User login
For MD-IQ only
BCR is unreliable surrogate for overall survival in prostate cancer
TOPLINE
METHODOLOGY
- In trials of localized prostate cancer, BCR remains a controversial surrogate endpoint for overall survival.
- The meta-analysis included 10,741 patients from 11 randomized clinical trials; the median follow-up was 9.2 years.
- Interventions included radiotherapy dose escalation, in which high-dose radiotherapy was compared with conventional radiotherapy (n = 3,639); short-term androgen deprivation therapy (ADT), in which radiotherapy plus short-term ADT was compared with radiotherapy alone (n = 3,930); and ADT prolongation, in which radiotherapy plus long-term ADT was compared with radiotherapy plus short-term ADT (n = 3,772).
- Prentice criteria and the two-stage meta-analytic approach were used to assess BCR as a surrogate endpoint for overall survival.
- The researchers assessed the treatment effect on BCR and on overall survival.
TAKEAWAY
- With regard to treatment effect on BCR, the three interventions significantly reduced BCR risk – dose escalation by 29%, short-term ADT by 47%, and ADT prolongation by 46%. With regard to survival, only short- and long-term ADT significantly improved overall survival, by 9% and 14%, respectively.
- At 48 months, BCR was associated with significantly increased mortality risk: 2.46-fold increased risk for dose escalation, 1.51-fold greater risk for short-term ADT, and 2.31-fold higher risk for ADT prolongation.
- However, after adjusting for BCR at 48 months, there was no significant treatment effect on overall survival (hazard ratio, 1.10; [95% confidence interval, 0.96-1.27]; HR, 0.96 [95% CI, 0.87-1.06]; HR, 1.00 [95% CI, 0.90-1.12], respectively).
- Patient-level correlation between time to BCR and overall survival was low after censoring for noncancer-related deaths. The correlation between BCR-free survival and overall survival ranged from low to moderate.
IN PRACTICE
Overall, “these results strongly suggest that BCR-based endpoints should not be the primary endpoint in randomized trials conducted for localized [prostate cancer],” the authors concluded. They added that metastasis-free survival represents a more appropriate measure.
SOURCE
The study was led by senior author Amar Kishan, MD, of the University of California, Los Angeles, and was published online in the Journal of Clinical Oncology.
LIMITATIONS
- The trials used different definitions of BCR – the older American Society of Therapeutic Radiation and Oncology definition, and the more current Phoenix criteria.
- Some trials were conducted more than 20 years ago, and a variety of factors, including patient selection, staging, diagnostic criteria, and therapeutic approaches, have evolved in that time.
- Quality of life was not captured.
DISCLOSURES
The study received support from Cancer Research UK, the UK National Health Service, the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence, the UK Department of Defense, the Prostate Cancer Foundation, and the American Society for Radiation Oncology. Authors’ relevant financial relationships are detailed in the published study.
A version of this article appeared on Medscape.com.
TOPLINE
METHODOLOGY
- In trials of localized prostate cancer, BCR remains a controversial surrogate endpoint for overall survival.
- The meta-analysis included 10,741 patients from 11 randomized clinical trials; the median follow-up was 9.2 years.
- Interventions included radiotherapy dose escalation, in which high-dose radiotherapy was compared with conventional radiotherapy (n = 3,639); short-term androgen deprivation therapy (ADT), in which radiotherapy plus short-term ADT was compared with radiotherapy alone (n = 3,930); and ADT prolongation, in which radiotherapy plus long-term ADT was compared with radiotherapy plus short-term ADT (n = 3,772).
- Prentice criteria and the two-stage meta-analytic approach were used to assess BCR as a surrogate endpoint for overall survival.
- The researchers assessed the treatment effect on BCR and on overall survival.
TAKEAWAY
- With regard to treatment effect on BCR, the three interventions significantly reduced BCR risk – dose escalation by 29%, short-term ADT by 47%, and ADT prolongation by 46%. With regard to survival, only short- and long-term ADT significantly improved overall survival, by 9% and 14%, respectively.
- At 48 months, BCR was associated with significantly increased mortality risk: 2.46-fold increased risk for dose escalation, 1.51-fold greater risk for short-term ADT, and 2.31-fold higher risk for ADT prolongation.
- However, after adjusting for BCR at 48 months, there was no significant treatment effect on overall survival (hazard ratio, 1.10; [95% confidence interval, 0.96-1.27]; HR, 0.96 [95% CI, 0.87-1.06]; HR, 1.00 [95% CI, 0.90-1.12], respectively).
- Patient-level correlation between time to BCR and overall survival was low after censoring for noncancer-related deaths. The correlation between BCR-free survival and overall survival ranged from low to moderate.
IN PRACTICE
Overall, “these results strongly suggest that BCR-based endpoints should not be the primary endpoint in randomized trials conducted for localized [prostate cancer],” the authors concluded. They added that metastasis-free survival represents a more appropriate measure.
SOURCE
The study was led by senior author Amar Kishan, MD, of the University of California, Los Angeles, and was published online in the Journal of Clinical Oncology.
LIMITATIONS
- The trials used different definitions of BCR – the older American Society of Therapeutic Radiation and Oncology definition, and the more current Phoenix criteria.
- Some trials were conducted more than 20 years ago, and a variety of factors, including patient selection, staging, diagnostic criteria, and therapeutic approaches, have evolved in that time.
- Quality of life was not captured.
DISCLOSURES
The study received support from Cancer Research UK, the UK National Health Service, the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence, the UK Department of Defense, the Prostate Cancer Foundation, and the American Society for Radiation Oncology. Authors’ relevant financial relationships are detailed in the published study.
A version of this article appeared on Medscape.com.
TOPLINE
METHODOLOGY
- In trials of localized prostate cancer, BCR remains a controversial surrogate endpoint for overall survival.
- The meta-analysis included 10,741 patients from 11 randomized clinical trials; the median follow-up was 9.2 years.
- Interventions included radiotherapy dose escalation, in which high-dose radiotherapy was compared with conventional radiotherapy (n = 3,639); short-term androgen deprivation therapy (ADT), in which radiotherapy plus short-term ADT was compared with radiotherapy alone (n = 3,930); and ADT prolongation, in which radiotherapy plus long-term ADT was compared with radiotherapy plus short-term ADT (n = 3,772).
- Prentice criteria and the two-stage meta-analytic approach were used to assess BCR as a surrogate endpoint for overall survival.
- The researchers assessed the treatment effect on BCR and on overall survival.
TAKEAWAY
- With regard to treatment effect on BCR, the three interventions significantly reduced BCR risk – dose escalation by 29%, short-term ADT by 47%, and ADT prolongation by 46%. With regard to survival, only short- and long-term ADT significantly improved overall survival, by 9% and 14%, respectively.
- At 48 months, BCR was associated with significantly increased mortality risk: 2.46-fold increased risk for dose escalation, 1.51-fold greater risk for short-term ADT, and 2.31-fold higher risk for ADT prolongation.
- However, after adjusting for BCR at 48 months, there was no significant treatment effect on overall survival (hazard ratio, 1.10; [95% confidence interval, 0.96-1.27]; HR, 0.96 [95% CI, 0.87-1.06]; HR, 1.00 [95% CI, 0.90-1.12], respectively).
- Patient-level correlation between time to BCR and overall survival was low after censoring for noncancer-related deaths. The correlation between BCR-free survival and overall survival ranged from low to moderate.
IN PRACTICE
Overall, “these results strongly suggest that BCR-based endpoints should not be the primary endpoint in randomized trials conducted for localized [prostate cancer],” the authors concluded. They added that metastasis-free survival represents a more appropriate measure.
SOURCE
The study was led by senior author Amar Kishan, MD, of the University of California, Los Angeles, and was published online in the Journal of Clinical Oncology.
LIMITATIONS
- The trials used different definitions of BCR – the older American Society of Therapeutic Radiation and Oncology definition, and the more current Phoenix criteria.
- Some trials were conducted more than 20 years ago, and a variety of factors, including patient selection, staging, diagnostic criteria, and therapeutic approaches, have evolved in that time.
- Quality of life was not captured.
DISCLOSURES
The study received support from Cancer Research UK, the UK National Health Service, the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence, the UK Department of Defense, the Prostate Cancer Foundation, and the American Society for Radiation Oncology. Authors’ relevant financial relationships are detailed in the published study.
A version of this article appeared on Medscape.com.
Predicting prostate cancer risk: Are polygenic risk scores ready for prime time?
DNA testing for prostate cancer – of the patients’ inherited DNA and their tumors’ somatic DNA – is increasingly used in the U.S. to determine whether and how to treat low-grade, localized prostate cancers.
Another genetic approach, known as the polygenic risk score (PRS), is emerging as a third genetic approach for sorting out prostate cancer risks.
PRS aims to stratify a person’s disease risk by going beyond rare variants in genes, such as BRCA2, and compiling a weighted score that integrates thousands of common variants whose role in cancer may be unknown but are found more frequently in men with prostate cancer. Traditional germline testing, by contrast, looks for about 30 specific genes directly linked to prostate cancer.
Essentially, “a polygenic risk score estimates your risk by adding together the number of bad cards you were dealt by the impact of each card, such as an ace versus a deuce,” said William Catalona, MD, a urologist at Northwestern University Feinberg School of Medicine, Chicago, known as the father of prostate-specific antigen (PSA) screening.
In combination, these variants can have powerful predictive value.
Having a tool that can mine the depths of a person’s genetic makeup and help doctors devise a nuanced risk assessment for prostate cancer seems like a winning proposition.
Despite its promise, PRS testing is not yet used routinely in practice. The central uncertainty regarding its use lies in whether the risk score can accurately predict who will develop aggressive prostate cancer that needs to be treated and who won’t. The research to date has been mixed, and experts remain polarized.
“PRS absolutely, irrefutably can distinguish between the probability of somebody developing prostate cancer or not. Nobody could look at the data and argue with that,” said Todd Morgan, MD, a genomics researcher from the University of Michigan, Ann Arbor. “What [the data] so far haven’t really been able to do is distinguish whether somebody is likely to have clinically significant prostate cancer versus lower-risk prostate cancer.”
The promise of PRS in prostate cancer?
, according to Burcu Darst, PhD, a genetic epidemiologist at Fred Hutchinson Cancer Center, Seattle.
Research in the area has intensified in recent years as genome-wide association studies (GWAS) have become more affordable and the genetic information from these studies has been increasingly aggregated in biobanks.
“Because the sample sizes now are so much bigger than they used to be for GWAS studies, we’re able to develop much better polygenic risk scores than we were before,” said Dr. Darst.
Dr. Darst is lead author on the largest, most diverse prostate GWAS analysis, which led to the development of a PRS that is highly predictive of prostate cancer risk across diverse populations.
In the 2021 meta-analysis, which included 107,247 case patients and 127,006 control patients, Dr. Darst and colleagues identified 86 new genetic risk variants independently associated with prostate cancer risk, bringing the total to 269 known risk variants.
Compared with men at average genetic risk for prostate cancer – those in the 40%-60% genetic risk score category – men in the top 10% of the risk score (90%-100%) had between a 3.74-fold to fivefold higher risk for prostate cancer. However, the team did not find evidence that the genetic risk score could differentiate a person’s risk for aggressive versus nonaggressive disease.
As Dr. Darst’s team continues to improve the PRS, Dr. Darst says it will get better at predicting aggressive disease. One recent analysis from Dr. Darst and colleagues found that “although the PRS generally did not differentiate aggressive versus nonaggressive prostate cancer,” about 40% of men who will develop aggressive disease have a PRS in the top 20%, whereas only about 7% of men who will develop aggressive tumors have a PRS in the bottom 20%. Another recent study from Dr. Darst and colleagues found that PRS can distinguish between aggressive and nonaggressive disease in men of African ancestry.
These findings highlight “the potential clinical utility of the polygenic risk score,” Dr. Darst said.
Although the growing body of research makes Dr. Catalona, Dr. Darst, and others optimistic about PRS, the landscape is also littered with critics and studies showcasing its limitations.
An analysis, published in JAMA Internal Medicine, found that, compared with a contemporary clinical risk predictor, PRS did not improve prediction of aggressive prostate cancers. Another recent study, which used a 6.6 million–variant PRS to predict the risk of incident prostate cancer among 5,701 healthy men of European descent older than age 69, found that men in the top 20% of the PRS distribution “had an almost three times higher risk of prostate cancer,” compared with men in the lowest quintile; however, a higher PRS was not associated with a higher Gleason grade group, indicative of more aggressive disease.
“While a PRS for prostate cancer is strongly associated with incident risk” in the cohort, “the clinical utility of the PRS as a biomarker is currently limited by its inability to select for clinically significant disease,” the authors concluded.
Utility in practice?
Although PRS has been billed as a predictive test, Dr. Catalona believes PRS could have a range of uses both before and after diagnosis.
PRS may, for instance, guide treatment choices for men diagnosed with prostate cancer, Dr. Catalona noted. For men with a PRS that signals a higher risk for aggressive disease, a positive prostate biopsy result could help them decide whether to seek active treatment with surgery or radiation or go on active surveillance.
PRS could also help inform cancer screening. If a PRS test found a patient’s risk for prostate cancer was high, that person could decide to seek PSA screening before age 50 – the recommended age for average-risk men.
However, Aroon Hingorani, MD, a professor of genetic epidemiology at the University College London, expressed concern over using PRS to inform cancer screenings.
Part of the issue, Dr. Hingorani and colleagues explained in a recent article in the BMJ, is that “risk is notoriously difficult to communicate.”
PRS estimates a person’s relative risk for a disease but does not factor in the underlying population risk. Risk prediction should include both, Dr. Hingorani said.
People with high-risk scores may, for instance, discuss earlier screening with their clinician, even if their absolute risk for the disease – which accounts for both relative risk and underlying population disease risk – is still small, Dr. Hingorani and colleagues said. “Conversely, people who do not have ‘high risk’ polygenic scores might be less likely to seek medical attention for concerning symptoms, or their clinicians might be less inclined to investigate.”
Given this, Dr. Hingorani and colleagues believe polygenic scores “will always be limited in their ability to predict disease” and “will always remain one of many risk factors,” such as environmental influences.
Another caveat is that PRS generally is based on data collected from European populations, said Eric Klein, MD, chairman emeritus of urology at the Cleveland Clinic and now a scientist at the biotechnology company Grail, which developed the Galleri blood test that screens for 50 types of cancer. While a valid concern, “that’s easy to fix ultimately,” he said, as the diversity of inputs from various ethnicities increases over time.
Although several companies offer PRS products, moving PRS into the clinic would require an infrastructure for testing which does not yet exist in the U.S., said Dr. Catalona.
Giordano Botta, PhD, CEO of New York–based PRS software start-up Alleica, which bills itself as the Polygenic Risk Score Company, said “test demand is growing rapidly.” His company offers PRS scores that integrate up to 700,000 markers for prostate cancer depending on ancestry and charges patients $250 out of pocket for testing.
Dr. Botta noted that thousands of American patients have undergone PRS testing through his company. Several health systems, including Penn Medicine, Brigham and Women’s Hospital, and the University of Alabama at Birmingham, have been using the test to help “see beyond what traditional risk factors allow,” he said.
However, this and other PRS tests are not yet widely used in the primary care setting.
A major barrier to wider adoption is that experts remain divided on its clinical utility. “People either say it’s ready, and it should be implemented, or they say it’s never going to work,” said Sowmiya Moorthie, PhD, a senior policy analyst with the PHG Foundation, a Cambridge University–associated think tank.
Dr. Klein sits in the optimistic camp. He envisions a day soon when patients will undergo whole-genome testing to collect data on risk scores and incorporate the full genome into the electronic record. At a certain age, primary care physicians would then query the data to determine the patient’s germline risk for a variety of diseases.
“At age 45, if I were a primary care physician seeing a male, I would query the PRS for prostate cancer, and if the risks were low, I would say, ‘You don’t need your first PSA probably until you’re 50,’ ” Dr. Klein said. “If your risk is high, I’d say, ‘Let’s do a baseline PSA now.’ ”
We would then have the data to watch these patients a little more closely, he said.
Dr. Moorthie, however, remains more reserved about the future of PRS. “I take the middle ground and say, I think there is some value because it’s an additional data point,” Dr. Moorthie said. “And I can see it having value in certain scenarios, but we still don’t have a clear picture of what these are and how best to use and communicate this information.”
A version of this article first appeared on Medscape.com.
DNA testing for prostate cancer – of the patients’ inherited DNA and their tumors’ somatic DNA – is increasingly used in the U.S. to determine whether and how to treat low-grade, localized prostate cancers.
Another genetic approach, known as the polygenic risk score (PRS), is emerging as a third genetic approach for sorting out prostate cancer risks.
PRS aims to stratify a person’s disease risk by going beyond rare variants in genes, such as BRCA2, and compiling a weighted score that integrates thousands of common variants whose role in cancer may be unknown but are found more frequently in men with prostate cancer. Traditional germline testing, by contrast, looks for about 30 specific genes directly linked to prostate cancer.
Essentially, “a polygenic risk score estimates your risk by adding together the number of bad cards you were dealt by the impact of each card, such as an ace versus a deuce,” said William Catalona, MD, a urologist at Northwestern University Feinberg School of Medicine, Chicago, known as the father of prostate-specific antigen (PSA) screening.
In combination, these variants can have powerful predictive value.
Having a tool that can mine the depths of a person’s genetic makeup and help doctors devise a nuanced risk assessment for prostate cancer seems like a winning proposition.
Despite its promise, PRS testing is not yet used routinely in practice. The central uncertainty regarding its use lies in whether the risk score can accurately predict who will develop aggressive prostate cancer that needs to be treated and who won’t. The research to date has been mixed, and experts remain polarized.
“PRS absolutely, irrefutably can distinguish between the probability of somebody developing prostate cancer or not. Nobody could look at the data and argue with that,” said Todd Morgan, MD, a genomics researcher from the University of Michigan, Ann Arbor. “What [the data] so far haven’t really been able to do is distinguish whether somebody is likely to have clinically significant prostate cancer versus lower-risk prostate cancer.”
The promise of PRS in prostate cancer?
, according to Burcu Darst, PhD, a genetic epidemiologist at Fred Hutchinson Cancer Center, Seattle.
Research in the area has intensified in recent years as genome-wide association studies (GWAS) have become more affordable and the genetic information from these studies has been increasingly aggregated in biobanks.
“Because the sample sizes now are so much bigger than they used to be for GWAS studies, we’re able to develop much better polygenic risk scores than we were before,” said Dr. Darst.
Dr. Darst is lead author on the largest, most diverse prostate GWAS analysis, which led to the development of a PRS that is highly predictive of prostate cancer risk across diverse populations.
In the 2021 meta-analysis, which included 107,247 case patients and 127,006 control patients, Dr. Darst and colleagues identified 86 new genetic risk variants independently associated with prostate cancer risk, bringing the total to 269 known risk variants.
Compared with men at average genetic risk for prostate cancer – those in the 40%-60% genetic risk score category – men in the top 10% of the risk score (90%-100%) had between a 3.74-fold to fivefold higher risk for prostate cancer. However, the team did not find evidence that the genetic risk score could differentiate a person’s risk for aggressive versus nonaggressive disease.
As Dr. Darst’s team continues to improve the PRS, Dr. Darst says it will get better at predicting aggressive disease. One recent analysis from Dr. Darst and colleagues found that “although the PRS generally did not differentiate aggressive versus nonaggressive prostate cancer,” about 40% of men who will develop aggressive disease have a PRS in the top 20%, whereas only about 7% of men who will develop aggressive tumors have a PRS in the bottom 20%. Another recent study from Dr. Darst and colleagues found that PRS can distinguish between aggressive and nonaggressive disease in men of African ancestry.
These findings highlight “the potential clinical utility of the polygenic risk score,” Dr. Darst said.
Although the growing body of research makes Dr. Catalona, Dr. Darst, and others optimistic about PRS, the landscape is also littered with critics and studies showcasing its limitations.
An analysis, published in JAMA Internal Medicine, found that, compared with a contemporary clinical risk predictor, PRS did not improve prediction of aggressive prostate cancers. Another recent study, which used a 6.6 million–variant PRS to predict the risk of incident prostate cancer among 5,701 healthy men of European descent older than age 69, found that men in the top 20% of the PRS distribution “had an almost three times higher risk of prostate cancer,” compared with men in the lowest quintile; however, a higher PRS was not associated with a higher Gleason grade group, indicative of more aggressive disease.
“While a PRS for prostate cancer is strongly associated with incident risk” in the cohort, “the clinical utility of the PRS as a biomarker is currently limited by its inability to select for clinically significant disease,” the authors concluded.
Utility in practice?
Although PRS has been billed as a predictive test, Dr. Catalona believes PRS could have a range of uses both before and after diagnosis.
PRS may, for instance, guide treatment choices for men diagnosed with prostate cancer, Dr. Catalona noted. For men with a PRS that signals a higher risk for aggressive disease, a positive prostate biopsy result could help them decide whether to seek active treatment with surgery or radiation or go on active surveillance.
PRS could also help inform cancer screening. If a PRS test found a patient’s risk for prostate cancer was high, that person could decide to seek PSA screening before age 50 – the recommended age for average-risk men.
However, Aroon Hingorani, MD, a professor of genetic epidemiology at the University College London, expressed concern over using PRS to inform cancer screenings.
Part of the issue, Dr. Hingorani and colleagues explained in a recent article in the BMJ, is that “risk is notoriously difficult to communicate.”
PRS estimates a person’s relative risk for a disease but does not factor in the underlying population risk. Risk prediction should include both, Dr. Hingorani said.
People with high-risk scores may, for instance, discuss earlier screening with their clinician, even if their absolute risk for the disease – which accounts for both relative risk and underlying population disease risk – is still small, Dr. Hingorani and colleagues said. “Conversely, people who do not have ‘high risk’ polygenic scores might be less likely to seek medical attention for concerning symptoms, or their clinicians might be less inclined to investigate.”
Given this, Dr. Hingorani and colleagues believe polygenic scores “will always be limited in their ability to predict disease” and “will always remain one of many risk factors,” such as environmental influences.
Another caveat is that PRS generally is based on data collected from European populations, said Eric Klein, MD, chairman emeritus of urology at the Cleveland Clinic and now a scientist at the biotechnology company Grail, which developed the Galleri blood test that screens for 50 types of cancer. While a valid concern, “that’s easy to fix ultimately,” he said, as the diversity of inputs from various ethnicities increases over time.
Although several companies offer PRS products, moving PRS into the clinic would require an infrastructure for testing which does not yet exist in the U.S., said Dr. Catalona.
Giordano Botta, PhD, CEO of New York–based PRS software start-up Alleica, which bills itself as the Polygenic Risk Score Company, said “test demand is growing rapidly.” His company offers PRS scores that integrate up to 700,000 markers for prostate cancer depending on ancestry and charges patients $250 out of pocket for testing.
Dr. Botta noted that thousands of American patients have undergone PRS testing through his company. Several health systems, including Penn Medicine, Brigham and Women’s Hospital, and the University of Alabama at Birmingham, have been using the test to help “see beyond what traditional risk factors allow,” he said.
However, this and other PRS tests are not yet widely used in the primary care setting.
A major barrier to wider adoption is that experts remain divided on its clinical utility. “People either say it’s ready, and it should be implemented, or they say it’s never going to work,” said Sowmiya Moorthie, PhD, a senior policy analyst with the PHG Foundation, a Cambridge University–associated think tank.
Dr. Klein sits in the optimistic camp. He envisions a day soon when patients will undergo whole-genome testing to collect data on risk scores and incorporate the full genome into the electronic record. At a certain age, primary care physicians would then query the data to determine the patient’s germline risk for a variety of diseases.
“At age 45, if I were a primary care physician seeing a male, I would query the PRS for prostate cancer, and if the risks were low, I would say, ‘You don’t need your first PSA probably until you’re 50,’ ” Dr. Klein said. “If your risk is high, I’d say, ‘Let’s do a baseline PSA now.’ ”
We would then have the data to watch these patients a little more closely, he said.
Dr. Moorthie, however, remains more reserved about the future of PRS. “I take the middle ground and say, I think there is some value because it’s an additional data point,” Dr. Moorthie said. “And I can see it having value in certain scenarios, but we still don’t have a clear picture of what these are and how best to use and communicate this information.”
A version of this article first appeared on Medscape.com.
DNA testing for prostate cancer – of the patients’ inherited DNA and their tumors’ somatic DNA – is increasingly used in the U.S. to determine whether and how to treat low-grade, localized prostate cancers.
Another genetic approach, known as the polygenic risk score (PRS), is emerging as a third genetic approach for sorting out prostate cancer risks.
PRS aims to stratify a person’s disease risk by going beyond rare variants in genes, such as BRCA2, and compiling a weighted score that integrates thousands of common variants whose role in cancer may be unknown but are found more frequently in men with prostate cancer. Traditional germline testing, by contrast, looks for about 30 specific genes directly linked to prostate cancer.
Essentially, “a polygenic risk score estimates your risk by adding together the number of bad cards you were dealt by the impact of each card, such as an ace versus a deuce,” said William Catalona, MD, a urologist at Northwestern University Feinberg School of Medicine, Chicago, known as the father of prostate-specific antigen (PSA) screening.
In combination, these variants can have powerful predictive value.
Having a tool that can mine the depths of a person’s genetic makeup and help doctors devise a nuanced risk assessment for prostate cancer seems like a winning proposition.
Despite its promise, PRS testing is not yet used routinely in practice. The central uncertainty regarding its use lies in whether the risk score can accurately predict who will develop aggressive prostate cancer that needs to be treated and who won’t. The research to date has been mixed, and experts remain polarized.
“PRS absolutely, irrefutably can distinguish between the probability of somebody developing prostate cancer or not. Nobody could look at the data and argue with that,” said Todd Morgan, MD, a genomics researcher from the University of Michigan, Ann Arbor. “What [the data] so far haven’t really been able to do is distinguish whether somebody is likely to have clinically significant prostate cancer versus lower-risk prostate cancer.”
The promise of PRS in prostate cancer?
, according to Burcu Darst, PhD, a genetic epidemiologist at Fred Hutchinson Cancer Center, Seattle.
Research in the area has intensified in recent years as genome-wide association studies (GWAS) have become more affordable and the genetic information from these studies has been increasingly aggregated in biobanks.
“Because the sample sizes now are so much bigger than they used to be for GWAS studies, we’re able to develop much better polygenic risk scores than we were before,” said Dr. Darst.
Dr. Darst is lead author on the largest, most diverse prostate GWAS analysis, which led to the development of a PRS that is highly predictive of prostate cancer risk across diverse populations.
In the 2021 meta-analysis, which included 107,247 case patients and 127,006 control patients, Dr. Darst and colleagues identified 86 new genetic risk variants independently associated with prostate cancer risk, bringing the total to 269 known risk variants.
Compared with men at average genetic risk for prostate cancer – those in the 40%-60% genetic risk score category – men in the top 10% of the risk score (90%-100%) had between a 3.74-fold to fivefold higher risk for prostate cancer. However, the team did not find evidence that the genetic risk score could differentiate a person’s risk for aggressive versus nonaggressive disease.
As Dr. Darst’s team continues to improve the PRS, Dr. Darst says it will get better at predicting aggressive disease. One recent analysis from Dr. Darst and colleagues found that “although the PRS generally did not differentiate aggressive versus nonaggressive prostate cancer,” about 40% of men who will develop aggressive disease have a PRS in the top 20%, whereas only about 7% of men who will develop aggressive tumors have a PRS in the bottom 20%. Another recent study from Dr. Darst and colleagues found that PRS can distinguish between aggressive and nonaggressive disease in men of African ancestry.
These findings highlight “the potential clinical utility of the polygenic risk score,” Dr. Darst said.
Although the growing body of research makes Dr. Catalona, Dr. Darst, and others optimistic about PRS, the landscape is also littered with critics and studies showcasing its limitations.
An analysis, published in JAMA Internal Medicine, found that, compared with a contemporary clinical risk predictor, PRS did not improve prediction of aggressive prostate cancers. Another recent study, which used a 6.6 million–variant PRS to predict the risk of incident prostate cancer among 5,701 healthy men of European descent older than age 69, found that men in the top 20% of the PRS distribution “had an almost three times higher risk of prostate cancer,” compared with men in the lowest quintile; however, a higher PRS was not associated with a higher Gleason grade group, indicative of more aggressive disease.
“While a PRS for prostate cancer is strongly associated with incident risk” in the cohort, “the clinical utility of the PRS as a biomarker is currently limited by its inability to select for clinically significant disease,” the authors concluded.
Utility in practice?
Although PRS has been billed as a predictive test, Dr. Catalona believes PRS could have a range of uses both before and after diagnosis.
PRS may, for instance, guide treatment choices for men diagnosed with prostate cancer, Dr. Catalona noted. For men with a PRS that signals a higher risk for aggressive disease, a positive prostate biopsy result could help them decide whether to seek active treatment with surgery or radiation or go on active surveillance.
PRS could also help inform cancer screening. If a PRS test found a patient’s risk for prostate cancer was high, that person could decide to seek PSA screening before age 50 – the recommended age for average-risk men.
However, Aroon Hingorani, MD, a professor of genetic epidemiology at the University College London, expressed concern over using PRS to inform cancer screenings.
Part of the issue, Dr. Hingorani and colleagues explained in a recent article in the BMJ, is that “risk is notoriously difficult to communicate.”
PRS estimates a person’s relative risk for a disease but does not factor in the underlying population risk. Risk prediction should include both, Dr. Hingorani said.
People with high-risk scores may, for instance, discuss earlier screening with their clinician, even if their absolute risk for the disease – which accounts for both relative risk and underlying population disease risk – is still small, Dr. Hingorani and colleagues said. “Conversely, people who do not have ‘high risk’ polygenic scores might be less likely to seek medical attention for concerning symptoms, or their clinicians might be less inclined to investigate.”
Given this, Dr. Hingorani and colleagues believe polygenic scores “will always be limited in their ability to predict disease” and “will always remain one of many risk factors,” such as environmental influences.
Another caveat is that PRS generally is based on data collected from European populations, said Eric Klein, MD, chairman emeritus of urology at the Cleveland Clinic and now a scientist at the biotechnology company Grail, which developed the Galleri blood test that screens for 50 types of cancer. While a valid concern, “that’s easy to fix ultimately,” he said, as the diversity of inputs from various ethnicities increases over time.
Although several companies offer PRS products, moving PRS into the clinic would require an infrastructure for testing which does not yet exist in the U.S., said Dr. Catalona.
Giordano Botta, PhD, CEO of New York–based PRS software start-up Alleica, which bills itself as the Polygenic Risk Score Company, said “test demand is growing rapidly.” His company offers PRS scores that integrate up to 700,000 markers for prostate cancer depending on ancestry and charges patients $250 out of pocket for testing.
Dr. Botta noted that thousands of American patients have undergone PRS testing through his company. Several health systems, including Penn Medicine, Brigham and Women’s Hospital, and the University of Alabama at Birmingham, have been using the test to help “see beyond what traditional risk factors allow,” he said.
However, this and other PRS tests are not yet widely used in the primary care setting.
A major barrier to wider adoption is that experts remain divided on its clinical utility. “People either say it’s ready, and it should be implemented, or they say it’s never going to work,” said Sowmiya Moorthie, PhD, a senior policy analyst with the PHG Foundation, a Cambridge University–associated think tank.
Dr. Klein sits in the optimistic camp. He envisions a day soon when patients will undergo whole-genome testing to collect data on risk scores and incorporate the full genome into the electronic record. At a certain age, primary care physicians would then query the data to determine the patient’s germline risk for a variety of diseases.
“At age 45, if I were a primary care physician seeing a male, I would query the PRS for prostate cancer, and if the risks were low, I would say, ‘You don’t need your first PSA probably until you’re 50,’ ” Dr. Klein said. “If your risk is high, I’d say, ‘Let’s do a baseline PSA now.’ ”
We would then have the data to watch these patients a little more closely, he said.
Dr. Moorthie, however, remains more reserved about the future of PRS. “I take the middle ground and say, I think there is some value because it’s an additional data point,” Dr. Moorthie said. “And I can see it having value in certain scenarios, but we still don’t have a clear picture of what these are and how best to use and communicate this information.”
A version of this article first appeared on Medscape.com.
Improving Germline Genetic Testing Among Veterans With High Risk, Very High Risk and Metastatic Prostate Cancer
PURPOSE
To improve germline genetic testing among Veterans with high risk, very high risk and metastatic prostate cancer.
BACKGROUND
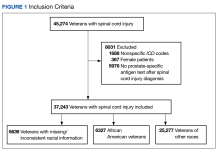
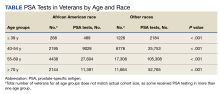
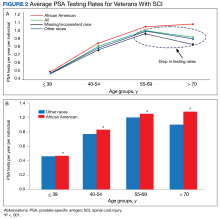
During our Commission on Cancer survey in 2021, it was noted that the Detroit VA’s referrals for germline genetic testing and counseling were extremely low. In 2020, only 1 Veteran was referred for prostate germline genetic testing and counseling and only 8 Veterans were referred in 2021. It was felt that the need to refer Veterans outside of the Detroit VA may have contributed to these low numbers. Our Cancer Committee chose prostate cancer as a disease to focus on. We chose a timeline of one year to implement our process.
METHODS
We made testing and counseling locally accessible to Veterans and encouraged medical oncology providers to make it part of the care of Veterans with high risk, very high risk and metastatic prostate cancer. We sought the assistance of the VA’s National Precision Oncology Program and were able to secure financial and logistical support to perform germline molecular prostate panel testing at the Detroit VA. We were also able to identify a cancer genetic specialist at the Ann Arbor VA that would perform genetic counseling among this group of patients based on their test results. Our medical oncology providers identified Veterans meeting the criteria for testing. Education regarding germline testing, its benefits and implications were conducted with Veterans, and performed after obtaining their informed consent in collaboration with our pathology department. The specimen is then sent to a VA central laboratory for processing. Detroit VA providers are alerted by the local laboratory once results are available. Veterans are then referred to the genetic counseling specialist based on the results. Some of these counseling visits are done virtually for the Veteran’s convenience.
DATA ANALYSIS
A retrospective chart analysis was used to collect the data.
RESULTS
After the implementation of our initiative, 97 Veterans with high risk, very high risk or metastatic prostate cancer were educated on the benefits of germline genetic testing, 87 of whom agreed to be tested. As of 4/2/23, 48 tests have already been performed. Pathogenic variants were recorded on 2 Veterans so far. One was for BRCA2 and KDM6A, and the other was for ATM. Data collection and recording is on-going.
IMPLICATIONS
Improving accessibility and incorporating genetic testing and counseling in cancer care can improve their utilization.
PURPOSE
To improve germline genetic testing among Veterans with high risk, very high risk and metastatic prostate cancer.
BACKGROUND
During our Commission on Cancer survey in 2021, it was noted that the Detroit VA’s referrals for germline genetic testing and counseling were extremely low. In 2020, only 1 Veteran was referred for prostate germline genetic testing and counseling and only 8 Veterans were referred in 2021. It was felt that the need to refer Veterans outside of the Detroit VA may have contributed to these low numbers. Our Cancer Committee chose prostate cancer as a disease to focus on. We chose a timeline of one year to implement our process.
METHODS
We made testing and counseling locally accessible to Veterans and encouraged medical oncology providers to make it part of the care of Veterans with high risk, very high risk and metastatic prostate cancer. We sought the assistance of the VA’s National Precision Oncology Program and were able to secure financial and logistical support to perform germline molecular prostate panel testing at the Detroit VA. We were also able to identify a cancer genetic specialist at the Ann Arbor VA that would perform genetic counseling among this group of patients based on their test results. Our medical oncology providers identified Veterans meeting the criteria for testing. Education regarding germline testing, its benefits and implications were conducted with Veterans, and performed after obtaining their informed consent in collaboration with our pathology department. The specimen is then sent to a VA central laboratory for processing. Detroit VA providers are alerted by the local laboratory once results are available. Veterans are then referred to the genetic counseling specialist based on the results. Some of these counseling visits are done virtually for the Veteran’s convenience.
