User login
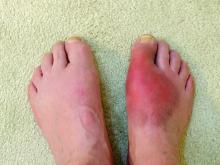
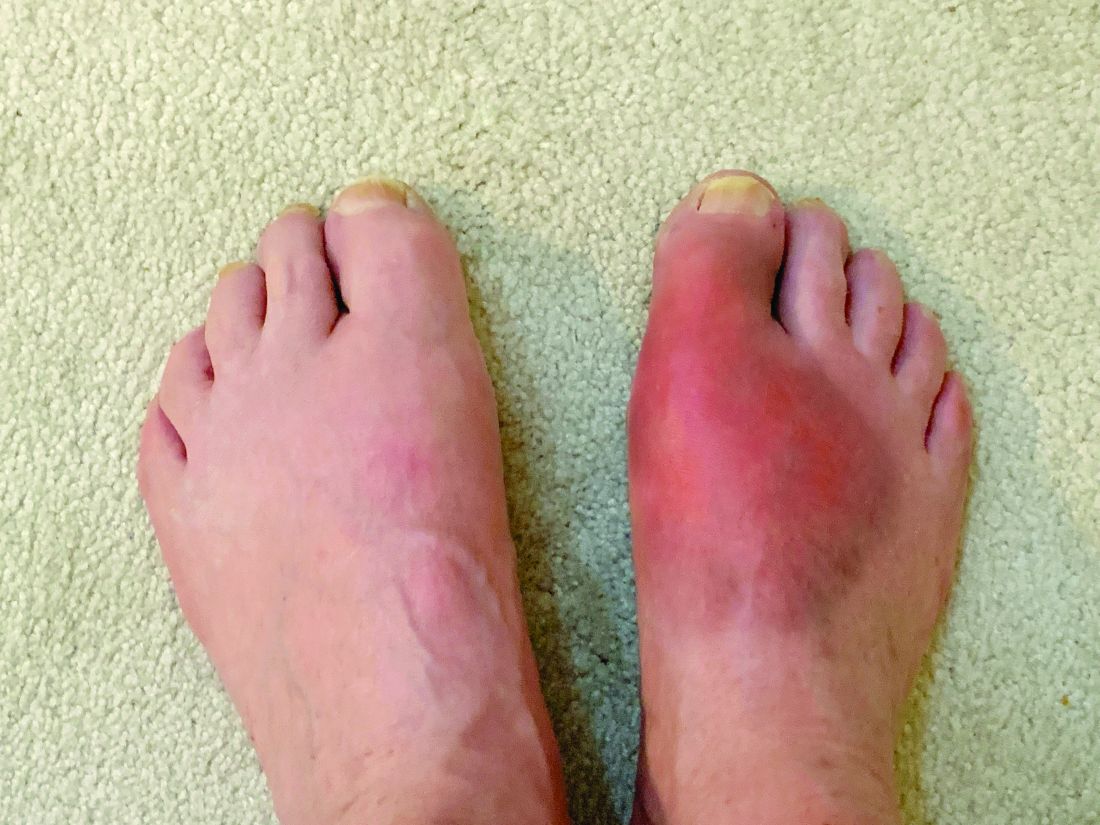
Care for patients with gout needs improvement, says doctor
said a presenter at the annual meeting of the American College of Physicians. Failure to understand the disease process and goals of urate-lowering therapy (ULT) is a key barrier to achieving optimal gout therapy.
“There’s too much focus on the flare and too little focus on the urate burden of the disease. Regardless of the clinical setting, the goal should be to manage [high] serum uric acid levels,” said Lawrence Edwards, MD, professor at the University of Florida, Gainesville, during his talk.
Dr. Edwards, who specializes in treating patients with gout and rheumatoid arthritis, discussed the role of primary care providers in the treatment of gout. “We can and must do better,” he said.
Understanding the pathology of gout is key to effective treatment
Knowledge of the molecular pathology of gout has advanced drastically over the last few years. “The improved understanding of the molecules involved in disease initiation and progression can help us make better treatment decisions depending on the stage of the disease,” Dr. Edwards said.
Gout is caused by the deposition of monosodium urate (MSU) crystals, which starts as asymptomatic hyperuricemia, he said. Inflammatory responses to MSU crystals are responsible for gout flares, the frequency of which increases as the disease progresses.
Innate immune responses driven by macrophages and neutrophils play a crucial role in acute gout attacks. In the molecular pathway, proinflammatory cytokines IL-1 beta and IL-6 are the mediators of gout flares, whereas IL-8 accumulates over time and contributes to disease progression and systemic illness. If left untreated or undertreated, the repeated inflammatory reaction leads to advanced gout. The urate burden also increases with disease progression.
“Physicians need to better educate themselves on the destructive nature of this inflammatory arthritis and the need for effective urate-lowering therapy in the management of gout,” Dr. Edwards said.
Management of acute gout attacks
The management of gout flares involves the use of pharmacological agents to control pain and inflammation. The three most common anti-inflammatory therapies are colchicine, NSAIDs, and corticosteroids (either oral or intramuscular).
The choice of which of these should be used alone or in combination for a flare is based on previous tolerance of the medication or the presence of diabetes, kidney disease, heart disease, or a history of upper gastrointestinal bleeding. Dr. Edwards referred internists to the 2020 American College of Rheumatology gout management guideline.
“Regardless of which therapy is chosen, the more important consideration is how quickly the patients can start treatment after the flare begins,” said Dr. Edwards when asked about priorities in the management of gout flares. “This means that the patient should have ready access to whichever the chosen approach is. We call this the ‘pill-in-the-pocket’ approach,” he added.
Reducing the urate burden is also important for effective treatment. The serum urate level is the primary marker of how well a patient’s gout is being managed. ULT should be initiated in patients with subcutaneous tophi, gout-related radiographic damage, or frequent flares (≥ 2 per year). Allopurinol is typically the first-line ULT of choice.
Dr. Edwards noted that far too much focus is placed on flare treatment rather than addressing the underlying sources of gouty symptoms – the elevated serum levels of urate.
Management of advanced gout
“The management of advanced gout is challenging, and the dissolution of MSU is slow unless you take an aggressive approach,” Dr. Edwards said.
Switching to pegloticase is recommended for patients with frequent flares, nonresolving tophi, or high serum urate levels that persist despite treatment with xanthine oxidase inhibitors or other ULT agents.
“The frequency and severity of gout flares are what patients focus on, but if that’s the only focus of the treating physicians, then they are leaving the job less than halfway done. Getting the serum urate to below a level of 6.0 mg/dL is the most important aspect in the lifelong management of gout,” said Dr. Edwards.
Barriers to effective gout treatment
When asked during an interview after the session about the most important barriers to successful gout management, Allison M. Mays, MD, a geriatric medicine subspecialist at Cedars-Sinai Medical Center, Los Angeles, said that “the fact that gout mostly impacts quality of life and not necessarily mortality means that other things may take precedent.” She explained that gout typically coexists with other comorbidities, often multiple ones. Patients may also defer taking an additional medication for a disease like gout, which has only episodic discomfort.
She added that gout management involves shared decision-making between patients and the medical team – including the primary care physician, rheumatologist, orthopedist, and emergency physician. Following a visit to the urgent care or the ED for an acute flare of gout, the patient may not follow up with their primary care doctor or bring it up at their next visit for chronic management, she noted.
Dr. Edwards serves as a consultant to Horizon Pharmaceuticals, Atom Biosciences, Shanton Biosciences, and Aclaris Therapeutics. Horizon marketed pegloticase up until last month when Amgen bought the drug. Dr. Edwards is also president of Gout Education Society, and he has no financial agreement with any of the multiple companies that produce colchicine and allopurinol. Dr. Mays reported no conflicts.
said a presenter at the annual meeting of the American College of Physicians. Failure to understand the disease process and goals of urate-lowering therapy (ULT) is a key barrier to achieving optimal gout therapy.
“There’s too much focus on the flare and too little focus on the urate burden of the disease. Regardless of the clinical setting, the goal should be to manage [high] serum uric acid levels,” said Lawrence Edwards, MD, professor at the University of Florida, Gainesville, during his talk.
Dr. Edwards, who specializes in treating patients with gout and rheumatoid arthritis, discussed the role of primary care providers in the treatment of gout. “We can and must do better,” he said.
Understanding the pathology of gout is key to effective treatment
Knowledge of the molecular pathology of gout has advanced drastically over the last few years. “The improved understanding of the molecules involved in disease initiation and progression can help us make better treatment decisions depending on the stage of the disease,” Dr. Edwards said.
Gout is caused by the deposition of monosodium urate (MSU) crystals, which starts as asymptomatic hyperuricemia, he said. Inflammatory responses to MSU crystals are responsible for gout flares, the frequency of which increases as the disease progresses.
Innate immune responses driven by macrophages and neutrophils play a crucial role in acute gout attacks. In the molecular pathway, proinflammatory cytokines IL-1 beta and IL-6 are the mediators of gout flares, whereas IL-8 accumulates over time and contributes to disease progression and systemic illness. If left untreated or undertreated, the repeated inflammatory reaction leads to advanced gout. The urate burden also increases with disease progression.
“Physicians need to better educate themselves on the destructive nature of this inflammatory arthritis and the need for effective urate-lowering therapy in the management of gout,” Dr. Edwards said.
Management of acute gout attacks
The management of gout flares involves the use of pharmacological agents to control pain and inflammation. The three most common anti-inflammatory therapies are colchicine, NSAIDs, and corticosteroids (either oral or intramuscular).
The choice of which of these should be used alone or in combination for a flare is based on previous tolerance of the medication or the presence of diabetes, kidney disease, heart disease, or a history of upper gastrointestinal bleeding. Dr. Edwards referred internists to the 2020 American College of Rheumatology gout management guideline.
“Regardless of which therapy is chosen, the more important consideration is how quickly the patients can start treatment after the flare begins,” said Dr. Edwards when asked about priorities in the management of gout flares. “This means that the patient should have ready access to whichever the chosen approach is. We call this the ‘pill-in-the-pocket’ approach,” he added.
Reducing the urate burden is also important for effective treatment. The serum urate level is the primary marker of how well a patient’s gout is being managed. ULT should be initiated in patients with subcutaneous tophi, gout-related radiographic damage, or frequent flares (≥ 2 per year). Allopurinol is typically the first-line ULT of choice.
Dr. Edwards noted that far too much focus is placed on flare treatment rather than addressing the underlying sources of gouty symptoms – the elevated serum levels of urate.
Management of advanced gout
“The management of advanced gout is challenging, and the dissolution of MSU is slow unless you take an aggressive approach,” Dr. Edwards said.
Switching to pegloticase is recommended for patients with frequent flares, nonresolving tophi, or high serum urate levels that persist despite treatment with xanthine oxidase inhibitors or other ULT agents.
“The frequency and severity of gout flares are what patients focus on, but if that’s the only focus of the treating physicians, then they are leaving the job less than halfway done. Getting the serum urate to below a level of 6.0 mg/dL is the most important aspect in the lifelong management of gout,” said Dr. Edwards.
Barriers to effective gout treatment
When asked during an interview after the session about the most important barriers to successful gout management, Allison M. Mays, MD, a geriatric medicine subspecialist at Cedars-Sinai Medical Center, Los Angeles, said that “the fact that gout mostly impacts quality of life and not necessarily mortality means that other things may take precedent.” She explained that gout typically coexists with other comorbidities, often multiple ones. Patients may also defer taking an additional medication for a disease like gout, which has only episodic discomfort.
She added that gout management involves shared decision-making between patients and the medical team – including the primary care physician, rheumatologist, orthopedist, and emergency physician. Following a visit to the urgent care or the ED for an acute flare of gout, the patient may not follow up with their primary care doctor or bring it up at their next visit for chronic management, she noted.
Dr. Edwards serves as a consultant to Horizon Pharmaceuticals, Atom Biosciences, Shanton Biosciences, and Aclaris Therapeutics. Horizon marketed pegloticase up until last month when Amgen bought the drug. Dr. Edwards is also president of Gout Education Society, and he has no financial agreement with any of the multiple companies that produce colchicine and allopurinol. Dr. Mays reported no conflicts.
said a presenter at the annual meeting of the American College of Physicians. Failure to understand the disease process and goals of urate-lowering therapy (ULT) is a key barrier to achieving optimal gout therapy.
“There’s too much focus on the flare and too little focus on the urate burden of the disease. Regardless of the clinical setting, the goal should be to manage [high] serum uric acid levels,” said Lawrence Edwards, MD, professor at the University of Florida, Gainesville, during his talk.
Dr. Edwards, who specializes in treating patients with gout and rheumatoid arthritis, discussed the role of primary care providers in the treatment of gout. “We can and must do better,” he said.
Understanding the pathology of gout is key to effective treatment
Knowledge of the molecular pathology of gout has advanced drastically over the last few years. “The improved understanding of the molecules involved in disease initiation and progression can help us make better treatment decisions depending on the stage of the disease,” Dr. Edwards said.
Gout is caused by the deposition of monosodium urate (MSU) crystals, which starts as asymptomatic hyperuricemia, he said. Inflammatory responses to MSU crystals are responsible for gout flares, the frequency of which increases as the disease progresses.
Innate immune responses driven by macrophages and neutrophils play a crucial role in acute gout attacks. In the molecular pathway, proinflammatory cytokines IL-1 beta and IL-6 are the mediators of gout flares, whereas IL-8 accumulates over time and contributes to disease progression and systemic illness. If left untreated or undertreated, the repeated inflammatory reaction leads to advanced gout. The urate burden also increases with disease progression.
“Physicians need to better educate themselves on the destructive nature of this inflammatory arthritis and the need for effective urate-lowering therapy in the management of gout,” Dr. Edwards said.
Management of acute gout attacks
The management of gout flares involves the use of pharmacological agents to control pain and inflammation. The three most common anti-inflammatory therapies are colchicine, NSAIDs, and corticosteroids (either oral or intramuscular).
The choice of which of these should be used alone or in combination for a flare is based on previous tolerance of the medication or the presence of diabetes, kidney disease, heart disease, or a history of upper gastrointestinal bleeding. Dr. Edwards referred internists to the 2020 American College of Rheumatology gout management guideline.
“Regardless of which therapy is chosen, the more important consideration is how quickly the patients can start treatment after the flare begins,” said Dr. Edwards when asked about priorities in the management of gout flares. “This means that the patient should have ready access to whichever the chosen approach is. We call this the ‘pill-in-the-pocket’ approach,” he added.
Reducing the urate burden is also important for effective treatment. The serum urate level is the primary marker of how well a patient’s gout is being managed. ULT should be initiated in patients with subcutaneous tophi, gout-related radiographic damage, or frequent flares (≥ 2 per year). Allopurinol is typically the first-line ULT of choice.
Dr. Edwards noted that far too much focus is placed on flare treatment rather than addressing the underlying sources of gouty symptoms – the elevated serum levels of urate.
Management of advanced gout
“The management of advanced gout is challenging, and the dissolution of MSU is slow unless you take an aggressive approach,” Dr. Edwards said.
Switching to pegloticase is recommended for patients with frequent flares, nonresolving tophi, or high serum urate levels that persist despite treatment with xanthine oxidase inhibitors or other ULT agents.
“The frequency and severity of gout flares are what patients focus on, but if that’s the only focus of the treating physicians, then they are leaving the job less than halfway done. Getting the serum urate to below a level of 6.0 mg/dL is the most important aspect in the lifelong management of gout,” said Dr. Edwards.
Barriers to effective gout treatment
When asked during an interview after the session about the most important barriers to successful gout management, Allison M. Mays, MD, a geriatric medicine subspecialist at Cedars-Sinai Medical Center, Los Angeles, said that “the fact that gout mostly impacts quality of life and not necessarily mortality means that other things may take precedent.” She explained that gout typically coexists with other comorbidities, often multiple ones. Patients may also defer taking an additional medication for a disease like gout, which has only episodic discomfort.
She added that gout management involves shared decision-making between patients and the medical team – including the primary care physician, rheumatologist, orthopedist, and emergency physician. Following a visit to the urgent care or the ED for an acute flare of gout, the patient may not follow up with their primary care doctor or bring it up at their next visit for chronic management, she noted.
Dr. Edwards serves as a consultant to Horizon Pharmaceuticals, Atom Biosciences, Shanton Biosciences, and Aclaris Therapeutics. Horizon marketed pegloticase up until last month when Amgen bought the drug. Dr. Edwards is also president of Gout Education Society, and he has no financial agreement with any of the multiple companies that produce colchicine and allopurinol. Dr. Mays reported no conflicts.
AT INTERNAL MEDICINE 2023
In families with gout, obesity and alcohol add to personal risk
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE AND RESEARCH
Teamwork guides cardio-rheumatology clinics that care for unique patient population
Clinical cardiologist Heba Wassif, MD, MPH, knows the value of working with her fellow rheumatologists, surgeons, and other clinicians to establish a care plan for her patients with cardiac conditions and autoimmune diseases.
She is the cofounder of the Cleveland Clinic’s new cardio-rheumatology program, which places an emphasis on multidisciplinary care. In her role, Dr. Wassif closely follows her patients, and if she sees any inflammation or any other condition that requires the rheumatologist, she reaches out to her colleagues to adjust medications if needed.
Collaboration with a rheumatologist was important when a patient with valvular disease was prepping for surgery. The patient was on significant immunosuppressants and the surgery had to be timed appropriately, accounting for any decreases in her immunosuppression, explained Dr. Wassif, director of inpatient clinical cardiology at Cleveland Clinic in Ohio.
Cardio-rheumatology programs are “the newest child” in a series of cardiology offshoots focusing on different populations. Cardio-oncology and cardio-obstetrics took off about 6 years ago, with cardio-rheumatology clinics and interested physicians rising in number over the last several years, Dr. Wassif noted.
The relationship between cardiovascular diseases and rheumatologic conditions is certainly recognized more often, “which means more literature is being published to discuss the link,” according to Rekha Mankad, MD, a trailblazer of this model of care. She directs the Women’s Heart Clinic at Mayo Clinic in Rochester, Minn., which was one of the earliest adopters of a cardio-rheumatology clinic.
Ten years ago, “nobody was talking about the link between rheumatologic conditions and cardiovascular disease,” Dr. Mankad said. “I’ve been asked to speak on this topic, and programs have asked me to speak about establishing cardio-rheumatology practices. So, there’s been an evolution as far as a recognition that these two conditions overlap.”
Patients have come to her independent of internal referrals, which means they have done Google searches on cardiology and rheumatology. “I think that it has made a splash, at least in the world of cardiology,” Dr. Mankad observed in an interview.
Other institutions such as NYU-Langone, Yale, Stanford, Brigham and Women’s Hospital in Boston, and Women’s College Hospital in Toronto have formed similar clinics whose focus is to address the specific needs of rheumatology patients with cardiac conditions through a teamwork approach.
Challenges of treating cardiac, rheumatologic conditions
The rise in clinics addresses the longstanding connection between autoimmune disorders and cardiac conditions.
Cardiologists have known that there is an element of inflammation that contributes to atherosclerosis, said Dr. Wassif, who has researched this topic extensively. A recent study she led found a strong association between rheumatic immune-mediated inflammatory diseases (IMIDs) and high risk of acute coronary syndrome in Medicare patients.
“This particular population has a very clear increased risk for cardiovascular conditions, including valve disease and heart failure,” she emphasized.
