User login
Omega-3 supplement sweet spot found for BP reduction
A meta-analysis of 71 randomized controlled trials has found the sweet spot for omega-3 fatty acid intake for lowering blood pressure: between 2 and 3 g/day. The investigators also reported that people at higher risk for cardiovascular disease may benefit from higher daily intake of omega-3.
The study analyzed data from randomized controlled trials involving 4,973 individuals and published from 1987 to 2020. Most of the trials used a combined supplementation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Outcomes analysis involved the impact of combined DHA-EPA at 1, 2, 3, 4, or 5 grams daily on average changes in both systolic and diastolic BP and compared them with the placebo or control groups who had a combined intake of 0 g/day.
“We found a significant nonlinear dose-response relationship for both SBP and DBP models,” wrote senior author Xinzhi Li, MD, PhD, and colleagues. Dr. Li is program director of the school of pharmacy at Macau University of Science and Technology in Taipa, China.
Most of the trials included in the meta-analysis evaluated fish oil supplements, but a number also included EPA and DHA omega-3 fatty acids consumed in food.
When the investigators analyzed studies that used an average baseline SBP of greater than 130 mm Hg, they found that increasing omega-3 supplementation resulted in strong reductions in SBP and DBP, but not so with people with baseline SBP below 130 mm Hg.
Across the entire cohort, average SBP and DBP changes averaged –2.61 (95% confidence interval, –3.57 to –1.65) and –1.64 (95% CI, –2.29 to –0.99) mm Hg for people taking 2 g/d omega-3 supplements, and –2.61 (95% CI, –3.52 to –1.69) and –1.80 (95% CI, –2.38 to –1.23) for those on 3 g/d. The changes weren’t as robust in higher and lower intake groups overall.
However, the higher the BP, the more robust the reductions. For those with SBP greater than 130 mm Hg, 3 g/d resulted in an average change of –3.22 mm Hg (95% CI, –5.21 to –1.23). In the greater than 80 mm Hg DBP group, 3 g/d of omega-3 resulted in an average –3.81 mm Hg reduction (95% CI, –4.48 to –1.87). In patients with BP greater than 140/90 and hypertension, the reductions were even more pronounced. And in patients with BP greater than 130/80, omega-3 intake of 4-5 g/d had a greater impact than 2-3 g/d, although that benefit didn’t carry over in the greater than 140/90 group.
High cholesterol was also a factor in determining the benefits of omega-3 supplementation on BP, as Dr. Li and colleagues wrote that they found “an approximately linear relationship” between hyperlipidemia and SBP, “suggesting that increasing supplementation was associated with greater reductions in SBP.” Likewise, the study found stronger effects on BP in studies with an average patient age greater than 45 years.
In 2019, the Food and Drug Administration issued an update that consuming combined EPA and DHA may lower BP in the general population and reduce the risk of hypertension, but that “the evidence is inconsistent and inconclusive.”
“However, while our study may add a layer of credible evidence, it does not meet the threshold to make an authorized health claim for omega-3 fatty acids in compliance with FDA regulations,” Dr. Li said.
The study addresses shortcomings of previous studies of omega-3 and BP and by identifying the optimal dose, Marc George, MRCP, PhD, of the Institute of Cardiovascular Science, University College, London, and Ajay Gupta, MD, PhD, of the William Harvey Research Institute at Queen Mary University, London, wrote in an accompanying editorial. “More importantly, they have demonstrated a significantly stronger and increased BP-lowering effect in higher cardiovascular risk groups, such as those with hypertension or hyperlipidemia.”
They also noted that the 2.61–mm Hg reduction in SBP the study reported is “likely to be significant” on a population level. “A 2–mm Hg reduction in SBP is estimated to reduce stroke mortality by 10% and deaths from ischemic heart disease by 7%,” they wrote. “Expressed another way, an analysis in the U.S. population using 2010 data estimates that a population-wide reduction in SBP of 2 mm Hg in those aged 45- 64 years would translate to 30,045 fewer cardiovascular events ([coronary heart disease], stroke, and heart failure).”
The investigators and editorialists have no disclosures.
A meta-analysis of 71 randomized controlled trials has found the sweet spot for omega-3 fatty acid intake for lowering blood pressure: between 2 and 3 g/day. The investigators also reported that people at higher risk for cardiovascular disease may benefit from higher daily intake of omega-3.
The study analyzed data from randomized controlled trials involving 4,973 individuals and published from 1987 to 2020. Most of the trials used a combined supplementation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Outcomes analysis involved the impact of combined DHA-EPA at 1, 2, 3, 4, or 5 grams daily on average changes in both systolic and diastolic BP and compared them with the placebo or control groups who had a combined intake of 0 g/day.
“We found a significant nonlinear dose-response relationship for both SBP and DBP models,” wrote senior author Xinzhi Li, MD, PhD, and colleagues. Dr. Li is program director of the school of pharmacy at Macau University of Science and Technology in Taipa, China.
Most of the trials included in the meta-analysis evaluated fish oil supplements, but a number also included EPA and DHA omega-3 fatty acids consumed in food.
When the investigators analyzed studies that used an average baseline SBP of greater than 130 mm Hg, they found that increasing omega-3 supplementation resulted in strong reductions in SBP and DBP, but not so with people with baseline SBP below 130 mm Hg.
Across the entire cohort, average SBP and DBP changes averaged –2.61 (95% confidence interval, –3.57 to –1.65) and –1.64 (95% CI, –2.29 to –0.99) mm Hg for people taking 2 g/d omega-3 supplements, and –2.61 (95% CI, –3.52 to –1.69) and –1.80 (95% CI, –2.38 to –1.23) for those on 3 g/d. The changes weren’t as robust in higher and lower intake groups overall.
However, the higher the BP, the more robust the reductions. For those with SBP greater than 130 mm Hg, 3 g/d resulted in an average change of –3.22 mm Hg (95% CI, –5.21 to –1.23). In the greater than 80 mm Hg DBP group, 3 g/d of omega-3 resulted in an average –3.81 mm Hg reduction (95% CI, –4.48 to –1.87). In patients with BP greater than 140/90 and hypertension, the reductions were even more pronounced. And in patients with BP greater than 130/80, omega-3 intake of 4-5 g/d had a greater impact than 2-3 g/d, although that benefit didn’t carry over in the greater than 140/90 group.
High cholesterol was also a factor in determining the benefits of omega-3 supplementation on BP, as Dr. Li and colleagues wrote that they found “an approximately linear relationship” between hyperlipidemia and SBP, “suggesting that increasing supplementation was associated with greater reductions in SBP.” Likewise, the study found stronger effects on BP in studies with an average patient age greater than 45 years.
In 2019, the Food and Drug Administration issued an update that consuming combined EPA and DHA may lower BP in the general population and reduce the risk of hypertension, but that “the evidence is inconsistent and inconclusive.”
“However, while our study may add a layer of credible evidence, it does not meet the threshold to make an authorized health claim for omega-3 fatty acids in compliance with FDA regulations,” Dr. Li said.
The study addresses shortcomings of previous studies of omega-3 and BP and by identifying the optimal dose, Marc George, MRCP, PhD, of the Institute of Cardiovascular Science, University College, London, and Ajay Gupta, MD, PhD, of the William Harvey Research Institute at Queen Mary University, London, wrote in an accompanying editorial. “More importantly, they have demonstrated a significantly stronger and increased BP-lowering effect in higher cardiovascular risk groups, such as those with hypertension or hyperlipidemia.”
They also noted that the 2.61–mm Hg reduction in SBP the study reported is “likely to be significant” on a population level. “A 2–mm Hg reduction in SBP is estimated to reduce stroke mortality by 10% and deaths from ischemic heart disease by 7%,” they wrote. “Expressed another way, an analysis in the U.S. population using 2010 data estimates that a population-wide reduction in SBP of 2 mm Hg in those aged 45- 64 years would translate to 30,045 fewer cardiovascular events ([coronary heart disease], stroke, and heart failure).”
The investigators and editorialists have no disclosures.
A meta-analysis of 71 randomized controlled trials has found the sweet spot for omega-3 fatty acid intake for lowering blood pressure: between 2 and 3 g/day. The investigators also reported that people at higher risk for cardiovascular disease may benefit from higher daily intake of omega-3.
The study analyzed data from randomized controlled trials involving 4,973 individuals and published from 1987 to 2020. Most of the trials used a combined supplementation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Outcomes analysis involved the impact of combined DHA-EPA at 1, 2, 3, 4, or 5 grams daily on average changes in both systolic and diastolic BP and compared them with the placebo or control groups who had a combined intake of 0 g/day.
“We found a significant nonlinear dose-response relationship for both SBP and DBP models,” wrote senior author Xinzhi Li, MD, PhD, and colleagues. Dr. Li is program director of the school of pharmacy at Macau University of Science and Technology in Taipa, China.
Most of the trials included in the meta-analysis evaluated fish oil supplements, but a number also included EPA and DHA omega-3 fatty acids consumed in food.
When the investigators analyzed studies that used an average baseline SBP of greater than 130 mm Hg, they found that increasing omega-3 supplementation resulted in strong reductions in SBP and DBP, but not so with people with baseline SBP below 130 mm Hg.
Across the entire cohort, average SBP and DBP changes averaged –2.61 (95% confidence interval, –3.57 to –1.65) and –1.64 (95% CI, –2.29 to –0.99) mm Hg for people taking 2 g/d omega-3 supplements, and –2.61 (95% CI, –3.52 to –1.69) and –1.80 (95% CI, –2.38 to –1.23) for those on 3 g/d. The changes weren’t as robust in higher and lower intake groups overall.
However, the higher the BP, the more robust the reductions. For those with SBP greater than 130 mm Hg, 3 g/d resulted in an average change of –3.22 mm Hg (95% CI, –5.21 to –1.23). In the greater than 80 mm Hg DBP group, 3 g/d of omega-3 resulted in an average –3.81 mm Hg reduction (95% CI, –4.48 to –1.87). In patients with BP greater than 140/90 and hypertension, the reductions were even more pronounced. And in patients with BP greater than 130/80, omega-3 intake of 4-5 g/d had a greater impact than 2-3 g/d, although that benefit didn’t carry over in the greater than 140/90 group.
High cholesterol was also a factor in determining the benefits of omega-3 supplementation on BP, as Dr. Li and colleagues wrote that they found “an approximately linear relationship” between hyperlipidemia and SBP, “suggesting that increasing supplementation was associated with greater reductions in SBP.” Likewise, the study found stronger effects on BP in studies with an average patient age greater than 45 years.
In 2019, the Food and Drug Administration issued an update that consuming combined EPA and DHA may lower BP in the general population and reduce the risk of hypertension, but that “the evidence is inconsistent and inconclusive.”
“However, while our study may add a layer of credible evidence, it does not meet the threshold to make an authorized health claim for omega-3 fatty acids in compliance with FDA regulations,” Dr. Li said.
The study addresses shortcomings of previous studies of omega-3 and BP and by identifying the optimal dose, Marc George, MRCP, PhD, of the Institute of Cardiovascular Science, University College, London, and Ajay Gupta, MD, PhD, of the William Harvey Research Institute at Queen Mary University, London, wrote in an accompanying editorial. “More importantly, they have demonstrated a significantly stronger and increased BP-lowering effect in higher cardiovascular risk groups, such as those with hypertension or hyperlipidemia.”
They also noted that the 2.61–mm Hg reduction in SBP the study reported is “likely to be significant” on a population level. “A 2–mm Hg reduction in SBP is estimated to reduce stroke mortality by 10% and deaths from ischemic heart disease by 7%,” they wrote. “Expressed another way, an analysis in the U.S. population using 2010 data estimates that a population-wide reduction in SBP of 2 mm Hg in those aged 45- 64 years would translate to 30,045 fewer cardiovascular events ([coronary heart disease], stroke, and heart failure).”
The investigators and editorialists have no disclosures.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
LDL lowering to specific targets may offset risk from high Lp(a)
MILAN – The increased risk for atherosclerotic cardiovascular disease events caused by elevated lipoprotein(a) levels can potentially be precisely offset by lowering LDL cholesterol to specific levels, suggests a novel study that underscores the importance or early intervention.
The results, derived from an analysis of data on Lp(a) and LDL cholesterol levels and associated genetic risk scores in almost 500,000 individuals from the United Kingdom, have been used to develop a series of age-related targets for lowering LDL cholesterol levels to counter the risk associated with lifetime Lp(a) exposure.
Measuring Lp(a) levels can “substantially refine individual estimates of absolute risk of atherosclerotic cardiovascular disease,” said study presenter Brian A. Ference, MD, Centre for Naturally Randomized Trials, University of Cambridge (England).
This can “directly inform treatment decisions about the intensity of LDL lowering or other risk-factor modification needed to overcome the increased risk caused by Lp(a).”
Dr. Ference said this will allow clinicians to personalize the prevention of atherosclerotic cardiovascular disease and identify people “who may benefit from potent Lp(a)-lowering therapies when they become available.”
The research was presented at the European Atherosclerosis Society (EAS) 2022 congress on May 24.
In addition to producing a tabular version of the intensification of LDL-cholesterol reduction needed to overcome the increased cardiovascular risk at different levels of Lp(a), stratified by age, Dr. Ference is working with the EAS to develop an app to further deliver on that personalized prevention.
It will display an individual’s lifetime risk for myocardial infarction or stroke, with and without the inclusion of Lp(a) levels, and determine not only the percentage of increased risk caused by Lp(a), but also the amount by which LDL cholesterol needs to be lowered to overcome that risk.
“The whole rationale for this study was to say, how can we give practical advice on how to use Lp(a) to inform clinical decisions about how to individualize personal risk reduction,” Dr. Ference told this news organization.
“What the app will do is make it very easy for clinicians to, first, understand how much Lp(a) increases risk, but specifically how they can use that information to directly inform their treatment decisions.”
In addition, Dr. Ference said that it will “show patients why it’s important for them” to intensify LDL lowering to overcome their particular level of Lp(a).
Other key takeaways from the results is the importance of intervention as early as possible to minimize the impact of lifetime exposure to increased Lp(a), and that the reduction in LDL cholesterol required to achieve that remains relatively modest.
For Dr. Ference, this means ideally beginning comprehensive health checks at 30 years of age and starting lipid-lowering interventions immediately for those at risk.
“The good thing about LDL and other causes of atherosclerotic cardiovascular disease is it doesn’t really matter how you lower it,” he said, noting that it could be with diet, lifestyle interventions, or medication.
Handy tool
The new app could be a “handy tool to counsel patients,” Florian Kronenberg, MD, Institute of Genetic Epidemiology, Medical University of Innsbruck, Austria, told this news organization.
“We can say, look, you have high Lp(a),” he said. “This is coming from nature, from your genetics, but here we have a point where we can act on your high risk by lowering LDL further. This is important to explain to the patient,” said Dr. Kronenberg, who was not involved in the study.
He emphasized that it is crucial to get across the idea of an individual’s global risk, with not just Lp(a) or cholesterol levels influencing their likelihood of cardiovascular events, but also their age, blood pressure, smoking status, and underlying genetic risk.
Dr. Kronenberg said the current data will be helpful in explaining to clinicians why they should lower LDL-cholesterol levels when a patients had high Lp(a), again centered on the idea of lowering their global risk.
During his presentation, Dr. Ference noted that an increase in Lp(a) levels is associated with a log-linear increase in atherosclerotic cardiovascular disease that is proportional to the absolute, rather than relative, magnitude of Lp(a) increase.
“Unfortunately, unlike other proteins,” he continued, diet and exercise do not affect levels, and there are currently no effective therapies to lower the risks associated with increased Lp(a) concentrations.
“For that reason,” he said, the 2019 ESC/EAS guidelines for the management of dyslipidemias, on which Dr. Ference was a coauthor, “recommend that we intensify life risk-factor modification in persons with elevated risks.”
However, he added, “this guidance is not specific enough to be useful, and that has created a great deal of inertia among clinicians,” with some concluding that they don’t need to measure Lp(a) “because there’s nothing they can do for it.”
Until the development of novel therapies that directly target Lp(a), the authors sought to quantify the amount of LDL lowering needed to “overcome the increased risk caused by Lp(a),” he said.
They studied data on 455,765 individuals from the UK Biobank who did not have a history of cardiovascular events, diabetes, or any cancer before the age of 30. They also had LDL cholesterol levels below 5 mmol/L at the time of enrollment to exclude people with presumed familial hypercholesterolemia.
The researchers used an Lp(a) genetic risk score based on the variants rs10455872 and rs3798220 and an LDL instrumental variable genetic score comprised of 100 variants to randomly categorize individuals with average Lp(a) levels, higher Lp(a) levels, or higher Lp(a) and lower LDL-cholesterol levels.
The data showed that, with elevated absolute levels of measured Lp(a) and with elevated genetic risk scores, there was a progressive increase in the lifetime risk for major coronary events.
When looking at the combination of both increased Lp(a) levels and lower LDL-cholesterol levels, they found that the increase in risk for major coronary events at Lp(a) of 123 nmol/L could be offset by a reduction in LDL-cholesterol levels of 19.5 mg/dL.
For people with an Lp(a) level of 251 nmol/L, the increase in risk for major coronary events was offset by a reduction in LDL-cholesterol levels of 36.1 mg/dL.
Furthermore, the researchers found that the magnitude of intensification of LDL-cholesterol lowering needed to overcome the risk caused by elevated Lp(a) levels varied by age.
For example, in individuals with an Lp(a) level of 220 nmol/L, the reduction in LDL-cholesterol levels needed to offset the risk for major coronary events was calculated to be 0.8 mmol/L if lipid-lowering was started at 30 years of age, rising to 0.9 mmol/L if started at 40 years, 1.2 mmol/L if started at 50 years, and 1.5 mmol/L if started at 60 years.
This, Dr. Ference said, suggests that “diet and lifestyle modification is unlikely to be an effective strategy if started later.”
No funding was declared. Dr. Ference declared relationships with Amgen, Novartis, Merck, Esperion Therapeutics, Pfizer, Regeneron, Sanofi, AstraZeneca, Eli Lilly, Novo Nordisk, The Medicines Company, Mylan, Daiichi Sankyo, Viatris, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, and KrKa Pharmaceuticals. Dr. Kronenberg declared relationships with Amgen, Novartis, and Kaneka.
A version of this article first appeared on Medscape.com.
MILAN – The increased risk for atherosclerotic cardiovascular disease events caused by elevated lipoprotein(a) levels can potentially be precisely offset by lowering LDL cholesterol to specific levels, suggests a novel study that underscores the importance or early intervention.
The results, derived from an analysis of data on Lp(a) and LDL cholesterol levels and associated genetic risk scores in almost 500,000 individuals from the United Kingdom, have been used to develop a series of age-related targets for lowering LDL cholesterol levels to counter the risk associated with lifetime Lp(a) exposure.
Measuring Lp(a) levels can “substantially refine individual estimates of absolute risk of atherosclerotic cardiovascular disease,” said study presenter Brian A. Ference, MD, Centre for Naturally Randomized Trials, University of Cambridge (England).
This can “directly inform treatment decisions about the intensity of LDL lowering or other risk-factor modification needed to overcome the increased risk caused by Lp(a).”
Dr. Ference said this will allow clinicians to personalize the prevention of atherosclerotic cardiovascular disease and identify people “who may benefit from potent Lp(a)-lowering therapies when they become available.”
The research was presented at the European Atherosclerosis Society (EAS) 2022 congress on May 24.
In addition to producing a tabular version of the intensification of LDL-cholesterol reduction needed to overcome the increased cardiovascular risk at different levels of Lp(a), stratified by age, Dr. Ference is working with the EAS to develop an app to further deliver on that personalized prevention.
It will display an individual’s lifetime risk for myocardial infarction or stroke, with and without the inclusion of Lp(a) levels, and determine not only the percentage of increased risk caused by Lp(a), but also the amount by which LDL cholesterol needs to be lowered to overcome that risk.
“The whole rationale for this study was to say, how can we give practical advice on how to use Lp(a) to inform clinical decisions about how to individualize personal risk reduction,” Dr. Ference told this news organization.
“What the app will do is make it very easy for clinicians to, first, understand how much Lp(a) increases risk, but specifically how they can use that information to directly inform their treatment decisions.”
In addition, Dr. Ference said that it will “show patients why it’s important for them” to intensify LDL lowering to overcome their particular level of Lp(a).
Other key takeaways from the results is the importance of intervention as early as possible to minimize the impact of lifetime exposure to increased Lp(a), and that the reduction in LDL cholesterol required to achieve that remains relatively modest.
For Dr. Ference, this means ideally beginning comprehensive health checks at 30 years of age and starting lipid-lowering interventions immediately for those at risk.
“The good thing about LDL and other causes of atherosclerotic cardiovascular disease is it doesn’t really matter how you lower it,” he said, noting that it could be with diet, lifestyle interventions, or medication.
Handy tool
The new app could be a “handy tool to counsel patients,” Florian Kronenberg, MD, Institute of Genetic Epidemiology, Medical University of Innsbruck, Austria, told this news organization.
“We can say, look, you have high Lp(a),” he said. “This is coming from nature, from your genetics, but here we have a point where we can act on your high risk by lowering LDL further. This is important to explain to the patient,” said Dr. Kronenberg, who was not involved in the study.
He emphasized that it is crucial to get across the idea of an individual’s global risk, with not just Lp(a) or cholesterol levels influencing their likelihood of cardiovascular events, but also their age, blood pressure, smoking status, and underlying genetic risk.
Dr. Kronenberg said the current data will be helpful in explaining to clinicians why they should lower LDL-cholesterol levels when a patients had high Lp(a), again centered on the idea of lowering their global risk.
During his presentation, Dr. Ference noted that an increase in Lp(a) levels is associated with a log-linear increase in atherosclerotic cardiovascular disease that is proportional to the absolute, rather than relative, magnitude of Lp(a) increase.
“Unfortunately, unlike other proteins,” he continued, diet and exercise do not affect levels, and there are currently no effective therapies to lower the risks associated with increased Lp(a) concentrations.
“For that reason,” he said, the 2019 ESC/EAS guidelines for the management of dyslipidemias, on which Dr. Ference was a coauthor, “recommend that we intensify life risk-factor modification in persons with elevated risks.”
However, he added, “this guidance is not specific enough to be useful, and that has created a great deal of inertia among clinicians,” with some concluding that they don’t need to measure Lp(a) “because there’s nothing they can do for it.”
Until the development of novel therapies that directly target Lp(a), the authors sought to quantify the amount of LDL lowering needed to “overcome the increased risk caused by Lp(a),” he said.
They studied data on 455,765 individuals from the UK Biobank who did not have a history of cardiovascular events, diabetes, or any cancer before the age of 30. They also had LDL cholesterol levels below 5 mmol/L at the time of enrollment to exclude people with presumed familial hypercholesterolemia.
The researchers used an Lp(a) genetic risk score based on the variants rs10455872 and rs3798220 and an LDL instrumental variable genetic score comprised of 100 variants to randomly categorize individuals with average Lp(a) levels, higher Lp(a) levels, or higher Lp(a) and lower LDL-cholesterol levels.
The data showed that, with elevated absolute levels of measured Lp(a) and with elevated genetic risk scores, there was a progressive increase in the lifetime risk for major coronary events.
When looking at the combination of both increased Lp(a) levels and lower LDL-cholesterol levels, they found that the increase in risk for major coronary events at Lp(a) of 123 nmol/L could be offset by a reduction in LDL-cholesterol levels of 19.5 mg/dL.
For people with an Lp(a) level of 251 nmol/L, the increase in risk for major coronary events was offset by a reduction in LDL-cholesterol levels of 36.1 mg/dL.
Furthermore, the researchers found that the magnitude of intensification of LDL-cholesterol lowering needed to overcome the risk caused by elevated Lp(a) levels varied by age.
For example, in individuals with an Lp(a) level of 220 nmol/L, the reduction in LDL-cholesterol levels needed to offset the risk for major coronary events was calculated to be 0.8 mmol/L if lipid-lowering was started at 30 years of age, rising to 0.9 mmol/L if started at 40 years, 1.2 mmol/L if started at 50 years, and 1.5 mmol/L if started at 60 years.
This, Dr. Ference said, suggests that “diet and lifestyle modification is unlikely to be an effective strategy if started later.”
No funding was declared. Dr. Ference declared relationships with Amgen, Novartis, Merck, Esperion Therapeutics, Pfizer, Regeneron, Sanofi, AstraZeneca, Eli Lilly, Novo Nordisk, The Medicines Company, Mylan, Daiichi Sankyo, Viatris, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, and KrKa Pharmaceuticals. Dr. Kronenberg declared relationships with Amgen, Novartis, and Kaneka.
A version of this article first appeared on Medscape.com.
MILAN – The increased risk for atherosclerotic cardiovascular disease events caused by elevated lipoprotein(a) levels can potentially be precisely offset by lowering LDL cholesterol to specific levels, suggests a novel study that underscores the importance or early intervention.
The results, derived from an analysis of data on Lp(a) and LDL cholesterol levels and associated genetic risk scores in almost 500,000 individuals from the United Kingdom, have been used to develop a series of age-related targets for lowering LDL cholesterol levels to counter the risk associated with lifetime Lp(a) exposure.
Measuring Lp(a) levels can “substantially refine individual estimates of absolute risk of atherosclerotic cardiovascular disease,” said study presenter Brian A. Ference, MD, Centre for Naturally Randomized Trials, University of Cambridge (England).
This can “directly inform treatment decisions about the intensity of LDL lowering or other risk-factor modification needed to overcome the increased risk caused by Lp(a).”
Dr. Ference said this will allow clinicians to personalize the prevention of atherosclerotic cardiovascular disease and identify people “who may benefit from potent Lp(a)-lowering therapies when they become available.”
The research was presented at the European Atherosclerosis Society (EAS) 2022 congress on May 24.
In addition to producing a tabular version of the intensification of LDL-cholesterol reduction needed to overcome the increased cardiovascular risk at different levels of Lp(a), stratified by age, Dr. Ference is working with the EAS to develop an app to further deliver on that personalized prevention.
It will display an individual’s lifetime risk for myocardial infarction or stroke, with and without the inclusion of Lp(a) levels, and determine not only the percentage of increased risk caused by Lp(a), but also the amount by which LDL cholesterol needs to be lowered to overcome that risk.
“The whole rationale for this study was to say, how can we give practical advice on how to use Lp(a) to inform clinical decisions about how to individualize personal risk reduction,” Dr. Ference told this news organization.
“What the app will do is make it very easy for clinicians to, first, understand how much Lp(a) increases risk, but specifically how they can use that information to directly inform their treatment decisions.”
In addition, Dr. Ference said that it will “show patients why it’s important for them” to intensify LDL lowering to overcome their particular level of Lp(a).
Other key takeaways from the results is the importance of intervention as early as possible to minimize the impact of lifetime exposure to increased Lp(a), and that the reduction in LDL cholesterol required to achieve that remains relatively modest.
For Dr. Ference, this means ideally beginning comprehensive health checks at 30 years of age and starting lipid-lowering interventions immediately for those at risk.
“The good thing about LDL and other causes of atherosclerotic cardiovascular disease is it doesn’t really matter how you lower it,” he said, noting that it could be with diet, lifestyle interventions, or medication.
Handy tool
The new app could be a “handy tool to counsel patients,” Florian Kronenberg, MD, Institute of Genetic Epidemiology, Medical University of Innsbruck, Austria, told this news organization.
“We can say, look, you have high Lp(a),” he said. “This is coming from nature, from your genetics, but here we have a point where we can act on your high risk by lowering LDL further. This is important to explain to the patient,” said Dr. Kronenberg, who was not involved in the study.
He emphasized that it is crucial to get across the idea of an individual’s global risk, with not just Lp(a) or cholesterol levels influencing their likelihood of cardiovascular events, but also their age, blood pressure, smoking status, and underlying genetic risk.
Dr. Kronenberg said the current data will be helpful in explaining to clinicians why they should lower LDL-cholesterol levels when a patients had high Lp(a), again centered on the idea of lowering their global risk.
During his presentation, Dr. Ference noted that an increase in Lp(a) levels is associated with a log-linear increase in atherosclerotic cardiovascular disease that is proportional to the absolute, rather than relative, magnitude of Lp(a) increase.
“Unfortunately, unlike other proteins,” he continued, diet and exercise do not affect levels, and there are currently no effective therapies to lower the risks associated with increased Lp(a) concentrations.
“For that reason,” he said, the 2019 ESC/EAS guidelines for the management of dyslipidemias, on which Dr. Ference was a coauthor, “recommend that we intensify life risk-factor modification in persons with elevated risks.”
However, he added, “this guidance is not specific enough to be useful, and that has created a great deal of inertia among clinicians,” with some concluding that they don’t need to measure Lp(a) “because there’s nothing they can do for it.”
Until the development of novel therapies that directly target Lp(a), the authors sought to quantify the amount of LDL lowering needed to “overcome the increased risk caused by Lp(a),” he said.
They studied data on 455,765 individuals from the UK Biobank who did not have a history of cardiovascular events, diabetes, or any cancer before the age of 30. They also had LDL cholesterol levels below 5 mmol/L at the time of enrollment to exclude people with presumed familial hypercholesterolemia.
The researchers used an Lp(a) genetic risk score based on the variants rs10455872 and rs3798220 and an LDL instrumental variable genetic score comprised of 100 variants to randomly categorize individuals with average Lp(a) levels, higher Lp(a) levels, or higher Lp(a) and lower LDL-cholesterol levels.
The data showed that, with elevated absolute levels of measured Lp(a) and with elevated genetic risk scores, there was a progressive increase in the lifetime risk for major coronary events.
When looking at the combination of both increased Lp(a) levels and lower LDL-cholesterol levels, they found that the increase in risk for major coronary events at Lp(a) of 123 nmol/L could be offset by a reduction in LDL-cholesterol levels of 19.5 mg/dL.
For people with an Lp(a) level of 251 nmol/L, the increase in risk for major coronary events was offset by a reduction in LDL-cholesterol levels of 36.1 mg/dL.
Furthermore, the researchers found that the magnitude of intensification of LDL-cholesterol lowering needed to overcome the risk caused by elevated Lp(a) levels varied by age.
For example, in individuals with an Lp(a) level of 220 nmol/L, the reduction in LDL-cholesterol levels needed to offset the risk for major coronary events was calculated to be 0.8 mmol/L if lipid-lowering was started at 30 years of age, rising to 0.9 mmol/L if started at 40 years, 1.2 mmol/L if started at 50 years, and 1.5 mmol/L if started at 60 years.
This, Dr. Ference said, suggests that “diet and lifestyle modification is unlikely to be an effective strategy if started later.”
No funding was declared. Dr. Ference declared relationships with Amgen, Novartis, Merck, Esperion Therapeutics, Pfizer, Regeneron, Sanofi, AstraZeneca, Eli Lilly, Novo Nordisk, The Medicines Company, Mylan, Daiichi Sankyo, Viatris, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, and KrKa Pharmaceuticals. Dr. Kronenberg declared relationships with Amgen, Novartis, and Kaneka.
A version of this article first appeared on Medscape.com.
AT EAS 2022
Very high HDL-C: Too much of a good thing?
A new study suggests that .
Investigators studied close to 10,000 patients with CAD in two separate cohorts. After adjusting for an array of covariates, they found that individuals with HDL-C levels greater than 80 mg/dL had a 96% higher risk for all-cause mortality and a 71% higher risk for cardiovascular mortality than those with HDL-C levels between 40 and 60 mg/dL.
A U-shaped association was found, with higher risk for all-cause and cardiovascular mortality in patients with both very low and very high, compared with midrange, HDL-C values.
“Very high HDL levels are associated with increased risk of adverse outcomes, not lower risk, as previously thought. This is true not only in the general population, but also in people with known coronary artery disease,” senior author Arshed A. Quyyumi, MD, professor of medicine, division of cardiology, Emory University, Atlanta, told this news organization.
