User login
Low Back Pain Triggered by Fatigue, Distractions, and Awkward Positions
New research reveals the physical and psychosocial factors that significantly increase the risk of low back pain onset. The trial results, which were published online ahead of print February 9 in Arthritis Care & Research, show that being engaged in manual tasks involving awkward positions will increase the risk of low back pain by 8 times. People who are distracted during activities or fatigued also significantly increase their risk of acute low back pain.
“Understanding which risk factors contribute to back pain and controlling exposure to these risks is an important first step in prevention,” said Manuela Ferreira, PhD, Associate Professor at the George Institute for Global Health and Sydney Medical School at the University of Sydney in New South Wales, Australia. “Our study is the first to examine brief exposure to a range of modifiable triggers for an acute episode of low back pain.”
For this case-crossover study, Dr. Ferreira and colleagues recruited 999 participants from 300 primary care clinics in Sydney, Australia, who had an acute low back pain episode between October 2011 and November 2012. Participants were asked to report exposure to 12 physical or psychosocial factors in the 96 hours prior to the onset of back pain.
The study found that:
• The risk of a new episode of low back pain significantly increased due to a range of triggers, from an odds ratio of 2.7 for moderate to vigorous physical activity to 25.0 for distraction during an activity.
• Back pain risk was highest between 7:00 am and at noontime.
• Age moderated the effect of exposure to heavy loads, with an odds ratio for individuals age 20, 40, or 60 at 13.6, 6.0, and 2.7, respectively.
“Understanding which modifiable risk factors lead to low back pain is an important step toward controlling a condition that affects so many worldwide,” said Dr. Ferreira. “Our findings enhance knowledge of low back pain triggers and will assist the development of new prevention programs that can reduce suffering from this potentially disabling condition.”
Suggested Reading
Steffens D, Ferreira ML, Latimer J, et al. What triggers an episode of acute low back pain? a case-crossover study. Arthritis Care Res. 2015 Feb 9. [Epub ahead of print]
New research reveals the physical and psychosocial factors that significantly increase the risk of low back pain onset. The trial results, which were published online ahead of print February 9 in Arthritis Care & Research, show that being engaged in manual tasks involving awkward positions will increase the risk of low back pain by 8 times. People who are distracted during activities or fatigued also significantly increase their risk of acute low back pain.
“Understanding which risk factors contribute to back pain and controlling exposure to these risks is an important first step in prevention,” said Manuela Ferreira, PhD, Associate Professor at the George Institute for Global Health and Sydney Medical School at the University of Sydney in New South Wales, Australia. “Our study is the first to examine brief exposure to a range of modifiable triggers for an acute episode of low back pain.”
For this case-crossover study, Dr. Ferreira and colleagues recruited 999 participants from 300 primary care clinics in Sydney, Australia, who had an acute low back pain episode between October 2011 and November 2012. Participants were asked to report exposure to 12 physical or psychosocial factors in the 96 hours prior to the onset of back pain.
The study found that:
• The risk of a new episode of low back pain significantly increased due to a range of triggers, from an odds ratio of 2.7 for moderate to vigorous physical activity to 25.0 for distraction during an activity.
• Back pain risk was highest between 7:00 am and at noontime.
• Age moderated the effect of exposure to heavy loads, with an odds ratio for individuals age 20, 40, or 60 at 13.6, 6.0, and 2.7, respectively.
“Understanding which modifiable risk factors lead to low back pain is an important step toward controlling a condition that affects so many worldwide,” said Dr. Ferreira. “Our findings enhance knowledge of low back pain triggers and will assist the development of new prevention programs that can reduce suffering from this potentially disabling condition.”
New research reveals the physical and psychosocial factors that significantly increase the risk of low back pain onset. The trial results, which were published online ahead of print February 9 in Arthritis Care & Research, show that being engaged in manual tasks involving awkward positions will increase the risk of low back pain by 8 times. People who are distracted during activities or fatigued also significantly increase their risk of acute low back pain.
“Understanding which risk factors contribute to back pain and controlling exposure to these risks is an important first step in prevention,” said Manuela Ferreira, PhD, Associate Professor at the George Institute for Global Health and Sydney Medical School at the University of Sydney in New South Wales, Australia. “Our study is the first to examine brief exposure to a range of modifiable triggers for an acute episode of low back pain.”
For this case-crossover study, Dr. Ferreira and colleagues recruited 999 participants from 300 primary care clinics in Sydney, Australia, who had an acute low back pain episode between October 2011 and November 2012. Participants were asked to report exposure to 12 physical or psychosocial factors in the 96 hours prior to the onset of back pain.
The study found that:
• The risk of a new episode of low back pain significantly increased due to a range of triggers, from an odds ratio of 2.7 for moderate to vigorous physical activity to 25.0 for distraction during an activity.
• Back pain risk was highest between 7:00 am and at noontime.
• Age moderated the effect of exposure to heavy loads, with an odds ratio for individuals age 20, 40, or 60 at 13.6, 6.0, and 2.7, respectively.
“Understanding which modifiable risk factors lead to low back pain is an important step toward controlling a condition that affects so many worldwide,” said Dr. Ferreira. “Our findings enhance knowledge of low back pain triggers and will assist the development of new prevention programs that can reduce suffering from this potentially disabling condition.”
Suggested Reading
Steffens D, Ferreira ML, Latimer J, et al. What triggers an episode of acute low back pain? a case-crossover study. Arthritis Care Res. 2015 Feb 9. [Epub ahead of print]
Suggested Reading
Steffens D, Ferreira ML, Latimer J, et al. What triggers an episode of acute low back pain? a case-crossover study. Arthritis Care Res. 2015 Feb 9. [Epub ahead of print]
Study: Osteoarthritis develops sooner than thought after ACL injury, repair
People who have had a knee reconstruction following trauma may be susceptible to osteoarthritis sooner than currently thought, according to new MRI findings at 1 year after anterior cruciate ligament reconstruction.
Almost a third of people studied had some evidence of early osteoarthritis (OA) at that early time point, challenging “existing dogma that degenerative joint disease does not become apparent for years post-ACLR,” reported Dr. Kay Crossley of the University of Queensland in Brisbane, Australia (Arthritis Rheumatol. 2015 Feb. 18 [doi:10.1002/art.39005]).
However, as they did not have access to preoperative images, they could not rule out that some OA features may have been preexisting and not related to knee trauma, they said.
“This is a sample that was taken after the injury and after the reconstruction, so they truly don’t know that what they’re finding is as a result of even the injury, surgery, or the meniscal damage or meniscal resection they had done at the time,” Dr. David J. Hunter, a leading OA expert from Sydney (Australia) University, said when asked to comment on the study’s findings.
“It may well be that these were people that had some underlying structural damage,” he added.
The researchers noted that radiographic knee OA was thought to be as high as 50%-90% a decade after anterior cruciate ligament reconstruction (ACLR). The issue is particularly important because ACL injuries typically occur in younger adults who are then prone to developing knee OA before they reach 40 years, they said.
“Early detection of knee OA after ACLR may permit early intervention such as load management, which is likely to be more effective prior to the development of advanced disease,” they wrote.
Their study included 111 patients aged 18-50 years who had undergone single-bundle hamstring-tendon autograft ACLR 1 year earlier.
MRI scans of their knees were compared with 20 uninjured asymptomatic matched controls. The researchers used the MRI Osteoarthritis Knee Score (MOAKS) to score specific OA features because the more recent Anterior Cruciate Ligament Osteoarthritis Score (ACLOAS) had not been published at the time of their study.
Results showed that 34 (31%) patients had MRI-defined knee OA following an ACLR a year earlier.
MRI-OA features were most frequently found in the patellofemoral compartment, particularly the medial femoral trochlea, a potentially underrecognized site of knee pathology following reconstruction, the researchers said.
Pathology in the patellofemoral joint included not only “early” features of OA, such as bone marrow lesions and partial-thickness cartilage loss, but also frank osteophytes on MRI, they noted.
None of the uninjured control knees had MRI-defined patellofemoral or tibiofemoral OA.
The authors acknowledged that a lack of access to preoperative knee images limited the conclusions they could reach in their study, but they noted that MRI-OA features were rarely seen in the small sample of uninjured matched control knees.
“Combined with the observation that six times as many reconstructed knees had radiographic osteophytes than uninjured contralateral knees, these findings suggest that knee trauma and/or reconstruction was strongly implicated in the development of OA features,” they wrote.
Another limitation that the authors acknowledged was that the MRI definition of OA was relatively new and was likely to be refined as the understanding of OA pathology evolved.
Dr. Hunter, who was the lead investigator involved in developing the MOAKS, agreed that the definition needed more validity and testing.
“This is the third study that uses that definition, and I do think that long-term clinical implications of what MRI definition means is unknown,” he said. “The challenge that we have is that we do kick up a lot of abnormalities, and we don’t truly know what the long-term clinical implications of those abnormalities are at this point.”
“There are a lot of problems with the way this study has been done, but I do think it is really helpful that it highlights how important injury is with regards to predisposing to early OA.”
“It’s something that a lot of people don’t really highlight or pay attention to,” he said.
The study was partly funded by the Queensland Orthopaedic Physiotherapy Network, a University of Melbourne Research Collaboration grant, and University of British Columbia’s Centre for Hip Health and Mobility via the Society for Mobility and Health. One study author is president of Boston Imaging Core Lab, LLC, and is a consultant to Merck Serono, Sanofi-Aventis, Genzyme and TissueGene. No other authors declared conflicts of interest.
People who have had a knee reconstruction following trauma may be susceptible to osteoarthritis sooner than currently thought, according to new MRI findings at 1 year after anterior cruciate ligament reconstruction.
Almost a third of people studied had some evidence of early osteoarthritis (OA) at that early time point, challenging “existing dogma that degenerative joint disease does not become apparent for years post-ACLR,” reported Dr. Kay Crossley of the University of Queensland in Brisbane, Australia (Arthritis Rheumatol. 2015 Feb. 18 [doi:10.1002/art.39005]).
However, as they did not have access to preoperative images, they could not rule out that some OA features may have been preexisting and not related to knee trauma, they said.
“This is a sample that was taken after the injury and after the reconstruction, so they truly don’t know that what they’re finding is as a result of even the injury, surgery, or the meniscal damage or meniscal resection they had done at the time,” Dr. David J. Hunter, a leading OA expert from Sydney (Australia) University, said when asked to comment on the study’s findings.
“It may well be that these were people that had some underlying structural damage,” he added.
The researchers noted that radiographic knee OA was thought to be as high as 50%-90% a decade after anterior cruciate ligament reconstruction (ACLR). The issue is particularly important because ACL injuries typically occur in younger adults who are then prone to developing knee OA before they reach 40 years, they said.
“Early detection of knee OA after ACLR may permit early intervention such as load management, which is likely to be more effective prior to the development of advanced disease,” they wrote.
Their study included 111 patients aged 18-50 years who had undergone single-bundle hamstring-tendon autograft ACLR 1 year earlier.
MRI scans of their knees were compared with 20 uninjured asymptomatic matched controls. The researchers used the MRI Osteoarthritis Knee Score (MOAKS) to score specific OA features because the more recent Anterior Cruciate Ligament Osteoarthritis Score (ACLOAS) had not been published at the time of their study.
Results showed that 34 (31%) patients had MRI-defined knee OA following an ACLR a year earlier.
MRI-OA features were most frequently found in the patellofemoral compartment, particularly the medial femoral trochlea, a potentially underrecognized site of knee pathology following reconstruction, the researchers said.
Pathology in the patellofemoral joint included not only “early” features of OA, such as bone marrow lesions and partial-thickness cartilage loss, but also frank osteophytes on MRI, they noted.
None of the uninjured control knees had MRI-defined patellofemoral or tibiofemoral OA.
The authors acknowledged that a lack of access to preoperative knee images limited the conclusions they could reach in their study, but they noted that MRI-OA features were rarely seen in the small sample of uninjured matched control knees.
“Combined with the observation that six times as many reconstructed knees had radiographic osteophytes than uninjured contralateral knees, these findings suggest that knee trauma and/or reconstruction was strongly implicated in the development of OA features,” they wrote.
Another limitation that the authors acknowledged was that the MRI definition of OA was relatively new and was likely to be refined as the understanding of OA pathology evolved.
Dr. Hunter, who was the lead investigator involved in developing the MOAKS, agreed that the definition needed more validity and testing.
“This is the third study that uses that definition, and I do think that long-term clinical implications of what MRI definition means is unknown,” he said. “The challenge that we have is that we do kick up a lot of abnormalities, and we don’t truly know what the long-term clinical implications of those abnormalities are at this point.”
“There are a lot of problems with the way this study has been done, but I do think it is really helpful that it highlights how important injury is with regards to predisposing to early OA.”
“It’s something that a lot of people don’t really highlight or pay attention to,” he said.
The study was partly funded by the Queensland Orthopaedic Physiotherapy Network, a University of Melbourne Research Collaboration grant, and University of British Columbia’s Centre for Hip Health and Mobility via the Society for Mobility and Health. One study author is president of Boston Imaging Core Lab, LLC, and is a consultant to Merck Serono, Sanofi-Aventis, Genzyme and TissueGene. No other authors declared conflicts of interest.
People who have had a knee reconstruction following trauma may be susceptible to osteoarthritis sooner than currently thought, according to new MRI findings at 1 year after anterior cruciate ligament reconstruction.
Almost a third of people studied had some evidence of early osteoarthritis (OA) at that early time point, challenging “existing dogma that degenerative joint disease does not become apparent for years post-ACLR,” reported Dr. Kay Crossley of the University of Queensland in Brisbane, Australia (Arthritis Rheumatol. 2015 Feb. 18 [doi:10.1002/art.39005]).
However, as they did not have access to preoperative images, they could not rule out that some OA features may have been preexisting and not related to knee trauma, they said.
“This is a sample that was taken after the injury and after the reconstruction, so they truly don’t know that what they’re finding is as a result of even the injury, surgery, or the meniscal damage or meniscal resection they had done at the time,” Dr. David J. Hunter, a leading OA expert from Sydney (Australia) University, said when asked to comment on the study’s findings.
“It may well be that these were people that had some underlying structural damage,” he added.
The researchers noted that radiographic knee OA was thought to be as high as 50%-90% a decade after anterior cruciate ligament reconstruction (ACLR). The issue is particularly important because ACL injuries typically occur in younger adults who are then prone to developing knee OA before they reach 40 years, they said.
“Early detection of knee OA after ACLR may permit early intervention such as load management, which is likely to be more effective prior to the development of advanced disease,” they wrote.
Their study included 111 patients aged 18-50 years who had undergone single-bundle hamstring-tendon autograft ACLR 1 year earlier.
MRI scans of their knees were compared with 20 uninjured asymptomatic matched controls. The researchers used the MRI Osteoarthritis Knee Score (MOAKS) to score specific OA features because the more recent Anterior Cruciate Ligament Osteoarthritis Score (ACLOAS) had not been published at the time of their study.
Results showed that 34 (31%) patients had MRI-defined knee OA following an ACLR a year earlier.
MRI-OA features were most frequently found in the patellofemoral compartment, particularly the medial femoral trochlea, a potentially underrecognized site of knee pathology following reconstruction, the researchers said.
Pathology in the patellofemoral joint included not only “early” features of OA, such as bone marrow lesions and partial-thickness cartilage loss, but also frank osteophytes on MRI, they noted.
None of the uninjured control knees had MRI-defined patellofemoral or tibiofemoral OA.
The authors acknowledged that a lack of access to preoperative knee images limited the conclusions they could reach in their study, but they noted that MRI-OA features were rarely seen in the small sample of uninjured matched control knees.
“Combined with the observation that six times as many reconstructed knees had radiographic osteophytes than uninjured contralateral knees, these findings suggest that knee trauma and/or reconstruction was strongly implicated in the development of OA features,” they wrote.
Another limitation that the authors acknowledged was that the MRI definition of OA was relatively new and was likely to be refined as the understanding of OA pathology evolved.
Dr. Hunter, who was the lead investigator involved in developing the MOAKS, agreed that the definition needed more validity and testing.
“This is the third study that uses that definition, and I do think that long-term clinical implications of what MRI definition means is unknown,” he said. “The challenge that we have is that we do kick up a lot of abnormalities, and we don’t truly know what the long-term clinical implications of those abnormalities are at this point.”
“There are a lot of problems with the way this study has been done, but I do think it is really helpful that it highlights how important injury is with regards to predisposing to early OA.”
“It’s something that a lot of people don’t really highlight or pay attention to,” he said.
The study was partly funded by the Queensland Orthopaedic Physiotherapy Network, a University of Melbourne Research Collaboration grant, and University of British Columbia’s Centre for Hip Health and Mobility via the Society for Mobility and Health. One study author is president of Boston Imaging Core Lab, LLC, and is a consultant to Merck Serono, Sanofi-Aventis, Genzyme and TissueGene. No other authors declared conflicts of interest.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: People who have undergone anterior cruciate ligament reconstruction following trauma may be susceptible to early OA sooner than previously thought, but the study authors did not have access to baseline images to rule out existing pathology.
Major finding: A third of the 111 patients studied had evidence of MRI-defined OA a year after their surgery.
Data source: MRI study of 111 patients who had undergone ACL surgery matched with 20 uninjured asymptomatic controls.
Disclosures: The study was partly funded by the Queensland Orthopaedic Physiotherapy Network, a University of Melbourne Research Collaboration grant, and University of British Columbia’s Centre for Hip Health and Mobility via the Society for Mobility and Health. One study author is president of Boston Imaging Core Lab, LLC, and is a consultant to Merck Serono, Sanofi-Aventis, Genzyme and TissueGene. No other authors declared conflicts of interest.
VIDEO: Ask patients about metal-on-metal hip implants
MAUI, HAWAII – Rheumatologists and other providers need to ask patients if they’ve had metal-on-metal hip implants.
That goes for hip resurfacing – which by definition is metal on metal – as well as actual metal-on-metal hips. Signs of trouble can be as subtle as mental status changes, and they go well beyond the traditional issues with worn-out artificial joints.
During a video interview at the 2015 Rheumatology Winter Clinical Symposium, Dr. Bill Bugbee, an orthopedic surgeon and professor at the University of California, San Diego, explained the problems and the warning signs for which physicians should watch.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MAUI, HAWAII – Rheumatologists and other providers need to ask patients if they’ve had metal-on-metal hip implants.
That goes for hip resurfacing – which by definition is metal on metal – as well as actual metal-on-metal hips. Signs of trouble can be as subtle as mental status changes, and they go well beyond the traditional issues with worn-out artificial joints.
During a video interview at the 2015 Rheumatology Winter Clinical Symposium, Dr. Bill Bugbee, an orthopedic surgeon and professor at the University of California, San Diego, explained the problems and the warning signs for which physicians should watch.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MAUI, HAWAII – Rheumatologists and other providers need to ask patients if they’ve had metal-on-metal hip implants.
That goes for hip resurfacing – which by definition is metal on metal – as well as actual metal-on-metal hips. Signs of trouble can be as subtle as mental status changes, and they go well beyond the traditional issues with worn-out artificial joints.
During a video interview at the 2015 Rheumatology Winter Clinical Symposium, Dr. Bill Bugbee, an orthopedic surgeon and professor at the University of California, San Diego, explained the problems and the warning signs for which physicians should watch.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT RWCS 2015
ACL repair: ‘We have to do better’
SNOWMASS, COLO. – A novel approach to repairing anterior cruciate ligament injuries – and perhaps thereby avoiding a downstream tidal wave of knee osteoarthritis – is creating major buzz in sports medicine circles.
“You’ll probably hear much more about this bioenhanced repair, with the expectation of achieving strength equal to that of ACL reconstruction and perhaps preventing the development of osteoarthritis 15 years down the road,” Dr. M. Timothy Hresko predicted at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
He cited research led by his colleague Dr. Martha M. Murray of Boston Children’s Hospital, which has resulted in development of a surgical technique combining a tissue-engineered composite scaffold with a suture repair of the torn ACL in what Dr. Murray has termed a bioenhanced repair.
Her work, to date preclinical, has garnered major awards from both the American Orthopaedic Society for Sports Medicine and the American Academy of Orthopaedic Surgeons. The Food and Drug Administration recently granted approval for the first clinical safety studies, to begin this year.
There is a major unmet need for better methods of repairing ACL injuries. They’re common, with an estimated 550,000 cases per year. The peak incidence occurs in 15- to 19-year-old female athletes. And the current gold standard therapy consisting of ACL reconstruction using an allograft or hamstring graft has a disturbingly high failure rate, both early and late. The graft failure rate is up to 20% in the first 2 years, climbing to 50% at 10 years.
“We just have to do better,” conceded Dr. Hresko, an orthopedic surgeon at Harvard Medical School, Boston, and Boston Children’s Hospital.
“One of the interesting and unfortunate facts,” he continued, “is that roughly 80% of people who have an ACL injury, with or without reconstruction, are still going to have osteoarthritis 14 years after the injury. So, if this is your 15-year-old daughter who plays basketball, she’ll only be 30 and will already have degenerative arthritis of the knee at what should still be a very active period of life.”
The bioenhanced repair now under study uses an extracellular matrix-based scaffold, which is loaded with a few milliliters of the patient’s own platelet-enriched plasma. The scaffold is applied between the torn ligament ends in order to stimulate collagen production and promote ligament healing. The suture repair of the ligament entails much less trauma than does standard reconstructive surgery.
In large-animal studies, the bioenhanced repair resulted in the same yield load, stiffness, and other desirable biomechanical properties at 1 year as with major reconstructive surgery. However, while premature posttraumatic osteoarthritis occurred in 80% of the knees treated with standard ACL reconstruction, there was no evidence of such damage 1 year following bioenhanced repair. Nor have adverse reactions to the scaffold been noted in the porcine model.
Dr. Hresko reported serving as a consultant to Depuy Spine.
SNOWMASS, COLO. – A novel approach to repairing anterior cruciate ligament injuries – and perhaps thereby avoiding a downstream tidal wave of knee osteoarthritis – is creating major buzz in sports medicine circles.
“You’ll probably hear much more about this bioenhanced repair, with the expectation of achieving strength equal to that of ACL reconstruction and perhaps preventing the development of osteoarthritis 15 years down the road,” Dr. M. Timothy Hresko predicted at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
He cited research led by his colleague Dr. Martha M. Murray of Boston Children’s Hospital, which has resulted in development of a surgical technique combining a tissue-engineered composite scaffold with a suture repair of the torn ACL in what Dr. Murray has termed a bioenhanced repair.
