User login
NAFLD less common, more severe in black children
ORLANDO – according to a review of 503 adolescents at the Yale University pediatric obesity clinic in New Haven, Conn.
As childhood obesity rates have climbed – the prevalence is now estimated to be around 20% – there’s been a corresponding increase in pediatric NAFLD, but it’s not very well characterized in children, and “there are many gaps in our knowledge,” said Nicola Santoro, MD, PhD, an assistant professor of pediatric endocrinology at Yale, and senior author of the review.
The goal of the work was to begin to plug the gaps. The children had baseline abdominal MRIs to quantify their hepatic fat content, along with oral glucose tolerance tests and genotyping for three single nucleotide polymorphisms (SNPs) strongly associated with the condition (PNPLA3 rs738409, GCKR rs1260326, and TM6SF2 rs58542926). MRI and metabolic testing were repeated at a mean of 2.27 years in 133 children.
The subjects were 13 years old on average, with a mean body mass index z-score of 2.52; 191 were white, 134 black, and 178 Hispanic. NAFLD was defined as a hepatic fat content of at least 5.5%.
The prevalence of fatty liver was 41.6% but ranged widely by ethnicity, with NAFLD diagnosed in 60% of Hispanic, 43% of white, but only 16% of black children. Among all three groups, prevalence was higher among boys.
Although NAFLD was least common among black children, when it was present, it was worse. Black children with NAFLD, compared with others, had the highest fasting glucose and 2-hour glucose levels; the highest insulin and C-peptide levels, and the highest hemoglobin A1c, despite similar age and gender distribution across the groups.
The findings translated to a higher prevalence of prediabetes and type 2 diabetes mellitus (66.6%), compared with white (24.4%) and Hispanic children (31.1%) with NAFLD.
Among 76 children who didn’t have NAFLD at baseline, 17 were diagnosed with the condition at follow-up. Progressors, compared with nonprogressors, showed higher baseline C-peptide levels (about 1,250 pmol/L versus 1,000 pmol/L) and greater weight gain (increase, versus a loss of, about 0.1 point on body mass index z-scores). Black children were the least likely to progress to NAFLD.
Increasing BMI z-score, higher baseline fasting C-peptide levels, and nonblack race strongly predicted progression (area under the curve = 0.887). The risk of progression was even higher when a NAFLD SNP was on board (AUC equal to or greater than 0.96).
Of 57 children with NAFLD at baseline, 13 didn’t meet the definition at follow-up, but regression turned out to be harder to predict. Regressors showed lower intrahepatic fat fractions at baseline (about 10% versus 20%), and a lowering of BMI z-scores at follow-up. Adding SNPs didn’t improve the model (AUC = 0.756).
As in adults, weight loss is the single most important factor to reverse NAFLD. “Even if you lose only a few kilos, fatty liver can go away. The liver cleans up pretty easily, but if you keep your weight, or you gain even a little bit, the disease keeps progressing,” Dr. Santoro said at the annual scientific sessions of the American Diabetes Association.
The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
*This story was updated on 7/20/2018.
SOURCE: Trico D et al. ADA 2018, Abstract 313-OR.
ORLANDO – according to a review of 503 adolescents at the Yale University pediatric obesity clinic in New Haven, Conn.
As childhood obesity rates have climbed – the prevalence is now estimated to be around 20% – there’s been a corresponding increase in pediatric NAFLD, but it’s not very well characterized in children, and “there are many gaps in our knowledge,” said Nicola Santoro, MD, PhD, an assistant professor of pediatric endocrinology at Yale, and senior author of the review.
The goal of the work was to begin to plug the gaps. The children had baseline abdominal MRIs to quantify their hepatic fat content, along with oral glucose tolerance tests and genotyping for three single nucleotide polymorphisms (SNPs) strongly associated with the condition (PNPLA3 rs738409, GCKR rs1260326, and TM6SF2 rs58542926). MRI and metabolic testing were repeated at a mean of 2.27 years in 133 children.
The subjects were 13 years old on average, with a mean body mass index z-score of 2.52; 191 were white, 134 black, and 178 Hispanic. NAFLD was defined as a hepatic fat content of at least 5.5%.
The prevalence of fatty liver was 41.6% but ranged widely by ethnicity, with NAFLD diagnosed in 60% of Hispanic, 43% of white, but only 16% of black children. Among all three groups, prevalence was higher among boys.
Although NAFLD was least common among black children, when it was present, it was worse. Black children with NAFLD, compared with others, had the highest fasting glucose and 2-hour glucose levels; the highest insulin and C-peptide levels, and the highest hemoglobin A1c, despite similar age and gender distribution across the groups.
The findings translated to a higher prevalence of prediabetes and type 2 diabetes mellitus (66.6%), compared with white (24.4%) and Hispanic children (31.1%) with NAFLD.
Among 76 children who didn’t have NAFLD at baseline, 17 were diagnosed with the condition at follow-up. Progressors, compared with nonprogressors, showed higher baseline C-peptide levels (about 1,250 pmol/L versus 1,000 pmol/L) and greater weight gain (increase, versus a loss of, about 0.1 point on body mass index z-scores). Black children were the least likely to progress to NAFLD.
Increasing BMI z-score, higher baseline fasting C-peptide levels, and nonblack race strongly predicted progression (area under the curve = 0.887). The risk of progression was even higher when a NAFLD SNP was on board (AUC equal to or greater than 0.96).
Of 57 children with NAFLD at baseline, 13 didn’t meet the definition at follow-up, but regression turned out to be harder to predict. Regressors showed lower intrahepatic fat fractions at baseline (about 10% versus 20%), and a lowering of BMI z-scores at follow-up. Adding SNPs didn’t improve the model (AUC = 0.756).
As in adults, weight loss is the single most important factor to reverse NAFLD. “Even if you lose only a few kilos, fatty liver can go away. The liver cleans up pretty easily, but if you keep your weight, or you gain even a little bit, the disease keeps progressing,” Dr. Santoro said at the annual scientific sessions of the American Diabetes Association.
The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
*This story was updated on 7/20/2018.
SOURCE: Trico D et al. ADA 2018, Abstract 313-OR.
ORLANDO – according to a review of 503 adolescents at the Yale University pediatric obesity clinic in New Haven, Conn.
As childhood obesity rates have climbed – the prevalence is now estimated to be around 20% – there’s been a corresponding increase in pediatric NAFLD, but it’s not very well characterized in children, and “there are many gaps in our knowledge,” said Nicola Santoro, MD, PhD, an assistant professor of pediatric endocrinology at Yale, and senior author of the review.
The goal of the work was to begin to plug the gaps. The children had baseline abdominal MRIs to quantify their hepatic fat content, along with oral glucose tolerance tests and genotyping for three single nucleotide polymorphisms (SNPs) strongly associated with the condition (PNPLA3 rs738409, GCKR rs1260326, and TM6SF2 rs58542926). MRI and metabolic testing were repeated at a mean of 2.27 years in 133 children.
The subjects were 13 years old on average, with a mean body mass index z-score of 2.52; 191 were white, 134 black, and 178 Hispanic. NAFLD was defined as a hepatic fat content of at least 5.5%.
The prevalence of fatty liver was 41.6% but ranged widely by ethnicity, with NAFLD diagnosed in 60% of Hispanic, 43% of white, but only 16% of black children. Among all three groups, prevalence was higher among boys.
Although NAFLD was least common among black children, when it was present, it was worse. Black children with NAFLD, compared with others, had the highest fasting glucose and 2-hour glucose levels; the highest insulin and C-peptide levels, and the highest hemoglobin A1c, despite similar age and gender distribution across the groups.
The findings translated to a higher prevalence of prediabetes and type 2 diabetes mellitus (66.6%), compared with white (24.4%) and Hispanic children (31.1%) with NAFLD.
Among 76 children who didn’t have NAFLD at baseline, 17 were diagnosed with the condition at follow-up. Progressors, compared with nonprogressors, showed higher baseline C-peptide levels (about 1,250 pmol/L versus 1,000 pmol/L) and greater weight gain (increase, versus a loss of, about 0.1 point on body mass index z-scores). Black children were the least likely to progress to NAFLD.
Increasing BMI z-score, higher baseline fasting C-peptide levels, and nonblack race strongly predicted progression (area under the curve = 0.887). The risk of progression was even higher when a NAFLD SNP was on board (AUC equal to or greater than 0.96).
Of 57 children with NAFLD at baseline, 13 didn’t meet the definition at follow-up, but regression turned out to be harder to predict. Regressors showed lower intrahepatic fat fractions at baseline (about 10% versus 20%), and a lowering of BMI z-scores at follow-up. Adding SNPs didn’t improve the model (AUC = 0.756).
As in adults, weight loss is the single most important factor to reverse NAFLD. “Even if you lose only a few kilos, fatty liver can go away. The liver cleans up pretty easily, but if you keep your weight, or you gain even a little bit, the disease keeps progressing,” Dr. Santoro said at the annual scientific sessions of the American Diabetes Association.
The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
*This story was updated on 7/20/2018.
SOURCE: Trico D et al. ADA 2018, Abstract 313-OR.
REPORTING FROM ADA 2018
Key clinical point: Obese black children are less likely than others to develop non-alcoholic fatty liver disease, but more likely to suffer its consequences if they do.
Major finding: Black children with NAFLD had a higher prevalence of prediabetes and type 2 diabetes (66.6%), compared with white (24.4%) and Hispanic children (31.1%).
Study details: Review of 503 obese adolescents
Disclosures: The investigators didn’t have any disclosures. The work was funded by the National Institutes of Health.
Source: Trico D et al. ADA 2018, Abstract 313-OR.
One practice’s experience with obesity treatment
WASHINGTON – “We have heard about a lot of really interesting new things in therapy, but the reality is that they are just not economically viable at present,” said David Feldshon, MD, of Minnesota Gastroenterology (MNGI), speaking about his experience with offering obesity treatment in his practice.
One problem that Dr. Feldshon and MNGI faced was that they could never get enough patients into their weight loss program, a meal-replacement program priced at about $200 a month. Part of this program, Optifast, a commercially available low-calorie diet and behavior modification program, cost about $360 a month. Most patients found the cost to be prohibitive according to Dr. Feldshon. The program that MNGI offered was fairly competitive when compared with a number of other commercial diet programs like Nutrisystem and Medifast, which cost $300 and $329 a month, respectively.
Because of the economic pressure these programs can exert on patients, Dr. Feldshon spoke to the difficulty of recruiting patients with major metabolic and digestive disorders. “I work in the liver clinic, so I see people with NASH, NAFLD, cirrhosis, near-cirrhosis, diabetes. These are people that would really benefit from this type of program, and I could not get a single patient to sign up.”
For Dr. Feldshon’s program, patients were required to come to MNGI once a week, which led to higher dropout rates. This is troubling because it is important that patients meet with doctors and other patients as part of a weight loss program. But the inconvenience for patients and doctors is not just in physically attending meetings, but in the medical billing. The Affordable Care Act and Medicare cover obesity screening and counseling, but this is only for primary care physicians, nurse practitioners, physician assistants, or clinical nurse specialists. These limits may not extend into private insurance, but they remain a barrier for those covered by Medicare, according to Dr. Feldshon.
Another effective but ultimately expensive option is weight loss drugs. The cost of drugs can vary wildly. Even well-established drugs like phentermine can cost anywhere from $5 to $35 out of pocket a month, according to Dr. Feldshon. Some drugs, like Saxenda, can cost as much as $1,414 per month if paid for out of pocket. While Saxenda is effective, there is a catch in the prescribing and billing for this drug: “The moment you go above 2.4 mg per day, the insurance company says, ‘Aha! He’s treating obesity,’ and they stop covering it.” One drug that Dr. Feldshon recommends is topiramate because it is a multiuse drug. “Often, the insurance company can’t figure out why you’re prescribing it, so they’ll okay it,” said Dr. Feldshon.
One of the most effective nonsurgical methods of weight loss is intragastric balloon. From 2016 to 2017, MNGI used gastric balloons in 22 patients, resulting in 11.4% total body weight loss in these patients. Unfortunately, it was a “financial loser,” according to Dr. Feldshon. The global case rate charge for one balloon is $8,200. MNGI then incurred $2,000 per balloon, $3,000 in hospital charges, $750 for the medical weight loss program, $1,350 for office personnel and visits, and a calculated opportunity cost of $3,140 resulting in a net loss of $2,040 per balloon. The most important factor in this calculation is the opportunity cost, which includes travel to and from the hospital and phone encounters with patients, which took away Dr. Feldshon’s ability to conduct colonoscopies and GI consults.
In an attempt to make balloons cost effective, Dr. Feldshon committed to doing these procedures on his day off, which reduced the opportunity cost to $0. This made the balloon procedure profitable, at $1,100 per balloon, but the volume was too low to make it worthwhile.
Despite the challenges his group faced with treating obesity, Dr. Feldshon offered some cost-saving solutions to help keep costs down for both patients and doctors. He suggested avoiding manufacturer weight loss programs. Identify an internal program that is reasonably priced or an external program like Weight Watchers. Physicians can utilize video conferencing for weekly meetings; this helps patients interact with doctors, and products like AdobeConnect cost physicians only about $50 a month. Patients can use free online journaling products like MyFitnessPal to track diet and exercise. Physicians can also recommend using generic and over-the-counter drugs and consider enlisting the help of a life coach or dietitian.
“All obese patients benefit from weight loss but we should be targeting those with metabolic syndrome, diabetes, heart disease, hypercholesterolemia, hyperlipidemia, and increased abdominal girth,” said Dr. Feldshon.
Dr. Feldshon has served on advisory committees and review panels and has worked with United Health Group as well as Prime Therapeutics.
AGA Resource
AGA has created an Obesity Practice Guide to provide gastroenterologists with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management, including a model for how to operationalize business issues.
The increase in the proportion of people who are overweight and obese presents gastroenterologists with new challenges and opportunities. Our internal medicine background, experience in nutrition, and role as endoscopists puts us in a unique position to manage obesity. In addition, many GI conditions are directly affected by obesity, including NASH, GERD, pancreatic diseases, and colon cancer. Good nutrition will always be the cornerstone of healthy weight, but nutritional advice alone results in modest (2%-3%) total weight loss. This can be augmented with medications, meal replacement, endobariatrics, and combinations of these.
Having said this, there are significant challenges to managing obesity as a gastroenterologist, and these stem almost entirely from the fact that there is poor coverage for these therapeutic options, as emphasized by Dr. Feldshon. However, it is still important to bring weight loss interventions into our clinical practice – for many reasons.
First, unlike other obesity management programs, we are typically not managing obesity in isolation. Usually, we are managing obesity in the setting of a disease state such as NASH. When we manage patients with NASH and stage 3 fibrosis, the patients’ decision making on how much to invest to prevent further progression is different; they’re more likely to take on some costs. Second, the degree of coverage for medications is improving. Similarly, although endobariatrics is not currently covered, with time it likely will be under certain criteria.
We need to build the clinical experience necessary to manage obesity and do so now, or other specialties will have become the main providers of weight loss interventions. This will become a lost opportunity for both medical and endobariatric management of these patients by us.
So despite the challenges raised by Dr. Feldshon, I would suggest that a practicing gastroenterologist interested in weight loss management focus on patients with obesity-related diseases first and expand their focus incrementally.
Wahajat Mehal, MD, DPhil, is a hepatologist and director of the Yale Weight Loss Program at Yale University, New Haven, Conn. He is an associate editor for GI & Hepatology News.
The increase in the proportion of people who are overweight and obese presents gastroenterologists with new challenges and opportunities. Our internal medicine background, experience in nutrition, and role as endoscopists puts us in a unique position to manage obesity. In addition, many GI conditions are directly affected by obesity, including NASH, GERD, pancreatic diseases, and colon cancer. Good nutrition will always be the cornerstone of healthy weight, but nutritional advice alone results in modest (2%-3%) total weight loss. This can be augmented with medications, meal replacement, endobariatrics, and combinations of these.
Having said this, there are significant challenges to managing obesity as a gastroenterologist, and these stem almost entirely from the fact that there is poor coverage for these therapeutic options, as emphasized by Dr. Feldshon. However, it is still important to bring weight loss interventions into our clinical practice – for many reasons.
First, unlike other obesity management programs, we are typically not managing obesity in isolation. Usually, we are managing obesity in the setting of a disease state such as NASH. When we manage patients with NASH and stage 3 fibrosis, the patients’ decision making on how much to invest to prevent further progression is different; they’re more likely to take on some costs. Second, the degree of coverage for medications is improving. Similarly, although endobariatrics is not currently covered, with time it likely will be under certain criteria.
We need to build the clinical experience necessary to manage obesity and do so now, or other specialties will have become the main providers of weight loss interventions. This will become a lost opportunity for both medical and endobariatric management of these patients by us.
So despite the challenges raised by Dr. Feldshon, I would suggest that a practicing gastroenterologist interested in weight loss management focus on patients with obesity-related diseases first and expand their focus incrementally.
Wahajat Mehal, MD, DPhil, is a hepatologist and director of the Yale Weight Loss Program at Yale University, New Haven, Conn. He is an associate editor for GI & Hepatology News.
The increase in the proportion of people who are overweight and obese presents gastroenterologists with new challenges and opportunities. Our internal medicine background, experience in nutrition, and role as endoscopists puts us in a unique position to manage obesity. In addition, many GI conditions are directly affected by obesity, including NASH, GERD, pancreatic diseases, and colon cancer. Good nutrition will always be the cornerstone of healthy weight, but nutritional advice alone results in modest (2%-3%) total weight loss. This can be augmented with medications, meal replacement, endobariatrics, and combinations of these.
Having said this, there are significant challenges to managing obesity as a gastroenterologist, and these stem almost entirely from the fact that there is poor coverage for these therapeutic options, as emphasized by Dr. Feldshon. However, it is still important to bring weight loss interventions into our clinical practice – for many reasons.
First, unlike other obesity management programs, we are typically not managing obesity in isolation. Usually, we are managing obesity in the setting of a disease state such as NASH. When we manage patients with NASH and stage 3 fibrosis, the patients’ decision making on how much to invest to prevent further progression is different; they’re more likely to take on some costs. Second, the degree of coverage for medications is improving. Similarly, although endobariatrics is not currently covered, with time it likely will be under certain criteria.
We need to build the clinical experience necessary to manage obesity and do so now, or other specialties will have become the main providers of weight loss interventions. This will become a lost opportunity for both medical and endobariatric management of these patients by us.
So despite the challenges raised by Dr. Feldshon, I would suggest that a practicing gastroenterologist interested in weight loss management focus on patients with obesity-related diseases first and expand their focus incrementally.
Wahajat Mehal, MD, DPhil, is a hepatologist and director of the Yale Weight Loss Program at Yale University, New Haven, Conn. He is an associate editor for GI & Hepatology News.
WASHINGTON – “We have heard about a lot of really interesting new things in therapy, but the reality is that they are just not economically viable at present,” said David Feldshon, MD, of Minnesota Gastroenterology (MNGI), speaking about his experience with offering obesity treatment in his practice.
One problem that Dr. Feldshon and MNGI faced was that they could never get enough patients into their weight loss program, a meal-replacement program priced at about $200 a month. Part of this program, Optifast, a commercially available low-calorie diet and behavior modification program, cost about $360 a month. Most patients found the cost to be prohibitive according to Dr. Feldshon. The program that MNGI offered was fairly competitive when compared with a number of other commercial diet programs like Nutrisystem and Medifast, which cost $300 and $329 a month, respectively.
Because of the economic pressure these programs can exert on patients, Dr. Feldshon spoke to the difficulty of recruiting patients with major metabolic and digestive disorders. “I work in the liver clinic, so I see people with NASH, NAFLD, cirrhosis, near-cirrhosis, diabetes. These are people that would really benefit from this type of program, and I could not get a single patient to sign up.”
For Dr. Feldshon’s program, patients were required to come to MNGI once a week, which led to higher dropout rates. This is troubling because it is important that patients meet with doctors and other patients as part of a weight loss program. But the inconvenience for patients and doctors is not just in physically attending meetings, but in the medical billing. The Affordable Care Act and Medicare cover obesity screening and counseling, but this is only for primary care physicians, nurse practitioners, physician assistants, or clinical nurse specialists. These limits may not extend into private insurance, but they remain a barrier for those covered by Medicare, according to Dr. Feldshon.
Another effective but ultimately expensive option is weight loss drugs. The cost of drugs can vary wildly. Even well-established drugs like phentermine can cost anywhere from $5 to $35 out of pocket a month, according to Dr. Feldshon. Some drugs, like Saxenda, can cost as much as $1,414 per month if paid for out of pocket. While Saxenda is effective, there is a catch in the prescribing and billing for this drug: “The moment you go above 2.4 mg per day, the insurance company says, ‘Aha! He’s treating obesity,’ and they stop covering it.” One drug that Dr. Feldshon recommends is topiramate because it is a multiuse drug. “Often, the insurance company can’t figure out why you’re prescribing it, so they’ll okay it,” said Dr. Feldshon.
One of the most effective nonsurgical methods of weight loss is intragastric balloon. From 2016 to 2017, MNGI used gastric balloons in 22 patients, resulting in 11.4% total body weight loss in these patients. Unfortunately, it was a “financial loser,” according to Dr. Feldshon. The global case rate charge for one balloon is $8,200. MNGI then incurred $2,000 per balloon, $3,000 in hospital charges, $750 for the medical weight loss program, $1,350 for office personnel and visits, and a calculated opportunity cost of $3,140 resulting in a net loss of $2,040 per balloon. The most important factor in this calculation is the opportunity cost, which includes travel to and from the hospital and phone encounters with patients, which took away Dr. Feldshon’s ability to conduct colonoscopies and GI consults.
In an attempt to make balloons cost effective, Dr. Feldshon committed to doing these procedures on his day off, which reduced the opportunity cost to $0. This made the balloon procedure profitable, at $1,100 per balloon, but the volume was too low to make it worthwhile.
Despite the challenges his group faced with treating obesity, Dr. Feldshon offered some cost-saving solutions to help keep costs down for both patients and doctors. He suggested avoiding manufacturer weight loss programs. Identify an internal program that is reasonably priced or an external program like Weight Watchers. Physicians can utilize video conferencing for weekly meetings; this helps patients interact with doctors, and products like AdobeConnect cost physicians only about $50 a month. Patients can use free online journaling products like MyFitnessPal to track diet and exercise. Physicians can also recommend using generic and over-the-counter drugs and consider enlisting the help of a life coach or dietitian.
“All obese patients benefit from weight loss but we should be targeting those with metabolic syndrome, diabetes, heart disease, hypercholesterolemia, hyperlipidemia, and increased abdominal girth,” said Dr. Feldshon.
Dr. Feldshon has served on advisory committees and review panels and has worked with United Health Group as well as Prime Therapeutics.
AGA Resource
AGA has created an Obesity Practice Guide to provide gastroenterologists with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management, including a model for how to operationalize business issues.
WASHINGTON – “We have heard about a lot of really interesting new things in therapy, but the reality is that they are just not economically viable at present,” said David Feldshon, MD, of Minnesota Gastroenterology (MNGI), speaking about his experience with offering obesity treatment in his practice.
One problem that Dr. Feldshon and MNGI faced was that they could never get enough patients into their weight loss program, a meal-replacement program priced at about $200 a month. Part of this program, Optifast, a commercially available low-calorie diet and behavior modification program, cost about $360 a month. Most patients found the cost to be prohibitive according to Dr. Feldshon. The program that MNGI offered was fairly competitive when compared with a number of other commercial diet programs like Nutrisystem and Medifast, which cost $300 and $329 a month, respectively.
Because of the economic pressure these programs can exert on patients, Dr. Feldshon spoke to the difficulty of recruiting patients with major metabolic and digestive disorders. “I work in the liver clinic, so I see people with NASH, NAFLD, cirrhosis, near-cirrhosis, diabetes. These are people that would really benefit from this type of program, and I could not get a single patient to sign up.”
For Dr. Feldshon’s program, patients were required to come to MNGI once a week, which led to higher dropout rates. This is troubling because it is important that patients meet with doctors and other patients as part of a weight loss program. But the inconvenience for patients and doctors is not just in physically attending meetings, but in the medical billing. The Affordable Care Act and Medicare cover obesity screening and counseling, but this is only for primary care physicians, nurse practitioners, physician assistants, or clinical nurse specialists. These limits may not extend into private insurance, but they remain a barrier for those covered by Medicare, according to Dr. Feldshon.
Another effective but ultimately expensive option is weight loss drugs. The cost of drugs can vary wildly. Even well-established drugs like phentermine can cost anywhere from $5 to $35 out of pocket a month, according to Dr. Feldshon. Some drugs, like Saxenda, can cost as much as $1,414 per month if paid for out of pocket. While Saxenda is effective, there is a catch in the prescribing and billing for this drug: “The moment you go above 2.4 mg per day, the insurance company says, ‘Aha! He’s treating obesity,’ and they stop covering it.” One drug that Dr. Feldshon recommends is topiramate because it is a multiuse drug. “Often, the insurance company can’t figure out why you’re prescribing it, so they’ll okay it,” said Dr. Feldshon.
One of the most effective nonsurgical methods of weight loss is intragastric balloon. From 2016 to 2017, MNGI used gastric balloons in 22 patients, resulting in 11.4% total body weight loss in these patients. Unfortunately, it was a “financial loser,” according to Dr. Feldshon. The global case rate charge for one balloon is $8,200. MNGI then incurred $2,000 per balloon, $3,000 in hospital charges, $750 for the medical weight loss program, $1,350 for office personnel and visits, and a calculated opportunity cost of $3,140 resulting in a net loss of $2,040 per balloon. The most important factor in this calculation is the opportunity cost, which includes travel to and from the hospital and phone encounters with patients, which took away Dr. Feldshon’s ability to conduct colonoscopies and GI consults.
In an attempt to make balloons cost effective, Dr. Feldshon committed to doing these procedures on his day off, which reduced the opportunity cost to $0. This made the balloon procedure profitable, at $1,100 per balloon, but the volume was too low to make it worthwhile.
Despite the challenges his group faced with treating obesity, Dr. Feldshon offered some cost-saving solutions to help keep costs down for both patients and doctors. He suggested avoiding manufacturer weight loss programs. Identify an internal program that is reasonably priced or an external program like Weight Watchers. Physicians can utilize video conferencing for weekly meetings; this helps patients interact with doctors, and products like AdobeConnect cost physicians only about $50 a month. Patients can use free online journaling products like MyFitnessPal to track diet and exercise. Physicians can also recommend using generic and over-the-counter drugs and consider enlisting the help of a life coach or dietitian.
“All obese patients benefit from weight loss but we should be targeting those with metabolic syndrome, diabetes, heart disease, hypercholesterolemia, hyperlipidemia, and increased abdominal girth,” said Dr. Feldshon.
Dr. Feldshon has served on advisory committees and review panels and has worked with United Health Group as well as Prime Therapeutics.
AGA Resource
AGA has created an Obesity Practice Guide to provide gastroenterologists with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management, including a model for how to operationalize business issues.
EXPERT ANALYSIS FROM DDW 2018
Maternal lifestyle affects child obesity
A study published in the British Medical Journal found that women who practiced five healthy habits had children who when they reached adolescence were 75% less likely to be overweight, compared with women who practiced none of the those healthy habits.
The healthy habits were maintaining a healthy weight, eating a nutritious diet, exercising regularly, not smoking, and consuming no more than a moderate amount of alcohol (BMJ 2018;362:k2486). I suspect you aren’t surprised by the core finding of this study of 16,945 female nurses and their 24,289 children. You’ve seen it scores of times. Mothers who lead unhealthy lifestyles seem to have children who are more likely to be obese. Now you have some numbers to support your decades of anecdotal observations. But the question is, what are we supposed to do with this new data? When and with whom should we share this unfortunate truth?
Evidence from previous studies makes it clear that by the time a child enters grade school the die is cast. Baby fat is neither cute nor temporary. This means that our target audience must be mothers-to-be and women whose children are infants and toddlers. On the other hand, telling the mother of an overweight teenager that her own unhealthy habits have probably contributed to her child’s weight problem is cruel and a waste of time. The mother already may have suspected her culpability. She also may feel that it is too late to do anything about it. While there have been some studies looking for an association between paternal body mass index and offspring BMI, I was unable to find any addressing paternal lifestyle and adolescent obesity.
This new study doesn’t address the unusual situation in which a mother of a teenager sheds all five of her unhealthy habits. I guess there may be examples in which a mother’s positive lifestyle change has helped reverse her adolescent child’s path to obesity. But I suspect these cases are rare.
So on one hand but on the other we must be careful to avoid playing the blame game and giving other mothers a one-way ticket on the guilt train. This is just one more example of the tightrope that we have been walking for generations. Every day in our offices we see children whose health is endangered by their parents’ behaviors and lifestyles. In cases in which the parental behavior is creating a serious short-term risk, such as failing to use an appropriate motor vehicle safety restraint system, we have no qualms about speaking out. We aren’t afraid to do a little shaming in hopes of sparing a family a serious guilt trip. When the threat to the child is more abstract and less dramatic – such as vaccine refusal – shaming and education don’t seem to be effective in changing parental behavior.
Obesity presents its own collection of complexities. It is like a car wreck seen in slow motion as the plots on the growth chart accumulate pound by pound. Unfortunately, parents often are among the last to notice or accept the reality. This new study doesn’t tell us whether we can make a difference. But it does suggest that when we first see the warning signs on the growth chart that we should engage the parents in a discussion of their lifestyle and its possible association with the child’s weight gain. The challenge, of course, is how one can cast the discussion without sounding judgmental.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
A study published in the British Medical Journal found that women who practiced five healthy habits had children who when they reached adolescence were 75% less likely to be overweight, compared with women who practiced none of the those healthy habits.
The healthy habits were maintaining a healthy weight, eating a nutritious diet, exercising regularly, not smoking, and consuming no more than a moderate amount of alcohol (BMJ 2018;362:k2486). I suspect you aren’t surprised by the core finding of this study of 16,945 female nurses and their 24,289 children. You’ve seen it scores of times. Mothers who lead unhealthy lifestyles seem to have children who are more likely to be obese. Now you have some numbers to support your decades of anecdotal observations. But the question is, what are we supposed to do with this new data? When and with whom should we share this unfortunate truth?
Evidence from previous studies makes it clear that by the time a child enters grade school the die is cast. Baby fat is neither cute nor temporary. This means that our target audience must be mothers-to-be and women whose children are infants and toddlers. On the other hand, telling the mother of an overweight teenager that her own unhealthy habits have probably contributed to her child’s weight problem is cruel and a waste of time. The mother already may have suspected her culpability. She also may feel that it is too late to do anything about it. While there have been some studies looking for an association between paternal body mass index and offspring BMI, I was unable to find any addressing paternal lifestyle and adolescent obesity.
This new study doesn’t address the unusual situation in which a mother of a teenager sheds all five of her unhealthy habits. I guess there may be examples in which a mother’s positive lifestyle change has helped reverse her adolescent child’s path to obesity. But I suspect these cases are rare.
So on one hand but on the other we must be careful to avoid playing the blame game and giving other mothers a one-way ticket on the guilt train. This is just one more example of the tightrope that we have been walking for generations. Every day in our offices we see children whose health is endangered by their parents’ behaviors and lifestyles. In cases in which the parental behavior is creating a serious short-term risk, such as failing to use an appropriate motor vehicle safety restraint system, we have no qualms about speaking out. We aren’t afraid to do a little shaming in hopes of sparing a family a serious guilt trip. When the threat to the child is more abstract and less dramatic – such as vaccine refusal – shaming and education don’t seem to be effective in changing parental behavior.
Obesity presents its own collection of complexities. It is like a car wreck seen in slow motion as the plots on the growth chart accumulate pound by pound. Unfortunately, parents often are among the last to notice or accept the reality. This new study doesn’t tell us whether we can make a difference. But it does suggest that when we first see the warning signs on the growth chart that we should engage the parents in a discussion of their lifestyle and its possible association with the child’s weight gain. The challenge, of course, is how one can cast the discussion without sounding judgmental.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
A study published in the British Medical Journal found that women who practiced five healthy habits had children who when they reached adolescence were 75% less likely to be overweight, compared with women who practiced none of the those healthy habits.
The healthy habits were maintaining a healthy weight, eating a nutritious diet, exercising regularly, not smoking, and consuming no more than a moderate amount of alcohol (BMJ 2018;362:k2486). I suspect you aren’t surprised by the core finding of this study of 16,945 female nurses and their 24,289 children. You’ve seen it scores of times. Mothers who lead unhealthy lifestyles seem to have children who are more likely to be obese. Now you have some numbers to support your decades of anecdotal observations. But the question is, what are we supposed to do with this new data? When and with whom should we share this unfortunate truth?
Evidence from previous studies makes it clear that by the time a child enters grade school the die is cast. Baby fat is neither cute nor temporary. This means that our target audience must be mothers-to-be and women whose children are infants and toddlers. On the other hand, telling the mother of an overweight teenager that her own unhealthy habits have probably contributed to her child’s weight problem is cruel and a waste of time. The mother already may have suspected her culpability. She also may feel that it is too late to do anything about it. While there have been some studies looking for an association between paternal body mass index and offspring BMI, I was unable to find any addressing paternal lifestyle and adolescent obesity.
This new study doesn’t address the unusual situation in which a mother of a teenager sheds all five of her unhealthy habits. I guess there may be examples in which a mother’s positive lifestyle change has helped reverse her adolescent child’s path to obesity. But I suspect these cases are rare.
So on one hand but on the other we must be careful to avoid playing the blame game and giving other mothers a one-way ticket on the guilt train. This is just one more example of the tightrope that we have been walking for generations. Every day in our offices we see children whose health is endangered by their parents’ behaviors and lifestyles. In cases in which the parental behavior is creating a serious short-term risk, such as failing to use an appropriate motor vehicle safety restraint system, we have no qualms about speaking out. We aren’t afraid to do a little shaming in hopes of sparing a family a serious guilt trip. When the threat to the child is more abstract and less dramatic – such as vaccine refusal – shaming and education don’t seem to be effective in changing parental behavior.
Obesity presents its own collection of complexities. It is like a car wreck seen in slow motion as the plots on the growth chart accumulate pound by pound. Unfortunately, parents often are among the last to notice or accept the reality. This new study doesn’t tell us whether we can make a difference. But it does suggest that when we first see the warning signs on the growth chart that we should engage the parents in a discussion of their lifestyle and its possible association with the child’s weight gain. The challenge, of course, is how one can cast the discussion without sounding judgmental.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Sleep may mediate healthy behavior in children
BALTIMORE – a 6-year follow-up of children in the Infant Feeding Practices Study II determined.
However, improving health in these children is more than a matter of simply seeing that they get more sleep, said lead investigator Jill Landsbaugh Kaar, PhD, of Children’s Hospital Colorado, Aurora, in presenting the results at the annual meeting of the Associated Professional Sleep Societies. “Perhaps there’s a potential pathway linking healthy eaters and obesity in children that may be mediated through sleep duration.”
The relationship between sleep, diet, and activity level may be more cyclical, rather than linear, Dr. Kaar said. “Poor sleep is typically linked to a poor diet or low levels of physical activity, and then linked to some outcome or disease,” she said. But her research indicates that those three factors – sleep, diet and activity – are more interrelated than one being causative of the others.
Noting that one in three adults and one in six children in the United States are either overweight or obese (JAMA. 2014 Feb 26;311[8]:806-14), Dr. Kaar said, “Childhood obesity prevention has really not been effective in reducing weight or preventing or limiting weight gain.” Such programs typically focus on one health behavior when each child has a unique pattern of health behaviors that influence weight.
Dr. Kaar’s research used data collected by the Centers for Disease Control and Prevention as part of a 6-year follow-up study of women from the Infant Feeding Practices Study II. Some 1,542 women completed mailed questionnaires about their 6-year-olds’ diet, activity, screen time, sleep duration, height, and weight. The statistical analysis grouped the children into health behavior patterns of diet, activity, and screen time and used a three-step mediation regression model to examine their hypothesis.
The analysis characterized children into three health behavior pattern groups: poorest eaters (22%), healthy children (37%), and active supereaters with the highest screen time (41%). The poorest eaters were more likely to be female (58%) and obese (18%) than the other groups, but even 10% of the healthy children group were obese.
In the first model, the poorest eaters had the highest risk of obesity. In the second model, both the poorest eaters and active supereaters had shorter sleep duration than healthy children – 9.46 and 9.59 hours a night, respectively, versus 9.97 hours for healthy children – “thus telling me that sleep was really driving that relationship,” Dr. Kaar said.
“Future interventions should consider that improving health behavior patterns by targeting someone’s diet or physical activity, that you’re also targeting them to improve sleep, and then through increasing sleep you will be influencing obesity,” she said. “Interventions and research studies in general really need to measure all of those health behaviors because they’re all related; it’s not just one of them leading to obesity risk.”
The next step for her research is to branch out beyond a one-center study, Dr. Kaar said.
Dr. Kaar reported having no financial relationships. An American Heart Association Scientist Development Award provided funding for the study.
BALTIMORE – a 6-year follow-up of children in the Infant Feeding Practices Study II determined.
However, improving health in these children is more than a matter of simply seeing that they get more sleep, said lead investigator Jill Landsbaugh Kaar, PhD, of Children’s Hospital Colorado, Aurora, in presenting the results at the annual meeting of the Associated Professional Sleep Societies. “Perhaps there’s a potential pathway linking healthy eaters and obesity in children that may be mediated through sleep duration.”
The relationship between sleep, diet, and activity level may be more cyclical, rather than linear, Dr. Kaar said. “Poor sleep is typically linked to a poor diet or low levels of physical activity, and then linked to some outcome or disease,” she said. But her research indicates that those three factors – sleep, diet and activity – are more interrelated than one being causative of the others.
Noting that one in three adults and one in six children in the United States are either overweight or obese (JAMA. 2014 Feb 26;311[8]:806-14), Dr. Kaar said, “Childhood obesity prevention has really not been effective in reducing weight or preventing or limiting weight gain.” Such programs typically focus on one health behavior when each child has a unique pattern of health behaviors that influence weight.
Dr. Kaar’s research used data collected by the Centers for Disease Control and Prevention as part of a 6-year follow-up study of women from the Infant Feeding Practices Study II. Some 1,542 women completed mailed questionnaires about their 6-year-olds’ diet, activity, screen time, sleep duration, height, and weight. The statistical analysis grouped the children into health behavior patterns of diet, activity, and screen time and used a three-step mediation regression model to examine their hypothesis.
The analysis characterized children into three health behavior pattern groups: poorest eaters (22%), healthy children (37%), and active supereaters with the highest screen time (41%). The poorest eaters were more likely to be female (58%) and obese (18%) than the other groups, but even 10% of the healthy children group were obese.
In the first model, the poorest eaters had the highest risk of obesity. In the second model, both the poorest eaters and active supereaters had shorter sleep duration than healthy children – 9.46 and 9.59 hours a night, respectively, versus 9.97 hours for healthy children – “thus telling me that sleep was really driving that relationship,” Dr. Kaar said.
“Future interventions should consider that improving health behavior patterns by targeting someone’s diet or physical activity, that you’re also targeting them to improve sleep, and then through increasing sleep you will be influencing obesity,” she said. “Interventions and research studies in general really need to measure all of those health behaviors because they’re all related; it’s not just one of them leading to obesity risk.”
The next step for her research is to branch out beyond a one-center study, Dr. Kaar said.
Dr. Kaar reported having no financial relationships. An American Heart Association Scientist Development Award provided funding for the study.
BALTIMORE – a 6-year follow-up of children in the Infant Feeding Practices Study II determined.
However, improving health in these children is more than a matter of simply seeing that they get more sleep, said lead investigator Jill Landsbaugh Kaar, PhD, of Children’s Hospital Colorado, Aurora, in presenting the results at the annual meeting of the Associated Professional Sleep Societies. “Perhaps there’s a potential pathway linking healthy eaters and obesity in children that may be mediated through sleep duration.”
The relationship between sleep, diet, and activity level may be more cyclical, rather than linear, Dr. Kaar said. “Poor sleep is typically linked to a poor diet or low levels of physical activity, and then linked to some outcome or disease,” she said. But her research indicates that those three factors – sleep, diet and activity – are more interrelated than one being causative of the others.
Noting that one in three adults and one in six children in the United States are either overweight or obese (JAMA. 2014 Feb 26;311[8]:806-14), Dr. Kaar said, “Childhood obesity prevention has really not been effective in reducing weight or preventing or limiting weight gain.” Such programs typically focus on one health behavior when each child has a unique pattern of health behaviors that influence weight.
Dr. Kaar’s research used data collected by the Centers for Disease Control and Prevention as part of a 6-year follow-up study of women from the Infant Feeding Practices Study II. Some 1,542 women completed mailed questionnaires about their 6-year-olds’ diet, activity, screen time, sleep duration, height, and weight. The statistical analysis grouped the children into health behavior patterns of diet, activity, and screen time and used a three-step mediation regression model to examine their hypothesis.
The analysis characterized children into three health behavior pattern groups: poorest eaters (22%), healthy children (37%), and active supereaters with the highest screen time (41%). The poorest eaters were more likely to be female (58%) and obese (18%) than the other groups, but even 10% of the healthy children group were obese.
In the first model, the poorest eaters had the highest risk of obesity. In the second model, both the poorest eaters and active supereaters had shorter sleep duration than healthy children – 9.46 and 9.59 hours a night, respectively, versus 9.97 hours for healthy children – “thus telling me that sleep was really driving that relationship,” Dr. Kaar said.
“Future interventions should consider that improving health behavior patterns by targeting someone’s diet or physical activity, that you’re also targeting them to improve sleep, and then through increasing sleep you will be influencing obesity,” she said. “Interventions and research studies in general really need to measure all of those health behaviors because they’re all related; it’s not just one of them leading to obesity risk.”
The next step for her research is to branch out beyond a one-center study, Dr. Kaar said.
Dr. Kaar reported having no financial relationships. An American Heart Association Scientist Development Award provided funding for the study.
REPORTING FROM SLEEP 2018
Key clinical point: Sleep may mediate how diet and activity influence weight in children.
Major finding: Healthy children had 9.97 hours of sleep per night versus 9.46 hours for poorest eaters.
Study details: A 6-year follow-up of 1,542 children in the Infant Feeding Practices Study II whose health behaviors were self-reported by mothers.
Disclosures: Dr. Kaar reported having no financial relationships. The study was funded through an American Heart Association Scientist Development Award.
Obesity triples post-MI sudden cardiac death risk
Obesity is associated with an increased risk of sudden cardiac death after myocardial infarction, although the so-called “obesity paradox” is still evident in a lower risk of all-cause mortality, a new analysis suggests.
Researchers reported the results of an observational cohort study using data from two Japanese cohort studies involving a total of 6,216 patients discharged alive after acute myocardial infarction. The study was published in the Journal of the American Heart Association.
They found that obese patients – those with a body mass index of at least 27.5 kg/m2 – had a nearly threefold higher risk of sudden cardiac death within 3 years, compared with patients who had a normal BMI, even after adjustment for age, sex, and risk factors such as multivessel disease, left ventricular ejection fraction, and medications.
However, the obese group also showed lower 3-year all-cause mortality, compared with the reference group, whose BMI was 18.5-22.9 kg/m2, while individuals with a BMI below 18.5 kg/m2 had a 61% higher risk of mortality.
The overall all-cause mortality in the cohort was 10.1%, and the incidence of sudden cardiac death was 1.2%.
“For the primary prevention of [coronary artery disease], obesity is recognized as a potent risk factor and an opportunity for therapeutic intervention to prevent cardiovascular disease,” wrote Tsuyoshi Shiga, MD, of Tokyo Women’s Medical University, and coauthors. “However, recent reports have shown that obesity (high BMI) itself does not present a mortality risk but is associated with a better prognosis (obesity paradox) in CAD patients receiving secondary care; these patients received appropriate therapy, including percutaneous coronary intervention and guideline-based medications such as aspirin, beta-blockers, and statins.”
The increased risk of sudden cardiac death in obese patients after MI was harder to explain.
The authors suggested that obesity itself may increase the risk of ventricular arrhythmias developing, and it is also linked with left ventricular hypertrophy, which can lead to cardiac remodeling. Other reports have found evidence in obese individuals of QT prolongation or an increased late potential, and autonomic disturbances that could trigger arrhythmias.
Although reduced left ventricular ejection fraction is the best available predictor of sudden cardiac death, the authors noted that their study found high BMI to be a risk factor independent of left ventricular ejection fraction.
The authors also raised the question of whether intentional weight loss might be effective in reducing the risk of sudden cardiac death in obese patients after MI, but suggested more research was needed to answer this.
The two cohort studies included in the analysis were funded by the Japan Heart Foundation, and the Japan Research Promotion Society for Cardiovascular Diseases. No conflicts of interest were declared.
SOURCE: Shiga T et al. J Am Heart Assoc, 2018; July 7. doi: 10.1161/JAHA.118.008633.
Obesity is associated with an increased risk of sudden cardiac death after myocardial infarction, although the so-called “obesity paradox” is still evident in a lower risk of all-cause mortality, a new analysis suggests.
Researchers reported the results of an observational cohort study using data from two Japanese cohort studies involving a total of 6,216 patients discharged alive after acute myocardial infarction. The study was published in the Journal of the American Heart Association.
They found that obese patients – those with a body mass index of at least 27.5 kg/m2 – had a nearly threefold higher risk of sudden cardiac death within 3 years, compared with patients who had a normal BMI, even after adjustment for age, sex, and risk factors such as multivessel disease, left ventricular ejection fraction, and medications.
However, the obese group also showed lower 3-year all-cause mortality, compared with the reference group, whose BMI was 18.5-22.9 kg/m2, while individuals with a BMI below 18.5 kg/m2 had a 61% higher risk of mortality.
The overall all-cause mortality in the cohort was 10.1%, and the incidence of sudden cardiac death was 1.2%.
“For the primary prevention of [coronary artery disease], obesity is recognized as a potent risk factor and an opportunity for therapeutic intervention to prevent cardiovascular disease,” wrote Tsuyoshi Shiga, MD, of Tokyo Women’s Medical University, and coauthors. “However, recent reports have shown that obesity (high BMI) itself does not present a mortality risk but is associated with a better prognosis (obesity paradox) in CAD patients receiving secondary care; these patients received appropriate therapy, including percutaneous coronary intervention and guideline-based medications such as aspirin, beta-blockers, and statins.”
The increased risk of sudden cardiac death in obese patients after MI was harder to explain.
The authors suggested that obesity itself may increase the risk of ventricular arrhythmias developing, and it is also linked with left ventricular hypertrophy, which can lead to cardiac remodeling. Other reports have found evidence in obese individuals of QT prolongation or an increased late potential, and autonomic disturbances that could trigger arrhythmias.
Although reduced left ventricular ejection fraction is the best available predictor of sudden cardiac death, the authors noted that their study found high BMI to be a risk factor independent of left ventricular ejection fraction.
The authors also raised the question of whether intentional weight loss might be effective in reducing the risk of sudden cardiac death in obese patients after MI, but suggested more research was needed to answer this.
The two cohort studies included in the analysis were funded by the Japan Heart Foundation, and the Japan Research Promotion Society for Cardiovascular Diseases. No conflicts of interest were declared.
SOURCE: Shiga T et al. J Am Heart Assoc, 2018; July 7. doi: 10.1161/JAHA.118.008633.
Obesity is associated with an increased risk of sudden cardiac death after myocardial infarction, although the so-called “obesity paradox” is still evident in a lower risk of all-cause mortality, a new analysis suggests.
Researchers reported the results of an observational cohort study using data from two Japanese cohort studies involving a total of 6,216 patients discharged alive after acute myocardial infarction. The study was published in the Journal of the American Heart Association.
They found that obese patients – those with a body mass index of at least 27.5 kg/m2 – had a nearly threefold higher risk of sudden cardiac death within 3 years, compared with patients who had a normal BMI, even after adjustment for age, sex, and risk factors such as multivessel disease, left ventricular ejection fraction, and medications.
However, the obese group also showed lower 3-year all-cause mortality, compared with the reference group, whose BMI was 18.5-22.9 kg/m2, while individuals with a BMI below 18.5 kg/m2 had a 61% higher risk of mortality.
The overall all-cause mortality in the cohort was 10.1%, and the incidence of sudden cardiac death was 1.2%.
“For the primary prevention of [coronary artery disease], obesity is recognized as a potent risk factor and an opportunity for therapeutic intervention to prevent cardiovascular disease,” wrote Tsuyoshi Shiga, MD, of Tokyo Women’s Medical University, and coauthors. “However, recent reports have shown that obesity (high BMI) itself does not present a mortality risk but is associated with a better prognosis (obesity paradox) in CAD patients receiving secondary care; these patients received appropriate therapy, including percutaneous coronary intervention and guideline-based medications such as aspirin, beta-blockers, and statins.”
The increased risk of sudden cardiac death in obese patients after MI was harder to explain.
The authors suggested that obesity itself may increase the risk of ventricular arrhythmias developing, and it is also linked with left ventricular hypertrophy, which can lead to cardiac remodeling. Other reports have found evidence in obese individuals of QT prolongation or an increased late potential, and autonomic disturbances that could trigger arrhythmias.
Although reduced left ventricular ejection fraction is the best available predictor of sudden cardiac death, the authors noted that their study found high BMI to be a risk factor independent of left ventricular ejection fraction.
The authors also raised the question of whether intentional weight loss might be effective in reducing the risk of sudden cardiac death in obese patients after MI, but suggested more research was needed to answer this.
The two cohort studies included in the analysis were funded by the Japan Heart Foundation, and the Japan Research Promotion Society for Cardiovascular Diseases. No conflicts of interest were declared.
SOURCE: Shiga T et al. J Am Heart Assoc, 2018; July 7. doi: 10.1161/JAHA.118.008633.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point: Obese MI patients have a significantly elevated risk of sudden cardiac death.
Major finding:
Study details: An Japanese observational cohort study of 6,216 patients with acute myocardial infarction.
Disclosures: The two cohort studies included in the analysis were funded by the Japan Heart Foundation, and the Japan Research Promotion Society for Cardiovascular Diseases. No conflicts of interest were declared.
Source: Shiga T et al. J Am Heart Assoc. 2018; July 7. doi: 10.1161/JAHA.118.008633.
New insights into sleep, pregnancy weight gain
BALTIMORE – Pregnant women who are overweight and obese are like the general population in that the less they sleep, the more weight they gain, particularly in the first half of pregnancy. However, unlike in the larger adult population, prolonged daily total eating time was not associated with gestational weight gain in these women, particularly early in pregnancy, according to findings from a small study presented at the Associated Professional Sleep Societies annual meeting.
Those findings point to a need to further study the , said Rachel P. Kolko, PhD, a postdoctoral scholar at the University of Pittsburgh, Western Psychiatric Institute and Clinic.
“The association with total sleep time was found to be significant, such that if you had less sleep, you had higher amounts of weight gain; we did not find a significant relation with our eating window variable,” Dr. Kolko said.
She reported on research involving 62 pregnant women, 53% of whom were overweight with a body mass index of 25-29.9 kg/m2 and 47% of whom were obese with BMI greater than 30. Forty-seven percent of the study population was nonwhite.
The research grew out of a need to identify potentially modifiable factors to curtail excessive gestational weight gain during pregnancy, she said. The study hypotheses were that both shorter total sleep time and longer total eating time would lead to higher gestational weight gain, but the study confirmed only the former as a contributing factor.
The women in the study were at 12-20 weeks of pregnancy. Gestational weight gain was calculated as the difference between self-reported prepregnancy weight and current weight. Total sleep time was based on the Pittsburgh Sleep Quality Index, and total eating time was calculated as the time difference between the day’s first meal or snack of more than 50 calories and the last, as self-reported.
Average total sleep time was 7.8 hours, with total eating time spanning 10.8 hours. On average, study participants gained 9.7 pounds through the first half of pregnancy, Dr. Kolko said. She noted that the Institute of Medicine, now known as the National Academy of Medicine, recommends that women who are overweight women gain 15-25 pounds during pregnancy and women who are obese gain 11-20 pounds (JAMA. 2017;317:2207-25). “Already about 20% of our sample has gained that amount of weight within the first half of pregnancy,” she said.
“Total sleep time was related to a higher early gestational weight in women with overweight and obesity, and it’s possible that addressing this may affect and hopefully improve women’s weight gain during early pregnancy to fit within those guidelines,” she said.
Future research should look at the entire gestational period – possibly targeting sleep patterns during pregnancy – and should expand to include women who are not overweight or obese, Dr. Kolko noted.
Dr. Kolko reported having no financial relationships to disclose.
SOURCE: Kolko RP, et al., SLEEP 2018, Abstract 0692.
BALTIMORE – Pregnant women who are overweight and obese are like the general population in that the less they sleep, the more weight they gain, particularly in the first half of pregnancy. However, unlike in the larger adult population, prolonged daily total eating time was not associated with gestational weight gain in these women, particularly early in pregnancy, according to findings from a small study presented at the Associated Professional Sleep Societies annual meeting.
Those findings point to a need to further study the , said Rachel P. Kolko, PhD, a postdoctoral scholar at the University of Pittsburgh, Western Psychiatric Institute and Clinic.
“The association with total sleep time was found to be significant, such that if you had less sleep, you had higher amounts of weight gain; we did not find a significant relation with our eating window variable,” Dr. Kolko said.
She reported on research involving 62 pregnant women, 53% of whom were overweight with a body mass index of 25-29.9 kg/m2 and 47% of whom were obese with BMI greater than 30. Forty-seven percent of the study population was nonwhite.
The research grew out of a need to identify potentially modifiable factors to curtail excessive gestational weight gain during pregnancy, she said. The study hypotheses were that both shorter total sleep time and longer total eating time would lead to higher gestational weight gain, but the study confirmed only the former as a contributing factor.
The women in the study were at 12-20 weeks of pregnancy. Gestational weight gain was calculated as the difference between self-reported prepregnancy weight and current weight. Total sleep time was based on the Pittsburgh Sleep Quality Index, and total eating time was calculated as the time difference between the day’s first meal or snack of more than 50 calories and the last, as self-reported.
Average total sleep time was 7.8 hours, with total eating time spanning 10.8 hours. On average, study participants gained 9.7 pounds through the first half of pregnancy, Dr. Kolko said. She noted that the Institute of Medicine, now known as the National Academy of Medicine, recommends that women who are overweight women gain 15-25 pounds during pregnancy and women who are obese gain 11-20 pounds (JAMA. 2017;317:2207-25). “Already about 20% of our sample has gained that amount of weight within the first half of pregnancy,” she said.
“Total sleep time was related to a higher early gestational weight in women with overweight and obesity, and it’s possible that addressing this may affect and hopefully improve women’s weight gain during early pregnancy to fit within those guidelines,” she said.
Future research should look at the entire gestational period – possibly targeting sleep patterns during pregnancy – and should expand to include women who are not overweight or obese, Dr. Kolko noted.
Dr. Kolko reported having no financial relationships to disclose.
SOURCE: Kolko RP, et al., SLEEP 2018, Abstract 0692.
BALTIMORE – Pregnant women who are overweight and obese are like the general population in that the less they sleep, the more weight they gain, particularly in the first half of pregnancy. However, unlike in the larger adult population, prolonged daily total eating time was not associated with gestational weight gain in these women, particularly early in pregnancy, according to findings from a small study presented at the Associated Professional Sleep Societies annual meeting.
Those findings point to a need to further study the , said Rachel P. Kolko, PhD, a postdoctoral scholar at the University of Pittsburgh, Western Psychiatric Institute and Clinic.
“The association with total sleep time was found to be significant, such that if you had less sleep, you had higher amounts of weight gain; we did not find a significant relation with our eating window variable,” Dr. Kolko said.
She reported on research involving 62 pregnant women, 53% of whom were overweight with a body mass index of 25-29.9 kg/m2 and 47% of whom were obese with BMI greater than 30. Forty-seven percent of the study population was nonwhite.
The research grew out of a need to identify potentially modifiable factors to curtail excessive gestational weight gain during pregnancy, she said. The study hypotheses were that both shorter total sleep time and longer total eating time would lead to higher gestational weight gain, but the study confirmed only the former as a contributing factor.
The women in the study were at 12-20 weeks of pregnancy. Gestational weight gain was calculated as the difference between self-reported prepregnancy weight and current weight. Total sleep time was based on the Pittsburgh Sleep Quality Index, and total eating time was calculated as the time difference between the day’s first meal or snack of more than 50 calories and the last, as self-reported.
Average total sleep time was 7.8 hours, with total eating time spanning 10.8 hours. On average, study participants gained 9.7 pounds through the first half of pregnancy, Dr. Kolko said. She noted that the Institute of Medicine, now known as the National Academy of Medicine, recommends that women who are overweight women gain 15-25 pounds during pregnancy and women who are obese gain 11-20 pounds (JAMA. 2017;317:2207-25). “Already about 20% of our sample has gained that amount of weight within the first half of pregnancy,” she said.
“Total sleep time was related to a higher early gestational weight in women with overweight and obesity, and it’s possible that addressing this may affect and hopefully improve women’s weight gain during early pregnancy to fit within those guidelines,” she said.
Future research should look at the entire gestational period – possibly targeting sleep patterns during pregnancy – and should expand to include women who are not overweight or obese, Dr. Kolko noted.
Dr. Kolko reported having no financial relationships to disclose.
SOURCE: Kolko RP, et al., SLEEP 2018, Abstract 0692.
REPORTING FROM SLEEP 2018
Key clinical point: In overweight/obese women, shorter sleep times are linked to early gestational weight gain.
Major finding: Overweight/obese women slept 30% less and had higher gestational weight gain in early pregnancy.
Study details: Study of 62 women between 12 and 20 weeks’ gestation with prepregnancy BMI greater than 25 kg/m2.
Disclosures: Dr. Kolko reported having no financial relationships.
Source: Kolko RP et al. SLEEP 2018, Abstract 0692.
Do carbs drive obesity? With evidence inconclusive, debate continues
While the debate continues, David S. Ludwig, MD, PhD, and Cara B. Ebbeling, PhD, argued in a recent clinical review that diet does indeed affect metabolism and body composition.
While evidence from human studies remains limited, animal research findings are consistent with a carbohydrate-insulin model of obesity, according to Dr. Ludwig and Dr. Ebbeling, who are with the New Balance Foundation Obesity Prevention Center at Boston Children’s Hospital and Harvard Medical School.
The carbohydrate-insulin model holds that eating processed, high–glycemic load carbohydrates causes hormonal changes that promote calorie deposition in fat tissue, aggravate hunger, and reduce energy expenditure, they said in JAMA Internal Medicine.
“The conventional way of thinking assumes that the individual has primary control over their calorie balance, and thus, bases conventional treatment on a target of establishing a negative energy balance – so that is 1,000 variations of the ‘eat less, move more’ recommendation,” Dr. Ludwig said in an interview.
The alternative to that established view has proven controversial. The Endocrine Society, in a recent scientific statement, said diet’s effect on obesity risk is largely explainable by calorie intake, rather than some special adverse effect on internal metabolism or energy expenditure.
“Stated differently, ‘a calorie is a calorie,’ ” the authors of the scientific statement said. “Thus, habitual consumption of highly palatable and energy-dense diets predispose to excess weight gain irrespective of macronutrient content.”
Others have sought to refute the carbohydrate-insulin hypothesis in recent reviews, such as an invited commentary in JAMA Internal Medicine by Kevin D. Hall, PhD, of the National Institute of Diabetes and Digestive and Kidney Diseases, and his coauthors.
“Although it is plausible that variables related to insulin signaling could be involved in obesity pathogenesis, the hypothesis that carbohydrate-stimulated insulin secretion is the primary cause of common obesity via direct effects on adipocytes is difficult to reconcile with current evidence,” Dr. Hall and his coauthors wrote in the commentary (JAMA Intern Med. 2018 Jul 2. doi: 10.1001/jamainternmed.2018.2920).
The conventional calorie balance model is a “straw man” that omits neuroendocrine mechanisms known to regulate homeostasis, added Dr. Hall and his coauthors, stating that accurate models of obesity should include physiological processes resisting weight loss and promoting weight gain.
“They might claim that this is a straw man argument, but I would claim that there is a case of the emperor’s new clothing,” Dr. Ludwig countered in the interview. “They argue that body weight is controlled by biology, and that that’s recognized in the conventional view, but how does that view inform treatment in any way? In the absence of any specific testable hypotheses for why the obesity epidemic has emerged so suddenly, conventional recommendations inevitably resort to advice to ‘eat less and move more.’ ”
Dr. Ludwig and Dr. Ebbeling have both conducted research studies examining the carbohydrate-insulin model, or the view that a high-carbohydrate diet results in postprandial hyperinsulinemia and promotes deposition of calories in adipocytes, leading to weight gain through slowing metabolism, increased hunger, or both.
In a study published in the Lancet, Dr. Ludwig and his coinvestigators found that rats fed a high–glycemic index (GI) diet for 18 weeks had more body fat (97.8 grams vs. 57.3 grams; P = .0152) and less lean body mass versus rats fed a low-GI diet. Rats on the high-GI diet also had greater increases over time in blood glucose and plasma insulin after oral glucose. Similarly, mice on a high-GI diet had nearly twice the body fat of mice on low-GI diet, after 9 weeks of feeding (Lancet. 2004 Aug 28. doi: 10.1016/S0140-6736(04)16937-7).
“There’s no way to explain that finding in view of the conventional view that all calories are alike to the body,” Dr. Ludwig said.
“Contrary to prediction of the conventional model, the inherently lower energy density of low-fat diets does not spontaneously produce sustained weight loss. In fact, several recent meta-analyses found that low-fat diets are inferior to all higher-fat [and thus low-glycemic] comparisons. However, these studies characteristically rely on dietary counseling, a method with limitations for testing mechanistic hypotheses owing to varying levels of noncompliance over the long-term,” Dr. Ludwig and Dr. Ebbeling wrote.
Criticisms that claim to refute the carbohydrate-insulin hypothesis are based in part on misinterpretation of recent feeding studies, according to Dr. Ludwig and Dr. Ebbeling. Multiple studies testing whether or not high–glycemic load meals lead to increased fat storage have reported no meaningful differences between low-fat and low-carbohydrate diets. However, these short-term studies, mostly 2 weeks in duration, preclude definitive findings, according to the review.
That’s because the process of adapting to a high-fat diet after having consumed a high-carbohydrate diet takes weeks, which is a well-recognized phenomenon, Dr. Ludwig said.
“If you put sedentary people into military boot camp and tested their biological state after 6 days, you’d probably find that they were fatigued, weak, and had higher inflammation in their muscles, but clearly, you wouldn’t conclude that fitness training is bad for your health,” he said in the interview. “But yet, these are the sort of data that are being used to ‘falsify’ the carbohydrate-insulin model.
“We acknowledge that there aren’t definitive human data,” he continued, “but the conventional model has failed to both explain the obesity epidemic and control it, and the latest public health data suggests that rates are higher today than ever before, despite 50 years of focusing on calorie balance.”
SOURCE: Ludwig DS et al. JAMA Intern Med. 2018 Jul 2. doi:10.1001/jamainternmed.2018.2933.
While the debate continues, David S. Ludwig, MD, PhD, and Cara B. Ebbeling, PhD, argued in a recent clinical review that diet does indeed affect metabolism and body composition.
While evidence from human studies remains limited, animal research findings are consistent with a carbohydrate-insulin model of obesity, according to Dr. Ludwig and Dr. Ebbeling, who are with the New Balance Foundation Obesity Prevention Center at Boston Children’s Hospital and Harvard Medical School.
The carbohydrate-insulin model holds that eating processed, high–glycemic load carbohydrates causes hormonal changes that promote calorie deposition in fat tissue, aggravate hunger, and reduce energy expenditure, they said in JAMA Internal Medicine.
“The conventional way of thinking assumes that the individual has primary control over their calorie balance, and thus, bases conventional treatment on a target of establishing a negative energy balance – so that is 1,000 variations of the ‘eat less, move more’ recommendation,” Dr. Ludwig said in an interview.
The alternative to that established view has proven controversial. The Endocrine Society, in a recent scientific statement, said diet’s effect on obesity risk is largely explainable by calorie intake, rather than some special adverse effect on internal metabolism or energy expenditure.
“Stated differently, ‘a calorie is a calorie,’ ” the authors of the scientific statement said. “Thus, habitual consumption of highly palatable and energy-dense diets predispose to excess weight gain irrespective of macronutrient content.”
Others have sought to refute the carbohydrate-insulin hypothesis in recent reviews, such as an invited commentary in JAMA Internal Medicine by Kevin D. Hall, PhD, of the National Institute of Diabetes and Digestive and Kidney Diseases, and his coauthors.
“Although it is plausible that variables related to insulin signaling could be involved in obesity pathogenesis, the hypothesis that carbohydrate-stimulated insulin secretion is the primary cause of common obesity via direct effects on adipocytes is difficult to reconcile with current evidence,” Dr. Hall and his coauthors wrote in the commentary (JAMA Intern Med. 2018 Jul 2. doi: 10.1001/jamainternmed.2018.2920).
The conventional calorie balance model is a “straw man” that omits neuroendocrine mechanisms known to regulate homeostasis, added Dr. Hall and his coauthors, stating that accurate models of obesity should include physiological processes resisting weight loss and promoting weight gain.
“They might claim that this is a straw man argument, but I would claim that there is a case of the emperor’s new clothing,” Dr. Ludwig countered in the interview. “They argue that body weight is controlled by biology, and that that’s recognized in the conventional view, but how does that view inform treatment in any way? In the absence of any specific testable hypotheses for why the obesity epidemic has emerged so suddenly, conventional recommendations inevitably resort to advice to ‘eat less and move more.’ ”
Dr. Ludwig and Dr. Ebbeling have both conducted research studies examining the carbohydrate-insulin model, or the view that a high-carbohydrate diet results in postprandial hyperinsulinemia and promotes deposition of calories in adipocytes, leading to weight gain through slowing metabolism, increased hunger, or both.
In a study published in the Lancet, Dr. Ludwig and his coinvestigators found that rats fed a high–glycemic index (GI) diet for 18 weeks had more body fat (97.8 grams vs. 57.3 grams; P = .0152) and less lean body mass versus rats fed a low-GI diet. Rats on the high-GI diet also had greater increases over time in blood glucose and plasma insulin after oral glucose. Similarly, mice on a high-GI diet had nearly twice the body fat of mice on low-GI diet, after 9 weeks of feeding (Lancet. 2004 Aug 28. doi: 10.1016/S0140-6736(04)16937-7).
“There’s no way to explain that finding in view of the conventional view that all calories are alike to the body,” Dr. Ludwig said.
“Contrary to prediction of the conventional model, the inherently lower energy density of low-fat diets does not spontaneously produce sustained weight loss. In fact, several recent meta-analyses found that low-fat diets are inferior to all higher-fat [and thus low-glycemic] comparisons. However, these studies characteristically rely on dietary counseling, a method with limitations for testing mechanistic hypotheses owing to varying levels of noncompliance over the long-term,” Dr. Ludwig and Dr. Ebbeling wrote.
Criticisms that claim to refute the carbohydrate-insulin hypothesis are based in part on misinterpretation of recent feeding studies, according to Dr. Ludwig and Dr. Ebbeling. Multiple studies testing whether or not high–glycemic load meals lead to increased fat storage have reported no meaningful differences between low-fat and low-carbohydrate diets. However, these short-term studies, mostly 2 weeks in duration, preclude definitive findings, according to the review.
That’s because the process of adapting to a high-fat diet after having consumed a high-carbohydrate diet takes weeks, which is a well-recognized phenomenon, Dr. Ludwig said.
“If you put sedentary people into military boot camp and tested their biological state after 6 days, you’d probably find that they were fatigued, weak, and had higher inflammation in their muscles, but clearly, you wouldn’t conclude that fitness training is bad for your health,” he said in the interview. “But yet, these are the sort of data that are being used to ‘falsify’ the carbohydrate-insulin model.
“We acknowledge that there aren’t definitive human data,” he continued, “but the conventional model has failed to both explain the obesity epidemic and control it, and the latest public health data suggests that rates are higher today than ever before, despite 50 years of focusing on calorie balance.”
SOURCE: Ludwig DS et al. JAMA Intern Med. 2018 Jul 2. doi:10.1001/jamainternmed.2018.2933.
While the debate continues, David S. Ludwig, MD, PhD, and Cara B. Ebbeling, PhD, argued in a recent clinical review that diet does indeed affect metabolism and body composition.
While evidence from human studies remains limited, animal research findings are consistent with a carbohydrate-insulin model of obesity, according to Dr. Ludwig and Dr. Ebbeling, who are with the New Balance Foundation Obesity Prevention Center at Boston Children’s Hospital and Harvard Medical School.
The carbohydrate-insulin model holds that eating processed, high–glycemic load carbohydrates causes hormonal changes that promote calorie deposition in fat tissue, aggravate hunger, and reduce energy expenditure, they said in JAMA Internal Medicine.
“The conventional way of thinking assumes that the individual has primary control over their calorie balance, and thus, bases conventional treatment on a target of establishing a negative energy balance – so that is 1,000 variations of the ‘eat less, move more’ recommendation,” Dr. Ludwig said in an interview.
The alternative to that established view has proven controversial. The Endocrine Society, in a recent scientific statement, said diet’s effect on obesity risk is largely explainable by calorie intake, rather than some special adverse effect on internal metabolism or energy expenditure.
“Stated differently, ‘a calorie is a calorie,’ ” the authors of the scientific statement said. “Thus, habitual consumption of highly palatable and energy-dense diets predispose to excess weight gain irrespective of macronutrient content.”
Others have sought to refute the carbohydrate-insulin hypothesis in recent reviews, such as an invited commentary in JAMA Internal Medicine by Kevin D. Hall, PhD, of the National Institute of Diabetes and Digestive and Kidney Diseases, and his coauthors.
“Although it is plausible that variables related to insulin signaling could be involved in obesity pathogenesis, the hypothesis that carbohydrate-stimulated insulin secretion is the primary cause of common obesity via direct effects on adipocytes is difficult to reconcile with current evidence,” Dr. Hall and his coauthors wrote in the commentary (JAMA Intern Med. 2018 Jul 2. doi: 10.1001/jamainternmed.2018.2920).
The conventional calorie balance model is a “straw man” that omits neuroendocrine mechanisms known to regulate homeostasis, added Dr. Hall and his coauthors, stating that accurate models of obesity should include physiological processes resisting weight loss and promoting weight gain.
“They might claim that this is a straw man argument, but I would claim that there is a case of the emperor’s new clothing,” Dr. Ludwig countered in the interview. “They argue that body weight is controlled by biology, and that that’s recognized in the conventional view, but how does that view inform treatment in any way? In the absence of any specific testable hypotheses for why the obesity epidemic has emerged so suddenly, conventional recommendations inevitably resort to advice to ‘eat less and move more.’ ”
Dr. Ludwig and Dr. Ebbeling have both conducted research studies examining the carbohydrate-insulin model, or the view that a high-carbohydrate diet results in postprandial hyperinsulinemia and promotes deposition of calories in adipocytes, leading to weight gain through slowing metabolism, increased hunger, or both.
In a study published in the Lancet, Dr. Ludwig and his coinvestigators found that rats fed a high–glycemic index (GI) diet for 18 weeks had more body fat (97.8 grams vs. 57.3 grams; P = .0152) and less lean body mass versus rats fed a low-GI diet. Rats on the high-GI diet also had greater increases over time in blood glucose and plasma insulin after oral glucose. Similarly, mice on a high-GI diet had nearly twice the body fat of mice on low-GI diet, after 9 weeks of feeding (Lancet. 2004 Aug 28. doi: 10.1016/S0140-6736(04)16937-7).
“There’s no way to explain that finding in view of the conventional view that all calories are alike to the body,” Dr. Ludwig said.
“Contrary to prediction of the conventional model, the inherently lower energy density of low-fat diets does not spontaneously produce sustained weight loss. In fact, several recent meta-analyses found that low-fat diets are inferior to all higher-fat [and thus low-glycemic] comparisons. However, these studies characteristically rely on dietary counseling, a method with limitations for testing mechanistic hypotheses owing to varying levels of noncompliance over the long-term,” Dr. Ludwig and Dr. Ebbeling wrote.
Criticisms that claim to refute the carbohydrate-insulin hypothesis are based in part on misinterpretation of recent feeding studies, according to Dr. Ludwig and Dr. Ebbeling. Multiple studies testing whether or not high–glycemic load meals lead to increased fat storage have reported no meaningful differences between low-fat and low-carbohydrate diets. However, these short-term studies, mostly 2 weeks in duration, preclude definitive findings, according to the review.
That’s because the process of adapting to a high-fat diet after having consumed a high-carbohydrate diet takes weeks, which is a well-recognized phenomenon, Dr. Ludwig said.
“If you put sedentary people into military boot camp and tested their biological state after 6 days, you’d probably find that they were fatigued, weak, and had higher inflammation in their muscles, but clearly, you wouldn’t conclude that fitness training is bad for your health,” he said in the interview. “But yet, these are the sort of data that are being used to ‘falsify’ the carbohydrate-insulin model.
“We acknowledge that there aren’t definitive human data,” he continued, “but the conventional model has failed to both explain the obesity epidemic and control it, and the latest public health data suggests that rates are higher today than ever before, despite 50 years of focusing on calorie balance.”
SOURCE: Ludwig DS et al. JAMA Intern Med. 2018 Jul 2. doi:10.1001/jamainternmed.2018.2933.
FROM JAMA INTERNAL MEDICINE
Obesity didn’t just happen overnight
Is it possible to get more exercise and still gain weight? In America it is.
The steady increase in obesity prevalence among adults in the United States has been exceeded over the last decade by the percentage of adults who are getting the recommended amount of exercise, according to the National Center for Health Statistics.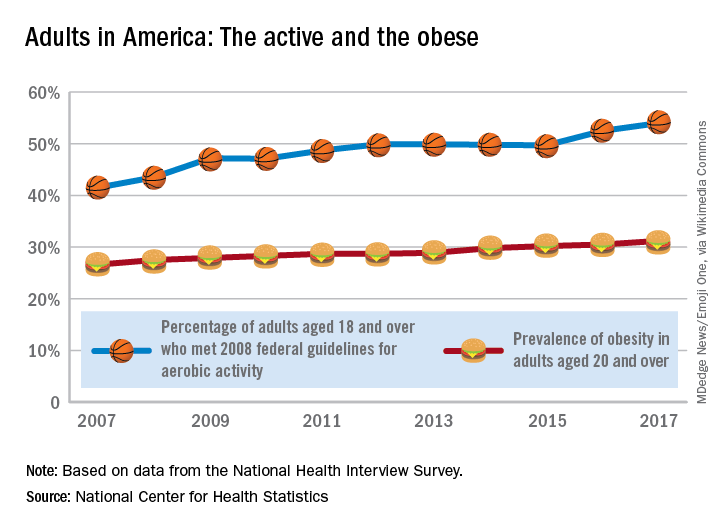
The 2008 guideline, “Physical Activity Guidelines for Americans” recommends that “adults perform at least 150 minutes a week of moderate-intensity aerobic physical activity, 75 minutes a week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic activity, performed in episodes of at least 10 minutes and preferably should be spread throughout the week,” the NCHS noted.
Is it possible to get more exercise and still gain weight? In America it is.
The steady increase in obesity prevalence among adults in the United States has been exceeded over the last decade by the percentage of adults who are getting the recommended amount of exercise, according to the National Center for Health Statistics.
The 2008 guideline, “Physical Activity Guidelines for Americans” recommends that “adults perform at least 150 minutes a week of moderate-intensity aerobic physical activity, 75 minutes a week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic activity, performed in episodes of at least 10 minutes and preferably should be spread throughout the week,” the NCHS noted.
Is it possible to get more exercise and still gain weight? In America it is.
The steady increase in obesity prevalence among adults in the United States has been exceeded over the last decade by the percentage of adults who are getting the recommended amount of exercise, according to the National Center for Health Statistics.
The 2008 guideline, “Physical Activity Guidelines for Americans” recommends that “adults perform at least 150 minutes a week of moderate-intensity aerobic physical activity, 75 minutes a week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic activity, performed in episodes of at least 10 minutes and preferably should be spread throughout the week,” the NCHS noted.
What underlies post–bariatric surgery bone fragility?
BOSTON – Charting a healthy path for patients after bariatric surgery can be complicated and addressing bone health is an important part of the endocrinologist’s role in keeping patients safe from postsurgical fractures, according to John Bilezikian, MD.
said Dr. Bilezikian, speaking during a bariatric surgery–focused session at the annual scientific & clinical congress of the American Academy of Clinical Endocrinologists.
It’s not easy to assess bone health, even before surgery, said Dr. Bilezikian. Even objective measures of bone density, such as dual-energy x-ray absorptiometry (DXA), may be skewed: very high fat mass causes artifact that interferes with accurate measurement of bone density, and DXA can’t distinguish between cortical and trabecular bone. The latter is a particular issue in high body mass index patients, since obesity is known to be associated with a more fragile bone microarchitecture, said Dr. Bilezikian, the Dorothy L. and Daniel H. Silberberg Professor of Medicine and director of the metabolic bone diseases unit at Columbia University, New York.
With these caveats in mind, Dr. Bilezikian said, there are some lessons to be learned from existing research to better manage bone health in bariatric patients.
After Roux-en-Y gastric bypass surgery (RYGB), bone turnover soon increases, with bone resorption markers increasing by up to 200% in the first 12-18 months after surgery. Bone formation markers also are elevated but to a lesser extent, said Dr. Bilezikian. Over time, the weight loss from RYGB is associated with a significant drop in bone mineral density (BMD) at weight-bearing sites. Weight loss was associated with bone loss at the total hip (r = 0.70; P less than .0003) and femoral neck (r = 0.47; P = .03 (J Clin Endocrinol Metab. 2013 Feb;98[2] 541-9).
A newer-technology, high-resolution peripheral quantitative CT (HR-pQCT) offers a noninvasive look not just at bone size and density but also at microarchitecture, including cortical thickness and details of trabecular structure. This technology “can help elucidate the structural basis for fragility,” said Dr. Bilezikian.
HR-pQCT was used in a recent study (J Bone Min Res. 2017 Dec. 27. doi: 10.1002/jbmr.3371) that followed 48 patients for 1 year after RYGB. Using HR-pQCT, DXA, and serum markers of bone turnover, the researchers found significant decrease in BMD and estimated decrease in bone strength after RYGB. Bone cortex became increasingly porous as well. Taken together, these changes may indicate an increased fracture risk, concluded the investigators.
A longer study that followed RYGB recipients for 2 years and used similar imaging and serum parameters also found that participants had decreased BMD. Tellingly, these investigators saw more marked increase in cortical porosity in the second year after bypass. Estimated bone strength continued to decline during the study period, even after weight loss had stopped.
All of these findings, said Dr. Bilezikian, point to a pathogenetic process other than weight loss that promotes the deteriorating bone microarchitecture seen years after RYGB. “Loss of bone mass and skeletal deterioration after gastric bypass surgery cannot be explained by weight loss alone,” said Dr. Bilezikian.
Another recent study was able to follow a small cohort of patients for a full 5 years, using DXA, lumbar CT, and Hr-pQCT. Though weight loss stabilized after 2 years and 25-OH D and calcium levels were unchanged from presurgical baseline, bone density continued to drop, and bone microarchitecture further deteriorated, said Dr. Bilezikian (Greenblatt L et al. ASBMR 2017, Abstract 1125).
Initially, post–bariatric surgery weight loss may induce bone changes because of skeletal unloading; further down the road, estrogen production by adipose tissue is decreased with ongoing fat loss, and sarcopenia may have an adverse effect on bone microarchitecture. Postsurgical malabsorption may also be an early mechanism of bone loss.
Other hormonal changes can include secondary hyperparathyroidism. Leptin, adiponectin, and peptide YY levels also may be altered.
Do these changes in BMD and bone architecture result in increased fracture risk? This question is difficult to answer, for the same reasons that other bariatric surgery research can be challenging, said Dr. Bilezikian. There is heterogeneity of procedures and supplement regimens, sample sizes can be small, follow-up times short, and adherence often is not tracked.
However, there are some clues that RYGB may be associated with an increased risk of all fractures and of fragility fractures, with appendicular fractures seen most frequently (Osteoporos Int. 2014 Jan; 25[1]:151-8). A larger study that tracked 12,676 patients receiving bariatric surgery, 38,028 patients with obesity, and 126,760 nonobese participants found that the bariatric patients had a 4.1% risk of fracture at 4 years post surgery, compared with 2.7% and 2.4% fracture rates in the participants with and without obesity, respectively (BMJ. 2016;354:i3794).
Other retrospective studies have found “a time-dependent increase in nonvertebral fractures with Roux-en-Y gastric bypass compared to gastric banding,” said Dr. Bilezikian.
How can these risks be managed after gastric bypass surgery? “Strive for nutritional adequacy” as the first step, said Dr. Bilezikian, meaning that calcium and vitamin D should be prescribed – and adherence encouraged – as indicated. Levels of 25-OH D should be checked regularly, with supplementation managed to keep levels over 30 ng/mL, he said.
All patients should be encouraged to develop and maintain an appropriate exercise regimen, and BMD should be followed over time. Those caring for post–gastric bypass patients can still use a bisphosphonate or other bone-health medication, if indicated using standard parameters. However, “You probably shouldn’t use an oral bisphosphonate in this population,” said Dr. Bilezikian.
Dr. Bilezikian reported that he has consulting or advisory relationships with Amgen, Radius Pharmaceuticals, Shire Pharmaceuticals, and Ultragenyx, and serves on a data safety monitoring board for Regeneron.
BOSTON – Charting a healthy path for patients after bariatric surgery can be complicated and addressing bone health is an important part of the endocrinologist’s role in keeping patients safe from postsurgical fractures, according to John Bilezikian, MD.
said Dr. Bilezikian, speaking during a bariatric surgery–focused session at the annual scientific & clinical congress of the American Academy of Clinical Endocrinologists.
It’s not easy to assess bone health, even before surgery, said Dr. Bilezikian. Even objective measures of bone density, such as dual-energy x-ray absorptiometry (DXA), may be skewed: very high fat mass causes artifact that interferes with accurate measurement of bone density, and DXA can’t distinguish between cortical and trabecular bone. The latter is a particular issue in high body mass index patients, since obesity is known to be associated with a more fragile bone microarchitecture, said Dr. Bilezikian, the Dorothy L. and Daniel H. Silberberg Professor of Medicine and director of the metabolic bone diseases unit at Columbia University, New York.
With these caveats in mind, Dr. Bilezikian said, there are some lessons to be learned from existing research to better manage bone health in bariatric patients.
After Roux-en-Y gastric bypass surgery (RYGB), bone turnover soon increases, with bone resorption markers increasing by up to 200% in the first 12-18 months after surgery. Bone formation markers also are elevated but to a lesser extent, said Dr. Bilezikian. Over time, the weight loss from RYGB is associated with a significant drop in bone mineral density (BMD) at weight-bearing sites. Weight loss was associated with bone loss at the total hip (r = 0.70; P less than .0003) and femoral neck (r = 0.47; P = .03 (J Clin Endocrinol Metab. 2013 Feb;98[2] 541-9).
A newer-technology, high-resolution peripheral quantitative CT (HR-pQCT) offers a noninvasive look not just at bone size and density but also at microarchitecture, including cortical thickness and details of trabecular structure. This technology “can help elucidate the structural basis for fragility,” said Dr. Bilezikian.
HR-pQCT was used in a recent study (J Bone Min Res. 2017 Dec. 27. doi: 10.1002/jbmr.3371) that followed 48 patients for 1 year after RYGB. Using HR-pQCT, DXA, and serum markers of bone turnover, the researchers found significant decrease in BMD and estimated decrease in bone strength after RYGB. Bone cortex became increasingly porous as well. Taken together, these changes may indicate an increased fracture risk, concluded the investigators.
A longer study that followed RYGB recipients for 2 years and used similar imaging and serum parameters also found that participants had decreased BMD. Tellingly, these investigators saw more marked increase in cortical porosity in the second year after bypass. Estimated bone strength continued to decline during the study period, even after weight loss had stopped.
All of these findings, said Dr. Bilezikian, point to a pathogenetic process other than weight loss that promotes the deteriorating bone microarchitecture seen years after RYGB. “Loss of bone mass and skeletal deterioration after gastric bypass surgery cannot be explained by weight loss alone,” said Dr. Bilezikian.
Another recent study was able to follow a small cohort of patients for a full 5 years, using DXA, lumbar CT, and Hr-pQCT. Though weight loss stabilized after 2 years and 25-OH D and calcium levels were unchanged from presurgical baseline, bone density continued to drop, and bone microarchitecture further deteriorated, said Dr. Bilezikian (Greenblatt L et al. ASBMR 2017, Abstract 1125).
Initially, post–bariatric surgery weight loss may induce bone changes because of skeletal unloading; further down the road, estrogen production by adipose tissue is decreased with ongoing fat loss, and sarcopenia may have an adverse effect on bone microarchitecture. Postsurgical malabsorption may also be an early mechanism of bone loss.
Other hormonal changes can include secondary hyperparathyroidism. Leptin, adiponectin, and peptide YY levels also may be altered.
Do these changes in BMD and bone architecture result in increased fracture risk? This question is difficult to answer, for the same reasons that other bariatric surgery research can be challenging, said Dr. Bilezikian. There is heterogeneity of procedures and supplement regimens, sample sizes can be small, follow-up times short, and adherence often is not tracked.
However, there are some clues that RYGB may be associated with an increased risk of all fractures and of fragility fractures, with appendicular fractures seen most frequently (Osteoporos Int. 2014 Jan; 25[1]:151-8). A larger study that tracked 12,676 patients receiving bariatric surgery, 38,028 patients with obesity, and 126,760 nonobese participants found that the bariatric patients had a 4.1% risk of fracture at 4 years post surgery, compared with 2.7% and 2.4% fracture rates in the participants with and without obesity, respectively (BMJ. 2016;354:i3794).
Other retrospective studies have found “a time-dependent increase in nonvertebral fractures with Roux-en-Y gastric bypass compared to gastric banding,” said Dr. Bilezikian.
How can these risks be managed after gastric bypass surgery? “Strive for nutritional adequacy” as the first step, said Dr. Bilezikian, meaning that calcium and vitamin D should be prescribed – and adherence encouraged – as indicated. Levels of 25-OH D should be checked regularly, with supplementation managed to keep levels over 30 ng/mL, he said.
All patients should be encouraged to develop and maintain an appropriate exercise regimen, and BMD should be followed over time. Those caring for post–gastric bypass patients can still use a bisphosphonate or other bone-health medication, if indicated using standard parameters. However, “You probably shouldn’t use an oral bisphosphonate in this population,” said Dr. Bilezikian.
Dr. Bilezikian reported that he has consulting or advisory relationships with Amgen, Radius Pharmaceuticals, Shire Pharmaceuticals, and Ultragenyx, and serves on a data safety monitoring board for Regeneron.
BOSTON – Charting a healthy path for patients after bariatric surgery can be complicated and addressing bone health is an important part of the endocrinologist’s role in keeping patients safe from postsurgical fractures, according to John Bilezikian, MD.
said Dr. Bilezikian, speaking during a bariatric surgery–focused session at the annual scientific & clinical congress of the American Academy of Clinical Endocrinologists.
It’s not easy to assess bone health, even before surgery, said Dr. Bilezikian. Even objective measures of bone density, such as dual-energy x-ray absorptiometry (DXA), may be skewed: very high fat mass causes artifact that interferes with accurate measurement of bone density, and DXA can’t distinguish between cortical and trabecular bone. The latter is a particular issue in high body mass index patients, since obesity is known to be associated with a more fragile bone microarchitecture, said Dr. Bilezikian, the Dorothy L. and Daniel H. Silberberg Professor of Medicine and director of the metabolic bone diseases unit at Columbia University, New York.
With these caveats in mind, Dr. Bilezikian said, there are some lessons to be learned from existing research to better manage bone health in bariatric patients.
After Roux-en-Y gastric bypass surgery (RYGB), bone turnover soon increases, with bone resorption markers increasing by up to 200% in the first 12-18 months after surgery. Bone formation markers also are elevated but to a lesser extent, said Dr. Bilezikian. Over time, the weight loss from RYGB is associated with a significant drop in bone mineral density (BMD) at weight-bearing sites. Weight loss was associated with bone loss at the total hip (r = 0.70; P less than .0003) and femoral neck (r = 0.47; P = .03 (J Clin Endocrinol Metab. 2013 Feb;98[2] 541-9).
A newer-technology, high-resolution peripheral quantitative CT (HR-pQCT) offers a noninvasive look not just at bone size and density but also at microarchitecture, including cortical thickness and details of trabecular structure. This technology “can help elucidate the structural basis for fragility,” said Dr. Bilezikian.
HR-pQCT was used in a recent study (J Bone Min Res. 2017 Dec. 27. doi: 10.1002/jbmr.3371) that followed 48 patients for 1 year after RYGB. Using HR-pQCT, DXA, and serum markers of bone turnover, the researchers found significant decrease in BMD and estimated decrease in bone strength after RYGB. Bone cortex became increasingly porous as well. Taken together, these changes may indicate an increased fracture risk, concluded the investigators.
A longer study that followed RYGB recipients for 2 years and used similar imaging and serum parameters also found that participants had decreased BMD. Tellingly, these investigators saw more marked increase in cortical porosity in the second year after bypass. Estimated bone strength continued to decline during the study period, even after weight loss had stopped.
All of these findings, said Dr. Bilezikian, point to a pathogenetic process other than weight loss that promotes the deteriorating bone microarchitecture seen years after RYGB. “Loss of bone mass and skeletal deterioration after gastric bypass surgery cannot be explained by weight loss alone,” said Dr. Bilezikian.
Another recent study was able to follow a small cohort of patients for a full 5 years, using DXA, lumbar CT, and Hr-pQCT. Though weight loss stabilized after 2 years and 25-OH D and calcium levels were unchanged from presurgical baseline, bone density continued to drop, and bone microarchitecture further deteriorated, said Dr. Bilezikian (Greenblatt L et al. ASBMR 2017, Abstract 1125).
Initially, post–bariatric surgery weight loss may induce bone changes because of skeletal unloading; further down the road, estrogen production by adipose tissue is decreased with ongoing fat loss, and sarcopenia may have an adverse effect on bone microarchitecture. Postsurgical malabsorption may also be an early mechanism of bone loss.
Other hormonal changes can include secondary hyperparathyroidism. Leptin, adiponectin, and peptide YY levels also may be altered.
Do these changes in BMD and bone architecture result in increased fracture risk? This question is difficult to answer, for the same reasons that other bariatric surgery research can be challenging, said Dr. Bilezikian. There is heterogeneity of procedures and supplement regimens, sample sizes can be small, follow-up times short, and adherence often is not tracked.
However, there are some clues that RYGB may be associated with an increased risk of all fractures and of fragility fractures, with appendicular fractures seen most frequently (Osteoporos Int. 2014 Jan; 25[1]:151-8). A larger study that tracked 12,676 patients receiving bariatric surgery, 38,028 patients with obesity, and 126,760 nonobese participants found that the bariatric patients had a 4.1% risk of fracture at 4 years post surgery, compared with 2.7% and 2.4% fracture rates in the participants with and without obesity, respectively (BMJ. 2016;354:i3794).
Other retrospective studies have found “a time-dependent increase in nonvertebral fractures with Roux-en-Y gastric bypass compared to gastric banding,” said Dr. Bilezikian.
How can these risks be managed after gastric bypass surgery? “Strive for nutritional adequacy” as the first step, said Dr. Bilezikian, meaning that calcium and vitamin D should be prescribed – and adherence encouraged – as indicated. Levels of 25-OH D should be checked regularly, with supplementation managed to keep levels over 30 ng/mL, he said.
All patients should be encouraged to develop and maintain an appropriate exercise regimen, and BMD should be followed over time. Those caring for post–gastric bypass patients can still use a bisphosphonate or other bone-health medication, if indicated using standard parameters. However, “You probably shouldn’t use an oral bisphosphonate in this population,” said Dr. Bilezikian.
Dr. Bilezikian reported that he has consulting or advisory relationships with Amgen, Radius Pharmaceuticals, Shire Pharmaceuticals, and Ultragenyx, and serves on a data safety monitoring board for Regeneron.
REPORTING FROM AACE 2018
Bariatric revision mortality linked to age, comorbidities
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
WASHINGTON – and appears to be rising in recent years, according to two studies presented at the annual Digestive Disease Week®.
Violeta B. Popov, MD, of New York University, and a team of researchers used the Nationwide Inpatient Sample (NIS) to look at mortality risk, costs, and risk factors for complications in revisional bariatric procedures.
In one presentation, Dr. Popov noted that revision after bariatric surgery occurred in approximately 8% of cases for a variety of reasons including lap band adjustment, weight regain, gastric reflux problems, and rarely, because of staple-line leaks. Referring to findings based on the Bariatric Outcomes Longitudinal Database (BOLD), Dr. Popov said that mortality after primary bariatric surgery is estimated at around 0.2% and revisional procedures carry nearly the same low level of mortality risk. BOLD was developed by the American Society of Metabolic and Bariatric Surgery and reflects outcomes from certified Bariatric Centers of Excellence from 2007 to 2012. However, Dr. Popov noted, the outcomes derived from BOLD may well be better than those from noncertified centers (Gastrointest Surg. 2015 Jan;19[1]:171-8).
Dr. Popov reported that the number of revisional procedures has doubled over recent years, from 6% of all bariatric procedures in 2011 to 13% in 2015. The reasons behind the increase could be related to the number of patients switching to a different bariatric approach, the removal of lap bands, and possibly the increase in the number of primary bariatric surgeries performed by less-skilled operators, Dr. Popov said.
The investigators aimed to determine the mortality trends for these procedures in addition to evaluating costs and risk factors for complications. They conducted a retrospective cohort study using the 2014 NIS, comprising 14,280 patients who underwent revisional bariatric surgery. The primary outcome was postoperative in-hospital mortality, with secondary outcomes of cost, length of hospital stay (LOS), and ICU stay. The variables included a variety of comorbidities, alcohol use, smoking, income, and insurance status.
The mean age of this sample was 68 years and 58.8% were female. Outcomes for revisional bariatric surgery were worse in several categories than were found in the BOLD study in terms of LOS, costs, and mortality, and postoperative in-hospital mortality was unexpectedly high at 2.1% (290 patients). A total of 3.3% of the patients had an ICU stay, one-quarter of whom died.
On univariate analysis, comorbidities (age, coagulopathy, chronic kidney disease, anemia, and chronic heart failure) and the combined number of chronic conditions were all significant predictors of mortality. Multivariate analysis identified age (odds ratio, 1.08; 95% confidence interval, 1.04-1.20; P less than .001), alcohol use (OR, 4.0; 95% CI, 1.3-11.7; P = .01), coagulopathy (OR, 5.4; 95% CI, 2.2-13.3; P less than .001), and insurance status (Medicaid vs. private; OR, 4.0; 95% CI, 1.7-9.9; P = .002) as the most significant predictors of mortality after a revisional bariatric procedure.
In a poster, Dr. Popov and her colleagues presented data from the NIS database looking at 10-year mortality and outcome trends for revisional surgery versus primary Roux-en-Y gastric bypass (RYGB) surgery. Inpatient mortality for RYGB decreased from 2.54% in 2003 to 1.80% in 2014, but was still substantially higher than the BOLD findings. But mortality for revisional surgery increased: 1.90% versus 2.03%. LOS for RYGB decreased from 5.9 days to 5.4 but increased for revisional surgery from 4.6 to 5.4 days. Cost for both procedures, adjusted for inflation, more than doubled between 2003 and 2014. And patients requiring ICU admission for both procedures went from 1% in 2003 to 3% in 2014.
The limitations of both analyses are their retrospective design, the NIS bias inferred by the inclusion of only inpatient procedures, and the lack of laboratory data or data on body mass index. In addition, during the study period, primary bariatric surgery began to be performed as an outpatient procedure. “Low-risk procedures performed in outpatient facilities will not be captured in the database and thus the higher mortality for these higher risk patients is expected,” Dr. Popov said. These patients are likely to be sicker and have more comorbidities. Revisional procedures are typically done in the hospital, but there are some low-risk revisional procedures such as lap band removal that could be done as outpatient procedures. Dr. Popov had confidence that the NIS database reflects real-world outcomes for revisional bariatric procedures.
She concluded that the explanation for the increase in mortality risk for revisional bariatric surgery may be because of more of these procedures being done outside centers of excellence and more, older patients with comorbidities having the surgery, and that nonsurgical alternatives should be explored for the older sicker patients.
Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
SOURCE: Popov VB et al. DDW 2018, Abstract 324.
REPORTING FROM DDW 2018
Key clinical point: Revisional bariatric procedures may carry a greater mortality risk than previous studies have suggested.
Major finding: The mortality rate in the sample was 2.1%.
Study details: The 2014 Nationwide Inpatient Sample database, comprising 14,280 patients who underwent revisional bariatric surgery.
Disclosures: Dr. Popova disclosed ownership of shares in Embarcadero Technologies but no conflicts of interest.
Source: Popov VB et al. DDW 2018, Abstract 324.












