User login
AGA updates guidelines for management of acute diverticulitis
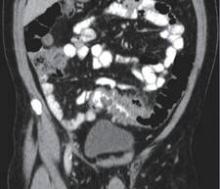
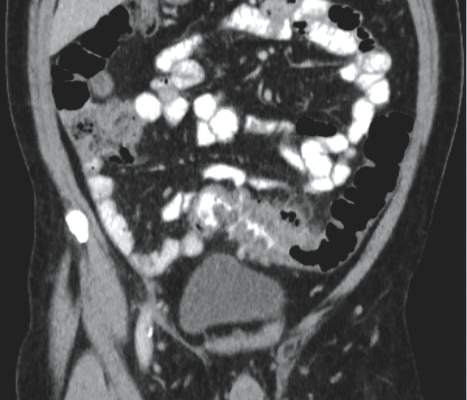
The American Gastroenterological Association (AGA) has announced new guidelines for the management of acute diverticulitis, the first practice guidelines for the disease since 1999, and they appear in the December issue of Gastroenterology.
“The management of acute diverticulitis has undergone meaningful change over the past decade, including a more judicious use of antibiotics and surgery, as well as preliminary and ongoing investigations into medical therapies to decrease symptoms and reduce recurrences,” the AGA noted. “The majority of the evidence currently, however, is of poor quality, and most of our recommendations are therefore conditional.”
The guidelines strongly recommend that patients not use mesalamine after acute uncomplicated diverticulitis, citing “moderate” evidence to this effect and saying that currently available evidence regarding the anti-inflammatory agent often used for ulcerative colitis “does not suggest efficacy in reducing recurrence risk, pain resolution, or need for surgery in this specific population.” All other recommendations, however, are conditional and offer either “low-” or “very low-quality” evidence to support them.
These additional recommendations include the following:
• Antibiotics should be used selectively, rather than routinely, in patients with acute uncomplicated diverticulitis.
• Colonoscopy should be performed after resolution of acute uncomplicated diverticulitis in appropriate candidates to exclude the misdiagnosis of a colonic neoplasm, if a high-quality exam of the colon has not been recently performed.
• Patients with a history of diverticulitis should consume a fiber-rich diet or consider fiber supplementation.
• Patients with diverticular disease should consider vigorous physical activity.
The AGA recommendations also advise against certain practices, all with conditional application and either “low-” or “very low-quality” supportive evidence. These are as follows:
• Elective colonic resection should not be done in patients with an initial episode of acute, uncomplicated diverticulitis; the decision to perform elective prophylactic colonic resection in this setting should be individualized.
• Patients with a history of diverticulitis should not be advised to avoid consumption of seeds, nuts, and popcorn.
• Patients with a history of diverticulitis should not be advised to avoid the use of aspirin.
• Patients with a history of diverticulitis should not be advised to avoid the use of nonselective nonsteroidal anti-inflammatory drugs.
• Do not advise the use of mesalamine after acute uncomplicated diverticulitis.
• Do not advise the use of rifaximin after acute uncomplicated diverticulitis.
• Do not advise the use of probiotics after acute uncomplicated diverticulitis.
“Acute diverticulitis is the third most common inpatient gastrointestinal diagnosis in the United States, costing over $2 billion annually, and is a common outpatient and emergency department diagnosis as well,” stated the AGA recommendations, which were developed by the AGA’s Clinical Guidelines Committee and approved by the AGA Institute Governing Board.
The statement notes that the condition “occurs in approximately 4% of patients with diverticulosis, roughly 15% of whom will have complicated disease, defined as an abscess, perforation, fistula or colon obstruction, and 15%-30% will experience recurrence.”
The new guidelines do not address other forms of diverticular disease, including symptomatic uncomplicated diverticular disease, diverticular bleeding, and segmental colitis associated with diverticulosis. It does not address the prevention of incident diverticulitis or the management of complicated disease.
Areas of further research, advises the AGA, should be in identifying patients who will benefit the most from antibiotics, identifying those in whom antibiotics can be withheld safely, identifying risk factors for recurrent diverticulitis, examining the risks of colonoscopy following acute diverticulitis, and taking a closer looks at anti-inflammatory drugs, antibiotics, probiotics, and dietary interventions as viable therapies.
The American Gastroenterological Association (AGA) has announced new guidelines for the management of acute diverticulitis, the first practice guidelines for the disease since 1999, and they appear in the December issue of Gastroenterology.
“The management of acute diverticulitis has undergone meaningful change over the past decade, including a more judicious use of antibiotics and surgery, as well as preliminary and ongoing investigations into medical therapies to decrease symptoms and reduce recurrences,” the AGA noted. “The majority of the evidence currently, however, is of poor quality, and most of our recommendations are therefore conditional.”
The guidelines strongly recommend that patients not use mesalamine after acute uncomplicated diverticulitis, citing “moderate” evidence to this effect and saying that currently available evidence regarding the anti-inflammatory agent often used for ulcerative colitis “does not suggest efficacy in reducing recurrence risk, pain resolution, or need for surgery in this specific population.” All other recommendations, however, are conditional and offer either “low-” or “very low-quality” evidence to support them.
These additional recommendations include the following:
• Antibiotics should be used selectively, rather than routinely, in patients with acute uncomplicated diverticulitis.
• Colonoscopy should be performed after resolution of acute uncomplicated diverticulitis in appropriate candidates to exclude the misdiagnosis of a colonic neoplasm, if a high-quality exam of the colon has not been recently performed.
• Patients with a history of diverticulitis should consume a fiber-rich diet or consider fiber supplementation.
• Patients with diverticular disease should consider vigorous physical activity.
The AGA recommendations also advise against certain practices, all with conditional application and either “low-” or “very low-quality” supportive evidence. These are as follows:
• Elective colonic resection should not be done in patients with an initial episode of acute, uncomplicated diverticulitis; the decision to perform elective prophylactic colonic resection in this setting should be individualized.
• Patients with a history of diverticulitis should not be advised to avoid consumption of seeds, nuts, and popcorn.
• Patients with a history of diverticulitis should not be advised to avoid the use of aspirin.
• Patients with a history of diverticulitis should not be advised to avoid the use of nonselective nonsteroidal anti-inflammatory drugs.
• Do not advise the use of mesalamine after acute uncomplicated diverticulitis.
• Do not advise the use of rifaximin after acute uncomplicated diverticulitis.
• Do not advise the use of probiotics after acute uncomplicated diverticulitis.
“Acute diverticulitis is the third most common inpatient gastrointestinal diagnosis in the United States, costing over $2 billion annually, and is a common outpatient and emergency department diagnosis as well,” stated the AGA recommendations, which were developed by the AGA’s Clinical Guidelines Committee and approved by the AGA Institute Governing Board.
The statement notes that the condition “occurs in approximately 4% of patients with diverticulosis, roughly 15% of whom will have complicated disease, defined as an abscess, perforation, fistula or colon obstruction, and 15%-30% will experience recurrence.”
The new guidelines do not address other forms of diverticular disease, including symptomatic uncomplicated diverticular disease, diverticular bleeding, and segmental colitis associated with diverticulosis. It does not address the prevention of incident diverticulitis or the management of complicated disease.
Areas of further research, advises the AGA, should be in identifying patients who will benefit the most from antibiotics, identifying those in whom antibiotics can be withheld safely, identifying risk factors for recurrent diverticulitis, examining the risks of colonoscopy following acute diverticulitis, and taking a closer looks at anti-inflammatory drugs, antibiotics, probiotics, and dietary interventions as viable therapies.
The American Gastroenterological Association (AGA) has announced new guidelines for the management of acute diverticulitis, the first practice guidelines for the disease since 1999, and they appear in the December issue of Gastroenterology.
“The management of acute diverticulitis has undergone meaningful change over the past decade, including a more judicious use of antibiotics and surgery, as well as preliminary and ongoing investigations into medical therapies to decrease symptoms and reduce recurrences,” the AGA noted. “The majority of the evidence currently, however, is of poor quality, and most of our recommendations are therefore conditional.”
The guidelines strongly recommend that patients not use mesalamine after acute uncomplicated diverticulitis, citing “moderate” evidence to this effect and saying that currently available evidence regarding the anti-inflammatory agent often used for ulcerative colitis “does not suggest efficacy in reducing recurrence risk, pain resolution, or need for surgery in this specific population.” All other recommendations, however, are conditional and offer either “low-” or “very low-quality” evidence to support them.
These additional recommendations include the following:
• Antibiotics should be used selectively, rather than routinely, in patients with acute uncomplicated diverticulitis.
• Colonoscopy should be performed after resolution of acute uncomplicated diverticulitis in appropriate candidates to exclude the misdiagnosis of a colonic neoplasm, if a high-quality exam of the colon has not been recently performed.
• Patients with a history of diverticulitis should consume a fiber-rich diet or consider fiber supplementation.
• Patients with diverticular disease should consider vigorous physical activity.
The AGA recommendations also advise against certain practices, all with conditional application and either “low-” or “very low-quality” supportive evidence. These are as follows:
• Elective colonic resection should not be done in patients with an initial episode of acute, uncomplicated diverticulitis; the decision to perform elective prophylactic colonic resection in this setting should be individualized.
• Patients with a history of diverticulitis should not be advised to avoid consumption of seeds, nuts, and popcorn.
• Patients with a history of diverticulitis should not be advised to avoid the use of aspirin.
• Patients with a history of diverticulitis should not be advised to avoid the use of nonselective nonsteroidal anti-inflammatory drugs.
• Do not advise the use of mesalamine after acute uncomplicated diverticulitis.
• Do not advise the use of rifaximin after acute uncomplicated diverticulitis.
• Do not advise the use of probiotics after acute uncomplicated diverticulitis.
“Acute diverticulitis is the third most common inpatient gastrointestinal diagnosis in the United States, costing over $2 billion annually, and is a common outpatient and emergency department diagnosis as well,” stated the AGA recommendations, which were developed by the AGA’s Clinical Guidelines Committee and approved by the AGA Institute Governing Board.
The statement notes that the condition “occurs in approximately 4% of patients with diverticulosis, roughly 15% of whom will have complicated disease, defined as an abscess, perforation, fistula or colon obstruction, and 15%-30% will experience recurrence.”
The new guidelines do not address other forms of diverticular disease, including symptomatic uncomplicated diverticular disease, diverticular bleeding, and segmental colitis associated with diverticulosis. It does not address the prevention of incident diverticulitis or the management of complicated disease.
Areas of further research, advises the AGA, should be in identifying patients who will benefit the most from antibiotics, identifying those in whom antibiotics can be withheld safely, identifying risk factors for recurrent diverticulitis, examining the risks of colonoscopy following acute diverticulitis, and taking a closer looks at anti-inflammatory drugs, antibiotics, probiotics, and dietary interventions as viable therapies.
Duodenoscopes often cultured high-concern organisms
High-concern organisms were cultured from 92% of duodenoscopes immediately after endoscopic retrograde cholangiopancreatography (ERCP), and from 17% of scopes after reprocessing, based on a small single-center study reported at an annual scientific meeting on infectious diseases.
“We think the high percentage of high-concern organisms is key to highlight, because it shows the importance of vigilant reprocessing techniques,” said Dr. Michaela Gazdik, who led the study at Intermountain Healthcare in Salt Lake City. A 17% prevalence of clinically significant residual contamination after reprocessing “aligns with reports from the literature, indicating that reprocessing protocols are likely effective,” she added.
But “no growth” on a quantitative plate does not necessarily mean there are no microbes on a duodenoscope, Dr. Gazdik emphasized. Swabbing the duodenoscope, and particularly the notoriously hard to clean elevator mechanism, could improve sampling, she said.
In 2013 and 2014, the FDA received reports of about 135 multidrug resistant infections acquired after ERCP. “After investigation, many of these outbreaks were attributed to the duodenoscope’s elevator mechanism,” Dr. Gazdik noted. The elevator mechanism contains microscopic crevices that can remain contaminated even after cleaning with a manual brush as recommended by manufacturers. To study the efficacy of their reprocessing system, Dr. Gazdik and her associates cultured 12 scopes immediately after ERCP and again after reprocessing. They also took conducted surveillance cultures of 11 scopes stored at other Intermountain Healthcare facilities.
Immediately after ERCP, 91.7% (11 of 12) scopes cultured out high-concern organisms, including Pseudomonas aeruginosa, Enterococcus species, Klebsiella pneumoniae, Enterobacter asburiae, and E. cloacae, said Dr. Gazdik. Three-quarters of the scopes also yielded gram-negative organisms. No organisms were resistant to carbapenem or vancomycin, based on testing with selective media.
After reprocessing, 17% of the scopes still yielded high-concern organisms, and 9% had more quantitative growth than desired, Dr. Gazdik reported. “We did tell our reprocessing departments that their protocols seem to be as effective as others out there,” she said. “But two scopes showed growth in broth that matched the pre-reprocessing growth, indicating it was left over after reprocessing. We thought that was an interesting result, because the interim CDC guidance does not include a swab portion.”
To culture the scopes, investigators flushed 5 mL of sterile saline through the elevator channel, brushed the cantilevered elevator mechanism in both the up and down positions, and rapidly swirled the brush in the flush saline. They performed quantitative colony counts by serially diluting the collected saline. For the swab-broth culture, they passed a sterile swab over and under the distal elevator mechanism and inoculating the swab into 5 mL tryptic soy broth. They also subcultured growth from the broth to identify bacteria.
Surveillance cultures of the 11 stored scopes identified no organisms from quantitative plate counts, but swab-inoculated broth cultures grew Micrococcus from three of 11 scopes, and non-CRE [carbapenem-resistant Enterobacteriaceae] K. pneumonia from one scope, Dr. Gazdik also reported. “Routine cultures play a role, mainly to continue heightened vigilance during reprocessing,” she said. “The sampling method we are going to be using will be simplified from the CDC procedure so that we can train endoscopy technicians to do it and then send samples to us.”
Dr. Gazdik and her coauthors reported no funding sources and had no conflicts of interest.
High-concern organisms were cultured from 92% of duodenoscopes immediately after endoscopic retrograde cholangiopancreatography (ERCP), and from 17% of scopes after reprocessing, based on a small single-center study reported at an annual scientific meeting on infectious diseases.
“We think the high percentage of high-concern organisms is key to highlight, because it shows the importance of vigilant reprocessing techniques,” said Dr. Michaela Gazdik, who led the study at Intermountain Healthcare in Salt Lake City. A 17% prevalence of clinically significant residual contamination after reprocessing “aligns with reports from the literature, indicating that reprocessing protocols are likely effective,” she added.
But “no growth” on a quantitative plate does not necessarily mean there are no microbes on a duodenoscope, Dr. Gazdik emphasized. Swabbing the duodenoscope, and particularly the notoriously hard to clean elevator mechanism, could improve sampling, she said.
In 2013 and 2014, the FDA received reports of about 135 multidrug resistant infections acquired after ERCP. “After investigation, many of these outbreaks were attributed to the duodenoscope’s elevator mechanism,” Dr. Gazdik noted. The elevator mechanism contains microscopic crevices that can remain contaminated even after cleaning with a manual brush as recommended by manufacturers. To study the efficacy of their reprocessing system, Dr. Gazdik and her associates cultured 12 scopes immediately after ERCP and again after reprocessing. They also took conducted surveillance cultures of 11 scopes stored at other Intermountain Healthcare facilities.
Immediately after ERCP, 91.7% (11 of 12) scopes cultured out high-concern organisms, including Pseudomonas aeruginosa, Enterococcus species, Klebsiella pneumoniae, Enterobacter asburiae, and E. cloacae, said Dr. Gazdik. Three-quarters of the scopes also yielded gram-negative organisms. No organisms were resistant to carbapenem or vancomycin, based on testing with selective media.
After reprocessing, 17% of the scopes still yielded high-concern organisms, and 9% had more quantitative growth than desired, Dr. Gazdik reported. “We did tell our reprocessing departments that their protocols seem to be as effective as others out there,” she said. “But two scopes showed growth in broth that matched the pre-reprocessing growth, indicating it was left over after reprocessing. We thought that was an interesting result, because the interim CDC guidance does not include a swab portion.”
To culture the scopes, investigators flushed 5 mL of sterile saline through the elevator channel, brushed the cantilevered elevator mechanism in both the up and down positions, and rapidly swirled the brush in the flush saline. They performed quantitative colony counts by serially diluting the collected saline. For the swab-broth culture, they passed a sterile swab over and under the distal elevator mechanism and inoculating the swab into 5 mL tryptic soy broth. They also subcultured growth from the broth to identify bacteria.
Surveillance cultures of the 11 stored scopes identified no organisms from quantitative plate counts, but swab-inoculated broth cultures grew Micrococcus from three of 11 scopes, and non-CRE [carbapenem-resistant Enterobacteriaceae] K. pneumonia from one scope, Dr. Gazdik also reported. “Routine cultures play a role, mainly to continue heightened vigilance during reprocessing,” she said. “The sampling method we are going to be using will be simplified from the CDC procedure so that we can train endoscopy technicians to do it and then send samples to us.”
Dr. Gazdik and her coauthors reported no funding sources and had no conflicts of interest.
High-concern organisms were cultured from 92% of duodenoscopes immediately after endoscopic retrograde cholangiopancreatography (ERCP), and from 17% of scopes after reprocessing, based on a small single-center study reported at an annual scientific meeting on infectious diseases.
“We think the high percentage of high-concern organisms is key to highlight, because it shows the importance of vigilant reprocessing techniques,” said Dr. Michaela Gazdik, who led the study at Intermountain Healthcare in Salt Lake City. A 17% prevalence of clinically significant residual contamination after reprocessing “aligns with reports from the literature, indicating that reprocessing protocols are likely effective,” she added.
But “no growth” on a quantitative plate does not necessarily mean there are no microbes on a duodenoscope, Dr. Gazdik emphasized. Swabbing the duodenoscope, and particularly the notoriously hard to clean elevator mechanism, could improve sampling, she said.
In 2013 and 2014, the FDA received reports of about 135 multidrug resistant infections acquired after ERCP. “After investigation, many of these outbreaks were attributed to the duodenoscope’s elevator mechanism,” Dr. Gazdik noted. The elevator mechanism contains microscopic crevices that can remain contaminated even after cleaning with a manual brush as recommended by manufacturers. To study the efficacy of their reprocessing system, Dr. Gazdik and her associates cultured 12 scopes immediately after ERCP and again after reprocessing. They also took conducted surveillance cultures of 11 scopes stored at other Intermountain Healthcare facilities.
Immediately after ERCP, 91.7% (11 of 12) scopes cultured out high-concern organisms, including Pseudomonas aeruginosa, Enterococcus species, Klebsiella pneumoniae, Enterobacter asburiae, and E. cloacae, said Dr. Gazdik. Three-quarters of the scopes also yielded gram-negative organisms. No organisms were resistant to carbapenem or vancomycin, based on testing with selective media.
After reprocessing, 17% of the scopes still yielded high-concern organisms, and 9% had more quantitative growth than desired, Dr. Gazdik reported. “We did tell our reprocessing departments that their protocols seem to be as effective as others out there,” she said. “But two scopes showed growth in broth that matched the pre-reprocessing growth, indicating it was left over after reprocessing. We thought that was an interesting result, because the interim CDC guidance does not include a swab portion.”
To culture the scopes, investigators flushed 5 mL of sterile saline through the elevator channel, brushed the cantilevered elevator mechanism in both the up and down positions, and rapidly swirled the brush in the flush saline. They performed quantitative colony counts by serially diluting the collected saline. For the swab-broth culture, they passed a sterile swab over and under the distal elevator mechanism and inoculating the swab into 5 mL tryptic soy broth. They also subcultured growth from the broth to identify bacteria.
Surveillance cultures of the 11 stored scopes identified no organisms from quantitative plate counts, but swab-inoculated broth cultures grew Micrococcus from three of 11 scopes, and non-CRE [carbapenem-resistant Enterobacteriaceae] K. pneumonia from one scope, Dr. Gazdik also reported. “Routine cultures play a role, mainly to continue heightened vigilance during reprocessing,” she said. “The sampling method we are going to be using will be simplified from the CDC procedure so that we can train endoscopy technicians to do it and then send samples to us.”
Dr. Gazdik and her coauthors reported no funding sources and had no conflicts of interest.
AT IDWEEK 2015
Key clinical point: High-concern organisms often contaminated duodenoscopes after ERCP.
Major finding: Investigators cultured these bacteria from 92% of duodenoscopes immediately after ERCP, and from 17% of scopes after reprocessing.
Data source: Cultures of 12 duodenoscopes immediately after ERCP and again after reprocessing, and surveillance cultures of 11 stored duodenoscopes.
Disclosures: Dr. Gazdik and her coauthors reported no funding sources and had no conflicts of interest.
No link found between IBS and serologic markers for celiac disease
Irritable bowel syndrome did not increase the likelihood of having serologic markers of celiac disease, according to a study of more than 3,000 residents of Southeastern Minnesota reported in the November issue of Clinical Gastroenterology and Hepatology.
Although several current guidelines list IBS as a risk factor for celiac disease, “our results suggest that testing for celiac disease [CD] in IBS will not have a significantly increased yield over population-based serologic screening,” said Dr. Rok Seon Choung of the Mayo Clinic, Rochester, Minn., and his associates. “In terms of IBS and other major GI syndromes, undetected CD does not appear to be positively associated with GI symptoms in the United States community.”
Despite widely available screening tests for CD, at least 80% of cases go undiagnosed. Testing based only on the presence of malabsorptive signs and symptoms misses many cases because of the trend toward “nonclassic” CD, said the researchers. “Physicians are especially likely to encounter patients with CD who have no classic symptoms while investigating other GI disorders,” they noted. “We aimed to determine whether positive results of serologic testing for CD by using a highly sensitive and specific assaywere associated with IBS and other functional gastrointestinal disorders in a large representative U.S. white population” (Clin Gastroenterol Hepatol. 2015 May doi: 10.1016/j.cgh.2015.05.014).
The investigators sent validated self-report bowel disease questionnaires to randomly chosen adults living in Olmsted County in Southeastern Minnesota. They also performed CD testing on serum from a convenience sample of 47,000 county residents with no prior diagnosis of CD. In all, 3,202 subjects completed questionnaires and had serum available for testing. About 55% of this group reported at least one GI symptom (95% confidence interval, 53%-57%), while 13.6% met criteria for IBS (95% CI, 12%-15%), the researchers said. A total of 1% of respondents had serologic markers for CD (95% CI, 0.7%-1.4%), in keeping with other epidemiologic studies in the United States, they added.
Notably, IBS affected only 3% of CD patients, compared with 14% of patients without CD, although the difference was not statistically significant (OR, 0.2; 95% CI, 0.03-1.5), the investigators said. Seropositive CD patients most often reported abdominal pain, constipation, weight loss, and dyspepsia, but none of these GI symptoms and no functional GI disorders were significantly more prevalent in CD patients than in non-CD patients. “These results may have important management and screening implications,” said the researchers. “Cost-effectiveness data suggest that testing for CD in patients with diarrhea-predominant IBS has an acceptable cost when the prevalence is above 1%, and becomes the dominant strategy when the prevalence exceeds 8%. However, we cannot confirm whether CD testing is a cost-effective approach in our population.”
The findings should be generalizable to white Americans, but not to the U.S. population as a whole because most participants were white, the researchers noted. “The prevalence of CD may vary by ethnic group, but the disease has been shown to be more common in whites than in other races,” they added. Responder bias was also possible, but past studies of the same bowel disease questionnaire uncovered no significant differences in rates of GI symptoms between responders and nonresponders, they noted.
