User login
Cancer immunotherapy seen repigmenting gray hair
Patients on immunotherapy treatments for lung cancer have experienced repigmentation of their formerly gray hair, according to a new report. Moreover, researchers say, all but one of the patients experiencing this effect also responded well to the therapy, suggesting that hair repigmentation could potentially serve as a marker of treatment response.
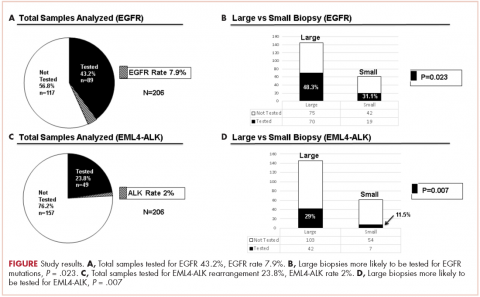
In a case series published online July 12 in JAMA Dermatology (2017. doi: 10.1001/jamadermatol.2017.2106), Noelia Rivera, MD, of the Universitat Autònoma de Barcelona and her colleagues report findings from 14 patients (13 male, mean age 65) receiving anti–programmed cell death 1 (anti–PD-1) and anti–programmed cell death ligand 1 (anti–PD-L1) therapies to treat squamous cell lung cancer or lung adenocarcinoma, with most (11) receiving nivolumab (Opdivo). All but one patient experienced a diffuse darkening of the hair to resemble its previous color, while the remaining patient’s repigmentation occurred in patches.
Dr. Rivera and her colleagues wrote in their analysis that gray hair follicles “still preserve a reduced number of differentiated and functioning melanocytes located in the hair bulb. This reduced number of melanocytes may explain the possibility of [repigmentation] under appropriate conditions.” But, there are competing theories as to why this should occur with cancer immunotherapy, they noted. One is that the drugs’ inhibition of proinflammatory cytokines acts as negative regulators of melanogenesis. Another is that melanocytes in hair follicles are activated through inflammatory mediators. Of the patients with hair repigmentation in the study, only one, who was being treated with nivolumab for lung squamous cell carcinoma, had disease progression. This patient was discontinued after four treatment sessions and died. The other 13 patients saw either stable disease or a partial response.
The study received no outside funding, but two investigators disclosed financial relationships with pharmaceutical manufacturers.
Patients on immunotherapy treatments for lung cancer have experienced repigmentation of their formerly gray hair, according to a new report. Moreover, researchers say, all but one of the patients experiencing this effect also responded well to the therapy, suggesting that hair repigmentation could potentially serve as a marker of treatment response.
In a case series published online July 12 in JAMA Dermatology (2017. doi: 10.1001/jamadermatol.2017.2106), Noelia Rivera, MD, of the Universitat Autònoma de Barcelona and her colleagues report findings from 14 patients (13 male, mean age 65) receiving anti–programmed cell death 1 (anti–PD-1) and anti–programmed cell death ligand 1 (anti–PD-L1) therapies to treat squamous cell lung cancer or lung adenocarcinoma, with most (11) receiving nivolumab (Opdivo). All but one patient experienced a diffuse darkening of the hair to resemble its previous color, while the remaining patient’s repigmentation occurred in patches.
Dr. Rivera and her colleagues wrote in their analysis that gray hair follicles “still preserve a reduced number of differentiated and functioning melanocytes located in the hair bulb. This reduced number of melanocytes may explain the possibility of [repigmentation] under appropriate conditions.” But, there are competing theories as to why this should occur with cancer immunotherapy, they noted. One is that the drugs’ inhibition of proinflammatory cytokines acts as negative regulators of melanogenesis. Another is that melanocytes in hair follicles are activated through inflammatory mediators. Of the patients with hair repigmentation in the study, only one, who was being treated with nivolumab for lung squamous cell carcinoma, had disease progression. This patient was discontinued after four treatment sessions and died. The other 13 patients saw either stable disease or a partial response.
The study received no outside funding, but two investigators disclosed financial relationships with pharmaceutical manufacturers.
Patients on immunotherapy treatments for lung cancer have experienced repigmentation of their formerly gray hair, according to a new report. Moreover, researchers say, all but one of the patients experiencing this effect also responded well to the therapy, suggesting that hair repigmentation could potentially serve as a marker of treatment response.
In a case series published online July 12 in JAMA Dermatology (2017. doi: 10.1001/jamadermatol.2017.2106), Noelia Rivera, MD, of the Universitat Autònoma de Barcelona and her colleagues report findings from 14 patients (13 male, mean age 65) receiving anti–programmed cell death 1 (anti–PD-1) and anti–programmed cell death ligand 1 (anti–PD-L1) therapies to treat squamous cell lung cancer or lung adenocarcinoma, with most (11) receiving nivolumab (Opdivo). All but one patient experienced a diffuse darkening of the hair to resemble its previous color, while the remaining patient’s repigmentation occurred in patches.
Dr. Rivera and her colleagues wrote in their analysis that gray hair follicles “still preserve a reduced number of differentiated and functioning melanocytes located in the hair bulb. This reduced number of melanocytes may explain the possibility of [repigmentation] under appropriate conditions.” But, there are competing theories as to why this should occur with cancer immunotherapy, they noted. One is that the drugs’ inhibition of proinflammatory cytokines acts as negative regulators of melanogenesis. Another is that melanocytes in hair follicles are activated through inflammatory mediators. Of the patients with hair repigmentation in the study, only one, who was being treated with nivolumab for lung squamous cell carcinoma, had disease progression. This patient was discontinued after four treatment sessions and died. The other 13 patients saw either stable disease or a partial response.
The study received no outside funding, but two investigators disclosed financial relationships with pharmaceutical manufacturers.
FROM JAMA DERMATOLOGY
Key clinical point:
Major finding: Of 52 patients, 14 patients saw a diffuse restoration of their original hair color during the course of treatment. All but 1 of these also saw a robust treatment response.
Data source: A case series drawn from a single-center cohort of 52 lung cancer patients treated with anti–PD-1 and anti–PD-L1 and monitored for cutaneous effects.
Disclosures: Two coauthors disclosed financial relationships with several drug manufacturers.
Molecular Markers and Targeted Therapies in the Management of Non-Small Cell Lung Cancer
INTRODUCTION
Lung cancer is the second most common type of cancer in the United States, with 222,500 estimated new cases in 2017, according to the American Cancer Society.1 However, it is by far the number one cause of death due to cancer, with an estimated 155,870 lung cancer–related deaths occurring in 2017, which is higher than the number of deaths due to breast cancer, prostate cancer, and colorectal cancer combined.1,2 Despite slightly decreasing incidence and mortality over the past decade, largely due to smoking cessation, the 5-year survival rate of lung cancer remains dismal at approximately 18%.2–4
Non-small cell lung cancer (NSCLC) accounts for 80% to 85% of all lung cancer cases.4 Traditionally, it is further divided based on histology: adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and not otherwise specified.5 Chemotherapy had been the cornerstone of treatment for stage IV NSCLC. It is not target-specific and is most effective against rapidly growing cells. Common adverse effects include alopecia, nausea/vomiting, myelosuppression, cardiotoxicity, neuropathy, and nephrotoxicity. However, this paradigm has shifted following the discovery of mutations of the epidermal growth factor receptor (EGFR) gene as an oncogenic driver that confers sensitivity to small molecule tyrosine kinase inhibitors (TKIs) targeting EGFR.6 The EGFR inhibitors are given orally and have a spectrum of toxicities (eg, such as rash, diarrhea, and elevated transaminases) different from that of systemic chemotherapy, which is often administered intravenously. Following the discovery of EGFR mutations, rearrangements of the anaplastic lymphoma kinase (ALK) gene7 and ROS1 gene8 were identified as targetable driver mutations in NSCLC. The frequency of both rearrangements is lower than that of EGFR mutations. Additionally, BRAF V600E mutation has been identified in NSCLC.9–12 This activation mutation is commonly seen in melanoma. Agents that have already been approved for the treatment of melanoma with the BRAF V600E mutation are being tested in NSCLC patients with this mutation.13–16
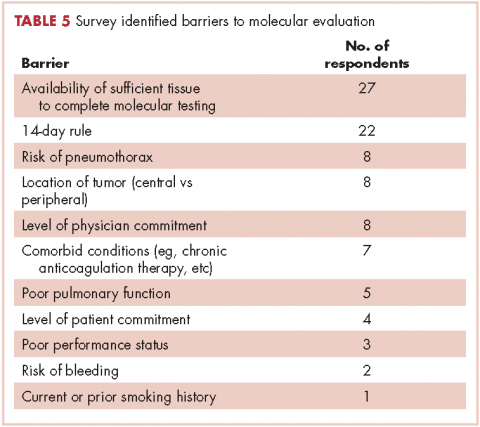
Given the effectiveness and tolerability of targeted therapy, identifying this distinct molecular subset of NSCLC patients is critical in treatment. Currently, molecular testing is mandatory in all stage IV patients with non-squamous cell carcinoma, as a preponderance of patients with driver mutations have this histology subtype.5,17–19 For patients with squamous cell carcinoma, molecular testing should be considered if the biopsy specimen is small, there is mixed histology, or the patient is a nonsmoker.5,20 Several techniques are commonly utilized in detecting these genetic alterations. EGFR mutation can be detected by polymerase chain reaction (PCR), ALK or ROS1 rearrangement can be detected by fluorescence in-situ hybridization (FISH), and immunohistochemistry (IHC) can also be used to detect ALK rearrangement. The current guideline is to use comprehensive genomic profiling to capture all the potential molecular targets simultaneously instead of running stepwise tests just for EGFR, ALK, and ROS1.5BRAF V600E mutation,13–16 MET exon 14 skipping mutation,21–24 RET rearrangements,25–27 and HER2 mutations28–30 are among the emergent genetic alterations with various responses to targeted therapy.31 Some of these targeted agents have been approved for other types of malignancy, and others are still in the development phase.
Several initiatives worldwide have reported better outcomes of patients with driver mutations treated with targeted therapy. For instance, the Lung Cancer Mutation Consortium in the United States demonstrated that the median survival of patients without driver mutations, with drivers mutations but not treated with targeted therapy, and with driver mutations and treated with targeted therapy was 2.08 years, 2.38 years, and 3.49 years, respectively.32 The French Cooperative Thoracic Intergroup-French National Cancer Institute demonstrated that the median survival for patients with driver mutations versus those without driver mutations was 16.5 months versus 11.8 months.33 The Spanish Lung Cancer Group demonstrated that the overall survival (OS) for patients with EGFR mutations treated with erlotinib was 27 months.34 The mutations in lung cancer, their frequencies, and the downstream signaling pathways are depicted in the Figure.35
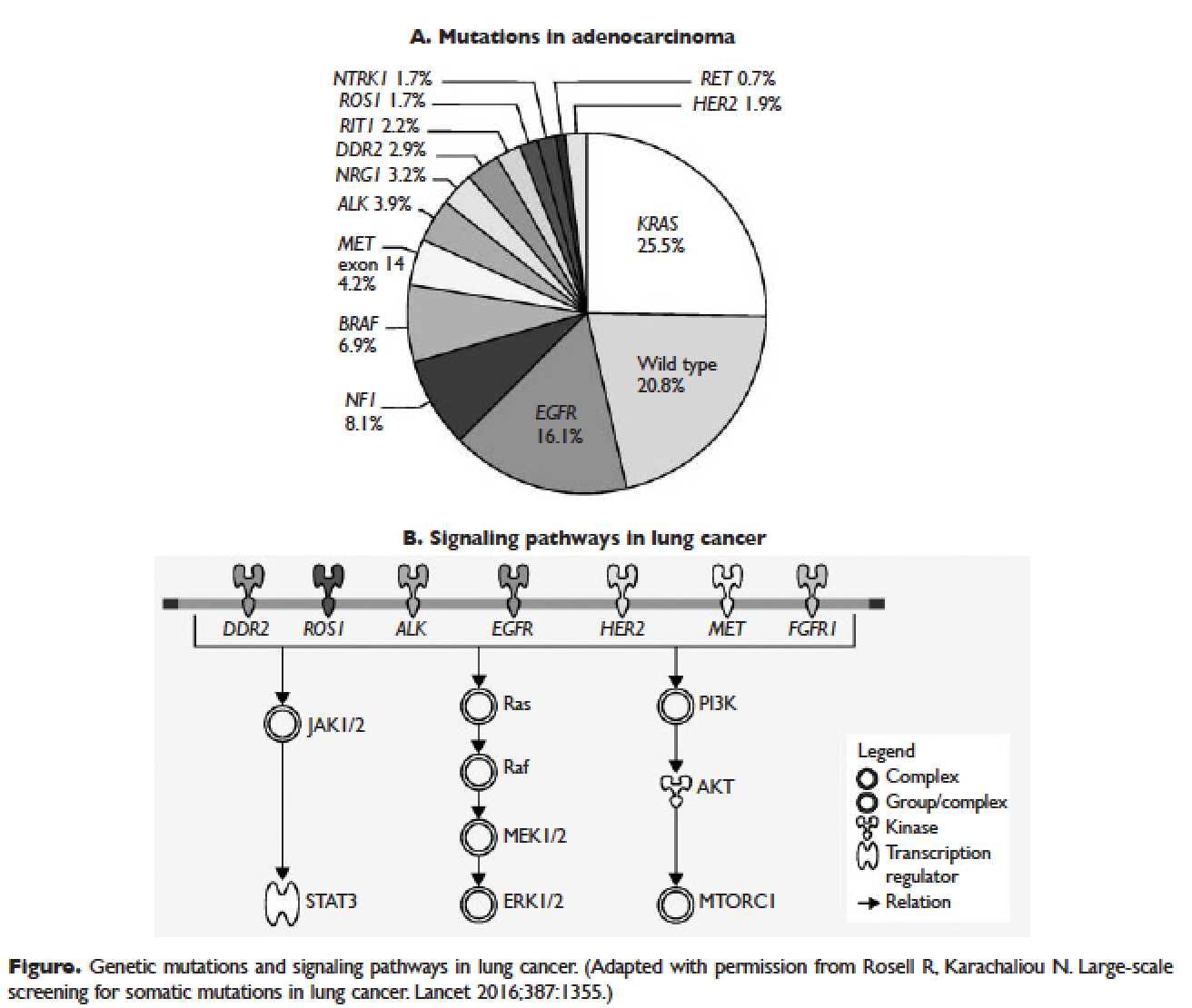
In this article, we discuss targeted therapy for patients with EGFR mutations, ALK rearrangements, ROS1 rearrangements, and BRAF V600E mutation. We also discuss the management of patients with EGFR mutations who develop a secondary mutation after TKI therapy. Almost all of the targeted agents discussed herein have been approved by the US Food and Drug Administration (FDA), so they are considered standard of care. All available phase 3 trials pertinent to these targeted therapies are included in the discussion.
EGFR MUTATIONS
CASE PRESENTATION 1
A 54-year-old Caucasian man who is a former smoker with a 10 pack-year history and past medical history of hypertension and dyslipidemia presents with progressive dyspnea for several weeks. A chest x-ray shows moderate pleural effusion on the left side with possible mass-like opacity on the left upper lung field. An ultrasound-guided thoracentesis is performed and cytology is positive for adenocarcinoma of likely pulmonary origin. Staging workup including positron emission tomography (PET)/computed tomography (CT) and magnetic resonance imaging of the brain with and without contrast is done. PET/CT shows a 5.5-cm mass in the left upper lobe of the lung with high fluorodeoxyglucose (FDG) uptake, several 1- to 2-cm mediastinal lymph nodes with moderate FDG uptake, and small pleural effusion on both sides with moderate FDG uptake. MRI-brain is negative for malignancy. The patient subsequently undergoes a CT-guided biopsy of the lung mass, which shows moderately differentiated adenocarcinoma. Comprehensive molecular profiling reveals EGFR L858R mutation only. The patient now presents for the initial consultation. Of note, his Eastern Cooperative Oncology Group performance status is 1.
What is the next step in the management of this patient?
FIRST-LINE TKI FOR SENSITIZING EGFR MUTATIONS
The 2 most common EGFR mutations are deletions in exon 19 and substitution of arginine for leucine in exon 21 (L858R), found in approximately 45% and 40% of patients with EGFR mutations, respectively.36 Both mutations are sensitive to EGFR TKIs. The benefit may be greater in patients with exon 19 deletions as compared to exon 21 L858R substitution,37,38 but this has not been demonstrated consistently in clinical trials.39-43 In the United States, EGFR mutations are found in approximately 10% of patients with NSCLC, while the incidence can be as high as 50% in Asia.44 Even though the cobas EGFR mutation test is the companion diagnostic approved by the US FDA, a positive test result from any laboratory with the Clinical Laboratory Improvement Amendments (CLIA) certificate should prompt the use of an EGFR TKI as the initial treatment.
Three EGFR TKIs that have been approved as first-line therapy in the United States are available: erlotinib, afatinib, and gefitinib.5 Both erlotinib and gefitinib are considered first-generation TKIs. They have higher binding affinity for the 2 common EGFR mutations than wild-type EGFR. In addition, they reversibly bind to the intracellular tyrosine kinase domain, resulting in inhibition of autophosphorylation of the tyrosine residues. Afatinib, a second-generation and irreversible TKI, targets EGFR (HER1) as well as HER2 and HER4.45
The superior efficacy of the EGFR TKIs over platinum doublet chemotherapy in treatment-naïve patients with EGFR mutations has been demonstrated in 7 randomized trials to date (Table).46 Erlotinib was the TKI arm for the OPTIMAL,41 EURTAC,42 and ENSURE trials;38 afatinib was the TKI arm for LUX-LUNG 337 and 6;43 gefitinib was the TKI arm for NEJ00239,47 and WJTOG3405.40 A meta-analysis of these 7 trials by Lee et al showed that progression-free survival (PFS) was significantly prolonged by EGFR TKIs (hazard ratio [HR] 0.37 [95% confidence interval {CI} 0.32 to 0.42]).46 For instance, in the EURTAC trial, median PFS was 9.7 months for patients treated with erlotinib as compared to 5.2 months for patients treated with platinum/gemcitabine or platinum/docetaxel.42 In this meta-analysis, prespecified subgroups included age, sex, ethnicity, smoking status, performance status, tumor histology, and EGFR mutation subtype. The superior outcome with TKIs was observed in all subgroups. Furthermore, patients with exon 19 deletions, nonsmokers, and women had even better outcomes.46
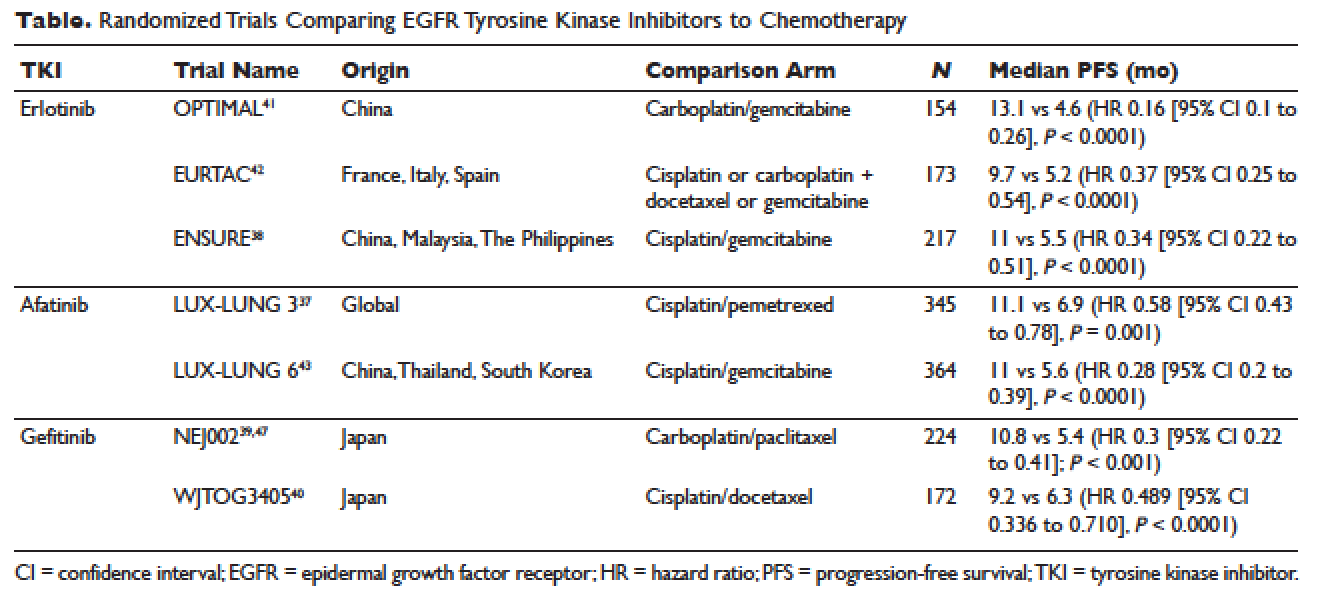
Erlotinib is the most commonly used TKI in the United States largely because gefitinib was off the market for some time until it was re-approved by the FDA in 2015. Interestingly, this “re-approval” was not based on either 1 of the 2 prospective trials (NEJ00239,47 and WJTOG340540), but rather was based on an exploratory analysis of the IPASS trial48,49 as well as a prospective phase 4, single-arm trial in Europe (IFUM).50 The superior efficacy of gefitinib over carboplatin/paclitaxel among patients with EGFR mutations in the IPASS trial was confirmed by blind independent central review, with longer PFS (HR 0.54 [95% CI 0.38 to 0.79] P = 0.0012) and higher objective response rate (ORR; odds ratio 3 [95% CI 1.63 to 5.54], P = 0.0004).49
CASE 1 CONTINUED
Based on the EGFR L858R mutation status, the patient is started on erlotinib. He is quite happy that he does not need intravenous chemotherapy but wants to know what toxicities he might potentially have with erlotinib.
What are the common adverse effects (AEs) of EGFR TKIs? How are AEs of TKIs managed?
Safety Profile
The important toxicities associated with EGFR TKIs are rash, gastrointestinal toxicity, hepatic toxicity, and pulmonary toxicity. Rash is an AE specific to all agents blocking the EGFR pathway, including small molecules and monoclonal antibodies such as cetuximab. The epidermis has a high level of expression of EGFR, which contributes to this toxicity.51 Rash usually presents as dry skin or acneiform eruption. Prophylactic treatment with oral tetracyclines and topical corticosteroids is generally recommended upon initiation of TKI therapy. Diarrhea is the most prevalent gastrointestinal toxicity. All patients starting treatment should be given prescriptions to manage diarrhea such as loperamide and be advised to call when it occurs. Hepatic toxicity is often manifested as elevated transaminases or bilirubin. Interstitial lung disease (ILD) is a rare but potentially fatal pulmonary toxicity.
Rash of any grade was reported in 49.2% of patients treated with erlotinib in clinical trials, while grade 3 rash occurred in 6% of patients and no grade 4 was reported. Diarrhea of any grade was reported in 20.3% of patients, grade 3 diarrhea occurred in 1.8%, and no grade 4 was reported. Grade 2 and 3 alanine aminotransferase (ALT) elevations were seen in 2% and 1% of patients, respectively. Grade 2 and 3 bilirubin elevations were seen in 4% and less than 1% of patients, respectively. The incidence of serious ILD-like events was less than 1%.52
Afatinib is associated with higher incidences of rash and diarrhea. Specifically, diarrhea and rash of all grades were reported in 96% and 90% of patients treated with afatinib, respectively. Paronychia of all grades occurred in 58% of patients. Elevated ALT of all grades was seen in 11% of patients. Approximately 1.5% of patients treated with afatinib across clinical trials had ILD or ILD-like AEs.53
Gefitinib, the most commonly used TKI outside United States, has a toxicity profile similar to erlotinib, except for hepatic toxicity. For instance, rash of all grades occurred in 47% of patients, diarrhea of all grades occurred in 29% of patients, and ILD or ILD-like AEs occurred in 1.3% of patients across clinical trials. In comparison, elevated ALT and aspartate aminotransferase (AST) of all grades was seen in 38% and 40% of patients, respectively.54 Therefore, close monitoring of liver function is clinically warranted. In particular, patients need to be advised to avoid concomitant use of herbal supplements, a common practice in Asian countries.
CASE 1 CONTINUED
The patient does well while on erlotinib at 150 mg orally once daily for about 8 months, until he develops increasing abdominal pain. A CT scan of the abdomen and pelvis with contrast shows a new 8-cm right adrenal mass. Additionally, a repeat CT scan of the chest with contrast shows a stable lung mass but enlarging mediastinal lymphadenopathy.
How would you manage the patient at this point?
MANAGEMENT OF T790M MUTATION AFTER PROGRESSION ON FIRST-LINE EGFR TKIS
As mentioned above, the median PFS of patients with EGFR mutations treated with 1 of the 3 TKIs is around 9 to 13 months.46 Of the various resistance mechanisms that have been described, the T790M mutation is found in approximately 60% of patients who progress after treatment with first-line TKIs.55,56 Other mechanisms, such as HER2 amplification, MET amplification, or rarely small cell transformation, have been reported.56 The first- and second-generation EGFR TKIs function by binding to the ATP-binding domain of mutated EGFR, leading to inhibition of the downstream signaling pathways (Figure, part B) and ultimately cell death.35 The T790M mutation hinders the interaction between the ATP-binding domain of EGFR kinase and TKIs, resulting in treatment resistance and disease progression.57,58
Osimertinib is a third-generation irreversible EGFR TKI with activity against both sensitizing EGFR and resistant T790M mutations. It has low affinity for wide-type EGFR as well as insulin receptor and insulin-like growth factor receptor.59 Osimertinib has been fully approved for NSCLC patients with EGFR mutations who have progressed on first-line EGFR TKIs with the development of T790M mutation. An international phase 3 trial (AURA3) randomly assigned 419 patients in a 2:1 ratio to either osimertinib or platinum/pemetrexed. Eligible patients all had the documented EGFR mutations and disease progression after first-line EGFR TKIs. Central confirmation of the T790M mutation was required. Median PFS by investigator assessment, the trial’s primary end point, was 10.1 months for osimertinib versus 4.4 months for chemotherapy (HR 0.3 [95% CI 0.23 to 0.41]; P < 0.001). ORR was 71% for osimertinib versus 31% for chemotherapy (HR 5.39 [95% CI 3.47 to 8.48], P < 0.001). A total of 144 patients with stable and asymptomatic brain metastases were also eligible. Median PFS for this subset of patients treated with osimertinib and chemotherapy was 8.5 months and 4.2 months, respectively (HR 0.32 [95% CI 0.21 to 0.49]). In the AURA3 trial, osimertinib was better tolerated than chemotherapy, with 23% of patients treated with osimertinib experiencing grade 3 or 4 AEs as compared to 47% of chemotherapy-treated patients. The most common AEs of any grade were diarrhea (41%), rash (34%), dry skin (23%), and paronychia (22%).60
For the case patient, a reasonable approach would be to obtain a tissue biopsy of the adrenal mass and more importantly to check for the T790M mutation. Similar to the companion diagnostic for EGFR mutations, the cobas EGFR mutation test v2 is the FDA-approved test for T790M. However, if this resistance mutation is detected by any CLIA-certified laboratories, osimertinib should be the recommended treatment option. If tissue biopsy is not feasible, plasma-based testing should be considered. A blood-based companion diagnostic also is FDA approved.
ALK REARRANGEMENTS
CASE 2 PRESENTATION
A 42-year-old Korean woman who is a non-smoker with no significant past medical history presents with fatigue, unintentional weight loss of 20 lb in the past 4 months, and vague abdominal pain. A CT can of the abdomen and pelvis without contrast shows multiple foci in the liver and an indeterminate nodule in the right lung base. She subsequently undergoes PET/CT, which confirms multiple liver nodules/masses ranging from 1 to 3 cm with moderate FDG uptake. In addition, there is a 3.5-cm pleura-based lung mass on the right side with moderate FDG uptake. MRI-brain with and without contrast is negative for malignancy. A CT-guided biopsy of 1 of the liver masses is ordered and pathology returns positive for poorly differentiated adenocarcinoma consistent with lung primary. Molecular analysis reveals an echinoderm microtubule-associated protein-like 4 (EML4)-ALK rearrangement. She is placed on crizotinib by an outside oncologist and after about 3 weeks of therapy is doing well. She is now in your clinic for a second opinion. She says that some of her friends told her about another medication called ceritinib and was wondering if she would need to switch her cancer treatment.
How would you respond to this patient’s inquiry?
FIRST-LINE TKIS FOR ALK REARRANGEMENTS
ALK rearrangements are found in 2% to 7% of NSCLC, with EML4-ALK being the most prevalent fusion variant.61 The inversion of chromosome 2p leads to the fusion of the EML4 gene and the ALK gene, which causes the constitutive activation of the fusion protein and ultimately increased transformation and tumorigenicity.7,61 Patients harboring ALK rearrangements tend to be non-smokers. Adenocarcinoma, especially signet ring cell subtype, is the predominant histology. Compared to EGFR mutations, patients with ALK mutations are significantly younger and more likely to be men.62ALK rearrangements can be detected by either FISH or IHC, and most next-generation sequencing (NGS) panels have the ability to identify this driver mutation.
Crizotinib is the first approved ALK inhibitor for the treatment of NSCLC in this molecular subset of patients.63 PROFILE 1014 is a phase 3 randomized trial that compared crizotinib with chemotherapy containing platinum/pemetrexed for up to 6 cycles. Crossover to crizotinib was allowed for patients with disease progression on chemotherapy. The primary end point was PFS by independent radiologic review. The crizotinib arm demonstrated superior PFS (10.9 months versus 7 months; HR 0.45 [95% CI 0.35 to 0.6], P < 0.001) and ORR (74% versus 45%, P < 0.001). Median survival was not reached in either arm (HR 0.82 [95% CI 0.54 to 1.26], P = 0.36).64 Based on this international trial, crizotinib is considered standard of care in the United States for treatment-naïve patients with advanced NSCLC harboring ALK rearrangements. The current recommended dose is 250 mg orally twice daily. Common treatment-related AEs of all grades include vision disorder (62%), nausea (53%), diarrhea (43%), vomiting (40%), edema (28%), and constipation (27%).65 PROFILE 1007 compared crizotinib with pemetrexed or docetaxel in ALK-rearranged NSCLC patients with prior exposure to 1 platinum-based chemotherapy. The median PFS was 7.7 months for crizotinib as compared to 3 months for chemotherapy (HR 0.49 [95% CI 0.37 to 0.64], P < 0.001). The response rates were 65% and 20% for crizotinib and chemotherapy, respectively (P < 0.001).66 In other countries, crizotinib following 1 prior platinum-based regimen may be considered standard of care based on this trial.
Ceritinib is an oral second-generation ALK inhibitor that is 20 times more potent than crizotinib based on enzymatic assays.67 It also targets ROS1 and insulin-like growth factor 1 receptor but not c-MET. It was first approved by the FDA in April 2014 for metastatic ALK-rearranged NSCLC following crizotinib.68 In May 2017, the FDA granted approval of ceritinib for treatment-naïve patients. This decision was based on the results of the ASCEND-4 trial, a randomized phase 3 trial assessing the efficacy and safety of ceritinib over chemotherapy in the first-line setting. The trial assigned 376 patients to either ceritinib at 750 mg once daily or platinum/pemetrexed for 4 cycles followed by maintenance pemetrexed. Median PFS was 16.6 months for ceritinib versus 8.1 months for chemotherapy (HR 0.55 [95% CI 0.42 to 0.73]; P < 0.00001).69 Toxicities of ceritinib are not negligible, with gastrointestinal toxicity being the most prevalent. For instance, diarrhea, nausea, vomiting, abdominal pain, and constipation of all grades were seen in 86%, 80%, 60%, 54%, and 29% of patients, respectively. Furthermore, fatigue and decreased appetite occurred in 52% and 34% of patients, respectively. In terms of laboratory abnormalities, 84% of patients experienced decreased hemoglobin of all grades; 80% increased ALT; 75% increased AST; 58% increased creatinine; 49% increased glucose; 36% decreased phosphate; and 28% increased lipase. Due to these AEs, the incidence of dose reduction was about 58% and the median onset was around 7 weeks.70
Alectinib is another oral second-generation ALK inhibitor that was approved by the FDA in December 2015 for the treatment of NSCLC patients with ALK rearrangements who have progressed on or are intolerant to crizotinib.71 Its indication will soon be broadened to the first-line setting based on the ALEX trial.72 Alectinib is a potent and highly selective TKI of ALK73 with activity against known resistant mutations to crizotinib.74,75 It also inhibits RET but not ROS1 or c-MET.76 ALEX, a randomized phase 3 study, compared alectinib with crizotinib in treatment-naïve patients with NSCLC harboring ALK rearrangements. The trial enrolled 303 patients and the median follow-up was approximately 18 months. The alectinib arm (600 mg twice daily) demonstrated significantly higher PFS by investigator-assessment, the trial’s primary end point. The 12-month event-free survival was 68.4% (95% CI 61% to 75.9%) versus 48.7% (95% CI 40.4% to 56.9%) for alectinib and crizotinib, respectively (HR 0.47 [95% CI 0.34 to 0.65], P < 0.001). The median PFS was not reached in the alectinib arm (95% CI 17.7 months to not estimable) as compared to 11.1 months in the crizotinib arm (95% CI 9.1 to 13.1 months).72 Alectinib is generally well tolerated. Common AEs of all grades include fatigue (41%), constipation (34%), edema (30%), and myalgia (29%). As alectinib can cause anemia, lymphopenia, hepatic toxicity, increased creatine phosphokinase, hyperglycemia, electrolyte abnormalities, and increased creatinine, periodic monitoring of these laboratory values is important, although most of these abnormalities are grade 1 or 2.77
Brigatinib, another oral second-generation ALK inhibitor, was granted accelerated approval by the FDA in April 2017 for ALK-rearranged and crizotinib-resistant NSCLC based on the ALTA trial. This randomized phase 2 study of brigatinib showed an ORR by investigator assessment of 54% (97.5% CI 43% to 65%) in the 180 mg once daily arm with lead-in of 90 mg once daily for 7 days. Median PFS was 12.9 months (95% CI 11.1 months to not reached [NR]).78 Currently, a phase 3 study of brigatinib versus crizotinib in ALK inhibitor–naïve patients is recruiting participants (ALTA-1L). It will be interesting to see if brigatinib can achieve a front-line indication.
Starting the case patient on crizotinib is well within the treatment guidelines. One may consider ceritinib or alectinib in the first-line setting, but both TKIs can be reserved upon disease progression. We would recommend a repeat biopsy at that point to look for resistant mechanisms, as certain secondary ALK mutations may be rescued by certain next-generation ALK inhibitors. For instance, the F1174V mutation has been reported to confer resistance to ceritinib but sensitivity to alectinib, while the opposite is true for I1171T. The G1202R mutation is resistant to ceritinib, alectinib, and brigatinib, but lorlatinib, a third-generation ALK inhibitor, has shown activity against this mutation.79 Furthermore, brain metastasis represents a treatment challenge for patients with ALK rearrangements. It is also an efficacy measure of next-generation ALK inhibitors, all of which have demonstrated better central nervous system activity than crizotinib.69,78,80 If the case patient were found to have brain metastasis at the initial diagnosis, either ceritinib or alectinib would be a reasonable choice since crizotinib has limited penetration of blood-brain barrier.81
ROS1 REARRANGEMENTS
CASE PRESENTATION 3
A 66-year-old Chinese woman who is a non-smoker with a past medical history of hypertension and hypothyroidism presents to the emergency department for worsening lower back pain. Initial workup includes x-ray of the lumbar spine followed by MRI with contrast, which shows a soft tissue mass at L3-4 without cord compression. CT of the chest, abdomen, and pelvis with contrast shows a 7-cm right hilar mass, bilateral small lung nodules, mediastinal lymphadenopathy, and multiple lytic lesions in ribs, lumbar spine, and pelvis. MRI-brain with and without contrast is negative for malignancy. She undergoes endo-bronchial ultrasound and biopsy of the right hilar mass, which shows poorly differentiated adenocarcinoma. While waiting for the result of the molecular analysis, the patient undergoes palliative radiation therapy to L2-5 with good pain relief. She is discharged from the hospital and presents to your clinic for follow up. Molecular analysis now reveals ROS1 rearrangement with CD74-ROS1 fusion.
What treatment plan should be put in place for this patient?
FIRST-LINE THERAPY FOR ROS1 REARRANGEMENTS
Approximately 2.4% of lung adenocarcinomas harbor ROS1 rearrangements.82 This distinct genetic alteration occurs more frequently in NSCLC patients who are younger, female, and never-smokers, and who have adenocarcinomas.8 It has been shown that ROS1 rearrangements rarely overlap with other genetic alterations including KRAS mutations, EGFR mutations, and ALK rearrangements.83 As a receptor tyrosine kinase, ROS1 is similar to ALK and insulin receptor family members.84 Crizotinib, which targets ALK, ROS1, and c-MET, was approved by the FDA on March 11, 2016, for the treatment of metastatic ROS1-rearranged NSCLC.85 The approval was based on a phase 2 expansion cohort of the original phase 1 study. Among 50 US patients enrolled in this expansion cohort, 3 had complete responses and 33 had partial responses with ORR of 72% (95% CI 58% to 84%). Median PFS was 19.2 months (95% CI 14.4 months to NR) and median duration of response (DOR) was 17.6 months (95% CI 14.5 months to NR).86 During longer follow-up, independent radiology review confirmed high ORR of 66% and median DOR of 18.3 months.85
Interestingly, no companion diagnostic assay has been approved for the detection of ROS1 rearrangements with the approval of crizotinib. In the United States, break apart FISH is the most common detection method. In fact, in the above mentioned phase 2 study, ROS1 rearrangements were detected in 49 out of 50 patients by this method.86 FISH can be technically challenging when dealing with high volume and multiple targets. Reverse transcriptase-PCR is another detection method, but it requires knowledge of the fusion partners. To date, at least 14 ROS1 fusion partners have been reported, with CD74 being the most common.87 NGS with appropriate design and validation can also be used to detect ROS1 rearrangements.
For the case patient, the recommendation would be to start her on crizotinib at 250 mg twice daily. Monitoring for vision disturbance, gastrointestinal complaints, and edema is warranted. Because the estimated onset of response is around 7.9 weeks,86 plans should be made to repeat her scans in approximately 2 months.
BRAF V600E MUTATIONS
CASE PRESENTATION 4
A 71-year-old Caucasian man with a past medical history of hypertension, dyslipidemia, and ischemic cerebrovascular accident without residual deficits was diagnosed with stage IV adenocarcinoma of the lung about 8 months ago. He has a 40 pack-year smoking history and quit smoking when he was diagnosed with lung cancer. His disease burden involved a large mediastinal mass, scattered pleural nodules, multiple lymphadenopathy, and several soft tissue masses. His outside oncologist started him on chemotherapy containing carboplatin and pemetrexed for 6 cycles followed by maintenance pemetrexed. The most recent restaging scans show disease progression with enlarging soft tissue masses and several new lytic bone lesions. MRI-brain with and without contrast shows 2 subcentimeter enhancing lesions. He transferred care to you approximately 4 weeks ago. You ordered a repeat biopsy of 1 of the enlarging soft tissue masses. Molecular analysis revealed BRAF V600E mutation. In the interim, he underwent stereotactic radiosurgery for the 2 brain lesions without any complications. The patient is now in your clinic for follow up.
What would be your recommended systemic treatment?
TARGETED THERAPIES FOR BRAF V600E MUTATION
BRAF mutations were first recognized as activating mutations in advanced melanomas, with BRAF V600E, resulting from the substitution of glutamic acid for valine at amino acid 600, being the most common. BRAF plays an important role in the mitogen-activated protein kinase (MAPK) signaling pathway. Briefly, the activation of MAPK pathway occurs upon ligand binding of receptor tyrosine kinases, which then involves RAS/BRAF/MEK/ERK in a stepwise manner, ultimately leading to cell survival. BRAF mutations have been increasingly recognized also as driver mutations in NSCLC.9–12 They can be detected by PCR or NGS method. The characteristics of NSCLC patients harboring BRAF mutations have been described by various groups.9–12 For instance, 1 case series showed that the incidence was 2.2% among patients with advanced lung adenocarcinoma; 50% of mutations were V600E, while G469A and D594G accounted for the remaining 39% and 11% of patients, respectively. All patients were either current or former smokers. The median OS of patients with BRAF mutations in this case series was NR, while it was 37 months for patients with EGFR mutations (P = 0.73) and NR for patients with ALK rearrangements (P = 0.64).9
For patients with BRAF V600E–mutant NSCLC who have progressed on platinum-based chemotherapy, the combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) may represent a new treatment paradigm. This was illustrated in a phase 2, nonrandomized, open-label study. A total of 57 patients were enrolled and 36 patients (63.2% [95% CI 49.3% to 75.6%]) achieved an overall response by investigator assessment, the trial’s primary end point. Disease control rate was 78.9% (95% CI 66.1% to 88.6%), with 4% complete response, 60% partial response, and 16% stable disease. PFS was 9.7 months (95% CI [6.9 to 19.6 months]). The safety profile was comparable to what had been observed in patients with melanoma treated with this regimen. More specifically, 56% of patients on this trial reported serious AEs, including pyrexia (16%), anemia (5%), confusional state (4%), decreased appetite (4%), hemoptysis (4%), hypercalcemia (4%), nausea (4%), and cutaneous squamous cell carcinoma (4%). In addition, neutropenia (9%) and hyponatremia (7%) were the most common grade 3-4 AEs.16
The case patient has experienced disease progression after 1 line of platinum-based chemotherapy, so the combination of dabrafenib and trametinib would be a robust systemic treatment option. dabrafenib as a single agent has also been studied in BRAF V600E–mutant NSCLC in a phase 2 trial. The overall response by investigator assessment among 84 patients was 33% (95% CI 23% to 45%).14 Vemurafenib, another oral BRAF TKI, has demonstrated efficacy for NSCLC patients harboring BRAF V600E mutation. In the cohort of 20 patients with NSCLC, the response rate was 42% (95% CI 20% to 67%) and median PFS was 7.3 months (95% CI 3.5 to 10.8 months).13 Patients with non-V600E mutations have shown variable responses to targeted therapies. MEK TKIs may be considered in this setting; however, the details of this discussion are beyond the scope of this review.
CONCLUSION
The management of advanced NSCLC with driver mutations has seen revolutionary changes over the past decade. Tremendous research has been done in order to first understand the molecular pathogenesis of NSCLC and then discover driver mutations that would lead to development of targeted therapies with clinically significant efficacy as well as tolerability. More recently, increasing efforts have focused on how to conquer acquired resistance in patients with disease progression after first-line TKIs. The field of EGFR-mutant NSCLC has set a successful example, but the work is nowhere near finished. The goals are to search for more driver mutations and to design agents that could potentially block cell survival signals once and for all.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1–19.
- Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S–29S.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-3013, based on November 2015 SEER data submission, posted to the SEER website, April 2016. Bethesda (MD): National Cancer Institute; 2016.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer: 1–190.
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–39.
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–6.
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancer. J Clin Oncol 2012;30:863–70.
- Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011;29:2046–51.
- Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol 2014;25:138–42.
- Litvak AM, Paik PK, Woo KM, et al. Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. J Thorac Oncol 2014;9:1669–74.
- Villaruz LC, Socinski MA, Abberbock S, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer 2015;121:448–56.
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015;373:726–36.
- Planchard D, Kim TM, Mazieres J, et al. DaBRAFenib in patients with BRAF V600E-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:642–50.
- Gautschi O, Milia J, Cabarrou B, et al. Targeted therapy for patients with BRAF-mutant lung cancer: results from the European EURAF cohort. J Thorac Oncol 2015;10:1451–7.
- Planchard D, Besse B, Groen HJ, et al. DaBRAFenib plus trametinib in patients with previously treated BRAF V600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984–93.
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer and Association for Molecular Pathology. J Thorac Oncol 2013;8:823–59.
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer and Association for Molecular Pathology. Arch Pathol Lab Med 2013;137:828–60.
- Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol 2014;32:3673–9.
- Paik PK, Varghese AM, Sima CS, et al. Response to erlotinib in patients with EGFR mutant advanced non-small cell lung cancers with a squamous or squamous-like component. Mol Cancer Ther 2012;11:2535–40.
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842–9.
- Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 mutations in non-small-cell lung cancer are associated with advanced age, and stage-dependent MET genomic amplification, and c-MET overexpression. J Clin Oncol 2016;34:721–30.
- Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol 2016;11:1493–502.
- Reungwetwattana T, Liang Y, Zhu V, et al. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: The why, the how, the who, the unknown, and the inevitable. Lung Cancer 2017;103:27–37.
- Drilon A, Wang L, Hasanovic A, et al. Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013;3:630–5.
- Lin JJ, Kennedy E, Sequist LV, et al. Clinical activity of alectinib in advanced RET-rearranged non-small cell lung cancer. J Thorac Oncol 2016;11:2027–32.
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653–60.
- Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med 2006;354:2619–21.
- Mazieres J. Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targted drugs: results from the European EUHER2 cohort. Annal Oncol 2016;27:281–6.
- Ou SH, Schrock AB, Bocharov EV, et al. HER2 transmembrane (TMD) mutations (V659/G660) that stabilize homo- and heterodimerization are rare oncogenic drivers in lung adenocarcinoma that respond to afatinib. J Thorac Oncol 2017;12:446–57.
- Jordan EJ, Kim HR, Arcila ME, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emergent therapies. Cancer Discov 2017;7:596–609.
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006.
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415–26.
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958–67.
- Rosell R, Karachaliou N, et al. Large-scale screening for somatic mutations in lung cancer. Lancet 2016;387:1354–6.
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339–46.
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34.
- Wu YL, Chou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883–9.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Eng J Med 2010;362:2380–8.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46.
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22.
- Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009;10:432–3.
- Nelson V, Ziehr J, Aqulnik M, et al. Afatinib: emerging next-generation tyrosine kinase inhibitor for NSCLC. Onco Targets Ther 2013;5:135–43.
- Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol 2015;33:1958–65.
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54–9.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57.
- Wu YL, Saijo N, Thongprasert S, et al. Efficacy according to blind independent central review: post-hoc analyses from the phase III, randomized, multicenter, IPASS study of first-line gefitinib versus carboplatin/paclitaxel in Asian patients with EFGR mutation-positive advanced NSCLC. Lung Cancer 2017;104:119–25.
- Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR-mutation positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer 2014;110:55–62.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol 2007;56:317–26.
- Tarceva [package insert]. South San Francisco (CA): Genentech, Inc; 2010. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021743s14s16lbl.pdf. Accessed April 23, 2017.
- Gilotrif [package insert.] Ridgefield (CT): Boehringer Ingelheim, Inc; 2013. www.accessdata.fda.gov/drugsatfda_docs/label/2013/201292s000lbl.pdf. Accessed April 23, 2017.
- Iressa [package insert]. Wilmington (DE): AstraZeneca, Inc; 2015. Error! Hyperlink reference not valid. Accessed April 23, 2017.
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–22.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR TKI therapy in 155 patients with EGFR mutant lung cancers. Clin Cancer Res 2013;19:2240–7.
- Yun CH, Mengwasser KE, Tom AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070–5.
- Sos ML, Rode HB, Heynck S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer Res 2010;70:868–74.
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T190M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046–61.
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629–40.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small cell lung cancer. N Engl J Med 2010;363:1693–703.
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247–53.
- Kazandjian D, Blumenthal GM, Chen HY, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist 2014;19:e5–11.
- Solomon BJ, Mok T, Kim DW, et al. First-ling crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–77.
- Xalkori [package insert]. New York: Pfizer, Inc; 2011. www.accessdata.fda.gov/drugsatfda_docs/label/2012/202570s002lbl.pdf. Accessed April 23, 2017.
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94.
- Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem 2013;56:5675–90.
- Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res 2015;21:2436–9.
- Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917–29.
- Zykadia [package insert]. East Hanover (NJ): Novartis Pharmaceuticals Corporation, Inc; 2016. www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/zykadia.pdf. Accessed April 23, 2017.
- Larkins E, Blumenthal GM, Chen H, et al. FDA approval: alectinib for the treatment of metastatic, ALK-positive non-small cell lung cancer following crizotinib. Clin Cancer Res 2016;22:5171–6.
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. New Engl J Med 2017 June 6 [Epub ahead of print].
- Kinoshita K, Asoh K, Furuichi N, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem 2012;20:1271–80.
- Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011;19:679–90.
- Kodama T, Tsukaguchi T, Yoshida M, et al. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett 2014;351:215–21.
- Kodama T, Tsukaguchi T, Satoh T, et al. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther 2014;13:2910–8.
- Alecensa [package insert]. South San Francisco (CA): Genentech, Inc; 2015. www.accessdata.fda.gov/drugsatfda_docs/label/2015/208434s000lbl.pdf. Accessed April 23, 2017.
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol 2017 May 5 [Epub ahead of print].
- Zhu V, Ou SH. Safety of alectinib for the treatment of metastatic ALK-rearranged non-small cell lung cancer. Expert Opin Drug Saf 2017;16:509–14.
- Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small cell lung cancer. J Clin Oncol 2016;34:4079–85.
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443–5.
- Zhu Q, Zhan P, Zhang X, et al. Clinicopathologic characteristics of patients with ROS1 fusion gene in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2015;4:300–9.
- Lin JJ, Ritterhouse LL, Ali SM, et al. ROS1 fusions rarely overlap with other oncogenic drivers in non-small cell lung cancer. J Thorac Oncol 2017;12:872–7.
- Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta 2009;1795:37–52.
- Kazandjian D, Blumenthal G, Luo L, et al. Benefit-Risk summary of crizotinib for the treatment of patients with ROS1 alteration-positive metastatic NSCLC. Oncologist 2016;21:974–80.
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963–71.
- Zhu VW, Upadhyay D, Schrock AB, et al. TPD52L1-ROS1, a new ROS1 fusion variant in lung adenosquamous cell carcinoma identified by comprehensive genomic profiling. Lung Cancer 2016;97:48–50.
INTRODUCTION
Lung cancer is the second most common type of cancer in the United States, with 222,500 estimated new cases in 2017, according to the American Cancer Society.1 However, it is by far the number one cause of death due to cancer, with an estimated 155,870 lung cancer–related deaths occurring in 2017, which is higher than the number of deaths due to breast cancer, prostate cancer, and colorectal cancer combined.1,2 Despite slightly decreasing incidence and mortality over the past decade, largely due to smoking cessation, the 5-year survival rate of lung cancer remains dismal at approximately 18%.2–4
Non-small cell lung cancer (NSCLC) accounts for 80% to 85% of all lung cancer cases.4 Traditionally, it is further divided based on histology: adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and not otherwise specified.5 Chemotherapy had been the cornerstone of treatment for stage IV NSCLC. It is not target-specific and is most effective against rapidly growing cells. Common adverse effects include alopecia, nausea/vomiting, myelosuppression, cardiotoxicity, neuropathy, and nephrotoxicity. However, this paradigm has shifted following the discovery of mutations of the epidermal growth factor receptor (EGFR) gene as an oncogenic driver that confers sensitivity to small molecule tyrosine kinase inhibitors (TKIs) targeting EGFR.6 The EGFR inhibitors are given orally and have a spectrum of toxicities (eg, such as rash, diarrhea, and elevated transaminases) different from that of systemic chemotherapy, which is often administered intravenously. Following the discovery of EGFR mutations, rearrangements of the anaplastic lymphoma kinase (ALK) gene7 and ROS1 gene8 were identified as targetable driver mutations in NSCLC. The frequency of both rearrangements is lower than that of EGFR mutations. Additionally, BRAF V600E mutation has been identified in NSCLC.9–12 This activation mutation is commonly seen in melanoma. Agents that have already been approved for the treatment of melanoma with the BRAF V600E mutation are being tested in NSCLC patients with this mutation.13–16
Given the effectiveness and tolerability of targeted therapy, identifying this distinct molecular subset of NSCLC patients is critical in treatment. Currently, molecular testing is mandatory in all stage IV patients with non-squamous cell carcinoma, as a preponderance of patients with driver mutations have this histology subtype.5,17–19 For patients with squamous cell carcinoma, molecular testing should be considered if the biopsy specimen is small, there is mixed histology, or the patient is a nonsmoker.5,20 Several techniques are commonly utilized in detecting these genetic alterations. EGFR mutation can be detected by polymerase chain reaction (PCR), ALK or ROS1 rearrangement can be detected by fluorescence in-situ hybridization (FISH), and immunohistochemistry (IHC) can also be used to detect ALK rearrangement. The current guideline is to use comprehensive genomic profiling to capture all the potential molecular targets simultaneously instead of running stepwise tests just for EGFR, ALK, and ROS1.5BRAF V600E mutation,13–16 MET exon 14 skipping mutation,21–24 RET rearrangements,25–27 and HER2 mutations28–30 are among the emergent genetic alterations with various responses to targeted therapy.31 Some of these targeted agents have been approved for other types of malignancy, and others are still in the development phase.
Several initiatives worldwide have reported better outcomes of patients with driver mutations treated with targeted therapy. For instance, the Lung Cancer Mutation Consortium in the United States demonstrated that the median survival of patients without driver mutations, with drivers mutations but not treated with targeted therapy, and with driver mutations and treated with targeted therapy was 2.08 years, 2.38 years, and 3.49 years, respectively.32 The French Cooperative Thoracic Intergroup-French National Cancer Institute demonstrated that the median survival for patients with driver mutations versus those without driver mutations was 16.5 months versus 11.8 months.33 The Spanish Lung Cancer Group demonstrated that the overall survival (OS) for patients with EGFR mutations treated with erlotinib was 27 months.34 The mutations in lung cancer, their frequencies, and the downstream signaling pathways are depicted in the Figure.35

In this article, we discuss targeted therapy for patients with EGFR mutations, ALK rearrangements, ROS1 rearrangements, and BRAF V600E mutation. We also discuss the management of patients with EGFR mutations who develop a secondary mutation after TKI therapy. Almost all of the targeted agents discussed herein have been approved by the US Food and Drug Administration (FDA), so they are considered standard of care. All available phase 3 trials pertinent to these targeted therapies are included in the discussion.
EGFR MUTATIONS
CASE PRESENTATION 1
A 54-year-old Caucasian man who is a former smoker with a 10 pack-year history and past medical history of hypertension and dyslipidemia presents with progressive dyspnea for several weeks. A chest x-ray shows moderate pleural effusion on the left side with possible mass-like opacity on the left upper lung field. An ultrasound-guided thoracentesis is performed and cytology is positive for adenocarcinoma of likely pulmonary origin. Staging workup including positron emission tomography (PET)/computed tomography (CT) and magnetic resonance imaging of the brain with and without contrast is done. PET/CT shows a 5.5-cm mass in the left upper lobe of the lung with high fluorodeoxyglucose (FDG) uptake, several 1- to 2-cm mediastinal lymph nodes with moderate FDG uptake, and small pleural effusion on both sides with moderate FDG uptake. MRI-brain is negative for malignancy. The patient subsequently undergoes a CT-guided biopsy of the lung mass, which shows moderately differentiated adenocarcinoma. Comprehensive molecular profiling reveals EGFR L858R mutation only. The patient now presents for the initial consultation. Of note, his Eastern Cooperative Oncology Group performance status is 1.
What is the next step in the management of this patient?
FIRST-LINE TKI FOR SENSITIZING EGFR MUTATIONS
The 2 most common EGFR mutations are deletions in exon 19 and substitution of arginine for leucine in exon 21 (L858R), found in approximately 45% and 40% of patients with EGFR mutations, respectively.36 Both mutations are sensitive to EGFR TKIs. The benefit may be greater in patients with exon 19 deletions as compared to exon 21 L858R substitution,37,38 but this has not been demonstrated consistently in clinical trials.39-43 In the United States, EGFR mutations are found in approximately 10% of patients with NSCLC, while the incidence can be as high as 50% in Asia.44 Even though the cobas EGFR mutation test is the companion diagnostic approved by the US FDA, a positive test result from any laboratory with the Clinical Laboratory Improvement Amendments (CLIA) certificate should prompt the use of an EGFR TKI as the initial treatment.
Three EGFR TKIs that have been approved as first-line therapy in the United States are available: erlotinib, afatinib, and gefitinib.5 Both erlotinib and gefitinib are considered first-generation TKIs. They have higher binding affinity for the 2 common EGFR mutations than wild-type EGFR. In addition, they reversibly bind to the intracellular tyrosine kinase domain, resulting in inhibition of autophosphorylation of the tyrosine residues. Afatinib, a second-generation and irreversible TKI, targets EGFR (HER1) as well as HER2 and HER4.45
The superior efficacy of the EGFR TKIs over platinum doublet chemotherapy in treatment-naïve patients with EGFR mutations has been demonstrated in 7 randomized trials to date (Table).46 Erlotinib was the TKI arm for the OPTIMAL,41 EURTAC,42 and ENSURE trials;38 afatinib was the TKI arm for LUX-LUNG 337 and 6;43 gefitinib was the TKI arm for NEJ00239,47 and WJTOG3405.40 A meta-analysis of these 7 trials by Lee et al showed that progression-free survival (PFS) was significantly prolonged by EGFR TKIs (hazard ratio [HR] 0.37 [95% confidence interval {CI} 0.32 to 0.42]).46 For instance, in the EURTAC trial, median PFS was 9.7 months for patients treated with erlotinib as compared to 5.2 months for patients treated with platinum/gemcitabine or platinum/docetaxel.42 In this meta-analysis, prespecified subgroups included age, sex, ethnicity, smoking status, performance status, tumor histology, and EGFR mutation subtype. The superior outcome with TKIs was observed in all subgroups. Furthermore, patients with exon 19 deletions, nonsmokers, and women had even better outcomes.46

Erlotinib is the most commonly used TKI in the United States largely because gefitinib was off the market for some time until it was re-approved by the FDA in 2015. Interestingly, this “re-approval” was not based on either 1 of the 2 prospective trials (NEJ00239,47 and WJTOG340540), but rather was based on an exploratory analysis of the IPASS trial48,49 as well as a prospective phase 4, single-arm trial in Europe (IFUM).50 The superior efficacy of gefitinib over carboplatin/paclitaxel among patients with EGFR mutations in the IPASS trial was confirmed by blind independent central review, with longer PFS (HR 0.54 [95% CI 0.38 to 0.79] P = 0.0012) and higher objective response rate (ORR; odds ratio 3 [95% CI 1.63 to 5.54], P = 0.0004).49
CASE 1 CONTINUED
Based on the EGFR L858R mutation status, the patient is started on erlotinib. He is quite happy that he does not need intravenous chemotherapy but wants to know what toxicities he might potentially have with erlotinib.
What are the common adverse effects (AEs) of EGFR TKIs? How are AEs of TKIs managed?
Safety Profile
The important toxicities associated with EGFR TKIs are rash, gastrointestinal toxicity, hepatic toxicity, and pulmonary toxicity. Rash is an AE specific to all agents blocking the EGFR pathway, including small molecules and monoclonal antibodies such as cetuximab. The epidermis has a high level of expression of EGFR, which contributes to this toxicity.51 Rash usually presents as dry skin or acneiform eruption. Prophylactic treatment with oral tetracyclines and topical corticosteroids is generally recommended upon initiation of TKI therapy. Diarrhea is the most prevalent gastrointestinal toxicity. All patients starting treatment should be given prescriptions to manage diarrhea such as loperamide and be advised to call when it occurs. Hepatic toxicity is often manifested as elevated transaminases or bilirubin. Interstitial lung disease (ILD) is a rare but potentially fatal pulmonary toxicity.
Rash of any grade was reported in 49.2% of patients treated with erlotinib in clinical trials, while grade 3 rash occurred in 6% of patients and no grade 4 was reported. Diarrhea of any grade was reported in 20.3% of patients, grade 3 diarrhea occurred in 1.8%, and no grade 4 was reported. Grade 2 and 3 alanine aminotransferase (ALT) elevations were seen in 2% and 1% of patients, respectively. Grade 2 and 3 bilirubin elevations were seen in 4% and less than 1% of patients, respectively. The incidence of serious ILD-like events was less than 1%.52
Afatinib is associated with higher incidences of rash and diarrhea. Specifically, diarrhea and rash of all grades were reported in 96% and 90% of patients treated with afatinib, respectively. Paronychia of all grades occurred in 58% of patients. Elevated ALT of all grades was seen in 11% of patients. Approximately 1.5% of patients treated with afatinib across clinical trials had ILD or ILD-like AEs.53
Gefitinib, the most commonly used TKI outside United States, has a toxicity profile similar to erlotinib, except for hepatic toxicity. For instance, rash of all grades occurred in 47% of patients, diarrhea of all grades occurred in 29% of patients, and ILD or ILD-like AEs occurred in 1.3% of patients across clinical trials. In comparison, elevated ALT and aspartate aminotransferase (AST) of all grades was seen in 38% and 40% of patients, respectively.54 Therefore, close monitoring of liver function is clinically warranted. In particular, patients need to be advised to avoid concomitant use of herbal supplements, a common practice in Asian countries.
CASE 1 CONTINUED
The patient does well while on erlotinib at 150 mg orally once daily for about 8 months, until he develops increasing abdominal pain. A CT scan of the abdomen and pelvis with contrast shows a new 8-cm right adrenal mass. Additionally, a repeat CT scan of the chest with contrast shows a stable lung mass but enlarging mediastinal lymphadenopathy.
How would you manage the patient at this point?
MANAGEMENT OF T790M MUTATION AFTER PROGRESSION ON FIRST-LINE EGFR TKIS
As mentioned above, the median PFS of patients with EGFR mutations treated with 1 of the 3 TKIs is around 9 to 13 months.46 Of the various resistance mechanisms that have been described, the T790M mutation is found in approximately 60% of patients who progress after treatment with first-line TKIs.55,56 Other mechanisms, such as HER2 amplification, MET amplification, or rarely small cell transformation, have been reported.56 The first- and second-generation EGFR TKIs function by binding to the ATP-binding domain of mutated EGFR, leading to inhibition of the downstream signaling pathways (Figure, part B) and ultimately cell death.35 The T790M mutation hinders the interaction between the ATP-binding domain of EGFR kinase and TKIs, resulting in treatment resistance and disease progression.57,58
Osimertinib is a third-generation irreversible EGFR TKI with activity against both sensitizing EGFR and resistant T790M mutations. It has low affinity for wide-type EGFR as well as insulin receptor and insulin-like growth factor receptor.59 Osimertinib has been fully approved for NSCLC patients with EGFR mutations who have progressed on first-line EGFR TKIs with the development of T790M mutation. An international phase 3 trial (AURA3) randomly assigned 419 patients in a 2:1 ratio to either osimertinib or platinum/pemetrexed. Eligible patients all had the documented EGFR mutations and disease progression after first-line EGFR TKIs. Central confirmation of the T790M mutation was required. Median PFS by investigator assessment, the trial’s primary end point, was 10.1 months for osimertinib versus 4.4 months for chemotherapy (HR 0.3 [95% CI 0.23 to 0.41]; P < 0.001). ORR was 71% for osimertinib versus 31% for chemotherapy (HR 5.39 [95% CI 3.47 to 8.48], P < 0.001). A total of 144 patients with stable and asymptomatic brain metastases were also eligible. Median PFS for this subset of patients treated with osimertinib and chemotherapy was 8.5 months and 4.2 months, respectively (HR 0.32 [95% CI 0.21 to 0.49]). In the AURA3 trial, osimertinib was better tolerated than chemotherapy, with 23% of patients treated with osimertinib experiencing grade 3 or 4 AEs as compared to 47% of chemotherapy-treated patients. The most common AEs of any grade were diarrhea (41%), rash (34%), dry skin (23%), and paronychia (22%).60
For the case patient, a reasonable approach would be to obtain a tissue biopsy of the adrenal mass and more importantly to check for the T790M mutation. Similar to the companion diagnostic for EGFR mutations, the cobas EGFR mutation test v2 is the FDA-approved test for T790M. However, if this resistance mutation is detected by any CLIA-certified laboratories, osimertinib should be the recommended treatment option. If tissue biopsy is not feasible, plasma-based testing should be considered. A blood-based companion diagnostic also is FDA approved.
ALK REARRANGEMENTS
CASE 2 PRESENTATION
A 42-year-old Korean woman who is a non-smoker with no significant past medical history presents with fatigue, unintentional weight loss of 20 lb in the past 4 months, and vague abdominal pain. A CT can of the abdomen and pelvis without contrast shows multiple foci in the liver and an indeterminate nodule in the right lung base. She subsequently undergoes PET/CT, which confirms multiple liver nodules/masses ranging from 1 to 3 cm with moderate FDG uptake. In addition, there is a 3.5-cm pleura-based lung mass on the right side with moderate FDG uptake. MRI-brain with and without contrast is negative for malignancy. A CT-guided biopsy of 1 of the liver masses is ordered and pathology returns positive for poorly differentiated adenocarcinoma consistent with lung primary. Molecular analysis reveals an echinoderm microtubule-associated protein-like 4 (EML4)-ALK rearrangement. She is placed on crizotinib by an outside oncologist and after about 3 weeks of therapy is doing well. She is now in your clinic for a second opinion. She says that some of her friends told her about another medication called ceritinib and was wondering if she would need to switch her cancer treatment.
How would you respond to this patient’s inquiry?
FIRST-LINE TKIS FOR ALK REARRANGEMENTS
ALK rearrangements are found in 2% to 7% of NSCLC, with EML4-ALK being the most prevalent fusion variant.61 The inversion of chromosome 2p leads to the fusion of the EML4 gene and the ALK gene, which causes the constitutive activation of the fusion protein and ultimately increased transformation and tumorigenicity.7,61 Patients harboring ALK rearrangements tend to be non-smokers. Adenocarcinoma, especially signet ring cell subtype, is the predominant histology. Compared to EGFR mutations, patients with ALK mutations are significantly younger and more likely to be men.62ALK rearrangements can be detected by either FISH or IHC, and most next-generation sequencing (NGS) panels have the ability to identify this driver mutation.
Crizotinib is the first approved ALK inhibitor for the treatment of NSCLC in this molecular subset of patients.63 PROFILE 1014 is a phase 3 randomized trial that compared crizotinib with chemotherapy containing platinum/pemetrexed for up to 6 cycles. Crossover to crizotinib was allowed for patients with disease progression on chemotherapy. The primary end point was PFS by independent radiologic review. The crizotinib arm demonstrated superior PFS (10.9 months versus 7 months; HR 0.45 [95% CI 0.35 to 0.6], P < 0.001) and ORR (74% versus 45%, P < 0.001). Median survival was not reached in either arm (HR 0.82 [95% CI 0.54 to 1.26], P = 0.36).64 Based on this international trial, crizotinib is considered standard of care in the United States for treatment-naïve patients with advanced NSCLC harboring ALK rearrangements. The current recommended dose is 250 mg orally twice daily. Common treatment-related AEs of all grades include vision disorder (62%), nausea (53%), diarrhea (43%), vomiting (40%), edema (28%), and constipation (27%).65 PROFILE 1007 compared crizotinib with pemetrexed or docetaxel in ALK-rearranged NSCLC patients with prior exposure to 1 platinum-based chemotherapy. The median PFS was 7.7 months for crizotinib as compared to 3 months for chemotherapy (HR 0.49 [95% CI 0.37 to 0.64], P < 0.001). The response rates were 65% and 20% for crizotinib and chemotherapy, respectively (P < 0.001).66 In other countries, crizotinib following 1 prior platinum-based regimen may be considered standard of care based on this trial.
Ceritinib is an oral second-generation ALK inhibitor that is 20 times more potent than crizotinib based on enzymatic assays.67 It also targets ROS1 and insulin-like growth factor 1 receptor but not c-MET. It was first approved by the FDA in April 2014 for metastatic ALK-rearranged NSCLC following crizotinib.68 In May 2017, the FDA granted approval of ceritinib for treatment-naïve patients. This decision was based on the results of the ASCEND-4 trial, a randomized phase 3 trial assessing the efficacy and safety of ceritinib over chemotherapy in the first-line setting. The trial assigned 376 patients to either ceritinib at 750 mg once daily or platinum/pemetrexed for 4 cycles followed by maintenance pemetrexed. Median PFS was 16.6 months for ceritinib versus 8.1 months for chemotherapy (HR 0.55 [95% CI 0.42 to 0.73]; P < 0.00001).69 Toxicities of ceritinib are not negligible, with gastrointestinal toxicity being the most prevalent. For instance, diarrhea, nausea, vomiting, abdominal pain, and constipation of all grades were seen in 86%, 80%, 60%, 54%, and 29% of patients, respectively. Furthermore, fatigue and decreased appetite occurred in 52% and 34% of patients, respectively. In terms of laboratory abnormalities, 84% of patients experienced decreased hemoglobin of all grades; 80% increased ALT; 75% increased AST; 58% increased creatinine; 49% increased glucose; 36% decreased phosphate; and 28% increased lipase. Due to these AEs, the incidence of dose reduction was about 58% and the median onset was around 7 weeks.70
Alectinib is another oral second-generation ALK inhibitor that was approved by the FDA in December 2015 for the treatment of NSCLC patients with ALK rearrangements who have progressed on or are intolerant to crizotinib.71 Its indication will soon be broadened to the first-line setting based on the ALEX trial.72 Alectinib is a potent and highly selective TKI of ALK73 with activity against known resistant mutations to crizotinib.74,75 It also inhibits RET but not ROS1 or c-MET.76 ALEX, a randomized phase 3 study, compared alectinib with crizotinib in treatment-naïve patients with NSCLC harboring ALK rearrangements. The trial enrolled 303 patients and the median follow-up was approximately 18 months. The alectinib arm (600 mg twice daily) demonstrated significantly higher PFS by investigator-assessment, the trial’s primary end point. The 12-month event-free survival was 68.4% (95% CI 61% to 75.9%) versus 48.7% (95% CI 40.4% to 56.9%) for alectinib and crizotinib, respectively (HR 0.47 [95% CI 0.34 to 0.65], P < 0.001). The median PFS was not reached in the alectinib arm (95% CI 17.7 months to not estimable) as compared to 11.1 months in the crizotinib arm (95% CI 9.1 to 13.1 months).72 Alectinib is generally well tolerated. Common AEs of all grades include fatigue (41%), constipation (34%), edema (30%), and myalgia (29%). As alectinib can cause anemia, lymphopenia, hepatic toxicity, increased creatine phosphokinase, hyperglycemia, electrolyte abnormalities, and increased creatinine, periodic monitoring of these laboratory values is important, although most of these abnormalities are grade 1 or 2.77
Brigatinib, another oral second-generation ALK inhibitor, was granted accelerated approval by the FDA in April 2017 for ALK-rearranged and crizotinib-resistant NSCLC based on the ALTA trial. This randomized phase 2 study of brigatinib showed an ORR by investigator assessment of 54% (97.5% CI 43% to 65%) in the 180 mg once daily arm with lead-in of 90 mg once daily for 7 days. Median PFS was 12.9 months (95% CI 11.1 months to not reached [NR]).78 Currently, a phase 3 study of brigatinib versus crizotinib in ALK inhibitor–naïve patients is recruiting participants (ALTA-1L). It will be interesting to see if brigatinib can achieve a front-line indication.
Starting the case patient on crizotinib is well within the treatment guidelines. One may consider ceritinib or alectinib in the first-line setting, but both TKIs can be reserved upon disease progression. We would recommend a repeat biopsy at that point to look for resistant mechanisms, as certain secondary ALK mutations may be rescued by certain next-generation ALK inhibitors. For instance, the F1174V mutation has been reported to confer resistance to ceritinib but sensitivity to alectinib, while the opposite is true for I1171T. The G1202R mutation is resistant to ceritinib, alectinib, and brigatinib, but lorlatinib, a third-generation ALK inhibitor, has shown activity against this mutation.79 Furthermore, brain metastasis represents a treatment challenge for patients with ALK rearrangements. It is also an efficacy measure of next-generation ALK inhibitors, all of which have demonstrated better central nervous system activity than crizotinib.69,78,80 If the case patient were found to have brain metastasis at the initial diagnosis, either ceritinib or alectinib would be a reasonable choice since crizotinib has limited penetration of blood-brain barrier.81
ROS1 REARRANGEMENTS
CASE PRESENTATION 3
A 66-year-old Chinese woman who is a non-smoker with a past medical history of hypertension and hypothyroidism presents to the emergency department for worsening lower back pain. Initial workup includes x-ray of the lumbar spine followed by MRI with contrast, which shows a soft tissue mass at L3-4 without cord compression. CT of the chest, abdomen, and pelvis with contrast shows a 7-cm right hilar mass, bilateral small lung nodules, mediastinal lymphadenopathy, and multiple lytic lesions in ribs, lumbar spine, and pelvis. MRI-brain with and without contrast is negative for malignancy. She undergoes endo-bronchial ultrasound and biopsy of the right hilar mass, which shows poorly differentiated adenocarcinoma. While waiting for the result of the molecular analysis, the patient undergoes palliative radiation therapy to L2-5 with good pain relief. She is discharged from the hospital and presents to your clinic for follow up. Molecular analysis now reveals ROS1 rearrangement with CD74-ROS1 fusion.
What treatment plan should be put in place for this patient?
FIRST-LINE THERAPY FOR ROS1 REARRANGEMENTS
Approximately 2.4% of lung adenocarcinomas harbor ROS1 rearrangements.82 This distinct genetic alteration occurs more frequently in NSCLC patients who are younger, female, and never-smokers, and who have adenocarcinomas.8 It has been shown that ROS1 rearrangements rarely overlap with other genetic alterations including KRAS mutations, EGFR mutations, and ALK rearrangements.83 As a receptor tyrosine kinase, ROS1 is similar to ALK and insulin receptor family members.84 Crizotinib, which targets ALK, ROS1, and c-MET, was approved by the FDA on March 11, 2016, for the treatment of metastatic ROS1-rearranged NSCLC.85 The approval was based on a phase 2 expansion cohort of the original phase 1 study. Among 50 US patients enrolled in this expansion cohort, 3 had complete responses and 33 had partial responses with ORR of 72% (95% CI 58% to 84%). Median PFS was 19.2 months (95% CI 14.4 months to NR) and median duration of response (DOR) was 17.6 months (95% CI 14.5 months to NR).86 During longer follow-up, independent radiology review confirmed high ORR of 66% and median DOR of 18.3 months.85
Interestingly, no companion diagnostic assay has been approved for the detection of ROS1 rearrangements with the approval of crizotinib. In the United States, break apart FISH is the most common detection method. In fact, in the above mentioned phase 2 study, ROS1 rearrangements were detected in 49 out of 50 patients by this method.86 FISH can be technically challenging when dealing with high volume and multiple targets. Reverse transcriptase-PCR is another detection method, but it requires knowledge of the fusion partners. To date, at least 14 ROS1 fusion partners have been reported, with CD74 being the most common.87 NGS with appropriate design and validation can also be used to detect ROS1 rearrangements.
For the case patient, the recommendation would be to start her on crizotinib at 250 mg twice daily. Monitoring for vision disturbance, gastrointestinal complaints, and edema is warranted. Because the estimated onset of response is around 7.9 weeks,86 plans should be made to repeat her scans in approximately 2 months.
BRAF V600E MUTATIONS
CASE PRESENTATION 4
A 71-year-old Caucasian man with a past medical history of hypertension, dyslipidemia, and ischemic cerebrovascular accident without residual deficits was diagnosed with stage IV adenocarcinoma of the lung about 8 months ago. He has a 40 pack-year smoking history and quit smoking when he was diagnosed with lung cancer. His disease burden involved a large mediastinal mass, scattered pleural nodules, multiple lymphadenopathy, and several soft tissue masses. His outside oncologist started him on chemotherapy containing carboplatin and pemetrexed for 6 cycles followed by maintenance pemetrexed. The most recent restaging scans show disease progression with enlarging soft tissue masses and several new lytic bone lesions. MRI-brain with and without contrast shows 2 subcentimeter enhancing lesions. He transferred care to you approximately 4 weeks ago. You ordered a repeat biopsy of 1 of the enlarging soft tissue masses. Molecular analysis revealed BRAF V600E mutation. In the interim, he underwent stereotactic radiosurgery for the 2 brain lesions without any complications. The patient is now in your clinic for follow up.
What would be your recommended systemic treatment?
TARGETED THERAPIES FOR BRAF V600E MUTATION
BRAF mutations were first recognized as activating mutations in advanced melanomas, with BRAF V600E, resulting from the substitution of glutamic acid for valine at amino acid 600, being the most common. BRAF plays an important role in the mitogen-activated protein kinase (MAPK) signaling pathway. Briefly, the activation of MAPK pathway occurs upon ligand binding of receptor tyrosine kinases, which then involves RAS/BRAF/MEK/ERK in a stepwise manner, ultimately leading to cell survival. BRAF mutations have been increasingly recognized also as driver mutations in NSCLC.9–12 They can be detected by PCR or NGS method. The characteristics of NSCLC patients harboring BRAF mutations have been described by various groups.9–12 For instance, 1 case series showed that the incidence was 2.2% among patients with advanced lung adenocarcinoma; 50% of mutations were V600E, while G469A and D594G accounted for the remaining 39% and 11% of patients, respectively. All patients were either current or former smokers. The median OS of patients with BRAF mutations in this case series was NR, while it was 37 months for patients with EGFR mutations (P = 0.73) and NR for patients with ALK rearrangements (P = 0.64).9
For patients with BRAF V600E–mutant NSCLC who have progressed on platinum-based chemotherapy, the combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) may represent a new treatment paradigm. This was illustrated in a phase 2, nonrandomized, open-label study. A total of 57 patients were enrolled and 36 patients (63.2% [95% CI 49.3% to 75.6%]) achieved an overall response by investigator assessment, the trial’s primary end point. Disease control rate was 78.9% (95% CI 66.1% to 88.6%), with 4% complete response, 60% partial response, and 16% stable disease. PFS was 9.7 months (95% CI [6.9 to 19.6 months]). The safety profile was comparable to what had been observed in patients with melanoma treated with this regimen. More specifically, 56% of patients on this trial reported serious AEs, including pyrexia (16%), anemia (5%), confusional state (4%), decreased appetite (4%), hemoptysis (4%), hypercalcemia (4%), nausea (4%), and cutaneous squamous cell carcinoma (4%). In addition, neutropenia (9%) and hyponatremia (7%) were the most common grade 3-4 AEs.16
The case patient has experienced disease progression after 1 line of platinum-based chemotherapy, so the combination of dabrafenib and trametinib would be a robust systemic treatment option. dabrafenib as a single agent has also been studied in BRAF V600E–mutant NSCLC in a phase 2 trial. The overall response by investigator assessment among 84 patients was 33% (95% CI 23% to 45%).14 Vemurafenib, another oral BRAF TKI, has demonstrated efficacy for NSCLC patients harboring BRAF V600E mutation. In the cohort of 20 patients with NSCLC, the response rate was 42% (95% CI 20% to 67%) and median PFS was 7.3 months (95% CI 3.5 to 10.8 months).13 Patients with non-V600E mutations have shown variable responses to targeted therapies. MEK TKIs may be considered in this setting; however, the details of this discussion are beyond the scope of this review.
CONCLUSION
The management of advanced NSCLC with driver mutations has seen revolutionary changes over the past decade. Tremendous research has been done in order to first understand the molecular pathogenesis of NSCLC and then discover driver mutations that would lead to development of targeted therapies with clinically significant efficacy as well as tolerability. More recently, increasing efforts have focused on how to conquer acquired resistance in patients with disease progression after first-line TKIs. The field of EGFR-mutant NSCLC has set a successful example, but the work is nowhere near finished. The goals are to search for more driver mutations and to design agents that could potentially block cell survival signals once and for all.
INTRODUCTION
Lung cancer is the second most common type of cancer in the United States, with 222,500 estimated new cases in 2017, according to the American Cancer Society.1 However, it is by far the number one cause of death due to cancer, with an estimated 155,870 lung cancer–related deaths occurring in 2017, which is higher than the number of deaths due to breast cancer, prostate cancer, and colorectal cancer combined.1,2 Despite slightly decreasing incidence and mortality over the past decade, largely due to smoking cessation, the 5-year survival rate of lung cancer remains dismal at approximately 18%.2–4
Non-small cell lung cancer (NSCLC) accounts for 80% to 85% of all lung cancer cases.4 Traditionally, it is further divided based on histology: adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and not otherwise specified.5 Chemotherapy had been the cornerstone of treatment for stage IV NSCLC. It is not target-specific and is most effective against rapidly growing cells. Common adverse effects include alopecia, nausea/vomiting, myelosuppression, cardiotoxicity, neuropathy, and nephrotoxicity. However, this paradigm has shifted following the discovery of mutations of the epidermal growth factor receptor (EGFR) gene as an oncogenic driver that confers sensitivity to small molecule tyrosine kinase inhibitors (TKIs) targeting EGFR.6 The EGFR inhibitors are given orally and have a spectrum of toxicities (eg, such as rash, diarrhea, and elevated transaminases) different from that of systemic chemotherapy, which is often administered intravenously. Following the discovery of EGFR mutations, rearrangements of the anaplastic lymphoma kinase (ALK) gene7 and ROS1 gene8 were identified as targetable driver mutations in NSCLC. The frequency of both rearrangements is lower than that of EGFR mutations. Additionally, BRAF V600E mutation has been identified in NSCLC.9–12 This activation mutation is commonly seen in melanoma. Agents that have already been approved for the treatment of melanoma with the BRAF V600E mutation are being tested in NSCLC patients with this mutation.13–16
Given the effectiveness and tolerability of targeted therapy, identifying this distinct molecular subset of NSCLC patients is critical in treatment. Currently, molecular testing is mandatory in all stage IV patients with non-squamous cell carcinoma, as a preponderance of patients with driver mutations have this histology subtype.5,17–19 For patients with squamous cell carcinoma, molecular testing should be considered if the biopsy specimen is small, there is mixed histology, or the patient is a nonsmoker.5,20 Several techniques are commonly utilized in detecting these genetic alterations. EGFR mutation can be detected by polymerase chain reaction (PCR), ALK or ROS1 rearrangement can be detected by fluorescence in-situ hybridization (FISH), and immunohistochemistry (IHC) can also be used to detect ALK rearrangement. The current guideline is to use comprehensive genomic profiling to capture all the potential molecular targets simultaneously instead of running stepwise tests just for EGFR, ALK, and ROS1.5BRAF V600E mutation,13–16 MET exon 14 skipping mutation,21–24 RET rearrangements,25–27 and HER2 mutations28–30 are among the emergent genetic alterations with various responses to targeted therapy.31 Some of these targeted agents have been approved for other types of malignancy, and others are still in the development phase.
Several initiatives worldwide have reported better outcomes of patients with driver mutations treated with targeted therapy. For instance, the Lung Cancer Mutation Consortium in the United States demonstrated that the median survival of patients without driver mutations, with drivers mutations but not treated with targeted therapy, and with driver mutations and treated with targeted therapy was 2.08 years, 2.38 years, and 3.49 years, respectively.32 The French Cooperative Thoracic Intergroup-French National Cancer Institute demonstrated that the median survival for patients with driver mutations versus those without driver mutations was 16.5 months versus 11.8 months.33 The Spanish Lung Cancer Group demonstrated that the overall survival (OS) for patients with EGFR mutations treated with erlotinib was 27 months.34 The mutations in lung cancer, their frequencies, and the downstream signaling pathways are depicted in the Figure.35

In this article, we discuss targeted therapy for patients with EGFR mutations, ALK rearrangements, ROS1 rearrangements, and BRAF V600E mutation. We also discuss the management of patients with EGFR mutations who develop a secondary mutation after TKI therapy. Almost all of the targeted agents discussed herein have been approved by the US Food and Drug Administration (FDA), so they are considered standard of care. All available phase 3 trials pertinent to these targeted therapies are included in the discussion.
EGFR MUTATIONS
CASE PRESENTATION 1
A 54-year-old Caucasian man who is a former smoker with a 10 pack-year history and past medical history of hypertension and dyslipidemia presents with progressive dyspnea for several weeks. A chest x-ray shows moderate pleural effusion on the left side with possible mass-like opacity on the left upper lung field. An ultrasound-guided thoracentesis is performed and cytology is positive for adenocarcinoma of likely pulmonary origin. Staging workup including positron emission tomography (PET)/computed tomography (CT) and magnetic resonance imaging of the brain with and without contrast is done. PET/CT shows a 5.5-cm mass in the left upper lobe of the lung with high fluorodeoxyglucose (FDG) uptake, several 1- to 2-cm mediastinal lymph nodes with moderate FDG uptake, and small pleural effusion on both sides with moderate FDG uptake. MRI-brain is negative for malignancy. The patient subsequently undergoes a CT-guided biopsy of the lung mass, which shows moderately differentiated adenocarcinoma. Comprehensive molecular profiling reveals EGFR L858R mutation only. The patient now presents for the initial consultation. Of note, his Eastern Cooperative Oncology Group performance status is 1.
What is the next step in the management of this patient?
FIRST-LINE TKI FOR SENSITIZING EGFR MUTATIONS
The 2 most common EGFR mutations are deletions in exon 19 and substitution of arginine for leucine in exon 21 (L858R), found in approximately 45% and 40% of patients with EGFR mutations, respectively.36 Both mutations are sensitive to EGFR TKIs. The benefit may be greater in patients with exon 19 deletions as compared to exon 21 L858R substitution,37,38 but this has not been demonstrated consistently in clinical trials.39-43 In the United States, EGFR mutations are found in approximately 10% of patients with NSCLC, while the incidence can be as high as 50% in Asia.44 Even though the cobas EGFR mutation test is the companion diagnostic approved by the US FDA, a positive test result from any laboratory with the Clinical Laboratory Improvement Amendments (CLIA) certificate should prompt the use of an EGFR TKI as the initial treatment.
Three EGFR TKIs that have been approved as first-line therapy in the United States are available: erlotinib, afatinib, and gefitinib.5 Both erlotinib and gefitinib are considered first-generation TKIs. They have higher binding affinity for the 2 common EGFR mutations than wild-type EGFR. In addition, they reversibly bind to the intracellular tyrosine kinase domain, resulting in inhibition of autophosphorylation of the tyrosine residues. Afatinib, a second-generation and irreversible TKI, targets EGFR (HER1) as well as HER2 and HER4.45
The superior efficacy of the EGFR TKIs over platinum doublet chemotherapy in treatment-naïve patients with EGFR mutations has been demonstrated in 7 randomized trials to date (Table).46 Erlotinib was the TKI arm for the OPTIMAL,41 EURTAC,42 and ENSURE trials;38 afatinib was the TKI arm for LUX-LUNG 337 and 6;43 gefitinib was the TKI arm for NEJ00239,47 and WJTOG3405.40 A meta-analysis of these 7 trials by Lee et al showed that progression-free survival (PFS) was significantly prolonged by EGFR TKIs (hazard ratio [HR] 0.37 [95% confidence interval {CI} 0.32 to 0.42]).46 For instance, in the EURTAC trial, median PFS was 9.7 months for patients treated with erlotinib as compared to 5.2 months for patients treated with platinum/gemcitabine or platinum/docetaxel.42 In this meta-analysis, prespecified subgroups included age, sex, ethnicity, smoking status, performance status, tumor histology, and EGFR mutation subtype. The superior outcome with TKIs was observed in all subgroups. Furthermore, patients with exon 19 deletions, nonsmokers, and women had even better outcomes.46

Erlotinib is the most commonly used TKI in the United States largely because gefitinib was off the market for some time until it was re-approved by the FDA in 2015. Interestingly, this “re-approval” was not based on either 1 of the 2 prospective trials (NEJ00239,47 and WJTOG340540), but rather was based on an exploratory analysis of the IPASS trial48,49 as well as a prospective phase 4, single-arm trial in Europe (IFUM).50 The superior efficacy of gefitinib over carboplatin/paclitaxel among patients with EGFR mutations in the IPASS trial was confirmed by blind independent central review, with longer PFS (HR 0.54 [95% CI 0.38 to 0.79] P = 0.0012) and higher objective response rate (ORR; odds ratio 3 [95% CI 1.63 to 5.54], P = 0.0004).49
CASE 1 CONTINUED
Based on the EGFR L858R mutation status, the patient is started on erlotinib. He is quite happy that he does not need intravenous chemotherapy but wants to know what toxicities he might potentially have with erlotinib.
What are the common adverse effects (AEs) of EGFR TKIs? How are AEs of TKIs managed?
Safety Profile
The important toxicities associated with EGFR TKIs are rash, gastrointestinal toxicity, hepatic toxicity, and pulmonary toxicity. Rash is an AE specific to all agents blocking the EGFR pathway, including small molecules and monoclonal antibodies such as cetuximab. The epidermis has a high level of expression of EGFR, which contributes to this toxicity.51 Rash usually presents as dry skin or acneiform eruption. Prophylactic treatment with oral tetracyclines and topical corticosteroids is generally recommended upon initiation of TKI therapy. Diarrhea is the most prevalent gastrointestinal toxicity. All patients starting treatment should be given prescriptions to manage diarrhea such as loperamide and be advised to call when it occurs. Hepatic toxicity is often manifested as elevated transaminases or bilirubin. Interstitial lung disease (ILD) is a rare but potentially fatal pulmonary toxicity.
Rash of any grade was reported in 49.2% of patients treated with erlotinib in clinical trials, while grade 3 rash occurred in 6% of patients and no grade 4 was reported. Diarrhea of any grade was reported in 20.3% of patients, grade 3 diarrhea occurred in 1.8%, and no grade 4 was reported. Grade 2 and 3 alanine aminotransferase (ALT) elevations were seen in 2% and 1% of patients, respectively. Grade 2 and 3 bilirubin elevations were seen in 4% and less than 1% of patients, respectively. The incidence of serious ILD-like events was less than 1%.52
Afatinib is associated with higher incidences of rash and diarrhea. Specifically, diarrhea and rash of all grades were reported in 96% and 90% of patients treated with afatinib, respectively. Paronychia of all grades occurred in 58% of patients. Elevated ALT of all grades was seen in 11% of patients. Approximately 1.5% of patients treated with afatinib across clinical trials had ILD or ILD-like AEs.53
Gefitinib, the most commonly used TKI outside United States, has a toxicity profile similar to erlotinib, except for hepatic toxicity. For instance, rash of all grades occurred in 47% of patients, diarrhea of all grades occurred in 29% of patients, and ILD or ILD-like AEs occurred in 1.3% of patients across clinical trials. In comparison, elevated ALT and aspartate aminotransferase (AST) of all grades was seen in 38% and 40% of patients, respectively.54 Therefore, close monitoring of liver function is clinically warranted. In particular, patients need to be advised to avoid concomitant use of herbal supplements, a common practice in Asian countries.
CASE 1 CONTINUED
The patient does well while on erlotinib at 150 mg orally once daily for about 8 months, until he develops increasing abdominal pain. A CT scan of the abdomen and pelvis with contrast shows a new 8-cm right adrenal mass. Additionally, a repeat CT scan of the chest with contrast shows a stable lung mass but enlarging mediastinal lymphadenopathy.
How would you manage the patient at this point?
MANAGEMENT OF T790M MUTATION AFTER PROGRESSION ON FIRST-LINE EGFR TKIS
As mentioned above, the median PFS of patients with EGFR mutations treated with 1 of the 3 TKIs is around 9 to 13 months.46 Of the various resistance mechanisms that have been described, the T790M mutation is found in approximately 60% of patients who progress after treatment with first-line TKIs.55,56 Other mechanisms, such as HER2 amplification, MET amplification, or rarely small cell transformation, have been reported.56 The first- and second-generation EGFR TKIs function by binding to the ATP-binding domain of mutated EGFR, leading to inhibition of the downstream signaling pathways (Figure, part B) and ultimately cell death.35 The T790M mutation hinders the interaction between the ATP-binding domain of EGFR kinase and TKIs, resulting in treatment resistance and disease progression.57,58
Osimertinib is a third-generation irreversible EGFR TKI with activity against both sensitizing EGFR and resistant T790M mutations. It has low affinity for wide-type EGFR as well as insulin receptor and insulin-like growth factor receptor.59 Osimertinib has been fully approved for NSCLC patients with EGFR mutations who have progressed on first-line EGFR TKIs with the development of T790M mutation. An international phase 3 trial (AURA3) randomly assigned 419 patients in a 2:1 ratio to either osimertinib or platinum/pemetrexed. Eligible patients all had the documented EGFR mutations and disease progression after first-line EGFR TKIs. Central confirmation of the T790M mutation was required. Median PFS by investigator assessment, the trial’s primary end point, was 10.1 months for osimertinib versus 4.4 months for chemotherapy (HR 0.3 [95% CI 0.23 to 0.41]; P < 0.001). ORR was 71% for osimertinib versus 31% for chemotherapy (HR 5.39 [95% CI 3.47 to 8.48], P < 0.001). A total of 144 patients with stable and asymptomatic brain metastases were also eligible. Median PFS for this subset of patients treated with osimertinib and chemotherapy was 8.5 months and 4.2 months, respectively (HR 0.32 [95% CI 0.21 to 0.49]). In the AURA3 trial, osimertinib was better tolerated than chemotherapy, with 23% of patients treated with osimertinib experiencing grade 3 or 4 AEs as compared to 47% of chemotherapy-treated patients. The most common AEs of any grade were diarrhea (41%), rash (34%), dry skin (23%), and paronychia (22%).60
For the case patient, a reasonable approach would be to obtain a tissue biopsy of the adrenal mass and more importantly to check for the T790M mutation. Similar to the companion diagnostic for EGFR mutations, the cobas EGFR mutation test v2 is the FDA-approved test for T790M. However, if this resistance mutation is detected by any CLIA-certified laboratories, osimertinib should be the recommended treatment option. If tissue biopsy is not feasible, plasma-based testing should be considered. A blood-based companion diagnostic also is FDA approved.
ALK REARRANGEMENTS
CASE 2 PRESENTATION
A 42-year-old Korean woman who is a non-smoker with no significant past medical history presents with fatigue, unintentional weight loss of 20 lb in the past 4 months, and vague abdominal pain. A CT can of the abdomen and pelvis without contrast shows multiple foci in the liver and an indeterminate nodule in the right lung base. She subsequently undergoes PET/CT, which confirms multiple liver nodules/masses ranging from 1 to 3 cm with moderate FDG uptake. In addition, there is a 3.5-cm pleura-based lung mass on the right side with moderate FDG uptake. MRI-brain with and without contrast is negative for malignancy. A CT-guided biopsy of 1 of the liver masses is ordered and pathology returns positive for poorly differentiated adenocarcinoma consistent with lung primary. Molecular analysis reveals an echinoderm microtubule-associated protein-like 4 (EML4)-ALK rearrangement. She is placed on crizotinib by an outside oncologist and after about 3 weeks of therapy is doing well. She is now in your clinic for a second opinion. She says that some of her friends told her about another medication called ceritinib and was wondering if she would need to switch her cancer treatment.
How would you respond to this patient’s inquiry?
FIRST-LINE TKIS FOR ALK REARRANGEMENTS
ALK rearrangements are found in 2% to 7% of NSCLC, with EML4-ALK being the most prevalent fusion variant.61 The inversion of chromosome 2p leads to the fusion of the EML4 gene and the ALK gene, which causes the constitutive activation of the fusion protein and ultimately increased transformation and tumorigenicity.7,61 Patients harboring ALK rearrangements tend to be non-smokers. Adenocarcinoma, especially signet ring cell subtype, is the predominant histology. Compared to EGFR mutations, patients with ALK mutations are significantly younger and more likely to be men.62ALK rearrangements can be detected by either FISH or IHC, and most next-generation sequencing (NGS) panels have the ability to identify this driver mutation.
Crizotinib is the first approved ALK inhibitor for the treatment of NSCLC in this molecular subset of patients.63 PROFILE 1014 is a phase 3 randomized trial that compared crizotinib with chemotherapy containing platinum/pemetrexed for up to 6 cycles. Crossover to crizotinib was allowed for patients with disease progression on chemotherapy. The primary end point was PFS by independent radiologic review. The crizotinib arm demonstrated superior PFS (10.9 months versus 7 months; HR 0.45 [95% CI 0.35 to 0.6], P < 0.001) and ORR (74% versus 45%, P < 0.001). Median survival was not reached in either arm (HR 0.82 [95% CI 0.54 to 1.26], P = 0.36).64 Based on this international trial, crizotinib is considered standard of care in the United States for treatment-naïve patients with advanced NSCLC harboring ALK rearrangements. The current recommended dose is 250 mg orally twice daily. Common treatment-related AEs of all grades include vision disorder (62%), nausea (53%), diarrhea (43%), vomiting (40%), edema (28%), and constipation (27%).65 PROFILE 1007 compared crizotinib with pemetrexed or docetaxel in ALK-rearranged NSCLC patients with prior exposure to 1 platinum-based chemotherapy. The median PFS was 7.7 months for crizotinib as compared to 3 months for chemotherapy (HR 0.49 [95% CI 0.37 to 0.64], P < 0.001). The response rates were 65% and 20% for crizotinib and chemotherapy, respectively (P < 0.001).66 In other countries, crizotinib following 1 prior platinum-based regimen may be considered standard of care based on this trial.
Ceritinib is an oral second-generation ALK inhibitor that is 20 times more potent than crizotinib based on enzymatic assays.67 It also targets ROS1 and insulin-like growth factor 1 receptor but not c-MET. It was first approved by the FDA in April 2014 for metastatic ALK-rearranged NSCLC following crizotinib.68 In May 2017, the FDA granted approval of ceritinib for treatment-naïve patients. This decision was based on the results of the ASCEND-4 trial, a randomized phase 3 trial assessing the efficacy and safety of ceritinib over chemotherapy in the first-line setting. The trial assigned 376 patients to either ceritinib at 750 mg once daily or platinum/pemetrexed for 4 cycles followed by maintenance pemetrexed. Median PFS was 16.6 months for ceritinib versus 8.1 months for chemotherapy (HR 0.55 [95% CI 0.42 to 0.73]; P < 0.00001).69 Toxicities of ceritinib are not negligible, with gastrointestinal toxicity being the most prevalent. For instance, diarrhea, nausea, vomiting, abdominal pain, and constipation of all grades were seen in 86%, 80%, 60%, 54%, and 29% of patients, respectively. Furthermore, fatigue and decreased appetite occurred in 52% and 34% of patients, respectively. In terms of laboratory abnormalities, 84% of patients experienced decreased hemoglobin of all grades; 80% increased ALT; 75% increased AST; 58% increased creatinine; 49% increased glucose; 36% decreased phosphate; and 28% increased lipase. Due to these AEs, the incidence of dose reduction was about 58% and the median onset was around 7 weeks.70
Alectinib is another oral second-generation ALK inhibitor that was approved by the FDA in December 2015 for the treatment of NSCLC patients with ALK rearrangements who have progressed on or are intolerant to crizotinib.71 Its indication will soon be broadened to the first-line setting based on the ALEX trial.72 Alectinib is a potent and highly selective TKI of ALK73 with activity against known resistant mutations to crizotinib.74,75 It also inhibits RET but not ROS1 or c-MET.76 ALEX, a randomized phase 3 study, compared alectinib with crizotinib in treatment-naïve patients with NSCLC harboring ALK rearrangements. The trial enrolled 303 patients and the median follow-up was approximately 18 months. The alectinib arm (600 mg twice daily) demonstrated significantly higher PFS by investigator-assessment, the trial’s primary end point. The 12-month event-free survival was 68.4% (95% CI 61% to 75.9%) versus 48.7% (95% CI 40.4% to 56.9%) for alectinib and crizotinib, respectively (HR 0.47 [95% CI 0.34 to 0.65], P < 0.001). The median PFS was not reached in the alectinib arm (95% CI 17.7 months to not estimable) as compared to 11.1 months in the crizotinib arm (95% CI 9.1 to 13.1 months).72 Alectinib is generally well tolerated. Common AEs of all grades include fatigue (41%), constipation (34%), edema (30%), and myalgia (29%). As alectinib can cause anemia, lymphopenia, hepatic toxicity, increased creatine phosphokinase, hyperglycemia, electrolyte abnormalities, and increased creatinine, periodic monitoring of these laboratory values is important, although most of these abnormalities are grade 1 or 2.77
Brigatinib, another oral second-generation ALK inhibitor, was granted accelerated approval by the FDA in April 2017 for ALK-rearranged and crizotinib-resistant NSCLC based on the ALTA trial. This randomized phase 2 study of brigatinib showed an ORR by investigator assessment of 54% (97.5% CI 43% to 65%) in the 180 mg once daily arm with lead-in of 90 mg once daily for 7 days. Median PFS was 12.9 months (95% CI 11.1 months to not reached [NR]).78 Currently, a phase 3 study of brigatinib versus crizotinib in ALK inhibitor–naïve patients is recruiting participants (ALTA-1L). It will be interesting to see if brigatinib can achieve a front-line indication.
Starting the case patient on crizotinib is well within the treatment guidelines. One may consider ceritinib or alectinib in the first-line setting, but both TKIs can be reserved upon disease progression. We would recommend a repeat biopsy at that point to look for resistant mechanisms, as certain secondary ALK mutations may be rescued by certain next-generation ALK inhibitors. For instance, the F1174V mutation has been reported to confer resistance to ceritinib but sensitivity to alectinib, while the opposite is true for I1171T. The G1202R mutation is resistant to ceritinib, alectinib, and brigatinib, but lorlatinib, a third-generation ALK inhibitor, has shown activity against this mutation.79 Furthermore, brain metastasis represents a treatment challenge for patients with ALK rearrangements. It is also an efficacy measure of next-generation ALK inhibitors, all of which have demonstrated better central nervous system activity than crizotinib.69,78,80 If the case patient were found to have brain metastasis at the initial diagnosis, either ceritinib or alectinib would be a reasonable choice since crizotinib has limited penetration of blood-brain barrier.81
ROS1 REARRANGEMENTS
CASE PRESENTATION 3
A 66-year-old Chinese woman who is a non-smoker with a past medical history of hypertension and hypothyroidism presents to the emergency department for worsening lower back pain. Initial workup includes x-ray of the lumbar spine followed by MRI with contrast, which shows a soft tissue mass at L3-4 without cord compression. CT of the chest, abdomen, and pelvis with contrast shows a 7-cm right hilar mass, bilateral small lung nodules, mediastinal lymphadenopathy, and multiple lytic lesions in ribs, lumbar spine, and pelvis. MRI-brain with and without contrast is negative for malignancy. She undergoes endo-bronchial ultrasound and biopsy of the right hilar mass, which shows poorly differentiated adenocarcinoma. While waiting for the result of the molecular analysis, the patient undergoes palliative radiation therapy to L2-5 with good pain relief. She is discharged from the hospital and presents to your clinic for follow up. Molecular analysis now reveals ROS1 rearrangement with CD74-ROS1 fusion.
What treatment plan should be put in place for this patient?
FIRST-LINE THERAPY FOR ROS1 REARRANGEMENTS
Approximately 2.4% of lung adenocarcinomas harbor ROS1 rearrangements.82 This distinct genetic alteration occurs more frequently in NSCLC patients who are younger, female, and never-smokers, and who have adenocarcinomas.8 It has been shown that ROS1 rearrangements rarely overlap with other genetic alterations including KRAS mutations, EGFR mutations, and ALK rearrangements.83 As a receptor tyrosine kinase, ROS1 is similar to ALK and insulin receptor family members.84 Crizotinib, which targets ALK, ROS1, and c-MET, was approved by the FDA on March 11, 2016, for the treatment of metastatic ROS1-rearranged NSCLC.85 The approval was based on a phase 2 expansion cohort of the original phase 1 study. Among 50 US patients enrolled in this expansion cohort, 3 had complete responses and 33 had partial responses with ORR of 72% (95% CI 58% to 84%). Median PFS was 19.2 months (95% CI 14.4 months to NR) and median duration of response (DOR) was 17.6 months (95% CI 14.5 months to NR).86 During longer follow-up, independent radiology review confirmed high ORR of 66% and median DOR of 18.3 months.85
Interestingly, no companion diagnostic assay has been approved for the detection of ROS1 rearrangements with the approval of crizotinib. In the United States, break apart FISH is the most common detection method. In fact, in the above mentioned phase 2 study, ROS1 rearrangements were detected in 49 out of 50 patients by this method.86 FISH can be technically challenging when dealing with high volume and multiple targets. Reverse transcriptase-PCR is another detection method, but it requires knowledge of the fusion partners. To date, at least 14 ROS1 fusion partners have been reported, with CD74 being the most common.87 NGS with appropriate design and validation can also be used to detect ROS1 rearrangements.
For the case patient, the recommendation would be to start her on crizotinib at 250 mg twice daily. Monitoring for vision disturbance, gastrointestinal complaints, and edema is warranted. Because the estimated onset of response is around 7.9 weeks,86 plans should be made to repeat her scans in approximately 2 months.
BRAF V600E MUTATIONS
CASE PRESENTATION 4
A 71-year-old Caucasian man with a past medical history of hypertension, dyslipidemia, and ischemic cerebrovascular accident without residual deficits was diagnosed with stage IV adenocarcinoma of the lung about 8 months ago. He has a 40 pack-year smoking history and quit smoking when he was diagnosed with lung cancer. His disease burden involved a large mediastinal mass, scattered pleural nodules, multiple lymphadenopathy, and several soft tissue masses. His outside oncologist started him on chemotherapy containing carboplatin and pemetrexed for 6 cycles followed by maintenance pemetrexed. The most recent restaging scans show disease progression with enlarging soft tissue masses and several new lytic bone lesions. MRI-brain with and without contrast shows 2 subcentimeter enhancing lesions. He transferred care to you approximately 4 weeks ago. You ordered a repeat biopsy of 1 of the enlarging soft tissue masses. Molecular analysis revealed BRAF V600E mutation. In the interim, he underwent stereotactic radiosurgery for the 2 brain lesions without any complications. The patient is now in your clinic for follow up.
What would be your recommended systemic treatment?
TARGETED THERAPIES FOR BRAF V600E MUTATION
BRAF mutations were first recognized as activating mutations in advanced melanomas, with BRAF V600E, resulting from the substitution of glutamic acid for valine at amino acid 600, being the most common. BRAF plays an important role in the mitogen-activated protein kinase (MAPK) signaling pathway. Briefly, the activation of MAPK pathway occurs upon ligand binding of receptor tyrosine kinases, which then involves RAS/BRAF/MEK/ERK in a stepwise manner, ultimately leading to cell survival. BRAF mutations have been increasingly recognized also as driver mutations in NSCLC.9–12 They can be detected by PCR or NGS method. The characteristics of NSCLC patients harboring BRAF mutations have been described by various groups.9–12 For instance, 1 case series showed that the incidence was 2.2% among patients with advanced lung adenocarcinoma; 50% of mutations were V600E, while G469A and D594G accounted for the remaining 39% and 11% of patients, respectively. All patients were either current or former smokers. The median OS of patients with BRAF mutations in this case series was NR, while it was 37 months for patients with EGFR mutations (P = 0.73) and NR for patients with ALK rearrangements (P = 0.64).9
For patients with BRAF V600E–mutant NSCLC who have progressed on platinum-based chemotherapy, the combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) may represent a new treatment paradigm. This was illustrated in a phase 2, nonrandomized, open-label study. A total of 57 patients were enrolled and 36 patients (63.2% [95% CI 49.3% to 75.6%]) achieved an overall response by investigator assessment, the trial’s primary end point. Disease control rate was 78.9% (95% CI 66.1% to 88.6%), with 4% complete response, 60% partial response, and 16% stable disease. PFS was 9.7 months (95% CI [6.9 to 19.6 months]). The safety profile was comparable to what had been observed in patients with melanoma treated with this regimen. More specifically, 56% of patients on this trial reported serious AEs, including pyrexia (16%), anemia (5%), confusional state (4%), decreased appetite (4%), hemoptysis (4%), hypercalcemia (4%), nausea (4%), and cutaneous squamous cell carcinoma (4%). In addition, neutropenia (9%) and hyponatremia (7%) were the most common grade 3-4 AEs.16
The case patient has experienced disease progression after 1 line of platinum-based chemotherapy, so the combination of dabrafenib and trametinib would be a robust systemic treatment option. dabrafenib as a single agent has also been studied in BRAF V600E–mutant NSCLC in a phase 2 trial. The overall response by investigator assessment among 84 patients was 33% (95% CI 23% to 45%).14 Vemurafenib, another oral BRAF TKI, has demonstrated efficacy for NSCLC patients harboring BRAF V600E mutation. In the cohort of 20 patients with NSCLC, the response rate was 42% (95% CI 20% to 67%) and median PFS was 7.3 months (95% CI 3.5 to 10.8 months).13 Patients with non-V600E mutations have shown variable responses to targeted therapies. MEK TKIs may be considered in this setting; however, the details of this discussion are beyond the scope of this review.
CONCLUSION
The management of advanced NSCLC with driver mutations has seen revolutionary changes over the past decade. Tremendous research has been done in order to first understand the molecular pathogenesis of NSCLC and then discover driver mutations that would lead to development of targeted therapies with clinically significant efficacy as well as tolerability. More recently, increasing efforts have focused on how to conquer acquired resistance in patients with disease progression after first-line TKIs. The field of EGFR-mutant NSCLC has set a successful example, but the work is nowhere near finished. The goals are to search for more driver mutations and to design agents that could potentially block cell survival signals once and for all.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1–19.
- Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S–29S.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-3013, based on November 2015 SEER data submission, posted to the SEER website, April 2016. Bethesda (MD): National Cancer Institute; 2016.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer: 1–190.
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–39.
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–6.
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancer. J Clin Oncol 2012;30:863–70.
- Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011;29:2046–51.
- Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol 2014;25:138–42.
- Litvak AM, Paik PK, Woo KM, et al. Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. J Thorac Oncol 2014;9:1669–74.
- Villaruz LC, Socinski MA, Abberbock S, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer 2015;121:448–56.
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015;373:726–36.
- Planchard D, Kim TM, Mazieres J, et al. DaBRAFenib in patients with BRAF V600E-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:642–50.
- Gautschi O, Milia J, Cabarrou B, et al. Targeted therapy for patients with BRAF-mutant lung cancer: results from the European EURAF cohort. J Thorac Oncol 2015;10:1451–7.
- Planchard D, Besse B, Groen HJ, et al. DaBRAFenib plus trametinib in patients with previously treated BRAF V600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984–93.
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer and Association for Molecular Pathology. J Thorac Oncol 2013;8:823–59.
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer and Association for Molecular Pathology. Arch Pathol Lab Med 2013;137:828–60.
- Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol 2014;32:3673–9.
- Paik PK, Varghese AM, Sima CS, et al. Response to erlotinib in patients with EGFR mutant advanced non-small cell lung cancers with a squamous or squamous-like component. Mol Cancer Ther 2012;11:2535–40.
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842–9.
- Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 mutations in non-small-cell lung cancer are associated with advanced age, and stage-dependent MET genomic amplification, and c-MET overexpression. J Clin Oncol 2016;34:721–30.
- Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol 2016;11:1493–502.
- Reungwetwattana T, Liang Y, Zhu V, et al. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: The why, the how, the who, the unknown, and the inevitable. Lung Cancer 2017;103:27–37.
- Drilon A, Wang L, Hasanovic A, et al. Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013;3:630–5.
- Lin JJ, Kennedy E, Sequist LV, et al. Clinical activity of alectinib in advanced RET-rearranged non-small cell lung cancer. J Thorac Oncol 2016;11:2027–32.
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653–60.
- Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med 2006;354:2619–21.
- Mazieres J. Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targted drugs: results from the European EUHER2 cohort. Annal Oncol 2016;27:281–6.
- Ou SH, Schrock AB, Bocharov EV, et al. HER2 transmembrane (TMD) mutations (V659/G660) that stabilize homo- and heterodimerization are rare oncogenic drivers in lung adenocarcinoma that respond to afatinib. J Thorac Oncol 2017;12:446–57.
- Jordan EJ, Kim HR, Arcila ME, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emergent therapies. Cancer Discov 2017;7:596–609.
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006.
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415–26.
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958–67.
- Rosell R, Karachaliou N, et al. Large-scale screening for somatic mutations in lung cancer. Lancet 2016;387:1354–6.
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339–46.
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34.
- Wu YL, Chou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883–9.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Eng J Med 2010;362:2380–8.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46.
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22.
- Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009;10:432–3.
- Nelson V, Ziehr J, Aqulnik M, et al. Afatinib: emerging next-generation tyrosine kinase inhibitor for NSCLC. Onco Targets Ther 2013;5:135–43.
- Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol 2015;33:1958–65.
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54–9.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57.
- Wu YL, Saijo N, Thongprasert S, et al. Efficacy according to blind independent central review: post-hoc analyses from the phase III, randomized, multicenter, IPASS study of first-line gefitinib versus carboplatin/paclitaxel in Asian patients with EFGR mutation-positive advanced NSCLC. Lung Cancer 2017;104:119–25.
- Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR-mutation positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer 2014;110:55–62.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol 2007;56:317–26.
- Tarceva [package insert]. South San Francisco (CA): Genentech, Inc; 2010. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021743s14s16lbl.pdf. Accessed April 23, 2017.
- Gilotrif [package insert.] Ridgefield (CT): Boehringer Ingelheim, Inc; 2013. www.accessdata.fda.gov/drugsatfda_docs/label/2013/201292s000lbl.pdf. Accessed April 23, 2017.
- Iressa [package insert]. Wilmington (DE): AstraZeneca, Inc; 2015. Error! Hyperlink reference not valid. Accessed April 23, 2017.
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–22.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR TKI therapy in 155 patients with EGFR mutant lung cancers. Clin Cancer Res 2013;19:2240–7.
- Yun CH, Mengwasser KE, Tom AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070–5.
- Sos ML, Rode HB, Heynck S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer Res 2010;70:868–74.
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T190M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046–61.
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629–40.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small cell lung cancer. N Engl J Med 2010;363:1693–703.
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247–53.
- Kazandjian D, Blumenthal GM, Chen HY, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist 2014;19:e5–11.
- Solomon BJ, Mok T, Kim DW, et al. First-ling crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–77.
- Xalkori [package insert]. New York: Pfizer, Inc; 2011. www.accessdata.fda.gov/drugsatfda_docs/label/2012/202570s002lbl.pdf. Accessed April 23, 2017.
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94.
- Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem 2013;56:5675–90.
- Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res 2015;21:2436–9.
- Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917–29.
- Zykadia [package insert]. East Hanover (NJ): Novartis Pharmaceuticals Corporation, Inc; 2016. www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/zykadia.pdf. Accessed April 23, 2017.
- Larkins E, Blumenthal GM, Chen H, et al. FDA approval: alectinib for the treatment of metastatic, ALK-positive non-small cell lung cancer following crizotinib. Clin Cancer Res 2016;22:5171–6.
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. New Engl J Med 2017 June 6 [Epub ahead of print].
- Kinoshita K, Asoh K, Furuichi N, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem 2012;20:1271–80.
- Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011;19:679–90.
- Kodama T, Tsukaguchi T, Yoshida M, et al. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett 2014;351:215–21.
- Kodama T, Tsukaguchi T, Satoh T, et al. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther 2014;13:2910–8.
- Alecensa [package insert]. South San Francisco (CA): Genentech, Inc; 2015. www.accessdata.fda.gov/drugsatfda_docs/label/2015/208434s000lbl.pdf. Accessed April 23, 2017.
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol 2017 May 5 [Epub ahead of print].
- Zhu V, Ou SH. Safety of alectinib for the treatment of metastatic ALK-rearranged non-small cell lung cancer. Expert Opin Drug Saf 2017;16:509–14.
- Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small cell lung cancer. J Clin Oncol 2016;34:4079–85.
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443–5.
- Zhu Q, Zhan P, Zhang X, et al. Clinicopathologic characteristics of patients with ROS1 fusion gene in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2015;4:300–9.
- Lin JJ, Ritterhouse LL, Ali SM, et al. ROS1 fusions rarely overlap with other oncogenic drivers in non-small cell lung cancer. J Thorac Oncol 2017;12:872–7.
- Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta 2009;1795:37–52.
- Kazandjian D, Blumenthal G, Luo L, et al. Benefit-Risk summary of crizotinib for the treatment of patients with ROS1 alteration-positive metastatic NSCLC. Oncologist 2016;21:974–80.
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963–71.
- Zhu VW, Upadhyay D, Schrock AB, et al. TPD52L1-ROS1, a new ROS1 fusion variant in lung adenosquamous cell carcinoma identified by comprehensive genomic profiling. Lung Cancer 2016;97:48–50.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1–19.
- Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S–29S.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-3013, based on November 2015 SEER data submission, posted to the SEER website, April 2016. Bethesda (MD): National Cancer Institute; 2016.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer: 1–190.
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–39.
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–6.
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancer. J Clin Oncol 2012;30:863–70.
- Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011;29:2046–51.
- Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol 2014;25:138–42.
- Litvak AM, Paik PK, Woo KM, et al. Clinical characteristics and course of 63 patients with BRAF mutant lung cancers. J Thorac Oncol 2014;9:1669–74.
- Villaruz LC, Socinski MA, Abberbock S, et al. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer 2015;121:448–56.
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015;373:726–36.
- Planchard D, Kim TM, Mazieres J, et al. DaBRAFenib in patients with BRAF V600E-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:642–50.
- Gautschi O, Milia J, Cabarrou B, et al. Targeted therapy for patients with BRAF-mutant lung cancer: results from the European EURAF cohort. J Thorac Oncol 2015;10:1451–7.
- Planchard D, Besse B, Groen HJ, et al. DaBRAFenib plus trametinib in patients with previously treated BRAF V600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984–93.
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer and Association for Molecular Pathology. J Thorac Oncol 2013;8:823–59.
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer and Association for Molecular Pathology. Arch Pathol Lab Med 2013;137:828–60.
- Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guideline. J Clin Oncol 2014;32:3673–9.
- Paik PK, Varghese AM, Sima CS, et al. Response to erlotinib in patients with EGFR mutant advanced non-small cell lung cancers with a squamous or squamous-like component. Mol Cancer Ther 2012;11:2535–40.
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842–9.
- Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 mutations in non-small-cell lung cancer are associated with advanced age, and stage-dependent MET genomic amplification, and c-MET overexpression. J Clin Oncol 2016;34:721–30.
- Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol 2016;11:1493–502.
- Reungwetwattana T, Liang Y, Zhu V, et al. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: The why, the how, the who, the unknown, and the inevitable. Lung Cancer 2017;103:27–37.
- Drilon A, Wang L, Hasanovic A, et al. Response to cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 2013;3:630–5.
- Lin JJ, Kennedy E, Sequist LV, et al. Clinical activity of alectinib in advanced RET-rearranged non-small cell lung cancer. J Thorac Oncol 2016;11:2027–32.
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016;17:1653–60.
- Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med 2006;354:2619–21.
- Mazieres J. Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targted drugs: results from the European EUHER2 cohort. Annal Oncol 2016;27:281–6.
- Ou SH, Schrock AB, Bocharov EV, et al. HER2 transmembrane (TMD) mutations (V659/G660) that stabilize homo- and heterodimerization are rare oncogenic drivers in lung adenocarcinoma that respond to afatinib. J Thorac Oncol 2017;12:446–57.
- Jordan EJ, Kim HR, Arcila ME, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emergent therapies. Cancer Discov 2017;7:596–609.
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006.
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415–26.
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958–67.
- Rosell R, Karachaliou N, et al. Large-scale screening for somatic mutations in lung cancer. Lancet 2016;387:1354–6.
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339–46.
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34.
- Wu YL, Chou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883–9.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Eng J Med 2010;362:2380–8.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46.
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22.
- Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009;10:432–3.
- Nelson V, Ziehr J, Aqulnik M, et al. Afatinib: emerging next-generation tyrosine kinase inhibitor for NSCLC. Onco Targets Ther 2013;5:135–43.
- Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol 2015;33:1958–65.
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54–9.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57.
- Wu YL, Saijo N, Thongprasert S, et al. Efficacy according to blind independent central review: post-hoc analyses from the phase III, randomized, multicenter, IPASS study of first-line gefitinib versus carboplatin/paclitaxel in Asian patients with EFGR mutation-positive advanced NSCLC. Lung Cancer 2017;104:119–25.
- Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR-mutation positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer 2014;110:55–62.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol 2007;56:317–26.
- Tarceva [package insert]. South San Francisco (CA): Genentech, Inc; 2010. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021743s14s16lbl.pdf. Accessed April 23, 2017.
- Gilotrif [package insert.] Ridgefield (CT): Boehringer Ingelheim, Inc; 2013. www.accessdata.fda.gov/drugsatfda_docs/label/2013/201292s000lbl.pdf. Accessed April 23, 2017.
- Iressa [package insert]. Wilmington (DE): AstraZeneca, Inc; 2015. Error! Hyperlink reference not valid. Accessed April 23, 2017.
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–22.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR TKI therapy in 155 patients with EGFR mutant lung cancers. Clin Cancer Res 2013;19:2240–7.
- Yun CH, Mengwasser KE, Tom AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070–5.
- Sos ML, Rode HB, Heynck S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer Res 2010;70:868–74.
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T190M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046–61.
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629–40.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small cell lung cancer. N Engl J Med 2010;363:1693–703.
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247–53.
- Kazandjian D, Blumenthal GM, Chen HY, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist 2014;19:e5–11.
- Solomon BJ, Mok T, Kim DW, et al. First-ling crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–77.
- Xalkori [package insert]. New York: Pfizer, Inc; 2011. www.accessdata.fda.gov/drugsatfda_docs/label/2012/202570s002lbl.pdf. Accessed April 23, 2017.
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94.
- Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem 2013;56:5675–90.
- Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res 2015;21:2436–9.
- Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917–29.
- Zykadia [package insert]. East Hanover (NJ): Novartis Pharmaceuticals Corporation, Inc; 2016. www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/zykadia.pdf. Accessed April 23, 2017.
- Larkins E, Blumenthal GM, Chen H, et al. FDA approval: alectinib for the treatment of metastatic, ALK-positive non-small cell lung cancer following crizotinib. Clin Cancer Res 2016;22:5171–6.
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. New Engl J Med 2017 June 6 [Epub ahead of print].
- Kinoshita K, Asoh K, Furuichi N, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem 2012;20:1271–80.
- Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011;19:679–90.
- Kodama T, Tsukaguchi T, Yoshida M, et al. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett 2014;351:215–21.
- Kodama T, Tsukaguchi T, Satoh T, et al. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther 2014;13:2910–8.
- Alecensa [package insert]. South San Francisco (CA): Genentech, Inc; 2015. www.accessdata.fda.gov/drugsatfda_docs/label/2015/208434s000lbl.pdf. Accessed April 23, 2017.
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol 2017 May 5 [Epub ahead of print].
- Zhu V, Ou SH. Safety of alectinib for the treatment of metastatic ALK-rearranged non-small cell lung cancer. Expert Opin Drug Saf 2017;16:509–14.
- Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small cell lung cancer. J Clin Oncol 2016;34:4079–85.
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443–5.
- Zhu Q, Zhan P, Zhang X, et al. Clinicopathologic characteristics of patients with ROS1 fusion gene in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2015;4:300–9.
- Lin JJ, Ritterhouse LL, Ali SM, et al. ROS1 fusions rarely overlap with other oncogenic drivers in non-small cell lung cancer. J Thorac Oncol 2017;12:872–7.
- Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta 2009;1795:37–52.
- Kazandjian D, Blumenthal G, Luo L, et al. Benefit-Risk summary of crizotinib for the treatment of patients with ROS1 alteration-positive metastatic NSCLC. Oncologist 2016;21:974–80.
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963–71.
- Zhu VW, Upadhyay D, Schrock AB, et al. TPD52L1-ROS1, a new ROS1 fusion variant in lung adenosquamous cell carcinoma identified by comprehensive genomic profiling. Lung Cancer 2016;97:48–50.
Dacomitinib boosts PFS in advanced NSCLC
CHICAGO – The clear advantage goes to the second-generation tyrosine kinase inhibitor in a new trial comparing dacomitinib to gefitinib for advanced non–small cell lung cancer.
In a randomized, open-label phase III trial designed as a head-to-head comparison of the two drugs for the first-line treatment of advanced non–small cell lung cancer (NSCLC), “the blinded, independent review showed that we have a median progression-free survival (PFS) of 14.7 months versus 9.2 months,” said first author Tony Mok, MD, professor and chair of the department of clinical oncology at the Chinese University of Hong Kong. This PFS rate, he said, “is among the highest of randomized phase III trials in the first-line setting.”
Two years into the study, those taking dacomitinib had triple the PFS rate of those on gefitinib (30.6% versus 9.6%). The overall hazard ratio (HR) for PFS with dacomitinib compared to gefitinib was 0.59 (95% confidence interval [CI], 0.47-0.74, P less than .0001).
A previous single-arm phase II trial of the drug, ARCHER 2017, showed a response rate of 75.6% and a median PFS of 18.2 months for patients with NSCLC and an EGFR-activating mutation.
“Based on these data, we thought it was likely that we could have a hypothesis for dacomitinib to be superior to gefitinib, a first-generation TKI [tyrosine kinase inhibitor], in terms of progression-free survival,” Dr. Mok said in a press conference at the annual meeting of the American Society of Clinical Oncology. Dacomitinib is a second-generation TKI.
Patients in the new study, ARCHER 1050, had advanced NSCLC with EGFR-activating mutations and no prior systemic treatment for their advanced disease. In addition, patients had good performance status, could not have had prior TKI exposure, and could not have CNS metastases. This last exclusion, explained Dr. Mok, was because investigators were uncertain about dacomitinib’s CNS penetration at the time of study design, and because gefitinib may also not be the best therapeutic choice for CNS metastases.
Patients were randomized 1:1 to receive either dacomitinib 45 mg orally daily (n = 227), or gefitinib 250 mg orally daily (n = 225). Patients were stratified by whether or not they were ethnically Asian, and by whether they had EGFR mutation of exon 19 or exon 21. Patients were balanced in terms of age, gender, ethnicity, smoking, and performance status between arms. About 75% of the patients were Asian, and 65% were nonsmokers.
The international study enrolled patients from 71 centers in Asia and Europe. At the time of the data cutoff, investigators saw PFS events totaling 59.9% in the dacomitinib arm and 79.6% in the gefitinib arm. Patients were followed for PFS for a median of 22.1 months. “We have relatively mature data,” said Dr. Mok, except for overall survival, with only 36.9% of events occurring at the time of the data cutoff.
The primary endpoint in the open-label trial was PFS in the intention-to-treat population, as assessed by an independent, blinded reviewer. Dr. Mok said that the study was powered to see at least 256 PFS events, and to see an improvement in PFS for dacomitinib that equated to an HR of no more than 0.667. This would translate to median PFS for dacomitinib of 14.3 months versus 9.5 months for gefitinib, values Dr. Mok said were “reasonable.” And, he pointed out, the study results fell almost exactly in line with these predictions, though the actual HR was a bit lower than predicted.
An analysis of PFS by subgroup, also conducted by independent review, found that dacomitinib was favored for all subgroups except for non-Asian patients, for whom the HR was 0.89 but did not reach statistical significance. Since these patients made up about one-fourth of the study population, said Dr. Mok, small sample size was a potential issue. “But we have to ask ourselves the question, do they really perform worse than the Asians, if they have a response?”
To attempt to answer this question, the investigators performed an exploratory analysis of the 72 non-Asian patients who had responded to therapy. Among this group, they saw data similar to that of the overall group, with an HR of 0.547 (95% CI, 0.321-0.933, P less than .0123).
Secondary endpoints included investigator-assessed PFS, overall survival, objective response rate, duration of response, quality of life, and safety assessments.
Objective response rates were similar between arms, at 74.9% for dacomitinib and 71.6% for gefitinib (P = .3883). However, the median duration of response was significantly longer for those on dacomitinib (14.8 versus 8.3 months, P less than .0001).
“This may be best explained by looking into the depth of the response,” said Dr. Mok. Patient-level data showed that dacomitinib patients had a larger reduction in target lesion size; “this may reflect a more potent inhibition of EGFR,” he said.
With the more potent inhibition, however, came more frequent grade 3 adverse events involving diarrhea, dermatitis, stomatitis, and paronychia for those on dacomitinib; however, noted Dr. Mok, serious transaminase elevations were more common in the gefitinib group. “There is no new signal” for concerning toxicity, he said. Dose reductions were more common in dacomitinib than in gefitinib (66.1% versus 18%), but there are two tiers of dose reductions permissible with dacomitinib, giving some flexibility.
Dr. Mok reported financial relationships with multiple pharmaceutical companies, including Pfizer and SFJ Pharmaceuticals, which sponsored the study.
koakes@frontlinemedcom.com
On Twitter @karioakes
CHICAGO – The clear advantage goes to the second-generation tyrosine kinase inhibitor in a new trial comparing dacomitinib to gefitinib for advanced non–small cell lung cancer.
In a randomized, open-label phase III trial designed as a head-to-head comparison of the two drugs for the first-line treatment of advanced non–small cell lung cancer (NSCLC), “the blinded, independent review showed that we have a median progression-free survival (PFS) of 14.7 months versus 9.2 months,” said first author Tony Mok, MD, professor and chair of the department of clinical oncology at the Chinese University of Hong Kong. This PFS rate, he said, “is among the highest of randomized phase III trials in the first-line setting.”
Two years into the study, those taking dacomitinib had triple the PFS rate of those on gefitinib (30.6% versus 9.6%). The overall hazard ratio (HR) for PFS with dacomitinib compared to gefitinib was 0.59 (95% confidence interval [CI], 0.47-0.74, P less than .0001).
A previous single-arm phase II trial of the drug, ARCHER 2017, showed a response rate of 75.6% and a median PFS of 18.2 months for patients with NSCLC and an EGFR-activating mutation.
“Based on these data, we thought it was likely that we could have a hypothesis for dacomitinib to be superior to gefitinib, a first-generation TKI [tyrosine kinase inhibitor], in terms of progression-free survival,” Dr. Mok said in a press conference at the annual meeting of the American Society of Clinical Oncology. Dacomitinib is a second-generation TKI.
Patients in the new study, ARCHER 1050, had advanced NSCLC with EGFR-activating mutations and no prior systemic treatment for their advanced disease. In addition, patients had good performance status, could not have had prior TKI exposure, and could not have CNS metastases. This last exclusion, explained Dr. Mok, was because investigators were uncertain about dacomitinib’s CNS penetration at the time of study design, and because gefitinib may also not be the best therapeutic choice for CNS metastases.
Patients were randomized 1:1 to receive either dacomitinib 45 mg orally daily (n = 227), or gefitinib 250 mg orally daily (n = 225). Patients were stratified by whether or not they were ethnically Asian, and by whether they had EGFR mutation of exon 19 or exon 21. Patients were balanced in terms of age, gender, ethnicity, smoking, and performance status between arms. About 75% of the patients were Asian, and 65% were nonsmokers.
The international study enrolled patients from 71 centers in Asia and Europe. At the time of the data cutoff, investigators saw PFS events totaling 59.9% in the dacomitinib arm and 79.6% in the gefitinib arm. Patients were followed for PFS for a median of 22.1 months. “We have relatively mature data,” said Dr. Mok, except for overall survival, with only 36.9% of events occurring at the time of the data cutoff.
The primary endpoint in the open-label trial was PFS in the intention-to-treat population, as assessed by an independent, blinded reviewer. Dr. Mok said that the study was powered to see at least 256 PFS events, and to see an improvement in PFS for dacomitinib that equated to an HR of no more than 0.667. This would translate to median PFS for dacomitinib of 14.3 months versus 9.5 months for gefitinib, values Dr. Mok said were “reasonable.” And, he pointed out, the study results fell almost exactly in line with these predictions, though the actual HR was a bit lower than predicted.
An analysis of PFS by subgroup, also conducted by independent review, found that dacomitinib was favored for all subgroups except for non-Asian patients, for whom the HR was 0.89 but did not reach statistical significance. Since these patients made up about one-fourth of the study population, said Dr. Mok, small sample size was a potential issue. “But we have to ask ourselves the question, do they really perform worse than the Asians, if they have a response?”
To attempt to answer this question, the investigators performed an exploratory analysis of the 72 non-Asian patients who had responded to therapy. Among this group, they saw data similar to that of the overall group, with an HR of 0.547 (95% CI, 0.321-0.933, P less than .0123).
Secondary endpoints included investigator-assessed PFS, overall survival, objective response rate, duration of response, quality of life, and safety assessments.
Objective response rates were similar between arms, at 74.9% for dacomitinib and 71.6% for gefitinib (P = .3883). However, the median duration of response was significantly longer for those on dacomitinib (14.8 versus 8.3 months, P less than .0001).
“This may be best explained by looking into the depth of the response,” said Dr. Mok. Patient-level data showed that dacomitinib patients had a larger reduction in target lesion size; “this may reflect a more potent inhibition of EGFR,” he said.
With the more potent inhibition, however, came more frequent grade 3 adverse events involving diarrhea, dermatitis, stomatitis, and paronychia for those on dacomitinib; however, noted Dr. Mok, serious transaminase elevations were more common in the gefitinib group. “There is no new signal” for concerning toxicity, he said. Dose reductions were more common in dacomitinib than in gefitinib (66.1% versus 18%), but there are two tiers of dose reductions permissible with dacomitinib, giving some flexibility.
Dr. Mok reported financial relationships with multiple pharmaceutical companies, including Pfizer and SFJ Pharmaceuticals, which sponsored the study.
koakes@frontlinemedcom.com
On Twitter @karioakes
CHICAGO – The clear advantage goes to the second-generation tyrosine kinase inhibitor in a new trial comparing dacomitinib to gefitinib for advanced non–small cell lung cancer.
In a randomized, open-label phase III trial designed as a head-to-head comparison of the two drugs for the first-line treatment of advanced non–small cell lung cancer (NSCLC), “the blinded, independent review showed that we have a median progression-free survival (PFS) of 14.7 months versus 9.2 months,” said first author Tony Mok, MD, professor and chair of the department of clinical oncology at the Chinese University of Hong Kong. This PFS rate, he said, “is among the highest of randomized phase III trials in the first-line setting.”
Two years into the study, those taking dacomitinib had triple the PFS rate of those on gefitinib (30.6% versus 9.6%). The overall hazard ratio (HR) for PFS with dacomitinib compared to gefitinib was 0.59 (95% confidence interval [CI], 0.47-0.74, P less than .0001).
A previous single-arm phase II trial of the drug, ARCHER 2017, showed a response rate of 75.6% and a median PFS of 18.2 months for patients with NSCLC and an EGFR-activating mutation.
“Based on these data, we thought it was likely that we could have a hypothesis for dacomitinib to be superior to gefitinib, a first-generation TKI [tyrosine kinase inhibitor], in terms of progression-free survival,” Dr. Mok said in a press conference at the annual meeting of the American Society of Clinical Oncology. Dacomitinib is a second-generation TKI.
Patients in the new study, ARCHER 1050, had advanced NSCLC with EGFR-activating mutations and no prior systemic treatment for their advanced disease. In addition, patients had good performance status, could not have had prior TKI exposure, and could not have CNS metastases. This last exclusion, explained Dr. Mok, was because investigators were uncertain about dacomitinib’s CNS penetration at the time of study design, and because gefitinib may also not be the best therapeutic choice for CNS metastases.
Patients were randomized 1:1 to receive either dacomitinib 45 mg orally daily (n = 227), or gefitinib 250 mg orally daily (n = 225). Patients were stratified by whether or not they were ethnically Asian, and by whether they had EGFR mutation of exon 19 or exon 21. Patients were balanced in terms of age, gender, ethnicity, smoking, and performance status between arms. About 75% of the patients were Asian, and 65% were nonsmokers.
The international study enrolled patients from 71 centers in Asia and Europe. At the time of the data cutoff, investigators saw PFS events totaling 59.9% in the dacomitinib arm and 79.6% in the gefitinib arm. Patients were followed for PFS for a median of 22.1 months. “We have relatively mature data,” said Dr. Mok, except for overall survival, with only 36.9% of events occurring at the time of the data cutoff.
The primary endpoint in the open-label trial was PFS in the intention-to-treat population, as assessed by an independent, blinded reviewer. Dr. Mok said that the study was powered to see at least 256 PFS events, and to see an improvement in PFS for dacomitinib that equated to an HR of no more than 0.667. This would translate to median PFS for dacomitinib of 14.3 months versus 9.5 months for gefitinib, values Dr. Mok said were “reasonable.” And, he pointed out, the study results fell almost exactly in line with these predictions, though the actual HR was a bit lower than predicted.
An analysis of PFS by subgroup, also conducted by independent review, found that dacomitinib was favored for all subgroups except for non-Asian patients, for whom the HR was 0.89 but did not reach statistical significance. Since these patients made up about one-fourth of the study population, said Dr. Mok, small sample size was a potential issue. “But we have to ask ourselves the question, do they really perform worse than the Asians, if they have a response?”
To attempt to answer this question, the investigators performed an exploratory analysis of the 72 non-Asian patients who had responded to therapy. Among this group, they saw data similar to that of the overall group, with an HR of 0.547 (95% CI, 0.321-0.933, P less than .0123).
Secondary endpoints included investigator-assessed PFS, overall survival, objective response rate, duration of response, quality of life, and safety assessments.
Objective response rates were similar between arms, at 74.9% for dacomitinib and 71.6% for gefitinib (P = .3883). However, the median duration of response was significantly longer for those on dacomitinib (14.8 versus 8.3 months, P less than .0001).
“This may be best explained by looking into the depth of the response,” said Dr. Mok. Patient-level data showed that dacomitinib patients had a larger reduction in target lesion size; “this may reflect a more potent inhibition of EGFR,” he said.
With the more potent inhibition, however, came more frequent grade 3 adverse events involving diarrhea, dermatitis, stomatitis, and paronychia for those on dacomitinib; however, noted Dr. Mok, serious transaminase elevations were more common in the gefitinib group. “There is no new signal” for concerning toxicity, he said. Dose reductions were more common in dacomitinib than in gefitinib (66.1% versus 18%), but there are two tiers of dose reductions permissible with dacomitinib, giving some flexibility.
Dr. Mok reported financial relationships with multiple pharmaceutical companies, including Pfizer and SFJ Pharmaceuticals, which sponsored the study.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT ASCO 2017
Key clinical point:
Major finding: At 2 years, the dacomitinib arm had triple the PFS rate of the gefitinib arm (30.6% versus 9.6%).
Data source: Randomized, open-label phase III clinical trial of 452 patients who received dacomitinib or gefitinib for first-line therapy for advanced non–small cell lung cancer.
Disclosures: Dr. Mok reported financial relationships with multiple pharmaceutical companies, including Pfizer and SFJ Pharmaceuticals, which funded the study.
Physician attitudes and prevalence of molecular testing in lung cancer
Lung cancer is the leading cause of cancer death in the United States. It is estimated that there will be 222,500 new cases of lung cancer and 155,870 deaths from lung cancer in 2017. Non–small-cell lung carcinoma (NSCLC) accounts for 80%-85% of lung cancers, with adenocarcinoma being the most common histologic subtype. Other less common subtypes include squamous-cell carcinoma, large-cell carcinoma, and NSCLC that cannot be further classified.1 Nearly 70% of patients present with locally advanced or metastatic disease at the time of diagnosis and are not candidates for surgical resection.2 For that group of patients, the mainstay of treatment is platinum-based chemotherapy with or without radiation therapy. Patients who are chemotherapy naive often experience a modest response, however; durable remission is short lived, and the 5-year survival rate remains staggeringly low.3 Improved understanding of the molecular pathways that drive malignancy in NSCLC has led to the development of drugs that target specific molecular pathways.4 By definition, these driver mutations facilitate oncogenesis by conferring a selective advantage during clonal evolution.5 Moreover, agents targeting these pathways are extremely active and induce durable responses in many patients.6,7,8
Predictive biomarkers in NSCLC include anaplastic lymphoma kinase (ALK) fusion oncogene and sensitizing epidermal growth factor receptor (EGFR) mutations. Mutations in the EGFR tyrosine kinase are observed in about 15%-20% of NSCLC adenocarcinomas in the United States and upward of 60% in Asian populations. They are also found more frequently in nonsmokers and women.6 The two most prevalent mutations in the EGFR tyrosine kinase domain are in-frame deletions of exon 19 and L858R substitution in exon 21, representing about 45% and 40% of mutations, respectively.9 Both mutations result in activation of the tyrosine kinase domain, and both are associated with sensitivity to the small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib.10 Other drug-sensitive mutations include point mutations at exon 21 (L861Q) and exon 18 (G719X).11 Targeted therapy produces durable responses in the majority of patients.12,13,14 Unfortunately, most patients develop acquired resistance to these therapies, which leads to disease progression.4,15-17
ALK gene rearrangements, although less prevalent, are another important molecular target in NSCLC and are seen in 2%-7% of cases in the United States.7 As with EGFR mutations, these mutations are more prevalent in nonsmokers, and they are found more commonly in younger patients and in men.8
Identification of driver mutations early in the course of disease and acquired resistance mutations later are crucial for the optimal management of advanced NSCLC. DNA analysis using polymerase chain reaction (PCR) and next-generation sequencing is the preferred method for testing for EGFR mutations, and ALK rearrangements are generally tested either by flourescence in situ hybridization (FISH) or immunohistochemistry.18,19 Newer blood-based assays have shown great promise, and clinicians may soon have the ability to monitor subtle genetic changes, identify resistance patterns, and change therapy when acquired resistance occurs.20
The American College of Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology have proposed guidelines for molecular testing in lung cancer. It is recommended that all advanced squamous and nonsquamous cell lung cancers with an adenocarcinoma component should be tested for EGFR and ALK mutations independent of age, sex, ethnicity, or smoking history. In the setting of smaller lung cancer specimens (eg, from biopsies, cytology) where an adenocarcinoma component cannot be completely excluded, EGFR and ALK testing may be performed in cases showing squamous or small cell histology but clinical criteria (eg, young age, lack of smoking history) may be useful in selecting a subset of these samples for testing. Samples obtained through surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, fine-needle aspiration, and thoracentesis are all considered suitable for testing, but large biopsy samples are generally preferred over small biopsy samples, cell-blocks, and cytology samples.21 Despite this recommendation, not all patients who are eligible for mutation analysis are tested. At our institution, preliminary observations suggested that the percentage of patients being tested and the prevalence of driver mutations were significantly lower compared with published data. The purpose of this study was to evaluate physician attitudes about molecular testing, and to determine the rate of testing, the effect of biopsy sample size on rate of testing, and the prevalence of driver mutations at our institution.
Methods
In this retrospective clinical study, we identified 206 cases of advanced nsNSCLC from the tumor registry (February 2011-February 2013). Registry data was obtained from three hospitals within our health network – two academic tertiary care centers, and one community-based hospital. The other hospitals in the network were excluded because their EHR systems were not integrated with the rest of the hospitals and/or there was a lack of registry data. The testing rates for driver mutations, prevalence of driver mutations, and the tissue procurement techniques were obtained from individual chart review. Surgical specimens, core biopsy samples, and large volume thoracentesis specimens were categorized as large biopsy samples, and samples obtained by fine-needle aspiration, bronchial washing, and bronchial brushing were considered small biopsy samples. We used a chi-square analysis to compare mutation testing rates between the large and small biopsy sample groups. The prevalence of driver mutations was determined, excluding unknown or inadequate samples.
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.
In a similar study, the Association for Community Cancer Centers conducted a project aimed at understanding the landscape and current challenges for molecular profiling in NSCLC. Eight institutions participated in the study, and baseline testing rates were analyzed. The findings demonstrated that high-volume institutions (treating >100 lung cancer patients a year tested 62% and 60% of advanced lung cancer patients for EGFR and EML4-ALK, respectively, and low-volume institutions (treating <100 lung cancer patients a year tested 52% and 47% for EGFR and EML4-ALK, respectively.25,26 In a recent international physician self-reported survey, Spicer and colleagues found that EGFR testing was requested before first-line therapy in patients with stage IIIB or IV disease in 81% of cases, and mutation results were available before start of therapy in 77% of the cases.27 Those percentages are relatively low, given that current guidelines recommend that molecular testing should be done for all patients with stage IIIB or IV nsNSCLC. This highlights the need for objective performance feedback so oncologists can make the necessary practice changes so that molecular testing is done before the start of therapy to ensure high-quality cancer care that will translate into better, cost-effective outcomes and improved patient quality of life.
Our study findings showed that the prevalence of EGFR and ALK mutations is substantially lower among the patients we treat in our network compared with other published data on prevalence. The reason for those low rates is not clear, but it is likely multifactorial. First, Western Pennsylvania, the region our network serves, has a large proportion of older adults – 17.3% of the population is older than 65 years (national average, 14.5%) and advanced age might have contributed to the lower EGFR and ALK rates measured in our study.28 Second, the smoking rate in Pennsylvania is higher than the national average, 20%-24% compared with 18%, respectively.29 Third, the air quality in Western Pennsylvania has historically been very poor as a result of the large steel and coal mining industries. Even though the air quality has improved in recent decades, the American Lung Association’s 2017 State of the Air report ranked Pittsburgh and surrounding areas in Western Pennsylvania among the top 25 most air polluted areas in the United States.30 It is not certain whether air pollution and air quality have any impact on driver mutation rates, but the correlation with smoking, ethnicity, and geographic distribution highlight the need for further epidemiologic studies.
Biopsy sufficiency – getting an adequate amount of sample tissue during biopsy – is a known challenge to molecular profiling, and we found that biopsy sample size had an impact on the testing rates in a large percentage of our cases. To fully understand the impact of biopsy sufficiency, we conducted a subset analysis and compared the testing rates between our large and small biopsy samples. Our analysis showed that larger-sample biopsies were more likely to be tested for mutations than were smaller-sample biopsies (EGFR: P = .023; ALK: P = .007).
Those results suggest that larger-sample biopsies should be encouraged, but procedural risks, tumor location, and patient age and wishes need to be considered before tissue acquisition.21 Furthermore, clinicians who are responsible for tissue procurement need to be properly educated on the tissue sample requirements and the impact these results have on treatment decisions.31 Our institution, like many others, has adopted rapid onsite evaluation (ROSE) of biopsy samples, whereby a trained cytopathologist reviews sample adequacy at the time of tissue procurement. Although there is scant data directly comparing molecular testing success rates with and without the ROSE protocol, a meta-analysis conducted by Schmidt and colleagues concluded that ROSE improved the adequacy rate of fine-needle aspiration cytology by 12%.32,33 Given that molecular profiling depends on both the absolute and relative amount of tumor cells present in the sample, the ROSE protocol likely enhances the procedural success rate and reduces the need for repeat and subsequent biopsies.
It is interesting to note that our data also demonstrated that we are obtaining large-sample biopsies in most of our patients (about 70%). However, we are still failing to test more than half of our cases for driver mutations (Figure, A and C). This strongly suggests there are additional factors beyond tissue adequacy that are contributing to our high failure rate. It is essential to understand the dynamics and system practices that influence testing rates if we are to improve the care and outcomes of our cancer patients. To better understand those barriers, we surveyed 110 practitioners (including medical oncologists, pulmonologists, thoracic surgeons, and interventional radiologists) about the molecular profiling process and their responses highlighted several important areas that deserve special attention (Tables 1, 4, 5).
In our institution, testing initiation is primarily the responsibility of the treating medical oncologist. This presents a challenge because there is often a significant delay between tissue acquisition, histologic confirmation, and oncologic review. Many institutions have adopted pathology-driven reflex testing to help overcome such delays. Automatic testing after pathologic confirmation streamlines the process, increases testing rates, and eliminates unnecessary delay between the time of diagnosis and the time of test ordering.34 It also allows for the molecular and histologic diagnosis to be integrated into a single pathology report before therapy is initiated.
Another barrier to timely testing according to the respondents, was the CMS’s 14-day rule. The 14-day rule requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing and was frequently identified as a cause for significant delay in testing and having an impact on first-line treatment decisions.35,36
Often clinicians will choose to defer testing until this time has elapsed to reduce the financial burden placed on the hospital but by that time, they might well have initiated treatment without knowing if the patient has a mutation. This is a significant challenge identified by many of our oncologists, and is a limitation to our analysis above as it is unclear what percentage of patients received follow up testing once care was established at an outside facility and once the 14-day time period had elapsed.
The data from our institution suggests there is discordance between physician attitudes and molecular testing practices. However, there are several limitations in our study. First, most of the survey respondents agreed that molecular testing is an important aspect of treating advanced lung cancer patients, but the retrospective nature of the study made it difficult to identify why testing was deferred or never conducted. Second, the absence of a centralized reporting system for molecular testing results at our institution, may have resulted in an overestimation of our testing failure rate in cases where results were not integrated our electronic medical record.
Third, the low survey response rate only allowed us to make generalizations regarding the conclusions, although it does provide a framework for future process improvements.
We believe the poor testing rates observed in our study are not isolated to our institution and reflect a significant challenge within the broader oncology community.27 A system of best practices is essential for capturing this subset of patients who are never tested. There is agreement among oncologists that improving our current testing rates will require a multidisciplinary approach, a refined process for molecular evaluation, a push toward reflex testing, and standardization of biopsy techniques and tissue handling procedures. In our institution, we have initiated a Lean Six Sigma and PDSA (plan, do, study, act) initiative to improve our current molecular testing process. In addition, because obtaining larger-sample biopsies or additional biopsies is often not feasible for many of our advanced cancer patients, we have started using whole blood circulating tumor cells (CTC) and plasma ctDNA (cell-free circulating DNA) for molecular testing. Recent studies have shown high concordance (89%) between tissue biopsies and blood-based mutation testing, which will likely have a positive impact on the cancer care of our patients and help to capture a subset of patients who are not candidates for traditional biopsies.37
Conclusions
Despite current guidelines for testing driver mutations in advanced nsNSCLC, a large segment of our patients are not being tested for those genetic aberrations. There are several barriers that continue to thwart the recommendation, including failure to integrate driver mutation testing into routine pathology practice (ie, reflex testing), insufficient tissue obtained from biopsy, and difficulty in obtaining tissue because of tumor location or risk of complications from the biopsy procedure. More important, these trends are not isolated to our institution and reflect a significant challenge within the oncology community. Our data show that for the purpose of driver mutation testing, larger-sample biopsies, such as surgical/core biopsies, are better than small-sample biopsies, such as needle aspiration. We have also demonstrated that the prevalence of driver mutations is lower in Western Pennsylvania, which is served by our network, than elsewhere in the United States.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
3. Kim TE, Murren JR. Therapy for stage IIIB and stage IV non-small cell lung cancer. Clin Chest Med. 2002;23(1):209-224.
4. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013). 2015;98(10):25-28.
5. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874.
8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
9. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24-31.
10. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306.
11. Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232-7241.
12. Shi Y, Siu-Kie JA, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
14. Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779.
15. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229.
16. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73.
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247.
18. Ellison G, Zhu G, Moulis A, Dearden S, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79-89.
19. Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449-1158.
20. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472-484.
21. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
22. Kwak EL, Bany YJ, Cambridge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
23. Shaw A, Yeap BY, Kenudson MM, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
24. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
25. Association of Community Cancer Centers. Molecular Testing in the Community Setting. In: Molecular testing: resources and tools for the multidisciplinary team. http://accc-cancer.org/resources/molecularTesting-Overview.asp. Accessed November 15, 2015.
26. Association of Community Cancer Centers. Molecular testing: ACCC peer-to-peer webinars. The tissue issue: sampling and testing with Gail Probst, RN, MS, AOCN. https://www.youtube.com/watch?v=lapmni938Mc&feature=youtu.be. Published September 14, 2015. Accessed November 2015.
27. Spicer J S, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i60.
28. West L, Cole S, Goodkind D. US Census Bureau, 65+ in the United States: 2010, U.S. Government Printing Office, Washington, DC, 2014
29. Centers for Disease Control and Prevention. State tobacco activities tracking and evaluation system. Current cigarette use among adults (Behavior Risk Factor Surveillance System) 2015. https://www.cdc.gov/statesystem/cigaretteuseadult.html. Last updated September 16, 2016. Accessed May 26, 2017.
30. The American Lung Association. State of the Air 2017. http://www.lung.org/assets/documents/healthy-air/state-of-the-air/state-of-the-air-2017.pdf. Published 2017. Accessed May 26, 2017.
31. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188(4):503-507.
32. Ferguson PE, Sales CM, Hodges DC, et al. Effects of a multidisciplinary approach to improve volume of diagnostic material in CT-guided lung biopsies. PLoS One. 2015 Oct 19;10(10).
33. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300-309.
34. Cengiz Inal, Yilmaz E, Chenget H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J Clin Oncol. 2014;32(suppl):5s. [abstract 8098].
35. Grzegorz K, Leighl, M. Challenges in NSCLC molecular testing barriers to implementation. Oncology Exchange. 2012;11(4):8-10.
36. Lynch JA, Khoury MJ, Ann Borzecket A, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the United States: a case study of T3 translational research. Genet Med. 2013;15(8):630-638.
37. Reck M. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (NSCLC): ASSESS study. Presented at the European Lung Cancer Conference (ELCC) Annual Meeting, Geneva; 15-18 April 2015.
Lung cancer is the leading cause of cancer death in the United States. It is estimated that there will be 222,500 new cases of lung cancer and 155,870 deaths from lung cancer in 2017. Non–small-cell lung carcinoma (NSCLC) accounts for 80%-85% of lung cancers, with adenocarcinoma being the most common histologic subtype. Other less common subtypes include squamous-cell carcinoma, large-cell carcinoma, and NSCLC that cannot be further classified.1 Nearly 70% of patients present with locally advanced or metastatic disease at the time of diagnosis and are not candidates for surgical resection.2 For that group of patients, the mainstay of treatment is platinum-based chemotherapy with or without radiation therapy. Patients who are chemotherapy naive often experience a modest response, however; durable remission is short lived, and the 5-year survival rate remains staggeringly low.3 Improved understanding of the molecular pathways that drive malignancy in NSCLC has led to the development of drugs that target specific molecular pathways.4 By definition, these driver mutations facilitate oncogenesis by conferring a selective advantage during clonal evolution.5 Moreover, agents targeting these pathways are extremely active and induce durable responses in many patients.6,7,8
Predictive biomarkers in NSCLC include anaplastic lymphoma kinase (ALK) fusion oncogene and sensitizing epidermal growth factor receptor (EGFR) mutations. Mutations in the EGFR tyrosine kinase are observed in about 15%-20% of NSCLC adenocarcinomas in the United States and upward of 60% in Asian populations. They are also found more frequently in nonsmokers and women.6 The two most prevalent mutations in the EGFR tyrosine kinase domain are in-frame deletions of exon 19 and L858R substitution in exon 21, representing about 45% and 40% of mutations, respectively.9 Both mutations result in activation of the tyrosine kinase domain, and both are associated with sensitivity to the small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib.10 Other drug-sensitive mutations include point mutations at exon 21 (L861Q) and exon 18 (G719X).11 Targeted therapy produces durable responses in the majority of patients.12,13,14 Unfortunately, most patients develop acquired resistance to these therapies, which leads to disease progression.4,15-17
ALK gene rearrangements, although less prevalent, are another important molecular target in NSCLC and are seen in 2%-7% of cases in the United States.7 As with EGFR mutations, these mutations are more prevalent in nonsmokers, and they are found more commonly in younger patients and in men.8
Identification of driver mutations early in the course of disease and acquired resistance mutations later are crucial for the optimal management of advanced NSCLC. DNA analysis using polymerase chain reaction (PCR) and next-generation sequencing is the preferred method for testing for EGFR mutations, and ALK rearrangements are generally tested either by flourescence in situ hybridization (FISH) or immunohistochemistry.18,19 Newer blood-based assays have shown great promise, and clinicians may soon have the ability to monitor subtle genetic changes, identify resistance patterns, and change therapy when acquired resistance occurs.20
The American College of Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology have proposed guidelines for molecular testing in lung cancer. It is recommended that all advanced squamous and nonsquamous cell lung cancers with an adenocarcinoma component should be tested for EGFR and ALK mutations independent of age, sex, ethnicity, or smoking history. In the setting of smaller lung cancer specimens (eg, from biopsies, cytology) where an adenocarcinoma component cannot be completely excluded, EGFR and ALK testing may be performed in cases showing squamous or small cell histology but clinical criteria (eg, young age, lack of smoking history) may be useful in selecting a subset of these samples for testing. Samples obtained through surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, fine-needle aspiration, and thoracentesis are all considered suitable for testing, but large biopsy samples are generally preferred over small biopsy samples, cell-blocks, and cytology samples.21 Despite this recommendation, not all patients who are eligible for mutation analysis are tested. At our institution, preliminary observations suggested that the percentage of patients being tested and the prevalence of driver mutations were significantly lower compared with published data. The purpose of this study was to evaluate physician attitudes about molecular testing, and to determine the rate of testing, the effect of biopsy sample size on rate of testing, and the prevalence of driver mutations at our institution.
Methods
In this retrospective clinical study, we identified 206 cases of advanced nsNSCLC from the tumor registry (February 2011-February 2013). Registry data was obtained from three hospitals within our health network – two academic tertiary care centers, and one community-based hospital. The other hospitals in the network were excluded because their EHR systems were not integrated with the rest of the hospitals and/or there was a lack of registry data. The testing rates for driver mutations, prevalence of driver mutations, and the tissue procurement techniques were obtained from individual chart review. Surgical specimens, core biopsy samples, and large volume thoracentesis specimens were categorized as large biopsy samples, and samples obtained by fine-needle aspiration, bronchial washing, and bronchial brushing were considered small biopsy samples. We used a chi-square analysis to compare mutation testing rates between the large and small biopsy sample groups. The prevalence of driver mutations was determined, excluding unknown or inadequate samples.
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.
In a similar study, the Association for Community Cancer Centers conducted a project aimed at understanding the landscape and current challenges for molecular profiling in NSCLC. Eight institutions participated in the study, and baseline testing rates were analyzed. The findings demonstrated that high-volume institutions (treating >100 lung cancer patients a year tested 62% and 60% of advanced lung cancer patients for EGFR and EML4-ALK, respectively, and low-volume institutions (treating <100 lung cancer patients a year tested 52% and 47% for EGFR and EML4-ALK, respectively.25,26 In a recent international physician self-reported survey, Spicer and colleagues found that EGFR testing was requested before first-line therapy in patients with stage IIIB or IV disease in 81% of cases, and mutation results were available before start of therapy in 77% of the cases.27 Those percentages are relatively low, given that current guidelines recommend that molecular testing should be done for all patients with stage IIIB or IV nsNSCLC. This highlights the need for objective performance feedback so oncologists can make the necessary practice changes so that molecular testing is done before the start of therapy to ensure high-quality cancer care that will translate into better, cost-effective outcomes and improved patient quality of life.
Our study findings showed that the prevalence of EGFR and ALK mutations is substantially lower among the patients we treat in our network compared with other published data on prevalence. The reason for those low rates is not clear, but it is likely multifactorial. First, Western Pennsylvania, the region our network serves, has a large proportion of older adults – 17.3% of the population is older than 65 years (national average, 14.5%) and advanced age might have contributed to the lower EGFR and ALK rates measured in our study.28 Second, the smoking rate in Pennsylvania is higher than the national average, 20%-24% compared with 18%, respectively.29 Third, the air quality in Western Pennsylvania has historically been very poor as a result of the large steel and coal mining industries. Even though the air quality has improved in recent decades, the American Lung Association’s 2017 State of the Air report ranked Pittsburgh and surrounding areas in Western Pennsylvania among the top 25 most air polluted areas in the United States.30 It is not certain whether air pollution and air quality have any impact on driver mutation rates, but the correlation with smoking, ethnicity, and geographic distribution highlight the need for further epidemiologic studies.
Biopsy sufficiency – getting an adequate amount of sample tissue during biopsy – is a known challenge to molecular profiling, and we found that biopsy sample size had an impact on the testing rates in a large percentage of our cases. To fully understand the impact of biopsy sufficiency, we conducted a subset analysis and compared the testing rates between our large and small biopsy samples. Our analysis showed that larger-sample biopsies were more likely to be tested for mutations than were smaller-sample biopsies (EGFR: P = .023; ALK: P = .007).
Those results suggest that larger-sample biopsies should be encouraged, but procedural risks, tumor location, and patient age and wishes need to be considered before tissue acquisition.21 Furthermore, clinicians who are responsible for tissue procurement need to be properly educated on the tissue sample requirements and the impact these results have on treatment decisions.31 Our institution, like many others, has adopted rapid onsite evaluation (ROSE) of biopsy samples, whereby a trained cytopathologist reviews sample adequacy at the time of tissue procurement. Although there is scant data directly comparing molecular testing success rates with and without the ROSE protocol, a meta-analysis conducted by Schmidt and colleagues concluded that ROSE improved the adequacy rate of fine-needle aspiration cytology by 12%.32,33 Given that molecular profiling depends on both the absolute and relative amount of tumor cells present in the sample, the ROSE protocol likely enhances the procedural success rate and reduces the need for repeat and subsequent biopsies.
It is interesting to note that our data also demonstrated that we are obtaining large-sample biopsies in most of our patients (about 70%). However, we are still failing to test more than half of our cases for driver mutations (Figure, A and C). This strongly suggests there are additional factors beyond tissue adequacy that are contributing to our high failure rate. It is essential to understand the dynamics and system practices that influence testing rates if we are to improve the care and outcomes of our cancer patients. To better understand those barriers, we surveyed 110 practitioners (including medical oncologists, pulmonologists, thoracic surgeons, and interventional radiologists) about the molecular profiling process and their responses highlighted several important areas that deserve special attention (Tables 1, 4, 5).
In our institution, testing initiation is primarily the responsibility of the treating medical oncologist. This presents a challenge because there is often a significant delay between tissue acquisition, histologic confirmation, and oncologic review. Many institutions have adopted pathology-driven reflex testing to help overcome such delays. Automatic testing after pathologic confirmation streamlines the process, increases testing rates, and eliminates unnecessary delay between the time of diagnosis and the time of test ordering.34 It also allows for the molecular and histologic diagnosis to be integrated into a single pathology report before therapy is initiated.
Another barrier to timely testing according to the respondents, was the CMS’s 14-day rule. The 14-day rule requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing and was frequently identified as a cause for significant delay in testing and having an impact on first-line treatment decisions.35,36
Often clinicians will choose to defer testing until this time has elapsed to reduce the financial burden placed on the hospital but by that time, they might well have initiated treatment without knowing if the patient has a mutation. This is a significant challenge identified by many of our oncologists, and is a limitation to our analysis above as it is unclear what percentage of patients received follow up testing once care was established at an outside facility and once the 14-day time period had elapsed.
The data from our institution suggests there is discordance between physician attitudes and molecular testing practices. However, there are several limitations in our study. First, most of the survey respondents agreed that molecular testing is an important aspect of treating advanced lung cancer patients, but the retrospective nature of the study made it difficult to identify why testing was deferred or never conducted. Second, the absence of a centralized reporting system for molecular testing results at our institution, may have resulted in an overestimation of our testing failure rate in cases where results were not integrated our electronic medical record.
Third, the low survey response rate only allowed us to make generalizations regarding the conclusions, although it does provide a framework for future process improvements.
We believe the poor testing rates observed in our study are not isolated to our institution and reflect a significant challenge within the broader oncology community.27 A system of best practices is essential for capturing this subset of patients who are never tested. There is agreement among oncologists that improving our current testing rates will require a multidisciplinary approach, a refined process for molecular evaluation, a push toward reflex testing, and standardization of biopsy techniques and tissue handling procedures. In our institution, we have initiated a Lean Six Sigma and PDSA (plan, do, study, act) initiative to improve our current molecular testing process. In addition, because obtaining larger-sample biopsies or additional biopsies is often not feasible for many of our advanced cancer patients, we have started using whole blood circulating tumor cells (CTC) and plasma ctDNA (cell-free circulating DNA) for molecular testing. Recent studies have shown high concordance (89%) between tissue biopsies and blood-based mutation testing, which will likely have a positive impact on the cancer care of our patients and help to capture a subset of patients who are not candidates for traditional biopsies.37
Conclusions
Despite current guidelines for testing driver mutations in advanced nsNSCLC, a large segment of our patients are not being tested for those genetic aberrations. There are several barriers that continue to thwart the recommendation, including failure to integrate driver mutation testing into routine pathology practice (ie, reflex testing), insufficient tissue obtained from biopsy, and difficulty in obtaining tissue because of tumor location or risk of complications from the biopsy procedure. More important, these trends are not isolated to our institution and reflect a significant challenge within the oncology community. Our data show that for the purpose of driver mutation testing, larger-sample biopsies, such as surgical/core biopsies, are better than small-sample biopsies, such as needle aspiration. We have also demonstrated that the prevalence of driver mutations is lower in Western Pennsylvania, which is served by our network, than elsewhere in the United States.
Lung cancer is the leading cause of cancer death in the United States. It is estimated that there will be 222,500 new cases of lung cancer and 155,870 deaths from lung cancer in 2017. Non–small-cell lung carcinoma (NSCLC) accounts for 80%-85% of lung cancers, with adenocarcinoma being the most common histologic subtype. Other less common subtypes include squamous-cell carcinoma, large-cell carcinoma, and NSCLC that cannot be further classified.1 Nearly 70% of patients present with locally advanced or metastatic disease at the time of diagnosis and are not candidates for surgical resection.2 For that group of patients, the mainstay of treatment is platinum-based chemotherapy with or without radiation therapy. Patients who are chemotherapy naive often experience a modest response, however; durable remission is short lived, and the 5-year survival rate remains staggeringly low.3 Improved understanding of the molecular pathways that drive malignancy in NSCLC has led to the development of drugs that target specific molecular pathways.4 By definition, these driver mutations facilitate oncogenesis by conferring a selective advantage during clonal evolution.5 Moreover, agents targeting these pathways are extremely active and induce durable responses in many patients.6,7,8
Predictive biomarkers in NSCLC include anaplastic lymphoma kinase (ALK) fusion oncogene and sensitizing epidermal growth factor receptor (EGFR) mutations. Mutations in the EGFR tyrosine kinase are observed in about 15%-20% of NSCLC adenocarcinomas in the United States and upward of 60% in Asian populations. They are also found more frequently in nonsmokers and women.6 The two most prevalent mutations in the EGFR tyrosine kinase domain are in-frame deletions of exon 19 and L858R substitution in exon 21, representing about 45% and 40% of mutations, respectively.9 Both mutations result in activation of the tyrosine kinase domain, and both are associated with sensitivity to the small-molecule tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib.10 Other drug-sensitive mutations include point mutations at exon 21 (L861Q) and exon 18 (G719X).11 Targeted therapy produces durable responses in the majority of patients.12,13,14 Unfortunately, most patients develop acquired resistance to these therapies, which leads to disease progression.4,15-17
ALK gene rearrangements, although less prevalent, are another important molecular target in NSCLC and are seen in 2%-7% of cases in the United States.7 As with EGFR mutations, these mutations are more prevalent in nonsmokers, and they are found more commonly in younger patients and in men.8
Identification of driver mutations early in the course of disease and acquired resistance mutations later are crucial for the optimal management of advanced NSCLC. DNA analysis using polymerase chain reaction (PCR) and next-generation sequencing is the preferred method for testing for EGFR mutations, and ALK rearrangements are generally tested either by flourescence in situ hybridization (FISH) or immunohistochemistry.18,19 Newer blood-based assays have shown great promise, and clinicians may soon have the ability to monitor subtle genetic changes, identify resistance patterns, and change therapy when acquired resistance occurs.20
The American College of Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology have proposed guidelines for molecular testing in lung cancer. It is recommended that all advanced squamous and nonsquamous cell lung cancers with an adenocarcinoma component should be tested for EGFR and ALK mutations independent of age, sex, ethnicity, or smoking history. In the setting of smaller lung cancer specimens (eg, from biopsies, cytology) where an adenocarcinoma component cannot be completely excluded, EGFR and ALK testing may be performed in cases showing squamous or small cell histology but clinical criteria (eg, young age, lack of smoking history) may be useful in selecting a subset of these samples for testing. Samples obtained through surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, fine-needle aspiration, and thoracentesis are all considered suitable for testing, but large biopsy samples are generally preferred over small biopsy samples, cell-blocks, and cytology samples.21 Despite this recommendation, not all patients who are eligible for mutation analysis are tested. At our institution, preliminary observations suggested that the percentage of patients being tested and the prevalence of driver mutations were significantly lower compared with published data. The purpose of this study was to evaluate physician attitudes about molecular testing, and to determine the rate of testing, the effect of biopsy sample size on rate of testing, and the prevalence of driver mutations at our institution.
Methods
In this retrospective clinical study, we identified 206 cases of advanced nsNSCLC from the tumor registry (February 2011-February 2013). Registry data was obtained from three hospitals within our health network – two academic tertiary care centers, and one community-based hospital. The other hospitals in the network were excluded because their EHR systems were not integrated with the rest of the hospitals and/or there was a lack of registry data. The testing rates for driver mutations, prevalence of driver mutations, and the tissue procurement techniques were obtained from individual chart review. Surgical specimens, core biopsy samples, and large volume thoracentesis specimens were categorized as large biopsy samples, and samples obtained by fine-needle aspiration, bronchial washing, and bronchial brushing were considered small biopsy samples. We used a chi-square analysis to compare mutation testing rates between the large and small biopsy sample groups. The prevalence of driver mutations was determined, excluding unknown or inadequate samples.
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.
In a similar study, the Association for Community Cancer Centers conducted a project aimed at understanding the landscape and current challenges for molecular profiling in NSCLC. Eight institutions participated in the study, and baseline testing rates were analyzed. The findings demonstrated that high-volume institutions (treating >100 lung cancer patients a year tested 62% and 60% of advanced lung cancer patients for EGFR and EML4-ALK, respectively, and low-volume institutions (treating <100 lung cancer patients a year tested 52% and 47% for EGFR and EML4-ALK, respectively.25,26 In a recent international physician self-reported survey, Spicer and colleagues found that EGFR testing was requested before first-line therapy in patients with stage IIIB or IV disease in 81% of cases, and mutation results were available before start of therapy in 77% of the cases.27 Those percentages are relatively low, given that current guidelines recommend that molecular testing should be done for all patients with stage IIIB or IV nsNSCLC. This highlights the need for objective performance feedback so oncologists can make the necessary practice changes so that molecular testing is done before the start of therapy to ensure high-quality cancer care that will translate into better, cost-effective outcomes and improved patient quality of life.
Our study findings showed that the prevalence of EGFR and ALK mutations is substantially lower among the patients we treat in our network compared with other published data on prevalence. The reason for those low rates is not clear, but it is likely multifactorial. First, Western Pennsylvania, the region our network serves, has a large proportion of older adults – 17.3% of the population is older than 65 years (national average, 14.5%) and advanced age might have contributed to the lower EGFR and ALK rates measured in our study.28 Second, the smoking rate in Pennsylvania is higher than the national average, 20%-24% compared with 18%, respectively.29 Third, the air quality in Western Pennsylvania has historically been very poor as a result of the large steel and coal mining industries. Even though the air quality has improved in recent decades, the American Lung Association’s 2017 State of the Air report ranked Pittsburgh and surrounding areas in Western Pennsylvania among the top 25 most air polluted areas in the United States.30 It is not certain whether air pollution and air quality have any impact on driver mutation rates, but the correlation with smoking, ethnicity, and geographic distribution highlight the need for further epidemiologic studies.
Biopsy sufficiency – getting an adequate amount of sample tissue during biopsy – is a known challenge to molecular profiling, and we found that biopsy sample size had an impact on the testing rates in a large percentage of our cases. To fully understand the impact of biopsy sufficiency, we conducted a subset analysis and compared the testing rates between our large and small biopsy samples. Our analysis showed that larger-sample biopsies were more likely to be tested for mutations than were smaller-sample biopsies (EGFR: P = .023; ALK: P = .007).
Those results suggest that larger-sample biopsies should be encouraged, but procedural risks, tumor location, and patient age and wishes need to be considered before tissue acquisition.21 Furthermore, clinicians who are responsible for tissue procurement need to be properly educated on the tissue sample requirements and the impact these results have on treatment decisions.31 Our institution, like many others, has adopted rapid onsite evaluation (ROSE) of biopsy samples, whereby a trained cytopathologist reviews sample adequacy at the time of tissue procurement. Although there is scant data directly comparing molecular testing success rates with and without the ROSE protocol, a meta-analysis conducted by Schmidt and colleagues concluded that ROSE improved the adequacy rate of fine-needle aspiration cytology by 12%.32,33 Given that molecular profiling depends on both the absolute and relative amount of tumor cells present in the sample, the ROSE protocol likely enhances the procedural success rate and reduces the need for repeat and subsequent biopsies.
It is interesting to note that our data also demonstrated that we are obtaining large-sample biopsies in most of our patients (about 70%). However, we are still failing to test more than half of our cases for driver mutations (Figure, A and C). This strongly suggests there are additional factors beyond tissue adequacy that are contributing to our high failure rate. It is essential to understand the dynamics and system practices that influence testing rates if we are to improve the care and outcomes of our cancer patients. To better understand those barriers, we surveyed 110 practitioners (including medical oncologists, pulmonologists, thoracic surgeons, and interventional radiologists) about the molecular profiling process and their responses highlighted several important areas that deserve special attention (Tables 1, 4, 5).
In our institution, testing initiation is primarily the responsibility of the treating medical oncologist. This presents a challenge because there is often a significant delay between tissue acquisition, histologic confirmation, and oncologic review. Many institutions have adopted pathology-driven reflex testing to help overcome such delays. Automatic testing after pathologic confirmation streamlines the process, increases testing rates, and eliminates unnecessary delay between the time of diagnosis and the time of test ordering.34 It also allows for the molecular and histologic diagnosis to be integrated into a single pathology report before therapy is initiated.
Another barrier to timely testing according to the respondents, was the CMS’s 14-day rule. The 14-day rule requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing and was frequently identified as a cause for significant delay in testing and having an impact on first-line treatment decisions.35,36
Often clinicians will choose to defer testing until this time has elapsed to reduce the financial burden placed on the hospital but by that time, they might well have initiated treatment without knowing if the patient has a mutation. This is a significant challenge identified by many of our oncologists, and is a limitation to our analysis above as it is unclear what percentage of patients received follow up testing once care was established at an outside facility and once the 14-day time period had elapsed.
The data from our institution suggests there is discordance between physician attitudes and molecular testing practices. However, there are several limitations in our study. First, most of the survey respondents agreed that molecular testing is an important aspect of treating advanced lung cancer patients, but the retrospective nature of the study made it difficult to identify why testing was deferred or never conducted. Second, the absence of a centralized reporting system for molecular testing results at our institution, may have resulted in an overestimation of our testing failure rate in cases where results were not integrated our electronic medical record.
Third, the low survey response rate only allowed us to make generalizations regarding the conclusions, although it does provide a framework for future process improvements.
We believe the poor testing rates observed in our study are not isolated to our institution and reflect a significant challenge within the broader oncology community.27 A system of best practices is essential for capturing this subset of patients who are never tested. There is agreement among oncologists that improving our current testing rates will require a multidisciplinary approach, a refined process for molecular evaluation, a push toward reflex testing, and standardization of biopsy techniques and tissue handling procedures. In our institution, we have initiated a Lean Six Sigma and PDSA (plan, do, study, act) initiative to improve our current molecular testing process. In addition, because obtaining larger-sample biopsies or additional biopsies is often not feasible for many of our advanced cancer patients, we have started using whole blood circulating tumor cells (CTC) and plasma ctDNA (cell-free circulating DNA) for molecular testing. Recent studies have shown high concordance (89%) between tissue biopsies and blood-based mutation testing, which will likely have a positive impact on the cancer care of our patients and help to capture a subset of patients who are not candidates for traditional biopsies.37
Conclusions
Despite current guidelines for testing driver mutations in advanced nsNSCLC, a large segment of our patients are not being tested for those genetic aberrations. There are several barriers that continue to thwart the recommendation, including failure to integrate driver mutation testing into routine pathology practice (ie, reflex testing), insufficient tissue obtained from biopsy, and difficulty in obtaining tissue because of tumor location or risk of complications from the biopsy procedure. More important, these trends are not isolated to our institution and reflect a significant challenge within the oncology community. Our data show that for the purpose of driver mutation testing, larger-sample biopsies, such as surgical/core biopsies, are better than small-sample biopsies, such as needle aspiration. We have also demonstrated that the prevalence of driver mutations is lower in Western Pennsylvania, which is served by our network, than elsewhere in the United States.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
3. Kim TE, Murren JR. Therapy for stage IIIB and stage IV non-small cell lung cancer. Clin Chest Med. 2002;23(1):209-224.
4. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013). 2015;98(10):25-28.
5. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874.
8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
9. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24-31.
10. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306.
11. Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232-7241.
12. Shi Y, Siu-Kie JA, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
14. Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779.
15. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229.
16. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73.
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247.
18. Ellison G, Zhu G, Moulis A, Dearden S, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79-89.
19. Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449-1158.
20. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472-484.
21. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
22. Kwak EL, Bany YJ, Cambridge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
23. Shaw A, Yeap BY, Kenudson MM, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
24. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
25. Association of Community Cancer Centers. Molecular Testing in the Community Setting. In: Molecular testing: resources and tools for the multidisciplinary team. http://accc-cancer.org/resources/molecularTesting-Overview.asp. Accessed November 15, 2015.
26. Association of Community Cancer Centers. Molecular testing: ACCC peer-to-peer webinars. The tissue issue: sampling and testing with Gail Probst, RN, MS, AOCN. https://www.youtube.com/watch?v=lapmni938Mc&feature=youtu.be. Published September 14, 2015. Accessed November 2015.
27. Spicer J S, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i60.
28. West L, Cole S, Goodkind D. US Census Bureau, 65+ in the United States: 2010, U.S. Government Printing Office, Washington, DC, 2014
29. Centers for Disease Control and Prevention. State tobacco activities tracking and evaluation system. Current cigarette use among adults (Behavior Risk Factor Surveillance System) 2015. https://www.cdc.gov/statesystem/cigaretteuseadult.html. Last updated September 16, 2016. Accessed May 26, 2017.
30. The American Lung Association. State of the Air 2017. http://www.lung.org/assets/documents/healthy-air/state-of-the-air/state-of-the-air-2017.pdf. Published 2017. Accessed May 26, 2017.
31. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188(4):503-507.
32. Ferguson PE, Sales CM, Hodges DC, et al. Effects of a multidisciplinary approach to improve volume of diagnostic material in CT-guided lung biopsies. PLoS One. 2015 Oct 19;10(10).
33. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300-309.
34. Cengiz Inal, Yilmaz E, Chenget H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J Clin Oncol. 2014;32(suppl):5s. [abstract 8098].
35. Grzegorz K, Leighl, M. Challenges in NSCLC molecular testing barriers to implementation. Oncology Exchange. 2012;11(4):8-10.
36. Lynch JA, Khoury MJ, Ann Borzecket A, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the United States: a case study of T3 translational research. Genet Med. 2013;15(8):630-638.
37. Reck M. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (NSCLC): ASSESS study. Presented at the European Lung Cancer Conference (ELCC) Annual Meeting, Geneva; 15-18 April 2015.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
3. Kim TE, Murren JR. Therapy for stage IIIB and stage IV non-small cell lung cancer. Clin Chest Med. 2002;23(1):209-224.
4. Black RC, Khurshid H. NSCLC: An update of driver mutations, their role in pathogenesis and clinical significance. R I Med J (2013). 2015;98(10):25-28.
5. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306-313.
6. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334.
7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874.
8. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
9. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24-31.
10. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306.
11. Riely GJ, Politi KA, Miller VA, et al. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12(24):7232-7241.
12. Shi Y, Siu-Kie JA, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154-162.
13. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
14. Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19(7):774-779.
15. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220-229.
16. Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73.
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247.
18. Ellison G, Zhu G, Moulis A, Dearden S, et al. EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol. 2013;66(2):79-89.
19. Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med. 2014;138(11):1449-1158.
20. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472-484.
21. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
22. Kwak EL, Bany YJ, Cambridge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
23. Shaw A, Yeap BY, Kenudson MM, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247-4253.
24. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
25. Association of Community Cancer Centers. Molecular Testing in the Community Setting. In: Molecular testing: resources and tools for the multidisciplinary team. http://accc-cancer.org/resources/molecularTesting-Overview.asp. Accessed November 15, 2015.
26. Association of Community Cancer Centers. Molecular testing: ACCC peer-to-peer webinars. The tissue issue: sampling and testing with Gail Probst, RN, MS, AOCN. https://www.youtube.com/watch?v=lapmni938Mc&feature=youtu.be. Published September 14, 2015. Accessed November 2015.
27. Spicer J S, Tischer B, Peters M. EGFR mutation testing and oncologist treatment choice in advanced NSCLC: global trends and differences. Ann Oncol. 2015;26(suppl 1):i60.
28. West L, Cole S, Goodkind D. US Census Bureau, 65+ in the United States: 2010, U.S. Government Printing Office, Washington, DC, 2014
29. Centers for Disease Control and Prevention. State tobacco activities tracking and evaluation system. Current cigarette use among adults (Behavior Risk Factor Surveillance System) 2015. https://www.cdc.gov/statesystem/cigaretteuseadult.html. Last updated September 16, 2016. Accessed May 26, 2017.
30. The American Lung Association. State of the Air 2017. http://www.lung.org/assets/documents/healthy-air/state-of-the-air/state-of-the-air-2017.pdf. Published 2017. Accessed May 26, 2017.
31. Gaga M, Powell CA, Schraufnagel DE, Schönfeld N, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013;188(4):503-507.
32. Ferguson PE, Sales CM, Hodges DC, et al. Effects of a multidisciplinary approach to improve volume of diagnostic material in CT-guided lung biopsies. PLoS One. 2015 Oct 19;10(10).
33. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300-309.
34. Cengiz Inal, Yilmaz E, Chenget H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: Experience from a community-based academic center. J Clin Oncol. 2014;32(suppl):5s. [abstract 8098].
35. Grzegorz K, Leighl, M. Challenges in NSCLC molecular testing barriers to implementation. Oncology Exchange. 2012;11(4):8-10.
36. Lynch JA, Khoury MJ, Ann Borzecket A, et al. Utilization of epidermal growth factor receptor (EGFR) testing in the United States: a case study of T3 translational research. Genet Med. 2013;15(8):630-638.
37. Reck M. Investigating the utility of circulating-free tumour-derived DNA (ctDNA) in plasma for the detection of epidermal growth factor receptor (EGFR) mutation status in European and Japanese patients (pts) with advanced non-small-cell lung cancer (NSCLC): ASSESS study. Presented at the European Lung Cancer Conference (ELCC) Annual Meeting, Geneva; 15-18 April 2015.
FDA approves dabrafenib and trametinib for BRAF V600E+ metastatic NSCLC
The FDA also approved a diagnostic, the Oncomine Dx Target Test, a next-generation sequencing test to detect gene mutations or alterations, including BRAF, from a single tissue specimen, the FDA reported in a statement.
The approvals are based on overall response rate (ORR) for the combination in a phase II, nonrandomized, noncomparative, open-label trial of patients with locally confirmed BRAF V600E mutation-positive metastatic NSCLC. The ORR for the combination treatment was 61% (95% confidence interval, 44%-77%) among 36 patients who had received no prior systemic therapy for metastatic NSCLC, and 63% (95% CI, 49%-76%) among 57 patients who had received at least one platinum-based chemotherapy regimen with demonstrated disease progression before enrollment. Those 93 patients were all treated with the combination of dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily).
The ORR was 27% (95% CI, 18%-38%) among a third cohort of 78 patients with previously treated BRAF V600E mutation-positive NSCLC who received single-agent dabrafenib.
The most common adverse reactions were similar to those reported in prior approvals for patients with melanoma, including pyrexia, fatigue, nausea, vomiting, diarrhea, dry skin, decreased appetite, edema, rash, chills, hemorrhage, cough, and dyspnea. The most common grade 3-4 adverse reactions were pyrexia, fatigue, dyspnea, vomiting, rash, hemorrhage, and diarrhea. The most common grade 3-4 laboratory abnormalities were hyponatremia, lymphopenia, anemia, hyperglycemia, neutropenia, leukopenia, hypophosphatemia, and increased alanine aminotransferase. Dabrafenib and trametinib were discontinued for adverse reactions in 18% and 19% of patients, respectively, the FDA said.
Novartis is marketing Dabrafenib as Tafinlar and trametinib as Mekinist.
The recommended doses are dabrafenib 150 mg orally twice daily, approximately 12 hours apart, with trametinib 2 mg orally once daily, following confirmation of BRAF V600E mutation in a tumor specimen by an FDA-approved test.
The FDA also approved a diagnostic, the Oncomine Dx Target Test, a next-generation sequencing test to detect gene mutations or alterations, including BRAF, from a single tissue specimen, the FDA reported in a statement.
The approvals are based on overall response rate (ORR) for the combination in a phase II, nonrandomized, noncomparative, open-label trial of patients with locally confirmed BRAF V600E mutation-positive metastatic NSCLC. The ORR for the combination treatment was 61% (95% confidence interval, 44%-77%) among 36 patients who had received no prior systemic therapy for metastatic NSCLC, and 63% (95% CI, 49%-76%) among 57 patients who had received at least one platinum-based chemotherapy regimen with demonstrated disease progression before enrollment. Those 93 patients were all treated with the combination of dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily).
The ORR was 27% (95% CI, 18%-38%) among a third cohort of 78 patients with previously treated BRAF V600E mutation-positive NSCLC who received single-agent dabrafenib.
The most common adverse reactions were similar to those reported in prior approvals for patients with melanoma, including pyrexia, fatigue, nausea, vomiting, diarrhea, dry skin, decreased appetite, edema, rash, chills, hemorrhage, cough, and dyspnea. The most common grade 3-4 adverse reactions were pyrexia, fatigue, dyspnea, vomiting, rash, hemorrhage, and diarrhea. The most common grade 3-4 laboratory abnormalities were hyponatremia, lymphopenia, anemia, hyperglycemia, neutropenia, leukopenia, hypophosphatemia, and increased alanine aminotransferase. Dabrafenib and trametinib were discontinued for adverse reactions in 18% and 19% of patients, respectively, the FDA said.
Novartis is marketing Dabrafenib as Tafinlar and trametinib as Mekinist.
The recommended doses are dabrafenib 150 mg orally twice daily, approximately 12 hours apart, with trametinib 2 mg orally once daily, following confirmation of BRAF V600E mutation in a tumor specimen by an FDA-approved test.
The FDA also approved a diagnostic, the Oncomine Dx Target Test, a next-generation sequencing test to detect gene mutations or alterations, including BRAF, from a single tissue specimen, the FDA reported in a statement.
The approvals are based on overall response rate (ORR) for the combination in a phase II, nonrandomized, noncomparative, open-label trial of patients with locally confirmed BRAF V600E mutation-positive metastatic NSCLC. The ORR for the combination treatment was 61% (95% confidence interval, 44%-77%) among 36 patients who had received no prior systemic therapy for metastatic NSCLC, and 63% (95% CI, 49%-76%) among 57 patients who had received at least one platinum-based chemotherapy regimen with demonstrated disease progression before enrollment. Those 93 patients were all treated with the combination of dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily).
The ORR was 27% (95% CI, 18%-38%) among a third cohort of 78 patients with previously treated BRAF V600E mutation-positive NSCLC who received single-agent dabrafenib.
The most common adverse reactions were similar to those reported in prior approvals for patients with melanoma, including pyrexia, fatigue, nausea, vomiting, diarrhea, dry skin, decreased appetite, edema, rash, chills, hemorrhage, cough, and dyspnea. The most common grade 3-4 adverse reactions were pyrexia, fatigue, dyspnea, vomiting, rash, hemorrhage, and diarrhea. The most common grade 3-4 laboratory abnormalities were hyponatremia, lymphopenia, anemia, hyperglycemia, neutropenia, leukopenia, hypophosphatemia, and increased alanine aminotransferase. Dabrafenib and trametinib were discontinued for adverse reactions in 18% and 19% of patients, respectively, the FDA said.
Novartis is marketing Dabrafenib as Tafinlar and trametinib as Mekinist.
The recommended doses are dabrafenib 150 mg orally twice daily, approximately 12 hours apart, with trametinib 2 mg orally once daily, following confirmation of BRAF V600E mutation in a tumor specimen by an FDA-approved test.
T-DMI doesn’t wow in HER2-overexpressing NSCLC
CHICAGO –Beyond its role in advanced breast cancer, the antibody-drug conjugate ado-trastuzumab-emtansine (T-DM1; Kadcyla) showed modest activity in some patients with metastatic non–small cell lung cancer (mNSCLC) overexpressing the human epidermal growth factor-2 (HER2) receptor, investigators reported.
In an ongoing phase II study, 4 of 20 patients with mNSCLC whose tumors expressed the highest levels of HER2 had partial responses. In contrast, none of the 20 patients with tumors overexpressing HER2 at lower levels had responses, reported Thomas E. Stinchcombe, MD, of Duke University, Durham, N.C.
Previous studies have shown that HER2 overexpression by immunohistochemistry (IHC) is associated with poor prognosis in patients with NSCLC, but in contrast to breast and gastric cancers, HER2 overexpression in NSCLC is not always accompanied by HER2 amplifications.
“HER2 amplifications and HER2 mutations are generally mutually exclusive in NSCLC. Given the known mechanism of action of T-DM1, HER2 overexpression was chosen as an inclusion criterion for this study,” Dr. Stinchcombe said.
They enrolled patients with HER2-overexpressing mNSCLC who had disease progression following platinum-based chemotherapy. The patients were assigned to one of two 20-patient cohorts based on IHC2+ (10% or more of cells stained with 2+ intensity), or IHC3+ (10% or more of cells stained with 3+intensity).
For the primary endpoint of treatment response none of the patients in the IHC2+ cohort had objective responses by Response Evaluation Criteria in Solid Tumors (RECIST), although eight patients in this cohort had stable disease, including one patient who remained in stable disease status on treatment out to 21 months at last follow-up.
In the IHC3+ cohort, four patients had partial responses, for an objective response rate of 20%. The median duration of response was 7.3 months. Two patients in this cohort had stable disease.
Median PFS was similar between the cohorts, at 2.6 months for IHC2+ and 2.7 months for IHC3+. The respective median OS durations were 12.2 and 12.1 months.
The safety profile of T-DM1 in this population was similar to that seen in breast cancer. There were two grade 3 serious adverse events: one infusion-related hypersensitivity reaction, and one case of thrombocytopenia. There were 10 grade 3 events of any kind, 1 grade 4 event, and 1 treatment withdrawal due to a grade 2 infusion reaction.
“I think it’s honestly a little hard to tell from this study, which is very small, whether there is really a role of [HER2] overexpression in actually driving oncogenesis and being a target for T-DM1,” she said.
Dr. Stinchcombe reported institutional research funding from Hoffmann-LaRoche, which sponsored the trial, and several coauthors are employees of the company.
CHICAGO –Beyond its role in advanced breast cancer, the antibody-drug conjugate ado-trastuzumab-emtansine (T-DM1; Kadcyla) showed modest activity in some patients with metastatic non–small cell lung cancer (mNSCLC) overexpressing the human epidermal growth factor-2 (HER2) receptor, investigators reported.
In an ongoing phase II study, 4 of 20 patients with mNSCLC whose tumors expressed the highest levels of HER2 had partial responses. In contrast, none of the 20 patients with tumors overexpressing HER2 at lower levels had responses, reported Thomas E. Stinchcombe, MD, of Duke University, Durham, N.C.
Previous studies have shown that HER2 overexpression by immunohistochemistry (IHC) is associated with poor prognosis in patients with NSCLC, but in contrast to breast and gastric cancers, HER2 overexpression in NSCLC is not always accompanied by HER2 amplifications.
“HER2 amplifications and HER2 mutations are generally mutually exclusive in NSCLC. Given the known mechanism of action of T-DM1, HER2 overexpression was chosen as an inclusion criterion for this study,” Dr. Stinchcombe said.
They enrolled patients with HER2-overexpressing mNSCLC who had disease progression following platinum-based chemotherapy. The patients were assigned to one of two 20-patient cohorts based on IHC2+ (10% or more of cells stained with 2+ intensity), or IHC3+ (10% or more of cells stained with 3+intensity).
For the primary endpoint of treatment response none of the patients in the IHC2+ cohort had objective responses by Response Evaluation Criteria in Solid Tumors (RECIST), although eight patients in this cohort had stable disease, including one patient who remained in stable disease status on treatment out to 21 months at last follow-up.
In the IHC3+ cohort, four patients had partial responses, for an objective response rate of 20%. The median duration of response was 7.3 months. Two patients in this cohort had stable disease.
Median PFS was similar between the cohorts, at 2.6 months for IHC2+ and 2.7 months for IHC3+. The respective median OS durations were 12.2 and 12.1 months.
The safety profile of T-DM1 in this population was similar to that seen in breast cancer. There were two grade 3 serious adverse events: one infusion-related hypersensitivity reaction, and one case of thrombocytopenia. There were 10 grade 3 events of any kind, 1 grade 4 event, and 1 treatment withdrawal due to a grade 2 infusion reaction.
“I think it’s honestly a little hard to tell from this study, which is very small, whether there is really a role of [HER2] overexpression in actually driving oncogenesis and being a target for T-DM1,” she said.
Dr. Stinchcombe reported institutional research funding from Hoffmann-LaRoche, which sponsored the trial, and several coauthors are employees of the company.
CHICAGO –Beyond its role in advanced breast cancer, the antibody-drug conjugate ado-trastuzumab-emtansine (T-DM1; Kadcyla) showed modest activity in some patients with metastatic non–small cell lung cancer (mNSCLC) overexpressing the human epidermal growth factor-2 (HER2) receptor, investigators reported.
In an ongoing phase II study, 4 of 20 patients with mNSCLC whose tumors expressed the highest levels of HER2 had partial responses. In contrast, none of the 20 patients with tumors overexpressing HER2 at lower levels had responses, reported Thomas E. Stinchcombe, MD, of Duke University, Durham, N.C.
Previous studies have shown that HER2 overexpression by immunohistochemistry (IHC) is associated with poor prognosis in patients with NSCLC, but in contrast to breast and gastric cancers, HER2 overexpression in NSCLC is not always accompanied by HER2 amplifications.
“HER2 amplifications and HER2 mutations are generally mutually exclusive in NSCLC. Given the known mechanism of action of T-DM1, HER2 overexpression was chosen as an inclusion criterion for this study,” Dr. Stinchcombe said.
They enrolled patients with HER2-overexpressing mNSCLC who had disease progression following platinum-based chemotherapy. The patients were assigned to one of two 20-patient cohorts based on IHC2+ (10% or more of cells stained with 2+ intensity), or IHC3+ (10% or more of cells stained with 3+intensity).
For the primary endpoint of treatment response none of the patients in the IHC2+ cohort had objective responses by Response Evaluation Criteria in Solid Tumors (RECIST), although eight patients in this cohort had stable disease, including one patient who remained in stable disease status on treatment out to 21 months at last follow-up.
In the IHC3+ cohort, four patients had partial responses, for an objective response rate of 20%. The median duration of response was 7.3 months. Two patients in this cohort had stable disease.
Median PFS was similar between the cohorts, at 2.6 months for IHC2+ and 2.7 months for IHC3+. The respective median OS durations were 12.2 and 12.1 months.
The safety profile of T-DM1 in this population was similar to that seen in breast cancer. There were two grade 3 serious adverse events: one infusion-related hypersensitivity reaction, and one case of thrombocytopenia. There were 10 grade 3 events of any kind, 1 grade 4 event, and 1 treatment withdrawal due to a grade 2 infusion reaction.
“I think it’s honestly a little hard to tell from this study, which is very small, whether there is really a role of [HER2] overexpression in actually driving oncogenesis and being a target for T-DM1,” she said.
Dr. Stinchcombe reported institutional research funding from Hoffmann-LaRoche, which sponsored the trial, and several coauthors are employees of the company.
AT ASCO 2017
Key clinical point: Some non–small cell lung cancer (NSCLC) tumors overexpress HER2, suggesting the use of T-DM1, an anti-HER2 antibody drug conjugate.
Major finding: Four of 20 patients with HER2-overexpressing NSCLC has partial responses to T-DM1 therapy.
Data source: Open-label prospective trial of 40 patients with NSCLC positive for HER2.
Disclosures: Dr. Stinchcombe reported institutional research funding from Hoffmann-LaRoche, which sponsored the trial, and several coauthors are employees of the company.
Lung cancer linked to suicide
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: During 1973-2013, suicide among U.S. lung cancer patients was four times higher than the general adult U.S. population.
Data source: Statistics on more than 3.6 million U.S. cancer patients in the SEER Program.
Disclosures: Dr. Rahouma had no disclosures.
Alectinib in ALK+ NSCLC is a watershed moment
CHICAGO – In what’s being hailed as practice-changing findings, the anaplastic lymphoma kinase inhibitor alectinib (Alecensa) was associated with more than doubled progression-free survival (PFS), compared with crizotinib (Xalkori), the current standard of care, in patients with treatment-naive non–small cell lung cancer (NSCLC) positive for ALK.
Additionally, in the global, phase III trial, alectinib was associated with a significantly lower risk of progression to CNS metastases, a common complication of advanced ALK+ NSCLC, reported Alice T. Shaw, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, on behalf of investigators in the ALEX trial.
“I view this as a watershed moment for the treatment of ALK mutant–positive lung cancer,” commented ASCO expert John Heymach, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
Unlike other head-to-head studies of similar drugs that frequently show only incremental benefit, the ALEX results showed a dramatic difference in outcomes for patients treated with alectinib, he said.
By comparison, the median PFS difference between chemotherapy and crizotinib in the PROFILE 1014 in patients with ALK-positive NSCLC trial was 10.9 vs. 7.0 months, Dr. Heymach pointed out.
The ALEX investigators enrolled 303 patients with untreated ALK-positive NSCLC confirmed by a central immunohistochemistry lab and randomly assigned them to treatment with either oral alectinib 600 mg twice daily or crizotinib 250 mg b.i.d.
At the primary data cutoff in February 2017, median PFS, the primary endpoint, was 11.1 months for patients treated with crizotinib, versus not reached for those treated with alectinib, translating into a hazard ratio for alectinib of 0.47 (P less than .0001).
Based on an independent review, the median PFS was determined to be 10.4 months for crizotinib, vs. 25.7 months with alectinib (HR, 0.50; P not shown).
The cumulative incidence of CNS progression, a secondary endpoint, was 41.4% in the crizotinib arm, vs. 9.41% in the alectinib arm (cause-specific HR, 0.16; P not shown).
In each arm, 97% of patients had any adverse event, and the incidence of serious adverse events was similar between the arms, at 29% for crizotinib and 28% for alectinib.
Adverse events leading to treatment discontinuation, dose reduction, or dose interruption were more frequent with crizotinib.
In the question and answer portion of the briefing, Dr. Shaw was asked whether crizotinib still had a role in this population.
“Going forward, I think that it’s pretty clear, if you have a newly diagnosed patient with metastatic ALK-positive lung cancer, that likely alectinib would be the preferred first choice,” she said.
The ALEX trial is supported by Roche. Dr. Shaw disclosed consulting or an advisory role with the company, and multiple coauthors disclosed similar relationships.
CHICAGO – In what’s being hailed as practice-changing findings, the anaplastic lymphoma kinase inhibitor alectinib (Alecensa) was associated with more than doubled progression-free survival (PFS), compared with crizotinib (Xalkori), the current standard of care, in patients with treatment-naive non–small cell lung cancer (NSCLC) positive for ALK.
Additionally, in the global, phase III trial, alectinib was associated with a significantly lower risk of progression to CNS metastases, a common complication of advanced ALK+ NSCLC, reported Alice T. Shaw, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, on behalf of investigators in the ALEX trial.
“I view this as a watershed moment for the treatment of ALK mutant–positive lung cancer,” commented ASCO expert John Heymach, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
Unlike other head-to-head studies of similar drugs that frequently show only incremental benefit, the ALEX results showed a dramatic difference in outcomes for patients treated with alectinib, he said.
By comparison, the median PFS difference between chemotherapy and crizotinib in the PROFILE 1014 in patients with ALK-positive NSCLC trial was 10.9 vs. 7.0 months, Dr. Heymach pointed out.
The ALEX investigators enrolled 303 patients with untreated ALK-positive NSCLC confirmed by a central immunohistochemistry lab and randomly assigned them to treatment with either oral alectinib 600 mg twice daily or crizotinib 250 mg b.i.d.
At the primary data cutoff in February 2017, median PFS, the primary endpoint, was 11.1 months for patients treated with crizotinib, versus not reached for those treated with alectinib, translating into a hazard ratio for alectinib of 0.47 (P less than .0001).
Based on an independent review, the median PFS was determined to be 10.4 months for crizotinib, vs. 25.7 months with alectinib (HR, 0.50; P not shown).
The cumulative incidence of CNS progression, a secondary endpoint, was 41.4% in the crizotinib arm, vs. 9.41% in the alectinib arm (cause-specific HR, 0.16; P not shown).
In each arm, 97% of patients had any adverse event, and the incidence of serious adverse events was similar between the arms, at 29% for crizotinib and 28% for alectinib.
Adverse events leading to treatment discontinuation, dose reduction, or dose interruption were more frequent with crizotinib.
In the question and answer portion of the briefing, Dr. Shaw was asked whether crizotinib still had a role in this population.
“Going forward, I think that it’s pretty clear, if you have a newly diagnosed patient with metastatic ALK-positive lung cancer, that likely alectinib would be the preferred first choice,” she said.
The ALEX trial is supported by Roche. Dr. Shaw disclosed consulting or an advisory role with the company, and multiple coauthors disclosed similar relationships.
CHICAGO – In what’s being hailed as practice-changing findings, the anaplastic lymphoma kinase inhibitor alectinib (Alecensa) was associated with more than doubled progression-free survival (PFS), compared with crizotinib (Xalkori), the current standard of care, in patients with treatment-naive non–small cell lung cancer (NSCLC) positive for ALK.
Additionally, in the global, phase III trial, alectinib was associated with a significantly lower risk of progression to CNS metastases, a common complication of advanced ALK+ NSCLC, reported Alice T. Shaw, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, on behalf of investigators in the ALEX trial.
“I view this as a watershed moment for the treatment of ALK mutant–positive lung cancer,” commented ASCO expert John Heymach, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
Unlike other head-to-head studies of similar drugs that frequently show only incremental benefit, the ALEX results showed a dramatic difference in outcomes for patients treated with alectinib, he said.
By comparison, the median PFS difference between chemotherapy and crizotinib in the PROFILE 1014 in patients with ALK-positive NSCLC trial was 10.9 vs. 7.0 months, Dr. Heymach pointed out.
The ALEX investigators enrolled 303 patients with untreated ALK-positive NSCLC confirmed by a central immunohistochemistry lab and randomly assigned them to treatment with either oral alectinib 600 mg twice daily or crizotinib 250 mg b.i.d.
At the primary data cutoff in February 2017, median PFS, the primary endpoint, was 11.1 months for patients treated with crizotinib, versus not reached for those treated with alectinib, translating into a hazard ratio for alectinib of 0.47 (P less than .0001).
Based on an independent review, the median PFS was determined to be 10.4 months for crizotinib, vs. 25.7 months with alectinib (HR, 0.50; P not shown).
The cumulative incidence of CNS progression, a secondary endpoint, was 41.4% in the crizotinib arm, vs. 9.41% in the alectinib arm (cause-specific HR, 0.16; P not shown).
In each arm, 97% of patients had any adverse event, and the incidence of serious adverse events was similar between the arms, at 29% for crizotinib and 28% for alectinib.
Adverse events leading to treatment discontinuation, dose reduction, or dose interruption were more frequent with crizotinib.
In the question and answer portion of the briefing, Dr. Shaw was asked whether crizotinib still had a role in this population.
“Going forward, I think that it’s pretty clear, if you have a newly diagnosed patient with metastatic ALK-positive lung cancer, that likely alectinib would be the preferred first choice,” she said.
The ALEX trial is supported by Roche. Dr. Shaw disclosed consulting or an advisory role with the company, and multiple coauthors disclosed similar relationships.
AT ASCO 2017
Key clinical point: Alectinib was associated with more than double the progression-free survival of standard of care crizotinib in patients with non–small cell lung cancer positive for the anaplastic lymphoma kinase.
Major finding: Median PFS by independent review was 10.4 months with crizotinib vs. 25.7 months with alectinib.
Data source: The ALEX trial, a phase III trial of 303 patients with ALK-positive NSCLC.
Disclosures: The ALEX trial is supported by Roche. Dr. Shaw disclosed consulting or an advisory role with the company, and multiple coauthors disclosed similar relationships
VIDEO: Immunotherapy ups disease control rate in relapsed mesothelioma
CHICAGO – Early data from a phase II trial of immune checkpoint inhibitors to treat relapsed mesothelioma give hope that immunotherapy may be an effective therapeutic option for the rapidly progressive, currently incurable cancer.
Reporting on 12 weeks of data from the randomized multicenter trial, Arnaud Scherpereel, MD, the study’s first author, said in a video interview, “We were very pleased to see that we were able to increase ... the disease control rate to 44% with nivolumab, and 50% with nivolumab plus ipilimumab. This was translated into a overall survival gain for these patients.” The best previous disease control rate seen with other therapies was less than 30%, said Dr. Scherpereel at the annual meeting of the American Society of Clinical Oncology.
Discussing the early results in a video interview, Dr. Scherpereel, head of the pulmonary and thoracic oncology department at the University Hospital of Lille, France noted that the median overall survival for the nivolumab patients was 10.4 months, and has not yet been reached for the nivolumab plus ipilimumab patients. Further, he said in a press briefing, “Tumors shrunk in 18% of patients treated with nivolumab and 26% of those treated with nivolumab plus ipilimumab.”
The French MAPS-2 study has enrolled 125 adult patients with malignant pleural mesothelioma who had measurable disease progression after one or two prior lines of chemotherapy, including pemetrexed/platinum doublet. Patients were randomized 1:1 to receive either nivolumab or nivolumab plus ipilimumab, until disease control or unacceptable toxicity was reached, for a maximum of 2 years. Patients were mostly (80%) male, with a median age of 71.8 years, and most had the epithelioid malignant pleural mesothelioma subtype.
In commentary at the press briefing announcing the findings, ASCO expert Michael Sabel, MD, said, “I need to emphasize that this is amazing, in that we are seeing [the use of] checkpoint inhibitors expanding beyond melanoma, to other cancers that we thought were not amenable to immunotherapy approaches.”
“This is a great example of how basic cancer research in one field can expand across others,” said Dr. Sabel of the departments of surgery and surgical oncology at the University of Michigan, Ann Arbor.
Most side effects were not severe, but there were three potentially drug-related deaths in the nivolumab-ipilimumab combo arm: one patient died of fulminant hepatitis, one from metabolic encephalitis, and one from acute renal failure. “There is no identified factor that is predictive” in terms of which patients will have the more significant known adverse effects of checkpoint inhibitors, said Dr. Scherpereel. Patients, caregivers, and health care professionals all need to be alert to the possibility of adverse events and act promptly if concerning symptoms crop up, he said.
Dr. Scherpereel said that though his study group has not yet reported the quality of life findings from MAPS-2, he sees that his patients who are study participants are doing better. “In my patients, they have a very good tolerance to this treatment compared to chemotherapy. They have less dyspnea, less chest pain. Clearly, we hope to get these drugs into the routine very quickly for them.”
Bristol-Myers Squibb manufactures both nivolumab and ipilimumab and provided the study drugs. Dr. Sabel disclosed a financial relationship with Merck. Dr. Scherpereel has no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
koakes@frontlinemedcom.com
On Twitter @karioakes
CHICAGO – Early data from a phase II trial of immune checkpoint inhibitors to treat relapsed mesothelioma give hope that immunotherapy may be an effective therapeutic option for the rapidly progressive, currently incurable cancer.
Reporting on 12 weeks of data from the randomized multicenter trial, Arnaud Scherpereel, MD, the study’s first author, said in a video interview, “We were very pleased to see that we were able to increase ... the disease control rate to 44% with nivolumab, and 50% with nivolumab plus ipilimumab. This was translated into a overall survival gain for these patients.” The best previous disease control rate seen with other therapies was less than 30%, said Dr. Scherpereel at the annual meeting of the American Society of Clinical Oncology.
Discussing the early results in a video interview, Dr. Scherpereel, head of the pulmonary and thoracic oncology department at the University Hospital of Lille, France noted that the median overall survival for the nivolumab patients was 10.4 months, and has not yet been reached for the nivolumab plus ipilimumab patients. Further, he said in a press briefing, “Tumors shrunk in 18% of patients treated with nivolumab and 26% of those treated with nivolumab plus ipilimumab.”
The French MAPS-2 study has enrolled 125 adult patients with malignant pleural mesothelioma who had measurable disease progression after one or two prior lines of chemotherapy, including pemetrexed/platinum doublet. Patients were randomized 1:1 to receive either nivolumab or nivolumab plus ipilimumab, until disease control or unacceptable toxicity was reached, for a maximum of 2 years. Patients were mostly (80%) male, with a median age of 71.8 years, and most had the epithelioid malignant pleural mesothelioma subtype.
In commentary at the press briefing announcing the findings, ASCO expert Michael Sabel, MD, said, “I need to emphasize that this is amazing, in that we are seeing [the use of] checkpoint inhibitors expanding beyond melanoma, to other cancers that we thought were not amenable to immunotherapy approaches.”
“This is a great example of how basic cancer research in one field can expand across others,” said Dr. Sabel of the departments of surgery and surgical oncology at the University of Michigan, Ann Arbor.
Most side effects were not severe, but there were three potentially drug-related deaths in the nivolumab-ipilimumab combo arm: one patient died of fulminant hepatitis, one from metabolic encephalitis, and one from acute renal failure. “There is no identified factor that is predictive” in terms of which patients will have the more significant known adverse effects of checkpoint inhibitors, said Dr. Scherpereel. Patients, caregivers, and health care professionals all need to be alert to the possibility of adverse events and act promptly if concerning symptoms crop up, he said.
Dr. Scherpereel said that though his study group has not yet reported the quality of life findings from MAPS-2, he sees that his patients who are study participants are doing better. “In my patients, they have a very good tolerance to this treatment compared to chemotherapy. They have less dyspnea, less chest pain. Clearly, we hope to get these drugs into the routine very quickly for them.”
Bristol-Myers Squibb manufactures both nivolumab and ipilimumab and provided the study drugs. Dr. Sabel disclosed a financial relationship with Merck. Dr. Scherpereel has no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
koakes@frontlinemedcom.com
On Twitter @karioakes
CHICAGO – Early data from a phase II trial of immune checkpoint inhibitors to treat relapsed mesothelioma give hope that immunotherapy may be an effective therapeutic option for the rapidly progressive, currently incurable cancer.
Reporting on 12 weeks of data from the randomized multicenter trial, Arnaud Scherpereel, MD, the study’s first author, said in a video interview, “We were very pleased to see that we were able to increase ... the disease control rate to 44% with nivolumab, and 50% with nivolumab plus ipilimumab. This was translated into a overall survival gain for these patients.” The best previous disease control rate seen with other therapies was less than 30%, said Dr. Scherpereel at the annual meeting of the American Society of Clinical Oncology.
Discussing the early results in a video interview, Dr. Scherpereel, head of the pulmonary and thoracic oncology department at the University Hospital of Lille, France noted that the median overall survival for the nivolumab patients was 10.4 months, and has not yet been reached for the nivolumab plus ipilimumab patients. Further, he said in a press briefing, “Tumors shrunk in 18% of patients treated with nivolumab and 26% of those treated with nivolumab plus ipilimumab.”
The French MAPS-2 study has enrolled 125 adult patients with malignant pleural mesothelioma who had measurable disease progression after one or two prior lines of chemotherapy, including pemetrexed/platinum doublet. Patients were randomized 1:1 to receive either nivolumab or nivolumab plus ipilimumab, until disease control or unacceptable toxicity was reached, for a maximum of 2 years. Patients were mostly (80%) male, with a median age of 71.8 years, and most had the epithelioid malignant pleural mesothelioma subtype.
In commentary at the press briefing announcing the findings, ASCO expert Michael Sabel, MD, said, “I need to emphasize that this is amazing, in that we are seeing [the use of] checkpoint inhibitors expanding beyond melanoma, to other cancers that we thought were not amenable to immunotherapy approaches.”
“This is a great example of how basic cancer research in one field can expand across others,” said Dr. Sabel of the departments of surgery and surgical oncology at the University of Michigan, Ann Arbor.
Most side effects were not severe, but there were three potentially drug-related deaths in the nivolumab-ipilimumab combo arm: one patient died of fulminant hepatitis, one from metabolic encephalitis, and one from acute renal failure. “There is no identified factor that is predictive” in terms of which patients will have the more significant known adverse effects of checkpoint inhibitors, said Dr. Scherpereel. Patients, caregivers, and health care professionals all need to be alert to the possibility of adverse events and act promptly if concerning symptoms crop up, he said.
Dr. Scherpereel said that though his study group has not yet reported the quality of life findings from MAPS-2, he sees that his patients who are study participants are doing better. “In my patients, they have a very good tolerance to this treatment compared to chemotherapy. They have less dyspnea, less chest pain. Clearly, we hope to get these drugs into the routine very quickly for them.”
Bristol-Myers Squibb manufactures both nivolumab and ipilimumab and provided the study drugs. Dr. Sabel disclosed a financial relationship with Merck. Dr. Scherpereel has no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
koakes@frontlinemedcom.com
On Twitter @karioakes
AT ASCO 2017
VIDEO: Alectinib doubles PFS and then some over crizotinib in ALK+ NSCLC
CHICAGO – The standard of care for patients with non–small cell lung cancer positive for the anaplastic lymphoma kinase (ALK) is the ALK inhibitor crizotinib (Xalkori). However, many patients on crizotinib will have disease progression within the first year of therapy, and many will go on to have central nervous system (CNS) metastases.
The multicenter international ALEX trial compared crizotinib with the second-generation ALK inhibitor alectinib (Alecensa). The investigators found that alectinib reduced the risk of progression by 53% and the time to CNS progression by 84%.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Alice T. Shaw, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, outlines the ALEX trial results, which are being hailed as “practice changing.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The standard of care for patients with non–small cell lung cancer positive for the anaplastic lymphoma kinase (ALK) is the ALK inhibitor crizotinib (Xalkori). However, many patients on crizotinib will have disease progression within the first year of therapy, and many will go on to have central nervous system (CNS) metastases.
The multicenter international ALEX trial compared crizotinib with the second-generation ALK inhibitor alectinib (Alecensa). The investigators found that alectinib reduced the risk of progression by 53% and the time to CNS progression by 84%.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Alice T. Shaw, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, outlines the ALEX trial results, which are being hailed as “practice changing.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The standard of care for patients with non–small cell lung cancer positive for the anaplastic lymphoma kinase (ALK) is the ALK inhibitor crizotinib (Xalkori). However, many patients on crizotinib will have disease progression within the first year of therapy, and many will go on to have central nervous system (CNS) metastases.
The multicenter international ALEX trial compared crizotinib with the second-generation ALK inhibitor alectinib (Alecensa). The investigators found that alectinib reduced the risk of progression by 53% and the time to CNS progression by 84%.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Alice T. Shaw, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, outlines the ALEX trial results, which are being hailed as “practice changing.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASCO 2017