User login
Inhibitor gets breakthrough designation for CLL
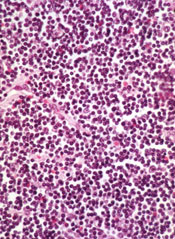
Though a safety issue previously slowed development of the BCL-2 inhibitor venetoclax (ABT-199), the drug is now moving through the pipeline.
The US Food and Drug Administration (FDA) has granted venetoclax breakthrough therapy designation to treat patients with relapsed or refractory chronic lymphocytic leukemia (CLL), including those with 17p deletion.
The drug has proven active against CLL and other hematologic malignancies.
However, it is also known to induce tumor lysis syndrome (TLS). In fact, TLS-related deaths temporarily halted enrollment in trials of venetoclax. But researchers discovered ways to reduce the risk of TLS, and the trials continued.
Now, the FDA has granted the drug breakthrough designation, which is intended to expedite the development and review of drugs indicated for serious or life-threatening conditions.
The criteria for breakthrough designation include preliminary clinical evidence suggesting the drug may offer substantial improvement on at least one clinically significant endpoint compared to available therapy.
Venetoclax in CLL/SLL
Results presented at the 2014 EHA Congress suggested that venetoclax can be effective in patients with CLL/small lymphocytic lymphoma (SLL), and certain measures can reduce the risk of TLS.
Researchers reported that modifying the dosing schedule of venetoclax, administering TLS prophylaxis, and monitoring patients can decrease or eliminate the risk of TLS. And venetoclax can produce responses in patients with high-risk disease.
In a phase 1 trial, the researchers tested venetoclax monotherapy in 105 patients with high-risk CLL/SLL. Seventy-eight patients were evaluable for treatment response as of April 2014. Nineteen of these patients had del (17p), 41 were fludarabine-refractory, and 24 had unmutated IGHV.
The response rate was 77% overall, 79% among patients with del (17p), 76% in patients who were fludarabine-refractory, and 75% in those with unmutated IGHV. The complete response rates were 23%, 26%, 22%, and 29%, respectively.
The median progression-free survival was about 18 months overall, but the median progression-free survival had not been reached for patients treated at or above 400 mg.
Seven patients developed TLS. One of these patients died, and 1 required dialysis. At the time of analysis, there were no cases of TLS among the 49 patients who received TLS prophylaxis and were given venetoclax according to the modified dosing schedule.
Common treatment-emergent adverse events included diarrhea (40%), neutropenia (36%), and nausea (35%). Grade 3/4 neutropenia occurred in 33% of patients, and febrile neutropenia occurred in 4%.
Thirty-seven patients discontinued treatment—22 due to progressive disease, 12 due to adverse events, and 3 for other reasons (1 required warfarin, and 2 proceeded to transplant).
Venetoclax is now being tested in phase 2 and 3 trials of CLL, as well as trials in other hematologic malignancies. Venetoclax is under development by AbbVie and Genentech/Roche. ![]()

Though a safety issue previously slowed development of the BCL-2 inhibitor venetoclax (ABT-199), the drug is now moving through the pipeline.
The US Food and Drug Administration (FDA) has granted venetoclax breakthrough therapy designation to treat patients with relapsed or refractory chronic lymphocytic leukemia (CLL), including those with 17p deletion.
The drug has proven active against CLL and other hematologic malignancies.
However, it is also known to induce tumor lysis syndrome (TLS). In fact, TLS-related deaths temporarily halted enrollment in trials of venetoclax. But researchers discovered ways to reduce the risk of TLS, and the trials continued.
Now, the FDA has granted the drug breakthrough designation, which is intended to expedite the development and review of drugs indicated for serious or life-threatening conditions.
The criteria for breakthrough designation include preliminary clinical evidence suggesting the drug may offer substantial improvement on at least one clinically significant endpoint compared to available therapy.
Venetoclax in CLL/SLL
Results presented at the 2014 EHA Congress suggested that venetoclax can be effective in patients with CLL/small lymphocytic lymphoma (SLL), and certain measures can reduce the risk of TLS.
Researchers reported that modifying the dosing schedule of venetoclax, administering TLS prophylaxis, and monitoring patients can decrease or eliminate the risk of TLS. And venetoclax can produce responses in patients with high-risk disease.
In a phase 1 trial, the researchers tested venetoclax monotherapy in 105 patients with high-risk CLL/SLL. Seventy-eight patients were evaluable for treatment response as of April 2014. Nineteen of these patients had del (17p), 41 were fludarabine-refractory, and 24 had unmutated IGHV.
The response rate was 77% overall, 79% among patients with del (17p), 76% in patients who were fludarabine-refractory, and 75% in those with unmutated IGHV. The complete response rates were 23%, 26%, 22%, and 29%, respectively.
The median progression-free survival was about 18 months overall, but the median progression-free survival had not been reached for patients treated at or above 400 mg.
Seven patients developed TLS. One of these patients died, and 1 required dialysis. At the time of analysis, there were no cases of TLS among the 49 patients who received TLS prophylaxis and were given venetoclax according to the modified dosing schedule.
Common treatment-emergent adverse events included diarrhea (40%), neutropenia (36%), and nausea (35%). Grade 3/4 neutropenia occurred in 33% of patients, and febrile neutropenia occurred in 4%.
Thirty-seven patients discontinued treatment—22 due to progressive disease, 12 due to adverse events, and 3 for other reasons (1 required warfarin, and 2 proceeded to transplant).
Venetoclax is now being tested in phase 2 and 3 trials of CLL, as well as trials in other hematologic malignancies. Venetoclax is under development by AbbVie and Genentech/Roche. ![]()

Though a safety issue previously slowed development of the BCL-2 inhibitor venetoclax (ABT-199), the drug is now moving through the pipeline.
The US Food and Drug Administration (FDA) has granted venetoclax breakthrough therapy designation to treat patients with relapsed or refractory chronic lymphocytic leukemia (CLL), including those with 17p deletion.
The drug has proven active against CLL and other hematologic malignancies.
However, it is also known to induce tumor lysis syndrome (TLS). In fact, TLS-related deaths temporarily halted enrollment in trials of venetoclax. But researchers discovered ways to reduce the risk of TLS, and the trials continued.
Now, the FDA has granted the drug breakthrough designation, which is intended to expedite the development and review of drugs indicated for serious or life-threatening conditions.
The criteria for breakthrough designation include preliminary clinical evidence suggesting the drug may offer substantial improvement on at least one clinically significant endpoint compared to available therapy.
Venetoclax in CLL/SLL
Results presented at the 2014 EHA Congress suggested that venetoclax can be effective in patients with CLL/small lymphocytic lymphoma (SLL), and certain measures can reduce the risk of TLS.
Researchers reported that modifying the dosing schedule of venetoclax, administering TLS prophylaxis, and monitoring patients can decrease or eliminate the risk of TLS. And venetoclax can produce responses in patients with high-risk disease.
In a phase 1 trial, the researchers tested venetoclax monotherapy in 105 patients with high-risk CLL/SLL. Seventy-eight patients were evaluable for treatment response as of April 2014. Nineteen of these patients had del (17p), 41 were fludarabine-refractory, and 24 had unmutated IGHV.
The response rate was 77% overall, 79% among patients with del (17p), 76% in patients who were fludarabine-refractory, and 75% in those with unmutated IGHV. The complete response rates were 23%, 26%, 22%, and 29%, respectively.
The median progression-free survival was about 18 months overall, but the median progression-free survival had not been reached for patients treated at or above 400 mg.
Seven patients developed TLS. One of these patients died, and 1 required dialysis. At the time of analysis, there were no cases of TLS among the 49 patients who received TLS prophylaxis and were given venetoclax according to the modified dosing schedule.
Common treatment-emergent adverse events included diarrhea (40%), neutropenia (36%), and nausea (35%). Grade 3/4 neutropenia occurred in 33% of patients, and febrile neutropenia occurred in 4%.
Thirty-seven patients discontinued treatment—22 due to progressive disease, 12 due to adverse events, and 3 for other reasons (1 required warfarin, and 2 proceeded to transplant).
Venetoclax is now being tested in phase 2 and 3 trials of CLL, as well as trials in other hematologic malignancies. Venetoclax is under development by AbbVie and Genentech/Roche. ![]()
Defining the role of TAMs in DLBCL

pseudopodia to engulf particles
PHILADELPHIA—New research suggests the prognostic value of tumor-associated macrophages (TAMs) is disease-specific as well as treatment-specific.
Investigators set out to determine if TAMs have a negative prognostic impact in diffuse large B-cell lymphoma (DLBCL), as previous studies produced conflicting results.
The team found that higher TAM levels are associated with worse survival in DLBCL, but only in patients who do not receive rituximab. The drug can overcome the poor prognosis TAMs confer in DLBCL.
Eri Matsuki, MD, PhD, of Memorial Sloan-Kettering Cancer Center in New York, New York, and her colleagues presented these findings in a poster at the AACR Annual Meeting 2015 (abstract 2371*).
To ascertain the role of TAMs in DLBCL, the investigators analyzed specimens from 103 DLBCL patients, 61 of whom received rituximab, 33 who did not, and 9 whose rituximab status was unknown. The team first looked at the expression of CD163 as a marker of TAMs.
“CD163 is more specific to M2-type macrophages, which have pro-tumor effects, compared to a more pan-macrophage marker which is widely used—CD68,” Dr Matsuki noted.
She and her colleagues found that a high level of CD163-positive cells (more than 150) was significantly associated with advanced-stage disease (P=0.016), non-GCB DLBCL (P=0.0071), and higher expression of c-Myc (P=0.0022).
“Our interpretation of that at this point is that, the more aggressive the tumor, the more likely that it would induce macrophages into the tissue,” Dr Matsuki said.
The investigators then assessed the impact of rituximab use. Among patients who didn’t receive rituximab, having a high level of CD163-positive cells (more than 150) was associated with inferior overall survival (OS, P=0.022). However, if patients did receive rituximab, there was no significant difference in OS.
Dr Matsuki said this supports previous studies showing that the prognostic effect of TAMs diminishes with rituximab use, as well as the in vitro finding that M2 macrophages exhibit increased phagocytosis of rituximab-opsonized tumor cells.
“So overall, what we’re seeing is that . . . the negative influence of TAMs can be overcome with rituximab use,” she summarized.
Dr Matsuki and her colleagues also looked at the patients’ lymphocyte-to-monocyte-ratio (LMR) because TAMs partly arise from peripheral blood monocytes.
The team found that having an LMR higher than 2.77 was significantly associated with superior OS (P=0.03) in patients who did not receive rituximab. And there was a trend toward improved OS with a higher LMR in patients who did receive the drug (P=0.07).
The investigators believe the differences they observed in the prognostic value of CD163 and LMR could be explained by the fact that TAMs are derived from both circulating monocytes and resident macrophages.
Dr Matsuki said this research has improved her group’s understanding of DLBCL, but they are still working to identify additional biomarkers associated with prognosis in this disease. ![]()
*Information in the abstract differs from that presented at the meeting.

