User login
Cementing Multihole, Metal, Modular Acetabular Shells Into Cages in Revision Total Hip Arthroplasty
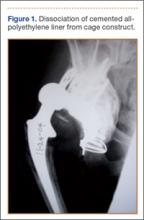
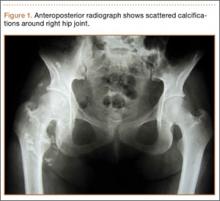
Although the number of total hip arthroplasties (THAs) being performed in the United States is increasing, revision THAs are more common.1 Many acetabular revisions can be successfully performed with standard or jumbo cementless acetabular cups, but major osseous deficiencies typically require reconstruction with a cage or cup/cage that bridges gaps in the pelvis and obtains fixation of the arthroplasty components.2,3 Cages and rings have been combined with all-polyethylene acetabular components (ie, all-polyethylene cups, or APCs) to reconstruct pelvic bone defects, but complications, including APC dissociation (Figure 1) and postoperative instability, can occur despite stable fixation of cage to pelvis.4 The incidence of dislocations with pelvic reconstruction rings using APCs has been reported to be 11%.4 If an APC has to be replaced because of wear, then major surgery may be required to extract the worn cup and cement a new cup in its place.
In this article, we describe a technique in which a metal, multihole acetabular shell is cemented into the cage or ring construct, avoiding some of the complications associated with traditional techniques by permitting use of a variety of liners.
Materials and Methods
We retrospectively reviewed the cases of all of Dr. Bolanos’ patients who underwent acetabular revision THA with cage reconstruction between February 1, 1998 and October 9, 2006. During this period, we were cementing a modular metal shell into the cage instead of an APC or polyethylene liner. All patients who underwent revision THA with cage reconstruction during the study period were included. Bone defects were treated with structural or morselized bone allograft. Every reconstruction involved use of an antiprotrusio cage or ring secured to the pelvis with screws, and a multihole acetabular shell cemented into place with a polyethylene liner applied. Elevated rims, lateralized liners, and constrained liners were used as needed to optimize stability. Femoral components were retained. Cage size was based on matching the osseous deficiencies. Shell size was determined by the inner diameter of the corresponding cage. Liner size was based on matching the shell and femoral head. During this period, none of the patients had other reconstructive techniques, such as trabecular metal augmentation, in combination with a modular acetabular shell, cup/cage reconstruction, or custom triflange components.
Patients engaged in protected weight-bearing ambulation for 3 months after surgery and were then permitted full, unrestricted activity. The primary outcome was mechanical failure of the reconstruction, or reoperation (Table). All reconstructions in this series consisted of acetabular revisions for aseptic loosening.
Surgical Technique
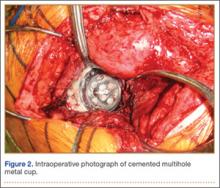
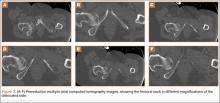
Six consecutive cases of pelvic discontinuity and 7 cases of segmental acetabular bone loss required use of cages or rings. Reconstruction cages were used to secure fixation to the ilium and ischium. With the technique described in this article, we used screws with rounded, prominent heads rather than flat heads between the cup and the cage or ring (Synthes, 6.5 mm) to ensure adequate cement mantle. The rounded screw heads were left prominent to approximate the function of cement pegs found on APCs. Screws were placed into the anterior, superior, medial, and posterior aspects of the cage to ensure adequate cement mantle between cup and cage. This was confirmed with trial placement of the cup into the cage before cementation and observation of the uniformity of the space between cup and cage. Trial placement also confirmed that the screws did not interfere with appropriate positioning of the cup. A multihole, metal acetabular cup was then cemented in the cage or ring such that cement extruded around the shell and into the holes of the cup and the cage, securing the cup to the cage. Use of a multihole, metal shell resulted in excellent cement fixation because the multiple holes created multiple circumferential cement pegs. Various liner options could then be used to optimize stability of the reconstruction. In some cases, excessive cement extruded into the interior aspect of the shell and hardened before curettage. If the excess cement could interfere with complete seating/locking of the liner, then a high-speed burr was used to easily remove cement (Figure 2). Polyethylene liners were then inserted into the shell. Femoral reconstruction was then performed, if needed, and stability of the arthroplasty checked. This technique allows the surgeon to then select from a variety of polyethylene liners as needed to optimize stability. Liners with elevated rims, lateralized liners, and constrained liners could be interchangeable options with this technique.
Results
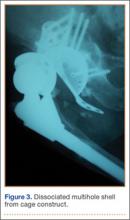
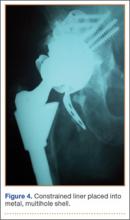
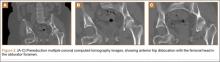
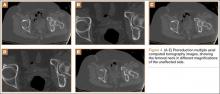
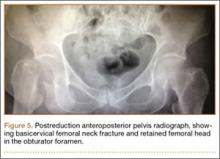
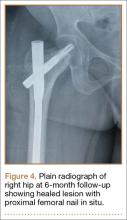
Thirteen patients with major osseous deficiencies of the pelvis were treated using this technique. At mean follow-up of 64.2 months (range, 3-133 months), 10 of the 13 patients had favorable outcomes without further surgery. One patient developed recurrent aseptic loosening that required re-revision, another patient developed recurrent instability that required acetabular liner and femoral head exchange, and a third patient with poor balance fell multiple times. This patient’s ninth fall resulted in dissociation of the acetabular shell from the cage (Figure 3), treated with placement of another cemented multihole metal shell with a standard liner. As dislocations recurred, the liner was changed to a constrained liner (Figure 4). The patient did not have any further dislocations or other hip-related problems. Integrity of cemented shell-cage fixation was maintained in 12 of the 13 patients at final follow-up.
Discussion
We have described a novel technique that facilitates reconstruction of major osseous deficiencies of the pelvis. The technique involves cementation of a multihole, metal acetabular shell into a cage or ring, permitting use of modular liners. The modularity in this approach to major hip reconstruction provides stability-optimization options that are not available with APCs. So far, the technique has demonstrated more advantages than disadvantages, so the indications for its use would be whenever a cage is used for pelvic reconstruction. Traditional techniques involve cementing an APC into the cage or ring. Use of multihole, metal shells for this purpose has several theoretical advantages. Multiple holes and the textured surface allow more interdigitation of cement with cup than APCs do; this interdigitation may improve the durability of the cemented interface. Cement also extrudes through the holes of the cage to secure the cup to the pelvis, as is done with cementation of APCs. Introduction of trabecular metal shells may also provide an even more secure bond to the shell, compared with APCs, though durability of a cemented trabecular metal interface has not been established. In addition, mechanical alignment guides cannot fasten as securely onto some APCs.
Nonmodular, cemented, metal-backed acetabular components, which were commonly used in hip arthroplasties at one time, were abandoned because of their relatively high loosening rate and because of advantages noted with modular components.5 The nonmodular components had been developed because of their theoretical advantages of improved distribution of forces into the cement mantle.5,6 However, those models had a relatively smooth metallic surface, which probably did not bond as well to cement as the shells used with the technique described in this article.
Dislocations can occur because of inadequately placed cups. Metallic cups can be improperly positioned, as can APCs. An advantage of the technique we have described over APCs is that liners with raised rims can be inserted with the apex placed wherever needed to best address instability. Dislocations can also occur because of factors such as inadequate offset and cognitive impairments. Our technique allows use of offset liners and constrained liners. Although these options may not prevent further dislocations, they often mitigate instability issues. Constrained liners and lateralized liners can be easily placed, and elevated rims can be swiveled as needed for stability. As use of cementless, metal-backed, modular acetabular components is common in primary THAs, most surgeons are familiar with the modular liner options available with use of the technique described in this article.
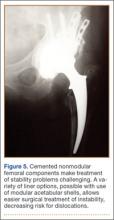
In this setting, modular, metal acetabular shells have the advantage of allowing surgeons to use the alignment guides they are accustomed to using. Modularity is another significant advantage over APCs. When an APC wears down, the component must be extracted to permit implantation of a new APC. With metal shells, a worn liner can be exchanged relatively easily. Modularity also gives surgeons many more options for addressing instability. Elevated rims can be moved, head sizes can be changed, and lateralized or constrained liners can be implanted easily. By comparison, with APCs, stability can be addressed only by modifying the femoral component or taking hip precautions which restrict range of motion of the hip. Modification of the femoral component is not possible with nonmodular femoral components in place (Figure 5). A potential disadvantage of this technique is increased cost associated with use of another component.
This small series of patients has had an excellent rate of success with cementation of multihole, metal-backed acetabular components into a cage or ring. These components may offer more secure fixation than APCs to cement extruded into the multiple holes, and improved metallurgy, such as trabecular metal. Surgeons who want to use modular components may prefer this technique because it allows them to select from various liner options. Surgeons should consider this technique for patients who need major pelvic reconstruction, though a larger study with longer follow-up is needed to determine its long-term durability.
Although the novel technique we have described has been helpful in our experience, this study had several limitations—small series, retrospective study, relatively short follow-up, lack of control group and functional data—that may have affected its conclusions. Further study and follow-up are needed to better determine the utility of this technique in clinical practice.
1. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
2. Berry DJ, Lewallen DG, Hanssen AD, Cabanela ME. Pelvic discontinuity in revision total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1692-1702.
3. Pieringer H, Auersperg V, Böhler N. Reconstruction of severe acetabular bone-deficiency: the Burch-Schneider antiprotrusio cage in primary and revision total hip arthroplasty. J Arthroplasty. 2006;21(4):489-496.
4. Goodman S, Saastamoinen H, Shasha N, Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19(4):436-446.
5. Cates HE, Faris PM, Keating EM, Ritter MA. Polyethylene wear in cemented metal-backed acetabular cups. J Bone Joint Surg Br. 1993;75(2):249-253.
6. Vasu R, Carter DR, Harris WH. Stress distribution in the acetabular region—I. Before and after total joint replacement. J Biomech. 1982;15(3):155-164.
Although the number of total hip arthroplasties (THAs) being performed in the United States is increasing, revision THAs are more common.1 Many acetabular revisions can be successfully performed with standard or jumbo cementless acetabular cups, but major osseous deficiencies typically require reconstruction with a cage or cup/cage that bridges gaps in the pelvis and obtains fixation of the arthroplasty components.2,3 Cages and rings have been combined with all-polyethylene acetabular components (ie, all-polyethylene cups, or APCs) to reconstruct pelvic bone defects, but complications, including APC dissociation (Figure 1) and postoperative instability, can occur despite stable fixation of cage to pelvis.4 The incidence of dislocations with pelvic reconstruction rings using APCs has been reported to be 11%.4 If an APC has to be replaced because of wear, then major surgery may be required to extract the worn cup and cement a new cup in its place.
In this article, we describe a technique in which a metal, multihole acetabular shell is cemented into the cage or ring construct, avoiding some of the complications associated with traditional techniques by permitting use of a variety of liners.
Materials and Methods
We retrospectively reviewed the cases of all of Dr. Bolanos’ patients who underwent acetabular revision THA with cage reconstruction between February 1, 1998 and October 9, 2006. During this period, we were cementing a modular metal shell into the cage instead of an APC or polyethylene liner. All patients who underwent revision THA with cage reconstruction during the study period were included. Bone defects were treated with structural or morselized bone allograft. Every reconstruction involved use of an antiprotrusio cage or ring secured to the pelvis with screws, and a multihole acetabular shell cemented into place with a polyethylene liner applied. Elevated rims, lateralized liners, and constrained liners were used as needed to optimize stability. Femoral components were retained. Cage size was based on matching the osseous deficiencies. Shell size was determined by the inner diameter of the corresponding cage. Liner size was based on matching the shell and femoral head. During this period, none of the patients had other reconstructive techniques, such as trabecular metal augmentation, in combination with a modular acetabular shell, cup/cage reconstruction, or custom triflange components.
Patients engaged in protected weight-bearing ambulation for 3 months after surgery and were then permitted full, unrestricted activity. The primary outcome was mechanical failure of the reconstruction, or reoperation (Table). All reconstructions in this series consisted of acetabular revisions for aseptic loosening.
Surgical Technique
Six consecutive cases of pelvic discontinuity and 7 cases of segmental acetabular bone loss required use of cages or rings. Reconstruction cages were used to secure fixation to the ilium and ischium. With the technique described in this article, we used screws with rounded, prominent heads rather than flat heads between the cup and the cage or ring (Synthes, 6.5 mm) to ensure adequate cement mantle. The rounded screw heads were left prominent to approximate the function of cement pegs found on APCs. Screws were placed into the anterior, superior, medial, and posterior aspects of the cage to ensure adequate cement mantle between cup and cage. This was confirmed with trial placement of the cup into the cage before cementation and observation of the uniformity of the space between cup and cage. Trial placement also confirmed that the screws did not interfere with appropriate positioning of the cup. A multihole, metal acetabular cup was then cemented in the cage or ring such that cement extruded around the shell and into the holes of the cup and the cage, securing the cup to the cage. Use of a multihole, metal shell resulted in excellent cement fixation because the multiple holes created multiple circumferential cement pegs. Various liner options could then be used to optimize stability of the reconstruction. In some cases, excessive cement extruded into the interior aspect of the shell and hardened before curettage. If the excess cement could interfere with complete seating/locking of the liner, then a high-speed burr was used to easily remove cement (Figure 2). Polyethylene liners were then inserted into the shell. Femoral reconstruction was then performed, if needed, and stability of the arthroplasty checked. This technique allows the surgeon to then select from a variety of polyethylene liners as needed to optimize stability. Liners with elevated rims, lateralized liners, and constrained liners could be interchangeable options with this technique.
Results
Thirteen patients with major osseous deficiencies of the pelvis were treated using this technique. At mean follow-up of 64.2 months (range, 3-133 months), 10 of the 13 patients had favorable outcomes without further surgery. One patient developed recurrent aseptic loosening that required re-revision, another patient developed recurrent instability that required acetabular liner and femoral head exchange, and a third patient with poor balance fell multiple times. This patient’s ninth fall resulted in dissociation of the acetabular shell from the cage (Figure 3), treated with placement of another cemented multihole metal shell with a standard liner. As dislocations recurred, the liner was changed to a constrained liner (Figure 4). The patient did not have any further dislocations or other hip-related problems. Integrity of cemented shell-cage fixation was maintained in 12 of the 13 patients at final follow-up.
Discussion
We have described a novel technique that facilitates reconstruction of major osseous deficiencies of the pelvis. The technique involves cementation of a multihole, metal acetabular shell into a cage or ring, permitting use of modular liners. The modularity in this approach to major hip reconstruction provides stability-optimization options that are not available with APCs. So far, the technique has demonstrated more advantages than disadvantages, so the indications for its use would be whenever a cage is used for pelvic reconstruction. Traditional techniques involve cementing an APC into the cage or ring. Use of multihole, metal shells for this purpose has several theoretical advantages. Multiple holes and the textured surface allow more interdigitation of cement with cup than APCs do; this interdigitation may improve the durability of the cemented interface. Cement also extrudes through the holes of the cage to secure the cup to the pelvis, as is done with cementation of APCs. Introduction of trabecular metal shells may also provide an even more secure bond to the shell, compared with APCs, though durability of a cemented trabecular metal interface has not been established. In addition, mechanical alignment guides cannot fasten as securely onto some APCs.
Nonmodular, cemented, metal-backed acetabular components, which were commonly used in hip arthroplasties at one time, were abandoned because of their relatively high loosening rate and because of advantages noted with modular components.5 The nonmodular components had been developed because of their theoretical advantages of improved distribution of forces into the cement mantle.5,6 However, those models had a relatively smooth metallic surface, which probably did not bond as well to cement as the shells used with the technique described in this article.
Dislocations can occur because of inadequately placed cups. Metallic cups can be improperly positioned, as can APCs. An advantage of the technique we have described over APCs is that liners with raised rims can be inserted with the apex placed wherever needed to best address instability. Dislocations can also occur because of factors such as inadequate offset and cognitive impairments. Our technique allows use of offset liners and constrained liners. Although these options may not prevent further dislocations, they often mitigate instability issues. Constrained liners and lateralized liners can be easily placed, and elevated rims can be swiveled as needed for stability. As use of cementless, metal-backed, modular acetabular components is common in primary THAs, most surgeons are familiar with the modular liner options available with use of the technique described in this article.
In this setting, modular, metal acetabular shells have the advantage of allowing surgeons to use the alignment guides they are accustomed to using. Modularity is another significant advantage over APCs. When an APC wears down, the component must be extracted to permit implantation of a new APC. With metal shells, a worn liner can be exchanged relatively easily. Modularity also gives surgeons many more options for addressing instability. Elevated rims can be moved, head sizes can be changed, and lateralized or constrained liners can be implanted easily. By comparison, with APCs, stability can be addressed only by modifying the femoral component or taking hip precautions which restrict range of motion of the hip. Modification of the femoral component is not possible with nonmodular femoral components in place (Figure 5). A potential disadvantage of this technique is increased cost associated with use of another component.
This small series of patients has had an excellent rate of success with cementation of multihole, metal-backed acetabular components into a cage or ring. These components may offer more secure fixation than APCs to cement extruded into the multiple holes, and improved metallurgy, such as trabecular metal. Surgeons who want to use modular components may prefer this technique because it allows them to select from various liner options. Surgeons should consider this technique for patients who need major pelvic reconstruction, though a larger study with longer follow-up is needed to determine its long-term durability.
Although the novel technique we have described has been helpful in our experience, this study had several limitations—small series, retrospective study, relatively short follow-up, lack of control group and functional data—that may have affected its conclusions. Further study and follow-up are needed to better determine the utility of this technique in clinical practice.
Although the number of total hip arthroplasties (THAs) being performed in the United States is increasing, revision THAs are more common.1 Many acetabular revisions can be successfully performed with standard or jumbo cementless acetabular cups, but major osseous deficiencies typically require reconstruction with a cage or cup/cage that bridges gaps in the pelvis and obtains fixation of the arthroplasty components.2,3 Cages and rings have been combined with all-polyethylene acetabular components (ie, all-polyethylene cups, or APCs) to reconstruct pelvic bone defects, but complications, including APC dissociation (Figure 1) and postoperative instability, can occur despite stable fixation of cage to pelvis.4 The incidence of dislocations with pelvic reconstruction rings using APCs has been reported to be 11%.4 If an APC has to be replaced because of wear, then major surgery may be required to extract the worn cup and cement a new cup in its place.
In this article, we describe a technique in which a metal, multihole acetabular shell is cemented into the cage or ring construct, avoiding some of the complications associated with traditional techniques by permitting use of a variety of liners.
Materials and Methods
We retrospectively reviewed the cases of all of Dr. Bolanos’ patients who underwent acetabular revision THA with cage reconstruction between February 1, 1998 and October 9, 2006. During this period, we were cementing a modular metal shell into the cage instead of an APC or polyethylene liner. All patients who underwent revision THA with cage reconstruction during the study period were included. Bone defects were treated with structural or morselized bone allograft. Every reconstruction involved use of an antiprotrusio cage or ring secured to the pelvis with screws, and a multihole acetabular shell cemented into place with a polyethylene liner applied. Elevated rims, lateralized liners, and constrained liners were used as needed to optimize stability. Femoral components were retained. Cage size was based on matching the osseous deficiencies. Shell size was determined by the inner diameter of the corresponding cage. Liner size was based on matching the shell and femoral head. During this period, none of the patients had other reconstructive techniques, such as trabecular metal augmentation, in combination with a modular acetabular shell, cup/cage reconstruction, or custom triflange components.
Patients engaged in protected weight-bearing ambulation for 3 months after surgery and were then permitted full, unrestricted activity. The primary outcome was mechanical failure of the reconstruction, or reoperation (Table). All reconstructions in this series consisted of acetabular revisions for aseptic loosening.
Surgical Technique
Six consecutive cases of pelvic discontinuity and 7 cases of segmental acetabular bone loss required use of cages or rings. Reconstruction cages were used to secure fixation to the ilium and ischium. With the technique described in this article, we used screws with rounded, prominent heads rather than flat heads between the cup and the cage or ring (Synthes, 6.5 mm) to ensure adequate cement mantle. The rounded screw heads were left prominent to approximate the function of cement pegs found on APCs. Screws were placed into the anterior, superior, medial, and posterior aspects of the cage to ensure adequate cement mantle between cup and cage. This was confirmed with trial placement of the cup into the cage before cementation and observation of the uniformity of the space between cup and cage. Trial placement also confirmed that the screws did not interfere with appropriate positioning of the cup. A multihole, metal acetabular cup was then cemented in the cage or ring such that cement extruded around the shell and into the holes of the cup and the cage, securing the cup to the cage. Use of a multihole, metal shell resulted in excellent cement fixation because the multiple holes created multiple circumferential cement pegs. Various liner options could then be used to optimize stability of the reconstruction. In some cases, excessive cement extruded into the interior aspect of the shell and hardened before curettage. If the excess cement could interfere with complete seating/locking of the liner, then a high-speed burr was used to easily remove cement (Figure 2). Polyethylene liners were then inserted into the shell. Femoral reconstruction was then performed, if needed, and stability of the arthroplasty checked. This technique allows the surgeon to then select from a variety of polyethylene liners as needed to optimize stability. Liners with elevated rims, lateralized liners, and constrained liners could be interchangeable options with this technique.
Results
Thirteen patients with major osseous deficiencies of the pelvis were treated using this technique. At mean follow-up of 64.2 months (range, 3-133 months), 10 of the 13 patients had favorable outcomes without further surgery. One patient developed recurrent aseptic loosening that required re-revision, another patient developed recurrent instability that required acetabular liner and femoral head exchange, and a third patient with poor balance fell multiple times. This patient’s ninth fall resulted in dissociation of the acetabular shell from the cage (Figure 3), treated with placement of another cemented multihole metal shell with a standard liner. As dislocations recurred, the liner was changed to a constrained liner (Figure 4). The patient did not have any further dislocations or other hip-related problems. Integrity of cemented shell-cage fixation was maintained in 12 of the 13 patients at final follow-up.
Discussion
We have described a novel technique that facilitates reconstruction of major osseous deficiencies of the pelvis. The technique involves cementation of a multihole, metal acetabular shell into a cage or ring, permitting use of modular liners. The modularity in this approach to major hip reconstruction provides stability-optimization options that are not available with APCs. So far, the technique has demonstrated more advantages than disadvantages, so the indications for its use would be whenever a cage is used for pelvic reconstruction. Traditional techniques involve cementing an APC into the cage or ring. Use of multihole, metal shells for this purpose has several theoretical advantages. Multiple holes and the textured surface allow more interdigitation of cement with cup than APCs do; this interdigitation may improve the durability of the cemented interface. Cement also extrudes through the holes of the cage to secure the cup to the pelvis, as is done with cementation of APCs. Introduction of trabecular metal shells may also provide an even more secure bond to the shell, compared with APCs, though durability of a cemented trabecular metal interface has not been established. In addition, mechanical alignment guides cannot fasten as securely onto some APCs.
Nonmodular, cemented, metal-backed acetabular components, which were commonly used in hip arthroplasties at one time, were abandoned because of their relatively high loosening rate and because of advantages noted with modular components.5 The nonmodular components had been developed because of their theoretical advantages of improved distribution of forces into the cement mantle.5,6 However, those models had a relatively smooth metallic surface, which probably did not bond as well to cement as the shells used with the technique described in this article.
Dislocations can occur because of inadequately placed cups. Metallic cups can be improperly positioned, as can APCs. An advantage of the technique we have described over APCs is that liners with raised rims can be inserted with the apex placed wherever needed to best address instability. Dislocations can also occur because of factors such as inadequate offset and cognitive impairments. Our technique allows use of offset liners and constrained liners. Although these options may not prevent further dislocations, they often mitigate instability issues. Constrained liners and lateralized liners can be easily placed, and elevated rims can be swiveled as needed for stability. As use of cementless, metal-backed, modular acetabular components is common in primary THAs, most surgeons are familiar with the modular liner options available with use of the technique described in this article.
In this setting, modular, metal acetabular shells have the advantage of allowing surgeons to use the alignment guides they are accustomed to using. Modularity is another significant advantage over APCs. When an APC wears down, the component must be extracted to permit implantation of a new APC. With metal shells, a worn liner can be exchanged relatively easily. Modularity also gives surgeons many more options for addressing instability. Elevated rims can be moved, head sizes can be changed, and lateralized or constrained liners can be implanted easily. By comparison, with APCs, stability can be addressed only by modifying the femoral component or taking hip precautions which restrict range of motion of the hip. Modification of the femoral component is not possible with nonmodular femoral components in place (Figure 5). A potential disadvantage of this technique is increased cost associated with use of another component.
This small series of patients has had an excellent rate of success with cementation of multihole, metal-backed acetabular components into a cage or ring. These components may offer more secure fixation than APCs to cement extruded into the multiple holes, and improved metallurgy, such as trabecular metal. Surgeons who want to use modular components may prefer this technique because it allows them to select from various liner options. Surgeons should consider this technique for patients who need major pelvic reconstruction, though a larger study with longer follow-up is needed to determine its long-term durability.
Although the novel technique we have described has been helpful in our experience, this study had several limitations—small series, retrospective study, relatively short follow-up, lack of control group and functional data—that may have affected its conclusions. Further study and follow-up are needed to better determine the utility of this technique in clinical practice.
1. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
2. Berry DJ, Lewallen DG, Hanssen AD, Cabanela ME. Pelvic discontinuity in revision total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1692-1702.
3. Pieringer H, Auersperg V, Böhler N. Reconstruction of severe acetabular bone-deficiency: the Burch-Schneider antiprotrusio cage in primary and revision total hip arthroplasty. J Arthroplasty. 2006;21(4):489-496.
4. Goodman S, Saastamoinen H, Shasha N, Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19(4):436-446.
5. Cates HE, Faris PM, Keating EM, Ritter MA. Polyethylene wear in cemented metal-backed acetabular cups. J Bone Joint Surg Br. 1993;75(2):249-253.
6. Vasu R, Carter DR, Harris WH. Stress distribution in the acetabular region—I. Before and after total joint replacement. J Biomech. 1982;15(3):155-164.
1. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
2. Berry DJ, Lewallen DG, Hanssen AD, Cabanela ME. Pelvic discontinuity in revision total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1692-1702.
3. Pieringer H, Auersperg V, Böhler N. Reconstruction of severe acetabular bone-deficiency: the Burch-Schneider antiprotrusio cage in primary and revision total hip arthroplasty. J Arthroplasty. 2006;21(4):489-496.
4. Goodman S, Saastamoinen H, Shasha N, Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19(4):436-446.
5. Cates HE, Faris PM, Keating EM, Ritter MA. Polyethylene wear in cemented metal-backed acetabular cups. J Bone Joint Surg Br. 1993;75(2):249-253.
6. Vasu R, Carter DR, Harris WH. Stress distribution in the acetabular region—I. Before and after total joint replacement. J Biomech. 1982;15(3):155-164.
Role of Surgical Dressings in Total Joint Arthroplasty: A Randomized Controlled Trial
Wound complications (eg, delayed wound healing, blisters, prolonged drainage) have been reported in up to 30% of patients who undergo elective total joint arthroplasty (TJA).1-6 Wound complications increase resource utilization, lengthen hospital stays, and increase costs.7-9 Prolonged wound healing and persistent wound drainage are also harbingers of both superficial and deep surgical site infections.5-11
In several studies, wound complications after TJA were the primary reason for hospital readmissions.12-15 As part of the Patient Protection and Affordable Care Act, hospitals will be penalized by the Centers for Medicaid & Medicare Services for unplanned hospital readmissions within 30 days after TJA. It is imperative, then, to reduce the risk factors and complications associated with surgical site infections to decrease unplanned readmissions.
Historically, little attention has been given to the role of surgical dressings and the effect of dressings on wound healing. Although many subspecialties (eg, cardiothoracic surgery, general surgery) have reported benefits in using occlusive dressings, adoption in TJA has been slow.16-18 At our institution about 5 years ago, we began using an occlusive silver-impregnated barrier dressing based on preliminary data from studies showing benefits of occlusive dressings in TJA.19,20
We conducted a study to determine if use of occlusive antimicrobial barrier dressings decreases rates of wound complications in TJA. We had 3 research questions: Compared with standard surgical dressings, are occlusive dressings associated with decreased rates of wound complications after TJA? Is there a difference in number of dressing changes required between the 2 dressing types? Is satisfaction higher for patients with occlusive dressings than for patients with standard dressings?
Patients and Methods
This randomized controlled trial (RCT) was reviewed and approved by the Institutional Review Board at Carolinas Healthcare. Patients were randomized by the research staff using a parallel, 1:1 allocation method. The randomization table was generated using a random number generator.
An a priori sample size estimate was made using a 2-tailed Fisher exact test with a .05 level of significance. Based on a study by Clarke and colleagues,21 we estimated the incidence of wound problems at 3% in the occlusive dressing (study) group and 13% in the standard dressing (control) group. We determined that 260 participants (130 per group) would be needed to achieve 80% power. We considered a 15% attrition rate for a total enrollment goal of 300 study participants (150 per group).
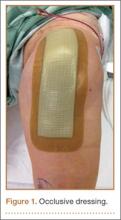
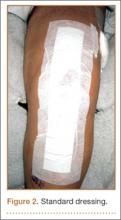
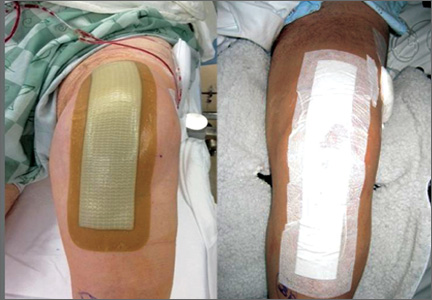
Between December 2010 and January 2013, patients presenting for either primary total hip arthroplasty (THA) or primary total knee arthroplasty (TKA) were recruited to participate in the study. Eligibility criteria (Table 1) were reviewed, and patients were enrolled by the senior surgeons, Dr. Springer, Dr. Beaver, Dr. Griffin, and Dr. Mason. All eligible participants who provided informed consent were randomized to receive either an occlusive antimicrobial barrier dressing (Aquacel Ag, ConvaTec) or standard surgical dressing (Primapore, Smith & Nephew). The occlusive dressing (Figure 1) consists of an outer barrier layer of hydrocolloid and a central island of hydrofiber, which absorbs and locks in any wound exudate within the fibers and prevents the creation of an overly moist wound environment that can lead to skin maceration and wound breakdown. In addition, the hydrofibers are embedded with ionic silver, which is released only at the site of wound exudate, or drainage; thus, there is no continuous exposure of the entire wound to silver. The standard dressing (Figure 2) consists of a central island of gauze enclosed in low-allergy acrylic adhesive tape.
All surgical dressings were placed over a closed incision in a sterile environment in the operating room after the procedure. The groups’ wound closures were identical.
A posterior approach was used for all THAs. The deep fascia was closed with a running barbed suture (Quill, Angiotech), the deep subcutaneous tissue with No. 1 Vicryl suture (Ethicon), and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch (Ethicon) was placed in the subcuticular layer and was followed with a skin adhesive (Dermabond, Ethicon). A closed suction drain, removed on postoperative day (POD) 1, was used for all THAs.
A standard medial parapatellar arthrotomy was used for all TKAs. The arthrotomy was closed with a running barbed suture, the deep subcutaneous tissue with No. 1 Vicryl suture, and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch was placed in the subcuticular layer and was followed with a skin adhesive. A closed suction drain was also used. In addition, a compressive wrap was placed over the dressing in the operating room and was removed the next morning. During the hospital stay, the surgical site was evaluated daily with a standard wound evaluation form.
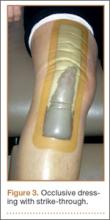
In the standard dressing group, the bandage was removed for wound evaluation on POD 2, and the dressing was changed every other day during the hospital stay. The dressing was also changed as needed for wound drainage (Figure 3) or other minor wound-healing concerns.
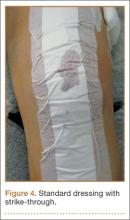
In the occlusive dressing group, the dressing design allowed the dressing to remain in place for about 7 days. It was removed by a home health nurse during a visit closest to but not before the 7-day mark. In addition, it was changed at surgeon discretion if there were concerns about wound drainage or wound healing. For the occlusive barrier, wound drainage was evaluated by strike-through of drainage on the back side of the dressing (Figure 4). If more than 50% of the dressing was saturated, the bandage was changed and the wound evaluated. If there were no immediate concerns about wound complications (eg, infection, blistering), a new occlusive dressing was placed. Because the occlusive dressing was waterproof, patients in the study group were able to shower immediately after surgery. In the control group, patients were allowed to shower if the surgical dressing was kept dry, as the bandage was not waterproof.
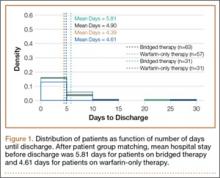
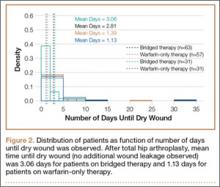
Per the study protocol, all patients were discharged home and followed by a single home health agency. Mean hospital stay was 3 days (range, 0-8 days), which did not differ significantly between groups (P = .133). All home health nurses were trained in evaluation of postsurgical wounds and were aware of the study requirements. The nurses visited all patients 3 days a week until the scheduled 4-week postoperative follow-up with the treating physician or physician assistant. At each visit, the nurse evaluated the wound and surrounding skin using a standard wound document. Dressings were changed based on the criteria we have described. Concerns about wound status (eg, drainage, blistering, erythema) prompted removal of the dressing for further evaluation. The physician was notified of concerns about wound healing, which prompted an office visit for evaluation. The dressing remained in place for a minimum of 7 days but in all cases was removed as close to 7 days as possible, depending on the scheduled nursing visits. Once uneventful wound healing was complete, no further dressing was required. A final wound evaluation was conducted by the surgeon at the 4-week postoperative evaluation.
The primary outcome measure was wound complication (dichotomous variable). Wounds were assessed by describing the amount, type, and color of exudate (Figure 5). The appearance of the wound margins and the surrounding skin was also assessed. Because wounds could not be directly visualized in the occlusive dressing group, drainage (indicated by strike-through) was used as a measure of possible wound complications, prompting removal and full evaluation.
Secondary endpoints included additional wound treatment or surgical procedures for wound complications, number of dressing changes, and patient satisfaction. Patients completed a satisfaction questionnaire at each wound assessment (Figure 6). Using a visual analog scale (VAS), they rated their satisfaction with their ability to perform activities of daily living (personal hygiene, change clothes, sit comfortably, sleep comfortably), drawing a line on the VAS at a point between 0 (totally unsatisfied) and 100 (totally satisfied) for each satisfaction measure. This line was measured and recorded by the study coordinator. The 4 satisfaction measures were averaged for a composite satisfaction measure.
All statistical analyses were conducted using SAS Version 9.2 (SAS Institute). Standard univariate descriptive statistics (means, standard deviations, frequencies, proportions) were calculated and reported. Differences in mean values for continuous data were assessed with independent t test or Wilcoxon rank sum test. Chi-square test and Fisher exact test were used to determine differences between groups for categorical or dichotomous variables. A significance level of .05 was used for all statistical tests.
Results
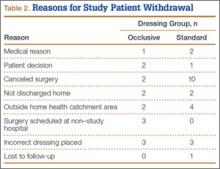
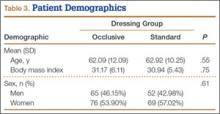
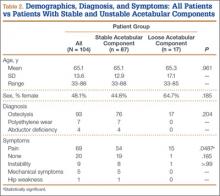
The 300 patients who consented to participate in the study were randomized to receive either occlusive dressing or standard dressing. After randomization, 38 patients (15 occlusive, 23 standard) were withdrawn from the study (Table 2), leaving a final dataset of 262 patients, 141 in the occlusive group (67 THAs, 74 TKAs) and 121 in the standard group (49 THAs, 72 TKAs). There were no differences in proportion of THAs or TKAs, age, sex, or body mass index between the occlusive and standard groups (Table 3).
There were statistically significantly (P = .015) fewer wound complications in the occlusive dressing group (10%) than in the standard dressing group (22%). Blisters at or around the wound site were reported in significantly (P = .026) fewer patients with occlusive dressing (1/141, 0.7%) than standard dressing (7/121, 6%). Additional wound care was required in 9 patients (7%) in the standard group and 6 patients (4%) in the occlusive group (P = .27). Two patients (1.7%) in the standard group were readmitted for treatment of wound dehiscence; no one in the occlusive group was readmitted to the hospital or had to return to the operating room for treatment of a wound complication. The difference was not statistically significant (P = .13). There were also no significant (P = .81) differences in rate of wound complications between THA and TKA patients.
There were statistically significantly (P < .0001) fewer dressing changes in the occlusive dressing group. Mean number of dressing changes was 0.14 (median, 0; interquartile range, 0-0) in the occlusive group and 2.8 (median, 2; interquartile range, 1-3) in the standard group.
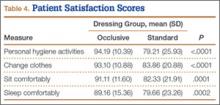
Compared with patients in the standard dressing group, patients in the occlusive dressing group reported significantly higher satisfaction scores. Mean overall patient satisfaction score was 92 in the occlusive group and 81 in the standard group (P < .0001). Patients in the occlusive group were more satisfied with their ability to take care of their personal hygiene, to change clothes, and to sit and sleep comfortably (Table 4).
Discussion
Wound complications after TJA are common, occurring in up to 30% of patients,1-6 and are associated with development of superficial and deep surgical site infections, increased resource utilization, and longer hospital stays.5-11 Although the role of surgical dressings has received little attention in TJA practice, other subspecialties have found that occlusive barrier dressings can reduce wound complications and promote wound healing.16,17 Mitotic cell division and leukocyte activity, which are critical in wound healing, increase under occlusive dressings. This cellular activity is disrupted with every dressing change, delaying wound healing (biological activity takes 3-4 hours to resume).22 In addition, occlusive dressings increase hypoxia, which promotes angiogenesis and accelerates wound healing.23
Despite being a prospective RCT, this study had several limitations. Because of the need to evaluate wounds and obvious differences between the 2 dressings (eg, color, ability to shower), it was not possible to blind the patient or surgeon to the dressing used. When rating satisfaction, patients were not able to directly compare the 2 dressings. The primary endpoint of the study was the complication rate; however, the deep periprosthetic infection rate may be a superior endpoint and would require a much larger study. Although we assumed that wound complications may be harbingers for periprosthetic infections, no patient in either group developed periprosthetic infection. Therefore, we cannot conclude that surgical dressings play a role in reducing infections. In addition, as the standard dressing was changed on POD 2 (per standard protocol) and the occlusive dressing could remain in place for up to 7 days, there was a selection bias in the evaluation of the number of dressing changes. However, given the characteristics of the standard dressing (eg, tape, gauze, nonocclusive), leaving it in place after POD 2 is not optimal. Therefore, we would expect to see a difference in the number of dressing changes. We think this comparison remains valid, as occlusive dressings were changed when there were indications of wound problems (eg, excessive drainage [strike-through], surrounding erythema, blistering). With an average of less than 1 dressing change in the occlusive group, we think this is a surrogate for uneventful wound healing and decreased wound complication, and our data support this. It is also important to test both dressing durability and patient tolerance for wearing a single dressing for 7 days.
Our RCT results showed that, compared with a standard dressing, an occlusive antimicrobial dressing was associated with a significant decrease in overall wound complications and blisters. These findings are similar to those of other studies of occlusive dressings in a number of surgical subspecialties.16,18 In an RCT of 200 patients who underwent elective and nonelective hip and knee surgery and were randomized to either absorbent perforated dressing with adhesive border (Cutiplast, Smith & Nephew) or Aquacel (ConvaTec) covered with vapor-permeable dressing (Tegaderm, 3M), Ravenscroft and colleagues20 found that Aquacel-plus-Tegaderm was 5.8 times more likely than Cutiplast to produce an uncompromised wound. Similarly, in an RCT of hydrofiber (Aquacel) and central pad (Mepore, Mölnlycke) dressings after primary THA and TKA, Abuzakuk and colleagues19 found significantly fewer dressing changes (43% vs 77%) and blisters (13% vs 26%) in the hydrofiber group than in the pad group.
Hopper and colleagues24 compared 50 consecutive patients treated with modern dressings (Aquacel) with 50 historical control patients treated with traditional surgical dressings (Mepore). Blisters developed in 20% of the patients in the traditional group and 4% of patients in the modern group (P = .028). The authors concluded that adverse outcomes of wound healing can be minimized with modern dressings.
A recent retrospective study by Cai and colleagues25 evaluated the incidence of acute periprosthetic infection (≤3 months after surgery) with use of occlusive (Aquacel) and standard dressings. Incidence of acute periprosthetic infection was 0.44% in the occlusive group and 1.7% in the standard group (P = .005). Incidence of wound-healing problems was not evaluated.
Our second aim in the present study was to evaluate the number of dressing changes required. There were significantly fewer dressing changes in the occlusive dressing group than in the standard dressing group. Therefore, wear time (amount of time a single dressing remains in place) was substantially longer for the occlusive group. In the study by Hopper and colleagues,24 wear time was significantly shorter for the traditional dressing than for the modern dressing (2 vs 7 days; P < .001), and the traditional dressing required more changes (3 vs 0; P < .001).
These findings are important for several reasons. Standard surgical dressings often require frequent changes. If left in place, they create an excessively moist wound environment that promotes blistering and delays wound healing. However, frequent dressing changes expose the wound and increase the risk for surgical site infection.26 A barrier dressing left in place from time of surgery prevents bacteria from entering and contaminating a healing wound. A study by Clarke and colleagues21 demonstrated higher skin colonization rates for patients who had dressings changed on POD 1 than for patients who had their first dressing change on POD 6.
Our third study aim was to evaluate patient satisfaction with surgical dressings. The orthopedic literature has little on this topic.23 Blisters and other wound complications can negatively affect satisfaction.2,3 Our data showed significant improvement in satisfaction, particularly regarding sterility and hygiene.
Other surgical subspecialties have found similar improvement in patient satisfaction with occlusive barrier dressings. In an RCT of 88 pediatric patients, Rasmussen and colleagues27 found that patients reported significantly less pain during changes of an occlusive adhesive dressing (Duoderm, ConvaTec) than during changes of a conventional Steristrip (3M) plus Cutiplast. According to the authors, the occlusive wound dressing seemed to minimize the physical and psychological trauma to the infant or child and lessen disruption of the child’s and the parents’ daily routines, because the children could be bathed immediately after surgery.
Our study did not specifically address cost. Cai and colleagues25 estimated that, if the Aquacel dressing were routinely used in every hip and knee arthroplasty, it would add about $27 million in cost. However, this must be balanced by the cost of managing infection after TJA. In the United States, at an estimated $50,000 to $100,000 per case and an annual incidence of 1% to 2%, the low-end cost for the treatment of periprosthetic infection would be $500 million.28 Cai and colleagues25 found a 4-fold reduction in periprosthetic infection when use of occlusive dressings was implemented. In addition, wound complications remain the number one reason for hospital readmission after TJA.12,13 Cost of hospital readmission, as well as financial penalties to institutions for unplanned readmission for wound complications, must be considered.
Conclusion
Our RCT results demonstrated that use of occlusive antimicrobial barrier dressings (vs standard surgical dressings) significantly reduced wound complications and dressing changes and improved overall patient satisfaction. These findings are similar to those in the literature on TJA and other surgical subspecialties. We conclude that occlusive surgical dressings reduce wound complications after TJA.
1. Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. J Wound Care. 2005;14(1):27-29.
2. Koval KJ, Egol KA, Hiebert R, Spratt KF. Tape blisters after hip surgery: can they be eliminated completely? Am J Orthop. 2007;36(5):261-265.
3. Lawrentschuk N, Falkenberg MP, Pirpiris M. Wound blisters post hip surgery: a prospective trial comparing dressings. ANZ J Surg. 2002;72(10):716-719.
4. Mihalko WM, Manaswi A, Brown TE, Parvizi J, Schmalzried TP, Saleh KJ. Infection in primary total knee arthroplasty: contributing factors. Instr Course Lect. 2008;57:317-325.
5. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
6. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
7. Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91(1):48-54.
8. Gordon SM, Culver DH, Simmons BP, Jarvis WR. Risk factors for wound infections after total knee arthroplasty. Am J Epidemiol. 1990;131(5):905-916.
9. Jaberi FM, Parvizi J, Haytmanek CT, Joshi A, Purtill J. Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Clin Orthop Relat Res. 2008;466(6):1368-1371.
10. Schmalzried TP. The infected hip: telltale signs and treatment options. J Arthroplasty. 2006;21(4 suppl 1):97-100.
11. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289.
12. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468.
13. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
14. Jordan CJ, Goldstein RY, Michels RF, Hutzler L, Slover JD, Bosco JA 3rd. Comprehensive program reduces hospital readmission rates after total joint arthroplasty. Am J Orthop. 2012;41(11):E147-E151.
15. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
16. Shinohara T, Yamashita Y, Satoh K, et al. Prospective evaluation of occlusive hydrocolloid dressing versus conventional gauze dressing regarding the healing effect after abdominal operations: randomized controlled trial. Asian J Surg. 2008;31(1):1-5.
17. Siah CJ, Yatim J. Efficacy of a total occlusive ionic silver-containing dressing combination in decreasing risk of surgical site infection: an RCT. J Wound Care. 2011;20(12):561-568.
18. Teshima H, Kawano H, Kashikie H, et al. A new hydrocolloid dressing prevents surgical site infection of median sternotomy wounds. Surg Today. 2009;39(10):848-854.
19. Abuzakuk TM, Coward P, Shenava Y, Kumar VS, Skinner JA. The management of wounds following primary lower limb arthroplasty: a prospective, randomised study comparing hydrofibre and central pad dressings. Int Wound J. 2006;3(2):133-137.
20. Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: Aquacel and Tegaderm versus Cutiplast. Ann R Coll Surg Engl. 2006;88(1):18-22.
21. Clarke JV, Deakin AH, Dillon JM, Emmerson S, Kinninmonth AW. A prospective clinical audit of a new dressing design for lower limb arthroplasty wounds. J Wound Care. 2009;18(1):5-8, 10-11.
22. Kloeters O. The use of a semi-occlusive dressing reduces epidermal inflammatory cytokine expression and mitigates dermal proliferation and inflammation in a rat incisional model. Wound Repair Regen. 2008;16(4):568-575.
23. Michie DD, Hugill JV. Influence of occlusive and impregnated gauze dressings on incisional healing: a prospective, randomized, controlled study. Ann Plast Surg. 1994;32(1):57-64.
24. Hopper GP, Deakin AH, Crane EO, Clarke JV. Enhancing patient recovery following lower limb arthroplasty with a modern wound dressing: a prospective, comparative audit. J Wound Care. 2012;21(4):200-203.
25. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
26. Berg A, Fleischer S, Kuss O, Unverzagt S, Langer G. Timing of dressing removal in the healing of surgical wounds by primary intention: quantitative systematic review protocol. J Adv Nurs. 2012;68(2):264-270.
27. Rasmussen H, Larsen MJ, Skeie E. Surgical wound dressing in outpatient paediatric surgery. A randomised study. Dan Med Bull. 1993;40(2):252-254.
28. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
Wound complications (eg, delayed wound healing, blisters, prolonged drainage) have been reported in up to 30% of patients who undergo elective total joint arthroplasty (TJA).1-6 Wound complications increase resource utilization, lengthen hospital stays, and increase costs.7-9 Prolonged wound healing and persistent wound drainage are also harbingers of both superficial and deep surgical site infections.5-11
In several studies, wound complications after TJA were the primary reason for hospital readmissions.12-15 As part of the Patient Protection and Affordable Care Act, hospitals will be penalized by the Centers for Medicaid & Medicare Services for unplanned hospital readmissions within 30 days after TJA. It is imperative, then, to reduce the risk factors and complications associated with surgical site infections to decrease unplanned readmissions.
Historically, little attention has been given to the role of surgical dressings and the effect of dressings on wound healing. Although many subspecialties (eg, cardiothoracic surgery, general surgery) have reported benefits in using occlusive dressings, adoption in TJA has been slow.16-18 At our institution about 5 years ago, we began using an occlusive silver-impregnated barrier dressing based on preliminary data from studies showing benefits of occlusive dressings in TJA.19,20
We conducted a study to determine if use of occlusive antimicrobial barrier dressings decreases rates of wound complications in TJA. We had 3 research questions: Compared with standard surgical dressings, are occlusive dressings associated with decreased rates of wound complications after TJA? Is there a difference in number of dressing changes required between the 2 dressing types? Is satisfaction higher for patients with occlusive dressings than for patients with standard dressings?
Patients and Methods
This randomized controlled trial (RCT) was reviewed and approved by the Institutional Review Board at Carolinas Healthcare. Patients were randomized by the research staff using a parallel, 1:1 allocation method. The randomization table was generated using a random number generator.
An a priori sample size estimate was made using a 2-tailed Fisher exact test with a .05 level of significance. Based on a study by Clarke and colleagues,21 we estimated the incidence of wound problems at 3% in the occlusive dressing (study) group and 13% in the standard dressing (control) group. We determined that 260 participants (130 per group) would be needed to achieve 80% power. We considered a 15% attrition rate for a total enrollment goal of 300 study participants (150 per group).
Between December 2010 and January 2013, patients presenting for either primary total hip arthroplasty (THA) or primary total knee arthroplasty (TKA) were recruited to participate in the study. Eligibility criteria (Table 1) were reviewed, and patients were enrolled by the senior surgeons, Dr. Springer, Dr. Beaver, Dr. Griffin, and Dr. Mason. All eligible participants who provided informed consent were randomized to receive either an occlusive antimicrobial barrier dressing (Aquacel Ag, ConvaTec) or standard surgical dressing (Primapore, Smith & Nephew). The occlusive dressing (Figure 1) consists of an outer barrier layer of hydrocolloid and a central island of hydrofiber, which absorbs and locks in any wound exudate within the fibers and prevents the creation of an overly moist wound environment that can lead to skin maceration and wound breakdown. In addition, the hydrofibers are embedded with ionic silver, which is released only at the site of wound exudate, or drainage; thus, there is no continuous exposure of the entire wound to silver. The standard dressing (Figure 2) consists of a central island of gauze enclosed in low-allergy acrylic adhesive tape.
All surgical dressings were placed over a closed incision in a sterile environment in the operating room after the procedure. The groups’ wound closures were identical.
A posterior approach was used for all THAs. The deep fascia was closed with a running barbed suture (Quill, Angiotech), the deep subcutaneous tissue with No. 1 Vicryl suture (Ethicon), and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch (Ethicon) was placed in the subcuticular layer and was followed with a skin adhesive (Dermabond, Ethicon). A closed suction drain, removed on postoperative day (POD) 1, was used for all THAs.
A standard medial parapatellar arthrotomy was used for all TKAs. The arthrotomy was closed with a running barbed suture, the deep subcutaneous tissue with No. 1 Vicryl suture, and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch was placed in the subcuticular layer and was followed with a skin adhesive. A closed suction drain was also used. In addition, a compressive wrap was placed over the dressing in the operating room and was removed the next morning. During the hospital stay, the surgical site was evaluated daily with a standard wound evaluation form.
In the standard dressing group, the bandage was removed for wound evaluation on POD 2, and the dressing was changed every other day during the hospital stay. The dressing was also changed as needed for wound drainage (Figure 3) or other minor wound-healing concerns.
In the occlusive dressing group, the dressing design allowed the dressing to remain in place for about 7 days. It was removed by a home health nurse during a visit closest to but not before the 7-day mark. In addition, it was changed at surgeon discretion if there were concerns about wound drainage or wound healing. For the occlusive barrier, wound drainage was evaluated by strike-through of drainage on the back side of the dressing (Figure 4). If more than 50% of the dressing was saturated, the bandage was changed and the wound evaluated. If there were no immediate concerns about wound complications (eg, infection, blistering), a new occlusive dressing was placed. Because the occlusive dressing was waterproof, patients in the study group were able to shower immediately after surgery. In the control group, patients were allowed to shower if the surgical dressing was kept dry, as the bandage was not waterproof.
Per the study protocol, all patients were discharged home and followed by a single home health agency. Mean hospital stay was 3 days (range, 0-8 days), which did not differ significantly between groups (P = .133). All home health nurses were trained in evaluation of postsurgical wounds and were aware of the study requirements. The nurses visited all patients 3 days a week until the scheduled 4-week postoperative follow-up with the treating physician or physician assistant. At each visit, the nurse evaluated the wound and surrounding skin using a standard wound document. Dressings were changed based on the criteria we have described. Concerns about wound status (eg, drainage, blistering, erythema) prompted removal of the dressing for further evaluation. The physician was notified of concerns about wound healing, which prompted an office visit for evaluation. The dressing remained in place for a minimum of 7 days but in all cases was removed as close to 7 days as possible, depending on the scheduled nursing visits. Once uneventful wound healing was complete, no further dressing was required. A final wound evaluation was conducted by the surgeon at the 4-week postoperative evaluation.
The primary outcome measure was wound complication (dichotomous variable). Wounds were assessed by describing the amount, type, and color of exudate (Figure 5). The appearance of the wound margins and the surrounding skin was also assessed. Because wounds could not be directly visualized in the occlusive dressing group, drainage (indicated by strike-through) was used as a measure of possible wound complications, prompting removal and full evaluation.
Secondary endpoints included additional wound treatment or surgical procedures for wound complications, number of dressing changes, and patient satisfaction. Patients completed a satisfaction questionnaire at each wound assessment (Figure 6). Using a visual analog scale (VAS), they rated their satisfaction with their ability to perform activities of daily living (personal hygiene, change clothes, sit comfortably, sleep comfortably), drawing a line on the VAS at a point between 0 (totally unsatisfied) and 100 (totally satisfied) for each satisfaction measure. This line was measured and recorded by the study coordinator. The 4 satisfaction measures were averaged for a composite satisfaction measure.
All statistical analyses were conducted using SAS Version 9.2 (SAS Institute). Standard univariate descriptive statistics (means, standard deviations, frequencies, proportions) were calculated and reported. Differences in mean values for continuous data were assessed with independent t test or Wilcoxon rank sum test. Chi-square test and Fisher exact test were used to determine differences between groups for categorical or dichotomous variables. A significance level of .05 was used for all statistical tests.
Results
The 300 patients who consented to participate in the study were randomized to receive either occlusive dressing or standard dressing. After randomization, 38 patients (15 occlusive, 23 standard) were withdrawn from the study (Table 2), leaving a final dataset of 262 patients, 141 in the occlusive group (67 THAs, 74 TKAs) and 121 in the standard group (49 THAs, 72 TKAs). There were no differences in proportion of THAs or TKAs, age, sex, or body mass index between the occlusive and standard groups (Table 3).
There were statistically significantly (P = .015) fewer wound complications in the occlusive dressing group (10%) than in the standard dressing group (22%). Blisters at or around the wound site were reported in significantly (P = .026) fewer patients with occlusive dressing (1/141, 0.7%) than standard dressing (7/121, 6%). Additional wound care was required in 9 patients (7%) in the standard group and 6 patients (4%) in the occlusive group (P = .27). Two patients (1.7%) in the standard group were readmitted for treatment of wound dehiscence; no one in the occlusive group was readmitted to the hospital or had to return to the operating room for treatment of a wound complication. The difference was not statistically significant (P = .13). There were also no significant (P = .81) differences in rate of wound complications between THA and TKA patients.
There were statistically significantly (P < .0001) fewer dressing changes in the occlusive dressing group. Mean number of dressing changes was 0.14 (median, 0; interquartile range, 0-0) in the occlusive group and 2.8 (median, 2; interquartile range, 1-3) in the standard group.
Compared with patients in the standard dressing group, patients in the occlusive dressing group reported significantly higher satisfaction scores. Mean overall patient satisfaction score was 92 in the occlusive group and 81 in the standard group (P < .0001). Patients in the occlusive group were more satisfied with their ability to take care of their personal hygiene, to change clothes, and to sit and sleep comfortably (Table 4).
Discussion
Wound complications after TJA are common, occurring in up to 30% of patients,1-6 and are associated with development of superficial and deep surgical site infections, increased resource utilization, and longer hospital stays.5-11 Although the role of surgical dressings has received little attention in TJA practice, other subspecialties have found that occlusive barrier dressings can reduce wound complications and promote wound healing.16,17 Mitotic cell division and leukocyte activity, which are critical in wound healing, increase under occlusive dressings. This cellular activity is disrupted with every dressing change, delaying wound healing (biological activity takes 3-4 hours to resume).22 In addition, occlusive dressings increase hypoxia, which promotes angiogenesis and accelerates wound healing.23
Despite being a prospective RCT, this study had several limitations. Because of the need to evaluate wounds and obvious differences between the 2 dressings (eg, color, ability to shower), it was not possible to blind the patient or surgeon to the dressing used. When rating satisfaction, patients were not able to directly compare the 2 dressings. The primary endpoint of the study was the complication rate; however, the deep periprosthetic infection rate may be a superior endpoint and would require a much larger study. Although we assumed that wound complications may be harbingers for periprosthetic infections, no patient in either group developed periprosthetic infection. Therefore, we cannot conclude that surgical dressings play a role in reducing infections. In addition, as the standard dressing was changed on POD 2 (per standard protocol) and the occlusive dressing could remain in place for up to 7 days, there was a selection bias in the evaluation of the number of dressing changes. However, given the characteristics of the standard dressing (eg, tape, gauze, nonocclusive), leaving it in place after POD 2 is not optimal. Therefore, we would expect to see a difference in the number of dressing changes. We think this comparison remains valid, as occlusive dressings were changed when there were indications of wound problems (eg, excessive drainage [strike-through], surrounding erythema, blistering). With an average of less than 1 dressing change in the occlusive group, we think this is a surrogate for uneventful wound healing and decreased wound complication, and our data support this. It is also important to test both dressing durability and patient tolerance for wearing a single dressing for 7 days.
Our RCT results showed that, compared with a standard dressing, an occlusive antimicrobial dressing was associated with a significant decrease in overall wound complications and blisters. These findings are similar to those of other studies of occlusive dressings in a number of surgical subspecialties.16,18 In an RCT of 200 patients who underwent elective and nonelective hip and knee surgery and were randomized to either absorbent perforated dressing with adhesive border (Cutiplast, Smith & Nephew) or Aquacel (ConvaTec) covered with vapor-permeable dressing (Tegaderm, 3M), Ravenscroft and colleagues20 found that Aquacel-plus-Tegaderm was 5.8 times more likely than Cutiplast to produce an uncompromised wound. Similarly, in an RCT of hydrofiber (Aquacel) and central pad (Mepore, Mölnlycke) dressings after primary THA and TKA, Abuzakuk and colleagues19 found significantly fewer dressing changes (43% vs 77%) and blisters (13% vs 26%) in the hydrofiber group than in the pad group.
Hopper and colleagues24 compared 50 consecutive patients treated with modern dressings (Aquacel) with 50 historical control patients treated with traditional surgical dressings (Mepore). Blisters developed in 20% of the patients in the traditional group and 4% of patients in the modern group (P = .028). The authors concluded that adverse outcomes of wound healing can be minimized with modern dressings.
A recent retrospective study by Cai and colleagues25 evaluated the incidence of acute periprosthetic infection (≤3 months after surgery) with use of occlusive (Aquacel) and standard dressings. Incidence of acute periprosthetic infection was 0.44% in the occlusive group and 1.7% in the standard group (P = .005). Incidence of wound-healing problems was not evaluated.
Our second aim in the present study was to evaluate the number of dressing changes required. There were significantly fewer dressing changes in the occlusive dressing group than in the standard dressing group. Therefore, wear time (amount of time a single dressing remains in place) was substantially longer for the occlusive group. In the study by Hopper and colleagues,24 wear time was significantly shorter for the traditional dressing than for the modern dressing (2 vs 7 days; P < .001), and the traditional dressing required more changes (3 vs 0; P < .001).
These findings are important for several reasons. Standard surgical dressings often require frequent changes. If left in place, they create an excessively moist wound environment that promotes blistering and delays wound healing. However, frequent dressing changes expose the wound and increase the risk for surgical site infection.26 A barrier dressing left in place from time of surgery prevents bacteria from entering and contaminating a healing wound. A study by Clarke and colleagues21 demonstrated higher skin colonization rates for patients who had dressings changed on POD 1 than for patients who had their first dressing change on POD 6.
Our third study aim was to evaluate patient satisfaction with surgical dressings. The orthopedic literature has little on this topic.23 Blisters and other wound complications can negatively affect satisfaction.2,3 Our data showed significant improvement in satisfaction, particularly regarding sterility and hygiene.
Other surgical subspecialties have found similar improvement in patient satisfaction with occlusive barrier dressings. In an RCT of 88 pediatric patients, Rasmussen and colleagues27 found that patients reported significantly less pain during changes of an occlusive adhesive dressing (Duoderm, ConvaTec) than during changes of a conventional Steristrip (3M) plus Cutiplast. According to the authors, the occlusive wound dressing seemed to minimize the physical and psychological trauma to the infant or child and lessen disruption of the child’s and the parents’ daily routines, because the children could be bathed immediately after surgery.
Our study did not specifically address cost. Cai and colleagues25 estimated that, if the Aquacel dressing were routinely used in every hip and knee arthroplasty, it would add about $27 million in cost. However, this must be balanced by the cost of managing infection after TJA. In the United States, at an estimated $50,000 to $100,000 per case and an annual incidence of 1% to 2%, the low-end cost for the treatment of periprosthetic infection would be $500 million.28 Cai and colleagues25 found a 4-fold reduction in periprosthetic infection when use of occlusive dressings was implemented. In addition, wound complications remain the number one reason for hospital readmission after TJA.12,13 Cost of hospital readmission, as well as financial penalties to institutions for unplanned readmission for wound complications, must be considered.
Conclusion
Our RCT results demonstrated that use of occlusive antimicrobial barrier dressings (vs standard surgical dressings) significantly reduced wound complications and dressing changes and improved overall patient satisfaction. These findings are similar to those in the literature on TJA and other surgical subspecialties. We conclude that occlusive surgical dressings reduce wound complications after TJA.
Wound complications (eg, delayed wound healing, blisters, prolonged drainage) have been reported in up to 30% of patients who undergo elective total joint arthroplasty (TJA).1-6 Wound complications increase resource utilization, lengthen hospital stays, and increase costs.7-9 Prolonged wound healing and persistent wound drainage are also harbingers of both superficial and deep surgical site infections.5-11
In several studies, wound complications after TJA were the primary reason for hospital readmissions.12-15 As part of the Patient Protection and Affordable Care Act, hospitals will be penalized by the Centers for Medicaid & Medicare Services for unplanned hospital readmissions within 30 days after TJA. It is imperative, then, to reduce the risk factors and complications associated with surgical site infections to decrease unplanned readmissions.
Historically, little attention has been given to the role of surgical dressings and the effect of dressings on wound healing. Although many subspecialties (eg, cardiothoracic surgery, general surgery) have reported benefits in using occlusive dressings, adoption in TJA has been slow.16-18 At our institution about 5 years ago, we began using an occlusive silver-impregnated barrier dressing based on preliminary data from studies showing benefits of occlusive dressings in TJA.19,20
We conducted a study to determine if use of occlusive antimicrobial barrier dressings decreases rates of wound complications in TJA. We had 3 research questions: Compared with standard surgical dressings, are occlusive dressings associated with decreased rates of wound complications after TJA? Is there a difference in number of dressing changes required between the 2 dressing types? Is satisfaction higher for patients with occlusive dressings than for patients with standard dressings?
