User login
Closing the racial gap in minimally invasive gyn hysterectomy and myomectomy
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
Placental allograft, cytology processor, cell-free RNA testing, and male infertility
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Human placental allograft
For case reports involving Revita and for more information, visit https://www.stimlabs.com/revita.
FDA approval for cytology processor
For more information, visit: https://www.hologic.com/.
Cell-free RNA testing for pregnancy complications
Currently, Mirvie is recruiting for their Miracle of Life study, which requests that single gestation pregnant mothers who are not scheduled for cesarean delivery provide a blood sample during their second trimester. Women can see if they are eligible for study participation by visiting https://www.curebase.com/study/miracle/home.
For more information, visit: https://mirvie.com/.
Male fertility platform
For more information, visit: https://posterityhealth.com/.
Hepatitis in pregnancy: Sorting through the alphabet
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
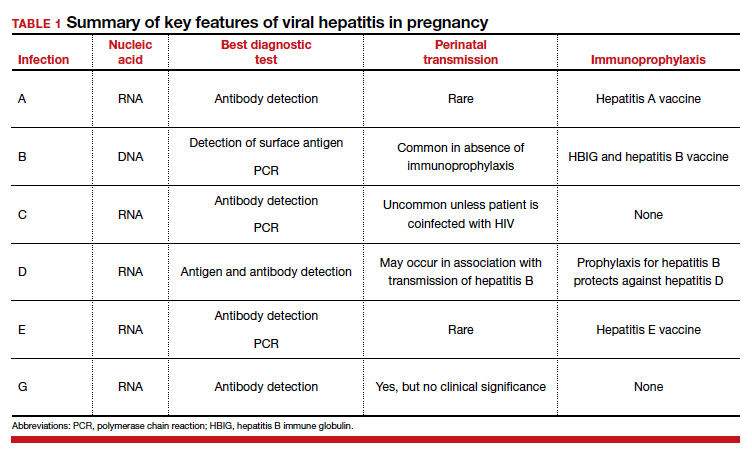
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).
Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).
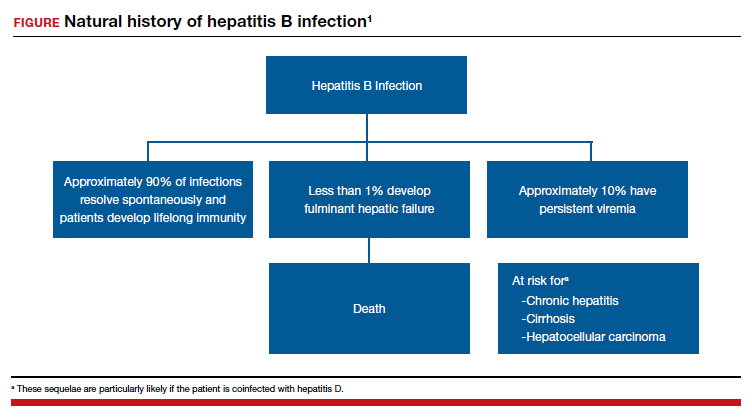

All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
A 27-year-old primigravida at 9 weeks 3 days of gestation tests positive for the hepatitis B surface antigen at her first prenatal appointment. She is completely asymptomatic.
- What additional tests are indicated?
- Does she pose a risk to her sexual partner, and is her newborn at risk for acquiring hepatitis B?
- Can anything be done to protect her partner and newborn from infection?
Meet our perpetrator
Hepatitis is one of the more common viral infections that may occur during pregnancy. Two forms of hepatitis, notably hepatitis A and E, pose a primary threat to the mother. Three forms (B, C, and D) present dangers for the mother, fetus, and newborn. This article will review the epidemiology, clinical manifestations, perinatal implications, and management of the various forms of viral hepatitis. (TABLE 1).

Hepatitis A
Hepatitis A is caused by an RNA virus that is transmitted by fecal-oral contact. The disease is most prevalent in areas with poor sanitation and close living conditions. The incubation period ranges from 15 to 50 days. Most children who acquire this disease are asymptomatic. By contrast, most infected adults are acutely symptomatic. Clinical manifestations typically include low-grade fever, malaise, anorexia, right upper quadrant pain and tenderness, jaundice, and claycolored stools.1,2
The diagnosis of acute hepatitis A infection is best confirmed by detection of immunoglobulin M (IgM)-specific antibodies. The serum transaminase concentrations and the serum bilirubin concentrations usually are significantly elevated. The international normalized ratio, prothrombin time, and partial thromboplastin time also may be elevated.1,2
The treatment for acute hepatitis A largely is supportive care: maintaining hydration, optimizing nutrition, and correcting coagulation abnormalities. The appropriate measures for prevention of hepatitis A are adoption of sound sanitation practices, particularly water purification; minimizing overcrowded living conditions; and administering the hepatitis A vaccine for both pre and postexposure prophylaxis.3,4 The hepatitis A vaccine is preferred over administration of immune globulin because it provides lifelong immunity.
The hepatitis A vaccine is produced in 2 monovalent formulations: Havrix (GlaxoSmithKline) and Vaqta (Merck & Co, Inc). The vaccine should be administered intramuscularly in 2 doses 6 to 12 months apart. The wholesale cost of the vaccine varies from $66 to $119 (according to http://www.goodrx.com). The vaccine also is available in a bivalent form, with recombinant hepatitis B vaccine (Twinrix, GlaxoSmithKline). When used in this form, 3 vaccine administrations are given—at 0, 1, and 6 months apart. The cost of the vaccine is approximately $150 (according to http://www.goodrx.com). TABLE 2 lists the individuals who are appropriate candidates for the hepatitis A vaccine.3,4

Hepatitis B
Hepatitis B is caused by a DNA virus that is transmitted parenterally or perinatally or through
Acute hepatitis B affects 1 to 2 of 1,000 pregnancies in the United States. Approximately 6 to 10 patients per 1,000 pregnancies are asymptomatic but chronically infected.4 The natural history of hepatitis B infection is shown in the FIGURE. The diagnosis of acute and chronic hepatitis B is best established by serology and polymerase chain reaction (PCR; TABLE 3).


All pregnant women should be routinely screened for the hepatitis B surface antigen.5,6 If they are seropositive for the surface antigen alone and receive no immunoprophylaxis, they have a 20% to 30% risk of transmitting infection to their neonate. Subsequently, if they also test positive for the hepatitis Be antigen, the risk of perinatal transmission increases to approximately 90%. Fortunately, 2 forms of immunoprophylaxis are highly effective in preventing perinatal transmission. Infants delivered to seropositive mothers should receive hepatitis B immune globulin within 12 hours of birth. Prior to discharge, the infant also should receive the first dose of the hepatitis B vaccine. Subsequent doses should be administered at 1 and 6 months of age. Infants delivered to seronegative mothers require only the vaccine series.1
Although immunoprophylaxis is highly effective, some neonates still acquire infection perinatally. Pan and colleagues7 and Jourdain et al8 demonstrated that administration of tenofovir 200 mg orally each day from 32 weeks’ gestation until delivery provided further protection against perinatal transmission in patients with a high viral load (defined as >1 million copies/mL). In 2016, the Society for Maternal-Fetal Medicine endorsed the use of tenofovir in women with a high viral load.6
Following delivery, women with chronic hepatitis B infection should be referred to a hepatology specialist for consideration of direct antiviral treatment. Multiple drugs are now available that are highly active against this micro-organism. These drugs include several forms of interferon, lamivudine, adefovir, entecavir, telbivudine, and tenofovir.1
Continue to: Hepatitis C...
Hepatitis C
Hepatitis C is caused by an RNA virus that has 6 genotypes. The most common genotype is HCV1, which affects 79% of patients; approximately 13% of patients have HCV2, and 6% have HCV3.9 Of note, the 3 individuals who discovered this virus—Drs. Harvey Alter, Michael Houghton, and Charles Rice—received the 2020 Nobel Prize in Medicine.10
Hepatitis C is transmitted via sexual contact, parenterally, and perinatally. In many patient populations in the United States, hepatitis C is now more prevalent than hepatitis B. Only about half of all infected persons are aware of their infection. If patients go untreated, approximately 15% to 30% eventually develop cirrhosis. Of these individuals, 1% to 3% develop hepatocellular cancer. Chronic hepatitis C is now the most common indication for liver transplantation in the United States.1,9
In the initial stages of infection, hepatitis C usually is asymptomatic. The best screening test is detection of hepatitis C antibody. Because of the increasing prevalence of this disease, the seriousness of the infection, and the recent availability of remarkably effective treatment, routine screening, rather than screening on the basis of risk factors, for hepatitis C in pregnancy is now indicated.11,12
The best tests for confirmation of infection are detection of antibody by enzyme immunoassay and recombinant immuno-blot assay and detection of viral RNA in serum by PCR. Seroconversion may not occur for up to 16 weeks after infection. Therefore, in at-risk patients who initially test negative, retesting is advisable. Patients with positive test results should have tests to identify the specific genotype, determine the viral load, and assess liver function.1
In patients who have undetectable viral loads and who do not have coexisting HIV infection, the risk of perinatal transmission of hepatitis C is less than 5%. If HIV infection is present, the risk of perinatal transmission approaches 20%.1,13,14
If the patient is coinfected with HIV, a scheduled cesarean delivery should be performed at 38 weeks’ gestation.1 If the viral load is undetectable, vaginal delivery is appropriate. If the viral load is high, however (arbitrarily defined as >2.5 millioncopies/mL), the optimal method of delivery is controversial. Several small, nonrandomized noncontrolled cohort studies support elective cesarean delivery in such patients.14
There is no contraindication to breastfeeding in women with hepatitis C unless they are coinfected with HIV. In such a circumstance, formula feeding should be chosen. After delivery, patients with hepatitis C should be referred to a gastroenterology specialist to receive antiviral treatment. Multiple new single-agent and combination regimens have produced cures in more than 90% of patients. These regimens usually require 8 to 12 weeks of treatment, and they are very expensive. They have not been widely tested in pregnant women.1
Hepatitis D
Hepatitis D, or delta hepatitis, is caused by an RNA virus. This virus is unique because it is incapable of independent replication. It must be present in association with hepatitis B to replicate and cause clinical infection. Therefore, the epidemiology of hepatitis D closely mirrors that of hepatitis B.1,2
Patients with hepatitis D typically present in one of two ways. Some individuals are acutely infected with hepatitis D at the same time that they acquire hepatitis B (coinfection). The natural history of this infection usually is spontaneous resolution without sequelae. Other patients have chronic hepatitis D superimposed on chronic hepatitis B (superinfection). Unfortunately, patients with the latter condition are at a notably increased risk for developing severe persistent liver disease.1,2
The diagnosis of hepatitis D may be confirmed by identifying the delta antigen in serum or in liver tissue obtained by biopsy or by identifying IgM- and IgG-specific antibodies in serum. In conjunction with hepatitis B, the delta virus can cause a chronic carrier state. Perinatal transmission is possible but uncommon. Of greatest importance, the immunoprophylaxis described for hepatitis B is almost perfectly protective against perinatal transmission of hepatitis D.1,2
Continue to: Hepatitis E...
