may be elevated in early vitamin B12 deficiency.
Celiac antibody panel
Celiac disease, a T-cell mediated enteropathy characterized by gluten intolerance and a herpetiform-like rash, can be associated with small fiber neuropathy.25 In some cases, neuropathy symptoms are preceded by the onset of gastrointestinal symptoms, or they may occur in isolation.25
Inflammatory disease testing
Sjögren syndrome accounts for nearly 10% of cases of small fiber neuropathy. Associated neuropathic symptoms are often non–length-dependent, can precede sicca symptoms for up to 6 years, and in some cases are the sole manifestation of the disease.10 Small fiber neuropathy may also be associated with vasculitis, systemic lupus erythematosus, and other connective tissue disorders.
Testing should include:
- Erythrocyte sedimentation rate, C-reactive protein, and antinuclear antibodies: though these are nonspecific markers of inflammation, they may support an immune-mediated etiology if positive
- Extractable nuclear antigen panel: Sjögren syndrome A and B autoantibodies are the most important components in this setting5,11
- The Schirmer test or salivary gland biopsy should be considered for seronegative patients with sicca or a suspected immune-mediated etiology, as the sensitivity of antibody testing ranges from only 10% to 55%.10
Thyroid function testing
Hypothyroidism, and less commonly hyperthyroidism, are associated with small fiber neuropathy.
Metabolic tests for liver and kidney disease
Renal insufficiency and liver impairment are well-known causes of small nerve fiber dysfunction. Testing should include:
- Comprehensive metabolic panel
- Gamma-glutamyltransferase if alcohol abuse is suspected, since heavy alcohol use is one of the most common causes of both large and small fiber neuropathy.
HIV and hepatitis C testing
For patients with relevant risk factors, HIV and hepatitis C testing should be part of the initial workup (and as second-tier testing for others). Patients who test positive for hepatitis C should undergo further testing for cryoglobulinemia, which can present with painful small fiber neuropathy.26
Serum and urine immunoelectrophoresis
Paraproteinemia, with causes ranging from monoclonal gammopathy of uncertain significance to multiple myeloma, has been associated with small fiber neuropathy. An abnormal serum or urine immunoelectrophoresis test warrants further investigation and possibly referral to a hematology-oncology specialist.
SECOND-TIER TESTING
Less common treatable causes of small fiber neuropathy may also be evaluated.
Copper, vitamin B1 (thiamine), or vitamin B6 (pyridoxine) deficiency testing. Although vitamin B6 toxicity may also result in neuropathy due to its toxic effect on the dorsal root ganglia, the mildly elevated vitamin B6 levels often found in patients being evaluated for neuropathy are unlikely to be the primary cause of symptoms. Many laboratories require fasting samples for accurate vitamin B6 levels.
Angiotensin-converting enzyme levels for sarcoidosis. Small fiber neuropathy is common in sarcoidosis, occurring in more than 30% of patients with systemic disease.27 However, screening for sarcoidosis by measuring serum levels is often falsely positive and is not cost-effective. In a study of 195 patients with idiopathic small fiber neuropathy,11 44% had an elevated serum level, but no evidence of sarcoidosis was seen on further testing, which included computed tomography of the chest in 29 patients.12 Thus, this test is best used for patients with evidence of systemic disease.
Amyloid testing for amyloidosis. Fat pad or bone marrow biopsy should be considered in the appropriate clinical setting.
Paraneoplastic autoantibody panel for occult cancer. Such testing may also be considered if clinically warranted. However, if a patient is found to have low positive titers of paraneoplastic antibodies and suspicion is low for an occult cancer (eg, no weight loss or early satiety), repeat confirmatory testing at another laboratory should be done before embarking on an extensive search for malignancy.
Ganglionic acetylcholine receptor antibody testing for autoimmune autonomic ganglionopathy. This should be ordered for patients with prominent autonomic dysfunction. The antibody test can be ordered separately or as part of an autoantibody panel. The antibody may indicate a primary immune-mediated process or a paraneoplastic disease.28
Genetic mutation testing. Recent discoveries of gene mutations leading to peripheral nerve hyperexcitability of voltage-gated sodium channels have elucidated a hereditary cause of small fiber neuropathy in nearly 30% of cases that were once thought to be idiopathic.29,30 Genetic testing for mutations in SCN9A and SCN10 (which code for the Nav1.7 and Nav1.8 sodium channels, respectively) is commercially available and may be considered for those with a family history of neuropathic pain in the feet or for young, otherwise healthy patients.
Fabry disease is an X-linked lysosomal disorder characterized by angiokeratomas, cardiac and renal impairment, and small fiber neuropathy. Treatment is now available, but screening is not cost-efficient and should only be pursued in patients with other symptoms of the disease.31,32
OTHER POSSIBLE CAUSES
Guillain-Barré syndrome
A Guillain-Barré syndrome variant has been reported that is characterized by ascending limb paresthesias and cerebrospinal fluid albuminocytologic dissociation in the setting of preserved deep tendon reflexes and normal findings on EMG.12 The clinical course is similar to that of typical Guillain-Barré syndrome, in that symptoms follow an upper respiratory or gastrointestinal tract infection, reach their nadir at 4 weeks, and then gradually improve. Some patients respond to intravenous immune globulin.
Vaccine-associated
Postvaccination small fiber neuropathy has also been reported. The nature of the association is unclear.33
Parkinson disease
Small fiber neuropathy is associated with Parkinson disease. It is attributed to a number of proposed factors, including neurodegeneration that occurs parallel to central nervous system decline, as well as intestinal malabsorption with resultant vitamin deficiency.34,35
Rapid glycemic lowering
Aggressive treatment of diabetes, defined as at least a 2-point reduction of serum hemoglobin A1c level over 3 months, may result in acute small fiber neuropathy. It manifests as severe distal extremity pain and dysautonomia.
In a retrospective study,36 104 (10.9%) of 954 patients presenting to a tertiary diabetic clinic developed treatment-induced diabetic neuropathy with symptoms occurring within 8 weeks of rapid glycemic control. The severity of neuropathy correlated with the degree and rate of glycemic lowering. The condition was reversible in some cases.
TREATING SPECIFIC DISORDERS
For patients with an identified cause of neuropathy, targeted treatment offers the best chance of halting progression and possibly improving symptoms. Below are recommendations for addressing neuropathy associated with the common diagnoses.
Diabetes, impaired glucose tolerance, and metabolic syndrome. In addition to glycemic- and lipid-lowering therapies, lifestyle modifications with a specific focus on exercise and nutrition are integral to treating diabetes and related disorders.
In the Look AHEAD (Action for Health in Diabetes) study,37 which evaluated the effects of intensive lifestyle intervention on neuropathy in 5,145 overweight patients with type 2 diabetes, patients in the intervention group had lower pain scores and better touch sensation in the toes compared with controls at 1 year. Differences correlated with the degree of weight loss and reduction of hemoglobin A1c and lipid levels.
As running and walking may not be feasible for many patients owing to pain, stationary cycling, aqua therapy, and swimming are other options. A stationary recumbent bike may be useful for older patients with balance issues.
Vitamin B12 deficiency. As reduced absorption rather than low dietary intake is the primary cause of vitamin B12 deficiency for many patients, parenteral rather than oral supplementation may be best. A suggested regimen is subcutaneous or intramuscular methylcobalamin injection of 1,000 µg given daily for 1 week, then once weekly for 1 month, followed by a maintenance dose once a month for at least 6 to 12 months. Alternatively, a daily dose of vitamin B12 1,000 µg can be taken sublingually.
Sjögren syndrome. According to anecdotal case reports, intravenous immune globulin, corticosteroids, and other immunosuppressants help painful small fiber neuropathy and dysautonomia associated with Sjögren syndrome.10
Sarcoidosis. Sarcoidosis-associated small fiber neuropathy may also respond to intravenous immune globulin, as well as infliximab and combination therapy.9 Culver et al38 found that cibinetide, an experimental erythropoetin agonist, resulted in improved corneal nerve fiber measures in patients with small fiber neuropathy associated with sarcoidosis.
Celiac disease. A gluten-free diet is the treatment for celiac disease and can help some patients.
GENERAL MANAGEMENT
For all patients, regardless of whether the cause of small fiber neuropathy has been identified, managing symptoms remains key, as pain and autonomic dysfunction can markedly impair quality of life. A multidisciplinary approach that incorporates pain medications, physical therapy, and lifestyle modifications is ideal. Integrative holistic treatments such as natural supplements, yoga, and other mind-body therapies may also help.
