User login
Late risks of breast cancer RT are higher for smokers
SAN ANTONIO – The late side effects of modern radiation therapy for breast cancer depend in part on a woman’s smoking status, suggests a meta-analysis of data from more than 40,000 women presented at the San Antonio Breast Cancer Symposium.
For nonsmokers, radiation therapy had little impact on the absolute risks of lung cancer or cardiac death, the main risks identified, which combined totaled less than 1%, Dr. Carolyn Taylor reported on behalf of the Early Breast Cancer Trialists’ Collaborative Group. But for women who had smoked throughout their adult life and continued to do so during and after treatment, it increased that absolute risk to roughly 2%.
“Smoking status can determine the net long-term effects of breast cancer radiotherapy on mortality. Stopping smoking at the time of radiotherapy may avoid much of the risk, and that’s because most of the risk of lung cancer starts more than 10 years after radiotherapy,” said Dr. Taylor, a radiation oncologist at the University of Oxford (England).
Radiation therapy remains an important tool in treating breast cancer, ultimately reducing the likelihood of death from the disease, she reminded symposium attendees. “The absolute benefit in women treated according to current guidelines is a few percent. Let’s remember the magnitude of that benefit as we think about the risks of radiotherapy.”
Attendee Dr. Steven Vogl of Montefiore Medical Center, New York, asked whether information was available on the location of the lung cancers that occurred in the trials.
“We didn’t have location of lung cancers. We didn’t even know if it was ipsilateral or contralateral to the previous breast cancer in this study,” Dr. Taylor replied. “But we’ve done other studies where we have known the location of the lung cancer, and there were similar findings in those studies.”
“In the last 4 years, we’ve had very good information that annual CT screening substantially and very quickly reduces the mortality from lung cancer,” Dr. Vogl added as a comment. “Any of us who care for patients who have been radiated where, really, any lung has been treated, who continue to smoke, should be screened – and screened and screened and screened again,” he recommended.
The investigators analyzed data from 40,781 women with breast cancer from 75 randomized trials conducted worldwide that compared outcomes with versus without radiation therapy. The median year of trial entry was 1983. On average, women in the trials received 10 Gy to both lungs combined and 6 Gy to the heart.
Comparing women who did and did not receive radiation therapy, the rate ratio for lung cancer was 2.10 at 10 or more years out, and the rate ratio for cardiac mortality was 1.30 overall. Given the mean radiation doses in the trials, the excess risk translated to 12% per Gray for lung cancer and 4% per Gray for cardiac mortality. “These rate ratios are likely to apply today,” Dr. Taylor maintained.
However, she noted, contemporary breast cancer radiation therapy techniques are much better at sparing normal tissues. To derive absolute risk estimates that are relevant today, she and her colleagues reviewed the literature for 2010-2015 and determined that women now receive an average of 5 Gy to both lungs combined and 4 Gy to the heart, with some centers achieving even lower values.
Among nonsmokers, the estimated cumulative 30-year risk of lung cancer was 0.5% for women who did not receive radiation therapy and 0.8% for those who received radiation therapy with a mean dose of 5 Gy to both lungs combined, Dr. Taylor reported. However, among long-term smokers, it was 9.4% without radiation and a substantially higher 13.8% with it.
Similarly, among nonsmokers, the estimated cumulative 30-year risk of ischemic heart disease death was 1.8% for women who did not receive radiation therapy and 2.0% for women who received radiation therapy with a mean dose of 2 Gy to the heart. Among long-term smokers, it was 8.0% without radiation and a slightly higher 8.6% with it.
Additional analyses looking at other late side effects showed no radiation therapy–related excess risk of sarcomas, according to Dr. Taylor. The risk of leukemia was increased with radiation, but actual numbers of cases were very small, she cautioned.
Attendee Dr. Pamela Goodwin, University of Toronto, said, “I’m just wondering whether you considered if it was valid to assume that there was a linear relationship between radiation dose and the risk of lung cancer in the range of radiation doses that you looked at, so, from the higher range in the earlier studies to the much lower dose now.”
Numbers of heart disease events were sufficient to establish a linear relationship, according to Dr. Taylor. Numbers of lung cancers were not, but case-control studies in the literature with adequate numbers have identified a linear relationship there, too. “We use what we can, and we have got now several hundred events, if you combine all of the literature together. And they do suggest the dose-response relationship is linear, but we can’t know that for certain,” she said.
SAN ANTONIO – The late side effects of modern radiation therapy for breast cancer depend in part on a woman’s smoking status, suggests a meta-analysis of data from more than 40,000 women presented at the San Antonio Breast Cancer Symposium.
For nonsmokers, radiation therapy had little impact on the absolute risks of lung cancer or cardiac death, the main risks identified, which combined totaled less than 1%, Dr. Carolyn Taylor reported on behalf of the Early Breast Cancer Trialists’ Collaborative Group. But for women who had smoked throughout their adult life and continued to do so during and after treatment, it increased that absolute risk to roughly 2%.
“Smoking status can determine the net long-term effects of breast cancer radiotherapy on mortality. Stopping smoking at the time of radiotherapy may avoid much of the risk, and that’s because most of the risk of lung cancer starts more than 10 years after radiotherapy,” said Dr. Taylor, a radiation oncologist at the University of Oxford (England).
Radiation therapy remains an important tool in treating breast cancer, ultimately reducing the likelihood of death from the disease, she reminded symposium attendees. “The absolute benefit in women treated according to current guidelines is a few percent. Let’s remember the magnitude of that benefit as we think about the risks of radiotherapy.”
Attendee Dr. Steven Vogl of Montefiore Medical Center, New York, asked whether information was available on the location of the lung cancers that occurred in the trials.
“We didn’t have location of lung cancers. We didn’t even know if it was ipsilateral or contralateral to the previous breast cancer in this study,” Dr. Taylor replied. “But we’ve done other studies where we have known the location of the lung cancer, and there were similar findings in those studies.”
“In the last 4 years, we’ve had very good information that annual CT screening substantially and very quickly reduces the mortality from lung cancer,” Dr. Vogl added as a comment. “Any of us who care for patients who have been radiated where, really, any lung has been treated, who continue to smoke, should be screened – and screened and screened and screened again,” he recommended.
The investigators analyzed data from 40,781 women with breast cancer from 75 randomized trials conducted worldwide that compared outcomes with versus without radiation therapy. The median year of trial entry was 1983. On average, women in the trials received 10 Gy to both lungs combined and 6 Gy to the heart.
Comparing women who did and did not receive radiation therapy, the rate ratio for lung cancer was 2.10 at 10 or more years out, and the rate ratio for cardiac mortality was 1.30 overall. Given the mean radiation doses in the trials, the excess risk translated to 12% per Gray for lung cancer and 4% per Gray for cardiac mortality. “These rate ratios are likely to apply today,” Dr. Taylor maintained.
However, she noted, contemporary breast cancer radiation therapy techniques are much better at sparing normal tissues. To derive absolute risk estimates that are relevant today, she and her colleagues reviewed the literature for 2010-2015 and determined that women now receive an average of 5 Gy to both lungs combined and 4 Gy to the heart, with some centers achieving even lower values.
Among nonsmokers, the estimated cumulative 30-year risk of lung cancer was 0.5% for women who did not receive radiation therapy and 0.8% for those who received radiation therapy with a mean dose of 5 Gy to both lungs combined, Dr. Taylor reported. However, among long-term smokers, it was 9.4% without radiation and a substantially higher 13.8% with it.
Similarly, among nonsmokers, the estimated cumulative 30-year risk of ischemic heart disease death was 1.8% for women who did not receive radiation therapy and 2.0% for women who received radiation therapy with a mean dose of 2 Gy to the heart. Among long-term smokers, it was 8.0% without radiation and a slightly higher 8.6% with it.
Additional analyses looking at other late side effects showed no radiation therapy–related excess risk of sarcomas, according to Dr. Taylor. The risk of leukemia was increased with radiation, but actual numbers of cases were very small, she cautioned.
Attendee Dr. Pamela Goodwin, University of Toronto, said, “I’m just wondering whether you considered if it was valid to assume that there was a linear relationship between radiation dose and the risk of lung cancer in the range of radiation doses that you looked at, so, from the higher range in the earlier studies to the much lower dose now.”
Numbers of heart disease events were sufficient to establish a linear relationship, according to Dr. Taylor. Numbers of lung cancers were not, but case-control studies in the literature with adequate numbers have identified a linear relationship there, too. “We use what we can, and we have got now several hundred events, if you combine all of the literature together. And they do suggest the dose-response relationship is linear, but we can’t know that for certain,” she said.
SAN ANTONIO – The late side effects of modern radiation therapy for breast cancer depend in part on a woman’s smoking status, suggests a meta-analysis of data from more than 40,000 women presented at the San Antonio Breast Cancer Symposium.
For nonsmokers, radiation therapy had little impact on the absolute risks of lung cancer or cardiac death, the main risks identified, which combined totaled less than 1%, Dr. Carolyn Taylor reported on behalf of the Early Breast Cancer Trialists’ Collaborative Group. But for women who had smoked throughout their adult life and continued to do so during and after treatment, it increased that absolute risk to roughly 2%.
“Smoking status can determine the net long-term effects of breast cancer radiotherapy on mortality. Stopping smoking at the time of radiotherapy may avoid much of the risk, and that’s because most of the risk of lung cancer starts more than 10 years after radiotherapy,” said Dr. Taylor, a radiation oncologist at the University of Oxford (England).
Radiation therapy remains an important tool in treating breast cancer, ultimately reducing the likelihood of death from the disease, she reminded symposium attendees. “The absolute benefit in women treated according to current guidelines is a few percent. Let’s remember the magnitude of that benefit as we think about the risks of radiotherapy.”
Attendee Dr. Steven Vogl of Montefiore Medical Center, New York, asked whether information was available on the location of the lung cancers that occurred in the trials.
“We didn’t have location of lung cancers. We didn’t even know if it was ipsilateral or contralateral to the previous breast cancer in this study,” Dr. Taylor replied. “But we’ve done other studies where we have known the location of the lung cancer, and there were similar findings in those studies.”
“In the last 4 years, we’ve had very good information that annual CT screening substantially and very quickly reduces the mortality from lung cancer,” Dr. Vogl added as a comment. “Any of us who care for patients who have been radiated where, really, any lung has been treated, who continue to smoke, should be screened – and screened and screened and screened again,” he recommended.
