User login
Mohs Micrographic Surgery for Digital Melanoma and Nonmelanoma Skin Cancers
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.
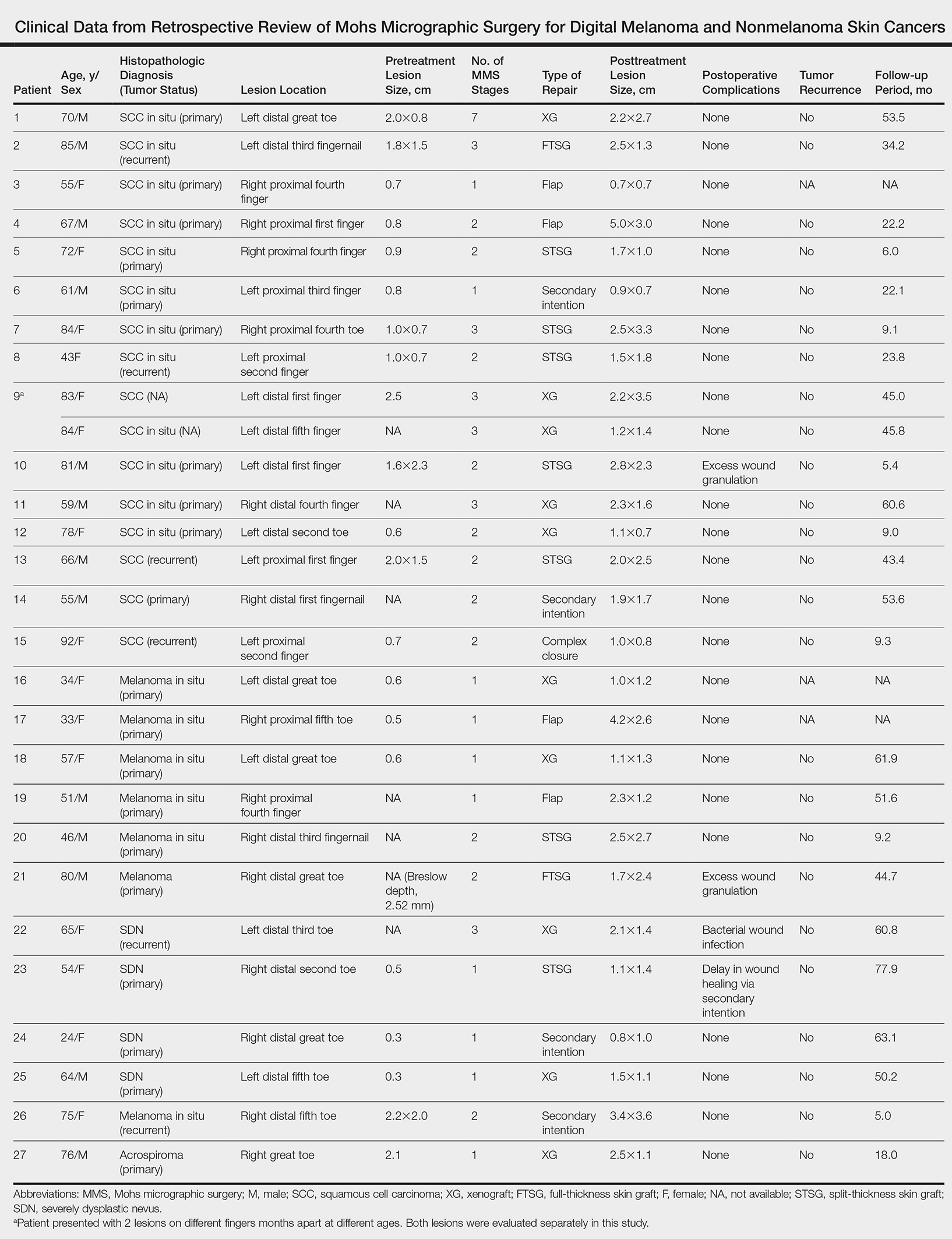
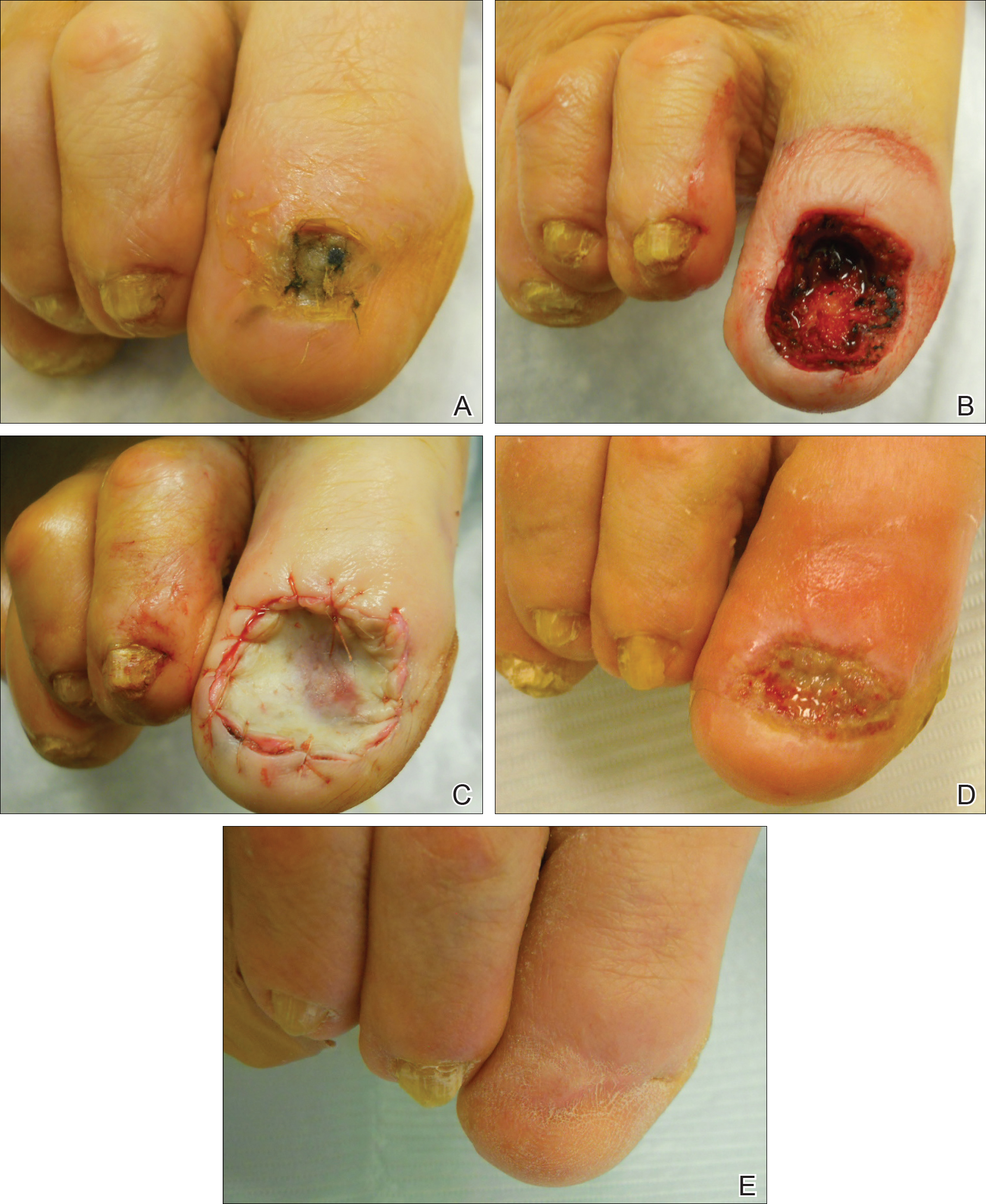
Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.
Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.
Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.

Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.
Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.

Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
Practice Points
- Melanoma and nonmelanoma skin cancers of the digits traditionally have been treated with wide local surgical excision and even amputation.
- Conservative tissue sparing techniques such as Mohs micrographic surgery can be used to treat digital skin cancers with high cure rates and improved functional and cosmetic results.
Penile Squamous Cell Carcinoma With Urethral Extension Treated With Mohs Micrographic Surgery
Penile squamous cell carcinoma (SCC) with considerable urethral extension is uncommon and difficult to manage. It often is resistant to less invasive and nonsurgical treatments and frequently results in partial or total penectomy, which can lead to cosmetic disfigurement, functional issues, and psychological distress. We report a case of penile SCC in situ with considerable urethral extension with a focus of cells suspicious for moderately well-differentiated and invasive SCC that was treated with
Mohs micrographic surgery with distal urethrectomy and reconstruction is a valuable treatment technique for cases of SCC involving the glans penis and distal urethra. It offers equivalent or better overall cure rates compared to more radical interventions. Additionally, preservation of the penis with MMS spares patients from considerable physical and psychosocial morbidity. Our case, along with growing body of literature,1-4 calls on dermatologists and urologists to consider MMS as a treatment for penile SCC with or without urethral involvement.
Case Report
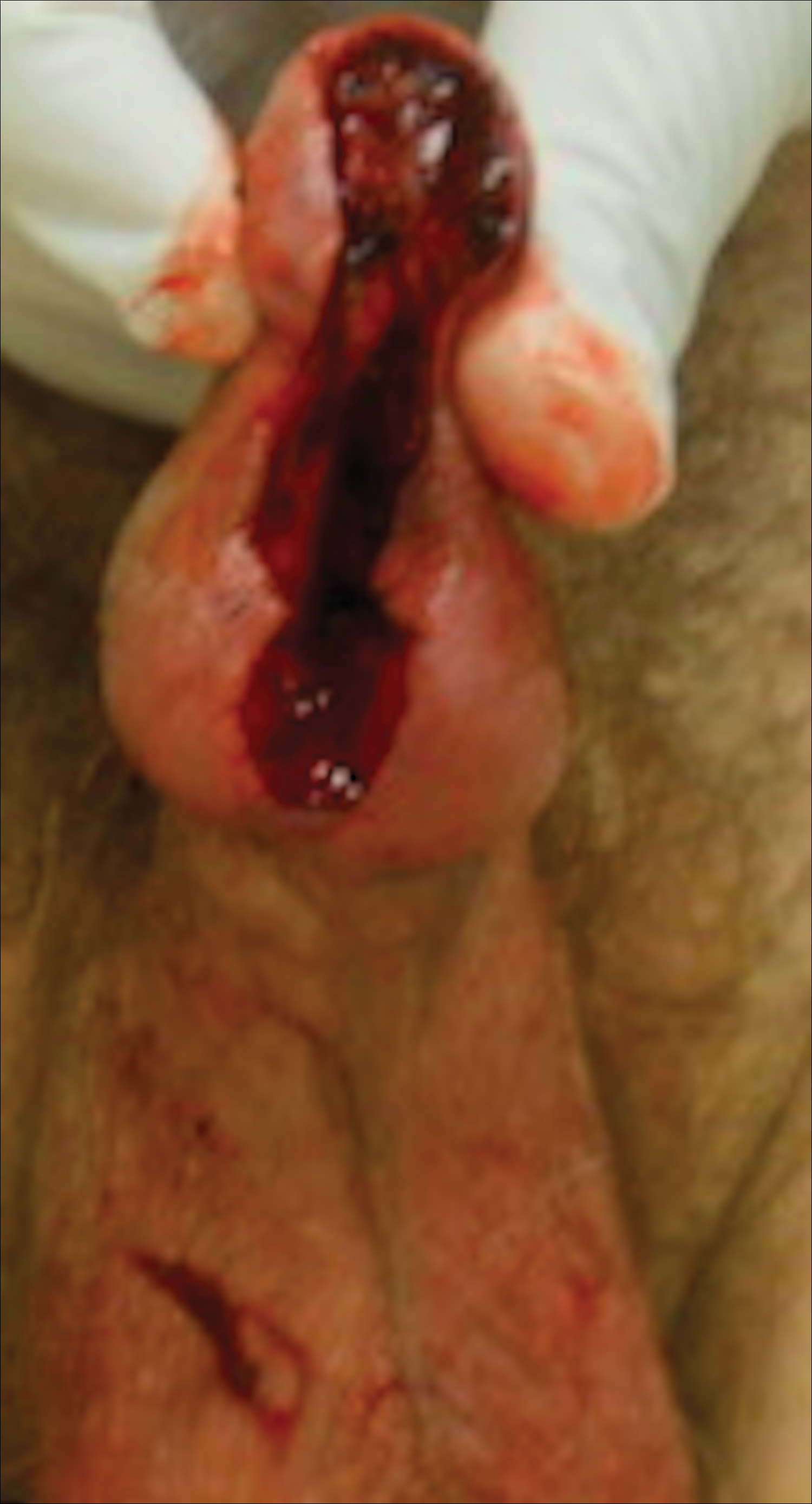
A 61-year-old man presented to the dermatology department with a pruritic lesion on the penis that had been present for 6 years. Shave biopsy demonstrated SCC in situ with a focus of cells suspicious for moderately well-differentiated and invasive SCC. Physical examination revealed an ill-defined, 2.2×1.9-cm, pink, eroded plaque involving the tip of the penis and surrounding the external urinary meatus (Figure 1). There was no palpable inguinal lymphadenopathy.
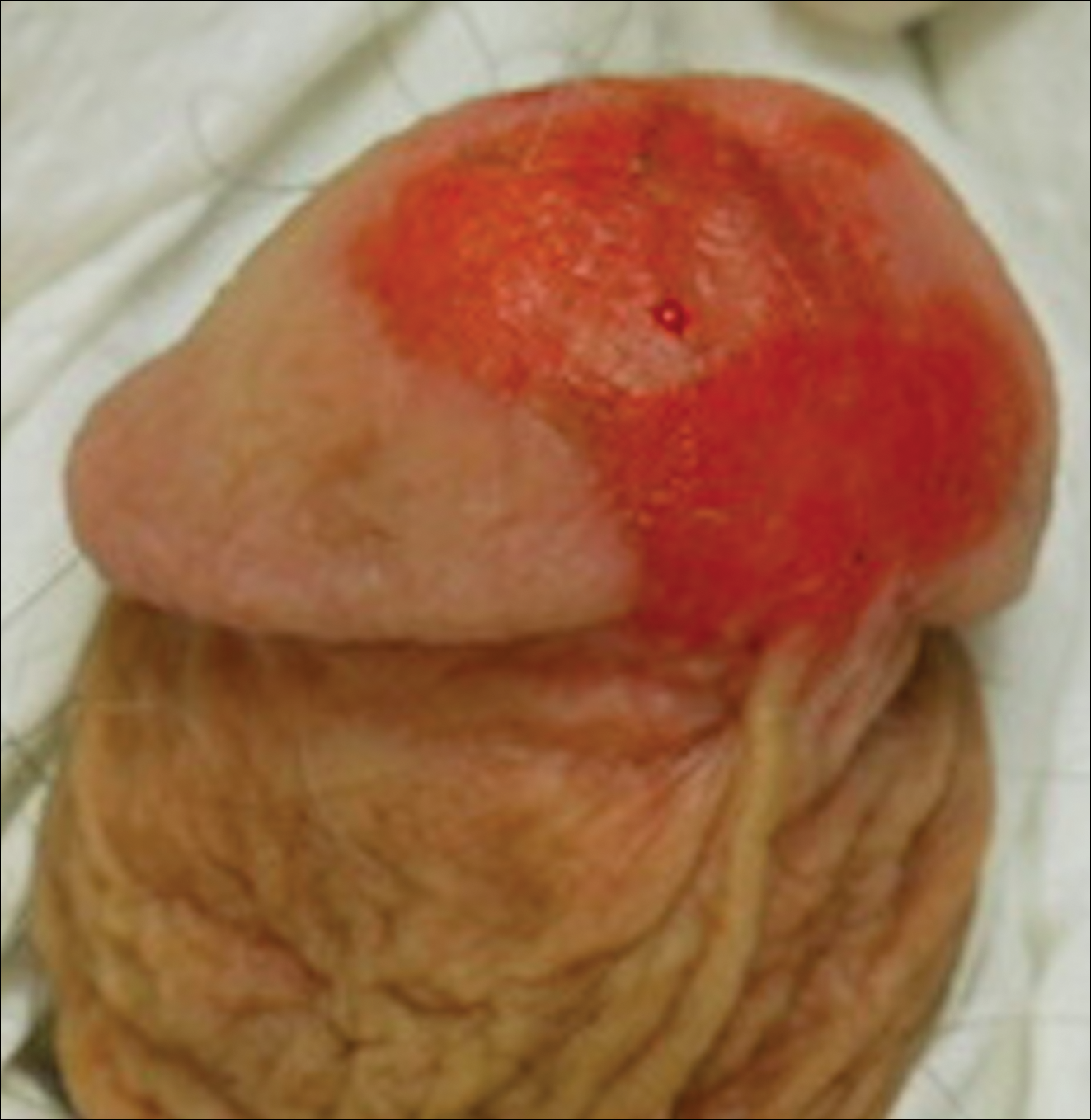
Distal penectomy and lymph node biopsy was recommended following evaluation by the urologic oncology department, but the patient declined these interventions and presented to our dermatology department (A.H.) for a second opinion. The tumor, including the invasive perineural portion, was removed using MMS several weeks after initially presenting to urologic oncology. Ventral meatotomy allowed access to the SCC in situ portion extending proximally up the pendulous urethra (Figure 2). Clear margins were obtained after the eighth stage of MMS, which required removal of 4 to 5 cm of the distal urethra (Figure 3). Reconstruction of the wound required urethral advancement, urethrostomy, and meatoplasty. A positive outcome was achieved with preservation of the length and shape of the penis as well as the cosmetic appearance of the glans penis (Figure 4). The patient was satisfied with the outcome. At 49 months’ follow-up, no evidence of local recurrence or disease progression was noted, and the distal urethrostomy remained intact and functional.


Comment
Penile SCC is a rare malignancy that represents between 0.4% and 0.6% of all malignant tumors in the United States and occurs most commonly in men aged 50 to 70 years.4 The incidence is higher in developing countries, approaching 10% of malignancies in men. It occurs most commonly on the glans penis, prepuce, and coronal sulcus, and has multiple possible appearances, including erythematous and indurated, warty and exophytic, or flat and ulcerated lesions.5 Some reports indicate that more than 40% of penile SCCs are attributable to human papilloma virus,6 while lack of circumcision, chronic inflammation, poor hygiene, balanitis xerotica obliterans, penile trauma, human immunodeficiency virus, UVA treatment of penile psoriasis, and tobacco use are known risk factors.5
Invasive penile SCC generally is treated with penectomy (partial or total), radiation therapy, or MMS; SCC in situ can be treated with topical chemotherapy, laser therapy, and wide local excision (2-cm margins) including circumcision, complete glansectomy, or MMS.5 Squamous cell carcinoma in situ with urethral involvement treated with nonsurgical therapies is associated with higher recurrence rates, ultimately necessitating more aggressive treatments, most commonly partial penectomy.7 The high local recurrence rate of SCC in situ with urethral involvement treated with nonsurgical therapies reflects the fact that determining the presence of urethral extension is difficult and, if present, is inherently inaccessible to these local therapies because the urethra is not an outward-facing tissue surface; MMS represents one possible solution to these issues.
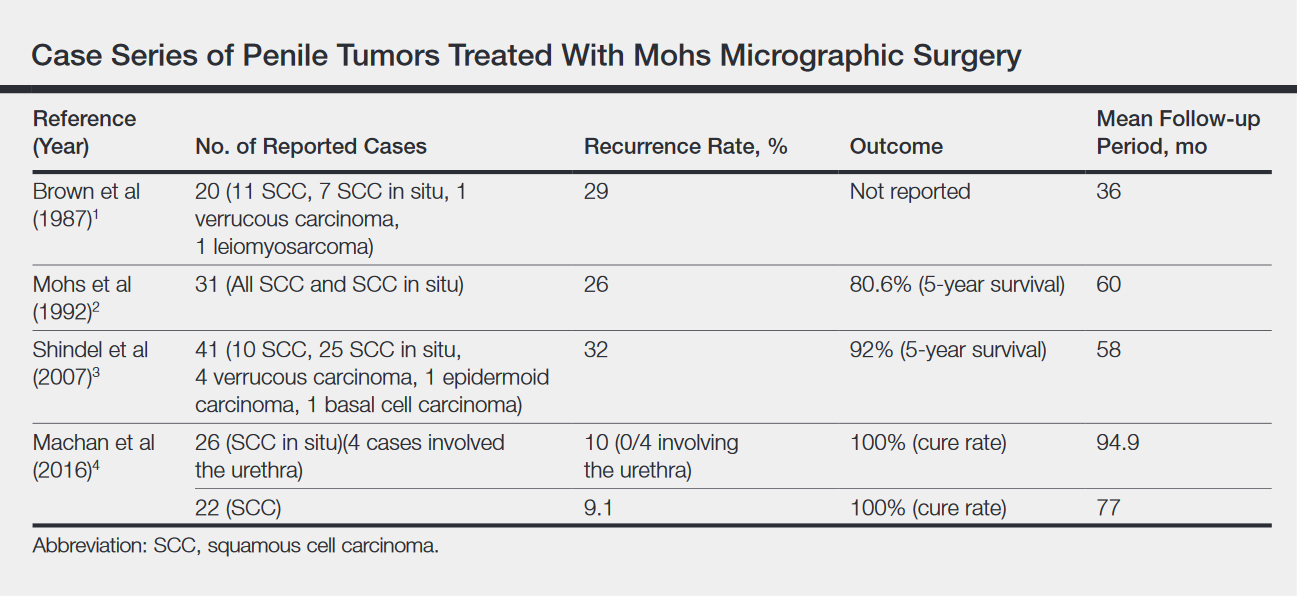
Across all treatment modalities, the most prognostic factor of cancer-specific survival in patients with penile SCC is pelvic lymph node involvement. Some reports cite 5-year survival rates as low as 0% in the setting of pelvic lymph node involvement,5 whereas others had cited rates of 29% to 40%4; 5-year survival rates of higher than 85% have been reported in node-negative patients.4 Recurrence rates vary widely by treatment modality, ranging from less than 10% with partial penectomy and long-term follow-up8 and up to 50% within 2 years with penile-preserving approaches (eg, topical chemotherapy, laser therapy, radiotherapy).5 Multiple case series of penile cancer (the most common of which was SCC/SCC in situ) treated with MMS report comparable and at times superior survival and recurrence data (Table).1-4 Slightly higher recurrences of penile SCC treated with MMS compared to penectomy have been reported, along with considerably higher recurrence rates compared to nonpenile cutaneous SCC treated with MMS (reported to be less than 3%).4 The elastic and expansile nature of penile tissue may lead to distortion from swelling/local anesthesia when taking individual Mohs layers. Additionally, as a large percentage of penile SCCs are attributable to human papillomavirus, difficulty in detecting human papilloma virus–infected cells (which may have oncogenic potential) with the naked eye or histologically with typical staining techniques may help explain the higher recurrence rate of penile SCC treated with MMS compared to penectomy. Despite the higher recurrence rates, survival is comparable or higher in cases treated with MMS (Table).
Partial penectomy also has a negative impact on health-related quality of life. Kieffer et al9 compared the impact of penile-sparing surgery (PSS)(including MMS) versus partial or total penectomy on sexual function and health-related quality of life in 90 patients with penile cancer. Although the association between the extent of surgery (partial penectomy/total penectomy/PSS) surgery type and extent and most outcome measures was not statistically significant, partial penectomy was associated with significantly more problems with orgasm (P=.031), concerns about appearance (P=.008), interference in daily life (P=.032), and urinary function (P<.0001) when compared to patients treated with PSS.9 Although this study included only laser/local excision with or without circumcision or glans penis amputation with or without reconstruction as PSSs and did not explicitly include MMS, MMS is clearly a tissue-sparing technique and the study results are generaliz
Conclusion
Penile SCC with considerable urethral extension is uncommon, difficult to manage, and often is resistant to less invasive and nonsurgical treatments. As a result, partial or total penectomy is sometimes necessary. Such cases benefit from MMS with distal urethrectomy and reconstruction because MMS provides equivalent or better overall cure rates compared to more radical interventions.1-4 Importantly, preservation of the penis with MMS can spare patients considerable physical and psychosocial morbidity. Partial penectomy is associated with more health-related quality-of-life problems with orgasm, concerns about appearance, interference in daily life, and urinary function compared to PSSs such as MMS.9 This case, and a growing body of literature, are a call to dermatologists and urologists to consider MMS as a treatment for penile SCC, even with involvement of the urethra.
- Brown MD, Zachary CB, Grekin RC, et al. Penile tumors: their management by Mohs micrographic surgery. J Dermatol Surg Oncol. 1987;13:1163-1167.
- Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am. 1992;19:291-304.
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol. 2007;178:1980-1985.
- Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936-944.
- Spiess PE, Horenblas S, Pagliaro LC, et al. Current concepts in penile cancer. J Natl Compr Canc Netw. 2013;11:617-624.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(10 suppl):2883-2891.
- Nash PA, Bihrle R, Gleason PE, et al. Mohs micrographic surgery and distal urethrectomy with immediate urethral reconstruction for glanular carcinoma in situ with significant urethral extension. Urology. 1996;47:108-110.
- Djordjevic ML, Palminteri E, Martins F. Male genital reconstruction for the penile cancer survivor. Curr Opin Urol. 2014;24:427-433.
- Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol. 2014;192:1105-1110.
Penile squamous cell carcinoma (SCC) with considerable urethral extension is uncommon and difficult to manage. It often is resistant to less invasive and nonsurgical treatments and frequently results in partial or total penectomy, which can lead to cosmetic disfigurement, functional issues, and psychological distress. We report a case of penile SCC in situ with considerable urethral extension with a focus of cells suspicious for moderately well-differentiated and invasive SCC that was treated with
Mohs micrographic surgery with distal urethrectomy and reconstruction is a valuable treatment technique for cases of SCC involving the glans penis and distal urethra. It offers equivalent or better overall cure rates compared to more radical interventions. Additionally, preservation of the penis with MMS spares patients from considerable physical and psychosocial morbidity. Our case, along with growing body of literature,1-4 calls on dermatologists and urologists to consider MMS as a treatment for penile SCC with or without urethral involvement.
Case Report
A 61-year-old man presented to the dermatology department with a pruritic lesion on the penis that had been present for 6 years. Shave biopsy demonstrated SCC in situ with a focus of cells suspicious for moderately well-differentiated and invasive SCC. Physical examination revealed an ill-defined, 2.2×1.9-cm, pink, eroded plaque involving the tip of the penis and surrounding the external urinary meatus (Figure 1). There was no palpable inguinal lymphadenopathy.

Distal penectomy and lymph node biopsy was recommended following evaluation by the urologic oncology department, but the patient declined these interventions and presented to our dermatology department (A.H.) for a second opinion. The tumor, including the invasive perineural portion, was removed using MMS several weeks after initially presenting to urologic oncology. Ventral meatotomy allowed access to the SCC in situ portion extending proximally up the pendulous urethra (Figure 2). Clear margins were obtained after the eighth stage of MMS, which required removal of 4 to 5 cm of the distal urethra (Figure 3). Reconstruction of the wound required urethral advancement, urethrostomy, and meatoplasty. A positive outcome was achieved with preservation of the length and shape of the penis as well as the cosmetic appearance of the glans penis (Figure 4). The patient was satisfied with the outcome. At 49 months’ follow-up, no evidence of local recurrence or disease progression was noted, and the distal urethrostomy remained intact and functional.



Comment
Penile SCC is a rare malignancy that represents between 0.4% and 0.6% of all malignant tumors in the United States and occurs most commonly in men aged 50 to 70 years.4 The incidence is higher in developing countries, approaching 10% of malignancies in men. It occurs most commonly on the glans penis, prepuce, and coronal sulcus, and has multiple possible appearances, including erythematous and indurated, warty and exophytic, or flat and ulcerated lesions.5 Some reports indicate that more than 40% of penile SCCs are attributable to human papilloma virus,6 while lack of circumcision, chronic inflammation, poor hygiene, balanitis xerotica obliterans, penile trauma, human immunodeficiency virus, UVA treatment of penile psoriasis, and tobacco use are known risk factors.5
Invasive penile SCC generally is treated with penectomy (partial or total), radiation therapy, or MMS; SCC in situ can be treated with topical chemotherapy, laser therapy, and wide local excision (2-cm margins) including circumcision, complete glansectomy, or MMS.5 Squamous cell carcinoma in situ with urethral involvement treated with nonsurgical therapies is associated with higher recurrence rates, ultimately necessitating more aggressive treatments, most commonly partial penectomy.7 The high local recurrence rate of SCC in situ with urethral involvement treated with nonsurgical therapies reflects the fact that determining the presence of urethral extension is difficult and, if present, is inherently inaccessible to these local therapies because the urethra is not an outward-facing tissue surface; MMS represents one possible solution to these issues.
Across all treatment modalities, the most prognostic factor of cancer-specific survival in patients with penile SCC is pelvic lymph node involvement. Some reports cite 5-year survival rates as low as 0% in the setting of pelvic lymph node involvement,5 whereas others had cited rates of 29% to 40%4; 5-year survival rates of higher than 85% have been reported in node-negative patients.4 Recurrence rates vary widely by treatment modality, ranging from less than 10% with partial penectomy and long-term follow-up8 and up to 50% within 2 years with penile-preserving approaches (eg, topical chemotherapy, laser therapy, radiotherapy).5 Multiple case series of penile cancer (the most common of which was SCC/SCC in situ) treated with MMS report comparable and at times superior survival and recurrence data (Table).1-4 Slightly higher recurrences of penile SCC treated with MMS compared to penectomy have been reported, along with considerably higher recurrence rates compared to nonpenile cutaneous SCC treated with MMS (reported to be less than 3%).4 The elastic and expansile nature of penile tissue may lead to distortion from swelling/local anesthesia when taking individual Mohs layers. Additionally, as a large percentage of penile SCCs are attributable to human papillomavirus, difficulty in detecting human papilloma virus–infected cells (which may have oncogenic potential) with the naked eye or histologically with typical staining techniques may help explain the higher recurrence rate of penile SCC treated with MMS compared to penectomy. Despite the higher recurrence rates, survival is comparable or higher in cases treated with MMS (Table).

Partial penectomy also has a negative impact on health-related quality of life. Kieffer et al9 compared the impact of penile-sparing surgery (PSS)(including MMS) versus partial or total penectomy on sexual function and health-related quality of life in 90 patients with penile cancer. Although the association between the extent of surgery (partial penectomy/total penectomy/PSS) surgery type and extent and most outcome measures was not statistically significant, partial penectomy was associated with significantly more problems with orgasm (P=.031), concerns about appearance (P=.008), interference in daily life (P=.032), and urinary function (P<.0001) when compared to patients treated with PSS.9 Although this study included only laser/local excision with or without circumcision or glans penis amputation with or without reconstruction as PSSs and did not explicitly include MMS, MMS is clearly a tissue-sparing technique and the study results are generaliz
Conclusion
Penile SCC with considerable urethral extension is uncommon, difficult to manage, and often is resistant to less invasive and nonsurgical treatments. As a result, partial or total penectomy is sometimes necessary. Such cases benefit from MMS with distal urethrectomy and reconstruction because MMS provides equivalent or better overall cure rates compared to more radical interventions.1-4 Importantly, preservation of the penis with MMS can spare patients considerable physical and psychosocial morbidity. Partial penectomy is associated with more health-related quality-of-life problems with orgasm, concerns about appearance, interference in daily life, and urinary function compared to PSSs such as MMS.9 This case, and a growing body of literature, are a call to dermatologists and urologists to consider MMS as a treatment for penile SCC, even with involvement of the urethra.
Penile squamous cell carcinoma (SCC) with considerable urethral extension is uncommon and difficult to manage. It often is resistant to less invasive and nonsurgical treatments and frequently results in partial or total penectomy, which can lead to cosmetic disfigurement, functional issues, and psychological distress. We report a case of penile SCC in situ with considerable urethral extension with a focus of cells suspicious for moderately well-differentiated and invasive SCC that was treated with
Mohs micrographic surgery with distal urethrectomy and reconstruction is a valuable treatment technique for cases of SCC involving the glans penis and distal urethra. It offers equivalent or better overall cure rates compared to more radical interventions. Additionally, preservation of the penis with MMS spares patients from considerable physical and psychosocial morbidity. Our case, along with growing body of literature,1-4 calls on dermatologists and urologists to consider MMS as a treatment for penile SCC with or without urethral involvement.
Case Report
A 61-year-old man presented to the dermatology department with a pruritic lesion on the penis that had been present for 6 years. Shave biopsy demonstrated SCC in situ with a focus of cells suspicious for moderately well-differentiated and invasive SCC. Physical examination revealed an ill-defined, 2.2×1.9-cm, pink, eroded plaque involving the tip of the penis and surrounding the external urinary meatus (Figure 1). There was no palpable inguinal lymphadenopathy.

Distal penectomy and lymph node biopsy was recommended following evaluation by the urologic oncology department, but the patient declined these interventions and presented to our dermatology department (A.H.) for a second opinion. The tumor, including the invasive perineural portion, was removed using MMS several weeks after initially presenting to urologic oncology. Ventral meatotomy allowed access to the SCC in situ portion extending proximally up the pendulous urethra (Figure 2). Clear margins were obtained after the eighth stage of MMS, which required removal of 4 to 5 cm of the distal urethra (Figure 3). Reconstruction of the wound required urethral advancement, urethrostomy, and meatoplasty. A positive outcome was achieved with preservation of the length and shape of the penis as well as the cosmetic appearance of the glans penis (Figure 4). The patient was satisfied with the outcome. At 49 months’ follow-up, no evidence of local recurrence or disease progression was noted, and the distal urethrostomy remained intact and functional.



