User login
Obesity pegged as source of marked increased risk of diabetes in PCOS
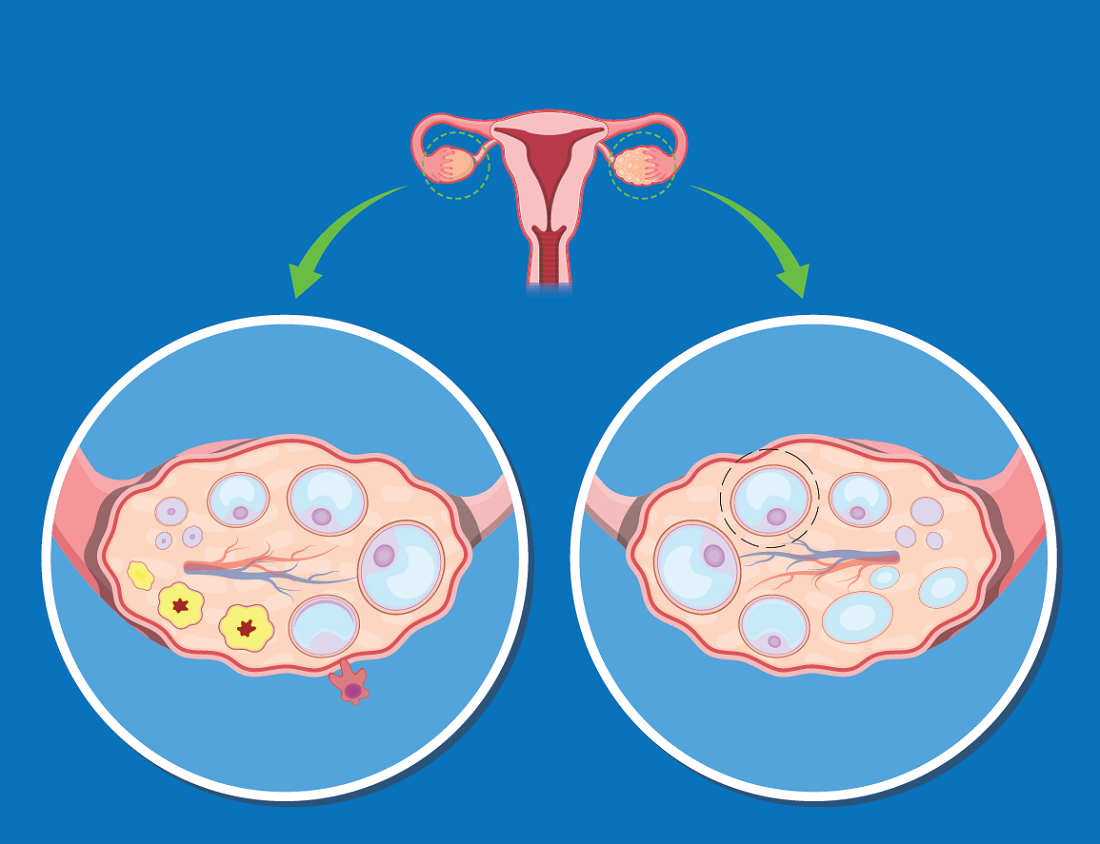
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
FROM ENDO 2021
High-intensity interval training cuts cardiometabolic risks in women with PCOS
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
FROM ENDO 2021
Women with PCOS at increased risk for COVID-19
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is the WHO’s HPV vaccination target within reach?
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
FROM PREVENTIVE MEDICINE
Reinstating in-person mifepristone administration requirements is harmful to patients and providers
In May 2020, the American College of Obstetricians and Gynecologists (ACOG), along with other organizations and physicians (Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong Women of Color Reproductive Justice Collective, Honor MacNaughton, MD), filed a civil action against the US Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS) challenging the requirements of in-person mifepristone dispensing, which was one of the 3 restrictions placed on the medicine as part of mifepristone’s risk evaluation and mitigation strategy (REMS). The requirements, which also include provider certification and patient signatures on specified consent forms, specifically target dosages of mifepristone for use related to abortions and miscarriages but do not apply when prescribing mifepristone for other medical conditions, even with higher doses. During the pandemic, the FDA suspended the REMS requirements for many other medications, including those more toxic than mifepristone. Additionally, the HHS activated a “telemedicine exception” that allows physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids, while minimizing the patient’s and provider’s risk of exposure to COVID-19 with in-person appointments. Notably, mifepristone for abortion and miscarriage management was excluded from this relaxation of the REMS requirement.
On July 13, 2020, a Federal District Court concluded that the in-person requirements were a “substantial obstacle” for women seeking abortions during the COVID-19 pandemic and granted a preliminary injunction to temporarily stop the FDA’s enforcement of the in-person requirements for mifepristone. We wrote about what that decision meant for ObGyns and urged clinicians to advocate to make the injunction permanent (OBG Manag. 2020;32(12):13-14, 23, 38. doi: 10.12788/obgm.0034.)
From there, however, the FDA worked to reverse that decision, which included applications to the District Court and to the Supreme Court for a stay of the injunction. If successful, this would suspend the injunction while the case was pending. In October, after the Supreme Court deferred review of the application (preferring a review by the lower courts), the District Court upheld the injunction of the in-person requirements citing the worsening pandemic crisis.
In-person requirement re-instated
On January 12, 2021, the United States Supreme Court granted the stay of the District Court’s injunction, which allowed the federal government to enforce the in-person requirement for mifepristone once again. The decision came down to a vote of 6 to 3. As is typical for decisions on stay orders, the court did not release a majority opinion explaining the reasoning behind this decision. In a concurring opinion, Chief Justice John Roberts wrote that the decision was not a judgment of if the requirements for in-person dispensing of mifepristone imposed an undue burden on women seeking an abortion. Instead, the Chief Justice explained that the decision came down to if a District Court could order the FDA to change their regulations based on “the court’s own evaluations of the COVID-19 pandemic,” maintaining that the court could not overrule “the politically accountable entities with the ‘background, competence, and expertise to assess public health.’”1 No other justices joined his opinion.
A worrisome pattern of a conservative supermajority
In her dissent, Justice Sonia Sotomayor criticized the government’s “statistically insignificant, cherry-picked data” and argued that the government did not provide any explanation from an FDA or HHS official explaining why mifepristone’s in-person requirement is more important than the in-person requirements of other drugs that have been waived during the pandemic.2 Therefore, she explained, there is “no reasoned decision” by any health official anywhere on which they can base the decision to grant the stay.