Patients with rheumatoid arthritis and lupus have up to a twofold and eightfold higher risk of heart disease, respectively, noted Michael S. Garshick, MD, a cardiovascular disease specialist who directs the cardio-rheumatology program at NYU-Langone Health, in New York. Cardiologists “have really developed an understanding that the immune system can impact the heart, and that there’s a need for people to understand the nuance behind how the immune system can affect them and what to do about it,” Dr. Garshick said.
Caring for patients with both afflictions comes with specific challenges. Many physicians are not well trained on managing and treating patients with these dual conditions.
The “lipid paradox,” in which lipids are reduced with active inflammation in some rheumatologic conditions, can make treatment more nuanced. In addition, the traditional ASCVD (atherosclerotic cardiovascular disease) score often underestimates the cardiovascular risk of these patients, noted cardiologist Margaret Furman, MD, MPH, assistant professor and codirector of Yale’s Cardio-Rheumatology Program, New Haven, Conn.
Newer biologic medications used to treat rheumatologic diseases can alter a patient’s lipid profile, she said in an interview.
“It can be difficult to assess each individual patient’s cardiovascular risk as their disease state and treatment can vary throughout their lifetime based on their degree of inflammation. The importance of aggressive lipid management is often underestimated,” Dr. Furman added.
Cardiology and rheumatology partnerships can address gaps in care of this unique group of patients, said Vaidehi R. Chowdhary, MBBS, MD, clinical chief of the Yale Section of Rheumatology, Allergy, and Immunology at Yale University.
“The role of the rheumatologist in this dyad is to educate patients on this risk, work toward adequate control of inflammation, and minimize use of medications that contribute to increased cardiovascular risks,” said Dr. Chowdhary, who cofounded Yale’s cardio-rheumatology program with Dr. Furman.
Cardiologists in turn can assert their knowledge about medications and their impact on lipids and inflammation, Dr. Wassif said.
Many anti-inflammatory therapies are now within the cardiologist’s purview, Dr. Garshick noted. “For example, specifically with pericarditis, there’s [Food and Drug Administration]–approved anti-inflammatories or biologics. We’re the ones who feel the most comfortable giving them right now.” Cardiologists quite often are consulted about medications that are efficacious in rheumatologic conditions but could negatively impact the cardiovascular system, such as Janus kinase inhibitors, he added.
‘Reading the tea leaves’
Each program has its own unique story. For the Cleveland Clinic, the concept of a cardio-rheumatology program began during the COVID-19 pandemic in 2020. Developing such a concept and gaining institutional acceptance is always a work in process, Dr. Wassif said. “It’s not that you decide one day that you’re going to build a center, and that center is going to come into fruition overnight. You first gauge interest within your division. Who are the individuals that are interested in this area?”
Cleveland Clinic’s center is seeking to build relations between medical disciplines while spotlighting the concept of cardio-rheumatology, said Dr. Wassif, who has been providing education within the clinic and at other health institutions to ensure that patients receive appropriate attention early.
NYU-Langone launched its program amid this heightened awareness that the immune system could affect atherosclerosis, “kind of reading of the tea leaves, so to speak,” Dr. Garshick said.
Several clinical trials served as a catalyst for this movement. “A lot of clinical cardiologists were never 100% convinced that targeting the immune system reduced cardiovascular disease,” he said. Then the CANTOS clinical trial came along and showed for the first time that a therapeutic monoclonal antibody targeting interleukin-1beta, a cytokine central to inflammatory response, could in fact reduce cardiovascular disease.
Trials like this, along with epidemiologic literature connecting the rheumatologic and the autoimmune conditions with cardiovascular disease, pushed this concept to the forefront, Dr. Garshick said.
The notion that a clinic could successfully address cardiac problems in patients with rheumatic diseases yielded promising returns at Women’s College Hospital in Toronto, according to a report presented at the 2018 American College of Rheumatology annual meeting. Researchers reported that patients with rheumatologic conditions who attended a cardio-rheumatology clinic at this center saw improvements in care. The clinic identified increased cardiovascular risk and early atherosclerosis, and 53.8% of patients altered their medications after being seen in the clinic.
A total of 39.7% and 32.1% received lipid lowering and antiplatelet therapies, respectively, and 14% received antihypertensive therapy. A small percentage were treated for heart failure or placed on lifelong anticoagulation therapy for atrial fibrillation, and one patient received a percutaneous coronary stent.
Ins and outs of the referral process
Initially designed for preventive cardiac risk assessment, Yale’s program evolved into a multidisciplinary, patient-centered approach for the management of complex cardiovascular conditions in patients with autoimmune rheumatologic diseases.
The program is open to anyone who carries a diagnosis of rheumatologic disease or has elevated inflammatory markers. “Every patient, regardless of the reason for the referral, receives a cardiovascular risk assessment,” Dr. Furman said.
Most referrals come from rheumatologists, although cardiology colleagues and pulmonologists have also sent referrals. A pulmonologist, for example, may want to rule out a cardiac cause to shortness of breath. The patient’s workup, care, and follow-up are based on the reason for referral.
“We are currently referring patients with established cardiac disease, traditional risk factors, or for better risk assessment for primary prevention of coronary artery disease,” Dr. Chowdhary said. “We communicate very frequently about medication changes, and patients are aware of goals of care from both sides.”
Dr. Furman works closely with several of the rheumatology specialists taking care of patients with rheumatoid arthritis, systemic lupus erythematosus, and scleroderma.
Rheumatology follows patients every 3-6 months or more frequently based on their disease activity.
Dr. Mankad uses her sleuthing skills at Mayo Clinic to determine what the patients need. If they come in for a preventive assessment, she looks more closely at their cardiovascular risks and may order additional imaging to look for subclinical atherosclerosis. “We’re more aggressive with statin therapy in this population because of that,” she said.
If it’s valve disease, she pays extra attention to the patients’ valves in the echocardiograms and follows them a bit more regularly than someone without a rheumatologic condition and valve disease.
For patients with heart failure signs or symptoms, “it depends on how symptomatic they are,” Dr. Mankad said. In some instances, she may look for evidence of heart failure with preserved ejection fraction in patients who have rheumatoid arthritis who happen to be short of breath. “There’s so many different manifestations that patients with rheumatologic conditions can have as far as what could be affected in the heart,” she noted.
Quite frequently, Dr. Mankad identifies subclinical disease in her patients with rheumatoid arthritis. “I’ve seen many patients whose risk scores would not dictate statin therapy. But I went looking for subclinical disease by either doing coronary assessment or carotid assessment and have found atherosclerosis that would be enough to warrant statin therapy.”
A personalized assessment to reduce cardiac risk
NYU-Langone’s program offers opportunities to educate patients about the link between cardiac and rheumatologic disease.
“Their rheumatologist or their dermatologist will say, ‘Hey, have you heard about the connection between psoriasis, psoriatic or rheumatoid arthritis, and heart disease and the risk of heart attack or stroke?’ ” Dr. Garshick said.
The patients will often say they know nothing about these connections and want to learn more about how to treat it.
“We’ll say, ‘we have someone here that can help you.’ They’ll send them to myself or other colleagues like me across the country. We’ll assess blood pressure, weight, lipids, hemoglobin A1c, and other serologic and oftentimes imaging biomarkers of cardiovascular risk.” The patients will receive a personalized assessment, listing things they can do to lower their risk, whether it’s diet, exercise, or lifestyle. “Many times it can involve medications to reduce heart disease risk,” said Dr. Garshick.
In some instances, a rheumatologist or dermatologist may be concerned about starting a patient on a specific medication for the disease such as a JAK inhibitor. “We’ll help assess their risk because there’s been a lot of literature out in the rheumatology world about the risk of JAK inhibitors and heart disease and blood clots,” said Dr. Garshick.
Dr. Garshick also sees patients with rheumatologic conditions who have a specific cardiovascular concern or complaint such as shortness of breath or chest pain. “We’ll work that up with a specific knowledge of the underlying immune condition and how that may impact their heart,” he said.
Advances in research
As they continue to see patients and devise specific care plans, developers of cardio-rheumatology programs have been supplementing their work with ongoing research.
Yale’s clinic is expanding this year to include a new attending physician, Attila Feher, MD, PhD, who has conducted research in autoimmunity and microcirculation using molecular imaging and multimodality imaging techniques. Prevalence of coronary microvascular dysfunction appears to be increased in this patient population, Dr. Furman said.
Dr. Wassif recently coauthored a paper that examined patients with underlying rheumatologic conditions who undergo valvular and aortic valve replacement. “To our surprise, there was really no difference between patients with autoimmune conditions and others with nonautoimmune conditions,” she said, adding that the study had its limitations.
Other work includes data on Medicare patients with ST- and non-ST-elevation myocardial infarctions who have an underlying autoimmune disorder. Dr. Wassif and her colleagues found that their long-term outcomes are worse than those of patients without these conditions. “It’s unclear if worse outcomes are related to complications of autoimmunity versus the extent of their underlying disease. This is a work in progress and certainly an area that is ripe for research.”
Dr. Garshick and other collaborators at NYU have been focusing on the endothelium, specifically platelet biology in patients with psoriasis, psoriatic arthritis, and lupus. “We’re about to start the same research with gout as well,” he said.
“The process we’re most interested in is understanding how these diseases impact the early stages of cholesterol. And the way we’re doing that is evaluating the vasculature, specifically the endothelium,” he said.
He has finished two clinical trials that evaluate how standard heart disease medications such as aspirin and statins impact or can potentially benefit patients with psoriasis and/or psoriatic arthritis. “We have a whole list of other trials in the pipeline with other institutions across the country.”
Through a grant, Dr. Mankad is assessing whether a PET scan could detect inflammation in the hearts of rheumatoid arthritis patients. “We’re looking to see if the reason these patients have heart failure later in life is because their heart muscle actually shows evidence of inflammation, even when they have no symptoms,” she explained.
Other tests such as echocardiogram and CT scans will be used to evaluate coronary disease in about 40-50 patients. The goal of using these multiple imaging tools is to find markers indicating that the heart is affected by rheumatoid arthritis, which may indicate a higher likelihood of developing heart failure, she said.
Clinics are popping up
Through these new clinics, some collaborations have emerged. Dr. Garshick works closely with Brigham and Women’s Hospital, which has a similar cardio-rheumatology program, run by Brittany Weber, MD, to exchange ideas, discuss challenging cases, and collaborate.
“There are a lot of clinics like us popping up across the country,” he observed. Every so often, he hears from other institutions that are interested in starting their own cardio-rheumatology programs. “They ask us: How do you start, what should we look for?”
It’s an education process for both patients and providers, Dr. Garshick emphasized. “I also think it’s a bandwidth issue. Many of our rheumatology and dermatology colleagues are acutely aware of the connection, but there may not be enough time at a clinic visit to really go in depth” with these dual conditions, he said.
NYU-Langone Health for the past several years has been holding a symposium to educate people on the cardio-rheumatology connection and treating inflammation in cardiovascular disease. This year’s symposium, held in conjunction with Brigham and Women’s Hospital, is scheduled for April 28. For more information, visit the course website: nyulmc.org/cvinflammationcme.
“What we’re trying to do is help [other institutions] get that bandwidth” to adequately help and serve these patients, he said.
Dr. Garshick has received consultant fees from Abbvie and Horizon therapeutics and an unrestricted research grant from Pfizer. No other sources had relevant financial disclosures.
Clinical cardiologist Heba Wassif, MD, MPH, knows the value of working with her fellow rheumatologists, surgeons, and other clinicians to establish a care plan for her patients with cardiac conditions and autoimmune diseases.
She is the cofounder of the Cleveland Clinic’s new cardio-rheumatology program, which places an emphasis on multidisciplinary care. In her role, Dr. Wassif closely follows her patients, and if she sees any inflammation or any other condition that requires the rheumatologist, she reaches out to her colleagues to adjust medications if needed.
Collaboration with a rheumatologist was important when a patient with valvular disease was prepping for surgery. The patient was on significant immunosuppressants and the surgery had to be timed appropriately, accounting for any decreases in her immunosuppression, explained Dr. Wassif, director of inpatient clinical cardiology at Cleveland Clinic in Ohio.
Cardio-rheumatology programs are “the newest child” in a series of cardiology offshoots focusing on different populations. Cardio-oncology and cardio-obstetrics took off about 6 years ago, with cardio-rheumatology clinics and interested physicians rising in number over the last several years, Dr. Wassif noted.
The relationship between cardiovascular diseases and rheumatologic conditions is certainly recognized more often, “which means more literature is being published to discuss the link,” according to Rekha Mankad, MD, a trailblazer of this model of care. She directs the Women’s Heart Clinic at Mayo Clinic in Rochester, Minn., which was one of the earliest adopters of a cardio-rheumatology clinic.
Ten years ago, “nobody was talking about the link between rheumatologic conditions and cardiovascular disease,” Dr. Mankad said. “I’ve been asked to speak on this topic, and programs have asked me to speak about establishing cardio-rheumatology practices. So, there’s been an evolution as far as a recognition that these two conditions overlap.”
Patients have come to her independent of internal referrals, which means they have done Google searches on cardiology and rheumatology. “I think that it has made a splash, at least in the world of cardiology,” Dr. Mankad observed in an interview.
Other institutions such as NYU-Langone, Yale, Stanford, Brigham and Women’s Hospital in Boston, and Women’s College Hospital in Toronto have formed similar clinics whose focus is to address the specific needs of rheumatology patients with cardiac conditions through a teamwork approach.
Challenges of treating cardiac, rheumatologic conditions
The rise in clinics addresses the longstanding connection between autoimmune disorders and cardiac conditions.
Cardiologists have known that there is an element of inflammation that contributes to atherosclerosis, said Dr. Wassif, who has researched this topic extensively. A recent study she led found a strong association between rheumatic immune-mediated inflammatory diseases (IMIDs) and high risk of acute coronary syndrome in Medicare patients.
“This particular population has a very clear increased risk for cardiovascular conditions, including valve disease and heart failure,” she emphasized.
Patients with rheumatoid arthritis and lupus have up to a twofold and eightfold higher risk of heart disease, respectively, noted Michael S. Garshick, MD, a cardiovascular disease specialist who directs the cardio-rheumatology program at NYU-Langone Health, in New York. Cardiologists “have really developed an understanding that the immune system can impact the heart, and that there’s a need for people to understand the nuance behind how the immune system can affect them and what to do about it,” Dr. Garshick said.
Caring for patients with both afflictions comes with specific challenges. Many physicians are not well trained on managing and treating patients with these dual conditions.
The “lipid paradox,” in which lipids are reduced with active inflammation in some rheumatologic conditions, can make treatment more nuanced. In addition, the traditional ASCVD (atherosclerotic cardiovascular disease) score often underestimates the cardiovascular risk of these patients, noted cardiologist Margaret Furman, MD, MPH, assistant professor and codirector of Yale’s Cardio-Rheumatology Program, New Haven, Conn.
Newer biologic medications used to treat rheumatologic diseases can alter a patient’s lipid profile, she said in an interview.
“It can be difficult to assess each individual patient’s cardiovascular risk as their disease state and treatment can vary throughout their lifetime based on their degree of inflammation. The importance of aggressive lipid management is often underestimated,” Dr. Furman added.
Cardiology and rheumatology partnerships can address gaps in care of this unique group of patients, said Vaidehi R. Chowdhary, MBBS, MD, clinical chief of the Yale Section of Rheumatology, Allergy, and Immunology at Yale University.
“The role of the rheumatologist in this dyad is to educate patients on this risk, work toward adequate control of inflammation, and minimize use of medications that contribute to increased cardiovascular risks,” said Dr. Chowdhary, who cofounded Yale’s cardio-rheumatology program with Dr. Furman.
Cardiologists in turn can assert their knowledge about medications and their impact on lipids and inflammation, Dr. Wassif said.
Many anti-inflammatory therapies are now within the cardiologist’s purview, Dr. Garshick noted. “For example, specifically with pericarditis, there’s [Food and Drug Administration]–approved anti-inflammatories or biologics. We’re the ones who feel the most comfortable giving them right now.” Cardiologists quite often are consulted about medications that are efficacious in rheumatologic conditions but could negatively impact the cardiovascular system, such as Janus kinase inhibitors, he added.
‘Reading the tea leaves’
Each program has its own unique story. For the Cleveland Clinic, the concept of a cardio-rheumatology program began during the COVID-19 pandemic in 2020. Developing such a concept and gaining institutional acceptance is always a work in process, Dr. Wassif said. “It’s not that you decide one day that you’re going to build a center, and that center is going to come into fruition overnight. You first gauge interest within your division. Who are the individuals that are interested in this area?”
Cleveland Clinic’s center is seeking to build relations between medical disciplines while spotlighting the concept of cardio-rheumatology, said Dr. Wassif, who has been providing education within the clinic and at other health institutions to ensure that patients receive appropriate attention early.
NYU-Langone launched its program amid this heightened awareness that the immune system could affect atherosclerosis, “kind of reading of the tea leaves, so to speak,” Dr. Garshick said.
Several clinical trials served as a catalyst for this movement. “A lot of clinical cardiologists were never 100% convinced that targeting the immune system reduced cardiovascular disease,” he said. Then the CANTOS clinical trial came along and showed for the first time that a therapeutic monoclonal antibody targeting interleukin-1beta, a cytokine central to inflammatory response, could in fact reduce cardiovascular disease.
Trials like this, along with epidemiologic literature connecting the rheumatologic and the autoimmune conditions with cardiovascular disease, pushed this concept to the forefront, Dr. Garshick said.
The notion that a clinic could successfully address cardiac problems in patients with rheumatic diseases yielded promising returns at Women’s College Hospital in Toronto, according to a report presented at the 2018 American College of Rheumatology annual meeting. Researchers reported that patients with rheumatologic conditions who attended a cardio-rheumatology clinic at this center saw improvements in care. The clinic identified increased cardiovascular risk and early atherosclerosis, and 53.8% of patients altered their medications after being seen in the clinic.
A total of 39.7% and 32.1% received lipid lowering and antiplatelet therapies, respectively, and 14% received antihypertensive therapy. A small percentage were treated for heart failure or placed on lifelong anticoagulation therapy for atrial fibrillation, and one patient received a percutaneous coronary stent.
Ins and outs of the referral process
Initially designed for preventive cardiac risk assessment, Yale’s program evolved into a multidisciplinary, patient-centered approach for the management of complex cardiovascular conditions in patients with autoimmune rheumatologic diseases.
The program is open to anyone who carries a diagnosis of rheumatologic disease or has elevated inflammatory markers. “Every patient, regardless of the reason for the referral, receives a cardiovascular risk assessment,” Dr. Furman said.
Most referrals come from rheumatologists, although cardiology colleagues and pulmonologists have also sent referrals. A pulmonologist, for example, may want to rule out a cardiac cause to shortness of breath. The patient’s workup, care, and follow-up are based on the reason for referral.
“We are currently referring patients with established cardiac disease, traditional risk factors, or for better risk assessment for primary prevention of coronary artery disease,” Dr. Chowdhary said. “We communicate very frequently about medication changes, and patients are aware of goals of care from both sides.”
Dr. Furman works closely with several of the rheumatology specialists taking care of patients with rheumatoid arthritis, systemic lupus erythematosus, and scleroderma.
Rheumatology follows patients every 3-6 months or more frequently based on their disease activity.
Dr. Mankad uses her sleuthing skills at Mayo Clinic to determine what the patients need. If they come in for a preventive assessment, she looks more closely at their cardiovascular risks and may order additional imaging to look for subclinical atherosclerosis. “We’re more aggressive with statin therapy in this population because of that,” she said.