Pain control
Mexiletine, a voltage-gated sodium channel blocker used as an antiarrhythmic, may help refractory pain or hereditary small fiber neuropathy related to sodium channel dysfunction. However, it is not recommended for diabetic neuropathy.39
Combination regimens that use drugs with different mechanisms of action can be effective. In one study, combined gabapentin and nortriptyline were more effective than either drug alone for neuropathic pain.40
Inhaled cannabis reduced pain in patients with HIV and diabetic neuropathy in a number of studies. Side effects included euphoria, somnolence, and cognitive impairment.41,42 The use of medical marijuana is not yet legal nationwide and may affect employability even in states in which it has been legalized.
Owing to the opioid epidemic and high addiction potential, opioids are no longer a preferred recommendation for chronic treatment of noncancer-related neuropathy. A population-based study of 2,892 patients with neuropathy found that those on chronic opioid therapy (≥ 90 days) had worse functional outcomes and higher rates of addiction and overdose than those on short-term therapy.43 However, the opioid agonist tramadol was found to be effective in reducing neuropathic pain and may be a safer option for patients with chronic small fiber neuropathy.44
Integrative, holistic therapies
PROGNOSIS
For many patients, small fiber neuropathy is a slowly progressive disorder that reaches a clinical plateau lasting for years, with progression to large fiber involvement reported in 13% to 36% of cases; over half of patients in one series either improved or remained stable over a period of 2 years.5,57 Long-term studies are needed to fully understand the natural disease course. In the meantime, treating underlying disease and managing symptoms are imperative to patient care.
Peripheral neuropathy is the most common reason for an outpatient neurology visit in the United States and accounts for over $10 billion in healthcare spending each year.1,2 When the disorder affects only small, thinly myelinated or unmyelinated nerve fibers, it is referred to as small fiber neuropathy, which commonly presents as numbness and burning pain in the feet.
This article details the manifestations and evaluation of small fiber neuropathy, with an eye toward diagnosing an underlying cause amenable to treatment.
OLDER PATIENTS MOST AFFECTED
The epidemiology of small fiber neuropathy is not well established. It occurs more commonly in older patients, but data are mixed on prevalence by sex.3–6 In a Dutch study,3 the overall prevalence was at least 53 cases per 100,000, with the highest rate in men over age 65.
CHARACTERISTIC SENSORY DISTURBANCES
Sensations vary in quality and time
Patients with small fiber neuropathy typically present with a symmetric length-dependent (“stocking-glove”) distribution of sensory changes, starting in the feet and gradually ascending up the legs and then to the hands.
Commonly reported neuropathic symptoms include various combinations of burning, numbness, tingling, itching, sunburn-like, and frostbite-like sensations. Nonneuropathic symptoms may include tightness, a vise-like squeezing of the feet, and the sensation of a sock rolled up at the end of the shoe. Cramps or spasms may also be reported but rarely occur in isolation.7
Symptoms are typically worse at the end of the day and while sitting or lying down at night. They can arise spontaneously but may also be triggered by something as minor as the touch of clothing or cool air against the skin. Bedsheet sensitivity of the feet is reported so often that it is used as an outcome measure in clinical trials. Symptoms can also be exacerbated by extremes in ambient temperature and are especially worse in cold weather.
Random patterns suggest an immune cause
Symptoms may also have a non–length-dependent distribution that is asymmetric, patchy, intermittent, and migratory, and can involve the face, proximal limbs, and trunk. Symptoms may vary throughout the day, eg, starting with electric-shock sensations on one side of the face, followed by perineal numbness and then tingling in the arms lasting for a few minutes to several hours. While such patterns may be seen with diabetes and other common etiologies, they often suggest an underlying immune-mediated disorder such as Sjögren syndrome or sarcoidosis.8–10 Although large fiber polyneuropathy may also be non–length-dependent, the deficits are usually fixed, with no migratory component.
Autonomic features may be prominent
Autonomic symptoms occur in nearly half of patients and can be as troublesome as neuropathic pain.3 Small nerve fibers mediate somatic and autonomic functions, an evolutionary link that may reflect visceral defense mechanisms responding to pain as a signal of danger.11 This may help explain the multisystemic nature of symptoms, which can include sweating abnormalities, bowel and bladder disturbances, dry eyes, dry mouth, gastrointestinal dysmotility, skin changes (eg, discoloration, loss of hair, shiny skin), sexual dysfunction, orthostatic hypotension, and palpitations. In some cases, isolated dysautonomia may be seen.
TARGETED EXAMINATION
History: Medications, alcohol, infections
When a patient presents with neuropathic pain in the feet, a detailed history should be obtained, including alcohol use, family history of neuropathy, and use of neurotoxic medications such as metronidazole, colchicine, and chemotherapeutic agents.
Human immunodeficiency virus (HIV) and hepatitis C infection are well known to be associated with small fiber neuropathy, so relevant risk factors (eg, blood transfusions, sexual history, intravenous drug use) should be asked about. Recent illnesses and vaccinations are another important line of questioning, as a small-fiber variant of Guillain-Barré syndrome has been described.12
Assess reflexes, strength, sensation
On physical examination, particular attention should be focused on searching for abnormalities indicating large nerve fiber involvement (eg, absent deep tendon reflexes, weakness of the toes). However, absent ankle deep tendon reflexes and reduced vibratory sense may also occur in healthy elderly people.
Similarly, proprioception, motor strength, balance, and vibratory sensation are functions of large myelinated nerve fibers, and thus remain unaffected in patients with only small fiber neuropathy.
Evidence of a systemic disorder should also be sought, as it may indicate an underlying etiology.
DIAGNOSTIC TESTING
Although patients with either large or small fiber neuropathy may have subjective hyperesthesia or numbness of the distal lower extremities, the absence of significant abnormalities on neurologic examination should prompt consideration of small fiber neuropathy.
Electromyography worthwhile
Nerve conduction studies and needle electrode examination evaluate only large nerve fiber conditions. While electromyographic results are normal in patients with isolated small fiber neuropathy, the test can help evaluate subclinical large nerve fiber involvement and alternative diagnoses such as bilateral S1 radiculopathy. Nerve conduction studies may be less useful in patients over age 75, as they may lack sural sensory responses because of aging changes.13
Skin biopsy easy to do
Skin biopsy for evaluating intraepidermal nerve fiber density is one of the most widely used tests for small fiber neuropathy. This minimally invasive procedure can now be performed in a primary care office using readily available tools or prepackaged kits and analyzed by several commercial laboratories.
Reduced intraepidermal nerve fiber density on skin biopsy has been described in various other conditions such as fibromyalgia and chronic pain syndromes.16,17 The clinical significance of these findings remains uncertain.
Quantitative sudomotor axon reflex testing
Quantitative sudomotor axon reflex testing (QSART) is a noninvasive autonomic study that assesses the volume of sweat produced by the limbs in response to acetylcholine. A measure of postganglionic sympathetic sudomotor nerve function, QSART has a sensitivity of up to 80% and can be used to diagnose small fiber neuropathy.18 In a series of 115 patients with sarcoidosis small fiber neuropathy,9 the QSART and skin biopsy findings were concordant in 17 cases and complementary in 29, allowing for confirmation of small fiber neuropathy in patients whose condition would have remained undiagnosed had only one test been performed. QSART can also be considered in cases where skin biopsy may be contraindicated (eg, patient use of anticoagulation). Of note, the study may be affected by a number of external factors, including caffeine, tobacco, antihistamines, and tricyclic antidepressants; these should be held before testing.
Other diagnostic studies
Other tests may be helpful, as follows:
Tilt-table and cardiovagal testing may be useful for patients with orthostasis and palpitations.
Thermoregulatory sweat testing can be used to evaluate patients with abnormal patterns of sweating, eg, hyperhidrosis of the face and head.
INITIAL TESTING FOR AN UNDERLYING CAUSE
Glucose tolerance test for diabetes
Diabetes is the most common identifiable cause of small fiber neuropathy and accounts for about a third of all cases.5 Impaired glucose tolerance is also thought to be a risk factor and has been found in up to 50% of idiopathic cases, but the association is still being debated.21
While testing for hemoglobin A1c is more convenient for the patient, especially because it does not require fasting, a 2-hour oral glucose tolerance test is more sensitive for detecting glucose dysmetabolism.22
Lipid panel for metabolic syndrome
Small fiber neuropathy is associated with individual components of the metabolic syndrome, which include obesity, hyperglycemia, and dyslipidemia. Of these, dyslipidemia has emerged as the primary factor involved in the development of small fiber neuropathy, via an inflammatory pathway or oxidative stress mechanism.23,24
Vitamin B12 deficiency testing
Vitamin B12 deficiency, a potentially correctable cause of small fiber neuropathy, may be underdiagnosed, especially as values obtained by blood testing may not reflect tissue uptake. Causes of vitamin B12 deficiency include reduced intake, pernicious anemia, and medications that can affect absorption of vitamin B12 (eg, proton pump inhibitors, histamine 2 receptor antagonists, metformin).
Testing should include:
- Complete blood cell count to evaluate for vitamin B12-related macrocytic anemia and other hematologic abnormalities
- Serum vitamin B12 level
- Methylmalonic acid or homocysteine level in patients with subclinical or mild vitamin B12 deficiency, manifested as low to normal vitamin B12 levels (< 400 pg/mL); methylmalonic acid and homocysteine require vitamin B12 as a cofactor for enzymatic conversion, and either or both may be elevated in early vitamin B12 deficiency.
Celiac antibody panel
Celiac disease, a T-cell mediated enteropathy characterized by gluten intolerance and a herpetiform-like rash, can be associated with small fiber neuropathy.25 In some cases, neuropathy symptoms are preceded by the onset of gastrointestinal symptoms, or they may occur in isolation.25
Inflammatory disease testing
Sjögren syndrome accounts for nearly 10% of cases of small fiber neuropathy. Associated neuropathic symptoms are often non–length-dependent, can precede sicca symptoms for up to 6 years, and in some cases are the sole manifestation of the disease.10 Small fiber neuropathy may also be associated with vasculitis, systemic lupus erythematosus, and other connective tissue disorders.
Testing should include:
- Erythrocyte sedimentation rate, C-reactive protein, and antinuclear antibodies: though these are nonspecific markers of inflammation, they may support an immune-mediated etiology if positive
- Extractable nuclear antigen panel: Sjögren syndrome A and B autoantibodies are the most important components in this setting5,11
- The Schirmer test or salivary gland biopsy should be considered for seronegative patients with sicca or a suspected immune-mediated etiology, as the sensitivity of antibody testing ranges from only 10% to 55%.10
Thyroid function testing
Hypothyroidism, and less commonly hyperthyroidism, are associated with small fiber neuropathy.
Metabolic tests for liver and kidney disease
Renal insufficiency and liver impairment are well-known causes of small nerve fiber dysfunction. Testing should include:
- Comprehensive metabolic panel
- Gamma-glutamyltransferase if alcohol abuse is suspected, since heavy alcohol use is one of the most common causes of both large and small fiber neuropathy.
HIV and hepatitis C testing
For patients with relevant risk factors, HIV and hepatitis C testing should be part of the initial workup (and as second-tier testing for others). Patients who test positive for hepatitis C should undergo further testing for cryoglobulinemia, which can present with painful small fiber neuropathy.26
Serum and urine immunoelectrophoresis
Paraproteinemia, with causes ranging from monoclonal gammopathy of uncertain significance to multiple myeloma, has been associated with small fiber neuropathy. An abnormal serum or urine immunoelectrophoresis test warrants further investigation and possibly referral to a hematology-oncology specialist.
SECOND-TIER TESTING
Less common treatable causes of small fiber neuropathy may also be evaluated.
Copper, vitamin B1 (thiamine), or vitamin B6 (pyridoxine) deficiency testing. Although vitamin B6 toxicity may also result in neuropathy due to its toxic effect on the dorsal root ganglia, the mildly elevated vitamin B6 levels often found in patients being evaluated for neuropathy are unlikely to be the primary cause of symptoms. Many laboratories require fasting samples for accurate vitamin B6 levels.
Angiotensin-converting enzyme levels for sarcoidosis. Small fiber neuropathy is common in sarcoidosis, occurring in more than 30% of patients with systemic disease.27 However, screening for sarcoidosis by measuring serum levels is often falsely positive and is not cost-effective. In a study of 195 patients with idiopathic small fiber neuropathy,11 44% had an elevated serum level, but no evidence of sarcoidosis was seen on further testing, which included computed tomography of the chest in 29 patients.12 Thus, this test is best used for patients with evidence of systemic disease.
Amyloid testing for amyloidosis. Fat pad or bone marrow biopsy should be considered in the appropriate clinical setting.
Paraneoplastic autoantibody panel for occult cancer. Such testing may also be considered if clinically warranted. However, if a patient is found to have low positive titers of paraneoplastic antibodies and suspicion is low for an occult cancer (eg, no weight loss or early satiety), repeat confirmatory testing at another laboratory should be done before embarking on an extensive search for malignancy.
Ganglionic acetylcholine receptor antibody testing for autoimmune autonomic ganglionopathy. This should be ordered for patients with prominent autonomic dysfunction. The antibody test can be ordered separately or as part of an autoantibody panel. The antibody may indicate a primary immune-mediated process or a paraneoplastic disease.28
Genetic mutation testing. Recent discoveries of gene mutations leading to peripheral nerve hyperexcitability of voltage-gated sodium channels have elucidated a hereditary cause of small fiber neuropathy in nearly 30% of cases that were once thought to be idiopathic.29,30 Genetic testing for mutations in SCN9A and SCN10 (which code for the Nav1.7 and Nav1.8 sodium channels, respectively) is commercially available and may be considered for those with a family history of neuropathic pain in the feet or for young, otherwise healthy patients.
Fabry disease is an X-linked lysosomal disorder characterized by angiokeratomas, cardiac and renal impairment, and small fiber neuropathy. Treatment is now available, but screening is not cost-efficient and should only be pursued in patients with other symptoms of the disease.31,32
OTHER POSSIBLE CAUSES
Guillain-Barré syndrome
A Guillain-Barré syndrome variant has been reported that is characterized by ascending limb paresthesias and cerebrospinal fluid albuminocytologic dissociation in the setting of preserved deep tendon reflexes and normal findings on EMG.12 The clinical course is similar to that of typical Guillain-Barré syndrome, in that symptoms follow an upper respiratory or gastrointestinal tract infection, reach their nadir at 4 weeks, and then gradually improve. Some patients respond to intravenous immune globulin.
Vaccine-associated
Postvaccination small fiber neuropathy has also been reported. The nature of the association is unclear.33
Parkinson disease
Small fiber neuropathy is associated with Parkinson disease. It is attributed to a number of proposed factors, including neurodegeneration that occurs parallel to central nervous system decline, as well as intestinal malabsorption with resultant vitamin deficiency.34,35
Rapid glycemic lowering
Aggressive treatment of diabetes, defined as at least a 2-point reduction of serum hemoglobin A1c level over 3 months, may result in acute small fiber neuropathy. It manifests as severe distal extremity pain and dysautonomia.
In a retrospective study,36 104 (10.9%) of 954 patients presenting to a tertiary diabetic clinic developed treatment-induced diabetic neuropathy with symptoms occurring within 8 weeks of rapid glycemic control. The severity of neuropathy correlated with the degree and rate of glycemic lowering. The condition was reversible in some cases.
TREATING SPECIFIC DISORDERS
For patients with an identified cause of neuropathy, targeted treatment offers the best chance of halting progression and possibly improving symptoms. Below are recommendations for addressing neuropathy associated with the common diagnoses.
Diabetes, impaired glucose tolerance, and metabolic syndrome. In addition to glycemic- and lipid-lowering therapies, lifestyle modifications with a specific focus on exercise and nutrition are integral to treating diabetes and related disorders.
In the Look AHEAD (Action for Health in Diabetes) study,37 which evaluated the effects of intensive lifestyle intervention on neuropathy in 5,145 overweight patients with type 2 diabetes, patients in the intervention group had lower pain scores and better touch sensation in the toes compared with controls at 1 year. Differences correlated with the degree of weight loss and reduction of hemoglobin A1c and lipid levels.
As running and walking may not be feasible for many patients owing to pain, stationary cycling, aqua therapy, and swimming are other options. A stationary recumbent bike may be useful for older patients with balance issues.
Vitamin B12 deficiency. As reduced absorption rather than low dietary intake is the primary cause of vitamin B12 deficiency for many patients, parenteral rather than oral supplementation may be best. A suggested regimen is subcutaneous or intramuscular methylcobalamin injection of 1,000 µg given daily for 1 week, then once weekly for 1 month, followed by a maintenance dose once a month for at least 6 to 12 months. Alternatively, a daily dose of vitamin B12 1,000 µg can be taken sublingually.
Sjögren syndrome. According to anecdotal case reports, intravenous immune globulin, corticosteroids, and other immunosuppressants help painful small fiber neuropathy and dysautonomia associated with Sjögren syndrome.10
Sarcoidosis. Sarcoidosis-associated small fiber neuropathy may also respond to intravenous immune globulin, as well as infliximab and combination therapy.9 Culver et al38 found that cibinetide, an experimental erythropoetin agonist, resulted in improved corneal nerve fiber measures in patients with small fiber neuropathy associated with sarcoidosis.
Celiac disease. A gluten-free diet is the treatment for celiac disease and can help some patients.
GENERAL MANAGEMENT
For all patients, regardless of whether the cause of small fiber neuropathy has been identified, managing symptoms remains key, as pain and autonomic dysfunction can markedly impair quality of life. A multidisciplinary approach that incorporates pain medications, physical therapy, and lifestyle modifications is ideal. Integrative holistic treatments such as natural supplements, yoga, and other mind-body therapies may also help.
Pain control
Mexiletine, a voltage-gated sodium channel blocker used as an antiarrhythmic, may help refractory pain or hereditary small fiber neuropathy related to sodium channel dysfunction. However, it is not recommended for diabetic neuropathy.39
Combination regimens that use drugs with different mechanisms of action can be effective. In one study, combined gabapentin and nortriptyline were more effective than either drug alone for neuropathic pain.40
Inhaled cannabis reduced pain in patients with HIV and diabetic neuropathy in a number of studies. Side effects included euphoria, somnolence, and cognitive impairment.41,42 The use of medical marijuana is not yet legal nationwide and may affect employability even in states in which it has been legalized.
Owing to the opioid epidemic and high addiction potential, opioids are no longer a preferred recommendation for chronic treatment of noncancer-related neuropathy. A population-based study of 2,892 patients with neuropathy found that those on chronic opioid therapy (≥ 90 days) had worse functional outcomes and higher rates of addiction and overdose than those on short-term therapy.43 However, the opioid agonist tramadol was found to be effective in reducing neuropathic pain and may be a safer option for patients with chronic small fiber neuropathy.44
Integrative, holistic therapies
PROGNOSIS
For many patients, small fiber neuropathy is a slowly progressive disorder that reaches a clinical plateau lasting for years, with progression to large fiber involvement reported in 13% to 36% of cases; over half of patients in one series either improved or remained stable over a period of 2 years.5,57 Long-term studies are needed to fully understand the natural disease course. In the meantime, treating underlying disease and managing symptoms are imperative to patient care.
- Burke JF, Skolarus LE, Callaghan BC, Kerber KA. Choosing Wisely: highest-cost tests in outpatient neurology. Ann Neurol 2013; 73(5):679–683. doi:10.1002/ana.23865
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26(6):1790–1795. pmid:12766111
- Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: a survey in the Netherlands. Neurology 2013; 81(15):1356–1360. doi:10.1212/WNL.0b013e3182a8236e
- Periquet MI, Novak V, Collins MP, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology 1999; 53(8):1641–1647. pmid:10563606
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131(pt 7):1912–1925. doi:10.1093/brain/awn093
- Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002; 26(2):173–188. doi:10.1002/mus.10181
- Lopate G, Streif E, Harms M, Weihl C, Pestronk A. Cramps and small-fiber neuropathy. Muscle Nerve 2013; 48(2):252–255. doi:10.1002/mus.23757
- Khan S, Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve 2012; 45(1):86–91. doi:10.1002/mus.22255
- Tavee JO, Karwa K, Ahmed Z, Thompson N, Parambil J, Culver DA. Sarcoidosis-associated small fiber neuropathy in a large cohort: clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir Med 2017; 126:135–138. doi:10.1016/j.rmed.2017.03.011
- Berkowitz AL, Samuels MA. The neurology of Sjogren’s syndrome and the rheumatology of peripheral neuropathy and myelitis. Pract Neurol 2014; 14(1):14–22. doi:10.1136/practneurol-2013-000651
- Lang M, Treister R, Oaklander AL. Diagnostic value of blood tests for occult causes of initially idiopathic small-fiber polyneuropathy. J Neurol 2016; 263(12):2515–2527. doi:10.1007/s00415-016-8270-5
- Seneviratne U, Gunasekera S. Acute small fibre sensory neuropathy: another variant of Guillain-Barré syndrome? J Neurol Neurosurg Psychiatry 2002; 72(4):540–542. pmid:11909922
- Tavee JO, Polston D, Zhou L, Shields RW, Butler RS, Levin KH. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve 2014; 49(4):564–569. doi:10.1002/mus.23971
- Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med 2009; 76(5):297–305. doi:10.3949/ccjm.76a.08070
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999; 53(8):1634–1640. pmid:10563605
- Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013; 154(11):2310–2316. doi:10.1016/j.pain.2013.06.001
- Üçeyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136(pt 6):1857–1867. doi:10.1093/brain/awt053
- Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992; 15(6):661–665. doi:10.1002/mus.880150605
- Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003; 46(5):683–688. doi:10.1007/s00125-003-1086-8
- de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy—a large cohort study and review of the literature. Eur J Neurol 2018; 25(2):348–355. doi:10.1111/ene.13508
- Smith AG. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst 2012; 17(suppl 2):15–21. doi:10.1111/j.1529-8027.2012.00390.x
- Hoffman-Snyder C, Smith BE, Ross MA, Hernandez J, Bosch EP. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006; 63(8):1075–1079. doi:10.1001/archneur.63.8.noc50336
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 2009; 14(4):257–267. doi:10.1111/j.1529-8027.2009.00237.x
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009; 58(7):1634–1640. doi:10.2337/db08-1771
- Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology 2003; 60(10):1581–1585. pmid:12771245
- Gemignani F, Brindani F, Alfieri S, et al. Clinical spectrum of cryoglobulinaemic neuropathy. J Neurol Neurosurg Psychiatry 2005; 76(10):1410–1414. doi:10.1136/jnnp.2004.057620
- Bakkers M, Merkies IS, Lauria G, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology 2009; 73(14):1142–1148. doi:10.1212/WNL.0b013e3181bacf05
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012; 71(1):26–39. doi:10.1002/ana.22485
- Brouwer BA, Merkies IS, Gerrits MM, Waxman SG, Hoeijmakers JG, Faber CG. Painful neuropathies: the emerging role of sodium channelopathies. J Peripher Nerv Syst 2014; 19(2):53–65. doi:10.1111/jns5.12071
- Samuelsson K, Kostulas K, Vrethem M, Rolfs A, Press R. Idiopathic small fiber neuropathy: phenotype, etiologies, and the search for Fabry disease. J Clin Neurol 2014; 10(2):108–118. doi:10.3988/jcn.2014.10.2.108
- de Greef BT, Hoeijmakers JG, Wolters EE, et al. No Fabry disease in patients presenting with isolated small fiber neuropathy. PLoS One 2016; 11(2):e0148316. doi:10.1371/journal.pone.0148316
- Souayah N, Ajroud-Driss S, Sander HW, Brannagan TH, Hays AP, Chin RL. Small fiber neuropathy following vaccination for rabies, varicella or Lyme disease. Vaccine 2009; 27(52):7322–7325. doi:10.1016/j.vaccine.2009.09.077
- Nolano M, Provitera V, Manganelli F, et al. Loss of cutaneous large and small fibers in naive and l-dopa–treated PD patients. Neurology 2017; 89(8):776–784. doi:10.1212/WNL.0000000000004274
- Zis P, Grünewald RA, Chaudhuri RK, Hadjivassiliou M. Peripheral neuropathy in idiopathic Parkinson’s disease: a systematic review. J Neurol Sci 2017; 378:204–209. doi:10.1016/j.jns.2017.05.023
- Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015; 138(pt 1):43–52. doi:10.1093/brain/awu307
- Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia 2017; 60(6):980–988. doi:10.1007/s00125-017-4253-z
- Culver DA, Dahan A, Bajorunas D, et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci 2017; 58(6):BIO52–BIO60. doi:10.1167/iovs.16-21291
- Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2011; 3(4):345–352.e21. doi:10.1016/j.pmrj.2011.03.008
- Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009; 374(9697):1252–1261. doi:10.1016/S0140-6736(09)61081-3
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ. Association of long-term opioid therapy with functional status, adverse outcomes, and mortality among patients with polyneuropathy. JAMA Neurol 2017; 74(7):773–779. doi:10.1001/jamaneurol.2017.0486
- Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998; 50(6):1842–1846. pmid:9633738
- Sima AA, Calvani M, Mehra M, Amato A; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care 2005; 28(1):89–94. pmid:15616239
- Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995; 38(12):1425–1433. pmid:8786016
- Scarpini E, Sacilotto G, Baron P, Cusini M, Scarlato G. Effect of acetyl-L-carnitine in the treatment of painful peripheral neuropathies in HIV+ patients. J Peripher Nerv Syst 1997; 2(3):250-252. pmid: 10975731
- Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 2013; 31(20):2627-2633. doi:10.1200/JCO.2012.44.8738
- Amara S. Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann Pharmacother 2008; 42(10):1481-1485. doi:10.1345/aph.1L179
- Huang JS, Wu CL, Fan CW, Chen WH, Yeh KY, Chang PH. Intravenous glutamine appears to reduce the severity of symptomatic platinum-induced neuropathy: a prospective randomized study. J Chemother 2015; 27(4):235-240. doi:10.1179/1973947815Y.0000000011
- Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 2014; 723:202-206. doi:10.1016/j.ejphar.2013.11.033
- Mendonça LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi MD, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology 2013; 34:205-211. doi:10.1016/j.neuro.2012.09.011
- Wagner K, Lee KS, Yang J, Hammock BD. Epoxy fatty acids mediate analgesia in murine diabetic neuropathy. Eur J Pain 2017; 21(3):456-465. doi:10.1002/ejp.939
- Lewis EJ, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: a 12-month pilot trial. Neurology 2017; 88(24):2294–2301. doi:10.1212/WNL.0000000000004033
- Hu D, Wang C, Li F, et al. A combined water extract of frankincense and myrrh alleviates neuropathic pain in mice via modulation of TRPV1. Neural Plast 2017; 2017:3710821. doi:10.1155/2017/3710821
- Tavee J, Rensel M, Planchon SM, Butler RS, Stone L. Effects of meditation on pain and quality of life in multiple sclerosis and peripheral neuropathy: a pilot study. Int J MS Care 2011; 13(4):163–168. doi:10.7224/1537-2073-13.4.163
- Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol 2016; 73(6):684–690. doi:10.1001/jamaneurol.2016.0057
- Burke JF, Skolarus LE, Callaghan BC, Kerber KA. Choosing Wisely: highest-cost tests in outpatient neurology. Ann Neurol 2013; 73(5):679–683. doi:10.1002/ana.23865
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26(6):1790–1795. pmid:12766111
- Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: a survey in the Netherlands. Neurology 2013; 81(15):1356–1360. doi:10.1212/WNL.0b013e3182a8236e
- Periquet MI, Novak V, Collins MP, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology 1999; 53(8):1641–1647. pmid:10563606
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131(pt 7):1912–1925. doi:10.1093/brain/awn093
- Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002; 26(2):173–188. doi:10.1002/mus.10181
- Lopate G, Streif E, Harms M, Weihl C, Pestronk A. Cramps and small-fiber neuropathy. Muscle Nerve 2013; 48(2):252–255. doi:10.1002/mus.23757
- Khan S, Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve 2012; 45(1):86–91. doi:10.1002/mus.22255
- Tavee JO, Karwa K, Ahmed Z, Thompson N, Parambil J, Culver DA. Sarcoidosis-associated small fiber neuropathy in a large cohort: clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir Med 2017; 126:135–138. doi:10.1016/j.rmed.2017.03.011
- Berkowitz AL, Samuels MA. The neurology of Sjogren’s syndrome and the rheumatology of peripheral neuropathy and myelitis. Pract Neurol 2014; 14(1):14–22. doi:10.1136/practneurol-2013-000651
- Lang M, Treister R, Oaklander AL. Diagnostic value of blood tests for occult causes of initially idiopathic small-fiber polyneuropathy. J Neurol 2016; 263(12):2515–2527. doi:10.1007/s00415-016-8270-5
- Seneviratne U, Gunasekera S. Acute small fibre sensory neuropathy: another variant of Guillain-Barré syndrome? J Neurol Neurosurg Psychiatry 2002; 72(4):540–542. pmid:11909922
- Tavee JO, Polston D, Zhou L, Shields RW, Butler RS, Levin KH. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve 2014; 49(4):564–569. doi:10.1002/mus.23971
- Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med 2009; 76(5):297–305. doi:10.3949/ccjm.76a.08070
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999; 53(8):1634–1640. pmid:10563605
- Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013; 154(11):2310–2316. doi:10.1016/j.pain.2013.06.001
- Üçeyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136(pt 6):1857–1867. doi:10.1093/brain/awt053
- Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992; 15(6):661–665. doi:10.1002/mus.880150605
- Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003; 46(5):683–688. doi:10.1007/s00125-003-1086-8
- de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy—a large cohort study and review of the literature. Eur J Neurol 2018; 25(2):348–355. doi:10.1111/ene.13508
- Smith AG. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripher Nerv Syst 2012; 17(suppl 2):15–21. doi:10.1111/j.1529-8027.2012.00390.x
- Hoffman-Snyder C, Smith BE, Ross MA, Hernandez J, Bosch EP. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006; 63(8):1075–1079. doi:10.1001/archneur.63.8.noc50336
- Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 2009; 14(4):257–267. doi:10.1111/j.1529-8027.2009.00237.x
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009; 58(7):1634–1640. doi:10.2337/db08-1771
- Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology 2003; 60(10):1581–1585. pmid:12771245
- Gemignani F, Brindani F, Alfieri S, et al. Clinical spectrum of cryoglobulinaemic neuropathy. J Neurol Neurosurg Psychiatry 2005; 76(10):1410–1414. doi:10.1136/jnnp.2004.057620
- Bakkers M, Merkies IS, Lauria G, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology 2009; 73(14):1142–1148. doi:10.1212/WNL.0b013e3181bacf05
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000; 343(12):847–855. doi:10.1056/NEJM200009213431204
- Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012; 71(1):26–39. doi:10.1002/ana.22485
- Brouwer BA, Merkies IS, Gerrits MM, Waxman SG, Hoeijmakers JG, Faber CG. Painful neuropathies: the emerging role of sodium channelopathies. J Peripher Nerv Syst 2014; 19(2):53–65. doi:10.1111/jns5.12071
- Samuelsson K, Kostulas K, Vrethem M, Rolfs A, Press R. Idiopathic small fiber neuropathy: phenotype, etiologies, and the search for Fabry disease. J Clin Neurol 2014; 10(2):108–118. doi:10.3988/jcn.2014.10.2.108
- de Greef BT, Hoeijmakers JG, Wolters EE, et al. No Fabry disease in patients presenting with isolated small fiber neuropathy. PLoS One 2016; 11(2):e0148316. doi:10.1371/journal.pone.0148316
- Souayah N, Ajroud-Driss S, Sander HW, Brannagan TH, Hays AP, Chin RL. Small fiber neuropathy following vaccination for rabies, varicella or Lyme disease. Vaccine 2009; 27(52):7322–7325. doi:10.1016/j.vaccine.2009.09.077
- Nolano M, Provitera V, Manganelli F, et al. Loss of cutaneous large and small fibers in naive and l-dopa–treated PD patients. Neurology 2017; 89(8):776–784. doi:10.1212/WNL.0000000000004274
- Zis P, Grünewald RA, Chaudhuri RK, Hadjivassiliou M. Peripheral neuropathy in idiopathic Parkinson’s disease: a systematic review. J Neurol Sci 2017; 378:204–209. doi:10.1016/j.jns.2017.05.023
- Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015; 138(pt 1):43–52. doi:10.1093/brain/awu307
- Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia 2017; 60(6):980–988. doi:10.1007/s00125-017-4253-z
- Culver DA, Dahan A, Bajorunas D, et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci 2017; 58(6):BIO52–BIO60. doi:10.1167/iovs.16-21291
- Bril V, England J, Franklin GM, et al; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2011; 3(4):345–352.e21. doi:10.1016/j.pmrj.2011.03.008
- Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009; 374(9697):1252–1261. doi:10.1016/S0140-6736(09)61081-3
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ. Association of long-term opioid therapy with functional status, adverse outcomes, and mortality among patients with polyneuropathy. JAMA Neurol 2017; 74(7):773–779. doi:10.1001/jamaneurol.2017.0486
- Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998; 50(6):1842–1846. pmid:9633738
- Sima AA, Calvani M, Mehra M, Amato A; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care 2005; 28(1):89–94. pmid:15616239
- Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995; 38(12):1425–1433. pmid:8786016
- Scarpini E, Sacilotto G, Baron P, Cusini M, Scarlato G. Effect of acetyl-L-carnitine in the treatment of painful peripheral neuropathies in HIV+ patients. J Peripher Nerv Syst 1997; 2(3):250-252. pmid: 10975731
- Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 2013; 31(20):2627-2633. doi:10.1200/JCO.2012.44.8738
- Amara S. Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann Pharmacother 2008; 42(10):1481-1485. doi:10.1345/aph.1L179
- Huang JS, Wu CL, Fan CW, Chen WH, Yeh KY, Chang PH. Intravenous glutamine appears to reduce the severity of symptomatic platinum-induced neuropathy: a prospective randomized study. J Chemother 2015; 27(4):235-240. doi:10.1179/1973947815Y.0000000011
- Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 2014; 723:202-206. doi:10.1016/j.ejphar.2013.11.033
- Mendonça LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi MD, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology 2013; 34:205-211. doi:10.1016/j.neuro.2012.09.011
- Wagner K, Lee KS, Yang J, Hammock BD. Epoxy fatty acids mediate analgesia in murine diabetic neuropathy. Eur J Pain 2017; 21(3):456-465. doi:10.1002/ejp.939
- Lewis EJ, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: a 12-month pilot trial. Neurology 2017; 88(24):2294–2301. doi:10.1212/WNL.0000000000004033
- Hu D, Wang C, Li F, et al. A combined water extract of frankincense and myrrh alleviates neuropathic pain in mice via modulation of TRPV1. Neural Plast 2017; 2017:3710821. doi:10.1155/2017/3710821
- Tavee J, Rensel M, Planchon SM, Butler RS, Stone L. Effects of meditation on pain and quality of life in multiple sclerosis and peripheral neuropathy: a pilot study. Int J MS Care 2011; 13(4):163–168. doi:10.7224/1537-2073-13.4.163
- Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol 2016; 73(6):684–690. doi:10.1001/jamaneurol.2016.0057
KEY POINTS
- Patients typically develop a symmetric “stocking-glove” pattern of sensory loss in the feet and hands.
