User login
Frozen noninferior to fresh fecal microbiota transplantation
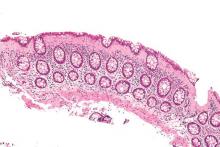
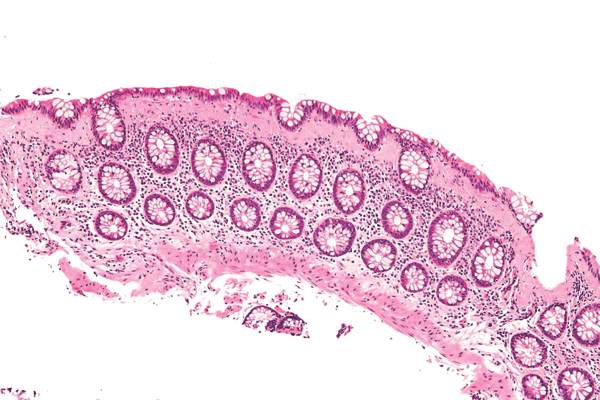
Fecal microbiota transplantation using frozen-then-thawed fecal material proved noninferior to that using fresh material for treating recurrent or refractory Clostridium difficile infection, according to a report published online Jan. 12 in JAMA.
Using frozen rather than fresh fecal material offers many advantages, such as allowing much more widespread and immediate accessibility of the treatment; reducing the number and frequency of donor screenings, which in turn would reduce costs; and ameliorating concern about potential transmission of pathogens from the donor to the recipient, since samples could be stored in quarantine until screening results are known, said Dr. Christine H. Lee of the department of pathology and molecular medicine, McMaster University, Hamilton (Ont.), and her associates.
They performed a 2-year randomized, double-blind noninferiority trial at six academic medical centers in Canada to compare frozen with fresh donor material in 232 adults with recurrent or refractory C. difficile infection. These study participants had “an extensive burden of comorbidity”: most had inflammatory bowel diseases and approximately 85% were immunocompromised, having undergone chronic hemodialysis or kidney transplantation, or having had metastatic solid tumors or hematologic malignancies. Half of the study subjects were inpatients, and approximately 75% were aged 65 years and older.
The patients were assigned to receive 50 mL of frozen-then-thawed FMT (114 participants) or fresh FMT (118 participants) by retention enema, delivered using 60-mL syringes. Those who didn’t improve by day 4 were given an additional FMT from the same donor. “Administration by enema is significantly less invasive than colonoscopy or nasojejunal/gastric administration and can be performed outside an acute care facility,” Dr. Lee and her associates noted.
The primary efficacy endpoint was clinical resolution, defined as no recurrence of C. difficile–related diarrhea at 13 weeks and no need for antibiotics. In the per-protocol population, 83.5% of the frozen FMT group and 85.1% of the fresh FMT group achieved this endpoint. In the intention-to-treat population, 75.0% of the frozen FMT group and 70.3% of the fresh FMT group achieved it. Both results demonstrate the noninferiority of frozen FMT, the investigators said (JAMA. 2016;315[2]:142-9. doi:10.1001/jama.201518098).
The proportion of adverse events and severe adverse events was deemed low and did not differ between the two study groups. The most common adverse events that may possibly have been related to treatment occurred in similar numbers of each group and included transient diarrhea, abdominal cramps, and nausea during the first 24 hours after transplantation and constipation and excess flatulence during the 13-week follow-up.
Even though this follow-up was longer than that in most clinical trials of FMT for C. difficile infection, which only tracked patients for 40 days, it is still insufficient to assess the long-term safety of the treatment. Ten-year follow-up of the participants in this trial is currently under way to examine any beneficial effects the treatment might exert on the metabolic syndrome, diabetes, or autoimmune disease, as well as any negative effects such as the development of autoimmune disorders or cancer.
The findings of Dr. Lee and her associates offer the best evidence to date supporting the use of frozen stool material in FMT, which would eliminate many of the logistical burdens associated with the treatment. For example, stool collection and processing would no longer have to be tied to the date and time of each individual transplantation.
These results also support the use of centralized stool banks, which would offer clinicians access to safe, screened stool material that could be shipped and stored frozen, then thawed for use as needed.
Dr. Preeti N. Malani and Dr. Krishna Rao are with the division of infectious diseases at the University of Michigan Health System, Ann Arbor. Dr. Malani is also an associate editor at JAMA and Dr. Rao is also with the Veterans Affairs Ann Arbor Healthcare System. Dr. Rao’s work is supported in part by the Claude D. Pepper Older Americans Independence Center. Dr. Malani and Dr. Rao reported having no relevant financial disclosures. They made these remarks in an editorial (JAMA. 2016;315[2]:137-8) accompanying Dr. Lee’s report.
The findings of Dr. Lee and her associates offer the best evidence to date supporting the use of frozen stool material in FMT, which would eliminate many of the logistical burdens associated with the treatment. For example, stool collection and processing would no longer have to be tied to the date and time of each individual transplantation.
These results also support the use of centralized stool banks, which would offer clinicians access to safe, screened stool material that could be shipped and stored frozen, then thawed for use as needed.
Dr. Preeti N. Malani and Dr. Krishna Rao are with the division of infectious diseases at the University of Michigan Health System, Ann Arbor. Dr. Malani is also an associate editor at JAMA and Dr. Rao is also with the Veterans Affairs Ann Arbor Healthcare System. Dr. Rao’s work is supported in part by the Claude D. Pepper Older Americans Independence Center. Dr. Malani and Dr. Rao reported having no relevant financial disclosures. They made these remarks in an editorial (JAMA. 2016;315[2]:137-8) accompanying Dr. Lee’s report.
The findings of Dr. Lee and her associates offer the best evidence to date supporting the use of frozen stool material in FMT, which would eliminate many of the logistical burdens associated with the treatment. For example, stool collection and processing would no longer have to be tied to the date and time of each individual transplantation.
These results also support the use of centralized stool banks, which would offer clinicians access to safe, screened stool material that could be shipped and stored frozen, then thawed for use as needed.
Dr. Preeti N. Malani and Dr. Krishna Rao are with the division of infectious diseases at the University of Michigan Health System, Ann Arbor. Dr. Malani is also an associate editor at JAMA and Dr. Rao is also with the Veterans Affairs Ann Arbor Healthcare System. Dr. Rao’s work is supported in part by the Claude D. Pepper Older Americans Independence Center. Dr. Malani and Dr. Rao reported having no relevant financial disclosures. They made these remarks in an editorial (JAMA. 2016;315[2]:137-8) accompanying Dr. Lee’s report.
Fecal microbiota transplantation using frozen-then-thawed fecal material proved noninferior to that using fresh material for treating recurrent or refractory Clostridium difficile infection, according to a report published online Jan. 12 in JAMA.
Using frozen rather than fresh fecal material offers many advantages, such as allowing much more widespread and immediate accessibility of the treatment; reducing the number and frequency of donor screenings, which in turn would reduce costs; and ameliorating concern about potential transmission of pathogens from the donor to the recipient, since samples could be stored in quarantine until screening results are known, said Dr. Christine H. Lee of the department of pathology and molecular medicine, McMaster University, Hamilton (Ont.), and her associates.
They performed a 2-year randomized, double-blind noninferiority trial at six academic medical centers in Canada to compare frozen with fresh donor material in 232 adults with recurrent or refractory C. difficile infection. These study participants had “an extensive burden of comorbidity”: most had inflammatory bowel diseases and approximately 85% were immunocompromised, having undergone chronic hemodialysis or kidney transplantation, or having had metastatic solid tumors or hematologic malignancies. Half of the study subjects were inpatients, and approximately 75% were aged 65 years and older.
The patients were assigned to receive 50 mL of frozen-then-thawed FMT (114 participants) or fresh FMT (118 participants) by retention enema, delivered using 60-mL syringes. Those who didn’t improve by day 4 were given an additional FMT from the same donor. “Administration by enema is significantly less invasive than colonoscopy or nasojejunal/gastric administration and can be performed outside an acute care facility,” Dr. Lee and her associates noted.
The primary efficacy endpoint was clinical resolution, defined as no recurrence of C. difficile–related diarrhea at 13 weeks and no need for antibiotics. In the per-protocol population, 83.5% of the frozen FMT group and 85.1% of the fresh FMT group achieved this endpoint. In the intention-to-treat population, 75.0% of the frozen FMT group and 70.3% of the fresh FMT group achieved it. Both results demonstrate the noninferiority of frozen FMT, the investigators said (JAMA. 2016;315[2]:142-9. doi:10.1001/jama.201518098).
The proportion of adverse events and severe adverse events was deemed low and did not differ between the two study groups. The most common adverse events that may possibly have been related to treatment occurred in similar numbers of each group and included transient diarrhea, abdominal cramps, and nausea during the first 24 hours after transplantation and constipation and excess flatulence during the 13-week follow-up.
Even though this follow-up was longer than that in most clinical trials of FMT for C. difficile infection, which only tracked patients for 40 days, it is still insufficient to assess the long-term safety of the treatment. Ten-year follow-up of the participants in this trial is currently under way to examine any beneficial effects the treatment might exert on the metabolic syndrome, diabetes, or autoimmune disease, as well as any negative effects such as the development of autoimmune disorders or cancer.
Fecal microbiota transplantation using frozen-then-thawed fecal material proved noninferior to that using fresh material for treating recurrent or refractory Clostridium difficile infection, according to a report published online Jan. 12 in JAMA.
Using frozen rather than fresh fecal material offers many advantages, such as allowing much more widespread and immediate accessibility of the treatment; reducing the number and frequency of donor screenings, which in turn would reduce costs; and ameliorating concern about potential transmission of pathogens from the donor to the recipient, since samples could be stored in quarantine until screening results are known, said Dr. Christine H. Lee of the department of pathology and molecular medicine, McMaster University, Hamilton (Ont.), and her associates.
They performed a 2-year randomized, double-blind noninferiority trial at six academic medical centers in Canada to compare frozen with fresh donor material in 232 adults with recurrent or refractory C. difficile infection. These study participants had “an extensive burden of comorbidity”: most had inflammatory bowel diseases and approximately 85% were immunocompromised, having undergone chronic hemodialysis or kidney transplantation, or having had metastatic solid tumors or hematologic malignancies. Half of the study subjects were inpatients, and approximately 75% were aged 65 years and older.
The patients were assigned to receive 50 mL of frozen-then-thawed FMT (114 participants) or fresh FMT (118 participants) by retention enema, delivered using 60-mL syringes. Those who didn’t improve by day 4 were given an additional FMT from the same donor. “Administration by enema is significantly less invasive than colonoscopy or nasojejunal/gastric administration and can be performed outside an acute care facility,” Dr. Lee and her associates noted.
The primary efficacy endpoint was clinical resolution, defined as no recurrence of C. difficile–related diarrhea at 13 weeks and no need for antibiotics. In the per-protocol population, 83.5% of the frozen FMT group and 85.1% of the fresh FMT group achieved this endpoint. In the intention-to-treat population, 75.0% of the frozen FMT group and 70.3% of the fresh FMT group achieved it. Both results demonstrate the noninferiority of frozen FMT, the investigators said (JAMA. 2016;315[2]:142-9. doi:10.1001/jama.201518098).
The proportion of adverse events and severe adverse events was deemed low and did not differ between the two study groups. The most common adverse events that may possibly have been related to treatment occurred in similar numbers of each group and included transient diarrhea, abdominal cramps, and nausea during the first 24 hours after transplantation and constipation and excess flatulence during the 13-week follow-up.
Even though this follow-up was longer than that in most clinical trials of FMT for C. difficile infection, which only tracked patients for 40 days, it is still insufficient to assess the long-term safety of the treatment. Ten-year follow-up of the participants in this trial is currently under way to examine any beneficial effects the treatment might exert on the metabolic syndrome, diabetes, or autoimmune disease, as well as any negative effects such as the development of autoimmune disorders or cancer.
FROM JAMA
Key clinical point: Fecal microbiota transplantation using frozen-then-thawed fecal material proved noninferior to using fresh material for recurrent/refractory Clostridium difficile infection.
Major finding: In the per-protocol population, 83.5% of the frozen FMT group and 85.1% of the fresh FMT group achieved clinical resolution.
Data source: A 2-year randomized, double-blind noninferiority trial involving 232 patients treated at six academic medical centers in Canada and followed for 13 weeks.
Disclosures: This study was funded by Physicians Services Inc., the Natural Sciences and Engineering Council, the National Science Foundation, and the gastrointestinal diseases research unit at Kingston (Ont.) General Hospital. Dr. Lee reported participating in clinical trials for ViroPharma, Actelion, Cubist, and Merck, and serving on the advisory boards of Rebiotix and Merck. Her associates reported numerous ties to industry sources.
Future IBD treatments out of sync with patient needs, expert says
ORLANDO – A range of novel therapies are set to come online for the treatment of inflammatory bowel disease (IBD), but they will miss the mark when it comes to meeting the need for better, longer-lasting outcomes, according to one expert.
“We’re positioning our therapies all wrong,” Dr. Stephen Hanauer said at a conference on inflammatory bowel diseases sponsored by the Crohn’s and Colitis Foundation of America.
“In psoriasis, patients are immediately given effective treatment with ustekinumab, but in IBD, we have to wait for patients to fail multiple therapies before they are given an effective one,” said Dr. Hanauer, who is the Clifford Joseph Barborka Professor of Medicine in the division of gastroenterology and hepatology at Northwestern University, Chicago. The result, he said, is that after first-line therapies in IBD have been used to diminishing effect, patients typically have waited up to between 6 and 10 years before they are given the treatment that works best for them. By then, when a patient likely has experienced penetrating disease and fistulas, even the most effective therapy has less than a 50% chance of working well.
This upside down state of affairs in the field is due to a combination of factors. Since the 1998 introduction to the U.S. market of infliximab for treating Crohn’s disease, much has been learned about the characteristics of IBD, including that it is a chronic, progressive condition with a higher burden of inflammation than is necessarily indicated by clinical symptoms.
But at the time infliximab was introduced, it was viewed not as a first-line treatment that could prevent further disease, but as a last ditch effort to halt an already rampant disease state that typically had its roots of destruction well planted long before the patient presented clinically, according to Dr. Hanauer.
In the last 2 decades, however, numerous studies have shown that earlier intervention with biologics results in higher treatment response rates and less structural trauma. []
In addition, data indicate that administering the correct choice of anti–tumor necrosis factor (TNF) agent in biologic-naive patients will yield as much as a 20% greater response rate, which could lead to lower costs. “Long-term pharmacoeconomic data are needed to include not only direct costs of care, but also indirect costs that capture lost income, productivity, [and] disability,” Dr. Hanauer said in an interview.
As it stands now, even vedolizumab, indicated in 2014 for use in both Crohn’s disease and ulcerative colitis, is kept for an even later stage of disease in the standard algorithm, coming behind not only “severe stage of disease” but placed after a patient has failed other anti-TNF treatment.

Biologics are far safer than the corticosteroids, which precede them in the typical treatment algorithm, but because cost is king, according Dr. Hanauer, “it’s the cost that drives our later-stage intervention … if anti-TNFs cost a dollar, we’d use them in everything.”
“We would see more impact if we move these therapies earlier into treatment,” he said. “Now we give the patients least likely to respond to these treatments, the [costliest] therapies,” Dr. Hanauer said during his presentation.
Meanwhile, the several novel IBD therapies in development are primarily being tested for late-stage disease. Changing the criteria for inclusion in such clinical trials could mean future treatment is more cost effective. “Study sponsors could go after ‘earlier’ indications in the mild to moderate range,” he said in the interview.
To improve the treatment algorithms already in use, Dr. Hanauer said clinicians should learn more about the pharmacokinetics and pharmacodynamics of their armamentarium, something he said most clinical trials tend to ignore. “Most [IBD] drugs were developed for rheumatoid arthritis where these kinds of considerations are not as important since they have many more ‘tools’ [to choose from] in rheumatology. In IBD, we have had to make the best of our limited tools. Industry would prefer to keep things simple, but it isn’t.”
Also availing themselves of therapeutic drug monitoring would help clinicians ensure that patients have high enough blood levels of their treatment at the time they complete induction therapy, making the management phase easier, Dr. Hanauer said.
Redefining disease severity will mean that new treatments will have more efficacy, and can lead to more opportunities for novel treatments to work. Until then, leaving biologics to be the agent of last resort means, according to Dr. Hanauer, there are more patients “who have none of the benefit and all of the risk.”
Dr. Hanauer reported several relevant financial disclosures, including AbbVie, Actavis, Hospira, Janssen, Novo Nordisk, and Pfizer.
Potential new inflammatory bowel disease treatments
Adhesion molecule–based therapies
• PF-00547659 (Pfizer), an anti-MAdCam S1P1 agonist; an oral agent; for ulcerative colitis
• Ozanimod (Celgene); for ulcerative colitis; now in phase III
• Etrolizumab (Hoffmann-La Roche); for Crohn’s disease; now in phase III
Biologics for IL-12/23 pathways
MEDI2070 (MedImmune/Amgen), an anti-p19 antibody; targets IL-23 for Crohn’s disease
Agents for the JAK-STAT pathway
• Mongersen (Celgene), an oral SMAD7 antisense oligonucleotide; for Crohn’s disease; in phase III
• Tofacitinib (Pfizer)
Sources: Dr. Brian Feagan, University of Western Ontario; Dr. Bruce Sands, Mount Sinai School of Medicine; Dr. Bill Sandborn, University of California, San Diego
On Twitter @whitneymcknight
ORLANDO – A range of novel therapies are set to come online for the treatment of inflammatory bowel disease (IBD), but they will miss the mark when it comes to meeting the need for better, longer-lasting outcomes, according to one expert.
“We’re positioning our therapies all wrong,” Dr. Stephen Hanauer said at a conference on inflammatory bowel diseases sponsored by the Crohn’s and Colitis Foundation of America.
“In psoriasis, patients are immediately given effective treatment with ustekinumab, but in IBD, we have to wait for patients to fail multiple therapies before they are given an effective one,” said Dr. Hanauer, who is the Clifford Joseph Barborka Professor of Medicine in the division of gastroenterology and hepatology at Northwestern University, Chicago. The result, he said, is that after first-line therapies in IBD have been used to diminishing effect, patients typically have waited up to between 6 and 10 years before they are given the treatment that works best for them. By then, when a patient likely has experienced penetrating disease and fistulas, even the most effective therapy has less than a 50% chance of working well.
This upside down state of affairs in the field is due to a combination of factors. Since the 1998 introduction to the U.S. market of infliximab for treating Crohn’s disease, much has been learned about the characteristics of IBD, including that it is a chronic, progressive condition with a higher burden of inflammation than is necessarily indicated by clinical symptoms.
But at the time infliximab was introduced, it was viewed not as a first-line treatment that could prevent further disease, but as a last ditch effort to halt an already rampant disease state that typically had its roots of destruction well planted long before the patient presented clinically, according to Dr. Hanauer.
In the last 2 decades, however, numerous studies have shown that earlier intervention with biologics results in higher treatment response rates and less structural trauma. []
In addition, data indicate that administering the correct choice of anti–tumor necrosis factor (TNF) agent in biologic-naive patients will yield as much as a 20% greater response rate, which could lead to lower costs. “Long-term pharmacoeconomic data are needed to include not only direct costs of care, but also indirect costs that capture lost income, productivity, [and] disability,” Dr. Hanauer said in an interview.
As it stands now, even vedolizumab, indicated in 2014 for use in both Crohn’s disease and ulcerative colitis, is kept for an even later stage of disease in the standard algorithm, coming behind not only “severe stage of disease” but placed after a patient has failed other anti-TNF treatment.

Biologics are far safer than the corticosteroids, which precede them in the typical treatment algorithm, but because cost is king, according Dr. Hanauer, “it’s the cost that drives our later-stage intervention … if anti-TNFs cost a dollar, we’d use them in everything.”
“We would see more impact if we move these therapies earlier into treatment,” he said. “Now we give the patients least likely to respond to these treatments, the [costliest] therapies,” Dr. Hanauer said during his presentation.
Meanwhile, the several novel IBD therapies in development are primarily being tested for late-stage disease. Changing the criteria for inclusion in such clinical trials could mean future treatment is more cost effective. “Study sponsors could go after ‘earlier’ indications in the mild to moderate range,” he said in the interview.
To improve the treatment algorithms already in use, Dr. Hanauer said clinicians should learn more about the pharmacokinetics and pharmacodynamics of their armamentarium, something he said most clinical trials tend to ignore. “Most [IBD] drugs were developed for rheumatoid arthritis where these kinds of considerations are not as important since they have many more ‘tools’ [to choose from] in rheumatology. In IBD, we have had to make the best of our limited tools. Industry would prefer to keep things simple, but it isn’t.”
Also availing themselves of therapeutic drug monitoring would help clinicians ensure that patients have high enough blood levels of their treatment at the time they complete induction therapy, making the management phase easier, Dr. Hanauer said.
Redefining disease severity will mean that new treatments will have more efficacy, and can lead to more opportunities for novel treatments to work. Until then, leaving biologics to be the agent of last resort means, according to Dr. Hanauer, there are more patients “who have none of the benefit and all of the risk.”
Dr. Hanauer reported several relevant financial disclosures, including AbbVie, Actavis, Hospira, Janssen, Novo Nordisk, and Pfizer.
Potential new inflammatory bowel disease treatments
Adhesion molecule–based therapies
• PF-00547659 (Pfizer), an anti-MAdCam S1P1 agonist; an oral agent; for ulcerative colitis
• Ozanimod (Celgene); for ulcerative colitis; now in phase III
• Etrolizumab (Hoffmann-La Roche); for Crohn’s disease; now in phase III
Biologics for IL-12/23 pathways
MEDI2070 (MedImmune/Amgen), an anti-p19 antibody; targets IL-23 for Crohn’s disease
Agents for the JAK-STAT pathway
• Mongersen (Celgene), an oral SMAD7 antisense oligonucleotide; for Crohn’s disease; in phase III
• Tofacitinib (Pfizer)
Sources: Dr. Brian Feagan, University of Western Ontario; Dr. Bruce Sands, Mount Sinai School of Medicine; Dr. Bill Sandborn, University of California, San Diego
On Twitter @whitneymcknight
ORLANDO – A range of novel therapies are set to come online for the treatment of inflammatory bowel disease (IBD), but they will miss the mark when it comes to meeting the need for better, longer-lasting outcomes, according to one expert.
“We’re positioning our therapies all wrong,” Dr. Stephen Hanauer said at a conference on inflammatory bowel diseases sponsored by the Crohn’s and Colitis Foundation of America.
“In psoriasis, patients are immediately given effective treatment with ustekinumab, but in IBD, we have to wait for patients to fail multiple therapies before they are given an effective one,” said Dr. Hanauer, who is the Clifford Joseph Barborka Professor of Medicine in the division of gastroenterology and hepatology at Northwestern University, Chicago. The result, he said, is that after first-line therapies in IBD have been used to diminishing effect, patients typically have waited up to between 6 and 10 years before they are given the treatment that works best for them. By then, when a patient likely has experienced penetrating disease and fistulas, even the most effective therapy has less than a 50% chance of working well.
This upside down state of affairs in the field is due to a combination of factors. Since the 1998 introduction to the U.S. market of infliximab for treating Crohn’s disease, much has been learned about the characteristics of IBD, including that it is a chronic, progressive condition with a higher burden of inflammation than is necessarily indicated by clinical symptoms.
But at the time infliximab was introduced, it was viewed not as a first-line treatment that could prevent further disease, but as a last ditch effort to halt an already rampant disease state that typically had its roots of destruction well planted long before the patient presented clinically, according to Dr. Hanauer.
In the last 2 decades, however, numerous studies have shown that earlier intervention with biologics results in higher treatment response rates and less structural trauma. []
In addition, data indicate that administering the correct choice of anti–tumor necrosis factor (TNF) agent in biologic-naive patients will yield as much as a 20% greater response rate, which could lead to lower costs. “Long-term pharmacoeconomic data are needed to include not only direct costs of care, but also indirect costs that capture lost income, productivity, [and] disability,” Dr. Hanauer said in an interview.
As it stands now, even vedolizumab, indicated in 2014 for use in both Crohn’s disease and ulcerative colitis, is kept for an even later stage of disease in the standard algorithm, coming behind not only “severe stage of disease” but placed after a patient has failed other anti-TNF treatment.

Biologics are far safer than the corticosteroids, which precede them in the typical treatment algorithm, but because cost is king, according Dr. Hanauer, “it’s the cost that drives our later-stage intervention … if anti-TNFs cost a dollar, we’d use them in everything.”
“We would see more impact if we move these therapies earlier into treatment,” he said. “Now we give the patients least likely to respond to these treatments, the [costliest] therapies,” Dr. Hanauer said during his presentation.
Meanwhile, the several novel IBD therapies in development are primarily being tested for late-stage disease. Changing the criteria for inclusion in such clinical trials could mean future treatment is more cost effective. “Study sponsors could go after ‘earlier’ indications in the mild to moderate range,” he said in the interview.
To improve the treatment algorithms already in use, Dr. Hanauer said clinicians should learn more about the pharmacokinetics and pharmacodynamics of their armamentarium, something he said most clinical trials tend to ignore. “Most [IBD] drugs were developed for rheumatoid arthritis where these kinds of considerations are not as important since they have many more ‘tools’ [to choose from] in rheumatology. In IBD, we have had to make the best of our limited tools. Industry would prefer to keep things simple, but it isn’t.”
Also availing themselves of therapeutic drug monitoring would help clinicians ensure that patients have high enough blood levels of their treatment at the time they complete induction therapy, making the management phase easier, Dr. Hanauer said.
Redefining disease severity will mean that new treatments will have more efficacy, and can lead to more opportunities for novel treatments to work. Until then, leaving biologics to be the agent of last resort means, according to Dr. Hanauer, there are more patients “who have none of the benefit and all of the risk.”
Dr. Hanauer reported several relevant financial disclosures, including AbbVie, Actavis, Hospira, Janssen, Novo Nordisk, and Pfizer.
Potential new inflammatory bowel disease treatments
Adhesion molecule–based therapies
• PF-00547659 (Pfizer), an anti-MAdCam S1P1 agonist; an oral agent; for ulcerative colitis
• Ozanimod (Celgene); for ulcerative colitis; now in phase III
• Etrolizumab (Hoffmann-La Roche); for Crohn’s disease; now in phase III
Biologics for IL-12/23 pathways
MEDI2070 (MedImmune/Amgen), an anti-p19 antibody; targets IL-23 for Crohn’s disease
Agents for the JAK-STAT pathway
• Mongersen (Celgene), an oral SMAD7 antisense oligonucleotide; for Crohn’s disease; in phase III
• Tofacitinib (Pfizer)
Sources: Dr. Brian Feagan, University of Western Ontario; Dr. Bruce Sands, Mount Sinai School of Medicine; Dr. Bill Sandborn, University of California, San Diego
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM 2015 ADVANCES IN IBD
Jury still out on appendectomy vs. antibiotics-first approach
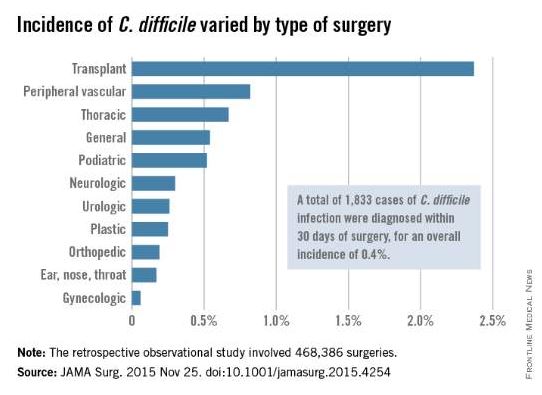
Despite a growing movement toward the antibiotics-first approach instead of surgical intervention for uncomplicated appendicitis, a new review of existing literature shows that the jury is still out on how to advise patients about their choices, according to Dr. Anne P. Ehlers and her colleagues who undertook the study.
The findings show that surgical intervention should continue to be considered a viable option and that appendectomy is not necessarily any better or worse in terms of long-term complication rates and length of hospital stay (J Am Coll Surg. 2015. doi:10.1016/j.jamcollsurg.2015.11.009).
“What we found is that treatment of acute, uncomplicated appendicitis with antibiotics first is probably a safe approach, but that there are many questions that need to be answered before we can fully inform our patients about the long-term outcomes of this treatment strategy,” Dr. Ehlers of the department of surgery at the University of Washington, Seattle, said in an interview.
Dr. Ehlers and her coinvestigators combed the PubMed and EMBASE databases for all English-language randomized controlled trials involving comparisons of antibiotic and appendectomy-based treatments for acute appendicitis. Studies were excluded if they lacked adult population investigation.
“Our study is a critical review of the literature [available] on this topic to understand the current state of evidence,” explained Dr. Ehlers, adding that the study’s main goal was to answer the most common questions she and her coauthors encountered when talking to physicians and patients about the benefits and risks of antibiotics-first over appendectomy; namely, “Is my appendicitis going to come back?” “Am I going to have a lot of extra trips to the hospital?” and “What’s my quality of life going to be?”
Ultimately, six trials, comprising 1,720 patients, were selected for inclusion. These studies were led by Dr. S. Eriksson (40 subjects), Dr. J. Styrud (252 subjects), Dr. A.N. Turhan (290 subjects), Dr. J. Hansson (369 subjects), Dr. C. Vons (239 subjects), and Dr. P. Salminen (530 subjects). The Styrud study did not enroll any women, and no study enrolled subjects older than 75 years of age, but the average age of each study’s patients ranged from 26 to 38 years.
Length-of-stay comparisons between appendectomy and antibiotics-first cohorts varied among the studies, but most showed the same or longer LOS for antibiotics-first subjects. Mean LOS was 3.3 for surgical patients vs. 3.1 for antibiotics (Eriksson), 2.6 surgical vs. 3.0 antibiotics (Styrud), 2.4 surgical vs. 3.14 antibiotics (Turhan), 3.0 for both (Hansson), 3.04 surgical vs. 3.96 antibiotics (Vons), and 3.0 for both (Salminen).
Rates of appendectomy among patients treated with an antibiotics-first approach varied as well, with a 35% rate in the Eriksson study (7 out of the 20 subjects treated with antibiotics-first) and a 24% rate in the Styrud and Turhan studies. The Hansson trial reported an unusually high crossover between cohorts of 60%, the investigators noted.
Two studies – those led by Turhan and Vons – noted higher rates of complications in antibiotics-first patients versus those who received surgical intervention: 4.7% vs. 4.4%, and 2.5% vs. less than 1%, respectively. All other studies had high rates of complications in the surgical cohorts, with the Eriksson study reporting no complications whatsoever among antibiotics-first subjects.
These findings, Dr. Ehlers stressed, are just a first step. This review’s relatively low sample size and limiting factors require that further studies be done to more firmly ascertain which option for appendicitis treatment is the most beneficial. To that end, Dr. Ehlers said that she and her coinvestigators are working on that next step.
“Our group is currently working on a study called the CODA [Comparing Outcomes of Drugs in Appendectomy] Study,” she said, with the goal of answering “questions about long-term patient-centered outcomes related to the antibiotics-first approach.” These questions include patients’ quality of life, whether patients have decisional regret about choosing one treatment strategy over another, if they develop long-term anxiety any time they experience even a small abdominal pain, how much time they miss from school or work by choosing one treatment option over another, and so on.
The goal of this study is that physicians “will be able to give [patients] a more informed view of what each treatment entails,” she said. Funded by the Patient-Centered Outcomes Research Institute, the study has no firm date of publication.
Dr. Ehlers disclosed receiving support via a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. No other conflicts of interest were reported.
Despite a growing movement toward the antibiotics-first approach instead of surgical intervention for uncomplicated appendicitis, a new review of existing literature shows that the jury is still out on how to advise patients about their choices, according to Dr. Anne P. Ehlers and her colleagues who undertook the study.
The findings show that surgical intervention should continue to be considered a viable option and that appendectomy is not necessarily any better or worse in terms of long-term complication rates and length of hospital stay (J Am Coll Surg. 2015. doi:10.1016/j.jamcollsurg.2015.11.009).
“What we found is that treatment of acute, uncomplicated appendicitis with antibiotics first is probably a safe approach, but that there are many questions that need to be answered before we can fully inform our patients about the long-term outcomes of this treatment strategy,” Dr. Ehlers of the department of surgery at the University of Washington, Seattle, said in an interview.
Dr. Ehlers and her coinvestigators combed the PubMed and EMBASE databases for all English-language randomized controlled trials involving comparisons of antibiotic and appendectomy-based treatments for acute appendicitis. Studies were excluded if they lacked adult population investigation.
“Our study is a critical review of the literature [available] on this topic to understand the current state of evidence,” explained Dr. Ehlers, adding that the study’s main goal was to answer the most common questions she and her coauthors encountered when talking to physicians and patients about the benefits and risks of antibiotics-first over appendectomy; namely, “Is my appendicitis going to come back?” “Am I going to have a lot of extra trips to the hospital?” and “What’s my quality of life going to be?”
Ultimately, six trials, comprising 1,720 patients, were selected for inclusion. These studies were led by Dr. S. Eriksson (40 subjects), Dr. J. Styrud (252 subjects), Dr. A.N. Turhan (290 subjects), Dr. J. Hansson (369 subjects), Dr. C. Vons (239 subjects), and Dr. P. Salminen (530 subjects). The Styrud study did not enroll any women, and no study enrolled subjects older than 75 years of age, but the average age of each study’s patients ranged from 26 to 38 years.
Length-of-stay comparisons between appendectomy and antibiotics-first cohorts varied among the studies, but most showed the same or longer LOS for antibiotics-first subjects. Mean LOS was 3.3 for surgical patients vs. 3.1 for antibiotics (Eriksson), 2.6 surgical vs. 3.0 antibiotics (Styrud), 2.4 surgical vs. 3.14 antibiotics (Turhan), 3.0 for both (Hansson), 3.04 surgical vs. 3.96 antibiotics (Vons), and 3.0 for both (Salminen).
Rates of appendectomy among patients treated with an antibiotics-first approach varied as well, with a 35% rate in the Eriksson study (7 out of the 20 subjects treated with antibiotics-first) and a 24% rate in the Styrud and Turhan studies. The Hansson trial reported an unusually high crossover between cohorts of 60%, the investigators noted.
Two studies – those led by Turhan and Vons – noted higher rates of complications in antibiotics-first patients versus those who received surgical intervention: 4.7% vs. 4.4%, and 2.5% vs. less than 1%, respectively. All other studies had high rates of complications in the surgical cohorts, with the Eriksson study reporting no complications whatsoever among antibiotics-first subjects.
These findings, Dr. Ehlers stressed, are just a first step. This review’s relatively low sample size and limiting factors require that further studies be done to more firmly ascertain which option for appendicitis treatment is the most beneficial. To that end, Dr. Ehlers said that she and her coinvestigators are working on that next step.
“Our group is currently working on a study called the CODA [Comparing Outcomes of Drugs in Appendectomy] Study,” she said, with the goal of answering “questions about long-term patient-centered outcomes related to the antibiotics-first approach.” These questions include patients’ quality of life, whether patients have decisional regret about choosing one treatment strategy over another, if they develop long-term anxiety any time they experience even a small abdominal pain, how much time they miss from school or work by choosing one treatment option over another, and so on.
The goal of this study is that physicians “will be able to give [patients] a more informed view of what each treatment entails,” she said. Funded by the Patient-Centered Outcomes Research Institute, the study has no firm date of publication.
Dr. Ehlers disclosed receiving support via a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. No other conflicts of interest were reported.
Despite a growing movement toward the antibiotics-first approach instead of surgical intervention for uncomplicated appendicitis, a new review of existing literature shows that the jury is still out on how to advise patients about their choices, according to Dr. Anne P. Ehlers and her colleagues who undertook the study.
The findings show that surgical intervention should continue to be considered a viable option and that appendectomy is not necessarily any better or worse in terms of long-term complication rates and length of hospital stay (J Am Coll Surg. 2015. doi:10.1016/j.jamcollsurg.2015.11.009).
“What we found is that treatment of acute, uncomplicated appendicitis with antibiotics first is probably a safe approach, but that there are many questions that need to be answered before we can fully inform our patients about the long-term outcomes of this treatment strategy,” Dr. Ehlers of the department of surgery at the University of Washington, Seattle, said in an interview.
Dr. Ehlers and her coinvestigators combed the PubMed and EMBASE databases for all English-language randomized controlled trials involving comparisons of antibiotic and appendectomy-based treatments for acute appendicitis. Studies were excluded if they lacked adult population investigation.
“Our study is a critical review of the literature [available] on this topic to understand the current state of evidence,” explained Dr. Ehlers, adding that the study’s main goal was to answer the most common questions she and her coauthors encountered when talking to physicians and patients about the benefits and risks of antibiotics-first over appendectomy; namely, “Is my appendicitis going to come back?” “Am I going to have a lot of extra trips to the hospital?” and “What’s my quality of life going to be?”
Ultimately, six trials, comprising 1,720 patients, were selected for inclusion. These studies were led by Dr. S. Eriksson (40 subjects), Dr. J. Styrud (252 subjects), Dr. A.N. Turhan (290 subjects), Dr. J. Hansson (369 subjects), Dr. C. Vons (239 subjects), and Dr. P. Salminen (530 subjects). The Styrud study did not enroll any women, and no study enrolled subjects older than 75 years of age, but the average age of each study’s patients ranged from 26 to 38 years.
Length-of-stay comparisons between appendectomy and antibiotics-first cohorts varied among the studies, but most showed the same or longer LOS for antibiotics-first subjects. Mean LOS was 3.3 for surgical patients vs. 3.1 for antibiotics (Eriksson), 2.6 surgical vs. 3.0 antibiotics (Styrud), 2.4 surgical vs. 3.14 antibiotics (Turhan), 3.0 for both (Hansson), 3.04 surgical vs. 3.96 antibiotics (Vons), and 3.0 for both (Salminen).
Rates of appendectomy among patients treated with an antibiotics-first approach varied as well, with a 35% rate in the Eriksson study (7 out of the 20 subjects treated with antibiotics-first) and a 24% rate in the Styrud and Turhan studies. The Hansson trial reported an unusually high crossover between cohorts of 60%, the investigators noted.
Two studies – those led by Turhan and Vons – noted higher rates of complications in antibiotics-first patients versus those who received surgical intervention: 4.7% vs. 4.4%, and 2.5% vs. less than 1%, respectively. All other studies had high rates of complications in the surgical cohorts, with the Eriksson study reporting no complications whatsoever among antibiotics-first subjects.
These findings, Dr. Ehlers stressed, are just a first step. This review’s relatively low sample size and limiting factors require that further studies be done to more firmly ascertain which option for appendicitis treatment is the most beneficial. To that end, Dr. Ehlers said that she and her coinvestigators are working on that next step.
“Our group is currently working on a study called the CODA [Comparing Outcomes of Drugs in Appendectomy] Study,” she said, with the goal of answering “questions about long-term patient-centered outcomes related to the antibiotics-first approach.” These questions include patients’ quality of life, whether patients have decisional regret about choosing one treatment strategy over another, if they develop long-term anxiety any time they experience even a small abdominal pain, how much time they miss from school or work by choosing one treatment option over another, and so on.
The goal of this study is that physicians “will be able to give [patients] a more informed view of what each treatment entails,” she said. Funded by the Patient-Centered Outcomes Research Institute, the study has no firm date of publication.
Dr. Ehlers disclosed receiving support via a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. No other conflicts of interest were reported.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: While the antibiotics-first approach to uncomplicated appendicitis has become increasingly popular in recent years, existing evidence shows that appendectomy is not inferior in terms of hospital stay and rates of complications.
Major finding: Length-of-stay and complication rates vary among all six included studies between antibiotics-first and surgical cohorts, indicating that neither one is definitively better or worse than the other.
Data source: Literature review of 1,720 appendicitis patients across six studies selected from PubMed and EMBASE databases.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases supported the study. No other conflicts of interest were reported.
Biosimilars primer: What you need to know now
ORLANDO – Regardless of what you think about using biosimilars, chances are you won’t be able to avoid using them if you already use biologics.
That’s according to Dr. David T. Rubin, codirector of the Digestive Diseases Center at the University of Chicago. “They’re coming and they will influence our practice, ” he told a clinical track audience at a conference on inflammatory bowel disease.
Before exploring how these medications may change how you treat patients, here’s a look at what they are and how they’re brought to market.
First, a little basic science review of small-molecule medications vs. biologic ones. Small-molecule agents are simple structures, which are stable enough to be replicated, do not tend to cause immunogenicity, and require very little in the way of testing for quality assurance.
By contrast, biologic medicines, including monoclonal antibodies, are complex structures – in some cases, highly complex – that are replicable, but often with a high degree of difficulty. Unlike small-molecule medicines, biologic drugs cannot be mass produced and require almost 250 sophisticated tests to ensure quality. They are less stable and can trigger an immunogenic response. The manufacturing process for biologics is so precise that the slightest disturbance in development can affect whether the medication is functional.
As a result, the typical development timeline for these medications is between 7 and 8 years, with costs running as high as $250 million each. Currently, there are more than 650 recombinant therapeutics in development worldwide, more than half of which are in the preclinical stage. The top original products being copied are adalimumab at 13, and infliximab with 9. Meanwhile, at least five as-of-yet unpublished studies are looking at how these potential adalimumab and infliximab biosimilars perform in inflammatory bowel disease (IBD), Dr. Rubin said. Biosimilars are used worldwide, primarily in Europe and Asia.
But when these biosimilars reach our shores, don’t call them generics. “I encourage you to not use that term, even when discussing them with patients,” Dr. Rubin said. Still, because the Food and Drug Administration says that a biosimilar should have no greater risk for adverse events or diminished efficacy compared with the original biologic just as with generics, pharmacists are within their rights to substitute biosimilars for original biologics without prescriber intervention.
That’s why, “There must be pharmacovigilance with biosimilars, just like with generics, after the drug is brought to market,” Dr. Rubin said.
Biosimilars are also not “biobetters,” medications that have modifications added to the original biologic product in order to improve their clinical performance. While biobetters can be patented, they do not have legal or regulatory status, however, because they are considered new drugs.
The FDA defines biosimilars as a biological product that is highly similar to the reference product, with no clinically meaningful safety, purity, or potency differences from the reference product.
Early in 2015, the biosimilar filgrastim-sndz (Zarxio TM, Sandoz-Novartis), which has been available in Europe since 2009, entered the U.S. market. With its biosimilarity to Filgrastim (Sandoz-Novartis), the medication’s primary indications are for various cancers and chronic neutropenia.
To help expedite bringing the medications to market, FDA guidance data requirements for biosimilars is abbreviated when compared with that for biologics. Rather than ask developers to conduct clinical trials, developers must provide at least one comparative study between the biosimilar and its original, according to the original drug’s indication. There also is what Dr. Rubin called a “weighted reliance” on analytical similarity to the original. In addition, no phase II dose-ranging studies are required. Indication extrapolation is also possible, meaning that safety and efficacy data leading to a biosimilar being approved for say, rheumatoid arthritis, could also be applied to Crohn’s disease.