“Physicians have to be cognizant of the fact that, at levels of HDL-C above 80 mg/dL, they [should be] more aggressive with risk reduction and not believe that the patient is at ‘low risk’ because of high levels of ‘good’ cholesterol,” said Dr. Quyyumi, director of the Emory Clinical Cardiovascular Research Institute.
The study was published online in JAMA Cardiology.
Inverse association?
HDL-C levels have “historically been inversely associated with increased cardiovascular disease (CVD) risk; however, recent studies have questioned the efficacy of therapies designed to increase HDL-C levels,” the authors wrote. Moreover, genetic variants associated with HDL-C have not been found to be linked to CVD risk.
Whether “very high HDL-C levels in patients with coronary artery disease (CAD) are associated with mortality risk remains unknown,” they wrote. In this study, the researchers investigated not only the potential risk of elevated HDL-C levels in these patients, but also the association of known HDL-C genetic variants with this risk.
To do so, they analyzed data from a subset of patients with CAD in two independent study groups: the UK Biobank (UKB; n = 14,478; mean [standard deviation] age, 61.2 [5.8] years; 76.2% male; 93.8% White) and the Emory Cardiovascular Biobank (EmCAB; n = 5,467; mean age, 63.8 [12.3] years; 66.4% male; 73.2% White). Participants were followed prospectively for a median of 8.9 (interquartile range, 8.0-9.7) years and 6.7 (IQR, 4.0-10.8) years, respectively.
Additional data collected included medical and medication history and demographic characteristics, which were used as covariates, as well as genomic information.
Of the UKB cohort, 12.4% and 7.9% sustained all-cause or cardiovascular death, respectively, during the follow-up period, and 1.8% of participants had an HDL-C level above 80 mg/dL.
Among these participants with very high HDL-C levels, 16.9% and 8.6% had all-cause or cardiovascular death, respectively. Compared with the reference category (HDL-C level of 40-60 mg/dL), those with low HDL-C levels (≤ 30 mg/dL) had an expected higher risk for both all-cause and cardiovascular mortality, even after adjustment for covariates (hazard ratio, 1.33; 95% confidence interval, 1.07-1.64 and HR, 1.42; 95% CI, 1.09-1.85, respectively; P = .009).
“Importantly,” the authors stated, “compared with the reference category, individuals with very high HDL-C levels (>80 mg/dL) also had a higher risk of all-cause death (HR, 1.58 [1.16-2.14], P = .004).”
Although cardiovascular death rates were not significantly greater in unadjusted analyses, after adjustment, the highest HDL-C group had an increased risk for all-cause and cardiovascular death (HR, 1.96; 95% CI, 1.42-2.71; P < .001 and HR, 1.71; 95% CI, 1.09-2.68, respectively; P = .02).
Compared with females, males with HDL-C levels above 80 mg/dL had a higher risk for all-cause and cardiovascular death.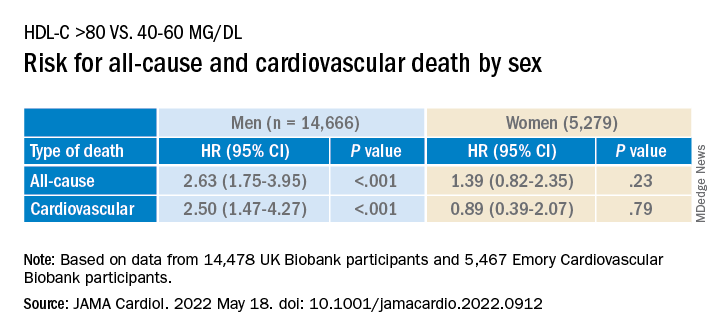
Similar findings were obtained in the EmCAB patients, 1.6% of whom had HDL-C levels above 80 mg/dL. During the follow-up period, 26.9% and 13.8% of participants sustained all-cause and cardiovascular death, respectively. Of those with HDL-C levels above 80 mg/dL, 30.0% and 16.7% experienced all-cause and cardiovascular death, respectively.
Compared with those with HDL-C levels of 40-60 mg/dL, those in the lowest (≤30 mg/dL) and highest (>80 mg/dL) groups had a “significant or near-significant greater risk for all-cause death in both unadjusted and fully adjusted models.
“Using adjusted HR curves, a U-shaped association between HDL-C and adverse events was evident with higher mortality at both very high and low HDL-C levels,” the authors noted.
Compared with patients without diabetes, those with diabetes and an HDL-C level above 80 mg/dL had a higher risk for all-cause and cardiovascular death, and patients younger than 65 years had a higher risk for cardiovascular death than patients 65 years and older.
The researchers found a “positive linear association” between the HDL-C genetic risk score (GRS) and HDL levels, wherein a 1-SD higher HDL-C GRS was associated with a 3.03 mg/dL higher HDL-C level (2.83-3.22; P < .001; R 2 = 0.06).
The HDL-C GRS was not associated with the risk for all-cause or cardiovascular death in unadjusted models, and after the HDL-C GRS was added to the fully adjusted models, the association with HDL-C level above 80 mg/dL was not attenuated, “indicating that HDL-C genetic variations in the GRS do not contribute substantially to the risk.”
“Potential mechanisms through which very high HDL-C might cause adverse cardiovascular outcomes in patients with CAD need to be studied,” Dr. Quyyumi said. “Whether the functional capacity of the HDL particle is altered when the level is very high remains unknown. Whether it is more able to oxidize and thus shift from being protective to harmful also needs to be investigated.”
Red flag
Commenting for this news organization, Sadiya Sana Khan, MD, MSc, assistant professor of medicine (cardiology) and preventive medicine (epidemiology), Northwestern University, Chicago, said: “I think the most important point [of the study] is to identify people with very high HDL-C. This can serve as a reminder to discuss heart-healthy lifestyles and discussion of statin therapy if needed, based on LDL-C.”
In an accompanying editorial coauthored with Gregg Fonarow, MD, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles, the pair wrote: “Although the present findings may be related to residual confounding, high HDL-C levels should not automatically be assumed to be protective.”
They advised clinicians to “use HDL-C levels as a surrogate marker, with very low and very high levels as a red flag to target for more intensive primary and secondary prevention, as the maxim for HDL-C as ‘good’ cholesterol only holds for HDL-C levels of 80 mg/dL or less.”
This study was supported in part by grants from the National Institutes of Health, the American Heart Association, and the Abraham J. & Phyllis Katz Foundation. Dr. Quyyumi and coauthors report no relevant financial relationships. Dr. Khan reports receiving grants from the American Heart Association and the National Institutes of Health outside the submitted work. Dr. Fonarow reports receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
A new study suggests that .
Investigators studied close to 10,000 patients with CAD in two separate cohorts. After adjusting for an array of covariates, they found that individuals with HDL-C levels greater than 80 mg/dL had a 96% higher risk for all-cause mortality and a 71% higher risk for cardiovascular mortality than those with HDL-C levels between 40 and 60 mg/dL.
A U-shaped association was found, with higher risk for all-cause and cardiovascular mortality in patients with both very low and very high, compared with midrange, HDL-C values.
“Very high HDL levels are associated with increased risk of adverse outcomes, not lower risk, as previously thought. This is true not only in the general population, but also in people with known coronary artery disease,” senior author Arshed A. Quyyumi, MD, professor of medicine, division of cardiology, Emory University, Atlanta, told this news organization.
“Physicians have to be cognizant of the fact that, at levels of HDL-C above 80 mg/dL, they [should be] more aggressive with risk reduction and not believe that the patient is at ‘low risk’ because of high levels of ‘good’ cholesterol,” said Dr. Quyyumi, director of the Emory Clinical Cardiovascular Research Institute.
The study was published online in JAMA Cardiology.
Inverse association?
HDL-C levels have “historically been inversely associated with increased cardiovascular disease (CVD) risk; however, recent studies have questioned the efficacy of therapies designed to increase HDL-C levels,” the authors wrote. Moreover, genetic variants associated with HDL-C have not been found to be linked to CVD risk.
Whether “very high HDL-C levels in patients with coronary artery disease (CAD) are associated with mortality risk remains unknown,” they wrote. In this study, the researchers investigated not only the potential risk of elevated HDL-C levels in these patients, but also the association of known HDL-C genetic variants with this risk.
To do so, they analyzed data from a subset of patients with CAD in two independent study groups: the UK Biobank (UKB; n = 14,478; mean [standard deviation] age, 61.2 [5.8] years; 76.2% male; 93.8% White) and the Emory Cardiovascular Biobank (EmCAB; n = 5,467; mean age, 63.8 [12.3] years; 66.4% male; 73.2% White). Participants were followed prospectively for a median of 8.9 (interquartile range, 8.0-9.7) years and 6.7 (IQR, 4.0-10.8) years, respectively.
Additional data collected included medical and medication history and demographic characteristics, which were used as covariates, as well as genomic information.
Of the UKB cohort, 12.4% and 7.9% sustained all-cause or cardiovascular death, respectively, during the follow-up period, and 1.8% of participants had an HDL-C level above 80 mg/dL.
Among these participants with very high HDL-C levels, 16.9% and 8.6% had all-cause or cardiovascular death, respectively. Compared with the reference category (HDL-C level of 40-60 mg/dL), those with low HDL-C levels (≤ 30 mg/dL) had an expected higher risk for both all-cause and cardiovascular mortality, even after adjustment for covariates (hazard ratio, 1.33; 95% confidence interval, 1.07-1.64 and HR, 1.42; 95% CI, 1.09-1.85, respectively; P = .009).
“Importantly,” the authors stated, “compared with the reference category, individuals with very high HDL-C levels (>80 mg/dL) also had a higher risk of all-cause death (HR, 1.58 [1.16-2.14], P = .004).”
Although cardiovascular death rates were not significantly greater in unadjusted analyses, after adjustment, the highest HDL-C group had an increased risk for all-cause and cardiovascular death (HR, 1.96; 95% CI, 1.42-2.71; P < .001 and HR, 1.71; 95% CI, 1.09-2.68, respectively; P = .02).
Compared with females, males with HDL-C levels above 80 mg/dL had a higher risk for all-cause and cardiovascular death.
Similar findings were obtained in the EmCAB patients, 1.6% of whom had HDL-C levels above 80 mg/dL. During the follow-up period, 26.9% and 13.8% of participants sustained all-cause and cardiovascular death, respectively. Of those with HDL-C levels above 80 mg/dL, 30.0% and 16.7% experienced all-cause and cardiovascular death, respectively.
Compared with those with HDL-C levels of 40-60 mg/dL, those in the lowest (≤30 mg/dL) and highest (>80 mg/dL) groups had a “significant or near-significant greater risk for all-cause death in both unadjusted and fully adjusted models.
“Using adjusted HR curves, a U-shaped association between HDL-C and adverse events was evident with higher mortality at both very high and low HDL-C levels,” the authors noted.
Compared with patients without diabetes, those with diabetes and an HDL-C level above 80 mg/dL had a higher risk for all-cause and cardiovascular death, and patients younger than 65 years had a higher risk for cardiovascular death than patients 65 years and older.
The researchers found a “positive linear association” between the HDL-C genetic risk score (GRS) and HDL levels, wherein a 1-SD higher HDL-C GRS was associated with a 3.03 mg/dL higher HDL-C level (2.83-3.22; P < .001; R 2 = 0.06).
The HDL-C GRS was not associated with the risk for all-cause or cardiovascular death in unadjusted models, and after the HDL-C GRS was added to the fully adjusted models, the association with HDL-C level above 80 mg/dL was not attenuated, “indicating that HDL-C genetic variations in the GRS do not contribute substantially to the risk.”
“Potential mechanisms through which very high HDL-C might cause adverse cardiovascular outcomes in patients with CAD need to be studied,” Dr. Quyyumi said. “Whether the functional capacity of the HDL particle is altered when the level is very high remains unknown. Whether it is more able to oxidize and thus shift from being protective to harmful also needs to be investigated.”
Red flag
Commenting for this news organization, Sadiya Sana Khan, MD, MSc, assistant professor of medicine (cardiology) and preventive medicine (epidemiology), Northwestern University, Chicago, said: “I think the most important point [of the study] is to identify people with very high HDL-C. This can serve as a reminder to discuss heart-healthy lifestyles and discussion of statin therapy if needed, based on LDL-C.”
In an accompanying editorial coauthored with Gregg Fonarow, MD, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles, the pair wrote: “Although the present findings may be related to residual confounding, high HDL-C levels should not automatically be assumed to be protective.”
They advised clinicians to “use HDL-C levels as a surrogate marker, with very low and very high levels as a red flag to target for more intensive primary and secondary prevention, as the maxim for HDL-C as ‘good’ cholesterol only holds for HDL-C levels of 80 mg/dL or less.”
This study was supported in part by grants from the National Institutes of Health, the American Heart Association, and the Abraham J. & Phyllis Katz Foundation. Dr. Quyyumi and coauthors report no relevant financial relationships. Dr. Khan reports receiving grants from the American Heart Association and the National Institutes of Health outside the submitted work. Dr. Fonarow reports receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
A new study suggests that .
Investigators studied close to 10,000 patients with CAD in two separate cohorts. After adjusting for an array of covariates, they found that individuals with HDL-C levels greater than 80 mg/dL had a 96% higher risk for all-cause mortality and a 71% higher risk for cardiovascular mortality than those with HDL-C levels between 40 and 60 mg/dL.
A U-shaped association was found, with higher risk for all-cause and cardiovascular mortality in patients with both very low and very high, compared with midrange, HDL-C values.
“Very high HDL levels are associated with increased risk of adverse outcomes, not lower risk, as previously thought. This is true not only in the general population, but also in people with known coronary artery disease,” senior author Arshed A. Quyyumi, MD, professor of medicine, division of cardiology, Emory University, Atlanta, told this news organization.
“Physicians have to be cognizant of the fact that, at levels of HDL-C above 80 mg/dL, they [should be] more aggressive with risk reduction and not believe that the patient is at ‘low risk’ because of high levels of ‘good’ cholesterol,” said Dr. Quyyumi, director of the Emory Clinical Cardiovascular Research Institute.
The study was published online in JAMA Cardiology.
Inverse association?
HDL-C levels have “historically been inversely associated with increased cardiovascular disease (CVD) risk; however, recent studies have questioned the efficacy of therapies designed to increase HDL-C levels,” the authors wrote. Moreover, genetic variants associated with HDL-C have not been found to be linked to CVD risk.
Whether “very high HDL-C levels in patients with coronary artery disease (CAD) are associated with mortality risk remains unknown,” they wrote. In this study, the researchers investigated not only the potential risk of elevated HDL-C levels in these patients, but also the association of known HDL-C genetic variants with this risk.
To do so, they analyzed data from a subset of patients with CAD in two independent study groups: the UK Biobank (UKB; n = 14,478; mean [standard deviation] age, 61.2 [5.8] years; 76.2% male; 93.8% White) and the Emory Cardiovascular Biobank (EmCAB; n = 5,467; mean age, 63.8 [12.3] years; 66.4% male; 73.2% White). Participants were followed prospectively for a median of 8.9 (interquartile range, 8.0-9.7) years and 6.7 (IQR, 4.0-10.8) years, respectively.
Additional data collected included medical and medication history and demographic characteristics, which were used as covariates, as well as genomic information.
Of the UKB cohort, 12.4% and 7.9% sustained all-cause or cardiovascular death, respectively, during the follow-up period, and 1.8% of participants had an HDL-C level above 80 mg/dL.
Among these participants with very high HDL-C levels, 16.9% and 8.6% had all-cause or cardiovascular death, respectively. Compared with the reference category (HDL-C level of 40-60 mg/dL), those with low HDL-C levels (≤ 30 mg/dL) had an expected higher risk for both all-cause and cardiovascular mortality, even after adjustment for covariates (hazard ratio, 1.33; 95% confidence interval, 1.07-1.64 and HR, 1.42; 95% CI, 1.09-1.85, respectively; P = .009).
“Importantly,” the authors stated, “compared with the reference category, individuals with very high HDL-C levels (>80 mg/dL) also had a higher risk of all-cause death (HR, 1.58 [1.16-2.14], P = .004).”
Although cardiovascular death rates were not significantly greater in unadjusted analyses, after adjustment, the highest HDL-C group had an increased risk for all-cause and cardiovascular death (HR, 1.96; 95% CI, 1.42-2.71; P < .001 and HR, 1.71; 95% CI, 1.09-2.68, respectively; P = .02).
Compared with females, males with HDL-C levels above 80 mg/dL had a higher risk for all-cause and cardiovascular death.
Similar findings were obtained in the EmCAB patients, 1.6% of whom had HDL-C levels above 80 mg/dL. During the follow-up period, 26.9% and 13.8% of participants sustained all-cause and cardiovascular death, respectively. Of those with HDL-C levels above 80 mg/dL, 30.0% and 16.7% experienced all-cause and cardiovascular death, respectively.
Compared with those with HDL-C levels of 40-60 mg/dL, those in the lowest (≤30 mg/dL) and highest (>80 mg/dL) groups had a “significant or near-significant greater risk for all-cause death in both unadjusted and fully adjusted models.
“Using adjusted HR curves, a U-shaped association between HDL-C and adverse events was evident with higher mortality at both very high and low HDL-C levels,” the authors noted.
Compared with patients without diabetes, those with diabetes and an HDL-C level above 80 mg/dL had a higher risk for all-cause and cardiovascular death, and patients younger than 65 years had a higher risk for cardiovascular death than patients 65 years and older.
The researchers found a “positive linear association” between the HDL-C genetic risk score (GRS) and HDL levels, wherein a 1-SD higher HDL-C GRS was associated with a 3.03 mg/dL higher HDL-C level (2.83-3.22; P < .001; R 2 = 0.06).
The HDL-C GRS was not associated with the risk for all-cause or cardiovascular death in unadjusted models, and after the HDL-C GRS was added to the fully adjusted models, the association with HDL-C level above 80 mg/dL was not attenuated, “indicating that HDL-C genetic variations in the GRS do not contribute substantially to the risk.”
“Potential mechanisms through which very high HDL-C might cause adverse cardiovascular outcomes in patients with CAD need to be studied,” Dr. Quyyumi said. “Whether the functional capacity of the HDL particle is altered when the level is very high remains unknown. Whether it is more able to oxidize and thus shift from being protective to harmful also needs to be investigated.”
Red flag
Commenting for this news organization, Sadiya Sana Khan, MD, MSc, assistant professor of medicine (cardiology) and preventive medicine (epidemiology), Northwestern University, Chicago, said: “I think the most important point [of the study] is to identify people with very high HDL-C. This can serve as a reminder to discuss heart-healthy lifestyles and discussion of statin therapy if needed, based on LDL-C.”
In an accompanying editorial coauthored with Gregg Fonarow, MD, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles, the pair wrote: “Although the present findings may be related to residual confounding, high HDL-C levels should not automatically be assumed to be protective.”
They advised clinicians to “use HDL-C levels as a surrogate marker, with very low and very high levels as a red flag to target for more intensive primary and secondary prevention, as the maxim for HDL-C as ‘good’ cholesterol only holds for HDL-C levels of 80 mg/dL or less.”
This study was supported in part by grants from the National Institutes of Health, the American Heart Association, and the Abraham J. & Phyllis Katz Foundation. Dr. Quyyumi and coauthors report no relevant financial relationships. Dr. Khan reports receiving grants from the American Heart Association and the National Institutes of Health outside the submitted work. Dr. Fonarow reports receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
SGLT2 inhibitors as first-line therapy in type 2 diabetes?
Use of sodium–glucose cotransporter-2 (SGLT-2) inhibitors rather than metformin as first-line treatment for type 2 diabetes appears to cut the risk for heart failure hospitalization but not myocardial infarction, stroke, or all-cause mortality, a new analysis of real-world data suggests.
Safety findings were similar, except for the fact that genital infections were more common with SGLT-2 inhibitors.
The study was conducted using claims data from two large U.S. insurance databases and Medicare. Propensity score matching was used to account for baseline differences.
The study was conducted by HoJin Shin, BPharm, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues. The findings were published online in Annals of Internal Medicine.
“Those who start SGLT-2 inhibitors as first line show similar risks, compared with metformin in MI, stroke, and all-cause mortality outcomes. Strikingly and consistently, SGLT-2 inhibitors show lower risk for hospitalization for heart failure, which is consistent with the findings from cardiovascular outcomes trials,” Dr. Shin said in an interview.
Just a beginning step, although trial probably wasn’t long enough
However, she added, “I don’t want to overstate anything. ... We aren’t powered enough to investigate who would benefit the most. ... As a pharmacoepidemiologist, I think it’s my duty to provide high-quality evidence so we can actually help physicians and patients make better decisions on their medication. Our current research is just a beginning step.”
Asked to comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, told this news organization, “This study generally confirmed conclusions from published RCTs [randomized clinical trials]. No real surprises, albeit the conclusions may not fully support some of the most enthusiastic claims for SGLT-2 inhibitors with respect to MI, stroke, and cardiovascular death.”
Indeed, Dr. Taylor noted that only two SGLT-2 inhibitors, canagliflozin and empagliflozin, were shown to have a statistically significant association with decreased major adverse cardiovascular events.
In contrast, neither dapagliflozin nor ertugliflozin showed significant benefit regarding those outcomes.
He also pointed out that those four major SLGT-2 inhibitor cardiovascular outcomes trials were placebo-controlled rather than head-to-head trials in which they were compared to an active comparator such as metformin.
“Viewed in this light, it’s probably not surprising that the present study did not demonstrate a robust benefit for SGLT-2 inhibitors to decrease [major adverse CV events].”
The duration of follow-up in the current study is also a limitation, he added.
“The majority of patients were followed for a year or less. This is probably sufficient to assess the impact of some pharmacological mechanisms, for example, the beneficial impact to decrease risk of heart failure by promoting urinary sodium excretion. However, it’s probably insufficient time to observe a beneficial impact on atherosclerosis. For example, there is typically a lag of several years before statins demonstrate efficacy with respect to adverse cardiovascular events.”
Nevertheless, he said, “it provides strong support for benefit with respect to decreasing risk of hospitalization for heart failure.”
He noted that while metformin is currently significantly cheaper than any SGLT-2 inhibitors, once the latter become available as generics, they will be cheaper, and this will likely have a bearing on prescribing decisions.
“Availability of generic SGLT-2 inhibitors offers potential to transform prescribing patterns for type 2 diabetes,” he noted.
First-line SGLT2 inhibitors versus metformin: Most outcomes similar
The study data came from two commercial U.S. health insurance databases, Optum Clinfomatics Data Mart and IBM Marketscan, and from Medicare fee-for-service enrollees.
From April 2013 through March 2020, a total of 9,334 patients began treatment with first-line SGLT-2 inhibitors; 819,973 patients began taking metformin. After 1:2 propensity score matching for confounders, there were 8,613 participants in the SGLT-2 inhibitor group and 17,226 in the group that began treatment with metformin.
The mean follow-up times were 10.7 months for patients taking SGLT-2 inhibitors and 12.2 months for patients taking metformin.
Incidence rates per 1,000 person-years for the composite of hospitalization for MI, hospitalization for ischemic or hemorrhagic stroke, or all-cause mortality (MI/stroke/mortality) were 15.0 versus 16.2 for SLGT-2 inhibitors versus metformin, not a significant difference (hazard ratio, 0.96).
However, for the composite of heart failure hospitalization or all-cause mortality, the rates were 18.3 versus 23.5, a significant difference, with an HR of 0.80. The benefit was seen beginning at about 6 months.
Compared with metformin, SGLT-2 inhibitors showed a significantly lower risk for heart failure hospitalization (HR, 0.78), a numerically (but not significantly) lower risk for MI (HR, 0.70), and similar risks for stroke, mortality, and MI/stroke/HHF/mortality.
Genital infections were significantly more common with SGLT-2 inhibitors (54.1 vs. 23.7 per 1,000 person-years; HR, 2.19). Other safety measures were similar, including acute kidney injury, bone fractures, severe hypoglycemia, diabetic ketoacidosis, and lower-limb amputations.
How does cost factor in?
A sensitivity analysis aimed at examining the possible effect of unmeasured socioeconomic status showed no difference in cardiovascular benefit for first-line SGLT-2 inhibitors and metformin, compared with first-line dipeptidyl peptidase–4 (DPP-4) inhibitors, which cost more than metformin; it is not known what effect DPP-4 inhibitors have on the cardiovascular outcomes of interest.
Cost and insurance coverage factor into the benefit/risk calculation. Metformin is far less costly than any of the SGLT-2 inhibitors – roughly $10 to $20 per month, compared with more than $500 a month.
However, “for some fortunate patients with the most generous pharmacy benefit insurance coverage, the out-of-pocket cost of brand name drugs like SGLT-2 inhibitors is substantially lower,” Dr. Taylor noted.
He said that the current study “raises questions about whether the clinical benefits of SGLT-2 inhibitors as initial monotherapy justify the higher price relative to metformin. The data in this paper suggest that the value case for SGLT-2 inhibitors is strongest for patients with the greatest risk to be hospitalized for heart failure.”
Indeed, Dr. Shin said, “Once we get more information, it may just help in extending the coverage from insurance companies and Medicare/Medicaid, to lower the barrier to access.”
Dr. Taylor reiterated that patents on some of the early SGLT-2 inhibitors are expected to expire in the next few years, which would make it possible for generic versions to be approved. “At that point, prices would likely fall, possibly to levels similar to metformin.”
The study was funded by grant support from the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, and Harvard Medical School, the National Institute on Aging, and the Patient-Centered Outcomes Research Institute. Dr. Shin has disclosed no relevant financial relationships. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Use of sodium–glucose cotransporter-2 (SGLT-2) inhibitors rather than metformin as first-line treatment for type 2 diabetes appears to cut the risk for heart failure hospitalization but not myocardial infarction, stroke, or all-cause mortality, a new analysis of real-world data suggests.
Safety findings were similar, except for the fact that genital infections were more common with SGLT-2 inhibitors.
The study was conducted using claims data from two large U.S. insurance databases and Medicare. Propensity score matching was used to account for baseline differences.
The study was conducted by HoJin Shin, BPharm, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues. The findings were published online in Annals of Internal Medicine.
“Those who start SGLT-2 inhibitors as first line show similar risks, compared with metformin in MI, stroke, and all-cause mortality outcomes. Strikingly and consistently, SGLT-2 inhibitors show lower risk for hospitalization for heart failure, which is consistent with the findings from cardiovascular outcomes trials,” Dr. Shin said in an interview.
Just a beginning step, although trial probably wasn’t long enough
However, she added, “I don’t want to overstate anything. ... We aren’t powered enough to investigate who would benefit the most. ... As a pharmacoepidemiologist, I think it’s my duty to provide high-quality evidence so we can actually help physicians and patients make better decisions on their medication. Our current research is just a beginning step.”
Asked to comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, told this news organization, “This study generally confirmed conclusions from published RCTs [randomized clinical trials]. No real surprises, albeit the conclusions may not fully support some of the most enthusiastic claims for SGLT-2 inhibitors with respect to MI, stroke, and cardiovascular death.”
Indeed, Dr. Taylor noted that only two SGLT-2 inhibitors, canagliflozin and empagliflozin, were shown to have a statistically significant association with decreased major adverse cardiovascular events.
In contrast, neither dapagliflozin nor ertugliflozin showed significant benefit regarding those outcomes.
He also pointed out that those four major SLGT-2 inhibitor cardiovascular outcomes trials were placebo-controlled rather than head-to-head trials in which they were compared to an active comparator such as metformin.
“Viewed in this light, it’s probably not surprising that the present study did not demonstrate a robust benefit for SGLT-2 inhibitors to decrease [major adverse CV events].”
The duration of follow-up in the current study is also a limitation, he added.
“The majority of patients were followed for a year or less. This is probably sufficient to assess the impact of some pharmacological mechanisms, for example, the beneficial impact to decrease risk of heart failure by promoting urinary sodium excretion. However, it’s probably insufficient time to observe a beneficial impact on atherosclerosis. For example, there is typically a lag of several years before statins demonstrate efficacy with respect to adverse cardiovascular events.”
Nevertheless, he said, “it provides strong support for benefit with respect to decreasing risk of hospitalization for heart failure.”
He noted that while metformin is currently significantly cheaper than any SGLT-2 inhibitors, once the latter become available as generics, they will be cheaper, and this will likely have a bearing on prescribing decisions.
“Availability of generic SGLT-2 inhibitors offers potential to transform prescribing patterns for type 2 diabetes,” he noted.
First-line SGLT2 inhibitors versus metformin: Most outcomes similar
The study data came from two commercial U.S. health insurance databases, Optum Clinfomatics Data Mart and IBM Marketscan, and from Medicare fee-for-service enrollees.
From April 2013 through March 2020, a total of 9,334 patients began treatment with first-line SGLT-2 inhibitors; 819,973 patients began taking metformin. After 1:2 propensity score matching for confounders, there were 8,613 participants in the SGLT-2 inhibitor group and 17,226 in the group that began treatment with metformin.
The mean follow-up times were 10.7 months for patients taking SGLT-2 inhibitors and 12.2 months for patients taking metformin.
Incidence rates per 1,000 person-years for the composite of hospitalization for MI, hospitalization for ischemic or hemorrhagic stroke, or all-cause mortality (MI/stroke/mortality) were 15.0 versus 16.2 for SLGT-2 inhibitors versus metformin, not a significant difference (hazard ratio, 0.96).
However, for the composite of heart failure hospitalization or all-cause mortality, the rates were 18.3 versus 23.5, a significant difference, with an HR of 0.80. The benefit was seen beginning at about 6 months.
Compared with metformin, SGLT-2 inhibitors showed a significantly lower risk for heart failure hospitalization (HR, 0.78), a numerically (but not significantly) lower risk for MI (HR, 0.70), and similar risks for stroke, mortality, and MI/stroke/HHF/mortality.
Genital infections were significantly more common with SGLT-2 inhibitors (54.1 vs. 23.7 per 1,000 person-years; HR, 2.19). Other safety measures were similar, including acute kidney injury, bone fractures, severe hypoglycemia, diabetic ketoacidosis, and lower-limb amputations.
How does cost factor in?
A sensitivity analysis aimed at examining the possible effect of unmeasured socioeconomic status showed no difference in cardiovascular benefit for first-line SGLT-2 inhibitors and metformin, compared with first-line dipeptidyl peptidase–4 (DPP-4) inhibitors, which cost more than metformin; it is not known what effect DPP-4 inhibitors have on the cardiovascular outcomes of interest.
Cost and insurance coverage factor into the benefit/risk calculation. Metformin is far less costly than any of the SGLT-2 inhibitors – roughly $10 to $20 per month, compared with more than $500 a month.
However, “for some fortunate patients with the most generous pharmacy benefit insurance coverage, the out-of-pocket cost of brand name drugs like SGLT-2 inhibitors is substantially lower,” Dr. Taylor noted.
He said that the current study “raises questions about whether the clinical benefits of SGLT-2 inhibitors as initial monotherapy justify the higher price relative to metformin. The data in this paper suggest that the value case for SGLT-2 inhibitors is strongest for patients with the greatest risk to be hospitalized for heart failure.”
Indeed, Dr. Shin said, “Once we get more information, it may just help in extending the coverage from insurance companies and Medicare/Medicaid, to lower the barrier to access.”
Dr. Taylor reiterated that patents on some of the early SGLT-2 inhibitors are expected to expire in the next few years, which would make it possible for generic versions to be approved. “At that point, prices would likely fall, possibly to levels similar to metformin.”
The study was funded by grant support from the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, and Harvard Medical School, the National Institute on Aging, and the Patient-Centered Outcomes Research Institute. Dr. Shin has disclosed no relevant financial relationships. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Use of sodium–glucose cotransporter-2 (SGLT-2) inhibitors rather than metformin as first-line treatment for type 2 diabetes appears to cut the risk for heart failure hospitalization but not myocardial infarction, stroke, or all-cause mortality, a new analysis of real-world data suggests.
Safety findings were similar, except for the fact that genital infections were more common with SGLT-2 inhibitors.
The study was conducted using claims data from two large U.S. insurance databases and Medicare. Propensity score matching was used to account for baseline differences.
The study was conducted by HoJin Shin, BPharm, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues. The findings were published online in Annals of Internal Medicine.
“Those who start SGLT-2 inhibitors as first line show similar risks, compared with metformin in MI, stroke, and all-cause mortality outcomes. Strikingly and consistently, SGLT-2 inhibitors show lower risk for hospitalization for heart failure, which is consistent with the findings from cardiovascular outcomes trials,” Dr. Shin said in an interview.
Just a beginning step, although trial probably wasn’t long enough
However, she added, “I don’t want to overstate anything. ... We aren’t powered enough to investigate who would benefit the most. ... As a pharmacoepidemiologist, I think it’s my duty to provide high-quality evidence so we can actually help physicians and patients make better decisions on their medication. Our current research is just a beginning step.”
Asked to comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, told this news organization, “This study generally confirmed conclusions from published RCTs [randomized clinical trials]. No real surprises, albeit the conclusions may not fully support some of the most enthusiastic claims for SGLT-2 inhibitors with respect to MI, stroke, and cardiovascular death.”
Indeed, Dr. Taylor noted that only two SGLT-2 inhibitors, canagliflozin and empagliflozin, were shown to have a statistically significant association with decreased major adverse cardiovascular events.
In contrast, neither dapagliflozin nor ertugliflozin showed significant benefit regarding those outcomes.
He also pointed out that those four major SLGT-2 inhibitor cardiovascular outcomes trials were placebo-controlled rather than head-to-head trials in which they were compared to an active comparator such as metformin.
“Viewed in this light, it’s probably not surprising that the present study did not demonstrate a robust benefit for SGLT-2 inhibitors to decrease [major adverse CV events].”
The duration of follow-up in the current study is also a limitation, he added.
“The majority of patients were followed for a year or less. This is probably sufficient to assess the impact of some pharmacological mechanisms, for example, the beneficial impact to decrease risk of heart failure by promoting urinary sodium excretion. However, it’s probably insufficient time to observe a beneficial impact on atherosclerosis. For example, there is typically a lag of several years before statins demonstrate efficacy with respect to adverse cardiovascular events.”
Nevertheless, he said, “it provides strong support for benefit with respect to decreasing risk of hospitalization for heart failure.”
He noted that while metformin is currently significantly cheaper than any SGLT-2 inhibitors, once the latter become available as generics, they will be cheaper, and this will likely have a bearing on prescribing decisions.
“Availability of generic SGLT-2 inhibitors offers potential to transform prescribing patterns for type 2 diabetes,” he noted.
First-line SGLT2 inhibitors versus metformin: Most outcomes similar
The study data came from two commercial U.S. health insurance databases, Optum Clinfomatics Data Mart and IBM Marketscan, and from Medicare fee-for-service enrollees.
From April 2013 through March 2020, a total of 9,334 patients began treatment with first-line SGLT-2 inhibitors; 819,973 patients began taking metformin. After 1:2 propensity score matching for confounders, there were 8,613 participants in the SGLT-2 inhibitor group and 17,226 in the group that began treatment with metformin.
The mean follow-up times were 10.7 months for patients taking SGLT-2 inhibitors and 12.2 months for patients taking metformin.
Incidence rates per 1,000 person-years for the composite of hospitalization for MI, hospitalization for ischemic or hemorrhagic stroke, or all-cause mortality (MI/stroke/mortality) were 15.0 versus 16.2 for SLGT-2 inhibitors versus metformin, not a significant difference (hazard ratio, 0.96).
However, for the composite of heart failure hospitalization or all-cause mortality, the rates were 18.3 versus 23.5, a significant difference, with an HR of 0.80. The benefit was seen beginning at about 6 months.
Compared with metformin, SGLT-2 inhibitors showed a significantly lower risk for heart failure hospitalization (HR, 0.78), a numerically (but not significantly) lower risk for MI (HR, 0.70), and similar risks for stroke, mortality, and MI/stroke/HHF/mortality.
Genital infections were significantly more common with SGLT-2 inhibitors (54.1 vs. 23.7 per 1,000 person-years; HR, 2.19). Other safety measures were similar, including acute kidney injury, bone fractures, severe hypoglycemia, diabetic ketoacidosis, and lower-limb amputations.
How does cost factor in?
A sensitivity analysis aimed at examining the possible effect of unmeasured socioeconomic status showed no difference in cardiovascular benefit for first-line SGLT-2 inhibitors and metformin, compared with first-line dipeptidyl peptidase–4 (DPP-4) inhibitors, which cost more than metformin; it is not known what effect DPP-4 inhibitors have on the cardiovascular outcomes of interest.
Cost and insurance coverage factor into the benefit/risk calculation. Metformin is far less costly than any of the SGLT-2 inhibitors – roughly $10 to $20 per month, compared with more than $500 a month.
However, “for some fortunate patients with the most generous pharmacy benefit insurance coverage, the out-of-pocket cost of brand name drugs like SGLT-2 inhibitors is substantially lower,” Dr. Taylor noted.
He said that the current study “raises questions about whether the clinical benefits of SGLT-2 inhibitors as initial monotherapy justify the higher price relative to metformin. The data in this paper suggest that the value case for SGLT-2 inhibitors is strongest for patients with the greatest risk to be hospitalized for heart failure.”
Indeed, Dr. Shin said, “Once we get more information, it may just help in extending the coverage from insurance companies and Medicare/Medicaid, to lower the barrier to access.”
Dr. Taylor reiterated that patents on some of the early SGLT-2 inhibitors are expected to expire in the next few years, which would make it possible for generic versions to be approved. “At that point, prices would likely fall, possibly to levels similar to metformin.”
The study was funded by grant support from the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, and Harvard Medical School, the National Institute on Aging, and the Patient-Centered Outcomes Research Institute. Dr. Shin has disclosed no relevant financial relationships. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
ESG’s cardiometabolic benefits last 5 years
SAN DIEGO – Endoscopic sleeve gastroplasty (ESG) led to sustained weight loss and a reduction of cardiometabolic syndrome comorbidities at 5 years, according to a new retrospective analysis of prospectively collected data.
Improved cardiometabolic outcomes following bariatric surgery have been well documented, but ESG is relatively new, so its outcomes haven’t been as well described. The outcomes are encouraging, though not as good as those of bariatric surgery. “It’s still better, but only one percent of the patients undergo the surgery, even though they’re candidates,” said Donevan Westerveld, MD, who presented the study at the annual Digestive Disease Week® (DDW).
Improvements included weight, HbA1c percentage, hypertension, and low-density lipoprotein. “I was surprised that the LDL decreased numerically, not so much HbA1c and hypertension. I knew [those] would come down with weight loss,” said Dr. Westerveld, a second-year fellow at Weill Cornell Medicine, New York.
He also called for guidelines for ESG. “Given the fact there’s an improvement of comorbid conditions, it’s something we should look at,” said Dr. Westerveld.
“It’s fascinating because it tells us two important things about endoscopic sleeve gastroplasty. One, [the benefit] in the majority of cases lasts at least 5 years. The weight loss is durable. And then it tells us that there’s improvement in all the cardiometabolic factors that matter, and those effects are seen all the way up to 5 years. So very important findings that support the benefits of the endoscopic gastroplasty in obesity and cardiometabolic risks and metabolic syndrome,” said Andres Acosta, MD, PhD, a comoderator of the session where the study was presented. He is assistant professor of medicine and a consultant in gastroenterology and hepatology at Mayo Clinic in Rochester, Minn.
The findings should also encourage more innovation. “Doing these endoscopic procedures, having successful results that hold for 5 years, opens the path for new and better procedures, so we have better weight loss,” said Dr. Acosta.
Previous work by Dr. Westerveld’s group found benefits of ESG at 12 months, including improvements in mean HbA1c levels in all patients (6.1%-5.5%; P = .05) and those with diabetes or prediabetes (6.6%-5.6%; P = .02), reduction in mean waist circumference (119.66-92.75 cm; P < .001), reduction in systolic blood pressure (129.02-122.23 mg/dL; P = .023), triglycerides (131.84-92.36 mg/dL; P = .017), and alanine aminotransferase (ALT, 32.26-20.68 mg/dL; P < .001).
In the new study, the group followed 255 patients at 1, 3, and 5 years post procedure who were treated consecutively at Weill Cornell Medicine from 2013 to 2021. Among the patients were those who had failed weight loss measures and were either not candidates for surgery or had refused surgery.
The mean age was 45.5 years, 69% were female, and the mean body mass index was 38.6. Overall, 40.3% had prediabetes or diabetes, 26.7% had hypertension, 60.8% had low-density lipoprotein (LDL) above 100 mg/dL, and 29.3% had elevated ALT. Sixty-six percent had been followed up at 1 year, 78% at 3 years, and 87% at 5 years.
Weight loss averaged 15.7% at 1 year and 15.3% at year 5, and the values were statistically significant. Among patients with diabetes and prediabetes, HbA1c percentage dropped from a baseline value of 6.4% to 5.7% at year 1, 6.1% at year 3, and 5.8% at year 5 (P < .05 for all). For all patients, the value dropped from 5.8% at baseline to 5.6% at year 1, 5.7% at year 3, and 5.4% at year 5. These changes were not statistically significant.
Systolic blood pressure went down among patients with stage 1 hypertension, from 135 mm Hg at baseline to 122 at year 1 and 121 at year 3 (P < .05 or both), but the mean value increased to 129 at year 5 and was not statistically significant. LDL among all patients declined from 136 mg/dL at baseline to 125 at year 1 (nonsignificant), 115 at year 3 (P < .05), and 109 at year 5 (P < .05). Alanine transaminase values declined from about 29 at baseline to 25 at year 1, 26 at year 3, and 24 at year 5 (P < .05 for all).
Serious adverse events were rare, occurring in just two cases (< 1%).
The study was limited by lack of a sham control, and its retrospective data may have included bias because many of the procedures were not paid for by insurance, leading to high rates of self-pay.
Dr. Westerveld has no relevant financial disclosures. Dr. Acosta is a founder of Gila Therapeutics and Phenomix Sciences. Dr. Acosta consults for Amgen, Gila Therapeutics, Rhythm Pharmaceuticals, and General Mills. He has received funding from Rhythm, Novo Nordisk, Apollo Endosurgery, and USGI Medical.
SAN DIEGO – Endoscopic sleeve gastroplasty (ESG) led to sustained weight loss and a reduction of cardiometabolic syndrome comorbidities at 5 years, according to a new retrospective analysis of prospectively collected data.
Improved cardiometabolic outcomes following bariatric surgery have been well documented, but ESG is relatively new, so its outcomes haven’t been as well described. The outcomes are encouraging, though not as good as those of bariatric surgery. “It’s still better, but only one percent of the patients undergo the surgery, even though they’re candidates,” said Donevan Westerveld, MD, who presented the study at the annual Digestive Disease Week® (DDW).
Improvements included weight, HbA1c percentage, hypertension, and low-density lipoprotein. “I was surprised that the LDL decreased numerically, not so much HbA1c and hypertension. I knew [those] would come down with weight loss,” said Dr. Westerveld, a second-year fellow at Weill Cornell Medicine, New York.
He also called for guidelines for ESG. “Given the fact there’s an improvement of comorbid conditions, it’s something we should look at,” said Dr. Westerveld.
“It’s fascinating because it tells us two important things about endoscopic sleeve gastroplasty. One, [the benefit] in the majority of cases lasts at least 5 years. The weight loss is durable. And then it tells us that there’s improvement in all the cardiometabolic factors that matter, and those effects are seen all the way up to 5 years. So very important findings that support the benefits of the endoscopic gastroplasty in obesity and cardiometabolic risks and metabolic syndrome,” said Andres Acosta, MD, PhD, a comoderator of the session where the study was presented. He is assistant professor of medicine and a consultant in gastroenterology and hepatology at Mayo Clinic in Rochester, Minn.
The findings should also encourage more innovation. “Doing these endoscopic procedures, having successful results that hold for 5 years, opens the path for new and better procedures, so we have better weight loss,” said Dr. Acosta.
Previous work by Dr. Westerveld’s group found benefits of ESG at 12 months, including improvements in mean HbA1c levels in all patients (6.1%-5.5%; P = .05) and those with diabetes or prediabetes (6.6%-5.6%; P = .02), reduction in mean waist circumference (119.66-92.75 cm; P < .001), reduction in systolic blood pressure (129.02-122.23 mg/dL; P = .023), triglycerides (131.84-92.36 mg/dL; P = .017), and alanine aminotransferase (ALT, 32.26-20.68 mg/dL; P < .001).
In the new study, the group followed 255 patients at 1, 3, and 5 years post procedure who were treated consecutively at Weill Cornell Medicine from 2013 to 2021. Among the patients were those who had failed weight loss measures and were either not candidates for surgery or had refused surgery.
The mean age was 45.5 years, 69% were female, and the mean body mass index was 38.6. Overall, 40.3% had prediabetes or diabetes, 26.7% had hypertension, 60.8% had low-density lipoprotein (LDL) above 100 mg/dL, and 29.3% had elevated ALT. Sixty-six percent had been followed up at 1 year, 78% at 3 years, and 87% at 5 years.
Weight loss averaged 15.7% at 1 year and 15.3% at year 5, and the values were statistically significant. Among patients with diabetes and prediabetes, HbA1c percentage dropped from a baseline value of 6.4% to 5.7% at year 1, 6.1% at year 3, and 5.8% at year 5 (P < .05 for all). For all patients, the value dropped from 5.8% at baseline to 5.6% at year 1, 5.7% at year 3, and 5.4% at year 5. These changes were not statistically significant.
Systolic blood pressure went down among patients with stage 1 hypertension, from 135 mm Hg at baseline to 122 at year 1 and 121 at year 3 (P < .05 or both), but the mean value increased to 129 at year 5 and was not statistically significant. LDL among all patients declined from 136 mg/dL at baseline to 125 at year 1 (nonsignificant), 115 at year 3 (P < .05), and 109 at year 5 (P < .05). Alanine transaminase values declined from about 29 at baseline to 25 at year 1, 26 at year 3, and 24 at year 5 (P < .05 for all).
Serious adverse events were rare, occurring in just two cases (< 1%).
The study was limited by lack of a sham control, and its retrospective data may have included bias because many of the procedures were not paid for by insurance, leading to high rates of self-pay.
Dr. Westerveld has no relevant financial disclosures. Dr. Acosta is a founder of Gila Therapeutics and Phenomix Sciences. Dr. Acosta consults for Amgen, Gila Therapeutics, Rhythm Pharmaceuticals, and General Mills. He has received funding from Rhythm, Novo Nordisk, Apollo Endosurgery, and USGI Medical.
SAN DIEGO – Endoscopic sleeve gastroplasty (ESG) led to sustained weight loss and a reduction of cardiometabolic syndrome comorbidities at 5 years, according to a new retrospective analysis of prospectively collected data.
Improved cardiometabolic outcomes following bariatric surgery have been well documented, but ESG is relatively new, so its outcomes haven’t been as well described. The outcomes are encouraging, though not as good as those of bariatric surgery. “It’s still better, but only one percent of the patients undergo the surgery, even though they’re candidates,” said Donevan Westerveld, MD, who presented the study at the annual Digestive Disease Week® (DDW).
Improvements included weight, HbA1c percentage, hypertension, and low-density lipoprotein. “I was surprised that the LDL decreased numerically, not so much HbA1c and hypertension. I knew [those] would come down with weight loss,” said Dr. Westerveld, a second-year fellow at Weill Cornell Medicine, New York.
He also called for guidelines for ESG. “Given the fact there’s an improvement of comorbid conditions, it’s something we should look at,” said Dr. Westerveld.
“It’s fascinating because it tells us two important things about endoscopic sleeve gastroplasty. One, [the benefit] in the majority of cases lasts at least 5 years. The weight loss is durable. And then it tells us that there’s improvement in all the cardiometabolic factors that matter, and those effects are seen all the way up to 5 years. So very important findings that support the benefits of the endoscopic gastroplasty in obesity and cardiometabolic risks and metabolic syndrome,” said Andres Acosta, MD, PhD, a comoderator of the session where the study was presented. He is assistant professor of medicine and a consultant in gastroenterology and hepatology at Mayo Clinic in Rochester, Minn.
The findings should also encourage more innovation. “Doing these endoscopic procedures, having successful results that hold for 5 years, opens the path for new and better procedures, so we have better weight loss,” said Dr. Acosta.
Previous work by Dr. Westerveld’s group found benefits of ESG at 12 months, including improvements in mean HbA1c levels in all patients (6.1%-5.5%; P = .05) and those with diabetes or prediabetes (6.6%-5.6%; P = .02), reduction in mean waist circumference (119.66-92.75 cm; P < .001), reduction in systolic blood pressure (129.02-122.23 mg/dL; P = .023), triglycerides (131.84-92.36 mg/dL; P = .017), and alanine aminotransferase (ALT, 32.26-20.68 mg/dL; P < .001).
In the new study, the group followed 255 patients at 1, 3, and 5 years post procedure who were treated consecutively at Weill Cornell Medicine from 2013 to 2021. Among the patients were those who had failed weight loss measures and were either not candidates for surgery or had refused surgery.
The mean age was 45.5 years, 69% were female, and the mean body mass index was 38.6. Overall, 40.3% had prediabetes or diabetes, 26.7% had hypertension, 60.8% had low-density lipoprotein (LDL) above 100 mg/dL, and 29.3% had elevated ALT. Sixty-six percent had been followed up at 1 year, 78% at 3 years, and 87% at 5 years.
Weight loss averaged 15.7% at 1 year and 15.3% at year 5, and the values were statistically significant. Among patients with diabetes and prediabetes, HbA1c percentage dropped from a baseline value of 6.4% to 5.7% at year 1, 6.1% at year 3, and 5.8% at year 5 (P < .05 for all). For all patients, the value dropped from 5.8% at baseline to 5.6% at year 1, 5.7% at year 3, and 5.4% at year 5. These changes were not statistically significant.
Systolic blood pressure went down among patients with stage 1 hypertension, from 135 mm Hg at baseline to 122 at year 1 and 121 at year 3 (P < .05 or both), but the mean value increased to 129 at year 5 and was not statistically significant. LDL among all patients declined from 136 mg/dL at baseline to 125 at year 1 (nonsignificant), 115 at year 3 (P < .05), and 109 at year 5 (P < .05). Alanine transaminase values declined from about 29 at baseline to 25 at year 1, 26 at year 3, and 24 at year 5 (P < .05 for all).
Serious adverse events were rare, occurring in just two cases (< 1%).
The study was limited by lack of a sham control, and its retrospective data may have included bias because many of the procedures were not paid for by insurance, leading to high rates of self-pay.
Dr. Westerveld has no relevant financial disclosures. Dr. Acosta is a founder of Gila Therapeutics and Phenomix Sciences. Dr. Acosta consults for Amgen, Gila Therapeutics, Rhythm Pharmaceuticals, and General Mills. He has received funding from Rhythm, Novo Nordisk, Apollo Endosurgery, and USGI Medical.
At DDW 2022
Experts endorse plant-based diet for type 2 diabetes remission
Many adults can achieve remission of type 2 diabetes with a primary intervention consisting of a diet that emphasizes whole, plant-based foods, according to a new publication from the American College of Lifestyle Medicine (ACLM).
The document was developed to assist clinicians treating adults with type 2 diabetes, with the goal of remission using diet as a primary intervention. A panel of 15 experts from seven societies reached consensus on 69 statements.
“A healthy diet is a foundational component of current lifestyle guidelines for treatment of type 2 diabetes, but it is often overlooked because of the lack of physician training and patient awareness,” Felice A. Caldarella, MD, president of the American Association of Clinical Endocrinology (AACE), said in a press release from ACLM.
“The consensus statements produced by this panel of experts are invaluable in bringing awareness to the value of diet for diabetes remission in addition to management,” he summarized.
The initiative was cosponsored by the Endocrine Society, endorsed by AACE, and supported by the Academy of Nutrition and Dietetics. The expert panel also included representatives from the American College of Cardiology, the American Heart Association, and the American Academy of Family Physicians. It was published in the American Journal of Lifestyle Medicine.
“I think many patients would do the challenging work of making lifestyle modifications if it meant remission of [type 2 diabetes] and sparing them the burden and cost of medications or surgery,” said Amy E. Rothberg, MD, PhD, who represented the Endocrine Society on the panel.
“By changing the course of the disease, i.e., if in remission, they are unlikely to get the complications related to [type 2 diabetes],” Dr. Rothberg, professor of nutritional sciences at the University of Michigan, Ann Arbor, told this news organization.
Consensus on 69 statements
The panel members used a modified Delphi process to develop the consensus statement. They identified 49 articles from the literature regarding dietary interventions in adults with type 2 diabetes. They reached consensus on 69 statements that cover seven topics: definitions and basic concepts; diet and remission of type 2 diabetes; dietary specifics and types of diets; adjuvant and alternative interventions; support, monitoring, and adherence to therapy; weight loss; and payment and policy.
Dr. Rothberg identified six key areas:
- Definition of remission: Type 2 diabetes remission is defined as A1c < 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for lowering blood glucose, consistent with the diabetes remission timeline published in 2021 by the American Diabetes Association. Remission does not exclude the possibility of recurrence. Remission is a realistic and achievable goal for some adults with type 2 diabetes.
- High-intensity diet, short duration of diabetes: Patients are more likely to attain remission with a high-intensity diet (e.g., high level of restrictions plus frequent patient contact or counseling) accompanied by physical activity and if the patient has had diabetes for 4 years or less. A high-fiber diet is essential.
- Fewer calories, focus on plant-based foods: Calorie reduction could be achieved by reducing food volume, portion sizes, or energy density, or by using liquid meal replacements, or by a combination of these approaches. It should mainly include whole, plant-based foods (whole grains, vegetables, legumes, fruits, nuts, and seeds) and avoid or minimize meat and other animal products, refined foods, ultra-processed foods, and foods with added fats.
- A very low energy diet as initial intervention is optional: There was consensus that this approach can achieve remission, but there was not agreement that low calorie content was essential for achieving remission, Dr. Rothberg noted.
- Beyond type 2 remission: Diet as a primary intervention can also lower the risk of cardiovascular disease and improve lipoprotein profile.
- Self-management, support, and monitoring: The group recognizes the importance of patient education and support. “This can play a vital role and should be part of any comprehensive lifestyle treatment,” said Dr. Rothberg. The diet and lifestyle strategies should be acceptable to most patients, easy to adhere to, accommodate patient preferences and values, and be culturally sensitive.
Intensive lifestyle change can equate to bariatric surgery
Also invited to comment, Yehuda Handelsman, MD, who coauthored a 2020 type 2 diabetes management algorithm by AACE and the American College of Endocrinology, and was not involved with the current initiative, agrees with the importance of lifestyle in the management of type 2 diabetes but takes issue with a few points.
Most clinicians and experts do not believe that diabetes can be reversed, as such, only controlled, noted Dr. Handelsman, medical director of the Metabolic Institute of America, Tarzana, Calif.
“We always have approached type 2 diabetes treatment with lifestyle – diet, exercise, and (as of late) sleep – as the mainstay of therapy,” he said.
However, most patients do not adhere to diet modifications by 6 months and especially by 1 year, which has led to universal recommendations to add medication to lifestyle from inception, he continued.
Most clinicians have not been trained in lifestyle modalities. And many patients with type 2 diabetes are not adherent to medications, which “led to the relative success of bariatric surgery leading to remission (at least for 3-5 years).”
“Remission, which in broad terms implies the disappearance of signs and symptoms, should be a top priority for individuals with type 2 diabetes,” the consensus statement authors wrote.
“While [bariatric surgery] can induce remission in 25% to 80% of targeted patients, it carries risk and its effectiveness wanes as subjects regain lost weight,” and “more dramatic and intensive [lifestyle] change produces remission rates equivalent to bariatric surgery,” they noted.
Need for more randomized trials
Dr. Handelsman also stressed that remission may be temporary. “Three months or 6 months cannot be a measure of success. We must have at least 1 year,” he added. “In fact, there are data to show that remission requires 3 years.”
Nevertheless, the consensus statement does highlight the importance of lifestyle in remission of diabetes, he agreed.
The expert panel also noted that patients can benefit from a healthy lifestyle, even if they do not attain remission, Dr. Rothberg pointed out.
Moving forward, the statement concludes that “there is ... an ongoing need for additional randomized controlled trials to assess sustainable plant-based dietary interventions with whole or minimally processed foods, as a primary means of treating [type 2 diabetes] with the goal of remission, as well as factors that lead to successful patient adherence and effective dissemination and implementation of such interventions.”
This study was supported by the Lisa Wendel Memorial Foundation. Dr. Rothberg has disclosed being the medical director of Rewind, a virtual platform created for weight control with the goal to “defeat” type 2 diabetes, and a consultant for a study for which Nestle provides product. Dr. Handelsman has disclosed receiving research grants and consultant and speaker honoraria from Amarin, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Corcept, Esperion, Ionis, Mankind, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Regor, Sanofi, and Vertis.
A version of this article first appeared on Medscape.com.
Many adults can achieve remission of type 2 diabetes with a primary intervention consisting of a diet that emphasizes whole, plant-based foods, according to a new publication from the American College of Lifestyle Medicine (ACLM).
The document was developed to assist clinicians treating adults with type 2 diabetes, with the goal of remission using diet as a primary intervention. A panel of 15 experts from seven societies reached consensus on 69 statements.
“A healthy diet is a foundational component of current lifestyle guidelines for treatment of type 2 diabetes, but it is often overlooked because of the lack of physician training and patient awareness,” Felice A. Caldarella, MD, president of the American Association of Clinical Endocrinology (AACE), said in a press release from ACLM.
“The consensus statements produced by this panel of experts are invaluable in bringing awareness to the value of diet for diabetes remission in addition to management,” he summarized.
The initiative was cosponsored by the Endocrine Society, endorsed by AACE, and supported by the Academy of Nutrition and Dietetics. The expert panel also included representatives from the American College of Cardiology, the American Heart Association, and the American Academy of Family Physicians. It was published in the American Journal of Lifestyle Medicine.
“I think many patients would do the challenging work of making lifestyle modifications if it meant remission of [type 2 diabetes] and sparing them the burden and cost of medications or surgery,” said Amy E. Rothberg, MD, PhD, who represented the Endocrine Society on the panel.
“By changing the course of the disease, i.e., if in remission, they are unlikely to get the complications related to [type 2 diabetes],” Dr. Rothberg, professor of nutritional sciences at the University of Michigan, Ann Arbor, told this news organization.
Consensus on 69 statements
The panel members used a modified Delphi process to develop the consensus statement. They identified 49 articles from the literature regarding dietary interventions in adults with type 2 diabetes. They reached consensus on 69 statements that cover seven topics: definitions and basic concepts; diet and remission of type 2 diabetes; dietary specifics and types of diets; adjuvant and alternative interventions; support, monitoring, and adherence to therapy; weight loss; and payment and policy.
Dr. Rothberg identified six key areas:
- Definition of remission: Type 2 diabetes remission is defined as A1c < 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for lowering blood glucose, consistent with the diabetes remission timeline published in 2021 by the American Diabetes Association. Remission does not exclude the possibility of recurrence. Remission is a realistic and achievable goal for some adults with type 2 diabetes.
- High-intensity diet, short duration of diabetes: Patients are more likely to attain remission with a high-intensity diet (e.g., high level of restrictions plus frequent patient contact or counseling) accompanied by physical activity and if the patient has had diabetes for 4 years or less. A high-fiber diet is essential.
- Fewer calories, focus on plant-based foods: Calorie reduction could be achieved by reducing food volume, portion sizes, or energy density, or by using liquid meal replacements, or by a combination of these approaches. It should mainly include whole, plant-based foods (whole grains, vegetables, legumes, fruits, nuts, and seeds) and avoid or minimize meat and other animal products, refined foods, ultra-processed foods, and foods with added fats.
- A very low energy diet as initial intervention is optional: There was consensus that this approach can achieve remission, but there was not agreement that low calorie content was essential for achieving remission, Dr. Rothberg noted.
- Beyond type 2 remission: Diet as a primary intervention can also lower the risk of cardiovascular disease and improve lipoprotein profile.
- Self-management, support, and monitoring: The group recognizes the importance of patient education and support. “This can play a vital role and should be part of any comprehensive lifestyle treatment,” said Dr. Rothberg. The diet and lifestyle strategies should be acceptable to most patients, easy to adhere to, accommodate patient preferences and values, and be culturally sensitive.
Intensive lifestyle change can equate to bariatric surgery
Also invited to comment, Yehuda Handelsman, MD, who coauthored a 2020 type 2 diabetes management algorithm by AACE and the American College of Endocrinology, and was not involved with the current initiative, agrees with the importance of lifestyle in the management of type 2 diabetes but takes issue with a few points.
Most clinicians and experts do not believe that diabetes can be reversed, as such, only controlled, noted Dr. Handelsman, medical director of the Metabolic Institute of America, Tarzana, Calif.
“We always have approached type 2 diabetes treatment with lifestyle – diet, exercise, and (as of late) sleep – as the mainstay of therapy,” he said.
However, most patients do not adhere to diet modifications by 6 months and especially by 1 year, which has led to universal recommendations to add medication to lifestyle from inception, he continued.
Most clinicians have not been trained in lifestyle modalities. And many patients with type 2 diabetes are not adherent to medications, which “led to the relative success of bariatric surgery leading to remission (at least for 3-5 years).”
“Remission, which in broad terms implies the disappearance of signs and symptoms, should be a top priority for individuals with type 2 diabetes,” the consensus statement authors wrote.
“While [bariatric surgery] can induce remission in 25% to 80% of targeted patients, it carries risk and its effectiveness wanes as subjects regain lost weight,” and “more dramatic and intensive [lifestyle] change produces remission rates equivalent to bariatric surgery,” they noted.
Need for more randomized trials
Dr. Handelsman also stressed that remission may be temporary. “Three months or 6 months cannot be a measure of success. We must have at least 1 year,” he added. “In fact, there are data to show that remission requires 3 years.”
Nevertheless, the consensus statement does highlight the importance of lifestyle in remission of diabetes, he agreed.
The expert panel also noted that patients can benefit from a healthy lifestyle, even if they do not attain remission, Dr. Rothberg pointed out.
Moving forward, the statement concludes that “there is ... an ongoing need for additional randomized controlled trials to assess sustainable plant-based dietary interventions with whole or minimally processed foods, as a primary means of treating [type 2 diabetes] with the goal of remission, as well as factors that lead to successful patient adherence and effective dissemination and implementation of such interventions.”
This study was supported by the Lisa Wendel Memorial Foundation. Dr. Rothberg has disclosed being the medical director of Rewind, a virtual platform created for weight control with the goal to “defeat” type 2 diabetes, and a consultant for a study for which Nestle provides product. Dr. Handelsman has disclosed receiving research grants and consultant and speaker honoraria from Amarin, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Corcept, Esperion, Ionis, Mankind, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Regor, Sanofi, and Vertis.
A version of this article first appeared on Medscape.com.
Many adults can achieve remission of type 2 diabetes with a primary intervention consisting of a diet that emphasizes whole, plant-based foods, according to a new publication from the American College of Lifestyle Medicine (ACLM).
The document was developed to assist clinicians treating adults with type 2 diabetes, with the goal of remission using diet as a primary intervention. A panel of 15 experts from seven societies reached consensus on 69 statements.
“A healthy diet is a foundational component of current lifestyle guidelines for treatment of type 2 diabetes, but it is often overlooked because of the lack of physician training and patient awareness,” Felice A. Caldarella, MD, president of the American Association of Clinical Endocrinology (AACE), said in a press release from ACLM.
“The consensus statements produced by this panel of experts are invaluable in bringing awareness to the value of diet for diabetes remission in addition to management,” he summarized.
The initiative was cosponsored by the Endocrine Society, endorsed by AACE, and supported by the Academy of Nutrition and Dietetics. The expert panel also included representatives from the American College of Cardiology, the American Heart Association, and the American Academy of Family Physicians. It was published in the American Journal of Lifestyle Medicine.
“I think many patients would do the challenging work of making lifestyle modifications if it meant remission of [type 2 diabetes] and sparing them the burden and cost of medications or surgery,” said Amy E. Rothberg, MD, PhD, who represented the Endocrine Society on the panel.
“By changing the course of the disease, i.e., if in remission, they are unlikely to get the complications related to [type 2 diabetes],” Dr. Rothberg, professor of nutritional sciences at the University of Michigan, Ann Arbor, told this news organization.
Consensus on 69 statements
The panel members used a modified Delphi process to develop the consensus statement. They identified 49 articles from the literature regarding dietary interventions in adults with type 2 diabetes. They reached consensus on 69 statements that cover seven topics: definitions and basic concepts; diet and remission of type 2 diabetes; dietary specifics and types of diets; adjuvant and alternative interventions; support, monitoring, and adherence to therapy; weight loss; and payment and policy.
Dr. Rothberg identified six key areas:
- Definition of remission: Type 2 diabetes remission is defined as A1c < 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for lowering blood glucose, consistent with the diabetes remission timeline published in 2021 by the American Diabetes Association. Remission does not exclude the possibility of recurrence. Remission is a realistic and achievable goal for some adults with type 2 diabetes.
- High-intensity diet, short duration of diabetes: Patients are more likely to attain remission with a high-intensity diet (e.g., high level of restrictions plus frequent patient contact or counseling) accompanied by physical activity and if the patient has had diabetes for 4 years or less. A high-fiber diet is essential.
- Fewer calories, focus on plant-based foods: Calorie reduction could be achieved by reducing food volume, portion sizes, or energy density, or by using liquid meal replacements, or by a combination of these approaches. It should mainly include whole, plant-based foods (whole grains, vegetables, legumes, fruits, nuts, and seeds) and avoid or minimize meat and other animal products, refined foods, ultra-processed foods, and foods with added fats.
- A very low energy diet as initial intervention is optional: There was consensus that this approach can achieve remission, but there was not agreement that low calorie content was essential for achieving remission, Dr. Rothberg noted.
- Beyond type 2 remission: Diet as a primary intervention can also lower the risk of cardiovascular disease and improve lipoprotein profile.
- Self-management, support, and monitoring: The group recognizes the importance of patient education and support. “This can play a vital role and should be part of any comprehensive lifestyle treatment,” said Dr. Rothberg. The diet and lifestyle strategies should be acceptable to most patients, easy to adhere to, accommodate patient preferences and values, and be culturally sensitive.
Intensive lifestyle change can equate to bariatric surgery
Also invited to comment, Yehuda Handelsman, MD, who coauthored a 2020 type 2 diabetes management algorithm by AACE and the American College of Endocrinology, and was not involved with the current initiative, agrees with the importance of lifestyle in the management of type 2 diabetes but takes issue with a few points.
Most clinicians and experts do not believe that diabetes can be reversed, as such, only controlled, noted Dr. Handelsman, medical director of the Metabolic Institute of America, Tarzana, Calif.
“We always have approached type 2 diabetes treatment with lifestyle – diet, exercise, and (as of late) sleep – as the mainstay of therapy,” he said.
However, most patients do not adhere to diet modifications by 6 months and especially by 1 year, which has led to universal recommendations to add medication to lifestyle from inception, he continued.
Most clinicians have not been trained in lifestyle modalities. And many patients with type 2 diabetes are not adherent to medications, which “led to the relative success of bariatric surgery leading to remission (at least for 3-5 years).”
“Remission, which in broad terms implies the disappearance of signs and symptoms, should be a top priority for individuals with type 2 diabetes,” the consensus statement authors wrote.
“While [bariatric surgery] can induce remission in 25% to 80% of targeted patients, it carries risk and its effectiveness wanes as subjects regain lost weight,” and “more dramatic and intensive [lifestyle] change produces remission rates equivalent to bariatric surgery,” they noted.
Need for more randomized trials
Dr. Handelsman also stressed that remission may be temporary. “Three months or 6 months cannot be a measure of success. We must have at least 1 year,” he added. “In fact, there are data to show that remission requires 3 years.”
Nevertheless, the consensus statement does highlight the importance of lifestyle in remission of diabetes, he agreed.
The expert panel also noted that patients can benefit from a healthy lifestyle, even if they do not attain remission, Dr. Rothberg pointed out.
Moving forward, the statement concludes that “there is ... an ongoing need for additional randomized controlled trials to assess sustainable plant-based dietary interventions with whole or minimally processed foods, as a primary means of treating [type 2 diabetes] with the goal of remission, as well as factors that lead to successful patient adherence and effective dissemination and implementation of such interventions.”
This study was supported by the Lisa Wendel Memorial Foundation. Dr. Rothberg has disclosed being the medical director of Rewind, a virtual platform created for weight control with the goal to “defeat” type 2 diabetes, and a consultant for a study for which Nestle provides product. Dr. Handelsman has disclosed receiving research grants and consultant and speaker honoraria from Amarin, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Corcept, Esperion, Ionis, Mankind, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Regor, Sanofi, and Vertis.
A version of this article first appeared on Medscape.com.
Could new therapy for food ‘cues’ improve weight loss?
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Hormones account for 10% of lipid changes after menopause
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
ISCHEMIA substudy data don’t add up, cardiac surgeons say
A recent ISCHEMIA trial substudy is under scrutiny from surgeons for a data discrepancy, rekindling concerns about reliance on the landmark trial data in the latest coronary revascularization guidelines.
As previously reported, the main ISCHEMIA findings showed no significant benefit for an initial strategy of percutaneous coronary intervention (PCI) or coronary bypass graft surgery (CABG) over medical therapy in patients with stable moderate to severe ischemic heart disease.
The 2021 substudy by Reynolds et al. showed that coronary artery disease (CAD) severity, classified using the modified Duke Prognostic Index score, predicted 4-year mortality and myocardial infarction in the trial, whereas ischemia severity did not.
Cardiac surgeons Joseph F. Sabik III, MD, and Faisal Bakaeen, MD, however, spotted that only 40 patients are in the Duke category 6 group (three-vessel severe stenosis of at least 70% or two-vessel severe stenosis with a proximal left anterior descending lesion) in Supplemental tables 1 and 2, whereas 659 are in the main paper.
In addition, the Supplemental tables list the following:
- 659 patients in Duke group 5, not 894 as in the paper.
- 894 patients in Duke group 4, not 743 as in the paper.
- 743 patients in Duke group 3, not 179 as in the paper.
The surgeons penned a letter to Circulation early in April flagging the discrepancies, but say it was rejected April 15 because it was submitted outside the journal’s 6-week window for letters. They posted a public comment on the Remarq research platform, as advised by Circulation’s editorial office, and reached out directly to the authors and ISCHEMIA leadership.
“They just keep saying it’s a simple formatting error. Well, if it is a simple formatting error, then fix it,” Dr. Sabik, chair of surgery at University Hospitals Cleveland Medical Center, said in an interview. “But here we are now, a month later, and they still haven’t published our letter. Why? We’re the ones who identified the problem.”
Dr. Sabik said the accuracy of the data has important implications because the recent AHA/ACC/SCAI coronary revascularization guidelines used the ISCHEMIA data to downgrade the CABG recommendation for complex multivessel disease from class 1 to class 2B. Patients with a Duke 6 score are also typically the ones referred for CABG by today’s heart teams.
Several surgical societies have contested the guidelines, questioning whether the ISCHEMIA patients are truly reflective of those seen in clinical practice and questioning the decision to treat PCI and surgery as equivalent strategies to decrease ischemic events.
Dr. Bakaeen, from the Cleveland Clinic, told this news organization they don’t want a public battle over the data like the one that befell the EXCEL trial, and that it’s entirely possible the investigators might have inadvertently upgraded all the Duke score assignments by 1.
A systematic error, however, is more plausible than a formatting error, he said, because Supplemental tables 1 and 2 correspond exactly to the Duke 1 to Duke 7 sequence, suggesting the tables are correct and that the error might have occurred downstream, including in the manuscript.
The numbers should be consistent across all the ISCHEMIA manuscripts, Dr. Bakaeen added, but currently “don’t add up,” even after adjustment for different denominators, and especially for participants with left main disease.
They hope that publication of their letter, he said, will convince the authors to publicly share the data for patients in each of the seven modified Duke categories.
Lead author of the ISCHEMIA substudy, Harmony Reynolds, MD, New York (N.Y.) University Langone Health, told this news organization via email that as a result of a “formatting error in the transfer of data from the statistical output file to a Word document, data in Supplemental tables 1 and 2 were incorrect.”
She explained that they planned to present six, not seven, rows for the Duke score in the tables, collapsing the first two categories of nonobstructive disease (Duke 1-2), as they were in all other tables and figures. However, the Supplemental tables had incorrect row headings and because the Word program is designed to fill all available rows, it inserted the data from the output file into a seven-row table shell, duplicating the values for row 1 in the last row for left main disease of at least 50%.
“The data were correctly presented in the main manuscript tables and figures and in the remainder of the supplement, with a total of 659 patients in the subset with modified Duke prognostic index category 6 on coronary CT angiography,” Dr. Reynolds said.
She noted that Circulation will issue a correction. In addition, “we are in the process of preparing the data for public sharing soon. The data will include the Duke prognostic score at all levels.”
Circulation editor-in-chief Joseph A. Hill, MD, PhD, chief of cardiology at UT Southwestern Medical Center, Dallas, declined to be interviewed but confirmed via email that Dr. Bakaeen and Dr. Sabik’s letter and the correction will be published the week of May 16.
As for the delay, he said, “I received their reach-out just over 1 week ago, and per protocol, we conducted an internal evaluation of their allegations, which took a bit of time.”
A version of this article first appeared on Medscape.com.
A recent ISCHEMIA trial substudy is under scrutiny from surgeons for a data discrepancy, rekindling concerns about reliance on the landmark trial data in the latest coronary revascularization guidelines.
As previously reported, the main ISCHEMIA findings showed no significant benefit for an initial strategy of percutaneous coronary intervention (PCI) or coronary bypass graft surgery (CABG) over medical therapy in patients with stable moderate to severe ischemic heart disease.
The 2021 substudy by Reynolds et al. showed that coronary artery disease (CAD) severity, classified using the modified Duke Prognostic Index score, predicted 4-year mortality and myocardial infarction in the trial, whereas ischemia severity did not.
Cardiac surgeons Joseph F. Sabik III, MD, and Faisal Bakaeen, MD, however, spotted that only 40 patients are in the Duke category 6 group (three-vessel severe stenosis of at least 70% or two-vessel severe stenosis with a proximal left anterior descending lesion) in Supplemental tables 1 and 2, whereas 659 are in the main paper.
In addition, the Supplemental tables list the following:
- 659 patients in Duke group 5, not 894 as in the paper.
- 894 patients in Duke group 4, not 743 as in the paper.
- 743 patients in Duke group 3, not 179 as in the paper.
The surgeons penned a letter to Circulation early in April flagging the discrepancies, but say it was rejected April 15 because it was submitted outside the journal’s 6-week window for letters. They posted a public comment on the Remarq research platform, as advised by Circulation’s editorial office, and reached out directly to the authors and ISCHEMIA leadership.
“They just keep saying it’s a simple formatting error. Well, if it is a simple formatting error, then fix it,” Dr. Sabik, chair of surgery at University Hospitals Cleveland Medical Center, said in an interview. “But here we are now, a month later, and they still haven’t published our letter. Why? We’re the ones who identified the problem.”
Dr. Sabik said the accuracy of the data has important implications because the recent AHA/ACC/SCAI coronary revascularization guidelines used the ISCHEMIA data to downgrade the CABG recommendation for complex multivessel disease from class 1 to class 2B. Patients with a Duke 6 score are also typically the ones referred for CABG by today’s heart teams.
Several surgical societies have contested the guidelines, questioning whether the ISCHEMIA patients are truly reflective of those seen in clinical practice and questioning the decision to treat PCI and surgery as equivalent strategies to decrease ischemic events.
Dr. Bakaeen, from the Cleveland Clinic, told this news organization they don’t want a public battle over the data like the one that befell the EXCEL trial, and that it’s entirely possible the investigators might have inadvertently upgraded all the Duke score assignments by 1.
A systematic error, however, is more plausible than a formatting error, he said, because Supplemental tables 1 and 2 correspond exactly to the Duke 1 to Duke 7 sequence, suggesting the tables are correct and that the error might have occurred downstream, including in the manuscript.
The numbers should be consistent across all the ISCHEMIA manuscripts, Dr. Bakaeen added, but currently “don’t add up,” even after adjustment for different denominators, and especially for participants with left main disease.
They hope that publication of their letter, he said, will convince the authors to publicly share the data for patients in each of the seven modified Duke categories.
Lead author of the ISCHEMIA substudy, Harmony Reynolds, MD, New York (N.Y.) University Langone Health, told this news organization via email that as a result of a “formatting error in the transfer of data from the statistical output file to a Word document, data in Supplemental tables 1 and 2 were incorrect.”
She explained that they planned to present six, not seven, rows for the Duke score in the tables, collapsing the first two categories of nonobstructive disease (Duke 1-2), as they were in all other tables and figures. However, the Supplemental tables had incorrect row headings and because the Word program is designed to fill all available rows, it inserted the data from the output file into a seven-row table shell, duplicating the values for row 1 in the last row for left main disease of at least 50%.
“The data were correctly presented in the main manuscript tables and figures and in the remainder of the supplement, with a total of 659 patients in the subset with modified Duke prognostic index category 6 on coronary CT angiography,” Dr. Reynolds said.
She noted that Circulation will issue a correction. In addition, “we are in the process of preparing the data for public sharing soon. The data will include the Duke prognostic score at all levels.”
Circulation editor-in-chief Joseph A. Hill, MD, PhD, chief of cardiology at UT Southwestern Medical Center, Dallas, declined to be interviewed but confirmed via email that Dr. Bakaeen and Dr. Sabik’s letter and the correction will be published the week of May 16.
As for the delay, he said, “I received their reach-out just over 1 week ago, and per protocol, we conducted an internal evaluation of their allegations, which took a bit of time.”
A version of this article first appeared on Medscape.com.
A recent ISCHEMIA trial substudy is under scrutiny from surgeons for a data discrepancy, rekindling concerns about reliance on the landmark trial data in the latest coronary revascularization guidelines.
As previously reported, the main ISCHEMIA findings showed no significant benefit for an initial strategy of percutaneous coronary intervention (PCI) or coronary bypass graft surgery (CABG) over medical therapy in patients with stable moderate to severe ischemic heart disease.
The 2021 substudy by Reynolds et al. showed that coronary artery disease (CAD) severity, classified using the modified Duke Prognostic Index score, predicted 4-year mortality and myocardial infarction in the trial, whereas ischemia severity did not.
Cardiac surgeons Joseph F. Sabik III, MD, and Faisal Bakaeen, MD, however, spotted that only 40 patients are in the Duke category 6 group (three-vessel severe stenosis of at least 70% or two-vessel severe stenosis with a proximal left anterior descending lesion) in Supplemental tables 1 and 2, whereas 659 are in the main paper.
In addition, the Supplemental tables list the following:
- 659 patients in Duke group 5, not 894 as in the paper.
- 894 patients in Duke group 4, not 743 as in the paper.
- 743 patients in Duke group 3, not 179 as in the paper.
The surgeons penned a letter to Circulation early in April flagging the discrepancies, but say it was rejected April 15 because it was submitted outside the journal’s 6-week window for letters. They posted a public comment on the Remarq research platform, as advised by Circulation’s editorial office, and reached out directly to the authors and ISCHEMIA leadership.
“They just keep saying it’s a simple formatting error. Well, if it is a simple formatting error, then fix it,” Dr. Sabik, chair of surgery at University Hospitals Cleveland Medical Center, said in an interview. “But here we are now, a month later, and they still haven’t published our letter. Why? We’re the ones who identified the problem.”
Dr. Sabik said the accuracy of the data has important implications because the recent AHA/ACC/SCAI coronary revascularization guidelines used the ISCHEMIA data to downgrade the CABG recommendation for complex multivessel disease from class 1 to class 2B. Patients with a Duke 6 score are also typically the ones referred for CABG by today’s heart teams.
Several surgical societies have contested the guidelines, questioning whether the ISCHEMIA patients are truly reflective of those seen in clinical practice and questioning the decision to treat PCI and surgery as equivalent strategies to decrease ischemic events.
Dr. Bakaeen, from the Cleveland Clinic, told this news organization they don’t want a public battle over the data like the one that befell the EXCEL trial, and that it’s entirely possible the investigators might have inadvertently upgraded all the Duke score assignments by 1.
A systematic error, however, is more plausible than a formatting error, he said, because Supplemental tables 1 and 2 correspond exactly to the Duke 1 to Duke 7 sequence, suggesting the tables are correct and that the error might have occurred downstream, including in the manuscript.
The numbers should be consistent across all the ISCHEMIA manuscripts, Dr. Bakaeen added, but currently “don’t add up,” even after adjustment for different denominators, and especially for participants with left main disease.
They hope that publication of their letter, he said, will convince the authors to publicly share the data for patients in each of the seven modified Duke categories.
Lead author of the ISCHEMIA substudy, Harmony Reynolds, MD, New York (N.Y.) University Langone Health, told this news organization via email that as a result of a “formatting error in the transfer of data from the statistical output file to a Word document, data in Supplemental tables 1 and 2 were incorrect.”
She explained that they planned to present six, not seven, rows for the Duke score in the tables, collapsing the first two categories of nonobstructive disease (Duke 1-2), as they were in all other tables and figures. However, the Supplemental tables had incorrect row headings and because the Word program is designed to fill all available rows, it inserted the data from the output file into a seven-row table shell, duplicating the values for row 1 in the last row for left main disease of at least 50%.
“The data were correctly presented in the main manuscript tables and figures and in the remainder of the supplement, with a total of 659 patients in the subset with modified Duke prognostic index category 6 on coronary CT angiography,” Dr. Reynolds said.
She noted that Circulation will issue a correction. In addition, “we are in the process of preparing the data for public sharing soon. The data will include the Duke prognostic score at all levels.”
Circulation editor-in-chief Joseph A. Hill, MD, PhD, chief of cardiology at UT Southwestern Medical Center, Dallas, declined to be interviewed but confirmed via email that Dr. Bakaeen and Dr. Sabik’s letter and the correction will be published the week of May 16.
As for the delay, he said, “I received their reach-out just over 1 week ago, and per protocol, we conducted an internal evaluation of their allegations, which took a bit of time.”
A version of this article first appeared on Medscape.com.
A surprise and a mystery: NAFLD in lean patients linked to CVD risk
People with nonalcoholic fatty liver disease (NAFLD) and a lean or healthy body mass index are at increased risk for peripheral vascular disease, stroke, and cardiovascular disease, a surprise finding from a new study reveals.
“Our team had expected to see that those with a normal BMI would have a lower prevalence of any metabolic or cardiovascular conditions,” lead researcher Karn Wijarnpreecha, MD, MPH, said during a media briefing that previewed select research for Digestive Disease Week® (DDW) 2022. “So, we were very surprised to find this link to cardiovascular disease.”
The investigators saw this increased risk of cardiovascular disease despite this group having a lower prevalence of atherosclerotic risk factors and metabolic disease.
This first study of its kind suggests physicians should consider the risk of cardiovascular disease in all patients with NAFLD, not just in those who are overweight or living with obesity – groups traditionally thought to carry more risk.
NAFLD in lean individuals is not a benign disease.
“NAFLD patients with a normal BMI are often overlooked because we assume that the risk for more serious conditions is lower than for those who are overweight or obese. But this way of thinking may be putting these patients at risk,” added Dr. Wijarnpreecha, who is a transplant hepatology fellow at the University of Michigan, Ann Arbor.
Key findings
Approximately 25% of U.S. adults live with NAFLD, an umbrella term for liver conditions in people who drink little to no alcohol. It is characterized by too much fat stored in the liver. Although most people have no symptoms, the condition can lead to other dangerous conditions, such as diabetes, cardiovascular disease, and cirrhosis of the liver, Dr. Wijarnpreecha said.
The investigators retrospectively studied a cohort of 18,793 adults diagnosed with NAFLD at the University of Michigan Hospital from 2012-2021. One aim was to compare the prevalence of cirrhosis, cardiovascular disease, metabolic diseases, and chronic kidney disease in relation to BMI.
They also classified people into four BMI categories: lean, overweight, obesity class 1, and obesity class 2-3.
Compared with non-lean patients, lean patients had a higher prevalence of peripheral arterial disease and stroke and a similar rate of cardiovascular disease based on identification of ICD codes.
Almost 6% of lean patients had peripheral arterial disease, compared with rates of approximately 4%-5% in overweight people and people with obesity. Similarly, more than 6% of the lean group experienced a stroke compared with 5% or less of the other BMI groups.
“We found that lean patients with NAFLD also had a significant higher prevalence of cardiovascular disease, independent of age, sex, race, smoking status, diabetes, hypertension, and dyslipidemia,” Dr. Wijarnpreecha said.
At the same time, compared with non-lean patients, lean patients had a lower prevalence of cirrhosis, diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease in an analysis that adjusted for confounders.
Exploring the unknown
Researchers now have a mystery on their hands: What is causing this unexpected higher risk of cardiovascular disease in lean people with NAFLD?
Loren Laine, MD, chief of the section of digestive diseases at Yale University School of Medicine, New Haven, Conn., and moderator of the media briefing, asked Wijarnpreecha for his leading theory behind this connection.
“We think that could be from a difference in lifestyle, diet, exercise, genetics, or even gut microbiota,” Dr. Wijarnpreecha replied. “But these are factors that we did not capture from this current study.”
“We are preparing to conduct additional research with longitudinal data to better understand NAFLD in lean patients,” Dr. Wijarnpreecha added.
“It’s an interesting finding, but there are some questions from this retrospective study,” said Arun J. Sanyal, MD, when asked to comment on the study.
Identifying and quantifying any alcohol use, smoking, or hypertension that could also have contributed to increased cardiovascular risk would be useful. Another question relates to how the population with NAFLD was identified. Was NAFLD an incidental finding in their diagnosis, asked Dr. Sanyal, director of the Stravitz-Sanyal Institute for Liver Disease & Metabolic Health at Virginia Commonwealth University, Richmond.
“I’m not dissing the study,” he said, “But like all the observations like this, I think we have to kick the tires.”
It’s an “important new observation” that requires further study to fully understand what it means and what the therapeutic implications might be. It is also important to assess any possible confounders and any causal relationship among these factors, Dr. Sanyal added.
“There’s no question it is important to continue to do these types of studies,” he added. “Through this kind of research we find new things that lead to the science that can then significantly change how we approach these issues.”
A version of this article first appeared on Medscape.com. This article was updated on May 18, 2022.
People with nonalcoholic fatty liver disease (NAFLD) and a lean or healthy body mass index are at increased risk for peripheral vascular disease, stroke, and cardiovascular disease, a surprise finding from a new study reveals.
“Our team had expected to see that those with a normal BMI would have a lower prevalence of any metabolic or cardiovascular conditions,” lead researcher Karn Wijarnpreecha, MD, MPH, said during a media briefing that previewed select research for Digestive Disease Week® (DDW) 2022. “So, we were very surprised to find this link to cardiovascular disease.”
The investigators saw this increased risk of cardiovascular disease despite this group having a lower prevalence of atherosclerotic risk factors and metabolic disease.
This first study of its kind suggests physicians should consider the risk of cardiovascular disease in all patients with NAFLD, not just in those who are overweight or living with obesity – groups traditionally thought to carry more risk.
NAFLD in lean individuals is not a benign disease.
“NAFLD patients with a normal BMI are often overlooked because we assume that the risk for more serious conditions is lower than for those who are overweight or obese. But this way of thinking may be putting these patients at risk,” added Dr. Wijarnpreecha, who is a transplant hepatology fellow at the University of Michigan, Ann Arbor.
Key findings
Approximately 25% of U.S. adults live with NAFLD, an umbrella term for liver conditions in people who drink little to no alcohol. It is characterized by too much fat stored in the liver. Although most people have no symptoms, the condition can lead to other dangerous conditions, such as diabetes, cardiovascular disease, and cirrhosis of the liver, Dr. Wijarnpreecha said.
The investigators retrospectively studied a cohort of 18,793 adults diagnosed with NAFLD at the University of Michigan Hospital from 2012-2021. One aim was to compare the prevalence of cirrhosis, cardiovascular disease, metabolic diseases, and chronic kidney disease in relation to BMI.
They also classified people into four BMI categories: lean, overweight, obesity class 1, and obesity class 2-3.
Compared with non-lean patients, lean patients had a higher prevalence of peripheral arterial disease and stroke and a similar rate of cardiovascular disease based on identification of ICD codes.
Almost 6% of lean patients had peripheral arterial disease, compared with rates of approximately 4%-5% in overweight people and people with obesity. Similarly, more than 6% of the lean group experienced a stroke compared with 5% or less of the other BMI groups.
“We found that lean patients with NAFLD also had a significant higher prevalence of cardiovascular disease, independent of age, sex, race, smoking status, diabetes, hypertension, and dyslipidemia,” Dr. Wijarnpreecha said.
At the same time, compared with non-lean patients, lean patients had a lower prevalence of cirrhosis, diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease in an analysis that adjusted for confounders.
Exploring the unknown
Researchers now have a mystery on their hands: What is causing this unexpected higher risk of cardiovascular disease in lean people with NAFLD?
Loren Laine, MD, chief of the section of digestive diseases at Yale University School of Medicine, New Haven, Conn., and moderator of the media briefing, asked Wijarnpreecha for his leading theory behind this connection.
“We think that could be from a difference in lifestyle, diet, exercise, genetics, or even gut microbiota,” Dr. Wijarnpreecha replied. “But these are factors that we did not capture from this current study.”
“We are preparing to conduct additional research with longitudinal data to better understand NAFLD in lean patients,” Dr. Wijarnpreecha added.
“It’s an interesting finding, but there are some questions from this retrospective study,” said Arun J. Sanyal, MD, when asked to comment on the study.
Identifying and quantifying any alcohol use, smoking, or hypertension that could also have contributed to increased cardiovascular risk would be useful. Another question relates to how the population with NAFLD was identified. Was NAFLD an incidental finding in their diagnosis, asked Dr. Sanyal, director of the Stravitz-Sanyal Institute for Liver Disease & Metabolic Health at Virginia Commonwealth University, Richmond.
“I’m not dissing the study,” he said, “But like all the observations like this, I think we have to kick the tires.”
It’s an “important new observation” that requires further study to fully understand what it means and what the therapeutic implications might be. It is also important to assess any possible confounders and any causal relationship among these factors, Dr. Sanyal added.
“There’s no question it is important to continue to do these types of studies,” he added. “Through this kind of research we find new things that lead to the science that can then significantly change how we approach these issues.”
A version of this article first appeared on Medscape.com. This article was updated on May 18, 2022.
People with nonalcoholic fatty liver disease (NAFLD) and a lean or healthy body mass index are at increased risk for peripheral vascular disease, stroke, and cardiovascular disease, a surprise finding from a new study reveals.
“Our team had expected to see that those with a normal BMI would have a lower prevalence of any metabolic or cardiovascular conditions,” lead researcher Karn Wijarnpreecha, MD, MPH, said during a media briefing that previewed select research for Digestive Disease Week® (DDW) 2022. “So, we were very surprised to find this link to cardiovascular disease.”
The investigators saw this increased risk of cardiovascular disease despite this group having a lower prevalence of atherosclerotic risk factors and metabolic disease.
This first study of its kind suggests physicians should consider the risk of cardiovascular disease in all patients with NAFLD, not just in those who are overweight or living with obesity – groups traditionally thought to carry more risk.
NAFLD in lean individuals is not a benign disease.
“NAFLD patients with a normal BMI are often overlooked because we assume that the risk for more serious conditions is lower than for those who are overweight or obese. But this way of thinking may be putting these patients at risk,” added Dr. Wijarnpreecha, who is a transplant hepatology fellow at the University of Michigan, Ann Arbor.
Key findings
Approximately 25% of U.S. adults live with NAFLD, an umbrella term for liver conditions in people who drink little to no alcohol. It is characterized by too much fat stored in the liver. Although most people have no symptoms, the condition can lead to other dangerous conditions, such as diabetes, cardiovascular disease, and cirrhosis of the liver, Dr. Wijarnpreecha said.
The investigators retrospectively studied a cohort of 18,793 adults diagnosed with NAFLD at the University of Michigan Hospital from 2012-2021. One aim was to compare the prevalence of cirrhosis, cardiovascular disease, metabolic diseases, and chronic kidney disease in relation to BMI.
They also classified people into four BMI categories: lean, overweight, obesity class 1, and obesity class 2-3.
Compared with non-lean patients, lean patients had a higher prevalence of peripheral arterial disease and stroke and a similar rate of cardiovascular disease based on identification of ICD codes.
Almost 6% of lean patients had peripheral arterial disease, compared with rates of approximately 4%-5% in overweight people and people with obesity. Similarly, more than 6% of the lean group experienced a stroke compared with 5% or less of the other BMI groups.
“We found that lean patients with NAFLD also had a significant higher prevalence of cardiovascular disease, independent of age, sex, race, smoking status, diabetes, hypertension, and dyslipidemia,” Dr. Wijarnpreecha said.
At the same time, compared with non-lean patients, lean patients had a lower prevalence of cirrhosis, diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease in an analysis that adjusted for confounders.
Exploring the unknown
Researchers now have a mystery on their hands: What is causing this unexpected higher risk of cardiovascular disease in lean people with NAFLD?
Loren Laine, MD, chief of the section of digestive diseases at Yale University School of Medicine, New Haven, Conn., and moderator of the media briefing, asked Wijarnpreecha for his leading theory behind this connection.
“We think that could be from a difference in lifestyle, diet, exercise, genetics, or even gut microbiota,” Dr. Wijarnpreecha replied. “But these are factors that we did not capture from this current study.”
“We are preparing to conduct additional research with longitudinal data to better understand NAFLD in lean patients,” Dr. Wijarnpreecha added.
“It’s an interesting finding, but there are some questions from this retrospective study,” said Arun J. Sanyal, MD, when asked to comment on the study.
Identifying and quantifying any alcohol use, smoking, or hypertension that could also have contributed to increased cardiovascular risk would be useful. Another question relates to how the population with NAFLD was identified. Was NAFLD an incidental finding in their diagnosis, asked Dr. Sanyal, director of the Stravitz-Sanyal Institute for Liver Disease & Metabolic Health at Virginia Commonwealth University, Richmond.
“I’m not dissing the study,” he said, “But like all the observations like this, I think we have to kick the tires.”
It’s an “important new observation” that requires further study to fully understand what it means and what the therapeutic implications might be. It is also important to assess any possible confounders and any causal relationship among these factors, Dr. Sanyal added.
“There’s no question it is important to continue to do these types of studies,” he added. “Through this kind of research we find new things that lead to the science that can then significantly change how we approach these issues.”
A version of this article first appeared on Medscape.com. This article was updated on May 18, 2022.