Her work, to date preclinical, has garnered major awards from both the American Orthopaedic Society for Sports Medicine and the American Academy of Orthopaedic Surgeons. The Food and Drug Administration recently granted approval for the first clinical safety studies, to begin this year.
There is a major unmet need for better methods of repairing ACL injuries. They’re common, with an estimated 550,000 cases per year. The peak incidence occurs in 15- to 19-year-old female athletes. And the current gold standard therapy consisting of ACL reconstruction using an allograft or hamstring graft has a disturbingly high failure rate, both early and late. The graft failure rate is up to 20% in the first 2 years, climbing to 50% at 10 years.
“We just have to do better,” conceded Dr. Hresko, an orthopedic surgeon at Harvard Medical School, Boston, and Boston Children’s Hospital.
“One of the interesting and unfortunate facts,” he continued, “is that roughly 80% of people who have an ACL injury, with or without reconstruction, are still going to have osteoarthritis 14 years after the injury. So, if this is your 15-year-old daughter who plays basketball, she’ll only be 30 and will already have degenerative arthritis of the knee at what should still be a very active period of life.”
The bioenhanced repair now under study uses an extracellular matrix-based scaffold, which is loaded with a few milliliters of the patient’s own platelet-enriched plasma. The scaffold is applied between the torn ligament ends in order to stimulate collagen production and promote ligament healing. The suture repair of the ligament entails much less trauma than does standard reconstructive surgery.
In large-animal studies, the bioenhanced repair resulted in the same yield load, stiffness, and other desirable biomechanical properties at 1 year as with major reconstructive surgery. However, while premature posttraumatic osteoarthritis occurred in 80% of the knees treated with standard ACL reconstruction, there was no evidence of such damage 1 year following bioenhanced repair. Nor have adverse reactions to the scaffold been noted in the porcine model.
Dr. Hresko reported serving as a consultant to Depuy Spine.
SNOWMASS, COLO. – A novel approach to repairing anterior cruciate ligament injuries – and perhaps thereby avoiding a downstream tidal wave of knee osteoarthritis – is creating major buzz in sports medicine circles.
“You’ll probably hear much more about this bioenhanced repair, with the expectation of achieving strength equal to that of ACL reconstruction and perhaps preventing the development of osteoarthritis 15 years down the road,” Dr. M. Timothy Hresko predicted at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
He cited research led by his colleague Dr. Martha M. Murray of Boston Children’s Hospital, which has resulted in development of a surgical technique combining a tissue-engineered composite scaffold with a suture repair of the torn ACL in what Dr. Murray has termed a bioenhanced repair.
Her work, to date preclinical, has garnered major awards from both the American Orthopaedic Society for Sports Medicine and the American Academy of Orthopaedic Surgeons. The Food and Drug Administration recently granted approval for the first clinical safety studies, to begin this year.
There is a major unmet need for better methods of repairing ACL injuries. They’re common, with an estimated 550,000 cases per year. The peak incidence occurs in 15- to 19-year-old female athletes. And the current gold standard therapy consisting of ACL reconstruction using an allograft or hamstring graft has a disturbingly high failure rate, both early and late. The graft failure rate is up to 20% in the first 2 years, climbing to 50% at 10 years.
“We just have to do better,” conceded Dr. Hresko, an orthopedic surgeon at Harvard Medical School, Boston, and Boston Children’s Hospital.
“One of the interesting and unfortunate facts,” he continued, “is that roughly 80% of people who have an ACL injury, with or without reconstruction, are still going to have osteoarthritis 14 years after the injury. So, if this is your 15-year-old daughter who plays basketball, she’ll only be 30 and will already have degenerative arthritis of the knee at what should still be a very active period of life.”
The bioenhanced repair now under study uses an extracellular matrix-based scaffold, which is loaded with a few milliliters of the patient’s own platelet-enriched plasma. The scaffold is applied between the torn ligament ends in order to stimulate collagen production and promote ligament healing. The suture repair of the ligament entails much less trauma than does standard reconstructive surgery.
In large-animal studies, the bioenhanced repair resulted in the same yield load, stiffness, and other desirable biomechanical properties at 1 year as with major reconstructive surgery. However, while premature posttraumatic osteoarthritis occurred in 80% of the knees treated with standard ACL reconstruction, there was no evidence of such damage 1 year following bioenhanced repair. Nor have adverse reactions to the scaffold been noted in the porcine model.
Dr. Hresko reported serving as a consultant to Depuy Spine.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Risk Factors for Postoperative Complications in Trigger Finger Release
Stenosing tenosynovitis, or trigger finger, is a pathology commonly referred to the plastic and hand surgery service of the North Florida/South Georgia Veterans Health System (NFSGVHS). Patients usually present to their primary care provider with symptoms of the finger being temporarily locked or stuck in the flexed position. This can be a painful problem due to the size mismatch between the flexor tendon and the pulley under which it glides.
Patients are typically referred to surgery after failing ≥ 1 attempt at nonoperative management. The surgery is relatively quick and straightforward; however, postoperative complications can lead to an unexpected costly and lengthy recovery. The objective of this study was to identify potential risk factors that can predispose patients to postoperative complications so that those risk factors may be better anticipated and modified, if possible.
Methods
A retrospective chart review of trigger finger release surgery was performed on-site at the Malcom Randall VAMC in Gainesville, Florida, from January 2005 to December 2010 to identify risk factors associated with postoperative complications. The study was approved by both the NFSGVHS Internal Review Board and the University of Florida Institutional Review Board. Patients who underwent surgery exclusively for ≥ 1 trigger fingers by the plastic surgery service were included in the study.
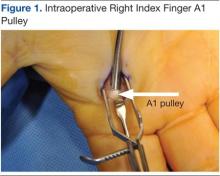
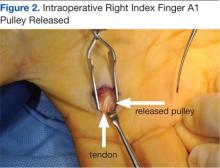
The surgery involves making an incision over the affected A1 pulley in the hand (Figure 1) and sharply releasing it (Figure 2) under direct vision. Potential risk factors for postoperative complications were recorded. These risk factors included smoking status, diabetic status, type of incision, and number of digits released during the surgical procedure.
Results
Ninety-eight digits (on 81 hands) were identified as meeting inclusion criteria. Surgeries were performed using a longitudinal (43), transverse (48), oblique (5), or Brunner (2) incision. There were 10 complications: cellulitis (3), pyogenic flexor tenosynovitis (3), scar adhesion (1), delayed healing (2), and incomplete release (1). The overall complication rate was 10.2%. The authors compared risk factors with complications, using the chi square test and a determining of P < .05.
Related: Making the Case for Minimally Invasive Surgery
There was no link found between overall postoperative complications and diabetic status, incision type, or smoking status. There was a statistically significant link between diabetic patients and the incidence of postoperative infection (P = .002) and between 2 digits operated on during the same surgery and postoperative infection (P = .027)
Discussion
The routine practice of the NFSGVHS hand clinic is to offer a steroid injection as the initial treatment for trigger finger. Health care providers (HCPs) allow no more than 3 injections to the same digit to avoid the rare but potentially serious complication of a tendon rupture.1 Due to the large NFSGVHS catchment area, wait time for elective trigger finger surgery is several months. This 3-injection plan has been well received by patients and referring providers due to these wait times. However, a recent article by Kerrigan and Stanwix concluded that the most cost-efficient treatment strategy is 2 steroid injections before surgery.2
More often than not, trigger finger release is a short, outpatient surgery with a quick recovery. To minimize the risk of stiffness and scar adhesions, the NFSGVHS practice is to refer all postoperative hand cases for ≥ 1 hand therapy appointment on the same day as their first postoperative visit.
Cost Estimates
When complications occur, they can be costly to patients due to both time spent away from home and work and additional expenses. When the current procedural terminology (CPT) codes are run through the VistA integrated billing system, based on the VHA Chief Business Office Reasonable Charges, a complication can more than double the charges associated with A1 pulley surgery.
A flexor sheath incision and drainage (I+D) (CPT 26020) charges $8,935.35 (facility charge, $6,911.95 plus professional fee, $2,023.40), compared with open trigger finger release (CPT 26055) at $8,365.66 (facility charge, $6,911.95 plus professional fee, $1,453.71). According to a conversation with the finance service officer at NFSGVHS (2/11/2014), the anesthesia bill ($490.56/15 min), anticipated level 3 emergency department visits (facility charge, $889.22 plus professional fee $493.40), and inpatient stays (daily floor bed $786.19) can make an infectious complication costly.
Trigger finger can also be released percutaneously. This is a reasonable option that avoids the operating room, but NFSGVHS surgeons prefer the open surgery due to concerns for tendon and nerve injury that can result from a blind sweep of the needle.3,4
Related: Prevention of Venous Thromboembolism After Total Joint Replacement
Existing studies found complications for trigger finger release ranging from 1% to 31%.5,6 Wound complications and joint stiffness are known complications.5-7 In this study, 60% of the complications were infections, and 80% of the complications were wound complications. Six of 8 patients with wound-healing complications received perioperative antibiotics. Three patients returned to the operating room for an I+D of the flexor sheath. The results showed a statistically significant link between > 1 digit treated at the same surgery and postoperative complications (P = .027). A PubMed search revealed no existing hand literature with this association.
Risk Factors
Diabetes, tobacco use, type of incision, and number of digits treated were assessed as risk factors for complications after trigger finger surgery. Nicotine is widely accepted as increasing the risk for wound complications.8 Almost 20% of the U.S. population smokes, compared with 22% of the VA population and 32% of active-duty military personnel.9 One in 4 veterans has been diagnosed with diabetes, a well-known predisposing factor in delayed wound healing and infection.10,11 No prior studies were found comparing type of incision or multiple digits treated as complications risk factors.
There is also a well-known association between trigger finger and diabetes. Chronic hyperglycemia results in the accumulation of collagen within tendon sheaths due to impairment of collagen breakdown. Patients with diabetes tend to present with multiple digit involvement and respond less favorably to steroid injections compared with patients without diabetes.12 Wound healing is also impaired in patients with diabetes. All 6 wound infections in this study were in patients with diabetes. Proposed etiologies for wound-healing complications include pathologic angiogenesis, impaired fibroblast proliferation and migration, impaired circulation, decreased oxygenation, and a defective immune response to the injured site.13
Trigger finger may develop in multiple digits. Once surgery has been planned for 1 digit, patients may request surgery on another digit on the same hand that has not had an attempt at nonoperative intervention. The NFSGVHS plastic surgeons have raised the threshold to offer multiple surgical procedures on the same hand at the same operative visit to minimize recovery time and number of visits, particularly when patients are travelling long distances. This may be less convenient; however, the overall cost to the patient and the health care system in the event of a complication is significant. Plastic surgery providers also run an alcohol prep pad over the incision site to prevent inoculation of the flexor sheath during suture removal.
Current recommendations to ameliorate the postoperative risks to the patient and costs to the system include endorsing a more conservative approach to treating trigger finger than was previously practiced at NFSGVHS. The known, less favorable response of patients with diabetes to steroid injections plus their elevated risk of postoperative infection create a catch-22 for the treatment plan. Given the low risk of a single steroid injection to the flexor sheath, this procedure is still recommended as a first-line treatment.
Related: Experience Tells in Hip Arthroplasty
During the 5-year study there was a lower threshold for surgical management and for treatment of multiple digits during the same surgery than the one currently practiced, with an overall consensus of the hospital’s HCPs. The authors recommend that all patients start with a steroid injection before committing to surgery. Patients with diabetes are informed that the injection will cause a temporary rise in their blood glucose.14 If they are resistant to the injection, high-dose oral nonsteroidal anti-inflammatory drugs and/or proximal interphalangeal joint splinting is ordered.
Verification of A1C values showing better chronic management of blood sugar is a procedure HCPs from the NFSGVHS will begin to follow. Preoperative A1C values between 6.5% and 8% in patients known to have diabetes has been recommended.15 A1C values > 7% have been found to be an independent risk factor for stenosing tenosynovitis.16 The total number of trigger finger surgeries may drop with the benefit of improved utilization of resources.
Conclusion
The authors found a statistically significant association between postoperative infection and 2 patient populations: patients with diabetes (P = .002) and patients having > 1 digit released during the same surgery (P = .027). This outcome suggests using caution when offering A1 pulley release in select patient populations.
Acknowledgement
Justine Pierson, BS, research coordinator at University of Florida, for statistical analysis. Funding is through salary.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Yamada K, Masuko, T, Iwasaki N. Rupture of the flexor digitorum profundus tendon after injections of insoluble steroid for a trigger finger. J Hand Surg Eur. 2011;36(1):77-78.
2. Kerrigan CL, Stanwix MG. Using evidence to minimize the cost of trigger finger care. J Hand Surg Am. 2009;34(6):997-1005.
3. Habbu R, Putnam MD, Adams JE. Percutaneous Release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
4. Guler F, Kose O, Ercan EC, Turan A, Canbora K. Open vs percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36(10):e1290-e1294.
5. Lim M-H, Lim K-K, Rasheed MZ, Narayana S, Tan B-H. Outcome of open trigger digit release. J Hand Surg Eur. 2007;32(4):457-479.
6. Will R, Lubahn J. Complications of open trigger finger release. J Hand Surg Am. 2010;35(4):594-596.
7. Lee WT, Chong AK. Outcome study of open trigger digit release. J Hand Surg Eur. 2011;36(4):339.
8. Rinker B. The evils of nicotine: An evidence-based guide to smoking and plastic surgery. Ann Plast Surg. 2013;70(5):599-605.
9. Bondurant S, Wedge R, eds. Combating Tobacco Use in Military and Veteran Populations. Washington, DC: The National Academies; 2009.
10. Shilling AM, Raphael J. Diabetes, hyperglycemia, and infections. Best Pract Res Clin Anaesthesiol. 2008;22(3):519-535.
11. Kuppersmith J, Francis J, Kerr E, et al. Advancing evidence-based care for diabetes: Lessons from the Veterans Health Administration. Health Aff. 2007;26(2):156-158.
12. Brown E, Genoway KA. Impact of diabetes on outcomes in hand surgery. J Hand Surg Am. 2011;36(12):2067-2072.
13. Francis-Goforth KN, Harken AH, Saba JD. Normalization of diabetic wound healing. Surgery. 2010;147(3):446-449.
14. Wang AA, Hutchinson DT. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. J Hand Surg Am. 2006;31(6):979-981.
15. Underwood P, Askari R, Hurwitz S, Chamarthi B, Garg R. Preoperative A1C and Clinical Outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014; 37(3): 611-616.
16. Vance MC, Tucker JJ, Harness NG. The association of hemoglobin A1c with the prevalence of stenosing tenosynovitis. J Hand Surg Am. 2012;37(9):1765-1769.
Stenosing tenosynovitis, or trigger finger, is a pathology commonly referred to the plastic and hand surgery service of the North Florida/South Georgia Veterans Health System (NFSGVHS). Patients usually present to their primary care provider with symptoms of the finger being temporarily locked or stuck in the flexed position. This can be a painful problem due to the size mismatch between the flexor tendon and the pulley under which it glides.
Patients are typically referred to surgery after failing ≥ 1 attempt at nonoperative management. The surgery is relatively quick and straightforward; however, postoperative complications can lead to an unexpected costly and lengthy recovery. The objective of this study was to identify potential risk factors that can predispose patients to postoperative complications so that those risk factors may be better anticipated and modified, if possible.
Methods
A retrospective chart review of trigger finger release surgery was performed on-site at the Malcom Randall VAMC in Gainesville, Florida, from January 2005 to December 2010 to identify risk factors associated with postoperative complications. The study was approved by both the NFSGVHS Internal Review Board and the University of Florida Institutional Review Board. Patients who underwent surgery exclusively for ≥ 1 trigger fingers by the plastic surgery service were included in the study.
The surgery involves making an incision over the affected A1 pulley in the hand (Figure 1) and sharply releasing it (Figure 2) under direct vision. Potential risk factors for postoperative complications were recorded. These risk factors included smoking status, diabetic status, type of incision, and number of digits released during the surgical procedure.
Results
Ninety-eight digits (on 81 hands) were identified as meeting inclusion criteria. Surgeries were performed using a longitudinal (43), transverse (48), oblique (5), or Brunner (2) incision. There were 10 complications: cellulitis (3), pyogenic flexor tenosynovitis (3), scar adhesion (1), delayed healing (2), and incomplete release (1). The overall complication rate was 10.2%. The authors compared risk factors with complications, using the chi square test and a determining of P < .05.
Related: Making the Case for Minimally Invasive Surgery
There was no link found between overall postoperative complications and diabetic status, incision type, or smoking status. There was a statistically significant link between diabetic patients and the incidence of postoperative infection (P = .002) and between 2 digits operated on during the same surgery and postoperative infection (P = .027)
Discussion
The routine practice of the NFSGVHS hand clinic is to offer a steroid injection as the initial treatment for trigger finger. Health care providers (HCPs) allow no more than 3 injections to the same digit to avoid the rare but potentially serious complication of a tendon rupture.1 Due to the large NFSGVHS catchment area, wait time for elective trigger finger surgery is several months. This 3-injection plan has been well received by patients and referring providers due to these wait times. However, a recent article by Kerrigan and Stanwix concluded that the most cost-efficient treatment strategy is 2 steroid injections before surgery.2
More often than not, trigger finger release is a short, outpatient surgery with a quick recovery. To minimize the risk of stiffness and scar adhesions, the NFSGVHS practice is to refer all postoperative hand cases for ≥ 1 hand therapy appointment on the same day as their first postoperative visit.
Cost Estimates
When complications occur, they can be costly to patients due to both time spent away from home and work and additional expenses. When the current procedural terminology (CPT) codes are run through the VistA integrated billing system, based on the VHA Chief Business Office Reasonable Charges, a complication can more than double the charges associated with A1 pulley surgery.
A flexor sheath incision and drainage (I+D) (CPT 26020) charges $8,935.35 (facility charge, $6,911.95 plus professional fee, $2,023.40), compared with open trigger finger release (CPT 26055) at $8,365.66 (facility charge, $6,911.95 plus professional fee, $1,453.71). According to a conversation with the finance service officer at NFSGVHS (2/11/2014), the anesthesia bill ($490.56/15 min), anticipated level 3 emergency department visits (facility charge, $889.22 plus professional fee $493.40), and inpatient stays (daily floor bed $786.19) can make an infectious complication costly.
Trigger finger can also be released percutaneously. This is a reasonable option that avoids the operating room, but NFSGVHS surgeons prefer the open surgery due to concerns for tendon and nerve injury that can result from a blind sweep of the needle.3,4
Related: Prevention of Venous Thromboembolism After Total Joint Replacement
Existing studies found complications for trigger finger release ranging from 1% to 31%.5,6 Wound complications and joint stiffness are known complications.5-7 In this study, 60% of the complications were infections, and 80% of the complications were wound complications. Six of 8 patients with wound-healing complications received perioperative antibiotics. Three patients returned to the operating room for an I+D of the flexor sheath. The results showed a statistically significant link between > 1 digit treated at the same surgery and postoperative complications (P = .027). A PubMed search revealed no existing hand literature with this association.
Risk Factors
Diabetes, tobacco use, type of incision, and number of digits treated were assessed as risk factors for complications after trigger finger surgery. Nicotine is widely accepted as increasing the risk for wound complications.8 Almost 20% of the U.S. population smokes, compared with 22% of the VA population and 32% of active-duty military personnel.9 One in 4 veterans has been diagnosed with diabetes, a well-known predisposing factor in delayed wound healing and infection.10,11 No prior studies were found comparing type of incision or multiple digits treated as complications risk factors.
There is also a well-known association between trigger finger and diabetes. Chronic hyperglycemia results in the accumulation of collagen within tendon sheaths due to impairment of collagen breakdown. Patients with diabetes tend to present with multiple digit involvement and respond less favorably to steroid injections compared with patients without diabetes.12 Wound healing is also impaired in patients with diabetes. All 6 wound infections in this study were in patients with diabetes. Proposed etiologies for wound-healing complications include pathologic angiogenesis, impaired fibroblast proliferation and migration, impaired circulation, decreased oxygenation, and a defective immune response to the injured site.13
Trigger finger may develop in multiple digits. Once surgery has been planned for 1 digit, patients may request surgery on another digit on the same hand that has not had an attempt at nonoperative intervention. The NFSGVHS plastic surgeons have raised the threshold to offer multiple surgical procedures on the same hand at the same operative visit to minimize recovery time and number of visits, particularly when patients are travelling long distances. This may be less convenient; however, the overall cost to the patient and the health care system in the event of a complication is significant. Plastic surgery providers also run an alcohol prep pad over the incision site to prevent inoculation of the flexor sheath during suture removal.
Current recommendations to ameliorate the postoperative risks to the patient and costs to the system include endorsing a more conservative approach to treating trigger finger than was previously practiced at NFSGVHS. The known, less favorable response of patients with diabetes to steroid injections plus their elevated risk of postoperative infection create a catch-22 for the treatment plan. Given the low risk of a single steroid injection to the flexor sheath, this procedure is still recommended as a first-line treatment.
Related: Experience Tells in Hip Arthroplasty
During the 5-year study there was a lower threshold for surgical management and for treatment of multiple digits during the same surgery than the one currently practiced, with an overall consensus of the hospital’s HCPs. The authors recommend that all patients start with a steroid injection before committing to surgery. Patients with diabetes are informed that the injection will cause a temporary rise in their blood glucose.14 If they are resistant to the injection, high-dose oral nonsteroidal anti-inflammatory drugs and/or proximal interphalangeal joint splinting is ordered.
Verification of A1C values showing better chronic management of blood sugar is a procedure HCPs from the NFSGVHS will begin to follow. Preoperative A1C values between 6.5% and 8% in patients known to have diabetes has been recommended.15 A1C values > 7% have been found to be an independent risk factor for stenosing tenosynovitis.16 The total number of trigger finger surgeries may drop with the benefit of improved utilization of resources.
Conclusion
The authors found a statistically significant association between postoperative infection and 2 patient populations: patients with diabetes (P = .002) and patients having > 1 digit released during the same surgery (P = .027). This outcome suggests using caution when offering A1 pulley release in select patient populations.
Acknowledgement
Justine Pierson, BS, research coordinator at University of Florida, for statistical analysis. Funding is through salary.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Stenosing tenosynovitis, or trigger finger, is a pathology commonly referred to the plastic and hand surgery service of the North Florida/South Georgia Veterans Health System (NFSGVHS). Patients usually present to their primary care provider with symptoms of the finger being temporarily locked or stuck in the flexed position. This can be a painful problem due to the size mismatch between the flexor tendon and the pulley under which it glides.
Patients are typically referred to surgery after failing ≥ 1 attempt at nonoperative management. The surgery is relatively quick and straightforward; however, postoperative complications can lead to an unexpected costly and lengthy recovery. The objective of this study was to identify potential risk factors that can predispose patients to postoperative complications so that those risk factors may be better anticipated and modified, if possible.
Methods
A retrospective chart review of trigger finger release surgery was performed on-site at the Malcom Randall VAMC in Gainesville, Florida, from January 2005 to December 2010 to identify risk factors associated with postoperative complications. The study was approved by both the NFSGVHS Internal Review Board and the University of Florida Institutional Review Board. Patients who underwent surgery exclusively for ≥ 1 trigger fingers by the plastic surgery service were included in the study.
The surgery involves making an incision over the affected A1 pulley in the hand (Figure 1) and sharply releasing it (Figure 2) under direct vision. Potential risk factors for postoperative complications were recorded. These risk factors included smoking status, diabetic status, type of incision, and number of digits released during the surgical procedure.
Results
Ninety-eight digits (on 81 hands) were identified as meeting inclusion criteria. Surgeries were performed using a longitudinal (43), transverse (48), oblique (5), or Brunner (2) incision. There were 10 complications: cellulitis (3), pyogenic flexor tenosynovitis (3), scar adhesion (1), delayed healing (2), and incomplete release (1). The overall complication rate was 10.2%. The authors compared risk factors with complications, using the chi square test and a determining of P < .05.
Related: Making the Case for Minimally Invasive Surgery
There was no link found between overall postoperative complications and diabetic status, incision type, or smoking status. There was a statistically significant link between diabetic patients and the incidence of postoperative infection (P = .002) and between 2 digits operated on during the same surgery and postoperative infection (P = .027)
Discussion
The routine practice of the NFSGVHS hand clinic is to offer a steroid injection as the initial treatment for trigger finger. Health care providers (HCPs) allow no more than 3 injections to the same digit to avoid the rare but potentially serious complication of a tendon rupture.1 Due to the large NFSGVHS catchment area, wait time for elective trigger finger surgery is several months. This 3-injection plan has been well received by patients and referring providers due to these wait times. However, a recent article by Kerrigan and Stanwix concluded that the most cost-efficient treatment strategy is 2 steroid injections before surgery.2
More often than not, trigger finger release is a short, outpatient surgery with a quick recovery. To minimize the risk of stiffness and scar adhesions, the NFSGVHS practice is to refer all postoperative hand cases for ≥ 1 hand therapy appointment on the same day as their first postoperative visit.
Cost Estimates
When complications occur, they can be costly to patients due to both time spent away from home and work and additional expenses. When the current procedural terminology (CPT) codes are run through the VistA integrated billing system, based on the VHA Chief Business Office Reasonable Charges, a complication can more than double the charges associated with A1 pulley surgery.
A flexor sheath incision and drainage (I+D) (CPT 26020) charges $8,935.35 (facility charge, $6,911.95 plus professional fee, $2,023.40), compared with open trigger finger release (CPT 26055) at $8,365.66 (facility charge, $6,911.95 plus professional fee, $1,453.71). According to a conversation with the finance service officer at NFSGVHS (2/11/2014), the anesthesia bill ($490.56/15 min), anticipated level 3 emergency department visits (facility charge, $889.22 plus professional fee $493.40), and inpatient stays (daily floor bed $786.19) can make an infectious complication costly.
Trigger finger can also be released percutaneously. This is a reasonable option that avoids the operating room, but NFSGVHS surgeons prefer the open surgery due to concerns for tendon and nerve injury that can result from a blind sweep of the needle.3,4
Related: Prevention of Venous Thromboembolism After Total Joint Replacement
Existing studies found complications for trigger finger release ranging from 1% to 31%.5,6 Wound complications and joint stiffness are known complications.5-7 In this study, 60% of the complications were infections, and 80% of the complications were wound complications. Six of 8 patients with wound-healing complications received perioperative antibiotics. Three patients returned to the operating room for an I+D of the flexor sheath. The results showed a statistically significant link between > 1 digit treated at the same surgery and postoperative complications (P = .027). A PubMed search revealed no existing hand literature with this association.
Risk Factors
Diabetes, tobacco use, type of incision, and number of digits treated were assessed as risk factors for complications after trigger finger surgery. Nicotine is widely accepted as increasing the risk for wound complications.8 Almost 20% of the U.S. population smokes, compared with 22% of the VA population and 32% of active-duty military personnel.9 One in 4 veterans has been diagnosed with diabetes, a well-known predisposing factor in delayed wound healing and infection.10,11 No prior studies were found comparing type of incision or multiple digits treated as complications risk factors.
There is also a well-known association between trigger finger and diabetes. Chronic hyperglycemia results in the accumulation of collagen within tendon sheaths due to impairment of collagen breakdown. Patients with diabetes tend to present with multiple digit involvement and respond less favorably to steroid injections compared with patients without diabetes.12 Wound healing is also impaired in patients with diabetes. All 6 wound infections in this study were in patients with diabetes. Proposed etiologies for wound-healing complications include pathologic angiogenesis, impaired fibroblast proliferation and migration, impaired circulation, decreased oxygenation, and a defective immune response to the injured site.13
Trigger finger may develop in multiple digits. Once surgery has been planned for 1 digit, patients may request surgery on another digit on the same hand that has not had an attempt at nonoperative intervention. The NFSGVHS plastic surgeons have raised the threshold to offer multiple surgical procedures on the same hand at the same operative visit to minimize recovery time and number of visits, particularly when patients are travelling long distances. This may be less convenient; however, the overall cost to the patient and the health care system in the event of a complication is significant. Plastic surgery providers also run an alcohol prep pad over the incision site to prevent inoculation of the flexor sheath during suture removal.
Current recommendations to ameliorate the postoperative risks to the patient and costs to the system include endorsing a more conservative approach to treating trigger finger than was previously practiced at NFSGVHS. The known, less favorable response of patients with diabetes to steroid injections plus their elevated risk of postoperative infection create a catch-22 for the treatment plan. Given the low risk of a single steroid injection to the flexor sheath, this procedure is still recommended as a first-line treatment.
Related: Experience Tells in Hip Arthroplasty
During the 5-year study there was a lower threshold for surgical management and for treatment of multiple digits during the same surgery than the one currently practiced, with an overall consensus of the hospital’s HCPs. The authors recommend that all patients start with a steroid injection before committing to surgery. Patients with diabetes are informed that the injection will cause a temporary rise in their blood glucose.14 If they are resistant to the injection, high-dose oral nonsteroidal anti-inflammatory drugs and/or proximal interphalangeal joint splinting is ordered.
Verification of A1C values showing better chronic management of blood sugar is a procedure HCPs from the NFSGVHS will begin to follow. Preoperative A1C values between 6.5% and 8% in patients known to have diabetes has been recommended.15 A1C values > 7% have been found to be an independent risk factor for stenosing tenosynovitis.16 The total number of trigger finger surgeries may drop with the benefit of improved utilization of resources.
Conclusion
The authors found a statistically significant association between postoperative infection and 2 patient populations: patients with diabetes (P = .002) and patients having > 1 digit released during the same surgery (P = .027). This outcome suggests using caution when offering A1 pulley release in select patient populations.
Acknowledgement
Justine Pierson, BS, research coordinator at University of Florida, for statistical analysis. Funding is through salary.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Yamada K, Masuko, T, Iwasaki N. Rupture of the flexor digitorum profundus tendon after injections of insoluble steroid for a trigger finger. J Hand Surg Eur. 2011;36(1):77-78.
2. Kerrigan CL, Stanwix MG. Using evidence to minimize the cost of trigger finger care. J Hand Surg Am. 2009;34(6):997-1005.
3. Habbu R, Putnam MD, Adams JE. Percutaneous Release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
4. Guler F, Kose O, Ercan EC, Turan A, Canbora K. Open vs percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36(10):e1290-e1294.
5. Lim M-H, Lim K-K, Rasheed MZ, Narayana S, Tan B-H. Outcome of open trigger digit release. J Hand Surg Eur. 2007;32(4):457-479.
6. Will R, Lubahn J. Complications of open trigger finger release. J Hand Surg Am. 2010;35(4):594-596.
7. Lee WT, Chong AK. Outcome study of open trigger digit release. J Hand Surg Eur. 2011;36(4):339.
8. Rinker B. The evils of nicotine: An evidence-based guide to smoking and plastic surgery. Ann Plast Surg. 2013;70(5):599-605.
9. Bondurant S, Wedge R, eds. Combating Tobacco Use in Military and Veteran Populations. Washington, DC: The National Academies; 2009.
10. Shilling AM, Raphael J. Diabetes, hyperglycemia, and infections. Best Pract Res Clin Anaesthesiol. 2008;22(3):519-535.
11. Kuppersmith J, Francis J, Kerr E, et al. Advancing evidence-based care for diabetes: Lessons from the Veterans Health Administration. Health Aff. 2007;26(2):156-158.
12. Brown E, Genoway KA. Impact of diabetes on outcomes in hand surgery. J Hand Surg Am. 2011;36(12):2067-2072.
13. Francis-Goforth KN, Harken AH, Saba JD. Normalization of diabetic wound healing. Surgery. 2010;147(3):446-449.
14. Wang AA, Hutchinson DT. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. J Hand Surg Am. 2006;31(6):979-981.
15. Underwood P, Askari R, Hurwitz S, Chamarthi B, Garg R. Preoperative A1C and Clinical Outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014; 37(3): 611-616.
16. Vance MC, Tucker JJ, Harness NG. The association of hemoglobin A1c with the prevalence of stenosing tenosynovitis. J Hand Surg Am. 2012;37(9):1765-1769.
1. Yamada K, Masuko, T, Iwasaki N. Rupture of the flexor digitorum profundus tendon after injections of insoluble steroid for a trigger finger. J Hand Surg Eur. 2011;36(1):77-78.
2. Kerrigan CL, Stanwix MG. Using evidence to minimize the cost of trigger finger care. J Hand Surg Am. 2009;34(6):997-1005.
3. Habbu R, Putnam MD, Adams JE. Percutaneous Release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
4. Guler F, Kose O, Ercan EC, Turan A, Canbora K. Open vs percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36(10):e1290-e1294.
5. Lim M-H, Lim K-K, Rasheed MZ, Narayana S, Tan B-H. Outcome of open trigger digit release. J Hand Surg Eur. 2007;32(4):457-479.
6. Will R, Lubahn J. Complications of open trigger finger release. J Hand Surg Am. 2010;35(4):594-596.
7. Lee WT, Chong AK. Outcome study of open trigger digit release. J Hand Surg Eur. 2011;36(4):339.
8. Rinker B. The evils of nicotine: An evidence-based guide to smoking and plastic surgery. Ann Plast Surg. 2013;70(5):599-605.
9. Bondurant S, Wedge R, eds. Combating Tobacco Use in Military and Veteran Populations. Washington, DC: The National Academies; 2009.
10. Shilling AM, Raphael J. Diabetes, hyperglycemia, and infections. Best Pract Res Clin Anaesthesiol. 2008;22(3):519-535.
11. Kuppersmith J, Francis J, Kerr E, et al. Advancing evidence-based care for diabetes: Lessons from the Veterans Health Administration. Health Aff. 2007;26(2):156-158.
12. Brown E, Genoway KA. Impact of diabetes on outcomes in hand surgery. J Hand Surg Am. 2011;36(12):2067-2072.
13. Francis-Goforth KN, Harken AH, Saba JD. Normalization of diabetic wound healing. Surgery. 2010;147(3):446-449.
14. Wang AA, Hutchinson DT. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. J Hand Surg Am. 2006;31(6):979-981.
15. Underwood P, Askari R, Hurwitz S, Chamarthi B, Garg R. Preoperative A1C and Clinical Outcomes in patients with diabetes undergoing major noncardiac surgical procedures. Diabetes Care. 2014; 37(3): 611-616.
16. Vance MC, Tucker JJ, Harness NG. The association of hemoglobin A1c with the prevalence of stenosing tenosynovitis. J Hand Surg Am. 2012;37(9):1765-1769.
Arthritis, Infectious Tenosynovitis, and Tendon Rupture in a Patient With Rheumatoid Arthritis and Psoriasis
Compared with monoarticular arthritis, polyarticular arthritis may yield an initially narrower differential diagnosis that focuses on systemic inflammatory conditions, such as rheumatoid arthritis (RA). Approximately 15% to 30% of septic arthritis is polyarticular, of which about 45% is associated with underlying RA.1,2 Regardless of the number of joints involved, septic (infectious) arthritis is a valid consideration given the morbidity and mortality.
In a retrospective study in the United Kingdom (UK) between 1982 and 1991, the morbidity and mortality of septic arthritis was 31.6% and 11.5%, respectively, and 16% of the study population had RA.3 A review of the literature by Dubost and colleagues found that polyarticular septic arthritis (PASA) has a mortality of 31% to 42% compared with 4% to 8% for monoarticular septic arthritis, and RA was present in 67% of the PASA fatalities.1
Related: The Golden Era of Treatment in Rheumatology
Rheumatoid arthritis and its treatment predispose patients to septic arthritis. Septic arthritis in the UK general population is 0.42 per 100 patient-years for patients with RA on antitumor necrosis factor therapy.3,4 In a retrospective study in the U.S., the incidence of septic arthritis was 0.40 per 100 patient-years for patients with RA compared with 0.02 per 100 patient-years for patients without RA.5
Other complications of RA include infectious tenosynovitis and tendon rupture. The incidence and prevalence of infectious tenosynovitis and tendon rupture in RA are not firmly established in the literature.
We present a patient with RA and psoriasis who responded initially to acute management for RA but subsequently was diagnosed with culture-negative polyarticular arthritis and infectious tenosynovitis associated with beta hemolytic group G Streptococcus (GGS), a part of Streptococcus milleri (S. milleri). During surgery, he was also found to have bilateral extensor pollicus longus (EPL) tendon rupture. Given the possible morbidity, the authors believe this patient may be of interest to the medical community.
Case Presentation
A 69-year-old African American male presented with 3 to 4 days of swelling and pain of bilateral wrists, bilateral hands, and the left ankle with subjective, but resolved, fevers and chills. His medical history was significant for seropositive erosive RA, psoriasis, hypertension, hyperlipidemia, alcohol abuse, chronic tobacco use, osteoporosis, and glaucoma. He did not have diabetes, reported no IV drug abuse, and except for the immunosuppressive effects of his medications, was not otherwise immunocompromised.
For 2 years in the outpatient setting, the rheumatology clinic had been managing the patient’s rheumatoid factor (RF) positive and anti-cyclic citrullinated peptide (CCP) antibody positive erosive RA with etanercept 25 mg subcutaneously twice a week. The RA affected his hands, wrists, shoulders, and ankles bilaterally but was successfully controlled. The dermatology clinic was managing the patient’s psoriasis with calcipotriene cream 0.005% twice a week and clobetasol ointment 0.05% twice a week. Psoriatic plaques were noted on bilateral elbows, bilateral dorsal hands, and bilateral dorsal feet.
Initial Evaluation
At evaluation, the patient’s vital signs revealed a temperature of 36.3°C (97.3°F), pulse of 102 beats per minute, respiratory rate of 16 breaths per minute, oxygen saturation of 99% on room air, and blood pressure of 102/70 mm Hg. He was found to have edema, tenderness, and erythema of the wrists bilaterally and left metacarpophalangeal joints (MCPs) and edematous right MCPs and left medial ankle.
The patient had been nonadherent with etanercept for 5 monthsand restarted taking the medication only 2 weeks before presentation. He had noticed worsening arthritis for at least 1 month. His last RA flare was approximately 1 year before presentation. Additional symptoms included 4 days of nausea, nonbloody and nonbilious emesis, left lower quadrant pain, and diarrhea without melena or hematochezia.
Initial laboratory studies found 3.2 k/μL white blood cells (WBCs) with a differential of 11.9% lymphocytes, 4.2% monocytes, 83.3% neutrophils, 0.5% eosinophils, and 0.1% basophils; 165 k/μL platelets; 96 mm/h erythrocyte sedimentation rate (ESR); and 45 mg/dL C-reactive protein. The patient was diagnosed with viral gastroenteritis and RA flare and was admitted for inpatient management secondary to limited ability to care for himself.
Related: Infliximab-Induced Complications
The patient was started on prednisone 40 mg orally once a day (for 5 days) for empiric treatment of an RA flare and continued on etanercept. The inpatient rheumatology service was consulted. Further evaluation later that day found involvement of the proximal interphalangeal joints and elbows and tenderness of the tendons of the dorsal hand bilaterally. Over the next 2 days, the patient remained afebrile and WBCs were within normal limits. Edema, erythema, and tenderness of the involved joints somewhat improved, but tenderness along the tendons of the dorsal hand worsened, which concerned the managing teams for infectious tenosynovitis.
By day 4, the patient was afebrile and had a leukocytosis of 12.9 k/μLwith neutrophils 86.7%, but improvement of erythema, pain, and range of motion of involved joints and no tenderness to palpation of tendons was noted. The inpatient orthopedic surgery service evaluated the patient and did not find sufficient evidence necessitating surgical intervention.
Worsening Condition
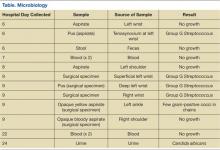
On day 6, arthrocentesis of the left wrist was performed secondary to worsening of erythema and edema. The patient experienced new edema of the left shoulder and leukocytosis continued to trend upward (15.7 k/μL on day 6). Purulent aspirate (1.5 mL) was obtained from the fluctuance and tenosynovium of the left wrist. Empiric vancomycin 1 g IV twice daily and ceftriaxone 2 g IV daily were started and continued for 3 days. By this point in his hospital course, the patient had received 1 dose of etanercept. Prednisone and etanercept were previously discontinued because of the discovered infection. Blood cultures were drawn and had no growth (Table). Gastroenterology studies were limited to stool cultures and did not include colonoscopy. Leukocytosis began trending down.
On day 8, antibiotics were tailored to penicillin G 4 million units IV every 4 hours following growth of GGS from the sample of the left wrist. Subsequently, synovial fluid (3 mL) from the left shoulder was obtained following initiation of antibiotic therapy and had no growth. Magnetic resonance imaging (MRI) found tenosynovitis of the left ankle and right wrist.
On day 9, transthoracic echocardiography was performed and found no evidence of infectious endocarditis. Later that night, the patient was taken to surgery for incision and drainage/debridement of bilateral wrists and left ankle, synovectomy of right wrist, and aspiration of right shoulder. Findings included abscess in the left wrist and inflammatory synovitis and bilateral EPL tendon rupture consistent with RA. Pus from the left ankle had few gram-positive cocci in chains with no growth, and the specimens from both wrists grew GGS. Aspirate from the left ankle was an opaque yellow fluid with 14,900/mm3 WBC, 30,000/mm3 red blood cells (RBC), 97% neutrophils, 1% macrophages, 2% lymphocytes, and 0% monocytes. Aspirate from the right shoulder was an opaque bloody fluid with 10,100/mm3 WBC, 40,000/mm3 RBC, 95% neutrophils, 2% macrophages, 1% lymphocytes, and 1% monocytes. On day 10, sulfasalazine 500 mg twice a day was initiated for RA.
Following surgery and continued antibiotics, the patient’s leukocytosis resolved, and improvement was seen in all joints with decreased edema, erythema, and pain and increased range of motion. Postoperative recovery was complicated by ileus, urinary retention, and fungal (Candida albicans) urinary tract infection, all of which resolved without significant complications. The inpatient rheumatology service restarted prednisone at a lower dose of 20 mg. The patient became afebrile and sufficiently stable for transfer to a lower level of care with continued physical therapy and IV antibiotics for another 3 weeks.
Discussion
The patient had 2 underlying systemic inflammatory conditions: RA and psoriasis. The underlying chronic arthritis was likely caused by RA, not psoriatic arthritis (PsA). The patient met the 2010 American College of Rheumatology criteria but failed to meet the classification criteria for PsA.6,7 However, the clinical features of RA and PsA overlap. Rheumatoid factor and CCP can be positive laboratory findings in both RA and PsA.8-14 Tenosynovitis is found in about half of RA patients and PsA patients (P > .05).15 In its evaluation of the patient, the inpatient rheumatology service suspected that the patient may have had RA with components of PsA.
Rheumatoid arthritis complicates the diagnosis of septic arthritis. In a study by Nolla and colleagues, a mean of 7.3 days (range 3 to 18 days) elapsed before a diagnosis of septic arthritis was made in 10 patients with RA on corticosteroids.2 Consideration of risk factors such as increasing age, male sex, tobacco use, extra-articular manifestations of RA, positive RF, rheumatoid nodules, poor functional capacity, high ESR, leukopenia, comorbidities (chronic lung disease, alcoholism, organic brain disease, and diabetes), and the use of corticosteroids may expedite the diagnosis of infections in patients with RA.16 In this case, the patient had some of these risk factors: age, male sex, alcoholism, chronic tobacco use, positive RF, high ESR, and leukopenia (at presentation).
Related: Trend Toward Concomitant Supplements and Medications
The history of medication nonadherence of etanercept with progressively worsening arthritis and early clinical improvement (reduction in erythema, edema, and pain and temporary loss of signs of tenosynovitis on examination) while on prednisone suggested that the patient had a RA flare. The prednisone likely alleviated the inflammatory process but created an immunosuppressed state that allowed GGS to invade and possibly disseminate. Alternately, the patient may have been infected before presentation. The lack of a definitive time line for his case prevented the authors from forming conclusions about a possible causal relationship between the infection and medications. The subjective fevers before admission were nonspecific and could have been caused by RA, presumed gastroenteritis, or other undiagnosed infectious processes. The observed leukocytosis may have been initially corticosteroid-induced.17
Septic Arthritis
The suspicion of septic arthritis and infectious tenosynovitis substantially increased on day 6 with worsening symptoms, involvement of additional joints, and spiking fevers. Group G Streptococcus was obtained from the aspirate of the left wrist and from the surgical specimens from the bilateral wrists. The clinical presentation, MRI imaging studies, and surgical and nonsurgical specimens supported a diagnosis of GGS tenosynovitis. However, there was no clear evidence (ie, positive culture with identified organism) of septic arthritis, likely secondary to early septic arthritis and initiation of antibiotics before joint aspirations. The aspirate from the left ankle was yellow and opaque, but the culture was negative.
The pathogenic organism in the patient was GGS. Group G Streptococcus is normal flora of the oral cavity, gastrointestinal (GI) tract, upper respiratory tract, genital tract, and skin, which were all possible sources of seeding.18 Streptococcal species account for about 20% of septic arthritis, and GGS arthritis accounts for 4% to 19% of streptococcal arthritis.19-22 From a review of the literature, 2 cases of GGS tenosynovitis have been published.23,24 However, in an ultrasound study and MRI study, 49% and 43%, respectively, of patients with RA had tenosynovitis of the tendons of the hands.15,25
GGS Demographics
About three-quarters (71%) of patients with GGS arthritis are male.19 The analysis of the literature by Bronze and colleagues found that chronic joint disease and alcoholism are present in 34% and 14% of patients with GGS arthritis, respectively. One-quarter (23% from Dubost and colleagues) to one-third (32% from Schattner and colleagues) of patients with GGS arthritis have RA.19,26
Fever is present in less than half (43%) of patients with GGS arthritis.19 Positive synovial fluid is expected in 90% of patients.19 Leukocytosis and elevated ESR need not be present.27,28 The arthritis is polyarticular in one-quarter of patients (24% from Bronze and colleagues and 26% from Dubost and colleagues).19,26
Positive blood cultures can be expected in one-fourth (26%) of patients with GGS arthritis.19 The patient’s blood cultures were negative. Blood cultures drawn before initiation of antibiotics yielded no growth, so if the spread was hematogenous, the bacteremia was transient or intermittent. Before and after initiation of antibiotics, specimens from the shoulders did not grow colonies, whereas specimens from the wrists did. If the shoulders were truly infected, these findings and the notably later involvement of the shoulders suggest that the shoulders may have been seeded later in the hospital course.
Trenkner and colleagues proposed that GI abnormalities provide a portal of entry for GGS, which is under the umbrella of S. milleri.29S. milleri is associated with abscess formation, usually of the GI tract.30-32 In the study patient, the possible gastroenteritis may have provided such a portal of entry and subsequent seeding to the joints, and an abscess was found in the left wrist.
Tendon Rupture
Additionally, bilateral EPL tendon rupture likely occurred as a consequence of the inflammatory process from RA and infectious tenosynovitis in the patient. According to Zheng and colleagues, tenosynovitis is an inflammatory process of the synovial tendon sheath that may result in degeneration and rupture of the tendons and may contribute to bone erosions, development of joint deformities, and loss of functional capacity.33 In a histologic study of a ruptured EPL tendon from a patient with RA, Harris observed a chronic inflammatory cellular reaction.34 Harris also described a male with RA with unconfirmed bilateral EPL rupture.34 Björkman and colleague identified previous injury, RA, and local or systemic steroids as important etiologic factors for EPL tendon rupture.35
As in the case of this patient, the utilization of both medical and surgical therapy is not uncommon for treating GGS infection. Antibiotic therapy typically consists of penicillin (74%).26 Surgical intervention is necessary in 16% to 37% of patients.19,26 This patient required both penicillin and incision and drainage/debridement before significant clinical improvement was noted. Prognosis of GGS arthritis is favorable with 5% mortality.26
Conclusion
Septic arthritis and infectious tenosynovitis are readily treatable with low mortality if promptly identified. Identification can be masked by other medical conditions, such as RA and psoriasis, and their associated immunosuppressive treatment. Bilateral EPL tendon rupture may be a complication of RA, particularly with an underlying septic arthritis and infectious tenosynovitis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dubost JJ, Fis I, Denis P, et al. Polyarticular septic arthritis. Medicine (Baltimore). 1993;72(5):296-310.
2. Nolla JM, Gómez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: A detailed analysis of 10 cases and literature review. Semin Arthritis Rheum. 2000;30(2):121-126.
3.Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
4. Galloway JB, Hyrich KL, Mercer LK, et al; BSR Biologics Register. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: Results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(10):1810-1814.
5. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002;46(9):2287-2293.
6. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
7. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665-2673.
8. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)—An analysis of 220 patients. Q J Med. 1987;62(238):127-141.
9. Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol. 2005;32(3):511-515.
10. Vander Cruyssen B, Hoffman IE, Zmierczak H, et al. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis. 2005;64(8):1145-1149.
11. Alenius GM, Berglin E, Rantapää Dahlgvist S. Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation. Ann Rheum Dis. 2006;65(3):398-400.
12. Candia L, Marquez J, Gonzalez C, et al. Low frequency of anticyclic citrullinated peptide antibodies in psoriatic arthritis but not in cutaneous psoriasis. J Clin Rheumatol. 2006;12(5):226-229.
13. Inanc N, Dalkilic E, Kamali S, et al. Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol. 2007;26(1):17-23.
14. Popescu C, Zofota S, Bojinca V, Ionescu R. Anti-cyclic citrullinated peptide antibodies in psoriatic arthritis—Cross-sectional study and literature review. J Med Life. 2013;6(4):376-382.
15. Schoellnast H, Deutschmann HA, Hermann J, et al. Psoriatic arthritis and rheumatoid arthritis: Findings in contrast-enhanced MRI. AJR Am J Roentgenol. 2006;187(2):351-357.
16. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2294-2300.
17. Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71(5):773-778.
18. Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10(2):257-285.
19. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Sauvezie B. Streptococcal septic arthritis in adults. A study of 55 cases with a literature review. Joint Bone Spine. 2004;71(4):303-311.
20. Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: Analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36(3):370-373.
21. Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117(3):423-428.
22. Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: A community based prospective survey. Ann Rheum Dis. 1997;56(8):470-475.
23. Bradlow A, Mitchell RG, Mowat AG. Group G streptococcal arthritis. Rheumatol Rehabil. 1982;21(4):206-210.
24. Meier JL, Gerster JC. Bursitis and tenosynovitis caused by group G streptococci. J Rheumatol. 1983;10(5):817-818.
25. Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. Hand tendon involvement in rheumatoid arthritis: An ultrasound study. Semin Arthritis Rheum. 2012;41(6):752-760.
26. Bronze MS, Whitby S, Schaberg DR. Group G streptococcal arthritis: Case report and review of the literature. Am J Med Sci. 1997;313(4):239-243.
27. Schattner A, Vosti KL. Bacterial arthritis due to beta-hemolytic streptococci of serogroups A, B, C, F, and G. Analysis of 23 cases and a review of the literature. Medicine (Baltimore). 1998;77(2):122-139.
28. Gaunt PN, Seal DV. Group G streptococcal infection of joints and joint prostheses. J Infect. 1986;13(2):115-123.
29. Trenkner SW, Braunstein EM, Lynn MD, Ike RW. Group G streptococcal arthritis and bowel disease: A rare enteropathic arthropathy. Gastrointest Radiol. 1987;12(3):265-267.
30. Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998;27(2):385-387.
31. Casariego E, Rodriguez A, Corredoira JC, et al. Prospective study of Streptococcus milleri bacteremia. Eur J Clin Microbiol Infect Dis. 1996;15(3):194-200.
32. Jacobs JA, Pietersen HG, Stobberingh EE, Soeters PB. Bacteremia involving the “Streptococcus milleri” group: Analysis of 19 cases. Clin Infect Dis. 1994;19(4):704-713.
33. Zheng S, Robinson E, Yeoman S, et al. MRI bone oedema predicts eight year tendon function at the wrist but not the requirement for orthopaedic surgery in rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):607-611.
34. Harris R. Spontaneous rupture of the tendon of extensor pollicis longus as a complication of rheumatoid arthritis. Ann Rheum Dis. 1951;10(3):298-306.
35. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: A study of aetiological factors. Scand J Plast Reconstr Surg Hand Surg. 2004;38(1):32-35.
Compared with monoarticular arthritis, polyarticular arthritis may yield an initially narrower differential diagnosis that focuses on systemic inflammatory conditions, such as rheumatoid arthritis (RA). Approximately 15% to 30% of septic arthritis is polyarticular, of which about 45% is associated with underlying RA.1,2 Regardless of the number of joints involved, septic (infectious) arthritis is a valid consideration given the morbidity and mortality.
In a retrospective study in the United Kingdom (UK) between 1982 and 1991, the morbidity and mortality of septic arthritis was 31.6% and 11.5%, respectively, and 16% of the study population had RA.3 A review of the literature by Dubost and colleagues found that polyarticular septic arthritis (PASA) has a mortality of 31% to 42% compared with 4% to 8% for monoarticular septic arthritis, and RA was present in 67% of the PASA fatalities.1
Related: The Golden Era of Treatment in Rheumatology
Rheumatoid arthritis and its treatment predispose patients to septic arthritis. Septic arthritis in the UK general population is 0.42 per 100 patient-years for patients with RA on antitumor necrosis factor therapy.3,4 In a retrospective study in the U.S., the incidence of septic arthritis was 0.40 per 100 patient-years for patients with RA compared with 0.02 per 100 patient-years for patients without RA.5
Other complications of RA include infectious tenosynovitis and tendon rupture. The incidence and prevalence of infectious tenosynovitis and tendon rupture in RA are not firmly established in the literature.
We present a patient with RA and psoriasis who responded initially to acute management for RA but subsequently was diagnosed with culture-negative polyarticular arthritis and infectious tenosynovitis associated with beta hemolytic group G Streptococcus (GGS), a part of Streptococcus milleri (S. milleri). During surgery, he was also found to have bilateral extensor pollicus longus (EPL) tendon rupture. Given the possible morbidity, the authors believe this patient may be of interest to the medical community.
Case Presentation
A 69-year-old African American male presented with 3 to 4 days of swelling and pain of bilateral wrists, bilateral hands, and the left ankle with subjective, but resolved, fevers and chills. His medical history was significant for seropositive erosive RA, psoriasis, hypertension, hyperlipidemia, alcohol abuse, chronic tobacco use, osteoporosis, and glaucoma. He did not have diabetes, reported no IV drug abuse, and except for the immunosuppressive effects of his medications, was not otherwise immunocompromised.
For 2 years in the outpatient setting, the rheumatology clinic had been managing the patient’s rheumatoid factor (RF) positive and anti-cyclic citrullinated peptide (CCP) antibody positive erosive RA with etanercept 25 mg subcutaneously twice a week. The RA affected his hands, wrists, shoulders, and ankles bilaterally but was successfully controlled. The dermatology clinic was managing the patient’s psoriasis with calcipotriene cream 0.005% twice a week and clobetasol ointment 0.05% twice a week. Psoriatic plaques were noted on bilateral elbows, bilateral dorsal hands, and bilateral dorsal feet.
Initial Evaluation
At evaluation, the patient’s vital signs revealed a temperature of 36.3°C (97.3°F), pulse of 102 beats per minute, respiratory rate of 16 breaths per minute, oxygen saturation of 99% on room air, and blood pressure of 102/70 mm Hg. He was found to have edema, tenderness, and erythema of the wrists bilaterally and left metacarpophalangeal joints (MCPs) and edematous right MCPs and left medial ankle.
The patient had been nonadherent with etanercept for 5 monthsand restarted taking the medication only 2 weeks before presentation. He had noticed worsening arthritis for at least 1 month. His last RA flare was approximately 1 year before presentation. Additional symptoms included 4 days of nausea, nonbloody and nonbilious emesis, left lower quadrant pain, and diarrhea without melena or hematochezia.
Initial laboratory studies found 3.2 k/μL white blood cells (WBCs) with a differential of 11.9% lymphocytes, 4.2% monocytes, 83.3% neutrophils, 0.5% eosinophils, and 0.1% basophils; 165 k/μL platelets; 96 mm/h erythrocyte sedimentation rate (ESR); and 45 mg/dL C-reactive protein. The patient was diagnosed with viral gastroenteritis and RA flare and was admitted for inpatient management secondary to limited ability to care for himself.
Related: Infliximab-Induced Complications
The patient was started on prednisone 40 mg orally once a day (for 5 days) for empiric treatment of an RA flare and continued on etanercept. The inpatient rheumatology service was consulted. Further evaluation later that day found involvement of the proximal interphalangeal joints and elbows and tenderness of the tendons of the dorsal hand bilaterally. Over the next 2 days, the patient remained afebrile and WBCs were within normal limits. Edema, erythema, and tenderness of the involved joints somewhat improved, but tenderness along the tendons of the dorsal hand worsened, which concerned the managing teams for infectious tenosynovitis.
By day 4, the patient was afebrile and had a leukocytosis of 12.9 k/μLwith neutrophils 86.7%, but improvement of erythema, pain, and range of motion of involved joints and no tenderness to palpation of tendons was noted. The inpatient orthopedic surgery service evaluated the patient and did not find sufficient evidence necessitating surgical intervention.
Worsening Condition
On day 6, arthrocentesis of the left wrist was performed secondary to worsening of erythema and edema. The patient experienced new edema of the left shoulder and leukocytosis continued to trend upward (15.7 k/μL on day 6). Purulent aspirate (1.5 mL) was obtained from the fluctuance and tenosynovium of the left wrist. Empiric vancomycin 1 g IV twice daily and ceftriaxone 2 g IV daily were started and continued for 3 days. By this point in his hospital course, the patient had received 1 dose of etanercept. Prednisone and etanercept were previously discontinued because of the discovered infection. Blood cultures were drawn and had no growth (Table). Gastroenterology studies were limited to stool cultures and did not include colonoscopy. Leukocytosis began trending down.
On day 8, antibiotics were tailored to penicillin G 4 million units IV every 4 hours following growth of GGS from the sample of the left wrist. Subsequently, synovial fluid (3 mL) from the left shoulder was obtained following initiation of antibiotic therapy and had no growth. Magnetic resonance imaging (MRI) found tenosynovitis of the left ankle and right wrist.
On day 9, transthoracic echocardiography was performed and found no evidence of infectious endocarditis. Later that night, the patient was taken to surgery for incision and drainage/debridement of bilateral wrists and left ankle, synovectomy of right wrist, and aspiration of right shoulder. Findings included abscess in the left wrist and inflammatory synovitis and bilateral EPL tendon rupture consistent with RA. Pus from the left ankle had few gram-positive cocci in chains with no growth, and the specimens from both wrists grew GGS. Aspirate from the left ankle was an opaque yellow fluid with 14,900/mm3 WBC, 30,000/mm3 red blood cells (RBC), 97% neutrophils, 1% macrophages, 2% lymphocytes, and 0% monocytes. Aspirate from the right shoulder was an opaque bloody fluid with 10,100/mm3 WBC, 40,000/mm3 RBC, 95% neutrophils, 2% macrophages, 1% lymphocytes, and 1% monocytes. On day 10, sulfasalazine 500 mg twice a day was initiated for RA.
Following surgery and continued antibiotics, the patient’s leukocytosis resolved, and improvement was seen in all joints with decreased edema, erythema, and pain and increased range of motion. Postoperative recovery was complicated by ileus, urinary retention, and fungal (Candida albicans) urinary tract infection, all of which resolved without significant complications. The inpatient rheumatology service restarted prednisone at a lower dose of 20 mg. The patient became afebrile and sufficiently stable for transfer to a lower level of care with continued physical therapy and IV antibiotics for another 3 weeks.
Discussion
The patient had 2 underlying systemic inflammatory conditions: RA and psoriasis. The underlying chronic arthritis was likely caused by RA, not psoriatic arthritis (PsA). The patient met the 2010 American College of Rheumatology criteria but failed to meet the classification criteria for PsA.6,7 However, the clinical features of RA and PsA overlap. Rheumatoid factor and CCP can be positive laboratory findings in both RA and PsA.8-14 Tenosynovitis is found in about half of RA patients and PsA patients (P > .05).15 In its evaluation of the patient, the inpatient rheumatology service suspected that the patient may have had RA with components of PsA.
Rheumatoid arthritis complicates the diagnosis of septic arthritis. In a study by Nolla and colleagues, a mean of 7.3 days (range 3 to 18 days) elapsed before a diagnosis of septic arthritis was made in 10 patients with RA on corticosteroids.2 Consideration of risk factors such as increasing age, male sex, tobacco use, extra-articular manifestations of RA, positive RF, rheumatoid nodules, poor functional capacity, high ESR, leukopenia, comorbidities (chronic lung disease, alcoholism, organic brain disease, and diabetes), and the use of corticosteroids may expedite the diagnosis of infections in patients with RA.16 In this case, the patient had some of these risk factors: age, male sex, alcoholism, chronic tobacco use, positive RF, high ESR, and leukopenia (at presentation).
Related: Trend Toward Concomitant Supplements and Medications
The history of medication nonadherence of etanercept with progressively worsening arthritis and early clinical improvement (reduction in erythema, edema, and pain and temporary loss of signs of tenosynovitis on examination) while on prednisone suggested that the patient had a RA flare. The prednisone likely alleviated the inflammatory process but created an immunosuppressed state that allowed GGS to invade and possibly disseminate. Alternately, the patient may have been infected before presentation. The lack of a definitive time line for his case prevented the authors from forming conclusions about a possible causal relationship between the infection and medications. The subjective fevers before admission were nonspecific and could have been caused by RA, presumed gastroenteritis, or other undiagnosed infectious processes. The observed leukocytosis may have been initially corticosteroid-induced.17
Septic Arthritis
The suspicion of septic arthritis and infectious tenosynovitis substantially increased on day 6 with worsening symptoms, involvement of additional joints, and spiking fevers. Group G Streptococcus was obtained from the aspirate of the left wrist and from the surgical specimens from the bilateral wrists. The clinical presentation, MRI imaging studies, and surgical and nonsurgical specimens supported a diagnosis of GGS tenosynovitis. However, there was no clear evidence (ie, positive culture with identified organism) of septic arthritis, likely secondary to early septic arthritis and initiation of antibiotics before joint aspirations. The aspirate from the left ankle was yellow and opaque, but the culture was negative.
The pathogenic organism in the patient was GGS. Group G Streptococcus is normal flora of the oral cavity, gastrointestinal (GI) tract, upper respiratory tract, genital tract, and skin, which were all possible sources of seeding.18 Streptococcal species account for about 20% of septic arthritis, and GGS arthritis accounts for 4% to 19% of streptococcal arthritis.19-22 From a review of the literature, 2 cases of GGS tenosynovitis have been published.23,24 However, in an ultrasound study and MRI study, 49% and 43%, respectively, of patients with RA had tenosynovitis of the tendons of the hands.15,25
GGS Demographics
About three-quarters (71%) of patients with GGS arthritis are male.19 The analysis of the literature by Bronze and colleagues found that chronic joint disease and alcoholism are present in 34% and 14% of patients with GGS arthritis, respectively. One-quarter (23% from Dubost and colleagues) to one-third (32% from Schattner and colleagues) of patients with GGS arthritis have RA.19,26
Fever is present in less than half (43%) of patients with GGS arthritis.19 Positive synovial fluid is expected in 90% of patients.19 Leukocytosis and elevated ESR need not be present.27,28 The arthritis is polyarticular in one-quarter of patients (24% from Bronze and colleagues and 26% from Dubost and colleagues).19,26
Positive blood cultures can be expected in one-fourth (26%) of patients with GGS arthritis.19 The patient’s blood cultures were negative. Blood cultures drawn before initiation of antibiotics yielded no growth, so if the spread was hematogenous, the bacteremia was transient or intermittent. Before and after initiation of antibiotics, specimens from the shoulders did not grow colonies, whereas specimens from the wrists did. If the shoulders were truly infected, these findings and the notably later involvement of the shoulders suggest that the shoulders may have been seeded later in the hospital course.
Trenkner and colleagues proposed that GI abnormalities provide a portal of entry for GGS, which is under the umbrella of S. milleri.29S. milleri is associated with abscess formation, usually of the GI tract.30-32 In the study patient, the possible gastroenteritis may have provided such a portal of entry and subsequent seeding to the joints, and an abscess was found in the left wrist.
Tendon Rupture
Additionally, bilateral EPL tendon rupture likely occurred as a consequence of the inflammatory process from RA and infectious tenosynovitis in the patient. According to Zheng and colleagues, tenosynovitis is an inflammatory process of the synovial tendon sheath that may result in degeneration and rupture of the tendons and may contribute to bone erosions, development of joint deformities, and loss of functional capacity.33 In a histologic study of a ruptured EPL tendon from a patient with RA, Harris observed a chronic inflammatory cellular reaction.34 Harris also described a male with RA with unconfirmed bilateral EPL rupture.34 Björkman and colleague identified previous injury, RA, and local or systemic steroids as important etiologic factors for EPL tendon rupture.35
As in the case of this patient, the utilization of both medical and surgical therapy is not uncommon for treating GGS infection. Antibiotic therapy typically consists of penicillin (74%).26 Surgical intervention is necessary in 16% to 37% of patients.19,26 This patient required both penicillin and incision and drainage/debridement before significant clinical improvement was noted. Prognosis of GGS arthritis is favorable with 5% mortality.26
Conclusion
Septic arthritis and infectious tenosynovitis are readily treatable with low mortality if promptly identified. Identification can be masked by other medical conditions, such as RA and psoriasis, and their associated immunosuppressive treatment. Bilateral EPL tendon rupture may be a complication of RA, particularly with an underlying septic arthritis and infectious tenosynovitis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Compared with monoarticular arthritis, polyarticular arthritis may yield an initially narrower differential diagnosis that focuses on systemic inflammatory conditions, such as rheumatoid arthritis (RA). Approximately 15% to 30% of septic arthritis is polyarticular, of which about 45% is associated with underlying RA.1,2 Regardless of the number of joints involved, septic (infectious) arthritis is a valid consideration given the morbidity and mortality.
In a retrospective study in the United Kingdom (UK) between 1982 and 1991, the morbidity and mortality of septic arthritis was 31.6% and 11.5%, respectively, and 16% of the study population had RA.3 A review of the literature by Dubost and colleagues found that polyarticular septic arthritis (PASA) has a mortality of 31% to 42% compared with 4% to 8% for monoarticular septic arthritis, and RA was present in 67% of the PASA fatalities.1
Related: The Golden Era of Treatment in Rheumatology
Rheumatoid arthritis and its treatment predispose patients to septic arthritis. Septic arthritis in the UK general population is 0.42 per 100 patient-years for patients with RA on antitumor necrosis factor therapy.3,4 In a retrospective study in the U.S., the incidence of septic arthritis was 0.40 per 100 patient-years for patients with RA compared with 0.02 per 100 patient-years for patients without RA.5
Other complications of RA include infectious tenosynovitis and tendon rupture. The incidence and prevalence of infectious tenosynovitis and tendon rupture in RA are not firmly established in the literature.
We present a patient with RA and psoriasis who responded initially to acute management for RA but subsequently was diagnosed with culture-negative polyarticular arthritis and infectious tenosynovitis associated with beta hemolytic group G Streptococcus (GGS), a part of Streptococcus milleri (S. milleri). During surgery, he was also found to have bilateral extensor pollicus longus (EPL) tendon rupture. Given the possible morbidity, the authors believe this patient may be of interest to the medical community.
Case Presentation
A 69-year-old African American male presented with 3 to 4 days of swelling and pain of bilateral wrists, bilateral hands, and the left ankle with subjective, but resolved, fevers and chills. His medical history was significant for seropositive erosive RA, psoriasis, hypertension, hyperlipidemia, alcohol abuse, chronic tobacco use, osteoporosis, and glaucoma. He did not have diabetes, reported no IV drug abuse, and except for the immunosuppressive effects of his medications, was not otherwise immunocompromised.
For 2 years in the outpatient setting, the rheumatology clinic had been managing the patient’s rheumatoid factor (RF) positive and anti-cyclic citrullinated peptide (CCP) antibody positive erosive RA with etanercept 25 mg subcutaneously twice a week. The RA affected his hands, wrists, shoulders, and ankles bilaterally but was successfully controlled. The dermatology clinic was managing the patient’s psoriasis with calcipotriene cream 0.005% twice a week and clobetasol ointment 0.05% twice a week. Psoriatic plaques were noted on bilateral elbows, bilateral dorsal hands, and bilateral dorsal feet.
Initial Evaluation
At evaluation, the patient’s vital signs revealed a temperature of 36.3°C (97.3°F), pulse of 102 beats per minute, respiratory rate of 16 breaths per minute, oxygen saturation of 99% on room air, and blood pressure of 102/70 mm Hg. He was found to have edema, tenderness, and erythema of the wrists bilaterally and left metacarpophalangeal joints (MCPs) and edematous right MCPs and left medial ankle.
The patient had been nonadherent with etanercept for 5 monthsand restarted taking the medication only 2 weeks before presentation. He had noticed worsening arthritis for at least 1 month. His last RA flare was approximately 1 year before presentation. Additional symptoms included 4 days of nausea, nonbloody and nonbilious emesis, left lower quadrant pain, and diarrhea without melena or hematochezia.
Initial laboratory studies found 3.2 k/μL white blood cells (WBCs) with a differential of 11.9% lymphocytes, 4.2% monocytes, 83.3% neutrophils, 0.5% eosinophils, and 0.1% basophils; 165 k/μL platelets; 96 mm/h erythrocyte sedimentation rate (ESR); and 45 mg/dL C-reactive protein. The patient was diagnosed with viral gastroenteritis and RA flare and was admitted for inpatient management secondary to limited ability to care for himself.
Related: Infliximab-Induced Complications
The patient was started on prednisone 40 mg orally once a day (for 5 days) for empiric treatment of an RA flare and continued on etanercept. The inpatient rheumatology service was consulted. Further evaluation later that day found involvement of the proximal interphalangeal joints and elbows and tenderness of the tendons of the dorsal hand bilaterally. Over the next 2 days, the patient remained afebrile and WBCs were within normal limits. Edema, erythema, and tenderness of the involved joints somewhat improved, but tenderness along the tendons of the dorsal hand worsened, which concerned the managing teams for infectious tenosynovitis.
By day 4, the patient was afebrile and had a leukocytosis of 12.9 k/μLwith neutrophils 86.7%, but improvement of erythema, pain, and range of motion of involved joints and no tenderness to palpation of tendons was noted. The inpatient orthopedic surgery service evaluated the patient and did not find sufficient evidence necessitating surgical intervention.
Worsening Condition
On day 6, arthrocentesis of the left wrist was performed secondary to worsening of erythema and edema. The patient experienced new edema of the left shoulder and leukocytosis continued to trend upward (15.7 k/μL on day 6). Purulent aspirate (1.5 mL) was obtained from the fluctuance and tenosynovium of the left wrist. Empiric vancomycin 1 g IV twice daily and ceftriaxone 2 g IV daily were started and continued for 3 days. By this point in his hospital course, the patient had received 1 dose of etanercept. Prednisone and etanercept were previously discontinued because of the discovered infection. Blood cultures were drawn and had no growth (Table). Gastroenterology studies were limited to stool cultures and did not include colonoscopy. Leukocytosis began trending down.
On day 8, antibiotics were tailored to penicillin G 4 million units IV every 4 hours following growth of GGS from the sample of the left wrist. Subsequently, synovial fluid (3 mL) from the left shoulder was obtained following initiation of antibiotic therapy and had no growth. Magnetic resonance imaging (MRI) found tenosynovitis of the left ankle and right wrist.
On day 9, transthoracic echocardiography was performed and found no evidence of infectious endocarditis. Later that night, the patient was taken to surgery for incision and drainage/debridement of bilateral wrists and left ankle, synovectomy of right wrist, and aspiration of right shoulder. Findings included abscess in the left wrist and inflammatory synovitis and bilateral EPL tendon rupture consistent with RA. Pus from the left ankle had few gram-positive cocci in chains with no growth, and the specimens from both wrists grew GGS. Aspirate from the left ankle was an opaque yellow fluid with 14,900/mm3 WBC, 30,000/mm3 red blood cells (RBC), 97% neutrophils, 1% macrophages, 2% lymphocytes, and 0% monocytes. Aspirate from the right shoulder was an opaque bloody fluid with 10,100/mm3 WBC, 40,000/mm3 RBC, 95% neutrophils, 2% macrophages, 1% lymphocytes, and 1% monocytes. On day 10, sulfasalazine 500 mg twice a day was initiated for RA.
Following surgery and continued antibiotics, the patient’s leukocytosis resolved, and improvement was seen in all joints with decreased edema, erythema, and pain and increased range of motion. Postoperative recovery was complicated by ileus, urinary retention, and fungal (Candida albicans) urinary tract infection, all of which resolved without significant complications. The inpatient rheumatology service restarted prednisone at a lower dose of 20 mg. The patient became afebrile and sufficiently stable for transfer to a lower level of care with continued physical therapy and IV antibiotics for another 3 weeks.
Discussion
The patient had 2 underlying systemic inflammatory conditions: RA and psoriasis. The underlying chronic arthritis was likely caused by RA, not psoriatic arthritis (PsA). The patient met the 2010 American College of Rheumatology criteria but failed to meet the classification criteria for PsA.6,7 However, the clinical features of RA and PsA overlap. Rheumatoid factor and CCP can be positive laboratory findings in both RA and PsA.8-14 Tenosynovitis is found in about half of RA patients and PsA patients (P > .05).15 In its evaluation of the patient, the inpatient rheumatology service suspected that the patient may have had RA with components of PsA.
Rheumatoid arthritis complicates the diagnosis of septic arthritis. In a study by Nolla and colleagues, a mean of 7.3 days (range 3 to 18 days) elapsed before a diagnosis of septic arthritis was made in 10 patients with RA on corticosteroids.2 Consideration of risk factors such as increasing age, male sex, tobacco use, extra-articular manifestations of RA, positive RF, rheumatoid nodules, poor functional capacity, high ESR, leukopenia, comorbidities (chronic lung disease, alcoholism, organic brain disease, and diabetes), and the use of corticosteroids may expedite the diagnosis of infections in patients with RA.16 In this case, the patient had some of these risk factors: age, male sex, alcoholism, chronic tobacco use, positive RF, high ESR, and leukopenia (at presentation).
Related: Trend Toward Concomitant Supplements and Medications
The history of medication nonadherence of etanercept with progressively worsening arthritis and early clinical improvement (reduction in erythema, edema, and pain and temporary loss of signs of tenosynovitis on examination) while on prednisone suggested that the patient had a RA flare. The prednisone likely alleviated the inflammatory process but created an immunosuppressed state that allowed GGS to invade and possibly disseminate. Alternately, the patient may have been infected before presentation. The lack of a definitive time line for his case prevented the authors from forming conclusions about a possible causal relationship between the infection and medications. The subjective fevers before admission were nonspecific and could have been caused by RA, presumed gastroenteritis, or other undiagnosed infectious processes. The observed leukocytosis may have been initially corticosteroid-induced.17
Septic Arthritis
The suspicion of septic arthritis and infectious tenosynovitis substantially increased on day 6 with worsening symptoms, involvement of additional joints, and spiking fevers. Group G Streptococcus was obtained from the aspirate of the left wrist and from the surgical specimens from the bilateral wrists. The clinical presentation, MRI imaging studies, and surgical and nonsurgical specimens supported a diagnosis of GGS tenosynovitis. However, there was no clear evidence (ie, positive culture with identified organism) of septic arthritis, likely secondary to early septic arthritis and initiation of antibiotics before joint aspirations. The aspirate from the left ankle was yellow and opaque, but the culture was negative.
The pathogenic organism in the patient was GGS. Group G Streptococcus is normal flora of the oral cavity, gastrointestinal (GI) tract, upper respiratory tract, genital tract, and skin, which were all possible sources of seeding.18 Streptococcal species account for about 20% of septic arthritis, and GGS arthritis accounts for 4% to 19% of streptococcal arthritis.19-22 From a review of the literature, 2 cases of GGS tenosynovitis have been published.23,24 However, in an ultrasound study and MRI study, 49% and 43%, respectively, of patients with RA had tenosynovitis of the tendons of the hands.15,25
GGS Demographics
About three-quarters (71%) of patients with GGS arthritis are male.19 The analysis of the literature by Bronze and colleagues found that chronic joint disease and alcoholism are present in 34% and 14% of patients with GGS arthritis, respectively. One-quarter (23% from Dubost and colleagues) to one-third (32% from Schattner and colleagues) of patients with GGS arthritis have RA.19,26
Fever is present in less than half (43%) of patients with GGS arthritis.19 Positive synovial fluid is expected in 90% of patients.19 Leukocytosis and elevated ESR need not be present.27,28 The arthritis is polyarticular in one-quarter of patients (24% from Bronze and colleagues and 26% from Dubost and colleagues).19,26
Positive blood cultures can be expected in one-fourth (26%) of patients with GGS arthritis.19 The patient’s blood cultures were negative. Blood cultures drawn before initiation of antibiotics yielded no growth, so if the spread was hematogenous, the bacteremia was transient or intermittent. Before and after initiation of antibiotics, specimens from the shoulders did not grow colonies, whereas specimens from the wrists did. If the shoulders were truly infected, these findings and the notably later involvement of the shoulders suggest that the shoulders may have been seeded later in the hospital course.
Trenkner and colleagues proposed that GI abnormalities provide a portal of entry for GGS, which is under the umbrella of S. milleri.29S. milleri is associated with abscess formation, usually of the GI tract.30-32 In the study patient, the possible gastroenteritis may have provided such a portal of entry and subsequent seeding to the joints, and an abscess was found in the left wrist.
Tendon Rupture
Additionally, bilateral EPL tendon rupture likely occurred as a consequence of the inflammatory process from RA and infectious tenosynovitis in the patient. According to Zheng and colleagues, tenosynovitis is an inflammatory process of the synovial tendon sheath that may result in degeneration and rupture of the tendons and may contribute to bone erosions, development of joint deformities, and loss of functional capacity.33 In a histologic study of a ruptured EPL tendon from a patient with RA, Harris observed a chronic inflammatory cellular reaction.34 Harris also described a male with RA with unconfirmed bilateral EPL rupture.34 Björkman and colleague identified previous injury, RA, and local or systemic steroids as important etiologic factors for EPL tendon rupture.35
As in the case of this patient, the utilization of both medical and surgical therapy is not uncommon for treating GGS infection. Antibiotic therapy typically consists of penicillin (74%).26 Surgical intervention is necessary in 16% to 37% of patients.19,26 This patient required both penicillin and incision and drainage/debridement before significant clinical improvement was noted. Prognosis of GGS arthritis is favorable with 5% mortality.26
Conclusion
Septic arthritis and infectious tenosynovitis are readily treatable with low mortality if promptly identified. Identification can be masked by other medical conditions, such as RA and psoriasis, and their associated immunosuppressive treatment. Bilateral EPL tendon rupture may be a complication of RA, particularly with an underlying septic arthritis and infectious tenosynovitis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dubost JJ, Fis I, Denis P, et al. Polyarticular septic arthritis. Medicine (Baltimore). 1993;72(5):296-310.
2. Nolla JM, Gómez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: A detailed analysis of 10 cases and literature review. Semin Arthritis Rheum. 2000;30(2):121-126.
3.Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
4. Galloway JB, Hyrich KL, Mercer LK, et al; BSR Biologics Register. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: Results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(10):1810-1814.
5. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002;46(9):2287-2293.
6. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
7. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665-2673.
8. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)—An analysis of 220 patients. Q J Med. 1987;62(238):127-141.
9. Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol. 2005;32(3):511-515.
10. Vander Cruyssen B, Hoffman IE, Zmierczak H, et al. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis. 2005;64(8):1145-1149.
11. Alenius GM, Berglin E, Rantapää Dahlgvist S. Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation. Ann Rheum Dis. 2006;65(3):398-400.
12. Candia L, Marquez J, Gonzalez C, et al. Low frequency of anticyclic citrullinated peptide antibodies in psoriatic arthritis but not in cutaneous psoriasis. J Clin Rheumatol. 2006;12(5):226-229.
13. Inanc N, Dalkilic E, Kamali S, et al. Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol. 2007;26(1):17-23.
14. Popescu C, Zofota S, Bojinca V, Ionescu R. Anti-cyclic citrullinated peptide antibodies in psoriatic arthritis—Cross-sectional study and literature review. J Med Life. 2013;6(4):376-382.
15. Schoellnast H, Deutschmann HA, Hermann J, et al. Psoriatic arthritis and rheumatoid arthritis: Findings in contrast-enhanced MRI. AJR Am J Roentgenol. 2006;187(2):351-357.
16. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2294-2300.
17. Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71(5):773-778.
18. Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10(2):257-285.
19. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Sauvezie B. Streptococcal septic arthritis in adults. A study of 55 cases with a literature review. Joint Bone Spine. 2004;71(4):303-311.
20. Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: Analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36(3):370-373.
21. Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117(3):423-428.
22. Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: A community based prospective survey. Ann Rheum Dis. 1997;56(8):470-475.
23. Bradlow A, Mitchell RG, Mowat AG. Group G streptococcal arthritis. Rheumatol Rehabil. 1982;21(4):206-210.
24. Meier JL, Gerster JC. Bursitis and tenosynovitis caused by group G streptococci. J Rheumatol. 1983;10(5):817-818.
25. Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. Hand tendon involvement in rheumatoid arthritis: An ultrasound study. Semin Arthritis Rheum. 2012;41(6):752-760.
26. Bronze MS, Whitby S, Schaberg DR. Group G streptococcal arthritis: Case report and review of the literature. Am J Med Sci. 1997;313(4):239-243.
27. Schattner A, Vosti KL. Bacterial arthritis due to beta-hemolytic streptococci of serogroups A, B, C, F, and G. Analysis of 23 cases and a review of the literature. Medicine (Baltimore). 1998;77(2):122-139.
28. Gaunt PN, Seal DV. Group G streptococcal infection of joints and joint prostheses. J Infect. 1986;13(2):115-123.
29. Trenkner SW, Braunstein EM, Lynn MD, Ike RW. Group G streptococcal arthritis and bowel disease: A rare enteropathic arthropathy. Gastrointest Radiol. 1987;12(3):265-267.
30. Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998;27(2):385-387.
31. Casariego E, Rodriguez A, Corredoira JC, et al. Prospective study of Streptococcus milleri bacteremia. Eur J Clin Microbiol Infect Dis. 1996;15(3):194-200.
32. Jacobs JA, Pietersen HG, Stobberingh EE, Soeters PB. Bacteremia involving the “Streptococcus milleri” group: Analysis of 19 cases. Clin Infect Dis. 1994;19(4):704-713.
33. Zheng S, Robinson E, Yeoman S, et al. MRI bone oedema predicts eight year tendon function at the wrist but not the requirement for orthopaedic surgery in rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):607-611.
34. Harris R. Spontaneous rupture of the tendon of extensor pollicis longus as a complication of rheumatoid arthritis. Ann Rheum Dis. 1951;10(3):298-306.
35. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: A study of aetiological factors. Scand J Plast Reconstr Surg Hand Surg. 2004;38(1):32-35.
1. Dubost JJ, Fis I, Denis P, et al. Polyarticular septic arthritis. Medicine (Baltimore). 1993;72(5):296-310.
2. Nolla JM, Gómez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: A detailed analysis of 10 cases and literature review. Semin Arthritis Rheum. 2000;30(2):121-126.
3.Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
4. Galloway JB, Hyrich KL, Mercer LK, et al; BSR Biologics Register. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: Results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(10):1810-1814.
5. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002;46(9):2287-2293.
6. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
7. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665-2673.
8. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)—An analysis of 220 patients. Q J Med. 1987;62(238):127-141.
9. Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol. 2005;32(3):511-515.
10. Vander Cruyssen B, Hoffman IE, Zmierczak H, et al. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis. 2005;64(8):1145-1149.
11. Alenius GM, Berglin E, Rantapää Dahlgvist S. Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation. Ann Rheum Dis. 2006;65(3):398-400.
12. Candia L, Marquez J, Gonzalez C, et al. Low frequency of anticyclic citrullinated peptide antibodies in psoriatic arthritis but not in cutaneous psoriasis. J Clin Rheumatol. 2006;12(5):226-229.
13. Inanc N, Dalkilic E, Kamali S, et al. Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol. 2007;26(1):17-23.
14. Popescu C, Zofota S, Bojinca V, Ionescu R. Anti-cyclic citrullinated peptide antibodies in psoriatic arthritis—Cross-sectional study and literature review. J Med Life. 2013;6(4):376-382.
15. Schoellnast H, Deutschmann HA, Hermann J, et al. Psoriatic arthritis and rheumatoid arthritis: Findings in contrast-enhanced MRI. AJR Am J Roentgenol. 2006;187(2):351-357.
16. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2294-2300.
17. Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71(5):773-778.
18. Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10(2):257-285.
19. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Sauvezie B. Streptococcal septic arthritis in adults. A study of 55 cases with a literature review. Joint Bone Spine. 2004;71(4):303-311.
20. Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: Analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36(3):370-373.
21. Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117(3):423-428.
22. Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: A community based prospective survey. Ann Rheum Dis. 1997;56(8):470-475.
23. Bradlow A, Mitchell RG, Mowat AG. Group G streptococcal arthritis. Rheumatol Rehabil. 1982;21(4):206-210.
24. Meier JL, Gerster JC. Bursitis and tenosynovitis caused by group G streptococci. J Rheumatol. 1983;10(5):817-818.
25. Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. Hand tendon involvement in rheumatoid arthritis: An ultrasound study. Semin Arthritis Rheum. 2012;41(6):752-760.
26. Bronze MS, Whitby S, Schaberg DR. Group G streptococcal arthritis: Case report and review of the literature. Am J Med Sci. 1997;313(4):239-243.
27. Schattner A, Vosti KL. Bacterial arthritis due to beta-hemolytic streptococci of serogroups A, B, C, F, and G. Analysis of 23 cases and a review of the literature. Medicine (Baltimore). 1998;77(2):122-139.
28. Gaunt PN, Seal DV. Group G streptococcal infection of joints and joint prostheses. J Infect. 1986;13(2):115-123.
29. Trenkner SW, Braunstein EM, Lynn MD, Ike RW. Group G streptococcal arthritis and bowel disease: A rare enteropathic arthropathy. Gastrointest Radiol. 1987;12(3):265-267.
30. Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998;27(2):385-387.
31. Casariego E, Rodriguez A, Corredoira JC, et al. Prospective study of Streptococcus milleri bacteremia. Eur J Clin Microbiol Infect Dis. 1996;15(3):194-200.
32. Jacobs JA, Pietersen HG, Stobberingh EE, Soeters PB. Bacteremia involving the “Streptococcus milleri” group: Analysis of 19 cases. Clin Infect Dis. 1994;19(4):704-713.
33. Zheng S, Robinson E, Yeoman S, et al. MRI bone oedema predicts eight year tendon function at the wrist but not the requirement for orthopaedic surgery in rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):607-611.
34. Harris R. Spontaneous rupture of the tendon of extensor pollicis longus as a complication of rheumatoid arthritis. Ann Rheum Dis. 1951;10(3):298-306.
35. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: A study of aetiological factors. Scand J Plast Reconstr Surg Hand Surg. 2004;38(1):32-35.
Cutaneous Burn Caused by Radiofrequency Ablation Probe During Shoulder Arthroscopy
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Biomechanical Evaluation of Proximally Placed Femoral Less-Invasive Stabilization System Plates
Several surgical options are available for treatment of supracondylar and intercondylar distal femur fractures, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 33. Preserving the osseous blood supply via indirect reduction techniques has been shown to increase union rates without the need for bone grafting.1,2 The Less-Invasive Stabilization System (LISS) made by Synthes (Paoli, Pennsylvania) melds minimally invasive internal fixation with multiple fixed-angle distal screws. It allows for submuscular placement, percutaneous unicortical screws in the diaphysis, and preservation of the metaphyseal fracture soft-tissue envelope.3
Proper lateral placement of the plate on the femur proximally can be difficult. Kregor and colleagues3 noted that 6% of cases did not have ideal placement on the lateral shaft of the femur when the 13-hole LISS plate was used. They advocated making a small incision at the proximal end of the LISS plate to aid in proper lateral placement. Kolb and colleagues4 noted that 2 of 31 patients had a “cutting out” of the proximal screws on LISS plates with anterior placement on the femur that eventually required repeat surgery in order to heal. This malpositioned plate was present at the end of the operation. These authors also recommended a proximal incision to avoid the issue. Schütz and colleagues5 noted that there were 4 cases of implant loosening among 107 distal femur fractures treated with LISS plating and that the unicortical screws in the diaphysis had loosened. They suggested anterior placement of the plate as a possible reason for fixation failure.
Although several studies have noted proximal screw pull-out, and proximal anterior malposition in the sagittal plane of the LISS plate has been suggested as a possible cause, we found no studies comparing incorrect proximal positioning on the femoral shaft with correct lateral placement of the LISS plate. Therefore, we used a previously established biomechanical model to compare LISS plates proximally placed either too anterior or too posterior to the direct lateral position on the femoral shaft. The constructs were tested in axial, torsional, and cyclical axial modes to assess plastic and total deformation, stiffness, and fixation failure.
Materials and Methods
Using fourth-generation femoral synthetic composite bones (Sawbones; Pacific Research Laboratories, Vashon, Washington) and a 13-hole Synthes femoral LISS plate, we made 3 groups of 9 specimens each, for a total of 27 femurs. The number of specimens was based on a power assessment in a study by Khalafi and colleagues.8 Several studies have validated use of Sawbones instead of cadavers in biomechanical testing to prevent variability.6-9 Proximal fixation was achieved with 5 unicortical screws (26 mm long) at screw holes 13, 11, 9, 7, and 4. All distal screw holes were filled for distal fixation with 75-mm-long screws to achieve bicortical fixation.
After application of the LISS plate, an AO/OTA 33-A3 fracture model was created in each specimen. A 1-cm gap was made 6 cm proximal to the intercondylar notch to create an unstable distal femur fracture pattern. In the method described by Zlowodzki and colleagues,10 an additional 3-cm cut was made diagonally in the medial cortex to prevent contact of the bone during mechanical testing.
Three different plate positions were used. The correct group was placed directly laterally proximally (Figure 1A). One incorrect group was plated with the proximal aspect of the plate 1 cm anterior (anterior group) (Figure 1B), and another incorrect group was plated with the proximal aspect of the plate 1 cm posterior (posterior group) (Figure 1C). Anterior or posterior plate placement resulted in some of the proximal screws having a more tangential placement, with fewer screws engaged compared with the properly placed plate.
The distal and proximal ends of each specimen were held to simulate the mechanical axis of the femur. This design was based on a model by Cordey and colleagues.11 A materials testing system (MTS, Minneapolis, Minnesota) was used for mechanical testing of the model.
Based on the protocol of Khalafi and colleagues,8 the models were tested in axial, torsional, and cyclical axial modes (Figures 2, 3). Axial loading consisted of a preload of 100 N followed by a compressive loading rate of 100 mm per minute in a displacement control mode. Testing was considered completed when 1 of 3 events occurred: 500 N was reached, the medial fracture gap closed, or fixation was lost. Torsional loading involved a preload of 5 Nm and subsequent torqueing at 20° per minute up to 20 Nm or loss of fixation or screw pull-out.8 Cyclical axial loading was based on protocols described by Marti and colleagues2 and Zlowodzki and colleagues.10 The initial load was 10 cycles of 300 N. Each subsequent load increment was increased by 100 N up to 1000 N, providing 10-second rest increments. This loading was conducted in a displacement control mode at 0.75 mm per second. Testing was aborted on fixation loss or complete closure of the medial fracture gap.
After testing was completed, statistically significant between-groups differences in plastic deformation and axial and torsional stiffness were determined by performing a Tukey-Kramer honestly significant difference test. Significance was set at P ≤ .05.
Results
During axial loading, there was no visual loss of fixation or change in displacement of the fracture gap for any group, and there was no screw cut-out or pull-out from the cortex during testing. In 1 plate in the posterior group, the most proximal screw made only loose contact with the cortex at only the distal portion of the screw. There was no significant difference (P = .9762) in stiffness in axial loading between the anterior group and the correct group. There was a significant (P = .0261) 16.4% increase in stiffness in the posterior group compared with the correct group (Table).
There was no screw cut-out, fixation failure, or change in displacement of the fracture gap for any group during torsional loading. There was a statistically significant (P = .0062) 12% increase in mean torsional stiffness in the anterior group compared with the correct group. There was no statistically significant difference (P = .1623) between the posterior group and the correct group (Table).
For cyclical axial testing, total deformation and plastic deformation were obtained by determining displacement under the initial 100 N load in the static/resting state. That number was then subtracted from maximum displacement, the peak value on the time-versus-displacement graph, to obtain the value for total deformation. Plastic deformation was calculated by subtracting initial displacement from final displacement in the static/resting state. The static/resting state is represented by the dips in displacement after each cycle on the time-versus-displacement graph (Figure 4).
There was a statistically significant (P = .0207) 14% increase in total deformation of the anteriorly positioned plate compared with the correctly positioned plate. There was no statistically significant difference in total deformation between the posteriorly placed plates and the correctly placed plates (Table).
There was no significant difference in plastic deformation between any of the groups in this study. There was no screw cut-out or fixation loss in any group to suggest a clinically relevant difference based on proximal placement of the LISS plate.
Discussion
In evaluating the stability of various constructs for fixation of distal femur fractures, the literature is consistent in reporting stiffness as the key factor. Stiffness is determined most often in terms of motion at the fracture site, as measured by displacement under axial and torsional loads.2,8,10,13 The LISS plate, which acts essentially as an “internal fixator” with proximal unicortical fixed-angle locking screws, has been shown to be comparable to other established methods of fixation.10,12 Zlowodzki and colleagues10 reported that the LISS plate had a higher load to failure when compared with angled blade plating and intramedullary nailing. Their study used fresh-frozen cadaver specimens from patients 70 years old or older. They concluded that, for distal femur fractures in osteoporotic bone, the LISS plate provided improved distal fixation.
In the present study, the posteriorly placed LISS plate outperformed the correctly placed plate in axial stiffness by 16.4%. However, there was no statistically significant difference in torsional stiffness and cyclical axial loading. This result is difficult to explain given that there was no screw cut-out or fixation loss for any of the constructs. Theoretically, with less proximal screw purchase in the posteriorly placed plate, the overall construct should be more susceptible to screw cut-out and fixation loss resulting in less axial stiffness overall.
Khalafi and colleagues8 created a distal femur fracture model using Sawbones with a 1-cm fracture gap. Using the 9-hole LISS plate for fixation, they tested this construct under axial, torsional, and cyclical axial loads. They tested 2 groups of 9 femurs. For group 1, the LISS plate was placed in the correct position on the distal femur, with the proximal end in the correct position on the femoral shaft. In group 2, the LISS plate was rotated 1 cm anteriorly. They found that axial stiffness (N/mm) was 21.5% greater in the correctly positioned plate. The anteriorly positioned group demonstrated 55% more irreversible or plastic deformation. The authors concluded that correct positioning of the femoral LISS plate provided improved mechanical stability.
Overall, our study results did not agree with those of Khalafi and colleagues8 in terms of the mechanical stability of a malpositioned LISS plate. Our construct showed a significant increase in torsional stiffness in the anteriorly placed plate. However, our construct also showed a significant increase in total deformation in cyclical axial loading in the anteriorly placed plate. There was no increased plastic deformation in either of the incorrectly placed groups in our study. The difference in results between studies can best be explained by the difference in plate lengths. We used a 13-hole plate, and Khalafi and colleagues8 used a 9-hole plate. Our theory is that the longer plate provided more resistance to relatively minor variations in plate position at the proximal end and thus resulted in less change in stiffness and stability around the fracture site.
Our model differed from that used in other biomechanical studies using Sawbones to simulate distal femur fractures in that it used the entire femur, including the proximal portion.8,13 This setup theoretically resulted in a more anatomical weight distribution compared with other models, in which the proximal portion of the femur was potted in polymethylmethacrylate. This difference in weight distribution could explain the variation in our results compared with other biomechanical studies. In addition, with use of different boundary conditions, the distal femur had unconstrained distal motion similar to the native environment of the femur.
This study had several limitations. First is its relatively low power (9 femurs per group). Although groups of 9 specimens in 2 groups were used in the study by Khalafi and colleagues8, testing a larger number of femurs could potentially identify more subtle differences between the 3 groups in our study. Second, given that femoral LISS plates come in different lengths, this study could be expanded to include the other plate sizes, as plate length could potentially play a role in stability at the fracture site. Third, though this Sawbones model has consistently reproduced the stability characteristics of human bone without variation between specimens, an osteoporotic model could be explored, as the femoral LISS plate is often used in osteoporotic fractures.7,14
Conclusion
Overall, our study results showed that 1-cm variations, anterior or posterior, had little effect on axial or torsional stiffness or plastic deformation under cyclical axial loading. Although these data can be promising for clinical application, the anterior placement of the LISS plate noted in failed fixation in other studies necessitates cautious interpretation of this study. Our use of a 13-hole (longer) plate, versus the 9-hole plate used in other studies, could explain the lack of variation between the 2 groups as well as the stability and tolerance of inappropriate placement. An osteoporotic model could help clinicians further discern the importance of accurate proximal placement of the femoral LISS plate.
1. Bolhofner BR, Carmen B, Clifford P. The results of open reduction and internal fixation of distal femur fractures using a biologic (indirect) reduction technique. J Orthop Trauma. 1996;10(6):372-377.
2. Marti A, Frankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma. 2001;15(7):482-487.
3. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system. J Orthop Trauma. 2004;18(8):509-520.
4. Kolb W, Guhlmann H, Windisch C, Marx F, Kolb K, Koller H. Fixation of distal femoral fractures with the less invasive stabilization system: a minimally invasive treatment with locked fixed-angle screws. J Trauma. 2008;65(6):1425-1434.
5. Schütz M, Müller M, Krettek C, et al. Minimally invasive fracture stabilization of distal femoral fractures with the LISS: a prospective multicenter study. Results of a clinical study with special emphasis on difficult cases. Injury. 2001;32(suppl 3):SC55-SC63.
6. Chong AC, Friis EA, Ballard GP, Czuwala PJ, Cooke FW. Fatigue performance of composite analogue femur constructs under high activity loading. Ann Biomed Eng. 2007;35(7):1196-1205.
7. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
8. Khalafi A, Curtiss S, Hazelwood S, Wolinsky P. The effect of plate rotation on the stiffness of femoral LISS: a mechanical study. J Orthop Trauma. 2006;20(8):542-546.
9. Zdero R, Olsen M, Bougherara H, Schemitsch EH. Cancellous bone screw purchase: a comparison of synthetic femurs, human femurs, and finite element analysis. Proc Inst Mech Eng H. 2008;222(8):1175-1183.
10. Zlowodzki M, Williamson S, Cole PA, Zardiackas LD, Kregor PJ. Biomechanical evaluation of the less invasive stabilization system, angled blade plate, and retrograde intramedullary nail for the internal fixation of distal femur fractures. J Orthop Trauma. 2004;18(8):494-502.
11. Cordey J, Borgeaud M, Frankle, M, Harder Y, Martinet O. Loading model for the human femur taking the tension band effect of the ilio-tibial tract into account. Injury. 1999;30(suppl 1):A26-A30.
12. Weight M, Collinge C. Early results of the less invasive stabilization system for mechanically unstable fractures of the distal femur (AO/OTA types A2, A3, C2, and C3). J Orthop Trauma. 2004;18(8):503-508.
13. Otto RJ, Moed BR, Bledsoe JG. Biomechanical comparison of polyaxial-type locking plates and a fixed-angle locking plate for internal fixation of distal femur fractures. J Orthop Trauma. 2009;23(9):645–652.
14. Wong M, Leung F, Chow SP. Treatment of distal femoral fractures in the elderly using a less-invasive plating technique. Int Orthop. 2005;29(2):
117-120.
Several surgical options are available for treatment of supracondylar and intercondylar distal femur fractures, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 33. Preserving the osseous blood supply via indirect reduction techniques has been shown to increase union rates without the need for bone grafting.1,2 The Less-Invasive Stabilization System (LISS) made by Synthes (Paoli, Pennsylvania) melds minimally invasive internal fixation with multiple fixed-angle distal screws. It allows for submuscular placement, percutaneous unicortical screws in the diaphysis, and preservation of the metaphyseal fracture soft-tissue envelope.3
Proper lateral placement of the plate on the femur proximally can be difficult. Kregor and colleagues3 noted that 6% of cases did not have ideal placement on the lateral shaft of the femur when the 13-hole LISS plate was used. They advocated making a small incision at the proximal end of the LISS plate to aid in proper lateral placement. Kolb and colleagues4 noted that 2 of 31 patients had a “cutting out” of the proximal screws on LISS plates with anterior placement on the femur that eventually required repeat surgery in order to heal. This malpositioned plate was present at the end of the operation. These authors also recommended a proximal incision to avoid the issue. Schütz and colleagues5 noted that there were 4 cases of implant loosening among 107 distal femur fractures treated with LISS plating and that the unicortical screws in the diaphysis had loosened. They suggested anterior placement of the plate as a possible reason for fixation failure.
Although several studies have noted proximal screw pull-out, and proximal anterior malposition in the sagittal plane of the LISS plate has been suggested as a possible cause, we found no studies comparing incorrect proximal positioning on the femoral shaft with correct lateral placement of the LISS plate. Therefore, we used a previously established biomechanical model to compare LISS plates proximally placed either too anterior or too posterior to the direct lateral position on the femoral shaft. The constructs were tested in axial, torsional, and cyclical axial modes to assess plastic and total deformation, stiffness, and fixation failure.
Materials and Methods
Using fourth-generation femoral synthetic composite bones (Sawbones; Pacific Research Laboratories, Vashon, Washington) and a 13-hole Synthes femoral LISS plate, we made 3 groups of 9 specimens each, for a total of 27 femurs. The number of specimens was based on a power assessment in a study by Khalafi and colleagues.8 Several studies have validated use of Sawbones instead of cadavers in biomechanical testing to prevent variability.6-9 Proximal fixation was achieved with 5 unicortical screws (26 mm long) at screw holes 13, 11, 9, 7, and 4. All distal screw holes were filled for distal fixation with 75-mm-long screws to achieve bicortical fixation.
After application of the LISS plate, an AO/OTA 33-A3 fracture model was created in each specimen. A 1-cm gap was made 6 cm proximal to the intercondylar notch to create an unstable distal femur fracture pattern. In the method described by Zlowodzki and colleagues,10 an additional 3-cm cut was made diagonally in the medial cortex to prevent contact of the bone during mechanical testing.
Three different plate positions were used. The correct group was placed directly laterally proximally (Figure 1A). One incorrect group was plated with the proximal aspect of the plate 1 cm anterior (anterior group) (Figure 1B), and another incorrect group was plated with the proximal aspect of the plate 1 cm posterior (posterior group) (Figure 1C). Anterior or posterior plate placement resulted in some of the proximal screws having a more tangential placement, with fewer screws engaged compared with the properly placed plate.
The distal and proximal ends of each specimen were held to simulate the mechanical axis of the femur. This design was based on a model by Cordey and colleagues.11 A materials testing system (MTS, Minneapolis, Minnesota) was used for mechanical testing of the model.
Based on the protocol of Khalafi and colleagues,8 the models were tested in axial, torsional, and cyclical axial modes (Figures 2, 3). Axial loading consisted of a preload of 100 N followed by a compressive loading rate of 100 mm per minute in a displacement control mode. Testing was considered completed when 1 of 3 events occurred: 500 N was reached, the medial fracture gap closed, or fixation was lost. Torsional loading involved a preload of 5 Nm and subsequent torqueing at 20° per minute up to 20 Nm or loss of fixation or screw pull-out.8 Cyclical axial loading was based on protocols described by Marti and colleagues2 and Zlowodzki and colleagues.10 The initial load was 10 cycles of 300 N. Each subsequent load increment was increased by 100 N up to 1000 N, providing 10-second rest increments. This loading was conducted in a displacement control mode at 0.75 mm per second. Testing was aborted on fixation loss or complete closure of the medial fracture gap.
After testing was completed, statistically significant between-groups differences in plastic deformation and axial and torsional stiffness were determined by performing a Tukey-Kramer honestly significant difference test. Significance was set at P ≤ .05.
Results
During axial loading, there was no visual loss of fixation or change in displacement of the fracture gap for any group, and there was no screw cut-out or pull-out from the cortex during testing. In 1 plate in the posterior group, the most proximal screw made only loose contact with the cortex at only the distal portion of the screw. There was no significant difference (P = .9762) in stiffness in axial loading between the anterior group and the correct group. There was a significant (P = .0261) 16.4% increase in stiffness in the posterior group compared with the correct group (Table).
There was no screw cut-out, fixation failure, or change in displacement of the fracture gap for any group during torsional loading. There was a statistically significant (P = .0062) 12% increase in mean torsional stiffness in the anterior group compared with the correct group. There was no statistically significant difference (P = .1623) between the posterior group and the correct group (Table).
For cyclical axial testing, total deformation and plastic deformation were obtained by determining displacement under the initial 100 N load in the static/resting state. That number was then subtracted from maximum displacement, the peak value on the time-versus-displacement graph, to obtain the value for total deformation. Plastic deformation was calculated by subtracting initial displacement from final displacement in the static/resting state. The static/resting state is represented by the dips in displacement after each cycle on the time-versus-displacement graph (Figure 4).
There was a statistically significant (P = .0207) 14% increase in total deformation of the anteriorly positioned plate compared with the correctly positioned plate. There was no statistically significant difference in total deformation between the posteriorly placed plates and the correctly placed plates (Table).
There was no significant difference in plastic deformation between any of the groups in this study. There was no screw cut-out or fixation loss in any group to suggest a clinically relevant difference based on proximal placement of the LISS plate.
Discussion
In evaluating the stability of various constructs for fixation of distal femur fractures, the literature is consistent in reporting stiffness as the key factor. Stiffness is determined most often in terms of motion at the fracture site, as measured by displacement under axial and torsional loads.2,8,10,13 The LISS plate, which acts essentially as an “internal fixator” with proximal unicortical fixed-angle locking screws, has been shown to be comparable to other established methods of fixation.10,12 Zlowodzki and colleagues10 reported that the LISS plate had a higher load to failure when compared with angled blade plating and intramedullary nailing. Their study used fresh-frozen cadaver specimens from patients 70 years old or older. They concluded that, for distal femur fractures in osteoporotic bone, the LISS plate provided improved distal fixation.
In the present study, the posteriorly placed LISS plate outperformed the correctly placed plate in axial stiffness by 16.4%. However, there was no statistically significant difference in torsional stiffness and cyclical axial loading. This result is difficult to explain given that there was no screw cut-out or fixation loss for any of the constructs. Theoretically, with less proximal screw purchase in the posteriorly placed plate, the overall construct should be more susceptible to screw cut-out and fixation loss resulting in less axial stiffness overall.
Khalafi and colleagues8 created a distal femur fracture model using Sawbones with a 1-cm fracture gap. Using the 9-hole LISS plate for fixation, they tested this construct under axial, torsional, and cyclical axial loads. They tested 2 groups of 9 femurs. For group 1, the LISS plate was placed in the correct position on the distal femur, with the proximal end in the correct position on the femoral shaft. In group 2, the LISS plate was rotated 1 cm anteriorly. They found that axial stiffness (N/mm) was 21.5% greater in the correctly positioned plate. The anteriorly positioned group demonstrated 55% more irreversible or plastic deformation. The authors concluded that correct positioning of the femoral LISS plate provided improved mechanical stability.
Overall, our study results did not agree with those of Khalafi and colleagues8 in terms of the mechanical stability of a malpositioned LISS plate. Our construct showed a significant increase in torsional stiffness in the anteriorly placed plate. However, our construct also showed a significant increase in total deformation in cyclical axial loading in the anteriorly placed plate. There was no increased plastic deformation in either of the incorrectly placed groups in our study. The difference in results between studies can best be explained by the difference in plate lengths. We used a 13-hole plate, and Khalafi and colleagues8 used a 9-hole plate. Our theory is that the longer plate provided more resistance to relatively minor variations in plate position at the proximal end and thus resulted in less change in stiffness and stability around the fracture site.
Our model differed from that used in other biomechanical studies using Sawbones to simulate distal femur fractures in that it used the entire femur, including the proximal portion.8,13 This setup theoretically resulted in a more anatomical weight distribution compared with other models, in which the proximal portion of the femur was potted in polymethylmethacrylate. This difference in weight distribution could explain the variation in our results compared with other biomechanical studies. In addition, with use of different boundary conditions, the distal femur had unconstrained distal motion similar to the native environment of the femur.
This study had several limitations. First is its relatively low power (9 femurs per group). Although groups of 9 specimens in 2 groups were used in the study by Khalafi and colleagues8, testing a larger number of femurs could potentially identify more subtle differences between the 3 groups in our study. Second, given that femoral LISS plates come in different lengths, this study could be expanded to include the other plate sizes, as plate length could potentially play a role in stability at the fracture site. Third, though this Sawbones model has consistently reproduced the stability characteristics of human bone without variation between specimens, an osteoporotic model could be explored, as the femoral LISS plate is often used in osteoporotic fractures.7,14
Conclusion
Overall, our study results showed that 1-cm variations, anterior or posterior, had little effect on axial or torsional stiffness or plastic deformation under cyclical axial loading. Although these data can be promising for clinical application, the anterior placement of the LISS plate noted in failed fixation in other studies necessitates cautious interpretation of this study. Our use of a 13-hole (longer) plate, versus the 9-hole plate used in other studies, could explain the lack of variation between the 2 groups as well as the stability and tolerance of inappropriate placement. An osteoporotic model could help clinicians further discern the importance of accurate proximal placement of the femoral LISS plate.
Several surgical options are available for treatment of supracondylar and intercondylar distal femur fractures, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 33. Preserving the osseous blood supply via indirect reduction techniques has been shown to increase union rates without the need for bone grafting.1,2 The Less-Invasive Stabilization System (LISS) made by Synthes (Paoli, Pennsylvania) melds minimally invasive internal fixation with multiple fixed-angle distal screws. It allows for submuscular placement, percutaneous unicortical screws in the diaphysis, and preservation of the metaphyseal fracture soft-tissue envelope.3
Proper lateral placement of the plate on the femur proximally can be difficult. Kregor and colleagues3 noted that 6% of cases did not have ideal placement on the lateral shaft of the femur when the 13-hole LISS plate was used. They advocated making a small incision at the proximal end of the LISS plate to aid in proper lateral placement. Kolb and colleagues4 noted that 2 of 31 patients had a “cutting out” of the proximal screws on LISS plates with anterior placement on the femur that eventually required repeat surgery in order to heal. This malpositioned plate was present at the end of the operation. These authors also recommended a proximal incision to avoid the issue. Schütz and colleagues5 noted that there were 4 cases of implant loosening among 107 distal femur fractures treated with LISS plating and that the unicortical screws in the diaphysis had loosened. They suggested anterior placement of the plate as a possible reason for fixation failure.
Although several studies have noted proximal screw pull-out, and proximal anterior malposition in the sagittal plane of the LISS plate has been suggested as a possible cause, we found no studies comparing incorrect proximal positioning on the femoral shaft with correct lateral placement of the LISS plate. Therefore, we used a previously established biomechanical model to compare LISS plates proximally placed either too anterior or too posterior to the direct lateral position on the femoral shaft. The constructs were tested in axial, torsional, and cyclical axial modes to assess plastic and total deformation, stiffness, and fixation failure.
Materials and Methods
Using fourth-generation femoral synthetic composite bones (Sawbones; Pacific Research Laboratories, Vashon, Washington) and a 13-hole Synthes femoral LISS plate, we made 3 groups of 9 specimens each, for a total of 27 femurs. The number of specimens was based on a power assessment in a study by Khalafi and colleagues.8 Several studies have validated use of Sawbones instead of cadavers in biomechanical testing to prevent variability.6-9 Proximal fixation was achieved with 5 unicortical screws (26 mm long) at screw holes 13, 11, 9, 7, and 4. All distal screw holes were filled for distal fixation with 75-mm-long screws to achieve bicortical fixation.
After application of the LISS plate, an AO/OTA 33-A3 fracture model was created in each specimen. A 1-cm gap was made 6 cm proximal to the intercondylar notch to create an unstable distal femur fracture pattern. In the method described by Zlowodzki and colleagues,10 an additional 3-cm cut was made diagonally in the medial cortex to prevent contact of the bone during mechanical testing.
Three different plate positions were used. The correct group was placed directly laterally proximally (Figure 1A). One incorrect group was plated with the proximal aspect of the plate 1 cm anterior (anterior group) (Figure 1B), and another incorrect group was plated with the proximal aspect of the plate 1 cm posterior (posterior group) (Figure 1C). Anterior or posterior plate placement resulted in some of the proximal screws having a more tangential placement, with fewer screws engaged compared with the properly placed plate.
The distal and proximal ends of each specimen were held to simulate the mechanical axis of the femur. This design was based on a model by Cordey and colleagues.11 A materials testing system (MTS, Minneapolis, Minnesota) was used for mechanical testing of the model.
Based on the protocol of Khalafi and colleagues,8 the models were tested in axial, torsional, and cyclical axial modes (Figures 2, 3). Axial loading consisted of a preload of 100 N followed by a compressive loading rate of 100 mm per minute in a displacement control mode. Testing was considered completed when 1 of 3 events occurred: 500 N was reached, the medial fracture gap closed, or fixation was lost. Torsional loading involved a preload of 5 Nm and subsequent torqueing at 20° per minute up to 20 Nm or loss of fixation or screw pull-out.8 Cyclical axial loading was based on protocols described by Marti and colleagues2 and Zlowodzki and colleagues.10 The initial load was 10 cycles of 300 N. Each subsequent load increment was increased by 100 N up to 1000 N, providing 10-second rest increments. This loading was conducted in a displacement control mode at 0.75 mm per second. Testing was aborted on fixation loss or complete closure of the medial fracture gap.
After testing was completed, statistically significant between-groups differences in plastic deformation and axial and torsional stiffness were determined by performing a Tukey-Kramer honestly significant difference test. Significance was set at P ≤ .05.
Results
During axial loading, there was no visual loss of fixation or change in displacement of the fracture gap for any group, and there was no screw cut-out or pull-out from the cortex during testing. In 1 plate in the posterior group, the most proximal screw made only loose contact with the cortex at only the distal portion of the screw. There was no significant difference (P = .9762) in stiffness in axial loading between the anterior group and the correct group. There was a significant (P = .0261) 16.4% increase in stiffness in the posterior group compared with the correct group (Table).
There was no screw cut-out, fixation failure, or change in displacement of the fracture gap for any group during torsional loading. There was a statistically significant (P = .0062) 12% increase in mean torsional stiffness in the anterior group compared with the correct group. There was no statistically significant difference (P = .1623) between the posterior group and the correct group (Table).
For cyclical axial testing, total deformation and plastic deformation were obtained by determining displacement under the initial 100 N load in the static/resting state. That number was then subtracted from maximum displacement, the peak value on the time-versus-displacement graph, to obtain the value for total deformation. Plastic deformation was calculated by subtracting initial displacement from final displacement in the static/resting state. The static/resting state is represented by the dips in displacement after each cycle on the time-versus-displacement graph (Figure 4).
There was a statistically significant (P = .0207) 14% increase in total deformation of the anteriorly positioned plate compared with the correctly positioned plate. There was no statistically significant difference in total deformation between the posteriorly placed plates and the correctly placed plates (Table).
There was no significant difference in plastic deformation between any of the groups in this study. There was no screw cut-out or fixation loss in any group to suggest a clinically relevant difference based on proximal placement of the LISS plate.
Discussion
In evaluating the stability of various constructs for fixation of distal femur fractures, the literature is consistent in reporting stiffness as the key factor. Stiffness is determined most often in terms of motion at the fracture site, as measured by displacement under axial and torsional loads.2,8,10,13 The LISS plate, which acts essentially as an “internal fixator” with proximal unicortical fixed-angle locking screws, has been shown to be comparable to other established methods of fixation.10,12 Zlowodzki and colleagues10 reported that the LISS plate had a higher load to failure when compared with angled blade plating and intramedullary nailing. Their study used fresh-frozen cadaver specimens from patients 70 years old or older. They concluded that, for distal femur fractures in osteoporotic bone, the LISS plate provided improved distal fixation.
In the present study, the posteriorly placed LISS plate outperformed the correctly placed plate in axial stiffness by 16.4%. However, there was no statistically significant difference in torsional stiffness and cyclical axial loading. This result is difficult to explain given that there was no screw cut-out or fixation loss for any of the constructs. Theoretically, with less proximal screw purchase in the posteriorly placed plate, the overall construct should be more susceptible to screw cut-out and fixation loss resulting in less axial stiffness overall.
Khalafi and colleagues8 created a distal femur fracture model using Sawbones with a 1-cm fracture gap. Using the 9-hole LISS plate for fixation, they tested this construct under axial, torsional, and cyclical axial loads. They tested 2 groups of 9 femurs. For group 1, the LISS plate was placed in the correct position on the distal femur, with the proximal end in the correct position on the femoral shaft. In group 2, the LISS plate was rotated 1 cm anteriorly. They found that axial stiffness (N/mm) was 21.5% greater in the correctly positioned plate. The anteriorly positioned group demonstrated 55% more irreversible or plastic deformation. The authors concluded that correct positioning of the femoral LISS plate provided improved mechanical stability.
Overall, our study results did not agree with those of Khalafi and colleagues8 in terms of the mechanical stability of a malpositioned LISS plate. Our construct showed a significant increase in torsional stiffness in the anteriorly placed plate. However, our construct also showed a significant increase in total deformation in cyclical axial loading in the anteriorly placed plate. There was no increased plastic deformation in either of the incorrectly placed groups in our study. The difference in results between studies can best be explained by the difference in plate lengths. We used a 13-hole plate, and Khalafi and colleagues8 used a 9-hole plate. Our theory is that the longer plate provided more resistance to relatively minor variations in plate position at the proximal end and thus resulted in less change in stiffness and stability around the fracture site.
Our model differed from that used in other biomechanical studies using Sawbones to simulate distal femur fractures in that it used the entire femur, including the proximal portion.8,13 This setup theoretically resulted in a more anatomical weight distribution compared with other models, in which the proximal portion of the femur was potted in polymethylmethacrylate. This difference in weight distribution could explain the variation in our results compared with other biomechanical studies. In addition, with use of different boundary conditions, the distal femur had unconstrained distal motion similar to the native environment of the femur.
This study had several limitations. First is its relatively low power (9 femurs per group). Although groups of 9 specimens in 2 groups were used in the study by Khalafi and colleagues8, testing a larger number of femurs could potentially identify more subtle differences between the 3 groups in our study. Second, given that femoral LISS plates come in different lengths, this study could be expanded to include the other plate sizes, as plate length could potentially play a role in stability at the fracture site. Third, though this Sawbones model has consistently reproduced the stability characteristics of human bone without variation between specimens, an osteoporotic model could be explored, as the femoral LISS plate is often used in osteoporotic fractures.7,14
Conclusion
Overall, our study results showed that 1-cm variations, anterior or posterior, had little effect on axial or torsional stiffness or plastic deformation under cyclical axial loading. Although these data can be promising for clinical application, the anterior placement of the LISS plate noted in failed fixation in other studies necessitates cautious interpretation of this study. Our use of a 13-hole (longer) plate, versus the 9-hole plate used in other studies, could explain the lack of variation between the 2 groups as well as the stability and tolerance of inappropriate placement. An osteoporotic model could help clinicians further discern the importance of accurate proximal placement of the femoral LISS plate.
1. Bolhofner BR, Carmen B, Clifford P. The results of open reduction and internal fixation of distal femur fractures using a biologic (indirect) reduction technique. J Orthop Trauma. 1996;10(6):372-377.
2. Marti A, Frankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma. 2001;15(7):482-487.
3. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system. J Orthop Trauma. 2004;18(8):509-520.
4. Kolb W, Guhlmann H, Windisch C, Marx F, Kolb K, Koller H. Fixation of distal femoral fractures with the less invasive stabilization system: a minimally invasive treatment with locked fixed-angle screws. J Trauma. 2008;65(6):1425-1434.
5. Schütz M, Müller M, Krettek C, et al. Minimally invasive fracture stabilization of distal femoral fractures with the LISS: a prospective multicenter study. Results of a clinical study with special emphasis on difficult cases. Injury. 2001;32(suppl 3):SC55-SC63.
6. Chong AC, Friis EA, Ballard GP, Czuwala PJ, Cooke FW. Fatigue performance of composite analogue femur constructs under high activity loading. Ann Biomed Eng. 2007;35(7):1196-1205.
7. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
8. Khalafi A, Curtiss S, Hazelwood S, Wolinsky P. The effect of plate rotation on the stiffness of femoral LISS: a mechanical study. J Orthop Trauma. 2006;20(8):542-546.
9. Zdero R, Olsen M, Bougherara H, Schemitsch EH. Cancellous bone screw purchase: a comparison of synthetic femurs, human femurs, and finite element analysis. Proc Inst Mech Eng H. 2008;222(8):1175-1183.
10. Zlowodzki M, Williamson S, Cole PA, Zardiackas LD, Kregor PJ. Biomechanical evaluation of the less invasive stabilization system, angled blade plate, and retrograde intramedullary nail for the internal fixation of distal femur fractures. J Orthop Trauma. 2004;18(8):494-502.
11. Cordey J, Borgeaud M, Frankle, M, Harder Y, Martinet O. Loading model for the human femur taking the tension band effect of the ilio-tibial tract into account. Injury. 1999;30(suppl 1):A26-A30.
12. Weight M, Collinge C. Early results of the less invasive stabilization system for mechanically unstable fractures of the distal femur (AO/OTA types A2, A3, C2, and C3). J Orthop Trauma. 2004;18(8):503-508.
13. Otto RJ, Moed BR, Bledsoe JG. Biomechanical comparison of polyaxial-type locking plates and a fixed-angle locking plate for internal fixation of distal femur fractures. J Orthop Trauma. 2009;23(9):645–652.
14. Wong M, Leung F, Chow SP. Treatment of distal femoral fractures in the elderly using a less-invasive plating technique. Int Orthop. 2005;29(2):
117-120.
1. Bolhofner BR, Carmen B, Clifford P. The results of open reduction and internal fixation of distal femur fractures using a biologic (indirect) reduction technique. J Orthop Trauma. 1996;10(6):372-377.
2. Marti A, Frankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma. 2001;15(7):482-487.
3. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system. J Orthop Trauma. 2004;18(8):509-520.
4. Kolb W, Guhlmann H, Windisch C, Marx F, Kolb K, Koller H. Fixation of distal femoral fractures with the less invasive stabilization system: a minimally invasive treatment with locked fixed-angle screws. J Trauma. 2008;65(6):1425-1434.
5. Schütz M, Müller M, Krettek C, et al. Minimally invasive fracture stabilization of distal femoral fractures with the LISS: a prospective multicenter study. Results of a clinical study with special emphasis on difficult cases. Injury. 2001;32(suppl 3):SC55-SC63.
6. Chong AC, Friis EA, Ballard GP, Czuwala PJ, Cooke FW. Fatigue performance of composite analogue femur constructs under high activity loading. Ann Biomed Eng. 2007;35(7):1196-1205.
7. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
8. Khalafi A, Curtiss S, Hazelwood S, Wolinsky P. The effect of plate rotation on the stiffness of femoral LISS: a mechanical study. J Orthop Trauma. 2006;20(8):542-546.
9. Zdero R, Olsen M, Bougherara H, Schemitsch EH. Cancellous bone screw purchase: a comparison of synthetic femurs, human femurs, and finite element analysis. Proc Inst Mech Eng H. 2008;222(8):1175-1183.
10. Zlowodzki M, Williamson S, Cole PA, Zardiackas LD, Kregor PJ. Biomechanical evaluation of the less invasive stabilization system, angled blade plate, and retrograde intramedullary nail for the internal fixation of distal femur fractures. J Orthop Trauma. 2004;18(8):494-502.
11. Cordey J, Borgeaud M, Frankle, M, Harder Y, Martinet O. Loading model for the human femur taking the tension band effect of the ilio-tibial tract into account. Injury. 1999;30(suppl 1):A26-A30.
12. Weight M, Collinge C. Early results of the less invasive stabilization system for mechanically unstable fractures of the distal femur (AO/OTA types A2, A3, C2, and C3). J Orthop Trauma. 2004;18(8):503-508.
13. Otto RJ, Moed BR, Bledsoe JG. Biomechanical comparison of polyaxial-type locking plates and a fixed-angle locking plate for internal fixation of distal femur fractures. J Orthop Trauma. 2009;23(9):645–652.
14. Wong M, Leung F, Chow SP. Treatment of distal femoral fractures in the elderly using a less-invasive plating technique. Int Orthop. 2005;29(2):
117-120.
Antibiotic Cement-Coated Plates for Management of Infected Fractures
Deep infection in the presence of an implant after open reduction and internal fixation (ORIF) is usually treated with removal of the implant, serial débridement procedures, lavage, intravenously administered antibiotics, and, in some cases, placement of antibiotic-impregnated beads. If infection occurs during the early stages of bone healing, stabilization of the fractures might be compromised after removal of the implant. Although antibiotic-impregnated beads offer local delivery of antibiotics, they do not provide structural support of the fracture site. The beads often are difficult to remove after in-growth of granulation tissue. In areas of subcutaneous bone, an antibiotic bead pouch might be preferred to an open wound. Published research regarding the use of antibiotic-coated plates during the acute or chronic stages of infection is scarce. Plates offer the versatility of fracture stabilization, and the addition of antibiotic cement to the plates might aid in eradication of infection without necessitating a second surgery for removal. The patients provided written informed consent for print and electronic publication of these case reports.
Technique
After removal of implants, we perform débridement of the soft tissues with a hydroscalpel (Versajet; Smith & Nephew, London, United Kingdom), mechanical débridement of bone, and curettage with high speed burr. The wound is then irrigated with pulse pressure lavage and a minimum of 3 L sterile normal saline. The extremity is re-prepped and re-draped; the entire surgical team’s gowns and gloves are changed; and new instrumentation, including cautery and suction equipment, is used. The cement is prepared with tobramycin (3.6 g) and vancomycin (1 g) per 40-g bag of cement. The plate is placed in silicon tubing, and the antibiotic-prepared cement is injected into the tubing and molded until dry. Care is taken to mold the locations of the screw holes by making incisions in the tubing at the appropriate locations. Screws are placed through the screw holes to ensure locking capability, and Kirschner wires are placed through temporary fixation holes (Figure 1). Once dry, the screws and wires are removed from the plate, and the cement-coated plate is removed from the tubing. The antibiotic-coated plate is applied to the fracture or osteotomy site and is seated with screws as appropriate (Figure 2). The wound is closed primarily. Wound drains or vacuum-assisted closure devices are not routinely used unless there is high risk for hematoma formation. The authors prefer to have high local concentrations of antibiotic in the surrounding tissues and wound.
Clinical Series
Case 1
A 31-year-old man fell from a ladder and sustained a bimalleolar ankle fracture-dislocation that was treated with ORIF. Three weeks after initial injury, the patient presented with an infected lateral wound with purulent discharge. He was taken to the operating room for initial débridement, irrigation, and fracture stabilization with an antibiotic-coated plate and tension-band wiring of the medial malleoli. He was discharged from the hospital on day 4 after admission. Cultures of the wound grew beta-hemolytic strep group G and coagulase-negative staphylococci in broth that was sensitive to oxacillin, vancomycin, and gentamycin. The patient was treated with a 6-week regimen of Unasyn (Roerig, New York, New York), developed bony union, and has been free of clinical signs of infection for 2 years (Figures 3, 4).
Case 2
A 27-year-old male carpenter fell from a height of 12 feet and sustained a fracture of the distal radius that was treated with external fixation. The proximal pin site became clinically infected and subsequently developed osteomyelitis. The patient had a draining wound with a fracture for 2 months. He underwent débridement with partial resection of the radius and placement of an antibiotic cement–coated plate and calcium phosphate bone-void filler impregnated with antibiotics. Pathology specimens were positive for osteomyelitis, and bone cultures showed methicillin-sensitive Staphylococcus aureus (MSSA). He received intravenously administered antibiotic therapy for 6 weeks after surgery. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 5A, 5B).
Case 3
A 44-year-old woman with insulin-dependent diabetes mellitus and venous stasis sustained a trimalleolar ankle fracture after a low-energy fall that was initially treated with ORIF. She underwent revision ORIF to treat a malunion 3 months after initial treatment. At 8 months, the patient developed a draining sinus communicating with the plate. Computed tomography revealed nonunion and indicated infection. The patient underwent resection of the osteomyelitis and repair of the fibular nonunion with an antibiotic-coated plate. Tissue cultures were positive for coagulase-negative staphylococcus, and pathology specimens were positive for osteomyelitis. She received postoperative antibiotics intravenously and 6 weeks of antibiotic therapy after discharge from the hospital. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 6, 7).
Case 4
A 48-year-old man sustained an open olecranon fracture in another country. The fracture was initially treated with 1 dose of intravenously administered antibiotics and 5 days of orally administered antibiotics. The patient returned to the United States and was treated with intravenously administered antibiotics for cellulitis of the elbow for 11 days before referral to our institution, where he underwent ORIF with placement of an antibiotic-coated plate and tension-band wiring. Soft-tissue and bone cultures had no growth. He received intravenously administered antibiotics for 6 weeks. At 5 months postoperatively, the plate was removed because of pain. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 8A-8C).
Discussion
Acute infections of fractures have recently been treated with success by Berkes and colleagues,1 who reported a 71% union rate achieved with operative débridement, antibiotic suppression, and retention of fixation until fracture union occurs. The study by Berkes and colleagues1 had a small patient population, and larger cohorts are needed to show more reliable results; however, this treatment maintains structural support for the fracture during healing but requires multiple trips to the operating room for débridements as well as the use of systemic intravenous antibiotic therapy.
A technique that was developed by the primary author (Janet D. Conway, MD) and has not been described in the literature allows for use of antibiotic cement–coated plates to treat early postoperative infections and osteomyelitic nonunions. This approach permits fracture stabilization and local delivery of high concentrations of broad-spectrum antibiotics and can reduce the number of débridement procedures required in the operating room. We present a technique that includes the use of antibiotic cement–coated plates to treat early postoperative infections associated with fractures and nonunions in order to provide eradication of infection and bony stabilization.
Our approach parallels the current theory that treating infection at a site of union is preferable to treating infection at a site of nonunion.1 Fixation devices should remain in place until osseous union is achieved. With the addition of antibiotics to the plate, removal might not be necessary unless a device is loose, nonfunctional, or, ultimately, causing pain. Other options, such as external fixation, can be burdensome to patients and can be associated with other risks. One of our 4 patients required fixation removal because of pain at the elbow; however, even noncoated olecranon plates typically are removed because of pain after fracture healing. Antibiotic cement adds bulk to the construct and can become very prominent in areas of little soft-tissue coverage (Figure 9).
Studies, assessing variables that correlate with higher likelihood of failure for primary repairs, have shown that open fracture, use of an intramedullary nail, and smoking are the highest risk factors for infected nonunion.1−4 Among our 4 patients, 3 were smokers and 1 originally had an open fracture. Smokers have been found to have a 37% higher nonunion rate and are 2 times more likely to develop wound infection and osteomyelitis.1,5 More than 60% of the time, infections are caused by S aureus or coagulase-negative staphylococci.1,5,6 In our study population, 3 of the 4 patients had coagulase-negative staphylococci grow in the cultures. Implants infected with S aureus or Candida require surgical removal. Those with less virulent coagulase-negative staphylococci might not necessitate removal; however, our population had had antibiotic therapy and continued draining sinus.5 Rightmire and colleagues7 reported that those who develop infection earlier than 16 weeks postoperatively have a 68% success rate and that smoking is a major risk factor for infection. Development of Pseudomonas in the wound has been shown to have a positive correlation with amputation.1,2 Infection with Pseudomonas, smoking, and involvement of the femur, tibia, ankle, or foot tended to result in failure.1,2 Being clinically free of signs of infection after 3 months offers a 50% cure rate, with 78% at 6 months and 95% after 1 year.2
When determining an antibiotic to use with the polymethylmethacrylate (PMMA) cement, many factors must be considered, including spectrum, heat stability, and elution characteristics.8 A synergistic effect has been seen with combinations of antibiotics (eg, vancomycin and tobramycin used together). Vancomycin concentrations increased by 103% and tobramycin by 68% when used together compared with their elution rates when used alone, showing passive opportunism.9 This will, in essence, increase concentrations of antibiotics at the site locally, which will increase the bacteriocidal potential but also create a larger antimicrobial spectrum.9
The authors used Cobalt Bone Cement (Biomet Orthopedics, Inc, Warsaw, Indiana) which been shown to have higher elution properties than Simplex P Bone Cement (Stryker, Kalamazoo, Michigan).3,10 The majority of elution occurs in the first 3 to 5 days but can continue for weeks after implantation. We place the cement on the plate allowing for its retention, hoping to eliminate a second surgery for removal.8 We recommend 3.6 g of tobramycin, and 1 g of vancomycin per 40-g bag of PMMA.3 This dose has been shown to be safe in respect to renal toxicity, plus the entire dose is not administered in a single setting because only a small portion of the cement is used when coating the plate. We close all wounds primarily, and do not regularly use drains or vacuum-assisted closures to help prevent a decrease in the local concentration of the antibiotics.11
Broad-spectrum antibiotics are used to coat the plate in order to cover as many microbial organisms as possible without knowing the final offending organism. In our experience, this current technique provides antibiotic delivery with bony stability, therefore eliminating the need for multiple sequential surgical procedures. This difficult patient problem does not occur with enough frequency to warrant a large randomized clinical trial. However, this technique has been effective in these cases and may be useful to orthopedic surgeons in the future.
Conclusion
Based on our experience, early aggressive débridement, coupled with broad-spectrum antibiotic cement–coated plate insertion, provides fracture stability and helps eradicate the infection with 1 surgical procedure.
1. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823-828.
2. Tice AD, Hoaglund PA, Shoultz, DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261-1268.
3. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417-427.
4. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
5. Liporace FA, Yoon RS, Frank MA, et al. Use of an “antibiotic plate” for infected periprosthetic fracture in total hip arthroplasty. J Orthop Trauma. 2012;26(3):e18-e23.
6. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429.
7. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466(2):466-472.
8. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244-252.
9. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944.
10. Greene N, Holtom PD, Warren CA, et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201-205.
11. Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother. 2012;46(7-8):929-934.
Deep infection in the presence of an implant after open reduction and internal fixation (ORIF) is usually treated with removal of the implant, serial débridement procedures, lavage, intravenously administered antibiotics, and, in some cases, placement of antibiotic-impregnated beads. If infection occurs during the early stages of bone healing, stabilization of the fractures might be compromised after removal of the implant. Although antibiotic-impregnated beads offer local delivery of antibiotics, they do not provide structural support of the fracture site. The beads often are difficult to remove after in-growth of granulation tissue. In areas of subcutaneous bone, an antibiotic bead pouch might be preferred to an open wound. Published research regarding the use of antibiotic-coated plates during the acute or chronic stages of infection is scarce. Plates offer the versatility of fracture stabilization, and the addition of antibiotic cement to the plates might aid in eradication of infection without necessitating a second surgery for removal. The patients provided written informed consent for print and electronic publication of these case reports.
Technique
After removal of implants, we perform débridement of the soft tissues with a hydroscalpel (Versajet; Smith & Nephew, London, United Kingdom), mechanical débridement of bone, and curettage with high speed burr. The wound is then irrigated with pulse pressure lavage and a minimum of 3 L sterile normal saline. The extremity is re-prepped and re-draped; the entire surgical team’s gowns and gloves are changed; and new instrumentation, including cautery and suction equipment, is used. The cement is prepared with tobramycin (3.6 g) and vancomycin (1 g) per 40-g bag of cement. The plate is placed in silicon tubing, and the antibiotic-prepared cement is injected into the tubing and molded until dry. Care is taken to mold the locations of the screw holes by making incisions in the tubing at the appropriate locations. Screws are placed through the screw holes to ensure locking capability, and Kirschner wires are placed through temporary fixation holes (Figure 1). Once dry, the screws and wires are removed from the plate, and the cement-coated plate is removed from the tubing. The antibiotic-coated plate is applied to the fracture or osteotomy site and is seated with screws as appropriate (Figure 2). The wound is closed primarily. Wound drains or vacuum-assisted closure devices are not routinely used unless there is high risk for hematoma formation. The authors prefer to have high local concentrations of antibiotic in the surrounding tissues and wound.
Clinical Series
Case 1
A 31-year-old man fell from a ladder and sustained a bimalleolar ankle fracture-dislocation that was treated with ORIF. Three weeks after initial injury, the patient presented with an infected lateral wound with purulent discharge. He was taken to the operating room for initial débridement, irrigation, and fracture stabilization with an antibiotic-coated plate and tension-band wiring of the medial malleoli. He was discharged from the hospital on day 4 after admission. Cultures of the wound grew beta-hemolytic strep group G and coagulase-negative staphylococci in broth that was sensitive to oxacillin, vancomycin, and gentamycin. The patient was treated with a 6-week regimen of Unasyn (Roerig, New York, New York), developed bony union, and has been free of clinical signs of infection for 2 years (Figures 3, 4).
Case 2
A 27-year-old male carpenter fell from a height of 12 feet and sustained a fracture of the distal radius that was treated with external fixation. The proximal pin site became clinically infected and subsequently developed osteomyelitis. The patient had a draining wound with a fracture for 2 months. He underwent débridement with partial resection of the radius and placement of an antibiotic cement–coated plate and calcium phosphate bone-void filler impregnated with antibiotics. Pathology specimens were positive for osteomyelitis, and bone cultures showed methicillin-sensitive Staphylococcus aureus (MSSA). He received intravenously administered antibiotic therapy for 6 weeks after surgery. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 5A, 5B).
Case 3
A 44-year-old woman with insulin-dependent diabetes mellitus and venous stasis sustained a trimalleolar ankle fracture after a low-energy fall that was initially treated with ORIF. She underwent revision ORIF to treat a malunion 3 months after initial treatment. At 8 months, the patient developed a draining sinus communicating with the plate. Computed tomography revealed nonunion and indicated infection. The patient underwent resection of the osteomyelitis and repair of the fibular nonunion with an antibiotic-coated plate. Tissue cultures were positive for coagulase-negative staphylococcus, and pathology specimens were positive for osteomyelitis. She received postoperative antibiotics intravenously and 6 weeks of antibiotic therapy after discharge from the hospital. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 6, 7).
Case 4
A 48-year-old man sustained an open olecranon fracture in another country. The fracture was initially treated with 1 dose of intravenously administered antibiotics and 5 days of orally administered antibiotics. The patient returned to the United States and was treated with intravenously administered antibiotics for cellulitis of the elbow for 11 days before referral to our institution, where he underwent ORIF with placement of an antibiotic-coated plate and tension-band wiring. Soft-tissue and bone cultures had no growth. He received intravenously administered antibiotics for 6 weeks. At 5 months postoperatively, the plate was removed because of pain. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 8A-8C).
Discussion
Acute infections of fractures have recently been treated with success by Berkes and colleagues,1 who reported a 71% union rate achieved with operative débridement, antibiotic suppression, and retention of fixation until fracture union occurs. The study by Berkes and colleagues1 had a small patient population, and larger cohorts are needed to show more reliable results; however, this treatment maintains structural support for the fracture during healing but requires multiple trips to the operating room for débridements as well as the use of systemic intravenous antibiotic therapy.
A technique that was developed by the primary author (Janet D. Conway, MD) and has not been described in the literature allows for use of antibiotic cement–coated plates to treat early postoperative infections and osteomyelitic nonunions. This approach permits fracture stabilization and local delivery of high concentrations of broad-spectrum antibiotics and can reduce the number of débridement procedures required in the operating room. We present a technique that includes the use of antibiotic cement–coated plates to treat early postoperative infections associated with fractures and nonunions in order to provide eradication of infection and bony stabilization.
Our approach parallels the current theory that treating infection at a site of union is preferable to treating infection at a site of nonunion.1 Fixation devices should remain in place until osseous union is achieved. With the addition of antibiotics to the plate, removal might not be necessary unless a device is loose, nonfunctional, or, ultimately, causing pain. Other options, such as external fixation, can be burdensome to patients and can be associated with other risks. One of our 4 patients required fixation removal because of pain at the elbow; however, even noncoated olecranon plates typically are removed because of pain after fracture healing. Antibiotic cement adds bulk to the construct and can become very prominent in areas of little soft-tissue coverage (Figure 9).
Studies, assessing variables that correlate with higher likelihood of failure for primary repairs, have shown that open fracture, use of an intramedullary nail, and smoking are the highest risk factors for infected nonunion.1−4 Among our 4 patients, 3 were smokers and 1 originally had an open fracture. Smokers have been found to have a 37% higher nonunion rate and are 2 times more likely to develop wound infection and osteomyelitis.1,5 More than 60% of the time, infections are caused by S aureus or coagulase-negative staphylococci.1,5,6 In our study population, 3 of the 4 patients had coagulase-negative staphylococci grow in the cultures. Implants infected with S aureus or Candida require surgical removal. Those with less virulent coagulase-negative staphylococci might not necessitate removal; however, our population had had antibiotic therapy and continued draining sinus.5 Rightmire and colleagues7 reported that those who develop infection earlier than 16 weeks postoperatively have a 68% success rate and that smoking is a major risk factor for infection. Development of Pseudomonas in the wound has been shown to have a positive correlation with amputation.1,2 Infection with Pseudomonas, smoking, and involvement of the femur, tibia, ankle, or foot tended to result in failure.1,2 Being clinically free of signs of infection after 3 months offers a 50% cure rate, with 78% at 6 months and 95% after 1 year.2
When determining an antibiotic to use with the polymethylmethacrylate (PMMA) cement, many factors must be considered, including spectrum, heat stability, and elution characteristics.8 A synergistic effect has been seen with combinations of antibiotics (eg, vancomycin and tobramycin used together). Vancomycin concentrations increased by 103% and tobramycin by 68% when used together compared with their elution rates when used alone, showing passive opportunism.9 This will, in essence, increase concentrations of antibiotics at the site locally, which will increase the bacteriocidal potential but also create a larger antimicrobial spectrum.9
The authors used Cobalt Bone Cement (Biomet Orthopedics, Inc, Warsaw, Indiana) which been shown to have higher elution properties than Simplex P Bone Cement (Stryker, Kalamazoo, Michigan).3,10 The majority of elution occurs in the first 3 to 5 days but can continue for weeks after implantation. We place the cement on the plate allowing for its retention, hoping to eliminate a second surgery for removal.8 We recommend 3.6 g of tobramycin, and 1 g of vancomycin per 40-g bag of PMMA.3 This dose has been shown to be safe in respect to renal toxicity, plus the entire dose is not administered in a single setting because only a small portion of the cement is used when coating the plate. We close all wounds primarily, and do not regularly use drains or vacuum-assisted closures to help prevent a decrease in the local concentration of the antibiotics.11
Broad-spectrum antibiotics are used to coat the plate in order to cover as many microbial organisms as possible without knowing the final offending organism. In our experience, this current technique provides antibiotic delivery with bony stability, therefore eliminating the need for multiple sequential surgical procedures. This difficult patient problem does not occur with enough frequency to warrant a large randomized clinical trial. However, this technique has been effective in these cases and may be useful to orthopedic surgeons in the future.
Conclusion
Based on our experience, early aggressive débridement, coupled with broad-spectrum antibiotic cement–coated plate insertion, provides fracture stability and helps eradicate the infection with 1 surgical procedure.
Deep infection in the presence of an implant after open reduction and internal fixation (ORIF) is usually treated with removal of the implant, serial débridement procedures, lavage, intravenously administered antibiotics, and, in some cases, placement of antibiotic-impregnated beads. If infection occurs during the early stages of bone healing, stabilization of the fractures might be compromised after removal of the implant. Although antibiotic-impregnated beads offer local delivery of antibiotics, they do not provide structural support of the fracture site. The beads often are difficult to remove after in-growth of granulation tissue. In areas of subcutaneous bone, an antibiotic bead pouch might be preferred to an open wound. Published research regarding the use of antibiotic-coated plates during the acute or chronic stages of infection is scarce. Plates offer the versatility of fracture stabilization, and the addition of antibiotic cement to the plates might aid in eradication of infection without necessitating a second surgery for removal. The patients provided written informed consent for print and electronic publication of these case reports.
Technique
After removal of implants, we perform débridement of the soft tissues with a hydroscalpel (Versajet; Smith & Nephew, London, United Kingdom), mechanical débridement of bone, and curettage with high speed burr. The wound is then irrigated with pulse pressure lavage and a minimum of 3 L sterile normal saline. The extremity is re-prepped and re-draped; the entire surgical team’s gowns and gloves are changed; and new instrumentation, including cautery and suction equipment, is used. The cement is prepared with tobramycin (3.6 g) and vancomycin (1 g) per 40-g bag of cement. The plate is placed in silicon tubing, and the antibiotic-prepared cement is injected into the tubing and molded until dry. Care is taken to mold the locations of the screw holes by making incisions in the tubing at the appropriate locations. Screws are placed through the screw holes to ensure locking capability, and Kirschner wires are placed through temporary fixation holes (Figure 1). Once dry, the screws and wires are removed from the plate, and the cement-coated plate is removed from the tubing. The antibiotic-coated plate is applied to the fracture or osteotomy site and is seated with screws as appropriate (Figure 2). The wound is closed primarily. Wound drains or vacuum-assisted closure devices are not routinely used unless there is high risk for hematoma formation. The authors prefer to have high local concentrations of antibiotic in the surrounding tissues and wound.
Clinical Series
Case 1
A 31-year-old man fell from a ladder and sustained a bimalleolar ankle fracture-dislocation that was treated with ORIF. Three weeks after initial injury, the patient presented with an infected lateral wound with purulent discharge. He was taken to the operating room for initial débridement, irrigation, and fracture stabilization with an antibiotic-coated plate and tension-band wiring of the medial malleoli. He was discharged from the hospital on day 4 after admission. Cultures of the wound grew beta-hemolytic strep group G and coagulase-negative staphylococci in broth that was sensitive to oxacillin, vancomycin, and gentamycin. The patient was treated with a 6-week regimen of Unasyn (Roerig, New York, New York), developed bony union, and has been free of clinical signs of infection for 2 years (Figures 3, 4).
Case 2
A 27-year-old male carpenter fell from a height of 12 feet and sustained a fracture of the distal radius that was treated with external fixation. The proximal pin site became clinically infected and subsequently developed osteomyelitis. The patient had a draining wound with a fracture for 2 months. He underwent débridement with partial resection of the radius and placement of an antibiotic cement–coated plate and calcium phosphate bone-void filler impregnated with antibiotics. Pathology specimens were positive for osteomyelitis, and bone cultures showed methicillin-sensitive Staphylococcus aureus (MSSA). He received intravenously administered antibiotic therapy for 6 weeks after surgery. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 5A, 5B).
Case 3
A 44-year-old woman with insulin-dependent diabetes mellitus and venous stasis sustained a trimalleolar ankle fracture after a low-energy fall that was initially treated with ORIF. She underwent revision ORIF to treat a malunion 3 months after initial treatment. At 8 months, the patient developed a draining sinus communicating with the plate. Computed tomography revealed nonunion and indicated infection. The patient underwent resection of the osteomyelitis and repair of the fibular nonunion with an antibiotic-coated plate. Tissue cultures were positive for coagulase-negative staphylococcus, and pathology specimens were positive for osteomyelitis. She received postoperative antibiotics intravenously and 6 weeks of antibiotic therapy after discharge from the hospital. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 6, 7).
Case 4
A 48-year-old man sustained an open olecranon fracture in another country. The fracture was initially treated with 1 dose of intravenously administered antibiotics and 5 days of orally administered antibiotics. The patient returned to the United States and was treated with intravenously administered antibiotics for cellulitis of the elbow for 11 days before referral to our institution, where he underwent ORIF with placement of an antibiotic-coated plate and tension-band wiring. Soft-tissue and bone cultures had no growth. He received intravenously administered antibiotics for 6 weeks. At 5 months postoperatively, the plate was removed because of pain. The patient has remained free of clinical signs of infection for more than 1 year and has achieved bony union (Figures 8A-8C).
Discussion
Acute infections of fractures have recently been treated with success by Berkes and colleagues,1 who reported a 71% union rate achieved with operative débridement, antibiotic suppression, and retention of fixation until fracture union occurs. The study by Berkes and colleagues1 had a small patient population, and larger cohorts are needed to show more reliable results; however, this treatment maintains structural support for the fracture during healing but requires multiple trips to the operating room for débridements as well as the use of systemic intravenous antibiotic therapy.
A technique that was developed by the primary author (Janet D. Conway, MD) and has not been described in the literature allows for use of antibiotic cement–coated plates to treat early postoperative infections and osteomyelitic nonunions. This approach permits fracture stabilization and local delivery of high concentrations of broad-spectrum antibiotics and can reduce the number of débridement procedures required in the operating room. We present a technique that includes the use of antibiotic cement–coated plates to treat early postoperative infections associated with fractures and nonunions in order to provide eradication of infection and bony stabilization.
Our approach parallels the current theory that treating infection at a site of union is preferable to treating infection at a site of nonunion.1 Fixation devices should remain in place until osseous union is achieved. With the addition of antibiotics to the plate, removal might not be necessary unless a device is loose, nonfunctional, or, ultimately, causing pain. Other options, such as external fixation, can be burdensome to patients and can be associated with other risks. One of our 4 patients required fixation removal because of pain at the elbow; however, even noncoated olecranon plates typically are removed because of pain after fracture healing. Antibiotic cement adds bulk to the construct and can become very prominent in areas of little soft-tissue coverage (Figure 9).
Studies, assessing variables that correlate with higher likelihood of failure for primary repairs, have shown that open fracture, use of an intramedullary nail, and smoking are the highest risk factors for infected nonunion.1−4 Among our 4 patients, 3 were smokers and 1 originally had an open fracture. Smokers have been found to have a 37% higher nonunion rate and are 2 times more likely to develop wound infection and osteomyelitis.1,5 More than 60% of the time, infections are caused by S aureus or coagulase-negative staphylococci.1,5,6 In our study population, 3 of the 4 patients had coagulase-negative staphylococci grow in the cultures. Implants infected with S aureus or Candida require surgical removal. Those with less virulent coagulase-negative staphylococci might not necessitate removal; however, our population had had antibiotic therapy and continued draining sinus.5 Rightmire and colleagues7 reported that those who develop infection earlier than 16 weeks postoperatively have a 68% success rate and that smoking is a major risk factor for infection. Development of Pseudomonas in the wound has been shown to have a positive correlation with amputation.1,2 Infection with Pseudomonas, smoking, and involvement of the femur, tibia, ankle, or foot tended to result in failure.1,2 Being clinically free of signs of infection after 3 months offers a 50% cure rate, with 78% at 6 months and 95% after 1 year.2
When determining an antibiotic to use with the polymethylmethacrylate (PMMA) cement, many factors must be considered, including spectrum, heat stability, and elution characteristics.8 A synergistic effect has been seen with combinations of antibiotics (eg, vancomycin and tobramycin used together). Vancomycin concentrations increased by 103% and tobramycin by 68% when used together compared with their elution rates when used alone, showing passive opportunism.9 This will, in essence, increase concentrations of antibiotics at the site locally, which will increase the bacteriocidal potential but also create a larger antimicrobial spectrum.9
The authors used Cobalt Bone Cement (Biomet Orthopedics, Inc, Warsaw, Indiana) which been shown to have higher elution properties than Simplex P Bone Cement (Stryker, Kalamazoo, Michigan).3,10 The majority of elution occurs in the first 3 to 5 days but can continue for weeks after implantation. We place the cement on the plate allowing for its retention, hoping to eliminate a second surgery for removal.8 We recommend 3.6 g of tobramycin, and 1 g of vancomycin per 40-g bag of PMMA.3 This dose has been shown to be safe in respect to renal toxicity, plus the entire dose is not administered in a single setting because only a small portion of the cement is used when coating the plate. We close all wounds primarily, and do not regularly use drains or vacuum-assisted closures to help prevent a decrease in the local concentration of the antibiotics.11
Broad-spectrum antibiotics are used to coat the plate in order to cover as many microbial organisms as possible without knowing the final offending organism. In our experience, this current technique provides antibiotic delivery with bony stability, therefore eliminating the need for multiple sequential surgical procedures. This difficult patient problem does not occur with enough frequency to warrant a large randomized clinical trial. However, this technique has been effective in these cases and may be useful to orthopedic surgeons in the future.
Conclusion
Based on our experience, early aggressive débridement, coupled with broad-spectrum antibiotic cement–coated plate insertion, provides fracture stability and helps eradicate the infection with 1 surgical procedure.
1. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823-828.
2. Tice AD, Hoaglund PA, Shoultz, DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261-1268.
3. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417-427.
4. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
5. Liporace FA, Yoon RS, Frank MA, et al. Use of an “antibiotic plate” for infected periprosthetic fracture in total hip arthroplasty. J Orthop Trauma. 2012;26(3):e18-e23.
6. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429.
7. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466(2):466-472.
8. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244-252.
9. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944.
10. Greene N, Holtom PD, Warren CA, et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201-205.
11. Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother. 2012;46(7-8):929-934.
1. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92(4):823-828.
2. Tice AD, Hoaglund PA, Shoultz, DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother. 2003;51(5):1261-1268.
3. Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417-427.
4. Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM; LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151-157.
5. Liporace FA, Yoon RS, Frank MA, et al. Use of an “antibiotic plate” for infected periprosthetic fracture in total hip arthroplasty. J Orthop Trauma. 2012;26(3):e18-e23.
6. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429.
7. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466(2):466-472.
8. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop. 1992;(278):244-252.
9. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11(8):939-944.
10. Greene N, Holtom PD, Warren CA, et al. In vitro elution of tobramycin and vancomycin polymethylmethacrylate beads and spacers from Simplex and Palacos. Am J Orthop. 1998;27(3):201-205.
11. Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother. 2012;46(7-8):929-934.
Mycobacterium bovis Infection of Total Knee Arthroplasty After Bacillus Calmette-Guérin Therapy for Bladder Cancer
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.