If it’s valve disease, she pays extra attention to the patients’ valves in the echocardiograms and follows them a bit more regularly than someone without a rheumatologic condition and valve disease.
For patients with heart failure signs or symptoms, “it depends on how symptomatic they are,” Dr. Mankad said. In some instances, she may look for evidence of heart failure with preserved ejection fraction in patients who have rheumatoid arthritis who happen to be short of breath. “There’s so many different manifestations that patients with rheumatologic conditions can have as far as what could be affected in the heart,” she noted.
Quite frequently, Dr. Mankad identifies subclinical disease in her patients with rheumatoid arthritis. “I’ve seen many patients whose risk scores would not dictate statin therapy. But I went looking for subclinical disease by either doing coronary assessment or carotid assessment and have found atherosclerosis that would be enough to warrant statin therapy.”
A personalized assessment to reduce cardiac risk
NYU-Langone’s program offers opportunities to educate patients about the link between cardiac and rheumatologic disease.
“Their rheumatologist or their dermatologist will say, ‘Hey, have you heard about the connection between psoriasis, psoriatic or rheumatoid arthritis, and heart disease and the risk of heart attack or stroke?’ ” Dr. Garshick said.
The patients will often say they know nothing about these connections and want to learn more about how to treat it.
“We’ll say, ‘we have someone here that can help you.’ They’ll send them to myself or other colleagues like me across the country. We’ll assess blood pressure, weight, lipids, hemoglobin A1c, and other serologic and oftentimes imaging biomarkers of cardiovascular risk.” The patients will receive a personalized assessment, listing things they can do to lower their risk, whether it’s diet, exercise, or lifestyle. “Many times it can involve medications to reduce heart disease risk,” said Dr. Garshick.
In some instances, a rheumatologist or dermatologist may be concerned about starting a patient on a specific medication for the disease such as a JAK inhibitor. “We’ll help assess their risk because there’s been a lot of literature out in the rheumatology world about the risk of JAK inhibitors and heart disease and blood clots,” said Dr. Garshick.
Dr. Garshick also sees patients with rheumatologic conditions who have a specific cardiovascular concern or complaint such as shortness of breath or chest pain. “We’ll work that up with a specific knowledge of the underlying immune condition and how that may impact their heart,” he said.
Advances in research
As they continue to see patients and devise specific care plans, developers of cardio-rheumatology programs have been supplementing their work with ongoing research.
Yale’s clinic is expanding this year to include a new attending physician, Attila Feher, MD, PhD, who has conducted research in autoimmunity and microcirculation using molecular imaging and multimodality imaging techniques. Prevalence of coronary microvascular dysfunction appears to be increased in this patient population, Dr. Furman said.
Dr. Wassif recently coauthored a paper that examined patients with underlying rheumatologic conditions who undergo valvular and aortic valve replacement. “To our surprise, there was really no difference between patients with autoimmune conditions and others with nonautoimmune conditions,” she said, adding that the study had its limitations.
Other work includes data on Medicare patients with ST- and non-ST-elevation myocardial infarctions who have an underlying autoimmune disorder. Dr. Wassif and her colleagues found that their long-term outcomes are worse than those of patients without these conditions. “It’s unclear if worse outcomes are related to complications of autoimmunity versus the extent of their underlying disease. This is a work in progress and certainly an area that is ripe for research.”
Dr. Garshick and other collaborators at NYU have been focusing on the endothelium, specifically platelet biology in patients with psoriasis, psoriatic arthritis, and lupus. “We’re about to start the same research with gout as well,” he said.
“The process we’re most interested in is understanding how these diseases impact the early stages of cholesterol. And the way we’re doing that is evaluating the vasculature, specifically the endothelium,” he said.
He has finished two clinical trials that evaluate how standard heart disease medications such as aspirin and statins impact or can potentially benefit patients with psoriasis and/or psoriatic arthritis. “We have a whole list of other trials in the pipeline with other institutions across the country.”
Through a grant, Dr. Mankad is assessing whether a PET scan could detect inflammation in the hearts of rheumatoid arthritis patients. “We’re looking to see if the reason these patients have heart failure later in life is because their heart muscle actually shows evidence of inflammation, even when they have no symptoms,” she explained.
Other tests such as echocardiogram and CT scans will be used to evaluate coronary disease in about 40-50 patients. The goal of using these multiple imaging tools is to find markers indicating that the heart is affected by rheumatoid arthritis, which may indicate a higher likelihood of developing heart failure, she said.
Clinics are popping up
Through these new clinics, some collaborations have emerged. Dr. Garshick works closely with Brigham and Women’s Hospital, which has a similar cardio-rheumatology program, run by Brittany Weber, MD, to exchange ideas, discuss challenging cases, and collaborate.
“There are a lot of clinics like us popping up across the country,” he observed. Every so often, he hears from other institutions that are interested in starting their own cardio-rheumatology programs. “They ask us: How do you start, what should we look for?”
It’s an education process for both patients and providers, Dr. Garshick emphasized. “I also think it’s a bandwidth issue. Many of our rheumatology and dermatology colleagues are acutely aware of the connection, but there may not be enough time at a clinic visit to really go in depth” with these dual conditions, he said.
NYU-Langone Health for the past several years has been holding a symposium to educate people on the cardio-rheumatology connection and treating inflammation in cardiovascular disease. This year’s symposium, held in conjunction with Brigham and Women’s Hospital, is scheduled for April 28. For more information, visit the course website: nyulmc.org/cvinflammationcme.
“What we’re trying to do is help [other institutions] get that bandwidth” to adequately help and serve these patients, he said.
Dr. Garshick has received consultant fees from Abbvie and Horizon therapeutics and an unrestricted research grant from Pfizer. No other sources had relevant financial disclosures.
Clinical cardiologist Heba Wassif, MD, MPH, knows the value of working with her fellow rheumatologists, surgeons, and other clinicians to establish a care plan for her patients with cardiac conditions and autoimmune diseases.
She is the cofounder of the Cleveland Clinic’s new cardio-rheumatology program, which places an emphasis on multidisciplinary care. In her role, Dr. Wassif closely follows her patients, and if she sees any inflammation or any other condition that requires the rheumatologist, she reaches out to her colleagues to adjust medications if needed.
Collaboration with a rheumatologist was important when a patient with valvular disease was prepping for surgery. The patient was on significant immunosuppressants and the surgery had to be timed appropriately, accounting for any decreases in her immunosuppression, explained Dr. Wassif, director of inpatient clinical cardiology at Cleveland Clinic in Ohio.
Cardio-rheumatology programs are “the newest child” in a series of cardiology offshoots focusing on different populations. Cardio-oncology and cardio-obstetrics took off about 6 years ago, with cardio-rheumatology clinics and interested physicians rising in number over the last several years, Dr. Wassif noted.
The relationship between cardiovascular diseases and rheumatologic conditions is certainly recognized more often, “which means more literature is being published to discuss the link,” according to Rekha Mankad, MD, a trailblazer of this model of care. She directs the Women’s Heart Clinic at Mayo Clinic in Rochester, Minn., which was one of the earliest adopters of a cardio-rheumatology clinic.
Ten years ago, “nobody was talking about the link between rheumatologic conditions and cardiovascular disease,” Dr. Mankad said. “I’ve been asked to speak on this topic, and programs have asked me to speak about establishing cardio-rheumatology practices. So, there’s been an evolution as far as a recognition that these two conditions overlap.”
Patients have come to her independent of internal referrals, which means they have done Google searches on cardiology and rheumatology. “I think that it has made a splash, at least in the world of cardiology,” Dr. Mankad observed in an interview.
Other institutions such as NYU-Langone, Yale, Stanford, Brigham and Women’s Hospital in Boston, and Women’s College Hospital in Toronto have formed similar clinics whose focus is to address the specific needs of rheumatology patients with cardiac conditions through a teamwork approach.
Challenges of treating cardiac, rheumatologic conditions
The rise in clinics addresses the longstanding connection between autoimmune disorders and cardiac conditions.
Cardiologists have known that there is an element of inflammation that contributes to atherosclerosis, said Dr. Wassif, who has researched this topic extensively. A recent study she led found a strong association between rheumatic immune-mediated inflammatory diseases (IMIDs) and high risk of acute coronary syndrome in Medicare patients.
“This particular population has a very clear increased risk for cardiovascular conditions, including valve disease and heart failure,” she emphasized.
Patients with rheumatoid arthritis and lupus have up to a twofold and eightfold higher risk of heart disease, respectively, noted Michael S. Garshick, MD, a cardiovascular disease specialist who directs the cardio-rheumatology program at NYU-Langone Health, in New York. Cardiologists “have really developed an understanding that the immune system can impact the heart, and that there’s a need for people to understand the nuance behind how the immune system can affect them and what to do about it,” Dr. Garshick said.
Caring for patients with both afflictions comes with specific challenges. Many physicians are not well trained on managing and treating patients with these dual conditions.
The “lipid paradox,” in which lipids are reduced with active inflammation in some rheumatologic conditions, can make treatment more nuanced. In addition, the traditional ASCVD (atherosclerotic cardiovascular disease) score often underestimates the cardiovascular risk of these patients, noted cardiologist Margaret Furman, MD, MPH, assistant professor and codirector of Yale’s Cardio-Rheumatology Program, New Haven, Conn.
Newer biologic medications used to treat rheumatologic diseases can alter a patient’s lipid profile, she said in an interview.
“It can be difficult to assess each individual patient’s cardiovascular risk as their disease state and treatment can vary throughout their lifetime based on their degree of inflammation. The importance of aggressive lipid management is often underestimated,” Dr. Furman added.
Cardiology and rheumatology partnerships can address gaps in care of this unique group of patients, said Vaidehi R. Chowdhary, MBBS, MD, clinical chief of the Yale Section of Rheumatology, Allergy, and Immunology at Yale University.
“The role of the rheumatologist in this dyad is to educate patients on this risk, work toward adequate control of inflammation, and minimize use of medications that contribute to increased cardiovascular risks,” said Dr. Chowdhary, who cofounded Yale’s cardio-rheumatology program with Dr. Furman.
Cardiologists in turn can assert their knowledge about medications and their impact on lipids and inflammation, Dr. Wassif said.
Many anti-inflammatory therapies are now within the cardiologist’s purview, Dr. Garshick noted. “For example, specifically with pericarditis, there’s [Food and Drug Administration]–approved anti-inflammatories or biologics. We’re the ones who feel the most comfortable giving them right now.” Cardiologists quite often are consulted about medications that are efficacious in rheumatologic conditions but could negatively impact the cardiovascular system, such as Janus kinase inhibitors, he added.
‘Reading the tea leaves’
Each program has its own unique story. For the Cleveland Clinic, the concept of a cardio-rheumatology program began during the COVID-19 pandemic in 2020. Developing such a concept and gaining institutional acceptance is always a work in process, Dr. Wassif said. “It’s not that you decide one day that you’re going to build a center, and that center is going to come into fruition overnight. You first gauge interest within your division. Who are the individuals that are interested in this area?”
Cleveland Clinic’s center is seeking to build relations between medical disciplines while spotlighting the concept of cardio-rheumatology, said Dr. Wassif, who has been providing education within the clinic and at other health institutions to ensure that patients receive appropriate attention early.
NYU-Langone launched its program amid this heightened awareness that the immune system could affect atherosclerosis, “kind of reading of the tea leaves, so to speak,” Dr. Garshick said.
Several clinical trials served as a catalyst for this movement. “A lot of clinical cardiologists were never 100% convinced that targeting the immune system reduced cardiovascular disease,” he said. Then the CANTOS clinical trial came along and showed for the first time that a therapeutic monoclonal antibody targeting interleukin-1beta, a cytokine central to inflammatory response, could in fact reduce cardiovascular disease.
Trials like this, along with epidemiologic literature connecting the rheumatologic and the autoimmune conditions with cardiovascular disease, pushed this concept to the forefront, Dr. Garshick said.
The notion that a clinic could successfully address cardiac problems in patients with rheumatic diseases yielded promising returns at Women’s College Hospital in Toronto, according to a report presented at the 2018 American College of Rheumatology annual meeting. Researchers reported that patients with rheumatologic conditions who attended a cardio-rheumatology clinic at this center saw improvements in care. The clinic identified increased cardiovascular risk and early atherosclerosis, and 53.8% of patients altered their medications after being seen in the clinic.
A total of 39.7% and 32.1% received lipid lowering and antiplatelet therapies, respectively, and 14% received antihypertensive therapy. A small percentage were treated for heart failure or placed on lifelong anticoagulation therapy for atrial fibrillation, and one patient received a percutaneous coronary stent.
Ins and outs of the referral process
Initially designed for preventive cardiac risk assessment, Yale’s program evolved into a multidisciplinary, patient-centered approach for the management of complex cardiovascular conditions in patients with autoimmune rheumatologic diseases.
The program is open to anyone who carries a diagnosis of rheumatologic disease or has elevated inflammatory markers. “Every patient, regardless of the reason for the referral, receives a cardiovascular risk assessment,” Dr. Furman said.
Most referrals come from rheumatologists, although cardiology colleagues and pulmonologists have also sent referrals. A pulmonologist, for example, may want to rule out a cardiac cause to shortness of breath. The patient’s workup, care, and follow-up are based on the reason for referral.
“We are currently referring patients with established cardiac disease, traditional risk factors, or for better risk assessment for primary prevention of coronary artery disease,” Dr. Chowdhary said. “We communicate very frequently about medication changes, and patients are aware of goals of care from both sides.”
Dr. Furman works closely with several of the rheumatology specialists taking care of patients with rheumatoid arthritis, systemic lupus erythematosus, and scleroderma.
Rheumatology follows patients every 3-6 months or more frequently based on their disease activity.
Dr. Mankad uses her sleuthing skills at Mayo Clinic to determine what the patients need. If they come in for a preventive assessment, she looks more closely at their cardiovascular risks and may order additional imaging to look for subclinical atherosclerosis. “We’re more aggressive with statin therapy in this population because of that,” she said.
If it’s valve disease, she pays extra attention to the patients’ valves in the echocardiograms and follows them a bit more regularly than someone without a rheumatologic condition and valve disease.
For patients with heart failure signs or symptoms, “it depends on how symptomatic they are,” Dr. Mankad said. In some instances, she may look for evidence of heart failure with preserved ejection fraction in patients who have rheumatoid arthritis who happen to be short of breath. “There’s so many different manifestations that patients with rheumatologic conditions can have as far as what could be affected in the heart,” she noted.
Quite frequently, Dr. Mankad identifies subclinical disease in her patients with rheumatoid arthritis. “I’ve seen many patients whose risk scores would not dictate statin therapy. But I went looking for subclinical disease by either doing coronary assessment or carotid assessment and have found atherosclerosis that would be enough to warrant statin therapy.”
A personalized assessment to reduce cardiac risk
NYU-Langone’s program offers opportunities to educate patients about the link between cardiac and rheumatologic disease.
“Their rheumatologist or their dermatologist will say, ‘Hey, have you heard about the connection between psoriasis, psoriatic or rheumatoid arthritis, and heart disease and the risk of heart attack or stroke?’ ” Dr. Garshick said.
The patients will often say they know nothing about these connections and want to learn more about how to treat it.
“We’ll say, ‘we have someone here that can help you.’ They’ll send them to myself or other colleagues like me across the country. We’ll assess blood pressure, weight, lipids, hemoglobin A1c, and other serologic and oftentimes imaging biomarkers of cardiovascular risk.” The patients will receive a personalized assessment, listing things they can do to lower their risk, whether it’s diet, exercise, or lifestyle. “Many times it can involve medications to reduce heart disease risk,” said Dr. Garshick.
In some instances, a rheumatologist or dermatologist may be concerned about starting a patient on a specific medication for the disease such as a JAK inhibitor. “We’ll help assess their risk because there’s been a lot of literature out in the rheumatology world about the risk of JAK inhibitors and heart disease and blood clots,” said Dr. Garshick.
Dr. Garshick also sees patients with rheumatologic conditions who have a specific cardiovascular concern or complaint such as shortness of breath or chest pain. “We’ll work that up with a specific knowledge of the underlying immune condition and how that may impact their heart,” he said.
Advances in research
As they continue to see patients and devise specific care plans, developers of cardio-rheumatology programs have been supplementing their work with ongoing research.
Yale’s clinic is expanding this year to include a new attending physician, Attila Feher, MD, PhD, who has conducted research in autoimmunity and microcirculation using molecular imaging and multimodality imaging techniques. Prevalence of coronary microvascular dysfunction appears to be increased in this patient population, Dr. Furman said.
Dr. Wassif recently coauthored a paper that examined patients with underlying rheumatologic conditions who undergo valvular and aortic valve replacement. “To our surprise, there was really no difference between patients with autoimmune conditions and others with nonautoimmune conditions,” she said, adding that the study had its limitations.
Other work includes data on Medicare patients with ST- and non-ST-elevation myocardial infarctions who have an underlying autoimmune disorder. Dr. Wassif and her colleagues found that their long-term outcomes are worse than those of patients without these conditions. “It’s unclear if worse outcomes are related to complications of autoimmunity versus the extent of their underlying disease. This is a work in progress and certainly an area that is ripe for research.”
Dr. Garshick and other collaborators at NYU have been focusing on the endothelium, specifically platelet biology in patients with psoriasis, psoriatic arthritis, and lupus. “We’re about to start the same research with gout as well,” he said.
“The process we’re most interested in is understanding how these diseases impact the early stages of cholesterol. And the way we’re doing that is evaluating the vasculature, specifically the endothelium,” he said.
He has finished two clinical trials that evaluate how standard heart disease medications such as aspirin and statins impact or can potentially benefit patients with psoriasis and/or psoriatic arthritis. “We have a whole list of other trials in the pipeline with other institutions across the country.”
Through a grant, Dr. Mankad is assessing whether a PET scan could detect inflammation in the hearts of rheumatoid arthritis patients. “We’re looking to see if the reason these patients have heart failure later in life is because their heart muscle actually shows evidence of inflammation, even when they have no symptoms,” she explained.
Other tests such as echocardiogram and CT scans will be used to evaluate coronary disease in about 40-50 patients. The goal of using these multiple imaging tools is to find markers indicating that the heart is affected by rheumatoid arthritis, which may indicate a higher likelihood of developing heart failure, she said.
Clinics are popping up
Through these new clinics, some collaborations have emerged. Dr. Garshick works closely with Brigham and Women’s Hospital, which has a similar cardio-rheumatology program, run by Brittany Weber, MD, to exchange ideas, discuss challenging cases, and collaborate.
“There are a lot of clinics like us popping up across the country,” he observed. Every so often, he hears from other institutions that are interested in starting their own cardio-rheumatology programs. “They ask us: How do you start, what should we look for?”
It’s an education process for both patients and providers, Dr. Garshick emphasized. “I also think it’s a bandwidth issue. Many of our rheumatology and dermatology colleagues are acutely aware of the connection, but there may not be enough time at a clinic visit to really go in depth” with these dual conditions, he said.
NYU-Langone Health for the past several years has been holding a symposium to educate people on the cardio-rheumatology connection and treating inflammation in cardiovascular disease. This year’s symposium, held in conjunction with Brigham and Women’s Hospital, is scheduled for April 28. For more information, visit the course website: nyulmc.org/cvinflammationcme.
“What we’re trying to do is help [other institutions] get that bandwidth” to adequately help and serve these patients, he said.
