User login
Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life
Background There is a paucity of literature comparing quality of life (QoL) before and after surgery in stage IA lung cancer, where surgical resection is the recommended curative treatment.
Objective To assess the impact of surgery on physical and mental health-related QoL in patients with stage IA lung cancer treated with surgical resection.
Methods Participants in the I-ELCAP cohort who were diagnosed with their first primary pathologic stage IA non-small-cell lung cancer, underwent surgery, and provided follow-up information on QoL 1 year later were included in the present analysis (N = 107). QoL information was collected using the SF-12 (12-item Short Form Health Survey), which generates 2 component scores related to mental health and physical health.
Results Statistical analyses indicated that physical health QoL was significantly worsened from before surgery to after surgery, whereas mental health QoL marginally improved from before to after surgery. Physical health QoL worsened for women from baseline to follow-up, but not for men. Only lobectomy (not limited resection) had an impact on QoL from before to after surgery.
Limitations Results are considered preliminary given the small sample size and multiple comparisons.
Conclusions The current study findings have implications for lung cancer health care professionals in regard to how they can most effectively present the possible impact of surgery on quality of life to this subset of patients in which disease has not yet significantly progressed.
Funding/sponsorship Gift from Sonia Lasry Gardner, in memory of her father, Moise Lasry.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a paucity of literature comparing quality of life (QoL) before and after surgery in stage IA lung cancer, where surgical resection is the recommended curative treatment.
Objective To assess the impact of surgery on physical and mental health-related QoL in patients with stage IA lung cancer treated with surgical resection.
Methods Participants in the I-ELCAP cohort who were diagnosed with their first primary pathologic stage IA non-small-cell lung cancer, underwent surgery, and provided follow-up information on QoL 1 year later were included in the present analysis (N = 107). QoL information was collected using the SF-12 (12-item Short Form Health Survey), which generates 2 component scores related to mental health and physical health.
Results Statistical analyses indicated that physical health QoL was significantly worsened from before surgery to after surgery, whereas mental health QoL marginally improved from before to after surgery. Physical health QoL worsened for women from baseline to follow-up, but not for men. Only lobectomy (not limited resection) had an impact on QoL from before to after surgery.
Limitations Results are considered preliminary given the small sample size and multiple comparisons.
Conclusions The current study findings have implications for lung cancer health care professionals in regard to how they can most effectively present the possible impact of surgery on quality of life to this subset of patients in which disease has not yet significantly progressed.
Funding/sponsorship Gift from Sonia Lasry Gardner, in memory of her father, Moise Lasry.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a paucity of literature comparing quality of life (QoL) before and after surgery in stage IA lung cancer, where surgical resection is the recommended curative treatment.
Objective To assess the impact of surgery on physical and mental health-related QoL in patients with stage IA lung cancer treated with surgical resection.
Methods Participants in the I-ELCAP cohort who were diagnosed with their first primary pathologic stage IA non-small-cell lung cancer, underwent surgery, and provided follow-up information on QoL 1 year later were included in the present analysis (N = 107). QoL information was collected using the SF-12 (12-item Short Form Health Survey), which generates 2 component scores related to mental health and physical health.
Results Statistical analyses indicated that physical health QoL was significantly worsened from before surgery to after surgery, whereas mental health QoL marginally improved from before to after surgery. Physical health QoL worsened for women from baseline to follow-up, but not for men. Only lobectomy (not limited resection) had an impact on QoL from before to after surgery.
Limitations Results are considered preliminary given the small sample size and multiple comparisons.
Conclusions The current study findings have implications for lung cancer health care professionals in regard to how they can most effectively present the possible impact of surgery on quality of life to this subset of patients in which disease has not yet significantly progressed.
Funding/sponsorship Gift from Sonia Lasry Gardner, in memory of her father, Moise Lasry.
Click on the PDF icon at the top of this introduction to read the full article.
PROCLAIM: Pemetrexed combo no better than standard tx in NSCLC
A combination of the antifolate agent pemetrexed plus cisplatin with thoracic radiation followed by consolidation with pemetrexed was no better than standard etoposide-cisplatin chemoradiotherapy and platinum doublet consolidation in patients with unresectable stage III nonsquamous, non–small cell lung cancer, results of a randomized clinical trial indicate.
In the PROCLAIM trial, results of which were reported at the American Society of Clinical Oncology’s 2015 annual meeting, there was no significant difference in overall survival between patients randomized to receive pemetrexed-cisplatin or etoposide-cisplatin, leading to early discontinuation of the trial because it had met the prespecified definition of futility, reported Dr. Suresh Senan from the Vrije University Medical Center in Amsterdam and colleagues.
They noted, however, that pemetrexed-cisplatin was less toxic than the current platinum doublet standard.
“A significantly lower incidence of drug-related grade 3 to 4 [adverse events], including neutropenia, was observed during the overall study period for pemetrexed-cisplatin. Grade 3 to 4 neutropenia and febrile neutropenia were also lower for pemetrexed-cisplatin during concurrent therapy. The PROCLAIM trial showed that pemetrexed-cisplatin has an acceptable safety profile, in a scenario in which concurrent chemoradiation remains the standard of care,” the investigators reported in the Journal of Clinical Oncology.
Investigators in the multicenter international trial chose to compare pemetrexed in combination with cisplatin because the antifolate has selective activity against nonsquamous NSCLC and is known to be a radiosensitizer. Additionally, maintenance pemetrexed has been shown in at least two randomized trials to offer a survival advantage following first-line therapy, they noted (J Clin Onc. 2016 Jan 25. doi: 10.1200/JCO.2015.64.8824).
In the PROCLAIM trial, patients with stage IIIA or IIIB unresectable nonsquamous NSCLC were randomly assigned to receive either pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 intravenously every 3 weeks for three cycles with concurrent thoracic radiation at doses of 60 to 66 Gy, followed by pemetrexed consolidation every 3 weeks for four cycles, or to standard therapy with etoposide 50 mg/m2 and cisplatin 50 mg/m2 intravenously every 4 weeks for two cycles with concurrent thoracic radiation at the same doses, followed by two cycles of consolidation with platinum-based doublet chemotherapy.
The trial was designed as a superiority study, with planned enrollment of about 600 patients, with 80% power to detect a hazard ratio of 0.74 for the experimental arm. The trial was stopped early for futility after an interim analysis was performed. At that time, 173 of 552 randomized patients had died.
Median overall survival was 26.8 months for the pemetrexed group vs. 25 months for the standard therapy group (HR, 0.98; P = .831).
A combination of the antifolate agent pemetrexed plus cisplatin with thoracic radiation followed by consolidation with pemetrexed was no better than standard etoposide-cisplatin chemoradiotherapy and platinum doublet consolidation in patients with unresectable stage III nonsquamous, non–small cell lung cancer, results of a randomized clinical trial indicate.
In the PROCLAIM trial, results of which were reported at the American Society of Clinical Oncology’s 2015 annual meeting, there was no significant difference in overall survival between patients randomized to receive pemetrexed-cisplatin or etoposide-cisplatin, leading to early discontinuation of the trial because it had met the prespecified definition of futility, reported Dr. Suresh Senan from the Vrije University Medical Center in Amsterdam and colleagues.
They noted, however, that pemetrexed-cisplatin was less toxic than the current platinum doublet standard.
“A significantly lower incidence of drug-related grade 3 to 4 [adverse events], including neutropenia, was observed during the overall study period for pemetrexed-cisplatin. Grade 3 to 4 neutropenia and febrile neutropenia were also lower for pemetrexed-cisplatin during concurrent therapy. The PROCLAIM trial showed that pemetrexed-cisplatin has an acceptable safety profile, in a scenario in which concurrent chemoradiation remains the standard of care,” the investigators reported in the Journal of Clinical Oncology.
Investigators in the multicenter international trial chose to compare pemetrexed in combination with cisplatin because the antifolate has selective activity against nonsquamous NSCLC and is known to be a radiosensitizer. Additionally, maintenance pemetrexed has been shown in at least two randomized trials to offer a survival advantage following first-line therapy, they noted (J Clin Onc. 2016 Jan 25. doi: 10.1200/JCO.2015.64.8824).
In the PROCLAIM trial, patients with stage IIIA or IIIB unresectable nonsquamous NSCLC were randomly assigned to receive either pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 intravenously every 3 weeks for three cycles with concurrent thoracic radiation at doses of 60 to 66 Gy, followed by pemetrexed consolidation every 3 weeks for four cycles, or to standard therapy with etoposide 50 mg/m2 and cisplatin 50 mg/m2 intravenously every 4 weeks for two cycles with concurrent thoracic radiation at the same doses, followed by two cycles of consolidation with platinum-based doublet chemotherapy.
The trial was designed as a superiority study, with planned enrollment of about 600 patients, with 80% power to detect a hazard ratio of 0.74 for the experimental arm. The trial was stopped early for futility after an interim analysis was performed. At that time, 173 of 552 randomized patients had died.
Median overall survival was 26.8 months for the pemetrexed group vs. 25 months for the standard therapy group (HR, 0.98; P = .831).
A combination of the antifolate agent pemetrexed plus cisplatin with thoracic radiation followed by consolidation with pemetrexed was no better than standard etoposide-cisplatin chemoradiotherapy and platinum doublet consolidation in patients with unresectable stage III nonsquamous, non–small cell lung cancer, results of a randomized clinical trial indicate.
In the PROCLAIM trial, results of which were reported at the American Society of Clinical Oncology’s 2015 annual meeting, there was no significant difference in overall survival between patients randomized to receive pemetrexed-cisplatin or etoposide-cisplatin, leading to early discontinuation of the trial because it had met the prespecified definition of futility, reported Dr. Suresh Senan from the Vrije University Medical Center in Amsterdam and colleagues.
They noted, however, that pemetrexed-cisplatin was less toxic than the current platinum doublet standard.
“A significantly lower incidence of drug-related grade 3 to 4 [adverse events], including neutropenia, was observed during the overall study period for pemetrexed-cisplatin. Grade 3 to 4 neutropenia and febrile neutropenia were also lower for pemetrexed-cisplatin during concurrent therapy. The PROCLAIM trial showed that pemetrexed-cisplatin has an acceptable safety profile, in a scenario in which concurrent chemoradiation remains the standard of care,” the investigators reported in the Journal of Clinical Oncology.
Investigators in the multicenter international trial chose to compare pemetrexed in combination with cisplatin because the antifolate has selective activity against nonsquamous NSCLC and is known to be a radiosensitizer. Additionally, maintenance pemetrexed has been shown in at least two randomized trials to offer a survival advantage following first-line therapy, they noted (J Clin Onc. 2016 Jan 25. doi: 10.1200/JCO.2015.64.8824).
In the PROCLAIM trial, patients with stage IIIA or IIIB unresectable nonsquamous NSCLC were randomly assigned to receive either pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 intravenously every 3 weeks for three cycles with concurrent thoracic radiation at doses of 60 to 66 Gy, followed by pemetrexed consolidation every 3 weeks for four cycles, or to standard therapy with etoposide 50 mg/m2 and cisplatin 50 mg/m2 intravenously every 4 weeks for two cycles with concurrent thoracic radiation at the same doses, followed by two cycles of consolidation with platinum-based doublet chemotherapy.
The trial was designed as a superiority study, with planned enrollment of about 600 patients, with 80% power to detect a hazard ratio of 0.74 for the experimental arm. The trial was stopped early for futility after an interim analysis was performed. At that time, 173 of 552 randomized patients had died.
Median overall survival was 26.8 months for the pemetrexed group vs. 25 months for the standard therapy group (HR, 0.98; P = .831).
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Pemetrexed-cisplatin and pemetrexed consolidation were not superior to etoposide-cisplatin and consolidation for advanced NSCLC.
Major finding: Median OS was 26.8 months for the pemetrexed group vs. 25 for the standard therapy group (HR, 0.98; P = .831).
Data source: Randomized phase III trial planned for 598 patients with advanced nonsquamous, non–small cell lung cancer.
Disclosures: The study was supported by Eli Lilly. Multiple authors reported research funding, consulting, or honoraria from the company, and six are Eli Lilly employees.
VIDEO: Novel imaging technique helps hunt for pulmonary lesions
PHOENIX – Each year more than 250,000 patients present with ground-glass opacities and other solitary pulmonary nodules, and they are difficult to locate.
“There’s been a need for our field to develop new technologies to find these nodules in the OR,” Dr. Sunil Singhal said in a video interview at the annual meeting of the Society of Thoracic Surgeons. “The fallback plan has always been that we can make a thoracotomy. Some studies have shown that in about one out of every two cases you end up opening a patient just to find a tiny little nodule.”
Dr. Singhal of the division of cardiothoracic surgery at the University of Pennsylvania School of Medicine, Philadelphia, discussed preoperative and intraoperative localization methods, including an investigational technology in which patients receive an intravascular dye that localizes the pulmonary tumor. “When we put our video-assisted thoracoscopic surgery camera in, the tumors are glowing,” he said. “We can then do a localized wedge excision and confirm margins of the staple line. We’ve done this [in] about 80 patients, and it’s been non-toxic, very safe, and very effective. Our biggest limitation has been our depth of penetration.”
Dr. Singhal reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – Each year more than 250,000 patients present with ground-glass opacities and other solitary pulmonary nodules, and they are difficult to locate.
“There’s been a need for our field to develop new technologies to find these nodules in the OR,” Dr. Sunil Singhal said in a video interview at the annual meeting of the Society of Thoracic Surgeons. “The fallback plan has always been that we can make a thoracotomy. Some studies have shown that in about one out of every two cases you end up opening a patient just to find a tiny little nodule.”
Dr. Singhal of the division of cardiothoracic surgery at the University of Pennsylvania School of Medicine, Philadelphia, discussed preoperative and intraoperative localization methods, including an investigational technology in which patients receive an intravascular dye that localizes the pulmonary tumor. “When we put our video-assisted thoracoscopic surgery camera in, the tumors are glowing,” he said. “We can then do a localized wedge excision and confirm margins of the staple line. We’ve done this [in] about 80 patients, and it’s been non-toxic, very safe, and very effective. Our biggest limitation has been our depth of penetration.”
Dr. Singhal reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – Each year more than 250,000 patients present with ground-glass opacities and other solitary pulmonary nodules, and they are difficult to locate.
“There’s been a need for our field to develop new technologies to find these nodules in the OR,” Dr. Sunil Singhal said in a video interview at the annual meeting of the Society of Thoracic Surgeons. “The fallback plan has always been that we can make a thoracotomy. Some studies have shown that in about one out of every two cases you end up opening a patient just to find a tiny little nodule.”
Dr. Singhal of the division of cardiothoracic surgery at the University of Pennsylvania School of Medicine, Philadelphia, discussed preoperative and intraoperative localization methods, including an investigational technology in which patients receive an intravascular dye that localizes the pulmonary tumor. “When we put our video-assisted thoracoscopic surgery camera in, the tumors are glowing,” he said. “We can then do a localized wedge excision and confirm margins of the staple line. We’ve done this [in] about 80 patients, and it’s been non-toxic, very safe, and very effective. Our biggest limitation has been our depth of penetration.”
Dr. Singhal reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING
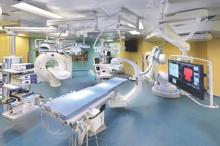
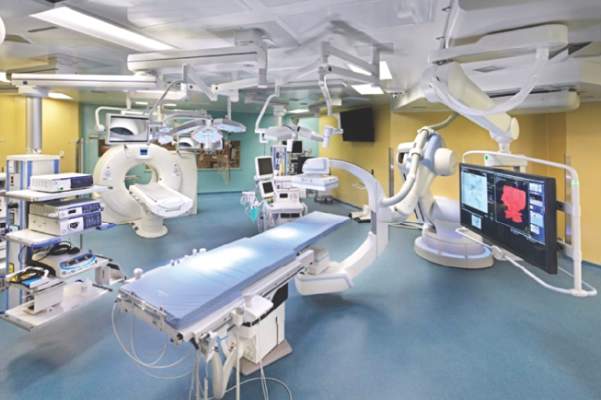
STS: Hybrid thoracic suite leverages CT’s imaging sensitivity
PHOENIX – Using CT imaging to detect lung cancers in people at high risk for developing it has made it possible to find small tumors with substantially increased sensitivity than is possible with radiography, However, this approach has posed a new challenge to thoracic surgeons: How to visualize these nodules – subcentimeter and nonpalpable – for biopsy or for resection?
The answer may be the hybrid thoracic operating room developed by Dr. Kazuhiro Yasufuku and his associates at Toronto General Hospital, a novel surgical suite that he described at the annual meeting of the Society of Thoracic Surgeons.
Dr. Yasufuku and his team began using the hybrid operating room on an investigational basis in 2013 and have now done about 50 cases as part of several research protocols. The trials address the feasibility of resection, biopsy, and nodule localization, as well as whether the hybrid approach reduces the amount of radiation exposure to both patients and to the surgical team, he said. They plan to report some of their initial results later this year.
The Toronto group assembled the hybrid array of equipment into a single operating room that includes both a dual-source, dual-energy CT scanner and a robotic cone-beam CT scanner, equipment for minimally invasive procedures including video-assisted thoracoscopic and robotic surgery, and advanced endoscopic technology including endobronchial ultrasound and navigational bronchoscopy. “We use innovative methods that we already know about, but bring them all together” within a single space, Dr. Yasufuku explained. “Rather than having patients go to several locations, we can do everything at the same time in one room.”
Perhaps the most novel aspect of this operating room is inclusion of a robotic cone-beam CT scanner, which uses mobile, flat CT-imaging panels that overcome the limitations of a conventional, fixed CT scanner. “They scan the patient and then we can retract them and get them out of the way” to better facilitate surgery, he said in an interview.
“We do not have a culture in thoracic surgery of using imaging during surgery,” said Dr. Yasufuku, director of the interventional thoracic surgery program at the University of Toronto. Hybrid operating rooms using noninvasive or minimally invasive equipment and procedures have become commonplace for cardiovascular surgeons and cardiac interventionalists, but this approach has generally not yet been applied to thoracic surgery for cancer, in large part because of the imaging limitations, he said. “It is difficult to perform video-assisted thorascopic surgery using fixed CT.”
Bronchoscopic technologies provide additional, important tools for minimally invasive thoracic surgery. “We use the hybrid operating room to mark small [nonpalpable] lesions.” One approach to marking is to place a microcoil within the nodule with a percutaneous needle. Another approach is to tag the nodule with a fluorescent dye using navigational bronchoscopy.
Dr. Yasufuku also emphasized that the hybrid operating room will also be valuable when new, minimally invasive, nonsurgical therapeutic options for treatment of lung cancer become available in the near future.
Dr. Yasufuku said that he had no relevant disclosures.
On Twitter @mitchelzoler
PHOENIX – Using CT imaging to detect lung cancers in people at high risk for developing it has made it possible to find small tumors with substantially increased sensitivity than is possible with radiography, However, this approach has posed a new challenge to thoracic surgeons: How to visualize these nodules – subcentimeter and nonpalpable – for biopsy or for resection?
The answer may be the hybrid thoracic operating room developed by Dr. Kazuhiro Yasufuku and his associates at Toronto General Hospital, a novel surgical suite that he described at the annual meeting of the Society of Thoracic Surgeons.
Dr. Yasufuku and his team began using the hybrid operating room on an investigational basis in 2013 and have now done about 50 cases as part of several research protocols. The trials address the feasibility of resection, biopsy, and nodule localization, as well as whether the hybrid approach reduces the amount of radiation exposure to both patients and to the surgical team, he said. They plan to report some of their initial results later this year.
The Toronto group assembled the hybrid array of equipment into a single operating room that includes both a dual-source, dual-energy CT scanner and a robotic cone-beam CT scanner, equipment for minimally invasive procedures including video-assisted thoracoscopic and robotic surgery, and advanced endoscopic technology including endobronchial ultrasound and navigational bronchoscopy. “We use innovative methods that we already know about, but bring them all together” within a single space, Dr. Yasufuku explained. “Rather than having patients go to several locations, we can do everything at the same time in one room.”
Perhaps the most novel aspect of this operating room is inclusion of a robotic cone-beam CT scanner, which uses mobile, flat CT-imaging panels that overcome the limitations of a conventional, fixed CT scanner. “They scan the patient and then we can retract them and get them out of the way” to better facilitate surgery, he said in an interview.
“We do not have a culture in thoracic surgery of using imaging during surgery,” said Dr. Yasufuku, director of the interventional thoracic surgery program at the University of Toronto. Hybrid operating rooms using noninvasive or minimally invasive equipment and procedures have become commonplace for cardiovascular surgeons and cardiac interventionalists, but this approach has generally not yet been applied to thoracic surgery for cancer, in large part because of the imaging limitations, he said. “It is difficult to perform video-assisted thorascopic surgery using fixed CT.”
Bronchoscopic technologies provide additional, important tools for minimally invasive thoracic surgery. “We use the hybrid operating room to mark small [nonpalpable] lesions.” One approach to marking is to place a microcoil within the nodule with a percutaneous needle. Another approach is to tag the nodule with a fluorescent dye using navigational bronchoscopy.
Dr. Yasufuku also emphasized that the hybrid operating room will also be valuable when new, minimally invasive, nonsurgical therapeutic options for treatment of lung cancer become available in the near future.
Dr. Yasufuku said that he had no relevant disclosures.
On Twitter @mitchelzoler
PHOENIX – Using CT imaging to detect lung cancers in people at high risk for developing it has made it possible to find small tumors with substantially increased sensitivity than is possible with radiography, However, this approach has posed a new challenge to thoracic surgeons: How to visualize these nodules – subcentimeter and nonpalpable – for biopsy or for resection?
The answer may be the hybrid thoracic operating room developed by Dr. Kazuhiro Yasufuku and his associates at Toronto General Hospital, a novel surgical suite that he described at the annual meeting of the Society of Thoracic Surgeons.
Dr. Yasufuku and his team began using the hybrid operating room on an investigational basis in 2013 and have now done about 50 cases as part of several research protocols. The trials address the feasibility of resection, biopsy, and nodule localization, as well as whether the hybrid approach reduces the amount of radiation exposure to both patients and to the surgical team, he said. They plan to report some of their initial results later this year.
The Toronto group assembled the hybrid array of equipment into a single operating room that includes both a dual-source, dual-energy CT scanner and a robotic cone-beam CT scanner, equipment for minimally invasive procedures including video-assisted thoracoscopic and robotic surgery, and advanced endoscopic technology including endobronchial ultrasound and navigational bronchoscopy. “We use innovative methods that we already know about, but bring them all together” within a single space, Dr. Yasufuku explained. “Rather than having patients go to several locations, we can do everything at the same time in one room.”
Perhaps the most novel aspect of this operating room is inclusion of a robotic cone-beam CT scanner, which uses mobile, flat CT-imaging panels that overcome the limitations of a conventional, fixed CT scanner. “They scan the patient and then we can retract them and get them out of the way” to better facilitate surgery, he said in an interview.
“We do not have a culture in thoracic surgery of using imaging during surgery,” said Dr. Yasufuku, director of the interventional thoracic surgery program at the University of Toronto. Hybrid operating rooms using noninvasive or minimally invasive equipment and procedures have become commonplace for cardiovascular surgeons and cardiac interventionalists, but this approach has generally not yet been applied to thoracic surgery for cancer, in large part because of the imaging limitations, he said. “It is difficult to perform video-assisted thorascopic surgery using fixed CT.”
Bronchoscopic technologies provide additional, important tools for minimally invasive thoracic surgery. “We use the hybrid operating room to mark small [nonpalpable] lesions.” One approach to marking is to place a microcoil within the nodule with a percutaneous needle. Another approach is to tag the nodule with a fluorescent dye using navigational bronchoscopy.
Dr. Yasufuku also emphasized that the hybrid operating room will also be valuable when new, minimally invasive, nonsurgical therapeutic options for treatment of lung cancer become available in the near future.
Dr. Yasufuku said that he had no relevant disclosures.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING
VIDEO: Which lesions are best for bronchoscopic endoluminal treatment?
PHOENIX – According to Dr. Moishe Liberman, promising lesions for bronchoscopic endoluminal treatment include endobronchial lesions and intraluminal exophytic tumors within the trachea or main bronchus, provided that the distal airway lumen is visible and you can get past the tumor with a flexible endoscope.
“We always teach the fellows that if you get pus back when you’re trying to get around the tumor or play with the tumor, you’re usually going to have a very good result,” said Dr. Liberman, a thoracic surgeon who directs the endoscopic tracheo-bronchial and oesophageal center at the Centre hospitalier de l’Université de Montréal, Quebec, Canada. “If you play with the tumor and you get the tumor out and you get nothing back, usually the CT scan or the X-ray postoperatively is going to look just like it did preoperatively, even though endoscopically you might have a good result.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Central lesions are also excellent candidates for endoluminal therapy, he said in a video interview at the meeting. Distal lesions in the small bronchi “are candidates but are much more difficult and require more specialized tools. The shorter the lesion, the more likely you are to have good success.”
Available options for delivering energy endoscopically include electrocautery, argon plasma coagulation, laser, and cryotherapy. A disadvantage of all of the thermal modalities except for cryotherapy “include the potential for airway fire and you have to work with low FiO2s [fraction of inspired oxygen],” Dr. Liberman noted. “A lot of these patients need high FiO2s to saturate, so I think that’s always an issue. We never go on cardiopulmonary bypass to do these cases and we never cannulate patients to do these cases. You also have to worry about gas emboli, especially when you open up big vessels. These modalities can also cause inadvertent airway injury, delayed effects, and bronchoscope damage.”
In general, he continued, laser-tissue interactions depend on the power and the wavelength of the laser as well as the color and the water content of the target tissue. “The power density of the wavelength you choose determines its ability to cut, coagulate, or vaporize the tissue,” he said.
“As the power density increases, the laser fiber approaches the target tissue. Power density is more important than the energy delivered.”
The Nd:YAG (neodymium-doped yttrium aluminium garnet) laser, which causes more destruction in the deep tissue than on the surface, is the most common laser used in interventional airway procedures, he said. Two other commonly used lasers include the KTP (potassium titanyl phosphate) and the CO2. “I like CO2s a lot for upper airway and subglottic problems as well as vocal cord problems,” Dr. Liberman said. “It’s very precise and has low penetration. The Nd:YAG is very good for deep penetration. You need familiarity with these. I don’t think you can just take one of these off the shelf if you’ve never used it before. Sometimes your ENT [ear nose and throat] or urology colleagues can help you, because they’re using a lot more of these lasers than we are.”
Contraindications for laser bronchoscopy include operable lesions. Dr. Liberman said that while he and his associates use lasers in a preoperative setting, “we’re very careful not to damage proximal or distal airway when we know we’re going to do a sleeve resection or pneumonectomy.”
Other contraindications for laser bronchoscopy include patients with a poor short-term prognosis, severe coagulation disorder, extrinsic airway obstruction, tracheoesophageal fistula or T-Med fistula, those with extensive submucosal disease causing obstruction, and those with lesion adjacent to the esophagus or to a major vessel.
Dr. Liberman reported having received research grants from Ethicon, Boston Scientific, Olympus, Covidien, and Baxter.
PHOENIX – According to Dr. Moishe Liberman, promising lesions for bronchoscopic endoluminal treatment include endobronchial lesions and intraluminal exophytic tumors within the trachea or main bronchus, provided that the distal airway lumen is visible and you can get past the tumor with a flexible endoscope.
“We always teach the fellows that if you get pus back when you’re trying to get around the tumor or play with the tumor, you’re usually going to have a very good result,” said Dr. Liberman, a thoracic surgeon who directs the endoscopic tracheo-bronchial and oesophageal center at the Centre hospitalier de l’Université de Montréal, Quebec, Canada. “If you play with the tumor and you get the tumor out and you get nothing back, usually the CT scan or the X-ray postoperatively is going to look just like it did preoperatively, even though endoscopically you might have a good result.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Central lesions are also excellent candidates for endoluminal therapy, he said in a video interview at the meeting. Distal lesions in the small bronchi “are candidates but are much more difficult and require more specialized tools. The shorter the lesion, the more likely you are to have good success.”
Available options for delivering energy endoscopically include electrocautery, argon plasma coagulation, laser, and cryotherapy. A disadvantage of all of the thermal modalities except for cryotherapy “include the potential for airway fire and you have to work with low FiO2s [fraction of inspired oxygen],” Dr. Liberman noted. “A lot of these patients need high FiO2s to saturate, so I think that’s always an issue. We never go on cardiopulmonary bypass to do these cases and we never cannulate patients to do these cases. You also have to worry about gas emboli, especially when you open up big vessels. These modalities can also cause inadvertent airway injury, delayed effects, and bronchoscope damage.”
In general, he continued, laser-tissue interactions depend on the power and the wavelength of the laser as well as the color and the water content of the target tissue. “The power density of the wavelength you choose determines its ability to cut, coagulate, or vaporize the tissue,” he said.
“As the power density increases, the laser fiber approaches the target tissue. Power density is more important than the energy delivered.”
The Nd:YAG (neodymium-doped yttrium aluminium garnet) laser, which causes more destruction in the deep tissue than on the surface, is the most common laser used in interventional airway procedures, he said. Two other commonly used lasers include the KTP (potassium titanyl phosphate) and the CO2. “I like CO2s a lot for upper airway and subglottic problems as well as vocal cord problems,” Dr. Liberman said. “It’s very precise and has low penetration. The Nd:YAG is very good for deep penetration. You need familiarity with these. I don’t think you can just take one of these off the shelf if you’ve never used it before. Sometimes your ENT [ear nose and throat] or urology colleagues can help you, because they’re using a lot more of these lasers than we are.”
Contraindications for laser bronchoscopy include operable lesions. Dr. Liberman said that while he and his associates use lasers in a preoperative setting, “we’re very careful not to damage proximal or distal airway when we know we’re going to do a sleeve resection or pneumonectomy.”
Other contraindications for laser bronchoscopy include patients with a poor short-term prognosis, severe coagulation disorder, extrinsic airway obstruction, tracheoesophageal fistula or T-Med fistula, those with extensive submucosal disease causing obstruction, and those with lesion adjacent to the esophagus or to a major vessel.
Dr. Liberman reported having received research grants from Ethicon, Boston Scientific, Olympus, Covidien, and Baxter.
PHOENIX – According to Dr. Moishe Liberman, promising lesions for bronchoscopic endoluminal treatment include endobronchial lesions and intraluminal exophytic tumors within the trachea or main bronchus, provided that the distal airway lumen is visible and you can get past the tumor with a flexible endoscope.
“We always teach the fellows that if you get pus back when you’re trying to get around the tumor or play with the tumor, you’re usually going to have a very good result,” said Dr. Liberman, a thoracic surgeon who directs the endoscopic tracheo-bronchial and oesophageal center at the Centre hospitalier de l’Université de Montréal, Quebec, Canada. “If you play with the tumor and you get the tumor out and you get nothing back, usually the CT scan or the X-ray postoperatively is going to look just like it did preoperatively, even though endoscopically you might have a good result.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Central lesions are also excellent candidates for endoluminal therapy, he said in a video interview at the meeting. Distal lesions in the small bronchi “are candidates but are much more difficult and require more specialized tools. The shorter the lesion, the more likely you are to have good success.”
Available options for delivering energy endoscopically include electrocautery, argon plasma coagulation, laser, and cryotherapy. A disadvantage of all of the thermal modalities except for cryotherapy “include the potential for airway fire and you have to work with low FiO2s [fraction of inspired oxygen],” Dr. Liberman noted. “A lot of these patients need high FiO2s to saturate, so I think that’s always an issue. We never go on cardiopulmonary bypass to do these cases and we never cannulate patients to do these cases. You also have to worry about gas emboli, especially when you open up big vessels. These modalities can also cause inadvertent airway injury, delayed effects, and bronchoscope damage.”
In general, he continued, laser-tissue interactions depend on the power and the wavelength of the laser as well as the color and the water content of the target tissue. “The power density of the wavelength you choose determines its ability to cut, coagulate, or vaporize the tissue,” he said.
“As the power density increases, the laser fiber approaches the target tissue. Power density is more important than the energy delivered.”
The Nd:YAG (neodymium-doped yttrium aluminium garnet) laser, which causes more destruction in the deep tissue than on the surface, is the most common laser used in interventional airway procedures, he said. Two other commonly used lasers include the KTP (potassium titanyl phosphate) and the CO2. “I like CO2s a lot for upper airway and subglottic problems as well as vocal cord problems,” Dr. Liberman said. “It’s very precise and has low penetration. The Nd:YAG is very good for deep penetration. You need familiarity with these. I don’t think you can just take one of these off the shelf if you’ve never used it before. Sometimes your ENT [ear nose and throat] or urology colleagues can help you, because they’re using a lot more of these lasers than we are.”
Contraindications for laser bronchoscopy include operable lesions. Dr. Liberman said that while he and his associates use lasers in a preoperative setting, “we’re very careful not to damage proximal or distal airway when we know we’re going to do a sleeve resection or pneumonectomy.”
Other contraindications for laser bronchoscopy include patients with a poor short-term prognosis, severe coagulation disorder, extrinsic airway obstruction, tracheoesophageal fistula or T-Med fistula, those with extensive submucosal disease causing obstruction, and those with lesion adjacent to the esophagus or to a major vessel.
Dr. Liberman reported having received research grants from Ethicon, Boston Scientific, Olympus, Covidien, and Baxter.
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING
Research refining radiomic features for lung cancer screening
SAN DIEGO – A series of radiomics-derived imaging features may improve the diagnostic accuracy of low-dose CT lung cancer screening and help predict which nodules are at risk of becoming cancers.
“We are providing pretty compelling evidence that there is some utility in this science,” Matthew Schabath, Ph.D., said at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Radiomics is an emerging field that uses high-throughput extraction to identify hundreds of quantitative features from standard computed tomography (CT) images and mines that data to develop diagnostic, predictive, or prognostic models.
Radiologists first identify a region of interest (ROI) on the CT scan containing either the whole tumor or spatially explicit regions of the tumor called “habitats.” These ROIs are then segmented via computer software before being rendered in three dimensions. Quantitative features are extracted from the rendered volumes and entered into the models, along with other clinical and patient data.
“Right now our tool box is about 219, but by the end of the year we are hoping to have close to 1,000 radiomic features we can extract from a 3-D rendered nodule or tumor,” said Dr. Schabath, of the Moffitt Cancer Center in Tampa, Fla.
Although not without its own challenges, radiomics is a far cry from the current practice that relies on a single CT feature, nodule size, and clinical guidelines to evaluate and follow-up pulmonary nodules, none of which provides clinicians tools to accurately predict the risk or probability of lung cancer development.
CT images are typically thought of as pictures, but in radiomics, “the images are data. That’s really the underlying principle,” he said.
Led by Dr. Robert Gillies, often referred to as the father of radiomics, the researchers extracted and analyzed the 219 radiomic features from nodules in 196 lung cancer cases and in 392 controls who had a positive but benign nodule at the baseline scan and were matched for age, sex, smoking status, and race.
The post hoc, nested case-control study used images and data from the pivotal National Lung Screening Trial, which identified a 20% reduction in lung cancer mortality for low-dose CT screening compared with chest x-rays, but with a 96% false-positive rate, which also highlighted the challenges of LDCT as a screening tool.
Two classes of features were extracted from the images: semantic features, which are commonly used in radiology to describe ROIs, and agnostic features, which are mathematically extracted quantitative descriptors that capture lesion heterogeneity.
Univariable analyses were used to identify statistically significant features (threshold P value less than .05) and a backward elimination process (threshold P less than 0.1) performed to generate the final set of features, Dr. Schabath said.
Separate analyses were performed for predictive and diagnostic features.
In the risk prediction model, eight “highly informative features” were identified, Dr. Schabath said. Five were agnostic and three were semantic – circularity of the nodule, volume, and distance from or pleural attachment.
The receiver operating characteristic (ROC) area under the curve for the model was 0.92, with 75% sensitivity and 89% specificity. When the model included only patient demographics, it was no better than flipping a coin for predicting nodules at risk of becoming cancerous (ROC 0.58), he said.
Six highly informative features were identified in the agnostic model, which extracted features from the nodules found at the first and second follow-up interval, Dr. Schabath said. Three were agnostic and three semantic – longest diameter, volume, and distance from or pleural attachment.
The ROC for the diagnostic model was 0.89, with 74% sensitivity and 89% specificity.
When an additional analysis was performed using a nodule threshold of less than 15 mm to account for nodule growth over time and smaller nodule size at baseline in controls, the ROC and specificity held steady, but sensitivity dropped off to 59%, he said.
“I think we’re showing a rigorous [statistical] approach by identifying really unique, highly informative features,” Dr. Schabath concluded.
The overlap of volume and distance from or pleural attachment in both the diagnostic and predictive models suggests “there might be something very important about these two features,” he added.
Dr. Schabath stressed that the findings are preliminary and said additional analyses will be run before the results are ready for prime time. Long-term goals are to implement radiomic-based decision support tools and models into radiology reading rooms.
“In the future, we envision that all medical images will be converted to mineable data with the process of radiomics as part of standard of care,” Dr. Gillies said in an interview. “Such data have already shown promise to increase the precision and accuracy of diagnostic images, and hence, will increasingly be used in therapy decision support.”
Among the many challenges that first need to be resolved are that images are often captured with settings and filters that can be different even within a single institution. The inconsistency adds noise to the data that are extracted by computers.
“Hence, the most robust data we have today are generated by radiologists themselves, although this has its own challenges of being time-consuming with inter-reader variability,” Dr. Gillies noted.
Another major challenge is sharing of the image data. Right now, radiomics is practiced at only a few research hospitals and thus, building large cohort studies requires that the images be moved across site. In the future, the researchers anticipate that software can be deployed across sites to enable radiomic feature extraction, which would mean that only the extracted data will have to be shared, he said.
SAN DIEGO – A series of radiomics-derived imaging features may improve the diagnostic accuracy of low-dose CT lung cancer screening and help predict which nodules are at risk of becoming cancers.
“We are providing pretty compelling evidence that there is some utility in this science,” Matthew Schabath, Ph.D., said at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Radiomics is an emerging field that uses high-throughput extraction to identify hundreds of quantitative features from standard computed tomography (CT) images and mines that data to develop diagnostic, predictive, or prognostic models.
Radiologists first identify a region of interest (ROI) on the CT scan containing either the whole tumor or spatially explicit regions of the tumor called “habitats.” These ROIs are then segmented via computer software before being rendered in three dimensions. Quantitative features are extracted from the rendered volumes and entered into the models, along with other clinical and patient data.
“Right now our tool box is about 219, but by the end of the year we are hoping to have close to 1,000 radiomic features we can extract from a 3-D rendered nodule or tumor,” said Dr. Schabath, of the Moffitt Cancer Center in Tampa, Fla.
Although not without its own challenges, radiomics is a far cry from the current practice that relies on a single CT feature, nodule size, and clinical guidelines to evaluate and follow-up pulmonary nodules, none of which provides clinicians tools to accurately predict the risk or probability of lung cancer development.
CT images are typically thought of as pictures, but in radiomics, “the images are data. That’s really the underlying principle,” he said.
Led by Dr. Robert Gillies, often referred to as the father of radiomics, the researchers extracted and analyzed the 219 radiomic features from nodules in 196 lung cancer cases and in 392 controls who had a positive but benign nodule at the baseline scan and were matched for age, sex, smoking status, and race.
The post hoc, nested case-control study used images and data from the pivotal National Lung Screening Trial, which identified a 20% reduction in lung cancer mortality for low-dose CT screening compared with chest x-rays, but with a 96% false-positive rate, which also highlighted the challenges of LDCT as a screening tool.
Two classes of features were extracted from the images: semantic features, which are commonly used in radiology to describe ROIs, and agnostic features, which are mathematically extracted quantitative descriptors that capture lesion heterogeneity.
Univariable analyses were used to identify statistically significant features (threshold P value less than .05) and a backward elimination process (threshold P less than 0.1) performed to generate the final set of features, Dr. Schabath said.
Separate analyses were performed for predictive and diagnostic features.
In the risk prediction model, eight “highly informative features” were identified, Dr. Schabath said. Five were agnostic and three were semantic – circularity of the nodule, volume, and distance from or pleural attachment.
The receiver operating characteristic (ROC) area under the curve for the model was 0.92, with 75% sensitivity and 89% specificity. When the model included only patient demographics, it was no better than flipping a coin for predicting nodules at risk of becoming cancerous (ROC 0.58), he said.
Six highly informative features were identified in the agnostic model, which extracted features from the nodules found at the first and second follow-up interval, Dr. Schabath said. Three were agnostic and three semantic – longest diameter, volume, and distance from or pleural attachment.
The ROC for the diagnostic model was 0.89, with 74% sensitivity and 89% specificity.
When an additional analysis was performed using a nodule threshold of less than 15 mm to account for nodule growth over time and smaller nodule size at baseline in controls, the ROC and specificity held steady, but sensitivity dropped off to 59%, he said.
“I think we’re showing a rigorous [statistical] approach by identifying really unique, highly informative features,” Dr. Schabath concluded.
The overlap of volume and distance from or pleural attachment in both the diagnostic and predictive models suggests “there might be something very important about these two features,” he added.
Dr. Schabath stressed that the findings are preliminary and said additional analyses will be run before the results are ready for prime time. Long-term goals are to implement radiomic-based decision support tools and models into radiology reading rooms.
“In the future, we envision that all medical images will be converted to mineable data with the process of radiomics as part of standard of care,” Dr. Gillies said in an interview. “Such data have already shown promise to increase the precision and accuracy of diagnostic images, and hence, will increasingly be used in therapy decision support.”
Among the many challenges that first need to be resolved are that images are often captured with settings and filters that can be different even within a single institution. The inconsistency adds noise to the data that are extracted by computers.
“Hence, the most robust data we have today are generated by radiologists themselves, although this has its own challenges of being time-consuming with inter-reader variability,” Dr. Gillies noted.
Another major challenge is sharing of the image data. Right now, radiomics is practiced at only a few research hospitals and thus, building large cohort studies requires that the images be moved across site. In the future, the researchers anticipate that software can be deployed across sites to enable radiomic feature extraction, which would mean that only the extracted data will have to be shared, he said.
SAN DIEGO – A series of radiomics-derived imaging features may improve the diagnostic accuracy of low-dose CT lung cancer screening and help predict which nodules are at risk of becoming cancers.
“We are providing pretty compelling evidence that there is some utility in this science,” Matthew Schabath, Ph.D., said at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Radiomics is an emerging field that uses high-throughput extraction to identify hundreds of quantitative features from standard computed tomography (CT) images and mines that data to develop diagnostic, predictive, or prognostic models.
Radiologists first identify a region of interest (ROI) on the CT scan containing either the whole tumor or spatially explicit regions of the tumor called “habitats.” These ROIs are then segmented via computer software before being rendered in three dimensions. Quantitative features are extracted from the rendered volumes and entered into the models, along with other clinical and patient data.
“Right now our tool box is about 219, but by the end of the year we are hoping to have close to 1,000 radiomic features we can extract from a 3-D rendered nodule or tumor,” said Dr. Schabath, of the Moffitt Cancer Center in Tampa, Fla.
Although not without its own challenges, radiomics is a far cry from the current practice that relies on a single CT feature, nodule size, and clinical guidelines to evaluate and follow-up pulmonary nodules, none of which provides clinicians tools to accurately predict the risk or probability of lung cancer development.
CT images are typically thought of as pictures, but in radiomics, “the images are data. That’s really the underlying principle,” he said.
Led by Dr. Robert Gillies, often referred to as the father of radiomics, the researchers extracted and analyzed the 219 radiomic features from nodules in 196 lung cancer cases and in 392 controls who had a positive but benign nodule at the baseline scan and were matched for age, sex, smoking status, and race.
The post hoc, nested case-control study used images and data from the pivotal National Lung Screening Trial, which identified a 20% reduction in lung cancer mortality for low-dose CT screening compared with chest x-rays, but with a 96% false-positive rate, which also highlighted the challenges of LDCT as a screening tool.
Two classes of features were extracted from the images: semantic features, which are commonly used in radiology to describe ROIs, and agnostic features, which are mathematically extracted quantitative descriptors that capture lesion heterogeneity.
Univariable analyses were used to identify statistically significant features (threshold P value less than .05) and a backward elimination process (threshold P less than 0.1) performed to generate the final set of features, Dr. Schabath said.
Separate analyses were performed for predictive and diagnostic features.
In the risk prediction model, eight “highly informative features” were identified, Dr. Schabath said. Five were agnostic and three were semantic – circularity of the nodule, volume, and distance from or pleural attachment.
The receiver operating characteristic (ROC) area under the curve for the model was 0.92, with 75% sensitivity and 89% specificity. When the model included only patient demographics, it was no better than flipping a coin for predicting nodules at risk of becoming cancerous (ROC 0.58), he said.
Six highly informative features were identified in the agnostic model, which extracted features from the nodules found at the first and second follow-up interval, Dr. Schabath said. Three were agnostic and three semantic – longest diameter, volume, and distance from or pleural attachment.
The ROC for the diagnostic model was 0.89, with 74% sensitivity and 89% specificity.
When an additional analysis was performed using a nodule threshold of less than 15 mm to account for nodule growth over time and smaller nodule size at baseline in controls, the ROC and specificity held steady, but sensitivity dropped off to 59%, he said.
“I think we’re showing a rigorous [statistical] approach by identifying really unique, highly informative features,” Dr. Schabath concluded.
The overlap of volume and distance from or pleural attachment in both the diagnostic and predictive models suggests “there might be something very important about these two features,” he added.
Dr. Schabath stressed that the findings are preliminary and said additional analyses will be run before the results are ready for prime time. Long-term goals are to implement radiomic-based decision support tools and models into radiology reading rooms.
“In the future, we envision that all medical images will be converted to mineable data with the process of radiomics as part of standard of care,” Dr. Gillies said in an interview. “Such data have already shown promise to increase the precision and accuracy of diagnostic images, and hence, will increasingly be used in therapy decision support.”
Among the many challenges that first need to be resolved are that images are often captured with settings and filters that can be different even within a single institution. The inconsistency adds noise to the data that are extracted by computers.
“Hence, the most robust data we have today are generated by radiologists themselves, although this has its own challenges of being time-consuming with inter-reader variability,” Dr. Gillies noted.
Another major challenge is sharing of the image data. Right now, radiomics is practiced at only a few research hospitals and thus, building large cohort studies requires that the images be moved across site. In the future, the researchers anticipate that software can be deployed across sites to enable radiomic feature extraction, which would mean that only the extracted data will have to be shared, he said.
AT AN AACR/IASLC JOINT CONFERENCE
Key clinical point: Non-invasive radiomics is showing potential for reducing false positives by differentiating between benign and cancerous lung nodules and quantitatively predicting future risk of lung cancer incidence.
Major finding: The receiver operating characteristic area under the curve for the risk prediction model was 0.92, with 75% sensitivity and 89% specificity.
Data source: Post hoc case-control analysis in 588 persons at high risk for lung cancer in the National Lung Screening Trial.
Disclosures: Dr. Schabath reported having no relevant conflicts of interest. Dr. Gillies reported serving as a speaker for HealthMyne.
Presurgery radiation shows benefit in lung cancer
The popularity of extrapleural pneumonectomy to treat asbestos-related thoracic mesothelioma has yielded to extended pleurectomy/decortication in recent years, but a recent study suggests that the extrapleural pneumonectomy procedure can achieve good results in a new protocol that involves administering radiation therapy before surgery as opposed the more conventional approach of radiation after surgery.
Researchers at the University of Toronto reported on their protocol that uses accelerated intensity modulated radiation therapy (IMRT) for malignant pleural mesothelioma (MPM) (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.09.129). They call the protocol SMART, for Surgery for Mesothelioma After Radiation Therapy.
“The rationale to develop this protocol was to optimize the delivery of radiation to the whole tumor bed, sterilize the edges of the tumor to limit the risk of spillage at the time of surgery, develop a shorter treatment plan and potentiate the activation of the immune system by using a hypofractionated regimen,” wrote Dr. Marc de Perrot and colleagues.
The protocol involves delivering 25 Gy of radiation in five daily fractions over a week to the entire side of the thorax with 5 Gy boosts based on imaging, followed by extrapleural pneumonectomy (EPP) 4-6 days later. Patients with three or more positive lymph notes (ypN2 disease) also are offered adjuvant chemotherapy.
The researchers performed the protocol on 62 patients from November 2008 to October 2014, which represents 24% of all patients with MPM seen at the institution in that period. Fifty-two patients were men and ages ranged from 41 to 75 years. Clinical stage of cancer ranged from T1N0 in 10 patients, to T2N0 in 35 and T3N0 in 13 (two had T4N0 and two had T3N2). Forty-five had right-side cancers. Six patients received an extended protocol for various reasons, including tumor extending to the chest wall.
All 62 patients completed IMRT and EPP. All but one had resection and reconstruction of the diaphragm, and all but four had resection and reconstruction of the pericardium.
Overall death rate was 4.8% (three patients). Results were better in patients with epithelioid tumors, with a median survival of 51 months and disease-free survival of 47 months. Those with biphasic subtypes had median survival of 10 months and disease-free survival of 8 months. Eight patients had ipsilateral chest recurrence. “This analysis demonstrates that the SMART approach is particularly encouraging for patients with epithelial subtype,” Dr. de Perrot and coauthors said. They no longer perform the SMART protocol on patients with biphasic subtype.
The protocol was not without complications. Twenty-four patients, about 38%, had serious complications that required intervention or worse. Twelve had atrial fibrillation, but none advanced to life-threatening disease. Among other complications, four had empyema – one resulting in death – and three had pulmonary emboli. One other patient in the complications group died from pneumonia, and another died from a heart attack at home.
This is the Toronto researchers’ second attempt at studying the three-modality approach. In their first attempt, only half the patients who started with preoperative chemotherapy went onto complete the radiation after surgery because of difficulties administering it (J Thorac Cardiovasc Surg. 2007;133:111-6; J Clin Oncol. 2009;27:1413-8). Also, about 25% of patients had disease progression during induction chemotherapy and could not go onto surgery.
They designed the most recent trial to deliver radiation before surgery because of the excellent local control of cancer along with evidence that MPM tumors were radio-sensitive. “Considering the risk of disease progression on induction chemotherapy, we felt that switching the order of therapy was potentially a better option for patients with surgically resectable disease,” Dr. de Perrott and colleagues said.
The researchers cited the study’s single-center nature with a single treatment arm, and the lack of longer-term follow-up, as limitations. “However, in our own experience, this approach has been very encouraging and has become our primary option for patients with surgically resectable MPM,” they noted.
The study authors had no conflicts to disclose.
Implementing the treatment regimen for malignant pleural mesothelioma (MPM) that the Toronto researchers studied poses “several high stakes challenges,” Dr. Valerie Rusch and coauthors at Memorial Sloan-Kettering Cancer Center, New York, said in their invited commentary (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.10.038).
 |
Dr. Valerie W. Rusch |
But they noted challenges involved with conventional multi-modality treatment for MPM, namely the 6 months of intensive treatment. However, the experience of the Toronto researchers will be difficult to replicate, they said. “Such outstanding results reflect the expertise of Dr. de Perrot and colleagues in the surgical care of MPM and the excellence of their multidisciplinary program,” Dr. Rusch and coauthors said.
The study results are among the best reported for MPM to date, they added, but they asked why. “Are they solely related to patient selection or do they reflect the true impact of a novel approach to treatment?”
Patients selected for the treatment need to be able to undergo the extrapleural pneumonectomy (EPP) and the surgeon has to be able to predict the resectability of the tumor. But limitations in existing staging methods for MPM make it difficult to predict tumor resectability. “To avoid bronchial stump leaks and other serious complications after EPP requires experience along with meticulous surgical technique and postoperative care,” Dr. Rusch and colleagues said. “Only high-volume centers of excellence could potentially reproduce these results.”
Despite the waning in popularity of EPP, the study results underscore its effectiveness in carefully selected patients – “those with epithelioid tumor histology and no tumor metastases.” To corroborate the findings, reports on other centers’ experience along with human and animal studies rather than a randomized clinical trial are needed. “Dr. de Perrot and colleagues may have been not only bold but SMART,” Dr. Rusch and colleagues said.
Implementing the treatment regimen for malignant pleural mesothelioma (MPM) that the Toronto researchers studied poses “several high stakes challenges,” Dr. Valerie Rusch and coauthors at Memorial Sloan-Kettering Cancer Center, New York, said in their invited commentary (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.10.038).
 |
Dr. Valerie W. Rusch |
But they noted challenges involved with conventional multi-modality treatment for MPM, namely the 6 months of intensive treatment. However, the experience of the Toronto researchers will be difficult to replicate, they said. “Such outstanding results reflect the expertise of Dr. de Perrot and colleagues in the surgical care of MPM and the excellence of their multidisciplinary program,” Dr. Rusch and coauthors said.
The study results are among the best reported for MPM to date, they added, but they asked why. “Are they solely related to patient selection or do they reflect the true impact of a novel approach to treatment?”
Patients selected for the treatment need to be able to undergo the extrapleural pneumonectomy (EPP) and the surgeon has to be able to predict the resectability of the tumor. But limitations in existing staging methods for MPM make it difficult to predict tumor resectability. “To avoid bronchial stump leaks and other serious complications after EPP requires experience along with meticulous surgical technique and postoperative care,” Dr. Rusch and colleagues said. “Only high-volume centers of excellence could potentially reproduce these results.”
Despite the waning in popularity of EPP, the study results underscore its effectiveness in carefully selected patients – “those with epithelioid tumor histology and no tumor metastases.” To corroborate the findings, reports on other centers’ experience along with human and animal studies rather than a randomized clinical trial are needed. “Dr. de Perrot and colleagues may have been not only bold but SMART,” Dr. Rusch and colleagues said.
Implementing the treatment regimen for malignant pleural mesothelioma (MPM) that the Toronto researchers studied poses “several high stakes challenges,” Dr. Valerie Rusch and coauthors at Memorial Sloan-Kettering Cancer Center, New York, said in their invited commentary (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.10.038).
 |
Dr. Valerie W. Rusch |
But they noted challenges involved with conventional multi-modality treatment for MPM, namely the 6 months of intensive treatment. However, the experience of the Toronto researchers will be difficult to replicate, they said. “Such outstanding results reflect the expertise of Dr. de Perrot and colleagues in the surgical care of MPM and the excellence of their multidisciplinary program,” Dr. Rusch and coauthors said.
The study results are among the best reported for MPM to date, they added, but they asked why. “Are they solely related to patient selection or do they reflect the true impact of a novel approach to treatment?”
Patients selected for the treatment need to be able to undergo the extrapleural pneumonectomy (EPP) and the surgeon has to be able to predict the resectability of the tumor. But limitations in existing staging methods for MPM make it difficult to predict tumor resectability. “To avoid bronchial stump leaks and other serious complications after EPP requires experience along with meticulous surgical technique and postoperative care,” Dr. Rusch and colleagues said. “Only high-volume centers of excellence could potentially reproduce these results.”
Despite the waning in popularity of EPP, the study results underscore its effectiveness in carefully selected patients – “those with epithelioid tumor histology and no tumor metastases.” To corroborate the findings, reports on other centers’ experience along with human and animal studies rather than a randomized clinical trial are needed. “Dr. de Perrot and colleagues may have been not only bold but SMART,” Dr. Rusch and colleagues said.
The popularity of extrapleural pneumonectomy to treat asbestos-related thoracic mesothelioma has yielded to extended pleurectomy/decortication in recent years, but a recent study suggests that the extrapleural pneumonectomy procedure can achieve good results in a new protocol that involves administering radiation therapy before surgery as opposed the more conventional approach of radiation after surgery.
Researchers at the University of Toronto reported on their protocol that uses accelerated intensity modulated radiation therapy (IMRT) for malignant pleural mesothelioma (MPM) (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.09.129). They call the protocol SMART, for Surgery for Mesothelioma After Radiation Therapy.
“The rationale to develop this protocol was to optimize the delivery of radiation to the whole tumor bed, sterilize the edges of the tumor to limit the risk of spillage at the time of surgery, develop a shorter treatment plan and potentiate the activation of the immune system by using a hypofractionated regimen,” wrote Dr. Marc de Perrot and colleagues.
The protocol involves delivering 25 Gy of radiation in five daily fractions over a week to the entire side of the thorax with 5 Gy boosts based on imaging, followed by extrapleural pneumonectomy (EPP) 4-6 days later. Patients with three or more positive lymph notes (ypN2 disease) also are offered adjuvant chemotherapy.
The researchers performed the protocol on 62 patients from November 2008 to October 2014, which represents 24% of all patients with MPM seen at the institution in that period. Fifty-two patients were men and ages ranged from 41 to 75 years. Clinical stage of cancer ranged from T1N0 in 10 patients, to T2N0 in 35 and T3N0 in 13 (two had T4N0 and two had T3N2). Forty-five had right-side cancers. Six patients received an extended protocol for various reasons, including tumor extending to the chest wall.
All 62 patients completed IMRT and EPP. All but one had resection and reconstruction of the diaphragm, and all but four had resection and reconstruction of the pericardium.
Overall death rate was 4.8% (three patients). Results were better in patients with epithelioid tumors, with a median survival of 51 months and disease-free survival of 47 months. Those with biphasic subtypes had median survival of 10 months and disease-free survival of 8 months. Eight patients had ipsilateral chest recurrence. “This analysis demonstrates that the SMART approach is particularly encouraging for patients with epithelial subtype,” Dr. de Perrot and coauthors said. They no longer perform the SMART protocol on patients with biphasic subtype.
The protocol was not without complications. Twenty-four patients, about 38%, had serious complications that required intervention or worse. Twelve had atrial fibrillation, but none advanced to life-threatening disease. Among other complications, four had empyema – one resulting in death – and three had pulmonary emboli. One other patient in the complications group died from pneumonia, and another died from a heart attack at home.
This is the Toronto researchers’ second attempt at studying the three-modality approach. In their first attempt, only half the patients who started with preoperative chemotherapy went onto complete the radiation after surgery because of difficulties administering it (J Thorac Cardiovasc Surg. 2007;133:111-6; J Clin Oncol. 2009;27:1413-8). Also, about 25% of patients had disease progression during induction chemotherapy and could not go onto surgery.
They designed the most recent trial to deliver radiation before surgery because of the excellent local control of cancer along with evidence that MPM tumors were radio-sensitive. “Considering the risk of disease progression on induction chemotherapy, we felt that switching the order of therapy was potentially a better option for patients with surgically resectable disease,” Dr. de Perrott and colleagues said.
The researchers cited the study’s single-center nature with a single treatment arm, and the lack of longer-term follow-up, as limitations. “However, in our own experience, this approach has been very encouraging and has become our primary option for patients with surgically resectable MPM,” they noted.
The study authors had no conflicts to disclose.
The popularity of extrapleural pneumonectomy to treat asbestos-related thoracic mesothelioma has yielded to extended pleurectomy/decortication in recent years, but a recent study suggests that the extrapleural pneumonectomy procedure can achieve good results in a new protocol that involves administering radiation therapy before surgery as opposed the more conventional approach of radiation after surgery.
Researchers at the University of Toronto reported on their protocol that uses accelerated intensity modulated radiation therapy (IMRT) for malignant pleural mesothelioma (MPM) (J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2015.09.129). They call the protocol SMART, for Surgery for Mesothelioma After Radiation Therapy.
“The rationale to develop this protocol was to optimize the delivery of radiation to the whole tumor bed, sterilize the edges of the tumor to limit the risk of spillage at the time of surgery, develop a shorter treatment plan and potentiate the activation of the immune system by using a hypofractionated regimen,” wrote Dr. Marc de Perrot and colleagues.
The protocol involves delivering 25 Gy of radiation in five daily fractions over a week to the entire side of the thorax with 5 Gy boosts based on imaging, followed by extrapleural pneumonectomy (EPP) 4-6 days later. Patients with three or more positive lymph notes (ypN2 disease) also are offered adjuvant chemotherapy.
The researchers performed the protocol on 62 patients from November 2008 to October 2014, which represents 24% of all patients with MPM seen at the institution in that period. Fifty-two patients were men and ages ranged from 41 to 75 years. Clinical stage of cancer ranged from T1N0 in 10 patients, to T2N0 in 35 and T3N0 in 13 (two had T4N0 and two had T3N2). Forty-five had right-side cancers. Six patients received an extended protocol for various reasons, including tumor extending to the chest wall.
All 62 patients completed IMRT and EPP. All but one had resection and reconstruction of the diaphragm, and all but four had resection and reconstruction of the pericardium.
Overall death rate was 4.8% (three patients). Results were better in patients with epithelioid tumors, with a median survival of 51 months and disease-free survival of 47 months. Those with biphasic subtypes had median survival of 10 months and disease-free survival of 8 months. Eight patients had ipsilateral chest recurrence. “This analysis demonstrates that the SMART approach is particularly encouraging for patients with epithelial subtype,” Dr. de Perrot and coauthors said. They no longer perform the SMART protocol on patients with biphasic subtype.
The protocol was not without complications. Twenty-four patients, about 38%, had serious complications that required intervention or worse. Twelve had atrial fibrillation, but none advanced to life-threatening disease. Among other complications, four had empyema – one resulting in death – and three had pulmonary emboli. One other patient in the complications group died from pneumonia, and another died from a heart attack at home.
This is the Toronto researchers’ second attempt at studying the three-modality approach. In their first attempt, only half the patients who started with preoperative chemotherapy went onto complete the radiation after surgery because of difficulties administering it (J Thorac Cardiovasc Surg. 2007;133:111-6; J Clin Oncol. 2009;27:1413-8). Also, about 25% of patients had disease progression during induction chemotherapy and could not go onto surgery.
They designed the most recent trial to deliver radiation before surgery because of the excellent local control of cancer along with evidence that MPM tumors were radio-sensitive. “Considering the risk of disease progression on induction chemotherapy, we felt that switching the order of therapy was potentially a better option for patients with surgically resectable disease,” Dr. de Perrott and colleagues said.
The researchers cited the study’s single-center nature with a single treatment arm, and the lack of longer-term follow-up, as limitations. “However, in our own experience, this approach has been very encouraging and has become our primary option for patients with surgically resectable MPM,” they noted.
The study authors had no conflicts to disclose.
Key clinical point: A new protocol that involves accelerated hemithoracic intensity modulated radiation before surgery for lung mesothelioma delivered encouraging results in patients with epithelioid tumors.
Major finding: Disease-free survival was 47 months in epithelial subtypes compared with 8 months in biphasic subtypes.
Data source: A single-center population of 62 patients with malignant pleural mesothelioma treated between November 2008 and October 2014.
Disclosures: The study authors had no relationships to disclose.
Deciphering lung cancer biomarkers shrouded in tobacco smoke
SAN DIEGO – Tobacco researchers are getting one step closer to identifying young smokers particularly susceptible to the carcinogenic effects of smoking, by using large-scale epidemiology studies and DNA adducts data.
“This is not lung cancer screening; this is not early detection of lung cancer; this is detection of susceptible, high-risk individuals, so they can be targeted for cessation, surveillance, and prevention,” said Stephen S. Hecht, Ph.D., professor of cancer prevention at the University of Minnesota Masonic Cancer Center in Minneapolis.
While nicotine itself is not a carcinogen, each puff of tobacco smoke delivers a mixture of more than 70 established carcinogens, including the potent lung-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Some of these carcinogens are excreted, but many will undergo metabolic activation and interact with a patient’s DNA.
The product of this interaction, called DNA adducts, causes persistent miscoding during DNA replication, leading to the thousands of mutations we see in lung cancer, he explained at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
The biomarkers of tobacco exposure and metabolism are very well developed, with blood and urinary biomarker panels applied in many studies and validated by mass spectrometry.
Using the Shanghai cohort study, the researchers identified three urinary biomarkers – PheT (r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene), total NNAL (a metabolite of NNK and its glucuronides), and total cotinine (a surrogate for nicotine that includes cotinine and its glucuronides) – that were significantly associated with lung cancer in 950 smokers, even after adjustment for smoking duration and intensity (Cancer Res. 2011;71:6749-57).
A more recent, deeper dive of 735 of those urinary samples, still stable about 20 years after they were taken, looked specifically at polymorphisms in cytochrome P450 2A6 (CYP2A6), the primary enzyme responsible for the oxidation of nicotine and cotinine.
Data currently in press show that CYP2A6 poor metabolizers had a lower risk of lung cancer than the combined group of normal, intermediate, or slow metabolizers (odds ratio, 0.64; P value for trend = .034).
“This is logical because poor metabolizers of nicotine have more unchanged nicotine on board, so they don’t need to smoke as intensely in order to get more nicotine because that nicotine is remaining to a greater extent in its natural form, rather than being metabolized into cotinine, which is not addictive,” Dr. Hecht said.
Recent genomewide-association studies by Dr. Hecht and his colleagues of smokers in the Multiethnic Cohort Study revealed that Japanese Americans had the highest number of low nicotine CYP2A6 metabolizing genotypes of the five ethnic groups analyzed, consistent with their low lung cancer risk.
Urinary biomarker studies showed that levels of total NNAL, 3-hydroxy phenanthrene, a biomarker of polycyclic aromatic hydrocarbon uptake, and S-phenylmercapturic acid (SPMA), a biomarker of benzene exposure, were also significantly higher among African Americans than whites and significantly lower in Japanese Americans than whites, he said.
The findings are consistent with the initial study results published a decade ago (N Engl J Med. 2006;354:333-342), showing that at low levels of smoking (10 cigarettes per day), Japanese Americans and Hispanics had one-third the risk of lung cancer of African Americans or Native Hawaiians (P less than .001). The differences disappeared at higher levels of smoking (30 cigarettes per day).
Results from the new analyses for Native Hawaiians and Hispanics, however, did not fit this pattern, and “we’re not sure what’s going on there and need to do more work,” Dr. Hecht said.
Nicotine equivalent levels in Native Hawaiians were actually lower than would be expected based on their high lung cancer risk, while Hispanics had higher levels than would be expected based on their low risk.
Interestingly, urinary levels of 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of acrolein, were found to be unusually high in Native Hawaiians and relatively low in Hispanics, “which may play into their lung cancer risk because acrolein is a very strong toxicant and there is evidence to suggest it is involved in lung cancer,” he said.
While tobacco exposure and metabolism biomarkers have hit their stride, less well developed are the DNA adducts and repair biomarkers that can predict lung cancer susceptibility. This is a critical step because of the role DNA adducts play in the formation of lung cancer mutations, Dr. Hecht said.
A smoker may have a high tobacco exposure as indicated by the urinary biomarkers, but have a low metabolism to form DNA adducts or high DNA repair, which would mean their DNA adduct levels would be low. Conversely, another smoker with low exposure and poor DNA repair may have higher DNA adduct levels, and thus, a greater risk for developing lung cancer.
In addition, the exposure biomarkers are not entirely predictive and thus, will need to be combined with multiple DNA adducts and repair if susceptible smokers are to be identified and targeted for state-of-the art cessation approaches and surveillance, he said.
Using high-level mass spectrometry, the researchers have been able to readily measure formaldehyde DNA adducts and tobacco-specific 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)–releasing DNA adducts in human oral cells. Levels of the HPB adduct were unusually high at 12-45 pmol/mg, which is similar to what is seen in animals when exposed to NNK at a much higher dose than smokers take in.
“So this is a real lead,” said Dr. Hecht, who reported having no relevant conflicts of interest.
SAN DIEGO – Tobacco researchers are getting one step closer to identifying young smokers particularly susceptible to the carcinogenic effects of smoking, by using large-scale epidemiology studies and DNA adducts data.
“This is not lung cancer screening; this is not early detection of lung cancer; this is detection of susceptible, high-risk individuals, so they can be targeted for cessation, surveillance, and prevention,” said Stephen S. Hecht, Ph.D., professor of cancer prevention at the University of Minnesota Masonic Cancer Center in Minneapolis.
While nicotine itself is not a carcinogen, each puff of tobacco smoke delivers a mixture of more than 70 established carcinogens, including the potent lung-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Some of these carcinogens are excreted, but many will undergo metabolic activation and interact with a patient’s DNA.
The product of this interaction, called DNA adducts, causes persistent miscoding during DNA replication, leading to the thousands of mutations we see in lung cancer, he explained at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
The biomarkers of tobacco exposure and metabolism are very well developed, with blood and urinary biomarker panels applied in many studies and validated by mass spectrometry.
Using the Shanghai cohort study, the researchers identified three urinary biomarkers – PheT (r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene), total NNAL (a metabolite of NNK and its glucuronides), and total cotinine (a surrogate for nicotine that includes cotinine and its glucuronides) – that were significantly associated with lung cancer in 950 smokers, even after adjustment for smoking duration and intensity (Cancer Res. 2011;71:6749-57).
A more recent, deeper dive of 735 of those urinary samples, still stable about 20 years after they were taken, looked specifically at polymorphisms in cytochrome P450 2A6 (CYP2A6), the primary enzyme responsible for the oxidation of nicotine and cotinine.
Data currently in press show that CYP2A6 poor metabolizers had a lower risk of lung cancer than the combined group of normal, intermediate, or slow metabolizers (odds ratio, 0.64; P value for trend = .034).
“This is logical because poor metabolizers of nicotine have more unchanged nicotine on board, so they don’t need to smoke as intensely in order to get more nicotine because that nicotine is remaining to a greater extent in its natural form, rather than being metabolized into cotinine, which is not addictive,” Dr. Hecht said.
Recent genomewide-association studies by Dr. Hecht and his colleagues of smokers in the Multiethnic Cohort Study revealed that Japanese Americans had the highest number of low nicotine CYP2A6 metabolizing genotypes of the five ethnic groups analyzed, consistent with their low lung cancer risk.
Urinary biomarker studies showed that levels of total NNAL, 3-hydroxy phenanthrene, a biomarker of polycyclic aromatic hydrocarbon uptake, and S-phenylmercapturic acid (SPMA), a biomarker of benzene exposure, were also significantly higher among African Americans than whites and significantly lower in Japanese Americans than whites, he said.
The findings are consistent with the initial study results published a decade ago (N Engl J Med. 2006;354:333-342), showing that at low levels of smoking (10 cigarettes per day), Japanese Americans and Hispanics had one-third the risk of lung cancer of African Americans or Native Hawaiians (P less than .001). The differences disappeared at higher levels of smoking (30 cigarettes per day).
Results from the new analyses for Native Hawaiians and Hispanics, however, did not fit this pattern, and “we’re not sure what’s going on there and need to do more work,” Dr. Hecht said.
Nicotine equivalent levels in Native Hawaiians were actually lower than would be expected based on their high lung cancer risk, while Hispanics had higher levels than would be expected based on their low risk.
Interestingly, urinary levels of 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of acrolein, were found to be unusually high in Native Hawaiians and relatively low in Hispanics, “which may play into their lung cancer risk because acrolein is a very strong toxicant and there is evidence to suggest it is involved in lung cancer,” he said.
While tobacco exposure and metabolism biomarkers have hit their stride, less well developed are the DNA adducts and repair biomarkers that can predict lung cancer susceptibility. This is a critical step because of the role DNA adducts play in the formation of lung cancer mutations, Dr. Hecht said.
A smoker may have a high tobacco exposure as indicated by the urinary biomarkers, but have a low metabolism to form DNA adducts or high DNA repair, which would mean their DNA adduct levels would be low. Conversely, another smoker with low exposure and poor DNA repair may have higher DNA adduct levels, and thus, a greater risk for developing lung cancer.
In addition, the exposure biomarkers are not entirely predictive and thus, will need to be combined with multiple DNA adducts and repair if susceptible smokers are to be identified and targeted for state-of-the art cessation approaches and surveillance, he said.
Using high-level mass spectrometry, the researchers have been able to readily measure formaldehyde DNA adducts and tobacco-specific 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)–releasing DNA adducts in human oral cells. Levels of the HPB adduct were unusually high at 12-45 pmol/mg, which is similar to what is seen in animals when exposed to NNK at a much higher dose than smokers take in.
“So this is a real lead,” said Dr. Hecht, who reported having no relevant conflicts of interest.
SAN DIEGO – Tobacco researchers are getting one step closer to identifying young smokers particularly susceptible to the carcinogenic effects of smoking, by using large-scale epidemiology studies and DNA adducts data.
“This is not lung cancer screening; this is not early detection of lung cancer; this is detection of susceptible, high-risk individuals, so they can be targeted for cessation, surveillance, and prevention,” said Stephen S. Hecht, Ph.D., professor of cancer prevention at the University of Minnesota Masonic Cancer Center in Minneapolis.
While nicotine itself is not a carcinogen, each puff of tobacco smoke delivers a mixture of more than 70 established carcinogens, including the potent lung-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Some of these carcinogens are excreted, but many will undergo metabolic activation and interact with a patient’s DNA.
The product of this interaction, called DNA adducts, causes persistent miscoding during DNA replication, leading to the thousands of mutations we see in lung cancer, he explained at a conference on lung cancer translational science sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
The biomarkers of tobacco exposure and metabolism are very well developed, with blood and urinary biomarker panels applied in many studies and validated by mass spectrometry.
Using the Shanghai cohort study, the researchers identified three urinary biomarkers – PheT (r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene), total NNAL (a metabolite of NNK and its glucuronides), and total cotinine (a surrogate for nicotine that includes cotinine and its glucuronides) – that were significantly associated with lung cancer in 950 smokers, even after adjustment for smoking duration and intensity (Cancer Res. 2011;71:6749-57).
A more recent, deeper dive of 735 of those urinary samples, still stable about 20 years after they were taken, looked specifically at polymorphisms in cytochrome P450 2A6 (CYP2A6), the primary enzyme responsible for the oxidation of nicotine and cotinine.
Data currently in press show that CYP2A6 poor metabolizers had a lower risk of lung cancer than the combined group of normal, intermediate, or slow metabolizers (odds ratio, 0.64; P value for trend = .034).
“This is logical because poor metabolizers of nicotine have more unchanged nicotine on board, so they don’t need to smoke as intensely in order to get more nicotine because that nicotine is remaining to a greater extent in its natural form, rather than being metabolized into cotinine, which is not addictive,” Dr. Hecht said.
Recent genomewide-association studies by Dr. Hecht and his colleagues of smokers in the Multiethnic Cohort Study revealed that Japanese Americans had the highest number of low nicotine CYP2A6 metabolizing genotypes of the five ethnic groups analyzed, consistent with their low lung cancer risk.
Urinary biomarker studies showed that levels of total NNAL, 3-hydroxy phenanthrene, a biomarker of polycyclic aromatic hydrocarbon uptake, and S-phenylmercapturic acid (SPMA), a biomarker of benzene exposure, were also significantly higher among African Americans than whites and significantly lower in Japanese Americans than whites, he said.
The findings are consistent with the initial study results published a decade ago (N Engl J Med. 2006;354:333-342), showing that at low levels of smoking (10 cigarettes per day), Japanese Americans and Hispanics had one-third the risk of lung cancer of African Americans or Native Hawaiians (P less than .001). The differences disappeared at higher levels of smoking (30 cigarettes per day).
Results from the new analyses for Native Hawaiians and Hispanics, however, did not fit this pattern, and “we’re not sure what’s going on there and need to do more work,” Dr. Hecht said.
Nicotine equivalent levels in Native Hawaiians were actually lower than would be expected based on their high lung cancer risk, while Hispanics had higher levels than would be expected based on their low risk.
Interestingly, urinary levels of 3-hydroxypropylmercapturic acid (3-HPMA), a metabolite of acrolein, were found to be unusually high in Native Hawaiians and relatively low in Hispanics, “which may play into their lung cancer risk because acrolein is a very strong toxicant and there is evidence to suggest it is involved in lung cancer,” he said.
While tobacco exposure and metabolism biomarkers have hit their stride, less well developed are the DNA adducts and repair biomarkers that can predict lung cancer susceptibility. This is a critical step because of the role DNA adducts play in the formation of lung cancer mutations, Dr. Hecht said.
A smoker may have a high tobacco exposure as indicated by the urinary biomarkers, but have a low metabolism to form DNA adducts or high DNA repair, which would mean their DNA adduct levels would be low. Conversely, another smoker with low exposure and poor DNA repair may have higher DNA adduct levels, and thus, a greater risk for developing lung cancer.
In addition, the exposure biomarkers are not entirely predictive and thus, will need to be combined with multiple DNA adducts and repair if susceptible smokers are to be identified and targeted for state-of-the art cessation approaches and surveillance, he said.
Using high-level mass spectrometry, the researchers have been able to readily measure formaldehyde DNA adducts and tobacco-specific 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)–releasing DNA adducts in human oral cells. Levels of the HPB adduct were unusually high at 12-45 pmol/mg, which is similar to what is seen in animals when exposed to NNK at a much higher dose than smokers take in.
“So this is a real lead,” said Dr. Hecht, who reported having no relevant conflicts of interest.
AT AN AACR/IASLC JOINT CONFERENCE
Families perceive few benefits from aggressive end-of-life care
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Bereaved families were substantially more satisfied with end-of-life cancer care when patients did not die in hospital, received more than 3 days of hospice care, and did not enter the ICU within 30 days of dying, according to a multicenter, prospective study published online Jan. 19 in JAMA.
The analysis is one of the first of its type to assess these end-of-life care indicators, said Dr. Alexi Wright of Harvard Medical School, Boston, and her associates. The findings could affect health policy as electronic health records expand under the Health Information Technology for Economic and Clinical Health Act, they said.
End-of-life cancer care has become increasingly aggressive, belying evidence that this approach does not improve patient outcomes, quality of life, or caregiver bereavement. To explore alternatives, the researchers analyzed 1,146 interviews of family members of Medicare patients who died of lung or colorectal cancer by 2011. Their data source was the multiregional, prospective, observational Cancer Care Outcomes Research and Surveillance (CanCORS) study (JAMA 2016;315:284-92).
Family members described end-of-life care as “excellent” 59% of the time when hospice care lasted more 3 days, but 43% of the time otherwise (95% confidence interval for adjusted difference, 11% to 22%). Notably, 73% of patients who received more than 3 days of hospice care died in their preferred location, compared with 40% of patients who received less or no hospice care. Care was rated as excellent 52% of the time when ICU admission was avoided within 30 days of death, and 57% of the time when patients died outside the hospital, compared with 45% and 42% of the time otherwise.
The results support “advance care planning consistent with the preferences of patients,” said the investigators. They recommended more extensive counseling of cancer patients and families, earlier palliative care referrals, and an audit and feedback system to monitor the use of aggressive end-of-life care.
The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the study. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum, also of Harvard Medical School, reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
FROM JAMA
Key clinical point: Bereaved family members were more satisfied with end-of-life cancer care when patients spent more than 3 days in hospice, died outside the hospital, and were not admitted to the ICU within 30 days of dying.
Major finding: Care was described as “excellent” about 9%-17% more often when these end-of-life quality indicators were met.
Data source: A multicenter, prospective, observational study of 1,146 family members of patients who died of lung or colorectal cancer.
Disclosures: The National Cancer Institute and the Cancer Care Outcomes Research and Surveillance Consortium funded the analysis. One coinvestigator reported financial relationships with the American Academy of Hospice and Palliative Medicine, National Institute of Nursing Research, National Institute on Aging, Retirement Research Retirement Foundation, California Healthcare Foundation, Commonwealth Fund, West Health Institute, University of Wisconsin, and UpToDate.com. Senior author Dr. Mary Landrum reported grant funding from Pfizer and personal fees from McKinsey and Company and Greylock McKinnon Associates. The other authors had no disclosures.
Adjuvant therapy extends survival in early SCLC
Surgical resection followed by adjuvant chemotherapy, with or without prophylactic cranial irradiation, extends overall survival beyond that achieved with surgery alone in early-stage small-cell lung cancer, according to a report published online Jan. 18 in Journal of Clinical Oncology.
National Comprehensive Cancer Network guidelines recommend surgery with adjuvant chemotherapy for patients with stage I SCLC, and also recommend prophylactic cranial irradiation. But these recommendations are based on “limited” data, and the roles of both adjuvant chemotherapy and irradiation for this patient population are “not yet well characterized,” said Dr. Chi-Fu Jeffrey Yang of Duke University Medical Center, Durham, N.C., and his associates.
In what they described as the first population-based study to assess adjuvant therapy in such patients, the investigators analyzed information in the National Cancer Database concerning 954 patients treated across the country during 2003-2011. A total of 566 patients (59.3%) received adjuvant chemotherapy and the remaining 388 did not. A total of 190 patients who received adjuvant chemotherapy also had prophylactic radiotherapy to the brain (99 patients), the lung (87 patients), or to an unrecorded site (4 patients).
The median follow-up for the entire cohort was 43 months. Overall median survival time was 55.6 months, and 5-year survival was 47.4%.
Compared with no adjuvant therapy, treatment with adjuvant chemotherapy, with or without radiotherapy, significantly extended both overall survival (66.0 months vs 42.1 months) and the 5-year overall survival rate (52.7% vs 40.4%) in the primary analysis of the study. After the data were adjusted to account for multiple confounding variables, the use of adjuvant chemotherapy alone and of adjuvant chemotherapy plus radiotherapy both were associated with improved survival, Dr. Yang and his associates said (J Clin Oncol. 2016 Jan. 18. doi:10.1200/jco.2015.63.8171).
In contrast, adjuvant chemotherapy with thoracic irradiation and thoracic irradiation alone were not associated with improved survival. However, it is possible that this study was affected by selection bias in that patients who received both chemotherapy and cranial irradiation may have been healthier than those who did not. The researchers attempted to control for possible bias in the multivariable analysis, but further assessment in prospective randomized clinical trials is warranted, they added.
Surgical resection followed by adjuvant chemotherapy, with or without prophylactic cranial irradiation, extends overall survival beyond that achieved with surgery alone in early-stage small-cell lung cancer, according to a report published online Jan. 18 in Journal of Clinical Oncology.
National Comprehensive Cancer Network guidelines recommend surgery with adjuvant chemotherapy for patients with stage I SCLC, and also recommend prophylactic cranial irradiation. But these recommendations are based on “limited” data, and the roles of both adjuvant chemotherapy and irradiation for this patient population are “not yet well characterized,” said Dr. Chi-Fu Jeffrey Yang of Duke University Medical Center, Durham, N.C., and his associates.
In what they described as the first population-based study to assess adjuvant therapy in such patients, the investigators analyzed information in the National Cancer Database concerning 954 patients treated across the country during 2003-2011. A total of 566 patients (59.3%) received adjuvant chemotherapy and the remaining 388 did not. A total of 190 patients who received adjuvant chemotherapy also had prophylactic radiotherapy to the brain (99 patients), the lung (87 patients), or to an unrecorded site (4 patients).
The median follow-up for the entire cohort was 43 months. Overall median survival time was 55.6 months, and 5-year survival was 47.4%.
Compared with no adjuvant therapy, treatment with adjuvant chemotherapy, with or without radiotherapy, significantly extended both overall survival (66.0 months vs 42.1 months) and the 5-year overall survival rate (52.7% vs 40.4%) in the primary analysis of the study. After the data were adjusted to account for multiple confounding variables, the use of adjuvant chemotherapy alone and of adjuvant chemotherapy plus radiotherapy both were associated with improved survival, Dr. Yang and his associates said (J Clin Oncol. 2016 Jan. 18. doi:10.1200/jco.2015.63.8171).
In contrast, adjuvant chemotherapy with thoracic irradiation and thoracic irradiation alone were not associated with improved survival. However, it is possible that this study was affected by selection bias in that patients who received both chemotherapy and cranial irradiation may have been healthier than those who did not. The researchers attempted to control for possible bias in the multivariable analysis, but further assessment in prospective randomized clinical trials is warranted, they added.
Surgical resection followed by adjuvant chemotherapy, with or without prophylactic cranial irradiation, extends overall survival beyond that achieved with surgery alone in early-stage small-cell lung cancer, according to a report published online Jan. 18 in Journal of Clinical Oncology.
National Comprehensive Cancer Network guidelines recommend surgery with adjuvant chemotherapy for patients with stage I SCLC, and also recommend prophylactic cranial irradiation. But these recommendations are based on “limited” data, and the roles of both adjuvant chemotherapy and irradiation for this patient population are “not yet well characterized,” said Dr. Chi-Fu Jeffrey Yang of Duke University Medical Center, Durham, N.C., and his associates.
In what they described as the first population-based study to assess adjuvant therapy in such patients, the investigators analyzed information in the National Cancer Database concerning 954 patients treated across the country during 2003-2011. A total of 566 patients (59.3%) received adjuvant chemotherapy and the remaining 388 did not. A total of 190 patients who received adjuvant chemotherapy also had prophylactic radiotherapy to the brain (99 patients), the lung (87 patients), or to an unrecorded site (4 patients).
The median follow-up for the entire cohort was 43 months. Overall median survival time was 55.6 months, and 5-year survival was 47.4%.
Compared with no adjuvant therapy, treatment with adjuvant chemotherapy, with or without radiotherapy, significantly extended both overall survival (66.0 months vs 42.1 months) and the 5-year overall survival rate (52.7% vs 40.4%) in the primary analysis of the study. After the data were adjusted to account for multiple confounding variables, the use of adjuvant chemotherapy alone and of adjuvant chemotherapy plus radiotherapy both were associated with improved survival, Dr. Yang and his associates said (J Clin Oncol. 2016 Jan. 18. doi:10.1200/jco.2015.63.8171).
In contrast, adjuvant chemotherapy with thoracic irradiation and thoracic irradiation alone were not associated with improved survival. However, it is possible that this study was affected by selection bias in that patients who received both chemotherapy and cranial irradiation may have been healthier than those who did not. The researchers attempted to control for possible bias in the multivariable analysis, but further assessment in prospective randomized clinical trials is warranted, they added.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Surgical resection followed by adjuvant chemotherapy, with or without prophylactic cranial irradiation, extends overall survival in early-stage small-cell lung cancer.
Major finding: Compared with no adjuvant therapy, treatment with adjuvant chemotherapy, with or without radiotherapy, significantly extended overall survival (66.0 months vs 42.1 months).
Data source: A retrospective analysis of information in the National Cancer Database concerning 954 patients treated during 2003-2011 and followed up for a median of 43 months.
Disclosures: The National Institutes of Health Cardiothoracic Surgical Trials Network and the American College of Surgeons Resident Research Scholarship supported the study. Dr. Yang had no financial relationships to disclose.