Hepatitis E
Hepatitis E is an RNA virus that has 1 serotype and 4 genotypes. Its epidemiology is similar to that of hepatitis A. It is the most common waterborne illness in the world. The incubation period varies from 21 to 56 days. This disease is quite rare in the United States but is endemic in developing nations. In those countries, maternal infection has an alarmingly high mortality rate (5%–25%). For example, in Bangladesh, hepatitis E is responsible for more than 1,000 deaths per year in pregnant women. When hepatitis E is identified in more affluent countries, the individual cases and small outbreaks usually are linked to consumption of undercooked pork or wild game.1,15-17
The clinical presentation of acute hepatitis E also is similar to that of hepatitis A. The usual manifestations are fever, malaise, anorexia, nausea, right upper quadrant pain and tenderness, jaundice, darkened urine, and clay-colored stools. The most useful diagnostic tests are serologic detection of viral-specific antibodies (positive IgM or a 4-fold increase in the prior IgG titer) and PCR-RNA.1,17
Hepatitis E usually does not cause a chronic carrier state, and perinatal transmission is rare. Fortunately, a highly effective vaccine was recently developed (Hecolin, Xiamen Innovax Biotech). This recombinant vaccine is specifically directed against the hepatitis E genotype 1. In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine or the hepatitis B vaccine. The vaccine was administered at time point 0, and 1 and 6 months later. Patients were followed for up to 4.5 years to assess efficacy, immunogenicity, and safety. During the study period, 7 cases of hepatitis E occurred in the vaccine group, compared with 53 in the control group. Approximately 56,000 patients were included in each group. The efficacy of the vaccine was 86.8% (P<.001).18
Hepatitis G
Hepatitis G is caused by 2 single-stranded RNA viruses that are virtually identical—hepatitis G virus and GB virus type C. The viruses share approximately 30% homology with hepatitis C virus. The organism is present throughout the world and infects approximately 1.5% to 2.0% of the population. The virus is transmitted by blood and sexual contact. It replicates preferentially in mononuclear cells and the bone marrow rather than in the liver.19-21
Hepatitis G is much less virulent than hepatitis C. Hepatitis G often coexists with hepatitis A, B, and C, as well as with HIV. Coinfection with hepatitis G does not adversely affect the clinical course of the other conditions.22,23
Most patients with hepatitis G are asymptomatic, and no treatment is indicated. The virus can cause a chronic carrier state. Perinatal transmission is distinctly uncommon. When it does occur, however, injury to mother, fetus, or neonate is unlikely.1,24
The diagnosis of hepatitis G can be established by detection of virus with PCR and by the identification of antibody by enzyme immunoassay. Routine screening for this infection in pregnancy is not indicated.1,2
Hepatitis B is highly contagious and can be transmitted from the patient to her sexual partner and neonate. Testing for hepatitis B surface antigen and antibody is indicated in her partner. If these tests are negative, the partner should immediately receive hepatitis B immune globulin and then be started on the 3-dose hepatitis B vaccination series. The patient’s newborn also should receive hepatitis B immune globulin within 12 hours of delivery and should receive the first dose of the hepatitis B vaccine prior to discharge from the hospital. The second and third doses should be administered 1 and 6 months after delivery.
The patient also should have the following tests:
• liver function tests
-serum transaminases
-direct and indirect bilirubin
-coagulation profile
• hepatitis D antigen
• hepatitis B genotype
• hepatitis B viral load
• HIV serology.
If the hepatitis B viral load exceeds 1 million copies/mL, the patient should be treated with tenofovir 200 mg daily from 28 weeks’ gestation until delivery. In addition, she should be referred to a liver disease specialist after delivery for consideration of treatment with directly-acting antiviral agents. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TB, et al, eds. Creasy & Resnik’s MaternalFetal Medicine Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Hepatitis in pregnancy. In: Queenan JR, Spong CY, Lockwood CJ, eds. Management of HighRisk Pregnancy. An EvidenceBased Approach. 5th ed. Blackwell; 2007:238-241.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471.
- Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med. 2007;367:1685-1694.
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
- Society for MaternalFetal Medicine (SMFM); Dionne-Odom J, Tita ATN, Silverman NS. #38. Hepatitis B in pregnancy: screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14.
- Pan CQ, Duan Z, Dai E, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429-2438.
- Hoofnagle JH, Feinstore SM. The discovery of hepatitis C—the 2020 Nobel Prize in Physiology or Medicine. N Engl J Med. 2020;384:2297-2299.
- Hughes BL, Page CM, Juller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Saab S, Kullar R, Gounder P. The urgent need for hepatitis C screening in pregnant women: a call to action. Obstet Gynecol. 2020;135:773-777.
- Berkley EMF, Leslie KK, Arora S, et al. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112:304-310.
- Brazel M, Duff P. Considerations on the mode of delivery for pregnant women with hepatitis C infection [published online November 22, 2019]. OBG Manag. 2020;32:39-44.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145-154.
- Khuroo MS, Teli MR, Skidmore S, et al. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255.
- Hoofnangle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237-1244.
- Zhang J, Zhang XF, Huang SJ, et al. Longterm efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922.
- Pickering L, ed. Red Book 2000 Report of Committee on Infectious Diseases. 25th ed. American Academy of Pediatrics; 2000.
- Chopra S. GB virus C (hepatitis G) infection. UpToDate website. Updated January 16, 2020. Accessed June 3, 2021. https://www.uptodate.com/contents/gb-virus-c-hepatitis-g-infection.
- Reshetnyak VI, Karlovich TI, Ilchenko LU. Hepatitis G virus. World J Gastroenterol. 2008;14:4725-4734.
- Kew MC, Kassianides C. HGV: hepatitis G virus or harmless G virus. Lancet. 1996;348(suppl II):10.
- Jarvis LM, Davidson F, Hanley JP, et al. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348;1352-1355.
- Feucht HH, Zollner B, Polywka S, et al. Vertical transmission of hepatitis G. Lancet. 1996;347;615-616.
3 cases of hormone therapy optimized to match the patient problem
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
Greater travel distance reduces rates of abortion
Travel distance is an important determinant of access to abortion care in the United States, new findings show.
Increases in median travel distance to the nearest abortion care facility were associated with significant reductions in median abortion rate.
The abortion rate was 21.1 per 1,000 female residents of reproductive age among those who lived less than 5 miles from a facility, but that number dropped to 3.9/1,000 for those living 120 miles or further away.
Overall, in a model of 3,107 U.S. counties that included 62.5 million women of reproductive age, there were an estimated 696,760 abortions (at a mean rate of 11.1/1,000). The authors estimate that if abortion services were integrated into primary care, an additional 18,190 abortions would be performed (mean rate, 11.4/1,000).
Similarly, if telemedicine became widely available in this setting, this would allow approximately 70,920 abortions (mean rate 12.3/1,000). The study was published online in JAMA Open Network.
Reducing travel distances to abortion facilities would increase access, but additional clinics and providers would be needed to meet the demand. But as the population density of many counties with poor access is low, innovative strategies are also needed.
Integrating abortion into primary care or making medication abortion care available by telemedicine may decrease this unmet need, and lead author Kirsten Thompson, MPH, noted that there is growing evidence that both solutions are quite feasible to implement.
“A study published in 2018 has led primary care providers to adopt the same regimen for miscarriage care, showing that they are interested and capable, despite the barriers posed by the mifepristone [Risk Evaluation and Mitigation Strategy] program for these patients,” said Ms. Thompson, who is program and communications director, Bixby Center for Global Reproductive Health, University of California, San Francisco. “Medical education programs designed specifically for primary care providers have trained family medicine and other clinicians in abortion care for over a decade.”
As for telemedicine, Ms. Thompson explained that, during the pandemic, a preliminary injunction in a federal court case and then the Food and Drug Administration suspended enforcement of the in-person requirements of the mifepristone REMS. “In states that allow medical abortion care by telemedicine, providers have been able to offer remote care when medically appropriate, including mailing medical abortion pills to patients at home,” she said. “Researchers have already published evidence on the safety of and patient satisfaction with this approach.”
However, there are two main barriers to the widespread adoption of medical abortion by telemedicine in the United States. “One is the potentially temporary nature of the FDA’s enforcement discretion and second, are the 19 states with laws that ban it, singling out medical abortion as somehow different from other forms of care by telemedicine,” she said.
Study details
About one in four women in the United States will terminate a pregnancy during their lifetime, but the issue is highly contentious and many states have implemented policies that restrict access to abortion care. The authors pointed out that studies have documented clinic closures and women being unable to obtain abortion care, with low-income women and non-White women being disproportionately affected. Increased travel to a provider has also been associated with delays in care as well as increased costs and stress.
Prior research has shown that the further a woman lives from a facility, the less likely she is to obtain abortion care. In this study, Ms. Thompson and colleagues examined the association between travel distance to the nearest abortion care facility and the abortion rate, and then modeled the effect of reduced travel distance on rates.
They first conducted a cross-sectional geographic analysis using the American Community Survey and the U.S. Census to calculate county-level abortion rates per 1,000 women aged between 15 and 44 years. The 2015 data covered 1,948 counties in 27 states.
Abortion rates were then estimated for 3,107 counties in 48 states and the effect of different travel distance scenarios on the abortion rate was also estimated by multivariable model. Data were collected from April 2018 to October 2019.
There were 37.3 million women of reproductive age residing in the 27 states, and a total of 428,720 reported abortions (mean rate, 11.5/1,000; median rate, 9.9/1,000 women).
When looking at all 48 states, the population-weighted mean travel distance to the nearest facility was 25.6 miles, with a median travel distance of 8.2 miles.
A multivariable model showed that a greater travel distance was associated with lower abortion rates. When compared with traveling less than 5 miles, the abortion rate declined by 0.05/1,000 for women traveling between 5 to less than 15 miles for care, 0.22 for those traveling 15 to less than 30 miles, 0.34 for 30 to less than 60 miles, 0.43 for 60 to less than 120 miles, and 0.73 for those traveling 120 miles or more.
They estimated that, if all travel was under 30 miles, there would be a 2.6% increase or 18,190 additional abortions. A simulation also showed that there would be a 10.2% increase (70,920 additional abortions) using medication via telemedicine.
Solutions are feasible
Approached for an independent comment, Sarah W. Prager, MD, MAS, professor of obstetrics and gynecology and division chief, complex family planning, at the University of Washington, Seattle, agreed that the solutions proposed by the authors were feasible.
“More than a third of abortions that are eligible are now done with medication,” she said, “And 89% of abortions are done in the first trimester.”
What this means is that early first-trimester abortions can conceivably be performed in the primary care setting. “Any primary care clinician – whether it’s a family practice or internal medicine physician, or nurse practitioner or nurse midwife – can all be trained to do aspiration or prescribe medication in the first trimester,” said Dr. Prager. “So it could easily be integrated into primary care settings if there was motivation for that to happen.”
However, she emphasized that more is involved than just training the provider. “The whole clinic has to buy into it,” Dr. Prager explained. “The nurses have to be willing to assist, you need the medical assistants, the scheduler or person who works the front desk – the whole clinic system has to buy into it and that’s where it becomes more challenging.”
The individual provider may be willing, but the system may still not be allowing that to happen. “This is also where telemedicine can come in, where the medication can be mailed so it can circumvent the problem to a certain extent,” Dr. Prager added. “You don’t have to have the infrastructure in the same way.”
But many states already have laws in place to make that illegal, especially for abortion care even if they allow it for similar types of care.
Another expert also weighed in and agreed that these two solutions can potentially be implemented.
“The concept of decreased rates of abortion associated with greater distances traveled is not new, but what is unique to this manuscript is the estimations that the authors conducted in understanding the impact of expanding access to abortion among primary care and telehealth providers,” said Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology, University of California, Davis.
“The study provides convincing evidence regarding the need to strengthen infrastructures that support expansion of these services in primary care settings, among physicians and advanced care practitioners,” she said. “Training to provide medical abortion and first-trimester surgical abortion is simple. Many primary care providers are already doing gynecologic procedures – IUD insertions, colposcopies, endometrial biopsies.”
Thus, she noted, adding abortion care “to their toolkit isn’t too far of a stretch.”
As for telemedicine, Dr. Cansino pointed out how the COVID-19 pandemic has also expanded what both patients and providers think are safe options for providing and receiving good care. “Consultations through telemedicine coupled with access to medications for medical abortion through local pharmacies or express mail is definitely safe and feasible.”
The study was supported by the William and Flora Hewlett Foundation and by an anonymous foundation for general operating support (Ms Thompson). Ms. Thompson reported receiving personal fees from GenBioPro outside the submitted work. Dr. Cansino and Dr. Prager have no disclosures.
Travel distance is an important determinant of access to abortion care in the United States, new findings show.
Increases in median travel distance to the nearest abortion care facility were associated with significant reductions in median abortion rate.
The abortion rate was 21.1 per 1,000 female residents of reproductive age among those who lived less than 5 miles from a facility, but that number dropped to 3.9/1,000 for those living 120 miles or further away.
Overall, in a model of 3,107 U.S. counties that included 62.5 million women of reproductive age, there were an estimated 696,760 abortions (at a mean rate of 11.1/1,000). The authors estimate that if abortion services were integrated into primary care, an additional 18,190 abortions would be performed (mean rate, 11.4/1,000).
Similarly, if telemedicine became widely available in this setting, this would allow approximately 70,920 abortions (mean rate 12.3/1,000). The study was published online in JAMA Open Network.
Reducing travel distances to abortion facilities would increase access, but additional clinics and providers would be needed to meet the demand. But as the population density of many counties with poor access is low, innovative strategies are also needed.
Integrating abortion into primary care or making medication abortion care available by telemedicine may decrease this unmet need, and lead author Kirsten Thompson, MPH, noted that there is growing evidence that both solutions are quite feasible to implement.
“A study published in 2018 has led primary care providers to adopt the same regimen for miscarriage care, showing that they are interested and capable, despite the barriers posed by the mifepristone [Risk Evaluation and Mitigation Strategy] program for these patients,” said Ms. Thompson, who is program and communications director, Bixby Center for Global Reproductive Health, University of California, San Francisco. “Medical education programs designed specifically for primary care providers have trained family medicine and other clinicians in abortion care for over a decade.”
As for telemedicine, Ms. Thompson explained that, during the pandemic, a preliminary injunction in a federal court case and then the Food and Drug Administration suspended enforcement of the in-person requirements of the mifepristone REMS. “In states that allow medical abortion care by telemedicine, providers have been able to offer remote care when medically appropriate, including mailing medical abortion pills to patients at home,” she said. “Researchers have already published evidence on the safety of and patient satisfaction with this approach.”
However, there are two main barriers to the widespread adoption of medical abortion by telemedicine in the United States. “One is the potentially temporary nature of the FDA’s enforcement discretion and second, are the 19 states with laws that ban it, singling out medical abortion as somehow different from other forms of care by telemedicine,” she said.
Study details
About one in four women in the United States will terminate a pregnancy during their lifetime, but the issue is highly contentious and many states have implemented policies that restrict access to abortion care. The authors pointed out that studies have documented clinic closures and women being unable to obtain abortion care, with low-income women and non-White women being disproportionately affected. Increased travel to a provider has also been associated with delays in care as well as increased costs and stress.
Prior research has shown that the further a woman lives from a facility, the less likely she is to obtain abortion care. In this study, Ms. Thompson and colleagues examined the association between travel distance to the nearest abortion care facility and the abortion rate, and then modeled the effect of reduced travel distance on rates.
They first conducted a cross-sectional geographic analysis using the American Community Survey and the U.S. Census to calculate county-level abortion rates per 1,000 women aged between 15 and 44 years. The 2015 data covered 1,948 counties in 27 states.
Abortion rates were then estimated for 3,107 counties in 48 states and the effect of different travel distance scenarios on the abortion rate was also estimated by multivariable model. Data were collected from April 2018 to October 2019.
There were 37.3 million women of reproductive age residing in the 27 states, and a total of 428,720 reported abortions (mean rate, 11.5/1,000; median rate, 9.9/1,000 women).
When looking at all 48 states, the population-weighted mean travel distance to the nearest facility was 25.6 miles, with a median travel distance of 8.2 miles.
A multivariable model showed that a greater travel distance was associated with lower abortion rates. When compared with traveling less than 5 miles, the abortion rate declined by 0.05/1,000 for women traveling between 5 to less than 15 miles for care, 0.22 for those traveling 15 to less than 30 miles, 0.34 for 30 to less than 60 miles, 0.43 for 60 to less than 120 miles, and 0.73 for those traveling 120 miles or more.
They estimated that, if all travel was under 30 miles, there would be a 2.6% increase or 18,190 additional abortions. A simulation also showed that there would be a 10.2% increase (70,920 additional abortions) using medication via telemedicine.
Solutions are feasible
Approached for an independent comment, Sarah W. Prager, MD, MAS, professor of obstetrics and gynecology and division chief, complex family planning, at the University of Washington, Seattle, agreed that the solutions proposed by the authors were feasible.
“More than a third of abortions that are eligible are now done with medication,” she said, “And 89% of abortions are done in the first trimester.”
What this means is that early first-trimester abortions can conceivably be performed in the primary care setting. “Any primary care clinician – whether it’s a family practice or internal medicine physician, or nurse practitioner or nurse midwife – can all be trained to do aspiration or prescribe medication in the first trimester,” said Dr. Prager. “So it could easily be integrated into primary care settings if there was motivation for that to happen.”
However, she emphasized that more is involved than just training the provider. “The whole clinic has to buy into it,” Dr. Prager explained. “The nurses have to be willing to assist, you need the medical assistants, the scheduler or person who works the front desk – the whole clinic system has to buy into it and that’s where it becomes more challenging.”
The individual provider may be willing, but the system may still not be allowing that to happen. “This is also where telemedicine can come in, where the medication can be mailed so it can circumvent the problem to a certain extent,” Dr. Prager added. “You don’t have to have the infrastructure in the same way.”
But many states already have laws in place to make that illegal, especially for abortion care even if they allow it for similar types of care.
Another expert also weighed in and agreed that these two solutions can potentially be implemented.
“The concept of decreased rates of abortion associated with greater distances traveled is not new, but what is unique to this manuscript is the estimations that the authors conducted in understanding the impact of expanding access to abortion among primary care and telehealth providers,” said Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology, University of California, Davis.
“The study provides convincing evidence regarding the need to strengthen infrastructures that support expansion of these services in primary care settings, among physicians and advanced care practitioners,” she said. “Training to provide medical abortion and first-trimester surgical abortion is simple. Many primary care providers are already doing gynecologic procedures – IUD insertions, colposcopies, endometrial biopsies.”
Thus, she noted, adding abortion care “to their toolkit isn’t too far of a stretch.”
As for telemedicine, Dr. Cansino pointed out how the COVID-19 pandemic has also expanded what both patients and providers think are safe options for providing and receiving good care. “Consultations through telemedicine coupled with access to medications for medical abortion through local pharmacies or express mail is definitely safe and feasible.”
The study was supported by the William and Flora Hewlett Foundation and by an anonymous foundation for general operating support (Ms Thompson). Ms. Thompson reported receiving personal fees from GenBioPro outside the submitted work. Dr. Cansino and Dr. Prager have no disclosures.
Travel distance is an important determinant of access to abortion care in the United States, new findings show.
Increases in median travel distance to the nearest abortion care facility were associated with significant reductions in median abortion rate.
The abortion rate was 21.1 per 1,000 female residents of reproductive age among those who lived less than 5 miles from a facility, but that number dropped to 3.9/1,000 for those living 120 miles or further away.
Overall, in a model of 3,107 U.S. counties that included 62.5 million women of reproductive age, there were an estimated 696,760 abortions (at a mean rate of 11.1/1,000). The authors estimate that if abortion services were integrated into primary care, an additional 18,190 abortions would be performed (mean rate, 11.4/1,000).
Similarly, if telemedicine became widely available in this setting, this would allow approximately 70,920 abortions (mean rate 12.3/1,000). The study was published online in JAMA Open Network.
Reducing travel distances to abortion facilities would increase access, but additional clinics and providers would be needed to meet the demand. But as the population density of many counties with poor access is low, innovative strategies are also needed.
Integrating abortion into primary care or making medication abortion care available by telemedicine may decrease this unmet need, and lead author Kirsten Thompson, MPH, noted that there is growing evidence that both solutions are quite feasible to implement.
“A study published in 2018 has led primary care providers to adopt the same regimen for miscarriage care, showing that they are interested and capable, despite the barriers posed by the mifepristone [Risk Evaluation and Mitigation Strategy] program for these patients,” said Ms. Thompson, who is program and communications director, Bixby Center for Global Reproductive Health, University of California, San Francisco. “Medical education programs designed specifically for primary care providers have trained family medicine and other clinicians in abortion care for over a decade.”
As for telemedicine, Ms. Thompson explained that, during the pandemic, a preliminary injunction in a federal court case and then the Food and Drug Administration suspended enforcement of the in-person requirements of the mifepristone REMS. “In states that allow medical abortion care by telemedicine, providers have been able to offer remote care when medically appropriate, including mailing medical abortion pills to patients at home,” she said. “Researchers have already published evidence on the safety of and patient satisfaction with this approach.”
However, there are two main barriers to the widespread adoption of medical abortion by telemedicine in the United States. “One is the potentially temporary nature of the FDA’s enforcement discretion and second, are the 19 states with laws that ban it, singling out medical abortion as somehow different from other forms of care by telemedicine,” she said.
Study details
About one in four women in the United States will terminate a pregnancy during their lifetime, but the issue is highly contentious and many states have implemented policies that restrict access to abortion care. The authors pointed out that studies have documented clinic closures and women being unable to obtain abortion care, with low-income women and non-White women being disproportionately affected. Increased travel to a provider has also been associated with delays in care as well as increased costs and stress.
Prior research has shown that the further a woman lives from a facility, the less likely she is to obtain abortion care. In this study, Ms. Thompson and colleagues examined the association between travel distance to the nearest abortion care facility and the abortion rate, and then modeled the effect of reduced travel distance on rates.
They first conducted a cross-sectional geographic analysis using the American Community Survey and the U.S. Census to calculate county-level abortion rates per 1,000 women aged between 15 and 44 years. The 2015 data covered 1,948 counties in 27 states.
Abortion rates were then estimated for 3,107 counties in 48 states and the effect of different travel distance scenarios on the abortion rate was also estimated by multivariable model. Data were collected from April 2018 to October 2019.
There were 37.3 million women of reproductive age residing in the 27 states, and a total of 428,720 reported abortions (mean rate, 11.5/1,000; median rate, 9.9/1,000 women).
When looking at all 48 states, the population-weighted mean travel distance to the nearest facility was 25.6 miles, with a median travel distance of 8.2 miles.
A multivariable model showed that a greater travel distance was associated with lower abortion rates. When compared with traveling less than 5 miles, the abortion rate declined by 0.05/1,000 for women traveling between 5 to less than 15 miles for care, 0.22 for those traveling 15 to less than 30 miles, 0.34 for 30 to less than 60 miles, 0.43 for 60 to less than 120 miles, and 0.73 for those traveling 120 miles or more.
They estimated that, if all travel was under 30 miles, there would be a 2.6% increase or 18,190 additional abortions. A simulation also showed that there would be a 10.2% increase (70,920 additional abortions) using medication via telemedicine.
Solutions are feasible
Approached for an independent comment, Sarah W. Prager, MD, MAS, professor of obstetrics and gynecology and division chief, complex family planning, at the University of Washington, Seattle, agreed that the solutions proposed by the authors were feasible.
“More than a third of abortions that are eligible are now done with medication,” she said, “And 89% of abortions are done in the first trimester.”
What this means is that early first-trimester abortions can conceivably be performed in the primary care setting. “Any primary care clinician – whether it’s a family practice or internal medicine physician, or nurse practitioner or nurse midwife – can all be trained to do aspiration or prescribe medication in the first trimester,” said Dr. Prager. “So it could easily be integrated into primary care settings if there was motivation for that to happen.”
However, she emphasized that more is involved than just training the provider. “The whole clinic has to buy into it,” Dr. Prager explained. “The nurses have to be willing to assist, you need the medical assistants, the scheduler or person who works the front desk – the whole clinic system has to buy into it and that’s where it becomes more challenging.”
The individual provider may be willing, but the system may still not be allowing that to happen. “This is also where telemedicine can come in, where the medication can be mailed so it can circumvent the problem to a certain extent,” Dr. Prager added. “You don’t have to have the infrastructure in the same way.”
But many states already have laws in place to make that illegal, especially for abortion care even if they allow it for similar types of care.
Another expert also weighed in and agreed that these two solutions can potentially be implemented.
“The concept of decreased rates of abortion associated with greater distances traveled is not new, but what is unique to this manuscript is the estimations that the authors conducted in understanding the impact of expanding access to abortion among primary care and telehealth providers,” said Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology, University of California, Davis.
“The study provides convincing evidence regarding the need to strengthen infrastructures that support expansion of these services in primary care settings, among physicians and advanced care practitioners,” she said. “Training to provide medical abortion and first-trimester surgical abortion is simple. Many primary care providers are already doing gynecologic procedures – IUD insertions, colposcopies, endometrial biopsies.”
Thus, she noted, adding abortion care “to their toolkit isn’t too far of a stretch.”
As for telemedicine, Dr. Cansino pointed out how the COVID-19 pandemic has also expanded what both patients and providers think are safe options for providing and receiving good care. “Consultations through telemedicine coupled with access to medications for medical abortion through local pharmacies or express mail is definitely safe and feasible.”
The study was supported by the William and Flora Hewlett Foundation and by an anonymous foundation for general operating support (Ms Thompson). Ms. Thompson reported receiving personal fees from GenBioPro outside the submitted work. Dr. Cansino and Dr. Prager have no disclosures.
FROM JAMA NETWORK OPEN
Drug effective in treating symptoms of postpartum depression
Those suffering from postpartum depression may have a more convenient treatment option, compared with the only drug approved by the Food and Drug Administration to specifically treat this mood disorder.
Observations from phase 3 of a clinical trial published in JAMA Psychiatry shows that zuranolone, an oral drug, improved the core symptoms of postpartum depression after just 3 days.
Postpartum depression affects approximately one in eight women, according to the Centers for Disease Control and Prevention. Brexanolone (Zulresso), which was approved by the FDA in 2019 to treat this condition, is administered intravenously over a 60-hour period with medical supervision.
“Many women don’t have child care and are unable to go to a hospital setting for 72 hours to receive treatment,” study author Kristina Deligiannidis, MD, associate professor at the Feinstein Institutes for Medical Research, Manhasset, N.Y., said in an interview. “The field really does need a variety of new and novel treatments that are fast acting. It is of utmost importance that we treat [postpartum depression] as quickly as possible because it has significant effects on maternal function, mood, and the ability to care for infants.”
Dr. Deligiannidis and colleagues randomly placed 153 volunteers between the ages of 18 and 45 years, who were 6 months or less post partum, into a group that would receive either a placebo or 30 mg of zuranolone daily for 2 weeks. The participants were followed for 45 days to test the effect of the drug.
Researchers measured depression using the Hamilton Rating Scale for Depression (HAMD-17) – where a score of 10-13 means a patient has mild symptoms, 14-17 means mild to moderate symptoms, and anything over 17 equals moderate to severe symptoms. At the baseline of the study, the average HAMD-17 score of those in the zuranolone and placebo groups were 28.4 and 28.8, respectively.
Researchers found that after day 3, 41% of those in the zuranolone group had a 50% or greater reduction in HAMD-17 score from baseline. By day 15, the day after their last dose, 72% of those who had taken zuranolone had a reduction in HAMD-17 compared with 56% of those who had taken the placebo. By day 45, that increased to 75% in the zuranolone group and 57% in the placebo group.
Dr. Deligiannidis, who initially wasn’t sure how long it would take for patients to see the beneficial effects of zuranolone, was surprised by how fast-acting the oral drug appeared to be in the clinical trial. Unlike brexanolone, which is infused into the veins and has rapid access to the brain and nervous system, zuranolone is an oral medicine that has to go through the stomach and the gastrointestinal tract, and then it has to go into the blood system and then has to cross the blood-brain barrier, she explained.
By day 15, 45% of women who took zuranolone received a HAMD-17 score of 7 or under, meaning they have remitted depression. By day 45, 53% of women who had taken the drug were in remission.
Although the zuranolone was well tolerated, about 5% of the group experienced adverse events. Of those who experienced side effects, 15% experienced drowsiness, 9% suffered from headaches, and 8% experienced dizziness and developed an upper respiratory infection. Participants also suffered diarrhea and sedation.
Lissette Tanner, MD, MPH, FACOG, who was not involved with the study, thought the current study’s findings were promising and would be a great alternative to brexanolone.
“You have the additional benefit that it’s an oral agent as opposed to injection, which I know a lot of patients often have concerns about,” said Dr. Tanner, assistant professor of gynecology and obstetrics at Emory University, Atlanta. “[It’s] an exciting prospect for clinical care to be able to prescribe an oral agent patients can feel comfortable taking at home.”
When it comes to the study’s method, Dr. Tanner noted that the researchers used the HAMD-17 scale as opposed to the Edinburgh Postnatal Depression Scale (EPDS), something that is used “a lot more in clinical situations and providers are a lot more familiar with.” Using the EPDS score would be more applicable “in terms of introducing these medications into true clinical care.”
In terms of follow-up, Dr. Tanner said there may be a need for ongoing research that follows the study participants for more than 45 days.
“For depressive symptoms in particular, oftentimes those symptoms ebb and flow. So seeing if there is a long-term response to these medications or just kind of an immediate onset then wane will be important in the future,” she added.
Dr. Tanner is also interested in pharmacokinetic studies involving zuranolone to see how much of the medication may potentially pass into breast milk.
Dr. Deligiannidis and Dr. Tanner had no financial disclosures.
Those suffering from postpartum depression may have a more convenient treatment option, compared with the only drug approved by the Food and Drug Administration to specifically treat this mood disorder.
Observations from phase 3 of a clinical trial published in JAMA Psychiatry shows that zuranolone, an oral drug, improved the core symptoms of postpartum depression after just 3 days.
Postpartum depression affects approximately one in eight women, according to the Centers for Disease Control and Prevention. Brexanolone (Zulresso), which was approved by the FDA in 2019 to treat this condition, is administered intravenously over a 60-hour period with medical supervision.
“Many women don’t have child care and are unable to go to a hospital setting for 72 hours to receive treatment,” study author Kristina Deligiannidis, MD, associate professor at the Feinstein Institutes for Medical Research, Manhasset, N.Y., said in an interview. “The field really does need a variety of new and novel treatments that are fast acting. It is of utmost importance that we treat [postpartum depression] as quickly as possible because it has significant effects on maternal function, mood, and the ability to care for infants.”
Dr. Deligiannidis and colleagues randomly placed 153 volunteers between the ages of 18 and 45 years, who were 6 months or less post partum, into a group that would receive either a placebo or 30 mg of zuranolone daily for 2 weeks. The participants were followed for 45 days to test the effect of the drug.
Researchers measured depression using the Hamilton Rating Scale for Depression (HAMD-17) – where a score of 10-13 means a patient has mild symptoms, 14-17 means mild to moderate symptoms, and anything over 17 equals moderate to severe symptoms. At the baseline of the study, the average HAMD-17 score of those in the zuranolone and placebo groups were 28.4 and 28.8, respectively.
Researchers found that after day 3, 41% of those in the zuranolone group had a 50% or greater reduction in HAMD-17 score from baseline. By day 15, the day after their last dose, 72% of those who had taken zuranolone had a reduction in HAMD-17 compared with 56% of those who had taken the placebo. By day 45, that increased to 75% in the zuranolone group and 57% in the placebo group.
Dr. Deligiannidis, who initially wasn’t sure how long it would take for patients to see the beneficial effects of zuranolone, was surprised by how fast-acting the oral drug appeared to be in the clinical trial. Unlike brexanolone, which is infused into the veins and has rapid access to the brain and nervous system, zuranolone is an oral medicine that has to go through the stomach and the gastrointestinal tract, and then it has to go into the blood system and then has to cross the blood-brain barrier, she explained.
By day 15, 45% of women who took zuranolone received a HAMD-17 score of 7 or under, meaning they have remitted depression. By day 45, 53% of women who had taken the drug were in remission.
Although the zuranolone was well tolerated, about 5% of the group experienced adverse events. Of those who experienced side effects, 15% experienced drowsiness, 9% suffered from headaches, and 8% experienced dizziness and developed an upper respiratory infection. Participants also suffered diarrhea and sedation.
Lissette Tanner, MD, MPH, FACOG, who was not involved with the study, thought the current study’s findings were promising and would be a great alternative to brexanolone.
“You have the additional benefit that it’s an oral agent as opposed to injection, which I know a lot of patients often have concerns about,” said Dr. Tanner, assistant professor of gynecology and obstetrics at Emory University, Atlanta. “[It’s] an exciting prospect for clinical care to be able to prescribe an oral agent patients can feel comfortable taking at home.”
When it comes to the study’s method, Dr. Tanner noted that the researchers used the HAMD-17 scale as opposed to the Edinburgh Postnatal Depression Scale (EPDS), something that is used “a lot more in clinical situations and providers are a lot more familiar with.” Using the EPDS score would be more applicable “in terms of introducing these medications into true clinical care.”
In terms of follow-up, Dr. Tanner said there may be a need for ongoing research that follows the study participants for more than 45 days.
“For depressive symptoms in particular, oftentimes those symptoms ebb and flow. So seeing if there is a long-term response to these medications or just kind of an immediate onset then wane will be important in the future,” she added.
Dr. Tanner is also interested in pharmacokinetic studies involving zuranolone to see how much of the medication may potentially pass into breast milk.
Dr. Deligiannidis and Dr. Tanner had no financial disclosures.
Those suffering from postpartum depression may have a more convenient treatment option, compared with the only drug approved by the Food and Drug Administration to specifically treat this mood disorder.
Observations from phase 3 of a clinical trial published in JAMA Psychiatry shows that zuranolone, an oral drug, improved the core symptoms of postpartum depression after just 3 days.
Postpartum depression affects approximately one in eight women, according to the Centers for Disease Control and Prevention. Brexanolone (Zulresso), which was approved by the FDA in 2019 to treat this condition, is administered intravenously over a 60-hour period with medical supervision.
“Many women don’t have child care and are unable to go to a hospital setting for 72 hours to receive treatment,” study author Kristina Deligiannidis, MD, associate professor at the Feinstein Institutes for Medical Research, Manhasset, N.Y., said in an interview. “The field really does need a variety of new and novel treatments that are fast acting. It is of utmost importance that we treat [postpartum depression] as quickly as possible because it has significant effects on maternal function, mood, and the ability to care for infants.”
Dr. Deligiannidis and colleagues randomly placed 153 volunteers between the ages of 18 and 45 years, who were 6 months or less post partum, into a group that would receive either a placebo or 30 mg of zuranolone daily for 2 weeks. The participants were followed for 45 days to test the effect of the drug.
Researchers measured depression using the Hamilton Rating Scale for Depression (HAMD-17) – where a score of 10-13 means a patient has mild symptoms, 14-17 means mild to moderate symptoms, and anything over 17 equals moderate to severe symptoms. At the baseline of the study, the average HAMD-17 score of those in the zuranolone and placebo groups were 28.4 and 28.8, respectively.
Researchers found that after day 3, 41% of those in the zuranolone group had a 50% or greater reduction in HAMD-17 score from baseline. By day 15, the day after their last dose, 72% of those who had taken zuranolone had a reduction in HAMD-17 compared with 56% of those who had taken the placebo. By day 45, that increased to 75% in the zuranolone group and 57% in the placebo group.
Dr. Deligiannidis, who initially wasn’t sure how long it would take for patients to see the beneficial effects of zuranolone, was surprised by how fast-acting the oral drug appeared to be in the clinical trial. Unlike brexanolone, which is infused into the veins and has rapid access to the brain and nervous system, zuranolone is an oral medicine that has to go through the stomach and the gastrointestinal tract, and then it has to go into the blood system and then has to cross the blood-brain barrier, she explained.
By day 15, 45% of women who took zuranolone received a HAMD-17 score of 7 or under, meaning they have remitted depression. By day 45, 53% of women who had taken the drug were in remission.
Although the zuranolone was well tolerated, about 5% of the group experienced adverse events. Of those who experienced side effects, 15% experienced drowsiness, 9% suffered from headaches, and 8% experienced dizziness and developed an upper respiratory infection. Participants also suffered diarrhea and sedation.
Lissette Tanner, MD, MPH, FACOG, who was not involved with the study, thought the current study’s findings were promising and would be a great alternative to brexanolone.
“You have the additional benefit that it’s an oral agent as opposed to injection, which I know a lot of patients often have concerns about,” said Dr. Tanner, assistant professor of gynecology and obstetrics at Emory University, Atlanta. “[It’s] an exciting prospect for clinical care to be able to prescribe an oral agent patients can feel comfortable taking at home.”
When it comes to the study’s method, Dr. Tanner noted that the researchers used the HAMD-17 scale as opposed to the Edinburgh Postnatal Depression Scale (EPDS), something that is used “a lot more in clinical situations and providers are a lot more familiar with.” Using the EPDS score would be more applicable “in terms of introducing these medications into true clinical care.”
In terms of follow-up, Dr. Tanner said there may be a need for ongoing research that follows the study participants for more than 45 days.
“For depressive symptoms in particular, oftentimes those symptoms ebb and flow. So seeing if there is a long-term response to these medications or just kind of an immediate onset then wane will be important in the future,” she added.
Dr. Tanner is also interested in pharmacokinetic studies involving zuranolone to see how much of the medication may potentially pass into breast milk.
Dr. Deligiannidis and Dr. Tanner had no financial disclosures.
FROM JAMA PSYCHIATRY
Secnidazole gets FDA nod for trichomoniasis
The Food and Drug Administration has expanded the approval of secnidazole to include treatment of trichomoniasis in adults, according to a statement from manufacturer Lupin Pharmaceuticals.
Trichomoniasis vaginalis is a common, nonviral, curable sexually transmitted disease that affects approximately 3 million to 5 million adults in the United States each year; the infection can linger for months or years if left untreated, and may have a negative impact on reproductive health. The drug was approved for the treatment of bacterial vaginosis in 2017.
The availability of a single-dose oral treatment for both trichomoniasis and bacterial vaginosis may help improve adherence and reduce risk factors associated with these conditions, including pelvic inflammatory disease and other sexually transmitted infections, according to the statement.
The approval for the new indication was based primarily on data from a phase 3 clinical trial in which women with a confirmed trichomoniasis diagnosis were randomized to a single dose of 2 g oral secnidazole or a placebo. Secnidazole showed a 92.2% cure rate for patients with trichomoniasis, compared with placebo, based on cultures collected 6-12 days after dosing. Cure rates in subsets of patients with HIV and bacterial vaginosis were 100% and 95%, respectively.
The most common treatment-related adverse events were vulvovaginal candidiasis and nausea, each reported in 2.7% of study participants. The study findings were published in March 2021 in Clinical Infections Diseases.
Secnidazole also is approved for treatment of trichomoniasis in men, based on data from four open-label studies, one with men only and three including both men and women, according to the statement.
Full prescribing information for secnidazole is available here.
The Food and Drug Administration has expanded the approval of secnidazole to include treatment of trichomoniasis in adults, according to a statement from manufacturer Lupin Pharmaceuticals.
Trichomoniasis vaginalis is a common, nonviral, curable sexually transmitted disease that affects approximately 3 million to 5 million adults in the United States each year; the infection can linger for months or years if left untreated, and may have a negative impact on reproductive health. The drug was approved for the treatment of bacterial vaginosis in 2017.
The availability of a single-dose oral treatment for both trichomoniasis and bacterial vaginosis may help improve adherence and reduce risk factors associated with these conditions, including pelvic inflammatory disease and other sexually transmitted infections, according to the statement.
The approval for the new indication was based primarily on data from a phase 3 clinical trial in which women with a confirmed trichomoniasis diagnosis were randomized to a single dose of 2 g oral secnidazole or a placebo. Secnidazole showed a 92.2% cure rate for patients with trichomoniasis, compared with placebo, based on cultures collected 6-12 days after dosing. Cure rates in subsets of patients with HIV and bacterial vaginosis were 100% and 95%, respectively.
The most common treatment-related adverse events were vulvovaginal candidiasis and nausea, each reported in 2.7% of study participants. The study findings were published in March 2021 in Clinical Infections Diseases.
Secnidazole also is approved for treatment of trichomoniasis in men, based on data from four open-label studies, one with men only and three including both men and women, according to the statement.
Full prescribing information for secnidazole is available here.
The Food and Drug Administration has expanded the approval of secnidazole to include treatment of trichomoniasis in adults, according to a statement from manufacturer Lupin Pharmaceuticals.
Trichomoniasis vaginalis is a common, nonviral, curable sexually transmitted disease that affects approximately 3 million to 5 million adults in the United States each year; the infection can linger for months or years if left untreated, and may have a negative impact on reproductive health. The drug was approved for the treatment of bacterial vaginosis in 2017.
The availability of a single-dose oral treatment for both trichomoniasis and bacterial vaginosis may help improve adherence and reduce risk factors associated with these conditions, including pelvic inflammatory disease and other sexually transmitted infections, according to the statement.
The approval for the new indication was based primarily on data from a phase 3 clinical trial in which women with a confirmed trichomoniasis diagnosis were randomized to a single dose of 2 g oral secnidazole or a placebo. Secnidazole showed a 92.2% cure rate for patients with trichomoniasis, compared with placebo, based on cultures collected 6-12 days after dosing. Cure rates in subsets of patients with HIV and bacterial vaginosis were 100% and 95%, respectively.
The most common treatment-related adverse events were vulvovaginal candidiasis and nausea, each reported in 2.7% of study participants. The study findings were published in March 2021 in Clinical Infections Diseases.
Secnidazole also is approved for treatment of trichomoniasis in men, based on data from four open-label studies, one with men only and three including both men and women, according to the statement.
Full prescribing information for secnidazole is available here.
Daily reporting from the 2021 Society of Gynecologic Surgeons Annual Meeting
TUESDAY, 6/29/21. DAY 3 AT SGS
The third day of the annual SGS meeting started with several academic roundtables hosted by experts in the field. These authorities shared their knowledge on a range of topics including endometriosis, building an academic career, diversity and equity in the workplace, and scientific publishing. The general session got underway with additional oral and video presentations highlighting advancements in our field. This year’s SGS President Dr. Miles Murphy gave the annual presidential address. He spoke genuinely and humbly about our field. Whitney Ross, MD, (@WRossMD), referred to his speech on Twitter as “Best. Presidential. Address. Ever.” –a sentiment felt by many in the crowd!
This year’s Telinde Lecture was given by Janet Dombrowski, the first ever non-physician to present this lecture. She spoke on resiliency in a lecture titled, “Cultivating Resilience: The Power in Connection & Collaboration.” It was an insightful and wise presentation on the power of connection and how connection bolsters our resiliency. She challenged us to all break down “thinking habits” that isolate us into silos and get in the way of powerful connection and collaboration. She reminded us of the African greeting “Sawubona” (I see you) and “Sikhona” (Because you see me, I am here). A gentle reminder that we feel our existence most tangibly when we are seen by others—an idea consistent with other important themes of this conference, focused on diversity, equity, and inclusion of all. The morning session was rounded out with a panel discussion on “Novel GYN Office Procedure,” featuring Drs. Cecile Ferrando (@CFerrandoMD), Abbas Shobeiri (@ShobeiriAbbas), Andrea Pezzela, and Eric Sokol.
The afternoon was filled with leisure activities in beautiful Palm Springs, including the SGS Golf Tournament, mountain biking, aerial tramway tour, and hike. The weather even cooperated with slightly cooler temperatures (think 100℉ instead of 120℉)! The evening was filled with food, drinks, and the excitement around the annual “SGS’ Got Talent” show! Everyone was able to let down, show off their dance moves, and enjoy some of that much needed connection time!
Tomorrow is the last day of #SGS2021! Excited to round out the conference with continued learning.
MONDAY, 6/28/21. DAY 2 AT SGS
The sun is up and working hard here in Palm Springs, and so are we!
Welcome and introduction of new members
The general session started with a warm welcome to the 12 new SGS members. A special shout out to Dr. Kelly Wright who is a new SGS Member and won the #SGS2021 tweetup! She ranked as a top influencer, prolific tweeter, and made more than 250K impressions leading up to SGS! Way to represent @MigsRunner.
General scientific sessions
There were several excellent oral and video presentations throughout the morning session. A range of topics were discussed, including postoperative pain management, strategies for cost-effective surgery, and how racial and ethnic disparities play into our medical education and patient outcomes. Dr. Eva Welch gave a stellar video presentation on straight-stick sacrocolpopexy techniques for the savvy surgeon. I personally will be incorporating some of her needle management tricks!
After a brief break with some refreshments and a stroll around the exhibit hall, the second scientific session initiated with a transformative lecture. Dr. Mark Walters presented "Insights on Surgical Education: How Can I Help You Get Better" in the inaugural Mark D. Walters Lectureship. Dr. Walters shared his experience and insights on how to transform oneself from a good surgeon to an expert and from a teacher to a coach in the operating room. His dedication to our field, years of experience, and wisdom earned him a standing ovation! Additional oral and video presentations followed. Dr. J. Wong shared correlations between surgeon gender and ergonomic strain with laparoscopic devices. Female surgeons more often reported inappropriate fit and expressed physical discomfort compared with male surgeons. Injuries and ergonomic strain lead to less operating and even disability for some surgeons. It is past time for us to have better--we need instruments that fit our hands!
The afternoon session started with a panel on "Perspectives on Race in GYN Surgery." It was another insightful discussion with thought- and action-provoking knowledge. The afternoon session included the SGS Prize Video by Dr. Angela DiCarlo-Meacham on excision of a vulvar cyst.
Fellows' Pelvic Research Network
After adjourning of the scientific sessions, the fellow-ran, multicenter research network (FPRN) met to give updates. This diverse group of both AUGS-SGS and FMIGS-SGS offers mentorship and relationships that are important for future careers and research. The collaboration allows the study of rare outcomes that may not be feasible at single sites. Dr. Amanda Yunker, fellowship director at Vanderbilt University, gave an amazing history lesson on the fields of OB and GYN, and the evolution of gynecologic surgery. We then had fun assigning a "report card grade" on how MIGS is doing comparatively with other subspecialties in the realms of academics and research.
VideoFest
The late afternoon was concluded with a surgical video session. What an amazing and talented group we are here at SGS!
President's awards ceremony and reception
The scientific focused day was rounded out with an evening of honors, awards, and social time as we celebrated all the achievements of our peers and colleagues. The president's reception was filled with food, laughter, networking, and reconnecting with friends and colleagues. We are looking forward to another day of education tomorrow!
Follow @JennaRehmerMD, @GynSurgery, and #SGS2021 on Twitter for updates.
SUNDAY, 6/27/21. DAY 1 AT SGS
Hello live from sunny Palm Spring, CA, and the Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS)! This year’s conference balances the long-awaited return to in-person events while simultaneously embracing virtual learning with their hybrid meeting format. You can follow me, @JennaRehmerMD, and #SGS2021 in real-time on Twitter.
Dismantling racism
We were incredibly fortunate to take a deep dive into dismantling racism in our personal and professional spheres. The postgraduate course was well researched and presented by Drs. Oluwateniola “Teni” Brown, Cassandra Carberry, Olivia Cardenas-Trowers (@otrowers_md), Annetta Madsen, Moiuri Siddique, and Blair Washington (@Dr_B_Washington). Each presentation provided a succinct and cohesive flow, taking us through what racism is, the historical and active structural racism in medicine, and the actions and steps of becoming anti-racist.
Dr. Brown discussed critical race theory. We learned that the engineered system of oppression is so advanced that it is often hidden in plain sight, and that one’s conscious awareness is not necessary in order to uphold the system of oppression. It is reinforced and supported with minimal effort. This is why not being racist is not enough; active anti-racism is needed to bring about change.
Fibroid management
Across the hall, Drs. Linda Bradley (@BradlelMD), Kimberly Kho (@KimberlyKho1), Cara King (@drcaraking), and Kelly Wright (@MigsRunner) broadened our armamentarium for uterine conservation in fibroid management. Dr. Bradley reviewed medical therapies, including novel treatments, as first-line or adjunct treatment options. Next, the course focused on surgical techniques for hysteroscopic myomectomies, optimization of minilaparotomy for myomectomy, and tissue extraction. Dr. King displayed true grit when giving her lecture from the airport after flight delays prevented her from being in person with us.
