User login
Transplant recipients need dermatologists both pre- and post-transplant
Boston – Shared medical appointments can work well for patients undergoing solid organ transplants and for the clinical staff treating them – serving as an efficient way to educate several patients in a common setting and giving them the opportunity to interact with others going through a similar experience.
“Having those shared medical appointments where they’re sitting next to somebody who’s going through the same thought process and information gathering ... and the ability to talk with similar folks is a huge benefit for those patients,” said Allison Vidimos, MD, chair of the department of dermatology at the Cleveland Clinic. Dr. Vidimos and her colleagues have been so focused on skin cancer diagnosis and treatment in this group of patients that “we’ve kind of lost sight of the human aspect of it and the fear that these patients have,” she added.
These shared medical appointments at the Cleveland Clinic also provide the dermatology staff an efficient means to educate a group of patients about the risks of developing skin cancers and benign conditions associated with immunosuppressive treatments. They also discuss sun protection and avoidance practices, and how to perform skin self exams, all of which are “really important for their well being after their transplant,” Dr. Vidimos said during an interview at the American Academy of Dermatology summer meeting.
Patients learn how frequently they will need a clinical skin exam post-transplant, depending on the occurrence of skin cancers, and that biopsies may be necessary at times.
Dr. Vidimos said it is up to the transplant surgeons to discuss with patients the very low risk of transmission of malignancies from the donor to the recipient. In her presentation at the meeting, she cited a 1.4% risk of an undetected skin or internal malignancy in a donor being transmitted to the recipient.
Because of changes in criteria, patients who have relatively low risk skin cancers or higher risk skin cancers and have been treated, and are at “a defined interval post-treatment where we feel it’s safe to do that transplant,” may be considered a transplant candidate, she said noting that previously, such patients would be excluded from a transplant.
The appropriate time intervals to wait for a transplant for candidates with a history of cutaneous squamous cell carcinoma, malignant melanoma, or Merkel cell carcinoma are spelled out in a recently published consensus paper Dr. Vidimos coauthored with other members of the International Transplant Skin Cancer Collaborative (Am J Transplant. 2016 Feb;16[2]:407-13).
Dr. Vidimos said she is sometimes called to the bedside to perform a skin exam in a pretransplant patient who is very ill. The most common scenario is a liver transplant candidate awaiting transport to the operating room, who has not had a skin exam and needs to be cleared by a dermatologist to rule out a melanoma or another type of skin cancer that could be “fertilized” by postoperative immunosuppressant therapy.
After a transplant, patients need to be seen frequently enough to detect malignant transformation of precancerous skin lesions. Dermatologists should have a low threshold for biopsying any suspicious lesions early. “A lot of times we get biopsies back of skin cancer that do not match ... the clinical picture,” Dr. Vidimos said. Patients should be referred when the dermatologist feels that he or she can not deliver the appropriate treatment.
As transplants have become fairly routine and patients are living longer, community dermatologists will most likely be seeing solid organ transplant recipients more frequently. With longer lifespans, those patients will have more opportunity to develop more skin cancers.
Dr. Vidimos disclosed having received grants and research funding from Genentech.
Boston – Shared medical appointments can work well for patients undergoing solid organ transplants and for the clinical staff treating them – serving as an efficient way to educate several patients in a common setting and giving them the opportunity to interact with others going through a similar experience.
“Having those shared medical appointments where they’re sitting next to somebody who’s going through the same thought process and information gathering ... and the ability to talk with similar folks is a huge benefit for those patients,” said Allison Vidimos, MD, chair of the department of dermatology at the Cleveland Clinic. Dr. Vidimos and her colleagues have been so focused on skin cancer diagnosis and treatment in this group of patients that “we’ve kind of lost sight of the human aspect of it and the fear that these patients have,” she added.
These shared medical appointments at the Cleveland Clinic also provide the dermatology staff an efficient means to educate a group of patients about the risks of developing skin cancers and benign conditions associated with immunosuppressive treatments. They also discuss sun protection and avoidance practices, and how to perform skin self exams, all of which are “really important for their well being after their transplant,” Dr. Vidimos said during an interview at the American Academy of Dermatology summer meeting.
Patients learn how frequently they will need a clinical skin exam post-transplant, depending on the occurrence of skin cancers, and that biopsies may be necessary at times.
Dr. Vidimos said it is up to the transplant surgeons to discuss with patients the very low risk of transmission of malignancies from the donor to the recipient. In her presentation at the meeting, she cited a 1.4% risk of an undetected skin or internal malignancy in a donor being transmitted to the recipient.
Because of changes in criteria, patients who have relatively low risk skin cancers or higher risk skin cancers and have been treated, and are at “a defined interval post-treatment where we feel it’s safe to do that transplant,” may be considered a transplant candidate, she said noting that previously, such patients would be excluded from a transplant.
The appropriate time intervals to wait for a transplant for candidates with a history of cutaneous squamous cell carcinoma, malignant melanoma, or Merkel cell carcinoma are spelled out in a recently published consensus paper Dr. Vidimos coauthored with other members of the International Transplant Skin Cancer Collaborative (Am J Transplant. 2016 Feb;16[2]:407-13).
Dr. Vidimos said she is sometimes called to the bedside to perform a skin exam in a pretransplant patient who is very ill. The most common scenario is a liver transplant candidate awaiting transport to the operating room, who has not had a skin exam and needs to be cleared by a dermatologist to rule out a melanoma or another type of skin cancer that could be “fertilized” by postoperative immunosuppressant therapy.
After a transplant, patients need to be seen frequently enough to detect malignant transformation of precancerous skin lesions. Dermatologists should have a low threshold for biopsying any suspicious lesions early. “A lot of times we get biopsies back of skin cancer that do not match ... the clinical picture,” Dr. Vidimos said. Patients should be referred when the dermatologist feels that he or she can not deliver the appropriate treatment.
As transplants have become fairly routine and patients are living longer, community dermatologists will most likely be seeing solid organ transplant recipients more frequently. With longer lifespans, those patients will have more opportunity to develop more skin cancers.
Dr. Vidimos disclosed having received grants and research funding from Genentech.
Boston – Shared medical appointments can work well for patients undergoing solid organ transplants and for the clinical staff treating them – serving as an efficient way to educate several patients in a common setting and giving them the opportunity to interact with others going through a similar experience.
“Having those shared medical appointments where they’re sitting next to somebody who’s going through the same thought process and information gathering ... and the ability to talk with similar folks is a huge benefit for those patients,” said Allison Vidimos, MD, chair of the department of dermatology at the Cleveland Clinic. Dr. Vidimos and her colleagues have been so focused on skin cancer diagnosis and treatment in this group of patients that “we’ve kind of lost sight of the human aspect of it and the fear that these patients have,” she added.
These shared medical appointments at the Cleveland Clinic also provide the dermatology staff an efficient means to educate a group of patients about the risks of developing skin cancers and benign conditions associated with immunosuppressive treatments. They also discuss sun protection and avoidance practices, and how to perform skin self exams, all of which are “really important for their well being after their transplant,” Dr. Vidimos said during an interview at the American Academy of Dermatology summer meeting.
Patients learn how frequently they will need a clinical skin exam post-transplant, depending on the occurrence of skin cancers, and that biopsies may be necessary at times.
Dr. Vidimos said it is up to the transplant surgeons to discuss with patients the very low risk of transmission of malignancies from the donor to the recipient. In her presentation at the meeting, she cited a 1.4% risk of an undetected skin or internal malignancy in a donor being transmitted to the recipient.
Because of changes in criteria, patients who have relatively low risk skin cancers or higher risk skin cancers and have been treated, and are at “a defined interval post-treatment where we feel it’s safe to do that transplant,” may be considered a transplant candidate, she said noting that previously, such patients would be excluded from a transplant.
The appropriate time intervals to wait for a transplant for candidates with a history of cutaneous squamous cell carcinoma, malignant melanoma, or Merkel cell carcinoma are spelled out in a recently published consensus paper Dr. Vidimos coauthored with other members of the International Transplant Skin Cancer Collaborative (Am J Transplant. 2016 Feb;16[2]:407-13).
Dr. Vidimos said she is sometimes called to the bedside to perform a skin exam in a pretransplant patient who is very ill. The most common scenario is a liver transplant candidate awaiting transport to the operating room, who has not had a skin exam and needs to be cleared by a dermatologist to rule out a melanoma or another type of skin cancer that could be “fertilized” by postoperative immunosuppressant therapy.
After a transplant, patients need to be seen frequently enough to detect malignant transformation of precancerous skin lesions. Dermatologists should have a low threshold for biopsying any suspicious lesions early. “A lot of times we get biopsies back of skin cancer that do not match ... the clinical picture,” Dr. Vidimos said. Patients should be referred when the dermatologist feels that he or she can not deliver the appropriate treatment.
As transplants have become fairly routine and patients are living longer, community dermatologists will most likely be seeing solid organ transplant recipients more frequently. With longer lifespans, those patients will have more opportunity to develop more skin cancers.
Dr. Vidimos disclosed having received grants and research funding from Genentech.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2016
Effectiveness of an Employee Skin Cancer Screening Program for Secondary Prevention
The incidence of skin cancer, along with its effects on patients and the economy, has continued to increase and therefore requires particular attention from dermatologists. UV light has been shown to be of etiopathologic importance in the development of various types of skin cancer.1-3 Studies have shown that there is a direct correlation between the incidence of skin cancer and average annual amounts of UV radiation exposure.3 Accordingly, in 2009 the International Agency for Research on Cancer classified UV light as carcinogenic to humans.4 Therefore, the general public must be made aware of the danger of exposure to UV radiation.
In Australia, government initiatives to educate the population on causes of skin cancer development and its relationship to UV radiation have already caused the public to change their way of thinking and to deal with sunlight in a conscious and responsible manner.5 A large proportion of the Australian population with light skin is at a particularly high risk for developing skin cancer due to intense exposure to UV radiation. Numerous campaigns in Germany and other countries have attempted to sensitize the public to this issue by emphasizing a reduction in UV exposure (primary prevention) or highlighting the importance of early diagnosis (secondary prevention).6,7
For a good prognosis, it is crucial that skin cancer, particularly melanoma, is discovered at an early or precancerous stage.8 For this reason, self-examination of the skin and skin cancer screening are important factors that can contribute to ensuring early and curative treatment.9-11 Since July 1, 2008, skin cancer screenings have been included in the preventative health care program by statutory health insurance providers in Germany. As part of this program, the cost of screening once every 2 years for individuals 35 years and older is covered by statutory health insurance.12 Several studies have shown a decline in the melanoma mortality rate since the introduction of skin cancer screening programs in Germany.11,13,14
Employee skin cancer screening programs are an important method of examining high numbers of individuals quickly and effectively. These programs have been carried out in Germany and other countries.15,16 Studies have shown that skin cancer screening carried out selectively on defined groups can be an effective form of secondary prevention, particularly for those who work outdoors.17
An employee skin cancer screening program was carried out as part of this study. The findings are interpreted and discussed in relation to other employee screening programs that have been reported as well as those introduced by statutory health insurance providers in Germany. The aim of this study was to determine the importance and effectiveness of employee skin cancer screening programs and the role they play in secondary prevention of skin cancer.
Methods
Study Population
Employees of a technical company in Bavaria, Germany, were offered a skin cancer screening program by the employer’s occupational health service and health insurance provider in collaboration with the Department of Dermatology at the University Hospital Erlangen (Erlangen, Germany). Skin examinations were performed exclusively by 5 trained dermatologists. Only direct employees of the company at 3 of its locations in the Erlangen area were eligible to participate. The total number of employees varied by location (1072–5126 employees). The majority of employees had a university education or had completed technical training. Family members and other individuals who were not members of the company were excluded. There were no further inclusion or exclusion criteria. Over a period of 13 days, 783 of 7823 total employees (10.0%) were examined and included in the study. The study was approved by the Responsible Ethics Commission of the Faculty of Medicine at Friedrich-Alexander-University Erlangen-Nürnberg, Germany.
Study Design
Employees signed a consent form for participation in the study and completed a standardized questionnaire. The questionnaire was based on surveys used in a prior study18 and collected information on current and prior skin lesions, prior dermatological screening, personal and family history of skin tumors, frequency of UV exposure, and type of UV protection used. For the question on measures taken for protection from UV radiation, possible answers included with sunscreen cream, with suitable sun-protective clothing, and by staying in the shade, or no measures were taken. In contrast to the other questions, multiple answers were accepted for this question. Answering no automatically excluded other possible answers. Participants also were asked to assess their own Fitzpatrick skin type19; the questionnaire included explanations of each skin type (I–IV).
The participants were then called in for examination by the dermatologist at 15-minute intervals. All clothing was removed and the skin was examined. Dermatoscopes were used for closer examination of suspicious skin lesions. The clinical results of the examinations were recorded on a standardized form.
An estimation of the number of melanocytic nevi—≤20, 21–49, or ≥50—was recorded for each patient. Suspicious skin lesions were assigned to one of the following categories: nevus requiring future checkup (Nc), nevus requiring excision (Ne), suspected malignant melanoma (MM), suspected squamous cell carcinoma, suspected basal cell carcinoma (BCC), suspected other skin tumor, and precancerous lesion. Fitzpatrick skin type also was assessed for all participants and recorded by the dermatologist carrying out the examination. Each participant was assigned to a risk group—low, moderate, or high risk—based on their individual risk for developing a skin tumor. Factors that were considered when determining participants’ risk for developing skin cancer included Fitzpatrick skin type, number of melanocytic nevi, personal and family history, leisure activities, UV protection used, and current clinical diagnosis of skin lesions.
After the skin examination, participants were informed of recommended treatment but were not given any additional dermatologic advice. Participants could arrange an appointment at the Department of Dermatology, University Hospital Erlangen, for the excision and histological analysis of the skin lesions. All recorded data were collected in a computerized spreadsheet program. When evaluating the questionnaires, questions that were not answered or were answered incorrectly (participant chose more than 1 answer) were ignored.
Statistical Analysis
Statistical analysis was carried out using SPSS software version 16.0. The majority of the data were nominal or ordinal. Metric data were checked for normal distribution using the Shapiro-Wilk test before carrying out parametric tests. Statistical tests were carried out using the χ2 test and the t test for independent samples. Non-nominal distributed data were checked using the Mann-Whitney U test. P<.05 was considered statistically significant in the exploratory data analysis.
Results
Of 783 employees included in the study, 288 (36.8%) were female and 495 (63.2%) were male (Table 1). In comparison with the total workforce, a significantly higher proportion of women than men took part in the cross-sectional study (P<.01). The average age (SD) was 42.3 (9.5) years (range, 18–64 years). Female participants (average age [SD], 39.8 [10.2] years) were significantly younger than male participants (average age [SD], 43.8 [8.8] years; P<.01). Forty-one percent of participants had a prior skin cancer screening. One percent of participants had a personal history of skin cancer, with 1 participant reporting a history of MM; 6.5% had a family history of skin cancer, of which 39.2% had a family history of MM.
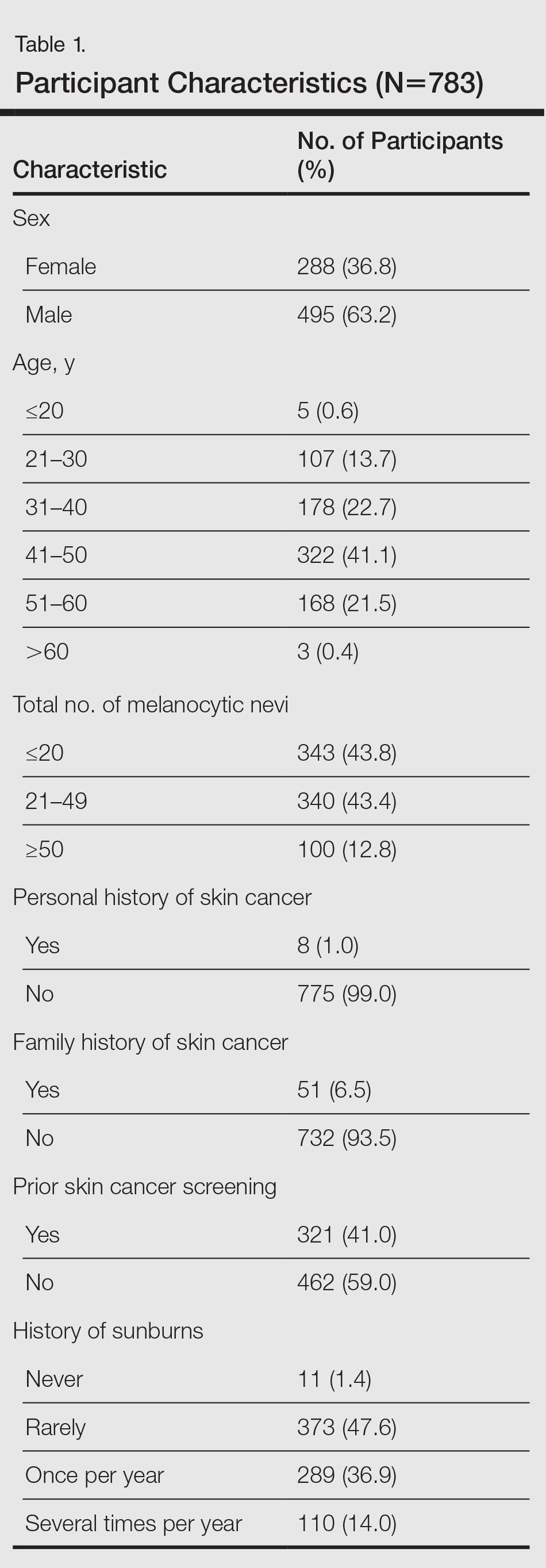
The results of the clinical examinations showed that 43.8% of participants had 20 or fewer melanocytic nevi, 43.4% had 21 to 49 melanocytic nevi, and 12.8% had 50 or more melanocytic nevi. Significantly more women than men had 20 or fewer melanocytic nevi (P<.05).
Approximately 92% of participants assessed themselves as having Fitzpatrick skin types II (35.2%) or III (56.7%), while only approximately 3.6% and 4.5% assessed themselves as having skin types I and IV, respectively. The results of the Fitzpatrick skin type assessments made by dermatologists were similar: 96.9% of participants were assessed as having Fitzpatrick skin types II (43.0%) and III (53.8%); approximately 1.9% and 1.3% were assessed as having Fitzpatrick skin types I and IV, respectively. Results showed that 80.2% of all participants assessed their skin type in the same way as the dermatologist; 13.5% assessed their skin type as darker and 6.3% (49/783) assessed it as lighter. A quantitative analysis of Fitzpatrick skin type and sex showed that significantly more male participants than female participants assessed their Fitzpatrick skin type darker than their actual skin type (P<.01).
Overall, 47.6% of participants reported having had sunburn rarely in the past, while 36.9% and 14.0% had experienced sunburn once per year and several times per year, respectively. Approximately 1.4% of participants reported never having a sunburn. More of the male participants made use of comprehensive sun protection using all methods listed (34.5%; P<.05) or a combination of sunscreen and sun-protective clothing (14.9%; P<.01) than the female participants who relied more frequently on sunscreen alone (29.5%; P<.01) or a combination of sunscreen and staying in the shade (29.5%; P<.01)
In general it was clear that sunscreen, either alone or in combination with other sun-protection methods, was used most frequently (88.0%); 58.0% protected themselves by staying in the shade, while 48.0% used suitable sun-protective clothing. Only 3.6% of participants did not protect themselves using any of the suggested methods.
A total of 661 categorized skin lesions were found in 377 participants. Of these lesions, 491 were Nc and 121 were Ne. Twenty-four of the skin lesions were suspected precancerous lesions, 13 were suspected BCC, 2 were suspected MM, and 10 were suspected other skin tumor (Table 2). Overall, male participants who were diagnosed with at least 1 skin lesion (average age, 44.0 years) were significantly older than the women (average age, 39.3 years)(P<.01). Similar findings were observed in participants with at least 1 Nc (men, 43.3 years; women, 38.7 years; P<.01) and at least 1 Ne (men, 44.2 years; women, 38.0 years; P<.05). With regard to the individual risk for developing skin cancer, 32.6% of participants were considered to be at low risk, 64.9% were at moderate risk, and 2.6% were at high risk.
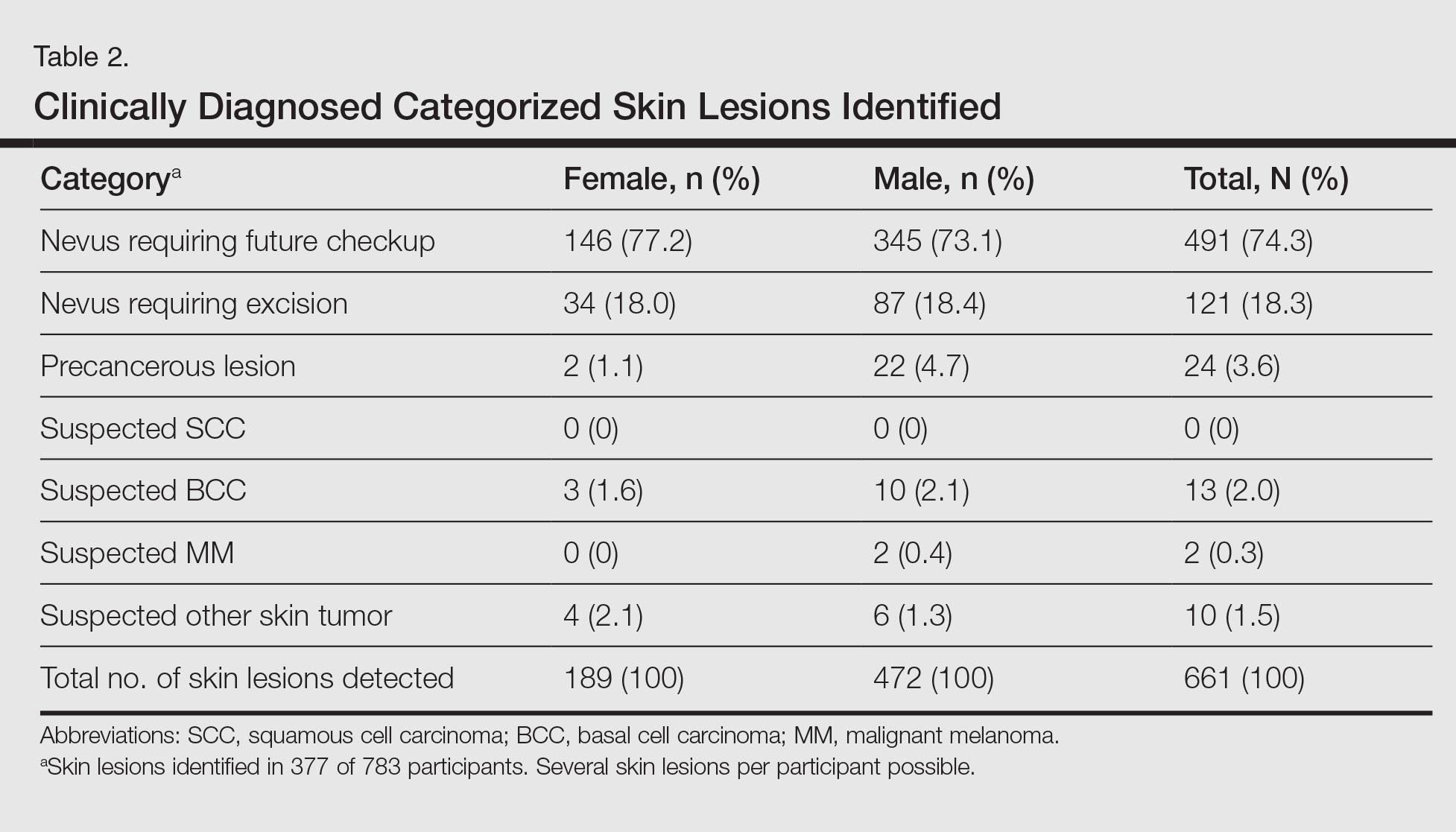
Approximately 61.5% of 377 participants who were diagnosed with at least 1 categorized skin lesion were advised to have a specific skin lesion checked by a dermatologist or to have a full examination for skin cancer once every 12 months. Furthermore, 22.5% were advised to follow-up biannually and 11.7% were advised to follow-up once every 2 years. Of the remaining participants who were advised to have follow-ups, 0.3% were advised to have a skin examination once every 3 months after having had MM, and 4.0% were advised to have follow-up once every 18 months. Overall, follow-up was recommended within 1 year in 84.4% of cases and within 1 to 2 years in 15.6% (Table 3).
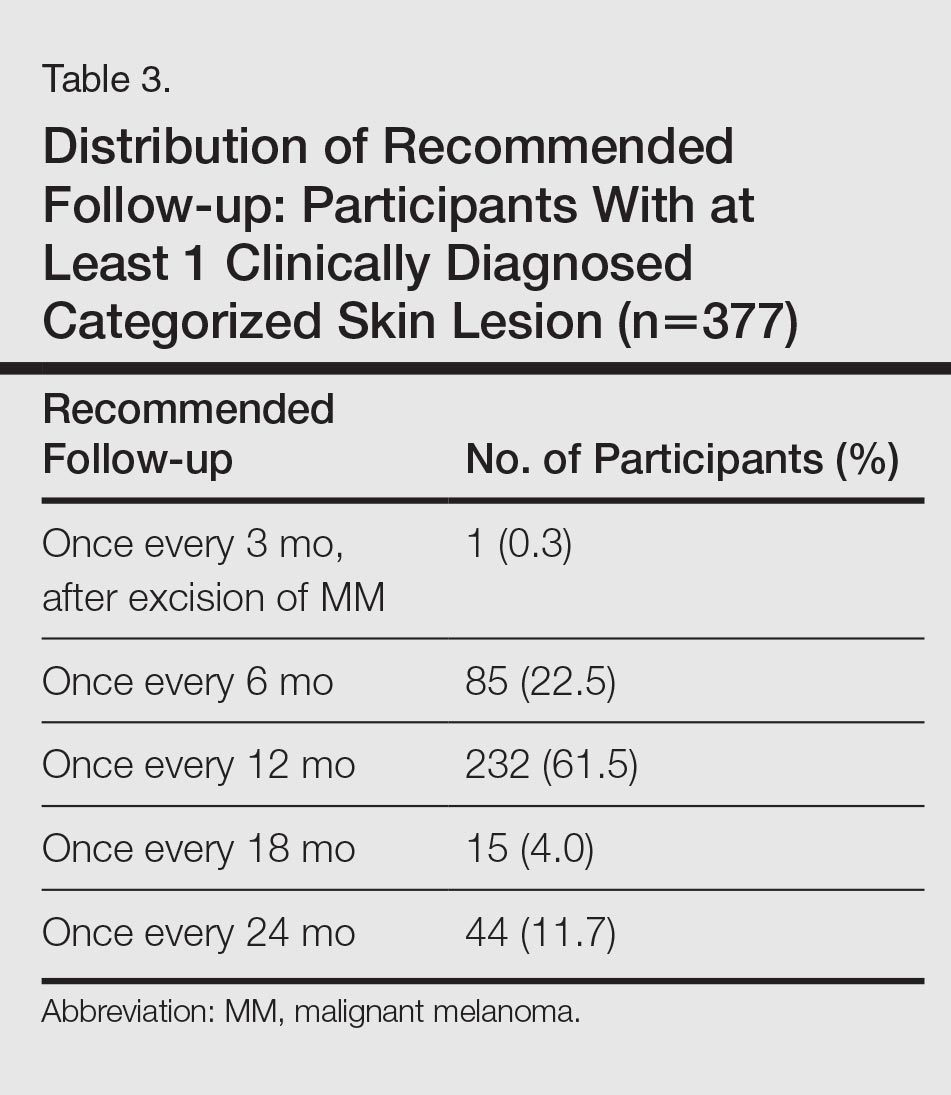
Subsequent histological analysis of the excised tissue resulted in a diagnosis of only 21 clinically significant skin conditions. One case of Bowen disease and 1 case of BCC was confirmed. Histological analysis identified the remaining 19 excised skin lesions, which included the 2 suspected MMs, as dysplastic nevi.
Comment
The aim of this cross-sectional study was to examine the importance and effectiveness of employee skin cancer screening programs. In comparison with the total workforce, significantly more women took part than men. Female participants were significantly younger than male participants, which mirrors the findings of prior studies showing that screening programs reach women more frequently than men and that women who participate in screenings are also younger on average in comparison to men.7-13 Men and older individuals usually are underrepresented.7,13 The average age of participants in our study was 42.3 years, which is lower than in the SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) study (average age, 49.7 years).13 The average age in our study also is likely to be lower than patients who undergo skin cancer screenings offered by statutory health insurance providers in Germany, which has a minimum age restriction of 35 years; however, it is comparable to the average age of participants in other employee screening programs and therefore represents the average age of individuals employed in Germany.15,16
The employee skin cancer screening program in this study generated a high level of interest, indicated by the fact that all available appointments had been booked just 36 hours after the screening was announced. Furthermore, there was a waiting list of approximately 300 employees who were not able to undergo a skin examination. For logistical reasons, the number of participants was limited to 10% of the workforce. The high level of interest is an indication of increased awareness of the importance of recognizing skin tumors early and the associated need for information as well as the need to undergo screening for skin cancer as a precaution. This observation also can be made with regard to the skin cancer screening introduced by statutory health insurance providers in Germany. Studies published by Augustin et al20 and Kornek et al21,22 confirm that skin cancer screenings have gained wide acceptance in Germany because they were introduced by statutory health insurance providers in 2008. The number of skin cancer screenings carried out by dermatologists in Germany also is increasing.20-22 Although approximately 19% of those eligible to participate took part in the SCREEN pilot project,13 approximately 31% of individuals who were eligible to participate took part in skin cancer screenings offered by statutory health insurance providers in Germany in 2012, and the percentage is rising.23 Two important factors affecting the high level of interest in the employee screening program used in our study were undoubtedly the advantages of the examination taking place during working hours and being held on the occupational health services’ premises in the workplace, which helped participants avoid the cost of travel and wait times associated with visiting a medical practice.
Of 783 participants included in this study, 377 displayed at least 1 categorized skin lesion; the majority were suspicious melanocytic nevi. This high incidence rate suggested that regular skin cancer screenings are useful, as it has been shown that there is a correlation between higher numbers of melanocytic nevi and increased risk for developing melanoma.24
In a study by Winkler et al,25 a skin cancer screening of 1658 bank and insurance employees found that 33.8% of those examined displayed at least 1 atypical melanocytic nevus and 27.2% displayed more than 50 melanocytic nevi (compared to 12.8% with ≥50 melanocytic nevi in the current study). The risk for developing skin cancer was classified as intermediate or high in 54.5% (compared to 67.5% at moderate or high risk in the current study).25 Therefore, the rate of suspicious skin lesions was lower in the population of the study by Winkler et al25 in comparison to the population of the current study. As the overall number of melanocytic nevi and the individual risk for skin cancer, however, was underestimated by the majority of the bank and insurance employees,25 employee skin cancer screening programs can be used as a potentially effective tool to make employees aware of the issue and sensitizing them to it. Employee screening in addition to a final diagnosis can contribute to ensuring suitable treatment is started. For example, in the large-scale employee screening published by Schaefer et al15 and Augustin et al,16 48,665 and 90,880 employees, respectively, were screened for inflammatory and noninflammatory skin diseases, and 19% and 27% of participants, respectively, were diagnosed with skin lesions that required treatment.
Participants in the current study were given no further treatment or advice. Recommendations were made that participants monitor suspicious skin lesions or have them removed. With regard to future screening, 84.4% of participants with at least 1 categorized skin lesion were advised to have a regular follow-up within 1 year, while 15.6% were advised to follow-up within 1 to 2 years. Therefore, a period of 2 years before the next checkup, the period between screenings offered by statutory health insurance providers in Germany,12 was considered too long for the majority of participants, according to the dermatologists involved with our study.
Conclusion
The high rate of suspicious skin lesions diagnosed demonstrated the effectiveness of skin cancer screenings organized in the workplace, which should be recommended for all employees, not only those who are at high risk for developing skin cancer due to the nature of their work, such as those who work outdoors. It should be noted that the study group examined in the current study was a homogeneous group of employees of a technical company only and is therefore relatively selective. Nevertheless, despite the comparatively selective and young participant group, these examinations provide evidence of the importance of skin cancer screening programs for a wider population.
Acknowledgments
The authors thank Heidi Seybold, MD; Petra Wörl, MD; Sybille Thoma-Uszynski, MD; and Jens Bussmann, MD (all from Erlangen, Germany), for their support and active assistance in the practical implementation of this study.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58:129-132.
- El Ghissassi F, Baan R, Straif K, et al; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10:751-752.
- MacLennan R, Green AC, McLeod GR, et al. Increasing incidence of cutaneous melanoma in Queensland, Australia. J Natl Cancer Inst. 1992;84:1427-1432.
- Heinzerling LM, Dummer R, Panizzon RG, et al. Prevention campaign against skin cancer. Dermatology. 2002;205:229-233.
- Stratigos A, Nikolaou V, Kedicoglou S, et al. Melanoma/skin cancer screening in a Mediterranean country: results of the Euromelanoma Screening Day Campaign in Greece. J Eur Acad Dermatol Venereol. 2007;21:56-62.
- Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17:393-399.
- Choudhury K, Volkmer B, Greinert R, et al. Effectiveness of skin cancer screening programmes. Br J Dermatol. 2012;167:94-98.
- Eisemann N, Waldmann A, Geller AC, et al. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol. 2014;134:43-50.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
- Bekanntmachung (1430 A) eines Beschlusses des Gemeinsamen Bundeausschusses über eine Änderung der Krebsfrüherkennungs-Richtlinien: Hautkrebs-Screening [press release]. Berlin, Germany: Bundesministerium für Gesundheit (Federal Ministry of Health, Germany); vom 15. November 2007.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
- Schaefer I, Rustenbach SJ, Zimmer L, et al. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatology. 2008;217:169-172.
- Augustin M, Herberger K, Hintzen S, et al. Prevalence of skin lesions and need for treatment in a cohort of 90880 workers. Br J Dermatol. 2011;165:865-873.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Harbauer A, Binder M, Pehamberger H, et al. Validity of an unsupervised self-administered questionnaire for self-assessment of melanoma risk. Melanoma Res. 2003;13:537-542.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Augustin M, Stadler R, Reusch M, et al. Skin cancer screening in Germany—perception by the public. J Dtsch Dermatol Ges. 2012;10:42-49.
- Kornek T, Augustin M. Skin cancer prevention. J Dtsch Dermatol Ges. 2013;11:283-296.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists’ perspective. Dermatology. 2012;225:289-293.
- Barmer GEK Arztreport 2014 [press release]. Berlin, Germany: Barmer GEK; February 4, 2014.
- Bauer J, Garbe C. Acquired melanocytic nevi as riskfactor for melanoma development. a comprehensive review of epidemiological data. Pigment Cell Res. 2003;16:297-306.
- Winkler A, Plugfelder A, Weide B, et al. Screening for skin cancer in bank and insurance employees: risk profile and correlation of self and physician’s assessment. Int J Dermatol. 2015;54:419-423.
The incidence of skin cancer, along with its effects on patients and the economy, has continued to increase and therefore requires particular attention from dermatologists. UV light has been shown to be of etiopathologic importance in the development of various types of skin cancer.1-3 Studies have shown that there is a direct correlation between the incidence of skin cancer and average annual amounts of UV radiation exposure.3 Accordingly, in 2009 the International Agency for Research on Cancer classified UV light as carcinogenic to humans.4 Therefore, the general public must be made aware of the danger of exposure to UV radiation.
In Australia, government initiatives to educate the population on causes of skin cancer development and its relationship to UV radiation have already caused the public to change their way of thinking and to deal with sunlight in a conscious and responsible manner.5 A large proportion of the Australian population with light skin is at a particularly high risk for developing skin cancer due to intense exposure to UV radiation. Numerous campaigns in Germany and other countries have attempted to sensitize the public to this issue by emphasizing a reduction in UV exposure (primary prevention) or highlighting the importance of early diagnosis (secondary prevention).6,7
For a good prognosis, it is crucial that skin cancer, particularly melanoma, is discovered at an early or precancerous stage.8 For this reason, self-examination of the skin and skin cancer screening are important factors that can contribute to ensuring early and curative treatment.9-11 Since July 1, 2008, skin cancer screenings have been included in the preventative health care program by statutory health insurance providers in Germany. As part of this program, the cost of screening once every 2 years for individuals 35 years and older is covered by statutory health insurance.12 Several studies have shown a decline in the melanoma mortality rate since the introduction of skin cancer screening programs in Germany.11,13,14
Employee skin cancer screening programs are an important method of examining high numbers of individuals quickly and effectively. These programs have been carried out in Germany and other countries.15,16 Studies have shown that skin cancer screening carried out selectively on defined groups can be an effective form of secondary prevention, particularly for those who work outdoors.17
An employee skin cancer screening program was carried out as part of this study. The findings are interpreted and discussed in relation to other employee screening programs that have been reported as well as those introduced by statutory health insurance providers in Germany. The aim of this study was to determine the importance and effectiveness of employee skin cancer screening programs and the role they play in secondary prevention of skin cancer.
Methods
Study Population
Employees of a technical company in Bavaria, Germany, were offered a skin cancer screening program by the employer’s occupational health service and health insurance provider in collaboration with the Department of Dermatology at the University Hospital Erlangen (Erlangen, Germany). Skin examinations were performed exclusively by 5 trained dermatologists. Only direct employees of the company at 3 of its locations in the Erlangen area were eligible to participate. The total number of employees varied by location (1072–5126 employees). The majority of employees had a university education or had completed technical training. Family members and other individuals who were not members of the company were excluded. There were no further inclusion or exclusion criteria. Over a period of 13 days, 783 of 7823 total employees (10.0%) were examined and included in the study. The study was approved by the Responsible Ethics Commission of the Faculty of Medicine at Friedrich-Alexander-University Erlangen-Nürnberg, Germany.
Study Design
Employees signed a consent form for participation in the study and completed a standardized questionnaire. The questionnaire was based on surveys used in a prior study18 and collected information on current and prior skin lesions, prior dermatological screening, personal and family history of skin tumors, frequency of UV exposure, and type of UV protection used. For the question on measures taken for protection from UV radiation, possible answers included with sunscreen cream, with suitable sun-protective clothing, and by staying in the shade, or no measures were taken. In contrast to the other questions, multiple answers were accepted for this question. Answering no automatically excluded other possible answers. Participants also were asked to assess their own Fitzpatrick skin type19; the questionnaire included explanations of each skin type (I–IV).
The participants were then called in for examination by the dermatologist at 15-minute intervals. All clothing was removed and the skin was examined. Dermatoscopes were used for closer examination of suspicious skin lesions. The clinical results of the examinations were recorded on a standardized form.
An estimation of the number of melanocytic nevi—≤20, 21–49, or ≥50—was recorded for each patient. Suspicious skin lesions were assigned to one of the following categories: nevus requiring future checkup (Nc), nevus requiring excision (Ne), suspected malignant melanoma (MM), suspected squamous cell carcinoma, suspected basal cell carcinoma (BCC), suspected other skin tumor, and precancerous lesion. Fitzpatrick skin type also was assessed for all participants and recorded by the dermatologist carrying out the examination. Each participant was assigned to a risk group—low, moderate, or high risk—based on their individual risk for developing a skin tumor. Factors that were considered when determining participants’ risk for developing skin cancer included Fitzpatrick skin type, number of melanocytic nevi, personal and family history, leisure activities, UV protection used, and current clinical diagnosis of skin lesions.
After the skin examination, participants were informed of recommended treatment but were not given any additional dermatologic advice. Participants could arrange an appointment at the Department of Dermatology, University Hospital Erlangen, for the excision and histological analysis of the skin lesions. All recorded data were collected in a computerized spreadsheet program. When evaluating the questionnaires, questions that were not answered or were answered incorrectly (participant chose more than 1 answer) were ignored.
Statistical Analysis
Statistical analysis was carried out using SPSS software version 16.0. The majority of the data were nominal or ordinal. Metric data were checked for normal distribution using the Shapiro-Wilk test before carrying out parametric tests. Statistical tests were carried out using the χ2 test and the t test for independent samples. Non-nominal distributed data were checked using the Mann-Whitney U test. P<.05 was considered statistically significant in the exploratory data analysis.
Results
Of 783 employees included in the study, 288 (36.8%) were female and 495 (63.2%) were male (Table 1). In comparison with the total workforce, a significantly higher proportion of women than men took part in the cross-sectional study (P<.01). The average age (SD) was 42.3 (9.5) years (range, 18–64 years). Female participants (average age [SD], 39.8 [10.2] years) were significantly younger than male participants (average age [SD], 43.8 [8.8] years; P<.01). Forty-one percent of participants had a prior skin cancer screening. One percent of participants had a personal history of skin cancer, with 1 participant reporting a history of MM; 6.5% had a family history of skin cancer, of which 39.2% had a family history of MM.
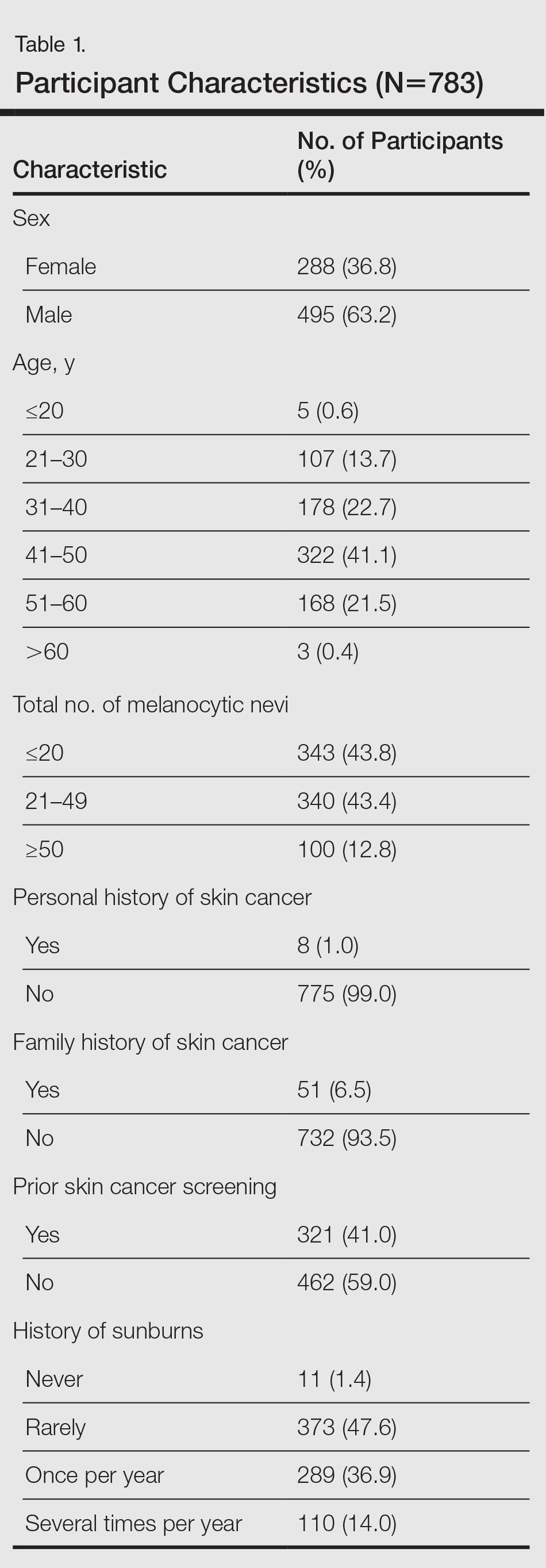
The results of the clinical examinations showed that 43.8% of participants had 20 or fewer melanocytic nevi, 43.4% had 21 to 49 melanocytic nevi, and 12.8% had 50 or more melanocytic nevi. Significantly more women than men had 20 or fewer melanocytic nevi (P<.05).
Approximately 92% of participants assessed themselves as having Fitzpatrick skin types II (35.2%) or III (56.7%), while only approximately 3.6% and 4.5% assessed themselves as having skin types I and IV, respectively. The results of the Fitzpatrick skin type assessments made by dermatologists were similar: 96.9% of participants were assessed as having Fitzpatrick skin types II (43.0%) and III (53.8%); approximately 1.9% and 1.3% were assessed as having Fitzpatrick skin types I and IV, respectively. Results showed that 80.2% of all participants assessed their skin type in the same way as the dermatologist; 13.5% assessed their skin type as darker and 6.3% (49/783) assessed it as lighter. A quantitative analysis of Fitzpatrick skin type and sex showed that significantly more male participants than female participants assessed their Fitzpatrick skin type darker than their actual skin type (P<.01).
Overall, 47.6% of participants reported having had sunburn rarely in the past, while 36.9% and 14.0% had experienced sunburn once per year and several times per year, respectively. Approximately 1.4% of participants reported never having a sunburn. More of the male participants made use of comprehensive sun protection using all methods listed (34.5%; P<.05) or a combination of sunscreen and sun-protective clothing (14.9%; P<.01) than the female participants who relied more frequently on sunscreen alone (29.5%; P<.01) or a combination of sunscreen and staying in the shade (29.5%; P<.01)
In general it was clear that sunscreen, either alone or in combination with other sun-protection methods, was used most frequently (88.0%); 58.0% protected themselves by staying in the shade, while 48.0% used suitable sun-protective clothing. Only 3.6% of participants did not protect themselves using any of the suggested methods.
A total of 661 categorized skin lesions were found in 377 participants. Of these lesions, 491 were Nc and 121 were Ne. Twenty-four of the skin lesions were suspected precancerous lesions, 13 were suspected BCC, 2 were suspected MM, and 10 were suspected other skin tumor (Table 2). Overall, male participants who were diagnosed with at least 1 skin lesion (average age, 44.0 years) were significantly older than the women (average age, 39.3 years)(P<.01). Similar findings were observed in participants with at least 1 Nc (men, 43.3 years; women, 38.7 years; P<.01) and at least 1 Ne (men, 44.2 years; women, 38.0 years; P<.05). With regard to the individual risk for developing skin cancer, 32.6% of participants were considered to be at low risk, 64.9% were at moderate risk, and 2.6% were at high risk.

Approximately 61.5% of 377 participants who were diagnosed with at least 1 categorized skin lesion were advised to have a specific skin lesion checked by a dermatologist or to have a full examination for skin cancer once every 12 months. Furthermore, 22.5% were advised to follow-up biannually and 11.7% were advised to follow-up once every 2 years. Of the remaining participants who were advised to have follow-ups, 0.3% were advised to have a skin examination once every 3 months after having had MM, and 4.0% were advised to have follow-up once every 18 months. Overall, follow-up was recommended within 1 year in 84.4% of cases and within 1 to 2 years in 15.6% (Table 3).

Subsequent histological analysis of the excised tissue resulted in a diagnosis of only 21 clinically significant skin conditions. One case of Bowen disease and 1 case of BCC was confirmed. Histological analysis identified the remaining 19 excised skin lesions, which included the 2 suspected MMs, as dysplastic nevi.
Comment
The aim of this cross-sectional study was to examine the importance and effectiveness of employee skin cancer screening programs. In comparison with the total workforce, significantly more women took part than men. Female participants were significantly younger than male participants, which mirrors the findings of prior studies showing that screening programs reach women more frequently than men and that women who participate in screenings are also younger on average in comparison to men.7-13 Men and older individuals usually are underrepresented.7,13 The average age of participants in our study was 42.3 years, which is lower than in the SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) study (average age, 49.7 years).13 The average age in our study also is likely to be lower than patients who undergo skin cancer screenings offered by statutory health insurance providers in Germany, which has a minimum age restriction of 35 years; however, it is comparable to the average age of participants in other employee screening programs and therefore represents the average age of individuals employed in Germany.15,16
The employee skin cancer screening program in this study generated a high level of interest, indicated by the fact that all available appointments had been booked just 36 hours after the screening was announced. Furthermore, there was a waiting list of approximately 300 employees who were not able to undergo a skin examination. For logistical reasons, the number of participants was limited to 10% of the workforce. The high level of interest is an indication of increased awareness of the importance of recognizing skin tumors early and the associated need for information as well as the need to undergo screening for skin cancer as a precaution. This observation also can be made with regard to the skin cancer screening introduced by statutory health insurance providers in Germany. Studies published by Augustin et al20 and Kornek et al21,22 confirm that skin cancer screenings have gained wide acceptance in Germany because they were introduced by statutory health insurance providers in 2008. The number of skin cancer screenings carried out by dermatologists in Germany also is increasing.20-22 Although approximately 19% of those eligible to participate took part in the SCREEN pilot project,13 approximately 31% of individuals who were eligible to participate took part in skin cancer screenings offered by statutory health insurance providers in Germany in 2012, and the percentage is rising.23 Two important factors affecting the high level of interest in the employee screening program used in our study were undoubtedly the advantages of the examination taking place during working hours and being held on the occupational health services’ premises in the workplace, which helped participants avoid the cost of travel and wait times associated with visiting a medical practice.
Of 783 participants included in this study, 377 displayed at least 1 categorized skin lesion; the majority were suspicious melanocytic nevi. This high incidence rate suggested that regular skin cancer screenings are useful, as it has been shown that there is a correlation between higher numbers of melanocytic nevi and increased risk for developing melanoma.24
In a study by Winkler et al,25 a skin cancer screening of 1658 bank and insurance employees found that 33.8% of those examined displayed at least 1 atypical melanocytic nevus and 27.2% displayed more than 50 melanocytic nevi (compared to 12.8% with ≥50 melanocytic nevi in the current study). The risk for developing skin cancer was classified as intermediate or high in 54.5% (compared to 67.5% at moderate or high risk in the current study).25 Therefore, the rate of suspicious skin lesions was lower in the population of the study by Winkler et al25 in comparison to the population of the current study. As the overall number of melanocytic nevi and the individual risk for skin cancer, however, was underestimated by the majority of the bank and insurance employees,25 employee skin cancer screening programs can be used as a potentially effective tool to make employees aware of the issue and sensitizing them to it. Employee screening in addition to a final diagnosis can contribute to ensuring suitable treatment is started. For example, in the large-scale employee screening published by Schaefer et al15 and Augustin et al,16 48,665 and 90,880 employees, respectively, were screened for inflammatory and noninflammatory skin diseases, and 19% and 27% of participants, respectively, were diagnosed with skin lesions that required treatment.
Participants in the current study were given no further treatment or advice. Recommendations were made that participants monitor suspicious skin lesions or have them removed. With regard to future screening, 84.4% of participants with at least 1 categorized skin lesion were advised to have a regular follow-up within 1 year, while 15.6% were advised to follow-up within 1 to 2 years. Therefore, a period of 2 years before the next checkup, the period between screenings offered by statutory health insurance providers in Germany,12 was considered too long for the majority of participants, according to the dermatologists involved with our study.
Conclusion
The high rate of suspicious skin lesions diagnosed demonstrated the effectiveness of skin cancer screenings organized in the workplace, which should be recommended for all employees, not only those who are at high risk for developing skin cancer due to the nature of their work, such as those who work outdoors. It should be noted that the study group examined in the current study was a homogeneous group of employees of a technical company only and is therefore relatively selective. Nevertheless, despite the comparatively selective and young participant group, these examinations provide evidence of the importance of skin cancer screening programs for a wider population.
Acknowledgments
The authors thank Heidi Seybold, MD; Petra Wörl, MD; Sybille Thoma-Uszynski, MD; and Jens Bussmann, MD (all from Erlangen, Germany), for their support and active assistance in the practical implementation of this study.
The incidence of skin cancer, along with its effects on patients and the economy, has continued to increase and therefore requires particular attention from dermatologists. UV light has been shown to be of etiopathologic importance in the development of various types of skin cancer.1-3 Studies have shown that there is a direct correlation between the incidence of skin cancer and average annual amounts of UV radiation exposure.3 Accordingly, in 2009 the International Agency for Research on Cancer classified UV light as carcinogenic to humans.4 Therefore, the general public must be made aware of the danger of exposure to UV radiation.
In Australia, government initiatives to educate the population on causes of skin cancer development and its relationship to UV radiation have already caused the public to change their way of thinking and to deal with sunlight in a conscious and responsible manner.5 A large proportion of the Australian population with light skin is at a particularly high risk for developing skin cancer due to intense exposure to UV radiation. Numerous campaigns in Germany and other countries have attempted to sensitize the public to this issue by emphasizing a reduction in UV exposure (primary prevention) or highlighting the importance of early diagnosis (secondary prevention).6,7
For a good prognosis, it is crucial that skin cancer, particularly melanoma, is discovered at an early or precancerous stage.8 For this reason, self-examination of the skin and skin cancer screening are important factors that can contribute to ensuring early and curative treatment.9-11 Since July 1, 2008, skin cancer screenings have been included in the preventative health care program by statutory health insurance providers in Germany. As part of this program, the cost of screening once every 2 years for individuals 35 years and older is covered by statutory health insurance.12 Several studies have shown a decline in the melanoma mortality rate since the introduction of skin cancer screening programs in Germany.11,13,14
Employee skin cancer screening programs are an important method of examining high numbers of individuals quickly and effectively. These programs have been carried out in Germany and other countries.15,16 Studies have shown that skin cancer screening carried out selectively on defined groups can be an effective form of secondary prevention, particularly for those who work outdoors.17
An employee skin cancer screening program was carried out as part of this study. The findings are interpreted and discussed in relation to other employee screening programs that have been reported as well as those introduced by statutory health insurance providers in Germany. The aim of this study was to determine the importance and effectiveness of employee skin cancer screening programs and the role they play in secondary prevention of skin cancer.
Methods
Study Population
Employees of a technical company in Bavaria, Germany, were offered a skin cancer screening program by the employer’s occupational health service and health insurance provider in collaboration with the Department of Dermatology at the University Hospital Erlangen (Erlangen, Germany). Skin examinations were performed exclusively by 5 trained dermatologists. Only direct employees of the company at 3 of its locations in the Erlangen area were eligible to participate. The total number of employees varied by location (1072–5126 employees). The majority of employees had a university education or had completed technical training. Family members and other individuals who were not members of the company were excluded. There were no further inclusion or exclusion criteria. Over a period of 13 days, 783 of 7823 total employees (10.0%) were examined and included in the study. The study was approved by the Responsible Ethics Commission of the Faculty of Medicine at Friedrich-Alexander-University Erlangen-Nürnberg, Germany.
Study Design
Employees signed a consent form for participation in the study and completed a standardized questionnaire. The questionnaire was based on surveys used in a prior study18 and collected information on current and prior skin lesions, prior dermatological screening, personal and family history of skin tumors, frequency of UV exposure, and type of UV protection used. For the question on measures taken for protection from UV radiation, possible answers included with sunscreen cream, with suitable sun-protective clothing, and by staying in the shade, or no measures were taken. In contrast to the other questions, multiple answers were accepted for this question. Answering no automatically excluded other possible answers. Participants also were asked to assess their own Fitzpatrick skin type19; the questionnaire included explanations of each skin type (I–IV).
The participants were then called in for examination by the dermatologist at 15-minute intervals. All clothing was removed and the skin was examined. Dermatoscopes were used for closer examination of suspicious skin lesions. The clinical results of the examinations were recorded on a standardized form.
An estimation of the number of melanocytic nevi—≤20, 21–49, or ≥50—was recorded for each patient. Suspicious skin lesions were assigned to one of the following categories: nevus requiring future checkup (Nc), nevus requiring excision (Ne), suspected malignant melanoma (MM), suspected squamous cell carcinoma, suspected basal cell carcinoma (BCC), suspected other skin tumor, and precancerous lesion. Fitzpatrick skin type also was assessed for all participants and recorded by the dermatologist carrying out the examination. Each participant was assigned to a risk group—low, moderate, or high risk—based on their individual risk for developing a skin tumor. Factors that were considered when determining participants’ risk for developing skin cancer included Fitzpatrick skin type, number of melanocytic nevi, personal and family history, leisure activities, UV protection used, and current clinical diagnosis of skin lesions.
After the skin examination, participants were informed of recommended treatment but were not given any additional dermatologic advice. Participants could arrange an appointment at the Department of Dermatology, University Hospital Erlangen, for the excision and histological analysis of the skin lesions. All recorded data were collected in a computerized spreadsheet program. When evaluating the questionnaires, questions that were not answered or were answered incorrectly (participant chose more than 1 answer) were ignored.
Statistical Analysis
Statistical analysis was carried out using SPSS software version 16.0. The majority of the data were nominal or ordinal. Metric data were checked for normal distribution using the Shapiro-Wilk test before carrying out parametric tests. Statistical tests were carried out using the χ2 test and the t test for independent samples. Non-nominal distributed data were checked using the Mann-Whitney U test. P<.05 was considered statistically significant in the exploratory data analysis.
Results
Of 783 employees included in the study, 288 (36.8%) were female and 495 (63.2%) were male (Table 1). In comparison with the total workforce, a significantly higher proportion of women than men took part in the cross-sectional study (P<.01). The average age (SD) was 42.3 (9.5) years (range, 18–64 years). Female participants (average age [SD], 39.8 [10.2] years) were significantly younger than male participants (average age [SD], 43.8 [8.8] years; P<.01). Forty-one percent of participants had a prior skin cancer screening. One percent of participants had a personal history of skin cancer, with 1 participant reporting a history of MM; 6.5% had a family history of skin cancer, of which 39.2% had a family history of MM.
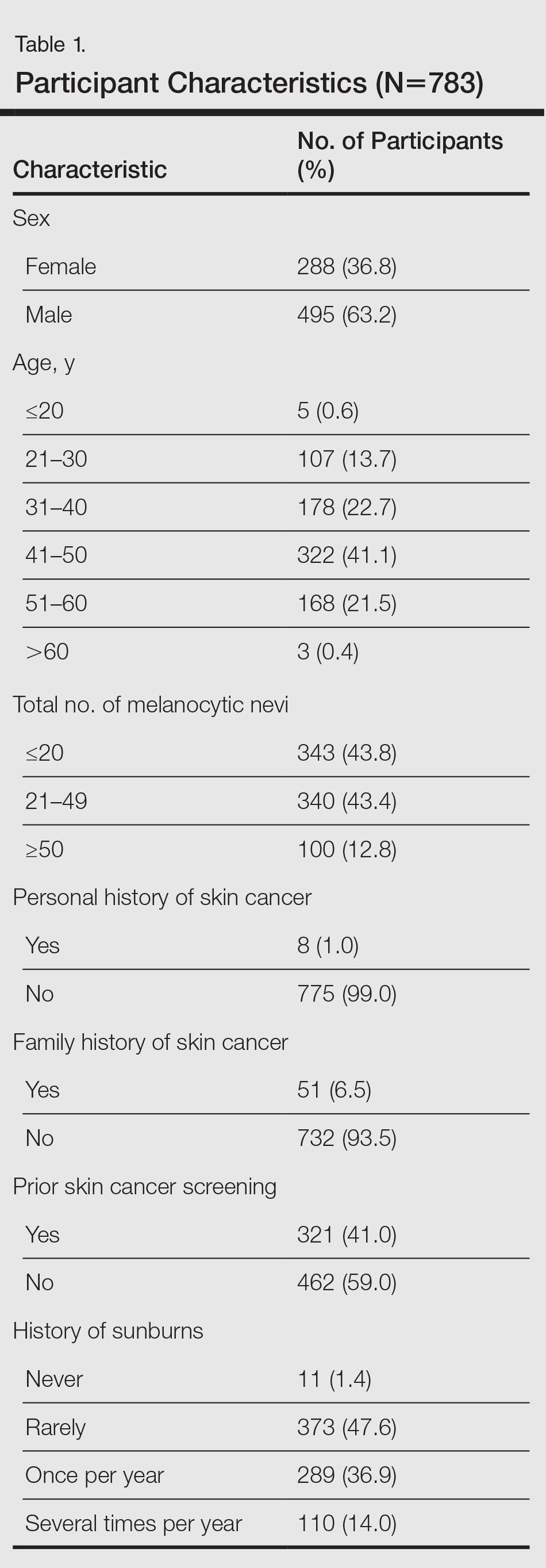
The results of the clinical examinations showed that 43.8% of participants had 20 or fewer melanocytic nevi, 43.4% had 21 to 49 melanocytic nevi, and 12.8% had 50 or more melanocytic nevi. Significantly more women than men had 20 or fewer melanocytic nevi (P<.05).
Approximately 92% of participants assessed themselves as having Fitzpatrick skin types II (35.2%) or III (56.7%), while only approximately 3.6% and 4.5% assessed themselves as having skin types I and IV, respectively. The results of the Fitzpatrick skin type assessments made by dermatologists were similar: 96.9% of participants were assessed as having Fitzpatrick skin types II (43.0%) and III (53.8%); approximately 1.9% and 1.3% were assessed as having Fitzpatrick skin types I and IV, respectively. Results showed that 80.2% of all participants assessed their skin type in the same way as the dermatologist; 13.5% assessed their skin type as darker and 6.3% (49/783) assessed it as lighter. A quantitative analysis of Fitzpatrick skin type and sex showed that significantly more male participants than female participants assessed their Fitzpatrick skin type darker than their actual skin type (P<.01).
Overall, 47.6% of participants reported having had sunburn rarely in the past, while 36.9% and 14.0% had experienced sunburn once per year and several times per year, respectively. Approximately 1.4% of participants reported never having a sunburn. More of the male participants made use of comprehensive sun protection using all methods listed (34.5%; P<.05) or a combination of sunscreen and sun-protective clothing (14.9%; P<.01) than the female participants who relied more frequently on sunscreen alone (29.5%; P<.01) or a combination of sunscreen and staying in the shade (29.5%; P<.01)
In general it was clear that sunscreen, either alone or in combination with other sun-protection methods, was used most frequently (88.0%); 58.0% protected themselves by staying in the shade, while 48.0% used suitable sun-protective clothing. Only 3.6% of participants did not protect themselves using any of the suggested methods.
A total of 661 categorized skin lesions were found in 377 participants. Of these lesions, 491 were Nc and 121 were Ne. Twenty-four of the skin lesions were suspected precancerous lesions, 13 were suspected BCC, 2 were suspected MM, and 10 were suspected other skin tumor (Table 2). Overall, male participants who were diagnosed with at least 1 skin lesion (average age, 44.0 years) were significantly older than the women (average age, 39.3 years)(P<.01). Similar findings were observed in participants with at least 1 Nc (men, 43.3 years; women, 38.7 years; P<.01) and at least 1 Ne (men, 44.2 years; women, 38.0 years; P<.05). With regard to the individual risk for developing skin cancer, 32.6% of participants were considered to be at low risk, 64.9% were at moderate risk, and 2.6% were at high risk.

Approximately 61.5% of 377 participants who were diagnosed with at least 1 categorized skin lesion were advised to have a specific skin lesion checked by a dermatologist or to have a full examination for skin cancer once every 12 months. Furthermore, 22.5% were advised to follow-up biannually and 11.7% were advised to follow-up once every 2 years. Of the remaining participants who were advised to have follow-ups, 0.3% were advised to have a skin examination once every 3 months after having had MM, and 4.0% were advised to have follow-up once every 18 months. Overall, follow-up was recommended within 1 year in 84.4% of cases and within 1 to 2 years in 15.6% (Table 3).

Subsequent histological analysis of the excised tissue resulted in a diagnosis of only 21 clinically significant skin conditions. One case of Bowen disease and 1 case of BCC was confirmed. Histological analysis identified the remaining 19 excised skin lesions, which included the 2 suspected MMs, as dysplastic nevi.
Comment
The aim of this cross-sectional study was to examine the importance and effectiveness of employee skin cancer screening programs. In comparison with the total workforce, significantly more women took part than men. Female participants were significantly younger than male participants, which mirrors the findings of prior studies showing that screening programs reach women more frequently than men and that women who participate in screenings are also younger on average in comparison to men.7-13 Men and older individuals usually are underrepresented.7,13 The average age of participants in our study was 42.3 years, which is lower than in the SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) study (average age, 49.7 years).13 The average age in our study also is likely to be lower than patients who undergo skin cancer screenings offered by statutory health insurance providers in Germany, which has a minimum age restriction of 35 years; however, it is comparable to the average age of participants in other employee screening programs and therefore represents the average age of individuals employed in Germany.15,16
The employee skin cancer screening program in this study generated a high level of interest, indicated by the fact that all available appointments had been booked just 36 hours after the screening was announced. Furthermore, there was a waiting list of approximately 300 employees who were not able to undergo a skin examination. For logistical reasons, the number of participants was limited to 10% of the workforce. The high level of interest is an indication of increased awareness of the importance of recognizing skin tumors early and the associated need for information as well as the need to undergo screening for skin cancer as a precaution. This observation also can be made with regard to the skin cancer screening introduced by statutory health insurance providers in Germany. Studies published by Augustin et al20 and Kornek et al21,22 confirm that skin cancer screenings have gained wide acceptance in Germany because they were introduced by statutory health insurance providers in 2008. The number of skin cancer screenings carried out by dermatologists in Germany also is increasing.20-22 Although approximately 19% of those eligible to participate took part in the SCREEN pilot project,13 approximately 31% of individuals who were eligible to participate took part in skin cancer screenings offered by statutory health insurance providers in Germany in 2012, and the percentage is rising.23 Two important factors affecting the high level of interest in the employee screening program used in our study were undoubtedly the advantages of the examination taking place during working hours and being held on the occupational health services’ premises in the workplace, which helped participants avoid the cost of travel and wait times associated with visiting a medical practice.
Of 783 participants included in this study, 377 displayed at least 1 categorized skin lesion; the majority were suspicious melanocytic nevi. This high incidence rate suggested that regular skin cancer screenings are useful, as it has been shown that there is a correlation between higher numbers of melanocytic nevi and increased risk for developing melanoma.24
In a study by Winkler et al,25 a skin cancer screening of 1658 bank and insurance employees found that 33.8% of those examined displayed at least 1 atypical melanocytic nevus and 27.2% displayed more than 50 melanocytic nevi (compared to 12.8% with ≥50 melanocytic nevi in the current study). The risk for developing skin cancer was classified as intermediate or high in 54.5% (compared to 67.5% at moderate or high risk in the current study).25 Therefore, the rate of suspicious skin lesions was lower in the population of the study by Winkler et al25 in comparison to the population of the current study. As the overall number of melanocytic nevi and the individual risk for skin cancer, however, was underestimated by the majority of the bank and insurance employees,25 employee skin cancer screening programs can be used as a potentially effective tool to make employees aware of the issue and sensitizing them to it. Employee screening in addition to a final diagnosis can contribute to ensuring suitable treatment is started. For example, in the large-scale employee screening published by Schaefer et al15 and Augustin et al,16 48,665 and 90,880 employees, respectively, were screened for inflammatory and noninflammatory skin diseases, and 19% and 27% of participants, respectively, were diagnosed with skin lesions that required treatment.
Participants in the current study were given no further treatment or advice. Recommendations were made that participants monitor suspicious skin lesions or have them removed. With regard to future screening, 84.4% of participants with at least 1 categorized skin lesion were advised to have a regular follow-up within 1 year, while 15.6% were advised to follow-up within 1 to 2 years. Therefore, a period of 2 years before the next checkup, the period between screenings offered by statutory health insurance providers in Germany,12 was considered too long for the majority of participants, according to the dermatologists involved with our study.
Conclusion
The high rate of suspicious skin lesions diagnosed demonstrated the effectiveness of skin cancer screenings organized in the workplace, which should be recommended for all employees, not only those who are at high risk for developing skin cancer due to the nature of their work, such as those who work outdoors. It should be noted that the study group examined in the current study was a homogeneous group of employees of a technical company only and is therefore relatively selective. Nevertheless, despite the comparatively selective and young participant group, these examinations provide evidence of the importance of skin cancer screening programs for a wider population.
Acknowledgments
The authors thank Heidi Seybold, MD; Petra Wörl, MD; Sybille Thoma-Uszynski, MD; and Jens Bussmann, MD (all from Erlangen, Germany), for their support and active assistance in the practical implementation of this study.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58:129-132.
- El Ghissassi F, Baan R, Straif K, et al; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10:751-752.
- MacLennan R, Green AC, McLeod GR, et al. Increasing incidence of cutaneous melanoma in Queensland, Australia. J Natl Cancer Inst. 1992;84:1427-1432.
- Heinzerling LM, Dummer R, Panizzon RG, et al. Prevention campaign against skin cancer. Dermatology. 2002;205:229-233.
- Stratigos A, Nikolaou V, Kedicoglou S, et al. Melanoma/skin cancer screening in a Mediterranean country: results of the Euromelanoma Screening Day Campaign in Greece. J Eur Acad Dermatol Venereol. 2007;21:56-62.
- Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17:393-399.
- Choudhury K, Volkmer B, Greinert R, et al. Effectiveness of skin cancer screening programmes. Br J Dermatol. 2012;167:94-98.
- Eisemann N, Waldmann A, Geller AC, et al. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol. 2014;134:43-50.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
- Bekanntmachung (1430 A) eines Beschlusses des Gemeinsamen Bundeausschusses über eine Änderung der Krebsfrüherkennungs-Richtlinien: Hautkrebs-Screening [press release]. Berlin, Germany: Bundesministerium für Gesundheit (Federal Ministry of Health, Germany); vom 15. November 2007.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
- Schaefer I, Rustenbach SJ, Zimmer L, et al. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatology. 2008;217:169-172.
- Augustin M, Herberger K, Hintzen S, et al. Prevalence of skin lesions and need for treatment in a cohort of 90880 workers. Br J Dermatol. 2011;165:865-873.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Harbauer A, Binder M, Pehamberger H, et al. Validity of an unsupervised self-administered questionnaire for self-assessment of melanoma risk. Melanoma Res. 2003;13:537-542.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Augustin M, Stadler R, Reusch M, et al. Skin cancer screening in Germany—perception by the public. J Dtsch Dermatol Ges. 2012;10:42-49.
- Kornek T, Augustin M. Skin cancer prevention. J Dtsch Dermatol Ges. 2013;11:283-296.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists’ perspective. Dermatology. 2012;225:289-293.
- Barmer GEK Arztreport 2014 [press release]. Berlin, Germany: Barmer GEK; February 4, 2014.
- Bauer J, Garbe C. Acquired melanocytic nevi as riskfactor for melanoma development. a comprehensive review of epidemiological data. Pigment Cell Res. 2003;16:297-306.
- Winkler A, Plugfelder A, Weide B, et al. Screening for skin cancer in bank and insurance employees: risk profile and correlation of self and physician’s assessment. Int J Dermatol. 2015;54:419-423.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58:129-132.
- El Ghissassi F, Baan R, Straif K, et al; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10:751-752.
- MacLennan R, Green AC, McLeod GR, et al. Increasing incidence of cutaneous melanoma in Queensland, Australia. J Natl Cancer Inst. 1992;84:1427-1432.
- Heinzerling LM, Dummer R, Panizzon RG, et al. Prevention campaign against skin cancer. Dermatology. 2002;205:229-233.
- Stratigos A, Nikolaou V, Kedicoglou S, et al. Melanoma/skin cancer screening in a Mediterranean country: results of the Euromelanoma Screening Day Campaign in Greece. J Eur Acad Dermatol Venereol. 2007;21:56-62.
- Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17:393-399.
- Choudhury K, Volkmer B, Greinert R, et al. Effectiveness of skin cancer screening programmes. Br J Dermatol. 2012;167:94-98.
- Eisemann N, Waldmann A, Geller AC, et al. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol. 2014;134:43-50.
- Katalinic A, Waldmann A, Weinstock MA, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
- Bekanntmachung (1430 A) eines Beschlusses des Gemeinsamen Bundeausschusses über eine Änderung der Krebsfrüherkennungs-Richtlinien: Hautkrebs-Screening [press release]. Berlin, Germany: Bundesministerium für Gesundheit (Federal Ministry of Health, Germany); vom 15. November 2007.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
- Schaefer I, Rustenbach SJ, Zimmer L, et al. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatology. 2008;217:169-172.
- Augustin M, Herberger K, Hintzen S, et al. Prevalence of skin lesions and need for treatment in a cohort of 90880 workers. Br J Dermatol. 2011;165:865-873.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Harbauer A, Binder M, Pehamberger H, et al. Validity of an unsupervised self-administered questionnaire for self-assessment of melanoma risk. Melanoma Res. 2003;13:537-542.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Augustin M, Stadler R, Reusch M, et al. Skin cancer screening in Germany—perception by the public. J Dtsch Dermatol Ges. 2012;10:42-49.
- Kornek T, Augustin M. Skin cancer prevention. J Dtsch Dermatol Ges. 2013;11:283-296.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists’ perspective. Dermatology. 2012;225:289-293.
- Barmer GEK Arztreport 2014 [press release]. Berlin, Germany: Barmer GEK; February 4, 2014.
- Bauer J, Garbe C. Acquired melanocytic nevi as riskfactor for melanoma development. a comprehensive review of epidemiological data. Pigment Cell Res. 2003;16:297-306.
- Winkler A, Plugfelder A, Weide B, et al. Screening for skin cancer in bank and insurance employees: risk profile and correlation of self and physician’s assessment. Int J Dermatol. 2015;54:419-423.
Practice Points
- Employee skin cancer screening programs are an important method of examining high numbers of individuals quickly and efficiently and should be used as an important tool for secondary skin cancer prevention.
- The high rate of suspicious skin lesions diagnosed in this study demonstrates the effectiveness of skin cancer screenings organized in the workplace and provides evidence of the importance of skin cancer screening programs for a wider population.
- Employee skin cancer screening programs should be recommended for all employees, not only those who are at high risk for developing skin cancer due to the nature of their work, such as those who work outdoors.
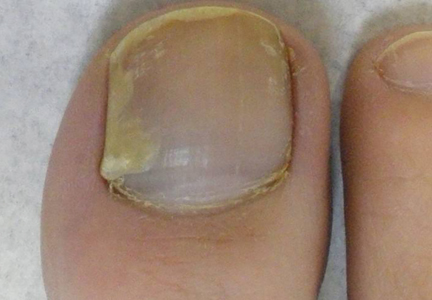
Subungual Onycholemmal Cyst of the Toenail Mimicking Subungual Melanoma
Case Report
A 23-year-old woman presented with a horizontal split along the midline of the right great toenail associated with some tenderness of 2 to 3 months’ duration. Approximately 5 years prior, she noticed a bluish-colored area under the nail that had been steadily increasing in size. She denied a history of trauma, drainage, or bleeding. There was no history of other nail abnormalities. Her medications and personal, family, and social history were noncontributory.
Physical examination of the right great toenail revealed a horizontal split of the nail plate with a bluish hue visible under the nail plate (Figure 1A). The remaining toenails and fingernails were normal. A punch biopsy of the nail bed was performed with a presumptive clinical diagnosis of subungual melanoma versus melanocytic nevus versus cyst (Figure 1B). Nail plate avulsion revealed a blackened nail bed dotted with areas of bluish color and a red friable nodule present focally. Upon further inspection, extension was apparent into the distal matrix.
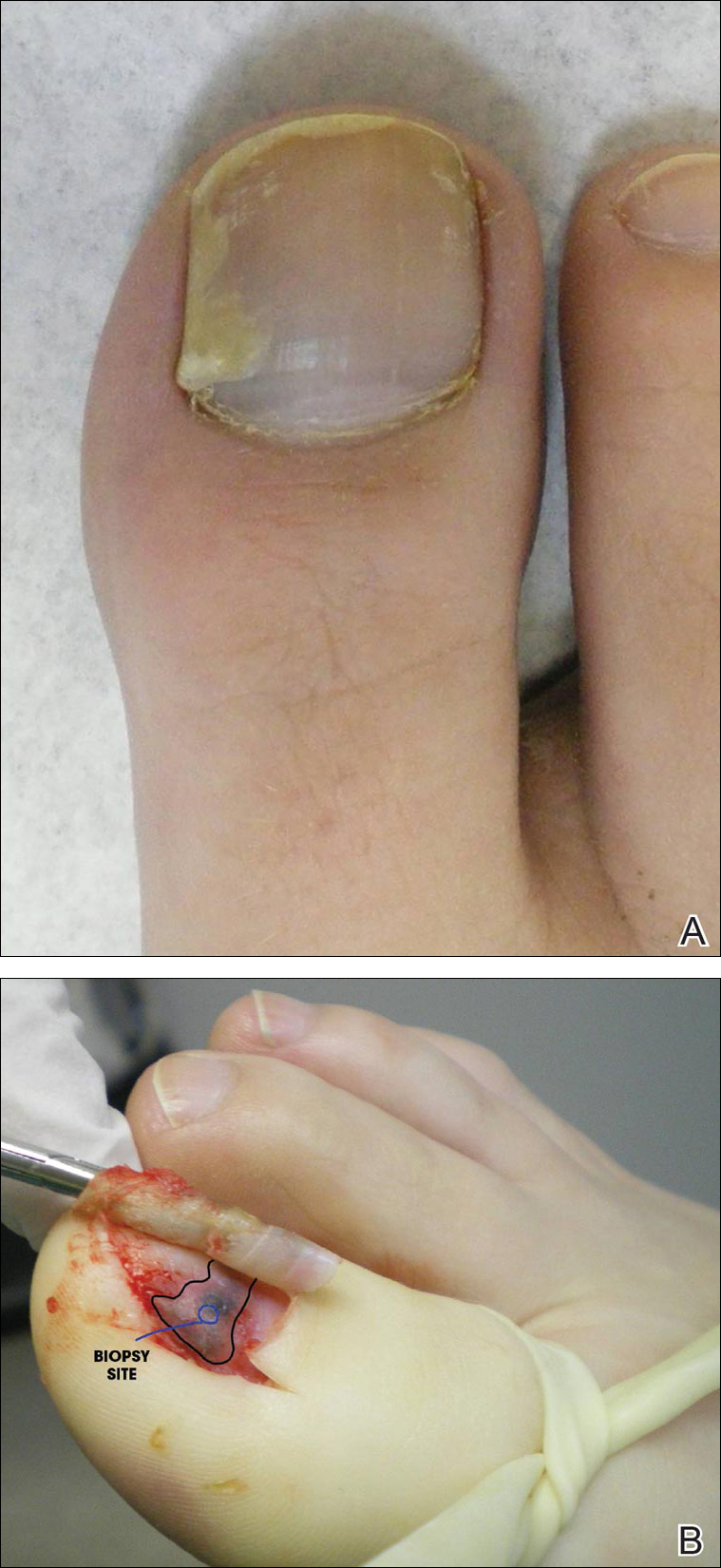
Histopathologic examination revealed a cystic structure with an epithelial lining mostly reminiscent of an isthmus catagen cyst admixed with the presence of both an intermittent focal granular layer and an eosinophilic cuticle surrounding pink laminated keratin, most consistent with a diagnosis of subungual onycholemmal cyst (SOC)(Figure 2). A reexcision was performed with removal of half of the nail bed, including a portion of the distal matrix extending inferiorly to the bone. Variably sized, epithelium-lined, keratin-filled cystic structures emanated from the nail bed epithelium. There were foci of hemorrhage and granulation tissue secondary to cyst rupture (Figure 3). The defect healed by secondary intention. No clinical evidence of recurrence was seen at 6-month follow-up.
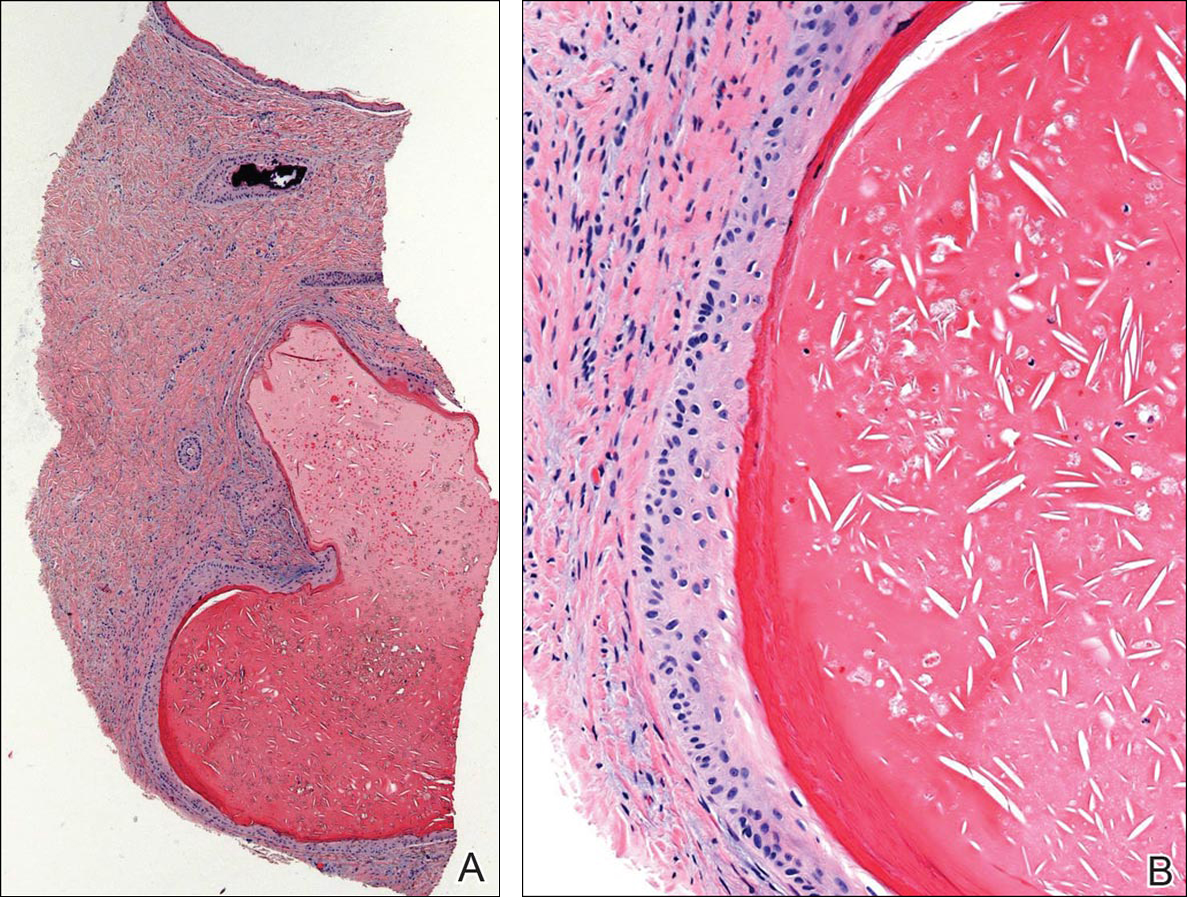

Subungual onycholemmal cysts, also known as subungual epidermoid cysts or subungual epidermoid inclusions, are rare and distinctive nail abnormalities occurring in the dermis of the nail bed. We present a case of an SOC in a toenail mimicking subungual malignant melanoma.
Originally described by Samman1 in 1959, SOCs were attributed to trauma to the nail with resultant implantation of the epidermis into the deeper tissue. Lewin2,3 examined 90 postmortem fingernail and nail bed samples and found 8 subungual epidermoid cysts associated with clubbing of the fingernails. He postulated that the early pathogenesis of clubbing involved dermal fibroblast proliferation in the nail bed, leading to sequestration of nail bed epithelium into the dermis with resultant cyst formation. Microscopic subungual cysts also were identified in normal-appearing nails without evidence of trauma, thought to have arisen from the tips of the nail bed rete ridges by a process of bulbous proliferation rather than sequestration. These findings in normal nails suggest that SOCs may represent a more common entity than previously recognized.
It is imperative to recognize the presence of nail unit tumors early because of the risk for permanent nail plate dystrophy and the possibility of a malignant tumor.4,5 Subungual onycholemmal cysts may present with a wide spectrum of clinical findings including marked subungual hyperkeratosis, onychodystrophy, ridging, nail bed pigmentation, clubbing, thickening, or less often a normal-appearing nail. Based on reported cases, several trends are evident. Although nail dystrophy is most often asymptomatic, pain is not uncommon.5,6 It most commonly involves single digits, predominantly thumbs and great toenails.7,8 This predilection suggests that trauma or other local factors may be involved in its pathogenesis. Of note, trauma to the nail may occur years before the development of the lesions or it may not be recalled at all.
Diagnosis requires a degree of clinical suspicion and a nail bed biopsy with partial or total nail plate avulsion to visualize the pathologic portion of the nail bed. Because surgical intervention may lead to the implantation of epithelium, recurrences after nail biopsy or excision may occur.
In contrast to epidermal inclusion cysts arising in the skin, most SOCs do not have a granular layer.9 Hair and nails represent analogous differentiation products of the ectoderm. The nail matrix is homologous to portions of the hair matrix, while the nail bed epithelium is comparable to the outer root sheath of the hair follicle.7 Subungual onycholemmal cysts originate from the nail bed epithelium, which keratinizes in the absence of a granular layer, similar to the follicular isthmus outer root sheath. Thus, SOCs are comparable to the outer root sheath–derived isthmus-catagen cysts because of their abrupt central keratinization.8
Subungual onycholemmal cysts also must be distinguished from slowly growing malignant tumors of the nail bed epithelium, referred to as onycholemmal carcinomas by Alessi et al.10 This entity characteristically presents in elderly patients as a slowly growing, circumscribed, subungual discoloration that may ulcerate, destroying the nail apparatus and penetrating the phalangeal bone. On histopathology, it is characterized by small cysts filled with eosinophilic keratin devoid of a granular layer and lined by atypical squamous epithelium accompanied by solid nests and strands of atypical keratinocytes within the dermis.11 When a cystic component and clear cells predominate, the designation of malignant proliferating onycholemmal cyst has been applied. Its infiltrative growth pattern with destruction of the underlying bone makes it an important entity to exclude when considering the differential diagnosis of tumors of the nail bed.
Subungual melanomas comprise only 1% to 3% of malignant melanomas and 85% are initially misdiagnosed due to their rarity and nonspecific variable presentation. Aside from clinical evidence of Hutchinson sign in the early stages in almost all cases, accurate diagnosis of subungual melanoma and differentiation from SOCs relies on histopathology. A biopsy is necessary to make the diagnosis, but even microscopic findings may be nonspecific during the early stages.
Conclusion
We report a case of a 23-year-old woman with horizontal ridging and tenderness of the right great toenail associated with pigmentation of 5 years’ duration due to an SOC. The etiology of these subungual cysts, with or without nail abnormalities, still remains unclear. Its predilection for the thumbs and great toenails suggests that trauma or other local factors may be involved in its pathogenesis. Because of the rarity of this entity, there are no guidelines for surgical treatment. Subungual onycholemmal cysts may be an underrecognized and more common entity that must be considered when discussing tumors of the nail unit.
- Samman PD. The human toe nail. its genesis and blood supply. Br J Dermatol. 1959;71:296-302.
- Lewin K. The normal fingernail. Br J Dermatol. 1965;77:421-430.
- Lewin K. Subungual epidermoid inclusions. Br J Dermatol. 1969;81:671-675.
- Dominguez-Cherit J, Chanussot-Deprez C, Maria-Sarti H, et al. Nail unit tumors: a study of 234 patients in the dermatology department of the “Dr. Manuel Gea González” General Hospital in Mexico City. Dermatol Surg. 2008;34:1363-1371.
- Sáez-de-Ocariz MM, Domínguez-Cherit J, García-Corona C. Subungual epidermoid cysts. Int J Dermatol. 2001;40:524-526.
- Molly DO, Herbert K. Subungual epidermoid cyst. J Hand Surg Br. 2006;31:345.
- Telang GH, Jellinek N. Multiple calcified subungual epidermoid inclusions. J Am Acad Dermatol. 2007;56:336-339.
- Fanti PA, Tosti A. Subungual epidermoid inclusions: report of 8 cases. Dermatologica. 1989;178:209-212.
- Takiyoshi N, Nakano H, Matsuzaki T, et al. An eclipse in the subungual space: a diagnostic sign for a subungual epidermal cyst? Br J Dermatol. 2009;161:962-963.
- Alessi E, Coggi A, Gianotti R, et al. Onycholemmal carcinoma. Am J Dermatopathol. 2004;26:397-402.
- Inaoki M, Makino E, Adachi M, et al. Onycholemmal carcinoma. J Cutan Pathol. 2006;33:577-580.
Case Report
A 23-year-old woman presented with a horizontal split along the midline of the right great toenail associated with some tenderness of 2 to 3 months’ duration. Approximately 5 years prior, she noticed a bluish-colored area under the nail that had been steadily increasing in size. She denied a history of trauma, drainage, or bleeding. There was no history of other nail abnormalities. Her medications and personal, family, and social history were noncontributory.
Physical examination of the right great toenail revealed a horizontal split of the nail plate with a bluish hue visible under the nail plate (Figure 1A). The remaining toenails and fingernails were normal. A punch biopsy of the nail bed was performed with a presumptive clinical diagnosis of subungual melanoma versus melanocytic nevus versus cyst (Figure 1B). Nail plate avulsion revealed a blackened nail bed dotted with areas of bluish color and a red friable nodule present focally. Upon further inspection, extension was apparent into the distal matrix.

Histopathologic examination revealed a cystic structure with an epithelial lining mostly reminiscent of an isthmus catagen cyst admixed with the presence of both an intermittent focal granular layer and an eosinophilic cuticle surrounding pink laminated keratin, most consistent with a diagnosis of subungual onycholemmal cyst (SOC)(Figure 2). A reexcision was performed with removal of half of the nail bed, including a portion of the distal matrix extending inferiorly to the bone. Variably sized, epithelium-lined, keratin-filled cystic structures emanated from the nail bed epithelium. There were foci of hemorrhage and granulation tissue secondary to cyst rupture (Figure 3). The defect healed by secondary intention. No clinical evidence of recurrence was seen at 6-month follow-up.


Subungual onycholemmal cysts, also known as subungual epidermoid cysts or subungual epidermoid inclusions, are rare and distinctive nail abnormalities occurring in the dermis of the nail bed. We present a case of an SOC in a toenail mimicking subungual malignant melanoma.
Originally described by Samman1 in 1959, SOCs were attributed to trauma to the nail with resultant implantation of the epidermis into the deeper tissue. Lewin2,3 examined 90 postmortem fingernail and nail bed samples and found 8 subungual epidermoid cysts associated with clubbing of the fingernails. He postulated that the early pathogenesis of clubbing involved dermal fibroblast proliferation in the nail bed, leading to sequestration of nail bed epithelium into the dermis with resultant cyst formation. Microscopic subungual cysts also were identified in normal-appearing nails without evidence of trauma, thought to have arisen from the tips of the nail bed rete ridges by a process of bulbous proliferation rather than sequestration. These findings in normal nails suggest that SOCs may represent a more common entity than previously recognized.
It is imperative to recognize the presence of nail unit tumors early because of the risk for permanent nail plate dystrophy and the possibility of a malignant tumor.4,5 Subungual onycholemmal cysts may present with a wide spectrum of clinical findings including marked subungual hyperkeratosis, onychodystrophy, ridging, nail bed pigmentation, clubbing, thickening, or less often a normal-appearing nail. Based on reported cases, several trends are evident. Although nail dystrophy is most often asymptomatic, pain is not uncommon.5,6 It most commonly involves single digits, predominantly thumbs and great toenails.7,8 This predilection suggests that trauma or other local factors may be involved in its pathogenesis. Of note, trauma to the nail may occur years before the development of the lesions or it may not be recalled at all.
Diagnosis requires a degree of clinical suspicion and a nail bed biopsy with partial or total nail plate avulsion to visualize the pathologic portion of the nail bed. Because surgical intervention may lead to the implantation of epithelium, recurrences after nail biopsy or excision may occur.
In contrast to epidermal inclusion cysts arising in the skin, most SOCs do not have a granular layer.9 Hair and nails represent analogous differentiation products of the ectoderm. The nail matrix is homologous to portions of the hair matrix, while the nail bed epithelium is comparable to the outer root sheath of the hair follicle.7 Subungual onycholemmal cysts originate from the nail bed epithelium, which keratinizes in the absence of a granular layer, similar to the follicular isthmus outer root sheath. Thus, SOCs are comparable to the outer root sheath–derived isthmus-catagen cysts because of their abrupt central keratinization.8
Subungual onycholemmal cysts also must be distinguished from slowly growing malignant tumors of the nail bed epithelium, referred to as onycholemmal carcinomas by Alessi et al.10 This entity characteristically presents in elderly patients as a slowly growing, circumscribed, subungual discoloration that may ulcerate, destroying the nail apparatus and penetrating the phalangeal bone. On histopathology, it is characterized by small cysts filled with eosinophilic keratin devoid of a granular layer and lined by atypical squamous epithelium accompanied by solid nests and strands of atypical keratinocytes within the dermis.11 When a cystic component and clear cells predominate, the designation of malignant proliferating onycholemmal cyst has been applied. Its infiltrative growth pattern with destruction of the underlying bone makes it an important entity to exclude when considering the differential diagnosis of tumors of the nail bed.
Subungual melanomas comprise only 1% to 3% of malignant melanomas and 85% are initially misdiagnosed due to their rarity and nonspecific variable presentation. Aside from clinical evidence of Hutchinson sign in the early stages in almost all cases, accurate diagnosis of subungual melanoma and differentiation from SOCs relies on histopathology. A biopsy is necessary to make the diagnosis, but even microscopic findings may be nonspecific during the early stages.
Conclusion
We report a case of a 23-year-old woman with horizontal ridging and tenderness of the right great toenail associated with pigmentation of 5 years’ duration due to an SOC. The etiology of these subungual cysts, with or without nail abnormalities, still remains unclear. Its predilection for the thumbs and great toenails suggests that trauma or other local factors may be involved in its pathogenesis. Because of the rarity of this entity, there are no guidelines for surgical treatment. Subungual onycholemmal cysts may be an underrecognized and more common entity that must be considered when discussing tumors of the nail unit.
Case Report
A 23-year-old woman presented with a horizontal split along the midline of the right great toenail associated with some tenderness of 2 to 3 months’ duration. Approximately 5 years prior, she noticed a bluish-colored area under the nail that had been steadily increasing in size. She denied a history of trauma, drainage, or bleeding. There was no history of other nail abnormalities. Her medications and personal, family, and social history were noncontributory.
Physical examination of the right great toenail revealed a horizontal split of the nail plate with a bluish hue visible under the nail plate (Figure 1A). The remaining toenails and fingernails were normal. A punch biopsy of the nail bed was performed with a presumptive clinical diagnosis of subungual melanoma versus melanocytic nevus versus cyst (Figure 1B). Nail plate avulsion revealed a blackened nail bed dotted with areas of bluish color and a red friable nodule present focally. Upon further inspection, extension was apparent into the distal matrix.

Histopathologic examination revealed a cystic structure with an epithelial lining mostly reminiscent of an isthmus catagen cyst admixed with the presence of both an intermittent focal granular layer and an eosinophilic cuticle surrounding pink laminated keratin, most consistent with a diagnosis of subungual onycholemmal cyst (SOC)(Figure 2). A reexcision was performed with removal of half of the nail bed, including a portion of the distal matrix extending inferiorly to the bone. Variably sized, epithelium-lined, keratin-filled cystic structures emanated from the nail bed epithelium. There were foci of hemorrhage and granulation tissue secondary to cyst rupture (Figure 3). The defect healed by secondary intention. No clinical evidence of recurrence was seen at 6-month follow-up.


Subungual onycholemmal cysts, also known as subungual epidermoid cysts or subungual epidermoid inclusions, are rare and distinctive nail abnormalities occurring in the dermis of the nail bed. We present a case of an SOC in a toenail mimicking subungual malignant melanoma.
Originally described by Samman1 in 1959, SOCs were attributed to trauma to the nail with resultant implantation of the epidermis into the deeper tissue. Lewin2,3 examined 90 postmortem fingernail and nail bed samples and found 8 subungual epidermoid cysts associated with clubbing of the fingernails. He postulated that the early pathogenesis of clubbing involved dermal fibroblast proliferation in the nail bed, leading to sequestration of nail bed epithelium into the dermis with resultant cyst formation. Microscopic subungual cysts also were identified in normal-appearing nails without evidence of trauma, thought to have arisen from the tips of the nail bed rete ridges by a process of bulbous proliferation rather than sequestration. These findings in normal nails suggest that SOCs may represent a more common entity than previously recognized.
It is imperative to recognize the presence of nail unit tumors early because of the risk for permanent nail plate dystrophy and the possibility of a malignant tumor.4,5 Subungual onycholemmal cysts may present with a wide spectrum of clinical findings including marked subungual hyperkeratosis, onychodystrophy, ridging, nail bed pigmentation, clubbing, thickening, or less often a normal-appearing nail. Based on reported cases, several trends are evident. Although nail dystrophy is most often asymptomatic, pain is not uncommon.5,6 It most commonly involves single digits, predominantly thumbs and great toenails.7,8 This predilection suggests that trauma or other local factors may be involved in its pathogenesis. Of note, trauma to the nail may occur years before the development of the lesions or it may not be recalled at all.
Diagnosis requires a degree of clinical suspicion and a nail bed biopsy with partial or total nail plate avulsion to visualize the pathologic portion of the nail bed. Because surgical intervention may lead to the implantation of epithelium, recurrences after nail biopsy or excision may occur.
In contrast to epidermal inclusion cysts arising in the skin, most SOCs do not have a granular layer.9 Hair and nails represent analogous differentiation products of the ectoderm. The nail matrix is homologous to portions of the hair matrix, while the nail bed epithelium is comparable to the outer root sheath of the hair follicle.7 Subungual onycholemmal cysts originate from the nail bed epithelium, which keratinizes in the absence of a granular layer, similar to the follicular isthmus outer root sheath. Thus, SOCs are comparable to the outer root sheath–derived isthmus-catagen cysts because of their abrupt central keratinization.8
Subungual onycholemmal cysts also must be distinguished from slowly growing malignant tumors of the nail bed epithelium, referred to as onycholemmal carcinomas by Alessi et al.10 This entity characteristically presents in elderly patients as a slowly growing, circumscribed, subungual discoloration that may ulcerate, destroying the nail apparatus and penetrating the phalangeal bone. On histopathology, it is characterized by small cysts filled with eosinophilic keratin devoid of a granular layer and lined by atypical squamous epithelium accompanied by solid nests and strands of atypical keratinocytes within the dermis.11 When a cystic component and clear cells predominate, the designation of malignant proliferating onycholemmal cyst has been applied. Its infiltrative growth pattern with destruction of the underlying bone makes it an important entity to exclude when considering the differential diagnosis of tumors of the nail bed.
Subungual melanomas comprise only 1% to 3% of malignant melanomas and 85% are initially misdiagnosed due to their rarity and nonspecific variable presentation. Aside from clinical evidence of Hutchinson sign in the early stages in almost all cases, accurate diagnosis of subungual melanoma and differentiation from SOCs relies on histopathology. A biopsy is necessary to make the diagnosis, but even microscopic findings may be nonspecific during the early stages.
Conclusion
We report a case of a 23-year-old woman with horizontal ridging and tenderness of the right great toenail associated with pigmentation of 5 years’ duration due to an SOC. The etiology of these subungual cysts, with or without nail abnormalities, still remains unclear. Its predilection for the thumbs and great toenails suggests that trauma or other local factors may be involved in its pathogenesis. Because of the rarity of this entity, there are no guidelines for surgical treatment. Subungual onycholemmal cysts may be an underrecognized and more common entity that must be considered when discussing tumors of the nail unit.
- Samman PD. The human toe nail. its genesis and blood supply. Br J Dermatol. 1959;71:296-302.
- Lewin K. The normal fingernail. Br J Dermatol. 1965;77:421-430.
- Lewin K. Subungual epidermoid inclusions. Br J Dermatol. 1969;81:671-675.
- Dominguez-Cherit J, Chanussot-Deprez C, Maria-Sarti H, et al. Nail unit tumors: a study of 234 patients in the dermatology department of the “Dr. Manuel Gea González” General Hospital in Mexico City. Dermatol Surg. 2008;34:1363-1371.
- Sáez-de-Ocariz MM, Domínguez-Cherit J, García-Corona C. Subungual epidermoid cysts. Int J Dermatol. 2001;40:524-526.
- Molly DO, Herbert K. Subungual epidermoid cyst. J Hand Surg Br. 2006;31:345.
- Telang GH, Jellinek N. Multiple calcified subungual epidermoid inclusions. J Am Acad Dermatol. 2007;56:336-339.
- Fanti PA, Tosti A. Subungual epidermoid inclusions: report of 8 cases. Dermatologica. 1989;178:209-212.
- Takiyoshi N, Nakano H, Matsuzaki T, et al. An eclipse in the subungual space: a diagnostic sign for a subungual epidermal cyst? Br J Dermatol. 2009;161:962-963.
- Alessi E, Coggi A, Gianotti R, et al. Onycholemmal carcinoma. Am J Dermatopathol. 2004;26:397-402.
- Inaoki M, Makino E, Adachi M, et al. Onycholemmal carcinoma. J Cutan Pathol. 2006;33:577-580.
- Samman PD. The human toe nail. its genesis and blood supply. Br J Dermatol. 1959;71:296-302.
- Lewin K. The normal fingernail. Br J Dermatol. 1965;77:421-430.
- Lewin K. Subungual epidermoid inclusions. Br J Dermatol. 1969;81:671-675.
- Dominguez-Cherit J, Chanussot-Deprez C, Maria-Sarti H, et al. Nail unit tumors: a study of 234 patients in the dermatology department of the “Dr. Manuel Gea González” General Hospital in Mexico City. Dermatol Surg. 2008;34:1363-1371.
- Sáez-de-Ocariz MM, Domínguez-Cherit J, García-Corona C. Subungual epidermoid cysts. Int J Dermatol. 2001;40:524-526.
- Molly DO, Herbert K. Subungual epidermoid cyst. J Hand Surg Br. 2006;31:345.
- Telang GH, Jellinek N. Multiple calcified subungual epidermoid inclusions. J Am Acad Dermatol. 2007;56:336-339.
- Fanti PA, Tosti A. Subungual epidermoid inclusions: report of 8 cases. Dermatologica. 1989;178:209-212.
- Takiyoshi N, Nakano H, Matsuzaki T, et al. An eclipse in the subungual space: a diagnostic sign for a subungual epidermal cyst? Br J Dermatol. 2009;161:962-963.
- Alessi E, Coggi A, Gianotti R, et al. Onycholemmal carcinoma. Am J Dermatopathol. 2004;26:397-402.
- Inaoki M, Makino E, Adachi M, et al. Onycholemmal carcinoma. J Cutan Pathol. 2006;33:577-580.
Practice Points
- Trauma to the nail may occur years before the development of subungual onycholemmal cysts or it may not be recalled at all.
- Diagnosis requires a degree of clinical suspicion and a nail bed biopsy.
- Subungual onycholemmal cysts must be distinguished from slowly growing malignant tumors of the nail bed epithelium.
Skip SNL biopsy for desmoplastic melanoma
SEATTLE – Sentinel lymph node biopsy in patients with head or neck desmoplastic melanoma is positive only 6% of the time, and it doesn’t change the risk of recurrence.
Although sentinel lymph node biopsy (SLNB) is routine in more common forms of cutaneous melanoma, findings from a retrospective case-control study suggest that it’s “not really necessary” for desmoplastic melanoma (DM) of the head or neck, said lead investigator Dylan Roden, MD, of the department of otolaryngology, New York University. General surgeons have pretty much come to that conclusion for DM elsewhere on the body, but it hasn’t been shown before for neck and head lesions, he said.
DM, an invasive form of melanoma in which malignant cells are surrounded by fibrous tissue, accounts for maybe 2% of cutaneous melanomas, with half or so presenting on the head or neck. The reason SLNB is of less use than with other melanomas is that DM “doesn’t often spread through the lymphatics. It’s not that patients won’t ever have metastases, but maybe it will be through the blood. Removing a lymph node won’t necessarily” detect it, Dr. Roden said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Forgoing SLNB has the added benefit of shaving an hour and a half or more off surgery, which is important since DM patients tend to be older, he added.
The NYU team matched 32 of their cases with 60 controls with more common superficial spreading or nodular melanoma of the head and neck, based on age, gender, ulceration status, and tumor stage. Mean tumor thickness in both groups was more than 4 mm.
SLNB was performed in 16 DM patients (50%) and 36 control patients (60%); it was positive in one DM patient (6.3%) versus 8 of 28 controls with reported results (28.6%).
Eleven DM patients (34%) had a recurrence, which was less frequent then in controls, where 33 patients (55%) had a recurrence (P = .05). “SNLB did not change the risk of overall or regional recurrence” in DM, Dr. Roden said.
Recurrence was more than twice as likely in control patients (odds ratio, 2.33; P = .06). Meanwhile, recurrence in DM was linked to perineural invasion (P = .02), but not ulceration status (P = .12) or mitoses (P = .40).
DM patients also had better 5-year overall survival (79% versus 62%) and disease-free survival (70% versus 42%; P for both = .06). In general, DM “has a more favorable prognosis,” Dr. Roden said.
Cases and controls were in their mid-60s, on average, and most were men. Ulceration was present in about a quarter of patients. Mitosis was more common in superficial spreading and nodular patients (92% versus 53%; P less than .001), while perineural invasion was more common in DM (40% versus 7%; P less than.001).
Although outcomes were more favorable for DM, previous studies have found a higher rate of sentinel lymph node metastases – above 20% – for DM lesions with mixed, rather than pure, pathology. The 6.3% positive SLNB rate at NYU is more in line with what’s been reported before for pure lesions. The team plans to look into the matter.
There was no outside funding for the work, and Dr. Roden had no disclosures.
SEATTLE – Sentinel lymph node biopsy in patients with head or neck desmoplastic melanoma is positive only 6% of the time, and it doesn’t change the risk of recurrence.
Although sentinel lymph node biopsy (SLNB) is routine in more common forms of cutaneous melanoma, findings from a retrospective case-control study suggest that it’s “not really necessary” for desmoplastic melanoma (DM) of the head or neck, said lead investigator Dylan Roden, MD, of the department of otolaryngology, New York University. General surgeons have pretty much come to that conclusion for DM elsewhere on the body, but it hasn’t been shown before for neck and head lesions, he said.
DM, an invasive form of melanoma in which malignant cells are surrounded by fibrous tissue, accounts for maybe 2% of cutaneous melanomas, with half or so presenting on the head or neck. The reason SLNB is of less use than with other melanomas is that DM “doesn’t often spread through the lymphatics. It’s not that patients won’t ever have metastases, but maybe it will be through the blood. Removing a lymph node won’t necessarily” detect it, Dr. Roden said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Forgoing SLNB has the added benefit of shaving an hour and a half or more off surgery, which is important since DM patients tend to be older, he added.
The NYU team matched 32 of their cases with 60 controls with more common superficial spreading or nodular melanoma of the head and neck, based on age, gender, ulceration status, and tumor stage. Mean tumor thickness in both groups was more than 4 mm.
SLNB was performed in 16 DM patients (50%) and 36 control patients (60%); it was positive in one DM patient (6.3%) versus 8 of 28 controls with reported results (28.6%).
Eleven DM patients (34%) had a recurrence, which was less frequent then in controls, where 33 patients (55%) had a recurrence (P = .05). “SNLB did not change the risk of overall or regional recurrence” in DM, Dr. Roden said.
Recurrence was more than twice as likely in control patients (odds ratio, 2.33; P = .06). Meanwhile, recurrence in DM was linked to perineural invasion (P = .02), but not ulceration status (P = .12) or mitoses (P = .40).
DM patients also had better 5-year overall survival (79% versus 62%) and disease-free survival (70% versus 42%; P for both = .06). In general, DM “has a more favorable prognosis,” Dr. Roden said.
Cases and controls were in their mid-60s, on average, and most were men. Ulceration was present in about a quarter of patients. Mitosis was more common in superficial spreading and nodular patients (92% versus 53%; P less than .001), while perineural invasion was more common in DM (40% versus 7%; P less than.001).
Although outcomes were more favorable for DM, previous studies have found a higher rate of sentinel lymph node metastases – above 20% – for DM lesions with mixed, rather than pure, pathology. The 6.3% positive SLNB rate at NYU is more in line with what’s been reported before for pure lesions. The team plans to look into the matter.
There was no outside funding for the work, and Dr. Roden had no disclosures.
SEATTLE – Sentinel lymph node biopsy in patients with head or neck desmoplastic melanoma is positive only 6% of the time, and it doesn’t change the risk of recurrence.
Although sentinel lymph node biopsy (SLNB) is routine in more common forms of cutaneous melanoma, findings from a retrospective case-control study suggest that it’s “not really necessary” for desmoplastic melanoma (DM) of the head or neck, said lead investigator Dylan Roden, MD, of the department of otolaryngology, New York University. General surgeons have pretty much come to that conclusion for DM elsewhere on the body, but it hasn’t been shown before for neck and head lesions, he said.
DM, an invasive form of melanoma in which malignant cells are surrounded by fibrous tissue, accounts for maybe 2% of cutaneous melanomas, with half or so presenting on the head or neck. The reason SLNB is of less use than with other melanomas is that DM “doesn’t often spread through the lymphatics. It’s not that patients won’t ever have metastases, but maybe it will be through the blood. Removing a lymph node won’t necessarily” detect it, Dr. Roden said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Forgoing SLNB has the added benefit of shaving an hour and a half or more off surgery, which is important since DM patients tend to be older, he added.
The NYU team matched 32 of their cases with 60 controls with more common superficial spreading or nodular melanoma of the head and neck, based on age, gender, ulceration status, and tumor stage. Mean tumor thickness in both groups was more than 4 mm.
SLNB was performed in 16 DM patients (50%) and 36 control patients (60%); it was positive in one DM patient (6.3%) versus 8 of 28 controls with reported results (28.6%).
Eleven DM patients (34%) had a recurrence, which was less frequent then in controls, where 33 patients (55%) had a recurrence (P = .05). “SNLB did not change the risk of overall or regional recurrence” in DM, Dr. Roden said.
Recurrence was more than twice as likely in control patients (odds ratio, 2.33; P = .06). Meanwhile, recurrence in DM was linked to perineural invasion (P = .02), but not ulceration status (P = .12) or mitoses (P = .40).
DM patients also had better 5-year overall survival (79% versus 62%) and disease-free survival (70% versus 42%; P for both = .06). In general, DM “has a more favorable prognosis,” Dr. Roden said.
Cases and controls were in their mid-60s, on average, and most were men. Ulceration was present in about a quarter of patients. Mitosis was more common in superficial spreading and nodular patients (92% versus 53%; P less than .001), while perineural invasion was more common in DM (40% versus 7%; P less than.001).
Although outcomes were more favorable for DM, previous studies have found a higher rate of sentinel lymph node metastases – above 20% – for DM lesions with mixed, rather than pure, pathology. The 6.3% positive SLNB rate at NYU is more in line with what’s been reported before for pure lesions. The team plans to look into the matter.
There was no outside funding for the work, and Dr. Roden had no disclosures.
AT AHNS 2016
Key clinical point: Sentinel lymph node biopsy in patients with head or neck desmoplastic melanoma is positive only 6% of the time, and it doesn’t change the risk of recurrence.
Major finding: SLNB was performed in 16 DM patients (50%) and 36 control patients (60%); it was positive in one DM patient (6.3%) versus 8 of 28 controls with reported results (28.6%).
Data source: Retrospective case-control study with 92 patients
Disclosures: There was no outside funding for the work, and the presenter had no disclosures.
Enhanced Radiation Dermatitis Associated With Concurrent Palliative Radiation and Vemurafenib Therapy
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.
The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.
We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.

The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.

We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.

The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.

We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
Practice Points
- Given the increased frequency of palliative and adjuvant radiation therapy in patients with metastatic melanoma, it is critical to understand the potential cutaneous side effects of vemurafenib when used in conjunction with radiotherapy.
- Clinicians should be aware of the increased risk for severe radiation dermatitis in patients on vemurafenib who are receiving concurrent palliative radiation therapy.
Certain skin cancers respond to nonsurgical treatments
BOSTON – Surgery is the standard of care for most skin cancers, but nonsurgical and adjuvant treatments can be good options for certain skin cancers when surgery would be neither curative nor feasible, according to Anthony Rossi, MD.
“Whether you’re treating a superficial basal cell or superficial squamous cell carcinoma, I think first and foremost, if you’re going to use nonsurgical treatment options, it’s important to have a good biopsy diagnosis,” Dr. Rossi said at the American Academy of Dermatology summer meeting. He also advised that the biopsy capture the entire lesion or a good portion of it to get a good representation. “You don’t want to be surprised by any hiding, high-risk subtypes,” said Dr. Rossi, of the Memorial Sloan Kettering Cancer Center in New York.
Deciding on a nonsurgical treatment option should be based on knowing the patient. For example, know the patient’s concerns about cosmetic deformities, willingness to undergo surgery or not, and ability and willingness to do follow-up self-care.
For superficial basal cell or squamous cell carcinomas in situ and even lentigo maligna in situ not amenable to surgery, imiquimod may be an appropriate treatment. Dr. Rossi said his practice is to use it in an incremental fashion, starting with application five times per week, going to every day if there is no response after 1 to 2 weeks. If the response remains inadequate, he recommended adding a topical retinoid, such as tazarotene, in an effort to increase penetration.
For basal cell carcinomas and squamous cell carcinoma in situ, he uses imiquimod for a total of 6 to 8 weeks, starting from the time of the first reaction. For melanoma in situ, he uses it for more than 60 applications (12 weeks).
To show patients where they should be applying any topical treatment, Dr. Rossi marks the skin, photographs it, and prints out a picture for the patient. Sometimes he uses the patient’s phone to take the picture. For a basal cell or squamous cell carcinoma in situ, he indicates an area at least 5 to 10 mm beyond the margin of the tumor. The area is even larger for melanomas.
Dr. Rossi studies confocal microscopy to detect skin cancers. He uses it before treating a lesion to define the clinical boundaries of the lesion and the boundaries for the nonsurgical treatments, and then he uses it on follow up to look for any recurrences.
New anti-tumor agents
Two oral inhibitors of the sonic hedgehog pathway have been approved within the past 5 years for locally advanced or metastatic basal cell carcinomas. “In the right person, they can be quite beneficial if surgery would leave them with a very large cosmetic deformity or if surgery would be not curative,” Dr. Rossi said. “We’re seeing good results” with acceptable adverse events, specifically taste disturbances, muscle cramps, and hair loss. The first such drug, vismodegib (Erivedge), was approved in 2012, and sonidegib (Odomzo) came on the market about 1 year ago.
Besides oral agents, photodynamic therapy (PDT) with photosensitizers are another option for certain skin tumors. Dr. Rossi said his practice is to keep the treatment room fairly warm to assure good blood flow to the skin and thus good penetration of the drug. Because PDT acts by generating singlet oxygen to kill tumors, good blood flow to the tumor is necessary. To minimize discomfort, he uses pretreatment acetaminophen if patients can take it. After a skin reaction occurs, cool compresses are used, along with dilute acetic acid soaks on crusted or scaling lesions in an effort to prevent infection.
And while these treatments can produce quite angry-looking lesions in the short term, very good healing usually occurs if patients are diligent about wound care. However, Dr. Rossi cautioned that they may need “more hand holding with these nonsurgical treatments, because it is a longer duration of treatment.”
In general for counseling patients on nonsurgical treatments, Dr. Rossi said it is advisable to have good pretreatment and post-treatment plans. “They have to know that they will need to be following up to make sure that there is no recurrence,” he said. “We don’t have clear surgical margins if we’re using these topical treatments, so we have to make sure that they have good, constant follow-up.”
Dr. Rossi reported consulting relationships with Merz, DynaMed, and Novartis.
BOSTON – Surgery is the standard of care for most skin cancers, but nonsurgical and adjuvant treatments can be good options for certain skin cancers when surgery would be neither curative nor feasible, according to Anthony Rossi, MD.
“Whether you’re treating a superficial basal cell or superficial squamous cell carcinoma, I think first and foremost, if you’re going to use nonsurgical treatment options, it’s important to have a good biopsy diagnosis,” Dr. Rossi said at the American Academy of Dermatology summer meeting. He also advised that the biopsy capture the entire lesion or a good portion of it to get a good representation. “You don’t want to be surprised by any hiding, high-risk subtypes,” said Dr. Rossi, of the Memorial Sloan Kettering Cancer Center in New York.
Deciding on a nonsurgical treatment option should be based on knowing the patient. For example, know the patient’s concerns about cosmetic deformities, willingness to undergo surgery or not, and ability and willingness to do follow-up self-care.
For superficial basal cell or squamous cell carcinomas in situ and even lentigo maligna in situ not amenable to surgery, imiquimod may be an appropriate treatment. Dr. Rossi said his practice is to use it in an incremental fashion, starting with application five times per week, going to every day if there is no response after 1 to 2 weeks. If the response remains inadequate, he recommended adding a topical retinoid, such as tazarotene, in an effort to increase penetration.
For basal cell carcinomas and squamous cell carcinoma in situ, he uses imiquimod for a total of 6 to 8 weeks, starting from the time of the first reaction. For melanoma in situ, he uses it for more than 60 applications (12 weeks).
To show patients where they should be applying any topical treatment, Dr. Rossi marks the skin, photographs it, and prints out a picture for the patient. Sometimes he uses the patient’s phone to take the picture. For a basal cell or squamous cell carcinoma in situ, he indicates an area at least 5 to 10 mm beyond the margin of the tumor. The area is even larger for melanomas.
Dr. Rossi studies confocal microscopy to detect skin cancers. He uses it before treating a lesion to define the clinical boundaries of the lesion and the boundaries for the nonsurgical treatments, and then he uses it on follow up to look for any recurrences.
New anti-tumor agents
Two oral inhibitors of the sonic hedgehog pathway have been approved within the past 5 years for locally advanced or metastatic basal cell carcinomas. “In the right person, they can be quite beneficial if surgery would leave them with a very large cosmetic deformity or if surgery would be not curative,” Dr. Rossi said. “We’re seeing good results” with acceptable adverse events, specifically taste disturbances, muscle cramps, and hair loss. The first such drug, vismodegib (Erivedge), was approved in 2012, and sonidegib (Odomzo) came on the market about 1 year ago.
Besides oral agents, photodynamic therapy (PDT) with photosensitizers are another option for certain skin tumors. Dr. Rossi said his practice is to keep the treatment room fairly warm to assure good blood flow to the skin and thus good penetration of the drug. Because PDT acts by generating singlet oxygen to kill tumors, good blood flow to the tumor is necessary. To minimize discomfort, he uses pretreatment acetaminophen if patients can take it. After a skin reaction occurs, cool compresses are used, along with dilute acetic acid soaks on crusted or scaling lesions in an effort to prevent infection.
And while these treatments can produce quite angry-looking lesions in the short term, very good healing usually occurs if patients are diligent about wound care. However, Dr. Rossi cautioned that they may need “more hand holding with these nonsurgical treatments, because it is a longer duration of treatment.”
In general for counseling patients on nonsurgical treatments, Dr. Rossi said it is advisable to have good pretreatment and post-treatment plans. “They have to know that they will need to be following up to make sure that there is no recurrence,” he said. “We don’t have clear surgical margins if we’re using these topical treatments, so we have to make sure that they have good, constant follow-up.”
Dr. Rossi reported consulting relationships with Merz, DynaMed, and Novartis.
BOSTON – Surgery is the standard of care for most skin cancers, but nonsurgical and adjuvant treatments can be good options for certain skin cancers when surgery would be neither curative nor feasible, according to Anthony Rossi, MD.
“Whether you’re treating a superficial basal cell or superficial squamous cell carcinoma, I think first and foremost, if you’re going to use nonsurgical treatment options, it’s important to have a good biopsy diagnosis,” Dr. Rossi said at the American Academy of Dermatology summer meeting. He also advised that the biopsy capture the entire lesion or a good portion of it to get a good representation. “You don’t want to be surprised by any hiding, high-risk subtypes,” said Dr. Rossi, of the Memorial Sloan Kettering Cancer Center in New York.
Deciding on a nonsurgical treatment option should be based on knowing the patient. For example, know the patient’s concerns about cosmetic deformities, willingness to undergo surgery or not, and ability and willingness to do follow-up self-care.
For superficial basal cell or squamous cell carcinomas in situ and even lentigo maligna in situ not amenable to surgery, imiquimod may be an appropriate treatment. Dr. Rossi said his practice is to use it in an incremental fashion, starting with application five times per week, going to every day if there is no response after 1 to 2 weeks. If the response remains inadequate, he recommended adding a topical retinoid, such as tazarotene, in an effort to increase penetration.
For basal cell carcinomas and squamous cell carcinoma in situ, he uses imiquimod for a total of 6 to 8 weeks, starting from the time of the first reaction. For melanoma in situ, he uses it for more than 60 applications (12 weeks).
To show patients where they should be applying any topical treatment, Dr. Rossi marks the skin, photographs it, and prints out a picture for the patient. Sometimes he uses the patient’s phone to take the picture. For a basal cell or squamous cell carcinoma in situ, he indicates an area at least 5 to 10 mm beyond the margin of the tumor. The area is even larger for melanomas.
Dr. Rossi studies confocal microscopy to detect skin cancers. He uses it before treating a lesion to define the clinical boundaries of the lesion and the boundaries for the nonsurgical treatments, and then he uses it on follow up to look for any recurrences.
New anti-tumor agents
Two oral inhibitors of the sonic hedgehog pathway have been approved within the past 5 years for locally advanced or metastatic basal cell carcinomas. “In the right person, they can be quite beneficial if surgery would leave them with a very large cosmetic deformity or if surgery would be not curative,” Dr. Rossi said. “We’re seeing good results” with acceptable adverse events, specifically taste disturbances, muscle cramps, and hair loss. The first such drug, vismodegib (Erivedge), was approved in 2012, and sonidegib (Odomzo) came on the market about 1 year ago.
Besides oral agents, photodynamic therapy (PDT) with photosensitizers are another option for certain skin tumors. Dr. Rossi said his practice is to keep the treatment room fairly warm to assure good blood flow to the skin and thus good penetration of the drug. Because PDT acts by generating singlet oxygen to kill tumors, good blood flow to the tumor is necessary. To minimize discomfort, he uses pretreatment acetaminophen if patients can take it. After a skin reaction occurs, cool compresses are used, along with dilute acetic acid soaks on crusted or scaling lesions in an effort to prevent infection.
And while these treatments can produce quite angry-looking lesions in the short term, very good healing usually occurs if patients are diligent about wound care. However, Dr. Rossi cautioned that they may need “more hand holding with these nonsurgical treatments, because it is a longer duration of treatment.”
In general for counseling patients on nonsurgical treatments, Dr. Rossi said it is advisable to have good pretreatment and post-treatment plans. “They have to know that they will need to be following up to make sure that there is no recurrence,” he said. “We don’t have clear surgical margins if we’re using these topical treatments, so we have to make sure that they have good, constant follow-up.”
Dr. Rossi reported consulting relationships with Merz, DynaMed, and Novartis.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2016
Skin Examinations for Early Melanoma Detection
USPSTF: Visual skin cancer screening lacks supporting evidence
The benefits and harms of visual screen cancer screening exams for asymptomatic adults can’t be adequately assessed with current evidence, according to a new recommendation from the U.S. Preventive Services Task Force.
“Evidence is inadequate to reliably conclude that early detection of skin cancer through visual skin examination by a clinician reduces morbidity or mortality,” according to the statement published online July 26 in JAMA (2016;316[4]:429-435. doi:10.1001/jama.2016.8465).
Approximately 76,400 adults in the United States will develop melanoma, and more than 10,000 will die from it, according to the USPSTF. However, more than 98% of skin cancer cases in the United States are basal and squamous cell carcinoma, which have much lower morbidity and mortality rates, noted the USPSTF researchers, led by Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco.
The current statement updates the USPSTF’s 2009 recommendation, which also found insufficient evidence to assess the harms and benefits of visual skin cancer screening in asymptomatic adults with no history of premalignant or malignant skin lesions. However, the current recommendation eliminates a statement about patients’ skin self-exams.
According to the USPSTF, evidence is “adequate” that a clinician’s visual skin exam has “modest sensitivity and specificity for detecting melanoma,” but evidence is inconsistent to support the ability of a visual skin exam to detect nonmelanoma skin cancer.
The USPSTF commissioned an evidence review that included 11 studies previously reviewed and 2 additional studies conducted since 2009. The two new studies included one that evaluated skin cancer screening performed by dermatologists or plastic surgeons and one that evaluated skin cancer screening performed by primary care physicians. Sensitivity and specificity in the two studies ranged from 40% to 70% and from 86% to 98%, respectively.
“None of the studies could draw reliable conclusions as to whether screening performed by any of the clinical specialties differed in diagnostic accuracy,” the researchers noted. In addition, “no [randomized controlled trial] has directly evaluated the effectiveness of the clinical visual skin examination for reducing skin cancer morbidity and mortality,” they wrote.
The recommendation was accompanied by several editorials published online July 26 in JAMA journals.
In JAMA, Hensin Tsao, MD, PhD, of Massachusetts General Hospital, Boston, and Martin Weinstock, MD, PhD, of Brown University, Providence, R.I., noted that the USPSTF considered the possibility of including information from high-quality case-control studies in lieu of randomized controlled trials, which have been difficult to conduct in skin cancer screening. “The evidentiary standard needs to be further refined to be appropriate to the modest magnitude of potential harms of a properly performed skin cancer screening,” they wrote (JAMA. 2016;316:398-400). Dr. Tsao disclosed an honorarium from Lubax.
In JAMA Dermatology, Susan Swetter, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System; Alan C. Geller, MPH, of Harvard School of Public Health, Boston; and Allan C. Halpern, MD, of Memorial Sloan Kettering Cancer Center, New York, wrote about ways to promote broader uptake of skin cancer screening. “Alternative models should be explored to bundle skin screening with other preventive services (e.g., blood pressure measurements or flu shots) and to engage advanced practice providers (e.g., nurse practitioners and physician assistants) to promote screening among individuals with less access to dermatologists,” they wrote (JAMA Dermatol. 2016. doi: 10.1001/jamadermatol.2016.2606).
In JAMA Oncology, Vinayak K. Nahar, MD, of the University of Mississippi Medical Center, Jackson; Jonathan E. Mayer, MD, of Johns Hopkins University, Baltimore; and Jane M. Grant-Kels, MD, of the University of Connecticut, Farmington, addressed concerns over performing more biopsies. “The USPSTF also raises concern over the number needed to biopsy to detect 1 case of melanoma. In weighing these data, one must also consider that many of the nonmelanomas biopsied were likely severely atypical nevi that have their own risk of malignant transformation. Although difficult to quantify, there is some benefit to removing a severely atypical nevus, both for risk of transformation and for a patient’s peace of mind,” they wrote (JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.2440).
In JAMA Internal Medicine, Eleni Linos, MD, of the University of California, San Francisco; Kenneth A. Katz, MD, of Kaiser Permanente, San Francisco; and Graham A. Colditz, MD, of Washington University, St. Louis, cautioned that the USPSTF recommendations shouldn’t be interpreted as minimizing the importance of skin cancer. “Instead, the report should motivate us to improve the evidence base for identifying groups of people in whom the benefits of screening might outweigh risks,” they wrote. “Meanwhile, we should also fully implement skin cancer primary prevention by eliminating indoor tanning exposure, especially among youths, and increasing the use of sun-protection strategies that work” (JAMA Intern. Med. 2016. doi: 10.1001/jamaintermed.2016.5008).
The recommendations are not an official position of the U.S. Department of Health and Human Services or the Agency for Healthcare Research and Quality.
“The American Academy of Dermatology is disappointed with this recommendation, as dermatologists know that skin cancer screenings can save lives, yet we acknowledge the need for additional research on the benefits and harms of skin cancer screening in the primary care setting,” Dr. Abel Torres, president of the American Academy of Dermatology, said in a statement responding to the USPSTF skin cancer screening recommendations.
“It is important for the public to understand that the USPSTF is not recommending against skin cancer screenings; it means the group did not find conclusive evidence to make a recommendation one way or another,” Dr. Torres said. “The public should know that this recommendation does not apply to individuals with suspicious skin lesions and those with an increased skin cancer risk, and it does not address the practice of skin self-exams.”
“The AAD encourages everyone to serve as their own health advocate by regularly conducting skin self-exams. Individuals who notice any unusual spots on their skin, including those that are changing, itching, or bleeding, should make an appointment with a board-certified dermatologist. In addition, individuals with an increased risk of melanoma – including men older than 50; people with more than 50 moles, or large or unusual moles; individuals with fair skin; and those with a history of skin cancer – should talk to a dermatologist about how often they should receive a skin exam from a doctor.”
Dr. Abel Torres is president of the American Academy of Dermatology. The comments are taken from his AAD statement on USPSTF Recommendation on Skin Cancer Screening issued on July 26, 2016.
“The American Academy of Dermatology is disappointed with this recommendation, as dermatologists know that skin cancer screenings can save lives, yet we acknowledge the need for additional research on the benefits and harms of skin cancer screening in the primary care setting,” Dr. Abel Torres, president of the American Academy of Dermatology, said in a statement responding to the USPSTF skin cancer screening recommendations.
“It is important for the public to understand that the USPSTF is not recommending against skin cancer screenings; it means the group did not find conclusive evidence to make a recommendation one way or another,” Dr. Torres said. “The public should know that this recommendation does not apply to individuals with suspicious skin lesions and those with an increased skin cancer risk, and it does not address the practice of skin self-exams.”
“The AAD encourages everyone to serve as their own health advocate by regularly conducting skin self-exams. Individuals who notice any unusual spots on their skin, including those that are changing, itching, or bleeding, should make an appointment with a board-certified dermatologist. In addition, individuals with an increased risk of melanoma – including men older than 50; people with more than 50 moles, or large or unusual moles; individuals with fair skin; and those with a history of skin cancer – should talk to a dermatologist about how often they should receive a skin exam from a doctor.”
Dr. Abel Torres is president of the American Academy of Dermatology. The comments are taken from his AAD statement on USPSTF Recommendation on Skin Cancer Screening issued on July 26, 2016.
“The American Academy of Dermatology is disappointed with this recommendation, as dermatologists know that skin cancer screenings can save lives, yet we acknowledge the need for additional research on the benefits and harms of skin cancer screening in the primary care setting,” Dr. Abel Torres, president of the American Academy of Dermatology, said in a statement responding to the USPSTF skin cancer screening recommendations.
“It is important for the public to understand that the USPSTF is not recommending against skin cancer screenings; it means the group did not find conclusive evidence to make a recommendation one way or another,” Dr. Torres said. “The public should know that this recommendation does not apply to individuals with suspicious skin lesions and those with an increased skin cancer risk, and it does not address the practice of skin self-exams.”
“The AAD encourages everyone to serve as their own health advocate by regularly conducting skin self-exams. Individuals who notice any unusual spots on their skin, including those that are changing, itching, or bleeding, should make an appointment with a board-certified dermatologist. In addition, individuals with an increased risk of melanoma – including men older than 50; people with more than 50 moles, or large or unusual moles; individuals with fair skin; and those with a history of skin cancer – should talk to a dermatologist about how often they should receive a skin exam from a doctor.”
Dr. Abel Torres is president of the American Academy of Dermatology. The comments are taken from his AAD statement on USPSTF Recommendation on Skin Cancer Screening issued on July 26, 2016.
The benefits and harms of visual screen cancer screening exams for asymptomatic adults can’t be adequately assessed with current evidence, according to a new recommendation from the U.S. Preventive Services Task Force.
“Evidence is inadequate to reliably conclude that early detection of skin cancer through visual skin examination by a clinician reduces morbidity or mortality,” according to the statement published online July 26 in JAMA (2016;316[4]:429-435. doi:10.1001/jama.2016.8465).
Approximately 76,400 adults in the United States will develop melanoma, and more than 10,000 will die from it, according to the USPSTF. However, more than 98% of skin cancer cases in the United States are basal and squamous cell carcinoma, which have much lower morbidity and mortality rates, noted the USPSTF researchers, led by Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco.
The current statement updates the USPSTF’s 2009 recommendation, which also found insufficient evidence to assess the harms and benefits of visual skin cancer screening in asymptomatic adults with no history of premalignant or malignant skin lesions. However, the current recommendation eliminates a statement about patients’ skin self-exams.
According to the USPSTF, evidence is “adequate” that a clinician’s visual skin exam has “modest sensitivity and specificity for detecting melanoma,” but evidence is inconsistent to support the ability of a visual skin exam to detect nonmelanoma skin cancer.
The USPSTF commissioned an evidence review that included 11 studies previously reviewed and 2 additional studies conducted since 2009. The two new studies included one that evaluated skin cancer screening performed by dermatologists or plastic surgeons and one that evaluated skin cancer screening performed by primary care physicians. Sensitivity and specificity in the two studies ranged from 40% to 70% and from 86% to 98%, respectively.
“None of the studies could draw reliable conclusions as to whether screening performed by any of the clinical specialties differed in diagnostic accuracy,” the researchers noted. In addition, “no [randomized controlled trial] has directly evaluated the effectiveness of the clinical visual skin examination for reducing skin cancer morbidity and mortality,” they wrote.
The recommendation was accompanied by several editorials published online July 26 in JAMA journals.
In JAMA, Hensin Tsao, MD, PhD, of Massachusetts General Hospital, Boston, and Martin Weinstock, MD, PhD, of Brown University, Providence, R.I., noted that the USPSTF considered the possibility of including information from high-quality case-control studies in lieu of randomized controlled trials, which have been difficult to conduct in skin cancer screening. “The evidentiary standard needs to be further refined to be appropriate to the modest magnitude of potential harms of a properly performed skin cancer screening,” they wrote (JAMA. 2016;316:398-400). Dr. Tsao disclosed an honorarium from Lubax.
In JAMA Dermatology, Susan Swetter, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System; Alan C. Geller, MPH, of Harvard School of Public Health, Boston; and Allan C. Halpern, MD, of Memorial Sloan Kettering Cancer Center, New York, wrote about ways to promote broader uptake of skin cancer screening. “Alternative models should be explored to bundle skin screening with other preventive services (e.g., blood pressure measurements or flu shots) and to engage advanced practice providers (e.g., nurse practitioners and physician assistants) to promote screening among individuals with less access to dermatologists,” they wrote (JAMA Dermatol. 2016. doi: 10.1001/jamadermatol.2016.2606).
In JAMA Oncology, Vinayak K. Nahar, MD, of the University of Mississippi Medical Center, Jackson; Jonathan E. Mayer, MD, of Johns Hopkins University, Baltimore; and Jane M. Grant-Kels, MD, of the University of Connecticut, Farmington, addressed concerns over performing more biopsies. “The USPSTF also raises concern over the number needed to biopsy to detect 1 case of melanoma. In weighing these data, one must also consider that many of the nonmelanomas biopsied were likely severely atypical nevi that have their own risk of malignant transformation. Although difficult to quantify, there is some benefit to removing a severely atypical nevus, both for risk of transformation and for a patient’s peace of mind,” they wrote (JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.2440).
In JAMA Internal Medicine, Eleni Linos, MD, of the University of California, San Francisco; Kenneth A. Katz, MD, of Kaiser Permanente, San Francisco; and Graham A. Colditz, MD, of Washington University, St. Louis, cautioned that the USPSTF recommendations shouldn’t be interpreted as minimizing the importance of skin cancer. “Instead, the report should motivate us to improve the evidence base for identifying groups of people in whom the benefits of screening might outweigh risks,” they wrote. “Meanwhile, we should also fully implement skin cancer primary prevention by eliminating indoor tanning exposure, especially among youths, and increasing the use of sun-protection strategies that work” (JAMA Intern. Med. 2016. doi: 10.1001/jamaintermed.2016.5008).
The recommendations are not an official position of the U.S. Department of Health and Human Services or the Agency for Healthcare Research and Quality.
The benefits and harms of visual screen cancer screening exams for asymptomatic adults can’t be adequately assessed with current evidence, according to a new recommendation from the U.S. Preventive Services Task Force.
“Evidence is inadequate to reliably conclude that early detection of skin cancer through visual skin examination by a clinician reduces morbidity or mortality,” according to the statement published online July 26 in JAMA (2016;316[4]:429-435. doi:10.1001/jama.2016.8465).
Approximately 76,400 adults in the United States will develop melanoma, and more than 10,000 will die from it, according to the USPSTF. However, more than 98% of skin cancer cases in the United States are basal and squamous cell carcinoma, which have much lower morbidity and mortality rates, noted the USPSTF researchers, led by Kirsten Bibbins-Domingo, MD, PhD, of the University of California, San Francisco.
The current statement updates the USPSTF’s 2009 recommendation, which also found insufficient evidence to assess the harms and benefits of visual skin cancer screening in asymptomatic adults with no history of premalignant or malignant skin lesions. However, the current recommendation eliminates a statement about patients’ skin self-exams.
According to the USPSTF, evidence is “adequate” that a clinician’s visual skin exam has “modest sensitivity and specificity for detecting melanoma,” but evidence is inconsistent to support the ability of a visual skin exam to detect nonmelanoma skin cancer.
The USPSTF commissioned an evidence review that included 11 studies previously reviewed and 2 additional studies conducted since 2009. The two new studies included one that evaluated skin cancer screening performed by dermatologists or plastic surgeons and one that evaluated skin cancer screening performed by primary care physicians. Sensitivity and specificity in the two studies ranged from 40% to 70% and from 86% to 98%, respectively.
“None of the studies could draw reliable conclusions as to whether screening performed by any of the clinical specialties differed in diagnostic accuracy,” the researchers noted. In addition, “no [randomized controlled trial] has directly evaluated the effectiveness of the clinical visual skin examination for reducing skin cancer morbidity and mortality,” they wrote.
The recommendation was accompanied by several editorials published online July 26 in JAMA journals.
In JAMA, Hensin Tsao, MD, PhD, of Massachusetts General Hospital, Boston, and Martin Weinstock, MD, PhD, of Brown University, Providence, R.I., noted that the USPSTF considered the possibility of including information from high-quality case-control studies in lieu of randomized controlled trials, which have been difficult to conduct in skin cancer screening. “The evidentiary standard needs to be further refined to be appropriate to the modest magnitude of potential harms of a properly performed skin cancer screening,” they wrote (JAMA. 2016;316:398-400). Dr. Tsao disclosed an honorarium from Lubax.
In JAMA Dermatology, Susan Swetter, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System; Alan C. Geller, MPH, of Harvard School of Public Health, Boston; and Allan C. Halpern, MD, of Memorial Sloan Kettering Cancer Center, New York, wrote about ways to promote broader uptake of skin cancer screening. “Alternative models should be explored to bundle skin screening with other preventive services (e.g., blood pressure measurements or flu shots) and to engage advanced practice providers (e.g., nurse practitioners and physician assistants) to promote screening among individuals with less access to dermatologists,” they wrote (JAMA Dermatol. 2016. doi: 10.1001/jamadermatol.2016.2606).
In JAMA Oncology, Vinayak K. Nahar, MD, of the University of Mississippi Medical Center, Jackson; Jonathan E. Mayer, MD, of Johns Hopkins University, Baltimore; and Jane M. Grant-Kels, MD, of the University of Connecticut, Farmington, addressed concerns over performing more biopsies. “The USPSTF also raises concern over the number needed to biopsy to detect 1 case of melanoma. In weighing these data, one must also consider that many of the nonmelanomas biopsied were likely severely atypical nevi that have their own risk of malignant transformation. Although difficult to quantify, there is some benefit to removing a severely atypical nevus, both for risk of transformation and for a patient’s peace of mind,” they wrote (JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.2440).
In JAMA Internal Medicine, Eleni Linos, MD, of the University of California, San Francisco; Kenneth A. Katz, MD, of Kaiser Permanente, San Francisco; and Graham A. Colditz, MD, of Washington University, St. Louis, cautioned that the USPSTF recommendations shouldn’t be interpreted as minimizing the importance of skin cancer. “Instead, the report should motivate us to improve the evidence base for identifying groups of people in whom the benefits of screening might outweigh risks,” they wrote. “Meanwhile, we should also fully implement skin cancer primary prevention by eliminating indoor tanning exposure, especially among youths, and increasing the use of sun-protection strategies that work” (JAMA Intern. Med. 2016. doi: 10.1001/jamaintermed.2016.5008).
The recommendations are not an official position of the U.S. Department of Health and Human Services or the Agency for Healthcare Research and Quality.
FROM JAMA
Here’s how to tackle teenage tanning
MINNEAPOLIS, MINN. – Indoor tanning is a significant contributor to the U.S. skin cancer epidemic and represents a 100% preventable source of exposure to these cancers. Understanding exactly who is using indoor tanning – and why – can provide insight and leverage to help change behavior, according to Cindy Firkins Smith, MD, adjunct professor of dermatology at the University of Minnesota, Minneapolis.
Dr. Smith noted that the typical indoor tanning bed user is female and between 17 and 30 years old. Other aspects of her lifestyle may be unhealthy; for example, she may smoke cigarettes, have an unhealthy pattern of alcohol consumption, and make unhealthy food choices (Cancer Causes Control 2006 June. doi:10.1007/s10552-005-0453-9). She also is likely to watch beauty-focused reality TV shows (J Am Acad Dermatol 2013 May. doi:10.1016/j.jaad.2012.09.055) and is likely to objectify her own body, seeing it as something to be viewed and judged (Arch Dermatol 2009 Sep 1. doi:10.1001/archdermatol.2009.190).
Tanning is a behavior that provides relaxation and positive emotions, and she receives support for this behavior from family and friends. “Tanning often starts with mom, which is one reason that parental permission legislation doesn’t work,” she said at the annual meeting of the Society for Pediatric Dermatology.
Dr. Smith said that up to 30 million people tan indoors every year. Rates of tanning for teenage girls are very high: up to 40% of American teenagers use indoor tanning, and 20%-30% of all 18- to 29-year-olds have used a tanning bed in the previous year (Jama Dermatol 2014 April doi: 10.1001/jamadermatol.2013.6896).
The ubiquity of tanning salons contributes to the problem, said Dr. Smith. “More is not better; in the largest U.S. cities, tanning salons outnumber [both] Starbucks and McDonald’s,” she said, noting that studies have shown that both proximity to tanning salons and the low cost of tanning encourage their use (Am J Prev Med. 2009 Mar 36[3]:243-6).
A 2015 study surveyed 125 colleges, finding that 48% had indoor tanning facilities in their campus or off-campus housing. College cash cards were acceptable payment at 14.4% of colleges, and 96% of off-campus housing facilities that offered tanning provided it as a free “perk” to residents (JAMA Dermatol 2015 Jan. doi: 10.1001/jamadermatol.2014.3590).
Further, some data suggest that tanning really can be addictive for some patients. Ultraviolet light exposure has been shown to “light up” pleasure centers in PET-CT studies, and some frequent tanners report relaxation and pleasure from tanning as well as craving and feelings of withdrawal when they miss sessions. Dr. Smith said she does not hesitate to refer teen patients to mental health providers if there are concerns about mood and depression.
Having an understanding of patient motivations to tan can help in getting patients to take steps toward change, said Dr. Smith. “What can we do? We can actually do a lot. We have a lot more influence than we think we do.”
At the level of the individual patient, just opening up a conversation can make a big difference. “We assume we know why teenagers go to a tanning booth. But do we? When you notice a young woman who’s been to a tanning booth, ask why,” using a nonjudgmental approach to begin a dialogue about the near-term and long-term dangers of tanning. A positive approach is key, noted Dr. Smith. “Focusing on the benefits of avoiding UV tanning is more effective than a heavy reliance on scare tactics,” she said. Also, “multiple interventions work better.”
Before-and-after photos of celebrities whose appearance has been affected by photoaging can be effective. Another tactic with a more positive spin is to share images of celebrities who have chosen not to tan and who celebrate their fair skin. These conversations are particularly important at prom time, peak tanning season for many young women, said Dr. Smith. She has a portfolio of photos showing fair-skinned women wearing high-contrast gowns, which she says are more flattering for pale skin than white or nude colors.
For patients who still want that tan look, “Tanning for reasons of appearance can be satisfied with sunless tanners,” said Dr. Smith. The most common ingredient in sunless tanners is dihydroxyacetone (DHA), which was approved in the 1970s for topical use. However, the Food and Drug Administration issued a warning in 2011 about spray tanning, noting that the “industry has not provided safety data to FDA in order for the agency to consider approving it for … ‘misting’ from tanning booths.” The FDA’s specific concern had to do with the unknown safety of ingestion, inhalation, and mucous membrane exposure that can result from spray tanning.
Moving to the legislative and policy level, change can be achieved when stakeholders band together to “ban the tan,” said Dr. Smith. “The best way to do it is with a village. It really takes a lot of people to do this.”
There are solid epidemiologic and economic reasons to focus on the skin cancer epidemic, said Dr. Smith. Skin cancer is now the most common cancer in the United States, and more new cases “are diagnosed each year than breast, prostate, lung, and colon cancers combined,” she said. Of nonmelanoma skin cancers, 90% are thought to be UV-related, and “the vast majority of mutations found in melanoma are caused by UV radiation.”
Skin cancers cost the United States over $8 billion annually, and although promising new immunotherapies are extending the lives of those with melanoma, these treatments cost hundreds of thousands of dollars a year. “This type of treatment is unaffordable for our system,” said Dr. Smith.
Progress is being made, she said, despite industry opposition. Individual states have regulated or banned tanning for minors, and at the federal level, tanning beds are now considered by the FDA to be Class II (moderate-risk) medical devices, a step up from their previous classification as the lowest-risk Class I devices, “The same as a tongue blade,” she said.
Dr. Smith had no relevant financial disclosures.
On Twitter @karioakes
MINNEAPOLIS, MINN. – Indoor tanning is a significant contributor to the U.S. skin cancer epidemic and represents a 100% preventable source of exposure to these cancers. Understanding exactly who is using indoor tanning – and why – can provide insight and leverage to help change behavior, according to Cindy Firkins Smith, MD, adjunct professor of dermatology at the University of Minnesota, Minneapolis.
Dr. Smith noted that the typical indoor tanning bed user is female and between 17 and 30 years old. Other aspects of her lifestyle may be unhealthy; for example, she may smoke cigarettes, have an unhealthy pattern of alcohol consumption, and make unhealthy food choices (Cancer Causes Control 2006 June. doi:10.1007/s10552-005-0453-9). She also is likely to watch beauty-focused reality TV shows (J Am Acad Dermatol 2013 May. doi:10.1016/j.jaad.2012.09.055) and is likely to objectify her own body, seeing it as something to be viewed and judged (Arch Dermatol 2009 Sep 1. doi:10.1001/archdermatol.2009.190).
Tanning is a behavior that provides relaxation and positive emotions, and she receives support for this behavior from family and friends. “Tanning often starts with mom, which is one reason that parental permission legislation doesn’t work,” she said at the annual meeting of the Society for Pediatric Dermatology.
Dr. Smith said that up to 30 million people tan indoors every year. Rates of tanning for teenage girls are very high: up to 40% of American teenagers use indoor tanning, and 20%-30% of all 18- to 29-year-olds have used a tanning bed in the previous year (Jama Dermatol 2014 April doi: 10.1001/jamadermatol.2013.6896).
The ubiquity of tanning salons contributes to the problem, said Dr. Smith. “More is not better; in the largest U.S. cities, tanning salons outnumber [both] Starbucks and McDonald’s,” she said, noting that studies have shown that both proximity to tanning salons and the low cost of tanning encourage their use (Am J Prev Med. 2009 Mar 36[3]:243-6).
A 2015 study surveyed 125 colleges, finding that 48% had indoor tanning facilities in their campus or off-campus housing. College cash cards were acceptable payment at 14.4% of colleges, and 96% of off-campus housing facilities that offered tanning provided it as a free “perk” to residents (JAMA Dermatol 2015 Jan. doi: 10.1001/jamadermatol.2014.3590).
Further, some data suggest that tanning really can be addictive for some patients. Ultraviolet light exposure has been shown to “light up” pleasure centers in PET-CT studies, and some frequent tanners report relaxation and pleasure from tanning as well as craving and feelings of withdrawal when they miss sessions. Dr. Smith said she does not hesitate to refer teen patients to mental health providers if there are concerns about mood and depression.
Having an understanding of patient motivations to tan can help in getting patients to take steps toward change, said Dr. Smith. “What can we do? We can actually do a lot. We have a lot more influence than we think we do.”
At the level of the individual patient, just opening up a conversation can make a big difference. “We assume we know why teenagers go to a tanning booth. But do we? When you notice a young woman who’s been to a tanning booth, ask why,” using a nonjudgmental approach to begin a dialogue about the near-term and long-term dangers of tanning. A positive approach is key, noted Dr. Smith. “Focusing on the benefits of avoiding UV tanning is more effective than a heavy reliance on scare tactics,” she said. Also, “multiple interventions work better.”
Before-and-after photos of celebrities whose appearance has been affected by photoaging can be effective. Another tactic with a more positive spin is to share images of celebrities who have chosen not to tan and who celebrate their fair skin. These conversations are particularly important at prom time, peak tanning season for many young women, said Dr. Smith. She has a portfolio of photos showing fair-skinned women wearing high-contrast gowns, which she says are more flattering for pale skin than white or nude colors.
For patients who still want that tan look, “Tanning for reasons of appearance can be satisfied with sunless tanners,” said Dr. Smith. The most common ingredient in sunless tanners is dihydroxyacetone (DHA), which was approved in the 1970s for topical use. However, the Food and Drug Administration issued a warning in 2011 about spray tanning, noting that the “industry has not provided safety data to FDA in order for the agency to consider approving it for … ‘misting’ from tanning booths.” The FDA’s specific concern had to do with the unknown safety of ingestion, inhalation, and mucous membrane exposure that can result from spray tanning.
Moving to the legislative and policy level, change can be achieved when stakeholders band together to “ban the tan,” said Dr. Smith. “The best way to do it is with a village. It really takes a lot of people to do this.”
There are solid epidemiologic and economic reasons to focus on the skin cancer epidemic, said Dr. Smith. Skin cancer is now the most common cancer in the United States, and more new cases “are diagnosed each year than breast, prostate, lung, and colon cancers combined,” she said. Of nonmelanoma skin cancers, 90% are thought to be UV-related, and “the vast majority of mutations found in melanoma are caused by UV radiation.”
Skin cancers cost the United States over $8 billion annually, and although promising new immunotherapies are extending the lives of those with melanoma, these treatments cost hundreds of thousands of dollars a year. “This type of treatment is unaffordable for our system,” said Dr. Smith.
Progress is being made, she said, despite industry opposition. Individual states have regulated or banned tanning for minors, and at the federal level, tanning beds are now considered by the FDA to be Class II (moderate-risk) medical devices, a step up from their previous classification as the lowest-risk Class I devices, “The same as a tongue blade,” she said.
Dr. Smith had no relevant financial disclosures.
On Twitter @karioakes
MINNEAPOLIS, MINN. – Indoor tanning is a significant contributor to the U.S. skin cancer epidemic and represents a 100% preventable source of exposure to these cancers. Understanding exactly who is using indoor tanning – and why – can provide insight and leverage to help change behavior, according to Cindy Firkins Smith, MD, adjunct professor of dermatology at the University of Minnesota, Minneapolis.
Dr. Smith noted that the typical indoor tanning bed user is female and between 17 and 30 years old. Other aspects of her lifestyle may be unhealthy; for example, she may smoke cigarettes, have an unhealthy pattern of alcohol consumption, and make unhealthy food choices (Cancer Causes Control 2006 June. doi:10.1007/s10552-005-0453-9). She also is likely to watch beauty-focused reality TV shows (J Am Acad Dermatol 2013 May. doi:10.1016/j.jaad.2012.09.055) and is likely to objectify her own body, seeing it as something to be viewed and judged (Arch Dermatol 2009 Sep 1. doi:10.1001/archdermatol.2009.190).
Tanning is a behavior that provides relaxation and positive emotions, and she receives support for this behavior from family and friends. “Tanning often starts with mom, which is one reason that parental permission legislation doesn’t work,” she said at the annual meeting of the Society for Pediatric Dermatology.
Dr. Smith said that up to 30 million people tan indoors every year. Rates of tanning for teenage girls are very high: up to 40% of American teenagers use indoor tanning, and 20%-30% of all 18- to 29-year-olds have used a tanning bed in the previous year (Jama Dermatol 2014 April doi: 10.1001/jamadermatol.2013.6896).
The ubiquity of tanning salons contributes to the problem, said Dr. Smith. “More is not better; in the largest U.S. cities, tanning salons outnumber [both] Starbucks and McDonald’s,” she said, noting that studies have shown that both proximity to tanning salons and the low cost of tanning encourage their use (Am J Prev Med. 2009 Mar 36[3]:243-6).
A 2015 study surveyed 125 colleges, finding that 48% had indoor tanning facilities in their campus or off-campus housing. College cash cards were acceptable payment at 14.4% of colleges, and 96% of off-campus housing facilities that offered tanning provided it as a free “perk” to residents (JAMA Dermatol 2015 Jan. doi: 10.1001/jamadermatol.2014.3590).
Further, some data suggest that tanning really can be addictive for some patients. Ultraviolet light exposure has been shown to “light up” pleasure centers in PET-CT studies, and some frequent tanners report relaxation and pleasure from tanning as well as craving and feelings of withdrawal when they miss sessions. Dr. Smith said she does not hesitate to refer teen patients to mental health providers if there are concerns about mood and depression.
Having an understanding of patient motivations to tan can help in getting patients to take steps toward change, said Dr. Smith. “What can we do? We can actually do a lot. We have a lot more influence than we think we do.”
At the level of the individual patient, just opening up a conversation can make a big difference. “We assume we know why teenagers go to a tanning booth. But do we? When you notice a young woman who’s been to a tanning booth, ask why,” using a nonjudgmental approach to begin a dialogue about the near-term and long-term dangers of tanning. A positive approach is key, noted Dr. Smith. “Focusing on the benefits of avoiding UV tanning is more effective than a heavy reliance on scare tactics,” she said. Also, “multiple interventions work better.”
Before-and-after photos of celebrities whose appearance has been affected by photoaging can be effective. Another tactic with a more positive spin is to share images of celebrities who have chosen not to tan and who celebrate their fair skin. These conversations are particularly important at prom time, peak tanning season for many young women, said Dr. Smith. She has a portfolio of photos showing fair-skinned women wearing high-contrast gowns, which she says are more flattering for pale skin than white or nude colors.
For patients who still want that tan look, “Tanning for reasons of appearance can be satisfied with sunless tanners,” said Dr. Smith. The most common ingredient in sunless tanners is dihydroxyacetone (DHA), which was approved in the 1970s for topical use. However, the Food and Drug Administration issued a warning in 2011 about spray tanning, noting that the “industry has not provided safety data to FDA in order for the agency to consider approving it for … ‘misting’ from tanning booths.” The FDA’s specific concern had to do with the unknown safety of ingestion, inhalation, and mucous membrane exposure that can result from spray tanning.
Moving to the legislative and policy level, change can be achieved when stakeholders band together to “ban the tan,” said Dr. Smith. “The best way to do it is with a village. It really takes a lot of people to do this.”
There are solid epidemiologic and economic reasons to focus on the skin cancer epidemic, said Dr. Smith. Skin cancer is now the most common cancer in the United States, and more new cases “are diagnosed each year than breast, prostate, lung, and colon cancers combined,” she said. Of nonmelanoma skin cancers, 90% are thought to be UV-related, and “the vast majority of mutations found in melanoma are caused by UV radiation.”
Skin cancers cost the United States over $8 billion annually, and although promising new immunotherapies are extending the lives of those with melanoma, these treatments cost hundreds of thousands of dollars a year. “This type of treatment is unaffordable for our system,” said Dr. Smith.
Progress is being made, she said, despite industry opposition. Individual states have regulated or banned tanning for minors, and at the federal level, tanning beds are now considered by the FDA to be Class II (moderate-risk) medical devices, a step up from their previous classification as the lowest-risk Class I devices, “The same as a tongue blade,” she said.
Dr. Smith had no relevant financial disclosures.
On Twitter @karioakes
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Pembrolizumab-ipilimumab combo is highly active in advanced melanoma
CHICAGO – The combination of pembrolizumab, an antibody to the human cell surface receptor programmed death-1 (PD-1), and ipilimumab, an antibody to the human T-cell receptor cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), is highly active against advanced melanoma and has acceptable safety, finds the KEYNOTE 029 trial’s expansion cohort.
The 153 patients in the cohort received a standard dose of pembrolizumab (2 mg/kg every 3 weeks) with a reduced dose of ipilimumab (1 mg/kg every 3 weeks for four doses) on the basis of earlier data showing substantial toxicity when a standard dose of ipilimumab was combined with other immune checkpoint inhibitors.
Results reported at the annual meeting of the American Society of Clinical Oncology showed that the overall response rate was 57%, and the disease control rate was 78%. Although 42% of patients experienced grade 3 or 4 treatment-related adverse events, most of these events resolved, and there were no treatment-related deaths.
“Pembrolizumab 2 mg/kg in combination with four doses of ipilimumab 1 mg/kg has a manageable toxicity profile and provides robust antitumor activity in patients with advanced melanoma,” concluded the investigators, who were led by Georgina Long, PhD, MBBS, chair of Melanoma Medical Oncology and Translational Research at the Melanoma Institute Australia and Royal North Shore Hospital, University of Sydney.
The response rate seen in KEYNOTE 029 was almost identical to that seen in the CheckMate 067 trial with the combination of nivolumab and standard-dose ipilimumab (3 mg/kg every 3 weeks for four doses), noted invited discussant Marc S. Ernstoff, MD, professor and chair of the department of medicine at the Roswell Park Cancer Institute in Buffalo, N.Y. It was also “remarkably comparable” to the 69% seen in the COMBI-d melanoma trial with the combination of dabrafenib (a BRAF inhibitor) and trametinib (an inhibitor of MEK MAPK/ERK kinase).
“There is a significant amount of grade 3 and 4 toxicity, but the dose of ipilimumab appeared to decrease this in the pembrolizumab-ipilimumab study compared to the nivolumab-ipilimumab study,” he noted. “There was a high percent of low-grade toxicities reported in all of these studies, and I would argue that as we are seeing patients survive longer, these low-grade toxicities are going to become more of an issue for us as oncologists to be able to deal with in terms of quality of life for patients surviving.”
There is good rationale for combining CTLA4 blockade and PD1 (or PD-L1) blockade in melanoma, Dr. Long maintained when introducing the research. “We know that CTLA inhibition at the priming phase in the periphery, at antigen presentation, is effective, as is PD-1 or PD-L1 [inhibition] at the effector phase down in the tumor bed,” she elaborated.
The patients with advanced melanoma enrolled in the expansion cohort could have received any number of prior therapies other than immune checkpoint inhibitors. However, in 87%, the study regimen was their first therapy.
At the time of data cutoff, 72% of patients had received all four planned doses of ipilimumab (Yervoy), and 56% were continuing on pembrolizumab (Keytruda).
The rates of any-grade and grade 3 or 4 treatment-related adverse events were 95% and 42%, respectively. The corresponding rates of immune-mediated adverse events were 58% and 25%.
The most common grade 3 or 4 treatment-related adverse events were lipase elevation (14%) and rash (3%). The former was asymptomatic and had no sequelae in the majority of cases, Dr. Long reported.
Hepatitis, colitis, and skin reactions were the most common grade 3 or 4 immune-mediated adverse events. The majority of immune-mediated adverse events were managed with systemic treatment, usually corticosteroids, and resolved.
When it came to efficacy, the overall response rate with the combination was similar across subgroups of patients stratified by PD-L1 status in the tumor and adjacent immune tissue, treatment history, baseline lactate dehydrogenase level, and BRAF mutational status.
Responses were ongoing in 98% of patients at data cutoff, with the duration of response ranging from about 6 weeks to 43 weeks, Dr. Long said. The disease control rate was 78%.
With a median follow-up of 10.0 months, median progression-free survival and overall survival were not yet reached. However, the 6-month rates of these outcomes were 70% and 93%, respectively.
Dr. Long disclosed that she is a consultant/adviser to Amgen, Bristol-Myers Squibb, Merck, Novartis, Provectus, and Roche, and that she has received honoraria from Bristol-Myers Squibb, Merck, and Novartis. The trial was supported by Merck.
CHICAGO – The combination of pembrolizumab, an antibody to the human cell surface receptor programmed death-1 (PD-1), and ipilimumab, an antibody to the human T-cell receptor cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), is highly active against advanced melanoma and has acceptable safety, finds the KEYNOTE 029 trial’s expansion cohort.
The 153 patients in the cohort received a standard dose of pembrolizumab (2 mg/kg every 3 weeks) with a reduced dose of ipilimumab (1 mg/kg every 3 weeks for four doses) on the basis of earlier data showing substantial toxicity when a standard dose of ipilimumab was combined with other immune checkpoint inhibitors.
Results reported at the annual meeting of the American Society of Clinical Oncology showed that the overall response rate was 57%, and the disease control rate was 78%. Although 42% of patients experienced grade 3 or 4 treatment-related adverse events, most of these events resolved, and there were no treatment-related deaths.
“Pembrolizumab 2 mg/kg in combination with four doses of ipilimumab 1 mg/kg has a manageable toxicity profile and provides robust antitumor activity in patients with advanced melanoma,” concluded the investigators, who were led by Georgina Long, PhD, MBBS, chair of Melanoma Medical Oncology and Translational Research at the Melanoma Institute Australia and Royal North Shore Hospital, University of Sydney.
The response rate seen in KEYNOTE 029 was almost identical to that seen in the CheckMate 067 trial with the combination of nivolumab and standard-dose ipilimumab (3 mg/kg every 3 weeks for four doses), noted invited discussant Marc S. Ernstoff, MD, professor and chair of the department of medicine at the Roswell Park Cancer Institute in Buffalo, N.Y. It was also “remarkably comparable” to the 69% seen in the COMBI-d melanoma trial with the combination of dabrafenib (a BRAF inhibitor) and trametinib (an inhibitor of MEK MAPK/ERK kinase).
“There is a significant amount of grade 3 and 4 toxicity, but the dose of ipilimumab appeared to decrease this in the pembrolizumab-ipilimumab study compared to the nivolumab-ipilimumab study,” he noted. “There was a high percent of low-grade toxicities reported in all of these studies, and I would argue that as we are seeing patients survive longer, these low-grade toxicities are going to become more of an issue for us as oncologists to be able to deal with in terms of quality of life for patients surviving.”
There is good rationale for combining CTLA4 blockade and PD1 (or PD-L1) blockade in melanoma, Dr. Long maintained when introducing the research. “We know that CTLA inhibition at the priming phase in the periphery, at antigen presentation, is effective, as is PD-1 or PD-L1 [inhibition] at the effector phase down in the tumor bed,” she elaborated.
The patients with advanced melanoma enrolled in the expansion cohort could have received any number of prior therapies other than immune checkpoint inhibitors. However, in 87%, the study regimen was their first therapy.
At the time of data cutoff, 72% of patients had received all four planned doses of ipilimumab (Yervoy), and 56% were continuing on pembrolizumab (Keytruda).
The rates of any-grade and grade 3 or 4 treatment-related adverse events were 95% and 42%, respectively. The corresponding rates of immune-mediated adverse events were 58% and 25%.
The most common grade 3 or 4 treatment-related adverse events were lipase elevation (14%) and rash (3%). The former was asymptomatic and had no sequelae in the majority of cases, Dr. Long reported.
Hepatitis, colitis, and skin reactions were the most common grade 3 or 4 immune-mediated adverse events. The majority of immune-mediated adverse events were managed with systemic treatment, usually corticosteroids, and resolved.
When it came to efficacy, the overall response rate with the combination was similar across subgroups of patients stratified by PD-L1 status in the tumor and adjacent immune tissue, treatment history, baseline lactate dehydrogenase level, and BRAF mutational status.
Responses were ongoing in 98% of patients at data cutoff, with the duration of response ranging from about 6 weeks to 43 weeks, Dr. Long said. The disease control rate was 78%.
With a median follow-up of 10.0 months, median progression-free survival and overall survival were not yet reached. However, the 6-month rates of these outcomes were 70% and 93%, respectively.
Dr. Long disclosed that she is a consultant/adviser to Amgen, Bristol-Myers Squibb, Merck, Novartis, Provectus, and Roche, and that she has received honoraria from Bristol-Myers Squibb, Merck, and Novartis. The trial was supported by Merck.
CHICAGO – The combination of pembrolizumab, an antibody to the human cell surface receptor programmed death-1 (PD-1), and ipilimumab, an antibody to the human T-cell receptor cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), is highly active against advanced melanoma and has acceptable safety, finds the KEYNOTE 029 trial’s expansion cohort.
The 153 patients in the cohort received a standard dose of pembrolizumab (2 mg/kg every 3 weeks) with a reduced dose of ipilimumab (1 mg/kg every 3 weeks for four doses) on the basis of earlier data showing substantial toxicity when a standard dose of ipilimumab was combined with other immune checkpoint inhibitors.
Results reported at the annual meeting of the American Society of Clinical Oncology showed that the overall response rate was 57%, and the disease control rate was 78%. Although 42% of patients experienced grade 3 or 4 treatment-related adverse events, most of these events resolved, and there were no treatment-related deaths.
“Pembrolizumab 2 mg/kg in combination with four doses of ipilimumab 1 mg/kg has a manageable toxicity profile and provides robust antitumor activity in patients with advanced melanoma,” concluded the investigators, who were led by Georgina Long, PhD, MBBS, chair of Melanoma Medical Oncology and Translational Research at the Melanoma Institute Australia and Royal North Shore Hospital, University of Sydney.
The response rate seen in KEYNOTE 029 was almost identical to that seen in the CheckMate 067 trial with the combination of nivolumab and standard-dose ipilimumab (3 mg/kg every 3 weeks for four doses), noted invited discussant Marc S. Ernstoff, MD, professor and chair of the department of medicine at the Roswell Park Cancer Institute in Buffalo, N.Y. It was also “remarkably comparable” to the 69% seen in the COMBI-d melanoma trial with the combination of dabrafenib (a BRAF inhibitor) and trametinib (an inhibitor of MEK MAPK/ERK kinase).
“There is a significant amount of grade 3 and 4 toxicity, but the dose of ipilimumab appeared to decrease this in the pembrolizumab-ipilimumab study compared to the nivolumab-ipilimumab study,” he noted. “There was a high percent of low-grade toxicities reported in all of these studies, and I would argue that as we are seeing patients survive longer, these low-grade toxicities are going to become more of an issue for us as oncologists to be able to deal with in terms of quality of life for patients surviving.”
There is good rationale for combining CTLA4 blockade and PD1 (or PD-L1) blockade in melanoma, Dr. Long maintained when introducing the research. “We know that CTLA inhibition at the priming phase in the periphery, at antigen presentation, is effective, as is PD-1 or PD-L1 [inhibition] at the effector phase down in the tumor bed,” she elaborated.
The patients with advanced melanoma enrolled in the expansion cohort could have received any number of prior therapies other than immune checkpoint inhibitors. However, in 87%, the study regimen was their first therapy.
At the time of data cutoff, 72% of patients had received all four planned doses of ipilimumab (Yervoy), and 56% were continuing on pembrolizumab (Keytruda).
The rates of any-grade and grade 3 or 4 treatment-related adverse events were 95% and 42%, respectively. The corresponding rates of immune-mediated adverse events were 58% and 25%.
The most common grade 3 or 4 treatment-related adverse events were lipase elevation (14%) and rash (3%). The former was asymptomatic and had no sequelae in the majority of cases, Dr. Long reported.
Hepatitis, colitis, and skin reactions were the most common grade 3 or 4 immune-mediated adverse events. The majority of immune-mediated adverse events were managed with systemic treatment, usually corticosteroids, and resolved.
When it came to efficacy, the overall response rate with the combination was similar across subgroups of patients stratified by PD-L1 status in the tumor and adjacent immune tissue, treatment history, baseline lactate dehydrogenase level, and BRAF mutational status.
Responses were ongoing in 98% of patients at data cutoff, with the duration of response ranging from about 6 weeks to 43 weeks, Dr. Long said. The disease control rate was 78%.
With a median follow-up of 10.0 months, median progression-free survival and overall survival were not yet reached. However, the 6-month rates of these outcomes were 70% and 93%, respectively.
Dr. Long disclosed that she is a consultant/adviser to Amgen, Bristol-Myers Squibb, Merck, Novartis, Provectus, and Roche, and that she has received honoraria from Bristol-Myers Squibb, Merck, and Novartis. The trial was supported by Merck.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Dual immune checkpoint blockade with pembrolizumab and ipilimumab is efficacious in advanced melanoma.
Major finding: The overall response rate was 57%, and the disease control rate was 78%.
Data source: An expansion cohort from a phase I/II trial among 153 patients with advanced melanoma (KEYNOTE 029).
Disclosures: Dr. Long disclosed that she is a consultant/advisor to Amgen, Bristol-Myers Squibb, Merck, Novartis, Provectus, and Roche, and that she has received honoraria from Bristol-Myers Squibb, Merck, and Novartis. The trial was supported by Merck.