So, what does all this mean for your patients? It depends upon in which state you practice: Even when a recombinant product meets the FDA criteria, whether or not a patient can be placed on a biosimilar comes down to state regulation. At present, 19 states have passed laws as to how and when biosimilars can be swapped out, most of them stipulating that a patient be notified when it occurs. Physicians maintain their rights to ask pharmacists to “dispense as written,” but since biosimilars are cheaper than their originals, you won’t necessarily get around mounting pressure from third-party payers to contain costs.
Dr. Rubin said this potential friction between insurers and physicians could result in delays with adverse effect on the patient. “If the insurance company says they prefer another agent over the one a patient is receiving, we all know about the unexpected delays that can occur when switching.”
Dr. Rubin predicted that the logistics of prescribing will be complicated by pharmaceutical marketing efforts. He noted the similar names of Remsima (Hospira) vs. Remicade (Janssen), Inflectra (Hospira) vs. infliximab generic. “It could be confusing for all of us.”
The question of how the drug will fare once inside the patient is still a matter of debate.
“The major issue is immunogenicity ... it’s impossible to predict in vitro,” said Dr. Brian Feagan, a copanelist with Dr. Rubin, and a professor of medicine at the University of Western Ontario in London, Canada. “Immunogenicity is determined by product-related factors, and a lot of clinical ones such as immunosuppression, coadministration, route of administration, disease-specific factors.”
The only way to truly determine the impact on immunogenicity of interchangeability, whether because of third-party payer stipulations or physician’s choice, is to do multiple switching trials, said Dr. Feagan.
But Dr. Stephen B. Hanauer, the Clifford Joseph Barborka Professor of Medicine in Gastroenterology and Hepatology at Northwestern University (Chicago), said that’s not likely to happen. “There’s no time for that as the FDA regulatory evaluation proceeds,” he said in an interview.
“The trial would take 2 years to accomplish, would need large numbers of patients in order to identify potential small differences, and would be too expensive.” All of which would defeat the purpose of the expedited approval process, Dr. Hanauer said, because the decision by Congress to give biosimilars the green light was to reduce cost.
On the other hand, said Dr. Feagan, the experiment on switching probably has already been done. That’s because despite what he referred to as efforts by pharmaceutical manufacturers to reassure physicians there is no drift from the original product, heterogeneity is inevitable.
These iterative qualities, according to Dr. Hanauer, essentially make the original products into biosimilars of themselves. Add to that, he said that depending upon the company used to perform the assays to determine immunogenicity, the range of results can vary widely, and you end up having to learn to live with a certain amount of uncertainty. “I’m not afraid of biosimilars,” Dr. Hanauer said while discussing biosimilars during an audience question time at the meeting.
“We are a little bit timid about biosimilars, but my sense is we will find our comfort level in the next few years, and we will start using them frequently,” Dr. Miguel Regueiro, medical director of the IBD Center at the University of Pittsburgh, said in an interview. “I think immunogenicity to biosimilars will be the same immunogenicity to the innovative biologics, but I don’t think we’re going to be comfortable with interchanging a biosimilar with a[n] original biologic because of the potential immunogenicity that can occur by switching between agents.”
Whether biosimilars can be used in place of their originals, said both Dr. Feagan and Dr. Hanauer, will come down to how extrapolated data is interpreted.
“I think the biggest debate the FDA is going to have [when indicating biosimilars for IBD] is overextrapolation,” Dr. Hanauer said in the interview. Since the FDA does not require clinical trials for biosimilars, but relies upon analytics instead, and because there are far less clinical data for biologics in IBD than there are for diseases such as rheumatoid or psoriatic arthritis, manufacturers will turn to those studies to demonstrate efficacy between originals and recombinants. “If 99.9% of the analytic assays and the clinical data in rheumatoid arthritis are virtually the same, I would assume that the data in inflammatory bowel disease is going to be virtually the same.”
However, at least in Canada, that was not the opinion of regulators who decided against approving the extrapolation of infliximab clinical data for indicating its biosimilar in IBD, but did allow extrapolation of the data for rheumatoid arthritis. “Health Canada decided that the antibody-dependent, cell-mediated cytotoxicity was different for IBD,” said Dr. Feagan.
Predicting the primacy of cost over keeping patients in remission, but at least for now, Dr. Rubin said the question of cost is “huge. Based on the European and Asian experience, the day one of these new products becomes available, the price of the existing therapies drops anywhere from 15% to 30%.”
Recent data places the cost of remission in the United States using infliximab at about $15,000.
Dr. Regueiro said these market forces are a good thing. “I don’t look at biosimilars in a negative context whatsoever. I think they are a necessary part of health care reform. Cost is definitely a driver, and that’s not bad.”
The meeting was sponsored by the Crohn’s & Colitis Foundation of America. Dr. Rubin has financial and consulting relationships with AbbVie, Janssen, Takeda, and numerous other pharmaceutical companies. Dr. Feagan has numerous relationships with pharmaceutical companies, including Abbott, Janssen, Teva, and others. Dr. Hanauer has served on the board of AbbVie and has financial relationships with numerous other pharmaceutical manufacturers. Dr. Regueiro did not have any disclosures relevant to this story.
On Twitter @whitneymcknight
This article was updated 1/6/16.
ORLANDO – Regardless of what you think about using biosimilars, chances are you won’t be able to avoid using them if you already use biologics.
That’s according to Dr. David T. Rubin, codirector of the Digestive Diseases Center at the University of Chicago. “They’re coming and they will influence our practice, ” he told a clinical track audience at a conference on inflammatory bowel disease.
Before exploring how these medications may change how you treat patients, here’s a look at what they are and how they’re brought to market.
First, a little basic science review of small-molecule medications vs. biologic ones. Small-molecule agents are simple structures, which are stable enough to be replicated, do not tend to cause immunogenicity, and require very little in the way of testing for quality assurance.
By contrast, biologic medicines, including monoclonal antibodies, are complex structures – in some cases, highly complex – that are replicable, but often with a high degree of difficulty. Unlike small-molecule medicines, biologic drugs cannot be mass produced and require almost 250 sophisticated tests to ensure quality. They are less stable and can trigger an immunogenic response. The manufacturing process for biologics is so precise that the slightest disturbance in development can affect whether the medication is functional.
As a result, the typical development timeline for these medications is between 7 and 8 years, with costs running as high as $250 million each. Currently, there are more than 650 recombinant therapeutics in development worldwide, more than half of which are in the preclinical stage. The top original products being copied are adalimumab at 13, and infliximab with 9. Meanwhile, at least five as-of-yet unpublished studies are looking at how these potential adalimumab and infliximab biosimilars perform in inflammatory bowel disease (IBD), Dr. Rubin said. Biosimilars are used worldwide, primarily in Europe and Asia.
But when these biosimilars reach our shores, don’t call them generics. “I encourage you to not use that term, even when discussing them with patients,” Dr. Rubin said. Still, because the Food and Drug Administration says that a biosimilar should have no greater risk for adverse events or diminished efficacy compared with the original biologic just as with generics, pharmacists are within their rights to substitute biosimilars for original biologics without prescriber intervention.
That’s why, “There must be pharmacovigilance with biosimilars, just like with generics, after the drug is brought to market,” Dr. Rubin said.
Biosimilars are also not “biobetters,” medications that have modifications added to the original biologic product in order to improve their clinical performance. While biobetters can be patented, they do not have legal or regulatory status, however, because they are considered new drugs.
The FDA defines biosimilars as a biological product that is highly similar to the reference product, with no clinically meaningful safety, purity, or potency differences from the reference product.
Early in 2015, the biosimilar filgrastim-sndz (Zarxio TM, Sandoz-Novartis), which has been available in Europe since 2009, entered the U.S. market. With its biosimilarity to Filgrastim (Sandoz-Novartis), the medication’s primary indications are for various cancers and chronic neutropenia.
To help expedite bringing the medications to market, FDA guidance data requirements for biosimilars is abbreviated when compared with that for biologics. Rather than ask developers to conduct clinical trials, developers must provide at least one comparative study between the biosimilar and its original, according to the original drug’s indication. There also is what Dr. Rubin called a “weighted reliance” on analytical similarity to the original. In addition, no phase II dose-ranging studies are required. Indication extrapolation is also possible, meaning that safety and efficacy data leading to a biosimilar being approved for say, rheumatoid arthritis, could also be applied to Crohn’s disease.
So, what does all this mean for your patients? It depends upon in which state you practice: Even when a recombinant product meets the FDA criteria, whether or not a patient can be placed on a biosimilar comes down to state regulation. At present, 19 states have passed laws as to how and when biosimilars can be swapped out, most of them stipulating that a patient be notified when it occurs. Physicians maintain their rights to ask pharmacists to “dispense as written,” but since biosimilars are cheaper than their originals, you won’t necessarily get around mounting pressure from third-party payers to contain costs.
Dr. Rubin said this potential friction between insurers and physicians could result in delays with adverse effect on the patient. “If the insurance company says they prefer another agent over the one a patient is receiving, we all know about the unexpected delays that can occur when switching.”
Dr. Rubin predicted that the logistics of prescribing will be complicated by pharmaceutical marketing efforts. He noted the similar names of Remsima (Hospira) vs. Remicade (Janssen), Inflectra (Hospira) vs. infliximab generic. “It could be confusing for all of us.”
The question of how the drug will fare once inside the patient is still a matter of debate.
“The major issue is immunogenicity ... it’s impossible to predict in vitro,” said Dr. Brian Feagan, a copanelist with Dr. Rubin, and a professor of medicine at the University of Western Ontario in London, Canada. “Immunogenicity is determined by product-related factors, and a lot of clinical ones such as immunosuppression, coadministration, route of administration, disease-specific factors.”
The only way to truly determine the impact on immunogenicity of interchangeability, whether because of third-party payer stipulations or physician’s choice, is to do multiple switching trials, said Dr. Feagan.
But Dr. Stephen B. Hanauer, the Clifford Joseph Barborka Professor of Medicine in Gastroenterology and Hepatology at Northwestern University (Chicago), said that’s not likely to happen. “There’s no time for that as the FDA regulatory evaluation proceeds,” he said in an interview.
“The trial would take 2 years to accomplish, would need large numbers of patients in order to identify potential small differences, and would be too expensive.” All of which would defeat the purpose of the expedited approval process, Dr. Hanauer said, because the decision by Congress to give biosimilars the green light was to reduce cost.
On the other hand, said Dr. Feagan, the experiment on switching probably has already been done. That’s because despite what he referred to as efforts by pharmaceutical manufacturers to reassure physicians there is no drift from the original product, heterogeneity is inevitable.
These iterative qualities, according to Dr. Hanauer, essentially make the original products into biosimilars of themselves. Add to that, he said that depending upon the company used to perform the assays to determine immunogenicity, the range of results can vary widely, and you end up having to learn to live with a certain amount of uncertainty. “I’m not afraid of biosimilars,” Dr. Hanauer said while discussing biosimilars during an audience question time at the meeting.
“We are a little bit timid about biosimilars, but my sense is we will find our comfort level in the next few years, and we will start using them frequently,” Dr. Miguel Regueiro, medical director of the IBD Center at the University of Pittsburgh, said in an interview. “I think immunogenicity to biosimilars will be the same immunogenicity to the innovative biologics, but I don’t think we’re going to be comfortable with interchanging a biosimilar with a[n] original biologic because of the potential immunogenicity that can occur by switching between agents.”
Whether biosimilars can be used in place of their originals, said both Dr. Feagan and Dr. Hanauer, will come down to how extrapolated data is interpreted.
“I think the biggest debate the FDA is going to have [when indicating biosimilars for IBD] is overextrapolation,” Dr. Hanauer said in the interview. Since the FDA does not require clinical trials for biosimilars, but relies upon analytics instead, and because there are far less clinical data for biologics in IBD than there are for diseases such as rheumatoid or psoriatic arthritis, manufacturers will turn to those studies to demonstrate efficacy between originals and recombinants. “If 99.9% of the analytic assays and the clinical data in rheumatoid arthritis are virtually the same, I would assume that the data in inflammatory bowel disease is going to be virtually the same.”
However, at least in Canada, that was not the opinion of regulators who decided against approving the extrapolation of infliximab clinical data for indicating its biosimilar in IBD, but did allow extrapolation of the data for rheumatoid arthritis. “Health Canada decided that the antibody-dependent, cell-mediated cytotoxicity was different for IBD,” said Dr. Feagan.
Predicting the primacy of cost over keeping patients in remission, but at least for now, Dr. Rubin said the question of cost is “huge. Based on the European and Asian experience, the day one of these new products becomes available, the price of the existing therapies drops anywhere from 15% to 30%.”
Recent data places the cost of remission in the United States using infliximab at about $15,000.
Dr. Regueiro said these market forces are a good thing. “I don’t look at biosimilars in a negative context whatsoever. I think they are a necessary part of health care reform. Cost is definitely a driver, and that’s not bad.”
The meeting was sponsored by the Crohn’s & Colitis Foundation of America. Dr. Rubin has financial and consulting relationships with AbbVie, Janssen, Takeda, and numerous other pharmaceutical companies. Dr. Feagan has numerous relationships with pharmaceutical companies, including Abbott, Janssen, Teva, and others. Dr. Hanauer has served on the board of AbbVie and has financial relationships with numerous other pharmaceutical manufacturers. Dr. Regueiro did not have any disclosures relevant to this story.
On Twitter @whitneymcknight
This article was updated 1/6/16.
ORLANDO – Regardless of what you think about using biosimilars, chances are you won’t be able to avoid using them if you already use biologics.
That’s according to Dr. David T. Rubin, codirector of the Digestive Diseases Center at the University of Chicago. “They’re coming and they will influence our practice, ” he told a clinical track audience at a conference on inflammatory bowel disease.
Before exploring how these medications may change how you treat patients, here’s a look at what they are and how they’re brought to market.
First, a little basic science review of small-molecule medications vs. biologic ones. Small-molecule agents are simple structures, which are stable enough to be replicated, do not tend to cause immunogenicity, and require very little in the way of testing for quality assurance.
By contrast, biologic medicines, including monoclonal antibodies, are complex structures – in some cases, highly complex – that are replicable, but often with a high degree of difficulty. Unlike small-molecule medicines, biologic drugs cannot be mass produced and require almost 250 sophisticated tests to ensure quality. They are less stable and can trigger an immunogenic response. The manufacturing process for biologics is so precise that the slightest disturbance in development can affect whether the medication is functional.
As a result, the typical development timeline for these medications is between 7 and 8 years, with costs running as high as $250 million each. Currently, there are more than 650 recombinant therapeutics in development worldwide, more than half of which are in the preclinical stage. The top original products being copied are adalimumab at 13, and infliximab with 9. Meanwhile, at least five as-of-yet unpublished studies are looking at how these potential adalimumab and infliximab biosimilars perform in inflammatory bowel disease (IBD), Dr. Rubin said. Biosimilars are used worldwide, primarily in Europe and Asia.
But when these biosimilars reach our shores, don’t call them generics. “I encourage you to not use that term, even when discussing them with patients,” Dr. Rubin said. Still, because the Food and Drug Administration says that a biosimilar should have no greater risk for adverse events or diminished efficacy compared with the original biologic just as with generics, pharmacists are within their rights to substitute biosimilars for original biologics without prescriber intervention.
That’s why, “There must be pharmacovigilance with biosimilars, just like with generics, after the drug is brought to market,” Dr. Rubin said.
Biosimilars are also not “biobetters,” medications that have modifications added to the original biologic product in order to improve their clinical performance. While biobetters can be patented, they do not have legal or regulatory status, however, because they are considered new drugs.
The FDA defines biosimilars as a biological product that is highly similar to the reference product, with no clinically meaningful safety, purity, or potency differences from the reference product.
Early in 2015, the biosimilar filgrastim-sndz (Zarxio TM, Sandoz-Novartis), which has been available in Europe since 2009, entered the U.S. market. With its biosimilarity to Filgrastim (Sandoz-Novartis), the medication’s primary indications are for various cancers and chronic neutropenia.
To help expedite bringing the medications to market, FDA guidance data requirements for biosimilars is abbreviated when compared with that for biologics. Rather than ask developers to conduct clinical trials, developers must provide at least one comparative study between the biosimilar and its original, according to the original drug’s indication. There also is what Dr. Rubin called a “weighted reliance” on analytical similarity to the original. In addition, no phase II dose-ranging studies are required. Indication extrapolation is also possible, meaning that safety and efficacy data leading to a biosimilar being approved for say, rheumatoid arthritis, could also be applied to Crohn’s disease.
So, what does all this mean for your patients? It depends upon in which state you practice: Even when a recombinant product meets the FDA criteria, whether or not a patient can be placed on a biosimilar comes down to state regulation. At present, 19 states have passed laws as to how and when biosimilars can be swapped out, most of them stipulating that a patient be notified when it occurs. Physicians maintain their rights to ask pharmacists to “dispense as written,” but since biosimilars are cheaper than their originals, you won’t necessarily get around mounting pressure from third-party payers to contain costs.
Dr. Rubin said this potential friction between insurers and physicians could result in delays with adverse effect on the patient. “If the insurance company says they prefer another agent over the one a patient is receiving, we all know about the unexpected delays that can occur when switching.”
Dr. Rubin predicted that the logistics of prescribing will be complicated by pharmaceutical marketing efforts. He noted the similar names of Remsima (Hospira) vs. Remicade (Janssen), Inflectra (Hospira) vs. infliximab generic. “It could be confusing for all of us.”
The question of how the drug will fare once inside the patient is still a matter of debate.
“The major issue is immunogenicity ... it’s impossible to predict in vitro,” said Dr. Brian Feagan, a copanelist with Dr. Rubin, and a professor of medicine at the University of Western Ontario in London, Canada. “Immunogenicity is determined by product-related factors, and a lot of clinical ones such as immunosuppression, coadministration, route of administration, disease-specific factors.”
The only way to truly determine the impact on immunogenicity of interchangeability, whether because of third-party payer stipulations or physician’s choice, is to do multiple switching trials, said Dr. Feagan.
But Dr. Stephen B. Hanauer, the Clifford Joseph Barborka Professor of Medicine in Gastroenterology and Hepatology at Northwestern University (Chicago), said that’s not likely to happen. “There’s no time for that as the FDA regulatory evaluation proceeds,” he said in an interview.
“The trial would take 2 years to accomplish, would need large numbers of patients in order to identify potential small differences, and would be too expensive.” All of which would defeat the purpose of the expedited approval process, Dr. Hanauer said, because the decision by Congress to give biosimilars the green light was to reduce cost.
On the other hand, said Dr. Feagan, the experiment on switching probably has already been done. That’s because despite what he referred to as efforts by pharmaceutical manufacturers to reassure physicians there is no drift from the original product, heterogeneity is inevitable.
These iterative qualities, according to Dr. Hanauer, essentially make the original products into biosimilars of themselves. Add to that, he said that depending upon the company used to perform the assays to determine immunogenicity, the range of results can vary widely, and you end up having to learn to live with a certain amount of uncertainty. “I’m not afraid of biosimilars,” Dr. Hanauer said while discussing biosimilars during an audience question time at the meeting.
“We are a little bit timid about biosimilars, but my sense is we will find our comfort level in the next few years, and we will start using them frequently,” Dr. Miguel Regueiro, medical director of the IBD Center at the University of Pittsburgh, said in an interview. “I think immunogenicity to biosimilars will be the same immunogenicity to the innovative biologics, but I don’t think we’re going to be comfortable with interchanging a biosimilar with a[n] original biologic because of the potential immunogenicity that can occur by switching between agents.”
Whether biosimilars can be used in place of their originals, said both Dr. Feagan and Dr. Hanauer, will come down to how extrapolated data is interpreted.
“I think the biggest debate the FDA is going to have [when indicating biosimilars for IBD] is overextrapolation,” Dr. Hanauer said in the interview. Since the FDA does not require clinical trials for biosimilars, but relies upon analytics instead, and because there are far less clinical data for biologics in IBD than there are for diseases such as rheumatoid or psoriatic arthritis, manufacturers will turn to those studies to demonstrate efficacy between originals and recombinants. “If 99.9% of the analytic assays and the clinical data in rheumatoid arthritis are virtually the same, I would assume that the data in inflammatory bowel disease is going to be virtually the same.”
However, at least in Canada, that was not the opinion of regulators who decided against approving the extrapolation of infliximab clinical data for indicating its biosimilar in IBD, but did allow extrapolation of the data for rheumatoid arthritis. “Health Canada decided that the antibody-dependent, cell-mediated cytotoxicity was different for IBD,” said Dr. Feagan.
Predicting the primacy of cost over keeping patients in remission, but at least for now, Dr. Rubin said the question of cost is “huge. Based on the European and Asian experience, the day one of these new products becomes available, the price of the existing therapies drops anywhere from 15% to 30%.”
Recent data places the cost of remission in the United States using infliximab at about $15,000.
Dr. Regueiro said these market forces are a good thing. “I don’t look at biosimilars in a negative context whatsoever. I think they are a necessary part of health care reform. Cost is definitely a driver, and that’s not bad.”
The meeting was sponsored by the Crohn’s & Colitis Foundation of America. Dr. Rubin has financial and consulting relationships with AbbVie, Janssen, Takeda, and numerous other pharmaceutical companies. Dr. Feagan has numerous relationships with pharmaceutical companies, including Abbott, Janssen, Teva, and others. Dr. Hanauer has served on the board of AbbVie and has financial relationships with numerous other pharmaceutical manufacturers. Dr. Regueiro did not have any disclosures relevant to this story.
On Twitter @whitneymcknight
This article was updated 1/6/16.
EXPERT ANALYSIS FROM 2015 ADVANCES IN IBD
Anti-TNF therapy can continue for IBD patients with skin lesions
Inflammatory bowel disease (IBD) patients who experience skin lesions during anti–tumor necrosis factor therapy do not usually need to stop treatment, according to Isabelle Cleynen, Ph.D., of the University of Leuven (Belgium) and her associates.
In their retrospective study of 917 IBD patients who started treatment with infliximab at the University Hospitals Leuven between December 1994 and January 2009, 264 developed skin lesions during the follow-up period. The most common type was psoriasiform eczema, in 30.6% of the patients with lesions. Other common types included eczema (in 23.5%), xerosis cutis (10.6%), palmoplantar pustulosis (5.3%), and psoriasis (3.8%). Median cumulative doses and trough levels of infliximab were similar in people who developed skin lesions and those who did not.
Just over half of patients with skin lesions received only topical treatment, 1.9% received only systemic treatment, 28% received both, and 19.3% of patients required no specific treatment. Almost 11% of patients who developed skin lesions were forced to stop therapy. Reasons for stopping treatment included an intolerable location of lesions, concomitant itching or pain, recurring episodes, and concomitant arthralgia.
“Knowledge of the diagnostic and therapeutic criteria and the clinical course of these lesions should assist in their management. With referral to a dedicated dermatologist, most lesions can be treated and the need for interruption of anti-TNF therapy is rare,” the investigators concluded.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M15-0729).
Inflammatory bowel disease (IBD) patients who experience skin lesions during anti–tumor necrosis factor therapy do not usually need to stop treatment, according to Isabelle Cleynen, Ph.D., of the University of Leuven (Belgium) and her associates.
In their retrospective study of 917 IBD patients who started treatment with infliximab at the University Hospitals Leuven between December 1994 and January 2009, 264 developed skin lesions during the follow-up period. The most common type was psoriasiform eczema, in 30.6% of the patients with lesions. Other common types included eczema (in 23.5%), xerosis cutis (10.6%), palmoplantar pustulosis (5.3%), and psoriasis (3.8%). Median cumulative doses and trough levels of infliximab were similar in people who developed skin lesions and those who did not.
Just over half of patients with skin lesions received only topical treatment, 1.9% received only systemic treatment, 28% received both, and 19.3% of patients required no specific treatment. Almost 11% of patients who developed skin lesions were forced to stop therapy. Reasons for stopping treatment included an intolerable location of lesions, concomitant itching or pain, recurring episodes, and concomitant arthralgia.
“Knowledge of the diagnostic and therapeutic criteria and the clinical course of these lesions should assist in their management. With referral to a dedicated dermatologist, most lesions can be treated and the need for interruption of anti-TNF therapy is rare,” the investigators concluded.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M15-0729).
Inflammatory bowel disease (IBD) patients who experience skin lesions during anti–tumor necrosis factor therapy do not usually need to stop treatment, according to Isabelle Cleynen, Ph.D., of the University of Leuven (Belgium) and her associates.
In their retrospective study of 917 IBD patients who started treatment with infliximab at the University Hospitals Leuven between December 1994 and January 2009, 264 developed skin lesions during the follow-up period. The most common type was psoriasiform eczema, in 30.6% of the patients with lesions. Other common types included eczema (in 23.5%), xerosis cutis (10.6%), palmoplantar pustulosis (5.3%), and psoriasis (3.8%). Median cumulative doses and trough levels of infliximab were similar in people who developed skin lesions and those who did not.
Just over half of patients with skin lesions received only topical treatment, 1.9% received only systemic treatment, 28% received both, and 19.3% of patients required no specific treatment. Almost 11% of patients who developed skin lesions were forced to stop therapy. Reasons for stopping treatment included an intolerable location of lesions, concomitant itching or pain, recurring episodes, and concomitant arthralgia.
“Knowledge of the diagnostic and therapeutic criteria and the clinical course of these lesions should assist in their management. With referral to a dedicated dermatologist, most lesions can be treated and the need for interruption of anti-TNF therapy is rare,” the investigators concluded.
Find the full study in Annals of Internal Medicine (doi: 10.7326/M15-0729).
AGA publishes microscopic colitis guideline
A microscopic colitis treatment guideline from the American Gastroenterological Association was published in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2015.11.008).
Microscopic colitis is most common in persons aged 60 years and over, typically presents as chronic watery diarrhea with nausea or abdominal pain caused by inflammation in the colon, and is diagnosed by colonic biopsy. It is more of a nuisance than life threatening, with no evidence that the persistent histologic inflammation predicts colorectal cancer or the need for surgical intervention.
The guideline strongly recommends, with moderate-quality evidence, treating symptomatic patients with budesonide rather than foregoing any treatment. The AGA also strongly recommends, with high-quality evidence, that budesonide be used over mesalamine to induce remission, unless such treatment is not feasible in a patient because of a drug reaction or its high price. In that case, the AGA recommends (with moderate-quality evidence) the use of mesalamine. Once induction therapy is discontinued, the AGA strongly recommends, with moderate-quality evidence, maintenance treatment using budesonide, if possible.
In patients for whom budesonide is not feasible, the AGA recommends that rather than forgo treatment, patients may be induced with bismuth subsalicylate (low-quality evidence) or prednisone (very-low-quality evidence).
Other recommendations made with low-quality evidence for treating symptomatic microscopic colitis include not using combination therapy with cholestyramine and mesalamine over mesalamine alone for induction therapy, nor Boswellia serrata to induce remission. The AGA also does not recommend (low-quality evidence) using probiotics over no treatment for the condition.
Because the meta-analysis of evidence conducted by the AGA only included data derived from clinical trials, the guideline does not include antidiarrheals, cholestyramine monotherapy, a variety of combination therapies, nor the medical treatment of steroid-refractory microscopic colitis, although the AGA did note in the guideline that very limited evidence from a series of cases suggests certain immunosuppressive therapies may benefit these patients.
On Twitter @whitneymcknight
A microscopic colitis treatment guideline from the American Gastroenterological Association was published in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2015.11.008).
Microscopic colitis is most common in persons aged 60 years and over, typically presents as chronic watery diarrhea with nausea or abdominal pain caused by inflammation in the colon, and is diagnosed by colonic biopsy. It is more of a nuisance than life threatening, with no evidence that the persistent histologic inflammation predicts colorectal cancer or the need for surgical intervention.
The guideline strongly recommends, with moderate-quality evidence, treating symptomatic patients with budesonide rather than foregoing any treatment. The AGA also strongly recommends, with high-quality evidence, that budesonide be used over mesalamine to induce remission, unless such treatment is not feasible in a patient because of a drug reaction or its high price. In that case, the AGA recommends (with moderate-quality evidence) the use of mesalamine. Once induction therapy is discontinued, the AGA strongly recommends, with moderate-quality evidence, maintenance treatment using budesonide, if possible.
In patients for whom budesonide is not feasible, the AGA recommends that rather than forgo treatment, patients may be induced with bismuth subsalicylate (low-quality evidence) or prednisone (very-low-quality evidence).
Other recommendations made with low-quality evidence for treating symptomatic microscopic colitis include not using combination therapy with cholestyramine and mesalamine over mesalamine alone for induction therapy, nor Boswellia serrata to induce remission. The AGA also does not recommend (low-quality evidence) using probiotics over no treatment for the condition.
Because the meta-analysis of evidence conducted by the AGA only included data derived from clinical trials, the guideline does not include antidiarrheals, cholestyramine monotherapy, a variety of combination therapies, nor the medical treatment of steroid-refractory microscopic colitis, although the AGA did note in the guideline that very limited evidence from a series of cases suggests certain immunosuppressive therapies may benefit these patients.
On Twitter @whitneymcknight
A microscopic colitis treatment guideline from the American Gastroenterological Association was published in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2015.11.008).
Microscopic colitis is most common in persons aged 60 years and over, typically presents as chronic watery diarrhea with nausea or abdominal pain caused by inflammation in the colon, and is diagnosed by colonic biopsy. It is more of a nuisance than life threatening, with no evidence that the persistent histologic inflammation predicts colorectal cancer or the need for surgical intervention.
The guideline strongly recommends, with moderate-quality evidence, treating symptomatic patients with budesonide rather than foregoing any treatment. The AGA also strongly recommends, with high-quality evidence, that budesonide be used over mesalamine to induce remission, unless such treatment is not feasible in a patient because of a drug reaction or its high price. In that case, the AGA recommends (with moderate-quality evidence) the use of mesalamine. Once induction therapy is discontinued, the AGA strongly recommends, with moderate-quality evidence, maintenance treatment using budesonide, if possible.
In patients for whom budesonide is not feasible, the AGA recommends that rather than forgo treatment, patients may be induced with bismuth subsalicylate (low-quality evidence) or prednisone (very-low-quality evidence).
Other recommendations made with low-quality evidence for treating symptomatic microscopic colitis include not using combination therapy with cholestyramine and mesalamine over mesalamine alone for induction therapy, nor Boswellia serrata to induce remission. The AGA also does not recommend (low-quality evidence) using probiotics over no treatment for the condition.
Because the meta-analysis of evidence conducted by the AGA only included data derived from clinical trials, the guideline does not include antidiarrheals, cholestyramine monotherapy, a variety of combination therapies, nor the medical treatment of steroid-refractory microscopic colitis, although the AGA did note in the guideline that very limited evidence from a series of cases suggests certain immunosuppressive therapies may benefit these patients.
On Twitter @whitneymcknight
FROM THE AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Postop C. diff infection associated with presurgical antibiotics
A hospital’s rate of postoperative Clostridium difficile infection is related to the number of preoperative antibiotics patients have taken, the complexity of their procedures, and the complexity of the hospital’s surgical program, in addition to known risk factors for the infection, according to a report published online in JAMA Surgery.
Several risk factors for postoperative C. difficile infection have already been identified, including advanced age and comorbidity. To examine known risk factors and identify possible new ones, researchers analyzed information from the Veterans Affairs Surgical Quality Improvement Program’s database, which documents all noncardiac operations at 134 VA medical centers each year.
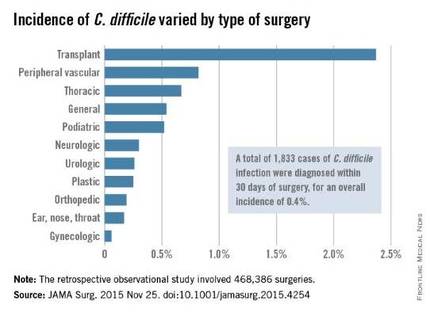
The investigators focused on 468,386 procedures performed during a 4-year period. A total of 1,833 cases of C. difficile infection were diagnosed within 30 days of surgery, for an overall incidence of 0.4% in this predominantly male, elderly population, said Xinli Li, Ph.D., of the Veterans Health Administration, Washington, and associates.
As expected, patients who developed postoperative C. difficile infection were significantly older than those who didn’t (mean age, 67.4 vs. 60.6 years) and were significantly more likely to have comorbidities such as impaired functional status, heart failure, chronic obstructive pulmonary disease, ascites, renal failure, bleeding disorders, wound infection, and recent weight loss.
Unexpectedly, the number of different antibiotics taken during the 60 days preceding surgery also was significantly associated with C. difficile infection. Patients who had taken three or more antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics, the investigators reported (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4263).In addition, patients who underwent more complex surgical procedures were at increased risk of this complication, as were patients at hospitals that frequently handled complex procedures. “These factors reflect the illness of patients, duration of operation, and hospital setting; each is an established risk factor for C. difficile infection,” Dr. Li and associates wrote.
Patients with C. difficile infection had higher rates of postoperative other morbidity (86.0% vs. 7.1%) and 30-day mortality (5.3% vs. 1.0%) and longer postoperative hospital stays (17.9 days vs. 3.6 days).
Contrary to previous studies, this study did not show a temporal increase in C. difficile infection. The overall incidence, as well as the incidences at individual hospitals, remained constant during the entire 4-year study period, the investigators added.
The incidence of C. difficile varied substantially among the 134 VA medical centers, from 0% to 1.35% of all surgical patients. “Surgical administrators and clinical teams may consider the results of this study to target interventions for specific patients undergoing high-risk procedures. Such interventions include selective antibiotic administration, early testing of at-risk patients, hand hygiene with nonalcohol agents, early contact precautions, and specific environmental cleaning protocols,” Dr. Li and associates wrote.
This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
The most important finding to highlight in the report by Li et al. is the 12-fold increase in morbidity and 5-fold increase in mortality among patients who developed postoperative C. difficile infection.
The study results underscore the importance of infection control and prevention efforts. They also show how important it is to develop prophylactic strategies, expeditious recognition of C. difficile, adequate supportive care, and improved therapies.
Dr. Paul K. Waltz and Dr. Brian S. Zuckerbraun are at the VA Pittsburgh Healthcare System and the University of Pennsylvania, Pittsburgh. They made these remarks in an invited commentary accompanying Dr. Li’s report (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4254).
The most important finding to highlight in the report by Li et al. is the 12-fold increase in morbidity and 5-fold increase in mortality among patients who developed postoperative C. difficile infection.
The study results underscore the importance of infection control and prevention efforts. They also show how important it is to develop prophylactic strategies, expeditious recognition of C. difficile, adequate supportive care, and improved therapies.
Dr. Paul K. Waltz and Dr. Brian S. Zuckerbraun are at the VA Pittsburgh Healthcare System and the University of Pennsylvania, Pittsburgh. They made these remarks in an invited commentary accompanying Dr. Li’s report (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4254).
The most important finding to highlight in the report by Li et al. is the 12-fold increase in morbidity and 5-fold increase in mortality among patients who developed postoperative C. difficile infection.
The study results underscore the importance of infection control and prevention efforts. They also show how important it is to develop prophylactic strategies, expeditious recognition of C. difficile, adequate supportive care, and improved therapies.
Dr. Paul K. Waltz and Dr. Brian S. Zuckerbraun are at the VA Pittsburgh Healthcare System and the University of Pennsylvania, Pittsburgh. They made these remarks in an invited commentary accompanying Dr. Li’s report (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4254).
A hospital’s rate of postoperative Clostridium difficile infection is related to the number of preoperative antibiotics patients have taken, the complexity of their procedures, and the complexity of the hospital’s surgical program, in addition to known risk factors for the infection, according to a report published online in JAMA Surgery.
Several risk factors for postoperative C. difficile infection have already been identified, including advanced age and comorbidity. To examine known risk factors and identify possible new ones, researchers analyzed information from the Veterans Affairs Surgical Quality Improvement Program’s database, which documents all noncardiac operations at 134 VA medical centers each year.

The investigators focused on 468,386 procedures performed during a 4-year period. A total of 1,833 cases of C. difficile infection were diagnosed within 30 days of surgery, for an overall incidence of 0.4% in this predominantly male, elderly population, said Xinli Li, Ph.D., of the Veterans Health Administration, Washington, and associates.
As expected, patients who developed postoperative C. difficile infection were significantly older than those who didn’t (mean age, 67.4 vs. 60.6 years) and were significantly more likely to have comorbidities such as impaired functional status, heart failure, chronic obstructive pulmonary disease, ascites, renal failure, bleeding disorders, wound infection, and recent weight loss.
Unexpectedly, the number of different antibiotics taken during the 60 days preceding surgery also was significantly associated with C. difficile infection. Patients who had taken three or more antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics, the investigators reported (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4263).In addition, patients who underwent more complex surgical procedures were at increased risk of this complication, as were patients at hospitals that frequently handled complex procedures. “These factors reflect the illness of patients, duration of operation, and hospital setting; each is an established risk factor for C. difficile infection,” Dr. Li and associates wrote.
Patients with C. difficile infection had higher rates of postoperative other morbidity (86.0% vs. 7.1%) and 30-day mortality (5.3% vs. 1.0%) and longer postoperative hospital stays (17.9 days vs. 3.6 days).
Contrary to previous studies, this study did not show a temporal increase in C. difficile infection. The overall incidence, as well as the incidences at individual hospitals, remained constant during the entire 4-year study period, the investigators added.
The incidence of C. difficile varied substantially among the 134 VA medical centers, from 0% to 1.35% of all surgical patients. “Surgical administrators and clinical teams may consider the results of this study to target interventions for specific patients undergoing high-risk procedures. Such interventions include selective antibiotic administration, early testing of at-risk patients, hand hygiene with nonalcohol agents, early contact precautions, and specific environmental cleaning protocols,” Dr. Li and associates wrote.
This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
A hospital’s rate of postoperative Clostridium difficile infection is related to the number of preoperative antibiotics patients have taken, the complexity of their procedures, and the complexity of the hospital’s surgical program, in addition to known risk factors for the infection, according to a report published online in JAMA Surgery.
Several risk factors for postoperative C. difficile infection have already been identified, including advanced age and comorbidity. To examine known risk factors and identify possible new ones, researchers analyzed information from the Veterans Affairs Surgical Quality Improvement Program’s database, which documents all noncardiac operations at 134 VA medical centers each year.

The investigators focused on 468,386 procedures performed during a 4-year period. A total of 1,833 cases of C. difficile infection were diagnosed within 30 days of surgery, for an overall incidence of 0.4% in this predominantly male, elderly population, said Xinli Li, Ph.D., of the Veterans Health Administration, Washington, and associates.
As expected, patients who developed postoperative C. difficile infection were significantly older than those who didn’t (mean age, 67.4 vs. 60.6 years) and were significantly more likely to have comorbidities such as impaired functional status, heart failure, chronic obstructive pulmonary disease, ascites, renal failure, bleeding disorders, wound infection, and recent weight loss.
Unexpectedly, the number of different antibiotics taken during the 60 days preceding surgery also was significantly associated with C. difficile infection. Patients who had taken three or more antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics, the investigators reported (JAMA Surg. 2015 Nov 25. doi: 10.1001/jamasurg.2015.4263).In addition, patients who underwent more complex surgical procedures were at increased risk of this complication, as were patients at hospitals that frequently handled complex procedures. “These factors reflect the illness of patients, duration of operation, and hospital setting; each is an established risk factor for C. difficile infection,” Dr. Li and associates wrote.
Patients with C. difficile infection had higher rates of postoperative other morbidity (86.0% vs. 7.1%) and 30-day mortality (5.3% vs. 1.0%) and longer postoperative hospital stays (17.9 days vs. 3.6 days).
Contrary to previous studies, this study did not show a temporal increase in C. difficile infection. The overall incidence, as well as the incidences at individual hospitals, remained constant during the entire 4-year study period, the investigators added.
The incidence of C. difficile varied substantially among the 134 VA medical centers, from 0% to 1.35% of all surgical patients. “Surgical administrators and clinical teams may consider the results of this study to target interventions for specific patients undergoing high-risk procedures. Such interventions include selective antibiotic administration, early testing of at-risk patients, hand hygiene with nonalcohol agents, early contact precautions, and specific environmental cleaning protocols,” Dr. Li and associates wrote.
This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point: A hospital’s rate of postoperative C. difficile infection is related to the number of preoperative antibiotics patients took and the complexity of their surgeries.
Major finding: Patients who had taken three or more preoperative antibiotics from different classes were nearly six times more likely to develop C. difficile than patients who had taken only one or no antibiotics.
Data source: A retrospective observational study involving 468,386 surgeries at 134 VA medical centers during a 4-year period.
Disclosures: This study was supported by the Veterans Health Administration. Dr. Li and associates reported having no relevant financial disclosures.
Roux-en-Y improves NASH in obese patients
SAN FRANCISCO – Bariatric surgery is an effective treatment for nonalcoholic steatohepatitis (NASH) in obese patients, a Markov model suggests.
Patients with all classes of obesity, including, mild, moderate, and severe, with all stages of fibrosis, experienced gains in life years following laparoscopic Roux-en-Y gastric bypass, compared with standard management and intensive lifestyle changes, based on the model, Dr. Kathleen Corey reported at the annual meeting of the American Association for the Study of Liver Diseases.
Surgery also increased quality-adjusted life years (QALY) in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis, said Dr Corey of Massachusetts General Hospital, Boston.
The number of F3 patients needed to treat to prevent one liver-related death was favorable across all body mass indexes for those with stage F3 fibrosis. The number needed to treat to prevent one case of cirrhosis in these patients was six to seven, and to prevent one liver-related death was eight to 11, she said.
A cost-effectiveness analysis, with a willingness-to-pay threshold of less than $100,000, also showed that surgery was cost effective for all fibrosis stages for those with severe and moderate obesity.
“The [incremental cost-effectiveness ratio] for BMI of 35-39.9 [kg/m2] was $34,000, for those with a BMI of 40 or greater it was $26,000, and we found that with overweight patients with F3 fibrosis, the ratio was also favorable at $59,000,” she said.
More than 78 million American adults suffer from obesity, and the prevalence of obesity is rising nationwide, she said, adding that obesity results in annual medical costs exceeding $147 billion. Treatment options for obesity are limited, and weight regain after weight loss is frequent, Dr. Corey noted.
“However, bariatric surgery has been shown to be a very effective treatment for obesity. Surgery has been shown to significantly reduce mortality in patients, and reduces progression of many comorbidities, including diabetes,” she said.
Guidelines suggest that bariatric surgery is appropriate for patients with a body mass index of 40 or greater and for those with a BMI of 35 or greater if obesity-related comorbidities such as diabetes or obstructive sleep apnea are present, and data suggest that surgery is also of benefit in those with BMI below 35 with diabetes.
The question remains, though, whether nonalcoholic fatty liver disease (NAFLD) – the most common cause of liver disease in the United States – is an indication in itself for bariatric surgery.
NAFLD is strongly associated with obesity, and its progressive form – NASH – can lead to cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.
“However, there are no FDA-approved therapies for NASH and no randomized controlled trials have been conducted to evaluate the role of bariatric surgery in NASH ... and appropriate BMI cutoffs in those with NASH have not been established,” Dr. Corey said.
Several nonrandomized prospective trials have, however, evaluated bariatric surgery in NASH patients with BMIs of 35 or greater with comorbidities, and in patients with BMIs of 40 or greater, and these have shown that NASH resolution can occur in up to 85% of patients at 1-5 years with fibrosis reduction seen in nearly a third, she said, adding that the risk-benefit ratio for surgery is unknown, as is the value of surgery in NASH patients with BMIs less than 35.
The current study looked at three treatment strategies, including standard management with lifestyle counseling; intensive lifestyle intervention based on a diabetes prevention program with lifestyle, nutrition, and exercise counseling; and bariatric Roux-en-Y gastric bypass in a hypothetical simulated cohort of 45-year-olds with NASH, F0-F3 fibrosis, and varying BMI values.
The findings, though limited by a lack of data on the impact of surgery on patients with a BMI of less than 35, and by evaluation of only one type of surgery, showed that bariatric surgery increased incremental life years at all weight and fibrosis stages vs. standard management and intensive lifestyle intervention, and that surgery increased QALY in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis.
“We found that surgery was cost effective for F3 fibrosis in those with moderate and severe obesity and those with overweight. Randomized trials, though, are needed to assess the management of weight loss surgery for NASH before this can be recommended, certainly in patients with a BMI of less than 35,” she concluded.
Dr. Corey reported serving on an advisory committee or review panel for Gilead, and serving in a speaking or teaching role for Synageva.
SAN FRANCISCO – Bariatric surgery is an effective treatment for nonalcoholic steatohepatitis (NASH) in obese patients, a Markov model suggests.
Patients with all classes of obesity, including, mild, moderate, and severe, with all stages of fibrosis, experienced gains in life years following laparoscopic Roux-en-Y gastric bypass, compared with standard management and intensive lifestyle changes, based on the model, Dr. Kathleen Corey reported at the annual meeting of the American Association for the Study of Liver Diseases.
Surgery also increased quality-adjusted life years (QALY) in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis, said Dr Corey of Massachusetts General Hospital, Boston.
The number of F3 patients needed to treat to prevent one liver-related death was favorable across all body mass indexes for those with stage F3 fibrosis. The number needed to treat to prevent one case of cirrhosis in these patients was six to seven, and to prevent one liver-related death was eight to 11, she said.
A cost-effectiveness analysis, with a willingness-to-pay threshold of less than $100,000, also showed that surgery was cost effective for all fibrosis stages for those with severe and moderate obesity.
“The [incremental cost-effectiveness ratio] for BMI of 35-39.9 [kg/m2] was $34,000, for those with a BMI of 40 or greater it was $26,000, and we found that with overweight patients with F3 fibrosis, the ratio was also favorable at $59,000,” she said.
More than 78 million American adults suffer from obesity, and the prevalence of obesity is rising nationwide, she said, adding that obesity results in annual medical costs exceeding $147 billion. Treatment options for obesity are limited, and weight regain after weight loss is frequent, Dr. Corey noted.
“However, bariatric surgery has been shown to be a very effective treatment for obesity. Surgery has been shown to significantly reduce mortality in patients, and reduces progression of many comorbidities, including diabetes,” she said.
Guidelines suggest that bariatric surgery is appropriate for patients with a body mass index of 40 or greater and for those with a BMI of 35 or greater if obesity-related comorbidities such as diabetes or obstructive sleep apnea are present, and data suggest that surgery is also of benefit in those with BMI below 35 with diabetes.
The question remains, though, whether nonalcoholic fatty liver disease (NAFLD) – the most common cause of liver disease in the United States – is an indication in itself for bariatric surgery.
NAFLD is strongly associated with obesity, and its progressive form – NASH – can lead to cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.
“However, there are no FDA-approved therapies for NASH and no randomized controlled trials have been conducted to evaluate the role of bariatric surgery in NASH ... and appropriate BMI cutoffs in those with NASH have not been established,” Dr. Corey said.
Several nonrandomized prospective trials have, however, evaluated bariatric surgery in NASH patients with BMIs of 35 or greater with comorbidities, and in patients with BMIs of 40 or greater, and these have shown that NASH resolution can occur in up to 85% of patients at 1-5 years with fibrosis reduction seen in nearly a third, she said, adding that the risk-benefit ratio for surgery is unknown, as is the value of surgery in NASH patients with BMIs less than 35.
The current study looked at three treatment strategies, including standard management with lifestyle counseling; intensive lifestyle intervention based on a diabetes prevention program with lifestyle, nutrition, and exercise counseling; and bariatric Roux-en-Y gastric bypass in a hypothetical simulated cohort of 45-year-olds with NASH, F0-F3 fibrosis, and varying BMI values.
The findings, though limited by a lack of data on the impact of surgery on patients with a BMI of less than 35, and by evaluation of only one type of surgery, showed that bariatric surgery increased incremental life years at all weight and fibrosis stages vs. standard management and intensive lifestyle intervention, and that surgery increased QALY in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis.
“We found that surgery was cost effective for F3 fibrosis in those with moderate and severe obesity and those with overweight. Randomized trials, though, are needed to assess the management of weight loss surgery for NASH before this can be recommended, certainly in patients with a BMI of less than 35,” she concluded.
Dr. Corey reported serving on an advisory committee or review panel for Gilead, and serving in a speaking or teaching role for Synageva.
SAN FRANCISCO – Bariatric surgery is an effective treatment for nonalcoholic steatohepatitis (NASH) in obese patients, a Markov model suggests.
Patients with all classes of obesity, including, mild, moderate, and severe, with all stages of fibrosis, experienced gains in life years following laparoscopic Roux-en-Y gastric bypass, compared with standard management and intensive lifestyle changes, based on the model, Dr. Kathleen Corey reported at the annual meeting of the American Association for the Study of Liver Diseases.
Surgery also increased quality-adjusted life years (QALY) in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis, said Dr Corey of Massachusetts General Hospital, Boston.
The number of F3 patients needed to treat to prevent one liver-related death was favorable across all body mass indexes for those with stage F3 fibrosis. The number needed to treat to prevent one case of cirrhosis in these patients was six to seven, and to prevent one liver-related death was eight to 11, she said.
A cost-effectiveness analysis, with a willingness-to-pay threshold of less than $100,000, also showed that surgery was cost effective for all fibrosis stages for those with severe and moderate obesity.
“The [incremental cost-effectiveness ratio] for BMI of 35-39.9 [kg/m2] was $34,000, for those with a BMI of 40 or greater it was $26,000, and we found that with overweight patients with F3 fibrosis, the ratio was also favorable at $59,000,” she said.
More than 78 million American adults suffer from obesity, and the prevalence of obesity is rising nationwide, she said, adding that obesity results in annual medical costs exceeding $147 billion. Treatment options for obesity are limited, and weight regain after weight loss is frequent, Dr. Corey noted.
“However, bariatric surgery has been shown to be a very effective treatment for obesity. Surgery has been shown to significantly reduce mortality in patients, and reduces progression of many comorbidities, including diabetes,” she said.
Guidelines suggest that bariatric surgery is appropriate for patients with a body mass index of 40 or greater and for those with a BMI of 35 or greater if obesity-related comorbidities such as diabetes or obstructive sleep apnea are present, and data suggest that surgery is also of benefit in those with BMI below 35 with diabetes.
The question remains, though, whether nonalcoholic fatty liver disease (NAFLD) – the most common cause of liver disease in the United States – is an indication in itself for bariatric surgery.
NAFLD is strongly associated with obesity, and its progressive form – NASH – can lead to cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.
“However, there are no FDA-approved therapies for NASH and no randomized controlled trials have been conducted to evaluate the role of bariatric surgery in NASH ... and appropriate BMI cutoffs in those with NASH have not been established,” Dr. Corey said.
Several nonrandomized prospective trials have, however, evaluated bariatric surgery in NASH patients with BMIs of 35 or greater with comorbidities, and in patients with BMIs of 40 or greater, and these have shown that NASH resolution can occur in up to 85% of patients at 1-5 years with fibrosis reduction seen in nearly a third, she said, adding that the risk-benefit ratio for surgery is unknown, as is the value of surgery in NASH patients with BMIs less than 35.
The current study looked at three treatment strategies, including standard management with lifestyle counseling; intensive lifestyle intervention based on a diabetes prevention program with lifestyle, nutrition, and exercise counseling; and bariatric Roux-en-Y gastric bypass in a hypothetical simulated cohort of 45-year-olds with NASH, F0-F3 fibrosis, and varying BMI values.
The findings, though limited by a lack of data on the impact of surgery on patients with a BMI of less than 35, and by evaluation of only one type of surgery, showed that bariatric surgery increased incremental life years at all weight and fibrosis stages vs. standard management and intensive lifestyle intervention, and that surgery increased QALY in those with moderate and severe obesity with all fibrosis stages, those with mild obesity and F2-F3 fibrosis, and in overweight patients with F3 fibrosis.
“We found that surgery was cost effective for F3 fibrosis in those with moderate and severe obesity and those with overweight. Randomized trials, though, are needed to assess the management of weight loss surgery for NASH before this can be recommended, certainly in patients with a BMI of less than 35,” she concluded.
Dr. Corey reported serving on an advisory committee or review panel for Gilead, and serving in a speaking or teaching role for Synageva.
AT THE LIVER MEETING 2015
Key clinical point: Bariatric surgery is an effective treatment for nonalcoholic steatohepatitis (NASH) in obese patients, a Markov model suggests.
Major finding: The number needed to treat to prevent 1 case of cirrhosis was 6-7; to prevent 1 liver-related death, it was 8-11.
Data source: A Markov modeling study involving a hypothetical simulated cohort of patients.
Disclosures: Dr. Corey reported serving on an advisory committee or review panel for Gilead, and serving in a speaking or teaching role for Synageva.
Crohn’s study found no reason to continue immunomodulators after starting anti-TNFs
Baseline exposure to an immunomodulator did not improve the odds of clinical response or remission when starting anti–tumor necrosis factor (anti-TNF) therapy for Crohn’s disease (CD), said authors of a meta-analysis of 11 randomized, controlled trials. Pending better trials, patients with CD and their clinicians will need to carefully weigh the risks and benefits of continuing an immunomodulator when starting anti-TNF therapy, Dr. Jennifer Jones of Dalhousie University in Halifax, Canada and her associates wrote in the December issue of Clinical Gastroenterology and Hepatology.
Intense debate persists about whether patients with CD who have already been exposed to immunomodulators such as azathioprine, 6-mercaptopurine, and methotrexate should stay on them when starting anti-TNF agents. The landmark 2010 SONIC trial could not answer this question because it only enrolled patients who had never received an immunomodulator, and more recent studies (Clin Gastroenterol Hepatol. 2011;9:36-41) have raised concerns about the safety of immunomodulators, the researchers noted. To compare combination immunomodulators and anti-TNF treatment with anti-TNF monotherapy in luminal and fistulizing CD, they analyzed original datasets from 11 randomized, controlled trials published between 1980 and 2008. A total of 625 patients with CD had received an immunomodulator, while 976 patients had not. The investigators excluded trials in which patients were naive to both immunomodulators and anti-TNF agents (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.034]).
In the overall analysis, combination therapy was no better than anti-TNF monotherapy in terms of 6-month remission, maintenance of response, or partial or full fistula closure, Dr. Jones and her associates reported. The same was true for subgroup analyses, but the odds ratio for infliximab reached statistical significance in a sensitivity analysis that included data from the ACCENT 2 (Clin Gastroenterol Hepatol. 2004;2:912-20) trial. “For the infliximab-only analysis, adding ACCENT 2 resulted in minimal change in the point estimate but, as expected, increased the precision of the 95% CIs (the lower CI increased from 0.97 to 1.06), which led to a statistically significant difference in the comparison between infliximab monotherapy and combination therapy,” the researchers commented. While sensitivity analyses have limitations, the finding “does raise the question” of whether the benefits of staying on an immunomodulator depend on the anti-TNF agent, they said.
Combination therapy did not heighten the chances of infusion reactions, malignancies, serious infections, or death, said the investigators. In fact, baseline immunomodulator exposure was associated with fewer injection site reactions among infliximab patients (OR, 0.46; 95% CI, 0.26-0.79). The researchers did not uncover publication bias, and found significant heterogeneity among studies only for the 6-month clinical response endpoint, they added.
The findings “challenge the clinical importance of combination therapy” in the setting of baseline immunomodulator exposure, but “it is hard to ignore the preponderance of data” on anti-TNF pharmacokinetics that support combination therapy over monotherapy, the investigators emphasized. “Whether combination therapy has a greater protective effect against anti-drug antibody development and lower trough levels for all anti-TNF agents or for patients previously exposed to anti-TNF agents is still in question,” they added. They called for a well-designed, randomized, placebo-controlled trial that uses objective measures of disease activity and follow patients long enough to assess efficacy.
The investigators reported no funding sources for the study. Dr. Jones reported having been a speaker for Jansen, Merck, Schering-Plough, Abbott, and AbbVie, and having served on advisory boards for Janssen, Abbott, and Takeda. Nine co-authors reported financial and consulting relationships with Jansen, Merck, Schering-Plough, Abbott, and a number of other pharmaceutical companies.
Source: American Gastroenterological Association
Baseline exposure to an immunomodulator did not improve the odds of clinical response or remission when starting anti–tumor necrosis factor (anti-TNF) therapy for Crohn’s disease (CD), said authors of a meta-analysis of 11 randomized, controlled trials. Pending better trials, patients with CD and their clinicians will need to carefully weigh the risks and benefits of continuing an immunomodulator when starting anti-TNF therapy, Dr. Jennifer Jones of Dalhousie University in Halifax, Canada and her associates wrote in the December issue of Clinical Gastroenterology and Hepatology.
Intense debate persists about whether patients with CD who have already been exposed to immunomodulators such as azathioprine, 6-mercaptopurine, and methotrexate should stay on them when starting anti-TNF agents. The landmark 2010 SONIC trial could not answer this question because it only enrolled patients who had never received an immunomodulator, and more recent studies (Clin Gastroenterol Hepatol. 2011;9:36-41) have raised concerns about the safety of immunomodulators, the researchers noted. To compare combination immunomodulators and anti-TNF treatment with anti-TNF monotherapy in luminal and fistulizing CD, they analyzed original datasets from 11 randomized, controlled trials published between 1980 and 2008. A total of 625 patients with CD had received an immunomodulator, while 976 patients had not. The investigators excluded trials in which patients were naive to both immunomodulators and anti-TNF agents (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.034]).
In the overall analysis, combination therapy was no better than anti-TNF monotherapy in terms of 6-month remission, maintenance of response, or partial or full fistula closure, Dr. Jones and her associates reported. The same was true for subgroup analyses, but the odds ratio for infliximab reached statistical significance in a sensitivity analysis that included data from the ACCENT 2 (Clin Gastroenterol Hepatol. 2004;2:912-20) trial. “For the infliximab-only analysis, adding ACCENT 2 resulted in minimal change in the point estimate but, as expected, increased the precision of the 95% CIs (the lower CI increased from 0.97 to 1.06), which led to a statistically significant difference in the comparison between infliximab monotherapy and combination therapy,” the researchers commented. While sensitivity analyses have limitations, the finding “does raise the question” of whether the benefits of staying on an immunomodulator depend on the anti-TNF agent, they said.
Combination therapy did not heighten the chances of infusion reactions, malignancies, serious infections, or death, said the investigators. In fact, baseline immunomodulator exposure was associated with fewer injection site reactions among infliximab patients (OR, 0.46; 95% CI, 0.26-0.79). The researchers did not uncover publication bias, and found significant heterogeneity among studies only for the 6-month clinical response endpoint, they added.
The findings “challenge the clinical importance of combination therapy” in the setting of baseline immunomodulator exposure, but “it is hard to ignore the preponderance of data” on anti-TNF pharmacokinetics that support combination therapy over monotherapy, the investigators emphasized. “Whether combination therapy has a greater protective effect against anti-drug antibody development and lower trough levels for all anti-TNF agents or for patients previously exposed to anti-TNF agents is still in question,” they added. They called for a well-designed, randomized, placebo-controlled trial that uses objective measures of disease activity and follow patients long enough to assess efficacy.
The investigators reported no funding sources for the study. Dr. Jones reported having been a speaker for Jansen, Merck, Schering-Plough, Abbott, and AbbVie, and having served on advisory boards for Janssen, Abbott, and Takeda. Nine co-authors reported financial and consulting relationships with Jansen, Merck, Schering-Plough, Abbott, and a number of other pharmaceutical companies.
Source: American Gastroenterological Association
Baseline exposure to an immunomodulator did not improve the odds of clinical response or remission when starting anti–tumor necrosis factor (anti-TNF) therapy for Crohn’s disease (CD), said authors of a meta-analysis of 11 randomized, controlled trials. Pending better trials, patients with CD and their clinicians will need to carefully weigh the risks and benefits of continuing an immunomodulator when starting anti-TNF therapy, Dr. Jennifer Jones of Dalhousie University in Halifax, Canada and her associates wrote in the December issue of Clinical Gastroenterology and Hepatology.
Intense debate persists about whether patients with CD who have already been exposed to immunomodulators such as azathioprine, 6-mercaptopurine, and methotrexate should stay on them when starting anti-TNF agents. The landmark 2010 SONIC trial could not answer this question because it only enrolled patients who had never received an immunomodulator, and more recent studies (Clin Gastroenterol Hepatol. 2011;9:36-41) have raised concerns about the safety of immunomodulators, the researchers noted. To compare combination immunomodulators and anti-TNF treatment with anti-TNF monotherapy in luminal and fistulizing CD, they analyzed original datasets from 11 randomized, controlled trials published between 1980 and 2008. A total of 625 patients with CD had received an immunomodulator, while 976 patients had not. The investigators excluded trials in which patients were naive to both immunomodulators and anti-TNF agents (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.034]).
In the overall analysis, combination therapy was no better than anti-TNF monotherapy in terms of 6-month remission, maintenance of response, or partial or full fistula closure, Dr. Jones and her associates reported. The same was true for subgroup analyses, but the odds ratio for infliximab reached statistical significance in a sensitivity analysis that included data from the ACCENT 2 (Clin Gastroenterol Hepatol. 2004;2:912-20) trial. “For the infliximab-only analysis, adding ACCENT 2 resulted in minimal change in the point estimate but, as expected, increased the precision of the 95% CIs (the lower CI increased from 0.97 to 1.06), which led to a statistically significant difference in the comparison between infliximab monotherapy and combination therapy,” the researchers commented. While sensitivity analyses have limitations, the finding “does raise the question” of whether the benefits of staying on an immunomodulator depend on the anti-TNF agent, they said.
Combination therapy did not heighten the chances of infusion reactions, malignancies, serious infections, or death, said the investigators. In fact, baseline immunomodulator exposure was associated with fewer injection site reactions among infliximab patients (OR, 0.46; 95% CI, 0.26-0.79). The researchers did not uncover publication bias, and found significant heterogeneity among studies only for the 6-month clinical response endpoint, they added.
The findings “challenge the clinical importance of combination therapy” in the setting of baseline immunomodulator exposure, but “it is hard to ignore the preponderance of data” on anti-TNF pharmacokinetics that support combination therapy over monotherapy, the investigators emphasized. “Whether combination therapy has a greater protective effect against anti-drug antibody development and lower trough levels for all anti-TNF agents or for patients previously exposed to anti-TNF agents is still in question,” they added. They called for a well-designed, randomized, placebo-controlled trial that uses objective measures of disease activity and follow patients long enough to assess efficacy.
The investigators reported no funding sources for the study. Dr. Jones reported having been a speaker for Jansen, Merck, Schering-Plough, Abbott, and AbbVie, and having served on advisory boards for Janssen, Abbott, and Takeda. Nine co-authors reported financial and consulting relationships with Jansen, Merck, Schering-Plough, Abbott, and a number of other pharmaceutical companies.
Source: American Gastroenterological Association
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Continuing an immunomodulator was no more effective than switching to anti-TNF monotherapy in a meta-analysis of patients with Crohn’s disease.
Major finding: Combination therapy was no more effective than anti-TNF monotherapy in terms of clinical response, remission induction, or fistula closure.
Data source: Meta-analysis of 11 randomized, controlled trials of 1,601 patients with luminal or fistulizing CD.
Disclosures: The investigators reported no funding sources for the study. Dr. Jones reported having been a speaker for Jansen, Merck, Schering-Plough, Abbott, and AbbVie, and serving on advisory boards for Janssen, Abbott, and Takeda. Nine coauthors reported financial and consulting relationships with Jansen, Merck, Schering-Plough, Abbott, and a number of other pharmaceutical companies.
Norovirus sends 1.6 million to doctors every year
SAN DIEGO – Norovirus infections send about 1.6 million people to the doctor every year in the United States, according to the first active surveillance study to cover all age groups here.
“This provides a baseline burden of norovirus disease to assess the potential impact of future vaccines,” said Dr. Aron Hall of the Centers for Disease Control and Prevention in Atlanta. Estimated infection rates generally resembled those from past studies, but were more robust and granular, Dr. Hall said at an annual scientific meeting on infectious diseases.
About 19-21 million Americans suffer acute gastroenteritis because of norovirus infection every year, Dr. Hall noted. About 400,000 of these patients seek urgent care, at least 56,000 are hospitalized, and 570-800 ultimately die from associated complications. However, several factors have impeded large epidemiologic studies of norovirus disease, he added. Most infected patients do not seek care, those who do often do not undergo stool testing, there is no national norovirus case reporting system, turnaround times and the sensitivity of clinical assays remain suboptimal, and there are no specific ICD codes for norovirus gastroenteritis, he said.
To overcome these obstacles, Dr. Hall and his associates spent a year studying 480,000 Kaiser Permanente Northwest enrollees in the Portland, Ore. area. Enrollees seek care almost entirely in-network and demographically resemble other residents in the region, Dr. Hall said. The researchers used automated software to identify about 19,000 patients with ICD-9 codes for acute gastroenteritis. They called about half of these patients to request stool samples within 3 days of their appointments, while they were likely to still be shedding pathogens. They aimed to test all patients who were younger than 5 years or older than 65 years, and called a random sample of 35% of the other patients.
In all, the researchers tested stool specimens for 84% (1,467) of patients whom they reached within the 3-day window, said Dr. Hall. About 13% of these patients tested positive for norovirus, of which 85% were genotype II, while the rest were genotype I. The overall incidence of medically attended acute gastroenteritis due to norovirus was 5 cases per 100,00 person-years, but rates were four to five times higher among infants and children under the age of 2 years (22.7 and 29.1 cases per 100,000 person-years, respectively), and about 50% higher among adults aged 85 years and older (7.4 cases per 100,000 person-years).
“Extrapolation to the U.S. population would yield 1.6 million norovirus-associated MAAGE [medically attended acute gastroenteritis] encounters per year,” Dr. Hall concluded. The research team is now sequencing norovirus genotypes, testing stool samples for other enteric pathogens, and studying high-risk subgroups, clinical severity, and cases of potential household transmission, he added.
Dr. Hall spoke at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. He had no disclosures. One of his coauthors reported receiving research support from GlaxoSmithKline.
SAN DIEGO – Norovirus infections send about 1.6 million people to the doctor every year in the United States, according to the first active surveillance study to cover all age groups here.
“This provides a baseline burden of norovirus disease to assess the potential impact of future vaccines,” said Dr. Aron Hall of the Centers for Disease Control and Prevention in Atlanta. Estimated infection rates generally resembled those from past studies, but were more robust and granular, Dr. Hall said at an annual scientific meeting on infectious diseases.
About 19-21 million Americans suffer acute gastroenteritis because of norovirus infection every year, Dr. Hall noted. About 400,000 of these patients seek urgent care, at least 56,000 are hospitalized, and 570-800 ultimately die from associated complications. However, several factors have impeded large epidemiologic studies of norovirus disease, he added. Most infected patients do not seek care, those who do often do not undergo stool testing, there is no national norovirus case reporting system, turnaround times and the sensitivity of clinical assays remain suboptimal, and there are no specific ICD codes for norovirus gastroenteritis, he said.
To overcome these obstacles, Dr. Hall and his associates spent a year studying 480,000 Kaiser Permanente Northwest enrollees in the Portland, Ore. area. Enrollees seek care almost entirely in-network and demographically resemble other residents in the region, Dr. Hall said. The researchers used automated software to identify about 19,000 patients with ICD-9 codes for acute gastroenteritis. They called about half of these patients to request stool samples within 3 days of their appointments, while they were likely to still be shedding pathogens. They aimed to test all patients who were younger than 5 years or older than 65 years, and called a random sample of 35% of the other patients.
In all, the researchers tested stool specimens for 84% (1,467) of patients whom they reached within the 3-day window, said Dr. Hall. About 13% of these patients tested positive for norovirus, of which 85% were genotype II, while the rest were genotype I. The overall incidence of medically attended acute gastroenteritis due to norovirus was 5 cases per 100,00 person-years, but rates were four to five times higher among infants and children under the age of 2 years (22.7 and 29.1 cases per 100,000 person-years, respectively), and about 50% higher among adults aged 85 years and older (7.4 cases per 100,000 person-years).
“Extrapolation to the U.S. population would yield 1.6 million norovirus-associated MAAGE [medically attended acute gastroenteritis] encounters per year,” Dr. Hall concluded. The research team is now sequencing norovirus genotypes, testing stool samples for other enteric pathogens, and studying high-risk subgroups, clinical severity, and cases of potential household transmission, he added.
Dr. Hall spoke at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. He had no disclosures. One of his coauthors reported receiving research support from GlaxoSmithKline.
SAN DIEGO – Norovirus infections send about 1.6 million people to the doctor every year in the United States, according to the first active surveillance study to cover all age groups here.
“This provides a baseline burden of norovirus disease to assess the potential impact of future vaccines,” said Dr. Aron Hall of the Centers for Disease Control and Prevention in Atlanta. Estimated infection rates generally resembled those from past studies, but were more robust and granular, Dr. Hall said at an annual scientific meeting on infectious diseases.
About 19-21 million Americans suffer acute gastroenteritis because of norovirus infection every year, Dr. Hall noted. About 400,000 of these patients seek urgent care, at least 56,000 are hospitalized, and 570-800 ultimately die from associated complications. However, several factors have impeded large epidemiologic studies of norovirus disease, he added. Most infected patients do not seek care, those who do often do not undergo stool testing, there is no national norovirus case reporting system, turnaround times and the sensitivity of clinical assays remain suboptimal, and there are no specific ICD codes for norovirus gastroenteritis, he said.
To overcome these obstacles, Dr. Hall and his associates spent a year studying 480,000 Kaiser Permanente Northwest enrollees in the Portland, Ore. area. Enrollees seek care almost entirely in-network and demographically resemble other residents in the region, Dr. Hall said. The researchers used automated software to identify about 19,000 patients with ICD-9 codes for acute gastroenteritis. They called about half of these patients to request stool samples within 3 days of their appointments, while they were likely to still be shedding pathogens. They aimed to test all patients who were younger than 5 years or older than 65 years, and called a random sample of 35% of the other patients.
In all, the researchers tested stool specimens for 84% (1,467) of patients whom they reached within the 3-day window, said Dr. Hall. About 13% of these patients tested positive for norovirus, of which 85% were genotype II, while the rest were genotype I. The overall incidence of medically attended acute gastroenteritis due to norovirus was 5 cases per 100,00 person-years, but rates were four to five times higher among infants and children under the age of 2 years (22.7 and 29.1 cases per 100,000 person-years, respectively), and about 50% higher among adults aged 85 years and older (7.4 cases per 100,000 person-years).
“Extrapolation to the U.S. population would yield 1.6 million norovirus-associated MAAGE [medically attended acute gastroenteritis] encounters per year,” Dr. Hall concluded. The research team is now sequencing norovirus genotypes, testing stool samples for other enteric pathogens, and studying high-risk subgroups, clinical severity, and cases of potential household transmission, he added.
Dr. Hall spoke at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. He had no disclosures. One of his coauthors reported receiving research support from GlaxoSmithKline.
AT IDWEEK 2015
Key clinical point: Norovirus infection is a common cause of medically attended acute gastroenteritis.
Major finding: Every year, about 1.6 million patients in the United States seek medical care for norovirus-associated MAAGE.
Data source: Active surveillance of 480,000 health maintenance organization enrollees in the urban Pacific Northwest between March 2014 and April 2015.
Disclosures: Dr. Hall had no disclosures. A coauthor reported receiving research support from GlaxoSmithKline.