User login
SU2C announces researcher-industry collaboration on immunotherapy
Stand Up To Cancer is calling for proposals to investigate additional uses for nivolumab, ipilimumab, elotuzumab, and urelumab, as part of a new researcher-industry collaborative program.
As many as four projects will be funded by Bristol-Myers Squibb, maker of the four agents, in the range of $1 million to $3 million each, according to a written statement from the American Association for Cancer Research (AACR).
The company will provide access to the three drugs already approved for the treatement of various cancers –nivolumab, ipilimumab, and elotuzumab– and to urelumab, an investigational agent that is currently in early clinical trials.
Proposals can include the study of one or more of the products, alone or in combination with other treatments, and may include products from other companies, as well as explore potential new uses for the drug(s), AACR said in the statement.
Nivolumab (Opdivo) is currently approved to treat advanced melanoma, non-small cell lung cancer, renal cell carcinoma, and classical Hodgkin lymphoma; Ipilimumab (Yervoy) is approved to treat melanoma; and elotuzumab (Empliciti) is approved to treat multiple myeloma, in conjunction with other drugs. Urelumab is being evaluated as a treatment for a range of cancers, including some hematological cancers, advanced colorectal cancer, and head and neck cancers.
The Stand Up To Cancer (SU2C) Catalyst program was launched in April to “use funding and materials from the pharmaceutical, biotechnology, diagnostic, and medical devices industries to accelerate research on cancer prevention, detection, and treatment,” according to a written statement from SU2C. Founding collaborators in addition to Bristol-Myers Squibb include Merck and Genentech.
The Catalyst projects must follow the SU2C model be carried out by a collaborative team, and be designed to accelerate the clinical use of therapeutic agents within the 3-year term of the grant, and to deliver near-term patient benefit.
The Request for Proposal for the Bristol-Myers Squibb agents is available at proposalCENTRAL, with proposals due by noon ET Monday, Aug. 15.
lnikolaides@frontlinemedcom.com
On Twitter @NikolaidesLaura
Stand Up To Cancer is calling for proposals to investigate additional uses for nivolumab, ipilimumab, elotuzumab, and urelumab, as part of a new researcher-industry collaborative program.
As many as four projects will be funded by Bristol-Myers Squibb, maker of the four agents, in the range of $1 million to $3 million each, according to a written statement from the American Association for Cancer Research (AACR).
The company will provide access to the three drugs already approved for the treatement of various cancers –nivolumab, ipilimumab, and elotuzumab– and to urelumab, an investigational agent that is currently in early clinical trials.
Proposals can include the study of one or more of the products, alone or in combination with other treatments, and may include products from other companies, as well as explore potential new uses for the drug(s), AACR said in the statement.
Nivolumab (Opdivo) is currently approved to treat advanced melanoma, non-small cell lung cancer, renal cell carcinoma, and classical Hodgkin lymphoma; Ipilimumab (Yervoy) is approved to treat melanoma; and elotuzumab (Empliciti) is approved to treat multiple myeloma, in conjunction with other drugs. Urelumab is being evaluated as a treatment for a range of cancers, including some hematological cancers, advanced colorectal cancer, and head and neck cancers.
The Stand Up To Cancer (SU2C) Catalyst program was launched in April to “use funding and materials from the pharmaceutical, biotechnology, diagnostic, and medical devices industries to accelerate research on cancer prevention, detection, and treatment,” according to a written statement from SU2C. Founding collaborators in addition to Bristol-Myers Squibb include Merck and Genentech.
The Catalyst projects must follow the SU2C model be carried out by a collaborative team, and be designed to accelerate the clinical use of therapeutic agents within the 3-year term of the grant, and to deliver near-term patient benefit.
The Request for Proposal for the Bristol-Myers Squibb agents is available at proposalCENTRAL, with proposals due by noon ET Monday, Aug. 15.
lnikolaides@frontlinemedcom.com
On Twitter @NikolaidesLaura
Stand Up To Cancer is calling for proposals to investigate additional uses for nivolumab, ipilimumab, elotuzumab, and urelumab, as part of a new researcher-industry collaborative program.
As many as four projects will be funded by Bristol-Myers Squibb, maker of the four agents, in the range of $1 million to $3 million each, according to a written statement from the American Association for Cancer Research (AACR).
The company will provide access to the three drugs already approved for the treatement of various cancers –nivolumab, ipilimumab, and elotuzumab– and to urelumab, an investigational agent that is currently in early clinical trials.
Proposals can include the study of one or more of the products, alone or in combination with other treatments, and may include products from other companies, as well as explore potential new uses for the drug(s), AACR said in the statement.
Nivolumab (Opdivo) is currently approved to treat advanced melanoma, non-small cell lung cancer, renal cell carcinoma, and classical Hodgkin lymphoma; Ipilimumab (Yervoy) is approved to treat melanoma; and elotuzumab (Empliciti) is approved to treat multiple myeloma, in conjunction with other drugs. Urelumab is being evaluated as a treatment for a range of cancers, including some hematological cancers, advanced colorectal cancer, and head and neck cancers.
The Stand Up To Cancer (SU2C) Catalyst program was launched in April to “use funding and materials from the pharmaceutical, biotechnology, diagnostic, and medical devices industries to accelerate research on cancer prevention, detection, and treatment,” according to a written statement from SU2C. Founding collaborators in addition to Bristol-Myers Squibb include Merck and Genentech.
The Catalyst projects must follow the SU2C model be carried out by a collaborative team, and be designed to accelerate the clinical use of therapeutic agents within the 3-year term of the grant, and to deliver near-term patient benefit.
The Request for Proposal for the Bristol-Myers Squibb agents is available at proposalCENTRAL, with proposals due by noon ET Monday, Aug. 15.
lnikolaides@frontlinemedcom.com
On Twitter @NikolaidesLaura
Sunscreens May Fail to Meet SPF Claims on Product Labels
New data from Consumer Reports indicate that 48% of all sunscreens tested (N=104) over 4 years did not provide the sun protection factor (SPF) promised on product labels, leaving consumers with insufficient sun protection, which could lead to long-term sun damage including wrinkles or skin cancer. Furthermore, 42% of chemical sunscreens (n=85) and 74% of mineral sunscreens (n=19) did not meet their SPF claims.
The study also reveals that more than one-third (35%) of sunscreens registered below SPF 30, which is the minimum recommended by the American Academy of Dermatology (AAD). Although product labels featured claims of water resistance, nearly half of the sunscreens tested failed to meet their SPF claim following water immersion.
Dermatologists can educate patients about correct sunscreen use and product labels to ensure the highest level of protection against melanoma and other skin cancers. According to a 2016 AAD survey, only 32% of respondents knew that an SPF 30 sunscreen does not provide twice as much protection as an SPF 15 sunscreen. Furthermore, only 45% of respondents knew that a higher-SPF sunscreen does not protect skin from sun exposure longer than a lower-SPF sunscreen.
The AAD has issued a list of talking points highlighting key messages that dermatologists can share with patients and/or the media when asked about sun-protection techniques and the recent sunscreen data released by Consumer Reports.
New data from Consumer Reports indicate that 48% of all sunscreens tested (N=104) over 4 years did not provide the sun protection factor (SPF) promised on product labels, leaving consumers with insufficient sun protection, which could lead to long-term sun damage including wrinkles or skin cancer. Furthermore, 42% of chemical sunscreens (n=85) and 74% of mineral sunscreens (n=19) did not meet their SPF claims.
The study also reveals that more than one-third (35%) of sunscreens registered below SPF 30, which is the minimum recommended by the American Academy of Dermatology (AAD). Although product labels featured claims of water resistance, nearly half of the sunscreens tested failed to meet their SPF claim following water immersion.
Dermatologists can educate patients about correct sunscreen use and product labels to ensure the highest level of protection against melanoma and other skin cancers. According to a 2016 AAD survey, only 32% of respondents knew that an SPF 30 sunscreen does not provide twice as much protection as an SPF 15 sunscreen. Furthermore, only 45% of respondents knew that a higher-SPF sunscreen does not protect skin from sun exposure longer than a lower-SPF sunscreen.
