User login
A Case Report on Bortezomib- Induced Hypotension: Rare Adverse Effect in Proteasome Inhibitor Therapy
Case Presentation
A 75-year-old man with chronic kidney disease, hypertension and diabetes mellitus presented with acute kidney injury (creatinine 5.2 from baseline 4.2) and a two-week history of increased urinary frequency. Labs revealed high anion gap metabolic acidosis, proteinuria, hematuria, pyuria, and acute on chronic anemia. He was diagnosed with kappa light chain nephropathy and multiple myeloma with 32% plasma cells on bone marrow biopsy. He began treatment with bortezomib, cyclophosphamide, and dexamethasone (Cy- BorD). Three days after cyclophosphamide and five days after bortezomib, the patient developed persistent hypotension with systolic BP in the 50s, unresponsive to fluids and Trendelenburg position. Due to end-stage renal disease with anuria, fluid resuscitation was limited. He required norepinephrine and was transferred to the ICU. Given instability, hemodialysis was deferred, and continuous renal replacement therapy was initiated. Shock evaluation included a CT abdomen showing enteritis versus ileus; however, infectious workup was negative. Cardiogenic shock was ruled out with a serial echocardiogram showing normal ejection fractions of 59-67% without significant valvular disease. The workup for adrenal insufficiency was negative. After the exclusion of other potential causes of shock, severe refractory hypotension was attributed to bortezomib toxicity.Hypotension is a known adverse effect of bortezomib. Orthostatic hypotension may occur in 8 to 9% of patients, and rarely, patients may experience heart failure, conduction disorders and arrhythmias, or cardiogenic shock. The pathologic mechanism of this toxicity is still poorly understood. Proposed mechanisms include direct endothelial toxicity as evidenced by thrombotic microangiopathy or impairment of sympathetic and parasympathetic nerve fibres. Most commonly, patients experience neurotoxicity, which may manifest as autonomic dysfunction or peripheral neuropathy. Cardiovascular complications are typically reversible. Our patient’s cardiac function remained within normal limits; therefore, his persistent hypotension was felt to be the result of direct toxicity from bortezomib rather than cardiogenic shock. Ultimately, blood pressure did improve, and vasopressors were discontinued. However, he continued to have orthostatic hypotension and continued to require supportive fludrocortisone, midodrine, and pyridostigmine. Goals of care have been discussed, and he wished to continue pursuing restorative care, with a plan for transition to carfilzomib versus daratumumab outpatient.
Case Presentation
A 75-year-old man with chronic kidney disease, hypertension and diabetes mellitus presented with acute kidney injury (creatinine 5.2 from baseline 4.2) and a two-week history of increased urinary frequency. Labs revealed high anion gap metabolic acidosis, proteinuria, hematuria, pyuria, and acute on chronic anemia. He was diagnosed with kappa light chain nephropathy and multiple myeloma with 32% plasma cells on bone marrow biopsy. He began treatment with bortezomib, cyclophosphamide, and dexamethasone (Cy- BorD). Three days after cyclophosphamide and five days after bortezomib, the patient developed persistent hypotension with systolic BP in the 50s, unresponsive to fluids and Trendelenburg position. Due to end-stage renal disease with anuria, fluid resuscitation was limited. He required norepinephrine and was transferred to the ICU. Given instability, hemodialysis was deferred, and continuous renal replacement therapy was initiated. Shock evaluation included a CT abdomen showing enteritis versus ileus; however, infectious workup was negative. Cardiogenic shock was ruled out with a serial echocardiogram showing normal ejection fractions of 59-67% without significant valvular disease. The workup for adrenal insufficiency was negative. After the exclusion of other potential causes of shock, severe refractory hypotension was attributed to bortezomib toxicity.Hypotension is a known adverse effect of bortezomib. Orthostatic hypotension may occur in 8 to 9% of patients, and rarely, patients may experience heart failure, conduction disorders and arrhythmias, or cardiogenic shock. The pathologic mechanism of this toxicity is still poorly understood. Proposed mechanisms include direct endothelial toxicity as evidenced by thrombotic microangiopathy or impairment of sympathetic and parasympathetic nerve fibres. Most commonly, patients experience neurotoxicity, which may manifest as autonomic dysfunction or peripheral neuropathy. Cardiovascular complications are typically reversible. Our patient’s cardiac function remained within normal limits; therefore, his persistent hypotension was felt to be the result of direct toxicity from bortezomib rather than cardiogenic shock. Ultimately, blood pressure did improve, and vasopressors were discontinued. However, he continued to have orthostatic hypotension and continued to require supportive fludrocortisone, midodrine, and pyridostigmine. Goals of care have been discussed, and he wished to continue pursuing restorative care, with a plan for transition to carfilzomib versus daratumumab outpatient.
Case Presentation
A 75-year-old man with chronic kidney disease, hypertension and diabetes mellitus presented with acute kidney injury (creatinine 5.2 from baseline 4.2) and a two-week history of increased urinary frequency. Labs revealed high anion gap metabolic acidosis, proteinuria, hematuria, pyuria, and acute on chronic anemia. He was diagnosed with kappa light chain nephropathy and multiple myeloma with 32% plasma cells on bone marrow biopsy. He began treatment with bortezomib, cyclophosphamide, and dexamethasone (Cy- BorD). Three days after cyclophosphamide and five days after bortezomib, the patient developed persistent hypotension with systolic BP in the 50s, unresponsive to fluids and Trendelenburg position. Due to end-stage renal disease with anuria, fluid resuscitation was limited. He required norepinephrine and was transferred to the ICU. Given instability, hemodialysis was deferred, and continuous renal replacement therapy was initiated. Shock evaluation included a CT abdomen showing enteritis versus ileus; however, infectious workup was negative. Cardiogenic shock was ruled out with a serial echocardiogram showing normal ejection fractions of 59-67% without significant valvular disease. The workup for adrenal insufficiency was negative. After the exclusion of other potential causes of shock, severe refractory hypotension was attributed to bortezomib toxicity.Hypotension is a known adverse effect of bortezomib. Orthostatic hypotension may occur in 8 to 9% of patients, and rarely, patients may experience heart failure, conduction disorders and arrhythmias, or cardiogenic shock. The pathologic mechanism of this toxicity is still poorly understood. Proposed mechanisms include direct endothelial toxicity as evidenced by thrombotic microangiopathy or impairment of sympathetic and parasympathetic nerve fibres. Most commonly, patients experience neurotoxicity, which may manifest as autonomic dysfunction or peripheral neuropathy. Cardiovascular complications are typically reversible. Our patient’s cardiac function remained within normal limits; therefore, his persistent hypotension was felt to be the result of direct toxicity from bortezomib rather than cardiogenic shock. Ultimately, blood pressure did improve, and vasopressors were discontinued. However, he continued to have orthostatic hypotension and continued to require supportive fludrocortisone, midodrine, and pyridostigmine. Goals of care have been discussed, and he wished to continue pursuing restorative care, with a plan for transition to carfilzomib versus daratumumab outpatient.
An Uncommon Presentation of Marginal Zone Lymphoma Involving the Sciatic Foramen
Background
Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma most commonly arising in mucosal, nodal, or splenic tissues. While extranodal presentations are recognized, involvement of the sciatic foramen is exceedingly rare. We present a unique case of stage IV MZL with primary involvement of the left sciatic foramen, identified incidentally during urologic evaluation.
Case Presentation
A 74-year-old male patient was referred for hematologic evaluation after imaging revealed a left sciatic foraminal mass during work-up for elevated PSA. CT abdomen/pelvis revealed a 4.7 cm mass in the left sciatic foramen. Follow-up PET-CT confirmed hypermetabolic activity in the mass, with additional areas of uptake in the right ilium and pleural- pericardial regions. The patient was asymptomatic and denied B-symptoms. CT-guided biopsy of the sciatic mass revealed low-grade B-cell lymphoma. Flow cytometry showed a CD20-positive, CD5-negative, CD10-negative, lambda light chain–restricted population consistent with marginal zone lymphoma. Laboratory studies demonstrated iron deficiency anemia, with otherwise unremarkable counts and chemistries. He was started on monotherapy with rituximab for four cycles. He tolerated treatment well. Interval PET imaging in April 2025 showed stable disease in the sciatic foramen and mild improvement in pleural- pericardial uptake. He is planned to start obinutuzumab in the upcoming month.
Discussion
This case illustrates a rare anatomic presentation of MZL, likely representing primary sciatic foramen involvement. The presence of additional PETavid lesions complicates staging, raising consideration of stage I vs. III/IV disease. Biopsy was limited to the sciatic lesion, and no bone marrow sampling was performed. Given the patient’s excellent performance status, absence of symptoms, and low tumor burden, single-agent rituximab was chosen initially in accordance with NCCN guidelines.
Conclusions
Sciatic foramen involvement by MZL is an extremely rare occurrence and may mimic more common soft tissue or neurogenic tumors radiographically. This case underscores the importance of biopsy for diagnosis and the value of multidisciplinary care. In the veteran population, such incidental findings on imaging warrant comprehensive evaluation, particularly in atypical anatomical sites.
Background
Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma most commonly arising in mucosal, nodal, or splenic tissues. While extranodal presentations are recognized, involvement of the sciatic foramen is exceedingly rare. We present a unique case of stage IV MZL with primary involvement of the left sciatic foramen, identified incidentally during urologic evaluation.
Case Presentation
A 74-year-old male patient was referred for hematologic evaluation after imaging revealed a left sciatic foraminal mass during work-up for elevated PSA. CT abdomen/pelvis revealed a 4.7 cm mass in the left sciatic foramen. Follow-up PET-CT confirmed hypermetabolic activity in the mass, with additional areas of uptake in the right ilium and pleural- pericardial regions. The patient was asymptomatic and denied B-symptoms. CT-guided biopsy of the sciatic mass revealed low-grade B-cell lymphoma. Flow cytometry showed a CD20-positive, CD5-negative, CD10-negative, lambda light chain–restricted population consistent with marginal zone lymphoma. Laboratory studies demonstrated iron deficiency anemia, with otherwise unremarkable counts and chemistries. He was started on monotherapy with rituximab for four cycles. He tolerated treatment well. Interval PET imaging in April 2025 showed stable disease in the sciatic foramen and mild improvement in pleural- pericardial uptake. He is planned to start obinutuzumab in the upcoming month.
Discussion
This case illustrates a rare anatomic presentation of MZL, likely representing primary sciatic foramen involvement. The presence of additional PETavid lesions complicates staging, raising consideration of stage I vs. III/IV disease. Biopsy was limited to the sciatic lesion, and no bone marrow sampling was performed. Given the patient’s excellent performance status, absence of symptoms, and low tumor burden, single-agent rituximab was chosen initially in accordance with NCCN guidelines.
Conclusions
Sciatic foramen involvement by MZL is an extremely rare occurrence and may mimic more common soft tissue or neurogenic tumors radiographically. This case underscores the importance of biopsy for diagnosis and the value of multidisciplinary care. In the veteran population, such incidental findings on imaging warrant comprehensive evaluation, particularly in atypical anatomical sites.
Background
Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma most commonly arising in mucosal, nodal, or splenic tissues. While extranodal presentations are recognized, involvement of the sciatic foramen is exceedingly rare. We present a unique case of stage IV MZL with primary involvement of the left sciatic foramen, identified incidentally during urologic evaluation.
Case Presentation
A 74-year-old male patient was referred for hematologic evaluation after imaging revealed a left sciatic foraminal mass during work-up for elevated PSA. CT abdomen/pelvis revealed a 4.7 cm mass in the left sciatic foramen. Follow-up PET-CT confirmed hypermetabolic activity in the mass, with additional areas of uptake in the right ilium and pleural- pericardial regions. The patient was asymptomatic and denied B-symptoms. CT-guided biopsy of the sciatic mass revealed low-grade B-cell lymphoma. Flow cytometry showed a CD20-positive, CD5-negative, CD10-negative, lambda light chain–restricted population consistent with marginal zone lymphoma. Laboratory studies demonstrated iron deficiency anemia, with otherwise unremarkable counts and chemistries. He was started on monotherapy with rituximab for four cycles. He tolerated treatment well. Interval PET imaging in April 2025 showed stable disease in the sciatic foramen and mild improvement in pleural- pericardial uptake. He is planned to start obinutuzumab in the upcoming month.
Discussion
This case illustrates a rare anatomic presentation of MZL, likely representing primary sciatic foramen involvement. The presence of additional PETavid lesions complicates staging, raising consideration of stage I vs. III/IV disease. Biopsy was limited to the sciatic lesion, and no bone marrow sampling was performed. Given the patient’s excellent performance status, absence of symptoms, and low tumor burden, single-agent rituximab was chosen initially in accordance with NCCN guidelines.
Conclusions
Sciatic foramen involvement by MZL is an extremely rare occurrence and may mimic more common soft tissue or neurogenic tumors radiographically. This case underscores the importance of biopsy for diagnosis and the value of multidisciplinary care. In the veteran population, such incidental findings on imaging warrant comprehensive evaluation, particularly in atypical anatomical sites.
Agent Orange Exposure and Genetic Factors Independently Raise Risk for Multiple Lymphoma Types
TOPLINE: A large-scale case-control study using the Million Veteran Program (MVP) found The study found independent associations of both genetic predisposition and Agent Orange (AO) exposure for several lymphoid malignant neoplasm subtypes.
METHODOLOGY:
A case-control study included 255,155 US veterans enrolled in the MVP with available genotype, Agent Orange exposure information, and lymphoid malignant neoplasm diagnosis from January 1, 1965, through June T1, 2024.
Analysis focused on non-Hispanic White veterans (median age 67 years; 92.5% male) due to ancestry distribution requirements for genome-wide association studies data availability.
Researchers excluded 628 samples across all lymphoid malignant neoplasm groups and 61,343 control samples due to unavailability of AO exposure information.
Investigators analyzed risk for chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, marginal zone lymphoma, and multiple myeloma as primary outcomes.
TAKEAWAY:
Agent Orange exposure was associated with increased risk for chronic lymphocytic leukemia (odds ratio [OR], 1.61; 95% confidence interval [CI], 1.40-1.84), diffuse large B-cell lymphoma (OR, 1.26; 95% CI, 1.03-1.53), follicular lymphoma (OR, 1.71; 95% CI, 1.39-2.11), and multiple myeloma (OR, 1.58; 95% CI, 1.35-1.86).
Polygenic risk scores showed significant associations with all subtypes: chronic lymphocytic leukemia (OR, 1.81; 95% CI, 1.70-1.93), diffuse large B-cell lymphoma (OR, 1.12; 95% CI, 1.02-1.21), follicular lymphoma (OR, 1.33; 95% CI, 1.21-1.47), marginal zone lymphoma (OR, 1.17; 95% CI, 1.04-1.32), and multiple myeloma (OR, 1.41; 95% CI, 1.31-1.52).
No significant polygenic risk score and AO exposure interactions were observed in the development of any lymphoid malignant neoplasm subtypes.
The researchers found independent associations of both genetic predisposition and Agent Orange exposure on several lymphoid malignant neoplasm subtypes.
IN PRACTICE:
"Our study addressed the public health concerns surrounding AO exposure and lymphoid malignant neoplasms, finding that both AO exposure and polygenic risk are independently associated with disease, suggesting potentially distinct and additive pathways that merit further investigation,” the authors wrote.
SOURCE: The study was led by Xueyi Teng, PhD, Department of Biological Chemistry, School of Medicine, University of California in Irvine, and Helen Ma, MD, Tibor Rubin Veterans Affairs Medical Center in Long Beach. It was published online in JAMA Network Open.
LIMITATIONS: According to the authors, while this represents the largest study of Agent Orange exposure and genetic risk in lymphoid malignant neoplasm development, the power to find interaction associations in specific subtypes might be limited. Self-reported AO exposure may have introduced survival bias, especially in aggressive subtypes, as patients with aggressive tumors might have died before joining the MVP. Additionally, approximately half of the patients were diagnosed with lymphoid malignant neoplasm before self-reporting AO exposure in the survey, potentially introducing recall bias.
DISCLOSURES: Xueyi Teng, PhD, reported receiving grants from the George E. Hewitt Foundation for Medical Research Postdoc Fellowship during the conduct of the study. The research was supported by grant MVPOOO and Veterans Affairs Career Development Award 1IK2CX002437-O1A1. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: A large-scale case-control study using the Million Veteran Program (MVP) found The study found independent associations of both genetic predisposition and Agent Orange (AO) exposure for several lymphoid malignant neoplasm subtypes.
METHODOLOGY:
A case-control study included 255,155 US veterans enrolled in the MVP with available genotype, Agent Orange exposure information, and lymphoid malignant neoplasm diagnosis from January 1, 1965, through June T1, 2024.
Analysis focused on non-Hispanic White veterans (median age 67 years; 92.5% male) due to ancestry distribution requirements for genome-wide association studies data availability.
Researchers excluded 628 samples across all lymphoid malignant neoplasm groups and 61,343 control samples due to unavailability of AO exposure information.
Investigators analyzed risk for chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, marginal zone lymphoma, and multiple myeloma as primary outcomes.
TAKEAWAY:
Agent Orange exposure was associated with increased risk for chronic lymphocytic leukemia (odds ratio [OR], 1.61; 95% confidence interval [CI], 1.40-1.84), diffuse large B-cell lymphoma (OR, 1.26; 95% CI, 1.03-1.53), follicular lymphoma (OR, 1.71; 95% CI, 1.39-2.11), and multiple myeloma (OR, 1.58; 95% CI, 1.35-1.86).
Polygenic risk scores showed significant associations with all subtypes: chronic lymphocytic leukemia (OR, 1.81; 95% CI, 1.70-1.93), diffuse large B-cell lymphoma (OR, 1.12; 95% CI, 1.02-1.21), follicular lymphoma (OR, 1.33; 95% CI, 1.21-1.47), marginal zone lymphoma (OR, 1.17; 95% CI, 1.04-1.32), and multiple myeloma (OR, 1.41; 95% CI, 1.31-1.52).
No significant polygenic risk score and AO exposure interactions were observed in the development of any lymphoid malignant neoplasm subtypes.
The researchers found independent associations of both genetic predisposition and Agent Orange exposure on several lymphoid malignant neoplasm subtypes.
IN PRACTICE:
"Our study addressed the public health concerns surrounding AO exposure and lymphoid malignant neoplasms, finding that both AO exposure and polygenic risk are independently associated with disease, suggesting potentially distinct and additive pathways that merit further investigation,” the authors wrote.
SOURCE: The study was led by Xueyi Teng, PhD, Department of Biological Chemistry, School of Medicine, University of California in Irvine, and Helen Ma, MD, Tibor Rubin Veterans Affairs Medical Center in Long Beach. It was published online in JAMA Network Open.
LIMITATIONS: According to the authors, while this represents the largest study of Agent Orange exposure and genetic risk in lymphoid malignant neoplasm development, the power to find interaction associations in specific subtypes might be limited. Self-reported AO exposure may have introduced survival bias, especially in aggressive subtypes, as patients with aggressive tumors might have died before joining the MVP. Additionally, approximately half of the patients were diagnosed with lymphoid malignant neoplasm before self-reporting AO exposure in the survey, potentially introducing recall bias.
DISCLOSURES: Xueyi Teng, PhD, reported receiving grants from the George E. Hewitt Foundation for Medical Research Postdoc Fellowship during the conduct of the study. The research was supported by grant MVPOOO and Veterans Affairs Career Development Award 1IK2CX002437-O1A1. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: A large-scale case-control study using the Million Veteran Program (MVP) found The study found independent associations of both genetic predisposition and Agent Orange (AO) exposure for several lymphoid malignant neoplasm subtypes.
METHODOLOGY:
A case-control study included 255,155 US veterans enrolled in the MVP with available genotype, Agent Orange exposure information, and lymphoid malignant neoplasm diagnosis from January 1, 1965, through June T1, 2024.
Analysis focused on non-Hispanic White veterans (median age 67 years; 92.5% male) due to ancestry distribution requirements for genome-wide association studies data availability.
Researchers excluded 628 samples across all lymphoid malignant neoplasm groups and 61,343 control samples due to unavailability of AO exposure information.
Investigators analyzed risk for chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, marginal zone lymphoma, and multiple myeloma as primary outcomes.
TAKEAWAY:
Agent Orange exposure was associated with increased risk for chronic lymphocytic leukemia (odds ratio [OR], 1.61; 95% confidence interval [CI], 1.40-1.84), diffuse large B-cell lymphoma (OR, 1.26; 95% CI, 1.03-1.53), follicular lymphoma (OR, 1.71; 95% CI, 1.39-2.11), and multiple myeloma (OR, 1.58; 95% CI, 1.35-1.86).
Polygenic risk scores showed significant associations with all subtypes: chronic lymphocytic leukemia (OR, 1.81; 95% CI, 1.70-1.93), diffuse large B-cell lymphoma (OR, 1.12; 95% CI, 1.02-1.21), follicular lymphoma (OR, 1.33; 95% CI, 1.21-1.47), marginal zone lymphoma (OR, 1.17; 95% CI, 1.04-1.32), and multiple myeloma (OR, 1.41; 95% CI, 1.31-1.52).
No significant polygenic risk score and AO exposure interactions were observed in the development of any lymphoid malignant neoplasm subtypes.
The researchers found independent associations of both genetic predisposition and Agent Orange exposure on several lymphoid malignant neoplasm subtypes.
IN PRACTICE:
"Our study addressed the public health concerns surrounding AO exposure and lymphoid malignant neoplasms, finding that both AO exposure and polygenic risk are independently associated with disease, suggesting potentially distinct and additive pathways that merit further investigation,” the authors wrote.
SOURCE: The study was led by Xueyi Teng, PhD, Department of Biological Chemistry, School of Medicine, University of California in Irvine, and Helen Ma, MD, Tibor Rubin Veterans Affairs Medical Center in Long Beach. It was published online in JAMA Network Open.
LIMITATIONS: According to the authors, while this represents the largest study of Agent Orange exposure and genetic risk in lymphoid malignant neoplasm development, the power to find interaction associations in specific subtypes might be limited. Self-reported AO exposure may have introduced survival bias, especially in aggressive subtypes, as patients with aggressive tumors might have died before joining the MVP. Additionally, approximately half of the patients were diagnosed with lymphoid malignant neoplasm before self-reporting AO exposure in the survey, potentially introducing recall bias.
DISCLOSURES: Xueyi Teng, PhD, reported receiving grants from the George E. Hewitt Foundation for Medical Research Postdoc Fellowship during the conduct of the study. The research was supported by grant MVPOOO and Veterans Affairs Career Development Award 1IK2CX002437-O1A1. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Study Investigates Non-Hodgkin Lymphoma in Air Force Missileers
Individuals working near intercontinental ballistic missiles (ICBMs), may be at higher risk of developing non-Hodgkin lymphoma (NHL) according to a preprint analysis conducted on missileers at Malmstrom Air Force Base in Montana. The study, which has not undergone peer review, found higher rates of NHL diagnosis at younger ages compared with the general population. The study also found a statistically significant increase in NHL diagnoses among older missileers, with such rates surpassing expected benchmarks.
The findings build on anecdotal and evidentiary data gathered in the last 50-plus years, including from the Torchlight Initiative, established in 2023 to collect self-reported cancer diagnoses and related fatalities from personnel and family members associated with the ICBM community.
The report shows patterns that “warranted a detailed statistical analysis,” leading to a granular examination of the registry and categorization of the data by cancer type, geographical location, and specific demographics. This narrowed the focus to 18 missileers who served at Malmstrom and were diagnosed with NHL.
In 2001, the Air Force Institute for Operational Health did a site evaluation and sampled for potential chemical and biological contaminants at Malmstrom following various reports of cancers from missileers, including 2 who died after being diagnosed with NHL. In a 2005 review, the Air Force said, “there is not sufficient evidence to consider the possibility of a cancer clustering to justify further investigation.”
In 2022, Lt. Col. Daniel Sebeck, a vice commander of Space Delta 8 in Colorado who served at Malmstrom and a close friend and fellow missileer were diagnosed with NHL. Sebeck discovered 36 cancer cases among missileers who had been stationed at Malmstrom. Ten developed NHL, 2 developed Hodgkin lymphoma, and 24 developed another form of cancer. The Air Force has acknowledged the concerns.
In 2023, US Air Force School of Aerospace Medicine (USAFSAM) approved the Missile Community Cancer Study (MCCS) to assess “specific cancer concerns raised by missile community members across related career fields and also examines the possibility of clusters of non-Hodgkin’s lymphoma at intercontinental ballistic missile bases.” The study compares 14 common cancers in the general population with that of missile-related career fields. The USAFSAM is reviewing records from former and current Missile Community members on active duty from 1976-2010, as well as state and national cancer data from multiple registries.
Early results from the MCCS suggested elevated rates of some cancers—mainly breast and prostate cancer—among missileers, maintainers, and other ICMB-related job positions, which aligns with other national cancer data.
At a June 2024 AFGSC town hall, officials announced that missileers would now have their information submitted to the Defense Occupational and Environmental Health Readiness System (DOEHRS), a Pentagon database for reporting occupational and exposure hazards.
"This info from DOEHRS flows into the recently developed Individual Longitudinal Exposure Record, a system that compiles occupational and environmental health data throughout a person's career," Lt. Col. John Severns, a spokesperson for Air Force Global Strike Command, said. DOEHRS, which has tracked Air Force records since 2010, allows US Department of Defense and US Department of Veterans Affairs clinical staff to access the information.
MCCS considers potential PCB exposures an occupational hazard. The Air Force says researchers are working with the System Program Offices and leadership to determine the timeframe of PCB removal from bases.
The lack of incontrovertible evidence connecting workplace toxins to NHL has often stymied patients and their family members from receiving appropriate benefits. An “informal talk” in April led by Rep. Mark Takano (D-CA) and Sen. Richard Blumenthal (D-CT) focused on exposures to hazardous materials at US military bases. Participants included various advocacy groups like the Torchlight Initiative, the Invisible Enemy, and Burn Pits 360.
More than a dozen veterans spoke about serving at military bases where they were exposed to a variety of harmful substances, and issues they faced in receiving coverage. David Crete, a veteran and chairman of The Invisible Enemy, said, “I am asking Congress to please allow us to get the benefits every other veteran earned. We are not asking to be special but to be treated equal.”
Rep. Takano called for greater focus on toxic exposures at US military bases: “We must push back against the idea that service members are only in harm’s way in war zones.”
Individuals working near intercontinental ballistic missiles (ICBMs), may be at higher risk of developing non-Hodgkin lymphoma (NHL) according to a preprint analysis conducted on missileers at Malmstrom Air Force Base in Montana. The study, which has not undergone peer review, found higher rates of NHL diagnosis at younger ages compared with the general population. The study also found a statistically significant increase in NHL diagnoses among older missileers, with such rates surpassing expected benchmarks.
The findings build on anecdotal and evidentiary data gathered in the last 50-plus years, including from the Torchlight Initiative, established in 2023 to collect self-reported cancer diagnoses and related fatalities from personnel and family members associated with the ICBM community.
The report shows patterns that “warranted a detailed statistical analysis,” leading to a granular examination of the registry and categorization of the data by cancer type, geographical location, and specific demographics. This narrowed the focus to 18 missileers who served at Malmstrom and were diagnosed with NHL.
In 2001, the Air Force Institute for Operational Health did a site evaluation and sampled for potential chemical and biological contaminants at Malmstrom following various reports of cancers from missileers, including 2 who died after being diagnosed with NHL. In a 2005 review, the Air Force said, “there is not sufficient evidence to consider the possibility of a cancer clustering to justify further investigation.”
In 2022, Lt. Col. Daniel Sebeck, a vice commander of Space Delta 8 in Colorado who served at Malmstrom and a close friend and fellow missileer were diagnosed with NHL. Sebeck discovered 36 cancer cases among missileers who had been stationed at Malmstrom. Ten developed NHL, 2 developed Hodgkin lymphoma, and 24 developed another form of cancer. The Air Force has acknowledged the concerns.
In 2023, US Air Force School of Aerospace Medicine (USAFSAM) approved the Missile Community Cancer Study (MCCS) to assess “specific cancer concerns raised by missile community members across related career fields and also examines the possibility of clusters of non-Hodgkin’s lymphoma at intercontinental ballistic missile bases.” The study compares 14 common cancers in the general population with that of missile-related career fields. The USAFSAM is reviewing records from former and current Missile Community members on active duty from 1976-2010, as well as state and national cancer data from multiple registries.
Early results from the MCCS suggested elevated rates of some cancers—mainly breast and prostate cancer—among missileers, maintainers, and other ICMB-related job positions, which aligns with other national cancer data.
At a June 2024 AFGSC town hall, officials announced that missileers would now have their information submitted to the Defense Occupational and Environmental Health Readiness System (DOEHRS), a Pentagon database for reporting occupational and exposure hazards.
"This info from DOEHRS flows into the recently developed Individual Longitudinal Exposure Record, a system that compiles occupational and environmental health data throughout a person's career," Lt. Col. John Severns, a spokesperson for Air Force Global Strike Command, said. DOEHRS, which has tracked Air Force records since 2010, allows US Department of Defense and US Department of Veterans Affairs clinical staff to access the information.
MCCS considers potential PCB exposures an occupational hazard. The Air Force says researchers are working with the System Program Offices and leadership to determine the timeframe of PCB removal from bases.
The lack of incontrovertible evidence connecting workplace toxins to NHL has often stymied patients and their family members from receiving appropriate benefits. An “informal talk” in April led by Rep. Mark Takano (D-CA) and Sen. Richard Blumenthal (D-CT) focused on exposures to hazardous materials at US military bases. Participants included various advocacy groups like the Torchlight Initiative, the Invisible Enemy, and Burn Pits 360.
More than a dozen veterans spoke about serving at military bases where they were exposed to a variety of harmful substances, and issues they faced in receiving coverage. David Crete, a veteran and chairman of The Invisible Enemy, said, “I am asking Congress to please allow us to get the benefits every other veteran earned. We are not asking to be special but to be treated equal.”
Rep. Takano called for greater focus on toxic exposures at US military bases: “We must push back against the idea that service members are only in harm’s way in war zones.”
Individuals working near intercontinental ballistic missiles (ICBMs), may be at higher risk of developing non-Hodgkin lymphoma (NHL) according to a preprint analysis conducted on missileers at Malmstrom Air Force Base in Montana. The study, which has not undergone peer review, found higher rates of NHL diagnosis at younger ages compared with the general population. The study also found a statistically significant increase in NHL diagnoses among older missileers, with such rates surpassing expected benchmarks.
The findings build on anecdotal and evidentiary data gathered in the last 50-plus years, including from the Torchlight Initiative, established in 2023 to collect self-reported cancer diagnoses and related fatalities from personnel and family members associated with the ICBM community.
The report shows patterns that “warranted a detailed statistical analysis,” leading to a granular examination of the registry and categorization of the data by cancer type, geographical location, and specific demographics. This narrowed the focus to 18 missileers who served at Malmstrom and were diagnosed with NHL.
In 2001, the Air Force Institute for Operational Health did a site evaluation and sampled for potential chemical and biological contaminants at Malmstrom following various reports of cancers from missileers, including 2 who died after being diagnosed with NHL. In a 2005 review, the Air Force said, “there is not sufficient evidence to consider the possibility of a cancer clustering to justify further investigation.”
In 2022, Lt. Col. Daniel Sebeck, a vice commander of Space Delta 8 in Colorado who served at Malmstrom and a close friend and fellow missileer were diagnosed with NHL. Sebeck discovered 36 cancer cases among missileers who had been stationed at Malmstrom. Ten developed NHL, 2 developed Hodgkin lymphoma, and 24 developed another form of cancer. The Air Force has acknowledged the concerns.
In 2023, US Air Force School of Aerospace Medicine (USAFSAM) approved the Missile Community Cancer Study (MCCS) to assess “specific cancer concerns raised by missile community members across related career fields and also examines the possibility of clusters of non-Hodgkin’s lymphoma at intercontinental ballistic missile bases.” The study compares 14 common cancers in the general population with that of missile-related career fields. The USAFSAM is reviewing records from former and current Missile Community members on active duty from 1976-2010, as well as state and national cancer data from multiple registries.
Early results from the MCCS suggested elevated rates of some cancers—mainly breast and prostate cancer—among missileers, maintainers, and other ICMB-related job positions, which aligns with other national cancer data.
At a June 2024 AFGSC town hall, officials announced that missileers would now have their information submitted to the Defense Occupational and Environmental Health Readiness System (DOEHRS), a Pentagon database for reporting occupational and exposure hazards.
"This info from DOEHRS flows into the recently developed Individual Longitudinal Exposure Record, a system that compiles occupational and environmental health data throughout a person's career," Lt. Col. John Severns, a spokesperson for Air Force Global Strike Command, said. DOEHRS, which has tracked Air Force records since 2010, allows US Department of Defense and US Department of Veterans Affairs clinical staff to access the information.
MCCS considers potential PCB exposures an occupational hazard. The Air Force says researchers are working with the System Program Offices and leadership to determine the timeframe of PCB removal from bases.
The lack of incontrovertible evidence connecting workplace toxins to NHL has often stymied patients and their family members from receiving appropriate benefits. An “informal talk” in April led by Rep. Mark Takano (D-CA) and Sen. Richard Blumenthal (D-CT) focused on exposures to hazardous materials at US military bases. Participants included various advocacy groups like the Torchlight Initiative, the Invisible Enemy, and Burn Pits 360.
More than a dozen veterans spoke about serving at military bases where they were exposed to a variety of harmful substances, and issues they faced in receiving coverage. David Crete, a veteran and chairman of The Invisible Enemy, said, “I am asking Congress to please allow us to get the benefits every other veteran earned. We are not asking to be special but to be treated equal.”
Rep. Takano called for greater focus on toxic exposures at US military bases: “We must push back against the idea that service members are only in harm’s way in war zones.”
RVD With Weekly Bortezomib Has a Favorable Toxicity and Effectiveness Profile in a Large Cohort of US Veterans With Multiple Myeloma
Background
Lenalidomide, bortezomib, and dexamethasone (RVD) is standard triplet induction for fit newly-diagnosed myeloma (NDMM) patients, with response rate (RR)>90%. A 21-day cycle with bortezomib given days 1, 4, 8, and 11 (2x/w) is standard. However, up to 80% of patients develop neuropathy. Weekly bortezomib dosing (1x/w), subcutaneous route, and 28- to 35-day cycle length may optimize tolerance. We present an effectiveness and toxicity analysis of Veterans who received RVD with 1x/w and 2x/w bortezomib for NDMM.
Methods
The VA Corporate Data Warehouse identified 1499 Veterans with NDMM given RVD ≤42 days of treatment start. 840 Veterans were grouped for initial analysis based on criteria: 1) lenalidomide and ≥ 3 bortezomib doses during cycle 1; 2) ≥6 mean days between bortezomib treatments=1x/w); and 3) number of lenalidomide days informed cycle length (21d, 28d, or 35d; default 7-day rest). Investigators reviewed algorithm results to finalize group assignments. Endpoints included depth of response, time to next treatment (TTNT), overall survival (OS), and neuropathy. Neuropathy was defined as use of neuropathy medications and neuropathy ICD-10 codes.
Results
Our algorithm correctly assigned 82% of 840 cycle 1 RVD schedules. The largest groups were 21d 1x/w (n=291), 21d 2x/w (n=193), 28d 1x/w (n=188), and 28d 2x/w (n=82). Median age was 68.3; 53% were non-Hispanic White. Demographics and ISS stage of groups were similar. 30% underwent autologous transplant. Tolerability. Median number of bortezomib doses ranged from 22.5-25.5 (p=0.57). Neuropathy favored 1x/w, 17.7 vs 30.2% (p=0.0001) and was highest (34.7%) in 21d 2x/w. Effectiveness. Response was assessable for 28% of patients. RR (72%, p=0.68) and median TTNT (19.3 months, p=0.20) were similar, including 1x/w vs 2x/w comparison (p=0.79). 21d regimens optimized TTNT (21.4 vs 13.9 months, p=0.045) and trended to better OS (73 vs 65 months, p=0.06).
Conclusions
1x/w RVD preserved effectiveness compared to “standard” RVD in a large Veteran cohort. 1x/w reduced neuropathy incidence. 28d regimens demonstrated inferior longer-term outcomes. Certain endpoints, such as RR and neuropathy, appear underestimated due to data source limitations. 21d 1x/w RVD optimizes effectiveness, tolerability, and administration and should be considered for broader utilization in Veterans with NDMM.
Background
Lenalidomide, bortezomib, and dexamethasone (RVD) is standard triplet induction for fit newly-diagnosed myeloma (NDMM) patients, with response rate (RR)>90%. A 21-day cycle with bortezomib given days 1, 4, 8, and 11 (2x/w) is standard. However, up to 80% of patients develop neuropathy. Weekly bortezomib dosing (1x/w), subcutaneous route, and 28- to 35-day cycle length may optimize tolerance. We present an effectiveness and toxicity analysis of Veterans who received RVD with 1x/w and 2x/w bortezomib for NDMM.
Methods
The VA Corporate Data Warehouse identified 1499 Veterans with NDMM given RVD ≤42 days of treatment start. 840 Veterans were grouped for initial analysis based on criteria: 1) lenalidomide and ≥ 3 bortezomib doses during cycle 1; 2) ≥6 mean days between bortezomib treatments=1x/w); and 3) number of lenalidomide days informed cycle length (21d, 28d, or 35d; default 7-day rest). Investigators reviewed algorithm results to finalize group assignments. Endpoints included depth of response, time to next treatment (TTNT), overall survival (OS), and neuropathy. Neuropathy was defined as use of neuropathy medications and neuropathy ICD-10 codes.
Results
Our algorithm correctly assigned 82% of 840 cycle 1 RVD schedules. The largest groups were 21d 1x/w (n=291), 21d 2x/w (n=193), 28d 1x/w (n=188), and 28d 2x/w (n=82). Median age was 68.3; 53% were non-Hispanic White. Demographics and ISS stage of groups were similar. 30% underwent autologous transplant. Tolerability. Median number of bortezomib doses ranged from 22.5-25.5 (p=0.57). Neuropathy favored 1x/w, 17.7 vs 30.2% (p=0.0001) and was highest (34.7%) in 21d 2x/w. Effectiveness. Response was assessable for 28% of patients. RR (72%, p=0.68) and median TTNT (19.3 months, p=0.20) were similar, including 1x/w vs 2x/w comparison (p=0.79). 21d regimens optimized TTNT (21.4 vs 13.9 months, p=0.045) and trended to better OS (73 vs 65 months, p=0.06).
Conclusions
1x/w RVD preserved effectiveness compared to “standard” RVD in a large Veteran cohort. 1x/w reduced neuropathy incidence. 28d regimens demonstrated inferior longer-term outcomes. Certain endpoints, such as RR and neuropathy, appear underestimated due to data source limitations. 21d 1x/w RVD optimizes effectiveness, tolerability, and administration and should be considered for broader utilization in Veterans with NDMM.
Background
Lenalidomide, bortezomib, and dexamethasone (RVD) is standard triplet induction for fit newly-diagnosed myeloma (NDMM) patients, with response rate (RR)>90%. A 21-day cycle with bortezomib given days 1, 4, 8, and 11 (2x/w) is standard. However, up to 80% of patients develop neuropathy. Weekly bortezomib dosing (1x/w), subcutaneous route, and 28- to 35-day cycle length may optimize tolerance. We present an effectiveness and toxicity analysis of Veterans who received RVD with 1x/w and 2x/w bortezomib for NDMM.
Methods
The VA Corporate Data Warehouse identified 1499 Veterans with NDMM given RVD ≤42 days of treatment start. 840 Veterans were grouped for initial analysis based on criteria: 1) lenalidomide and ≥ 3 bortezomib doses during cycle 1; 2) ≥6 mean days between bortezomib treatments=1x/w); and 3) number of lenalidomide days informed cycle length (21d, 28d, or 35d; default 7-day rest). Investigators reviewed algorithm results to finalize group assignments. Endpoints included depth of response, time to next treatment (TTNT), overall survival (OS), and neuropathy. Neuropathy was defined as use of neuropathy medications and neuropathy ICD-10 codes.
Results
Our algorithm correctly assigned 82% of 840 cycle 1 RVD schedules. The largest groups were 21d 1x/w (n=291), 21d 2x/w (n=193), 28d 1x/w (n=188), and 28d 2x/w (n=82). Median age was 68.3; 53% were non-Hispanic White. Demographics and ISS stage of groups were similar. 30% underwent autologous transplant. Tolerability. Median number of bortezomib doses ranged from 22.5-25.5 (p=0.57). Neuropathy favored 1x/w, 17.7 vs 30.2% (p=0.0001) and was highest (34.7%) in 21d 2x/w. Effectiveness. Response was assessable for 28% of patients. RR (72%, p=0.68) and median TTNT (19.3 months, p=0.20) were similar, including 1x/w vs 2x/w comparison (p=0.79). 21d regimens optimized TTNT (21.4 vs 13.9 months, p=0.045) and trended to better OS (73 vs 65 months, p=0.06).
Conclusions
1x/w RVD preserved effectiveness compared to “standard” RVD in a large Veteran cohort. 1x/w reduced neuropathy incidence. 28d regimens demonstrated inferior longer-term outcomes. Certain endpoints, such as RR and neuropathy, appear underestimated due to data source limitations. 21d 1x/w RVD optimizes effectiveness, tolerability, and administration and should be considered for broader utilization in Veterans with NDMM.
Chronic Myeloid Leukemia Presenting as Priapism: A Rare and Acute Initial Presentation in a Young Male
Introduction
Priapism, defined as a prolonged and often painful penile erection without sexual arousal, constitutes a urological emergency requiring immediate intervention. While commonly associated with conditions like sickle cell anemia and certain medications, malignancy-related priapism is rare and frequently overlooked. Herein, we present a unique case of a 31-year-old male with no significant medical history, who developed persistent priapism as the initial presentation of chronic myeloid leukemia (CML).
Case Presentation
A 31-year-old male without significant medical history, presented to the emergency department with painless priapism, was evaluated by urology and discharged home with precautions. He returned the following day with persistent, now painful priapism. Upon examination, his vital signs were stable. Urology performed aspiration and injection with Sudafed, resulting in mild symptom improvement. Laboratory findings revealed elevated white blood cell count (563.64 k/mcL), anemia (hemoglobin 8.4 g/dL), and a peripheral blood smear showed immature circulating cells with blast forms. He was transferred to a tertiary care center where conservative management addressed bleeding from the penile injection site, with subsequent treatment including leukapheresis and hydroxyurea for cytoreduction. Imaging revealed severe splenomegaly (36 cm) with abdominal mass effect. Peripheral flow cytometry didn’t show malignancy, but cytogenetic analysis showed a BCR/ABL1 fusion gene, confirming chronic myeloid leukemia (CML). Bone marrow biopsy showed hypercellularity without increased blasts. Treatment with dasatinib reduced the white count to 52,000 k/mcL, and was discharged home.
Discussion
Priapism is a urological emergency necessitating immediate intervention to prevent erectile dysfunction and permanent impotence. Management aims to achieve detumescence and typically involves methods such as irrigation or injection of vasoconstrictors into the penis. Malignancy-associated priapism (MAP) often results from venous obstruction due to hyperviscosity. Studies show that CML accounts for approximately 50% cases presenting with MAP, predominantly affecting younger individuals with a mean onset around 27 years of age. Priapism can occur before, during, or after treatment initiation or splenectomy in these patients. Providers should keep a high threshold of suspicion for MAP in patients with no other risk factors as prompt identification and treatment are needed to avoid permanent injury.
Introduction
Priapism, defined as a prolonged and often painful penile erection without sexual arousal, constitutes a urological emergency requiring immediate intervention. While commonly associated with conditions like sickle cell anemia and certain medications, malignancy-related priapism is rare and frequently overlooked. Herein, we present a unique case of a 31-year-old male with no significant medical history, who developed persistent priapism as the initial presentation of chronic myeloid leukemia (CML).
Case Presentation
A 31-year-old male without significant medical history, presented to the emergency department with painless priapism, was evaluated by urology and discharged home with precautions. He returned the following day with persistent, now painful priapism. Upon examination, his vital signs were stable. Urology performed aspiration and injection with Sudafed, resulting in mild symptom improvement. Laboratory findings revealed elevated white blood cell count (563.64 k/mcL), anemia (hemoglobin 8.4 g/dL), and a peripheral blood smear showed immature circulating cells with blast forms. He was transferred to a tertiary care center where conservative management addressed bleeding from the penile injection site, with subsequent treatment including leukapheresis and hydroxyurea for cytoreduction. Imaging revealed severe splenomegaly (36 cm) with abdominal mass effect. Peripheral flow cytometry didn’t show malignancy, but cytogenetic analysis showed a BCR/ABL1 fusion gene, confirming chronic myeloid leukemia (CML). Bone marrow biopsy showed hypercellularity without increased blasts. Treatment with dasatinib reduced the white count to 52,000 k/mcL, and was discharged home.
Discussion
Priapism is a urological emergency necessitating immediate intervention to prevent erectile dysfunction and permanent impotence. Management aims to achieve detumescence and typically involves methods such as irrigation or injection of vasoconstrictors into the penis. Malignancy-associated priapism (MAP) often results from venous obstruction due to hyperviscosity. Studies show that CML accounts for approximately 50% cases presenting with MAP, predominantly affecting younger individuals with a mean onset around 27 years of age. Priapism can occur before, during, or after treatment initiation or splenectomy in these patients. Providers should keep a high threshold of suspicion for MAP in patients with no other risk factors as prompt identification and treatment are needed to avoid permanent injury.
Introduction
Priapism, defined as a prolonged and often painful penile erection without sexual arousal, constitutes a urological emergency requiring immediate intervention. While commonly associated with conditions like sickle cell anemia and certain medications, malignancy-related priapism is rare and frequently overlooked. Herein, we present a unique case of a 31-year-old male with no significant medical history, who developed persistent priapism as the initial presentation of chronic myeloid leukemia (CML).
Case Presentation
A 31-year-old male without significant medical history, presented to the emergency department with painless priapism, was evaluated by urology and discharged home with precautions. He returned the following day with persistent, now painful priapism. Upon examination, his vital signs were stable. Urology performed aspiration and injection with Sudafed, resulting in mild symptom improvement. Laboratory findings revealed elevated white blood cell count (563.64 k/mcL), anemia (hemoglobin 8.4 g/dL), and a peripheral blood smear showed immature circulating cells with blast forms. He was transferred to a tertiary care center where conservative management addressed bleeding from the penile injection site, with subsequent treatment including leukapheresis and hydroxyurea for cytoreduction. Imaging revealed severe splenomegaly (36 cm) with abdominal mass effect. Peripheral flow cytometry didn’t show malignancy, but cytogenetic analysis showed a BCR/ABL1 fusion gene, confirming chronic myeloid leukemia (CML). Bone marrow biopsy showed hypercellularity without increased blasts. Treatment with dasatinib reduced the white count to 52,000 k/mcL, and was discharged home.
Discussion
Priapism is a urological emergency necessitating immediate intervention to prevent erectile dysfunction and permanent impotence. Management aims to achieve detumescence and typically involves methods such as irrigation or injection of vasoconstrictors into the penis. Malignancy-associated priapism (MAP) often results from venous obstruction due to hyperviscosity. Studies show that CML accounts for approximately 50% cases presenting with MAP, predominantly affecting younger individuals with a mean onset around 27 years of age. Priapism can occur before, during, or after treatment initiation or splenectomy in these patients. Providers should keep a high threshold of suspicion for MAP in patients with no other risk factors as prompt identification and treatment are needed to avoid permanent injury.
The First Female Patient in the Veteran Affairs System to Receive Chimeric Antigen Receptors (CAR) T-cell Therapy for Refractory Multiple Myeloma and the Role of CAR T-cell Therapy in Penta-refractory Disease
Background
In 2024, the first two veterans, both from the Michael E. DeBakey Veteran Affairs (VA) Medical Center, received chimeric antigen receptors (CAR) T-cell therapy for refractory multiple myeloma through the Tennessee Valley Healthcare System (TVHS). Currently, TVHS is the only VA where this treatment is available. One of these patients also had penta-refractory multiple myeloma (P-RMM), which is associated with significantly worse progression-free survival and overall survival (OS) (Gill et al, 2021). P-RMM is defined as resistance to at least two immunomodulatory drugs, two different proteasome inhibitors, and one CD38 monoclonal antibody.
Case Presentation
A 71-year-old female veteran was diagnosed with high-risk multiple myeloma and received induction therapy with carfilzomib, lenalidomide, and dexamethasone in 2017. She underwent autologous stem cell transplant (SCT) in 4/2018. The veteran subsequently received maintenance therapy with lenalidomide, bortezomib, and dexamethasone. Her disease recurred in 1/2022. The patient then received two more lines of treatments with daratumumab and pomalidomide followed by selinexor. She had another autologous SCT in 5/2023, to which she was refractory. Her fifth line therapy included addition of bortezomib to her selinexor regimen. She eventually underwent CAR T-cell therapy at THVS on 5/1/2024 with good tolerance of therapy. At her follow-up visit, the patient had significant response to CAR T-cell treatment, based on her symptoms and improvement in free light chains and serum protein electrophoresis.
Discussion
CAR T-cell therapy is one of the newest and most cutting-edge therapies for patients with refractory multiple myeloma. Access to this therapy has been limited throughout the country. However, as shown by our case, this life-saving treatment is now available to patients within the VA. According to a retrospective study on P-RMM patients, the OS in patients who received B-cell maturation antigen (BCMA) targeted therapy was significantly higher than in those who did not (17 vs. 6 months, p < 0.0001). Among the BCMA-targeted therapies, CAR T-cell therapy is associated with the highest OS (29 months) compared to antibody-drug conjugates and bispecific T-cell engagers (Atrash et al, 2023). Thus, accessibility to CAR T-cell therapy was essential in our patient with P-RMM in ensuring her best survival outcomes.
Background
In 2024, the first two veterans, both from the Michael E. DeBakey Veteran Affairs (VA) Medical Center, received chimeric antigen receptors (CAR) T-cell therapy for refractory multiple myeloma through the Tennessee Valley Healthcare System (TVHS). Currently, TVHS is the only VA where this treatment is available. One of these patients also had penta-refractory multiple myeloma (P-RMM), which is associated with significantly worse progression-free survival and overall survival (OS) (Gill et al, 2021). P-RMM is defined as resistance to at least two immunomodulatory drugs, two different proteasome inhibitors, and one CD38 monoclonal antibody.
Case Presentation
A 71-year-old female veteran was diagnosed with high-risk multiple myeloma and received induction therapy with carfilzomib, lenalidomide, and dexamethasone in 2017. She underwent autologous stem cell transplant (SCT) in 4/2018. The veteran subsequently received maintenance therapy with lenalidomide, bortezomib, and dexamethasone. Her disease recurred in 1/2022. The patient then received two more lines of treatments with daratumumab and pomalidomide followed by selinexor. She had another autologous SCT in 5/2023, to which she was refractory. Her fifth line therapy included addition of bortezomib to her selinexor regimen. She eventually underwent CAR T-cell therapy at THVS on 5/1/2024 with good tolerance of therapy. At her follow-up visit, the patient had significant response to CAR T-cell treatment, based on her symptoms and improvement in free light chains and serum protein electrophoresis.
Discussion
CAR T-cell therapy is one of the newest and most cutting-edge therapies for patients with refractory multiple myeloma. Access to this therapy has been limited throughout the country. However, as shown by our case, this life-saving treatment is now available to patients within the VA. According to a retrospective study on P-RMM patients, the OS in patients who received B-cell maturation antigen (BCMA) targeted therapy was significantly higher than in those who did not (17 vs. 6 months, p < 0.0001). Among the BCMA-targeted therapies, CAR T-cell therapy is associated with the highest OS (29 months) compared to antibody-drug conjugates and bispecific T-cell engagers (Atrash et al, 2023). Thus, accessibility to CAR T-cell therapy was essential in our patient with P-RMM in ensuring her best survival outcomes.
Background
In 2024, the first two veterans, both from the Michael E. DeBakey Veteran Affairs (VA) Medical Center, received chimeric antigen receptors (CAR) T-cell therapy for refractory multiple myeloma through the Tennessee Valley Healthcare System (TVHS). Currently, TVHS is the only VA where this treatment is available. One of these patients also had penta-refractory multiple myeloma (P-RMM), which is associated with significantly worse progression-free survival and overall survival (OS) (Gill et al, 2021). P-RMM is defined as resistance to at least two immunomodulatory drugs, two different proteasome inhibitors, and one CD38 monoclonal antibody.
Case Presentation
A 71-year-old female veteran was diagnosed with high-risk multiple myeloma and received induction therapy with carfilzomib, lenalidomide, and dexamethasone in 2017. She underwent autologous stem cell transplant (SCT) in 4/2018. The veteran subsequently received maintenance therapy with lenalidomide, bortezomib, and dexamethasone. Her disease recurred in 1/2022. The patient then received two more lines of treatments with daratumumab and pomalidomide followed by selinexor. She had another autologous SCT in 5/2023, to which she was refractory. Her fifth line therapy included addition of bortezomib to her selinexor regimen. She eventually underwent CAR T-cell therapy at THVS on 5/1/2024 with good tolerance of therapy. At her follow-up visit, the patient had significant response to CAR T-cell treatment, based on her symptoms and improvement in free light chains and serum protein electrophoresis.
Discussion
CAR T-cell therapy is one of the newest and most cutting-edge therapies for patients with refractory multiple myeloma. Access to this therapy has been limited throughout the country. However, as shown by our case, this life-saving treatment is now available to patients within the VA. According to a retrospective study on P-RMM patients, the OS in patients who received B-cell maturation antigen (BCMA) targeted therapy was significantly higher than in those who did not (17 vs. 6 months, p < 0.0001). Among the BCMA-targeted therapies, CAR T-cell therapy is associated with the highest OS (29 months) compared to antibody-drug conjugates and bispecific T-cell engagers (Atrash et al, 2023). Thus, accessibility to CAR T-cell therapy was essential in our patient with P-RMM in ensuring her best survival outcomes.
Is Location a Risk Factor for Early-Onset Cancer?
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Cancer Data Trends 2024
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
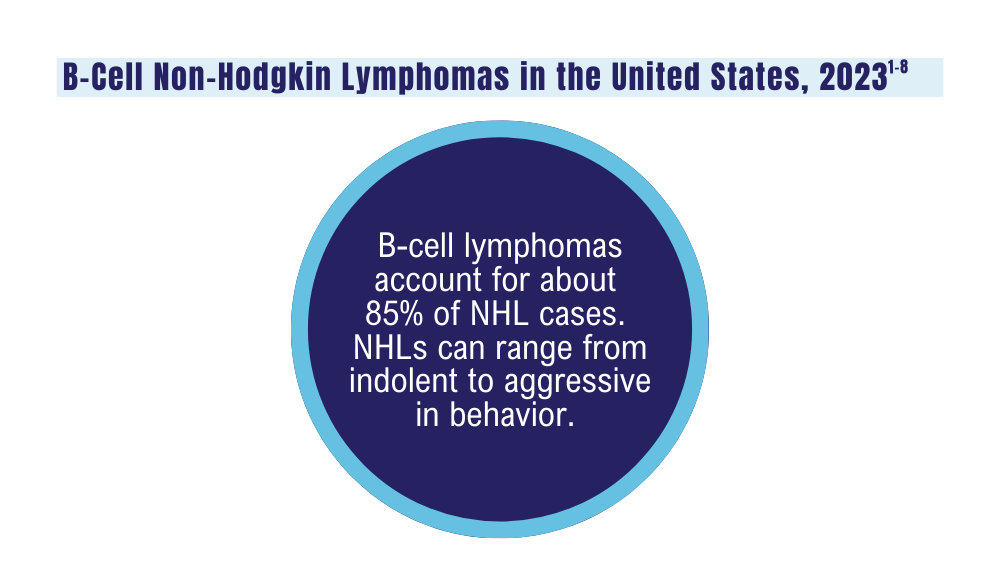
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
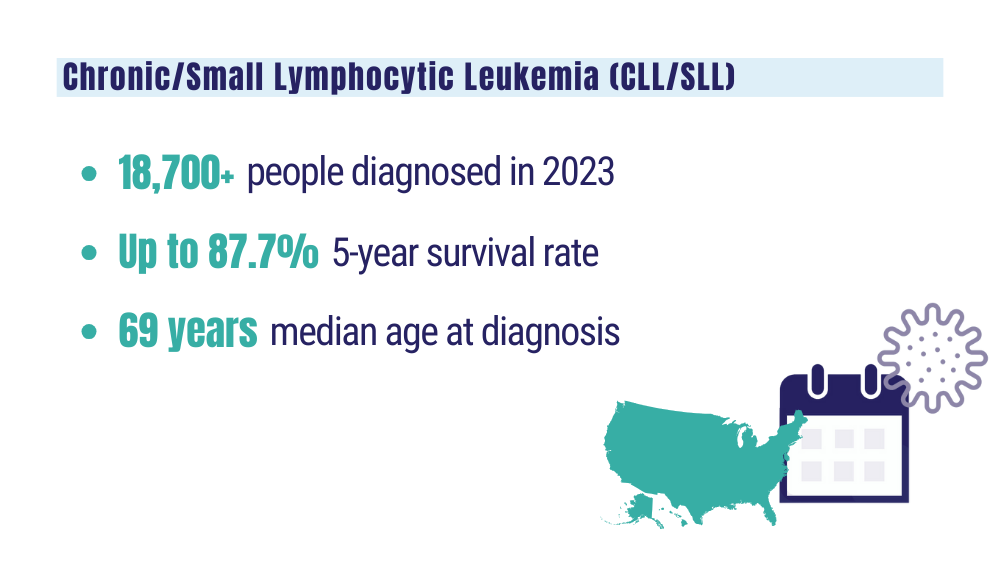
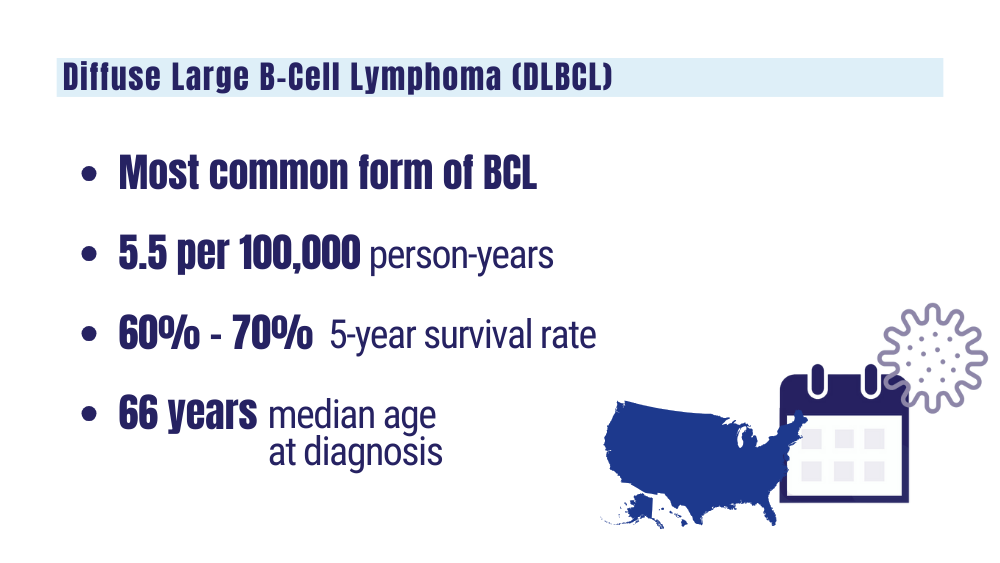
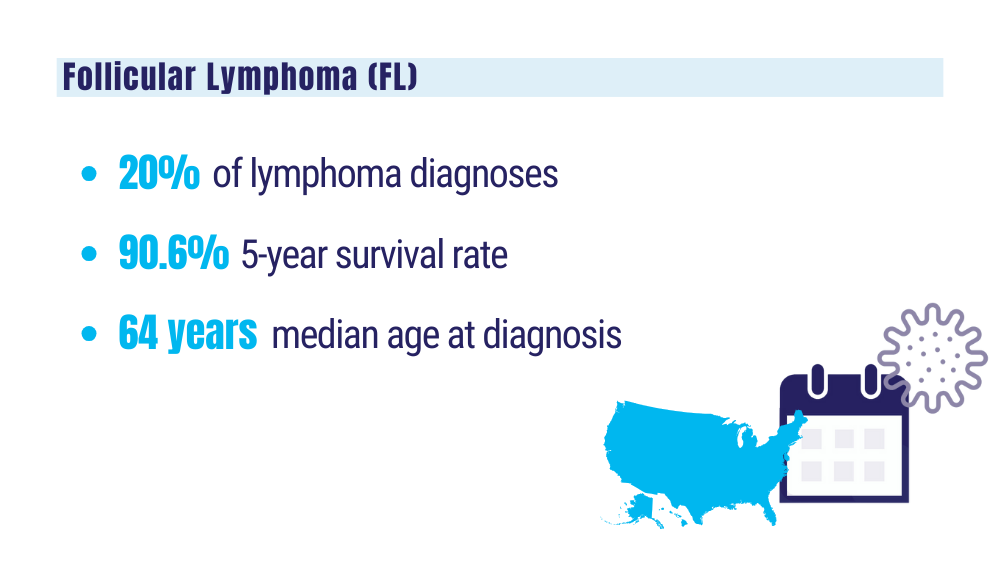
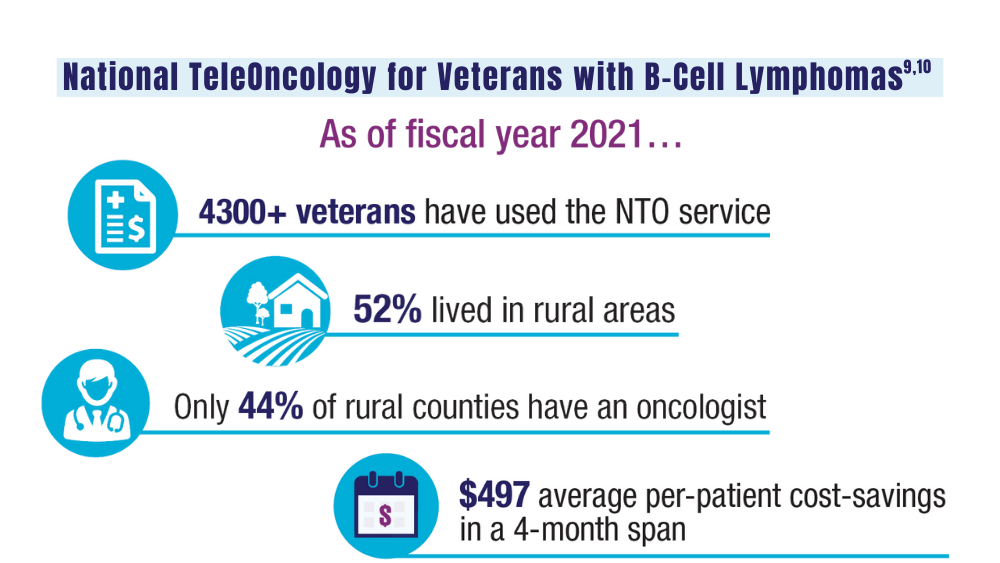
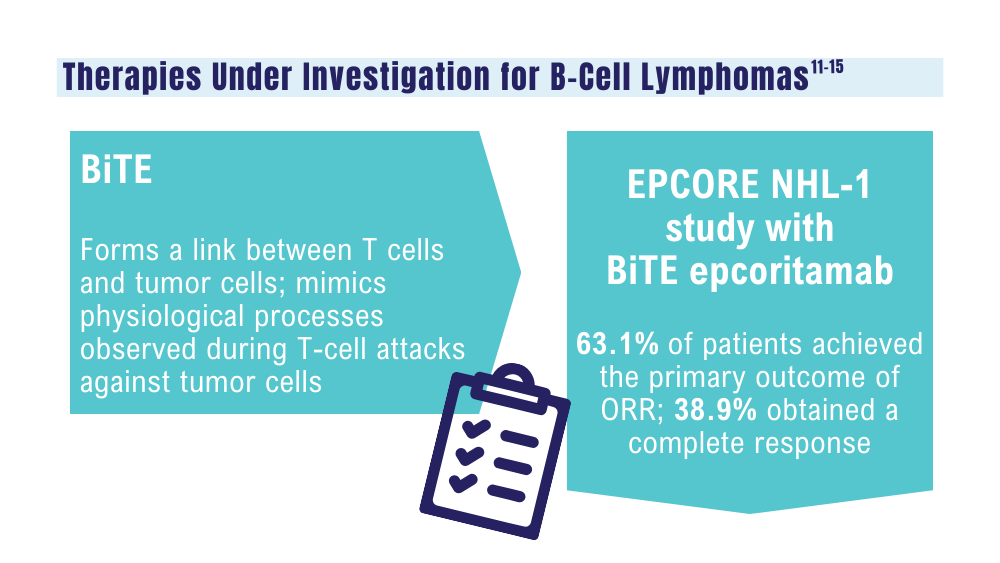
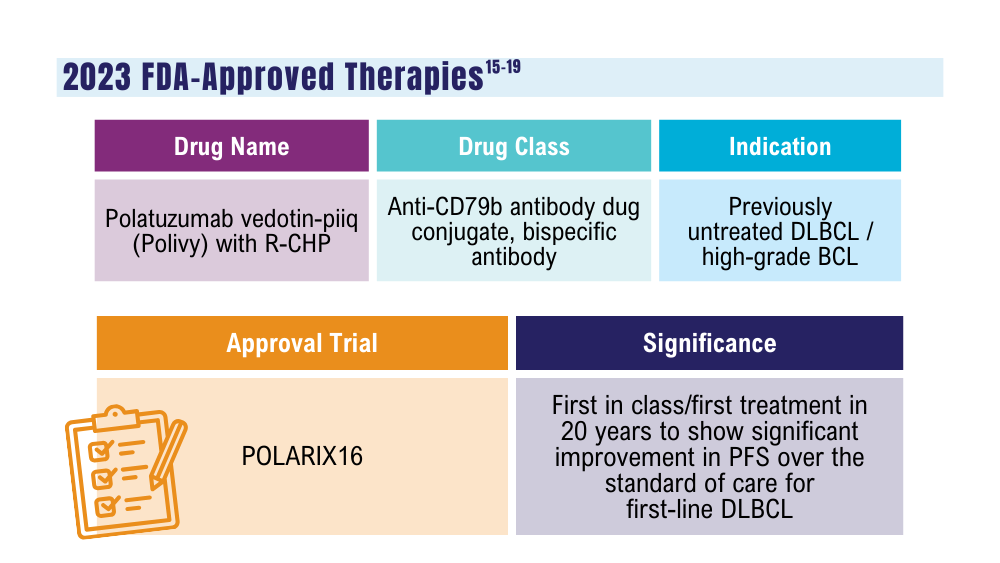
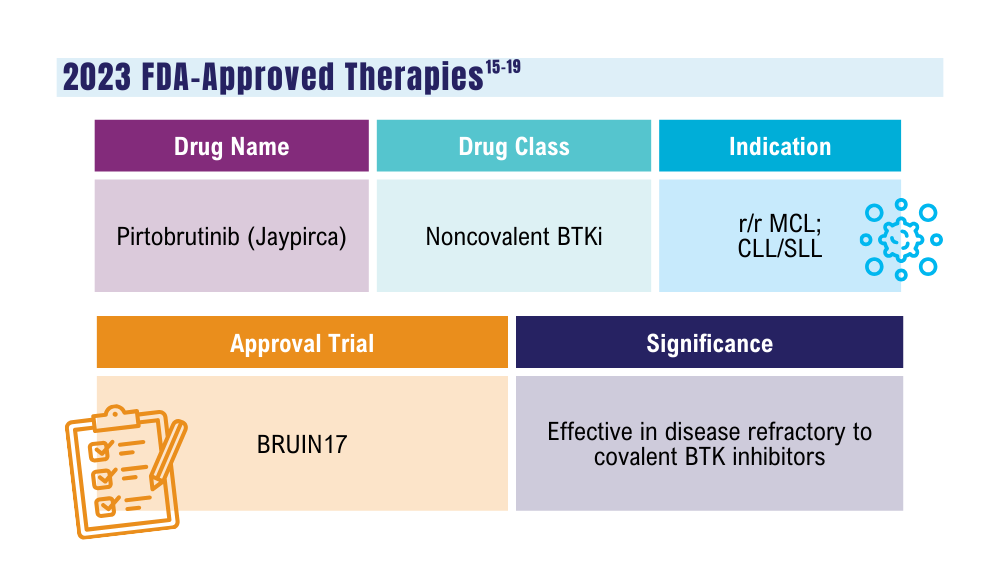
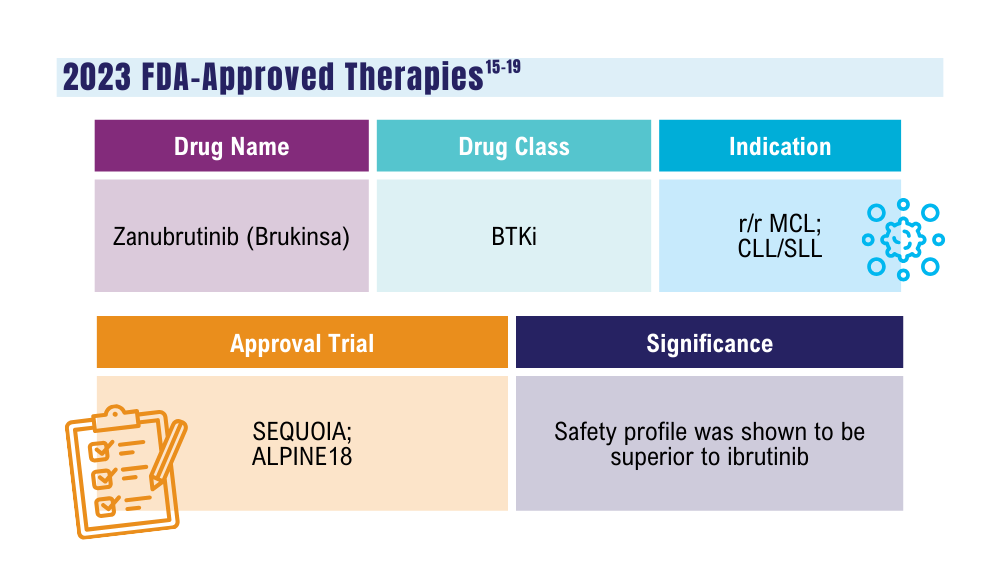
Cancer Data Trends 2024: B-Cell Lymphoma
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
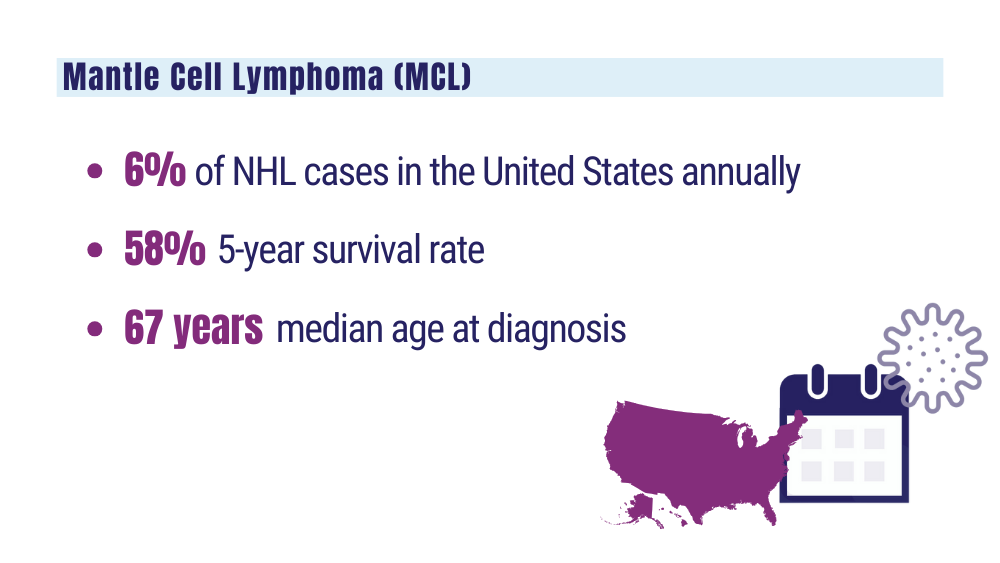
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
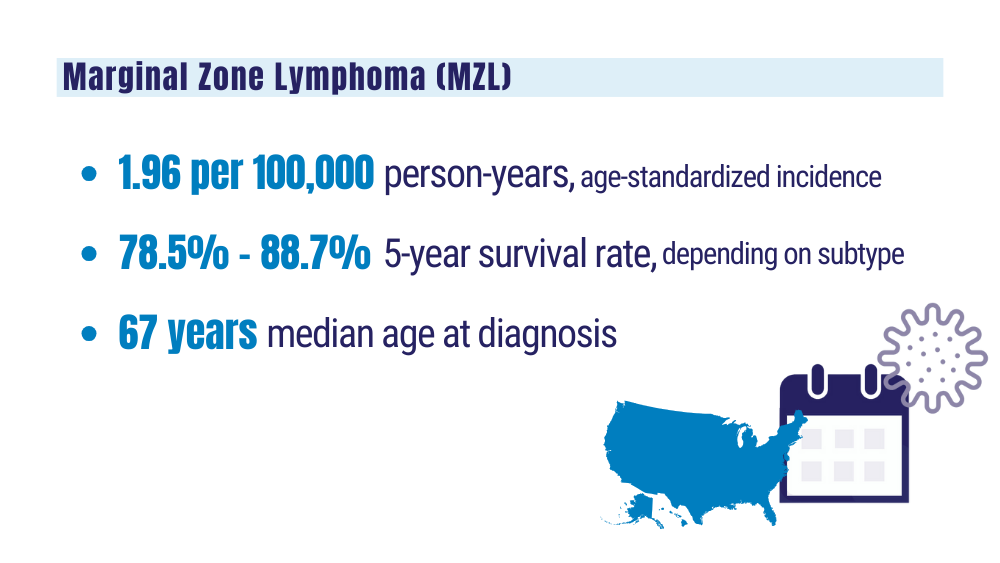
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
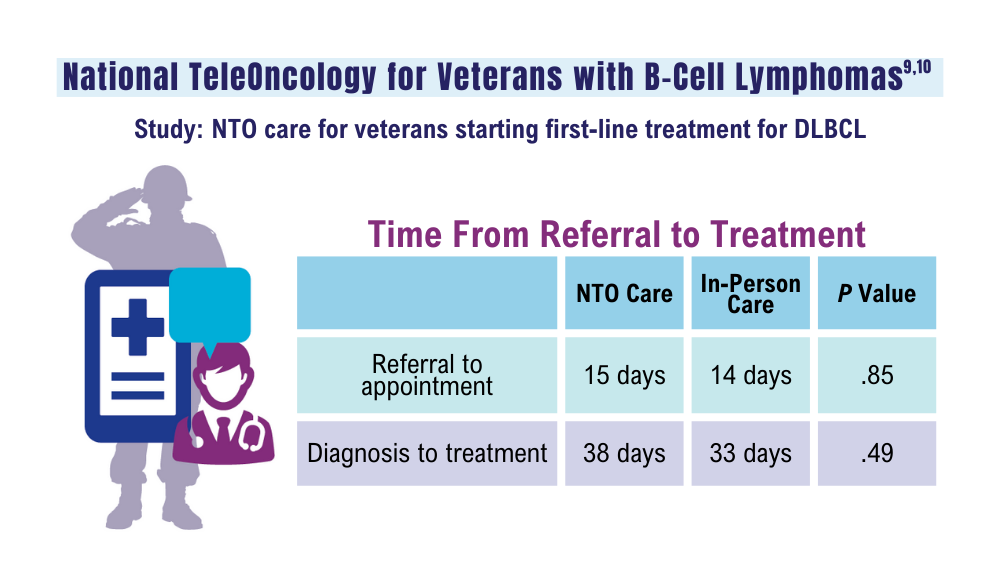
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
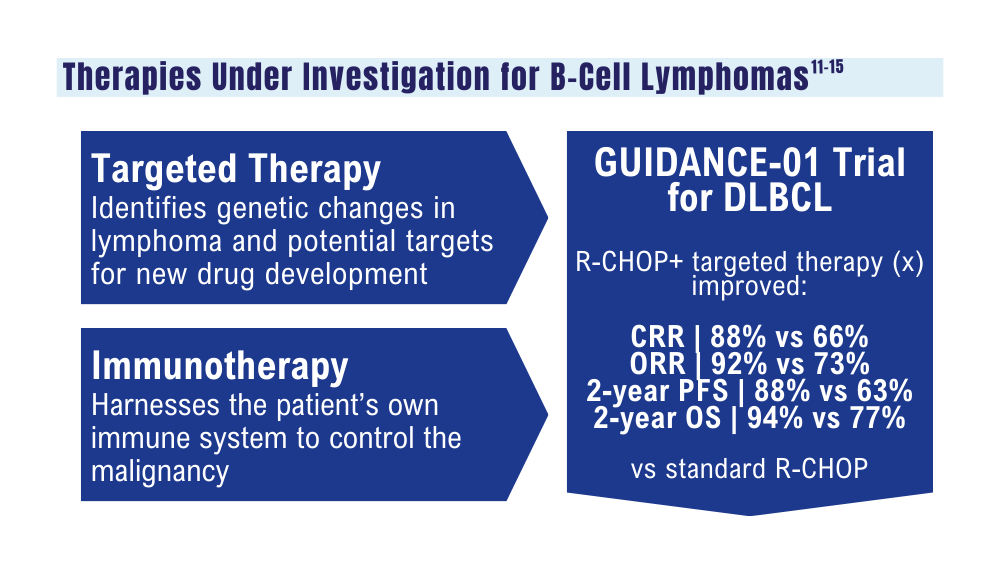
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
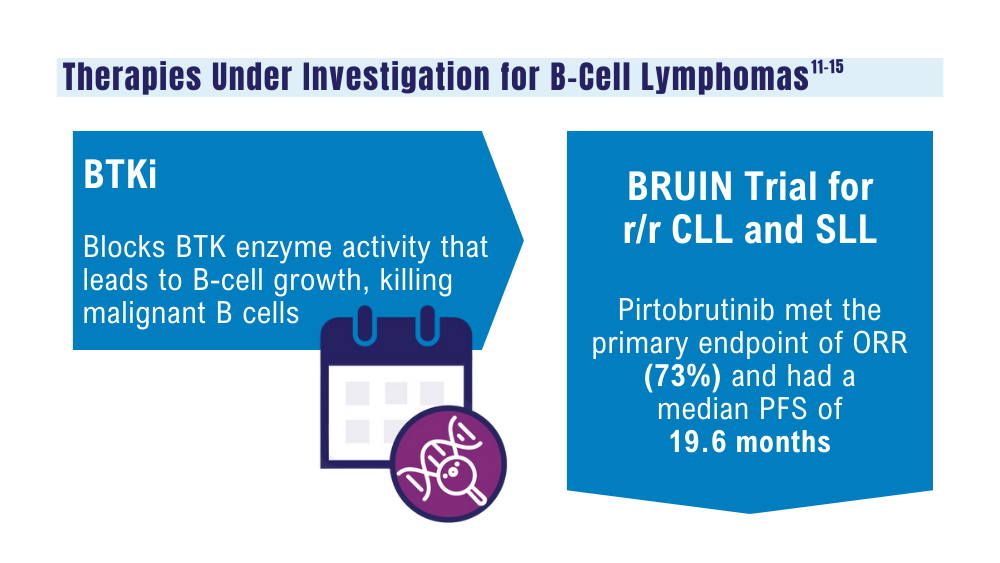
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
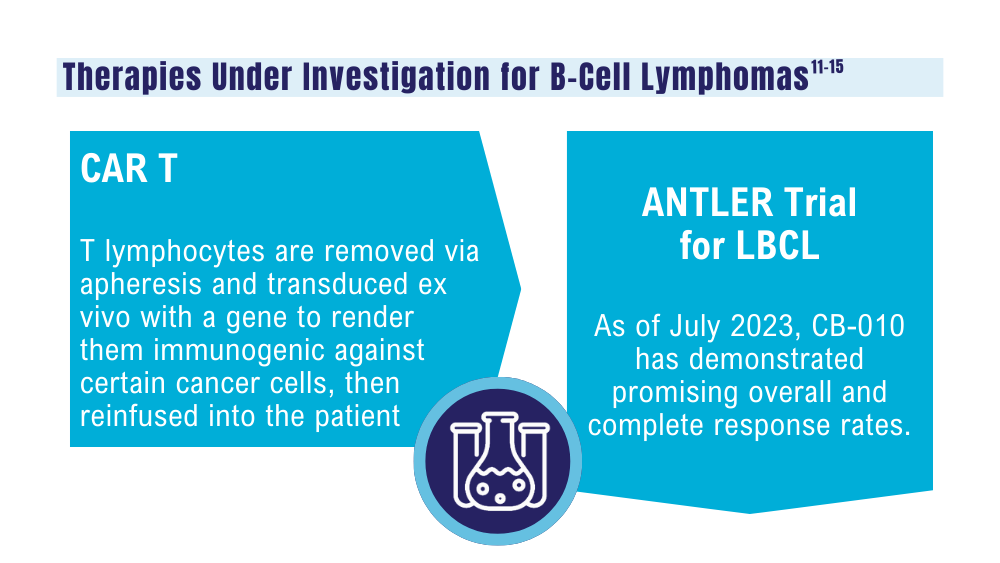
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
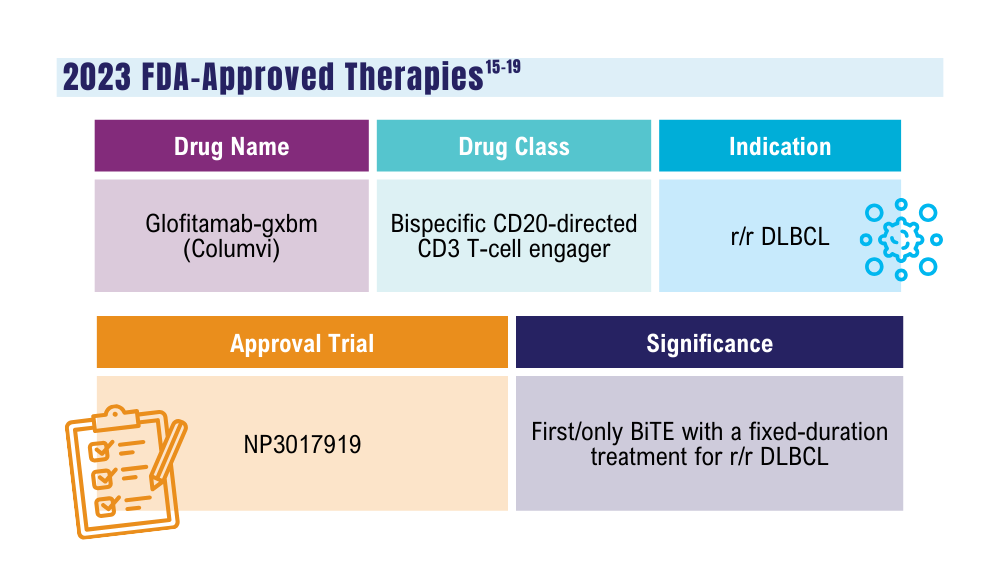
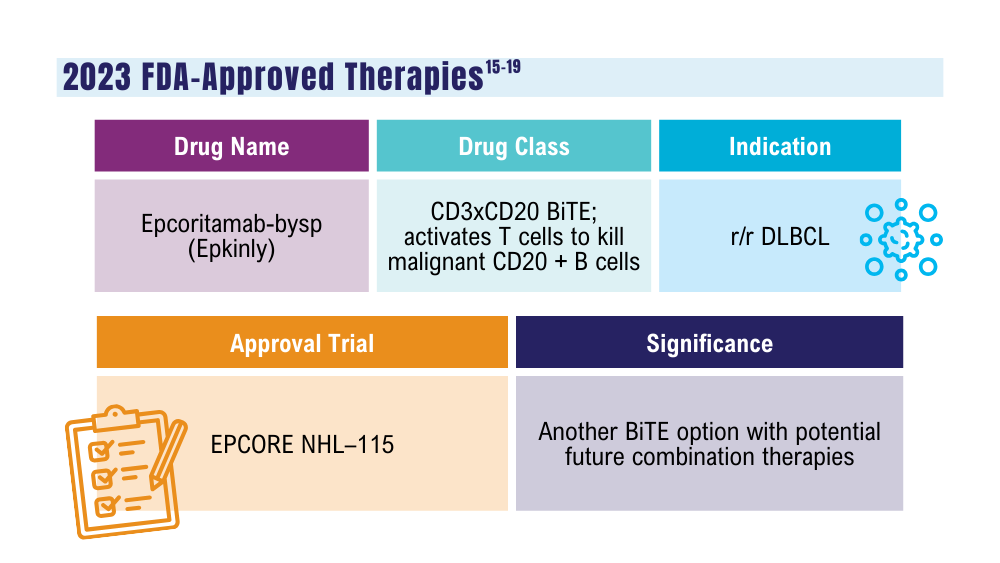
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell