User login
Risk Factors for Thromboembolic Events After Surgery for Ankle Fractures
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
Venous thromboembolic events (VTEs), encompassing both deep vein thrombosis (DVT) and pulmonary embolism (PE), are potentially fatal events that can occur after orthopedic surgery.1 In patients who do not receive prophylaxis, VTE incidence can be as high as 70% for total hip arthroplasty,2 26% for hip fracture,3 and 5% for ankle fracture.4 Based on the relatively low incidence of VTE after ankle fractures and insufficient evidence for VTE prophylaxis in this population, the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures.1,5 Nevertheless, certain patients may be at increased risk for VTE after open reduction and internal fixation (ORIF) of an ankle fracture. In such cases, further consideration for prophylaxis may be warranted.
Other studies of VTEs have identified general risk factors of increased age, obesity, prior thromboembolic disease, oral contraceptive use, multitrauma, varicose veins, and prolonged immobilization, among others.1,6,7 In orthopedics, most of this research comes from total joint arthroplasty and hip fracture studies. However, there is relatively limited data for ankle fracture. The best studies directly addressing VTE after ORIF of ankle fractures have had important limitations, including missing patient data and suboptimal capture of VTE occurrences,8-10 possibly leading to underestimates of the incidence of VTEs.
Given the limited data available, we conducted a retrospective national-cohort study to determine the incidence of and independent risk factors for VTEs after ankle fracture ORIF. If patients who are at higher risk for VTE can be identified, they can and should be carefully monitored and be considered for VTE prophylaxis. This information is needed for patient counseling and clinical decision-making.
Materials and Methods
This retrospective study used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which captures data from more than 370 participating US hospitals.11 In ACS-NSQIP, 150 patient variables are collected from operative reports, medical records, and patient interviews by trained clinical reviewers.11,12 Patients are identified prospectively and randomly sampled at participating hospitals. Routine auditing is performed to ensure high-quality data. Clinical data are collected for the entire 30-day postoperative period, regardless of discharge status during this time.
Patients who underwent ankle fracture ORIF between 2005 and 2012 were identified in the ACS-NSQIP database. They were initially selected by the postoperative diagnosis of ankle fracture (International Classification of Diseases, Ninth Revision codes 824.0-824.9). Of these patients, only those with primary Current Procedural Terminology codes 27766 (ORIF of medial malleolus fracture), 27769 (ORIF of posterior malleolus fracture), 27792 (ORIF of lateral malleolus fracture), 27814 (ORIF of bimalleollar fracture), and 27822/27823 (ORIF of trimalleollar fracture) were included in the analysis. Patients with incomplete perioperative data were excluded, leaving 4412 patients (out of the initial 4785) for analysis.
Patient characteristics, including sex, age, height, weight, and history of smoking, were collected from the ACS-NSQIP database. Body mass index (BMI) was calculated from each patient’s height and weight. Age was divided into approximately 20-year increments, beginning with age 18 years, in order to compare younger, middle-aged, and elderly groups of patients with ankle fractures. BMI was divided into categories based on the World Health Organization definitions of obesity: under 25 kg/m2 (normal weight), 25 to 30 kg/m2 (overweight), 30 to 35 kg/m2 (class I obesity), and 35 kg/m2 or over (class II and class III obesity).13
Information about medical comorbidities is also available in the ACS-NSQIP database. History of pulmonary disease was defined as a history of dyspnea, severe chronic obstructive pulmonary disease, ventilator-assisted respiration within 48 hours before surgery, or current pneumonia. History of heart disease was defined as a history of congestive heart failure (CHF) or angina within 1 month before admission, myocardial infarction within 6 months before admission, cardiac surgery, or percutaneous coronary intervention. American Society of Anesthesiologists (ASA) classes 3 and above signify severe systemic disease. Steroid use was defined as requiring regular administration of corticosteroid medications within 1 month before surgery. Disseminated cancer was defined as a malignancy that has spread to 1 or more sites besides the primary site.
Functional status was defined as the ability to perform activities of daily living (ADLs) within 30 days before surgery. Best functional status during this period was recorded. ACS-NSQIP defines ADLs as the “activities usually performed in the course of a normal day in a person’s life,” including bathing, feeding, dressing, toileting, and mobility. An independent patient does not require assistance for any ADLs; a partially dependent patient requires assistance for some ADLs; and a totally dependent patient requires assistance in all ADLs. Partially and totally dependent patients were grouped for analysis. Anesthesia type was separated into general and nongeneral, which includes monitored anesthesia care, spinal anesthesia, and regional anesthesia.
ACS-NSQIP also records the occurrence of multiple events up to 30 days after surgery. For our study, VTE was defined as the occurrence of a DVT or a PE during this period. ACS-NSQIP defines DVT as a new blood clot or thrombus identified within a vein—with confirmation by duplex ultrasonography, venogram, or computed tomography (CT)—that required therapy (anticoagulation, placement of vena cava filter, and/or clipping of vena cava). PE is recorded if ventilation/perfusion (VQ) scan, CT examination, transesophageal echocardiogram, pulmonary arteriogram, CT angiogram, or any other definitive modality is positive.
Statistical analyses were performed with Stata Version 11.2 (StataCorp). Demographic and comorbidity variables were tested for association with occurrence of VTE using bivariate and multivariate logistic regression.
Final multivariate models were constructed with a backward stepwise process that initially included all potential variables and sequentially excluded variables with the highest P value until only those with P < .200 remained. Variables with .050 < P < .200 were left in the model to control for potential confounding but are not considered significantly associated with the outcome. Statistical significance was established at a 2-sided α of 0.050 (P < .050). The fitness of the final logistic regression model was assessed with the C statistic and the Hosmer-Lemeshow goodness-of-fit test.
Results
For the 4412 ankle fracture patients who met the inclusion criteria, mean (SD) age was 50.9 (18.2) years, and mean (SD) BMI was 30.4 (7.6) kg/m2. The cohort was 40.4% male. Surgery was performed on 235 patients (5.3%) with medial malleolus fracture, 1143 patients (25.9%) with lateral malleolus fracture, 1705 patients (38.6%) with bimalleollar fracture, and 1329 patients (30.1%) with trimalleollar fracture. Table 1 summarizes the patient characteristics.
Of the 33 patients (0.8%) with a VTE recorded within the first 30 postoperative days, 16 (0.4% of all patients) had a DVT recorded, 14 (0.3% of all patients) had a PE recorded, and 3 (0.1% of all patients) had both a DVT and a PE recorded. In 13 (39.4%) of the 33 patients with a VTE, the event occurred after discharge. VTEs were reported a mean (SD) of 11.5 (9.6) days after surgery. No patient in this study died of VTE.
Bivariate logistic regressions were performed to test the association of each patient variable with the occurrence of a VTE. Results are listed in Table 2. The bivariate analyses revealed significant associations between VTE after ankle fracture ORIF and the patient variables of age 60 years or older (odds ratio [OR], 2.40; 95% confidence interval [CI], 1.01-5.72), class I obesity (BMI, 30-35 kg/m2: OR, 5.15, 95% CI, 1.14-23.28), class II and class III obesity (BMI, ≥35 kg/m2: OR, 6.33, 95% CI, 1.41-28.38), ASA classes 3 and 4 (OR, 3.05; 95% CI, 1.53-6.08), history of heart disease (OR, 5.10; 95% CI, 2.08-12.49), history of hypertension (OR, 2.81; 95% CI, 1.39-5.66), and dependent functional status (OR, 3.39; 95% CI, 1.52-7.56).
Multivariate logistic regression was used to control for potential confounding variables and determine which factors were independently associated with VTEs. Results of this analysis are listed in Table 2 as well. The multivariate analysis revealed that the patient variables of class I obesity (BMI, 30-35 kg/m2: OR, 4.77; 95% CI, 1.05-21.72; P = .044), class II and class III obesity (BMI, ≥35 kg/m2: OR, 4.71; 95% CI, 1.03-21.68; P = .046), history of heart disease (OR, 3.28; 95% CI, 1.20-8.97; P = .020), and dependent functional status (OR, 2.59; 95% CI, 1.11-6.04; P = .028) were independently associated with an increased rate of VTEs. Of note, anesthesia type was not significantly associated with occurrence of VTE on bivariate or multivariate analysis.
The C statistic of the final multivariate model was 0.76, indicating very good distinguishing ability. The Hosmer-Lemeshow goodness-of-fit test showed no evidence of lack of fit.
Discussion
Citing the lack of conclusive evidence and the low incidence of VTE after ankle fracture surgery, current recommendations are to avoid routine VTE prophylaxis in the postoperative management of patients who undergo this surgery.1,5 However, it is important to identify patients who are at increased risk, as some may benefit from VTE prophylaxis. In the present study, we used the large, high-quality ACS-NSQIP database collecting information from multiple US hospitals to examine risk factors for VTE after ankle fracture ORIF. We identified 4412 patients who underwent ankle fracture ORIF between 2005 and 2012, and found an overall VTE incidence of 0.8%. Multivariate analysis identified obesity, history of heart disease, and dependent functional status as independent risk factors for VTE after ankle fracture ORIF.
This study’s 0.8% incidence of VTE after ankle fracture ORIF is consistent with the range (0.29%-5%) reported in other ankle fracture studies.4,8-10,14-18 We found that VTEs occurred a mean of about 11 days after surgery, and no patient died of VTE.
Obesity (BMI, ≥30 kg/m2) had the strongest association with VTEs in this study. Obesity, which is a growing public health concern, can make postoperative care and mobilization more difficult.19 Obesity has previously been associated with VTEs after ankle fractures, and BMI of over 25 kg/m2 is one of the Caprini criteria for thrombosis risk factor assessment.6,10 In our study, however, BMI of 25 to 30 kg/m2 was not associated with an increased VTE rate, indicating that moderately overweight patients may not be at significantly higher risk for VTE (compared with patients with normal BMI) and may not need VTE prophylaxis. VTE prophylaxis after ankle fracture surgery may be considered in patients with BMI over 30 kg/m2.
History of heart disease was also associated with VTEs in this study. Patients with a history of heart disease were at 3 times the risk for VTE within 30 days of ankle fracture surgery. This association is also consistent with the Caprini criteria, which include acute myocardial infarction and CHF as risk factors for venous thrombosis.6 Other studies have found associations between CHF and VTE and between cardiovascular risk factors and VTE.7,20 The association between cardiovascular disease and VTE may derive from the decreased venous flow rate associated with CHF or an overall vascular disease state. These patients may benefit from heightened surveillance and postoperative prophylaxis for VTE.
Dependent functional status was the final risk factor found to be associated with VTE after ankle fracture ORIF. This association likely derives from an inability to mobilize independently, leading to increased venous stasis. Immobilization has been previously associated with increased risk for VTE after ankle surgery.7,14,16,20 Caretakers should be aware of this increased risk during the postoperative period and diligently monitor these patients for signs and symptoms of VTE. Prophylaxis may also be considered in this patient population.
Several risk factors that were significant on bivariate analysis (increased age; increased ASA class; history of diabetes, pulmonary disease, hypertension) were not significant in the final multivariate model. This finding suggests covariance between these factors and those that were significant in the final multivariate model. In particular, age and increased overall comorbidity (represented by increased ASA class) were not significant in our multivariate model—contrary to findings of other studies.8-10 It is possible that history of heart disease alone was responsible for the association between overall comorbidity and VTE in those studies. In the present study, separating and controlling for individual comorbidities could have allowed this association to be more precisely characterized.
The characteristics of the ACS-NSQIP database limited our study in several ways. First, although ACS-NSQIP makes significant efforts to collect as many patient variables as possible, some information is not captured. Data about additional factors that may affect VTE risk (eg, history of previous VTE, hypercoagulable state, history of malignancy other than disseminated cancer, tourniquet time, patient position in operating room) were not available. Second, data are collected only on those postoperative adverse events that occur within 30 days after surgery; data on VTEs that occur later are not captured. However, it has been shown that the majority of VTEs occur within the first 30 days after lower extremity trauma and surgery,21,22 so this follow-up interval was deemed adequate for capture of VTE data. Third, the database does not include information on the prophylactic regimens used for these patients—which may have weakened the associations between predictor variables and VTE risk and led to an underestimated effect size. VTE incidence, as well as the odds of developing a VTE with one of the identified risk factors, may actually be higher than reported in this study.
Conclusion
VTEs are serious complications that can occur after ORIF of ankle fractures. In this study, the overall incidence of VTE after ankle fracture ORIF was 0.8%. Although the American Orthopaedic Foot and Ankle Society and the American College of Chest Physicians do not recommend routine screening or prophylaxis for VTE in patients with ankle fractures,1,5 the results of this study showed there may be a benefit in emphasizing VTE prophylaxis after ankle fracture ORIF in patients with obesity, history of heart disease, or dependent functional status. At minimum, these patients should be more carefully monitored for development of VTEs.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
1. American Orthopaedic Foot and Ankle Society. Position statement: the use of VTED prophylaxis in foot and ankle surgery. http://www.aofas.org/medical-community/health-policy/Documents/VTED-Position-Statement-Approv-7-9-13-FINAL.pdf. Updated 2013. Accessed May 10, 2015.
2. Grady-Benson JC, Oishi CS, Hanson PB, Colwell CW Jr, Otis SM, Walker RH. Routine postoperative duplex ultrasonography screening and monitoring for the detection of deep vein thrombosis. A survey of 110 total hip arthroplasties. Clin Orthop Relat Res. 1994;(307):130-141.
3. Salzman EW, Harris WH, DeSanctis RW. Anticoagulation for prevention of thromboembolism following fractures of the hip. New Engl J Med. 1966;275(3):122-130.
4. Patil S, Gandhi J, Curzon I, Hui AC. Incidence of deep-vein thrombosis in patients with fractures of the ankle treated in a plaster cast. J Bone Joint Surg Br. 2007;89(10):1340-1343.
5. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
6. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51(2-3):70-78.
7. Mayle RE Jr, DiGiovanni CW, Lin SS, Tabrizi P, Chou LB. Current concepts review: venous thromboembolic disease in foot and ankle surgery. Foot Ankle Int. 2007;28(11):1207-1216.
8. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497.
9. SooHoo NF, Eagan M, Krenek L, Zingmond DS. Incidence and factors predicting pulmonary embolism and deep venous thrombosis following surgical treatment of ankle fractures. Foot Ankle Surg. 2011;17(4):259-262.
10. Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012;51(1):63-68.
11. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2012 ACS NSQIP Participant Use Data File. http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. Published October 2013. Accessed May 10, 2015.
12. Khuri SF, Henderson WG, Daley J, et al; Principal Investigators of Patient Safety in Surgery Study. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg. 2008;248(2):329-336.
13. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523-1529.
14. Mizel MS, Temple HT, Michelson JD, et al. Thromboembolism after foot and ankle surgery. A multicenter study. Clin Orthop Relat Res. 1998;(348):180-185.
15. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414.
16. Hanslow SS, Grujic L, Slater HK, Chen D. Thromboembolic disease after foot and ankle surgery. Foot Ankle Int. 2006;27(9):693-695.
17. Pelet S, Roger ME, Belzile EL, Bouchard M. The incidence of thromboembolic events in surgically treated ankle fracture. J Bone Joint Surg Am. 2012;94(6):502-506.
18. Manafi Rasi A, Kazemian G, Emami Moghadam M, et al. Deep vein thrombosis following below knee immobilization: the need for chemoprophylaxis. Trauma Mon. 2013;17(4):367-369.
19. Sabharwal S, Root MZ. Impact of obesity on orthopaedics. J Bone Joint Surg Am. 2012;94(11):1045-1052.
20. Kadous A, Abdelgawad AA, Kanlic E. Deep venous thrombosis and pulmonary embolism after surgical treatment of ankle fractures: a case report and review of literature. J Foot Ankle Surg. 2012;51(4):457-463.
21. Forsythe RM, Peitzman AB, DeCato T, et al. Early lower extremity fracture fixation and the risk of early pulmonary embolus: filter before fixation? J Trauma. 2011;70(6):1381-1388.
22. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
Total Hip Arthroplasty for Posttraumatic Osteoarthritis of the Hip Fares Worse Than THA for Primary Osteoarthritis
The incidence of hip fractures decreased between 1995 and 2005, but these injuries continue to occur in large numbers. Between 1986 and 2005, the mean annual number of hip fractures was 957.3/100,000, and the majority of these occurred in patients 75 to 84 years old.1 Investigators have described total hip arthroplasty (THA) performed after initial surgical treatment in patients who developed osteoarthritis (OA) of the hip secondary to a fracture.2-7 Only 1 of these studies compared these patients with a control group of patients who had THA for primary hip OA.2 No study included both previous proximal femur and acetabular fractures.
Postfracture OA may occur when there is residual articular incongruity after fracture or osteonecrosis of the femoral head. THA is commonly used to treat OA when more conservative treatments have failed.6 Other indications for conversion to THA include femoral neck nonunion, significant leg-length discrepancy, and femoral head damage caused by previous internal fixation.4
Given these conditions and previous study findings, THA performed in patients with previous hip fracture fixation is potentially more complicated than THA for primary OA. We therefore conducted a study to evaluate differences in sociodemographic factors, surgical details, and outcomes between patients who had THA for posttraumatic OA and patients who had THA for primary OA.
Materials and Methods
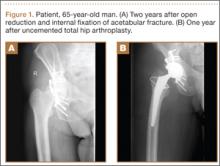
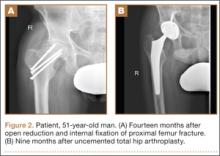
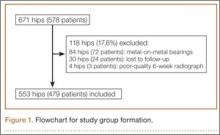
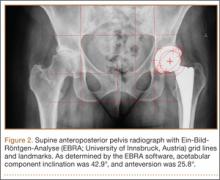
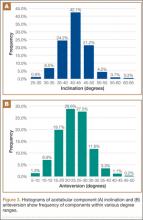
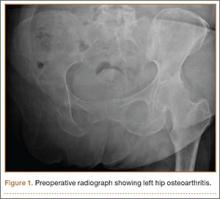
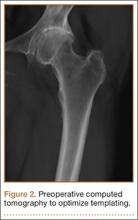
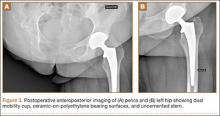
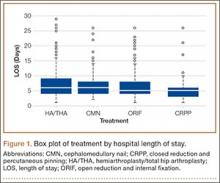
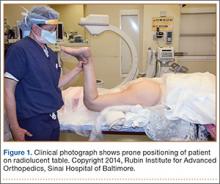
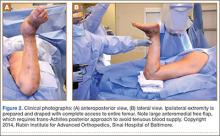
After obtaining institutional review board approval and patient consent, we used a prospective database to follow 3844 patients who had THA performed for OA by 1 of 17 different surgeons at a single center over an 8-year period. Patients who had THA for secondary causes of hip OA, developmental hip dysplasia, or inflammatory processes were excluded. Of the remaining 1199 patients, 62 (5.2%) had THA for posttraumatic OA after previous acetabular or proximal femur fracture fixation (Figures 1, 2) (no THA was performed at time of initial fracture treatment), and 1137 had THA for primary OA and served as the control group.
We collected data on age, sex, fracture location, reason for THA, time between open reduction and internal fixation (ORIF) and THA, type of components, cement use, leg-length discrepancy, intraoperative complications, blood loss, operating room time, and postoperative complications. All patients were aseptic at time of THA. All posttraumatic OA patients had previous hardware removed; the extent of hardware removal was dictated by the exposure required for prosthesis implantation. These patients were contacted, and clinical follow-up was assessed with modified Harris Hip Score (HHS).8 HHS was determined by Dr. Khurana. Statistical analysis was performed with Student t test and Pearson χ2 test using PASW Statistics 18 (SPSS, Chicago, Illinois).
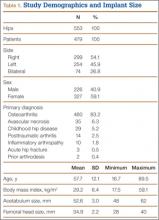
The 62 posttraumatic OA patients had 63 fractures, 41 of the proximal femur (femoral neck and intertrochanteric; 65%) and 22 acetabular (35%). This group consisted of 33 females and 29 males. Their mean age at time of THA surgery was 58 years (range, 31-90 years). Mean age of the control patients was 59.4 years (range, 18-95 years). There were 35 right hips and 27 left hips in the posttrauma group. Mean body mass index (BMI) was 28.4 for the posttrauma group and 28.9 for the control group. There were no differences in age (P = .451), sex (P = .674), or BMI (P = .592) between the 2 groups (Table 1).
All 62 posttraumatic OA patients had complete hospital data, and 32 (52%) of the 62 underwent long-term follow-up (mean, 4.3 years; range, 4 months–10.5 years). At time of attempted contact (mean, 6.79 years after THA), 7 patients were deceased; cause of death was an unrelated medical condition (1) or unknown (6). The rest of the patients did not respond to multiple telephone and mail summons. Primary reasons for conversion to THA included OA (34 patients, 54%), development of osteonecrosis (12 patients, 19%), and nonunion (12 patients, 19%). The rest of the patients had fixation failure. The mechanisms of injury were motor vehicle accidents (30 patients), falls (20), and other causes (15).
Results
Thirty-two (52%) of the posttraumatic OA patients had a preoperative leg-length discrepancy. For these patients, mean time between initial fracture fixation and conversion to THA was 74 months (range, 1-480 months). Four patients required grafting with cancellous autogenous bone graft or allograft chips to fill a bony defect. Mean acetabular component diameter was 54 mm. Nineteen patients had acetabular fixation supplemented with screws. (Screw supplementation data were not recorded for control patients.) Three patients (4.7%) with an acetabular fracture had heterotopic bone removed at time of THA. Two patients underwent neurolysis of the sciatic nerve at time of surgery for preexisting nerve palsy.
Mean postoperative hemoglobin was 109 g/L in the posttraumatic OA group and 121 g/L in the control group (P <. 001). Mean postoperative hematocrit was 0.327 and 0.367, respectively (P < .001). Mean amount of Cell Saver (Haemonetics) used by patients was 176.2 and 72.9 mL, respectively (P < .001). Posttrauma patients lost a mean of 360 mL of blood more than control patients did (P < .001) and were transfused a mean of 1.59 units of blood, compared with 0.85 unit in the controls (P < .001). Patients with acetabular fractures required a mean of only 0.65 unit of transfused blood. Mean operating room time was 240.5 minutes for posttrauma patients and 135.6 minutes for control patients (P < .001). In the posttrauma group, mean size of the head of the femoral component was 29 mm (head size was not recorded for the control group). Posttrauma patients had 18 (29%) hybrid cemented hip replacements (femoral component only) and 44 uncemented hip replacements. Data on femoral stem size and type were not reported for either group.
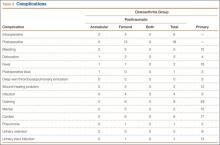
Twenty-four posttrauma patients (39%) had a total of 63 perioperative complications, and 131 control patients (11.5%) had a total of 160 complications (P < .001). Complications in posttrauma patients with proximal femur fractures included excess bleeding (5 patients), in-hospital dislocations (2), and postoperative infections (4: 2 superficial wound infections, 1 implant infection requiring explant, 1 Clostridium difficile infection); in patients with acetabular fractures, there was only 1 dislocation (no infections). The posttraumatic OA group did not develop any symptomatic venous thromboembolic complications. One patient developed a sciatic nerve palsy after surgery. Of the 3 patients who sustained dislocations, 2 were treated with closed reduction and maintenance of implants, and 1 with revision THA. Complications in the control group included 3 infections, 4 dislocations, and 12 cases of extensive blood loss (Table 2).
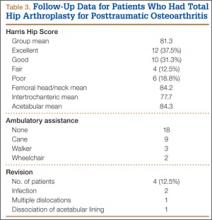
In patients with long-term follow-up, mean postoperative modified HHS was 81.33 (range, 34.1-100.1). Twelve patients had an excellent score (>90), 10 a good score (80-89), 4 a fair score (70-79), and 6 a poor score (<70). Mean HHS was 84.2 for the 16 patients with a femoral head or neck fracture, 77.7 for the 6 patients with an intertrochanteric fracture, and 84.3 for the 9 patients with an acetabular fracture. Nine patients reported using a cane, 3 required walkers, 2 required wheelchairs, and 18 did not require any walking support. Four (12.5%) of the 32 patients required THA revision a mean of 3.5 years (range, 2 months–8 years) after initial arthroplasty. Reasons for revision were infections (2 patients), multiple dislocations (1), and dissociation of acetabular lining (1) (Table 3). Two of the patients who underwent THA revision had a cemented femoral stem, and 2 did not have any cemented implants. Additional details of the femoral stem components were not available for either group.
Discussion
Patients who develop posttraumatic OA of the hip have limited options. THA has emerged as an excellent option in cases of failed repair of fractures about the hip joint. The results of the present study are consistent with earlier findings of the effectiveness of THA in salvaging posttraumatic hips.2-7 THA for patients with posttraumatic arthritis of the hip after acetabular or proximal femur fracture is longer and more complicated than THA for primary OA, and there is significantly more blood loss. In addition, the rate of early failure appears to be higher.9
In this study, mean amount of blood transfused for patients with previous acetabular fracture was 0.65 unit, much less than the mean of 3.5 units noted by Weber and colleagues.6 In their study, complications associated with THA were increased in patients with posttraumatic OA from acetabular fractures. The authors attributed these complications to scarring from previous surgery, retained hardware, heterotopic bone, and residual osseous deformity and deficiency. Our results support their conclusion. Operating times were longer, as well as blood loss and the need for blood transfusions and other blood products were increased in the patients with posttraumatic OA, as compared with patients with primary OA. Fifteen percent of patients with an acetabular fracture had undergone removal of heterotopic bone at time of surgery—similar to the rate of 18% noted in the Weber study.6
Our results showed that the rate of revision THA was also higher than in patients with primary THA within the general population—reported to be about 4%.9 The higher rate may be the result of the additional surgeries performed on patients with fractures, or hardware retention increasing the infection risk over the years. Our revision rate of 12.5% was similar to the 19% found by Ranawat and colleagues7 in their study.
A majority of the patients in our study had favorable long-term HHS. Mean overall HHS was 83, slightly better than the 79 reported by Srivastav and colleagues.4 We found that patients with intertrochanteric fractures ultimately had worse outcome scores than patients with acetabular or femoral neck fractures. These results are consistent with findings reported by Mehlhoff and colleagues5 in a study comparing patients with femoral neck and intertrochanteric fractures. Mean HHS for the intertrochanteric fracture patients in our study was 77.7, comparable to the mean of 78 reported by Mehlhoff and colleagues.5 Mean HHS for the femoral neck or head fractures in our study was 84.2, similar to the mean of 81 they noted. Patients with a previous acetabular fracture in our study had a mean HHS of 84.3, consistent with the 84 reported by Ranawat and colleagues7 for patients who had initially undergone ORIF for acetabular fracture. Mean HHS in our study (83) was slightly less than the 88.5 reported by Shi and colleagues10 in their study of primary THAs.
Few studies have been conducted exclusively on one type of hip fracture (acetabular) or another (proximal femur), and all except 1 did not perform a comparison. Tabsh and colleagues2 compared similar cohorts but focused solely on patients with previous proximal femur fractures. The present study included a control group and both acetabular and proximal femur fractures, which allowed us to compare patients with and without previous fracture fixation and to consider the 2 different fracture types and see if they affected outcomes.
The strengths of this study include the large control group and the relatively short data-collection period. The shorter period decreased the influence of improvements in implants on patient outcomes. In addition, the control group was our own population, as we did not compare our cohort of patients with previous internal fixation and patients who had primary THAs in other studies, aside from comparisons for revision rates and HHS.
Although the ultimate long-term follow-up rate for patients with previous internal fixation was 50%, our sample size was still larger than that in most reported studies. Another weakness of our study was the large number of surgeons (17), representing an array of techniques, approaches, and surgical experience. All these factors could have influenced patient outcomes and operative data. In addition, data on revision rates and HHS were not available for our control group, so we could not directly compare these outcomes with those of the posttraumatic group. However, we used previously reported data on revision rates and HHS in primary THAs for comparison with the posttraumatic group.9,10
Conclusion
In this study, THA was a viable option for patients with posttraumatic arthritis from a previous acetabular or proximal femur fracture. The outcomes, however, were less reliable than the outcomes of primary THA for degenerative arthritis, and the complication rates were higher. Surgeons should counsel patients about the complexity of the procedure as well as its ultimately favorable outcomes. Surgeons should expect additional technical difficulties in the operating room when treating this patient population.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579.
2. Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166-169.
3. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85(5):899-904.
4. Srivastav S, Mittal V, Agarwal S. Total hip arthroplasty following failed fixation of proximal hip fractures. Indian J Orthop. 2008;42(3):279-286.
5. Mehlhoff T, Landon GC, Tullos HS. Total hip arthroplasty following failed internal fixation of hip fractures. Clin Orthop Relat Res. 1991;(269):32-37.
6. Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80(9):1295-1305.
7. Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24(5):759-767.
8. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
9. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
10. Shi HY, Mau LW, Chang JK, Wang JW, Chiu HC. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18(8):1053-1060.
The incidence of hip fractures decreased between 1995 and 2005, but these injuries continue to occur in large numbers. Between 1986 and 2005, the mean annual number of hip fractures was 957.3/100,000, and the majority of these occurred in patients 75 to 84 years old.1 Investigators have described total hip arthroplasty (THA) performed after initial surgical treatment in patients who developed osteoarthritis (OA) of the hip secondary to a fracture.2-7 Only 1 of these studies compared these patients with a control group of patients who had THA for primary hip OA.2 No study included both previous proximal femur and acetabular fractures.
Postfracture OA may occur when there is residual articular incongruity after fracture or osteonecrosis of the femoral head. THA is commonly used to treat OA when more conservative treatments have failed.6 Other indications for conversion to THA include femoral neck nonunion, significant leg-length discrepancy, and femoral head damage caused by previous internal fixation.4
Given these conditions and previous study findings, THA performed in patients with previous hip fracture fixation is potentially more complicated than THA for primary OA. We therefore conducted a study to evaluate differences in sociodemographic factors, surgical details, and outcomes between patients who had THA for posttraumatic OA and patients who had THA for primary OA.
Materials and Methods
After obtaining institutional review board approval and patient consent, we used a prospective database to follow 3844 patients who had THA performed for OA by 1 of 17 different surgeons at a single center over an 8-year period. Patients who had THA for secondary causes of hip OA, developmental hip dysplasia, or inflammatory processes were excluded. Of the remaining 1199 patients, 62 (5.2%) had THA for posttraumatic OA after previous acetabular or proximal femur fracture fixation (Figures 1, 2) (no THA was performed at time of initial fracture treatment), and 1137 had THA for primary OA and served as the control group.
We collected data on age, sex, fracture location, reason for THA, time between open reduction and internal fixation (ORIF) and THA, type of components, cement use, leg-length discrepancy, intraoperative complications, blood loss, operating room time, and postoperative complications. All patients were aseptic at time of THA. All posttraumatic OA patients had previous hardware removed; the extent of hardware removal was dictated by the exposure required for prosthesis implantation. These patients were contacted, and clinical follow-up was assessed with modified Harris Hip Score (HHS).8 HHS was determined by Dr. Khurana. Statistical analysis was performed with Student t test and Pearson χ2 test using PASW Statistics 18 (SPSS, Chicago, Illinois).
The 62 posttraumatic OA patients had 63 fractures, 41 of the proximal femur (femoral neck and intertrochanteric; 65%) and 22 acetabular (35%). This group consisted of 33 females and 29 males. Their mean age at time of THA surgery was 58 years (range, 31-90 years). Mean age of the control patients was 59.4 years (range, 18-95 years). There were 35 right hips and 27 left hips in the posttrauma group. Mean body mass index (BMI) was 28.4 for the posttrauma group and 28.9 for the control group. There were no differences in age (P = .451), sex (P = .674), or BMI (P = .592) between the 2 groups (Table 1).
All 62 posttraumatic OA patients had complete hospital data, and 32 (52%) of the 62 underwent long-term follow-up (mean, 4.3 years; range, 4 months–10.5 years). At time of attempted contact (mean, 6.79 years after THA), 7 patients were deceased; cause of death was an unrelated medical condition (1) or unknown (6). The rest of the patients did not respond to multiple telephone and mail summons. Primary reasons for conversion to THA included OA (34 patients, 54%), development of osteonecrosis (12 patients, 19%), and nonunion (12 patients, 19%). The rest of the patients had fixation failure. The mechanisms of injury were motor vehicle accidents (30 patients), falls (20), and other causes (15).
Results
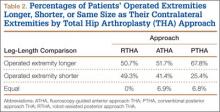
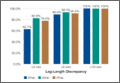
Thirty-two (52%) of the posttraumatic OA patients had a preoperative leg-length discrepancy. For these patients, mean time between initial fracture fixation and conversion to THA was 74 months (range, 1-480 months). Four patients required grafting with cancellous autogenous bone graft or allograft chips to fill a bony defect. Mean acetabular component diameter was 54 mm. Nineteen patients had acetabular fixation supplemented with screws. (Screw supplementation data were not recorded for control patients.) Three patients (4.7%) with an acetabular fracture had heterotopic bone removed at time of THA. Two patients underwent neurolysis of the sciatic nerve at time of surgery for preexisting nerve palsy.
Mean postoperative hemoglobin was 109 g/L in the posttraumatic OA group and 121 g/L in the control group (P <. 001). Mean postoperative hematocrit was 0.327 and 0.367, respectively (P < .001). Mean amount of Cell Saver (Haemonetics) used by patients was 176.2 and 72.9 mL, respectively (P < .001). Posttrauma patients lost a mean of 360 mL of blood more than control patients did (P < .001) and were transfused a mean of 1.59 units of blood, compared with 0.85 unit in the controls (P < .001). Patients with acetabular fractures required a mean of only 0.65 unit of transfused blood. Mean operating room time was 240.5 minutes for posttrauma patients and 135.6 minutes for control patients (P < .001). In the posttrauma group, mean size of the head of the femoral component was 29 mm (head size was not recorded for the control group). Posttrauma patients had 18 (29%) hybrid cemented hip replacements (femoral component only) and 44 uncemented hip replacements. Data on femoral stem size and type were not reported for either group.
Twenty-four posttrauma patients (39%) had a total of 63 perioperative complications, and 131 control patients (11.5%) had a total of 160 complications (P < .001). Complications in posttrauma patients with proximal femur fractures included excess bleeding (5 patients), in-hospital dislocations (2), and postoperative infections (4: 2 superficial wound infections, 1 implant infection requiring explant, 1 Clostridium difficile infection); in patients with acetabular fractures, there was only 1 dislocation (no infections). The posttraumatic OA group did not develop any symptomatic venous thromboembolic complications. One patient developed a sciatic nerve palsy after surgery. Of the 3 patients who sustained dislocations, 2 were treated with closed reduction and maintenance of implants, and 1 with revision THA. Complications in the control group included 3 infections, 4 dislocations, and 12 cases of extensive blood loss (Table 2).
In patients with long-term follow-up, mean postoperative modified HHS was 81.33 (range, 34.1-100.1). Twelve patients had an excellent score (>90), 10 a good score (80-89), 4 a fair score (70-79), and 6 a poor score (<70). Mean HHS was 84.2 for the 16 patients with a femoral head or neck fracture, 77.7 for the 6 patients with an intertrochanteric fracture, and 84.3 for the 9 patients with an acetabular fracture. Nine patients reported using a cane, 3 required walkers, 2 required wheelchairs, and 18 did not require any walking support. Four (12.5%) of the 32 patients required THA revision a mean of 3.5 years (range, 2 months–8 years) after initial arthroplasty. Reasons for revision were infections (2 patients), multiple dislocations (1), and dissociation of acetabular lining (1) (Table 3). Two of the patients who underwent THA revision had a cemented femoral stem, and 2 did not have any cemented implants. Additional details of the femoral stem components were not available for either group.
Discussion
Patients who develop posttraumatic OA of the hip have limited options. THA has emerged as an excellent option in cases of failed repair of fractures about the hip joint. The results of the present study are consistent with earlier findings of the effectiveness of THA in salvaging posttraumatic hips.2-7 THA for patients with posttraumatic arthritis of the hip after acetabular or proximal femur fracture is longer and more complicated than THA for primary OA, and there is significantly more blood loss. In addition, the rate of early failure appears to be higher.9
In this study, mean amount of blood transfused for patients with previous acetabular fracture was 0.65 unit, much less than the mean of 3.5 units noted by Weber and colleagues.6 In their study, complications associated with THA were increased in patients with posttraumatic OA from acetabular fractures. The authors attributed these complications to scarring from previous surgery, retained hardware, heterotopic bone, and residual osseous deformity and deficiency. Our results support their conclusion. Operating times were longer, as well as blood loss and the need for blood transfusions and other blood products were increased in the patients with posttraumatic OA, as compared with patients with primary OA. Fifteen percent of patients with an acetabular fracture had undergone removal of heterotopic bone at time of surgery—similar to the rate of 18% noted in the Weber study.6
Our results showed that the rate of revision THA was also higher than in patients with primary THA within the general population—reported to be about 4%.9 The higher rate may be the result of the additional surgeries performed on patients with fractures, or hardware retention increasing the infection risk over the years. Our revision rate of 12.5% was similar to the 19% found by Ranawat and colleagues7 in their study.
A majority of the patients in our study had favorable long-term HHS. Mean overall HHS was 83, slightly better than the 79 reported by Srivastav and colleagues.4 We found that patients with intertrochanteric fractures ultimately had worse outcome scores than patients with acetabular or femoral neck fractures. These results are consistent with findings reported by Mehlhoff and colleagues5 in a study comparing patients with femoral neck and intertrochanteric fractures. Mean HHS for the intertrochanteric fracture patients in our study was 77.7, comparable to the mean of 78 reported by Mehlhoff and colleagues.5 Mean HHS for the femoral neck or head fractures in our study was 84.2, similar to the mean of 81 they noted. Patients with a previous acetabular fracture in our study had a mean HHS of 84.3, consistent with the 84 reported by Ranawat and colleagues7 for patients who had initially undergone ORIF for acetabular fracture. Mean HHS in our study (83) was slightly less than the 88.5 reported by Shi and colleagues10 in their study of primary THAs.
Few studies have been conducted exclusively on one type of hip fracture (acetabular) or another (proximal femur), and all except 1 did not perform a comparison. Tabsh and colleagues2 compared similar cohorts but focused solely on patients with previous proximal femur fractures. The present study included a control group and both acetabular and proximal femur fractures, which allowed us to compare patients with and without previous fracture fixation and to consider the 2 different fracture types and see if they affected outcomes.
The strengths of this study include the large control group and the relatively short data-collection period. The shorter period decreased the influence of improvements in implants on patient outcomes. In addition, the control group was our own population, as we did not compare our cohort of patients with previous internal fixation and patients who had primary THAs in other studies, aside from comparisons for revision rates and HHS.
Although the ultimate long-term follow-up rate for patients with previous internal fixation was 50%, our sample size was still larger than that in most reported studies. Another weakness of our study was the large number of surgeons (17), representing an array of techniques, approaches, and surgical experience. All these factors could have influenced patient outcomes and operative data. In addition, data on revision rates and HHS were not available for our control group, so we could not directly compare these outcomes with those of the posttraumatic group. However, we used previously reported data on revision rates and HHS in primary THAs for comparison with the posttraumatic group.9,10
Conclusion
In this study, THA was a viable option for patients with posttraumatic arthritis from a previous acetabular or proximal femur fracture. The outcomes, however, were less reliable than the outcomes of primary THA for degenerative arthritis, and the complication rates were higher. Surgeons should counsel patients about the complexity of the procedure as well as its ultimately favorable outcomes. Surgeons should expect additional technical difficulties in the operating room when treating this patient population.
The incidence of hip fractures decreased between 1995 and 2005, but these injuries continue to occur in large numbers. Between 1986 and 2005, the mean annual number of hip fractures was 957.3/100,000, and the majority of these occurred in patients 75 to 84 years old.1 Investigators have described total hip arthroplasty (THA) performed after initial surgical treatment in patients who developed osteoarthritis (OA) of the hip secondary to a fracture.2-7 Only 1 of these studies compared these patients with a control group of patients who had THA for primary hip OA.2 No study included both previous proximal femur and acetabular fractures.
Postfracture OA may occur when there is residual articular incongruity after fracture or osteonecrosis of the femoral head. THA is commonly used to treat OA when more conservative treatments have failed.6 Other indications for conversion to THA include femoral neck nonunion, significant leg-length discrepancy, and femoral head damage caused by previous internal fixation.4
Given these conditions and previous study findings, THA performed in patients with previous hip fracture fixation is potentially more complicated than THA for primary OA. We therefore conducted a study to evaluate differences in sociodemographic factors, surgical details, and outcomes between patients who had THA for posttraumatic OA and patients who had THA for primary OA.
Materials and Methods
After obtaining institutional review board approval and patient consent, we used a prospective database to follow 3844 patients who had THA performed for OA by 1 of 17 different surgeons at a single center over an 8-year period. Patients who had THA for secondary causes of hip OA, developmental hip dysplasia, or inflammatory processes were excluded. Of the remaining 1199 patients, 62 (5.2%) had THA for posttraumatic OA after previous acetabular or proximal femur fracture fixation (Figures 1, 2) (no THA was performed at time of initial fracture treatment), and 1137 had THA for primary OA and served as the control group.
We collected data on age, sex, fracture location, reason for THA, time between open reduction and internal fixation (ORIF) and THA, type of components, cement use, leg-length discrepancy, intraoperative complications, blood loss, operating room time, and postoperative complications. All patients were aseptic at time of THA. All posttraumatic OA patients had previous hardware removed; the extent of hardware removal was dictated by the exposure required for prosthesis implantation. These patients were contacted, and clinical follow-up was assessed with modified Harris Hip Score (HHS).8 HHS was determined by Dr. Khurana. Statistical analysis was performed with Student t test and Pearson χ2 test using PASW Statistics 18 (SPSS, Chicago, Illinois).
The 62 posttraumatic OA patients had 63 fractures, 41 of the proximal femur (femoral neck and intertrochanteric; 65%) and 22 acetabular (35%). This group consisted of 33 females and 29 males. Their mean age at time of THA surgery was 58 years (range, 31-90 years). Mean age of the control patients was 59.4 years (range, 18-95 years). There were 35 right hips and 27 left hips in the posttrauma group. Mean body mass index (BMI) was 28.4 for the posttrauma group and 28.9 for the control group. There were no differences in age (P = .451), sex (P = .674), or BMI (P = .592) between the 2 groups (Table 1).
All 62 posttraumatic OA patients had complete hospital data, and 32 (52%) of the 62 underwent long-term follow-up (mean, 4.3 years; range, 4 months–10.5 years). At time of attempted contact (mean, 6.79 years after THA), 7 patients were deceased; cause of death was an unrelated medical condition (1) or unknown (6). The rest of the patients did not respond to multiple telephone and mail summons. Primary reasons for conversion to THA included OA (34 patients, 54%), development of osteonecrosis (12 patients, 19%), and nonunion (12 patients, 19%). The rest of the patients had fixation failure. The mechanisms of injury were motor vehicle accidents (30 patients), falls (20), and other causes (15).
Results
Thirty-two (52%) of the posttraumatic OA patients had a preoperative leg-length discrepancy. For these patients, mean time between initial fracture fixation and conversion to THA was 74 months (range, 1-480 months). Four patients required grafting with cancellous autogenous bone graft or allograft chips to fill a bony defect. Mean acetabular component diameter was 54 mm. Nineteen patients had acetabular fixation supplemented with screws. (Screw supplementation data were not recorded for control patients.) Three patients (4.7%) with an acetabular fracture had heterotopic bone removed at time of THA. Two patients underwent neurolysis of the sciatic nerve at time of surgery for preexisting nerve palsy.
Mean postoperative hemoglobin was 109 g/L in the posttraumatic OA group and 121 g/L in the control group (P <. 001). Mean postoperative hematocrit was 0.327 and 0.367, respectively (P < .001). Mean amount of Cell Saver (Haemonetics) used by patients was 176.2 and 72.9 mL, respectively (P < .001). Posttrauma patients lost a mean of 360 mL of blood more than control patients did (P < .001) and were transfused a mean of 1.59 units of blood, compared with 0.85 unit in the controls (P < .001). Patients with acetabular fractures required a mean of only 0.65 unit of transfused blood. Mean operating room time was 240.5 minutes for posttrauma patients and 135.6 minutes for control patients (P < .001). In the posttrauma group, mean size of the head of the femoral component was 29 mm (head size was not recorded for the control group). Posttrauma patients had 18 (29%) hybrid cemented hip replacements (femoral component only) and 44 uncemented hip replacements. Data on femoral stem size and type were not reported for either group.
Twenty-four posttrauma patients (39%) had a total of 63 perioperative complications, and 131 control patients (11.5%) had a total of 160 complications (P < .001). Complications in posttrauma patients with proximal femur fractures included excess bleeding (5 patients), in-hospital dislocations (2), and postoperative infections (4: 2 superficial wound infections, 1 implant infection requiring explant, 1 Clostridium difficile infection); in patients with acetabular fractures, there was only 1 dislocation (no infections). The posttraumatic OA group did not develop any symptomatic venous thromboembolic complications. One patient developed a sciatic nerve palsy after surgery. Of the 3 patients who sustained dislocations, 2 were treated with closed reduction and maintenance of implants, and 1 with revision THA. Complications in the control group included 3 infections, 4 dislocations, and 12 cases of extensive blood loss (Table 2).
In patients with long-term follow-up, mean postoperative modified HHS was 81.33 (range, 34.1-100.1). Twelve patients had an excellent score (>90), 10 a good score (80-89), 4 a fair score (70-79), and 6 a poor score (<70). Mean HHS was 84.2 for the 16 patients with a femoral head or neck fracture, 77.7 for the 6 patients with an intertrochanteric fracture, and 84.3 for the 9 patients with an acetabular fracture. Nine patients reported using a cane, 3 required walkers, 2 required wheelchairs, and 18 did not require any walking support. Four (12.5%) of the 32 patients required THA revision a mean of 3.5 years (range, 2 months–8 years) after initial arthroplasty. Reasons for revision were infections (2 patients), multiple dislocations (1), and dissociation of acetabular lining (1) (Table 3). Two of the patients who underwent THA revision had a cemented femoral stem, and 2 did not have any cemented implants. Additional details of the femoral stem components were not available for either group.
Discussion
Patients who develop posttraumatic OA of the hip have limited options. THA has emerged as an excellent option in cases of failed repair of fractures about the hip joint. The results of the present study are consistent with earlier findings of the effectiveness of THA in salvaging posttraumatic hips.2-7 THA for patients with posttraumatic arthritis of the hip after acetabular or proximal femur fracture is longer and more complicated than THA for primary OA, and there is significantly more blood loss. In addition, the rate of early failure appears to be higher.9
In this study, mean amount of blood transfused for patients with previous acetabular fracture was 0.65 unit, much less than the mean of 3.5 units noted by Weber and colleagues.6 In their study, complications associated with THA were increased in patients with posttraumatic OA from acetabular fractures. The authors attributed these complications to scarring from previous surgery, retained hardware, heterotopic bone, and residual osseous deformity and deficiency. Our results support their conclusion. Operating times were longer, as well as blood loss and the need for blood transfusions and other blood products were increased in the patients with posttraumatic OA, as compared with patients with primary OA. Fifteen percent of patients with an acetabular fracture had undergone removal of heterotopic bone at time of surgery—similar to the rate of 18% noted in the Weber study.6
Our results showed that the rate of revision THA was also higher than in patients with primary THA within the general population—reported to be about 4%.9 The higher rate may be the result of the additional surgeries performed on patients with fractures, or hardware retention increasing the infection risk over the years. Our revision rate of 12.5% was similar to the 19% found by Ranawat and colleagues7 in their study.
A majority of the patients in our study had favorable long-term HHS. Mean overall HHS was 83, slightly better than the 79 reported by Srivastav and colleagues.4 We found that patients with intertrochanteric fractures ultimately had worse outcome scores than patients with acetabular or femoral neck fractures. These results are consistent with findings reported by Mehlhoff and colleagues5 in a study comparing patients with femoral neck and intertrochanteric fractures. Mean HHS for the intertrochanteric fracture patients in our study was 77.7, comparable to the mean of 78 reported by Mehlhoff and colleagues.5 Mean HHS for the femoral neck or head fractures in our study was 84.2, similar to the mean of 81 they noted. Patients with a previous acetabular fracture in our study had a mean HHS of 84.3, consistent with the 84 reported by Ranawat and colleagues7 for patients who had initially undergone ORIF for acetabular fracture. Mean HHS in our study (83) was slightly less than the 88.5 reported by Shi and colleagues10 in their study of primary THAs.
Few studies have been conducted exclusively on one type of hip fracture (acetabular) or another (proximal femur), and all except 1 did not perform a comparison. Tabsh and colleagues2 compared similar cohorts but focused solely on patients with previous proximal femur fractures. The present study included a control group and both acetabular and proximal femur fractures, which allowed us to compare patients with and without previous fracture fixation and to consider the 2 different fracture types and see if they affected outcomes.
The strengths of this study include the large control group and the relatively short data-collection period. The shorter period decreased the influence of improvements in implants on patient outcomes. In addition, the control group was our own population, as we did not compare our cohort of patients with previous internal fixation and patients who had primary THAs in other studies, aside from comparisons for revision rates and HHS.
Although the ultimate long-term follow-up rate for patients with previous internal fixation was 50%, our sample size was still larger than that in most reported studies. Another weakness of our study was the large number of surgeons (17), representing an array of techniques, approaches, and surgical experience. All these factors could have influenced patient outcomes and operative data. In addition, data on revision rates and HHS were not available for our control group, so we could not directly compare these outcomes with those of the posttraumatic group. However, we used previously reported data on revision rates and HHS in primary THAs for comparison with the posttraumatic group.9,10
Conclusion
In this study, THA was a viable option for patients with posttraumatic arthritis from a previous acetabular or proximal femur fracture. The outcomes, however, were less reliable than the outcomes of primary THA for degenerative arthritis, and the complication rates were higher. Surgeons should counsel patients about the complexity of the procedure as well as its ultimately favorable outcomes. Surgeons should expect additional technical difficulties in the operating room when treating this patient population.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579.
2. Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166-169.
3. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85(5):899-904.
4. Srivastav S, Mittal V, Agarwal S. Total hip arthroplasty following failed fixation of proximal hip fractures. Indian J Orthop. 2008;42(3):279-286.
5. Mehlhoff T, Landon GC, Tullos HS. Total hip arthroplasty following failed internal fixation of hip fractures. Clin Orthop Relat Res. 1991;(269):32-37.
6. Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80(9):1295-1305.
7. Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24(5):759-767.
8. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
9. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
10. Shi HY, Mau LW, Chang JK, Wang JW, Chiu HC. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18(8):1053-1060.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579.
2. Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166-169.
3. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85(5):899-904.
4. Srivastav S, Mittal V, Agarwal S. Total hip arthroplasty following failed fixation of proximal hip fractures. Indian J Orthop. 2008;42(3):279-286.
5. Mehlhoff T, Landon GC, Tullos HS. Total hip arthroplasty following failed internal fixation of hip fractures. Clin Orthop Relat Res. 1991;(269):32-37.
6. Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80(9):1295-1305.
7. Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24(5):759-767.
8. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
9. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
10. Shi HY, Mau LW, Chang JK, Wang JW, Chiu HC. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18(8):1053-1060.
The Role of Vitamin C in Orthopedic Trauma and Bone Health
L-ascorbic acid, more commonly know as vitamin C, is an essential micronutrient used in numerous metabolic pathways. It functions physiologically as a water-soluble antioxidant by virtue of its high reducing power, playing a key role in the function of leukocytes, protein metabolism, and production of neurotransmitters.1-3 Vitamin C also contributes to musculoskeletal health through biosynthesis of carnitine and collagen4 and enhancement of intestinal absorption of dietary iron5 from plants and vegetables. Unlike most animals, humans are unable to synthesize this essential vitamin and therefore require intake from natural dietary sources or supplements.6 The ability of vitamin C to prevent or treat disease has been an area of research interest since the vitamin was identified and isolated by Szent-Györgyi in the 1930s.7-16 Research in orthopedic surgery has focused on the effects of vitamin C on fracture healing, its potential use in preventing complex regional pain syndrome (CRPS), and its role in the pathophysiology of osteoarthritis. In this article, we review the basics of vitamin C metabolism and summarize the evidence surrounding the role of vitamin C supplementation in orthopedics.
Sources and Metabolism
Vitamin C is found naturally in many fruits and vegetables (Table 1) and is a common fortification in cereals, juices, and multivitamins. Daily recommended intake (Table 2) depends on age and smoking status. Absorption occurs in the distal small intestine, with blood plasma vitamin C concentrations reflecting dietary intake. Pharmacokinetic studies have shown that vitamin C concentrations are tightly regulated through absorption, tissue accumulation, and renal resorption, with plasma concentrations rarely exceeding 100 μmol/L without additional supplementation.17 Although the usual dietary doses of 100 mg/d (adult) are almost completely absorbed, producing a plasma concentration of 60 μmol/L, higher intake results in an increasingly smaller fraction absorbed.1,18 Intake of more than 1000 mg/d results in less than 50% absorption19 (unmetabolized vitamin C is excreted in stool and urine1). Even at higher doses, vitamin C has low toxicity3; the most common complaints are diarrhea, nausea, and abdominal cramps caused by the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract.1
Vitamin C Deficiency
The relationship between vitamin C deficiency and the development of scurvy has been documented for centuries. Symptoms are described in the ancient Egyptian, Greek, and Roman literature.20 Ascorbic acid is essential for normal collagen function, as it is a required cofactor for enzymatic transfer of hydroxyl groups to select proline and lysine residues during procollagen formation. Hydroxylysine contributes to the intermolecular cross-links in collagen, and hydroxyproline stabilizes the triple-helix structure of collagen.21 Insufficient vitamin C during this process results in collagen that is non-cross-linked, nonhelical, structurally unstable, and weak.21 Clinical manifestations of scurvy stem from an underlying impairment of collagen production causing a systemic decrease in connective tissue integrity, capillary fragility, poor wound healing, fatigue, myalgias, arthritis, and even death.22 Vitamin C deficiency has also been implicated as a cause of diffuse bleeding in surgical patients with normal coagulation parameters secondary to capillary fragility.23 In the United States, the 2003–2004 National Health and Nutrition Examination Survey (NHANES) measured serum vitamin C concentrations in 7277 noninstitutionalized patients 6 years old or older.24 Age-adjusted incidence of subnormal serum vitamin C levels (<28 μmol/L) was 19.6%, and incidence of frank vitamin C deficiency (<11.4 μmol/L) was 7.1%. Reported rates of vitamin C deficiency in hospitalized patients are much higher, with 47% to 60% having subnormal values (<28 μmol/L) and 17% to 19% being vitamin C–deficient (<11.4 μmol/L).22,25 Identified risk factors for hypovitaminosis C include advanced age, obesity, low socioeconomic status, unemployment, male sex, and concomitant alcohol and tobacco consumption.22,24,25
Fracture Healing and Prevention
The effects of vitamin C deficiency on bone healing have been studied with animal models as early as the 1940s.26,27 Early experiments using guinea pigs demonstrated failure of bone graft incorporation, delayed collagen maturation, and decreased collagen and callus formation in scorbutic animals compared with controls that received vitamin C supplementation.26,27 Based on his work with guinea pigs, Bourne26 reported in 1942 that vitamin C deficiency significantly inhibited the reparative process in damaged bone and that patients with fractures should receive vitamin C supplementation. Building on this early research, Yilmaz and colleagues28 found faster histologic healing for tibia fractures in a rat model for animals that received a single injection of vitamin C 0.5 mg/kg compared with a nonscorbutic control group, and Sarisözen and colleagues29 showed significantly accelerated histologic bone formation and mineralization at the fracture site for rats that received vitamin C supplementation. Moreover, Kipp and colleagues30 found that scorbutic guinea pigs had lower bone mineral density (BMD), decreased bone mineral content, and impaired collagen synthesis of articular cartilage and tendons compared with nondeficient controls.
Besides promoting bone formation, vitamin C improves the mechanical strength of callus formation. Alcantara-Martos and colleagues31 used an osteogenic disorder Shionogi (ODS) rat model to examine the effects of vitamin C intake on femoral fracture healing. This particular animal model is unable to produce its own vitamin C. The groups with lower serum vitamin C levels demonstrated lower mechanical resistance of the fracture callus to torsional loads 5 weeks after fracture. Moreover, the group that received vitamin C supplementation showed higher histologic grade of callus formation and demonstrated faster healing rates. The authors suggested that subclinical vitamin C deficiency can delay fracture healing and that vitamin C supplementation in nondeficient patients would improve bone healing.
Other research has demonstrated a link between vitamin C and mesenchymal cell differentiation. Mohan and colleagues32 used an sfx mouse model to show that vitamin C deficiency results in decreased bone formation secondary to impaired osteoblast differentiation, diminished bone density, and development of spontaneous fractures. The authors indicated that not only is vitamin C essential for maintenance of differentiated functions of osteoblasts, but deficiency during early active growth may affect peak BMD levels in humans. Additional studies have demonstrated the role of vitamin C in endochondral bone formation through both induction of osteoblast differentiation and modulation of gene expression in hypertrophic chondrocytes.33-36 Chronic vitamin C deficiency has been found to depress osteoblast function and differentiation of chondrocytes.37 More recently, Kim and colleagues38 examined the effect of vitamin C insufficiency in Gulo-deficient mice, which are unable to synthesize ascorbic acid. Ascorbic acid insufficiency over 4 weeks led to decreased plasma levels of osteocalcin and bone formation in vivo as well as significantly diminished metaphyseal trabecular bone. Despite all the evidence demonstrating the importance of vitamin C in bone formation and maintenance, many of the underlying processes in this relationship have yet to be determined.
Bone Mineral Density
Several observational studies have found a positive association between vitamin C intake and BMD in postmenopausal women. In a retrospective, cross-sectional study by Hall and Greendale,39 a positive association was found between vitamin C intake and BMD of the femoral neck in 775 participants in the Postmenopausal Estrogen/Progestin Interventions trial. After calcium intake, physical activity level, smoking, estrogen use, age, and body mass index were adjusted for, each 100-mg increase in dietary vitamin C was associated with a 0.017 g/cm2 increase in BMD. Wang and colleagues40 found a positive association between dietary vitamin C intake and femoral neck BMD in a retrospective analysis of 125 postmenopausal Mexican American women. Other observational studies have reported that decreased intake of vitamin C is associated with osteoporosis41 and increased rates of BMD loss42 and that supplementation with vitamin C may suppress bone resorption in postmenopausal women.43
The results of these studies contrast with the findings of Leveille and colleagues,44 who examined the relationship between dietary vitamin C and hip BMD in 1892 postmenopausal women. Although the authors found that women (age, 55-64 years) using vitamin C supplements for more than 10 years had an average BMD 6.7% higher than that of nonusers, they did not find any association between dietary vitamin C intake and BMD. Moreover, NHANES III also found inconsistent associations between vitamin C and BMD among 13,080 adults surveyed in the United States.45 Although for premenopausal women dietary ascorbic acid was associated with increased BMD, for postmenopausal women with a history of smoking and estrogen replacement, it was actually associated with lower BMD values. For other subgroups in the study, the relationship was also inconsistent or nonlinear.
The exact mechanism by which ascorbic acid contributes to BMD is not fully delineated. However, it likely is related to the known role of vitamin C in collagen formation, bone matrix development, osteoblast differentiation, and its antioxidant effects limiting bone resorption.44,46
Hip Fractures
Besides demonstrating positive effects of vitamin C on bone healing and BMD, epidemiologic studies have found evidence of a protective effect of vitamin C on hip fracture risk. In a study of the Swedish Mammography cohort, 66,651 women (age, 40-76 years) were prospectively followed.47 The authors found that the odds ratio (OR) for hip fractures among smokers with a low intake of vitamin E (median intake, ≤6.2 mg/d) was 3.0 (95% CI, 1.6-5.4) and for vitamin C (median intake, ≤67 mg/d) was 3.0 (95% CI, 1.6-5.6). Moreover, in smokers with a low intake of both vitamins E and C, OR increased to 4.9 (95% CI, 2.2-11.0). In addition, the Utah Study of Nutrition and Bone Health matched 1215 cases of hip fractures in patients who had ever smoked (age, >50 years) with 1349 controls and found that vitamin C intake above 159 mg/d had a significant protective effect on the incidence of hip fracture; however, a graded relationship was not observed.48 Despite the inconsistencies in the NHANES III study regarding the relationship between vitamin C and BMD, Simon and Hudes45 found that serum vitamin C was associated with lower risk for self-reported fracture in postmenopausal women who had ever smoked and had a history of estrogen therapy (OR, 0.51; 95% CI, 0.36-0.70). Finally, Sahni and colleagues49 followed 958 Framingham cohort men and women (mean age, 75 years) over 17 years and found that those in the highest tertile of total vitamin C intake (median, 313 mg/d) had significantly fewer hip fractures and nonvertebral fractures compared with those in the lowest tertile of intake (median, 94 mg/d). Dietary vitamin C intake was not associated with fracture risk in this study.
Complex Regional Pain Syndrome
Type 1 CRPS is a debilitating condition characterized by severe pain, swelling, and vasomotor instability. It is commonly precipitated by an injury or surgery to an extremity and is a dreaded sequelae in orthopedics,50 with incidence rates of 10% to 22% in wrist fractures51-53 and 10% after foot and ankle surgery.54 Although the pathophysiology of CRPS remains unknown, dysregulation and increased permeability of the vasculature caused by free radicals are thought to play an important role.55 In dermal burns, high doses of vitamin C therapy slowed progression of vascular permeability and therefore reduced extravascular leakage of fluids and protein.56,57 The ability of vitamin C to prevent CRPS has been studied in only a handful of trials.
In a double-blind trial, Zollinger and colleagues51 randomized 127 conservatively treated distal radius fractures to receive either vitamin C 500 mg or placebo daily for 50 days starting on day of injury. Incidence of CRPS (using the diagnostic criteria proposed by Veldman and colleagues58) at 1-year follow-up was 22% in the placebo group and 7% in the vitamin C group (95% CI for difference, 2%-26%). Complaints while wearing the cast and fracture type increased the risk for developing CRPS. This initial study was followed up by a prospective, randomized, double-blind multicenter trial by the same authors,52 who had 416 patients with 427 wrist fractures receive either placebo or vitamin C 200 mg/d, 500 mg/d, or 1500 mg/d for 50 days. This follow-up study included both operative (11%) and nonoperative (89%) distal radius fractures. Incidence of CRPS was 10.1% in the placebo group and 2.4% in the vitamin C group (P < .002). Although there was an appreciable drop in the relative risk (RR) of developing CRPS between the vitamin C 200-mg/d and 500-mg/d groups (0.41-0.17), there was no additional benefit in the 1500-mg/d group. Pooling the data for these 2 randomized trials showed that the overall RR for developing CRPS was lower with vitamin C supplementation (RR, 0.28; 95% CI, 0.14-0.56; P = .0003).59
Results of the 2 trials by Zollinger and colleagues51,52 have been met with several concerns.60-62 As a corollary to the unclear etiology of CRPS, several different sets of diagnostic criteria exist, and the criteria are somewhat subjective and imprecise. Although both trials used the Veldman criteria,58 the incidence of CRPS in the placebo group dropped unexpectedly between trials, from 22% to 10.1%, and the results may have been different had other criteria been used. Moreover, the idea that toxic oxygen radicals have a role in CRPS and that vitamin C can scavenge these radicals is based on limited data.61 In the absence of a clear pathophysiologic explanation, some surgeons have been reluctant to treat patients with vitamin C supplementation.
Cazeneuve and colleagues53 also studied the effect of vitamin C supplementation on CRPS in patients with distal radius fractures treated with reduction and intrafocal pinning. Group 1 consisted of 100 patients (treated from 1995 to 1998) who did not receive vitamin C supplementation, and group 2 consisted of 95 patients (treated from 1998 to 2002) who received vitamin C 1000 mg/d for 45 days starting on day of fracture. Patients were followed for up to 90 days after surgery. Incidence of CRPS type 1 was 10% in the untreated group and 2.1% in the group that received vitamin C supplementation.
Vitamin C prophylaxis for CRPS has also been studied in foot and ankle surgery. Besse and colleagues54 prospectively compared 2 chronologically successive groups that received (235 feet) or did not receive (185 feet) vitamin C 1000-mg/d supplementation for 45 days. Incidence of CRPS type 1 as diagnosed with International Association for the Study of Pain (IASP) criteria dropped from 9.6% to 1.7% with vitamin C supplementation. In a case series, Zollinger and colleagues63 examined CRPS type 1 rates after performing cementless total trapeziometacarpal semiconstrained joint prosthesis implantations for trapeziometacarpal arthritis. Forty implantations were performed in 34 patients. All patients received vitamin C 500 mg/d for CRPS prevention starting 2 days before surgery for 50 days. There were no cases of CRPS in the postoperative period, according to Veldman or IASP criteria. Although the results of the studies by Cazeneuve and colleagues53 and Besse and colleagues54 agree with those of the distal radius fracture trials by Zollinger and colleagues,51,52 the quasi-experimental design and the lack of blinding and randomization temper the conclusions that can be drawn because of the risk for significant bias.
In a recent systematic review examining the effectiveness of vitamin C supplementation in preventing CRPS in trauma and surgery in the extremities, Shibuya and colleagues64 concluded that taking at least 500 mg of vitamin C daily for 45 to 50 days after injury or surgery may help decrease the incidence of CRPS after a traumatic event.
Osteoarthritis
Damage caused by free radicals has long been thought to play an important role in osteoarthritis (OA).65-67 A cross-sectional study in knee OA found that amounts of joint fluid antioxidants were lower in patients with severe arthritis than in those with intact cartilage, further implicating free radicals in the pathophysiology of OA.68 Use of vitamin C for prophylaxis against development or progression of OA is therefore a hot research topic. Thus far, animal studies have had mixed results—several showing a chondroprotective effect of vitamin C69,70 and others finding either no effect or even a positive association with the development of arthritis.71
The literature on human subjects, chiefly observational studies, is just as controversial. Wang and colleagues40 found vitamin C intake associated with both a 50% risk reduction of bone marrow lesions on magnetic resonance imaging over a 10-year interval (OR, 0.5; 95% CI, 0.29-0.87) and inversely associated with the tibial plateau bone area. Similarly, the Clearwater Osteoarthritis Study, which followed 1023 patients (age, >40 years), showed that participants who took vitamin C supplements were 11% less likely to develop radiographic evidence of OA (RR, 0.89; 95% CI, 0.85-0.93).72 Nonetheless, other studies have failed to show such associations73 or have demonstrated the opposite effect. Chaganti and colleagues74 analyzed levels of vitamins C and E in the Multicenter Osteoarthritis Study (MOST) cohort of 3026 men and women (age, 50-79 years) and found higher vitamin levels were not protective against incidence of radiographic whole-knee OA and may even have been associated with increased risk.
Conclusion
Vitamin C is an essential micronutrient and a powerful water-soluble antioxidant in numerous biochemical pathways that influence bone health. It has been implicated in the biology of fracture healing, and vitamin C supplementation has been proposed as prophylaxis against hip fractures based on observational data. Results of 2 high-quality double-blind randomized trials support use of vitamin C as prophylaxis against CRPS in wrist fractures treated conservatively and operatively; the evidence for foot and ankle surgery is weaker. Use of vitamin C in OA prevention has tremendous potential, though animal and human study results are controversial. Heterogeneous results and lack of prospective trials preclude any recommendation at this time.
1. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5(2):66-74.
2. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377-6381.
3. Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637-640.
4. Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65-70.
5. Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993;51(11):313-326.
6. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184.
7. Szent-Györgyi A. On the function of hexuronic acid in the respiration of the cabbage leaf. J Biol Chem. 1931;90(1):385-393.
8. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1933;27(1):279-285.
9. Pauling L. Vitamin C and the Common Cold. San Francisco, CA: Freeman; 1970.
10. Spittle CR. Atherosclerosis and vitamin C. Lancet. 1971;2(7737):1280-1281.
11. Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810-816.
12. Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53(1 suppl):270S-282S.
13. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687-690.
14. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980.
15. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145-1154.
16. Roberts JM, Myatt L, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
17. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
18. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423.
19. Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357-377.
20. Carpenter KJ. The History of Scurvy and Vitamin C. New York, NY: Cambridge University Press; 1986.
21. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879-2882.
22. Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14(7):419-425.
23. Blee TH, Cogbill TH, Lambert PJ. Hemorrhage associated with vitamin C deficiency in surgical patients. Surgery. 2002;131(4):408-412.
24. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263.
25. Gan R, Eintracht S, Hoffer LJ. Vitamin C deficiency in a university teaching hospital. J Am Coll Nutr. 2008;27(3):428-433.
26. Bourne G. The effect of graded doses of vitamin C upon the regeneration of bone in guinea-pigs on a scorbutic diet. J Physiol. 1942;101(3):327-336.
27. Bourne GH. The relative importance of periosteum and endosteum in bone healing and the relationship of vitamin C to their activities. Proc R Soc Med. 1944;37(6):275-279.
28. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426-428.
29. Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309-313.
30. Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281-288.
31. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder Shionogi rats. J Bone Joint Surg Br. 2007;89(3):402-407.
32. Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597-1610.
33. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143(2):213-221.
34. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235-246.
35. Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264(29):17281-17286.
36. Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid–dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11(8):1103-1113.
37. Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. Anat Rec. 2002;268(2):93-104.
38. Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013;46(4):254-261.
39. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63(3):183-189.
40. Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66.
41. Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523-1527.
42. Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. 2003;14(5):418-428.
43. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health. 2006;15(3):295-300.
44. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington state, USA. J Epidemiol Community Health. 1997;51(5):479-485.
45. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154(5):427-433.
46. Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):581-588.
47. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14(1):129-135.
48. Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. 2006;163(1):9-17.
49. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24(6):1086-1094.
50. Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clin Proc. 2002;77(2):174-180.
51. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354(9195):2025-2028.
52. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose–response study. J Bone Joint Surg Am. 2007;89(7):1424-1431.
53. Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures [in French]. Acta Orthop Belg. 2002;68(5):481-484.
54. Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15(4):179-182.
55. Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3(1-5):13-18.
56. Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18(2):127-131.
57. Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560-566.
58. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016.
59. Zollinger PE. The administration of vitamin C in prevention of CRPS-I after distal radial fractures and hand surgery—a review of two RCTs and one observational prospective study. Open Conference Proc J. 2011;2:1-4.
60. Rogers BA, Ricketts DM. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2008;90(2):447-448.
61. Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82(6):873.
62. Frolke JP. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2007;89(11):2550-2551.
63. Zollinger PE, Unal H, Ellis ML, Tuinebreijer WE. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop J. 2010;4:62-66.
64. Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery—systematic review and meta-analysis. J Foot Ankle Surg. 2013;52(1):62-66.
65. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-567.
66. McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
67. Kaiki G, Tsuji H, Yonezawa T, et al. Osteoarthrosis induced by intra-articular hydrogen peroxide injection and running load. J Orthop Res. 1990;8(5):731-740.
68. Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515-521.
69. Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol. 1990;71(2):279-293.
70. Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10(2):119-126.
71. Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50(6):1822-1831.
72. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14(4):709-715.
73. Hill J, Bird HA. Failure of selenium-ace to improve osteoarthritis. Br J Rheumatol. 1990;29(3):211-213.
74. Chaganti RK, Tolstykh I, Javaid MK, et al; Multicenter Osteoarthritis Study Group (MOST). High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190-196.
75. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 26. http://www.ars.usda.gov/Services/docs.htm?docid=24936. Published August 2013. Revised November 2013. Accessed May 14, 2015.
76. National Institutes of Health, Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. National Institutes of Health website. http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Reviewed June 5, 2013. Accessed May 14, 2015.
77. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000.
L-ascorbic acid, more commonly know as vitamin C, is an essential micronutrient used in numerous metabolic pathways. It functions physiologically as a water-soluble antioxidant by virtue of its high reducing power, playing a key role in the function of leukocytes, protein metabolism, and production of neurotransmitters.1-3 Vitamin C also contributes to musculoskeletal health through biosynthesis of carnitine and collagen4 and enhancement of intestinal absorption of dietary iron5 from plants and vegetables. Unlike most animals, humans are unable to synthesize this essential vitamin and therefore require intake from natural dietary sources or supplements.6 The ability of vitamin C to prevent or treat disease has been an area of research interest since the vitamin was identified and isolated by Szent-Györgyi in the 1930s.7-16 Research in orthopedic surgery has focused on the effects of vitamin C on fracture healing, its potential use in preventing complex regional pain syndrome (CRPS), and its role in the pathophysiology of osteoarthritis. In this article, we review the basics of vitamin C metabolism and summarize the evidence surrounding the role of vitamin C supplementation in orthopedics.
Sources and Metabolism
Vitamin C is found naturally in many fruits and vegetables (Table 1) and is a common fortification in cereals, juices, and multivitamins. Daily recommended intake (Table 2) depends on age and smoking status. Absorption occurs in the distal small intestine, with blood plasma vitamin C concentrations reflecting dietary intake. Pharmacokinetic studies have shown that vitamin C concentrations are tightly regulated through absorption, tissue accumulation, and renal resorption, with plasma concentrations rarely exceeding 100 μmol/L without additional supplementation.17 Although the usual dietary doses of 100 mg/d (adult) are almost completely absorbed, producing a plasma concentration of 60 μmol/L, higher intake results in an increasingly smaller fraction absorbed.1,18 Intake of more than 1000 mg/d results in less than 50% absorption19 (unmetabolized vitamin C is excreted in stool and urine1). Even at higher doses, vitamin C has low toxicity3; the most common complaints are diarrhea, nausea, and abdominal cramps caused by the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract.1
Vitamin C Deficiency
The relationship between vitamin C deficiency and the development of scurvy has been documented for centuries. Symptoms are described in the ancient Egyptian, Greek, and Roman literature.20 Ascorbic acid is essential for normal collagen function, as it is a required cofactor for enzymatic transfer of hydroxyl groups to select proline and lysine residues during procollagen formation. Hydroxylysine contributes to the intermolecular cross-links in collagen, and hydroxyproline stabilizes the triple-helix structure of collagen.21 Insufficient vitamin C during this process results in collagen that is non-cross-linked, nonhelical, structurally unstable, and weak.21 Clinical manifestations of scurvy stem from an underlying impairment of collagen production causing a systemic decrease in connective tissue integrity, capillary fragility, poor wound healing, fatigue, myalgias, arthritis, and even death.22 Vitamin C deficiency has also been implicated as a cause of diffuse bleeding in surgical patients with normal coagulation parameters secondary to capillary fragility.23 In the United States, the 2003–2004 National Health and Nutrition Examination Survey (NHANES) measured serum vitamin C concentrations in 7277 noninstitutionalized patients 6 years old or older.24 Age-adjusted incidence of subnormal serum vitamin C levels (<28 μmol/L) was 19.6%, and incidence of frank vitamin C deficiency (<11.4 μmol/L) was 7.1%. Reported rates of vitamin C deficiency in hospitalized patients are much higher, with 47% to 60% having subnormal values (<28 μmol/L) and 17% to 19% being vitamin C–deficient (<11.4 μmol/L).22,25 Identified risk factors for hypovitaminosis C include advanced age, obesity, low socioeconomic status, unemployment, male sex, and concomitant alcohol and tobacco consumption.22,24,25
Fracture Healing and Prevention
The effects of vitamin C deficiency on bone healing have been studied with animal models as early as the 1940s.26,27 Early experiments using guinea pigs demonstrated failure of bone graft incorporation, delayed collagen maturation, and decreased collagen and callus formation in scorbutic animals compared with controls that received vitamin C supplementation.26,27 Based on his work with guinea pigs, Bourne26 reported in 1942 that vitamin C deficiency significantly inhibited the reparative process in damaged bone and that patients with fractures should receive vitamin C supplementation. Building on this early research, Yilmaz and colleagues28 found faster histologic healing for tibia fractures in a rat model for animals that received a single injection of vitamin C 0.5 mg/kg compared with a nonscorbutic control group, and Sarisözen and colleagues29 showed significantly accelerated histologic bone formation and mineralization at the fracture site for rats that received vitamin C supplementation. Moreover, Kipp and colleagues30 found that scorbutic guinea pigs had lower bone mineral density (BMD), decreased bone mineral content, and impaired collagen synthesis of articular cartilage and tendons compared with nondeficient controls.
Besides promoting bone formation, vitamin C improves the mechanical strength of callus formation. Alcantara-Martos and colleagues31 used an osteogenic disorder Shionogi (ODS) rat model to examine the effects of vitamin C intake on femoral fracture healing. This particular animal model is unable to produce its own vitamin C. The groups with lower serum vitamin C levels demonstrated lower mechanical resistance of the fracture callus to torsional loads 5 weeks after fracture. Moreover, the group that received vitamin C supplementation showed higher histologic grade of callus formation and demonstrated faster healing rates. The authors suggested that subclinical vitamin C deficiency can delay fracture healing and that vitamin C supplementation in nondeficient patients would improve bone healing.
Other research has demonstrated a link between vitamin C and mesenchymal cell differentiation. Mohan and colleagues32 used an sfx mouse model to show that vitamin C deficiency results in decreased bone formation secondary to impaired osteoblast differentiation, diminished bone density, and development of spontaneous fractures. The authors indicated that not only is vitamin C essential for maintenance of differentiated functions of osteoblasts, but deficiency during early active growth may affect peak BMD levels in humans. Additional studies have demonstrated the role of vitamin C in endochondral bone formation through both induction of osteoblast differentiation and modulation of gene expression in hypertrophic chondrocytes.33-36 Chronic vitamin C deficiency has been found to depress osteoblast function and differentiation of chondrocytes.37 More recently, Kim and colleagues38 examined the effect of vitamin C insufficiency in Gulo-deficient mice, which are unable to synthesize ascorbic acid. Ascorbic acid insufficiency over 4 weeks led to decreased plasma levels of osteocalcin and bone formation in vivo as well as significantly diminished metaphyseal trabecular bone. Despite all the evidence demonstrating the importance of vitamin C in bone formation and maintenance, many of the underlying processes in this relationship have yet to be determined.
Bone Mineral Density
Several observational studies have found a positive association between vitamin C intake and BMD in postmenopausal women. In a retrospective, cross-sectional study by Hall and Greendale,39 a positive association was found between vitamin C intake and BMD of the femoral neck in 775 participants in the Postmenopausal Estrogen/Progestin Interventions trial. After calcium intake, physical activity level, smoking, estrogen use, age, and body mass index were adjusted for, each 100-mg increase in dietary vitamin C was associated with a 0.017 g/cm2 increase in BMD. Wang and colleagues40 found a positive association between dietary vitamin C intake and femoral neck BMD in a retrospective analysis of 125 postmenopausal Mexican American women. Other observational studies have reported that decreased intake of vitamin C is associated with osteoporosis41 and increased rates of BMD loss42 and that supplementation with vitamin C may suppress bone resorption in postmenopausal women.43
The results of these studies contrast with the findings of Leveille and colleagues,44 who examined the relationship between dietary vitamin C and hip BMD in 1892 postmenopausal women. Although the authors found that women (age, 55-64 years) using vitamin C supplements for more than 10 years had an average BMD 6.7% higher than that of nonusers, they did not find any association between dietary vitamin C intake and BMD. Moreover, NHANES III also found inconsistent associations between vitamin C and BMD among 13,080 adults surveyed in the United States.45 Although for premenopausal women dietary ascorbic acid was associated with increased BMD, for postmenopausal women with a history of smoking and estrogen replacement, it was actually associated with lower BMD values. For other subgroups in the study, the relationship was also inconsistent or nonlinear.
The exact mechanism by which ascorbic acid contributes to BMD is not fully delineated. However, it likely is related to the known role of vitamin C in collagen formation, bone matrix development, osteoblast differentiation, and its antioxidant effects limiting bone resorption.44,46
Hip Fractures
Besides demonstrating positive effects of vitamin C on bone healing and BMD, epidemiologic studies have found evidence of a protective effect of vitamin C on hip fracture risk. In a study of the Swedish Mammography cohort, 66,651 women (age, 40-76 years) were prospectively followed.47 The authors found that the odds ratio (OR) for hip fractures among smokers with a low intake of vitamin E (median intake, ≤6.2 mg/d) was 3.0 (95% CI, 1.6-5.4) and for vitamin C (median intake, ≤67 mg/d) was 3.0 (95% CI, 1.6-5.6). Moreover, in smokers with a low intake of both vitamins E and C, OR increased to 4.9 (95% CI, 2.2-11.0). In addition, the Utah Study of Nutrition and Bone Health matched 1215 cases of hip fractures in patients who had ever smoked (age, >50 years) with 1349 controls and found that vitamin C intake above 159 mg/d had a significant protective effect on the incidence of hip fracture; however, a graded relationship was not observed.48 Despite the inconsistencies in the NHANES III study regarding the relationship between vitamin C and BMD, Simon and Hudes45 found that serum vitamin C was associated with lower risk for self-reported fracture in postmenopausal women who had ever smoked and had a history of estrogen therapy (OR, 0.51; 95% CI, 0.36-0.70). Finally, Sahni and colleagues49 followed 958 Framingham cohort men and women (mean age, 75 years) over 17 years and found that those in the highest tertile of total vitamin C intake (median, 313 mg/d) had significantly fewer hip fractures and nonvertebral fractures compared with those in the lowest tertile of intake (median, 94 mg/d). Dietary vitamin C intake was not associated with fracture risk in this study.
Complex Regional Pain Syndrome
Type 1 CRPS is a debilitating condition characterized by severe pain, swelling, and vasomotor instability. It is commonly precipitated by an injury or surgery to an extremity and is a dreaded sequelae in orthopedics,50 with incidence rates of 10% to 22% in wrist fractures51-53 and 10% after foot and ankle surgery.54 Although the pathophysiology of CRPS remains unknown, dysregulation and increased permeability of the vasculature caused by free radicals are thought to play an important role.55 In dermal burns, high doses of vitamin C therapy slowed progression of vascular permeability and therefore reduced extravascular leakage of fluids and protein.56,57 The ability of vitamin C to prevent CRPS has been studied in only a handful of trials.
In a double-blind trial, Zollinger and colleagues51 randomized 127 conservatively treated distal radius fractures to receive either vitamin C 500 mg or placebo daily for 50 days starting on day of injury. Incidence of CRPS (using the diagnostic criteria proposed by Veldman and colleagues58) at 1-year follow-up was 22% in the placebo group and 7% in the vitamin C group (95% CI for difference, 2%-26%). Complaints while wearing the cast and fracture type increased the risk for developing CRPS. This initial study was followed up by a prospective, randomized, double-blind multicenter trial by the same authors,52 who had 416 patients with 427 wrist fractures receive either placebo or vitamin C 200 mg/d, 500 mg/d, or 1500 mg/d for 50 days. This follow-up study included both operative (11%) and nonoperative (89%) distal radius fractures. Incidence of CRPS was 10.1% in the placebo group and 2.4% in the vitamin C group (P < .002). Although there was an appreciable drop in the relative risk (RR) of developing CRPS between the vitamin C 200-mg/d and 500-mg/d groups (0.41-0.17), there was no additional benefit in the 1500-mg/d group. Pooling the data for these 2 randomized trials showed that the overall RR for developing CRPS was lower with vitamin C supplementation (RR, 0.28; 95% CI, 0.14-0.56; P = .0003).59
Results of the 2 trials by Zollinger and colleagues51,52 have been met with several concerns.60-62 As a corollary to the unclear etiology of CRPS, several different sets of diagnostic criteria exist, and the criteria are somewhat subjective and imprecise. Although both trials used the Veldman criteria,58 the incidence of CRPS in the placebo group dropped unexpectedly between trials, from 22% to 10.1%, and the results may have been different had other criteria been used. Moreover, the idea that toxic oxygen radicals have a role in CRPS and that vitamin C can scavenge these radicals is based on limited data.61 In the absence of a clear pathophysiologic explanation, some surgeons have been reluctant to treat patients with vitamin C supplementation.
Cazeneuve and colleagues53 also studied the effect of vitamin C supplementation on CRPS in patients with distal radius fractures treated with reduction and intrafocal pinning. Group 1 consisted of 100 patients (treated from 1995 to 1998) who did not receive vitamin C supplementation, and group 2 consisted of 95 patients (treated from 1998 to 2002) who received vitamin C 1000 mg/d for 45 days starting on day of fracture. Patients were followed for up to 90 days after surgery. Incidence of CRPS type 1 was 10% in the untreated group and 2.1% in the group that received vitamin C supplementation.
Vitamin C prophylaxis for CRPS has also been studied in foot and ankle surgery. Besse and colleagues54 prospectively compared 2 chronologically successive groups that received (235 feet) or did not receive (185 feet) vitamin C 1000-mg/d supplementation for 45 days. Incidence of CRPS type 1 as diagnosed with International Association for the Study of Pain (IASP) criteria dropped from 9.6% to 1.7% with vitamin C supplementation. In a case series, Zollinger and colleagues63 examined CRPS type 1 rates after performing cementless total trapeziometacarpal semiconstrained joint prosthesis implantations for trapeziometacarpal arthritis. Forty implantations were performed in 34 patients. All patients received vitamin C 500 mg/d for CRPS prevention starting 2 days before surgery for 50 days. There were no cases of CRPS in the postoperative period, according to Veldman or IASP criteria. Although the results of the studies by Cazeneuve and colleagues53 and Besse and colleagues54 agree with those of the distal radius fracture trials by Zollinger and colleagues,51,52 the quasi-experimental design and the lack of blinding and randomization temper the conclusions that can be drawn because of the risk for significant bias.
In a recent systematic review examining the effectiveness of vitamin C supplementation in preventing CRPS in trauma and surgery in the extremities, Shibuya and colleagues64 concluded that taking at least 500 mg of vitamin C daily for 45 to 50 days after injury or surgery may help decrease the incidence of CRPS after a traumatic event.
Osteoarthritis
Damage caused by free radicals has long been thought to play an important role in osteoarthritis (OA).65-67 A cross-sectional study in knee OA found that amounts of joint fluid antioxidants were lower in patients with severe arthritis than in those with intact cartilage, further implicating free radicals in the pathophysiology of OA.68 Use of vitamin C for prophylaxis against development or progression of OA is therefore a hot research topic. Thus far, animal studies have had mixed results—several showing a chondroprotective effect of vitamin C69,70 and others finding either no effect or even a positive association with the development of arthritis.71
The literature on human subjects, chiefly observational studies, is just as controversial. Wang and colleagues40 found vitamin C intake associated with both a 50% risk reduction of bone marrow lesions on magnetic resonance imaging over a 10-year interval (OR, 0.5; 95% CI, 0.29-0.87) and inversely associated with the tibial plateau bone area. Similarly, the Clearwater Osteoarthritis Study, which followed 1023 patients (age, >40 years), showed that participants who took vitamin C supplements were 11% less likely to develop radiographic evidence of OA (RR, 0.89; 95% CI, 0.85-0.93).72 Nonetheless, other studies have failed to show such associations73 or have demonstrated the opposite effect. Chaganti and colleagues74 analyzed levels of vitamins C and E in the Multicenter Osteoarthritis Study (MOST) cohort of 3026 men and women (age, 50-79 years) and found higher vitamin levels were not protective against incidence of radiographic whole-knee OA and may even have been associated with increased risk.
Conclusion
Vitamin C is an essential micronutrient and a powerful water-soluble antioxidant in numerous biochemical pathways that influence bone health. It has been implicated in the biology of fracture healing, and vitamin C supplementation has been proposed as prophylaxis against hip fractures based on observational data. Results of 2 high-quality double-blind randomized trials support use of vitamin C as prophylaxis against CRPS in wrist fractures treated conservatively and operatively; the evidence for foot and ankle surgery is weaker. Use of vitamin C in OA prevention has tremendous potential, though animal and human study results are controversial. Heterogeneous results and lack of prospective trials preclude any recommendation at this time.
L-ascorbic acid, more commonly know as vitamin C, is an essential micronutrient used in numerous metabolic pathways. It functions physiologically as a water-soluble antioxidant by virtue of its high reducing power, playing a key role in the function of leukocytes, protein metabolism, and production of neurotransmitters.1-3 Vitamin C also contributes to musculoskeletal health through biosynthesis of carnitine and collagen4 and enhancement of intestinal absorption of dietary iron5 from plants and vegetables. Unlike most animals, humans are unable to synthesize this essential vitamin and therefore require intake from natural dietary sources or supplements.6 The ability of vitamin C to prevent or treat disease has been an area of research interest since the vitamin was identified and isolated by Szent-Györgyi in the 1930s.7-16 Research in orthopedic surgery has focused on the effects of vitamin C on fracture healing, its potential use in preventing complex regional pain syndrome (CRPS), and its role in the pathophysiology of osteoarthritis. In this article, we review the basics of vitamin C metabolism and summarize the evidence surrounding the role of vitamin C supplementation in orthopedics.
Sources and Metabolism
Vitamin C is found naturally in many fruits and vegetables (Table 1) and is a common fortification in cereals, juices, and multivitamins. Daily recommended intake (Table 2) depends on age and smoking status. Absorption occurs in the distal small intestine, with blood plasma vitamin C concentrations reflecting dietary intake. Pharmacokinetic studies have shown that vitamin C concentrations are tightly regulated through absorption, tissue accumulation, and renal resorption, with plasma concentrations rarely exceeding 100 μmol/L without additional supplementation.17 Although the usual dietary doses of 100 mg/d (adult) are almost completely absorbed, producing a plasma concentration of 60 μmol/L, higher intake results in an increasingly smaller fraction absorbed.1,18 Intake of more than 1000 mg/d results in less than 50% absorption19 (unmetabolized vitamin C is excreted in stool and urine1). Even at higher doses, vitamin C has low toxicity3; the most common complaints are diarrhea, nausea, and abdominal cramps caused by the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract.1
Vitamin C Deficiency
The relationship between vitamin C deficiency and the development of scurvy has been documented for centuries. Symptoms are described in the ancient Egyptian, Greek, and Roman literature.20 Ascorbic acid is essential for normal collagen function, as it is a required cofactor for enzymatic transfer of hydroxyl groups to select proline and lysine residues during procollagen formation. Hydroxylysine contributes to the intermolecular cross-links in collagen, and hydroxyproline stabilizes the triple-helix structure of collagen.21 Insufficient vitamin C during this process results in collagen that is non-cross-linked, nonhelical, structurally unstable, and weak.21 Clinical manifestations of scurvy stem from an underlying impairment of collagen production causing a systemic decrease in connective tissue integrity, capillary fragility, poor wound healing, fatigue, myalgias, arthritis, and even death.22 Vitamin C deficiency has also been implicated as a cause of diffuse bleeding in surgical patients with normal coagulation parameters secondary to capillary fragility.23 In the United States, the 2003–2004 National Health and Nutrition Examination Survey (NHANES) measured serum vitamin C concentrations in 7277 noninstitutionalized patients 6 years old or older.24 Age-adjusted incidence of subnormal serum vitamin C levels (<28 μmol/L) was 19.6%, and incidence of frank vitamin C deficiency (<11.4 μmol/L) was 7.1%. Reported rates of vitamin C deficiency in hospitalized patients are much higher, with 47% to 60% having subnormal values (<28 μmol/L) and 17% to 19% being vitamin C–deficient (<11.4 μmol/L).22,25 Identified risk factors for hypovitaminosis C include advanced age, obesity, low socioeconomic status, unemployment, male sex, and concomitant alcohol and tobacco consumption.22,24,25
Fracture Healing and Prevention
The effects of vitamin C deficiency on bone healing have been studied with animal models as early as the 1940s.26,27 Early experiments using guinea pigs demonstrated failure of bone graft incorporation, delayed collagen maturation, and decreased collagen and callus formation in scorbutic animals compared with controls that received vitamin C supplementation.26,27 Based on his work with guinea pigs, Bourne26 reported in 1942 that vitamin C deficiency significantly inhibited the reparative process in damaged bone and that patients with fractures should receive vitamin C supplementation. Building on this early research, Yilmaz and colleagues28 found faster histologic healing for tibia fractures in a rat model for animals that received a single injection of vitamin C 0.5 mg/kg compared with a nonscorbutic control group, and Sarisözen and colleagues29 showed significantly accelerated histologic bone formation and mineralization at the fracture site for rats that received vitamin C supplementation. Moreover, Kipp and colleagues30 found that scorbutic guinea pigs had lower bone mineral density (BMD), decreased bone mineral content, and impaired collagen synthesis of articular cartilage and tendons compared with nondeficient controls.
Besides promoting bone formation, vitamin C improves the mechanical strength of callus formation. Alcantara-Martos and colleagues31 used an osteogenic disorder Shionogi (ODS) rat model to examine the effects of vitamin C intake on femoral fracture healing. This particular animal model is unable to produce its own vitamin C. The groups with lower serum vitamin C levels demonstrated lower mechanical resistance of the fracture callus to torsional loads 5 weeks after fracture. Moreover, the group that received vitamin C supplementation showed higher histologic grade of callus formation and demonstrated faster healing rates. The authors suggested that subclinical vitamin C deficiency can delay fracture healing and that vitamin C supplementation in nondeficient patients would improve bone healing.
Other research has demonstrated a link between vitamin C and mesenchymal cell differentiation. Mohan and colleagues32 used an sfx mouse model to show that vitamin C deficiency results in decreased bone formation secondary to impaired osteoblast differentiation, diminished bone density, and development of spontaneous fractures. The authors indicated that not only is vitamin C essential for maintenance of differentiated functions of osteoblasts, but deficiency during early active growth may affect peak BMD levels in humans. Additional studies have demonstrated the role of vitamin C in endochondral bone formation through both induction of osteoblast differentiation and modulation of gene expression in hypertrophic chondrocytes.33-36 Chronic vitamin C deficiency has been found to depress osteoblast function and differentiation of chondrocytes.37 More recently, Kim and colleagues38 examined the effect of vitamin C insufficiency in Gulo-deficient mice, which are unable to synthesize ascorbic acid. Ascorbic acid insufficiency over 4 weeks led to decreased plasma levels of osteocalcin and bone formation in vivo as well as significantly diminished metaphyseal trabecular bone. Despite all the evidence demonstrating the importance of vitamin C in bone formation and maintenance, many of the underlying processes in this relationship have yet to be determined.
Bone Mineral Density
Several observational studies have found a positive association between vitamin C intake and BMD in postmenopausal women. In a retrospective, cross-sectional study by Hall and Greendale,39 a positive association was found between vitamin C intake and BMD of the femoral neck in 775 participants in the Postmenopausal Estrogen/Progestin Interventions trial. After calcium intake, physical activity level, smoking, estrogen use, age, and body mass index were adjusted for, each 100-mg increase in dietary vitamin C was associated with a 0.017 g/cm2 increase in BMD. Wang and colleagues40 found a positive association between dietary vitamin C intake and femoral neck BMD in a retrospective analysis of 125 postmenopausal Mexican American women. Other observational studies have reported that decreased intake of vitamin C is associated with osteoporosis41 and increased rates of BMD loss42 and that supplementation with vitamin C may suppress bone resorption in postmenopausal women.43
The results of these studies contrast with the findings of Leveille and colleagues,44 who examined the relationship between dietary vitamin C and hip BMD in 1892 postmenopausal women. Although the authors found that women (age, 55-64 years) using vitamin C supplements for more than 10 years had an average BMD 6.7% higher than that of nonusers, they did not find any association between dietary vitamin C intake and BMD. Moreover, NHANES III also found inconsistent associations between vitamin C and BMD among 13,080 adults surveyed in the United States.45 Although for premenopausal women dietary ascorbic acid was associated with increased BMD, for postmenopausal women with a history of smoking and estrogen replacement, it was actually associated with lower BMD values. For other subgroups in the study, the relationship was also inconsistent or nonlinear.
The exact mechanism by which ascorbic acid contributes to BMD is not fully delineated. However, it likely is related to the known role of vitamin C in collagen formation, bone matrix development, osteoblast differentiation, and its antioxidant effects limiting bone resorption.44,46
Hip Fractures
Besides demonstrating positive effects of vitamin C on bone healing and BMD, epidemiologic studies have found evidence of a protective effect of vitamin C on hip fracture risk. In a study of the Swedish Mammography cohort, 66,651 women (age, 40-76 years) were prospectively followed.47 The authors found that the odds ratio (OR) for hip fractures among smokers with a low intake of vitamin E (median intake, ≤6.2 mg/d) was 3.0 (95% CI, 1.6-5.4) and for vitamin C (median intake, ≤67 mg/d) was 3.0 (95% CI, 1.6-5.6). Moreover, in smokers with a low intake of both vitamins E and C, OR increased to 4.9 (95% CI, 2.2-11.0). In addition, the Utah Study of Nutrition and Bone Health matched 1215 cases of hip fractures in patients who had ever smoked (age, >50 years) with 1349 controls and found that vitamin C intake above 159 mg/d had a significant protective effect on the incidence of hip fracture; however, a graded relationship was not observed.48 Despite the inconsistencies in the NHANES III study regarding the relationship between vitamin C and BMD, Simon and Hudes45 found that serum vitamin C was associated with lower risk for self-reported fracture in postmenopausal women who had ever smoked and had a history of estrogen therapy (OR, 0.51; 95% CI, 0.36-0.70). Finally, Sahni and colleagues49 followed 958 Framingham cohort men and women (mean age, 75 years) over 17 years and found that those in the highest tertile of total vitamin C intake (median, 313 mg/d) had significantly fewer hip fractures and nonvertebral fractures compared with those in the lowest tertile of intake (median, 94 mg/d). Dietary vitamin C intake was not associated with fracture risk in this study.
Complex Regional Pain Syndrome
Type 1 CRPS is a debilitating condition characterized by severe pain, swelling, and vasomotor instability. It is commonly precipitated by an injury or surgery to an extremity and is a dreaded sequelae in orthopedics,50 with incidence rates of 10% to 22% in wrist fractures51-53 and 10% after foot and ankle surgery.54 Although the pathophysiology of CRPS remains unknown, dysregulation and increased permeability of the vasculature caused by free radicals are thought to play an important role.55 In dermal burns, high doses of vitamin C therapy slowed progression of vascular permeability and therefore reduced extravascular leakage of fluids and protein.56,57 The ability of vitamin C to prevent CRPS has been studied in only a handful of trials.
In a double-blind trial, Zollinger and colleagues51 randomized 127 conservatively treated distal radius fractures to receive either vitamin C 500 mg or placebo daily for 50 days starting on day of injury. Incidence of CRPS (using the diagnostic criteria proposed by Veldman and colleagues58) at 1-year follow-up was 22% in the placebo group and 7% in the vitamin C group (95% CI for difference, 2%-26%). Complaints while wearing the cast and fracture type increased the risk for developing CRPS. This initial study was followed up by a prospective, randomized, double-blind multicenter trial by the same authors,52 who had 416 patients with 427 wrist fractures receive either placebo or vitamin C 200 mg/d, 500 mg/d, or 1500 mg/d for 50 days. This follow-up study included both operative (11%) and nonoperative (89%) distal radius fractures. Incidence of CRPS was 10.1% in the placebo group and 2.4% in the vitamin C group (P < .002). Although there was an appreciable drop in the relative risk (RR) of developing CRPS between the vitamin C 200-mg/d and 500-mg/d groups (0.41-0.17), there was no additional benefit in the 1500-mg/d group. Pooling the data for these 2 randomized trials showed that the overall RR for developing CRPS was lower with vitamin C supplementation (RR, 0.28; 95% CI, 0.14-0.56; P = .0003).59
Results of the 2 trials by Zollinger and colleagues51,52 have been met with several concerns.60-62 As a corollary to the unclear etiology of CRPS, several different sets of diagnostic criteria exist, and the criteria are somewhat subjective and imprecise. Although both trials used the Veldman criteria,58 the incidence of CRPS in the placebo group dropped unexpectedly between trials, from 22% to 10.1%, and the results may have been different had other criteria been used. Moreover, the idea that toxic oxygen radicals have a role in CRPS and that vitamin C can scavenge these radicals is based on limited data.61 In the absence of a clear pathophysiologic explanation, some surgeons have been reluctant to treat patients with vitamin C supplementation.
Cazeneuve and colleagues53 also studied the effect of vitamin C supplementation on CRPS in patients with distal radius fractures treated with reduction and intrafocal pinning. Group 1 consisted of 100 patients (treated from 1995 to 1998) who did not receive vitamin C supplementation, and group 2 consisted of 95 patients (treated from 1998 to 2002) who received vitamin C 1000 mg/d for 45 days starting on day of fracture. Patients were followed for up to 90 days after surgery. Incidence of CRPS type 1 was 10% in the untreated group and 2.1% in the group that received vitamin C supplementation.
Vitamin C prophylaxis for CRPS has also been studied in foot and ankle surgery. Besse and colleagues54 prospectively compared 2 chronologically successive groups that received (235 feet) or did not receive (185 feet) vitamin C 1000-mg/d supplementation for 45 days. Incidence of CRPS type 1 as diagnosed with International Association for the Study of Pain (IASP) criteria dropped from 9.6% to 1.7% with vitamin C supplementation. In a case series, Zollinger and colleagues63 examined CRPS type 1 rates after performing cementless total trapeziometacarpal semiconstrained joint prosthesis implantations for trapeziometacarpal arthritis. Forty implantations were performed in 34 patients. All patients received vitamin C 500 mg/d for CRPS prevention starting 2 days before surgery for 50 days. There were no cases of CRPS in the postoperative period, according to Veldman or IASP criteria. Although the results of the studies by Cazeneuve and colleagues53 and Besse and colleagues54 agree with those of the distal radius fracture trials by Zollinger and colleagues,51,52 the quasi-experimental design and the lack of blinding and randomization temper the conclusions that can be drawn because of the risk for significant bias.
In a recent systematic review examining the effectiveness of vitamin C supplementation in preventing CRPS in trauma and surgery in the extremities, Shibuya and colleagues64 concluded that taking at least 500 mg of vitamin C daily for 45 to 50 days after injury or surgery may help decrease the incidence of CRPS after a traumatic event.
Osteoarthritis
Damage caused by free radicals has long been thought to play an important role in osteoarthritis (OA).65-67 A cross-sectional study in knee OA found that amounts of joint fluid antioxidants were lower in patients with severe arthritis than in those with intact cartilage, further implicating free radicals in the pathophysiology of OA.68 Use of vitamin C for prophylaxis against development or progression of OA is therefore a hot research topic. Thus far, animal studies have had mixed results—several showing a chondroprotective effect of vitamin C69,70 and others finding either no effect or even a positive association with the development of arthritis.71
The literature on human subjects, chiefly observational studies, is just as controversial. Wang and colleagues40 found vitamin C intake associated with both a 50% risk reduction of bone marrow lesions on magnetic resonance imaging over a 10-year interval (OR, 0.5; 95% CI, 0.29-0.87) and inversely associated with the tibial plateau bone area. Similarly, the Clearwater Osteoarthritis Study, which followed 1023 patients (age, >40 years), showed that participants who took vitamin C supplements were 11% less likely to develop radiographic evidence of OA (RR, 0.89; 95% CI, 0.85-0.93).72 Nonetheless, other studies have failed to show such associations73 or have demonstrated the opposite effect. Chaganti and colleagues74 analyzed levels of vitamins C and E in the Multicenter Osteoarthritis Study (MOST) cohort of 3026 men and women (age, 50-79 years) and found higher vitamin levels were not protective against incidence of radiographic whole-knee OA and may even have been associated with increased risk.
Conclusion
Vitamin C is an essential micronutrient and a powerful water-soluble antioxidant in numerous biochemical pathways that influence bone health. It has been implicated in the biology of fracture healing, and vitamin C supplementation has been proposed as prophylaxis against hip fractures based on observational data. Results of 2 high-quality double-blind randomized trials support use of vitamin C as prophylaxis against CRPS in wrist fractures treated conservatively and operatively; the evidence for foot and ankle surgery is weaker. Use of vitamin C in OA prevention has tremendous potential, though animal and human study results are controversial. Heterogeneous results and lack of prospective trials preclude any recommendation at this time.
1. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5(2):66-74.
2. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377-6381.
3. Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637-640.
4. Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65-70.
5. Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993;51(11):313-326.
6. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184.
7. Szent-Györgyi A. On the function of hexuronic acid in the respiration of the cabbage leaf. J Biol Chem. 1931;90(1):385-393.
8. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1933;27(1):279-285.
9. Pauling L. Vitamin C and the Common Cold. San Francisco, CA: Freeman; 1970.
10. Spittle CR. Atherosclerosis and vitamin C. Lancet. 1971;2(7737):1280-1281.
11. Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810-816.
12. Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53(1 suppl):270S-282S.
13. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687-690.
14. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980.
15. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145-1154.
16. Roberts JM, Myatt L, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
17. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
18. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423.
19. Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357-377.
20. Carpenter KJ. The History of Scurvy and Vitamin C. New York, NY: Cambridge University Press; 1986.
21. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879-2882.
22. Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14(7):419-425.
23. Blee TH, Cogbill TH, Lambert PJ. Hemorrhage associated with vitamin C deficiency in surgical patients. Surgery. 2002;131(4):408-412.
24. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263.
25. Gan R, Eintracht S, Hoffer LJ. Vitamin C deficiency in a university teaching hospital. J Am Coll Nutr. 2008;27(3):428-433.
26. Bourne G. The effect of graded doses of vitamin C upon the regeneration of bone in guinea-pigs on a scorbutic diet. J Physiol. 1942;101(3):327-336.
27. Bourne GH. The relative importance of periosteum and endosteum in bone healing and the relationship of vitamin C to their activities. Proc R Soc Med. 1944;37(6):275-279.
28. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426-428.
29. Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309-313.
30. Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281-288.
31. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder Shionogi rats. J Bone Joint Surg Br. 2007;89(3):402-407.
32. Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597-1610.
33. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143(2):213-221.
34. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235-246.
35. Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264(29):17281-17286.
36. Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid–dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11(8):1103-1113.
37. Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. Anat Rec. 2002;268(2):93-104.
38. Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013;46(4):254-261.
39. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63(3):183-189.
40. Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66.
41. Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523-1527.
42. Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. 2003;14(5):418-428.
43. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health. 2006;15(3):295-300.
44. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington state, USA. J Epidemiol Community Health. 1997;51(5):479-485.
45. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154(5):427-433.
46. Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):581-588.
47. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14(1):129-135.
48. Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. 2006;163(1):9-17.
49. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24(6):1086-1094.
50. Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clin Proc. 2002;77(2):174-180.
51. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354(9195):2025-2028.
52. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose–response study. J Bone Joint Surg Am. 2007;89(7):1424-1431.
53. Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures [in French]. Acta Orthop Belg. 2002;68(5):481-484.
54. Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15(4):179-182.
55. Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3(1-5):13-18.
56. Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18(2):127-131.
57. Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560-566.
58. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016.
59. Zollinger PE. The administration of vitamin C in prevention of CRPS-I after distal radial fractures and hand surgery—a review of two RCTs and one observational prospective study. Open Conference Proc J. 2011;2:1-4.
60. Rogers BA, Ricketts DM. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2008;90(2):447-448.
61. Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82(6):873.
62. Frolke JP. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2007;89(11):2550-2551.
63. Zollinger PE, Unal H, Ellis ML, Tuinebreijer WE. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop J. 2010;4:62-66.
64. Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery—systematic review and meta-analysis. J Foot Ankle Surg. 2013;52(1):62-66.
65. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-567.
66. McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
67. Kaiki G, Tsuji H, Yonezawa T, et al. Osteoarthrosis induced by intra-articular hydrogen peroxide injection and running load. J Orthop Res. 1990;8(5):731-740.
68. Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515-521.
69. Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol. 1990;71(2):279-293.
70. Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10(2):119-126.
71. Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50(6):1822-1831.
72. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14(4):709-715.
73. Hill J, Bird HA. Failure of selenium-ace to improve osteoarthritis. Br J Rheumatol. 1990;29(3):211-213.
74. Chaganti RK, Tolstykh I, Javaid MK, et al; Multicenter Osteoarthritis Study Group (MOST). High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190-196.
75. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 26. http://www.ars.usda.gov/Services/docs.htm?docid=24936. Published August 2013. Revised November 2013. Accessed May 14, 2015.
76. National Institutes of Health, Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. National Institutes of Health website. http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Reviewed June 5, 2013. Accessed May 14, 2015.
77. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000.
1. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5(2):66-74.
2. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377-6381.
3. Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637-640.
4. Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65-70.
5. Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993;51(11):313-326.
6. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184.
7. Szent-Györgyi A. On the function of hexuronic acid in the respiration of the cabbage leaf. J Biol Chem. 1931;90(1):385-393.
8. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1933;27(1):279-285.
9. Pauling L. Vitamin C and the Common Cold. San Francisco, CA: Freeman; 1970.
10. Spittle CR. Atherosclerosis and vitamin C. Lancet. 1971;2(7737):1280-1281.
11. Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810-816.
12. Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53(1 suppl):270S-282S.
13. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687-690.
14. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980.
15. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145-1154.
16. Roberts JM, Myatt L, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
17. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
18. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423.
19. Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357-377.
20. Carpenter KJ. The History of Scurvy and Vitamin C. New York, NY: Cambridge University Press; 1986.
21. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879-2882.
22. Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14(7):419-425.
23. Blee TH, Cogbill TH, Lambert PJ. Hemorrhage associated with vitamin C deficiency in surgical patients. Surgery. 2002;131(4):408-412.
24. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263.
25. Gan R, Eintracht S, Hoffer LJ. Vitamin C deficiency in a university teaching hospital. J Am Coll Nutr. 2008;27(3):428-433.
26. Bourne G. The effect of graded doses of vitamin C upon the regeneration of bone in guinea-pigs on a scorbutic diet. J Physiol. 1942;101(3):327-336.
27. Bourne GH. The relative importance of periosteum and endosteum in bone healing and the relationship of vitamin C to their activities. Proc R Soc Med. 1944;37(6):275-279.
28. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426-428.
29. Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309-313.
30. Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281-288.
31. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder Shionogi rats. J Bone Joint Surg Br. 2007;89(3):402-407.
32. Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597-1610.
33. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143(2):213-221.
34. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235-246.
35. Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264(29):17281-17286.
36. Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid–dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11(8):1103-1113.
37. Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. Anat Rec. 2002;268(2):93-104.
38. Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013;46(4):254-261.
39. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63(3):183-189.
40. Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66.
41. Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523-1527.
42. Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. 2003;14(5):418-428.
43. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health. 2006;15(3):295-300.
44. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington state, USA. J Epidemiol Community Health. 1997;51(5):479-485.
45. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154(5):427-433.
46. Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):581-588.
47. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14(1):129-135.
48. Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. 2006;163(1):9-17.
49. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24(6):1086-1094.
50. Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clin Proc. 2002;77(2):174-180.
51. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354(9195):2025-2028.
52. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose–response study. J Bone Joint Surg Am. 2007;89(7):1424-1431.
53. Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures [in French]. Acta Orthop Belg. 2002;68(5):481-484.
54. Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15(4):179-182.
55. Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3(1-5):13-18.
56. Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18(2):127-131.
57. Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560-566.
58. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016.
59. Zollinger PE. The administration of vitamin C in prevention of CRPS-I after distal radial fractures and hand surgery—a review of two RCTs and one observational prospective study. Open Conference Proc J. 2011;2:1-4.
60. Rogers BA, Ricketts DM. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2008;90(2):447-448.
61. Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82(6):873.
62. Frolke JP. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2007;89(11):2550-2551.
63. Zollinger PE, Unal H, Ellis ML, Tuinebreijer WE. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop J. 2010;4:62-66.
64. Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery—systematic review and meta-analysis. J Foot Ankle Surg. 2013;52(1):62-66.
65. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-567.
66. McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
67. Kaiki G, Tsuji H, Yonezawa T, et al. Osteoarthrosis induced by intra-articular hydrogen peroxide injection and running load. J Orthop Res. 1990;8(5):731-740.
68. Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515-521.
69. Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol. 1990;71(2):279-293.
70. Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10(2):119-126.
71. Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50(6):1822-1831.
72. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14(4):709-715.
73. Hill J, Bird HA. Failure of selenium-ace to improve osteoarthritis. Br J Rheumatol. 1990;29(3):211-213.
74. Chaganti RK, Tolstykh I, Javaid MK, et al; Multicenter Osteoarthritis Study Group (MOST). High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190-196.
75. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 26. http://www.ars.usda.gov/Services/docs.htm?docid=24936. Published August 2013. Revised November 2013. Accessed May 14, 2015.
76. National Institutes of Health, Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. National Institutes of Health website. http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Reviewed June 5, 2013. Accessed May 14, 2015.
77. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000.
Targeting a New Safe Zone: A Step in the Development of Patient-Specific Component Positioning for Total Hip Arthroplasty
Postoperative dislocation remains a common complication of primary total hip arthroplasties (THAs), affecting less than 1% to more than 10% in reported series.1,2 In large datasets for modern implants, the incidence of dislocation is 2% to 4%.3,4 Given that more than 200,000 THAs are performed in the United States each year,5 these low percentages represent a large number of patients. The multiplex patient variables that affect THA stability include age, sex, body mass index (BMI), and comorbid conditions.6-8 Surgical approach, restoration of leg length and femoral offset, femoral head size, and component positioning are also important surgical factors that can increase or decrease the incidence of dislocation.3,8,9 In particular, appropriate acetabular component orientation is crucial; surgeons can control this factor and thereby limit the occurrence of dislocation.10 Furthermore, acetabular malpositioning can increase the risk of liner fractures and accelerate bearing-surface wear.11-14
To minimize the risk of postoperative dislocation, surgeons traditionally have targeted the Lewinnek safe zone, with its mean (SD) inclination of 40° (10°) and mean (SD) anteversion of 15° (10°), for acetabular component orientation.15 However, the applicability of this target zone to preventing hip instability using modern implant designs, components, and surgical techniques remains unknown. Achieving acetabular orientation based on maximizing range of motion (ROM) before impingement may be optimal, with anteversion from 20° to 30° and inclination from 40° to 45°.16,17 Furthermore, mean (SD) native acetabular anteversion ranges from 21.3° (6.2°) for men to 24.6° (6.6°) for women.18 Placing THA acetabular components near the native range for anteversion may best provide impingement-free ROM and thus optimize THA stability,16,19 but this has not been proved in a clinical study.
Early dislocation is typically classified as occurring within 6 months after surgery,9 with almost 80% of dislocations occurring within 3 months after surgery.10 Surgeon-specific factors, such as acetabular component positioning, are thought to have a predominant effect on dislocations in the early postoperative period.10 Computer-assisted surgery (CAS), such as imageless navigation, is more accurate than conventional methods for acetabular component placement,20-23 but the clinical relevance of improving accuracy for acetabular component placement has not been shown with respect to altering patient outcomes.23
We conducted a study in a large single-surgeon patient cohort to determine the incidence of early postoperative dislocation with target anteversion increased to 25°, approximating mean native acetabular anteversion.16,19 In addition, we sought to determine the accuracy of imageless navigation in achieving target acetabular component placement.
Materials and Methods
After obtaining institutional review board approval for this retrospective clinical study, we reviewed 671 consecutive cases of primary THA performed by a single surgeon using an imageless CAS system (AchieveCAS; Smith & Nephew, Memphis, Tennessee) between July 2006 and October 2012. THAs were excluded if a metal-on-metal bearing surface was used, if an adequate 6-week postoperative supine anteroposterior (AP) pelvis radiograph was unavailable, or if 6-month clinical follow-up findings were not available (Figure 1). The quality of AP radiographs was deemed poor if they were not centered on the symphysis pubis and if the sacrococcygeal joint was not centered over the symphysis pubis. After exclusion criteria were applied, 553 arthroplasties (479 patients) with a mean (SD) follow-up of 2.4 (1.4) years remained. Perioperative demographic data and component sizes are listed in Table 1.
During surgery, the anterior pelvic plane, defined by the anterior-superior iliac spines and pubic tubercle, was registered with the CAS system with the patient in the supine position. THA was performed with the patient in the lateral decubitus position using a posterolateral technique. For all patients, the surgeon used a hemispherical acetabular component (R3 Acetabular System; Smith & Nephew); bearings that were either metal on highly cross-linked polyethylene (XLPE) or Oxinium (Smith & Nephew) on XLPE; and neutral XLPE acetabular inserts. The goals for acetabular inclination and anteversion were 40° and 25°, respectively, with ±10° each for the target zone. The CAS system was used to adjust target anteversion for sagittal pelvic tilt.24 Uncemented femoral components were used for all patients, and the goal for femoral component anteversion was 15°. Transosseous repair of the posterior capsule and short external rotators was performed after component implantation.25
On each 6-week postoperative radiograph, acetabular orientation was measured with Ein-Bild-Röntgen-Analyse (EBRA; University of Innsbruck, Austria) software, which provides a validated method for measuring acetabular inclination and anteversion on supine AP pelvis radiographs.10,26 Pelvic boundaries were delineated with grid lines defining pelvic position. Reference points around the projections of the prosthetic femoral head, the hemispherical cup, and the rim of the cup were marked (Figure 2). EBRA calculated radiographic inclination and anteversion of the acetabular component based on the spatial position of the cup center in relation to the plane of the radiograph and the pelvic position.26
Charts were reviewed to identify patients with early postoperative dislocations, as well as dislocation timing, recurrence, and other characteristics. We defined early dislocation as instability occurring within 6 months after surgery. Revision surgery for instability was also identified.
For the statistical analysis, orientation error was defined as the absolute value of the difference between target orientation (40° inclination, 25° anteversion) and radiographic measurements. Repeated-measures multiple regression with the generalized estimating equations approach was used to identify baseline patient characteristics (age, sex, BMI, primary diagnosis, laterality) associated with component positioning outside of our targeted ranges for inclination and anteversion. Fisher exact tests were used to examine the relationship between dislocation and component placement in either the Lewinnek safe zone or our targeted zone. All tests were 2-sided with a significance level of .05. All analyses were performed with SAS for Windows 9.3 (SAS Institute, Cary, North Carolina).
Results
Mean (SD) acetabular inclination was 42.2° (4.9°) (range, 27.6°-65.0°), with a mean (SD) orientation error of 4.2° (3.4°) (Figure 3A). Mean (SD) anteversion was 23.9° (6.5°) (range, 6.2°-48.0°), with a mean (SD) orientation error of 5.2° (4.1°) (Figure 3B). Components were placed outside the Lewinnek safe zone for inclination or anteversion in 46.5% of cases and outside the target zone in 17.7% of cases (Figure 4). Variation in acetabular anteversion alone accounted for 67.3% of target zone outliers (Table 2). Only 0.9% of components were placed outside the target ranges for both inclination and anteversion.
Regression analysis was performed separately for inclination and anteversion to determine the risk factors for placing the acetabular component outside the target orientation ranges. Only higher BMI was associated with malposition with respect to inclination (hazard ratio [HR], 1.059; 95% confidence interval [CI], 1.011-1.111; P = .017). Of obese patients with inclination outside the target range, 90.9% had an inclination angle of more than 50°. Associations between inclination outside the target range and age (P = .769), sex (P = .217), preoperative diagnosis (P > .99), and laterality (P = .106) were statistically insignificant. Only female sex was associated with position of the acetabular component outside the target range for anteversion (HR, 1.871; 95% CI, 1.061-3.299; P = .030). Of female patients with anteversion outside the target range, 70.0% had anteversion of less than 15°. Associations between anteversion outside the target range and age (P = .762), BMI (P = .583), preoperative diagnosis (P > .99), and laterality (P = .235) were statistically insignificant.
Six THAs (1.1%) in 6 patients experienced dislocation within 6 months after surgery (Table 3); mean (SD) time of dislocation was 58.3 (13.8) days after surgery. There was no relationship between dislocation incidence and component placement in the Lewinnek zone (P = .224) or our target zone (P = .287). Of the dislocation cases, 50% involved female patients, and 50% involved right hips. Mean (SD) age of these patients was 53.3 (7.6) years. Mean (SD) BMI was 25.4 (0.9) kg/m2. Osteoarthritis was the primary diagnosis for all patients with early dislocation; 32- or 36-mm femoral heads were used in these cases. Two patients had acetabular components placed outside of our target zone. One patient, who had abnormal pelvic obliquity and sagittal tilt from scoliosis (Figures 5A, 5B), had an acetabular component placed outside both the target zone and the Lewinnek safe zone. Mean (SD) acetabular inclination was 39.8° (3.6°), and mean (SD) anteversion was 21.8° (7.3°) (Figure 5C). Two dislocations resulted from trauma, 1 dislocation was related to hyperlaxity, 1 patient had cerebral palsy, and 1 patient had no evident predisposing risk factors. Three patients (0.54%) had multiple episodes of instability requiring revision during the follow-up period.
Discussion
To our knowledge, this study represents the largest cohort of primary THAs performed with an imageless navigation system. Our results showed that increasing targeted acetabular anteversion to 25° using a posterolateral surgical approach and modern implants resulted in a 1.1% incidence of early dislocation and a 0.54% incidence of recurrent instability requiring reoperation. Of the patients with a dislocation, only 1 did not experience trauma and did not have a risk factor for dislocation. Only 1 patient with a dislocation had acetabular components positioned outside both the target zone and the Lewinnek safe zone. The acetabular component was placed within the target zone in 82.3% of cases in which the imageless navigation system was used. In our cohort, BMI was the only risk factor for placement of the acetabular component outside our target range for inclination, and sex was associated with components outside the target range for anteversion.
Early dislocation after THA is often related to improper implant orientation, inadequate restoration of offset and myofascial tension, and decreased femoral head–neck ratio.8 Although dislocation rates in the literature vary widely,1,2 Medicare data suggest that the rate for the first 6 months after surgery can be as high as 4.21%.3,4 Although use of femoral heads with a diameter of 32 mm or larger may decrease this rate to 2.14%,3 accurate acetabular component orientation helps prevent postoperative dislocation.10 Using an imageless navigation system to target 25° of anteversion and 40° of inclination resulted in an early-dislocation rate about 49% less than the rate in a Medicare population treated with similar, modern implants.3
Callanan and colleagues11 found that freehand techniques were inaccurate for acetabular positioning in up to 50% of cases, and several studies have demonstrated that imageless navigation systems were more accurate than conventional guides.20,21,27-29 Higher BMI has been implicated as a risk factor for acetabular malpositioning in several studies of the accuracy of freehand techniques11 and imageless navigation techniques.23,30 Soft-tissue impediment to the component insertion handle poses a risk of increased inclination and inadequate anteversion, regardless of method used (conventional, CAS). When the acetabular component is placed freehand in obese patients, it is difficult to judge the position of the pelvis on the operating room table. For imageless navigation, a larger amount of adipose tissue over bony landmarks may limit the accuracy of anterior pelvic plane registration.30 Sex typically is not cited as a risk factor for inaccurate acetabular component positioning. We speculate that omitted-variable bias may explain the observed association between female sex and anteversion. For example, changes in postoperative pelvic tilt alter apparent anteversion on plain radiographs,31-34 but preoperative and postoperative sagittal pelvic tilt was not recorded in this study.
The proper position of the acetabular component has been debated.15,16,35,36 Although it is generally agreed that inclination of 40° ± 10° balances ROM, stability, and bearing-surface wear,12,13,15,16 proposed targets for anteversion vary widely, from 0° to 40°.35,36 Patel and colleagues16 formulated computer models based on cadaveric specimens to determine that THA impingement was minimized when the acetabular component was placed to match the native anteversion of the acetabulum.In their study model, 20° of anteversion paralleled native acetabular orientation. Tohtz and colleagues18 reviewed computed tomography scans of 144 female hips and 192 male hips and found that mean (SD) anteversion was 24.6° (6.6°) for women and 21.3° (6.2°) for men. Whether native anatomy is a valid reference for acetabular anteversion is controversial,19 and definitive recommendations for target anteversion cannot be made, as the effect of acetabular anteversion on the wear of various bearing materials is unknown.14 Yet, as with inclination, ideal anteversion is likely a compromise between maximizing impingement-free ROM and minimizing wear.
The present study had several limitations. A single-surgeon patient series was reviewed retrospectively, and there was no control group. We determined the incidence only of early dislocation, and 5.3% of THAs that were not metal-on-metal were either lost to follow-up or had inadequate radiographs. However, of the patients excluded for inadequate radiographs, none had an early dislocation. The effects of our surgical techniques on long-term outcomes, bearing wear, and dislocation are unknown. We were not able to comment on the direction of dislocation for any of the 6 patients with early dislocation, as all dislocations were reduced at facilities other than our hospital. Therefore, we cannot determine whether increasing acetabular anteversion resulted in a larger number of anterior versus posterior dislocations.15
We did not use CAS to place any of the femoral components. Therefore, we could not accurately target combined anteversion, defined as the sum of acetabular and femoral version, which may be an important determinant of THA stability.28 Although restoration of femoral offset and leg length is important in preventing THA dislocation,8 the CAS techniques used did not influence these parameters, and they were not measured.
As an imageless navigation system was used, there were no preoperative axial images, which could have been used to assess native acetabular orientation. This limited our assessment with respect to matching each patient’s natural anteversion. Imageless navigation, which references only the anterior pelvic plane, may not be reliable in patients with excessive sagittal pelvic tilt.37 Furthermore, changes in the functional position of the pelvis from supine to sitting to standing were not accounted for, and changes in sagittal tilt between these positions can be significant.38 Changes in sagittal pelvic tilt affect measurement of acetabular anteversion on plain radiographs, with anterior tilt reducing apparent anteversion and posterior tilt increasing it.32,34 Although postoperative computed tomography is the gold standard for assessing acetabular component orientation, EBRA significantly reduces errors of measurement on plain radiographs.10 Some variability in measured anteversion may be explained by our surgical technique. In particular, if the cup was uncovered anteriorly, additional anteversion was usually accepted during surgery to minimize anterior impingement and limit the risk of iliopsoas tendonitis.16,39
Our study results suggested that increasing target acetabular anteversion to 25° may reduce the incidence of early postoperative instability relative to rates reported in the literature. Despite the higher accuracy of component placement with an imageless navigation system, dislocations occurred in patients with acetabular components positioned in our target zone and in the historical safe zone. These dislocations support the notion that there likely is no absolute safe range for acetabular component positioning, as THA stability depends on many factors. Ideal targets for implant orientation for acetabulum and femur may be patient-specific.16,19 Investigators should prospectively evaluate patient-specific THA component positioning and determine its effect on postoperative dislocation and bearing-surface wear. As specific implant targets are further defined, tools that are more precise and accurate than conventional techniques will be needed to achieve goal component positioning. Our study results confirmed that imageless navigation is an accurate method for achieving acetabular orientation targets.
1. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop. 2006;(447):34-38.
2. Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;(441):262-267.
3. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
4. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
5. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21(suppl 1):S1-S6.
6. Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141-147.
7. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867-872.
8. Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop. 1997;(344):179-187.
9. Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132(7):1031-1036.
10. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87(6):762-769.
11. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469(2):319-329.
12. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34(1):19-26.
13. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259-2265.
14. Esposito CI, Walter WL, Roques A, et al. Wear in alumina-on-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012;94(7):901-907.
15. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
16. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. 2010;25(8):1275-1281.e1.
17. Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815-821.
18. Tohtz SW, Sassy D, Matziolis G, Preininger B, Perka C, Hasart O. CT evaluation of native acetabular orientation and localization: sex-specific data comparison on 336 hip joints. Technol Health Care. 2010;18(2):129-136.
19. Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95(22):e172.
20. Nogler M, Kessler O, Prassl A, et al. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop. 2004;(426):159-163.
21. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51-56.
22. Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop. 2004;(426):174-179.
23. Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786-791.
24. Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90(2):357-365.
25. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop. 1998;(355):224-228.
26. Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28(10):1225-1236.
27. Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89(3):494-499.
28. Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;(465):92-99.
29. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15-21.
30. Hohmann E, Bryant A, Tetsworth K. Anterior pelvic soft tissue thickness influences acetabular cup positioning with imageless navigation. J Arthroplasty. 2012;27(6):945-952.
31. Nguyen AD, Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389-398.
32. Malik A, Wan Z, Jaramaz B, Bowman G, Dorr LD. A validation model for measurement of acetabular component position. J Arthroplasty. 2010;25(5):812-819.
33. Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays—a comparison of six parameters. Skeletal Radiol. 2006;35(3):149-155.
34. Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield Award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop. 2009;467(1):43-49.
35. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
36. Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-513.
37. Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26(4):596-605.
38. Lazennec JY, Riwan A, Gravez F, et al. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(suppl 5):S91-S104.
39. Trousdale RT, Cabanela ME, Berry DJ. Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty. 1995;10(4):546-549.
Postoperative dislocation remains a common complication of primary total hip arthroplasties (THAs), affecting less than 1% to more than 10% in reported series.1,2 In large datasets for modern implants, the incidence of dislocation is 2% to 4%.3,4 Given that more than 200,000 THAs are performed in the United States each year,5 these low percentages represent a large number of patients. The multiplex patient variables that affect THA stability include age, sex, body mass index (BMI), and comorbid conditions.6-8 Surgical approach, restoration of leg length and femoral offset, femoral head size, and component positioning are also important surgical factors that can increase or decrease the incidence of dislocation.3,8,9 In particular, appropriate acetabular component orientation is crucial; surgeons can control this factor and thereby limit the occurrence of dislocation.10 Furthermore, acetabular malpositioning can increase the risk of liner fractures and accelerate bearing-surface wear.11-14
To minimize the risk of postoperative dislocation, surgeons traditionally have targeted the Lewinnek safe zone, with its mean (SD) inclination of 40° (10°) and mean (SD) anteversion of 15° (10°), for acetabular component orientation.15 However, the applicability of this target zone to preventing hip instability using modern implant designs, components, and surgical techniques remains unknown. Achieving acetabular orientation based on maximizing range of motion (ROM) before impingement may be optimal, with anteversion from 20° to 30° and inclination from 40° to 45°.16,17 Furthermore, mean (SD) native acetabular anteversion ranges from 21.3° (6.2°) for men to 24.6° (6.6°) for women.18 Placing THA acetabular components near the native range for anteversion may best provide impingement-free ROM and thus optimize THA stability,16,19 but this has not been proved in a clinical study.
Early dislocation is typically classified as occurring within 6 months after surgery,9 with almost 80% of dislocations occurring within 3 months after surgery.10 Surgeon-specific factors, such as acetabular component positioning, are thought to have a predominant effect on dislocations in the early postoperative period.10 Computer-assisted surgery (CAS), such as imageless navigation, is more accurate than conventional methods for acetabular component placement,20-23 but the clinical relevance of improving accuracy for acetabular component placement has not been shown with respect to altering patient outcomes.23
We conducted a study in a large single-surgeon patient cohort to determine the incidence of early postoperative dislocation with target anteversion increased to 25°, approximating mean native acetabular anteversion.16,19 In addition, we sought to determine the accuracy of imageless navigation in achieving target acetabular component placement.
Materials and Methods
After obtaining institutional review board approval for this retrospective clinical study, we reviewed 671 consecutive cases of primary THA performed by a single surgeon using an imageless CAS system (AchieveCAS; Smith & Nephew, Memphis, Tennessee) between July 2006 and October 2012. THAs were excluded if a metal-on-metal bearing surface was used, if an adequate 6-week postoperative supine anteroposterior (AP) pelvis radiograph was unavailable, or if 6-month clinical follow-up findings were not available (Figure 1). The quality of AP radiographs was deemed poor if they were not centered on the symphysis pubis and if the sacrococcygeal joint was not centered over the symphysis pubis. After exclusion criteria were applied, 553 arthroplasties (479 patients) with a mean (SD) follow-up of 2.4 (1.4) years remained. Perioperative demographic data and component sizes are listed in Table 1.
During surgery, the anterior pelvic plane, defined by the anterior-superior iliac spines and pubic tubercle, was registered with the CAS system with the patient in the supine position. THA was performed with the patient in the lateral decubitus position using a posterolateral technique. For all patients, the surgeon used a hemispherical acetabular component (R3 Acetabular System; Smith & Nephew); bearings that were either metal on highly cross-linked polyethylene (XLPE) or Oxinium (Smith & Nephew) on XLPE; and neutral XLPE acetabular inserts. The goals for acetabular inclination and anteversion were 40° and 25°, respectively, with ±10° each for the target zone. The CAS system was used to adjust target anteversion for sagittal pelvic tilt.24 Uncemented femoral components were used for all patients, and the goal for femoral component anteversion was 15°. Transosseous repair of the posterior capsule and short external rotators was performed after component implantation.25
On each 6-week postoperative radiograph, acetabular orientation was measured with Ein-Bild-Röntgen-Analyse (EBRA; University of Innsbruck, Austria) software, which provides a validated method for measuring acetabular inclination and anteversion on supine AP pelvis radiographs.10,26 Pelvic boundaries were delineated with grid lines defining pelvic position. Reference points around the projections of the prosthetic femoral head, the hemispherical cup, and the rim of the cup were marked (Figure 2). EBRA calculated radiographic inclination and anteversion of the acetabular component based on the spatial position of the cup center in relation to the plane of the radiograph and the pelvic position.26
Charts were reviewed to identify patients with early postoperative dislocations, as well as dislocation timing, recurrence, and other characteristics. We defined early dislocation as instability occurring within 6 months after surgery. Revision surgery for instability was also identified.
For the statistical analysis, orientation error was defined as the absolute value of the difference between target orientation (40° inclination, 25° anteversion) and radiographic measurements. Repeated-measures multiple regression with the generalized estimating equations approach was used to identify baseline patient characteristics (age, sex, BMI, primary diagnosis, laterality) associated with component positioning outside of our targeted ranges for inclination and anteversion. Fisher exact tests were used to examine the relationship between dislocation and component placement in either the Lewinnek safe zone or our targeted zone. All tests were 2-sided with a significance level of .05. All analyses were performed with SAS for Windows 9.3 (SAS Institute, Cary, North Carolina).
Results
Mean (SD) acetabular inclination was 42.2° (4.9°) (range, 27.6°-65.0°), with a mean (SD) orientation error of 4.2° (3.4°) (Figure 3A). Mean (SD) anteversion was 23.9° (6.5°) (range, 6.2°-48.0°), with a mean (SD) orientation error of 5.2° (4.1°) (Figure 3B). Components were placed outside the Lewinnek safe zone for inclination or anteversion in 46.5% of cases and outside the target zone in 17.7% of cases (Figure 4). Variation in acetabular anteversion alone accounted for 67.3% of target zone outliers (Table 2). Only 0.9% of components were placed outside the target ranges for both inclination and anteversion.
Regression analysis was performed separately for inclination and anteversion to determine the risk factors for placing the acetabular component outside the target orientation ranges. Only higher BMI was associated with malposition with respect to inclination (hazard ratio [HR], 1.059; 95% confidence interval [CI], 1.011-1.111; P = .017). Of obese patients with inclination outside the target range, 90.9% had an inclination angle of more than 50°. Associations between inclination outside the target range and age (P = .769), sex (P = .217), preoperative diagnosis (P > .99), and laterality (P = .106) were statistically insignificant. Only female sex was associated with position of the acetabular component outside the target range for anteversion (HR, 1.871; 95% CI, 1.061-3.299; P = .030). Of female patients with anteversion outside the target range, 70.0% had anteversion of less than 15°. Associations between anteversion outside the target range and age (P = .762), BMI (P = .583), preoperative diagnosis (P > .99), and laterality (P = .235) were statistically insignificant.
Six THAs (1.1%) in 6 patients experienced dislocation within 6 months after surgery (Table 3); mean (SD) time of dislocation was 58.3 (13.8) days after surgery. There was no relationship between dislocation incidence and component placement in the Lewinnek zone (P = .224) or our target zone (P = .287). Of the dislocation cases, 50% involved female patients, and 50% involved right hips. Mean (SD) age of these patients was 53.3 (7.6) years. Mean (SD) BMI was 25.4 (0.9) kg/m2. Osteoarthritis was the primary diagnosis for all patients with early dislocation; 32- or 36-mm femoral heads were used in these cases. Two patients had acetabular components placed outside of our target zone. One patient, who had abnormal pelvic obliquity and sagittal tilt from scoliosis (Figures 5A, 5B), had an acetabular component placed outside both the target zone and the Lewinnek safe zone. Mean (SD) acetabular inclination was 39.8° (3.6°), and mean (SD) anteversion was 21.8° (7.3°) (Figure 5C). Two dislocations resulted from trauma, 1 dislocation was related to hyperlaxity, 1 patient had cerebral palsy, and 1 patient had no evident predisposing risk factors. Three patients (0.54%) had multiple episodes of instability requiring revision during the follow-up period.
Discussion
To our knowledge, this study represents the largest cohort of primary THAs performed with an imageless navigation system. Our results showed that increasing targeted acetabular anteversion to 25° using a posterolateral surgical approach and modern implants resulted in a 1.1% incidence of early dislocation and a 0.54% incidence of recurrent instability requiring reoperation. Of the patients with a dislocation, only 1 did not experience trauma and did not have a risk factor for dislocation. Only 1 patient with a dislocation had acetabular components positioned outside both the target zone and the Lewinnek safe zone. The acetabular component was placed within the target zone in 82.3% of cases in which the imageless navigation system was used. In our cohort, BMI was the only risk factor for placement of the acetabular component outside our target range for inclination, and sex was associated with components outside the target range for anteversion.
Early dislocation after THA is often related to improper implant orientation, inadequate restoration of offset and myofascial tension, and decreased femoral head–neck ratio.8 Although dislocation rates in the literature vary widely,1,2 Medicare data suggest that the rate for the first 6 months after surgery can be as high as 4.21%.3,4 Although use of femoral heads with a diameter of 32 mm or larger may decrease this rate to 2.14%,3 accurate acetabular component orientation helps prevent postoperative dislocation.10 Using an imageless navigation system to target 25° of anteversion and 40° of inclination resulted in an early-dislocation rate about 49% less than the rate in a Medicare population treated with similar, modern implants.3
Callanan and colleagues11 found that freehand techniques were inaccurate for acetabular positioning in up to 50% of cases, and several studies have demonstrated that imageless navigation systems were more accurate than conventional guides.20,21,27-29 Higher BMI has been implicated as a risk factor for acetabular malpositioning in several studies of the accuracy of freehand techniques11 and imageless navigation techniques.23,30 Soft-tissue impediment to the component insertion handle poses a risk of increased inclination and inadequate anteversion, regardless of method used (conventional, CAS). When the acetabular component is placed freehand in obese patients, it is difficult to judge the position of the pelvis on the operating room table. For imageless navigation, a larger amount of adipose tissue over bony landmarks may limit the accuracy of anterior pelvic plane registration.30 Sex typically is not cited as a risk factor for inaccurate acetabular component positioning. We speculate that omitted-variable bias may explain the observed association between female sex and anteversion. For example, changes in postoperative pelvic tilt alter apparent anteversion on plain radiographs,31-34 but preoperative and postoperative sagittal pelvic tilt was not recorded in this study.
The proper position of the acetabular component has been debated.15,16,35,36 Although it is generally agreed that inclination of 40° ± 10° balances ROM, stability, and bearing-surface wear,12,13,15,16 proposed targets for anteversion vary widely, from 0° to 40°.35,36 Patel and colleagues16 formulated computer models based on cadaveric specimens to determine that THA impingement was minimized when the acetabular component was placed to match the native anteversion of the acetabulum.In their study model, 20° of anteversion paralleled native acetabular orientation. Tohtz and colleagues18 reviewed computed tomography scans of 144 female hips and 192 male hips and found that mean (SD) anteversion was 24.6° (6.6°) for women and 21.3° (6.2°) for men. Whether native anatomy is a valid reference for acetabular anteversion is controversial,19 and definitive recommendations for target anteversion cannot be made, as the effect of acetabular anteversion on the wear of various bearing materials is unknown.14 Yet, as with inclination, ideal anteversion is likely a compromise between maximizing impingement-free ROM and minimizing wear.
The present study had several limitations. A single-surgeon patient series was reviewed retrospectively, and there was no control group. We determined the incidence only of early dislocation, and 5.3% of THAs that were not metal-on-metal were either lost to follow-up or had inadequate radiographs. However, of the patients excluded for inadequate radiographs, none had an early dislocation. The effects of our surgical techniques on long-term outcomes, bearing wear, and dislocation are unknown. We were not able to comment on the direction of dislocation for any of the 6 patients with early dislocation, as all dislocations were reduced at facilities other than our hospital. Therefore, we cannot determine whether increasing acetabular anteversion resulted in a larger number of anterior versus posterior dislocations.15
We did not use CAS to place any of the femoral components. Therefore, we could not accurately target combined anteversion, defined as the sum of acetabular and femoral version, which may be an important determinant of THA stability.28 Although restoration of femoral offset and leg length is important in preventing THA dislocation,8 the CAS techniques used did not influence these parameters, and they were not measured.
As an imageless navigation system was used, there were no preoperative axial images, which could have been used to assess native acetabular orientation. This limited our assessment with respect to matching each patient’s natural anteversion. Imageless navigation, which references only the anterior pelvic plane, may not be reliable in patients with excessive sagittal pelvic tilt.37 Furthermore, changes in the functional position of the pelvis from supine to sitting to standing were not accounted for, and changes in sagittal tilt between these positions can be significant.38 Changes in sagittal pelvic tilt affect measurement of acetabular anteversion on plain radiographs, with anterior tilt reducing apparent anteversion and posterior tilt increasing it.32,34 Although postoperative computed tomography is the gold standard for assessing acetabular component orientation, EBRA significantly reduces errors of measurement on plain radiographs.10 Some variability in measured anteversion may be explained by our surgical technique. In particular, if the cup was uncovered anteriorly, additional anteversion was usually accepted during surgery to minimize anterior impingement and limit the risk of iliopsoas tendonitis.16,39
Our study results suggested that increasing target acetabular anteversion to 25° may reduce the incidence of early postoperative instability relative to rates reported in the literature. Despite the higher accuracy of component placement with an imageless navigation system, dislocations occurred in patients with acetabular components positioned in our target zone and in the historical safe zone. These dislocations support the notion that there likely is no absolute safe range for acetabular component positioning, as THA stability depends on many factors. Ideal targets for implant orientation for acetabulum and femur may be patient-specific.16,19 Investigators should prospectively evaluate patient-specific THA component positioning and determine its effect on postoperative dislocation and bearing-surface wear. As specific implant targets are further defined, tools that are more precise and accurate than conventional techniques will be needed to achieve goal component positioning. Our study results confirmed that imageless navigation is an accurate method for achieving acetabular orientation targets.
Postoperative dislocation remains a common complication of primary total hip arthroplasties (THAs), affecting less than 1% to more than 10% in reported series.1,2 In large datasets for modern implants, the incidence of dislocation is 2% to 4%.3,4 Given that more than 200,000 THAs are performed in the United States each year,5 these low percentages represent a large number of patients. The multiplex patient variables that affect THA stability include age, sex, body mass index (BMI), and comorbid conditions.6-8 Surgical approach, restoration of leg length and femoral offset, femoral head size, and component positioning are also important surgical factors that can increase or decrease the incidence of dislocation.3,8,9 In particular, appropriate acetabular component orientation is crucial; surgeons can control this factor and thereby limit the occurrence of dislocation.10 Furthermore, acetabular malpositioning can increase the risk of liner fractures and accelerate bearing-surface wear.11-14
To minimize the risk of postoperative dislocation, surgeons traditionally have targeted the Lewinnek safe zone, with its mean (SD) inclination of 40° (10°) and mean (SD) anteversion of 15° (10°), for acetabular component orientation.15 However, the applicability of this target zone to preventing hip instability using modern implant designs, components, and surgical techniques remains unknown. Achieving acetabular orientation based on maximizing range of motion (ROM) before impingement may be optimal, with anteversion from 20° to 30° and inclination from 40° to 45°.16,17 Furthermore, mean (SD) native acetabular anteversion ranges from 21.3° (6.2°) for men to 24.6° (6.6°) for women.18 Placing THA acetabular components near the native range for anteversion may best provide impingement-free ROM and thus optimize THA stability,16,19 but this has not been proved in a clinical study.
Early dislocation is typically classified as occurring within 6 months after surgery,9 with almost 80% of dislocations occurring within 3 months after surgery.10 Surgeon-specific factors, such as acetabular component positioning, are thought to have a predominant effect on dislocations in the early postoperative period.10 Computer-assisted surgery (CAS), such as imageless navigation, is more accurate than conventional methods for acetabular component placement,20-23 but the clinical relevance of improving accuracy for acetabular component placement has not been shown with respect to altering patient outcomes.23
We conducted a study in a large single-surgeon patient cohort to determine the incidence of early postoperative dislocation with target anteversion increased to 25°, approximating mean native acetabular anteversion.16,19 In addition, we sought to determine the accuracy of imageless navigation in achieving target acetabular component placement.
Materials and Methods
After obtaining institutional review board approval for this retrospective clinical study, we reviewed 671 consecutive cases of primary THA performed by a single surgeon using an imageless CAS system (AchieveCAS; Smith & Nephew, Memphis, Tennessee) between July 2006 and October 2012. THAs were excluded if a metal-on-metal bearing surface was used, if an adequate 6-week postoperative supine anteroposterior (AP) pelvis radiograph was unavailable, or if 6-month clinical follow-up findings were not available (Figure 1). The quality of AP radiographs was deemed poor if they were not centered on the symphysis pubis and if the sacrococcygeal joint was not centered over the symphysis pubis. After exclusion criteria were applied, 553 arthroplasties (479 patients) with a mean (SD) follow-up of 2.4 (1.4) years remained. Perioperative demographic data and component sizes are listed in Table 1.
During surgery, the anterior pelvic plane, defined by the anterior-superior iliac spines and pubic tubercle, was registered with the CAS system with the patient in the supine position. THA was performed with the patient in the lateral decubitus position using a posterolateral technique. For all patients, the surgeon used a hemispherical acetabular component (R3 Acetabular System; Smith & Nephew); bearings that were either metal on highly cross-linked polyethylene (XLPE) or Oxinium (Smith & Nephew) on XLPE; and neutral XLPE acetabular inserts. The goals for acetabular inclination and anteversion were 40° and 25°, respectively, with ±10° each for the target zone. The CAS system was used to adjust target anteversion for sagittal pelvic tilt.24 Uncemented femoral components were used for all patients, and the goal for femoral component anteversion was 15°. Transosseous repair of the posterior capsule and short external rotators was performed after component implantation.25
On each 6-week postoperative radiograph, acetabular orientation was measured with Ein-Bild-Röntgen-Analyse (EBRA; University of Innsbruck, Austria) software, which provides a validated method for measuring acetabular inclination and anteversion on supine AP pelvis radiographs.10,26 Pelvic boundaries were delineated with grid lines defining pelvic position. Reference points around the projections of the prosthetic femoral head, the hemispherical cup, and the rim of the cup were marked (Figure 2). EBRA calculated radiographic inclination and anteversion of the acetabular component based on the spatial position of the cup center in relation to the plane of the radiograph and the pelvic position.26
Charts were reviewed to identify patients with early postoperative dislocations, as well as dislocation timing, recurrence, and other characteristics. We defined early dislocation as instability occurring within 6 months after surgery. Revision surgery for instability was also identified.
For the statistical analysis, orientation error was defined as the absolute value of the difference between target orientation (40° inclination, 25° anteversion) and radiographic measurements. Repeated-measures multiple regression with the generalized estimating equations approach was used to identify baseline patient characteristics (age, sex, BMI, primary diagnosis, laterality) associated with component positioning outside of our targeted ranges for inclination and anteversion. Fisher exact tests were used to examine the relationship between dislocation and component placement in either the Lewinnek safe zone or our targeted zone. All tests were 2-sided with a significance level of .05. All analyses were performed with SAS for Windows 9.3 (SAS Institute, Cary, North Carolina).
Results
Mean (SD) acetabular inclination was 42.2° (4.9°) (range, 27.6°-65.0°), with a mean (SD) orientation error of 4.2° (3.4°) (Figure 3A). Mean (SD) anteversion was 23.9° (6.5°) (range, 6.2°-48.0°), with a mean (SD) orientation error of 5.2° (4.1°) (Figure 3B). Components were placed outside the Lewinnek safe zone for inclination or anteversion in 46.5% of cases and outside the target zone in 17.7% of cases (Figure 4). Variation in acetabular anteversion alone accounted for 67.3% of target zone outliers (Table 2). Only 0.9% of components were placed outside the target ranges for both inclination and anteversion.
Regression analysis was performed separately for inclination and anteversion to determine the risk factors for placing the acetabular component outside the target orientation ranges. Only higher BMI was associated with malposition with respect to inclination (hazard ratio [HR], 1.059; 95% confidence interval [CI], 1.011-1.111; P = .017). Of obese patients with inclination outside the target range, 90.9% had an inclination angle of more than 50°. Associations between inclination outside the target range and age (P = .769), sex (P = .217), preoperative diagnosis (P > .99), and laterality (P = .106) were statistically insignificant. Only female sex was associated with position of the acetabular component outside the target range for anteversion (HR, 1.871; 95% CI, 1.061-3.299; P = .030). Of female patients with anteversion outside the target range, 70.0% had anteversion of less than 15°. Associations between anteversion outside the target range and age (P = .762), BMI (P = .583), preoperative diagnosis (P > .99), and laterality (P = .235) were statistically insignificant.
Six THAs (1.1%) in 6 patients experienced dislocation within 6 months after surgery (Table 3); mean (SD) time of dislocation was 58.3 (13.8) days after surgery. There was no relationship between dislocation incidence and component placement in the Lewinnek zone (P = .224) or our target zone (P = .287). Of the dislocation cases, 50% involved female patients, and 50% involved right hips. Mean (SD) age of these patients was 53.3 (7.6) years. Mean (SD) BMI was 25.4 (0.9) kg/m2. Osteoarthritis was the primary diagnosis for all patients with early dislocation; 32- or 36-mm femoral heads were used in these cases. Two patients had acetabular components placed outside of our target zone. One patient, who had abnormal pelvic obliquity and sagittal tilt from scoliosis (Figures 5A, 5B), had an acetabular component placed outside both the target zone and the Lewinnek safe zone. Mean (SD) acetabular inclination was 39.8° (3.6°), and mean (SD) anteversion was 21.8° (7.3°) (Figure 5C). Two dislocations resulted from trauma, 1 dislocation was related to hyperlaxity, 1 patient had cerebral palsy, and 1 patient had no evident predisposing risk factors. Three patients (0.54%) had multiple episodes of instability requiring revision during the follow-up period.
Discussion
To our knowledge, this study represents the largest cohort of primary THAs performed with an imageless navigation system. Our results showed that increasing targeted acetabular anteversion to 25° using a posterolateral surgical approach and modern implants resulted in a 1.1% incidence of early dislocation and a 0.54% incidence of recurrent instability requiring reoperation. Of the patients with a dislocation, only 1 did not experience trauma and did not have a risk factor for dislocation. Only 1 patient with a dislocation had acetabular components positioned outside both the target zone and the Lewinnek safe zone. The acetabular component was placed within the target zone in 82.3% of cases in which the imageless navigation system was used. In our cohort, BMI was the only risk factor for placement of the acetabular component outside our target range for inclination, and sex was associated with components outside the target range for anteversion.
Early dislocation after THA is often related to improper implant orientation, inadequate restoration of offset and myofascial tension, and decreased femoral head–neck ratio.8 Although dislocation rates in the literature vary widely,1,2 Medicare data suggest that the rate for the first 6 months after surgery can be as high as 4.21%.3,4 Although use of femoral heads with a diameter of 32 mm or larger may decrease this rate to 2.14%,3 accurate acetabular component orientation helps prevent postoperative dislocation.10 Using an imageless navigation system to target 25° of anteversion and 40° of inclination resulted in an early-dislocation rate about 49% less than the rate in a Medicare population treated with similar, modern implants.3
Callanan and colleagues11 found that freehand techniques were inaccurate for acetabular positioning in up to 50% of cases, and several studies have demonstrated that imageless navigation systems were more accurate than conventional guides.20,21,27-29 Higher BMI has been implicated as a risk factor for acetabular malpositioning in several studies of the accuracy of freehand techniques11 and imageless navigation techniques.23,30 Soft-tissue impediment to the component insertion handle poses a risk of increased inclination and inadequate anteversion, regardless of method used (conventional, CAS). When the acetabular component is placed freehand in obese patients, it is difficult to judge the position of the pelvis on the operating room table. For imageless navigation, a larger amount of adipose tissue over bony landmarks may limit the accuracy of anterior pelvic plane registration.30 Sex typically is not cited as a risk factor for inaccurate acetabular component positioning. We speculate that omitted-variable bias may explain the observed association between female sex and anteversion. For example, changes in postoperative pelvic tilt alter apparent anteversion on plain radiographs,31-34 but preoperative and postoperative sagittal pelvic tilt was not recorded in this study.
The proper position of the acetabular component has been debated.15,16,35,36 Although it is generally agreed that inclination of 40° ± 10° balances ROM, stability, and bearing-surface wear,12,13,15,16 proposed targets for anteversion vary widely, from 0° to 40°.35,36 Patel and colleagues16 formulated computer models based on cadaveric specimens to determine that THA impingement was minimized when the acetabular component was placed to match the native anteversion of the acetabulum.In their study model, 20° of anteversion paralleled native acetabular orientation. Tohtz and colleagues18 reviewed computed tomography scans of 144 female hips and 192 male hips and found that mean (SD) anteversion was 24.6° (6.6°) for women and 21.3° (6.2°) for men. Whether native anatomy is a valid reference for acetabular anteversion is controversial,19 and definitive recommendations for target anteversion cannot be made, as the effect of acetabular anteversion on the wear of various bearing materials is unknown.14 Yet, as with inclination, ideal anteversion is likely a compromise between maximizing impingement-free ROM and minimizing wear.
The present study had several limitations. A single-surgeon patient series was reviewed retrospectively, and there was no control group. We determined the incidence only of early dislocation, and 5.3% of THAs that were not metal-on-metal were either lost to follow-up or had inadequate radiographs. However, of the patients excluded for inadequate radiographs, none had an early dislocation. The effects of our surgical techniques on long-term outcomes, bearing wear, and dislocation are unknown. We were not able to comment on the direction of dislocation for any of the 6 patients with early dislocation, as all dislocations were reduced at facilities other than our hospital. Therefore, we cannot determine whether increasing acetabular anteversion resulted in a larger number of anterior versus posterior dislocations.15
We did not use CAS to place any of the femoral components. Therefore, we could not accurately target combined anteversion, defined as the sum of acetabular and femoral version, which may be an important determinant of THA stability.28 Although restoration of femoral offset and leg length is important in preventing THA dislocation,8 the CAS techniques used did not influence these parameters, and they were not measured.
As an imageless navigation system was used, there were no preoperative axial images, which could have been used to assess native acetabular orientation. This limited our assessment with respect to matching each patient’s natural anteversion. Imageless navigation, which references only the anterior pelvic plane, may not be reliable in patients with excessive sagittal pelvic tilt.37 Furthermore, changes in the functional position of the pelvis from supine to sitting to standing were not accounted for, and changes in sagittal tilt between these positions can be significant.38 Changes in sagittal pelvic tilt affect measurement of acetabular anteversion on plain radiographs, with anterior tilt reducing apparent anteversion and posterior tilt increasing it.32,34 Although postoperative computed tomography is the gold standard for assessing acetabular component orientation, EBRA significantly reduces errors of measurement on plain radiographs.10 Some variability in measured anteversion may be explained by our surgical technique. In particular, if the cup was uncovered anteriorly, additional anteversion was usually accepted during surgery to minimize anterior impingement and limit the risk of iliopsoas tendonitis.16,39
Our study results suggested that increasing target acetabular anteversion to 25° may reduce the incidence of early postoperative instability relative to rates reported in the literature. Despite the higher accuracy of component placement with an imageless navigation system, dislocations occurred in patients with acetabular components positioned in our target zone and in the historical safe zone. These dislocations support the notion that there likely is no absolute safe range for acetabular component positioning, as THA stability depends on many factors. Ideal targets for implant orientation for acetabulum and femur may be patient-specific.16,19 Investigators should prospectively evaluate patient-specific THA component positioning and determine its effect on postoperative dislocation and bearing-surface wear. As specific implant targets are further defined, tools that are more precise and accurate than conventional techniques will be needed to achieve goal component positioning. Our study results confirmed that imageless navigation is an accurate method for achieving acetabular orientation targets.
1. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop. 2006;(447):34-38.
2. Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;(441):262-267.
3. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
4. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
5. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21(suppl 1):S1-S6.
6. Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141-147.
7. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867-872.
8. Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop. 1997;(344):179-187.
9. Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132(7):1031-1036.
10. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87(6):762-769.
11. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469(2):319-329.
12. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34(1):19-26.
13. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259-2265.
14. Esposito CI, Walter WL, Roques A, et al. Wear in alumina-on-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012;94(7):901-907.
15. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
16. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. 2010;25(8):1275-1281.e1.
17. Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815-821.
18. Tohtz SW, Sassy D, Matziolis G, Preininger B, Perka C, Hasart O. CT evaluation of native acetabular orientation and localization: sex-specific data comparison on 336 hip joints. Technol Health Care. 2010;18(2):129-136.
19. Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95(22):e172.
20. Nogler M, Kessler O, Prassl A, et al. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop. 2004;(426):159-163.
21. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51-56.
22. Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop. 2004;(426):174-179.
23. Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786-791.
24. Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90(2):357-365.
25. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop. 1998;(355):224-228.
26. Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28(10):1225-1236.
27. Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89(3):494-499.
28. Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;(465):92-99.
29. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15-21.
30. Hohmann E, Bryant A, Tetsworth K. Anterior pelvic soft tissue thickness influences acetabular cup positioning with imageless navigation. J Arthroplasty. 2012;27(6):945-952.
31. Nguyen AD, Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389-398.
32. Malik A, Wan Z, Jaramaz B, Bowman G, Dorr LD. A validation model for measurement of acetabular component position. J Arthroplasty. 2010;25(5):812-819.
33. Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays—a comparison of six parameters. Skeletal Radiol. 2006;35(3):149-155.
34. Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield Award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop. 2009;467(1):43-49.
35. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
36. Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-513.
37. Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26(4):596-605.
38. Lazennec JY, Riwan A, Gravez F, et al. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(suppl 5):S91-S104.
39. Trousdale RT, Cabanela ME, Berry DJ. Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty. 1995;10(4):546-549.
1. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop. 2006;(447):34-38.
2. Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;(441):262-267.
3. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
4. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
5. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21(suppl 1):S1-S6.
6. Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141-147.
7. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867-872.
8. Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop. 1997;(344):179-187.
9. Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132(7):1031-1036.
10. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87(6):762-769.
11. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469(2):319-329.
12. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34(1):19-26.
13. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259-2265.
14. Esposito CI, Walter WL, Roques A, et al. Wear in alumina-on-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012;94(7):901-907.
15. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
16. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. 2010;25(8):1275-1281.e1.
17. Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815-821.
18. Tohtz SW, Sassy D, Matziolis G, Preininger B, Perka C, Hasart O. CT evaluation of native acetabular orientation and localization: sex-specific data comparison on 336 hip joints. Technol Health Care. 2010;18(2):129-136.
19. Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95(22):e172.
20. Nogler M, Kessler O, Prassl A, et al. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop. 2004;(426):159-163.
21. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51-56.
22. Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop. 2004;(426):174-179.
23. Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786-791.
24. Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90(2):357-365.
25. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop. 1998;(355):224-228.
26. Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28(10):1225-1236.
27. Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89(3):494-499.
28. Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;(465):92-99.
29. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15-21.
30. Hohmann E, Bryant A, Tetsworth K. Anterior pelvic soft tissue thickness influences acetabular cup positioning with imageless navigation. J Arthroplasty. 2012;27(6):945-952.
31. Nguyen AD, Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389-398.
32. Malik A, Wan Z, Jaramaz B, Bowman G, Dorr LD. A validation model for measurement of acetabular component position. J Arthroplasty. 2010;25(5):812-819.
33. Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays—a comparison of six parameters. Skeletal Radiol. 2006;35(3):149-155.
34. Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield Award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop. 2009;467(1):43-49.
35. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
36. Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-513.
37. Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26(4):596-605.
38. Lazennec JY, Riwan A, Gravez F, et al. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(suppl 5):S91-S104.
39. Trousdale RT, Cabanela ME, Berry DJ. Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty. 1995;10(4):546-549.
Leg-Length Discrepancy After Total Hip Arthroplasty: Comparison of Robot-Assisted Posterior, Fluoroscopy-Guided Anterior, and Conventional Posterior Approaches
Total hip arthroplasty (THA) effectively provides adequate pain relief and favorable outcomes in patients with hip osteoarthritis (OA). However, leg-length discrepancy (LLD) is still a significant cause of morbidity,1 including nerve damage,2,3 low back pain,2,4,5 and abnormal gait.2,6,7 Although most of the LLD values reported in the literature fall under the acceptable threshold of 10 mm,8 some patients report dissatisfaction,9 leading to litigation against orthopedic surgeons.2 However, lower extremity lengthening is sometimes needed to achieve adequate hip joint stability and prevent dislocations.2,10
Several methods have been developed to help surgeons estimate the change in leg length during surgery in an attempt to improve clinical outcomes. Use of guide pins as a reference on the pelvis decreased LLD and improved outcomes in some published studies.11,12 Preoperative templating of implant size, cup position, and level of femoral neck cut is very important in helping minimize clinically significant LLD after THA.2,13,14 Computer-assisted THA has also been introduced to try to improve component positioning, restoration of hip center of rotation, and minimizing of LLD.15-17 However, cost and increased operative time have prevented widespread adoption of computer-assisted surgery in THA.
Proponents of different surgical approaches have argued about the superiority of one approach over another. The posterior approach is the gold standard in THA because it is safe, easy to perform, and, if needed, extensile.11 However, exact determination of the intraoperative 3-dimensional (3-D) orientation of the pelvis, and subsequently of LLD, is challenging when the patient lies in the lateral position. The anterior approach has gained in popularity because of its advantages in accelerating postoperative rehabilitation and decreasing hospital length of stay.18 Placing the patient supine is advantageous because it allows leveling of the pelvis and estimation of LLD (by comparing the positions of the lower extremities).19 The anterior approach also allows for radiographic measurements on the operating table.19,20 However, this approach has a high learning curve21 and is not extensile.21 To date, no study has shown superiority of the anterior approach over either the conventional posterior approach or the robot-assisted posterior approach in minimizing LLD after THA.
We conducted a study to compare LLD in patients who underwent THA performed with a robot-assisted posterior approach (RTHA), a fluoroscopy-guided anterior approach (ATHA), or a conventional posterior approach (PTHA). We hypothesized that, compared with PTHA, both RTHA and ATHA would result in reduced LLD.
Materials and Methods
We reviewed all RTHAs, ATHAs, and PTHAs performed by Dr. Domb between September 2008 and December 2012. Study inclusion criteria were a diagnosis of hip OA and the availability of postoperative supine anteroposterior pelvis radiographs. Exclusion criteria were a diagnosis other than hip OA, missing or improper postoperative radiographs (radiographs with rotated or tilted pelvis),22 and radiographs on which at least one of the lesser trochanters was difficult to define. Of the 155 cases included in the study, 67 were RTHAs, 29 were ATHAs, and 59 were PTHAs.
All patients scheduled for THA underwent preoperative planning; plain radiographs were used to determine component size and position, level of neck cut, and amount of leg lengthening or shortening needed. In all RTHA cases, computed tomography of the involved hip was performed before surgery. The MAKO system (MAKO Surgical Corporation, Davie, Florida) was used to develop a patient-specific 3-D model of the pelvis and proximal femur, and this model was used to guide THA execution. The system was then used to detect patient-specific landmarks during surgery, to register the femur and the acetabulum, and to help determine the position of the pelvis and proximal femur during surgery. This system, which uses a haptic robotic arm that guides acetabular reaming and cup placement, provides feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the MAKO software, and all provided measurements were made on the coronal (functional) plane of the body, as described by Murray.23 ATHA was performed with the patient in the supine position on a Hana table (Mizuho OSI, Union City, California) with fluoroscopic guidance. PTHA was performed in the conventional way, with the patient in the lateral position.
Radiographic measurements of LLD were made with TraumaCad software (Build 2.2.535.0; Voyant Health, Petah-Tikva, Israel). The accuracy of this software has been studied and reported in the literature.24-26 Radiographs were calibrated using the known size of each femoral head as a marker. The reference on the pelvis was the interobturator line (line tangent to inferior border of obturator foramina), and the reference on the femurs was the most superior and medial aspect of each lesser trochanter. Two lines were drawn, each perpendicular to the interobturator line, starting from the previously defined reference point on each lesser trochanter. The difference in length between these 2 lines was recorded as the LLD. Values were recorded relative to the operative extremity. For example, if the operative extremity was longer than the nonoperative extremity, the LLD was given a positive value.
To eliminate bias and increase measurement accuracy, the study had each of 2 observers collect the LLD data twice, 2 months apart. These observers were blinded to each other’s results and to the type of surgery performed. (Neither observer was Dr. Domb, the senior surgeon.) IBM SPSS Statistics software (Version 20; IBM, Armonk, New York) was used for statistical analysis. Each patient’s 4 measurements were averaged into a single number for LLD, and the absolute LLD values were used in all statistical analyses. Means, standard deviations (SDs), and 95% confidence intervals (CIs) were calculated for LLD in each of the 3 groups. Pearson correlation coefficient was used to determine interobserver and intraobserver reliability. One-way analysis of variance (ANOVA) was used to compare group means for age, body mass index (BMI), and LLD. In each group, number of outliers was determined with outliers set at LLDs of more than 3 mm and more than 5 mm. Fischer exact test was used to compare number of outliers in each group. P < .05 was considered statistically significant.
Results
Table 1 lists the demographic data, including age, sex, and BMI, and compares the means. There were strong interobserver and intraobserver correlations for all LLD measurements (r > 0.9; P < .001). Mean (SD) LLD was 2.7 (1.8) mm (95% CI, 2.3-3.2) in the RTHA group, 1.8 (1.6) mm (95% CI, 1.2-2.4) in the ATHA group, and 1.9 (1.6) mm (95% CI, 1.5-2.4) in the PTHA group (P = .01). When LLD of more than 3 mm was set as an outlier, percentage of outliers was 37.3% (RTHA), 17.2% (ATHA), and 22% (PTHA) (P = .06-.78). When LLD of more than 5 mm was set as an outlier, percentage of outliers was 10.4% (RTHA), 6.9% (ATHA), and 8.5% (PTHA) (P = .72 to >.99). No patient in any group had LLD of 10 mm or more (Figure). Table 2 lists percentages of patients’ operated extremities that were longer, shorter, or the same size as their contralateral extremities. Six (9.0%) of the 67 RTHA patients, 4 (13.8%) of the 29 ATHA patients, and 3 (5.1%) of the 59 PTHA patients had a contralateral THA.
Discussion
Our study results showed that RTHA, ATHA, and PTHA were equally effective in minimizing LLD. There was a statistically significant difference in mean LLD among the 3 groups studied. The RTHA group had the largest mean (SD) LLD: 2.7 (1.8) mm. However, statistically significant differences do not always indicate clinical significance.27 Therefore, comparison of the 3 groups’ means is not enough for drawing significant conclusions. The more important point to consider is the number of cases of LLD of 10 mm or more—a discrepancy that would be perceptible to patients and thus become a source of dissatisfaction with painless THA.28 Patients perceive LLD when shortening exceeds 10 mm and lengthening exceeds 6 mm,29 or when LLD is more than 10 mm.16,19,20 Despite significant differences in means, all our cases came in under the 10-mm threshold. When the threshold was decreased to 5 mm (and to 3 mm), there was no statistically significant difference among the groups in the number of cases above the threshold.
LLD remains a source of significant post-THA comorbidity and patient dissatisfaction.1-7,19 Despite surgeons’ efforts to minimize LLD, some patients can detect even a subtle LLD after surgery.1,8,29 Most LLD values reported in the literature fall under the 10-mm threshold.16,19,20 In some cases, however, postoperative LLD is more than 1 cm, enough to prompt litigation against orthopedic surgeons.2 Surgeons have tried to improve LLD with use of multiple techniques, including use of intraoperative measuring devices,30 patient positioning during surgery,20 use of computer-assisted surgery,19 and use of intraoperative fluoroscopy.20
Proponents of computer-assisted THA have argued that this technique improves accuracy in placing the acetabular cup in the safe zone,31 minimizes LLD, and restores femoral offset.32,33 Manzotti and colleagues16 reported on 48 cases of computer-assisted THA matched to 48 cases of conventional THA using the posterior approach. Mean (SD) LLD was 5.06 (2.99) mm in the computer-assisted group and 7.64 (4.36) mm in the conventional group; there was a statistically significant difference in favor of the computer-assisted group (P = .04). However, 5 patients in the computer-assisted group and 13 in the conventional group had LLD of more than 10 mm, and the difference was statistically significant.16 Moreover, the study population was heterogeneous, with 12 patients in both groups having developmental dysplasia as a primary diagnosis.16 All the cases in our study had a diagnosis of OA, and no case had LLD of 10 mm or more.
Several advantages have been proposed for the anterior approach. The supine position (with direct comparison of leg lengths) and the use of fluoroscopy have been described as advantageous in minimizing LLD.20,21 In their study of 494 primary THAs performed with the anterior approach, Matta and colleagues20 reported mean (SD) postoperative LLD of 3 (2) mm (range, 0-26 mm) and concluded that the anterior approach was effective in restoring leg lengths and ensuring proper cup placement while not increasing the dislocation rate. However, they did not compare this approach with others or with computer-assisted THA with respect to LLD.
In another study, Nam and colleagues19 compared LLD after THA performed with 3 different approaches (anterior, conventional posterior, posterior-navigated) and found no statistically significant difference in LLD among the groups. However, LLD was more than 10 mm in 2.2% of anterior cases, 4.4% of conventional posterior cases, and 4.4% of posterior-navigated cases. When 5 mm was used as a cutoff, percentage of patients who were outliers was 31.1% (anterior), 20% (conventional posterior), and 23.3% (navigated-posterior). Our data showed superior results in using 5 mm as a cutoff, with percentage of outliers of 6.9% with ATHA, 8.5% with PTHA, and 10.4% with RTHA. However, Nam and colleagues19 used a larger patient cohort and different techniques for measuring LLD on anteroposterior pelvis radiographs.
The most likely reason that the groups in our study were comparable in terms of LLD accuracy and lack of outliers over the 10-mm cutoff was Dr. Domb’s high accuracy in minimizing LLD using each of the 3 techniques. For ATHA, mean (SD) LLD was 1.8 (1.6) mm (no LLD of ≥10 mm), better than the 3 (2) mm (0.9% with LLD of >10 mm) reported by Matta and colleagues20 and the 3.8 (3.9) mm (2.2% with LLD of >10 mm) reported by Nam and colleagues.19 For PTHA, mean (SD) LLD was 1.9 (1.6) mm (no LLD of ≥10 mm), comparable to some of the best results reported in the literature—for example, the 1 mm (3% with LLD of >10 mm) reported by Woolson and colleagues.34 For RTHA, mean (SD) LLD was 2.7 (1.8) mm (no LLD of ≥10 mm), superior to the 3.9 (2.7) mm (4.4% with LLD of >10 mm) reported by Nam and colleagues19 for posterior-navigated THA and the 5.06 (2.99) mm (10.4% with LLD of >10 mm) reported by Manzotti and colleagues16 for computer-assisted THA.
This study had several notable strengths. All patients had a diagnosis of hip OA and were operated on by a single surgeon. Radiographs were calibrated using the size of the implanted femoral head. Radiographic data were measured using the same technique in all cases and were collected twice by 2 observers (not the senior surgeon) to decrease bias and determine interobserver and intraobserver reliability. In addition, surgeon experience might have played an important role in minimizing LLD regardless of technique and approach used for THA.
Study limitations were different number of cases in each group, lack of matching, lack of clinical follow-up, and lack of long-term assessment of clinical outcomes and complications.
Conclusion
As performed by an experienced surgeon, RTHA, ATHA, and PTHA did not differ in obtaining minimal LLD. All 3 groups had a low frequency of outliers, using thresholds of 3 mm and 5 mm, and no patient in any group had LLD of 10 mm or more. All 3 techniques are effective in achieving accuracy in LLD.
1. Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19(4 suppl 1):108-110.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop. 1987;(218):136-141.
4. Giles LG, Taylor JR. Low-back pain associated with leg length inequality. Spine. 1981;6(5):510-521.
5. Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2310-2317.
6. Edeen J, Sharkey PF, Alexander AH. Clinical significance of leg-length inequality after total hip arthroplasty. Am J Orthop. 1995;24(4):347-351.
7. Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83(6):907-915.
8. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
9. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
10. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
11. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
12. McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop. 1985;(194):269-270.
13. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13(7):455-462.
14. Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20(1):51-58.
15. Confalonieri N, Manzotti A, Montironi F, Pullen C. Leg length discrepancy, dislocation rate, and offset in total hip replacement using a short modular stem: navigation vs conventional freehand. Orthopedics. 2008;31(10 suppl 1).
16. Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19-24.
17. Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16(2):171-176.
18. Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849-854.
19. Nam D, Sculco PK, Abdel MP, Alexiades MM, Figgie MP, Mayman DJ. Leg-length inequalities following THA based on surgical technique. Orthopedics. 2013;36(4):e395-e400.
20. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
21. Yi C, Agudelo JF, Dayton MR, Morgan SJ. Early complications of anterior supine intermuscular total hip arthroplasty. Orthopedics. 2013;36(3):e276-e281.
22. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop. 2003;(407):241-248.
23. Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228-232.
24. Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32(11):815.
25. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130(12):1429-1432.
26. Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8.
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
28. Abraham WD, Dimon JH 3rd. Leg length discrepancy in total hip arthroplasty. Orthop Clin North Am. 1992;23(2):201-209.
29. Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155-157.
30. Matsuda K, Nakamura S, Matsushita T. A simple method to minimize limb-length discrepancy after hip arthroplasty. Acta Orthop. 2006;77(3):375-379.
31. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22(2):151-159.
32. Renkawitz T, Schuster T, Herold T, et al. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5(2):192-197.
33. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop. 2010;468(4):1072-1081.
34. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159-164.
Total hip arthroplasty (THA) effectively provides adequate pain relief and favorable outcomes in patients with hip osteoarthritis (OA). However, leg-length discrepancy (LLD) is still a significant cause of morbidity,1 including nerve damage,2,3 low back pain,2,4,5 and abnormal gait.2,6,7 Although most of the LLD values reported in the literature fall under the acceptable threshold of 10 mm,8 some patients report dissatisfaction,9 leading to litigation against orthopedic surgeons.2 However, lower extremity lengthening is sometimes needed to achieve adequate hip joint stability and prevent dislocations.2,10
Several methods have been developed to help surgeons estimate the change in leg length during surgery in an attempt to improve clinical outcomes. Use of guide pins as a reference on the pelvis decreased LLD and improved outcomes in some published studies.11,12 Preoperative templating of implant size, cup position, and level of femoral neck cut is very important in helping minimize clinically significant LLD after THA.2,13,14 Computer-assisted THA has also been introduced to try to improve component positioning, restoration of hip center of rotation, and minimizing of LLD.15-17 However, cost and increased operative time have prevented widespread adoption of computer-assisted surgery in THA.
Proponents of different surgical approaches have argued about the superiority of one approach over another. The posterior approach is the gold standard in THA because it is safe, easy to perform, and, if needed, extensile.11 However, exact determination of the intraoperative 3-dimensional (3-D) orientation of the pelvis, and subsequently of LLD, is challenging when the patient lies in the lateral position. The anterior approach has gained in popularity because of its advantages in accelerating postoperative rehabilitation and decreasing hospital length of stay.18 Placing the patient supine is advantageous because it allows leveling of the pelvis and estimation of LLD (by comparing the positions of the lower extremities).19 The anterior approach also allows for radiographic measurements on the operating table.19,20 However, this approach has a high learning curve21 and is not extensile.21 To date, no study has shown superiority of the anterior approach over either the conventional posterior approach or the robot-assisted posterior approach in minimizing LLD after THA.
We conducted a study to compare LLD in patients who underwent THA performed with a robot-assisted posterior approach (RTHA), a fluoroscopy-guided anterior approach (ATHA), or a conventional posterior approach (PTHA). We hypothesized that, compared with PTHA, both RTHA and ATHA would result in reduced LLD.
Materials and Methods
We reviewed all RTHAs, ATHAs, and PTHAs performed by Dr. Domb between September 2008 and December 2012. Study inclusion criteria were a diagnosis of hip OA and the availability of postoperative supine anteroposterior pelvis radiographs. Exclusion criteria were a diagnosis other than hip OA, missing or improper postoperative radiographs (radiographs with rotated or tilted pelvis),22 and radiographs on which at least one of the lesser trochanters was difficult to define. Of the 155 cases included in the study, 67 were RTHAs, 29 were ATHAs, and 59 were PTHAs.
All patients scheduled for THA underwent preoperative planning; plain radiographs were used to determine component size and position, level of neck cut, and amount of leg lengthening or shortening needed. In all RTHA cases, computed tomography of the involved hip was performed before surgery. The MAKO system (MAKO Surgical Corporation, Davie, Florida) was used to develop a patient-specific 3-D model of the pelvis and proximal femur, and this model was used to guide THA execution. The system was then used to detect patient-specific landmarks during surgery, to register the femur and the acetabulum, and to help determine the position of the pelvis and proximal femur during surgery. This system, which uses a haptic robotic arm that guides acetabular reaming and cup placement, provides feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the MAKO software, and all provided measurements were made on the coronal (functional) plane of the body, as described by Murray.23 ATHA was performed with the patient in the supine position on a Hana table (Mizuho OSI, Union City, California) with fluoroscopic guidance. PTHA was performed in the conventional way, with the patient in the lateral position.
Radiographic measurements of LLD were made with TraumaCad software (Build 2.2.535.0; Voyant Health, Petah-Tikva, Israel). The accuracy of this software has been studied and reported in the literature.24-26 Radiographs were calibrated using the known size of each femoral head as a marker. The reference on the pelvis was the interobturator line (line tangent to inferior border of obturator foramina), and the reference on the femurs was the most superior and medial aspect of each lesser trochanter. Two lines were drawn, each perpendicular to the interobturator line, starting from the previously defined reference point on each lesser trochanter. The difference in length between these 2 lines was recorded as the LLD. Values were recorded relative to the operative extremity. For example, if the operative extremity was longer than the nonoperative extremity, the LLD was given a positive value.
To eliminate bias and increase measurement accuracy, the study had each of 2 observers collect the LLD data twice, 2 months apart. These observers were blinded to each other’s results and to the type of surgery performed. (Neither observer was Dr. Domb, the senior surgeon.) IBM SPSS Statistics software (Version 20; IBM, Armonk, New York) was used for statistical analysis. Each patient’s 4 measurements were averaged into a single number for LLD, and the absolute LLD values were used in all statistical analyses. Means, standard deviations (SDs), and 95% confidence intervals (CIs) were calculated for LLD in each of the 3 groups. Pearson correlation coefficient was used to determine interobserver and intraobserver reliability. One-way analysis of variance (ANOVA) was used to compare group means for age, body mass index (BMI), and LLD. In each group, number of outliers was determined with outliers set at LLDs of more than 3 mm and more than 5 mm. Fischer exact test was used to compare number of outliers in each group. P < .05 was considered statistically significant.
Results
Table 1 lists the demographic data, including age, sex, and BMI, and compares the means. There were strong interobserver and intraobserver correlations for all LLD measurements (r > 0.9; P < .001). Mean (SD) LLD was 2.7 (1.8) mm (95% CI, 2.3-3.2) in the RTHA group, 1.8 (1.6) mm (95% CI, 1.2-2.4) in the ATHA group, and 1.9 (1.6) mm (95% CI, 1.5-2.4) in the PTHA group (P = .01). When LLD of more than 3 mm was set as an outlier, percentage of outliers was 37.3% (RTHA), 17.2% (ATHA), and 22% (PTHA) (P = .06-.78). When LLD of more than 5 mm was set as an outlier, percentage of outliers was 10.4% (RTHA), 6.9% (ATHA), and 8.5% (PTHA) (P = .72 to >.99). No patient in any group had LLD of 10 mm or more (Figure). Table 2 lists percentages of patients’ operated extremities that were longer, shorter, or the same size as their contralateral extremities. Six (9.0%) of the 67 RTHA patients, 4 (13.8%) of the 29 ATHA patients, and 3 (5.1%) of the 59 PTHA patients had a contralateral THA.
Discussion
Our study results showed that RTHA, ATHA, and PTHA were equally effective in minimizing LLD. There was a statistically significant difference in mean LLD among the 3 groups studied. The RTHA group had the largest mean (SD) LLD: 2.7 (1.8) mm. However, statistically significant differences do not always indicate clinical significance.27 Therefore, comparison of the 3 groups’ means is not enough for drawing significant conclusions. The more important point to consider is the number of cases of LLD of 10 mm or more—a discrepancy that would be perceptible to patients and thus become a source of dissatisfaction with painless THA.28 Patients perceive LLD when shortening exceeds 10 mm and lengthening exceeds 6 mm,29 or when LLD is more than 10 mm.16,19,20 Despite significant differences in means, all our cases came in under the 10-mm threshold. When the threshold was decreased to 5 mm (and to 3 mm), there was no statistically significant difference among the groups in the number of cases above the threshold.
LLD remains a source of significant post-THA comorbidity and patient dissatisfaction.1-7,19 Despite surgeons’ efforts to minimize LLD, some patients can detect even a subtle LLD after surgery.1,8,29 Most LLD values reported in the literature fall under the 10-mm threshold.16,19,20 In some cases, however, postoperative LLD is more than 1 cm, enough to prompt litigation against orthopedic surgeons.2 Surgeons have tried to improve LLD with use of multiple techniques, including use of intraoperative measuring devices,30 patient positioning during surgery,20 use of computer-assisted surgery,19 and use of intraoperative fluoroscopy.20
Proponents of computer-assisted THA have argued that this technique improves accuracy in placing the acetabular cup in the safe zone,31 minimizes LLD, and restores femoral offset.32,33 Manzotti and colleagues16 reported on 48 cases of computer-assisted THA matched to 48 cases of conventional THA using the posterior approach. Mean (SD) LLD was 5.06 (2.99) mm in the computer-assisted group and 7.64 (4.36) mm in the conventional group; there was a statistically significant difference in favor of the computer-assisted group (P = .04). However, 5 patients in the computer-assisted group and 13 in the conventional group had LLD of more than 10 mm, and the difference was statistically significant.16 Moreover, the study population was heterogeneous, with 12 patients in both groups having developmental dysplasia as a primary diagnosis.16 All the cases in our study had a diagnosis of OA, and no case had LLD of 10 mm or more.
Several advantages have been proposed for the anterior approach. The supine position (with direct comparison of leg lengths) and the use of fluoroscopy have been described as advantageous in minimizing LLD.20,21 In their study of 494 primary THAs performed with the anterior approach, Matta and colleagues20 reported mean (SD) postoperative LLD of 3 (2) mm (range, 0-26 mm) and concluded that the anterior approach was effective in restoring leg lengths and ensuring proper cup placement while not increasing the dislocation rate. However, they did not compare this approach with others or with computer-assisted THA with respect to LLD.
In another study, Nam and colleagues19 compared LLD after THA performed with 3 different approaches (anterior, conventional posterior, posterior-navigated) and found no statistically significant difference in LLD among the groups. However, LLD was more than 10 mm in 2.2% of anterior cases, 4.4% of conventional posterior cases, and 4.4% of posterior-navigated cases. When 5 mm was used as a cutoff, percentage of patients who were outliers was 31.1% (anterior), 20% (conventional posterior), and 23.3% (navigated-posterior). Our data showed superior results in using 5 mm as a cutoff, with percentage of outliers of 6.9% with ATHA, 8.5% with PTHA, and 10.4% with RTHA. However, Nam and colleagues19 used a larger patient cohort and different techniques for measuring LLD on anteroposterior pelvis radiographs.
The most likely reason that the groups in our study were comparable in terms of LLD accuracy and lack of outliers over the 10-mm cutoff was Dr. Domb’s high accuracy in minimizing LLD using each of the 3 techniques. For ATHA, mean (SD) LLD was 1.8 (1.6) mm (no LLD of ≥10 mm), better than the 3 (2) mm (0.9% with LLD of >10 mm) reported by Matta and colleagues20 and the 3.8 (3.9) mm (2.2% with LLD of >10 mm) reported by Nam and colleagues.19 For PTHA, mean (SD) LLD was 1.9 (1.6) mm (no LLD of ≥10 mm), comparable to some of the best results reported in the literature—for example, the 1 mm (3% with LLD of >10 mm) reported by Woolson and colleagues.34 For RTHA, mean (SD) LLD was 2.7 (1.8) mm (no LLD of ≥10 mm), superior to the 3.9 (2.7) mm (4.4% with LLD of >10 mm) reported by Nam and colleagues19 for posterior-navigated THA and the 5.06 (2.99) mm (10.4% with LLD of >10 mm) reported by Manzotti and colleagues16 for computer-assisted THA.
This study had several notable strengths. All patients had a diagnosis of hip OA and were operated on by a single surgeon. Radiographs were calibrated using the size of the implanted femoral head. Radiographic data were measured using the same technique in all cases and were collected twice by 2 observers (not the senior surgeon) to decrease bias and determine interobserver and intraobserver reliability. In addition, surgeon experience might have played an important role in minimizing LLD regardless of technique and approach used for THA.
Study limitations were different number of cases in each group, lack of matching, lack of clinical follow-up, and lack of long-term assessment of clinical outcomes and complications.
Conclusion
As performed by an experienced surgeon, RTHA, ATHA, and PTHA did not differ in obtaining minimal LLD. All 3 groups had a low frequency of outliers, using thresholds of 3 mm and 5 mm, and no patient in any group had LLD of 10 mm or more. All 3 techniques are effective in achieving accuracy in LLD.
Total hip arthroplasty (THA) effectively provides adequate pain relief and favorable outcomes in patients with hip osteoarthritis (OA). However, leg-length discrepancy (LLD) is still a significant cause of morbidity,1 including nerve damage,2,3 low back pain,2,4,5 and abnormal gait.2,6,7 Although most of the LLD values reported in the literature fall under the acceptable threshold of 10 mm,8 some patients report dissatisfaction,9 leading to litigation against orthopedic surgeons.2 However, lower extremity lengthening is sometimes needed to achieve adequate hip joint stability and prevent dislocations.2,10
Several methods have been developed to help surgeons estimate the change in leg length during surgery in an attempt to improve clinical outcomes. Use of guide pins as a reference on the pelvis decreased LLD and improved outcomes in some published studies.11,12 Preoperative templating of implant size, cup position, and level of femoral neck cut is very important in helping minimize clinically significant LLD after THA.2,13,14 Computer-assisted THA has also been introduced to try to improve component positioning, restoration of hip center of rotation, and minimizing of LLD.15-17 However, cost and increased operative time have prevented widespread adoption of computer-assisted surgery in THA.
Proponents of different surgical approaches have argued about the superiority of one approach over another. The posterior approach is the gold standard in THA because it is safe, easy to perform, and, if needed, extensile.11 However, exact determination of the intraoperative 3-dimensional (3-D) orientation of the pelvis, and subsequently of LLD, is challenging when the patient lies in the lateral position. The anterior approach has gained in popularity because of its advantages in accelerating postoperative rehabilitation and decreasing hospital length of stay.18 Placing the patient supine is advantageous because it allows leveling of the pelvis and estimation of LLD (by comparing the positions of the lower extremities).19 The anterior approach also allows for radiographic measurements on the operating table.19,20 However, this approach has a high learning curve21 and is not extensile.21 To date, no study has shown superiority of the anterior approach over either the conventional posterior approach or the robot-assisted posterior approach in minimizing LLD after THA.
We conducted a study to compare LLD in patients who underwent THA performed with a robot-assisted posterior approach (RTHA), a fluoroscopy-guided anterior approach (ATHA), or a conventional posterior approach (PTHA). We hypothesized that, compared with PTHA, both RTHA and ATHA would result in reduced LLD.
Materials and Methods
We reviewed all RTHAs, ATHAs, and PTHAs performed by Dr. Domb between September 2008 and December 2012. Study inclusion criteria were a diagnosis of hip OA and the availability of postoperative supine anteroposterior pelvis radiographs. Exclusion criteria were a diagnosis other than hip OA, missing or improper postoperative radiographs (radiographs with rotated or tilted pelvis),22 and radiographs on which at least one of the lesser trochanters was difficult to define. Of the 155 cases included in the study, 67 were RTHAs, 29 were ATHAs, and 59 were PTHAs.
All patients scheduled for THA underwent preoperative planning; plain radiographs were used to determine component size and position, level of neck cut, and amount of leg lengthening or shortening needed. In all RTHA cases, computed tomography of the involved hip was performed before surgery. The MAKO system (MAKO Surgical Corporation, Davie, Florida) was used to develop a patient-specific 3-D model of the pelvis and proximal femur, and this model was used to guide THA execution. The system was then used to detect patient-specific landmarks during surgery, to register the femur and the acetabulum, and to help determine the position of the pelvis and proximal femur during surgery. This system, which uses a haptic robotic arm that guides acetabular reaming and cup placement, provides feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the MAKO software, and all provided measurements were made on the coronal (functional) plane of the body, as described by Murray.23 ATHA was performed with the patient in the supine position on a Hana table (Mizuho OSI, Union City, California) with fluoroscopic guidance. PTHA was performed in the conventional way, with the patient in the lateral position.
Radiographic measurements of LLD were made with TraumaCad software (Build 2.2.535.0; Voyant Health, Petah-Tikva, Israel). The accuracy of this software has been studied and reported in the literature.24-26 Radiographs were calibrated using the known size of each femoral head as a marker. The reference on the pelvis was the interobturator line (line tangent to inferior border of obturator foramina), and the reference on the femurs was the most superior and medial aspect of each lesser trochanter. Two lines were drawn, each perpendicular to the interobturator line, starting from the previously defined reference point on each lesser trochanter. The difference in length between these 2 lines was recorded as the LLD. Values were recorded relative to the operative extremity. For example, if the operative extremity was longer than the nonoperative extremity, the LLD was given a positive value.
To eliminate bias and increase measurement accuracy, the study had each of 2 observers collect the LLD data twice, 2 months apart. These observers were blinded to each other’s results and to the type of surgery performed. (Neither observer was Dr. Domb, the senior surgeon.) IBM SPSS Statistics software (Version 20; IBM, Armonk, New York) was used for statistical analysis. Each patient’s 4 measurements were averaged into a single number for LLD, and the absolute LLD values were used in all statistical analyses. Means, standard deviations (SDs), and 95% confidence intervals (CIs) were calculated for LLD in each of the 3 groups. Pearson correlation coefficient was used to determine interobserver and intraobserver reliability. One-way analysis of variance (ANOVA) was used to compare group means for age, body mass index (BMI), and LLD. In each group, number of outliers was determined with outliers set at LLDs of more than 3 mm and more than 5 mm. Fischer exact test was used to compare number of outliers in each group. P < .05 was considered statistically significant.
Results
Table 1 lists the demographic data, including age, sex, and BMI, and compares the means. There were strong interobserver and intraobserver correlations for all LLD measurements (r > 0.9; P < .001). Mean (SD) LLD was 2.7 (1.8) mm (95% CI, 2.3-3.2) in the RTHA group, 1.8 (1.6) mm (95% CI, 1.2-2.4) in the ATHA group, and 1.9 (1.6) mm (95% CI, 1.5-2.4) in the PTHA group (P = .01). When LLD of more than 3 mm was set as an outlier, percentage of outliers was 37.3% (RTHA), 17.2% (ATHA), and 22% (PTHA) (P = .06-.78). When LLD of more than 5 mm was set as an outlier, percentage of outliers was 10.4% (RTHA), 6.9% (ATHA), and 8.5% (PTHA) (P = .72 to >.99). No patient in any group had LLD of 10 mm or more (Figure). Table 2 lists percentages of patients’ operated extremities that were longer, shorter, or the same size as their contralateral extremities. Six (9.0%) of the 67 RTHA patients, 4 (13.8%) of the 29 ATHA patients, and 3 (5.1%) of the 59 PTHA patients had a contralateral THA.
Discussion
Our study results showed that RTHA, ATHA, and PTHA were equally effective in minimizing LLD. There was a statistically significant difference in mean LLD among the 3 groups studied. The RTHA group had the largest mean (SD) LLD: 2.7 (1.8) mm. However, statistically significant differences do not always indicate clinical significance.27 Therefore, comparison of the 3 groups’ means is not enough for drawing significant conclusions. The more important point to consider is the number of cases of LLD of 10 mm or more—a discrepancy that would be perceptible to patients and thus become a source of dissatisfaction with painless THA.28 Patients perceive LLD when shortening exceeds 10 mm and lengthening exceeds 6 mm,29 or when LLD is more than 10 mm.16,19,20 Despite significant differences in means, all our cases came in under the 10-mm threshold. When the threshold was decreased to 5 mm (and to 3 mm), there was no statistically significant difference among the groups in the number of cases above the threshold.
LLD remains a source of significant post-THA comorbidity and patient dissatisfaction.1-7,19 Despite surgeons’ efforts to minimize LLD, some patients can detect even a subtle LLD after surgery.1,8,29 Most LLD values reported in the literature fall under the 10-mm threshold.16,19,20 In some cases, however, postoperative LLD is more than 1 cm, enough to prompt litigation against orthopedic surgeons.2 Surgeons have tried to improve LLD with use of multiple techniques, including use of intraoperative measuring devices,30 patient positioning during surgery,20 use of computer-assisted surgery,19 and use of intraoperative fluoroscopy.20
Proponents of computer-assisted THA have argued that this technique improves accuracy in placing the acetabular cup in the safe zone,31 minimizes LLD, and restores femoral offset.32,33 Manzotti and colleagues16 reported on 48 cases of computer-assisted THA matched to 48 cases of conventional THA using the posterior approach. Mean (SD) LLD was 5.06 (2.99) mm in the computer-assisted group and 7.64 (4.36) mm in the conventional group; there was a statistically significant difference in favor of the computer-assisted group (P = .04). However, 5 patients in the computer-assisted group and 13 in the conventional group had LLD of more than 10 mm, and the difference was statistically significant.16 Moreover, the study population was heterogeneous, with 12 patients in both groups having developmental dysplasia as a primary diagnosis.16 All the cases in our study had a diagnosis of OA, and no case had LLD of 10 mm or more.
Several advantages have been proposed for the anterior approach. The supine position (with direct comparison of leg lengths) and the use of fluoroscopy have been described as advantageous in minimizing LLD.20,21 In their study of 494 primary THAs performed with the anterior approach, Matta and colleagues20 reported mean (SD) postoperative LLD of 3 (2) mm (range, 0-26 mm) and concluded that the anterior approach was effective in restoring leg lengths and ensuring proper cup placement while not increasing the dislocation rate. However, they did not compare this approach with others or with computer-assisted THA with respect to LLD.
In another study, Nam and colleagues19 compared LLD after THA performed with 3 different approaches (anterior, conventional posterior, posterior-navigated) and found no statistically significant difference in LLD among the groups. However, LLD was more than 10 mm in 2.2% of anterior cases, 4.4% of conventional posterior cases, and 4.4% of posterior-navigated cases. When 5 mm was used as a cutoff, percentage of patients who were outliers was 31.1% (anterior), 20% (conventional posterior), and 23.3% (navigated-posterior). Our data showed superior results in using 5 mm as a cutoff, with percentage of outliers of 6.9% with ATHA, 8.5% with PTHA, and 10.4% with RTHA. However, Nam and colleagues19 used a larger patient cohort and different techniques for measuring LLD on anteroposterior pelvis radiographs.
The most likely reason that the groups in our study were comparable in terms of LLD accuracy and lack of outliers over the 10-mm cutoff was Dr. Domb’s high accuracy in minimizing LLD using each of the 3 techniques. For ATHA, mean (SD) LLD was 1.8 (1.6) mm (no LLD of ≥10 mm), better than the 3 (2) mm (0.9% with LLD of >10 mm) reported by Matta and colleagues20 and the 3.8 (3.9) mm (2.2% with LLD of >10 mm) reported by Nam and colleagues.19 For PTHA, mean (SD) LLD was 1.9 (1.6) mm (no LLD of ≥10 mm), comparable to some of the best results reported in the literature—for example, the 1 mm (3% with LLD of >10 mm) reported by Woolson and colleagues.34 For RTHA, mean (SD) LLD was 2.7 (1.8) mm (no LLD of ≥10 mm), superior to the 3.9 (2.7) mm (4.4% with LLD of >10 mm) reported by Nam and colleagues19 for posterior-navigated THA and the 5.06 (2.99) mm (10.4% with LLD of >10 mm) reported by Manzotti and colleagues16 for computer-assisted THA.
This study had several notable strengths. All patients had a diagnosis of hip OA and were operated on by a single surgeon. Radiographs were calibrated using the size of the implanted femoral head. Radiographic data were measured using the same technique in all cases and were collected twice by 2 observers (not the senior surgeon) to decrease bias and determine interobserver and intraobserver reliability. In addition, surgeon experience might have played an important role in minimizing LLD regardless of technique and approach used for THA.
Study limitations were different number of cases in each group, lack of matching, lack of clinical follow-up, and lack of long-term assessment of clinical outcomes and complications.
Conclusion
As performed by an experienced surgeon, RTHA, ATHA, and PTHA did not differ in obtaining minimal LLD. All 3 groups had a low frequency of outliers, using thresholds of 3 mm and 5 mm, and no patient in any group had LLD of 10 mm or more. All 3 techniques are effective in achieving accuracy in LLD.
1. Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19(4 suppl 1):108-110.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop. 1987;(218):136-141.
4. Giles LG, Taylor JR. Low-back pain associated with leg length inequality. Spine. 1981;6(5):510-521.
5. Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2310-2317.
6. Edeen J, Sharkey PF, Alexander AH. Clinical significance of leg-length inequality after total hip arthroplasty. Am J Orthop. 1995;24(4):347-351.
7. Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83(6):907-915.
8. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
9. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
10. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
11. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
12. McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop. 1985;(194):269-270.
13. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13(7):455-462.
14. Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20(1):51-58.
15. Confalonieri N, Manzotti A, Montironi F, Pullen C. Leg length discrepancy, dislocation rate, and offset in total hip replacement using a short modular stem: navigation vs conventional freehand. Orthopedics. 2008;31(10 suppl 1).
16. Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19-24.
17. Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16(2):171-176.
18. Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849-854.
19. Nam D, Sculco PK, Abdel MP, Alexiades MM, Figgie MP, Mayman DJ. Leg-length inequalities following THA based on surgical technique. Orthopedics. 2013;36(4):e395-e400.
20. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
21. Yi C, Agudelo JF, Dayton MR, Morgan SJ. Early complications of anterior supine intermuscular total hip arthroplasty. Orthopedics. 2013;36(3):e276-e281.
22. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop. 2003;(407):241-248.
23. Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228-232.
24. Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32(11):815.
25. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130(12):1429-1432.
26. Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8.
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
28. Abraham WD, Dimon JH 3rd. Leg length discrepancy in total hip arthroplasty. Orthop Clin North Am. 1992;23(2):201-209.
29. Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155-157.
30. Matsuda K, Nakamura S, Matsushita T. A simple method to minimize limb-length discrepancy after hip arthroplasty. Acta Orthop. 2006;77(3):375-379.
31. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22(2):151-159.
32. Renkawitz T, Schuster T, Herold T, et al. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5(2):192-197.
33. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop. 2010;468(4):1072-1081.
34. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159-164.
1. Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19(4 suppl 1):108-110.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop. 1987;(218):136-141.
4. Giles LG, Taylor JR. Low-back pain associated with leg length inequality. Spine. 1981;6(5):510-521.
5. Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2310-2317.
6. Edeen J, Sharkey PF, Alexander AH. Clinical significance of leg-length inequality after total hip arthroplasty. Am J Orthop. 1995;24(4):347-351.
7. Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83(6):907-915.
8. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
9. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
10. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
11. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
12. McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop. 1985;(194):269-270.
13. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13(7):455-462.
14. Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20(1):51-58.
15. Confalonieri N, Manzotti A, Montironi F, Pullen C. Leg length discrepancy, dislocation rate, and offset in total hip replacement using a short modular stem: navigation vs conventional freehand. Orthopedics. 2008;31(10 suppl 1).
16. Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19-24.
17. Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16(2):171-176.
18. Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849-854.
19. Nam D, Sculco PK, Abdel MP, Alexiades MM, Figgie MP, Mayman DJ. Leg-length inequalities following THA based on surgical technique. Orthopedics. 2013;36(4):e395-e400.
20. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
21. Yi C, Agudelo JF, Dayton MR, Morgan SJ. Early complications of anterior supine intermuscular total hip arthroplasty. Orthopedics. 2013;36(3):e276-e281.
22. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop. 2003;(407):241-248.
23. Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228-232.
24. Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32(11):815.
25. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130(12):1429-1432.
26. Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8.
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
28. Abraham WD, Dimon JH 3rd. Leg length discrepancy in total hip arthroplasty. Orthop Clin North Am. 1992;23(2):201-209.
29. Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155-157.
30. Matsuda K, Nakamura S, Matsushita T. A simple method to minimize limb-length discrepancy after hip arthroplasty. Acta Orthop. 2006;77(3):375-379.
31. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22(2):151-159.
32. Renkawitz T, Schuster T, Herold T, et al. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5(2):192-197.
33. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop. 2010;468(4):1072-1081.
34. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159-164.
Patients’ Perceptions of the Costs of Total Hip and Knee Arthroplasty
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
Total Hip Arthroplasty After Contralateral Hip Disarticulation: A Challenging “Simple Primary”
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Operative Intervention for Geriatric Hip Fracture: Does Type of Surgery Affect Hospital Length of Stay?
Hip fractures, the most severe and costly fall-related fractures, account for 350,000 hospital admissions per year.1 The majority of hip fractures result from low-impact falls, typically in patients over age 60 years. In fact, the increase in hip fracture with age is nearly exponential.2,3 With the predicted aging of our population, hip fractures will continue to increase in volume. Between 2000 and 2050, the elderly US population will increase by 135%,4 proportionately increasing the number of projected hip fractures. Considering that hip fractures account for 72% of total costs in terms of orthopedic fracture care in the elderly, the dramatic rise in hip fractures is of great concern for future costs of health care delivery in this field.5-7
In an effort to move toward a value-based system in which costs are reduced while quality of care is maintained, Medicare recently unveiled a new bundled payment system of reimbursement. Through this system, hospitals will be reimbursed for treatment provided to Medicare beneficiaries based on the expected costs of care, instead of through the traditional fee-for-service model. Given this development, orthopedic surgeons will need to develop interventions that reduce costs while maintaining quality of care after hip fracture surgery.
One of the most significant ramifications of a value-based system is that reimbursement for hip fractures may be standardized based on a single diagnosis regardless of the actual costs associated with treatment.8 In hip fracture cases, however, a wide range of factors, including degree of communition of the bone, presence of medical comorbidities,9 and amount of soft-tissue injury, can dramatically increase recovery time. In fact, one of the most important determinants of treatment costs related to hospital length of stay (LOS) is whether the fracture is a femoral neck or intertrochanteric fracture.10,11 Type of fracture is a significant determinant of surgical options, and these can dramatically change patient outcomes and costs of surgical care.12-16 In addition, hospital recovery time or LOS can vary widely based on type of surgery. As hospitalization costs account for 44% of the direct medical costs for hip fractures,17 differences in LOS can have major financial implications in a value-based system of reimbursement in which all forms of hip fracture are reimbursed a standard amount.
We conducted a study to analyze differences in hospital LOS for different forms of hip fracture repair to determine the potential financial repercussions of a bundled payment model of reimbursement. By performing a retrospective chart review at a large, level I trauma center, we were able to compare LOS and associated costs for total hip arthroplasty (THA), hemiarthroplasty (HA), cephalomedullary nailing (CMN), open reduction and internal fixation (ORIF), and closed reduction and percutaneous pinning (CRPP).
Materials and Methods
After receiving institutional review board approval for this study, we retrospectively reviewed all hip fracture cases treated at a level I trauma center between January 2000 and December 2009. Current Procedural Terminology (CPT) codes were searched for cases of low-energy falls that caused hip fractures that were resolved with THA, HA, CMN, ORIF, or CRPP. Patients who underwent HA or THA were grouped for analysis. Patients who were over age 60 years and had acetabular, proximal femoral, trochanteric, or femoral neck fractures were included in our search. Patients who had incomplete medical records or did not meet the age criterion were excluded from analysis.
We reviewed patient charts in our institutional electronic medical records database to collect these data: date of birth, age, sex, date of admission, date of discharge, American Society of Anesthesiologists (ASA) Physical Status score, complications, height, weight, start and stop times of procedure, whether or not the procedure was an emergent procedure, days from admission to surgery, 90-day readmissions, days from surgery to discharge, and general category of operation. We also recorded individual comorbidities, including prior myocardial infarction, dysrhythmia, atrial fibrillation, congestive heart failure, heart block, cerebrovascular disease, chronic obstructive pulmonary disease, emphysema, current smoking status, smoking history, renal disease, dialysis, cancer, and diabetes. Duration of surgery was calculated from recorded start and stop times. Body mass index was calculated using height and weight recorded during initial stay. LOS was recorded as the difference between the admission and discharge dates.
Mean total cost to the hospital ($4530/d patient was hospitalized) was obtained from the institution’s financial services. All fractional LOS values were rounded to the nearest whole number and multiplied by the per diem cost. Student t test was used to compare mean LOS and costs of HA/THA with those of all the other procedures. Additional tests were run to analyze differences in LOS and type of surgeries performed throughout the 9-year period. A multivariate regression model controlling for ASA score, body mass index, age, sex, and comorbidities was developed to analyze differences in LOS and costs for patients who underwent HA/THA versus CMN, ORIF, and CRPP. Significance was set at P = .05.
Results
Our search identified 720 patients who were over age 60 years and underwent operative fixation for hip fracture at our level I trauma center between 2000 and 2009. Of these 720 patients, 105 who had incomplete charts or did not meet the age criteria were excluded, leaving 615 patients (with complete records of isolated low-energy hip fractures) for analysis.
Table 1 lists the demographics of our patient population. The majority of patients had undergone ORIF (30.24%) or HA/THA (45.69%). CRPP was the least common procedure (9.92%) after CMN (14.15%). Mean age was 78.4 years; the majority of patients were between 75 and 89 years of age. Mean hospital LOS was 6.91 days. The majority of patients (n = 414; 67.32%) were female. ASA scores had a narrow distribution, with most patients assigned a score of 3. The readmission rate was significantly higher for HA/THA (39.1%) than for ORIF (28.5%; P = .02) and CRPP (24.6%; P = .04).
Table 2 lists mean LOS and associated costs for each procedure compared with HA/THA. Mean LOS for all patients was 6.91 days, with associated hospitalization costs of $30,011.25. Patients who underwent HA/THA had the longest mean LOS (7.43 days) and highest mean hospitalization costs ($33,657.90). In comparison, patients who underwent ORIF had a mean LOS of 6.59 days with $29,852.70 in costs (P = .04). CRPP also had a significantly (P < .003) shorter LOS (5.59 days) and lower costs ($25,322.70). Although CMN had a mean LOS of 6.89 days and $31,211.70 in costs, the difference in LOS was not significantly different from that of HA/THA. The proportion of surgeries that were HA/THA, CMN, ORIF, and CRPP did not change significantly through the 9-year period (P = .19). Similarly, mean LOS did not change significantly for any of the types of surgery through this period (Table 3).
Figure 1 provides the distribution of LOS for all 4 procedures. The interquartile range (IQR) for patients who underwent HA/THA was 4 to 9 days (median, 6 days). Patients who underwent CMN also had a median LOS of 6 days and an IQR of 4 to 8 days. Both ORIF (IQR, 4-8 days) and CRPP (IQR, 3-6 days) were associated with a median LOS of 5 days.
Figure 2 shows mean hospitalization costs based on type of procedure. HA/THA had the highest mean cost, $33,657.90, or $8335.20 more than CRPP ($25,322.70). Patients who underwent CMN had a mean cost of $31,211.70, versus $29,852.70 for patients who underwent ORIF.
Table 4 summarizes the multivariate analysis results. After ASA score, sex, age, and comorbidities were controlled for, there was an overall significant relationship involving surgical treatment, LOS, and associated hospitalization costs for HA/THA, ORIF, and CRPP. Compared with HA/THA, ORIF had $3805.20 less in costs (P = .042) and 0.84 fewer hospital days. Patients who underwent CRPP were hospitalized for significantly fewer days (1.63) and associated costs ($7383.90) (P = .0076). There was no significant difference in LOS and costs between HA/THA and CMN. Of the controlled variables, only ASA score (P < .001) and male sex (P = .001) were significantly associated with changes in LOS and costs. There was no significant association with comorbidities, LOS, or costs.
Discussion
In this study of surgical intervention in patients with hip fractures, we determined that HA/THA was associated with significantly increased hospital LOS and costs than ORIF and CRPP. Although arthroplasty had an increased mean LOS compared with CMN, the difference was not statistically significant. In addition to type of procedure, both male sex (P = .001) and preoperative ASA score (P < .001) were significant predictors of LOS and costs. These findings are supported by other studies in which preoperative functioning was found to be a strong predictor of increased LOS and costs among hip fracture patients,18 most likely because of increased risk for complications.19
Although our study was the first to directly compare LOS and costs for HA/THA and CMN, other investigators have analyzed the effect of surgical complications on LOS for patients treated with THA, HA, and CMN. In a study on the effects of surgical complications on LOS after hip fracture surgery, Foss and colleagues17 reported that the proportion of CMN patients (31%) with complications was larger than that of HA patients (19%) and THA patients (0%). They also reported that surgical complications were associated with significantly increased LOS during primary admission. Similarly, Edwards and colleagues20 found that the infection risk was higher with CMN (3.1%) than with THA (0%) and HA (0%-2.3%) and that infections were associated with increased LOS (P > .001). However, further statistical analysis revealed that the odds of developing an infection were not significantly higher with CMN than with other studies.20 Similarly, other studies have reported low rates of complications, including nonunion, with CMN.21,22 In our study, we found no significant difference in LOS and costs for CMN and HA/THA after controlling for ASA score, which is known to be associated with a higher risk for complications.18,19
The largest difference in LOS and costs after controlling for potential confounding variables was between HA/THA and CRPP ($7383.90). To our knowledge, only one study has performed a comparative analysis of LOS for CRPP and other surgical treatments for hip fractures. For femoral neck fractures treated between 1990 and 1994, Fekete and colleagues23 found that LOS was 14.9 days for ORIF cases and 12.1 days for CRPP cases—a difference of 2.8 days. In comparison, we found a 1-day difference in mean LOS between ORIF cases (6.59 days) and CRPP cases (5.59 days).
Other studies of LOS and associated costs over a 2-year period have found that ORIF is overall more costly than HA/THA. For example, Keating and colleagues13 compared total costs of care, including LOS, for healthy older patients with displaced intracapsular hip fractures treated with ORIF, bipolar HA, or THA. Although ORIF was initially less costly than HA/THA, overall ORIF costs over 2 years were significantly higher because of readmissions, which increased overall LOS. Similarly, in cases of displaced femoral fractures, Iorio and colleagues15 found that LOS was 6.4 days for ORIF, 4.9 days for unipolar HA, 6.2 days for bipolar HA, and 5.5 days for cemented and hybrid THA. However, when overall projected costs were estimated, including the costs of rehabilitation and of (probable) revision arthroplasty, ORIF was estimated to cost more over a 2-year period because of the need for additional care and in-patient stays. In contrast, we found that hospitalization costs were $3805.20 lower for ORIF than for HA/THA, even after adjusting for comorbidities, and that ORIF had a lower overall readmission rate. Early discharge of patients who are at risk for subsequent complications may have played a significant role in increasing readmission rates for arthroplasty patients. These findings indicate the complexities involved in a bundled payment system of reimbursement, in which a single payment for both initial stay and related readmissions will force orthopedists to consider long-term hospitalization costs when deciding on length of postoperative care and the most cost-effective surgical treatment.
One of the limitations of this study is its retrospective design. Although selection of our sample from a single level I trauma center reduced differences in cost and patient care protocols between institutions, it also reduced the generalizability of our actual costs. In addition, for some patients, LOS may have increased because of delays in surgery or discharge, lack of operating room availability, or need for further medical clearance for additional procedures. Day of admission could also have significantly affected LOS. However, the effects of these confounding factors were reduced because of the large sample analyzed. As stated earlier, overall LOS depends on both initial in-patient stays and readmissions. Therefore, long-term prospective studies that compare LOS and associated costs for patients with hip fractures treated with ORIF, CRPP, HA/THA, and CMN are needed.