The AAD has issued a list of talking points highlighting key messages that dermatologists can share with patients and/or the media when asked about sun-protection techniques and the recent sunscreen data released by Consumer Reports.
New data from Consumer Reports indicate that 48% of all sunscreens tested (N=104) over 4 years did not provide the sun protection factor (SPF) promised on product labels, leaving consumers with insufficient sun protection, which could lead to long-term sun damage including wrinkles or skin cancer. Furthermore, 42% of chemical sunscreens (n=85) and 74% of mineral sunscreens (n=19) did not meet their SPF claims.
The study also reveals that more than one-third (35%) of sunscreens registered below SPF 30, which is the minimum recommended by the American Academy of Dermatology (AAD). Although product labels featured claims of water resistance, nearly half of the sunscreens tested failed to meet their SPF claim following water immersion.
Dermatologists can educate patients about correct sunscreen use and product labels to ensure the highest level of protection against melanoma and other skin cancers. According to a 2016 AAD survey, only 32% of respondents knew that an SPF 30 sunscreen does not provide twice as much protection as an SPF 15 sunscreen. Furthermore, only 45% of respondents knew that a higher-SPF sunscreen does not protect skin from sun exposure longer than a lower-SPF sunscreen.
The AAD has issued a list of talking points highlighting key messages that dermatologists can share with patients and/or the media when asked about sun-protection techniques and the recent sunscreen data released by Consumer Reports.
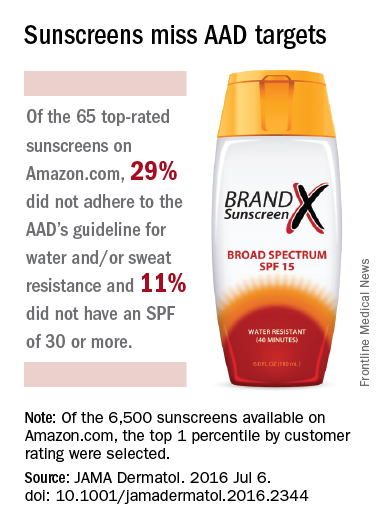
40% of top-rated sunscreens fall short of AAD guidelines
Customer satisfaction ratings of sunscreens do not always reflect the products’ effectiveness, as 40% of the 65 top-rated sunscreens available on Amazon.com did not adhere to all three of the American Academy of Dermatology’s recommended criteria.
The AAD recommends the following for all sunscreens: sun protection factor (SPF) of 30 more, broad-spectrum protection, and water and/or sweat resistance. Of those criteria, water/sweat resistance was missing in 19 (29%), compared with SPF less than 30 in 7 (11%) products and lack of broad-spectrum protection in 4 (6%). Some products missed more than one criterion, said Shuai Xu, MD, of Northwestern University, Chicago, and his associates (JAMA Dermatolol. 2016 Jul 6. doi: 10.1001/jamadermatol.2016.2344).

Of the qualities besides performance that were analyzed, “cosmetic elegance,” which the investigators “defined as any feature associated with skin sensation on application, color, or scent,” was the positive feature most often mentioned in the customer reviews. On the other hand, they noted, “dermatologist recommendations were not a significantly cited positive feature.”
The sunscreens in the analysis represented the top 1 percentile by customer rating of the 6,500 products categorized as sunscreens on Amazon as of December 2015. The 65 products included in the study had more than 24,400 customer reviews and a median rating of 4.5 out of 5 stars, Dr. Xu and his associates said.
The investigators did not report any conflicts of interest.
Customer satisfaction ratings of sunscreens do not always reflect the products’ effectiveness, as 40% of the 65 top-rated sunscreens available on Amazon.com did not adhere to all three of the American Academy of Dermatology’s recommended criteria.
The AAD recommends the following for all sunscreens: sun protection factor (SPF) of 30 more, broad-spectrum protection, and water and/or sweat resistance. Of those criteria, water/sweat resistance was missing in 19 (29%), compared with SPF less than 30 in 7 (11%) products and lack of broad-spectrum protection in 4 (6%). Some products missed more than one criterion, said Shuai Xu, MD, of Northwestern University, Chicago, and his associates (JAMA Dermatolol. 2016 Jul 6. doi: 10.1001/jamadermatol.2016.2344).

Of the qualities besides performance that were analyzed, “cosmetic elegance,” which the investigators “defined as any feature associated with skin sensation on application, color, or scent,” was the positive feature most often mentioned in the customer reviews. On the other hand, they noted, “dermatologist recommendations were not a significantly cited positive feature.”
The sunscreens in the analysis represented the top 1 percentile by customer rating of the 6,500 products categorized as sunscreens on Amazon as of December 2015. The 65 products included in the study had more than 24,400 customer reviews and a median rating of 4.5 out of 5 stars, Dr. Xu and his associates said.
The investigators did not report any conflicts of interest.
Customer satisfaction ratings of sunscreens do not always reflect the products’ effectiveness, as 40% of the 65 top-rated sunscreens available on Amazon.com did not adhere to all three of the American Academy of Dermatology’s recommended criteria.
The AAD recommends the following for all sunscreens: sun protection factor (SPF) of 30 more, broad-spectrum protection, and water and/or sweat resistance. Of those criteria, water/sweat resistance was missing in 19 (29%), compared with SPF less than 30 in 7 (11%) products and lack of broad-spectrum protection in 4 (6%). Some products missed more than one criterion, said Shuai Xu, MD, of Northwestern University, Chicago, and his associates (JAMA Dermatolol. 2016 Jul 6. doi: 10.1001/jamadermatol.2016.2344).

Of the qualities besides performance that were analyzed, “cosmetic elegance,” which the investigators “defined as any feature associated with skin sensation on application, color, or scent,” was the positive feature most often mentioned in the customer reviews. On the other hand, they noted, “dermatologist recommendations were not a significantly cited positive feature.”
The sunscreens in the analysis represented the top 1 percentile by customer rating of the 6,500 products categorized as sunscreens on Amazon as of December 2015. The 65 products included in the study had more than 24,400 customer reviews and a median rating of 4.5 out of 5 stars, Dr. Xu and his associates said.
The investigators did not report any conflicts of interest.
FROM JAMA DERMATOLOGY
Study tracks distant metastatic patterns of Merkel cell carcinoma
SCOTTSDALE, ARIZ. – Distant metastatic sites of Merkel cell carcinoma most often involved the supraclavicular, retroperitoneal, and iliac lymph nodes, in a single center retrospective study of 305 patients.
Merkel cell carcinoma metastases “clearly favored distant nodes, but the distribution of other metastatic sites was distinct from other cancers, such as melanoma,” Jamiluddin Qazi, an undergraduate student at the University of Washington, Seattle, said at the annual meeting of the Society for Investigative Dermatology. The findings could help guide imaging and other surveillance of patients after they develop a primary Merkel cell tumor, he added.
About 2,000 individuals in the United States are diagnosed with Merkel cell carcinoma every year. About 40% of these patients develop metastatic disease, which has a 5-year survival rate of less than 25%, Mr. Qazi noted. Indeed, median survival after diagnosis of metastatic Merkel cell carcinoma was only 9.5 months in one recent study (J Cutan Pathol. 2010;37:20-7). Programmed death 1 (PD-1) blockade with pembrolizumab (Keytruda) can potentially improve survival (N Engl J Med. 2016; 374:2542-52), “but there is no consensus regarding follow-up for Merkel cell carcinoma. The 2016 National Comprehensive Cancer Network (NCCN) guidelines recommend ‘follow up as clinically indicated,’ and a lack of data has led to ambiguity,” Mr. Qazi said.