Dr. Garshick has received consultant fees from Abbvie and Horizon therapeutics and an unrestricted research grant from Pfizer. No other sources had relevant financial disclosures.
Phil Robinson: Rheumatologist, colleague, huntsman spider rescuer
Helen Tanner remembers stealing glimpses of her husband, Philip (“Phil”) Robinson, MBChB, PhD, associate professor at the University of Queensland (Australia), catching and rehoming huge Huntsman spiders. Robinson made the extra effort because he didn’t want to hurt them; he wasn’t a big fan of the large spider with a potential leg span of 6 inches that’s commonly found in Australia, per the Australian Museum.
Robinson also relished taking his children, Eddie, 4, and Tommy, 7, on roller coaster rides, which they enjoyed, despite the experience typically giving him motion sickness, Tanner said.
“He would do anything to make the children happy,” she said. “His children meant the world to him.”
Robinson died Jan. 3 as a result of diffuse gastric adenocarcinoma, according to his wife, who added that it was a short, 2-week-long illness.
A leader of global effort to understand COVID-19 and rheumatic disease
Jinoos Yazdany, MD, MPH, chief of the division of rheumatology at Zuckerberg San Francisco General Hospital and professor of medicine at the University of California, San Francisco, described Robinson as “one of the hardest-working people I have ever known. ... [still] his deep love and dedication for his family and kids was always present.”
Robinson would wake up early, even on weekends, to lead international calls across multiple time zones, Yazdany said. “He was driven by a deep curiosity and an intense desire to generate scholarship that would help people with rheumatic diseases.”
Yazdany added that Robinson had a full research portfolio in gout and spondyloarthritis and a busy clinical practice.
She often caught glimpses of Robinson’s young children during Zoom calls. “He was also a talented baker and loved to bake with his kids, often posting pictures of his creations on social media,” Yazdany said.
A mutual colleague compiled some of Robinson’s baking successes. That includes “ ‘probably about to be locked down’ cookies” on July 17, 2021, and “Queensland lockdown cookies!!!” on July 2, 2021. Reuters reported on July 21, 2021, that Australia was witnessing an alarming increase in COVID-19 cases.
Robinson also worked his social media skills to rally support for the COVID-19 Global Rheumatology Alliance, according to an article published by his colleagues in The Rheumatologist. Yazdany collaborated with him in this effort.
Launched on March 12, 2020, the Global Rheumatology Alliance’s mission is to “collect, analyze, and disseminate information about COVID-19 and rheumatology to patients, physicians, and other relevant groups to improve the care of patients with rheumatic disease.” Robinson served as chair of governance and policy for the collaborative effort.
Inspired by a conversation on Twitter by Leonard Calabrese, DO, a rheumatologist at the Cleveland Clinic in Ohio, about an outcomes registry created by gastroenterologists specific to patients with inflammatory bowel disease, Robinson launched a discussion about a similar effort for rheumatology on Twitter, they write.
Along with colleagues and within a single Zoom conference call, Yazdany and Robinson had a plan to organize the registry, Robinson’s colleagues write.
Two projects of the Global Rheumatology Alliance are a health care provider–entered registry for providers to enter data about rheumatology patients with COVID-19 infections, and their COVID-19 Vax Survey, which is available in 12 languages, including English.
Yazdany had never met Robinson before she started working with him on the Global Rheumatology Alliance. They started chatting on Twitter, then moved to Zoom conference calls, and subsequently had weeks when they talked by phone and “emailed constantly,” she said.
“As I reflect on our initial interactions, I am struck by how brilliantly we got along and trusted each other,” Yazdany told this news organization. “We both liked to think big, believed in inclusive collaborations, and were committed to helping people with rheumatic diseases during a scary and uncertain time.”
Still, Yazdany noted that she and Phil brought different strengths to their collaborations. She brought her skills related to the technical aspects of research databases, while “Phil worked his magic in mobilizing friends and colleagues from all over the world,” she said. “He served as a wonderful leader, one whom people believed in and would follow.”
The two colleagues, who spent much of their collaborations over Zoom calls, email, and Slack, while living more than 7,000 miles apart, finally met in person at ACR Convergence 2022, which took place in Philadelphia that year. “It felt like the best kind of reunion with a dear friend,” Yazdany remembered.
A mentor who created a platform for ‘good people to do great things’
David Liew, MBBS, PhD, consultant rheumatologist and clinical pharmacologist at Austin Health in Melbourne, marveled at Robinson’s ability to “distill things simply and cleanly, with clarity but without losing detail.” Liew, who collaborated with Robinson throughout the COVID-19 pandemic, likens the experience to “jamming with [jazz musician John] Coltrane.”
“What I think was particularly remarkable was the capacity to not only have those thoughts himself, but to facilitate others to have that springboard,” said Liew, who added that Robinson “took enormous pleasure in facilitating others’ success.”
“I think the greatest joy he drew out of the COVID-19 Global Rheumatology Alliance, apart from facing up to the challenge that needed to be faced, was creating a platform for good people to do great things,” said Liew, who recalled one situation where Robinson gently challenged him. Looking back now, Liew can “now see he very clearly was laying me up, giving me the best chance to shine.”
He describes Robinson as “a deep soul who loved his wife and two sons enormously” and “a whiskey aficionado.” “[Whiskey] suited his contemplative style,” Liew recalled. “Some of my fondest conversations with him were over a whiskey, either in person or virtually, pondering the ‘big issues.’”
A ‘friend and a colleague and so much more’
Claire Barrett, MBBS, president of the Australian Rheumatology Association, described Robinson as a “friend and a colleague and so much more. ... [He was] someone who I worked, laughed, ate, drank, danced, and had fun with,” she told this news organization. They served together as volunteers for the Australian Rheumatology Association and its Queensland branch, as well as Arthritis Queensland; they were also colleagues at Metro North Hospital and Health Service in Brisbane, Queensland.
Robinson was her “go to” for insightful comment on a variety of topics, she said. That could be advice on managing a patient with difficult gout, challenging spondyloarthritis, or the best treatment for a patient with COVID-19.
“[Phil] was a friend I could ask about anything, knowing I would not be judged,” Barrett said. “His kids and our grandkids are similar ages, so we would swap stories and photos and laugh about how cute/funny/cuddly/busy/etc. they were. My heart is broken he won’t get the chance to enjoy their future and the excitement of having Phil continuing to be such an active dad.”
Tanner, Robinson’s wife, said, “he loved everything.” That included the academic side of medicine, and working out what was wrong with his patients and helping them get better. “He was dedicated to this,” she added.
“He also loved the camaraderie of the job – all the people he met and interacted with. [Phil] loved sharing his ideas for research and also discussing complex patients with colleagues. He was driven by finding the answers to problems and doing this as part of a team of researchers/clinicians. He wasn’t interested in personal success.”
Robinson received his medical degree from Otago Medical School in Dunedin, New Zealand, according to the University of Queensland. His specialty training in general and acute care medicine and rheumatology was completed in Wellington, New Zealand, and Dunedin. Robinson also achieved a PhD in human genetics at the University of Queensland Diamantina Institute and had a postdoctoral fellowship at the Queensland Brain Institute at the University of Queensland.
Before his death, he worked at the Royal Brisbane and Women’s Hospital in Herston, Queensland, and at St. Andrew’s War Memorial Hospital in Spring Hill in Brisbane.
A version of this article first appeared on Medscape.com.
Helen Tanner remembers stealing glimpses of her husband, Philip (“Phil”) Robinson, MBChB, PhD, associate professor at the University of Queensland (Australia), catching and rehoming huge Huntsman spiders. Robinson made the extra effort because he didn’t want to hurt them; he wasn’t a big fan of the large spider with a potential leg span of 6 inches that’s commonly found in Australia, per the Australian Museum.
Robinson also relished taking his children, Eddie, 4, and Tommy, 7, on roller coaster rides, which they enjoyed, despite the experience typically giving him motion sickness, Tanner said.
“He would do anything to make the children happy,” she said. “His children meant the world to him.”
Robinson died Jan. 3 as a result of diffuse gastric adenocarcinoma, according to his wife, who added that it was a short, 2-week-long illness.
A leader of global effort to understand COVID-19 and rheumatic disease
Jinoos Yazdany, MD, MPH, chief of the division of rheumatology at Zuckerberg San Francisco General Hospital and professor of medicine at the University of California, San Francisco, described Robinson as “one of the hardest-working people I have ever known. ... [still] his deep love and dedication for his family and kids was always present.”
Robinson would wake up early, even on weekends, to lead international calls across multiple time zones, Yazdany said. “He was driven by a deep curiosity and an intense desire to generate scholarship that would help people with rheumatic diseases.”
Yazdany added that Robinson had a full research portfolio in gout and spondyloarthritis and a busy clinical practice.
She often caught glimpses of Robinson’s young children during Zoom calls. “He was also a talented baker and loved to bake with his kids, often posting pictures of his creations on social media,” Yazdany said.
A mutual colleague compiled some of Robinson’s baking successes. That includes “ ‘probably about to be locked down’ cookies” on July 17, 2021, and “Queensland lockdown cookies!!!” on July 2, 2021. Reuters reported on July 21, 2021, that Australia was witnessing an alarming increase in COVID-19 cases.
Robinson also worked his social media skills to rally support for the COVID-19 Global Rheumatology Alliance, according to an article published by his colleagues in The Rheumatologist. Yazdany collaborated with him in this effort.
Launched on March 12, 2020, the Global Rheumatology Alliance’s mission is to “collect, analyze, and disseminate information about COVID-19 and rheumatology to patients, physicians, and other relevant groups to improve the care of patients with rheumatic disease.” Robinson served as chair of governance and policy for the collaborative effort.
Inspired by a conversation on Twitter by Leonard Calabrese, DO, a rheumatologist at the Cleveland Clinic in Ohio, about an outcomes registry created by gastroenterologists specific to patients with inflammatory bowel disease, Robinson launched a discussion about a similar effort for rheumatology on Twitter, they write.
Along with colleagues and within a single Zoom conference call, Yazdany and Robinson had a plan to organize the registry, Robinson’s colleagues write.
Two projects of the Global Rheumatology Alliance are a health care provider–entered registry for providers to enter data about rheumatology patients with COVID-19 infections, and their COVID-19 Vax Survey, which is available in 12 languages, including English.
Yazdany had never met Robinson before she started working with him on the Global Rheumatology Alliance. They started chatting on Twitter, then moved to Zoom conference calls, and subsequently had weeks when they talked by phone and “emailed constantly,” she said.
“As I reflect on our initial interactions, I am struck by how brilliantly we got along and trusted each other,” Yazdany told this news organization. “We both liked to think big, believed in inclusive collaborations, and were committed to helping people with rheumatic diseases during a scary and uncertain time.”
Still, Yazdany noted that she and Phil brought different strengths to their collaborations. She brought her skills related to the technical aspects of research databases, while “Phil worked his magic in mobilizing friends and colleagues from all over the world,” she said. “He served as a wonderful leader, one whom people believed in and would follow.”
The two colleagues, who spent much of their collaborations over Zoom calls, email, and Slack, while living more than 7,000 miles apart, finally met in person at ACR Convergence 2022, which took place in Philadelphia that year. “It felt like the best kind of reunion with a dear friend,” Yazdany remembered.
A mentor who created a platform for ‘good people to do great things’
David Liew, MBBS, PhD, consultant rheumatologist and clinical pharmacologist at Austin Health in Melbourne, marveled at Robinson’s ability to “distill things simply and cleanly, with clarity but without losing detail.” Liew, who collaborated with Robinson throughout the COVID-19 pandemic, likens the experience to “jamming with [jazz musician John] Coltrane.”
“What I think was particularly remarkable was the capacity to not only have those thoughts himself, but to facilitate others to have that springboard,” said Liew, who added that Robinson “took enormous pleasure in facilitating others’ success.”
“I think the greatest joy he drew out of the COVID-19 Global Rheumatology Alliance, apart from facing up to the challenge that needed to be faced, was creating a platform for good people to do great things,” said Liew, who recalled one situation where Robinson gently challenged him. Looking back now, Liew can “now see he very clearly was laying me up, giving me the best chance to shine.”
He describes Robinson as “a deep soul who loved his wife and two sons enormously” and “a whiskey aficionado.” “[Whiskey] suited his contemplative style,” Liew recalled. “Some of my fondest conversations with him were over a whiskey, either in person or virtually, pondering the ‘big issues.’”
A ‘friend and a colleague and so much more’
Claire Barrett, MBBS, president of the Australian Rheumatology Association, described Robinson as a “friend and a colleague and so much more. ... [He was] someone who I worked, laughed, ate, drank, danced, and had fun with,” she told this news organization. They served together as volunteers for the Australian Rheumatology Association and its Queensland branch, as well as Arthritis Queensland; they were also colleagues at Metro North Hospital and Health Service in Brisbane, Queensland.
Robinson was her “go to” for insightful comment on a variety of topics, she said. That could be advice on managing a patient with difficult gout, challenging spondyloarthritis, or the best treatment for a patient with COVID-19.
“[Phil] was a friend I could ask about anything, knowing I would not be judged,” Barrett said. “His kids and our grandkids are similar ages, so we would swap stories and photos and laugh about how cute/funny/cuddly/busy/etc. they were. My heart is broken he won’t get the chance to enjoy their future and the excitement of having Phil continuing to be such an active dad.”
Tanner, Robinson’s wife, said, “he loved everything.” That included the academic side of medicine, and working out what was wrong with his patients and helping them get better. “He was dedicated to this,” she added.
“He also loved the camaraderie of the job – all the people he met and interacted with. [Phil] loved sharing his ideas for research and also discussing complex patients with colleagues. He was driven by finding the answers to problems and doing this as part of a team of researchers/clinicians. He wasn’t interested in personal success.”
Robinson received his medical degree from Otago Medical School in Dunedin, New Zealand, according to the University of Queensland. His specialty training in general and acute care medicine and rheumatology was completed in Wellington, New Zealand, and Dunedin. Robinson also achieved a PhD in human genetics at the University of Queensland Diamantina Institute and had a postdoctoral fellowship at the Queensland Brain Institute at the University of Queensland.
Before his death, he worked at the Royal Brisbane and Women’s Hospital in Herston, Queensland, and at St. Andrew’s War Memorial Hospital in Spring Hill in Brisbane.
A version of this article first appeared on Medscape.com.
Helen Tanner remembers stealing glimpses of her husband, Philip (“Phil”) Robinson, MBChB, PhD, associate professor at the University of Queensland (Australia), catching and rehoming huge Huntsman spiders. Robinson made the extra effort because he didn’t want to hurt them; he wasn’t a big fan of the large spider with a potential leg span of 6 inches that’s commonly found in Australia, per the Australian Museum.
Robinson also relished taking his children, Eddie, 4, and Tommy, 7, on roller coaster rides, which they enjoyed, despite the experience typically giving him motion sickness, Tanner said.
“He would do anything to make the children happy,” she said. “His children meant the world to him.”
Robinson died Jan. 3 as a result of diffuse gastric adenocarcinoma, according to his wife, who added that it was a short, 2-week-long illness.
A leader of global effort to understand COVID-19 and rheumatic disease
Jinoos Yazdany, MD, MPH, chief of the division of rheumatology at Zuckerberg San Francisco General Hospital and professor of medicine at the University of California, San Francisco, described Robinson as “one of the hardest-working people I have ever known. ... [still] his deep love and dedication for his family and kids was always present.”
Robinson would wake up early, even on weekends, to lead international calls across multiple time zones, Yazdany said. “He was driven by a deep curiosity and an intense desire to generate scholarship that would help people with rheumatic diseases.”
Yazdany added that Robinson had a full research portfolio in gout and spondyloarthritis and a busy clinical practice.
She often caught glimpses of Robinson’s young children during Zoom calls. “He was also a talented baker and loved to bake with his kids, often posting pictures of his creations on social media,” Yazdany said.
A mutual colleague compiled some of Robinson’s baking successes. That includes “ ‘probably about to be locked down’ cookies” on July 17, 2021, and “Queensland lockdown cookies!!!” on July 2, 2021. Reuters reported on July 21, 2021, that Australia was witnessing an alarming increase in COVID-19 cases.
Robinson also worked his social media skills to rally support for the COVID-19 Global Rheumatology Alliance, according to an article published by his colleagues in The Rheumatologist. Yazdany collaborated with him in this effort.
Launched on March 12, 2020, the Global Rheumatology Alliance’s mission is to “collect, analyze, and disseminate information about COVID-19 and rheumatology to patients, physicians, and other relevant groups to improve the care of patients with rheumatic disease.” Robinson served as chair of governance and policy for the collaborative effort.
Inspired by a conversation on Twitter by Leonard Calabrese, DO, a rheumatologist at the Cleveland Clinic in Ohio, about an outcomes registry created by gastroenterologists specific to patients with inflammatory bowel disease, Robinson launched a discussion about a similar effort for rheumatology on Twitter, they write.
Along with colleagues and within a single Zoom conference call, Yazdany and Robinson had a plan to organize the registry, Robinson’s colleagues write.
Two projects of the Global Rheumatology Alliance are a health care provider–entered registry for providers to enter data about rheumatology patients with COVID-19 infections, and their COVID-19 Vax Survey, which is available in 12 languages, including English.
Yazdany had never met Robinson before she started working with him on the Global Rheumatology Alliance. They started chatting on Twitter, then moved to Zoom conference calls, and subsequently had weeks when they talked by phone and “emailed constantly,” she said.
“As I reflect on our initial interactions, I am struck by how brilliantly we got along and trusted each other,” Yazdany told this news organization. “We both liked to think big, believed in inclusive collaborations, and were committed to helping people with rheumatic diseases during a scary and uncertain time.”
Still, Yazdany noted that she and Phil brought different strengths to their collaborations. She brought her skills related to the technical aspects of research databases, while “Phil worked his magic in mobilizing friends and colleagues from all over the world,” she said. “He served as a wonderful leader, one whom people believed in and would follow.”
The two colleagues, who spent much of their collaborations over Zoom calls, email, and Slack, while living more than 7,000 miles apart, finally met in person at ACR Convergence 2022, which took place in Philadelphia that year. “It felt like the best kind of reunion with a dear friend,” Yazdany remembered.
A mentor who created a platform for ‘good people to do great things’
David Liew, MBBS, PhD, consultant rheumatologist and clinical pharmacologist at Austin Health in Melbourne, marveled at Robinson’s ability to “distill things simply and cleanly, with clarity but without losing detail.” Liew, who collaborated with Robinson throughout the COVID-19 pandemic, likens the experience to “jamming with [jazz musician John] Coltrane.”
“What I think was particularly remarkable was the capacity to not only have those thoughts himself, but to facilitate others to have that springboard,” said Liew, who added that Robinson “took enormous pleasure in facilitating others’ success.”
“I think the greatest joy he drew out of the COVID-19 Global Rheumatology Alliance, apart from facing up to the challenge that needed to be faced, was creating a platform for good people to do great things,” said Liew, who recalled one situation where Robinson gently challenged him. Looking back now, Liew can “now see he very clearly was laying me up, giving me the best chance to shine.”
He describes Robinson as “a deep soul who loved his wife and two sons enormously” and “a whiskey aficionado.” “[Whiskey] suited his contemplative style,” Liew recalled. “Some of my fondest conversations with him were over a whiskey, either in person or virtually, pondering the ‘big issues.’”