Patients and Methods
This randomized controlled trial (RCT) was reviewed and approved by the Institutional Review Board at Carolinas Healthcare. Patients were randomized by the research staff using a parallel, 1:1 allocation method. The randomization table was generated using a random number generator.
An a priori sample size estimate was made using a 2-tailed Fisher exact test with a .05 level of significance. Based on a study by Clarke and colleagues,21 we estimated the incidence of wound problems at 3% in the occlusive dressing (study) group and 13% in the standard dressing (control) group. We determined that 260 participants (130 per group) would be needed to achieve 80% power. We considered a 15% attrition rate for a total enrollment goal of 300 study participants (150 per group).
Between December 2010 and January 2013, patients presenting for either primary total hip arthroplasty (THA) or primary total knee arthroplasty (TKA) were recruited to participate in the study. Eligibility criteria (Table 1) were reviewed, and patients were enrolled by the senior surgeons, Dr. Springer, Dr. Beaver, Dr. Griffin, and Dr. Mason. All eligible participants who provided informed consent were randomized to receive either an occlusive antimicrobial barrier dressing (Aquacel Ag, ConvaTec) or standard surgical dressing (Primapore, Smith & Nephew). The occlusive dressing (Figure 1) consists of an outer barrier layer of hydrocolloid and a central island of hydrofiber, which absorbs and locks in any wound exudate within the fibers and prevents the creation of an overly moist wound environment that can lead to skin maceration and wound breakdown. In addition, the hydrofibers are embedded with ionic silver, which is released only at the site of wound exudate, or drainage; thus, there is no continuous exposure of the entire wound to silver. The standard dressing (Figure 2) consists of a central island of gauze enclosed in low-allergy acrylic adhesive tape.
All surgical dressings were placed over a closed incision in a sterile environment in the operating room after the procedure. The groups’ wound closures were identical.
A posterior approach was used for all THAs. The deep fascia was closed with a running barbed suture (Quill, Angiotech), the deep subcutaneous tissue with No. 1 Vicryl suture (Ethicon), and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch (Ethicon) was placed in the subcuticular layer and was followed with a skin adhesive (Dermabond, Ethicon). A closed suction drain, removed on postoperative day (POD) 1, was used for all THAs.
A standard medial parapatellar arthrotomy was used for all TKAs. The arthrotomy was closed with a running barbed suture, the deep subcutaneous tissue with No. 1 Vicryl suture, and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch was placed in the subcuticular layer and was followed with a skin adhesive. A closed suction drain was also used. In addition, a compressive wrap was placed over the dressing in the operating room and was removed the next morning. During the hospital stay, the surgical site was evaluated daily with a standard wound evaluation form.
In the standard dressing group, the bandage was removed for wound evaluation on POD 2, and the dressing was changed every other day during the hospital stay. The dressing was also changed as needed for wound drainage (Figure 3) or other minor wound-healing concerns.
In the occlusive dressing group, the dressing design allowed the dressing to remain in place for about 7 days. It was removed by a home health nurse during a visit closest to but not before the 7-day mark. In addition, it was changed at surgeon discretion if there were concerns about wound drainage or wound healing. For the occlusive barrier, wound drainage was evaluated by strike-through of drainage on the back side of the dressing (Figure 4). If more than 50% of the dressing was saturated, the bandage was changed and the wound evaluated. If there were no immediate concerns about wound complications (eg, infection, blistering), a new occlusive dressing was placed. Because the occlusive dressing was waterproof, patients in the study group were able to shower immediately after surgery. In the control group, patients were allowed to shower if the surgical dressing was kept dry, as the bandage was not waterproof.
Per the study protocol, all patients were discharged home and followed by a single home health agency. Mean hospital stay was 3 days (range, 0-8 days), which did not differ significantly between groups (P = .133). All home health nurses were trained in evaluation of postsurgical wounds and were aware of the study requirements. The nurses visited all patients 3 days a week until the scheduled 4-week postoperative follow-up with the treating physician or physician assistant. At each visit, the nurse evaluated the wound and surrounding skin using a standard wound document. Dressings were changed based on the criteria we have described. Concerns about wound status (eg, drainage, blistering, erythema) prompted removal of the dressing for further evaluation. The physician was notified of concerns about wound healing, which prompted an office visit for evaluation. The dressing remained in place for a minimum of 7 days but in all cases was removed as close to 7 days as possible, depending on the scheduled nursing visits. Once uneventful wound healing was complete, no further dressing was required. A final wound evaluation was conducted by the surgeon at the 4-week postoperative evaluation.
The primary outcome measure was wound complication (dichotomous variable). Wounds were assessed by describing the amount, type, and color of exudate (Figure 5). The appearance of the wound margins and the surrounding skin was also assessed. Because wounds could not be directly visualized in the occlusive dressing group, drainage (indicated by strike-through) was used as a measure of possible wound complications, prompting removal and full evaluation.
Secondary endpoints included additional wound treatment or surgical procedures for wound complications, number of dressing changes, and patient satisfaction. Patients completed a satisfaction questionnaire at each wound assessment (Figure 6). Using a visual analog scale (VAS), they rated their satisfaction with their ability to perform activities of daily living (personal hygiene, change clothes, sit comfortably, sleep comfortably), drawing a line on the VAS at a point between 0 (totally unsatisfied) and 100 (totally satisfied) for each satisfaction measure. This line was measured and recorded by the study coordinator. The 4 satisfaction measures were averaged for a composite satisfaction measure.
All statistical analyses were conducted using SAS Version 9.2 (SAS Institute). Standard univariate descriptive statistics (means, standard deviations, frequencies, proportions) were calculated and reported. Differences in mean values for continuous data were assessed with independent t test or Wilcoxon rank sum test. Chi-square test and Fisher exact test were used to determine differences between groups for categorical or dichotomous variables. A significance level of .05 was used for all statistical tests.
Results
The 300 patients who consented to participate in the study were randomized to receive either occlusive dressing or standard dressing. After randomization, 38 patients (15 occlusive, 23 standard) were withdrawn from the study (Table 2), leaving a final dataset of 262 patients, 141 in the occlusive group (67 THAs, 74 TKAs) and 121 in the standard group (49 THAs, 72 TKAs). There were no differences in proportion of THAs or TKAs, age, sex, or body mass index between the occlusive and standard groups (Table 3).
There were statistically significantly (P = .015) fewer wound complications in the occlusive dressing group (10%) than in the standard dressing group (22%). Blisters at or around the wound site were reported in significantly (P = .026) fewer patients with occlusive dressing (1/141, 0.7%) than standard dressing (7/121, 6%). Additional wound care was required in 9 patients (7%) in the standard group and 6 patients (4%) in the occlusive group (P = .27). Two patients (1.7%) in the standard group were readmitted for treatment of wound dehiscence; no one in the occlusive group was readmitted to the hospital or had to return to the operating room for treatment of a wound complication. The difference was not statistically significant (P = .13). There were also no significant (P = .81) differences in rate of wound complications between THA and TKA patients.
There were statistically significantly (P < .0001) fewer dressing changes in the occlusive dressing group. Mean number of dressing changes was 0.14 (median, 0; interquartile range, 0-0) in the occlusive group and 2.8 (median, 2; interquartile range, 1-3) in the standard group.
Compared with patients in the standard dressing group, patients in the occlusive dressing group reported significantly higher satisfaction scores. Mean overall patient satisfaction score was 92 in the occlusive group and 81 in the standard group (P < .0001). Patients in the occlusive group were more satisfied with their ability to take care of their personal hygiene, to change clothes, and to sit and sleep comfortably (Table 4).
Discussion
Wound complications after TJA are common, occurring in up to 30% of patients,1-6 and are associated with development of superficial and deep surgical site infections, increased resource utilization, and longer hospital stays.5-11 Although the role of surgical dressings has received little attention in TJA practice, other subspecialties have found that occlusive barrier dressings can reduce wound complications and promote wound healing.16,17 Mitotic cell division and leukocyte activity, which are critical in wound healing, increase under occlusive dressings. This cellular activity is disrupted with every dressing change, delaying wound healing (biological activity takes 3-4 hours to resume).22 In addition, occlusive dressings increase hypoxia, which promotes angiogenesis and accelerates wound healing.23
Despite being a prospective RCT, this study had several limitations. Because of the need to evaluate wounds and obvious differences between the 2 dressings (eg, color, ability to shower), it was not possible to blind the patient or surgeon to the dressing used. When rating satisfaction, patients were not able to directly compare the 2 dressings. The primary endpoint of the study was the complication rate; however, the deep periprosthetic infection rate may be a superior endpoint and would require a much larger study. Although we assumed that wound complications may be harbingers for periprosthetic infections, no patient in either group developed periprosthetic infection. Therefore, we cannot conclude that surgical dressings play a role in reducing infections. In addition, as the standard dressing was changed on POD 2 (per standard protocol) and the occlusive dressing could remain in place for up to 7 days, there was a selection bias in the evaluation of the number of dressing changes. However, given the characteristics of the standard dressing (eg, tape, gauze, nonocclusive), leaving it in place after POD 2 is not optimal. Therefore, we would expect to see a difference in the number of dressing changes. We think this comparison remains valid, as occlusive dressings were changed when there were indications of wound problems (eg, excessive drainage [strike-through], surrounding erythema, blistering). With an average of less than 1 dressing change in the occlusive group, we think this is a surrogate for uneventful wound healing and decreased wound complication, and our data support this. It is also important to test both dressing durability and patient tolerance for wearing a single dressing for 7 days.
Our RCT results showed that, compared with a standard dressing, an occlusive antimicrobial dressing was associated with a significant decrease in overall wound complications and blisters. These findings are similar to those of other studies of occlusive dressings in a number of surgical subspecialties.16,18 In an RCT of 200 patients who underwent elective and nonelective hip and knee surgery and were randomized to either absorbent perforated dressing with adhesive border (Cutiplast, Smith & Nephew) or Aquacel (ConvaTec) covered with vapor-permeable dressing (Tegaderm, 3M), Ravenscroft and colleagues20 found that Aquacel-plus-Tegaderm was 5.8 times more likely than Cutiplast to produce an uncompromised wound. Similarly, in an RCT of hydrofiber (Aquacel) and central pad (Mepore, Mölnlycke) dressings after primary THA and TKA, Abuzakuk and colleagues19 found significantly fewer dressing changes (43% vs 77%) and blisters (13% vs 26%) in the hydrofiber group than in the pad group.
Hopper and colleagues24 compared 50 consecutive patients treated with modern dressings (Aquacel) with 50 historical control patients treated with traditional surgical dressings (Mepore). Blisters developed in 20% of the patients in the traditional group and 4% of patients in the modern group (P = .028). The authors concluded that adverse outcomes of wound healing can be minimized with modern dressings.
A recent retrospective study by Cai and colleagues25 evaluated the incidence of acute periprosthetic infection (≤3 months after surgery) with use of occlusive (Aquacel) and standard dressings. Incidence of acute periprosthetic infection was 0.44% in the occlusive group and 1.7% in the standard group (P = .005). Incidence of wound-healing problems was not evaluated.
Our second aim in the present study was to evaluate the number of dressing changes required. There were significantly fewer dressing changes in the occlusive dressing group than in the standard dressing group. Therefore, wear time (amount of time a single dressing remains in place) was substantially longer for the occlusive group. In the study by Hopper and colleagues,24 wear time was significantly shorter for the traditional dressing than for the modern dressing (2 vs 7 days; P < .001), and the traditional dressing required more changes (3 vs 0; P < .001).
These findings are important for several reasons. Standard surgical dressings often require frequent changes. If left in place, they create an excessively moist wound environment that promotes blistering and delays wound healing. However, frequent dressing changes expose the wound and increase the risk for surgical site infection.26 A barrier dressing left in place from time of surgery prevents bacteria from entering and contaminating a healing wound. A study by Clarke and colleagues21 demonstrated higher skin colonization rates for patients who had dressings changed on POD 1 than for patients who had their first dressing change on POD 6.
Our third study aim was to evaluate patient satisfaction with surgical dressings. The orthopedic literature has little on this topic.23 Blisters and other wound complications can negatively affect satisfaction.2,3 Our data showed significant improvement in satisfaction, particularly regarding sterility and hygiene.
Other surgical subspecialties have found similar improvement in patient satisfaction with occlusive barrier dressings. In an RCT of 88 pediatric patients, Rasmussen and colleagues27 found that patients reported significantly less pain during changes of an occlusive adhesive dressing (Duoderm, ConvaTec) than during changes of a conventional Steristrip (3M) plus Cutiplast. According to the authors, the occlusive wound dressing seemed to minimize the physical and psychological trauma to the infant or child and lessen disruption of the child’s and the parents’ daily routines, because the children could be bathed immediately after surgery.
Our study did not specifically address cost. Cai and colleagues25 estimated that, if the Aquacel dressing were routinely used in every hip and knee arthroplasty, it would add about $27 million in cost. However, this must be balanced by the cost of managing infection after TJA. In the United States, at an estimated $50,000 to $100,000 per case and an annual incidence of 1% to 2%, the low-end cost for the treatment of periprosthetic infection would be $500 million.28 Cai and colleagues25 found a 4-fold reduction in periprosthetic infection when use of occlusive dressings was implemented. In addition, wound complications remain the number one reason for hospital readmission after TJA.12,13 Cost of hospital readmission, as well as financial penalties to institutions for unplanned readmission for wound complications, must be considered.
Conclusion
Our RCT results demonstrated that use of occlusive antimicrobial barrier dressings (vs standard surgical dressings) significantly reduced wound complications and dressing changes and improved overall patient satisfaction. These findings are similar to those in the literature on TJA and other surgical subspecialties. We conclude that occlusive surgical dressings reduce wound complications after TJA.
1. Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. J Wound Care. 2005;14(1):27-29.
2. Koval KJ, Egol KA, Hiebert R, Spratt KF. Tape blisters after hip surgery: can they be eliminated completely? Am J Orthop. 2007;36(5):261-265.
3. Lawrentschuk N, Falkenberg MP, Pirpiris M. Wound blisters post hip surgery: a prospective trial comparing dressings. ANZ J Surg. 2002;72(10):716-719.
4. Mihalko WM, Manaswi A, Brown TE, Parvizi J, Schmalzried TP, Saleh KJ. Infection in primary total knee arthroplasty: contributing factors. Instr Course Lect. 2008;57:317-325.
5. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
6. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
7. Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91(1):48-54.
8. Gordon SM, Culver DH, Simmons BP, Jarvis WR. Risk factors for wound infections after total knee arthroplasty. Am J Epidemiol. 1990;131(5):905-916.
9. Jaberi FM, Parvizi J, Haytmanek CT, Joshi A, Purtill J. Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Clin Orthop Relat Res. 2008;466(6):1368-1371.
10. Schmalzried TP. The infected hip: telltale signs and treatment options. J Arthroplasty. 2006;21(4 suppl 1):97-100.
11. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289.
12. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468.
13. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
14. Jordan CJ, Goldstein RY, Michels RF, Hutzler L, Slover JD, Bosco JA 3rd. Comprehensive program reduces hospital readmission rates after total joint arthroplasty. Am J Orthop. 2012;41(11):E147-E151.
15. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
16. Shinohara T, Yamashita Y, Satoh K, et al. Prospective evaluation of occlusive hydrocolloid dressing versus conventional gauze dressing regarding the healing effect after abdominal operations: randomized controlled trial. Asian J Surg. 2008;31(1):1-5.
17. Siah CJ, Yatim J. Efficacy of a total occlusive ionic silver-containing dressing combination in decreasing risk of surgical site infection: an RCT. J Wound Care. 2011;20(12):561-568.
18. Teshima H, Kawano H, Kashikie H, et al. A new hydrocolloid dressing prevents surgical site infection of median sternotomy wounds. Surg Today. 2009;39(10):848-854.
19. Abuzakuk TM, Coward P, Shenava Y, Kumar VS, Skinner JA. The management of wounds following primary lower limb arthroplasty: a prospective, randomised study comparing hydrofibre and central pad dressings. Int Wound J. 2006;3(2):133-137.
20. Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: Aquacel and Tegaderm versus Cutiplast. Ann R Coll Surg Engl. 2006;88(1):18-22.
21. Clarke JV, Deakin AH, Dillon JM, Emmerson S, Kinninmonth AW. A prospective clinical audit of a new dressing design for lower limb arthroplasty wounds. J Wound Care. 2009;18(1):5-8, 10-11.
22. Kloeters O. The use of a semi-occlusive dressing reduces epidermal inflammatory cytokine expression and mitigates dermal proliferation and inflammation in a rat incisional model. Wound Repair Regen. 2008;16(4):568-575.
23. Michie DD, Hugill JV. Influence of occlusive and impregnated gauze dressings on incisional healing: a prospective, randomized, controlled study. Ann Plast Surg. 1994;32(1):57-64.
24. Hopper GP, Deakin AH, Crane EO, Clarke JV. Enhancing patient recovery following lower limb arthroplasty with a modern wound dressing: a prospective, comparative audit. J Wound Care. 2012;21(4):200-203.
25. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
26. Berg A, Fleischer S, Kuss O, Unverzagt S, Langer G. Timing of dressing removal in the healing of surgical wounds by primary intention: quantitative systematic review protocol. J Adv Nurs. 2012;68(2):264-270.
27. Rasmussen H, Larsen MJ, Skeie E. Surgical wound dressing in outpatient paediatric surgery. A randomised study. Dan Med Bull. 1993;40(2):252-254.
28. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
1. Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. J Wound Care. 2005;14(1):27-29.
2. Koval KJ, Egol KA, Hiebert R, Spratt KF. Tape blisters after hip surgery: can they be eliminated completely? Am J Orthop. 2007;36(5):261-265.
3. Lawrentschuk N, Falkenberg MP, Pirpiris M. Wound blisters post hip surgery: a prospective trial comparing dressings. ANZ J Surg. 2002;72(10):716-719.
4. Mihalko WM, Manaswi A, Brown TE, Parvizi J, Schmalzried TP, Saleh KJ. Infection in primary total knee arthroplasty: contributing factors. Instr Course Lect. 2008;57:317-325.
5. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
6. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
7. Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91(1):48-54.
8. Gordon SM, Culver DH, Simmons BP, Jarvis WR. Risk factors for wound infections after total knee arthroplasty. Am J Epidemiol. 1990;131(5):905-916.
9. Jaberi FM, Parvizi J, Haytmanek CT, Joshi A, Purtill J. Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Clin Orthop Relat Res. 2008;466(6):1368-1371.
10. Schmalzried TP. The infected hip: telltale signs and treatment options. J Arthroplasty. 2006;21(4 suppl 1):97-100.
11. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289.
12. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468.
13. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
14. Jordan CJ, Goldstein RY, Michels RF, Hutzler L, Slover JD, Bosco JA 3rd. Comprehensive program reduces hospital readmission rates after total joint arthroplasty. Am J Orthop. 2012;41(11):E147-E151.
15. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
16. Shinohara T, Yamashita Y, Satoh K, et al. Prospective evaluation of occlusive hydrocolloid dressing versus conventional gauze dressing regarding the healing effect after abdominal operations: randomized controlled trial. Asian J Surg. 2008;31(1):1-5.
17. Siah CJ, Yatim J. Efficacy of a total occlusive ionic silver-containing dressing combination in decreasing risk of surgical site infection: an RCT. J Wound Care. 2011;20(12):561-568.
18. Teshima H, Kawano H, Kashikie H, et al. A new hydrocolloid dressing prevents surgical site infection of median sternotomy wounds. Surg Today. 2009;39(10):848-854.
19. Abuzakuk TM, Coward P, Shenava Y, Kumar VS, Skinner JA. The management of wounds following primary lower limb arthroplasty: a prospective, randomised study comparing hydrofibre and central pad dressings. Int Wound J. 2006;3(2):133-137.
20. Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: Aquacel and Tegaderm versus Cutiplast. Ann R Coll Surg Engl. 2006;88(1):18-22.
21. Clarke JV, Deakin AH, Dillon JM, Emmerson S, Kinninmonth AW. A prospective clinical audit of a new dressing design for lower limb arthroplasty wounds. J Wound Care. 2009;18(1):5-8, 10-11.
22. Kloeters O. The use of a semi-occlusive dressing reduces epidermal inflammatory cytokine expression and mitigates dermal proliferation and inflammation in a rat incisional model. Wound Repair Regen. 2008;16(4):568-575.
23. Michie DD, Hugill JV. Influence of occlusive and impregnated gauze dressings on incisional healing: a prospective, randomized, controlled study. Ann Plast Surg. 1994;32(1):57-64.
24. Hopper GP, Deakin AH, Crane EO, Clarke JV. Enhancing patient recovery following lower limb arthroplasty with a modern wound dressing: a prospective, comparative audit. J Wound Care. 2012;21(4):200-203.
25. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
26. Berg A, Fleischer S, Kuss O, Unverzagt S, Langer G. Timing of dressing removal in the healing of surgical wounds by primary intention: quantitative systematic review protocol. J Adv Nurs. 2012;68(2):264-270.
27. Rasmussen H, Larsen MJ, Skeie E. Surgical wound dressing in outpatient paediatric surgery. A randomised study. Dan Med Bull. 1993;40(2):252-254.
28. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
Modular Versus Nonmodular Femoral Necks for Primary Total Hip Arthroplasty
Femoral stem modularity in total hip arthroplasty (THA) has a checkered past. Developments such as the modular head–trunnion interface, which allows for placement of femoral heads of different sizes and offsets, and the modular midstem, which allows for version adjustments independent of patient anatomy (S-ROM, Depuy) and for bypassing proximal bone defects in the revision setting (Restoration Modular, Stryker; ZMR-XL, Zimmer), have proved very successful.1-10 However, even these successful advances have been associated with failures at the modular junction.11-13 Proximal femoral neck–stem modularity (PFNSM) has had mixed results, with notable failures and recalls associated with the neck–stem junction.14,15 Failures at this junction have occurred secondary to corrosion and breakage of the modular neck.16-18 Nevertheless, proximal modular stems remain available for implantation. One such system, the M/L Taper stem with Kinectiv technology (Zimmer), is an all-titanium construct that allows for adjustment of several variables (length, offset, version), providing numerous combinations beyond those of the original M/L Taper offerings. Advantages of these offerings include closer reconstruction of patient anatomy, stability improvements, and easing of the process of revision in polyethylene/femoral head exchanges or in infections in which single-staged irrigation and débridement and polyethylene/head exchange are chosen.
These theoretic advantages must be judged in the context of the possible disadvantages of the modular neck junction. The mechanical environment of the junction places it at risk for failure as well as for metallosis from fretting, crevice corrosion, and recurrent repassivation.19 Although the titanium necks are at less risk for degradation than their cobalt-chromium counterparts, they are at higher risk for breakage.13,19 For one of the surgeons in our practice, the M/L Taper stem with Kinectiv technology is the stem of choice for primary THA.
We conducted a study to determine, in the setting of primary THA, how often a neck–stem combination choice resulted in a reconstructive geometry that would not have been possible had the surgeon opted for the traditional M/L Taper stem. Every Kinectiv stem has numerous neck options with a head center position that would not be possible with the nonmodular M/L Taper. However, in a high-volume community practice, how often is a modular neck that results in an otherwise unavailable head center being used for the reconstruction?
Materials and Methods
This study was approved by our local institutional review board. The Kinectiv stem is used by 1 of the 4 high-volume joint replacement surgeons in our practice (not one of the authors). From our community practice joint registry, we identified every stem–neck combination used since the Kinectiv stem became available in 2006.20 Each case was performed using a posterior approach. A trabecular metal acetabular component (Zimmer) secured with 2 screws was used, and an M/L Taper stem with Kinectiv technology was implanted in each case.
Once the neck–stem combination was determined, its position on the head centers map was compared with that of the standard M/L Taper head centers (Figures 1, 2) for each stem size as the relationship of the Kinectiv head center varies with each stem size compared with the head center of the M/L Taper stems. If the head centers were in contact on the map, the geometry was considered identical. If the head centers were not in contact, we noted where the nearest standard M/L Taper head center lay in terms of length and offset. As the head centers are laid out in regular, 4-mm increments, this estimation was relatively easy. Any anteverted or retroverted neck was considered to have no adequate substitution in the standard M/L Taper stem offerings. This initial evaluation was performed by Dr. Carothers.
We then reviewed the head center comparisons independently. For every Kinectiv head center that did not contact an M/L Taper counterpart, the difference between those head centers was reviewed. Each of us noted whether the difference between the head centers was clinically relevant, as many of the head center positions are extremely close. The head centers that were so close as to be deemed clinically irrelevant were recorded.
Results
Between January 2008 and October 2013, 463 primary THAs were performed using the M/L Taper femoral stem with Kinectiv technology. Of the neck options used, 205 (44%) had a head center identical to that of a nonmodular M/L Taper stem. In another 56 cases (12%), all 3 reviewing surgeons agreed that the M/L Taper head center was so close to the Kinectiv head center as to be clinically indistinguishable. Of these 56 cases, 54 had a head center difference of less than 1 mm in length or offset; the other 2 had a 2-mm difference in offset.
Thus, a total of 261 stems (56%) had a standard M/L Taper option that offered an identical head center or one so close as to be clinically indistinguishable. Interestingly, in the group of 202 stems that did not have an identical head center and were not clinically indistinguishable, 132 (65%) of these modular stems were within 4 mm in length and 2 mm of offset of the closest Kinectiv head center. A verted neck was used in 12 cases (11 anteverted, 1 retroverted).
Nine of the 463 cases required revision surgery, 3 for recurrent instability. In 1 of these 3 cases, the acetabulum was revised for malposition, and the neck was converted from standard offset, +0 mm length (head center identical to nonmodular stem), to extended offset, +4 mm length (2 mm shorter with 1 mm less offset than closest nonmodular head center). The second case had complete deficiency of the abductor tendons and was converted to a constrained liner, though at the time of the THA a head center identical to that of the nonmodular stem was used. The third case was revised to convert a standard offset, +0 mm length, straight neck (head center identical to nonmodular stem), to extended offset, +4 mm length, anteverted neck (anteversion making this a unique head center position). Of the other 6 cases, 1 was treated for corrosion at the head–neck junction by changing the head from cobalt-chromium to ceramic (the junction was noted to be pristine), 1 underwent revision of the acetabular component for loosening, 2 femoral stems were revised for periprosthetic femur fracture, and 2 cases underwent 2-stage revision for late infection. There were no failures secondary to metallosis at the neck–stem junction and no modular breakages. The 3 cases of recurrent instability had no dislocation episodes after revision.
Discussion
PFNSM was developed to help more closely reconstruct patient anatomy. PFNSM allows for individualization of offset, length, and version—and thus for optimization of component interaction to avoid impingement and dislocation while promoting range of motion and normal gait.21 These benefits must be judged in light of the disadvantages of proximal stem modularity, including corrosion and breakage of the modular neck.14-18
In the present study, conducted in a high-volume private practice setting, 44% of necks used in a proximally modular construct had a head center identical to that of a nonmodular alternative. In the opinion of the 3 authors (high-volume hip surgeons), an additional 12% of the modular stems had a head center so close to that of the nonmodular stem as to be clinically indistinguishable. In addition, 132 of the modular necks had a femoral head center within 4 mm in length and 2 mm of offset of the nonmodular stem. These findings call into question the theoretical benefits of regular use of this modular femoral stem for primary THA. Certainly there are extreme femoral neck–shaft angle cases in which the standard nonmodular stem may be inadequate and this proximal modularity would be helpful, but our study showed such cases are relatively less frequent. We caution against routine use of this proximal modularity in primary THA and suggest restricting it to cases in which the standard stem offerings are unacceptable. These findings are not surprising given that the standard M/L Taper stem is based on a historically successful model with neck angle and length options designed to meet the goals of restoring length, offset, range of motion, and stability. We would expect that a well-designed stem will meet these goals in the majority of cases.
Of our 463 cases with the modular neck, 9 required revision surgery. Of these 9 revisions, 2 were for recurrent dislocation in which the modular neck was revised to one that enhanced stability, and there were no further dislocations. The ability to change the geometry of the proximal femur resulted in a stability solution that avoided revision of the entire femoral component, as might otherwise be required. One case of acetabular loosening and 1 case that required placement of a constrained liner were potentially benefited by the modular neck in that the surgeries may have been expedited by being able to remove the neck to ease exposure for placement of the acetabular components. The other 5 revisions—2 for periprosthetic femur fracture, 2 two-stage revisions for infection, and 1 femoral head exchange for metallosis at the head–neck junction—saw no benefit from the modularity in the revision setting.
This study had several limitations. First, as it was primarily an evaluation of use of a modular femoral system, there was no attempt to account for the fact that acetabular component orientation can affect stability and, thus, the perceived need for additional offset or changes in version. The habit of all 3 of the reviewing surgeons is to consider the position of the acetabular component and to reposition the component, if necessary, to achieve appropriate stability. Therefore, the need for the modularity may be even less than suggested by this study. In addition, the idea that (in 12 cases) no standard stem option would be acceptable because of the use of a verted neck ignores the possibility that cup repositioning could have obviated the need for additional version. Furthermore, use of a 36-mm head results in an additional 3.5 mm of offset in the polyethylene liner, and this study did not account for the option of increasing head size—and for the potential increase in stability from a larger head and the increased offset gained from the liner.
A second limitation is that a significant number of Kinectiv stems (132) had a head center within 4 mm in length and 2 mm of offset of the nearest M/L Taper stem. We carefully template every primary THA to determine the plan that will optimize component size and position and restore length and offset. More options for achieving these goals are available when templating with the intention of using the Kinectiv modular neck. The neck cut and position of the stem proximally or distally in the proximal femur may not need to be so exact, as the additional options may be able to accommodate minor inaccuracies. Thus, the reported percentage of clinically indistinguishable head centers (12%) may underestimate the actual number of modular stems that could have been replaced with a nonmodular stem.
Third, this study did not evaluate the effect of the modular junction on ease of irrigation and débridement with head/neck and polyethylene exchange in cases of infection, or on ease of head/neck and polyethylene exchange for revision. In addition, the study did not evaluate other cases of instability involving a nonmodular stem that otherwise could have been solved with simple revision of the head/neck combination, avoiding revision of the entire stem and/or the acetabular component. We reported revisions for infection and for instability, but comprehensive assessment and comparison were beyond the scope of this study. Certainly ease of revision of the head and neck is a factor that could favor use of the modularity.
Fourth, this was not a clinical outcome study comparing 2 different femoral stems. We sought only to determine how often a modular neck was chosen that resulted in a head center that would have been unavailable to the non-modular stem suggesting that the patient was receiving a reconstructive benefit in exchange for the modularity. However, 2 recent reports have noted no clinical benefit at 2-year follow-up with use of the modular neck compared with the nonmodular stem.22,23
Though the M/L Taper with Kinectiv technology has, thus far, performed well, PFNSM should be used with caution in light of recently reported failures at the neck–stem junction.14,16-18 Our study results suggest that most (≥56%) of the modular stems used could have been reconstructed as acceptably with a nonmodular stem, and therefore a reconstructive benefit was not realized in trade for the potential risks of proximal modularity. Only 2 of the 9 revision cases saw a clear advantage in being able to change the modular neck geometry in the revision setting. Given the recently reported failures and the high-profile recall of a modular stem,14 we recommend restricting the modular stem to cases that cannot be adequately reconstructed with the nonmodular option.
1. Barrack RL. Modularity of prosthetic implants. J Am Acad Orthop Surg. 1994;2(1):16-25.
2. Cameron HU. Modularity in primary total hip arthroplasty. J Arthroplasty. 1996;11(3):332-334.
3. Hozack WJ, Mesa JJ, Rothman RF. Head–neck modularity for total hip arthroplasty. Is it necessary? J Arthroplasty. 1996;11(4):397-399.
4. Holt GE, Christie MJ, Schwartz HS. Trabecular metal endoprosthetic limb salvage reconstruction of the lower limb. J Arthroplasty. 2009;24(7):1079-1089.
5. Sporer SM, Obar RJ, Bernini PM. Primary total hip arthroplasty using a modular proximally coated prosthesis in patients older than 70: two to eight year results. J Arthroplasty. 2004;19(2):197-203.
6. Spitzer AI. The S-ROM cementless femoral stem: history and literature review. Orthopedics. 2005;28(9 suppl):s1117-s1124.
7. Mumme T, Müller-Rath R, Andereya S, Wirtz DC. Uncemented femoral revision arthroplasty using the modular revision prosthesis MRP-TITAN revision stem. Oper Orthop Traumatol. 2007;19(1):56-77.
8. Wirtz DC, Heller KD, Holzwarth U, et al. A modular femoral implant for uncemented stem revision in THR. Int Orthop. 2000;24(3):134-138.
9. Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Revision total hip arthroplasty with a porous-coated modular stem: 5 to 10 years follow-up. Clin Orthop Relat Res. 2010;468(5):1310-1315.
10. Bolognesi MP, Pietrobon R, Clifford PE, Vail TP. Comparison of a hydroxyapatite-coated sleeve and a porous-coated sleeve with a modular revision hip stem. A prospective, randomized study. J Bone Joint Surg Am. 2004;86(12):2720-2725.
11. Huot Carlson JC, Van Citters DW, Currier JH, Bryant AM, Mayor MB, Collier JP. Femoral stem fracture and in vivo corrosion of retrieved modular femoral hips. J Arthroplasty. 2012;27(7):1389-1396.e1.
12. Gilbert JL, Mehta M, Pinder B. Fretting crevice corrosion of stainless steel stem–CoCr femoral head connections: comparisons of materials, initial moisture, and offset length. J Biomed Mater Res B Appl Biomater. 2009;88(1):162-173.
13. Kop AM, Keogh C, Swarts E. Proximal component modularity in THA—at what cost? An implant retrieval study. Clin Orthop Relat Res. 2012;470(7):1885-1894.
14. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck–body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
15. Vundelinckx BJ, Verhelst LA, De Schepper J. Taper corrosion in modular hip prostheses: analysis of serum metal ions in 19 patients. J Arthroplasty. 2013;28(7):1218-1223.
16. Kouzelis A, Georgiou CS, Megas P. Dissociation of modular total hip arthroplasty at the neck–stem interface without dislocation. J Orthop Traumatol. 2012;13(4):221-224.
17. Sotereanos NG, Sauber TJ, Tupis TT. Modular femoral neck fracture after primary total hip arthroplasty. J Arthroplasty. 2013;28(1):196.e7-e9.
18. Wodecki P, Sabbah D, Kermarrec G, Semaan I. New type of hip arthroplasty failure related to modular femoral components: breakage at the neck–stem junction. Orthop Traumatol Surg Res. 2013;99(6):741-744.
19. Dorn U, Neumann D, Frank M. Corrosion behavior of tantalum-coated cobalt-chromium modular necks compared to titanium modular necks in a simulator test. J Arthroplasty. 2014;29(4):831-835.
20. Carothers JT, White RE, Tripuraneni KR, Hattab MW, Archibeck MJ. Lessons learned from managing a prospective, private practice joint replacement registry: a 25-year experience. Clin Orthop Relat Res. 2013;471(2):537-543.
21. Archibeck MJ, Cummins T, Carothers J, Junick DW, White RE Jr. A comparison of two implant systems in restoration of hip geometry in arthroplasty. Clin Orthop Rel Res. 2011;469(2):443-446.
22. Duwelius PJ, Hartzband MA, Burkhart R, et al. Clinical results of a modular neck hip system: hitting the “bull’s-eye” more accurately. Am J Orthop. 2010;39(10 suppl):2-6.
23. Duwelius PJ, Burkhart B, Carnahan C, et al. Modular versus nonmodular neck femoral implants in primary total hip arthroplasty: which is better? Clin Orthop Relat Res. 2014;472(4):1240-1245.
Femoral stem modularity in total hip arthroplasty (THA) has a checkered past. Developments such as the modular head–trunnion interface, which allows for placement of femoral heads of different sizes and offsets, and the modular midstem, which allows for version adjustments independent of patient anatomy (S-ROM, Depuy) and for bypassing proximal bone defects in the revision setting (Restoration Modular, Stryker; ZMR-XL, Zimmer), have proved very successful.1-10 However, even these successful advances have been associated with failures at the modular junction.11-13 Proximal femoral neck–stem modularity (PFNSM) has had mixed results, with notable failures and recalls associated with the neck–stem junction.14,15 Failures at this junction have occurred secondary to corrosion and breakage of the modular neck.16-18 Nevertheless, proximal modular stems remain available for implantation. One such system, the M/L Taper stem with Kinectiv technology (Zimmer), is an all-titanium construct that allows for adjustment of several variables (length, offset, version), providing numerous combinations beyond those of the original M/L Taper offerings. Advantages of these offerings include closer reconstruction of patient anatomy, stability improvements, and easing of the process of revision in polyethylene/femoral head exchanges or in infections in which single-staged irrigation and débridement and polyethylene/head exchange are chosen.
These theoretic advantages must be judged in the context of the possible disadvantages of the modular neck junction. The mechanical environment of the junction places it at risk for failure as well as for metallosis from fretting, crevice corrosion, and recurrent repassivation.19 Although the titanium necks are at less risk for degradation than their cobalt-chromium counterparts, they are at higher risk for breakage.13,19 For one of the surgeons in our practice, the M/L Taper stem with Kinectiv technology is the stem of choice for primary THA.
We conducted a study to determine, in the setting of primary THA, how often a neck–stem combination choice resulted in a reconstructive geometry that would not have been possible had the surgeon opted for the traditional M/L Taper stem. Every Kinectiv stem has numerous neck options with a head center position that would not be possible with the nonmodular M/L Taper. However, in a high-volume community practice, how often is a modular neck that results in an otherwise unavailable head center being used for the reconstruction?
Materials and Methods
This study was approved by our local institutional review board. The Kinectiv stem is used by 1 of the 4 high-volume joint replacement surgeons in our practice (not one of the authors). From our community practice joint registry, we identified every stem–neck combination used since the Kinectiv stem became available in 2006.20 Each case was performed using a posterior approach. A trabecular metal acetabular component (Zimmer) secured with 2 screws was used, and an M/L Taper stem with Kinectiv technology was implanted in each case.
Once the neck–stem combination was determined, its position on the head centers map was compared with that of the standard M/L Taper head centers (Figures 1, 2) for each stem size as the relationship of the Kinectiv head center varies with each stem size compared with the head center of the M/L Taper stems. If the head centers were in contact on the map, the geometry was considered identical. If the head centers were not in contact, we noted where the nearest standard M/L Taper head center lay in terms of length and offset. As the head centers are laid out in regular, 4-mm increments, this estimation was relatively easy. Any anteverted or retroverted neck was considered to have no adequate substitution in the standard M/L Taper stem offerings. This initial evaluation was performed by Dr. Carothers.
We then reviewed the head center comparisons independently. For every Kinectiv head center that did not contact an M/L Taper counterpart, the difference between those head centers was reviewed. Each of us noted whether the difference between the head centers was clinically relevant, as many of the head center positions are extremely close. The head centers that were so close as to be deemed clinically irrelevant were recorded.
Results
Between January 2008 and October 2013, 463 primary THAs were performed using the M/L Taper femoral stem with Kinectiv technology. Of the neck options used, 205 (44%) had a head center identical to that of a nonmodular M/L Taper stem. In another 56 cases (12%), all 3 reviewing surgeons agreed that the M/L Taper head center was so close to the Kinectiv head center as to be clinically indistinguishable. Of these 56 cases, 54 had a head center difference of less than 1 mm in length or offset; the other 2 had a 2-mm difference in offset.
Thus, a total of 261 stems (56%) had a standard M/L Taper option that offered an identical head center or one so close as to be clinically indistinguishable. Interestingly, in the group of 202 stems that did not have an identical head center and were not clinically indistinguishable, 132 (65%) of these modular stems were within 4 mm in length and 2 mm of offset of the closest Kinectiv head center. A verted neck was used in 12 cases (11 anteverted, 1 retroverted).
Nine of the 463 cases required revision surgery, 3 for recurrent instability. In 1 of these 3 cases, the acetabulum was revised for malposition, and the neck was converted from standard offset, +0 mm length (head center identical to nonmodular stem), to extended offset, +4 mm length (2 mm shorter with 1 mm less offset than closest nonmodular head center). The second case had complete deficiency of the abductor tendons and was converted to a constrained liner, though at the time of the THA a head center identical to that of the nonmodular stem was used. The third case was revised to convert a standard offset, +0 mm length, straight neck (head center identical to nonmodular stem), to extended offset, +4 mm length, anteverted neck (anteversion making this a unique head center position). Of the other 6 cases, 1 was treated for corrosion at the head–neck junction by changing the head from cobalt-chromium to ceramic (the junction was noted to be pristine), 1 underwent revision of the acetabular component for loosening, 2 femoral stems were revised for periprosthetic femur fracture, and 2 cases underwent 2-stage revision for late infection. There were no failures secondary to metallosis at the neck–stem junction and no modular breakages. The 3 cases of recurrent instability had no dislocation episodes after revision.
Discussion
PFNSM was developed to help more closely reconstruct patient anatomy. PFNSM allows for individualization of offset, length, and version—and thus for optimization of component interaction to avoid impingement and dislocation while promoting range of motion and normal gait.21 These benefits must be judged in light of the disadvantages of proximal stem modularity, including corrosion and breakage of the modular neck.14-18
In the present study, conducted in a high-volume private practice setting, 44% of necks used in a proximally modular construct had a head center identical to that of a nonmodular alternative. In the opinion of the 3 authors (high-volume hip surgeons), an additional 12% of the modular stems had a head center so close to that of the nonmodular stem as to be clinically indistinguishable. In addition, 132 of the modular necks had a femoral head center within 4 mm in length and 2 mm of offset of the nonmodular stem. These findings call into question the theoretical benefits of regular use of this modular femoral stem for primary THA. Certainly there are extreme femoral neck–shaft angle cases in which the standard nonmodular stem may be inadequate and this proximal modularity would be helpful, but our study showed such cases are relatively less frequent. We caution against routine use of this proximal modularity in primary THA and suggest restricting it to cases in which the standard stem offerings are unacceptable. These findings are not surprising given that the standard M/L Taper stem is based on a historically successful model with neck angle and length options designed to meet the goals of restoring length, offset, range of motion, and stability. We would expect that a well-designed stem will meet these goals in the majority of cases.
Of our 463 cases with the modular neck, 9 required revision surgery. Of these 9 revisions, 2 were for recurrent dislocation in which the modular neck was revised to one that enhanced stability, and there were no further dislocations. The ability to change the geometry of the proximal femur resulted in a stability solution that avoided revision of the entire femoral component, as might otherwise be required. One case of acetabular loosening and 1 case that required placement of a constrained liner were potentially benefited by the modular neck in that the surgeries may have been expedited by being able to remove the neck to ease exposure for placement of the acetabular components. The other 5 revisions—2 for periprosthetic femur fracture, 2 two-stage revisions for infection, and 1 femoral head exchange for metallosis at the head–neck junction—saw no benefit from the modularity in the revision setting.
This study had several limitations. First, as it was primarily an evaluation of use of a modular femoral system, there was no attempt to account for the fact that acetabular component orientation can affect stability and, thus, the perceived need for additional offset or changes in version. The habit of all 3 of the reviewing surgeons is to consider the position of the acetabular component and to reposition the component, if necessary, to achieve appropriate stability. Therefore, the need for the modularity may be even less than suggested by this study. In addition, the idea that (in 12 cases) no standard stem option would be acceptable because of the use of a verted neck ignores the possibility that cup repositioning could have obviated the need for additional version. Furthermore, use of a 36-mm head results in an additional 3.5 mm of offset in the polyethylene liner, and this study did not account for the option of increasing head size—and for the potential increase in stability from a larger head and the increased offset gained from the liner.
A second limitation is that a significant number of Kinectiv stems (132) had a head center within 4 mm in length and 2 mm of offset of the nearest M/L Taper stem. We carefully template every primary THA to determine the plan that will optimize component size and position and restore length and offset. More options for achieving these goals are available when templating with the intention of using the Kinectiv modular neck. The neck cut and position of the stem proximally or distally in the proximal femur may not need to be so exact, as the additional options may be able to accommodate minor inaccuracies. Thus, the reported percentage of clinically indistinguishable head centers (12%) may underestimate the actual number of modular stems that could have been replaced with a nonmodular stem.
Third, this study did not evaluate the effect of the modular junction on ease of irrigation and débridement with head/neck and polyethylene exchange in cases of infection, or on ease of head/neck and polyethylene exchange for revision. In addition, the study did not evaluate other cases of instability involving a nonmodular stem that otherwise could have been solved with simple revision of the head/neck combination, avoiding revision of the entire stem and/or the acetabular component. We reported revisions for infection and for instability, but comprehensive assessment and comparison were beyond the scope of this study. Certainly ease of revision of the head and neck is a factor that could favor use of the modularity.
Fourth, this was not a clinical outcome study comparing 2 different femoral stems. We sought only to determine how often a modular neck was chosen that resulted in a head center that would have been unavailable to the non-modular stem suggesting that the patient was receiving a reconstructive benefit in exchange for the modularity. However, 2 recent reports have noted no clinical benefit at 2-year follow-up with use of the modular neck compared with the nonmodular stem.22,23
Though the M/L Taper with Kinectiv technology has, thus far, performed well, PFNSM should be used with caution in light of recently reported failures at the neck–stem junction.14,16-18 Our study results suggest that most (≥56%) of the modular stems used could have been reconstructed as acceptably with a nonmodular stem, and therefore a reconstructive benefit was not realized in trade for the potential risks of proximal modularity. Only 2 of the 9 revision cases saw a clear advantage in being able to change the modular neck geometry in the revision setting. Given the recently reported failures and the high-profile recall of a modular stem,14 we recommend restricting the modular stem to cases that cannot be adequately reconstructed with the nonmodular option.
Femoral stem modularity in total hip arthroplasty (THA) has a checkered past. Developments such as the modular head–trunnion interface, which allows for placement of femoral heads of different sizes and offsets, and the modular midstem, which allows for version adjustments independent of patient anatomy (S-ROM, Depuy) and for bypassing proximal bone defects in the revision setting (Restoration Modular, Stryker; ZMR-XL, Zimmer), have proved very successful.1-10 However, even these successful advances have been associated with failures at the modular junction.11-13 Proximal femoral neck–stem modularity (PFNSM) has had mixed results, with notable failures and recalls associated with the neck–stem junction.14,15 Failures at this junction have occurred secondary to corrosion and breakage of the modular neck.16-18 Nevertheless, proximal modular stems remain available for implantation. One such system, the M/L Taper stem with Kinectiv technology (Zimmer), is an all-titanium construct that allows for adjustment of several variables (length, offset, version), providing numerous combinations beyond those of the original M/L Taper offerings. Advantages of these offerings include closer reconstruction of patient anatomy, stability improvements, and easing of the process of revision in polyethylene/femoral head exchanges or in infections in which single-staged irrigation and débridement and polyethylene/head exchange are chosen.
These theoretic advantages must be judged in the context of the possible disadvantages of the modular neck junction. The mechanical environment of the junction places it at risk for failure as well as for metallosis from fretting, crevice corrosion, and recurrent repassivation.19 Although the titanium necks are at less risk for degradation than their cobalt-chromium counterparts, they are at higher risk for breakage.13,19 For one of the surgeons in our practice, the M/L Taper stem with Kinectiv technology is the stem of choice for primary THA.
We conducted a study to determine, in the setting of primary THA, how often a neck–stem combination choice resulted in a reconstructive geometry that would not have been possible had the surgeon opted for the traditional M/L Taper stem. Every Kinectiv stem has numerous neck options with a head center position that would not be possible with the nonmodular M/L Taper. However, in a high-volume community practice, how often is a modular neck that results in an otherwise unavailable head center being used for the reconstruction?
Materials and Methods
This study was approved by our local institutional review board. The Kinectiv stem is used by 1 of the 4 high-volume joint replacement surgeons in our practice (not one of the authors). From our community practice joint registry, we identified every stem–neck combination used since the Kinectiv stem became available in 2006.20 Each case was performed using a posterior approach. A trabecular metal acetabular component (Zimmer) secured with 2 screws was used, and an M/L Taper stem with Kinectiv technology was implanted in each case.
Once the neck–stem combination was determined, its position on the head centers map was compared with that of the standard M/L Taper head centers (Figures 1, 2) for each stem size as the relationship of the Kinectiv head center varies with each stem size compared with the head center of the M/L Taper stems. If the head centers were in contact on the map, the geometry was considered identical. If the head centers were not in contact, we noted where the nearest standard M/L Taper head center lay in terms of length and offset. As the head centers are laid out in regular, 4-mm increments, this estimation was relatively easy. Any anteverted or retroverted neck was considered to have no adequate substitution in the standard M/L Taper stem offerings. This initial evaluation was performed by Dr. Carothers.
We then reviewed the head center comparisons independently. For every Kinectiv head center that did not contact an M/L Taper counterpart, the difference between those head centers was reviewed. Each of us noted whether the difference between the head centers was clinically relevant, as many of the head center positions are extremely close. The head centers that were so close as to be deemed clinically irrelevant were recorded.
Results
Between January 2008 and October 2013, 463 primary THAs were performed using the M/L Taper femoral stem with Kinectiv technology. Of the neck options used, 205 (44%) had a head center identical to that of a nonmodular M/L Taper stem. In another 56 cases (12%), all 3 reviewing surgeons agreed that the M/L Taper head center was so close to the Kinectiv head center as to be clinically indistinguishable. Of these 56 cases, 54 had a head center difference of less than 1 mm in length or offset; the other 2 had a 2-mm difference in offset.
Thus, a total of 261 stems (56%) had a standard M/L Taper option that offered an identical head center or one so close as to be clinically indistinguishable. Interestingly, in the group of 202 stems that did not have an identical head center and were not clinically indistinguishable, 132 (65%) of these modular stems were within 4 mm in length and 2 mm of offset of the closest Kinectiv head center. A verted neck was used in 12 cases (11 anteverted, 1 retroverted).
Nine of the 463 cases required revision surgery, 3 for recurrent instability. In 1 of these 3 cases, the acetabulum was revised for malposition, and the neck was converted from standard offset, +0 mm length (head center identical to nonmodular stem), to extended offset, +4 mm length (2 mm shorter with 1 mm less offset than closest nonmodular head center). The second case had complete deficiency of the abductor tendons and was converted to a constrained liner, though at the time of the THA a head center identical to that of the nonmodular stem was used. The third case was revised to convert a standard offset, +0 mm length, straight neck (head center identical to nonmodular stem), to extended offset, +4 mm length, anteverted neck (anteversion making this a unique head center position). Of the other 6 cases, 1 was treated for corrosion at the head–neck junction by changing the head from cobalt-chromium to ceramic (the junction was noted to be pristine), 1 underwent revision of the acetabular component for loosening, 2 femoral stems were revised for periprosthetic femur fracture, and 2 cases underwent 2-stage revision for late infection. There were no failures secondary to metallosis at the neck–stem junction and no modular breakages. The 3 cases of recurrent instability had no dislocation episodes after revision.
Discussion
PFNSM was developed to help more closely reconstruct patient anatomy. PFNSM allows for individualization of offset, length, and version—and thus for optimization of component interaction to avoid impingement and dislocation while promoting range of motion and normal gait.21 These benefits must be judged in light of the disadvantages of proximal stem modularity, including corrosion and breakage of the modular neck.14-18
In the present study, conducted in a high-volume private practice setting, 44% of necks used in a proximally modular construct had a head center identical to that of a nonmodular alternative. In the opinion of the 3 authors (high-volume hip surgeons), an additional 12% of the modular stems had a head center so close to that of the nonmodular stem as to be clinically indistinguishable. In addition, 132 of the modular necks had a femoral head center within 4 mm in length and 2 mm of offset of the nonmodular stem. These findings call into question the theoretical benefits of regular use of this modular femoral stem for primary THA. Certainly there are extreme femoral neck–shaft angle cases in which the standard nonmodular stem may be inadequate and this proximal modularity would be helpful, but our study showed such cases are relatively less frequent. We caution against routine use of this proximal modularity in primary THA and suggest restricting it to cases in which the standard stem offerings are unacceptable. These findings are not surprising given that the standard M/L Taper stem is based on a historically successful model with neck angle and length options designed to meet the goals of restoring length, offset, range of motion, and stability. We would expect that a well-designed stem will meet these goals in the majority of cases.
Of our 463 cases with the modular neck, 9 required revision surgery. Of these 9 revisions, 2 were for recurrent dislocation in which the modular neck was revised to one that enhanced stability, and there were no further dislocations. The ability to change the geometry of the proximal femur resulted in a stability solution that avoided revision of the entire femoral component, as might otherwise be required. One case of acetabular loosening and 1 case that required placement of a constrained liner were potentially benefited by the modular neck in that the surgeries may have been expedited by being able to remove the neck to ease exposure for placement of the acetabular components. The other 5 revisions—2 for periprosthetic femur fracture, 2 two-stage revisions for infection, and 1 femoral head exchange for metallosis at the head–neck junction—saw no benefit from the modularity in the revision setting.
This study had several limitations. First, as it was primarily an evaluation of use of a modular femoral system, there was no attempt to account for the fact that acetabular component orientation can affect stability and, thus, the perceived need for additional offset or changes in version. The habit of all 3 of the reviewing surgeons is to consider the position of the acetabular component and to reposition the component, if necessary, to achieve appropriate stability. Therefore, the need for the modularity may be even less than suggested by this study. In addition, the idea that (in 12 cases) no standard stem option would be acceptable because of the use of a verted neck ignores the possibility that cup repositioning could have obviated the need for additional version. Furthermore, use of a 36-mm head results in an additional 3.5 mm of offset in the polyethylene liner, and this study did not account for the option of increasing head size—and for the potential increase in stability from a larger head and the increased offset gained from the liner.
A second limitation is that a significant number of Kinectiv stems (132) had a head center within 4 mm in length and 2 mm of offset of the nearest M/L Taper stem. We carefully template every primary THA to determine the plan that will optimize component size and position and restore length and offset. More options for achieving these goals are available when templating with the intention of using the Kinectiv modular neck. The neck cut and position of the stem proximally or distally in the proximal femur may not need to be so exact, as the additional options may be able to accommodate minor inaccuracies. Thus, the reported percentage of clinically indistinguishable head centers (12%) may underestimate the actual number of modular stems that could have been replaced with a nonmodular stem.
Third, this study did not evaluate the effect of the modular junction on ease of irrigation and débridement with head/neck and polyethylene exchange in cases of infection, or on ease of head/neck and polyethylene exchange for revision. In addition, the study did not evaluate other cases of instability involving a nonmodular stem that otherwise could have been solved with simple revision of the head/neck combination, avoiding revision of the entire stem and/or the acetabular component. We reported revisions for infection and for instability, but comprehensive assessment and comparison were beyond the scope of this study. Certainly ease of revision of the head and neck is a factor that could favor use of the modularity.
Fourth, this was not a clinical outcome study comparing 2 different femoral stems. We sought only to determine how often a modular neck was chosen that resulted in a head center that would have been unavailable to the non-modular stem suggesting that the patient was receiving a reconstructive benefit in exchange for the modularity. However, 2 recent reports have noted no clinical benefit at 2-year follow-up with use of the modular neck compared with the nonmodular stem.22,23
Though the M/L Taper with Kinectiv technology has, thus far, performed well, PFNSM should be used with caution in light of recently reported failures at the neck–stem junction.14,16-18 Our study results suggest that most (≥56%) of the modular stems used could have been reconstructed as acceptably with a nonmodular stem, and therefore a reconstructive benefit was not realized in trade for the potential risks of proximal modularity. Only 2 of the 9 revision cases saw a clear advantage in being able to change the modular neck geometry in the revision setting. Given the recently reported failures and the high-profile recall of a modular stem,14 we recommend restricting the modular stem to cases that cannot be adequately reconstructed with the nonmodular option.
1. Barrack RL. Modularity of prosthetic implants. J Am Acad Orthop Surg. 1994;2(1):16-25.
2. Cameron HU. Modularity in primary total hip arthroplasty. J Arthroplasty. 1996;11(3):332-334.
3. Hozack WJ, Mesa JJ, Rothman RF. Head–neck modularity for total hip arthroplasty. Is it necessary? J Arthroplasty. 1996;11(4):397-399.
4. Holt GE, Christie MJ, Schwartz HS. Trabecular metal endoprosthetic limb salvage reconstruction of the lower limb. J Arthroplasty. 2009;24(7):1079-1089.
5. Sporer SM, Obar RJ, Bernini PM. Primary total hip arthroplasty using a modular proximally coated prosthesis in patients older than 70: two to eight year results. J Arthroplasty. 2004;19(2):197-203.
6. Spitzer AI. The S-ROM cementless femoral stem: history and literature review. Orthopedics. 2005;28(9 suppl):s1117-s1124.
7. Mumme T, Müller-Rath R, Andereya S, Wirtz DC. Uncemented femoral revision arthroplasty using the modular revision prosthesis MRP-TITAN revision stem. Oper Orthop Traumatol. 2007;19(1):56-77.
8. Wirtz DC, Heller KD, Holzwarth U, et al. A modular femoral implant for uncemented stem revision in THR. Int Orthop. 2000;24(3):134-138.
9. Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Revision total hip arthroplasty with a porous-coated modular stem: 5 to 10 years follow-up. Clin Orthop Relat Res. 2010;468(5):1310-1315.
10. Bolognesi MP, Pietrobon R, Clifford PE, Vail TP. Comparison of a hydroxyapatite-coated sleeve and a porous-coated sleeve with a modular revision hip stem. A prospective, randomized study. J Bone Joint Surg Am. 2004;86(12):2720-2725.
11. Huot Carlson JC, Van Citters DW, Currier JH, Bryant AM, Mayor MB, Collier JP. Femoral stem fracture and in vivo corrosion of retrieved modular femoral hips. J Arthroplasty. 2012;27(7):1389-1396.e1.
12. Gilbert JL, Mehta M, Pinder B. Fretting crevice corrosion of stainless steel stem–CoCr femoral head connections: comparisons of materials, initial moisture, and offset length. J Biomed Mater Res B Appl Biomater. 2009;88(1):162-173.
13. Kop AM, Keogh C, Swarts E. Proximal component modularity in THA—at what cost? An implant retrieval study. Clin Orthop Relat Res. 2012;470(7):1885-1894.
14. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck–body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
15. Vundelinckx BJ, Verhelst LA, De Schepper J. Taper corrosion in modular hip prostheses: analysis of serum metal ions in 19 patients. J Arthroplasty. 2013;28(7):1218-1223.
16. Kouzelis A, Georgiou CS, Megas P. Dissociation of modular total hip arthroplasty at the neck–stem interface without dislocation. J Orthop Traumatol. 2012;13(4):221-224.
17. Sotereanos NG, Sauber TJ, Tupis TT. Modular femoral neck fracture after primary total hip arthroplasty. J Arthroplasty. 2013;28(1):196.e7-e9.
18. Wodecki P, Sabbah D, Kermarrec G, Semaan I. New type of hip arthroplasty failure related to modular femoral components: breakage at the neck–stem junction. Orthop Traumatol Surg Res. 2013;99(6):741-744.
19. Dorn U, Neumann D, Frank M. Corrosion behavior of tantalum-coated cobalt-chromium modular necks compared to titanium modular necks in a simulator test. J Arthroplasty. 2014;29(4):831-835.
20. Carothers JT, White RE, Tripuraneni KR, Hattab MW, Archibeck MJ. Lessons learned from managing a prospective, private practice joint replacement registry: a 25-year experience. Clin Orthop Relat Res. 2013;471(2):537-543.
21. Archibeck MJ, Cummins T, Carothers J, Junick DW, White RE Jr. A comparison of two implant systems in restoration of hip geometry in arthroplasty. Clin Orthop Rel Res. 2011;469(2):443-446.
22. Duwelius PJ, Hartzband MA, Burkhart R, et al. Clinical results of a modular neck hip system: hitting the “bull’s-eye” more accurately. Am J Orthop. 2010;39(10 suppl):2-6.
23. Duwelius PJ, Burkhart B, Carnahan C, et al. Modular versus nonmodular neck femoral implants in primary total hip arthroplasty: which is better? Clin Orthop Relat Res. 2014;472(4):1240-1245.
1. Barrack RL. Modularity of prosthetic implants. J Am Acad Orthop Surg. 1994;2(1):16-25.
2. Cameron HU. Modularity in primary total hip arthroplasty. J Arthroplasty. 1996;11(3):332-334.
3. Hozack WJ, Mesa JJ, Rothman RF. Head–neck modularity for total hip arthroplasty. Is it necessary? J Arthroplasty. 1996;11(4):397-399.
4. Holt GE, Christie MJ, Schwartz HS. Trabecular metal endoprosthetic limb salvage reconstruction of the lower limb. J Arthroplasty. 2009;24(7):1079-1089.
5. Sporer SM, Obar RJ, Bernini PM. Primary total hip arthroplasty using a modular proximally coated prosthesis in patients older than 70: two to eight year results. J Arthroplasty. 2004;19(2):197-203.
6. Spitzer AI. The S-ROM cementless femoral stem: history and literature review. Orthopedics. 2005;28(9 suppl):s1117-s1124.
7. Mumme T, Müller-Rath R, Andereya S, Wirtz DC. Uncemented femoral revision arthroplasty using the modular revision prosthesis MRP-TITAN revision stem. Oper Orthop Traumatol. 2007;19(1):56-77.
8. Wirtz DC, Heller KD, Holzwarth U, et al. A modular femoral implant for uncemented stem revision in THR. Int Orthop. 2000;24(3):134-138.
9. Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross AE. Revision total hip arthroplasty with a porous-coated modular stem: 5 to 10 years follow-up. Clin Orthop Relat Res. 2010;468(5):1310-1315.
10. Bolognesi MP, Pietrobon R, Clifford PE, Vail TP. Comparison of a hydroxyapatite-coated sleeve and a porous-coated sleeve with a modular revision hip stem. A prospective, randomized study. J Bone Joint Surg Am. 2004;86(12):2720-2725.
11. Huot Carlson JC, Van Citters DW, Currier JH, Bryant AM, Mayor MB, Collier JP. Femoral stem fracture and in vivo corrosion of retrieved modular femoral hips. J Arthroplasty. 2012;27(7):1389-1396.e1.
12. Gilbert JL, Mehta M, Pinder B. Fretting crevice corrosion of stainless steel stem–CoCr femoral head connections: comparisons of materials, initial moisture, and offset length. J Biomed Mater Res B Appl Biomater. 2009;88(1):162-173.
13. Kop AM, Keogh C, Swarts E. Proximal component modularity in THA—at what cost? An implant retrieval study. Clin Orthop Relat Res. 2012;470(7):1885-1894.
14. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck–body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
15. Vundelinckx BJ, Verhelst LA, De Schepper J. Taper corrosion in modular hip prostheses: analysis of serum metal ions in 19 patients. J Arthroplasty. 2013;28(7):1218-1223.
16. Kouzelis A, Georgiou CS, Megas P. Dissociation of modular total hip arthroplasty at the neck–stem interface without dislocation. J Orthop Traumatol. 2012;13(4):221-224.
17. Sotereanos NG, Sauber TJ, Tupis TT. Modular femoral neck fracture after primary total hip arthroplasty. J Arthroplasty. 2013;28(1):196.e7-e9.
18. Wodecki P, Sabbah D, Kermarrec G, Semaan I. New type of hip arthroplasty failure related to modular femoral components: breakage at the neck–stem junction. Orthop Traumatol Surg Res. 2013;99(6):741-744.
19. Dorn U, Neumann D, Frank M. Corrosion behavior of tantalum-coated cobalt-chromium modular necks compared to titanium modular necks in a simulator test. J Arthroplasty. 2014;29(4):831-835.
20. Carothers JT, White RE, Tripuraneni KR, Hattab MW, Archibeck MJ. Lessons learned from managing a prospective, private practice joint replacement registry: a 25-year experience. Clin Orthop Relat Res. 2013;471(2):537-543.
21. Archibeck MJ, Cummins T, Carothers J, Junick DW, White RE Jr. A comparison of two implant systems in restoration of hip geometry in arthroplasty. Clin Orthop Rel Res. 2011;469(2):443-446.
22. Duwelius PJ, Hartzband MA, Burkhart R, et al. Clinical results of a modular neck hip system: hitting the “bull’s-eye” more accurately. Am J Orthop. 2010;39(10 suppl):2-6.
23. Duwelius PJ, Burkhart B, Carnahan C, et al. Modular versus nonmodular neck femoral implants in primary total hip arthroplasty: which is better? Clin Orthop Relat Res. 2014;472(4):1240-1245.
Radiographically Silent Loosening of the Acetabular Component in Hip Arthroplasty
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
Total hip arthroplasty (THA) is an excellent option for the treatment of osteoarthritis of the hip. In numerous studies, modern implants have shown survivorship of more than 90% at 10 years.1,2 Polyethylene wear and subsequent osteolysis are major obstacles to the long-term success of THA.3-5 Polyethylene wear particles are phagocytized by macrophages, inducing an inflammatory response that can ultimately lead to osteolysis of the bony architecture surrounding the bone–implant interface.6,7 As modern implants often rely on direct implant-to-bone ingrowth to maintain fixation, wear at this junction can lead to aseptic component loosening and ultimately require revision surgery.8-10 Osteolysis can be diagnosed with plain radiography or computed tomography (CT).11 CT is more sensitive than plain radiography for the diagnosis of osteolysis and is better able to determine the size and location of osteolytic lesions.12,13
Although diagnosis of osteolysis is well defined in the literature, what is more challenging is radiographic diagnosis of a loose acetabular component.11 The most commonly described criteria for loosening are presence of a complete radiolucent line of more than 2 mm in width at the bone–implant interface and any progressive tilting or migration of the component.14,15 CT-based criteria for component loosening remain largely undefined, though Egawa and colleagues16 showed that acetabular osteolysis involving less than 40% of the total cup surface is not associated with component loosening. Although a patient may show signs of osteolysis on postoperative imaging, this finding does not necessitate immediate revision surgery.17 Osteolysis may be monitored clinically and followed radiographically to determine when intervention is necessary.13
The goals of revision surgery are to eliminate the wear generator and bone-graft lytic lesions where needed to help maintain the bone–implant interface.17 The timing of such surgery is important, as the surgeon must balance the risk for gross component migration against the morbidity and mortality associated with acetabular component revision.18 This is in contrast to the settings of infection, periprosthetic fracture, recurrent instability, and component fracture/loosening, in which revision is urgently indicated and the case cannot be managed conservatively.
We conducted a study to determine the incidence of loose acetabular components without radiographic or clinical findings that would necessitate urgent revision THA. Radiographically silent loosening (RSL) was defined as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Although these patients make up a small minority of the revision population, knowing the incidence of RSL can help raise surgeon awareness of this potentially dangerous situation. We further sought to determine whether patients with RSL present with different demographic characteristics or clinical symptoms than patients with stable acetabular components.
Materials and Methods
In this retrospective, case–control, institutional review board–approved study, we evaluated patients who had undergone revision THA and had preoperative plain radiographs and CT images. We identified patients by International Classification of Diseases, Ninth Revision (ICD‑9) codes (00.70, 00.71, 00.72, 00.73, 80.05, 81.53, 84.56, 84.57) and searched for those cases treated between 2000 and 2012.
Inclusion criteria were confirmed revision THA and confirmed plain radiography and CT of the THA performed before revision. When osteolysis was diagnosed by plain radiography, CT was ordered to determine the extent of bony lesions or to evaluate for eccentric head position or component malposition. Last, all patients included in the study had a detailed operative report clearly indicating acetabular component stability at time of revision. Acetabular component stability at time of surgery was determined according to the criteria defined by Berger and colleagues.19 Cups were evaluated for gross motion during both hip dislocation and during edge loading of the component after thorough scar and capsular débridement.
Patients who did not have CT performed before revision surgery were excluded from the study. These patients had been diagnosed by clinical history and/or plain radiography. Cases revised for periprosthetic infection or periprosthetic fracture were also excluded. Patients with metal-on-metal bearings were excluded, as were any cases revised from hemiarthroplasty to THA, as well as cases revised for recurrent dislocations or component malposition.
All plain radiographs and CT images were evaluated by the orthopedic surgeon who performed the revision and by a radiologist. Images were inspected for signs of gross component migration, tilting, and concentric lucency of the bone–implant interface. Patients with imaging that showed signs of component movement or migration (as seen by the attending surgeon or the radiologist) were excluded. Patients with radiographic evidence of femoral stem loosening were also excluded, as they had an indication to undergo revision arthroplasty. The remaining patients were then stratified into 2 groups: those with stable acetabular components at time of revision and those with loose acetabular components. Stable acetabular shells showed no gross motion of the implant with dislocation, edge loading with an impactor, or pulling with a Kocher clamp after débridement and screw removal.15,19 The 2 groups were then compared with respect to age, sex, and most common presenting symptoms and diagnoses. Fischer exact test and Student t test were used to statistically compare the groups.
Results
Overall, 393 patients underwent revision arthroplasty for the diagnoses (ICD-9 codes) indicated (Figure). One hundred eighty-nine patients (48.1%) had CT performed before revision. Of these 189 patients, 85 were excluded for diagnoses that were evident on either plain radiography or CT, that necessitated urgent revision, or for procedures beyond the scope of the study (Table 1). CT showed a loose cup in 28 patients; 6 of these cups were also seen on CT. Thirteen patients were diagnosed with a loose femoral stem, 10 with a periprosthetic infection, and 8 with a periprosthetic fracture.
One hundred four patients (54 men, 50 women) met the study inclusion criteria. Mean age was 65.1 years. Of these 104 patients, 87 (83.7%) had a stable acetabular shell at time of revision surgery; the other 17 (16.3%) were diagnosed with RSL of the acetabular shell. Osteolysis was the most common diagnosis (89.4%) in the overall population, and pain was the most common complaint at time of presentation (66.6%). Lack of symptoms was the second most common presentation at time of revision (19.2%) (Table 2). Patients without symptoms underwent revision surgery because of concern about impending compromise of the bone–implant interface and progressive osteolysis.
The 2 groups (stable vs unstable acetabular shells) were not significantly different with respect to age (P = .961) or sex distribution (P = .185). All patients in the RSL group were diagnosed with osteolysis radiographically, and 15 (88%) of 17 patients presented with pain as the primary complaint, compared with only 54 (62%) of 87 patients in the group with stable shells. Patients with RSL were significantly more likely to present with pain as the primary complaint (P = .0487). Nineteen patients in the stable implant group and only 1 patient in the RSL group were asymptomatic, but this was not statistically significant (P = .185) when compared against all other diagnoses.
Discussion
We defined RSL as an acetabular component that was loose at time of revision surgery but that did not show frank signs of loosening on either plain radiography or CT. Patients with RSL and the surgeons who treat them are in a difficult position. In the setting of osteolysis, patients can be treated with serial radiographic imaging and clinical monitoring to determine if and when revision arthroplasty should be performed.17 However, given the complexity and risks associated with revision THA, surgeons should be aware that the acetabular shell may necessitate revision even if it does not appear to be frankly unstable on radiographic imaging.18
Of the 393 patients who underwent revision THA at our institution, 48.1% were evaluated with CT. Eighty-five of the 189 patients who underwent CT were diagnosed with radiographic loosening, or were diagnosed as needing urgent revision THA in the setting of loose components, periprosthetic infection, periprosthetic fracture, or catastrophic implant failure. Of the remaining 104 patients, 17 (16.3%) met the diagnosis of RSL of the acetabular component. The most common complaint was pain, and the most common diagnoses were osteolysis and polyethylene wear. Age and sex were not associated with increased likelihood of RSL.
Our study has several limitations. We defined the radiographic diagnosis of loose acetabular components as components showing frank migration, tilting, or a 2-mm concentric lucency on plain radiography or CT. Although these are common definitions of loose acetabular components, more sensitive radiographic measures have been described.16 We also excluded patients with recurrent dislocations and metal-on-metal prostheses, as these cases increase the metal artifact on CT and limit the ability to evaluate the bone–implant interface. Metal artifact remains an ongoing challenge to use of CT for post-THA imaging. However, tailored imaging protocols are helping to eliminate metal artifact. Bone scan was not used to evaluate for possible component loosening. Although sensitivity and specificity are about 67% and 76%, respectively,20 Temmerman and colleagues21 also found poor intraobserver reliability (0.53) for bone scans in the setting of uncemented acetabular components. Last, our study did not evaluate the bony ingrowth patterns that corresponded to stable or unstable fixation and did not evaluate the volumetric size or anatomical location of the osteolytic lesions on CT. Careful assessment of these variables is clinically relevant when trying to determine if revision arthroplasty is warranted.
Although we used relatively simple radiographic criteria to define loose components, more sensitive and specific techniques have been described for both plain radiography and CT. Moore and colleagues22 described 5 radiographic signs of bony ingrowth; when 3 or more were present, sensitivity was 89.6% and specificity 76.9%. Mehin and colleagues23 suggested that osteolysis involving more than 50% of the circumference of the shell on a standard pelvic radiograph might require revision arthroplasty. However, more recent studies have found that anteroposterior and lateral radiographs are less able to evaluate the anterior and posterior rims of the bone–implant interface, and it is this ingrowth area that may be the most crucial for stable osseointegration.12,16
CT has expanded our ability to evaluate the bone–implant interface in 3 dimensions. Egawa and colleagues16 described using CT to evaluate the surface area involved with osteolysis and found that less than 40% involvement of the surface area generally corresponded to well-fixed components. Furthermore, they found that osteolysis generally creates lesions inferior and superior to the acetabular component and less often involves the anterior and posterior rims, which may be more important for stable fixation. The authors noted that volumetric analysis and CT were not as cost-effective as plain radiography and were more time- and skill-intensive.
Osteolysis itself remains a common indication for revision THA. However, the most appropriate procedure remains controversial. Mallory and colleagues24 recommended explanting all acetabular shells in the setting of revision arthroplasty. They indicated that full assessment of the bony pelvis and any lytic defects was possible only with the wide exposure gained by acetabular component removal. More recent studies have begun to justify isolated component revision in the setting of well-fixed acetabular shells. Studies by Maloney and colleagues,10 Park and colleagues,15 and Beaulé and colleagues25 have shown excellent outcomes with retention of well-fixed acetabular shells and removal of the wear generator in the setting of osteolysis. Haidukewych17 wrote that the goals in addressing osteolysis in revision THA are to eliminate the wear generator, débride osteolytic lesions, and restore bone stock. Surgeons should weigh the benefits of component retention and isolated liner exchange against the morbidity associated with explantation and preparation for implanting a new component. Good outcomes have been achieved with isolated component exchange, but surgeons should be aware that instability remains the most common complication after isolated liner exchange.8
The majority of our patients with RSL presented with complaints of pain and the diagnosis of osteolysis. One patient who had the diagnosis but was clinically asymptomatic was found to have a loose acetabular shell at time of revision surgery. Given the increased morbidity associated with acetabular component revision, this patient’s condition represents a dangerous combination of RSL and clinically asymptomatic component loosening. By raising awareness about RSL and its incidence, we should be able to increase our ability to detect RSL. A surgeon who detects RSL before gross migration or movement of the acetabular component may be better able to plan for revision arthroplasty before a catastrophic event that may necessitate a larger, more complex procedure. With the number of patients who require revision THA continuing to rise, surgeons should be aware of the incidence of RSL and the potential of RSL to affect patient care and potential surgical options.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
1. Milošev I, Kovač S, Trebše R, Levašič V, Pišot V. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am. 2012;94(19):1756-1763.
2. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
3. Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am. 2000;82(8):1102-1107.
4. Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85(6):1095-1099.
5. Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;(393):66-70.
6. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;(460):240-252.
7. Catelas I, Jacobs JJ. Biologic activity of wear particles. Instr Course Lect. 2010;59:3-16.
8. Paprosky WG, Nourbash P, Gill P. Treatment of progressive periacetabular osteolysis: cup revision versus liner exchange and bone grafting. Paper presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 4-8, 1999; Anaheim, CA.
9. Engh CA Jr, Claus AM, Hopper RH Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;(393):137-146.
10. Maloney WJ, Peters P, Engh CA, Chandler H. Severe osteolysis of the pelvic in association with acetabular replacement without cement. J Bone Joint Surg Am. 1993;75(11):1627-1635.
11. Claus AM, Engh CA Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA Sr. Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(8):1519-1526.
12. Puri L, Wixson RL, Stern SH, Kohli J, Hendrix RW, Stulberg SD. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609-614.
13. Stulberg SD, Wixson RL, Adams AD, Hendrix RW, Bernfield JB. Monitoring pelvic osteolysis following total hip replacement surgery: an algorithm for surveillance. J Bone Joint Surg Am. 2002;84(suppl 2):116-122.
14. Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4(3):245-251.
15. Park KS, Yoon TR, Song EK, Lee KB. Results of isolated femoral component revision with well-fixed acetabular implant retention. J Arthroplasty. 2010;25(8):1188-1195.
16. Egawa H, Ho H, Hopper RH Jr, Engh CA Jr, Engh CA. Computed tomography assessment of pelvic osteolysis and cup–lesion interface involvement with a press-fit porous-coated acetabular cup. J Arthroplasty. 2009;24(2):233-239.
17. Haidukewych GJ. Osteolysis in the well-fixed socket: cup retention or revision? J Bone Joint Surg Br. 2012;94(12):65-69.
18. Stulberg BN, Della Valle AG. What are the guidelines for the surgical and nonsurgical treatment of periprosthetic osteolysis? J Am Acad Orthop Surg. 2008;16(suppl 1):S20-S25.
19. Berger RA, Quigley LR, Jacobs JJ, Sheinkop MB, Rosenberg AG, Galante JO. The fate of stable cemented acetabular components retained during revision of a femoral component of a total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1682-1691.
20. Temmerman OP, Raijmakers PG, Deville WL, Berkhof J, Hooft L, Heyligers IC. The use of plain radiography, subtraction arthrography, nuclear arthrography, and bone scintigraphy in the diagnosis of a loose acetabular component of a total hip prosthesis: a systematic review. J Arthroplasty. 2007;22(6):818-827.
21. Temmerman OP, Raijmakers PG, David EF, et al. A comparison of radiographic and scintigraphic techniques to assess aseptic loosening of the acetabular component in a total hip replacement. J Bone Joint Surg Am. 2004;86(11):2456-2463.
22. Moore MS, McAuley JP, Young AM, Engh CA Sr. Radiographic signs of osseointegration in porous-coated acetabular components. Clin Orthop Relat Res. 2006;(444):176-183.
23. Mehin R, Yuan X, Haydon C, et al. Retroacetabular osteolysis: when to operate? Clin Orthop Relat Res. 2004;(428):247-255.
24. Mallory TH, Lombardi AV Jr, Fada RA, Adams JB, Kefauver CA, Eberle RW. Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop Relat Res. 2000;(381):120-128.
25. Beaulé PE, Le Duff MJ, Dorey FJ, Amstutz HC. Fate of cementless acetabular components retained during revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2288-2293.
Causes and Rates of Unplanned Readmissions After Elective Primary Total Joint Arthroplasty: A Systematic Review and Meta-Analysis
Total joint arthroplasty (TJA) is a clinically effective, cost-effective treatment for symptomatic arthritis.1,2 After TJA, patients report reduced pain, restored range of motion, high satisfaction, and ability to return to a more active lifestyle.3-7 The number of total hip arthroplasties (THAs) performed in the United States is expected to reach 572,000 by 2030, a 174% increase, and the number of total knee arthroplasties (TKAs) 3.5 million, nearly a 7-fold increase.8,9 Since 2005, the cost of THA has risen more than 4 times, to $13.43 billion, and the cost of TKA has risen more than 5 times, to $40.8 billion.8,9 Given the demand and price tag, TJA is the single largest cost in the Medicare budget.10
Given its potential to improve care and reduce costs, reducing readmission rates in the surgical setting is a priority for physicians and policymakers.11 Readmissions for TJA are highly scrutinized as a performance indicator—the Centers for Medicare & Medicaid Services (CMS) started including them in its readmissions penalty program in 2013—and were recently validated as a measure of surgical quality.12-14 Accurate assessments of readmissions after TJA are unclear, with rates ranging from 1% to 8.5% between 7 and 90 days after surgery.2,15-17 The early success of TJA as an elective (and more frequently outpatient) procedure has paradoxically translated to less tolerance for readmissions. Post-TJA complications resulting in readmission are subject to financial penalties, and there is an implicit judgment of inadequate surgical management.12
Not only is the readmission rate poorly characterized, but there is no consensus on the leading reasons for readmissions after primary elective unilateral TJAs. The range of rates, reasons, and follow-up periods reported in the literature is wide.18,19 CMS plans to monitor readmissions over 7 to 90 days after surgery (the period depends on the complication), whereas a significant portion of the orthopedic literature documents 90-day rates.19 In 2012, the Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation prepared for CMS a comprehensive report identifying rates of post-TJA complications and readmissions.20 The report, however, is limited to US hospitals and Medicare patients and therefore may overstate the rates, given this population’s documented comorbidities and the reimbursement variations between Medicare and commercial insurance.21 Lack of consensus on readmissions after primary elective unilateral TJAs requires that we synthesize available data to answer several questions: What is the overall readmission rate 30 and 90 days after TJA? What are the primary reasons for readmission 30 and 90 days after TJA? What are the cause-specific readmission rates? We performed a systematic review and a meta-analysis to answer these questions and to add clarity to the literature in order to help guide policy.
Materials and Methods
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.22 Two reviewers independently completed structured searches of the Medline and Cochrane Central Register of Controlled Trials databases. Search terms were: (total hip replacement OR hip arthroplasty OR total hip arthroplasty OR total knee replacement OR knee arthroplasty OR total knee arthroplasty) AND (readmission OR complication OR discharge). They updated the search June 1, 2013. Four limits were applied: publication between January 1, 1982 and December 12, 2012; human subjects only; age 19+ years; and English-language articles. Study eligibility was determined by using standardized criteria as defined by the inclusion and exclusion criteria described in 3 stages: title review, abstract review, and full-article review. The reviewers also performed ancestry searches, including searches for major review articles and bibliographies of all retrieved studies, to identify additional studies not identified in the keyword searches. Discrepancies were resolved by author consensus.
Inclusion criteria were original studies that presented level I to III evidence and that were identified in structured online searches; published in English between January 1, 1982 and December 31, 2012; involved patients older than 19 years; and reported both readmission rates and reasons at follow-up 30 or 90 days after elective primary unilateral TJA, regardless of indication. Exclusion criteria were studies that reported data from hip fracture, knee fracture, and pelvis fracture cases; those that reported data from hemiarthroplasty, Birmingham hip resurfacing procedures, other resurfacing procedures, simultaneous bilateral hip or knee arthroplasties, unicompartmental knee arthroplasty, patellofemoral arthroplasty, metastatic or bone cancer, or revision hip or knee arthroplasty; those that did not report extractable reasons for readmission; those that reported complications but did not specify readmission rates; and those that reported readmission data only from after the 90-day follow-up window. In cases in which multiple studies reported data from the same patient population, only the largest or most recent report was used.
Two reviewers extracted the quantitative data from eligible studies. The 2 primary outcomes of interests were all-cause readmission rates, and reasons for readmission 30 and 90 days after TJA. Other extracted data were evidence level; publication journal, year, and country; data source (academic institution, Medicare); study design; number of patients; patient characteristics; surgical approach; follow-up period; overall readmission rate; anticoagulant use; tourniquet use; and compression stocking use. In addition, all post-TJA readmissions were assumed to be unplanned, except for staged sequential bilateral arthroplasty for osteoarthritis (excluded from analysis).
Readmission reasons were divided into 4 major categories as defined by the literature and the authors: thromboembolic disease, joint-specific reasons, surgical site infection, and surgical sequelae. The diagnoses in these categories are listed in Table 1. Other extracted reasons were cardiac dysrhythmia and pneumonia.
In cases in which there were at least 2 comparable studies, a meta-analysis was performed to obtain pooled estimates of the proportion of patients readmitted at 30 or 90 days. We calculated a Higgins I2 measure for between-study heterogeneity and random-effects analysis, using the method of DerSimonian and Laird23 if I2 was greater than 0.5. Pooled estimates were obtained for both overall and cause-specific reasons for readmission for all reasons reported in at least 3 studies. Small-study or publication bias was assessed using funnel plot asymmetry when at least 5 studies were analyzed as recommended.24 The meta-analytic findings for both overall and cause-specific readmission are presented as pooled proportions with 95% confidence intervals (CIs). All meta-analyses were performed using Stata 10.0.
Results
Fifteen unique TJA studies (12 THA, 10 TKA) met the criteria for the meta-analysis.20,25-38Figure 1 depicts the PRISMA flowchart for study identification.22
Of the 12 studies eligible for the THA analysis (Table 2), 6 were conducted in the United States,20,26,27,30,33,34 5 in Europe,25,28,29,32,35 and 1 in Canada.31 Seven of the 12 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (2 reported rates at both follow-ups). We analyzed a total of 113,396 patients at the 30-day window and 192,380 patients at the 90-day window. Mean age was 74.2 years. The included studies were variable and sparse in their reporting of specific characteristics (Table 3).
Of the 10 studies (2 prospective, 8 retrospective) eligible for the TKA analysis (Table 4), 6 were conducted in the United States,20,26,27,34,36,37 3 in Europe,25,29,35 and 1 in Asia.38 Four of the 10 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (1 reported rates at both follow-ups).27 We analyzed a total of 3,278,635 patients at the 30-day window and 272,419 patients at the 90-day window. Mean age was 74.3 years. The included studies were quite variable and sparse in their reporting of specific characteristics (Table 5).
We performed random-effects meta-analyses of all unplanned readmissions at both 30 and 90 days (all I2s > 0.5). Among 5 THA studies that reported overall rates at 30 days,20,27,28,32,33 the estimated overall unplanned rate among the 120,272 index surgeries was 5.6% (95% CI, 3.2%-8.0%). Among 5 THA studies that reported overall rates at 90 days,20,25-27,31 the estimated overall unplanned rate among the 192,380 index surgeries was 7.7% (95% CI, 3.2%-12.2%) (I2 = 1.00). Among 3 TKA studies that reported overall rates at 30 days,27,37,38 the estimated overall unplanned rate among the 3,278,635 index surgeries was 3.3% (95% CI, 0.7%-5.9%). Among 5 TKA studies that reported overall rates at 90 days,20,25-27,36 the estimated overall unplanned rate among the 272,419 index surgeries was 9.7% (95% CI, 7.1%-12.4%) (I2 = 0.97).
30-Day Readmission Rates
The most common reason for readmission 30 days after THA discharge was joint-specific. This reason accounted for 39.3% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.2% (95% CI, 0.0%-4.6%; P < .001; I2 = 1.00) among 4 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 0.8%-2.5%; P < .001; I2 = 0.95) and thromboembolic disease (1.5%; 95% CI, 1.0%-1.9%; P < .001; I2 = 0.95). See Figure 2 for 30-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.1%; P < .001; I2 = 0.94). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported in only 1 study each.
The most common reason for readmission 30 days after TKA discharge was surgical site infection. This reason accounted for 12.1% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.4% (95% CI, 0.3%-0.6%; P < .001; I2 = 0.61) among 3 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.3% of the time. Joint-specific reasons were reported in 2 studies (95% CI, 0.0%-0.8%; P = .259; I2 = 0.94). Thromboembolic disease was reported in 4 studies (95% CI, 0.0%-0.7%; P = .067; I2 = 0.98) (Figure 3). Only these 3 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and “sequelae” were reported in only 1 study each.
90-Day Readmission Rates
Consistent with the 30-day THA results, the most common reason for readmission 90 days after THA discharge was joint-specific. This reason accounted for 31.2% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.4% (95% CI, 0.0%-4.9%; P < .001; I2 = 1.00) among 5 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 1.0%-2.2%; P < .003; I2 = 0.83) and thromboembolic disease (1.0%; 95% CI, 0.7%-1.4%; P < .001; I2 = 0.97). See Figure 4 for 90-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.0%; P < .001; I2 = 0.99). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported by only 1 study each.
Consistent with the 30-day TKA results, the most common reason for readmission 90 days after TKA discharge was surgical site infection. This reason accounted for 9.3% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.9% (95% CI, 0.4%-1.4%; P < .001; I2 = 0.93) among 5 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.7% of the time. Joint-specific reasons were reported in 5 studies (95% CI, 0.2%-1.1%; P =.003; I2 = 0.94). Thromboembolic disease was reported in 7 studies (95% CI, 0.3%-1.1%; P < .001; I2 = 0.97) (Figure 5). Bleeding was reported in 3 studies, with a pooled rate of 0.4% (95% CI, 0.0%-0.9%; P = .128; I2 = 0.83). Cardiac dysrhythmia was reported in 2 studies, with an estimated pooled rate of 0.3% (95% CI, 0.2%-0.5%; P < .001). Only these 5 reasons could be pooled, as pneumonia and “sequelae” were reported in only 1 study each.
Discussion
This study is the first systematic review and meta-analysis of the literature to identify overall and cause-specific readmission rates after TJA.
For THA, 30- and 90-day readmission rates were 5.6% and 7.7%, respectively. Joint-specific causes were the most common reason for readmission at both 30 and 90 days after THA. For TKA, 30- and 90-day rates were 3.3% and 9.7%, respectively. Surgical site infection was the most common reason for readmission at both 30 and 90 days after TKA.
Hospital readmissions are an important area of scrutiny for Medicare and the health care systems broadly. Readmissions after surgery are deemed quality indicators potentially suggesting incomplete management of active issues and inadequate preparation for discharge.39 Unplanned readmissions also place a significant economic burden on Medicare: $17.5 billion in 2010.40 Given their association with quality of overall surgical care, improved readmission rates have the potential to improve the standard of care and reduce costs.
Higher readmission rates will significantly affect hospitals as CMS shifts to bundling payments for acute-care episodes, such as TJA.41-43 Further, private and public health care payers are increasingly using unplanned 30- and 90-day readmission rates as a marker of quality of care. However, there is little agreement about readmission rates and reasons, let alone what follow-up window should be used to define orthopedic readmissions. One study involving the MEDPAR (Medicare Provider Analysis and Review) database found that a common reason for readmission after major hip or knee surgery was “aftercare” for surgical sequelae (10.3%)15; another study found a 15% increase in post-THA hospitalizations, most commonly for a mechanical complication (joint-related).44 There are no prior complete systematic reviews or meta-analyses of overall rates of readmissions after primary unilateral TJAs, or of the reasons for these readmissions. The closest such report, the Yale report to CMS, was skewed to a proportion of US hospitals treating a population prone to significant comorbidities.20
Although the strength of this study lies in its rigorous identification and extraction of data, notable clarifications must be made when synthesizing the information. First, the definitions of various thromboembolic events varied greatly. Some studies reported deep vein thrombosis (DVT) and pulmonary embolism (PE) separately, whereas others reported only DVT or only PE. Some studies reported rates of readmission for “thromboembolic disorder,” and one25 reported rates for DVT, PE, and thromboembolic disorder. To pool these related events, we created a composite definition that included DVT, PE, and thromboembolic disorders, which we termed thromboembolic disease. We also created a composite measure for joint-specific reasons for readmission. This category included joint infection that definitely required reentry into the joint, but using this category may have led to underestimation of surgical site infection rates, which were defined separately. Third, there was significant variation in documentation of surgical site infection among the studies included in this review. Some studies specified superficial wounds, whereas others did not categorize complications as superficial, deep, or intracapsular, which would qualify as a “joint-specific” cause. Despite this variation, surgical site infection after TJA was found to be the most common reason for readmission.
Our systematic review and meta-analysis were limited, as any others are, by the quality of studies investigated. Few studies reported cause-specific rates and reasons for readmission. Given the small sample, formal tests for small-study or publication bias could not be performed. Some studies included tremendous amounts of data, and International Classification of Diseases, Ninth Revision (ICD-9) codes were used without physician review of readmission diagnoses. In the absence of oversight, many readmissions could have been misinterpreted and incorrectly logged, or simply miscoded. Saucedo and colleagues27,45 found that readmission diagnostic codes were often unverified. Numerous other studies corroborated this lack of correlation with physician-derived readmission diagnoses in just 25% of cases.46-54 Another study limitation is the unknown number of patients who had TJA but presented and were subsequently readmitted to a different hospital. Last, as this review included patients who had surgery performed within a 30-year period, it could not address the shifts in postoperative management that occurred in that time, particularly with respect to anticoagulation. This limitation was partially addressed in THA by dividing final studies into 3 decades. Of these studies, only 1 was from the first decade, 3 were from the second, and the rest were from the third. Of the 3 from the second decade, only the study by Warwick and colleagues29 (1995) explicitly did not use anticoagulation, but compression stockings were used, and consequently there was a 4.0% rate of readmission for thromboembolic disease alone, compared with the study by White and colleagues34 (1998), which explicitly used anticoagulation and boasted a 1.7% rate of readmission for thromboembolic disease. This isolated comparison illustrates the effect of routine anticoagulation and the changes in surgical standards over the 3 decades.
The numbers from this systematic review and meta-analysis represent an international benchmark for TJA as a procedure. Knowing the top reasons for readmission will lead to more focus on joint-related and medical issues (surgical site infection, thromboembolic disease) before discharge to avoid readmission after elective unilateral primary TJA. Although readmission rates have received attention in the United States as a primary means of combating soaring health care costs, knowing the rates for a common procedure applies broadly as an indicator for standard of care worldwide, according to the World Health Organization.55 This study is the first systematic review and meta-analysis of documented readmission rates and reasons for readmission to identify overall and cause-specific rates after TJA. The hope is that our findings will add clarity to the literature and help guide the decisions of physicians and policymakers.
Conclusion
Readmission rates are an increasingly important metric in the United States and around the world, yet there is no consensus regarding overall readmission rates and reasons for readmission after primary unilateral TJAs. Our systematic review and meta-analysis of the literature found overall unplanned readmission rates of 5.6% (30 days) and 7.7% (90 days) for THA and 3.3% (30 days) and 9.7% (90 days) for TKA. At both 30 and 90 days, the most common readmission reasons were joint-specific (THA) and surgical site infection (TKA). New investigations should be directed toward developing countermeasures to lower the rates of readmission.
1. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
2. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2001. JAMA. 2011;305(15):1560-1567.
3. de Vries LM, Sturkenboom MC, Verhaar JA, Kingma JH, Stricker BH. Complications after hip arthroplasty and the association with hospital procedure volume. Acta Orthop. 2011;82(5):545-552.
4. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
5. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869-1876.
6. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533.
7. Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12(4):387-396.
8. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
9. Kurtz SM, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of Medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133-138.
11. Li LT, Mills WL, White DL, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175-1181.
12. Readmissions Reduction Program. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed July 27, 2015.
13. Tsai TC, Joynt KE, Orav J, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134-1142.
14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428.
15. Zmistowski B, Hozack WJ, Parvizi J. Readmission rates after total hip arthroplasty. JAMA. 2011;306(8):825.
16. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
17. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
18. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369.
19. Atkinson JG. Flaws in the Medicare readmission penalty. N Engl J Med. 2012;367(21):2056-2057.
20. Grosso LM, Curtis JP, Lin Z, et al. Hospital-level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty (THA) And/Or Total Knee Arthroplasty (TKA): Measure Methodology Report. Report prepared for Centers for Medicare & Medicaid Services. QualityNet website. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228772504368. Submitted June 25, 2012. Accessed August 4, 2015.
21. Robinson JC. Analysis of Medicare and commercial insurer–paid total knee replacement reveals opportunities for cost reduction. Health Care Incentives Improvement Institute website. http://www.hci3.org/sites/default/files/files/HCI-2012-IssueBrief-L6-2.pdf. Published 2012. Accessed July 27, 2015.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
24. Higgins JP, Thompson SG. Quantifying heterogeniety in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
25. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
26. Keeney JA, Adelani MA, Nunley RM, Clohisy JC, Barrack RL. Assessing readmission databases: how reliable is the information? J Arthroplasty. 2012;27(8 suppl):72-76.e1-e2.
27. Saucedo JM, Marecek GS, Wanke TR, Lee J, Stulberg SD, Puri L. Understanding readmissions after primary total hip and knee arthroplasty: who’s at risk? J Arthroplasty. 2014;29(2):256-260.
28. Seagroatt V, Tan HS, Goldacre M, Bulstrode C, Nugent I, Gill L. Elective total hip replacement: incidence, emergency readmission rate, and postoperative mortality. BMJ. 1991;303(6815):1431-1435.
29. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77(1):6-10.
30. Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79(4):485-494.
31. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
32. Cullen C, Johnson DS, Cook G. Re-admission rates within 28 days of total hip replacement. Ann R Coll Surg Engl. 2006;88(5):475-478.
33. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
34. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531.
35. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
36. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449.
37. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
38. Seah VW, Singh G, Yang KY, Yeo SJ, Lo NN, Seow KH. Thirty-day mortality and morbidity after total knee arthroplasty. Ann Acad Med Singapore. 2007;36(12):1010-1012.
39. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
40. The Revolving Door: A Report on U.S. Hospital Readmissions. An Analysis of Medicare Data by the Dartmouth Atlas Project. Stories From Patients and Health Care Providers by PerryUndem Research & Communication. Robert Wood Johnson Foundation. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Published February 2013. Accessed July 27, 2015.
41. Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM R. 2010;2(9):806-810.
42. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
43. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty-day readmission rates as a measure of quality: causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag. 2013;58(1):64-76.
44. Bohm ER, Dunbar MJ, Frood JJ, Johnson TM, Morris KA. Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J Arthroplasty. 2012;27(2)232-237.
45. Saucedo J, Marecek GS, Lee J, Huminiak L, Stulberg SD, Puri L. How accurately are we coding readmission diagnoses after total joint arthroplasty? J Arthroplasty. 2013;28(7):1076-1079.
46. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
47. Bozic KJ, Chiu VW, Takemoto SK, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25(6 suppl):58-61.
48. Cram P, Ibrahim SA, Lu X, Wolf BR. Impact of alternative coding schemes on incidence rates of key complications after total hip arthroplasty: a risk-adjusted analysis of a national data set. Geriatr Orthop Surg Rehabil. 2012;3(1):17-26.
49. Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981.
50. Cima RR, Lackore KA, Nehring SA, et al. How best to measure surgical quality? Comparison of the Agency for Healthcare Research and Quality Patient Safety Indicators (AHRQ-PSI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) postoperative adverse events at a single institution. Surgery. 2011;150(5):943-949.
51. Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery. 2008;144(4):662-667.
52. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health. 1996;86(1):70-72.
53. HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov. Accessed July 27, 2015.
54. Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J. 2011;5:80-85.
55. Parker SG. Do Current Discharge Arrangements From Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status? Health Evidence Network report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2005. http://www.euro.who.int/__data/assets/pdf_file/0006/74670/E87542.pdf. Accessed July 27, 2015.
Total joint arthroplasty (TJA) is a clinically effective, cost-effective treatment for symptomatic arthritis.1,2 After TJA, patients report reduced pain, restored range of motion, high satisfaction, and ability to return to a more active lifestyle.3-7 The number of total hip arthroplasties (THAs) performed in the United States is expected to reach 572,000 by 2030, a 174% increase, and the number of total knee arthroplasties (TKAs) 3.5 million, nearly a 7-fold increase.8,9 Since 2005, the cost of THA has risen more than 4 times, to $13.43 billion, and the cost of TKA has risen more than 5 times, to $40.8 billion.8,9 Given the demand and price tag, TJA is the single largest cost in the Medicare budget.10
Given its potential to improve care and reduce costs, reducing readmission rates in the surgical setting is a priority for physicians and policymakers.11 Readmissions for TJA are highly scrutinized as a performance indicator—the Centers for Medicare & Medicaid Services (CMS) started including them in its readmissions penalty program in 2013—and were recently validated as a measure of surgical quality.12-14 Accurate assessments of readmissions after TJA are unclear, with rates ranging from 1% to 8.5% between 7 and 90 days after surgery.2,15-17 The early success of TJA as an elective (and more frequently outpatient) procedure has paradoxically translated to less tolerance for readmissions. Post-TJA complications resulting in readmission are subject to financial penalties, and there is an implicit judgment of inadequate surgical management.12
Not only is the readmission rate poorly characterized, but there is no consensus on the leading reasons for readmissions after primary elective unilateral TJAs. The range of rates, reasons, and follow-up periods reported in the literature is wide.18,19 CMS plans to monitor readmissions over 7 to 90 days after surgery (the period depends on the complication), whereas a significant portion of the orthopedic literature documents 90-day rates.19 In 2012, the Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation prepared for CMS a comprehensive report identifying rates of post-TJA complications and readmissions.20 The report, however, is limited to US hospitals and Medicare patients and therefore may overstate the rates, given this population’s documented comorbidities and the reimbursement variations between Medicare and commercial insurance.21 Lack of consensus on readmissions after primary elective unilateral TJAs requires that we synthesize available data to answer several questions: What is the overall readmission rate 30 and 90 days after TJA? What are the primary reasons for readmission 30 and 90 days after TJA? What are the cause-specific readmission rates? We performed a systematic review and a meta-analysis to answer these questions and to add clarity to the literature in order to help guide policy.
Materials and Methods
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.22 Two reviewers independently completed structured searches of the Medline and Cochrane Central Register of Controlled Trials databases. Search terms were: (total hip replacement OR hip arthroplasty OR total hip arthroplasty OR total knee replacement OR knee arthroplasty OR total knee arthroplasty) AND (readmission OR complication OR discharge). They updated the search June 1, 2013. Four limits were applied: publication between January 1, 1982 and December 12, 2012; human subjects only; age 19+ years; and English-language articles. Study eligibility was determined by using standardized criteria as defined by the inclusion and exclusion criteria described in 3 stages: title review, abstract review, and full-article review. The reviewers also performed ancestry searches, including searches for major review articles and bibliographies of all retrieved studies, to identify additional studies not identified in the keyword searches. Discrepancies were resolved by author consensus.
Inclusion criteria were original studies that presented level I to III evidence and that were identified in structured online searches; published in English between January 1, 1982 and December 31, 2012; involved patients older than 19 years; and reported both readmission rates and reasons at follow-up 30 or 90 days after elective primary unilateral TJA, regardless of indication. Exclusion criteria were studies that reported data from hip fracture, knee fracture, and pelvis fracture cases; those that reported data from hemiarthroplasty, Birmingham hip resurfacing procedures, other resurfacing procedures, simultaneous bilateral hip or knee arthroplasties, unicompartmental knee arthroplasty, patellofemoral arthroplasty, metastatic or bone cancer, or revision hip or knee arthroplasty; those that did not report extractable reasons for readmission; those that reported complications but did not specify readmission rates; and those that reported readmission data only from after the 90-day follow-up window. In cases in which multiple studies reported data from the same patient population, only the largest or most recent report was used.
Two reviewers extracted the quantitative data from eligible studies. The 2 primary outcomes of interests were all-cause readmission rates, and reasons for readmission 30 and 90 days after TJA. Other extracted data were evidence level; publication journal, year, and country; data source (academic institution, Medicare); study design; number of patients; patient characteristics; surgical approach; follow-up period; overall readmission rate; anticoagulant use; tourniquet use; and compression stocking use. In addition, all post-TJA readmissions were assumed to be unplanned, except for staged sequential bilateral arthroplasty for osteoarthritis (excluded from analysis).
Readmission reasons were divided into 4 major categories as defined by the literature and the authors: thromboembolic disease, joint-specific reasons, surgical site infection, and surgical sequelae. The diagnoses in these categories are listed in Table 1. Other extracted reasons were cardiac dysrhythmia and pneumonia.
In cases in which there were at least 2 comparable studies, a meta-analysis was performed to obtain pooled estimates of the proportion of patients readmitted at 30 or 90 days. We calculated a Higgins I2 measure for between-study heterogeneity and random-effects analysis, using the method of DerSimonian and Laird23 if I2 was greater than 0.5. Pooled estimates were obtained for both overall and cause-specific reasons for readmission for all reasons reported in at least 3 studies. Small-study or publication bias was assessed using funnel plot asymmetry when at least 5 studies were analyzed as recommended.24 The meta-analytic findings for both overall and cause-specific readmission are presented as pooled proportions with 95% confidence intervals (CIs). All meta-analyses were performed using Stata 10.0.
Results
Fifteen unique TJA studies (12 THA, 10 TKA) met the criteria for the meta-analysis.20,25-38Figure 1 depicts the PRISMA flowchart for study identification.22
Of the 12 studies eligible for the THA analysis (Table 2), 6 were conducted in the United States,20,26,27,30,33,34 5 in Europe,25,28,29,32,35 and 1 in Canada.31 Seven of the 12 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (2 reported rates at both follow-ups). We analyzed a total of 113,396 patients at the 30-day window and 192,380 patients at the 90-day window. Mean age was 74.2 years. The included studies were variable and sparse in their reporting of specific characteristics (Table 3).
Of the 10 studies (2 prospective, 8 retrospective) eligible for the TKA analysis (Table 4), 6 were conducted in the United States,20,26,27,34,36,37 3 in Europe,25,29,35 and 1 in Asia.38 Four of the 10 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (1 reported rates at both follow-ups).27 We analyzed a total of 3,278,635 patients at the 30-day window and 272,419 patients at the 90-day window. Mean age was 74.3 years. The included studies were quite variable and sparse in their reporting of specific characteristics (Table 5).
We performed random-effects meta-analyses of all unplanned readmissions at both 30 and 90 days (all I2s > 0.5). Among 5 THA studies that reported overall rates at 30 days,20,27,28,32,33 the estimated overall unplanned rate among the 120,272 index surgeries was 5.6% (95% CI, 3.2%-8.0%). Among 5 THA studies that reported overall rates at 90 days,20,25-27,31 the estimated overall unplanned rate among the 192,380 index surgeries was 7.7% (95% CI, 3.2%-12.2%) (I2 = 1.00). Among 3 TKA studies that reported overall rates at 30 days,27,37,38 the estimated overall unplanned rate among the 3,278,635 index surgeries was 3.3% (95% CI, 0.7%-5.9%). Among 5 TKA studies that reported overall rates at 90 days,20,25-27,36 the estimated overall unplanned rate among the 272,419 index surgeries was 9.7% (95% CI, 7.1%-12.4%) (I2 = 0.97).
30-Day Readmission Rates
The most common reason for readmission 30 days after THA discharge was joint-specific. This reason accounted for 39.3% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.2% (95% CI, 0.0%-4.6%; P < .001; I2 = 1.00) among 4 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 0.8%-2.5%; P < .001; I2 = 0.95) and thromboembolic disease (1.5%; 95% CI, 1.0%-1.9%; P < .001; I2 = 0.95). See Figure 2 for 30-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.1%; P < .001; I2 = 0.94). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported in only 1 study each.
The most common reason for readmission 30 days after TKA discharge was surgical site infection. This reason accounted for 12.1% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.4% (95% CI, 0.3%-0.6%; P < .001; I2 = 0.61) among 3 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.3% of the time. Joint-specific reasons were reported in 2 studies (95% CI, 0.0%-0.8%; P = .259; I2 = 0.94). Thromboembolic disease was reported in 4 studies (95% CI, 0.0%-0.7%; P = .067; I2 = 0.98) (Figure 3). Only these 3 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and “sequelae” were reported in only 1 study each.
90-Day Readmission Rates
Consistent with the 30-day THA results, the most common reason for readmission 90 days after THA discharge was joint-specific. This reason accounted for 31.2% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.4% (95% CI, 0.0%-4.9%; P < .001; I2 = 1.00) among 5 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 1.0%-2.2%; P < .003; I2 = 0.83) and thromboembolic disease (1.0%; 95% CI, 0.7%-1.4%; P < .001; I2 = 0.97). See Figure 4 for 90-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.0%; P < .001; I2 = 0.99). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported by only 1 study each.
Consistent with the 30-day TKA results, the most common reason for readmission 90 days after TKA discharge was surgical site infection. This reason accounted for 9.3% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.9% (95% CI, 0.4%-1.4%; P < .001; I2 = 0.93) among 5 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.7% of the time. Joint-specific reasons were reported in 5 studies (95% CI, 0.2%-1.1%; P =.003; I2 = 0.94). Thromboembolic disease was reported in 7 studies (95% CI, 0.3%-1.1%; P < .001; I2 = 0.97) (Figure 5). Bleeding was reported in 3 studies, with a pooled rate of 0.4% (95% CI, 0.0%-0.9%; P = .128; I2 = 0.83). Cardiac dysrhythmia was reported in 2 studies, with an estimated pooled rate of 0.3% (95% CI, 0.2%-0.5%; P < .001). Only these 5 reasons could be pooled, as pneumonia and “sequelae” were reported in only 1 study each.
Discussion
This study is the first systematic review and meta-analysis of the literature to identify overall and cause-specific readmission rates after TJA.
For THA, 30- and 90-day readmission rates were 5.6% and 7.7%, respectively. Joint-specific causes were the most common reason for readmission at both 30 and 90 days after THA. For TKA, 30- and 90-day rates were 3.3% and 9.7%, respectively. Surgical site infection was the most common reason for readmission at both 30 and 90 days after TKA.
Hospital readmissions are an important area of scrutiny for Medicare and the health care systems broadly. Readmissions after surgery are deemed quality indicators potentially suggesting incomplete management of active issues and inadequate preparation for discharge.39 Unplanned readmissions also place a significant economic burden on Medicare: $17.5 billion in 2010.40 Given their association with quality of overall surgical care, improved readmission rates have the potential to improve the standard of care and reduce costs.
Higher readmission rates will significantly affect hospitals as CMS shifts to bundling payments for acute-care episodes, such as TJA.41-43 Further, private and public health care payers are increasingly using unplanned 30- and 90-day readmission rates as a marker of quality of care. However, there is little agreement about readmission rates and reasons, let alone what follow-up window should be used to define orthopedic readmissions. One study involving the MEDPAR (Medicare Provider Analysis and Review) database found that a common reason for readmission after major hip or knee surgery was “aftercare” for surgical sequelae (10.3%)15; another study found a 15% increase in post-THA hospitalizations, most commonly for a mechanical complication (joint-related).44 There are no prior complete systematic reviews or meta-analyses of overall rates of readmissions after primary unilateral TJAs, or of the reasons for these readmissions. The closest such report, the Yale report to CMS, was skewed to a proportion of US hospitals treating a population prone to significant comorbidities.20
Although the strength of this study lies in its rigorous identification and extraction of data, notable clarifications must be made when synthesizing the information. First, the definitions of various thromboembolic events varied greatly. Some studies reported deep vein thrombosis (DVT) and pulmonary embolism (PE) separately, whereas others reported only DVT or only PE. Some studies reported rates of readmission for “thromboembolic disorder,” and one25 reported rates for DVT, PE, and thromboembolic disorder. To pool these related events, we created a composite definition that included DVT, PE, and thromboembolic disorders, which we termed thromboembolic disease. We also created a composite measure for joint-specific reasons for readmission. This category included joint infection that definitely required reentry into the joint, but using this category may have led to underestimation of surgical site infection rates, which were defined separately. Third, there was significant variation in documentation of surgical site infection among the studies included in this review. Some studies specified superficial wounds, whereas others did not categorize complications as superficial, deep, or intracapsular, which would qualify as a “joint-specific” cause. Despite this variation, surgical site infection after TJA was found to be the most common reason for readmission.
Our systematic review and meta-analysis were limited, as any others are, by the quality of studies investigated. Few studies reported cause-specific rates and reasons for readmission. Given the small sample, formal tests for small-study or publication bias could not be performed. Some studies included tremendous amounts of data, and International Classification of Diseases, Ninth Revision (ICD-9) codes were used without physician review of readmission diagnoses. In the absence of oversight, many readmissions could have been misinterpreted and incorrectly logged, or simply miscoded. Saucedo and colleagues27,45 found that readmission diagnostic codes were often unverified. Numerous other studies corroborated this lack of correlation with physician-derived readmission diagnoses in just 25% of cases.46-54 Another study limitation is the unknown number of patients who had TJA but presented and were subsequently readmitted to a different hospital. Last, as this review included patients who had surgery performed within a 30-year period, it could not address the shifts in postoperative management that occurred in that time, particularly with respect to anticoagulation. This limitation was partially addressed in THA by dividing final studies into 3 decades. Of these studies, only 1 was from the first decade, 3 were from the second, and the rest were from the third. Of the 3 from the second decade, only the study by Warwick and colleagues29 (1995) explicitly did not use anticoagulation, but compression stockings were used, and consequently there was a 4.0% rate of readmission for thromboembolic disease alone, compared with the study by White and colleagues34 (1998), which explicitly used anticoagulation and boasted a 1.7% rate of readmission for thromboembolic disease. This isolated comparison illustrates the effect of routine anticoagulation and the changes in surgical standards over the 3 decades.
The numbers from this systematic review and meta-analysis represent an international benchmark for TJA as a procedure. Knowing the top reasons for readmission will lead to more focus on joint-related and medical issues (surgical site infection, thromboembolic disease) before discharge to avoid readmission after elective unilateral primary TJA. Although readmission rates have received attention in the United States as a primary means of combating soaring health care costs, knowing the rates for a common procedure applies broadly as an indicator for standard of care worldwide, according to the World Health Organization.55 This study is the first systematic review and meta-analysis of documented readmission rates and reasons for readmission to identify overall and cause-specific rates after TJA. The hope is that our findings will add clarity to the literature and help guide the decisions of physicians and policymakers.
Conclusion
Readmission rates are an increasingly important metric in the United States and around the world, yet there is no consensus regarding overall readmission rates and reasons for readmission after primary unilateral TJAs. Our systematic review and meta-analysis of the literature found overall unplanned readmission rates of 5.6% (30 days) and 7.7% (90 days) for THA and 3.3% (30 days) and 9.7% (90 days) for TKA. At both 30 and 90 days, the most common readmission reasons were joint-specific (THA) and surgical site infection (TKA). New investigations should be directed toward developing countermeasures to lower the rates of readmission.
Total joint arthroplasty (TJA) is a clinically effective, cost-effective treatment for symptomatic arthritis.1,2 After TJA, patients report reduced pain, restored range of motion, high satisfaction, and ability to return to a more active lifestyle.3-7 The number of total hip arthroplasties (THAs) performed in the United States is expected to reach 572,000 by 2030, a 174% increase, and the number of total knee arthroplasties (TKAs) 3.5 million, nearly a 7-fold increase.8,9 Since 2005, the cost of THA has risen more than 4 times, to $13.43 billion, and the cost of TKA has risen more than 5 times, to $40.8 billion.8,9 Given the demand and price tag, TJA is the single largest cost in the Medicare budget.10
Given its potential to improve care and reduce costs, reducing readmission rates in the surgical setting is a priority for physicians and policymakers.11 Readmissions for TJA are highly scrutinized as a performance indicator—the Centers for Medicare & Medicaid Services (CMS) started including them in its readmissions penalty program in 2013—and were recently validated as a measure of surgical quality.12-14 Accurate assessments of readmissions after TJA are unclear, with rates ranging from 1% to 8.5% between 7 and 90 days after surgery.2,15-17 The early success of TJA as an elective (and more frequently outpatient) procedure has paradoxically translated to less tolerance for readmissions. Post-TJA complications resulting in readmission are subject to financial penalties, and there is an implicit judgment of inadequate surgical management.12
Not only is the readmission rate poorly characterized, but there is no consensus on the leading reasons for readmissions after primary elective unilateral TJAs. The range of rates, reasons, and follow-up periods reported in the literature is wide.18,19 CMS plans to monitor readmissions over 7 to 90 days after surgery (the period depends on the complication), whereas a significant portion of the orthopedic literature documents 90-day rates.19 In 2012, the Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation prepared for CMS a comprehensive report identifying rates of post-TJA complications and readmissions.20 The report, however, is limited to US hospitals and Medicare patients and therefore may overstate the rates, given this population’s documented comorbidities and the reimbursement variations between Medicare and commercial insurance.21 Lack of consensus on readmissions after primary elective unilateral TJAs requires that we synthesize available data to answer several questions: What is the overall readmission rate 30 and 90 days after TJA? What are the primary reasons for readmission 30 and 90 days after TJA? What are the cause-specific readmission rates? We performed a systematic review and a meta-analysis to answer these questions and to add clarity to the literature in order to help guide policy.
Materials and Methods
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.22 Two reviewers independently completed structured searches of the Medline and Cochrane Central Register of Controlled Trials databases. Search terms were: (total hip replacement OR hip arthroplasty OR total hip arthroplasty OR total knee replacement OR knee arthroplasty OR total knee arthroplasty) AND (readmission OR complication OR discharge). They updated the search June 1, 2013. Four limits were applied: publication between January 1, 1982 and December 12, 2012; human subjects only; age 19+ years; and English-language articles. Study eligibility was determined by using standardized criteria as defined by the inclusion and exclusion criteria described in 3 stages: title review, abstract review, and full-article review. The reviewers also performed ancestry searches, including searches for major review articles and bibliographies of all retrieved studies, to identify additional studies not identified in the keyword searches. Discrepancies were resolved by author consensus.
Inclusion criteria were original studies that presented level I to III evidence and that were identified in structured online searches; published in English between January 1, 1982 and December 31, 2012; involved patients older than 19 years; and reported both readmission rates and reasons at follow-up 30 or 90 days after elective primary unilateral TJA, regardless of indication. Exclusion criteria were studies that reported data from hip fracture, knee fracture, and pelvis fracture cases; those that reported data from hemiarthroplasty, Birmingham hip resurfacing procedures, other resurfacing procedures, simultaneous bilateral hip or knee arthroplasties, unicompartmental knee arthroplasty, patellofemoral arthroplasty, metastatic or bone cancer, or revision hip or knee arthroplasty; those that did not report extractable reasons for readmission; those that reported complications but did not specify readmission rates; and those that reported readmission data only from after the 90-day follow-up window. In cases in which multiple studies reported data from the same patient population, only the largest or most recent report was used.
Two reviewers extracted the quantitative data from eligible studies. The 2 primary outcomes of interests were all-cause readmission rates, and reasons for readmission 30 and 90 days after TJA. Other extracted data were evidence level; publication journal, year, and country; data source (academic institution, Medicare); study design; number of patients; patient characteristics; surgical approach; follow-up period; overall readmission rate; anticoagulant use; tourniquet use; and compression stocking use. In addition, all post-TJA readmissions were assumed to be unplanned, except for staged sequential bilateral arthroplasty for osteoarthritis (excluded from analysis).
Readmission reasons were divided into 4 major categories as defined by the literature and the authors: thromboembolic disease, joint-specific reasons, surgical site infection, and surgical sequelae. The diagnoses in these categories are listed in Table 1. Other extracted reasons were cardiac dysrhythmia and pneumonia.
In cases in which there were at least 2 comparable studies, a meta-analysis was performed to obtain pooled estimates of the proportion of patients readmitted at 30 or 90 days. We calculated a Higgins I2 measure for between-study heterogeneity and random-effects analysis, using the method of DerSimonian and Laird23 if I2 was greater than 0.5. Pooled estimates were obtained for both overall and cause-specific reasons for readmission for all reasons reported in at least 3 studies. Small-study or publication bias was assessed using funnel plot asymmetry when at least 5 studies were analyzed as recommended.24 The meta-analytic findings for both overall and cause-specific readmission are presented as pooled proportions with 95% confidence intervals (CIs). All meta-analyses were performed using Stata 10.0.
Results
Fifteen unique TJA studies (12 THA, 10 TKA) met the criteria for the meta-analysis.20,25-38Figure 1 depicts the PRISMA flowchart for study identification.22
Of the 12 studies eligible for the THA analysis (Table 2), 6 were conducted in the United States,20,26,27,30,33,34 5 in Europe,25,28,29,32,35 and 1 in Canada.31 Seven of the 12 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (2 reported rates at both follow-ups). We analyzed a total of 113,396 patients at the 30-day window and 192,380 patients at the 90-day window. Mean age was 74.2 years. The included studies were variable and sparse in their reporting of specific characteristics (Table 3).
Of the 10 studies (2 prospective, 8 retrospective) eligible for the TKA analysis (Table 4), 6 were conducted in the United States,20,26,27,34,36,37 3 in Europe,25,29,35 and 1 in Asia.38 Four of the 10 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (1 reported rates at both follow-ups).27 We analyzed a total of 3,278,635 patients at the 30-day window and 272,419 patients at the 90-day window. Mean age was 74.3 years. The included studies were quite variable and sparse in their reporting of specific characteristics (Table 5).
We performed random-effects meta-analyses of all unplanned readmissions at both 30 and 90 days (all I2s > 0.5). Among 5 THA studies that reported overall rates at 30 days,20,27,28,32,33 the estimated overall unplanned rate among the 120,272 index surgeries was 5.6% (95% CI, 3.2%-8.0%). Among 5 THA studies that reported overall rates at 90 days,20,25-27,31 the estimated overall unplanned rate among the 192,380 index surgeries was 7.7% (95% CI, 3.2%-12.2%) (I2 = 1.00). Among 3 TKA studies that reported overall rates at 30 days,27,37,38 the estimated overall unplanned rate among the 3,278,635 index surgeries was 3.3% (95% CI, 0.7%-5.9%). Among 5 TKA studies that reported overall rates at 90 days,20,25-27,36 the estimated overall unplanned rate among the 272,419 index surgeries was 9.7% (95% CI, 7.1%-12.4%) (I2 = 0.97).
30-Day Readmission Rates
The most common reason for readmission 30 days after THA discharge was joint-specific. This reason accounted for 39.3% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.2% (95% CI, 0.0%-4.6%; P < .001; I2 = 1.00) among 4 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 0.8%-2.5%; P < .001; I2 = 0.95) and thromboembolic disease (1.5%; 95% CI, 1.0%-1.9%; P < .001; I2 = 0.95). See Figure 2 for 30-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.1%; P < .001; I2 = 0.94). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported in only 1 study each.
The most common reason for readmission 30 days after TKA discharge was surgical site infection. This reason accounted for 12.1% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.4% (95% CI, 0.3%-0.6%; P < .001; I2 = 0.61) among 3 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.3% of the time. Joint-specific reasons were reported in 2 studies (95% CI, 0.0%-0.8%; P = .259; I2 = 0.94). Thromboembolic disease was reported in 4 studies (95% CI, 0.0%-0.7%; P = .067; I2 = 0.98) (Figure 3). Only these 3 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and “sequelae” were reported in only 1 study each.
90-Day Readmission Rates
Consistent with the 30-day THA results, the most common reason for readmission 90 days after THA discharge was joint-specific. This reason accounted for 31.2% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.4% (95% CI, 0.0%-4.9%; P < .001; I2 = 1.00) among 5 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 1.0%-2.2%; P < .003; I2 = 0.83) and thromboembolic disease (1.0%; 95% CI, 0.7%-1.4%; P < .001; I2 = 0.97). See Figure 4 for 90-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.0%; P < .001; I2 = 0.99). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported by only 1 study each.
Consistent with the 30-day TKA results, the most common reason for readmission 90 days after TKA discharge was surgical site infection. This reason accounted for 9.3% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.9% (95% CI, 0.4%-1.4%; P < .001; I2 = 0.93) among 5 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.7% of the time. Joint-specific reasons were reported in 5 studies (95% CI, 0.2%-1.1%; P =.003; I2 = 0.94). Thromboembolic disease was reported in 7 studies (95% CI, 0.3%-1.1%; P < .001; I2 = 0.97) (Figure 5). Bleeding was reported in 3 studies, with a pooled rate of 0.4% (95% CI, 0.0%-0.9%; P = .128; I2 = 0.83). Cardiac dysrhythmia was reported in 2 studies, with an estimated pooled rate of 0.3% (95% CI, 0.2%-0.5%; P < .001). Only these 5 reasons could be pooled, as pneumonia and “sequelae” were reported in only 1 study each.
Discussion
This study is the first systematic review and meta-analysis of the literature to identify overall and cause-specific readmission rates after TJA.
For THA, 30- and 90-day readmission rates were 5.6% and 7.7%, respectively. Joint-specific causes were the most common reason for readmission at both 30 and 90 days after THA. For TKA, 30- and 90-day rates were 3.3% and 9.7%, respectively. Surgical site infection was the most common reason for readmission at both 30 and 90 days after TKA.
Hospital readmissions are an important area of scrutiny for Medicare and the health care systems broadly. Readmissions after surgery are deemed quality indicators potentially suggesting incomplete management of active issues and inadequate preparation for discharge.39 Unplanned readmissions also place a significant economic burden on Medicare: $17.5 billion in 2010.40 Given their association with quality of overall surgical care, improved readmission rates have the potential to improve the standard of care and reduce costs.
Higher readmission rates will significantly affect hospitals as CMS shifts to bundling payments for acute-care episodes, such as TJA.41-43 Further, private and public health care payers are increasingly using unplanned 30- and 90-day readmission rates as a marker of quality of care. However, there is little agreement about readmission rates and reasons, let alone what follow-up window should be used to define orthopedic readmissions. One study involving the MEDPAR (Medicare Provider Analysis and Review) database found that a common reason for readmission after major hip or knee surgery was “aftercare” for surgical sequelae (10.3%)15; another study found a 15% increase in post-THA hospitalizations, most commonly for a mechanical complication (joint-related).44 There are no prior complete systematic reviews or meta-analyses of overall rates of readmissions after primary unilateral TJAs, or of the reasons for these readmissions. The closest such report, the Yale report to CMS, was skewed to a proportion of US hospitals treating a population prone to significant comorbidities.20
Although the strength of this study lies in its rigorous identification and extraction of data, notable clarifications must be made when synthesizing the information. First, the definitions of various thromboembolic events varied greatly. Some studies reported deep vein thrombosis (DVT) and pulmonary embolism (PE) separately, whereas others reported only DVT or only PE. Some studies reported rates of readmission for “thromboembolic disorder,” and one25 reported rates for DVT, PE, and thromboembolic disorder. To pool these related events, we created a composite definition that included DVT, PE, and thromboembolic disorders, which we termed thromboembolic disease. We also created a composite measure for joint-specific reasons for readmission. This category included joint infection that definitely required reentry into the joint, but using this category may have led to underestimation of surgical site infection rates, which were defined separately. Third, there was significant variation in documentation of surgical site infection among the studies included in this review. Some studies specified superficial wounds, whereas others did not categorize complications as superficial, deep, or intracapsular, which would qualify as a “joint-specific” cause. Despite this variation, surgical site infection after TJA was found to be the most common reason for readmission.
Our systematic review and meta-analysis were limited, as any others are, by the quality of studies investigated. Few studies reported cause-specific rates and reasons for readmission. Given the small sample, formal tests for small-study or publication bias could not be performed. Some studies included tremendous amounts of data, and International Classification of Diseases, Ninth Revision (ICD-9) codes were used without physician review of readmission diagnoses. In the absence of oversight, many readmissions could have been misinterpreted and incorrectly logged, or simply miscoded. Saucedo and colleagues27,45 found that readmission diagnostic codes were often unverified. Numerous other studies corroborated this lack of correlation with physician-derived readmission diagnoses in just 25% of cases.46-54 Another study limitation is the unknown number of patients who had TJA but presented and were subsequently readmitted to a different hospital. Last, as this review included patients who had surgery performed within a 30-year period, it could not address the shifts in postoperative management that occurred in that time, particularly with respect to anticoagulation. This limitation was partially addressed in THA by dividing final studies into 3 decades. Of these studies, only 1 was from the first decade, 3 were from the second, and the rest were from the third. Of the 3 from the second decade, only the study by Warwick and colleagues29 (1995) explicitly did not use anticoagulation, but compression stockings were used, and consequently there was a 4.0% rate of readmission for thromboembolic disease alone, compared with the study by White and colleagues34 (1998), which explicitly used anticoagulation and boasted a 1.7% rate of readmission for thromboembolic disease. This isolated comparison illustrates the effect of routine anticoagulation and the changes in surgical standards over the 3 decades.
The numbers from this systematic review and meta-analysis represent an international benchmark for TJA as a procedure. Knowing the top reasons for readmission will lead to more focus on joint-related and medical issues (surgical site infection, thromboembolic disease) before discharge to avoid readmission after elective unilateral primary TJA. Although readmission rates have received attention in the United States as a primary means of combating soaring health care costs, knowing the rates for a common procedure applies broadly as an indicator for standard of care worldwide, according to the World Health Organization.55 This study is the first systematic review and meta-analysis of documented readmission rates and reasons for readmission to identify overall and cause-specific rates after TJA. The hope is that our findings will add clarity to the literature and help guide the decisions of physicians and policymakers.
Conclusion
Readmission rates are an increasingly important metric in the United States and around the world, yet there is no consensus regarding overall readmission rates and reasons for readmission after primary unilateral TJAs. Our systematic review and meta-analysis of the literature found overall unplanned readmission rates of 5.6% (30 days) and 7.7% (90 days) for THA and 3.3% (30 days) and 9.7% (90 days) for TKA. At both 30 and 90 days, the most common readmission reasons were joint-specific (THA) and surgical site infection (TKA). New investigations should be directed toward developing countermeasures to lower the rates of readmission.
1. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
2. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2001. JAMA. 2011;305(15):1560-1567.
3. de Vries LM, Sturkenboom MC, Verhaar JA, Kingma JH, Stricker BH. Complications after hip arthroplasty and the association with hospital procedure volume. Acta Orthop. 2011;82(5):545-552.
4. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
5. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869-1876.
6. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533.
7. Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12(4):387-396.
8. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
9. Kurtz SM, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of Medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133-138.
11. Li LT, Mills WL, White DL, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175-1181.
12. Readmissions Reduction Program. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed July 27, 2015.
13. Tsai TC, Joynt KE, Orav J, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134-1142.
14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428.
15. Zmistowski B, Hozack WJ, Parvizi J. Readmission rates after total hip arthroplasty. JAMA. 2011;306(8):825.
16. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
17. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
18. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369.
19. Atkinson JG. Flaws in the Medicare readmission penalty. N Engl J Med. 2012;367(21):2056-2057.
20. Grosso LM, Curtis JP, Lin Z, et al. Hospital-level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty (THA) And/Or Total Knee Arthroplasty (TKA): Measure Methodology Report. Report prepared for Centers for Medicare & Medicaid Services. QualityNet website. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228772504368. Submitted June 25, 2012. Accessed August 4, 2015.
21. Robinson JC. Analysis of Medicare and commercial insurer–paid total knee replacement reveals opportunities for cost reduction. Health Care Incentives Improvement Institute website. http://www.hci3.org/sites/default/files/files/HCI-2012-IssueBrief-L6-2.pdf. Published 2012. Accessed July 27, 2015.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
24. Higgins JP, Thompson SG. Quantifying heterogeniety in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
25. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
26. Keeney JA, Adelani MA, Nunley RM, Clohisy JC, Barrack RL. Assessing readmission databases: how reliable is the information? J Arthroplasty. 2012;27(8 suppl):72-76.e1-e2.
27. Saucedo JM, Marecek GS, Wanke TR, Lee J, Stulberg SD, Puri L. Understanding readmissions after primary total hip and knee arthroplasty: who’s at risk? J Arthroplasty. 2014;29(2):256-260.
28. Seagroatt V, Tan HS, Goldacre M, Bulstrode C, Nugent I, Gill L. Elective total hip replacement: incidence, emergency readmission rate, and postoperative mortality. BMJ. 1991;303(6815):1431-1435.
29. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77(1):6-10.
30. Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79(4):485-494.
31. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
32. Cullen C, Johnson DS, Cook G. Re-admission rates within 28 days of total hip replacement. Ann R Coll Surg Engl. 2006;88(5):475-478.
33. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
34. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531.
35. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
36. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449.
37. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
38. Seah VW, Singh G, Yang KY, Yeo SJ, Lo NN, Seow KH. Thirty-day mortality and morbidity after total knee arthroplasty. Ann Acad Med Singapore. 2007;36(12):1010-1012.
39. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
40. The Revolving Door: A Report on U.S. Hospital Readmissions. An Analysis of Medicare Data by the Dartmouth Atlas Project. Stories From Patients and Health Care Providers by PerryUndem Research & Communication. Robert Wood Johnson Foundation. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Published February 2013. Accessed July 27, 2015.
41. Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM R. 2010;2(9):806-810.
42. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
43. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty-day readmission rates as a measure of quality: causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag. 2013;58(1):64-76.
44. Bohm ER, Dunbar MJ, Frood JJ, Johnson TM, Morris KA. Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J Arthroplasty. 2012;27(2)232-237.
45. Saucedo J, Marecek GS, Lee J, Huminiak L, Stulberg SD, Puri L. How accurately are we coding readmission diagnoses after total joint arthroplasty? J Arthroplasty. 2013;28(7):1076-1079.
46. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
47. Bozic KJ, Chiu VW, Takemoto SK, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25(6 suppl):58-61.
48. Cram P, Ibrahim SA, Lu X, Wolf BR. Impact of alternative coding schemes on incidence rates of key complications after total hip arthroplasty: a risk-adjusted analysis of a national data set. Geriatr Orthop Surg Rehabil. 2012;3(1):17-26.
49. Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981.
50. Cima RR, Lackore KA, Nehring SA, et al. How best to measure surgical quality? Comparison of the Agency for Healthcare Research and Quality Patient Safety Indicators (AHRQ-PSI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) postoperative adverse events at a single institution. Surgery. 2011;150(5):943-949.
51. Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery. 2008;144(4):662-667.
52. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health. 1996;86(1):70-72.
53. HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov. Accessed July 27, 2015.
54. Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J. 2011;5:80-85.
55. Parker SG. Do Current Discharge Arrangements From Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status? Health Evidence Network report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2005. http://www.euro.who.int/__data/assets/pdf_file/0006/74670/E87542.pdf. Accessed July 27, 2015.
1. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
2. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2001. JAMA. 2011;305(15):1560-1567.
3. de Vries LM, Sturkenboom MC, Verhaar JA, Kingma JH, Stricker BH. Complications after hip arthroplasty and the association with hospital procedure volume. Acta Orthop. 2011;82(5):545-552.
4. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
5. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869-1876.
6. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533.
7. Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12(4):387-396.
8. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
9. Kurtz SM, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of Medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133-138.
11. Li LT, Mills WL, White DL, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175-1181.
12. Readmissions Reduction Program. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed July 27, 2015.
13. Tsai TC, Joynt KE, Orav J, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134-1142.
14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428.
15. Zmistowski B, Hozack WJ, Parvizi J. Readmission rates after total hip arthroplasty. JAMA. 2011;306(8):825.
16. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
17. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
18. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369.
19. Atkinson JG. Flaws in the Medicare readmission penalty. N Engl J Med. 2012;367(21):2056-2057.
20. Grosso LM, Curtis JP, Lin Z, et al. Hospital-level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty (THA) And/Or Total Knee Arthroplasty (TKA): Measure Methodology Report. Report prepared for Centers for Medicare & Medicaid Services. QualityNet website. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228772504368. Submitted June 25, 2012. Accessed August 4, 2015.
21. Robinson JC. Analysis of Medicare and commercial insurer–paid total knee replacement reveals opportunities for cost reduction. Health Care Incentives Improvement Institute website. http://www.hci3.org/sites/default/files/files/HCI-2012-IssueBrief-L6-2.pdf. Published 2012. Accessed July 27, 2015.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
24. Higgins JP, Thompson SG. Quantifying heterogeniety in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
25. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
26. Keeney JA, Adelani MA, Nunley RM, Clohisy JC, Barrack RL. Assessing readmission databases: how reliable is the information? J Arthroplasty. 2012;27(8 suppl):72-76.e1-e2.
27. Saucedo JM, Marecek GS, Wanke TR, Lee J, Stulberg SD, Puri L. Understanding readmissions after primary total hip and knee arthroplasty: who’s at risk? J Arthroplasty. 2014;29(2):256-260.
28. Seagroatt V, Tan HS, Goldacre M, Bulstrode C, Nugent I, Gill L. Elective total hip replacement: incidence, emergency readmission rate, and postoperative mortality. BMJ. 1991;303(6815):1431-1435.
29. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77(1):6-10.
30. Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79(4):485-494.
31. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
32. Cullen C, Johnson DS, Cook G. Re-admission rates within 28 days of total hip replacement. Ann R Coll Surg Engl. 2006;88(5):475-478.
33. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
34. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531.
35. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
36. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449.
37. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
38. Seah VW, Singh G, Yang KY, Yeo SJ, Lo NN, Seow KH. Thirty-day mortality and morbidity after total knee arthroplasty. Ann Acad Med Singapore. 2007;36(12):1010-1012.
39. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
40. The Revolving Door: A Report on U.S. Hospital Readmissions. An Analysis of Medicare Data by the Dartmouth Atlas Project. Stories From Patients and Health Care Providers by PerryUndem Research & Communication. Robert Wood Johnson Foundation. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Published February 2013. Accessed July 27, 2015.
41. Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM R. 2010;2(9):806-810.
42. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
43. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty-day readmission rates as a measure of quality: causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag. 2013;58(1):64-76.
44. Bohm ER, Dunbar MJ, Frood JJ, Johnson TM, Morris KA. Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J Arthroplasty. 2012;27(2)232-237.
45. Saucedo J, Marecek GS, Lee J, Huminiak L, Stulberg SD, Puri L. How accurately are we coding readmission diagnoses after total joint arthroplasty? J Arthroplasty. 2013;28(7):1076-1079.
46. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
47. Bozic KJ, Chiu VW, Takemoto SK, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25(6 suppl):58-61.
48. Cram P, Ibrahim SA, Lu X, Wolf BR. Impact of alternative coding schemes on incidence rates of key complications after total hip arthroplasty: a risk-adjusted analysis of a national data set. Geriatr Orthop Surg Rehabil. 2012;3(1):17-26.
49. Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981.
50. Cima RR, Lackore KA, Nehring SA, et al. How best to measure surgical quality? Comparison of the Agency for Healthcare Research and Quality Patient Safety Indicators (AHRQ-PSI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) postoperative adverse events at a single institution. Surgery. 2011;150(5):943-949.
51. Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery. 2008;144(4):662-667.
52. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health. 1996;86(1):70-72.
53. HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov. Accessed July 27, 2015.
54. Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J. 2011;5:80-85.
55. Parker SG. Do Current Discharge Arrangements From Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status? Health Evidence Network report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2005. http://www.euro.who.int/__data/assets/pdf_file/0006/74670/E87542.pdf. Accessed July 27, 2015.
Giant Solitary Synovial Chondromatosis Mimicking Chondrosarcoma: Report of a Rare Histologic Presentation and Literature Review
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
Iatrogenic Femoral Neck Fracture After Closed Reduction of Anterior Hip Dislocation in the Emergency Department
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
Xanthogranulomatous Osteomyelitis of Proximal Femur Masquerading as Benign Bone Tumor
Xanthogranulomatous osteomyelitis (XO) is a type of chronic inflammatory process that is characterized by the collection of foamy macrophages along with mononuclear cells in the tissue.1 Xanthogranulomatous osteomyelitis is characterized by the presence of granular, eosinophilic, periodic acid–Schiff–positive histiocytes in the initial stages, followed by the mixture of foamy macrophages and activated plasma cells and, last, by the presence of suppurative foci and hemorrhage. This is an uncommon process best known to occur in the gallbladder, kidney, urinary bladder, fallopian tube, ovary, vagina, prostate, testis, epididymis, colon, and appendix.2-4 Very rarely, it can affect lungs, brain, or bone. Only 5 cases of XO have been reported in the literature.5-8
We report XO of the proximal femur in a 65-year-old woman who initially had a clinical and radiologic diagnosis of aneurysmal bone cyst; however, histopathologic examination confirmed the diagnosis of XO. Xanthogranulomatous osteomyelitis mimics a neoplastic pathology in gallbladder, kidney, and prostrate on gross clinical and radiologic examination.9 The pathogenesis of XO is best characterized by a delayed type of hypersensitivity reaction.10 The differential diagnosis includes chronic recurrent multifocal osteomyelitis, xanthoma, infiltrative storage disorder, malakoplakia, Langerhans cell histiocytosis, fibrohistiocytic tumor, Erdheim-Chester disease, and metastatic renal cell carcinoma.11-14 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old hypertensive woman presented with complaints of pain in the right hip for a duration of 6 months. Pain was radiating from the right hip region to the anteromedial aspect of the knee and progressively increasing, with a history of pain at rest suggestive of a nonmechanical pathology in the hip. There was no history of fever, weight loss, loss of appetite, pain in any other joint, or morning stiffness. The patient was mobile without support and was able to squat and sit cross-legged; however, the stance phase on the right side was less than on the left side, suggestive of an antalgic component in the gait.
On examining the patient, there was anterior hip joint tenderness with no local sign of any infective or inflammatory pathology. Trochanteric tenderness was present, but there was no irregularity, broadening, or thickening of the trochanter. There was no restriction in the range of motion, and no coronal or sagittal plane deformity in the right hip. There was no limb-length discrepancy. However, the patient was not able to raise her leg actively, probably because of pain in the right hip.
On plain radiographs of the pelvis with bilateral hips, a well-defined nonexpansile uniloculated lytic lesion with sclerotic margins was present in the neck of the right femur, extending to the intertrochanteric area (Figure 1). Ground-glass appearance was also noted. Considering the benign nature of the lesion radiologically and clinically, a differential diagnosis of hyperparathyroidism, renal osteodystrophy, multiple myeloma, and fibrous dysplasia was considered. Hematologic investigations, skeletal survey, and magnetic resonance imaging (MRI) of the bilateral hips were performed to rule out the differential diagnosis.
The patient’s hemoglobin level was 11.8 g/dL with total white blood cell count of 10,300/µL. Renal and hepatic functions were within normal limit. Serum erythrocyte sedimentation rate (ESR) was 12 mm/h and C-reactive protein level was normal. Serum parathyroid level was 32 pg/mL, which was within normal limits, with an alkaline phosphatase level of 101 U/L. The skeletal survey showed no other bony lesion in the body. T1-weighted MRI of both hips showed a well-defined hypointense lesion in the neck and intertrochanteric area of the right hip, which was hyperintense on T2-weighted MRI, suggestive of aneurysmal bone cyst (Figure 2).
Normal ESR, hemoglobin, alkaline phosphatase, and serum parathyroid levels and normal skeletal survey almost ruled out multiple myeloma and hyperparathyroidism. Normal renal profile ruled out renal osteodystrophy and the osteitis fibrosa cystica lesion associated with it. We planned for prophylactic internal fixation of the lesion to prevent a pathologic fracture. According to Mirels,15 if there is a lytic lesion covering more than two-thirds of the circumference of the bone in the peritrochanteric area, the chances of a pathologic fracture are high and such fractures should be fixed.
We planned for curettage of the lesion with bone grafting and in situ intramedullary fixation of the lesion. Curettage was done according to the plan and the sample was sent for histopathologic examination. In situ internal fixation and bone grafting were performed by using a proximal femoral intramedullary nail. To our surprise, the biopsy sample was reported as xanthogranuloma, with multiple foamy macrophages mixed with inflammatory cells and aggregates of lymphocytes (Figure 3). Mycobacterial and routine bacterial cultures were reported as negative. The patient was kept on oral antibiotics (cefixime and moxifloxacin) for 6 weeks, and she made an uneventful recovery. At 6-month follow-up, a radiograph of the right hip showed a healed lesion with proximal femoral nail in situ (Figure 4).
Discussion
To the best of our knowledge, a total of 5 cases of XO have been reported in the literature. The earliest of these reports were by Cozzutto and Carbone,1 who reported 2 cases of XO of the first rib and of the epiphysis of the tibia, respectively. The importance of these lesions to diagnosis is their confusion with a neoplastic disease, as XO is itself a benign disorder. These lesions can mimic a neoplastic lesion in clinical and radiologic presentation and the only way to differentiate the lesion from a neoplastic disease is by histopathologic examination of the tissue. Hypothetically, xanthogranulomatous disorders can be related to trauma or infection.
In 2007, Vankalakunti and colleagues6 reported XO of the ulna in a 50-year-old postmenopausal woman. In that case, progressive swelling was present on the extensor aspect of her right forearm for a period of 2 years, for which curettage and bone grafting were performed, using autograft from the ipsilateral iliac crest. The tissue culture was sterile, and XO was diagnosed as a result of the histopathologic examination. In 2009, Cennimo and colleagues7 reported XO of the index finger and wrist of a man complaining of pain and swelling for 1 year, which was unresponsive to antibiotics. The diagnosis of XO was confirmed histopathologically, when the culture of the same tissue grew Mycobacterium marinum. Radical synovectomy of the lesion was performed, after which minocycline, clarithromycin, and ethambutol were administered. In 2012, Borjian and colleagues8 reported a case of XO of the proximal humerus and proximal fibula in a 14-year-old child. The child, who presented with fever, pain, and restriction of shoulder movements, was started on oral antibiotics as the tissue culture grew Staphylococcus aureus; the patient did not complete the course of treatment in the hospital. No surgical intervention was done in this case. The diagnosis of XO was confirmed by microscopic examination of the tissue.
An association between bacterial infection and xanthogranulomatous inflammation has existed in several organs, such as the kidneys, and in the gastrointestinal system, but such an association of the 2 is yet to be determined for bone.5,10,16-19 Because of the paucity of literature on the disease, a management protocol for XO of bone has not been defined, and decisions have to be made considering the natural history of the disease in other organs. We present this case primarily because of its rarity, curability, and its close resemblance to bone tumors. While XO is benign, it can mimic a neoplastic bone lesion in its imaging and clinical manifestations, and appropriate differentiation is crucial. Currently, histopathologic examination of lesions is the most specific and is the gold standard for diagnosis.
Conclusion
Xanthogranulomatous osteomyelitis is a very rare entity, and only a few cases have been reported in the English-language literature. Though rare, XO warrants greater emphasis than it receives in the literature. It is a chronic inflammatory disease having a close resemblance to bone tumors. A high index of suspicion must be practiced to differentiate XO from tumors. Histopathologic examination is mandatory to establish definitive diagnosis and correct treatment.
1. Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183(4):395-402.
2. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101(11):869-875.
3. Nistal M, Gonzalez-Peramato P, Serrano A, Regadera J. Xanthogranulomatous funiculitis and orchiepididymitis: report of 2 cases with immunohistochemical study and literature review. Arch Pathol Lab Med. 2004;128(8):911-914.
4. Oh YH, Seong SS, Jang KS, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55(7):440-444.
5. Cozzutto C. Xanthogranulomatous osteomyelitis. Arch Pathol Lab Med. 1984;108(12):973-6.
6. Vankalakunti M, Saikia UN, Mathew M, Kang M. Xanthogranulomatous osteomyelitis of ulna mimicking neoplasm. World J Surg Oncol. 2007;30(5):46.
7. Cennimo DJ, Agag R, Fleegler E, et al. Mycobacterium marinum hand infection in a “sushi chef.” Eplasty. 2009;14(9):e43.
8. Borjian A, Rezaei F, Eshaghi MA, Shemshaki H. Xanthogranulomatous osteomyelitis. J Orthop Traumatol. 2012;13(4):217-220.
9. Rafique M, Yaqoob N. Xanthogranulomatous prostatitis: a mimic of carcinoma of prostate. World J Surg Oncol. 2006;4:30.
10. Nakashiro H, Haraoka S, Fujiwara K, Harada S, Hisatsugu T, Watanabe T. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191(11):1078-1086.
11. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
12. Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378.
13. Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol. 1999;30(1):59-65.
14. Kayser R, Mahlfeld K, Grasshoff H. Vertebral Langerhans-cell histiocytosis in childhood – a differential diagnosis of spinal osteomyelitis. Klin Padiatr. 1999;211(5):399-402.
15. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
16. Machiz S, Gordon J, Block N, Politano VA. Salmonella typhosa urinary tract infection and xanthogranulomatous pyelonephritis. Case report and review of literature. J Fla Med Assoc. 1974;61(9):703-705.
17. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. 1977;11(2):189-191.
18. Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine. 1979;58(2):171-181.
19. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46(1):88-90.
Xanthogranulomatous osteomyelitis (XO) is a type of chronic inflammatory process that is characterized by the collection of foamy macrophages along with mononuclear cells in the tissue.1 Xanthogranulomatous osteomyelitis is characterized by the presence of granular, eosinophilic, periodic acid–Schiff–positive histiocytes in the initial stages, followed by the mixture of foamy macrophages and activated plasma cells and, last, by the presence of suppurative foci and hemorrhage. This is an uncommon process best known to occur in the gallbladder, kidney, urinary bladder, fallopian tube, ovary, vagina, prostate, testis, epididymis, colon, and appendix.2-4 Very rarely, it can affect lungs, brain, or bone. Only 5 cases of XO have been reported in the literature.5-8
We report XO of the proximal femur in a 65-year-old woman who initially had a clinical and radiologic diagnosis of aneurysmal bone cyst; however, histopathologic examination confirmed the diagnosis of XO. Xanthogranulomatous osteomyelitis mimics a neoplastic pathology in gallbladder, kidney, and prostrate on gross clinical and radiologic examination.9 The pathogenesis of XO is best characterized by a delayed type of hypersensitivity reaction.10 The differential diagnosis includes chronic recurrent multifocal osteomyelitis, xanthoma, infiltrative storage disorder, malakoplakia, Langerhans cell histiocytosis, fibrohistiocytic tumor, Erdheim-Chester disease, and metastatic renal cell carcinoma.11-14 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old hypertensive woman presented with complaints of pain in the right hip for a duration of 6 months. Pain was radiating from the right hip region to the anteromedial aspect of the knee and progressively increasing, with a history of pain at rest suggestive of a nonmechanical pathology in the hip. There was no history of fever, weight loss, loss of appetite, pain in any other joint, or morning stiffness. The patient was mobile without support and was able to squat and sit cross-legged; however, the stance phase on the right side was less than on the left side, suggestive of an antalgic component in the gait.
On examining the patient, there was anterior hip joint tenderness with no local sign of any infective or inflammatory pathology. Trochanteric tenderness was present, but there was no irregularity, broadening, or thickening of the trochanter. There was no restriction in the range of motion, and no coronal or sagittal plane deformity in the right hip. There was no limb-length discrepancy. However, the patient was not able to raise her leg actively, probably because of pain in the right hip.
On plain radiographs of the pelvis with bilateral hips, a well-defined nonexpansile uniloculated lytic lesion with sclerotic margins was present in the neck of the right femur, extending to the intertrochanteric area (Figure 1). Ground-glass appearance was also noted. Considering the benign nature of the lesion radiologically and clinically, a differential diagnosis of hyperparathyroidism, renal osteodystrophy, multiple myeloma, and fibrous dysplasia was considered. Hematologic investigations, skeletal survey, and magnetic resonance imaging (MRI) of the bilateral hips were performed to rule out the differential diagnosis.
The patient’s hemoglobin level was 11.8 g/dL with total white blood cell count of 10,300/µL. Renal and hepatic functions were within normal limit. Serum erythrocyte sedimentation rate (ESR) was 12 mm/h and C-reactive protein level was normal. Serum parathyroid level was 32 pg/mL, which was within normal limits, with an alkaline phosphatase level of 101 U/L. The skeletal survey showed no other bony lesion in the body. T1-weighted MRI of both hips showed a well-defined hypointense lesion in the neck and intertrochanteric area of the right hip, which was hyperintense on T2-weighted MRI, suggestive of aneurysmal bone cyst (Figure 2).
Normal ESR, hemoglobin, alkaline phosphatase, and serum parathyroid levels and normal skeletal survey almost ruled out multiple myeloma and hyperparathyroidism. Normal renal profile ruled out renal osteodystrophy and the osteitis fibrosa cystica lesion associated with it. We planned for prophylactic internal fixation of the lesion to prevent a pathologic fracture. According to Mirels,15 if there is a lytic lesion covering more than two-thirds of the circumference of the bone in the peritrochanteric area, the chances of a pathologic fracture are high and such fractures should be fixed.
We planned for curettage of the lesion with bone grafting and in situ intramedullary fixation of the lesion. Curettage was done according to the plan and the sample was sent for histopathologic examination. In situ internal fixation and bone grafting were performed by using a proximal femoral intramedullary nail. To our surprise, the biopsy sample was reported as xanthogranuloma, with multiple foamy macrophages mixed with inflammatory cells and aggregates of lymphocytes (Figure 3). Mycobacterial and routine bacterial cultures were reported as negative. The patient was kept on oral antibiotics (cefixime and moxifloxacin) for 6 weeks, and she made an uneventful recovery. At 6-month follow-up, a radiograph of the right hip showed a healed lesion with proximal femoral nail in situ (Figure 4).
Discussion
To the best of our knowledge, a total of 5 cases of XO have been reported in the literature. The earliest of these reports were by Cozzutto and Carbone,1 who reported 2 cases of XO of the first rib and of the epiphysis of the tibia, respectively. The importance of these lesions to diagnosis is their confusion with a neoplastic disease, as XO is itself a benign disorder. These lesions can mimic a neoplastic lesion in clinical and radiologic presentation and the only way to differentiate the lesion from a neoplastic disease is by histopathologic examination of the tissue. Hypothetically, xanthogranulomatous disorders can be related to trauma or infection.
In 2007, Vankalakunti and colleagues6 reported XO of the ulna in a 50-year-old postmenopausal woman. In that case, progressive swelling was present on the extensor aspect of her right forearm for a period of 2 years, for which curettage and bone grafting were performed, using autograft from the ipsilateral iliac crest. The tissue culture was sterile, and XO was diagnosed as a result of the histopathologic examination. In 2009, Cennimo and colleagues7 reported XO of the index finger and wrist of a man complaining of pain and swelling for 1 year, which was unresponsive to antibiotics. The diagnosis of XO was confirmed histopathologically, when the culture of the same tissue grew Mycobacterium marinum. Radical synovectomy of the lesion was performed, after which minocycline, clarithromycin, and ethambutol were administered. In 2012, Borjian and colleagues8 reported a case of XO of the proximal humerus and proximal fibula in a 14-year-old child. The child, who presented with fever, pain, and restriction of shoulder movements, was started on oral antibiotics as the tissue culture grew Staphylococcus aureus; the patient did not complete the course of treatment in the hospital. No surgical intervention was done in this case. The diagnosis of XO was confirmed by microscopic examination of the tissue.
An association between bacterial infection and xanthogranulomatous inflammation has existed in several organs, such as the kidneys, and in the gastrointestinal system, but such an association of the 2 is yet to be determined for bone.5,10,16-19 Because of the paucity of literature on the disease, a management protocol for XO of bone has not been defined, and decisions have to be made considering the natural history of the disease in other organs. We present this case primarily because of its rarity, curability, and its close resemblance to bone tumors. While XO is benign, it can mimic a neoplastic bone lesion in its imaging and clinical manifestations, and appropriate differentiation is crucial. Currently, histopathologic examination of lesions is the most specific and is the gold standard for diagnosis.
Conclusion
Xanthogranulomatous osteomyelitis is a very rare entity, and only a few cases have been reported in the English-language literature. Though rare, XO warrants greater emphasis than it receives in the literature. It is a chronic inflammatory disease having a close resemblance to bone tumors. A high index of suspicion must be practiced to differentiate XO from tumors. Histopathologic examination is mandatory to establish definitive diagnosis and correct treatment.
Xanthogranulomatous osteomyelitis (XO) is a type of chronic inflammatory process that is characterized by the collection of foamy macrophages along with mononuclear cells in the tissue.1 Xanthogranulomatous osteomyelitis is characterized by the presence of granular, eosinophilic, periodic acid–Schiff–positive histiocytes in the initial stages, followed by the mixture of foamy macrophages and activated plasma cells and, last, by the presence of suppurative foci and hemorrhage. This is an uncommon process best known to occur in the gallbladder, kidney, urinary bladder, fallopian tube, ovary, vagina, prostate, testis, epididymis, colon, and appendix.2-4 Very rarely, it can affect lungs, brain, or bone. Only 5 cases of XO have been reported in the literature.5-8
We report XO of the proximal femur in a 65-year-old woman who initially had a clinical and radiologic diagnosis of aneurysmal bone cyst; however, histopathologic examination confirmed the diagnosis of XO. Xanthogranulomatous osteomyelitis mimics a neoplastic pathology in gallbladder, kidney, and prostrate on gross clinical and radiologic examination.9 The pathogenesis of XO is best characterized by a delayed type of hypersensitivity reaction.10 The differential diagnosis includes chronic recurrent multifocal osteomyelitis, xanthoma, infiltrative storage disorder, malakoplakia, Langerhans cell histiocytosis, fibrohistiocytic tumor, Erdheim-Chester disease, and metastatic renal cell carcinoma.11-14 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old hypertensive woman presented with complaints of pain in the right hip for a duration of 6 months. Pain was radiating from the right hip region to the anteromedial aspect of the knee and progressively increasing, with a history of pain at rest suggestive of a nonmechanical pathology in the hip. There was no history of fever, weight loss, loss of appetite, pain in any other joint, or morning stiffness. The patient was mobile without support and was able to squat and sit cross-legged; however, the stance phase on the right side was less than on the left side, suggestive of an antalgic component in the gait.
On examining the patient, there was anterior hip joint tenderness with no local sign of any infective or inflammatory pathology. Trochanteric tenderness was present, but there was no irregularity, broadening, or thickening of the trochanter. There was no restriction in the range of motion, and no coronal or sagittal plane deformity in the right hip. There was no limb-length discrepancy. However, the patient was not able to raise her leg actively, probably because of pain in the right hip.
On plain radiographs of the pelvis with bilateral hips, a well-defined nonexpansile uniloculated lytic lesion with sclerotic margins was present in the neck of the right femur, extending to the intertrochanteric area (Figure 1). Ground-glass appearance was also noted. Considering the benign nature of the lesion radiologically and clinically, a differential diagnosis of hyperparathyroidism, renal osteodystrophy, multiple myeloma, and fibrous dysplasia was considered. Hematologic investigations, skeletal survey, and magnetic resonance imaging (MRI) of the bilateral hips were performed to rule out the differential diagnosis.
The patient’s hemoglobin level was 11.8 g/dL with total white blood cell count of 10,300/µL. Renal and hepatic functions were within normal limit. Serum erythrocyte sedimentation rate (ESR) was 12 mm/h and C-reactive protein level was normal. Serum parathyroid level was 32 pg/mL, which was within normal limits, with an alkaline phosphatase level of 101 U/L. The skeletal survey showed no other bony lesion in the body. T1-weighted MRI of both hips showed a well-defined hypointense lesion in the neck and intertrochanteric area of the right hip, which was hyperintense on T2-weighted MRI, suggestive of aneurysmal bone cyst (Figure 2).
