User login
Surgery after immunotherapy effective in advanced melanoma
CHICAGO – Surgical resection is an effective treatment in selected patients with advanced melanoma treated with checkpoint blockade immunotherapy, according to a study of an institutional database at Memorial Sloan Kettering Cancer Center in New York presented at the Society of Surgical Oncology Annual Cancer Symposium.
“In the era of improved systemic therapy, checkpoint blockade for metastatic melanoma and the ability to surgically resect all disease after treatment is associated with an estimated survival of 75%, better than what’s been previously reported,” said Danielle M. Bello, MD, of Memorial Sloan Kettering.
The study analyzed a cohort of 237 patients who had unresectable stage III and IV melanoma and were treated with checkpoint blockade, including CTLA-4, programmed cell death protein 1 (PD-1), and programmed death-ligand 1 inhibitors, and then had surgical resection during 2003-2017.
Dr. Bello noted two previous studies that had reported encouraging outcomes in advanced melanoma. The first highlighted the role for surgery in stage IV melanoma. In that phase 3 clinical trial, patients had resection of up to five sites of metastatic disease and were then randomized to one of two treatment arms: bacillus Calmette-Guérin and allogeneic whole-cell vaccine (Canvaxin) or bacillus Calmette-Guérin and placebo. While this trial found no difference in overall survival between groups, it did report a 5-year overall survival exceeding 40% in both treatment arms, which highlighted that Stage IV patients who underwent resection of all their disease had survival outcomes superior to outcomes previously reported (Ann Surg Onc. 2017 Dec;24[13]:3991-4000). The second trial, the recent Checkmate 067 trial, emphasized the role of effective systemic checkpoint blockade in advanced stage III and IV melanoma. It reported that patients treated with combined nivolumab/ipilimumab therapy had not reached median overall survival at minimum 36 months of follow-up (N Engl J Med. 2017 Oct 5;377[14]:1345-56).
“We know that checkpoint inhibitor therapy has revolutionized the landscape of unresectable stage III and IV melanoma,” Dr. Bello said. However, despite encouraging trial readouts of overall survival, progression-free survival is a different story. “We know that the median progression-free survival even in our best combination therapy is 11.5 months, meaning that 50% of patients will go on to progress in a year and many will go on to surgical resection of their disease and do quite well,” she said.
Dr. Bello and her coauthors set out to describe outcomes of a “highly selective group” of patients who had surgical resection after checkpoint inhibitor therapy. “The majority of patients in our study had a cutaneous primary melanoma,” she said. Median age was 63 years, and 88% had stage IV disease. Regarding checkpoint blockade regimen, 62% received anti–CTLA-4, and 29% received combination anti–PD-1 and anti–CLTA-4 either sequentially or concomitantly prior to resection.
The median time from the start of immunotherapy to the first operation was 7 months. Forty-six percent had no further postoperative treatment after resection. In those, who did require further treatment, the majority received anti–PD-1 followed by targeted BRAF/MEK therapy, she said.
The analysis stratified patients into the following three categories based on radiological response to immunotherapy:
- Overall response to checkpoint blockade and the index lesion was either smaller since initiation of therapy or stabilized (12; 5.1%). Half of this group had a pathological complete response.
- Isolated site of progressive disease with residual stable disease elsewhere or as the only site of progressive disease (106; 44.7%).
- Multiple sites of progressive and palliative operations (119; 50.2%).
Median overall survival was 21 months in the entire study cohort with a median follow-up of 23 months, Dr. Bello said. “Those resected to no evidence of disease (NED) – 87 patients – had an estimated 5-year overall survival of 75%.” The NED group did not reach median OS.
The analysis also stratified overall survival by response to immunotherapy. “Patients with responding or stable disease had an estimated 90% 5-year overall survival,” Dr. Bellow said. “Those with one isolated progressive lesion that was resected had a 60% 5-year overall survival.” A more detailed analysis of the latter group found that those who had a resection to NED had an improved overall survival of 75% at 5 years. Resected patients who had residual remaining disease had a 30% 5-year overall survival.
“Further follow-up is needed to assess the durability and contributions of surgery, and further studies are underway to identify biomarkers associated with improved survival after immunotherapy and surgery,” Dr. Bello said.
SOURCE: Bello DM et al. SSO 2018, Abstract 5.
CHICAGO – Surgical resection is an effective treatment in selected patients with advanced melanoma treated with checkpoint blockade immunotherapy, according to a study of an institutional database at Memorial Sloan Kettering Cancer Center in New York presented at the Society of Surgical Oncology Annual Cancer Symposium.
“In the era of improved systemic therapy, checkpoint blockade for metastatic melanoma and the ability to surgically resect all disease after treatment is associated with an estimated survival of 75%, better than what’s been previously reported,” said Danielle M. Bello, MD, of Memorial Sloan Kettering.
The study analyzed a cohort of 237 patients who had unresectable stage III and IV melanoma and were treated with checkpoint blockade, including CTLA-4, programmed cell death protein 1 (PD-1), and programmed death-ligand 1 inhibitors, and then had surgical resection during 2003-2017.
Dr. Bello noted two previous studies that had reported encouraging outcomes in advanced melanoma. The first highlighted the role for surgery in stage IV melanoma. In that phase 3 clinical trial, patients had resection of up to five sites of metastatic disease and were then randomized to one of two treatment arms: bacillus Calmette-Guérin and allogeneic whole-cell vaccine (Canvaxin) or bacillus Calmette-Guérin and placebo. While this trial found no difference in overall survival between groups, it did report a 5-year overall survival exceeding 40% in both treatment arms, which highlighted that Stage IV patients who underwent resection of all their disease had survival outcomes superior to outcomes previously reported (Ann Surg Onc. 2017 Dec;24[13]:3991-4000). The second trial, the recent Checkmate 067 trial, emphasized the role of effective systemic checkpoint blockade in advanced stage III and IV melanoma. It reported that patients treated with combined nivolumab/ipilimumab therapy had not reached median overall survival at minimum 36 months of follow-up (N Engl J Med. 2017 Oct 5;377[14]:1345-56).
“We know that checkpoint inhibitor therapy has revolutionized the landscape of unresectable stage III and IV melanoma,” Dr. Bello said. However, despite encouraging trial readouts of overall survival, progression-free survival is a different story. “We know that the median progression-free survival even in our best combination therapy is 11.5 months, meaning that 50% of patients will go on to progress in a year and many will go on to surgical resection of their disease and do quite well,” she said.
Dr. Bello and her coauthors set out to describe outcomes of a “highly selective group” of patients who had surgical resection after checkpoint inhibitor therapy. “The majority of patients in our study had a cutaneous primary melanoma,” she said. Median age was 63 years, and 88% had stage IV disease. Regarding checkpoint blockade regimen, 62% received anti–CTLA-4, and 29% received combination anti–PD-1 and anti–CLTA-4 either sequentially or concomitantly prior to resection.
The median time from the start of immunotherapy to the first operation was 7 months. Forty-six percent had no further postoperative treatment after resection. In those, who did require further treatment, the majority received anti–PD-1 followed by targeted BRAF/MEK therapy, she said.
The analysis stratified patients into the following three categories based on radiological response to immunotherapy:
- Overall response to checkpoint blockade and the index lesion was either smaller since initiation of therapy or stabilized (12; 5.1%). Half of this group had a pathological complete response.
- Isolated site of progressive disease with residual stable disease elsewhere or as the only site of progressive disease (106; 44.7%).
- Multiple sites of progressive and palliative operations (119; 50.2%).
Median overall survival was 21 months in the entire study cohort with a median follow-up of 23 months, Dr. Bello said. “Those resected to no evidence of disease (NED) – 87 patients – had an estimated 5-year overall survival of 75%.” The NED group did not reach median OS.
The analysis also stratified overall survival by response to immunotherapy. “Patients with responding or stable disease had an estimated 90% 5-year overall survival,” Dr. Bellow said. “Those with one isolated progressive lesion that was resected had a 60% 5-year overall survival.” A more detailed analysis of the latter group found that those who had a resection to NED had an improved overall survival of 75% at 5 years. Resected patients who had residual remaining disease had a 30% 5-year overall survival.
“Further follow-up is needed to assess the durability and contributions of surgery, and further studies are underway to identify biomarkers associated with improved survival after immunotherapy and surgery,” Dr. Bello said.
SOURCE: Bello DM et al. SSO 2018, Abstract 5.
CHICAGO – Surgical resection is an effective treatment in selected patients with advanced melanoma treated with checkpoint blockade immunotherapy, according to a study of an institutional database at Memorial Sloan Kettering Cancer Center in New York presented at the Society of Surgical Oncology Annual Cancer Symposium.
“In the era of improved systemic therapy, checkpoint blockade for metastatic melanoma and the ability to surgically resect all disease after treatment is associated with an estimated survival of 75%, better than what’s been previously reported,” said Danielle M. Bello, MD, of Memorial Sloan Kettering.
The study analyzed a cohort of 237 patients who had unresectable stage III and IV melanoma and were treated with checkpoint blockade, including CTLA-4, programmed cell death protein 1 (PD-1), and programmed death-ligand 1 inhibitors, and then had surgical resection during 2003-2017.
Dr. Bello noted two previous studies that had reported encouraging outcomes in advanced melanoma. The first highlighted the role for surgery in stage IV melanoma. In that phase 3 clinical trial, patients had resection of up to five sites of metastatic disease and were then randomized to one of two treatment arms: bacillus Calmette-Guérin and allogeneic whole-cell vaccine (Canvaxin) or bacillus Calmette-Guérin and placebo. While this trial found no difference in overall survival between groups, it did report a 5-year overall survival exceeding 40% in both treatment arms, which highlighted that Stage IV patients who underwent resection of all their disease had survival outcomes superior to outcomes previously reported (Ann Surg Onc. 2017 Dec;24[13]:3991-4000). The second trial, the recent Checkmate 067 trial, emphasized the role of effective systemic checkpoint blockade in advanced stage III and IV melanoma. It reported that patients treated with combined nivolumab/ipilimumab therapy had not reached median overall survival at minimum 36 months of follow-up (N Engl J Med. 2017 Oct 5;377[14]:1345-56).
“We know that checkpoint inhibitor therapy has revolutionized the landscape of unresectable stage III and IV melanoma,” Dr. Bello said. However, despite encouraging trial readouts of overall survival, progression-free survival is a different story. “We know that the median progression-free survival even in our best combination therapy is 11.5 months, meaning that 50% of patients will go on to progress in a year and many will go on to surgical resection of their disease and do quite well,” she said.
Dr. Bello and her coauthors set out to describe outcomes of a “highly selective group” of patients who had surgical resection after checkpoint inhibitor therapy. “The majority of patients in our study had a cutaneous primary melanoma,” she said. Median age was 63 years, and 88% had stage IV disease. Regarding checkpoint blockade regimen, 62% received anti–CTLA-4, and 29% received combination anti–PD-1 and anti–CLTA-4 either sequentially or concomitantly prior to resection.
The median time from the start of immunotherapy to the first operation was 7 months. Forty-six percent had no further postoperative treatment after resection. In those, who did require further treatment, the majority received anti–PD-1 followed by targeted BRAF/MEK therapy, she said.
The analysis stratified patients into the following three categories based on radiological response to immunotherapy:
- Overall response to checkpoint blockade and the index lesion was either smaller since initiation of therapy or stabilized (12; 5.1%). Half of this group had a pathological complete response.
- Isolated site of progressive disease with residual stable disease elsewhere or as the only site of progressive disease (106; 44.7%).
- Multiple sites of progressive and palliative operations (119; 50.2%).
Median overall survival was 21 months in the entire study cohort with a median follow-up of 23 months, Dr. Bello said. “Those resected to no evidence of disease (NED) – 87 patients – had an estimated 5-year overall survival of 75%.” The NED group did not reach median OS.
The analysis also stratified overall survival by response to immunotherapy. “Patients with responding or stable disease had an estimated 90% 5-year overall survival,” Dr. Bellow said. “Those with one isolated progressive lesion that was resected had a 60% 5-year overall survival.” A more detailed analysis of the latter group found that those who had a resection to NED had an improved overall survival of 75% at 5 years. Resected patients who had residual remaining disease had a 30% 5-year overall survival.
“Further follow-up is needed to assess the durability and contributions of surgery, and further studies are underway to identify biomarkers associated with improved survival after immunotherapy and surgery,” Dr. Bello said.
SOURCE: Bello DM et al. SSO 2018, Abstract 5.
REPORTING FROM SSO 2018
Key clinical point: Surgery after immunotherapy can achieve good outcomes in advanced melanoma.
Major findings: Complete resection achieved an estimated 5-year overall survival of 75%.
Study details: Analysis of a cohort of 237 patients from a prospectively maintained institutional melanoma database who had surgery after immunotherapy for unresectable stage III and IV melanoma during 2003-2017.
Disclosures: Dr. Bello reported having no financial disclosures. Some coauthors reported financial relationships with various pharmaceutical companies.
Source: Bello DM et al. SSO 2018, Abstract 5.
Tanning is the new tobacco
I was driving to work the other day, perched up in my pickup truck (somehow you knew that) and noticed a fancy race car in front of me with a vanity tag. It read HRTATTK 4. Well, I thought after four heart attacks maybe I would splurge on a special car too (more likely a newer truck). Then I noticed smoke coming out of the driver’s window, and I could see this guy in his side view mirror, presumably Mr. “Heart Attack 4,” puffing away on a cigarette. Wow.
Then I got to work and saw my secretary, who works with her oxygen on, out back puffing a cigarette. Wow.
It turns out that cigarette smoke contains substances that act as a monoamine oxidase (MAO) A inhibitor, prolonging the dopamine high in the brain (Proc Natl Acad Sci U S A. 1996 Nov 26;93[24]:14065-9). Makes sense and may explain the above smoking behavior. I truly believe cigarettes are as or more addictive than any other dopamine enhancing drug.
More than 50 years ago, a national campaign against smoking was launched after the 1964 Surgeon General’s report concluded that smoking was a major health hazard. (Looking back, one of the few losses of not having to pull journal articles from the stacks in the library, is that medical students and residents can’t shake their heads in wonder at the cigarette ads in old medical journals.) The impact of the national antismoking campaign has been dramatic, but smoking remains the leading preventable cause of death in the United States and globally, according to the Centers for Disease Control and Prevention.
in 2006, from 1992 (Arch Dermatol. 2010;146[3]:283-7), dermatologists had good footing on which to start a major prevention campaign. The American Cancer Society got on board, and in 2014, acting surgeon general Boris Lushniak, MD, issued a call to action to prevent skin cancer along with Howard Koh, MD, the assistant secretary of health, in “The Surgeon General’s Call to Action to Prevent Skin Cancer” in 2014, and the campaign was on.
Well, I am delighted to pass on a report from Leonard Lichtenfeld, MD, deputy chief medical officer for the American Cancer Society, who recently described in his March 2018 blog what may the first signs of the effectiveness of efforts to promote protection from ultraviolet ray exposure (JAMA Dermatol. 2018;154[3]:361-2). He writes: “In young white women ages 15 to 24, the incidence of melanoma has declined an average of 5.5% per year from January 2005 through December 2014. Not 5.5% over those ten years but 5.5 % PER YEAR. That’s remarkable, to say the least.”
As for the reasons behind these trends, he says, “no one can say for certain,” but he refers to national data indicating that indoor tanning has decreased in the past few years, especially among adolescents and young adults.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
I was driving to work the other day, perched up in my pickup truck (somehow you knew that) and noticed a fancy race car in front of me with a vanity tag. It read HRTATTK 4. Well, I thought after four heart attacks maybe I would splurge on a special car too (more likely a newer truck). Then I noticed smoke coming out of the driver’s window, and I could see this guy in his side view mirror, presumably Mr. “Heart Attack 4,” puffing away on a cigarette. Wow.
Then I got to work and saw my secretary, who works with her oxygen on, out back puffing a cigarette. Wow.
It turns out that cigarette smoke contains substances that act as a monoamine oxidase (MAO) A inhibitor, prolonging the dopamine high in the brain (Proc Natl Acad Sci U S A. 1996 Nov 26;93[24]:14065-9). Makes sense and may explain the above smoking behavior. I truly believe cigarettes are as or more addictive than any other dopamine enhancing drug.
More than 50 years ago, a national campaign against smoking was launched after the 1964 Surgeon General’s report concluded that smoking was a major health hazard. (Looking back, one of the few losses of not having to pull journal articles from the stacks in the library, is that medical students and residents can’t shake their heads in wonder at the cigarette ads in old medical journals.) The impact of the national antismoking campaign has been dramatic, but smoking remains the leading preventable cause of death in the United States and globally, according to the Centers for Disease Control and Prevention.
in 2006, from 1992 (Arch Dermatol. 2010;146[3]:283-7), dermatologists had good footing on which to start a major prevention campaign. The American Cancer Society got on board, and in 2014, acting surgeon general Boris Lushniak, MD, issued a call to action to prevent skin cancer along with Howard Koh, MD, the assistant secretary of health, in “The Surgeon General’s Call to Action to Prevent Skin Cancer” in 2014, and the campaign was on.
Well, I am delighted to pass on a report from Leonard Lichtenfeld, MD, deputy chief medical officer for the American Cancer Society, who recently described in his March 2018 blog what may the first signs of the effectiveness of efforts to promote protection from ultraviolet ray exposure (JAMA Dermatol. 2018;154[3]:361-2). He writes: “In young white women ages 15 to 24, the incidence of melanoma has declined an average of 5.5% per year from January 2005 through December 2014. Not 5.5% over those ten years but 5.5 % PER YEAR. That’s remarkable, to say the least.”
As for the reasons behind these trends, he says, “no one can say for certain,” but he refers to national data indicating that indoor tanning has decreased in the past few years, especially among adolescents and young adults.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
I was driving to work the other day, perched up in my pickup truck (somehow you knew that) and noticed a fancy race car in front of me with a vanity tag. It read HRTATTK 4. Well, I thought after four heart attacks maybe I would splurge on a special car too (more likely a newer truck). Then I noticed smoke coming out of the driver’s window, and I could see this guy in his side view mirror, presumably Mr. “Heart Attack 4,” puffing away on a cigarette. Wow.
Then I got to work and saw my secretary, who works with her oxygen on, out back puffing a cigarette. Wow.
It turns out that cigarette smoke contains substances that act as a monoamine oxidase (MAO) A inhibitor, prolonging the dopamine high in the brain (Proc Natl Acad Sci U S A. 1996 Nov 26;93[24]:14065-9). Makes sense and may explain the above smoking behavior. I truly believe cigarettes are as or more addictive than any other dopamine enhancing drug.
More than 50 years ago, a national campaign against smoking was launched after the 1964 Surgeon General’s report concluded that smoking was a major health hazard. (Looking back, one of the few losses of not having to pull journal articles from the stacks in the library, is that medical students and residents can’t shake their heads in wonder at the cigarette ads in old medical journals.) The impact of the national antismoking campaign has been dramatic, but smoking remains the leading preventable cause of death in the United States and globally, according to the Centers for Disease Control and Prevention.
in 2006, from 1992 (Arch Dermatol. 2010;146[3]:283-7), dermatologists had good footing on which to start a major prevention campaign. The American Cancer Society got on board, and in 2014, acting surgeon general Boris Lushniak, MD, issued a call to action to prevent skin cancer along with Howard Koh, MD, the assistant secretary of health, in “The Surgeon General’s Call to Action to Prevent Skin Cancer” in 2014, and the campaign was on.
Well, I am delighted to pass on a report from Leonard Lichtenfeld, MD, deputy chief medical officer for the American Cancer Society, who recently described in his March 2018 blog what may the first signs of the effectiveness of efforts to promote protection from ultraviolet ray exposure (JAMA Dermatol. 2018;154[3]:361-2). He writes: “In young white women ages 15 to 24, the incidence of melanoma has declined an average of 5.5% per year from January 2005 through December 2014. Not 5.5% over those ten years but 5.5 % PER YEAR. That’s remarkable, to say the least.”
As for the reasons behind these trends, he says, “no one can say for certain,” but he refers to national data indicating that indoor tanning has decreased in the past few years, especially among adolescents and young adults.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Diabetes from checkpoint inhibitors probably means lifelong insulin
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
REPORTING FROM ENDO 2018
Key clinical point: Be on the lookout for new-onset diabetes when patients start immune checkpoint inhibitors.
Major finding: In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs.
Study details: Review of 24 cases.
Disclosures: The investigators had no disclosures. A funding source was not reported.
Source: Iyer PC et al. Abstract OR05-5.
Unknown primary melanoma looks a lot like known
Stage IV melanoma of unknown primary (MUP) origin, in which a primary tumor has either resolved or remains undiscovered, shares similar outcomes and prognostic factors to melanoma of known primary (MKP) origin, according to a new analysis of the nationwide Surveillance, Epidemiology, and End Results (SEER)-18 registries spanning from 1973 to 2014.
Previous studies of MUP have been single institutional or multi-institutional studies. The current work is the first population-level study.
MUP is uncommon, representing 2.5-5% of melanoma cases. As with MKP, worse survival of MUP patients was tied to age greater than 50 years and not undergoing a surgical procedure. The researchers did find a slight advantage in one-year survival for MUP patients compared to MKP, which could be because many of the MUP patients had experienced an immune response that eliminated the primary tumor.
“You could imagine that if the body attacks the primary tumor and it goes away, you’re set up to fight off the metastatic melanoma better,” said lead study author Jeffrey Scott, MD, a micrographic surgery and dermatologic oncology fellow at University Hospitals Cleveland Medical Center, Case Western Reserve University.
The study appeared online March 23 in the Journal of the American Academy of Dermatology.
The researchers analyzed 322 stage IV MUP cases and 12,796 stage IV MKP cases. The incidence of stage IV MUP increased over time, from 1.52 per 100,000 between 1973 and 1984, to 5.83 per 100,000 from 2005 to 2014. MUP patients were more likely to be recommended for surgery than MKP patients (surgery not recommended for 47.7% of MKP cases, compared to 37.7% of MUP cases), and they had better 1-year survival rates compared to the general U.S. population than did MKP patients (0.54, 95% CI, 0.48-0.60 versus 0.41, 95% CI 0.39-0.42). The improved survival of MUP over MKP remained steady at each measured time point out to 5 years.
However, there was no difference in 5-year disease-specific survival (DSS) in MUP versus MKP (HR, 0.91; 95% CI, 0.79-1.04; P =.16), or in the 5-year DSS Kaplan-Meier curve after adjustment for year of diagnosis, age, sex, race, and surgical treatment (log-rank P = .93).
A multivariate analysis showed increased 5-year DSS among patients who received surgery (HR, 0.41; 95% CI, 0.30-0.56; P less than .001) and decreased 5-year DSS among patients over 50 (HR 3.27, 95% CI, 1.17-9.17; P = .02 for age 50-59).
“The prognostic factors are very similar, so you should treat these patients (with MUP) similarly to patients with melanoma of known primary, the same treatments, the same clinical trials,” Dr. Scott and associates said.
The results also raise the possibility of gaining a better understanding of how immune response affects the course of metastatic melanoma.
“If MUP is due to the fact that your immune system is attacking the primary tumor, then what characteristics of the person would cause that to happen? Are younger patients (exhibiting) a more robust immune response? Could that explain why their prognosis is better? More molecular studies of the actual tumors and the immune characteristics of these patients would help us answer that,” they added.
A resolved primary tumor isn’t the only explanation for MUP, however. It’s also possible that melanocytes are found in unexpected sites, perhaps because they did not complete their migration during development. “They could give rise to melanoma that would present (as MUP). Maybe there never was a skin tumor,” the authors wrote.
The investigators recommend a thorough search for a primary tumor, employing ophthalmologists, gynecologists, and other specialists if necessary. “You could argue that once you have metastatic disease, what’s the point of finding the primary tumor? But it’s important to correctly classify these patients, in terms of what clinical trials and treatments they may be eligible for,” Dr. Scott and associates said.
SOURCE: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
Stage IV melanoma of unknown primary (MUP) origin, in which a primary tumor has either resolved or remains undiscovered, shares similar outcomes and prognostic factors to melanoma of known primary (MKP) origin, according to a new analysis of the nationwide Surveillance, Epidemiology, and End Results (SEER)-18 registries spanning from 1973 to 2014.
Previous studies of MUP have been single institutional or multi-institutional studies. The current work is the first population-level study.
MUP is uncommon, representing 2.5-5% of melanoma cases. As with MKP, worse survival of MUP patients was tied to age greater than 50 years and not undergoing a surgical procedure. The researchers did find a slight advantage in one-year survival for MUP patients compared to MKP, which could be because many of the MUP patients had experienced an immune response that eliminated the primary tumor.
“You could imagine that if the body attacks the primary tumor and it goes away, you’re set up to fight off the metastatic melanoma better,” said lead study author Jeffrey Scott, MD, a micrographic surgery and dermatologic oncology fellow at University Hospitals Cleveland Medical Center, Case Western Reserve University.
The study appeared online March 23 in the Journal of the American Academy of Dermatology.
The researchers analyzed 322 stage IV MUP cases and 12,796 stage IV MKP cases. The incidence of stage IV MUP increased over time, from 1.52 per 100,000 between 1973 and 1984, to 5.83 per 100,000 from 2005 to 2014. MUP patients were more likely to be recommended for surgery than MKP patients (surgery not recommended for 47.7% of MKP cases, compared to 37.7% of MUP cases), and they had better 1-year survival rates compared to the general U.S. population than did MKP patients (0.54, 95% CI, 0.48-0.60 versus 0.41, 95% CI 0.39-0.42). The improved survival of MUP over MKP remained steady at each measured time point out to 5 years.
However, there was no difference in 5-year disease-specific survival (DSS) in MUP versus MKP (HR, 0.91; 95% CI, 0.79-1.04; P =.16), or in the 5-year DSS Kaplan-Meier curve after adjustment for year of diagnosis, age, sex, race, and surgical treatment (log-rank P = .93).
A multivariate analysis showed increased 5-year DSS among patients who received surgery (HR, 0.41; 95% CI, 0.30-0.56; P less than .001) and decreased 5-year DSS among patients over 50 (HR 3.27, 95% CI, 1.17-9.17; P = .02 for age 50-59).
“The prognostic factors are very similar, so you should treat these patients (with MUP) similarly to patients with melanoma of known primary, the same treatments, the same clinical trials,” Dr. Scott and associates said.
The results also raise the possibility of gaining a better understanding of how immune response affects the course of metastatic melanoma.
“If MUP is due to the fact that your immune system is attacking the primary tumor, then what characteristics of the person would cause that to happen? Are younger patients (exhibiting) a more robust immune response? Could that explain why their prognosis is better? More molecular studies of the actual tumors and the immune characteristics of these patients would help us answer that,” they added.
A resolved primary tumor isn’t the only explanation for MUP, however. It’s also possible that melanocytes are found in unexpected sites, perhaps because they did not complete their migration during development. “They could give rise to melanoma that would present (as MUP). Maybe there never was a skin tumor,” the authors wrote.
The investigators recommend a thorough search for a primary tumor, employing ophthalmologists, gynecologists, and other specialists if necessary. “You could argue that once you have metastatic disease, what’s the point of finding the primary tumor? But it’s important to correctly classify these patients, in terms of what clinical trials and treatments they may be eligible for,” Dr. Scott and associates said.
SOURCE: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
Stage IV melanoma of unknown primary (MUP) origin, in which a primary tumor has either resolved or remains undiscovered, shares similar outcomes and prognostic factors to melanoma of known primary (MKP) origin, according to a new analysis of the nationwide Surveillance, Epidemiology, and End Results (SEER)-18 registries spanning from 1973 to 2014.
Previous studies of MUP have been single institutional or multi-institutional studies. The current work is the first population-level study.
MUP is uncommon, representing 2.5-5% of melanoma cases. As with MKP, worse survival of MUP patients was tied to age greater than 50 years and not undergoing a surgical procedure. The researchers did find a slight advantage in one-year survival for MUP patients compared to MKP, which could be because many of the MUP patients had experienced an immune response that eliminated the primary tumor.
“You could imagine that if the body attacks the primary tumor and it goes away, you’re set up to fight off the metastatic melanoma better,” said lead study author Jeffrey Scott, MD, a micrographic surgery and dermatologic oncology fellow at University Hospitals Cleveland Medical Center, Case Western Reserve University.
The study appeared online March 23 in the Journal of the American Academy of Dermatology.
The researchers analyzed 322 stage IV MUP cases and 12,796 stage IV MKP cases. The incidence of stage IV MUP increased over time, from 1.52 per 100,000 between 1973 and 1984, to 5.83 per 100,000 from 2005 to 2014. MUP patients were more likely to be recommended for surgery than MKP patients (surgery not recommended for 47.7% of MKP cases, compared to 37.7% of MUP cases), and they had better 1-year survival rates compared to the general U.S. population than did MKP patients (0.54, 95% CI, 0.48-0.60 versus 0.41, 95% CI 0.39-0.42). The improved survival of MUP over MKP remained steady at each measured time point out to 5 years.
However, there was no difference in 5-year disease-specific survival (DSS) in MUP versus MKP (HR, 0.91; 95% CI, 0.79-1.04; P =.16), or in the 5-year DSS Kaplan-Meier curve after adjustment for year of diagnosis, age, sex, race, and surgical treatment (log-rank P = .93).
A multivariate analysis showed increased 5-year DSS among patients who received surgery (HR, 0.41; 95% CI, 0.30-0.56; P less than .001) and decreased 5-year DSS among patients over 50 (HR 3.27, 95% CI, 1.17-9.17; P = .02 for age 50-59).
“The prognostic factors are very similar, so you should treat these patients (with MUP) similarly to patients with melanoma of known primary, the same treatments, the same clinical trials,” Dr. Scott and associates said.
The results also raise the possibility of gaining a better understanding of how immune response affects the course of metastatic melanoma.
“If MUP is due to the fact that your immune system is attacking the primary tumor, then what characteristics of the person would cause that to happen? Are younger patients (exhibiting) a more robust immune response? Could that explain why their prognosis is better? More molecular studies of the actual tumors and the immune characteristics of these patients would help us answer that,” they added.
A resolved primary tumor isn’t the only explanation for MUP, however. It’s also possible that melanocytes are found in unexpected sites, perhaps because they did not complete their migration during development. “They could give rise to melanoma that would present (as MUP). Maybe there never was a skin tumor,” the authors wrote.
The investigators recommend a thorough search for a primary tumor, employing ophthalmologists, gynecologists, and other specialists if necessary. “You could argue that once you have metastatic disease, what’s the point of finding the primary tumor? But it’s important to correctly classify these patients, in terms of what clinical trials and treatments they may be eligible for,” Dr. Scott and associates said.
SOURCE: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Unknown primary melanomas should be approached similar to melanomas with known primaries.
Major finding: The 5-year DSS rate was lower in patients age 50-59 (HR, 3.27).
Study details: Retrospective analysis of 322 stage IV MUP cases and 12,796 stage IV MKP.
Disclosures: The study was funded by the Char and Chuck Fowler Family Foundation. Dr. Scott reported no relevant financial relationships.
Source: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
Melanoma in young children may be biologically distinct from that in teens
Pediatric melanomas appear to be more progressive in adolescents than in young children, based on data from a retrospective study of 32 cases.
Few young children with melanoma die, despite a greater likelihood of thicker tumors, lymph node metastasis, and later diagnosis, which suggests that melanoma in young children may be biologically distinct from melanoma in adolescents, wrote Diana W. Bartenstein, of Harvard University Medical School, Boston, and her colleagues.
Overall, significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01). In addition, children were more likely than adolescents to present with stage 3 or 4 cancer (58% vs. 25%) and with Clark level IV and V tumors (42% vs. 35%), although these differences were not significant. The median Breslow thickness of lesions was greater in children than in adolescents (3.5 mm vs. 1.5 mm) as was the median mitotic index (5 mitotic figures per mm2 vs. 2 mitotic figures per mm2) and children were more likely than adolescents to have neural invasion, but these differences were not significant either.
During the study period of more than 20 years, none of the children younger than 11 years died, compared with four deaths in adolescents, a statistically significant difference (P = .04). The follow-up for surviving individuals ranged from 9-37 months with a median of 44 months.
The study findings were limited by several factors including the small sample size and difficulty in assessing spitzoid tumors, the researchers noted.
However, “these results support the hypothesis that melanoma in young children may be biologically distinct from melanoma in adults,” they said. “Alternatively, melanoma subtype may drive survival differences between children and adolescents.”
No conflicts of interest were reported. The study was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance.
SOURCE: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
Pediatric melanomas appear to be more progressive in adolescents than in young children, based on data from a retrospective study of 32 cases.
Few young children with melanoma die, despite a greater likelihood of thicker tumors, lymph node metastasis, and later diagnosis, which suggests that melanoma in young children may be biologically distinct from melanoma in adolescents, wrote Diana W. Bartenstein, of Harvard University Medical School, Boston, and her colleagues.
Overall, significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01). In addition, children were more likely than adolescents to present with stage 3 or 4 cancer (58% vs. 25%) and with Clark level IV and V tumors (42% vs. 35%), although these differences were not significant. The median Breslow thickness of lesions was greater in children than in adolescents (3.5 mm vs. 1.5 mm) as was the median mitotic index (5 mitotic figures per mm2 vs. 2 mitotic figures per mm2) and children were more likely than adolescents to have neural invasion, but these differences were not significant either.
During the study period of more than 20 years, none of the children younger than 11 years died, compared with four deaths in adolescents, a statistically significant difference (P = .04). The follow-up for surviving individuals ranged from 9-37 months with a median of 44 months.
The study findings were limited by several factors including the small sample size and difficulty in assessing spitzoid tumors, the researchers noted.
However, “these results support the hypothesis that melanoma in young children may be biologically distinct from melanoma in adults,” they said. “Alternatively, melanoma subtype may drive survival differences between children and adolescents.”
No conflicts of interest were reported. The study was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance.
SOURCE: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
Pediatric melanomas appear to be more progressive in adolescents than in young children, based on data from a retrospective study of 32 cases.
Few young children with melanoma die, despite a greater likelihood of thicker tumors, lymph node metastasis, and later diagnosis, which suggests that melanoma in young children may be biologically distinct from melanoma in adolescents, wrote Diana W. Bartenstein, of Harvard University Medical School, Boston, and her colleagues.
Overall, significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01). In addition, children were more likely than adolescents to present with stage 3 or 4 cancer (58% vs. 25%) and with Clark level IV and V tumors (42% vs. 35%), although these differences were not significant. The median Breslow thickness of lesions was greater in children than in adolescents (3.5 mm vs. 1.5 mm) as was the median mitotic index (5 mitotic figures per mm2 vs. 2 mitotic figures per mm2) and children were more likely than adolescents to have neural invasion, but these differences were not significant either.
During the study period of more than 20 years, none of the children younger than 11 years died, compared with four deaths in adolescents, a statistically significant difference (P = .04). The follow-up for surviving individuals ranged from 9-37 months with a median of 44 months.
The study findings were limited by several factors including the small sample size and difficulty in assessing spitzoid tumors, the researchers noted.
However, “these results support the hypothesis that melanoma in young children may be biologically distinct from melanoma in adults,” they said. “Alternatively, melanoma subtype may drive survival differences between children and adolescents.”
No conflicts of interest were reported. The study was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance.
SOURCE: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
FROM PEDIATRIC DERMATOLOGY
Key clinical point:
Major finding: Significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01).
Study details: A retrospective cohort study of 32 children and adolescents with melanoma.
Disclosures: The study was supported by the Alpha Omega Alpha–Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance. No conflicts of interest were reported.
Source: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
Melanoma in US Hispanics: Recommended Strategies to Reduce Disparities in Outcomes
Cutaneous melanoma is a considerable public health concern. In the United States, an estimated 87,110 cases were diagnosed in 2017, and more than 9000 deaths are expected as result of this disease in 2018.1 Early diagnosis of melanoma is associated with favorable survival rates (5-year overall survival rates for melanoma in situ and stage IA melanoma, 99% and 97%, respectively).2 In contrast, the prognosis for advanced-stage melanoma is poor, with a 5-year survival rate of 16% for patients with stage IV disease. Therefore, early detection is critical to reducing mortality in melanoma patients.3
The term Hispanic refers to a panethnic category primarily encompassing Mexican-Americans, Cubans, and Puerto Ricans, as well as individuals from the Caribbean and Central and South America. These populations are diverse in birth origin, primary language, acculturation, distinct ethnic traditions, education level, and occupation. Hispanics in the United States are heterogeneous in many dimensions related to health risks, health care use, and health outcomes.4 Genetic predisposition, lifestyle risks, and access to and use of health care services can shape melanoma diagnosis, treatment, and progression across Hispanic populations differently than in other populations.
In this review, the epidemiology and clinical presentation of melanoma in US Hispanics is summarized, and recommendations for a research agenda to advance understanding of this disease in the most rapidly growing segment of the US population is provided.
In the period from 2008 to 2012, the age-adjusted incidence of melanoma in US Hispanics (4.6 per 100,00 men and 4.2 per 100,00 women) was lower than in NHWs.5 Garnett et al5 reported a decline in melanoma incidence in US Hispanics between 2003 and 2012—an observation that stands in contrast to state-level studies in California and Florida, in which small but substantial increases in melanoma incidence among Hispanics were reported.6,7 The rising incidence of melanomas thicker than 1.5 mm at presentation among Hispanic men living in California is particularly worrisome.6 Discrepancies in incidence trends might reflect changes in incidence over time or differences in state-level registry reporting of melanoma.5
Despite a lower overall incidence of melanoma in US Hispanics, those who do develop the disease are 2.4 times more likely (age-adjusted odds ratio) to present with stage III disease (confidence interval, 1.89-3.05)8 and are 3.64 times more likely to develop distant metastases (confidence interval, 2.65-5.0) than NHWs.3,7,9-13 Disparities also exist in the diagnosis of childhood melanoma: Hispanic children and adolescents who have a diagnosis of melanoma are 3 times more likely to present with advanced disease than NHW counterparts.14 Survival analyses by age and stage show considerably lower survival among Hispanic patients compared to NHW patients with stage I and II disease. In part, worse survival outcomes among Hispanics are the result of the pattern of more advanced disease at presentation.8,14,15
Late presentation for evaluation of melanomas in Hispanics has been attributed to a number of variables, including a lack of skin cancer awareness and knowledge,9,16 a lower rate of self- and physician-performed skin examinations,10 differences in tumor biology,9 and socioeconomic forces.7,17
In a previous study investigating the relationship between neighborhood characteristics and tumor stage at melanoma diagnosis in Hispanic men in California, Texas, and Florida, several key findings emerged.17 First, residency in a census tract with a high density of immigrants (California, Texas) and a high composition of Hispanics (California, Florida) was an important predictor of a late-stage melanoma diagnosis in fully adjusted models. Additionally, the strength of association between measures of socioeconomic status (ie, poverty and education) and tumor stage at melanoma diagnosis was attenuated in multivariate models when enclaves and availability of primary care resources were taken into account. Hispanic melanoma cases in areas with a low density of primary care physicians had an increased likelihood of late-stage diagnosis in California and Texas. The probability of late-stage diagnosis was concentrated in specific regions along the United States–Mexico border, in south central California, and along the southeastern coast of Florida. Lastly, in Texas, Hispanic men aged 18 to 34 years and 35 to 49 years were at an increased risk of late-stage melanoma diagnosis compared to men 65 years and older.17
Demographic and Clinical Characteristics of Melanoma in Hispanic Patients
Among Hispanics, white Hispanics comprise the majority of melanoma cases.5 Median age at diagnosis is younger in Hispanics compared to whites.5,6 Hispanic men typically are older (median age, 61 years) than Hispanic women (median age, 52 years) at diagnosis.5 Similar to what is seen in NHWs, young Hispanic women experience a higher melanoma incidence than young Hispanic men.5 Among older Hispanics, melanoma is more common in men.5,8
Melanomas located on the lower extremities and hips are more prevalent in Hispanics than in NHWs.5,8,18 Among Hispanics, there are age- and sex-based variations in the anatomic location of primary tumors: in Hispanic men, truncal tumors predominate, and in Hispanic women, tumors of the lower extremities are most common across all age groups.5 The incidence of melanomas located in the head and neck region increases with age for both Hispanic men and women.
For melanomas in which the histologic type is known, superficial spreading melanoma is the most common subtype among Hispanics.5,17,19 Acral lentiginous melanomas and nodular melanomas are more common among Hispanics than among NHWs.5,17,19
The observation that Hispanics with melanoma are more prone to lower-extremity tumors and nodular and acral lentiginous melanoma subtypes than NHWs suggests that UV exposure history may be of less importance in this population. Although numerous studies have explored melanoma risk factors in NHWs, there is a striking paucity of such studies in Hispanics. For example, there are conflicting data regarding the role of UV exposure in melanoma risk among Hispanics. Hu et al20 found that UV index and latitude correlated with melanoma risk in this population, whereas Eide et al21 found no association between UV exposure and melanoma incidence in Hispanics. A prospective study involving a multiethnic cohort (of whom 40 of the 107 participants were Hispanic) found no clear association between a history of sunburn and melanoma risk in Hispanics.18
Strategies for Reducing Disparities in Outcomes
Our knowledge of melanoma epidemiology in Hispanics derives mainly from secondary analyses of state-level and national cancer registry data sets.5-8,13-15,17,19,20 These administrative data sources often are limited by missing data (eg, tumor thickness, histologic subtype) or lack important patient-level information (eg, self-identified race and ethnicity, health insurance status). Additionally, the manner in which data are collected and integrated into research varies; for example, socioeconomic measures often are reported as either area-based or composite measures.
The host phenotypic characteristics of melanoma in NHWs are well understood, but the biological and environmental determinants of melanoma risk in Hispanics and other minorities are unknown. For example, fair complexion, red hair, blue eyes, increased freckling density, and the presence of numerous dysplastic and common melanocytic nevi indicate a propensity toward cutaneous melanoma.23,24 However, the relevance of such risk factors in Hispanics is unknown and has not been widely investigated in this patient population. Park et al18 found that a person’s sunburn susceptibility phenotype (defined as hair and eye color, ability to tan, and skin reaction to sunlight) was associated with an increased risk of melanoma among nonwhite, multiracial individuals. However, this study was limited by a small number of minority cases, which included only 40 Hispanic participants with melanoma.18 There is a need for rigorous observational studies to clearly define the phenotypic characteristics, sun-exposure behavior patterns, and genetic contributors to melanoma genesis in Hispanics.
The biologic determinants of postdiagnosis survival in Hispanics with melanoma are not well understood. It is unknown if genetic predisposition modifies melanoma risk in Hispanics. For example, the frequency of BRAF gene mutation or other driver mutations in US Hispanics has been understudied. It is important to know if mutation frequency patterns differ in Hispanics patients compared to NHWs because this knowledge could have considerable implications for treatment. Several recommendations should be considered to address these knowledge gaps. First, there is a need for development or enhancement of melanoma biorepositories, which should include tumor and nontumor specimens from a diverse sample of melanoma patients.
Conclusion
- American Cancer Society. Key statistics for melanoma skin cancer. www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html. Accessed January 13, 2018.
- Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? Cancer. 2012;118:5395-5402.
- Bergad LW, Klein HS. Hispanics in the United States: A Demographic, Social, and Economic History, 1980-2005. New York, NY: Cambridge University Press; 2010.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Pollitt RA, Clarke CA, Swetter SM, et al. The expanding melanoma burden in California Hispanics: importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer. 2011;117:152-161.
- Hu S, Parmet, Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites,Hispanics, and blacks in Florida. JAMA Dermatology. 2010;145:1369-1374.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Pollitt RA, Swetter SM, Johnson TM, et al. Examining the pathways linking lower socioeconomic status and advanced melanoma. Cancer. 2012;118:4004-4013.
- Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit. 2005;11:RA163-RA172.
- Singh SD, Ajani UA, Johnson CJ, et al. Association of cutaneous melanoma incidence with area-based socioeconomic indicators-United States, 2004-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S58-S68.
- Pollitt RA, Clarke CA, Shema SJ, et al. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7-13.
- Clairwood M, Ricketts J, Grant-Kels J, et al. Melanoma in skin of color in Connecticut: an analysis of melanoma incidence and stage at diagnosis in non-Hispanic blacks, non-Hispanic whites, and Hispanics. Int J Dermatol. 2014;53:425-433.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Harvey VM, Enos CW, Chen JT, et al. The role of neighborhood characteristics in late stage melanoma diagnosis among Hispanic men in California, Texas, and Florida, 1996-2012 [published online June 18, 2017]. J Cancer Epidemiol. 2017;2017:8418904.
- Park SL, Le Marchand L, Wilkens LR, et al. Risk factors for malignant melanoma in white and non-white/non-African American populations: the multiethnic cohort. Cancer Prev Res. 2012;5:423-434.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
- Hu S, Ma F, Collado-Mesa F, et al. UV radiation, latitude, and melanoma in US Hispanics and blacks. Arch Dermatol. 2004;140:819-824.
- Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations—US Surveillance, Epidemiology, and End Results (SEER) program, 1992 to 2001. Arch Dermatol. 2005;141:477-481.
- Polite BN, Adams-Campbell LL, Brawley OW, et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. Cancer Res. 2017;77:4548-4555.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040-2059.
- Chang YM, Newton-Bishop JA, Bishop DT, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int J Cancer. 2009;124:420-428.
- Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2014;25:309-319.
- Rapkin BD, Weiss E, Lounsbury D, et al. Reducing disparities in cancer screening and prevention through community-based participatory research partnerships with local libraries: a comprehensive dynamic trial. Am J Community Psychol. 2017;60:145-159.
Cutaneous melanoma is a considerable public health concern. In the United States, an estimated 87,110 cases were diagnosed in 2017, and more than 9000 deaths are expected as result of this disease in 2018.1 Early diagnosis of melanoma is associated with favorable survival rates (5-year overall survival rates for melanoma in situ and stage IA melanoma, 99% and 97%, respectively).2 In contrast, the prognosis for advanced-stage melanoma is poor, with a 5-year survival rate of 16% for patients with stage IV disease. Therefore, early detection is critical to reducing mortality in melanoma patients.3
The term Hispanic refers to a panethnic category primarily encompassing Mexican-Americans, Cubans, and Puerto Ricans, as well as individuals from the Caribbean and Central and South America. These populations are diverse in birth origin, primary language, acculturation, distinct ethnic traditions, education level, and occupation. Hispanics in the United States are heterogeneous in many dimensions related to health risks, health care use, and health outcomes.4 Genetic predisposition, lifestyle risks, and access to and use of health care services can shape melanoma diagnosis, treatment, and progression across Hispanic populations differently than in other populations.
In this review, the epidemiology and clinical presentation of melanoma in US Hispanics is summarized, and recommendations for a research agenda to advance understanding of this disease in the most rapidly growing segment of the US population is provided.
In the period from 2008 to 2012, the age-adjusted incidence of melanoma in US Hispanics (4.6 per 100,00 men and 4.2 per 100,00 women) was lower than in NHWs.5 Garnett et al5 reported a decline in melanoma incidence in US Hispanics between 2003 and 2012—an observation that stands in contrast to state-level studies in California and Florida, in which small but substantial increases in melanoma incidence among Hispanics were reported.6,7 The rising incidence of melanomas thicker than 1.5 mm at presentation among Hispanic men living in California is particularly worrisome.6 Discrepancies in incidence trends might reflect changes in incidence over time or differences in state-level registry reporting of melanoma.5
Despite a lower overall incidence of melanoma in US Hispanics, those who do develop the disease are 2.4 times more likely (age-adjusted odds ratio) to present with stage III disease (confidence interval, 1.89-3.05)8 and are 3.64 times more likely to develop distant metastases (confidence interval, 2.65-5.0) than NHWs.3,7,9-13 Disparities also exist in the diagnosis of childhood melanoma: Hispanic children and adolescents who have a diagnosis of melanoma are 3 times more likely to present with advanced disease than NHW counterparts.14 Survival analyses by age and stage show considerably lower survival among Hispanic patients compared to NHW patients with stage I and II disease. In part, worse survival outcomes among Hispanics are the result of the pattern of more advanced disease at presentation.8,14,15
Late presentation for evaluation of melanomas in Hispanics has been attributed to a number of variables, including a lack of skin cancer awareness and knowledge,9,16 a lower rate of self- and physician-performed skin examinations,10 differences in tumor biology,9 and socioeconomic forces.7,17
In a previous study investigating the relationship between neighborhood characteristics and tumor stage at melanoma diagnosis in Hispanic men in California, Texas, and Florida, several key findings emerged.17 First, residency in a census tract with a high density of immigrants (California, Texas) and a high composition of Hispanics (California, Florida) was an important predictor of a late-stage melanoma diagnosis in fully adjusted models. Additionally, the strength of association between measures of socioeconomic status (ie, poverty and education) and tumor stage at melanoma diagnosis was attenuated in multivariate models when enclaves and availability of primary care resources were taken into account. Hispanic melanoma cases in areas with a low density of primary care physicians had an increased likelihood of late-stage diagnosis in California and Texas. The probability of late-stage diagnosis was concentrated in specific regions along the United States–Mexico border, in south central California, and along the southeastern coast of Florida. Lastly, in Texas, Hispanic men aged 18 to 34 years and 35 to 49 years were at an increased risk of late-stage melanoma diagnosis compared to men 65 years and older.17
Demographic and Clinical Characteristics of Melanoma in Hispanic Patients
Among Hispanics, white Hispanics comprise the majority of melanoma cases.5 Median age at diagnosis is younger in Hispanics compared to whites.5,6 Hispanic men typically are older (median age, 61 years) than Hispanic women (median age, 52 years) at diagnosis.5 Similar to what is seen in NHWs, young Hispanic women experience a higher melanoma incidence than young Hispanic men.5 Among older Hispanics, melanoma is more common in men.5,8
Melanomas located on the lower extremities and hips are more prevalent in Hispanics than in NHWs.5,8,18 Among Hispanics, there are age- and sex-based variations in the anatomic location of primary tumors: in Hispanic men, truncal tumors predominate, and in Hispanic women, tumors of the lower extremities are most common across all age groups.5 The incidence of melanomas located in the head and neck region increases with age for both Hispanic men and women.
For melanomas in which the histologic type is known, superficial spreading melanoma is the most common subtype among Hispanics.5,17,19 Acral lentiginous melanomas and nodular melanomas are more common among Hispanics than among NHWs.5,17,19
The observation that Hispanics with melanoma are more prone to lower-extremity tumors and nodular and acral lentiginous melanoma subtypes than NHWs suggests that UV exposure history may be of less importance in this population. Although numerous studies have explored melanoma risk factors in NHWs, there is a striking paucity of such studies in Hispanics. For example, there are conflicting data regarding the role of UV exposure in melanoma risk among Hispanics. Hu et al20 found that UV index and latitude correlated with melanoma risk in this population, whereas Eide et al21 found no association between UV exposure and melanoma incidence in Hispanics. A prospective study involving a multiethnic cohort (of whom 40 of the 107 participants were Hispanic) found no clear association between a history of sunburn and melanoma risk in Hispanics.18
Strategies for Reducing Disparities in Outcomes
Our knowledge of melanoma epidemiology in Hispanics derives mainly from secondary analyses of state-level and national cancer registry data sets.5-8,13-15,17,19,20 These administrative data sources often are limited by missing data (eg, tumor thickness, histologic subtype) or lack important patient-level information (eg, self-identified race and ethnicity, health insurance status). Additionally, the manner in which data are collected and integrated into research varies; for example, socioeconomic measures often are reported as either area-based or composite measures.
The host phenotypic characteristics of melanoma in NHWs are well understood, but the biological and environmental determinants of melanoma risk in Hispanics and other minorities are unknown. For example, fair complexion, red hair, blue eyes, increased freckling density, and the presence of numerous dysplastic and common melanocytic nevi indicate a propensity toward cutaneous melanoma.23,24 However, the relevance of such risk factors in Hispanics is unknown and has not been widely investigated in this patient population. Park et al18 found that a person’s sunburn susceptibility phenotype (defined as hair and eye color, ability to tan, and skin reaction to sunlight) was associated with an increased risk of melanoma among nonwhite, multiracial individuals. However, this study was limited by a small number of minority cases, which included only 40 Hispanic participants with melanoma.18 There is a need for rigorous observational studies to clearly define the phenotypic characteristics, sun-exposure behavior patterns, and genetic contributors to melanoma genesis in Hispanics.
The biologic determinants of postdiagnosis survival in Hispanics with melanoma are not well understood. It is unknown if genetic predisposition modifies melanoma risk in Hispanics. For example, the frequency of BRAF gene mutation or other driver mutations in US Hispanics has been understudied. It is important to know if mutation frequency patterns differ in Hispanics patients compared to NHWs because this knowledge could have considerable implications for treatment. Several recommendations should be considered to address these knowledge gaps. First, there is a need for development or enhancement of melanoma biorepositories, which should include tumor and nontumor specimens from a diverse sample of melanoma patients.
Conclusion
Cutaneous melanoma is a considerable public health concern. In the United States, an estimated 87,110 cases were diagnosed in 2017, and more than 9000 deaths are expected as result of this disease in 2018.1 Early diagnosis of melanoma is associated with favorable survival rates (5-year overall survival rates for melanoma in situ and stage IA melanoma, 99% and 97%, respectively).2 In contrast, the prognosis for advanced-stage melanoma is poor, with a 5-year survival rate of 16% for patients with stage IV disease. Therefore, early detection is critical to reducing mortality in melanoma patients.3
The term Hispanic refers to a panethnic category primarily encompassing Mexican-Americans, Cubans, and Puerto Ricans, as well as individuals from the Caribbean and Central and South America. These populations are diverse in birth origin, primary language, acculturation, distinct ethnic traditions, education level, and occupation. Hispanics in the United States are heterogeneous in many dimensions related to health risks, health care use, and health outcomes.4 Genetic predisposition, lifestyle risks, and access to and use of health care services can shape melanoma diagnosis, treatment, and progression across Hispanic populations differently than in other populations.
In this review, the epidemiology and clinical presentation of melanoma in US Hispanics is summarized, and recommendations for a research agenda to advance understanding of this disease in the most rapidly growing segment of the US population is provided.
In the period from 2008 to 2012, the age-adjusted incidence of melanoma in US Hispanics (4.6 per 100,00 men and 4.2 per 100,00 women) was lower than in NHWs.5 Garnett et al5 reported a decline in melanoma incidence in US Hispanics between 2003 and 2012—an observation that stands in contrast to state-level studies in California and Florida, in which small but substantial increases in melanoma incidence among Hispanics were reported.6,7 The rising incidence of melanomas thicker than 1.5 mm at presentation among Hispanic men living in California is particularly worrisome.6 Discrepancies in incidence trends might reflect changes in incidence over time or differences in state-level registry reporting of melanoma.5
Despite a lower overall incidence of melanoma in US Hispanics, those who do develop the disease are 2.4 times more likely (age-adjusted odds ratio) to present with stage III disease (confidence interval, 1.89-3.05)8 and are 3.64 times more likely to develop distant metastases (confidence interval, 2.65-5.0) than NHWs.3,7,9-13 Disparities also exist in the diagnosis of childhood melanoma: Hispanic children and adolescents who have a diagnosis of melanoma are 3 times more likely to present with advanced disease than NHW counterparts.14 Survival analyses by age and stage show considerably lower survival among Hispanic patients compared to NHW patients with stage I and II disease. In part, worse survival outcomes among Hispanics are the result of the pattern of more advanced disease at presentation.8,14,15
Late presentation for evaluation of melanomas in Hispanics has been attributed to a number of variables, including a lack of skin cancer awareness and knowledge,9,16 a lower rate of self- and physician-performed skin examinations,10 differences in tumor biology,9 and socioeconomic forces.7,17
In a previous study investigating the relationship between neighborhood characteristics and tumor stage at melanoma diagnosis in Hispanic men in California, Texas, and Florida, several key findings emerged.17 First, residency in a census tract with a high density of immigrants (California, Texas) and a high composition of Hispanics (California, Florida) was an important predictor of a late-stage melanoma diagnosis in fully adjusted models. Additionally, the strength of association between measures of socioeconomic status (ie, poverty and education) and tumor stage at melanoma diagnosis was attenuated in multivariate models when enclaves and availability of primary care resources were taken into account. Hispanic melanoma cases in areas with a low density of primary care physicians had an increased likelihood of late-stage diagnosis in California and Texas. The probability of late-stage diagnosis was concentrated in specific regions along the United States–Mexico border, in south central California, and along the southeastern coast of Florida. Lastly, in Texas, Hispanic men aged 18 to 34 years and 35 to 49 years were at an increased risk of late-stage melanoma diagnosis compared to men 65 years and older.17
Demographic and Clinical Characteristics of Melanoma in Hispanic Patients
Among Hispanics, white Hispanics comprise the majority of melanoma cases.5 Median age at diagnosis is younger in Hispanics compared to whites.5,6 Hispanic men typically are older (median age, 61 years) than Hispanic women (median age, 52 years) at diagnosis.5 Similar to what is seen in NHWs, young Hispanic women experience a higher melanoma incidence than young Hispanic men.5 Among older Hispanics, melanoma is more common in men.5,8
Melanomas located on the lower extremities and hips are more prevalent in Hispanics than in NHWs.5,8,18 Among Hispanics, there are age- and sex-based variations in the anatomic location of primary tumors: in Hispanic men, truncal tumors predominate, and in Hispanic women, tumors of the lower extremities are most common across all age groups.5 The incidence of melanomas located in the head and neck region increases with age for both Hispanic men and women.
For melanomas in which the histologic type is known, superficial spreading melanoma is the most common subtype among Hispanics.5,17,19 Acral lentiginous melanomas and nodular melanomas are more common among Hispanics than among NHWs.5,17,19
The observation that Hispanics with melanoma are more prone to lower-extremity tumors and nodular and acral lentiginous melanoma subtypes than NHWs suggests that UV exposure history may be of less importance in this population. Although numerous studies have explored melanoma risk factors in NHWs, there is a striking paucity of such studies in Hispanics. For example, there are conflicting data regarding the role of UV exposure in melanoma risk among Hispanics. Hu et al20 found that UV index and latitude correlated with melanoma risk in this population, whereas Eide et al21 found no association between UV exposure and melanoma incidence in Hispanics. A prospective study involving a multiethnic cohort (of whom 40 of the 107 participants were Hispanic) found no clear association between a history of sunburn and melanoma risk in Hispanics.18
Strategies for Reducing Disparities in Outcomes
Our knowledge of melanoma epidemiology in Hispanics derives mainly from secondary analyses of state-level and national cancer registry data sets.5-8,13-15,17,19,20 These administrative data sources often are limited by missing data (eg, tumor thickness, histologic subtype) or lack important patient-level information (eg, self-identified race and ethnicity, health insurance status). Additionally, the manner in which data are collected and integrated into research varies; for example, socioeconomic measures often are reported as either area-based or composite measures.
The host phenotypic characteristics of melanoma in NHWs are well understood, but the biological and environmental determinants of melanoma risk in Hispanics and other minorities are unknown. For example, fair complexion, red hair, blue eyes, increased freckling density, and the presence of numerous dysplastic and common melanocytic nevi indicate a propensity toward cutaneous melanoma.23,24 However, the relevance of such risk factors in Hispanics is unknown and has not been widely investigated in this patient population. Park et al18 found that a person’s sunburn susceptibility phenotype (defined as hair and eye color, ability to tan, and skin reaction to sunlight) was associated with an increased risk of melanoma among nonwhite, multiracial individuals. However, this study was limited by a small number of minority cases, which included only 40 Hispanic participants with melanoma.18 There is a need for rigorous observational studies to clearly define the phenotypic characteristics, sun-exposure behavior patterns, and genetic contributors to melanoma genesis in Hispanics.
The biologic determinants of postdiagnosis survival in Hispanics with melanoma are not well understood. It is unknown if genetic predisposition modifies melanoma risk in Hispanics. For example, the frequency of BRAF gene mutation or other driver mutations in US Hispanics has been understudied. It is important to know if mutation frequency patterns differ in Hispanics patients compared to NHWs because this knowledge could have considerable implications for treatment. Several recommendations should be considered to address these knowledge gaps. First, there is a need for development or enhancement of melanoma biorepositories, which should include tumor and nontumor specimens from a diverse sample of melanoma patients.
Conclusion
- American Cancer Society. Key statistics for melanoma skin cancer. www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html. Accessed January 13, 2018.
- Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? Cancer. 2012;118:5395-5402.
- Bergad LW, Klein HS. Hispanics in the United States: A Demographic, Social, and Economic History, 1980-2005. New York, NY: Cambridge University Press; 2010.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Pollitt RA, Clarke CA, Swetter SM, et al. The expanding melanoma burden in California Hispanics: importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer. 2011;117:152-161.
- Hu S, Parmet, Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites,Hispanics, and blacks in Florida. JAMA Dermatology. 2010;145:1369-1374.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Pollitt RA, Swetter SM, Johnson TM, et al. Examining the pathways linking lower socioeconomic status and advanced melanoma. Cancer. 2012;118:4004-4013.
- Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit. 2005;11:RA163-RA172.
- Singh SD, Ajani UA, Johnson CJ, et al. Association of cutaneous melanoma incidence with area-based socioeconomic indicators-United States, 2004-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S58-S68.
- Pollitt RA, Clarke CA, Shema SJ, et al. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7-13.
- Clairwood M, Ricketts J, Grant-Kels J, et al. Melanoma in skin of color in Connecticut: an analysis of melanoma incidence and stage at diagnosis in non-Hispanic blacks, non-Hispanic whites, and Hispanics. Int J Dermatol. 2014;53:425-433.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Harvey VM, Enos CW, Chen JT, et al. The role of neighborhood characteristics in late stage melanoma diagnosis among Hispanic men in California, Texas, and Florida, 1996-2012 [published online June 18, 2017]. J Cancer Epidemiol. 2017;2017:8418904.
- Park SL, Le Marchand L, Wilkens LR, et al. Risk factors for malignant melanoma in white and non-white/non-African American populations: the multiethnic cohort. Cancer Prev Res. 2012;5:423-434.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
- Hu S, Ma F, Collado-Mesa F, et al. UV radiation, latitude, and melanoma in US Hispanics and blacks. Arch Dermatol. 2004;140:819-824.
- Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations—US Surveillance, Epidemiology, and End Results (SEER) program, 1992 to 2001. Arch Dermatol. 2005;141:477-481.
- Polite BN, Adams-Campbell LL, Brawley OW, et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. Cancer Res. 2017;77:4548-4555.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040-2059.
- Chang YM, Newton-Bishop JA, Bishop DT, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int J Cancer. 2009;124:420-428.
- Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2014;25:309-319.
- Rapkin BD, Weiss E, Lounsbury D, et al. Reducing disparities in cancer screening and prevention through community-based participatory research partnerships with local libraries: a comprehensive dynamic trial. Am J Community Psychol. 2017;60:145-159.
- American Cancer Society. Key statistics for melanoma skin cancer. www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html. Accessed January 13, 2018.
- Balch CM, Gershenwald JE, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? Cancer. 2012;118:5395-5402.
- Bergad LW, Klein HS. Hispanics in the United States: A Demographic, Social, and Economic History, 1980-2005. New York, NY: Cambridge University Press; 2010.
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Pollitt RA, Clarke CA, Swetter SM, et al. The expanding melanoma burden in California Hispanics: importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer. 2011;117:152-161.
- Hu S, Parmet, Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites,Hispanics, and blacks in Florida. JAMA Dermatology. 2010;145:1369-1374.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Pollitt RA, Swetter SM, Johnson TM, et al. Examining the pathways linking lower socioeconomic status and advanced melanoma. Cancer. 2012;118:4004-4013.
- Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit. 2005;11:RA163-RA172.
- Singh SD, Ajani UA, Johnson CJ, et al. Association of cutaneous melanoma incidence with area-based socioeconomic indicators-United States, 2004-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S58-S68.
- Pollitt RA, Clarke CA, Shema SJ, et al. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7-13.
- Clairwood M, Ricketts J, Grant-Kels J, et al. Melanoma in skin of color in Connecticut: an analysis of melanoma incidence and stage at diagnosis in non-Hispanic blacks, non-Hispanic whites, and Hispanics. Int J Dermatol. 2014;53:425-433.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Harvey VM, Enos CW, Chen JT, et al. The role of neighborhood characteristics in late stage melanoma diagnosis among Hispanic men in California, Texas, and Florida, 1996-2012 [published online June 18, 2017]. J Cancer Epidemiol. 2017;2017:8418904.
- Park SL, Le Marchand L, Wilkens LR, et al. Risk factors for malignant melanoma in white and non-white/non-African American populations: the multiethnic cohort. Cancer Prev Res. 2012;5:423-434.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
- Hu S, Ma F, Collado-Mesa F, et al. UV radiation, latitude, and melanoma in US Hispanics and blacks. Arch Dermatol. 2004;140:819-824.
- Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations—US Surveillance, Epidemiology, and End Results (SEER) program, 1992 to 2001. Arch Dermatol. 2005;141:477-481.
- Polite BN, Adams-Campbell LL, Brawley OW, et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. Cancer Res. 2017;77:4548-4555.
- Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040-2059.
- Chang YM, Newton-Bishop JA, Bishop DT, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int J Cancer. 2009;124:420-428.
- Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2014;25:309-319.
- Rapkin BD, Weiss E, Lounsbury D, et al. Reducing disparities in cancer screening and prevention through community-based participatory research partnerships with local libraries: a comprehensive dynamic trial. Am J Community Psychol. 2017;60:145-159.
Practice Points
- Although the age-adjusted incidence of melanoma among US Hispanics is lower than among non-Hispanic whites, Hispanics with melanoma are more likely to present with stage III disease and have distant metastases.
- Late presentation of melanoma in Hispanics is not completely understood but may be attributed to socioeconomic factors, lack of skin cancer awareness and knowledge, lower rate of self- and physician-performed skin examinations, and differences in tumor biology, among other variables.
- Research is needed to address gaps in knowledge about the risk of melanoma and comparatively poor outcomes among Hispanics so interventional efforts for prevention, early detection, and treatment can be implemented.
U.S. adolescent malignant melanoma nearly halved during 2000-2014
SAN DIEGO – based on information from a National Cancer Institute database.
The substantial drop in new cases of malignant melanoma in Americans aged 10-19 years over the most recent 15-year period with data available contrasts with a stable rate among children aged 0-9 years, and a steadily rising rate among adults during the same period, Ryan C. Kelm said at the annual meeting of the American Academy of Dermatology.
Mr. Kelm and his associates studied U.S. data compiled from 2000 to 2014 by the SEER Program, maintained by the National Cancer Institute. They identified 1,796 patients aged 0-19 years diagnosed with malignant melanoma (218 children and 1,578 adolescents). The overall incidence rate for the entire 15-year period was just over 1 case per million among children and just under 9 cases per million among adolescents. In contrast, the adult U.S. incidence rate estimates for 2018 are pegged at 260 per million among non-Hispanic whites, 40 per million among Hispanics, and 10 per million among black Americans, according to the American Cancer Society.
An additional analysis showed a notable difference in incidence rates over the 15-year period studied, depending on age. In children aged 0-9 years, the annual incidence rate held roughly steady at just under 2 cases per million throughout the 15 years. But among adolescents, the rate fell over time, from about 10-12 cases per million during 2000-2004 to about 5-7 cases per million during 2010-2014. In 2001, the rate was about 11 cases per million, and in 2013, the rate was about 6 cases per million. This contrasts with the adult rate, which has “risen rapidly over the past 30 years,” according to the American Cancer Society’s 2018 report.
The SEER data also showed that distribution of melanoma histologic types differed by age. Among adolescents the most common identified form was “superficial spreading,” in 32%, with nodular in 6%, mixed epithelioid and spindle cell in 2%, and “not otherwise specified” in 54%. In children, the most commonly identified form was mixed epithelioid and spindle cell, in 10%, followed by nodular in 9%, and superficial spreading in 9%, with 63% not otherwise specified.
SOURCE: Kelm RC et al. AAD 18, Abstract 6722.
SAN DIEGO – based on information from a National Cancer Institute database.
The substantial drop in new cases of malignant melanoma in Americans aged 10-19 years over the most recent 15-year period with data available contrasts with a stable rate among children aged 0-9 years, and a steadily rising rate among adults during the same period, Ryan C. Kelm said at the annual meeting of the American Academy of Dermatology.
Mr. Kelm and his associates studied U.S. data compiled from 2000 to 2014 by the SEER Program, maintained by the National Cancer Institute. They identified 1,796 patients aged 0-19 years diagnosed with malignant melanoma (218 children and 1,578 adolescents). The overall incidence rate for the entire 15-year period was just over 1 case per million among children and just under 9 cases per million among adolescents. In contrast, the adult U.S. incidence rate estimates for 2018 are pegged at 260 per million among non-Hispanic whites, 40 per million among Hispanics, and 10 per million among black Americans, according to the American Cancer Society.
An additional analysis showed a notable difference in incidence rates over the 15-year period studied, depending on age. In children aged 0-9 years, the annual incidence rate held roughly steady at just under 2 cases per million throughout the 15 years. But among adolescents, the rate fell over time, from about 10-12 cases per million during 2000-2004 to about 5-7 cases per million during 2010-2014. In 2001, the rate was about 11 cases per million, and in 2013, the rate was about 6 cases per million. This contrasts with the adult rate, which has “risen rapidly over the past 30 years,” according to the American Cancer Society’s 2018 report.
The SEER data also showed that distribution of melanoma histologic types differed by age. Among adolescents the most common identified form was “superficial spreading,” in 32%, with nodular in 6%, mixed epithelioid and spindle cell in 2%, and “not otherwise specified” in 54%. In children, the most commonly identified form was mixed epithelioid and spindle cell, in 10%, followed by nodular in 9%, and superficial spreading in 9%, with 63% not otherwise specified.
SOURCE: Kelm RC et al. AAD 18, Abstract 6722.
SAN DIEGO – based on information from a National Cancer Institute database.
The substantial drop in new cases of malignant melanoma in Americans aged 10-19 years over the most recent 15-year period with data available contrasts with a stable rate among children aged 0-9 years, and a steadily rising rate among adults during the same period, Ryan C. Kelm said at the annual meeting of the American Academy of Dermatology.
Mr. Kelm and his associates studied U.S. data compiled from 2000 to 2014 by the SEER Program, maintained by the National Cancer Institute. They identified 1,796 patients aged 0-19 years diagnosed with malignant melanoma (218 children and 1,578 adolescents). The overall incidence rate for the entire 15-year period was just over 1 case per million among children and just under 9 cases per million among adolescents. In contrast, the adult U.S. incidence rate estimates for 2018 are pegged at 260 per million among non-Hispanic whites, 40 per million among Hispanics, and 10 per million among black Americans, according to the American Cancer Society.
An additional analysis showed a notable difference in incidence rates over the 15-year period studied, depending on age. In children aged 0-9 years, the annual incidence rate held roughly steady at just under 2 cases per million throughout the 15 years. But among adolescents, the rate fell over time, from about 10-12 cases per million during 2000-2004 to about 5-7 cases per million during 2010-2014. In 2001, the rate was about 11 cases per million, and in 2013, the rate was about 6 cases per million. This contrasts with the adult rate, which has “risen rapidly over the past 30 years,” according to the American Cancer Society’s 2018 report.
The SEER data also showed that distribution of melanoma histologic types differed by age. Among adolescents the most common identified form was “superficial spreading,” in 32%, with nodular in 6%, mixed epithelioid and spindle cell in 2%, and “not otherwise specified” in 54%. In children, the most commonly identified form was mixed epithelioid and spindle cell, in 10%, followed by nodular in 9%, and superficial spreading in 9%, with 63% not otherwise specified.
SOURCE: Kelm RC et al. AAD 18, Abstract 6722.
REPORTING FROM AAD 18
Key clinical point: U.S. incident malignant melanoma in adolescents dropped by nearly 50% during 2000-2014.
Major finding: Malignant melanoma occurred in about 11 adolescents per million in 2001 and about 6 per million in 2013.
Study details: Review of data collected in the SEER database of the National Cancer Institute.
Disclosures: Mr. Kelm had no disclosures.
Source: Kelm RC et al. AAD 18, Abstract 6722.
Diffuse Cutaneous Breast Cancer Metastases Resembling Subcutaneous Nodules With No Surface Changes
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.
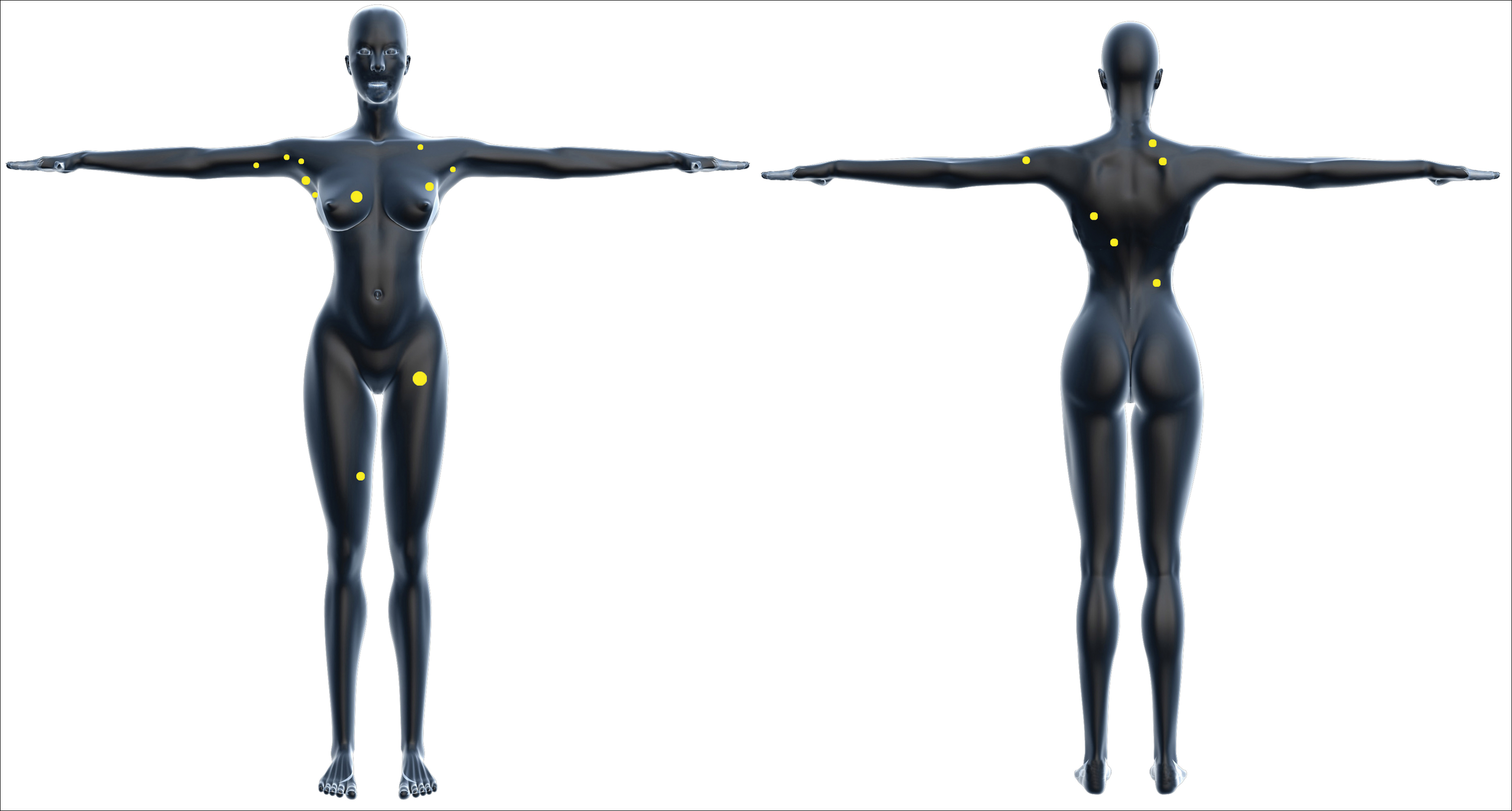
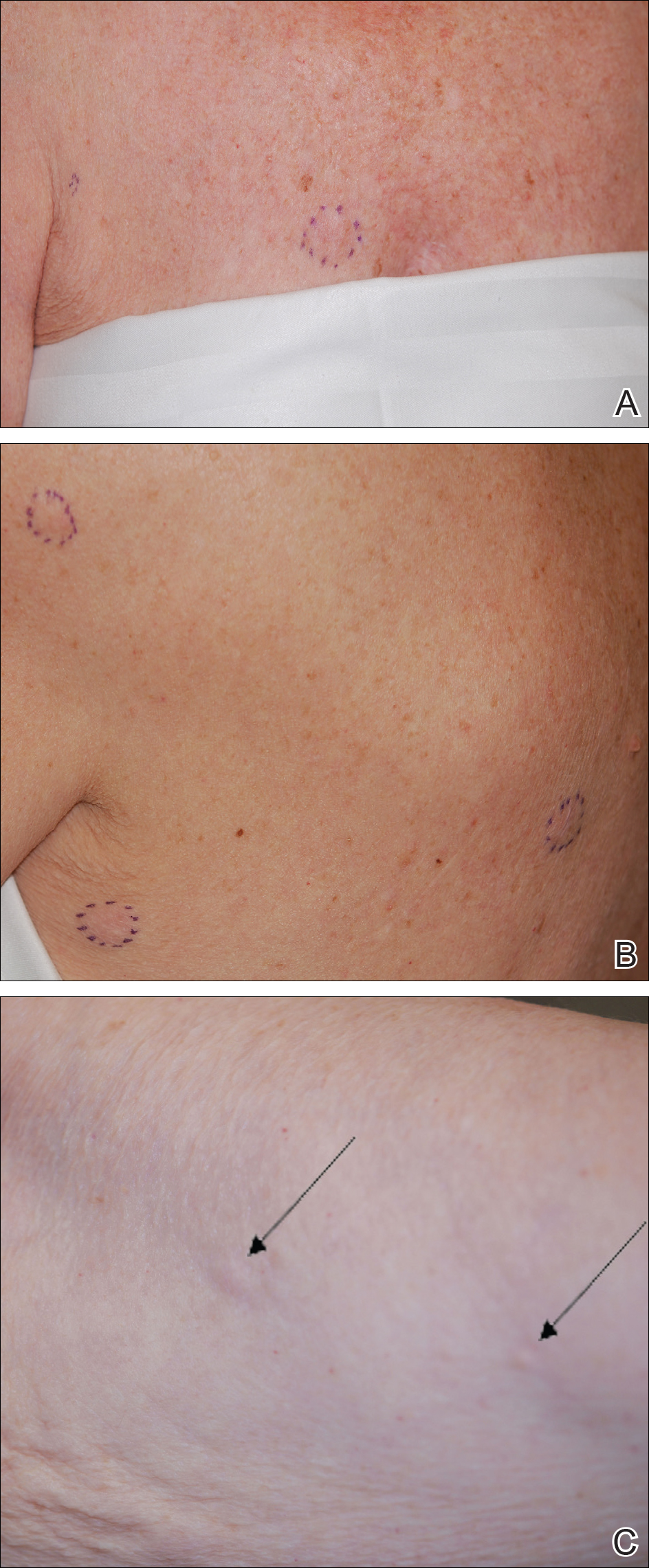
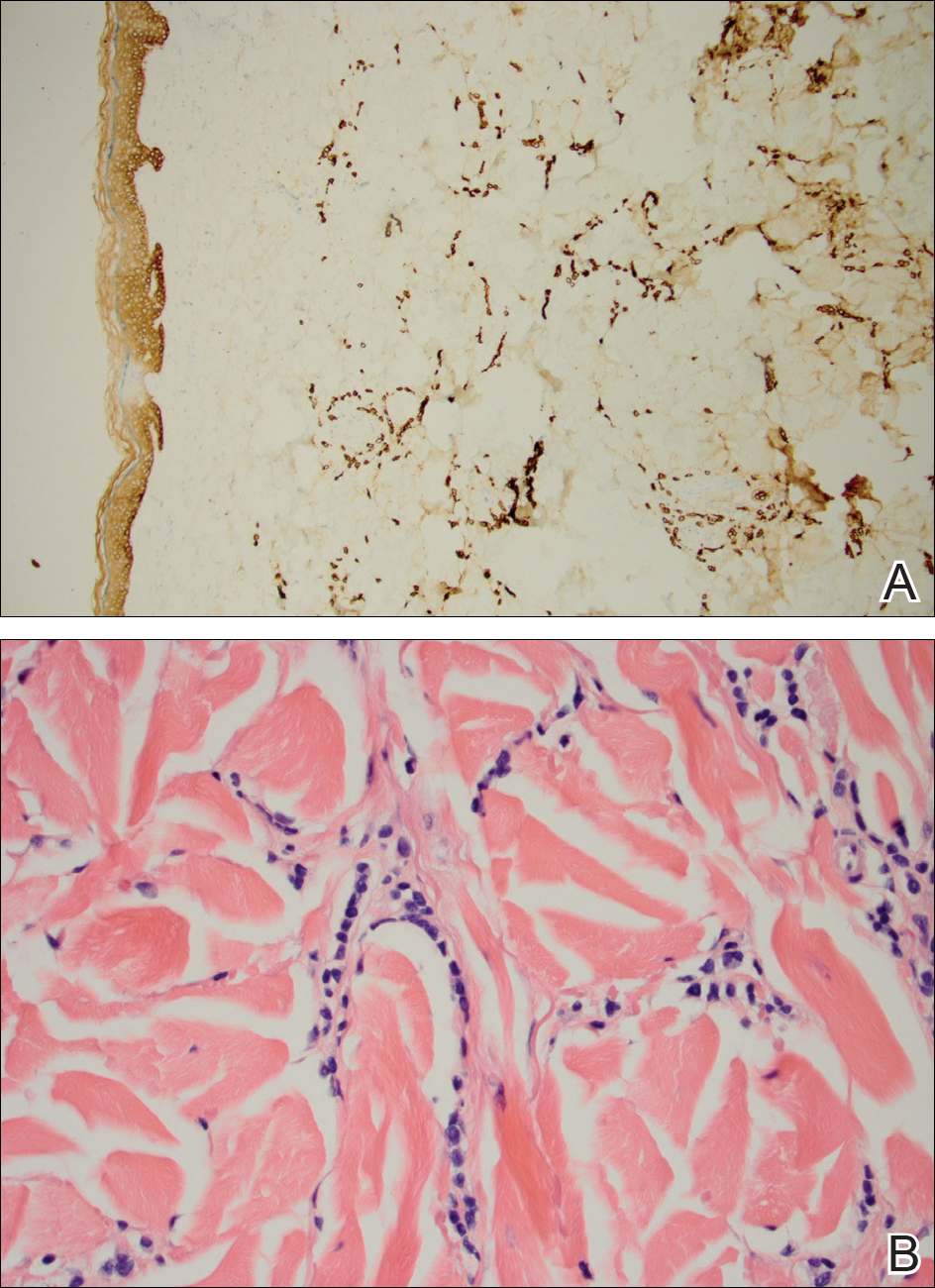
After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.
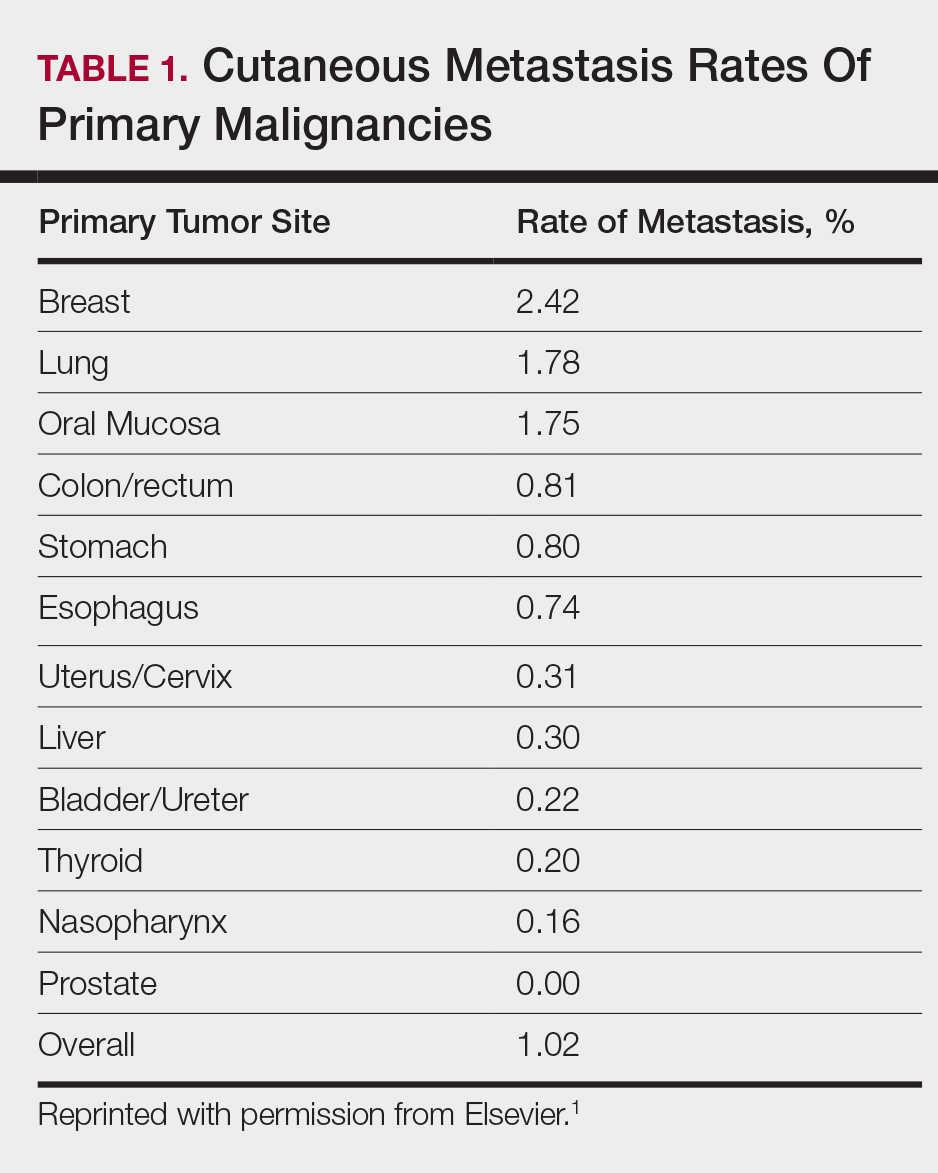
The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.
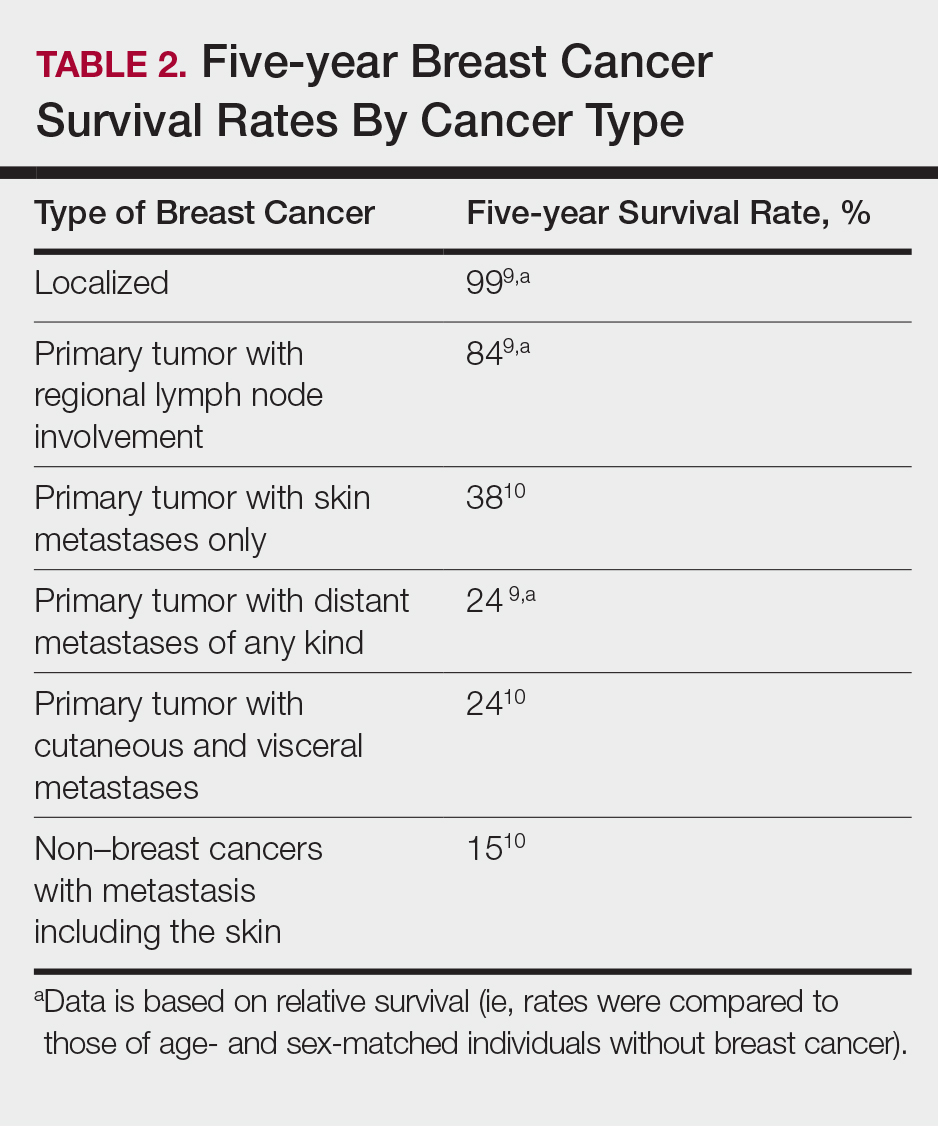
Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.



After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.

The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.

Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.



After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.

The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.

Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
Practice Points
- Although breast cancer has the highest rate of cutaneous metastasis among internal malignancies, cutaneous metastases occur in only a small minority of breast cancer patients.
- Cutaneous metastases from breast cancer typically do not carry as poor a prognosis as those in other internal malignancies.
- The clinical presentation of cutaneous metastases from breast cancer can be varied. In our patient, the metastases were subtle and resembled subcutaneous nodules lacking surface changes, thus making them best detectable by palpation.
- While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response, the cutaneous response may be poorer due to the skin’s relatively weaker blood supply.
Immunotherapy regimen influences inflammatory arthritis presentation
Variations in the clinical presentation of immunotherapy-induced inflammatory arthritis is partly explained by which treatment regimen was used to treat the cancer, a single-center study suggests.
While immune checkpoint inhibitors (ICI) have revolutionized the field of oncology, their use for an ever-widening range of indications had created an increasing population of patients referred to rheumatologists for the management of immune-related adverse events (IrAEs), according to Laura C. Cappelli, MD, and her colleagues at John Hopkins University, Baltimore.
Well-established guidelines exist for managing adverse events such as colitis and pneumonitis, but there are only preliminary guidelines for evaluating and treating immunotherapy-induced inflammatory arthritis (IA). “This may stem from a lack of consistent reporting of rheumatologic IrAEs in clinical trials, the non–life threatening nature of [inflammatory arthritis], or lack of recognition of musculoskeletal symptoms by treating providers,” they wrote in Seminars in Arthritis and Rheumatism.
Clinical trials have reported ranges of arthralgia in 1%-43% of patients treated with ICIs, but no accurate estimate of the incidence of IA exists.
The researchers noted that treating patients with ICI-induced IA is complicated by a history of active or recently treated cancer and concerns over using immunosuppression in the context of ICI therapy.
They set out to evaluate the clinical presentations of 30 patients seen in their clinic with ICI-induced IA. Patients were a median of 59 years old and 12 (40%) were female. Tumor types included metastatic melanoma, non–small cell lung cancer, small cell lung cancer, colorectal cancer, Hodgkin lymphoma, cutaneous lymphoma, renal cell carcinoma, duodenal carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and cutaneous squamous cell carcinoma.
Sixteen patients were treated with anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 monotherapy, and 14 were treated with combination anti–CTLA-4/PD-1 therapy.
Patients on combination therapy were significantly younger (7.5 years, P = 0.01) and were more likely to have metastatic melanoma as their underlying cancer.
Patients who received combination therapy were more likely to present first with knee IA (n = 10) and none had small joint involvement. In contrast, initial small joint involvement was more common in the monotherapy group (n = 6).
C-reactive protein levels were significantly higher in the combination therapy group (4mg/dL vs. 0.5mg/dL, P = 0.03). Only monotherapy patients were positive for anti–citrullinated peptide antibodies, rheumatoid factor, or antinuclear antibodies.
Most of the patients in the study had an additional IrAE, with colitis being the most common (n=10), followed by thyroid disease, pneumonitis, and rash. Patients on PD-1 or programmed death ligand 1 monotherapy were more likely to have IA as their first IrAE.
The research team noted that the median time to symptom onset was 5 months after ICI initiation.
Diagnosis of IA following patient-reported symptoms was an average of 5.2 months, with a significant difference in lag time to diagnosis depending on initial joint presentation. For example, patients with initial small joint involvement had a 10 month longer lag time to IA diagnosis than those with knees as the initial joint involved.
In terms of treatment, 24 patients were treated with systemic corticosteroids and 10 required additional immunosuppression. The need for corticosteroids did not differ by ICI treatment regimen, but those treated with combination therapy were more likely to require additional immunosuppression (P = 0.02).
Tumor necrosis factor inhibitors with or without methotrexate were prescribed for seven patients. All of the patients had a clinical improvement in their arthritis symptoms. Four had a complete tumor response at the time of tumor necrosis factor inhibitor initiation with none having tumor progression.
The three patients treated with methotrexate monotherapy had a complete or sustained partial tumor response to ICI therapy and their cancer did not develop during IA management follow-up.
The authors went on to look at the persistence of IA after cessation of therapy in a subset of 21 patients. They found that 18 of these patients still had IA symptoms months after stopping treatment. They suggested that the delay in diagnosis and treatment seen in their study might explain the finding.
The study provides “critical information, not just for rheumatologists as they try to recognize subgroups in ICI-induced IA and diagnose patients with this new entity, but also for oncology providers who are usually first to encounter patients with ICI-induced IA and subsequently refer patients to rheumatology,” Dr. Cappelli and colleagues wrote.
The experience so far with using immunosuppression in ICI-induced IA “has been reassuring in terms of cancer outcomes, but more studies are needed to confirm this finding,” they concluded.
SOURCE: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
Variations in the clinical presentation of immunotherapy-induced inflammatory arthritis is partly explained by which treatment regimen was used to treat the cancer, a single-center study suggests.
While immune checkpoint inhibitors (ICI) have revolutionized the field of oncology, their use for an ever-widening range of indications had created an increasing population of patients referred to rheumatologists for the management of immune-related adverse events (IrAEs), according to Laura C. Cappelli, MD, and her colleagues at John Hopkins University, Baltimore.
Well-established guidelines exist for managing adverse events such as colitis and pneumonitis, but there are only preliminary guidelines for evaluating and treating immunotherapy-induced inflammatory arthritis (IA). “This may stem from a lack of consistent reporting of rheumatologic IrAEs in clinical trials, the non–life threatening nature of [inflammatory arthritis], or lack of recognition of musculoskeletal symptoms by treating providers,” they wrote in Seminars in Arthritis and Rheumatism.
Clinical trials have reported ranges of arthralgia in 1%-43% of patients treated with ICIs, but no accurate estimate of the incidence of IA exists.
The researchers noted that treating patients with ICI-induced IA is complicated by a history of active or recently treated cancer and concerns over using immunosuppression in the context of ICI therapy.
They set out to evaluate the clinical presentations of 30 patients seen in their clinic with ICI-induced IA. Patients were a median of 59 years old and 12 (40%) were female. Tumor types included metastatic melanoma, non–small cell lung cancer, small cell lung cancer, colorectal cancer, Hodgkin lymphoma, cutaneous lymphoma, renal cell carcinoma, duodenal carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and cutaneous squamous cell carcinoma.
Sixteen patients were treated with anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 monotherapy, and 14 were treated with combination anti–CTLA-4/PD-1 therapy.
Patients on combination therapy were significantly younger (7.5 years, P = 0.01) and were more likely to have metastatic melanoma as their underlying cancer.
Patients who received combination therapy were more likely to present first with knee IA (n = 10) and none had small joint involvement. In contrast, initial small joint involvement was more common in the monotherapy group (n = 6).
C-reactive protein levels were significantly higher in the combination therapy group (4mg/dL vs. 0.5mg/dL, P = 0.03). Only monotherapy patients were positive for anti–citrullinated peptide antibodies, rheumatoid factor, or antinuclear antibodies.
Most of the patients in the study had an additional IrAE, with colitis being the most common (n=10), followed by thyroid disease, pneumonitis, and rash. Patients on PD-1 or programmed death ligand 1 monotherapy were more likely to have IA as their first IrAE.
The research team noted that the median time to symptom onset was 5 months after ICI initiation.
Diagnosis of IA following patient-reported symptoms was an average of 5.2 months, with a significant difference in lag time to diagnosis depending on initial joint presentation. For example, patients with initial small joint involvement had a 10 month longer lag time to IA diagnosis than those with knees as the initial joint involved.
In terms of treatment, 24 patients were treated with systemic corticosteroids and 10 required additional immunosuppression. The need for corticosteroids did not differ by ICI treatment regimen, but those treated with combination therapy were more likely to require additional immunosuppression (P = 0.02).
Tumor necrosis factor inhibitors with or without methotrexate were prescribed for seven patients. All of the patients had a clinical improvement in their arthritis symptoms. Four had a complete tumor response at the time of tumor necrosis factor inhibitor initiation with none having tumor progression.
The three patients treated with methotrexate monotherapy had a complete or sustained partial tumor response to ICI therapy and their cancer did not develop during IA management follow-up.
The authors went on to look at the persistence of IA after cessation of therapy in a subset of 21 patients. They found that 18 of these patients still had IA symptoms months after stopping treatment. They suggested that the delay in diagnosis and treatment seen in their study might explain the finding.
The study provides “critical information, not just for rheumatologists as they try to recognize subgroups in ICI-induced IA and diagnose patients with this new entity, but also for oncology providers who are usually first to encounter patients with ICI-induced IA and subsequently refer patients to rheumatology,” Dr. Cappelli and colleagues wrote.
The experience so far with using immunosuppression in ICI-induced IA “has been reassuring in terms of cancer outcomes, but more studies are needed to confirm this finding,” they concluded.
SOURCE: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
Variations in the clinical presentation of immunotherapy-induced inflammatory arthritis is partly explained by which treatment regimen was used to treat the cancer, a single-center study suggests.
While immune checkpoint inhibitors (ICI) have revolutionized the field of oncology, their use for an ever-widening range of indications had created an increasing population of patients referred to rheumatologists for the management of immune-related adverse events (IrAEs), according to Laura C. Cappelli, MD, and her colleagues at John Hopkins University, Baltimore.
Well-established guidelines exist for managing adverse events such as colitis and pneumonitis, but there are only preliminary guidelines for evaluating and treating immunotherapy-induced inflammatory arthritis (IA). “This may stem from a lack of consistent reporting of rheumatologic IrAEs in clinical trials, the non–life threatening nature of [inflammatory arthritis], or lack of recognition of musculoskeletal symptoms by treating providers,” they wrote in Seminars in Arthritis and Rheumatism.
Clinical trials have reported ranges of arthralgia in 1%-43% of patients treated with ICIs, but no accurate estimate of the incidence of IA exists.
The researchers noted that treating patients with ICI-induced IA is complicated by a history of active or recently treated cancer and concerns over using immunosuppression in the context of ICI therapy.
They set out to evaluate the clinical presentations of 30 patients seen in their clinic with ICI-induced IA. Patients were a median of 59 years old and 12 (40%) were female. Tumor types included metastatic melanoma, non–small cell lung cancer, small cell lung cancer, colorectal cancer, Hodgkin lymphoma, cutaneous lymphoma, renal cell carcinoma, duodenal carcinoma, Merkel cell carcinoma, cutaneous basal cell carcinoma, and cutaneous squamous cell carcinoma.
Sixteen patients were treated with anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 monotherapy, and 14 were treated with combination anti–CTLA-4/PD-1 therapy.
Patients on combination therapy were significantly younger (7.5 years, P = 0.01) and were more likely to have metastatic melanoma as their underlying cancer.
Patients who received combination therapy were more likely to present first with knee IA (n = 10) and none had small joint involvement. In contrast, initial small joint involvement was more common in the monotherapy group (n = 6).
C-reactive protein levels were significantly higher in the combination therapy group (4mg/dL vs. 0.5mg/dL, P = 0.03). Only monotherapy patients were positive for anti–citrullinated peptide antibodies, rheumatoid factor, or antinuclear antibodies.
Most of the patients in the study had an additional IrAE, with colitis being the most common (n=10), followed by thyroid disease, pneumonitis, and rash. Patients on PD-1 or programmed death ligand 1 monotherapy were more likely to have IA as their first IrAE.
The research team noted that the median time to symptom onset was 5 months after ICI initiation.
Diagnosis of IA following patient-reported symptoms was an average of 5.2 months, with a significant difference in lag time to diagnosis depending on initial joint presentation. For example, patients with initial small joint involvement had a 10 month longer lag time to IA diagnosis than those with knees as the initial joint involved.
In terms of treatment, 24 patients were treated with systemic corticosteroids and 10 required additional immunosuppression. The need for corticosteroids did not differ by ICI treatment regimen, but those treated with combination therapy were more likely to require additional immunosuppression (P = 0.02).
Tumor necrosis factor inhibitors with or without methotrexate were prescribed for seven patients. All of the patients had a clinical improvement in their arthritis symptoms. Four had a complete tumor response at the time of tumor necrosis factor inhibitor initiation with none having tumor progression.
The three patients treated with methotrexate monotherapy had a complete or sustained partial tumor response to ICI therapy and their cancer did not develop during IA management follow-up.
The authors went on to look at the persistence of IA after cessation of therapy in a subset of 21 patients. They found that 18 of these patients still had IA symptoms months after stopping treatment. They suggested that the delay in diagnosis and treatment seen in their study might explain the finding.
The study provides “critical information, not just for rheumatologists as they try to recognize subgroups in ICI-induced IA and diagnose patients with this new entity, but also for oncology providers who are usually first to encounter patients with ICI-induced IA and subsequently refer patients to rheumatology,” Dr. Cappelli and colleagues wrote.
The experience so far with using immunosuppression in ICI-induced IA “has been reassuring in terms of cancer outcomes, but more studies are needed to confirm this finding,” they concluded.
SOURCE: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Key clinical point: The clinical features of patients with immunotherapy-induced inflammatory arthritis differ according to the treatment regimen used.
Major findings: Combination immune checkpoint inhibitor therapy was associated with higher C-reactive protein levels and a higher likelihood of having a large joint affected first.
Study details: A single-center, retrospective cohort study of 30 patients with rheumatologist-confirmed inflammatory arthritis after receiving immune checkpoint inhibitor therapy.
Disclosures: The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Disease and the Jerome L. Greene Foundation.
Source: Cappelli LC et al. Semin Arthritis Rheum. doi: 10.1016/j.semarthrit. 2018.02.011.
When to worry about congenital melanocytic nevi
KAUAI, HAWAII – according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital.
Two or more nevi increase the risk of CNS involvement, which in turn increases the risk of malignant conversion by more than 16-fold.
Among the studies she cited was a 2017 literature review of 448 children with congenital nevi, 10 of whom developed melanoma: It arose in the skin in 2, the brain in 6, and an unknown location in 2. All 10 children were born with two or more nevi, and not all of them had large or giant nevi, which is a known risk factor for malignant conversion (Br J Dermatol. 2017 May;176[5]:1131-43).
“If the scanning brain MRI is normal, [children] might not have congenital melanocytic nevus syndrome, and would be at low risk for melanoma,” Dr. Huang said. “If it’s abnormal, they might be at high risk for melanoma.” In the 2017 study, the odds ratio for melanoma with an abnormal MRI was 16.7 (P = .001).
Both melanocytes and neuronal cells arise from the embryonic neural crest, which explains the link between congenital nevi and brain lesions. Almost all congenital nevi are associated with early postzygotic mutations in the NRAS gene, and it’s possible the mutations affect other neural crest cell lines, including in the CNS, she said.
It’s also important to remember that childhood melanoma often doesn’t follow the ABCDE (asymmetry, border irregularity, color not uniform, diameter greater than 6 mm, and evolving) signs of melanoma common in adults.
In a retrospective study of 70 children with melanoma or ambiguous melanocytic tumors, 40% of pubertal subjects and 60% of prepubertal participants did not meet conventional adult ABCDE criteria. The majority of cases were raised, even in color, less than 6 mm across, symmetric, and de novo (J Am Acad Dermatol. 2013 Jun;68[6]:913-25).
It turns out that rapid evolution in size, shape, and color is the number one, unifying factor in childhood melanomas. Other key clues include raised lesions with uniform color or no pigmentation at all. A modified ABCDE for pediatric melanoma has been proposed: amelanotic, bump/bleeding, color uniform, diameter variable, de novo, and evolution.
“The lesson to learn is not to ignore the traditional ABCDEs of melanoma, but to recognize that pediatric melanoma may present with different clinical characteristics, and to incorporate this awareness into our practice,” Dr. Huang said.
She did not have any disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital.
Two or more nevi increase the risk of CNS involvement, which in turn increases the risk of malignant conversion by more than 16-fold.
Among the studies she cited was a 2017 literature review of 448 children with congenital nevi, 10 of whom developed melanoma: It arose in the skin in 2, the brain in 6, and an unknown location in 2. All 10 children were born with two or more nevi, and not all of them had large or giant nevi, which is a known risk factor for malignant conversion (Br J Dermatol. 2017 May;176[5]:1131-43).
“If the scanning brain MRI is normal, [children] might not have congenital melanocytic nevus syndrome, and would be at low risk for melanoma,” Dr. Huang said. “If it’s abnormal, they might be at high risk for melanoma.” In the 2017 study, the odds ratio for melanoma with an abnormal MRI was 16.7 (P = .001).
Both melanocytes and neuronal cells arise from the embryonic neural crest, which explains the link between congenital nevi and brain lesions. Almost all congenital nevi are associated with early postzygotic mutations in the NRAS gene, and it’s possible the mutations affect other neural crest cell lines, including in the CNS, she said.
It’s also important to remember that childhood melanoma often doesn’t follow the ABCDE (asymmetry, border irregularity, color not uniform, diameter greater than 6 mm, and evolving) signs of melanoma common in adults.
In a retrospective study of 70 children with melanoma or ambiguous melanocytic tumors, 40% of pubertal subjects and 60% of prepubertal participants did not meet conventional adult ABCDE criteria. The majority of cases were raised, even in color, less than 6 mm across, symmetric, and de novo (J Am Acad Dermatol. 2013 Jun;68[6]:913-25).
It turns out that rapid evolution in size, shape, and color is the number one, unifying factor in childhood melanomas. Other key clues include raised lesions with uniform color or no pigmentation at all. A modified ABCDE for pediatric melanoma has been proposed: amelanotic, bump/bleeding, color uniform, diameter variable, de novo, and evolution.
“The lesson to learn is not to ignore the traditional ABCDEs of melanoma, but to recognize that pediatric melanoma may present with different clinical characteristics, and to incorporate this awareness into our practice,” Dr. Huang said.
She did not have any disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – according to Jennifer Huang, MD, a pediatric dermatologist at Boston Children’s Hospital.
Two or more nevi increase the risk of CNS involvement, which in turn increases the risk of malignant conversion by more than 16-fold.
Among the studies she cited was a 2017 literature review of 448 children with congenital nevi, 10 of whom developed melanoma: It arose in the skin in 2, the brain in 6, and an unknown location in 2. All 10 children were born with two or more nevi, and not all of them had large or giant nevi, which is a known risk factor for malignant conversion (Br J Dermatol. 2017 May;176[5]:1131-43).
“If the scanning brain MRI is normal, [children] might not have congenital melanocytic nevus syndrome, and would be at low risk for melanoma,” Dr. Huang said. “If it’s abnormal, they might be at high risk for melanoma.” In the 2017 study, the odds ratio for melanoma with an abnormal MRI was 16.7 (P = .001).
Both melanocytes and neuronal cells arise from the embryonic neural crest, which explains the link between congenital nevi and brain lesions. Almost all congenital nevi are associated with early postzygotic mutations in the NRAS gene, and it’s possible the mutations affect other neural crest cell lines, including in the CNS, she said.
It’s also important to remember that childhood melanoma often doesn’t follow the ABCDE (asymmetry, border irregularity, color not uniform, diameter greater than 6 mm, and evolving) signs of melanoma common in adults.
In a retrospective study of 70 children with melanoma or ambiguous melanocytic tumors, 40% of pubertal subjects and 60% of prepubertal participants did not meet conventional adult ABCDE criteria. The majority of cases were raised, even in color, less than 6 mm across, symmetric, and de novo (J Am Acad Dermatol. 2013 Jun;68[6]:913-25).
It turns out that rapid evolution in size, shape, and color is the number one, unifying factor in childhood melanomas. Other key clues include raised lesions with uniform color or no pigmentation at all. A modified ABCDE for pediatric melanoma has been proposed: amelanotic, bump/bleeding, color uniform, diameter variable, de novo, and evolution.
“The lesson to learn is not to ignore the traditional ABCDEs of melanoma, but to recognize that pediatric melanoma may present with different clinical characteristics, and to incorporate this awareness into our practice,” Dr. Huang said.
She did not have any disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR