User login
Nationwide implementation of MIS reduced complications and increased survival in early-stage endometrial cancer
To determine if a nationwide implementation of robotic minimally invasive surgery (MIS) influenced the risk of severe complications and survival among women with early-stage endometrial cancer, a group of researchers from the University of Southern Denmark studied the Danish Gynecological Cancer Database, a nationwide, mandatory prospective registration of new cases of women with endometrial cancer who received their surgical treatment in a public hospital.1 Siv Joergensen, MD, reported results at the 47th AAGL Global Congress on Minimally Invasive Gynecology annual meeting on November 13, 2018, in Las Vegas, Nevada.
The transition to robotic MIS was undertaken in Denmark from 2008 to 2013, with the centralization of endometrial cancer treatment in 2012. Over the span of 10 years, the surgical approach to treatment changed from 97% open access surgery to 95% MIS.
For the prospective cohort study, more than 7,000 women with endometrial cancer who received a hysterectomy from January 2005 to June 2015 were grouped by those receiving surgical care before (group 1) and after (group 2) robotic MIS implementation in Denmark. A total of 5,654 women with FIGO Stage I–II endometrial cancer were included in the final study.
Severe complications were 7.3% in group 1 and 6.2% in group 2 (odds ratio, 1.38; 95% confidence interval [CI], 1.10–1.73). Five-year survival rates were significantly lower before robotic MIS was implemented (hazard ratio, 1.22; 95% CI, 1.05–1.41), and no difference was found between laparoscopic and robotic MIS.
The authors concluded that nationwide implementation of robotic MIS enabled a shift toward all types of MIS (with a 73% reduction in hysterectomies performed by laparotomy) and translated into reduced risk of severe complications and increased survival.
How do these results compare with those in the United States?
According to Erica Dun, MD, MPH, who provided commentary for Dr. Joergensen’s study, the United States adopted robotic MIS in the early 2000s. Around 2008, 14% of hysterectomies performed for early-stage endometrial cancer were done through a MIS approach.2 In 2014, after a study in which Walker and colleagues found that laparoscopy was safe and feasible compared with laparotomy,3 the American College of Obstetricians and Gynecologists, jointly with the Society of Gynecologic Oncologists, stated that “MIS should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”4
Dr. Dun pointed out that Casarin and colleagues found in 2018 that 71.4% of surgeries performed in the United States for endometrial cancer were performed through MIS.5 That number rose to 86.5% MIS (72.5% robot-assisted) for centers of the National Comprehensive Cancer Network.6
Dr. Dun concluded that nationwide implementation of robotic MIS is feasible for gynecologic oncologists, and it is beneficial for patients.
1. Joergensen SL. Nationwide implementation of robotic minimally invasive surgery for endometrial cancer increases survival and reduces complications. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
2. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041-1048.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
4. American College of Obstetricians and Gynecologists, Society of Gynecologic Oncologists. Practice bulletin no. 149: endometrial cancer. Obstet Gynecol. 2015;125:1006-1026.
5. Casarin J, et al. Adaptation of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet Gynecol. 2018;131:304-311.
6. Bergstrom, Aloisi A, Armbruster S, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480-484.
To determine if a nationwide implementation of robotic minimally invasive surgery (MIS) influenced the risk of severe complications and survival among women with early-stage endometrial cancer, a group of researchers from the University of Southern Denmark studied the Danish Gynecological Cancer Database, a nationwide, mandatory prospective registration of new cases of women with endometrial cancer who received their surgical treatment in a public hospital.1 Siv Joergensen, MD, reported results at the 47th AAGL Global Congress on Minimally Invasive Gynecology annual meeting on November 13, 2018, in Las Vegas, Nevada.
The transition to robotic MIS was undertaken in Denmark from 2008 to 2013, with the centralization of endometrial cancer treatment in 2012. Over the span of 10 years, the surgical approach to treatment changed from 97% open access surgery to 95% MIS.
For the prospective cohort study, more than 7,000 women with endometrial cancer who received a hysterectomy from January 2005 to June 2015 were grouped by those receiving surgical care before (group 1) and after (group 2) robotic MIS implementation in Denmark. A total of 5,654 women with FIGO Stage I–II endometrial cancer were included in the final study.
Severe complications were 7.3% in group 1 and 6.2% in group 2 (odds ratio, 1.38; 95% confidence interval [CI], 1.10–1.73). Five-year survival rates were significantly lower before robotic MIS was implemented (hazard ratio, 1.22; 95% CI, 1.05–1.41), and no difference was found between laparoscopic and robotic MIS.
The authors concluded that nationwide implementation of robotic MIS enabled a shift toward all types of MIS (with a 73% reduction in hysterectomies performed by laparotomy) and translated into reduced risk of severe complications and increased survival.
How do these results compare with those in the United States?
According to Erica Dun, MD, MPH, who provided commentary for Dr. Joergensen’s study, the United States adopted robotic MIS in the early 2000s. Around 2008, 14% of hysterectomies performed for early-stage endometrial cancer were done through a MIS approach.2 In 2014, after a study in which Walker and colleagues found that laparoscopy was safe and feasible compared with laparotomy,3 the American College of Obstetricians and Gynecologists, jointly with the Society of Gynecologic Oncologists, stated that “MIS should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”4
Dr. Dun pointed out that Casarin and colleagues found in 2018 that 71.4% of surgeries performed in the United States for endometrial cancer were performed through MIS.5 That number rose to 86.5% MIS (72.5% robot-assisted) for centers of the National Comprehensive Cancer Network.6
Dr. Dun concluded that nationwide implementation of robotic MIS is feasible for gynecologic oncologists, and it is beneficial for patients.
To determine if a nationwide implementation of robotic minimally invasive surgery (MIS) influenced the risk of severe complications and survival among women with early-stage endometrial cancer, a group of researchers from the University of Southern Denmark studied the Danish Gynecological Cancer Database, a nationwide, mandatory prospective registration of new cases of women with endometrial cancer who received their surgical treatment in a public hospital.1 Siv Joergensen, MD, reported results at the 47th AAGL Global Congress on Minimally Invasive Gynecology annual meeting on November 13, 2018, in Las Vegas, Nevada.
The transition to robotic MIS was undertaken in Denmark from 2008 to 2013, with the centralization of endometrial cancer treatment in 2012. Over the span of 10 years, the surgical approach to treatment changed from 97% open access surgery to 95% MIS.
For the prospective cohort study, more than 7,000 women with endometrial cancer who received a hysterectomy from January 2005 to June 2015 were grouped by those receiving surgical care before (group 1) and after (group 2) robotic MIS implementation in Denmark. A total of 5,654 women with FIGO Stage I–II endometrial cancer were included in the final study.
Severe complications were 7.3% in group 1 and 6.2% in group 2 (odds ratio, 1.38; 95% confidence interval [CI], 1.10–1.73). Five-year survival rates were significantly lower before robotic MIS was implemented (hazard ratio, 1.22; 95% CI, 1.05–1.41), and no difference was found between laparoscopic and robotic MIS.
The authors concluded that nationwide implementation of robotic MIS enabled a shift toward all types of MIS (with a 73% reduction in hysterectomies performed by laparotomy) and translated into reduced risk of severe complications and increased survival.
How do these results compare with those in the United States?
According to Erica Dun, MD, MPH, who provided commentary for Dr. Joergensen’s study, the United States adopted robotic MIS in the early 2000s. Around 2008, 14% of hysterectomies performed for early-stage endometrial cancer were done through a MIS approach.2 In 2014, after a study in which Walker and colleagues found that laparoscopy was safe and feasible compared with laparotomy,3 the American College of Obstetricians and Gynecologists, jointly with the Society of Gynecologic Oncologists, stated that “MIS should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”4
Dr. Dun pointed out that Casarin and colleagues found in 2018 that 71.4% of surgeries performed in the United States for endometrial cancer were performed through MIS.5 That number rose to 86.5% MIS (72.5% robot-assisted) for centers of the National Comprehensive Cancer Network.6
Dr. Dun concluded that nationwide implementation of robotic MIS is feasible for gynecologic oncologists, and it is beneficial for patients.
1. Joergensen SL. Nationwide implementation of robotic minimally invasive surgery for endometrial cancer increases survival and reduces complications. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
2. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041-1048.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
4. American College of Obstetricians and Gynecologists, Society of Gynecologic Oncologists. Practice bulletin no. 149: endometrial cancer. Obstet Gynecol. 2015;125:1006-1026.
5. Casarin J, et al. Adaptation of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet Gynecol. 2018;131:304-311.
6. Bergstrom, Aloisi A, Armbruster S, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480-484.
1. Joergensen SL. Nationwide implementation of robotic minimally invasive surgery for endometrial cancer increases survival and reduces complications. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
2. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041-1048.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
4. American College of Obstetricians and Gynecologists, Society of Gynecologic Oncologists. Practice bulletin no. 149: endometrial cancer. Obstet Gynecol. 2015;125:1006-1026.
5. Casarin J, et al. Adaptation of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet Gynecol. 2018;131:304-311.
6. Bergstrom, Aloisi A, Armbruster S, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480-484.
Patient-centric pain management decision aid reduces opioid use posthysterectomy
Investigators at the University of Michigan, Ann Arbor, found that a simple patient decision aid can be a useful tool in providing adequate postsurgical pain control to patients while reducing the number of opioid tablets in the community. The shared decision-making aid focuses on educating the patient about opioid use and engages her in an appropriate postoperative pain management plan. Results from this prospective quality improvement study were presented in a poster at the 47th AAGL Global Congress on Minimally Invasive Gynecology (Las Vegas, Nevada, November 11–15, 2018).1
Annmarie Vilkins, DO, and colleagues’ aim was to evaluate the impact of shared decision-making through the use of a patient decision aid targeting posthysterectomy pain management and opioid use. Can such a targeted strategy help decrease posthysterectomy opioid distribution in the community without compromising patient pain control or satisfaction?
The authors noted that more than 46 people die each day from an overdose involving prescription opioids.2 Studies have shown that patients actually use significantly fewer opioid tablets than the amount clinicians generally prescribe following ObGyn surgeries.3,4 Unused prescription opioid availability has the potential for accidental use or intentional misuse of the unneeded drugs by others.
Study methods
The investigators included all English-speaking patients undergoing hysterectomy for benign disease at their institution from March 1 through July 31, 2018. Data were analyzed from women undergoing laparoscopic, vaginal, or abdominal hysterectomy before (n = 195) and after (n = 177) the decision aid was implemented.
Preoperative education. In the preoperative area, patients were uniformly educated regarding postoperative pain expectations (for example, it is normal to have some pain; the goal is to manage your pain so you can function; some women do not require opioid medications after surgery), risks of opioid medications (such as dependence or addiction; misuse of leftover pills by others), adverse effects (drowsiness; confusion), and the recommended postoperative pain management schedule.
Postoperatively, pain medications included ibuprofen around the clock, acetaminophen as needed (used with caution when hydrocodone with acetaminophen was also prescribed), and opioids only if needed.
Discharge medication planning. Using a visual scale, the investigators then educated patients regarding the maximum number of opioid tablets permitted to be prescribed according to department guidelines and the average number of opioid tablets that a typical patient uses. The number of opioid tablets prescribed varied based on route of hysterectomy (laparoscopic, abdominal, or vaginal). For example, for a laparoscopic hysterectomy, the maximum allowed prescription for oxycodone was 20 tablets, while patients used an average number of 10 tablets.
The patient was then asked to choose her desired number of tablets with which she would like to be discharged.
Structured telephone calls were made to patients 2 weeks postoperatively.
Impact of the decision aid on opioid prescribing
Before implementation of the decision aid, the average number of opioid pills prescribed at discharge was 25 (median, 20–35), while that number dropped to 10 (median, 10–15) after the aid’s implementation. Similarly, the average oral morphine equivalents (OMEs) at time of discharge was 150 (interquartile range [IQR], 120–200) before decision aid implementation and 75 (IQR, 25–150) after decision aid implementation. Similar reductions in average OMEs were observed before and after the aid’s implementation across the 3 hysterectomy routes.
Continue to: According to the type of opioid...
According to the type of opioid prescribed at discharge, hydrocodone 5 mg was prescribed in 99 cases (50.8%) before decision aid implementation and in 14 cases (7.9%) after implementation. By contrast, oxycodone 5 mg was prescribed in 85 cases (43.6%) before implementation and in 149 cases (84.2%) after implementation.
The number of refill requests was similar before (n = 11 [5.6%]) and after (n = 12 [6.8%]) the aid’s implementation.
Tool reduced opioid availability in the community
The use of a simple patient decision aid—which focuses on opioid education and engages patients in an appropriate postoperative pain management plan—can result in fewer opioid tablets in the community while still providing adequate pain control, the authors concluded.
Online resource. For more on targeted strategies to optimize opioid prescribing after surgery, visit the University of Michigan’s Opioid Prescribing Engagement Network (OPEN) at http://michigan-open.org.
- Vilkins A, Till S, Lim R, et al. The impact of shared decision making on post-hysterectomy opioid prescribing. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349-358.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- As-Sanie S, Till S, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261-1268.
Investigators at the University of Michigan, Ann Arbor, found that a simple patient decision aid can be a useful tool in providing adequate postsurgical pain control to patients while reducing the number of opioid tablets in the community. The shared decision-making aid focuses on educating the patient about opioid use and engages her in an appropriate postoperative pain management plan. Results from this prospective quality improvement study were presented in a poster at the 47th AAGL Global Congress on Minimally Invasive Gynecology (Las Vegas, Nevada, November 11–15, 2018).1
Annmarie Vilkins, DO, and colleagues’ aim was to evaluate the impact of shared decision-making through the use of a patient decision aid targeting posthysterectomy pain management and opioid use. Can such a targeted strategy help decrease posthysterectomy opioid distribution in the community without compromising patient pain control or satisfaction?
The authors noted that more than 46 people die each day from an overdose involving prescription opioids.2 Studies have shown that patients actually use significantly fewer opioid tablets than the amount clinicians generally prescribe following ObGyn surgeries.3,4 Unused prescription opioid availability has the potential for accidental use or intentional misuse of the unneeded drugs by others.
Study methods
The investigators included all English-speaking patients undergoing hysterectomy for benign disease at their institution from March 1 through July 31, 2018. Data were analyzed from women undergoing laparoscopic, vaginal, or abdominal hysterectomy before (n = 195) and after (n = 177) the decision aid was implemented.
Preoperative education. In the preoperative area, patients were uniformly educated regarding postoperative pain expectations (for example, it is normal to have some pain; the goal is to manage your pain so you can function; some women do not require opioid medications after surgery), risks of opioid medications (such as dependence or addiction; misuse of leftover pills by others), adverse effects (drowsiness; confusion), and the recommended postoperative pain management schedule.
Postoperatively, pain medications included ibuprofen around the clock, acetaminophen as needed (used with caution when hydrocodone with acetaminophen was also prescribed), and opioids only if needed.
Discharge medication planning. Using a visual scale, the investigators then educated patients regarding the maximum number of opioid tablets permitted to be prescribed according to department guidelines and the average number of opioid tablets that a typical patient uses. The number of opioid tablets prescribed varied based on route of hysterectomy (laparoscopic, abdominal, or vaginal). For example, for a laparoscopic hysterectomy, the maximum allowed prescription for oxycodone was 20 tablets, while patients used an average number of 10 tablets.
The patient was then asked to choose her desired number of tablets with which she would like to be discharged.
Structured telephone calls were made to patients 2 weeks postoperatively.
Impact of the decision aid on opioid prescribing
Before implementation of the decision aid, the average number of opioid pills prescribed at discharge was 25 (median, 20–35), while that number dropped to 10 (median, 10–15) after the aid’s implementation. Similarly, the average oral morphine equivalents (OMEs) at time of discharge was 150 (interquartile range [IQR], 120–200) before decision aid implementation and 75 (IQR, 25–150) after decision aid implementation. Similar reductions in average OMEs were observed before and after the aid’s implementation across the 3 hysterectomy routes.
Continue to: According to the type of opioid...
According to the type of opioid prescribed at discharge, hydrocodone 5 mg was prescribed in 99 cases (50.8%) before decision aid implementation and in 14 cases (7.9%) after implementation. By contrast, oxycodone 5 mg was prescribed in 85 cases (43.6%) before implementation and in 149 cases (84.2%) after implementation.
The number of refill requests was similar before (n = 11 [5.6%]) and after (n = 12 [6.8%]) the aid’s implementation.
Tool reduced opioid availability in the community
The use of a simple patient decision aid—which focuses on opioid education and engages patients in an appropriate postoperative pain management plan—can result in fewer opioid tablets in the community while still providing adequate pain control, the authors concluded.
Online resource. For more on targeted strategies to optimize opioid prescribing after surgery, visit the University of Michigan’s Opioid Prescribing Engagement Network (OPEN) at http://michigan-open.org.
Investigators at the University of Michigan, Ann Arbor, found that a simple patient decision aid can be a useful tool in providing adequate postsurgical pain control to patients while reducing the number of opioid tablets in the community. The shared decision-making aid focuses on educating the patient about opioid use and engages her in an appropriate postoperative pain management plan. Results from this prospective quality improvement study were presented in a poster at the 47th AAGL Global Congress on Minimally Invasive Gynecology (Las Vegas, Nevada, November 11–15, 2018).1
Annmarie Vilkins, DO, and colleagues’ aim was to evaluate the impact of shared decision-making through the use of a patient decision aid targeting posthysterectomy pain management and opioid use. Can such a targeted strategy help decrease posthysterectomy opioid distribution in the community without compromising patient pain control or satisfaction?
The authors noted that more than 46 people die each day from an overdose involving prescription opioids.2 Studies have shown that patients actually use significantly fewer opioid tablets than the amount clinicians generally prescribe following ObGyn surgeries.3,4 Unused prescription opioid availability has the potential for accidental use or intentional misuse of the unneeded drugs by others.
Study methods
The investigators included all English-speaking patients undergoing hysterectomy for benign disease at their institution from March 1 through July 31, 2018. Data were analyzed from women undergoing laparoscopic, vaginal, or abdominal hysterectomy before (n = 195) and after (n = 177) the decision aid was implemented.
Preoperative education. In the preoperative area, patients were uniformly educated regarding postoperative pain expectations (for example, it is normal to have some pain; the goal is to manage your pain so you can function; some women do not require opioid medications after surgery), risks of opioid medications (such as dependence or addiction; misuse of leftover pills by others), adverse effects (drowsiness; confusion), and the recommended postoperative pain management schedule.
Postoperatively, pain medications included ibuprofen around the clock, acetaminophen as needed (used with caution when hydrocodone with acetaminophen was also prescribed), and opioids only if needed.
Discharge medication planning. Using a visual scale, the investigators then educated patients regarding the maximum number of opioid tablets permitted to be prescribed according to department guidelines and the average number of opioid tablets that a typical patient uses. The number of opioid tablets prescribed varied based on route of hysterectomy (laparoscopic, abdominal, or vaginal). For example, for a laparoscopic hysterectomy, the maximum allowed prescription for oxycodone was 20 tablets, while patients used an average number of 10 tablets.
The patient was then asked to choose her desired number of tablets with which she would like to be discharged.
Structured telephone calls were made to patients 2 weeks postoperatively.
Impact of the decision aid on opioid prescribing
Before implementation of the decision aid, the average number of opioid pills prescribed at discharge was 25 (median, 20–35), while that number dropped to 10 (median, 10–15) after the aid’s implementation. Similarly, the average oral morphine equivalents (OMEs) at time of discharge was 150 (interquartile range [IQR], 120–200) before decision aid implementation and 75 (IQR, 25–150) after decision aid implementation. Similar reductions in average OMEs were observed before and after the aid’s implementation across the 3 hysterectomy routes.
Continue to: According to the type of opioid...
According to the type of opioid prescribed at discharge, hydrocodone 5 mg was prescribed in 99 cases (50.8%) before decision aid implementation and in 14 cases (7.9%) after implementation. By contrast, oxycodone 5 mg was prescribed in 85 cases (43.6%) before implementation and in 149 cases (84.2%) after implementation.
The number of refill requests was similar before (n = 11 [5.6%]) and after (n = 12 [6.8%]) the aid’s implementation.
Tool reduced opioid availability in the community
The use of a simple patient decision aid—which focuses on opioid education and engages patients in an appropriate postoperative pain management plan—can result in fewer opioid tablets in the community while still providing adequate pain control, the authors concluded.
Online resource. For more on targeted strategies to optimize opioid prescribing after surgery, visit the University of Michigan’s Opioid Prescribing Engagement Network (OPEN) at http://michigan-open.org.
- Vilkins A, Till S, Lim R, et al. The impact of shared decision making on post-hysterectomy opioid prescribing. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349-358.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- As-Sanie S, Till S, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261-1268.
- Vilkins A, Till S, Lim R, et al. The impact of shared decision making on post-hysterectomy opioid prescribing. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349-358.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- As-Sanie S, Till S, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261-1268.
Most oral HRT linked to increased VTE risk
Transdermal hormone replacement therapy is associated with the lowest risk of venous thromboembolism, yet still is relatively underused compared to oral preparations, researchers say.
Writing in the BMJ, Yana Vinogradova, PhD, of the University of Nottingham (England) and her associates reported the results of two nested case-control studies of hormone replacement therapy (HRT) and venous thromboembolism (VTE) from Jan. 1998 to Feb. 2017 that altogether included 80,396 women aged 40-79 years with a primary diagnosis of VTE matched to 391,494 female controls.
Overall, 7% of the women with VTE had been exposed to HRT in the 90 days before the index date versus 5.5% of controls.
The greatest increase in risk of VTE, compared with no exposure, was seen with oral conjugated equine estrogen with medroxyprogesterone acetate, which was associated with a more than twofold increase in risk (odds ratio, 2.10; 95% confidence interval, 1.92-2.31; P less than .01).
However transdermal HRT use was not associated with any increase in risk, compared with no HRT exposure. The data even pointed to a slight decrease in risk, which the authors suggested may be the result of some residual confounding or indication bias.
Oral HRT generally was associated with a 58% increased risk of VTE, which amounted to a number needed to harm of 1,076 and nine extra cases of VTE per 10,000 women taking oral HRT.
Dr. Vinogradova and her colleagues noted that the vast majority of women in the study were being prescribed oral HRT for menopausal symptoms despite previous studies showing transdermal HRT has much lower risk.
“When women with menopausal symptoms already have an increased VTE risk because of comorbidities or obesity, these women and their doctors should give greater consideration to transdermal HRT,” they wrote.
Lubna Pal, MBBS, director of the menopause program and professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., commented in an interview, “These data are tremendously reassuring. The reported findings are: 1) reaffirm what we have already known , i.e. that advancing age, higher body mass index, and higher doses of exogenous systemic estrogen therapy are associated with increased risk for VTE; 2) offer greater granularity in risk for VTE with different formulations of estrogens and progestins and different regimens than understood thus far, 3) reaffirm that, unlike oral estrogen, transdermal estrogen formulations in doses commonly utilized in clinical practice are not associated with VTE risk, and 4) provide reassurance that the absolute risk, while exaggerated with oral estrogen or combination estrogen and progestin use, is nonetheless small as reflected in the number needed to harm with oral hormone therapy being 1,076, and the number of extra VTE cases attributable to oral HT being 9 per 10,000 woman years.
“The authors are to be commended on this massive analytic undertaking that allows an improved understanding of HRT-related risk for VTE and offers meaningful guidance to the practitioner,” said Dr. Pal, who was not involved in the study.*
Estrogen-only preparations had a 40% higher risk and combined preparations had a 73% higher risk, compared with no exposure.
In estrogen-only preparations, the lowest risk was seen with estradiol, compared with conjugated equine estrogens or combined preparations.
The lowest risk of VTE among oral preparations was seen with estradiol plus dydrogesterone, which only showed a nonsignificant 18% increase in risk.
In an attempt to account for possible increased risk of VTE, the authors conducted a sensitivity analysis in a subgroup of women who had not previously used anticoagulants, but they found similar results to the main analysis.
“This sensitivity analysis indicates that most of the excluded women had probably used anticoagulants because of atrial fibrillation or hip replacement operations rather than an earlier unrecorded VTE,” they wrote.
One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
SOURCE: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810.
*This article was updated 1/11/19.
Transdermal hormone replacement therapy is associated with the lowest risk of venous thromboembolism, yet still is relatively underused compared to oral preparations, researchers say.
Writing in the BMJ, Yana Vinogradova, PhD, of the University of Nottingham (England) and her associates reported the results of two nested case-control studies of hormone replacement therapy (HRT) and venous thromboembolism (VTE) from Jan. 1998 to Feb. 2017 that altogether included 80,396 women aged 40-79 years with a primary diagnosis of VTE matched to 391,494 female controls.
Overall, 7% of the women with VTE had been exposed to HRT in the 90 days before the index date versus 5.5% of controls.
The greatest increase in risk of VTE, compared with no exposure, was seen with oral conjugated equine estrogen with medroxyprogesterone acetate, which was associated with a more than twofold increase in risk (odds ratio, 2.10; 95% confidence interval, 1.92-2.31; P less than .01).
However transdermal HRT use was not associated with any increase in risk, compared with no HRT exposure. The data even pointed to a slight decrease in risk, which the authors suggested may be the result of some residual confounding or indication bias.
Oral HRT generally was associated with a 58% increased risk of VTE, which amounted to a number needed to harm of 1,076 and nine extra cases of VTE per 10,000 women taking oral HRT.
Dr. Vinogradova and her colleagues noted that the vast majority of women in the study were being prescribed oral HRT for menopausal symptoms despite previous studies showing transdermal HRT has much lower risk.
“When women with menopausal symptoms already have an increased VTE risk because of comorbidities or obesity, these women and their doctors should give greater consideration to transdermal HRT,” they wrote.
Lubna Pal, MBBS, director of the menopause program and professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., commented in an interview, “These data are tremendously reassuring. The reported findings are: 1) reaffirm what we have already known , i.e. that advancing age, higher body mass index, and higher doses of exogenous systemic estrogen therapy are associated with increased risk for VTE; 2) offer greater granularity in risk for VTE with different formulations of estrogens and progestins and different regimens than understood thus far, 3) reaffirm that, unlike oral estrogen, transdermal estrogen formulations in doses commonly utilized in clinical practice are not associated with VTE risk, and 4) provide reassurance that the absolute risk, while exaggerated with oral estrogen or combination estrogen and progestin use, is nonetheless small as reflected in the number needed to harm with oral hormone therapy being 1,076, and the number of extra VTE cases attributable to oral HT being 9 per 10,000 woman years.
“The authors are to be commended on this massive analytic undertaking that allows an improved understanding of HRT-related risk for VTE and offers meaningful guidance to the practitioner,” said Dr. Pal, who was not involved in the study.*
Estrogen-only preparations had a 40% higher risk and combined preparations had a 73% higher risk, compared with no exposure.
In estrogen-only preparations, the lowest risk was seen with estradiol, compared with conjugated equine estrogens or combined preparations.
The lowest risk of VTE among oral preparations was seen with estradiol plus dydrogesterone, which only showed a nonsignificant 18% increase in risk.
In an attempt to account for possible increased risk of VTE, the authors conducted a sensitivity analysis in a subgroup of women who had not previously used anticoagulants, but they found similar results to the main analysis.
“This sensitivity analysis indicates that most of the excluded women had probably used anticoagulants because of atrial fibrillation or hip replacement operations rather than an earlier unrecorded VTE,” they wrote.
One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
SOURCE: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810.
*This article was updated 1/11/19.
Transdermal hormone replacement therapy is associated with the lowest risk of venous thromboembolism, yet still is relatively underused compared to oral preparations, researchers say.
Writing in the BMJ, Yana Vinogradova, PhD, of the University of Nottingham (England) and her associates reported the results of two nested case-control studies of hormone replacement therapy (HRT) and venous thromboembolism (VTE) from Jan. 1998 to Feb. 2017 that altogether included 80,396 women aged 40-79 years with a primary diagnosis of VTE matched to 391,494 female controls.
Overall, 7% of the women with VTE had been exposed to HRT in the 90 days before the index date versus 5.5% of controls.
The greatest increase in risk of VTE, compared with no exposure, was seen with oral conjugated equine estrogen with medroxyprogesterone acetate, which was associated with a more than twofold increase in risk (odds ratio, 2.10; 95% confidence interval, 1.92-2.31; P less than .01).
However transdermal HRT use was not associated with any increase in risk, compared with no HRT exposure. The data even pointed to a slight decrease in risk, which the authors suggested may be the result of some residual confounding or indication bias.
Oral HRT generally was associated with a 58% increased risk of VTE, which amounted to a number needed to harm of 1,076 and nine extra cases of VTE per 10,000 women taking oral HRT.
Dr. Vinogradova and her colleagues noted that the vast majority of women in the study were being prescribed oral HRT for menopausal symptoms despite previous studies showing transdermal HRT has much lower risk.
“When women with menopausal symptoms already have an increased VTE risk because of comorbidities or obesity, these women and their doctors should give greater consideration to transdermal HRT,” they wrote.
Lubna Pal, MBBS, director of the menopause program and professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., commented in an interview, “These data are tremendously reassuring. The reported findings are: 1) reaffirm what we have already known , i.e. that advancing age, higher body mass index, and higher doses of exogenous systemic estrogen therapy are associated with increased risk for VTE; 2) offer greater granularity in risk for VTE with different formulations of estrogens and progestins and different regimens than understood thus far, 3) reaffirm that, unlike oral estrogen, transdermal estrogen formulations in doses commonly utilized in clinical practice are not associated with VTE risk, and 4) provide reassurance that the absolute risk, while exaggerated with oral estrogen or combination estrogen and progestin use, is nonetheless small as reflected in the number needed to harm with oral hormone therapy being 1,076, and the number of extra VTE cases attributable to oral HT being 9 per 10,000 woman years.
“The authors are to be commended on this massive analytic undertaking that allows an improved understanding of HRT-related risk for VTE and offers meaningful guidance to the practitioner,” said Dr. Pal, who was not involved in the study.*
Estrogen-only preparations had a 40% higher risk and combined preparations had a 73% higher risk, compared with no exposure.
In estrogen-only preparations, the lowest risk was seen with estradiol, compared with conjugated equine estrogens or combined preparations.
The lowest risk of VTE among oral preparations was seen with estradiol plus dydrogesterone, which only showed a nonsignificant 18% increase in risk.
In an attempt to account for possible increased risk of VTE, the authors conducted a sensitivity analysis in a subgroup of women who had not previously used anticoagulants, but they found similar results to the main analysis.
“This sensitivity analysis indicates that most of the excluded women had probably used anticoagulants because of atrial fibrillation or hip replacement operations rather than an earlier unrecorded VTE,” they wrote.
One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
SOURCE: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810.
*This article was updated 1/11/19.
FROM THE BMJ
Key clinical point: Transdermal HRT is not associated with any increase in VTE risk.
Major finding: Conjugated equine estrogen with medroxyprogesterone shows a twofold increase in VTE risk.
Study details: Nested case-control study in 80,396 women and 391,494 female controls.
Disclosures: One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
Source: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810
Should we abandon minimally invasive surgery for cervical cancer?
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
Managing menopausal vasomotor and genitourinary symptoms after breast cancer
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.
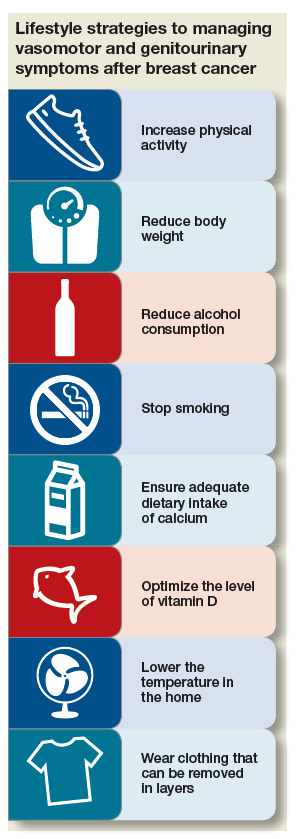
The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
The HPV vaccine is now recommended for adults aged 27–45: Counseling implications
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.
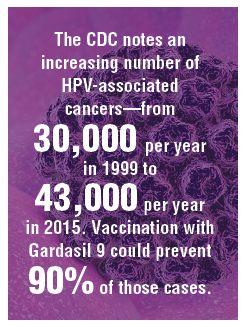
HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.
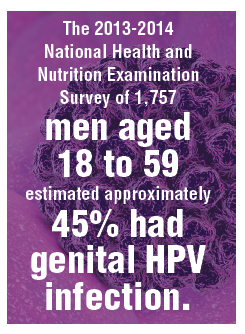
The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.

HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.

The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.

HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.

The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
Don’t leave vaginal hysterectomies behind, surgeon urges
LAS VEGAS –
While “younger trainees are seeing fewer vaginal procedures being done and have less confidence to do the procedure,” research suggests that the vaginal approach can offer major benefits, compared with the alternatives, Rosanne M. Kho, MD, of the Cleveland Clinic, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Kho pointed to several studies suggesting a decline in vaginal hysterectomies as laparoscopic and robot procedures become more common. One study compared hysterectomy surgery approaches during 2007-2010 and found a sharp rise in robotic procedures (0.5% to 10%) and a big decrease in abdominal procedures (from 54% to 40%). The rate of laparoscopic procedures grew (from 24% to 30%), while vaginal procedures dipped slightly (22% to 20%) (JAMA. 2013 Feb 20;309[7]:689-98). Another study tracked hysterectomy strategies at Pittsburgh’s Magee-Womens Hospital in almost 14,000 women during 2000-2010. It found that vaginal hysterectomy rates fell from 22% to 17% while laparoscopic rates grew remarkably from 3% to 43%. Open procedures fell dramatically from 75% to 36% (Am J Obstet Gynecol. 2013 Apr. doi: 10.1016/j.ajog.2013.01.022).
These findings are “telling me that surgeons are steering away from the vaginal approach because the laparoscopic and robotic approach are much more appealing,” Dr. Koh said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Specifically, it appears that surgeons think the vaginal hysterectomy is more “challenging” and “cumbersome,” Dr. Kho said, and they lack inadequate training.
Why should vaginal hysterectomy still be considered? Dr. Kho pointed to two pieces of evidence:
- Expert opinion. A 2017 committee opinion from the American College of Obstetricians and Gynecologists examined routes of hysterectomy in benign disease and declared that, despite the decrease in its use, “evidence supports the opinion that [when feasible] vaginal hysterectomy is associated with better outcomes” than are laparoscopic or abdominal hysterectomy. Also, the decision to perform a salpingo-oophorectomy is not necessarily a contraindication to performing a vaginal hysterectomy, according to the committee opinion (Obstet Gynecol. 2017 Jun;129[6]:e155-e9).The opinion also says, “the vaginal approach is preferred among the minimally invasive approaches. Laparoscopic hysterectomy is a preferable alternative to open abdominal hysterectomy for those patients in whom a vaginal hysterectomy is not indicated or feasible. Although minimally invasive approaches to hysterectomy are the preferred route, open abdominal hysterectomy remains an important surgical option for some patients.”
- Randomized, controlled studies. A 2015 Cochrane Library systematic review examined 47 randomized, controlled trials and found that “vaginal hysterectomy should be performed whenever possible. Where vaginal hysterectomy is not possible, both a laparoscopic approach and abdominal hysterectomy have their pros and cons, and these should be incorporated in the decision-making process” (Cochrane Database Syst Rev. 2015 Aug 12. doi: 10.1002/14651858.CD003677.pub5).
What if a patient has an enlarged uterus? Dr. Kho coauthored a 2017 review that suggested that vaginal hysterectomy may be appropriate in this case. Her report found that in women with large uteri, “vaginal hysterectomy is preferred over laparoscopic and laparoscopic assistance with less operative time and hospital cost. In morbidly obese patients with large uteri, total laparoscopic hysterectomy is superior to vaginal hysterectomy with lesser odds of blood transfusion and lower length of hospital stay” (Clin Obstet Gynecol. 2017 Jun;60[2]:286-95).
What about the removal of fallopian tubes – salpingectomy – during vaginal hysterectomy? Dr. Kho highlighted a 2017 decision analysis that said these procedures are frequently performed for cancer prevention during laparoscopic and open hysterectomies “but [fallopian tubes] are not routinely removed during vaginal hysterectomy because of perceptions of increased morbidity, difficulty, or inadequate surgical training.”
The analysis, however, determined that “salpingectomy should routinely be performed with vaginal hysterectomy because it was the dominant and therefore cost-effective strategy. Complications are minimally increased, but the trade-off with cancer prevention is highly favorable.” (Am J Obstet Gynecol. 2017 Nov;217[5]:603.e1-603.e6).
Dr. Kho reported consulting for AbbVie, Olympus, and Applied Medical.
LAS VEGAS –
While “younger trainees are seeing fewer vaginal procedures being done and have less confidence to do the procedure,” research suggests that the vaginal approach can offer major benefits, compared with the alternatives, Rosanne M. Kho, MD, of the Cleveland Clinic, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Kho pointed to several studies suggesting a decline in vaginal hysterectomies as laparoscopic and robot procedures become more common. One study compared hysterectomy surgery approaches during 2007-2010 and found a sharp rise in robotic procedures (0.5% to 10%) and a big decrease in abdominal procedures (from 54% to 40%). The rate of laparoscopic procedures grew (from 24% to 30%), while vaginal procedures dipped slightly (22% to 20%) (JAMA. 2013 Feb 20;309[7]:689-98). Another study tracked hysterectomy strategies at Pittsburgh’s Magee-Womens Hospital in almost 14,000 women during 2000-2010. It found that vaginal hysterectomy rates fell from 22% to 17% while laparoscopic rates grew remarkably from 3% to 43%. Open procedures fell dramatically from 75% to 36% (Am J Obstet Gynecol. 2013 Apr. doi: 10.1016/j.ajog.2013.01.022).
These findings are “telling me that surgeons are steering away from the vaginal approach because the laparoscopic and robotic approach are much more appealing,” Dr. Koh said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Specifically, it appears that surgeons think the vaginal hysterectomy is more “challenging” and “cumbersome,” Dr. Kho said, and they lack inadequate training.
Why should vaginal hysterectomy still be considered? Dr. Kho pointed to two pieces of evidence:
- Expert opinion. A 2017 committee opinion from the American College of Obstetricians and Gynecologists examined routes of hysterectomy in benign disease and declared that, despite the decrease in its use, “evidence supports the opinion that [when feasible] vaginal hysterectomy is associated with better outcomes” than are laparoscopic or abdominal hysterectomy. Also, the decision to perform a salpingo-oophorectomy is not necessarily a contraindication to performing a vaginal hysterectomy, according to the committee opinion (Obstet Gynecol. 2017 Jun;129[6]:e155-e9).The opinion also says, “the vaginal approach is preferred among the minimally invasive approaches. Laparoscopic hysterectomy is a preferable alternative to open abdominal hysterectomy for those patients in whom a vaginal hysterectomy is not indicated or feasible. Although minimally invasive approaches to hysterectomy are the preferred route, open abdominal hysterectomy remains an important surgical option for some patients.”
- Randomized, controlled studies. A 2015 Cochrane Library systematic review examined 47 randomized, controlled trials and found that “vaginal hysterectomy should be performed whenever possible. Where vaginal hysterectomy is not possible, both a laparoscopic approach and abdominal hysterectomy have their pros and cons, and these should be incorporated in the decision-making process” (Cochrane Database Syst Rev. 2015 Aug 12. doi: 10.1002/14651858.CD003677.pub5).
What if a patient has an enlarged uterus? Dr. Kho coauthored a 2017 review that suggested that vaginal hysterectomy may be appropriate in this case. Her report found that in women with large uteri, “vaginal hysterectomy is preferred over laparoscopic and laparoscopic assistance with less operative time and hospital cost. In morbidly obese patients with large uteri, total laparoscopic hysterectomy is superior to vaginal hysterectomy with lesser odds of blood transfusion and lower length of hospital stay” (Clin Obstet Gynecol. 2017 Jun;60[2]:286-95).
What about the removal of fallopian tubes – salpingectomy – during vaginal hysterectomy? Dr. Kho highlighted a 2017 decision analysis that said these procedures are frequently performed for cancer prevention during laparoscopic and open hysterectomies “but [fallopian tubes] are not routinely removed during vaginal hysterectomy because of perceptions of increased morbidity, difficulty, or inadequate surgical training.”
The analysis, however, determined that “salpingectomy should routinely be performed with vaginal hysterectomy because it was the dominant and therefore cost-effective strategy. Complications are minimally increased, but the trade-off with cancer prevention is highly favorable.” (Am J Obstet Gynecol. 2017 Nov;217[5]:603.e1-603.e6).
Dr. Kho reported consulting for AbbVie, Olympus, and Applied Medical.
LAS VEGAS –
While “younger trainees are seeing fewer vaginal procedures being done and have less confidence to do the procedure,” research suggests that the vaginal approach can offer major benefits, compared with the alternatives, Rosanne M. Kho, MD, of the Cleveland Clinic, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Kho pointed to several studies suggesting a decline in vaginal hysterectomies as laparoscopic and robot procedures become more common. One study compared hysterectomy surgery approaches during 2007-2010 and found a sharp rise in robotic procedures (0.5% to 10%) and a big decrease in abdominal procedures (from 54% to 40%). The rate of laparoscopic procedures grew (from 24% to 30%), while vaginal procedures dipped slightly (22% to 20%) (JAMA. 2013 Feb 20;309[7]:689-98). Another study tracked hysterectomy strategies at Pittsburgh’s Magee-Womens Hospital in almost 14,000 women during 2000-2010. It found that vaginal hysterectomy rates fell from 22% to 17% while laparoscopic rates grew remarkably from 3% to 43%. Open procedures fell dramatically from 75% to 36% (Am J Obstet Gynecol. 2013 Apr. doi: 10.1016/j.ajog.2013.01.022).
These findings are “telling me that surgeons are steering away from the vaginal approach because the laparoscopic and robotic approach are much more appealing,” Dr. Koh said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Specifically, it appears that surgeons think the vaginal hysterectomy is more “challenging” and “cumbersome,” Dr. Kho said, and they lack inadequate training.
Why should vaginal hysterectomy still be considered? Dr. Kho pointed to two pieces of evidence:
- Expert opinion. A 2017 committee opinion from the American College of Obstetricians and Gynecologists examined routes of hysterectomy in benign disease and declared that, despite the decrease in its use, “evidence supports the opinion that [when feasible] vaginal hysterectomy is associated with better outcomes” than are laparoscopic or abdominal hysterectomy. Also, the decision to perform a salpingo-oophorectomy is not necessarily a contraindication to performing a vaginal hysterectomy, according to the committee opinion (Obstet Gynecol. 2017 Jun;129[6]:e155-e9).The opinion also says, “the vaginal approach is preferred among the minimally invasive approaches. Laparoscopic hysterectomy is a preferable alternative to open abdominal hysterectomy for those patients in whom a vaginal hysterectomy is not indicated or feasible. Although minimally invasive approaches to hysterectomy are the preferred route, open abdominal hysterectomy remains an important surgical option for some patients.”
- Randomized, controlled studies. A 2015 Cochrane Library systematic review examined 47 randomized, controlled trials and found that “vaginal hysterectomy should be performed whenever possible. Where vaginal hysterectomy is not possible, both a laparoscopic approach and abdominal hysterectomy have their pros and cons, and these should be incorporated in the decision-making process” (Cochrane Database Syst Rev. 2015 Aug 12. doi: 10.1002/14651858.CD003677.pub5).
What if a patient has an enlarged uterus? Dr. Kho coauthored a 2017 review that suggested that vaginal hysterectomy may be appropriate in this case. Her report found that in women with large uteri, “vaginal hysterectomy is preferred over laparoscopic and laparoscopic assistance with less operative time and hospital cost. In morbidly obese patients with large uteri, total laparoscopic hysterectomy is superior to vaginal hysterectomy with lesser odds of blood transfusion and lower length of hospital stay” (Clin Obstet Gynecol. 2017 Jun;60[2]:286-95).
What about the removal of fallopian tubes – salpingectomy – during vaginal hysterectomy? Dr. Kho highlighted a 2017 decision analysis that said these procedures are frequently performed for cancer prevention during laparoscopic and open hysterectomies “but [fallopian tubes] are not routinely removed during vaginal hysterectomy because of perceptions of increased morbidity, difficulty, or inadequate surgical training.”
The analysis, however, determined that “salpingectomy should routinely be performed with vaginal hysterectomy because it was the dominant and therefore cost-effective strategy. Complications are minimally increased, but the trade-off with cancer prevention is highly favorable.” (Am J Obstet Gynecol. 2017 Nov;217[5]:603.e1-603.e6).
Dr. Kho reported consulting for AbbVie, Olympus, and Applied Medical.
EXPERT ANALYSIS FROM PAGS
No change in postoperative pain with restrictive opioid protocol
Opioid prescriptions after gynecologic surgery can be significantly reduced without impacting postoperative pain scores or complication rates, according to a paper published in JAMA Network Open.
A tertiary care comprehensive care center implemented an ultrarestrictive opioid prescription protocol (UROPP) then evaluated the outcomes in a case-control study involving 605 women undergoing gynecologic surgery, compared with 626 controls treated before implementation of the new protocol.
The ultrarestrictive protocol was prompted by frequent inquiries from patients who had used very little of their prescribed opioids after surgery and wanted to know what to do with the unused pills.
The new protocol involved a short preoperative counseling session about postoperative pain management. Following that, ambulatory surgery, minimally invasive surgery, or laparotomy patients were prescribed a 7-day supply of nonopioid pain relief. Laparotomy patients were also prescribed a 3-day supply of an oral opioid.
Any patients who required more than five opioid doses in the 24 hours before discharge were also prescribed a 3-day supply of opioid pain medication as needed, and all patients had the option of requesting an additional 3-day opioid refill.
Researchers saw no significant differences between the two groups in mean postoperative pain scores 2 weeks after surgery, and a similar number of patients in each group requested an opioid refill. There was also no significant difference in the number of postoperative complications between groups.
Implementation of the ultrarestrictive protocol was associated with significant declines in the mean number of opioid pills prescribed dropped from 31.7 to 3.5 in all surgical cases, from 43.6 to 12.1 in the laparotomy group, from 38.4 to 1.3 in the minimally invasive surgery group, and from 13.9 to 0.2 in patients who underwent ambulatory surgery.
“These data suggest that the implementation of a UROPP in a large surgical service is feasible and safe and was associated with a significantly decreased number of opioids dispensed during the perioperative period, particularly among opioid-naive patients,” wrote Jaron Mark, MD, of the department of gynecologic oncology at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and his coauthors. “The opioid-sparing effect was also marked and statistically significant in the laparotomy group, where most patients remained physically active and recovered well with no negative sequelae or elevated pain score after surgery.”
The researchers also noted that patients who were discharged home with an opioid prescription were more likely to call and request a refill within 30 days, compared with patients who did not receive opioids at discharge.
The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
SOURCE: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
The ultrarestrictive postoperative opioid prescribing protocol described in this study is a promising strategy for reducing opioid prescribing without increasing pain and limiting the potential for diversion and misuse of opioids. An important element of this protocol is the preoperative counseling, because setting patient expectations is likely to be an important factor in improving postoperative outcomes.
It is also worth noting that this study focused on patients undergoing major and minor gynecologic surgery, so more research is needed to explore these outcomes particularly among patients undergoing procedures that may be associated with a higher risk of persistent postoperative pain and/or opioid use. It is also a management strategy explored in patients at low risk of chronic postoperative opioid use, but a similar pathway should be developed and explored in more high-risk patients.
Dr. Jennifer M. Hah is from the department of anesthesiology, perioperative, and pain management at Stanford University (Calif.). These comments are taken from an accompanying editorial (JAMA Network Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5432). No conflicts of interest were reported.
The ultrarestrictive postoperative opioid prescribing protocol described in this study is a promising strategy for reducing opioid prescribing without increasing pain and limiting the potential for diversion and misuse of opioids. An important element of this protocol is the preoperative counseling, because setting patient expectations is likely to be an important factor in improving postoperative outcomes.
It is also worth noting that this study focused on patients undergoing major and minor gynecologic surgery, so more research is needed to explore these outcomes particularly among patients undergoing procedures that may be associated with a higher risk of persistent postoperative pain and/or opioid use. It is also a management strategy explored in patients at low risk of chronic postoperative opioid use, but a similar pathway should be developed and explored in more high-risk patients.
Dr. Jennifer M. Hah is from the department of anesthesiology, perioperative, and pain management at Stanford University (Calif.). These comments are taken from an accompanying editorial (JAMA Network Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5432). No conflicts of interest were reported.
The ultrarestrictive postoperative opioid prescribing protocol described in this study is a promising strategy for reducing opioid prescribing without increasing pain and limiting the potential for diversion and misuse of opioids. An important element of this protocol is the preoperative counseling, because setting patient expectations is likely to be an important factor in improving postoperative outcomes.
It is also worth noting that this study focused on patients undergoing major and minor gynecologic surgery, so more research is needed to explore these outcomes particularly among patients undergoing procedures that may be associated with a higher risk of persistent postoperative pain and/or opioid use. It is also a management strategy explored in patients at low risk of chronic postoperative opioid use, but a similar pathway should be developed and explored in more high-risk patients.
Dr. Jennifer M. Hah is from the department of anesthesiology, perioperative, and pain management at Stanford University (Calif.). These comments are taken from an accompanying editorial (JAMA Network Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5432). No conflicts of interest were reported.
Opioid prescriptions after gynecologic surgery can be significantly reduced without impacting postoperative pain scores or complication rates, according to a paper published in JAMA Network Open.
A tertiary care comprehensive care center implemented an ultrarestrictive opioid prescription protocol (UROPP) then evaluated the outcomes in a case-control study involving 605 women undergoing gynecologic surgery, compared with 626 controls treated before implementation of the new protocol.
The ultrarestrictive protocol was prompted by frequent inquiries from patients who had used very little of their prescribed opioids after surgery and wanted to know what to do with the unused pills.
The new protocol involved a short preoperative counseling session about postoperative pain management. Following that, ambulatory surgery, minimally invasive surgery, or laparotomy patients were prescribed a 7-day supply of nonopioid pain relief. Laparotomy patients were also prescribed a 3-day supply of an oral opioid.
Any patients who required more than five opioid doses in the 24 hours before discharge were also prescribed a 3-day supply of opioid pain medication as needed, and all patients had the option of requesting an additional 3-day opioid refill.
Researchers saw no significant differences between the two groups in mean postoperative pain scores 2 weeks after surgery, and a similar number of patients in each group requested an opioid refill. There was also no significant difference in the number of postoperative complications between groups.
Implementation of the ultrarestrictive protocol was associated with significant declines in the mean number of opioid pills prescribed dropped from 31.7 to 3.5 in all surgical cases, from 43.6 to 12.1 in the laparotomy group, from 38.4 to 1.3 in the minimally invasive surgery group, and from 13.9 to 0.2 in patients who underwent ambulatory surgery.
“These data suggest that the implementation of a UROPP in a large surgical service is feasible and safe and was associated with a significantly decreased number of opioids dispensed during the perioperative period, particularly among opioid-naive patients,” wrote Jaron Mark, MD, of the department of gynecologic oncology at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and his coauthors. “The opioid-sparing effect was also marked and statistically significant in the laparotomy group, where most patients remained physically active and recovered well with no negative sequelae or elevated pain score after surgery.”
The researchers also noted that patients who were discharged home with an opioid prescription were more likely to call and request a refill within 30 days, compared with patients who did not receive opioids at discharge.
The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
SOURCE: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
Opioid prescriptions after gynecologic surgery can be significantly reduced without impacting postoperative pain scores or complication rates, according to a paper published in JAMA Network Open.
A tertiary care comprehensive care center implemented an ultrarestrictive opioid prescription protocol (UROPP) then evaluated the outcomes in a case-control study involving 605 women undergoing gynecologic surgery, compared with 626 controls treated before implementation of the new protocol.
The ultrarestrictive protocol was prompted by frequent inquiries from patients who had used very little of their prescribed opioids after surgery and wanted to know what to do with the unused pills.
The new protocol involved a short preoperative counseling session about postoperative pain management. Following that, ambulatory surgery, minimally invasive surgery, or laparotomy patients were prescribed a 7-day supply of nonopioid pain relief. Laparotomy patients were also prescribed a 3-day supply of an oral opioid.
Any patients who required more than five opioid doses in the 24 hours before discharge were also prescribed a 3-day supply of opioid pain medication as needed, and all patients had the option of requesting an additional 3-day opioid refill.
Researchers saw no significant differences between the two groups in mean postoperative pain scores 2 weeks after surgery, and a similar number of patients in each group requested an opioid refill. There was also no significant difference in the number of postoperative complications between groups.
Implementation of the ultrarestrictive protocol was associated with significant declines in the mean number of opioid pills prescribed dropped from 31.7 to 3.5 in all surgical cases, from 43.6 to 12.1 in the laparotomy group, from 38.4 to 1.3 in the minimally invasive surgery group, and from 13.9 to 0.2 in patients who underwent ambulatory surgery.
“These data suggest that the implementation of a UROPP in a large surgical service is feasible and safe and was associated with a significantly decreased number of opioids dispensed during the perioperative period, particularly among opioid-naive patients,” wrote Jaron Mark, MD, of the department of gynecologic oncology at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and his coauthors. “The opioid-sparing effect was also marked and statistically significant in the laparotomy group, where most patients remained physically active and recovered well with no negative sequelae or elevated pain score after surgery.”
The researchers also noted that patients who were discharged home with an opioid prescription were more likely to call and request a refill within 30 days, compared with patients who did not receive opioids at discharge.
The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
SOURCE: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
Key clinical point: A ultrarestrictive postoperative opioid protocol is not associated with higher postoperative pain scores.
Major finding: The protocol achieves significant reductions in opioid use.
Study details: A case-control study in 1,231 women undergoing gynecologic surgery.
Disclosures: The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute, and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
Source: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
Black women more likely to have open hysterectomies
Black women were more likely than white women to undergo open hysterectomy, according to a recent analysis of national surgical data by Amy L. Alexander, MD, MS, of Northwestern University, Chicago, and her colleagues.
Even after the researchers controlled for many factors that might influence surgical approach, such as comorbidities and body mass index, black women had an odds ratio of 2.02 to receive open, rather than laparoscopic, hysterectomy (95% confidence interval, 1.85-2.20), according to a study in Obstetrics & Gynecology.
The analysis of the targeted hysterectomy file in the National Surgical Quality Improvement Program (NSQIP) database showed that, of 15,316 women who had hysterectomy for nonmalignant indications, the 25% who were black also were more likely to have major complications with open procedures. Such complications as sepsis, wound dehiscence, prolonged intubation, and death were seen in 4% of the black women, versus 2% of the white women receiving open hysterectomy (P less than .001). Minor complications, such as urinary tract infections, superficial wound infections, and blood transfusions, also were more common for black women having open procedures (11% vs. 7%; P less than .001).
The study used a large national database with detailed information about comorbidities and patient characteristics to look at racial disparities in surgical route and complications for the second-most-common surgical procedure women receive on the United States. The results, said Dr. Alexander and her coauthors, confirm and extend previous work showing these disparities.
Black women are known to have more diabetes and hypertension, as well as higher rates of obesity, compared with white women, wrote Dr. Alexander and her coauthors. Even after they controlled for all these variables, black women still had significantly higher odds of having complications from hysterectomy: The odds ratios for major and minor complications were 1.56 and 1.27, respectively.
Uterine weight was included and tracked as a binary variable, with large uteri considered those weighing 250 g or more. Making uterine weight a binary, rather than continuous or categorical variable, didn’t significantly change results, and realistically mirrors a surgeon’s assessment of a uterus as “large” or “small” when making treatment decisions, Dr. Alexander and her coauthors said.
Because the median weight of uteri from black women was more than double the weight of those from white women (262 g vs. 123 g), the investigators also performed an analysis looking just at women with uterine weight less than 250 g, to ensure that uterine weight alone was not accounting for much of the disparity. In this analysis, the black patients still had an adjusted odds ratio of 1.84 for receiving an open procedure.
“Some of the postoperative complications experienced by black women are likely attributable to the fact that black women are more likely to undergo an open hysterectomy,” noted Dr. Alexander and her colleagues. “However, because black race is still associated with a higher odds of complications, even when adjusting for hysterectomy route, there are other contributing factors that warrant further investigation.” Among these factors, they said, may be access to care and quality of care while hospitalized.
The study’s strengths include the use of the NSQIP database’s prospectively collected data to construct the cohort study; the data base included “important patient-level factors such as uterine size, obesity, and comorbidities not previously available in other secondary data set studies,” noted Dr. Alexander and her colleagues. But the possibility of unmeasured bias persists, they said, and such variables as regional practice patterns and surgeon experience and procedure volume could not be detected from the NSQIP data on hand.
“This study suggests that an important step to reduce the disparity in route of surgery and postoperative complications is to increase access to and use of minimally invasive surgery,” wrote Dr. Alexander and her coauthors.
The study was funded by the National Institutes of Health. Dr. Alexander and her colleagues reported no conflicts of interest.
koakes@mdedge.com
SOURCE: Alexander AL et al. Obstet Gynecol. 2018 Dec 4. doi: 10.1097/AOG.0000000000002990
The discrepancies reported by Dr. Alexander and her colleagues between black and white women undergoing hysterectomy for nonmalignant reasons mirror discrepancies found for other oncologic procedures, including colectomy and prostatectomy.
Findings of the current study show that black women continue to be less likely to receive more modern, minimally invasive procedures, and that outcomes are worse for these women, even after controlling for factors that may contribute to complications.
The American College of Obstetricians and Gynecologists (ACOG) has looked at this issue. In a 2015 opinion, an ACOG committee looked at three categories of factors contributing to racial disparities in women’s health care. Patient-level factors include such things as genetics, medical comorbidities, and patient preferences and adherence. Health care system–level factors include insurance status and geographic barriers to accessing care, while stereotyping and implicit bias on the part of practitioners constitute the third set of factors.
All these factors are likely in play for the discrepancies seen in hysterectomy rates. Those who care for black women need to understand the long, shameful history of how black Americans were mistreated, undertreated, and used for medical experimentation without consent. This past shapes present care and contributes to the difficulty black patients have trusting today’s health care systems.
The important question today is how individual health care providers will address their own biases, learn from the past, and move forward to do better for our black patients.
Shanna N. Wingo, MD , is an ob.gyn. and a gynecologic oncologist in private practice in Phoenix. She had no potential conflicts of interest. These remarks were drawn from an editorial accompanying the study by Dr. Alexander et al.( Obstet Gynecol. 2018 Dec 4;133[1]:4-5 ).
The discrepancies reported by Dr. Alexander and her colleagues between black and white women undergoing hysterectomy for nonmalignant reasons mirror discrepancies found for other oncologic procedures, including colectomy and prostatectomy.
Findings of the current study show that black women continue to be less likely to receive more modern, minimally invasive procedures, and that outcomes are worse for these women, even after controlling for factors that may contribute to complications.
The American College of Obstetricians and Gynecologists (ACOG) has looked at this issue. In a 2015 opinion, an ACOG committee looked at three categories of factors contributing to racial disparities in women’s health care. Patient-level factors include such things as genetics, medical comorbidities, and patient preferences and adherence. Health care system–level factors include insurance status and geographic barriers to accessing care, while stereotyping and implicit bias on the part of practitioners constitute the third set of factors.
All these factors are likely in play for the discrepancies seen in hysterectomy rates. Those who care for black women need to understand the long, shameful history of how black Americans were mistreated, undertreated, and used for medical experimentation without consent. This past shapes present care and contributes to the difficulty black patients have trusting today’s health care systems.
The important question today is how individual health care providers will address their own biases, learn from the past, and move forward to do better for our black patients.
Shanna N. Wingo, MD , is an ob.gyn. and a gynecologic oncologist in private practice in Phoenix. She had no potential conflicts of interest. These remarks were drawn from an editorial accompanying the study by Dr. Alexander et al.( Obstet Gynecol. 2018 Dec 4;133[1]:4-5 ).
The discrepancies reported by Dr. Alexander and her colleagues between black and white women undergoing hysterectomy for nonmalignant reasons mirror discrepancies found for other oncologic procedures, including colectomy and prostatectomy.
Findings of the current study show that black women continue to be less likely to receive more modern, minimally invasive procedures, and that outcomes are worse for these women, even after controlling for factors that may contribute to complications.
The American College of Obstetricians and Gynecologists (ACOG) has looked at this issue. In a 2015 opinion, an ACOG committee looked at three categories of factors contributing to racial disparities in women’s health care. Patient-level factors include such things as genetics, medical comorbidities, and patient preferences and adherence. Health care system–level factors include insurance status and geographic barriers to accessing care, while stereotyping and implicit bias on the part of practitioners constitute the third set of factors.
All these factors are likely in play for the discrepancies seen in hysterectomy rates. Those who care for black women need to understand the long, shameful history of how black Americans were mistreated, undertreated, and used for medical experimentation without consent. This past shapes present care and contributes to the difficulty black patients have trusting today’s health care systems.
The important question today is how individual health care providers will address their own biases, learn from the past, and move forward to do better for our black patients.
Shanna N. Wingo, MD , is an ob.gyn. and a gynecologic oncologist in private practice in Phoenix. She had no potential conflicts of interest. These remarks were drawn from an editorial accompanying the study by Dr. Alexander et al.( Obstet Gynecol. 2018 Dec 4;133[1]:4-5 ).
Black women were more likely than white women to undergo open hysterectomy, according to a recent analysis of national surgical data by Amy L. Alexander, MD, MS, of Northwestern University, Chicago, and her colleagues.
Even after the researchers controlled for many factors that might influence surgical approach, such as comorbidities and body mass index, black women had an odds ratio of 2.02 to receive open, rather than laparoscopic, hysterectomy (95% confidence interval, 1.85-2.20), according to a study in Obstetrics & Gynecology.
The analysis of the targeted hysterectomy file in the National Surgical Quality Improvement Program (NSQIP) database showed that, of 15,316 women who had hysterectomy for nonmalignant indications, the 25% who were black also were more likely to have major complications with open procedures. Such complications as sepsis, wound dehiscence, prolonged intubation, and death were seen in 4% of the black women, versus 2% of the white women receiving open hysterectomy (P less than .001). Minor complications, such as urinary tract infections, superficial wound infections, and blood transfusions, also were more common for black women having open procedures (11% vs. 7%; P less than .001).
The study used a large national database with detailed information about comorbidities and patient characteristics to look at racial disparities in surgical route and complications for the second-most-common surgical procedure women receive on the United States. The results, said Dr. Alexander and her coauthors, confirm and extend previous work showing these disparities.
Black women are known to have more diabetes and hypertension, as well as higher rates of obesity, compared with white women, wrote Dr. Alexander and her coauthors. Even after they controlled for all these variables, black women still had significantly higher odds of having complications from hysterectomy: The odds ratios for major and minor complications were 1.56 and 1.27, respectively.
Uterine weight was included and tracked as a binary variable, with large uteri considered those weighing 250 g or more. Making uterine weight a binary, rather than continuous or categorical variable, didn’t significantly change results, and realistically mirrors a surgeon’s assessment of a uterus as “large” or “small” when making treatment decisions, Dr. Alexander and her coauthors said.
Because the median weight of uteri from black women was more than double the weight of those from white women (262 g vs. 123 g), the investigators also performed an analysis looking just at women with uterine weight less than 250 g, to ensure that uterine weight alone was not accounting for much of the disparity. In this analysis, the black patients still had an adjusted odds ratio of 1.84 for receiving an open procedure.
“Some of the postoperative complications experienced by black women are likely attributable to the fact that black women are more likely to undergo an open hysterectomy,” noted Dr. Alexander and her colleagues. “However, because black race is still associated with a higher odds of complications, even when adjusting for hysterectomy route, there are other contributing factors that warrant further investigation.” Among these factors, they said, may be access to care and quality of care while hospitalized.
The study’s strengths include the use of the NSQIP database’s prospectively collected data to construct the cohort study; the data base included “important patient-level factors such as uterine size, obesity, and comorbidities not previously available in other secondary data set studies,” noted Dr. Alexander and her colleagues. But the possibility of unmeasured bias persists, they said, and such variables as regional practice patterns and surgeon experience and procedure volume could not be detected from the NSQIP data on hand.
“This study suggests that an important step to reduce the disparity in route of surgery and postoperative complications is to increase access to and use of minimally invasive surgery,” wrote Dr. Alexander and her coauthors.
The study was funded by the National Institutes of Health. Dr. Alexander and her colleagues reported no conflicts of interest.
koakes@mdedge.com
SOURCE: Alexander AL et al. Obstet Gynecol. 2018 Dec 4. doi: 10.1097/AOG.0000000000002990
Black women were more likely than white women to undergo open hysterectomy, according to a recent analysis of national surgical data by Amy L. Alexander, MD, MS, of Northwestern University, Chicago, and her colleagues.
Even after the researchers controlled for many factors that might influence surgical approach, such as comorbidities and body mass index, black women had an odds ratio of 2.02 to receive open, rather than laparoscopic, hysterectomy (95% confidence interval, 1.85-2.20), according to a study in Obstetrics & Gynecology.
The analysis of the targeted hysterectomy file in the National Surgical Quality Improvement Program (NSQIP) database showed that, of 15,316 women who had hysterectomy for nonmalignant indications, the 25% who were black also were more likely to have major complications with open procedures. Such complications as sepsis, wound dehiscence, prolonged intubation, and death were seen in 4% of the black women, versus 2% of the white women receiving open hysterectomy (P less than .001). Minor complications, such as urinary tract infections, superficial wound infections, and blood transfusions, also were more common for black women having open procedures (11% vs. 7%; P less than .001).
The study used a large national database with detailed information about comorbidities and patient characteristics to look at racial disparities in surgical route and complications for the second-most-common surgical procedure women receive on the United States. The results, said Dr. Alexander and her coauthors, confirm and extend previous work showing these disparities.
Black women are known to have more diabetes and hypertension, as well as higher rates of obesity, compared with white women, wrote Dr. Alexander and her coauthors. Even after they controlled for all these variables, black women still had significantly higher odds of having complications from hysterectomy: The odds ratios for major and minor complications were 1.56 and 1.27, respectively.
Uterine weight was included and tracked as a binary variable, with large uteri considered those weighing 250 g or more. Making uterine weight a binary, rather than continuous or categorical variable, didn’t significantly change results, and realistically mirrors a surgeon’s assessment of a uterus as “large” or “small” when making treatment decisions, Dr. Alexander and her coauthors said.
Because the median weight of uteri from black women was more than double the weight of those from white women (262 g vs. 123 g), the investigators also performed an analysis looking just at women with uterine weight less than 250 g, to ensure that uterine weight alone was not accounting for much of the disparity. In this analysis, the black patients still had an adjusted odds ratio of 1.84 for receiving an open procedure.
“Some of the postoperative complications experienced by black women are likely attributable to the fact that black women are more likely to undergo an open hysterectomy,” noted Dr. Alexander and her colleagues. “However, because black race is still associated with a higher odds of complications, even when adjusting for hysterectomy route, there are other contributing factors that warrant further investigation.” Among these factors, they said, may be access to care and quality of care while hospitalized.
The study’s strengths include the use of the NSQIP database’s prospectively collected data to construct the cohort study; the data base included “important patient-level factors such as uterine size, obesity, and comorbidities not previously available in other secondary data set studies,” noted Dr. Alexander and her colleagues. But the possibility of unmeasured bias persists, they said, and such variables as regional practice patterns and surgeon experience and procedure volume could not be detected from the NSQIP data on hand.
“This study suggests that an important step to reduce the disparity in route of surgery and postoperative complications is to increase access to and use of minimally invasive surgery,” wrote Dr. Alexander and her coauthors.
The study was funded by the National Institutes of Health. Dr. Alexander and her colleagues reported no conflicts of interest.
koakes@mdedge.com
SOURCE: Alexander AL et al. Obstet Gynecol. 2018 Dec 4. doi: 10.1097/AOG.0000000000002990
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Black women were more likely than white women were to have open hysterectomies.
Major finding:
Study details: Analysis of prospectively collected hysterectomy data of 15,136 women in the NSQIP database.
Disclosures: The study was funded by the National Institutes of Health. Dr. Alexander and her coauthors reported no conflicts of interest.
Source: Alexander AL et al. Obstet Gynecol. 2018 Dec 4. doi: 10.1097/AOG0000000000002990.
FDA expands Essure’s postmarketing surveillance study
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”