Normal ESR, hemoglobin, alkaline phosphatase, and serum parathyroid levels and normal skeletal survey almost ruled out multiple myeloma and hyperparathyroidism. Normal renal profile ruled out renal osteodystrophy and the osteitis fibrosa cystica lesion associated with it. We planned for prophylactic internal fixation of the lesion to prevent a pathologic fracture. According to Mirels,15 if there is a lytic lesion covering more than two-thirds of the circumference of the bone in the peritrochanteric area, the chances of a pathologic fracture are high and such fractures should be fixed.
We planned for curettage of the lesion with bone grafting and in situ intramedullary fixation of the lesion. Curettage was done according to the plan and the sample was sent for histopathologic examination. In situ internal fixation and bone grafting were performed by using a proximal femoral intramedullary nail. To our surprise, the biopsy sample was reported as xanthogranuloma, with multiple foamy macrophages mixed with inflammatory cells and aggregates of lymphocytes (Figure 3). Mycobacterial and routine bacterial cultures were reported as negative. The patient was kept on oral antibiotics (cefixime and moxifloxacin) for 6 weeks, and she made an uneventful recovery. At 6-month follow-up, a radiograph of the right hip showed a healed lesion with proximal femoral nail in situ (Figure 4).
Discussion
To the best of our knowledge, a total of 5 cases of XO have been reported in the literature. The earliest of these reports were by Cozzutto and Carbone,1 who reported 2 cases of XO of the first rib and of the epiphysis of the tibia, respectively. The importance of these lesions to diagnosis is their confusion with a neoplastic disease, as XO is itself a benign disorder. These lesions can mimic a neoplastic lesion in clinical and radiologic presentation and the only way to differentiate the lesion from a neoplastic disease is by histopathologic examination of the tissue. Hypothetically, xanthogranulomatous disorders can be related to trauma or infection.
In 2007, Vankalakunti and colleagues6 reported XO of the ulna in a 50-year-old postmenopausal woman. In that case, progressive swelling was present on the extensor aspect of her right forearm for a period of 2 years, for which curettage and bone grafting were performed, using autograft from the ipsilateral iliac crest. The tissue culture was sterile, and XO was diagnosed as a result of the histopathologic examination. In 2009, Cennimo and colleagues7 reported XO of the index finger and wrist of a man complaining of pain and swelling for 1 year, which was unresponsive to antibiotics. The diagnosis of XO was confirmed histopathologically, when the culture of the same tissue grew Mycobacterium marinum. Radical synovectomy of the lesion was performed, after which minocycline, clarithromycin, and ethambutol were administered. In 2012, Borjian and colleagues8 reported a case of XO of the proximal humerus and proximal fibula in a 14-year-old child. The child, who presented with fever, pain, and restriction of shoulder movements, was started on oral antibiotics as the tissue culture grew Staphylococcus aureus; the patient did not complete the course of treatment in the hospital. No surgical intervention was done in this case. The diagnosis of XO was confirmed by microscopic examination of the tissue.
An association between bacterial infection and xanthogranulomatous inflammation has existed in several organs, such as the kidneys, and in the gastrointestinal system, but such an association of the 2 is yet to be determined for bone.5,10,16-19 Because of the paucity of literature on the disease, a management protocol for XO of bone has not been defined, and decisions have to be made considering the natural history of the disease in other organs. We present this case primarily because of its rarity, curability, and its close resemblance to bone tumors. While XO is benign, it can mimic a neoplastic bone lesion in its imaging and clinical manifestations, and appropriate differentiation is crucial. Currently, histopathologic examination of lesions is the most specific and is the gold standard for diagnosis.
Conclusion
Xanthogranulomatous osteomyelitis is a very rare entity, and only a few cases have been reported in the English-language literature. Though rare, XO warrants greater emphasis than it receives in the literature. It is a chronic inflammatory disease having a close resemblance to bone tumors. A high index of suspicion must be practiced to differentiate XO from tumors. Histopathologic examination is mandatory to establish definitive diagnosis and correct treatment.
1. Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183(4):395-402.
2. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101(11):869-875.
3. Nistal M, Gonzalez-Peramato P, Serrano A, Regadera J. Xanthogranulomatous funiculitis and orchiepididymitis: report of 2 cases with immunohistochemical study and literature review. Arch Pathol Lab Med. 2004;128(8):911-914.
4. Oh YH, Seong SS, Jang KS, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55(7):440-444.
5. Cozzutto C. Xanthogranulomatous osteomyelitis. Arch Pathol Lab Med. 1984;108(12):973-6.
6. Vankalakunti M, Saikia UN, Mathew M, Kang M. Xanthogranulomatous osteomyelitis of ulna mimicking neoplasm. World J Surg Oncol. 2007;30(5):46.
7. Cennimo DJ, Agag R, Fleegler E, et al. Mycobacterium marinum hand infection in a “sushi chef.” Eplasty. 2009;14(9):e43.
8. Borjian A, Rezaei F, Eshaghi MA, Shemshaki H. Xanthogranulomatous osteomyelitis. J Orthop Traumatol. 2012;13(4):217-220.
9. Rafique M, Yaqoob N. Xanthogranulomatous prostatitis: a mimic of carcinoma of prostate. World J Surg Oncol. 2006;4:30.
10. Nakashiro H, Haraoka S, Fujiwara K, Harada S, Hisatsugu T, Watanabe T. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191(11):1078-1086.
11. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
12. Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378.
13. Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol. 1999;30(1):59-65.
14. Kayser R, Mahlfeld K, Grasshoff H. Vertebral Langerhans-cell histiocytosis in childhood – a differential diagnosis of spinal osteomyelitis. Klin Padiatr. 1999;211(5):399-402.
15. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
16. Machiz S, Gordon J, Block N, Politano VA. Salmonella typhosa urinary tract infection and xanthogranulomatous pyelonephritis. Case report and review of literature. J Fla Med Assoc. 1974;61(9):703-705.
17. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. 1977;11(2):189-191.
18. Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine. 1979;58(2):171-181.
19. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46(1):88-90.
1. Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183(4):395-402.
2. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101(11):869-875.
3. Nistal M, Gonzalez-Peramato P, Serrano A, Regadera J. Xanthogranulomatous funiculitis and orchiepididymitis: report of 2 cases with immunohistochemical study and literature review. Arch Pathol Lab Med. 2004;128(8):911-914.
4. Oh YH, Seong SS, Jang KS, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55(7):440-444.
5. Cozzutto C. Xanthogranulomatous osteomyelitis. Arch Pathol Lab Med. 1984;108(12):973-6.
6. Vankalakunti M, Saikia UN, Mathew M, Kang M. Xanthogranulomatous osteomyelitis of ulna mimicking neoplasm. World J Surg Oncol. 2007;30(5):46.
7. Cennimo DJ, Agag R, Fleegler E, et al. Mycobacterium marinum hand infection in a “sushi chef.” Eplasty. 2009;14(9):e43.
8. Borjian A, Rezaei F, Eshaghi MA, Shemshaki H. Xanthogranulomatous osteomyelitis. J Orthop Traumatol. 2012;13(4):217-220.
9. Rafique M, Yaqoob N. Xanthogranulomatous prostatitis: a mimic of carcinoma of prostate. World J Surg Oncol. 2006;4:30.
10. Nakashiro H, Haraoka S, Fujiwara K, Harada S, Hisatsugu T, Watanabe T. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191(11):1078-1086.
11. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
12. Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378.
13. Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol. 1999;30(1):59-65.
14. Kayser R, Mahlfeld K, Grasshoff H. Vertebral Langerhans-cell histiocytosis in childhood – a differential diagnosis of spinal osteomyelitis. Klin Padiatr. 1999;211(5):399-402.
15. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
16. Machiz S, Gordon J, Block N, Politano VA. Salmonella typhosa urinary tract infection and xanthogranulomatous pyelonephritis. Case report and review of literature. J Fla Med Assoc. 1974;61(9):703-705.
17. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. 1977;11(2):189-191.
18. Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine. 1979;58(2):171-181.
19. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46(1):88-90.
CMS Proposes Major Initiative for Hip and Knee Replacements
In an attempt to reduce the rate and cost of complications following hip and knee replacements among Medicare beneficiaries, the Centers for Medicare & Medicaid Services (CMS) announced a new Comprehensive Care for Joint Replacement payment model. With this new measure, the CMS proposes to hold hospitals accountable for the quality of care they deliver to Medicare fee-for-service beneficiaries for hip and knee replacements from surgery through recovery.
“We are committed to changing our health care system to pay for quality over quantity, so that we spend our dollars more wisely and improve care for patients,” said Sylvia M. Burwell, Secretary of Health and Human Services.
Through the proposed 5-year payment model, health care providers in 75 geographic areas would continue to be paid under existing Medicare payment systems. However, the hospital where the hip or knee replacement is performed would be held liable for the quality and costs of care for the duration of care, from the time of the surgery through 90 days after discharge.
Depending on the hospital’s quality and cost performance during the episode, the hospital may receive an additional payment or be required to repay Medicare for a portion of the episode costs. As a result, hospitals would have an incentive to work with physicians, home health agencies, and nursing facilities to ensure that beneficiaries receive the coordinated care they need, with the goal of reducing avoidable hospitalizations and complications. Hospitals would receive tools, such as spending and utilization data and sharing of best practices, to improve the effectiveness of care coordination.
These bundled payments for joint replacement surgeries would build upon successful demonstration programs already underway in Medicare. This model is also consistent with the private sector, where major employers and leading providers and care systems are moving towards bundled payments for orthopedic services.
“Today, we are taking another important step to improve the quality of care for the hundreds of thousands of Americans who have hip and knee replacements through Medicare every year. By focusing on episodes of care, rather than a piecemeal system, hospitals and physicians have an incentive to work together to deliver more effective and efficient care. This model will incentivize providing patients with the right care the first time and finding better ways to help them recover successfully. It will reward providers and doctors for helping patients get and stay healthy, ” stated Ms. Burwell.
In an attempt to reduce the rate and cost of complications following hip and knee replacements among Medicare beneficiaries, the Centers for Medicare & Medicaid Services (CMS) announced a new Comprehensive Care for Joint Replacement payment model. With this new measure, the CMS proposes to hold hospitals accountable for the quality of care they deliver to Medicare fee-for-service beneficiaries for hip and knee replacements from surgery through recovery.
“We are committed to changing our health care system to pay for quality over quantity, so that we spend our dollars more wisely and improve care for patients,” said Sylvia M. Burwell, Secretary of Health and Human Services.
Through the proposed 5-year payment model, health care providers in 75 geographic areas would continue to be paid under existing Medicare payment systems. However, the hospital where the hip or knee replacement is performed would be held liable for the quality and costs of care for the duration of care, from the time of the surgery through 90 days after discharge.
Depending on the hospital’s quality and cost performance during the episode, the hospital may receive an additional payment or be required to repay Medicare for a portion of the episode costs. As a result, hospitals would have an incentive to work with physicians, home health agencies, and nursing facilities to ensure that beneficiaries receive the coordinated care they need, with the goal of reducing avoidable hospitalizations and complications. Hospitals would receive tools, such as spending and utilization data and sharing of best practices, to improve the effectiveness of care coordination.
These bundled payments for joint replacement surgeries would build upon successful demonstration programs already underway in Medicare. This model is also consistent with the private sector, where major employers and leading providers and care systems are moving towards bundled payments for orthopedic services.
“Today, we are taking another important step to improve the quality of care for the hundreds of thousands of Americans who have hip and knee replacements through Medicare every year. By focusing on episodes of care, rather than a piecemeal system, hospitals and physicians have an incentive to work together to deliver more effective and efficient care. This model will incentivize providing patients with the right care the first time and finding better ways to help them recover successfully. It will reward providers and doctors for helping patients get and stay healthy, ” stated Ms. Burwell.
In an attempt to reduce the rate and cost of complications following hip and knee replacements among Medicare beneficiaries, the Centers for Medicare & Medicaid Services (CMS) announced a new Comprehensive Care for Joint Replacement payment model. With this new measure, the CMS proposes to hold hospitals accountable for the quality of care they deliver to Medicare fee-for-service beneficiaries for hip and knee replacements from surgery through recovery.
“We are committed to changing our health care system to pay for quality over quantity, so that we spend our dollars more wisely and improve care for patients,” said Sylvia M. Burwell, Secretary of Health and Human Services.
Through the proposed 5-year payment model, health care providers in 75 geographic areas would continue to be paid under existing Medicare payment systems. However, the hospital where the hip or knee replacement is performed would be held liable for the quality and costs of care for the duration of care, from the time of the surgery through 90 days after discharge.
Depending on the hospital’s quality and cost performance during the episode, the hospital may receive an additional payment or be required to repay Medicare for a portion of the episode costs. As a result, hospitals would have an incentive to work with physicians, home health agencies, and nursing facilities to ensure that beneficiaries receive the coordinated care they need, with the goal of reducing avoidable hospitalizations and complications. Hospitals would receive tools, such as spending and utilization data and sharing of best practices, to improve the effectiveness of care coordination.
These bundled payments for joint replacement surgeries would build upon successful demonstration programs already underway in Medicare. This model is also consistent with the private sector, where major employers and leading providers and care systems are moving towards bundled payments for orthopedic services.
“Today, we are taking another important step to improve the quality of care for the hundreds of thousands of Americans who have hip and knee replacements through Medicare every year. By focusing on episodes of care, rather than a piecemeal system, hospitals and physicians have an incentive to work together to deliver more effective and efficient care. This model will incentivize providing patients with the right care the first time and finding better ways to help them recover successfully. It will reward providers and doctors for helping patients get and stay healthy, ” stated Ms. Burwell.
Enoxaparin and Warfarin for Venous Thromboembolism Prophylaxis in Total Hip Arthroplasty: To Bridge or Not to Bridge?
According to the literature, the rate of deep venous thrombosis after total hip arthroplasty (THA) can be high (45%-63%) without prophylactic anticoagulation.1-6 A meta-analysis of 13 studies found a rate of 51%.7 As lower extremity deep venous thrombi are the initial source of symptomatic pulmonary emboli in about 90% of cases,8 THA patients are usually given medication postoperatively focused on prevention of these thromboembolic events.9 Chemoprophylaxis may involve warfarin, enoxaparin, or their combination in an anticoagulation bridge. Enoxaparin is one of many low-molecular-weight heparins (LMWHs). All LMWHs exert their anticoagulant effect by binding to antithrombin III.10 The binding of LMWH to antithrombin III catalyzes the inhibition of factor Xa by antithrombin III, disrupting clot formation.11
In its hydroquinone form, vitamin K is essential as a cofactor for carboxylation of the glutamic acid residues of the amino-terminals of the coagulation proteins II, VII, IX, and X, leading to their activation. Anticoagulation by warfarin is achieved by the inhibition of the reductase enzymes that produce vitamin K hydroquinone in the liver from vitamin K epoxide.12 This inhibition prevents activation of the clotting proteins.12,13 Prophylaxis with enoxaparin or warfarin can reduce the rate of venous thromboembolic disease to 3.6% and 3.7%, respectively.2 However, these medications inhibit the clotting cascade, and their use risks prolonging the healing process.9 The delay increases the risk for wound infection,14 which can lead to a longer hospital stay and therefore higher costs.
We conducted a study to compare patients who received warfarin only with patients who received warfarin bridged with enoxaparin as antithrombotic chemoprophylaxis after THA. Outcomes of interest were number of days until a dry wound was observed and length of hospital stay. We hypothesized that, compared with warfarin-only therapy, bridged therapy would increase the risk for prolonged wound healing and result in longer hospital stays.
Materials and Methods
At our 746-bed academic medical center, 121 THAs were performed between January 1, 2008 and December 31, 2009. This study was approved by the center’s Office for Human Subjects Protections institutional review board (IRB). The research involved collecting or studying existing data, documents, and records recorded anonymously by the investigator in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects, and therefore patient consent was not needed. Therefore, the IRB waived the need for consent. Relevant data included in this study were extracted from patient medical records, given within 35 days of surgery. For each patient, discharge notes provided data on the hospital course, and nurses’ notes provided data on wound status after THA.
Propensity Score Matching
For accurate analysis, it was important to consider confounding factors in both patient groups. Some covariates that may influence accurate analysis are age,15 diabetes,16 sex,15,17 hypertension,18 and body mass index.15,19Propensity score, defined as the conditional probability of receiving treatment, given the observed background covariates, was initially defined by Rosenbaum20 and Rubin.21 The motivation behind propensity scores can be understood by considering an idealized situation in which the 2 groups are similar on all background characteristics. In nonexperimental studies, researchers aim to find for each treated individual a comparison individual who looks exactly the same as the treated individual with respect to observed pretreatment covariates. Thus, assuming no hidden bias, any difference in outcomes within these pairs can be attributed to the variable of interest and not to any other differences between the treated and comparison individuals. Our study is a typical nonexperimental retrospective study in which the 2 groups being compared are patients receiving warfarin only or warfarin bridged with enoxaparin. To minimize the influence of background covariates, we used matching procedures and present our results both with and without the use of matching techniques.
Data and Results
There are different matching algorithms aimed at matching groups. In our study, the optimal matching procedure alone could not produce adequately matched data, so we used both optimal matching20 and genetic matching.22,23 Genetic matching procedure with replacement22 can produce well-matched data—it matched each patient in the warfarin-only group with a patient in the bridged-therapy group and allowed different patients to be matched with 1 similar patient in the control group. However, as the same patients in the bridged-therapy group might be matched multiple times, it would complicate the after-matching analysis. We therefore used a 2-step matching procedure to obtain well-matched data, and a simplified analysis procedure after matching. In the first step, we implemented genetic matching with replacement, as introduced by Abadie and Imbens,22 to match each warfarin-only patient with 1 bridged-therapy patient. In the second step, we applied optimal matching to the 2 groups. This 2-step matching turned out to produce better matched pairs, as denoted by Rubin.21 Both matching steps were implemented using the MatchIt function in R.24
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.
1. Bergqvist D, Benoni G, Björgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696-700.
2. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81(7):932-940.
3. Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res. 1989;(242):212-231.
4. Johnson R, Carmichael JH, Almond HG, Loynes RP. Deep venous thrombosis following Charnley arthroplasty. Clin Orthop Relat Res. 1978;(132):24-30.
5. Stamatakis JD, Kakkar VV, Sagar S, Lawrence D, Nairn D, Bentley PG. Femoral vein thrombosis and total hip replacement. Br Med J. 1977;2(6081):223-225.
6. Turpie AG, Levine MN, Hirsh J, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315(15):925-929.
7. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S-334S.
8. Westrich GH, Sánchez PM. Prevention and treatment of thromboembolic disease: an overview. Instr Course Lect. 2002;51:471-480.
9. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
10. Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L-10L.
11. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20(7):771-775.
12. Kamali F, Wood P, Ward A. Vitamin K deficiency amplifies anticoagulation response to ximelagatran: possible implications for direct thrombin inhibitors and their clinical safety. Ann Hematol. 2009;88(2):141-149.
13. Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1-7.
14. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515.
15. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
16. Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22(5):651-656.
17. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
18. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
19. Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57(2):327-334.
20. Rosenbaum PR. A characterization of optimal designs for observational studies. J R Stat Soc Ser B. 1991;53(3):597-610.
21. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(1):169-188.
22. Abadie A, Imbens GW. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. Berkeley, CA: Department of Economics, University of California; 2002.
23. Diamond A, Sekhon J. Genetic matching for estimating causal effects: a new method of achieving balance in observational studies. Paper presented at: Annual Meeting of the Midwest Political Science Association; April 2005; Chicago, IL.
24. Imai K, King G, Lau O. logit: logistic regression for dichotomous dependent variables. In: Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2011; 238-244. http://gking.harvard.edu/zelig. Accessed May 26, 2015.
25. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
26. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008;65(9):1169-1173.
27. Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day [2010]. http://kff.org/other/state-indicator/expenses-per-inpatient-day/#table. Accessed May 26, 2015.
According to the literature, the rate of deep venous thrombosis after total hip arthroplasty (THA) can be high (45%-63%) without prophylactic anticoagulation.1-6 A meta-analysis of 13 studies found a rate of 51%.7 As lower extremity deep venous thrombi are the initial source of symptomatic pulmonary emboli in about 90% of cases,8 THA patients are usually given medication postoperatively focused on prevention of these thromboembolic events.9 Chemoprophylaxis may involve warfarin, enoxaparin, or their combination in an anticoagulation bridge. Enoxaparin is one of many low-molecular-weight heparins (LMWHs). All LMWHs exert their anticoagulant effect by binding to antithrombin III.10 The binding of LMWH to antithrombin III catalyzes the inhibition of factor Xa by antithrombin III, disrupting clot formation.11
In its hydroquinone form, vitamin K is essential as a cofactor for carboxylation of the glutamic acid residues of the amino-terminals of the coagulation proteins II, VII, IX, and X, leading to their activation. Anticoagulation by warfarin is achieved by the inhibition of the reductase enzymes that produce vitamin K hydroquinone in the liver from vitamin K epoxide.12 This inhibition prevents activation of the clotting proteins.12,13 Prophylaxis with enoxaparin or warfarin can reduce the rate of venous thromboembolic disease to 3.6% and 3.7%, respectively.2 However, these medications inhibit the clotting cascade, and their use risks prolonging the healing process.9 The delay increases the risk for wound infection,14 which can lead to a longer hospital stay and therefore higher costs.
We conducted a study to compare patients who received warfarin only with patients who received warfarin bridged with enoxaparin as antithrombotic chemoprophylaxis after THA. Outcomes of interest were number of days until a dry wound was observed and length of hospital stay. We hypothesized that, compared with warfarin-only therapy, bridged therapy would increase the risk for prolonged wound healing and result in longer hospital stays.
Materials and Methods
At our 746-bed academic medical center, 121 THAs were performed between January 1, 2008 and December 31, 2009. This study was approved by the center’s Office for Human Subjects Protections institutional review board (IRB). The research involved collecting or studying existing data, documents, and records recorded anonymously by the investigator in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects, and therefore patient consent was not needed. Therefore, the IRB waived the need for consent. Relevant data included in this study were extracted from patient medical records, given within 35 days of surgery. For each patient, discharge notes provided data on the hospital course, and nurses’ notes provided data on wound status after THA.
Propensity Score Matching
For accurate analysis, it was important to consider confounding factors in both patient groups. Some covariates that may influence accurate analysis are age,15 diabetes,16 sex,15,17 hypertension,18 and body mass index.15,19Propensity score, defined as the conditional probability of receiving treatment, given the observed background covariates, was initially defined by Rosenbaum20 and Rubin.21 The motivation behind propensity scores can be understood by considering an idealized situation in which the 2 groups are similar on all background characteristics. In nonexperimental studies, researchers aim to find for each treated individual a comparison individual who looks exactly the same as the treated individual with respect to observed pretreatment covariates. Thus, assuming no hidden bias, any difference in outcomes within these pairs can be attributed to the variable of interest and not to any other differences between the treated and comparison individuals. Our study is a typical nonexperimental retrospective study in which the 2 groups being compared are patients receiving warfarin only or warfarin bridged with enoxaparin. To minimize the influence of background covariates, we used matching procedures and present our results both with and without the use of matching techniques.
Data and Results
There are different matching algorithms aimed at matching groups. In our study, the optimal matching procedure alone could not produce adequately matched data, so we used both optimal matching20 and genetic matching.22,23 Genetic matching procedure with replacement22 can produce well-matched data—it matched each patient in the warfarin-only group with a patient in the bridged-therapy group and allowed different patients to be matched with 1 similar patient in the control group. However, as the same patients in the bridged-therapy group might be matched multiple times, it would complicate the after-matching analysis. We therefore used a 2-step matching procedure to obtain well-matched data, and a simplified analysis procedure after matching. In the first step, we implemented genetic matching with replacement, as introduced by Abadie and Imbens,22 to match each warfarin-only patient with 1 bridged-therapy patient. In the second step, we applied optimal matching to the 2 groups. This 2-step matching turned out to produce better matched pairs, as denoted by Rubin.21 Both matching steps were implemented using the MatchIt function in R.24
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.
According to the literature, the rate of deep venous thrombosis after total hip arthroplasty (THA) can be high (45%-63%) without prophylactic anticoagulation.1-6 A meta-analysis of 13 studies found a rate of 51%.7 As lower extremity deep venous thrombi are the initial source of symptomatic pulmonary emboli in about 90% of cases,8 THA patients are usually given medication postoperatively focused on prevention of these thromboembolic events.9 Chemoprophylaxis may involve warfarin, enoxaparin, or their combination in an anticoagulation bridge. Enoxaparin is one of many low-molecular-weight heparins (LMWHs). All LMWHs exert their anticoagulant effect by binding to antithrombin III.10 The binding of LMWH to antithrombin III catalyzes the inhibition of factor Xa by antithrombin III, disrupting clot formation.11
In its hydroquinone form, vitamin K is essential as a cofactor for carboxylation of the glutamic acid residues of the amino-terminals of the coagulation proteins II, VII, IX, and X, leading to their activation. Anticoagulation by warfarin is achieved by the inhibition of the reductase enzymes that produce vitamin K hydroquinone in the liver from vitamin K epoxide.12 This inhibition prevents activation of the clotting proteins.12,13 Prophylaxis with enoxaparin or warfarin can reduce the rate of venous thromboembolic disease to 3.6% and 3.7%, respectively.2 However, these medications inhibit the clotting cascade, and their use risks prolonging the healing process.9 The delay increases the risk for wound infection,14 which can lead to a longer hospital stay and therefore higher costs.
We conducted a study to compare patients who received warfarin only with patients who received warfarin bridged with enoxaparin as antithrombotic chemoprophylaxis after THA. Outcomes of interest were number of days until a dry wound was observed and length of hospital stay. We hypothesized that, compared with warfarin-only therapy, bridged therapy would increase the risk for prolonged wound healing and result in longer hospital stays.
Materials and Methods
At our 746-bed academic medical center, 121 THAs were performed between January 1, 2008 and December 31, 2009. This study was approved by the center’s Office for Human Subjects Protections institutional review board (IRB). The research involved collecting or studying existing data, documents, and records recorded anonymously by the investigator in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects, and therefore patient consent was not needed. Therefore, the IRB waived the need for consent. Relevant data included in this study were extracted from patient medical records, given within 35 days of surgery. For each patient, discharge notes provided data on the hospital course, and nurses’ notes provided data on wound status after THA.
Propensity Score Matching
For accurate analysis, it was important to consider confounding factors in both patient groups. Some covariates that may influence accurate analysis are age,15 diabetes,16 sex,15,17 hypertension,18 and body mass index.15,19Propensity score, defined as the conditional probability of receiving treatment, given the observed background covariates, was initially defined by Rosenbaum20 and Rubin.21 The motivation behind propensity scores can be understood by considering an idealized situation in which the 2 groups are similar on all background characteristics. In nonexperimental studies, researchers aim to find for each treated individual a comparison individual who looks exactly the same as the treated individual with respect to observed pretreatment covariates. Thus, assuming no hidden bias, any difference in outcomes within these pairs can be attributed to the variable of interest and not to any other differences between the treated and comparison individuals. Our study is a typical nonexperimental retrospective study in which the 2 groups being compared are patients receiving warfarin only or warfarin bridged with enoxaparin. To minimize the influence of background covariates, we used matching procedures and present our results both with and without the use of matching techniques.
Data and Results
There are different matching algorithms aimed at matching groups. In our study, the optimal matching procedure alone could not produce adequately matched data, so we used both optimal matching20 and genetic matching.22,23 Genetic matching procedure with replacement22 can produce well-matched data—it matched each patient in the warfarin-only group with a patient in the bridged-therapy group and allowed different patients to be matched with 1 similar patient in the control group. However, as the same patients in the bridged-therapy group might be matched multiple times, it would complicate the after-matching analysis. We therefore used a 2-step matching procedure to obtain well-matched data, and a simplified analysis procedure after matching. In the first step, we implemented genetic matching with replacement, as introduced by Abadie and Imbens,22 to match each warfarin-only patient with 1 bridged-therapy patient. In the second step, we applied optimal matching to the 2 groups. This 2-step matching turned out to produce better matched pairs, as denoted by Rubin.21 Both matching steps were implemented using the MatchIt function in R.24
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.
1. Bergqvist D, Benoni G, Björgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696-700.
2. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81(7):932-940.
3. Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res. 1989;(242):212-231.
4. Johnson R, Carmichael JH, Almond HG, Loynes RP. Deep venous thrombosis following Charnley arthroplasty. Clin Orthop Relat Res. 1978;(132):24-30.
5. Stamatakis JD, Kakkar VV, Sagar S, Lawrence D, Nairn D, Bentley PG. Femoral vein thrombosis and total hip replacement. Br Med J. 1977;2(6081):223-225.
6. Turpie AG, Levine MN, Hirsh J, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315(15):925-929.
7. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S-334S.
8. Westrich GH, Sánchez PM. Prevention and treatment of thromboembolic disease: an overview. Instr Course Lect. 2002;51:471-480.
9. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
10. Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L-10L.
11. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20(7):771-775.
12. Kamali F, Wood P, Ward A. Vitamin K deficiency amplifies anticoagulation response to ximelagatran: possible implications for direct thrombin inhibitors and their clinical safety. Ann Hematol. 2009;88(2):141-149.
13. Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1-7.
14. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515.
15. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
16. Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22(5):651-656.
17. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
18. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
19. Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57(2):327-334.
20. Rosenbaum PR. A characterization of optimal designs for observational studies. J R Stat Soc Ser B. 1991;53(3):597-610.
21. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(1):169-188.
22. Abadie A, Imbens GW. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. Berkeley, CA: Department of Economics, University of California; 2002.
23. Diamond A, Sekhon J. Genetic matching for estimating causal effects: a new method of achieving balance in observational studies. Paper presented at: Annual Meeting of the Midwest Political Science Association; April 2005; Chicago, IL.
24. Imai K, King G, Lau O. logit: logistic regression for dichotomous dependent variables. In: Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2011; 238-244. http://gking.harvard.edu/zelig. Accessed May 26, 2015.
25. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
26. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008;65(9):1169-1173.
27. Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day [2010]. http://kff.org/other/state-indicator/expenses-per-inpatient-day/#table. Accessed May 26, 2015.
1. Bergqvist D, Benoni G, Björgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696-700.
2. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81(7):932-940.
3. Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res. 1989;(242):212-231.
4. Johnson R, Carmichael JH, Almond HG, Loynes RP. Deep venous thrombosis following Charnley arthroplasty. Clin Orthop Relat Res. 1978;(132):24-30.
5. Stamatakis JD, Kakkar VV, Sagar S, Lawrence D, Nairn D, Bentley PG. Femoral vein thrombosis and total hip replacement. Br Med J. 1977;2(6081):223-225.
6. Turpie AG, Levine MN, Hirsh J, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315(15):925-929.
7. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S-334S.
8. Westrich GH, Sánchez PM. Prevention and treatment of thromboembolic disease: an overview. Instr Course Lect. 2002;51:471-480.
9. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
10. Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L-10L.
11. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20(7):771-775.
12. Kamali F, Wood P, Ward A. Vitamin K deficiency amplifies anticoagulation response to ximelagatran: possible implications for direct thrombin inhibitors and their clinical safety. Ann Hematol. 2009;88(2):141-149.
13. Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1-7.
14. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515.
15. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
16. Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22(5):651-656.
17. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
18. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
19. Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57(2):327-334.
20. Rosenbaum PR. A characterization of optimal designs for observational studies. J R Stat Soc Ser B. 1991;53(3):597-610.
21. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(1):169-188.
22. Abadie A, Imbens GW. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. Berkeley, CA: Department of Economics, University of California; 2002.
23. Diamond A, Sekhon J. Genetic matching for estimating causal effects: a new method of achieving balance in observational studies. Paper presented at: Annual Meeting of the Midwest Political Science Association; April 2005; Chicago, IL.
24. Imai K, King G, Lau O. logit: logistic regression for dichotomous dependent variables. In: Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2011; 238-244. http://gking.harvard.edu/zelig. Accessed May 26, 2015.
25. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
26. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008;65(9):1169-1173.
27. Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day [2010]. http://kff.org/other/state-indicator/expenses-per-inpatient-day/#table. Accessed May 26, 2015.