Conclusion
It has been recently suggested that hip fracture repair be included in the National Pilot Program on Payment Bundling, which will potentially reimburse orthopedic surgeons a standardized amount for hip fracture surgery regardless of actual treatment costs.8 In this model, it will be essential to understand how type of fracture and surgical procedure can influence LOS and therefore hip fracture treatment costs. We found that, based on these factors, mean LOS ranged from 5.59 to 7.43 days, which translates to a cost range of $25,322.70 to $33,657.90. Before a standardized bundled payment system is implemented, further studies are needed to identify other factors that can significantly affect the cost of hip fracture repair.
1. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
2. Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham study. Am J Public Health. 2002;92(5):858-862.
3. Scott JC. Osteoporosis and hip fractures. Rheum Dis Clin North Am. 1990;16(3):717-740.
4. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776-781.
5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465-475.
6. Burge RT, King AB, Balda E, Worley D. Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health. 2003;6(5):574-583.
7. Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605-615.
8. Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff. 2011;30(9):1708-1717.
9. Shah A, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop. 2002;(399):28-34.
10. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
11. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
12. Carroll C, Stevenson M, Scope A, Evans P, Buckley S. Hemiarthroplasty and total hip arthroplasty for treating primary intracapsular fracture of the hip: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15(36):1-74.
13. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
14. Rogmark C, Carlsson A, Johnell O, Sembo I. Costs of internal fixation and arthroplasty for displaced femoral neck fractures: a randomized study of 68 patients. Acta Orthop Scand. 2003;74(3):293-298.
15. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop. 2001;(383):229-242.
16. Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24(6):854-860.
17. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
18. Garcia AE, Bonnaig JV, Yoneda ZT. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
19. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
20. Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770-777.
21. Jain P, Maini L, Mishra P, Upadhyay A, Agarwal A. Cephalomedullary interlocked nail for ipsilateral hip and femoral shaft fractures. Injury. 2004;35(10):1031-1038.
22. Matre K, Havelin LI, Gjertsen JE, Espehaug B, Fevang JM. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop. 2013;471(4):1379-1386.
23. Fekete K, Manninger J, Kazár G, Cserháti P, Bosch U. Percutaneous internal fixation of femoral neck fractures with cannulated screws and a small tension band plate. Orthop Traumatol. 2000;8(4):250-263.
Hip fractures, the most severe and costly fall-related fractures, account for 350,000 hospital admissions per year.1 The majority of hip fractures result from low-impact falls, typically in patients over age 60 years. In fact, the increase in hip fracture with age is nearly exponential.2,3 With the predicted aging of our population, hip fractures will continue to increase in volume. Between 2000 and 2050, the elderly US population will increase by 135%,4 proportionately increasing the number of projected hip fractures. Considering that hip fractures account for 72% of total costs in terms of orthopedic fracture care in the elderly, the dramatic rise in hip fractures is of great concern for future costs of health care delivery in this field.5-7
In an effort to move toward a value-based system in which costs are reduced while quality of care is maintained, Medicare recently unveiled a new bundled payment system of reimbursement. Through this system, hospitals will be reimbursed for treatment provided to Medicare beneficiaries based on the expected costs of care, instead of through the traditional fee-for-service model. Given this development, orthopedic surgeons will need to develop interventions that reduce costs while maintaining quality of care after hip fracture surgery.
One of the most significant ramifications of a value-based system is that reimbursement for hip fractures may be standardized based on a single diagnosis regardless of the actual costs associated with treatment.8 In hip fracture cases, however, a wide range of factors, including degree of communition of the bone, presence of medical comorbidities,9 and amount of soft-tissue injury, can dramatically increase recovery time. In fact, one of the most important determinants of treatment costs related to hospital length of stay (LOS) is whether the fracture is a femoral neck or intertrochanteric fracture.10,11 Type of fracture is a significant determinant of surgical options, and these can dramatically change patient outcomes and costs of surgical care.12-16 In addition, hospital recovery time or LOS can vary widely based on type of surgery. As hospitalization costs account for 44% of the direct medical costs for hip fractures,17 differences in LOS can have major financial implications in a value-based system of reimbursement in which all forms of hip fracture are reimbursed a standard amount.
We conducted a study to analyze differences in hospital LOS for different forms of hip fracture repair to determine the potential financial repercussions of a bundled payment model of reimbursement. By performing a retrospective chart review at a large, level I trauma center, we were able to compare LOS and associated costs for total hip arthroplasty (THA), hemiarthroplasty (HA), cephalomedullary nailing (CMN), open reduction and internal fixation (ORIF), and closed reduction and percutaneous pinning (CRPP).
Materials and Methods
After receiving institutional review board approval for this study, we retrospectively reviewed all hip fracture cases treated at a level I trauma center between January 2000 and December 2009. Current Procedural Terminology (CPT) codes were searched for cases of low-energy falls that caused hip fractures that were resolved with THA, HA, CMN, ORIF, or CRPP. Patients who underwent HA or THA were grouped for analysis. Patients who were over age 60 years and had acetabular, proximal femoral, trochanteric, or femoral neck fractures were included in our search. Patients who had incomplete medical records or did not meet the age criterion were excluded from analysis.
We reviewed patient charts in our institutional electronic medical records database to collect these data: date of birth, age, sex, date of admission, date of discharge, American Society of Anesthesiologists (ASA) Physical Status score, complications, height, weight, start and stop times of procedure, whether or not the procedure was an emergent procedure, days from admission to surgery, 90-day readmissions, days from surgery to discharge, and general category of operation. We also recorded individual comorbidities, including prior myocardial infarction, dysrhythmia, atrial fibrillation, congestive heart failure, heart block, cerebrovascular disease, chronic obstructive pulmonary disease, emphysema, current smoking status, smoking history, renal disease, dialysis, cancer, and diabetes. Duration of surgery was calculated from recorded start and stop times. Body mass index was calculated using height and weight recorded during initial stay. LOS was recorded as the difference between the admission and discharge dates.
Mean total cost to the hospital ($4530/d patient was hospitalized) was obtained from the institution’s financial services. All fractional LOS values were rounded to the nearest whole number and multiplied by the per diem cost. Student t test was used to compare mean LOS and costs of HA/THA with those of all the other procedures. Additional tests were run to analyze differences in LOS and type of surgeries performed throughout the 9-year period. A multivariate regression model controlling for ASA score, body mass index, age, sex, and comorbidities was developed to analyze differences in LOS and costs for patients who underwent HA/THA versus CMN, ORIF, and CRPP. Significance was set at P = .05.
Results
Our search identified 720 patients who were over age 60 years and underwent operative fixation for hip fracture at our level I trauma center between 2000 and 2009. Of these 720 patients, 105 who had incomplete charts or did not meet the age criteria were excluded, leaving 615 patients (with complete records of isolated low-energy hip fractures) for analysis.
Table 1 lists the demographics of our patient population. The majority of patients had undergone ORIF (30.24%) or HA/THA (45.69%). CRPP was the least common procedure (9.92%) after CMN (14.15%). Mean age was 78.4 years; the majority of patients were between 75 and 89 years of age. Mean hospital LOS was 6.91 days. The majority of patients (n = 414; 67.32%) were female. ASA scores had a narrow distribution, with most patients assigned a score of 3. The readmission rate was significantly higher for HA/THA (39.1%) than for ORIF (28.5%; P = .02) and CRPP (24.6%; P = .04).
Table 2 lists mean LOS and associated costs for each procedure compared with HA/THA. Mean LOS for all patients was 6.91 days, with associated hospitalization costs of $30,011.25. Patients who underwent HA/THA had the longest mean LOS (7.43 days) and highest mean hospitalization costs ($33,657.90). In comparison, patients who underwent ORIF had a mean LOS of 6.59 days with $29,852.70 in costs (P = .04). CRPP also had a significantly (P < .003) shorter LOS (5.59 days) and lower costs ($25,322.70). Although CMN had a mean LOS of 6.89 days and $31,211.70 in costs, the difference in LOS was not significantly different from that of HA/THA. The proportion of surgeries that were HA/THA, CMN, ORIF, and CRPP did not change significantly through the 9-year period (P = .19). Similarly, mean LOS did not change significantly for any of the types of surgery through this period (Table 3).
Figure 1 provides the distribution of LOS for all 4 procedures. The interquartile range (IQR) for patients who underwent HA/THA was 4 to 9 days (median, 6 days). Patients who underwent CMN also had a median LOS of 6 days and an IQR of 4 to 8 days. Both ORIF (IQR, 4-8 days) and CRPP (IQR, 3-6 days) were associated with a median LOS of 5 days.
Figure 2 shows mean hospitalization costs based on type of procedure. HA/THA had the highest mean cost, $33,657.90, or $8335.20 more than CRPP ($25,322.70). Patients who underwent CMN had a mean cost of $31,211.70, versus $29,852.70 for patients who underwent ORIF.
Table 4 summarizes the multivariate analysis results. After ASA score, sex, age, and comorbidities were controlled for, there was an overall significant relationship involving surgical treatment, LOS, and associated hospitalization costs for HA/THA, ORIF, and CRPP. Compared with HA/THA, ORIF had $3805.20 less in costs (P = .042) and 0.84 fewer hospital days. Patients who underwent CRPP were hospitalized for significantly fewer days (1.63) and associated costs ($7383.90) (P = .0076). There was no significant difference in LOS and costs between HA/THA and CMN. Of the controlled variables, only ASA score (P < .001) and male sex (P = .001) were significantly associated with changes in LOS and costs. There was no significant association with comorbidities, LOS, or costs.
Discussion
In this study of surgical intervention in patients with hip fractures, we determined that HA/THA was associated with significantly increased hospital LOS and costs than ORIF and CRPP. Although arthroplasty had an increased mean LOS compared with CMN, the difference was not statistically significant. In addition to type of procedure, both male sex (P = .001) and preoperative ASA score (P < .001) were significant predictors of LOS and costs. These findings are supported by other studies in which preoperative functioning was found to be a strong predictor of increased LOS and costs among hip fracture patients,18 most likely because of increased risk for complications.19
Although our study was the first to directly compare LOS and costs for HA/THA and CMN, other investigators have analyzed the effect of surgical complications on LOS for patients treated with THA, HA, and CMN. In a study on the effects of surgical complications on LOS after hip fracture surgery, Foss and colleagues17 reported that the proportion of CMN patients (31%) with complications was larger than that of HA patients (19%) and THA patients (0%). They also reported that surgical complications were associated with significantly increased LOS during primary admission. Similarly, Edwards and colleagues20 found that the infection risk was higher with CMN (3.1%) than with THA (0%) and HA (0%-2.3%) and that infections were associated with increased LOS (P > .001). However, further statistical analysis revealed that the odds of developing an infection were not significantly higher with CMN than with other studies.20 Similarly, other studies have reported low rates of complications, including nonunion, with CMN.21,22 In our study, we found no significant difference in LOS and costs for CMN and HA/THA after controlling for ASA score, which is known to be associated with a higher risk for complications.18,19
The largest difference in LOS and costs after controlling for potential confounding variables was between HA/THA and CRPP ($7383.90). To our knowledge, only one study has performed a comparative analysis of LOS for CRPP and other surgical treatments for hip fractures. For femoral neck fractures treated between 1990 and 1994, Fekete and colleagues23 found that LOS was 14.9 days for ORIF cases and 12.1 days for CRPP cases—a difference of 2.8 days. In comparison, we found a 1-day difference in mean LOS between ORIF cases (6.59 days) and CRPP cases (5.59 days).
Other studies of LOS and associated costs over a 2-year period have found that ORIF is overall more costly than HA/THA. For example, Keating and colleagues13 compared total costs of care, including LOS, for healthy older patients with displaced intracapsular hip fractures treated with ORIF, bipolar HA, or THA. Although ORIF was initially less costly than HA/THA, overall ORIF costs over 2 years were significantly higher because of readmissions, which increased overall LOS. Similarly, in cases of displaced femoral fractures, Iorio and colleagues15 found that LOS was 6.4 days for ORIF, 4.9 days for unipolar HA, 6.2 days for bipolar HA, and 5.5 days for cemented and hybrid THA. However, when overall projected costs were estimated, including the costs of rehabilitation and of (probable) revision arthroplasty, ORIF was estimated to cost more over a 2-year period because of the need for additional care and in-patient stays. In contrast, we found that hospitalization costs were $3805.20 lower for ORIF than for HA/THA, even after adjusting for comorbidities, and that ORIF had a lower overall readmission rate. Early discharge of patients who are at risk for subsequent complications may have played a significant role in increasing readmission rates for arthroplasty patients. These findings indicate the complexities involved in a bundled payment system of reimbursement, in which a single payment for both initial stay and related readmissions will force orthopedists to consider long-term hospitalization costs when deciding on length of postoperative care and the most cost-effective surgical treatment.
One of the limitations of this study is its retrospective design. Although selection of our sample from a single level I trauma center reduced differences in cost and patient care protocols between institutions, it also reduced the generalizability of our actual costs. In addition, for some patients, LOS may have increased because of delays in surgery or discharge, lack of operating room availability, or need for further medical clearance for additional procedures. Day of admission could also have significantly affected LOS. However, the effects of these confounding factors were reduced because of the large sample analyzed. As stated earlier, overall LOS depends on both initial in-patient stays and readmissions. Therefore, long-term prospective studies that compare LOS and associated costs for patients with hip fractures treated with ORIF, CRPP, HA/THA, and CMN are needed.
Conclusion
It has been recently suggested that hip fracture repair be included in the National Pilot Program on Payment Bundling, which will potentially reimburse orthopedic surgeons a standardized amount for hip fracture surgery regardless of actual treatment costs.8 In this model, it will be essential to understand how type of fracture and surgical procedure can influence LOS and therefore hip fracture treatment costs. We found that, based on these factors, mean LOS ranged from 5.59 to 7.43 days, which translates to a cost range of $25,322.70 to $33,657.90. Before a standardized bundled payment system is implemented, further studies are needed to identify other factors that can significantly affect the cost of hip fracture repair.
Hip fractures, the most severe and costly fall-related fractures, account for 350,000 hospital admissions per year.1 The majority of hip fractures result from low-impact falls, typically in patients over age 60 years. In fact, the increase in hip fracture with age is nearly exponential.2,3 With the predicted aging of our population, hip fractures will continue to increase in volume. Between 2000 and 2050, the elderly US population will increase by 135%,4 proportionately increasing the number of projected hip fractures. Considering that hip fractures account for 72% of total costs in terms of orthopedic fracture care in the elderly, the dramatic rise in hip fractures is of great concern for future costs of health care delivery in this field.5-7
In an effort to move toward a value-based system in which costs are reduced while quality of care is maintained, Medicare recently unveiled a new bundled payment system of reimbursement. Through this system, hospitals will be reimbursed for treatment provided to Medicare beneficiaries based on the expected costs of care, instead of through the traditional fee-for-service model. Given this development, orthopedic surgeons will need to develop interventions that reduce costs while maintaining quality of care after hip fracture surgery.
One of the most significant ramifications of a value-based system is that reimbursement for hip fractures may be standardized based on a single diagnosis regardless of the actual costs associated with treatment.8 In hip fracture cases, however, a wide range of factors, including degree of communition of the bone, presence of medical comorbidities,9 and amount of soft-tissue injury, can dramatically increase recovery time. In fact, one of the most important determinants of treatment costs related to hospital length of stay (LOS) is whether the fracture is a femoral neck or intertrochanteric fracture.10,11 Type of fracture is a significant determinant of surgical options, and these can dramatically change patient outcomes and costs of surgical care.12-16 In addition, hospital recovery time or LOS can vary widely based on type of surgery. As hospitalization costs account for 44% of the direct medical costs for hip fractures,17 differences in LOS can have major financial implications in a value-based system of reimbursement in which all forms of hip fracture are reimbursed a standard amount.
We conducted a study to analyze differences in hospital LOS for different forms of hip fracture repair to determine the potential financial repercussions of a bundled payment model of reimbursement. By performing a retrospective chart review at a large, level I trauma center, we were able to compare LOS and associated costs for total hip arthroplasty (THA), hemiarthroplasty (HA), cephalomedullary nailing (CMN), open reduction and internal fixation (ORIF), and closed reduction and percutaneous pinning (CRPP).
Materials and Methods
After receiving institutional review board approval for this study, we retrospectively reviewed all hip fracture cases treated at a level I trauma center between January 2000 and December 2009. Current Procedural Terminology (CPT) codes were searched for cases of low-energy falls that caused hip fractures that were resolved with THA, HA, CMN, ORIF, or CRPP. Patients who underwent HA or THA were grouped for analysis. Patients who were over age 60 years and had acetabular, proximal femoral, trochanteric, or femoral neck fractures were included in our search. Patients who had incomplete medical records or did not meet the age criterion were excluded from analysis.
We reviewed patient charts in our institutional electronic medical records database to collect these data: date of birth, age, sex, date of admission, date of discharge, American Society of Anesthesiologists (ASA) Physical Status score, complications, height, weight, start and stop times of procedure, whether or not the procedure was an emergent procedure, days from admission to surgery, 90-day readmissions, days from surgery to discharge, and general category of operation. We also recorded individual comorbidities, including prior myocardial infarction, dysrhythmia, atrial fibrillation, congestive heart failure, heart block, cerebrovascular disease, chronic obstructive pulmonary disease, emphysema, current smoking status, smoking history, renal disease, dialysis, cancer, and diabetes. Duration of surgery was calculated from recorded start and stop times. Body mass index was calculated using height and weight recorded during initial stay. LOS was recorded as the difference between the admission and discharge dates.
Mean total cost to the hospital ($4530/d patient was hospitalized) was obtained from the institution’s financial services. All fractional LOS values were rounded to the nearest whole number and multiplied by the per diem cost. Student t test was used to compare mean LOS and costs of HA/THA with those of all the other procedures. Additional tests were run to analyze differences in LOS and type of surgeries performed throughout the 9-year period. A multivariate regression model controlling for ASA score, body mass index, age, sex, and comorbidities was developed to analyze differences in LOS and costs for patients who underwent HA/THA versus CMN, ORIF, and CRPP. Significance was set at P = .05.
Results
Our search identified 720 patients who were over age 60 years and underwent operative fixation for hip fracture at our level I trauma center between 2000 and 2009. Of these 720 patients, 105 who had incomplete charts or did not meet the age criteria were excluded, leaving 615 patients (with complete records of isolated low-energy hip fractures) for analysis.
Table 1 lists the demographics of our patient population. The majority of patients had undergone ORIF (30.24%) or HA/THA (45.69%). CRPP was the least common procedure (9.92%) after CMN (14.15%). Mean age was 78.4 years; the majority of patients were between 75 and 89 years of age. Mean hospital LOS was 6.91 days. The majority of patients (n = 414; 67.32%) were female. ASA scores had a narrow distribution, with most patients assigned a score of 3. The readmission rate was significantly higher for HA/THA (39.1%) than for ORIF (28.5%; P = .02) and CRPP (24.6%; P = .04).
Table 2 lists mean LOS and associated costs for each procedure compared with HA/THA. Mean LOS for all patients was 6.91 days, with associated hospitalization costs of $30,011.25. Patients who underwent HA/THA had the longest mean LOS (7.43 days) and highest mean hospitalization costs ($33,657.90). In comparison, patients who underwent ORIF had a mean LOS of 6.59 days with $29,852.70 in costs (P = .04). CRPP also had a significantly (P < .003) shorter LOS (5.59 days) and lower costs ($25,322.70). Although CMN had a mean LOS of 6.89 days and $31,211.70 in costs, the difference in LOS was not significantly different from that of HA/THA. The proportion of surgeries that were HA/THA, CMN, ORIF, and CRPP did not change significantly through the 9-year period (P = .19). Similarly, mean LOS did not change significantly for any of the types of surgery through this period (Table 3).
Figure 1 provides the distribution of LOS for all 4 procedures. The interquartile range (IQR) for patients who underwent HA/THA was 4 to 9 days (median, 6 days). Patients who underwent CMN also had a median LOS of 6 days and an IQR of 4 to 8 days. Both ORIF (IQR, 4-8 days) and CRPP (IQR, 3-6 days) were associated with a median LOS of 5 days.
Figure 2 shows mean hospitalization costs based on type of procedure. HA/THA had the highest mean cost, $33,657.90, or $8335.20 more than CRPP ($25,322.70). Patients who underwent CMN had a mean cost of $31,211.70, versus $29,852.70 for patients who underwent ORIF.
Table 4 summarizes the multivariate analysis results. After ASA score, sex, age, and comorbidities were controlled for, there was an overall significant relationship involving surgical treatment, LOS, and associated hospitalization costs for HA/THA, ORIF, and CRPP. Compared with HA/THA, ORIF had $3805.20 less in costs (P = .042) and 0.84 fewer hospital days. Patients who underwent CRPP were hospitalized for significantly fewer days (1.63) and associated costs ($7383.90) (P = .0076). There was no significant difference in LOS and costs between HA/THA and CMN. Of the controlled variables, only ASA score (P < .001) and male sex (P = .001) were significantly associated with changes in LOS and costs. There was no significant association with comorbidities, LOS, or costs.
Discussion
In this study of surgical intervention in patients with hip fractures, we determined that HA/THA was associated with significantly increased hospital LOS and costs than ORIF and CRPP. Although arthroplasty had an increased mean LOS compared with CMN, the difference was not statistically significant. In addition to type of procedure, both male sex (P = .001) and preoperative ASA score (P < .001) were significant predictors of LOS and costs. These findings are supported by other studies in which preoperative functioning was found to be a strong predictor of increased LOS and costs among hip fracture patients,18 most likely because of increased risk for complications.19
Although our study was the first to directly compare LOS and costs for HA/THA and CMN, other investigators have analyzed the effect of surgical complications on LOS for patients treated with THA, HA, and CMN. In a study on the effects of surgical complications on LOS after hip fracture surgery, Foss and colleagues17 reported that the proportion of CMN patients (31%) with complications was larger than that of HA patients (19%) and THA patients (0%). They also reported that surgical complications were associated with significantly increased LOS during primary admission. Similarly, Edwards and colleagues20 found that the infection risk was higher with CMN (3.1%) than with THA (0%) and HA (0%-2.3%) and that infections were associated with increased LOS (P > .001). However, further statistical analysis revealed that the odds of developing an infection were not significantly higher with CMN than with other studies.20 Similarly, other studies have reported low rates of complications, including nonunion, with CMN.21,22 In our study, we found no significant difference in LOS and costs for CMN and HA/THA after controlling for ASA score, which is known to be associated with a higher risk for complications.18,19
The largest difference in LOS and costs after controlling for potential confounding variables was between HA/THA and CRPP ($7383.90). To our knowledge, only one study has performed a comparative analysis of LOS for CRPP and other surgical treatments for hip fractures. For femoral neck fractures treated between 1990 and 1994, Fekete and colleagues23 found that LOS was 14.9 days for ORIF cases and 12.1 days for CRPP cases—a difference of 2.8 days. In comparison, we found a 1-day difference in mean LOS between ORIF cases (6.59 days) and CRPP cases (5.59 days).
Other studies of LOS and associated costs over a 2-year period have found that ORIF is overall more costly than HA/THA. For example, Keating and colleagues13 compared total costs of care, including LOS, for healthy older patients with displaced intracapsular hip fractures treated with ORIF, bipolar HA, or THA. Although ORIF was initially less costly than HA/THA, overall ORIF costs over 2 years were significantly higher because of readmissions, which increased overall LOS. Similarly, in cases of displaced femoral fractures, Iorio and colleagues15 found that LOS was 6.4 days for ORIF, 4.9 days for unipolar HA, 6.2 days for bipolar HA, and 5.5 days for cemented and hybrid THA. However, when overall projected costs were estimated, including the costs of rehabilitation and of (probable) revision arthroplasty, ORIF was estimated to cost more over a 2-year period because of the need for additional care and in-patient stays. In contrast, we found that hospitalization costs were $3805.20 lower for ORIF than for HA/THA, even after adjusting for comorbidities, and that ORIF had a lower overall readmission rate. Early discharge of patients who are at risk for subsequent complications may have played a significant role in increasing readmission rates for arthroplasty patients. These findings indicate the complexities involved in a bundled payment system of reimbursement, in which a single payment for both initial stay and related readmissions will force orthopedists to consider long-term hospitalization costs when deciding on length of postoperative care and the most cost-effective surgical treatment.
One of the limitations of this study is its retrospective design. Although selection of our sample from a single level I trauma center reduced differences in cost and patient care protocols between institutions, it also reduced the generalizability of our actual costs. In addition, for some patients, LOS may have increased because of delays in surgery or discharge, lack of operating room availability, or need for further medical clearance for additional procedures. Day of admission could also have significantly affected LOS. However, the effects of these confounding factors were reduced because of the large sample analyzed. As stated earlier, overall LOS depends on both initial in-patient stays and readmissions. Therefore, long-term prospective studies that compare LOS and associated costs for patients with hip fractures treated with ORIF, CRPP, HA/THA, and CMN are needed.
Conclusion
It has been recently suggested that hip fracture repair be included in the National Pilot Program on Payment Bundling, which will potentially reimburse orthopedic surgeons a standardized amount for hip fracture surgery regardless of actual treatment costs.8 In this model, it will be essential to understand how type of fracture and surgical procedure can influence LOS and therefore hip fracture treatment costs. We found that, based on these factors, mean LOS ranged from 5.59 to 7.43 days, which translates to a cost range of $25,322.70 to $33,657.90. Before a standardized bundled payment system is implemented, further studies are needed to identify other factors that can significantly affect the cost of hip fracture repair.
1. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
2. Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham study. Am J Public Health. 2002;92(5):858-862.
3. Scott JC. Osteoporosis and hip fractures. Rheum Dis Clin North Am. 1990;16(3):717-740.
4. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776-781.
5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465-475.
6. Burge RT, King AB, Balda E, Worley D. Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health. 2003;6(5):574-583.
7. Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605-615.
8. Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff. 2011;30(9):1708-1717.
9. Shah A, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop. 2002;(399):28-34.
10. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
11. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
12. Carroll C, Stevenson M, Scope A, Evans P, Buckley S. Hemiarthroplasty and total hip arthroplasty for treating primary intracapsular fracture of the hip: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15(36):1-74.
13. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
14. Rogmark C, Carlsson A, Johnell O, Sembo I. Costs of internal fixation and arthroplasty for displaced femoral neck fractures: a randomized study of 68 patients. Acta Orthop Scand. 2003;74(3):293-298.
15. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop. 2001;(383):229-242.
16. Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24(6):854-860.
17. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
18. Garcia AE, Bonnaig JV, Yoneda ZT. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
19. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
20. Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770-777.
21. Jain P, Maini L, Mishra P, Upadhyay A, Agarwal A. Cephalomedullary interlocked nail for ipsilateral hip and femoral shaft fractures. Injury. 2004;35(10):1031-1038.
22. Matre K, Havelin LI, Gjertsen JE, Espehaug B, Fevang JM. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop. 2013;471(4):1379-1386.
23. Fekete K, Manninger J, Kazár G, Cserháti P, Bosch U. Percutaneous internal fixation of femoral neck fractures with cannulated screws and a small tension band plate. Orthop Traumatol. 2000;8(4):250-263.
1. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
2. Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham study. Am J Public Health. 2002;92(5):858-862.
3. Scott JC. Osteoporosis and hip fractures. Rheum Dis Clin North Am. 1990;16(3):717-740.
4. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776-781.
5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465-475.
6. Burge RT, King AB, Balda E, Worley D. Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health. 2003;6(5):574-583.
7. Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605-615.
8. Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff. 2011;30(9):1708-1717.
9. Shah A, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop. 2002;(399):28-34.
10. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
11. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
12. Carroll C, Stevenson M, Scope A, Evans P, Buckley S. Hemiarthroplasty and total hip arthroplasty for treating primary intracapsular fracture of the hip: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15(36):1-74.
13. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
14. Rogmark C, Carlsson A, Johnell O, Sembo I. Costs of internal fixation and arthroplasty for displaced femoral neck fractures: a randomized study of 68 patients. Acta Orthop Scand. 2003;74(3):293-298.
15. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop. 2001;(383):229-242.
16. Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24(6):854-860.
17. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
18. Garcia AE, Bonnaig JV, Yoneda ZT. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
19. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
20. Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770-777.
21. Jain P, Maini L, Mishra P, Upadhyay A, Agarwal A. Cephalomedullary interlocked nail for ipsilateral hip and femoral shaft fractures. Injury. 2004;35(10):1031-1038.
22. Matre K, Havelin LI, Gjertsen JE, Espehaug B, Fevang JM. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop. 2013;471(4):1379-1386.
23. Fekete K, Manninger J, Kazár G, Cserháti P, Bosch U. Percutaneous internal fixation of femoral neck fractures with cannulated screws and a small tension band plate. Orthop Traumatol. 2000;8(4):250-263.
Retrograde Reamer/Irrigator/Aspirator Technique for Autologous Bone Graft Harvesting With the Patient in the Prone Position
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
Women Fare Better Than Men Following Total Knee, Hip Replacement
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”
LAS VEGAS—While women may have their first total joint replacement (TJR) at an older age, they are less likely to have complications related to their surgery or require revision surgery, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS). The findings contradict the theory that TJR is underutilized in female patients because they have worse outcomes than men.
Researchers reviewed patient databases from an Ontario hospital for first-time primary total hip replacement (THR) and total knee replacement (TKR) patients between 2002 and 2009. There were 37,881 THR surgeries (53.8% female) and 59,564 TKR surgeries (60.5% female). Women who underwent THR were significantly older than males (70 years vs. 65 years); however, there was no difference in age between male and female patients undergoing TKR (median age 68 years for both). A greater proportion of female patients undergoing TJR were defined as frail (6.6% vs. 3.5% for THR; and, 6.7% vs. 4% for TKR).
Following surgery, men were:
• 15% more likely to return to the emergency department within 30 days of hospital discharge following either THR or TKR.
• 60% and 70% more likely to have an acute myocardial infarction within 3 months following THR and TKR, respectively.
• 50% more likely to require a revision arthroplasty within 2 years of TKR.
• 25% more likely to be readmitted to the hospital and 70% more likely to experience an infection or revision surgery within 2 years of TKR, compared to women.
“Despite the fact that women have a higher prevalence of advanced hip and knee arthritis, prior research indicates that North American women with arthritis are less likely to receive joint replacement than men,” said lead study author Bheeshma Ravi, MD, PhD, an orthopedic surgery resident at the University of Toronto. “One possible explanation is that women are less often offered or accept surgery because their risk of serious complications following surgery is greater than that of men.
“In this study, we found that while overall rates of serious complications were low for both groups, they were lower for women than for men for both hip and knee replacement, particularly the latter” said Dr. Ravi. “Thus, the previously documented sex difference utilization of TJR cannot be explained by differential risks of complications following surgery.”