- The diagnosis may be confirmed with skin biopsy for nerve fiber density, which can easily be done in a clinic setting with commercially available kits.
- Diabetes is the most common identifiable cause of small fiber neuropathy.
- Serologic testing can help uncover a vitamin deficiency or other potentially treatable condition.
- Antiepileptics, antidepressants, and topical agents are first-line drugs for managing pain.
Ablation of atrial fibrillation: Facts for the referring physician
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation. Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins. Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors. Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice. We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time
Retrospective studies have reported a low risk of stroke in patients who discontinue anticoagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Contact force-sensing catheters. Radiofrequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure. It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure. The current guidelines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radiofrequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2):56–61. pmid:15785146
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370–2375. pmid:11343485
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012; 5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220. pmid:14118488
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101(3):402–405. pmid:1999933
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101(4):569–583. pmid:2008095
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95(3):572–576. pmid:9024141
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10):659–666. doi:10.1056/NEJM199809033391003
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972; 34(5):520–525. pmid:5031645
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7):1954–1968. pmid:7671380
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415(6868):219–226. doi:10.1038/415219a
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011; 22(2):137–141. doi:10.1111/j.1540-8167.2010.01885.x
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4(3):271–278. doi:10.1161/CIRCEP.111.962100
- Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016; 9(1):e003669. doi:10.1161/CIRCEP.115.003669
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41(10):1690–1696. pmid:12767648
- Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347(23):1834–1840. doi:10.1056/NEJMoa021375
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825–1833. doi:10.1056/NEJMoa021328
- Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005; 149(6):1106–111. doi:10.1016/j.ahj.2004.11.030
- Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001; 104(3):292–296. pmid:11457747
- Guiot A, Jongnarangsin K, Chugh A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012; 23(1):36–43. doi:10.1111/j.1540-8167.2011.02141.x
- Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006; 114(8):759–765. doi:10.1161/CIRCULATIONAHA.106.641225
- Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55(8):735–743. doi:10.1016/j.jacc.2009.11.039
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017; 33(5):369–409. doi:10.1016/j.joa.2017.08.001
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23):2235–2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015; 132(10):907–915. doi:10.1161/CIRCULATIONAHA.114.014092
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014; 64(7):647–656. doi:10.1016/j.jacc.2014.04.072
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol 2017; 28(5):483–488. doi:10.1111/jce.13179
- Deshmukh A, Patel NJ, Pant I, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128(19):2104–2112. doi:10.1161/CIRCULATIONAHA.113.003862
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111(9):1100–1105. doi:10.1161/01.CIR.0000157153.30978.67
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3(1):32–38. doi:10.1161/CIRCEP.109.859116
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293(21):2634–2640. doi:10.1001/jama.293.21.2634
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118(24):2498–2505. doi:10.1161/CIRCULATIONAHA.108.772582
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010; 7(6):835–846. doi:10.1016/j.hrthm.2010.01.017
- Packer DL, Lee KL, Mark DB, Robb RA. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial, CABANA. http://cabanatrial.org/. Accessed September 10, 2018.
- Scherr D, Sharma K, Dalal D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(12):1357–1363. doi:10.1111/j.1540-8167.2009.01540.x
- Wazni OM, Rossillo A, Marrouche NF, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005; 16(6):576–581. doi:10.1111/j.1540-8167.2005.40480.x
- Hussein AA, Martin DO, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009; 6(10):1425–1429. doi:10.1016/j.hrthm.2009.07.007
- Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013; 6(3):460–466. doi:10.1161/CIRCEP.113.000320
- Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010; 56(18):1463–1472. doi:10.1016/j.jacc.2010.04.057
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002; 40(1):100–104. pmid:12103262
- Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation—a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016; 11(5):e0156121. doi:10.1371/journal.pone.0156121
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011; 4(1):11–14. doi:10.1161/CIRCEP.110.955393
- Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study). Circulation 2009; 120(12):1036–1040. doi:10.1161/CIRCULATIONAHA.108.839639
- Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013; 111(4):552–556. doi:10.1016/j.amjcard.2012.10.040
- Lee SH, Tai CT, Hsieh MH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004 Jun;10(3):221-6. doi:10.1023/B:JICE.0000026915.02503.92
- Zhang XD, Gu J, Jiang WF, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J 2014; 35(20):1327–1334. doi:10.1093/eurheartj/ehu017
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation. Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins. Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors. Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice. We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time
Retrospective studies have reported a low risk of stroke in patients who discontinue anticoagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Contact force-sensing catheters. Radiofrequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure. It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure. The current guidelines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radiofrequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation. Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins. Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors. Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice. We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time
Retrospective studies have reported a low risk of stroke in patients who discontinue anticoagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Contact force-sensing catheters. Radiofrequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure. It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure. The current guidelines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radiofrequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2):56–61. pmid:15785146
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370–2375. pmid:11343485
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012; 5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220. pmid:14118488
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101(3):402–405. pmid:1999933
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101(4):569–583. pmid:2008095
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95(3):572–576. pmid:9024141
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10):659–666. doi:10.1056/NEJM199809033391003
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972; 34(5):520–525. pmid:5031645
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7):1954–1968. pmid:7671380
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415(6868):219–226. doi:10.1038/415219a
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011; 22(2):137–141. doi:10.1111/j.1540-8167.2010.01885.x
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4(3):271–278. doi:10.1161/CIRCEP.111.962100
- Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016; 9(1):e003669. doi:10.1161/CIRCEP.115.003669
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41(10):1690–1696. pmid:12767648
- Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347(23):1834–1840. doi:10.1056/NEJMoa021375
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825–1833. doi:10.1056/NEJMoa021328
- Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005; 149(6):1106–111. doi:10.1016/j.ahj.2004.11.030
- Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001; 104(3):292–296. pmid:11457747
- Guiot A, Jongnarangsin K, Chugh A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012; 23(1):36–43. doi:10.1111/j.1540-8167.2011.02141.x
- Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006; 114(8):759–765. doi:10.1161/CIRCULATIONAHA.106.641225
- Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55(8):735–743. doi:10.1016/j.jacc.2009.11.039
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017; 33(5):369–409. doi:10.1016/j.joa.2017.08.001
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23):2235–2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015; 132(10):907–915. doi:10.1161/CIRCULATIONAHA.114.014092
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014; 64(7):647–656. doi:10.1016/j.jacc.2014.04.072
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol 2017; 28(5):483–488. doi:10.1111/jce.13179
- Deshmukh A, Patel NJ, Pant I, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128(19):2104–2112. doi:10.1161/CIRCULATIONAHA.113.003862
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111(9):1100–1105. doi:10.1161/01.CIR.0000157153.30978.67
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3(1):32–38. doi:10.1161/CIRCEP.109.859116
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293(21):2634–2640. doi:10.1001/jama.293.21.2634
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118(24):2498–2505. doi:10.1161/CIRCULATIONAHA.108.772582
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010; 7(6):835–846. doi:10.1016/j.hrthm.2010.01.017
- Packer DL, Lee KL, Mark DB, Robb RA. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial, CABANA. http://cabanatrial.org/. Accessed September 10, 2018.
- Scherr D, Sharma K, Dalal D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(12):1357–1363. doi:10.1111/j.1540-8167.2009.01540.x
- Wazni OM, Rossillo A, Marrouche NF, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005; 16(6):576–581. doi:10.1111/j.1540-8167.2005.40480.x
- Hussein AA, Martin DO, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009; 6(10):1425–1429. doi:10.1016/j.hrthm.2009.07.007
- Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013; 6(3):460–466. doi:10.1161/CIRCEP.113.000320
- Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010; 56(18):1463–1472. doi:10.1016/j.jacc.2010.04.057
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002; 40(1):100–104. pmid:12103262
- Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation—a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016; 11(5):e0156121. doi:10.1371/journal.pone.0156121
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011; 4(1):11–14. doi:10.1161/CIRCEP.110.955393
- Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study). Circulation 2009; 120(12):1036–1040. doi:10.1161/CIRCULATIONAHA.108.839639
- Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013; 111(4):552–556. doi:10.1016/j.amjcard.2012.10.040
- Lee SH, Tai CT, Hsieh MH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004 Jun;10(3):221-6. doi:10.1023/B:JICE.0000026915.02503.92
- Zhang XD, Gu J, Jiang WF, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J 2014; 35(20):1327–1334. doi:10.1093/eurheartj/ehu017
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 2005; 14(2):56–61. pmid:15785146
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370–2375. pmid:11343485
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012; 5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964; 67:200–220. pmid:14118488
- Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101(3):402–405. pmid:1999933
- Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101(4):569–583. pmid:2008095
- Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997; 95(3):572–576. pmid:9024141
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339(10):659–666. doi:10.1056/NEJM199809033391003
- Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972; 34(5):520–525. pmid:5031645
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995; 92(7):1954–1968. pmid:7671380
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002; 415(6868):219–226. doi:10.1038/415219a
- Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol 2011; 22(2):137–141. doi:10.1111/j.1540-8167.2010.01885.x
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4(3):271–278. doi:10.1161/CIRCEP.111.962100
- Hussein AA, Saliba WI, Barakat A, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016; 9(1):e003669. doi:10.1161/CIRCEP.115.003669
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003; 41(10):1690–1696. pmid:12767648
- Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002; 347(23):1834–1840. doi:10.1056/NEJMoa021375
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347(23):1825–1833. doi:10.1056/NEJMoa021328
- Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al; RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005; 149(6):1106–111. doi:10.1016/j.ahj.2004.11.030
- Pedersen OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001; 104(3):292–296. pmid:11457747
- Guiot A, Jongnarangsin K, Chugh A, et al. Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 2012; 23(1):36–43. doi:10.1111/j.1540-8167.2011.02141.x
- Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006; 114(8):759–765. doi:10.1161/CIRCULATIONAHA.106.641225
- Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010; 55(8):735–743. doi:10.1016/j.jacc.2009.11.039
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017; 33(5):369–409. doi:10.1016/j.joa.2017.08.001
- Kuck KH, Brugada J, Fürnkranz A, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374(23):2235–2245. doi:10.1056/NEJMoa1602014
- Reddy VY, Dukkipati SR, Neuzil P, et al. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015; 132(10):907–915. doi:10.1161/CIRCULATIONAHA.114.014092
- Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014; 64(7):647–656. doi:10.1016/j.jacc.2014.04.072
- Hussein AA, Barakat AF, Saliba WI, et al. Persistent atrial fibrillation ablation with or without contact force sensing. J Cardiovasc Electrophysiol 2017; 28(5):483–488. doi:10.1111/jce.13179
- Deshmukh A, Patel NJ, Pant I, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 2013; 128(19):2104–2112. doi:10.1161/CIRCULATIONAHA.113.003862
- Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005; 111(9):1100–1105. doi:10.1161/01.CIR.0000157153.30978.67
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3(1):32–38. doi:10.1161/CIRCEP.109.859116
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005; 293(21):2634–2640. doi:10.1001/jama.293.21.2634
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008; 118(24):2498–2505. doi:10.1161/CIRCULATIONAHA.108.772582
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010; 7(6):835–846. doi:10.1016/j.hrthm.2010.01.017
- Packer DL, Lee KL, Mark DB, Robb RA. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial, CABANA. http://cabanatrial.org/. Accessed September 10, 2018.
- Scherr D, Sharma K, Dalal D, et al. Incidence and predictors of periprocedural cerebrovascular accident in patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(12):1357–1363. doi:10.1111/j.1540-8167.2009.01540.x
- Wazni OM, Rossillo A, Marrouche NF, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol 2005; 16(6):576–581. doi:10.1111/j.1540-8167.2005.40480.x
- Hussein AA, Martin DO, Saliba W, et al. Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm 2009; 6(10):1425–1429. doi:10.1016/j.hrthm.2009.07.007
- Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013; 6(3):460–466. doi:10.1161/CIRCEP.113.000320
- Koyama T, Tada H, Sekiguchi Y, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol 2010; 56(18):1463–1472. doi:10.1016/j.jacc.2010.04.057
- Oral H, Knight BP, Ozaydin M, et al. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J Am Coll Cardiol 2002; 40(1):100–104. pmid:12103262
- Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation—a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One 2016; 11(5):e0156121. doi:10.1371/journal.pone.0156121
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol 2011; 4(1):11–14. doi:10.1161/CIRCEP.110.955393
- Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study). Circulation 2009; 120(12):1036–1040. doi:10.1161/CIRCULATIONAHA.108.839639
- Sotomi Y, Inoue K, Ito N, et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013; 111(4):552–556. doi:10.1016/j.amjcard.2012.10.040
- Lee SH, Tai CT, Hsieh MH, et al. Predictors of early and late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2004 Jun;10(3):221-6. doi:10.1023/B:JICE.0000026915.02503.92
- Zhang XD, Gu J, Jiang WF, et al. Optimal rhythm-control strategy for recurrent atrial tachycardia after catheter ablation of persistent atrial fibrillation: a randomized clinical trial. Eur Heart J 2014; 35(20):1327–1334. doi:10.1093/eurheartj/ehu017
KEY POINTS
- Atrial fibrillation is increasing in prevalence with the aging of the US population and is associated with worsening quality of life and increased risk of stroke, heart failure, and death.
- Atrial fibrillation results in adverse atrial remodeling and fibrosis, eventually leading to persistence of the arrhythmia and making rhythm control difficult.
- Catheter ablation has evolved to be a safe procedure with technologic advancements, especially in experienced tertiary care centers.
- The primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, it could also decrease the risk of stroke, heart failure, and death, but these outcomes have not been systematically evaluated in a large randomized controlled trial.
Stroke Increases the Risk of All-Cause Dementia
Protecting the blood supply to the brain could reduce the risk of incident dementia.
Stroke is a strong independent risk factor for all-cause dementia, according to research published online ahead of print August 25 in Alzheimer’s & Dementia. Clinicians should incorporate stroke-prevention strategies into their health interventions to reduce patients’ risk of dementia, said the authors.
“Around a third of dementia cases are thought to be potentially preventable, though this estimate does not take into account the risk associated with stroke,” said David Llewellyn, PhD, Senior Research Fellow at University of Exeter Medical School in the United Kingdom. “Our findings indicate that this figure could be even higher and reinforce the importance of protecting the blood supply to the brain when attempting to reduce the global burden of dementia.”
Meta-Analysis of Previous Research
Stroke is a recognized risk factor for all-cause dementia, but no researchers had previously performed a meta-analysis to quantify the risk. Dr. Llewellyn and colleagues searched Medline, PsycINFO, and Embase databases for prospective studies that investigated the association between prevalent or incident stroke and incident all-cause dementia. They excluded studies that lacked a comparison group or that had a comparison group other than a stroke-free group. The investigators pooled adjusted estimates across studies using random effects meta-analysis and evaluated potential effect modifiers with meta-regression.
Dr. Llewellyn and colleagues identified 11,129 articles, 26 of which were eligible for analysis. They also included 16 studies from a previous systematic review and four studies identified through backward and forward citation searches. In all, 36 studies examined prevalent stroke (1.9 million participants), and 12 studies examined incident stroke (1.3 million participants). The studies were conducted in America, Europe, Asia, and Australia and included more than three million participants. Follow-up periods ranged from nine months to 25 years.
Stroke Affected Dementia Risk
When the researchers pooled results from 22 cohorts of participants who were cognitively normal at baseline, they found that those with prevalent stroke had a higher adjusted risk of incident dementia, compared with those without stroke (hazard ratio [HR], 1.69). Sensitivity analyses did not change the results significantly. Prevalent stroke was associated with a higher risk of incident dementia among men than among women. Sex explained 50.2% of heterogeneity between studies for prevalent stroke.
After combining the adjusted results from eight studies, Dr. Llewellyn and colleagues found that incident stroke more than doubled the risk of incident all-cause dementia, compared with no incident stroke (risk ratio [RR], 2.18). For a sensitivity analysis, the investigators excluded three studies that combined stroke with transient ischemic attack; this adjustment strengthened the association.