Working with oncologists and radiologists at the Seattle Cancer Care Alliance, he analyzed a tissue and clinical database of 442 initial distant Merkel cell carcinoma metastases among 305 patients. Initial distant metastases were defined as the first lesions detected beyond the regional lymph nodes of the primary tumor. A total of 69% of patients had one initial distant metastasis, 19% had two concurrently identified lesions, 9% had three lesions, and 4% had at least four lesions, Mr. Qazi reported.
“Merkel cell carcinoma seemed to metastasize to unusual places, but clearly preferred the distant lymph nodes. In all, 26% of metastases localized there, most commonly to the supraclavicular, retroperitoneal, and iliac nodes,” he said. The next most common site of distant metastasis was the liver (15% of lesions), followed by the skin and bone (13% of lesions each), lung (6%), and pancreas (5%). Less common sites included the heart, spleen, abdominal muscle, brain, kidneys, adrenal glands, gonad, chest wall, and stomach.
Comparing these findings with a similar study in melanoma (J Oncol 2012. doi: 10.1155/2012/647684) showed that both cancers have about the same chances of metastasizing to the liver, bone, kidneys, adrenal glands, and stomach, Mr. Qazi said. However, Merkel cell carcinoma was less likely to metastasize to the brain (3% of lesions, vs. 12% for melanoma) and lung (6% vs. 14%), and was more likely to metastasize to the pancreas (5% vs. 1%).
Now the investigators are working to link metastatic sites with factors such as the location of the primary tumor, the presence or absence of lymphovascular invasion, and the status of the immune system and Merkel polyomavirus infection, said Mr. Qazi. They also are analyzing time from diagnosis or treatment to metastasis to help guide decisions about when to order follow-up imaging. Ultimately, they hope to create an online tool that enables clinicians to describe a primary Merkel cell carcinoma and rapidly receive automated information about the most likely timing and location of metastasis.
The National Institutes of Health supported the study. Mr. Qazi had no conflicts of interest.
SCOTTSDALE, ARIZ. – Distant metastatic sites of Merkel cell carcinoma most often involved the supraclavicular, retroperitoneal, and iliac lymph nodes, in a single center retrospective study of 305 patients.
Merkel cell carcinoma metastases “clearly favored distant nodes, but the distribution of other metastatic sites was distinct from other cancers, such as melanoma,” Jamiluddin Qazi, an undergraduate student at the University of Washington, Seattle, said at the annual meeting of the Society for Investigative Dermatology. The findings could help guide imaging and other surveillance of patients after they develop a primary Merkel cell tumor, he added.
About 2,000 individuals in the United States are diagnosed with Merkel cell carcinoma every year. About 40% of these patients develop metastatic disease, which has a 5-year survival rate of less than 25%, Mr. Qazi noted. Indeed, median survival after diagnosis of metastatic Merkel cell carcinoma was only 9.5 months in one recent study (J Cutan Pathol. 2010;37:20-7). Programmed death 1 (PD-1) blockade with pembrolizumab (Keytruda) can potentially improve survival (N Engl J Med. 2016; 374:2542-52), “but there is no consensus regarding follow-up for Merkel cell carcinoma. The 2016 National Comprehensive Cancer Network (NCCN) guidelines recommend ‘follow up as clinically indicated,’ and a lack of data has led to ambiguity,” Mr. Qazi said.
Working with oncologists and radiologists at the Seattle Cancer Care Alliance, he analyzed a tissue and clinical database of 442 initial distant Merkel cell carcinoma metastases among 305 patients. Initial distant metastases were defined as the first lesions detected beyond the regional lymph nodes of the primary tumor. A total of 69% of patients had one initial distant metastasis, 19% had two concurrently identified lesions, 9% had three lesions, and 4% had at least four lesions, Mr. Qazi reported.
“Merkel cell carcinoma seemed to metastasize to unusual places, but clearly preferred the distant lymph nodes. In all, 26% of metastases localized there, most commonly to the supraclavicular, retroperitoneal, and iliac nodes,” he said. The next most common site of distant metastasis was the liver (15% of lesions), followed by the skin and bone (13% of lesions each), lung (6%), and pancreas (5%). Less common sites included the heart, spleen, abdominal muscle, brain, kidneys, adrenal glands, gonad, chest wall, and stomach.
Comparing these findings with a similar study in melanoma (J Oncol 2012. doi: 10.1155/2012/647684) showed that both cancers have about the same chances of metastasizing to the liver, bone, kidneys, adrenal glands, and stomach, Mr. Qazi said. However, Merkel cell carcinoma was less likely to metastasize to the brain (3% of lesions, vs. 12% for melanoma) and lung (6% vs. 14%), and was more likely to metastasize to the pancreas (5% vs. 1%).
Now the investigators are working to link metastatic sites with factors such as the location of the primary tumor, the presence or absence of lymphovascular invasion, and the status of the immune system and Merkel polyomavirus infection, said Mr. Qazi. They also are analyzing time from diagnosis or treatment to metastasis to help guide decisions about when to order follow-up imaging. Ultimately, they hope to create an online tool that enables clinicians to describe a primary Merkel cell carcinoma and rapidly receive automated information about the most likely timing and location of metastasis.
The National Institutes of Health supported the study. Mr. Qazi had no conflicts of interest.
SCOTTSDALE, ARIZ. – Distant metastatic sites of Merkel cell carcinoma most often involved the supraclavicular, retroperitoneal, and iliac lymph nodes, in a single center retrospective study of 305 patients.
Merkel cell carcinoma metastases “clearly favored distant nodes, but the distribution of other metastatic sites was distinct from other cancers, such as melanoma,” Jamiluddin Qazi, an undergraduate student at the University of Washington, Seattle, said at the annual meeting of the Society for Investigative Dermatology. The findings could help guide imaging and other surveillance of patients after they develop a primary Merkel cell tumor, he added.
About 2,000 individuals in the United States are diagnosed with Merkel cell carcinoma every year. About 40% of these patients develop metastatic disease, which has a 5-year survival rate of less than 25%, Mr. Qazi noted. Indeed, median survival after diagnosis of metastatic Merkel cell carcinoma was only 9.5 months in one recent study (J Cutan Pathol. 2010;37:20-7). Programmed death 1 (PD-1) blockade with pembrolizumab (Keytruda) can potentially improve survival (N Engl J Med. 2016; 374:2542-52), “but there is no consensus regarding follow-up for Merkel cell carcinoma. The 2016 National Comprehensive Cancer Network (NCCN) guidelines recommend ‘follow up as clinically indicated,’ and a lack of data has led to ambiguity,” Mr. Qazi said.
Working with oncologists and radiologists at the Seattle Cancer Care Alliance, he analyzed a tissue and clinical database of 442 initial distant Merkel cell carcinoma metastases among 305 patients. Initial distant metastases were defined as the first lesions detected beyond the regional lymph nodes of the primary tumor. A total of 69% of patients had one initial distant metastasis, 19% had two concurrently identified lesions, 9% had three lesions, and 4% had at least four lesions, Mr. Qazi reported.
“Merkel cell carcinoma seemed to metastasize to unusual places, but clearly preferred the distant lymph nodes. In all, 26% of metastases localized there, most commonly to the supraclavicular, retroperitoneal, and iliac nodes,” he said. The next most common site of distant metastasis was the liver (15% of lesions), followed by the skin and bone (13% of lesions each), lung (6%), and pancreas (5%). Less common sites included the heart, spleen, abdominal muscle, brain, kidneys, adrenal glands, gonad, chest wall, and stomach.
Comparing these findings with a similar study in melanoma (J Oncol 2012. doi: 10.1155/2012/647684) showed that both cancers have about the same chances of metastasizing to the liver, bone, kidneys, adrenal glands, and stomach, Mr. Qazi said. However, Merkel cell carcinoma was less likely to metastasize to the brain (3% of lesions, vs. 12% for melanoma) and lung (6% vs. 14%), and was more likely to metastasize to the pancreas (5% vs. 1%).
Now the investigators are working to link metastatic sites with factors such as the location of the primary tumor, the presence or absence of lymphovascular invasion, and the status of the immune system and Merkel polyomavirus infection, said Mr. Qazi. They also are analyzing time from diagnosis or treatment to metastasis to help guide decisions about when to order follow-up imaging. Ultimately, they hope to create an online tool that enables clinicians to describe a primary Merkel cell carcinoma and rapidly receive automated information about the most likely timing and location of metastasis.
The National Institutes of Health supported the study. Mr. Qazi had no conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Distant metastases of Merkel cell carcinoma most often involve the lymph nodes, followed by the liver, skin, and bone.
Major finding: Distant lymph node metastases comprised 26% of lesions, the liver comprised 15%, and skin and bone made up 13% each.
Data source: A single-center retrospective study of 442 initial distant metastases of Merkel cell carcinoma among 305 patients.
Disclosures: The National Institutes of Health supported the study. Mr. Qazi had no conflicts of interest.
Study tracks distant metastatic patterns of Merkel cell carcinoma
SCOTTSDALE, ARIZ. – Distant metastatic sites of Merkel cell carcinoma most often involved the supraclavicular, retroperitoneal, and iliac lymph nodes, in a single center retrospective study of 305 patients.
Merkel cell carcinoma metastases “clearly favored distant nodes, but the distribution of other metastatic sites was distinct from other cancers, such as melanoma,” Jamiluddin Qazi, an undergraduate student at the University of Washington, Seattle, said at the annual meeting of the Society for Investigative Dermatology. The findings could help guide imaging and other surveillance of patients after they develop a primary Merkel cell tumor, he added.
About 2,000 individuals in the United States are diagnosed with Merkel cell carcinoma every year. About 40% of these patients develop metastatic disease, which has a 5-year survival rate of less than 25%, Mr. Qazi noted. Indeed, median survival after diagnosis of metastatic Merkel cell carcinoma was only 9.5 months in one recent study (J Cutan Pathol. 2010;37:20-7). Programmed death 1 (PD-1) blockade with pembrolizumab (Keytruda) can potentially improve survival (N Engl J Med. 2016; 374:2542-52), “but there is no consensus regarding follow-up for Merkel cell carcinoma. The 2016 National Comprehensive Cancer Network (NCCN) guidelines recommend ‘follow up as clinically indicated,’ and a lack of data has led to ambiguity,” Mr. Qazi said.
Working with oncologists and radiologists at the Seattle Cancer Care Alliance, he analyzed a tissue and clinical database of 442 initial distant Merkel cell carcinoma metastases among 305 patients. Initial distant metastases were defined as the first lesions detected beyond the regional lymph nodes of the primary tumor. A total of 69% of patients had one initial distant metastasis, 19% had two concurrently identified lesions, 9% had three lesions, and 4% had at least four lesions, Mr. Qazi reported.
“Merkel cell carcinoma seemed to metastasize to unusual places, but clearly preferred the distant lymph nodes. In all, 26% of metastases localized there, most commonly to the supraclavicular, retroperitoneal, and iliac nodes,” he said. The next most common site of distant metastasis was the liver (15% of lesions), followed by the skin and bone (13% of lesions each), lung (6%), and pancreas (5%). Less common sites included the heart, spleen, abdominal muscle, brain, kidneys, adrenal glands, gonad, chest wall, and stomach.
Comparing these findings with a similar study in melanoma (J Oncol 2012. doi: 10.1155/2012/647684) showed that both cancers have about the same chances of metastasizing to the liver, bone, kidneys, adrenal glands, and stomach, Mr. Qazi said. However, Merkel cell carcinoma was less likely to metastasize to the brain (3% of lesions, vs. 12% for melanoma) and lung (6% vs. 14%), and was more likely to metastasize to the pancreas (5% vs. 1%).
Now the investigators are working to link metastatic sites with factors such as the location of the primary tumor, the presence or absence of lymphovascular invasion, and the status of the immune system and Merkel polyomavirus infection, said Mr. Qazi. They also are analyzing time from diagnosis or treatment to metastasis to help guide decisions about when to order follow-up imaging. Ultimately, they hope to create an online tool that enables clinicians to describe a primary Merkel cell carcinoma and rapidly receive automated information about the most likely timing and location of metastasis.
The National Institutes of Health supported the study. Mr. Qazi had no conflicts of interest.
SCOTTSDALE, ARIZ. – Distant metastatic sites of Merkel cell carcinoma most often involved the supraclavicular, retroperitoneal, and iliac lymph nodes, in a single center retrospective study of 305 patients.
Merkel cell carcinoma metastases “clearly favored distant nodes, but the distribution of other metastatic sites was distinct from other cancers, such as melanoma,” Jamiluddin Qazi, an undergraduate student at the University of Washington, Seattle, said at the annual meeting of the Society for Investigative Dermatology. The findings could help guide imaging and other surveillance of patients after they develop a primary Merkel cell tumor, he added.
About 2,000 individuals in the United States are diagnosed with Merkel cell carcinoma every year. About 40% of these patients develop metastatic disease, which has a 5-year survival rate of less than 25%, Mr. Qazi noted. Indeed, median survival after diagnosis of metastatic Merkel cell carcinoma was only 9.5 months in one recent study (J Cutan Pathol. 2010;37:20-7). Programmed death 1 (PD-1) blockade with pembrolizumab (Keytruda) can potentially improve survival (N Engl J Med. 2016; 374:2542-52), “but there is no consensus regarding follow-up for Merkel cell carcinoma. The 2016 National Comprehensive Cancer Network (NCCN) guidelines recommend ‘follow up as clinically indicated,’ and a lack of data has led to ambiguity,” Mr. Qazi said.
Working with oncologists and radiologists at the Seattle Cancer Care Alliance, he analyzed a tissue and clinical database of 442 initial distant Merkel cell carcinoma metastases among 305 patients. Initial distant metastases were defined as the first lesions detected beyond the regional lymph nodes of the primary tumor. A total of 69% of patients had one initial distant metastasis, 19% had two concurrently identified lesions, 9% had three lesions, and 4% had at least four lesions, Mr. Qazi reported.
“Merkel cell carcinoma seemed to metastasize to unusual places, but clearly preferred the distant lymph nodes. In all, 26% of metastases localized there, most commonly to the supraclavicular, retroperitoneal, and iliac nodes,” he said. The next most common site of distant metastasis was the liver (15% of lesions), followed by the skin and bone (13% of lesions each), lung (6%), and pancreas (5%). Less common sites included the heart, spleen, abdominal muscle, brain, kidneys, adrenal glands, gonad, chest wall, and stomach.
Comparing these findings with a similar study in melanoma (J Oncol 2012. doi: 10.1155/2012/647684) showed that both cancers have about the same chances of metastasizing to the liver, bone, kidneys, adrenal glands, and stomach, Mr. Qazi said. However, Merkel cell carcinoma was less likely to metastasize to the brain (3% of lesions, vs. 12% for melanoma) and lung (6% vs. 14%), and was more likely to metastasize to the pancreas (5% vs. 1%).
Now the investigators are working to link metastatic sites with factors such as the location of the primary tumor, the presence or absence of lymphovascular invasion, and the status of the immune system and Merkel polyomavirus infection, said Mr. Qazi. They also are analyzing time from diagnosis or treatment to metastasis to help guide decisions about when to order follow-up imaging. Ultimately, they hope to create an online tool that enables clinicians to describe a primary Merkel cell carcinoma and rapidly receive automated information about the most likely timing and location of metastasis.
The National Institutes of Health supported the study. Mr. Qazi had no conflicts of interest.
SCOTTSDALE, ARIZ. – Distant metastatic sites of Merkel cell carcinoma most often involved the supraclavicular, retroperitoneal, and iliac lymph nodes, in a single center retrospective study of 305 patients.
Merkel cell carcinoma metastases “clearly favored distant nodes, but the distribution of other metastatic sites was distinct from other cancers, such as melanoma,” Jamiluddin Qazi, an undergraduate student at the University of Washington, Seattle, said at the annual meeting of the Society for Investigative Dermatology. The findings could help guide imaging and other surveillance of patients after they develop a primary Merkel cell tumor, he added.
About 2,000 individuals in the United States are diagnosed with Merkel cell carcinoma every year. About 40% of these patients develop metastatic disease, which has a 5-year survival rate of less than 25%, Mr. Qazi noted. Indeed, median survival after diagnosis of metastatic Merkel cell carcinoma was only 9.5 months in one recent study (J Cutan Pathol. 2010;37:20-7). Programmed death 1 (PD-1) blockade with pembrolizumab (Keytruda) can potentially improve survival (N Engl J Med. 2016; 374:2542-52), “but there is no consensus regarding follow-up for Merkel cell carcinoma. The 2016 National Comprehensive Cancer Network (NCCN) guidelines recommend ‘follow up as clinically indicated,’ and a lack of data has led to ambiguity,” Mr. Qazi said.
Working with oncologists and radiologists at the Seattle Cancer Care Alliance, he analyzed a tissue and clinical database of 442 initial distant Merkel cell carcinoma metastases among 305 patients. Initial distant metastases were defined as the first lesions detected beyond the regional lymph nodes of the primary tumor. A total of 69% of patients had one initial distant metastasis, 19% had two concurrently identified lesions, 9% had three lesions, and 4% had at least four lesions, Mr. Qazi reported.
“Merkel cell carcinoma seemed to metastasize to unusual places, but clearly preferred the distant lymph nodes. In all, 26% of metastases localized there, most commonly to the supraclavicular, retroperitoneal, and iliac nodes,” he said. The next most common site of distant metastasis was the liver (15% of lesions), followed by the skin and bone (13% of lesions each), lung (6%), and pancreas (5%). Less common sites included the heart, spleen, abdominal muscle, brain, kidneys, adrenal glands, gonad, chest wall, and stomach.
Comparing these findings with a similar study in melanoma (J Oncol 2012. doi: 10.1155/2012/647684) showed that both cancers have about the same chances of metastasizing to the liver, bone, kidneys, adrenal glands, and stomach, Mr. Qazi said. However, Merkel cell carcinoma was less likely to metastasize to the brain (3% of lesions, vs. 12% for melanoma) and lung (6% vs. 14%), and was more likely to metastasize to the pancreas (5% vs. 1%).
Now the investigators are working to link metastatic sites with factors such as the location of the primary tumor, the presence or absence of lymphovascular invasion, and the status of the immune system and Merkel polyomavirus infection, said Mr. Qazi. They also are analyzing time from diagnosis or treatment to metastasis to help guide decisions about when to order follow-up imaging. Ultimately, they hope to create an online tool that enables clinicians to describe a primary Merkel cell carcinoma and rapidly receive automated information about the most likely timing and location of metastasis.
The National Institutes of Health supported the study. Mr. Qazi had no conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Distant metastases of Merkel cell carcinoma most often involve the lymph nodes, followed by the liver, skin, and bone.
Major finding: Distant lymph node metastases comprised 26% of lesions, the liver comprised 15%, and skin and bone made up 13% each.
Data source: A single-center retrospective study of 442 initial distant metastases of Merkel cell carcinoma among 305 patients.
Disclosures: The National Institutes of Health supported the study. Mr. Qazi had no conflicts of interest.
Dual immune checkpoint blockade found durable in melanoma
CHICAGO – Immune checkpoint blockade, especially with a combination of agents having complementary mechanisms of action, has durable efficacy when used as initial therapy for advanced melanoma, according to an update of the CheckMate 067 trial.
The trial randomized 945 treatment-naive patients with unresectable stage III or IV melanoma evenly to double-blind treatment with nivolumab, an antibody to the cell surface receptor programmed death 1 (PD-1); ipilimumab, an antibody to the T-cell receptor cytotoxic T-lymphocyte–associated antigen 4 (CTLA4); or the combination.
Initial results, after a median follow-up of about 12.4 months, showed that the risk of progression-free survival events was 58% lower with the combination and 43% lower with nivolumab alone as compared with ipilimumab alone (N Engl J Med. 2015;373[1]:23-34).
The update, now with a median follow-up of 20.7 months, showed that these results held up, with respective 58% and 45% reductions in the risk of events, researchers reported at the annual meeting of the American Society of Clinical Oncology. The combination was also superior to nivolumab alone, netting a 24% lower risk of events. Additionally, no cumulative or new toxicities were seen.
“Based on available evidence, the combination of nivolumab and ipilimumab represents a means to improve outcomes versus nivolumab alone,” said first author Jedd D. Wolchok, MD, PhD, chief of the Melanoma & Immunotherapeutics Service at the Memorial Sloan Kettering Cancer Center in New York. “Additional insights will be gained with the emergence of overall survival data.”
Neither tumor expression of PD-L1, a ligand of PD-1, nor presence of a BRAF mutation was very helpful in identifying patients who would benefit to a greater extent from these therapies.
The findings add to evidence establishing the efficacy of combination immunotherapy in melanoma, according to invited discussant Marc S. Ernstoff, MD, professor and chair of the department of medicine at the Roswell Park Cancer Institute in Buffalo, N.Y. At the same time, the trial left unanswered questions such as what strategy should be used after progression on either or both agents, and what are the appropriate doses and durations of therapy. Also unclear is which type of therapy to use first line in patients with BRAF mutations, he added. “Whether you start with immunotherapy or targeted therapy in BRAF-mutated patients is still in equipoise, and I would encourage everyone here to participate in the ECOG 6134 trial looking at the randomization of immune checkpoint therapy versus targeted therapy in BRAF-mutated patients,” he said. “The biomarker studies are still provocative, and we still need a lot more data to be able to preselect patients who might benefit from either of these therapies.”
“One has to recognize that these agents are costly,” Dr. Ernstoff maintained, with the acquisition cost of the checkpoint inhibitors ranging from roughly $140,000 to $290,000 per year depending on the agent(s) used. This issue will also have to be addressed going forward.
“The future is very bright. There are now 76 trials listed in PDQ [Physician Data Query] of combination PD-1 therapies in melanoma alone,” he concluded. “Immunotherapy continues to capture our imagination.”
The updated intent-to-treat analyses of CheckMate 067 – conducted after all patients had at least 18 months of follow-up – showed that median progression-free survival, one of the trial’s primary endpoints, was now 11.5 months with the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), 6.9 months with nivolumab alone, and 2.9 months with ipilimumab alone, Dr. Wolchok reported at the meeting.
The differences translated to significantly better outcomes with the combination (hazard ratio, 0.42) and with nivolumab (HR, 0.55) as compared with ipilimumab. Moreover, the combination was superior to nivolumab (HR, 0.76).
The overall response rate, the trial’s other primary endpoint, was 57.6% with the combination and 43.7% with nivolumab alone, both of which were superior to the 19.0% with ipilimumab alone.
“While the response rates have not changed, some partial responses have evolved into complete responses over time,” Dr. Wolchok noted.
Findings were similar when patients were stratified by BRAF mutational status. And in exploratory analyses, outcomes were numerically better with the combination than with nivolumab alone regardless of whether tumors had high or low PD-L1 expression.
Safety results were much the same as previously reported. The rate of grade 3 or 4 treatment-related adverse events was 56.5% with the combination, 19.8% with nivolumab monotherapy, and 27.0% with ipilimumab monotherapy. There were no treatment-related deaths with the combination and one with each of the monotherapies.
“There is no common signature adverse event with this combination,” Dr. Wolchok pointed out. “The majority of grade 3 or 4 adverse events resolved in all of the groups with the use of established algorithms. However, as observed in prior studies, most of the endocrine events did not resolve and required hormone replacement.”
About 40% of the combination therapy group stopped treatment because of adverse events. “Interestingly, 68% of these patients who discontinued due to treatment-related adverse events developed a response, and 50% of these responses occurred after treatment had ended,” he reported. “This is very important information for us as we talk to patients and their families about the difficulties of stopping treatment.”
Dr. Wolchok disclosed that he is a consultant for Bristol-Myers Squibb, Genentech, Jounce Therapeutics, Medimmune, Merck, Polaris, Polynoma, Potenza, Tizona, Ziopharm, F-Star, Beigene, Lilly, Advaxis, and Sellas, and that he receives grant/research support from Bristol-Myers Squibb. The trial was sponsored by Bristol-Myers Squibb. Dako collaborated on development of the automated anti–PD-L1 immunohistochemistry assay.
CHICAGO – Immune checkpoint blockade, especially with a combination of agents having complementary mechanisms of action, has durable efficacy when used as initial therapy for advanced melanoma, according to an update of the CheckMate 067 trial.
The trial randomized 945 treatment-naive patients with unresectable stage III or IV melanoma evenly to double-blind treatment with nivolumab, an antibody to the cell surface receptor programmed death 1 (PD-1); ipilimumab, an antibody to the T-cell receptor cytotoxic T-lymphocyte–associated antigen 4 (CTLA4); or the combination.
Initial results, after a median follow-up of about 12.4 months, showed that the risk of progression-free survival events was 58% lower with the combination and 43% lower with nivolumab alone as compared with ipilimumab alone (N Engl J Med. 2015;373[1]:23-34).
The update, now with a median follow-up of 20.7 months, showed that these results held up, with respective 58% and 45% reductions in the risk of events, researchers reported at the annual meeting of the American Society of Clinical Oncology. The combination was also superior to nivolumab alone, netting a 24% lower risk of events. Additionally, no cumulative or new toxicities were seen.
“Based on available evidence, the combination of nivolumab and ipilimumab represents a means to improve outcomes versus nivolumab alone,” said first author Jedd D. Wolchok, MD, PhD, chief of the Melanoma & Immunotherapeutics Service at the Memorial Sloan Kettering Cancer Center in New York. “Additional insights will be gained with the emergence of overall survival data.”
Neither tumor expression of PD-L1, a ligand of PD-1, nor presence of a BRAF mutation was very helpful in identifying patients who would benefit to a greater extent from these therapies.
The findings add to evidence establishing the efficacy of combination immunotherapy in melanoma, according to invited discussant Marc S. Ernstoff, MD, professor and chair of the department of medicine at the Roswell Park Cancer Institute in Buffalo, N.Y. At the same time, the trial left unanswered questions such as what strategy should be used after progression on either or both agents, and what are the appropriate doses and durations of therapy. Also unclear is which type of therapy to use first line in patients with BRAF mutations, he added. “Whether you start with immunotherapy or targeted therapy in BRAF-mutated patients is still in equipoise, and I would encourage everyone here to participate in the ECOG 6134 trial looking at the randomization of immune checkpoint therapy versus targeted therapy in BRAF-mutated patients,” he said. “The biomarker studies are still provocative, and we still need a lot more data to be able to preselect patients who might benefit from either of these therapies.”
“One has to recognize that these agents are costly,” Dr. Ernstoff maintained, with the acquisition cost of the checkpoint inhibitors ranging from roughly $140,000 to $290,000 per year depending on the agent(s) used. This issue will also have to be addressed going forward.
“The future is very bright. There are now 76 trials listed in PDQ [Physician Data Query] of combination PD-1 therapies in melanoma alone,” he concluded. “Immunotherapy continues to capture our imagination.”
The updated intent-to-treat analyses of CheckMate 067 – conducted after all patients had at least 18 months of follow-up – showed that median progression-free survival, one of the trial’s primary endpoints, was now 11.5 months with the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), 6.9 months with nivolumab alone, and 2.9 months with ipilimumab alone, Dr. Wolchok reported at the meeting.
The differences translated to significantly better outcomes with the combination (hazard ratio, 0.42) and with nivolumab (HR, 0.55) as compared with ipilimumab. Moreover, the combination was superior to nivolumab (HR, 0.76).
The overall response rate, the trial’s other primary endpoint, was 57.6% with the combination and 43.7% with nivolumab alone, both of which were superior to the 19.0% with ipilimumab alone.
“While the response rates have not changed, some partial responses have evolved into complete responses over time,” Dr. Wolchok noted.
Findings were similar when patients were stratified by BRAF mutational status. And in exploratory analyses, outcomes were numerically better with the combination than with nivolumab alone regardless of whether tumors had high or low PD-L1 expression.
Safety results were much the same as previously reported. The rate of grade 3 or 4 treatment-related adverse events was 56.5% with the combination, 19.8% with nivolumab monotherapy, and 27.0% with ipilimumab monotherapy. There were no treatment-related deaths with the combination and one with each of the monotherapies.
“There is no common signature adverse event with this combination,” Dr. Wolchok pointed out. “The majority of grade 3 or 4 adverse events resolved in all of the groups with the use of established algorithms. However, as observed in prior studies, most of the endocrine events did not resolve and required hormone replacement.”
About 40% of the combination therapy group stopped treatment because of adverse events. “Interestingly, 68% of these patients who discontinued due to treatment-related adverse events developed a response, and 50% of these responses occurred after treatment had ended,” he reported. “This is very important information for us as we talk to patients and their families about the difficulties of stopping treatment.”
Dr. Wolchok disclosed that he is a consultant for Bristol-Myers Squibb, Genentech, Jounce Therapeutics, Medimmune, Merck, Polaris, Polynoma, Potenza, Tizona, Ziopharm, F-Star, Beigene, Lilly, Advaxis, and Sellas, and that he receives grant/research support from Bristol-Myers Squibb. The trial was sponsored by Bristol-Myers Squibb. Dako collaborated on development of the automated anti–PD-L1 immunohistochemistry assay.
CHICAGO – Immune checkpoint blockade, especially with a combination of agents having complementary mechanisms of action, has durable efficacy when used as initial therapy for advanced melanoma, according to an update of the CheckMate 067 trial.
The trial randomized 945 treatment-naive patients with unresectable stage III or IV melanoma evenly to double-blind treatment with nivolumab, an antibody to the cell surface receptor programmed death 1 (PD-1); ipilimumab, an antibody to the T-cell receptor cytotoxic T-lymphocyte–associated antigen 4 (CTLA4); or the combination.
Initial results, after a median follow-up of about 12.4 months, showed that the risk of progression-free survival events was 58% lower with the combination and 43% lower with nivolumab alone as compared with ipilimumab alone (N Engl J Med. 2015;373[1]:23-34).
The update, now with a median follow-up of 20.7 months, showed that these results held up, with respective 58% and 45% reductions in the risk of events, researchers reported at the annual meeting of the American Society of Clinical Oncology. The combination was also superior to nivolumab alone, netting a 24% lower risk of events. Additionally, no cumulative or new toxicities were seen.
“Based on available evidence, the combination of nivolumab and ipilimumab represents a means to improve outcomes versus nivolumab alone,” said first author Jedd D. Wolchok, MD, PhD, chief of the Melanoma & Immunotherapeutics Service at the Memorial Sloan Kettering Cancer Center in New York. “Additional insights will be gained with the emergence of overall survival data.”
Neither tumor expression of PD-L1, a ligand of PD-1, nor presence of a BRAF mutation was very helpful in identifying patients who would benefit to a greater extent from these therapies.
The findings add to evidence establishing the efficacy of combination immunotherapy in melanoma, according to invited discussant Marc S. Ernstoff, MD, professor and chair of the department of medicine at the Roswell Park Cancer Institute in Buffalo, N.Y. At the same time, the trial left unanswered questions such as what strategy should be used after progression on either or both agents, and what are the appropriate doses and durations of therapy. Also unclear is which type of therapy to use first line in patients with BRAF mutations, he added. “Whether you start with immunotherapy or targeted therapy in BRAF-mutated patients is still in equipoise, and I would encourage everyone here to participate in the ECOG 6134 trial looking at the randomization of immune checkpoint therapy versus targeted therapy in BRAF-mutated patients,” he said. “The biomarker studies are still provocative, and we still need a lot more data to be able to preselect patients who might benefit from either of these therapies.”
“One has to recognize that these agents are costly,” Dr. Ernstoff maintained, with the acquisition cost of the checkpoint inhibitors ranging from roughly $140,000 to $290,000 per year depending on the agent(s) used. This issue will also have to be addressed going forward.
“The future is very bright. There are now 76 trials listed in PDQ [Physician Data Query] of combination PD-1 therapies in melanoma alone,” he concluded. “Immunotherapy continues to capture our imagination.”
The updated intent-to-treat analyses of CheckMate 067 – conducted after all patients had at least 18 months of follow-up – showed that median progression-free survival, one of the trial’s primary endpoints, was now 11.5 months with the combination of nivolumab (Opdivo) and ipilimumab (Yervoy), 6.9 months with nivolumab alone, and 2.9 months with ipilimumab alone, Dr. Wolchok reported at the meeting.
The differences translated to significantly better outcomes with the combination (hazard ratio, 0.42) and with nivolumab (HR, 0.55) as compared with ipilimumab. Moreover, the combination was superior to nivolumab (HR, 0.76).
The overall response rate, the trial’s other primary endpoint, was 57.6% with the combination and 43.7% with nivolumab alone, both of which were superior to the 19.0% with ipilimumab alone.
“While the response rates have not changed, some partial responses have evolved into complete responses over time,” Dr. Wolchok noted.
Findings were similar when patients were stratified by BRAF mutational status. And in exploratory analyses, outcomes were numerically better with the combination than with nivolumab alone regardless of whether tumors had high or low PD-L1 expression.
Safety results were much the same as previously reported. The rate of grade 3 or 4 treatment-related adverse events was 56.5% with the combination, 19.8% with nivolumab monotherapy, and 27.0% with ipilimumab monotherapy. There were no treatment-related deaths with the combination and one with each of the monotherapies.
“There is no common signature adverse event with this combination,” Dr. Wolchok pointed out. “The majority of grade 3 or 4 adverse events resolved in all of the groups with the use of established algorithms. However, as observed in prior studies, most of the endocrine events did not resolve and required hormone replacement.”
About 40% of the combination therapy group stopped treatment because of adverse events. “Interestingly, 68% of these patients who discontinued due to treatment-related adverse events developed a response, and 50% of these responses occurred after treatment had ended,” he reported. “This is very important information for us as we talk to patients and their families about the difficulties of stopping treatment.”
Dr. Wolchok disclosed that he is a consultant for Bristol-Myers Squibb, Genentech, Jounce Therapeutics, Medimmune, Merck, Polaris, Polynoma, Potenza, Tizona, Ziopharm, F-Star, Beigene, Lilly, Advaxis, and Sellas, and that he receives grant/research support from Bristol-Myers Squibb. The trial was sponsored by Bristol-Myers Squibb. Dako collaborated on development of the automated anti–PD-L1 immunohistochemistry assay.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Nivolumab-ipilimumab combination therapy and nivolumab monotherapy are more efficacious than ipilimumab monotherapy when used in the first line for advanced melanoma.
Major finding: The risk of progression-free survival events was lower with nivolumab plus ipilimumab (HR, 0.42) and with nivolumab alone (HR, 0.55) as compared with ipilimumab alone.
Data source: A phase III randomized trial among 945 treatment-naive patients with advanced melanoma (CheckMate 067).
Disclosures: Dr. Wolchok disclosed that he is a consultant for Bristol-Myers Squibb, Genentech, Jounce Therapeutics, Medimmune, Merck, Polaris, Polynoma, Potenza, Tizona, Ziopharm, F-Star, Beigene, Lilly, Advaxis, and Sellas, and that he receives grant/research support from Bristol-Myers Squibb. The trial was sponsored by Bristol-Myers Squibb. Dako collaborated on development of the automated anti-PD-L1 immunohistochemistry assay.
Case series describes melanoma-associated leukoderma presenting as atypical vitiligo
Consider melanoma-associated leukoderma (MAL) as a possible diagnosis in patients presenting with atypical vitiligo-like skin depigmentation that is refractory to standard treatment, advised the authors of a series of seven such cases.
In a research letter published online in the British Journal of Dermatology, Dr. H.E. Teulings and colleagues from the department of dermatology and the Netherlands Institute for Pigment Disorders at the University of Amsterdam, presented a retrospective analysis of seven patients diagnosed with MAL from 2009-2014, who had been initially diagnosed with nonsegmental vitiligo.
The authors defined MAL as “depigmentation that developed within 1 year before the detection of a primary melanoma or within 3 years before the detection of melanoma metastases with an unknown primary tumour.”
The five women and two men were white and were older (aged 45 to 72 years). They had experienced a sudden onset of highly progressive hypo- and depigmentation, which the authors described as often consisting of “round, patchy, confetti-like lesions” measuring 4-5 mm in diameter; most were scattered symmetrically over the trunk, extremities, and/or face (Br J Dermatol. 2016 Jun 7. doi: 10.1111/bjd.14790).
The authors noted that this presentation was unlike typical vitiligo, “which is often bilaterally distributed in an acrofacial pattern, or scattered symmetrically over the entire body with a predilection for extensor surfaces with a relatively early onset in life and a slowly evolving disease course over time.”
The lesions were also generally resistant to topical steroids and UV phototherapy, and six of the seven patients had no family history of vitiligo. The patients were either diagnosed with a primary melanoma at first presentation or were later diagnosed with metastatic melanoma. The majority responded well to immunotherapy, although one patient died.
“In conclusion, although MAL only constitutes a small percentage of patients presenting with vitiligo-like depigmentation, awareness of this phenomenon and correct diagnosis of these patients is crucial to limit further melanoma treatment delay,” the authors wrote. “Many dermatologists are not aware of the diagnosis MAL and may easily diagnose and treat these patients as having nonsegmental vitiligo, thereby overlooking the underlying (metastatic) melanoma,” they added.
Dr. Teulings is supported by a grant from the Dutch Cancer Society. The authors had no conflicts of interest to declare.
Consider melanoma-associated leukoderma (MAL) as a possible diagnosis in patients presenting with atypical vitiligo-like skin depigmentation that is refractory to standard treatment, advised the authors of a series of seven such cases.
In a research letter published online in the British Journal of Dermatology, Dr. H.E. Teulings and colleagues from the department of dermatology and the Netherlands Institute for Pigment Disorders at the University of Amsterdam, presented a retrospective analysis of seven patients diagnosed with MAL from 2009-2014, who had been initially diagnosed with nonsegmental vitiligo.
The authors defined MAL as “depigmentation that developed within 1 year before the detection of a primary melanoma or within 3 years before the detection of melanoma metastases with an unknown primary tumour.”
The five women and two men were white and were older (aged 45 to 72 years). They had experienced a sudden onset of highly progressive hypo- and depigmentation, which the authors described as often consisting of “round, patchy, confetti-like lesions” measuring 4-5 mm in diameter; most were scattered symmetrically over the trunk, extremities, and/or face (Br J Dermatol. 2016 Jun 7. doi: 10.1111/bjd.14790).
The authors noted that this presentation was unlike typical vitiligo, “which is often bilaterally distributed in an acrofacial pattern, or scattered symmetrically over the entire body with a predilection for extensor surfaces with a relatively early onset in life and a slowly evolving disease course over time.”
The lesions were also generally resistant to topical steroids and UV phototherapy, and six of the seven patients had no family history of vitiligo. The patients were either diagnosed with a primary melanoma at first presentation or were later diagnosed with metastatic melanoma. The majority responded well to immunotherapy, although one patient died.
“In conclusion, although MAL only constitutes a small percentage of patients presenting with vitiligo-like depigmentation, awareness of this phenomenon and correct diagnosis of these patients is crucial to limit further melanoma treatment delay,” the authors wrote. “Many dermatologists are not aware of the diagnosis MAL and may easily diagnose and treat these patients as having nonsegmental vitiligo, thereby overlooking the underlying (metastatic) melanoma,” they added.
Dr. Teulings is supported by a grant from the Dutch Cancer Society. The authors had no conflicts of interest to declare.
Consider melanoma-associated leukoderma (MAL) as a possible diagnosis in patients presenting with atypical vitiligo-like skin depigmentation that is refractory to standard treatment, advised the authors of a series of seven such cases.
In a research letter published online in the British Journal of Dermatology, Dr. H.E. Teulings and colleagues from the department of dermatology and the Netherlands Institute for Pigment Disorders at the University of Amsterdam, presented a retrospective analysis of seven patients diagnosed with MAL from 2009-2014, who had been initially diagnosed with nonsegmental vitiligo.
The authors defined MAL as “depigmentation that developed within 1 year before the detection of a primary melanoma or within 3 years before the detection of melanoma metastases with an unknown primary tumour.”
The five women and two men were white and were older (aged 45 to 72 years). They had experienced a sudden onset of highly progressive hypo- and depigmentation, which the authors described as often consisting of “round, patchy, confetti-like lesions” measuring 4-5 mm in diameter; most were scattered symmetrically over the trunk, extremities, and/or face (Br J Dermatol. 2016 Jun 7. doi: 10.1111/bjd.14790).
The authors noted that this presentation was unlike typical vitiligo, “which is often bilaterally distributed in an acrofacial pattern, or scattered symmetrically over the entire body with a predilection for extensor surfaces with a relatively early onset in life and a slowly evolving disease course over time.”
The lesions were also generally resistant to topical steroids and UV phototherapy, and six of the seven patients had no family history of vitiligo. The patients were either diagnosed with a primary melanoma at first presentation or were later diagnosed with metastatic melanoma. The majority responded well to immunotherapy, although one patient died.
“In conclusion, although MAL only constitutes a small percentage of patients presenting with vitiligo-like depigmentation, awareness of this phenomenon and correct diagnosis of these patients is crucial to limit further melanoma treatment delay,” the authors wrote. “Many dermatologists are not aware of the diagnosis MAL and may easily diagnose and treat these patients as having nonsegmental vitiligo, thereby overlooking the underlying (metastatic) melanoma,” they added.
Dr. Teulings is supported by a grant from the Dutch Cancer Society. The authors had no conflicts of interest to declare.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Doctors should consider melanoma-associated leukoderma (MAL) as a possible diagnosis in patients presenting with atypical vitiligo-like depigmentation that is refractory to standard treatment.
Major finding: Seven patients with MAL, who were initially diagnosed with nonsegmental vitiligo, were older; had late onset, progressive symptoms; and had presentations that were not like typical vitiligo.
Data source: A retrospective case series of seven patients diagnosed with MAL at a tertiary vitiligo center.
Disclosures: One author was supported by a grant from the Dutch Cancer Society. No conflicts of interest were declared.
T-VEC plus ipilimumab safe, effective in advanced melanoma
Talimogene laherparepvec (T-VEC) combined with ipilimumab was more effective in treating advanced melanoma than either treatment alone, report Igor Puzanov, MD, of Vanderbilt University Medical Center in Nashville, Tenn., and coauthors.
In a phase Ib trial of 19 patients with advanced melanoma, the results showed that 50% had positive responses to the combined immunotherapy, and 44% had durable responses lasting 6 months or longer. At 18 months, 50% of patients showed no progression, and overall patient survival was 67%, the authors reported.
The study’s safety analysis showed no new signs of safety concerns, or of any dose-limiting toxicities.
Read the full study in The Journal of Clinical Oncology.
Talimogene laherparepvec (T-VEC) combined with ipilimumab was more effective in treating advanced melanoma than either treatment alone, report Igor Puzanov, MD, of Vanderbilt University Medical Center in Nashville, Tenn., and coauthors.
In a phase Ib trial of 19 patients with advanced melanoma, the results showed that 50% had positive responses to the combined immunotherapy, and 44% had durable responses lasting 6 months or longer. At 18 months, 50% of patients showed no progression, and overall patient survival was 67%, the authors reported.
The study’s safety analysis showed no new signs of safety concerns, or of any dose-limiting toxicities.
Read the full study in The Journal of Clinical Oncology.
Talimogene laherparepvec (T-VEC) combined with ipilimumab was more effective in treating advanced melanoma than either treatment alone, report Igor Puzanov, MD, of Vanderbilt University Medical Center in Nashville, Tenn., and coauthors.
In a phase Ib trial of 19 patients with advanced melanoma, the results showed that 50% had positive responses to the combined immunotherapy, and 44% had durable responses lasting 6 months or longer. At 18 months, 50% of patients showed no progression, and overall patient survival was 67%, the authors reported.
The study’s safety analysis showed no new signs of safety concerns, or of any dose-limiting toxicities.
Read the full study in The Journal of Clinical Oncology.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Evolving therapeutic strategies maintain clinical momentum in melanoma
The past 5 years have witnessed a watershed moment in the management of metastatic melanoma. The successes of molecularly targeted and immune-based therapies have transformed it from an aggressively lethal malignancy into one that is readily treatable. Here, we discuss continued efforts to find new therapies and broaden the clinical impact of existing options to maintain the unprecedented momentum of improving patient outcomes.
Click on the PDF icon at the top of this introduction to read the full article.
The past 5 years have witnessed a watershed moment in the management of metastatic melanoma. The successes of molecularly targeted and immune-based therapies have transformed it from an aggressively lethal malignancy into one that is readily treatable. Here, we discuss continued efforts to find new therapies and broaden the clinical impact of existing options to maintain the unprecedented momentum of improving patient outcomes.
Click on the PDF icon at the top of this introduction to read the full article.
The past 5 years have witnessed a watershed moment in the management of metastatic melanoma. The successes of molecularly targeted and immune-based therapies have transformed it from an aggressively lethal malignancy into one that is readily treatable. Here, we discuss continued efforts to find new therapies and broaden the clinical impact of existing options to maintain the unprecedented momentum of improving patient outcomes.
Click on the PDF icon at the top of this introduction to read the full article.
Implementation of ipilimumab therapy in a private practice oncology group: overcoming start-up and reimbursement issues related to expensive new cancer drugs
The monoclonal antibody ipilimumab was the first treatment in more than 30 years to improve long-term survival in metastatic melanoma patients. Offering expensive ipilimumab treatment presented significant business challenges and potential financial risks for our private oncology practice and for patients because of the high acquisition cost of this agent. There was initial uncertainty about the willingness of insurance companies to reimburse for this new drug based on previous experiences in our practice with other expensive new drugs. Here we describe how our multiphysician practice methodically introduced ipilimumab treatment into the practice.
Click on the PDF icon at the top of this introduction to read the full article.
The monoclonal antibody ipilimumab was the first treatment in more than 30 years to improve long-term survival in metastatic melanoma patients. Offering expensive ipilimumab treatment presented significant business challenges and potential financial risks for our private oncology practice and for patients because of the high acquisition cost of this agent. There was initial uncertainty about the willingness of insurance companies to reimburse for this new drug based on previous experiences in our practice with other expensive new drugs. Here we describe how our multiphysician practice methodically introduced ipilimumab treatment into the practice.
Click on the PDF icon at the top of this introduction to read the full article.
The monoclonal antibody ipilimumab was the first treatment in more than 30 years to improve long-term survival in metastatic melanoma patients. Offering expensive ipilimumab treatment presented significant business challenges and potential financial risks for our private oncology practice and for patients because of the high acquisition cost of this agent. There was initial uncertainty about the willingness of insurance companies to reimburse for this new drug based on previous experiences in our practice with other expensive new drugs. Here we describe how our multiphysician practice methodically introduced ipilimumab treatment into the practice.
Click on the PDF icon at the top of this introduction to read the full article.