A ‘friend and a colleague and so much more’
Claire Barrett, MBBS, president of the Australian Rheumatology Association, described Robinson as a “friend and a colleague and so much more. ... [He was] someone who I worked, laughed, ate, drank, danced, and had fun with,” she told this news organization. They served together as volunteers for the Australian Rheumatology Association and its Queensland branch, as well as Arthritis Queensland; they were also colleagues at Metro North Hospital and Health Service in Brisbane, Queensland.
Robinson was her “go to” for insightful comment on a variety of topics, she said. That could be advice on managing a patient with difficult gout, challenging spondyloarthritis, or the best treatment for a patient with COVID-19.
“[Phil] was a friend I could ask about anything, knowing I would not be judged,” Barrett said. “His kids and our grandkids are similar ages, so we would swap stories and photos and laugh about how cute/funny/cuddly/busy/etc. they were. My heart is broken he won’t get the chance to enjoy their future and the excitement of having Phil continuing to be such an active dad.”
Tanner, Robinson’s wife, said, “he loved everything.” That included the academic side of medicine, and working out what was wrong with his patients and helping them get better. “He was dedicated to this,” she added.
“He also loved the camaraderie of the job – all the people he met and interacted with. [Phil] loved sharing his ideas for research and also discussing complex patients with colleagues. He was driven by finding the answers to problems and doing this as part of a team of researchers/clinicians. He wasn’t interested in personal success.”
Robinson received his medical degree from Otago Medical School in Dunedin, New Zealand, according to the University of Queensland. His specialty training in general and acute care medicine and rheumatology was completed in Wellington, New Zealand, and Dunedin. Robinson also achieved a PhD in human genetics at the University of Queensland Diamantina Institute and had a postdoctoral fellowship at the Queensland Brain Institute at the University of Queensland.
Before his death, he worked at the Royal Brisbane and Women’s Hospital in Herston, Queensland, and at St. Andrew’s War Memorial Hospital in Spring Hill in Brisbane.
A version of this article first appeared on Medscape.com.
Metabolic syndrome may promote gout in young men
Metabolic syndrome is associated with a significantly increased risk for gout in young men, but the risk can be mitigated by improvement in individual components of the syndrome, based on data from a pair of population-based studies totaling more than 4 million individuals.
Gout remains the most common type of inflammatory arthritis in men, and the rate has been rising among younger adults, Yeonghee Eun, MD, PhD, of Sungkyunkwan University, Seoul, South Korea, and colleagues wrote. An increasing body of evidence suggests a link between gout and metabolic syndrome (MetS), but large studies have been lacking, especially in younger adults.
In a study published in Frontiers in Medicine, the researchers reviewed data from 3,569,104 men aged 20-39 years who underwent a health checkup between 2009 and 2012 in South Korea, based on the Korean National Health Insurance Service. The primary outcome of incident gout was identified using claims data. The mean age of the participants was 31.5 years.
Over a mean follow-up of 7.4 years, the incidence of gout was 3.36 per 1,000 person-years. The risk of developing gout was more than twice as high among individuals who met MetS criteria than in those who did not (adjusted hazard ratio, 2.44).
MetS was defined as the presence of at least two of the following components: hypertriglyceridemia, abdominal obesity, reduced HDL cholesterol, elevated blood pressure, and elevated fasting glucose.
Overall, individuals with all five MetS components had a fivefold increase in gout risk, compared with people who did not have MetS (aHR, 5.24). In an analysis of each component of MetS, hypertriglyceridemia and abdominal obesity showed the strongest association with gout (aHRs of 2.08 and 2.33, respectively).
The impact of MetS on risk of incident gout was greater in younger participants, which suggests that the management of MetS in young people should be emphasized, the researchers said.
In a further analysis of body mass index subgroups, MetS had the greatest impact on gout risk for individuals who were underweight (aHR, 3.82). “In particular, in the underweight group, the risk of gout increased 10-fold when abdominal obesity was present,” the researchers said.
The study was limited by several factors including potential selection bias and potential overestimation of gout incidence because of the use of diagnostic codes, the researchers noted. Other limitations included lack of control for nutritional or dietary risk factors and the inability to include cases that occurred after the study period.
However, findings were strengthened by the large number of participants with MetS who were underweight or normal weight, the researchers wrote. More research on the mechanism of action is needed, but the data suggest that MetS is a key risk factor in the development of gout in young men.
In a second study, published in Arthritis & Rheumatology, Dr. Eun and colleagues examined associations between MetS changes and incident gout in young men. Although previous studies have shown that changes in MetS status can alter the risk of cardiovascular events, atrial fibrillation, end-stage renal disease, and all-cause mortality, the impact of these changes on gout has not been well studied, they said. The researchers used the same study cohort, the National Health Insurance Service database in South Korea. They reviewed data from 1,293,166 individuals aged 20-39 years. Of these, 18,473 were diagnosed with gout for an incidence rate 3.36/1,000 person-years. The researchers compared gout incidence for men who met criteria for MetS at three health checkups and those without MetS.
Overall, patients with MetS at all three checkups had a nearly fourfold higher risk of gout than those who never had MetS, with an adjusted hazard ratio of 3.82, the researchers wrote. The development of MetS over the study period more than doubled the risk of gout, but recovery from MetS reduced incident gout risk by approximately 50% (aHR, 0.52).
In findings similar to the Frontiers in Medicine study, the greatest associations with gout were noted for changes in elevated triglycerides and changes in abdominal obesity; aHRs for development and recovery for elevated triglycerides were 1.74 and 0.56, respectively, and for abdominal obesity, 1.94 and 0.69, respectively.
More research is needed to explore the mechanism by which both abdominal obesity and elevated triglycerides drive the development of gout, the researchers wrote in their discussion.
Also similar to the Frontiers study, the associations among changes in MetS and incident gout were greater for the youngest participants (in their 20s) and in the underweight or normal weight BMI groups.
Limitations of the second study included possible selection bias because of the study population of workplace employees who participated in regular health checks and the lack of data on women or on men aged 40 years and older, the researchers noted. Other limitations included possible misclassification of MetS because of varying health checkup results and drug claims, and lack of data on serum urate, which prevented assessment of hyperuricemia as a cause of gout.
However, the results were strengthened by the large sample size and suggest that MetS is a modifiable risk factor for gout, the researchers concluded.
Neither of the studies received outside funding. The researchers had no financial conflicts to disclose.
Metabolic syndrome is associated with a significantly increased risk for gout in young men, but the risk can be mitigated by improvement in individual components of the syndrome, based on data from a pair of population-based studies totaling more than 4 million individuals.
Gout remains the most common type of inflammatory arthritis in men, and the rate has been rising among younger adults, Yeonghee Eun, MD, PhD, of Sungkyunkwan University, Seoul, South Korea, and colleagues wrote. An increasing body of evidence suggests a link between gout and metabolic syndrome (MetS), but large studies have been lacking, especially in younger adults.
In a study published in Frontiers in Medicine, the researchers reviewed data from 3,569,104 men aged 20-39 years who underwent a health checkup between 2009 and 2012 in South Korea, based on the Korean National Health Insurance Service. The primary outcome of incident gout was identified using claims data. The mean age of the participants was 31.5 years.
Over a mean follow-up of 7.4 years, the incidence of gout was 3.36 per 1,000 person-years. The risk of developing gout was more than twice as high among individuals who met MetS criteria than in those who did not (adjusted hazard ratio, 2.44).
MetS was defined as the presence of at least two of the following components: hypertriglyceridemia, abdominal obesity, reduced HDL cholesterol, elevated blood pressure, and elevated fasting glucose.
Overall, individuals with all five MetS components had a fivefold increase in gout risk, compared with people who did not have MetS (aHR, 5.24). In an analysis of each component of MetS, hypertriglyceridemia and abdominal obesity showed the strongest association with gout (aHRs of 2.08 and 2.33, respectively).
The impact of MetS on risk of incident gout was greater in younger participants, which suggests that the management of MetS in young people should be emphasized, the researchers said.
In a further analysis of body mass index subgroups, MetS had the greatest impact on gout risk for individuals who were underweight (aHR, 3.82). “In particular, in the underweight group, the risk of gout increased 10-fold when abdominal obesity was present,” the researchers said.
The study was limited by several factors including potential selection bias and potential overestimation of gout incidence because of the use of diagnostic codes, the researchers noted. Other limitations included lack of control for nutritional or dietary risk factors and the inability to include cases that occurred after the study period.
However, findings were strengthened by the large number of participants with MetS who were underweight or normal weight, the researchers wrote. More research on the mechanism of action is needed, but the data suggest that MetS is a key risk factor in the development of gout in young men.
In a second study, published in Arthritis & Rheumatology, Dr. Eun and colleagues examined associations between MetS changes and incident gout in young men. Although previous studies have shown that changes in MetS status can alter the risk of cardiovascular events, atrial fibrillation, end-stage renal disease, and all-cause mortality, the impact of these changes on gout has not been well studied, they said. The researchers used the same study cohort, the National Health Insurance Service database in South Korea. They reviewed data from 1,293,166 individuals aged 20-39 years. Of these, 18,473 were diagnosed with gout for an incidence rate 3.36/1,000 person-years. The researchers compared gout incidence for men who met criteria for MetS at three health checkups and those without MetS.
Overall, patients with MetS at all three checkups had a nearly fourfold higher risk of gout than those who never had MetS, with an adjusted hazard ratio of 3.82, the researchers wrote. The development of MetS over the study period more than doubled the risk of gout, but recovery from MetS reduced incident gout risk by approximately 50% (aHR, 0.52).
In findings similar to the Frontiers in Medicine study, the greatest associations with gout were noted for changes in elevated triglycerides and changes in abdominal obesity; aHRs for development and recovery for elevated triglycerides were 1.74 and 0.56, respectively, and for abdominal obesity, 1.94 and 0.69, respectively.
More research is needed to explore the mechanism by which both abdominal obesity and elevated triglycerides drive the development of gout, the researchers wrote in their discussion.
Also similar to the Frontiers study, the associations among changes in MetS and incident gout were greater for the youngest participants (in their 20s) and in the underweight or normal weight BMI groups.
Limitations of the second study included possible selection bias because of the study population of workplace employees who participated in regular health checks and the lack of data on women or on men aged 40 years and older, the researchers noted. Other limitations included possible misclassification of MetS because of varying health checkup results and drug claims, and lack of data on serum urate, which prevented assessment of hyperuricemia as a cause of gout.
However, the results were strengthened by the large sample size and suggest that MetS is a modifiable risk factor for gout, the researchers concluded.
Neither of the studies received outside funding. The researchers had no financial conflicts to disclose.
Metabolic syndrome is associated with a significantly increased risk for gout in young men, but the risk can be mitigated by improvement in individual components of the syndrome, based on data from a pair of population-based studies totaling more than 4 million individuals.
Gout remains the most common type of inflammatory arthritis in men, and the rate has been rising among younger adults, Yeonghee Eun, MD, PhD, of Sungkyunkwan University, Seoul, South Korea, and colleagues wrote. An increasing body of evidence suggests a link between gout and metabolic syndrome (MetS), but large studies have been lacking, especially in younger adults.
In a study published in Frontiers in Medicine, the researchers reviewed data from 3,569,104 men aged 20-39 years who underwent a health checkup between 2009 and 2012 in South Korea, based on the Korean National Health Insurance Service. The primary outcome of incident gout was identified using claims data. The mean age of the participants was 31.5 years.
Over a mean follow-up of 7.4 years, the incidence of gout was 3.36 per 1,000 person-years. The risk of developing gout was more than twice as high among individuals who met MetS criteria than in those who did not (adjusted hazard ratio, 2.44).
MetS was defined as the presence of at least two of the following components: hypertriglyceridemia, abdominal obesity, reduced HDL cholesterol, elevated blood pressure, and elevated fasting glucose.
Overall, individuals with all five MetS components had a fivefold increase in gout risk, compared with people who did not have MetS (aHR, 5.24). In an analysis of each component of MetS, hypertriglyceridemia and abdominal obesity showed the strongest association with gout (aHRs of 2.08 and 2.33, respectively).
The impact of MetS on risk of incident gout was greater in younger participants, which suggests that the management of MetS in young people should be emphasized, the researchers said.
In a further analysis of body mass index subgroups, MetS had the greatest impact on gout risk for individuals who were underweight (aHR, 3.82). “In particular, in the underweight group, the risk of gout increased 10-fold when abdominal obesity was present,” the researchers said.
The study was limited by several factors including potential selection bias and potential overestimation of gout incidence because of the use of diagnostic codes, the researchers noted. Other limitations included lack of control for nutritional or dietary risk factors and the inability to include cases that occurred after the study period.
However, findings were strengthened by the large number of participants with MetS who were underweight or normal weight, the researchers wrote. More research on the mechanism of action is needed, but the data suggest that MetS is a key risk factor in the development of gout in young men.
In a second study, published in Arthritis & Rheumatology, Dr. Eun and colleagues examined associations between MetS changes and incident gout in young men. Although previous studies have shown that changes in MetS status can alter the risk of cardiovascular events, atrial fibrillation, end-stage renal disease, and all-cause mortality, the impact of these changes on gout has not been well studied, they said. The researchers used the same study cohort, the National Health Insurance Service database in South Korea. They reviewed data from 1,293,166 individuals aged 20-39 years. Of these, 18,473 were diagnosed with gout for an incidence rate 3.36/1,000 person-years. The researchers compared gout incidence for men who met criteria for MetS at three health checkups and those without MetS.
Overall, patients with MetS at all three checkups had a nearly fourfold higher risk of gout than those who never had MetS, with an adjusted hazard ratio of 3.82, the researchers wrote. The development of MetS over the study period more than doubled the risk of gout, but recovery from MetS reduced incident gout risk by approximately 50% (aHR, 0.52).
In findings similar to the Frontiers in Medicine study, the greatest associations with gout were noted for changes in elevated triglycerides and changes in abdominal obesity; aHRs for development and recovery for elevated triglycerides were 1.74 and 0.56, respectively, and for abdominal obesity, 1.94 and 0.69, respectively.
More research is needed to explore the mechanism by which both abdominal obesity and elevated triglycerides drive the development of gout, the researchers wrote in their discussion.
Also similar to the Frontiers study, the associations among changes in MetS and incident gout were greater for the youngest participants (in their 20s) and in the underweight or normal weight BMI groups.
Limitations of the second study included possible selection bias because of the study population of workplace employees who participated in regular health checks and the lack of data on women or on men aged 40 years and older, the researchers noted. Other limitations included possible misclassification of MetS because of varying health checkup results and drug claims, and lack of data on serum urate, which prevented assessment of hyperuricemia as a cause of gout.
However, the results were strengthened by the large sample size and suggest that MetS is a modifiable risk factor for gout, the researchers concluded.
Neither of the studies received outside funding. The researchers had no financial conflicts to disclose.
FROM FRONTIERS IN MEDICINE AND ARTHRITIS & RHEUMATOLOGY
Intensive gout treatment meets urate goal, lowers tophi burden
PHILADELPHIA – Patients with gout who underwent an intensive treat-to-target regimen of monthly up-titration of urate-lowering therapy (ULT) to reach a target serum urate level were significantly more likely to reach that goal at 1 year than were patients who received conventional gout management in a randomized, controlled trial.
These results came from the TICOG (Tight Control of Gout) trial, one of a handful of recent trials to test a treat-to-target strategy with ULT in the management of gout. Beyond the primary outcome of reaching target serum urate level of < 5 mg/dL (< 300 micromol/L), the results also showed that the tight-control strategy significantly lowered urate to a greater extent than conventional management, reduced tophus size in the first metatarsophalangeal (MTP) joint, and improved gray scale synovitis on ultrasound significantly more than with conventional management, Sarah Black, MBBS, a rheumatology trainee at Musgrave Park Hospital, Belfast, Northern Ireland, reported at the American College of Rheumatology annual meeting.
“Based on these outcomes, we question whether gout is best managed in primary or secondary care. We think there is an argument for establishing specialist gout clinics with more time to focus on patient education to help improve outcomes. These clinics could be led by allied health care professionals, such as specialist nurses and pharmacists,” Dr. Black said at the meeting.
Gout management guidelines issued by the British Society for Rheumatology in 2017 call for a target serum urate level of < 5 mg/dL, whereas the ACR’s 2020 guideline for the management of gout endorses a treat-to-target management strategy that aims for a serum urate level of < 6 mg/dL.
The single-center, nonblinded trial recruited 110 patients aged 18-85 years over a 3-year period to take ULT with allopurinol as first-line therapy starting at 100 mg/day. Everyone received the same advice regarding ULT up-titration, lifestyle changes, and gout education at baseline. The second-line agent for ULT was febuxostat (Uloric) 80 mg daily, with uricosuric drugs as third-line agents. All patients received colchicine or NSAID prophylaxis for gout flares for the first 6 months, depending on their comorbidities.
The trial excluded patients who had been treated with ULT within the past 6 months or had experienced prior hypersensitivity to ULT, severe renal impairment (creatinine clearance < 30 mL/min as measured by estimated glomerular filtration rate), significant liver impairment, or any other significant medical disease affecting life expectancy shorter than 1 year.
Conventional management consisted of urate level review at 0, 6, and 12 months with up-titration at each visit and primary care management of ULT between reviews until the target serum urate level was reached. In the tight-control group, monthly up-titrations occurred at the Musgrave Park Hospital at visits with the study team that were led by a rheumatologist and a specialist pharmacist.
A total of 48 patients in the conventional arm and 47 in the tight-control arm completed the trial. At baseline, monosodium urate crystals were detected in joint aspirates in 56% of patients receiving tight control and in 58.5% of those receiving conventional management. The mean serum urate level was 490 micromol/L (8.24 mg/dL) for tight-control patients and 470 micromol/L (7.9 mg/dL) for conventionally managed patients.
By 1 year, 89.4% of patients in the tight-control group had achieved the target urate level, compared with 39.6% in the conventional-management group (P < .001). At 6 months, serum urate had declined by 37.6% with tight control vs. 18% with conventional management. By the end of the trial, the median allopurinol dose was 400 mg with tight control (range, 200-900 mg) and 200 mg with conventional management (range, 0-400 mg). A total of 89% of patients were taking allopurinol at the end of the trial.
As expected, tight control led to more flares per month on average (0.35 vs. 0.13) in the 79 patients for whom complete data on flare frequency were available.
On blinded ultrasound evaluations, the median diameter of the first MTP tophus declined significantly more with tight control than with conventional management (–4.65 mm vs. –0.30 mm; P = .003). Gray scale synovitis in the knee improved in 63% of patients undergoing tight control, compared with 14% of conventionally managed patients (P = .043). The researchers observed no difference in resolution of the double-contour sign or in the number of erosions between the groups, although the 1-year time frame may not have been long enough to see resolution and improvement, Dr. Black said.
Dr. Black said that a follow-up study is planned with the same patient cohort at 3 years.
When asked about the feasibility of monthly ULT titration visits for gout management, audience member Tuhina Neogi, MD, professor of epidemiology at Boston University and chief of rheumatology at Boston Medical Center, told this news organization, “We don’t have a lot of data to guide us in that regard, and I also think it depends on what the increment of the dose titration is, but we generally do recognize that therapeutic inertia is bad – keeping someone on a dose for a long time. For me, I don’t think monthly is unreasonable if you have good prophylaxis [against acute flares].”
Dr. Neogi also noted that such monthly assessments don’t have to take place at a hospital. “I think there are many different practice models in which it could be implemented [that are not physician-driven].”
The study had no outside funding. Dr. Black has disclosed no relevant financial relationships. Dr. Neogi has received consulting fees from a variety of pharmaceutical companies, including Alnylam, Regeneron, Eli Lilly, EMD Serono, Novartis, Pfizer, and GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Patients with gout who underwent an intensive treat-to-target regimen of monthly up-titration of urate-lowering therapy (ULT) to reach a target serum urate level were significantly more likely to reach that goal at 1 year than were patients who received conventional gout management in a randomized, controlled trial.
These results came from the TICOG (Tight Control of Gout) trial, one of a handful of recent trials to test a treat-to-target strategy with ULT in the management of gout. Beyond the primary outcome of reaching target serum urate level of < 5 mg/dL (< 300 micromol/L), the results also showed that the tight-control strategy significantly lowered urate to a greater extent than conventional management, reduced tophus size in the first metatarsophalangeal (MTP) joint, and improved gray scale synovitis on ultrasound significantly more than with conventional management, Sarah Black, MBBS, a rheumatology trainee at Musgrave Park Hospital, Belfast, Northern Ireland, reported at the American College of Rheumatology annual meeting.
“Based on these outcomes, we question whether gout is best managed in primary or secondary care. We think there is an argument for establishing specialist gout clinics with more time to focus on patient education to help improve outcomes. These clinics could be led by allied health care professionals, such as specialist nurses and pharmacists,” Dr. Black said at the meeting.
Gout management guidelines issued by the British Society for Rheumatology in 2017 call for a target serum urate level of < 5 mg/dL, whereas the ACR’s 2020 guideline for the management of gout endorses a treat-to-target management strategy that aims for a serum urate level of < 6 mg/dL.
The single-center, nonblinded trial recruited 110 patients aged 18-85 years over a 3-year period to take ULT with allopurinol as first-line therapy starting at 100 mg/day. Everyone received the same advice regarding ULT up-titration, lifestyle changes, and gout education at baseline. The second-line agent for ULT was febuxostat (Uloric) 80 mg daily, with uricosuric drugs as third-line agents. All patients received colchicine or NSAID prophylaxis for gout flares for the first 6 months, depending on their comorbidities.
The trial excluded patients who had been treated with ULT within the past 6 months or had experienced prior hypersensitivity to ULT, severe renal impairment (creatinine clearance < 30 mL/min as measured by estimated glomerular filtration rate), significant liver impairment, or any other significant medical disease affecting life expectancy shorter than 1 year.
Conventional management consisted of urate level review at 0, 6, and 12 months with up-titration at each visit and primary care management of ULT between reviews until the target serum urate level was reached. In the tight-control group, monthly up-titrations occurred at the Musgrave Park Hospital at visits with the study team that were led by a rheumatologist and a specialist pharmacist.
A total of 48 patients in the conventional arm and 47 in the tight-control arm completed the trial. At baseline, monosodium urate crystals were detected in joint aspirates in 56% of patients receiving tight control and in 58.5% of those receiving conventional management. The mean serum urate level was 490 micromol/L (8.24 mg/dL) for tight-control patients and 470 micromol/L (7.9 mg/dL) for conventionally managed patients.
By 1 year, 89.4% of patients in the tight-control group had achieved the target urate level, compared with 39.6% in the conventional-management group (P < .001). At 6 months, serum urate had declined by 37.6% with tight control vs. 18% with conventional management. By the end of the trial, the median allopurinol dose was 400 mg with tight control (range, 200-900 mg) and 200 mg with conventional management (range, 0-400 mg). A total of 89% of patients were taking allopurinol at the end of the trial.
As expected, tight control led to more flares per month on average (0.35 vs. 0.13) in the 79 patients for whom complete data on flare frequency were available.
On blinded ultrasound evaluations, the median diameter of the first MTP tophus declined significantly more with tight control than with conventional management (–4.65 mm vs. –0.30 mm; P = .003). Gray scale synovitis in the knee improved in 63% of patients undergoing tight control, compared with 14% of conventionally managed patients (P = .043). The researchers observed no difference in resolution of the double-contour sign or in the number of erosions between the groups, although the 1-year time frame may not have been long enough to see resolution and improvement, Dr. Black said.
Dr. Black said that a follow-up study is planned with the same patient cohort at 3 years.
When asked about the feasibility of monthly ULT titration visits for gout management, audience member Tuhina Neogi, MD, professor of epidemiology at Boston University and chief of rheumatology at Boston Medical Center, told this news organization, “We don’t have a lot of data to guide us in that regard, and I also think it depends on what the increment of the dose titration is, but we generally do recognize that therapeutic inertia is bad – keeping someone on a dose for a long time. For me, I don’t think monthly is unreasonable if you have good prophylaxis [against acute flares].”
Dr. Neogi also noted that such monthly assessments don’t have to take place at a hospital. “I think there are many different practice models in which it could be implemented [that are not physician-driven].”
The study had no outside funding. Dr. Black has disclosed no relevant financial relationships. Dr. Neogi has received consulting fees from a variety of pharmaceutical companies, including Alnylam, Regeneron, Eli Lilly, EMD Serono, Novartis, Pfizer, and GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Patients with gout who underwent an intensive treat-to-target regimen of monthly up-titration of urate-lowering therapy (ULT) to reach a target serum urate level were significantly more likely to reach that goal at 1 year than were patients who received conventional gout management in a randomized, controlled trial.
These results came from the TICOG (Tight Control of Gout) trial, one of a handful of recent trials to test a treat-to-target strategy with ULT in the management of gout. Beyond the primary outcome of reaching target serum urate level of < 5 mg/dL (< 300 micromol/L), the results also showed that the tight-control strategy significantly lowered urate to a greater extent than conventional management, reduced tophus size in the first metatarsophalangeal (MTP) joint, and improved gray scale synovitis on ultrasound significantly more than with conventional management, Sarah Black, MBBS, a rheumatology trainee at Musgrave Park Hospital, Belfast, Northern Ireland, reported at the American College of Rheumatology annual meeting.
“Based on these outcomes, we question whether gout is best managed in primary or secondary care. We think there is an argument for establishing specialist gout clinics with more time to focus on patient education to help improve outcomes. These clinics could be led by allied health care professionals, such as specialist nurses and pharmacists,” Dr. Black said at the meeting.
Gout management guidelines issued by the British Society for Rheumatology in 2017 call for a target serum urate level of < 5 mg/dL, whereas the ACR’s 2020 guideline for the management of gout endorses a treat-to-target management strategy that aims for a serum urate level of < 6 mg/dL.
The single-center, nonblinded trial recruited 110 patients aged 18-85 years over a 3-year period to take ULT with allopurinol as first-line therapy starting at 100 mg/day. Everyone received the same advice regarding ULT up-titration, lifestyle changes, and gout education at baseline. The second-line agent for ULT was febuxostat (Uloric) 80 mg daily, with uricosuric drugs as third-line agents. All patients received colchicine or NSAID prophylaxis for gout flares for the first 6 months, depending on their comorbidities.
The trial excluded patients who had been treated with ULT within the past 6 months or had experienced prior hypersensitivity to ULT, severe renal impairment (creatinine clearance < 30 mL/min as measured by estimated glomerular filtration rate), significant liver impairment, or any other significant medical disease affecting life expectancy shorter than 1 year.
Conventional management consisted of urate level review at 0, 6, and 12 months with up-titration at each visit and primary care management of ULT between reviews until the target serum urate level was reached. In the tight-control group, monthly up-titrations occurred at the Musgrave Park Hospital at visits with the study team that were led by a rheumatologist and a specialist pharmacist.
A total of 48 patients in the conventional arm and 47 in the tight-control arm completed the trial. At baseline, monosodium urate crystals were detected in joint aspirates in 56% of patients receiving tight control and in 58.5% of those receiving conventional management. The mean serum urate level was 490 micromol/L (8.24 mg/dL) for tight-control patients and 470 micromol/L (7.9 mg/dL) for conventionally managed patients.
By 1 year, 89.4% of patients in the tight-control group had achieved the target urate level, compared with 39.6% in the conventional-management group (P < .001). At 6 months, serum urate had declined by 37.6% with tight control vs. 18% with conventional management. By the end of the trial, the median allopurinol dose was 400 mg with tight control (range, 200-900 mg) and 200 mg with conventional management (range, 0-400 mg). A total of 89% of patients were taking allopurinol at the end of the trial.
As expected, tight control led to more flares per month on average (0.35 vs. 0.13) in the 79 patients for whom complete data on flare frequency were available.
On blinded ultrasound evaluations, the median diameter of the first MTP tophus declined significantly more with tight control than with conventional management (–4.65 mm vs. –0.30 mm; P = .003). Gray scale synovitis in the knee improved in 63% of patients undergoing tight control, compared with 14% of conventionally managed patients (P = .043). The researchers observed no difference in resolution of the double-contour sign or in the number of erosions between the groups, although the 1-year time frame may not have been long enough to see resolution and improvement, Dr. Black said.
Dr. Black said that a follow-up study is planned with the same patient cohort at 3 years.
When asked about the feasibility of monthly ULT titration visits for gout management, audience member Tuhina Neogi, MD, professor of epidemiology at Boston University and chief of rheumatology at Boston Medical Center, told this news organization, “We don’t have a lot of data to guide us in that regard, and I also think it depends on what the increment of the dose titration is, but we generally do recognize that therapeutic inertia is bad – keeping someone on a dose for a long time. For me, I don’t think monthly is unreasonable if you have good prophylaxis [against acute flares].”
Dr. Neogi also noted that such monthly assessments don’t have to take place at a hospital. “I think there are many different practice models in which it could be implemented [that are not physician-driven].”
The study had no outside funding. Dr. Black has disclosed no relevant financial relationships. Dr. Neogi has received consulting fees from a variety of pharmaceutical companies, including Alnylam, Regeneron, Eli Lilly, EMD Serono, Novartis, Pfizer, and GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
AT ACR 2022
Prednisone, colchicine equivalent in efficacy for CPP crystal arthritis
PHILADELPHIA – Prednisone appears to have the edge over colchicine for control of pain in patients with acute calcium pyrophosphate (CPP) crystal arthritis, an intensely painful rheumatic disease primarily affecting older patients.
Among 111 patients with acute CPP crystal arthritis randomized to receive either prednisone or colchicine for control of acute pain in a multicenter study, 2 days of therapy with the oral agents provided equivalent pain relief on the second day, and patients generally tolerated each agent well, reported Tristan Pascart, MD, from the Groupement Hospitalier de l’Institut Catholique de Lille (France).
“Almost three-fourths of patients are considered to be good responders to both drugs on day 3, and, maybe, safety is the key issue distinguishing the two treatments: Colchicine was generally well tolerated, but even with this very short time frame of treatment, one patient out of five had diarrhea, which is more of a concern in this elderly population at risk of dehydration,” he said in an oral abstract session at the annual meeting of the American College of Rheumatology.
In contrast, only about 6% of patients assigned to prednisone had diarrhea, and other adverse events that occurred more frequently with the corticosteroid, including hypertension, hyperglycemia, and insomnia all resolved after the therapy was stopped.
Common and acutely painful
Acute CPP crystal arthritis is a common complication that often occurs during hospitalization for primarily nonrheumatologic causes, Dr. Pascart said, and “in the absence of clinical trials, the management relies on expert opinion, which stems from extrapolated data from gap studies” primarily with prednisone or colchicine, Dr. Pascart said.
To fill in the knowledge gap, Dr. Pascart and colleagues conducted the COLCHICORT study to evaluate whether the two drugs were comparable in efficacy and safety for control of acute pain in a vulnerable population.
The multicenter, open-label trial included patients older than age 65 years with an estimated glomerular filtration rate above 30 mL/min per 1.73 m2 who presented with acute CPP deposition arthritis with symptoms occurring within the previous 36 hours. CPP arthritis was defined by the identification of CPP crystals on synovial fluid analysis or typical clinical presentation with evidence of chondrocalcinosis on x-rays or ultrasound.
Patients with a history of gout, cognitive decline that could impair pain assessment, or contraindications to either of the study drugs were excluded.
The participants were randomized to receive either colchicine 1.5 mg (1 mg to start, then 0.5 mg one hour later) at baseline and then 1 mg on day 1, or oral prednisone 30 mg at baseline and on day 1. The patients also received 1 g of systemic acetaminophen, and three 50-mg doses of tramadol during the first 24 hours.
Of the 111 patients randomized, 54 were assigned to receive prednisone, and 57 were assigned to receive colchicine. Baseline characteristics were similar between the groups, with a mean age of about 86 years, body mass index of around 25 kg/m2, and blood pressure in the range of 130/69 mm Hg.
For nearly half of all patients in study each arm the most painful joint was the knee, followed by wrists and ankles.
There was no difference between the groups in the primary efficacy outcome of a change at 24 hours over baseline in visual analog scale (VAS) (0-100 mm) scores, either in a per-protocol analysis or modified intention-to-treat analysis. The mean change in VAS at 24 hours in the colchicine group was –36.6 mm, compared with –37.7 mm in the prednisone group. The investigators had previously determined that any difference between the two drugs of less than 13 mm on pain VAS at 24 hours would meet the definition for equivalent efficacy.
In both groups, a majority of patients had either an improvement greater than 50% in pain VAS scores and/or a pain VAS score less than 40 mm at both 24 and 48 hours.
At 7 days of follow-up, 21.8% of patients assigned to colchicine had diarrhea, compared with 5.6% of those assigned to prednisone. Adverse events occurring more frequently with prednisone included hyperglycemia, hypertension, and insomnia.
Patients who received colchicine and were also on statins had a trend toward a higher risk for diarrhea, but the study was not adequately powered to detect an association, and the trend was not statistically significant, Dr. Pascart said.
“Taken together, safety issues suggest that prednisone should be considered as the first-line therapy in acute CPP crystal arthritis. Future research is warranted to determine factors increasing the risk of colchicine-induced diarrhea,” he concluded.
Both drugs are used
Sara K. Tedeschi, MD, from Brigham & Women’s Hospital in Boston, who attended the session where the data were presented, has a special clinical interest in CPP deposition disease. She applauded Dr. Pascart and colleagues for conducting a rare clinical trial in CPP crystal arthritis.
In an interview, she said that the study suggests “we can keep in mind shorter courses of treatment for acute CPP crystal arthritis; I think that’s one big takeaway from this study.”
Asked whether she would change her practice based on the findings, Dr. Tedeschi replied: “I personally am not sure that I would be moved to use prednisone more than colchicine; I actually take away from this that colchicine is equivalent to prednisone for short-term use for CPP arthritis, but I think it’s also really important to note that this is in the context of quite a lot of acetaminophen and quite a lot of tramadol, and frankly I don’t usually use tramadol with my patients, but I might consider doing that, especially as there were no delirium events in this population.”
Dr. Tedeschi was not involved in the study.
Asked the same question, Michael Toprover, MD, from New York University Langone Medical Center, a moderator of the session who was not involved in the study, said: “I usually use a combination of medications. I generally, in someone who is hospitalized in particular and is in such severe pain, use a combination of colchicine and prednisone, unless I’m worried about infection, in which case I’ll start colchicine until we’ve proven that it’s CPPD, and then I’ll add prednisone.”
The study was funded by PHRC-1 GIRCI Nord Ouest, a clinical research program funded by the Ministry of Health in France. Dr. Pascart, Dr. Tedeschi, and Dr. Toprover all reported having no relevant conflicts of interest.
PHILADELPHIA – Prednisone appears to have the edge over colchicine for control of pain in patients with acute calcium pyrophosphate (CPP) crystal arthritis, an intensely painful rheumatic disease primarily affecting older patients.
Among 111 patients with acute CPP crystal arthritis randomized to receive either prednisone or colchicine for control of acute pain in a multicenter study, 2 days of therapy with the oral agents provided equivalent pain relief on the second day, and patients generally tolerated each agent well, reported Tristan Pascart, MD, from the Groupement Hospitalier de l’Institut Catholique de Lille (France).
“Almost three-fourths of patients are considered to be good responders to both drugs on day 3, and, maybe, safety is the key issue distinguishing the two treatments: Colchicine was generally well tolerated, but even with this very short time frame of treatment, one patient out of five had diarrhea, which is more of a concern in this elderly population at risk of dehydration,” he said in an oral abstract session at the annual meeting of the American College of Rheumatology.
In contrast, only about 6% of patients assigned to prednisone had diarrhea, and other adverse events that occurred more frequently with the corticosteroid, including hypertension, hyperglycemia, and insomnia all resolved after the therapy was stopped.
Common and acutely painful
Acute CPP crystal arthritis is a common complication that often occurs during hospitalization for primarily nonrheumatologic causes, Dr. Pascart said, and “in the absence of clinical trials, the management relies on expert opinion, which stems from extrapolated data from gap studies” primarily with prednisone or colchicine, Dr. Pascart said.
To fill in the knowledge gap, Dr. Pascart and colleagues conducted the COLCHICORT study to evaluate whether the two drugs were comparable in efficacy and safety for control of acute pain in a vulnerable population.
The multicenter, open-label trial included patients older than age 65 years with an estimated glomerular filtration rate above 30 mL/min per 1.73 m2 who presented with acute CPP deposition arthritis with symptoms occurring within the previous 36 hours. CPP arthritis was defined by the identification of CPP crystals on synovial fluid analysis or typical clinical presentation with evidence of chondrocalcinosis on x-rays or ultrasound.
Patients with a history of gout, cognitive decline that could impair pain assessment, or contraindications to either of the study drugs were excluded.
The participants were randomized to receive either colchicine 1.5 mg (1 mg to start, then 0.5 mg one hour later) at baseline and then 1 mg on day 1, or oral prednisone 30 mg at baseline and on day 1. The patients also received 1 g of systemic acetaminophen, and three 50-mg doses of tramadol during the first 24 hours.
Of the 111 patients randomized, 54 were assigned to receive prednisone, and 57 were assigned to receive colchicine. Baseline characteristics were similar between the groups, with a mean age of about 86 years, body mass index of around 25 kg/m2, and blood pressure in the range of 130/69 mm Hg.
For nearly half of all patients in study each arm the most painful joint was the knee, followed by wrists and ankles.
There was no difference between the groups in the primary efficacy outcome of a change at 24 hours over baseline in visual analog scale (VAS) (0-100 mm) scores, either in a per-protocol analysis or modified intention-to-treat analysis. The mean change in VAS at 24 hours in the colchicine group was –36.6 mm, compared with –37.7 mm in the prednisone group. The investigators had previously determined that any difference between the two drugs of less than 13 mm on pain VAS at 24 hours would meet the definition for equivalent efficacy.
In both groups, a majority of patients had either an improvement greater than 50% in pain VAS scores and/or a pain VAS score less than 40 mm at both 24 and 48 hours.
At 7 days of follow-up, 21.8% of patients assigned to colchicine had diarrhea, compared with 5.6% of those assigned to prednisone. Adverse events occurring more frequently with prednisone included hyperglycemia, hypertension, and insomnia.
Patients who received colchicine and were also on statins had a trend toward a higher risk for diarrhea, but the study was not adequately powered to detect an association, and the trend was not statistically significant, Dr. Pascart said.
“Taken together, safety issues suggest that prednisone should be considered as the first-line therapy in acute CPP crystal arthritis. Future research is warranted to determine factors increasing the risk of colchicine-induced diarrhea,” he concluded.
Both drugs are used
Sara K. Tedeschi, MD, from Brigham & Women’s Hospital in Boston, who attended the session where the data were presented, has a special clinical interest in CPP deposition disease. She applauded Dr. Pascart and colleagues for conducting a rare clinical trial in CPP crystal arthritis.
In an interview, she said that the study suggests “we can keep in mind shorter courses of treatment for acute CPP crystal arthritis; I think that’s one big takeaway from this study.”
Asked whether she would change her practice based on the findings, Dr. Tedeschi replied: “I personally am not sure that I would be moved to use prednisone more than colchicine; I actually take away from this that colchicine is equivalent to prednisone for short-term use for CPP arthritis, but I think it’s also really important to note that this is in the context of quite a lot of acetaminophen and quite a lot of tramadol, and frankly I don’t usually use tramadol with my patients, but I might consider doing that, especially as there were no delirium events in this population.”
Dr. Tedeschi was not involved in the study.
Asked the same question, Michael Toprover, MD, from New York University Langone Medical Center, a moderator of the session who was not involved in the study, said: “I usually use a combination of medications. I generally, in someone who is hospitalized in particular and is in such severe pain, use a combination of colchicine and prednisone, unless I’m worried about infection, in which case I’ll start colchicine until we’ve proven that it’s CPPD, and then I’ll add prednisone.”
The study was funded by PHRC-1 GIRCI Nord Ouest, a clinical research program funded by the Ministry of Health in France. Dr. Pascart, Dr. Tedeschi, and Dr. Toprover all reported having no relevant conflicts of interest.
PHILADELPHIA – Prednisone appears to have the edge over colchicine for control of pain in patients with acute calcium pyrophosphate (CPP) crystal arthritis, an intensely painful rheumatic disease primarily affecting older patients.
Among 111 patients with acute CPP crystal arthritis randomized to receive either prednisone or colchicine for control of acute pain in a multicenter study, 2 days of therapy with the oral agents provided equivalent pain relief on the second day, and patients generally tolerated each agent well, reported Tristan Pascart, MD, from the Groupement Hospitalier de l’Institut Catholique de Lille (France).
“Almost three-fourths of patients are considered to be good responders to both drugs on day 3, and, maybe, safety is the key issue distinguishing the two treatments: Colchicine was generally well tolerated, but even with this very short time frame of treatment, one patient out of five had diarrhea, which is more of a concern in this elderly population at risk of dehydration,” he said in an oral abstract session at the annual meeting of the American College of Rheumatology.
In contrast, only about 6% of patients assigned to prednisone had diarrhea, and other adverse events that occurred more frequently with the corticosteroid, including hypertension, hyperglycemia, and insomnia all resolved after the therapy was stopped.
Common and acutely painful
Acute CPP crystal arthritis is a common complication that often occurs during hospitalization for primarily nonrheumatologic causes, Dr. Pascart said, and “in the absence of clinical trials, the management relies on expert opinion, which stems from extrapolated data from gap studies” primarily with prednisone or colchicine, Dr. Pascart said.
To fill in the knowledge gap, Dr. Pascart and colleagues conducted the COLCHICORT study to evaluate whether the two drugs were comparable in efficacy and safety for control of acute pain in a vulnerable population.
The multicenter, open-label trial included patients older than age 65 years with an estimated glomerular filtration rate above 30 mL/min per 1.73 m2 who presented with acute CPP deposition arthritis with symptoms occurring within the previous 36 hours. CPP arthritis was defined by the identification of CPP crystals on synovial fluid analysis or typical clinical presentation with evidence of chondrocalcinosis on x-rays or ultrasound.
Patients with a history of gout, cognitive decline that could impair pain assessment, or contraindications to either of the study drugs were excluded.
The participants were randomized to receive either colchicine 1.5 mg (1 mg to start, then 0.5 mg one hour later) at baseline and then 1 mg on day 1, or oral prednisone 30 mg at baseline and on day 1. The patients also received 1 g of systemic acetaminophen, and three 50-mg doses of tramadol during the first 24 hours.
Of the 111 patients randomized, 54 were assigned to receive prednisone, and 57 were assigned to receive colchicine. Baseline characteristics were similar between the groups, with a mean age of about 86 years, body mass index of around 25 kg/m2, and blood pressure in the range of 130/69 mm Hg.
For nearly half of all patients in study each arm the most painful joint was the knee, followed by wrists and ankles.
There was no difference between the groups in the primary efficacy outcome of a change at 24 hours over baseline in visual analog scale (VAS) (0-100 mm) scores, either in a per-protocol analysis or modified intention-to-treat analysis. The mean change in VAS at 24 hours in the colchicine group was –36.6 mm, compared with –37.7 mm in the prednisone group. The investigators had previously determined that any difference between the two drugs of less than 13 mm on pain VAS at 24 hours would meet the definition for equivalent efficacy.
In both groups, a majority of patients had either an improvement greater than 50% in pain VAS scores and/or a pain VAS score less than 40 mm at both 24 and 48 hours.
At 7 days of follow-up, 21.8% of patients assigned to colchicine had diarrhea, compared with 5.6% of those assigned to prednisone. Adverse events occurring more frequently with prednisone included hyperglycemia, hypertension, and insomnia.
Patients who received colchicine and were also on statins had a trend toward a higher risk for diarrhea, but the study was not adequately powered to detect an association, and the trend was not statistically significant, Dr. Pascart said.
“Taken together, safety issues suggest that prednisone should be considered as the first-line therapy in acute CPP crystal arthritis. Future research is warranted to determine factors increasing the risk of colchicine-induced diarrhea,” he concluded.
Both drugs are used
Sara K. Tedeschi, MD, from Brigham & Women’s Hospital in Boston, who attended the session where the data were presented, has a special clinical interest in CPP deposition disease. She applauded Dr. Pascart and colleagues for conducting a rare clinical trial in CPP crystal arthritis.
In an interview, she said that the study suggests “we can keep in mind shorter courses of treatment for acute CPP crystal arthritis; I think that’s one big takeaway from this study.”
Asked whether she would change her practice based on the findings, Dr. Tedeschi replied: “I personally am not sure that I would be moved to use prednisone more than colchicine; I actually take away from this that colchicine is equivalent to prednisone for short-term use for CPP arthritis, but I think it’s also really important to note that this is in the context of quite a lot of acetaminophen and quite a lot of tramadol, and frankly I don’t usually use tramadol with my patients, but I might consider doing that, especially as there were no delirium events in this population.”
Dr. Tedeschi was not involved in the study.
Asked the same question, Michael Toprover, MD, from New York University Langone Medical Center, a moderator of the session who was not involved in the study, said: “I usually use a combination of medications. I generally, in someone who is hospitalized in particular and is in such severe pain, use a combination of colchicine and prednisone, unless I’m worried about infection, in which case I’ll start colchicine until we’ve proven that it’s CPPD, and then I’ll add prednisone.”
The study was funded by PHRC-1 GIRCI Nord Ouest, a clinical research program funded by the Ministry of Health in France. Dr. Pascart, Dr. Tedeschi, and Dr. Toprover all reported having no relevant conflicts of interest.
AT ACR 2022
Gout too often treated only in emergency department
Only about one in three patients seen in the emergency department of an academic health system for acute gout had a follow-up visit that addressed this condition, Lesley Jackson, MD, of the University of Alabama at Birmingham, reported at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
Dr. Jackson presented research done on patients seen within her university’s health system, looking at 72 patients seen in the ED between September 2021 and February 2022. Medications prescribed at discharge from the ED included corticosteroids (46 patients, or 64%), opioids (45 patients, 63%), NSAIDs (31 patients, 43%), and colchicine (23 patients, 32%).
Only 26 patients, or about 36%, had a subsequent outpatient visit in the UAB health system addressing gout, she said. Of 33 patients with any outpatient follow-up visit within the UAB system, 21 were within 1 month after the index ED visit, followed by 3 more prior to 3 months, and 9 more after 3 months.
The limitations of the study includes its collection of data from a single institution. But the results highlight the need for improved quality of care for gout, with too many people being treated for this condition primarily in the ED, she said.
In an email exchange arranged by the Arthritis Foundation, Herbert S. B. Baraf, MD, said he agreed that patients too often limit their treatment for gout to seeking care for acute attacks in the ED.
Because of competing demands, physicians working there are more to take a “Band-Aid” approach and not impress upon patients that gout is a lifelong condition that needs follow-up and monitoring, said Dr. Baraf, clinical professor of medicine at George Washington University, Washington, and an associate clinical professor at the University of Maryland, Baltimore. He retired from private practice in 2022.
“This problem is akin to the patient who has a hip fracture due to osteoporosis who gets a surgical repair but is never referred for osteoporotic management,” wrote Dr. Baraf, who is a former board member of the Arthritis Foundation.
He suggested viewing gout as a form of arthritis that has two components.
“The first, that which brings the patient to seek medical care, is the often exquisitely painful attack of pain and swelling in a joint or joints that comes on acutely,” he wrote. “Calming these attacks are the focus of the patient and the doctor, who does the evaluation as relief of pain and inflammation is the most pressing task at hand.”
But equally important is the second element, addressing the cause of these flare ups of arthritis, he wrote. Elevated uric acid leads to crystalline deposits of urate in the joints, particularly in the feet, ankles, knees, and hands. Over time, these deposits generate seemingly random flare ups of acute joint pain in one or more of these areas.
“Thus, when a patient presents to an emergency room with a first or second attack of gout, pain relief is the primary focus of the visit,” Dr. Baraf wrote. “But if over time that is the only focus, and the elevation of serum uric acid is not addressed, deposits will continue to mount and flare ups will occur with increasing frequency and severity.”
This study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Jackson has no relevant financial disclosures.
Only about one in three patients seen in the emergency department of an academic health system for acute gout had a follow-up visit that addressed this condition, Lesley Jackson, MD, of the University of Alabama at Birmingham, reported at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
Dr. Jackson presented research done on patients seen within her university’s health system, looking at 72 patients seen in the ED between September 2021 and February 2022. Medications prescribed at discharge from the ED included corticosteroids (46 patients, or 64%), opioids (45 patients, 63%), NSAIDs (31 patients, 43%), and colchicine (23 patients, 32%).
Only 26 patients, or about 36%, had a subsequent outpatient visit in the UAB health system addressing gout, she said. Of 33 patients with any outpatient follow-up visit within the UAB system, 21 were within 1 month after the index ED visit, followed by 3 more prior to 3 months, and 9 more after 3 months.
The limitations of the study includes its collection of data from a single institution. But the results highlight the need for improved quality of care for gout, with too many people being treated for this condition primarily in the ED, she said.
In an email exchange arranged by the Arthritis Foundation, Herbert S. B. Baraf, MD, said he agreed that patients too often limit their treatment for gout to seeking care for acute attacks in the ED.
Because of competing demands, physicians working there are more to take a “Band-Aid” approach and not impress upon patients that gout is a lifelong condition that needs follow-up and monitoring, said Dr. Baraf, clinical professor of medicine at George Washington University, Washington, and an associate clinical professor at the University of Maryland, Baltimore. He retired from private practice in 2022.
“This problem is akin to the patient who has a hip fracture due to osteoporosis who gets a surgical repair but is never referred for osteoporotic management,” wrote Dr. Baraf, who is a former board member of the Arthritis Foundation.
He suggested viewing gout as a form of arthritis that has two components.
“The first, that which brings the patient to seek medical care, is the often exquisitely painful attack of pain and swelling in a joint or joints that comes on acutely,” he wrote. “Calming these attacks are the focus of the patient and the doctor, who does the evaluation as relief of pain and inflammation is the most pressing task at hand.”
But equally important is the second element, addressing the cause of these flare ups of arthritis, he wrote. Elevated uric acid leads to crystalline deposits of urate in the joints, particularly in the feet, ankles, knees, and hands. Over time, these deposits generate seemingly random flare ups of acute joint pain in one or more of these areas.
“Thus, when a patient presents to an emergency room with a first or second attack of gout, pain relief is the primary focus of the visit,” Dr. Baraf wrote. “But if over time that is the only focus, and the elevation of serum uric acid is not addressed, deposits will continue to mount and flare ups will occur with increasing frequency and severity.”
This study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Jackson has no relevant financial disclosures.
Only about one in three patients seen in the emergency department of an academic health system for acute gout had a follow-up visit that addressed this condition, Lesley Jackson, MD, of the University of Alabama at Birmingham, reported at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
Dr. Jackson presented research done on patients seen within her university’s health system, looking at 72 patients seen in the ED between September 2021 and February 2022. Medications prescribed at discharge from the ED included corticosteroids (46 patients, or 64%), opioids (45 patients, 63%), NSAIDs (31 patients, 43%), and colchicine (23 patients, 32%).
Only 26 patients, or about 36%, had a subsequent outpatient visit in the UAB health system addressing gout, she said. Of 33 patients with any outpatient follow-up visit within the UAB system, 21 were within 1 month after the index ED visit, followed by 3 more prior to 3 months, and 9 more after 3 months.
The limitations of the study includes its collection of data from a single institution. But the results highlight the need for improved quality of care for gout, with too many people being treated for this condition primarily in the ED, she said.
In an email exchange arranged by the Arthritis Foundation, Herbert S. B. Baraf, MD, said he agreed that patients too often limit their treatment for gout to seeking care for acute attacks in the ED.
Because of competing demands, physicians working there are more to take a “Band-Aid” approach and not impress upon patients that gout is a lifelong condition that needs follow-up and monitoring, said Dr. Baraf, clinical professor of medicine at George Washington University, Washington, and an associate clinical professor at the University of Maryland, Baltimore. He retired from private practice in 2022.
“This problem is akin to the patient who has a hip fracture due to osteoporosis who gets a surgical repair but is never referred for osteoporotic management,” wrote Dr. Baraf, who is a former board member of the Arthritis Foundation.
He suggested viewing gout as a form of arthritis that has two components.
“The first, that which brings the patient to seek medical care, is the often exquisitely painful attack of pain and swelling in a joint or joints that comes on acutely,” he wrote. “Calming these attacks are the focus of the patient and the doctor, who does the evaluation as relief of pain and inflammation is the most pressing task at hand.”
But equally important is the second element, addressing the cause of these flare ups of arthritis, he wrote. Elevated uric acid leads to crystalline deposits of urate in the joints, particularly in the feet, ankles, knees, and hands. Over time, these deposits generate seemingly random flare ups of acute joint pain in one or more of these areas.
“Thus, when a patient presents to an emergency room with a first or second attack of gout, pain relief is the primary focus of the visit,” Dr. Baraf wrote. “But if over time that is the only focus, and the elevation of serum uric acid is not addressed, deposits will continue to mount and flare ups will occur with increasing frequency and severity.”
This study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Jackson has no relevant financial disclosures.
FROM G-CAN 2022
Poor control of serum urate linked to cardiovascular risk in patients with gout
A new study based on U.S. veterans’ medical records adds to the evidence for a link between gout – especially poorly controlled cases – and cardiovascular disease (CVD) risk, Tate Johnson, MD, reported at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network.
Gout was associated with a 68% increased risk of heart failure (HF) hospitalization, 25% increased risk of HF-related death, and a 22% increased risk of major adverse cardiovascular events (MACE), said Dr. Johnson, of the division of rheumatology at the University of Nebraska, Omaha.
Poorly controlled serum urate was associated with a higher risk of cardiovascular events, regardless of the use of urate-lowering therapy (ULT). He said more research is needed to see if there is a causal link between gout, hyperuricemia – or its treatment – and CVD risk.
Dr. Johnson and colleagues used records from the Veterans Health Administration for this study. They created a retrospective, matched cohort study that looked at records dating from January 1999 to September 2015. Patients with gout (≥ 2 ICD-9 codes) were matched 1:10 on age, sex, and year of VHA enrollment to patients without a gout ICD-9 code or a record of receiving ULT. They matched 559,243 people with gout to 5,407,379 people who did not have a diagnosis or a recorded treatment for this condition.
Over 43,331,604 person-years, Dr. Johnson and colleagues observed 137,162 CVD events in gout (incidence rate 33.96 per 1,000 person-years) vs. 879,903 in non-gout patients (IR 22.37 per 1,000 person-years). Gout was most strongly associated with HF hospitalization, with a nearly threefold higher risk (hazard ratio, 2.78; 95% confidence interval, 2.73-2.83), which attenuated but persisted after adjustment for additional CVD risk factors (adjusted hazard ratio, 1.68; 95% CI, 1.65-1.70) and excluding patients with prevalent HF (aHR, 1.60; 95% CI, 1.57-1.64).
People with gout were also at higher risk of HF-related death (aHR, 1.25; 95% CI, 1.21-1.29), MACE (aHR, 1.22; 95% CI, 1.21-1.23), and coronary artery disease–related death (aHR, 1.21; 95% CI, 1.20-1.22).
Among people with gout in the study, poor serum urate control was associated with a higher risk of all CVD events, with the highest CVD risk occurring in patients with inadequately controlled serum urate despite receipt of ULT, particularly related to HF hospitalization (aHR, 1.43; 95% CI, 1.34-1.52) and HF-related death (aHR, 1.47; 95% CI, 1.34-1.61).
Limits of the study include the generalizability of the study population. Reflecting the VHA’s patient population, 99% of the cohort were men, with 62% of the gout group and 59.4% of the control group identifying as White and non-Hispanic.
The study provides evidence that may be found only by studying medical records, Richard J. Johnson, MD, of the University of Colorado at Denver, Aurora, said in an interview.
Dr. Richard Johnson, who is not related to the author, said that only about one-third of people with gout are adequately treated, and about another one-third take urate-lowering therapy (ULT) but fail to get their serum urate level under control. But it would be unethical to design a clinical trial to study CVD risk and poorly controlled serum urate without ULT treatment.
“The only way you can figure out if uric acid lowering is going to help these guys is to actually do a study like this where you see the ones who don’t get adequate treatment versus adequate treatment and you show that there’s going to be a difference in outcome,” he said.
Dr. Richard Johnson contrasted this approach with the one used in the recently reported study that appeared to cast doubt on the link between serum uric acid levels and cardiovascular disease. The ALL-HEART trial found that allopurinol, a drug commonly used to treat gout, provided no benefit in terms of reducing cardiovascular events in patients with ischemic heart disease. But these patients did not have gout, and that was a critical difference, he said.
He noted that it was not surprising that the results of ALL-HEART were negative, given the study design.
“The ALL-HEART study treated people regardless of their uric acid level, and they also excluded subjects who had a history of gout,” he said. “Yet the risk associated with uric acid occurs primarily among those with elevated serum uric acid levels and those with gout.”
The study received funding from the Rheumatology Research Foundation and the VHA. Neither Dr. Tate Johnson nor Dr. Richard Johnson had any relevant disclosures.
A new study based on U.S. veterans’ medical records adds to the evidence for a link between gout – especially poorly controlled cases – and cardiovascular disease (CVD) risk, Tate Johnson, MD, reported at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network.
Gout was associated with a 68% increased risk of heart failure (HF) hospitalization, 25% increased risk of HF-related death, and a 22% increased risk of major adverse cardiovascular events (MACE), said Dr. Johnson, of the division of rheumatology at the University of Nebraska, Omaha.
Poorly controlled serum urate was associated with a higher risk of cardiovascular events, regardless of the use of urate-lowering therapy (ULT). He said more research is needed to see if there is a causal link between gout, hyperuricemia – or its treatment – and CVD risk.
Dr. Johnson and colleagues used records from the Veterans Health Administration for this study. They created a retrospective, matched cohort study that looked at records dating from January 1999 to September 2015. Patients with gout (≥ 2 ICD-9 codes) were matched 1:10 on age, sex, and year of VHA enrollment to patients without a gout ICD-9 code or a record of receiving ULT. They matched 559,243 people with gout to 5,407,379 people who did not have a diagnosis or a recorded treatment for this condition.
Over 43,331,604 person-years, Dr. Johnson and colleagues observed 137,162 CVD events in gout (incidence rate 33.96 per 1,000 person-years) vs. 879,903 in non-gout patients (IR 22.37 per 1,000 person-years). Gout was most strongly associated with HF hospitalization, with a nearly threefold higher risk (hazard ratio, 2.78; 95% confidence interval, 2.73-2.83), which attenuated but persisted after adjustment for additional CVD risk factors (adjusted hazard ratio, 1.68; 95% CI, 1.65-1.70) and excluding patients with prevalent HF (aHR, 1.60; 95% CI, 1.57-1.64).
People with gout were also at higher risk of HF-related death (aHR, 1.25; 95% CI, 1.21-1.29), MACE (aHR, 1.22; 95% CI, 1.21-1.23), and coronary artery disease–related death (aHR, 1.21; 95% CI, 1.20-1.22).
Among people with gout in the study, poor serum urate control was associated with a higher risk of all CVD events, with the highest CVD risk occurring in patients with inadequately controlled serum urate despite receipt of ULT, particularly related to HF hospitalization (aHR, 1.43; 95% CI, 1.34-1.52) and HF-related death (aHR, 1.47; 95% CI, 1.34-1.61).
Limits of the study include the generalizability of the study population. Reflecting the VHA’s patient population, 99% of the cohort were men, with 62% of the gout group and 59.4% of the control group identifying as White and non-Hispanic.
The study provides evidence that may be found only by studying medical records, Richard J. Johnson, MD, of the University of Colorado at Denver, Aurora, said in an interview.
Dr. Richard Johnson, who is not related to the author, said that only about one-third of people with gout are adequately treated, and about another one-third take urate-lowering therapy (ULT) but fail to get their serum urate level under control. But it would be unethical to design a clinical trial to study CVD risk and poorly controlled serum urate without ULT treatment.
“The only way you can figure out if uric acid lowering is going to help these guys is to actually do a study like this where you see the ones who don’t get adequate treatment versus adequate treatment and you show that there’s going to be a difference in outcome,” he said.
Dr. Richard Johnson contrasted this approach with the one used in the recently reported study that appeared to cast doubt on the link between serum uric acid levels and cardiovascular disease. The ALL-HEART trial found that allopurinol, a drug commonly used to treat gout, provided no benefit in terms of reducing cardiovascular events in patients with ischemic heart disease. But these patients did not have gout, and that was a critical difference, he said.
He noted that it was not surprising that the results of ALL-HEART were negative, given the study design.
“The ALL-HEART study treated people regardless of their uric acid level, and they also excluded subjects who had a history of gout,” he said. “Yet the risk associated with uric acid occurs primarily among those with elevated serum uric acid levels and those with gout.”
The study received funding from the Rheumatology Research Foundation and the VHA. Neither Dr. Tate Johnson nor Dr. Richard Johnson had any relevant disclosures.
A new study based on U.S. veterans’ medical records adds to the evidence for a link between gout – especially poorly controlled cases – and cardiovascular disease (CVD) risk, Tate Johnson, MD, reported at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network.
Gout was associated with a 68% increased risk of heart failure (HF) hospitalization, 25% increased risk of HF-related death, and a 22% increased risk of major adverse cardiovascular events (MACE), said Dr. Johnson, of the division of rheumatology at the University of Nebraska, Omaha.
Poorly controlled serum urate was associated with a higher risk of cardiovascular events, regardless of the use of urate-lowering therapy (ULT). He said more research is needed to see if there is a causal link between gout, hyperuricemia – or its treatment – and CVD risk.
Dr. Johnson and colleagues used records from the Veterans Health Administration for this study. They created a retrospective, matched cohort study that looked at records dating from January 1999 to September 2015. Patients with gout (≥ 2 ICD-9 codes) were matched 1:10 on age, sex, and year of VHA enrollment to patients without a gout ICD-9 code or a record of receiving ULT. They matched 559,243 people with gout to 5,407,379 people who did not have a diagnosis or a recorded treatment for this condition.
Over 43,331,604 person-years, Dr. Johnson and colleagues observed 137,162 CVD events in gout (incidence rate 33.96 per 1,000 person-years) vs. 879,903 in non-gout patients (IR 22.37 per 1,000 person-years). Gout was most strongly associated with HF hospitalization, with a nearly threefold higher risk (hazard ratio, 2.78; 95% confidence interval, 2.73-2.83), which attenuated but persisted after adjustment for additional CVD risk factors (adjusted hazard ratio, 1.68; 95% CI, 1.65-1.70) and excluding patients with prevalent HF (aHR, 1.60; 95% CI, 1.57-1.64).
People with gout were also at higher risk of HF-related death (aHR, 1.25; 95% CI, 1.21-1.29), MACE (aHR, 1.22; 95% CI, 1.21-1.23), and coronary artery disease–related death (aHR, 1.21; 95% CI, 1.20-1.22).
Among people with gout in the study, poor serum urate control was associated with a higher risk of all CVD events, with the highest CVD risk occurring in patients with inadequately controlled serum urate despite receipt of ULT, particularly related to HF hospitalization (aHR, 1.43; 95% CI, 1.34-1.52) and HF-related death (aHR, 1.47; 95% CI, 1.34-1.61).
Limits of the study include the generalizability of the study population. Reflecting the VHA’s patient population, 99% of the cohort were men, with 62% of the gout group and 59.4% of the control group identifying as White and non-Hispanic.
The study provides evidence that may be found only by studying medical records, Richard J. Johnson, MD, of the University of Colorado at Denver, Aurora, said in an interview.
Dr. Richard Johnson, who is not related to the author, said that only about one-third of people with gout are adequately treated, and about another one-third take urate-lowering therapy (ULT) but fail to get their serum urate level under control. But it would be unethical to design a clinical trial to study CVD risk and poorly controlled serum urate without ULT treatment.
“The only way you can figure out if uric acid lowering is going to help these guys is to actually do a study like this where you see the ones who don’t get adequate treatment versus adequate treatment and you show that there’s going to be a difference in outcome,” he said.
Dr. Richard Johnson contrasted this approach with the one used in the recently reported study that appeared to cast doubt on the link between serum uric acid levels and cardiovascular disease. The ALL-HEART trial found that allopurinol, a drug commonly used to treat gout, provided no benefit in terms of reducing cardiovascular events in patients with ischemic heart disease. But these patients did not have gout, and that was a critical difference, he said.
He noted that it was not surprising that the results of ALL-HEART were negative, given the study design.
“The ALL-HEART study treated people regardless of their uric acid level, and they also excluded subjects who had a history of gout,” he said. “Yet the risk associated with uric acid occurs primarily among those with elevated serum uric acid levels and those with gout.”
The study received funding from the Rheumatology Research Foundation and the VHA. Neither Dr. Tate Johnson nor Dr. Richard Johnson had any relevant disclosures.
FROM G-CAN 2022
Research ties gout in women to comorbidities more than genetics
Comorbidities may play a greater role than genetics women with gout, although this appears not to be true for men, Nicholas Sumpter, MSc, of the University of Alabama at Birmingham said at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
Mr. Sumpter was among the authors of a recent paper in Arthritis & Rheumatology that suggested that earlier gout onset involves the accumulation of certain allelic variants in men. This genetic risk was shared across multiple ancestral groups in the study, conducted with men of European and Polynesian ancestry, Mr. Sumpter and colleagues reported.
“There might be more than one factor in gout in men, but in women we’ve been getting at this idea that comorbidities are the big thing,” he said.
During his presentation, Mr. Sumpter offered a hypothesis that in men there might be a kind of “two-pronged attack,” with increases in serum urate linked to genetic risk, but comorbidities also playing a role. “But that may not be the case for women.”
In his presentation, Mr. Sumpter noted a paper published in March 2022 from his University of Alabama at Birmingham colleagues, Aakash V. Patel, MD, and Angelo L. Gaffo, MD. In the article, Dr. Patel and Dr. Gaffo delved into the challenges of treating women with gout given “the paucity of appropriately well-powered, randomized-controlled trials investigating the efficacy” of commonly used treatments.
“This poses major challenges for the management of female gout patients since they carry a greater burden of cardiovascular and renal morbidity, which is known to modulate the pathophysiology of gout; as such, conclusions regarding the efficacy of treatments for females cannot be extrapolated from investigative studies that are predominantly male,” they wrote, calling for increased efforts to enroll women in studies of treatments for this condition.
There’s increased interest in how gout affects women, including findings in a paper published in September in Arthritis & Rheumatology that found people with gout, especially women, appear to be at higher risk for poor COVID-19 outcomes, including hospitalization and death, regardless of COVID-19 vaccination status.
Gout has become more common in women, although this remains a condition that is far more likely to strike men.
The age-standardized prevalence of gout among women rose from 233.52 per 100,000 in 1990 to 253.49 in 2017, a gain of about 9%, according to a systematic analysis of the Global Burden of Disease Study.
That topped the roughly 5% gain seen for men in the same time frame, with the rate going from 747.48 per 100,000 to 790.90. With the aging of the global population, gout’s burden in terms of prevalence and disability is expected to increase.
Impact of obesity and healthy eating patterns
Obesity, or excess adiposity, appears to be of particular concern for women in terms of gout risk.
While obesity and genetic predisposition both are strongly associated with a higher risk of gout, the excess risk of both combined was higher than the sum of each, particularly among women, Natalie McCormick, PhD, of Massachusetts General Hospital, Boston, and coauthors reported in Annals of the Rheumatic Diseases.
These findings suggested that “addressing excess adiposity could prevent a large proportion of female gout cases in particular, as well as its cardiometabolic comorbidities, and the benefit could be greater in genetically predisposed women,” they wrote.
In general, there’s a need to re-examine the advice given by many clinicians in the past that people with gout, or those at risk for it, should follow a low-protein diet to avoid purines, Dr. McCormick said in an interview.
“Now we’re finding that a healthier diet that balances protein as well as fat intake can actually be better both for cardiovascular health and for gout prevention,” she said.
Dr. McCormick’s research on this topic includes a 2022 JAMA Internal Medicine article, and a 2021 article in Current Rheumatology Reports. In the latter article, Dr. McCormick and colleagues examined the benefits of changing habits for patients, such as following one of several well-established healthy eating patterns, including the Mediterranean and DASH diets.
With excess weight and associated cardiovascular and endocrine risks already elevated among people with gout, especially women, the “conventional low-purine (i.e., low-protein) approach to gout dietary guidance is neither helpful nor sustainable and may lead to detrimental effects related to worsening insulin resistance as a result of substitution of healthy proteins with unhealthy carbohydrates or fats,” they wrote. “Rather, by focusing our dietary recommendations on healthy eating patterns which have been proven to reduce cardiometabolic risk factors, as opposed to singular ‘good’ or ‘bad’ food items or groups, the beneficial effects of such diets on relevant gout endpoints should naturally follow for the majority of typical gout cases, mediated through changes in insulin resistance.”
Mr. Sumpter and Dr. McCormick had no competing interests to declare.
Comorbidities may play a greater role than genetics women with gout, although this appears not to be true for men, Nicholas Sumpter, MSc, of the University of Alabama at Birmingham said at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
Mr. Sumpter was among the authors of a recent paper in Arthritis & Rheumatology that suggested that earlier gout onset involves the accumulation of certain allelic variants in men. This genetic risk was shared across multiple ancestral groups in the study, conducted with men of European and Polynesian ancestry, Mr. Sumpter and colleagues reported.
“There might be more than one factor in gout in men, but in women we’ve been getting at this idea that comorbidities are the big thing,” he said.
During his presentation, Mr. Sumpter offered a hypothesis that in men there might be a kind of “two-pronged attack,” with increases in serum urate linked to genetic risk, but comorbidities also playing a role. “But that may not be the case for women.”
In his presentation, Mr. Sumpter noted a paper published in March 2022 from his University of Alabama at Birmingham colleagues, Aakash V. Patel, MD, and Angelo L. Gaffo, MD. In the article, Dr. Patel and Dr. Gaffo delved into the challenges of treating women with gout given “the paucity of appropriately well-powered, randomized-controlled trials investigating the efficacy” of commonly used treatments.
“This poses major challenges for the management of female gout patients since they carry a greater burden of cardiovascular and renal morbidity, which is known to modulate the pathophysiology of gout; as such, conclusions regarding the efficacy of treatments for females cannot be extrapolated from investigative studies that are predominantly male,” they wrote, calling for increased efforts to enroll women in studies of treatments for this condition.
There’s increased interest in how gout affects women, including findings in a paper published in September in Arthritis & Rheumatology that found people with gout, especially women, appear to be at higher risk for poor COVID-19 outcomes, including hospitalization and death, regardless of COVID-19 vaccination status.
Gout has become more common in women, although this remains a condition that is far more likely to strike men.
The age-standardized prevalence of gout among women rose from 233.52 per 100,000 in 1990 to 253.49 in 2017, a gain of about 9%, according to a systematic analysis of the Global Burden of Disease Study.
That topped the roughly 5% gain seen for men in the same time frame, with the rate going from 747.48 per 100,000 to 790.90. With the aging of the global population, gout’s burden in terms of prevalence and disability is expected to increase.
Impact of obesity and healthy eating patterns
Obesity, or excess adiposity, appears to be of particular concern for women in terms of gout risk.
While obesity and genetic predisposition both are strongly associated with a higher risk of gout, the excess risk of both combined was higher than the sum of each, particularly among women, Natalie McCormick, PhD, of Massachusetts General Hospital, Boston, and coauthors reported in Annals of the Rheumatic Diseases.
These findings suggested that “addressing excess adiposity could prevent a large proportion of female gout cases in particular, as well as its cardiometabolic comorbidities, and the benefit could be greater in genetically predisposed women,” they wrote.
In general, there’s a need to re-examine the advice given by many clinicians in the past that people with gout, or those at risk for it, should follow a low-protein diet to avoid purines, Dr. McCormick said in an interview.
“Now we’re finding that a healthier diet that balances protein as well as fat intake can actually be better both for cardiovascular health and for gout prevention,” she said.
Dr. McCormick’s research on this topic includes a 2022 JAMA Internal Medicine article, and a 2021 article in Current Rheumatology Reports. In the latter article, Dr. McCormick and colleagues examined the benefits of changing habits for patients, such as following one of several well-established healthy eating patterns, including the Mediterranean and DASH diets.
With excess weight and associated cardiovascular and endocrine risks already elevated among people with gout, especially women, the “conventional low-purine (i.e., low-protein) approach to gout dietary guidance is neither helpful nor sustainable and may lead to detrimental effects related to worsening insulin resistance as a result of substitution of healthy proteins with unhealthy carbohydrates or fats,” they wrote. “Rather, by focusing our dietary recommendations on healthy eating patterns which have been proven to reduce cardiometabolic risk factors, as opposed to singular ‘good’ or ‘bad’ food items or groups, the beneficial effects of such diets on relevant gout endpoints should naturally follow for the majority of typical gout cases, mediated through changes in insulin resistance.”
Mr. Sumpter and Dr. McCormick had no competing interests to declare.
Comorbidities may play a greater role than genetics women with gout, although this appears not to be true for men, Nicholas Sumpter, MSc, of the University of Alabama at Birmingham said at the annual research symposium of the Gout, Hyperuricemia, and Crystal Associated Disease Network (G-CAN).
Mr. Sumpter was among the authors of a recent paper in Arthritis & Rheumatology that suggested that earlier gout onset involves the accumulation of certain allelic variants in men. This genetic risk was shared across multiple ancestral groups in the study, conducted with men of European and Polynesian ancestry, Mr. Sumpter and colleagues reported.
“There might be more than one factor in gout in men, but in women we’ve been getting at this idea that comorbidities are the big thing,” he said.
During his presentation, Mr. Sumpter offered a hypothesis that in men there might be a kind of “two-pronged attack,” with increases in serum urate linked to genetic risk, but comorbidities also playing a role. “But that may not be the case for women.”
In his presentation, Mr. Sumpter noted a paper published in March 2022 from his University of Alabama at Birmingham colleagues, Aakash V. Patel, MD, and Angelo L. Gaffo, MD. In the article, Dr. Patel and Dr. Gaffo delved into the challenges of treating women with gout given “the paucity of appropriately well-powered, randomized-controlled trials investigating the efficacy” of commonly used treatments.
“This poses major challenges for the management of female gout patients since they carry a greater burden of cardiovascular and renal morbidity, which is known to modulate the pathophysiology of gout; as such, conclusions regarding the efficacy of treatments for females cannot be extrapolated from investigative studies that are predominantly male,” they wrote, calling for increased efforts to enroll women in studies of treatments for this condition.
There’s increased interest in how gout affects women, including findings in a paper published in September in Arthritis & Rheumatology that found people with gout, especially women, appear to be at higher risk for poor COVID-19 outcomes, including hospitalization and death, regardless of COVID-19 vaccination status.
Gout has become more common in women, although this remains a condition that is far more likely to strike men.
The age-standardized prevalence of gout among women rose from 233.52 per 100,000 in 1990 to 253.49 in 2017, a gain of about 9%, according to a systematic analysis of the Global Burden of Disease Study.
That topped the roughly 5% gain seen for men in the same time frame, with the rate going from 747.48 per 100,000 to 790.90. With the aging of the global population, gout’s burden in terms of prevalence and disability is expected to increase.
Impact of obesity and healthy eating patterns
Obesity, or excess adiposity, appears to be of particular concern for women in terms of gout risk.
While obesity and genetic predisposition both are strongly associated with a higher risk of gout, the excess risk of both combined was higher than the sum of each, particularly among women, Natalie McCormick, PhD, of Massachusetts General Hospital, Boston, and coauthors reported in Annals of the Rheumatic Diseases.
These findings suggested that “addressing excess adiposity could prevent a large proportion of female gout cases in particular, as well as its cardiometabolic comorbidities, and the benefit could be greater in genetically predisposed women,” they wrote.
In general, there’s a need to re-examine the advice given by many clinicians in the past that people with gout, or those at risk for it, should follow a low-protein diet to avoid purines, Dr. McCormick said in an interview.
“Now we’re finding that a healthier diet that balances protein as well as fat intake can actually be better both for cardiovascular health and for gout prevention,” she said.
Dr. McCormick’s research on this topic includes a 2022 JAMA Internal Medicine article, and a 2021 article in Current Rheumatology Reports. In the latter article, Dr. McCormick and colleagues examined the benefits of changing habits for patients, such as following one of several well-established healthy eating patterns, including the Mediterranean and DASH diets.
With excess weight and associated cardiovascular and endocrine risks already elevated among people with gout, especially women, the “conventional low-purine (i.e., low-protein) approach to gout dietary guidance is neither helpful nor sustainable and may lead to detrimental effects related to worsening insulin resistance as a result of substitution of healthy proteins with unhealthy carbohydrates or fats,” they wrote. “Rather, by focusing our dietary recommendations on healthy eating patterns which have been proven to reduce cardiometabolic risk factors, as opposed to singular ‘good’ or ‘bad’ food items or groups, the beneficial effects of such diets on relevant gout endpoints should naturally follow for the majority of typical gout cases, mediated through changes in insulin resistance.”
Mr. Sumpter and Dr. McCormick had no competing interests to declare.
FROM G-CAN 2022