The study’s strengths include the investigators’ search of several major databases and their contacts with authors who provided relevant data. The analysis reflects the limitations of the original studies, however. These limitations include selective samples and differences in stroke assessment and dementia diagnosis criteria. In addition, dementia may develop years before it is diagnosed. “More detailed reporting of the interval between stroke occurrence and dementia diagnosis in future studies will help to better characterize the role of time since stroke in the risk of dementia,” said Dr. Llewellyn.
—Erik Greb
Kuz´ma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018 Aug 25 [Epub ahead of print].
Protecting the blood supply to the brain could reduce the risk of incident dementia.
Protecting the blood supply to the brain could reduce the risk of incident dementia.
Stroke is a strong independent risk factor for all-cause dementia, according to research published online ahead of print August 25 in Alzheimer’s & Dementia. Clinicians should incorporate stroke-prevention strategies into their health interventions to reduce patients’ risk of dementia, said the authors.
“Around a third of dementia cases are thought to be potentially preventable, though this estimate does not take into account the risk associated with stroke,” said David Llewellyn, PhD, Senior Research Fellow at University of Exeter Medical School in the United Kingdom. “Our findings indicate that this figure could be even higher and reinforce the importance of protecting the blood supply to the brain when attempting to reduce the global burden of dementia.”
Meta-Analysis of Previous Research
Stroke is a recognized risk factor for all-cause dementia, but no researchers had previously performed a meta-analysis to quantify the risk. Dr. Llewellyn and colleagues searched Medline, PsycINFO, and Embase databases for prospective studies that investigated the association between prevalent or incident stroke and incident all-cause dementia. They excluded studies that lacked a comparison group or that had a comparison group other than a stroke-free group. The investigators pooled adjusted estimates across studies using random effects meta-analysis and evaluated potential effect modifiers with meta-regression.
Dr. Llewellyn and colleagues identified 11,129 articles, 26 of which were eligible for analysis. They also included 16 studies from a previous systematic review and four studies identified through backward and forward citation searches. In all, 36 studies examined prevalent stroke (1.9 million participants), and 12 studies examined incident stroke (1.3 million participants). The studies were conducted in America, Europe, Asia, and Australia and included more than three million participants. Follow-up periods ranged from nine months to 25 years.
Stroke Affected Dementia Risk
When the researchers pooled results from 22 cohorts of participants who were cognitively normal at baseline, they found that those with prevalent stroke had a higher adjusted risk of incident dementia, compared with those without stroke (hazard ratio [HR], 1.69). Sensitivity analyses did not change the results significantly. Prevalent stroke was associated with a higher risk of incident dementia among men than among women. Sex explained 50.2% of heterogeneity between studies for prevalent stroke.
After combining the adjusted results from eight studies, Dr. Llewellyn and colleagues found that incident stroke more than doubled the risk of incident all-cause dementia, compared with no incident stroke (risk ratio [RR], 2.18). For a sensitivity analysis, the investigators excluded three studies that combined stroke with transient ischemic attack; this adjustment strengthened the association.
The study’s strengths include the investigators’ search of several major databases and their contacts with authors who provided relevant data. The analysis reflects the limitations of the original studies, however. These limitations include selective samples and differences in stroke assessment and dementia diagnosis criteria. In addition, dementia may develop years before it is diagnosed. “More detailed reporting of the interval between stroke occurrence and dementia diagnosis in future studies will help to better characterize the role of time since stroke in the risk of dementia,” said Dr. Llewellyn.
—Erik Greb
Kuz´ma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018 Aug 25 [Epub ahead of print].
Stroke is a strong independent risk factor for all-cause dementia, according to research published online ahead of print August 25 in Alzheimer’s & Dementia. Clinicians should incorporate stroke-prevention strategies into their health interventions to reduce patients’ risk of dementia, said the authors.
“Around a third of dementia cases are thought to be potentially preventable, though this estimate does not take into account the risk associated with stroke,” said David Llewellyn, PhD, Senior Research Fellow at University of Exeter Medical School in the United Kingdom. “Our findings indicate that this figure could be even higher and reinforce the importance of protecting the blood supply to the brain when attempting to reduce the global burden of dementia.”
Meta-Analysis of Previous Research
Stroke is a recognized risk factor for all-cause dementia, but no researchers had previously performed a meta-analysis to quantify the risk. Dr. Llewellyn and colleagues searched Medline, PsycINFO, and Embase databases for prospective studies that investigated the association between prevalent or incident stroke and incident all-cause dementia. They excluded studies that lacked a comparison group or that had a comparison group other than a stroke-free group. The investigators pooled adjusted estimates across studies using random effects meta-analysis and evaluated potential effect modifiers with meta-regression.
Dr. Llewellyn and colleagues identified 11,129 articles, 26 of which were eligible for analysis. They also included 16 studies from a previous systematic review and four studies identified through backward and forward citation searches. In all, 36 studies examined prevalent stroke (1.9 million participants), and 12 studies examined incident stroke (1.3 million participants). The studies were conducted in America, Europe, Asia, and Australia and included more than three million participants. Follow-up periods ranged from nine months to 25 years.
Stroke Affected Dementia Risk
When the researchers pooled results from 22 cohorts of participants who were cognitively normal at baseline, they found that those with prevalent stroke had a higher adjusted risk of incident dementia, compared with those without stroke (hazard ratio [HR], 1.69). Sensitivity analyses did not change the results significantly. Prevalent stroke was associated with a higher risk of incident dementia among men than among women. Sex explained 50.2% of heterogeneity between studies for prevalent stroke.
After combining the adjusted results from eight studies, Dr. Llewellyn and colleagues found that incident stroke more than doubled the risk of incident all-cause dementia, compared with no incident stroke (risk ratio [RR], 2.18). For a sensitivity analysis, the investigators excluded three studies that combined stroke with transient ischemic attack; this adjustment strengthened the association.
The study’s strengths include the investigators’ search of several major databases and their contacts with authors who provided relevant data. The analysis reflects the limitations of the original studies, however. These limitations include selective samples and differences in stroke assessment and dementia diagnosis criteria. In addition, dementia may develop years before it is diagnosed. “More detailed reporting of the interval between stroke occurrence and dementia diagnosis in future studies will help to better characterize the role of time since stroke in the risk of dementia,” said Dr. Llewellyn.
—Erik Greb
Kuz´ma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018 Aug 25 [Epub ahead of print].
Burden of dementia will shift more to minorities by 2060
, according to a study in Alzheimer’s and Dementia.
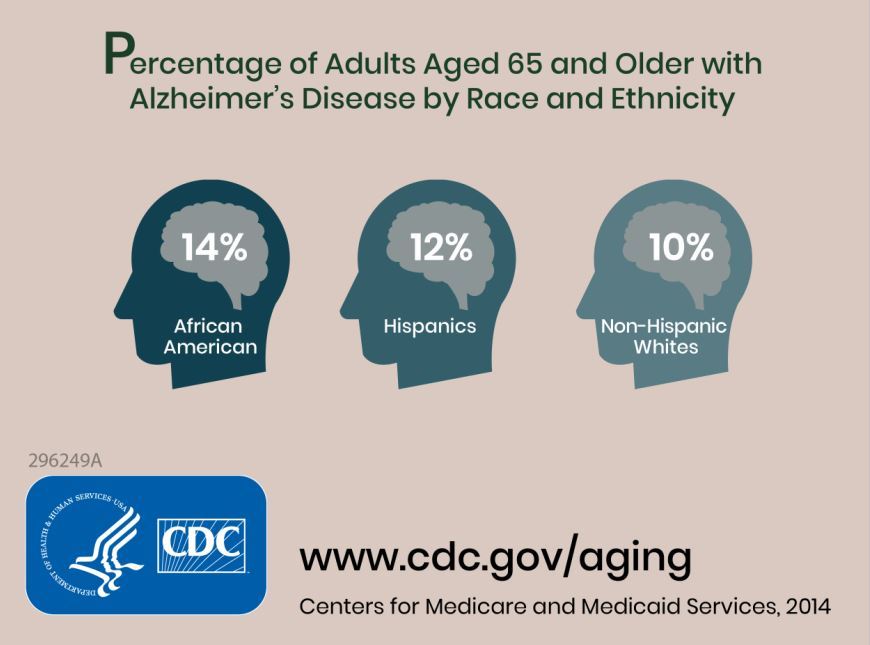
Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
, according to a study in Alzheimer’s and Dementia.

Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
, according to a study in Alzheimer’s and Dementia.

Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
FROM ALZHEIMER’S AND DEMENTIA