This ruling was the Supreme Court’s first major decision on reproductive health since the confirmation of Justice Amy Coney Barrett and may be an insight into future decisions of the new conservative supermajority on abortion and reproductive health issues. Particularly worrisome is what this decision could mean for stays in abortion cases that dictate whether or not the regulation is enforced during an active case. Even if cases are ruled in favor of patients and abortion providers, if the courts continue to allow enforcement of abortion restrictions during litigation, this could result in permanent closure of abortion clinics and prevent many individuals from accessing safe and legal abortion.
Looking toward the future
In the setting of almost 29 million cases of COVID-19 and more than 526,000 deaths, this stay order requires women seeking a medication abortion to make an appointment at a clinic, risking possible exposure to COVID-19, in order to access mifepristone.3,4 The Biden administration can and should remove the FDA requirement for in-person delivery of mifepristone, which would mitigate the effects of the stay order and allow women to obtain medication abortions during the pandemic.
Take action
- Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe mifepristone after this stay order
- Minimize a patient’s wait time for mifepristone administration by blocking time in your weekly schedule for patients seeking abortion care
- Work with other providers and health care professionals in your area to submit petitions to the FDA
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Roberts, CJ, concurring).
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Sotomayor, J, dissenting).
- COVID data tracker. Centers for Disease Control and Prevention website. https://covid.cdc.gov/covid-data-tracker. Accessed March 9, 2021.
- Fulcer IR, Neill S, Bharadwa S, et al. State and federal abortion restrictions increase risk of COVID-19 exposure by mandating unnecessary clinic visits. Contraception. 2020;102:385-391.
In May 2020, the American College of Obstetricians and Gynecologists (ACOG), along with other organizations and physicians (Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong Women of Color Reproductive Justice Collective, Honor MacNaughton, MD), filed a civil action against the US Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS) challenging the requirements of in-person mifepristone dispensing, which was one of the 3 restrictions placed on the medicine as part of mifepristone’s risk evaluation and mitigation strategy (REMS). The requirements, which also include provider certification and patient signatures on specified consent forms, specifically target dosages of mifepristone for use related to abortions and miscarriages but do not apply when prescribing mifepristone for other medical conditions, even with higher doses. During the pandemic, the FDA suspended the REMS requirements for many other medications, including those more toxic than mifepristone. Additionally, the HHS activated a “telemedicine exception” that allows physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids, while minimizing the patient’s and provider’s risk of exposure to COVID-19 with in-person appointments. Notably, mifepristone for abortion and miscarriage management was excluded from this relaxation of the REMS requirement.
On July 13, 2020, a Federal District Court concluded that the in-person requirements were a “substantial obstacle” for women seeking abortions during the COVID-19 pandemic and granted a preliminary injunction to temporarily stop the FDA’s enforcement of the in-person requirements for mifepristone. We wrote about what that decision meant for ObGyns and urged clinicians to advocate to make the injunction permanent (OBG Manag. 2020;32(12):13-14, 23, 38. doi: 10.12788/obgm.0034.)
From there, however, the FDA worked to reverse that decision, which included applications to the District Court and to the Supreme Court for a stay of the injunction. If successful, this would suspend the injunction while the case was pending. In October, after the Supreme Court deferred review of the application (preferring a review by the lower courts), the District Court upheld the injunction of the in-person requirements citing the worsening pandemic crisis.
In-person requirement re-instated
On January 12, 2021, the United States Supreme Court granted the stay of the District Court’s injunction, which allowed the federal government to enforce the in-person requirement for mifepristone once again. The decision came down to a vote of 6 to 3. As is typical for decisions on stay orders, the court did not release a majority opinion explaining the reasoning behind this decision. In a concurring opinion, Chief Justice John Roberts wrote that the decision was not a judgment of if the requirements for in-person dispensing of mifepristone imposed an undue burden on women seeking an abortion. Instead, the Chief Justice explained that the decision came down to if a District Court could order the FDA to change their regulations based on “the court’s own evaluations of the COVID-19 pandemic,” maintaining that the court could not overrule “the politically accountable entities with the ‘background, competence, and expertise to assess public health.’”1 No other justices joined his opinion.
A worrisome pattern of a conservative supermajority
In her dissent, Justice Sonia Sotomayor criticized the government’s “statistically insignificant, cherry-picked data” and argued that the government did not provide any explanation from an FDA or HHS official explaining why mifepristone’s in-person requirement is more important than the in-person requirements of other drugs that have been waived during the pandemic.2 Therefore, she explained, there is “no reasoned decision” by any health official anywhere on which they can base the decision to grant the stay.
This ruling was the Supreme Court’s first major decision on reproductive health since the confirmation of Justice Amy Coney Barrett and may be an insight into future decisions of the new conservative supermajority on abortion and reproductive health issues. Particularly worrisome is what this decision could mean for stays in abortion cases that dictate whether or not the regulation is enforced during an active case. Even if cases are ruled in favor of patients and abortion providers, if the courts continue to allow enforcement of abortion restrictions during litigation, this could result in permanent closure of abortion clinics and prevent many individuals from accessing safe and legal abortion.
Looking toward the future
In the setting of almost 29 million cases of COVID-19 and more than 526,000 deaths, this stay order requires women seeking a medication abortion to make an appointment at a clinic, risking possible exposure to COVID-19, in order to access mifepristone.3,4 The Biden administration can and should remove the FDA requirement for in-person delivery of mifepristone, which would mitigate the effects of the stay order and allow women to obtain medication abortions during the pandemic.
Take action
- Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe mifepristone after this stay order
- Minimize a patient’s wait time for mifepristone administration by blocking time in your weekly schedule for patients seeking abortion care
- Work with other providers and health care professionals in your area to submit petitions to the FDA
In May 2020, the American College of Obstetricians and Gynecologists (ACOG), along with other organizations and physicians (Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong Women of Color Reproductive Justice Collective, Honor MacNaughton, MD), filed a civil action against the US Food and Drug Administration (FDA) and the Department of Health and Human Services (HHS) challenging the requirements of in-person mifepristone dispensing, which was one of the 3 restrictions placed on the medicine as part of mifepristone’s risk evaluation and mitigation strategy (REMS). The requirements, which also include provider certification and patient signatures on specified consent forms, specifically target dosages of mifepristone for use related to abortions and miscarriages but do not apply when prescribing mifepristone for other medical conditions, even with higher doses. During the pandemic, the FDA suspended the REMS requirements for many other medications, including those more toxic than mifepristone. Additionally, the HHS activated a “telemedicine exception” that allows physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids, while minimizing the patient’s and provider’s risk of exposure to COVID-19 with in-person appointments. Notably, mifepristone for abortion and miscarriage management was excluded from this relaxation of the REMS requirement.
On July 13, 2020, a Federal District Court concluded that the in-person requirements were a “substantial obstacle” for women seeking abortions during the COVID-19 pandemic and granted a preliminary injunction to temporarily stop the FDA’s enforcement of the in-person requirements for mifepristone. We wrote about what that decision meant for ObGyns and urged clinicians to advocate to make the injunction permanent (OBG Manag. 2020;32(12):13-14, 23, 38. doi: 10.12788/obgm.0034.)
From there, however, the FDA worked to reverse that decision, which included applications to the District Court and to the Supreme Court for a stay of the injunction. If successful, this would suspend the injunction while the case was pending. In October, after the Supreme Court deferred review of the application (preferring a review by the lower courts), the District Court upheld the injunction of the in-person requirements citing the worsening pandemic crisis.
In-person requirement re-instated
On January 12, 2021, the United States Supreme Court granted the stay of the District Court’s injunction, which allowed the federal government to enforce the in-person requirement for mifepristone once again. The decision came down to a vote of 6 to 3. As is typical for decisions on stay orders, the court did not release a majority opinion explaining the reasoning behind this decision. In a concurring opinion, Chief Justice John Roberts wrote that the decision was not a judgment of if the requirements for in-person dispensing of mifepristone imposed an undue burden on women seeking an abortion. Instead, the Chief Justice explained that the decision came down to if a District Court could order the FDA to change their regulations based on “the court’s own evaluations of the COVID-19 pandemic,” maintaining that the court could not overrule “the politically accountable entities with the ‘background, competence, and expertise to assess public health.’”1 No other justices joined his opinion.
A worrisome pattern of a conservative supermajority
In her dissent, Justice Sonia Sotomayor criticized the government’s “statistically insignificant, cherry-picked data” and argued that the government did not provide any explanation from an FDA or HHS official explaining why mifepristone’s in-person requirement is more important than the in-person requirements of other drugs that have been waived during the pandemic.2 Therefore, she explained, there is “no reasoned decision” by any health official anywhere on which they can base the decision to grant the stay.
This ruling was the Supreme Court’s first major decision on reproductive health since the confirmation of Justice Amy Coney Barrett and may be an insight into future decisions of the new conservative supermajority on abortion and reproductive health issues. Particularly worrisome is what this decision could mean for stays in abortion cases that dictate whether or not the regulation is enforced during an active case. Even if cases are ruled in favor of patients and abortion providers, if the courts continue to allow enforcement of abortion restrictions during litigation, this could result in permanent closure of abortion clinics and prevent many individuals from accessing safe and legal abortion.
Looking toward the future
In the setting of almost 29 million cases of COVID-19 and more than 526,000 deaths, this stay order requires women seeking a medication abortion to make an appointment at a clinic, risking possible exposure to COVID-19, in order to access mifepristone.3,4 The Biden administration can and should remove the FDA requirement for in-person delivery of mifepristone, which would mitigate the effects of the stay order and allow women to obtain medication abortions during the pandemic.
Take action
- Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe mifepristone after this stay order
- Minimize a patient’s wait time for mifepristone administration by blocking time in your weekly schedule for patients seeking abortion care
- Work with other providers and health care professionals in your area to submit petitions to the FDA
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Roberts, CJ, concurring).
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Sotomayor, J, dissenting).
- COVID data tracker. Centers for Disease Control and Prevention website. https://covid.cdc.gov/covid-data-tracker. Accessed March 9, 2021.
- Fulcer IR, Neill S, Bharadwa S, et al. State and federal abortion restrictions increase risk of COVID-19 exposure by mandating unnecessary clinic visits. Contraception. 2020;102:385-391.
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Roberts, CJ, concurring).
- FDA v American College of Obstetricians and Gynecologists, 592 US __ (2021)(Sotomayor, J, dissenting).
- COVID data tracker. Centers for Disease Control and Prevention website. https://covid.cdc.gov/covid-data-tracker. Accessed March 9, 2021.
- Fulcer IR, Neill S, Bharadwa S, et al. State and federal abortion restrictions increase risk of COVID-19 exposure by mandating unnecessary clinic visits. Contraception. 2020;102:385-391.
Is there liability if you don’t test for BRCA?
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6
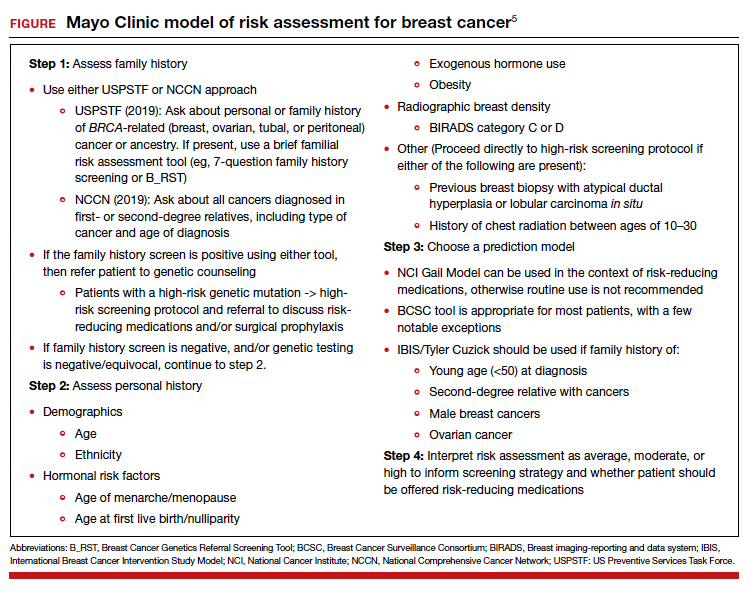
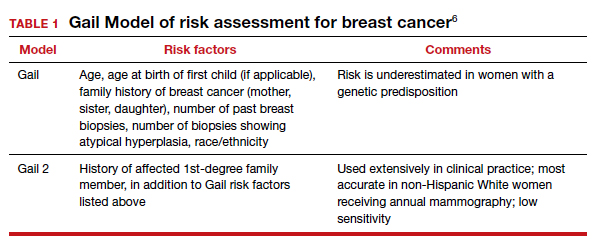
Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7
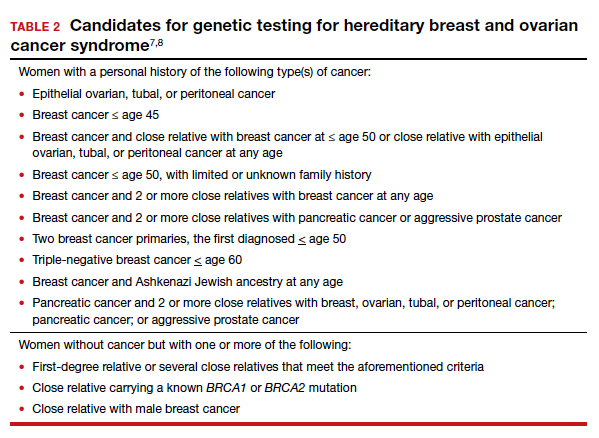
The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
2021 Update on gynecologic cancer
Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).
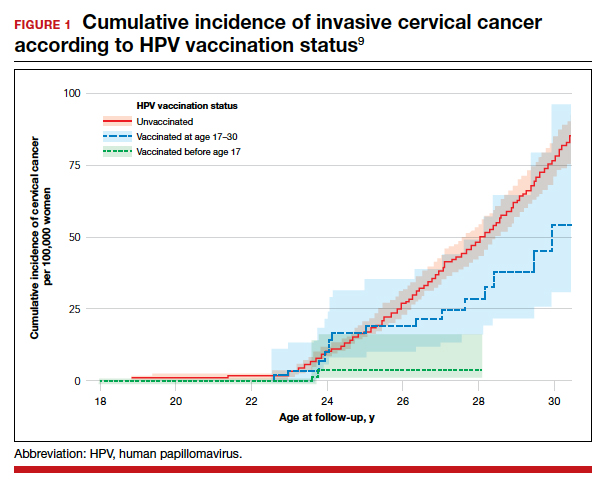
The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●
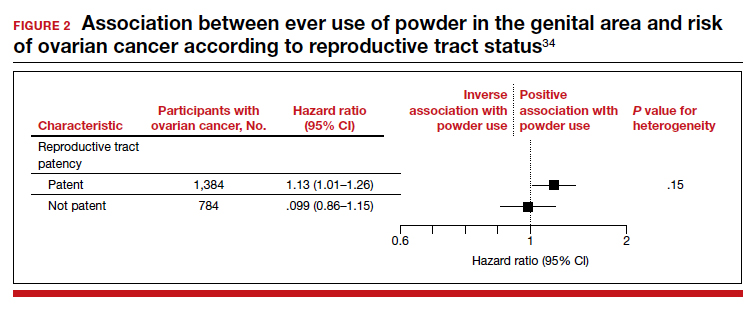
The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.

Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).

The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●

The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.

Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).

The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●

The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
For heavy menstrual bleeding, are long-term outcomes similar for treatment with the LNG-IUS and radiofrequency endometrial ablation?
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Type 3 von Willebrand a rare but serious bleeding disorder
Type 3 von Willebrand disease (VWD) is rare, but this form of the disease is associated with severe bleeding, particularly in muscles and joints, a bleeding disorders expert said.
“There’s a virtually complete deficiency in von Willebrand factor [in type 3 disease], so usually it’s defined as below 5 or in some studies below 3 IU/dL, but also due to the very low levels of von Willebrand factor, there’s also a very low level of factor VIII,” said Jeroen Eikenboom, MD, PhD, from Leiden (the Netherlands) University Medical Center.
“The inheritance pattern is autosomal recessive, and the prevalence is about 1 in a million,” he said during the annual congress of the European Association for Haemophilia and Allied Disorders.
Erik Adolf von Willenbrand, MD, PhD, first described this form of VWD in a family from the Åland Islands, an autonomous region of Finland. The disease, later discovered to be caused in this family by a cytosine deletion in exon 18 of the von Willebrand factor (VWF) gene, was associated with fatal bleeding events in several family members.
“As VWF is the carrier protein of factor VIII, the very low VWF level leads to a strongly reduced factor VIII level, comparable to the levels seen in mild to moderate hemophilia A. As a consequence, VWD type 3 has the combined characteristics of a primary as well as a secondary hemostasis defect,” Dr. Eikenboom explained in an abstract accompanying his talk.
Compared with VWD type 1 or 2, type 3 VWD is associated with bleeding episodes more commonly seen in patients with hemophilia A, notably mucocutaneous bleeding, bleeding after trauma or during surgery, and bleeding into joints and/or muscles.
Treatment
The goals of treatment for patients with type 3 VWD are to correct the dual hemostasis defects of impaired platelet adhesion because of low VWF levels, and the intrinsic coagulation defect because of levels of factor VIII.
Desmopressin is not effective in type 3 VWD, Dr. Eikenboom said, so treatment requires the use of either plasma-derived VWF, with or without factor VIII, or recombinant VWF.
In the United States, the only standalone VWF concentrate approved by the Food and Drug Administration is a recombinant product (Vonvendi), Three other human plasma–derived concentrates containing both VWF and factor VIII are also licensed (Alpanate, Humate-P, Wilate).
Clinicians prescribing the combined factor concentrates need to be aware of differences in pharmacokinetics between the products.
For example, following infusion of Wilate, which has equal amounts of von Willebrand factor and factor VIII, there is an increase in circulation of both von Willebrand factor and factor VIII and a similar decline in each factor over time.
In contrast, following an infusion of Humate-P, which contains lower levels of factor VIII, “interestingly, you see a secondary rise of factor VIII in Humate-P–infused patients, whereas the secondary rise is not visible in the Wilate patients,” he said.
Approximately 22% of patients with type 3 VWD also receive prophylaxis with VWF concentrate, which has been shown to decrease the median annualized bleeding rate from 25% to 6.1%.
Dr. Eikenboom cautioned that 5%-10% of patients with type 3 VWD may develop allo-antibodies against VWF concentrates, which can complicate treatment and carries risk of anaphylactic shock.
“It’s also been mentioned in literature that there may be an association with partial or complete von Willebrand factor gene deletions or nonsense mutations and the development of allo-antibodies,” he said.
Prophylaxis burdensome but helpful
Veronica H. Flood, MD, from the Medical College of Wisconsin, Milwaukee, who specializes in the treatment of patients with von Willebrand disease, follows a number of both girls and boys with type 3 VWD.
“Those are the people who will have bleeding into their joints, and for the girls, worse periods than some of those with other types of von Willebrand disease, and it is true that if you want to stop their bleeding, you cannot use desmopressin like we use in most other von Willebrand patients. They will need factor, although for the heavy menstrual bleeding you can use hormones or tranexamic acid – there are some other options for that,” she said in an interview.
She also noted that type 3 von Willebrand disease can be highly variable. For patients with especially frequent joint bleeding, her center recommends prophylaxis.
“Prophylaxis can be very burdensome for patients. You’re talking about IV therapy several times a week, but it’s very helpful for the joint bleeds. Episodic prophylaxis can be very helpful for heavy menstrual bleeding, and we actually have type 2, type 3, and some type 1 patients with bad enough nose bleeds that they end up on prophylaxis,” she said.
Patients with gastrointestinal bleeding are the most challenging to care for, she noted.
“You can put them on factor prophylaxis, but even that isn’t always enough to help some adults with bad GI bleeding, and we’re investigating other options for that,” she said.
Dr. Eikenboom disclosed research support from CSL Behring and honoraria (directed to his institution) for educational activities sponsored by Roche and Celgene. Dr. Flood reported having no conflicts of interest to disclose.
Type 3 von Willebrand disease (VWD) is rare, but this form of the disease is associated with severe bleeding, particularly in muscles and joints, a bleeding disorders expert said.
“There’s a virtually complete deficiency in von Willebrand factor [in type 3 disease], so usually it’s defined as below 5 or in some studies below 3 IU/dL, but also due to the very low levels of von Willebrand factor, there’s also a very low level of factor VIII,” said Jeroen Eikenboom, MD, PhD, from Leiden (the Netherlands) University Medical Center.
“The inheritance pattern is autosomal recessive, and the prevalence is about 1 in a million,” he said during the annual congress of the European Association for Haemophilia and Allied Disorders.
Erik Adolf von Willenbrand, MD, PhD, first described this form of VWD in a family from the Åland Islands, an autonomous region of Finland. The disease, later discovered to be caused in this family by a cytosine deletion in exon 18 of the von Willebrand factor (VWF) gene, was associated with fatal bleeding events in several family members.
“As VWF is the carrier protein of factor VIII, the very low VWF level leads to a strongly reduced factor VIII level, comparable to the levels seen in mild to moderate hemophilia A. As a consequence, VWD type 3 has the combined characteristics of a primary as well as a secondary hemostasis defect,” Dr. Eikenboom explained in an abstract accompanying his talk.
Compared with VWD type 1 or 2, type 3 VWD is associated with bleeding episodes more commonly seen in patients with hemophilia A, notably mucocutaneous bleeding, bleeding after trauma or during surgery, and bleeding into joints and/or muscles.
Treatment
The goals of treatment for patients with type 3 VWD are to correct the dual hemostasis defects of impaired platelet adhesion because of low VWF levels, and the intrinsic coagulation defect because of levels of factor VIII.
Desmopressin is not effective in type 3 VWD, Dr. Eikenboom said, so treatment requires the use of either plasma-derived VWF, with or without factor VIII, or recombinant VWF.
In the United States, the only standalone VWF concentrate approved by the Food and Drug Administration is a recombinant product (Vonvendi), Three other human plasma–derived concentrates containing both VWF and factor VIII are also licensed (Alpanate, Humate-P, Wilate).
Clinicians prescribing the combined factor concentrates need to be aware of differences in pharmacokinetics between the products.
For example, following infusion of Wilate, which has equal amounts of von Willebrand factor and factor VIII, there is an increase in circulation of both von Willebrand factor and factor VIII and a similar decline in each factor over time.
In contrast, following an infusion of Humate-P, which contains lower levels of factor VIII, “interestingly, you see a secondary rise of factor VIII in Humate-P–infused patients, whereas the secondary rise is not visible in the Wilate patients,” he said.
Approximately 22% of patients with type 3 VWD also receive prophylaxis with VWF concentrate, which has been shown to decrease the median annualized bleeding rate from 25% to 6.1%.
Dr. Eikenboom cautioned that 5%-10% of patients with type 3 VWD may develop allo-antibodies against VWF concentrates, which can complicate treatment and carries risk of anaphylactic shock.
“It’s also been mentioned in literature that there may be an association with partial or complete von Willebrand factor gene deletions or nonsense mutations and the development of allo-antibodies,” he said.
Prophylaxis burdensome but helpful
Veronica H. Flood, MD, from the Medical College of Wisconsin, Milwaukee, who specializes in the treatment of patients with von Willebrand disease, follows a number of both girls and boys with type 3 VWD.
“Those are the people who will have bleeding into their joints, and for the girls, worse periods than some of those with other types of von Willebrand disease, and it is true that if you want to stop their bleeding, you cannot use desmopressin like we use in most other von Willebrand patients. They will need factor, although for the heavy menstrual bleeding you can use hormones or tranexamic acid – there are some other options for that,” she said in an interview.
She also noted that type 3 von Willebrand disease can be highly variable. For patients with especially frequent joint bleeding, her center recommends prophylaxis.
“Prophylaxis can be very burdensome for patients. You’re talking about IV therapy several times a week, but it’s very helpful for the joint bleeds. Episodic prophylaxis can be very helpful for heavy menstrual bleeding, and we actually have type 2, type 3, and some type 1 patients with bad enough nose bleeds that they end up on prophylaxis,” she said.
Patients with gastrointestinal bleeding are the most challenging to care for, she noted.
“You can put them on factor prophylaxis, but even that isn’t always enough to help some adults with bad GI bleeding, and we’re investigating other options for that,” she said.
Dr. Eikenboom disclosed research support from CSL Behring and honoraria (directed to his institution) for educational activities sponsored by Roche and Celgene. Dr. Flood reported having no conflicts of interest to disclose.
Type 3 von Willebrand disease (VWD) is rare, but this form of the disease is associated with severe bleeding, particularly in muscles and joints, a bleeding disorders expert said.
“There’s a virtually complete deficiency in von Willebrand factor [in type 3 disease], so usually it’s defined as below 5 or in some studies below 3 IU/dL, but also due to the very low levels of von Willebrand factor, there’s also a very low level of factor VIII,” said Jeroen Eikenboom, MD, PhD, from Leiden (the Netherlands) University Medical Center.
“The inheritance pattern is autosomal recessive, and the prevalence is about 1 in a million,” he said during the annual congress of the European Association for Haemophilia and Allied Disorders.
Erik Adolf von Willenbrand, MD, PhD, first described this form of VWD in a family from the Åland Islands, an autonomous region of Finland. The disease, later discovered to be caused in this family by a cytosine deletion in exon 18 of the von Willebrand factor (VWF) gene, was associated with fatal bleeding events in several family members.
“As VWF is the carrier protein of factor VIII, the very low VWF level leads to a strongly reduced factor VIII level, comparable to the levels seen in mild to moderate hemophilia A. As a consequence, VWD type 3 has the combined characteristics of a primary as well as a secondary hemostasis defect,” Dr. Eikenboom explained in an abstract accompanying his talk.
Compared with VWD type 1 or 2, type 3 VWD is associated with bleeding episodes more commonly seen in patients with hemophilia A, notably mucocutaneous bleeding, bleeding after trauma or during surgery, and bleeding into joints and/or muscles.
Treatment
The goals of treatment for patients with type 3 VWD are to correct the dual hemostasis defects of impaired platelet adhesion because of low VWF levels, and the intrinsic coagulation defect because of levels of factor VIII.
Desmopressin is not effective in type 3 VWD, Dr. Eikenboom said, so treatment requires the use of either plasma-derived VWF, with or without factor VIII, or recombinant VWF.
In the United States, the only standalone VWF concentrate approved by the Food and Drug Administration is a recombinant product (Vonvendi), Three other human plasma–derived concentrates containing both VWF and factor VIII are also licensed (Alpanate, Humate-P, Wilate).
Clinicians prescribing the combined factor concentrates need to be aware of differences in pharmacokinetics between the products.
For example, following infusion of Wilate, which has equal amounts of von Willebrand factor and factor VIII, there is an increase in circulation of both von Willebrand factor and factor VIII and a similar decline in each factor over time.
In contrast, following an infusion of Humate-P, which contains lower levels of factor VIII, “interestingly, you see a secondary rise of factor VIII in Humate-P–infused patients, whereas the secondary rise is not visible in the Wilate patients,” he said.
Approximately 22% of patients with type 3 VWD also receive prophylaxis with VWF concentrate, which has been shown to decrease the median annualized bleeding rate from 25% to 6.1%.
Dr. Eikenboom cautioned that 5%-10% of patients with type 3 VWD may develop allo-antibodies against VWF concentrates, which can complicate treatment and carries risk of anaphylactic shock.
“It’s also been mentioned in literature that there may be an association with partial or complete von Willebrand factor gene deletions or nonsense mutations and the development of allo-antibodies,” he said.
Prophylaxis burdensome but helpful
Veronica H. Flood, MD, from the Medical College of Wisconsin, Milwaukee, who specializes in the treatment of patients with von Willebrand disease, follows a number of both girls and boys with type 3 VWD.
“Those are the people who will have bleeding into their joints, and for the girls, worse periods than some of those with other types of von Willebrand disease, and it is true that if you want to stop their bleeding, you cannot use desmopressin like we use in most other von Willebrand patients. They will need factor, although for the heavy menstrual bleeding you can use hormones or tranexamic acid – there are some other options for that,” she said in an interview.
She also noted that type 3 von Willebrand disease can be highly variable. For patients with especially frequent joint bleeding, her center recommends prophylaxis.
“Prophylaxis can be very burdensome for patients. You’re talking about IV therapy several times a week, but it’s very helpful for the joint bleeds. Episodic prophylaxis can be very helpful for heavy menstrual bleeding, and we actually have type 2, type 3, and some type 1 patients with bad enough nose bleeds that they end up on prophylaxis,” she said.
Patients with gastrointestinal bleeding are the most challenging to care for, she noted.
“You can put them on factor prophylaxis, but even that isn’t always enough to help some adults with bad GI bleeding, and we’re investigating other options for that,” she said.
Dr. Eikenboom disclosed research support from CSL Behring and honoraria (directed to his institution) for educational activities sponsored by Roche and Celgene. Dr. Flood reported having no conflicts of interest to disclose.
FROM EAHAD 2021
Vagisil offered teens a vaginal ‘glow up.’ Docs cry foul
Late one night in early February, Jen Gunter, MD, was scrolling online when she discovered a new “feminine hygiene” product being marketed for teen girls. The new vanilla clementine scented wipes and cleansers with confetti-colored packaging and a cute name (OMV!) irked Dr. Gunter because they are designed for girls to use to “freshen” their vaginal area.
Dr. Gunter, a San Francisco-based gynecologist and author of “The Vagina Bible,” has built a reputation as a fierce advocate for women’s health and debunker of pseudoscience. She has called out jade eggs and “detox pearls” and various other items that promise to improve the vagina but that she and other doctors warn could actually be harmful. And, in her view, this product is no different.
She fired off a tweet that became the first volley in a vociferous social media countercampaign: “Hey @vagisil going to call you out here for this predatory line of products aimed at teen girls. Why do you think teen vulvas need special cleaning? To be prepped for men? Because they are dirty. Anxiously awaiting your answer as are all my followers.”
Vagisil responded on Instagram that “we want to clarify any confusion or the underlying belief that OMV! was developed because there is something wrong with teens or that vulvas/vaginas are inherently dirty. That is not the case. All-Day Fresh Wash is an all-over body wash, that is safe, gentle, and pH-balanced for sensitive vulvar area skin.”
Dr. Gunter’s Feb. 4 tweet attracted more than 8,300 likes, 1,300 retweets and hundreds of comments, but that was just the beginning. Dr. Gunter has continued to tweet about the OMV! product line – and has inspired dozens of other gynecologists to join in.
‘Your vagina is fine’
Dr. Gunter and other gynecologists have long delivered the message that water alone is sufficient to cleanse the vulvar area and that the vagina itself is self-cleaning. Research into the vaginal microbiome reveals the role of lactobacilli in preventing urogenital diseases. “Disturbances in your vagina microbiome are hard to undo,” says Jocelyn Fitzgerald, MD, a urogynecologist and pelvic reconstructive surgeon at Magee-Womens Hospital at the University of Pittsburgh Medical Center.
To underscore that message, Dr. Fitzgerald recently tweeted in support of Dr. Gunter’s Twitter thread: “Honestly, the @vagisil marketing campaign is a brilliant one because using their products while your vagina is perfectly fine will destroy your microbiome, give you real Bacterial Vaginosis, and prompt you to buy more Vagisil. DON’T FALL FOR IT GIRLS YOUR VAGINA IS FINE.”
In an emailed response to this news organization, a Vagisil spokesperson said, “We follow industry best practices for testing and OMV! products are rigorously assessed for safety and quality. In addition, we work with respected, independent clinical labs that follow strict testing protocols, using board-certified gynecologists and dermatologists to test our products before launch.”
However, beyond the potential for irritation or misuse, the gynecologists zeroed in on the underlying message that girls would feel more confident if they used the wipes and cleanser. For example, the company suggested that teens could use the wipes to get rid of “period funk.”
“There is no such thing as period funk!” gynecologist Danielle Jones, MD, exclaimed in a video on YouTube, where she has a channel called Mama Doctor Jones – with 700,000 subscribers. “All you need is ordinary hygiene. Period funk is not a thing! And if you feel like something is going on because there’s an odor that is abnormal, you need to talk to your doctor.”
Adult women often use wipes and special cleansers in the vaginal area. An online survey of 1,435 Canadian women, published in BMC Women’s Health in 2018, found 42% had used vaginal wipes, 12% had used vaginal washes or cleansers – and 4% had used them internally.
When it launched OMV! in July, Vagisil said it had engaged 2,500 teens and their mothers in creating the product, which it said was “designed to meet the cleansing and care needs of a new generation of young women.”
That extension of a product most commonly used by adult women to teenagers – who often feel self-conscious about their bodies – is exactly what bothers Dr. Gunter. “BTW I am sorry I am subjecting you all to my @vagisil outrage, but preying on teens and amplifying patriarchal shame of normal bodily functions to sell an irritating product is not acceptable. I’m not stopping until they take that OMV! product line down everywhere,” she said in a Feb. 8 tweet that attracted more than 7,900 likes.
No ‘glow up’ needed
Dr. Gunter’s tweets tapped into collective anger over the shaming of women’s bodies. The OMV! marketing suggested that teens could get a “glow up” with the products.
“Your vulva doesn’t need a ‘glow up.’ It’s fine like it is. And if it’s not, talk to your doctor,” Dr. Jones said in her Feb. 8 video, which has had almost 350,000 views, with 28,000 likes and only 149 dislikes.
“They’re very clearly pathologizing normal physiology,” Dr. Jones says. “They’re creating language that makes people feel as though their normal bodily functions have to be somehow fixed or changed.”
Dr. Gunter says she specifically wanted to prevent Vagisil from leveraging social media to influence teen girls. With her stream of tweets and support from colleagues around the country, she has sparked a prolonged online conversation.
“I am encouraged by the strong response on social media from both other enraged ob.gyns. and health care professionals as well the response from a lot of women and men,” Dr. Gunter said in an interview. “We have effectively blocked [Vagisil] from using social media.”
In its response to this news organization, Vagisil noted, “We are a brand run by women with daughters of our own.” While defending the products, Vagisil acknowledged the criticisms: “We are always listening to our consumers and our expert partners so that we continuously evolve. We appreciate the perspective that our language choice surrounding periods may perpetuate an old idea and have already begun to make changes to address this.”
Dr. Gunter says she plans to stay on topic. “Given the number of people outraged, I suspect if they venture out on social media again the reaction will be swift,” she said. “Hopefully we have made OMV! toxic for influencers as well.”
In fact, she’s ready to take on “the entire predatory feminine hygiene market. I’m sick of their false claims about balancing pH and not-so-subtle suggestions that vaginas and vulvas and menstruation stink. These products cause psychological harm as well as physical harm from their irritants,” she said.
A version of this article first appeared on Medscape.com.
Late one night in early February, Jen Gunter, MD, was scrolling online when she discovered a new “feminine hygiene” product being marketed for teen girls. The new vanilla clementine scented wipes and cleansers with confetti-colored packaging and a cute name (OMV!) irked Dr. Gunter because they are designed for girls to use to “freshen” their vaginal area.
Dr. Gunter, a San Francisco-based gynecologist and author of “The Vagina Bible,” has built a reputation as a fierce advocate for women’s health and debunker of pseudoscience. She has called out jade eggs and “detox pearls” and various other items that promise to improve the vagina but that she and other doctors warn could actually be harmful. And, in her view, this product is no different.
She fired off a tweet that became the first volley in a vociferous social media countercampaign: “Hey @vagisil going to call you out here for this predatory line of products aimed at teen girls. Why do you think teen vulvas need special cleaning? To be prepped for men? Because they are dirty. Anxiously awaiting your answer as are all my followers.”
Vagisil responded on Instagram that “we want to clarify any confusion or the underlying belief that OMV! was developed because there is something wrong with teens or that vulvas/vaginas are inherently dirty. That is not the case. All-Day Fresh Wash is an all-over body wash, that is safe, gentle, and pH-balanced for sensitive vulvar area skin.”
Dr. Gunter’s Feb. 4 tweet attracted more than 8,300 likes, 1,300 retweets and hundreds of comments, but that was just the beginning. Dr. Gunter has continued to tweet about the OMV! product line – and has inspired dozens of other gynecologists to join in.
‘Your vagina is fine’
Dr. Gunter and other gynecologists have long delivered the message that water alone is sufficient to cleanse the vulvar area and that the vagina itself is self-cleaning. Research into the vaginal microbiome reveals the role of lactobacilli in preventing urogenital diseases. “Disturbances in your vagina microbiome are hard to undo,” says Jocelyn Fitzgerald, MD, a urogynecologist and pelvic reconstructive surgeon at Magee-Womens Hospital at the University of Pittsburgh Medical Center.
To underscore that message, Dr. Fitzgerald recently tweeted in support of Dr. Gunter’s Twitter thread: “Honestly, the @vagisil marketing campaign is a brilliant one because using their products while your vagina is perfectly fine will destroy your microbiome, give you real Bacterial Vaginosis, and prompt you to buy more Vagisil. DON’T FALL FOR IT GIRLS YOUR VAGINA IS FINE.”
In an emailed response to this news organization, a Vagisil spokesperson said, “We follow industry best practices for testing and OMV! products are rigorously assessed for safety and quality. In addition, we work with respected, independent clinical labs that follow strict testing protocols, using board-certified gynecologists and dermatologists to test our products before launch.”
However, beyond the potential for irritation or misuse, the gynecologists zeroed in on the underlying message that girls would feel more confident if they used the wipes and cleanser. For example, the company suggested that teens could use the wipes to get rid of “period funk.”
“There is no such thing as period funk!” gynecologist Danielle Jones, MD, exclaimed in a video on YouTube, where she has a channel called Mama Doctor Jones – with 700,000 subscribers. “All you need is ordinary hygiene. Period funk is not a thing! And if you feel like something is going on because there’s an odor that is abnormal, you need to talk to your doctor.”
Adult women often use wipes and special cleansers in the vaginal area. An online survey of 1,435 Canadian women, published in BMC Women’s Health in 2018, found 42% had used vaginal wipes, 12% had used vaginal washes or cleansers – and 4% had used them internally.
When it launched OMV! in July, Vagisil said it had engaged 2,500 teens and their mothers in creating the product, which it said was “designed to meet the cleansing and care needs of a new generation of young women.”
That extension of a product most commonly used by adult women to teenagers – who often feel self-conscious about their bodies – is exactly what bothers Dr. Gunter. “BTW I am sorry I am subjecting you all to my @vagisil outrage, but preying on teens and amplifying patriarchal shame of normal bodily functions to sell an irritating product is not acceptable. I’m not stopping until they take that OMV! product line down everywhere,” she said in a Feb. 8 tweet that attracted more than 7,900 likes.
No ‘glow up’ needed
Dr. Gunter’s tweets tapped into collective anger over the shaming of women’s bodies. The OMV! marketing suggested that teens could get a “glow up” with the products.
“Your vulva doesn’t need a ‘glow up.’ It’s fine like it is. And if it’s not, talk to your doctor,” Dr. Jones said in her Feb. 8 video, which has had almost 350,000 views, with 28,000 likes and only 149 dislikes.
“They’re very clearly pathologizing normal physiology,” Dr. Jones says. “They’re creating language that makes people feel as though their normal bodily functions have to be somehow fixed or changed.”
Dr. Gunter says she specifically wanted to prevent Vagisil from leveraging social media to influence teen girls. With her stream of tweets and support from colleagues around the country, she has sparked a prolonged online conversation.
“I am encouraged by the strong response on social media from both other enraged ob.gyns. and health care professionals as well the response from a lot of women and men,” Dr. Gunter said in an interview. “We have effectively blocked [Vagisil] from using social media.”
In its response to this news organization, Vagisil noted, “We are a brand run by women with daughters of our own.” While defending the products, Vagisil acknowledged the criticisms: “We are always listening to our consumers and our expert partners so that we continuously evolve. We appreciate the perspective that our language choice surrounding periods may perpetuate an old idea and have already begun to make changes to address this.”
Dr. Gunter says she plans to stay on topic. “Given the number of people outraged, I suspect if they venture out on social media again the reaction will be swift,” she said. “Hopefully we have made OMV! toxic for influencers as well.”
In fact, she’s ready to take on “the entire predatory feminine hygiene market. I’m sick of their false claims about balancing pH and not-so-subtle suggestions that vaginas and vulvas and menstruation stink. These products cause psychological harm as well as physical harm from their irritants,” she said.
A version of this article first appeared on Medscape.com.
Late one night in early February, Jen Gunter, MD, was scrolling online when she discovered a new “feminine hygiene” product being marketed for teen girls. The new vanilla clementine scented wipes and cleansers with confetti-colored packaging and a cute name (OMV!) irked Dr. Gunter because they are designed for girls to use to “freshen” their vaginal area.
Dr. Gunter, a San Francisco-based gynecologist and author of “The Vagina Bible,” has built a reputation as a fierce advocate for women’s health and debunker of pseudoscience. She has called out jade eggs and “detox pearls” and various other items that promise to improve the vagina but that she and other doctors warn could actually be harmful. And, in her view, this product is no different.
She fired off a tweet that became the first volley in a vociferous social media countercampaign: “Hey @vagisil going to call you out here for this predatory line of products aimed at teen girls. Why do you think teen vulvas need special cleaning? To be prepped for men? Because they are dirty. Anxiously awaiting your answer as are all my followers.”
Vagisil responded on Instagram that “we want to clarify any confusion or the underlying belief that OMV! was developed because there is something wrong with teens or that vulvas/vaginas are inherently dirty. That is not the case. All-Day Fresh Wash is an all-over body wash, that is safe, gentle, and pH-balanced for sensitive vulvar area skin.”
Dr. Gunter’s Feb. 4 tweet attracted more than 8,300 likes, 1,300 retweets and hundreds of comments, but that was just the beginning. Dr. Gunter has continued to tweet about the OMV! product line – and has inspired dozens of other gynecologists to join in.
‘Your vagina is fine’
Dr. Gunter and other gynecologists have long delivered the message that water alone is sufficient to cleanse the vulvar area and that the vagina itself is self-cleaning. Research into the vaginal microbiome reveals the role of lactobacilli in preventing urogenital diseases. “Disturbances in your vagina microbiome are hard to undo,” says Jocelyn Fitzgerald, MD, a urogynecologist and pelvic reconstructive surgeon at Magee-Womens Hospital at the University of Pittsburgh Medical Center.
To underscore that message, Dr. Fitzgerald recently tweeted in support of Dr. Gunter’s Twitter thread: “Honestly, the @vagisil marketing campaign is a brilliant one because using their products while your vagina is perfectly fine will destroy your microbiome, give you real Bacterial Vaginosis, and prompt you to buy more Vagisil. DON’T FALL FOR IT GIRLS YOUR VAGINA IS FINE.”
In an emailed response to this news organization, a Vagisil spokesperson said, “We follow industry best practices for testing and OMV! products are rigorously assessed for safety and quality. In addition, we work with respected, independent clinical labs that follow strict testing protocols, using board-certified gynecologists and dermatologists to test our products before launch.”
However, beyond the potential for irritation or misuse, the gynecologists zeroed in on the underlying message that girls would feel more confident if they used the wipes and cleanser. For example, the company suggested that teens could use the wipes to get rid of “period funk.”
“There is no such thing as period funk!” gynecologist Danielle Jones, MD, exclaimed in a video on YouTube, where she has a channel called Mama Doctor Jones – with 700,000 subscribers. “All you need is ordinary hygiene. Period funk is not a thing! And if you feel like something is going on because there’s an odor that is abnormal, you need to talk to your doctor.”
Adult women often use wipes and special cleansers in the vaginal area. An online survey of 1,435 Canadian women, published in BMC Women’s Health in 2018, found 42% had used vaginal wipes, 12% had used vaginal washes or cleansers – and 4% had used them internally.
When it launched OMV! in July, Vagisil said it had engaged 2,500 teens and their mothers in creating the product, which it said was “designed to meet the cleansing and care needs of a new generation of young women.”
That extension of a product most commonly used by adult women to teenagers – who often feel self-conscious about their bodies – is exactly what bothers Dr. Gunter. “BTW I am sorry I am subjecting you all to my @vagisil outrage, but preying on teens and amplifying patriarchal shame of normal bodily functions to sell an irritating product is not acceptable. I’m not stopping until they take that OMV! product line down everywhere,” she said in a Feb. 8 tweet that attracted more than 7,900 likes.
No ‘glow up’ needed
Dr. Gunter’s tweets tapped into collective anger over the shaming of women’s bodies. The OMV! marketing suggested that teens could get a “glow up” with the products.
“Your vulva doesn’t need a ‘glow up.’ It’s fine like it is. And if it’s not, talk to your doctor,” Dr. Jones said in her Feb. 8 video, which has had almost 350,000 views, with 28,000 likes and only 149 dislikes.
“They’re very clearly pathologizing normal physiology,” Dr. Jones says. “They’re creating language that makes people feel as though their normal bodily functions have to be somehow fixed or changed.”
Dr. Gunter says she specifically wanted to prevent Vagisil from leveraging social media to influence teen girls. With her stream of tweets and support from colleagues around the country, she has sparked a prolonged online conversation.
“I am encouraged by the strong response on social media from both other enraged ob.gyns. and health care professionals as well the response from a lot of women and men,” Dr. Gunter said in an interview. “We have effectively blocked [Vagisil] from using social media.”
In its response to this news organization, Vagisil noted, “We are a brand run by women with daughters of our own.” While defending the products, Vagisil acknowledged the criticisms: “We are always listening to our consumers and our expert partners so that we continuously evolve. We appreciate the perspective that our language choice surrounding periods may perpetuate an old idea and have already begun to make changes to address this.”
Dr. Gunter says she plans to stay on topic. “Given the number of people outraged, I suspect if they venture out on social media again the reaction will be swift,” she said. “Hopefully we have made OMV! toxic for influencers as well.”
In fact, she’s ready to take on “the entire predatory feminine hygiene market. I’m sick of their false claims about balancing pH and not-so-subtle suggestions that vaginas and vulvas and menstruation stink. These products cause psychological harm as well as physical harm from their irritants,” she said.
A version of this article first appeared on Medscape.com.