The National Institutes of Health funded part of the work. Coauthor Dr. Nicholas Talley reported having colicensed the questionnaire used in the study. The remaining authors disclosed no financial conflicts.
In the well-designed and rigorous study by Choung et al., the authors conducted a community-based, cross-sectional survey among residents of Olmsted County, Minn., collecting data on symptoms compatible with functional GI disorders, including irritable bowel syndrome; the authors linked these data to prevalence surveys testing for undiagnosed celiac disease using serologic tests conducted among more than 47,000 individuals within the same regio
 |
| Dr. Alexander Ford |
Patients with celiac disease may present with GI symptoms such as abdominal pain, bloating, and diarrhea, leading to confusion with IBS and diagnostic delay. Current guidelines, therefore, recommend screening patients consulting with IBS-type symptoms routinely for celiac disease. Despite this, in the study only 3% of individuals with positive celiac serology met the criteria for IBS, compared with 14% of those testing negative. Also of note is that subjects with positive serology were no more likely to report other GI symptoms felt to be typical presenting features of celiac disease, including abdominal pain, diarrhea, bloating, or abdominal distension. This suggests the yield of opportunistic screening of people reporting GI symptoms in the U.S. community is low.
However, current guidelines do not recommend screening people with IBS for celiac disease in the general population, and based their recommendations on studies conducted among patients consulting with GI symptoms. As a result, although the authors concluded, justifiably, that testing in the community is unlikely to have a significantly increased yield over population-based screening, it should not lead to a change in recommendations for practice in either primary or secondary care in other countries.
Dr. Alexander C. Ford is associate professor and honorary consultant gastroenterologist at Leeds Gastroenterology Institute, St. James’s University Hospital, and Leeds (England) Institute of Biomedical and Clinical Sciences, University of Leeds. He had no relevant financial conflicts of interest.
In the well-designed and rigorous study by Choung et al., the authors conducted a community-based, cross-sectional survey among residents of Olmsted County, Minn., collecting data on symptoms compatible with functional GI disorders, including irritable bowel syndrome; the authors linked these data to prevalence surveys testing for undiagnosed celiac disease using serologic tests conducted among more than 47,000 individuals within the same regio
 |
| Dr. Alexander Ford |
Patients with celiac disease may present with GI symptoms such as abdominal pain, bloating, and diarrhea, leading to confusion with IBS and diagnostic delay. Current guidelines, therefore, recommend screening patients consulting with IBS-type symptoms routinely for celiac disease. Despite this, in the study only 3% of individuals with positive celiac serology met the criteria for IBS, compared with 14% of those testing negative. Also of note is that subjects with positive serology were no more likely to report other GI symptoms felt to be typical presenting features of celiac disease, including abdominal pain, diarrhea, bloating, or abdominal distension. This suggests the yield of opportunistic screening of people reporting GI symptoms in the U.S. community is low.
However, current guidelines do not recommend screening people with IBS for celiac disease in the general population, and based their recommendations on studies conducted among patients consulting with GI symptoms. As a result, although the authors concluded, justifiably, that testing in the community is unlikely to have a significantly increased yield over population-based screening, it should not lead to a change in recommendations for practice in either primary or secondary care in other countries.
Dr. Alexander C. Ford is associate professor and honorary consultant gastroenterologist at Leeds Gastroenterology Institute, St. James’s University Hospital, and Leeds (England) Institute of Biomedical and Clinical Sciences, University of Leeds. He had no relevant financial conflicts of interest.
In the well-designed and rigorous study by Choung et al., the authors conducted a community-based, cross-sectional survey among residents of Olmsted County, Minn., collecting data on symptoms compatible with functional GI disorders, including irritable bowel syndrome; the authors linked these data to prevalence surveys testing for undiagnosed celiac disease using serologic tests conducted among more than 47,000 individuals within the same regio
 |
| Dr. Alexander Ford |
Patients with celiac disease may present with GI symptoms such as abdominal pain, bloating, and diarrhea, leading to confusion with IBS and diagnostic delay. Current guidelines, therefore, recommend screening patients consulting with IBS-type symptoms routinely for celiac disease. Despite this, in the study only 3% of individuals with positive celiac serology met the criteria for IBS, compared with 14% of those testing negative. Also of note is that subjects with positive serology were no more likely to report other GI symptoms felt to be typical presenting features of celiac disease, including abdominal pain, diarrhea, bloating, or abdominal distension. This suggests the yield of opportunistic screening of people reporting GI symptoms in the U.S. community is low.
However, current guidelines do not recommend screening people with IBS for celiac disease in the general population, and based their recommendations on studies conducted among patients consulting with GI symptoms. As a result, although the authors concluded, justifiably, that testing in the community is unlikely to have a significantly increased yield over population-based screening, it should not lead to a change in recommendations for practice in either primary or secondary care in other countries.
Dr. Alexander C. Ford is associate professor and honorary consultant gastroenterologist at Leeds Gastroenterology Institute, St. James’s University Hospital, and Leeds (England) Institute of Biomedical and Clinical Sciences, University of Leeds. He had no relevant financial conflicts of interest.
Irritable bowel syndrome did not increase the likelihood of having serologic markers of celiac disease, according to a study of more than 3,000 residents of Southeastern Minnesota reported in the November issue of Clinical Gastroenterology and Hepatology.
Although several current guidelines list IBS as a risk factor for celiac disease, “our results suggest that testing for celiac disease [CD] in IBS will not have a significantly increased yield over population-based serologic screening,” said Dr. Rok Seon Choung of the Mayo Clinic, Rochester, Minn., and his associates. “In terms of IBS and other major GI syndromes, undetected CD does not appear to be positively associated with GI symptoms in the United States community.”
Despite widely available screening tests for CD, at least 80% of cases go undiagnosed. Testing based only on the presence of malabsorptive signs and symptoms misses many cases because of the trend toward “nonclassic” CD, said the researchers. “Physicians are especially likely to encounter patients with CD who have no classic symptoms while investigating other GI disorders,” they noted. “We aimed to determine whether positive results of serologic testing for CD by using a highly sensitive and specific assaywere associated with IBS and other functional gastrointestinal disorders in a large representative U.S. white population” (Clin Gastroenterol Hepatol. 2015 May doi: 10.1016/j.cgh.2015.05.014).
The investigators sent validated self-report bowel disease questionnaires to randomly chosen adults living in Olmsted County in Southeastern Minnesota. They also performed CD testing on serum from a convenience sample of 47,000 county residents with no prior diagnosis of CD. In all, 3,202 subjects completed questionnaires and had serum available for testing. About 55% of this group reported at least one GI symptom (95% confidence interval, 53%-57%), while 13.6% met criteria for IBS (95% CI, 12%-15%), the researchers said. A total of 1% of respondents had serologic markers for CD (95% CI, 0.7%-1.4%), in keeping with other epidemiologic studies in the United States, they added.
Notably, IBS affected only 3% of CD patients, compared with 14% of patients without CD, although the difference was not statistically significant (OR, 0.2; 95% CI, 0.03-1.5), the investigators said. Seropositive CD patients most often reported abdominal pain, constipation, weight loss, and dyspepsia, but none of these GI symptoms and no functional GI disorders were significantly more prevalent in CD patients than in non-CD patients. “These results may have important management and screening implications,” said the researchers. “Cost-effectiveness data suggest that testing for CD in patients with diarrhea-predominant IBS has an acceptable cost when the prevalence is above 1%, and becomes the dominant strategy when the prevalence exceeds 8%. However, we cannot confirm whether CD testing is a cost-effective approach in our population.”
The findings should be generalizable to white Americans, but not to the U.S. population as a whole because most participants were white, the researchers noted. “The prevalence of CD may vary by ethnic group, but the disease has been shown to be more common in whites than in other races,” they added. Responder bias was also possible, but past studies of the same bowel disease questionnaire uncovered no significant differences in rates of GI symptoms between responders and nonresponders, they noted.
The National Institutes of Health funded part of the work. Coauthor Dr. Nicholas Talley reported having colicensed the questionnaire used in the study. The remaining authors disclosed no financial conflicts.
Irritable bowel syndrome did not increase the likelihood of having serologic markers of celiac disease, according to a study of more than 3,000 residents of Southeastern Minnesota reported in the November issue of Clinical Gastroenterology and Hepatology.
Although several current guidelines list IBS as a risk factor for celiac disease, “our results suggest that testing for celiac disease [CD] in IBS will not have a significantly increased yield over population-based serologic screening,” said Dr. Rok Seon Choung of the Mayo Clinic, Rochester, Minn., and his associates. “In terms of IBS and other major GI syndromes, undetected CD does not appear to be positively associated with GI symptoms in the United States community.”
Despite widely available screening tests for CD, at least 80% of cases go undiagnosed. Testing based only on the presence of malabsorptive signs and symptoms misses many cases because of the trend toward “nonclassic” CD, said the researchers. “Physicians are especially likely to encounter patients with CD who have no classic symptoms while investigating other GI disorders,” they noted. “We aimed to determine whether positive results of serologic testing for CD by using a highly sensitive and specific assaywere associated with IBS and other functional gastrointestinal disorders in a large representative U.S. white population” (Clin Gastroenterol Hepatol. 2015 May doi: 10.1016/j.cgh.2015.05.014).
The investigators sent validated self-report bowel disease questionnaires to randomly chosen adults living in Olmsted County in Southeastern Minnesota. They also performed CD testing on serum from a convenience sample of 47,000 county residents with no prior diagnosis of CD. In all, 3,202 subjects completed questionnaires and had serum available for testing. About 55% of this group reported at least one GI symptom (95% confidence interval, 53%-57%), while 13.6% met criteria for IBS (95% CI, 12%-15%), the researchers said. A total of 1% of respondents had serologic markers for CD (95% CI, 0.7%-1.4%), in keeping with other epidemiologic studies in the United States, they added.
Notably, IBS affected only 3% of CD patients, compared with 14% of patients without CD, although the difference was not statistically significant (OR, 0.2; 95% CI, 0.03-1.5), the investigators said. Seropositive CD patients most often reported abdominal pain, constipation, weight loss, and dyspepsia, but none of these GI symptoms and no functional GI disorders were significantly more prevalent in CD patients than in non-CD patients. “These results may have important management and screening implications,” said the researchers. “Cost-effectiveness data suggest that testing for CD in patients with diarrhea-predominant IBS has an acceptable cost when the prevalence is above 1%, and becomes the dominant strategy when the prevalence exceeds 8%. However, we cannot confirm whether CD testing is a cost-effective approach in our population.”
The findings should be generalizable to white Americans, but not to the U.S. population as a whole because most participants were white, the researchers noted. “The prevalence of CD may vary by ethnic group, but the disease has been shown to be more common in whites than in other races,” they added. Responder bias was also possible, but past studies of the same bowel disease questionnaire uncovered no significant differences in rates of GI symptoms between responders and nonresponders, they noted.
The National Institutes of Health funded part of the work. Coauthor Dr. Nicholas Talley reported having colicensed the questionnaire used in the study. The remaining authors disclosed no financial conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Irritable bowel syndrome did not increase the likelihood of seropositivity for celiac disease.
Major finding: Patients with IBS were no more likely than others to have serologic markers for celiac disease (odds ratio, 0.2; 95% confidence interval, 0.03-1.5).
Data source: An analysis of bowel symptom surveys and serum samples from 3,202 residents of one county.
Disclosures: The National Institutes of Health funded part of the work. Coauthor Dr. Nicholas Talley reported having colicensed the questionnaire used in the study. The remaining authors disclosed no conflicts.
Low-FODMAP and traditional IBS diets found equally effective for symptom reduction
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
FROM GASTROENTEROLOGY
Key clinical point: Diets low in fermentable short-chain carbohydrates cut irritable bowel disease symptoms as effectively as did “traditional” IBS diets.
Major finding: After 4 weeks, patients in both groups experienced similar and significant (P < .0001) decreases in IBS symptoms.
Data source: A randomized, multicenter, parallel-group, single-blinded study of 75 patients.
Disclosures: The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Low-FODMAP and traditional IBS diets found equally effective for symptom reduction
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
FROM GASTROENTEROLOGY
Key clinical point: Diets low in fermentable short-chain carbohydrates cut irritable bowel disease symptoms as effectively as did “traditional” IBS diets.
Major finding: After 4 weeks, patients in both groups experienced similar and significant (P < .0001) decreases in IBS symptoms.
Data source: A randomized, multicenter, parallel-group, single-blinded study of 75 patients.
Disclosures: The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
No link found between immunosuppression and anal dysplasia in IBD
Immunosuppression did not affect the probability of abnormal anal cytology among patients with inflammatory bowel disease (IBD), according to a prospective, single-center, cross-sectional study of 270 adults published in the November issue of Clinical Gastroenterology and Hepatology.
“Although our study suggested that immunosuppression may not play a role in the risk of dysplasia, a question remains as to whether it contributes to malignant transformation in patients with dysplasia. More research needs to be performed to identify the utility of wider anal dysplasia screening programs in high-risk populations, and the role of HPV vaccine in prevention,” Dr. Shamita Shah of Stanford (Calif.) University and her associates wrote in Clinical Gastroenterology and Hepatology.
Immunosuppression is a cornerstone of IBD management. Because immunosuppressive medications inhibit cell-mediated immunity, patients are at increased risk of opportunistic infections and neoplasias, the researchers noted. One study reported a greater risk of cervical dysplasia among immunosuppressed women with IBD, but the risk of anal dysplasia and cancer in IBD has not been well studied, they said (Clinical Gasteroenterol Hepatol. 2015. doi: 10.1016/j.cgh.2015.05.031).
To examine associations between anal dysplasia and IBD, human papillomavirus infection, and immunosuppression, the researchers analyzed anal Pap tests from 100 IBD patients who were immunosuppressed, 94 IBD patients who were not immunosuppressed, and 76 healthy controls. They identified 19 cases of atypical squamous cells of undetermined significance (ASCUS). The prevalence of ASCUS was somewhat higher among IBD patients (8.8%) than controls (2.6%; P = 0.1), but did not vary based on immunosuppression status or HPV infection. High-risk HPV occurred in 2% of the entire cohort, including 11% of patients with ASCUS and 1.5% of patients with normal Pap cytology (P = .01). High-resolution anoscopy of six patients with ASCUS revealed two cases of condylomatous disease, but no biopsy-positive dysplasia, the investigators reported.
Patients with Crohn’s disease had a significantly higher prevalence of ASCUS than other study participants (P = .02), but patients with ulcerative colitis or unspecified IBD did not, said the researchers. Having had Crohn’s disease for at least 10 years was associated with a fivefold increase in the odds of ASCUS in the multivariate analysis (95% confidence interval, 1.9-13.6), and female sex was also a risk factor (odds ratio, 3.3; P = .047). Notably, women with long-standing Crohn’s disease were almost five times more likely to have abnormal anal Pap cytology than other subjects (P = .0038), the investigators said. “One proposed mechanism for this was a reduction in human defensins in Crohn’s disease patients,” they added. Defensins – antiviral proteins that are found in immune cells – are known to inhibit cutaneous and mucosal HPV, and can be decreased in patients with Crohn’s disease for several reasons, they said. “Although more research is needed in this area, the hindered ability for an IBD patient to defend against viral illness may be responsible for their susceptibility to HPV-related cervical and anal neoplasias.”
Hologic donated the anal Pap tests used in the study. The researchers disclosed no conflicts of interest.
Immunosuppression did not affect the probability of abnormal anal cytology among patients with inflammatory bowel disease (IBD), according to a prospective, single-center, cross-sectional study of 270 adults published in the November issue of Clinical Gastroenterology and Hepatology.
“Although our study suggested that immunosuppression may not play a role in the risk of dysplasia, a question remains as to whether it contributes to malignant transformation in patients with dysplasia. More research needs to be performed to identify the utility of wider anal dysplasia screening programs in high-risk populations, and the role of HPV vaccine in prevention,” Dr. Shamita Shah of Stanford (Calif.) University and her associates wrote in Clinical Gastroenterology and Hepatology.
Immunosuppression is a cornerstone of IBD management. Because immunosuppressive medications inhibit cell-mediated immunity, patients are at increased risk of opportunistic infections and neoplasias, the researchers noted. One study reported a greater risk of cervical dysplasia among immunosuppressed women with IBD, but the risk of anal dysplasia and cancer in IBD has not been well studied, they said (Clinical Gasteroenterol Hepatol. 2015. doi: 10.1016/j.cgh.2015.05.031).
To examine associations between anal dysplasia and IBD, human papillomavirus infection, and immunosuppression, the researchers analyzed anal Pap tests from 100 IBD patients who were immunosuppressed, 94 IBD patients who were not immunosuppressed, and 76 healthy controls. They identified 19 cases of atypical squamous cells of undetermined significance (ASCUS). The prevalence of ASCUS was somewhat higher among IBD patients (8.8%) than controls (2.6%; P = 0.1), but did not vary based on immunosuppression status or HPV infection. High-risk HPV occurred in 2% of the entire cohort, including 11% of patients with ASCUS and 1.5% of patients with normal Pap cytology (P = .01). High-resolution anoscopy of six patients with ASCUS revealed two cases of condylomatous disease, but no biopsy-positive dysplasia, the investigators reported.
Patients with Crohn’s disease had a significantly higher prevalence of ASCUS than other study participants (P = .02), but patients with ulcerative colitis or unspecified IBD did not, said the researchers. Having had Crohn’s disease for at least 10 years was associated with a fivefold increase in the odds of ASCUS in the multivariate analysis (95% confidence interval, 1.9-13.6), and female sex was also a risk factor (odds ratio, 3.3; P = .047). Notably, women with long-standing Crohn’s disease were almost five times more likely to have abnormal anal Pap cytology than other subjects (P = .0038), the investigators said. “One proposed mechanism for this was a reduction in human defensins in Crohn’s disease patients,” they added. Defensins – antiviral proteins that are found in immune cells – are known to inhibit cutaneous and mucosal HPV, and can be decreased in patients with Crohn’s disease for several reasons, they said. “Although more research is needed in this area, the hindered ability for an IBD patient to defend against viral illness may be responsible for their susceptibility to HPV-related cervical and anal neoplasias.”
Hologic donated the anal Pap tests used in the study. The researchers disclosed no conflicts of interest.
Immunosuppression did not affect the probability of abnormal anal cytology among patients with inflammatory bowel disease (IBD), according to a prospective, single-center, cross-sectional study of 270 adults published in the November issue of Clinical Gastroenterology and Hepatology.
“Although our study suggested that immunosuppression may not play a role in the risk of dysplasia, a question remains as to whether it contributes to malignant transformation in patients with dysplasia. More research needs to be performed to identify the utility of wider anal dysplasia screening programs in high-risk populations, and the role of HPV vaccine in prevention,” Dr. Shamita Shah of Stanford (Calif.) University and her associates wrote in Clinical Gastroenterology and Hepatology.
Immunosuppression is a cornerstone of IBD management. Because immunosuppressive medications inhibit cell-mediated immunity, patients are at increased risk of opportunistic infections and neoplasias, the researchers noted. One study reported a greater risk of cervical dysplasia among immunosuppressed women with IBD, but the risk of anal dysplasia and cancer in IBD has not been well studied, they said (Clinical Gasteroenterol Hepatol. 2015. doi: 10.1016/j.cgh.2015.05.031).
To examine associations between anal dysplasia and IBD, human papillomavirus infection, and immunosuppression, the researchers analyzed anal Pap tests from 100 IBD patients who were immunosuppressed, 94 IBD patients who were not immunosuppressed, and 76 healthy controls. They identified 19 cases of atypical squamous cells of undetermined significance (ASCUS). The prevalence of ASCUS was somewhat higher among IBD patients (8.8%) than controls (2.6%; P = 0.1), but did not vary based on immunosuppression status or HPV infection. High-risk HPV occurred in 2% of the entire cohort, including 11% of patients with ASCUS and 1.5% of patients with normal Pap cytology (P = .01). High-resolution anoscopy of six patients with ASCUS revealed two cases of condylomatous disease, but no biopsy-positive dysplasia, the investigators reported.
Patients with Crohn’s disease had a significantly higher prevalence of ASCUS than other study participants (P = .02), but patients with ulcerative colitis or unspecified IBD did not, said the researchers. Having had Crohn’s disease for at least 10 years was associated with a fivefold increase in the odds of ASCUS in the multivariate analysis (95% confidence interval, 1.9-13.6), and female sex was also a risk factor (odds ratio, 3.3; P = .047). Notably, women with long-standing Crohn’s disease were almost five times more likely to have abnormal anal Pap cytology than other subjects (P = .0038), the investigators said. “One proposed mechanism for this was a reduction in human defensins in Crohn’s disease patients,” they added. Defensins – antiviral proteins that are found in immune cells – are known to inhibit cutaneous and mucosal HPV, and can be decreased in patients with Crohn’s disease for several reasons, they said. “Although more research is needed in this area, the hindered ability for an IBD patient to defend against viral illness may be responsible for their susceptibility to HPV-related cervical and anal neoplasias.”
Hologic donated the anal Pap tests used in the study. The researchers disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Immunosuppression did not affect the probability of abnormal anal cytology among patients with inflammatory bowel disease.
Major finding: Almost 9% of patients had atypical squamous cells on anal Pap testing, with no difference in prevalence based on immunosuppression.
Data source: A prospective, single-center, cross-sectional study of 270 subjects.
Disclosures: Hologic donated the anal Pap tests used in the study. The researchers disclosed no conflicts of interest.
VIDEO: Expert outlines new thinking on irritable bowel syndrome
HONOLULU – New medications and more patient-focused strategies are changing the treatment of irritable bowel syndrome, as physicians move away from simply treating symptoms and gain a better understanding of IBS processes and mechanisms of action.
“I think there’s kind of an explosion in medications for functional bowel disease, specifically irritable bowel syndrome, over the last couple years, because we’ve gotten a lot smarter about how we think about these patients and how we treat these patients,” explained Dr. Darren M. Brenner, director of the functional bowel program at Northwestern University, Chicago.
In an interview at the annual meeting of the American College of Gastroenterology, Dr. Brenner discussed new approaches to target the syndrome’s mechanisms of action, new thinking by the Food and Drug Administration on IBS drug approval, and the wide range of traditional, complementary and alternative, and diet-related therapeutic options now available to patients.
HONOLULU – New medications and more patient-focused strategies are changing the treatment of irritable bowel syndrome, as physicians move away from simply treating symptoms and gain a better understanding of IBS processes and mechanisms of action.
“I think there’s kind of an explosion in medications for functional bowel disease, specifically irritable bowel syndrome, over the last couple years, because we’ve gotten a lot smarter about how we think about these patients and how we treat these patients,” explained Dr. Darren M. Brenner, director of the functional bowel program at Northwestern University, Chicago.
In an interview at the annual meeting of the American College of Gastroenterology, Dr. Brenner discussed new approaches to target the syndrome’s mechanisms of action, new thinking by the Food and Drug Administration on IBS drug approval, and the wide range of traditional, complementary and alternative, and diet-related therapeutic options now available to patients.
HONOLULU – New medications and more patient-focused strategies are changing the treatment of irritable bowel syndrome, as physicians move away from simply treating symptoms and gain a better understanding of IBS processes and mechanisms of action.
“I think there’s kind of an explosion in medications for functional bowel disease, specifically irritable bowel syndrome, over the last couple years, because we’ve gotten a lot smarter about how we think about these patients and how we treat these patients,” explained Dr. Darren M. Brenner, director of the functional bowel program at Northwestern University, Chicago.
In an interview at the annual meeting of the American College of Gastroenterology, Dr. Brenner discussed new approaches to target the syndrome’s mechanisms of action, new thinking by the Food and Drug Administration on IBS drug approval, and the wide range of traditional, complementary and alternative, and diet-related therapeutic options now available to patients.
AT ACG 2015
VIDEO: Serum amyloid A could be IBD biomarker
HONOLULU – Serum amyloid A levels may offer an effective biomarker to gauge disease activity in inflammatory bowel disease, while baseline fecal calprotectin levels could help predict how patients with IBD will respond to anti-tumor necrosis factor therapy, according to two studies presented at the annual meeting of the American College of Gastroenterology.
“There’s a really high need for biomarkers in order to have a better assessment and better control of disease activity, before treatment and then throughout therapy,” explained the studies’ lead author, Dr. Andres Yarur of the University of Chicago.
In an interview at the meeting, Dr. Yarur discussed the two studies’ results, and how serum amyloid A levels, in combination with C-reactive protein levels, may improve clinicians’ ability to identify active endoscopic disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HONOLULU – Serum amyloid A levels may offer an effective biomarker to gauge disease activity in inflammatory bowel disease, while baseline fecal calprotectin levels could help predict how patients with IBD will respond to anti-tumor necrosis factor therapy, according to two studies presented at the annual meeting of the American College of Gastroenterology.
“There’s a really high need for biomarkers in order to have a better assessment and better control of disease activity, before treatment and then throughout therapy,” explained the studies’ lead author, Dr. Andres Yarur of the University of Chicago.
In an interview at the meeting, Dr. Yarur discussed the two studies’ results, and how serum amyloid A levels, in combination with C-reactive protein levels, may improve clinicians’ ability to identify active endoscopic disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HONOLULU – Serum amyloid A levels may offer an effective biomarker to gauge disease activity in inflammatory bowel disease, while baseline fecal calprotectin levels could help predict how patients with IBD will respond to anti-tumor necrosis factor therapy, according to two studies presented at the annual meeting of the American College of Gastroenterology.
“There’s a really high need for biomarkers in order to have a better assessment and better control of disease activity, before treatment and then throughout therapy,” explained the studies’ lead author, Dr. Andres Yarur of the University of Chicago.
In an interview at the meeting, Dr. Yarur discussed the two studies’ results, and how serum amyloid A levels, in combination with C-reactive protein levels, may improve clinicians’ ability to identify active endoscopic disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACG 2015
VIDEO: Gut microbiota may predict C. diff treatment response
HONOLULU – Could gut microbiota be a better predictor than clinical factors of a patient’s response to treatment for Clostridium difficile infection?
In a study of 88 patients with C. difficile, the overall treatment failure rate was 12.5% – but clinical factors such as age, sex, ongoing antibiotic exposure, and hospitalization status failed to predict which patients wouldn’t respond to treatment.
So, “we aimed to identify if there are any gut microbiota signatures to predict treatment response and treatment failure,” explained the study’s lead author, Dr. Sahil Khanna of the Mayo Clinic, Rochester, Minn.
In an interview at the annual meeting of the American College of Gastroenterology, Dr. Khanna discussed the study results and why gut microbiota may be an effective predictor of treatment responders and nonresponders.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HONOLULU – Could gut microbiota be a better predictor than clinical factors of a patient’s response to treatment for Clostridium difficile infection?
In a study of 88 patients with C. difficile, the overall treatment failure rate was 12.5% – but clinical factors such as age, sex, ongoing antibiotic exposure, and hospitalization status failed to predict which patients wouldn’t respond to treatment.
So, “we aimed to identify if there are any gut microbiota signatures to predict treatment response and treatment failure,” explained the study’s lead author, Dr. Sahil Khanna of the Mayo Clinic, Rochester, Minn.
In an interview at the annual meeting of the American College of Gastroenterology, Dr. Khanna discussed the study results and why gut microbiota may be an effective predictor of treatment responders and nonresponders.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HONOLULU – Could gut microbiota be a better predictor than clinical factors of a patient’s response to treatment for Clostridium difficile infection?
In a study of 88 patients with C. difficile, the overall treatment failure rate was 12.5% – but clinical factors such as age, sex, ongoing antibiotic exposure, and hospitalization status failed to predict which patients wouldn’t respond to treatment.
So, “we aimed to identify if there are any gut microbiota signatures to predict treatment response and treatment failure,” explained the study’s lead author, Dr. Sahil Khanna of the Mayo Clinic, Rochester, Minn.
In an interview at the annual meeting of the American College of Gastroenterology, Dr. Khanna discussed the study results and why gut microbiota may be an effective predictor of treatment responders and nonresponders.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At ACG 2015
New targeted Crohn’s therapy performs well in phase III trial
HONOLULU – Ustekinumab, a monoclonal antibody targeted against interleukins 12 and 23 (IL-12 and IL-23), met its primary endpoint for control of Crohn’s disease in a multinational phase III trial presented at the annual meeting of the American College of Gastroenterology.
The trial, called UNITI-2, enrolled patients with moderate to severe Crohn’s disease who had failed traditional therapies but were naive to or at least had not failed a tumor necrosis factor (TNF) inhibitor, reported Dr. Brian Feagan, professor of medicine, University of Western Ontario, London. Results of a second and parallel phase III trial with ustekinumab, called UNITI-1, which enrolled TNF inhibitor failures, have not yet been reported.
In UNITI-2, 628 patients were randomized to placebo, 130 mg of ustekinumab in a fixed subcutaneous dose of 130 mg, or a weight-based dose of 6 mg/kg of subcutaneous ustekinumab. Major enrollment criteria, other than failure of a non-TNF inhibitor therapy, included a Crohn’s disease activity index (CDAI) score between 220 and 450. The primary endpoint was a CDAI reduction of at least 100 points at 6 weeks. Clinical remission at 8 weeks, defined as CDAI less than 150, was a secondary endpoint.
The primary endpoint was reached by 28.7% randomized to placebo, 51.7% of those randomized to the fixed dose of ustekinumab, and 55.5% of those randomized to weight-based dosing. The advantage for the active treatment arms was statistically significant (both P less than .001). For the secondary endpoint of clinical remission at 8 weeks, the rates were 19.6% for placebo, 30.6% (P = .009 vs. placebo) for fixed-dose ustekinumab, and 40.2% (P less than .001 vs. placebo) for the weight-based dose.
The anti-inflammatory effect of ustekinumab, which inhibits signal transduction of IL-12 and IL-23 by binding to the p40 subunit that both cytokines employ for receptor binding, was reflected in the rapid reduction in C-reactive protein (CRP) concentrations observed with both doses but not with placebo, according to Dr. Feagan. He noted that the greater CRP reduction on the weight-based dosing, which nearly doubles ustekinumab exposure for some individuals, supports the dose-response anti-inflammatory mechanism of the drug.
Ustekinumab was well tolerated with similar rates and types of adverse events reported in the active treatment and placebo groups. This included infections, serious infections, and infusion reactions. There was a theoretical concern about an increased risk for cardiovascular events that was not substantiated in this study. No malignancies were observed in this short-term study.
Nearly 70% of the patients were naive to a biologic treatment. Of the 31.4% with prior exposure to a TNF inhibitor, none had met criteria for failure. About 80% had failed corticosteroids after what was considered to be an adequate course, while nearly 70% had failed one or more immunomodulators. About half had failed both.
Details about quality of life measures were not presented at the ACG, but Dr. Feagan said, summarizing the data, they “also confirmed the clinical efficacy of ustekinumab,” which was approved for the treatment of psoriatic arthritis more than 2 years ago.
The data, which suggest a level of efficacy comparable to other targeted therapies in Crohn’s disease, appear to encourage a filing for Food and Drug Administration approval in Crohn’s disease. However, data about the relative efficacy of this drug in those who have failed a TNF inhibitor is expected to provide greater insight about how this agent will add to current options.
“It is encouraging to see disease control with agents targeting novel cytokines in the inflammatory pathway, but we need to begin thinking about how these biologics fit with each other,” said Dr. David Rubin, chief, section of gastroenterology, hepatology, and nutrition, University of Chicago. Dr. Rubin was a moderator of the ACG session in which the UNITI-2 data were presented.
Dr. Feagan has financial relationships with Millennium, Merck, Centocor, Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, and many other pharmaceutical companies.
HONOLULU – Ustekinumab, a monoclonal antibody targeted against interleukins 12 and 23 (IL-12 and IL-23), met its primary endpoint for control of Crohn’s disease in a multinational phase III trial presented at the annual meeting of the American College of Gastroenterology.
The trial, called UNITI-2, enrolled patients with moderate to severe Crohn’s disease who had failed traditional therapies but were naive to or at least had not failed a tumor necrosis factor (TNF) inhibitor, reported Dr. Brian Feagan, professor of medicine, University of Western Ontario, London. Results of a second and parallel phase III trial with ustekinumab, called UNITI-1, which enrolled TNF inhibitor failures, have not yet been reported.
In UNITI-2, 628 patients were randomized to placebo, 130 mg of ustekinumab in a fixed subcutaneous dose of 130 mg, or a weight-based dose of 6 mg/kg of subcutaneous ustekinumab. Major enrollment criteria, other than failure of a non-TNF inhibitor therapy, included a Crohn’s disease activity index (CDAI) score between 220 and 450. The primary endpoint was a CDAI reduction of at least 100 points at 6 weeks. Clinical remission at 8 weeks, defined as CDAI less than 150, was a secondary endpoint.
The primary endpoint was reached by 28.7% randomized to placebo, 51.7% of those randomized to the fixed dose of ustekinumab, and 55.5% of those randomized to weight-based dosing. The advantage for the active treatment arms was statistically significant (both P less than .001). For the secondary endpoint of clinical remission at 8 weeks, the rates were 19.6% for placebo, 30.6% (P = .009 vs. placebo) for fixed-dose ustekinumab, and 40.2% (P less than .001 vs. placebo) for the weight-based dose.
The anti-inflammatory effect of ustekinumab, which inhibits signal transduction of IL-12 and IL-23 by binding to the p40 subunit that both cytokines employ for receptor binding, was reflected in the rapid reduction in C-reactive protein (CRP) concentrations observed with both doses but not with placebo, according to Dr. Feagan. He noted that the greater CRP reduction on the weight-based dosing, which nearly doubles ustekinumab exposure for some individuals, supports the dose-response anti-inflammatory mechanism of the drug.
Ustekinumab was well tolerated with similar rates and types of adverse events reported in the active treatment and placebo groups. This included infections, serious infections, and infusion reactions. There was a theoretical concern about an increased risk for cardiovascular events that was not substantiated in this study. No malignancies were observed in this short-term study.
Nearly 70% of the patients were naive to a biologic treatment. Of the 31.4% with prior exposure to a TNF inhibitor, none had met criteria for failure. About 80% had failed corticosteroids after what was considered to be an adequate course, while nearly 70% had failed one or more immunomodulators. About half had failed both.
Details about quality of life measures were not presented at the ACG, but Dr. Feagan said, summarizing the data, they “also confirmed the clinical efficacy of ustekinumab,” which was approved for the treatment of psoriatic arthritis more than 2 years ago.
The data, which suggest a level of efficacy comparable to other targeted therapies in Crohn’s disease, appear to encourage a filing for Food and Drug Administration approval in Crohn’s disease. However, data about the relative efficacy of this drug in those who have failed a TNF inhibitor is expected to provide greater insight about how this agent will add to current options.
“It is encouraging to see disease control with agents targeting novel cytokines in the inflammatory pathway, but we need to begin thinking about how these biologics fit with each other,” said Dr. David Rubin, chief, section of gastroenterology, hepatology, and nutrition, University of Chicago. Dr. Rubin was a moderator of the ACG session in which the UNITI-2 data were presented.
Dr. Feagan has financial relationships with Millennium, Merck, Centocor, Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, and many other pharmaceutical companies.
HONOLULU – Ustekinumab, a monoclonal antibody targeted against interleukins 12 and 23 (IL-12 and IL-23), met its primary endpoint for control of Crohn’s disease in a multinational phase III trial presented at the annual meeting of the American College of Gastroenterology.
The trial, called UNITI-2, enrolled patients with moderate to severe Crohn’s disease who had failed traditional therapies but were naive to or at least had not failed a tumor necrosis factor (TNF) inhibitor, reported Dr. Brian Feagan, professor of medicine, University of Western Ontario, London. Results of a second and parallel phase III trial with ustekinumab, called UNITI-1, which enrolled TNF inhibitor failures, have not yet been reported.
In UNITI-2, 628 patients were randomized to placebo, 130 mg of ustekinumab in a fixed subcutaneous dose of 130 mg, or a weight-based dose of 6 mg/kg of subcutaneous ustekinumab. Major enrollment criteria, other than failure of a non-TNF inhibitor therapy, included a Crohn’s disease activity index (CDAI) score between 220 and 450. The primary endpoint was a CDAI reduction of at least 100 points at 6 weeks. Clinical remission at 8 weeks, defined as CDAI less than 150, was a secondary endpoint.
The primary endpoint was reached by 28.7% randomized to placebo, 51.7% of those randomized to the fixed dose of ustekinumab, and 55.5% of those randomized to weight-based dosing. The advantage for the active treatment arms was statistically significant (both P less than .001). For the secondary endpoint of clinical remission at 8 weeks, the rates were 19.6% for placebo, 30.6% (P = .009 vs. placebo) for fixed-dose ustekinumab, and 40.2% (P less than .001 vs. placebo) for the weight-based dose.
The anti-inflammatory effect of ustekinumab, which inhibits signal transduction of IL-12 and IL-23 by binding to the p40 subunit that both cytokines employ for receptor binding, was reflected in the rapid reduction in C-reactive protein (CRP) concentrations observed with both doses but not with placebo, according to Dr. Feagan. He noted that the greater CRP reduction on the weight-based dosing, which nearly doubles ustekinumab exposure for some individuals, supports the dose-response anti-inflammatory mechanism of the drug.
Ustekinumab was well tolerated with similar rates and types of adverse events reported in the active treatment and placebo groups. This included infections, serious infections, and infusion reactions. There was a theoretical concern about an increased risk for cardiovascular events that was not substantiated in this study. No malignancies were observed in this short-term study.
Nearly 70% of the patients were naive to a biologic treatment. Of the 31.4% with prior exposure to a TNF inhibitor, none had met criteria for failure. About 80% had failed corticosteroids after what was considered to be an adequate course, while nearly 70% had failed one or more immunomodulators. About half had failed both.
Details about quality of life measures were not presented at the ACG, but Dr. Feagan said, summarizing the data, they “also confirmed the clinical efficacy of ustekinumab,” which was approved for the treatment of psoriatic arthritis more than 2 years ago.
The data, which suggest a level of efficacy comparable to other targeted therapies in Crohn’s disease, appear to encourage a filing for Food and Drug Administration approval in Crohn’s disease. However, data about the relative efficacy of this drug in those who have failed a TNF inhibitor is expected to provide greater insight about how this agent will add to current options.
“It is encouraging to see disease control with agents targeting novel cytokines in the inflammatory pathway, but we need to begin thinking about how these biologics fit with each other,” said Dr. David Rubin, chief, section of gastroenterology, hepatology, and nutrition, University of Chicago. Dr. Rubin was a moderator of the ACG session in which the UNITI-2 data were presented.
Dr. Feagan has financial relationships with Millennium, Merck, Centocor, Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, and many other pharmaceutical companies.
AT ACG 2015
Key clinical point: Ustekinumab, a novel monoclonal antibody for the treatment of Crohn’s disease, met its primary endpoint in a phase III trial.
Major finding: A reduction of 100 points or greater in the Crohn’s disease activity index (CDAI) was achieved by more than 50% patients on either dosing strategy of ustekinumab versus 28.7% of those in the placebo group (P less than .001).
Data source: Double-blind, placebo-controlled multicenter phase III trial.
Disclosures: The trial was sponsored by Janssen. Dr. Feagan has financial relationships with Millennium, Merck, Centocor, Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, and many others.