Comment
Penile SCC is a rare malignancy that represents between 0.4% and 0.6% of all malignant tumors in the United States and occurs most commonly in men aged 50 to 70 years.4 The incidence is higher in developing countries, approaching 10% of malignancies in men. It occurs most commonly on the glans penis, prepuce, and coronal sulcus, and has multiple possible appearances, including erythematous and indurated, warty and exophytic, or flat and ulcerated lesions.5 Some reports indicate that more than 40% of penile SCCs are attributable to human papilloma virus,6 while lack of circumcision, chronic inflammation, poor hygiene, balanitis xerotica obliterans, penile trauma, human immunodeficiency virus, UVA treatment of penile psoriasis, and tobacco use are known risk factors.5
Invasive penile SCC generally is treated with penectomy (partial or total), radiation therapy, or MMS; SCC in situ can be treated with topical chemotherapy, laser therapy, and wide local excision (2-cm margins) including circumcision, complete glansectomy, or MMS.5 Squamous cell carcinoma in situ with urethral involvement treated with nonsurgical therapies is associated with higher recurrence rates, ultimately necessitating more aggressive treatments, most commonly partial penectomy.7 The high local recurrence rate of SCC in situ with urethral involvement treated with nonsurgical therapies reflects the fact that determining the presence of urethral extension is difficult and, if present, is inherently inaccessible to these local therapies because the urethra is not an outward-facing tissue surface; MMS represents one possible solution to these issues.
Across all treatment modalities, the most prognostic factor of cancer-specific survival in patients with penile SCC is pelvic lymph node involvement. Some reports cite 5-year survival rates as low as 0% in the setting of pelvic lymph node involvement,5 whereas others had cited rates of 29% to 40%4; 5-year survival rates of higher than 85% have been reported in node-negative patients.4 Recurrence rates vary widely by treatment modality, ranging from less than 10% with partial penectomy and long-term follow-up8 and up to 50% within 2 years with penile-preserving approaches (eg, topical chemotherapy, laser therapy, radiotherapy).5 Multiple case series of penile cancer (the most common of which was SCC/SCC in situ) treated with MMS report comparable and at times superior survival and recurrence data (Table).1-4 Slightly higher recurrences of penile SCC treated with MMS compared to penectomy have been reported, along with considerably higher recurrence rates compared to nonpenile cutaneous SCC treated with MMS (reported to be less than 3%).4 The elastic and expansile nature of penile tissue may lead to distortion from swelling/local anesthesia when taking individual Mohs layers. Additionally, as a large percentage of penile SCCs are attributable to human papillomavirus, difficulty in detecting human papilloma virus–infected cells (which may have oncogenic potential) with the naked eye or histologically with typical staining techniques may help explain the higher recurrence rate of penile SCC treated with MMS compared to penectomy. Despite the higher recurrence rates, survival is comparable or higher in cases treated with MMS (Table).

Partial penectomy also has a negative impact on health-related quality of life. Kieffer et al9 compared the impact of penile-sparing surgery (PSS)(including MMS) versus partial or total penectomy on sexual function and health-related quality of life in 90 patients with penile cancer. Although the association between the extent of surgery (partial penectomy/total penectomy/PSS) surgery type and extent and most outcome measures was not statistically significant, partial penectomy was associated with significantly more problems with orgasm (P=.031), concerns about appearance (P=.008), interference in daily life (P=.032), and urinary function (P<.0001) when compared to patients treated with PSS.9 Although this study included only laser/local excision with or without circumcision or glans penis amputation with or without reconstruction as PSSs and did not explicitly include MMS, MMS is clearly a tissue-sparing technique and the study results are generaliz
Conclusion
Penile SCC with considerable urethral extension is uncommon, difficult to manage, and often is resistant to less invasive and nonsurgical treatments. As a result, partial or total penectomy is sometimes necessary. Such cases benefit from MMS with distal urethrectomy and reconstruction because MMS provides equivalent or better overall cure rates compared to more radical interventions.1-4 Importantly, preservation of the penis with MMS can spare patients considerable physical and psychosocial morbidity. Partial penectomy is associated with more health-related quality-of-life problems with orgasm, concerns about appearance, interference in daily life, and urinary function compared to PSSs such as MMS.9 This case, and a growing body of literature, are a call to dermatologists and urologists to consider MMS as a treatment for penile SCC, even with involvement of the urethra.
- Brown MD, Zachary CB, Grekin RC, et al. Penile tumors: their management by Mohs micrographic surgery. J Dermatol Surg Oncol. 1987;13:1163-1167.
- Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am. 1992;19:291-304.
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol. 2007;178:1980-1985.
- Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936-944.
- Spiess PE, Horenblas S, Pagliaro LC, et al. Current concepts in penile cancer. J Natl Compr Canc Netw. 2013;11:617-624.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(10 suppl):2883-2891.
- Nash PA, Bihrle R, Gleason PE, et al. Mohs micrographic surgery and distal urethrectomy with immediate urethral reconstruction for glanular carcinoma in situ with significant urethral extension. Urology. 1996;47:108-110.
- Djordjevic ML, Palminteri E, Martins F. Male genital reconstruction for the penile cancer survivor. Curr Opin Urol. 2014;24:427-433.
- Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol. 2014;192:1105-1110.
- Brown MD, Zachary CB, Grekin RC, et al. Penile tumors: their management by Mohs micrographic surgery. J Dermatol Surg Oncol. 1987;13:1163-1167.
- Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am. 1992;19:291-304.
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol. 2007;178:1980-1985.
- Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936-944.
- Spiess PE, Horenblas S, Pagliaro LC, et al. Current concepts in penile cancer. J Natl Compr Canc Netw. 2013;11:617-624.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(10 suppl):2883-2891.
- Nash PA, Bihrle R, Gleason PE, et al. Mohs micrographic surgery and distal urethrectomy with immediate urethral reconstruction for glanular carcinoma in situ with significant urethral extension. Urology. 1996;47:108-110.
- Djordjevic ML, Palminteri E, Martins F. Male genital reconstruction for the penile cancer survivor. Curr Opin Urol. 2014;24:427-433.
- Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol. 2014;192:1105-1110.
Resident Pearl
- Penile squamous cell carcinoma (SCC) often is treated with partial or total penectomy, especially when there is urethral extension. Mohs micrographic surgery for penile SCC results in equivalent or better overall cure rates and decreases morbidity.
Circulating biomarkers predicted melanoma survival
Among patients with metastatic melanoma, detectable circulating levels of BRAFV600 mutated circulating tumor DNA and elevated levels of circulating hepatocyte growth factor separately predicted significantly worse survival, regardless of treatment.
After adjusting for lactate dehydrogenase, ECOG (Eastern Cooperative Oncology Group) status, disease stage, and treatment, hazard ratios for overall survival were 1.75 (95% confidence interval, 1.35-2.28) for detectable versus undetectable circulating tumor DNA (ctDNA) and 1.24 (95% CI, 1.00-1.53) for high versus low circulating hepatocyte growth factor (cHGF), reported William Lu, PhD, of Genentech in South San Francisco, with his associates. Their retrospective, exploratory analysis of the phase 3 BRIM-3 trial was published in JCO Precision Oncology.
BRIM-3 was a multicenter, open-label trial of 675 patients with previously untreated unresectable stage IIIC or stage IV melanoma that tested positive for the BRAFV600 mutation. Patients received either vemurafenib or dacarbazine therapy.
Baseline ctDNA, baseline cHGF, and ECOG performance status most strongly predicted overall survival, said the investigators. Patients whose mutant ctDNA fraction exceeded 0.039 had an 11.5-month shorter median overall survival on vemurafenib (P less than .001) and a 13.9-month shorter median overall survival on dacarbazine (P less than .001) than patients whose mutant ctDNA fraction was less than 0.039, they explained. Similarly, median overall survival times were 5.4 months shorter for vemurafenib (P = .002) and 12.3 months shorter for dacarbazine (P less than .001) when patients had high (more than 438 pg/mL) versus low circulating cHGF.
Median overall survival for vemurafenib was shortest (7.3 months) in the subgroup of 64 patients with detectable BRAFV600 ctDNA and elevated cHGF, according to the researchers. In contrast, median overall survival for vemurafenib was longest (22.0 months) when patients had undetectable ctDNA and an ECOG status of 0.
“Although similar studies have been performed, to our knowledge, our study is unique in its size and in its design as a randomized trial,” Dr. Lu and associates concluded. “The biomarkers in this study can be readily acquired and measured, and require only small volumes of plasma.” They suggested validating the findings in a separate dataset.
F. Hoffman-La Roche provided funding. Roche Molecular Systems makes the Cobas 4800 BRAFV600 Mutation Test used in the study. Dr. Lu disclosed employment and research funding from Genentech/Roche.
SOURCE: Lu W et al. JCO Precis Oncol. 2018 Apr 25. doi: 10.1200/PO.17.00168.
Among patients with metastatic melanoma, detectable circulating levels of BRAFV600 mutated circulating tumor DNA and elevated levels of circulating hepatocyte growth factor separately predicted significantly worse survival, regardless of treatment.
After adjusting for lactate dehydrogenase, ECOG (Eastern Cooperative Oncology Group) status, disease stage, and treatment, hazard ratios for overall survival were 1.75 (95% confidence interval, 1.35-2.28) for detectable versus undetectable circulating tumor DNA (ctDNA) and 1.24 (95% CI, 1.00-1.53) for high versus low circulating hepatocyte growth factor (cHGF), reported William Lu, PhD, of Genentech in South San Francisco, with his associates. Their retrospective, exploratory analysis of the phase 3 BRIM-3 trial was published in JCO Precision Oncology.
BRIM-3 was a multicenter, open-label trial of 675 patients with previously untreated unresectable stage IIIC or stage IV melanoma that tested positive for the BRAFV600 mutation. Patients received either vemurafenib or dacarbazine therapy.
Baseline ctDNA, baseline cHGF, and ECOG performance status most strongly predicted overall survival, said the investigators. Patients whose mutant ctDNA fraction exceeded 0.039 had an 11.5-month shorter median overall survival on vemurafenib (P less than .001) and a 13.9-month shorter median overall survival on dacarbazine (P less than .001) than patients whose mutant ctDNA fraction was less than 0.039, they explained. Similarly, median overall survival times were 5.4 months shorter for vemurafenib (P = .002) and 12.3 months shorter for dacarbazine (P less than .001) when patients had high (more than 438 pg/mL) versus low circulating cHGF.
Median overall survival for vemurafenib was shortest (7.3 months) in the subgroup of 64 patients with detectable BRAFV600 ctDNA and elevated cHGF, according to the researchers. In contrast, median overall survival for vemurafenib was longest (22.0 months) when patients had undetectable ctDNA and an ECOG status of 0.
“Although similar studies have been performed, to our knowledge, our study is unique in its size and in its design as a randomized trial,” Dr. Lu and associates concluded. “The biomarkers in this study can be readily acquired and measured, and require only small volumes of plasma.” They suggested validating the findings in a separate dataset.
F. Hoffman-La Roche provided funding. Roche Molecular Systems makes the Cobas 4800 BRAFV600 Mutation Test used in the study. Dr. Lu disclosed employment and research funding from Genentech/Roche.
SOURCE: Lu W et al. JCO Precis Oncol. 2018 Apr 25. doi: 10.1200/PO.17.00168.
Among patients with metastatic melanoma, detectable circulating levels of BRAFV600 mutated circulating tumor DNA and elevated levels of circulating hepatocyte growth factor separately predicted significantly worse survival, regardless of treatment.
After adjusting for lactate dehydrogenase, ECOG (Eastern Cooperative Oncology Group) status, disease stage, and treatment, hazard ratios for overall survival were 1.75 (95% confidence interval, 1.35-2.28) for detectable versus undetectable circulating tumor DNA (ctDNA) and 1.24 (95% CI, 1.00-1.53) for high versus low circulating hepatocyte growth factor (cHGF), reported William Lu, PhD, of Genentech in South San Francisco, with his associates. Their retrospective, exploratory analysis of the phase 3 BRIM-3 trial was published in JCO Precision Oncology.
BRIM-3 was a multicenter, open-label trial of 675 patients with previously untreated unresectable stage IIIC or stage IV melanoma that tested positive for the BRAFV600 mutation. Patients received either vemurafenib or dacarbazine therapy.
Baseline ctDNA, baseline cHGF, and ECOG performance status most strongly predicted overall survival, said the investigators. Patients whose mutant ctDNA fraction exceeded 0.039 had an 11.5-month shorter median overall survival on vemurafenib (P less than .001) and a 13.9-month shorter median overall survival on dacarbazine (P less than .001) than patients whose mutant ctDNA fraction was less than 0.039, they explained. Similarly, median overall survival times were 5.4 months shorter for vemurafenib (P = .002) and 12.3 months shorter for dacarbazine (P less than .001) when patients had high (more than 438 pg/mL) versus low circulating cHGF.
Median overall survival for vemurafenib was shortest (7.3 months) in the subgroup of 64 patients with detectable BRAFV600 ctDNA and elevated cHGF, according to the researchers. In contrast, median overall survival for vemurafenib was longest (22.0 months) when patients had undetectable ctDNA and an ECOG status of 0.
“Although similar studies have been performed, to our knowledge, our study is unique in its size and in its design as a randomized trial,” Dr. Lu and associates concluded. “The biomarkers in this study can be readily acquired and measured, and require only small volumes of plasma.” They suggested validating the findings in a separate dataset.
F. Hoffman-La Roche provided funding. Roche Molecular Systems makes the Cobas 4800 BRAFV600 Mutation Test used in the study. Dr. Lu disclosed employment and research funding from Genentech/Roche.
SOURCE: Lu W et al. JCO Precis Oncol. 2018 Apr 25. doi: 10.1200/PO.17.00168.
FROM JCO PRECISION ONCOLOGY
Key clinical point: Among patients with metastatic melanoma, high circulating levels of BRAFV600 mutated circulating tumor DNA and circulating hepatocyte growth factor predicted significantly worse overall survival, irrespective of treatment.
Major finding: Adjusted hazard ratios for overall survival were 1.75 for high versus undetectable circulating tumor DNA and 1.24 for high versus low circulating hepatocyte growth factor.
Study details: A retrospective, exploratory analysis of 675 patients with metastatic BRAFV600 mutated advanced melanoma.
Disclosures: F. Hoffman-La Roche provided funding. Roche Molecular Systems makes the Cobas 4800 BRAFV600 Mutation Test used in the study. Dr. Lu disclosed employment and research funding from Genentech/Roche.
Source: Lu W et al. JCO Precis Oncol. 2018 Apr 25. doi: 10.1200/PO.17.00168.
World Trade Center responders face greater cancer burden, including greater risk of multiple myeloma
Rescue and recovery workers who were involved in the aftermath of the World Trade Center disaster may face a greater cancer burden than the general population, according to two studies published in JAMA Oncology.
In particular, they may be at risk of developing multiple myeloma at an earlier age.
The first study was a closed-cohort study of 14,474 employees of the Fire Department of the City of New York (FDNY) who were exposed to the World Trade Center disaster but were cancer-free as of Jan. 1, 2012. The aim was to project cancer incidence from 2012 through 2031, based on data from the FDNY World Trade Center Health Program, and compare those rates with age-, race-, and sex-specific New York cancer rates from the general population.
The modeling projected a “modestly” higher number of cancer cases in the white male subgroup of rescue and recovery workers exposed to the World Trade Center (2,714 vs. 2,596 for the general population of New York; P less than .001). Specifically, the investigators projected significantly higher case counts of prostate cancer (1,437 vs. 863), thyroid cancer (73 vs. 57), and melanoma (201 vs. 131), compared with the general population in New York, but fewer lung (237 vs. 373), colorectal (172 vs. 267), and kidney cancers (66 vs. 132) (P less than .001 for all).
“Our findings suggest that the FDNY WTC-exposed cohort may experience a greater burden of cancer than would be expected from a population with similar demographic characteristics,” wrote Rachel Zeig-Owens, DrPH, from the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, and coauthors, highlighting prostate cancer as a particular concern.
However, they also acknowledged that the elevated rates observed in people exposed to the World Trade Center disaster could be a result of increased surveillance, even though they did attempt to correct for that, and that firefighters in general might face higher risks.
“It is possible that firefighters have a higher risk of cancer than the general population owing to exposures associated with the occupation,” they wrote. However occupation could also have the opposite effect, as rescue and recovery workers tend to have lower smoking rates, which may explain the relatively low rates of certain cancers such as lung cancer, they said.
A second study examined the effect of the World Trade Center disaster on the risk of multiple myeloma and monoclonal gammopathies in exposed firefighters.
The seroprevalence study of monoclonal gammopathies of undetermined significance (MGUS) in 781 exposed firefighters revealed that the age-standardized prevalence of these was 76% higher in this population than it was in a white male reference population living in Minnesota.
In particular, the age-standardized prevalence of light-chain MGUS was more than threefold higher in exposed firefighters, compared with the reference population.
Researchers also analyzed a case series of 16 exposed white male firefighters who received a diagnosis of multiple myeloma after Sept. 11, 2001. Of the 14 patients for whom data on the monoclonal protein isotype was available, half had light-chain multiple myeloma.
“These findings are of interest due to previously observed associations between light-chain multiple myeloma and light-chain MGUS and exposure to toxins, and chronic immune stimulation,” wrote Ola Landgren, MD, PhD, from the Memorial Sloan Kettering Cancer Center and his coauthors.
Seven patients were also assessed for CD20 expression – a marker of poorer prognosis – and 71% were found to be CD20-positive, a prevalence around 3.5-fold higher than that seen in the general population.
The cohort with multiple myeloma was diagnosed on average 12 years younger than those in the general population. The authors commented that this was unlikely to be caused by lead-time bias because the time from first symptoms to clinical manifestation of the disease is usually around 1 year.
“Taken together, our results show that environmental exposure due to the WTC attacks is associated with myeloma precursor disease (MGUS and light-chain MGUS) and may be a risk factor for the development of multiple myeloma at an earlier age, particularly the light-chain subtype,” the authors wrote.
The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared.
The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
SOURCE: Zeig-Owens R et al. JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology. 2018 April 16. doi: 10.1001/jamaoncol.2018.0509.
When the heroes of the World Trade Center are diagnosed with even a common cancer, there is a natural tendency to assume that the diagnosis is the result of their service during the disaster. However, it is important to appreciate that the firefighting profession is known to be associated with higher risks of monoclonal gammopathy of undetermined significance and multiple myelomas, compared with the general population.
Given that, it would have been preferable to compare the World Trade Center–exposed populations with an equally intensively screened, age-matched cohort of firefighters from another major city.
If we apply Sir Richard Doll’s rule that a single epidemiologic study cannot be persuasive until the lower bound of the 95% confidence interval is greater than three, the relative risks in the study by Landgren and colleagues are too small to be persuasive.
The predicted increases in cancers of the prostate, thyroid, and myeloma are interesting, but these have also been previously reported in firefighters from other cities.
Despite this, we owe it to these men and women to find the truth and determine the illnesses that are associated with their service.
Otis W. Brawley, MD, is chief medical and scientific officer and executive vice president of the American Cancer Society and a professor at Emory University, Atlanta. These comments are taken from an accompanying editorial (JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0498.) No conflicts of interest were declared.
When the heroes of the World Trade Center are diagnosed with even a common cancer, there is a natural tendency to assume that the diagnosis is the result of their service during the disaster. However, it is important to appreciate that the firefighting profession is known to be associated with higher risks of monoclonal gammopathy of undetermined significance and multiple myelomas, compared with the general population.
Given that, it would have been preferable to compare the World Trade Center–exposed populations with an equally intensively screened, age-matched cohort of firefighters from another major city.
If we apply Sir Richard Doll’s rule that a single epidemiologic study cannot be persuasive until the lower bound of the 95% confidence interval is greater than three, the relative risks in the study by Landgren and colleagues are too small to be persuasive.
The predicted increases in cancers of the prostate, thyroid, and myeloma are interesting, but these have also been previously reported in firefighters from other cities.
Despite this, we owe it to these men and women to find the truth and determine the illnesses that are associated with their service.
Otis W. Brawley, MD, is chief medical and scientific officer and executive vice president of the American Cancer Society and a professor at Emory University, Atlanta. These comments are taken from an accompanying editorial (JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0498.) No conflicts of interest were declared.
When the heroes of the World Trade Center are diagnosed with even a common cancer, there is a natural tendency to assume that the diagnosis is the result of their service during the disaster. However, it is important to appreciate that the firefighting profession is known to be associated with higher risks of monoclonal gammopathy of undetermined significance and multiple myelomas, compared with the general population.
Given that, it would have been preferable to compare the World Trade Center–exposed populations with an equally intensively screened, age-matched cohort of firefighters from another major city.
If we apply Sir Richard Doll’s rule that a single epidemiologic study cannot be persuasive until the lower bound of the 95% confidence interval is greater than three, the relative risks in the study by Landgren and colleagues are too small to be persuasive.
The predicted increases in cancers of the prostate, thyroid, and myeloma are interesting, but these have also been previously reported in firefighters from other cities.
Despite this, we owe it to these men and women to find the truth and determine the illnesses that are associated with their service.
Otis W. Brawley, MD, is chief medical and scientific officer and executive vice president of the American Cancer Society and a professor at Emory University, Atlanta. These comments are taken from an accompanying editorial (JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0498.) No conflicts of interest were declared.
Rescue and recovery workers who were involved in the aftermath of the World Trade Center disaster may face a greater cancer burden than the general population, according to two studies published in JAMA Oncology.
In particular, they may be at risk of developing multiple myeloma at an earlier age.
The first study was a closed-cohort study of 14,474 employees of the Fire Department of the City of New York (FDNY) who were exposed to the World Trade Center disaster but were cancer-free as of Jan. 1, 2012. The aim was to project cancer incidence from 2012 through 2031, based on data from the FDNY World Trade Center Health Program, and compare those rates with age-, race-, and sex-specific New York cancer rates from the general population.
The modeling projected a “modestly” higher number of cancer cases in the white male subgroup of rescue and recovery workers exposed to the World Trade Center (2,714 vs. 2,596 for the general population of New York; P less than .001). Specifically, the investigators projected significantly higher case counts of prostate cancer (1,437 vs. 863), thyroid cancer (73 vs. 57), and melanoma (201 vs. 131), compared with the general population in New York, but fewer lung (237 vs. 373), colorectal (172 vs. 267), and kidney cancers (66 vs. 132) (P less than .001 for all).
“Our findings suggest that the FDNY WTC-exposed cohort may experience a greater burden of cancer than would be expected from a population with similar demographic characteristics,” wrote Rachel Zeig-Owens, DrPH, from the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, and coauthors, highlighting prostate cancer as a particular concern.
However, they also acknowledged that the elevated rates observed in people exposed to the World Trade Center disaster could be a result of increased surveillance, even though they did attempt to correct for that, and that firefighters in general might face higher risks.
“It is possible that firefighters have a higher risk of cancer than the general population owing to exposures associated with the occupation,” they wrote. However occupation could also have the opposite effect, as rescue and recovery workers tend to have lower smoking rates, which may explain the relatively low rates of certain cancers such as lung cancer, they said.
A second study examined the effect of the World Trade Center disaster on the risk of multiple myeloma and monoclonal gammopathies in exposed firefighters.
The seroprevalence study of monoclonal gammopathies of undetermined significance (MGUS) in 781 exposed firefighters revealed that the age-standardized prevalence of these was 76% higher in this population than it was in a white male reference population living in Minnesota.
In particular, the age-standardized prevalence of light-chain MGUS was more than threefold higher in exposed firefighters, compared with the reference population.
Researchers also analyzed a case series of 16 exposed white male firefighters who received a diagnosis of multiple myeloma after Sept. 11, 2001. Of the 14 patients for whom data on the monoclonal protein isotype was available, half had light-chain multiple myeloma.
“These findings are of interest due to previously observed associations between light-chain multiple myeloma and light-chain MGUS and exposure to toxins, and chronic immune stimulation,” wrote Ola Landgren, MD, PhD, from the Memorial Sloan Kettering Cancer Center and his coauthors.
Seven patients were also assessed for CD20 expression – a marker of poorer prognosis – and 71% were found to be CD20-positive, a prevalence around 3.5-fold higher than that seen in the general population.
The cohort with multiple myeloma was diagnosed on average 12 years younger than those in the general population. The authors commented that this was unlikely to be caused by lead-time bias because the time from first symptoms to clinical manifestation of the disease is usually around 1 year.
“Taken together, our results show that environmental exposure due to the WTC attacks is associated with myeloma precursor disease (MGUS and light-chain MGUS) and may be a risk factor for the development of multiple myeloma at an earlier age, particularly the light-chain subtype,” the authors wrote.
The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared.
The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
SOURCE: Zeig-Owens R et al. JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology. 2018 April 16. doi: 10.1001/jamaoncol.2018.0509.
Rescue and recovery workers who were involved in the aftermath of the World Trade Center disaster may face a greater cancer burden than the general population, according to two studies published in JAMA Oncology.
In particular, they may be at risk of developing multiple myeloma at an earlier age.
The first study was a closed-cohort study of 14,474 employees of the Fire Department of the City of New York (FDNY) who were exposed to the World Trade Center disaster but were cancer-free as of Jan. 1, 2012. The aim was to project cancer incidence from 2012 through 2031, based on data from the FDNY World Trade Center Health Program, and compare those rates with age-, race-, and sex-specific New York cancer rates from the general population.
The modeling projected a “modestly” higher number of cancer cases in the white male subgroup of rescue and recovery workers exposed to the World Trade Center (2,714 vs. 2,596 for the general population of New York; P less than .001). Specifically, the investigators projected significantly higher case counts of prostate cancer (1,437 vs. 863), thyroid cancer (73 vs. 57), and melanoma (201 vs. 131), compared with the general population in New York, but fewer lung (237 vs. 373), colorectal (172 vs. 267), and kidney cancers (66 vs. 132) (P less than .001 for all).
“Our findings suggest that the FDNY WTC-exposed cohort may experience a greater burden of cancer than would be expected from a population with similar demographic characteristics,” wrote Rachel Zeig-Owens, DrPH, from the Montefiore Medical Center and Albert Einstein College of Medicine, both in New York, and coauthors, highlighting prostate cancer as a particular concern.
However, they also acknowledged that the elevated rates observed in people exposed to the World Trade Center disaster could be a result of increased surveillance, even though they did attempt to correct for that, and that firefighters in general might face higher risks.
“It is possible that firefighters have a higher risk of cancer than the general population owing to exposures associated with the occupation,” they wrote. However occupation could also have the opposite effect, as rescue and recovery workers tend to have lower smoking rates, which may explain the relatively low rates of certain cancers such as lung cancer, they said.
A second study examined the effect of the World Trade Center disaster on the risk of multiple myeloma and monoclonal gammopathies in exposed firefighters.
The seroprevalence study of monoclonal gammopathies of undetermined significance (MGUS) in 781 exposed firefighters revealed that the age-standardized prevalence of these was 76% higher in this population than it was in a white male reference population living in Minnesota.
In particular, the age-standardized prevalence of light-chain MGUS was more than threefold higher in exposed firefighters, compared with the reference population.
Researchers also analyzed a case series of 16 exposed white male firefighters who received a diagnosis of multiple myeloma after Sept. 11, 2001. Of the 14 patients for whom data on the monoclonal protein isotype was available, half had light-chain multiple myeloma.
“These findings are of interest due to previously observed associations between light-chain multiple myeloma and light-chain MGUS and exposure to toxins, and chronic immune stimulation,” wrote Ola Landgren, MD, PhD, from the Memorial Sloan Kettering Cancer Center and his coauthors.
Seven patients were also assessed for CD20 expression – a marker of poorer prognosis – and 71% were found to be CD20-positive, a prevalence around 3.5-fold higher than that seen in the general population.
The cohort with multiple myeloma was diagnosed on average 12 years younger than those in the general population. The authors commented that this was unlikely to be caused by lead-time bias because the time from first symptoms to clinical manifestation of the disease is usually around 1 year.
“Taken together, our results show that environmental exposure due to the WTC attacks is associated with myeloma precursor disease (MGUS and light-chain MGUS) and may be a risk factor for the development of multiple myeloma at an earlier age, particularly the light-chain subtype,” the authors wrote.
The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared.
The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
SOURCE: Zeig-Owens R et al. JAMA Oncology. 2018 April 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology. 2018 April 16. doi: 10.1001/jamaoncol.2018.0509.
FROM JAMA ONCOLOGY
Key clinical point: Monoclonal gammopathies and multiple myeloma may occur more often and earlier in World Trade Center rescue workers.
Major finding: Prevalence of light-chain monoclonal gammopathies is threefold higher in exposed firefighters than in a reference population of white males.
Study details: A cohort study in 14,474 employees of the Fire Department of the City of New York exposed to the Sept. 11, 2001, World Trade Center disaster, a case series of 16 exposed white male firefighters diagnosed with multiple myeloma, and a seroprevalence study of monoclonal gammopathies of undetermined significance in 781 exposed firefighters.
Disclosures: The first study was supported by the National Institute of Occupational Safety and Health; no conflicts of interest were declared. The second study was supported by the V Foundation for Cancer Research, the Byrne Fund for the benefit of Memorial Sloan-Kettering Cancer Center, the National Cancer Institute, the Albert Einstein Cancer Center, and the National Institute for Occupational Safety and Health; no conflicts of interest were declared.
Source: Zeig-Owens R et al. JAMA Oncology 2018, Apr 26. doi: 10.1001/jamaoncol.2018.0504. Landgren O et al. JAMA Oncology 2018, Apr 26. doi: 10.1001/jamaoncol.2018.0509.
Ideal sun protection practices by parents low in Toronto
according to Marcus G. Tan, MD, of the University of Toronto, and his associates.
Too much sun exposure as a child is a known risk factor for skin cancer, and “it has been estimated that approximately half of cumulative ultraviolet exposure by age 60 occurs before age 20.” So it is important that children be protected from excessive sun exposure, the investigators wrote, underscoring the reason for their study of parental sun protection by Canadian parents in Toronto, which has a multiracial population.
Parental sun protection efforts were considered ideal if they used sun protection every day; on sunny days all year round; or during the summer, using at least SPF 30, and reapplying sunscreen at least every 2 hours. Parental efforts would be considered nonideal if they did not meet any of these three criteria.
Of 183 parents, 17% used ideal sun protection for their children; 28% were in the lighter-skinned group (Fitzpatrick phototype I-III) and 5% in the darker-skinned group (Fitzpatrick phototype IV-V), The greater likelihood of using ideal sun protection among those in the lighter-skinned group was statistically significant (odds ratio, 7.4; P less than .001). As each child grew 1 year older, parents were 31% less likely to use ideal sun protection (OR, 0.69; P = .007).
Parents whose children were in the lighter-skinned group were “more likely to believe that sun exposure was harmful” for their children (OR, 17.2), and “to perceive value in sun protection,” (OR, 11.4) the researchers said. The parents whose children were in the darker-skinned group were more likely to consider sun protection as having drawbacks (OR, 9.2), and to believe that darker skin tone provides more sun protection (OR, 12.4). Neither of the two groups was more likely to consider that sun exposure provided health benefits or that it improved appearance.
Parents whose children were in the lighter-skinned group reported significantly more frequent use of sunscreen (OR, 3.6), sunglasses (OR, 2.3), and sun suits (OR, 2.7). The parents of the children in this group reported similar or higher rates of use of hats, long-sleeved clothing, and umbrellas; seeking shade; and staying indoors between 12 pm and 2 pm, but these rates were not statistically significantly different from those reported by the parents of children in the darker-skinned group, the investigators said.
“Our study suggests that more targeted education guidelines are necessary to ensure sun safety for all children, especially in a multiracial population,” said Dr. Tan and his colleagues. “Dermatologists and primary care physicians are in ideal positions to initiate personalized efforts to improve overall rates of sun protection.”
Dr. Tan reported no relevant financial disclosures. Dr. Miriam Weinstein has worked as an advisory board member for Johnson & Johnson, which manufactures sunscreen; and Dr. Shudeshna Nag consults for Valeant, which manufactures sunscreen.
SOURCE: Tan MG et al. Pediatr Dermatol. 2018 Feb 13. doi: 10.1111/pde.13433.
according to Marcus G. Tan, MD, of the University of Toronto, and his associates.
Too much sun exposure as a child is a known risk factor for skin cancer, and “it has been estimated that approximately half of cumulative ultraviolet exposure by age 60 occurs before age 20.” So it is important that children be protected from excessive sun exposure, the investigators wrote, underscoring the reason for their study of parental sun protection by Canadian parents in Toronto, which has a multiracial population.
Parental sun protection efforts were considered ideal if they used sun protection every day; on sunny days all year round; or during the summer, using at least SPF 30, and reapplying sunscreen at least every 2 hours. Parental efforts would be considered nonideal if they did not meet any of these three criteria.
Of 183 parents, 17% used ideal sun protection for their children; 28% were in the lighter-skinned group (Fitzpatrick phototype I-III) and 5% in the darker-skinned group (Fitzpatrick phototype IV-V), The greater likelihood of using ideal sun protection among those in the lighter-skinned group was statistically significant (odds ratio, 7.4; P less than .001). As each child grew 1 year older, parents were 31% less likely to use ideal sun protection (OR, 0.69; P = .007).
Parents whose children were in the lighter-skinned group were “more likely to believe that sun exposure was harmful” for their children (OR, 17.2), and “to perceive value in sun protection,” (OR, 11.4) the researchers said. The parents whose children were in the darker-skinned group were more likely to consider sun protection as having drawbacks (OR, 9.2), and to believe that darker skin tone provides more sun protection (OR, 12.4). Neither of the two groups was more likely to consider that sun exposure provided health benefits or that it improved appearance.
Parents whose children were in the lighter-skinned group reported significantly more frequent use of sunscreen (OR, 3.6), sunglasses (OR, 2.3), and sun suits (OR, 2.7). The parents of the children in this group reported similar or higher rates of use of hats, long-sleeved clothing, and umbrellas; seeking shade; and staying indoors between 12 pm and 2 pm, but these rates were not statistically significantly different from those reported by the parents of children in the darker-skinned group, the investigators said.
“Our study suggests that more targeted education guidelines are necessary to ensure sun safety for all children, especially in a multiracial population,” said Dr. Tan and his colleagues. “Dermatologists and primary care physicians are in ideal positions to initiate personalized efforts to improve overall rates of sun protection.”
Dr. Tan reported no relevant financial disclosures. Dr. Miriam Weinstein has worked as an advisory board member for Johnson & Johnson, which manufactures sunscreen; and Dr. Shudeshna Nag consults for Valeant, which manufactures sunscreen.
SOURCE: Tan MG et al. Pediatr Dermatol. 2018 Feb 13. doi: 10.1111/pde.13433.
according to Marcus G. Tan, MD, of the University of Toronto, and his associates.
Too much sun exposure as a child is a known risk factor for skin cancer, and “it has been estimated that approximately half of cumulative ultraviolet exposure by age 60 occurs before age 20.” So it is important that children be protected from excessive sun exposure, the investigators wrote, underscoring the reason for their study of parental sun protection by Canadian parents in Toronto, which has a multiracial population.
Parental sun protection efforts were considered ideal if they used sun protection every day; on sunny days all year round; or during the summer, using at least SPF 30, and reapplying sunscreen at least every 2 hours. Parental efforts would be considered nonideal if they did not meet any of these three criteria.
Of 183 parents, 17% used ideal sun protection for their children; 28% were in the lighter-skinned group (Fitzpatrick phototype I-III) and 5% in the darker-skinned group (Fitzpatrick phototype IV-V), The greater likelihood of using ideal sun protection among those in the lighter-skinned group was statistically significant (odds ratio, 7.4; P less than .001). As each child grew 1 year older, parents were 31% less likely to use ideal sun protection (OR, 0.69; P = .007).
Parents whose children were in the lighter-skinned group were “more likely to believe that sun exposure was harmful” for their children (OR, 17.2), and “to perceive value in sun protection,” (OR, 11.4) the researchers said. The parents whose children were in the darker-skinned group were more likely to consider sun protection as having drawbacks (OR, 9.2), and to believe that darker skin tone provides more sun protection (OR, 12.4). Neither of the two groups was more likely to consider that sun exposure provided health benefits or that it improved appearance.
Parents whose children were in the lighter-skinned group reported significantly more frequent use of sunscreen (OR, 3.6), sunglasses (OR, 2.3), and sun suits (OR, 2.7). The parents of the children in this group reported similar or higher rates of use of hats, long-sleeved clothing, and umbrellas; seeking shade; and staying indoors between 12 pm and 2 pm, but these rates were not statistically significantly different from those reported by the parents of children in the darker-skinned group, the investigators said.
“Our study suggests that more targeted education guidelines are necessary to ensure sun safety for all children, especially in a multiracial population,” said Dr. Tan and his colleagues. “Dermatologists and primary care physicians are in ideal positions to initiate personalized efforts to improve overall rates of sun protection.”
Dr. Tan reported no relevant financial disclosures. Dr. Miriam Weinstein has worked as an advisory board member for Johnson & Johnson, which manufactures sunscreen; and Dr. Shudeshna Nag consults for Valeant, which manufactures sunscreen.
SOURCE: Tan MG et al. Pediatr Dermatol. 2018 Feb 13. doi: 10.1111/pde.13433.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: More targeted education of parents regarding sun protection practices may be needed.
Major finding: Of 183 parents, 17% used ideal sun protection; 28% were in the lighter-skinned group and 5% in the darker-skinned group (odds ratio, 7.4; P less than .001).
Study details: The parents of 183 children aged from 6 months to 6 years in Toronto completed a questionnaire about their sun protection practices.
Disclosures: Dr. Marcus G. Tan reported no relevant financial disclosures. Dr.Miriam Weinstein has worked as an advisory board member for Johnson & Johnson, which manufactures sunscreen; and Dr. Shudeshna Nag consults for Valeant, which manufactures sunscreen.
Source: Tan MG et al. Pediatr Dermatol. 2018 Feb 13. doi: 10.1111/pde.13433.
Early results favorable for combo TLR9 agonist + pembro in advanced melanoma
CHICAGO – The intratumoral Toll-Like Receptor 9 (TLR-9) agonist, CMP-001, in combination with pembrolizumab in advanced melanoma patients, was well tolerated with a durable systemic clinical response, according to early results from an ongoing phase 1 trial.
Objective response rates on weekly (n = 56) and every 3 weeks schedules (n = 13) were 23% (13%-36%) and 15% (2%-45%) respectively, reported Mohammed M. Milhem, MBBS, of the University of Iowa, Iowa City.
For those dosed weekly at low dose (less than 5 mL) and high dose (5 mL or more), the ORR was 19% (n = 43, 95% confidence interval, 8%-33%) and 27% (n = 26, 95% CI, 12%-48%), respectively. Activity was demonstrated in subjects regardless of tumor burden, Dr. Milhem said at the annual meeting of the American Association for Cancer Research.
In this phase 1b study with a 3+3 design of dose escalation and expansion, the researchers enrolled patients with advanced melanoma who did not respond or had progressed resistant on prior anti-PD-1 monotherapy or in combination. CMP-001 was injected intratumorally in combination with pembrolizumab as per label intravenously.
The study drug CMP-001 has two components, a 30-mer CpG-A DNA oligonucleotide and a nonvirulent virus-like particle (VLP). The CpG-A DNA is packaged within the VLP that protects it from degradation and also allows TLR9 receptor uptake. CpG-A DNA acts as a TLR9 agonist by binding to it, thereby activating plasmacytoid dendritic cells (pDCs) within the tumor microenvironment. The activation results in secretion of large amounts of type 1 interferon and Th1 chemokines, changing the microenvironment from a “cold/desert-like” immune suppressed state to a “hot” antitumor inflamed state, Dr. Milhem said.
“The T cells thus generated can mediate tumor rejection both in the injected and noninjected tumor,” he said. Two CMP-001 schedules were evaluated, weekly for 7 weeks or weekly for 2 weeks, followed thereafter by every 3 weeks until discontinuation (due to progression, toxicity, investigator decision, or withdrawal of consent). Scans were done every 12 weeks and tumor response was assessed by RECIST v1.1.
The CMP-001 dose escalation scheme ranged from 1 mg to 10 mg. The maximum tolerated dose was not reached and the dose of 5 mg/weekly plus pembrolizumab was used for the dose expansion phase. It was up to the investigator to increase the dose to 10 mg since maximum tolerated dose was not reached. The key inclusion criteria were metastatic or unresectable melanoma; in the dose escalation phase prior best response to anti-PD1-based therapy was disease progression or stable disease. In the dose expansion phase, patients who had progressed on anti-PD1 based therapy were allowed regardless of best response. There was no restriction on the number of prior lines of therapy.
A total of 69 subjects were treated, 44 subjects from dose escalation and 25 in the expansion phase (ongoing). Two subjects discontinued because of treatment-related adverse events. The rest of the patients had a manageable toxicity profile consisting predominantly of fever, nausea/vomiting, hypotension and rigors. Severe grade 3/4 treatment-related adverse events were reported in more than 1 subject, with hypotension (n = 9, 13%) being the most prominent AE, followed by anemia (n = 2, 3%), chills (n = 2, 3%), and hypertension (n = 2, 3%). Hypotension was manageable by responsive fluid resuscitation and in some patients required stress dose steroids. Most of these side effects occurred 1-4 hours after the CMP-001 injection.
Of the 18 responders, 1 progressed, 2 withdrew consent, and 13 remain on study with 2 subjects maintaining their response though week 72. The median duration of response was not reached. Regression of noninjected tumors occurred in cutaneous, nodal, hepatic, and splenic metastases.
“CMP-001 plus pembrolizumab induced systemic antitumor activity, and not just local efficacy since both injected and noninjected target lesions changed from baseline per RECIST,” Dr. Milhem said. Not only did the responders show a rapid reduction in target lesions from baseline, but also a durable tumor regression as usually seen with other immunotherapeutics.
Immunohistochemical analysis of tumor biopsies demonstrated increase in CD8 (greater than fivefold) and PD-L1 expression, 5 weeks after therapy in a subset of patients with pre- and posttreatment biopsies. Transcriptional analysis by RNA-seq revealed induction of T cell inflamed gene signature, notably significant upregulation of TLR, and IFN-responsive genes.
It would be interesting to further investigate how this combination therapy compares with other strategies in a similar clinical scenario, such as oncolytic virus, other TLR ligands or means of APC activation, discussant Jedd Wolchok, MD, PhD, pointed out. Understanding resistance mechanisms at an individual patient level and optimal patient selection for this combination therapy remains a challenge, he said.
Dr. Milhem had no financial relationships to disclose.
SOURCE: Milhem MD et al. AACR Annual Meeting Abstract CT144.
CHICAGO – The intratumoral Toll-Like Receptor 9 (TLR-9) agonist, CMP-001, in combination with pembrolizumab in advanced melanoma patients, was well tolerated with a durable systemic clinical response, according to early results from an ongoing phase 1 trial.
Objective response rates on weekly (n = 56) and every 3 weeks schedules (n = 13) were 23% (13%-36%) and 15% (2%-45%) respectively, reported Mohammed M. Milhem, MBBS, of the University of Iowa, Iowa City.
For those dosed weekly at low dose (less than 5 mL) and high dose (5 mL or more), the ORR was 19% (n = 43, 95% confidence interval, 8%-33%) and 27% (n = 26, 95% CI, 12%-48%), respectively. Activity was demonstrated in subjects regardless of tumor burden, Dr. Milhem said at the annual meeting of the American Association for Cancer Research.
In this phase 1b study with a 3+3 design of dose escalation and expansion, the researchers enrolled patients with advanced melanoma who did not respond or had progressed resistant on prior anti-PD-1 monotherapy or in combination. CMP-001 was injected intratumorally in combination with pembrolizumab as per label intravenously.
The study drug CMP-001 has two components, a 30-mer CpG-A DNA oligonucleotide and a nonvirulent virus-like particle (VLP). The CpG-A DNA is packaged within the VLP that protects it from degradation and also allows TLR9 receptor uptake. CpG-A DNA acts as a TLR9 agonist by binding to it, thereby activating plasmacytoid dendritic cells (pDCs) within the tumor microenvironment. The activation results in secretion of large amounts of type 1 interferon and Th1 chemokines, changing the microenvironment from a “cold/desert-like” immune suppressed state to a “hot” antitumor inflamed state, Dr. Milhem said.
“The T cells thus generated can mediate tumor rejection both in the injected and noninjected tumor,” he said. Two CMP-001 schedules were evaluated, weekly for 7 weeks or weekly for 2 weeks, followed thereafter by every 3 weeks until discontinuation (due to progression, toxicity, investigator decision, or withdrawal of consent). Scans were done every 12 weeks and tumor response was assessed by RECIST v1.1.
The CMP-001 dose escalation scheme ranged from 1 mg to 10 mg. The maximum tolerated dose was not reached and the dose of 5 mg/weekly plus pembrolizumab was used for the dose expansion phase. It was up to the investigator to increase the dose to 10 mg since maximum tolerated dose was not reached. The key inclusion criteria were metastatic or unresectable melanoma; in the dose escalation phase prior best response to anti-PD1-based therapy was disease progression or stable disease. In the dose expansion phase, patients who had progressed on anti-PD1 based therapy were allowed regardless of best response. There was no restriction on the number of prior lines of therapy.
A total of 69 subjects were treated, 44 subjects from dose escalation and 25 in the expansion phase (ongoing). Two subjects discontinued because of treatment-related adverse events. The rest of the patients had a manageable toxicity profile consisting predominantly of fever, nausea/vomiting, hypotension and rigors. Severe grade 3/4 treatment-related adverse events were reported in more than 1 subject, with hypotension (n = 9, 13%) being the most prominent AE, followed by anemia (n = 2, 3%), chills (n = 2, 3%), and hypertension (n = 2, 3%). Hypotension was manageable by responsive fluid resuscitation and in some patients required stress dose steroids. Most of these side effects occurred 1-4 hours after the CMP-001 injection.
Of the 18 responders, 1 progressed, 2 withdrew consent, and 13 remain on study with 2 subjects maintaining their response though week 72. The median duration of response was not reached. Regression of noninjected tumors occurred in cutaneous, nodal, hepatic, and splenic metastases.
“CMP-001 plus pembrolizumab induced systemic antitumor activity, and not just local efficacy since both injected and noninjected target lesions changed from baseline per RECIST,” Dr. Milhem said. Not only did the responders show a rapid reduction in target lesions from baseline, but also a durable tumor regression as usually seen with other immunotherapeutics.
Immunohistochemical analysis of tumor biopsies demonstrated increase in CD8 (greater than fivefold) and PD-L1 expression, 5 weeks after therapy in a subset of patients with pre- and posttreatment biopsies. Transcriptional analysis by RNA-seq revealed induction of T cell inflamed gene signature, notably significant upregulation of TLR, and IFN-responsive genes.
It would be interesting to further investigate how this combination therapy compares with other strategies in a similar clinical scenario, such as oncolytic virus, other TLR ligands or means of APC activation, discussant Jedd Wolchok, MD, PhD, pointed out. Understanding resistance mechanisms at an individual patient level and optimal patient selection for this combination therapy remains a challenge, he said.
Dr. Milhem had no financial relationships to disclose.
SOURCE: Milhem MD et al. AACR Annual Meeting Abstract CT144.
CHICAGO – The intratumoral Toll-Like Receptor 9 (TLR-9) agonist, CMP-001, in combination with pembrolizumab in advanced melanoma patients, was well tolerated with a durable systemic clinical response, according to early results from an ongoing phase 1 trial.
Objective response rates on weekly (n = 56) and every 3 weeks schedules (n = 13) were 23% (13%-36%) and 15% (2%-45%) respectively, reported Mohammed M. Milhem, MBBS, of the University of Iowa, Iowa City.
For those dosed weekly at low dose (less than 5 mL) and high dose (5 mL or more), the ORR was 19% (n = 43, 95% confidence interval, 8%-33%) and 27% (n = 26, 95% CI, 12%-48%), respectively. Activity was demonstrated in subjects regardless of tumor burden, Dr. Milhem said at the annual meeting of the American Association for Cancer Research.
In this phase 1b study with a 3+3 design of dose escalation and expansion, the researchers enrolled patients with advanced melanoma who did not respond or had progressed resistant on prior anti-PD-1 monotherapy or in combination. CMP-001 was injected intratumorally in combination with pembrolizumab as per label intravenously.
The study drug CMP-001 has two components, a 30-mer CpG-A DNA oligonucleotide and a nonvirulent virus-like particle (VLP). The CpG-A DNA is packaged within the VLP that protects it from degradation and also allows TLR9 receptor uptake. CpG-A DNA acts as a TLR9 agonist by binding to it, thereby activating plasmacytoid dendritic cells (pDCs) within the tumor microenvironment. The activation results in secretion of large amounts of type 1 interferon and Th1 chemokines, changing the microenvironment from a “cold/desert-like” immune suppressed state to a “hot” antitumor inflamed state, Dr. Milhem said.
“The T cells thus generated can mediate tumor rejection both in the injected and noninjected tumor,” he said. Two CMP-001 schedules were evaluated, weekly for 7 weeks or weekly for 2 weeks, followed thereafter by every 3 weeks until discontinuation (due to progression, toxicity, investigator decision, or withdrawal of consent). Scans were done every 12 weeks and tumor response was assessed by RECIST v1.1.
The CMP-001 dose escalation scheme ranged from 1 mg to 10 mg. The maximum tolerated dose was not reached and the dose of 5 mg/weekly plus pembrolizumab was used for the dose expansion phase. It was up to the investigator to increase the dose to 10 mg since maximum tolerated dose was not reached. The key inclusion criteria were metastatic or unresectable melanoma; in the dose escalation phase prior best response to anti-PD1-based therapy was disease progression or stable disease. In the dose expansion phase, patients who had progressed on anti-PD1 based therapy were allowed regardless of best response. There was no restriction on the number of prior lines of therapy.
A total of 69 subjects were treated, 44 subjects from dose escalation and 25 in the expansion phase (ongoing). Two subjects discontinued because of treatment-related adverse events. The rest of the patients had a manageable toxicity profile consisting predominantly of fever, nausea/vomiting, hypotension and rigors. Severe grade 3/4 treatment-related adverse events were reported in more than 1 subject, with hypotension (n = 9, 13%) being the most prominent AE, followed by anemia (n = 2, 3%), chills (n = 2, 3%), and hypertension (n = 2, 3%). Hypotension was manageable by responsive fluid resuscitation and in some patients required stress dose steroids. Most of these side effects occurred 1-4 hours after the CMP-001 injection.
Of the 18 responders, 1 progressed, 2 withdrew consent, and 13 remain on study with 2 subjects maintaining their response though week 72. The median duration of response was not reached. Regression of noninjected tumors occurred in cutaneous, nodal, hepatic, and splenic metastases.
“CMP-001 plus pembrolizumab induced systemic antitumor activity, and not just local efficacy since both injected and noninjected target lesions changed from baseline per RECIST,” Dr. Milhem said. Not only did the responders show a rapid reduction in target lesions from baseline, but also a durable tumor regression as usually seen with other immunotherapeutics.
Immunohistochemical analysis of tumor biopsies demonstrated increase in CD8 (greater than fivefold) and PD-L1 expression, 5 weeks after therapy in a subset of patients with pre- and posttreatment biopsies. Transcriptional analysis by RNA-seq revealed induction of T cell inflamed gene signature, notably significant upregulation of TLR, and IFN-responsive genes.
It would be interesting to further investigate how this combination therapy compares with other strategies in a similar clinical scenario, such as oncolytic virus, other TLR ligands or means of APC activation, discussant Jedd Wolchok, MD, PhD, pointed out. Understanding resistance mechanisms at an individual patient level and optimal patient selection for this combination therapy remains a challenge, he said.
Dr. Milhem had no financial relationships to disclose.
SOURCE: Milhem MD et al. AACR Annual Meeting Abstract CT144.
REPORTING FROM THE AACR ANNUAL MEETING
Key clinical point: The combination demonstrated a manageable toxicity profile with ORR of 22%.
Major finding: Objective response rates on weekly (n = 56) and every 3 weeks schedules (n = 13) were 23% (13%-36%) and 15% (2%-45%) respectively.
Study details: This phase 1b study comprised 69 patients (44 in escalation and 25 in expansion).
Disclosures: Dr. Milhem had no financial relationships to disclose.
Source: Milhem MD et al. AACR Annual Meeting. Abstract CT144.
KEYNOTE-054: Adjuvant pembrolizumab beat placebo in high-risk resected melanoma
CHICAGO – Adjuvant pembrolizumab for resected high-risk melanoma slowed the rate of recurrence or death by 43% compared with placebo in a phase 3 trial of 1,519 patients.
After 15 months of follow-up, 12-month rates of recurrence-free survival (RFS) were 75% for pembrolizumab and 61% for placebo (hazard ratio, 0.57; P less than .001), Alexander M.M. Eggermont, MD, PhD, reported at the annual meeting of the American Association for Cancer Research.
By 18 months, the RFS difference between the arms had widened even more (71% versus 53%), Dr. Eggermont and his associates said at the meeting. The report was published simultaneously in the New England Journal of Medicine.
Adjuvant pembrolizumab was effective irrespective of PD-L1 tumor expression status. In a subgroup of more than 800 patients with PD-L1-positive tumors, 12-month RFS rates were 77% for pembrolizumab and 63% for placebo (HR, 0.54; 95% CI, 0.42 to 0.69; P less than .001). Among 116 patients who were PD-L1-negative, these rates were 72% and 52%, respectively (HR, 0.47; P = .01).
Treatment produced no new safety signals, said Dr. Eggermont of Gustave Roussy Cancer Campus Grand Paris and University Paris-Saclay, Villejuif, France. Grade 3 or higher toxicities affected 15% of pembrolizumab patients. Myositis caused one pembrolizumab-related death.
The findings bolster data suggesting that adjuvant therapy can stop or delay recurrence in resected high-risk melanoma. Previously, adjuvant ipilimumab was approved after significantly extending RFS and overall survival in the placebo-controlled European Organization for Research and Treatment of Cancer 18071 trial. More recently, adjuvant dabrafenib plus trametinib reduced the risk of recurrence compared with placebo in completely resected stage III melanoma with BRAF mutations (COMBI-AD), and adjuvant nivolumab significantly improved RFS and was less toxic than was ipilimumab in patients with advanced resected BRAF-mutated and BRAF-wild-type melanomas (CheckMate 238).
Like the EORTC 18071 trial, KEYNOTE-054 (EORTC 1325) enrolled adults with completely resected stage III cutaneous melanoma. Patients with stage IIIa disease were high-risk, with sentinel node tumors exceeding 1-mm diameter per Rotterdam criteria. Stage IIIB or IIIC patients had no in-transit metastases. In all, 1,015 patients received up to 18 doses of pembrolizumab (200 mg infused every 3 weeks) or placebo for approximately 1 year. Relapsers could either repeat pembrolizumab or cross over to the pembrolizumab arm.
Treatment-related adverse events occurred in 78% of pembrolizumab patients and 66% of placebo recipients. As in prior studies, the most frequent adverse effects of pembrolizumab included fatigue or asthenia (37%), skin reactions (28%), diarrhea (19%), arthralgia (12%), nausea (11%), and dyspnea (6%). Rates of immune-related adverse events of any grade were 37% versus 9%. The most common immune-related adverse event was endocrinopathy (23%), specifically hypothyroidism (14%) and hyperthyroidism (10%). Grade 3 or higher toxicities affected 15% of pembrolizumab recipients and most often consisted of colitis (2%), endocrine disorders (1.8%), or hepatobiliary disorders (1.4%). Myositis caused the only pembrolizumab-related death.
Patients and clinicians await KEYNOTE-054 readouts on distant metastasis-free survival and overall survival. In past trials of adjuvant interferon alfa or ipilimumab for high-risk melanoma, RFS and overall survival closely correlated, Dr. Eggermont noted. KEYNOTE-54 can be expected to produce similar findings unless post-relapse therapy – including crossover to the pembrolizumab arm – narrows the survival advantage of adjuvant treatment, he added.
Merck makes pembrolizumab and funded the trial. Dr. Eggermont disclosed ties to Actelion, Agenus, Bayer, BMS, Incyte, ISA Pharmaceuticals, HalioDX, Merck-Serono, MSD, Nektar, Novartis, Pfizer, and Sanofi outside the submitted work.
SOURCE: Eggermont AMM et al. AACR Annual Meeting Abstract CT001.
CHICAGO – Adjuvant pembrolizumab for resected high-risk melanoma slowed the rate of recurrence or death by 43% compared with placebo in a phase 3 trial of 1,519 patients.
After 15 months of follow-up, 12-month rates of recurrence-free survival (RFS) were 75% for pembrolizumab and 61% for placebo (hazard ratio, 0.57; P less than .001), Alexander M.M. Eggermont, MD, PhD, reported at the annual meeting of the American Association for Cancer Research.
By 18 months, the RFS difference between the arms had widened even more (71% versus 53%), Dr. Eggermont and his associates said at the meeting. The report was published simultaneously in the New England Journal of Medicine.
Adjuvant pembrolizumab was effective irrespective of PD-L1 tumor expression status. In a subgroup of more than 800 patients with PD-L1-positive tumors, 12-month RFS rates were 77% for pembrolizumab and 63% for placebo (HR, 0.54; 95% CI, 0.42 to 0.69; P less than .001). Among 116 patients who were PD-L1-negative, these rates were 72% and 52%, respectively (HR, 0.47; P = .01).
Treatment produced no new safety signals, said Dr. Eggermont of Gustave Roussy Cancer Campus Grand Paris and University Paris-Saclay, Villejuif, France. Grade 3 or higher toxicities affected 15% of pembrolizumab patients. Myositis caused one pembrolizumab-related death.
The findings bolster data suggesting that adjuvant therapy can stop or delay recurrence in resected high-risk melanoma. Previously, adjuvant ipilimumab was approved after significantly extending RFS and overall survival in the placebo-controlled European Organization for Research and Treatment of Cancer 18071 trial. More recently, adjuvant dabrafenib plus trametinib reduced the risk of recurrence compared with placebo in completely resected stage III melanoma with BRAF mutations (COMBI-AD), and adjuvant nivolumab significantly improved RFS and was less toxic than was ipilimumab in patients with advanced resected BRAF-mutated and BRAF-wild-type melanomas (CheckMate 238).
Like the EORTC 18071 trial, KEYNOTE-054 (EORTC 1325) enrolled adults with completely resected stage III cutaneous melanoma. Patients with stage IIIa disease were high-risk, with sentinel node tumors exceeding 1-mm diameter per Rotterdam criteria. Stage IIIB or IIIC patients had no in-transit metastases. In all, 1,015 patients received up to 18 doses of pembrolizumab (200 mg infused every 3 weeks) or placebo for approximately 1 year. Relapsers could either repeat pembrolizumab or cross over to the pembrolizumab arm.
Treatment-related adverse events occurred in 78% of pembrolizumab patients and 66% of placebo recipients. As in prior studies, the most frequent adverse effects of pembrolizumab included fatigue or asthenia (37%), skin reactions (28%), diarrhea (19%), arthralgia (12%), nausea (11%), and dyspnea (6%). Rates of immune-related adverse events of any grade were 37% versus 9%. The most common immune-related adverse event was endocrinopathy (23%), specifically hypothyroidism (14%) and hyperthyroidism (10%). Grade 3 or higher toxicities affected 15% of pembrolizumab recipients and most often consisted of colitis (2%), endocrine disorders (1.8%), or hepatobiliary disorders (1.4%). Myositis caused the only pembrolizumab-related death.
Patients and clinicians await KEYNOTE-054 readouts on distant metastasis-free survival and overall survival. In past trials of adjuvant interferon alfa or ipilimumab for high-risk melanoma, RFS and overall survival closely correlated, Dr. Eggermont noted. KEYNOTE-54 can be expected to produce similar findings unless post-relapse therapy – including crossover to the pembrolizumab arm – narrows the survival advantage of adjuvant treatment, he added.
Merck makes pembrolizumab and funded the trial. Dr. Eggermont disclosed ties to Actelion, Agenus, Bayer, BMS, Incyte, ISA Pharmaceuticals, HalioDX, Merck-Serono, MSD, Nektar, Novartis, Pfizer, and Sanofi outside the submitted work.
SOURCE: Eggermont AMM et al. AACR Annual Meeting Abstract CT001.
CHICAGO – Adjuvant pembrolizumab for resected high-risk melanoma slowed the rate of recurrence or death by 43% compared with placebo in a phase 3 trial of 1,519 patients.
After 15 months of follow-up, 12-month rates of recurrence-free survival (RFS) were 75% for pembrolizumab and 61% for placebo (hazard ratio, 0.57; P less than .001), Alexander M.M. Eggermont, MD, PhD, reported at the annual meeting of the American Association for Cancer Research.
By 18 months, the RFS difference between the arms had widened even more (71% versus 53%), Dr. Eggermont and his associates said at the meeting. The report was published simultaneously in the New England Journal of Medicine.
Adjuvant pembrolizumab was effective irrespective of PD-L1 tumor expression status. In a subgroup of more than 800 patients with PD-L1-positive tumors, 12-month RFS rates were 77% for pembrolizumab and 63% for placebo (HR, 0.54; 95% CI, 0.42 to 0.69; P less than .001). Among 116 patients who were PD-L1-negative, these rates were 72% and 52%, respectively (HR, 0.47; P = .01).
Treatment produced no new safety signals, said Dr. Eggermont of Gustave Roussy Cancer Campus Grand Paris and University Paris-Saclay, Villejuif, France. Grade 3 or higher toxicities affected 15% of pembrolizumab patients. Myositis caused one pembrolizumab-related death.
The findings bolster data suggesting that adjuvant therapy can stop or delay recurrence in resected high-risk melanoma. Previously, adjuvant ipilimumab was approved after significantly extending RFS and overall survival in the placebo-controlled European Organization for Research and Treatment of Cancer 18071 trial. More recently, adjuvant dabrafenib plus trametinib reduced the risk of recurrence compared with placebo in completely resected stage III melanoma with BRAF mutations (COMBI-AD), and adjuvant nivolumab significantly improved RFS and was less toxic than was ipilimumab in patients with advanced resected BRAF-mutated and BRAF-wild-type melanomas (CheckMate 238).
Like the EORTC 18071 trial, KEYNOTE-054 (EORTC 1325) enrolled adults with completely resected stage III cutaneous melanoma. Patients with stage IIIa disease were high-risk, with sentinel node tumors exceeding 1-mm diameter per Rotterdam criteria. Stage IIIB or IIIC patients had no in-transit metastases. In all, 1,015 patients received up to 18 doses of pembrolizumab (200 mg infused every 3 weeks) or placebo for approximately 1 year. Relapsers could either repeat pembrolizumab or cross over to the pembrolizumab arm.
Treatment-related adverse events occurred in 78% of pembrolizumab patients and 66% of placebo recipients. As in prior studies, the most frequent adverse effects of pembrolizumab included fatigue or asthenia (37%), skin reactions (28%), diarrhea (19%), arthralgia (12%), nausea (11%), and dyspnea (6%). Rates of immune-related adverse events of any grade were 37% versus 9%. The most common immune-related adverse event was endocrinopathy (23%), specifically hypothyroidism (14%) and hyperthyroidism (10%). Grade 3 or higher toxicities affected 15% of pembrolizumab recipients and most often consisted of colitis (2%), endocrine disorders (1.8%), or hepatobiliary disorders (1.4%). Myositis caused the only pembrolizumab-related death.
Patients and clinicians await KEYNOTE-054 readouts on distant metastasis-free survival and overall survival. In past trials of adjuvant interferon alfa or ipilimumab for high-risk melanoma, RFS and overall survival closely correlated, Dr. Eggermont noted. KEYNOTE-54 can be expected to produce similar findings unless post-relapse therapy – including crossover to the pembrolizumab arm – narrows the survival advantage of adjuvant treatment, he added.
Merck makes pembrolizumab and funded the trial. Dr. Eggermont disclosed ties to Actelion, Agenus, Bayer, BMS, Incyte, ISA Pharmaceuticals, HalioDX, Merck-Serono, MSD, Nektar, Novartis, Pfizer, and Sanofi outside the submitted work.
SOURCE: Eggermont AMM et al. AACR Annual Meeting Abstract CT001.
REPORTING FROM THE AACR ANNUAL MEETING
Key clinical point: Adjuvant pembrolizumab (200 mg every 3 weeks) significantly extended recurrence-free survival in adults with high-risk, completely resected stage III melanoma.
Major finding: After 15 months of median follow-up, 12-month rates of recurrence-free survival were 75% for pembrolizumab and 61% for placebo (hazard ratio, 0.57; P less than .001). There was one treatment-related death in the pembrolizumab group.
Study details: KEYNOTE-054, a randomized, double-blind, phase 3 trial of 1,019 patients.
Disclosures: Merck makes pembrolizumab and funded the trial.
Source: Eggermont AMM et al. AACR Annual Meeting. Abstract CT001.
Gene strong predictor of metastasis in melanoma
CHICAGO – Investigators have identified four genes that are overexpressed in primary melanoma, including one, CXCL1, that holds promise as a strong predictor of future metastatic disease, according to study results presented at the Society of Surgical Oncology Annual Cancer Symposium.
The study implicated four genes strongly expressed in primary melanoma tumors of patients who develop distant metastases – CXCL1, CXCL2, CBL, and CD276 – said Jennifer Erdrich, MD, MPH, of Cedars Sinai Medical Center, Los Angeles. However, CXCL1 stood out. “CXCL1 overexpression is an independent predictor of developing metastatic disease. Patients with CXCL1 overexpression in the primary tumor in our study had decreased overall 5-year survival.” CXCL1 may be a useful predictive marker in primary melanoma and a potential target for immunotherapy, she said.
The rationale for analyzing the 79 genes implicated in cancer only rather than the entire array of 22,000 genes was to reduce the odds of a high false-discovery rate from 5% to 0.007%. “This is what strengthens our findings in a cohort of 37 patients,” Dr. Erdrich said.
The study analyzed pathological characteristics of the metastatic and nonmetastatic groups. Most characteristics were similar between the two groups, including location of the primary tumor in the trunk and extremities of 67% and 71%, respectively, and age of 60 years and older. The analysis noted two deviations: primary tumor size was thicker in the metastatic group (2.1 mm vs. 1.05 mm; P = .6), although Dr. Erdrich noted this was “not significantly different”; and a higher rate of ulceration in the metastatic group (50% vs. 13%; P = .05).
The genes CXCL1 and CXCL2 are both chemokines involved in growth and inflammation. “CXCL1 expression was 2.51 times greater in the metastatic group,” Dr. Erdrich said (P less than .001). Overexpression in the other three genes of interest was: CXCL2, 1.68 times greater (P less than .01); CD276, which is involved in T-cell immunity, 1.16 times greater (P = .04); and C-CBL, which is a photo-oncogene involved in the ubiquitin pathway, 1.15 times greater (P = .01). “The overexpression of all four of these was statistically significant,” she said.
Univariate analysis found ulceration of the primary along with overexpression of
the four genes to be significant predictors of metastasis. “However, in our multivariate model, three of the genes dropped out but CXCL1 remained robust,” she said.
Dr. Erdrich noted that CXCL1 is a cytokine located on chromosome 4, is secreted by macrophages, exerts its signal through CXCR2, and is one of five cytokines upregulated in lesions that respond to immunotherapy (Br J Dermatol. 2016;175:966-78).
CXCL1 compares favorably with S100, the existing blood-based biomarker for predicting recurrence in high-risk melanoma, as a predictor of metastases, Dr. Erdrich said, with an area under the curve of 0.80 versus 0.66; sensitivity of 67% versus 77%; specificity of 97% versus 61%; positive predictive value of 80% versus 40%; and negative predictive value of 94% versus 88% (Anticancer Res. 1999;19:2685-90; Cancer. 2003;97:1737-45).
The study also looked at overall survival in patients with low and high expression of CXCL1. “The patients with high expression had 5-year survival of only 50% compared to those of low expression, whose 5-year survival was 97%,” Dr. Erdrich said.
Dr. Erdrich and her coauthors reported having no financial relationships.
SOURCE: Erdrich J et al. SSO 2018, Abstract 82.
CHICAGO – Investigators have identified four genes that are overexpressed in primary melanoma, including one, CXCL1, that holds promise as a strong predictor of future metastatic disease, according to study results presented at the Society of Surgical Oncology Annual Cancer Symposium.
The study implicated four genes strongly expressed in primary melanoma tumors of patients who develop distant metastases – CXCL1, CXCL2, CBL, and CD276 – said Jennifer Erdrich, MD, MPH, of Cedars Sinai Medical Center, Los Angeles. However, CXCL1 stood out. “CXCL1 overexpression is an independent predictor of developing metastatic disease. Patients with CXCL1 overexpression in the primary tumor in our study had decreased overall 5-year survival.” CXCL1 may be a useful predictive marker in primary melanoma and a potential target for immunotherapy, she said.
The rationale for analyzing the 79 genes implicated in cancer only rather than the entire array of 22,000 genes was to reduce the odds of a high false-discovery rate from 5% to 0.007%. “This is what strengthens our findings in a cohort of 37 patients,” Dr. Erdrich said.
The study analyzed pathological characteristics of the metastatic and nonmetastatic groups. Most characteristics were similar between the two groups, including location of the primary tumor in the trunk and extremities of 67% and 71%, respectively, and age of 60 years and older. The analysis noted two deviations: primary tumor size was thicker in the metastatic group (2.1 mm vs. 1.05 mm; P = .6), although Dr. Erdrich noted this was “not significantly different”; and a higher rate of ulceration in the metastatic group (50% vs. 13%; P = .05).
The genes CXCL1 and CXCL2 are both chemokines involved in growth and inflammation. “CXCL1 expression was 2.51 times greater in the metastatic group,” Dr. Erdrich said (P less than .001). Overexpression in the other three genes of interest was: CXCL2, 1.68 times greater (P less than .01); CD276, which is involved in T-cell immunity, 1.16 times greater (P = .04); and C-CBL, which is a photo-oncogene involved in the ubiquitin pathway, 1.15 times greater (P = .01). “The overexpression of all four of these was statistically significant,” she said.
Univariate analysis found ulceration of the primary along with overexpression of
the four genes to be significant predictors of metastasis. “However, in our multivariate model, three of the genes dropped out but CXCL1 remained robust,” she said.
Dr. Erdrich noted that CXCL1 is a cytokine located on chromosome 4, is secreted by macrophages, exerts its signal through CXCR2, and is one of five cytokines upregulated in lesions that respond to immunotherapy (Br J Dermatol. 2016;175:966-78).
CXCL1 compares favorably with S100, the existing blood-based biomarker for predicting recurrence in high-risk melanoma, as a predictor of metastases, Dr. Erdrich said, with an area under the curve of 0.80 versus 0.66; sensitivity of 67% versus 77%; specificity of 97% versus 61%; positive predictive value of 80% versus 40%; and negative predictive value of 94% versus 88% (Anticancer Res. 1999;19:2685-90; Cancer. 2003;97:1737-45).
The study also looked at overall survival in patients with low and high expression of CXCL1. “The patients with high expression had 5-year survival of only 50% compared to those of low expression, whose 5-year survival was 97%,” Dr. Erdrich said.
Dr. Erdrich and her coauthors reported having no financial relationships.
SOURCE: Erdrich J et al. SSO 2018, Abstract 82.
CHICAGO – Investigators have identified four genes that are overexpressed in primary melanoma, including one, CXCL1, that holds promise as a strong predictor of future metastatic disease, according to study results presented at the Society of Surgical Oncology Annual Cancer Symposium.
The study implicated four genes strongly expressed in primary melanoma tumors of patients who develop distant metastases – CXCL1, CXCL2, CBL, and CD276 – said Jennifer Erdrich, MD, MPH, of Cedars Sinai Medical Center, Los Angeles. However, CXCL1 stood out. “CXCL1 overexpression is an independent predictor of developing metastatic disease. Patients with CXCL1 overexpression in the primary tumor in our study had decreased overall 5-year survival.” CXCL1 may be a useful predictive marker in primary melanoma and a potential target for immunotherapy, she said.
The rationale for analyzing the 79 genes implicated in cancer only rather than the entire array of 22,000 genes was to reduce the odds of a high false-discovery rate from 5% to 0.007%. “This is what strengthens our findings in a cohort of 37 patients,” Dr. Erdrich said.
The study analyzed pathological characteristics of the metastatic and nonmetastatic groups. Most characteristics were similar between the two groups, including location of the primary tumor in the trunk and extremities of 67% and 71%, respectively, and age of 60 years and older. The analysis noted two deviations: primary tumor size was thicker in the metastatic group (2.1 mm vs. 1.05 mm; P = .6), although Dr. Erdrich noted this was “not significantly different”; and a higher rate of ulceration in the metastatic group (50% vs. 13%; P = .05).
The genes CXCL1 and CXCL2 are both chemokines involved in growth and inflammation. “CXCL1 expression was 2.51 times greater in the metastatic group,” Dr. Erdrich said (P less than .001). Overexpression in the other three genes of interest was: CXCL2, 1.68 times greater (P less than .01); CD276, which is involved in T-cell immunity, 1.16 times greater (P = .04); and C-CBL, which is a photo-oncogene involved in the ubiquitin pathway, 1.15 times greater (P = .01). “The overexpression of all four of these was statistically significant,” she said.
Univariate analysis found ulceration of the primary along with overexpression of
the four genes to be significant predictors of metastasis. “However, in our multivariate model, three of the genes dropped out but CXCL1 remained robust,” she said.
Dr. Erdrich noted that CXCL1 is a cytokine located on chromosome 4, is secreted by macrophages, exerts its signal through CXCR2, and is one of five cytokines upregulated in lesions that respond to immunotherapy (Br J Dermatol. 2016;175:966-78).
CXCL1 compares favorably with S100, the existing blood-based biomarker for predicting recurrence in high-risk melanoma, as a predictor of metastases, Dr. Erdrich said, with an area under the curve of 0.80 versus 0.66; sensitivity of 67% versus 77%; specificity of 97% versus 61%; positive predictive value of 80% versus 40%; and negative predictive value of 94% versus 88% (Anticancer Res. 1999;19:2685-90; Cancer. 2003;97:1737-45).
The study also looked at overall survival in patients with low and high expression of CXCL1. “The patients with high expression had 5-year survival of only 50% compared to those of low expression, whose 5-year survival was 97%,” Dr. Erdrich said.
Dr. Erdrich and her coauthors reported having no financial relationships.
SOURCE: Erdrich J et al. SSO 2018, Abstract 82.
REPORTING FROM SSO 2018
Key clinical point: The CXCL1 gene may predict metastatic risk in primary melanoma.
Major findings: CXCL1 overexpression yielded 50% 5-year survival, almost half that of underexpression.
Study details: Gene analysis of samples from 37 patients with nonmetastatic primary melanoma who had surgical removal of primary lesion with median follow-up of 38 months.
Disclosures: Dr. Erdrich and her coauthors reported having no financial disclosures.
Source: Erdrich J et al. SSO 2018, Abstract 82.
Extracapsular spread predicts survival in SLN+ melanoma
CHICAGO – Extracapsular extension, or extracapsular spread (ECS), has been recognized as a risk factor in melanoma patients with macrometastatic (N+) disease, but a study from the United Kingdom has found it may also be an important indicator of progression-free and overall survival in patients who have sentinel node positive (SLN+) micrometastatic disease, a researcher reported at the Society of Surgical Oncology Annual Cancer Symposium.
“There is limited published data on ECS in micrometastatic disease, although there is progression-free survival data published in the literature,” Michelle Lo, MBCHB, MRCS, of Norfolk and Norwich University Hospitals, in Norwich, England, said in presenting the results. “The goal of the study was to determine the incidence of ECS in the micrometastatic group and to determine the prognostic significance of this.”
The study found that the incidence of ECS in the N+ group was significantly higher than the SLN+ group, 52.4% vs. 16.2% (P less than .0001). ECS proved to be a significant prognostic indicator of disease-specific survival and overall survival for both N+ and SLN+ disease. “There was no statistical difference in Breslow thickness between the two groups regardless of ECS,” she said.
Both the N+ and SLN+ groups with ECS had more lymph nodes than the ECS-absent subgroups, Dr. Lo said. However, in the ECS-absent subgroups, N+ patients had twice the number of lymph nodes than SLN+ patients. “This would suggest that ECS is a high-risk phenotype from the outset of metastases rather than something that’s developed over time,” she said. “Our data is in line with international staging data.”
The ECS-absent SLN– disease group had the most favorable survival outcomes, while those with ECS-present N+ disease had the worst outcomes. The prognosis of ECS-present, SLN+ patients was statistically similar to the ECS-absent, N+ group, she said.
In patients with SLN+ disease, Breslow thickness and N-stage were independent prognostic indicators for progression-free survival (hazard ratio 2.4, P less than .0001) and disease-free survival (HR 2.3, P less than .0001), Dr. Lo noted that median progression-free survival in SLN+ and N+ disease was 20 and 10 months, respectively. “Within our cohort of patients with ECS present in the micrometastatic group, their disease progressed within 3 years,” she said.
A multivariate analysis showed the survival data from this study was consistent with American Joint Committee on Cancer staging criteria, Dr. Lo said. “ECS is well recognized in the macrometastatic group, but we demonstrated from our data that the incidence of ECS in the micrometastatic group is one in six. It’s an independent risk factor for disease progression and an independent risk factor for worst disease-specific and overall survival, and it upstages micrometastatic disease.” ECS upstages stage III disease in a fashion similar to that of ulceration in primary melanoma, she said.
“In the absence of data to suggest otherwise, we would still recommend completion lymph node dissection in our micrometastatic group where ECS is present, and we would advocate that ECS should be included as an independent staging variable in the future,” Dr. Lo said.
Dr. Lo and her coauthors reported having no financial disclosures.
SOURCE: Lo M, et al. SSO 2018 Abstract 70.
CHICAGO – Extracapsular extension, or extracapsular spread (ECS), has been recognized as a risk factor in melanoma patients with macrometastatic (N+) disease, but a study from the United Kingdom has found it may also be an important indicator of progression-free and overall survival in patients who have sentinel node positive (SLN+) micrometastatic disease, a researcher reported at the Society of Surgical Oncology Annual Cancer Symposium.
“There is limited published data on ECS in micrometastatic disease, although there is progression-free survival data published in the literature,” Michelle Lo, MBCHB, MRCS, of Norfolk and Norwich University Hospitals, in Norwich, England, said in presenting the results. “The goal of the study was to determine the incidence of ECS in the micrometastatic group and to determine the prognostic significance of this.”
The study found that the incidence of ECS in the N+ group was significantly higher than the SLN+ group, 52.4% vs. 16.2% (P less than .0001). ECS proved to be a significant prognostic indicator of disease-specific survival and overall survival for both N+ and SLN+ disease. “There was no statistical difference in Breslow thickness between the two groups regardless of ECS,” she said.
Both the N+ and SLN+ groups with ECS had more lymph nodes than the ECS-absent subgroups, Dr. Lo said. However, in the ECS-absent subgroups, N+ patients had twice the number of lymph nodes than SLN+ patients. “This would suggest that ECS is a high-risk phenotype from the outset of metastases rather than something that’s developed over time,” she said. “Our data is in line with international staging data.”
The ECS-absent SLN– disease group had the most favorable survival outcomes, while those with ECS-present N+ disease had the worst outcomes. The prognosis of ECS-present, SLN+ patients was statistically similar to the ECS-absent, N+ group, she said.
In patients with SLN+ disease, Breslow thickness and N-stage were independent prognostic indicators for progression-free survival (hazard ratio 2.4, P less than .0001) and disease-free survival (HR 2.3, P less than .0001), Dr. Lo noted that median progression-free survival in SLN+ and N+ disease was 20 and 10 months, respectively. “Within our cohort of patients with ECS present in the micrometastatic group, their disease progressed within 3 years,” she said.
A multivariate analysis showed the survival data from this study was consistent with American Joint Committee on Cancer staging criteria, Dr. Lo said. “ECS is well recognized in the macrometastatic group, but we demonstrated from our data that the incidence of ECS in the micrometastatic group is one in six. It’s an independent risk factor for disease progression and an independent risk factor for worst disease-specific and overall survival, and it upstages micrometastatic disease.” ECS upstages stage III disease in a fashion similar to that of ulceration in primary melanoma, she said.
“In the absence of data to suggest otherwise, we would still recommend completion lymph node dissection in our micrometastatic group where ECS is present, and we would advocate that ECS should be included as an independent staging variable in the future,” Dr. Lo said.
Dr. Lo and her coauthors reported having no financial disclosures.
SOURCE: Lo M, et al. SSO 2018 Abstract 70.
CHICAGO – Extracapsular extension, or extracapsular spread (ECS), has been recognized as a risk factor in melanoma patients with macrometastatic (N+) disease, but a study from the United Kingdom has found it may also be an important indicator of progression-free and overall survival in patients who have sentinel node positive (SLN+) micrometastatic disease, a researcher reported at the Society of Surgical Oncology Annual Cancer Symposium.
“There is limited published data on ECS in micrometastatic disease, although there is progression-free survival data published in the literature,” Michelle Lo, MBCHB, MRCS, of Norfolk and Norwich University Hospitals, in Norwich, England, said in presenting the results. “The goal of the study was to determine the incidence of ECS in the micrometastatic group and to determine the prognostic significance of this.”
The study found that the incidence of ECS in the N+ group was significantly higher than the SLN+ group, 52.4% vs. 16.2% (P less than .0001). ECS proved to be a significant prognostic indicator of disease-specific survival and overall survival for both N+ and SLN+ disease. “There was no statistical difference in Breslow thickness between the two groups regardless of ECS,” she said.
Both the N+ and SLN+ groups with ECS had more lymph nodes than the ECS-absent subgroups, Dr. Lo said. However, in the ECS-absent subgroups, N+ patients had twice the number of lymph nodes than SLN+ patients. “This would suggest that ECS is a high-risk phenotype from the outset of metastases rather than something that’s developed over time,” she said. “Our data is in line with international staging data.”
The ECS-absent SLN– disease group had the most favorable survival outcomes, while those with ECS-present N+ disease had the worst outcomes. The prognosis of ECS-present, SLN+ patients was statistically similar to the ECS-absent, N+ group, she said.
In patients with SLN+ disease, Breslow thickness and N-stage were independent prognostic indicators for progression-free survival (hazard ratio 2.4, P less than .0001) and disease-free survival (HR 2.3, P less than .0001), Dr. Lo noted that median progression-free survival in SLN+ and N+ disease was 20 and 10 months, respectively. “Within our cohort of patients with ECS present in the micrometastatic group, their disease progressed within 3 years,” she said.
A multivariate analysis showed the survival data from this study was consistent with American Joint Committee on Cancer staging criteria, Dr. Lo said. “ECS is well recognized in the macrometastatic group, but we demonstrated from our data that the incidence of ECS in the micrometastatic group is one in six. It’s an independent risk factor for disease progression and an independent risk factor for worst disease-specific and overall survival, and it upstages micrometastatic disease.” ECS upstages stage III disease in a fashion similar to that of ulceration in primary melanoma, she said.
“In the absence of data to suggest otherwise, we would still recommend completion lymph node dissection in our micrometastatic group where ECS is present, and we would advocate that ECS should be included as an independent staging variable in the future,” Dr. Lo said.
Dr. Lo and her coauthors reported having no financial disclosures.
SOURCE: Lo M, et al. SSO 2018 Abstract 70.
REPORTING FROM SSO 2018
Key clinical point: Extracapsular spread (ECS) may be a reliable biomarker of survival in stage III melanoma.
Major finding: ECS status was an indicator of progression-free survival (hazard ratio 2.4; P less than .0001) in micrometastatic disease.
Study details: Retrospective cohort study of 515 patients who had micro- or macrometastatic lymphadenopathy at two U.K. centers from 2000 to 2017.
Disclosures: Dr. Lo and coauthors reported having no financial disclosures.
Source: Lo M et al. SSO 2018 Abstract 70.
What’s new in the latest melanoma guidelines
KAUAI, HAWAII – Melanoma , resulting in an evidence-based improved prognosis for many of them, Laura Korb Ferris, MD, PhD, said at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
Dr. Ferris, of the department of dermatology, University of Pittsburgh, highlighted some of the key changes in the eighth edition of the AJCC staging manual, which is now in effect. She also described the clinical implications of important updates introduced in the 2018 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and management of melanoma.
The AJCC eighth edition
The eighth edition is built upon an AJCC database of more than 46,000 patients with stage I-III melanoma diagnosed since 1998 at 10 academic medical centers. The AJCC panel made no changes in stage IV melanoma guidance because the newer targeted therapies have rapidly changed treatment outcomes in that setting and longer follow-up is needed to assess the full impact.
The current edition of the AJCC melanoma staging manual creates a new subcategory within pathologic stage III. In the melanoma staging world, that’s exciting news, especially because this change has important implications for prognosis.
This fourth subcategory, stage IIID, is for melanomas, which in the Tumor, Nodes, Metastasis (TNM) classification scheme, are primary tumor stage T4b, meaning greater than 4.0 mm in thickness and with ulceration; regional lymph node N3a, b, or c, based upon the number of metastatic nodes involved and whether they were clinically occult nodal metastases detected by sentinel lymph node biopsy (SLNB) or clinically detected; and M0, meaning no distant metastatic disease. In the 8th edition, the AJCC staging system can be applied in patients with T2 through T4 primary melanoma only if they have undergone SLNB.
This new approach to stage III disease makes for more homogeneous patient subgroups, which in turn provides much better stratification of prognosis than was possible in the seventh edition of the AJCC staging manual, which dates back to 2010. Most strikingly, the 5-year melanoma-specific survival rate for patients with stage IIIA disease was 78% in the seventh edition of AJCC, but it climbs to 93% in the eighth edition. For patients with stage IIIB melanoma, 5-year melanoma-specific survival improved from 59% in the seventh edition to 83% in the current iteration, while in stage IIIC, the jump is from 40% to 69%. All this is made possible because the eighth edition separates out patients with the new stage IIID, whose 5-year melanoma-specific survival is only 32%, Dr. Ferris explained.
Among the other key points to remember about the eighth edition of AJCC:
- Tumor thickness is now measured to the nearest 0.1 mm rather than to the nearest 0.01 mm, as previously. Thus, a 0.75-mm-thick melanoma is now rounded up to 0.8 mm, while a 0.74-mm melanoma becomes a 0.7-mm tumor.
- Based upon recent evidence, tumors that are 0.8-1.0 mm thick, with or without ulceration, are now classified at T1b. So are ulcerated lesions that are less than 0.8 mm.
- Dermal mitotic rate is no longer used in staging T1 tumors, although it’s still supposed to be included in pathology reports.
- The T category definitions of primary tumors have been clarified in the eighth edition. A tumor is now classified as T0 only if there is no evidence of a primary tumor. Tx is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained by curettage. Tis is utilized for melanoma in situ.
- The N subcategory definitions of regional nodal status have been revised. Microsatellites, clinical satellites, and in-transit metastases are now categorized as N1c, N2c, or N3c based upon the number of tumor-involved regional lymph nodes. These features are no longer defined by their size or distance from the primary tumor.
2018 NCCN melanoma guidelines
The guidelines have been revised to recommend against SLNB if a patient’s pretest probability of finding a positive SLN is less than 5%. This includes patients who have a clinical stage IA/T1a melanoma with a Breslow thickness of less than 0.8 mm without ulceration.
There is to be no SLNB in patients with microsatellites, clinical satellites, or in-transit metastases because SLN status has no prognostic significance in this situation.
Routine ordering of prognostic genetic tests for BRAF or the multigene test panels that are now commercially available is not recommended except to guide systemic therapy or to determine if a patient is a candidate for a specific clinical trial. “Basically, there is not a place to use this information in the NCCN guidelines,” according to the dermatologist.
What about completion lymphadenectomy in the SLN-positive melanoma patient?
Completion lymph node dissection looks increasingly like a procedure in search of an indication. Results of the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–II (MSLT-II) demonstrated not even a hint of a difference in 3-year melanoma-specific survival in 1,934 melanoma patients with sentinel lymph node metastases regardless of whether they were randomized to immediate completion lymph node dissection or ultrasound-based nodal monitoring. Moreover, completion lymphadenectomy was associated with significant morbidity: a 24.1% incidence of lymphedema, compared with a 6.3% rate in the observation group (N Engl J Med. 2017 Jun 8;376[23]:2211-22).
On the other hand, Dr. Ferris noted that many newer drugs are being approved for the treatment of stage III melanoma, and in all the pivotal clinical trials, patients had to have undergone completion lymph node dissection as a condition of participation. So the surgery becomes a consideration if physicians want to use the newer agents the way they were used successfully in the trials.
The full eighth edition of the AJCC cancer staging manual is available for purchase. For physicians with a specific interest in melanoma, Dr. Ferris recommended as an extremely useful alternative the AJCC expert writing panel’s free downloadable summary of the evidence-based changes made in melanoma staging (CA Cancer J Clin. 2017 Nov;67[6]:472-92). The 2018 NCCN guidelines (Melanoma. Version 1.2018 Oct. 11, 2017) are available for free (www.NCCN.org).
Dr. Ferris reported serving as a consultant to DermTech.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Melanoma , resulting in an evidence-based improved prognosis for many of them, Laura Korb Ferris, MD, PhD, said at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
Dr. Ferris, of the department of dermatology, University of Pittsburgh, highlighted some of the key changes in the eighth edition of the AJCC staging manual, which is now in effect. She also described the clinical implications of important updates introduced in the 2018 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and management of melanoma.
The AJCC eighth edition
The eighth edition is built upon an AJCC database of more than 46,000 patients with stage I-III melanoma diagnosed since 1998 at 10 academic medical centers. The AJCC panel made no changes in stage IV melanoma guidance because the newer targeted therapies have rapidly changed treatment outcomes in that setting and longer follow-up is needed to assess the full impact.
The current edition of the AJCC melanoma staging manual creates a new subcategory within pathologic stage III. In the melanoma staging world, that’s exciting news, especially because this change has important implications for prognosis.
This fourth subcategory, stage IIID, is for melanomas, which in the Tumor, Nodes, Metastasis (TNM) classification scheme, are primary tumor stage T4b, meaning greater than 4.0 mm in thickness and with ulceration; regional lymph node N3a, b, or c, based upon the number of metastatic nodes involved and whether they were clinically occult nodal metastases detected by sentinel lymph node biopsy (SLNB) or clinically detected; and M0, meaning no distant metastatic disease. In the 8th edition, the AJCC staging system can be applied in patients with T2 through T4 primary melanoma only if they have undergone SLNB.
This new approach to stage III disease makes for more homogeneous patient subgroups, which in turn provides much better stratification of prognosis than was possible in the seventh edition of the AJCC staging manual, which dates back to 2010. Most strikingly, the 5-year melanoma-specific survival rate for patients with stage IIIA disease was 78% in the seventh edition of AJCC, but it climbs to 93% in the eighth edition. For patients with stage IIIB melanoma, 5-year melanoma-specific survival improved from 59% in the seventh edition to 83% in the current iteration, while in stage IIIC, the jump is from 40% to 69%. All this is made possible because the eighth edition separates out patients with the new stage IIID, whose 5-year melanoma-specific survival is only 32%, Dr. Ferris explained.
Among the other key points to remember about the eighth edition of AJCC:
- Tumor thickness is now measured to the nearest 0.1 mm rather than to the nearest 0.01 mm, as previously. Thus, a 0.75-mm-thick melanoma is now rounded up to 0.8 mm, while a 0.74-mm melanoma becomes a 0.7-mm tumor.
- Based upon recent evidence, tumors that are 0.8-1.0 mm thick, with or without ulceration, are now classified at T1b. So are ulcerated lesions that are less than 0.8 mm.
- Dermal mitotic rate is no longer used in staging T1 tumors, although it’s still supposed to be included in pathology reports.
- The T category definitions of primary tumors have been clarified in the eighth edition. A tumor is now classified as T0 only if there is no evidence of a primary tumor. Tx is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained by curettage. Tis is utilized for melanoma in situ.
- The N subcategory definitions of regional nodal status have been revised. Microsatellites, clinical satellites, and in-transit metastases are now categorized as N1c, N2c, or N3c based upon the number of tumor-involved regional lymph nodes. These features are no longer defined by their size or distance from the primary tumor.
2018 NCCN melanoma guidelines
The guidelines have been revised to recommend against SLNB if a patient’s pretest probability of finding a positive SLN is less than 5%. This includes patients who have a clinical stage IA/T1a melanoma with a Breslow thickness of less than 0.8 mm without ulceration.
There is to be no SLNB in patients with microsatellites, clinical satellites, or in-transit metastases because SLN status has no prognostic significance in this situation.
Routine ordering of prognostic genetic tests for BRAF or the multigene test panels that are now commercially available is not recommended except to guide systemic therapy or to determine if a patient is a candidate for a specific clinical trial. “Basically, there is not a place to use this information in the NCCN guidelines,” according to the dermatologist.
What about completion lymphadenectomy in the SLN-positive melanoma patient?
Completion lymph node dissection looks increasingly like a procedure in search of an indication. Results of the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–II (MSLT-II) demonstrated not even a hint of a difference in 3-year melanoma-specific survival in 1,934 melanoma patients with sentinel lymph node metastases regardless of whether they were randomized to immediate completion lymph node dissection or ultrasound-based nodal monitoring. Moreover, completion lymphadenectomy was associated with significant morbidity: a 24.1% incidence of lymphedema, compared with a 6.3% rate in the observation group (N Engl J Med. 2017 Jun 8;376[23]:2211-22).
On the other hand, Dr. Ferris noted that many newer drugs are being approved for the treatment of stage III melanoma, and in all the pivotal clinical trials, patients had to have undergone completion lymph node dissection as a condition of participation. So the surgery becomes a consideration if physicians want to use the newer agents the way they were used successfully in the trials.
The full eighth edition of the AJCC cancer staging manual is available for purchase. For physicians with a specific interest in melanoma, Dr. Ferris recommended as an extremely useful alternative the AJCC expert writing panel’s free downloadable summary of the evidence-based changes made in melanoma staging (CA Cancer J Clin. 2017 Nov;67[6]:472-92). The 2018 NCCN guidelines (Melanoma. Version 1.2018 Oct. 11, 2017) are available for free (www.NCCN.org).
Dr. Ferris reported serving as a consultant to DermTech.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Melanoma , resulting in an evidence-based improved prognosis for many of them, Laura Korb Ferris, MD, PhD, said at the Hawaii Dermatology Seminar provided by Skin Disease Education Foundation/Global Academy for Medical Education.
Dr. Ferris, of the department of dermatology, University of Pittsburgh, highlighted some of the key changes in the eighth edition of the AJCC staging manual, which is now in effect. She also described the clinical implications of important updates introduced in the 2018 National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and management of melanoma.
The AJCC eighth edition
The eighth edition is built upon an AJCC database of more than 46,000 patients with stage I-III melanoma diagnosed since 1998 at 10 academic medical centers. The AJCC panel made no changes in stage IV melanoma guidance because the newer targeted therapies have rapidly changed treatment outcomes in that setting and longer follow-up is needed to assess the full impact.
The current edition of the AJCC melanoma staging manual creates a new subcategory within pathologic stage III. In the melanoma staging world, that’s exciting news, especially because this change has important implications for prognosis.
This fourth subcategory, stage IIID, is for melanomas, which in the Tumor, Nodes, Metastasis (TNM) classification scheme, are primary tumor stage T4b, meaning greater than 4.0 mm in thickness and with ulceration; regional lymph node N3a, b, or c, based upon the number of metastatic nodes involved and whether they were clinically occult nodal metastases detected by sentinel lymph node biopsy (SLNB) or clinically detected; and M0, meaning no distant metastatic disease. In the 8th edition, the AJCC staging system can be applied in patients with T2 through T4 primary melanoma only if they have undergone SLNB.
This new approach to stage III disease makes for more homogeneous patient subgroups, which in turn provides much better stratification of prognosis than was possible in the seventh edition of the AJCC staging manual, which dates back to 2010. Most strikingly, the 5-year melanoma-specific survival rate for patients with stage IIIA disease was 78% in the seventh edition of AJCC, but it climbs to 93% in the eighth edition. For patients with stage IIIB melanoma, 5-year melanoma-specific survival improved from 59% in the seventh edition to 83% in the current iteration, while in stage IIIC, the jump is from 40% to 69%. All this is made possible because the eighth edition separates out patients with the new stage IIID, whose 5-year melanoma-specific survival is only 32%, Dr. Ferris explained.
Among the other key points to remember about the eighth edition of AJCC:
- Tumor thickness is now measured to the nearest 0.1 mm rather than to the nearest 0.01 mm, as previously. Thus, a 0.75-mm-thick melanoma is now rounded up to 0.8 mm, while a 0.74-mm melanoma becomes a 0.7-mm tumor.
- Based upon recent evidence, tumors that are 0.8-1.0 mm thick, with or without ulceration, are now classified at T1b. So are ulcerated lesions that are less than 0.8 mm.
- Dermal mitotic rate is no longer used in staging T1 tumors, although it’s still supposed to be included in pathology reports.
- The T category definitions of primary tumors have been clarified in the eighth edition. A tumor is now classified as T0 only if there is no evidence of a primary tumor. Tx is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained by curettage. Tis is utilized for melanoma in situ.
- The N subcategory definitions of regional nodal status have been revised. Microsatellites, clinical satellites, and in-transit metastases are now categorized as N1c, N2c, or N3c based upon the number of tumor-involved regional lymph nodes. These features are no longer defined by their size or distance from the primary tumor.
2018 NCCN melanoma guidelines
The guidelines have been revised to recommend against SLNB if a patient’s pretest probability of finding a positive SLN is less than 5%. This includes patients who have a clinical stage IA/T1a melanoma with a Breslow thickness of less than 0.8 mm without ulceration.
There is to be no SLNB in patients with microsatellites, clinical satellites, or in-transit metastases because SLN status has no prognostic significance in this situation.
Routine ordering of prognostic genetic tests for BRAF or the multigene test panels that are now commercially available is not recommended except to guide systemic therapy or to determine if a patient is a candidate for a specific clinical trial. “Basically, there is not a place to use this information in the NCCN guidelines,” according to the dermatologist.
What about completion lymphadenectomy in the SLN-positive melanoma patient?
Completion lymph node dissection looks increasingly like a procedure in search of an indication. Results of the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–II (MSLT-II) demonstrated not even a hint of a difference in 3-year melanoma-specific survival in 1,934 melanoma patients with sentinel lymph node metastases regardless of whether they were randomized to immediate completion lymph node dissection or ultrasound-based nodal monitoring. Moreover, completion lymphadenectomy was associated with significant morbidity: a 24.1% incidence of lymphedema, compared with a 6.3% rate in the observation group (N Engl J Med. 2017 Jun 8;376[23]:2211-22).
On the other hand, Dr. Ferris noted that many newer drugs are being approved for the treatment of stage III melanoma, and in all the pivotal clinical trials, patients had to have undergone completion lymph node dissection as a condition of participation. So the surgery becomes a consideration if physicians want to use the newer agents the way they were used successfully in the trials.
The full eighth edition of the AJCC cancer staging manual is available for purchase. For physicians with a specific interest in melanoma, Dr. Ferris recommended as an extremely useful alternative the AJCC expert writing panel’s free downloadable summary of the evidence-based changes made in melanoma staging (CA Cancer J Clin. 2017 Nov;67[6]:472-92). The 2018 NCCN guidelines (Melanoma. Version 1.2018 Oct. 11, 2017) are available for free (www.NCCN.org).
Dr. Ferris reported serving as a consultant to DermTech.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR