User login
Similar brain atrophy in obesity and Alzheimer’s disease
Comparisons of MRI scans for more than 1,000 participants indicate correlations between the two conditions, especially in areas of gray matter thinning, suggesting that managing excess weight might slow cognitive decline and lower the risk for AD, according to the researchers.
However, brain maps of obesity did not correlate with maps of amyloid or tau protein accumulation.
“The fact that obesity-related brain atrophy did not correlate with the distribution of amyloid and tau proteins in AD was not what we expected,” study author Filip Morys, PhD, a postdoctoral researcher at McGill University, Montreal, said in an interview. “But it might just show that the specific mechanisms underpinning obesity- and Alzheimer’s disease–related neurodegeneration are different. This remains to be confirmed.”
The study was published in the Journal of Alzheimer’s Disease.
Cortical Thinning
The current study was prompted by the team’s earlier study, which showed that obesity-related neurodegeneration patterns were visually similar to those of AD, said Dr. Morys. “It was known previously that obesity is a risk factor for AD, but we wanted to directly compare brain atrophy patterns in both, which is what we did in this new study.”
The researchers analyzed data from a pooled sample of more than 1,300 participants. From the ADNI database, the researchers selected participants with AD and age- and sex-matched cognitively healthy controls. From the UK Biobank, the researchers drew a sample of lean, overweight, and obese participants without neurologic disease.
To determine how the weight status of patients with AD affects the correspondence between AD and obesity maps, they categorized participants with AD and healthy controls from the ADNI database into lean, overweight, and obese subgroups.
Then, to investigate mechanisms that might drive the similarities between obesity-related brain atrophy and AD-related amyloid-beta accumulation, they looked for overlapping areas in PET brain maps between patients with these outcomes.
The investigations showed that obesity maps were highly correlated with AD maps, but not with amyloid-beta or tau protein maps. The researchers also found significant correlations between obesity and the lean individuals with AD.
Brain regions with the highest similarities between obesity and AD were located mainly in the left temporal and bilateral prefrontal cortices.
“Our research confirms that obesity-related gray matter atrophy resembles that of AD,” the authors concluded. “Excess weight management could lead to improved health outcomes, slow down cognitive decline in aging, and lower the risk for AD.”
Upcoming research “will focus on investigating how weight loss can affect the risk for AD, other dementias, and cognitive decline in general,” said Dr. Morys. “At this point, our study suggests that obesity prevention, weight loss, but also decreasing other metabolic risk factors related to obesity, such as type-2 diabetes or hypertension, might reduce the risk for AD and have beneficial effects on cognition.”
Lifestyle habits
Commenting on the findings, Claire Sexton, DPhil, vice president of scientific programs and outreach at the Alzheimer’s Association, cautioned that a single cross-sectional study isn’t conclusive. “Previous studies have illustrated that the relationship between obesity and dementia is complex. Growing evidence indicates that people can reduce their risk of cognitive decline by adopting key lifestyle habits, like regular exercise, a heart-healthy diet and staying socially and cognitively engaged.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to study how targeting these risk factors in combination may reduce risk for cognitive decline in older adults.
The work was supported by a Foundation Scheme award from the Canadian Institutes of Health Research. Dr. Morys received a postdoctoral fellowship from Fonds de Recherche du Quebec – Santé. Data collection and sharing were funded by the Alzheimer’s Disease Neuroimaging Initiative, the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and multiple pharmaceutical companies and other private sector organizations. Dr. Morys and Dr. Sexton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comparisons of MRI scans for more than 1,000 participants indicate correlations between the two conditions, especially in areas of gray matter thinning, suggesting that managing excess weight might slow cognitive decline and lower the risk for AD, according to the researchers.
However, brain maps of obesity did not correlate with maps of amyloid or tau protein accumulation.
“The fact that obesity-related brain atrophy did not correlate with the distribution of amyloid and tau proteins in AD was not what we expected,” study author Filip Morys, PhD, a postdoctoral researcher at McGill University, Montreal, said in an interview. “But it might just show that the specific mechanisms underpinning obesity- and Alzheimer’s disease–related neurodegeneration are different. This remains to be confirmed.”
The study was published in the Journal of Alzheimer’s Disease.
Cortical Thinning
The current study was prompted by the team’s earlier study, which showed that obesity-related neurodegeneration patterns were visually similar to those of AD, said Dr. Morys. “It was known previously that obesity is a risk factor for AD, but we wanted to directly compare brain atrophy patterns in both, which is what we did in this new study.”
The researchers analyzed data from a pooled sample of more than 1,300 participants. From the ADNI database, the researchers selected participants with AD and age- and sex-matched cognitively healthy controls. From the UK Biobank, the researchers drew a sample of lean, overweight, and obese participants without neurologic disease.
To determine how the weight status of patients with AD affects the correspondence between AD and obesity maps, they categorized participants with AD and healthy controls from the ADNI database into lean, overweight, and obese subgroups.
Then, to investigate mechanisms that might drive the similarities between obesity-related brain atrophy and AD-related amyloid-beta accumulation, they looked for overlapping areas in PET brain maps between patients with these outcomes.
The investigations showed that obesity maps were highly correlated with AD maps, but not with amyloid-beta or tau protein maps. The researchers also found significant correlations between obesity and the lean individuals with AD.
Brain regions with the highest similarities between obesity and AD were located mainly in the left temporal and bilateral prefrontal cortices.
“Our research confirms that obesity-related gray matter atrophy resembles that of AD,” the authors concluded. “Excess weight management could lead to improved health outcomes, slow down cognitive decline in aging, and lower the risk for AD.”
Upcoming research “will focus on investigating how weight loss can affect the risk for AD, other dementias, and cognitive decline in general,” said Dr. Morys. “At this point, our study suggests that obesity prevention, weight loss, but also decreasing other metabolic risk factors related to obesity, such as type-2 diabetes or hypertension, might reduce the risk for AD and have beneficial effects on cognition.”
Lifestyle habits
Commenting on the findings, Claire Sexton, DPhil, vice president of scientific programs and outreach at the Alzheimer’s Association, cautioned that a single cross-sectional study isn’t conclusive. “Previous studies have illustrated that the relationship between obesity and dementia is complex. Growing evidence indicates that people can reduce their risk of cognitive decline by adopting key lifestyle habits, like regular exercise, a heart-healthy diet and staying socially and cognitively engaged.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to study how targeting these risk factors in combination may reduce risk for cognitive decline in older adults.
The work was supported by a Foundation Scheme award from the Canadian Institutes of Health Research. Dr. Morys received a postdoctoral fellowship from Fonds de Recherche du Quebec – Santé. Data collection and sharing were funded by the Alzheimer’s Disease Neuroimaging Initiative, the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and multiple pharmaceutical companies and other private sector organizations. Dr. Morys and Dr. Sexton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Comparisons of MRI scans for more than 1,000 participants indicate correlations between the two conditions, especially in areas of gray matter thinning, suggesting that managing excess weight might slow cognitive decline and lower the risk for AD, according to the researchers.
However, brain maps of obesity did not correlate with maps of amyloid or tau protein accumulation.
“The fact that obesity-related brain atrophy did not correlate with the distribution of amyloid and tau proteins in AD was not what we expected,” study author Filip Morys, PhD, a postdoctoral researcher at McGill University, Montreal, said in an interview. “But it might just show that the specific mechanisms underpinning obesity- and Alzheimer’s disease–related neurodegeneration are different. This remains to be confirmed.”
The study was published in the Journal of Alzheimer’s Disease.
Cortical Thinning
The current study was prompted by the team’s earlier study, which showed that obesity-related neurodegeneration patterns were visually similar to those of AD, said Dr. Morys. “It was known previously that obesity is a risk factor for AD, but we wanted to directly compare brain atrophy patterns in both, which is what we did in this new study.”
The researchers analyzed data from a pooled sample of more than 1,300 participants. From the ADNI database, the researchers selected participants with AD and age- and sex-matched cognitively healthy controls. From the UK Biobank, the researchers drew a sample of lean, overweight, and obese participants without neurologic disease.
To determine how the weight status of patients with AD affects the correspondence between AD and obesity maps, they categorized participants with AD and healthy controls from the ADNI database into lean, overweight, and obese subgroups.
Then, to investigate mechanisms that might drive the similarities between obesity-related brain atrophy and AD-related amyloid-beta accumulation, they looked for overlapping areas in PET brain maps between patients with these outcomes.
The investigations showed that obesity maps were highly correlated with AD maps, but not with amyloid-beta or tau protein maps. The researchers also found significant correlations between obesity and the lean individuals with AD.
Brain regions with the highest similarities between obesity and AD were located mainly in the left temporal and bilateral prefrontal cortices.
“Our research confirms that obesity-related gray matter atrophy resembles that of AD,” the authors concluded. “Excess weight management could lead to improved health outcomes, slow down cognitive decline in aging, and lower the risk for AD.”
Upcoming research “will focus on investigating how weight loss can affect the risk for AD, other dementias, and cognitive decline in general,” said Dr. Morys. “At this point, our study suggests that obesity prevention, weight loss, but also decreasing other metabolic risk factors related to obesity, such as type-2 diabetes or hypertension, might reduce the risk for AD and have beneficial effects on cognition.”
Lifestyle habits
Commenting on the findings, Claire Sexton, DPhil, vice president of scientific programs and outreach at the Alzheimer’s Association, cautioned that a single cross-sectional study isn’t conclusive. “Previous studies have illustrated that the relationship between obesity and dementia is complex. Growing evidence indicates that people can reduce their risk of cognitive decline by adopting key lifestyle habits, like regular exercise, a heart-healthy diet and staying socially and cognitively engaged.”
The Alzheimer’s Association is leading a 2-year clinical trial, U.S. Pointer, to study how targeting these risk factors in combination may reduce risk for cognitive decline in older adults.
The work was supported by a Foundation Scheme award from the Canadian Institutes of Health Research. Dr. Morys received a postdoctoral fellowship from Fonds de Recherche du Quebec – Santé. Data collection and sharing were funded by the Alzheimer’s Disease Neuroimaging Initiative, the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and multiple pharmaceutical companies and other private sector organizations. Dr. Morys and Dr. Sexton reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Psychiatric illnesses share common brain network
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.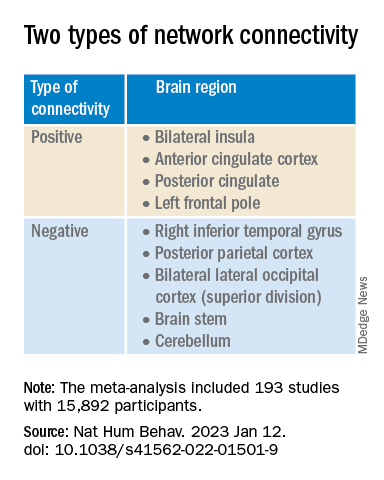
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE HUMAN BEHAVIOR
Six healthy lifestyle habits linked to slowed memory decline
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.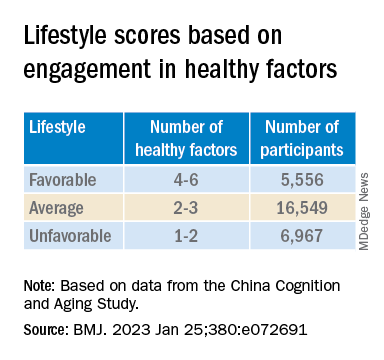
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.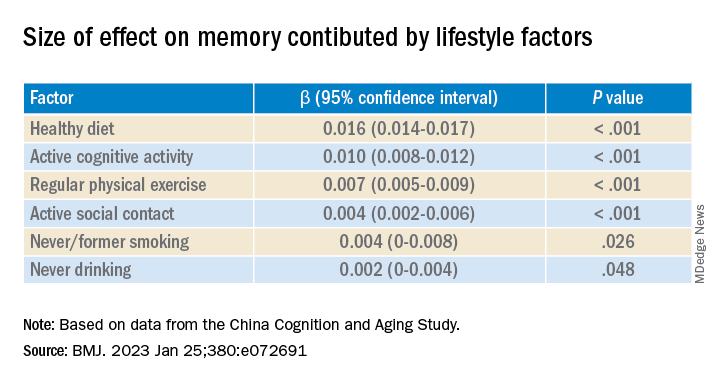
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Investigators found that a healthy diet, cognitive activity, regular physical exercise, not smoking, and abstaining from alcohol were significantly linked to slowed cognitive decline irrespective of APOE4 status.
After adjusting for health and socioeconomic factors, investigators found that each individual healthy behavior was associated with a slower-than-average decline in memory over a decade. A healthy diet emerged as the strongest deterrent, followed by cognitive activity and physical exercise.
“A healthy lifestyle is associated with slower memory decline, even in the presence of the APOE4 allele,” study investigators led by Jianping Jia, MD, PhD, of the Innovation Center for Neurological Disorders and the department of neurology, Xuan Wu Hospital, Capital Medical University, Beijing, write.
“This study might offer important information to protect older adults against memory decline,” they add.
The study was published online in the BMJ.
Preventing memory decline
Memory “continuously declines as people age,” but age-related memory decline is not necessarily a prodrome of dementia and can “merely be senescent forgetfulness,” the investigators note. This can be “reversed or [can] become stable,” instead of progressing to a pathologic state.
Factors affecting memory include aging, APOE4 genotype, chronic diseases, and lifestyle patterns, with lifestyle “receiving increasing attention as a modifiable behavior.”
Nevertheless, few studies have focused on the impact of lifestyle on memory, and those that have are mostly cross-sectional and also “did not consider the interaction between a healthy lifestyle and genetic risk,” the researchers note.
To investigate, the researchers conducted a longitudinal study, known as the China Cognition and Aging Study, that considered genetic risk as well as lifestyle factors.
The study began in 2009 and concluded in 2019. Participants were evaluated and underwent neuropsychological testing in 2012, 2014, 2016, and at the study’s conclusion.
Participants (n = 29,072; mean [SD] age, 72.23 [6.61] years; 48.54% women; 20.43% APOE4 carriers) were required to have normal cognitive function at baseline. Data on those whose condition progressed to mild cognitive impairment (MCI) or dementia during the follow-up period were excluded after their diagnosis.
The Mini–Mental State Examination was used to assess global cognitive function. Memory function was assessed using the World Health Organization/University of California, Los Angeles Auditory Verbal Learning Test.
“Lifestyle” consisted of six modifiable factors: physical exercise (weekly frequency and total time), smoking (current, former, or never-smokers), alcohol consumption (never drank, drank occasionally, low to excess drinking, and heavy drinking), diet (daily intake of 12 food items: fruits, vegetables, fish, meat, dairy products, salt, oil, eggs, cereals, legumes, nuts, tea), cognitive activity (writing, reading, playing cards, mahjong, other games), and social contact (participating in meetings, attending parties, visiting friends/relatives, traveling, chatting online).
Participants’ lifestyles were scored on the basis of the number of healthy factors they engaged in.
Participants were also stratified by APOE genotype into APOE4 carriers and noncarriers.
Demographic and other items of health information, including the presence of medical illness, were used as covariates. The researchers also included the “learning effect of each participant as a covariate, due to repeated cognitive assessments.”
Important for public health
During the 10-year period, 7,164 participants died, and 3,567 stopped participating.
Participants in the favorable and average groups showed slower memory decline per increased year of age (0.007 [0.005-0.009], P < .001; and 0.002 [0 .000-0.003], P = .033 points higher, respectively), compared with those in the unfavorable group.
Healthy diet had the strongest protective effect on memory.
Memory decline occurred faster in APOE4 vesus non-APOE4 carriers (0.002 points/year [95% confidence interval, 0.001-0.003]; P = .007).
But APOE4 carriers with favorable and average lifestyles showed slower memory decline (0.027 [0.023-0.031] and 0.014 [0.010-0.019], respectively), compared with those with unfavorable lifestyles. Similar findings were obtained in non-APOE4 carriers.
Those with favorable or average lifestyle were respectively almost 90% and 30% less likely to develop dementia or MCI, compared with those with an unfavorable lifestyle.
The authors acknowledge the study’s limitations, including its observational design and the potential for measurement errors, owing to self-reporting of lifestyle factors. Additionally, some participants did not return for follow-up evaluations, leading to potential selection bias.
Nevertheless, the findings “might offer important information for public health to protect older [people] against memory decline,” they note – especially since the study “provides evidence that these effects also include individuals with the APOE4 allele.”
‘Important, encouraging’ research
In a comment, Severine Sabia, PhD, a senior researcher at the Université Paris Cité, INSERM Institut National de la Santé et de la Recherche Medicalé, France, called the findings “important and encouraging.”
However, said Dr. Sabia, who was not involved with the study, “there remain important research questions that need to be investigated in order to identify key behaviors: which combination, the cutoff of risk, and when to intervene.”
Future research on prevention “should examine a wider range of possible risk factors” and should also “identify specific exposures associated with the greatest risk, while also considering the risk threshold and age at exposure for each one.”
In an accompanying editorial, Dr. Sabia and co-author Archana Singh-Manoux, PhD, note that the risk of cognitive decline and dementia are probably determined by multiple factors.
They liken it to the “multifactorial risk paradigm introduced by the Framingham study,” which has “led to a substantial reduction in cardiovascular disease.” A similar approach could be used with dementia prevention, they suggest.
The authors received support from the Xuanwu Hospital of Capital Medical University for the submitted work. One of the authors received a grant from the French National Research Agency. The other authors have disclosed no relevant financial relationships. Dr. Sabia received grant funding from the French National Research Agency. Dr. Singh-Manoux received grants from the National Institute on Aging of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Tips and tools to help you manage ADHD in children, adolescents
THE CASE
James B* is a 7-year-old Black child who presented to his primary care physician (PCP) for a well-child visit. During preventive health screening, James’ mother expressed concerns about his behavior, characterizing him as immature, aggressive, destructive, and occasionally self-loathing. She described him as physically uncoordinated, struggling to keep up with his peers in sports, and tiring after 20 minutes of activity. James slept 10 hours nightly but was often restless and snored intermittently. As a second grader, his academic achievement was not progressing, and he had become increasingly inattentive at home and at school. James’ mother offered several examples of his fighting with his siblings, noncompliance with morning routines, and avoidance of learning activities. Additionally, his mother expressed concern that James, as a Black child, might eventually be unfairly labeled as a problem child by his teachers or held back a grade level in school.
Although James did not have a family history of developmental delays or learning disorders, he had not met any milestones on time for gross or fine motor, language, cognitive, and social-emotional skills. James had a history of chronic otitis media, for which pressure equalizer tubes were inserted at age 2 years. He had not had any major physical injuries, psychological trauma, recent life transitions, or adverse childhood events. When asked, James’ mother acknowledged symptoms of maternal depression but alluded to faith-based reasons for not seeking treatment for herself.
James’ physical examination was unremarkable. His height, weight, and vitals were all within normal limits. However, he had some difficulty with verbal articulation and expression and showed signs of a possible vocal tic. Based on James’ presentation, his PCP suspected attention-deficit/hyperactivity disorder (ADHD), as well as neurodevelopmental delays.
The PCP gave James’ mother the Strengths and Difficulties Questionnaire to complete and the Vanderbilt Assessment Scales for her and James’ teacher to fill out independently and return to the clinic. The PCP also instructed James’ mother on how to use a sleep diary to maintain a 1-month log of his sleep patterns and habits. The PCP consulted the integrated behavioral health clinician (IBHC; a clinical social worker embedded in the primary care clinic) and made a warm handoff for the IBHC to further assess James’ maladaptive behaviors and interactions.
●
* The patient’s name has been changed to protect his identity.
James is one of more than 6 million children, ages 3 to 17 years, in the United States who live with ADHD.1,2 ADHD is the most common neurodevelopmental disorder among children, and it affects multiple cognitive and behavioral domains throughout the lifespan.3 Children with ADHD often initially present in primary care settings; thus, PCPs are well positioned to diagnose the disorder and provide longitudinal treatment. This Behavioral Health Consult reviews clinical assessment and practice guidelines, as well as treatment recommendations applicable across different areas of influence—individual, family, community, and systems—for PCPs and IBHCs to use in managing ADHD in children.
ADHD features can vary by age and sex
ADHD is a persistent pattern of inattention or hyperactivity and impulsivity interfering with functioning or development in childhood and functioning later in adulthood. ADHD symptoms manifest prior to age 12 years and must occur in 2 or more settings.4 Symptoms should not be better explained by another psychiatric disorder or occur exclusively during the course of another disorder (TABLE 1).4
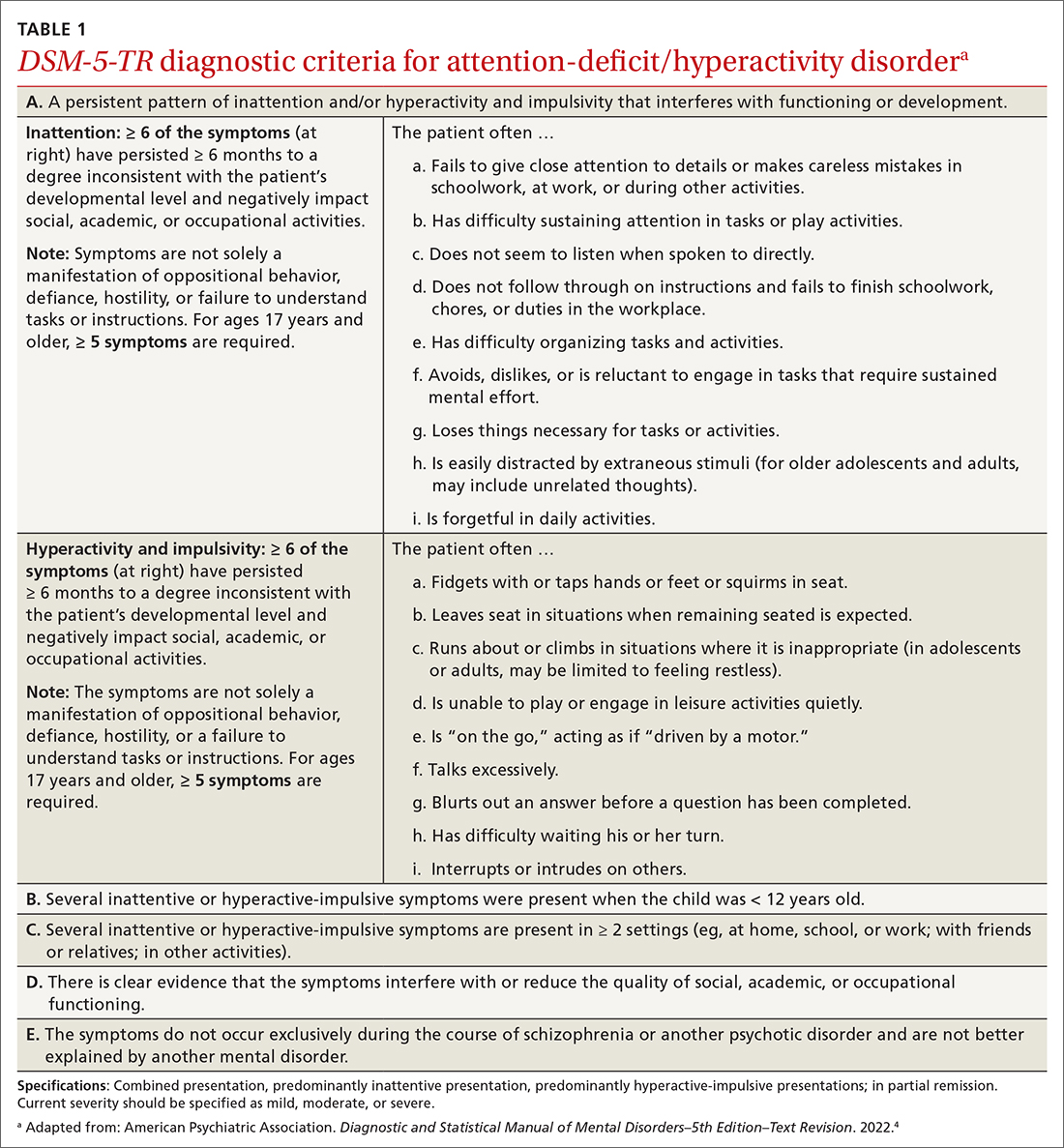
The rate of heritability is high, with significant incidence among first-degree relatives.4 Children with ADHD show executive functioning deficits in 1 or more cognitive domains (eg, visuospatial, memory, inhibitions, decision making, and reward regulation).4,5 The prevalence of ADHD nationally is approximately 9.8% (2.2%, ages 3-5 years; 10%, ages 6-11 years; 13.2%, ages 12-17 years) in children and adolescents; worldwide prevalence is 7.2%.1,6 It persists among 2.6% to 6.8% of adults worldwide.7
Research has shown that boys ages 6 to 11 years are significantly more likely than girls to exhibit attention-getting, externalizing behaviors or conduct problems (eg, hyperactivity, impulsivity, disruption, aggression).1,6 On the other hand, girls ages 12 to 17 years tend to display internalized (eg, depressed mood, anxiety, low self-esteem) or inattentive behaviors, which clinicians and educators may assess as less severe and warranting fewer supportive measures.1
The prevalence of ADHD and its associated factors, which evolve through maturation, underscore the importance of persistent, patient-centered, and collaborative PCP and IBHC clinical management.
Continue to: Begin with a screening tool, move to a clinical interview
Begin with a screening tool, move to a clinical interview
When caregivers express concerns about their child’s behavior, focus, mood, learning, and socialization, consider initiating a multimodal evaluation for ADHD.5,8 Embarking on an ADHD assessment can require extended or multiple visits to arrive at the diagnosis, followed by still more visits to confirm a course of care and adjust medications. The integrative care approach described in the patient case and elaborated on later in this article can help facilitate assessment and treatment of ADHD.9
Signs of ADHD may be observed at initial screening using a tool such as the Ages & Stages Questionnaire (https://agesandstages.com/products-pricing/asq3/) to reveal indications of norm deviations or delays commensurate with ADHD.10 However, to substantiate the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision criteria for an accurate diagnosis,4 the American Academy of Pediatrics (AAP) clinical practice guidelines require a thorough clinical interview, administration of a standardized assessment tool, and review of objective reports in conjunction with a physical examination and psychosocial evaluation.6 Standardized measures of psychological, neurocognitive, and academic achievement reported by caregivers and collateral contacts (eg, teachers, counselors, coaches, care providers) are needed to maximize data objectivity and symptom accuracy across settings (TABLE 210-17). Additionally, periodic reassessment is recommended to validate changes in diagnostic subtype and treatment plans due to the chronic and dynamic nature of ADHD.
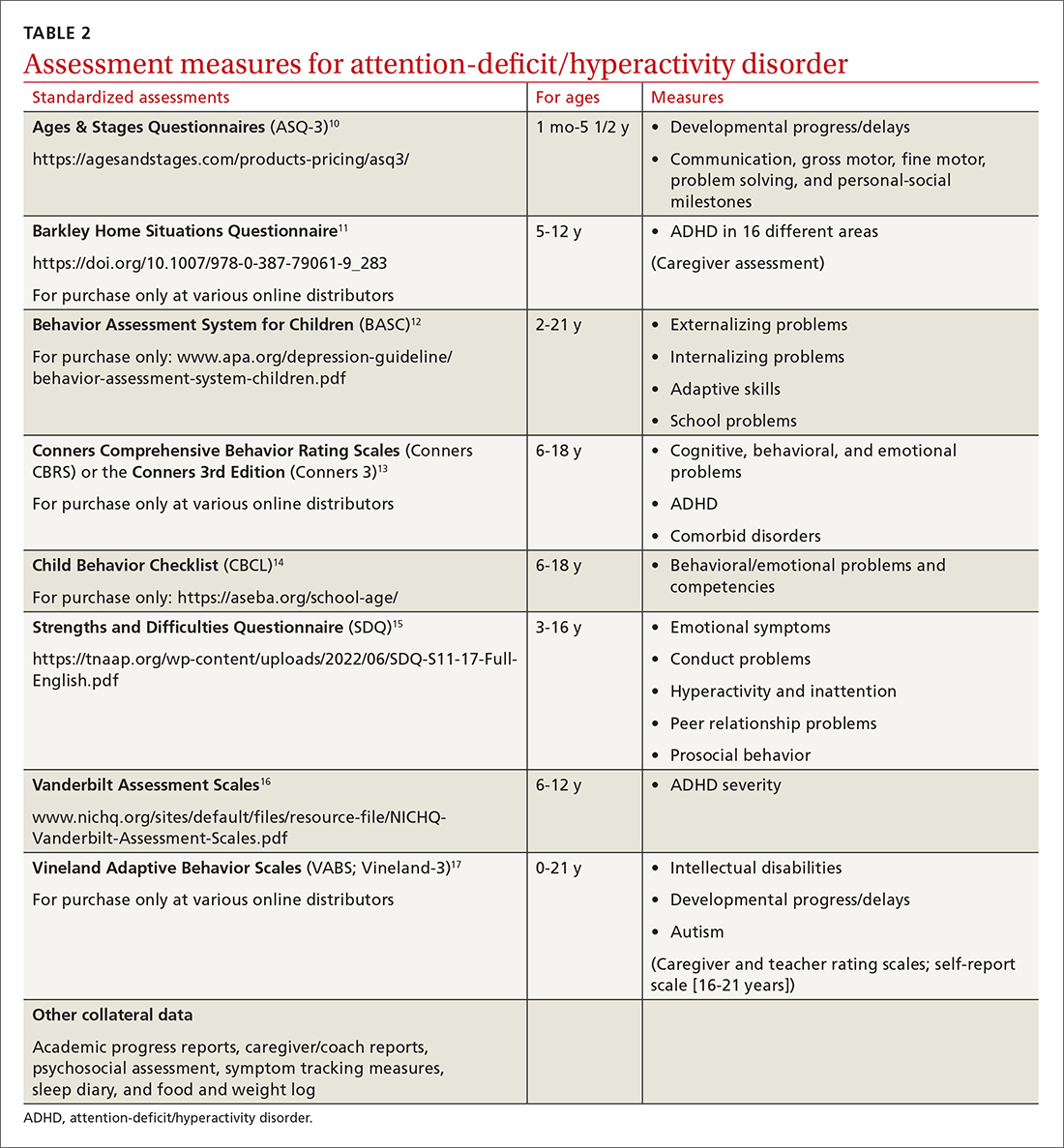
Consider comorbidities and alternate diagnoses
The diagnostic possibility of ADHD should also prompt consideration of other childhood disorders due to the high potential for comorbidities.4,6 In a 2016 study, approximately 64% of
Various medical disorders may manifest with similar signs or symptoms to ADHD, such as thyroid disorders, seizure disorders, adverse drug effects, anemia, genetic anomalies, and others.6,19
If there are behavioral concerns or developmental delays associated with tall stature for age or pubertal or testicular development anomalies, consult a geneticist and a developmental pediatrician for targeted testing and neurodevelopmental assessment, respectively. For example, ADHD is a common comorbidity among boys who also have XYY syndrome (Jacobs syndrome). However, due to the variability of symptoms and severity, XYY syndrome often goes undiagnosed, leaving a host of compounding pervasive and developmental problems untreated. Overall, more than two-thirds of patients with ADHD and a co-occurring condition are either inaccurately diagnosed or not referred for additional assessment and adjunct treatment.21
Continue to: Risks that arise over time
Risks that arise over time. As ADHD persists, adolescents are at greater risk for psychiatric comorbidities, suicidality, and functional impairments (eg, risky behaviors, occupational problems, truancy, delinquency, and poor self-esteem).4,8 Adolescents with internalized behaviors are more likely to experience comorbid depressive disorders with increased risk for self-harm.4,5,8 As adolescents age and their sense of autonomy increases, there is a tendency among those who have received a diagnosis of ADHD to minimize symptoms and decrease the frequency of routine clinic visits along with medication use and treatment compliance.3 Additionally, abuse, misuse, and misappropriation of stimulants among teens and young adults are commonplace.
Wide-scope, multidisciplinary evaluation and close clinical management reduce the potential for imprecise diagnoses, particularly at critical developmental junctures. AAP suggests that PCPs can treat mild and moderate cases of ADHD, but if the treating clinician does not have adequate training, experience, time, or clinical support to manage this condition, early referral is warranted.6
A guide to pharmacotherapy
Approximately 77% of children ages 2 to 17 years with a diagnosis of ADHD receive any form of treatment.2 Treatment for ADHD can include behavioral therapy and medication.2 AAP clinical practice guidelines caution against prescribing medications for children younger than 6 years, relying instead on caregiver-, teacher-, or clinician-administered behavioral strategies and parental training in behavioral modification. For children and adolescents between ages 6 and 18 years, first-line treatment includes pharmacotherapy balanced with behavioral therapy, academic modifications, and educational supports (eg, 504 Plan, individualized education plan [IEP]).6
Psychostimulants are preferred. These agents (eg, methylphenidate, amphetamine) remain the most efficacious class of medications to reduce hyperactivity and inattentiveness and to improve function. While long-acting psychostimulants are associated with better medication adherence and adverse-effect tolerance than are short-acting forms, the latter offer more flexibility in dosing. Start by titrating any stimulant to the lowest effective dose; reassess monthly until potential rebound effects stabilize.
Due to potential adverse effects of this class of medication, screen for any family history or personal risk for structural or electrical cardiac anomalies before starting pharmacotherapy. If any such risks exist, arrange for further cardiac evaluation before initiating medication.6 Adverse effects of stimulants include reduced appetite, gastrointestinal symptoms, headaches, anxiousness, parasomnia, tachycardia, and hypertension.
Continue to: Once medication is stabilized...
Once medication is stabilized, monitor treatment 2 to 3 times per year thereafter; watch for longer-term adverse effects such as weight loss, decreased growth rate, and psychiatric comorbidities including the Food and Drug Administration (FDA)’s black box warning of increased risk for suicidality.5,6,22
Other options. The optimal duration of psychostimulant use remains debatable, as existing evidence does not support its long-term use (10 years) over other interventions, such as nonstimulants and nonmedicinal therapies.22 Although backed by less evidence, additional medications indicated for the treatment of ADHD include: (1) atomoxetine, a selective norepinephrine reuptake inhibitor, and (2) the selective alpha-2 adrenergic agonists, extended-release guanfacine and extended-release clonidine (third-line agent).22
Adverse effects of these FDA-approved medications are similar to those observed in stimulant medications. Evaluation of cardiac risks is recommended before starting nonstimulant medications. The alpha-2 adrenergic agonists may also be used as adjunct therapies to stimulants. Before stopping an alpha-2 adrenergic agonist, taper the dosage slowly to avoid the risk for rebound hypertension.6,23 Given the wide variety of medication options and variability of effects, it may be necessary to try different medications as children grow and their symptoms and capacity to manage them change. Additional guidance on FDA-approved medications is available at www.ADHDMedicationGuide.com.
How multilevel care coordination can work
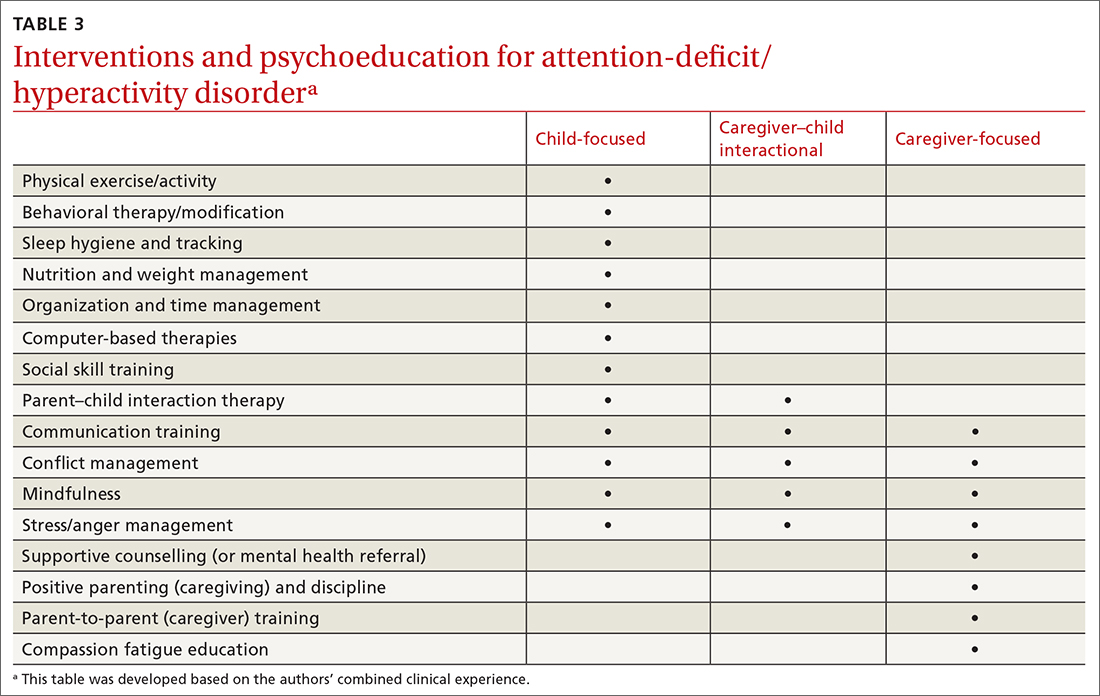
As with other chronic or developmental conditions, the treatment of ADHD requires an interdisciplinary perspective. Continuous, comprehensive case management can help patients overcome obstacles to wellness by balancing the resolution of problems with the development of resilience. Well-documented collaboration of subspecialists, educators, and other stakeholders engaged in ADHD care at multiple levels (individual, family, community, and health care system) increases the likelihood of meaningful, sustainable gains. Using a patient-centered medical home framework, IBHCs or other allied health professionals embedded in, or co-located with, primary care settings can be key to accessing evidence-based treatments that include: psycho-education and mindfulness-based stress reduction training for caregivers24,25; occupational,26 cognitive behavioral,27 or family therapies28,29; neuro-feedback; computer-based attention training; group- or community-based interventions; and academic and social supports.5,8
Treatment approaches that capitalize on children’s neurologic and psychological plasticity and fortify self-efficacy with developmentally appropriate tools empower them to surmount ADHD symptoms over time.23 Facilitating children’s resilience within a developmental framework and health system’s capacities with socio-culturally relevant approaches, consultation, and research can optimize outcomes and mitigate pervasiveness into adulthood. While the patient is at the center of treatment, it is important to consider the family, school, and communities in which the child lives, learns, and plays. PCPs and IBHCs together can consider a “try and track” method to follow progress, changes, and outcomes over time. With this method, the physician can employ approaches that focus on the patient, caregiver, or the caregiver–child interaction (TABLE 3).
Continue to: Assess patients' needs and the resources available
Assess patients’ needs and the resources available throughout the system of care beyond the primary care setting. Stay abreast of hospital policies, health care insurance coverage, and community- and school-based health programs, and any gaps in adequate and equitable assessment and treatment. For example, while clinical recommendations include psychiatric care, health insurance availability or limits in coverage may dissuade caregivers from seeking help or limit initial or long-term access to resources for help.30 Integrating or advocating for clinic support resources or staffing to assist patients in navigating and mitigating challenges may lessen the management burden and increase the likelihood and longevity of favorable health outcomes.
Steps to ensuring health care equity
Among children of historically marginalized and racial and ethnic minority groups or those of populations affected by health disparities, ADHD symptoms and needs are often masked by structural biases that lead to inequitable care and outcomes, as well as treatment misprioritization or delays.31 In particular, evidence has shown that recognition and diagnostic specificity of ADHD and comorbidities, not prevalence, vary more widely among minority than among nonminority populations,32 contributing to the 23% of children with ADHD who receive no treatment at all.2
Understand caregiver concerns. This diagnosis discrepancy is correlated with symptom rating sensitivities (eg, reliability, perception, accuracy) among informants and how caregivers observe, perceive, appreciate, understand, and report behaviors. This discrepancy is also related to cultural belief differences, physician–patient communication variants, and a litany of other socioeconomic determinants.2,4,31 Caregivers from some cultural, ethnic, or socioeconomic backgrounds may be doubtful of psychiatric assessment, diagnoses, treatment, or medication, and that can impact how children are engaged in clinical and educational settings from the outset.31 In the case we described, James’ mother was initially hesitant to explore psychotropic medications and was concerned about stigmatization within the school system. She also seemed to avoid psychiatric treatment for her own depressive symptoms due to cultural and religious beliefs.
Health care provider concerns. Some PCPs may hesitate to explore medications due to limited knowledge and skill in dosing and titrating based on a child’s age, stage, and symptoms, and a perceived lack of competence in managing ADHD. This, too, can indirectly perpetuate existing health disparities. Furthermore, ADHD symptoms may be deemed a secondary or tertiary concern if other complex or urgent medical or undifferentiated developmental problems manifest.
Compounding matters is the limited dissemination of empiric research articles (including randomized controlled trials with representative samples) and limited education on the effectiveness and safety of psychopharmacologic interventions across the lifespan and different cultural and ethnic groups.4 Consequently, patients who struggle with unmanaged ADHD symptoms are more likely to have chronic mental health disorders, maladaptive behaviors, and other co-occurring conditions contributing to the complexity of individual needs, health care burdens, or justice system involvement; this is particularly true for those of racial and ethnic minorities.33
Continue to: Impact of the COVID-19 pandemic
Impact of the COVID-19 pandemic. Patients—particularly those in minority or health disparity populations—who under normal circumstances might have been hesitant to seek help may have felt even more reluctant to do so during the COVID-19 pandemic. We have not yet learned the degree to which limited availability of preventive health care services, decreased routine visits, and fluctuating insurance coverage has impacted the diagnosis, management, or severity of childhood disorders during the past 2 years. Reports of national findings indicate that prolonged periods out of school and reduced daily structure were associated with increased disruptions in mood, sleep, and appetite, particularly among children with pre-existing pathologies. Evidence suggests that school-aged children experienced more anxiety, regressive behaviors, and parasomnias than they did before the pandemic, while adolescents experienced more isolation and depressive symptoms.34,35
However, there remains a paucity of large-scale or representative studies that use an intersectional lens to examine the influence of COVID-19 on children with ADHD. Therefore, PCPs and IBHCs should refocus attention on possibly undiagnosed, stagnated, or regressed ADHD cases, as well as the adults who care for them. (See “5 ways to overcome Tx barriers and promote health equity.”)
SIDEBAR
5 ways to overcome Tx barriers and promote health equitya
1. Inquire about cultural or ethnic beliefs and behaviors and socioeconomic barriers.
2. Establish trust or assuage mistrust by exploring and dispelling misinformation.
3. Offer accessible, feasible, and sustainable evidence-based interventions.
4. Encourage autonomy and selfdetermination throughout the health care process.
5. Connect caregivers and children with clinical, community, and school-based resources and coordinators.
a These recommendations are based on the authors’ combined clinical experience.
THE CASE
During a follow-up visit 1 month later, the PCP confirmed the clinical impression of ADHD combined presentation with a clinical interview and review of the Strengths and Difficulties Questionnaire completed by James’ mother and the Vanderbilt Assessment Scales completed by James’ mother and teacher. The sleep diary indicated potential problems and apneas worthy of consults for pulmonary function testing, a sleep study, and otolaryngology examination. The PCP informed James’ mother on sleep hygiene strategies and ADHD medication options. She indicated that she wanted to pursue the referrals and behavioral modifications before starting any medication trial.
The PCP referred James to a developmental pediatrician for in-depth assessment of his overall development, learning, and functioning. The developmental pediatrician ultimately confirmed the diagnosis of ADHD, as well as motor and speech delays warranting physical, occupational, and speech therapies. The developmental pediatrician also referred James for targeted genetic testing because she suspected a genetic disorder (eg, XYY syndrome).
The PCP reconnected James and his mother to the IBHC to facilitate subspecialty and school-based care coordination and to provide in-office and home-based interventions. The IBHC assessed James’ emotional dysregulation and impulsivity as adversely impacting his interpersonal relationships and planned to address these issues with behavioral and parent–child interaction therapies and skills training during the course of 6 to 12 visits. James’ mother was encouraged to engage his teacher on his academic performance and to initiate a 504 Plan or IEP for in-school accommodations and support. The IBHC aided in tracking his assessments, referrals, follow-ups, access barriers, and treatment goals.
After 6 months, James had made only modest progress, and his mother requested that he begin a trial of medication. Based on his weight, symptoms, behavior patterns, and sleep habits, the PCP prescribed extended-release dexmethylphenidate 10 mg each morning, then extended-release clonidine 0.1 mg nightly. With team-based clinical management of pharmacologic, behavioral, physical, speech, and occupational therapies, James’ behavior and sleep improved, and the signs of a vocal tic diminished.
By the next school year, James demonstrated a marked improvement in impulse control, attention, and academic functioning. He followed up with the PCP at least quarterly for reassessment of his symptoms, growth, and experience of adverse effects, and to titrate medications accordingly. James and his mother continued to work closely with the IBHC monthly to engage interventions and to monitor his progress at home and school.
CORRESPONDENCE
Sundania J. W. Wonnum, PhD, LCSW, National Institute on Minority Health and Health Disparities, 6707 Democracy Boulevard, Suite 800, Bethesda, MD 20892; sundania.wonnum@nih.gov
1. Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children—United States, 2013-2019. MMWR Suppl. 2022;71:1-42. doi: 10.15585/mmwr.su7102a1
2. Danielson ML, Holbrook JR, Blumberg SJ, et al. State-level estimates of the prevalence of parent-reported ADHD diagnosis and treatment among U.S. children and adolescents, 2016 to 2019. J Atten Disord. 2022;26:1685-1697. doi: 10.1177/10870547221099961
3. Faraone SV, Banaschewski T, Coghill D, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789-818. doi: 10.1016/j.neubiorev.2021.01.022
4. American Psychiatric Association
5. Brahmbhatt K, Hilty DM, Mina H, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143. doi: 10.1016/j.jadohealth.2016.03.025
6. Wolraich ML, Hagan JF, Allan C, et al. AAP Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528
7. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
8. Chang JG, Cimino FM, Gossa W. ADHD in children: common questions and answers. Am Fam Physician. 2020;102:592-602.
9. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169:929-937. doi: 10.1001/jamapediatrics.2015.1141
10. Squires J, Bricker D. Ages & Stages Questionnaires®. 3rd ed (ASQ®-3). Paul H. Brookes Publishing Co., Inc; 2009.
11. DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. J Clin Child Psychol. 1992;21:178-188. doi.org/10.1207/s15374424jccp2102_10
12. Merenda PF. BASC: behavior assessment system for children. Meas Eval Counsel Develop. 1996;28:229-232.
13. Conners CK. Conners, 3rd ed manual. Multi-Health Systems. 2008.
14. Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; 1999:429-466.
15. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791-799.
16. Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559-567. doi: 10.1093/jpepsy/jsg046
17. Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, ed. Major Psychological Assessment Instruments. Vol 2. Allyn & Bacon; 2003:199-231.
18. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199-212. doi: 10.1080/15374416.2017.1417860
19. Ghriwati NA, Langberg JM, Gardner W, et al. Impact of mental health comorbidities on the community-based pediatric treatment and outcomes of children with attention deficit hyperactivity disorder. J Dev Behav Ped. 2017;38:20-28. doi: 10.1097/DBP.0000000000000359
20. Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish Cohort. Int J Ped Otorhinolaryngol. 2016;84:12-20. doi: 10.1016/j.ijporl.2016.02.017
21. Ross JL Roeltgen DP Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. doi: 10.1542/peds.2011-0719
22. Mechler K, Banaschewski T, Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940
23. Mishra J, Merzenich MM, Sagar R. Accessible online neuroplasticity-targeted training for children with ADHD. Child Adolesc Psychiatry Ment Health. 2013;7:38. doi: 10.1186/1753-2000-7-38
24. Neece CL. Mindfulness-based stress reduction for parents of young children with developmental delays: implications for parental mental health and child behavior problems. J Applied Res Intellect Disabil. 2014;27:174-186. doi: 10.1111/jar.12064
25. Petcharat M, Liehr P. Mindfulness training for parents of children with special needs: guidance for nurses in mental health practice. J Child Adolesc Psychiatr Nursing. 2017;30:35-46. doi: 10.1111/jcap.12169
26. Hahn-Markowitz J, Burger I, Manor I, et al. Efficacy of cognitive-functional (Cog-Fun) occupational therapy intervention among children with ADHD: an RCT. J Atten Disord. 2020;24:655-666. doi: 10.1177/1087054716666955
27. Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Atten Disord. 2020;24:875-888.
28. Carr AW, Bean RA, Nelson KF. Childhood attention-deficit hyperactivity disorder: family therapy from an attachment based perspective. Child Youth Serv Rev. 2020;119:105666.
29. Robin AL. Family therapy for adolescents with ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23:747-756. doi: 10.1016/j.chc.2014.06.001
30. Cattoi B, Alpern I, Katz JS, et al. The adverse health outcomes, economic burden, and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): a call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J Atten Disord. 2022;26:807-808. doi: 10.1177/10870547211036754
31. Hinojosa MS, Hinojosa R, Nguyen J. Shared decision making and treatment for minority children with ADHD. J Transcult Nurs. 2020;31:135-143. doi: 10.1177/1043659619853021
32. Slobodin O, Masalha R. Challenges in ADHD care for ethnic minority children: a review of the current literature. Transcult Psychiatry. 2020;57:468-483. doi: 10.1177/1363461520902885
33. Retz W, Ginsberg Y, Turner D, et al. Attention-deficit/hyperactivity disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236-248. doi: 10.1016/j.neubiorev.2020.11.025
34. Del Sol Calderon P, Izquierdo A, Garcia Moreno M. Effects of the pandemic on the mental health of children and adolescents. Review and current scientific evidence of the SARS-COV2 pandemic. Eur Psychiatry. 2021;64:S223-S224. doi: 10.1192/j.eurpsy.2021.597
35. Insa I, Alda JA. Attention deficit hyperactivity disorder (ADHD) & COVID-19: attention deficit hyperactivity disorder: consequences of the 1st wave. Eur Psychiatry. 2021;64:S660. doi: 10.1192/j.eurpsy.2021.1752
THE CASE
James B* is a 7-year-old Black child who presented to his primary care physician (PCP) for a well-child visit. During preventive health screening, James’ mother expressed concerns about his behavior, characterizing him as immature, aggressive, destructive, and occasionally self-loathing. She described him as physically uncoordinated, struggling to keep up with his peers in sports, and tiring after 20 minutes of activity. James slept 10 hours nightly but was often restless and snored intermittently. As a second grader, his academic achievement was not progressing, and he had become increasingly inattentive at home and at school. James’ mother offered several examples of his fighting with his siblings, noncompliance with morning routines, and avoidance of learning activities. Additionally, his mother expressed concern that James, as a Black child, might eventually be unfairly labeled as a problem child by his teachers or held back a grade level in school.
Although James did not have a family history of developmental delays or learning disorders, he had not met any milestones on time for gross or fine motor, language, cognitive, and social-emotional skills. James had a history of chronic otitis media, for which pressure equalizer tubes were inserted at age 2 years. He had not had any major physical injuries, psychological trauma, recent life transitions, or adverse childhood events. When asked, James’ mother acknowledged symptoms of maternal depression but alluded to faith-based reasons for not seeking treatment for herself.
James’ physical examination was unremarkable. His height, weight, and vitals were all within normal limits. However, he had some difficulty with verbal articulation and expression and showed signs of a possible vocal tic. Based on James’ presentation, his PCP suspected attention-deficit/hyperactivity disorder (ADHD), as well as neurodevelopmental delays.
The PCP gave James’ mother the Strengths and Difficulties Questionnaire to complete and the Vanderbilt Assessment Scales for her and James’ teacher to fill out independently and return to the clinic. The PCP also instructed James’ mother on how to use a sleep diary to maintain a 1-month log of his sleep patterns and habits. The PCP consulted the integrated behavioral health clinician (IBHC; a clinical social worker embedded in the primary care clinic) and made a warm handoff for the IBHC to further assess James’ maladaptive behaviors and interactions.
●
* The patient’s name has been changed to protect his identity.
James is one of more than 6 million children, ages 3 to 17 years, in the United States who live with ADHD.1,2 ADHD is the most common neurodevelopmental disorder among children, and it affects multiple cognitive and behavioral domains throughout the lifespan.3 Children with ADHD often initially present in primary care settings; thus, PCPs are well positioned to diagnose the disorder and provide longitudinal treatment. This Behavioral Health Consult reviews clinical assessment and practice guidelines, as well as treatment recommendations applicable across different areas of influence—individual, family, community, and systems—for PCPs and IBHCs to use in managing ADHD in children.
ADHD features can vary by age and sex
ADHD is a persistent pattern of inattention or hyperactivity and impulsivity interfering with functioning or development in childhood and functioning later in adulthood. ADHD symptoms manifest prior to age 12 years and must occur in 2 or more settings.4 Symptoms should not be better explained by another psychiatric disorder or occur exclusively during the course of another disorder (TABLE 1).4

The rate of heritability is high, with significant incidence among first-degree relatives.4 Children with ADHD show executive functioning deficits in 1 or more cognitive domains (eg, visuospatial, memory, inhibitions, decision making, and reward regulation).4,5 The prevalence of ADHD nationally is approximately 9.8% (2.2%, ages 3-5 years; 10%, ages 6-11 years; 13.2%, ages 12-17 years) in children and adolescents; worldwide prevalence is 7.2%.1,6 It persists among 2.6% to 6.8% of adults worldwide.7
Research has shown that boys ages 6 to 11 years are significantly more likely than girls to exhibit attention-getting, externalizing behaviors or conduct problems (eg, hyperactivity, impulsivity, disruption, aggression).1,6 On the other hand, girls ages 12 to 17 years tend to display internalized (eg, depressed mood, anxiety, low self-esteem) or inattentive behaviors, which clinicians and educators may assess as less severe and warranting fewer supportive measures.1
The prevalence of ADHD and its associated factors, which evolve through maturation, underscore the importance of persistent, patient-centered, and collaborative PCP and IBHC clinical management.
Continue to: Begin with a screening tool, move to a clinical interview
Begin with a screening tool, move to a clinical interview
When caregivers express concerns about their child’s behavior, focus, mood, learning, and socialization, consider initiating a multimodal evaluation for ADHD.5,8 Embarking on an ADHD assessment can require extended or multiple visits to arrive at the diagnosis, followed by still more visits to confirm a course of care and adjust medications. The integrative care approach described in the patient case and elaborated on later in this article can help facilitate assessment and treatment of ADHD.9
Signs of ADHD may be observed at initial screening using a tool such as the Ages & Stages Questionnaire (https://agesandstages.com/products-pricing/asq3/) to reveal indications of norm deviations or delays commensurate with ADHD.10 However, to substantiate the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision criteria for an accurate diagnosis,4 the American Academy of Pediatrics (AAP) clinical practice guidelines require a thorough clinical interview, administration of a standardized assessment tool, and review of objective reports in conjunction with a physical examination and psychosocial evaluation.6 Standardized measures of psychological, neurocognitive, and academic achievement reported by caregivers and collateral contacts (eg, teachers, counselors, coaches, care providers) are needed to maximize data objectivity and symptom accuracy across settings (TABLE 210-17). Additionally, periodic reassessment is recommended to validate changes in diagnostic subtype and treatment plans due to the chronic and dynamic nature of ADHD.

Consider comorbidities and alternate diagnoses
The diagnostic possibility of ADHD should also prompt consideration of other childhood disorders due to the high potential for comorbidities.4,6 In a 2016 study, approximately 64% of
Various medical disorders may manifest with similar signs or symptoms to ADHD, such as thyroid disorders, seizure disorders, adverse drug effects, anemia, genetic anomalies, and others.6,19
If there are behavioral concerns or developmental delays associated with tall stature for age or pubertal or testicular development anomalies, consult a geneticist and a developmental pediatrician for targeted testing and neurodevelopmental assessment, respectively. For example, ADHD is a common comorbidity among boys who also have XYY syndrome (Jacobs syndrome). However, due to the variability of symptoms and severity, XYY syndrome often goes undiagnosed, leaving a host of compounding pervasive and developmental problems untreated. Overall, more than two-thirds of patients with ADHD and a co-occurring condition are either inaccurately diagnosed or not referred for additional assessment and adjunct treatment.21
Continue to: Risks that arise over time
Risks that arise over time. As ADHD persists, adolescents are at greater risk for psychiatric comorbidities, suicidality, and functional impairments (eg, risky behaviors, occupational problems, truancy, delinquency, and poor self-esteem).4,8 Adolescents with internalized behaviors are more likely to experience comorbid depressive disorders with increased risk for self-harm.4,5,8 As adolescents age and their sense of autonomy increases, there is a tendency among those who have received a diagnosis of ADHD to minimize symptoms and decrease the frequency of routine clinic visits along with medication use and treatment compliance.3 Additionally, abuse, misuse, and misappropriation of stimulants among teens and young adults are commonplace.
Wide-scope, multidisciplinary evaluation and close clinical management reduce the potential for imprecise diagnoses, particularly at critical developmental junctures. AAP suggests that PCPs can treat mild and moderate cases of ADHD, but if the treating clinician does not have adequate training, experience, time, or clinical support to manage this condition, early referral is warranted.6
A guide to pharmacotherapy
Approximately 77% of children ages 2 to 17 years with a diagnosis of ADHD receive any form of treatment.2 Treatment for ADHD can include behavioral therapy and medication.2 AAP clinical practice guidelines caution against prescribing medications for children younger than 6 years, relying instead on caregiver-, teacher-, or clinician-administered behavioral strategies and parental training in behavioral modification. For children and adolescents between ages 6 and 18 years, first-line treatment includes pharmacotherapy balanced with behavioral therapy, academic modifications, and educational supports (eg, 504 Plan, individualized education plan [IEP]).6
Psychostimulants are preferred. These agents (eg, methylphenidate, amphetamine) remain the most efficacious class of medications to reduce hyperactivity and inattentiveness and to improve function. While long-acting psychostimulants are associated with better medication adherence and adverse-effect tolerance than are short-acting forms, the latter offer more flexibility in dosing. Start by titrating any stimulant to the lowest effective dose; reassess monthly until potential rebound effects stabilize.
Due to potential adverse effects of this class of medication, screen for any family history or personal risk for structural or electrical cardiac anomalies before starting pharmacotherapy. If any such risks exist, arrange for further cardiac evaluation before initiating medication.6 Adverse effects of stimulants include reduced appetite, gastrointestinal symptoms, headaches, anxiousness, parasomnia, tachycardia, and hypertension.
Continue to: Once medication is stabilized...
Once medication is stabilized, monitor treatment 2 to 3 times per year thereafter; watch for longer-term adverse effects such as weight loss, decreased growth rate, and psychiatric comorbidities including the Food and Drug Administration (FDA)’s black box warning of increased risk for suicidality.5,6,22
Other options. The optimal duration of psychostimulant use remains debatable, as existing evidence does not support its long-term use (10 years) over other interventions, such as nonstimulants and nonmedicinal therapies.22 Although backed by less evidence, additional medications indicated for the treatment of ADHD include: (1) atomoxetine, a selective norepinephrine reuptake inhibitor, and (2) the selective alpha-2 adrenergic agonists, extended-release guanfacine and extended-release clonidine (third-line agent).22
Adverse effects of these FDA-approved medications are similar to those observed in stimulant medications. Evaluation of cardiac risks is recommended before starting nonstimulant medications. The alpha-2 adrenergic agonists may also be used as adjunct therapies to stimulants. Before stopping an alpha-2 adrenergic agonist, taper the dosage slowly to avoid the risk for rebound hypertension.6,23 Given the wide variety of medication options and variability of effects, it may be necessary to try different medications as children grow and their symptoms and capacity to manage them change. Additional guidance on FDA-approved medications is available at www.ADHDMedicationGuide.com.
How multilevel care coordination can work
As with other chronic or developmental conditions, the treatment of ADHD requires an interdisciplinary perspective. Continuous, comprehensive case management can help patients overcome obstacles to wellness by balancing the resolution of problems with the development of resilience. Well-documented collaboration of subspecialists, educators, and other stakeholders engaged in ADHD care at multiple levels (individual, family, community, and health care system) increases the likelihood of meaningful, sustainable gains. Using a patient-centered medical home framework, IBHCs or other allied health professionals embedded in, or co-located with, primary care settings can be key to accessing evidence-based treatments that include: psycho-education and mindfulness-based stress reduction training for caregivers24,25; occupational,26 cognitive behavioral,27 or family therapies28,29; neuro-feedback; computer-based attention training; group- or community-based interventions; and academic and social supports.5,8
Treatment approaches that capitalize on children’s neurologic and psychological plasticity and fortify self-efficacy with developmentally appropriate tools empower them to surmount ADHD symptoms over time.23 Facilitating children’s resilience within a developmental framework and health system’s capacities with socio-culturally relevant approaches, consultation, and research can optimize outcomes and mitigate pervasiveness into adulthood. While the patient is at the center of treatment, it is important to consider the family, school, and communities in which the child lives, learns, and plays. PCPs and IBHCs together can consider a “try and track” method to follow progress, changes, and outcomes over time. With this method, the physician can employ approaches that focus on the patient, caregiver, or the caregiver–child interaction (TABLE 3).

Continue to: Assess patients' needs and the resources available
Assess patients’ needs and the resources available throughout the system of care beyond the primary care setting. Stay abreast of hospital policies, health care insurance coverage, and community- and school-based health programs, and any gaps in adequate and equitable assessment and treatment. For example, while clinical recommendations include psychiatric care, health insurance availability or limits in coverage may dissuade caregivers from seeking help or limit initial or long-term access to resources for help.30 Integrating or advocating for clinic support resources or staffing to assist patients in navigating and mitigating challenges may lessen the management burden and increase the likelihood and longevity of favorable health outcomes.
Steps to ensuring health care equity
Among children of historically marginalized and racial and ethnic minority groups or those of populations affected by health disparities, ADHD symptoms and needs are often masked by structural biases that lead to inequitable care and outcomes, as well as treatment misprioritization or delays.31 In particular, evidence has shown that recognition and diagnostic specificity of ADHD and comorbidities, not prevalence, vary more widely among minority than among nonminority populations,32 contributing to the 23% of children with ADHD who receive no treatment at all.2
Understand caregiver concerns. This diagnosis discrepancy is correlated with symptom rating sensitivities (eg, reliability, perception, accuracy) among informants and how caregivers observe, perceive, appreciate, understand, and report behaviors. This discrepancy is also related to cultural belief differences, physician–patient communication variants, and a litany of other socioeconomic determinants.2,4,31 Caregivers from some cultural, ethnic, or socioeconomic backgrounds may be doubtful of psychiatric assessment, diagnoses, treatment, or medication, and that can impact how children are engaged in clinical and educational settings from the outset.31 In the case we described, James’ mother was initially hesitant to explore psychotropic medications and was concerned about stigmatization within the school system. She also seemed to avoid psychiatric treatment for her own depressive symptoms due to cultural and religious beliefs.
Health care provider concerns. Some PCPs may hesitate to explore medications due to limited knowledge and skill in dosing and titrating based on a child’s age, stage, and symptoms, and a perceived lack of competence in managing ADHD. This, too, can indirectly perpetuate existing health disparities. Furthermore, ADHD symptoms may be deemed a secondary or tertiary concern if other complex or urgent medical or undifferentiated developmental problems manifest.
Compounding matters is the limited dissemination of empiric research articles (including randomized controlled trials with representative samples) and limited education on the effectiveness and safety of psychopharmacologic interventions across the lifespan and different cultural and ethnic groups.4 Consequently, patients who struggle with unmanaged ADHD symptoms are more likely to have chronic mental health disorders, maladaptive behaviors, and other co-occurring conditions contributing to the complexity of individual needs, health care burdens, or justice system involvement; this is particularly true for those of racial and ethnic minorities.33
Continue to: Impact of the COVID-19 pandemic
Impact of the COVID-19 pandemic. Patients—particularly those in minority or health disparity populations—who under normal circumstances might have been hesitant to seek help may have felt even more reluctant to do so during the COVID-19 pandemic. We have not yet learned the degree to which limited availability of preventive health care services, decreased routine visits, and fluctuating insurance coverage has impacted the diagnosis, management, or severity of childhood disorders during the past 2 years. Reports of national findings indicate that prolonged periods out of school and reduced daily structure were associated with increased disruptions in mood, sleep, and appetite, particularly among children with pre-existing pathologies. Evidence suggests that school-aged children experienced more anxiety, regressive behaviors, and parasomnias than they did before the pandemic, while adolescents experienced more isolation and depressive symptoms.34,35
However, there remains a paucity of large-scale or representative studies that use an intersectional lens to examine the influence of COVID-19 on children with ADHD. Therefore, PCPs and IBHCs should refocus attention on possibly undiagnosed, stagnated, or regressed ADHD cases, as well as the adults who care for them. (See “5 ways to overcome Tx barriers and promote health equity.”)
SIDEBAR
5 ways to overcome Tx barriers and promote health equitya
1. Inquire about cultural or ethnic beliefs and behaviors and socioeconomic barriers.
2. Establish trust or assuage mistrust by exploring and dispelling misinformation.
3. Offer accessible, feasible, and sustainable evidence-based interventions.
4. Encourage autonomy and selfdetermination throughout the health care process.
5. Connect caregivers and children with clinical, community, and school-based resources and coordinators.
a These recommendations are based on the authors’ combined clinical experience.
THE CASE
During a follow-up visit 1 month later, the PCP confirmed the clinical impression of ADHD combined presentation with a clinical interview and review of the Strengths and Difficulties Questionnaire completed by James’ mother and the Vanderbilt Assessment Scales completed by James’ mother and teacher. The sleep diary indicated potential problems and apneas worthy of consults for pulmonary function testing, a sleep study, and otolaryngology examination. The PCP informed James’ mother on sleep hygiene strategies and ADHD medication options. She indicated that she wanted to pursue the referrals and behavioral modifications before starting any medication trial.
The PCP referred James to a developmental pediatrician for in-depth assessment of his overall development, learning, and functioning. The developmental pediatrician ultimately confirmed the diagnosis of ADHD, as well as motor and speech delays warranting physical, occupational, and speech therapies. The developmental pediatrician also referred James for targeted genetic testing because she suspected a genetic disorder (eg, XYY syndrome).
The PCP reconnected James and his mother to the IBHC to facilitate subspecialty and school-based care coordination and to provide in-office and home-based interventions. The IBHC assessed James’ emotional dysregulation and impulsivity as adversely impacting his interpersonal relationships and planned to address these issues with behavioral and parent–child interaction therapies and skills training during the course of 6 to 12 visits. James’ mother was encouraged to engage his teacher on his academic performance and to initiate a 504 Plan or IEP for in-school accommodations and support. The IBHC aided in tracking his assessments, referrals, follow-ups, access barriers, and treatment goals.
After 6 months, James had made only modest progress, and his mother requested that he begin a trial of medication. Based on his weight, symptoms, behavior patterns, and sleep habits, the PCP prescribed extended-release dexmethylphenidate 10 mg each morning, then extended-release clonidine 0.1 mg nightly. With team-based clinical management of pharmacologic, behavioral, physical, speech, and occupational therapies, James’ behavior and sleep improved, and the signs of a vocal tic diminished.
By the next school year, James demonstrated a marked improvement in impulse control, attention, and academic functioning. He followed up with the PCP at least quarterly for reassessment of his symptoms, growth, and experience of adverse effects, and to titrate medications accordingly. James and his mother continued to work closely with the IBHC monthly to engage interventions and to monitor his progress at home and school.
CORRESPONDENCE
Sundania J. W. Wonnum, PhD, LCSW, National Institute on Minority Health and Health Disparities, 6707 Democracy Boulevard, Suite 800, Bethesda, MD 20892; sundania.wonnum@nih.gov
THE CASE
James B* is a 7-year-old Black child who presented to his primary care physician (PCP) for a well-child visit. During preventive health screening, James’ mother expressed concerns about his behavior, characterizing him as immature, aggressive, destructive, and occasionally self-loathing. She described him as physically uncoordinated, struggling to keep up with his peers in sports, and tiring after 20 minutes of activity. James slept 10 hours nightly but was often restless and snored intermittently. As a second grader, his academic achievement was not progressing, and he had become increasingly inattentive at home and at school. James’ mother offered several examples of his fighting with his siblings, noncompliance with morning routines, and avoidance of learning activities. Additionally, his mother expressed concern that James, as a Black child, might eventually be unfairly labeled as a problem child by his teachers or held back a grade level in school.
Although James did not have a family history of developmental delays or learning disorders, he had not met any milestones on time for gross or fine motor, language, cognitive, and social-emotional skills. James had a history of chronic otitis media, for which pressure equalizer tubes were inserted at age 2 years. He had not had any major physical injuries, psychological trauma, recent life transitions, or adverse childhood events. When asked, James’ mother acknowledged symptoms of maternal depression but alluded to faith-based reasons for not seeking treatment for herself.
James’ physical examination was unremarkable. His height, weight, and vitals were all within normal limits. However, he had some difficulty with verbal articulation and expression and showed signs of a possible vocal tic. Based on James’ presentation, his PCP suspected attention-deficit/hyperactivity disorder (ADHD), as well as neurodevelopmental delays.
The PCP gave James’ mother the Strengths and Difficulties Questionnaire to complete and the Vanderbilt Assessment Scales for her and James’ teacher to fill out independently and return to the clinic. The PCP also instructed James’ mother on how to use a sleep diary to maintain a 1-month log of his sleep patterns and habits. The PCP consulted the integrated behavioral health clinician (IBHC; a clinical social worker embedded in the primary care clinic) and made a warm handoff for the IBHC to further assess James’ maladaptive behaviors and interactions.
●
* The patient’s name has been changed to protect his identity.
James is one of more than 6 million children, ages 3 to 17 years, in the United States who live with ADHD.1,2 ADHD is the most common neurodevelopmental disorder among children, and it affects multiple cognitive and behavioral domains throughout the lifespan.3 Children with ADHD often initially present in primary care settings; thus, PCPs are well positioned to diagnose the disorder and provide longitudinal treatment. This Behavioral Health Consult reviews clinical assessment and practice guidelines, as well as treatment recommendations applicable across different areas of influence—individual, family, community, and systems—for PCPs and IBHCs to use in managing ADHD in children.
ADHD features can vary by age and sex
ADHD is a persistent pattern of inattention or hyperactivity and impulsivity interfering with functioning or development in childhood and functioning later in adulthood. ADHD symptoms manifest prior to age 12 years and must occur in 2 or more settings.4 Symptoms should not be better explained by another psychiatric disorder or occur exclusively during the course of another disorder (TABLE 1).4

The rate of heritability is high, with significant incidence among first-degree relatives.4 Children with ADHD show executive functioning deficits in 1 or more cognitive domains (eg, visuospatial, memory, inhibitions, decision making, and reward regulation).4,5 The prevalence of ADHD nationally is approximately 9.8% (2.2%, ages 3-5 years; 10%, ages 6-11 years; 13.2%, ages 12-17 years) in children and adolescents; worldwide prevalence is 7.2%.1,6 It persists among 2.6% to 6.8% of adults worldwide.7
Research has shown that boys ages 6 to 11 years are significantly more likely than girls to exhibit attention-getting, externalizing behaviors or conduct problems (eg, hyperactivity, impulsivity, disruption, aggression).1,6 On the other hand, girls ages 12 to 17 years tend to display internalized (eg, depressed mood, anxiety, low self-esteem) or inattentive behaviors, which clinicians and educators may assess as less severe and warranting fewer supportive measures.1
The prevalence of ADHD and its associated factors, which evolve through maturation, underscore the importance of persistent, patient-centered, and collaborative PCP and IBHC clinical management.
Continue to: Begin with a screening tool, move to a clinical interview
Begin with a screening tool, move to a clinical interview
When caregivers express concerns about their child’s behavior, focus, mood, learning, and socialization, consider initiating a multimodal evaluation for ADHD.5,8 Embarking on an ADHD assessment can require extended or multiple visits to arrive at the diagnosis, followed by still more visits to confirm a course of care and adjust medications. The integrative care approach described in the patient case and elaborated on later in this article can help facilitate assessment and treatment of ADHD.9
Signs of ADHD may be observed at initial screening using a tool such as the Ages & Stages Questionnaire (https://agesandstages.com/products-pricing/asq3/) to reveal indications of norm deviations or delays commensurate with ADHD.10 However, to substantiate the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision criteria for an accurate diagnosis,4 the American Academy of Pediatrics (AAP) clinical practice guidelines require a thorough clinical interview, administration of a standardized assessment tool, and review of objective reports in conjunction with a physical examination and psychosocial evaluation.6 Standardized measures of psychological, neurocognitive, and academic achievement reported by caregivers and collateral contacts (eg, teachers, counselors, coaches, care providers) are needed to maximize data objectivity and symptom accuracy across settings (TABLE 210-17). Additionally, periodic reassessment is recommended to validate changes in diagnostic subtype and treatment plans due to the chronic and dynamic nature of ADHD.

Consider comorbidities and alternate diagnoses
The diagnostic possibility of ADHD should also prompt consideration of other childhood disorders due to the high potential for comorbidities.4,6 In a 2016 study, approximately 64% of
Various medical disorders may manifest with similar signs or symptoms to ADHD, such as thyroid disorders, seizure disorders, adverse drug effects, anemia, genetic anomalies, and others.6,19
If there are behavioral concerns or developmental delays associated with tall stature for age or pubertal or testicular development anomalies, consult a geneticist and a developmental pediatrician for targeted testing and neurodevelopmental assessment, respectively. For example, ADHD is a common comorbidity among boys who also have XYY syndrome (Jacobs syndrome). However, due to the variability of symptoms and severity, XYY syndrome often goes undiagnosed, leaving a host of compounding pervasive and developmental problems untreated. Overall, more than two-thirds of patients with ADHD and a co-occurring condition are either inaccurately diagnosed or not referred for additional assessment and adjunct treatment.21
Continue to: Risks that arise over time
Risks that arise over time. As ADHD persists, adolescents are at greater risk for psychiatric comorbidities, suicidality, and functional impairments (eg, risky behaviors, occupational problems, truancy, delinquency, and poor self-esteem).4,8 Adolescents with internalized behaviors are more likely to experience comorbid depressive disorders with increased risk for self-harm.4,5,8 As adolescents age and their sense of autonomy increases, there is a tendency among those who have received a diagnosis of ADHD to minimize symptoms and decrease the frequency of routine clinic visits along with medication use and treatment compliance.3 Additionally, abuse, misuse, and misappropriation of stimulants among teens and young adults are commonplace.
Wide-scope, multidisciplinary evaluation and close clinical management reduce the potential for imprecise diagnoses, particularly at critical developmental junctures. AAP suggests that PCPs can treat mild and moderate cases of ADHD, but if the treating clinician does not have adequate training, experience, time, or clinical support to manage this condition, early referral is warranted.6
A guide to pharmacotherapy
Approximately 77% of children ages 2 to 17 years with a diagnosis of ADHD receive any form of treatment.2 Treatment for ADHD can include behavioral therapy and medication.2 AAP clinical practice guidelines caution against prescribing medications for children younger than 6 years, relying instead on caregiver-, teacher-, or clinician-administered behavioral strategies and parental training in behavioral modification. For children and adolescents between ages 6 and 18 years, first-line treatment includes pharmacotherapy balanced with behavioral therapy, academic modifications, and educational supports (eg, 504 Plan, individualized education plan [IEP]).6
Psychostimulants are preferred. These agents (eg, methylphenidate, amphetamine) remain the most efficacious class of medications to reduce hyperactivity and inattentiveness and to improve function. While long-acting psychostimulants are associated with better medication adherence and adverse-effect tolerance than are short-acting forms, the latter offer more flexibility in dosing. Start by titrating any stimulant to the lowest effective dose; reassess monthly until potential rebound effects stabilize.
Due to potential adverse effects of this class of medication, screen for any family history or personal risk for structural or electrical cardiac anomalies before starting pharmacotherapy. If any such risks exist, arrange for further cardiac evaluation before initiating medication.6 Adverse effects of stimulants include reduced appetite, gastrointestinal symptoms, headaches, anxiousness, parasomnia, tachycardia, and hypertension.
Continue to: Once medication is stabilized...
Once medication is stabilized, monitor treatment 2 to 3 times per year thereafter; watch for longer-term adverse effects such as weight loss, decreased growth rate, and psychiatric comorbidities including the Food and Drug Administration (FDA)’s black box warning of increased risk for suicidality.5,6,22
Other options. The optimal duration of psychostimulant use remains debatable, as existing evidence does not support its long-term use (10 years) over other interventions, such as nonstimulants and nonmedicinal therapies.22 Although backed by less evidence, additional medications indicated for the treatment of ADHD include: (1) atomoxetine, a selective norepinephrine reuptake inhibitor, and (2) the selective alpha-2 adrenergic agonists, extended-release guanfacine and extended-release clonidine (third-line agent).22
Adverse effects of these FDA-approved medications are similar to those observed in stimulant medications. Evaluation of cardiac risks is recommended before starting nonstimulant medications. The alpha-2 adrenergic agonists may also be used as adjunct therapies to stimulants. Before stopping an alpha-2 adrenergic agonist, taper the dosage slowly to avoid the risk for rebound hypertension.6,23 Given the wide variety of medication options and variability of effects, it may be necessary to try different medications as children grow and their symptoms and capacity to manage them change. Additional guidance on FDA-approved medications is available at www.ADHDMedicationGuide.com.
How multilevel care coordination can work
As with other chronic or developmental conditions, the treatment of ADHD requires an interdisciplinary perspective. Continuous, comprehensive case management can help patients overcome obstacles to wellness by balancing the resolution of problems with the development of resilience. Well-documented collaboration of subspecialists, educators, and other stakeholders engaged in ADHD care at multiple levels (individual, family, community, and health care system) increases the likelihood of meaningful, sustainable gains. Using a patient-centered medical home framework, IBHCs or other allied health professionals embedded in, or co-located with, primary care settings can be key to accessing evidence-based treatments that include: psycho-education and mindfulness-based stress reduction training for caregivers24,25; occupational,26 cognitive behavioral,27 or family therapies28,29; neuro-feedback; computer-based attention training; group- or community-based interventions; and academic and social supports.5,8
Treatment approaches that capitalize on children’s neurologic and psychological plasticity and fortify self-efficacy with developmentally appropriate tools empower them to surmount ADHD symptoms over time.23 Facilitating children’s resilience within a developmental framework and health system’s capacities with socio-culturally relevant approaches, consultation, and research can optimize outcomes and mitigate pervasiveness into adulthood. While the patient is at the center of treatment, it is important to consider the family, school, and communities in which the child lives, learns, and plays. PCPs and IBHCs together can consider a “try and track” method to follow progress, changes, and outcomes over time. With this method, the physician can employ approaches that focus on the patient, caregiver, or the caregiver–child interaction (TABLE 3).

Continue to: Assess patients' needs and the resources available
Assess patients’ needs and the resources available throughout the system of care beyond the primary care setting. Stay abreast of hospital policies, health care insurance coverage, and community- and school-based health programs, and any gaps in adequate and equitable assessment and treatment. For example, while clinical recommendations include psychiatric care, health insurance availability or limits in coverage may dissuade caregivers from seeking help or limit initial or long-term access to resources for help.30 Integrating or advocating for clinic support resources or staffing to assist patients in navigating and mitigating challenges may lessen the management burden and increase the likelihood and longevity of favorable health outcomes.
Steps to ensuring health care equity
Among children of historically marginalized and racial and ethnic minority groups or those of populations affected by health disparities, ADHD symptoms and needs are often masked by structural biases that lead to inequitable care and outcomes, as well as treatment misprioritization or delays.31 In particular, evidence has shown that recognition and diagnostic specificity of ADHD and comorbidities, not prevalence, vary more widely among minority than among nonminority populations,32 contributing to the 23% of children with ADHD who receive no treatment at all.2
Understand caregiver concerns. This diagnosis discrepancy is correlated with symptom rating sensitivities (eg, reliability, perception, accuracy) among informants and how caregivers observe, perceive, appreciate, understand, and report behaviors. This discrepancy is also related to cultural belief differences, physician–patient communication variants, and a litany of other socioeconomic determinants.2,4,31 Caregivers from some cultural, ethnic, or socioeconomic backgrounds may be doubtful of psychiatric assessment, diagnoses, treatment, or medication, and that can impact how children are engaged in clinical and educational settings from the outset.31 In the case we described, James’ mother was initially hesitant to explore psychotropic medications and was concerned about stigmatization within the school system. She also seemed to avoid psychiatric treatment for her own depressive symptoms due to cultural and religious beliefs.
Health care provider concerns. Some PCPs may hesitate to explore medications due to limited knowledge and skill in dosing and titrating based on a child’s age, stage, and symptoms, and a perceived lack of competence in managing ADHD. This, too, can indirectly perpetuate existing health disparities. Furthermore, ADHD symptoms may be deemed a secondary or tertiary concern if other complex or urgent medical or undifferentiated developmental problems manifest.
Compounding matters is the limited dissemination of empiric research articles (including randomized controlled trials with representative samples) and limited education on the effectiveness and safety of psychopharmacologic interventions across the lifespan and different cultural and ethnic groups.4 Consequently, patients who struggle with unmanaged ADHD symptoms are more likely to have chronic mental health disorders, maladaptive behaviors, and other co-occurring conditions contributing to the complexity of individual needs, health care burdens, or justice system involvement; this is particularly true for those of racial and ethnic minorities.33
Continue to: Impact of the COVID-19 pandemic
Impact of the COVID-19 pandemic. Patients—particularly those in minority or health disparity populations—who under normal circumstances might have been hesitant to seek help may have felt even more reluctant to do so during the COVID-19 pandemic. We have not yet learned the degree to which limited availability of preventive health care services, decreased routine visits, and fluctuating insurance coverage has impacted the diagnosis, management, or severity of childhood disorders during the past 2 years. Reports of national findings indicate that prolonged periods out of school and reduced daily structure were associated with increased disruptions in mood, sleep, and appetite, particularly among children with pre-existing pathologies. Evidence suggests that school-aged children experienced more anxiety, regressive behaviors, and parasomnias than they did before the pandemic, while adolescents experienced more isolation and depressive symptoms.34,35
However, there remains a paucity of large-scale or representative studies that use an intersectional lens to examine the influence of COVID-19 on children with ADHD. Therefore, PCPs and IBHCs should refocus attention on possibly undiagnosed, stagnated, or regressed ADHD cases, as well as the adults who care for them. (See “5 ways to overcome Tx barriers and promote health equity.”)
SIDEBAR
5 ways to overcome Tx barriers and promote health equitya
1. Inquire about cultural or ethnic beliefs and behaviors and socioeconomic barriers.
2. Establish trust or assuage mistrust by exploring and dispelling misinformation.
3. Offer accessible, feasible, and sustainable evidence-based interventions.
4. Encourage autonomy and selfdetermination throughout the health care process.
5. Connect caregivers and children with clinical, community, and school-based resources and coordinators.
a These recommendations are based on the authors’ combined clinical experience.
THE CASE
During a follow-up visit 1 month later, the PCP confirmed the clinical impression of ADHD combined presentation with a clinical interview and review of the Strengths and Difficulties Questionnaire completed by James’ mother and the Vanderbilt Assessment Scales completed by James’ mother and teacher. The sleep diary indicated potential problems and apneas worthy of consults for pulmonary function testing, a sleep study, and otolaryngology examination. The PCP informed James’ mother on sleep hygiene strategies and ADHD medication options. She indicated that she wanted to pursue the referrals and behavioral modifications before starting any medication trial.
The PCP referred James to a developmental pediatrician for in-depth assessment of his overall development, learning, and functioning. The developmental pediatrician ultimately confirmed the diagnosis of ADHD, as well as motor and speech delays warranting physical, occupational, and speech therapies. The developmental pediatrician also referred James for targeted genetic testing because she suspected a genetic disorder (eg, XYY syndrome).
The PCP reconnected James and his mother to the IBHC to facilitate subspecialty and school-based care coordination and to provide in-office and home-based interventions. The IBHC assessed James’ emotional dysregulation and impulsivity as adversely impacting his interpersonal relationships and planned to address these issues with behavioral and parent–child interaction therapies and skills training during the course of 6 to 12 visits. James’ mother was encouraged to engage his teacher on his academic performance and to initiate a 504 Plan or IEP for in-school accommodations and support. The IBHC aided in tracking his assessments, referrals, follow-ups, access barriers, and treatment goals.
After 6 months, James had made only modest progress, and his mother requested that he begin a trial of medication. Based on his weight, symptoms, behavior patterns, and sleep habits, the PCP prescribed extended-release dexmethylphenidate 10 mg each morning, then extended-release clonidine 0.1 mg nightly. With team-based clinical management of pharmacologic, behavioral, physical, speech, and occupational therapies, James’ behavior and sleep improved, and the signs of a vocal tic diminished.
By the next school year, James demonstrated a marked improvement in impulse control, attention, and academic functioning. He followed up with the PCP at least quarterly for reassessment of his symptoms, growth, and experience of adverse effects, and to titrate medications accordingly. James and his mother continued to work closely with the IBHC monthly to engage interventions and to monitor his progress at home and school.
CORRESPONDENCE
Sundania J. W. Wonnum, PhD, LCSW, National Institute on Minority Health and Health Disparities, 6707 Democracy Boulevard, Suite 800, Bethesda, MD 20892; sundania.wonnum@nih.gov
1. Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children—United States, 2013-2019. MMWR Suppl. 2022;71:1-42. doi: 10.15585/mmwr.su7102a1
2. Danielson ML, Holbrook JR, Blumberg SJ, et al. State-level estimates of the prevalence of parent-reported ADHD diagnosis and treatment among U.S. children and adolescents, 2016 to 2019. J Atten Disord. 2022;26:1685-1697. doi: 10.1177/10870547221099961
3. Faraone SV, Banaschewski T, Coghill D, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789-818. doi: 10.1016/j.neubiorev.2021.01.022
4. American Psychiatric Association
5. Brahmbhatt K, Hilty DM, Mina H, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143. doi: 10.1016/j.jadohealth.2016.03.025
6. Wolraich ML, Hagan JF, Allan C, et al. AAP Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528
7. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
8. Chang JG, Cimino FM, Gossa W. ADHD in children: common questions and answers. Am Fam Physician. 2020;102:592-602.
9. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169:929-937. doi: 10.1001/jamapediatrics.2015.1141
10. Squires J, Bricker D. Ages & Stages Questionnaires®. 3rd ed (ASQ®-3). Paul H. Brookes Publishing Co., Inc; 2009.
11. DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. J Clin Child Psychol. 1992;21:178-188. doi.org/10.1207/s15374424jccp2102_10
12. Merenda PF. BASC: behavior assessment system for children. Meas Eval Counsel Develop. 1996;28:229-232.
13. Conners CK. Conners, 3rd ed manual. Multi-Health Systems. 2008.
14. Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; 1999:429-466.
15. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791-799.
16. Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559-567. doi: 10.1093/jpepsy/jsg046
17. Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, ed. Major Psychological Assessment Instruments. Vol 2. Allyn & Bacon; 2003:199-231.
18. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199-212. doi: 10.1080/15374416.2017.1417860
19. Ghriwati NA, Langberg JM, Gardner W, et al. Impact of mental health comorbidities on the community-based pediatric treatment and outcomes of children with attention deficit hyperactivity disorder. J Dev Behav Ped. 2017;38:20-28. doi: 10.1097/DBP.0000000000000359
20. Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish Cohort. Int J Ped Otorhinolaryngol. 2016;84:12-20. doi: 10.1016/j.ijporl.2016.02.017
21. Ross JL Roeltgen DP Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. doi: 10.1542/peds.2011-0719
22. Mechler K, Banaschewski T, Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940
23. Mishra J, Merzenich MM, Sagar R. Accessible online neuroplasticity-targeted training for children with ADHD. Child Adolesc Psychiatry Ment Health. 2013;7:38. doi: 10.1186/1753-2000-7-38
24. Neece CL. Mindfulness-based stress reduction for parents of young children with developmental delays: implications for parental mental health and child behavior problems. J Applied Res Intellect Disabil. 2014;27:174-186. doi: 10.1111/jar.12064
25. Petcharat M, Liehr P. Mindfulness training for parents of children with special needs: guidance for nurses in mental health practice. J Child Adolesc Psychiatr Nursing. 2017;30:35-46. doi: 10.1111/jcap.12169
26. Hahn-Markowitz J, Burger I, Manor I, et al. Efficacy of cognitive-functional (Cog-Fun) occupational therapy intervention among children with ADHD: an RCT. J Atten Disord. 2020;24:655-666. doi: 10.1177/1087054716666955
27. Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Atten Disord. 2020;24:875-888.
28. Carr AW, Bean RA, Nelson KF. Childhood attention-deficit hyperactivity disorder: family therapy from an attachment based perspective. Child Youth Serv Rev. 2020;119:105666.
29. Robin AL. Family therapy for adolescents with ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23:747-756. doi: 10.1016/j.chc.2014.06.001
30. Cattoi B, Alpern I, Katz JS, et al. The adverse health outcomes, economic burden, and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): a call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J Atten Disord. 2022;26:807-808. doi: 10.1177/10870547211036754
31. Hinojosa MS, Hinojosa R, Nguyen J. Shared decision making and treatment for minority children with ADHD. J Transcult Nurs. 2020;31:135-143. doi: 10.1177/1043659619853021
32. Slobodin O, Masalha R. Challenges in ADHD care for ethnic minority children: a review of the current literature. Transcult Psychiatry. 2020;57:468-483. doi: 10.1177/1363461520902885
33. Retz W, Ginsberg Y, Turner D, et al. Attention-deficit/hyperactivity disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236-248. doi: 10.1016/j.neubiorev.2020.11.025
34. Del Sol Calderon P, Izquierdo A, Garcia Moreno M. Effects of the pandemic on the mental health of children and adolescents. Review and current scientific evidence of the SARS-COV2 pandemic. Eur Psychiatry. 2021;64:S223-S224. doi: 10.1192/j.eurpsy.2021.597
35. Insa I, Alda JA. Attention deficit hyperactivity disorder (ADHD) & COVID-19: attention deficit hyperactivity disorder: consequences of the 1st wave. Eur Psychiatry. 2021;64:S660. doi: 10.1192/j.eurpsy.2021.1752
1. Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children—United States, 2013-2019. MMWR Suppl. 2022;71:1-42. doi: 10.15585/mmwr.su7102a1
2. Danielson ML, Holbrook JR, Blumberg SJ, et al. State-level estimates of the prevalence of parent-reported ADHD diagnosis and treatment among U.S. children and adolescents, 2016 to 2019. J Atten Disord. 2022;26:1685-1697. doi: 10.1177/10870547221099961
3. Faraone SV, Banaschewski T, Coghill D, et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789-818. doi: 10.1016/j.neubiorev.2021.01.022
4. American Psychiatric Association
5. Brahmbhatt K, Hilty DM, Mina H, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: a concise review. J Adolesc Health. 2016;59:135-143. doi: 10.1016/j.jadohealth.2016.03.025
6. Wolraich ML, Hagan JF, Allan C, et al. AAP Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. doi: 10.1542/peds.2019-2528
7. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
8. Chang JG, Cimino FM, Gossa W. ADHD in children: common questions and answers. Am Fam Physician. 2020;102:592-602.
9. Asarnow JR, Rozenman M, Wiblin J, et al. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169:929-937. doi: 10.1001/jamapediatrics.2015.1141
10. Squires J, Bricker D. Ages & Stages Questionnaires®. 3rd ed (ASQ®-3). Paul H. Brookes Publishing Co., Inc; 2009.
11. DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. J Clin Child Psychol. 1992;21:178-188. doi.org/10.1207/s15374424jccp2102_10
12. Merenda PF. BASC: behavior assessment system for children. Meas Eval Counsel Develop. 1996;28:229-232.
13. Conners CK. Conners, 3rd ed manual. Multi-Health Systems. 2008.
14. Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; 1999:429-466.
15. Goodman R. The extended version of the Strengths and Difficulties Questionnaire as a guide to child psychiatric caseness and consequent burden. J Child Psychol Psychiatry. 1999;40:791-799.
16. Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559-567. doi: 10.1093/jpepsy/jsg046
17. Sparrow SS, Cicchetti DV. The Vineland Adaptive Behavior Scales. In: Newmark CS, ed. Major Psychological Assessment Instruments. Vol 2. Allyn & Bacon; 2003:199-231.
18. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199-212. doi: 10.1080/15374416.2017.1417860
19. Ghriwati NA, Langberg JM, Gardner W, et al. Impact of mental health comorbidities on the community-based pediatric treatment and outcomes of children with attention deficit hyperactivity disorder. J Dev Behav Ped. 2017;38:20-28. doi: 10.1097/DBP.0000000000000359
20. Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish Cohort. Int J Ped Otorhinolaryngol. 2016;84:12-20. doi: 10.1016/j.ijporl.2016.02.017
21. Ross JL Roeltgen DP Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. doi: 10.1542/peds.2011-0719
22. Mechler K, Banaschewski T, Hohmann S, et al. Evidence-based pharmacological treatment options for ADHD in children and adolescents. Pharmacol Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940
23. Mishra J, Merzenich MM, Sagar R. Accessible online neuroplasticity-targeted training for children with ADHD. Child Adolesc Psychiatry Ment Health. 2013;7:38. doi: 10.1186/1753-2000-7-38
24. Neece CL. Mindfulness-based stress reduction for parents of young children with developmental delays: implications for parental mental health and child behavior problems. J Applied Res Intellect Disabil. 2014;27:174-186. doi: 10.1111/jar.12064
25. Petcharat M, Liehr P. Mindfulness training for parents of children with special needs: guidance for nurses in mental health practice. J Child Adolesc Psychiatr Nursing. 2017;30:35-46. doi: 10.1111/jcap.12169
26. Hahn-Markowitz J, Burger I, Manor I, et al. Efficacy of cognitive-functional (Cog-Fun) occupational therapy intervention among children with ADHD: an RCT. J Atten Disord. 2020;24:655-666. doi: 10.1177/1087054716666955
27. Young Z, Moghaddam N, Tickle A. The efficacy of cognitive behavioral therapy for adults with ADHD: a systematic review and meta-analysis of randomized controlled trials. J Atten Disord. 2020;24:875-888.
28. Carr AW, Bean RA, Nelson KF. Childhood attention-deficit hyperactivity disorder: family therapy from an attachment based perspective. Child Youth Serv Rev. 2020;119:105666.
29. Robin AL. Family therapy for adolescents with ADHD. Child Adolesc Psychiatr Clin N Am. 2014;23:747-756. doi: 10.1016/j.chc.2014.06.001
30. Cattoi B, Alpern I, Katz JS, et al. The adverse health outcomes, economic burden, and public health implications of unmanaged attention deficit hyperactivity disorder (ADHD): a call to action resulting from CHADD summit, Washington, DC, October 17, 2019. J Atten Disord. 2022;26:807-808. doi: 10.1177/10870547211036754
31. Hinojosa MS, Hinojosa R, Nguyen J. Shared decision making and treatment for minority children with ADHD. J Transcult Nurs. 2020;31:135-143. doi: 10.1177/1043659619853021
32. Slobodin O, Masalha R. Challenges in ADHD care for ethnic minority children: a review of the current literature. Transcult Psychiatry. 2020;57:468-483. doi: 10.1177/1363461520902885
33. Retz W, Ginsberg Y, Turner D, et al. Attention-deficit/hyperactivity disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236-248. doi: 10.1016/j.neubiorev.2020.11.025
34. Del Sol Calderon P, Izquierdo A, Garcia Moreno M. Effects of the pandemic on the mental health of children and adolescents. Review and current scientific evidence of the SARS-COV2 pandemic. Eur Psychiatry. 2021;64:S223-S224. doi: 10.1192/j.eurpsy.2021.597
35. Insa I, Alda JA. Attention deficit hyperactivity disorder (ADHD) & COVID-19: attention deficit hyperactivity disorder: consequences of the 1st wave. Eur Psychiatry. 2021;64:S660. doi: 10.1192/j.eurpsy.2021.1752
Don’t cross the friends line with patients
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
Nine more minutes a day of vigorous exercise tied to better cognition
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
such as running and cycling, plays in brain health.
“Even minor differences in daily behavior appeared meaningful for cognition in this study,” researcher John J. Mitchell, MSci and PhD candidate, Medical Research Council, London, told this news organization.
The findings were published online in the Journal of Epidemiology and Community Health.
Research gap
Previous research has linked physical activity (PA) with increased cognitive reserve, which delays the onset of cognitive decline in later life. But disentangling the most important components of PA for cognition – such as intensity and volume – has not been well researched.
Previous studies didn’t capture sleep time, which typically takes up the largest component of the day. Sleep is “acutely relevant” when examining cognition, the investigators noted.
In addition, studies in this area often focus on just one or two activity components of the day, which “neglects the growing awareness” that movements “are all tightly interlinked,” said Mr. Mitchell.
The new study included 4,481 participants in the British Cohort Study who were born in 1970 across England, Scotland, and Wales. The participants were followed throughout childhood and adulthood.
The median age of the participants was 47 years, and they were predominantly White, female (52%), married (66%), and well educated. Most were occasional or nonrisky alcohol consumers, and half had never smoked.
The researchers collected biometric measurements and health, demographic, and lifestyle information. Participants wore a thigh-mounted accelerometer at least 7 consecutive hours a day for up to 7 days to track PA, sedentary behavior (SB), and sleep time.
The device used in the study could detect subtle movements as well as speed of accelerations, said Mr. Mitchell. “From this, we can distinguish MVPA from slow walking, standing, and sitting. It’s the current best practice for detecting the more subtle movements we make, such as brisk walking and stair climbing, beyond just ‘exercise,’ “ he added.
Light intensity PA (LIPA) describes movement such as walking and moving around the house or office, while MVPA includes activities such as brisk walking and running that accelerate the heart rate. SB, defined as time spent sitting or lying, is distinguished from standing by the thigh inclination.
On an average day, the cohort spent 51 minutes in MVPA; 5 hours, 42 minutes in LIPA; 9 hours, 16 minutes in SB; and 8 hours, 11 minutes sleeping.
Researchers calculated an overall global score for verbal memory and executive function.
The study used “compositional data analysis,” a statistical method that can examine the associations of cognition and PA in the context of all components of daily movement.
The analysis revealed a positive association between MVPA and cognition relative to all other behaviors, after adjustment for sociodemographic factors that included sex, age, education, and marital status. But the relationship lessened after further adjustment for health status – for example, cardiovascular disease or disability – and lifestyle factors, such as alcohol consumption and smoking status.
SB relative to all other movements remained positively associated with cognition after full adjustment. This, the authors speculated, may reflect engagement in cognitively stimulating activities such as reading.
To better understand the associations, the researchers used a statistical method to reallocate time in the cohort’s average day from one activity component to another.
“We held two of the components static but moved time between the other two and monitored the theoretical ramifications of that change for cognition,” said Mr. Mitchell.
Real cognitive change
There was a 1.31% improvement in cognition ranking compared to the sample average after replacing 9 minutes of sedentary activity with MVPA (1.31; 95% confidence interval [CI], 0.09-2.50). There was a 1.27% improvement after replacing 7 minutes of LIPA with MVPA, and a 1.2% improvement after replacing 7 minutes of sleep with MVPA.
Individuals might move up from about the 50th percentile to the 51st or 52nd percentile after just 9 minutes of more moderate to vigorous movement in place of sitting, said Mr. Mitchell. “This highlights how even very modest differences in people’s daily movement – less than 10 minutes – is linked to quite real changes in our cognitive health.”
The impact of physical activity appeared greatest on working memory and mental processes, such as planning and organization.
On the other hand, cognition declined by 1%-2% after replacing MVPA with 8 minutes of SB, 6 minutes of LIPA, or 7 minutes of sleep.
The activity tracking device couldn’t determine how well participants slept, which is “a clear limitation” of the study, said Mr. Mitchell. “We have to be cautious when trying to interpret our findings surrounding sleep.”
Another limitation is that despite a large sample size, people of color were underrepresented, limiting the generalizability of the findings. As well, other healthy pursuits – for example, reading – might have contributed to improved cognition.
Important findings
In a comment, Jennifer J. Heisz, PhD, associate professor and Canada research chair in brain health and aging, department of kinesiology, McMaster University, Hamilton, Ont., said the findings from the study are important.
“Through the statistical modelling, the authors demonstrate that swapping just 9 minutes of sedentary behavior with moderate to vigorous physical activity, such as a brisk walk or bike ride, was associated with an increase in cognition.”
She added that this seemed to be especially true for people who sit while at work.
The findings “confer with the growing consensus” that some exercise is better than none when it comes to brain health, said Dr. Heisz.
“Clinicians should encourage their patients to add a brisk, 10-minute walk to their daily routine and break up prolonged sitting with short movement breaks.”
She noted the study was cross-sectional, “so it is not possible to infer causation.”
The study received funding from the Medical Research Council and the British Heart Foundation. Mr. Mitchell and Dr. Heisz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF EPIDEMIOLOGY AND COMMUNITY HEALTH
Severe health diagnoses drive suicide risk
Individuals diagnosed with a severe physical health condition were significantly more likely to commit suicide at 6 months and at 1 year later, based on data from more than 47 million individuals in a national database.
Previous smaller studies have shown a link between increased risk for suicide and a range of health conditions including cancer, coronary heart disease, neurologic conditions, diabetes, and osteoporosis, Vahé Nafilyan, PhD, of the Office for National Statistics, Newport, England, and colleagues wrote.
However, large-scale population-level studies of the association between specific diagnoses and suicide are lacking, they said.
In a study published in The Lancet Regional Health–Europe, the researchers reviewed a dataset that combined the 2011 Census, death registration records, and the Hospital Episode Statistics. The study population included 47,354,696 individuals aged 6 years and older living in England in 2017. The mean age of the study population was 39.6 years, and 52% were female. The researchers examined deaths that occurred between Jan. 1, 2017, and Dec. 31, 2021.
The health conditions included in the analysis were low-survival cancers, chronic ischemic heart disease, chronic obstructive pulmonary disease, and degenerative neurological disease.
The diagnosis of any of these conditions significantly increased the risk for suicide compared with controls. The highest risk appeared within 6 months of a diagnosis or first treatment, but the increased risk persisted at 1 year.
The suicide rate among low-survival cancer patients was 16.6 per 100,000 patients, compared with 5.7 per 100,000 controls; at 1 year, these rates were 21.6 and 9.5 per 100,000 patients and controls, respectively.
For COPD patients, the suicide rate at 6 months after diagnosis was 13.7 per 100,000 patients versus 5.6 per 100,000 matched controls; the suicide rates at 1 year were 22.4 per 100,000 patients and 10.6 per 100,000 matched controls.
The suicide rate at 6 months for individuals diagnosed with chronic ischemic heart disease was 11.0 per 100,000 patients and 4.2 per 100,000 matched controls; at 1 year, the suicide rates were 16.1 per 100,000 patients and 8.8 per 100,000 matched controls.
The 1-year suicide rate was especially high among patients with degenerative neurological conditions (114.5 per 100,000 patients); however, the estimate was considered imprecise because of the rarity of these diseases and subsequent low number of suicides, the researchers noted.
The results support data from previous studies showing links between increased risk of suicide and severe physical conditions, the researchers wrote. Patterns of suicide were similar between men and women and after adjusting for sociodemographic factors.
The findings were limited by the inability to fully control for a history of depression or self-harm, and by the imprecise estimates given the rare occurrence of suicide overall, the researchers noted. Other limitations included the late registration of deaths from external causes and the focus only on suicides that occurred in England and Wales, meaning that individuals who traveled abroad for assisted suicide were not captured in the dataset.
“Further research is needed to understand the mechanisms driving the elevated risk of suicide and help provide the best support to these patients,” the researchers concluded.
However, the current results enhance the literature with a large, population-based review of the elevated suicide risk among individuals newly diagnosed with severe health conditions, and reflect the need for better support for these patients to help with coping, they said.
The study was funded by the Office for National Statistics. The researchers reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals diagnosed with a severe physical health condition were significantly more likely to commit suicide at 6 months and at 1 year later, based on data from more than 47 million individuals in a national database.
Previous smaller studies have shown a link between increased risk for suicide and a range of health conditions including cancer, coronary heart disease, neurologic conditions, diabetes, and osteoporosis, Vahé Nafilyan, PhD, of the Office for National Statistics, Newport, England, and colleagues wrote.
However, large-scale population-level studies of the association between specific diagnoses and suicide are lacking, they said.
In a study published in The Lancet Regional Health–Europe, the researchers reviewed a dataset that combined the 2011 Census, death registration records, and the Hospital Episode Statistics. The study population included 47,354,696 individuals aged 6 years and older living in England in 2017. The mean age of the study population was 39.6 years, and 52% were female. The researchers examined deaths that occurred between Jan. 1, 2017, and Dec. 31, 2021.
The health conditions included in the analysis were low-survival cancers, chronic ischemic heart disease, chronic obstructive pulmonary disease, and degenerative neurological disease.
The diagnosis of any of these conditions significantly increased the risk for suicide compared with controls. The highest risk appeared within 6 months of a diagnosis or first treatment, but the increased risk persisted at 1 year.
The suicide rate among low-survival cancer patients was 16.6 per 100,000 patients, compared with 5.7 per 100,000 controls; at 1 year, these rates were 21.6 and 9.5 per 100,000 patients and controls, respectively.
For COPD patients, the suicide rate at 6 months after diagnosis was 13.7 per 100,000 patients versus 5.6 per 100,000 matched controls; the suicide rates at 1 year were 22.4 per 100,000 patients and 10.6 per 100,000 matched controls.
The suicide rate at 6 months for individuals diagnosed with chronic ischemic heart disease was 11.0 per 100,000 patients and 4.2 per 100,000 matched controls; at 1 year, the suicide rates were 16.1 per 100,000 patients and 8.8 per 100,000 matched controls.
The 1-year suicide rate was especially high among patients with degenerative neurological conditions (114.5 per 100,000 patients); however, the estimate was considered imprecise because of the rarity of these diseases and subsequent low number of suicides, the researchers noted.
The results support data from previous studies showing links between increased risk of suicide and severe physical conditions, the researchers wrote. Patterns of suicide were similar between men and women and after adjusting for sociodemographic factors.
The findings were limited by the inability to fully control for a history of depression or self-harm, and by the imprecise estimates given the rare occurrence of suicide overall, the researchers noted. Other limitations included the late registration of deaths from external causes and the focus only on suicides that occurred in England and Wales, meaning that individuals who traveled abroad for assisted suicide were not captured in the dataset.
“Further research is needed to understand the mechanisms driving the elevated risk of suicide and help provide the best support to these patients,” the researchers concluded.
However, the current results enhance the literature with a large, population-based review of the elevated suicide risk among individuals newly diagnosed with severe health conditions, and reflect the need for better support for these patients to help with coping, they said.
The study was funded by the Office for National Statistics. The researchers reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals diagnosed with a severe physical health condition were significantly more likely to commit suicide at 6 months and at 1 year later, based on data from more than 47 million individuals in a national database.
Previous smaller studies have shown a link between increased risk for suicide and a range of health conditions including cancer, coronary heart disease, neurologic conditions, diabetes, and osteoporosis, Vahé Nafilyan, PhD, of the Office for National Statistics, Newport, England, and colleagues wrote.
However, large-scale population-level studies of the association between specific diagnoses and suicide are lacking, they said.
In a study published in The Lancet Regional Health–Europe, the researchers reviewed a dataset that combined the 2011 Census, death registration records, and the Hospital Episode Statistics. The study population included 47,354,696 individuals aged 6 years and older living in England in 2017. The mean age of the study population was 39.6 years, and 52% were female. The researchers examined deaths that occurred between Jan. 1, 2017, and Dec. 31, 2021.
The health conditions included in the analysis were low-survival cancers, chronic ischemic heart disease, chronic obstructive pulmonary disease, and degenerative neurological disease.
The diagnosis of any of these conditions significantly increased the risk for suicide compared with controls. The highest risk appeared within 6 months of a diagnosis or first treatment, but the increased risk persisted at 1 year.
The suicide rate among low-survival cancer patients was 16.6 per 100,000 patients, compared with 5.7 per 100,000 controls; at 1 year, these rates were 21.6 and 9.5 per 100,000 patients and controls, respectively.
For COPD patients, the suicide rate at 6 months after diagnosis was 13.7 per 100,000 patients versus 5.6 per 100,000 matched controls; the suicide rates at 1 year were 22.4 per 100,000 patients and 10.6 per 100,000 matched controls.
The suicide rate at 6 months for individuals diagnosed with chronic ischemic heart disease was 11.0 per 100,000 patients and 4.2 per 100,000 matched controls; at 1 year, the suicide rates were 16.1 per 100,000 patients and 8.8 per 100,000 matched controls.
The 1-year suicide rate was especially high among patients with degenerative neurological conditions (114.5 per 100,000 patients); however, the estimate was considered imprecise because of the rarity of these diseases and subsequent low number of suicides, the researchers noted.
The results support data from previous studies showing links between increased risk of suicide and severe physical conditions, the researchers wrote. Patterns of suicide were similar between men and women and after adjusting for sociodemographic factors.
The findings were limited by the inability to fully control for a history of depression or self-harm, and by the imprecise estimates given the rare occurrence of suicide overall, the researchers noted. Other limitations included the late registration of deaths from external causes and the focus only on suicides that occurred in England and Wales, meaning that individuals who traveled abroad for assisted suicide were not captured in the dataset.
“Further research is needed to understand the mechanisms driving the elevated risk of suicide and help provide the best support to these patients,” the researchers concluded.
However, the current results enhance the literature with a large, population-based review of the elevated suicide risk among individuals newly diagnosed with severe health conditions, and reflect the need for better support for these patients to help with coping, they said.
The study was funded by the Office for National Statistics. The researchers reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE LANCET REGIONAL HEALTH–EUROPE
Concern grows over ‘medical assistance in dying for mental illness’ law
Canada already has the largest number of deaths by MAID of any nation, with 10,064 in 2021, a 32% increase from 2020. With the addition of serious mental illness (SMI) as an eligible category, the country is on track to have the most liberal assisted-death policy in the world.
Concerns about the additional number of patients who could become eligible for MAID, and a lack of evidence-backed standards from disability rights groups, mental health advocates, First Nations leaders, psychiatrists, and other mental health providers, seems to have led the Canadian government to give the proposed law some sober second thought.
“Listening to experts and Canadians, we believe this date needs to be temporarily delayed,” said David Lametti, Canada’s minister of Justice and attorney general of Canada; Jean-Yves Duclos, minister of Health; and Carolyn Bennett, minister of Mental Health and Addictions, in a Dec. 15, 2022, joint statement.
Canada’s Parliament – which approved the expansion – will now have to vote on whether to okay a pause on the legislation.
However, the Canadian Psychiatric Association has not been calling for a delay in the proposed legislation. In a November 2021 statement, the CPA said it “does not take a position on the legality or morality of MAID,” but added that to deny MAID to people with mental illness was discriminatory, and that, as it was the law, it must be followed.
“CPA has not taken a position about MAID,” the association’s president Gary Chaimowitz, MBChB, told this news organization. “We know this is coming and our organization is trying to get its members ready for what will be most likely the ability of people with mental conditions to be able to request MAID,” said Dr. Chaimowitz, who is also head of forensic psychiatry at St. Joseph’s Healthcare and a professor of psychiatry at McMaster University, both in Hamilton, Ont.
Dr. Chaimowitz acknowledges that “a majority of psychiatrists do not want to be involved in anything to do with MAID.”
“The idea, certainly in psychiatry, is to get people well and we’ve been taught that people dying from a major mental disorder is something that we’re trained to prevent,” he added.
A ‘clinical option’
Assisted medical death is especially fraught in psychiatry, said Rebecca Brendel, MD, president of the American Psychiatric Association. She noted a 25-year life expectancy gap between people with SMI and those who do not have such conditions.
“As a profession we have very serious obligations to advance treatment so that a person with serious mental illness can live [a] full, productive, and healthy [life],” Dr. Brendel, associate director of the Center for Bioethics at Harvard Medical School in Boston, said in an interview.
Under the Canadian proposal, psychiatrists would be allowed to suggest MAID as a “clinical option.”
Harold Braswell, PhD, a fellow with The Hastings Center, a bioethics research institute, calls that problematic.
“It’s not neutral to suggest to someone that it would be theoretically reasonable to end their lives,” Dr. Braswell, associate professor at the Albert Gnaegi Center for Health Care Ethics at Saint Louis University, told this news organization.
It also creates a double standard in the treatment of suicidal ideation, in which suicide prevention is absolute for some, but encouraging it as a possibility for others, he added.
“To have that come from an authority figure is something that’s very harsh and, in my opinion, very potentially destructive,” especially for vulnerable groups, like First Nations people, who already have elevated rates of suicide, said Dr. Braswell.
Fierce debate
Since 2016, Canada has allowed MAID for medical conditions and diseases that will not improve and in cases where the evidence shows that medical providers can accurately predict the condition will not improve.
However, in 2019, a Quebec court ruled that the law unconstitutionally barred euthanasia in people who were not terminally ill. In March 2021, Canada’s criminal code was amended to allow MAID for people whose natural death was not “reasonably foreseeable,” but it excluded SMI for a period of 2 years, ending in March 2023.
The 2-year stay was intended to allow for study and to give mental health providers and MAID assessors time to develop standards.
The federal government charged a 12-member expert panel with determining how to safely allow MAID for SMI. In its final report released in May 2022 it recommended that standards be developed.
The panel acknowledged that for many conditions it may be impossible to make predictions about whether an individual might improve. However, it did not mention SMI.
In those cases, when MAID is requested, “establishing incurability and irreversibility on the basis of the evolution and response to past interventions is necessary,” the panel noted, adding that these are the criteria used by psychiatrists assessing euthanasia requests in the Netherlands and Belgium.
But the notion that mental illness can be irremediable has been fiercely debated.
Soon after the expert report was released, the Center for Addiction and Mental Health in Toronto noted on its website that there are currently “no agreed upon standards for psychiatrists or other health care practitioners to use to determine if a person’s mental illness is ‘grievous and irremediable’ for the purposes of MAID.”
Dr. Chaimowitz acknowledged that “there’s no agreed-upon definition of incurability” in mental illness. Some psychiatrists “will argue that there’s always another treatment that can be attempted,” he said, adding that there has been a lack of consensus on irremediability among CPA members.
Protecting vulnerable populations
Matt Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora, said the question of irremediability is crucial. “Most people with mental illness do get better, especially if they’re in treatment,” Dr. Wynia said.
For MAID assessors it may be difficult to know when someone has tried all possible treatments, especially given the wide array of options, including psychedelics, said Dr. Wynia.
Dr. Braswell said there is not enough evidence that mental illness is incurable. With SMI, “there’s a lot more potential for the causes of the individual’s suffering to be ameliorated. By offering MAID, you’re going to kill people who might have been able to get out of this through other nonlethal means.”
Currently, MAID is provided for an irremediable medical condition, “in other words, a condition that will not improve and that we can predict will not improve,” said Karandeep S. Gaind, MD, chief of psychiatry at Toronto’s Humber River Hospital and physician chair of the hospital’s MAID team.
“If that’s the premise, then I think we cannot provide MAID for sole mental illness,” Dr. Gaind said. “Because we can’t honestly make those predictions” with mental illness, he added.
Dr. Gaind does not support MAID for mental illness and believes that it will put the vulnerable – including those living in poverty – at particular risk.
With the proposed expansion, MAID is “now becoming something which is being sought as a way to escape a painful life rather than to avoid a painful death,” said Dr. Gaind, who is also a past president of the CPA.
One member of the federal government’s expert panel – Ellen Cohen, who had a psychiatric condition – wrote in The Globe and Mail that she quit early on when it became apparent that the panel was not seriously considering her own experiences or the possibility that poverty and lack of access to care or social supports could strongly influence a request for MAID.
Social determinants of suffering
People with mental illness often are without homes, have substance use disorders, have been stigmatized and discriminated against, and have poor social supports, said Dr. Wynia. “You worry that it’s all of those things that are making them want to end their lives,” he said.
The Daily Mail ran a story in December 2022 about a 65-year-old Canadian who said he’d applied for MAID solely because of fears that his disability benefits for various chronic health conditions were being cut off and that he didn’t want to live in poverty.
A 51-year-old Ontario woman with multiple chemical sensitivities was granted MAID after she said she could not find housing that could keep her safe, according to an August report by CTV News.
Tarek Rajji, MD, chief of the Adult Neurodevelopment and Geriatric Psychiatry Division at CAMH, said social determinants of health need to be considered in standards created to guide MAID for mental illness.
“We’re very mindful of the fact that the suffering, that is, the grievousness that the person is living with, in the context of mental illness, many times is due to the social determinants of their illness and the social determinants of their suffering,” Dr. Rajji said.
Many are also concerned that it will be difficult to separate out suicidality from sheer hopelessness.
The CPA has advised a group that’s working on developing guidelines for MAID in SMI and is also developing a curriculum for mental health providers, Dr. Chaimowitz said. As part of that, there will be a process to ensure that someone who is actively suicidal is not granted MAID.
“I do not believe that it’s contemplated that MAID is going to accelerate or facilitate suicidal ideation,” he said. Someone who is suicidal will be referred to treatment, said Dr. Chaimowitz.
“People with depression often feel hopeless,” and may refuse treatments that have worked in the past, countered Dr. Gaind. Some of his patients “are absolutely convinced that nothing will help,” he said.
Troublesome cases
The expert panel said in its final report that “it is not possible to provide fixed rules for how many attempts at interventions, how many types of interventions, and over how much time,” are necessary to establish “irreversibility” of mental illness.
Dr. Chaimowitz said MAID will not be offered to anyone “refusing treatment for their condition without any good reason.” They will be “unlikely to meet criteria for incurable,” as they will have needed to avail themselves of the array of treatments available, he said.
That would be similar to rules in Belgium and the Netherlands, which allow euthanasia for psychiatric conditions.
An estimated 100-300 psychiatric patients receive euthanasia each year in those countries, according to a 2021 commentary in Psychiatric Times (Jun 7;38[6]) by Mark S. Komrad, MD, a Towson, Maryland-based psychiatrist.
There are still troublesome cases.
As previously reported by this news organization, many in Belgium were distressed recently at the news that a 23-year-old woman who had survived a terrorist attack, Shanti De Corte, requested and was granted euthanasia.
As the deadline for implementation of MAID grew closer, calls for delay grew louder, especially given the lack of concrete standards for providers.
During the waning months of 2022, Dr. Gaind – who said he was suspended from CPA for “unprofessional interactions” and allegedly misrepresenting CPA’s processes and governance matters – announced the launch of a new organization, the Society of Canadian Psychiatry, in November calling for a delay in MAID of at least 1 year so that evidence-based safeguards could be implemented. The petition has been signed by more than 200 psychiatrists, along with several dozen physicians, MAID assessors, and individuals with mental illness and family members.
The Association of Chairs of Psychiatry in Canada, the Canadian Association for Suicide Prevention, the Council of Canadians with Disabilities, a group of indigenous leaders, and the Ontario Association for ACT and FACT, psychiatrists who provide care to individuals with severe mental illness, among other groups, joined the call for a delay.
In its December announcement, the Canadian federal ministers said a factor in seeking a delay was that standards guiding clinicians would not be delivered until at least February – too close to when applications would be opened.
Upon hearing about the federal government’s intentions, the chair of the expert panel, Mona Gupta, MD, told The Canadian Press that she did not think it was necessary to put off implementation because necessary safeguards were already in place.
Dr. Chaimowitz awaits the standards but is optimistic that for mental illness, “the process will be tightly controlled, closely monitored, and open to scrutiny,” he said.
Dr. Braswell is not convinced. The concern is that adding people with mental illness is “going to overload the capacity of the government to monitor this practice,” he said.
Is the United States next?
Although Canada and the United States share a border, it’s unlikely that U.S. states will allow aid in dying for nonterminal illness, much less for psychiatric conditions any time soon, said Dr. Braswell and others.
Ten states – California, Colorado, Hawaii, Maine, Montana, New Jersey, New Mexico, Oregon, Vermont, and Washington – have laws allowing assistance in dying, but for terminal illness only.
In 2016, the APA adopted the American Medical Association policy on medical euthanasia, stating, “that a psychiatrist should not prescribe or administer any intervention to a nonterminally ill person for the purpose of causing death.”
Dr. Brendel said the field is acutely aware that people with mental illness do suffer, but that more work needs to be done – and is being done – on “distinguishing wishes to hasten death or end one’s life from these historical or traditional notions that any premature death is a suicide.”
There is also increasing discussion within the medical community, not just psychiatry, about a physician’s duty to relieve suffering, said Dr. Wynia. “There’s debate basically about whether we stand for preserving life essentially at all costs and never being involved in the taking of life, or whether we stand for reduction of suffering and being the advocate for the patients that we serve,” he said.
“Those are both legitimate,” said Dr. Wynia, adding, “there are good reasons to want both of those to be true.”
“I suspect that 20 years from now we will still be having conversations about how physicians, how psychiatrists ought to participate in preserving life and in shepherding death,” said Dr. Brendel.
But to Dr. Gaind, the debate is not just esoteric, it’s a soon-to-be reality in Canada. “When we’re providing death to people who aren’t dying, to me that’s like providing what amounts to a wrongful death,” he said.
A version of this article originally appeared on Medscape.com.
Canada already has the largest number of deaths by MAID of any nation, with 10,064 in 2021, a 32% increase from 2020. With the addition of serious mental illness (SMI) as an eligible category, the country is on track to have the most liberal assisted-death policy in the world.
Concerns about the additional number of patients who could become eligible for MAID, and a lack of evidence-backed standards from disability rights groups, mental health advocates, First Nations leaders, psychiatrists, and other mental health providers, seems to have led the Canadian government to give the proposed law some sober second thought.
“Listening to experts and Canadians, we believe this date needs to be temporarily delayed,” said David Lametti, Canada’s minister of Justice and attorney general of Canada; Jean-Yves Duclos, minister of Health; and Carolyn Bennett, minister of Mental Health and Addictions, in a Dec. 15, 2022, joint statement.
Canada’s Parliament – which approved the expansion – will now have to vote on whether to okay a pause on the legislation.
However, the Canadian Psychiatric Association has not been calling for a delay in the proposed legislation. In a November 2021 statement, the CPA said it “does not take a position on the legality or morality of MAID,” but added that to deny MAID to people with mental illness was discriminatory, and that, as it was the law, it must be followed.
“CPA has not taken a position about MAID,” the association’s president Gary Chaimowitz, MBChB, told this news organization. “We know this is coming and our organization is trying to get its members ready for what will be most likely the ability of people with mental conditions to be able to request MAID,” said Dr. Chaimowitz, who is also head of forensic psychiatry at St. Joseph’s Healthcare and a professor of psychiatry at McMaster University, both in Hamilton, Ont.
Dr. Chaimowitz acknowledges that “a majority of psychiatrists do not want to be involved in anything to do with MAID.”
“The idea, certainly in psychiatry, is to get people well and we’ve been taught that people dying from a major mental disorder is something that we’re trained to prevent,” he added.
A ‘clinical option’
Assisted medical death is especially fraught in psychiatry, said Rebecca Brendel, MD, president of the American Psychiatric Association. She noted a 25-year life expectancy gap between people with SMI and those who do not have such conditions.
“As a profession we have very serious obligations to advance treatment so that a person with serious mental illness can live [a] full, productive, and healthy [life],” Dr. Brendel, associate director of the Center for Bioethics at Harvard Medical School in Boston, said in an interview.
Under the Canadian proposal, psychiatrists would be allowed to suggest MAID as a “clinical option.”
Harold Braswell, PhD, a fellow with The Hastings Center, a bioethics research institute, calls that problematic.
“It’s not neutral to suggest to someone that it would be theoretically reasonable to end their lives,” Dr. Braswell, associate professor at the Albert Gnaegi Center for Health Care Ethics at Saint Louis University, told this news organization.
It also creates a double standard in the treatment of suicidal ideation, in which suicide prevention is absolute for some, but encouraging it as a possibility for others, he added.
“To have that come from an authority figure is something that’s very harsh and, in my opinion, very potentially destructive,” especially for vulnerable groups, like First Nations people, who already have elevated rates of suicide, said Dr. Braswell.
Fierce debate
Since 2016, Canada has allowed MAID for medical conditions and diseases that will not improve and in cases where the evidence shows that medical providers can accurately predict the condition will not improve.
However, in 2019, a Quebec court ruled that the law unconstitutionally barred euthanasia in people who were not terminally ill. In March 2021, Canada’s criminal code was amended to allow MAID for people whose natural death was not “reasonably foreseeable,” but it excluded SMI for a period of 2 years, ending in March 2023.
The 2-year stay was intended to allow for study and to give mental health providers and MAID assessors time to develop standards.
The federal government charged a 12-member expert panel with determining how to safely allow MAID for SMI. In its final report released in May 2022 it recommended that standards be developed.
The panel acknowledged that for many conditions it may be impossible to make predictions about whether an individual might improve. However, it did not mention SMI.
In those cases, when MAID is requested, “establishing incurability and irreversibility on the basis of the evolution and response to past interventions is necessary,” the panel noted, adding that these are the criteria used by psychiatrists assessing euthanasia requests in the Netherlands and Belgium.
But the notion that mental illness can be irremediable has been fiercely debated.
Soon after the expert report was released, the Center for Addiction and Mental Health in Toronto noted on its website that there are currently “no agreed upon standards for psychiatrists or other health care practitioners to use to determine if a person’s mental illness is ‘grievous and irremediable’ for the purposes of MAID.”
Dr. Chaimowitz acknowledged that “there’s no agreed-upon definition of incurability” in mental illness. Some psychiatrists “will argue that there’s always another treatment that can be attempted,” he said, adding that there has been a lack of consensus on irremediability among CPA members.
Protecting vulnerable populations
Matt Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora, said the question of irremediability is crucial. “Most people with mental illness do get better, especially if they’re in treatment,” Dr. Wynia said.
For MAID assessors it may be difficult to know when someone has tried all possible treatments, especially given the wide array of options, including psychedelics, said Dr. Wynia.
Dr. Braswell said there is not enough evidence that mental illness is incurable. With SMI, “there’s a lot more potential for the causes of the individual’s suffering to be ameliorated. By offering MAID, you’re going to kill people who might have been able to get out of this through other nonlethal means.”
Currently, MAID is provided for an irremediable medical condition, “in other words, a condition that will not improve and that we can predict will not improve,” said Karandeep S. Gaind, MD, chief of psychiatry at Toronto’s Humber River Hospital and physician chair of the hospital’s MAID team.
“If that’s the premise, then I think we cannot provide MAID for sole mental illness,” Dr. Gaind said. “Because we can’t honestly make those predictions” with mental illness, he added.
Dr. Gaind does not support MAID for mental illness and believes that it will put the vulnerable – including those living in poverty – at particular risk.
With the proposed expansion, MAID is “now becoming something which is being sought as a way to escape a painful life rather than to avoid a painful death,” said Dr. Gaind, who is also a past president of the CPA.
One member of the federal government’s expert panel – Ellen Cohen, who had a psychiatric condition – wrote in The Globe and Mail that she quit early on when it became apparent that the panel was not seriously considering her own experiences or the possibility that poverty and lack of access to care or social supports could strongly influence a request for MAID.
Social determinants of suffering
People with mental illness often are without homes, have substance use disorders, have been stigmatized and discriminated against, and have poor social supports, said Dr. Wynia. “You worry that it’s all of those things that are making them want to end their lives,” he said.
The Daily Mail ran a story in December 2022 about a 65-year-old Canadian who said he’d applied for MAID solely because of fears that his disability benefits for various chronic health conditions were being cut off and that he didn’t want to live in poverty.
A 51-year-old Ontario woman with multiple chemical sensitivities was granted MAID after she said she could not find housing that could keep her safe, according to an August report by CTV News.
Tarek Rajji, MD, chief of the Adult Neurodevelopment and Geriatric Psychiatry Division at CAMH, said social determinants of health need to be considered in standards created to guide MAID for mental illness.
“We’re very mindful of the fact that the suffering, that is, the grievousness that the person is living with, in the context of mental illness, many times is due to the social determinants of their illness and the social determinants of their suffering,” Dr. Rajji said.
Many are also concerned that it will be difficult to separate out suicidality from sheer hopelessness.
The CPA has advised a group that’s working on developing guidelines for MAID in SMI and is also developing a curriculum for mental health providers, Dr. Chaimowitz said. As part of that, there will be a process to ensure that someone who is actively suicidal is not granted MAID.
“I do not believe that it’s contemplated that MAID is going to accelerate or facilitate suicidal ideation,” he said. Someone who is suicidal will be referred to treatment, said Dr. Chaimowitz.
“People with depression often feel hopeless,” and may refuse treatments that have worked in the past, countered Dr. Gaind. Some of his patients “are absolutely convinced that nothing will help,” he said.
Troublesome cases
The expert panel said in its final report that “it is not possible to provide fixed rules for how many attempts at interventions, how many types of interventions, and over how much time,” are necessary to establish “irreversibility” of mental illness.
Dr. Chaimowitz said MAID will not be offered to anyone “refusing treatment for their condition without any good reason.” They will be “unlikely to meet criteria for incurable,” as they will have needed to avail themselves of the array of treatments available, he said.
That would be similar to rules in Belgium and the Netherlands, which allow euthanasia for psychiatric conditions.
An estimated 100-300 psychiatric patients receive euthanasia each year in those countries, according to a 2021 commentary in Psychiatric Times (Jun 7;38[6]) by Mark S. Komrad, MD, a Towson, Maryland-based psychiatrist.
There are still troublesome cases.
As previously reported by this news organization, many in Belgium were distressed recently at the news that a 23-year-old woman who had survived a terrorist attack, Shanti De Corte, requested and was granted euthanasia.
As the deadline for implementation of MAID grew closer, calls for delay grew louder, especially given the lack of concrete standards for providers.
During the waning months of 2022, Dr. Gaind – who said he was suspended from CPA for “unprofessional interactions” and allegedly misrepresenting CPA’s processes and governance matters – announced the launch of a new organization, the Society of Canadian Psychiatry, in November calling for a delay in MAID of at least 1 year so that evidence-based safeguards could be implemented. The petition has been signed by more than 200 psychiatrists, along with several dozen physicians, MAID assessors, and individuals with mental illness and family members.
The Association of Chairs of Psychiatry in Canada, the Canadian Association for Suicide Prevention, the Council of Canadians with Disabilities, a group of indigenous leaders, and the Ontario Association for ACT and FACT, psychiatrists who provide care to individuals with severe mental illness, among other groups, joined the call for a delay.
In its December announcement, the Canadian federal ministers said a factor in seeking a delay was that standards guiding clinicians would not be delivered until at least February – too close to when applications would be opened.
Upon hearing about the federal government’s intentions, the chair of the expert panel, Mona Gupta, MD, told The Canadian Press that she did not think it was necessary to put off implementation because necessary safeguards were already in place.
Dr. Chaimowitz awaits the standards but is optimistic that for mental illness, “the process will be tightly controlled, closely monitored, and open to scrutiny,” he said.
Dr. Braswell is not convinced. The concern is that adding people with mental illness is “going to overload the capacity of the government to monitor this practice,” he said.
Is the United States next?
Although Canada and the United States share a border, it’s unlikely that U.S. states will allow aid in dying for nonterminal illness, much less for psychiatric conditions any time soon, said Dr. Braswell and others.
Ten states – California, Colorado, Hawaii, Maine, Montana, New Jersey, New Mexico, Oregon, Vermont, and Washington – have laws allowing assistance in dying, but for terminal illness only.
In 2016, the APA adopted the American Medical Association policy on medical euthanasia, stating, “that a psychiatrist should not prescribe or administer any intervention to a nonterminally ill person for the purpose of causing death.”
Dr. Brendel said the field is acutely aware that people with mental illness do suffer, but that more work needs to be done – and is being done – on “distinguishing wishes to hasten death or end one’s life from these historical or traditional notions that any premature death is a suicide.”
There is also increasing discussion within the medical community, not just psychiatry, about a physician’s duty to relieve suffering, said Dr. Wynia. “There’s debate basically about whether we stand for preserving life essentially at all costs and never being involved in the taking of life, or whether we stand for reduction of suffering and being the advocate for the patients that we serve,” he said.
“Those are both legitimate,” said Dr. Wynia, adding, “there are good reasons to want both of those to be true.”
“I suspect that 20 years from now we will still be having conversations about how physicians, how psychiatrists ought to participate in preserving life and in shepherding death,” said Dr. Brendel.
But to Dr. Gaind, the debate is not just esoteric, it’s a soon-to-be reality in Canada. “When we’re providing death to people who aren’t dying, to me that’s like providing what amounts to a wrongful death,” he said.
A version of this article originally appeared on Medscape.com.
Canada already has the largest number of deaths by MAID of any nation, with 10,064 in 2021, a 32% increase from 2020. With the addition of serious mental illness (SMI) as an eligible category, the country is on track to have the most liberal assisted-death policy in the world.
Concerns about the additional number of patients who could become eligible for MAID, and a lack of evidence-backed standards from disability rights groups, mental health advocates, First Nations leaders, psychiatrists, and other mental health providers, seems to have led the Canadian government to give the proposed law some sober second thought.
“Listening to experts and Canadians, we believe this date needs to be temporarily delayed,” said David Lametti, Canada’s minister of Justice and attorney general of Canada; Jean-Yves Duclos, minister of Health; and Carolyn Bennett, minister of Mental Health and Addictions, in a Dec. 15, 2022, joint statement.
Canada’s Parliament – which approved the expansion – will now have to vote on whether to okay a pause on the legislation.
However, the Canadian Psychiatric Association has not been calling for a delay in the proposed legislation. In a November 2021 statement, the CPA said it “does not take a position on the legality or morality of MAID,” but added that to deny MAID to people with mental illness was discriminatory, and that, as it was the law, it must be followed.
“CPA has not taken a position about MAID,” the association’s president Gary Chaimowitz, MBChB, told this news organization. “We know this is coming and our organization is trying to get its members ready for what will be most likely the ability of people with mental conditions to be able to request MAID,” said Dr. Chaimowitz, who is also head of forensic psychiatry at St. Joseph’s Healthcare and a professor of psychiatry at McMaster University, both in Hamilton, Ont.
Dr. Chaimowitz acknowledges that “a majority of psychiatrists do not want to be involved in anything to do with MAID.”
“The idea, certainly in psychiatry, is to get people well and we’ve been taught that people dying from a major mental disorder is something that we’re trained to prevent,” he added.
A ‘clinical option’
Assisted medical death is especially fraught in psychiatry, said Rebecca Brendel, MD, president of the American Psychiatric Association. She noted a 25-year life expectancy gap between people with SMI and those who do not have such conditions.
“As a profession we have very serious obligations to advance treatment so that a person with serious mental illness can live [a] full, productive, and healthy [life],” Dr. Brendel, associate director of the Center for Bioethics at Harvard Medical School in Boston, said in an interview.
Under the Canadian proposal, psychiatrists would be allowed to suggest MAID as a “clinical option.”
Harold Braswell, PhD, a fellow with The Hastings Center, a bioethics research institute, calls that problematic.
“It’s not neutral to suggest to someone that it would be theoretically reasonable to end their lives,” Dr. Braswell, associate professor at the Albert Gnaegi Center for Health Care Ethics at Saint Louis University, told this news organization.
It also creates a double standard in the treatment of suicidal ideation, in which suicide prevention is absolute for some, but encouraging it as a possibility for others, he added.
“To have that come from an authority figure is something that’s very harsh and, in my opinion, very potentially destructive,” especially for vulnerable groups, like First Nations people, who already have elevated rates of suicide, said Dr. Braswell.
Fierce debate
Since 2016, Canada has allowed MAID for medical conditions and diseases that will not improve and in cases where the evidence shows that medical providers can accurately predict the condition will not improve.
However, in 2019, a Quebec court ruled that the law unconstitutionally barred euthanasia in people who were not terminally ill. In March 2021, Canada’s criminal code was amended to allow MAID for people whose natural death was not “reasonably foreseeable,” but it excluded SMI for a period of 2 years, ending in March 2023.
The 2-year stay was intended to allow for study and to give mental health providers and MAID assessors time to develop standards.
The federal government charged a 12-member expert panel with determining how to safely allow MAID for SMI. In its final report released in May 2022 it recommended that standards be developed.
The panel acknowledged that for many conditions it may be impossible to make predictions about whether an individual might improve. However, it did not mention SMI.
In those cases, when MAID is requested, “establishing incurability and irreversibility on the basis of the evolution and response to past interventions is necessary,” the panel noted, adding that these are the criteria used by psychiatrists assessing euthanasia requests in the Netherlands and Belgium.
But the notion that mental illness can be irremediable has been fiercely debated.
Soon after the expert report was released, the Center for Addiction and Mental Health in Toronto noted on its website that there are currently “no agreed upon standards for psychiatrists or other health care practitioners to use to determine if a person’s mental illness is ‘grievous and irremediable’ for the purposes of MAID.”
Dr. Chaimowitz acknowledged that “there’s no agreed-upon definition of incurability” in mental illness. Some psychiatrists “will argue that there’s always another treatment that can be attempted,” he said, adding that there has been a lack of consensus on irremediability among CPA members.
Protecting vulnerable populations
Matt Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora, said the question of irremediability is crucial. “Most people with mental illness do get better, especially if they’re in treatment,” Dr. Wynia said.
For MAID assessors it may be difficult to know when someone has tried all possible treatments, especially given the wide array of options, including psychedelics, said Dr. Wynia.
Dr. Braswell said there is not enough evidence that mental illness is incurable. With SMI, “there’s a lot more potential for the causes of the individual’s suffering to be ameliorated. By offering MAID, you’re going to kill people who might have been able to get out of this through other nonlethal means.”
Currently, MAID is provided for an irremediable medical condition, “in other words, a condition that will not improve and that we can predict will not improve,” said Karandeep S. Gaind, MD, chief of psychiatry at Toronto’s Humber River Hospital and physician chair of the hospital’s MAID team.
“If that’s the premise, then I think we cannot provide MAID for sole mental illness,” Dr. Gaind said. “Because we can’t honestly make those predictions” with mental illness, he added.
Dr. Gaind does not support MAID for mental illness and believes that it will put the vulnerable – including those living in poverty – at particular risk.
With the proposed expansion, MAID is “now becoming something which is being sought as a way to escape a painful life rather than to avoid a painful death,” said Dr. Gaind, who is also a past president of the CPA.
One member of the federal government’s expert panel – Ellen Cohen, who had a psychiatric condition – wrote in The Globe and Mail that she quit early on when it became apparent that the panel was not seriously considering her own experiences or the possibility that poverty and lack of access to care or social supports could strongly influence a request for MAID.
Social determinants of suffering
People with mental illness often are without homes, have substance use disorders, have been stigmatized and discriminated against, and have poor social supports, said Dr. Wynia. “You worry that it’s all of those things that are making them want to end their lives,” he said.
The Daily Mail ran a story in December 2022 about a 65-year-old Canadian who said he’d applied for MAID solely because of fears that his disability benefits for various chronic health conditions were being cut off and that he didn’t want to live in poverty.
A 51-year-old Ontario woman with multiple chemical sensitivities was granted MAID after she said she could not find housing that could keep her safe, according to an August report by CTV News.
Tarek Rajji, MD, chief of the Adult Neurodevelopment and Geriatric Psychiatry Division at CAMH, said social determinants of health need to be considered in standards created to guide MAID for mental illness.
“We’re very mindful of the fact that the suffering, that is, the grievousness that the person is living with, in the context of mental illness, many times is due to the social determinants of their illness and the social determinants of their suffering,” Dr. Rajji said.
Many are also concerned that it will be difficult to separate out suicidality from sheer hopelessness.
The CPA has advised a group that’s working on developing guidelines for MAID in SMI and is also developing a curriculum for mental health providers, Dr. Chaimowitz said. As part of that, there will be a process to ensure that someone who is actively suicidal is not granted MAID.
“I do not believe that it’s contemplated that MAID is going to accelerate or facilitate suicidal ideation,” he said. Someone who is suicidal will be referred to treatment, said Dr. Chaimowitz.
“People with depression often feel hopeless,” and may refuse treatments that have worked in the past, countered Dr. Gaind. Some of his patients “are absolutely convinced that nothing will help,” he said.
Troublesome cases
The expert panel said in its final report that “it is not possible to provide fixed rules for how many attempts at interventions, how many types of interventions, and over how much time,” are necessary to establish “irreversibility” of mental illness.
Dr. Chaimowitz said MAID will not be offered to anyone “refusing treatment for their condition without any good reason.” They will be “unlikely to meet criteria for incurable,” as they will have needed to avail themselves of the array of treatments available, he said.
That would be similar to rules in Belgium and the Netherlands, which allow euthanasia for psychiatric conditions.
An estimated 100-300 psychiatric patients receive euthanasia each year in those countries, according to a 2021 commentary in Psychiatric Times (Jun 7;38[6]) by Mark S. Komrad, MD, a Towson, Maryland-based psychiatrist.
There are still troublesome cases.
As previously reported by this news organization, many in Belgium were distressed recently at the news that a 23-year-old woman who had survived a terrorist attack, Shanti De Corte, requested and was granted euthanasia.
As the deadline for implementation of MAID grew closer, calls for delay grew louder, especially given the lack of concrete standards for providers.
During the waning months of 2022, Dr. Gaind – who said he was suspended from CPA for “unprofessional interactions” and allegedly misrepresenting CPA’s processes and governance matters – announced the launch of a new organization, the Society of Canadian Psychiatry, in November calling for a delay in MAID of at least 1 year so that evidence-based safeguards could be implemented. The petition has been signed by more than 200 psychiatrists, along with several dozen physicians, MAID assessors, and individuals with mental illness and family members.
The Association of Chairs of Psychiatry in Canada, the Canadian Association for Suicide Prevention, the Council of Canadians with Disabilities, a group of indigenous leaders, and the Ontario Association for ACT and FACT, psychiatrists who provide care to individuals with severe mental illness, among other groups, joined the call for a delay.
In its December announcement, the Canadian federal ministers said a factor in seeking a delay was that standards guiding clinicians would not be delivered until at least February – too close to when applications would be opened.
Upon hearing about the federal government’s intentions, the chair of the expert panel, Mona Gupta, MD, told The Canadian Press that she did not think it was necessary to put off implementation because necessary safeguards were already in place.
Dr. Chaimowitz awaits the standards but is optimistic that for mental illness, “the process will be tightly controlled, closely monitored, and open to scrutiny,” he said.
Dr. Braswell is not convinced. The concern is that adding people with mental illness is “going to overload the capacity of the government to monitor this practice,” he said.
Is the United States next?
Although Canada and the United States share a border, it’s unlikely that U.S. states will allow aid in dying for nonterminal illness, much less for psychiatric conditions any time soon, said Dr. Braswell and others.
Ten states – California, Colorado, Hawaii, Maine, Montana, New Jersey, New Mexico, Oregon, Vermont, and Washington – have laws allowing assistance in dying, but for terminal illness only.
In 2016, the APA adopted the American Medical Association policy on medical euthanasia, stating, “that a psychiatrist should not prescribe or administer any intervention to a nonterminally ill person for the purpose of causing death.”
Dr. Brendel said the field is acutely aware that people with mental illness do suffer, but that more work needs to be done – and is being done – on “distinguishing wishes to hasten death or end one’s life from these historical or traditional notions that any premature death is a suicide.”
There is also increasing discussion within the medical community, not just psychiatry, about a physician’s duty to relieve suffering, said Dr. Wynia. “There’s debate basically about whether we stand for preserving life essentially at all costs and never being involved in the taking of life, or whether we stand for reduction of suffering and being the advocate for the patients that we serve,” he said.
“Those are both legitimate,” said Dr. Wynia, adding, “there are good reasons to want both of those to be true.”
“I suspect that 20 years from now we will still be having conversations about how physicians, how psychiatrists ought to participate in preserving life and in shepherding death,” said Dr. Brendel.
But to Dr. Gaind, the debate is not just esoteric, it’s a soon-to-be reality in Canada. “When we’re providing death to people who aren’t dying, to me that’s like providing what amounts to a wrongful death,” he said.
A version of this article originally appeared on Medscape.com.
Childhood behavioral, emotional problems linked to poor economic and social outcomes in adulthood
Children with chronically elevated externalizing symptoms, such as behavioral problems, or internalizing symptoms, such as mental health concerns, have an increased risk for poor economic and social outcomes in adulthood, data from a new study suggest.
Children with comorbid externalizing and internalizing symptoms were especially vulnerable to long-term economic and social exclusion.
“Research has mostly studied the outcomes of children with either behavioral problems or depression-anxiety problems. However, comorbidity is the rule rather than the exception in clinical practice,” senior author Massimilliano Orri, PhD, an assistant professor of psychiatry at McGill University and clinical psychologist with the Douglas Mental Health University Institute, both in Montreal, said in an interview.
“Our findings are important, as they show that comorbidity between externalizing and internalizing problems is associated with real-life outcomes that profoundly influence a youth’s chances to participate in society later in life,” he said.
The study was published in JAMA Network Open.
Analyzing associations
Dr. Orri and colleagues analyzed data for 3,017 children in the Quebec Longitudinal Study of Kindergarten Children, a population-based birth cohort that enrolled participants in 1986-1987 and 1987-1988 while they were attending kindergarten. The sample included 2,000 children selected at random and 1,017 children who scored at or above the 80th percentile for disruptive behavior problems.
The research team looked at the association between childhood behavioral profiles and economic and social outcomes for ages 19-37 years, including employment earnings, receipt of welfare, intimate partnerships, and having children living in the household. They obtained the outcome data from participants’ tax returns for 1998-2017.
During enrollment in the study, the children’s teachers assessed behavioral symptoms annually for ages 6-12 years using the Social Behavior Questionnaire. Based on the assessments, the research team categorized the students as having no or low symptoms, high externalizing symptoms only (such as hyperactivity, impulsivity, aggression, and rule violation), high internalizing symptoms only (such as anxiety, depression, worry, and social withdrawal), or comorbid symptoms. They looked at other variables as well, including the child’s sex, the parents’ age at the birth of their first child, the parents’ years of education, family structure, and the parents’ household income.
Among the 3,017 participants, 45.4% of children had no or low symptoms, 29.2% had high externalizing symptoms, 11.7% had high internalizing symptoms, and 13.7% had comorbid symptoms. About 53% were boys, and 47% were girls.
In general, boys were more likely to exhibit high externalizing symptoms, and girls were more likely to exhibit high internalizing symptoms. In the comorbid group, about 82% were boys, and they were more likely to have younger mothers, come from households with lower earnings when they were ages 3-5 years, and have a nonintact family at age 6 years.
The average age at follow-up was 37 years. Participants earned an average of $32,800 per year at ages 33-37 years (between 2013 and 2017). During the 20 years of follow-up, participants received welfare support for about 1.5 years, had an intimate partner for 7.4 years, and had children living in the household for 11 years.
Overall, participants in the high externalizing and high internalizing symptom profiles – and especially those in the comorbid profile – had lower earnings and a higher incidence of annual welfare receipt across early adulthood, compared with participants with low or no symptoms. They were also less likely to have an intimate partner or have children living in the household. Participants with a comorbid symptom profile earned $15,031 less per year and had a 3.79-times higher incidence of annual welfare receipt.
Lower earnings
Across the sample, men were more likely to have higher earnings and less likely to receive welfare each year, but they also were less likely to have an intimate partner or have children in the household. Among those with the high externalizing profile, men were significantly less likely to receive welfare. Among the comorbid profile, men were less likely to have children in the household.
Compared with the no-symptom or low-symptom profile, those in the high externalizing profile earned $5,904 less per year and had a two-times–higher incidence of welfare receipt. Those in the high internalizing profile earned $8,473 less per year, had a 2.07-times higher incidence of welfare receipt, and had a lower incidence of intimate partnership.
Compared with the high externalizing profile, those in the comorbid profile earned $9,126 less per year, had a higher incidence of annual welfare receipt, had a lower incidence of intimate partnership, and were less likely to have children in the household. Similarly, compared with the high internalizing profile, those in the comorbid profile earned $6,558 less per year and were more likely to exhibit the other poor long-term outcomes. Participants in the high internalizing profile earned $2,568 less per year than those in the high externalizing profile.
During a 40-year working career, the estimated lost personal employment earnings were $140,515 for the high externalizing profile, $201,657 for the high internalizing profile, and $357,737 for the comorbid profile, compared with those in the no-symptom or low-symptom profile.
“We know that children with externalizing and internalizing symptoms can have many problems in the short term – like social difficulties and lower education attainment – but it’s important to also understand the potential long-term outcomes,” study author Francis Vergunst, DPhil/PhD, an associate professor of child psychosocial difficulties at the University of Oslo, told this news organization.
“For example, when people have insufficient income, are forced to seek welfare support, or lack the social support structure that comes from an intimate partnership, it can have profound consequences for their mental health and well-being – and for society as a whole,” he said. “Understanding this helps to build the case for early prevention programs that can reduce childhood externalizing and internalizing problems and improve long-term outcomes.”
Several mechanisms could explain the associations found across the childhood symptom profiles, the study authors wrote. For instance, children with early behavior problems may be more likely to engage in risky adolescent activities, such as substance use, delinquent peer affiliations, and academic underachievement, which affects their transition to adulthood and accumulation of social and economic capital throughout life. Those with comorbid symptoms likely experience a compounded effect.
Future studies should investigate how to intervene effectively to support children, particularly those with comorbid externalizing and internalizing symptoms, the study authors write.
“Currently, most published studies focus on children with either externalizing or internalizing problems (and these programs can be effective, especially for externalizing problems), but we know very little about how to improve long-term outcomes for children with comorbid symptoms,” Dr. Vergunst said. “Given the large costs of these problems for individuals and society, this is a critical area for further research.”
‘Solid evidence’
Commenting on the findings, Ian Colman, PhD, a professor of epidemiology and public health and director of the Applied Psychiatric Epidemiology Across the Life course (APEAL) lab at the University of Ottawa, said, “Research like this provides solid evidence that if we do not provide appropriate supports for children who are struggling with their mental health or related behaviors, then these children are more likely to face a life of social and economic exclusion.”
Dr. Colman, who wasn’t involved with this study, has researched long-term psychosocial outcomes among adolescents with depression, as well as those with externalizing behaviors. He and colleagues have found poorer outcomes among those who exhibit mild or severe difficulties during childhood.
“Studying the long-term outcomes associated with child and adolescent mental and behavioral disorders gives us an idea of how concerned we should be about their future,” he said.
Dr. Vergunst was funded by the Canadian Institute of Health Research and Fonds de Recherche du Quebec Santé postdoctoral fellowships. Dr. Orri and Dr. Colman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children with chronically elevated externalizing symptoms, such as behavioral problems, or internalizing symptoms, such as mental health concerns, have an increased risk for poor economic and social outcomes in adulthood, data from a new study suggest.
Children with comorbid externalizing and internalizing symptoms were especially vulnerable to long-term economic and social exclusion.
“Research has mostly studied the outcomes of children with either behavioral problems or depression-anxiety problems. However, comorbidity is the rule rather than the exception in clinical practice,” senior author Massimilliano Orri, PhD, an assistant professor of psychiatry at McGill University and clinical psychologist with the Douglas Mental Health University Institute, both in Montreal, said in an interview.
“Our findings are important, as they show that comorbidity between externalizing and internalizing problems is associated with real-life outcomes that profoundly influence a youth’s chances to participate in society later in life,” he said.
The study was published in JAMA Network Open.
Analyzing associations
Dr. Orri and colleagues analyzed data for 3,017 children in the Quebec Longitudinal Study of Kindergarten Children, a population-based birth cohort that enrolled participants in 1986-1987 and 1987-1988 while they were attending kindergarten. The sample included 2,000 children selected at random and 1,017 children who scored at or above the 80th percentile for disruptive behavior problems.
The research team looked at the association between childhood behavioral profiles and economic and social outcomes for ages 19-37 years, including employment earnings, receipt of welfare, intimate partnerships, and having children living in the household. They obtained the outcome data from participants’ tax returns for 1998-2017.
During enrollment in the study, the children’s teachers assessed behavioral symptoms annually for ages 6-12 years using the Social Behavior Questionnaire. Based on the assessments, the research team categorized the students as having no or low symptoms, high externalizing symptoms only (such as hyperactivity, impulsivity, aggression, and rule violation), high internalizing symptoms only (such as anxiety, depression, worry, and social withdrawal), or comorbid symptoms. They looked at other variables as well, including the child’s sex, the parents’ age at the birth of their first child, the parents’ years of education, family structure, and the parents’ household income.
Among the 3,017 participants, 45.4% of children had no or low symptoms, 29.2% had high externalizing symptoms, 11.7% had high internalizing symptoms, and 13.7% had comorbid symptoms. About 53% were boys, and 47% were girls.
In general, boys were more likely to exhibit high externalizing symptoms, and girls were more likely to exhibit high internalizing symptoms. In the comorbid group, about 82% were boys, and they were more likely to have younger mothers, come from households with lower earnings when they were ages 3-5 years, and have a nonintact family at age 6 years.
The average age at follow-up was 37 years. Participants earned an average of $32,800 per year at ages 33-37 years (between 2013 and 2017). During the 20 years of follow-up, participants received welfare support for about 1.5 years, had an intimate partner for 7.4 years, and had children living in the household for 11 years.
Overall, participants in the high externalizing and high internalizing symptom profiles – and especially those in the comorbid profile – had lower earnings and a higher incidence of annual welfare receipt across early adulthood, compared with participants with low or no symptoms. They were also less likely to have an intimate partner or have children living in the household. Participants with a comorbid symptom profile earned $15,031 less per year and had a 3.79-times higher incidence of annual welfare receipt.
Lower earnings
Across the sample, men were more likely to have higher earnings and less likely to receive welfare each year, but they also were less likely to have an intimate partner or have children in the household. Among those with the high externalizing profile, men were significantly less likely to receive welfare. Among the comorbid profile, men were less likely to have children in the household.
Compared with the no-symptom or low-symptom profile, those in the high externalizing profile earned $5,904 less per year and had a two-times–higher incidence of welfare receipt. Those in the high internalizing profile earned $8,473 less per year, had a 2.07-times higher incidence of welfare receipt, and had a lower incidence of intimate partnership.
Compared with the high externalizing profile, those in the comorbid profile earned $9,126 less per year, had a higher incidence of annual welfare receipt, had a lower incidence of intimate partnership, and were less likely to have children in the household. Similarly, compared with the high internalizing profile, those in the comorbid profile earned $6,558 less per year and were more likely to exhibit the other poor long-term outcomes. Participants in the high internalizing profile earned $2,568 less per year than those in the high externalizing profile.
During a 40-year working career, the estimated lost personal employment earnings were $140,515 for the high externalizing profile, $201,657 for the high internalizing profile, and $357,737 for the comorbid profile, compared with those in the no-symptom or low-symptom profile.
“We know that children with externalizing and internalizing symptoms can have many problems in the short term – like social difficulties and lower education attainment – but it’s important to also understand the potential long-term outcomes,” study author Francis Vergunst, DPhil/PhD, an associate professor of child psychosocial difficulties at the University of Oslo, told this news organization.
“For example, when people have insufficient income, are forced to seek welfare support, or lack the social support structure that comes from an intimate partnership, it can have profound consequences for their mental health and well-being – and for society as a whole,” he said. “Understanding this helps to build the case for early prevention programs that can reduce childhood externalizing and internalizing problems and improve long-term outcomes.”
Several mechanisms could explain the associations found across the childhood symptom profiles, the study authors wrote. For instance, children with early behavior problems may be more likely to engage in risky adolescent activities, such as substance use, delinquent peer affiliations, and academic underachievement, which affects their transition to adulthood and accumulation of social and economic capital throughout life. Those with comorbid symptoms likely experience a compounded effect.
Future studies should investigate how to intervene effectively to support children, particularly those with comorbid externalizing and internalizing symptoms, the study authors write.
“Currently, most published studies focus on children with either externalizing or internalizing problems (and these programs can be effective, especially for externalizing problems), but we know very little about how to improve long-term outcomes for children with comorbid symptoms,” Dr. Vergunst said. “Given the large costs of these problems for individuals and society, this is a critical area for further research.”
‘Solid evidence’
Commenting on the findings, Ian Colman, PhD, a professor of epidemiology and public health and director of the Applied Psychiatric Epidemiology Across the Life course (APEAL) lab at the University of Ottawa, said, “Research like this provides solid evidence that if we do not provide appropriate supports for children who are struggling with their mental health or related behaviors, then these children are more likely to face a life of social and economic exclusion.”
Dr. Colman, who wasn’t involved with this study, has researched long-term psychosocial outcomes among adolescents with depression, as well as those with externalizing behaviors. He and colleagues have found poorer outcomes among those who exhibit mild or severe difficulties during childhood.
“Studying the long-term outcomes associated with child and adolescent mental and behavioral disorders gives us an idea of how concerned we should be about their future,” he said.
Dr. Vergunst was funded by the Canadian Institute of Health Research and Fonds de Recherche du Quebec Santé postdoctoral fellowships. Dr. Orri and Dr. Colman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children with chronically elevated externalizing symptoms, such as behavioral problems, or internalizing symptoms, such as mental health concerns, have an increased risk for poor economic and social outcomes in adulthood, data from a new study suggest.
Children with comorbid externalizing and internalizing symptoms were especially vulnerable to long-term economic and social exclusion.
“Research has mostly studied the outcomes of children with either behavioral problems or depression-anxiety problems. However, comorbidity is the rule rather than the exception in clinical practice,” senior author Massimilliano Orri, PhD, an assistant professor of psychiatry at McGill University and clinical psychologist with the Douglas Mental Health University Institute, both in Montreal, said in an interview.
“Our findings are important, as they show that comorbidity between externalizing and internalizing problems is associated with real-life outcomes that profoundly influence a youth’s chances to participate in society later in life,” he said.
The study was published in JAMA Network Open.
Analyzing associations
Dr. Orri and colleagues analyzed data for 3,017 children in the Quebec Longitudinal Study of Kindergarten Children, a population-based birth cohort that enrolled participants in 1986-1987 and 1987-1988 while they were attending kindergarten. The sample included 2,000 children selected at random and 1,017 children who scored at or above the 80th percentile for disruptive behavior problems.
The research team looked at the association between childhood behavioral profiles and economic and social outcomes for ages 19-37 years, including employment earnings, receipt of welfare, intimate partnerships, and having children living in the household. They obtained the outcome data from participants’ tax returns for 1998-2017.
During enrollment in the study, the children’s teachers assessed behavioral symptoms annually for ages 6-12 years using the Social Behavior Questionnaire. Based on the assessments, the research team categorized the students as having no or low symptoms, high externalizing symptoms only (such as hyperactivity, impulsivity, aggression, and rule violation), high internalizing symptoms only (such as anxiety, depression, worry, and social withdrawal), or comorbid symptoms. They looked at other variables as well, including the child’s sex, the parents’ age at the birth of their first child, the parents’ years of education, family structure, and the parents’ household income.
Among the 3,017 participants, 45.4% of children had no or low symptoms, 29.2% had high externalizing symptoms, 11.7% had high internalizing symptoms, and 13.7% had comorbid symptoms. About 53% were boys, and 47% were girls.
In general, boys were more likely to exhibit high externalizing symptoms, and girls were more likely to exhibit high internalizing symptoms. In the comorbid group, about 82% were boys, and they were more likely to have younger mothers, come from households with lower earnings when they were ages 3-5 years, and have a nonintact family at age 6 years.
The average age at follow-up was 37 years. Participants earned an average of $32,800 per year at ages 33-37 years (between 2013 and 2017). During the 20 years of follow-up, participants received welfare support for about 1.5 years, had an intimate partner for 7.4 years, and had children living in the household for 11 years.
Overall, participants in the high externalizing and high internalizing symptom profiles – and especially those in the comorbid profile – had lower earnings and a higher incidence of annual welfare receipt across early adulthood, compared with participants with low or no symptoms. They were also less likely to have an intimate partner or have children living in the household. Participants with a comorbid symptom profile earned $15,031 less per year and had a 3.79-times higher incidence of annual welfare receipt.
Lower earnings
Across the sample, men were more likely to have higher earnings and less likely to receive welfare each year, but they also were less likely to have an intimate partner or have children in the household. Among those with the high externalizing profile, men were significantly less likely to receive welfare. Among the comorbid profile, men were less likely to have children in the household.
Compared with the no-symptom or low-symptom profile, those in the high externalizing profile earned $5,904 less per year and had a two-times–higher incidence of welfare receipt. Those in the high internalizing profile earned $8,473 less per year, had a 2.07-times higher incidence of welfare receipt, and had a lower incidence of intimate partnership.
Compared with the high externalizing profile, those in the comorbid profile earned $9,126 less per year, had a higher incidence of annual welfare receipt, had a lower incidence of intimate partnership, and were less likely to have children in the household. Similarly, compared with the high internalizing profile, those in the comorbid profile earned $6,558 less per year and were more likely to exhibit the other poor long-term outcomes. Participants in the high internalizing profile earned $2,568 less per year than those in the high externalizing profile.
During a 40-year working career, the estimated lost personal employment earnings were $140,515 for the high externalizing profile, $201,657 for the high internalizing profile, and $357,737 for the comorbid profile, compared with those in the no-symptom or low-symptom profile.
“We know that children with externalizing and internalizing symptoms can have many problems in the short term – like social difficulties and lower education attainment – but it’s important to also understand the potential long-term outcomes,” study author Francis Vergunst, DPhil/PhD, an associate professor of child psychosocial difficulties at the University of Oslo, told this news organization.
“For example, when people have insufficient income, are forced to seek welfare support, or lack the social support structure that comes from an intimate partnership, it can have profound consequences for their mental health and well-being – and for society as a whole,” he said. “Understanding this helps to build the case for early prevention programs that can reduce childhood externalizing and internalizing problems and improve long-term outcomes.”
Several mechanisms could explain the associations found across the childhood symptom profiles, the study authors wrote. For instance, children with early behavior problems may be more likely to engage in risky adolescent activities, such as substance use, delinquent peer affiliations, and academic underachievement, which affects their transition to adulthood and accumulation of social and economic capital throughout life. Those with comorbid symptoms likely experience a compounded effect.
Future studies should investigate how to intervene effectively to support children, particularly those with comorbid externalizing and internalizing symptoms, the study authors write.
“Currently, most published studies focus on children with either externalizing or internalizing problems (and these programs can be effective, especially for externalizing problems), but we know very little about how to improve long-term outcomes for children with comorbid symptoms,” Dr. Vergunst said. “Given the large costs of these problems for individuals and society, this is a critical area for further research.”
‘Solid evidence’
Commenting on the findings, Ian Colman, PhD, a professor of epidemiology and public health and director of the Applied Psychiatric Epidemiology Across the Life course (APEAL) lab at the University of Ottawa, said, “Research like this provides solid evidence that if we do not provide appropriate supports for children who are struggling with their mental health or related behaviors, then these children are more likely to face a life of social and economic exclusion.”
Dr. Colman, who wasn’t involved with this study, has researched long-term psychosocial outcomes among adolescents with depression, as well as those with externalizing behaviors. He and colleagues have found poorer outcomes among those who exhibit mild or severe difficulties during childhood.
“Studying the long-term outcomes associated with child and adolescent mental and behavioral disorders gives us an idea of how concerned we should be about their future,” he said.
Dr. Vergunst was funded by the Canadian Institute of Health Research and Fonds de Recherche du Quebec Santé postdoctoral fellowships. Dr. Orri and Dr. Colman report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Depression guidelines fall short in characterizing withdrawal
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Previous research suggests that approximately half of patients who discontinue or decrease dosage of antidepressants experience withdrawal symptoms, wrote Anders Sørensen, MD, of Copenhagen University Hospital, and colleagues. These symptoms are diverse and may include flulike symptoms, fatigue, anxiety, and sensations of electric shock, they noted. Most withdrawal effects last for a few weeks, but some persist for months or years, sometimes described as persistent postwithdrawal disorder, they added.
“Symptoms of withdrawal and depression overlap considerably but constitute two fundamentally different clinical conditions, which makes it important to distinguish between the two,” the researchers emphasized.
In a study published in the Journal of Affective Disorders, the researchers identified 21 clinical practice guidelines (CPGs) for depression published between 1998 and 2022. The guidelines were published in the United Kingdom, the United States, Canada, Australia, Singapore, Ireland, and New Zealand. They compared descriptions of withdrawal from antidepressants and calculated the proportion of CPGs with different information.
Overall, 15 of the 21 studies in the review (71%) noted that antidepressants are associated with withdrawal symptoms, but less than half (43%) used the term “withdrawal symptoms,” or similar. Of the nine guidelines that mentioned withdrawal symptoms, five used the term interchangeably with “discontinuation symptoms” and six used the term “discontinuation symptoms” only when discussing antidepressant withdrawal. In addition, six CPGs specifically stated that patients who stop antidepressants can experience withdrawal symptoms, and five stated that these symptoms also can occur in patients who are reducing or tapering their doses.
The type of withdrawal symptoms was mentioned in 10 CPGs, and the other 11 had no information on potential withdrawal symptoms, the researchers noted. Of the CPGs that mentioned symptoms specifically associated with withdrawal, the number of potential symptoms ranged from 4 to 39.
“None of the CPGs provided an exhaustive list of the potential withdrawal symptoms identified in the research literature,” the researchers wrote in their discussion.
Only four of the guidelines (19%) mentioned the overlap in symptoms between withdrawal from antidepressants and depression relapse, and only one provided guidance on distinguishing between the two conditions. Most of the symptoms of withdrawal, when described, were characterized as mild, brief, or self-limiting, the researchers noted.
“Being in withdrawal is a fundamentally different clinical situation than experiencing relapse, requiring two distinctly different treatment approaches,” the researchers emphasized. “Withdrawal reactions that are more severe and longer lasting than currently defined in the CPGs could risk getting misinterpreted as relapse, potentially leading to resumed unnecessary long-term antidepressant treatment in some patients,” they added.
The findings were limited by several factors including the inclusion only of guidelines from English-speaking countries, which may limit generalizability, the researchers noted. Other potential limitations include the subjective judgments involved in creating different guidelines, they said.
However, the results support the need for improved CPGs that help clinicians distinguish potential withdrawal reactions from depression relapse, and the need for more research on optimal dose reduction strategies for antidepressants, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS



