User login
Screening and Treating Hepatitis C in the VA: Achieving Excellence Using Lean and System Redesign
Hepatitis C virus (HCV) infection is a major public health problem in the US. Following the 2010 report of the Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) on hepatitis and liver cancer, the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan in 2011 with subsequent action plan updates for 2014-2016 and 2017-2020.1-3 A NASEM phase 2 report and the 2017-2020 HHS action plan outline a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities.3,4 The Department of Veterans Affairs (VA) is the single largest HCV care provider in the US with about 165,000 veterans in care diagnosed with HCV in the beginning of 2014 and is a national leader in the testing and treatment of HCV.5,6
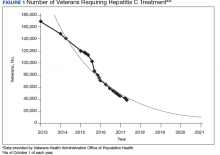
The VA’s recommendations for screening for HCV infection are in alignment with the United States Preventive Services Task Force (USPSTF) and Centers for Disease Control and Prevention (CDC) recommendations to test all veterans born between 1945 and 1965 and anyone with risk factors such as injection drug use.7-9 As of January 1, 2018, the VA had screened more than 80% of veterans in care within this highest risk birth cohort. As of January 1, 2018, more than 100,000 veterans in VA care have initiated treatment for HCV with direct-acting antivirals (DAAs) (Figure 1).
Several critical factors contributed to the VA success with HCV testing and treatment, including congressional appropriation of funding from fiscal year (FY) 2016 through FY 2018, unrestricted access to interferon-free DAA HCV treatments, and dedicated resources from the VA National Viral Hepatitis Program within the HIV, Hepatitis, and Related Conditions Programs (HHRC) in the Office of Specialty Care Services.5 In 2014, HHRC created and supported the Hepatitis Innovation Team (HIT) Collaborative, a VA process improvement initiative enabling
Veterans Integrated Service Network (VISN) -based, multidisciplinary teams to increase veterans’ access to HCV testing and treatment.
As the VA makes consistent progress toward eliminating HCV in veterans in VA care, it has become clear that achieving a cure is only a starting point in improving HCV care. Many patients with HCV infection also have advanced liver disease (ALD), or cirrhosis, which is a condition of permanent liver fibrosis that remains after the patient has been cured of HCV infection. In addition to hepatitis C, ALD also can be caused by excessive alcohol use, hepatitis B virus (HBV) infection, nonalcoholic fatty liver diseases, and several other inherited diseases. Advanced liver disease affects more than 80,000 veterans in VA care, and the HIT infrastructure provides an excellent framework to better understand and address facility-level and systemwide challenges in diagnosing, caring for, and treating veterans with ALD across the Veterans Health Administration (VHA) system.
This report will describe the elements that contributed to the success of the HIT Collaborative in redesigning care for patients affected by HCV in the VA and how these elements can be applied to improve the system of care for VHA ALD care.
Hepatitis Innovation Teams Collaborative Leadership
After the US Food and Drug Administration (FDA) approved new DAA medications to treat HCV, the VA recognized the need to mobilize the health care system quickly and allocate resources for these new, minimally toxic, and highly effective medications. Early in 2014, HHRC established the National Hepatitis C Resource Center (NHCRC), a successor program to the 4 regional hepatitis C resource centers that had addressed HCV care across the system.10 The NHCRC was charged with developing an operational strategy for VA to respond rapidly to the availability of DAAs. In collaboration with representatives from the Office of Strategic Integration | Veterans Engineering Resource Center (OSI|VERC), the NHCRC formed the HIT Collaborative Leadership Team (CLT).
The HIT CLT is responsible for executing the HIT Collaborative and uses a Lean process improvement framework focused on eliminating waste and maximizing value. Members of the CLT with expertise in facilitation, Lean process improvement, leadership, clinical knowledge, and population health management act as coaches for the VISN HITs. The CLT works to build and support the VISN HITs, identify opportunities for individual teams to improve and assist in finding the right local mix of “players” to be successful. The HIT CLT ensures all teams are functioning and working toward achieving their goals. The CLT obtains data from VA national databases, which are provided to the VISN HITs to inform and encourage continuous improvement of their strategies. Annual VA-wide aspirational goals are developed and disseminated to encourage a unified mission.
Catchment areas for each VISN include between 6 and 10 medical centers as well as outpatient and ambulatory care centers. Multidisciplinary HITs are composed of physicians, nurses, pharmacists, nurse practitioners, physician assistants, social workers, mental health and substance use providers, peer support specialists, administrators, information technology experts, and systems redesign professionals from medical centers within each VISN. Teams develop strong relationships across medical centers, implement context-specific strategies applicable to rural
and urban centers, and share expertise. In addition to intra-VISN process improvement, HITs collaborate monthly across VISNs via a virtual platform. They share strong practices, seek advice from one another, and compare outcomes on an established set of goals.
The HITs use process improvement tools to systematically assess the current steps involved in care. At the close of each year, the HITs analyze the current state of operations and set goals to improve over the following year guided by a target state map. Seed funding is provided to every VISN HIT annually to launch change initiatives. Many VISN HITs use these funds to support a VISN HIT coordinator, and HITs also use this financial support to conduct 2- to 3-day process improvement workshops and to purchase supplies, such as point-of-care testing kits. The HIT communication and work are predominantly executed virtually.
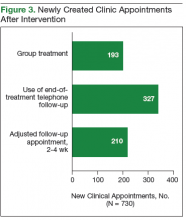
Each year, teams worked toward achieving goals set nationally. These included increasing HCV birth cohort testing and improving the percentage of patients who had SVR12 testing
(Table).
the percentage of patients who received SVR12 testing posttreatment completion was not included in the HIT Collaborative’s annual goals for the first year of the program. Recognizing this as a critical area for improvement, the HIT CLT set a goal to test 80% of all patients who completed treatment. The HITs applied Lean tools to identify and overcome gaps in the SVR12 testing process. By the end of the second year, 84% of all patients who completed treatment had been tested for SVR12.
The HITs also set specific local VISN and medical center goals, prioritizing projects that could have the greatest impact on local patient access and quality of care and build on existing strengths and address barriers. These projects encompass a wide range of areas that contribute to the overall national goals.
Focus on Lean
Lean process improvement is based on 2 key pillars: respect for people (those seeking service as customers and patients and those providing service as frontline staff and stakeholders) and continuous improvement. With Lean, personnel providing care should work to identify and eliminate waste in the system and to streamline care delivery to maximize process steps that are most valued by patients (eg, interaction with a clinical provider) and minimize those that are not valued (eg, time spent waiting to see a provider). With the knowledge that HHRC fully supports their work, HITs were encouraged to innovate based on local resources, context, and culture.
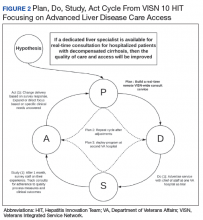
Teams receive basic training in Lean from the HIT CLT and local systems redesign specialists if available. The HITs apply the A3 structured approach to problem solving.11 The HITs follow prescribed problemsolving steps that help identify where to focus process improvement efforts, including analyzing the current state of care, outlining the target state, and prioritizing solution
approaches based on what will have the highest impact for patients.
to accommodate the outcomes they observe (Figure 2).
Innovations
Over the course of the HIT Collaborative, numerous innovations have emerged to address and mitigate barriers to HCV screening and treatment. Examples of successful innovations include the following:
- To address transportation issues, several teams developed programs specific to patients with HCV in rural locations or with limited mobility. Mobile vans and units traditionally used as mobile cardiology clinics were transformed into HCV clinics, bringing testing and treatment services directly to veterans;
- Pharmacists and social workers developed outreach strategies to locate homeless veterans, provide point-of-care testing and utilize mobile technology to concurrently enroll and link veterans to care; and
- Many liver care teams partnered with inpatient and outpatient substance use treatment clinics to provide patient education and coordinate HCV treatment.
Inter-VISN working groups developed systemwide tools to address common needs. In the program’s first year, a few medical facilities across a handful of VISNs shared local population health management systems, programming, and best practices. Over time, this working group combined the virtual networking capacity of the HIT Collaborative with technical expertise to promote rapid dissemination and uptake of a population health management system. Providers at medical centers across VA use the tools to identify veterans who should be screened and treated for HCV with the ability to continuously update information, identifying patients who do not respond to treatment or patients overdue for SVR12 testing.
Providers with experience using telehepatology formed another inter-VISN working group. These subject matter experts provided guidance to care teams interested in implementing telehealth in areas where limited local resources or knowledge had prevented them from moving forward. The ability to build a strong coalition across content areas fostered a collaborative learning environment, adaptable to implementing new processes and technologies.
In 2017, the VA made significant efforts to reach out to veterans eligible for VA care who had not yet been screened or remained untreated. In May, Hepatitis Awareness Month, HITs held HCV testing and community outreach events and participated in veteran stand-downs and veteran service organization activities.
Evaluation
Since 2014, the VA has increased its HCV treatment and screening rates. To assess the components contributing to these achievements and the role of the HIT Collaborative in driving this success, a team of implementation scientists have been working with the CLT to conduct a HIT program evaluation. The goal of the evaluation is to establish the impact of the HIT Collaborative. The evaluation team catalogs the activities of the Collaborative and the HITs and assesses implementation strategies (use of specific techniques) to increase the uptake of evidence-based practices specifically related to HCV treatment.12
At the close of each FY, HCV providers and members of the HIT Collaborative are queried through an online survey to determine which strategies have been used to improve HCV care and how these strategies were associated with the HIT Collaborative. The use of more strategies was associated with more HCV treatment initiations.13 All utilized strategies were identified whether or not they were associated with treatment starts. These data are being used to understand which combinations of strategies are most effective at increasing treatment for HCV in the VA and to inform future initiatives.
Expanding the Scope
Inspired by the successful results of the HIT work in HCV and in the spirit of continuously improving health care delivery, HHRC expanded the scope of the HIT Collaborative in FY 2018 to include ALD. There are about 80,000 veterans in VA care with advanced scarring of the liver and between 10,000 to 15,000 new diagnoses each year. In addition to HCV as an etiology for ALD, cases of cirrhosis are projected to increase among veterans in care due to metabolic syndrome and alcohol use. A recent review of VA data from fiscal year 2016 found that 88.6% of ALD patients had been seen in primary care within the past 2 years, with about half (51%) seen in a gastroenterology (GI) or hepatology clinic (Personal communication, HIV, Hepatitis, and Related Conditions Program Office March 16, 2018). For patients in VA care with ALD, GI visits are associated with a lower 5-year mortality.14 Annual mortality for all ALD patients in VA is 6.2%, and of those with a hospital admission, mortality rises to 31%.15 In FY 2016, there were about 52,000 ALD-related discharges (more than 2 per patient). Of those discharges, 24% were readmitted within 30 days, with an average length of stay of 1.9 days and an estimated cost per patient of $47,000 over 3 years.16
Hepatologists from across the VA convened to identify critical opportunities for improvement for patients with ALD. Base on available evidence presented in the literature and their clinical expertise, these subject matter experts identified several areas for quality improvement, with the overarching goal to improve identification of patients with early cirrhosis and ensure appropriate linkage to care for all cirrhotic patients, thus improving quality of life and reducing mortality. Although not finalized, candidate improvement targets include consistent linkage to care and treatment for HCV and HBV, comprehensive case management, post-discharge patient follow-up, and adherence to evidence-based standards of care.
Conclusion
The VA has made great strides in nearly eliminating HCV among veterans in VA care. The national effort to redesign hepatitis care using Lean management strategies and develop local and regional teams and centralized support allowed VA to maximize available resources to achieve higher rates of HCV birth cohort testing and treatment of patients infected with HCV than has any other health care system in the US.
The HIT Collaborative has been a unique and innovative mechanism to promote directed, patient-outcome driven change in a large and dynamic health care system. It has allowed rural and urban providers to work together to develop and spread quality improvement innovations and as an integrated system to achieve national priorities. The focus of this foundational HIT structure is expanding to identifying, treat, and care for VA’s ALD population.
1. Colvin HM, Mitchell AE, eds; and the Committee on the Prevention and Control of Viral Hepatitis Infections Board on Population Health and Public Health Practice. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: The National Academies Press; 2010.
2. US Department of Health and Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care and treatment of viral hepatitis. https://www.hhs.gov/sites/default/files/action-plan-viral-hepatitis-2011.pdf. Accessed April 27, 2018.
3. Wolitski R. National viral hepatitis action plan: 2017-2020. https://www.hhs.gov/hepatitis/action-plan/national-viralhepatitis-action-plan-overview/index.html. Updated February
21, 2018. Accessed May 8, 2018.
4. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: The National Academies Press; 2017.
5. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C infection: best practices from the Department of Veterans Affairs. Ann of Intern Med. 2017;167(7):499-504.
6. Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol. 2015;63(3):586-592.
7. US Department of Veterans Affairs, Veteran Health Administration. National Clinical Preventive Service Guidance Statements: Screening for Hepatitis C. http://www.prevention.va.gov/CPS/Screening_for_Hepatitis_C.asp. Published on June 20, 2017. [Nonpubic document; source not verified.]
8. Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
9. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
10. Garrard J, Choudary V, Groom H, et al. Organizational change in management of hepatitis C: evaluation of a CME program. J Contin Educ Health Prof. 2006;26(2):145-160.
11. Shook J. Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor, and Lead. Cambridge, MA: Lean Enterprise Institute; 2010.
12. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
13. Rogal SS, Yakovchenko V, Waltz TJ, et al. The association between implementation strategy use and the uptake of hepatitis C treatment in a national sample. Implement Sci.
2017;12(1):60.
14. Mellinger JL, Moser S, Welsh DE, et al. Access to subspecialty care and survival among patients with liver disease. Am J Gastroenterol. 2016;111(6):838-844.
15. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in the burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans from 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
16. Kaplan DE, Chapko MK, Mehta R, et al; VOCAL Study Group. Healthcare costs related to treatment of hepatocellular carcinoma among veterans with cirrhosis in the United States. Clin Gastroenterol Hepatol. 2018;16(1):106-114.