Multidisciplinary care within gyn surgery
In this virtual only postgraduate course, Drs. Risal Djohan (@DjohanMD), Cecile Ferrando (@CFerrandoMD), Marie Fidela Paraiso, Sandip Vasavada (@SandipVasavada), and Sarah Vogler showed us the importance of multidisciplinary care within gynecologic surgery practices. They explored how to streamline the approach so it complements your practice, how to co-bill for shared patient care, and tips and tricks for optimizing the surgical experience for the patient.
Industry presentations
Over lunch, Dr. Opoku-Akane presented on using ERAS (enhanced recovery after surgery) protocols for endometriosis and chronic pelvic pain and how to optimize the use of alternative surgical modalities for endometriosis. Following this, Drs. Albert Huany and Craig McCoy taught about a new technology using electrical stimulation to optimize visualization of the ureter.
Harnessing the power of social media
This workshop, organized by SGS Social Media Committee Chair Dr. Amy Park (@dramypark) showed us the importance of having an online identity for the sharing of ideas, networking, professional development, and education. We learned how to optimize our online bios, proper use of GYN ontology for hashtags, and how to maintain professionalism on social media. We reviewed the data on how sharing publications on social media improves altmetric scores and discussed how our social media influence may be tied to performance in the future.
Lessons in leadership
We rounded out the day with after-dinner dessert and drinks at the evening SGS Women’s Council presentation. We had the great honor of hearing from Lori Ryerker, CEO of Celanese Corporation, a Fortune 500 global company. She provided much wisdom on being a leader. She shared several keys to creating a successful work environment:
- being a leader that “provides an environment where people feel like they can bring their best selves every day” (and that being your best self is being your whole self, without reservations)
- allowing all genders, sexual orientations, races, ethnicities, and ages to show up together without reservations (because only then can people feel safe to be their best, because their best self is their true self).
It was a wonderful and successful kick-off to the meeting. I look forward to a full day tomorrow! Follow along as this year’s Fellow Scholars, Drs. Tara Brah (@TaraBrah), Amr El Haraki (@drharaki), Sheena Galhotra (@SheenaGalhotra), Meenal Misal (@meenalmisalMD), and yours truly, post live updates daily.
TUESDAY, 6/29/21. DAY 3 AT SGS
The third day of the annual SGS meeting started with several academic roundtables hosted by experts in the field. These authorities shared their knowledge on a range of topics including endometriosis, building an academic career, diversity and equity in the workplace, and scientific publishing. The general session got underway with additional oral and video presentations highlighting advancements in our field. This year’s SGS President Dr. Miles Murphy gave the annual presidential address. He spoke genuinely and humbly about our field. Whitney Ross, MD, (@WRossMD), referred to his speech on Twitter as “Best. Presidential. Address. Ever.” –a sentiment felt by many in the crowd!
This year’s Telinde Lecture was given by Janet Dombrowski, the first ever non-physician to present this lecture. She spoke on resiliency in a lecture titled, “Cultivating Resilience: The Power in Connection & Collaboration.” It was an insightful and wise presentation on the power of connection and how connection bolsters our resiliency. She challenged us to all break down “thinking habits” that isolate us into silos and get in the way of powerful connection and collaboration. She reminded us of the African greeting “Sawubona” (I see you) and “Sikhona” (Because you see me, I am here). A gentle reminder that we feel our existence most tangibly when we are seen by others—an idea consistent with other important themes of this conference, focused on diversity, equity, and inclusion of all. The morning session was rounded out with a panel discussion on “Novel GYN Office Procedure,” featuring Drs. Cecile Ferrando (@CFerrandoMD), Abbas Shobeiri (@ShobeiriAbbas), Andrea Pezzela, and Eric Sokol.
The afternoon was filled with leisure activities in beautiful Palm Springs, including the SGS Golf Tournament, mountain biking, aerial tramway tour, and hike. The weather even cooperated with slightly cooler temperatures (think 100℉ instead of 120℉)! The evening was filled with food, drinks, and the excitement around the annual “SGS’ Got Talent” show! Everyone was able to let down, show off their dance moves, and enjoy some of that much needed connection time!
Tomorrow is the last day of #SGS2021! Excited to round out the conference with continued learning.
MONDAY, 6/28/21. DAY 2 AT SGS
The sun is up and working hard here in Palm Springs, and so are we!
Welcome and introduction of new members
The general session started with a warm welcome to the 12 new SGS members. A special shout out to Dr. Kelly Wright who is a new SGS Member and won the #SGS2021 tweetup! She ranked as a top influencer, prolific tweeter, and made more than 250K impressions leading up to SGS! Way to represent @MigsRunner.
General scientific sessions
There were several excellent oral and video presentations throughout the morning session. A range of topics were discussed, including postoperative pain management, strategies for cost-effective surgery, and how racial and ethnic disparities play into our medical education and patient outcomes. Dr. Eva Welch gave a stellar video presentation on straight-stick sacrocolpopexy techniques for the savvy surgeon. I personally will be incorporating some of her needle management tricks!
After a brief break with some refreshments and a stroll around the exhibit hall, the second scientific session initiated with a transformative lecture. Dr. Mark Walters presented "Insights on Surgical Education: How Can I Help You Get Better" in the inaugural Mark D. Walters Lectureship. Dr. Walters shared his experience and insights on how to transform oneself from a good surgeon to an expert and from a teacher to a coach in the operating room. His dedication to our field, years of experience, and wisdom earned him a standing ovation! Additional oral and video presentations followed. Dr. J. Wong shared correlations between surgeon gender and ergonomic strain with laparoscopic devices. Female surgeons more often reported inappropriate fit and expressed physical discomfort compared with male surgeons. Injuries and ergonomic strain lead to less operating and even disability for some surgeons. It is past time for us to have better--we need instruments that fit our hands!
The afternoon session started with a panel on "Perspectives on Race in GYN Surgery." It was another insightful discussion with thought- and action-provoking knowledge. The afternoon session included the SGS Prize Video by Dr. Angela DiCarlo-Meacham on excision of a vulvar cyst.
Fellows' Pelvic Research Network
After adjourning of the scientific sessions, the fellow-ran, multicenter research network (FPRN) met to give updates. This diverse group of both AUGS-SGS and FMIGS-SGS offers mentorship and relationships that are important for future careers and research. The collaboration allows the study of rare outcomes that may not be feasible at single sites. Dr. Amanda Yunker, fellowship director at Vanderbilt University, gave an amazing history lesson on the fields of OB and GYN, and the evolution of gynecologic surgery. We then had fun assigning a "report card grade" on how MIGS is doing comparatively with other subspecialties in the realms of academics and research.
VideoFest
The late afternoon was concluded with a surgical video session. What an amazing and talented group we are here at SGS!
President's awards ceremony and reception
The scientific focused day was rounded out with an evening of honors, awards, and social time as we celebrated all the achievements of our peers and colleagues. The president's reception was filled with food, laughter, networking, and reconnecting with friends and colleagues. We are looking forward to another day of education tomorrow!
Follow @JennaRehmerMD, @GynSurgery, and #SGS2021 on Twitter for updates.
SUNDAY, 6/27/21. DAY 1 AT SGS
Hello live from sunny Palm Spring, CA, and the Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS)! This year’s conference balances the long-awaited return to in-person events while simultaneously embracing virtual learning with their hybrid meeting format. You can follow me, @JennaRehmerMD, and #SGS2021 in real-time on Twitter.
Dismantling racism
We were incredibly fortunate to take a deep dive into dismantling racism in our personal and professional spheres. The postgraduate course was well researched and presented by Drs. Oluwateniola “Teni” Brown, Cassandra Carberry, Olivia Cardenas-Trowers (@otrowers_md), Annetta Madsen, Moiuri Siddique, and Blair Washington (@Dr_B_Washington). Each presentation provided a succinct and cohesive flow, taking us through what racism is, the historical and active structural racism in medicine, and the actions and steps of becoming anti-racist.
Dr. Brown discussed critical race theory. We learned that the engineered system of oppression is so advanced that it is often hidden in plain sight, and that one’s conscious awareness is not necessary in order to uphold the system of oppression. It is reinforced and supported with minimal effort. This is why not being racist is not enough; active anti-racism is needed to bring about change.
Fibroid management
Across the hall, Drs. Linda Bradley (@BradlelMD), Kimberly Kho (@KimberlyKho1), Cara King (@drcaraking), and Kelly Wright (@MigsRunner) broadened our armamentarium for uterine conservation in fibroid management. Dr. Bradley reviewed medical therapies, including novel treatments, as first-line or adjunct treatment options. Next, the course focused on surgical techniques for hysteroscopic myomectomies, optimization of minilaparotomy for myomectomy, and tissue extraction. Dr. King displayed true grit when giving her lecture from the airport after flight delays prevented her from being in person with us.
Multidisciplinary care within gyn surgery
In this virtual only postgraduate course, Drs. Risal Djohan (@DjohanMD), Cecile Ferrando (@CFerrandoMD), Marie Fidela Paraiso, Sandip Vasavada (@SandipVasavada), and Sarah Vogler showed us the importance of multidisciplinary care within gynecologic surgery practices. They explored how to streamline the approach so it complements your practice, how to co-bill for shared patient care, and tips and tricks for optimizing the surgical experience for the patient.
Industry presentations
Over lunch, Dr. Opoku-Akane presented on using ERAS (enhanced recovery after surgery) protocols for endometriosis and chronic pelvic pain and how to optimize the use of alternative surgical modalities for endometriosis. Following this, Drs. Albert Huany and Craig McCoy taught about a new technology using electrical stimulation to optimize visualization of the ureter.
Harnessing the power of social media
This workshop, organized by SGS Social Media Committee Chair Dr. Amy Park (@dramypark) showed us the importance of having an online identity for the sharing of ideas, networking, professional development, and education. We learned how to optimize our online bios, proper use of GYN ontology for hashtags, and how to maintain professionalism on social media. We reviewed the data on how sharing publications on social media improves altmetric scores and discussed how our social media influence may be tied to performance in the future.
Lessons in leadership
We rounded out the day with after-dinner dessert and drinks at the evening SGS Women’s Council presentation. We had the great honor of hearing from Lori Ryerker, CEO of Celanese Corporation, a Fortune 500 global company. She provided much wisdom on being a leader. She shared several keys to creating a successful work environment:
- being a leader that “provides an environment where people feel like they can bring their best selves every day” (and that being your best self is being your whole self, without reservations)
- allowing all genders, sexual orientations, races, ethnicities, and ages to show up together without reservations (because only then can people feel safe to be their best, because their best self is their true self).
It was a wonderful and successful kick-off to the meeting. I look forward to a full day tomorrow! Follow along as this year’s Fellow Scholars, Drs. Tara Brah (@TaraBrah), Amr El Haraki (@drharaki), Sheena Galhotra (@SheenaGalhotra), Meenal Misal (@meenalmisalMD), and yours truly, post live updates daily.
TUESDAY, 6/29/21. DAY 3 AT SGS
The third day of the annual SGS meeting started with several academic roundtables hosted by experts in the field. These authorities shared their knowledge on a range of topics including endometriosis, building an academic career, diversity and equity in the workplace, and scientific publishing. The general session got underway with additional oral and video presentations highlighting advancements in our field. This year’s SGS President Dr. Miles Murphy gave the annual presidential address. He spoke genuinely and humbly about our field. Whitney Ross, MD, (@WRossMD), referred to his speech on Twitter as “Best. Presidential. Address. Ever.” –a sentiment felt by many in the crowd!
This year’s Telinde Lecture was given by Janet Dombrowski, the first ever non-physician to present this lecture. She spoke on resiliency in a lecture titled, “Cultivating Resilience: The Power in Connection & Collaboration.” It was an insightful and wise presentation on the power of connection and how connection bolsters our resiliency. She challenged us to all break down “thinking habits” that isolate us into silos and get in the way of powerful connection and collaboration. She reminded us of the African greeting “Sawubona” (I see you) and “Sikhona” (Because you see me, I am here). A gentle reminder that we feel our existence most tangibly when we are seen by others—an idea consistent with other important themes of this conference, focused on diversity, equity, and inclusion of all. The morning session was rounded out with a panel discussion on “Novel GYN Office Procedure,” featuring Drs. Cecile Ferrando (@CFerrandoMD), Abbas Shobeiri (@ShobeiriAbbas), Andrea Pezzela, and Eric Sokol.
