User login
Exploration of Modern Military Research Resources
Advances in medical biotechnologies, data-gathering techniques, and -omics technologies have resulted in the broader understanding of disease pathology and treatment and have facilitated the individualization of health care plans to meet the unique needs of each patient. Military medicine often has been on the forefront of medical technology, disease understanding, and clinical care both on and off the battlefield, in large part due to the unique resources available in the military health care system. These resources allow investigators the ability to integrate vast amounts of epidemiologic data with an extensive biological sample database of its service members, which in the modern age has translated into advances in the understanding of melanoma and the treatment of scars.
History of Research in the Military
Starting in the 1950s, the US Department of Defense (DoD) started to collect serum samples of its service members for the purpose of research.1 It was not until 1985 that the DoD implemented a long-term frozen storage system for serum samples obtained through mandatory screening for human immunodeficiency virus (HIV) in service members.2 Subsequently, the Department of Defense Serum Repository (DoDSR) was officially established in 1989 as a central archive for the long-term storage of serum obtained from active-duty and reserve service members in the US Navy, Army, and Marines.2,3 In the mid-1990s, the DoDSR expanded its capabilities to include the storage of serum samples from all military members, including the US Air Force, obtained predeployment and postdeployment.3,4 At that time, a records-keeping system was established, now known as the Defense Medical Surveillance System (DMSS). The creation of the DMSS provided an extensive epidemiologic database that provided valuable information such as demographic data, service records, deployment data, reportable medical events, exposure history, and vaccination records, which could be linked to the serum samples of each service member.2-4 Since 2008, the responsibilities of maintaining the DoDSR and the DMSS were transferred to the Armed Forces Health Surveillance Center (AFHSC).5
There have been several other databases created over the years that provide additional support and resources to military investigators. The Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services both help investigators to track the incidence of specific cancers in the military population and provide them with pathologic specimens. Additionally, electronic medical records including the composite health care system and the Armed Forces Health Longitudinal Technology Application supplemented with insurance claims data accessible from the Military Health System Management and Reporting Tool (M2) database have made it possible to track patient data.
Utilization of Military Research Resources
Today, the DoDSR is a secure facility that maintains more than 56 million serum specimens from more than 11 million individuals in –30°C freezers, making it one of the largest repositories in the world.3,6 Each serum sample is linked with an individual’s DMSS record, providing a way for investigators to study how external factors such as deployment history, occupation, and exposure history relate to an individual’s unique genetic and physiological makeup. Furthermore, these data can be used for seroepidemiologic investigations that contribute to all facets of clinical care. The AFHSC routinely publishes findings related to notifiable diseases, disease outbreaks, and disease trends in a monthly report.7
There are strict guidelines in place that limit access to the DoDSR and service members’ data. Use of the repository for information directly related to a patient’s health care is one reason for access, such as analyzing serum for antibodies and seroconversion to assist in the diagnosis of a disease such as HIV. Another reason would be to obtain information needed for criminal investigations and prosecution. Typically, these types of requests require a judge-issued court order and approval by the Assistant Secretary of Defense for Health Affairs.4 The DoDSR also is used to study force health protection issues, such as infectious disease incidence and disease prevalence in the military population.
Obtaining access to the DoDSR and service members’ data for research purposes requires that the principal investigator be a DoD employee. Each research proposal is reviewed by members of the AFHSC to determine if the DoDSR is able to meet the demands of the project, including having the appropriate number of serum samples and supporting epidemiologic data available. The AFHSC provides a letter of support if it deems the project to be in line with its current resources and capabilities. Each research proposal is then sent to an institutional review board (IRB) to determine if the study is exempt or needs to go through a full IRB review process. A study might be exempt if the investigators are not obtaining data through interaction with living individuals or not having access to any identifiable protected health information associated with the samples.6 Regardless of whether the study is exempt or not exempt, the AFHSC will de-identify each sample before releasing the samples to the investigators by using a coding system to shield the patient’s identity from the investigator.
Resources within the military medical research system provide investigators with access to an extensive biorepository of serum and linked epidemiological data. Samples from the DoDSR have been used in no less than 75 peer-reviewed publications since 1985.8,9 Several of these studies have been influential in expanding knowledge about conditions seen more commonly in the military population such as stress fractures, traumatic brain injuries, posttraumatic stress disorder, and suicide.8 Additionally, DoDSR samples have been used to form military vaccination policies and track both infectious and noninfectious conditions in the military; for example, during the H1N1 influenza virus outbreak of 2009, AFHSC was essential in helping to limit the spread of the virus within the military community by using its data and collaborating with groups such as the Centers for Disease Control and Prevention to develop a plan for disease surveillance and control.5
Several military research resources are currently being used for a melanoma study that aims to assess if specific phenotypic features, melanoma risk alleles, and environmental factors (eg, duty station location, occupation, amount of UV exposure) can be used to develop better screening models to identify individuals who are at risk for developing melanoma. Secondarily, the study aims to determine if recently developed multimarker diagnostic and prognostic assays for melanoma will prove useful in the diagnostic and prognostic assessment of melanocytic neoplasms in the military population. For this study, one of the authors (J.H.M) is utilizing DoDSR serum from 1700 retrospective cases of invasive melanoma and 1700 matched controls. Additionally, the Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services databases are being used to obtain tissue from more than 300 melanoma cases and nevi controls.
Limitations of the Current System
Despite the impressive capabilities of the current system, there are some issues that limit its potential. One such limitation is associated with the way that the serum samples at the DoDSR are utilized. Through 2012, the DoDSR had 54,542,658 serum specimens available, of which only 228,610 (0.42%) had ever been accessed for study.8 With such a wealth of information and relative availability, why are the serum samples not being accessed more frequently for studies? The inherent nature of the DoDSR being a restricted facility and only accessible to DoD-affiliated investigators may contribute, which allows the DoDSR to fulfill its primary purpose of contributing to military-relevant investigations but at the same time limits the number and type of investigations that can be performed. One idea that has been proposed is allowing civilian investigator access to the DoDSR if it can be proven that the research is targeted toward military-relevant issues.8 However, the current AFHSC access guidelines would need revision and would require additional safeguards to ensure that military-protected health information is not compromised. Nonetheless, such a change may result in more extensive use of DoDSR resources in the future.
An ethical issue that needs to be addressed pertains to how the DoDSR permits use of human serum samples for research purposes without getting consent from the individuals being studied. The serum samples are collected as part of mandatory predeployment and postdeployment examinations for HIV screening of all military members. These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Although it is true that military members must comply with specific requirements pertaining to military readiness (eg, receiving appropriate vaccinations, drug testing, regular medical screening), it is debated whether they still retain the right as patients to refuse participating in research and clinical trials.10 The AFHSC does have several regulatory steps in place to ensure that military members’ samples are used in an appropriate manner, including requiring a DoD primary investigator, IRB review of every research proposal, and de-identification of samples. At a minimum, giving military members the ability to provide informed consent would ensure that the military system is adhering to evolving human research standards.
The current lack of biological specimens other than serum in the DoDSR is another limitation of the current system. Recent advances in molecular analyses are impacted by expanding -omics techniques, such as epigenomics, transcriptomics, and proteomics. The field of epigenomics is the study of reversible changes to DNA (eg, methylation) associated with specific disease states or following specific environmental exposures.9,11 Transcriptomics, which analyzes messenger RNA transcript levels of expressed genes, and proteomics, which uses expression of proteins, are 2 techniques being used to develop biomarkers associated with specific diseases and environmental exposures.9,11 Serum alone does not provide the high-quality nucleic acids needed for many of these studies to take place. Adding whole-blood specimens or blood spot samples of military service members to the DoDSR would allow researchers to use these techniques to investigate many new biomarkers associated with military-relevant diseases and exposures. These techniques also can be used in the expanding field of personalized medicine so that health care providers are able to tailor all phases of care, including diagnosis and treatment, to an individual’s genetic profile.
Conclusion
The history of research in military medicine has been built on achieving the primary goal of serving those men and women who put their lives in danger to protect this country. In an evolving environment of new technologies that have led to changes in service members’ injuries, exposures, and diseases, military medicine also must adapt. Resources such as the DoDSR and DMSS, which provide investigators with the unique ability to link epidemiological data with serum samples, have been invaluable contributors to this overall mission. As with any large system, there are always improvements that can be made. Improving access to the DoDSR serum samples, educating and obtaining consent from military service members to use their samples in research, and adding specimens to the DoDSR that can be used for -omics techniques are 3 changes that should be considered to maximize
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900-1904.
- Department of Defense Serum Repository. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Department-of-Defense-Serum-Repository. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD serum repository. Mil Med. 2015;180(10 suppl):10-12.
- DeFraites RF. The Armed Forces Health Surveillance Center: enhancing the Military Health System’s public health capabilities. BMC Public Health. 2011;11(suppl 2):S1.
- Pavlin JA, Welch RA. Ethics, human use, and the department of defense serum repository. Mil Med. 2015;180:49-56.
- Defense Medical Surveillance System. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Surveillance-System. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV, et al. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. Plos One. 2015;10:1-16.
- Mancuso JD, Mallon TM, Gaydos JC. Maximizing the capabilities of the DoD serum repository to meet current and future needs: report of the needs panel. Mil Med. 2015;180:14-24.
- Department of Defense. Department of Defense Instruction. http://www.dtic.mil/whs/directives/corres/pdf/600014p.pdf. Posted September 26, 2001. Updated October 3, 2013. Accessed August 2, 2016.
- Lindler LE. Building a DoD biorepository for the future: potential benefits and way forward. Mil Med. 2015;180:90-94.
Advances in medical biotechnologies, data-gathering techniques, and -omics technologies have resulted in the broader understanding of disease pathology and treatment and have facilitated the individualization of health care plans to meet the unique needs of each patient. Military medicine often has been on the forefront of medical technology, disease understanding, and clinical care both on and off the battlefield, in large part due to the unique resources available in the military health care system. These resources allow investigators the ability to integrate vast amounts of epidemiologic data with an extensive biological sample database of its service members, which in the modern age has translated into advances in the understanding of melanoma and the treatment of scars.
History of Research in the Military
Starting in the 1950s, the US Department of Defense (DoD) started to collect serum samples of its service members for the purpose of research.1 It was not until 1985 that the DoD implemented a long-term frozen storage system for serum samples obtained through mandatory screening for human immunodeficiency virus (HIV) in service members.2 Subsequently, the Department of Defense Serum Repository (DoDSR) was officially established in 1989 as a central archive for the long-term storage of serum obtained from active-duty and reserve service members in the US Navy, Army, and Marines.2,3 In the mid-1990s, the DoDSR expanded its capabilities to include the storage of serum samples from all military members, including the US Air Force, obtained predeployment and postdeployment.3,4 At that time, a records-keeping system was established, now known as the Defense Medical Surveillance System (DMSS). The creation of the DMSS provided an extensive epidemiologic database that provided valuable information such as demographic data, service records, deployment data, reportable medical events, exposure history, and vaccination records, which could be linked to the serum samples of each service member.2-4 Since 2008, the responsibilities of maintaining the DoDSR and the DMSS were transferred to the Armed Forces Health Surveillance Center (AFHSC).5
There have been several other databases created over the years that provide additional support and resources to military investigators. The Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services both help investigators to track the incidence of specific cancers in the military population and provide them with pathologic specimens. Additionally, electronic medical records including the composite health care system and the Armed Forces Health Longitudinal Technology Application supplemented with insurance claims data accessible from the Military Health System Management and Reporting Tool (M2) database have made it possible to track patient data.
Utilization of Military Research Resources
Today, the DoDSR is a secure facility that maintains more than 56 million serum specimens from more than 11 million individuals in –30°C freezers, making it one of the largest repositories in the world.3,6 Each serum sample is linked with an individual’s DMSS record, providing a way for investigators to study how external factors such as deployment history, occupation, and exposure history relate to an individual’s unique genetic and physiological makeup. Furthermore, these data can be used for seroepidemiologic investigations that contribute to all facets of clinical care. The AFHSC routinely publishes findings related to notifiable diseases, disease outbreaks, and disease trends in a monthly report.7
There are strict guidelines in place that limit access to the DoDSR and service members’ data. Use of the repository for information directly related to a patient’s health care is one reason for access, such as analyzing serum for antibodies and seroconversion to assist in the diagnosis of a disease such as HIV. Another reason would be to obtain information needed for criminal investigations and prosecution. Typically, these types of requests require a judge-issued court order and approval by the Assistant Secretary of Defense for Health Affairs.4 The DoDSR also is used to study force health protection issues, such as infectious disease incidence and disease prevalence in the military population.
Obtaining access to the DoDSR and service members’ data for research purposes requires that the principal investigator be a DoD employee. Each research proposal is reviewed by members of the AFHSC to determine if the DoDSR is able to meet the demands of the project, including having the appropriate number of serum samples and supporting epidemiologic data available. The AFHSC provides a letter of support if it deems the project to be in line with its current resources and capabilities. Each research proposal is then sent to an institutional review board (IRB) to determine if the study is exempt or needs to go through a full IRB review process. A study might be exempt if the investigators are not obtaining data through interaction with living individuals or not having access to any identifiable protected health information associated with the samples.6 Regardless of whether the study is exempt or not exempt, the AFHSC will de-identify each sample before releasing the samples to the investigators by using a coding system to shield the patient’s identity from the investigator.
Resources within the military medical research system provide investigators with access to an extensive biorepository of serum and linked epidemiological data. Samples from the DoDSR have been used in no less than 75 peer-reviewed publications since 1985.8,9 Several of these studies have been influential in expanding knowledge about conditions seen more commonly in the military population such as stress fractures, traumatic brain injuries, posttraumatic stress disorder, and suicide.8 Additionally, DoDSR samples have been used to form military vaccination policies and track both infectious and noninfectious conditions in the military; for example, during the H1N1 influenza virus outbreak of 2009, AFHSC was essential in helping to limit the spread of the virus within the military community by using its data and collaborating with groups such as the Centers for Disease Control and Prevention to develop a plan for disease surveillance and control.5
Several military research resources are currently being used for a melanoma study that aims to assess if specific phenotypic features, melanoma risk alleles, and environmental factors (eg, duty station location, occupation, amount of UV exposure) can be used to develop better screening models to identify individuals who are at risk for developing melanoma. Secondarily, the study aims to determine if recently developed multimarker diagnostic and prognostic assays for melanoma will prove useful in the diagnostic and prognostic assessment of melanocytic neoplasms in the military population. For this study, one of the authors (J.H.M) is utilizing DoDSR serum from 1700 retrospective cases of invasive melanoma and 1700 matched controls. Additionally, the Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services databases are being used to obtain tissue from more than 300 melanoma cases and nevi controls.
Limitations of the Current System
Despite the impressive capabilities of the current system, there are some issues that limit its potential. One such limitation is associated with the way that the serum samples at the DoDSR are utilized. Through 2012, the DoDSR had 54,542,658 serum specimens available, of which only 228,610 (0.42%) had ever been accessed for study.8 With such a wealth of information and relative availability, why are the serum samples not being accessed more frequently for studies? The inherent nature of the DoDSR being a restricted facility and only accessible to DoD-affiliated investigators may contribute, which allows the DoDSR to fulfill its primary purpose of contributing to military-relevant investigations but at the same time limits the number and type of investigations that can be performed. One idea that has been proposed is allowing civilian investigator access to the DoDSR if it can be proven that the research is targeted toward military-relevant issues.8 However, the current AFHSC access guidelines would need revision and would require additional safeguards to ensure that military-protected health information is not compromised. Nonetheless, such a change may result in more extensive use of DoDSR resources in the future.
An ethical issue that needs to be addressed pertains to how the DoDSR permits use of human serum samples for research purposes without getting consent from the individuals being studied. The serum samples are collected as part of mandatory predeployment and postdeployment examinations for HIV screening of all military members. These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Although it is true that military members must comply with specific requirements pertaining to military readiness (eg, receiving appropriate vaccinations, drug testing, regular medical screening), it is debated whether they still retain the right as patients to refuse participating in research and clinical trials.10 The AFHSC does have several regulatory steps in place to ensure that military members’ samples are used in an appropriate manner, including requiring a DoD primary investigator, IRB review of every research proposal, and de-identification of samples. At a minimum, giving military members the ability to provide informed consent would ensure that the military system is adhering to evolving human research standards.
The current lack of biological specimens other than serum in the DoDSR is another limitation of the current system. Recent advances in molecular analyses are impacted by expanding -omics techniques, such as epigenomics, transcriptomics, and proteomics. The field of epigenomics is the study of reversible changes to DNA (eg, methylation) associated with specific disease states or following specific environmental exposures.9,11 Transcriptomics, which analyzes messenger RNA transcript levels of expressed genes, and proteomics, which uses expression of proteins, are 2 techniques being used to develop biomarkers associated with specific diseases and environmental exposures.9,11 Serum alone does not provide the high-quality nucleic acids needed for many of these studies to take place. Adding whole-blood specimens or blood spot samples of military service members to the DoDSR would allow researchers to use these techniques to investigate many new biomarkers associated with military-relevant diseases and exposures. These techniques also can be used in the expanding field of personalized medicine so that health care providers are able to tailor all phases of care, including diagnosis and treatment, to an individual’s genetic profile.
Conclusion
The history of research in military medicine has been built on achieving the primary goal of serving those men and women who put their lives in danger to protect this country. In an evolving environment of new technologies that have led to changes in service members’ injuries, exposures, and diseases, military medicine also must adapt. Resources such as the DoDSR and DMSS, which provide investigators with the unique ability to link epidemiological data with serum samples, have been invaluable contributors to this overall mission. As with any large system, there are always improvements that can be made. Improving access to the DoDSR serum samples, educating and obtaining consent from military service members to use their samples in research, and adding specimens to the DoDSR that can be used for -omics techniques are 3 changes that should be considered to maximize
Advances in medical biotechnologies, data-gathering techniques, and -omics technologies have resulted in the broader understanding of disease pathology and treatment and have facilitated the individualization of health care plans to meet the unique needs of each patient. Military medicine often has been on the forefront of medical technology, disease understanding, and clinical care both on and off the battlefield, in large part due to the unique resources available in the military health care system. These resources allow investigators the ability to integrate vast amounts of epidemiologic data with an extensive biological sample database of its service members, which in the modern age has translated into advances in the understanding of melanoma and the treatment of scars.
History of Research in the Military
Starting in the 1950s, the US Department of Defense (DoD) started to collect serum samples of its service members for the purpose of research.1 It was not until 1985 that the DoD implemented a long-term frozen storage system for serum samples obtained through mandatory screening for human immunodeficiency virus (HIV) in service members.2 Subsequently, the Department of Defense Serum Repository (DoDSR) was officially established in 1989 as a central archive for the long-term storage of serum obtained from active-duty and reserve service members in the US Navy, Army, and Marines.2,3 In the mid-1990s, the DoDSR expanded its capabilities to include the storage of serum samples from all military members, including the US Air Force, obtained predeployment and postdeployment.3,4 At that time, a records-keeping system was established, now known as the Defense Medical Surveillance System (DMSS). The creation of the DMSS provided an extensive epidemiologic database that provided valuable information such as demographic data, service records, deployment data, reportable medical events, exposure history, and vaccination records, which could be linked to the serum samples of each service member.2-4 Since 2008, the responsibilities of maintaining the DoDSR and the DMSS were transferred to the Armed Forces Health Surveillance Center (AFHSC).5
There have been several other databases created over the years that provide additional support and resources to military investigators. The Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services both help investigators to track the incidence of specific cancers in the military population and provide them with pathologic specimens. Additionally, electronic medical records including the composite health care system and the Armed Forces Health Longitudinal Technology Application supplemented with insurance claims data accessible from the Military Health System Management and Reporting Tool (M2) database have made it possible to track patient data.
Utilization of Military Research Resources
Today, the DoDSR is a secure facility that maintains more than 56 million serum specimens from more than 11 million individuals in –30°C freezers, making it one of the largest repositories in the world.3,6 Each serum sample is linked with an individual’s DMSS record, providing a way for investigators to study how external factors such as deployment history, occupation, and exposure history relate to an individual’s unique genetic and physiological makeup. Furthermore, these data can be used for seroepidemiologic investigations that contribute to all facets of clinical care. The AFHSC routinely publishes findings related to notifiable diseases, disease outbreaks, and disease trends in a monthly report.7
There are strict guidelines in place that limit access to the DoDSR and service members’ data. Use of the repository for information directly related to a patient’s health care is one reason for access, such as analyzing serum for antibodies and seroconversion to assist in the diagnosis of a disease such as HIV. Another reason would be to obtain information needed for criminal investigations and prosecution. Typically, these types of requests require a judge-issued court order and approval by the Assistant Secretary of Defense for Health Affairs.4 The DoDSR also is used to study force health protection issues, such as infectious disease incidence and disease prevalence in the military population.
Obtaining access to the DoDSR and service members’ data for research purposes requires that the principal investigator be a DoD employee. Each research proposal is reviewed by members of the AFHSC to determine if the DoDSR is able to meet the demands of the project, including having the appropriate number of serum samples and supporting epidemiologic data available. The AFHSC provides a letter of support if it deems the project to be in line with its current resources and capabilities. Each research proposal is then sent to an institutional review board (IRB) to determine if the study is exempt or needs to go through a full IRB review process. A study might be exempt if the investigators are not obtaining data through interaction with living individuals or not having access to any identifiable protected health information associated with the samples.6 Regardless of whether the study is exempt or not exempt, the AFHSC will de-identify each sample before releasing the samples to the investigators by using a coding system to shield the patient’s identity from the investigator.
Resources within the military medical research system provide investigators with access to an extensive biorepository of serum and linked epidemiological data. Samples from the DoDSR have been used in no less than 75 peer-reviewed publications since 1985.8,9 Several of these studies have been influential in expanding knowledge about conditions seen more commonly in the military population such as stress fractures, traumatic brain injuries, posttraumatic stress disorder, and suicide.8 Additionally, DoDSR samples have been used to form military vaccination policies and track both infectious and noninfectious conditions in the military; for example, during the H1N1 influenza virus outbreak of 2009, AFHSC was essential in helping to limit the spread of the virus within the military community by using its data and collaborating with groups such as the Centers for Disease Control and Prevention to develop a plan for disease surveillance and control.5
Several military research resources are currently being used for a melanoma study that aims to assess if specific phenotypic features, melanoma risk alleles, and environmental factors (eg, duty station location, occupation, amount of UV exposure) can be used to develop better screening models to identify individuals who are at risk for developing melanoma. Secondarily, the study aims to determine if recently developed multimarker diagnostic and prognostic assays for melanoma will prove useful in the diagnostic and prognostic assessment of melanocytic neoplasms in the military population. For this study, one of the authors (J.H.M) is utilizing DoDSR serum from 1700 retrospective cases of invasive melanoma and 1700 matched controls. Additionally, the Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services databases are being used to obtain tissue from more than 300 melanoma cases and nevi controls.
Limitations of the Current System
Despite the impressive capabilities of the current system, there are some issues that limit its potential. One such limitation is associated with the way that the serum samples at the DoDSR are utilized. Through 2012, the DoDSR had 54,542,658 serum specimens available, of which only 228,610 (0.42%) had ever been accessed for study.8 With such a wealth of information and relative availability, why are the serum samples not being accessed more frequently for studies? The inherent nature of the DoDSR being a restricted facility and only accessible to DoD-affiliated investigators may contribute, which allows the DoDSR to fulfill its primary purpose of contributing to military-relevant investigations but at the same time limits the number and type of investigations that can be performed. One idea that has been proposed is allowing civilian investigator access to the DoDSR if it can be proven that the research is targeted toward military-relevant issues.8 However, the current AFHSC access guidelines would need revision and would require additional safeguards to ensure that military-protected health information is not compromised. Nonetheless, such a change may result in more extensive use of DoDSR resources in the future.
An ethical issue that needs to be addressed pertains to how the DoDSR permits use of human serum samples for research purposes without getting consent from the individuals being studied. The serum samples are collected as part of mandatory predeployment and postdeployment examinations for HIV screening of all military members. These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Although it is true that military members must comply with specific requirements pertaining to military readiness (eg, receiving appropriate vaccinations, drug testing, regular medical screening), it is debated whether they still retain the right as patients to refuse participating in research and clinical trials.10 The AFHSC does have several regulatory steps in place to ensure that military members’ samples are used in an appropriate manner, including requiring a DoD primary investigator, IRB review of every research proposal, and de-identification of samples. At a minimum, giving military members the ability to provide informed consent would ensure that the military system is adhering to evolving human research standards.
The current lack of biological specimens other than serum in the DoDSR is another limitation of the current system. Recent advances in molecular analyses are impacted by expanding -omics techniques, such as epigenomics, transcriptomics, and proteomics. The field of epigenomics is the study of reversible changes to DNA (eg, methylation) associated with specific disease states or following specific environmental exposures.9,11 Transcriptomics, which analyzes messenger RNA transcript levels of expressed genes, and proteomics, which uses expression of proteins, are 2 techniques being used to develop biomarkers associated with specific diseases and environmental exposures.9,11 Serum alone does not provide the high-quality nucleic acids needed for many of these studies to take place. Adding whole-blood specimens or blood spot samples of military service members to the DoDSR would allow researchers to use these techniques to investigate many new biomarkers associated with military-relevant diseases and exposures. These techniques also can be used in the expanding field of personalized medicine so that health care providers are able to tailor all phases of care, including diagnosis and treatment, to an individual’s genetic profile.
Conclusion
The history of research in military medicine has been built on achieving the primary goal of serving those men and women who put their lives in danger to protect this country. In an evolving environment of new technologies that have led to changes in service members’ injuries, exposures, and diseases, military medicine also must adapt. Resources such as the DoDSR and DMSS, which provide investigators with the unique ability to link epidemiological data with serum samples, have been invaluable contributors to this overall mission. As with any large system, there are always improvements that can be made. Improving access to the DoDSR serum samples, educating and obtaining consent from military service members to use their samples in research, and adding specimens to the DoDSR that can be used for -omics techniques are 3 changes that should be considered to maximize
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900-1904.
- Department of Defense Serum Repository. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Department-of-Defense-Serum-Repository. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD serum repository. Mil Med. 2015;180(10 suppl):10-12.