Hepatitis C virus (HCV) infection is a major public health problem in the US. Following the 2010 report of the Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) on hepatitis and liver cancer, the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan in 2011 with subsequent action plan updates for 2014-2016 and 2017-2020.1-3 A NASEM phase 2 report and the 2017-2020 HHS action plan outline a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities.3,4 The Department of Veterans Affairs (VA) is the single largest HCV care provider in the US with about 165,000 veterans in care diagnosed with HCV in the beginning of 2014 and is a national leader in the testing and treatment of HCV.5,6
The VA’s recommendations for screening for HCV infection are in alignment with the United States Preventive Services Task Force (USPSTF) and Centers for Disease Control and Prevention (CDC) recommendations to test all veterans born between 1945 and 1965 and anyone with risk factors such as injection drug use.7-9 As of January 1, 2018, the VA had screened more than 80% of veterans in care within this highest risk birth cohort. As of January 1, 2018, more than 100,000 veterans in VA care have initiated treatment for HCV with direct-acting antivirals (DAAs) (Figure 1).
Several critical factors contributed to the VA success with HCV testing and treatment, including congressional appropriation of funding from fiscal year (FY) 2016 through FY 2018, unrestricted access to interferon-free DAA HCV treatments, and dedicated resources from the VA National Viral Hepatitis Program within the HIV, Hepatitis, and Related Conditions Programs (HHRC) in the Office of Specialty Care Services.5 In 2014, HHRC created and supported the Hepatitis Innovation Team (HIT) Collaborative, a VA process improvement initiative enabling
Veterans Integrated Service Network (VISN) -based, multidisciplinary teams to increase veterans’ access to HCV testing and treatment.
As the VA makes consistent progress toward eliminating HCV in veterans in VA care, it has become clear that achieving a cure is only a starting point in improving HCV care. Many patients with HCV infection also have advanced liver disease (ALD), or cirrhosis, which is a condition of permanent liver fibrosis that remains after the patient has been cured of HCV infection. In addition to hepatitis C, ALD also can be caused by excessive alcohol use, hepatitis B virus (HBV) infection, nonalcoholic fatty liver diseases, and several other inherited diseases. Advanced liver disease affects more than 80,000 veterans in VA care, and the HIT infrastructure provides an excellent framework to better understand and address facility-level and systemwide challenges in diagnosing, caring for, and treating veterans with ALD across the Veterans Health Administration (VHA) system.
This report will describe the elements that contributed to the success of the HIT Collaborative in redesigning care for patients affected by HCV in the VA and how these elements can be applied to improve the system of care for VHA ALD care.
Hepatitis Innovation Teams Collaborative Leadership
After the US Food and Drug Administration (FDA) approved new DAA medications to treat HCV, the VA recognized the need to mobilize the health care system quickly and allocate resources for these new, minimally toxic, and highly effective medications. Early in 2014, HHRC established the National Hepatitis C Resource Center (NHCRC), a successor program to the 4 regional hepatitis C resource centers that had addressed HCV care across the system.10 The NHCRC was charged with developing an operational strategy for VA to respond rapidly to the availability of DAAs. In collaboration with representatives from the Office of Strategic Integration | Veterans Engineering Resource Center (OSI|VERC), the NHCRC formed the HIT Collaborative Leadership Team (CLT).
The HIT CLT is responsible for executing the HIT Collaborative and uses a Lean process improvement framework focused on eliminating waste and maximizing value. Members of the CLT with expertise in facilitation, Lean process improvement, leadership, clinical knowledge, and population health management act as coaches for the VISN HITs. The CLT works to build and support the VISN HITs, identify opportunities for individual teams to improve and assist in finding the right local mix of “players” to be successful. The HIT CLT ensures all teams are functioning and working toward achieving their goals. The CLT obtains data from VA national databases, which are provided to the VISN HITs to inform and encourage continuous improvement of their strategies. Annual VA-wide aspirational goals are developed and disseminated to encourage a unified mission.
Catchment areas for each VISN include between 6 and 10 medical centers as well as outpatient and ambulatory care centers. Multidisciplinary HITs are composed of physicians, nurses, pharmacists, nurse practitioners, physician assistants, social workers, mental health and substance use providers, peer support specialists, administrators, information technology experts, and systems redesign professionals from medical centers within each VISN. Teams develop strong relationships across medical centers, implement context-specific strategies applicable to rural
and urban centers, and share expertise. In addition to intra-VISN process improvement, HITs collaborate monthly across VISNs via a virtual platform. They share strong practices, seek advice from one another, and compare outcomes on an established set of goals.
The HITs use process improvement tools to systematically assess the current steps involved in care. At the close of each year, the HITs analyze the current state of operations and set goals to improve over the following year guided by a target state map. Seed funding is provided to every VISN HIT annually to launch change initiatives. Many VISN HITs use these funds to support a VISN HIT coordinator, and HITs also use this financial support to conduct 2- to 3-day process improvement workshops and to purchase supplies, such as point-of-care testing kits. The HIT communication and work are predominantly executed virtually.
Each year, teams worked toward achieving goals set nationally. These included increasing HCV birth cohort testing and improving the percentage of patients who had SVR12 testing
(Table).
the percentage of patients who received SVR12 testing posttreatment completion was not included in the HIT Collaborative’s annual goals for the first year of the program. Recognizing this as a critical area for improvement, the HIT CLT set a goal to test 80% of all patients who completed treatment. The HITs applied Lean tools to identify and overcome gaps in the SVR12 testing process. By the end of the second year, 84% of all patients who completed treatment had been tested for SVR12.
The HITs also set specific local VISN and medical center goals, prioritizing projects that could have the greatest impact on local patient access and quality of care and build on existing strengths and address barriers. These projects encompass a wide range of areas that contribute to the overall national goals.
Focus on Lean
Lean process improvement is based on 2 key pillars: respect for people (those seeking service as customers and patients and those providing service as frontline staff and stakeholders) and continuous improvement. With Lean, personnel providing care should work to identify and eliminate waste in the system and to streamline care delivery to maximize process steps that are most valued by patients (eg, interaction with a clinical provider) and minimize those that are not valued (eg, time spent waiting to see a provider). With the knowledge that HHRC fully supports their work, HITs were encouraged to innovate based on local resources, context, and culture.
Teams receive basic training in Lean from the HIT CLT and local systems redesign specialists if available. The HITs apply the A3 structured approach to problem solving.11 The HITs follow prescribed problemsolving steps that help identify where to focus process improvement efforts, including analyzing the current state of care, outlining the target state, and prioritizing solution
approaches based on what will have the highest impact for patients.
to accommodate the outcomes they observe (Figure 2).
Innovations
Over the course of the HIT Collaborative, numerous innovations have emerged to address and mitigate barriers to HCV screening and treatment. Examples of successful innovations include the following:
- To address transportation issues, several teams developed programs specific to patients with HCV in rural locations or with limited mobility. Mobile vans and units traditionally used as mobile cardiology clinics were transformed into HCV clinics, bringing testing and treatment services directly to veterans;
- Pharmacists and social workers developed outreach strategies to locate homeless veterans, provide point-of-care testing and utilize mobile technology to concurrently enroll and link veterans to care; and
- Many liver care teams partnered with inpatient and outpatient substance use treatment clinics to provide patient education and coordinate HCV treatment.
Inter-VISN working groups developed systemwide tools to address common needs. In the program’s first year, a few medical facilities across a handful of VISNs shared local population health management systems, programming, and best practices. Over time, this working group combined the virtual networking capacity of the HIT Collaborative with technical expertise to promote rapid dissemination and uptake of a population health management system. Providers at medical centers across VA use the tools to identify veterans who should be screened and treated for HCV with the ability to continuously update information, identifying patients who do not respond to treatment or patients overdue for SVR12 testing.
Providers with experience using telehepatology formed another inter-VISN working group. These subject matter experts provided guidance to care teams interested in implementing telehealth in areas where limited local resources or knowledge had prevented them from moving forward. The ability to build a strong coalition across content areas fostered a collaborative learning environment, adaptable to implementing new processes and technologies.
In 2017, the VA made significant efforts to reach out to veterans eligible for VA care who had not yet been screened or remained untreated. In May, Hepatitis Awareness Month, HITs held HCV testing and community outreach events and participated in veteran stand-downs and veteran service organization activities.
Evaluation
Since 2014, the VA has increased its HCV treatment and screening rates. To assess the components contributing to these achievements and the role of the HIT Collaborative in driving this success, a team of implementation scientists have been working with the CLT to conduct a HIT program evaluation. The goal of the evaluation is to establish the impact of the HIT Collaborative. The evaluation team catalogs the activities of the Collaborative and the HITs and assesses implementation strategies (use of specific techniques) to increase the uptake of evidence-based practices specifically related to HCV treatment.12
At the close of each FY, HCV providers and members of the HIT Collaborative are queried through an online survey to determine which strategies have been used to improve HCV care and how these strategies were associated with the HIT Collaborative. The use of more strategies was associated with more HCV treatment initiations.13 All utilized strategies were identified whether or not they were associated with treatment starts. These data are being used to understand which combinations of strategies are most effective at increasing treatment for HCV in the VA and to inform future initiatives.
Expanding the Scope
Inspired by the successful results of the HIT work in HCV and in the spirit of continuously improving health care delivery, HHRC expanded the scope of the HIT Collaborative in FY 2018 to include ALD. There are about 80,000 veterans in VA care with advanced scarring of the liver and between 10,000 to 15,000 new diagnoses each year. In addition to HCV as an etiology for ALD, cases of cirrhosis are projected to increase among veterans in care due to metabolic syndrome and alcohol use. A recent review of VA data from fiscal year 2016 found that 88.6% of ALD patients had been seen in primary care within the past 2 years, with about half (51%) seen in a gastroenterology (GI) or hepatology clinic (Personal communication, HIV, Hepatitis, and Related Conditions Program Office March 16, 2018). For patients in VA care with ALD, GI visits are associated with a lower 5-year mortality.14 Annual mortality for all ALD patients in VA is 6.2%, and of those with a hospital admission, mortality rises to 31%.15 In FY 2016, there were about 52,000 ALD-related discharges (more than 2 per patient). Of those discharges, 24% were readmitted within 30 days, with an average length of stay of 1.9 days and an estimated cost per patient of $47,000 over 3 years.16
Hepatologists from across the VA convened to identify critical opportunities for improvement for patients with ALD. Base on available evidence presented in the literature and their clinical expertise, these subject matter experts identified several areas for quality improvement, with the overarching goal to improve identification of patients with early cirrhosis and ensure appropriate linkage to care for all cirrhotic patients, thus improving quality of life and reducing mortality. Although not finalized, candidate improvement targets include consistent linkage to care and treatment for HCV and HBV, comprehensive case management, post-discharge patient follow-up, and adherence to evidence-based standards of care.
Conclusion
The VA has made great strides in nearly eliminating HCV among veterans in VA care. The national effort to redesign hepatitis care using Lean management strategies and develop local and regional teams and centralized support allowed VA to maximize available resources to achieve higher rates of HCV birth cohort testing and treatment of patients infected with HCV than has any other health care system in the US.
The HIT Collaborative has been a unique and innovative mechanism to promote directed, patient-outcome driven change in a large and dynamic health care system. It has allowed rural and urban providers to work together to develop and spread quality improvement innovations and as an integrated system to achieve national priorities. The focus of this foundational HIT structure is expanding to identifying, treat, and care for VA’s ALD population.
Hepatitis C virus (HCV) infection is a major public health problem in the US. Following the 2010 report of the Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) on hepatitis and liver cancer, the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan in 2011 with subsequent action plan updates for 2014-2016 and 2017-2020.1-3 A NASEM phase 2 report and the 2017-2020 HHS action plan outline a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities.3,4 The Department of Veterans Affairs (VA) is the single largest HCV care provider in the US with about 165,000 veterans in care diagnosed with HCV in the beginning of 2014 and is a national leader in the testing and treatment of HCV.5,6
The VA’s recommendations for screening for HCV infection are in alignment with the United States Preventive Services Task Force (USPSTF) and Centers for Disease Control and Prevention (CDC) recommendations to test all veterans born between 1945 and 1965 and anyone with risk factors such as injection drug use.7-9 As of January 1, 2018, the VA had screened more than 80% of veterans in care within this highest risk birth cohort. As of January 1, 2018, more than 100,000 veterans in VA care have initiated treatment for HCV with direct-acting antivirals (DAAs) (Figure 1).
Several critical factors contributed to the VA success with HCV testing and treatment, including congressional appropriation of funding from fiscal year (FY) 2016 through FY 2018, unrestricted access to interferon-free DAA HCV treatments, and dedicated resources from the VA National Viral Hepatitis Program within the HIV, Hepatitis, and Related Conditions Programs (HHRC) in the Office of Specialty Care Services.5 In 2014, HHRC created and supported the Hepatitis Innovation Team (HIT) Collaborative, a VA process improvement initiative enabling
Veterans Integrated Service Network (VISN) -based, multidisciplinary teams to increase veterans’ access to HCV testing and treatment.
As the VA makes consistent progress toward eliminating HCV in veterans in VA care, it has become clear that achieving a cure is only a starting point in improving HCV care. Many patients with HCV infection also have advanced liver disease (ALD), or cirrhosis, which is a condition of permanent liver fibrosis that remains after the patient has been cured of HCV infection. In addition to hepatitis C, ALD also can be caused by excessive alcohol use, hepatitis B virus (HBV) infection, nonalcoholic fatty liver diseases, and several other inherited diseases. Advanced liver disease affects more than 80,000 veterans in VA care, and the HIT infrastructure provides an excellent framework to better understand and address facility-level and systemwide challenges in diagnosing, caring for, and treating veterans with ALD across the Veterans Health Administration (VHA) system.
This report will describe the elements that contributed to the success of the HIT Collaborative in redesigning care for patients affected by HCV in the VA and how these elements can be applied to improve the system of care for VHA ALD care.
Hepatitis Innovation Teams Collaborative Leadership
After the US Food and Drug Administration (FDA) approved new DAA medications to treat HCV, the VA recognized the need to mobilize the health care system quickly and allocate resources for these new, minimally toxic, and highly effective medications. Early in 2014, HHRC established the National Hepatitis C Resource Center (NHCRC), a successor program to the 4 regional hepatitis C resource centers that had addressed HCV care across the system.10 The NHCRC was charged with developing an operational strategy for VA to respond rapidly to the availability of DAAs. In collaboration with representatives from the Office of Strategic Integration | Veterans Engineering Resource Center (OSI|VERC), the NHCRC formed the HIT Collaborative Leadership Team (CLT).
The HIT CLT is responsible for executing the HIT Collaborative and uses a Lean process improvement framework focused on eliminating waste and maximizing value. Members of the CLT with expertise in facilitation, Lean process improvement, leadership, clinical knowledge, and population health management act as coaches for the VISN HITs. The CLT works to build and support the VISN HITs, identify opportunities for individual teams to improve and assist in finding the right local mix of “players” to be successful. The HIT CLT ensures all teams are functioning and working toward achieving their goals. The CLT obtains data from VA national databases, which are provided to the VISN HITs to inform and encourage continuous improvement of their strategies. Annual VA-wide aspirational goals are developed and disseminated to encourage a unified mission.
Catchment areas for each VISN include between 6 and 10 medical centers as well as outpatient and ambulatory care centers. Multidisciplinary HITs are composed of physicians, nurses, pharmacists, nurse practitioners, physician assistants, social workers, mental health and substance use providers, peer support specialists, administrators, information technology experts, and systems redesign professionals from medical centers within each VISN. Teams develop strong relationships across medical centers, implement context-specific strategies applicable to rural
and urban centers, and share expertise. In addition to intra-VISN process improvement, HITs collaborate monthly across VISNs via a virtual platform. They share strong practices, seek advice from one another, and compare outcomes on an established set of goals.
The HITs use process improvement tools to systematically assess the current steps involved in care. At the close of each year, the HITs analyze the current state of operations and set goals to improve over the following year guided by a target state map. Seed funding is provided to every VISN HIT annually to launch change initiatives. Many VISN HITs use these funds to support a VISN HIT coordinator, and HITs also use this financial support to conduct 2- to 3-day process improvement workshops and to purchase supplies, such as point-of-care testing kits. The HIT communication and work are predominantly executed virtually.
Each year, teams worked toward achieving goals set nationally. These included increasing HCV birth cohort testing and improving the percentage of patients who had SVR12 testing
(Table).
the percentage of patients who received SVR12 testing posttreatment completion was not included in the HIT Collaborative’s annual goals for the first year of the program. Recognizing this as a critical area for improvement, the HIT CLT set a goal to test 80% of all patients who completed treatment. The HITs applied Lean tools to identify and overcome gaps in the SVR12 testing process. By the end of the second year, 84% of all patients who completed treatment had been tested for SVR12.
The HITs also set specific local VISN and medical center goals, prioritizing projects that could have the greatest impact on local patient access and quality of care and build on existing strengths and address barriers. These projects encompass a wide range of areas that contribute to the overall national goals.
Focus on Lean
Lean process improvement is based on 2 key pillars: respect for people (those seeking service as customers and patients and those providing service as frontline staff and stakeholders) and continuous improvement. With Lean, personnel providing care should work to identify and eliminate waste in the system and to streamline care delivery to maximize process steps that are most valued by patients (eg, interaction with a clinical provider) and minimize those that are not valued (eg, time spent waiting to see a provider). With the knowledge that HHRC fully supports their work, HITs were encouraged to innovate based on local resources, context, and culture.
Teams receive basic training in Lean from the HIT CLT and local systems redesign specialists if available. The HITs apply the A3 structured approach to problem solving.11 The HITs follow prescribed problemsolving steps that help identify where to focus process improvement efforts, including analyzing the current state of care, outlining the target state, and prioritizing solution
approaches based on what will have the highest impact for patients.
to accommodate the outcomes they observe (Figure 2).
Innovations
Over the course of the HIT Collaborative, numerous innovations have emerged to address and mitigate barriers to HCV screening and treatment. Examples of successful innovations include the following:
- To address transportation issues, several teams developed programs specific to patients with HCV in rural locations or with limited mobility. Mobile vans and units traditionally used as mobile cardiology clinics were transformed into HCV clinics, bringing testing and treatment services directly to veterans;
- Pharmacists and social workers developed outreach strategies to locate homeless veterans, provide point-of-care testing and utilize mobile technology to concurrently enroll and link veterans to care; and
- Many liver care teams partnered with inpatient and outpatient substance use treatment clinics to provide patient education and coordinate HCV treatment.
Inter-VISN working groups developed systemwide tools to address common needs. In the program’s first year, a few medical facilities across a handful of VISNs shared local population health management systems, programming, and best practices. Over time, this working group combined the virtual networking capacity of the HIT Collaborative with technical expertise to promote rapid dissemination and uptake of a population health management system. Providers at medical centers across VA use the tools to identify veterans who should be screened and treated for HCV with the ability to continuously update information, identifying patients who do not respond to treatment or patients overdue for SVR12 testing.
Providers with experience using telehepatology formed another inter-VISN working group. These subject matter experts provided guidance to care teams interested in implementing telehealth in areas where limited local resources or knowledge had prevented them from moving forward. The ability to build a strong coalition across content areas fostered a collaborative learning environment, adaptable to implementing new processes and technologies.
In 2017, the VA made significant efforts to reach out to veterans eligible for VA care who had not yet been screened or remained untreated. In May, Hepatitis Awareness Month, HITs held HCV testing and community outreach events and participated in veteran stand-downs and veteran service organization activities.
Evaluation
Since 2014, the VA has increased its HCV treatment and screening rates. To assess the components contributing to these achievements and the role of the HIT Collaborative in driving this success, a team of implementation scientists have been working with the CLT to conduct a HIT program evaluation. The goal of the evaluation is to establish the impact of the HIT Collaborative. The evaluation team catalogs the activities of the Collaborative and the HITs and assesses implementation strategies (use of specific techniques) to increase the uptake of evidence-based practices specifically related to HCV treatment.12
At the close of each FY, HCV providers and members of the HIT Collaborative are queried through an online survey to determine which strategies have been used to improve HCV care and how these strategies were associated with the HIT Collaborative. The use of more strategies was associated with more HCV treatment initiations.13 All utilized strategies were identified whether or not they were associated with treatment starts. These data are being used to understand which combinations of strategies are most effective at increasing treatment for HCV in the VA and to inform future initiatives.
Expanding the Scope
Inspired by the successful results of the HIT work in HCV and in the spirit of continuously improving health care delivery, HHRC expanded the scope of the HIT Collaborative in FY 2018 to include ALD. There are about 80,000 veterans in VA care with advanced scarring of the liver and between 10,000 to 15,000 new diagnoses each year. In addition to HCV as an etiology for ALD, cases of cirrhosis are projected to increase among veterans in care due to metabolic syndrome and alcohol use. A recent review of VA data from fiscal year 2016 found that 88.6% of ALD patients had been seen in primary care within the past 2 years, with about half (51%) seen in a gastroenterology (GI) or hepatology clinic (Personal communication, HIV, Hepatitis, and Related Conditions Program Office March 16, 2018). For patients in VA care with ALD, GI visits are associated with a lower 5-year mortality.14 Annual mortality for all ALD patients in VA is 6.2%, and of those with a hospital admission, mortality rises to 31%.15 In FY 2016, there were about 52,000 ALD-related discharges (more than 2 per patient). Of those discharges, 24% were readmitted within 30 days, with an average length of stay of 1.9 days and an estimated cost per patient of $47,000 over 3 years.16
Hepatologists from across the VA convened to identify critical opportunities for improvement for patients with ALD. Base on available evidence presented in the literature and their clinical expertise, these subject matter experts identified several areas for quality improvement, with the overarching goal to improve identification of patients with early cirrhosis and ensure appropriate linkage to care for all cirrhotic patients, thus improving quality of life and reducing mortality. Although not finalized, candidate improvement targets include consistent linkage to care and treatment for HCV and HBV, comprehensive case management, post-discharge patient follow-up, and adherence to evidence-based standards of care.
Conclusion
The VA has made great strides in nearly eliminating HCV among veterans in VA care. The national effort to redesign hepatitis care using Lean management strategies and develop local and regional teams and centralized support allowed VA to maximize available resources to achieve higher rates of HCV birth cohort testing and treatment of patients infected with HCV than has any other health care system in the US.
The HIT Collaborative has been a unique and innovative mechanism to promote directed, patient-outcome driven change in a large and dynamic health care system. It has allowed rural and urban providers to work together to develop and spread quality improvement innovations and as an integrated system to achieve national priorities. The focus of this foundational HIT structure is expanding to identifying, treat, and care for VA’s ALD population.
1. Colvin HM, Mitchell AE, eds; and the Committee on the Prevention and Control of Viral Hepatitis Infections Board on Population Health and Public Health Practice. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: The National Academies Press; 2010.
2. US Department of Health and Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care and treatment of viral hepatitis. https://www.hhs.gov/sites/default/files/action-plan-viral-hepatitis-2011.pdf. Accessed April 27, 2018.
3. Wolitski R. National viral hepatitis action plan: 2017-2020. https://www.hhs.gov/hepatitis/action-plan/national-viralhepatitis-action-plan-overview/index.html. Updated February
21, 2018. Accessed May 8, 2018.
4. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: The National Academies Press; 2017.
5. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C infection: best practices from the Department of Veterans Affairs. Ann of Intern Med. 2017;167(7):499-504.
6. Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol. 2015;63(3):586-592.
7. US Department of Veterans Affairs, Veteran Health Administration. National Clinical Preventive Service Guidance Statements: Screening for Hepatitis C. http://www.prevention.va.gov/CPS/Screening_for_Hepatitis_C.asp. Published on June 20, 2017. [Nonpubic document; source not verified.]
8. Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
9. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
10. Garrard J, Choudary V, Groom H, et al. Organizational change in management of hepatitis C: evaluation of a CME program. J Contin Educ Health Prof. 2006;26(2):145-160.
11. Shook J. Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor, and Lead. Cambridge, MA: Lean Enterprise Institute; 2010.
12. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
13. Rogal SS, Yakovchenko V, Waltz TJ, et al. The association between implementation strategy use and the uptake of hepatitis C treatment in a national sample. Implement Sci.
2017;12(1):60.
14. Mellinger JL, Moser S, Welsh DE, et al. Access to subspecialty care and survival among patients with liver disease. Am J Gastroenterol. 2016;111(6):838-844.
15. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in the burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans from 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
16. Kaplan DE, Chapko MK, Mehta R, et al; VOCAL Study Group. Healthcare costs related to treatment of hepatocellular carcinoma among veterans with cirrhosis in the United States. Clin Gastroenterol Hepatol. 2018;16(1):106-114.
1. Colvin HM, Mitchell AE, eds; and the Committee on the Prevention and Control of Viral Hepatitis Infections Board on Population Health and Public Health Practice. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: The National Academies Press; 2010.
2. US Department of Health and Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care and treatment of viral hepatitis. https://www.hhs.gov/sites/default/files/action-plan-viral-hepatitis-2011.pdf. Accessed April 27, 2018.
3. Wolitski R. National viral hepatitis action plan: 2017-2020. https://www.hhs.gov/hepatitis/action-plan/national-viralhepatitis-action-plan-overview/index.html. Updated February
21, 2018. Accessed May 8, 2018.
4. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: The National Academies Press; 2017.
5. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C infection: best practices from the Department of Veterans Affairs. Ann of Intern Med. 2017;167(7):499-504.
6. Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol. 2015;63(3):586-592.
7. US Department of Veterans Affairs, Veteran Health Administration. National Clinical Preventive Service Guidance Statements: Screening for Hepatitis C. http://www.prevention.va.gov/CPS/Screening_for_Hepatitis_C.asp. Published on June 20, 2017. [Nonpubic document; source not verified.]
8. Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
9. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
10. Garrard J, Choudary V, Groom H, et al. Organizational change in management of hepatitis C: evaluation of a CME program. J Contin Educ Health Prof. 2006;26(2):145-160.
11. Shook J. Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor, and Lead. Cambridge, MA: Lean Enterprise Institute; 2010.
12. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
13. Rogal SS, Yakovchenko V, Waltz TJ, et al. The association between implementation strategy use and the uptake of hepatitis C treatment in a national sample. Implement Sci.
2017;12(1):60.
14. Mellinger JL, Moser S, Welsh DE, et al. Access to subspecialty care and survival among patients with liver disease. Am J Gastroenterol. 2016;111(6):838-844.
15. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in the burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans from 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
16. Kaplan DE, Chapko MK, Mehta R, et al; VOCAL Study Group. Healthcare costs related to treatment of hepatocellular carcinoma among veterans with cirrhosis in the United States. Clin Gastroenterol Hepatol. 2018;16(1):106-114.
HCV remodels lipid metabolism in infected cells
Hepatitis C virus infection often is associated with the accumulation of fat in hepatocytes, which shows a connection between the virus and the lipid metabolism of the liver, according to Sarah Hoffman, MD, of the Leibniz Institute for Experimental Virology, Hamburg, Germany, and her colleagues. “Our study provides a detailed analysis of the changes in the lipid composition in HCV-infected cells that revealed dependency on FA [fatty acid] elongation and desaturation for effective viral replication and virion production,” they reported.
Dr. Hoffman and her colleagues assessed lipid composition of infected cells in an in vitro study of cell lines, which were assessed 8-11 days post infection, according to the report published in BBA: Molecular and Cell Biology of Lipids. They determined the abundance of each major lipid class and compared the pattern of HCV-infected cells with that of controls.
The researchers found that HCV caused an accumulation of membrane phosopholipids but not neutral lipids and that cholesterol accumulated in the perinuclear region of HCV-infected cells. In addition, lipid species with longer fatty acyl chains were more abundant in HCV-infected cells and free polyunsaturated fatty acid (PUFA) levels were greatly increased.
In further confirmation of the critical role of lipid metabolism in HCV replication, they found that knockdown of fatty acid elongases and desaturases disrupted HCV replication, while overexpression of these enzymes showed a proviral effect.
“We identified several lipid-remodeling pathways that are required for distinct steps in viral infection. Future studies have to address the molecular function of longer fatty acyl chains in HCV RNA replication and why PUFAs are needed for HCV particle production,” the researchers concluded.
The authors reported government and institutional-only funding and no personal disclosures.
SOURCE: Hoffman S et al. Biochim Biophys Acta. 2018 Jun 6;1863(9):1041-56.
Hepatitis C virus infection often is associated with the accumulation of fat in hepatocytes, which shows a connection between the virus and the lipid metabolism of the liver, according to Sarah Hoffman, MD, of the Leibniz Institute for Experimental Virology, Hamburg, Germany, and her colleagues. “Our study provides a detailed analysis of the changes in the lipid composition in HCV-infected cells that revealed dependency on FA [fatty acid] elongation and desaturation for effective viral replication and virion production,” they reported.
Dr. Hoffman and her colleagues assessed lipid composition of infected cells in an in vitro study of cell lines, which were assessed 8-11 days post infection, according to the report published in BBA: Molecular and Cell Biology of Lipids. They determined the abundance of each major lipid class and compared the pattern of HCV-infected cells with that of controls.
The researchers found that HCV caused an accumulation of membrane phosopholipids but not neutral lipids and that cholesterol accumulated in the perinuclear region of HCV-infected cells. In addition, lipid species with longer fatty acyl chains were more abundant in HCV-infected cells and free polyunsaturated fatty acid (PUFA) levels were greatly increased.
In further confirmation of the critical role of lipid metabolism in HCV replication, they found that knockdown of fatty acid elongases and desaturases disrupted HCV replication, while overexpression of these enzymes showed a proviral effect.
“We identified several lipid-remodeling pathways that are required for distinct steps in viral infection. Future studies have to address the molecular function of longer fatty acyl chains in HCV RNA replication and why PUFAs are needed for HCV particle production,” the researchers concluded.
The authors reported government and institutional-only funding and no personal disclosures.
SOURCE: Hoffman S et al. Biochim Biophys Acta. 2018 Jun 6;1863(9):1041-56.
Hepatitis C virus infection often is associated with the accumulation of fat in hepatocytes, which shows a connection between the virus and the lipid metabolism of the liver, according to Sarah Hoffman, MD, of the Leibniz Institute for Experimental Virology, Hamburg, Germany, and her colleagues. “Our study provides a detailed analysis of the changes in the lipid composition in HCV-infected cells that revealed dependency on FA [fatty acid] elongation and desaturation for effective viral replication and virion production,” they reported.
Dr. Hoffman and her colleagues assessed lipid composition of infected cells in an in vitro study of cell lines, which were assessed 8-11 days post infection, according to the report published in BBA: Molecular and Cell Biology of Lipids. They determined the abundance of each major lipid class and compared the pattern of HCV-infected cells with that of controls.
The researchers found that HCV caused an accumulation of membrane phosopholipids but not neutral lipids and that cholesterol accumulated in the perinuclear region of HCV-infected cells. In addition, lipid species with longer fatty acyl chains were more abundant in HCV-infected cells and free polyunsaturated fatty acid (PUFA) levels were greatly increased.
In further confirmation of the critical role of lipid metabolism in HCV replication, they found that knockdown of fatty acid elongases and desaturases disrupted HCV replication, while overexpression of these enzymes showed a proviral effect.
“We identified several lipid-remodeling pathways that are required for distinct steps in viral infection. Future studies have to address the molecular function of longer fatty acyl chains in HCV RNA replication and why PUFAs are needed for HCV particle production,” the researchers concluded.
The authors reported government and institutional-only funding and no personal disclosures.
SOURCE: Hoffman S et al. Biochim Biophys Acta. 2018 Jun 6;1863(9):1041-56.
FROM BBA: MOLECULAR AND CELL BIOLOGY OF LIPIDS
Cigarette smoking epidemic among HCV-infected individuals
There is a cigarette smoking epidemic embedded within the hepatitis C virus epidemic in the United States, according to the results of an analysis of data between 1999 and 2014 from the National Health and Nutrition Examination Survey (NHANES).
Smoking and hepatitis C information were available for 90.1% of the NHANES adult population. Of the 39,472 individuals evaluated, 1.3% were hepatitis C+ and 22.3% were current smokers. Hepatitis C+ individuals were almost three times as likely to be smokers as were those who were hepatitis C– (62.4% vs. 22.9%, respectively), according to the report, published in The American Journal of Medicine (Am J Med. 2018 Jun;131[6]:699-75).
Ryung S. Kim, PhD, of Albert Einstein College of Medicine, New York, and his colleagues also found that hepatitis C+ smokers were more likely to be older, male, black, less educated, poor, and uninsured compared with their hepatitis C– smoking counterparts. They also were more likely to use drugs, including heroin, and to be depressed.
Multivariate analysis showed a significant association of both hepatitis C infection and smoking with current depression and hypertension, Dr. Kim and his colleagues wrote.
“It is public health folly to spend tens of millions of dollars annually” on treatment of hepatitis C patients, “and ignore the lethal addiction affecting more than 60% of them. As we enter a new era of hepatitis C treatment, it is a public health imperative to research, develop, and implement tobacco treatments for the hepatitis C+ community,” Dr. Kim and his colleagues concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Kim RS et al. Am J Med. 2018Jun;131[6]:669-75).
There is a cigarette smoking epidemic embedded within the hepatitis C virus epidemic in the United States, according to the results of an analysis of data between 1999 and 2014 from the National Health and Nutrition Examination Survey (NHANES).
Smoking and hepatitis C information were available for 90.1% of the NHANES adult population. Of the 39,472 individuals evaluated, 1.3% were hepatitis C+ and 22.3% were current smokers. Hepatitis C+ individuals were almost three times as likely to be smokers as were those who were hepatitis C– (62.4% vs. 22.9%, respectively), according to the report, published in The American Journal of Medicine (Am J Med. 2018 Jun;131[6]:699-75).
Ryung S. Kim, PhD, of Albert Einstein College of Medicine, New York, and his colleagues also found that hepatitis C+ smokers were more likely to be older, male, black, less educated, poor, and uninsured compared with their hepatitis C– smoking counterparts. They also were more likely to use drugs, including heroin, and to be depressed.
Multivariate analysis showed a significant association of both hepatitis C infection and smoking with current depression and hypertension, Dr. Kim and his colleagues wrote.
“It is public health folly to spend tens of millions of dollars annually” on treatment of hepatitis C patients, “and ignore the lethal addiction affecting more than 60% of them. As we enter a new era of hepatitis C treatment, it is a public health imperative to research, develop, and implement tobacco treatments for the hepatitis C+ community,” Dr. Kim and his colleagues concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Kim RS et al. Am J Med. 2018Jun;131[6]:669-75).
There is a cigarette smoking epidemic embedded within the hepatitis C virus epidemic in the United States, according to the results of an analysis of data between 1999 and 2014 from the National Health and Nutrition Examination Survey (NHANES).
Smoking and hepatitis C information were available for 90.1% of the NHANES adult population. Of the 39,472 individuals evaluated, 1.3% were hepatitis C+ and 22.3% were current smokers. Hepatitis C+ individuals were almost three times as likely to be smokers as were those who were hepatitis C– (62.4% vs. 22.9%, respectively), according to the report, published in The American Journal of Medicine (Am J Med. 2018 Jun;131[6]:699-75).
Ryung S. Kim, PhD, of Albert Einstein College of Medicine, New York, and his colleagues also found that hepatitis C+ smokers were more likely to be older, male, black, less educated, poor, and uninsured compared with their hepatitis C– smoking counterparts. They also were more likely to use drugs, including heroin, and to be depressed.
Multivariate analysis showed a significant association of both hepatitis C infection and smoking with current depression and hypertension, Dr. Kim and his colleagues wrote.
“It is public health folly to spend tens of millions of dollars annually” on treatment of hepatitis C patients, “and ignore the lethal addiction affecting more than 60% of them. As we enter a new era of hepatitis C treatment, it is a public health imperative to research, develop, and implement tobacco treatments for the hepatitis C+ community,” Dr. Kim and his colleagues concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Kim RS et al. Am J Med. 2018Jun;131[6]:669-75).
FROM THE AMERICAN JOURNAL OF MEDICINE
Acute Hepatitis E Superinfection Reactivates Chronic HBV
Many things can reactivate chronic hepatitis B virus (HBV) infection—withdrawal of antiviral therapy, pregnancy, and chemotherapy, to name a few. So when a patient with stable chronic HBV virus presented with significant hepatitis flare, clinicians from Beth Israel Deaconess Medical Center in Boston, Massachusetts, had a long list to check.
They first ruled out drug-associated hepatotoxicity and screened the patient for common causes of acute hepatitis. Beyond the HBV, the patient did not have other significant medical conditions, had not had close contact with anyone ill, and was not pregnant. Tests were negative for cytomegalovirus, Epstein-Barr syndrome, HIV, hepatitis A, C, and D. The patient tested negative for both antihepatitis E virus (HEV) IgM and IgG in a visit about 9 months before.
However, she reported regularly consuming pork, including a recent barbecue meal. Thus, the clinicians focused on HEV serology, which confirmed that she had an acute HEV infection.
Pigs act as a “natural reservoir” for HEV; contaminated meats and direct contact with animals are the most common causes of HEV human infection in industrialized countries. Recent data reveal the prevalence of HEV antibodies in the US is about 6%, illustrating that it is not as uncommon as it was thought to be. Although there was no direct evidence to confirm the source of her infection, it seemed likely due to the pork consumption.
The patient was started on tenofovir but stopped it 4 months later because she felt well. After a subsequent flare, “repeated counseling” persuaded the patient to start on entecavir, with successful viral suppression.
Hepatitis E superinfection on chronic HBV can contribute to significant morbidity and mortality, the clinicians say, particularly in patients with cirrhosis. Concurrent infection with another viral hepatitis should be considered in both immunodeficient and immunocompetent patients with chronic HBV reactivation.
Source:
Aslam A, Susheela A, Iriana S, Chan SS, Lau D. BMJ Case Rep. 2018;2018. pii: bcr-2017-223616.
doi: 10.1136/bcr-2017-223616.
Many things can reactivate chronic hepatitis B virus (HBV) infection—withdrawal of antiviral therapy, pregnancy, and chemotherapy, to name a few. So when a patient with stable chronic HBV virus presented with significant hepatitis flare, clinicians from Beth Israel Deaconess Medical Center in Boston, Massachusetts, had a long list to check.
They first ruled out drug-associated hepatotoxicity and screened the patient for common causes of acute hepatitis. Beyond the HBV, the patient did not have other significant medical conditions, had not had close contact with anyone ill, and was not pregnant. Tests were negative for cytomegalovirus, Epstein-Barr syndrome, HIV, hepatitis A, C, and D. The patient tested negative for both antihepatitis E virus (HEV) IgM and IgG in a visit about 9 months before.
However, she reported regularly consuming pork, including a recent barbecue meal. Thus, the clinicians focused on HEV serology, which confirmed that she had an acute HEV infection.
Pigs act as a “natural reservoir” for HEV; contaminated meats and direct contact with animals are the most common causes of HEV human infection in industrialized countries. Recent data reveal the prevalence of HEV antibodies in the US is about 6%, illustrating that it is not as uncommon as it was thought to be. Although there was no direct evidence to confirm the source of her infection, it seemed likely due to the pork consumption.
The patient was started on tenofovir but stopped it 4 months later because she felt well. After a subsequent flare, “repeated counseling” persuaded the patient to start on entecavir, with successful viral suppression.
Hepatitis E superinfection on chronic HBV can contribute to significant morbidity and mortality, the clinicians say, particularly in patients with cirrhosis. Concurrent infection with another viral hepatitis should be considered in both immunodeficient and immunocompetent patients with chronic HBV reactivation.
Source:
Aslam A, Susheela A, Iriana S, Chan SS, Lau D. BMJ Case Rep. 2018;2018. pii: bcr-2017-223616.
doi: 10.1136/bcr-2017-223616.
Many things can reactivate chronic hepatitis B virus (HBV) infection—withdrawal of antiviral therapy, pregnancy, and chemotherapy, to name a few. So when a patient with stable chronic HBV virus presented with significant hepatitis flare, clinicians from Beth Israel Deaconess Medical Center in Boston, Massachusetts, had a long list to check.
They first ruled out drug-associated hepatotoxicity and screened the patient for common causes of acute hepatitis. Beyond the HBV, the patient did not have other significant medical conditions, had not had close contact with anyone ill, and was not pregnant. Tests were negative for cytomegalovirus, Epstein-Barr syndrome, HIV, hepatitis A, C, and D. The patient tested negative for both antihepatitis E virus (HEV) IgM and IgG in a visit about 9 months before.
However, she reported regularly consuming pork, including a recent barbecue meal. Thus, the clinicians focused on HEV serology, which confirmed that she had an acute HEV infection.
Pigs act as a “natural reservoir” for HEV; contaminated meats and direct contact with animals are the most common causes of HEV human infection in industrialized countries. Recent data reveal the prevalence of HEV antibodies in the US is about 6%, illustrating that it is not as uncommon as it was thought to be. Although there was no direct evidence to confirm the source of her infection, it seemed likely due to the pork consumption.
The patient was started on tenofovir but stopped it 4 months later because she felt well. After a subsequent flare, “repeated counseling” persuaded the patient to start on entecavir, with successful viral suppression.
Hepatitis E superinfection on chronic HBV can contribute to significant morbidity and mortality, the clinicians say, particularly in patients with cirrhosis. Concurrent infection with another viral hepatitis should be considered in both immunodeficient and immunocompetent patients with chronic HBV reactivation.
Source:
Aslam A, Susheela A, Iriana S, Chan SS, Lau D. BMJ Case Rep. 2018;2018. pii: bcr-2017-223616.
doi: 10.1136/bcr-2017-223616.
Strategies to Improve Hepatocellular Carcinoma Surveillance in Veterans With Hepatitis B Infection (FULL)
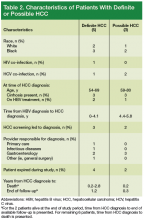
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
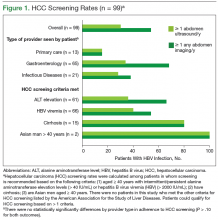
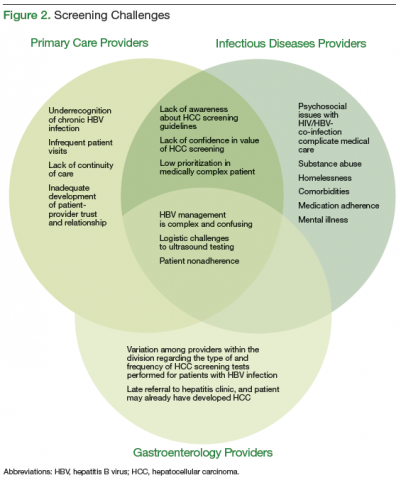
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
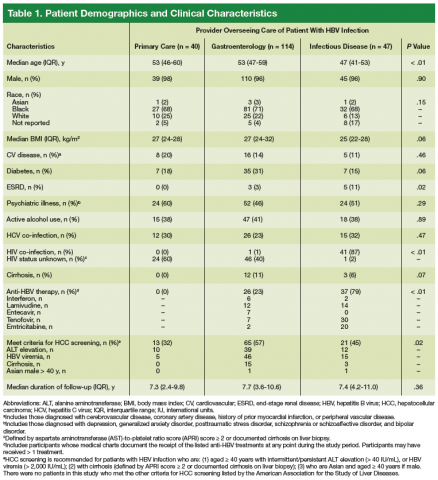
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
Improving Veteran Access to Treatment for Hepatitis C Virus Infection (FULL)
In the U.S., 2.7 to 3.9 million people are chronically infected with the hepatitis C virus (HCV).1 Survey data suggest that HCV infection is more prevalent in patients enrolled in the VA health care system than it is in civilian health care systems.2 Studies have shown that Vietnam veterans, veterans with mental health and substance abuse disorders, and veterans without stable housing are more likely to be infected with HCV.3 Data from the VA HCV Clinical Case Registry (CCR) for 2015 showed that 174,842 veterans with chronic HCV infection receieved care within the VHA, which makes the VA the single largest HCV care provider in the nation.4
The VA is dedicated to providing treatment to veterans with HCV infection. For fiscal year (FY) 2016, the VA allocated $1 billion to HCV care,and in February 2016 it began offering treatment to all veterans with HCV, regardless of degree of fibrosis or severity of underlying liver disease.3,5 Each VAMC was tasked with improving veterans’ access to HCV treatment.
In an effort to engage patients in HCV care, the multidisciplinary HCV team at the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, launched a 2-phase improvement process in 2016. The goal in phase 1 was to increase patient access to HCV clinics, and the goal in phase 2 was to recruit patients for direct-acting antiviral (DAA) therapy for HCV. These efforts were designed to increase screening, identification, and linkage to care for HCV and to expand clinic access for the treatment and cure of all identified veterans who pursued treatment.
Patients with HCV infection, referred from primary care clinics, initially were evaluated by HCV clinic providers (hepatologists, infectious disease specialists, gastroenterology fellows, or nurse practitioners) for eligibility to receive DAA therapy for HCV. Eligible patients then were referred to a pharmacist-run HCV clinic, which had been established at RLRVAMC in 2011. At the start of FY 2016, the clinic, staffed by 3 pharmacists, operated 5 half-days per week and accommodated up to 35 weekly patient appointments.
In this clinic, patients received initial education and medication reconciliation for potential drug interactions with DAAs. Once the HCV treatment was initiated, patients were evaluated in the clinic every 2 weeks for medication refills and assessment for tolerability, adherence, and laboratory abnormalities until end of treatment (8-24 weeks, depending on HCV genotype, experiences with prior HCV treatment, and presence/absence of cirrhosis). Twelve weeks after completion of treatment, viral load was obtained to determine sustained virologic response (SVR12).
Methods
Phase 1: Improve Clinic Access
During FY 2016, methods for expanding clinic access to accommodate a large influx of treatment-eligible patients were reviewed and implemented.
In the first intervention, unneeded follow-up visits were eliminated to make room for additional new patient appointments. In general, patients treated with ribavirin require close monitoring, given the risk for anemia.6 With the release of newer DAAs, however, more patients became eligible for treatment with ribavirin-free regimens.7 As a result, follow-up appointments for these patients were extended to 4-week intervals instead of every 2 weeks. A patient with a history of nonadherence to medication use or clinic visits was still maintained on a 2-week schedule of follow-up for close monitoring.
In the second intervention, opportunities for switching those who completed treatment from face-to-face clinic visits to telephone were identified. These patients historically were seen in clinic for a brief interview and for a blood test used to determine end-of-treatment viral load. Improving access for new patients in the clinic involved moving more existing patients from in-clinic visits to telephone. At the end of the treatment plan, existing patients received an order for laboratory tests that included viral load. When all laboratory results were ready, patients were contacted by telephone. Recruiting a registered nurse to the treatment team who assisted with telephone visits further improved clinic efficiency.
The third intervention was inspired by successful results at other VA sites and launched a group treatment clinic for patients who were starting ribavirin-free DAA regimens.7 Group visits were run by 2 pharmacists and accommodated up to 10 veterans. Patients underwent testing for HCV genotype and viral load before the initial group visit. At check-in, patients received a short questionnaire and consent form for group participation. The questionnaire reviewed patient history of drug and alcohol use and potential barriers to medication adherence. Patients also were encouraged to write down any questions they had about the treatment. During the initial group visit, pharmacists provided general education about the medications, potential adverse effects, treatment expectations, and the monitoring plan. Follow-up visits were conducted in a group setting as well.
Phase 2: Increase Recruitment
The records of 534 patients with advanced liver disease (F3-F4 fibrosis on the Fibrosis-4 Index for Liver Fibrosis) and HCV infection were identified in the CCR database for the period August 2015 to December 2015 (Figure 1).8 Patients were excluded if they were deceased, were receiving palliative care (n = 45), or if they had transferred their care to another VA facility (n = 69). Of the 420 patients in the study reviewed, 234 (56%) had not previously been referred to an HCV clinic or been started on treatment because of a variety of social issues, including active substance use (Figure 2).
Many of the patients were difficult to engage because the clinic could not effectively assist them in achieving sobriety and lacked support personnel who could address their complex social issues. Given the availability of all-oral HCV treatments, the VA Public Health Department issued guidance allowing all HCV-infected patients to receive DAA treatment regardless of ongoing drug or alcohol use disorders.9 Substance use was not to be considered a contraindication to therapy. It was suggested that health care providers determine these patients’ treatment eligibility on a case-by-case basis. An official VA memorandum supporting this initiative was released in September 2016.10
Interventions
In an effort to engage all HCV-infected patients, the CCR review was expanded to include patients without advanced liver disease. All patients were contacted by mail. Any patient registered for secure messaging through MyHealtheVet also received a secure message. Patients were informed about the newly approved DAA therapies and were connected directly with specialized HCV clinic schedulers at RLRVAMC. Patients who responded were scheduled for a group education class facilitated by 2 members of the HCV treatment team.
Unlike patients in the group treatment clinic, patients in the education class had not completed the necessary workup for treatment initiation. In the class, patients received education on new HCV treatments and were linked to social work care if needed to streamline the referral process. All baseline laboratory test results also were obtained.
Another intervention implemented to recruit patients in this difficult-to-treat population was the addition of a social worker to the treatment team. Beginning in late June 2016, high-risk patients were referred to the social worker by HCV providers or pharmacists. For each referred patient, the social worker performed a psychosocial assessment to identify potential barriers to successful treatment and then connected the patient with either VA or community resources for support.
The social worker linked patients to mental health or substance use-related services, empowered them to access transportation resources for clinic appointments, orchestrated assistance with medication adherence from a home health nurse, and reached out to patients in person or by telephone to address specific needs that might limit their ability to attend appointments. The social worker also provided harm reduction planning and goal setting support to help patients with substance use disorders achieve sobriety or reduce substance use while on HCV treatment. All efforts were made to ensure that patients adhered to their clinic visits and medication use. In addition, during social work assessment, factors such as housing concerns, travel barriers, and loss and grief were identified and promptly addressed.
Results
After the phase 1 intervention, 730 additional appointments were added in FY 2016 (Figure 3). As a result, 409 patients with HCV infection were started on treatment in FY 2016 compared with 192 in FY 2015. More important, the rapid increase in capacity and treatment initiation did not sacrifice the quality of care provided. Ninety-eight percent of patients who started treatment in FY 2016 successfully completed their treatment course. The overall SVR12 rate was 96% for all genotype 1 patients treated with ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, or elbasvir/grazoprevir with or without ribavirin. In addition, the SVR12 rate was 82% for genotype 2 patients (almost all cirrhotic) treated with sofosbuvir plus ribavirin and 93% for genotype 3 patients treated with daclatasvir, sofosbuvir, and ribavirin.
Phase 2: Increase Recruitment
The expanded CCR review identified 234 patients with advanced liver disease and 546 patients without advanced disease. As this was a rolling review, 58 patients were linked to care before being contacted. Of the 722 patients in the cohort, 528 were contacted by mail and 194 both by mail and by MyHealtheVet messaging. One hundred forty-one patients responded: 129 by mail and 12 by MyHealtheVet messaging (eFigure 1).
Of the 101 patients scheduled for group education, 43 attended education in FY 2016 (eFigure 2).
In June 2016, a social worker was added to the treatment team in an effort to improve recruitment in this difficult to treat population (Figure 2). Between June 2016 and end of FY 2016, 48 patients were referred to the social worker for evaluation. The primary reasons for referral were ongoing substance/alcohol use or high risk for relapse (n = 22); appointment adherence barriers, including problems with transportation (n = 16); underlying mental health disorders (n = 4); barriers to medication adherence (n = 3); and unstable housing (n = 3). Of these 48 patients, 31 received a single social worker intervention to connect with resources; the other 17 were recommended for intensive case management for ongoing support during preparation for HCV treatment and during therapy. As a result of social work involvement, 31 out of 48 referred patients were successfully started on treatment in FY 2016.
Discussion
The VA continues focusing its efforts and resources on treating HCV infection in FY 2017. To further expand outreach, RLRVAMC is working on several additional process improvements. One reason for the lower than expected number of patients who did not see a provider after attending the group education class is that these patients were difficult to reach for scheduling. A medical support assistant is now attending these classes; immediately after a class ends and before leaving the facility, this assistant schedules patients for appointments with HCV providers. The team social worker continues to help prepare patients for treatment and targets interventions for patients early in their HCV workup so that resources are allocated before treatment initiation. In the first 2 months of FY 2017, about 10 more patients who were referred to the social worker for assessment and support started treatment.
Outreach letter responses identified almost 600 potential candidates for treatment. Pharmacists telephoned these patients in another effort to connect them with VA services. Interested patients were scheduled for a group education visit. Also, pharmacists reached out to all primary care clinics and community-based outpatient clinics connected with the facility to provide education on VA policies regarding HCV treatment eligibility and to encourage providers to refer all patients with HCV infection to the HCV clinic. This education was provided at primary care team meetings, and providers not in attendance receive individual outreach by pharmacists. Primary care providers also received a pocket card that summarized recommendations for HCV screening and referrals. These efforts and initiatives are expected to increase veterans’ access to care for HCV infection within the catchment area.
Conclusion
Treatment team interventions in FY 2016 significantly increased veterans’ access to RLRVAMC HCV care. The number of patients who started treatment more than doubled since the previous year. Many of these patients had complex social issues or treatment barriers but successfully started therapy with the help of additional support staff.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Updated January 27, 2017. Accessed May 9, 2017.
2. U.S. Department of Veterans Affairs. Epidemiology of hepatitis C. http://www.hepatitis.va.gov/provider/reviews/epidemiology.asp. Updated August 26, 2016. Accessed May 9, 2017.
3. U.S. Department of Veterans Affairs, Office of Research and Development. VA research on hepatitis C. http://www.research.va.gov/topics/hep-c.cfm. Updated October 14, 2016. Accessed May 9, 2017.
4. U.S. Department of Veterans Affairs. HIV, hepatitis, and public health pathogens programs annual stakeholders report: 2015. https://www.hepatitis.va.gov/pdf/stakeholders-report-2015.pdf. Published May 2015. Accessed May 10, 2017.
5. Lynch TG, McCarthy MF; US Department of Veterans Affairs. Hepatitis C virus (HCV) funding and prioritization status update [memorandum]. http://www.hepatitis.va.gov/pdf/choice-prioritization-update.pdf. Published February 24, 2016. Accessed May 9, 2017.
6. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
7. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-954.
8. Vallet-Pichard A, Mallet V, Nalpas B, et al. Fib-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
9. U.S. Department of Veterans Affairs National Hepatitis C Resource Center Program and National Viral Hepatitis Program the HIV, Hepatitis, and Related Conditions Program in the Office of Specialty Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. https://www.hepatitis.va.gov/pdf/treatment-considerations-2017-03-08.pdf. Updated March 8, 2017. Accessed May 9, 2017.
10. Lynch TG; U.S. Department of Veterans Affairs. Evaluation and treatment of veterans with hepatitis C (HCV) and co-occurring substance use or mental health concerns [memorandum]. http://www.hepatitis.va.gov/pdf/memo-HCV-and -mental-health.pdf. Published September 9, 2016. Accessed May 9, 2017.
In the U.S., 2.7 to 3.9 million people are chronically infected with the hepatitis C virus (HCV).1 Survey data suggest that HCV infection is more prevalent in patients enrolled in the VA health care system than it is in civilian health care systems.2 Studies have shown that Vietnam veterans, veterans with mental health and substance abuse disorders, and veterans without stable housing are more likely to be infected with HCV.3 Data from the VA HCV Clinical Case Registry (CCR) for 2015 showed that 174,842 veterans with chronic HCV infection receieved care within the VHA, which makes the VA the single largest HCV care provider in the nation.4
The VA is dedicated to providing treatment to veterans with HCV infection. For fiscal year (FY) 2016, the VA allocated $1 billion to HCV care,and in February 2016 it began offering treatment to all veterans with HCV, regardless of degree of fibrosis or severity of underlying liver disease.3,5 Each VAMC was tasked with improving veterans’ access to HCV treatment.
In an effort to engage patients in HCV care, the multidisciplinary HCV team at the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, launched a 2-phase improvement process in 2016. The goal in phase 1 was to increase patient access to HCV clinics, and the goal in phase 2 was to recruit patients for direct-acting antiviral (DAA) therapy for HCV. These efforts were designed to increase screening, identification, and linkage to care for HCV and to expand clinic access for the treatment and cure of all identified veterans who pursued treatment.
Patients with HCV infection, referred from primary care clinics, initially were evaluated by HCV clinic providers (hepatologists, infectious disease specialists, gastroenterology fellows, or nurse practitioners) for eligibility to receive DAA therapy for HCV. Eligible patients then were referred to a pharmacist-run HCV clinic, which had been established at RLRVAMC in 2011. At the start of FY 2016, the clinic, staffed by 3 pharmacists, operated 5 half-days per week and accommodated up to 35 weekly patient appointments.
In this clinic, patients received initial education and medication reconciliation for potential drug interactions with DAAs. Once the HCV treatment was initiated, patients were evaluated in the clinic every 2 weeks for medication refills and assessment for tolerability, adherence, and laboratory abnormalities until end of treatment (8-24 weeks, depending on HCV genotype, experiences with prior HCV treatment, and presence/absence of cirrhosis). Twelve weeks after completion of treatment, viral load was obtained to determine sustained virologic response (SVR12).
Methods
Phase 1: Improve Clinic Access
During FY 2016, methods for expanding clinic access to accommodate a large influx of treatment-eligible patients were reviewed and implemented.
In the first intervention, unneeded follow-up visits were eliminated to make room for additional new patient appointments. In general, patients treated with ribavirin require close monitoring, given the risk for anemia.6 With the release of newer DAAs, however, more patients became eligible for treatment with ribavirin-free regimens.7 As a result, follow-up appointments for these patients were extended to 4-week intervals instead of every 2 weeks. A patient with a history of nonadherence to medication use or clinic visits was still maintained on a 2-week schedule of follow-up for close monitoring.
In the second intervention, opportunities for switching those who completed treatment from face-to-face clinic visits to telephone were identified. These patients historically were seen in clinic for a brief interview and for a blood test used to determine end-of-treatment viral load. Improving access for new patients in the clinic involved moving more existing patients from in-clinic visits to telephone. At the end of the treatment plan, existing patients received an order for laboratory tests that included viral load. When all laboratory results were ready, patients were contacted by telephone. Recruiting a registered nurse to the treatment team who assisted with telephone visits further improved clinic efficiency.
The third intervention was inspired by successful results at other VA sites and launched a group treatment clinic for patients who were starting ribavirin-free DAA regimens.7 Group visits were run by 2 pharmacists and accommodated up to 10 veterans. Patients underwent testing for HCV genotype and viral load before the initial group visit. At check-in, patients received a short questionnaire and consent form for group participation. The questionnaire reviewed patient history of drug and alcohol use and potential barriers to medication adherence. Patients also were encouraged to write down any questions they had about the treatment. During the initial group visit, pharmacists provided general education about the medications, potential adverse effects, treatment expectations, and the monitoring plan. Follow-up visits were conducted in a group setting as well.
Phase 2: Increase Recruitment
The records of 534 patients with advanced liver disease (F3-F4 fibrosis on the Fibrosis-4 Index for Liver Fibrosis) and HCV infection were identified in the CCR database for the period August 2015 to December 2015 (Figure 1).8 Patients were excluded if they were deceased, were receiving palliative care (n = 45), or if they had transferred their care to another VA facility (n = 69). Of the 420 patients in the study reviewed, 234 (56%) had not previously been referred to an HCV clinic or been started on treatment because of a variety of social issues, including active substance use (Figure 2).
Many of the patients were difficult to engage because the clinic could not effectively assist them in achieving sobriety and lacked support personnel who could address their complex social issues. Given the availability of all-oral HCV treatments, the VA Public Health Department issued guidance allowing all HCV-infected patients to receive DAA treatment regardless of ongoing drug or alcohol use disorders.9 Substance use was not to be considered a contraindication to therapy. It was suggested that health care providers determine these patients’ treatment eligibility on a case-by-case basis. An official VA memorandum supporting this initiative was released in September 2016.10
Interventions
In an effort to engage all HCV-infected patients, the CCR review was expanded to include patients without advanced liver disease. All patients were contacted by mail. Any patient registered for secure messaging through MyHealtheVet also received a secure message. Patients were informed about the newly approved DAA therapies and were connected directly with specialized HCV clinic schedulers at RLRVAMC. Patients who responded were scheduled for a group education class facilitated by 2 members of the HCV treatment team.
Unlike patients in the group treatment clinic, patients in the education class had not completed the necessary workup for treatment initiation. In the class, patients received education on new HCV treatments and were linked to social work care if needed to streamline the referral process. All baseline laboratory test results also were obtained.
Another intervention implemented to recruit patients in this difficult-to-treat population was the addition of a social worker to the treatment team. Beginning in late June 2016, high-risk patients were referred to the social worker by HCV providers or pharmacists. For each referred patient, the social worker performed a psychosocial assessment to identify potential barriers to successful treatment and then connected the patient with either VA or community resources for support.
The social worker linked patients to mental health or substance use-related services, empowered them to access transportation resources for clinic appointments, orchestrated assistance with medication adherence from a home health nurse, and reached out to patients in person or by telephone to address specific needs that might limit their ability to attend appointments. The social worker also provided harm reduction planning and goal setting support to help patients with substance use disorders achieve sobriety or reduce substance use while on HCV treatment. All efforts were made to ensure that patients adhered to their clinic visits and medication use. In addition, during social work assessment, factors such as housing concerns, travel barriers, and loss and grief were identified and promptly addressed.
Results
After the phase 1 intervention, 730 additional appointments were added in FY 2016 (Figure 3). As a result, 409 patients with HCV infection were started on treatment in FY 2016 compared with 192 in FY 2015. More important, the rapid increase in capacity and treatment initiation did not sacrifice the quality of care provided. Ninety-eight percent of patients who started treatment in FY 2016 successfully completed their treatment course. The overall SVR12 rate was 96% for all genotype 1 patients treated with ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, or elbasvir/grazoprevir with or without ribavirin. In addition, the SVR12 rate was 82% for genotype 2 patients (almost all cirrhotic) treated with sofosbuvir plus ribavirin and 93% for genotype 3 patients treated with daclatasvir, sofosbuvir, and ribavirin.
Phase 2: Increase Recruitment
The expanded CCR review identified 234 patients with advanced liver disease and 546 patients without advanced disease. As this was a rolling review, 58 patients were linked to care before being contacted. Of the 722 patients in the cohort, 528 were contacted by mail and 194 both by mail and by MyHealtheVet messaging. One hundred forty-one patients responded: 129 by mail and 12 by MyHealtheVet messaging (eFigure 1).
Of the 101 patients scheduled for group education, 43 attended education in FY 2016 (eFigure 2).
In June 2016, a social worker was added to the treatment team in an effort to improve recruitment in this difficult to treat population (Figure 2). Between June 2016 and end of FY 2016, 48 patients were referred to the social worker for evaluation. The primary reasons for referral were ongoing substance/alcohol use or high risk for relapse (n = 22); appointment adherence barriers, including problems with transportation (n = 16); underlying mental health disorders (n = 4); barriers to medication adherence (n = 3); and unstable housing (n = 3). Of these 48 patients, 31 received a single social worker intervention to connect with resources; the other 17 were recommended for intensive case management for ongoing support during preparation for HCV treatment and during therapy. As a result of social work involvement, 31 out of 48 referred patients were successfully started on treatment in FY 2016.
Discussion
The VA continues focusing its efforts and resources on treating HCV infection in FY 2017. To further expand outreach, RLRVAMC is working on several additional process improvements. One reason for the lower than expected number of patients who did not see a provider after attending the group education class is that these patients were difficult to reach for scheduling. A medical support assistant is now attending these classes; immediately after a class ends and before leaving the facility, this assistant schedules patients for appointments with HCV providers. The team social worker continues to help prepare patients for treatment and targets interventions for patients early in their HCV workup so that resources are allocated before treatment initiation. In the first 2 months of FY 2017, about 10 more patients who were referred to the social worker for assessment and support started treatment.
Outreach letter responses identified almost 600 potential candidates for treatment. Pharmacists telephoned these patients in another effort to connect them with VA services. Interested patients were scheduled for a group education visit. Also, pharmacists reached out to all primary care clinics and community-based outpatient clinics connected with the facility to provide education on VA policies regarding HCV treatment eligibility and to encourage providers to refer all patients with HCV infection to the HCV clinic. This education was provided at primary care team meetings, and providers not in attendance receive individual outreach by pharmacists. Primary care providers also received a pocket card that summarized recommendations for HCV screening and referrals. These efforts and initiatives are expected to increase veterans’ access to care for HCV infection within the catchment area.
Conclusion
Treatment team interventions in FY 2016 significantly increased veterans’ access to RLRVAMC HCV care. The number of patients who started treatment more than doubled since the previous year. Many of these patients had complex social issues or treatment barriers but successfully started therapy with the help of additional support staff.
Click here to read the digital edition.
In the U.S., 2.7 to 3.9 million people are chronically infected with the hepatitis C virus (HCV).1 Survey data suggest that HCV infection is more prevalent in patients enrolled in the VA health care system than it is in civilian health care systems.2 Studies have shown that Vietnam veterans, veterans with mental health and substance abuse disorders, and veterans without stable housing are more likely to be infected with HCV.3 Data from the VA HCV Clinical Case Registry (CCR) for 2015 showed that 174,842 veterans with chronic HCV infection receieved care within the VHA, which makes the VA the single largest HCV care provider in the nation.4
The VA is dedicated to providing treatment to veterans with HCV infection. For fiscal year (FY) 2016, the VA allocated $1 billion to HCV care,and in February 2016 it began offering treatment to all veterans with HCV, regardless of degree of fibrosis or severity of underlying liver disease.3,5 Each VAMC was tasked with improving veterans’ access to HCV treatment.
In an effort to engage patients in HCV care, the multidisciplinary HCV team at the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, launched a 2-phase improvement process in 2016. The goal in phase 1 was to increase patient access to HCV clinics, and the goal in phase 2 was to recruit patients for direct-acting antiviral (DAA) therapy for HCV. These efforts were designed to increase screening, identification, and linkage to care for HCV and to expand clinic access for the treatment and cure of all identified veterans who pursued treatment.
Patients with HCV infection, referred from primary care clinics, initially were evaluated by HCV clinic providers (hepatologists, infectious disease specialists, gastroenterology fellows, or nurse practitioners) for eligibility to receive DAA therapy for HCV. Eligible patients then were referred to a pharmacist-run HCV clinic, which had been established at RLRVAMC in 2011. At the start of FY 2016, the clinic, staffed by 3 pharmacists, operated 5 half-days per week and accommodated up to 35 weekly patient appointments.
In this clinic, patients received initial education and medication reconciliation for potential drug interactions with DAAs. Once the HCV treatment was initiated, patients were evaluated in the clinic every 2 weeks for medication refills and assessment for tolerability, adherence, and laboratory abnormalities until end of treatment (8-24 weeks, depending on HCV genotype, experiences with prior HCV treatment, and presence/absence of cirrhosis). Twelve weeks after completion of treatment, viral load was obtained to determine sustained virologic response (SVR12).
Methods
Phase 1: Improve Clinic Access
During FY 2016, methods for expanding clinic access to accommodate a large influx of treatment-eligible patients were reviewed and implemented.
In the first intervention, unneeded follow-up visits were eliminated to make room for additional new patient appointments. In general, patients treated with ribavirin require close monitoring, given the risk for anemia.6 With the release of newer DAAs, however, more patients became eligible for treatment with ribavirin-free regimens.7 As a result, follow-up appointments for these patients were extended to 4-week intervals instead of every 2 weeks. A patient with a history of nonadherence to medication use or clinic visits was still maintained on a 2-week schedule of follow-up for close monitoring.
In the second intervention, opportunities for switching those who completed treatment from face-to-face clinic visits to telephone were identified. These patients historically were seen in clinic for a brief interview and for a blood test used to determine end-of-treatment viral load. Improving access for new patients in the clinic involved moving more existing patients from in-clinic visits to telephone. At the end of the treatment plan, existing patients received an order for laboratory tests that included viral load. When all laboratory results were ready, patients were contacted by telephone. Recruiting a registered nurse to the treatment team who assisted with telephone visits further improved clinic efficiency.
The third intervention was inspired by successful results at other VA sites and launched a group treatment clinic for patients who were starting ribavirin-free DAA regimens.7 Group visits were run by 2 pharmacists and accommodated up to 10 veterans. Patients underwent testing for HCV genotype and viral load before the initial group visit. At check-in, patients received a short questionnaire and consent form for group participation. The questionnaire reviewed patient history of drug and alcohol use and potential barriers to medication adherence. Patients also were encouraged to write down any questions they had about the treatment. During the initial group visit, pharmacists provided general education about the medications, potential adverse effects, treatment expectations, and the monitoring plan. Follow-up visits were conducted in a group setting as well.
Phase 2: Increase Recruitment
The records of 534 patients with advanced liver disease (F3-F4 fibrosis on the Fibrosis-4 Index for Liver Fibrosis) and HCV infection were identified in the CCR database for the period August 2015 to December 2015 (Figure 1).8 Patients were excluded if they were deceased, were receiving palliative care (n = 45), or if they had transferred their care to another VA facility (n = 69). Of the 420 patients in the study reviewed, 234 (56%) had not previously been referred to an HCV clinic or been started on treatment because of a variety of social issues, including active substance use (Figure 2).
Many of the patients were difficult to engage because the clinic could not effectively assist them in achieving sobriety and lacked support personnel who could address their complex social issues. Given the availability of all-oral HCV treatments, the VA Public Health Department issued guidance allowing all HCV-infected patients to receive DAA treatment regardless of ongoing drug or alcohol use disorders.9 Substance use was not to be considered a contraindication to therapy. It was suggested that health care providers determine these patients’ treatment eligibility on a case-by-case basis. An official VA memorandum supporting this initiative was released in September 2016.10
Interventions
In an effort to engage all HCV-infected patients, the CCR review was expanded to include patients without advanced liver disease. All patients were contacted by mail. Any patient registered for secure messaging through MyHealtheVet also received a secure message. Patients were informed about the newly approved DAA therapies and were connected directly with specialized HCV clinic schedulers at RLRVAMC. Patients who responded were scheduled for a group education class facilitated by 2 members of the HCV treatment team.
Unlike patients in the group treatment clinic, patients in the education class had not completed the necessary workup for treatment initiation. In the class, patients received education on new HCV treatments and were linked to social work care if needed to streamline the referral process. All baseline laboratory test results also were obtained.
Another intervention implemented to recruit patients in this difficult-to-treat population was the addition of a social worker to the treatment team. Beginning in late June 2016, high-risk patients were referred to the social worker by HCV providers or pharmacists. For each referred patient, the social worker performed a psychosocial assessment to identify potential barriers to successful treatment and then connected the patient with either VA or community resources for support.
The social worker linked patients to mental health or substance use-related services, empowered them to access transportation resources for clinic appointments, orchestrated assistance with medication adherence from a home health nurse, and reached out to patients in person or by telephone to address specific needs that might limit their ability to attend appointments. The social worker also provided harm reduction planning and goal setting support to help patients with substance use disorders achieve sobriety or reduce substance use while on HCV treatment. All efforts were made to ensure that patients adhered to their clinic visits and medication use. In addition, during social work assessment, factors such as housing concerns, travel barriers, and loss and grief were identified and promptly addressed.
Results
After the phase 1 intervention, 730 additional appointments were added in FY 2016 (Figure 3). As a result, 409 patients with HCV infection were started on treatment in FY 2016 compared with 192 in FY 2015. More important, the rapid increase in capacity and treatment initiation did not sacrifice the quality of care provided. Ninety-eight percent of patients who started treatment in FY 2016 successfully completed their treatment course. The overall SVR12 rate was 96% for all genotype 1 patients treated with ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, or elbasvir/grazoprevir with or without ribavirin. In addition, the SVR12 rate was 82% for genotype 2 patients (almost all cirrhotic) treated with sofosbuvir plus ribavirin and 93% for genotype 3 patients treated with daclatasvir, sofosbuvir, and ribavirin.
Phase 2: Increase Recruitment
The expanded CCR review identified 234 patients with advanced liver disease and 546 patients without advanced disease. As this was a rolling review, 58 patients were linked to care before being contacted. Of the 722 patients in the cohort, 528 were contacted by mail and 194 both by mail and by MyHealtheVet messaging. One hundred forty-one patients responded: 129 by mail and 12 by MyHealtheVet messaging (eFigure 1).
Of the 101 patients scheduled for group education, 43 attended education in FY 2016 (eFigure 2).
In June 2016, a social worker was added to the treatment team in an effort to improve recruitment in this difficult to treat population (Figure 2). Between June 2016 and end of FY 2016, 48 patients were referred to the social worker for evaluation. The primary reasons for referral were ongoing substance/alcohol use or high risk for relapse (n = 22); appointment adherence barriers, including problems with transportation (n = 16); underlying mental health disorders (n = 4); barriers to medication adherence (n = 3); and unstable housing (n = 3). Of these 48 patients, 31 received a single social worker intervention to connect with resources; the other 17 were recommended for intensive case management for ongoing support during preparation for HCV treatment and during therapy. As a result of social work involvement, 31 out of 48 referred patients were successfully started on treatment in FY 2016.
Discussion
The VA continues focusing its efforts and resources on treating HCV infection in FY 2017. To further expand outreach, RLRVAMC is working on several additional process improvements. One reason for the lower than expected number of patients who did not see a provider after attending the group education class is that these patients were difficult to reach for scheduling. A medical support assistant is now attending these classes; immediately after a class ends and before leaving the facility, this assistant schedules patients for appointments with HCV providers. The team social worker continues to help prepare patients for treatment and targets interventions for patients early in their HCV workup so that resources are allocated before treatment initiation. In the first 2 months of FY 2017, about 10 more patients who were referred to the social worker for assessment and support started treatment.
Outreach letter responses identified almost 600 potential candidates for treatment. Pharmacists telephoned these patients in another effort to connect them with VA services. Interested patients were scheduled for a group education visit. Also, pharmacists reached out to all primary care clinics and community-based outpatient clinics connected with the facility to provide education on VA policies regarding HCV treatment eligibility and to encourage providers to refer all patients with HCV infection to the HCV clinic. This education was provided at primary care team meetings, and providers not in attendance receive individual outreach by pharmacists. Primary care providers also received a pocket card that summarized recommendations for HCV screening and referrals. These efforts and initiatives are expected to increase veterans’ access to care for HCV infection within the catchment area.
Conclusion
Treatment team interventions in FY 2016 significantly increased veterans’ access to RLRVAMC HCV care. The number of patients who started treatment more than doubled since the previous year. Many of these patients had complex social issues or treatment barriers but successfully started therapy with the help of additional support staff.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Updated January 27, 2017. Accessed May 9, 2017.
2. U.S. Department of Veterans Affairs. Epidemiology of hepatitis C. http://www.hepatitis.va.gov/provider/reviews/epidemiology.asp. Updated August 26, 2016. Accessed May 9, 2017.
3. U.S. Department of Veterans Affairs, Office of Research and Development. VA research on hepatitis C. http://www.research.va.gov/topics/hep-c.cfm. Updated October 14, 2016. Accessed May 9, 2017.
4. U.S. Department of Veterans Affairs. HIV, hepatitis, and public health pathogens programs annual stakeholders report: 2015. https://www.hepatitis.va.gov/pdf/stakeholders-report-2015.pdf. Published May 2015. Accessed May 10, 2017.
5. Lynch TG, McCarthy MF; US Department of Veterans Affairs. Hepatitis C virus (HCV) funding and prioritization status update [memorandum]. http://www.hepatitis.va.gov/pdf/choice-prioritization-update.pdf. Published February 24, 2016. Accessed May 9, 2017.
6. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
7. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-954.
8. Vallet-Pichard A, Mallet V, Nalpas B, et al. Fib-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
9. U.S. Department of Veterans Affairs National Hepatitis C Resource Center Program and National Viral Hepatitis Program the HIV, Hepatitis, and Related Conditions Program in the Office of Specialty Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. https://www.hepatitis.va.gov/pdf/treatment-considerations-2017-03-08.pdf. Updated March 8, 2017. Accessed May 9, 2017.
10. Lynch TG; U.S. Department of Veterans Affairs. Evaluation and treatment of veterans with hepatitis C (HCV) and co-occurring substance use or mental health concerns [memorandum]. http://www.hepatitis.va.gov/pdf/memo-HCV-and -mental-health.pdf. Published September 9, 2016. Accessed May 9, 2017.
1. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. https://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Updated January 27, 2017. Accessed May 9, 2017.
2. U.S. Department of Veterans Affairs. Epidemiology of hepatitis C. http://www.hepatitis.va.gov/provider/reviews/epidemiology.asp. Updated August 26, 2016. Accessed May 9, 2017.
3. U.S. Department of Veterans Affairs, Office of Research and Development. VA research on hepatitis C. http://www.research.va.gov/topics/hep-c.cfm. Updated October 14, 2016. Accessed May 9, 2017.
4. U.S. Department of Veterans Affairs. HIV, hepatitis, and public health pathogens programs annual stakeholders report: 2015. https://www.hepatitis.va.gov/pdf/stakeholders-report-2015.pdf. Published May 2015. Accessed May 10, 2017.
5. Lynch TG, McCarthy MF; US Department of Veterans Affairs. Hepatitis C virus (HCV) funding and prioritization status update [memorandum]. http://www.hepatitis.va.gov/pdf/choice-prioritization-update.pdf. Published February 24, 2016. Accessed May 9, 2017.
6. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
7. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-954.
8. Vallet-Pichard A, Mallet V, Nalpas B, et al. Fib-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
9. U.S. Department of Veterans Affairs National Hepatitis C Resource Center Program and National Viral Hepatitis Program the HIV, Hepatitis, and Related Conditions Program in the Office of Specialty Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. https://www.hepatitis.va.gov/pdf/treatment-considerations-2017-03-08.pdf. Updated March 8, 2017. Accessed May 9, 2017.
10. Lynch TG; U.S. Department of Veterans Affairs. Evaluation and treatment of veterans with hepatitis C (HCV) and co-occurring substance use or mental health concerns [memorandum]. http://www.hepatitis.va.gov/pdf/memo-HCV-and -mental-health.pdf. Published September 9, 2016. Accessed May 9, 2017.
High-dose opioid prescribing linked to heroin use risk among U.S. veterans
SAN DIEGO – High-dose opioid prescribing is associated with an increased risk of heroin use among U.S. veterans, a prospective study showed.
“Despite evidence linking increased risk of opioid use disorder with specific opioid prescribing patterns, the relationship between these practices and heroin use is less understood,” researchers led by Geetanjoli Banerjee wrote in an abstract presented during the annual meeting of the College on Problems of Drug Dependence. “Understanding the relationship between prescription opioid use and initiation of heroin use among veterans is particularly important, as this population has both a higher underlying prevalence of substance abuse disorders and pain conditions.”
Ms. Banerjee, a graduate student at Brown University, Providence, R.I., and her associates examined data from 3,570 individuals enrolled in the Veterans Aging Cohort Study without nonmedical use or prescription opioids or heroin use in the year prior to baseline, and who received more than one prescription opioid from the VA during follow-up. between 2002 and 2012.
Among the 3,833 eligible participants, the proportion who were prescribed high-dose opioids (defined as a morphine equivalent daily dose of 90 mg or greater) did not change significantly between 2002 and 2012, and ranged from 4.4% to 5.9% (P = .22). The researchers also found that the 10-year incidence rate of recent heroin use was 8.15 event per 1,000 person-years. The time to recent heroin use differed significantly between participants with and without prior receipt of a high-dose prescription at baseline (P less than .001). Multivariable Cox regression analysis showed that prior receipt of a high-dose opioid prescription was associated with recent heroin use (adjusted hazard ratio, 2.54).
“Among patients who report no past year illicit use of opioids at baseline and who are receiving prescription opioids from the VA, receipt of high-dose prescription opioid increased the risk of subsequent heroin use,” the researchers concluded. “Patients receiving high-dose opioids, ages 50-56, with a history of HCV infection, injecting, opioid use disorder, or recent stimulant use should be screened for heroin use.”
They acknowledged certain limitations of the study, including its self-reported design and the fact that VA pharmacy data do not capture opioid prescriptions filled outside of the VA.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. Ms. Banerjee reported having no financial disclosures.
SAN DIEGO – High-dose opioid prescribing is associated with an increased risk of heroin use among U.S. veterans, a prospective study showed.
“Despite evidence linking increased risk of opioid use disorder with specific opioid prescribing patterns, the relationship between these practices and heroin use is less understood,” researchers led by Geetanjoli Banerjee wrote in an abstract presented during the annual meeting of the College on Problems of Drug Dependence. “Understanding the relationship between prescription opioid use and initiation of heroin use among veterans is particularly important, as this population has both a higher underlying prevalence of substance abuse disorders and pain conditions.”
Ms. Banerjee, a graduate student at Brown University, Providence, R.I., and her associates examined data from 3,570 individuals enrolled in the Veterans Aging Cohort Study without nonmedical use or prescription opioids or heroin use in the year prior to baseline, and who received more than one prescription opioid from the VA during follow-up. between 2002 and 2012.
Among the 3,833 eligible participants, the proportion who were prescribed high-dose opioids (defined as a morphine equivalent daily dose of 90 mg or greater) did not change significantly between 2002 and 2012, and ranged from 4.4% to 5.9% (P = .22). The researchers also found that the 10-year incidence rate of recent heroin use was 8.15 event per 1,000 person-years. The time to recent heroin use differed significantly between participants with and without prior receipt of a high-dose prescription at baseline (P less than .001). Multivariable Cox regression analysis showed that prior receipt of a high-dose opioid prescription was associated with recent heroin use (adjusted hazard ratio, 2.54).
“Among patients who report no past year illicit use of opioids at baseline and who are receiving prescription opioids from the VA, receipt of high-dose prescription opioid increased the risk of subsequent heroin use,” the researchers concluded. “Patients receiving high-dose opioids, ages 50-56, with a history of HCV infection, injecting, opioid use disorder, or recent stimulant use should be screened for heroin use.”
They acknowledged certain limitations of the study, including its self-reported design and the fact that VA pharmacy data do not capture opioid prescriptions filled outside of the VA.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. Ms. Banerjee reported having no financial disclosures.
SAN DIEGO – High-dose opioid prescribing is associated with an increased risk of heroin use among U.S. veterans, a prospective study showed.
“Despite evidence linking increased risk of opioid use disorder with specific opioid prescribing patterns, the relationship between these practices and heroin use is less understood,” researchers led by Geetanjoli Banerjee wrote in an abstract presented during the annual meeting of the College on Problems of Drug Dependence. “Understanding the relationship between prescription opioid use and initiation of heroin use among veterans is particularly important, as this population has both a higher underlying prevalence of substance abuse disorders and pain conditions.”
Ms. Banerjee, a graduate student at Brown University, Providence, R.I., and her associates examined data from 3,570 individuals enrolled in the Veterans Aging Cohort Study without nonmedical use or prescription opioids or heroin use in the year prior to baseline, and who received more than one prescription opioid from the VA during follow-up. between 2002 and 2012.
Among the 3,833 eligible participants, the proportion who were prescribed high-dose opioids (defined as a morphine equivalent daily dose of 90 mg or greater) did not change significantly between 2002 and 2012, and ranged from 4.4% to 5.9% (P = .22). The researchers also found that the 10-year incidence rate of recent heroin use was 8.15 event per 1,000 person-years. The time to recent heroin use differed significantly between participants with and without prior receipt of a high-dose prescription at baseline (P less than .001). Multivariable Cox regression analysis showed that prior receipt of a high-dose opioid prescription was associated with recent heroin use (adjusted hazard ratio, 2.54).
“Among patients who report no past year illicit use of opioids at baseline and who are receiving prescription opioids from the VA, receipt of high-dose prescription opioid increased the risk of subsequent heroin use,” the researchers concluded. “Patients receiving high-dose opioids, ages 50-56, with a history of HCV infection, injecting, opioid use disorder, or recent stimulant use should be screened for heroin use.”
They acknowledged certain limitations of the study, including its self-reported design and the fact that VA pharmacy data do not capture opioid prescriptions filled outside of the VA.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. Ms. Banerjee reported having no financial disclosures.
REPORTING CPDD 2018
Key clinical point: Screen patients who receive high-dose opioids and meet several other criteria for heroin use.
Major finding:. On multivariate Cox regression, prior prescription of high-dose opioids was an independent predictor of recent heroin use (adjusted hazard ratio, 2.54).
Study details: A prospective analysis of 3,833 participants in the Veterans Aging Cohort Study.
Disclosures: The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. Ms. Banerjee reported having no financial disclosures.
HCV incidence is elevated in HIV-positive MSM
The incidence rate of HCV among HIV-positive men who have sex with men (MSM) was significantly higher than that found in HIV-negative MSM and the general population, according to a study published in Digestive and Liver Disease.
Sexual transmission of hepatitis C virus (HCV) is uncommon in the general population, but evidence indicates that its rate is higher in people living with HIV and that HIV-positive MSM who practice condomless sex have been shown to be at increased risk for sexually-acquired HCV, according to Gianluca Cuomo, MD, and his colleagues at the Azienda Ospedaliero-Universitaria di Modena (Italy), Infectious Diseases Clinic.
Dr. Cuomo and his colleagues assessed 442 HIV-positive MSM outpatients who were antibody negative to HCV-Ab at first observation who were entered into a Kaplan-Meier model in order to assess the HCV infection incidence rate. Prevalence analysis was performed with HIV-positive MSM who were on follow-up at 2016. An HIV-negative MSM population served as a control.
“Our study indicates an incidence rate of HCV among MSM living with HIV of 0.44 cases per 100 patient-years with a global prevalence of 9%, both of which are significantly higher than that of non-HIV MSM and the general population,” Dr. Cuomo and his colleagues found (Digestive and Liver Disease; 2018, [doi.org/10.1016/j.dld.2018.05.021]).
“Early management and treatment of HCV infection and behavioral interventions could reduce HCV transmission. Annual screenings for HCV and other sexually transmitted diseases should be performed for HIV MSM patients,” the researchers concluded.
Dr. Cuomo and his colleagues reported that they had no disclosures.
SOURCE: Cuomo, G., et al. Digestive and Liver Disease (2018), [doi.org/10.1016/j.dld.2018.05.021].
The incidence rate of HCV among HIV-positive men who have sex with men (MSM) was significantly higher than that found in HIV-negative MSM and the general population, according to a study published in Digestive and Liver Disease.
Sexual transmission of hepatitis C virus (HCV) is uncommon in the general population, but evidence indicates that its rate is higher in people living with HIV and that HIV-positive MSM who practice condomless sex have been shown to be at increased risk for sexually-acquired HCV, according to Gianluca Cuomo, MD, and his colleagues at the Azienda Ospedaliero-Universitaria di Modena (Italy), Infectious Diseases Clinic.
Dr. Cuomo and his colleagues assessed 442 HIV-positive MSM outpatients who were antibody negative to HCV-Ab at first observation who were entered into a Kaplan-Meier model in order to assess the HCV infection incidence rate. Prevalence analysis was performed with HIV-positive MSM who were on follow-up at 2016. An HIV-negative MSM population served as a control.
“Our study indicates an incidence rate of HCV among MSM living with HIV of 0.44 cases per 100 patient-years with a global prevalence of 9%, both of which are significantly higher than that of non-HIV MSM and the general population,” Dr. Cuomo and his colleagues found (Digestive and Liver Disease; 2018, [doi.org/10.1016/j.dld.2018.05.021]).
“Early management and treatment of HCV infection and behavioral interventions could reduce HCV transmission. Annual screenings for HCV and other sexually transmitted diseases should be performed for HIV MSM patients,” the researchers concluded.
Dr. Cuomo and his colleagues reported that they had no disclosures.
SOURCE: Cuomo, G., et al. Digestive and Liver Disease (2018), [doi.org/10.1016/j.dld.2018.05.021].
The incidence rate of HCV among HIV-positive men who have sex with men (MSM) was significantly higher than that found in HIV-negative MSM and the general population, according to a study published in Digestive and Liver Disease.
Sexual transmission of hepatitis C virus (HCV) is uncommon in the general population, but evidence indicates that its rate is higher in people living with HIV and that HIV-positive MSM who practice condomless sex have been shown to be at increased risk for sexually-acquired HCV, according to Gianluca Cuomo, MD, and his colleagues at the Azienda Ospedaliero-Universitaria di Modena (Italy), Infectious Diseases Clinic.
Dr. Cuomo and his colleagues assessed 442 HIV-positive MSM outpatients who were antibody negative to HCV-Ab at first observation who were entered into a Kaplan-Meier model in order to assess the HCV infection incidence rate. Prevalence analysis was performed with HIV-positive MSM who were on follow-up at 2016. An HIV-negative MSM population served as a control.
“Our study indicates an incidence rate of HCV among MSM living with HIV of 0.44 cases per 100 patient-years with a global prevalence of 9%, both of which are significantly higher than that of non-HIV MSM and the general population,” Dr. Cuomo and his colleagues found (Digestive and Liver Disease; 2018, [doi.org/10.1016/j.dld.2018.05.021]).
“Early management and treatment of HCV infection and behavioral interventions could reduce HCV transmission. Annual screenings for HCV and other sexually transmitted diseases should be performed for HIV MSM patients,” the researchers concluded.
Dr. Cuomo and his colleagues reported that they had no disclosures.
SOURCE: Cuomo, G., et al. Digestive and Liver Disease (2018), [doi.org/10.1016/j.dld.2018.05.021].
FROM DIGESTIVE AND LIVER DISEASE
HCV and alcohol use disorder – bad news for the liver
Patients infected with both hepatitis C virus (HCV) and alcohol use disorder (AUD) were twice as likely to present with advanced liver fibrosis at hospital admission, according to the results of a database study published in Drug and Alcohol Dependence (2018;188:180-6).
The study population consisted of 1,313 patients (80% men). Median age at admission was 45 years and the median alcohol consumption was 200 g/day. HCV infection was present in 236 patients (18%), according to Arantza Sanvisens, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain, and her colleagues.
After adjustment by sex, age and quantity of alcohol consumption, patients with HCV infection were two times more likely to have advanced liver fibrosis (odds ratio = 2.1, 95% confidence ratio,1.5–3.1).
“Successful evaluation of liver damage in this population includes the management of both excessive alcohol consumption and chronic HCV-related disease,” according to Dr. Sanvisens and her colleagues. “Furthermore, current guidelines from the American Association for the Study of Liver Disease, the European Association for the Study of the Liver, and the World Health Organization already recommend treatment of HCV infection in individuals with substance use disorder,” they concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Sanvisens, A et al., Drug and Alcohol Dependence (2018;188:180-6).
Patients infected with both hepatitis C virus (HCV) and alcohol use disorder (AUD) were twice as likely to present with advanced liver fibrosis at hospital admission, according to the results of a database study published in Drug and Alcohol Dependence (2018;188:180-6).
The study population consisted of 1,313 patients (80% men). Median age at admission was 45 years and the median alcohol consumption was 200 g/day. HCV infection was present in 236 patients (18%), according to Arantza Sanvisens, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain, and her colleagues.
After adjustment by sex, age and quantity of alcohol consumption, patients with HCV infection were two times more likely to have advanced liver fibrosis (odds ratio = 2.1, 95% confidence ratio,1.5–3.1).
“Successful evaluation of liver damage in this population includes the management of both excessive alcohol consumption and chronic HCV-related disease,” according to Dr. Sanvisens and her colleagues. “Furthermore, current guidelines from the American Association for the Study of Liver Disease, the European Association for the Study of the Liver, and the World Health Organization already recommend treatment of HCV infection in individuals with substance use disorder,” they concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Sanvisens, A et al., Drug and Alcohol Dependence (2018;188:180-6).
Patients infected with both hepatitis C virus (HCV) and alcohol use disorder (AUD) were twice as likely to present with advanced liver fibrosis at hospital admission, according to the results of a database study published in Drug and Alcohol Dependence (2018;188:180-6).
The study population consisted of 1,313 patients (80% men). Median age at admission was 45 years and the median alcohol consumption was 200 g/day. HCV infection was present in 236 patients (18%), according to Arantza Sanvisens, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain, and her colleagues.
After adjustment by sex, age and quantity of alcohol consumption, patients with HCV infection were two times more likely to have advanced liver fibrosis (odds ratio = 2.1, 95% confidence ratio,1.5–3.1).
“Successful evaluation of liver damage in this population includes the management of both excessive alcohol consumption and chronic HCV-related disease,” according to Dr. Sanvisens and her colleagues. “Furthermore, current guidelines from the American Association for the Study of Liver Disease, the European Association for the Study of the Liver, and the World Health Organization already recommend treatment of HCV infection in individuals with substance use disorder,” they concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Sanvisens, A et al., Drug and Alcohol Dependence (2018;188:180-6).
FROM DRUG AND ALCOHOL DEPENDENCE
Insurer denials of DAA therapy for HCV on the rise
About one in three patients had lack of fill approval by insurers, contributing to a continued lack of access to hepatitis C virus (HCV) therapy across insurance types, despite availability of new, highly effective regimens and relaxation of restrictions on reimbursement, according to Charitha Gowda, MD, of Ohio State University, Columbus, and her coauthors.
“To achieve the goal of HCV elimination, access to antiviral treatment must be improved,” they wrote.
The study by Dr. Gowda and colleagues included 9,025 patients in 45 states who had a DAA prescription submitted to one large, independent pharmacy provider between January 2016 and April 2017. Of those patients, most (4,702) were covered by Medicaid, while 2,502 were covered commercially, and 1,821 were covered by Medicare.
Over the 16-month study period, 3,200 patients (35.5%) had an absolute denial of treatment, defined as lack of fill approval by the insurer.
Absolute denials were significantly more frequent in patients with commercial insurance (52.4%), as compared with Medicaid (34.5%) and Medicare (14.7%).
Absolute denials increased significantly over the 16-month study period, from 27.7% in the first quarter evaluated to 43.8% in the last, researchers noted, adding that each insurance type had a significant increase in absolute denials over time.
While DAAs are associated with very high cure rates, their high costs have led to restrictions to access by both private and public insurers. However, over the past few years, restrictions in DAA reimbursement have been relaxed in a variety of settings because of advocacy efforts, greater price competition, and class action lawsuits/threats of legal action, Dr. Gowda and colleagues noted.
“The reason for this higher than expected denial rate is unclear, but may be due to attempts to treat chronic HCV-infected patients who have less advanced liver fibrosis, have not met sobriety restrictions, or have not had consultation with a specialist,” Dr. Gowda and colleagues wrote in their report.
The study was supported by the Penn Center for AIDS Research and the National Institutes of Health. Dr. Gowda had no conflicts of interest to report. Two coauthors reported grant support and/or advisory board fees from Gilead.
SOURCE: Gowda C et al. Open Forum Infect Dis. 2018 Jun 7 doi: 10.1093/ofid/ofy076.
About one in three patients had lack of fill approval by insurers, contributing to a continued lack of access to hepatitis C virus (HCV) therapy across insurance types, despite availability of new, highly effective regimens and relaxation of restrictions on reimbursement, according to Charitha Gowda, MD, of Ohio State University, Columbus, and her coauthors.
“To achieve the goal of HCV elimination, access to antiviral treatment must be improved,” they wrote.
The study by Dr. Gowda and colleagues included 9,025 patients in 45 states who had a DAA prescription submitted to one large, independent pharmacy provider between January 2016 and April 2017. Of those patients, most (4,702) were covered by Medicaid, while 2,502 were covered commercially, and 1,821 were covered by Medicare.
Over the 16-month study period, 3,200 patients (35.5%) had an absolute denial of treatment, defined as lack of fill approval by the insurer.
Absolute denials were significantly more frequent in patients with commercial insurance (52.4%), as compared with Medicaid (34.5%) and Medicare (14.7%).
Absolute denials increased significantly over the 16-month study period, from 27.7% in the first quarter evaluated to 43.8% in the last, researchers noted, adding that each insurance type had a significant increase in absolute denials over time.
While DAAs are associated with very high cure rates, their high costs have led to restrictions to access by both private and public insurers. However, over the past few years, restrictions in DAA reimbursement have been relaxed in a variety of settings because of advocacy efforts, greater price competition, and class action lawsuits/threats of legal action, Dr. Gowda and colleagues noted.
“The reason for this higher than expected denial rate is unclear, but may be due to attempts to treat chronic HCV-infected patients who have less advanced liver fibrosis, have not met sobriety restrictions, or have not had consultation with a specialist,” Dr. Gowda and colleagues wrote in their report.
The study was supported by the Penn Center for AIDS Research and the National Institutes of Health. Dr. Gowda had no conflicts of interest to report. Two coauthors reported grant support and/or advisory board fees from Gilead.
SOURCE: Gowda C et al. Open Forum Infect Dis. 2018 Jun 7 doi: 10.1093/ofid/ofy076.
About one in three patients had lack of fill approval by insurers, contributing to a continued lack of access to hepatitis C virus (HCV) therapy across insurance types, despite availability of new, highly effective regimens and relaxation of restrictions on reimbursement, according to Charitha Gowda, MD, of Ohio State University, Columbus, and her coauthors.
“To achieve the goal of HCV elimination, access to antiviral treatment must be improved,” they wrote.
The study by Dr. Gowda and colleagues included 9,025 patients in 45 states who had a DAA prescription submitted to one large, independent pharmacy provider between January 2016 and April 2017. Of those patients, most (4,702) were covered by Medicaid, while 2,502 were covered commercially, and 1,821 were covered by Medicare.
Over the 16-month study period, 3,200 patients (35.5%) had an absolute denial of treatment, defined as lack of fill approval by the insurer.
Absolute denials were significantly more frequent in patients with commercial insurance (52.4%), as compared with Medicaid (34.5%) and Medicare (14.7%).
Absolute denials increased significantly over the 16-month study period, from 27.7% in the first quarter evaluated to 43.8% in the last, researchers noted, adding that each insurance type had a significant increase in absolute denials over time.
While DAAs are associated with very high cure rates, their high costs have led to restrictions to access by both private and public insurers. However, over the past few years, restrictions in DAA reimbursement have been relaxed in a variety of settings because of advocacy efforts, greater price competition, and class action lawsuits/threats of legal action, Dr. Gowda and colleagues noted.
“The reason for this higher than expected denial rate is unclear, but may be due to attempts to treat chronic HCV-infected patients who have less advanced liver fibrosis, have not met sobriety restrictions, or have not had consultation with a specialist,” Dr. Gowda and colleagues wrote in their report.
The study was supported by the Penn Center for AIDS Research and the National Institutes of Health. Dr. Gowda had no conflicts of interest to report. Two coauthors reported grant support and/or advisory board fees from Gilead.
SOURCE: Gowda C et al. Open Forum Infect Dis. 2018 Jun 7 doi: 10.1093/ofid/ofy076.
FROM OPEN FORUM INFECTIOUS DISEASES
Key clinical point: Insurance denials of direct-acting antiviral (DAA) prescriptions have increased over time, contrary to expectations.
Major finding: A total of 35.5% of patients had an absolute denial of treatment, defined as lack of fill approval by the insurer.
Study details: A cohort study including 9,025 patients who had a DAA prescription submitted to a national specialty pharmacy between January 2016 and April 2017.
Disclosures: The study was supported by the Penn Center for AIDS Research and the National Institutes of Health. Two coauthors reported grant support and/or advisory board fees from Gilead.
Source: Gowda C et al. Open Forum Infect Dis. 2018 Jun 7.