User login
A patient with severe adenomyosis requests uterine-sparing surgery
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.
Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
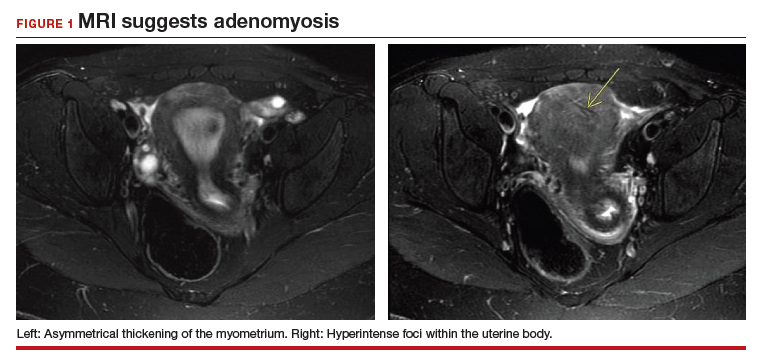
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25
Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
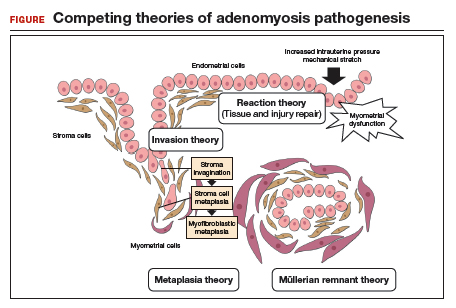
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9
References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
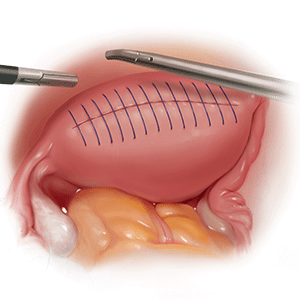
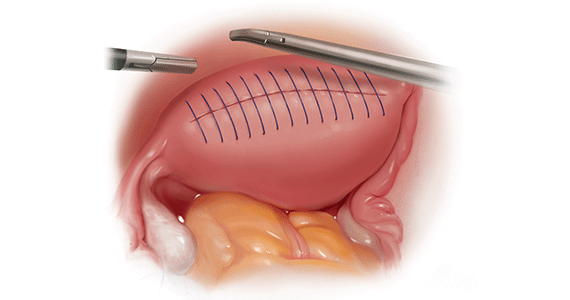
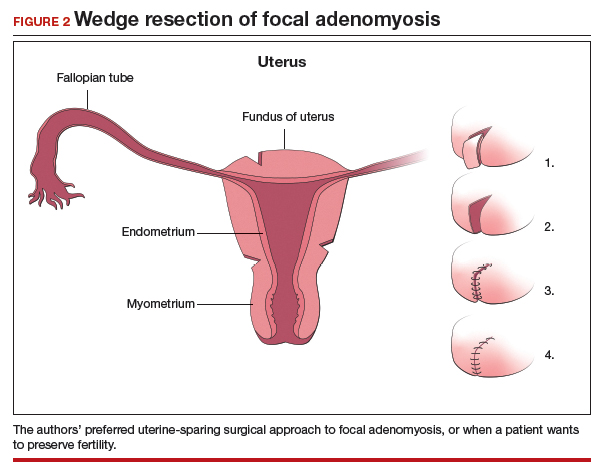
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.
CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.

Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25

Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9

References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.

CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.

Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25

Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9

References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.

CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
Product Update: Bijuva; Liletta; Aegea Vapor System; Natural Cycles
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
TREATMENT FOR VASOMOTOR SYMPTOMS
BIJUVA™ has been US Food and Drug Administration (FDA)–approved as the first oral treatment for moderate-to-severe vasomotor symptoms due to menopause in women with a uterus. BIJUVA offers a combination of bioidentical estradiol to reduce moderate-to-severe hot flashes and bioidentical progesterone to reduce the risk for endometrial hyperplasia.
TherapeuticsMD says that BIJUVA will be available Spring 2019 and is a proven treatment option for women who are experiencing bothersome symptoms of menopause, with clinical trial data demonstrating a statistically significant reduction in both the frequency and severity of moderate-to-severe vasomotor symptoms. The manufacturer also says that BIJUVA is developed to be identical in molecular structure to the hormones already produced by the body and is designed to help women restore what is lost during menopause.
BIJUVA estradiol and progesterone combination (1 mg/100 mg) will be available in capsule form.
FOR MORE INFORMATION, VISIT:https://bijuva.com/discover/
LILETTA USE EXTENDED
The FDA has approved LILETTA® (levonorgestrel-releasing intrauterine system) 52 mg for 5-year use. This approval is based on efficacy and safety data from ACCESS IUS, the largest ongoing intrauterine device (IUD) Phase 3 clinical trial in the United States. Previously, LILETTA was indicated for use up to 4 years.
LILETTA continues to be greater than 99% effective in preventing pregnancy in a broad range of women, regardless of age, race, body mass index, or parity, according to Allergan and Medicines360. The extended duration and proven efficacy across a diverse population enables more women in the United States to obtain effective birth control, as the IUD is now available for a low cost at public health clinics.
FOR MORE INFORMATION, VISIT: https://www.liletta.com
Continue to: ENDOMETRIAL ABLATION TECHNOLOGY
ENDOMETRIAL ABLATION TECHNOLOGY
AEGEA Medical introduces the AEGEA Vapor SystemTM, an innovative solution for endometrial ablation to treat menorrhagia.
The system uses Adaptive Vapor Ablation and is the first endometrial ablation system specifically designed for use in the doctor’s office, allowing minimal anesthesia/analgesia and rapid recovery, says AEGEA Medical.
AEGEA Medical describes the AEGEA Vapor System as a fully automated safety monitoring and vapor delivery system that uses a slender, flexible Vapor Probe with SmartSealTM technology and the Integrity ProTM safety feature, for an added level of confidence. The 4-minute procedure time includes 2 minutes of vapor treatment and can be performed in patients with a wider range of uterine anatomies than indicated for use with currently available treatments, says AEGEA Medical.
FOR MORE INFORMATION, VISIT: http://aegeamedical.com/
NATURAL CYCLES
The FDA has cleared Natural Cycles as the first digital method of birth control in the United States. Delivered in the form of an app, Natural Cycles is a fertility awareness–based contraceptive that uses a sophisticated algorithm to accurately and conveniently determine a woman’s daily fertility based on basal body temperature.
That data builds into a personalized fertility indicator that informs her when she needs to use protection to minimize the chance of conception. The app also can be used to help plan a pregnancy when the time is right, according to Natural Cycles.
A clinical study showed that the efficacy of a contraceptive mobile application is higher than usually reported for traditional fertility awareness–based methods. The application may contribute to reducing the unmet need for contraception, says Natural Cycles.
FOR MORE INFORMATION, VISIT: https://www.naturalcycles.com/en/hcp
HHS effort aims to end new HIV cases within 10 years
WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.
“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.
George Sigounas, PhD, administrator or the Health Resources and Services Administration, said that existing community health centers will be especially important in reaching rural underserved and marginalized populations. Currently, he said, HRSA supports 12,000 service delivery sites across the country that are already delivering care to 27 million individuals. “These sites will play a major expanded role in providing PrEP to those who are at the greatest risk of contracting HIV,” said Dr. Sigounas.
Among the currently existing resources that will be leveraged are services provided by the Ryan White HIV/AIDS program, which already provides HIV primary medical care and support services through a network of grants to states and local government and community organizations. About half of the people currently diagnosed with HIV in the United States receive services through this program now.
The NIH maintains a geographically distributed network of Centers for AIDS Research that also will be folded into the new initiative.
In his remarks, Anthony S. Fauci, MD, director of the NIH’s National Center for Allergy and Infectious Diseases, pointed out that, “Treatment and detection are wrapped together, because treated individuals can’t transmit HIV” if they are adherent to antiretroviral medication use and achieve an undetectable viral load, he said. “If you get everyone who’s infected on antiretrovirals and give those who need it PrEP, you can theoretically end the epidemic as we know it – and that is our goal.”
Dr. Fauci went on to say that implementation science will play a key role in achieving a targeted and coordinated approach. “We will work closely with our colleagues to make sure the implementation is done well. We have lessons learned; we will do better and better,” he said.
The nuts and bolts of the program include a four-pronged strategy to diagnose individuals as early as possible after infection, to initiate prompt, effective, and sustained treatment, to protect those at risk for HIV by proven means including PrEP, and to provide rapid response when new HIV clusters are identified. A reimagining of current and future personnel into an “HIV health force” will put teams on the ground in each jurisdiction to carry out the initiative.
Though the goal is to provide PrEP to every at-risk individual, Dr. Fauci said that current modeling shows that if PrEP reaches 50%-60% in the at-risk population, new infections can be reduced by 90%. He added, “PrEP works. The efficacy is well over 90%.”
Funding details were not released at the press briefing; Dr. Giroir said that figures will be released by the Office of Management and Budget as part of the 2020 budget cycle. He confirmed, however, that new funds will be allocated for the effort, rather than a mere reshuffling of existing fund and resources.
Several of the leaders acknowledged the problem of stigma and marginalization that many individuals living with or at risk for HIV face, since men who have sex with men, transgender people, sex workers, and those with opioid use disorder all fall into this category.
“Every American deserves to be treated with respect and dignity. We will vigorously enforce all laws on the books about discrimination,” said Rear Adm. Michael Weahkee, MD, principal deputy director of the Indian Health Service. This is especially important in Native American communities “where everybody knows everybody,” he said, and it’s vitally important to include individual and community education in the efforts.
Dr. Redfield concurred, adding that “Dr. Fauci and I have been engaged in HIV since 1981. We have witnessed firsthand the negative impact that stigma can have on our capacity to practice public health. The transgender population, in particular, needs to be reached out to. We need to be able to address in a comprehensive way how to destigmatize the HIV population.”
WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.
“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.
George Sigounas, PhD, administrator or the Health Resources and Services Administration, said that existing community health centers will be especially important in reaching rural underserved and marginalized populations. Currently, he said, HRSA supports 12,000 service delivery sites across the country that are already delivering care to 27 million individuals. “These sites will play a major expanded role in providing PrEP to those who are at the greatest risk of contracting HIV,” said Dr. Sigounas.
Among the currently existing resources that will be leveraged are services provided by the Ryan White HIV/AIDS program, which already provides HIV primary medical care and support services through a network of grants to states and local government and community organizations. About half of the people currently diagnosed with HIV in the United States receive services through this program now.
The NIH maintains a geographically distributed network of Centers for AIDS Research that also will be folded into the new initiative.
In his remarks, Anthony S. Fauci, MD, director of the NIH’s National Center for Allergy and Infectious Diseases, pointed out that, “Treatment and detection are wrapped together, because treated individuals can’t transmit HIV” if they are adherent to antiretroviral medication use and achieve an undetectable viral load, he said. “If you get everyone who’s infected on antiretrovirals and give those who need it PrEP, you can theoretically end the epidemic as we know it – and that is our goal.”
Dr. Fauci went on to say that implementation science will play a key role in achieving a targeted and coordinated approach. “We will work closely with our colleagues to make sure the implementation is done well. We have lessons learned; we will do better and better,” he said.
The nuts and bolts of the program include a four-pronged strategy to diagnose individuals as early as possible after infection, to initiate prompt, effective, and sustained treatment, to protect those at risk for HIV by proven means including PrEP, and to provide rapid response when new HIV clusters are identified. A reimagining of current and future personnel into an “HIV health force” will put teams on the ground in each jurisdiction to carry out the initiative.
Though the goal is to provide PrEP to every at-risk individual, Dr. Fauci said that current modeling shows that if PrEP reaches 50%-60% in the at-risk population, new infections can be reduced by 90%. He added, “PrEP works. The efficacy is well over 90%.”
Funding details were not released at the press briefing; Dr. Giroir said that figures will be released by the Office of Management and Budget as part of the 2020 budget cycle. He confirmed, however, that new funds will be allocated for the effort, rather than a mere reshuffling of existing fund and resources.
Several of the leaders acknowledged the problem of stigma and marginalization that many individuals living with or at risk for HIV face, since men who have sex with men, transgender people, sex workers, and those with opioid use disorder all fall into this category.
“Every American deserves to be treated with respect and dignity. We will vigorously enforce all laws on the books about discrimination,” said Rear Adm. Michael Weahkee, MD, principal deputy director of the Indian Health Service. This is especially important in Native American communities “where everybody knows everybody,” he said, and it’s vitally important to include individual and community education in the efforts.
Dr. Redfield concurred, adding that “Dr. Fauci and I have been engaged in HIV since 1981. We have witnessed firsthand the negative impact that stigma can have on our capacity to practice public health. The transgender population, in particular, needs to be reached out to. We need to be able to address in a comprehensive way how to destigmatize the HIV population.”
WASHINGTON – Leaders from five federal agencies came together to announce the framework for a bold new national initiative that aims to eliminate new cases of HIV infection in the United States within 10 years. The announcement came the day after President Trump’s State of the Union address, which highlighted the new effort.
“HIV has cost America too much for too long,” said Adm. Brett Giroir, MD, assistant secretary for health at the Department of Health & Human Services, in a press briefing. In addition to the 700,000 U.S. lives the disease has claimed since 1981, “We are at high risk of another 400,000 becoming infected over the next decade,” with about 40,000 new infections still occurring every year, he said.
Dr. Giroir will lead a coordinated effort among HHS, the Centers for Disease Control, the National Institutes of Health, the Health Resources and Services Administration, and the Indian Health Service. The goals are to reduce new cases of HIV by 50% within 5 years, and by 90% within 10 years.
These 48 counties, together with Washington and San Juan, Puerto Rico, accounted for more than half of the new HIV diagnoses in 2016 and 2017, said Dr. Giroir.
“This is a laser-focused program targeting counties where infection is the highest,” said CDC Director Robert R. Redfield, MD. “We propose to deploy personnel, resources, and strategies” in these targeted areas to maximize not just diagnosis and treatment but also to reach those at risk for HIV to enroll them in preexposure prophylaxis (PrEP) regimens, he said.
In addition to the targeted counties, seven states in the rural South as well as Native American and Alaskan Native populations also will receive intensified education, diagnostic, and treatment services. The targeted states are Alabama, Arkansas, Kentucky, Mississippi, Missouri, Oklahoma, and South Carolina.
George Sigounas, PhD, administrator or the Health Resources and Services Administration, said that existing community health centers will be especially important in reaching rural underserved and marginalized populations. Currently, he said, HRSA supports 12,000 service delivery sites across the country that are already delivering care to 27 million individuals. “These sites will play a major expanded role in providing PrEP to those who are at the greatest risk of contracting HIV,” said Dr. Sigounas.
Among the currently existing resources that will be leveraged are services provided by the Ryan White HIV/AIDS program, which already provides HIV primary medical care and support services through a network of grants to states and local government and community organizations. About half of the people currently diagnosed with HIV in the United States receive services through this program now.
The NIH maintains a geographically distributed network of Centers for AIDS Research that also will be folded into the new initiative.
In his remarks, Anthony S. Fauci, MD, director of the NIH’s National Center for Allergy and Infectious Diseases, pointed out that, “Treatment and detection are wrapped together, because treated individuals can’t transmit HIV” if they are adherent to antiretroviral medication use and achieve an undetectable viral load, he said. “If you get everyone who’s infected on antiretrovirals and give those who need it PrEP, you can theoretically end the epidemic as we know it – and that is our goal.”
Dr. Fauci went on to say that implementation science will play a key role in achieving a targeted and coordinated approach. “We will work closely with our colleagues to make sure the implementation is done well. We have lessons learned; we will do better and better,” he said.
The nuts and bolts of the program include a four-pronged strategy to diagnose individuals as early as possible after infection, to initiate prompt, effective, and sustained treatment, to protect those at risk for HIV by proven means including PrEP, and to provide rapid response when new HIV clusters are identified. A reimagining of current and future personnel into an “HIV health force” will put teams on the ground in each jurisdiction to carry out the initiative.
Though the goal is to provide PrEP to every at-risk individual, Dr. Fauci said that current modeling shows that if PrEP reaches 50%-60% in the at-risk population, new infections can be reduced by 90%. He added, “PrEP works. The efficacy is well over 90%.”
Funding details were not released at the press briefing; Dr. Giroir said that figures will be released by the Office of Management and Budget as part of the 2020 budget cycle. He confirmed, however, that new funds will be allocated for the effort, rather than a mere reshuffling of existing fund and resources.
Several of the leaders acknowledged the problem of stigma and marginalization that many individuals living with or at risk for HIV face, since men who have sex with men, transgender people, sex workers, and those with opioid use disorder all fall into this category.
“Every American deserves to be treated with respect and dignity. We will vigorously enforce all laws on the books about discrimination,” said Rear Adm. Michael Weahkee, MD, principal deputy director of the Indian Health Service. This is especially important in Native American communities “where everybody knows everybody,” he said, and it’s vitally important to include individual and community education in the efforts.
Dr. Redfield concurred, adding that “Dr. Fauci and I have been engaged in HIV since 1981. We have witnessed firsthand the negative impact that stigma can have on our capacity to practice public health. The transgender population, in particular, needs to be reached out to. We need to be able to address in a comprehensive way how to destigmatize the HIV population.”
FROM A HEALTH AND HUMAN SERVICES BRIEFING
Is vaginal estrogen used for GSM associated with a higher risk of CVD or cancer?
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
Expert Commentary
Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. December 17, 2018. doi: 10.1097/GME.0000000000001284.
GSM, a chronic and often progressive condition, occurs in almost 50% of postmenopausal women and has been shown to impair sexual function and quality of life.1 Symptoms include vaginal dryness, vulvar or vaginal itching, dyspareunia, urinary urgency or frequency, and increased urinary tract infections. Although lubricants or vaginal moisturizers may be sufficient to treat GSM, targeted hormonal therapy may be needed to improve the symptoms and resolve the underlying cause, due to vaginal hormone loss.
Despite lack of any observational or clinical trial evidence for chronic health disease risks related to low-dose vaginal estrogen use, there remains an US Food and Drug Administration boxed warning on the package label for low-dose vaginal estrogen related to risks of heart disease, stroke, venous thromboembolism, pdementia, and breast cancer. The objective of the investigation by Bhupathiraju and colleagues was to evaluate associations between vaginal estrogen use and health outcomes, including CVD (myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal), and hip fracture.
Details of the study
The prospective analysis included 896 postmenopausal current users of vaginal estrogen in the Nurses’ Health Study (NHS; 1982–2012), compared with 52,901 nonusers. Eighteen years of follow-up was evaluated. Users of systemic hormone therapy were excluded from the analysis. For the NHS, self-reported data were collected every 2 years on questionnaires for vaginal estrogen use and health outcomes. Investigators used medical records to confirm health outcomes.
After adjusting for covariates, no significant differences in risks were found for CVD, cancer, and hip fracture between users and nonusers of vaginal estrogen, regardless of hysterectomy status.
Key findings
After adjusting for multiple variables (including age, race, physical activity, age at menopause, hysterectomy, aspirin use, parental history of cancer, etc), health outcomes for CVDs, all cancers, and hip fracture were:
- myocardial infarction: hazard ratio (HR), 0.73 (95% confidence interval [CI], 0.47–1.13)
- stroke: HR, 0.85 (95% CI, 0.56–1.29)
- pulmonary embolism/deep vein thrombosis: HR, 1.06 (95% CI, 0.58–1.93)
- hip fracture: HR, 0.91 (95% CI, 0.60–1.38)
- all cancers: HR, 1.05 (95% CI, 0.89–1.25).
Continue to: Health outcomes for specific invasive cancers
Health outcomes for specific invasive cancers (risk for endometrial cancer included only women with an intact uterus) were:
- invasive breast cancer: HR, 1.07 (95% CI, 0.78–1.47)
- ovarian cancer: HR, 1.17 (95% CI, 0.52–2.65)
- endometrial cancer: HR, 1.62 (95% CI, 0.88–2.97)
- colorectal cancer: HR, 0.77 (95% CI, 0.45–1.34).
Study strengths and weaknesses
A causal relationship cannot be proven as the study was observational. However, a strength included the 18 years of follow-up. Women used vaginal estrogen for an average of 3 years, which provided longer-term safety data than available 12-month clinical trial data. Data were collected through self-report on questionnaires every 2 years, which is a drawback; however, participants were registered nurses, who have been shown to provide reliable health-related information. Comparisons between therapies were not possible as data were not collected about type or dosage of vaginal estrogen. Available therapies during the NHS included vaginal estrogen tablets, creams, and an estradiol ring, with higher doses available during earlier parts of the study than the lower doses commonly prescribed in current day.
Overall
The findings from this long-term follow-up of the NHS provide support for the safety of vaginal estrogen for treatment of GSM. No statistically significant increased health risks were found for users of vaginal estrogen, similar to earlier reported findings from the large Women’s Health Initiative.2 Low-dose vaginal estrogen is recommended for treatment of GSM by The North American Menopause Society, the American College of Obstetricians and Gynecologists, and the Endocrine Society.
Absorption of low-dose vaginal estrogen preparations appears minimal, and they are effective and generally safe for the treatment of GSM for women at any age. Progesterone is not recommended with low-dose vaginal estrogen therapies, based primarily on randomized clinical trial safety data of 12 months.3 Postmenopausal bleeding, however, needs to be thoroughly evaluated. For women with breast cancer, include the oncologist in decision making about the use of low-dose vaginal estrogen.
Despite the boxed warning on vaginal estrogen, the findings from this study support the safety of vaginal estrogen use for effective relief of GSM in women with and without a uterus.
JOANN V. PINKERTON, MD, NCMP
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;251:704-711.
- Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause. 2018;25:11-20.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
Uterine aspiration: From OR to office
CASE Patient with early pregnancy failure opts for surgical management
A 36-year-old woman (G3P2) at 9 weeks from her last menstrual period presents for an initial obstetric examination. On transvaginal ultrasound, her ObGyn notes an embryo measuring 9 weeks without cardiac activity. The ObGyn informs her of the early pregnancy failure diagnosis and offers bereavement support, and then reviews the available options: expectant management with follow-up in 2 weeks, medical management with mifepristone and misoprostol, and surgical management with a dilation and curettage (D&C). The patient is interested in expedited treatment and thus selects D&C, and the staff books the next available operating room (OR) slot for her the subsequent week. Over the weekend, the patient calls to report heavy bleeding and passage of clots, and the ObGyn’s practice partner takes her to the OR for a D&C for incomplete abortion.
Early pregnancy failure occurs in about 1 in 5 pregnancies. Treatment options include expectant, medical, or surgical management. Surgical management is classically offered in the OR via D&C. With the advent of manual vacuum aspiration (MVA) using a 60-mL handheld syringe aspirator, office-based treatment of pregnancy failure has become more widely available.
In this article we make the case for why, in appropriate clinical situations, office-based uterine aspiration, compared with uterine aspiration in the OR, should be the standard for surgical management of early pregnancy failure, for these reasons:
1. equivalent safety profile
2. reduced costs, and
3. patient-centered characteristics.
1 Office-based procedures are safe
Suction curettage is one of the most common surgical procedures for a woman to undergo during her lifetime, and it has an excellent safety profile. Authors of a recent systematic review found that major surgical complications, including transfusion and uterine perforation requiring repair, occurred in less than 0.1% of all uterine aspiration procedures.1 Importantly, this complication rate did not differ by inpatient or outpatient site of procedure.
Anesthesia-related complications at the time of aspiration also are extremely rare, and they are less likely to occur in the office setting than in surgical centers or hospital-based clinics (<0.2% and <0.5%, respectively).1 This may be a result of the types of anesthesia offered at varying locations, given that local analgesia or moderate sedation is likely used in office-based procedures while deep sedation or general anesthesia may be employed at other practice locations.
Studies specifically designed to determine the safety of suction aspiration by practice location have yielded similar results. Researchers who conducted a systematic review comparing the safety of procedures done at ambulatory surgical centers with office-based procedures found no difference in safety between procedures performed in these 2 settings.2 These findings were confirmed by results from a large retrospective cohort study that reviewed more than 50,000 aspiration procedures performed in ambulatory surgical centers versus private offices.3 In that study, only 0.32% of women had any major adverse event, and there were no statistically significant differences in complication rates between settings.3
Complication rates based on procedure type are similar for MVA and electric suction aspiration. Early studies revealed no difference in the need for reaspiration or other complications for MVA compared with electric suction.4 This was later confirmed by a systematic review that found no significant differences in safety by type of suction overall, and a possible trend toward fewer uterine perforations with MVA.5 When procedures were assessed by gestational age, additional trends toward the safety of MVA emerged. For example, in procedures performed at less than 50 days’ gestational age, estimated blood loss and severe pain occurred less commonly during procedures performed using MVA.5
Continue to: 2 Office-based procedures are less expensive
2 Office-based procedures are less expensive
There has been a trend in recent decades to obtain cost savings by moving appropriately selected gynecologic procedures from the operative suite to the outpatient setting. Because of MVA’s minimal up-front and ongoing costs, office-based suction aspiration is one of the most cost-effective procedures performed in the outpatient setting.
Dalton and colleagues, for example, demonstrated that in women diagnosed with early pregnancy failure, suction curettage is 50% less expensive when performed in the office as compared to in the operating suite.6 Likewise, in a cohort of patients who presented to the emergency department with an incomplete abortion, Blumenthal and colleagues showed a 41% procedural cost reduction by offering D&C in the outpatient setting instead of the OR.7 Waiting times and mean procedure times also were reduced by nearly half.
Recent studies have broadened cost analyses beyond the comparison of inpatient versus outpatient procedures. A multicenter trial of women with first-trimester pregnancy failure compared the costs of medication management with those of surgical procedures; as expected, the cost of D&C in the OR was significantly more expensive than medication management.8 However, MVA in the office was less expensive than medication management, due largely to the increased cost of managing medication failures.
In addition, a recent, well-designed decision model study demonstrated that offering women with early pregnancy failure a greater array of management options decreases costs.9 The study compared the costs when women were offered the most common options, expectant management or uterine evacuation in the OR, versus the costs when additional options were also offered. When options were expanded to include medication management and MVA in the office, costs decreased by nearly 20% overall.9
3 Office-based procedures are more patient centered
The benefits of surgical management of an early pregnancy failure include very high success rates (98%) and convenient timing. Among women who elect surgical management, a desire to expedite the process in a predictable fashion is a common factor in their decision.10,11 It is unsurprising then that 68% of patients will select an office-based procedure if they do not perceive that the clinician has a setting preference.6
When surgical management is performed in the OR, scheduling delays are common. Such delays can be clinically important: Women progressing to a miscarriage while awaiting surgical treatment may be at risk for urgent, unplanned interval procedures for incomplete abortion, and they may be dissatisfied with the inability to access the desired management. While women are highly satisfied after treatment for early pregnancy failure in general,6 OR treatment can cause dissatisfaction because patients miss more work days or need assistance at home.12 In a cross-sectional study, patients who elected office-based aspiration reported less delay to treatment (less than 2 hours) compared with women who elected OR procedures (more than 12 hours), and shorter time to procedure initiation was a satisfier.13
Women also note fear of the hospital setting and general anesthesia, and they tend to see hospital-based services as more invasive.11 Clinicians can offer anesthesia in the outpatient setting with nonsteroidal anti-inflammatory medications and a paracervical block, oral sedation with an anxiolytic, or in some cases intravenous (IV) sedation with conscious sedation.
Continue to: Our process for office-based uterine aspiration
Our process for office-based uterine aspiration
We follow the step-by-step process outlined below for performing office-based uterine aspiration. Clinicians should review their clinic’s protocols prior to implementing such a plan.
Review the patient history and pregnancy dating. Patients with serious medical conditions, such as history of postabortion hemorrhage or a bleeding disorder, may not be appropriate candidates for an office-based procedure. We perform bedside ultrasonography to confirm pregnancy dating and diagnosis of pregnancy failure.
Review consent for the procedure and sedation. Risks of office-based uterine aspiration are the same as those for D&C: bleeding, uterine perforation, and failure to fully evacuate the uterus. Benefits include rapid, safe evacuation of the pregnancy. Alternative treatments include expectant or medical management.
For pain management, we start by discussing expectations with the patient. Providing general anesthesia in the outpatient setting is not safe; many women are satisfied, however, with local anesthesia with or without sedation.
Local anesthesia may be given using a paracervical block with 2 mL of 1% lidocaine at the tenaculum site followed by 18 mL divided between the 4 and 8 o’clock positions. In our practice, we are trained providers of conscious sedation, so additionally we offer IV fentanyl 100 μg and IV midazolam 2 mg given prior to the procedure.
Provide antibiotic prophylaxis. The American College of Obstetricians and Gynecologists and the Society for Family Planning recommend doxycycline 200 mg orally as a preoperative prophylaxis for office-based uterine aspiration.14,15 Metronidazole is an acceptable alternative for patients who have medication allergies.
Prepare the surgical field. To complete this procedure, you will need the following equipment:
- one MVA kit that includes an aspirator, curettes, and dilators (FIGURE)
- 20 mL 1% lidocaine, divided into two 10-mL syringes with a 22-gauge 3.5-inch spinal needle
- speculum
- cervical antiseptic prep
- single-tooth tenaculum
- ring forceps.

Perform the MVA procedure. A full description of how to perform the MVA procedure using the Ipas MVA Plus Aspirator device is available online at http://provideaccess.org/wp-content/uploads/2012/09/4Performing-MVA-Us ing-the-Ipas-MVA-Plus.pdf.
A good option for many women
A D&C in the OR remains an appropriate option for patients who are clinically unstable due to heavy vaginal bleeding. With highly sensitive home urine pregnancy tests, pregnancies often are diagnosed before clinically apparent miscarriage. In fact, many such patients are diagnosed with pregnancy failure in the office, as was our patient in the case scenario. For such women, office-based management of early pregnancy failure is preferred because it is safe, cost-effective, and patient centered.
The “Break This Practice Habit” series is spearheaded by Dr. Lauren Demosthenes, who makes overarching high value cost decisions in her role as Medical Director of High Value Care and Innovation, Department of ObGyn at Greenville Health System in Greenville, South Carolina. Watch for quarterly case presentations of low value, low evidence practices that should be questioned in current day, followed by reasons why that practice should be abandoned. If you would like to contribute to this series, please submit your query to Dr. Demosthenes at ldemosthenes@mdedge.com.
- White K, Carroll E, Grossman D. Complications from first-trimester aspiration abortion: a systematic review of the literature. Contraception. 2015;92:422-438.
- Berglas NF, Battistelli MF, Nicholson WK, et al. The effect of facility characteristics on patient safety, patient experience, and service availability for procedures in non-hospital affiliated outpatient settings: a systematic review. PloS One. 2018;13:e0190975.
- Roberts SC, Upadhyay UD, Liu G, et al. Association of facility type with procedural-related morbidities and adverse events among patients undergoing induced abortions. JAMA. 2018;319:2497-2506.
- Goldberg AB, Dean G, Kang MS, et al. Manual versus electric vacuum aspiration for early first-trimester abortion: a controlled study of complication rates. Obstet Gynecol. 2004;103:101-107.
- Wen J, Cai QY, Deng F, et al. Manual versus electric vacuum aspiration for first-trimester abortion: a systematic review. BJOG. 2008;115:5-13.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Blumenthal PD, Remsburg RE. A time and cost analysis of the management of incomplete abortion with manual vacuum aspiration. Int J Gynaecol Obstet. 1994;45:261-267.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Dalton VK, Liang A, Hutton DW, et al. Beyond usual care: the economic consequences of expanding treatment options in early pregnancy loss. Am J Obstet Gynecol. 2015;212:177.e1-6.
- Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
- Smith LF, Frost J, Levitas R, et al. Women’s experiences of three early miscarriage management options: a qualitative study. Br J Gen Pract. 2006;56:198-205.
- Edwards S, Tureck R, Fredrick M, et al. Patient acceptability of manual versus electric vacuum aspiration for early pregnancy loss. J Womens Health (Larchmt). 2007;16:1429-1436.
- Dodge LE, Hofler LG, Hacker MR, et al. Patient satisfaction and wait times following outpatient manual vacuum aspiration compared to electric vacuum aspiration in the operating room: a cross-sectional study. Contracept Reprod Med. 2017;2:18.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 195: Prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Achilles SL, Reeves MF; Society of Family Planning. Prevention of infection after induced abortion. Contraception. 2011;837:295–309.
CASE Patient with early pregnancy failure opts for surgical management
A 36-year-old woman (G3P2) at 9 weeks from her last menstrual period presents for an initial obstetric examination. On transvaginal ultrasound, her ObGyn notes an embryo measuring 9 weeks without cardiac activity. The ObGyn informs her of the early pregnancy failure diagnosis and offers bereavement support, and then reviews the available options: expectant management with follow-up in 2 weeks, medical management with mifepristone and misoprostol, and surgical management with a dilation and curettage (D&C). The patient is interested in expedited treatment and thus selects D&C, and the staff books the next available operating room (OR) slot for her the subsequent week. Over the weekend, the patient calls to report heavy bleeding and passage of clots, and the ObGyn’s practice partner takes her to the OR for a D&C for incomplete abortion.
Early pregnancy failure occurs in about 1 in 5 pregnancies. Treatment options include expectant, medical, or surgical management. Surgical management is classically offered in the OR via D&C. With the advent of manual vacuum aspiration (MVA) using a 60-mL handheld syringe aspirator, office-based treatment of pregnancy failure has become more widely available.
In this article we make the case for why, in appropriate clinical situations, office-based uterine aspiration, compared with uterine aspiration in the OR, should be the standard for surgical management of early pregnancy failure, for these reasons:
1. equivalent safety profile
2. reduced costs, and
3. patient-centered characteristics.
1 Office-based procedures are safe
Suction curettage is one of the most common surgical procedures for a woman to undergo during her lifetime, and it has an excellent safety profile. Authors of a recent systematic review found that major surgical complications, including transfusion and uterine perforation requiring repair, occurred in less than 0.1% of all uterine aspiration procedures.1 Importantly, this complication rate did not differ by inpatient or outpatient site of procedure.
Anesthesia-related complications at the time of aspiration also are extremely rare, and they are less likely to occur in the office setting than in surgical centers or hospital-based clinics (<0.2% and <0.5%, respectively).1 This may be a result of the types of anesthesia offered at varying locations, given that local analgesia or moderate sedation is likely used in office-based procedures while deep sedation or general anesthesia may be employed at other practice locations.
Studies specifically designed to determine the safety of suction aspiration by practice location have yielded similar results. Researchers who conducted a systematic review comparing the safety of procedures done at ambulatory surgical centers with office-based procedures found no difference in safety between procedures performed in these 2 settings.2 These findings were confirmed by results from a large retrospective cohort study that reviewed more than 50,000 aspiration procedures performed in ambulatory surgical centers versus private offices.3 In that study, only 0.32% of women had any major adverse event, and there were no statistically significant differences in complication rates between settings.3
Complication rates based on procedure type are similar for MVA and electric suction aspiration. Early studies revealed no difference in the need for reaspiration or other complications for MVA compared with electric suction.4 This was later confirmed by a systematic review that found no significant differences in safety by type of suction overall, and a possible trend toward fewer uterine perforations with MVA.5 When procedures were assessed by gestational age, additional trends toward the safety of MVA emerged. For example, in procedures performed at less than 50 days’ gestational age, estimated blood loss and severe pain occurred less commonly during procedures performed using MVA.5
Continue to: 2 Office-based procedures are less expensive
2 Office-based procedures are less expensive
There has been a trend in recent decades to obtain cost savings by moving appropriately selected gynecologic procedures from the operative suite to the outpatient setting. Because of MVA’s minimal up-front and ongoing costs, office-based suction aspiration is one of the most cost-effective procedures performed in the outpatient setting.
Dalton and colleagues, for example, demonstrated that in women diagnosed with early pregnancy failure, suction curettage is 50% less expensive when performed in the office as compared to in the operating suite.6 Likewise, in a cohort of patients who presented to the emergency department with an incomplete abortion, Blumenthal and colleagues showed a 41% procedural cost reduction by offering D&C in the outpatient setting instead of the OR.7 Waiting times and mean procedure times also were reduced by nearly half.
Recent studies have broadened cost analyses beyond the comparison of inpatient versus outpatient procedures. A multicenter trial of women with first-trimester pregnancy failure compared the costs of medication management with those of surgical procedures; as expected, the cost of D&C in the OR was significantly more expensive than medication management.8 However, MVA in the office was less expensive than medication management, due largely to the increased cost of managing medication failures.
In addition, a recent, well-designed decision model study demonstrated that offering women with early pregnancy failure a greater array of management options decreases costs.9 The study compared the costs when women were offered the most common options, expectant management or uterine evacuation in the OR, versus the costs when additional options were also offered. When options were expanded to include medication management and MVA in the office, costs decreased by nearly 20% overall.9
3 Office-based procedures are more patient centered
The benefits of surgical management of an early pregnancy failure include very high success rates (98%) and convenient timing. Among women who elect surgical management, a desire to expedite the process in a predictable fashion is a common factor in their decision.10,11 It is unsurprising then that 68% of patients will select an office-based procedure if they do not perceive that the clinician has a setting preference.6
When surgical management is performed in the OR, scheduling delays are common. Such delays can be clinically important: Women progressing to a miscarriage while awaiting surgical treatment may be at risk for urgent, unplanned interval procedures for incomplete abortion, and they may be dissatisfied with the inability to access the desired management. While women are highly satisfied after treatment for early pregnancy failure in general,6 OR treatment can cause dissatisfaction because patients miss more work days or need assistance at home.12 In a cross-sectional study, patients who elected office-based aspiration reported less delay to treatment (less than 2 hours) compared with women who elected OR procedures (more than 12 hours), and shorter time to procedure initiation was a satisfier.13
Women also note fear of the hospital setting and general anesthesia, and they tend to see hospital-based services as more invasive.11 Clinicians can offer anesthesia in the outpatient setting with nonsteroidal anti-inflammatory medications and a paracervical block, oral sedation with an anxiolytic, or in some cases intravenous (IV) sedation with conscious sedation.
Continue to: Our process for office-based uterine aspiration
Our process for office-based uterine aspiration
We follow the step-by-step process outlined below for performing office-based uterine aspiration. Clinicians should review their clinic’s protocols prior to implementing such a plan.
Review the patient history and pregnancy dating. Patients with serious medical conditions, such as history of postabortion hemorrhage or a bleeding disorder, may not be appropriate candidates for an office-based procedure. We perform bedside ultrasonography to confirm pregnancy dating and diagnosis of pregnancy failure.
Review consent for the procedure and sedation. Risks of office-based uterine aspiration are the same as those for D&C: bleeding, uterine perforation, and failure to fully evacuate the uterus. Benefits include rapid, safe evacuation of the pregnancy. Alternative treatments include expectant or medical management.
For pain management, we start by discussing expectations with the patient. Providing general anesthesia in the outpatient setting is not safe; many women are satisfied, however, with local anesthesia with or without sedation.
Local anesthesia may be given using a paracervical block with 2 mL of 1% lidocaine at the tenaculum site followed by 18 mL divided between the 4 and 8 o’clock positions. In our practice, we are trained providers of conscious sedation, so additionally we offer IV fentanyl 100 μg and IV midazolam 2 mg given prior to the procedure.
Provide antibiotic prophylaxis. The American College of Obstetricians and Gynecologists and the Society for Family Planning recommend doxycycline 200 mg orally as a preoperative prophylaxis for office-based uterine aspiration.14,15 Metronidazole is an acceptable alternative for patients who have medication allergies.
Prepare the surgical field. To complete this procedure, you will need the following equipment:
- one MVA kit that includes an aspirator, curettes, and dilators (FIGURE)
- 20 mL 1% lidocaine, divided into two 10-mL syringes with a 22-gauge 3.5-inch spinal needle
- speculum
- cervical antiseptic prep
- single-tooth tenaculum
- ring forceps.

Perform the MVA procedure. A full description of how to perform the MVA procedure using the Ipas MVA Plus Aspirator device is available online at http://provideaccess.org/wp-content/uploads/2012/09/4Performing-MVA-Us ing-the-Ipas-MVA-Plus.pdf.
A good option for many women
A D&C in the OR remains an appropriate option for patients who are clinically unstable due to heavy vaginal bleeding. With highly sensitive home urine pregnancy tests, pregnancies often are diagnosed before clinically apparent miscarriage. In fact, many such patients are diagnosed with pregnancy failure in the office, as was our patient in the case scenario. For such women, office-based management of early pregnancy failure is preferred because it is safe, cost-effective, and patient centered.
The “Break This Practice Habit” series is spearheaded by Dr. Lauren Demosthenes, who makes overarching high value cost decisions in her role as Medical Director of High Value Care and Innovation, Department of ObGyn at Greenville Health System in Greenville, South Carolina. Watch for quarterly case presentations of low value, low evidence practices that should be questioned in current day, followed by reasons why that practice should be abandoned. If you would like to contribute to this series, please submit your query to Dr. Demosthenes at ldemosthenes@mdedge.com.
CASE Patient with early pregnancy failure opts for surgical management
A 36-year-old woman (G3P2) at 9 weeks from her last menstrual period presents for an initial obstetric examination. On transvaginal ultrasound, her ObGyn notes an embryo measuring 9 weeks without cardiac activity. The ObGyn informs her of the early pregnancy failure diagnosis and offers bereavement support, and then reviews the available options: expectant management with follow-up in 2 weeks, medical management with mifepristone and misoprostol, and surgical management with a dilation and curettage (D&C). The patient is interested in expedited treatment and thus selects D&C, and the staff books the next available operating room (OR) slot for her the subsequent week. Over the weekend, the patient calls to report heavy bleeding and passage of clots, and the ObGyn’s practice partner takes her to the OR for a D&C for incomplete abortion.
Early pregnancy failure occurs in about 1 in 5 pregnancies. Treatment options include expectant, medical, or surgical management. Surgical management is classically offered in the OR via D&C. With the advent of manual vacuum aspiration (MVA) using a 60-mL handheld syringe aspirator, office-based treatment of pregnancy failure has become more widely available.
In this article we make the case for why, in appropriate clinical situations, office-based uterine aspiration, compared with uterine aspiration in the OR, should be the standard for surgical management of early pregnancy failure, for these reasons:
1. equivalent safety profile
2. reduced costs, and
3. patient-centered characteristics.
1 Office-based procedures are safe
Suction curettage is one of the most common surgical procedures for a woman to undergo during her lifetime, and it has an excellent safety profile. Authors of a recent systematic review found that major surgical complications, including transfusion and uterine perforation requiring repair, occurred in less than 0.1% of all uterine aspiration procedures.1 Importantly, this complication rate did not differ by inpatient or outpatient site of procedure.
Anesthesia-related complications at the time of aspiration also are extremely rare, and they are less likely to occur in the office setting than in surgical centers or hospital-based clinics (<0.2% and <0.5%, respectively).1 This may be a result of the types of anesthesia offered at varying locations, given that local analgesia or moderate sedation is likely used in office-based procedures while deep sedation or general anesthesia may be employed at other practice locations.
Studies specifically designed to determine the safety of suction aspiration by practice location have yielded similar results. Researchers who conducted a systematic review comparing the safety of procedures done at ambulatory surgical centers with office-based procedures found no difference in safety between procedures performed in these 2 settings.2 These findings were confirmed by results from a large retrospective cohort study that reviewed more than 50,000 aspiration procedures performed in ambulatory surgical centers versus private offices.3 In that study, only 0.32% of women had any major adverse event, and there were no statistically significant differences in complication rates between settings.3
Complication rates based on procedure type are similar for MVA and electric suction aspiration. Early studies revealed no difference in the need for reaspiration or other complications for MVA compared with electric suction.4 This was later confirmed by a systematic review that found no significant differences in safety by type of suction overall, and a possible trend toward fewer uterine perforations with MVA.5 When procedures were assessed by gestational age, additional trends toward the safety of MVA emerged. For example, in procedures performed at less than 50 days’ gestational age, estimated blood loss and severe pain occurred less commonly during procedures performed using MVA.5
Continue to: 2 Office-based procedures are less expensive
2 Office-based procedures are less expensive
There has been a trend in recent decades to obtain cost savings by moving appropriately selected gynecologic procedures from the operative suite to the outpatient setting. Because of MVA’s minimal up-front and ongoing costs, office-based suction aspiration is one of the most cost-effective procedures performed in the outpatient setting.
Dalton and colleagues, for example, demonstrated that in women diagnosed with early pregnancy failure, suction curettage is 50% less expensive when performed in the office as compared to in the operating suite.6 Likewise, in a cohort of patients who presented to the emergency department with an incomplete abortion, Blumenthal and colleagues showed a 41% procedural cost reduction by offering D&C in the outpatient setting instead of the OR.7 Waiting times and mean procedure times also were reduced by nearly half.
Recent studies have broadened cost analyses beyond the comparison of inpatient versus outpatient procedures. A multicenter trial of women with first-trimester pregnancy failure compared the costs of medication management with those of surgical procedures; as expected, the cost of D&C in the OR was significantly more expensive than medication management.8 However, MVA in the office was less expensive than medication management, due largely to the increased cost of managing medication failures.
In addition, a recent, well-designed decision model study demonstrated that offering women with early pregnancy failure a greater array of management options decreases costs.9 The study compared the costs when women were offered the most common options, expectant management or uterine evacuation in the OR, versus the costs when additional options were also offered. When options were expanded to include medication management and MVA in the office, costs decreased by nearly 20% overall.9
3 Office-based procedures are more patient centered
The benefits of surgical management of an early pregnancy failure include very high success rates (98%) and convenient timing. Among women who elect surgical management, a desire to expedite the process in a predictable fashion is a common factor in their decision.10,11 It is unsurprising then that 68% of patients will select an office-based procedure if they do not perceive that the clinician has a setting preference.6
When surgical management is performed in the OR, scheduling delays are common. Such delays can be clinically important: Women progressing to a miscarriage while awaiting surgical treatment may be at risk for urgent, unplanned interval procedures for incomplete abortion, and they may be dissatisfied with the inability to access the desired management. While women are highly satisfied after treatment for early pregnancy failure in general,6 OR treatment can cause dissatisfaction because patients miss more work days or need assistance at home.12 In a cross-sectional study, patients who elected office-based aspiration reported less delay to treatment (less than 2 hours) compared with women who elected OR procedures (more than 12 hours), and shorter time to procedure initiation was a satisfier.13
Women also note fear of the hospital setting and general anesthesia, and they tend to see hospital-based services as more invasive.11 Clinicians can offer anesthesia in the outpatient setting with nonsteroidal anti-inflammatory medications and a paracervical block, oral sedation with an anxiolytic, or in some cases intravenous (IV) sedation with conscious sedation.
Continue to: Our process for office-based uterine aspiration
Our process for office-based uterine aspiration
We follow the step-by-step process outlined below for performing office-based uterine aspiration. Clinicians should review their clinic’s protocols prior to implementing such a plan.
Review the patient history and pregnancy dating. Patients with serious medical conditions, such as history of postabortion hemorrhage or a bleeding disorder, may not be appropriate candidates for an office-based procedure. We perform bedside ultrasonography to confirm pregnancy dating and diagnosis of pregnancy failure.
Review consent for the procedure and sedation. Risks of office-based uterine aspiration are the same as those for D&C: bleeding, uterine perforation, and failure to fully evacuate the uterus. Benefits include rapid, safe evacuation of the pregnancy. Alternative treatments include expectant or medical management.
For pain management, we start by discussing expectations with the patient. Providing general anesthesia in the outpatient setting is not safe; many women are satisfied, however, with local anesthesia with or without sedation.
Local anesthesia may be given using a paracervical block with 2 mL of 1% lidocaine at the tenaculum site followed by 18 mL divided between the 4 and 8 o’clock positions. In our practice, we are trained providers of conscious sedation, so additionally we offer IV fentanyl 100 μg and IV midazolam 2 mg given prior to the procedure.
Provide antibiotic prophylaxis. The American College of Obstetricians and Gynecologists and the Society for Family Planning recommend doxycycline 200 mg orally as a preoperative prophylaxis for office-based uterine aspiration.14,15 Metronidazole is an acceptable alternative for patients who have medication allergies.
Prepare the surgical field. To complete this procedure, you will need the following equipment:
- one MVA kit that includes an aspirator, curettes, and dilators (FIGURE)
- 20 mL 1% lidocaine, divided into two 10-mL syringes with a 22-gauge 3.5-inch spinal needle
- speculum
- cervical antiseptic prep
- single-tooth tenaculum
- ring forceps.

Perform the MVA procedure. A full description of how to perform the MVA procedure using the Ipas MVA Plus Aspirator device is available online at http://provideaccess.org/wp-content/uploads/2012/09/4Performing-MVA-Us ing-the-Ipas-MVA-Plus.pdf.
A good option for many women
A D&C in the OR remains an appropriate option for patients who are clinically unstable due to heavy vaginal bleeding. With highly sensitive home urine pregnancy tests, pregnancies often are diagnosed before clinically apparent miscarriage. In fact, many such patients are diagnosed with pregnancy failure in the office, as was our patient in the case scenario. For such women, office-based management of early pregnancy failure is preferred because it is safe, cost-effective, and patient centered.
The “Break This Practice Habit” series is spearheaded by Dr. Lauren Demosthenes, who makes overarching high value cost decisions in her role as Medical Director of High Value Care and Innovation, Department of ObGyn at Greenville Health System in Greenville, South Carolina. Watch for quarterly case presentations of low value, low evidence practices that should be questioned in current day, followed by reasons why that practice should be abandoned. If you would like to contribute to this series, please submit your query to Dr. Demosthenes at ldemosthenes@mdedge.com.
- White K, Carroll E, Grossman D. Complications from first-trimester aspiration abortion: a systematic review of the literature. Contraception. 2015;92:422-438.
- Berglas NF, Battistelli MF, Nicholson WK, et al. The effect of facility characteristics on patient safety, patient experience, and service availability for procedures in non-hospital affiliated outpatient settings: a systematic review. PloS One. 2018;13:e0190975.
- Roberts SC, Upadhyay UD, Liu G, et al. Association of facility type with procedural-related morbidities and adverse events among patients undergoing induced abortions. JAMA. 2018;319:2497-2506.
- Goldberg AB, Dean G, Kang MS, et al. Manual versus electric vacuum aspiration for early first-trimester abortion: a controlled study of complication rates. Obstet Gynecol. 2004;103:101-107.
- Wen J, Cai QY, Deng F, et al. Manual versus electric vacuum aspiration for first-trimester abortion: a systematic review. BJOG. 2008;115:5-13.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Blumenthal PD, Remsburg RE. A time and cost analysis of the management of incomplete abortion with manual vacuum aspiration. Int J Gynaecol Obstet. 1994;45:261-267.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Dalton VK, Liang A, Hutton DW, et al. Beyond usual care: the economic consequences of expanding treatment options in early pregnancy loss. Am J Obstet Gynecol. 2015;212:177.e1-6.
- Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
- Smith LF, Frost J, Levitas R, et al. Women’s experiences of three early miscarriage management options: a qualitative study. Br J Gen Pract. 2006;56:198-205.
- Edwards S, Tureck R, Fredrick M, et al. Patient acceptability of manual versus electric vacuum aspiration for early pregnancy loss. J Womens Health (Larchmt). 2007;16:1429-1436.
- Dodge LE, Hofler LG, Hacker MR, et al. Patient satisfaction and wait times following outpatient manual vacuum aspiration compared to electric vacuum aspiration in the operating room: a cross-sectional study. Contracept Reprod Med. 2017;2:18.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 195: Prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Achilles SL, Reeves MF; Society of Family Planning. Prevention of infection after induced abortion. Contraception. 2011;837:295–309.
- White K, Carroll E, Grossman D. Complications from first-trimester aspiration abortion: a systematic review of the literature. Contraception. 2015;92:422-438.
- Berglas NF, Battistelli MF, Nicholson WK, et al. The effect of facility characteristics on patient safety, patient experience, and service availability for procedures in non-hospital affiliated outpatient settings: a systematic review. PloS One. 2018;13:e0190975.
- Roberts SC, Upadhyay UD, Liu G, et al. Association of facility type with procedural-related morbidities and adverse events among patients undergoing induced abortions. JAMA. 2018;319:2497-2506.
- Goldberg AB, Dean G, Kang MS, et al. Manual versus electric vacuum aspiration for early first-trimester abortion: a controlled study of complication rates. Obstet Gynecol. 2004;103:101-107.
- Wen J, Cai QY, Deng F, et al. Manual versus electric vacuum aspiration for first-trimester abortion: a systematic review. BJOG. 2008;115:5-13.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Blumenthal PD, Remsburg RE. A time and cost analysis of the management of incomplete abortion with manual vacuum aspiration. Int J Gynaecol Obstet. 1994;45:261-267.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Dalton VK, Liang A, Hutton DW, et al. Beyond usual care: the economic consequences of expanding treatment options in early pregnancy loss. Am J Obstet Gynecol. 2015;212:177.e1-6.
- Schreiber CA, Chavez V, Whittaker PG, et al. Treatment decisions at the time of miscarriage diagnosis. Obstet Gynecol. 2016;128:1347-1356.
- Smith LF, Frost J, Levitas R, et al. Women’s experiences of three early miscarriage management options: a qualitative study. Br J Gen Pract. 2006;56:198-205.
- Edwards S, Tureck R, Fredrick M, et al. Patient acceptability of manual versus electric vacuum aspiration for early pregnancy loss. J Womens Health (Larchmt). 2007;16:1429-1436.
- Dodge LE, Hofler LG, Hacker MR, et al. Patient satisfaction and wait times following outpatient manual vacuum aspiration compared to electric vacuum aspiration in the operating room: a cross-sectional study. Contracept Reprod Med. 2017;2:18.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 195: Prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Achilles SL, Reeves MF; Society of Family Planning. Prevention of infection after induced abortion. Contraception. 2011;837:295–309.
Training in pathology and a good microscope help vulvar disorder diagnosis
LAS VEGAS –
In a presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium, Dr. Baggish ran through some tips about diagnosing and treating vulvar conditions. He discussed routine disorders (such as pubic lice), potentially dangerous disorders (such as lichen sclerosis, an inflammatory skin condition that can develop into squamous cell carcinoma), and rare disorders (such as Behçet’s syndrome, an inflammation of the blood vessels that can cause genital sores, and Fox-Fordyce disease of the vulva, which produces intense itching).
Dr. Baggish, a professor at the University of California, San Francisco, who treats patients in the Wine Country town of Saint Helena, elaborated on the treatment of vulvar disease in an interview at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company. The following are a few of his tips for gynecologists who want to expand their expertise and treat more patients with vulvar disorders.
- Get training in pathology. “That has made a big difference in my ability to intercept different kinds of vulvar and skin diseases,” he said. “You also need to see a lot of abnormalities so you can recognize the kinds of changes that you’re seeing.”
- Take a closer look with a microscope. “I have an operating microscope like an ophthalmologist would use, and it’s on a stand, not a table,” he said. “It always provides magnification with good light. This is a big advantage because misdiagnoses can be made when you can’t see the lesion well.” He added that he projects what he sees in the microscope onto a monitor so the patient can take a look herself. “I’ve found that very valuable,” he said.
- Be alert for chemical burns. “I’ve seen chemical burns when patients have had fungal infections and treated it with certain topical treatments like gentian violet. Somebody may also get a chemical burn from putting some kind of deodorant on their vulva,” Dr. Baggish said. “If you have a chemical burn, you’ll want to treat it with a cream to cover the lesion until it heals on its own. Silvadene is soothing, and patients find it very comfortable.”
- Get the right kind of biopsy. If you can’t identify a lesion, he said, “it’s better to do a biopsy.” He recommends asking pathologists for a reticulum stain. “It shows the support structure of the underlying tissue in the dermis of the layers of the skin, like the structure of a building before you put the covering on the girders,” he said. “The support structure is broken up in lichen planus [a common inflammatory condition that affects the skin and mucous membranes and can cause pain and itch]. You see that if you do a reticulum stain.” If a patient has an inflammatory condition, ask for relevant stains, he said. “For example, if there’s a question that this could be a viral disease like herpes simplex, I’m going to ask them to do a stain for viral inclusions,” he said. “Likewise, I will always ask for a stain for fungal particles, for yeast particles. Sometimes I’ll pick up something like an infection I otherwise would have missed.”
- Contact a specialist when needed. If a biopsy doesn’t help you identify a lesion, he said, “seek out an expert in this area who could be helpful.”
A number of gynecologists like Dr. Baggish specialize in vulvar disease, and several medical centers in the United States operate specialized vulvar clinics including Oregon Health & Science University, Portland; the University of Michigan, Ann Arbor; and Saint Louis University.
Dr. Baggish said he had no disclosures.
LAS VEGAS –
In a presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium, Dr. Baggish ran through some tips about diagnosing and treating vulvar conditions. He discussed routine disorders (such as pubic lice), potentially dangerous disorders (such as lichen sclerosis, an inflammatory skin condition that can develop into squamous cell carcinoma), and rare disorders (such as Behçet’s syndrome, an inflammation of the blood vessels that can cause genital sores, and Fox-Fordyce disease of the vulva, which produces intense itching).
Dr. Baggish, a professor at the University of California, San Francisco, who treats patients in the Wine Country town of Saint Helena, elaborated on the treatment of vulvar disease in an interview at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company. The following are a few of his tips for gynecologists who want to expand their expertise and treat more patients with vulvar disorders.
- Get training in pathology. “That has made a big difference in my ability to intercept different kinds of vulvar and skin diseases,” he said. “You also need to see a lot of abnormalities so you can recognize the kinds of changes that you’re seeing.”
- Take a closer look with a microscope. “I have an operating microscope like an ophthalmologist would use, and it’s on a stand, not a table,” he said. “It always provides magnification with good light. This is a big advantage because misdiagnoses can be made when you can’t see the lesion well.” He added that he projects what he sees in the microscope onto a monitor so the patient can take a look herself. “I’ve found that very valuable,” he said.
- Be alert for chemical burns. “I’ve seen chemical burns when patients have had fungal infections and treated it with certain topical treatments like gentian violet. Somebody may also get a chemical burn from putting some kind of deodorant on their vulva,” Dr. Baggish said. “If you have a chemical burn, you’ll want to treat it with a cream to cover the lesion until it heals on its own. Silvadene is soothing, and patients find it very comfortable.”
- Get the right kind of biopsy. If you can’t identify a lesion, he said, “it’s better to do a biopsy.” He recommends asking pathologists for a reticulum stain. “It shows the support structure of the underlying tissue in the dermis of the layers of the skin, like the structure of a building before you put the covering on the girders,” he said. “The support structure is broken up in lichen planus [a common inflammatory condition that affects the skin and mucous membranes and can cause pain and itch]. You see that if you do a reticulum stain.” If a patient has an inflammatory condition, ask for relevant stains, he said. “For example, if there’s a question that this could be a viral disease like herpes simplex, I’m going to ask them to do a stain for viral inclusions,” he said. “Likewise, I will always ask for a stain for fungal particles, for yeast particles. Sometimes I’ll pick up something like an infection I otherwise would have missed.”
- Contact a specialist when needed. If a biopsy doesn’t help you identify a lesion, he said, “seek out an expert in this area who could be helpful.”
A number of gynecologists like Dr. Baggish specialize in vulvar disease, and several medical centers in the United States operate specialized vulvar clinics including Oregon Health & Science University, Portland; the University of Michigan, Ann Arbor; and Saint Louis University.
Dr. Baggish said he had no disclosures.
LAS VEGAS –
In a presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium, Dr. Baggish ran through some tips about diagnosing and treating vulvar conditions. He discussed routine disorders (such as pubic lice), potentially dangerous disorders (such as lichen sclerosis, an inflammatory skin condition that can develop into squamous cell carcinoma), and rare disorders (such as Behçet’s syndrome, an inflammation of the blood vessels that can cause genital sores, and Fox-Fordyce disease of the vulva, which produces intense itching).
Dr. Baggish, a professor at the University of California, San Francisco, who treats patients in the Wine Country town of Saint Helena, elaborated on the treatment of vulvar disease in an interview at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company. The following are a few of his tips for gynecologists who want to expand their expertise and treat more patients with vulvar disorders.
- Get training in pathology. “That has made a big difference in my ability to intercept different kinds of vulvar and skin diseases,” he said. “You also need to see a lot of abnormalities so you can recognize the kinds of changes that you’re seeing.”
- Take a closer look with a microscope. “I have an operating microscope like an ophthalmologist would use, and it’s on a stand, not a table,” he said. “It always provides magnification with good light. This is a big advantage because misdiagnoses can be made when you can’t see the lesion well.” He added that he projects what he sees in the microscope onto a monitor so the patient can take a look herself. “I’ve found that very valuable,” he said.
- Be alert for chemical burns. “I’ve seen chemical burns when patients have had fungal infections and treated it with certain topical treatments like gentian violet. Somebody may also get a chemical burn from putting some kind of deodorant on their vulva,” Dr. Baggish said. “If you have a chemical burn, you’ll want to treat it with a cream to cover the lesion until it heals on its own. Silvadene is soothing, and patients find it very comfortable.”
- Get the right kind of biopsy. If you can’t identify a lesion, he said, “it’s better to do a biopsy.” He recommends asking pathologists for a reticulum stain. “It shows the support structure of the underlying tissue in the dermis of the layers of the skin, like the structure of a building before you put the covering on the girders,” he said. “The support structure is broken up in lichen planus [a common inflammatory condition that affects the skin and mucous membranes and can cause pain and itch]. You see that if you do a reticulum stain.” If a patient has an inflammatory condition, ask for relevant stains, he said. “For example, if there’s a question that this could be a viral disease like herpes simplex, I’m going to ask them to do a stain for viral inclusions,” he said. “Likewise, I will always ask for a stain for fungal particles, for yeast particles. Sometimes I’ll pick up something like an infection I otherwise would have missed.”
- Contact a specialist when needed. If a biopsy doesn’t help you identify a lesion, he said, “seek out an expert in this area who could be helpful.”
A number of gynecologists like Dr. Baggish specialize in vulvar disease, and several medical centers in the United States operate specialized vulvar clinics including Oregon Health & Science University, Portland; the University of Michigan, Ann Arbor; and Saint Louis University.
Dr. Baggish said he had no disclosures.
EXPERT ANALYSIS FROM PAGS
Vulvar disease treatment tips: From lice to lichen sclerosus
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS
When NOT to perform a Pap test
Pap tests have the reputation of being a simple, noninvasive, low-cost test to offer patients, and, therefore, it is understandable to believe there is no harm in offering it in all situations. However, if inappropriately applied in isolation, performing the Pap test may do more harm than good.
I recently saw a patient in consultation for cervical cancer. Her story was similar to one I’ve seen many times before. She was a 30-year-old non–English-speaking Hispanic woman who received regular care from the health department clinics.
In April of the prior year, she had noticed abnormal bleeding symptoms including intermenstrual and postcoital bleeding. She visited the health department and reported these symptoms to the provider who performed an examination. According to the provider’s notes, the cervix appeared “abnormal” and a Pap test was done. The result of this Pap test was high-grade dysplasia. The patient was promptly notified of the result and an appointment was arranged with the local ob.gyn. for a consultation, presumably for colposcopy and subsequent appropriate excisional procedure. Unfortunately, the patient did not attend that scheduled appointment. She later recounted to me that it was because she had not understood that it was important. She had a long history of abnormal Pap tests which, in the past, had only required repeat testing or minor interventions such as “freezing.”
Her bleeding symptoms became worse, and she developed abnormal discharge and pain. In November, she presented again for evaluation to the same provider. Now her cervix appeared very abnormal and was described as a “crater.” Again a Pap test was done. This time the Pap test showed “carcinoma,” and the patient was informed that she had cancer and was referred to gynecologic oncology. When I examined this unfortunate young woman, I discovered a 10 cm, stage IIB very locally advanced tumor. She is currently receiving primary chemotherapy/radiation with an approximately 60% probability of cure, and a high likelihood of lifelong sequelae of this toxic therapy.
This case highlights that, even when patients are engaged within our health care system, we can miss the opportunity to diagnose early-stage cancers if we are not utilizing screening versus diagnostic tests appropriately.
The purpose of a Pap test is as a screening test, which are designed to detect disease in asymptomatic individuals. The accuracy of these tests is determined in low-risk (asymptomatic) populations, which influences the pretest probability of disease. In asymptomatic patients with a normal screening test, it is safe to wait out the interval of time for the repeat screening test, because the combination of a low pretest probability and a high sensitivity of the test in finding disease means that there is a very low chance of missing disease.
Dysplasia rarely causes bleeding. However, invasive cervical cancer does. If a patient has a symptom such as abnormal bleeding, they no longer fit into the population with a low pretest probability for having cervical cancer. This same sensitivity of the Pap test in finding disease, combined with the now-higher pretest probability can raise the level of false-negative results to unacceptably high levels.
Patients with symptoms of cervical cancer should not receive screening tests exclusively; they should receive diagnostic tests. For example, Pap tests should not be used in isolation to diagnose pathology in patients with abnormal bleeding or discharge, just as screening mammograms should not be ordered in patients with symptomatic breast lumps, nipple discharge, retraction, etc. (these women should be referred for diagnostic mammography and ultrasound). It is not unusual for gynecologic oncologists to see patients with visible invasive cervical cancer who have only cervical intraepithelial neoplasia grade 3 on the preceding Pap test. There is a 34% positive predictive value that a cervical cancer will be found with a high-grade dysplastic Pap test.1 Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. It analyzes individual cells rather than a piece of tissue with intercellular relationships.
The take-home message for this column is that, if a provider sees an abnormal lesion on a cervix, they should biopsy the visible lesion to obtain a histologic diagnosis. Simply performing a Pap test alone may result in false reassurance and in underestimating the severity of disease.
Some providers will tell me that they have concerns about performing a biopsy on a grossly abnormal cervix for fear that the subsequent bleeding will be difficult to manage in the outpatient setting. This is understandable, although it is unlikely that an office equipped with the ability to perform colposcopy or excisional procedures would not have the necessary equipment to manage this. Prolonged pressure applied to the cervix with topical hemostatic agents or – in extreme cases – vaginal packing with gauze always has been effective for me in these circumstances.
The additional benefit of establishing histologic confirmation prior to referral is expediting care, including additional imaging and referrals to treating providers. If the diagnosis is inadequately established prior to their appointment with a gynecologic oncologist, it can add further delays before definitive surgical or nonsurgical management can be initiated, which is particularly problematic if the patient is experiencing severe bleeding. If the provider feels uncomfortable with proceeding with biopsy, they should inform the patient very clearly that they suspect that there is a cancer of the cervix, and it needs attention from a cancer specialist to confirm the diagnosis. This clear communication will minimize the likelihood that the patient may not show up for the subsequent appointments before her diagnosis is definitively established.
Another common scenario in which Pap tests are inappropriately applied is in the surveillance of endometrial cancer. In 2013, the Society of Gynecologic Oncology released its five “Choosing Wisely” recommendations. This included the recommendation to not perform Pap tests in the surveillance of endometrial cancer. This recommendation was based on a body of evidence that demonstrates screening for endometrial cancer recurrence with Pap smears does not detect vaginal mucosal recurrences any sooner than visualization of lesions on speculum examination.2,3 These Pap-positive recurrences almost always are visible on exam. Additionally, false positives are common in this population, particularly among women who have had radiation or have atrophic tissues.
Using Pap tests for the surveillance of cervical cancer is somewhat more complicated. Similarly, they do not detect cervical cancer recurrence any sooner than comprehensive examination does. However, this population may suffer from chronic human papillomavirus (HPV) infection, and there remains a role of the Pap test in screening for future, new HPV-related preinvasive vaginal disease. Therefore, Pap tests, and/or HPV testing can be offered to cervical cancer survivors in accordance with the American Society for Colposcopy and Cervical Pathology guidelines for noncervical cancer patients, with the caveat that, if radiation has been given, false positives are more likely.2
Pap tests clearly have an important role as a screening test in asymptomatic individuals. However, when the patient has a symptom that might be cervical cancer or a visibly suspicious lesion, she should receive a diagnostic test, and Pap tests are not designed for that purpose.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References
1. Cytopathology. 2016 Jun;27(3):201-9.
2. Gynecol Oncol. 2017 Jul;146(1):3-10.
3. Gynecol Oncol. 2011 Nov;123(2):205-7.
Pap tests have the reputation of being a simple, noninvasive, low-cost test to offer patients, and, therefore, it is understandable to believe there is no harm in offering it in all situations. However, if inappropriately applied in isolation, performing the Pap test may do more harm than good.
I recently saw a patient in consultation for cervical cancer. Her story was similar to one I’ve seen many times before. She was a 30-year-old non–English-speaking Hispanic woman who received regular care from the health department clinics.
In April of the prior year, she had noticed abnormal bleeding symptoms including intermenstrual and postcoital bleeding. She visited the health department and reported these symptoms to the provider who performed an examination. According to the provider’s notes, the cervix appeared “abnormal” and a Pap test was done. The result of this Pap test was high-grade dysplasia. The patient was promptly notified of the result and an appointment was arranged with the local ob.gyn. for a consultation, presumably for colposcopy and subsequent appropriate excisional procedure. Unfortunately, the patient did not attend that scheduled appointment. She later recounted to me that it was because she had not understood that it was important. She had a long history of abnormal Pap tests which, in the past, had only required repeat testing or minor interventions such as “freezing.”
Her bleeding symptoms became worse, and she developed abnormal discharge and pain. In November, she presented again for evaluation to the same provider. Now her cervix appeared very abnormal and was described as a “crater.” Again a Pap test was done. This time the Pap test showed “carcinoma,” and the patient was informed that she had cancer and was referred to gynecologic oncology. When I examined this unfortunate young woman, I discovered a 10 cm, stage IIB very locally advanced tumor. She is currently receiving primary chemotherapy/radiation with an approximately 60% probability of cure, and a high likelihood of lifelong sequelae of this toxic therapy.
This case highlights that, even when patients are engaged within our health care system, we can miss the opportunity to diagnose early-stage cancers if we are not utilizing screening versus diagnostic tests appropriately.
The purpose of a Pap test is as a screening test, which are designed to detect disease in asymptomatic individuals. The accuracy of these tests is determined in low-risk (asymptomatic) populations, which influences the pretest probability of disease. In asymptomatic patients with a normal screening test, it is safe to wait out the interval of time for the repeat screening test, because the combination of a low pretest probability and a high sensitivity of the test in finding disease means that there is a very low chance of missing disease.
Dysplasia rarely causes bleeding. However, invasive cervical cancer does. If a patient has a symptom such as abnormal bleeding, they no longer fit into the population with a low pretest probability for having cervical cancer. This same sensitivity of the Pap test in finding disease, combined with the now-higher pretest probability can raise the level of false-negative results to unacceptably high levels.
Patients with symptoms of cervical cancer should not receive screening tests exclusively; they should receive diagnostic tests. For example, Pap tests should not be used in isolation to diagnose pathology in patients with abnormal bleeding or discharge, just as screening mammograms should not be ordered in patients with symptomatic breast lumps, nipple discharge, retraction, etc. (these women should be referred for diagnostic mammography and ultrasound). It is not unusual for gynecologic oncologists to see patients with visible invasive cervical cancer who have only cervical intraepithelial neoplasia grade 3 on the preceding Pap test. There is a 34% positive predictive value that a cervical cancer will be found with a high-grade dysplastic Pap test.1 Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. It analyzes individual cells rather than a piece of tissue with intercellular relationships.
The take-home message for this column is that, if a provider sees an abnormal lesion on a cervix, they should biopsy the visible lesion to obtain a histologic diagnosis. Simply performing a Pap test alone may result in false reassurance and in underestimating the severity of disease.
Some providers will tell me that they have concerns about performing a biopsy on a grossly abnormal cervix for fear that the subsequent bleeding will be difficult to manage in the outpatient setting. This is understandable, although it is unlikely that an office equipped with the ability to perform colposcopy or excisional procedures would not have the necessary equipment to manage this. Prolonged pressure applied to the cervix with topical hemostatic agents or – in extreme cases – vaginal packing with gauze always has been effective for me in these circumstances.
The additional benefit of establishing histologic confirmation prior to referral is expediting care, including additional imaging and referrals to treating providers. If the diagnosis is inadequately established prior to their appointment with a gynecologic oncologist, it can add further delays before definitive surgical or nonsurgical management can be initiated, which is particularly problematic if the patient is experiencing severe bleeding. If the provider feels uncomfortable with proceeding with biopsy, they should inform the patient very clearly that they suspect that there is a cancer of the cervix, and it needs attention from a cancer specialist to confirm the diagnosis. This clear communication will minimize the likelihood that the patient may not show up for the subsequent appointments before her diagnosis is definitively established.
Another common scenario in which Pap tests are inappropriately applied is in the surveillance of endometrial cancer. In 2013, the Society of Gynecologic Oncology released its five “Choosing Wisely” recommendations. This included the recommendation to not perform Pap tests in the surveillance of endometrial cancer. This recommendation was based on a body of evidence that demonstrates screening for endometrial cancer recurrence with Pap smears does not detect vaginal mucosal recurrences any sooner than visualization of lesions on speculum examination.2,3 These Pap-positive recurrences almost always are visible on exam. Additionally, false positives are common in this population, particularly among women who have had radiation or have atrophic tissues.
Using Pap tests for the surveillance of cervical cancer is somewhat more complicated. Similarly, they do not detect cervical cancer recurrence any sooner than comprehensive examination does. However, this population may suffer from chronic human papillomavirus (HPV) infection, and there remains a role of the Pap test in screening for future, new HPV-related preinvasive vaginal disease. Therefore, Pap tests, and/or HPV testing can be offered to cervical cancer survivors in accordance with the American Society for Colposcopy and Cervical Pathology guidelines for noncervical cancer patients, with the caveat that, if radiation has been given, false positives are more likely.2
Pap tests clearly have an important role as a screening test in asymptomatic individuals. However, when the patient has a symptom that might be cervical cancer or a visibly suspicious lesion, she should receive a diagnostic test, and Pap tests are not designed for that purpose.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References
1. Cytopathology. 2016 Jun;27(3):201-9.
2. Gynecol Oncol. 2017 Jul;146(1):3-10.
3. Gynecol Oncol. 2011 Nov;123(2):205-7.
Pap tests have the reputation of being a simple, noninvasive, low-cost test to offer patients, and, therefore, it is understandable to believe there is no harm in offering it in all situations. However, if inappropriately applied in isolation, performing the Pap test may do more harm than good.
I recently saw a patient in consultation for cervical cancer. Her story was similar to one I’ve seen many times before. She was a 30-year-old non–English-speaking Hispanic woman who received regular care from the health department clinics.
In April of the prior year, she had noticed abnormal bleeding symptoms including intermenstrual and postcoital bleeding. She visited the health department and reported these symptoms to the provider who performed an examination. According to the provider’s notes, the cervix appeared “abnormal” and a Pap test was done. The result of this Pap test was high-grade dysplasia. The patient was promptly notified of the result and an appointment was arranged with the local ob.gyn. for a consultation, presumably for colposcopy and subsequent appropriate excisional procedure. Unfortunately, the patient did not attend that scheduled appointment. She later recounted to me that it was because she had not understood that it was important. She had a long history of abnormal Pap tests which, in the past, had only required repeat testing or minor interventions such as “freezing.”
Her bleeding symptoms became worse, and she developed abnormal discharge and pain. In November, she presented again for evaluation to the same provider. Now her cervix appeared very abnormal and was described as a “crater.” Again a Pap test was done. This time the Pap test showed “carcinoma,” and the patient was informed that she had cancer and was referred to gynecologic oncology. When I examined this unfortunate young woman, I discovered a 10 cm, stage IIB very locally advanced tumor. She is currently receiving primary chemotherapy/radiation with an approximately 60% probability of cure, and a high likelihood of lifelong sequelae of this toxic therapy.
This case highlights that, even when patients are engaged within our health care system, we can miss the opportunity to diagnose early-stage cancers if we are not utilizing screening versus diagnostic tests appropriately.
The purpose of a Pap test is as a screening test, which are designed to detect disease in asymptomatic individuals. The accuracy of these tests is determined in low-risk (asymptomatic) populations, which influences the pretest probability of disease. In asymptomatic patients with a normal screening test, it is safe to wait out the interval of time for the repeat screening test, because the combination of a low pretest probability and a high sensitivity of the test in finding disease means that there is a very low chance of missing disease.
Dysplasia rarely causes bleeding. However, invasive cervical cancer does. If a patient has a symptom such as abnormal bleeding, they no longer fit into the population with a low pretest probability for having cervical cancer. This same sensitivity of the Pap test in finding disease, combined with the now-higher pretest probability can raise the level of false-negative results to unacceptably high levels.
Patients with symptoms of cervical cancer should not receive screening tests exclusively; they should receive diagnostic tests. For example, Pap tests should not be used in isolation to diagnose pathology in patients with abnormal bleeding or discharge, just as screening mammograms should not be ordered in patients with symptomatic breast lumps, nipple discharge, retraction, etc. (these women should be referred for diagnostic mammography and ultrasound). It is not unusual for gynecologic oncologists to see patients with visible invasive cervical cancer who have only cervical intraepithelial neoplasia grade 3 on the preceding Pap test. There is a 34% positive predictive value that a cervical cancer will be found with a high-grade dysplastic Pap test.1 Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. It analyzes individual cells rather than a piece of tissue with intercellular relationships.
The take-home message for this column is that, if a provider sees an abnormal lesion on a cervix, they should biopsy the visible lesion to obtain a histologic diagnosis. Simply performing a Pap test alone may result in false reassurance and in underestimating the severity of disease.
Some providers will tell me that they have concerns about performing a biopsy on a grossly abnormal cervix for fear that the subsequent bleeding will be difficult to manage in the outpatient setting. This is understandable, although it is unlikely that an office equipped with the ability to perform colposcopy or excisional procedures would not have the necessary equipment to manage this. Prolonged pressure applied to the cervix with topical hemostatic agents or – in extreme cases – vaginal packing with gauze always has been effective for me in these circumstances.
The additional benefit of establishing histologic confirmation prior to referral is expediting care, including additional imaging and referrals to treating providers. If the diagnosis is inadequately established prior to their appointment with a gynecologic oncologist, it can add further delays before definitive surgical or nonsurgical management can be initiated, which is particularly problematic if the patient is experiencing severe bleeding. If the provider feels uncomfortable with proceeding with biopsy, they should inform the patient very clearly that they suspect that there is a cancer of the cervix, and it needs attention from a cancer specialist to confirm the diagnosis. This clear communication will minimize the likelihood that the patient may not show up for the subsequent appointments before her diagnosis is definitively established.
Another common scenario in which Pap tests are inappropriately applied is in the surveillance of endometrial cancer. In 2013, the Society of Gynecologic Oncology released its five “Choosing Wisely” recommendations. This included the recommendation to not perform Pap tests in the surveillance of endometrial cancer. This recommendation was based on a body of evidence that demonstrates screening for endometrial cancer recurrence with Pap smears does not detect vaginal mucosal recurrences any sooner than visualization of lesions on speculum examination.2,3 These Pap-positive recurrences almost always are visible on exam. Additionally, false positives are common in this population, particularly among women who have had radiation or have atrophic tissues.
Using Pap tests for the surveillance of cervical cancer is somewhat more complicated. Similarly, they do not detect cervical cancer recurrence any sooner than comprehensive examination does. However, this population may suffer from chronic human papillomavirus (HPV) infection, and there remains a role of the Pap test in screening for future, new HPV-related preinvasive vaginal disease. Therefore, Pap tests, and/or HPV testing can be offered to cervical cancer survivors in accordance with the American Society for Colposcopy and Cervical Pathology guidelines for noncervical cancer patients, with the caveat that, if radiation has been given, false positives are more likely.2
Pap tests clearly have an important role as a screening test in asymptomatic individuals. However, when the patient has a symptom that might be cervical cancer or a visibly suspicious lesion, she should receive a diagnostic test, and Pap tests are not designed for that purpose.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References
1. Cytopathology. 2016 Jun;27(3):201-9.
2. Gynecol Oncol. 2017 Jul;146(1):3-10.
3. Gynecol Oncol. 2011 Nov;123(2):205-7.
FDA permits marketing of first M. genitalium diagnostic test
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
Intimate partner violence, guns, and the ObGyn
On the afternoon of November 19, 2018, Dr. Tamara O’Neal was shot and killed by her ex-fiancé outside Mercy Hospital and Medical Center in Chicago, Illinois. After killing Dr. O’Neal, the gunman ran into the hospital where he exchanged gunfire with police, killing a pharmacy resident and a police officer, before he was killed by police.1
This horrific encounter between a woman and her former partner begs for a conversation about intimate partner violence (IPV). A data brief of The National Intimate Partner and Sexual Violence Survey was published in November 2018. According to this report, 30.6% of women experienced physical violence by an intimate partner in 2015, with 21.4% of women experiencing severe physical violence. In addition, 31.0% of men experienced physical violence by an intimate partner in 2015; 14.9% of men experienced severe physical violence.2

Intimate partner violence is “our lane”
The shooting at Mercy Hospital occurred amongst a backdrop of controversy between the National Rifle Association (NRA) and the medical community. On November 7, 2018, the NRA tweeted that doctors should “stay in their lane” with regard to gun control after a position paper from the American College of Physicians on reducing firearm deaths and injuries was published in the Annals of Internal Medicine.3 Doctors from every field and from all over the country responded through social media by stating that treating bullet wounds and caring for those affected by gun violence was “their lane.”4
It is time for us as a community to recognize that gun violence affects us all. The majority of mass shooters have a history of IPV and often target their current or prior partner during the shooting.5 At this intersection of IPV and gun control, the physician has a unique role. We not only treat those affected by gun violence and advocate for better gun control but we also have a duty to screen our patients for IPV. Part of the sacred patient-physician relationship is being present for our patients when they need us most. The American College of Obstetricians and Gynecologists (ACOG) recommends that ObGyns screen patients for IPV at regular intervals and recognizes that it may take several conversations before a patient discloses her history of IPV.6 Additionally, given the increased risk of gun injuries and death, it behooves us to also screen for gun safety in the home.
Ask patients about IPV, and ask again
The shooting at Mercy Hospital was a stark reminder that IPV can affect any of us. With nearly one-third of women and more than one-quarter of men experiencing IPV in their lifetime, action must be taken. The first step is to routinely screen patients for IPV, offering support and community resources. (see “Screening for intimate partner violence). The second step is to work to decrease the access perpetrators of IPV have to weapons with which to enact violence—through legislation, community engagement, and using our physician voices.
States that have passed legislation that prohibits persons with active restraining orders or a history of IPV or domestic violence from possessing firearms has seen a decrease in IPV firearm homicide rates.7 These policies can make a profound impact on the safety of our patients. Women who are in violent relationships are 5 times more likely to die if their partner has access to a firearm.5
Continue to: #BreakTheCycle...
#BreakTheCycle
The 116th Congress convened in January. We have an opportunity to make real gun legislation reform and work to keep our communities and our patients at risk for IPV safer. Tweet your representatives with #BreakTheCycle, and be on the lookout for important legislation to enact real change.
To sign the open letter from American Healthcare Professionals to the NRA regarding their recent comments and our medical experiences with gun violence, click here. Currently, there are more than 41,000 signatures.
There are numerous verified screening tools available to assess for intimate partner violence (IPV) for both pregnant and nonpregnant patients. Many recommended tools are accessible on the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf. In our office, the tool most commonly used is a 3-part question assessing domestic violence and IPV. It is important to recognize IPV can affect everyone—all races and religions regardless of socioeconomic background, sexual orientation, and pregnancy status. All patients deserve screening for IPV, and it should never be assumed a patient is not at risk. During an annual gynecology visit for return and new patients or a new obstetric intake visit, we use the following script obtained from ACOG’s Committee Opinion 518 on IPV1 :
Because violence is so common in many women’s lives and because there is help available for women being abused, I now ask every patient about domestic violence:
1. Within the past year (or since you have become pregnant) have you been hit, slapped, kicked, or otherwise physically hurt by someone?
2. Are you in a relationship with a person who threatens or physically hurts you?
3. Has anyone forced you to have sexual activities that made you feel uncomfortable?
If a patient screens positive, we assess their immediate safety. If a social worker is readily available, we arrange an urgent meeting with the patient. If offices do not have immediate access to this service, online information can be provided to patients, including the National Resource Center on Domestic Violence (https://nnedv.org/) and a toll-free number to the National Domestic Violence Hotline: 1-800-799-7233. Additionally, we ask patients about any history of verbal, physical, or sexual violence with prior partners, family members, acquaintances, coworkers, etc. Although the patient might not be at immediate risk, prior experiences with abuse can cause fear and anxiety around gynecologic and obstetric exams. Acknowledging this history can help the clinician adjust his or her physical exam and support the patient during, what may be, a triggering experience.
As an additional resource, Dr. Katherine Hicks-Courant, a resident at Tufts Medical Center, in Boston, Massachusetts, created a tool kit for providers working with pregnant patients with a history of sexual assault. It can be accessed without login online under the Junior Fellow Initiative Toolkit section at http://www.acog.org.
References
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119:412-417.
If you, or someone you know, needs help, please call The National Domestic Violence Hotline at 1-800-799-7233.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
1. Buckley M, Gorner J, Greene M. “Chicago hospital shooting: Young cop, doctor, pharmacy resident and gunman die in Mercy Hospital attack. Chicago Tribune. Nov. 20, 2018.
2. Smith SG, Zhang X, Basile KC, et al. The National Intimate Partner
and Sexual Violence Survey (NISVS): 2015 data brief – updated release. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; November 2018.
3. Butkus R, Doherty R, Bornstein SS; for the Health and Public Policy Committee of the American College of Physicians. Reducing firearm injuries and deaths in the United States: a position paper from the American College of Physicians. Ann Intern Med. 2018;169:704-707.
4. Papenfuss M. NRA Tweets Warning to Anti-Gun Doctors: ‘Stay In Your Lane’. The Huffington Post. November 8, 2018.
5. Everytown for Gun Safety website. Mass Shootings in the United States: 2009–2016. Available at https://everytownresearch.org/reports/mass-shootings-analysis/. Accessed January 17, 2019.
6. The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119(2 pt 1):412-417. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Intimate-Partner-Violence.
7. Zeoli AM, McCourt A, Buggs S, et al. Analysis of the strength of legal firearms restrictions for perpetrators of domestic violence and their associations with intimate partner homicide. Am J Epidemiol. 2018;187:2365-2371.
On the afternoon of November 19, 2018, Dr. Tamara O’Neal was shot and killed by her ex-fiancé outside Mercy Hospital and Medical Center in Chicago, Illinois. After killing Dr. O’Neal, the gunman ran into the hospital where he exchanged gunfire with police, killing a pharmacy resident and a police officer, before he was killed by police.1
This horrific encounter between a woman and her former partner begs for a conversation about intimate partner violence (IPV). A data brief of The National Intimate Partner and Sexual Violence Survey was published in November 2018. According to this report, 30.6% of women experienced physical violence by an intimate partner in 2015, with 21.4% of women experiencing severe physical violence. In addition, 31.0% of men experienced physical violence by an intimate partner in 2015; 14.9% of men experienced severe physical violence.2

Intimate partner violence is “our lane”
The shooting at Mercy Hospital occurred amongst a backdrop of controversy between the National Rifle Association (NRA) and the medical community. On November 7, 2018, the NRA tweeted that doctors should “stay in their lane” with regard to gun control after a position paper from the American College of Physicians on reducing firearm deaths and injuries was published in the Annals of Internal Medicine.3 Doctors from every field and from all over the country responded through social media by stating that treating bullet wounds and caring for those affected by gun violence was “their lane.”4
It is time for us as a community to recognize that gun violence affects us all. The majority of mass shooters have a history of IPV and often target their current or prior partner during the shooting.5 At this intersection of IPV and gun control, the physician has a unique role. We not only treat those affected by gun violence and advocate for better gun control but we also have a duty to screen our patients for IPV. Part of the sacred patient-physician relationship is being present for our patients when they need us most. The American College of Obstetricians and Gynecologists (ACOG) recommends that ObGyns screen patients for IPV at regular intervals and recognizes that it may take several conversations before a patient discloses her history of IPV.6 Additionally, given the increased risk of gun injuries and death, it behooves us to also screen for gun safety in the home.
Ask patients about IPV, and ask again
The shooting at Mercy Hospital was a stark reminder that IPV can affect any of us. With nearly one-third of women and more than one-quarter of men experiencing IPV in their lifetime, action must be taken. The first step is to routinely screen patients for IPV, offering support and community resources. (see “Screening for intimate partner violence). The second step is to work to decrease the access perpetrators of IPV have to weapons with which to enact violence—through legislation, community engagement, and using our physician voices.
States that have passed legislation that prohibits persons with active restraining orders or a history of IPV or domestic violence from possessing firearms has seen a decrease in IPV firearm homicide rates.7 These policies can make a profound impact on the safety of our patients. Women who are in violent relationships are 5 times more likely to die if their partner has access to a firearm.5
Continue to: #BreakTheCycle...
#BreakTheCycle
The 116th Congress convened in January. We have an opportunity to make real gun legislation reform and work to keep our communities and our patients at risk for IPV safer. Tweet your representatives with #BreakTheCycle, and be on the lookout for important legislation to enact real change.
To sign the open letter from American Healthcare Professionals to the NRA regarding their recent comments and our medical experiences with gun violence, click here. Currently, there are more than 41,000 signatures.
There are numerous verified screening tools available to assess for intimate partner violence (IPV) for both pregnant and nonpregnant patients. Many recommended tools are accessible on the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf. In our office, the tool most commonly used is a 3-part question assessing domestic violence and IPV. It is important to recognize IPV can affect everyone—all races and religions regardless of socioeconomic background, sexual orientation, and pregnancy status. All patients deserve screening for IPV, and it should never be assumed a patient is not at risk. During an annual gynecology visit for return and new patients or a new obstetric intake visit, we use the following script obtained from ACOG’s Committee Opinion 518 on IPV1 :
Because violence is so common in many women’s lives and because there is help available for women being abused, I now ask every patient about domestic violence:
1. Within the past year (or since you have become pregnant) have you been hit, slapped, kicked, or otherwise physically hurt by someone?
2. Are you in a relationship with a person who threatens or physically hurts you?
3. Has anyone forced you to have sexual activities that made you feel uncomfortable?
If a patient screens positive, we assess their immediate safety. If a social worker is readily available, we arrange an urgent meeting with the patient. If offices do not have immediate access to this service, online information can be provided to patients, including the National Resource Center on Domestic Violence (https://nnedv.org/) and a toll-free number to the National Domestic Violence Hotline: 1-800-799-7233. Additionally, we ask patients about any history of verbal, physical, or sexual violence with prior partners, family members, acquaintances, coworkers, etc. Although the patient might not be at immediate risk, prior experiences with abuse can cause fear and anxiety around gynecologic and obstetric exams. Acknowledging this history can help the clinician adjust his or her physical exam and support the patient during, what may be, a triggering experience.
As an additional resource, Dr. Katherine Hicks-Courant, a resident at Tufts Medical Center, in Boston, Massachusetts, created a tool kit for providers working with pregnant patients with a history of sexual assault. It can be accessed without login online under the Junior Fellow Initiative Toolkit section at http://www.acog.org.
References
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119:412-417.
If you, or someone you know, needs help, please call The National Domestic Violence Hotline at 1-800-799-7233.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
On the afternoon of November 19, 2018, Dr. Tamara O’Neal was shot and killed by her ex-fiancé outside Mercy Hospital and Medical Center in Chicago, Illinois. After killing Dr. O’Neal, the gunman ran into the hospital where he exchanged gunfire with police, killing a pharmacy resident and a police officer, before he was killed by police.1
This horrific encounter between a woman and her former partner begs for a conversation about intimate partner violence (IPV). A data brief of The National Intimate Partner and Sexual Violence Survey was published in November 2018. According to this report, 30.6% of women experienced physical violence by an intimate partner in 2015, with 21.4% of women experiencing severe physical violence. In addition, 31.0% of men experienced physical violence by an intimate partner in 2015; 14.9% of men experienced severe physical violence.2

Intimate partner violence is “our lane”
The shooting at Mercy Hospital occurred amongst a backdrop of controversy between the National Rifle Association (NRA) and the medical community. On November 7, 2018, the NRA tweeted that doctors should “stay in their lane” with regard to gun control after a position paper from the American College of Physicians on reducing firearm deaths and injuries was published in the Annals of Internal Medicine.3 Doctors from every field and from all over the country responded through social media by stating that treating bullet wounds and caring for those affected by gun violence was “their lane.”4
It is time for us as a community to recognize that gun violence affects us all. The majority of mass shooters have a history of IPV and often target their current or prior partner during the shooting.5 At this intersection of IPV and gun control, the physician has a unique role. We not only treat those affected by gun violence and advocate for better gun control but we also have a duty to screen our patients for IPV. Part of the sacred patient-physician relationship is being present for our patients when they need us most. The American College of Obstetricians and Gynecologists (ACOG) recommends that ObGyns screen patients for IPV at regular intervals and recognizes that it may take several conversations before a patient discloses her history of IPV.6 Additionally, given the increased risk of gun injuries and death, it behooves us to also screen for gun safety in the home.
Ask patients about IPV, and ask again
The shooting at Mercy Hospital was a stark reminder that IPV can affect any of us. With nearly one-third of women and more than one-quarter of men experiencing IPV in their lifetime, action must be taken. The first step is to routinely screen patients for IPV, offering support and community resources. (see “Screening for intimate partner violence). The second step is to work to decrease the access perpetrators of IPV have to weapons with which to enact violence—through legislation, community engagement, and using our physician voices.
States that have passed legislation that prohibits persons with active restraining orders or a history of IPV or domestic violence from possessing firearms has seen a decrease in IPV firearm homicide rates.7 These policies can make a profound impact on the safety of our patients. Women who are in violent relationships are 5 times more likely to die if their partner has access to a firearm.5
Continue to: #BreakTheCycle...
#BreakTheCycle
The 116th Congress convened in January. We have an opportunity to make real gun legislation reform and work to keep our communities and our patients at risk for IPV safer. Tweet your representatives with #BreakTheCycle, and be on the lookout for important legislation to enact real change.
To sign the open letter from American Healthcare Professionals to the NRA regarding their recent comments and our medical experiences with gun violence, click here. Currently, there are more than 41,000 signatures.
There are numerous verified screening tools available to assess for intimate partner violence (IPV) for both pregnant and nonpregnant patients. Many recommended tools are accessible on the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf. In our office, the tool most commonly used is a 3-part question assessing domestic violence and IPV. It is important to recognize IPV can affect everyone—all races and religions regardless of socioeconomic background, sexual orientation, and pregnancy status. All patients deserve screening for IPV, and it should never be assumed a patient is not at risk. During an annual gynecology visit for return and new patients or a new obstetric intake visit, we use the following script obtained from ACOG’s Committee Opinion 518 on IPV1 :
Because violence is so common in many women’s lives and because there is help available for women being abused, I now ask every patient about domestic violence:
1. Within the past year (or since you have become pregnant) have you been hit, slapped, kicked, or otherwise physically hurt by someone?
2. Are you in a relationship with a person who threatens or physically hurts you?
3. Has anyone forced you to have sexual activities that made you feel uncomfortable?
If a patient screens positive, we assess their immediate safety. If a social worker is readily available, we arrange an urgent meeting with the patient. If offices do not have immediate access to this service, online information can be provided to patients, including the National Resource Center on Domestic Violence (https://nnedv.org/) and a toll-free number to the National Domestic Violence Hotline: 1-800-799-7233. Additionally, we ask patients about any history of verbal, physical, or sexual violence with prior partners, family members, acquaintances, coworkers, etc. Although the patient might not be at immediate risk, prior experiences with abuse can cause fear and anxiety around gynecologic and obstetric exams. Acknowledging this history can help the clinician adjust his or her physical exam and support the patient during, what may be, a triggering experience.
As an additional resource, Dr. Katherine Hicks-Courant, a resident at Tufts Medical Center, in Boston, Massachusetts, created a tool kit for providers working with pregnant patients with a history of sexual assault. It can be accessed without login online under the Junior Fellow Initiative Toolkit section at http://www.acog.org.
References
1. American College of Obstetricians and Gynecologists. Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119:412-417.
If you, or someone you know, needs help, please call The National Domestic Violence Hotline at 1-800-799-7233.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
1. Buckley M, Gorner J, Greene M. “Chicago hospital shooting: Young cop, doctor, pharmacy resident and gunman die in Mercy Hospital attack. Chicago Tribune. Nov. 20, 2018.
2. Smith SG, Zhang X, Basile KC, et al. The National Intimate Partner
and Sexual Violence Survey (NISVS): 2015 data brief – updated release. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; November 2018.
3. Butkus R, Doherty R, Bornstein SS; for the Health and Public Policy Committee of the American College of Physicians. Reducing firearm injuries and deaths in the United States: a position paper from the American College of Physicians. Ann Intern Med. 2018;169:704-707.
4. Papenfuss M. NRA Tweets Warning to Anti-Gun Doctors: ‘Stay In Your Lane’. The Huffington Post. November 8, 2018.
5. Everytown for Gun Safety website. Mass Shootings in the United States: 2009–2016. Available at https://everytownresearch.org/reports/mass-shootings-analysis/. Accessed January 17, 2019.
6. The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119(2 pt 1):412-417. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Intimate-Partner-Violence.
7. Zeoli AM, McCourt A, Buggs S, et al. Analysis of the strength of legal firearms restrictions for perpetrators of domestic violence and their associations with intimate partner homicide. Am J Epidemiol. 2018;187:2365-2371.
1. Buckley M, Gorner J, Greene M. “Chicago hospital shooting: Young cop, doctor, pharmacy resident and gunman die in Mercy Hospital attack. Chicago Tribune. Nov. 20, 2018.
2. Smith SG, Zhang X, Basile KC, et al. The National Intimate Partner
and Sexual Violence Survey (NISVS): 2015 data brief – updated release. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; November 2018.
3. Butkus R, Doherty R, Bornstein SS; for the Health and Public Policy Committee of the American College of Physicians. Reducing firearm injuries and deaths in the United States: a position paper from the American College of Physicians. Ann Intern Med. 2018;169:704-707.
4. Papenfuss M. NRA Tweets Warning to Anti-Gun Doctors: ‘Stay In Your Lane’. The Huffington Post. November 8, 2018.
5. Everytown for Gun Safety website. Mass Shootings in the United States: 2009–2016. Available at https://everytownresearch.org/reports/mass-shootings-analysis/. Accessed January 17, 2019.
6. The American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 518: intimate partner violence. Obstet Gynecol. 2012;119(2 pt 1):412-417. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/Intimate-Partner-Violence.
7. Zeoli AM, McCourt A, Buggs S, et al. Analysis of the strength of legal firearms restrictions for perpetrators of domestic violence and their associations with intimate partner homicide. Am J Epidemiol. 2018;187:2365-2371.