The investigators analyzed data from 40,781 women with breast cancer from 75 randomized trials conducted worldwide that compared outcomes with versus without radiation therapy. The median year of trial entry was 1983. On average, women in the trials received 10 Gy to both lungs combined and 6 Gy to the heart.
Comparing women who did and did not receive radiation therapy, the rate ratio for lung cancer was 2.10 at 10 or more years out, and the rate ratio for cardiac mortality was 1.30 overall. Given the mean radiation doses in the trials, the excess risk translated to 12% per Gray for lung cancer and 4% per Gray for cardiac mortality. “These rate ratios are likely to apply today,” Dr. Taylor maintained.
However, she noted, contemporary breast cancer radiation therapy techniques are much better at sparing normal tissues. To derive absolute risk estimates that are relevant today, she and her colleagues reviewed the literature for 2010-2015 and determined that women now receive an average of 5 Gy to both lungs combined and 4 Gy to the heart, with some centers achieving even lower values.
Among nonsmokers, the estimated cumulative 30-year risk of lung cancer was 0.5% for women who did not receive radiation therapy and 0.8% for those who received radiation therapy with a mean dose of 5 Gy to both lungs combined, Dr. Taylor reported. However, among long-term smokers, it was 9.4% without radiation and a substantially higher 13.8% with it.
Similarly, among nonsmokers, the estimated cumulative 30-year risk of ischemic heart disease death was 1.8% for women who did not receive radiation therapy and 2.0% for women who received radiation therapy with a mean dose of 2 Gy to the heart. Among long-term smokers, it was 8.0% without radiation and a slightly higher 8.6% with it.
Additional analyses looking at other late side effects showed no radiation therapy–related excess risk of sarcomas, according to Dr. Taylor. The risk of leukemia was increased with radiation, but actual numbers of cases were very small, she cautioned.
Attendee Dr. Pamela Goodwin, University of Toronto, said, “I’m just wondering whether you considered if it was valid to assume that there was a linear relationship between radiation dose and the risk of lung cancer in the range of radiation doses that you looked at, so, from the higher range in the earlier studies to the much lower dose now.”
Numbers of heart disease events were sufficient to establish a linear relationship, according to Dr. Taylor. Numbers of lung cancers were not, but case-control studies in the literature with adequate numbers have identified a linear relationship there, too. “We use what we can, and we have got now several hundred events, if you combine all of the literature together. And they do suggest the dose-response relationship is linear, but we can’t know that for certain,” she said.
AT SABCS 2015
Key clinical point: Receipt of radiation therapy had little effect on risks among nonsmokers, but increased the risk of lung cancer and cardiac mortality among smokers.
Major finding: The cumulative 30-year risk of lung cancer differed little with radiation versus without radiation in nonsmokers (0.8% vs. 0.5%) but was much higher with radiation in smokers (13.8% vs. 9.4%).
Data source: A meta-analysis of 40,781 women with breast cancer in 75 randomized trials.
Disclosures: Dr. Taylor disclosed that she had no relevant conflicts of interest.
Former smokers turning to e-cigarettes
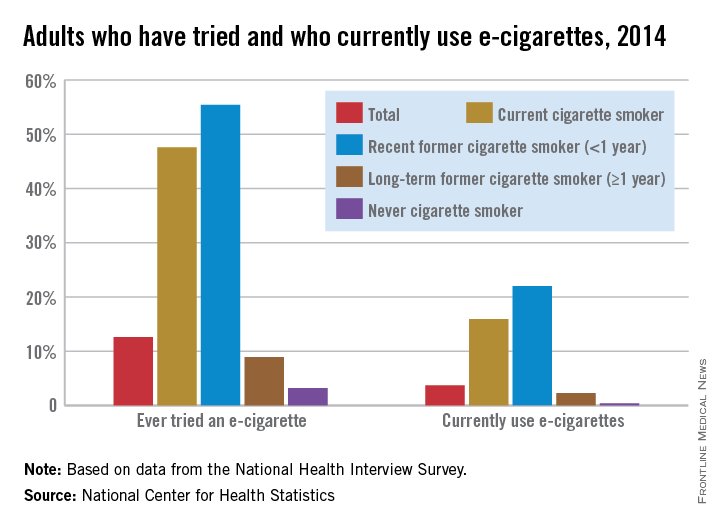
Just over 55% of adults who quit smoking cigarettes less than a year before had tried an electronic cigarette, and 22% were currently using them in 2014, the National Center for Health Statistics reports.
Overall, almost 13% of adults had ever tried an electronic cigarette, and close to 4% were using them regularly. Among current smokers, 47% had tried an e-cigarette, and nearly 16% were currently using them, according to data from the 2014 National Health Interview Survey.
Former smokers who quit more than a year before were much less likely to have tried an e-cigarette (9%) or to be a current user (2%), while 3% of never cigarette smokers reported that they had tried an e-cigarette and 0.4% were currently using them, the NCHS said.
Just over 55% of adults who quit smoking cigarettes less than a year before had tried an electronic cigarette, and 22% were currently using them in 2014, the National Center for Health Statistics reports.
Overall, almost 13% of adults had ever tried an electronic cigarette, and close to 4% were using them regularly. Among current smokers, 47% had tried an e-cigarette, and nearly 16% were currently using them, according to data from the 2014 National Health Interview Survey.
Former smokers who quit more than a year before were much less likely to have tried an e-cigarette (9%) or to be a current user (2%), while 3% of never cigarette smokers reported that they had tried an e-cigarette and 0.4% were currently using them, the NCHS said.
Just over 55% of adults who quit smoking cigarettes less than a year before had tried an electronic cigarette, and 22% were currently using them in 2014, the National Center for Health Statistics reports.
Overall, almost 13% of adults had ever tried an electronic cigarette, and close to 4% were using them regularly. Among current smokers, 47% had tried an e-cigarette, and nearly 16% were currently using them, according to data from the 2014 National Health Interview Survey.
Former smokers who quit more than a year before were much less likely to have tried an e-cigarette (9%) or to be a current user (2%), while 3% of never cigarette smokers reported that they had tried an e-cigarette and 0.4% were currently using them, the NCHS said.
Mutation in ALK-rearranged lung cancer confers resistance to lorlatinib, restores sensitivity to crizotinib
In a patient with metastatic ALK-rearranged non–small-cell lung cancer (NSCLC), resistance to ALK inhibitors began with a founder ALK C1156Y clone resistant to crizotinib, and progressed to a double-mutant C1156Y-L1198F clone that was resistant to lorlatinib but sensitive again to the less potent, first generation crizotinib, according to a case report published Dec. 23 in the New England Journal of Medicine.
Whole genome sequencing of tumor samples suggested that a minor subclone harboring the C1156Y mutation was enriched during crizotinib treatment, and under selective pressure during lorlatinib treatment, acquired the L1198F mutation that conferred resistance to the potent, third-generation ALK inhibitor lorlatinib but restored sensitivity to crizotinib. The patient relapsed after the second response to crizotinib, and tumor analysis did not detect the L1198F mutation (N Engl J Med. 2015 Dec 23. doi:10.1056/NEJMoa1508887).
“These results highlight the clinical usefulness of developing multiple, structurally distinct inhibitors that target the same oncogenic kinase,” wrote Dr. Alice Shaw, thoracic oncologist at Massachusetts General Hospital and professor at Harvard Medical School, Boston, and colleagues.
“Our results also highlight ALK L1198F as a novel resistance mechanism in ALK-rearranged NSCLC. Remarkably, this substitution changes the exact residue used to enhance selectivity of lorlatinib for ALK over other kinases,” they wrote.
Co-crystal structures of mutant and wild-type ALK kinase domains with bound inhibitors show that the leucine-to-phenylalanine mutation at residue 1198 leads to steric clash with lorlatinib but not with crizotinib. The presence of phenylalanine may result in more favorable binding of crizotinib, which may offset the increased kinase activity due to C1156Y.
The case study analyzed tumor samples from a woman with metastatic ALK-rearranged NSCLC who had received multiple therapies, including crizotinib, ceritinib, and lorlatinib.
Cell survival assays showed that the double mutant ALK C1156Y-L1198F was resistant to lorlatinib, as well as to second-generation ALK inhibitors, and it was sensitive to crizotinib; the single L1198F mutation increased sensitivity to crizotinib. Assays with cell lines expressing additional ALK mutations, including the highly refractory G1202R mutation, demonstrated that in almost all cases, L1198F increased sensitivity to crizotinib but promoted resistance to the other ALK inhibitors.
The study was funded in part by Pfizer. Dr. Shaw reported personal fees from Pfizer, Novartis, Genentech, Roche, and Ariad during the conduct of the study, and personal fees from Blueprint Medicine, Daiichi-Sankyo, and Ignyta outside the submitted work. Several of her coauthors reported ties to industry.
In a patient with metastatic ALK-rearranged non–small-cell lung cancer (NSCLC), resistance to ALK inhibitors began with a founder ALK C1156Y clone resistant to crizotinib, and progressed to a double-mutant C1156Y-L1198F clone that was resistant to lorlatinib but sensitive again to the less potent, first generation crizotinib, according to a case report published Dec. 23 in the New England Journal of Medicine.
Whole genome sequencing of tumor samples suggested that a minor subclone harboring the C1156Y mutation was enriched during crizotinib treatment, and under selective pressure during lorlatinib treatment, acquired the L1198F mutation that conferred resistance to the potent, third-generation ALK inhibitor lorlatinib but restored sensitivity to crizotinib. The patient relapsed after the second response to crizotinib, and tumor analysis did not detect the L1198F mutation (N Engl J Med. 2015 Dec 23. doi:10.1056/NEJMoa1508887).
“These results highlight the clinical usefulness of developing multiple, structurally distinct inhibitors that target the same oncogenic kinase,” wrote Dr. Alice Shaw, thoracic oncologist at Massachusetts General Hospital and professor at Harvard Medical School, Boston, and colleagues.
“Our results also highlight ALK L1198F as a novel resistance mechanism in ALK-rearranged NSCLC. Remarkably, this substitution changes the exact residue used to enhance selectivity of lorlatinib for ALK over other kinases,” they wrote.
Co-crystal structures of mutant and wild-type ALK kinase domains with bound inhibitors show that the leucine-to-phenylalanine mutation at residue 1198 leads to steric clash with lorlatinib but not with crizotinib. The presence of phenylalanine may result in more favorable binding of crizotinib, which may offset the increased kinase activity due to C1156Y.
The case study analyzed tumor samples from a woman with metastatic ALK-rearranged NSCLC who had received multiple therapies, including crizotinib, ceritinib, and lorlatinib.
Cell survival assays showed that the double mutant ALK C1156Y-L1198F was resistant to lorlatinib, as well as to second-generation ALK inhibitors, and it was sensitive to crizotinib; the single L1198F mutation increased sensitivity to crizotinib. Assays with cell lines expressing additional ALK mutations, including the highly refractory G1202R mutation, demonstrated that in almost all cases, L1198F increased sensitivity to crizotinib but promoted resistance to the other ALK inhibitors.
The study was funded in part by Pfizer. Dr. Shaw reported personal fees from Pfizer, Novartis, Genentech, Roche, and Ariad during the conduct of the study, and personal fees from Blueprint Medicine, Daiichi-Sankyo, and Ignyta outside the submitted work. Several of her coauthors reported ties to industry.
In a patient with metastatic ALK-rearranged non–small-cell lung cancer (NSCLC), resistance to ALK inhibitors began with a founder ALK C1156Y clone resistant to crizotinib, and progressed to a double-mutant C1156Y-L1198F clone that was resistant to lorlatinib but sensitive again to the less potent, first generation crizotinib, according to a case report published Dec. 23 in the New England Journal of Medicine.
Whole genome sequencing of tumor samples suggested that a minor subclone harboring the C1156Y mutation was enriched during crizotinib treatment, and under selective pressure during lorlatinib treatment, acquired the L1198F mutation that conferred resistance to the potent, third-generation ALK inhibitor lorlatinib but restored sensitivity to crizotinib. The patient relapsed after the second response to crizotinib, and tumor analysis did not detect the L1198F mutation (N Engl J Med. 2015 Dec 23. doi:10.1056/NEJMoa1508887).
“These results highlight the clinical usefulness of developing multiple, structurally distinct inhibitors that target the same oncogenic kinase,” wrote Dr. Alice Shaw, thoracic oncologist at Massachusetts General Hospital and professor at Harvard Medical School, Boston, and colleagues.
“Our results also highlight ALK L1198F as a novel resistance mechanism in ALK-rearranged NSCLC. Remarkably, this substitution changes the exact residue used to enhance selectivity of lorlatinib for ALK over other kinases,” they wrote.
Co-crystal structures of mutant and wild-type ALK kinase domains with bound inhibitors show that the leucine-to-phenylalanine mutation at residue 1198 leads to steric clash with lorlatinib but not with crizotinib. The presence of phenylalanine may result in more favorable binding of crizotinib, which may offset the increased kinase activity due to C1156Y.
The case study analyzed tumor samples from a woman with metastatic ALK-rearranged NSCLC who had received multiple therapies, including crizotinib, ceritinib, and lorlatinib.
Cell survival assays showed that the double mutant ALK C1156Y-L1198F was resistant to lorlatinib, as well as to second-generation ALK inhibitors, and it was sensitive to crizotinib; the single L1198F mutation increased sensitivity to crizotinib. Assays with cell lines expressing additional ALK mutations, including the highly refractory G1202R mutation, demonstrated that in almost all cases, L1198F increased sensitivity to crizotinib but promoted resistance to the other ALK inhibitors.
The study was funded in part by Pfizer. Dr. Shaw reported personal fees from Pfizer, Novartis, Genentech, Roche, and Ariad during the conduct of the study, and personal fees from Blueprint Medicine, Daiichi-Sankyo, and Ignyta outside the submitted work. Several of her coauthors reported ties to industry.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Cancer clinical trial enrollment of diverse and underserved patients within an urban safety net hospital
Background Enrollment rates onto cancer clinical trials are low and reflect a small subset of the population of which even fewer participants come from populations of racial or ethnic diversity or low socioeconomic status. There is a need to increase enrollment onto cancer clinical trials with a focus on recruitment of a diverse, underrepresented patient population.
Objective To use the electronic medical record (EMR) to understand the eligibility and enrollment rates for all available cancer trials in the ambulatory care setting at an urban safety net hospital to identify specific strategies for enhanced accrual onto cancer clinical trials of diverse and underserved patients.
Methods A clinical trial screening note was created for the EMR by the clinical trials office at an urban safety net hospital. 847 cancer clinical trial screening notes were extracted from the EMR between January 1, 2010 and December 31, 2010. During that time, 99 cancer trials were registered for accrual, including clinical treatment, survey, data repository, imaging, and symptom management trials. Data on eligibility, enrollment status, and relationship to sociodemographic status were compared.
Limitations This is a single-institution and retrospective study.
Conclusion The findings demonstrate that a formal process of tracking cancer clinical trial screens using an EMR can document baseline rates of institution-specific accrual patterns and identify targeted strategies for increasing cancer clinical trial enrollment among a vulnerable patient population. Offering nontreatment trials may be an important and strategic method of engaging this vulnerable population in clinical research.
Funding/sponsorship Boston Medical Center Minority-Based Community Clinical Oncology Program (NCI 1U-10CA129519- 01A1), Boston Me
Click on the PDF icon at the top of this introduction to read the full article.
Background Enrollment rates onto cancer clinical trials are low and reflect a small subset of the population of which even fewer participants come from populations of racial or ethnic diversity or low socioeconomic status. There is a need to increase enrollment onto cancer clinical trials with a focus on recruitment of a diverse, underrepresented patient population.
Objective To use the electronic medical record (EMR) to understand the eligibility and enrollment rates for all available cancer trials in the ambulatory care setting at an urban safety net hospital to identify specific strategies for enhanced accrual onto cancer clinical trials of diverse and underserved patients.
Methods A clinical trial screening note was created for the EMR by the clinical trials office at an urban safety net hospital. 847 cancer clinical trial screening notes were extracted from the EMR between January 1, 2010 and December 31, 2010. During that time, 99 cancer trials were registered for accrual, including clinical treatment, survey, data repository, imaging, and symptom management trials. Data on eligibility, enrollment status, and relationship to sociodemographic status were compared.
Limitations This is a single-institution and retrospective study.
Conclusion The findings demonstrate that a formal process of tracking cancer clinical trial screens using an EMR can document baseline rates of institution-specific accrual patterns and identify targeted strategies for increasing cancer clinical trial enrollment among a vulnerable patient population. Offering nontreatment trials may be an important and strategic method of engaging this vulnerable population in clinical research.
Funding/sponsorship Boston Medical Center Minority-Based Community Clinical Oncology Program (NCI 1U-10CA129519- 01A1), Boston Me
Click on the PDF icon at the top of this introduction to read the full article.
Background Enrollment rates onto cancer clinical trials are low and reflect a small subset of the population of which even fewer participants come from populations of racial or ethnic diversity or low socioeconomic status. There is a need to increase enrollment onto cancer clinical trials with a focus on recruitment of a diverse, underrepresented patient population.
Objective To use the electronic medical record (EMR) to understand the eligibility and enrollment rates for all available cancer trials in the ambulatory care setting at an urban safety net hospital to identify specific strategies for enhanced accrual onto cancer clinical trials of diverse and underserved patients.
Methods A clinical trial screening note was created for the EMR by the clinical trials office at an urban safety net hospital. 847 cancer clinical trial screening notes were extracted from the EMR between January 1, 2010 and December 31, 2010. During that time, 99 cancer trials were registered for accrual, including clinical treatment, survey, data repository, imaging, and symptom management trials. Data on eligibility, enrollment status, and relationship to sociodemographic status were compared.
Limitations This is a single-institution and retrospective study.
Conclusion The findings demonstrate that a formal process of tracking cancer clinical trial screens using an EMR can document baseline rates of institution-specific accrual patterns and identify targeted strategies for increasing cancer clinical trial enrollment among a vulnerable patient population. Offering nontreatment trials may be an important and strategic method of engaging this vulnerable population in clinical research.
Funding/sponsorship Boston Medical Center Minority-Based Community Clinical Oncology Program (NCI 1U-10CA129519- 01A1), Boston Me
Click on the PDF icon at the top of this introduction to read the full article.
Oncology 2015: new therapies and new transitions toward value-based cancer care
The past year has been an exciting one for new oncology and hematology drug approvals and the continued evolution of our oncology delivery system toward high quality and value. In all, at press time in mid-November, the US Food and Drug Administration (FDA) had approved or granted expanded indications for 24 drugs, compared with 19 in the 2 preceding years. Of those 24 approvals, 7 were accelerated and 6 were expanded approvals, and 3 alone were for the immunotherapeutic drug, nivolumab – 2 for non-small-cell lung cancer (NSCLC) and 1 for metastatic melanoma.
Click on the PDF icon at the top of this introduction to read the full article.
The past year has been an exciting one for new oncology and hematology drug approvals and the continued evolution of our oncology delivery system toward high quality and value. In all, at press time in mid-November, the US Food and Drug Administration (FDA) had approved or granted expanded indications for 24 drugs, compared with 19 in the 2 preceding years. Of those 24 approvals, 7 were accelerated and 6 were expanded approvals, and 3 alone were for the immunotherapeutic drug, nivolumab – 2 for non-small-cell lung cancer (NSCLC) and 1 for metastatic melanoma.
Click on the PDF icon at the top of this introduction to read the full article.
The past year has been an exciting one for new oncology and hematology drug approvals and the continued evolution of our oncology delivery system toward high quality and value. In all, at press time in mid-November, the US Food and Drug Administration (FDA) had approved or granted expanded indications for 24 drugs, compared with 19 in the 2 preceding years. Of those 24 approvals, 7 were accelerated and 6 were expanded approvals, and 3 alone were for the immunotherapeutic drug, nivolumab – 2 for non-small-cell lung cancer (NSCLC) and 1 for metastatic melanoma.
Click on the PDF icon at the top of this introduction to read the full article.
Alectinib FDA approved to treat metastatic ALK-positive NSCLC*
[UPDATE: 5:04 pm] Alectinib has been approved by the Food and Drug Administration for the treatment of patients with metastatic ALK-positive non–small cell lung cancer (NSCLC) who have been treated unsuccessfully with crizotinib.
Alectinib was approved based on data from two single-arm clinical trials of patients with crizotinib-refractory metastatic ALK-positive NSCLC.
One study, conducted in North America, included 87 patients who were treated with 600 mg of oral alectinib twice daily. A total of 38% of patients experienced a reduction in tumor size with a 7.5-month duration of response. In the second trial with the same treatment regimen, conducted globally, 44% of patients saw a reduction in tumor size with an 11.2-month duration of response.
Treatment with alectinib also reduced the size of brain metastases in 61% of patients in a pooled subset of patients in both trials, with a 9.1-month duration of response.
The most common adverse events were fatigue, constipation, edema, and myalgias.
“Today’s approval provides a new therapy for a group of patients who would have few treatment options once their disease no longer responds to treatment with [crizotinib],” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA Center for Drug Evaluation and Research, said in a written statement. “In addition to the primary effect on tumors in the lung, [alectinib] clinical trials provide evidence of an effect on tumors that had spread to the brain, which is an important effect for clinicians to understand.”
Alectinib will be marketed as Alecensa by Genentech. It was approved under several FDA accelerated approval programs. Treatment with alectinib is expected to cost approximately $12,500 per month, according to a Genentech spokesperson.*
On Twitter @denisefulton
*This story has been updated with cost information.
*Correction, 12/21/2015: An earlier version of this story misspelled metastatic.
[UPDATE: 5:04 pm] Alectinib has been approved by the Food and Drug Administration for the treatment of patients with metastatic ALK-positive non–small cell lung cancer (NSCLC) who have been treated unsuccessfully with crizotinib.
Alectinib was approved based on data from two single-arm clinical trials of patients with crizotinib-refractory metastatic ALK-positive NSCLC.
One study, conducted in North America, included 87 patients who were treated with 600 mg of oral alectinib twice daily. A total of 38% of patients experienced a reduction in tumor size with a 7.5-month duration of response. In the second trial with the same treatment regimen, conducted globally, 44% of patients saw a reduction in tumor size with an 11.2-month duration of response.
Treatment with alectinib also reduced the size of brain metastases in 61% of patients in a pooled subset of patients in both trials, with a 9.1-month duration of response.
The most common adverse events were fatigue, constipation, edema, and myalgias.
“Today’s approval provides a new therapy for a group of patients who would have few treatment options once their disease no longer responds to treatment with [crizotinib],” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA Center for Drug Evaluation and Research, said in a written statement. “In addition to the primary effect on tumors in the lung, [alectinib] clinical trials provide evidence of an effect on tumors that had spread to the brain, which is an important effect for clinicians to understand.”
Alectinib will be marketed as Alecensa by Genentech. It was approved under several FDA accelerated approval programs. Treatment with alectinib is expected to cost approximately $12,500 per month, according to a Genentech spokesperson.*
On Twitter @denisefulton
*This story has been updated with cost information.
*Correction, 12/21/2015: An earlier version of this story misspelled metastatic.
[UPDATE: 5:04 pm] Alectinib has been approved by the Food and Drug Administration for the treatment of patients with metastatic ALK-positive non–small cell lung cancer (NSCLC) who have been treated unsuccessfully with crizotinib.
Alectinib was approved based on data from two single-arm clinical trials of patients with crizotinib-refractory metastatic ALK-positive NSCLC.
One study, conducted in North America, included 87 patients who were treated with 600 mg of oral alectinib twice daily. A total of 38% of patients experienced a reduction in tumor size with a 7.5-month duration of response. In the second trial with the same treatment regimen, conducted globally, 44% of patients saw a reduction in tumor size with an 11.2-month duration of response.
Treatment with alectinib also reduced the size of brain metastases in 61% of patients in a pooled subset of patients in both trials, with a 9.1-month duration of response.
The most common adverse events were fatigue, constipation, edema, and myalgias.
“Today’s approval provides a new therapy for a group of patients who would have few treatment options once their disease no longer responds to treatment with [crizotinib],” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA Center for Drug Evaluation and Research, said in a written statement. “In addition to the primary effect on tumors in the lung, [alectinib] clinical trials provide evidence of an effect on tumors that had spread to the brain, which is an important effect for clinicians to understand.”
Alectinib will be marketed as Alecensa by Genentech. It was approved under several FDA accelerated approval programs. Treatment with alectinib is expected to cost approximately $12,500 per month, according to a Genentech spokesperson.*
On Twitter @denisefulton
*This story has been updated with cost information.
*Correction, 12/21/2015: An earlier version of this story misspelled metastatic.
Necitumumab approved as first-line treatment of metastatic squamous NSCLC
Necitumumab, an epidermal growth factor receptor (EGFR) antagonist, combined with two forms of chemotherapy, has been approved as a first-line treatment of patients with metastatic squamous non–small cell lung cancer (NSCLC). the Food and Drug Administration announced on Nov. 24.
Necitumumab is a “monoclonal antibody that blocks activity of EGFR, a protein commonly found on squamous NSCLC tumors,” the FDA statement said. The statement points put that necitumumab “was not found to be an effective treatment” in patients with nonsquamous NSCLC.
Eli Lilly will market necitumumab as Portrazza. It is administered as an intravenous infusion over 60 minutes on days 1 and 8 of each 3-week cycle.
“Today’s approval provides certain patients with squamous cell lung cancer a new option that may extend survival,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA Center for Drug Evaluation and Research, said in the statement.
Necitumumab was evaluated in multicenter, randomized, open-label study of 1,093 people with advanced squamous NSCLC, treated with gemcitabine and cisplatin with or without necitumumab. Median overall survival, the primary endpoint, was 11.5 months in the necitumumab-treated arm vs. 9.9 months among controls, a 1.6 month difference that was statistically significant.
At a July meeting of the FDA’s Oncologic Drugs Advisory Committee, 11 of the 12 panelists agreed that the efficacy and safety results of this study supported a “positive benefit risk assessment” of necitumumab combined with gemcitabine and cisplatin for this group of patients – despite a modest effect on overall survival, a very small but statistically significant effect on progression-free survival, and some safety concerns, the main question they were asked by the FDA to discuss.
Rash and hypomagnesemia are the most common adverse events associated with necitumumab, according to the FDA.
The prescribing information includes a boxed warning regarding the risk of cardiopulmonary arrest and hypomagnesemia associated with treatment, and points out that 83% of the patients treated with necitumumab, gemcitabine, and cisplatin developed hypomagnesemia, which was severe in 20%; and that the rate of cardiopulmonary arrest and/or sudden death occurred in 3% of those on the combination.
Serious adverse events associated with necitumumab should be reported to the FDA’s MedWatch program.
Necitumumab, an epidermal growth factor receptor (EGFR) antagonist, combined with two forms of chemotherapy, has been approved as a first-line treatment of patients with metastatic squamous non–small cell lung cancer (NSCLC). the Food and Drug Administration announced on Nov. 24.
Necitumumab is a “monoclonal antibody that blocks activity of EGFR, a protein commonly found on squamous NSCLC tumors,” the FDA statement said. The statement points put that necitumumab “was not found to be an effective treatment” in patients with nonsquamous NSCLC.
Eli Lilly will market necitumumab as Portrazza. It is administered as an intravenous infusion over 60 minutes on days 1 and 8 of each 3-week cycle.
“Today’s approval provides certain patients with squamous cell lung cancer a new option that may extend survival,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA Center for Drug Evaluation and Research, said in the statement.
Necitumumab was evaluated in multicenter, randomized, open-label study of 1,093 people with advanced squamous NSCLC, treated with gemcitabine and cisplatin with or without necitumumab. Median overall survival, the primary endpoint, was 11.5 months in the necitumumab-treated arm vs. 9.9 months among controls, a 1.6 month difference that was statistically significant.
At a July meeting of the FDA’s Oncologic Drugs Advisory Committee, 11 of the 12 panelists agreed that the efficacy and safety results of this study supported a “positive benefit risk assessment” of necitumumab combined with gemcitabine and cisplatin for this group of patients – despite a modest effect on overall survival, a very small but statistically significant effect on progression-free survival, and some safety concerns, the main question they were asked by the FDA to discuss.
Rash and hypomagnesemia are the most common adverse events associated with necitumumab, according to the FDA.
The prescribing information includes a boxed warning regarding the risk of cardiopulmonary arrest and hypomagnesemia associated with treatment, and points out that 83% of the patients treated with necitumumab, gemcitabine, and cisplatin developed hypomagnesemia, which was severe in 20%; and that the rate of cardiopulmonary arrest and/or sudden death occurred in 3% of those on the combination.
Serious adverse events associated with necitumumab should be reported to the FDA’s MedWatch program.
Necitumumab, an epidermal growth factor receptor (EGFR) antagonist, combined with two forms of chemotherapy, has been approved as a first-line treatment of patients with metastatic squamous non–small cell lung cancer (NSCLC). the Food and Drug Administration announced on Nov. 24.
Necitumumab is a “monoclonal antibody that blocks activity of EGFR, a protein commonly found on squamous NSCLC tumors,” the FDA statement said. The statement points put that necitumumab “was not found to be an effective treatment” in patients with nonsquamous NSCLC.
Eli Lilly will market necitumumab as Portrazza. It is administered as an intravenous infusion over 60 minutes on days 1 and 8 of each 3-week cycle.
“Today’s approval provides certain patients with squamous cell lung cancer a new option that may extend survival,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA Center for Drug Evaluation and Research, said in the statement.
Necitumumab was evaluated in multicenter, randomized, open-label study of 1,093 people with advanced squamous NSCLC, treated with gemcitabine and cisplatin with or without necitumumab. Median overall survival, the primary endpoint, was 11.5 months in the necitumumab-treated arm vs. 9.9 months among controls, a 1.6 month difference that was statistically significant.
At a July meeting of the FDA’s Oncologic Drugs Advisory Committee, 11 of the 12 panelists agreed that the efficacy and safety results of this study supported a “positive benefit risk assessment” of necitumumab combined with gemcitabine and cisplatin for this group of patients – despite a modest effect on overall survival, a very small but statistically significant effect on progression-free survival, and some safety concerns, the main question they were asked by the FDA to discuss.
Rash and hypomagnesemia are the most common adverse events associated with necitumumab, according to the FDA.
The prescribing information includes a boxed warning regarding the risk of cardiopulmonary arrest and hypomagnesemia associated with treatment, and points out that 83% of the patients treated with necitumumab, gemcitabine, and cisplatin developed hypomagnesemia, which was severe in 20%; and that the rate of cardiopulmonary arrest and/or sudden death occurred in 3% of those on the combination.
Serious adverse events associated with necitumumab should be reported to the FDA’s MedWatch program.
Alectinib shows promise in crizotinib-refractory ALK-rearranged NSCLC
Alectinib, an oral, small-molecule, ATP-competitive tyrosine kinase inhibitor of ALK, demonstrated good clinical activity in crizotinib-refractory patients with advanced non–small-cell lung cancer (NSCLC) harboring ALK rearrangements.
The overall response rate (ORR) for all 122 evaluable patients was 49%: 44% for the 96 patients who had received prior chemotherapy, and 69% for the 26 chemotherapy-naive patients. The median duration of response (DOR) for the 61 patients with a partial response was 11.2 months.
“The clinically meaningful ORR and DOR in patients with crizotinib-resistant disease and the sustained CNS response reported from this study, as well as the good tolerability profile, support the additional development of this promising new ALK inhibitor,” wrote Dr. Sai-Hong Ignatius Ou, clinical professor at the University of California, Irvine, and colleagues (J Clin Oncol. 2015 Nov. 23. doi:10.1200/JCO.2015.63.9443).
The observed clinical activity of alectinib in the CNS was consistent with preclinical data showing high CNS tissue penetration and earlier phase I/II results. Among 35 patients with baseline measurable CNS lesions, the ORR was 57%, including seven complete responses. CNS complete responses were observed in 10 of 23 patients (43%) who had no prior brain radiation.
The cumulative incidence rates of CNS progression were lower than were those of non-CNS progression, “which seems to suggest that alectinib can prevent or delay the emergence of CNS metastases,” the authors wrote. At 12 months, 33 patients had a CNS progression and 43 had a non-CNS progression.
Alectinib showed an acceptable safety profile. The most common adverse events were myalgia, fatigue, and gastrointestinal events, usually grade 1 or 2. Grade 3 or 4 adverse events occurred at a rate less than 5%. Most patients maintained therapeutic levels of alectinib throughout the study, indicated by a mean dose intensity of 97%.
The phase II study evaluated 122 patients who had locally advanced or metastatic NSCLC harboring an ALK rearrangement and who had progressed on crizotinib treatment.
Multiple oral doses at 600 mg twice daily produced an overall flat PK profile, supporting sustained alectinib exposure throughout the dosing interval. In contrast to results from the U.S. phase I/II study that reported differences in alectinib exposures between white and Asian patients, this study showed no marked differences between white (n = 6) and Asian (n = 20) patients evaluated.
F. Hoffmann-La Roche supported the study. Dr. Ou disclosed ties with Pfizer, F. Hoffmann-La Roche, Boehringer Ingelheim, ARIAD, AstraZeneca, Clovis Oncology, Astellas Pharma, Ignyta, and Daichi Sankyo. Several of his coauthors reported ties to industry.
Alectinib, an oral, small-molecule, ATP-competitive tyrosine kinase inhibitor of ALK, demonstrated good clinical activity in crizotinib-refractory patients with advanced non–small-cell lung cancer (NSCLC) harboring ALK rearrangements.
The overall response rate (ORR) for all 122 evaluable patients was 49%: 44% for the 96 patients who had received prior chemotherapy, and 69% for the 26 chemotherapy-naive patients. The median duration of response (DOR) for the 61 patients with a partial response was 11.2 months.
“The clinically meaningful ORR and DOR in patients with crizotinib-resistant disease and the sustained CNS response reported from this study, as well as the good tolerability profile, support the additional development of this promising new ALK inhibitor,” wrote Dr. Sai-Hong Ignatius Ou, clinical professor at the University of California, Irvine, and colleagues (J Clin Oncol. 2015 Nov. 23. doi:10.1200/JCO.2015.63.9443).
The observed clinical activity of alectinib in the CNS was consistent with preclinical data showing high CNS tissue penetration and earlier phase I/II results. Among 35 patients with baseline measurable CNS lesions, the ORR was 57%, including seven complete responses. CNS complete responses were observed in 10 of 23 patients (43%) who had no prior brain radiation.
The cumulative incidence rates of CNS progression were lower than were those of non-CNS progression, “which seems to suggest that alectinib can prevent or delay the emergence of CNS metastases,” the authors wrote. At 12 months, 33 patients had a CNS progression and 43 had a non-CNS progression.
Alectinib showed an acceptable safety profile. The most common adverse events were myalgia, fatigue, and gastrointestinal events, usually grade 1 or 2. Grade 3 or 4 adverse events occurred at a rate less than 5%. Most patients maintained therapeutic levels of alectinib throughout the study, indicated by a mean dose intensity of 97%.
The phase II study evaluated 122 patients who had locally advanced or metastatic NSCLC harboring an ALK rearrangement and who had progressed on crizotinib treatment.
Multiple oral doses at 600 mg twice daily produced an overall flat PK profile, supporting sustained alectinib exposure throughout the dosing interval. In contrast to results from the U.S. phase I/II study that reported differences in alectinib exposures between white and Asian patients, this study showed no marked differences between white (n = 6) and Asian (n = 20) patients evaluated.
F. Hoffmann-La Roche supported the study. Dr. Ou disclosed ties with Pfizer, F. Hoffmann-La Roche, Boehringer Ingelheim, ARIAD, AstraZeneca, Clovis Oncology, Astellas Pharma, Ignyta, and Daichi Sankyo. Several of his coauthors reported ties to industry.
Alectinib, an oral, small-molecule, ATP-competitive tyrosine kinase inhibitor of ALK, demonstrated good clinical activity in crizotinib-refractory patients with advanced non–small-cell lung cancer (NSCLC) harboring ALK rearrangements.
The overall response rate (ORR) for all 122 evaluable patients was 49%: 44% for the 96 patients who had received prior chemotherapy, and 69% for the 26 chemotherapy-naive patients. The median duration of response (DOR) for the 61 patients with a partial response was 11.2 months.
“The clinically meaningful ORR and DOR in patients with crizotinib-resistant disease and the sustained CNS response reported from this study, as well as the good tolerability profile, support the additional development of this promising new ALK inhibitor,” wrote Dr. Sai-Hong Ignatius Ou, clinical professor at the University of California, Irvine, and colleagues (J Clin Oncol. 2015 Nov. 23. doi:10.1200/JCO.2015.63.9443).
The observed clinical activity of alectinib in the CNS was consistent with preclinical data showing high CNS tissue penetration and earlier phase I/II results. Among 35 patients with baseline measurable CNS lesions, the ORR was 57%, including seven complete responses. CNS complete responses were observed in 10 of 23 patients (43%) who had no prior brain radiation.
The cumulative incidence rates of CNS progression were lower than were those of non-CNS progression, “which seems to suggest that alectinib can prevent or delay the emergence of CNS metastases,” the authors wrote. At 12 months, 33 patients had a CNS progression and 43 had a non-CNS progression.
Alectinib showed an acceptable safety profile. The most common adverse events were myalgia, fatigue, and gastrointestinal events, usually grade 1 or 2. Grade 3 or 4 adverse events occurred at a rate less than 5%. Most patients maintained therapeutic levels of alectinib throughout the study, indicated by a mean dose intensity of 97%.
The phase II study evaluated 122 patients who had locally advanced or metastatic NSCLC harboring an ALK rearrangement and who had progressed on crizotinib treatment.
Multiple oral doses at 600 mg twice daily produced an overall flat PK profile, supporting sustained alectinib exposure throughout the dosing interval. In contrast to results from the U.S. phase I/II study that reported differences in alectinib exposures between white and Asian patients, this study showed no marked differences between white (n = 6) and Asian (n = 20) patients evaluated.
F. Hoffmann-La Roche supported the study. Dr. Ou disclosed ties with Pfizer, F. Hoffmann-La Roche, Boehringer Ingelheim, ARIAD, AstraZeneca, Clovis Oncology, Astellas Pharma, Ignyta, and Daichi Sankyo. Several of his coauthors reported ties to industry.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Alectinib, an oral, small molecule inhibitor of ALK, showed promising clinical activity in crizotinib-refractory patients with non–small-cell lung cancer (NSCLC).
Major finding: The overall response rate was 49% for all evaluable patients, 44% for those who had received prior chemotherapy, and 69% for chemotherapy-naive patients.
Data source: The phase II study evaluated 122 patients with locally advanced or metastatic NSCLC harboring an ALK rearrangement and who had progressed on crizotinib treatment.
Disclosures: F. Hoffmann-La Roche supported the study. Dr. Ou disclosed ties with Pfizer, F. Hoffmann-La Roche, Boehringer Ingelheim, ARIAD, AstraZeneca, Clovis Oncology, Astellas Pharma, Ignyta, and Daichi Sankyo. Several of his coauthors reported ties to industry.
Hold the checkpoint inhibitors when pneumonitis symptoms occur
BOSTON – Treatment of pneumonitis associated with the PD-1 axis checkpoint inhibitors requires close monitoring of patients and rapid clinical response, an oncologist advised.
“We need to be vigilant when we treat our patients. If a patient has cough or shortness of breath, you take it seriously, even if [it is] a patient who has lung cancer and is a smoker,” said Dr. Scott Gettinger of Yale Cancer Center, New Haven, Conn.
He recommended that before starting patients on a programmed death-1 (PD-1) axis inhibitor such as pembrolizumab (Keytruda) or nivolumab (Opdivo), clinicians get baseline oxygen saturation levels to obtain an objective measure for following patients during therapy.
“When you suspect pneumonitis you have to start steroids right away, or patients can spiral down,” he said at the AACR/NCI/EORTC International Conference on Molecular Targets and Cancer Therapeutics.
Pneumonitis – characterized by cough, dyspnea, and hypoxia – is a common adverse event associated with PD-1 and PD-ligand 1 (PD-L1) inhibitors, but is rarely seen in patients treated with CTLA-4 inhibitors such as ipilimumab (Yervoy). Current evidence suggests that pneumonitis is more prevalent in patients with non–small-cell lung cancer, occurring in approximately 4%-6% of patients cases, than in melanoma (1%). The difference is probably due to a history of smoking common to the majority of patients with NSCLC, Dr. Gettinger said.
“The other theme that we’re beginning to see is that maybe pneumonitis is a bit more common with anti-PD-1 vs. anti-PD-L1 antibodies,” he said.
It’s theorized that PD-1 inhibitors may block binding of PD-L2 to one of its binding partners, thereby interfering with respiratory tolerance, he said.
Evidence from the pivotal clinical trial of nivolumab in lung cancer (Checkmate 057) suggests that the time to onset of pneumonitis was a median of 31.1 weeks (range 11.7 to 56.9 weeks). The pneumonitis resolved in about 5-7 weeks.
Investigators at Memorial Sloan Kettering Cancer Center in New York City reported at the European Cancer Congress 2015 on pneumonitis in 36 of 653 patients treated with an anti PD-1/PD-L1 monoclonal antibody from 2009 through 2014, 33 of whom had received a PD-1 inhibitor, and 3 of whom received a PD-L1 inhibitor.
They found that pneumonitis in patients with lung cancer tended to resemble chronic obstructive pneumonia, whereas patients with melanoma were more likely to have ground-glass opacifications on radiography. There was a trend, falling just short of significance, toward association of COP-like pneumonitis with development of grade 3 or greater, and with a requirement for more than one type of immunosuppression, compared to other subtypes.
Algorithms for management
Dr. Gettinger briefly outlined an algorithm offered by Bristol-Myers Squibb for management of suspected pulmonary toxicity with nivolumab. For management of patients with grade 1 toxicities (asymptomatic, with radiographic changes only), it recommends that clinicians consider delay of immuno-oncologic (I-O) therapy, monitor for symptoms every 2-3 days, and consider consultations with pulmonary and infectious disease specialists.
For grade 2 pneumonitis, marked by mild to moderate new symptoms, the algorithm calls for clinicians to delay I-O therapy per protocol, consult with pulmonary and ID specialists, monitor symptoms daily and consider hospitalization, start the patient on steroids with 1.0 mg/kg per day methylprednisolone IV or the oral equivalent, and consider bronchoscopy and lung biopsy.
For patients with grade 3 or 4 toxicities, marked by severe new symptoms, new or worsening hypoxia, or other life-threatening symptoms, the algorithm states that clinicians should discontinue I-O therapy, hospitalize the patient, consult with pulmonary and ID, give 2-4 mg/kg per day methylprednisolone IV or the oral equivalent, add prophylactic antibiotics for opportunistic infections, and consider bronchoscopy and lung biopsy.
At Yale, Dr. Gettinger and colleagues, when presented with a symptomatic patient on a PD-axis inhibitor, will first rule out other etiologies such as infection, chronic obstructive pulmonary disease exacerbation, or cancer progression), typically with bronchoscopy.
For patients with moderate pneumonitis, they may treat with prednisone for 1 or 2 weeks, with a 4-6 week taper begun as symptoms start to resolve.
“In a patient who has profound hypoxia and shortness of breath, we may want to go higher: We might give them 2 mg/kg twice a day of [methylprednisolone] in the hospital, wait until they get better, and then slowly taper them, whether it be over 4 or 6 weeks or longer. Occasionally our patients need to get even higher doses of steroids and there’s really nothing to guide us. Who knows if 1 gram of [methylprednisolone] may be better than 60 g of prednisone? But we do it, and patients do get better with the higher doses,” he said.
In rare instances, patients may required other immunosuppressive agents, such as infliximab (Remicade), cyclophosphamide, or mycophenolate mofetil.
Patients who require additional immunosuppressive agents to resolve severe pneumonitis tend to have poor outcomes, Dr. Gettinger said.
Re-challenge of patients with a PD-1 or PD-L1 inhibitor following resolution of pneumonitis appears to be inadvisable for all patients except possibly those with grade 1 (asymptomatic) toxicity, due to the high risk of recurrence, he said.
BOSTON – Treatment of pneumonitis associated with the PD-1 axis checkpoint inhibitors requires close monitoring of patients and rapid clinical response, an oncologist advised.
“We need to be vigilant when we treat our patients. If a patient has cough or shortness of breath, you take it seriously, even if [it is] a patient who has lung cancer and is a smoker,” said Dr. Scott Gettinger of Yale Cancer Center, New Haven, Conn.
He recommended that before starting patients on a programmed death-1 (PD-1) axis inhibitor such as pembrolizumab (Keytruda) or nivolumab (Opdivo), clinicians get baseline oxygen saturation levels to obtain an objective measure for following patients during therapy.
“When you suspect pneumonitis you have to start steroids right away, or patients can spiral down,” he said at the AACR/NCI/EORTC International Conference on Molecular Targets and Cancer Therapeutics.
Pneumonitis – characterized by cough, dyspnea, and hypoxia – is a common adverse event associated with PD-1 and PD-ligand 1 (PD-L1) inhibitors, but is rarely seen in patients treated with CTLA-4 inhibitors such as ipilimumab (Yervoy). Current evidence suggests that pneumonitis is more prevalent in patients with non–small-cell lung cancer, occurring in approximately 4%-6% of patients cases, than in melanoma (1%). The difference is probably due to a history of smoking common to the majority of patients with NSCLC, Dr. Gettinger said.
“The other theme that we’re beginning to see is that maybe pneumonitis is a bit more common with anti-PD-1 vs. anti-PD-L1 antibodies,” he said.
It’s theorized that PD-1 inhibitors may block binding of PD-L2 to one of its binding partners, thereby interfering with respiratory tolerance, he said.
Evidence from the pivotal clinical trial of nivolumab in lung cancer (Checkmate 057) suggests that the time to onset of pneumonitis was a median of 31.1 weeks (range 11.7 to 56.9 weeks). The pneumonitis resolved in about 5-7 weeks.
Investigators at Memorial Sloan Kettering Cancer Center in New York City reported at the European Cancer Congress 2015 on pneumonitis in 36 of 653 patients treated with an anti PD-1/PD-L1 monoclonal antibody from 2009 through 2014, 33 of whom had received a PD-1 inhibitor, and 3 of whom received a PD-L1 inhibitor.
They found that pneumonitis in patients with lung cancer tended to resemble chronic obstructive pneumonia, whereas patients with melanoma were more likely to have ground-glass opacifications on radiography. There was a trend, falling just short of significance, toward association of COP-like pneumonitis with development of grade 3 or greater, and with a requirement for more than one type of immunosuppression, compared to other subtypes.
Algorithms for management
Dr. Gettinger briefly outlined an algorithm offered by Bristol-Myers Squibb for management of suspected pulmonary toxicity with nivolumab. For management of patients with grade 1 toxicities (asymptomatic, with radiographic changes only), it recommends that clinicians consider delay of immuno-oncologic (I-O) therapy, monitor for symptoms every 2-3 days, and consider consultations with pulmonary and infectious disease specialists.
For grade 2 pneumonitis, marked by mild to moderate new symptoms, the algorithm calls for clinicians to delay I-O therapy per protocol, consult with pulmonary and ID specialists, monitor symptoms daily and consider hospitalization, start the patient on steroids with 1.0 mg/kg per day methylprednisolone IV or the oral equivalent, and consider bronchoscopy and lung biopsy.
For patients with grade 3 or 4 toxicities, marked by severe new symptoms, new or worsening hypoxia, or other life-threatening symptoms, the algorithm states that clinicians should discontinue I-O therapy, hospitalize the patient, consult with pulmonary and ID, give 2-4 mg/kg per day methylprednisolone IV or the oral equivalent, add prophylactic antibiotics for opportunistic infections, and consider bronchoscopy and lung biopsy.
At Yale, Dr. Gettinger and colleagues, when presented with a symptomatic patient on a PD-axis inhibitor, will first rule out other etiologies such as infection, chronic obstructive pulmonary disease exacerbation, or cancer progression), typically with bronchoscopy.
For patients with moderate pneumonitis, they may treat with prednisone for 1 or 2 weeks, with a 4-6 week taper begun as symptoms start to resolve.
“In a patient who has profound hypoxia and shortness of breath, we may want to go higher: We might give them 2 mg/kg twice a day of [methylprednisolone] in the hospital, wait until they get better, and then slowly taper them, whether it be over 4 or 6 weeks or longer. Occasionally our patients need to get even higher doses of steroids and there’s really nothing to guide us. Who knows if 1 gram of [methylprednisolone] may be better than 60 g of prednisone? But we do it, and patients do get better with the higher doses,” he said.
In rare instances, patients may required other immunosuppressive agents, such as infliximab (Remicade), cyclophosphamide, or mycophenolate mofetil.
Patients who require additional immunosuppressive agents to resolve severe pneumonitis tend to have poor outcomes, Dr. Gettinger said.
Re-challenge of patients with a PD-1 or PD-L1 inhibitor following resolution of pneumonitis appears to be inadvisable for all patients except possibly those with grade 1 (asymptomatic) toxicity, due to the high risk of recurrence, he said.
BOSTON – Treatment of pneumonitis associated with the PD-1 axis checkpoint inhibitors requires close monitoring of patients and rapid clinical response, an oncologist advised.
“We need to be vigilant when we treat our patients. If a patient has cough or shortness of breath, you take it seriously, even if [it is] a patient who has lung cancer and is a smoker,” said Dr. Scott Gettinger of Yale Cancer Center, New Haven, Conn.
He recommended that before starting patients on a programmed death-1 (PD-1) axis inhibitor such as pembrolizumab (Keytruda) or nivolumab (Opdivo), clinicians get baseline oxygen saturation levels to obtain an objective measure for following patients during therapy.
“When you suspect pneumonitis you have to start steroids right away, or patients can spiral down,” he said at the AACR/NCI/EORTC International Conference on Molecular Targets and Cancer Therapeutics.
Pneumonitis – characterized by cough, dyspnea, and hypoxia – is a common adverse event associated with PD-1 and PD-ligand 1 (PD-L1) inhibitors, but is rarely seen in patients treated with CTLA-4 inhibitors such as ipilimumab (Yervoy). Current evidence suggests that pneumonitis is more prevalent in patients with non–small-cell lung cancer, occurring in approximately 4%-6% of patients cases, than in melanoma (1%). The difference is probably due to a history of smoking common to the majority of patients with NSCLC, Dr. Gettinger said.
“The other theme that we’re beginning to see is that maybe pneumonitis is a bit more common with anti-PD-1 vs. anti-PD-L1 antibodies,” he said.
It’s theorized that PD-1 inhibitors may block binding of PD-L2 to one of its binding partners, thereby interfering with respiratory tolerance, he said.
Evidence from the pivotal clinical trial of nivolumab in lung cancer (Checkmate 057) suggests that the time to onset of pneumonitis was a median of 31.1 weeks (range 11.7 to 56.9 weeks). The pneumonitis resolved in about 5-7 weeks.
Investigators at Memorial Sloan Kettering Cancer Center in New York City reported at the European Cancer Congress 2015 on pneumonitis in 36 of 653 patients treated with an anti PD-1/PD-L1 monoclonal antibody from 2009 through 2014, 33 of whom had received a PD-1 inhibitor, and 3 of whom received a PD-L1 inhibitor.
They found that pneumonitis in patients with lung cancer tended to resemble chronic obstructive pneumonia, whereas patients with melanoma were more likely to have ground-glass opacifications on radiography. There was a trend, falling just short of significance, toward association of COP-like pneumonitis with development of grade 3 or greater, and with a requirement for more than one type of immunosuppression, compared to other subtypes.
Algorithms for management
Dr. Gettinger briefly outlined an algorithm offered by Bristol-Myers Squibb for management of suspected pulmonary toxicity with nivolumab. For management of patients with grade 1 toxicities (asymptomatic, with radiographic changes only), it recommends that clinicians consider delay of immuno-oncologic (I-O) therapy, monitor for symptoms every 2-3 days, and consider consultations with pulmonary and infectious disease specialists.
For grade 2 pneumonitis, marked by mild to moderate new symptoms, the algorithm calls for clinicians to delay I-O therapy per protocol, consult with pulmonary and ID specialists, monitor symptoms daily and consider hospitalization, start the patient on steroids with 1.0 mg/kg per day methylprednisolone IV or the oral equivalent, and consider bronchoscopy and lung biopsy.
For patients with grade 3 or 4 toxicities, marked by severe new symptoms, new or worsening hypoxia, or other life-threatening symptoms, the algorithm states that clinicians should discontinue I-O therapy, hospitalize the patient, consult with pulmonary and ID, give 2-4 mg/kg per day methylprednisolone IV or the oral equivalent, add prophylactic antibiotics for opportunistic infections, and consider bronchoscopy and lung biopsy.
At Yale, Dr. Gettinger and colleagues, when presented with a symptomatic patient on a PD-axis inhibitor, will first rule out other etiologies such as infection, chronic obstructive pulmonary disease exacerbation, or cancer progression), typically with bronchoscopy.
For patients with moderate pneumonitis, they may treat with prednisone for 1 or 2 weeks, with a 4-6 week taper begun as symptoms start to resolve.
“In a patient who has profound hypoxia and shortness of breath, we may want to go higher: We might give them 2 mg/kg twice a day of [methylprednisolone] in the hospital, wait until they get better, and then slowly taper them, whether it be over 4 or 6 weeks or longer. Occasionally our patients need to get even higher doses of steroids and there’s really nothing to guide us. Who knows if 1 gram of [methylprednisolone] may be better than 60 g of prednisone? But we do it, and patients do get better with the higher doses,” he said.
In rare instances, patients may required other immunosuppressive agents, such as infliximab (Remicade), cyclophosphamide, or mycophenolate mofetil.
Patients who require additional immunosuppressive agents to resolve severe pneumonitis tend to have poor outcomes, Dr. Gettinger said.
Re-challenge of patients with a PD-1 or PD-L1 inhibitor following resolution of pneumonitis appears to be inadvisable for all patients except possibly those with grade 1 (asymptomatic) toxicity, due to the high risk of recurrence, he said.
AT AACR–NCI–EORTC
Key clinical point: Pneumonitis is an uncommon but potentially serious adverse event associated with PD-1/PD-L1 checkpoint inhibitor therapy.
Major finding: Pneumonitis occurs in about 4%-6% of patients with non–small-cell lung cancer treated with a PD-1 axis inhibitor.
Data source: Review of current understanding of pneumonitis associated with anti-PD-1/PD-L1 therapeutic agents.
Disclosures: Dr. Gettinger disclosed serving as a consultant for BMS.
Immune-related events with checkpoint inhibitors are manageable
BOSTON– Immune-related adverse events associated with checkpoint inhibitor therapy are generally mild to moderate and transient, but some less common side effects can be serious or even fatal, according to an immunotherapy researcher.
“Rapid identification of these side effects and initiation of systemic immunosuppression can improve outcomes without compromising the efficacy of immune-checkpoint inhibition,” said Dr. Antoine Italiano from the Institut Bergonié in Bordeaux, France.
There is also evidence to suggest that immune-related adverse events (irAEs) associated with the programmed-death 1 (PD-1) inhibitors pembrolizumab (Keytruda) and nivolumab (Opdivo) may be predictive of favorable outcomes. In contrast, although there was early clinical evidence to suggest that adverse reactions to immune checkpoint inhibition with cytotoxic T-lymphocyte–associated protein 4 (anti-CTLA-4) antibodies such as ipilimumab (Yervoy) correlate with outcomes, more recent evidence suggests that toxicity with this class of agents is not predictive of efficacy, Dr. Italiano said at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics.
“Correlation between safety profile and outcome must be confirmed by further studies,” he said, adding that “further studies are also needed to identify patients at high risk of poor tolerability.”
Immune-related adverse events associated with anti-CTLA-4 therapy generally involve organ systems such as the skin, digestive tract, and endocrine system. Rare adverse events reported with these agents include renal injury, sarcoidosis, uveitis, and myelitis, among others.
The events tend to arise around 10 weeks of therapy, following three cycles with either ipilimumab or the investigational agent tremelimumab. Late-occurring events, defined as those that arise more than 70 days after the last infusion, are uncommon, occurring in less than 7% of patients.
Most irAEs seen with anti-CTLA-4 therapy are reversible within about 6 weeks, although some events, such as hypophysitis (autoimmune inflammation of the pituitary gland), can take significantly longer to resolve, Dr. Italiano said.
He cited a recent systematic review and meta-analysis showing that among patients treated with any anti-CTLA-4, the overall incidence of all-grade irAEs was 72%, and the overall incidence of high-grade irAEs was 24%. This study also showed that there was a dose-dependent risk of developing irAEs with ipilimumab, with the incidence of all grades of events at 61% for the 3 mg/kg dose, and 79% for the 10 mg/kg dose.
Two potential biomarkers for gastrointestinal irAEs, the neutrophil-activation markers CD177 and CEACAM1, were identified in a 2013 study. This finding suggests a possible role of neutrophils in ipilimumab-associated GI irAEs, Dr. Italiano noted.
Evidence from early clinical studies of ipilimumab in metastatic melanoma suggested that irAEs correlated with outcomes, but a study published in October 2015 seems to debunk this notion, showing that among 298 patients treated with ipilimumab, neither time to treatment failure nor overall survival were affected by the occurrence of irAEs, he added.
As to whether therapy with anti-CTLA-4 antibodies is safe for treatment of cancer for patients with autoimmune diseases or immunodeficient states, the jury is still out, because these patients were typically excluded from clinical trials.
“But there are a few recent case reports suggesting that treating patients with autoimmune disease with ipilimumab is safe and does not induce exacerbation of the symptoms of the underlying autoimmune disease,” Dr. Italiano said.
PD-1 inhibitors
Adverse events common to the PD-1 inhibitors pembrolizumab and nivolumab and occurring in more than 5% of patients with each include fatigue/asthenia, decreased appetite, diarrhea, rash, pruritus, nausea, and arthralgia.
In clinical trials of the agents for treatment of melanoma, vitiligo was the most common irAE, occurring in 7%-8% of patients. Other events, occurring in similar frequency across the various trials, included hypo- or hyperthyroidism, pneumonitis, colitis, hepatitis, renal failure/nephritis, uveitis/iritis, and hypophysitis.
The time to first occurrence and resolution of irAEs with the PD-1 inhibitors varies by organ system, with skin toxicity occurring within the few weeks of therapy, peaking at about 15 weeks, and resolving by about 25 weeks. Gastrointestinal toxicities crop up at about 10 weeks, but quickly resolve.
Among the less common (less than 10%) irAEs, hepatic and pulmonary events seen to occur around week 8 or 9 and resolve within 2-4 weeks, whereas endocrine events start showing up around week 10, peak at about 25 weeks, and resolve around 40 weeks.
Among patients treated with PD-1 inhibitors for non–small cell lung cancer, the adverse-event profile is similar to that seen in treatment of patients with melanoma, except for the absence of vitiligo, Dr. Italiano noted.
In contrast to the CTLA-4 inhibitors, irAEs seen with the PD-1 inhibitors, especially cutaneous events, appear to be associated with favorable outcomes.
For example, in a prospective, single-center observational study of pembrolizumab in 67 patients with metastatic melanoma, 17 developed vitiligo, and 12 of these patients had an objective response (18% complete and 53% partial responses). The objective response rate in this group was 71%, compared with 28% (14 of 50 patients) for those who did not develop vitiligo.
In a second, retrospective study of 83 patients enrolled in clinical trials of pembrolizumab for melanoma, non–small cell lung cancer, prostate cancer, and Merkel cell carcinoma, patients in each of three pembrolizumab dosing groups who developed cutaneous AEs had significantly longer progression-free intervals than patients who did not develop cutaneous AEs.
A similar correlation between cutaneous events with nivolumab and favorable outcomes was seen in a study of pooled data on 148 patients with resected or unresectable metastatic melanoma. The investigators found that both rash and vitiligo correlated significantly with better overall survival.
BOSTON– Immune-related adverse events associated with checkpoint inhibitor therapy are generally mild to moderate and transient, but some less common side effects can be serious or even fatal, according to an immunotherapy researcher.
“Rapid identification of these side effects and initiation of systemic immunosuppression can improve outcomes without compromising the efficacy of immune-checkpoint inhibition,” said Dr. Antoine Italiano from the Institut Bergonié in Bordeaux, France.
There is also evidence to suggest that immune-related adverse events (irAEs) associated with the programmed-death 1 (PD-1) inhibitors pembrolizumab (Keytruda) and nivolumab (Opdivo) may be predictive of favorable outcomes. In contrast, although there was early clinical evidence to suggest that adverse reactions to immune checkpoint inhibition with cytotoxic T-lymphocyte–associated protein 4 (anti-CTLA-4) antibodies such as ipilimumab (Yervoy) correlate with outcomes, more recent evidence suggests that toxicity with this class of agents is not predictive of efficacy, Dr. Italiano said at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics.
“Correlation between safety profile and outcome must be confirmed by further studies,” he said, adding that “further studies are also needed to identify patients at high risk of poor tolerability.”
Immune-related adverse events associated with anti-CTLA-4 therapy generally involve organ systems such as the skin, digestive tract, and endocrine system. Rare adverse events reported with these agents include renal injury, sarcoidosis, uveitis, and myelitis, among others.
The events tend to arise around 10 weeks of therapy, following three cycles with either ipilimumab or the investigational agent tremelimumab. Late-occurring events, defined as those that arise more than 70 days after the last infusion, are uncommon, occurring in less than 7% of patients.
Most irAEs seen with anti-CTLA-4 therapy are reversible within about 6 weeks, although some events, such as hypophysitis (autoimmune inflammation of the pituitary gland), can take significantly longer to resolve, Dr. Italiano said.
He cited a recent systematic review and meta-analysis showing that among patients treated with any anti-CTLA-4, the overall incidence of all-grade irAEs was 72%, and the overall incidence of high-grade irAEs was 24%. This study also showed that there was a dose-dependent risk of developing irAEs with ipilimumab, with the incidence of all grades of events at 61% for the 3 mg/kg dose, and 79% for the 10 mg/kg dose.
Two potential biomarkers for gastrointestinal irAEs, the neutrophil-activation markers CD177 and CEACAM1, were identified in a 2013 study. This finding suggests a possible role of neutrophils in ipilimumab-associated GI irAEs, Dr. Italiano noted.
Evidence from early clinical studies of ipilimumab in metastatic melanoma suggested that irAEs correlated with outcomes, but a study published in October 2015 seems to debunk this notion, showing that among 298 patients treated with ipilimumab, neither time to treatment failure nor overall survival were affected by the occurrence of irAEs, he added.
As to whether therapy with anti-CTLA-4 antibodies is safe for treatment of cancer for patients with autoimmune diseases or immunodeficient states, the jury is still out, because these patients were typically excluded from clinical trials.
“But there are a few recent case reports suggesting that treating patients with autoimmune disease with ipilimumab is safe and does not induce exacerbation of the symptoms of the underlying autoimmune disease,” Dr. Italiano said.
PD-1 inhibitors
Adverse events common to the PD-1 inhibitors pembrolizumab and nivolumab and occurring in more than 5% of patients with each include fatigue/asthenia, decreased appetite, diarrhea, rash, pruritus, nausea, and arthralgia.
In clinical trials of the agents for treatment of melanoma, vitiligo was the most common irAE, occurring in 7%-8% of patients. Other events, occurring in similar frequency across the various trials, included hypo- or hyperthyroidism, pneumonitis, colitis, hepatitis, renal failure/nephritis, uveitis/iritis, and hypophysitis.
The time to first occurrence and resolution of irAEs with the PD-1 inhibitors varies by organ system, with skin toxicity occurring within the few weeks of therapy, peaking at about 15 weeks, and resolving by about 25 weeks. Gastrointestinal toxicities crop up at about 10 weeks, but quickly resolve.
Among the less common (less than 10%) irAEs, hepatic and pulmonary events seen to occur around week 8 or 9 and resolve within 2-4 weeks, whereas endocrine events start showing up around week 10, peak at about 25 weeks, and resolve around 40 weeks.
Among patients treated with PD-1 inhibitors for non–small cell lung cancer, the adverse-event profile is similar to that seen in treatment of patients with melanoma, except for the absence of vitiligo, Dr. Italiano noted.
In contrast to the CTLA-4 inhibitors, irAEs seen with the PD-1 inhibitors, especially cutaneous events, appear to be associated with favorable outcomes.
For example, in a prospective, single-center observational study of pembrolizumab in 67 patients with metastatic melanoma, 17 developed vitiligo, and 12 of these patients had an objective response (18% complete and 53% partial responses). The objective response rate in this group was 71%, compared with 28% (14 of 50 patients) for those who did not develop vitiligo.
In a second, retrospective study of 83 patients enrolled in clinical trials of pembrolizumab for melanoma, non–small cell lung cancer, prostate cancer, and Merkel cell carcinoma, patients in each of three pembrolizumab dosing groups who developed cutaneous AEs had significantly longer progression-free intervals than patients who did not develop cutaneous AEs.
A similar correlation between cutaneous events with nivolumab and favorable outcomes was seen in a study of pooled data on 148 patients with resected or unresectable metastatic melanoma. The investigators found that both rash and vitiligo correlated significantly with better overall survival.
BOSTON– Immune-related adverse events associated with checkpoint inhibitor therapy are generally mild to moderate and transient, but some less common side effects can be serious or even fatal, according to an immunotherapy researcher.
“Rapid identification of these side effects and initiation of systemic immunosuppression can improve outcomes without compromising the efficacy of immune-checkpoint inhibition,” said Dr. Antoine Italiano from the Institut Bergonié in Bordeaux, France.
There is also evidence to suggest that immune-related adverse events (irAEs) associated with the programmed-death 1 (PD-1) inhibitors pembrolizumab (Keytruda) and nivolumab (Opdivo) may be predictive of favorable outcomes. In contrast, although there was early clinical evidence to suggest that adverse reactions to immune checkpoint inhibition with cytotoxic T-lymphocyte–associated protein 4 (anti-CTLA-4) antibodies such as ipilimumab (Yervoy) correlate with outcomes, more recent evidence suggests that toxicity with this class of agents is not predictive of efficacy, Dr. Italiano said at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics.
“Correlation between safety profile and outcome must be confirmed by further studies,” he said, adding that “further studies are also needed to identify patients at high risk of poor tolerability.”
Immune-related adverse events associated with anti-CTLA-4 therapy generally involve organ systems such as the skin, digestive tract, and endocrine system. Rare adverse events reported with these agents include renal injury, sarcoidosis, uveitis, and myelitis, among others.
The events tend to arise around 10 weeks of therapy, following three cycles with either ipilimumab or the investigational agent tremelimumab. Late-occurring events, defined as those that arise more than 70 days after the last infusion, are uncommon, occurring in less than 7% of patients.
Most irAEs seen with anti-CTLA-4 therapy are reversible within about 6 weeks, although some events, such as hypophysitis (autoimmune inflammation of the pituitary gland), can take significantly longer to resolve, Dr. Italiano said.
He cited a recent systematic review and meta-analysis showing that among patients treated with any anti-CTLA-4, the overall incidence of all-grade irAEs was 72%, and the overall incidence of high-grade irAEs was 24%. This study also showed that there was a dose-dependent risk of developing irAEs with ipilimumab, with the incidence of all grades of events at 61% for the 3 mg/kg dose, and 79% for the 10 mg/kg dose.
Two potential biomarkers for gastrointestinal irAEs, the neutrophil-activation markers CD177 and CEACAM1, were identified in a 2013 study. This finding suggests a possible role of neutrophils in ipilimumab-associated GI irAEs, Dr. Italiano noted.
Evidence from early clinical studies of ipilimumab in metastatic melanoma suggested that irAEs correlated with outcomes, but a study published in October 2015 seems to debunk this notion, showing that among 298 patients treated with ipilimumab, neither time to treatment failure nor overall survival were affected by the occurrence of irAEs, he added.
As to whether therapy with anti-CTLA-4 antibodies is safe for treatment of cancer for patients with autoimmune diseases or immunodeficient states, the jury is still out, because these patients were typically excluded from clinical trials.
“But there are a few recent case reports suggesting that treating patients with autoimmune disease with ipilimumab is safe and does not induce exacerbation of the symptoms of the underlying autoimmune disease,” Dr. Italiano said.
PD-1 inhibitors
Adverse events common to the PD-1 inhibitors pembrolizumab and nivolumab and occurring in more than 5% of patients with each include fatigue/asthenia, decreased appetite, diarrhea, rash, pruritus, nausea, and arthralgia.
In clinical trials of the agents for treatment of melanoma, vitiligo was the most common irAE, occurring in 7%-8% of patients. Other events, occurring in similar frequency across the various trials, included hypo- or hyperthyroidism, pneumonitis, colitis, hepatitis, renal failure/nephritis, uveitis/iritis, and hypophysitis.
The time to first occurrence and resolution of irAEs with the PD-1 inhibitors varies by organ system, with skin toxicity occurring within the few weeks of therapy, peaking at about 15 weeks, and resolving by about 25 weeks. Gastrointestinal toxicities crop up at about 10 weeks, but quickly resolve.
Among the less common (less than 10%) irAEs, hepatic and pulmonary events seen to occur around week 8 or 9 and resolve within 2-4 weeks, whereas endocrine events start showing up around week 10, peak at about 25 weeks, and resolve around 40 weeks.
Among patients treated with PD-1 inhibitors for non–small cell lung cancer, the adverse-event profile is similar to that seen in treatment of patients with melanoma, except for the absence of vitiligo, Dr. Italiano noted.
In contrast to the CTLA-4 inhibitors, irAEs seen with the PD-1 inhibitors, especially cutaneous events, appear to be associated with favorable outcomes.
For example, in a prospective, single-center observational study of pembrolizumab in 67 patients with metastatic melanoma, 17 developed vitiligo, and 12 of these patients had an objective response (18% complete and 53% partial responses). The objective response rate in this group was 71%, compared with 28% (14 of 50 patients) for those who did not develop vitiligo.
In a second, retrospective study of 83 patients enrolled in clinical trials of pembrolizumab for melanoma, non–small cell lung cancer, prostate cancer, and Merkel cell carcinoma, patients in each of three pembrolizumab dosing groups who developed cutaneous AEs had significantly longer progression-free intervals than patients who did not develop cutaneous AEs.
A similar correlation between cutaneous events with nivolumab and favorable outcomes was seen in a study of pooled data on 148 patients with resected or unresectable metastatic melanoma. The investigators found that both rash and vitiligo correlated significantly with better overall survival.
AT AACR–NCI–EORTC
Key clinical point: Inhibitors of CTLA-4 and PD-1/PD-L1 are associated with manageable immune-related adverse events.
Major finding: Cutaneous adverse events with PD-1 inhibitor therapy appear to be predictive of favorable outcomes.
Data source: Review of current knowledge of the immune-related adverse events associated with checkpoint inhibitors.
Disclosures: Dr. Italiano reported no conflicts of interest.