pseudopodia to engulf particles
PHILADELPHIA—New research suggests the prognostic value of tumor-associated macrophages (TAMs) is disease-specific as well as treatment-specific.
Investigators set out to determine if TAMs have a negative prognostic impact in diffuse large B-cell lymphoma (DLBCL), as previous studies produced conflicting results.
The team found that higher TAM levels are associated with worse survival in DLBCL, but only in patients who do not receive rituximab. The drug can overcome the poor prognosis TAMs confer in DLBCL.
Eri Matsuki, MD, PhD, of Memorial Sloan-Kettering Cancer Center in New York, New York, and her colleagues presented these findings in a poster at the AACR Annual Meeting 2015 (abstract 2371*).
To ascertain the role of TAMs in DLBCL, the investigators analyzed specimens from 103 DLBCL patients, 61 of whom received rituximab, 33 who did not, and 9 whose rituximab status was unknown. The team first looked at the expression of CD163 as a marker of TAMs.
“CD163 is more specific to M2-type macrophages, which have pro-tumor effects, compared to a more pan-macrophage marker which is widely used—CD68,” Dr Matsuki noted.
She and her colleagues found that a high level of CD163-positive cells (more than 150) was significantly associated with advanced-stage disease (P=0.016), non-GCB DLBCL (P=0.0071), and higher expression of c-Myc (P=0.0022).
“Our interpretation of that at this point is that, the more aggressive the tumor, the more likely that it would induce macrophages into the tissue,” Dr Matsuki said.
The investigators then assessed the impact of rituximab use. Among patients who didn’t receive rituximab, having a high level of CD163-positive cells (more than 150) was associated with inferior overall survival (OS, P=0.022). However, if patients did receive rituximab, there was no significant difference in OS.
Dr Matsuki said this supports previous studies showing that the prognostic effect of TAMs diminishes with rituximab use, as well as the in vitro finding that M2 macrophages exhibit increased phagocytosis of rituximab-opsonized tumor cells.
“So overall, what we’re seeing is that . . . the negative influence of TAMs can be overcome with rituximab use,” she summarized.
Dr Matsuki and her colleagues also looked at the patients’ lymphocyte-to-monocyte-ratio (LMR) because TAMs partly arise from peripheral blood monocytes.
The team found that having an LMR higher than 2.77 was significantly associated with superior OS (P=0.03) in patients who did not receive rituximab. And there was a trend toward improved OS with a higher LMR in patients who did receive the drug (P=0.07).
The investigators believe the differences they observed in the prognostic value of CD163 and LMR could be explained by the fact that TAMs are derived from both circulating monocytes and resident macrophages.
Dr Matsuki said this research has improved her group’s understanding of DLBCL, but they are still working to identify additional biomarkers associated with prognosis in this disease. ![]()
*Information in the abstract differs from that presented at the meeting.

pseudopodia to engulf particles
PHILADELPHIA—New research suggests the prognostic value of tumor-associated macrophages (TAMs) is disease-specific as well as treatment-specific.
Investigators set out to determine if TAMs have a negative prognostic impact in diffuse large B-cell lymphoma (DLBCL), as previous studies produced conflicting results.
The team found that higher TAM levels are associated with worse survival in DLBCL, but only in patients who do not receive rituximab. The drug can overcome the poor prognosis TAMs confer in DLBCL.
Eri Matsuki, MD, PhD, of Memorial Sloan-Kettering Cancer Center in New York, New York, and her colleagues presented these findings in a poster at the AACR Annual Meeting 2015 (abstract 2371*).
To ascertain the role of TAMs in DLBCL, the investigators analyzed specimens from 103 DLBCL patients, 61 of whom received rituximab, 33 who did not, and 9 whose rituximab status was unknown. The team first looked at the expression of CD163 as a marker of TAMs.
“CD163 is more specific to M2-type macrophages, which have pro-tumor effects, compared to a more pan-macrophage marker which is widely used—CD68,” Dr Matsuki noted.
She and her colleagues found that a high level of CD163-positive cells (more than 150) was significantly associated with advanced-stage disease (P=0.016), non-GCB DLBCL (P=0.0071), and higher expression of c-Myc (P=0.0022).
“Our interpretation of that at this point is that, the more aggressive the tumor, the more likely that it would induce macrophages into the tissue,” Dr Matsuki said.
The investigators then assessed the impact of rituximab use. Among patients who didn’t receive rituximab, having a high level of CD163-positive cells (more than 150) was associated with inferior overall survival (OS, P=0.022). However, if patients did receive rituximab, there was no significant difference in OS.
Dr Matsuki said this supports previous studies showing that the prognostic effect of TAMs diminishes with rituximab use, as well as the in vitro finding that M2 macrophages exhibit increased phagocytosis of rituximab-opsonized tumor cells.
“So overall, what we’re seeing is that . . . the negative influence of TAMs can be overcome with rituximab use,” she summarized.
Dr Matsuki and her colleagues also looked at the patients’ lymphocyte-to-monocyte-ratio (LMR) because TAMs partly arise from peripheral blood monocytes.
The team found that having an LMR higher than 2.77 was significantly associated with superior OS (P=0.03) in patients who did not receive rituximab. And there was a trend toward improved OS with a higher LMR in patients who did receive the drug (P=0.07).
The investigators believe the differences they observed in the prognostic value of CD163 and LMR could be explained by the fact that TAMs are derived from both circulating monocytes and resident macrophages.
Dr Matsuki said this research has improved her group’s understanding of DLBCL, but they are still working to identify additional biomarkers associated with prognosis in this disease. ![]()
*Information in the abstract differs from that presented at the meeting.
Cancer rate doubles in HCV patients
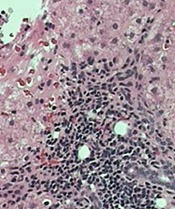
tissue with active HCV
Photo: Sutter Health
VIENNA, AUSTRIA—A 5-year retrospective study has shown that the cancer rate in patients with hepatitis C virus (HCV) is about double that for people without HCV, even when liver cancer is excluded.
And when liver cancer is included, the rate increases to 2.5 times higher in people with HCV.
Researchers presented these findings at the International Liver Congress 2015 as abstract 0058.
The team reviewed patient records from 2008 to 2012 at Kaiser Permanente Southern California, recording all cancer diagnoses in patients 18 years or older with or without HCV.
During the 5-year time period, there were 145,210 patient years in the HCV cohort and 13,948,826 patient years in the non-HCV cohort.
The mean age at cancer diagnosis was 61.8 in the HCV cohort and 63.5 in the non-HCV cohort.
Researchers recorded 2213 cancer diagnoses in the HCV cohort (1524/100,000). This number decreased to 1654 when they excluded liver cancer (1139/100,000).
In the non-HCV cohort, they recorded 84,419 cancer diagnoses (605/100,000), which decreased to 83,795 when liver cancer was excluded (601/100,000).
Cancer types known to be associated with HCV include non-Hodgkin lymphoma (NHL), renal and prostate cancers, and liver cancer.
NHL occurred 3.63 times more frequently in patients with HCV than in those without HCV, and myeloma occurred 2.93 times more frequently.
Renal cancer occurred 3.27 times and prostate cancer 1.98 times more frequently in patients with HCV than in those without.
“The results suggest that cancer rates are increased in the cohort of hepatitis C patients versus the non-hepatitis C patients, both including and excluding liver cancers,” said senior study author Lisa Nyberg, MD, MPH, of Kaiser Permanente.
“These findings certainly point to the suggestion that hepatitis C may be associated with an increased risk of cancer.”
However, she added that the findings “must be interpreted with caution, as the study also showed that confounding factors such as alcohol abuse, tobacco, obesity, and diabetes modified the results.” ![]()

tissue with active HCV
Photo: Sutter Health
VIENNA, AUSTRIA—A 5-year retrospective study has shown that the cancer rate in patients with hepatitis C virus (HCV) is about double that for people without HCV, even when liver cancer is excluded.
And when liver cancer is included, the rate increases to 2.5 times higher in people with HCV.
Researchers presented these findings at the International Liver Congress 2015 as abstract 0058.
The team reviewed patient records from 2008 to 2012 at Kaiser Permanente Southern California, recording all cancer diagnoses in patients 18 years or older with or without HCV.
During the 5-year time period, there were 145,210 patient years in the HCV cohort and 13,948,826 patient years in the non-HCV cohort.
The mean age at cancer diagnosis was 61.8 in the HCV cohort and 63.5 in the non-HCV cohort.
Researchers recorded 2213 cancer diagnoses in the HCV cohort (1524/100,000). This number decreased to 1654 when they excluded liver cancer (1139/100,000).
In the non-HCV cohort, they recorded 84,419 cancer diagnoses (605/100,000), which decreased to 83,795 when liver cancer was excluded (601/100,000).
Cancer types known to be associated with HCV include non-Hodgkin lymphoma (NHL), renal and prostate cancers, and liver cancer.
NHL occurred 3.63 times more frequently in patients with HCV than in those without HCV, and myeloma occurred 2.93 times more frequently.
Renal cancer occurred 3.27 times and prostate cancer 1.98 times more frequently in patients with HCV than in those without.
“The results suggest that cancer rates are increased in the cohort of hepatitis C patients versus the non-hepatitis C patients, both including and excluding liver cancers,” said senior study author Lisa Nyberg, MD, MPH, of Kaiser Permanente.
“These findings certainly point to the suggestion that hepatitis C may be associated with an increased risk of cancer.”
However, she added that the findings “must be interpreted with caution, as the study also showed that confounding factors such as alcohol abuse, tobacco, obesity, and diabetes modified the results.” ![]()

tissue with active HCV
Photo: Sutter Health
VIENNA, AUSTRIA—A 5-year retrospective study has shown that the cancer rate in patients with hepatitis C virus (HCV) is about double that for people without HCV, even when liver cancer is excluded.
And when liver cancer is included, the rate increases to 2.5 times higher in people with HCV.
Researchers presented these findings at the International Liver Congress 2015 as abstract 0058.
The team reviewed patient records from 2008 to 2012 at Kaiser Permanente Southern California, recording all cancer diagnoses in patients 18 years or older with or without HCV.
During the 5-year time period, there were 145,210 patient years in the HCV cohort and 13,948,826 patient years in the non-HCV cohort.
The mean age at cancer diagnosis was 61.8 in the HCV cohort and 63.5 in the non-HCV cohort.
Researchers recorded 2213 cancer diagnoses in the HCV cohort (1524/100,000). This number decreased to 1654 when they excluded liver cancer (1139/100,000).
In the non-HCV cohort, they recorded 84,419 cancer diagnoses (605/100,000), which decreased to 83,795 when liver cancer was excluded (601/100,000).
Cancer types known to be associated with HCV include non-Hodgkin lymphoma (NHL), renal and prostate cancers, and liver cancer.
NHL occurred 3.63 times more frequently in patients with HCV than in those without HCV, and myeloma occurred 2.93 times more frequently.
Renal cancer occurred 3.27 times and prostate cancer 1.98 times more frequently in patients with HCV than in those without.
“The results suggest that cancer rates are increased in the cohort of hepatitis C patients versus the non-hepatitis C patients, both including and excluding liver cancers,” said senior study author Lisa Nyberg, MD, MPH, of Kaiser Permanente.
“These findings certainly point to the suggestion that hepatitis C may be associated with an increased risk of cancer.”
However, she added that the findings “must be interpreted with caution, as the study also showed that confounding factors such as alcohol abuse, tobacco, obesity, and diabetes modified the results.” ![]()
Exercise may lower risk for non-Hodgkin’s lymphoma
Vigorous physical activity may help lower the risk of non-Hodgkin’s lymphoma, Terry Boyle, Ph.D., and his coauthors reported in Cancer Epidemiology, Biomarkers & Prevention.
In a case-control study of 820 non-Hodgkin’s lymphoma (NHL) patients and 848 controls, those in the higher (second, third, and fourth) quartiles of lifetime vigorous intensity exercise had a 25%-30% lower risk of NHL than did those in the lower quartile (odds ratios of 0.69; 0.68; and 0.75, respectively).
“Given this finding, more research on physical activity intensity and timing in relation to NHL risk is warranted,” Dr. Boyle and his associates wrote.
Read the full article here: Cancer Epidemiol. Biomarkers Prev. 2015;24:873-7 (doi:10.1158/1055-9965.EPI-14-1303).
Vigorous physical activity may help lower the risk of non-Hodgkin’s lymphoma, Terry Boyle, Ph.D., and his coauthors reported in Cancer Epidemiology, Biomarkers & Prevention.
In a case-control study of 820 non-Hodgkin’s lymphoma (NHL) patients and 848 controls, those in the higher (second, third, and fourth) quartiles of lifetime vigorous intensity exercise had a 25%-30% lower risk of NHL than did those in the lower quartile (odds ratios of 0.69; 0.68; and 0.75, respectively).
“Given this finding, more research on physical activity intensity and timing in relation to NHL risk is warranted,” Dr. Boyle and his associates wrote.
Read the full article here: Cancer Epidemiol. Biomarkers Prev. 2015;24:873-7 (doi:10.1158/1055-9965.EPI-14-1303).
Vigorous physical activity may help lower the risk of non-Hodgkin’s lymphoma, Terry Boyle, Ph.D., and his coauthors reported in Cancer Epidemiology, Biomarkers & Prevention.
In a case-control study of 820 non-Hodgkin’s lymphoma (NHL) patients and 848 controls, those in the higher (second, third, and fourth) quartiles of lifetime vigorous intensity exercise had a 25%-30% lower risk of NHL than did those in the lower quartile (odds ratios of 0.69; 0.68; and 0.75, respectively).
“Given this finding, more research on physical activity intensity and timing in relation to NHL risk is warranted,” Dr. Boyle and his associates wrote.
Read the full article here: Cancer Epidemiol. Biomarkers Prev. 2015;24:873-7 (doi:10.1158/1055-9965.EPI-14-1303).
Vigorous physical activity may lower risk of NHL

Photo by Shannon E. Renfroe
People who regularly engage in vigorous physical activity throughout their lifetime may have a lower risk of developing non-Hodgkin lymphoma (NHL), according to research published in Cancer Epidemiology, Biomarkers & Prevention.
“We know that being physically active reduces the risk of colon cancer and breast cancer, and also leads to a range of other physical and mental health benefits,” said study author Terry Boyle, PhD, of the University of British Columbia in Vancouver, Canada.
“Our findings suggest that people who do vigorous physical activity may also have a lower risk for NHL.”
Dr Boyle and his colleagues used data from a case-control study conducted between 2000 and 2004 in British Columbia. The team analyzed 749 NHL patients and 818 control subjects matched for age, gender, and residential location.
Study subjects recorded information on demographics and various risk factors for NHL, including lifetime recreational physical activity, on a questionnaire. Participants were asked to record the average number of days per week and average number of hours per day they performed mild, moderate, or vigorous physical activity for each decade of life.
The researchers defined “mild” activities as those that increase heart and breathing rates above resting level, “moderate” activities as those that increase heart rate moderately, and “vigorous” activities as those that increase breathing and heart rates to a high level. Mild and moderate activity were ultimately combined into a single category.
The team assigned a metabolic-equivalent (MET) value to the different types of physical activity.
Then, to assess the association between lifetime physical activity and NHL risk, the researchers calculated the average MET-hours per week over a lifetime for total physical activity, moderate-intensity activity, and vigorous-intensity activity. Finally, they classified participants into quartiles.
Participants who engaged in the most vigorously intense physical activity throughout their lifetime were classified in the second, third, and fourth quartiles. These subjects had about a 25% to 30% lower risk for NHL when compared to participants in the lowest (first) quartile of vigorously intense physical activity.
The adjusted odds ratio was 0.69 for the second quartile, 0.68 for the third, and 0.75 for the fourth (PTrend=0.072).
There was an inverse association between lifetime vigorous-intensity physical activity and overall NHL risk in males and females, as well as for all NHL subtypes. Furthermore, vigorous physical activity did not confer a greater benefit for any specific age group.
The researchers found no association between total lifetime physical activity and NHL risk or lifetime moderate-intensity physical activity and NHL risk.
Despite these results, Dr Boyle said there isn’t enough research on this topic to confirm that being physically active reduces the risk of NHL.
“So we are planning to pool data from several studies to investigate this topic further,” he said. “We know that different types of NHL may have different risk factors, so we are also planning to investigate whether physical activity influences the risk for different types of NHL in different ways.” ![]()

Photo by Shannon E. Renfroe
People who regularly engage in vigorous physical activity throughout their lifetime may have a lower risk of developing non-Hodgkin lymphoma (NHL), according to research published in Cancer Epidemiology, Biomarkers & Prevention.
“We know that being physically active reduces the risk of colon cancer and breast cancer, and also leads to a range of other physical and mental health benefits,” said study author Terry Boyle, PhD, of the University of British Columbia in Vancouver, Canada.
“Our findings suggest that people who do vigorous physical activity may also have a lower risk for NHL.”
Dr Boyle and his colleagues used data from a case-control study conducted between 2000 and 2004 in British Columbia. The team analyzed 749 NHL patients and 818 control subjects matched for age, gender, and residential location.
Study subjects recorded information on demographics and various risk factors for NHL, including lifetime recreational physical activity, on a questionnaire. Participants were asked to record the average number of days per week and average number of hours per day they performed mild, moderate, or vigorous physical activity for each decade of life.
The researchers defined “mild” activities as those that increase heart and breathing rates above resting level, “moderate” activities as those that increase heart rate moderately, and “vigorous” activities as those that increase breathing and heart rates to a high level. Mild and moderate activity were ultimately combined into a single category.
The team assigned a metabolic-equivalent (MET) value to the different types of physical activity.
Then, to assess the association between lifetime physical activity and NHL risk, the researchers calculated the average MET-hours per week over a lifetime for total physical activity, moderate-intensity activity, and vigorous-intensity activity. Finally, they classified participants into quartiles.
Participants who engaged in the most vigorously intense physical activity throughout their lifetime were classified in the second, third, and fourth quartiles. These subjects had about a 25% to 30% lower risk for NHL when compared to participants in the lowest (first) quartile of vigorously intense physical activity.
The adjusted odds ratio was 0.69 for the second quartile, 0.68 for the third, and 0.75 for the fourth (PTrend=0.072).
There was an inverse association between lifetime vigorous-intensity physical activity and overall NHL risk in males and females, as well as for all NHL subtypes. Furthermore, vigorous physical activity did not confer a greater benefit for any specific age group.
The researchers found no association between total lifetime physical activity and NHL risk or lifetime moderate-intensity physical activity and NHL risk.
Despite these results, Dr Boyle said there isn’t enough research on this topic to confirm that being physically active reduces the risk of NHL.
“So we are planning to pool data from several studies to investigate this topic further,” he said. “We know that different types of NHL may have different risk factors, so we are also planning to investigate whether physical activity influences the risk for different types of NHL in different ways.” ![]()

Photo by Shannon E. Renfroe
People who regularly engage in vigorous physical activity throughout their lifetime may have a lower risk of developing non-Hodgkin lymphoma (NHL), according to research published in Cancer Epidemiology, Biomarkers & Prevention.
“We know that being physically active reduces the risk of colon cancer and breast cancer, and also leads to a range of other physical and mental health benefits,” said study author Terry Boyle, PhD, of the University of British Columbia in Vancouver, Canada.
“Our findings suggest that people who do vigorous physical activity may also have a lower risk for NHL.”
Dr Boyle and his colleagues used data from a case-control study conducted between 2000 and 2004 in British Columbia. The team analyzed 749 NHL patients and 818 control subjects matched for age, gender, and residential location.
Study subjects recorded information on demographics and various risk factors for NHL, including lifetime recreational physical activity, on a questionnaire. Participants were asked to record the average number of days per week and average number of hours per day they performed mild, moderate, or vigorous physical activity for each decade of life.
The researchers defined “mild” activities as those that increase heart and breathing rates above resting level, “moderate” activities as those that increase heart rate moderately, and “vigorous” activities as those that increase breathing and heart rates to a high level. Mild and moderate activity were ultimately combined into a single category.
The team assigned a metabolic-equivalent (MET) value to the different types of physical activity.
Then, to assess the association between lifetime physical activity and NHL risk, the researchers calculated the average MET-hours per week over a lifetime for total physical activity, moderate-intensity activity, and vigorous-intensity activity. Finally, they classified participants into quartiles.
Participants who engaged in the most vigorously intense physical activity throughout their lifetime were classified in the second, third, and fourth quartiles. These subjects had about a 25% to 30% lower risk for NHL when compared to participants in the lowest (first) quartile of vigorously intense physical activity.
The adjusted odds ratio was 0.69 for the second quartile, 0.68 for the third, and 0.75 for the fourth (PTrend=0.072).
There was an inverse association between lifetime vigorous-intensity physical activity and overall NHL risk in males and females, as well as for all NHL subtypes. Furthermore, vigorous physical activity did not confer a greater benefit for any specific age group.
The researchers found no association between total lifetime physical activity and NHL risk or lifetime moderate-intensity physical activity and NHL risk.
Despite these results, Dr Boyle said there isn’t enough research on this topic to confirm that being physically active reduces the risk of NHL.
“So we are planning to pool data from several studies to investigate this topic further,” he said. “We know that different types of NHL may have different risk factors, so we are also planning to investigate whether physical activity influences the risk for different types of NHL in different ways.” ![]()
Symptoms confer higher-than-expected risk of HL, NHL
Results from two new studies indicate that lymphadenopathy and head and neck masses are associated with a higher risk of lymphoma than we thought.
These two factors proved to be the strongest predictors of Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL).
So unless these symptoms can be explained, general practitioners should refer affected patients to specialists as quickly as possible, study investigators said.
Both studies were published in the British Journal of General Practice.
“Cancer guidelines are based on the most robust evidence, and, up to now, this has been missing,” said Willie Hamilton, MD, of the University of Exeter Medical School in the UK.
“Our research has revealed the importance of persistent, swollen lymph glands, particularly in the neck, as part of cancer. Of course, swollen glands are common with throat infections, but in cancer, they are usually larger and painless. It’s been known for a long time that this could represent cancer. This study shows that the risk is higher than previously thought.”
The first study was a large-scale assessment of symptoms that are markers of NHL. Researchers assessed 4362 NHL patients (≥ 40 years of age) and 19,468 controls.
The 5 symptoms associated with the highest risk of developing NHL were lymphadenopathy (odds ratio [OR]=263), head and neck mass not described as lymphadenopathy (OR=49), other mass (OR=12), weight loss (OR=3.2), and abdominal pain (OR=2.5).
In the second study, investigators assessed 283 HL patients (≥ 40 years of age) and 1237 control subjects.
The team found that 6 features were independently associated with HL—lymphadenopathy (OR=280), head and neck mass not described as lymphadenopathy (OR=260), other mass (OR=12), thrombocytosis (OR=6.0), raised inflammatory markers (OR=5.2), and low full blood count (OR=2.8).
Combining the results of both studies, the investigators found that, for subjects older than 60 years of age, lymphadenopathy had a positive-predictive value of 18.6% for either NHL or HL. The positive-predictive value was 4.6% for head and neck mass and 1.1% for a mass elsewhere.
Therefore, the team said patients in this age group who present with lymphadenopathy or a head and neck mass should be referred to a specialist, unless there is a clear alternative explanation.
Referral is particularly urgent if either symptom has been present for 6 weeks or more, according to the investigators. They said that no blood test or other symptoms change that.
“Early diagnosis is vital to reducing cancer deaths,” said Liz Shephard, PhD, of the University of Exeter Medical School. “We now hope that this research will feed into guidelines to help GPs refer earlier and potentially to save lives.” ![]()

Results from two new studies indicate that lymphadenopathy and head and neck masses are associated with a higher risk of lymphoma than we thought.
These two factors proved to be the strongest predictors of Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL).
So unless these symptoms can be explained, general practitioners should refer affected patients to specialists as quickly as possible, study investigators said.
Both studies were published in the British Journal of General Practice.
“Cancer guidelines are based on the most robust evidence, and, up to now, this has been missing,” said Willie Hamilton, MD, of the University of Exeter Medical School in the UK.
“Our research has revealed the importance of persistent, swollen lymph glands, particularly in the neck, as part of cancer. Of course, swollen glands are common with throat infections, but in cancer, they are usually larger and painless. It’s been known for a long time that this could represent cancer. This study shows that the risk is higher than previously thought.”
The first study was a large-scale assessment of symptoms that are markers of NHL. Researchers assessed 4362 NHL patients (≥ 40 years of age) and 19,468 controls.
The 5 symptoms associated with the highest risk of developing NHL were lymphadenopathy (odds ratio [OR]=263), head and neck mass not described as lymphadenopathy (OR=49), other mass (OR=12), weight loss (OR=3.2), and abdominal pain (OR=2.5).
In the second study, investigators assessed 283 HL patients (≥ 40 years of age) and 1237 control subjects.
The team found that 6 features were independently associated with HL—lymphadenopathy (OR=280), head and neck mass not described as lymphadenopathy (OR=260), other mass (OR=12), thrombocytosis (OR=6.0), raised inflammatory markers (OR=5.2), and low full blood count (OR=2.8).
Combining the results of both studies, the investigators found that, for subjects older than 60 years of age, lymphadenopathy had a positive-predictive value of 18.6% for either NHL or HL. The positive-predictive value was 4.6% for head and neck mass and 1.1% for a mass elsewhere.
Therefore, the team said patients in this age group who present with lymphadenopathy or a head and neck mass should be referred to a specialist, unless there is a clear alternative explanation.
Referral is particularly urgent if either symptom has been present for 6 weeks or more, according to the investigators. They said that no blood test or other symptoms change that.
“Early diagnosis is vital to reducing cancer deaths,” said Liz Shephard, PhD, of the University of Exeter Medical School. “We now hope that this research will feed into guidelines to help GPs refer earlier and potentially to save lives.” ![]()

Results from two new studies indicate that lymphadenopathy and head and neck masses are associated with a higher risk of lymphoma than we thought.
These two factors proved to be the strongest predictors of Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL).
So unless these symptoms can be explained, general practitioners should refer affected patients to specialists as quickly as possible, study investigators said.
Both studies were published in the British Journal of General Practice.
“Cancer guidelines are based on the most robust evidence, and, up to now, this has been missing,” said Willie Hamilton, MD, of the University of Exeter Medical School in the UK.
“Our research has revealed the importance of persistent, swollen lymph glands, particularly in the neck, as part of cancer. Of course, swollen glands are common with throat infections, but in cancer, they are usually larger and painless. It’s been known for a long time that this could represent cancer. This study shows that the risk is higher than previously thought.”
The first study was a large-scale assessment of symptoms that are markers of NHL. Researchers assessed 4362 NHL patients (≥ 40 years of age) and 19,468 controls.
The 5 symptoms associated with the highest risk of developing NHL were lymphadenopathy (odds ratio [OR]=263), head and neck mass not described as lymphadenopathy (OR=49), other mass (OR=12), weight loss (OR=3.2), and abdominal pain (OR=2.5).
In the second study, investigators assessed 283 HL patients (≥ 40 years of age) and 1237 control subjects.
The team found that 6 features were independently associated with HL—lymphadenopathy (OR=280), head and neck mass not described as lymphadenopathy (OR=260), other mass (OR=12), thrombocytosis (OR=6.0), raised inflammatory markers (OR=5.2), and low full blood count (OR=2.8).
Combining the results of both studies, the investigators found that, for subjects older than 60 years of age, lymphadenopathy had a positive-predictive value of 18.6% for either NHL or HL. The positive-predictive value was 4.6% for head and neck mass and 1.1% for a mass elsewhere.
Therefore, the team said patients in this age group who present with lymphadenopathy or a head and neck mass should be referred to a specialist, unless there is a clear alternative explanation.
Referral is particularly urgent if either symptom has been present for 6 weeks or more, according to the investigators. They said that no blood test or other symptoms change that.
“Early diagnosis is vital to reducing cancer deaths,” said Liz Shephard, PhD, of the University of Exeter Medical School. “We now hope that this research will feed into guidelines to help GPs refer earlier and potentially to save lives.” ![]()
Susceptibility to 2nd cancers in WM/LPL survivors

PHILADELPHIA—A retrospective study has revealed factors that appear to influence a person’s susceptibility to Waldenström’s macroglobulinemia (WM)/lymphoplasmacytic lymphoma (LPL) and other malignancies.
Study investigators looked at patients diagnosed with WM or LPL over a 20-year period and found about a 50% excess of second primary cancers in this population.
The patients had a significantly increased risk of multiple hematologic and solid tumor malignancies, and a few of these malignancies had shared susceptibility factors with WM/LPL.
The investigators believe that identifying these factors may prove useful for determining genetic susceptibility to WM/LPL.
Mary L. McMaster, MD, of the National Cancer Institute in Bethesda, Maryland, and her colleagues presented these findings at the AACR Annual Meeting 2015 (abstract 3709).
The team used data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SSER) database to evaluate the risk of subsequent primary cancer in 3825 patients diagnosed with WM (n=2163) or LPL (n=1662) from 1992 to 2011. The patients’ median age was 70, most of them were male (n=2221), and most were white (n=3153).
Dr McMaster said she and her colleagues looked at both WM and LPL in this study because SEER does not include information about immunoglobulin subtype, which makes it difficult to identify all WM cases with absolute certainty.
“[D]epending on what information a pathologist has when they review a bone marrow biopsy, for example, they may or may not know whether there’s IgM present,” Dr McMaster said. “So you may have a diagnosis of LPL and not have the information required to make the diagnosis of WM. For that reason, we combined both entities for this study.”
Dr McMaster and her colleagues calculated the observed-to-expected standardized incidence ratios (SIRs) for invasive cancers. After adjusting for multiple comparisons, the team found that survivors of WM/LPL had a significantly increased risk of developing a second primary malignancy (SIR=1.49).
This increased risk was seen for males and females and persisted throughout follow-up. The risk was higher for patients younger than 65 years of age (SIR=1.95).
Hematologic malignancies
WM/LPL survivors had a significantly increased risk of several hematologic malignancies. The SIR was 4.09 for all hematologic malignancies, 4.29 for lymphomas, and 3.16 for leukemias.
Dr McMaster pointed out that several lymphoma subtypes can have lymphoplasmacytic differentiation, the most common being marginal zone lymphoma. And this could potentially result in misclassification.
“So we actually ran the study with and without marginal zone lymphoma and saw no difference in the results,” she said. “So we don’t think misclassification accounts for the majority of what we’re seeing.”
The investigators found that WM/LPL survivors had the highest risk of developing Burkitt lymphoma (SIR=13.45), followed by Hodgkin lymphoma (SIR=9.80), T-cell non-Hodgkin lymphoma (SIR=6.62), mantle cell lymphoma (SIR=5.37), diffuse large B-cell lymphoma (DLBCL, SIR=4.76), multiple myeloma (SIR=4.40), any non-Hodgkin lymphoma (SIR=4.08), and acute myeloid leukemia (AML, SIR=3.27).
“Waldenström’s is known to transform, on occasion, to DLBCL,” Dr McMaster said. “So that may well account for the excess of DLBCL that we see in this population.”
She also noted that, prior to the early 2000s, WM was typically treated with alkylating agents. And alkylating agents have been linked to an increased risk of AML.
In this population, the risk of AML peaked 5 to 10 years after WM/LPL diagnosis and was only present in patients treated prior to 2002. This suggests the AML observed in this study was likely treatment-related.
Dr McMaster and her colleagues also found that WM/LPL survivors did not have a significantly increased risk of developing acute lymphocytic leukemia (SIR=0), hairy cell leukemia (SIR=0), chronic lymphocytic leukemia/small lymphocytic lymphoma (SIR=0.97), or follicular lymphoma (SIR=2.25).
Solid tumors
WM/LPL survivors did have a significantly increased risk of certain solid tumor malignancies. The overall SIR for solid tumors was 1.21.
The risk was significant for non-epithelial skin cancers (SIR=5.15), thyroid cancers (SIR=3.13), melanoma (SIR=1.72), and cancers of the lung and bronchus (SIR=1.44) or respiratory system (SIR=1.42).
“Melanoma has an immunological basis, as does Waldenström’s, so we think there may be some shared etiology there,” Dr McMaster said.
She also noted that a strong risk factor for thyroid cancer, particularly papillary thyroid cancer, is a history of autoimmune thyroid disease.
“Autoimmune disease of any sort is a risk factor for Waldenström’s macroglobulinemia,” she said. “So again, we think there might be a basis for shared susceptibility there.”
Dr McMaster said this research suggests that multiple primary cancers may occur in a single individual because of shared genetic susceptibility, shared environmental exposures, treatment effects, or chance. She believes future research will show that both genetic and environmental factors contribute to WM.
Investigators are currently conducting whole-exome sequencing studies and genome-wide association studies in patients with familial and spontaneous WM, with the hopes of identifying genes that contribute to WM susceptibility. ![]()

PHILADELPHIA—A retrospective study has revealed factors that appear to influence a person’s susceptibility to Waldenström’s macroglobulinemia (WM)/lymphoplasmacytic lymphoma (LPL) and other malignancies.
Study investigators looked at patients diagnosed with WM or LPL over a 20-year period and found about a 50% excess of second primary cancers in this population.
The patients had a significantly increased risk of multiple hematologic and solid tumor malignancies, and a few of these malignancies had shared susceptibility factors with WM/LPL.
The investigators believe that identifying these factors may prove useful for determining genetic susceptibility to WM/LPL.
Mary L. McMaster, MD, of the National Cancer Institute in Bethesda, Maryland, and her colleagues presented these findings at the AACR Annual Meeting 2015 (abstract 3709).
The team used data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SSER) database to evaluate the risk of subsequent primary cancer in 3825 patients diagnosed with WM (n=2163) or LPL (n=1662) from 1992 to 2011. The patients’ median age was 70, most of them were male (n=2221), and most were white (n=3153).
Dr McMaster said she and her colleagues looked at both WM and LPL in this study because SEER does not include information about immunoglobulin subtype, which makes it difficult to identify all WM cases with absolute certainty.
“[D]epending on what information a pathologist has when they review a bone marrow biopsy, for example, they may or may not know whether there’s IgM present,” Dr McMaster said. “So you may have a diagnosis of LPL and not have the information required to make the diagnosis of WM. For that reason, we combined both entities for this study.”
Dr McMaster and her colleagues calculated the observed-to-expected standardized incidence ratios (SIRs) for invasive cancers. After adjusting for multiple comparisons, the team found that survivors of WM/LPL had a significantly increased risk of developing a second primary malignancy (SIR=1.49).
This increased risk was seen for males and females and persisted throughout follow-up. The risk was higher for patients younger than 65 years of age (SIR=1.95).
Hematologic malignancies
WM/LPL survivors had a significantly increased risk of several hematologic malignancies. The SIR was 4.09 for all hematologic malignancies, 4.29 for lymphomas, and 3.16 for leukemias.
Dr McMaster pointed out that several lymphoma subtypes can have lymphoplasmacytic differentiation, the most common being marginal zone lymphoma. And this could potentially result in misclassification.
“So we actually ran the study with and without marginal zone lymphoma and saw no difference in the results,” she said. “So we don’t think misclassification accounts for the majority of what we’re seeing.”
The investigators found that WM/LPL survivors had the highest risk of developing Burkitt lymphoma (SIR=13.45), followed by Hodgkin lymphoma (SIR=9.80), T-cell non-Hodgkin lymphoma (SIR=6.62), mantle cell lymphoma (SIR=5.37), diffuse large B-cell lymphoma (DLBCL, SIR=4.76), multiple myeloma (SIR=4.40), any non-Hodgkin lymphoma (SIR=4.08), and acute myeloid leukemia (AML, SIR=3.27).
“Waldenström’s is known to transform, on occasion, to DLBCL,” Dr McMaster said. “So that may well account for the excess of DLBCL that we see in this population.”
She also noted that, prior to the early 2000s, WM was typically treated with alkylating agents. And alkylating agents have been linked to an increased risk of AML.
In this population, the risk of AML peaked 5 to 10 years after WM/LPL diagnosis and was only present in patients treated prior to 2002. This suggests the AML observed in this study was likely treatment-related.
Dr McMaster and her colleagues also found that WM/LPL survivors did not have a significantly increased risk of developing acute lymphocytic leukemia (SIR=0), hairy cell leukemia (SIR=0), chronic lymphocytic leukemia/small lymphocytic lymphoma (SIR=0.97), or follicular lymphoma (SIR=2.25).
Solid tumors
WM/LPL survivors did have a significantly increased risk of certain solid tumor malignancies. The overall SIR for solid tumors was 1.21.
The risk was significant for non-epithelial skin cancers (SIR=5.15), thyroid cancers (SIR=3.13), melanoma (SIR=1.72), and cancers of the lung and bronchus (SIR=1.44) or respiratory system (SIR=1.42).
“Melanoma has an immunological basis, as does Waldenström’s, so we think there may be some shared etiology there,” Dr McMaster said.
She also noted that a strong risk factor for thyroid cancer, particularly papillary thyroid cancer, is a history of autoimmune thyroid disease.
“Autoimmune disease of any sort is a risk factor for Waldenström’s macroglobulinemia,” she said. “So again, we think there might be a basis for shared susceptibility there.”
Dr McMaster said this research suggests that multiple primary cancers may occur in a single individual because of shared genetic susceptibility, shared environmental exposures, treatment effects, or chance. She believes future research will show that both genetic and environmental factors contribute to WM.
Investigators are currently conducting whole-exome sequencing studies and genome-wide association studies in patients with familial and spontaneous WM, with the hopes of identifying genes that contribute to WM susceptibility. ![]()

PHILADELPHIA—A retrospective study has revealed factors that appear to influence a person’s susceptibility to Waldenström’s macroglobulinemia (WM)/lymphoplasmacytic lymphoma (LPL) and other malignancies.
Study investigators looked at patients diagnosed with WM or LPL over a 20-year period and found about a 50% excess of second primary cancers in this population.
The patients had a significantly increased risk of multiple hematologic and solid tumor malignancies, and a few of these malignancies had shared susceptibility factors with WM/LPL.
The investigators believe that identifying these factors may prove useful for determining genetic susceptibility to WM/LPL.
Mary L. McMaster, MD, of the National Cancer Institute in Bethesda, Maryland, and her colleagues presented these findings at the AACR Annual Meeting 2015 (abstract 3709).
The team used data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SSER) database to evaluate the risk of subsequent primary cancer in 3825 patients diagnosed with WM (n=2163) or LPL (n=1662) from 1992 to 2011. The patients’ median age was 70, most of them were male (n=2221), and most were white (n=3153).
Dr McMaster said she and her colleagues looked at both WM and LPL in this study because SEER does not include information about immunoglobulin subtype, which makes it difficult to identify all WM cases with absolute certainty.
“[D]epending on what information a pathologist has when they review a bone marrow biopsy, for example, they may or may not know whether there’s IgM present,” Dr McMaster said. “So you may have a diagnosis of LPL and not have the information required to make the diagnosis of WM. For that reason, we combined both entities for this study.”
Dr McMaster and her colleagues calculated the observed-to-expected standardized incidence ratios (SIRs) for invasive cancers. After adjusting for multiple comparisons, the team found that survivors of WM/LPL had a significantly increased risk of developing a second primary malignancy (SIR=1.49).
This increased risk was seen for males and females and persisted throughout follow-up. The risk was higher for patients younger than 65 years of age (SIR=1.95).
Hematologic malignancies
WM/LPL survivors had a significantly increased risk of several hematologic malignancies. The SIR was 4.09 for all hematologic malignancies, 4.29 for lymphomas, and 3.16 for leukemias.
Dr McMaster pointed out that several lymphoma subtypes can have lymphoplasmacytic differentiation, the most common being marginal zone lymphoma. And this could potentially result in misclassification.
“So we actually ran the study with and without marginal zone lymphoma and saw no difference in the results,” she said. “So we don’t think misclassification accounts for the majority of what we’re seeing.”
The investigators found that WM/LPL survivors had the highest risk of developing Burkitt lymphoma (SIR=13.45), followed by Hodgkin lymphoma (SIR=9.80), T-cell non-Hodgkin lymphoma (SIR=6.62), mantle cell lymphoma (SIR=5.37), diffuse large B-cell lymphoma (DLBCL, SIR=4.76), multiple myeloma (SIR=4.40), any non-Hodgkin lymphoma (SIR=4.08), and acute myeloid leukemia (AML, SIR=3.27).
“Waldenström’s is known to transform, on occasion, to DLBCL,” Dr McMaster said. “So that may well account for the excess of DLBCL that we see in this population.”
She also noted that, prior to the early 2000s, WM was typically treated with alkylating agents. And alkylating agents have been linked to an increased risk of AML.
In this population, the risk of AML peaked 5 to 10 years after WM/LPL diagnosis and was only present in patients treated prior to 2002. This suggests the AML observed in this study was likely treatment-related.
Dr McMaster and her colleagues also found that WM/LPL survivors did not have a significantly increased risk of developing acute lymphocytic leukemia (SIR=0), hairy cell leukemia (SIR=0), chronic lymphocytic leukemia/small lymphocytic lymphoma (SIR=0.97), or follicular lymphoma (SIR=2.25).
Solid tumors
WM/LPL survivors did have a significantly increased risk of certain solid tumor malignancies. The overall SIR for solid tumors was 1.21.
The risk was significant for non-epithelial skin cancers (SIR=5.15), thyroid cancers (SIR=3.13), melanoma (SIR=1.72), and cancers of the lung and bronchus (SIR=1.44) or respiratory system (SIR=1.42).
“Melanoma has an immunological basis, as does Waldenström’s, so we think there may be some shared etiology there,” Dr McMaster said.
She also noted that a strong risk factor for thyroid cancer, particularly papillary thyroid cancer, is a history of autoimmune thyroid disease.
“Autoimmune disease of any sort is a risk factor for Waldenström’s macroglobulinemia,” she said. “So again, we think there might be a basis for shared susceptibility there.”
Dr McMaster said this research suggests that multiple primary cancers may occur in a single individual because of shared genetic susceptibility, shared environmental exposures, treatment effects, or chance. She believes future research will show that both genetic and environmental factors contribute to WM.
Investigators are currently conducting whole-exome sequencing studies and genome-wide association studies in patients with familial and spontaneous WM, with the hopes of identifying genes that contribute to WM susceptibility. ![]()
Molecule increases TRAIL expression to fight NHL

Convention Center, site of the
AACR Annual Meeting 2015
PHILADELPHIA—When current treatment approaches failed to save a young patient with non-Hodgkin lymphoma (NHL), a researcher from The Children’s Hospital of Philadelphia was driven to investigate new therapeutic options.
The investigation led the researcher, Mala Talekar, MBBS, to ONC201 (formerly TIC10), a small molecule that induces apoptosis by increasing surface expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).
Preclinical experiments showed that ONC201 is active against NHL as a single agent, and it synergizes with chemotherapeutic drugs that are already used to treat NHL.
Dr Talekar and her colleagues described these experiments in a poster presented at the AACR Annual Meeting 2015 (abstract 5387). Some of the investigators involved in this research are employed by Oncoceutics, Inc., the company developing ONC201.
A researcher’s inspiration
“When I was doing my fellowship training, I had a teenage boy who had a rare form of non-Hodgkin’s lymphoma,” Dr Talekar explained. “He did not survive, despite receiving multiple treatments that are available for pediatric non-Hodgkin’s lymphoma.”
The boy’s death inspired Dr Talekar to seek new and better approaches to treat NHL. A search of the medical literature unearthed several articles detailing a TRAIL-based approach to treating lymphoma. So she decided to further investigate the effects of TRAIL in NHL.
“I first tried TRAIL in one lymphoma cell line,” she said. “And even though it did kill the cancer cells, it did not really give a satisfactory response.”
So Dr Talekar turned to the TRAIL agonist antibodies lexatumumab and mapatumumab, introducing each of them to human lymphoma cells. Although the antibodies caused more cell death than TRAIL itself, the response was still not satisfactory, she said.
“Fortunately for me, while I was working in the lab, one of the postdocs, Joshua Allen, discovered a new molecule called TRAIL-inducing compound 10, or TIC10,” Dr Talekar said. “So I tried TIC10—it is now called ONC201—and it gave a beautiful dose-response curve, causing complete cell death of the lymphoma cells.”
Dr Talekar was “very inspired” by this result and decided to test ONC201 in 8 different NHL cell lines—4 Burkitt lymphoma (Daudi, Raji, Ramos, and BJAB), 1 anaplastic large-cell lymphoma (Karpas299), and 3 mantle cell lymphoma (UPN2, Granta, and NCEB) cell lines.
“I found a beautiful dose-response curve,” Dr Talekar said, “suggesting that this molecule works in micromolar concentrations across all of the lymphoma cell lines.”
Elucidating the mechanism
Dr Talekar then set out to determine exactly how ONC201 causes cell death in NHL. Flow cytometry revealed that, as the dose of ONC201 increases, cell death increases, as does sub-G1 DNA content. This suggests the drug is causing cell death by apoptosis.
Next, Dr Talekar introduced ONC201 to NHL cell lines along with a pan-caspase inhibitor. She found the inhibitor blocked ONC201-induced apoptosis, which suggests ONC201 works via the caspase-mediated apoptotic pathway.
“The initial mechanism of action proposed for ONC201 was dual inactivation of two kinases, Akt and ERK,” Dr Talekar noted. “The dual inactivation causes dephosphorylation of Foxo3a. This causes its translocation to the nucleus and downstream upregulation of TRAIL, and, therefore, increased surface TRAIL expression. And we know increases in surface TRAIL cause cell death by apoptosis.”
With this in mind, Dr Talekar looked for increases in surface TRIAL after she incubated lymphoma cells with ONC201. She observed a dose-dependent increase in surface TRAIL and a linear correlation between the increase in TRAIL and apoptosis.
Then, she introduced ONC201 and a TRAIL-sequestering antibody, RIK-2, to lymphoma cells. RIK-2 inhibited apoptosis, which suggests ONC201 works as an anti-apoptotic agent via the TRAIL pathway.
Further testing
As a final step, Dr Talekar tested ONC201 in combination with chemotherapy drugs that are already used to treat pediatric NHL. She observed at least an additive effect, and sometimes a synergistic effect, between the drugs. The best responses occurred when she combined ONC201 with cytarabine, bortezomib, or doxorubicin.
Now, Dr Talekar is working on testing ONC201 in combination with cytarabine in a xenograft model of Burkitt lymphoma.
She noted that other in vivo research has suggested ONC201 has a “very benign safety profile.” In another poster presented at AACR 2015 (abstract 4479), researchers reported results indicating that ONC201 is safe.
“They have tested it in mice and dogs and found that, at 10-fold the therapeutic dose, you don’t see much toxicity at all,” Dr Talekar said.
ONC201 is also being tested in a phase 1 study of adults with advanced solid tumors. Phase 1 studies of the drug in relapsed or refractory NHL and relapsed or refractory acute leukemias and high-risk myelodysplastic syndromes are not yet recruiting patients. ![]()

Convention Center, site of the
AACR Annual Meeting 2015
PHILADELPHIA—When current treatment approaches failed to save a young patient with non-Hodgkin lymphoma (NHL), a researcher from The Children’s Hospital of Philadelphia was driven to investigate new therapeutic options.
The investigation led the researcher, Mala Talekar, MBBS, to ONC201 (formerly TIC10), a small molecule that induces apoptosis by increasing surface expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).
Preclinical experiments showed that ONC201 is active against NHL as a single agent, and it synergizes with chemotherapeutic drugs that are already used to treat NHL.
Dr Talekar and her colleagues described these experiments in a poster presented at the AACR Annual Meeting 2015 (abstract 5387). Some of the investigators involved in this research are employed by Oncoceutics, Inc., the company developing ONC201.
A researcher’s inspiration
“When I was doing my fellowship training, I had a teenage boy who had a rare form of non-Hodgkin’s lymphoma,” Dr Talekar explained. “He did not survive, despite receiving multiple treatments that are available for pediatric non-Hodgkin’s lymphoma.”
The boy’s death inspired Dr Talekar to seek new and better approaches to treat NHL. A search of the medical literature unearthed several articles detailing a TRAIL-based approach to treating lymphoma. So she decided to further investigate the effects of TRAIL in NHL.
“I first tried TRAIL in one lymphoma cell line,” she said. “And even though it did kill the cancer cells, it did not really give a satisfactory response.”
So Dr Talekar turned to the TRAIL agonist antibodies lexatumumab and mapatumumab, introducing each of them to human lymphoma cells. Although the antibodies caused more cell death than TRAIL itself, the response was still not satisfactory, she said.
“Fortunately for me, while I was working in the lab, one of the postdocs, Joshua Allen, discovered a new molecule called TRAIL-inducing compound 10, or TIC10,” Dr Talekar said. “So I tried TIC10—it is now called ONC201—and it gave a beautiful dose-response curve, causing complete cell death of the lymphoma cells.”
Dr Talekar was “very inspired” by this result and decided to test ONC201 in 8 different NHL cell lines—4 Burkitt lymphoma (Daudi, Raji, Ramos, and BJAB), 1 anaplastic large-cell lymphoma (Karpas299), and 3 mantle cell lymphoma (UPN2, Granta, and NCEB) cell lines.
“I found a beautiful dose-response curve,” Dr Talekar said, “suggesting that this molecule works in micromolar concentrations across all of the lymphoma cell lines.”
Elucidating the mechanism
Dr Talekar then set out to determine exactly how ONC201 causes cell death in NHL. Flow cytometry revealed that, as the dose of ONC201 increases, cell death increases, as does sub-G1 DNA content. This suggests the drug is causing cell death by apoptosis.
Next, Dr Talekar introduced ONC201 to NHL cell lines along with a pan-caspase inhibitor. She found the inhibitor blocked ONC201-induced apoptosis, which suggests ONC201 works via the caspase-mediated apoptotic pathway.
“The initial mechanism of action proposed for ONC201 was dual inactivation of two kinases, Akt and ERK,” Dr Talekar noted. “The dual inactivation causes dephosphorylation of Foxo3a. This causes its translocation to the nucleus and downstream upregulation of TRAIL, and, therefore, increased surface TRAIL expression. And we know increases in surface TRAIL cause cell death by apoptosis.”
With this in mind, Dr Talekar looked for increases in surface TRIAL after she incubated lymphoma cells with ONC201. She observed a dose-dependent increase in surface TRAIL and a linear correlation between the increase in TRAIL and apoptosis.
Then, she introduced ONC201 and a TRAIL-sequestering antibody, RIK-2, to lymphoma cells. RIK-2 inhibited apoptosis, which suggests ONC201 works as an anti-apoptotic agent via the TRAIL pathway.
Further testing
As a final step, Dr Talekar tested ONC201 in combination with chemotherapy drugs that are already used to treat pediatric NHL. She observed at least an additive effect, and sometimes a synergistic effect, between the drugs. The best responses occurred when she combined ONC201 with cytarabine, bortezomib, or doxorubicin.
Now, Dr Talekar is working on testing ONC201 in combination with cytarabine in a xenograft model of Burkitt lymphoma.
She noted that other in vivo research has suggested ONC201 has a “very benign safety profile.” In another poster presented at AACR 2015 (abstract 4479), researchers reported results indicating that ONC201 is safe.
“They have tested it in mice and dogs and found that, at 10-fold the therapeutic dose, you don’t see much toxicity at all,” Dr Talekar said.
ONC201 is also being tested in a phase 1 study of adults with advanced solid tumors. Phase 1 studies of the drug in relapsed or refractory NHL and relapsed or refractory acute leukemias and high-risk myelodysplastic syndromes are not yet recruiting patients. ![]()

Convention Center, site of the
AACR Annual Meeting 2015
PHILADELPHIA—When current treatment approaches failed to save a young patient with non-Hodgkin lymphoma (NHL), a researcher from The Children’s Hospital of Philadelphia was driven to investigate new therapeutic options.
The investigation led the researcher, Mala Talekar, MBBS, to ONC201 (formerly TIC10), a small molecule that induces apoptosis by increasing surface expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).
Preclinical experiments showed that ONC201 is active against NHL as a single agent, and it synergizes with chemotherapeutic drugs that are already used to treat NHL.
Dr Talekar and her colleagues described these experiments in a poster presented at the AACR Annual Meeting 2015 (abstract 5387). Some of the investigators involved in this research are employed by Oncoceutics, Inc., the company developing ONC201.
A researcher’s inspiration
“When I was doing my fellowship training, I had a teenage boy who had a rare form of non-Hodgkin’s lymphoma,” Dr Talekar explained. “He did not survive, despite receiving multiple treatments that are available for pediatric non-Hodgkin’s lymphoma.”
The boy’s death inspired Dr Talekar to seek new and better approaches to treat NHL. A search of the medical literature unearthed several articles detailing a TRAIL-based approach to treating lymphoma. So she decided to further investigate the effects of TRAIL in NHL.
“I first tried TRAIL in one lymphoma cell line,” she said. “And even though it did kill the cancer cells, it did not really give a satisfactory response.”
So Dr Talekar turned to the TRAIL agonist antibodies lexatumumab and mapatumumab, introducing each of them to human lymphoma cells. Although the antibodies caused more cell death than TRAIL itself, the response was still not satisfactory, she said.
“Fortunately for me, while I was working in the lab, one of the postdocs, Joshua Allen, discovered a new molecule called TRAIL-inducing compound 10, or TIC10,” Dr Talekar said. “So I tried TIC10—it is now called ONC201—and it gave a beautiful dose-response curve, causing complete cell death of the lymphoma cells.”
Dr Talekar was “very inspired” by this result and decided to test ONC201 in 8 different NHL cell lines—4 Burkitt lymphoma (Daudi, Raji, Ramos, and BJAB), 1 anaplastic large-cell lymphoma (Karpas299), and 3 mantle cell lymphoma (UPN2, Granta, and NCEB) cell lines.
“I found a beautiful dose-response curve,” Dr Talekar said, “suggesting that this molecule works in micromolar concentrations across all of the lymphoma cell lines.”
Elucidating the mechanism
Dr Talekar then set out to determine exactly how ONC201 causes cell death in NHL. Flow cytometry revealed that, as the dose of ONC201 increases, cell death increases, as does sub-G1 DNA content. This suggests the drug is causing cell death by apoptosis.
Next, Dr Talekar introduced ONC201 to NHL cell lines along with a pan-caspase inhibitor. She found the inhibitor blocked ONC201-induced apoptosis, which suggests ONC201 works via the caspase-mediated apoptotic pathway.
“The initial mechanism of action proposed for ONC201 was dual inactivation of two kinases, Akt and ERK,” Dr Talekar noted. “The dual inactivation causes dephosphorylation of Foxo3a. This causes its translocation to the nucleus and downstream upregulation of TRAIL, and, therefore, increased surface TRAIL expression. And we know increases in surface TRAIL cause cell death by apoptosis.”
With this in mind, Dr Talekar looked for increases in surface TRIAL after she incubated lymphoma cells with ONC201. She observed a dose-dependent increase in surface TRAIL and a linear correlation between the increase in TRAIL and apoptosis.
Then, she introduced ONC201 and a TRAIL-sequestering antibody, RIK-2, to lymphoma cells. RIK-2 inhibited apoptosis, which suggests ONC201 works as an anti-apoptotic agent via the TRAIL pathway.
Further testing
As a final step, Dr Talekar tested ONC201 in combination with chemotherapy drugs that are already used to treat pediatric NHL. She observed at least an additive effect, and sometimes a synergistic effect, between the drugs. The best responses occurred when she combined ONC201 with cytarabine, bortezomib, or doxorubicin.
Now, Dr Talekar is working on testing ONC201 in combination with cytarabine in a xenograft model of Burkitt lymphoma.
She noted that other in vivo research has suggested ONC201 has a “very benign safety profile.” In another poster presented at AACR 2015 (abstract 4479), researchers reported results indicating that ONC201 is safe.
“They have tested it in mice and dogs and found that, at 10-fold the therapeutic dose, you don’t see much toxicity at all,” Dr Talekar said.
ONC201 is also being tested in a phase 1 study of adults with advanced solid tumors. Phase 1 studies of the drug in relapsed or refractory NHL and relapsed or refractory acute leukemias and high-risk myelodysplastic syndromes are not yet recruiting patients.
HL survivors have long-term risk of cardiovascular disease

Photo by Rhoda Baer
Survivors of Hodgkin lymphoma (HL) have an increased risk of developing cardiovascular diseases throughout their lives, according to a study published in JAMA Internal Medicine.
Previous research suggested that HL treatment is associated with an increased risk of cardiovascular diseases.
However, those studies did not determine how long the increased risk persists or pinpoint the risk factors for various cardiovascular diseases.
So Flora E. van Leeuwen, PhD, of the Netherlands Cancer Institute in Amsterdam, and her colleagues decided to investigate.
The team examined the risk for cardiovascular disease in HL survivors up to 40 years after they received treatment and compared that with the risk for cardiovascular disease in the general population. The researchers also studied treatment-related risk factors.
The study included 2524 Dutch patients who were diagnosed with HL when they were younger than 51 years of age. The patients’ median age was 27.3 years.
The patients were treated from 1965 through 1995 and had survived for at least 5 years after diagnosis. In all, 2052 patients (81.3%) had received mediastinal radiotherapy, and 773 (30.6%) had received chemotherapy containing an anthracycline.
At a median of 20.3 years of follow-up, there were 1713 cardiovascular events in 797 patients (31.6%), and 410 of those patients (51.4%) had experienced 2 events or more.
The most frequently occurring cardiovascular disease was coronary heart disease (CHD), with 401 patients developing it as their first event. This was followed by valvular heart disease (VHD, 374 events) and heart failure (HF, 140 events).
HL survivors had a 3.2-fold increased risk of developing CHD and a 6.8-fold increased risk of developing HF compared to the general population.
HL survivors who had been treated before age 25 had a 4.6-fold to 7.5-fold increased risk of CHD and a 10.9-fold to 40.5-fold increased risk of HF, depending on the age they ultimately attained.
HL survivors treated at 35 to 50 years of age had a 2.0-fold to 2.3-fold increased risk of CHD and a 3.1-fold to 5.2-fold increased risk of HF, depending on their attained age.
The risks of CHD and HF remained significantly increased beyond 35 years after HL treatment. The standardized incidence ratios were 3.9 and 5.8, respectively.
The median times between HL treatment and first cardiovascular disease events were 18 years for CHD, 24 years for VHD, and 19 years for HF.
The cumulative risk of any type of cardiovascular disease was 50% at 40 years after HL diagnosis. For patients who were treated for HL before they were 25, the cumulative risk of developing a cardiovascular disease at 60 years of age or older was 20% for CHD, 31% for VHD, and 11% for HF.
The study also suggested that mediastinal radiotherapy increased the risk of CHD, VHD, and HF. But anthracycline-containing chemotherapy only increased the risk of VHD and HF.
Dr van Leeuwen and her colleagues concluded that both physicians and patients should be aware that HL survivors have a persistently increased risk of developing cardiovascular diseases throughout their lives. The team also believes the results of their study may direct guidelines for follow-up in HL survivors.
A commentary related to this research is available in JAMA Internal Medicine as well.

Photo by Rhoda Baer
Survivors of Hodgkin lymphoma (HL) have an increased risk of developing cardiovascular diseases throughout their lives, according to a study published in JAMA Internal Medicine.
Previous research suggested that HL treatment is associated with an increased risk of cardiovascular diseases.
However, those studies did not determine how long the increased risk persists or pinpoint the risk factors for various cardiovascular diseases.
So Flora E. van Leeuwen, PhD, of the Netherlands Cancer Institute in Amsterdam, and her colleagues decided to investigate.
The team examined the risk for cardiovascular disease in HL survivors up to 40 years after they received treatment and compared that with the risk for cardiovascular disease in the general population. The researchers also studied treatment-related risk factors.
The study included 2524 Dutch patients who were diagnosed with HL when they were younger than 51 years of age. The patients’ median age was 27.3 years.
The patients were treated from 1965 through 1995 and had survived for at least 5 years after diagnosis. In all, 2052 patients (81.3%) had received mediastinal radiotherapy, and 773 (30.6%) had received chemotherapy containing an anthracycline.
At a median of 20.3 years of follow-up, there were 1713 cardiovascular events in 797 patients (31.6%), and 410 of those patients (51.4%) had experienced 2 events or more.
The most frequently occurring cardiovascular disease was coronary heart disease (CHD), with 401 patients developing it as their first event. This was followed by valvular heart disease (VHD, 374 events) and heart failure (HF, 140 events).
HL survivors had a 3.2-fold increased risk of developing CHD and a 6.8-fold increased risk of developing HF compared to the general population.
HL survivors who had been treated before age 25 had a 4.6-fold to 7.5-fold increased risk of CHD and a 10.9-fold to 40.5-fold increased risk of HF, depending on the age they ultimately attained.
HL survivors treated at 35 to 50 years of age had a 2.0-fold to 2.3-fold increased risk of CHD and a 3.1-fold to 5.2-fold increased risk of HF, depending on their attained age.
The risks of CHD and HF remained significantly increased beyond 35 years after HL treatment. The standardized incidence ratios were 3.9 and 5.8, respectively.
The median times between HL treatment and first cardiovascular disease events were 18 years for CHD, 24 years for VHD, and 19 years for HF.
The cumulative risk of any type of cardiovascular disease was 50% at 40 years after HL diagnosis. For patients who were treated for HL before they were 25, the cumulative risk of developing a cardiovascular disease at 60 years of age or older was 20% for CHD, 31% for VHD, and 11% for HF.
The study also suggested that mediastinal radiotherapy increased the risk of CHD, VHD, and HF. But anthracycline-containing chemotherapy only increased the risk of VHD and HF.
Dr van Leeuwen and her colleagues concluded that both physicians and patients should be aware that HL survivors have a persistently increased risk of developing cardiovascular diseases throughout their lives. The team also believes the results of their study may direct guidelines for follow-up in HL survivors.
A commentary related to this research is available in JAMA Internal Medicine as well.

Photo by Rhoda Baer
Survivors of Hodgkin lymphoma (HL) have an increased risk of developing cardiovascular diseases throughout their lives, according to a study published in JAMA Internal Medicine.
Previous research suggested that HL treatment is associated with an increased risk of cardiovascular diseases.
However, those studies did not determine how long the increased risk persists or pinpoint the risk factors for various cardiovascular diseases.
So Flora E. van Leeuwen, PhD, of the Netherlands Cancer Institute in Amsterdam, and her colleagues decided to investigate.
The team examined the risk for cardiovascular disease in HL survivors up to 40 years after they received treatment and compared that with the risk for cardiovascular disease in the general population. The researchers also studied treatment-related risk factors.
The study included 2524 Dutch patients who were diagnosed with HL when they were younger than 51 years of age. The patients’ median age was 27.3 years.
The patients were treated from 1965 through 1995 and had survived for at least 5 years after diagnosis. In all, 2052 patients (81.3%) had received mediastinal radiotherapy, and 773 (30.6%) had received chemotherapy containing an anthracycline.
At a median of 20.3 years of follow-up, there were 1713 cardiovascular events in 797 patients (31.6%), and 410 of those patients (51.4%) had experienced 2 events or more.
The most frequently occurring cardiovascular disease was coronary heart disease (CHD), with 401 patients developing it as their first event. This was followed by valvular heart disease (VHD, 374 events) and heart failure (HF, 140 events).
HL survivors had a 3.2-fold increased risk of developing CHD and a 6.8-fold increased risk of developing HF compared to the general population.
HL survivors who had been treated before age 25 had a 4.6-fold to 7.5-fold increased risk of CHD and a 10.9-fold to 40.5-fold increased risk of HF, depending on the age they ultimately attained.
HL survivors treated at 35 to 50 years of age had a 2.0-fold to 2.3-fold increased risk of CHD and a 3.1-fold to 5.2-fold increased risk of HF, depending on their attained age.
The risks of CHD and HF remained significantly increased beyond 35 years after HL treatment. The standardized incidence ratios were 3.9 and 5.8, respectively.
The median times between HL treatment and first cardiovascular disease events were 18 years for CHD, 24 years for VHD, and 19 years for HF.
The cumulative risk of any type of cardiovascular disease was 50% at 40 years after HL diagnosis. For patients who were treated for HL before they were 25, the cumulative risk of developing a cardiovascular disease at 60 years of age or older was 20% for CHD, 31% for VHD, and 11% for HF.
The study also suggested that mediastinal radiotherapy increased the risk of CHD, VHD, and HF. But anthracycline-containing chemotherapy only increased the risk of VHD and HF.
Dr van Leeuwen and her colleagues concluded that both physicians and patients should be aware that HL survivors have a persistently increased risk of developing cardiovascular diseases throughout their lives. The team also believes the results of their study may direct guidelines for follow-up in HL survivors.
A commentary related to this research is available in JAMA Internal Medicine as well.
CVD risk persists for 40 years in Hodgkin’s survivors
People who survive Hodgkin’s lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease for at least 40 years – the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
Until now, follow-up studies of such patients “rarely exceeded 20-25 years,” before most survivors reached the age at which cardiovascular disease (CVD) becomes commonplace in the general population. To compare CVD rates between survivors and the general population at later ages, investigators examined the medical records of 2,524 individuals who survived 5 years or more after being treated for Hodgkin’s lymphoma as adolescents or young adults at five Dutch medical centers between 1965 and 1995.
A total of 81% of the cohort had received mediastinal radiotherapy and 31% had received anthracycline-containing chemotherapy. After 5-47 years of follow-up, 797 of these patients experienced 1,713 cardiovascular events. The most frequently occurring events included 401 coronary heart disease events (such as myocardial infarction and angina pectoris), 374 valvular heart disease events, and 140 heart failure events (such as cardiomyopathy and congestive heart failure), Frederika A. van Nimwegen of the department of epidemiology, the Netherlands Cancer Institute, Amsterdam, and her colleagues wrote in JAMA Internal Medicine on April 27 (doi:10.1001/jamainternmed.2015.1180).
Compared with the general population, Hodgkin’s survivors had a 3.2-fold higher standardized incidence ratio (SIR) of developing coronary heart disease and a 6.8-fold higher SIR of developing heart failure, corresponding to 70 excess cases of coronary heart disease and 58 excess cases of heart failure per 10,000 person-years.
These risks were significantly higher for survivors than for the general population at all ages, but patients who had been diagnosed and treated before the age of 25 years were at particularly elevated risk: they carried a 4.6- to 7.5-fold higher risk of coronary heart disease and a 10.9- to 40.5-fold higher risk of heart failure. At 40 years after Hodgkin’s diagnosis and treatment, the cumulative incidence of any type of CVD was 50%, the investigators wrote. Both survivors of Hodgkin’s lymphoma and their physicians should be aware that these patients remain at substantially increased cardiovascular risk throughout their lives, Ms. Van Nimwegen and her colleagues wrote.
This study was supported by the Dutch Cancer Society. Ms. van Nimwegen and her colleagues reported having no financial disclosures.
Primary care physicians must rise to the challenge of promoting the health of cancer survivors. Previous research suggests that many are not comfortable caring for this patient population and report knowledge gaps regarding the additional screening and surveillance they require. Most patients in the study by Ms. van Nimwegen and her colleagues were not screened for CVD.
Asking just a few key questions will identify these patients: What kind of cancer did you have? How old were you at diagnosis? Did you receive any chest radiotherapy? Did you receive doxorubicin (which they may know only by the brand name Adriamycin)? Our clinical experience has been that patients typically know the answers to these basic questions, which is a simple way of identifying those at increased risk.
Dr. Emily Tonorezos is with the department of medicine at Memorial Sloan Kettering Cancer Center and at Cornell University, both in New York. Dr. Linda Overholser is with the division of general internal medicine at the University of Colorado at Denver, Aurora. They reported having no relevant financial disclosures. These comments are adapted from an accompanying editorial written by Dr. Tonorezos and Dr. Overholser (JAMA Intern. Med. 2015 April 27 [doi:10.1001/jamainternmed.2015.1187]).
Primary care physicians must rise to the challenge of promoting the health of cancer survivors. Previous research suggests that many are not comfortable caring for this patient population and report knowledge gaps regarding the additional screening and surveillance they require. Most patients in the study by Ms. van Nimwegen and her colleagues were not screened for CVD.
Asking just a few key questions will identify these patients: What kind of cancer did you have? How old were you at diagnosis? Did you receive any chest radiotherapy? Did you receive doxorubicin (which they may know only by the brand name Adriamycin)? Our clinical experience has been that patients typically know the answers to these basic questions, which is a simple way of identifying those at increased risk.
Dr. Emily Tonorezos is with the department of medicine at Memorial Sloan Kettering Cancer Center and at Cornell University, both in New York. Dr. Linda Overholser is with the division of general internal medicine at the University of Colorado at Denver, Aurora. They reported having no relevant financial disclosures. These comments are adapted from an accompanying editorial written by Dr. Tonorezos and Dr. Overholser (JAMA Intern. Med. 2015 April 27 [doi:10.1001/jamainternmed.2015.1187]).
Primary care physicians must rise to the challenge of promoting the health of cancer survivors. Previous research suggests that many are not comfortable caring for this patient population and report knowledge gaps regarding the additional screening and surveillance they require. Most patients in the study by Ms. van Nimwegen and her colleagues were not screened for CVD.
Asking just a few key questions will identify these patients: What kind of cancer did you have? How old were you at diagnosis? Did you receive any chest radiotherapy? Did you receive doxorubicin (which they may know only by the brand name Adriamycin)? Our clinical experience has been that patients typically know the answers to these basic questions, which is a simple way of identifying those at increased risk.
Dr. Emily Tonorezos is with the department of medicine at Memorial Sloan Kettering Cancer Center and at Cornell University, both in New York. Dr. Linda Overholser is with the division of general internal medicine at the University of Colorado at Denver, Aurora. They reported having no relevant financial disclosures. These comments are adapted from an accompanying editorial written by Dr. Tonorezos and Dr. Overholser (JAMA Intern. Med. 2015 April 27 [doi:10.1001/jamainternmed.2015.1187]).
People who survive Hodgkin’s lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease for at least 40 years – the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
Until now, follow-up studies of such patients “rarely exceeded 20-25 years,” before most survivors reached the age at which cardiovascular disease (CVD) becomes commonplace in the general population. To compare CVD rates between survivors and the general population at later ages, investigators examined the medical records of 2,524 individuals who survived 5 years or more after being treated for Hodgkin’s lymphoma as adolescents or young adults at five Dutch medical centers between 1965 and 1995.
A total of 81% of the cohort had received mediastinal radiotherapy and 31% had received anthracycline-containing chemotherapy. After 5-47 years of follow-up, 797 of these patients experienced 1,713 cardiovascular events. The most frequently occurring events included 401 coronary heart disease events (such as myocardial infarction and angina pectoris), 374 valvular heart disease events, and 140 heart failure events (such as cardiomyopathy and congestive heart failure), Frederika A. van Nimwegen of the department of epidemiology, the Netherlands Cancer Institute, Amsterdam, and her colleagues wrote in JAMA Internal Medicine on April 27 (doi:10.1001/jamainternmed.2015.1180).
Compared with the general population, Hodgkin’s survivors had a 3.2-fold higher standardized incidence ratio (SIR) of developing coronary heart disease and a 6.8-fold higher SIR of developing heart failure, corresponding to 70 excess cases of coronary heart disease and 58 excess cases of heart failure per 10,000 person-years.
These risks were significantly higher for survivors than for the general population at all ages, but patients who had been diagnosed and treated before the age of 25 years were at particularly elevated risk: they carried a 4.6- to 7.5-fold higher risk of coronary heart disease and a 10.9- to 40.5-fold higher risk of heart failure. At 40 years after Hodgkin’s diagnosis and treatment, the cumulative incidence of any type of CVD was 50%, the investigators wrote. Both survivors of Hodgkin’s lymphoma and their physicians should be aware that these patients remain at substantially increased cardiovascular risk throughout their lives, Ms. Van Nimwegen and her colleagues wrote.
This study was supported by the Dutch Cancer Society. Ms. van Nimwegen and her colleagues reported having no financial disclosures.
People who survive Hodgkin’s lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease for at least 40 years – the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
Until now, follow-up studies of such patients “rarely exceeded 20-25 years,” before most survivors reached the age at which cardiovascular disease (CVD) becomes commonplace in the general population. To compare CVD rates between survivors and the general population at later ages, investigators examined the medical records of 2,524 individuals who survived 5 years or more after being treated for Hodgkin’s lymphoma as adolescents or young adults at five Dutch medical centers between 1965 and 1995.
A total of 81% of the cohort had received mediastinal radiotherapy and 31% had received anthracycline-containing chemotherapy. After 5-47 years of follow-up, 797 of these patients experienced 1,713 cardiovascular events. The most frequently occurring events included 401 coronary heart disease events (such as myocardial infarction and angina pectoris), 374 valvular heart disease events, and 140 heart failure events (such as cardiomyopathy and congestive heart failure), Frederika A. van Nimwegen of the department of epidemiology, the Netherlands Cancer Institute, Amsterdam, and her colleagues wrote in JAMA Internal Medicine on April 27 (doi:10.1001/jamainternmed.2015.1180).
Compared with the general population, Hodgkin’s survivors had a 3.2-fold higher standardized incidence ratio (SIR) of developing coronary heart disease and a 6.8-fold higher SIR of developing heart failure, corresponding to 70 excess cases of coronary heart disease and 58 excess cases of heart failure per 10,000 person-years.
These risks were significantly higher for survivors than for the general population at all ages, but patients who had been diagnosed and treated before the age of 25 years were at particularly elevated risk: they carried a 4.6- to 7.5-fold higher risk of coronary heart disease and a 10.9- to 40.5-fold higher risk of heart failure. At 40 years after Hodgkin’s diagnosis and treatment, the cumulative incidence of any type of CVD was 50%, the investigators wrote. Both survivors of Hodgkin’s lymphoma and their physicians should be aware that these patients remain at substantially increased cardiovascular risk throughout their lives, Ms. Van Nimwegen and her colleagues wrote.
This study was supported by the Dutch Cancer Society. Ms. van Nimwegen and her colleagues reported having no financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Hodgkin’s lymphoma survivors remain at high cardiovascular risk for at least 40 years, which is the longest they have been followed.
Major finding: At 40 years after Hodgkin’s diagnosis and treatment, the cumulative incidence of any type of cardiovascular disease was 50%.
Data source: Retrospective cohort study involved 2,524 Dutch patients who were first treated for Hodgkin’s lymphoma in 1965-1995 and followed for cardiovascular events for up to 47 years.
Disclosures: This study was supported by the Dutch Cancer Society. Ms. van Nimwegen and her colleagues reported having no financial disclosures.