The afternoon was filled with leisure activities in beautiful Palm Springs, including the SGS Golf Tournament, mountain biking, aerial tramway tour, and hike. The weather even cooperated with slightly cooler temperatures (think 100℉ instead of 120℉)! The evening was filled with food, drinks, and the excitement around the annual “SGS’ Got Talent” show! Everyone was able to let down, show off their dance moves, and enjoy some of that much needed connection time!
Tomorrow is the last day of #SGS2021! Excited to round out the conference with continued learning.
MONDAY, 6/28/21. DAY 2 AT SGS
The sun is up and working hard here in Palm Springs, and so are we!
Welcome and introduction of new members
The general session started with a warm welcome to the 12 new SGS members. A special shout out to Dr. Kelly Wright who is a new SGS Member and won the #SGS2021 tweetup! She ranked as a top influencer, prolific tweeter, and made more than 250K impressions leading up to SGS! Way to represent @MigsRunner.
General scientific sessions
There were several excellent oral and video presentations throughout the morning session. A range of topics were discussed, including postoperative pain management, strategies for cost-effective surgery, and how racial and ethnic disparities play into our medical education and patient outcomes. Dr. Eva Welch gave a stellar video presentation on straight-stick sacrocolpopexy techniques for the savvy surgeon. I personally will be incorporating some of her needle management tricks!
After a brief break with some refreshments and a stroll around the exhibit hall, the second scientific session initiated with a transformative lecture. Dr. Mark Walters presented "Insights on Surgical Education: How Can I Help You Get Better" in the inaugural Mark D. Walters Lectureship. Dr. Walters shared his experience and insights on how to transform oneself from a good surgeon to an expert and from a teacher to a coach in the operating room. His dedication to our field, years of experience, and wisdom earned him a standing ovation! Additional oral and video presentations followed. Dr. J. Wong shared correlations between surgeon gender and ergonomic strain with laparoscopic devices. Female surgeons more often reported inappropriate fit and expressed physical discomfort compared with male surgeons. Injuries and ergonomic strain lead to less operating and even disability for some surgeons. It is past time for us to have better--we need instruments that fit our hands!
The afternoon session started with a panel on "Perspectives on Race in GYN Surgery." It was another insightful discussion with thought- and action-provoking knowledge. The afternoon session included the SGS Prize Video by Dr. Angela DiCarlo-Meacham on excision of a vulvar cyst.
Fellows' Pelvic Research Network
After adjourning of the scientific sessions, the fellow-ran, multicenter research network (FPRN) met to give updates. This diverse group of both AUGS-SGS and FMIGS-SGS offers mentorship and relationships that are important for future careers and research. The collaboration allows the study of rare outcomes that may not be feasible at single sites. Dr. Amanda Yunker, fellowship director at Vanderbilt University, gave an amazing history lesson on the fields of OB and GYN, and the evolution of gynecologic surgery. We then had fun assigning a "report card grade" on how MIGS is doing comparatively with other subspecialties in the realms of academics and research.
VideoFest
The late afternoon was concluded with a surgical video session. What an amazing and talented group we are here at SGS!
President's awards ceremony and reception
The scientific focused day was rounded out with an evening of honors, awards, and social time as we celebrated all the achievements of our peers and colleagues. The president's reception was filled with food, laughter, networking, and reconnecting with friends and colleagues. We are looking forward to another day of education tomorrow!
Follow @JennaRehmerMD, @GynSurgery, and #SGS2021 on Twitter for updates.
SUNDAY, 6/27/21. DAY 1 AT SGS
Hello live from sunny Palm Spring, CA, and the Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS)! This year’s conference balances the long-awaited return to in-person events while simultaneously embracing virtual learning with their hybrid meeting format. You can follow me, @JennaRehmerMD, and #SGS2021 in real-time on Twitter.
Dismantling racism
We were incredibly fortunate to take a deep dive into dismantling racism in our personal and professional spheres. The postgraduate course was well researched and presented by Drs. Oluwateniola “Teni” Brown, Cassandra Carberry, Olivia Cardenas-Trowers (@otrowers_md), Annetta Madsen, Moiuri Siddique, and Blair Washington (@Dr_B_Washington). Each presentation provided a succinct and cohesive flow, taking us through what racism is, the historical and active structural racism in medicine, and the actions and steps of becoming anti-racist.
Dr. Brown discussed critical race theory. We learned that the engineered system of oppression is so advanced that it is often hidden in plain sight, and that one’s conscious awareness is not necessary in order to uphold the system of oppression. It is reinforced and supported with minimal effort. This is why not being racist is not enough; active anti-racism is needed to bring about change.
Fibroid management
Across the hall, Drs. Linda Bradley (@BradlelMD), Kimberly Kho (@KimberlyKho1), Cara King (@drcaraking), and Kelly Wright (@MigsRunner) broadened our armamentarium for uterine conservation in fibroid management. Dr. Bradley reviewed medical therapies, including novel treatments, as first-line or adjunct treatment options. Next, the course focused on surgical techniques for hysteroscopic myomectomies, optimization of minilaparotomy for myomectomy, and tissue extraction. Dr. King displayed true grit when giving her lecture from the airport after flight delays prevented her from being in person with us.
Multidisciplinary care within gyn surgery
In this virtual only postgraduate course, Drs. Risal Djohan (@DjohanMD), Cecile Ferrando (@CFerrandoMD), Marie Fidela Paraiso, Sandip Vasavada (@SandipVasavada), and Sarah Vogler showed us the importance of multidisciplinary care within gynecologic surgery practices. They explored how to streamline the approach so it complements your practice, how to co-bill for shared patient care, and tips and tricks for optimizing the surgical experience for the patient.
Industry presentations
Over lunch, Dr. Opoku-Akane presented on using ERAS (enhanced recovery after surgery) protocols for endometriosis and chronic pelvic pain and how to optimize the use of alternative surgical modalities for endometriosis. Following this, Drs. Albert Huany and Craig McCoy taught about a new technology using electrical stimulation to optimize visualization of the ureter.
Harnessing the power of social media
This workshop, organized by SGS Social Media Committee Chair Dr. Amy Park (@dramypark) showed us the importance of having an online identity for the sharing of ideas, networking, professional development, and education. We learned how to optimize our online bios, proper use of GYN ontology for hashtags, and how to maintain professionalism on social media. We reviewed the data on how sharing publications on social media improves altmetric scores and discussed how our social media influence may be tied to performance in the future.
Lessons in leadership
We rounded out the day with after-dinner dessert and drinks at the evening SGS Women’s Council presentation. We had the great honor of hearing from Lori Ryerker, CEO of Celanese Corporation, a Fortune 500 global company. She provided much wisdom on being a leader. She shared several keys to creating a successful work environment:
- being a leader that “provides an environment where people feel like they can bring their best selves every day” (and that being your best self is being your whole self, without reservations)
- allowing all genders, sexual orientations, races, ethnicities, and ages to show up together without reservations (because only then can people feel safe to be their best, because their best self is their true self).
It was a wonderful and successful kick-off to the meeting. I look forward to a full day tomorrow! Follow along as this year’s Fellow Scholars, Drs. Tara Brah (@TaraBrah), Amr El Haraki (@drharaki), Sheena Galhotra (@SheenaGalhotra), Meenal Misal (@meenalmisalMD), and yours truly, post live updates daily.
Dynamic ultrasonography: An idea whose time has come
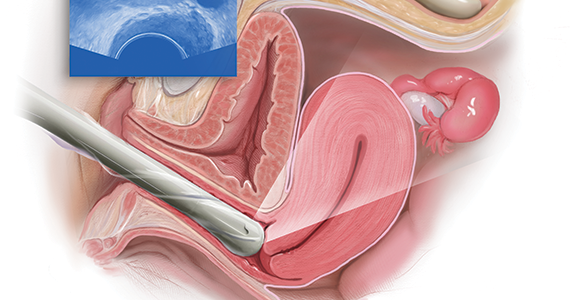
Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3
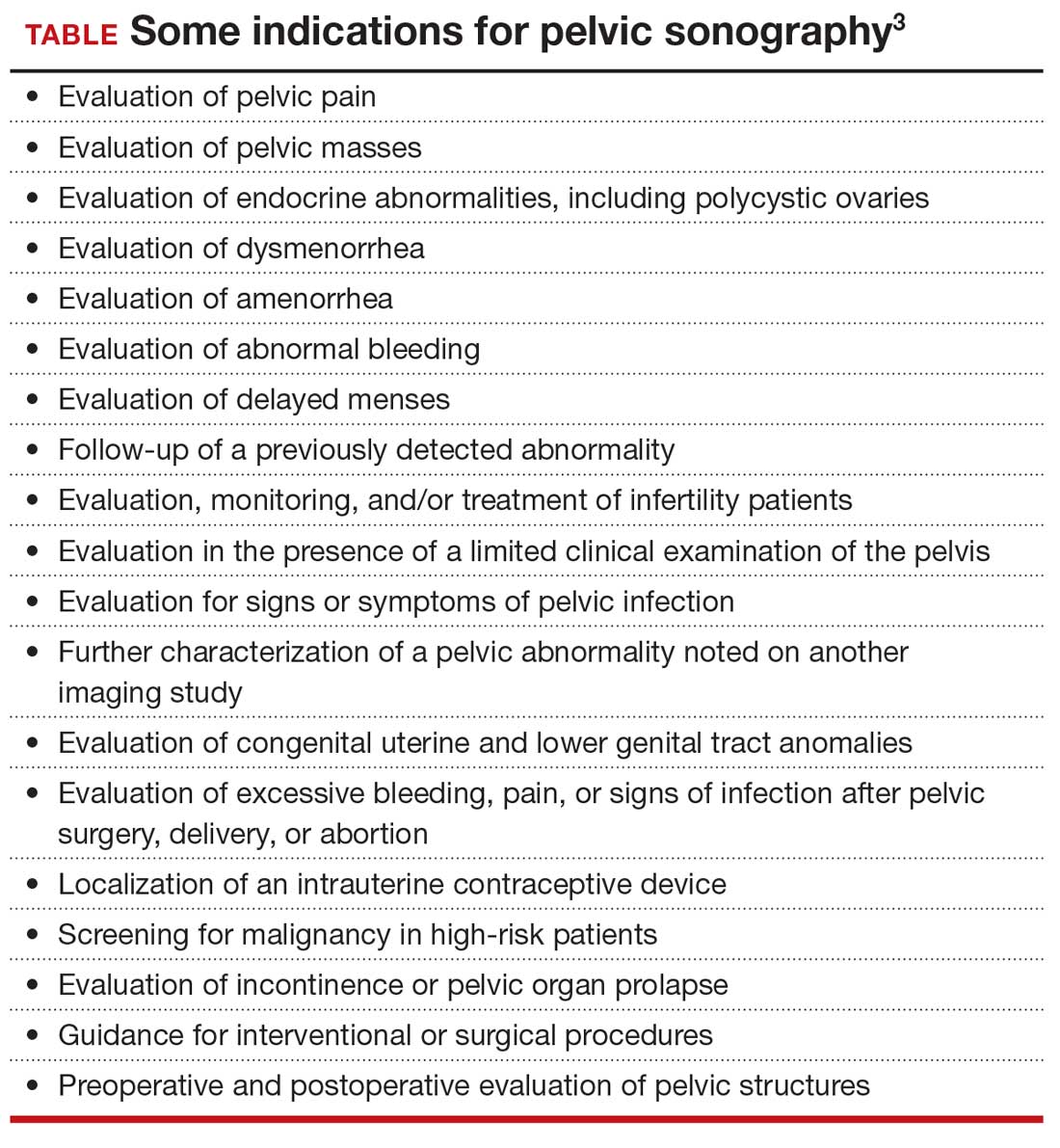
Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.
Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
How to choose the right vaginal moisturizer or lubricant for your patient
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.
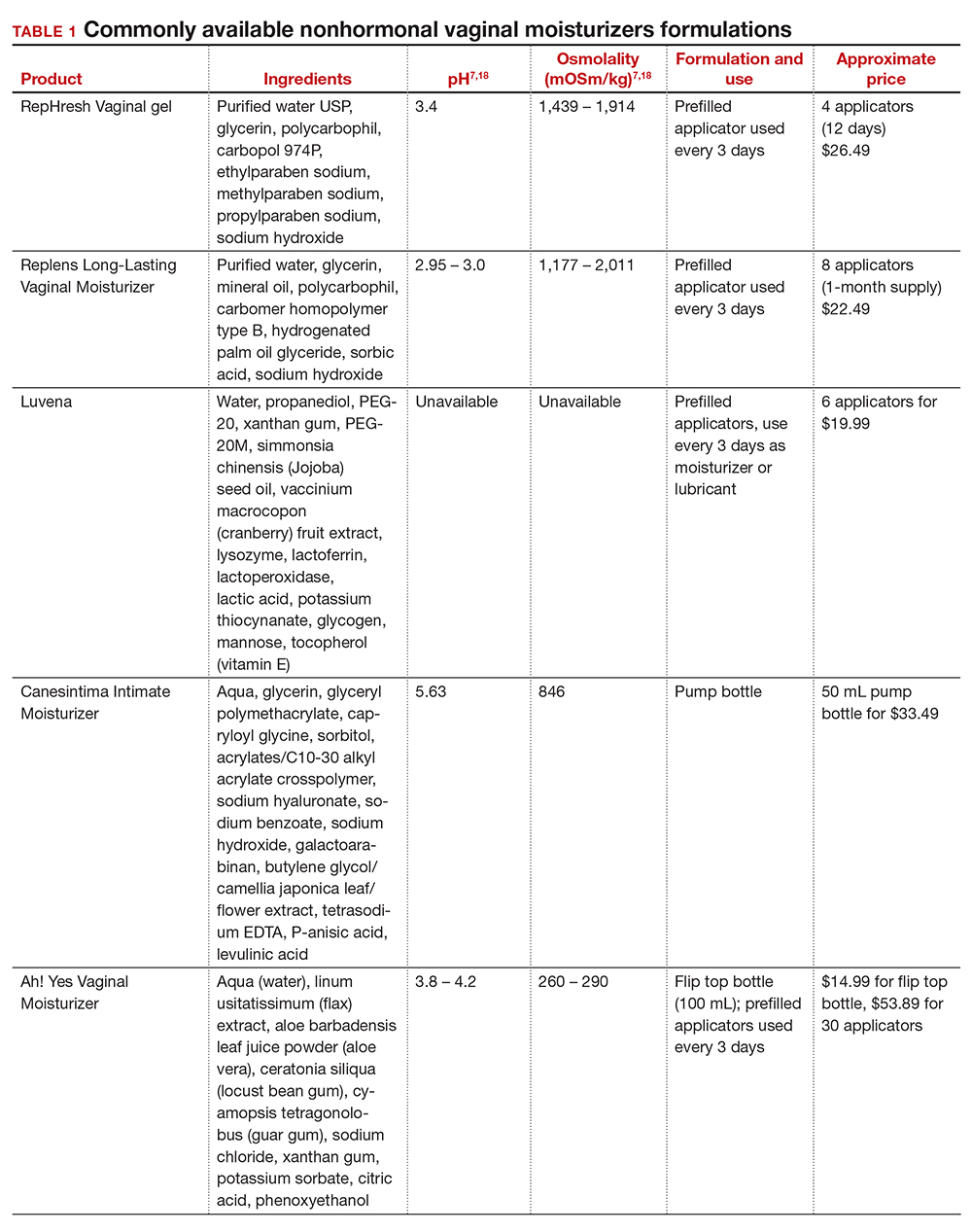
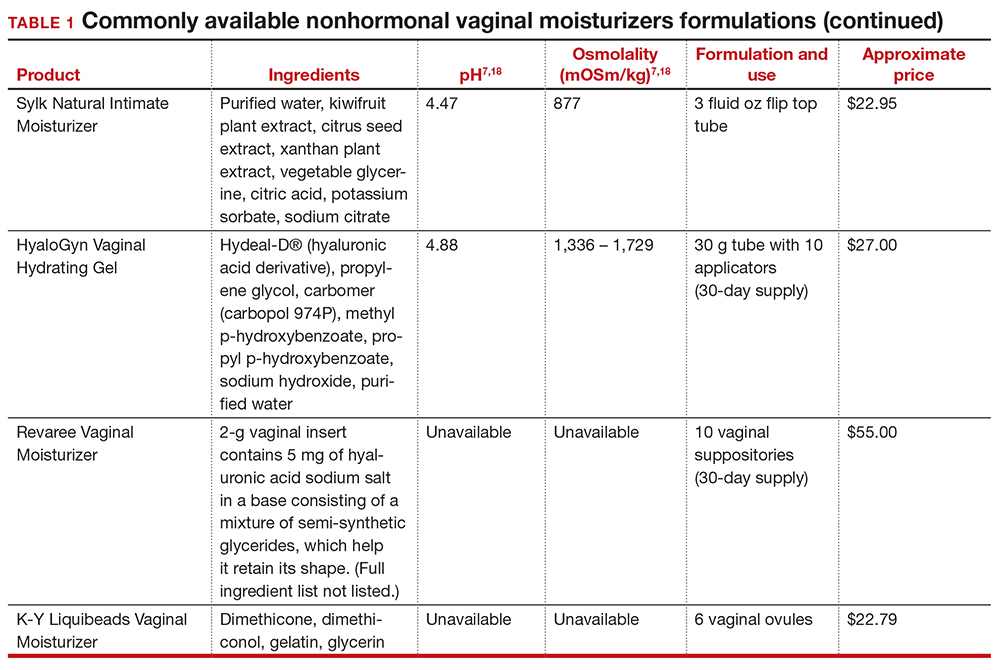
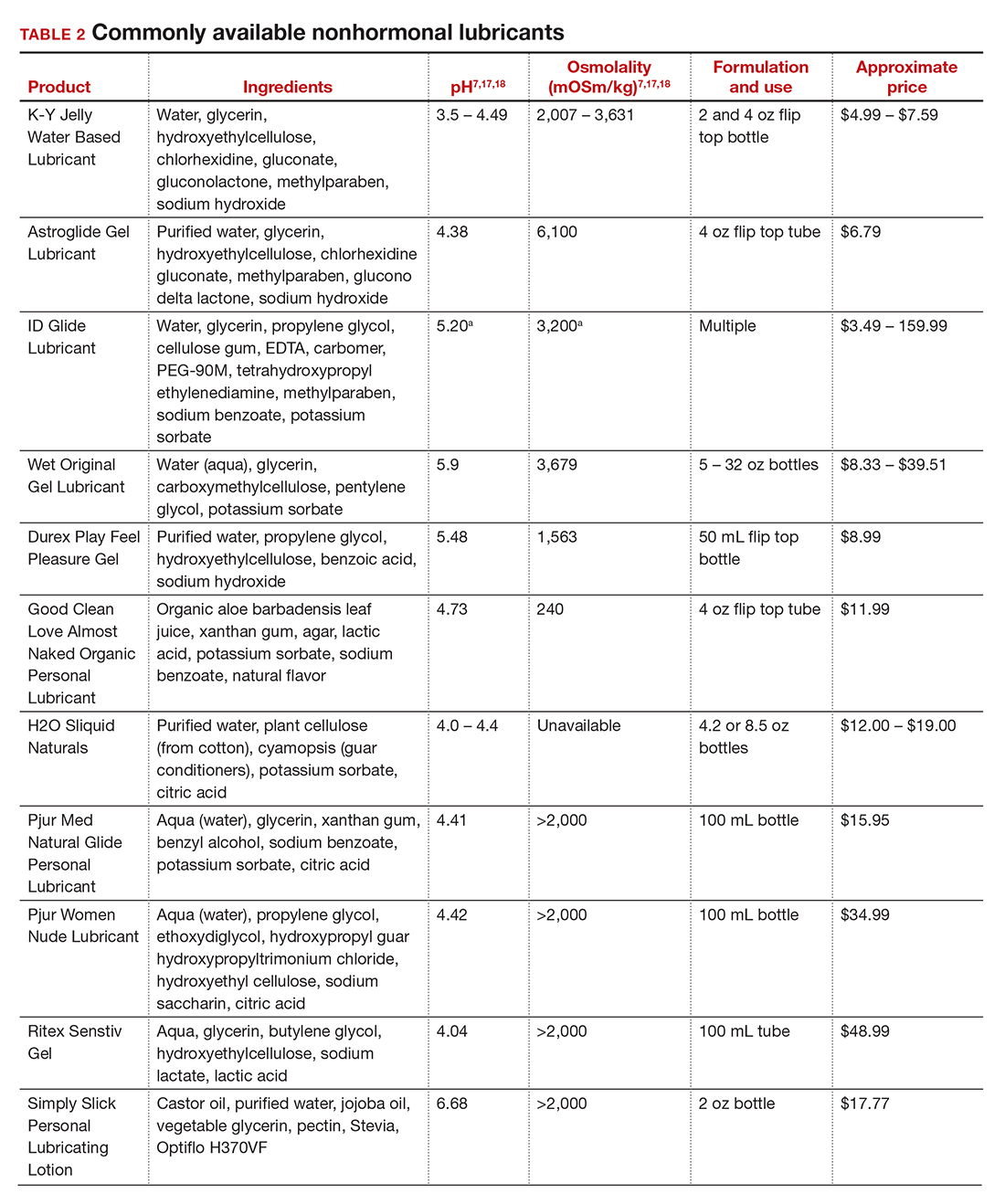
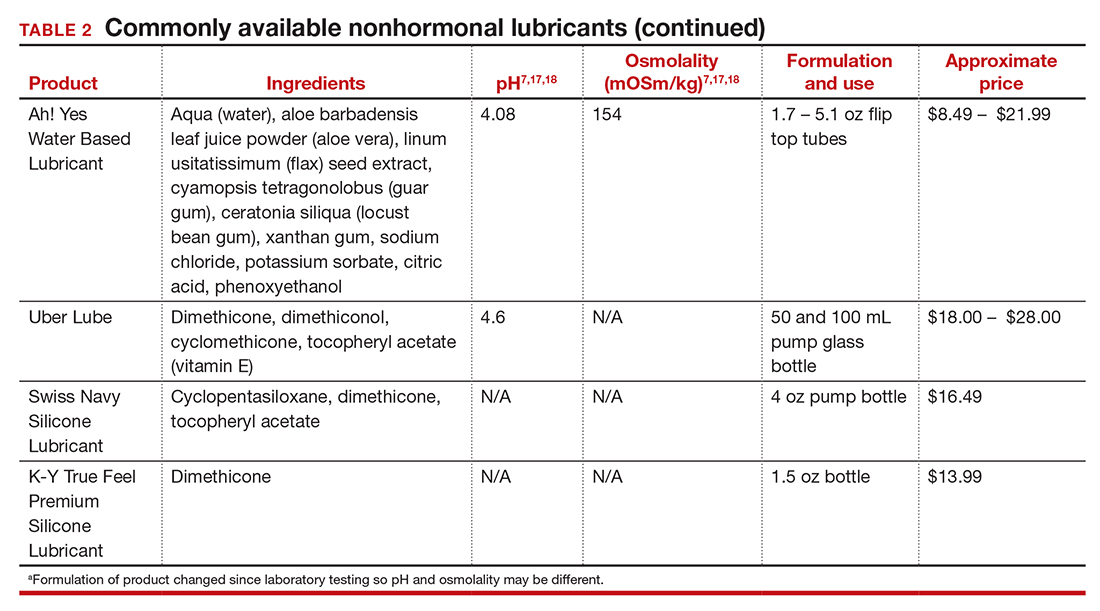
The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.