- DeFraites RF. The Armed Forces Health Surveillance Center: enhancing the Military Health System’s public health capabilities. BMC Public Health. 2011;11(suppl 2):S1.
- Pavlin JA, Welch RA. Ethics, human use, and the department of defense serum repository. Mil Med. 2015;180:49-56.
- Defense Medical Surveillance System. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Surveillance-System. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV, et al. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. Plos One. 2015;10:1-16.
- Mancuso JD, Mallon TM, Gaydos JC. Maximizing the capabilities of the DoD serum repository to meet current and future needs: report of the needs panel. Mil Med. 2015;180:14-24.
- Department of Defense. Department of Defense Instruction. http://www.dtic.mil/whs/directives/corres/pdf/600014p.pdf. Posted September 26, 2001. Updated October 3, 2013. Accessed August 2, 2016.
- Lindler LE. Building a DoD biorepository for the future: potential benefits and way forward. Mil Med. 2015;180:90-94.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900-1904.
- Department of Defense Serum Repository. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Department-of-Defense-Serum-Repository. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD serum repository. Mil Med. 2015;180(10 suppl):10-12.
- DeFraites RF. The Armed Forces Health Surveillance Center: enhancing the Military Health System’s public health capabilities. BMC Public Health. 2011;11(suppl 2):S1.
- Pavlin JA, Welch RA. Ethics, human use, and the department of defense serum repository. Mil Med. 2015;180:49-56.
- Defense Medical Surveillance System. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Surveillance-System. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV, et al. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. Plos One. 2015;10:1-16.
- Mancuso JD, Mallon TM, Gaydos JC. Maximizing the capabilities of the DoD serum repository to meet current and future needs: report of the needs panel. Mil Med. 2015;180:14-24.
- Department of Defense. Department of Defense Instruction. http://www.dtic.mil/whs/directives/corres/pdf/600014p.pdf. Posted September 26, 2001. Updated October 3, 2013. Accessed August 2, 2016.
- Lindler LE. Building a DoD biorepository for the future: potential benefits and way forward. Mil Med. 2015;180:90-94.
Practice Points
- Large patient databases and tissue repositories are increasingly being used to improve patient care through the use of clinical data, genomics, proteinomics, and metabolomics.
- The US Military has an established electronic medical record as well as tissue and serum repositories that can be leveraged to study melanoma and other dermatologic diseases.
Evaluating PD-1 Inhibitors
PD-1 antibodies are being touted as a promising immunotherapeutic approach for treating malignant melanoma among other cancers. The antibodies target proteins that promote programmed cell death and activate the immune system to attack tumors.
To find out more about the efficacy and safety of PD-1 antibody treatment, researchers from Xiamen University, Chinese Academy of Medical Sciences, and Peking Union Medical College, China, reviewed data from 5 multicenter, randomized clinical trials involving 2,828 patients. In 2 trials, patients were previously untreated; in the other 3, patients had progression after anti-CTLA-4 treatment or had received no more than 1 previous systemic therapy. Patients in the experimental groups received nivolumab or pembrolizumab; patients in the control groups received ipilimumab or chemotherapy.
In all 5 trials, the researchers noted significant differences between the anti-PD-1 groups and the control groups. The PD-1 antibody treatment was associated with a significantly better overall response rate (ORR): 40.0% in patients on nivolumab 3 mg/kg IV every 2 weeks as front-line therapy and 31.6% in those who received nivolumab at the same dosage after progression from anti-CTLA-4 treatment. Response was improved whether the drug was used as first-line treatment or for refractory/relapsed melanoma.
Related: Nivolumab Approved for Expanded Indication
Patients in the PD-1 groups also had a significantly greater rate of progression-free survival (PFS) compared with those who received other treatments, such as chemotherapy and ipilimumab. The median PFS was > 4.7 months in the nivolumab group and > 3.7 months in the pembrolizumab group. In 2 trials, the ORR among patients receiving pembrolizumab was between 23.3% and 33.2%; different dosages improved the overall response rate in both untreated and relapsed/refractory patients.
The most common adverse events (AEs) were fatigue, diarrhea, pruritus, rash, and nausea. Patients treated with nivolumab reported significantly fewer AEs. Although a high dosage or short intermission of pembrolizumab extended the median PFS, a subgroup analysis of different doses revealed a significant dose-dependent increase in AEs.
Source:
Lin Z, Chen X, Li Z, et al. PLoS One. 2016;11(8):e0160485.
doi: 10.1371/journal.pone.0160485.
PD-1 antibodies are being touted as a promising immunotherapeutic approach for treating malignant melanoma among other cancers. The antibodies target proteins that promote programmed cell death and activate the immune system to attack tumors.
To find out more about the efficacy and safety of PD-1 antibody treatment, researchers from Xiamen University, Chinese Academy of Medical Sciences, and Peking Union Medical College, China, reviewed data from 5 multicenter, randomized clinical trials involving 2,828 patients. In 2 trials, patients were previously untreated; in the other 3, patients had progression after anti-CTLA-4 treatment or had received no more than 1 previous systemic therapy. Patients in the experimental groups received nivolumab or pembrolizumab; patients in the control groups received ipilimumab or chemotherapy.
In all 5 trials, the researchers noted significant differences between the anti-PD-1 groups and the control groups. The PD-1 antibody treatment was associated with a significantly better overall response rate (ORR): 40.0% in patients on nivolumab 3 mg/kg IV every 2 weeks as front-line therapy and 31.6% in those who received nivolumab at the same dosage after progression from anti-CTLA-4 treatment. Response was improved whether the drug was used as first-line treatment or for refractory/relapsed melanoma.
Related: Nivolumab Approved for Expanded Indication
Patients in the PD-1 groups also had a significantly greater rate of progression-free survival (PFS) compared with those who received other treatments, such as chemotherapy and ipilimumab. The median PFS was > 4.7 months in the nivolumab group and > 3.7 months in the pembrolizumab group. In 2 trials, the ORR among patients receiving pembrolizumab was between 23.3% and 33.2%; different dosages improved the overall response rate in both untreated and relapsed/refractory patients.
The most common adverse events (AEs) were fatigue, diarrhea, pruritus, rash, and nausea. Patients treated with nivolumab reported significantly fewer AEs. Although a high dosage or short intermission of pembrolizumab extended the median PFS, a subgroup analysis of different doses revealed a significant dose-dependent increase in AEs.
Source:
Lin Z, Chen X, Li Z, et al. PLoS One. 2016;11(8):e0160485.
doi: 10.1371/journal.pone.0160485.
PD-1 antibodies are being touted as a promising immunotherapeutic approach for treating malignant melanoma among other cancers. The antibodies target proteins that promote programmed cell death and activate the immune system to attack tumors.
To find out more about the efficacy and safety of PD-1 antibody treatment, researchers from Xiamen University, Chinese Academy of Medical Sciences, and Peking Union Medical College, China, reviewed data from 5 multicenter, randomized clinical trials involving 2,828 patients. In 2 trials, patients were previously untreated; in the other 3, patients had progression after anti-CTLA-4 treatment or had received no more than 1 previous systemic therapy. Patients in the experimental groups received nivolumab or pembrolizumab; patients in the control groups received ipilimumab or chemotherapy.
In all 5 trials, the researchers noted significant differences between the anti-PD-1 groups and the control groups. The PD-1 antibody treatment was associated with a significantly better overall response rate (ORR): 40.0% in patients on nivolumab 3 mg/kg IV every 2 weeks as front-line therapy and 31.6% in those who received nivolumab at the same dosage after progression from anti-CTLA-4 treatment. Response was improved whether the drug was used as first-line treatment or for refractory/relapsed melanoma.
Related: Nivolumab Approved for Expanded Indication
Patients in the PD-1 groups also had a significantly greater rate of progression-free survival (PFS) compared with those who received other treatments, such as chemotherapy and ipilimumab. The median PFS was > 4.7 months in the nivolumab group and > 3.7 months in the pembrolizumab group. In 2 trials, the ORR among patients receiving pembrolizumab was between 23.3% and 33.2%; different dosages improved the overall response rate in both untreated and relapsed/refractory patients.
The most common adverse events (AEs) were fatigue, diarrhea, pruritus, rash, and nausea. Patients treated with nivolumab reported significantly fewer AEs. Although a high dosage or short intermission of pembrolizumab extended the median PFS, a subgroup analysis of different doses revealed a significant dose-dependent increase in AEs.
Source:
Lin Z, Chen X, Li Z, et al. PLoS One. 2016;11(8):e0160485.
doi: 10.1371/journal.pone.0160485.
FDA modifies dosage regimen for nivolumab
The Food and Drug Administration has modified the dosage regimen for nivolumab for indications of renal cell carcinoma, metastatic melanoma, and non–small cell lung cancer.
The single-dose regimen of nivolumab (3 mg/kg IV every 2 weeks) is replaced with the new recommended regimen of 240 mg IV every 2 weeks until disease progression or intolerable toxicity, the FDA said in a written statement.
The nivolumab (Opdivo) dosing regimen in combination with ipilimumab for melanoma will stay the same (nivolumab 1 mg/kg IV, followed by ipilimumab on the same day, every 3 weeks for four doses); however, after completion of ipilimumab, the recommended nivolumab dose is modified to 240 mg every 2 weeks until disease progression or intolerable toxicity. The recommended dose for classical Hodgkin lymphoma remains at 3 mg/kg IV every 2 weeks until disease progression or intolerable toxicity.
The change was made based on analyses demonstrating the comparability of the pharmacokinetics exposure, safety, and efficacy of the proposed new dosing regimen with the previously approved regimen. “Based on simulations by the population pharmacokinetics model, [the] FDA determined that the overall exposure at 240 mg every 2 weeks flat dose is similar (less than 6% difference) to 3 mg/kg every 2 weeks. These differences in exposure are not likely to have a clinically meaningful effect on safety and efficacy, since dose/exposure response relationships appear to be relatively flat in these three indications,” the FDA said.
The Food and Drug Administration has modified the dosage regimen for nivolumab for indications of renal cell carcinoma, metastatic melanoma, and non–small cell lung cancer.
The single-dose regimen of nivolumab (3 mg/kg IV every 2 weeks) is replaced with the new recommended regimen of 240 mg IV every 2 weeks until disease progression or intolerable toxicity, the FDA said in a written statement.
The nivolumab (Opdivo) dosing regimen in combination with ipilimumab for melanoma will stay the same (nivolumab 1 mg/kg IV, followed by ipilimumab on the same day, every 3 weeks for four doses); however, after completion of ipilimumab, the recommended nivolumab dose is modified to 240 mg every 2 weeks until disease progression or intolerable toxicity. The recommended dose for classical Hodgkin lymphoma remains at 3 mg/kg IV every 2 weeks until disease progression or intolerable toxicity.
The change was made based on analyses demonstrating the comparability of the pharmacokinetics exposure, safety, and efficacy of the proposed new dosing regimen with the previously approved regimen. “Based on simulations by the population pharmacokinetics model, [the] FDA determined that the overall exposure at 240 mg every 2 weeks flat dose is similar (less than 6% difference) to 3 mg/kg every 2 weeks. These differences in exposure are not likely to have a clinically meaningful effect on safety and efficacy, since dose/exposure response relationships appear to be relatively flat in these three indications,” the FDA said.
The Food and Drug Administration has modified the dosage regimen for nivolumab for indications of renal cell carcinoma, metastatic melanoma, and non–small cell lung cancer.
The single-dose regimen of nivolumab (3 mg/kg IV every 2 weeks) is replaced with the new recommended regimen of 240 mg IV every 2 weeks until disease progression or intolerable toxicity, the FDA said in a written statement.
The nivolumab (Opdivo) dosing regimen in combination with ipilimumab for melanoma will stay the same (nivolumab 1 mg/kg IV, followed by ipilimumab on the same day, every 3 weeks for four doses); however, after completion of ipilimumab, the recommended nivolumab dose is modified to 240 mg every 2 weeks until disease progression or intolerable toxicity. The recommended dose for classical Hodgkin lymphoma remains at 3 mg/kg IV every 2 weeks until disease progression or intolerable toxicity.
The change was made based on analyses demonstrating the comparability of the pharmacokinetics exposure, safety, and efficacy of the proposed new dosing regimen with the previously approved regimen. “Based on simulations by the population pharmacokinetics model, [the] FDA determined that the overall exposure at 240 mg every 2 weeks flat dose is similar (less than 6% difference) to 3 mg/kg every 2 weeks. These differences in exposure are not likely to have a clinically meaningful effect on safety and efficacy, since dose/exposure response relationships appear to be relatively flat in these three indications,” the FDA said.
Study suggests link between commonly used antihypertensive drug and skin cancer
Despite its widespread use as a guideline-recommended, first-line agent for the treatment of hypertension, hydrochlorothiazide (HCTZ) may contribute to an increased risk of skin cancer, according to Armand B. Cognetta Jr., MD, and his associates in the division of dermatology, Mohs Micrographic Surgery Unit, Florida State University, Tallahassee.
The common, long-term use of HCTZ for treatment of hypertension, combined with its known photosensitizing effects, makes it a potential candidate for increasing the risk of squamous cell carcinoma (SCC) and other skin cancers.
To further elucidate the association between longterm HCTZ exposure and skin cancer risk, Dr. Cognetta and his associates screened medication lists of 75 patients seen in their Mohs practice over a period of 5 days for lifetime SCCs and HCTZ exposure. For this study, patients with more than 20 lifetime SCCs were considered high risk. They also conducted a literature review of previous studies exploring this relationship, from 1966 to 2015.
Of the 75 patients screened, 4 met the criteria for inclusion in the high risk category. These four patients had a combined lifetime total of 288 SCC and 98 basal cell carcinomas (BCCs), including 10 that were lip SCC. All four patients were non-Hispanic white males and had been taking HCTZ alone or in combination for 3-15 years (Dermatol Surg. 2016 Sep;42[9]:1107-9).
The literature search produced three relevant studies, all of which had large patient populations, published between 2008 and 2012, “demonstrating an increased risk of SCC or lip cancer” associated with HCTZ use, with the highest risk associated with over 5 years of use, the researchers wrote.
“As cutaneous oncologists, it is our duty to look for ‘correctable’ causes of skin cancer,” they noted. “Hydrochlorothiazide, a known photosensitizer, when taken by white non-Hispanic patients with a history of multiple SCC, skin cancers may represent a ‘correctable’ cause.”
In their practice, they screen their high-risk SCC non-Hispanic patients for HCTZ use, “and send a standard letter explaining the association to the primary care provider with a request to change to a different antihypertensive if possible,” they wrote. “Many primary care providers are unaware of the association between HCTZ use and skin cancer,” they observed.
The authors had no disclosures.
Despite its widespread use as a guideline-recommended, first-line agent for the treatment of hypertension, hydrochlorothiazide (HCTZ) may contribute to an increased risk of skin cancer, according to Armand B. Cognetta Jr., MD, and his associates in the division of dermatology, Mohs Micrographic Surgery Unit, Florida State University, Tallahassee.
The common, long-term use of HCTZ for treatment of hypertension, combined with its known photosensitizing effects, makes it a potential candidate for increasing the risk of squamous cell carcinoma (SCC) and other skin cancers.
To further elucidate the association between longterm HCTZ exposure and skin cancer risk, Dr. Cognetta and his associates screened medication lists of 75 patients seen in their Mohs practice over a period of 5 days for lifetime SCCs and HCTZ exposure. For this study, patients with more than 20 lifetime SCCs were considered high risk. They also conducted a literature review of previous studies exploring this relationship, from 1966 to 2015.
Of the 75 patients screened, 4 met the criteria for inclusion in the high risk category. These four patients had a combined lifetime total of 288 SCC and 98 basal cell carcinomas (BCCs), including 10 that were lip SCC. All four patients were non-Hispanic white males and had been taking HCTZ alone or in combination for 3-15 years (Dermatol Surg. 2016 Sep;42[9]:1107-9).
The literature search produced three relevant studies, all of which had large patient populations, published between 2008 and 2012, “demonstrating an increased risk of SCC or lip cancer” associated with HCTZ use, with the highest risk associated with over 5 years of use, the researchers wrote.
“As cutaneous oncologists, it is our duty to look for ‘correctable’ causes of skin cancer,” they noted. “Hydrochlorothiazide, a known photosensitizer, when taken by white non-Hispanic patients with a history of multiple SCC, skin cancers may represent a ‘correctable’ cause.”
In their practice, they screen their high-risk SCC non-Hispanic patients for HCTZ use, “and send a standard letter explaining the association to the primary care provider with a request to change to a different antihypertensive if possible,” they wrote. “Many primary care providers are unaware of the association between HCTZ use and skin cancer,” they observed.
The authors had no disclosures.
Despite its widespread use as a guideline-recommended, first-line agent for the treatment of hypertension, hydrochlorothiazide (HCTZ) may contribute to an increased risk of skin cancer, according to Armand B. Cognetta Jr., MD, and his associates in the division of dermatology, Mohs Micrographic Surgery Unit, Florida State University, Tallahassee.
The common, long-term use of HCTZ for treatment of hypertension, combined with its known photosensitizing effects, makes it a potential candidate for increasing the risk of squamous cell carcinoma (SCC) and other skin cancers.
To further elucidate the association between longterm HCTZ exposure and skin cancer risk, Dr. Cognetta and his associates screened medication lists of 75 patients seen in their Mohs practice over a period of 5 days for lifetime SCCs and HCTZ exposure. For this study, patients with more than 20 lifetime SCCs were considered high risk. They also conducted a literature review of previous studies exploring this relationship, from 1966 to 2015.
Of the 75 patients screened, 4 met the criteria for inclusion in the high risk category. These four patients had a combined lifetime total of 288 SCC and 98 basal cell carcinomas (BCCs), including 10 that were lip SCC. All four patients were non-Hispanic white males and had been taking HCTZ alone or in combination for 3-15 years (Dermatol Surg. 2016 Sep;42[9]:1107-9).
The literature search produced three relevant studies, all of which had large patient populations, published between 2008 and 2012, “demonstrating an increased risk of SCC or lip cancer” associated with HCTZ use, with the highest risk associated with over 5 years of use, the researchers wrote.
“As cutaneous oncologists, it is our duty to look for ‘correctable’ causes of skin cancer,” they noted. “Hydrochlorothiazide, a known photosensitizer, when taken by white non-Hispanic patients with a history of multiple SCC, skin cancers may represent a ‘correctable’ cause.”
In their practice, they screen their high-risk SCC non-Hispanic patients for HCTZ use, “and send a standard letter explaining the association to the primary care provider with a request to change to a different antihypertensive if possible,” they wrote. “Many primary care providers are unaware of the association between HCTZ use and skin cancer,” they observed.
The authors had no disclosures.
Key clinical point: The antihypertensive agent hydrochlorothiazide (HCTZ ) is photosensitizing and may be associated with an increased risk for skin cancers in non-Hispanic white males with a history of squamous cell carcinoma (SCC).
Major finding: All four patients considered high risk based on lifetime SCC history were non-Hispanic white males with a history of HCTZ exposure, an association supported by three previously published studies.
Data sources: Medical records from 75 patients seen in a single dermatology practice over a 5-day period, and a literature search as well as relevant publications detected through a literature search spanning the years 1966-2015.
Disclosures: The authors had no disclosures. A funding source was not identified.
Sequential protocol benefits patients with field cancerization
BOSTON – Field cancerization poses unique treatment challenges, but useful approaches to managing patients with such extreme dermatoheliosis are emerging, according to Anokhi Jambusaria-Pahlajani, MD.
In patients with extensive sun damage to the head and neck, she said she performs initial curettage of any hyperkeratotic actinic keratoses, followed by topical 0.5% 5-fluorouracil applied twice daily on postcurettage days 1 through 5. On day 6, she performs aminolevulinic acid photodynamic therapy (PDT) with 1 hour of incubation.
“Basically, on day zero they come into my office, and I look for anything that’s hyperkeratotic, and I numb it up and curette it. The goal of that is to get rid of the scale so that when I do the topical 5-fluorouracil or I do the photodynamic therapy, the medicine is actually getting to where we want it to go,” Dr. Jambusaria-Pahlajani, a dermatologist in Round Rock, Tex., said at the American Academy of Dermatology summer meeting.
She discussed one such patient – a lung transplant recipient – in whom this approach worked particularly well. At 6 months’ follow-up, the patient had had a significant response, and during a follow-up of at least 2 years, he did not develop a single actinic keratosis.
Early results with this combination approach were so encouraging that she and her colleagues published a case series of four solid organ transplant recipients. “All four patients tolerated the approach well, demonstrated excellent response to treatment with complete or near complete resolution of actinic keratosis and squamous cell carcinoma in situ lesions,” she said. At 1-6 months following treatment, “basically patients were clear,” she added (Dermatol Surg. 2016 Jan;42:S66-72).
Dr. Jambusaria-Pahlajani also said she has seen “great results” in most of the 30-40 other patients she has treated using this approach. “I’ve followed up patients for years, and they really haven’t had a problem like what they had prior to getting this treatment,” she said.
She noted that, anecdotally, many patients who had been treated with multiple courses of topical 5-fluorouracil monotherapy or photodynamic therapy actually preferred the combination approach. They felt that they were out of commission for a shorter period of time, and most of the patients “tended to be kind of red and inflamed for about 7-10 days total from start to finish, which was a lot better than with, for example, 5-fluorouracil,” she said.
One patient – another lung transplant recipient – initially responded to combination therapy, but returned in 3 months with numerous recurrent lesions. Based on findings from a 2010 study, a cyclic approach to PDT was tried in this patient. In that study of 12 solid organ transplant recipients, cyclic PDT reduced the incidence of squamous cell carcinoma (Dermatol Surg. 2010 May;36[5]:652-8).
The study subjects had a high burden of squamous cell carcinoma in situ. The cyclic PDT they received included blue light PDT with 1 hour of incubation every 4-8 weeks for 2 years. The median number of new invasive and in situ squamous cell carcinomas declined from about 20 to 4 in the first year (a 79% reduction vs. 1 month prior to first treatment) and to 1 at 2 years (a 95% reduction).
“This patient happened to be a transplant patient as well, but I think you could also use [this approach] in nontransplant patients,” Dr. Jambusaria-Pahlajani said. His response was “pretty significant,” and after 18 months of treatment he was doing well.
“The main thing that you have to remember, though, is that these patients have to be willing to come in every 4-8 weeks to get the PDT,” which, she added, “is not for every patient.” It’s also not for every provider. Aside from staff or resource access limitations, these treatments can be very time consuming and costly, she noted. “Treating these patients can be a challenge, but it can be rewarding, as well,” she commented.
She reported having no relevant financial disclosures.
BOSTON – Field cancerization poses unique treatment challenges, but useful approaches to managing patients with such extreme dermatoheliosis are emerging, according to Anokhi Jambusaria-Pahlajani, MD.
In patients with extensive sun damage to the head and neck, she said she performs initial curettage of any hyperkeratotic actinic keratoses, followed by topical 0.5% 5-fluorouracil applied twice daily on postcurettage days 1 through 5. On day 6, she performs aminolevulinic acid photodynamic therapy (PDT) with 1 hour of incubation.
“Basically, on day zero they come into my office, and I look for anything that’s hyperkeratotic, and I numb it up and curette it. The goal of that is to get rid of the scale so that when I do the topical 5-fluorouracil or I do the photodynamic therapy, the medicine is actually getting to where we want it to go,” Dr. Jambusaria-Pahlajani, a dermatologist in Round Rock, Tex., said at the American Academy of Dermatology summer meeting.
She discussed one such patient – a lung transplant recipient – in whom this approach worked particularly well. At 6 months’ follow-up, the patient had had a significant response, and during a follow-up of at least 2 years, he did not develop a single actinic keratosis.
Early results with this combination approach were so encouraging that she and her colleagues published a case series of four solid organ transplant recipients. “All four patients tolerated the approach well, demonstrated excellent response to treatment with complete or near complete resolution of actinic keratosis and squamous cell carcinoma in situ lesions,” she said. At 1-6 months following treatment, “basically patients were clear,” she added (Dermatol Surg. 2016 Jan;42:S66-72).
Dr. Jambusaria-Pahlajani also said she has seen “great results” in most of the 30-40 other patients she has treated using this approach. “I’ve followed up patients for years, and they really haven’t had a problem like what they had prior to getting this treatment,” she said.
She noted that, anecdotally, many patients who had been treated with multiple courses of topical 5-fluorouracil monotherapy or photodynamic therapy actually preferred the combination approach. They felt that they were out of commission for a shorter period of time, and most of the patients “tended to be kind of red and inflamed for about 7-10 days total from start to finish, which was a lot better than with, for example, 5-fluorouracil,” she said.
One patient – another lung transplant recipient – initially responded to combination therapy, but returned in 3 months with numerous recurrent lesions. Based on findings from a 2010 study, a cyclic approach to PDT was tried in this patient. In that study of 12 solid organ transplant recipients, cyclic PDT reduced the incidence of squamous cell carcinoma (Dermatol Surg. 2010 May;36[5]:652-8).
The study subjects had a high burden of squamous cell carcinoma in situ. The cyclic PDT they received included blue light PDT with 1 hour of incubation every 4-8 weeks for 2 years. The median number of new invasive and in situ squamous cell carcinomas declined from about 20 to 4 in the first year (a 79% reduction vs. 1 month prior to first treatment) and to 1 at 2 years (a 95% reduction).
“This patient happened to be a transplant patient as well, but I think you could also use [this approach] in nontransplant patients,” Dr. Jambusaria-Pahlajani said. His response was “pretty significant,” and after 18 months of treatment he was doing well.
“The main thing that you have to remember, though, is that these patients have to be willing to come in every 4-8 weeks to get the PDT,” which, she added, “is not for every patient.” It’s also not for every provider. Aside from staff or resource access limitations, these treatments can be very time consuming and costly, she noted. “Treating these patients can be a challenge, but it can be rewarding, as well,” she commented.
She reported having no relevant financial disclosures.
BOSTON – Field cancerization poses unique treatment challenges, but useful approaches to managing patients with such extreme dermatoheliosis are emerging, according to Anokhi Jambusaria-Pahlajani, MD.
In patients with extensive sun damage to the head and neck, she said she performs initial curettage of any hyperkeratotic actinic keratoses, followed by topical 0.5% 5-fluorouracil applied twice daily on postcurettage days 1 through 5. On day 6, she performs aminolevulinic acid photodynamic therapy (PDT) with 1 hour of incubation.
“Basically, on day zero they come into my office, and I look for anything that’s hyperkeratotic, and I numb it up and curette it. The goal of that is to get rid of the scale so that when I do the topical 5-fluorouracil or I do the photodynamic therapy, the medicine is actually getting to where we want it to go,” Dr. Jambusaria-Pahlajani, a dermatologist in Round Rock, Tex., said at the American Academy of Dermatology summer meeting.
She discussed one such patient – a lung transplant recipient – in whom this approach worked particularly well. At 6 months’ follow-up, the patient had had a significant response, and during a follow-up of at least 2 years, he did not develop a single actinic keratosis.
Early results with this combination approach were so encouraging that she and her colleagues published a case series of four solid organ transplant recipients. “All four patients tolerated the approach well, demonstrated excellent response to treatment with complete or near complete resolution of actinic keratosis and squamous cell carcinoma in situ lesions,” she said. At 1-6 months following treatment, “basically patients were clear,” she added (Dermatol Surg. 2016 Jan;42:S66-72).
Dr. Jambusaria-Pahlajani also said she has seen “great results” in most of the 30-40 other patients she has treated using this approach. “I’ve followed up patients for years, and they really haven’t had a problem like what they had prior to getting this treatment,” she said.
She noted that, anecdotally, many patients who had been treated with multiple courses of topical 5-fluorouracil monotherapy or photodynamic therapy actually preferred the combination approach. They felt that they were out of commission for a shorter period of time, and most of the patients “tended to be kind of red and inflamed for about 7-10 days total from start to finish, which was a lot better than with, for example, 5-fluorouracil,” she said.
One patient – another lung transplant recipient – initially responded to combination therapy, but returned in 3 months with numerous recurrent lesions. Based on findings from a 2010 study, a cyclic approach to PDT was tried in this patient. In that study of 12 solid organ transplant recipients, cyclic PDT reduced the incidence of squamous cell carcinoma (Dermatol Surg. 2010 May;36[5]:652-8).
The study subjects had a high burden of squamous cell carcinoma in situ. The cyclic PDT they received included blue light PDT with 1 hour of incubation every 4-8 weeks for 2 years. The median number of new invasive and in situ squamous cell carcinomas declined from about 20 to 4 in the first year (a 79% reduction vs. 1 month prior to first treatment) and to 1 at 2 years (a 95% reduction).
“This patient happened to be a transplant patient as well, but I think you could also use [this approach] in nontransplant patients,” Dr. Jambusaria-Pahlajani said. His response was “pretty significant,” and after 18 months of treatment he was doing well.
“The main thing that you have to remember, though, is that these patients have to be willing to come in every 4-8 weeks to get the PDT,” which, she added, “is not for every patient.” It’s also not for every provider. Aside from staff or resource access limitations, these treatments can be very time consuming and costly, she noted. “Treating these patients can be a challenge, but it can be rewarding, as well,” she commented.
She reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM AAD SUMMER ACADEMY 2016
Veterans’ keratinocyte carcinoma, actinic keratosis care cost $356 million in 2012
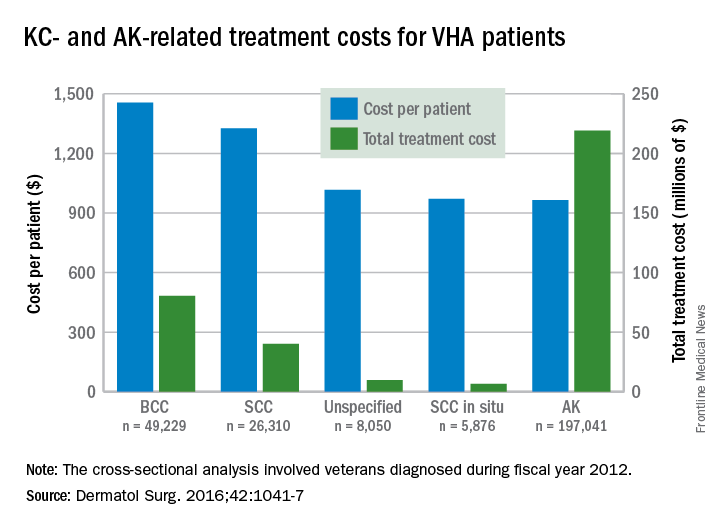
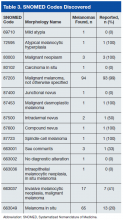
Almost 4% of the 5.9 million veterans treated by the Veterans Health Administration in 2012 had a diagnosis of keratinocyte carcinoma (KC) or actinic keratosis (AK), and their treatment cost $356 million, according to an analysis of VHA-provided or -contracted outpatient encounters.
Treatment costs per patient for KCs, also known as nonmelanoma skin cancers, were $1,456 for basal cell carcinoma (BCC), $1,326 for squamous cell carcinoma (SCC), $1,016 for unspecified, nongenital invasive KCs, $971 for squamous cell carcinoma in situ, and $906 for genital skin cancer (not shown on graph), reported Jean Yoon, PhD, of Veterans Affairs Palo Alto Health Care System, Menlo Park, Calif., and her associates (Dermatol Surg. 2016;42:1041-7).
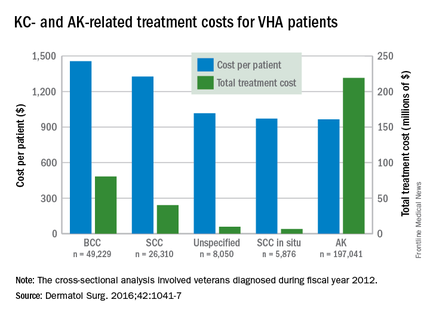
The VHA’s cost per patient for AK was relatively low – $965 per patient in 2012 – but the number of patients – 197,041 – was more than four times higher than any of the KCs. There were 49,229 veterans with BCC, 26,310 veterans with SCC, 8,050 veterans with unspecified KC, 5,876 veterans with SCC in situ, and 512 veterans with genital skin cancer, according to the analysis of administrative data on outpatient care and prescription drugs provided or paid by the VHA in fiscal year 2012. The total number of patients was 227,601, as some patients had more than one of the study diagnoses.
As a result of the high number of patients, actinic keratosis care totaled $219 million, compared with $80 million for BCC, $40 million for SCC, $9.6 million for nonspecified KC, $6.6 million for SCC in situ, and $582,000 for genital skin cancer, the investigators wrote.
The study was supported by a grant from the Department of Veterans Affairs. One of Dr. Yoon’s associates served as a consultant to several companies, but the remaining investigators had no conflicts to report.
Almost 4% of the 5.9 million veterans treated by the Veterans Health Administration in 2012 had a diagnosis of keratinocyte carcinoma (KC) or actinic keratosis (AK), and their treatment cost $356 million, according to an analysis of VHA-provided or -contracted outpatient encounters.
Treatment costs per patient for KCs, also known as nonmelanoma skin cancers, were $1,456 for basal cell carcinoma (BCC), $1,326 for squamous cell carcinoma (SCC), $1,016 for unspecified, nongenital invasive KCs, $971 for squamous cell carcinoma in situ, and $906 for genital skin cancer (not shown on graph), reported Jean Yoon, PhD, of Veterans Affairs Palo Alto Health Care System, Menlo Park, Calif., and her associates (Dermatol Surg. 2016;42:1041-7).

The VHA’s cost per patient for AK was relatively low – $965 per patient in 2012 – but the number of patients – 197,041 – was more than four times higher than any of the KCs. There were 49,229 veterans with BCC, 26,310 veterans with SCC, 8,050 veterans with unspecified KC, 5,876 veterans with SCC in situ, and 512 veterans with genital skin cancer, according to the analysis of administrative data on outpatient care and prescription drugs provided or paid by the VHA in fiscal year 2012. The total number of patients was 227,601, as some patients had more than one of the study diagnoses.
As a result of the high number of patients, actinic keratosis care totaled $219 million, compared with $80 million for BCC, $40 million for SCC, $9.6 million for nonspecified KC, $6.6 million for SCC in situ, and $582,000 for genital skin cancer, the investigators wrote.
The study was supported by a grant from the Department of Veterans Affairs. One of Dr. Yoon’s associates served as a consultant to several companies, but the remaining investigators had no conflicts to report.
Almost 4% of the 5.9 million veterans treated by the Veterans Health Administration in 2012 had a diagnosis of keratinocyte carcinoma (KC) or actinic keratosis (AK), and their treatment cost $356 million, according to an analysis of VHA-provided or -contracted outpatient encounters.
Treatment costs per patient for KCs, also known as nonmelanoma skin cancers, were $1,456 for basal cell carcinoma (BCC), $1,326 for squamous cell carcinoma (SCC), $1,016 for unspecified, nongenital invasive KCs, $971 for squamous cell carcinoma in situ, and $906 for genital skin cancer (not shown on graph), reported Jean Yoon, PhD, of Veterans Affairs Palo Alto Health Care System, Menlo Park, Calif., and her associates (Dermatol Surg. 2016;42:1041-7).

The VHA’s cost per patient for AK was relatively low – $965 per patient in 2012 – but the number of patients – 197,041 – was more than four times higher than any of the KCs. There were 49,229 veterans with BCC, 26,310 veterans with SCC, 8,050 veterans with unspecified KC, 5,876 veterans with SCC in situ, and 512 veterans with genital skin cancer, according to the analysis of administrative data on outpatient care and prescription drugs provided or paid by the VHA in fiscal year 2012. The total number of patients was 227,601, as some patients had more than one of the study diagnoses.
As a result of the high number of patients, actinic keratosis care totaled $219 million, compared with $80 million for BCC, $40 million for SCC, $9.6 million for nonspecified KC, $6.6 million for SCC in situ, and $582,000 for genital skin cancer, the investigators wrote.
The study was supported by a grant from the Department of Veterans Affairs. One of Dr. Yoon’s associates served as a consultant to several companies, but the remaining investigators had no conflicts to report.
FROM DERMATOLOGIC SURGERY
Pneumonitis with nivolumab treatment shows common radiographic patterns
A study of cancer patients enrolled in trials of the programmed cell death-1 inhibiting medicine nivolumab found that among a minority who developed pneumonitis during treatment, distinct radiographic patterns were significantly associated with the level of pneumonitis severity.
Investigators found that cryptic organizing pneumonia pattern (COP) was the most common, though not the most severe. Led by Mizuki Nishino, MD, of Brigham and Women’s Hospital, Boston, the researchers looked at the 20 patients out of a cohort of 170 (11.8%) who had developed pneumonitis, and found that radiologic patterns indicating acute interstitial pneumonia/acute respiratory distress syndrome (n = 2) had the highest severity grade on a scale of 1-5 (median 3), followed by those with COP pattern (n = 13, median grade 2), hypersensitivity pneumonitis (n = 2, median grade 1), and nonspecific interstitial pneumonia (n = 3, median grade 1). The pattern was significantly associated with severity (P = .0006).
The study cohort included patients being treated with nivolumab for lung cancer, melanoma, and lymphoma; the COP patten was the most common across tumor types and observed in patients receiving monotherapy and combination therapy alike. Therapy with nivolumab was suspended for all 20 pneumonitis patients, and most (n = 17) received treatment for pneumonitis with corticosteroids with or without infliximab, for a median treatment time of 6 weeks. Seven patients were able to restart nivolumab, though pneumonitis recurred in two, the investigators reported (Clin Cancer Res. 2016 Aug 17. doi: 10.1158/1078-0432.CCR-16-1320).
“Time from initiation of therapy to the development of pneumonitis had a wide range (0.5-11.5 months), indicating an importance of careful observation and follow-up for signs and symptoms of pneumonitis throughout treatment,” Dr. Nishino and colleagues wrote in their analysis, adding that shorter times were observed for lung cancer patients, possibly because of their higher pulmonary burden, a lower threshold for performing chest scans in these patients, or both. “In most patients, clinical and radiographic improvements were noted after treatment, indicating that [PD-1 inhibitor-related pneumonitis], although potentially serious, is treatable if diagnosed and managed appropriately. The observation emphasizes the importance of timely recognition, accurate diagnosis, and early intervention.”
The lead author and several coauthors disclosed funding from Bristol-Myers Squibb, which sponsored the trial, as well as from other manufacturers.
A study of cancer patients enrolled in trials of the programmed cell death-1 inhibiting medicine nivolumab found that among a minority who developed pneumonitis during treatment, distinct radiographic patterns were significantly associated with the level of pneumonitis severity.
Investigators found that cryptic organizing pneumonia pattern (COP) was the most common, though not the most severe. Led by Mizuki Nishino, MD, of Brigham and Women’s Hospital, Boston, the researchers looked at the 20 patients out of a cohort of 170 (11.8%) who had developed pneumonitis, and found that radiologic patterns indicating acute interstitial pneumonia/acute respiratory distress syndrome (n = 2) had the highest severity grade on a scale of 1-5 (median 3), followed by those with COP pattern (n = 13, median grade 2), hypersensitivity pneumonitis (n = 2, median grade 1), and nonspecific interstitial pneumonia (n = 3, median grade 1). The pattern was significantly associated with severity (P = .0006).
The study cohort included patients being treated with nivolumab for lung cancer, melanoma, and lymphoma; the COP patten was the most common across tumor types and observed in patients receiving monotherapy and combination therapy alike. Therapy with nivolumab was suspended for all 20 pneumonitis patients, and most (n = 17) received treatment for pneumonitis with corticosteroids with or without infliximab, for a median treatment time of 6 weeks. Seven patients were able to restart nivolumab, though pneumonitis recurred in two, the investigators reported (Clin Cancer Res. 2016 Aug 17. doi: 10.1158/1078-0432.CCR-16-1320).
“Time from initiation of therapy to the development of pneumonitis had a wide range (0.5-11.5 months), indicating an importance of careful observation and follow-up for signs and symptoms of pneumonitis throughout treatment,” Dr. Nishino and colleagues wrote in their analysis, adding that shorter times were observed for lung cancer patients, possibly because of their higher pulmonary burden, a lower threshold for performing chest scans in these patients, or both. “In most patients, clinical and radiographic improvements were noted after treatment, indicating that [PD-1 inhibitor-related pneumonitis], although potentially serious, is treatable if diagnosed and managed appropriately. The observation emphasizes the importance of timely recognition, accurate diagnosis, and early intervention.”
The lead author and several coauthors disclosed funding from Bristol-Myers Squibb, which sponsored the trial, as well as from other manufacturers.
A study of cancer patients enrolled in trials of the programmed cell death-1 inhibiting medicine nivolumab found that among a minority who developed pneumonitis during treatment, distinct radiographic patterns were significantly associated with the level of pneumonitis severity.
Investigators found that cryptic organizing pneumonia pattern (COP) was the most common, though not the most severe. Led by Mizuki Nishino, MD, of Brigham and Women’s Hospital, Boston, the researchers looked at the 20 patients out of a cohort of 170 (11.8%) who had developed pneumonitis, and found that radiologic patterns indicating acute interstitial pneumonia/acute respiratory distress syndrome (n = 2) had the highest severity grade on a scale of 1-5 (median 3), followed by those with COP pattern (n = 13, median grade 2), hypersensitivity pneumonitis (n = 2, median grade 1), and nonspecific interstitial pneumonia (n = 3, median grade 1). The pattern was significantly associated with severity (P = .0006).
The study cohort included patients being treated with nivolumab for lung cancer, melanoma, and lymphoma; the COP patten was the most common across tumor types and observed in patients receiving monotherapy and combination therapy alike. Therapy with nivolumab was suspended for all 20 pneumonitis patients, and most (n = 17) received treatment for pneumonitis with corticosteroids with or without infliximab, for a median treatment time of 6 weeks. Seven patients were able to restart nivolumab, though pneumonitis recurred in two, the investigators reported (Clin Cancer Res. 2016 Aug 17. doi: 10.1158/1078-0432.CCR-16-1320).
“Time from initiation of therapy to the development of pneumonitis had a wide range (0.5-11.5 months), indicating an importance of careful observation and follow-up for signs and symptoms of pneumonitis throughout treatment,” Dr. Nishino and colleagues wrote in their analysis, adding that shorter times were observed for lung cancer patients, possibly because of their higher pulmonary burden, a lower threshold for performing chest scans in these patients, or both. “In most patients, clinical and radiographic improvements were noted after treatment, indicating that [PD-1 inhibitor-related pneumonitis], although potentially serious, is treatable if diagnosed and managed appropriately. The observation emphasizes the importance of timely recognition, accurate diagnosis, and early intervention.”
The lead author and several coauthors disclosed funding from Bristol-Myers Squibb, which sponsored the trial, as well as from other manufacturers.
FROM CLINICAL CANCER RESEARCH
Key clinical point: Pneumonitis related to treatment with PD-1 inhibitors showed distinct radiographic patterns associated with severity; most cases resolved with corticosteroid treatment.
Major finding: Of 20 patients in nivolumab trials who developed pneumonitis, a COP pattern was seen in 13, and other patterns in 7; different patterns were significantly associated with pneumonitis severity (P = .006).
Data source: 170 patients with melanoma, lung cancer or lymphoma enrolled in single-site open-label clinical trial of nivolumab.
Disclosures: The lead author and several coauthors disclosed funding from Bristol-Myers Squibb, which sponsored the trial, as well as from other manufacturers.
Hat-Wearing Patterns in Spectators Attending Baseball Games: A 10-Year Retrospective Comparison
Spectators at baseball games may be exposed to excess solar UV radiation (UVR), which has been linked to the development of both melanoma and nonmelanoma skin cancers.1,2 Although baseball hats traditionally are worn to demonstrate team support, they also may provide some sun protection for the head and face where skin cancers are commonly found.
The importance of protecting the skin from solar UVR has led to sun-protection programs and community education as well as efforts to evaluate the impact of these programs. Major League Baseball (MLB) has partnered with the American Academy of Dermatology since 1999 to promote the importance of sun protection and raise skin cancer awareness through its Play Sun Smart program.3 A study conducted 10 years ago (N=2030) evaluated hat use in spectators at MLB games and noted that less than half of all spectators in seating sections exposed to direct sunlight wore hats.4 The purpose of the current study was to evaluate how public education about sun protection has impacted the use of hats by spectators at MLB games in 2015 compared to the prior study in 2006.
Methods
Data were collected during a 3-game series (2 day games, 1 night game) in August 2015 in New York, New York. During one of the day games, 18,000 fans received a free wide-brimmed hat. High-resolution digital photographs of seating sections were obtained using a camera with a 300-mm lens. Using the same methodology as the prior study,4 sunny and shaded seating sections were photographed during all 3 games (Figure). Photographs of each section were analyzed by an independent reviewer using a high-resolution computer screen. Spectators wearing head coverings—baseball hats, visors, or hats with circumferential brims—were defined as using hats. The number of spectators wearing hats versus not wearing hats was recorded for all identical sections of interest. Bleacher seating was analyzed separately, as spectators presumably knew in advance of the continuous direct sun exposure during day games, and a subset of young children in the bleachers (<10 years of age) also was assessed. A continuously sunny section also was evaluated at the second and sixth innings to see if hats were presumably purchased during exposure. Statistical significance was determined using χ2 tests with P<.05 indicating statistical significance.

Results
This analysis consisted of 3539 spectators. In both the sunny and shaded sections of a day game, there were more spectators wearing hats (49% and 37%, respectively)(P<.001) than in the same sections at night games (35% and 29%, respectively)(Table 1). During the day game, more spectators wore hats in the sunny section than in the adjacent shaded section (49% vs 37%; P<.001). Analysis of the same 2 sections during the night game revealed no significant differences.
Spectators sitting in the bleachers during a day game who presumably knew to anticipate direct sun exposure showed no significant differences in hat-wearing patterns versus the sunny section (44% vs 49%) but were more likely to wear hats compared to those sitting in the bleachers at the night game (44% vs 33%)(P<.001)(Table 1). There was no significant difference in the number of hats worn by spectators in the sunny section in the second inning (43%) versus the same section after continuous sun exposure at the sixth inning (44%)(Table 2). Significantly more children seated in the bleachers during the day game wore hats compared to adults in the same section (64% vs 42%; P<.001)(Table 3). During the hat giveaway day, significantly more spectators wore hats (the majority of which were the free giveaway hats) across all sections studied (P<.001)(Table 4).
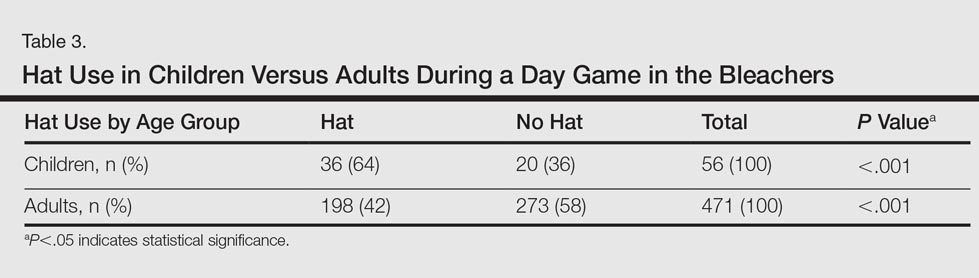

Comment
More than 23 million spectators attended daytime MLB games in 2015, with millions more attending minor league and amateur events.5Although sun-protection messages tend to be well understood and received by society, many choose to ignore them.6
In partnership with the American Academy of Dermatology, the MLB’s Play Sun Smart program has promoted UVR risk awareness at sporting events since 1999.3 Those affiliated with MLB teams also receive annual skin cancer screenings in conjunction with a public education effort in May of each season. However, despite the years of sun-protection education, our study found that less than half of attendees wore hats for UVR protection. In fact, there were no significant differences noted across all of the hat-wearing parameters studied (day vs night game, sunny vs shaded section, sunny section over course of game) between the current study compared to the results from 10 years prior4 (Tables 1 and 2). For spectators in the bleacher section, even presumably knowing in advance that seating would be in the sun did not significantly increase hat-wearing behavior. Although skin cancer rates continue to rise, hat-wearing trends remain stable, revealing a concerning trend.
Increased availability of sunscreen has led to improved sun-protective behaviors in many populations.7 In our study, the free hat giveaway had the greatest impact on hat wearing, which suggests that improved availability and access to hats can lead to an important opportunity for sun-protection programs to partner with hat manufacturers to augment their use and protective impact.
Sun avoidance during childhood and adolescence has been shown to decrease the risk for melanoma.1 Young children had the highest rate of hat usage in the current study, possibly due to parental example or dictates. Research has shown the importance of role models in promoting sun safety to young children,8,9 so perhaps use of hats by parents or MLB players contributed to the hat-wearing behavior observed in this subpopulation.
Given the limited change observed in hat-wearing behaviors over the last decade, a knowledge and behavioral gap appears to exist that may be able to be exploited to enhance future sun protection. Also, based on our findings, the MLB and other sun-protection education campaigns may wish to augment their UVR protective messages by offering hat giveaways, which appear to have a notable impact.
Acknowledgment
The authors thank Jessie Skapik, BS (New York, New York), for her independent review of the spectator photographs.
References
1. Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58(5, suppl 2):S129-S132.
2. Lim HW, James WD, Rigel DS, et al. Adverse effects of ultraviolet radiation from the use of indoor tanning equipment: time to ban the tan. J Am Acad Dermatol. 2011;64:893-902.
3. Play Sun Smart. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/programs/play-sun-smart. Accessed August 25, 2016.
4. Rigel AS, Lebwohl MG. Hat-wearing patterns in persons attending baseball games. J Am Acad Dermatol. 2006;54:918-919.
5. MLB attendance report - 2016. ESPN website. www.espn.go.com/mlb/attendance. Accessed May 20, 2016.
6. Turner D, Harrison SL, Buettner P, et al. Does being a “SunSmart School” influence hat-wearing compliance? an ecological study of hat-wearing rates at Australian primary schools in a region of high sun exposure [published online December 29, 2013]. Prev Med. 2014;60:107-114.
7. Dubas LE, Adams BB. Sunscreen use and availability among female collegiate athletes [published online February 3, 2012]. J Am Acad Dermatol. 2012;67:876.e1-876.e6.
8. O’Riodran DL, Geller AC, Brooks DR, et al. Sunburn reduction through parental role modeling and sunscreen vigilance. J Pediatr. 2003;142:67-72.
9. Turrisi R, Hillhouse J, Heavin S, et al. Examination of the short-term efficacy of a parent-based intervention to prevent skin cancer. J Behav Med. 2004;27:393-412.
Spectators at baseball games may be exposed to excess solar UV radiation (UVR), which has been linked to the development of both melanoma and nonmelanoma skin cancers.1,2 Although baseball hats traditionally are worn to demonstrate team support, they also may provide some sun protection for the head and face where skin cancers are commonly found.
The importance of protecting the skin from solar UVR has led to sun-protection programs and community education as well as efforts to evaluate the impact of these programs. Major League Baseball (MLB) has partnered with the American Academy of Dermatology since 1999 to promote the importance of sun protection and raise skin cancer awareness through its Play Sun Smart program.3 A study conducted 10 years ago (N=2030) evaluated hat use in spectators at MLB games and noted that less than half of all spectators in seating sections exposed to direct sunlight wore hats.4 The purpose of the current study was to evaluate how public education about sun protection has impacted the use of hats by spectators at MLB games in 2015 compared to the prior study in 2006.
Methods
Data were collected during a 3-game series (2 day games, 1 night game) in August 2015 in New York, New York. During one of the day games, 18,000 fans received a free wide-brimmed hat. High-resolution digital photographs of seating sections were obtained using a camera with a 300-mm lens. Using the same methodology as the prior study,4 sunny and shaded seating sections were photographed during all 3 games (Figure). Photographs of each section were analyzed by an independent reviewer using a high-resolution computer screen. Spectators wearing head coverings—baseball hats, visors, or hats with circumferential brims—were defined as using hats. The number of spectators wearing hats versus not wearing hats was recorded for all identical sections of interest. Bleacher seating was analyzed separately, as spectators presumably knew in advance of the continuous direct sun exposure during day games, and a subset of young children in the bleachers (<10 years of age) also was assessed. A continuously sunny section also was evaluated at the second and sixth innings to see if hats were presumably purchased during exposure. Statistical significance was determined using χ2 tests with P<.05 indicating statistical significance.

Results
This analysis consisted of 3539 spectators. In both the sunny and shaded sections of a day game, there were more spectators wearing hats (49% and 37%, respectively)(P<.001) than in the same sections at night games (35% and 29%, respectively)(Table 1). During the day game, more spectators wore hats in the sunny section than in the adjacent shaded section (49% vs 37%; P<.001). Analysis of the same 2 sections during the night game revealed no significant differences.

Spectators sitting in the bleachers during a day game who presumably knew to anticipate direct sun exposure showed no significant differences in hat-wearing patterns versus the sunny section (44% vs 49%) but were more likely to wear hats compared to those sitting in the bleachers at the night game (44% vs 33%)(P<.001)(Table 1). There was no significant difference in the number of hats worn by spectators in the sunny section in the second inning (43%) versus the same section after continuous sun exposure at the sixth inning (44%)(Table 2). Significantly more children seated in the bleachers during the day game wore hats compared to adults in the same section (64% vs 42%; P<.001)(Table 3). During the hat giveaway day, significantly more spectators wore hats (the majority of which were the free giveaway hats) across all sections studied (P<.001)(Table 4).



Comment
More than 23 million spectators attended daytime MLB games in 2015, with millions more attending minor league and amateur events.5Although sun-protection messages tend to be well understood and received by society, many choose to ignore them.6
In partnership with the American Academy of Dermatology, the MLB’s Play Sun Smart program has promoted UVR risk awareness at sporting events since 1999.3 Those affiliated with MLB teams also receive annual skin cancer screenings in conjunction with a public education effort in May of each season. However, despite the years of sun-protection education, our study found that less than half of attendees wore hats for UVR protection. In fact, there were no significant differences noted across all of the hat-wearing parameters studied (day vs night game, sunny vs shaded section, sunny section over course of game) between the current study compared to the results from 10 years prior4 (Tables 1 and 2). For spectators in the bleacher section, even presumably knowing in advance that seating would be in the sun did not significantly increase hat-wearing behavior. Although skin cancer rates continue to rise, hat-wearing trends remain stable, revealing a concerning trend.
Increased availability of sunscreen has led to improved sun-protective behaviors in many populations.7 In our study, the free hat giveaway had the greatest impact on hat wearing, which suggests that improved availability and access to hats can lead to an important opportunity for sun-protection programs to partner with hat manufacturers to augment their use and protective impact.
Sun avoidance during childhood and adolescence has been shown to decrease the risk for melanoma.1 Young children had the highest rate of hat usage in the current study, possibly due to parental example or dictates. Research has shown the importance of role models in promoting sun safety to young children,8,9 so perhaps use of hats by parents or MLB players contributed to the hat-wearing behavior observed in this subpopulation.
Given the limited change observed in hat-wearing behaviors over the last decade, a knowledge and behavioral gap appears to exist that may be able to be exploited to enhance future sun protection. Also, based on our findings, the MLB and other sun-protection education campaigns may wish to augment their UVR protective messages by offering hat giveaways, which appear to have a notable impact.
Acknowledgment
The authors thank Jessie Skapik, BS (New York, New York), for her independent review of the spectator photographs.
Spectators at baseball games may be exposed to excess solar UV radiation (UVR), which has been linked to the development of both melanoma and nonmelanoma skin cancers.1,2 Although baseball hats traditionally are worn to demonstrate team support, they also may provide some sun protection for the head and face where skin cancers are commonly found.
The importance of protecting the skin from solar UVR has led to sun-protection programs and community education as well as efforts to evaluate the impact of these programs. Major League Baseball (MLB) has partnered with the American Academy of Dermatology since 1999 to promote the importance of sun protection and raise skin cancer awareness through its Play Sun Smart program.3 A study conducted 10 years ago (N=2030) evaluated hat use in spectators at MLB games and noted that less than half of all spectators in seating sections exposed to direct sunlight wore hats.4 The purpose of the current study was to evaluate how public education about sun protection has impacted the use of hats by spectators at MLB games in 2015 compared to the prior study in 2006.
Methods
Data were collected during a 3-game series (2 day games, 1 night game) in August 2015 in New York, New York. During one of the day games, 18,000 fans received a free wide-brimmed hat. High-resolution digital photographs of seating sections were obtained using a camera with a 300-mm lens. Using the same methodology as the prior study,4 sunny and shaded seating sections were photographed during all 3 games (Figure). Photographs of each section were analyzed by an independent reviewer using a high-resolution computer screen. Spectators wearing head coverings—baseball hats, visors, or hats with circumferential brims—were defined as using hats. The number of spectators wearing hats versus not wearing hats was recorded for all identical sections of interest. Bleacher seating was analyzed separately, as spectators presumably knew in advance of the continuous direct sun exposure during day games, and a subset of young children in the bleachers (<10 years of age) also was assessed. A continuously sunny section also was evaluated at the second and sixth innings to see if hats were presumably purchased during exposure. Statistical significance was determined using χ2 tests with P<.05 indicating statistical significance.

Results
This analysis consisted of 3539 spectators. In both the sunny and shaded sections of a day game, there were more spectators wearing hats (49% and 37%, respectively)(P<.001) than in the same sections at night games (35% and 29%, respectively)(Table 1). During the day game, more spectators wore hats in the sunny section than in the adjacent shaded section (49% vs 37%; P<.001). Analysis of the same 2 sections during the night game revealed no significant differences.

Spectators sitting in the bleachers during a day game who presumably knew to anticipate direct sun exposure showed no significant differences in hat-wearing patterns versus the sunny section (44% vs 49%) but were more likely to wear hats compared to those sitting in the bleachers at the night game (44% vs 33%)(P<.001)(Table 1). There was no significant difference in the number of hats worn by spectators in the sunny section in the second inning (43%) versus the same section after continuous sun exposure at the sixth inning (44%)(Table 2). Significantly more children seated in the bleachers during the day game wore hats compared to adults in the same section (64% vs 42%; P<.001)(Table 3). During the hat giveaway day, significantly more spectators wore hats (the majority of which were the free giveaway hats) across all sections studied (P<.001)(Table 4).



Comment
More than 23 million spectators attended daytime MLB games in 2015, with millions more attending minor league and amateur events.5Although sun-protection messages tend to be well understood and received by society, many choose to ignore them.6
In partnership with the American Academy of Dermatology, the MLB’s Play Sun Smart program has promoted UVR risk awareness at sporting events since 1999.3 Those affiliated with MLB teams also receive annual skin cancer screenings in conjunction with a public education effort in May of each season. However, despite the years of sun-protection education, our study found that less than half of attendees wore hats for UVR protection. In fact, there were no significant differences noted across all of the hat-wearing parameters studied (day vs night game, sunny vs shaded section, sunny section over course of game) between the current study compared to the results from 10 years prior4 (Tables 1 and 2). For spectators in the bleacher section, even presumably knowing in advance that seating would be in the sun did not significantly increase hat-wearing behavior. Although skin cancer rates continue to rise, hat-wearing trends remain stable, revealing a concerning trend.
Increased availability of sunscreen has led to improved sun-protective behaviors in many populations.7 In our study, the free hat giveaway had the greatest impact on hat wearing, which suggests that improved availability and access to hats can lead to an important opportunity for sun-protection programs to partner with hat manufacturers to augment their use and protective impact.
Sun avoidance during childhood and adolescence has been shown to decrease the risk for melanoma.1 Young children had the highest rate of hat usage in the current study, possibly due to parental example or dictates. Research has shown the importance of role models in promoting sun safety to young children,8,9 so perhaps use of hats by parents or MLB players contributed to the hat-wearing behavior observed in this subpopulation.
Given the limited change observed in hat-wearing behaviors over the last decade, a knowledge and behavioral gap appears to exist that may be able to be exploited to enhance future sun protection. Also, based on our findings, the MLB and other sun-protection education campaigns may wish to augment their UVR protective messages by offering hat giveaways, which appear to have a notable impact.
Acknowledgment
The authors thank Jessie Skapik, BS (New York, New York), for her independent review of the spectator photographs.
References
1. Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58(5, suppl 2):S129-S132.
2. Lim HW, James WD, Rigel DS, et al. Adverse effects of ultraviolet radiation from the use of indoor tanning equipment: time to ban the tan. J Am Acad Dermatol. 2011;64:893-902.
3. Play Sun Smart. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/programs/play-sun-smart. Accessed August 25, 2016.
4. Rigel AS, Lebwohl MG. Hat-wearing patterns in persons attending baseball games. J Am Acad Dermatol. 2006;54:918-919.
5. MLB attendance report - 2016. ESPN website. www.espn.go.com/mlb/attendance. Accessed May 20, 2016.
6. Turner D, Harrison SL, Buettner P, et al. Does being a “SunSmart School” influence hat-wearing compliance? an ecological study of hat-wearing rates at Australian primary schools in a region of high sun exposure [published online December 29, 2013]. Prev Med. 2014;60:107-114.
7. Dubas LE, Adams BB. Sunscreen use and availability among female collegiate athletes [published online February 3, 2012]. J Am Acad Dermatol. 2012;67:876.e1-876.e6.
8. O’Riodran DL, Geller AC, Brooks DR, et al. Sunburn reduction through parental role modeling and sunscreen vigilance. J Pediatr. 2003;142:67-72.
9. Turrisi R, Hillhouse J, Heavin S, et al. Examination of the short-term efficacy of a parent-based intervention to prevent skin cancer. J Behav Med. 2004;27:393-412.
References
1. Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58(5, suppl 2):S129-S132.
2. Lim HW, James WD, Rigel DS, et al. Adverse effects of ultraviolet radiation from the use of indoor tanning equipment: time to ban the tan. J Am Acad Dermatol. 2011;64:893-902.
3. Play Sun Smart. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/programs/play-sun-smart. Accessed August 25, 2016.
4. Rigel AS, Lebwohl MG. Hat-wearing patterns in persons attending baseball games. J Am Acad Dermatol. 2006;54:918-919.
5. MLB attendance report - 2016. ESPN website. www.espn.go.com/mlb/attendance. Accessed May 20, 2016.
6. Turner D, Harrison SL, Buettner P, et al. Does being a “SunSmart School” influence hat-wearing compliance? an ecological study of hat-wearing rates at Australian primary schools in a region of high sun exposure [published online December 29, 2013]. Prev Med. 2014;60:107-114.
7. Dubas LE, Adams BB. Sunscreen use and availability among female collegiate athletes [published online February 3, 2012]. J Am Acad Dermatol. 2012;67:876.e1-876.e6.
8. O’Riodran DL, Geller AC, Brooks DR, et al. Sunburn reduction through parental role modeling and sunscreen vigilance. J Pediatr. 2003;142:67-72.
9. Turrisi R, Hillhouse J, Heavin S, et al. Examination of the short-term efficacy of a parent-based intervention to prevent skin cancer. J Behav Med. 2004;27:393-412.
Practice Points
- With less than half of attendees wearing hats to Major League Baseball games, there has been limited change in hat-wearing behavior over the last decade, possibly due to a knowledge or behavioral gap.
- Improved availability and access to hats can lead to improved sun-protective behaviors.
Nevus Spilus: Is the Presence of Hair Associated With an Increased Risk for Melanoma?
The term nevus spilus (NS), also known as speckled lentiginous nevus, was first used in the 19th century to describe lesions with background café au lait–like lentiginous melanocytic hyperplasia speckled with small, 1- to 3-mm, darker foci. The dark spots reflect lentigines; junctional, compound, and intradermal nevus cell nests; and more rarely Spitz and blue nevi. Both macular and papular subtypes have been described.1 This birthmark is quite common, occurring in 1.3% to 2.3% of the adult population worldwide.2 Hypertrichosis has been described in NS.3-9 Two subsequent cases of malignant melanoma in hairy NS suggested that lesions may be particularly prone to malignant degeneration.4,8 We report an additional case of hairy NS that was not associated with melanoma and consider whether dermatologists should warn their patients about this association.
Case Report
A 26-year-old woman presented with a stable 7×8-cm, tan-brown, macular, pigmented birthmark studded with darker 1- to 2-mm, irregular, brown-black and blue, confettilike macules on the left proximal lateral thigh that had been present since birth (Figure 1). Dark terminal hairs were present, arising from both the darker and lighter pigmented areas but not the surrounding normal skin.
A 4-mm punch biopsy from one of the dark blue macules demonstrated uniform lentiginous melanocytic hyperplasia and nevus cell nests adjacent to the sweat glands extending into the mid dermis (Figure 2). No clinical evidence of malignant degeneration was present.
Comment
The risk for melanoma is increased in classic nonspeckled congenital nevi and the risk correlates with the size of the lesion and most probably the number of nevus cells in the lesion that increase the risk for a random mutation.8,10,11 It is likely that NS with or without hair presages a small increased risk for melanoma,6,9,12 which is not surprising because NS is a subtype of congenital melanocytic nevus (CMN), a condition that is present at birth and results from a proliferation of melanocytes.6 Nevus spilus, however, appears to have a notably lower risk for malignant degeneration than other classic CMN of the same size. The following support for this hypothesis is offered: First, CMN have nevus cells broadly filling the dermis that extend more deeply into the dermis than NS (Figure 2A).10 In our estimation, CMN have at least 100 times the number of nevus cells per square centimeter compared to NS. The potential for malignant degeneration of any one melanocyte is greater when more are present. Second, although some NS lesions evolve, classic CMN are universally more proliferative than NS.10,13 The involved skin in CMN thickens over time with increased numbers of melanocytes and marked overgrowth of adjacent tissue. Melanocytes in a proliferative phase may be more likely to undergo malignant degeneration.10
A PubMed search of articles indexed for MEDLINE using the search term nevus spilus and melanoma yielded 2 cases4,8 of melanoma arising among 15 cases of hairy NS in the literature, which led to the suggestion that the presence of hair could be associated with an increased risk for malignant degeneration in NS (Table). This apparent high incidence of melanoma most likely reflects referral/publication bias rather than a statistically significant association. In fact, the clinical lesion most clinically similar to hairy NS is Becker nevus, with tan macules demonstrating lentiginous melanocytic hyperplasia associated with numerous coarse terminal hairs. There is no indication that Becker nevi have a considerable premalignant potential, though one case of melanoma arising in a Becker nevus has been reported.9 There is no evidence to suggest that classic CMN with hypertrichosis has a greater premalignant potential than similar lesions without hypertrichosis.

We noticed the presence of hair in our patient’s lesion only after reports in the literature caused us to look for this phenomenon.9 This occurrence may actually be quite common. We do not recommend prophylactic excision of NS and believe the risk for malignant degeneration is low in NS with or without hair, though larger NS (>4 cm), especially giant, zosteriform, or segmental lesions, may have a greater risk.1,6,9,10 It is prudent for physicians to carefully examine NS and sample suspicious foci, especially when patients describe a lesion as changing.
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology. 2006;212:53-58.
- Ly L, Christie M, Swain S, et al. Melanoma(s) arising in large segmental speckled lentiginous nevi: a case series. J Am Acad Dermatol. 2011;64:1190-1193.
- Prose NS, Heilman E, Felman YM, et al. Multiple benign juvenile melanoma. J Am Acad Dermatol. 1983;9:236-242.
- Grinspan D, Casala A, Abulafia J, et al. Melanoma on dysplastic nevus spilus. Int J Dermatol. 1997;36:499-502 .
- Langenbach N, Pfau A, Landthaler M, et al. Naevi spili, café-au-lait spots and melanocytic naevi aggregated alongside Blaschko’s lines, with a review of segmental melanocytic lesions. Acta Derm Venereol. 1998;78:378-380.
- Schaffer JV, Orlow SJ, Lazova R, et al. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch Dermatol. 2001;137:172-178.
- Saraswat A, Dogra S, Bansali A, et al. Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D–resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074-1076.
- Zeren-Bilgin
i , Gür S, Aydın O, et al. Melanoma arising in a hairy nevus spilus. Int J Dermatol. 2006;45:1362-1364. - Singh S, Jain N, Khanna N, et al. Hairy nevus spilus: a case series. Pediatr Dermatol. 2013;30:100-104.
- Price HN, Schaffer JV. Congenital melanocytic nevi—when to worry and how to treat: facts and controversies. Clin Dermatol. 2010;28:293-302.
- Alikhan Ali, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? J Am Acad Dermatol. 2012;67:495.e1-495.e17.
- Haenssle HA, Kaune KM, Buhl T, et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J Am Acad Dermatol. 2009;61:337-341.
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol. 2001;137:215-216.
The term nevus spilus (NS), also known as speckled lentiginous nevus, was first used in the 19th century to describe lesions with background café au lait–like lentiginous melanocytic hyperplasia speckled with small, 1- to 3-mm, darker foci. The dark spots reflect lentigines; junctional, compound, and intradermal nevus cell nests; and more rarely Spitz and blue nevi. Both macular and papular subtypes have been described.1 This birthmark is quite common, occurring in 1.3% to 2.3% of the adult population worldwide.2 Hypertrichosis has been described in NS.3-9 Two subsequent cases of malignant melanoma in hairy NS suggested that lesions may be particularly prone to malignant degeneration.4,8 We report an additional case of hairy NS that was not associated with melanoma and consider whether dermatologists should warn their patients about this association.
Case Report
A 26-year-old woman presented with a stable 7×8-cm, tan-brown, macular, pigmented birthmark studded with darker 1- to 2-mm, irregular, brown-black and blue, confettilike macules on the left proximal lateral thigh that had been present since birth (Figure 1). Dark terminal hairs were present, arising from both the darker and lighter pigmented areas but not the surrounding normal skin.

A 4-mm punch biopsy from one of the dark blue macules demonstrated uniform lentiginous melanocytic hyperplasia and nevus cell nests adjacent to the sweat glands extending into the mid dermis (Figure 2). No clinical evidence of malignant degeneration was present.

Comment
The risk for melanoma is increased in classic nonspeckled congenital nevi and the risk correlates with the size of the lesion and most probably the number of nevus cells in the lesion that increase the risk for a random mutation.8,10,11 It is likely that NS with or without hair presages a small increased risk for melanoma,6,9,12 which is not surprising because NS is a subtype of congenital melanocytic nevus (CMN), a condition that is present at birth and results from a proliferation of melanocytes.6 Nevus spilus, however, appears to have a notably lower risk for malignant degeneration than other classic CMN of the same size. The following support for this hypothesis is offered: First, CMN have nevus cells broadly filling the dermis that extend more deeply into the dermis than NS (Figure 2A).10 In our estimation, CMN have at least 100 times the number of nevus cells per square centimeter compared to NS. The potential for malignant degeneration of any one melanocyte is greater when more are present. Second, although some NS lesions evolve, classic CMN are universally more proliferative than NS.10,13 The involved skin in CMN thickens over time with increased numbers of melanocytes and marked overgrowth of adjacent tissue. Melanocytes in a proliferative phase may be more likely to undergo malignant degeneration.10
A PubMed search of articles indexed for MEDLINE using the search term nevus spilus and melanoma yielded 2 cases4,8 of melanoma arising among 15 cases of hairy NS in the literature, which led to the suggestion that the presence of hair could be associated with an increased risk for malignant degeneration in NS (Table). This apparent high incidence of melanoma most likely reflects referral/publication bias rather than a statistically significant association. In fact, the clinical lesion most clinically similar to hairy NS is Becker nevus, with tan macules demonstrating lentiginous melanocytic hyperplasia associated with numerous coarse terminal hairs. There is no indication that Becker nevi have a considerable premalignant potential, though one case of melanoma arising in a Becker nevus has been reported.9 There is no evidence to suggest that classic CMN with hypertrichosis has a greater premalignant potential than similar lesions without hypertrichosis.

We noticed the presence of hair in our patient’s lesion only after reports in the literature caused us to look for this phenomenon.9 This occurrence may actually be quite common. We do not recommend prophylactic excision of NS and believe the risk for malignant degeneration is low in NS with or without hair, though larger NS (>4 cm), especially giant, zosteriform, or segmental lesions, may have a greater risk.1,6,9,10 It is prudent for physicians to carefully examine NS and sample suspicious foci, especially when patients describe a lesion as changing.
The term nevus spilus (NS), also known as speckled lentiginous nevus, was first used in the 19th century to describe lesions with background café au lait–like lentiginous melanocytic hyperplasia speckled with small, 1- to 3-mm, darker foci. The dark spots reflect lentigines; junctional, compound, and intradermal nevus cell nests; and more rarely Spitz and blue nevi. Both macular and papular subtypes have been described.1 This birthmark is quite common, occurring in 1.3% to 2.3% of the adult population worldwide.2 Hypertrichosis has been described in NS.3-9 Two subsequent cases of malignant melanoma in hairy NS suggested that lesions may be particularly prone to malignant degeneration.4,8 We report an additional case of hairy NS that was not associated with melanoma and consider whether dermatologists should warn their patients about this association.
Case Report
A 26-year-old woman presented with a stable 7×8-cm, tan-brown, macular, pigmented birthmark studded with darker 1- to 2-mm, irregular, brown-black and blue, confettilike macules on the left proximal lateral thigh that had been present since birth (Figure 1). Dark terminal hairs were present, arising from both the darker and lighter pigmented areas but not the surrounding normal skin.

A 4-mm punch biopsy from one of the dark blue macules demonstrated uniform lentiginous melanocytic hyperplasia and nevus cell nests adjacent to the sweat glands extending into the mid dermis (Figure 2). No clinical evidence of malignant degeneration was present.

Comment
The risk for melanoma is increased in classic nonspeckled congenital nevi and the risk correlates with the size of the lesion and most probably the number of nevus cells in the lesion that increase the risk for a random mutation.8,10,11 It is likely that NS with or without hair presages a small increased risk for melanoma,6,9,12 which is not surprising because NS is a subtype of congenital melanocytic nevus (CMN), a condition that is present at birth and results from a proliferation of melanocytes.6 Nevus spilus, however, appears to have a notably lower risk for malignant degeneration than other classic CMN of the same size. The following support for this hypothesis is offered: First, CMN have nevus cells broadly filling the dermis that extend more deeply into the dermis than NS (Figure 2A).10 In our estimation, CMN have at least 100 times the number of nevus cells per square centimeter compared to NS. The potential for malignant degeneration of any one melanocyte is greater when more are present. Second, although some NS lesions evolve, classic CMN are universally more proliferative than NS.10,13 The involved skin in CMN thickens over time with increased numbers of melanocytes and marked overgrowth of adjacent tissue. Melanocytes in a proliferative phase may be more likely to undergo malignant degeneration.10
A PubMed search of articles indexed for MEDLINE using the search term nevus spilus and melanoma yielded 2 cases4,8 of melanoma arising among 15 cases of hairy NS in the literature, which led to the suggestion that the presence of hair could be associated with an increased risk for malignant degeneration in NS (Table). This apparent high incidence of melanoma most likely reflects referral/publication bias rather than a statistically significant association. In fact, the clinical lesion most clinically similar to hairy NS is Becker nevus, with tan macules demonstrating lentiginous melanocytic hyperplasia associated with numerous coarse terminal hairs. There is no indication that Becker nevi have a considerable premalignant potential, though one case of melanoma arising in a Becker nevus has been reported.9 There is no evidence to suggest that classic CMN with hypertrichosis has a greater premalignant potential than similar lesions without hypertrichosis.

We noticed the presence of hair in our patient’s lesion only after reports in the literature caused us to look for this phenomenon.9 This occurrence may actually be quite common. We do not recommend prophylactic excision of NS and believe the risk for malignant degeneration is low in NS with or without hair, though larger NS (>4 cm), especially giant, zosteriform, or segmental lesions, may have a greater risk.1,6,9,10 It is prudent for physicians to carefully examine NS and sample suspicious foci, especially when patients describe a lesion as changing.
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology. 2006;212:53-58.
- Ly L, Christie M, Swain S, et al. Melanoma(s) arising in large segmental speckled lentiginous nevi: a case series. J Am Acad Dermatol. 2011;64:1190-1193.
- Prose NS, Heilman E, Felman YM, et al. Multiple benign juvenile melanoma. J Am Acad Dermatol. 1983;9:236-242.
- Grinspan D, Casala A, Abulafia J, et al. Melanoma on dysplastic nevus spilus. Int J Dermatol. 1997;36:499-502 .
- Langenbach N, Pfau A, Landthaler M, et al. Naevi spili, café-au-lait spots and melanocytic naevi aggregated alongside Blaschko’s lines, with a review of segmental melanocytic lesions. Acta Derm Venereol. 1998;78:378-380.
- Schaffer JV, Orlow SJ, Lazova R, et al. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch Dermatol. 2001;137:172-178.
- Saraswat A, Dogra S, Bansali A, et al. Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D–resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074-1076.
- Zeren-Bilgin
i , Gür S, Aydın O, et al. Melanoma arising in a hairy nevus spilus. Int J Dermatol. 2006;45:1362-1364. - Singh S, Jain N, Khanna N, et al. Hairy nevus spilus: a case series. Pediatr Dermatol. 2013;30:100-104.
- Price HN, Schaffer JV. Congenital melanocytic nevi—when to worry and how to treat: facts and controversies. Clin Dermatol. 2010;28:293-302.
- Alikhan Ali, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? J Am Acad Dermatol. 2012;67:495.e1-495.e17.
- Haenssle HA, Kaune KM, Buhl T, et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J Am Acad Dermatol. 2009;61:337-341.
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol. 2001;137:215-216.
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology. 2006;212:53-58.
- Ly L, Christie M, Swain S, et al. Melanoma(s) arising in large segmental speckled lentiginous nevi: a case series. J Am Acad Dermatol. 2011;64:1190-1193.
- Prose NS, Heilman E, Felman YM, et al. Multiple benign juvenile melanoma. J Am Acad Dermatol. 1983;9:236-242.
- Grinspan D, Casala A, Abulafia J, et al. Melanoma on dysplastic nevus spilus. Int J Dermatol. 1997;36:499-502 .
- Langenbach N, Pfau A, Landthaler M, et al. Naevi spili, café-au-lait spots and melanocytic naevi aggregated alongside Blaschko’s lines, with a review of segmental melanocytic lesions. Acta Derm Venereol. 1998;78:378-380.
- Schaffer JV, Orlow SJ, Lazova R, et al. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch Dermatol. 2001;137:172-178.
- Saraswat A, Dogra S, Bansali A, et al. Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D–resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074-1076.
- Zeren-Bilgin
i , Gür S, Aydın O, et al. Melanoma arising in a hairy nevus spilus. Int J Dermatol. 2006;45:1362-1364. - Singh S, Jain N, Khanna N, et al. Hairy nevus spilus: a case series. Pediatr Dermatol. 2013;30:100-104.
- Price HN, Schaffer JV. Congenital melanocytic nevi—when to worry and how to treat: facts and controversies. Clin Dermatol. 2010;28:293-302.
- Alikhan Ali, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? J Am Acad Dermatol. 2012;67:495.e1-495.e17.
- Haenssle HA, Kaune KM, Buhl T, et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J Am Acad Dermatol. 2009;61:337-341.
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol. 2001;137:215-216.
Practice Points
- Nevus spilus (NS) appears as a café au lait macule studded with darker brown “moles.”
- Although melanoma has been described in NS, it is rare.
- There is no evidence that hairy NS are predisposed to melanoma.
Melanoma Registry Underreporting in the Veterans Health Administration
The National Cancer Data Base (NCDB) of the American College of Surgeons (ACS), the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI), and the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC) are among the leading registries for cancer surveillance, collecting cancer epidemiology data for the majority of the U.S. population.1 This national coverage aids researchers and policymakers in conducting epidemiologic studies and allocating health resources.1,2
U.S. federal law mandates the reporting of cancer.3,4 State laws require cancer reporting as well, but requirements vary slightly from state to state.5 However, all cancers with an ICD-O (International Classification of Diseases for Oncology) code of 2 or 3 are reportable. For Washington state, cancer must be reported unless it is basal or squamous cell carcinoma of nonmucoepidermoid skin or in situ cancer of the uterine cervix.5 In general, each facility that diagnoses or treats a melanoma is required to report it. Data are consolidated at the central registry level if necessary.
Cancer reporting often fails to meet states’ requirements.6-8 Since the inception of SEER and NPCR, many studies have assessed the accuracy of the cancer data reported to these registries and have found these data to be inaccurate or incomplete.6-8 Melanoma reporting, in particular,
seems to be prone to error. Studies have demonstrated melanoma underreporting ranging from 10% to 70%, with an increase in underreporting over time.9-11 Significant delays of up to 10 years have been found between initial diagnosis and reporting for melanomas.12 In general, these studies have focused on smaller facilities, such as private laboratories, which lack in-house reporting systems.
Cancer reporting is especially important in the VHA, the largest U.S. health care system. Health data on about 9 million enrolled veterans have been invaluable for understanding cancer epidemiology. Underreporting and misreporting of cancer cases in private medical offices and smaller treatment facilities may be attributable to lack of funding, personnel, administrative support, or knowledge of reporting requirements. In contrast, the VHA requires cancer reporting and provides funding, personnel, and administrative support.13
The VA Puget Sound Health Care System (VAPSHCS) traditionally has employed registrars to perform the majority of basic cancer registry tasks, including abstracting, case finding, and lifelong follow-up of the cancer patients listed in the registry. The registrars use OncoTraX software, which finds possible cancer cases from pathology, radiology, and patient treatment files, to accession cases. Unique cancer cases are reported to the VA Central Cancer Registry (VACCR), the Washington state registry, and the NCDB, which then transfer the data to the national registries. Accordingly, cases not accessioned would not be reported to the VA, state, and national registries.
The authors conducted a quality improvement project to ascertain whether primary cutaneous melanomas biopsied at VAPSHCS were underreported.
Materials
The VAPSHCS serves about 100,000 veterans and consists of 2 major treatment facilities, 2 community-based outpatient clinics, 1 outreach clinic, and 4 contract community-based outpatient clinics. Pathology cases for the entire VAPSHCS are accessioned in a central laboratory in Seattle.
Data Sources and Chart Abstraction
Data sources included the VA Corporate Data Warehouse (CDW), the VAPSHCS cancer registry, the Computerized Patient Record System (CPRS), VistA (Veterans Health Information Systems and Technology Architecture), VistA Web, and VistA Imaging.
Study Population
The study population consisted of veterans who had been diagnosed with primary cutaneous melanoma and had the diagnosis confirmed by biopsy performed at VAPSHCS between January 1, 2006 and December 31, 2012.
Statistical Analysis
Odds ratios were calculated using the MedCalc Odds Ratio Calculator.14
Methods
The authors identified SNOMED (Systematized Nomenclature of Medicine) codes that included the character string melanoma (Table 1). Using these codes, they queried VistA to identify melanoma cases diagnosed in the VAPSHCS Pathology and Laboratory Service during the period 2006 to 2012. To confirm the completeness of the local report, the authors performed the same search using the CDW. The SNOMED code case-finding was supplemented with cases ascertained using ICD-9 codes and problem list diagnoses.
A case must be reported to the local cancer registry if diagnosis or treatment takes place at the facility. All cases ascertained with the authors’ search criteria are, by definition, reportable to the local cancer registry. The authors then applied inclusion and exclusion criteria to determine
which cases were primary cutaneous melanomas and therefore candidates for this investigation (Table 2).
Having ascertained the primary cutaneous melanomas, the authors abstracted the pathology TNM (tumor, node, metastasis) staging (Breslow depth, mitotic index, presence of ulceration) and diagnosis dates from CPRS pathology reports. They then determined whether each case had been reported to the VACCR (OncoTraX was used to query for the accession status of each melanoma). If the melanoma was not accessioned, the authors tried to determine why.
Results
The authors discovered 193 primary cutaneous melanomas diagnosed by biopsy performed at VAPSHCS. Of these 193 melanomas, 71 (36.8%) had not been reported.
After the pathologist has completed a report, SNOMED codes are assigned by the pathology laboratory. Case finding with OncoTraX depends on SNOMED codes and other parameters (imaging, treatment, oncology consultation). OncoTraX is designed for case finding using World Health Organization (WHO) standardized 8000/X-9000/X series SNOMED codes. To understand the relationship between reporting and SNOMED codes, the authors ascertained the codes for all melanomas in the present study.
Table 3 lists the SNOMED codes assigned to melanomas biopsied at VAPSHCS and the percentage reported for each. Of the 106 melanomas that had been assigned WHO standardized codes, 101 (95.2%) had been reported. In contrast, only 21 (24.1%) of the 87 melanomas that had been assigned non-WHO standardized codes had been reported. In this study, non-WHO standardized codes are locally generated codes; they began with facility station number 663.
Use of locally generated codes may have contributed to nonreporting. Of the 71 melanomas not reported, 66 had a SNOMED code beginning with 663, and the other 5 had a WHO standardized SNOMED code. Odds of being nonreported were much higher for the melanomas with 663 codes than for the melanomas with WHO standardized codes (odds ratio [OR], 63.5; confidence interval [CI], 22.8-176.7; P ≤ .0001).
There was also a difference in coding between invasive and in situ melanomas. Of the 87 melanomas with a 663 code, 68 were in situ. Of the 106 melanomas with a national-level code, 11 were in situ. The odds of being assigned a local code were much higher for the in situ melanomas than they were for the invasive melanomas (OR, 30.9; CI, 13.8-69.1; P ≤ .0001).
Since 2000, the SNOMED code for melanoma in situ has been 87202, but no melanomas in situ were assigned this code. The 87202 code was not available in VistA for pathology laboratories to assign to melanomas at the time this study was conducted. Instead, most melanomas in situ were assigned a locally generated code. However, OncoTraX cannot recognize local codes, so melanomas assigned a local code might not have been accessionable.
The remaining 5 unreported melanomas were assigned WHO standardized codes. Secondary analysis revealed clerical errors, 4 made by the pathology laboratory and 1 by the registrar.
Discussion
Data from central cancer registries are used in a variety of fields, from research studies to health policymaking. They are used to “monitor cancer trends over time, show cancer patterns in various populations, identify high-risk groups, guide planning and evaluation of cancer control programs, help set priorities for allocating health resources, and advance clinical, epidemiologic, and health services research.”1
Melanoma underreporting has been demonstrated in previous studies, with the percentage of underreported cases varying from 10.4%11 to 70%.9 A longitudinal study of melanomas in Washington state found that underreporting of cutaneous melanomas increased from 2% to 21% over a 10-year period.10 This trend prompted examination of this study’s data for a similar temporal trend, and none was found.
A 2008 study found that more melanoma cases were being diagnosed or treated at outpatient facilities.9 Such facilities are prone to problems in reporting because they lack in-house reporting systems and knowledge of melanoma reporting requirements.9 A 2011 study of
practicing dermatologists found that many failed to report melanomas to a registry, and more than half were unaware of the requirement.12 Accordingly, underreporting is likely to continue. Results of the present study showed that melanoma underreporting was a major issue at VAPSHCS and that it could occur even in facilities that used in-house reporting systems and were aware of reporting requirements. The primary cause of underreporting was generation and use of local SNOMED codes that were unrecognizable by OncoTraX. A secondary cause was clerical error.
Discovery of unreported cases prompted facility review of procedures for reporting melanomas and expansion of current methods for melanoma discovery. All unreported cases have been entered into the VACCR, the Washington state registry, and the NCDB, which populate the national cancer registries. Contract registry staff were educated regarding melanoma reporting requirements, particularly requirements for melanoma in situ. The 87202 SNOMED code for melanoma in situ also has been added to VistA at VAPSHCS. A follow-up study will be conducted to ascertain whether the interventions have corrected the underreporting of melanoma.
Study Limitations
The cases used in the study were obtained by SNOMED codes, CDW problem lists, and ICD-9 codes. This method may have missed cases that were assigned incorrect SNOMED codes and were not assigned to the problem list, or that were assigned to the problem list after the study period. The authors used a subset of all reportable cases—namely, those biopsied at VAPSHCS. Although this subset constituted the significant majority of reportable cases, the authors do not know the extent of underreporting of cases that were not biopsied at VAPSHCS. The extent to which other VA facilities generate local SNOMED codes also is unknown.
Conclusion
Melanoma underreporting at VAPSHCS is an addressable concern. The primary cause of underreporting was the use of locally generated SNOMED codes that were not recognized by cancer registry software. The present study should be repeated at other VA facilities to determine the extent to which its findings are generalizable.
Acknowledgments
The authors thank Dr. Stevan Knezevich for reviewing cases, Pam Pehan for providing the list of VAPSHCS melanomas accessioned from VistA, and Eddie Alaniz and Eugene Gavrilenko for helping ascertain SNOMED codes.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Division of Cancer Prevention and Control, Centers for Disease Control and Prevention. National Program of Cancer Registries (NPCR). CDC website. http://www.cdc.gov/cancer/npcr/about.htm. Updated April 20, 2016. Accessed July 1, 2016.
2. National Cancer Institute. Overview of the SEER program. National Cancer Institute website. http://seer.cancer.gov/about/overview.html. Accessed July 18, 2016.
3. Cancer Registries Amendment Act. 42 USC §201-280e (2016).
4. Cancer Registry of Greater California. Cancer reporting. Cancer Registry of Greater California website. http://crgc-cancer.org/hospitals-and-physicians. Accessed July 18, 2016.
5. American Academy of Dermatology. State cancer registry laws and requirements. American Academy of Dermatology website. https://www.aad.org/file%20library/global%20navigatio/education%20and%20quality%20care/state%20cancer%20registries/state-cancer-registries-laws-and-requirements.pdf. Accessed July 18, 2016.
6. Craig BM, Rollison DE, List AF, Cogle CR. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol Biomarkers Prev. 2012;21(3):474-481.
7. Fanning J, Gangestad A, Andrews SJ. National Cancer Data Base/Surveillance Epidemiology and End Results: potential insensitive-measure bias. Gynecol Oncol. 2000;77(3):450-453.
8. Thoburn KK, German RR, Lewis M, Nichols PJ, Ahmed F, Jackson-Thompson J. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer. 2007;109(8):1607-1616.
9. Cockburn M, Swetter SM, Peng D, Keegan TH, Deapen D, Clarke CA. Melanoma underreporting: why does it happen, how big is the problem, and how do we fix it? J Am Acad Dermatol. 2008;59(6):1081-1085.
10. Karagas MR, Thomas DB, Roth GJ, Johnson LK, Weiss NS. The effects of changes in health care delivery on the reported incidence of cutaneous melanoma in western Washington state. Am J Epidemiol. 1991;133(1):58-62.
11. Merlino LA, Sullivan KJ, Whitaker DC, Lynch CF. The independent pathology laboratory as a reporting source for cutaneous melanoma incidence in Iowa, 1977–1994. J Am Acad Dermatol. 1997;37(4):578-585.
12. Cartee TV, Kini SP, Chen SC. Melanoma reporting to central cancer registries by US dermatologists: an analysis of the persistent knowledge and practice gap. J Am Acad Dermatol. 2011;65(5)(suppl 1):S124-S132.
13. Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537-1545.
14. Odds ratio calculator. MedCalc website. https://www.medcalc.org/calc/odds_ratio.php. Accessed June 16, 2016.
Note: Page numbers differ between the print issue and digital edition.
The National Cancer Data Base (NCDB) of the American College of Surgeons (ACS), the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI), and the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC) are among the leading registries for cancer surveillance, collecting cancer epidemiology data for the majority of the U.S. population.1 This national coverage aids researchers and policymakers in conducting epidemiologic studies and allocating health resources.1,2
U.S. federal law mandates the reporting of cancer.3,4 State laws require cancer reporting as well, but requirements vary slightly from state to state.5 However, all cancers with an ICD-O (International Classification of Diseases for Oncology) code of 2 or 3 are reportable. For Washington state, cancer must be reported unless it is basal or squamous cell carcinoma of nonmucoepidermoid skin or in situ cancer of the uterine cervix.5 In general, each facility that diagnoses or treats a melanoma is required to report it. Data are consolidated at the central registry level if necessary.
Cancer reporting often fails to meet states’ requirements.6-8 Since the inception of SEER and NPCR, many studies have assessed the accuracy of the cancer data reported to these registries and have found these data to be inaccurate or incomplete.6-8 Melanoma reporting, in particular,
seems to be prone to error. Studies have demonstrated melanoma underreporting ranging from 10% to 70%, with an increase in underreporting over time.9-11 Significant delays of up to 10 years have been found between initial diagnosis and reporting for melanomas.12 In general, these studies have focused on smaller facilities, such as private laboratories, which lack in-house reporting systems.
Cancer reporting is especially important in the VHA, the largest U.S. health care system. Health data on about 9 million enrolled veterans have been invaluable for understanding cancer epidemiology. Underreporting and misreporting of cancer cases in private medical offices and smaller treatment facilities may be attributable to lack of funding, personnel, administrative support, or knowledge of reporting requirements. In contrast, the VHA requires cancer reporting and provides funding, personnel, and administrative support.13
The VA Puget Sound Health Care System (VAPSHCS) traditionally has employed registrars to perform the majority of basic cancer registry tasks, including abstracting, case finding, and lifelong follow-up of the cancer patients listed in the registry. The registrars use OncoTraX software, which finds possible cancer cases from pathology, radiology, and patient treatment files, to accession cases. Unique cancer cases are reported to the VA Central Cancer Registry (VACCR), the Washington state registry, and the NCDB, which then transfer the data to the national registries. Accordingly, cases not accessioned would not be reported to the VA, state, and national registries.
The authors conducted a quality improvement project to ascertain whether primary cutaneous melanomas biopsied at VAPSHCS were underreported.
Materials
The VAPSHCS serves about 100,000 veterans and consists of 2 major treatment facilities, 2 community-based outpatient clinics, 1 outreach clinic, and 4 contract community-based outpatient clinics. Pathology cases for the entire VAPSHCS are accessioned in a central laboratory in Seattle.
Data Sources and Chart Abstraction
Data sources included the VA Corporate Data Warehouse (CDW), the VAPSHCS cancer registry, the Computerized Patient Record System (CPRS), VistA (Veterans Health Information Systems and Technology Architecture), VistA Web, and VistA Imaging.
Study Population
The study population consisted of veterans who had been diagnosed with primary cutaneous melanoma and had the diagnosis confirmed by biopsy performed at VAPSHCS between January 1, 2006 and December 31, 2012.
Statistical Analysis
Odds ratios were calculated using the MedCalc Odds Ratio Calculator.14
Methods
The authors identified SNOMED (Systematized Nomenclature of Medicine) codes that included the character string melanoma (Table 1). Using these codes, they queried VistA to identify melanoma cases diagnosed in the VAPSHCS Pathology and Laboratory Service during the period 2006 to 2012. To confirm the completeness of the local report, the authors performed the same search using the CDW. The SNOMED code case-finding was supplemented with cases ascertained using ICD-9 codes and problem list diagnoses.
A case must be reported to the local cancer registry if diagnosis or treatment takes place at the facility. All cases ascertained with the authors’ search criteria are, by definition, reportable to the local cancer registry. The authors then applied inclusion and exclusion criteria to determine
which cases were primary cutaneous melanomas and therefore candidates for this investigation (Table 2).
Having ascertained the primary cutaneous melanomas, the authors abstracted the pathology TNM (tumor, node, metastasis) staging (Breslow depth, mitotic index, presence of ulceration) and diagnosis dates from CPRS pathology reports. They then determined whether each case had been reported to the VACCR (OncoTraX was used to query for the accession status of each melanoma). If the melanoma was not accessioned, the authors tried to determine why.
Results
The authors discovered 193 primary cutaneous melanomas diagnosed by biopsy performed at VAPSHCS. Of these 193 melanomas, 71 (36.8%) had not been reported.
After the pathologist has completed a report, SNOMED codes are assigned by the pathology laboratory. Case finding with OncoTraX depends on SNOMED codes and other parameters (imaging, treatment, oncology consultation). OncoTraX is designed for case finding using World Health Organization (WHO) standardized 8000/X-9000/X series SNOMED codes. To understand the relationship between reporting and SNOMED codes, the authors ascertained the codes for all melanomas in the present study.
Table 3 lists the SNOMED codes assigned to melanomas biopsied at VAPSHCS and the percentage reported for each. Of the 106 melanomas that had been assigned WHO standardized codes, 101 (95.2%) had been reported. In contrast, only 21 (24.1%) of the 87 melanomas that had been assigned non-WHO standardized codes had been reported. In this study, non-WHO standardized codes are locally generated codes; they began with facility station number 663.
Use of locally generated codes may have contributed to nonreporting. Of the 71 melanomas not reported, 66 had a SNOMED code beginning with 663, and the other 5 had a WHO standardized SNOMED code. Odds of being nonreported were much higher for the melanomas with 663 codes than for the melanomas with WHO standardized codes (odds ratio [OR], 63.5; confidence interval [CI], 22.8-176.7; P ≤ .0001).
There was also a difference in coding between invasive and in situ melanomas. Of the 87 melanomas with a 663 code, 68 were in situ. Of the 106 melanomas with a national-level code, 11 were in situ. The odds of being assigned a local code were much higher for the in situ melanomas than they were for the invasive melanomas (OR, 30.9; CI, 13.8-69.1; P ≤ .0001).
Since 2000, the SNOMED code for melanoma in situ has been 87202, but no melanomas in situ were assigned this code. The 87202 code was not available in VistA for pathology laboratories to assign to melanomas at the time this study was conducted. Instead, most melanomas in situ were assigned a locally generated code. However, OncoTraX cannot recognize local codes, so melanomas assigned a local code might not have been accessionable.
The remaining 5 unreported melanomas were assigned WHO standardized codes. Secondary analysis revealed clerical errors, 4 made by the pathology laboratory and 1 by the registrar.
Discussion
Data from central cancer registries are used in a variety of fields, from research studies to health policymaking. They are used to “monitor cancer trends over time, show cancer patterns in various populations, identify high-risk groups, guide planning and evaluation of cancer control programs, help set priorities for allocating health resources, and advance clinical, epidemiologic, and health services research.”1
Melanoma underreporting has been demonstrated in previous studies, with the percentage of underreported cases varying from 10.4%11 to 70%.9 A longitudinal study of melanomas in Washington state found that underreporting of cutaneous melanomas increased from 2% to 21% over a 10-year period.10 This trend prompted examination of this study’s data for a similar temporal trend, and none was found.
A 2008 study found that more melanoma cases were being diagnosed or treated at outpatient facilities.9 Such facilities are prone to problems in reporting because they lack in-house reporting systems and knowledge of melanoma reporting requirements.9 A 2011 study of
practicing dermatologists found that many failed to report melanomas to a registry, and more than half were unaware of the requirement.12 Accordingly, underreporting is likely to continue. Results of the present study showed that melanoma underreporting was a major issue at VAPSHCS and that it could occur even in facilities that used in-house reporting systems and were aware of reporting requirements. The primary cause of underreporting was generation and use of local SNOMED codes that were unrecognizable by OncoTraX. A secondary cause was clerical error.
Discovery of unreported cases prompted facility review of procedures for reporting melanomas and expansion of current methods for melanoma discovery. All unreported cases have been entered into the VACCR, the Washington state registry, and the NCDB, which populate the national cancer registries. Contract registry staff were educated regarding melanoma reporting requirements, particularly requirements for melanoma in situ. The 87202 SNOMED code for melanoma in situ also has been added to VistA at VAPSHCS. A follow-up study will be conducted to ascertain whether the interventions have corrected the underreporting of melanoma.
Study Limitations
The cases used in the study were obtained by SNOMED codes, CDW problem lists, and ICD-9 codes. This method may have missed cases that were assigned incorrect SNOMED codes and were not assigned to the problem list, or that were assigned to the problem list after the study period. The authors used a subset of all reportable cases—namely, those biopsied at VAPSHCS. Although this subset constituted the significant majority of reportable cases, the authors do not know the extent of underreporting of cases that were not biopsied at VAPSHCS. The extent to which other VA facilities generate local SNOMED codes also is unknown.
Conclusion
Melanoma underreporting at VAPSHCS is an addressable concern. The primary cause of underreporting was the use of locally generated SNOMED codes that were not recognized by cancer registry software. The present study should be repeated at other VA facilities to determine the extent to which its findings are generalizable.
Acknowledgments
The authors thank Dr. Stevan Knezevich for reviewing cases, Pam Pehan for providing the list of VAPSHCS melanomas accessioned from VistA, and Eddie Alaniz and Eugene Gavrilenko for helping ascertain SNOMED codes.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
The National Cancer Data Base (NCDB) of the American College of Surgeons (ACS), the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI), and the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC) are among the leading registries for cancer surveillance, collecting cancer epidemiology data for the majority of the U.S. population.1 This national coverage aids researchers and policymakers in conducting epidemiologic studies and allocating health resources.1,2
U.S. federal law mandates the reporting of cancer.3,4 State laws require cancer reporting as well, but requirements vary slightly from state to state.5 However, all cancers with an ICD-O (International Classification of Diseases for Oncology) code of 2 or 3 are reportable. For Washington state, cancer must be reported unless it is basal or squamous cell carcinoma of nonmucoepidermoid skin or in situ cancer of the uterine cervix.5 In general, each facility that diagnoses or treats a melanoma is required to report it. Data are consolidated at the central registry level if necessary.
Cancer reporting often fails to meet states’ requirements.6-8 Since the inception of SEER and NPCR, many studies have assessed the accuracy of the cancer data reported to these registries and have found these data to be inaccurate or incomplete.6-8 Melanoma reporting, in particular,
seems to be prone to error. Studies have demonstrated melanoma underreporting ranging from 10% to 70%, with an increase in underreporting over time.9-11 Significant delays of up to 10 years have been found between initial diagnosis and reporting for melanomas.12 In general, these studies have focused on smaller facilities, such as private laboratories, which lack in-house reporting systems.
Cancer reporting is especially important in the VHA, the largest U.S. health care system. Health data on about 9 million enrolled veterans have been invaluable for understanding cancer epidemiology. Underreporting and misreporting of cancer cases in private medical offices and smaller treatment facilities may be attributable to lack of funding, personnel, administrative support, or knowledge of reporting requirements. In contrast, the VHA requires cancer reporting and provides funding, personnel, and administrative support.13
The VA Puget Sound Health Care System (VAPSHCS) traditionally has employed registrars to perform the majority of basic cancer registry tasks, including abstracting, case finding, and lifelong follow-up of the cancer patients listed in the registry. The registrars use OncoTraX software, which finds possible cancer cases from pathology, radiology, and patient treatment files, to accession cases. Unique cancer cases are reported to the VA Central Cancer Registry (VACCR), the Washington state registry, and the NCDB, which then transfer the data to the national registries. Accordingly, cases not accessioned would not be reported to the VA, state, and national registries.
The authors conducted a quality improvement project to ascertain whether primary cutaneous melanomas biopsied at VAPSHCS were underreported.
Materials
The VAPSHCS serves about 100,000 veterans and consists of 2 major treatment facilities, 2 community-based outpatient clinics, 1 outreach clinic, and 4 contract community-based outpatient clinics. Pathology cases for the entire VAPSHCS are accessioned in a central laboratory in Seattle.
Data Sources and Chart Abstraction
Data sources included the VA Corporate Data Warehouse (CDW), the VAPSHCS cancer registry, the Computerized Patient Record System (CPRS), VistA (Veterans Health Information Systems and Technology Architecture), VistA Web, and VistA Imaging.
Study Population
The study population consisted of veterans who had been diagnosed with primary cutaneous melanoma and had the diagnosis confirmed by biopsy performed at VAPSHCS between January 1, 2006 and December 31, 2012.
Statistical Analysis
Odds ratios were calculated using the MedCalc Odds Ratio Calculator.14
Methods
The authors identified SNOMED (Systematized Nomenclature of Medicine) codes that included the character string melanoma (Table 1). Using these codes, they queried VistA to identify melanoma cases diagnosed in the VAPSHCS Pathology and Laboratory Service during the period 2006 to 2012. To confirm the completeness of the local report, the authors performed the same search using the CDW. The SNOMED code case-finding was supplemented with cases ascertained using ICD-9 codes and problem list diagnoses.
A case must be reported to the local cancer registry if diagnosis or treatment takes place at the facility. All cases ascertained with the authors’ search criteria are, by definition, reportable to the local cancer registry. The authors then applied inclusion and exclusion criteria to determine
which cases were primary cutaneous melanomas and therefore candidates for this investigation (Table 2).
Having ascertained the primary cutaneous melanomas, the authors abstracted the pathology TNM (tumor, node, metastasis) staging (Breslow depth, mitotic index, presence of ulceration) and diagnosis dates from CPRS pathology reports. They then determined whether each case had been reported to the VACCR (OncoTraX was used to query for the accession status of each melanoma). If the melanoma was not accessioned, the authors tried to determine why.
Results
The authors discovered 193 primary cutaneous melanomas diagnosed by biopsy performed at VAPSHCS. Of these 193 melanomas, 71 (36.8%) had not been reported.
After the pathologist has completed a report, SNOMED codes are assigned by the pathology laboratory. Case finding with OncoTraX depends on SNOMED codes and other parameters (imaging, treatment, oncology consultation). OncoTraX is designed for case finding using World Health Organization (WHO) standardized 8000/X-9000/X series SNOMED codes. To understand the relationship between reporting and SNOMED codes, the authors ascertained the codes for all melanomas in the present study.
Table 3 lists the SNOMED codes assigned to melanomas biopsied at VAPSHCS and the percentage reported for each. Of the 106 melanomas that had been assigned WHO standardized codes, 101 (95.2%) had been reported. In contrast, only 21 (24.1%) of the 87 melanomas that had been assigned non-WHO standardized codes had been reported. In this study, non-WHO standardized codes are locally generated codes; they began with facility station number 663.
Use of locally generated codes may have contributed to nonreporting. Of the 71 melanomas not reported, 66 had a SNOMED code beginning with 663, and the other 5 had a WHO standardized SNOMED code. Odds of being nonreported were much higher for the melanomas with 663 codes than for the melanomas with WHO standardized codes (odds ratio [OR], 63.5; confidence interval [CI], 22.8-176.7; P ≤ .0001).
There was also a difference in coding between invasive and in situ melanomas. Of the 87 melanomas with a 663 code, 68 were in situ. Of the 106 melanomas with a national-level code, 11 were in situ. The odds of being assigned a local code were much higher for the in situ melanomas than they were for the invasive melanomas (OR, 30.9; CI, 13.8-69.1; P ≤ .0001).
Since 2000, the SNOMED code for melanoma in situ has been 87202, but no melanomas in situ were assigned this code. The 87202 code was not available in VistA for pathology laboratories to assign to melanomas at the time this study was conducted. Instead, most melanomas in situ were assigned a locally generated code. However, OncoTraX cannot recognize local codes, so melanomas assigned a local code might not have been accessionable.
The remaining 5 unreported melanomas were assigned WHO standardized codes. Secondary analysis revealed clerical errors, 4 made by the pathology laboratory and 1 by the registrar.
Discussion
Data from central cancer registries are used in a variety of fields, from research studies to health policymaking. They are used to “monitor cancer trends over time, show cancer patterns in various populations, identify high-risk groups, guide planning and evaluation of cancer control programs, help set priorities for allocating health resources, and advance clinical, epidemiologic, and health services research.”1
Melanoma underreporting has been demonstrated in previous studies, with the percentage of underreported cases varying from 10.4%11 to 70%.9 A longitudinal study of melanomas in Washington state found that underreporting of cutaneous melanomas increased from 2% to 21% over a 10-year period.10 This trend prompted examination of this study’s data for a similar temporal trend, and none was found.
A 2008 study found that more melanoma cases were being diagnosed or treated at outpatient facilities.9 Such facilities are prone to problems in reporting because they lack in-house reporting systems and knowledge of melanoma reporting requirements.9 A 2011 study of
practicing dermatologists found that many failed to report melanomas to a registry, and more than half were unaware of the requirement.12 Accordingly, underreporting is likely to continue. Results of the present study showed that melanoma underreporting was a major issue at VAPSHCS and that it could occur even in facilities that used in-house reporting systems and were aware of reporting requirements. The primary cause of underreporting was generation and use of local SNOMED codes that were unrecognizable by OncoTraX. A secondary cause was clerical error.
Discovery of unreported cases prompted facility review of procedures for reporting melanomas and expansion of current methods for melanoma discovery. All unreported cases have been entered into the VACCR, the Washington state registry, and the NCDB, which populate the national cancer registries. Contract registry staff were educated regarding melanoma reporting requirements, particularly requirements for melanoma in situ. The 87202 SNOMED code for melanoma in situ also has been added to VistA at VAPSHCS. A follow-up study will be conducted to ascertain whether the interventions have corrected the underreporting of melanoma.
Study Limitations
The cases used in the study were obtained by SNOMED codes, CDW problem lists, and ICD-9 codes. This method may have missed cases that were assigned incorrect SNOMED codes and were not assigned to the problem list, or that were assigned to the problem list after the study period. The authors used a subset of all reportable cases—namely, those biopsied at VAPSHCS. Although this subset constituted the significant majority of reportable cases, the authors do not know the extent of underreporting of cases that were not biopsied at VAPSHCS. The extent to which other VA facilities generate local SNOMED codes also is unknown.
Conclusion
Melanoma underreporting at VAPSHCS is an addressable concern. The primary cause of underreporting was the use of locally generated SNOMED codes that were not recognized by cancer registry software. The present study should be repeated at other VA facilities to determine the extent to which its findings are generalizable.
Acknowledgments
The authors thank Dr. Stevan Knezevich for reviewing cases, Pam Pehan for providing the list of VAPSHCS melanomas accessioned from VistA, and Eddie Alaniz and Eugene Gavrilenko for helping ascertain SNOMED codes.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Division of Cancer Prevention and Control, Centers for Disease Control and Prevention. National Program of Cancer Registries (NPCR). CDC website. http://www.cdc.gov/cancer/npcr/about.htm. Updated April 20, 2016. Accessed July 1, 2016.
2. National Cancer Institute. Overview of the SEER program. National Cancer Institute website. http://seer.cancer.gov/about/overview.html. Accessed July 18, 2016.
3. Cancer Registries Amendment Act. 42 USC §201-280e (2016).
4. Cancer Registry of Greater California. Cancer reporting. Cancer Registry of Greater California website. http://crgc-cancer.org/hospitals-and-physicians. Accessed July 18, 2016.
5. American Academy of Dermatology. State cancer registry laws and requirements. American Academy of Dermatology website. https://www.aad.org/file%20library/global%20navigatio/education%20and%20quality%20care/state%20cancer%20registries/state-cancer-registries-laws-and-requirements.pdf. Accessed July 18, 2016.
6. Craig BM, Rollison DE, List AF, Cogle CR. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol Biomarkers Prev. 2012;21(3):474-481.
7. Fanning J, Gangestad A, Andrews SJ. National Cancer Data Base/Surveillance Epidemiology and End Results: potential insensitive-measure bias. Gynecol Oncol. 2000;77(3):450-453.
8. Thoburn KK, German RR, Lewis M, Nichols PJ, Ahmed F, Jackson-Thompson J. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer. 2007;109(8):1607-1616.
9. Cockburn M, Swetter SM, Peng D, Keegan TH, Deapen D, Clarke CA. Melanoma underreporting: why does it happen, how big is the problem, and how do we fix it? J Am Acad Dermatol. 2008;59(6):1081-1085.
10. Karagas MR, Thomas DB, Roth GJ, Johnson LK, Weiss NS. The effects of changes in health care delivery on the reported incidence of cutaneous melanoma in western Washington state. Am J Epidemiol. 1991;133(1):58-62.
11. Merlino LA, Sullivan KJ, Whitaker DC, Lynch CF. The independent pathology laboratory as a reporting source for cutaneous melanoma incidence in Iowa, 1977–1994. J Am Acad Dermatol. 1997;37(4):578-585.
12. Cartee TV, Kini SP, Chen SC. Melanoma reporting to central cancer registries by US dermatologists: an analysis of the persistent knowledge and practice gap. J Am Acad Dermatol. 2011;65(5)(suppl 1):S124-S132.
13. Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537-1545.
14. Odds ratio calculator. MedCalc website. https://www.medcalc.org/calc/odds_ratio.php. Accessed June 16, 2016.
Note: Page numbers differ between the print issue and digital edition.
1. Division of Cancer Prevention and Control, Centers for Disease Control and Prevention. National Program of Cancer Registries (NPCR). CDC website. http://www.cdc.gov/cancer/npcr/about.htm. Updated April 20, 2016. Accessed July 1, 2016.
2. National Cancer Institute. Overview of the SEER program. National Cancer Institute website. http://seer.cancer.gov/about/overview.html. Accessed July 18, 2016.
3. Cancer Registries Amendment Act. 42 USC §201-280e (2016).
4. Cancer Registry of Greater California. Cancer reporting. Cancer Registry of Greater California website. http://crgc-cancer.org/hospitals-and-physicians. Accessed July 18, 2016.
5. American Academy of Dermatology. State cancer registry laws and requirements. American Academy of Dermatology website. https://www.aad.org/file%20library/global%20navigatio/education%20and%20quality%20care/state%20cancer%20registries/state-cancer-registries-laws-and-requirements.pdf. Accessed July 18, 2016.
6. Craig BM, Rollison DE, List AF, Cogle CR. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol Biomarkers Prev. 2012;21(3):474-481.
7. Fanning J, Gangestad A, Andrews SJ. National Cancer Data Base/Surveillance Epidemiology and End Results: potential insensitive-measure bias. Gynecol Oncol. 2000;77(3):450-453.
8. Thoburn KK, German RR, Lewis M, Nichols PJ, Ahmed F, Jackson-Thompson J. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer. 2007;109(8):1607-1616.
9. Cockburn M, Swetter SM, Peng D, Keegan TH, Deapen D, Clarke CA. Melanoma underreporting: why does it happen, how big is the problem, and how do we fix it? J Am Acad Dermatol. 2008;59(6):1081-1085.
10. Karagas MR, Thomas DB, Roth GJ, Johnson LK, Weiss NS. The effects of changes in health care delivery on the reported incidence of cutaneous melanoma in western Washington state. Am J Epidemiol. 1991;133(1):58-62.
11. Merlino LA, Sullivan KJ, Whitaker DC, Lynch CF. The independent pathology laboratory as a reporting source for cutaneous melanoma incidence in Iowa, 1977–1994. J Am Acad Dermatol. 1997;37(4):578-585.
12. Cartee TV, Kini SP, Chen SC. Melanoma reporting to central cancer registries by US dermatologists: an analysis of the persistent knowledge and practice gap. J Am Acad Dermatol. 2011;65(5)(suppl 1):S124-S132.
13. Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537-1545.
14. Odds ratio calculator. MedCalc website. https://www.medcalc.org/calc/odds_ratio.php. Accessed June 16, 2016.
Note: Page numbers differ between the print issue and digital edition.