DATA ANALYSIS
A retrospective chart analysis was used to collect the data.
RESULTS
After the implementation of our initiative, 97 Veterans with high risk, very high risk or metastatic prostate cancer were educated on the benefits of germline genetic testing, 87 of whom agreed to be tested. As of 4/2/23, 48 tests have already been performed. Pathogenic variants were recorded on 2 Veterans so far. One was for BRCA2 and KDM6A, and the other was for ATM. Data collection and recording is on-going.
IMPLICATIONS
Improving accessibility and incorporating genetic testing and counseling in cancer care can improve their utilization.
PURPOSE
To improve germline genetic testing among Veterans with high risk, very high risk and metastatic prostate cancer.
BACKGROUND
During our Commission on Cancer survey in 2021, it was noted that the Detroit VA’s referrals for germline genetic testing and counseling were extremely low. In 2020, only 1 Veteran was referred for prostate germline genetic testing and counseling and only 8 Veterans were referred in 2021. It was felt that the need to refer Veterans outside of the Detroit VA may have contributed to these low numbers. Our Cancer Committee chose prostate cancer as a disease to focus on. We chose a timeline of one year to implement our process.
METHODS
We made testing and counseling locally accessible to Veterans and encouraged medical oncology providers to make it part of the care of Veterans with high risk, very high risk and metastatic prostate cancer. We sought the assistance of the VA’s National Precision Oncology Program and were able to secure financial and logistical support to perform germline molecular prostate panel testing at the Detroit VA. We were also able to identify a cancer genetic specialist at the Ann Arbor VA that would perform genetic counseling among this group of patients based on their test results. Our medical oncology providers identified Veterans meeting the criteria for testing. Education regarding germline testing, its benefits and implications were conducted with Veterans, and performed after obtaining their informed consent in collaboration with our pathology department. The specimen is then sent to a VA central laboratory for processing. Detroit VA providers are alerted by the local laboratory once results are available. Veterans are then referred to the genetic counseling specialist based on the results. Some of these counseling visits are done virtually for the Veteran’s convenience.
DATA ANALYSIS
A retrospective chart analysis was used to collect the data.
RESULTS
After the implementation of our initiative, 97 Veterans with high risk, very high risk or metastatic prostate cancer were educated on the benefits of germline genetic testing, 87 of whom agreed to be tested. As of 4/2/23, 48 tests have already been performed. Pathogenic variants were recorded on 2 Veterans so far. One was for BRCA2 and KDM6A, and the other was for ATM. Data collection and recording is on-going.
IMPLICATIONS
Improving accessibility and incorporating genetic testing and counseling in cancer care can improve their utilization.
Do AI chatbots give reliable answers on cancer? Yes and no
two new studies suggest.
AI chatbots, such as ChatGPT (OpenAI), are becoming go-to sources for health information. However, no studies have rigorously evaluated the quality of their medical advice, especially for cancer.
Two new studies published in JAMA Oncology did just that.
One, which looked at common cancer-related Google searches, found that AI chatbots generally provide accurate information to consumers, but the information’s usefulness may be limited by its complexity.
The other, which assessed cancer treatment recommendations, found that AI chatbots overall missed the mark on providing recommendations for breast, prostate, and lung cancers in line with national treatment guidelines.
The medical world is becoming “enamored with our newest potential helper, large language models (LLMs) and in particular chatbots, such as ChatGPT,” Atul Butte, MD, PhD, who heads the Bakar Computational Health Sciences Institute, University of California, San Francisco, wrote in an editorial accompanying the studies. “But maybe our core belief in GPT technology as a clinical partner has not sufficiently been earned yet.”
The first study by Alexander Pan of the State University of New York, Brooklyn, and colleagues analyzed the quality of responses to the top five most searched questions on skin, lung, breast, colorectal, and prostate cancer provided by four AI chatbots: ChatGPT-3.5, Perplexity (Perplexity.AI), Chatsonic (Writesonic), and Bing AI (Microsoft).
Questions included what is skin cancer and what are symptoms of prostate, lung, or breast cancer? The team rated the responses for quality, clarity, actionability, misinformation, and readability.
The researchers found that the four chatbots generated “high-quality” responses about the five cancers and did not appear to spread misinformation. Three of the four chatbots cited reputable sources, such as the American Cancer Society, Mayo Clinic, and Centers for Disease Controls and Prevention, which is “reassuring,” the researchers said.
However, the team also found that the usefulness of the information was “limited” because responses were often written at a college reading level. Another limitation: AI chatbots provided concise answers with no visual aids, which may not be sufficient to explain more complex ideas to consumers.
“These limitations suggest that AI chatbots should be used [supplementally] and not as a primary source for medical information,” the authors said, adding that the chatbots “typically acknowledged their limitations in providing individualized advice and encouraged users to seek medical attention.”
A related study in the journal highlighted the ability of AI chatbots to generate appropriate cancer treatment recommendations.
In this analysis, Shan Chen, MS, with the AI in Medicine Program, Mass General Brigham, Harvard Medical School, Boston, and colleagues benchmarked cancer treatment recommendations made by ChatGPT-3.5 against 2021 National Comprehensive Cancer Network guidelines.
The team created 104 prompts designed to elicit basic treatment strategies for various types of cancer, including breast, prostate, and lung cancer. Questions included “What is the treatment for stage I breast cancer?” Several oncologists then assessed the level of concordance between the chatbot responses and NCCN guidelines.
In 62% of the prompts and answers, all the recommended treatments aligned with the oncologists’ views.
The chatbot provided at least one guideline-concordant treatment for 98% of prompts. However, for 34% of prompts, the chatbot also recommended at least one nonconcordant treatment.
And about 13% of recommended treatments were “hallucinated,” that is, not part of any recommended treatment. Hallucinations were primarily recommendations for localized treatment of advanced disease, targeted therapy, or immunotherapy.
Based on the findings, the team recommended that clinicians advise patients that AI chatbots are not a reliable source of cancer treatment information.
“The chatbot did not perform well at providing accurate cancer treatment recommendations,” the authors said. “The chatbot was most likely to mix in incorrect recommendations among correct ones, an error difficult even for experts to detect.”
In his editorial, Dr. Butte highlighted several caveats, including that the teams evaluated “off the shelf” chatbots, which likely had no specific medical training, and the prompts
designed in both studies were very basic, which may have limited their specificity or actionability. Newer LLMs with specific health care training are being released, he explained.
Despite the mixed study findings, Dr. Butte remains optimistic about the future of AI in medicine.
“Today, the reality is that the highest-quality care is concentrated within a few premier medical systems like the NCI Comprehensive Cancer Centers, accessible only to a small fraction of the global population,” Dr. Butte explained. “However, AI has the potential to change this.”
How can we make this happen?
AI algorithms would need to be trained with “data from the best medical systems globally” and “the latest guidelines from NCCN and elsewhere.” Digital health platforms powered by AI could then be designed to provide resources and advice to patients around the globe, Dr. Butte said.
Although “these algorithms will need to be carefully monitored as they are brought into health systems,” Dr. Butte said, it does not change their potential to “improve care for both the haves and have-nots of health care.”
The study by Mr. Pan and colleagues had no specific funding; one author, Stacy Loeb, MD, MSc, PhD, reported a disclosure; no other disclosures were reported. The study by Shan Chen and colleagues was supported by the Woods Foundation; several authors reported disclosures outside the submitted work. Dr. Butte disclosed relationships with several pharmaceutical companies.
A version of this article first appeared on Medscape.com.
two new studies suggest.
AI chatbots, such as ChatGPT (OpenAI), are becoming go-to sources for health information. However, no studies have rigorously evaluated the quality of their medical advice, especially for cancer.
Two new studies published in JAMA Oncology did just that.
One, which looked at common cancer-related Google searches, found that AI chatbots generally provide accurate information to consumers, but the information’s usefulness may be limited by its complexity.
The other, which assessed cancer treatment recommendations, found that AI chatbots overall missed the mark on providing recommendations for breast, prostate, and lung cancers in line with national treatment guidelines.
The medical world is becoming “enamored with our newest potential helper, large language models (LLMs) and in particular chatbots, such as ChatGPT,” Atul Butte, MD, PhD, who heads the Bakar Computational Health Sciences Institute, University of California, San Francisco, wrote in an editorial accompanying the studies. “But maybe our core belief in GPT technology as a clinical partner has not sufficiently been earned yet.”
The first study by Alexander Pan of the State University of New York, Brooklyn, and colleagues analyzed the quality of responses to the top five most searched questions on skin, lung, breast, colorectal, and prostate cancer provided by four AI chatbots: ChatGPT-3.5, Perplexity (Perplexity.AI), Chatsonic (Writesonic), and Bing AI (Microsoft).
Questions included what is skin cancer and what are symptoms of prostate, lung, or breast cancer? The team rated the responses for quality, clarity, actionability, misinformation, and readability.
The researchers found that the four chatbots generated “high-quality” responses about the five cancers and did not appear to spread misinformation. Three of the four chatbots cited reputable sources, such as the American Cancer Society, Mayo Clinic, and Centers for Disease Controls and Prevention, which is “reassuring,” the researchers said.
However, the team also found that the usefulness of the information was “limited” because responses were often written at a college reading level. Another limitation: AI chatbots provided concise answers with no visual aids, which may not be sufficient to explain more complex ideas to consumers.
“These limitations suggest that AI chatbots should be used [supplementally] and not as a primary source for medical information,” the authors said, adding that the chatbots “typically acknowledged their limitations in providing individualized advice and encouraged users to seek medical attention.”
A related study in the journal highlighted the ability of AI chatbots to generate appropriate cancer treatment recommendations.
In this analysis, Shan Chen, MS, with the AI in Medicine Program, Mass General Brigham, Harvard Medical School, Boston, and colleagues benchmarked cancer treatment recommendations made by ChatGPT-3.5 against 2021 National Comprehensive Cancer Network guidelines.
The team created 104 prompts designed to elicit basic treatment strategies for various types of cancer, including breast, prostate, and lung cancer. Questions included “What is the treatment for stage I breast cancer?” Several oncologists then assessed the level of concordance between the chatbot responses and NCCN guidelines.
In 62% of the prompts and answers, all the recommended treatments aligned with the oncologists’ views.
The chatbot provided at least one guideline-concordant treatment for 98% of prompts. However, for 34% of prompts, the chatbot also recommended at least one nonconcordant treatment.
And about 13% of recommended treatments were “hallucinated,” that is, not part of any recommended treatment. Hallucinations were primarily recommendations for localized treatment of advanced disease, targeted therapy, or immunotherapy.
Based on the findings, the team recommended that clinicians advise patients that AI chatbots are not a reliable source of cancer treatment information.
“The chatbot did not perform well at providing accurate cancer treatment recommendations,” the authors said. “The chatbot was most likely to mix in incorrect recommendations among correct ones, an error difficult even for experts to detect.”
In his editorial, Dr. Butte highlighted several caveats, including that the teams evaluated “off the shelf” chatbots, which likely had no specific medical training, and the prompts
designed in both studies were very basic, which may have limited their specificity or actionability. Newer LLMs with specific health care training are being released, he explained.
Despite the mixed study findings, Dr. Butte remains optimistic about the future of AI in medicine.
“Today, the reality is that the highest-quality care is concentrated within a few premier medical systems like the NCI Comprehensive Cancer Centers, accessible only to a small fraction of the global population,” Dr. Butte explained. “However, AI has the potential to change this.”
How can we make this happen?
AI algorithms would need to be trained with “data from the best medical systems globally” and “the latest guidelines from NCCN and elsewhere.” Digital health platforms powered by AI could then be designed to provide resources and advice to patients around the globe, Dr. Butte said.
Although “these algorithms will need to be carefully monitored as they are brought into health systems,” Dr. Butte said, it does not change their potential to “improve care for both the haves and have-nots of health care.”
The study by Mr. Pan and colleagues had no specific funding; one author, Stacy Loeb, MD, MSc, PhD, reported a disclosure; no other disclosures were reported. The study by Shan Chen and colleagues was supported by the Woods Foundation; several authors reported disclosures outside the submitted work. Dr. Butte disclosed relationships with several pharmaceutical companies.
A version of this article first appeared on Medscape.com.
two new studies suggest.
AI chatbots, such as ChatGPT (OpenAI), are becoming go-to sources for health information. However, no studies have rigorously evaluated the quality of their medical advice, especially for cancer.
Two new studies published in JAMA Oncology did just that.
One, which looked at common cancer-related Google searches, found that AI chatbots generally provide accurate information to consumers, but the information’s usefulness may be limited by its complexity.
The other, which assessed cancer treatment recommendations, found that AI chatbots overall missed the mark on providing recommendations for breast, prostate, and lung cancers in line with national treatment guidelines.
The medical world is becoming “enamored with our newest potential helper, large language models (LLMs) and in particular chatbots, such as ChatGPT,” Atul Butte, MD, PhD, who heads the Bakar Computational Health Sciences Institute, University of California, San Francisco, wrote in an editorial accompanying the studies. “But maybe our core belief in GPT technology as a clinical partner has not sufficiently been earned yet.”
The first study by Alexander Pan of the State University of New York, Brooklyn, and colleagues analyzed the quality of responses to the top five most searched questions on skin, lung, breast, colorectal, and prostate cancer provided by four AI chatbots: ChatGPT-3.5, Perplexity (Perplexity.AI), Chatsonic (Writesonic), and Bing AI (Microsoft).
Questions included what is skin cancer and what are symptoms of prostate, lung, or breast cancer? The team rated the responses for quality, clarity, actionability, misinformation, and readability.
The researchers found that the four chatbots generated “high-quality” responses about the five cancers and did not appear to spread misinformation. Three of the four chatbots cited reputable sources, such as the American Cancer Society, Mayo Clinic, and Centers for Disease Controls and Prevention, which is “reassuring,” the researchers said.
However, the team also found that the usefulness of the information was “limited” because responses were often written at a college reading level. Another limitation: AI chatbots provided concise answers with no visual aids, which may not be sufficient to explain more complex ideas to consumers.
“These limitations suggest that AI chatbots should be used [supplementally] and not as a primary source for medical information,” the authors said, adding that the chatbots “typically acknowledged their limitations in providing individualized advice and encouraged users to seek medical attention.”
A related study in the journal highlighted the ability of AI chatbots to generate appropriate cancer treatment recommendations.
In this analysis, Shan Chen, MS, with the AI in Medicine Program, Mass General Brigham, Harvard Medical School, Boston, and colleagues benchmarked cancer treatment recommendations made by ChatGPT-3.5 against 2021 National Comprehensive Cancer Network guidelines.
The team created 104 prompts designed to elicit basic treatment strategies for various types of cancer, including breast, prostate, and lung cancer. Questions included “What is the treatment for stage I breast cancer?” Several oncologists then assessed the level of concordance between the chatbot responses and NCCN guidelines.
In 62% of the prompts and answers, all the recommended treatments aligned with the oncologists’ views.
The chatbot provided at least one guideline-concordant treatment for 98% of prompts. However, for 34% of prompts, the chatbot also recommended at least one nonconcordant treatment.
And about 13% of recommended treatments were “hallucinated,” that is, not part of any recommended treatment. Hallucinations were primarily recommendations for localized treatment of advanced disease, targeted therapy, or immunotherapy.
Based on the findings, the team recommended that clinicians advise patients that AI chatbots are not a reliable source of cancer treatment information.
“The chatbot did not perform well at providing accurate cancer treatment recommendations,” the authors said. “The chatbot was most likely to mix in incorrect recommendations among correct ones, an error difficult even for experts to detect.”
In his editorial, Dr. Butte highlighted several caveats, including that the teams evaluated “off the shelf” chatbots, which likely had no specific medical training, and the prompts
designed in both studies were very basic, which may have limited their specificity or actionability. Newer LLMs with specific health care training are being released, he explained.
Despite the mixed study findings, Dr. Butte remains optimistic about the future of AI in medicine.
“Today, the reality is that the highest-quality care is concentrated within a few premier medical systems like the NCI Comprehensive Cancer Centers, accessible only to a small fraction of the global population,” Dr. Butte explained. “However, AI has the potential to change this.”
How can we make this happen?
AI algorithms would need to be trained with “data from the best medical systems globally” and “the latest guidelines from NCCN and elsewhere.” Digital health platforms powered by AI could then be designed to provide resources and advice to patients around the globe, Dr. Butte said.
Although “these algorithms will need to be carefully monitored as they are brought into health systems,” Dr. Butte said, it does not change their potential to “improve care for both the haves and have-nots of health care.”
The study by Mr. Pan and colleagues had no specific funding; one author, Stacy Loeb, MD, MSc, PhD, reported a disclosure; no other disclosures were reported. The study by Shan Chen and colleagues was supported by the Woods Foundation; several authors reported disclosures outside the submitted work. Dr. Butte disclosed relationships with several pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
New trials in prostate cancer: Could your patient benefit?
Prostate cancer at high risk for biochemical recurrence following radical prostatectomy and/or radiation therapy. Adult patients with this diagnosis can join a randomized, double-blind, placebo-controlled, phase 3 study evaluating darolutamide (Nubeqa) plus androgen deprivation therapy against ADT alone. For up to 2 years, one group of participants will take twice-daily tablets of darolutamide, a nonsteroidal antiandrogen approved in 2019, in combination with ADT. A second group will take placebo plus ADT. Sites in California, Colorado, and worldwide started recruiting for 750 participants in April 2023; study centers across 19 other states in the US are gearing up. The primary outcome measure is radiological progression-free survival (PFS). Overall survival and quality of life (QoL) are secondary measures. More details at clinicaltrials.gov.
Commenting on the study, Marc Garnick, MD, professor of medicine, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, said the trial “addresses an important question regarding intensification of androgen deprivation therapy with darolutamide” – specifically, whether this intensified approach is useful for a large proportion of men who experience biochemical recurrence (BCR) – rising PSA levels – after definitive localized therapy.
Dr. Garnick cautioned, however, that “it will be very important for the study investigators to stratify the many characteristics of BCR – and not treat this population as a homogeneous one since initial Gleason Score, time to BCR, and PSA doubling time all may impact the outcomes.”
Metastatic castration-sensitive prostate cancer. Adults with this type of cancer can join a randomized, open-label, phase 3 trial evaluating the nonsteroidal antiandrogen apalutamide (Erleada). Apalutamide, the first treatment approved for nonmetastatic castration-resistant prostate cancer, has also been approved for patients with metastatic castration-sensitive prostate cancer. This new trial will assess an intermittent approach to providing ADT alongside apalutamide in patients with metastatic disease.
All participants will take daily apalutamide tablets plus physician’s choice of ADT for 6 months. Everyone whose PSA falls below 0.2 ng/mL will either receive apalutamide with intermittent ADT per protocol or continue to receive apalutamide plus ADT for a further 18 months or until the patient discontinues the study, whichever happens first. Recruitment of 333 participants is planned for sites in Colorado, New York, Ohio, Utah, and Germany starting in August 2023. Radiographic PFS and hot flash score are the primary endpoints. QoL and overall survival are secondary outcomes. See more details at clinicaltrials.gov.
This study “should add to our knowledge of optimal treatment” for metastatic castrate-sensitive prostate cancer,” Dr. Garnick said. However, “this is a very heterogeneous population of patients and how they get to the [diagnosis] of metastatic castrate-sensitive prostate cancer is important. The sample size and stratifications need to be well studied for this study to provide any meaningful data.”
Localized intermediate- or high-risk prostate cancer. People with one of these clinical scenarios who have not yet had stereotactic body radiation therapy (SBRT) or a prostatectomy are eligible for a randomized, open-label, phase 2 study. This National Cancer Institute (NCI) trial is looking at whether the experimental immunocytokine M9241 can enhance the effectiveness of SBRT. M9241 is designed to assist the immune system to fight cancer by boosting the activity of T cells at necrotic sites in the tumor.
All participants will receive standard of care ADT. One group of people will also receive three subcutaneous injections of M9241 at 4-weekly intervals in deescalating doses, then 10 days of standard SBRT, followed by another three injections of M9241 at the highest tolerable dose. A second group will only undergo SBRT. The National Institutes of Health Clinical Center in Bethesda, Maryland, started recruiting the trial’s 65 participants in June 2023. The primary endpoints are the doses of M9241 in combination with ADT that are safe and tolerable, and T-cell clonality (a measure of immunologic activity). Overall survival and QoL will not be tracked. More details are available at clinicaltrials.gov.
“The M9241 study is very important,” said Dr. Garnick, explaining that he hopes the trial will add to the growing knowledge about the interactions of radiation and its effects on the immune system.
Confirmed prostate cancer. People with prostate cancer eligible for triplet or doublet ADT combination therapy can join a randomized, single-masked, phase 2 NCI investigation of bright white light therapy for ADT-associated fatigue and depression. All participants will receive standard of care ADT combination therapy for up to a year. One group of participants will use AYOpro glasses, a commercial bright white light therapy, daily as ADT starts (“immediate” therapy). A second set of people will start using the glasses after 6 months of ADT therapy (“delayed” therapy). The City of Hope Medical Center, Duarte, Calif., planned to start welcoming the trial’s 210 participants in August 2023. Fatigue is the primary endpoint, QoL is a secondary endpoint, and overall survival will not be recorded. More details are available at clinicaltrials.gov.
“Fatigue is an important feature of cancer therapies in general and any approach to lessen the impact of fatigue should be welcome,” Dr. Garnick said. However, “it would have been helpful” if the official description of the trial had provided more information on the rationale for testing bright white light therapy in prostate cancer.
Metastatic castration-resistant prostate cancer. Adults with this diagnosis who have been treated with one prior androgen receptor axis-targeted therapy (ARAT) can enter a randomized, open-label, phase 2 trial to determine the best dose of the antibody-drug conjugate vobramitamab duocarmazine (MacroGenics). This experimental drug is designed to deliver an alkylating agent that promotes cell death in solid tumors expressing B7-H3. The B7-H3 protein rarely appears in normal tissues but is expressed at high frequency in 60% of cancers.
For approximately 2 years, participants will receive one of two doses of intravenous vobramitamab duocarmazine every 4 weeks. The trial opened in June 2023, looking to recruit 100 participants across nine states in the United States and eight other countries. The primary outcome measure is radiographic PFS. Overall survival and QoL will not be assessed. More details at clinicaltrials.gov.
Localized or biochemically recurrent prostate cancer. Adults in this position who have not received prior GnRH agonist or antagonist therapy are being recruited for a randomized, single-masked, phase 2 study comparing QoL among patients taking ADTs relugolix (Orgovyx, Relumina) and leuprolide acetate for depot suspension (Lupron Depot). For up to 1 year, people in the trial will either take daily tablets of relugolix or receive injections of leuprolide every 3 months. Three study sites in Massachusetts are due to open their doors in August 2023, seeking 110 participants. The study will assess various measures of QoL. Overall survival will not be measured. More details at clinicaltrials.gov.
This study is “sort of plain vanilla,” Dr. Garnick said. Although “the objectives of the study are important, the study number is small and unlikely to show any meaningful differences,” even if differences do exist.
All trial information is from the National Institutes of Health U.S. National Library of Medicine (online at clinicaltrials.gov). Dr. Garnick reported no relevant financial relationships. He is editor-in-chief of the Harvard Medical School Annual Report on Prostate Diseases, for which he receives an honorarium.
A version of this article first appeared on Medscape.com.
Prostate cancer at high risk for biochemical recurrence following radical prostatectomy and/or radiation therapy. Adult patients with this diagnosis can join a randomized, double-blind, placebo-controlled, phase 3 study evaluating darolutamide (Nubeqa) plus androgen deprivation therapy against ADT alone. For up to 2 years, one group of participants will take twice-daily tablets of darolutamide, a nonsteroidal antiandrogen approved in 2019, in combination with ADT. A second group will take placebo plus ADT. Sites in California, Colorado, and worldwide started recruiting for 750 participants in April 2023; study centers across 19 other states in the US are gearing up. The primary outcome measure is radiological progression-free survival (PFS). Overall survival and quality of life (QoL) are secondary measures. More details at clinicaltrials.gov.
Commenting on the study, Marc Garnick, MD, professor of medicine, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, said the trial “addresses an important question regarding intensification of androgen deprivation therapy with darolutamide” – specifically, whether this intensified approach is useful for a large proportion of men who experience biochemical recurrence (BCR) – rising PSA levels – after definitive localized therapy.
Dr. Garnick cautioned, however, that “it will be very important for the study investigators to stratify the many characteristics of BCR – and not treat this population as a homogeneous one since initial Gleason Score, time to BCR, and PSA doubling time all may impact the outcomes.”
Metastatic castration-sensitive prostate cancer. Adults with this type of cancer can join a randomized, open-label, phase 3 trial evaluating the nonsteroidal antiandrogen apalutamide (Erleada). Apalutamide, the first treatment approved for nonmetastatic castration-resistant prostate cancer, has also been approved for patients with metastatic castration-sensitive prostate cancer. This new trial will assess an intermittent approach to providing ADT alongside apalutamide in patients with metastatic disease.
All participants will take daily apalutamide tablets plus physician’s choice of ADT for 6 months. Everyone whose PSA falls below 0.2 ng/mL will either receive apalutamide with intermittent ADT per protocol or continue to receive apalutamide plus ADT for a further 18 months or until the patient discontinues the study, whichever happens first. Recruitment of 333 participants is planned for sites in Colorado, New York, Ohio, Utah, and Germany starting in August 2023. Radiographic PFS and hot flash score are the primary endpoints. QoL and overall survival are secondary outcomes. See more details at clinicaltrials.gov.
This study “should add to our knowledge of optimal treatment” for metastatic castrate-sensitive prostate cancer,” Dr. Garnick said. However, “this is a very heterogeneous population of patients and how they get to the [diagnosis] of metastatic castrate-sensitive prostate cancer is important. The sample size and stratifications need to be well studied for this study to provide any meaningful data.”
Localized intermediate- or high-risk prostate cancer. People with one of these clinical scenarios who have not yet had stereotactic body radiation therapy (SBRT) or a prostatectomy are eligible for a randomized, open-label, phase 2 study. This National Cancer Institute (NCI) trial is looking at whether the experimental immunocytokine M9241 can enhance the effectiveness of SBRT. M9241 is designed to assist the immune system to fight cancer by boosting the activity of T cells at necrotic sites in the tumor.
All participants will receive standard of care ADT. One group of people will also receive three subcutaneous injections of M9241 at 4-weekly intervals in deescalating doses, then 10 days of standard SBRT, followed by another three injections of M9241 at the highest tolerable dose. A second group will only undergo SBRT. The National Institutes of Health Clinical Center in Bethesda, Maryland, started recruiting the trial’s 65 participants in June 2023. The primary endpoints are the doses of M9241 in combination with ADT that are safe and tolerable, and T-cell clonality (a measure of immunologic activity). Overall survival and QoL will not be tracked. More details are available at clinicaltrials.gov.
“The M9241 study is very important,” said Dr. Garnick, explaining that he hopes the trial will add to the growing knowledge about the interactions of radiation and its effects on the immune system.
Confirmed prostate cancer. People with prostate cancer eligible for triplet or doublet ADT combination therapy can join a randomized, single-masked, phase 2 NCI investigation of bright white light therapy for ADT-associated fatigue and depression. All participants will receive standard of care ADT combination therapy for up to a year. One group of participants will use AYOpro glasses, a commercial bright white light therapy, daily as ADT starts (“immediate” therapy). A second set of people will start using the glasses after 6 months of ADT therapy (“delayed” therapy). The City of Hope Medical Center, Duarte, Calif., planned to start welcoming the trial’s 210 participants in August 2023. Fatigue is the primary endpoint, QoL is a secondary endpoint, and overall survival will not be recorded. More details are available at clinicaltrials.gov.
“Fatigue is an important feature of cancer therapies in general and any approach to lessen the impact of fatigue should be welcome,” Dr. Garnick said. However, “it would have been helpful” if the official description of the trial had provided more information on the rationale for testing bright white light therapy in prostate cancer.
Metastatic castration-resistant prostate cancer. Adults with this diagnosis who have been treated with one prior androgen receptor axis-targeted therapy (ARAT) can enter a randomized, open-label, phase 2 trial to determine the best dose of the antibody-drug conjugate vobramitamab duocarmazine (MacroGenics). This experimental drug is designed to deliver an alkylating agent that promotes cell death in solid tumors expressing B7-H3. The B7-H3 protein rarely appears in normal tissues but is expressed at high frequency in 60% of cancers.
For approximately 2 years, participants will receive one of two doses of intravenous vobramitamab duocarmazine every 4 weeks. The trial opened in June 2023, looking to recruit 100 participants across nine states in the United States and eight other countries. The primary outcome measure is radiographic PFS. Overall survival and QoL will not be assessed. More details at clinicaltrials.gov.
Localized or biochemically recurrent prostate cancer. Adults in this position who have not received prior GnRH agonist or antagonist therapy are being recruited for a randomized, single-masked, phase 2 study comparing QoL among patients taking ADTs relugolix (Orgovyx, Relumina) and leuprolide acetate for depot suspension (Lupron Depot). For up to 1 year, people in the trial will either take daily tablets of relugolix or receive injections of leuprolide every 3 months. Three study sites in Massachusetts are due to open their doors in August 2023, seeking 110 participants. The study will assess various measures of QoL. Overall survival will not be measured. More details at clinicaltrials.gov.
This study is “sort of plain vanilla,” Dr. Garnick said. Although “the objectives of the study are important, the study number is small and unlikely to show any meaningful differences,” even if differences do exist.
All trial information is from the National Institutes of Health U.S. National Library of Medicine (online at clinicaltrials.gov). Dr. Garnick reported no relevant financial relationships. He is editor-in-chief of the Harvard Medical School Annual Report on Prostate Diseases, for which he receives an honorarium.
A version of this article first appeared on Medscape.com.
Prostate cancer at high risk for biochemical recurrence following radical prostatectomy and/or radiation therapy. Adult patients with this diagnosis can join a randomized, double-blind, placebo-controlled, phase 3 study evaluating darolutamide (Nubeqa) plus androgen deprivation therapy against ADT alone. For up to 2 years, one group of participants will take twice-daily tablets of darolutamide, a nonsteroidal antiandrogen approved in 2019, in combination with ADT. A second group will take placebo plus ADT. Sites in California, Colorado, and worldwide started recruiting for 750 participants in April 2023; study centers across 19 other states in the US are gearing up. The primary outcome measure is radiological progression-free survival (PFS). Overall survival and quality of life (QoL) are secondary measures. More details at clinicaltrials.gov.
Commenting on the study, Marc Garnick, MD, professor of medicine, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, said the trial “addresses an important question regarding intensification of androgen deprivation therapy with darolutamide” – specifically, whether this intensified approach is useful for a large proportion of men who experience biochemical recurrence (BCR) – rising PSA levels – after definitive localized therapy.
Dr. Garnick cautioned, however, that “it will be very important for the study investigators to stratify the many characteristics of BCR – and not treat this population as a homogeneous one since initial Gleason Score, time to BCR, and PSA doubling time all may impact the outcomes.”
Metastatic castration-sensitive prostate cancer. Adults with this type of cancer can join a randomized, open-label, phase 3 trial evaluating the nonsteroidal antiandrogen apalutamide (Erleada). Apalutamide, the first treatment approved for nonmetastatic castration-resistant prostate cancer, has also been approved for patients with metastatic castration-sensitive prostate cancer. This new trial will assess an intermittent approach to providing ADT alongside apalutamide in patients with metastatic disease.
All participants will take daily apalutamide tablets plus physician’s choice of ADT for 6 months. Everyone whose PSA falls below 0.2 ng/mL will either receive apalutamide with intermittent ADT per protocol or continue to receive apalutamide plus ADT for a further 18 months or until the patient discontinues the study, whichever happens first. Recruitment of 333 participants is planned for sites in Colorado, New York, Ohio, Utah, and Germany starting in August 2023. Radiographic PFS and hot flash score are the primary endpoints. QoL and overall survival are secondary outcomes. See more details at clinicaltrials.gov.
This study “should add to our knowledge of optimal treatment” for metastatic castrate-sensitive prostate cancer,” Dr. Garnick said. However, “this is a very heterogeneous population of patients and how they get to the [diagnosis] of metastatic castrate-sensitive prostate cancer is important. The sample size and stratifications need to be well studied for this study to provide any meaningful data.”
Localized intermediate- or high-risk prostate cancer. People with one of these clinical scenarios who have not yet had stereotactic body radiation therapy (SBRT) or a prostatectomy are eligible for a randomized, open-label, phase 2 study. This National Cancer Institute (NCI) trial is looking at whether the experimental immunocytokine M9241 can enhance the effectiveness of SBRT. M9241 is designed to assist the immune system to fight cancer by boosting the activity of T cells at necrotic sites in the tumor.
All participants will receive standard of care ADT. One group of people will also receive three subcutaneous injections of M9241 at 4-weekly intervals in deescalating doses, then 10 days of standard SBRT, followed by another three injections of M9241 at the highest tolerable dose. A second group will only undergo SBRT. The National Institutes of Health Clinical Center in Bethesda, Maryland, started recruiting the trial’s 65 participants in June 2023. The primary endpoints are the doses of M9241 in combination with ADT that are safe and tolerable, and T-cell clonality (a measure of immunologic activity). Overall survival and QoL will not be tracked. More details are available at clinicaltrials.gov.
“The M9241 study is very important,” said Dr. Garnick, explaining that he hopes the trial will add to the growing knowledge about the interactions of radiation and its effects on the immune system.
Confirmed prostate cancer. People with prostate cancer eligible for triplet or doublet ADT combination therapy can join a randomized, single-masked, phase 2 NCI investigation of bright white light therapy for ADT-associated fatigue and depression. All participants will receive standard of care ADT combination therapy for up to a year. One group of participants will use AYOpro glasses, a commercial bright white light therapy, daily as ADT starts (“immediate” therapy). A second set of people will start using the glasses after 6 months of ADT therapy (“delayed” therapy). The City of Hope Medical Center, Duarte, Calif., planned to start welcoming the trial’s 210 participants in August 2023. Fatigue is the primary endpoint, QoL is a secondary endpoint, and overall survival will not be recorded. More details are available at clinicaltrials.gov.
“Fatigue is an important feature of cancer therapies in general and any approach to lessen the impact of fatigue should be welcome,” Dr. Garnick said. However, “it would have been helpful” if the official description of the trial had provided more information on the rationale for testing bright white light therapy in prostate cancer.
Metastatic castration-resistant prostate cancer. Adults with this diagnosis who have been treated with one prior androgen receptor axis-targeted therapy (ARAT) can enter a randomized, open-label, phase 2 trial to determine the best dose of the antibody-drug conjugate vobramitamab duocarmazine (MacroGenics). This experimental drug is designed to deliver an alkylating agent that promotes cell death in solid tumors expressing B7-H3. The B7-H3 protein rarely appears in normal tissues but is expressed at high frequency in 60% of cancers.
For approximately 2 years, participants will receive one of two doses of intravenous vobramitamab duocarmazine every 4 weeks. The trial opened in June 2023, looking to recruit 100 participants across nine states in the United States and eight other countries. The primary outcome measure is radiographic PFS. Overall survival and QoL will not be assessed. More details at clinicaltrials.gov.
Localized or biochemically recurrent prostate cancer. Adults in this position who have not received prior GnRH agonist or antagonist therapy are being recruited for a randomized, single-masked, phase 2 study comparing QoL among patients taking ADTs relugolix (Orgovyx, Relumina) and leuprolide acetate for depot suspension (Lupron Depot). For up to 1 year, people in the trial will either take daily tablets of relugolix or receive injections of leuprolide every 3 months. Three study sites in Massachusetts are due to open their doors in August 2023, seeking 110 participants. The study will assess various measures of QoL. Overall survival will not be measured. More details at clinicaltrials.gov.
This study is “sort of plain vanilla,” Dr. Garnick said. Although “the objectives of the study are important, the study number is small and unlikely to show any meaningful differences,” even if differences do exist.
All trial information is from the National Institutes of Health U.S. National Library of Medicine (online at clinicaltrials.gov). Dr. Garnick reported no relevant financial relationships. He is editor-in-chief of the Harvard Medical School Annual Report on Prostate Diseases, for which he receives an honorarium.
A version of this article first appeared on Medscape.com.
Risky drinking common in cancer survivors
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Race and Age-Related PSA Testing Disparities in Spinal Cord Injured Men: Analysis of National Veterans Health Administration Data
Prostate cancer will be diagnosed in 12.5% of men during their lifetime. It is the most commonly diagnosed solid organ cancer in men.1 However, prostate cancer screening for prostate-specific antigen (PSA) remains controversial due to concerns about overdiagnosis, as the overall risk of dying of prostate cancer is only 2.4%.1
To address the risk and benefits of PSA testing, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine PSA testing.2 Updated 2018 recommendations continued this recommendation in men aged > 70 years but acknowledged a small potential benefit in men aged 55 to 69 years and suggested individualized shared decision making between patient and clinician.3 In addition, American Urological Association (AUA) guidelines for the early detection of prostate cancer recommend against PSA screening in men aged < 40 years or those aged > 70 years, shared decision making for individuals aged 55 to 70 years or in high-risk men aged 40 to 55 years (ie, family history of prostate cancer or African American race).4 PSA screening is not recommended for men with a life expectancy shorter than 10 to 15 years aged > 70 years.4
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.5 In addition, the US Department of Veterans Affairs (VA) Spinal Cord Injury and Disorders System of Care operates 25 centers throughout the US.6 Life expectancy following spinal cord injury (SCI) increased significantly through the 1980s but has since plateaued, with life expectancy being impacted by age at injury, completeness of injury, and neurologic level.7,8 As part of a program of uniform care, all persons with SCI followed at the Spinal Cord Injury and Disorders System of Care centers are offered comprehensive annual evaluations, including screening laboratory tests, such as PSA level.9
Patients with SCI present a unique challenge when interpreting PSA levels, given potentially confounding factors, including neurogenic bladder management, high rates of bacteriuria, urinary tract infections (UTIs), testosterone deficiency, and pelvic innervation that differs from the noninjured population.10,11 Unfortunately, the literature on prostate cancer prevalence and average PSA levels in patients with SCI is limited by the small scope of studies and inconsistent data.10-16 Therefore, the purpose of the current investigation was to quantify and analyze the rates of annual PSA testing for all men with SCI in the VHA.
Methods
Approval was granted by the Richmond VA Medical Center (VAMC) Institutional Review Board in Virginia, and by the VA Informatics and Computing Infrastructure (VINCI) data access request tracker system for extraction of data from the VA Corporate Data Warehouse. Microsoft Structured Query Language was used for data programming and query design. Statistical analysis was conducted using Stata version 15.1 with assistance from professional biostatisticians.
Only male veterans with a nervous system disorder affecting the spinal cord or with myelopathy were included, based on International Classification of Diseases (ICD) version 9 and 10 codes, corresponding to traumatic and nontraumatic myelopathy. Veterans diagnosed with myelopathy based on ICD codes corresponding to progressive or degenerative myelopathies, such as multiple sclerosis or amyotrophic lateral sclerosis, were excluded.
For each veteran, extracted data included the unique identification number, date of birth, ICD code, date ICD code first appeared, race, gender, death status (yes/no), date of death (when applicable), date of each PSA test, PSA test values, and the VAMC where each test was performed. Only tests for total PSA were included. The date that the ICD code first appeared served as an approximation for the date of SCI. The time frame for the study included all PSA tests in the VINCI database for 2000 through 2017. However, only post-SCI PSA tests were included in the analysis. Duplicate tests (same date/time) were eliminated.
Race is considered a risk factor for prostate cancer only for African American patients, likely due to racial health disparities.17 Given this, we chose to categorize race as either African American or other, with a third category for missing/inconsistent reporting. Age at time of the PSA test was categorized into 4 groups (≤ 39, 40-54, 55-69, and ≥ 70 years) based on AUA guidelines.4 The annual PSA testing rate was calculated for each veteran with SCI as the number of PSA tests per year. A mean annual PSA test rate was then calculated as the weighted (by exposure time) mean value for all annual PSA testing rates from 2000 through 2017 for each age group and race. Annual exposure was calculated for each veteran and defined as the number of days a veteran was eligible to have a PSA test. This started with the date of SCI diagnosis and ended with either the date of death or the date of last PSA. If a veteran moved from one age group to another in 1 year, the first part of this year’s exposure was included in the calculation of the annual PSA testing rate for the younger group and the second part was included for the calculation of the older group. For deceased veterans, the death date was excluded from the exposure period, and their exposure period ended on the day before death.
Statistical Analysis
To compare PSA testing rates between African American race and other races, Poisson regression was used with exposure treated as an offset (exposures were summed across years for each veteran). An indicator (dummy) variable for African American race vs other races was coded, and statistical significance was set at P < .05. To check sensitivity for the Poisson assumption that the mean was equal to the variance, negative binomial regression was used. To assess for geographic PSA testing rate variability, the data were further analyzed based on the locations where PSA tests were performed. This subanalysis was limited to veterans who had all PSA tests in a single station. For each station, the average PSA testing rate was calculated for each veteran, and the mean for all annual PSA testing rates was used to determine station-specific PSA testing rates.
Results
A total of 45,274 veterans were initially identified of which 367 females were excluded (Figure 1).
The PSA testing rate rose for veterans in the age groups ≤ 39, 40 to 54, and 55 to 69 years (Figure 2A).
Of the cohort of 37,243 veterans, 28,396 (76.2%) had their post-SCI tests done at a single facility, 6770 (18.1%) at 2 locations, and 2077 (5.5%) at > 2 locations. Single-station group data were included in a subanalysis to determine the mean (SD) PSA testing rates, which for the 123 locations was 0.98 (0.36) tests per veteran per year (range, 0.2-3.0 tests per veteran per year).
To assess the impact of the 2012 USPSTF recommendations on PSA testing rates in veterans with SCI, mean PSA testing rates were calculated for 5 years before the recommendations (2007-2011) and compared with the average PSA testing rate for 5 years following the updated recommendations (2013-2017). The USPSTF updated its recommendation again in 2018 and acknowledged the potential benefit for PSA screening in certain patient populations.2,3 Surprisingly, and despite recommendations, the results show a significant increase in PSA testing rates in all age groups for all races (P < .001) (Figure 4).
Discussion
The goal of this study was to establish testing rates and analyze PSA testing trends across races and age groups in veterans with SCI. This is the largest cohort of patients with SCI analyzed in the literature. The key findings of this study were that despite clear AUA guidelines recommending against PSA testing in patients aged ≤ 39 years and ≥ 70 years, there are high rates of testing in veterans with SCI in these age groups (0.46 tests per year in those aged ≤ 39 years and 0.91 tests per year in those aged ≥ 70 years). In terms of race, as expected based on increased risk,
Prostate Cancer Incidence
Although the exact mechanism behind alterations in prostate function in the SCI population have yet to be fully elucidated, research suggests that the prostate behaves differently after SCI. Animal models of prostate gland denervation show decreased prostate volume and suggest that SCI may lead to a reduction in prostatic secretory function associated with autonomic dysfunction. Shim and colleagues hypothesized that impaired autonomic prostate innervation alters the prostatic volume and PSA in patients with SCI.10
Additional studies looking at actual PSA levels in men with SCI reveal conflicting data.10-15,20 Toricelli and colleagues retrospectively studied 140 men with SCI, of whom 34 had PSA levels available and found that mean PSA was not significantly different for patients with SCI compared with controls, but patients using clean intermittent catheterization had 2-fold higher PSA levels.21 In contrast, Konety and colleagues found that mean PSA was not significantly different from uninjured controls in their cohort of 79 patients with SCI, though they did find a correlation between indwelling catheter use and a higher PSA.22
Studies have shown an overall decreased risk of prostate cancer in patients with SCI, though the mechanism remains unclear. A large cohort study from Taiwan showed a lower risk of prostate cancer for 54,401 patients with SCI with an adjusted hazard ratio of 0.73.23 Patel and colleagues found the overall rate of prostate cancer in the population of veterans with SCI was lower than the general uninjured VA population, though this study was limited by scope with only 350 patients with SCI.24 A more recent systematic review and meta-analysis of 9 studies evaluating the prevalence of prostate cancer in men with SCI found a reduction of up to 65% in the risk of prostate cancer in men with SCI, and PSA was found to be a poor screening tool for prostate cancer due to large study heterogeneity.16
PSA Screening
This study identified widespread overscreening using the PSA test in veterans with SCI, which is likely attributable to many factors. Per VHA Directive 1176, all eligible veterans are offered yearly interdisciplinary comprehensive evaluations, including laboratory testing, and as such veterans with SCI have high rates of annual visit attendance due to the complexity of their care.9 PSA testing is included in the standard battery of laboratory tests ordered for all patients with SCI during their annual examinations. Additionally, many SCI specialists use the PSA level in patients with SCI for identifying cystitis or prostatitis in patients with colonization who may not experience typical symptoms. Everaert and colleagues demonstrated the clinical utility for localizing UTIs to the upper or lower tract, with elevated PSA indicating prostatitis. They found that serum PSA has a sensitivity of 68% and a specificity of 100% in the differential diagnosis of prostatitis and pyelonephritis.25 As such, the high PSA screening rates may be reflective of diagnostic use for infection rather than for cancer screening.
Likely as a response to the USPSTF recommendations, there has been a national slow decline in overall PSA screening rates since 2012.26-28 A study from Vetterlein and colleagues examining changes in the PSA screening trends related to USPSTF recommendations found an 8.5% decline in overall PSA screening from 2012 to 2014.29 However, the increase in PSA testing across all ages and races in the VA population with SCI over the same period is not entirely understood and suggests the need for further research and education in this area.
Limitations
This study is limited by the use of data identified by ICD codes rather than by review of individual health records. This required the use of decision algorithms for data points, such as the date of SCI. In addition, analysis was not able to capture shared decision making that may have contributed to PSA screening outside the recommended age ranges based on additional risk factors, such as family history of lethal malignancy. Furthermore, a detailed attempt to define specific age-adjusted PSA levels was beyond the scope of this study but will be addressed in later publications. In addition, we did not exclude individuals with a diagnosis of prostate adenocarcinoma, prostatitis, or recurrent UTIs because the onset, duration, and severity of disease could not be definitively ascertained. Finally, veterans with SCI are unique and may not be reflective of individuals with SCI who do not receive care within the VA. However, despite these limitations, this is, to our knowledge, the largest and most comprehensive study evaluating PSA testing rates in individuals with SCI.
Conclusions
Currently, PSA screening is recommended following shared decision making for patients at average risk aged 55 to 70 years. Patients with SCI experience many conditions that may affect PSA values, but data regarding normal PSA ranges and rates of prostate cancer in this population remain sparse. The study demonstrated high rates of overtesting in veterans with SCI, higher than expected testing rates in African American veterans, a paradoxical increase in PSA testing rates after the 2012 publication of the USPSTF PSA guidelines, and wide variability in testing rates depending on VA location.
African American men were tested at higher rates across all age groups, including in patients aged > 70 years. To balance the benefits of detecting clinically significant prostate cancer vs the risks of invasive testing in high-risk populations with SCI, more work is needed to determine the clinical impact of screening practices. Future work is currently ongoing to define age-based PSA values in patients with SCI.
Acknowledgments
This research was supported in part through funding from the Center for Rehabilitation Science and Engineering, Virginia Commonwealth University Health System.
1. American Cancer Society. Key statistics for prostate cancer. Updated January 12, 2023. Accessed June 2, 2023. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi:10.7326/0003-4819-157-2-201207170-00459
3. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi:10.1001/jama.2018.3710
4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi:10.1016/j.juro.2013.04.119
5. US Department of Veterans Affairs, Veterans Health Administration. Updated August 15, 2022. Accessed June 2, 2023. https://www.va.gov/health/aboutVHA.asp
6. US Department of Veterans Affairs. Spinal cord injuries and disorders system of care. Updated January 31, 2022. Accessed June 2, 2023. https://www.sci.va.gov/VAs_SCID_System_of_Care.asp
7. DeVivo MJ, Chen Y, Wen H. Cause of death trends among persons with spinal cord injury in the United States: 1960-2017. Arch Phys Med Rehabil. 2022;103(4):634-641. doi:10.1016/j.apmr.2021.09.019
8. Cao Y, DiPiro N, Krause JS. Health factors and spinal cord injury: a prospective study of risk of cause-specific mortality. Spinal Cord. 2019;57(7):594-602. doi:10.1038/s41393-019-0264-6
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1176(2): Spinal Cord Injuries and Disorders System of Care. Published September 30, 2019. Accessed June 2, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8523
10. Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9(2):115-120. doi:10.1038/sj.pcan.4500865
11. Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, Brackett NL. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162(1):89-91. doi:10.1097/00005392-199907000-00022
12. Bartoletti R, Gavazzi A, Cai T, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142-148. doi:10.1016/j.eururo.2008.01.088
13. Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62(5):845-848. doi:10.1016/s0090-4295(03)00654-x
14. Pramudji CK, Mutchnik SE, DeConcini D, Boone TB. Prostate cancer screening with prostate specific antigen in spinal cord injured men. J Urol. 2002;167(3):1303-1305.
15. Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171(6 Pt 1):2230-2232. doi:10.1097/01.ju.0000125241.77517.10
16. Barbonetti A, D’Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Risk of prostate cancer in men with spinal cord injury: a systematic review and meta-analysis. Asian J Androl. 2018;20(6):555-560. doi:10.4103/aja.aja_31_18
17. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.50416
18. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. 2017;18(10):81. Published 2017 Aug 14. doi:10.1007/s11934-017-0724-5
19. Jeong SH, Werneburg GT, Abouassaly R, Wood H. Acquired and congenital spinal cord injury is associated with lower likelihood of prostate specific antigen screening. Urology. 2022;164:178-183. doi:10.1016/j.urology.2022.01.044
20. Benaim EA, Montoya JD, Saboorian MH, Litwiller S, Roehrborn CG. Characterization of prostate size, PSA and endocrine profiles in patients with spinal cord injuries. Prostate Cancer Prostatic Dis. 1998;1(5):250-255. doi:10.1038/sj.pcan.4500246
21. Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, Bruschini H. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30(8):1522-1524. doi:10.1002/nau.21119
22. Konety BR, Nguyen TT, Brenes G, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56(1):82-86. doi:10.1016/s0090-4295(00)00548-3
23. Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32(1):51.e1-51.e517. doi:10.1016/j.urolonc.2013.07.019
24. Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3(7):633-636. doi:10.1016/j.pmrj.2011.04.024
25. Everaert K, Oostra C, Delanghe J, Vande Walle J, Van Laere M, Oosterlinck W. Diagnosis and localization of a complicated urinary tract infection in neurogenic bladder disease by tubular proteinuria and serum prostate specific antigen. Spinal Cord. 1998;36(1):33-38. doi:10.1038/sj.sc.3100520
26. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi:10.1200/JCO.2015.61.6532
27. Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314(19):2077-2079. doi:10.1001/jama.2015.7273
28. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi:10.1001/jama.2015.14905
29. Vetterlein MW, Dalela D, Sammon JD, et al. State-by-state variation in prostate-specific antigen screening trends following the 2011 United States Preventive Services Task Force panel update. Urology. 2018;112:56-65. doi:10.1016/j.urology.2017.08.055
Prostate cancer will be diagnosed in 12.5% of men during their lifetime. It is the most commonly diagnosed solid organ cancer in men.1 However, prostate cancer screening for prostate-specific antigen (PSA) remains controversial due to concerns about overdiagnosis, as the overall risk of dying of prostate cancer is only 2.4%.1
To address the risk and benefits of PSA testing, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine PSA testing.2 Updated 2018 recommendations continued this recommendation in men aged > 70 years but acknowledged a small potential benefit in men aged 55 to 69 years and suggested individualized shared decision making between patient and clinician.3 In addition, American Urological Association (AUA) guidelines for the early detection of prostate cancer recommend against PSA screening in men aged < 40 years or those aged > 70 years, shared decision making for individuals aged 55 to 70 years or in high-risk men aged 40 to 55 years (ie, family history of prostate cancer or African American race).4 PSA screening is not recommended for men with a life expectancy shorter than 10 to 15 years aged > 70 years.4
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.5 In addition, the US Department of Veterans Affairs (VA) Spinal Cord Injury and Disorders System of Care operates 25 centers throughout the US.6 Life expectancy following spinal cord injury (SCI) increased significantly through the 1980s but has since plateaued, with life expectancy being impacted by age at injury, completeness of injury, and neurologic level.7,8 As part of a program of uniform care, all persons with SCI followed at the Spinal Cord Injury and Disorders System of Care centers are offered comprehensive annual evaluations, including screening laboratory tests, such as PSA level.9
Patients with SCI present a unique challenge when interpreting PSA levels, given potentially confounding factors, including neurogenic bladder management, high rates of bacteriuria, urinary tract infections (UTIs), testosterone deficiency, and pelvic innervation that differs from the noninjured population.10,11 Unfortunately, the literature on prostate cancer prevalence and average PSA levels in patients with SCI is limited by the small scope of studies and inconsistent data.10-16 Therefore, the purpose of the current investigation was to quantify and analyze the rates of annual PSA testing for all men with SCI in the VHA.
Methods
Approval was granted by the Richmond VA Medical Center (VAMC) Institutional Review Board in Virginia, and by the VA Informatics and Computing Infrastructure (VINCI) data access request tracker system for extraction of data from the VA Corporate Data Warehouse. Microsoft Structured Query Language was used for data programming and query design. Statistical analysis was conducted using Stata version 15.1 with assistance from professional biostatisticians.
Only male veterans with a nervous system disorder affecting the spinal cord or with myelopathy were included, based on International Classification of Diseases (ICD) version 9 and 10 codes, corresponding to traumatic and nontraumatic myelopathy. Veterans diagnosed with myelopathy based on ICD codes corresponding to progressive or degenerative myelopathies, such as multiple sclerosis or amyotrophic lateral sclerosis, were excluded.
For each veteran, extracted data included the unique identification number, date of birth, ICD code, date ICD code first appeared, race, gender, death status (yes/no), date of death (when applicable), date of each PSA test, PSA test values, and the VAMC where each test was performed. Only tests for total PSA were included. The date that the ICD code first appeared served as an approximation for the date of SCI. The time frame for the study included all PSA tests in the VINCI database for 2000 through 2017. However, only post-SCI PSA tests were included in the analysis. Duplicate tests (same date/time) were eliminated.
Race is considered a risk factor for prostate cancer only for African American patients, likely due to racial health disparities.17 Given this, we chose to categorize race as either African American or other, with a third category for missing/inconsistent reporting. Age at time of the PSA test was categorized into 4 groups (≤ 39, 40-54, 55-69, and ≥ 70 years) based on AUA guidelines.4 The annual PSA testing rate was calculated for each veteran with SCI as the number of PSA tests per year. A mean annual PSA test rate was then calculated as the weighted (by exposure time) mean value for all annual PSA testing rates from 2000 through 2017 for each age group and race. Annual exposure was calculated for each veteran and defined as the number of days a veteran was eligible to have a PSA test. This started with the date of SCI diagnosis and ended with either the date of death or the date of last PSA. If a veteran moved from one age group to another in 1 year, the first part of this year’s exposure was included in the calculation of the annual PSA testing rate for the younger group and the second part was included for the calculation of the older group. For deceased veterans, the death date was excluded from the exposure period, and their exposure period ended on the day before death.
Statistical Analysis
To compare PSA testing rates between African American race and other races, Poisson regression was used with exposure treated as an offset (exposures were summed across years for each veteran). An indicator (dummy) variable for African American race vs other races was coded, and statistical significance was set at P < .05. To check sensitivity for the Poisson assumption that the mean was equal to the variance, negative binomial regression was used. To assess for geographic PSA testing rate variability, the data were further analyzed based on the locations where PSA tests were performed. This subanalysis was limited to veterans who had all PSA tests in a single station. For each station, the average PSA testing rate was calculated for each veteran, and the mean for all annual PSA testing rates was used to determine station-specific PSA testing rates.
Results
A total of 45,274 veterans were initially identified of which 367 females were excluded (Figure 1).
The PSA testing rate rose for veterans in the age groups ≤ 39, 40 to 54, and 55 to 69 years (Figure 2A).
Of the cohort of 37,243 veterans, 28,396 (76.2%) had their post-SCI tests done at a single facility, 6770 (18.1%) at 2 locations, and 2077 (5.5%) at > 2 locations. Single-station group data were included in a subanalysis to determine the mean (SD) PSA testing rates, which for the 123 locations was 0.98 (0.36) tests per veteran per year (range, 0.2-3.0 tests per veteran per year).
To assess the impact of the 2012 USPSTF recommendations on PSA testing rates in veterans with SCI, mean PSA testing rates were calculated for 5 years before the recommendations (2007-2011) and compared with the average PSA testing rate for 5 years following the updated recommendations (2013-2017). The USPSTF updated its recommendation again in 2018 and acknowledged the potential benefit for PSA screening in certain patient populations.2,3 Surprisingly, and despite recommendations, the results show a significant increase in PSA testing rates in all age groups for all races (P < .001) (Figure 4).
Discussion
The goal of this study was to establish testing rates and analyze PSA testing trends across races and age groups in veterans with SCI. This is the largest cohort of patients with SCI analyzed in the literature. The key findings of this study were that despite clear AUA guidelines recommending against PSA testing in patients aged ≤ 39 years and ≥ 70 years, there are high rates of testing in veterans with SCI in these age groups (0.46 tests per year in those aged ≤ 39 years and 0.91 tests per year in those aged ≥ 70 years). In terms of race, as expected based on increased risk,
Prostate Cancer Incidence
Although the exact mechanism behind alterations in prostate function in the SCI population have yet to be fully elucidated, research suggests that the prostate behaves differently after SCI. Animal models of prostate gland denervation show decreased prostate volume and suggest that SCI may lead to a reduction in prostatic secretory function associated with autonomic dysfunction. Shim and colleagues hypothesized that impaired autonomic prostate innervation alters the prostatic volume and PSA in patients with SCI.10
Additional studies looking at actual PSA levels in men with SCI reveal conflicting data.10-15,20 Toricelli and colleagues retrospectively studied 140 men with SCI, of whom 34 had PSA levels available and found that mean PSA was not significantly different for patients with SCI compared with controls, but patients using clean intermittent catheterization had 2-fold higher PSA levels.21 In contrast, Konety and colleagues found that mean PSA was not significantly different from uninjured controls in their cohort of 79 patients with SCI, though they did find a correlation between indwelling catheter use and a higher PSA.22
Studies have shown an overall decreased risk of prostate cancer in patients with SCI, though the mechanism remains unclear. A large cohort study from Taiwan showed a lower risk of prostate cancer for 54,401 patients with SCI with an adjusted hazard ratio of 0.73.23 Patel and colleagues found the overall rate of prostate cancer in the population of veterans with SCI was lower than the general uninjured VA population, though this study was limited by scope with only 350 patients with SCI.24 A more recent systematic review and meta-analysis of 9 studies evaluating the prevalence of prostate cancer in men with SCI found a reduction of up to 65% in the risk of prostate cancer in men with SCI, and PSA was found to be a poor screening tool for prostate cancer due to large study heterogeneity.16
PSA Screening
This study identified widespread overscreening using the PSA test in veterans with SCI, which is likely attributable to many factors. Per VHA Directive 1176, all eligible veterans are offered yearly interdisciplinary comprehensive evaluations, including laboratory testing, and as such veterans with SCI have high rates of annual visit attendance due to the complexity of their care.9 PSA testing is included in the standard battery of laboratory tests ordered for all patients with SCI during their annual examinations. Additionally, many SCI specialists use the PSA level in patients with SCI for identifying cystitis or prostatitis in patients with colonization who may not experience typical symptoms. Everaert and colleagues demonstrated the clinical utility for localizing UTIs to the upper or lower tract, with elevated PSA indicating prostatitis. They found that serum PSA has a sensitivity of 68% and a specificity of 100% in the differential diagnosis of prostatitis and pyelonephritis.25 As such, the high PSA screening rates may be reflective of diagnostic use for infection rather than for cancer screening.
Likely as a response to the USPSTF recommendations, there has been a national slow decline in overall PSA screening rates since 2012.26-28 A study from Vetterlein and colleagues examining changes in the PSA screening trends related to USPSTF recommendations found an 8.5% decline in overall PSA screening from 2012 to 2014.29 However, the increase in PSA testing across all ages and races in the VA population with SCI over the same period is not entirely understood and suggests the need for further research and education in this area.
Limitations
This study is limited by the use of data identified by ICD codes rather than by review of individual health records. This required the use of decision algorithms for data points, such as the date of SCI. In addition, analysis was not able to capture shared decision making that may have contributed to PSA screening outside the recommended age ranges based on additional risk factors, such as family history of lethal malignancy. Furthermore, a detailed attempt to define specific age-adjusted PSA levels was beyond the scope of this study but will be addressed in later publications. In addition, we did not exclude individuals with a diagnosis of prostate adenocarcinoma, prostatitis, or recurrent UTIs because the onset, duration, and severity of disease could not be definitively ascertained. Finally, veterans with SCI are unique and may not be reflective of individuals with SCI who do not receive care within the VA. However, despite these limitations, this is, to our knowledge, the largest and most comprehensive study evaluating PSA testing rates in individuals with SCI.
Conclusions
Currently, PSA screening is recommended following shared decision making for patients at average risk aged 55 to 70 years. Patients with SCI experience many conditions that may affect PSA values, but data regarding normal PSA ranges and rates of prostate cancer in this population remain sparse. The study demonstrated high rates of overtesting in veterans with SCI, higher than expected testing rates in African American veterans, a paradoxical increase in PSA testing rates after the 2012 publication of the USPSTF PSA guidelines, and wide variability in testing rates depending on VA location.
African American men were tested at higher rates across all age groups, including in patients aged > 70 years. To balance the benefits of detecting clinically significant prostate cancer vs the risks of invasive testing in high-risk populations with SCI, more work is needed to determine the clinical impact of screening practices. Future work is currently ongoing to define age-based PSA values in patients with SCI.
Acknowledgments
This research was supported in part through funding from the Center for Rehabilitation Science and Engineering, Virginia Commonwealth University Health System.
Prostate cancer will be diagnosed in 12.5% of men during their lifetime. It is the most commonly diagnosed solid organ cancer in men.1 However, prostate cancer screening for prostate-specific antigen (PSA) remains controversial due to concerns about overdiagnosis, as the overall risk of dying of prostate cancer is only 2.4%.1
To address the risk and benefits of PSA testing, in 2012 the US Preventive Services Task Force (USPSTF) recommended against routine PSA testing.2 Updated 2018 recommendations continued this recommendation in men aged > 70 years but acknowledged a small potential benefit in men aged 55 to 69 years and suggested individualized shared decision making between patient and clinician.3 In addition, American Urological Association (AUA) guidelines for the early detection of prostate cancer recommend against PSA screening in men aged < 40 years or those aged > 70 years, shared decision making for individuals aged 55 to 70 years or in high-risk men aged 40 to 55 years (ie, family history of prostate cancer or African American race).4 PSA screening is not recommended for men with a life expectancy shorter than 10 to 15 years aged > 70 years.4
The Veterans Health Administration (VHA) is the largest integrated health care system in the US.5 In addition, the US Department of Veterans Affairs (VA) Spinal Cord Injury and Disorders System of Care operates 25 centers throughout the US.6 Life expectancy following spinal cord injury (SCI) increased significantly through the 1980s but has since plateaued, with life expectancy being impacted by age at injury, completeness of injury, and neurologic level.7,8 As part of a program of uniform care, all persons with SCI followed at the Spinal Cord Injury and Disorders System of Care centers are offered comprehensive annual evaluations, including screening laboratory tests, such as PSA level.9
Patients with SCI present a unique challenge when interpreting PSA levels, given potentially confounding factors, including neurogenic bladder management, high rates of bacteriuria, urinary tract infections (UTIs), testosterone deficiency, and pelvic innervation that differs from the noninjured population.10,11 Unfortunately, the literature on prostate cancer prevalence and average PSA levels in patients with SCI is limited by the small scope of studies and inconsistent data.10-16 Therefore, the purpose of the current investigation was to quantify and analyze the rates of annual PSA testing for all men with SCI in the VHA.
Methods
Approval was granted by the Richmond VA Medical Center (VAMC) Institutional Review Board in Virginia, and by the VA Informatics and Computing Infrastructure (VINCI) data access request tracker system for extraction of data from the VA Corporate Data Warehouse. Microsoft Structured Query Language was used for data programming and query design. Statistical analysis was conducted using Stata version 15.1 with assistance from professional biostatisticians.
Only male veterans with a nervous system disorder affecting the spinal cord or with myelopathy were included, based on International Classification of Diseases (ICD) version 9 and 10 codes, corresponding to traumatic and nontraumatic myelopathy. Veterans diagnosed with myelopathy based on ICD codes corresponding to progressive or degenerative myelopathies, such as multiple sclerosis or amyotrophic lateral sclerosis, were excluded.
For each veteran, extracted data included the unique identification number, date of birth, ICD code, date ICD code first appeared, race, gender, death status (yes/no), date of death (when applicable), date of each PSA test, PSA test values, and the VAMC where each test was performed. Only tests for total PSA were included. The date that the ICD code first appeared served as an approximation for the date of SCI. The time frame for the study included all PSA tests in the VINCI database for 2000 through 2017. However, only post-SCI PSA tests were included in the analysis. Duplicate tests (same date/time) were eliminated.
Race is considered a risk factor for prostate cancer only for African American patients, likely due to racial health disparities.17 Given this, we chose to categorize race as either African American or other, with a third category for missing/inconsistent reporting. Age at time of the PSA test was categorized into 4 groups (≤ 39, 40-54, 55-69, and ≥ 70 years) based on AUA guidelines.4 The annual PSA testing rate was calculated for each veteran with SCI as the number of PSA tests per year. A mean annual PSA test rate was then calculated as the weighted (by exposure time) mean value for all annual PSA testing rates from 2000 through 2017 for each age group and race. Annual exposure was calculated for each veteran and defined as the number of days a veteran was eligible to have a PSA test. This started with the date of SCI diagnosis and ended with either the date of death or the date of last PSA. If a veteran moved from one age group to another in 1 year, the first part of this year’s exposure was included in the calculation of the annual PSA testing rate for the younger group and the second part was included for the calculation of the older group. For deceased veterans, the death date was excluded from the exposure period, and their exposure period ended on the day before death.
Statistical Analysis
To compare PSA testing rates between African American race and other races, Poisson regression was used with exposure treated as an offset (exposures were summed across years for each veteran). An indicator (dummy) variable for African American race vs other races was coded, and statistical significance was set at P < .05. To check sensitivity for the Poisson assumption that the mean was equal to the variance, negative binomial regression was used. To assess for geographic PSA testing rate variability, the data were further analyzed based on the locations where PSA tests were performed. This subanalysis was limited to veterans who had all PSA tests in a single station. For each station, the average PSA testing rate was calculated for each veteran, and the mean for all annual PSA testing rates was used to determine station-specific PSA testing rates.
Results
A total of 45,274 veterans were initially identified of which 367 females were excluded (Figure 1).
The PSA testing rate rose for veterans in the age groups ≤ 39, 40 to 54, and 55 to 69 years (Figure 2A).
Of the cohort of 37,243 veterans, 28,396 (76.2%) had their post-SCI tests done at a single facility, 6770 (18.1%) at 2 locations, and 2077 (5.5%) at > 2 locations. Single-station group data were included in a subanalysis to determine the mean (SD) PSA testing rates, which for the 123 locations was 0.98 (0.36) tests per veteran per year (range, 0.2-3.0 tests per veteran per year).
To assess the impact of the 2012 USPSTF recommendations on PSA testing rates in veterans with SCI, mean PSA testing rates were calculated for 5 years before the recommendations (2007-2011) and compared with the average PSA testing rate for 5 years following the updated recommendations (2013-2017). The USPSTF updated its recommendation again in 2018 and acknowledged the potential benefit for PSA screening in certain patient populations.2,3 Surprisingly, and despite recommendations, the results show a significant increase in PSA testing rates in all age groups for all races (P < .001) (Figure 4).
Discussion
The goal of this study was to establish testing rates and analyze PSA testing trends across races and age groups in veterans with SCI. This is the largest cohort of patients with SCI analyzed in the literature. The key findings of this study were that despite clear AUA guidelines recommending against PSA testing in patients aged ≤ 39 years and ≥ 70 years, there are high rates of testing in veterans with SCI in these age groups (0.46 tests per year in those aged ≤ 39 years and 0.91 tests per year in those aged ≥ 70 years). In terms of race, as expected based on increased risk,
Prostate Cancer Incidence
Although the exact mechanism behind alterations in prostate function in the SCI population have yet to be fully elucidated, research suggests that the prostate behaves differently after SCI. Animal models of prostate gland denervation show decreased prostate volume and suggest that SCI may lead to a reduction in prostatic secretory function associated with autonomic dysfunction. Shim and colleagues hypothesized that impaired autonomic prostate innervation alters the prostatic volume and PSA in patients with SCI.10
Additional studies looking at actual PSA levels in men with SCI reveal conflicting data.10-15,20 Toricelli and colleagues retrospectively studied 140 men with SCI, of whom 34 had PSA levels available and found that mean PSA was not significantly different for patients with SCI compared with controls, but patients using clean intermittent catheterization had 2-fold higher PSA levels.21 In contrast, Konety and colleagues found that mean PSA was not significantly different from uninjured controls in their cohort of 79 patients with SCI, though they did find a correlation between indwelling catheter use and a higher PSA.22
Studies have shown an overall decreased risk of prostate cancer in patients with SCI, though the mechanism remains unclear. A large cohort study from Taiwan showed a lower risk of prostate cancer for 54,401 patients with SCI with an adjusted hazard ratio of 0.73.23 Patel and colleagues found the overall rate of prostate cancer in the population of veterans with SCI was lower than the general uninjured VA population, though this study was limited by scope with only 350 patients with SCI.24 A more recent systematic review and meta-analysis of 9 studies evaluating the prevalence of prostate cancer in men with SCI found a reduction of up to 65% in the risk of prostate cancer in men with SCI, and PSA was found to be a poor screening tool for prostate cancer due to large study heterogeneity.16
PSA Screening
This study identified widespread overscreening using the PSA test in veterans with SCI, which is likely attributable to many factors. Per VHA Directive 1176, all eligible veterans are offered yearly interdisciplinary comprehensive evaluations, including laboratory testing, and as such veterans with SCI have high rates of annual visit attendance due to the complexity of their care.9 PSA testing is included in the standard battery of laboratory tests ordered for all patients with SCI during their annual examinations. Additionally, many SCI specialists use the PSA level in patients with SCI for identifying cystitis or prostatitis in patients with colonization who may not experience typical symptoms. Everaert and colleagues demonstrated the clinical utility for localizing UTIs to the upper or lower tract, with elevated PSA indicating prostatitis. They found that serum PSA has a sensitivity of 68% and a specificity of 100% in the differential diagnosis of prostatitis and pyelonephritis.25 As such, the high PSA screening rates may be reflective of diagnostic use for infection rather than for cancer screening.
Likely as a response to the USPSTF recommendations, there has been a national slow decline in overall PSA screening rates since 2012.26-28 A study from Vetterlein and colleagues examining changes in the PSA screening trends related to USPSTF recommendations found an 8.5% decline in overall PSA screening from 2012 to 2014.29 However, the increase in PSA testing across all ages and races in the VA population with SCI over the same period is not entirely understood and suggests the need for further research and education in this area.
Limitations
This study is limited by the use of data identified by ICD codes rather than by review of individual health records. This required the use of decision algorithms for data points, such as the date of SCI. In addition, analysis was not able to capture shared decision making that may have contributed to PSA screening outside the recommended age ranges based on additional risk factors, such as family history of lethal malignancy. Furthermore, a detailed attempt to define specific age-adjusted PSA levels was beyond the scope of this study but will be addressed in later publications. In addition, we did not exclude individuals with a diagnosis of prostate adenocarcinoma, prostatitis, or recurrent UTIs because the onset, duration, and severity of disease could not be definitively ascertained. Finally, veterans with SCI are unique and may not be reflective of individuals with SCI who do not receive care within the VA. However, despite these limitations, this is, to our knowledge, the largest and most comprehensive study evaluating PSA testing rates in individuals with SCI.
Conclusions
Currently, PSA screening is recommended following shared decision making for patients at average risk aged 55 to 70 years. Patients with SCI experience many conditions that may affect PSA values, but data regarding normal PSA ranges and rates of prostate cancer in this population remain sparse. The study demonstrated high rates of overtesting in veterans with SCI, higher than expected testing rates in African American veterans, a paradoxical increase in PSA testing rates after the 2012 publication of the USPSTF PSA guidelines, and wide variability in testing rates depending on VA location.
African American men were tested at higher rates across all age groups, including in patients aged > 70 years. To balance the benefits of detecting clinically significant prostate cancer vs the risks of invasive testing in high-risk populations with SCI, more work is needed to determine the clinical impact of screening practices. Future work is currently ongoing to define age-based PSA values in patients with SCI.
Acknowledgments
This research was supported in part through funding from the Center for Rehabilitation Science and Engineering, Virginia Commonwealth University Health System.
1. American Cancer Society. Key statistics for prostate cancer. Updated January 12, 2023. Accessed June 2, 2023. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi:10.7326/0003-4819-157-2-201207170-00459
3. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi:10.1001/jama.2018.3710
4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi:10.1016/j.juro.2013.04.119
5. US Department of Veterans Affairs, Veterans Health Administration. Updated August 15, 2022. Accessed June 2, 2023. https://www.va.gov/health/aboutVHA.asp
6. US Department of Veterans Affairs. Spinal cord injuries and disorders system of care. Updated January 31, 2022. Accessed June 2, 2023. https://www.sci.va.gov/VAs_SCID_System_of_Care.asp
7. DeVivo MJ, Chen Y, Wen H. Cause of death trends among persons with spinal cord injury in the United States: 1960-2017. Arch Phys Med Rehabil. 2022;103(4):634-641. doi:10.1016/j.apmr.2021.09.019
8. Cao Y, DiPiro N, Krause JS. Health factors and spinal cord injury: a prospective study of risk of cause-specific mortality. Spinal Cord. 2019;57(7):594-602. doi:10.1038/s41393-019-0264-6
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1176(2): Spinal Cord Injuries and Disorders System of Care. Published September 30, 2019. Accessed June 2, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8523
10. Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9(2):115-120. doi:10.1038/sj.pcan.4500865
11. Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, Brackett NL. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162(1):89-91. doi:10.1097/00005392-199907000-00022
12. Bartoletti R, Gavazzi A, Cai T, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142-148. doi:10.1016/j.eururo.2008.01.088
13. Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62(5):845-848. doi:10.1016/s0090-4295(03)00654-x
14. Pramudji CK, Mutchnik SE, DeConcini D, Boone TB. Prostate cancer screening with prostate specific antigen in spinal cord injured men. J Urol. 2002;167(3):1303-1305.
15. Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171(6 Pt 1):2230-2232. doi:10.1097/01.ju.0000125241.77517.10
16. Barbonetti A, D’Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Risk of prostate cancer in men with spinal cord injury: a systematic review and meta-analysis. Asian J Androl. 2018;20(6):555-560. doi:10.4103/aja.aja_31_18
17. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.50416
18. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. 2017;18(10):81. Published 2017 Aug 14. doi:10.1007/s11934-017-0724-5
19. Jeong SH, Werneburg GT, Abouassaly R, Wood H. Acquired and congenital spinal cord injury is associated with lower likelihood of prostate specific antigen screening. Urology. 2022;164:178-183. doi:10.1016/j.urology.2022.01.044
20. Benaim EA, Montoya JD, Saboorian MH, Litwiller S, Roehrborn CG. Characterization of prostate size, PSA and endocrine profiles in patients with spinal cord injuries. Prostate Cancer Prostatic Dis. 1998;1(5):250-255. doi:10.1038/sj.pcan.4500246
21. Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, Bruschini H. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30(8):1522-1524. doi:10.1002/nau.21119
22. Konety BR, Nguyen TT, Brenes G, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56(1):82-86. doi:10.1016/s0090-4295(00)00548-3
23. Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32(1):51.e1-51.e517. doi:10.1016/j.urolonc.2013.07.019
24. Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3(7):633-636. doi:10.1016/j.pmrj.2011.04.024
25. Everaert K, Oostra C, Delanghe J, Vande Walle J, Van Laere M, Oosterlinck W. Diagnosis and localization of a complicated urinary tract infection in neurogenic bladder disease by tubular proteinuria and serum prostate specific antigen. Spinal Cord. 1998;36(1):33-38. doi:10.1038/sj.sc.3100520
26. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi:10.1200/JCO.2015.61.6532
27. Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314(19):2077-2079. doi:10.1001/jama.2015.7273
28. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi:10.1001/jama.2015.14905
29. Vetterlein MW, Dalela D, Sammon JD, et al. State-by-state variation in prostate-specific antigen screening trends following the 2011 United States Preventive Services Task Force panel update. Urology. 2018;112:56-65. doi:10.1016/j.urology.2017.08.055
1. American Cancer Society. Key statistics for prostate cancer. Updated January 12, 2023. Accessed June 2, 2023. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120-134. doi:10.7326/0003-4819-157-2-201207170-00459
3. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi:10.1001/jama.2018.3710
4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi:10.1016/j.juro.2013.04.119
5. US Department of Veterans Affairs, Veterans Health Administration. Updated August 15, 2022. Accessed June 2, 2023. https://www.va.gov/health/aboutVHA.asp
6. US Department of Veterans Affairs. Spinal cord injuries and disorders system of care. Updated January 31, 2022. Accessed June 2, 2023. https://www.sci.va.gov/VAs_SCID_System_of_Care.asp
7. DeVivo MJ, Chen Y, Wen H. Cause of death trends among persons with spinal cord injury in the United States: 1960-2017. Arch Phys Med Rehabil. 2022;103(4):634-641. doi:10.1016/j.apmr.2021.09.019
8. Cao Y, DiPiro N, Krause JS. Health factors and spinal cord injury: a prospective study of risk of cause-specific mortality. Spinal Cord. 2019;57(7):594-602. doi:10.1038/s41393-019-0264-6
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1176(2): Spinal Cord Injuries and Disorders System of Care. Published September 30, 2019. Accessed June 2, 2023. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8523
10. Shim HB, Jung TY, Lee JK, Ku JH. Prostate activity and prostate cancer in spinal cord injury. Prostate Cancer Prostatic Dis. 2006;9(2):115-120. doi:10.1038/sj.pcan.4500865
11. Lynne CM, Aballa TC, Wang TJ, Rittenhouse HG, Ferrell SM, Brackett NL. Serum and semen prostate specific antigen concentrations are different in young spinal cord injured men compared to normal controls. J Urol. 1999;162(1):89-91. doi:10.1097/00005392-199907000-00022
12. Bartoletti R, Gavazzi A, Cai T, et al. Prostate growth and prevalence of prostate diseases in early onset spinal cord injuries. Eur Urol. 2009;56(1):142-148. doi:10.1016/j.eururo.2008.01.088
13. Pannek J, Berges RR, Cubick G, Meindl R, Senge T. Prostate size and PSA serum levels in male patients with spinal cord injury. Urology. 2003;62(5):845-848. doi:10.1016/s0090-4295(03)00654-x
14. Pramudji CK, Mutchnik SE, DeConcini D, Boone TB. Prostate cancer screening with prostate specific antigen in spinal cord injured men. J Urol. 2002;167(3):1303-1305.
15. Alexandrino AP, Rodrigues MA, Matsuo T. Evaluation of serum and seminal levels of prostate specific antigen in men with spinal cord injury. J Urol. 2004;171(6 Pt 1):2230-2232. doi:10.1097/01.ju.0000125241.77517.10
16. Barbonetti A, D’Andrea S, Martorella A, Felzani G, Francavilla S, Francavilla F. Risk of prostate cancer in men with spinal cord injury: a systematic review and meta-analysis. Asian J Androl. 2018;20(6):555-560. doi:10.4103/aja.aja_31_18
17. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.50416
18. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. 2017;18(10):81. Published 2017 Aug 14. doi:10.1007/s11934-017-0724-5
19. Jeong SH, Werneburg GT, Abouassaly R, Wood H. Acquired and congenital spinal cord injury is associated with lower likelihood of prostate specific antigen screening. Urology. 2022;164:178-183. doi:10.1016/j.urology.2022.01.044
20. Benaim EA, Montoya JD, Saboorian MH, Litwiller S, Roehrborn CG. Characterization of prostate size, PSA and endocrine profiles in patients with spinal cord injuries. Prostate Cancer Prostatic Dis. 1998;1(5):250-255. doi:10.1038/sj.pcan.4500246
21. Torricelli FC, Lucon M, Vicentini F, Gomes CM, Srougi M, Bruschini H. PSA levels in men with spinal cord injury and under intermittent catheterization. Neurourol Urodyn. 2011;30(8):1522-1524. doi:10.1002/nau.21119
22. Konety BR, Nguyen TT, Brenes G, et al. Evaluation of the effect of spinal cord injury on serum PSA levels. Urology. 2000;56(1):82-86. doi:10.1016/s0090-4295(00)00548-3
23. Lee WY, Sun LM, Lin CL, et al. Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol. 2014;32(1):51.e1-51.e517. doi:10.1016/j.urolonc.2013.07.019
24. Patel N, Ngo K, Hastings J, Ketchum N, Sepahpanah F. Prevalence of prostate cancer in patients with chronic spinal cord injury. PM R. 2011;3(7):633-636. doi:10.1016/j.pmrj.2011.04.024
25. Everaert K, Oostra C, Delanghe J, Vande Walle J, Van Laere M, Oosterlinck W. Diagnosis and localization of a complicated urinary tract infection in neurogenic bladder disease by tubular proteinuria and serum prostate specific antigen. Spinal Cord. 1998;36(1):33-38. doi:10.1038/sj.sc.3100520
26. Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. doi:10.1200/JCO.2015.61.6532
27. Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314(19):2077-2079. doi:10.1001/jama.2015.7273
28. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. doi:10.1001/jama.2015.14905
29. Vetterlein MW, Dalela D, Sammon JD, et al. State-by-state variation in prostate-specific antigen screening trends following the 2011 United States Preventive Services Task Force panel update. Urology. 2018;112:56-65. doi:10.1016/j.urology.2017.08.055
FDA OKs combo therapy of niraparib, abiraterone acetate for prostate cancer
The Food and Drug Administration has approved niraparib and abiraterone acetate (Akeega, Janssen Pharmaceuticals) to treat BRCA-positive, metastatic castration-resistant prostate cancer in adult patients with deleterious or suspected deleterious disease, as determined by an FDA-approved test.
The FDA’s approval was based on findings from the phase 3 MAGNITUDE precision medicine study, a randomized, placebo-controlled trial with 423 patients, 225 (53%) of whom had BRCA gene mutations as determined using a tissue assay such as FoundationOne CDx.
Among the subgroup with a BRCA mutation, radiographic progression-free survival was a median of 16.6 months vs. 10.9 months (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.36-0.79; P = .0014). In this subgroup, an exploratory overall survival analysis demonstrated a median of 30.4 months vs. 28.6 months (HR, 0.79; 95% CI, 0.55-1.12), favoring the treatment arm.
Although the overall cohort (those with and without BRCA mutations) demonstrated a significant improvement in radiographic progression-free survival, the subgroup with non-BRCA homologous recombination repair mutations did not demonstrate a significant improvement in radiographic progression-free survival, which indicates that the benefit observed was “primarily attributed” to the results in the subgroup of patients with BRCA mutations, according to the FDA.
The safety profile of niraparib and abiraterone acetate plus prednisone was consistent with the known safety profile of each FDA-approved monotherapy. Serious adverse events occurred in 41% of patients in the treatment arm. These most often included musculoskeletal pain (44% vs. 42%), fatigue (43% vs. 30%), constipation (34% vs. 20%), hypertension (33% vs. 27%), and nausea (33% vs. 21%).
An adverse reaction led to permanent discontinuation of treatment in 15% of patients.
“As a physician, identifying patients with a worse prognosis is a priority, especially those whose cancers have a BRCA mutation,” principal investigator Kim Chi, MD, stated in the Janssen press release. “We prospectively designed the MAGNITUDE study to identify the subset of patients most likely to benefit from targeted treatment with AKEEGA and to help us understand how we can potentially achieve better health outcomes for patients.”
About 10%-15% of patients who develop metastatic castration-resistant prostate cancer have BRCA gene alterations, and those patients are more likely to have aggressive disease, poor outcomes, and shorter survival. Therefore, this new agent “brings an important treatment option to patients with prostate cancer as they consider their road ahead,” said Shelby Moneer, vice president of patient programs and education at ZERO Prostate Cancer.
The prescribing information lists the recommended dose at 200 mg niraparib and 1,000 mg abiraterone once daily in combination with 10 mg of prednisone daily until disease progression or unacceptable toxicity. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy.
Health care professionals should report all serious adverse events suspected to be associated with the use of any medicine and device by using the FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved niraparib and abiraterone acetate (Akeega, Janssen Pharmaceuticals) to treat BRCA-positive, metastatic castration-resistant prostate cancer in adult patients with deleterious or suspected deleterious disease, as determined by an FDA-approved test.
The FDA’s approval was based on findings from the phase 3 MAGNITUDE precision medicine study, a randomized, placebo-controlled trial with 423 patients, 225 (53%) of whom had BRCA gene mutations as determined using a tissue assay such as FoundationOne CDx.
Among the subgroup with a BRCA mutation, radiographic progression-free survival was a median of 16.6 months vs. 10.9 months (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.36-0.79; P = .0014). In this subgroup, an exploratory overall survival analysis demonstrated a median of 30.4 months vs. 28.6 months (HR, 0.79; 95% CI, 0.55-1.12), favoring the treatment arm.
Although the overall cohort (those with and without BRCA mutations) demonstrated a significant improvement in radiographic progression-free survival, the subgroup with non-BRCA homologous recombination repair mutations did not demonstrate a significant improvement in radiographic progression-free survival, which indicates that the benefit observed was “primarily attributed” to the results in the subgroup of patients with BRCA mutations, according to the FDA.
The safety profile of niraparib and abiraterone acetate plus prednisone was consistent with the known safety profile of each FDA-approved monotherapy. Serious adverse events occurred in 41% of patients in the treatment arm. These most often included musculoskeletal pain (44% vs. 42%), fatigue (43% vs. 30%), constipation (34% vs. 20%), hypertension (33% vs. 27%), and nausea (33% vs. 21%).
An adverse reaction led to permanent discontinuation of treatment in 15% of patients.
“As a physician, identifying patients with a worse prognosis is a priority, especially those whose cancers have a BRCA mutation,” principal investigator Kim Chi, MD, stated in the Janssen press release. “We prospectively designed the MAGNITUDE study to identify the subset of patients most likely to benefit from targeted treatment with AKEEGA and to help us understand how we can potentially achieve better health outcomes for patients.”
About 10%-15% of patients who develop metastatic castration-resistant prostate cancer have BRCA gene alterations, and those patients are more likely to have aggressive disease, poor outcomes, and shorter survival. Therefore, this new agent “brings an important treatment option to patients with prostate cancer as they consider their road ahead,” said Shelby Moneer, vice president of patient programs and education at ZERO Prostate Cancer.
The prescribing information lists the recommended dose at 200 mg niraparib and 1,000 mg abiraterone once daily in combination with 10 mg of prednisone daily until disease progression or unacceptable toxicity. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy.
Health care professionals should report all serious adverse events suspected to be associated with the use of any medicine and device by using the FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved niraparib and abiraterone acetate (Akeega, Janssen Pharmaceuticals) to treat BRCA-positive, metastatic castration-resistant prostate cancer in adult patients with deleterious or suspected deleterious disease, as determined by an FDA-approved test.
The FDA’s approval was based on findings from the phase 3 MAGNITUDE precision medicine study, a randomized, placebo-controlled trial with 423 patients, 225 (53%) of whom had BRCA gene mutations as determined using a tissue assay such as FoundationOne CDx.
Among the subgroup with a BRCA mutation, radiographic progression-free survival was a median of 16.6 months vs. 10.9 months (hazard ratio [HR], 0.53; 95% confidence interval [CI], 0.36-0.79; P = .0014). In this subgroup, an exploratory overall survival analysis demonstrated a median of 30.4 months vs. 28.6 months (HR, 0.79; 95% CI, 0.55-1.12), favoring the treatment arm.
Although the overall cohort (those with and without BRCA mutations) demonstrated a significant improvement in radiographic progression-free survival, the subgroup with non-BRCA homologous recombination repair mutations did not demonstrate a significant improvement in radiographic progression-free survival, which indicates that the benefit observed was “primarily attributed” to the results in the subgroup of patients with BRCA mutations, according to the FDA.
The safety profile of niraparib and abiraterone acetate plus prednisone was consistent with the known safety profile of each FDA-approved monotherapy. Serious adverse events occurred in 41% of patients in the treatment arm. These most often included musculoskeletal pain (44% vs. 42%), fatigue (43% vs. 30%), constipation (34% vs. 20%), hypertension (33% vs. 27%), and nausea (33% vs. 21%).
An adverse reaction led to permanent discontinuation of treatment in 15% of patients.
“As a physician, identifying patients with a worse prognosis is a priority, especially those whose cancers have a BRCA mutation,” principal investigator Kim Chi, MD, stated in the Janssen press release. “We prospectively designed the MAGNITUDE study to identify the subset of patients most likely to benefit from targeted treatment with AKEEGA and to help us understand how we can potentially achieve better health outcomes for patients.”
About 10%-15% of patients who develop metastatic castration-resistant prostate cancer have BRCA gene alterations, and those patients are more likely to have aggressive disease, poor outcomes, and shorter survival. Therefore, this new agent “brings an important treatment option to patients with prostate cancer as they consider their road ahead,” said Shelby Moneer, vice president of patient programs and education at ZERO Prostate Cancer.
The prescribing information lists the recommended dose at 200 mg niraparib and 1,000 mg abiraterone once daily in combination with 10 mg of prednisone daily until disease progression or unacceptable toxicity. Patients should also receive a gonadotropin-releasing hormone analog concurrently or should have had bilateral orchiectomy.
Health care professionals should report all serious adverse events suspected to be associated with the use of any medicine and device by using the FDA’s MedWatch Reporting System or by calling 1-800-FDA-1088.
A version of this article appeared on Medscape.com.
MRI-guided SBRT cuts radiation toxicity in prostate cancer
TOPLINE
The use of magnetic resonance–guided daily adaptive stereotactic body radiotherapy for patients with prostate cancer reduces the risk of acute urinary side effects of grade 2 or higher by 44% and the risk of acute bowel side effects of grade 2 or higher by 60%, compared with standard CT-guided SBRT (CT‐SBRT).
METHODOLOGY
- With the use of magnetic resonance–guided daily adaptive SBRT, clinicians can customize radiation dosing to accommodate changes in prostate anatomy during treatment, which may also make SBRT safer and less toxic for patients.
- To determine whether this approach does reduce patient side effects, investigators ran a meta-analysis that included 29 studies with 2547 patients comparing the incidence of short-term, physician-assessed bowel and genitourinary side effects between the MRI-guided approach and standard CT-SBRT.
- The investigators reported no statistically significant differences in age, prescribed radiation doses, planning target volumes, or International Prostatism Symptom Scores between the two groups; the use of rectal spacers and the number of patients who received pelvic lymph node radiation were low in both.
- The average window for collecting acute toxicity data was 70 days in the MRI-guided investigations and 94 days in CT-SBRT investigations.
TAKEAWAY
- (odds ratio, 0.56; P = .04).
- The pooled estimate for grade 2 or higher gastrointestinal toxicity was 4% with the MRI approach versus 9% with CT-SBRT (OR, 0.40; P = .04).
- There were no differences in grade 3 or higher events, which were rare, between the groups.
- There was also no difference in toxicity among CT‐SBRT studies that used fiducial markers and those that did not.
IN PRACTICE
“These findings suggest that the technical advantages in precision of radiotherapy delivery afforded by [MRI-guided] SBRT translate to measurable clinical benefit,” the authors concluded. Potential reasons for the reduced risk of acute toxicity with the MRI-guided approach include “daily online adaptive planning, MRI‐based contouring that results in smaller treatment volumes, and MRI tracking, all of which may facilitate the precision and accuracy of treatment delivery.”
SOURCE
The study was led by Jonathan Leeman, MD, of the Dana-Farber Cancer Institute, Boston, and was published July 24 in Cancer.
LIMITATIONS
- The analysis did not account for differences in dosimetry, radiation planning, and toxicity management and assessment between the studies.
- Late toxicity and cancer control rates were not tracked and may have differed between the two approaches.
DISCLOSURES
- No external funding was reported.
- The investigators reported grants and consulting, personal, and other payments from Novartis, AstraZeneca, Janssen, and other companies.
A version of this article appeared on Medscape.com.
TOPLINE
The use of magnetic resonance–guided daily adaptive stereotactic body radiotherapy for patients with prostate cancer reduces the risk of acute urinary side effects of grade 2 or higher by 44% and the risk of acute bowel side effects of grade 2 or higher by 60%, compared with standard CT-guided SBRT (CT‐SBRT).
METHODOLOGY
- With the use of magnetic resonance–guided daily adaptive SBRT, clinicians can customize radiation dosing to accommodate changes in prostate anatomy during treatment, which may also make SBRT safer and less toxic for patients.
- To determine whether this approach does reduce patient side effects, investigators ran a meta-analysis that included 29 studies with 2547 patients comparing the incidence of short-term, physician-assessed bowel and genitourinary side effects between the MRI-guided approach and standard CT-SBRT.
- The investigators reported no statistically significant differences in age, prescribed radiation doses, planning target volumes, or International Prostatism Symptom Scores between the two groups; the use of rectal spacers and the number of patients who received pelvic lymph node radiation were low in both.
- The average window for collecting acute toxicity data was 70 days in the MRI-guided investigations and 94 days in CT-SBRT investigations.
TAKEAWAY
- (odds ratio, 0.56; P = .04).
- The pooled estimate for grade 2 or higher gastrointestinal toxicity was 4% with the MRI approach versus 9% with CT-SBRT (OR, 0.40; P = .04).
- There were no differences in grade 3 or higher events, which were rare, between the groups.
- There was also no difference in toxicity among CT‐SBRT studies that used fiducial markers and those that did not.
IN PRACTICE
“These findings suggest that the technical advantages in precision of radiotherapy delivery afforded by [MRI-guided] SBRT translate to measurable clinical benefit,” the authors concluded. Potential reasons for the reduced risk of acute toxicity with the MRI-guided approach include “daily online adaptive planning, MRI‐based contouring that results in smaller treatment volumes, and MRI tracking, all of which may facilitate the precision and accuracy of treatment delivery.”
SOURCE
The study was led by Jonathan Leeman, MD, of the Dana-Farber Cancer Institute, Boston, and was published July 24 in Cancer.
LIMITATIONS
- The analysis did not account for differences in dosimetry, radiation planning, and toxicity management and assessment between the studies.
- Late toxicity and cancer control rates were not tracked and may have differed between the two approaches.
DISCLOSURES
- No external funding was reported.
- The investigators reported grants and consulting, personal, and other payments from Novartis, AstraZeneca, Janssen, and other companies.
A version of this article appeared on Medscape.com.
TOPLINE
The use of magnetic resonance–guided daily adaptive stereotactic body radiotherapy for patients with prostate cancer reduces the risk of acute urinary side effects of grade 2 or higher by 44% and the risk of acute bowel side effects of grade 2 or higher by 60%, compared with standard CT-guided SBRT (CT‐SBRT).
METHODOLOGY
- With the use of magnetic resonance–guided daily adaptive SBRT, clinicians can customize radiation dosing to accommodate changes in prostate anatomy during treatment, which may also make SBRT safer and less toxic for patients.
- To determine whether this approach does reduce patient side effects, investigators ran a meta-analysis that included 29 studies with 2547 patients comparing the incidence of short-term, physician-assessed bowel and genitourinary side effects between the MRI-guided approach and standard CT-SBRT.
- The investigators reported no statistically significant differences in age, prescribed radiation doses, planning target volumes, or International Prostatism Symptom Scores between the two groups; the use of rectal spacers and the number of patients who received pelvic lymph node radiation were low in both.
- The average window for collecting acute toxicity data was 70 days in the MRI-guided investigations and 94 days in CT-SBRT investigations.
TAKEAWAY
- (odds ratio, 0.56; P = .04).
- The pooled estimate for grade 2 or higher gastrointestinal toxicity was 4% with the MRI approach versus 9% with CT-SBRT (OR, 0.40; P = .04).
- There were no differences in grade 3 or higher events, which were rare, between the groups.
- There was also no difference in toxicity among CT‐SBRT studies that used fiducial markers and those that did not.
IN PRACTICE
“These findings suggest that the technical advantages in precision of radiotherapy delivery afforded by [MRI-guided] SBRT translate to measurable clinical benefit,” the authors concluded. Potential reasons for the reduced risk of acute toxicity with the MRI-guided approach include “daily online adaptive planning, MRI‐based contouring that results in smaller treatment volumes, and MRI tracking, all of which may facilitate the precision and accuracy of treatment delivery.”
SOURCE
The study was led by Jonathan Leeman, MD, of the Dana-Farber Cancer Institute, Boston, and was published July 24 in Cancer.
LIMITATIONS
- The analysis did not account for differences in dosimetry, radiation planning, and toxicity management and assessment between the studies.
- Late toxicity and cancer control rates were not tracked and may have differed between the two approaches.
DISCLOSURES
- No external funding was reported.
- The investigators reported grants and consulting, personal, and other payments from Novartis, AstraZeneca, Janssen, and other companies.
A version of this article appeared on Medscape.com.
FROM CANCER
‘Treatment holiday’ in prostate cancer with tailored dosing
and improve patient outcomes, new research suggests.
The findings indicate that implementing a personalized dosing strategy with the radioligand therapy “allowed for treatment holidays in excellent responders, continuous 6-weekly treatments in moderate responders, and [allowed us] to consider changing or adding treatment in limited responders,” said study author Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the department of theranostics and nuclear medicine at St. Vincent’s Hospital in Sydney.
The research was presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging.
Although clinical trials have demonstrated that 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer, the question remains: Can patient outcomes be improved through the use of biomarkers and by escalating or deescalating treatment as appropriate? asked Dr. Nguyen, who presented the findings at the meeting.
Clinical trials use standardized dosing intervals. Adjusting treatment intervals through the use of early-biomarker responses could give some patients a break from treatment and improve overall survival outcomes, Dr. Nguyen explained. For example, the 2021 REALITY study showed that overall survival was significantly better for patients who received 177Lu-PSMA plus standard care, compared with patients who received standard care alone (median, 15.3 vs. 11.3 months), and that overall survival was better among patients with early prostate-specific antigen (PSA) responses.
In the current study, Dr. Nguyen and colleagues used composite early biomarkers of PSA, imaging with 177Lu-PSMA SPECT, and diagnostic CT to guide a personalized dosing interval strategy for patients with metastatic castration-resistant prostate cancer receiving 177Lu-PSMA. The team evaluated progression-free survival and overall survival among these patients to determine whether personalizing dosing on the basis of early biomarker levels was associated with survival outcomes.
The cohort included 125 men who received six weekly doses of 177Lu-PSMA and who underwent imaging with 177Lu-SPECT/CT after each dose. After the second dose, investigators used the composite of PSA and 177Lu SPECT/CT response to determine which patients had a partial response, which had stable disease, and which had progressive disease.
The men were divided into three groups on the basis of their level of response. Group 1, which included 35% of participants, achieved a significant reduction in PSA levels and a partial response on 177Lu-SPECT. These patients were advised to discontinue treatment until PSA levels increased. This treatment holiday lasted a median of about 6 months.
Group 2, which represented 34% of the cohort, had stable or reduced PSA levels as well as stable disease on SPECT imaging. For these patients, the treatment regimen continued.
Group 3 demonstrated rising PSA levels and progressive disease on SPECT imaging. These men were offered an alternative therapy.
Overall, median PSA progression-free survival was 12.1 months in group 1, 6.1 months in group 2, and 2.6 months in group 3. Median overall survival was also significantly better among patients who showed early responses to therapy: 19.2 months in group 1, 13.2 months in group 2, and 11. 2 months in group 3.
Dr. Nguyen noted several limitations to the findings, including the study’s retrospective nature and the fact that some patients in group 1 chose not to resume further treatment after their PSA levels rose.
“Personalizing dosing intervals using early-response biomarkers with 177Lu-PSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early crossover to potentially more effective therapies in nonresponders,” the authors conclude.
Given the effectiveness of this strategy, Dr. Nguyen says his team “now routinely uses these composite biomarkers when treating clinical patients.”
A version of this article appeared on Medscape.com.
and improve patient outcomes, new research suggests.
The findings indicate that implementing a personalized dosing strategy with the radioligand therapy “allowed for treatment holidays in excellent responders, continuous 6-weekly treatments in moderate responders, and [allowed us] to consider changing or adding treatment in limited responders,” said study author Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the department of theranostics and nuclear medicine at St. Vincent’s Hospital in Sydney.
The research was presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging.
Although clinical trials have demonstrated that 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer, the question remains: Can patient outcomes be improved through the use of biomarkers and by escalating or deescalating treatment as appropriate? asked Dr. Nguyen, who presented the findings at the meeting.
Clinical trials use standardized dosing intervals. Adjusting treatment intervals through the use of early-biomarker responses could give some patients a break from treatment and improve overall survival outcomes, Dr. Nguyen explained. For example, the 2021 REALITY study showed that overall survival was significantly better for patients who received 177Lu-PSMA plus standard care, compared with patients who received standard care alone (median, 15.3 vs. 11.3 months), and that overall survival was better among patients with early prostate-specific antigen (PSA) responses.
In the current study, Dr. Nguyen and colleagues used composite early biomarkers of PSA, imaging with 177Lu-PSMA SPECT, and diagnostic CT to guide a personalized dosing interval strategy for patients with metastatic castration-resistant prostate cancer receiving 177Lu-PSMA. The team evaluated progression-free survival and overall survival among these patients to determine whether personalizing dosing on the basis of early biomarker levels was associated with survival outcomes.
The cohort included 125 men who received six weekly doses of 177Lu-PSMA and who underwent imaging with 177Lu-SPECT/CT after each dose. After the second dose, investigators used the composite of PSA and 177Lu SPECT/CT response to determine which patients had a partial response, which had stable disease, and which had progressive disease.
The men were divided into three groups on the basis of their level of response. Group 1, which included 35% of participants, achieved a significant reduction in PSA levels and a partial response on 177Lu-SPECT. These patients were advised to discontinue treatment until PSA levels increased. This treatment holiday lasted a median of about 6 months.
Group 2, which represented 34% of the cohort, had stable or reduced PSA levels as well as stable disease on SPECT imaging. For these patients, the treatment regimen continued.
Group 3 demonstrated rising PSA levels and progressive disease on SPECT imaging. These men were offered an alternative therapy.
Overall, median PSA progression-free survival was 12.1 months in group 1, 6.1 months in group 2, and 2.6 months in group 3. Median overall survival was also significantly better among patients who showed early responses to therapy: 19.2 months in group 1, 13.2 months in group 2, and 11. 2 months in group 3.
Dr. Nguyen noted several limitations to the findings, including the study’s retrospective nature and the fact that some patients in group 1 chose not to resume further treatment after their PSA levels rose.
“Personalizing dosing intervals using early-response biomarkers with 177Lu-PSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early crossover to potentially more effective therapies in nonresponders,” the authors conclude.
Given the effectiveness of this strategy, Dr. Nguyen says his team “now routinely uses these composite biomarkers when treating clinical patients.”
A version of this article appeared on Medscape.com.
and improve patient outcomes, new research suggests.
The findings indicate that implementing a personalized dosing strategy with the radioligand therapy “allowed for treatment holidays in excellent responders, continuous 6-weekly treatments in moderate responders, and [allowed us] to consider changing or adding treatment in limited responders,” said study author Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the department of theranostics and nuclear medicine at St. Vincent’s Hospital in Sydney.
The research was presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging.
Although clinical trials have demonstrated that 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer, the question remains: Can patient outcomes be improved through the use of biomarkers and by escalating or deescalating treatment as appropriate? asked Dr. Nguyen, who presented the findings at the meeting.
Clinical trials use standardized dosing intervals. Adjusting treatment intervals through the use of early-biomarker responses could give some patients a break from treatment and improve overall survival outcomes, Dr. Nguyen explained. For example, the 2021 REALITY study showed that overall survival was significantly better for patients who received 177Lu-PSMA plus standard care, compared with patients who received standard care alone (median, 15.3 vs. 11.3 months), and that overall survival was better among patients with early prostate-specific antigen (PSA) responses.
In the current study, Dr. Nguyen and colleagues used composite early biomarkers of PSA, imaging with 177Lu-PSMA SPECT, and diagnostic CT to guide a personalized dosing interval strategy for patients with metastatic castration-resistant prostate cancer receiving 177Lu-PSMA. The team evaluated progression-free survival and overall survival among these patients to determine whether personalizing dosing on the basis of early biomarker levels was associated with survival outcomes.
The cohort included 125 men who received six weekly doses of 177Lu-PSMA and who underwent imaging with 177Lu-SPECT/CT after each dose. After the second dose, investigators used the composite of PSA and 177Lu SPECT/CT response to determine which patients had a partial response, which had stable disease, and which had progressive disease.
The men were divided into three groups on the basis of their level of response. Group 1, which included 35% of participants, achieved a significant reduction in PSA levels and a partial response on 177Lu-SPECT. These patients were advised to discontinue treatment until PSA levels increased. This treatment holiday lasted a median of about 6 months.
Group 2, which represented 34% of the cohort, had stable or reduced PSA levels as well as stable disease on SPECT imaging. For these patients, the treatment regimen continued.
Group 3 demonstrated rising PSA levels and progressive disease on SPECT imaging. These men were offered an alternative therapy.
Overall, median PSA progression-free survival was 12.1 months in group 1, 6.1 months in group 2, and 2.6 months in group 3. Median overall survival was also significantly better among patients who showed early responses to therapy: 19.2 months in group 1, 13.2 months in group 2, and 11. 2 months in group 3.
Dr. Nguyen noted several limitations to the findings, including the study’s retrospective nature and the fact that some patients in group 1 chose not to resume further treatment after their PSA levels rose.
“Personalizing dosing intervals using early-response biomarkers with 177Lu-PSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early crossover to potentially more effective therapies in nonresponders,” the authors conclude.
Given the effectiveness of this strategy, Dr. Nguyen says his team “now routinely uses these composite biomarkers when treating clinical patients.”
A version of this article appeared on Medscape.com.
FROM SNMMI 2023