User login
Tonsillectomy and Psoriasis
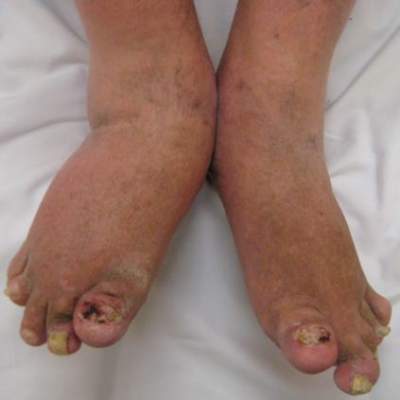
We are all aware that infections, particularly streptococcal infection, can be associated with psoriasis, especially the guttate variety. A logical question emanating from this fact is: Would tonsillectomy and adenoidectomy have any impact on psoriasis and its symptoms?
In a November 2014 article published online in the Journal of the American Academy of Dermatology, Rachakonda et al (doi:10.1016/j.jaad.2014.10.013) performed an extensive literature review to evaluate if tonsillectomy reduces psoriasis severity. The authors searched the following sources: MEDLINE, CINAHL, Cochrane, Embase, Web of Science, and Ovid databases (August 1, 1960, to September 12, 2013). In addition, they executed a manual search of selected references. Through this process, they identified observational studies and clinical trials examining psoriasis after tonsillectomy.
In the analysis, the authors included data from 20 articles from the 53 years they examined. From this literature, they included 545 patients with psoriasis who were either evaluated for or underwent tonsillectomy. Of 410 patients with psoriasis who actually underwent tonsillectomy, 290 experienced improvement in their psoriasis. Although some individuals who underwent tonsillectomy experienced sustained improvement in their disease, others experienced relapse following the procedure. The authors noted that their study was limited. Fifteen of 20 analyzed publications were case reports or series that lacked control groups. In addition, they noted that a publication bias that favored the reporting of improved cases needs to be considered.
Based on this comprehensive systematic review on the effect of tonsillectomy on psoriasis, the authors concluded that although tonsillectomy is effective in ameliorating psoriasis in a subpopulation of patients, there are insufficient data to describe the differences in clinical characteristics between responders versus nonresponders. Tonsillectomy may be a potential option for patients with recalcitrant psoriasis that is associated with occurrences of tonsillitis. Studies with long-term follow-up are needed to elucidate more clearly the extent and persistence of benefit of tonsillectomy in psoriasis.
What’s the issue?
Tonsillectomy represents an intriguing option not commonly considered for those with resistant disease. Based on the current data, will you discuss tonsillectomy with your patients?
We want to know your views! Tell us what you think.
Reader Comment
This concept so intrigued me when I heard Dr. Susan Katz discuss it at the NYU Advances in Medicine conference last June that I had the discussion with one of my patients, and she opted to have tonsillectomy this past fall. So far, she seems to improving, but I have not yet discontinued her long-term biologic therapy. At the same conference, Dr. Katz presented the idea that delaying antibiotic treatment of streptococcal pharyngitis by a few days might actually improve strep clearance rates by allowing the immune system to mount a greater response to the infection. Waiting for culture results before initiating antibiotic therapy might be prudent not only because it might reduce the unnecessary use of antibiotics in non-streptococcal pharyngitis, but because it might actually improve the long-term prognosis of patients (with or without psoriasis) who do have strep by reducing the odds of a chronic carrier state. Thanks for highlighting this area of study. Fascinating to consider that there might be a surgical cure for some psoriatic patients.
—Jennifer Goldwasser, MD (Scarsdale, New York)
We are all aware that infections, particularly streptococcal infection, can be associated with psoriasis, especially the guttate variety. A logical question emanating from this fact is: Would tonsillectomy and adenoidectomy have any impact on psoriasis and its symptoms?
In a November 2014 article published online in the Journal of the American Academy of Dermatology, Rachakonda et al (doi:10.1016/j.jaad.2014.10.013) performed an extensive literature review to evaluate if tonsillectomy reduces psoriasis severity. The authors searched the following sources: MEDLINE, CINAHL, Cochrane, Embase, Web of Science, and Ovid databases (August 1, 1960, to September 12, 2013). In addition, they executed a manual search of selected references. Through this process, they identified observational studies and clinical trials examining psoriasis after tonsillectomy.
In the analysis, the authors included data from 20 articles from the 53 years they examined. From this literature, they included 545 patients with psoriasis who were either evaluated for or underwent tonsillectomy. Of 410 patients with psoriasis who actually underwent tonsillectomy, 290 experienced improvement in their psoriasis. Although some individuals who underwent tonsillectomy experienced sustained improvement in their disease, others experienced relapse following the procedure. The authors noted that their study was limited. Fifteen of 20 analyzed publications were case reports or series that lacked control groups. In addition, they noted that a publication bias that favored the reporting of improved cases needs to be considered.
Based on this comprehensive systematic review on the effect of tonsillectomy on psoriasis, the authors concluded that although tonsillectomy is effective in ameliorating psoriasis in a subpopulation of patients, there are insufficient data to describe the differences in clinical characteristics between responders versus nonresponders. Tonsillectomy may be a potential option for patients with recalcitrant psoriasis that is associated with occurrences of tonsillitis. Studies with long-term follow-up are needed to elucidate more clearly the extent and persistence of benefit of tonsillectomy in psoriasis.
What’s the issue?
Tonsillectomy represents an intriguing option not commonly considered for those with resistant disease. Based on the current data, will you discuss tonsillectomy with your patients?
We want to know your views! Tell us what you think.
Reader Comment
This concept so intrigued me when I heard Dr. Susan Katz discuss it at the NYU Advances in Medicine conference last June that I had the discussion with one of my patients, and she opted to have tonsillectomy this past fall. So far, she seems to improving, but I have not yet discontinued her long-term biologic therapy. At the same conference, Dr. Katz presented the idea that delaying antibiotic treatment of streptococcal pharyngitis by a few days might actually improve strep clearance rates by allowing the immune system to mount a greater response to the infection. Waiting for culture results before initiating antibiotic therapy might be prudent not only because it might reduce the unnecessary use of antibiotics in non-streptococcal pharyngitis, but because it might actually improve the long-term prognosis of patients (with or without psoriasis) who do have strep by reducing the odds of a chronic carrier state. Thanks for highlighting this area of study. Fascinating to consider that there might be a surgical cure for some psoriatic patients.
—Jennifer Goldwasser, MD (Scarsdale, New York)
We are all aware that infections, particularly streptococcal infection, can be associated with psoriasis, especially the guttate variety. A logical question emanating from this fact is: Would tonsillectomy and adenoidectomy have any impact on psoriasis and its symptoms?
In a November 2014 article published online in the Journal of the American Academy of Dermatology, Rachakonda et al (doi:10.1016/j.jaad.2014.10.013) performed an extensive literature review to evaluate if tonsillectomy reduces psoriasis severity. The authors searched the following sources: MEDLINE, CINAHL, Cochrane, Embase, Web of Science, and Ovid databases (August 1, 1960, to September 12, 2013). In addition, they executed a manual search of selected references. Through this process, they identified observational studies and clinical trials examining psoriasis after tonsillectomy.
In the analysis, the authors included data from 20 articles from the 53 years they examined. From this literature, they included 545 patients with psoriasis who were either evaluated for or underwent tonsillectomy. Of 410 patients with psoriasis who actually underwent tonsillectomy, 290 experienced improvement in their psoriasis. Although some individuals who underwent tonsillectomy experienced sustained improvement in their disease, others experienced relapse following the procedure. The authors noted that their study was limited. Fifteen of 20 analyzed publications were case reports or series that lacked control groups. In addition, they noted that a publication bias that favored the reporting of improved cases needs to be considered.
Based on this comprehensive systematic review on the effect of tonsillectomy on psoriasis, the authors concluded that although tonsillectomy is effective in ameliorating psoriasis in a subpopulation of patients, there are insufficient data to describe the differences in clinical characteristics between responders versus nonresponders. Tonsillectomy may be a potential option for patients with recalcitrant psoriasis that is associated with occurrences of tonsillitis. Studies with long-term follow-up are needed to elucidate more clearly the extent and persistence of benefit of tonsillectomy in psoriasis.
What’s the issue?
Tonsillectomy represents an intriguing option not commonly considered for those with resistant disease. Based on the current data, will you discuss tonsillectomy with your patients?
We want to know your views! Tell us what you think.
Reader Comment
This concept so intrigued me when I heard Dr. Susan Katz discuss it at the NYU Advances in Medicine conference last June that I had the discussion with one of my patients, and she opted to have tonsillectomy this past fall. So far, she seems to improving, but I have not yet discontinued her long-term biologic therapy. At the same conference, Dr. Katz presented the idea that delaying antibiotic treatment of streptococcal pharyngitis by a few days might actually improve strep clearance rates by allowing the immune system to mount a greater response to the infection. Waiting for culture results before initiating antibiotic therapy might be prudent not only because it might reduce the unnecessary use of antibiotics in non-streptococcal pharyngitis, but because it might actually improve the long-term prognosis of patients (with or without psoriasis) who do have strep by reducing the odds of a chronic carrier state. Thanks for highlighting this area of study. Fascinating to consider that there might be a surgical cure for some psoriatic patients.
—Jennifer Goldwasser, MD (Scarsdale, New York)
Corticosteroid spray quickly, safely relieves psoriasis scaling
ORLANDO – A topical corticosteroid spray quickly and effectively reduced scaling in patients with plaque psoriasis, according to pooled phase III study results.
After 1 week of twice daily application of 0.25% Topicort spray (TaroPharma, Hawthorne, N.Y.), scaling was clear or almost clear in 29% of 120 treated patients, and was mild in an additional 40% of patients. At 4 weeks, scaling was clear or almost clear in 61% of patients, and mild in an additional 23%, Dr. Brian Keegan of East Windsor, N.J., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Additionally, 70% of patients experienced global improvement in psoriasis after 4 weeks of treatment; psoriasis was clear or almost clear in 42% of patients, and mild in 28%, Dr. Keegan said.
Study participants were adults with 10%-86% of body area affected by plaque psoriasis. Most of the patients (58%) were aged 41-to-64 years with a mean affected body surface area of 17%. Most had moderate scaling, but about a third of patients had severe or very severe scaling.
They applied Topicort 0.25% spray twice daily for 4 weeks.
The only adverse events that occurred in more than 1% of patients were dryness, irritation, and pruritus at the application site, which each occurred in 2%-3% of patients.
Treatment with Topicort – a class 1 super-potent corticosteroid indicated for up to 4 weeks of treatment of plaque psoriasis – quickly and safely relieved scaling in the majority of patients treated, Dr. Keegan concluded, noting that the findings are important given that scaling is very common among patients with plaque psoriasis, many of whom find it to be bothersome.
In fact, 94% of more than 17,000 participants in a National Psoriasis Foundation survey reported being bothered by scaling, he noted.
This study was sponsored by TaroPharma.
ORLANDO – A topical corticosteroid spray quickly and effectively reduced scaling in patients with plaque psoriasis, according to pooled phase III study results.
After 1 week of twice daily application of 0.25% Topicort spray (TaroPharma, Hawthorne, N.Y.), scaling was clear or almost clear in 29% of 120 treated patients, and was mild in an additional 40% of patients. At 4 weeks, scaling was clear or almost clear in 61% of patients, and mild in an additional 23%, Dr. Brian Keegan of East Windsor, N.J., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Additionally, 70% of patients experienced global improvement in psoriasis after 4 weeks of treatment; psoriasis was clear or almost clear in 42% of patients, and mild in 28%, Dr. Keegan said.
Study participants were adults with 10%-86% of body area affected by plaque psoriasis. Most of the patients (58%) were aged 41-to-64 years with a mean affected body surface area of 17%. Most had moderate scaling, but about a third of patients had severe or very severe scaling.
They applied Topicort 0.25% spray twice daily for 4 weeks.
The only adverse events that occurred in more than 1% of patients were dryness, irritation, and pruritus at the application site, which each occurred in 2%-3% of patients.
Treatment with Topicort – a class 1 super-potent corticosteroid indicated for up to 4 weeks of treatment of plaque psoriasis – quickly and safely relieved scaling in the majority of patients treated, Dr. Keegan concluded, noting that the findings are important given that scaling is very common among patients with plaque psoriasis, many of whom find it to be bothersome.
In fact, 94% of more than 17,000 participants in a National Psoriasis Foundation survey reported being bothered by scaling, he noted.
This study was sponsored by TaroPharma.
ORLANDO – A topical corticosteroid spray quickly and effectively reduced scaling in patients with plaque psoriasis, according to pooled phase III study results.
After 1 week of twice daily application of 0.25% Topicort spray (TaroPharma, Hawthorne, N.Y.), scaling was clear or almost clear in 29% of 120 treated patients, and was mild in an additional 40% of patients. At 4 weeks, scaling was clear or almost clear in 61% of patients, and mild in an additional 23%, Dr. Brian Keegan of East Windsor, N.J., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Additionally, 70% of patients experienced global improvement in psoriasis after 4 weeks of treatment; psoriasis was clear or almost clear in 42% of patients, and mild in 28%, Dr. Keegan said.
Study participants were adults with 10%-86% of body area affected by plaque psoriasis. Most of the patients (58%) were aged 41-to-64 years with a mean affected body surface area of 17%. Most had moderate scaling, but about a third of patients had severe or very severe scaling.
They applied Topicort 0.25% spray twice daily for 4 weeks.
The only adverse events that occurred in more than 1% of patients were dryness, irritation, and pruritus at the application site, which each occurred in 2%-3% of patients.
Treatment with Topicort – a class 1 super-potent corticosteroid indicated for up to 4 weeks of treatment of plaque psoriasis – quickly and safely relieved scaling in the majority of patients treated, Dr. Keegan concluded, noting that the findings are important given that scaling is very common among patients with plaque psoriasis, many of whom find it to be bothersome.
In fact, 94% of more than 17,000 participants in a National Psoriasis Foundation survey reported being bothered by scaling, he noted.
This study was sponsored by TaroPharma.
AT THE ODAC CONFERENCE
Key clinical point: Psoriasis patients with scaling are likely to respond to corticosteroid spray treatment.
Major finding: At 4 weeks, scaling was clear, almost clear, or mild in 84% of patients.
Data source: An analysis of pooled phase III trial data for 120 patients.
Disclosures: This study was sponsored by TaroPharma.
Apremilast looks good for psoriasis, psoriatic arthritis
New studies have led to a number of newly approved medications and therapies for patients suffering from psoriasis and psoriatic arthritis, and several more are on the horizon as clinicians and researchers continue finding new ways to effectively treat these conditions.
“This presentation will cover the new agents recently approved and nearing approval over the next 12 months,” Dr. J. Mark Jackson said in an interview. “It will highlight the general efficacy, safety, and dosing of each agent and will also highlight what is on the way.” Dr. Jackson, of the departments of medicine and dermatology at the University of Louisville (Ky.) discussed his findings at the meeting sponsored by Global Academy for Medical Education.
Among the new agents that Dr. Jackson discussed is apremilast (Otezla by Celgene), which has been approved for treatment of both psoriatic arthritis (PsA) and psoriasis (PsO), as of Sept. 23, 2014. The central mechanism of the medication is an intracellular phosphodieterase-4 (PDE-4) inhibitor, and it is administered orally with a titrated dose over the first 2 weeks of treatment from 10 mg once a day to the maintenance dosage of 30 mg twice a day.
“Apremilast is the newest agent approved for PsO and PsA and demonstrates efficacy for both,” explained Dr. Jackson, who also stated that apremilast should warrant further study to test its “utility in the treatment of atopic and endogenous dermatitis, with studies [currently] ongoing.”
Apremilast was tested against a placebo, with doses of apremilast titrated during the first week of administration, in the ESTEEM 1 trial (Study to Evaluate Safety and Effectiveness of Oral Apremilast in Patients With Moderate to Severe Plaque Psoriasis). After 16 weeks, psoriasis area and severity index (PASI) 75, PASI 50, and static Physicians Global Assessment (sPGA) levels were all significantly higher in subjects using apremilast than those randomized into the placebo cohort. “The new interleukin 17 agents have very robust PASI 75 responses and will be a great addition to the market,” said Dr. Jackson.
Similarly robust results were also seen in apremilast used to treat PsA in the PALACE 1 trial (Study of Apremilast to Treat Active Psoriatic Arthritis). In this randomized, double-blind, placebo-controlled study, ACR 20 (arthritis self management), ACR 50, and ACR 70 scores were significantly higher after 24 weeks in subjects given apremilast 30 mg twice daily and apremilast 20 mg twice daily, than in subjects who were on placebo.
Furthermore, Dr. Jackson explained that the aforementioned interleukin-17 agents are “also being investigated for PsA, and it will be interesting to see how they compare in efficacy to the currently approved agents.”
Treatment options that Dr. Jackson spoke about include certolizumab (Cimzia by UCB/Dermira) and golimumab (Simponi by Janssen), both of which are already approved for PsA, and the former of which is in a phase III trial for PsO. Dr. Jackson also discussed several treatments currently in phase III trials, such as secukinumab by Novartis, brodalumab by Amgen and AstraZeneca, and tofacitinib by Pfizer – all of which are for use against both PsO and PsA, the latter being topically applied for PsO – and tildrakizumab by Merck and Sun Pharma for PsO only.
Global Academy and this news organization are owned by the same parent company.
Dr. Jackson said he has received research, honoraria, consulting and/or other support from the following companies: Abbvie, Amgen, Celgene, Galderma, Genentech, Janssen, Lilly, Medicis, Medimetriks, Novartis, Pfizer, Promius, Topica, and TopMD.
New studies have led to a number of newly approved medications and therapies for patients suffering from psoriasis and psoriatic arthritis, and several more are on the horizon as clinicians and researchers continue finding new ways to effectively treat these conditions.
“This presentation will cover the new agents recently approved and nearing approval over the next 12 months,” Dr. J. Mark Jackson said in an interview. “It will highlight the general efficacy, safety, and dosing of each agent and will also highlight what is on the way.” Dr. Jackson, of the departments of medicine and dermatology at the University of Louisville (Ky.) discussed his findings at the meeting sponsored by Global Academy for Medical Education.
Among the new agents that Dr. Jackson discussed is apremilast (Otezla by Celgene), which has been approved for treatment of both psoriatic arthritis (PsA) and psoriasis (PsO), as of Sept. 23, 2014. The central mechanism of the medication is an intracellular phosphodieterase-4 (PDE-4) inhibitor, and it is administered orally with a titrated dose over the first 2 weeks of treatment from 10 mg once a day to the maintenance dosage of 30 mg twice a day.
“Apremilast is the newest agent approved for PsO and PsA and demonstrates efficacy for both,” explained Dr. Jackson, who also stated that apremilast should warrant further study to test its “utility in the treatment of atopic and endogenous dermatitis, with studies [currently] ongoing.”
Apremilast was tested against a placebo, with doses of apremilast titrated during the first week of administration, in the ESTEEM 1 trial (Study to Evaluate Safety and Effectiveness of Oral Apremilast in Patients With Moderate to Severe Plaque Psoriasis). After 16 weeks, psoriasis area and severity index (PASI) 75, PASI 50, and static Physicians Global Assessment (sPGA) levels were all significantly higher in subjects using apremilast than those randomized into the placebo cohort. “The new interleukin 17 agents have very robust PASI 75 responses and will be a great addition to the market,” said Dr. Jackson.
Similarly robust results were also seen in apremilast used to treat PsA in the PALACE 1 trial (Study of Apremilast to Treat Active Psoriatic Arthritis). In this randomized, double-blind, placebo-controlled study, ACR 20 (arthritis self management), ACR 50, and ACR 70 scores were significantly higher after 24 weeks in subjects given apremilast 30 mg twice daily and apremilast 20 mg twice daily, than in subjects who were on placebo.
Furthermore, Dr. Jackson explained that the aforementioned interleukin-17 agents are “also being investigated for PsA, and it will be interesting to see how they compare in efficacy to the currently approved agents.”
Treatment options that Dr. Jackson spoke about include certolizumab (Cimzia by UCB/Dermira) and golimumab (Simponi by Janssen), both of which are already approved for PsA, and the former of which is in a phase III trial for PsO. Dr. Jackson also discussed several treatments currently in phase III trials, such as secukinumab by Novartis, brodalumab by Amgen and AstraZeneca, and tofacitinib by Pfizer – all of which are for use against both PsO and PsA, the latter being topically applied for PsO – and tildrakizumab by Merck and Sun Pharma for PsO only.
Global Academy and this news organization are owned by the same parent company.
Dr. Jackson said he has received research, honoraria, consulting and/or other support from the following companies: Abbvie, Amgen, Celgene, Galderma, Genentech, Janssen, Lilly, Medicis, Medimetriks, Novartis, Pfizer, Promius, Topica, and TopMD.
New studies have led to a number of newly approved medications and therapies for patients suffering from psoriasis and psoriatic arthritis, and several more are on the horizon as clinicians and researchers continue finding new ways to effectively treat these conditions.
“This presentation will cover the new agents recently approved and nearing approval over the next 12 months,” Dr. J. Mark Jackson said in an interview. “It will highlight the general efficacy, safety, and dosing of each agent and will also highlight what is on the way.” Dr. Jackson, of the departments of medicine and dermatology at the University of Louisville (Ky.) discussed his findings at the meeting sponsored by Global Academy for Medical Education.
Among the new agents that Dr. Jackson discussed is apremilast (Otezla by Celgene), which has been approved for treatment of both psoriatic arthritis (PsA) and psoriasis (PsO), as of Sept. 23, 2014. The central mechanism of the medication is an intracellular phosphodieterase-4 (PDE-4) inhibitor, and it is administered orally with a titrated dose over the first 2 weeks of treatment from 10 mg once a day to the maintenance dosage of 30 mg twice a day.
“Apremilast is the newest agent approved for PsO and PsA and demonstrates efficacy for both,” explained Dr. Jackson, who also stated that apremilast should warrant further study to test its “utility in the treatment of atopic and endogenous dermatitis, with studies [currently] ongoing.”
Apremilast was tested against a placebo, with doses of apremilast titrated during the first week of administration, in the ESTEEM 1 trial (Study to Evaluate Safety and Effectiveness of Oral Apremilast in Patients With Moderate to Severe Plaque Psoriasis). After 16 weeks, psoriasis area and severity index (PASI) 75, PASI 50, and static Physicians Global Assessment (sPGA) levels were all significantly higher in subjects using apremilast than those randomized into the placebo cohort. “The new interleukin 17 agents have very robust PASI 75 responses and will be a great addition to the market,” said Dr. Jackson.
Similarly robust results were also seen in apremilast used to treat PsA in the PALACE 1 trial (Study of Apremilast to Treat Active Psoriatic Arthritis). In this randomized, double-blind, placebo-controlled study, ACR 20 (arthritis self management), ACR 50, and ACR 70 scores were significantly higher after 24 weeks in subjects given apremilast 30 mg twice daily and apremilast 20 mg twice daily, than in subjects who were on placebo.
Furthermore, Dr. Jackson explained that the aforementioned interleukin-17 agents are “also being investigated for PsA, and it will be interesting to see how they compare in efficacy to the currently approved agents.”
Treatment options that Dr. Jackson spoke about include certolizumab (Cimzia by UCB/Dermira) and golimumab (Simponi by Janssen), both of which are already approved for PsA, and the former of which is in a phase III trial for PsO. Dr. Jackson also discussed several treatments currently in phase III trials, such as secukinumab by Novartis, brodalumab by Amgen and AstraZeneca, and tofacitinib by Pfizer – all of which are for use against both PsO and PsA, the latter being topically applied for PsO – and tildrakizumab by Merck and Sun Pharma for PsO only.
Global Academy and this news organization are owned by the same parent company.
Dr. Jackson said he has received research, honoraria, consulting and/or other support from the following companies: Abbvie, Amgen, Celgene, Galderma, Genentech, Janssen, Lilly, Medicis, Medimetriks, Novartis, Pfizer, Promius, Topica, and TopMD.
EXPERT ANALYSIS FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Secukinumab earns FDA approval for plaque psoriasis
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
Psoriasis treatment recommendations address four clinical scenarios
New guidelines on nail psoriasis address four clinical manifestations of the disease. The recommendations by the Medical Board of the National Psoriasis Foundation appeared as a consensus statement in the January issue of JAMA Dermatology.
Limitations in clinical trial data make comparing treatments difficult, noted lead author Dr. Jeffrey J. Crowley of Bakersfield (Calif.) Dermatology and his associates. “There are limited data to evaluate or support the use of combination therapy in nail psoriasis. Thus, treatment options recommended in this review are monotherapy,” the guidelines authors added (JAMA Dermatol. 2015;151:87-94).
To develop the guidelines, the research team searched PubMed for articles on nail psoriasis dating from Jan. 1, 1947 through May 11, 2014. They evaluated these studies for level of evidence based on recommendations for writing guidelines from Dr. Paul G. Shekelle of the VA West Los Angeles Medical Center and his associates (BMJ 1999;318:593-6).
They also polled the Medical Board of the National Psoriasis Foundation regarding their treatment approach for four clinical presentations of nail psoriasis:
• For treatment-naive patients with psoriasis of the nails only (affecting at least 3 of 10 fingernails), the board recommended initial treatment with high-potency topical corticosteroids (with or without calcipotriol), with intralesional corticosteroids as a secondary option. Intralesional corticosteroids have been used for decades, but clinical data supporting their use are “extremely limited,” the guidelines state.
• For extensive nail psoriasis (affecting at least five fingernails and causing moderate to severe pain) that has failed topical treatment, the board recommended adalimumab most enthusiastically, followed by etanercept, intralesional corticosteroids, ustekinumab, methotrexate sodium, and acitretin in decreasing order.
• For concurrent skin and nail disease without joint involvement (defined as skin disease on at least 8% of the body surface and moderately to severely painful dystrophy of at least 5 of 10 nails), the board strongly recommended adalimumab, etanercept, and ustekinumab, and also recommended methotrexate, acitretin, infliximab, and apremilast.
• For concurrent nail, skin, and joint involvement (defined as skin disease on 8% of the body surface, a history of dactylitis and morning stiffness (psoriatic arthritis), and severe, painful involvement of at least 5 of 10 nails), the board most strongly recommended adalimumab, followed by etanercept, ustekinumab, infliximab, methotrexate, apremilast, and golimumab.
Because nails grow slowly, psoriatic joint and skin disease often improve before nail psoriasis does, the authors noted. “Few studies show any significant improvement before 12 weeks, and several studies with etanercept, infliximab, and ustekinumab demonstrate continued improvement beyond 6 months,” they wrote.
About half of patients with psoriasis have some amount of nail involvement, and about 70% of patients with psoriatic arthritis have nail disease, according to the literature review. Dermatophyte infections can further complicate treatment of nail psoriasis, and immunosuppressive therapies can lead to onychomycosis in patients whose psoriasis includes the toenails, the authors added.
Dr. Crowley reported speaker and consulting honoraria from AbbVie, Abbott, and Amgen, and research funding from Abbott, AbbVie, Amgen, AstraZeneca, Celgene, Eli Lilly, Janssen Pharmaceutica, Merck, Pfizer, and Regeneron Pharmaceuticals. Four coauthors reported advisory, consulting, or financial relationships with Amgen, Abbott, Janssen Biotech Inc., Celgene, Novartis International AG, Abbvie, Merck, Celgene, Leo Pharma, Eli Lilly, Pfizer, and the National Psoriasis Foundation.
New guidelines on nail psoriasis address four clinical manifestations of the disease. The recommendations by the Medical Board of the National Psoriasis Foundation appeared as a consensus statement in the January issue of JAMA Dermatology.
Limitations in clinical trial data make comparing treatments difficult, noted lead author Dr. Jeffrey J. Crowley of Bakersfield (Calif.) Dermatology and his associates. “There are limited data to evaluate or support the use of combination therapy in nail psoriasis. Thus, treatment options recommended in this review are monotherapy,” the guidelines authors added (JAMA Dermatol. 2015;151:87-94).
To develop the guidelines, the research team searched PubMed for articles on nail psoriasis dating from Jan. 1, 1947 through May 11, 2014. They evaluated these studies for level of evidence based on recommendations for writing guidelines from Dr. Paul G. Shekelle of the VA West Los Angeles Medical Center and his associates (BMJ 1999;318:593-6).
They also polled the Medical Board of the National Psoriasis Foundation regarding their treatment approach for four clinical presentations of nail psoriasis:
• For treatment-naive patients with psoriasis of the nails only (affecting at least 3 of 10 fingernails), the board recommended initial treatment with high-potency topical corticosteroids (with or without calcipotriol), with intralesional corticosteroids as a secondary option. Intralesional corticosteroids have been used for decades, but clinical data supporting their use are “extremely limited,” the guidelines state.
• For extensive nail psoriasis (affecting at least five fingernails and causing moderate to severe pain) that has failed topical treatment, the board recommended adalimumab most enthusiastically, followed by etanercept, intralesional corticosteroids, ustekinumab, methotrexate sodium, and acitretin in decreasing order.
• For concurrent skin and nail disease without joint involvement (defined as skin disease on at least 8% of the body surface and moderately to severely painful dystrophy of at least 5 of 10 nails), the board strongly recommended adalimumab, etanercept, and ustekinumab, and also recommended methotrexate, acitretin, infliximab, and apremilast.
• For concurrent nail, skin, and joint involvement (defined as skin disease on 8% of the body surface, a history of dactylitis and morning stiffness (psoriatic arthritis), and severe, painful involvement of at least 5 of 10 nails), the board most strongly recommended adalimumab, followed by etanercept, ustekinumab, infliximab, methotrexate, apremilast, and golimumab.
Because nails grow slowly, psoriatic joint and skin disease often improve before nail psoriasis does, the authors noted. “Few studies show any significant improvement before 12 weeks, and several studies with etanercept, infliximab, and ustekinumab demonstrate continued improvement beyond 6 months,” they wrote.
About half of patients with psoriasis have some amount of nail involvement, and about 70% of patients with psoriatic arthritis have nail disease, according to the literature review. Dermatophyte infections can further complicate treatment of nail psoriasis, and immunosuppressive therapies can lead to onychomycosis in patients whose psoriasis includes the toenails, the authors added.
Dr. Crowley reported speaker and consulting honoraria from AbbVie, Abbott, and Amgen, and research funding from Abbott, AbbVie, Amgen, AstraZeneca, Celgene, Eli Lilly, Janssen Pharmaceutica, Merck, Pfizer, and Regeneron Pharmaceuticals. Four coauthors reported advisory, consulting, or financial relationships with Amgen, Abbott, Janssen Biotech Inc., Celgene, Novartis International AG, Abbvie, Merck, Celgene, Leo Pharma, Eli Lilly, Pfizer, and the National Psoriasis Foundation.
New guidelines on nail psoriasis address four clinical manifestations of the disease. The recommendations by the Medical Board of the National Psoriasis Foundation appeared as a consensus statement in the January issue of JAMA Dermatology.
Limitations in clinical trial data make comparing treatments difficult, noted lead author Dr. Jeffrey J. Crowley of Bakersfield (Calif.) Dermatology and his associates. “There are limited data to evaluate or support the use of combination therapy in nail psoriasis. Thus, treatment options recommended in this review are monotherapy,” the guidelines authors added (JAMA Dermatol. 2015;151:87-94).
To develop the guidelines, the research team searched PubMed for articles on nail psoriasis dating from Jan. 1, 1947 through May 11, 2014. They evaluated these studies for level of evidence based on recommendations for writing guidelines from Dr. Paul G. Shekelle of the VA West Los Angeles Medical Center and his associates (BMJ 1999;318:593-6).
They also polled the Medical Board of the National Psoriasis Foundation regarding their treatment approach for four clinical presentations of nail psoriasis:
• For treatment-naive patients with psoriasis of the nails only (affecting at least 3 of 10 fingernails), the board recommended initial treatment with high-potency topical corticosteroids (with or without calcipotriol), with intralesional corticosteroids as a secondary option. Intralesional corticosteroids have been used for decades, but clinical data supporting their use are “extremely limited,” the guidelines state.
• For extensive nail psoriasis (affecting at least five fingernails and causing moderate to severe pain) that has failed topical treatment, the board recommended adalimumab most enthusiastically, followed by etanercept, intralesional corticosteroids, ustekinumab, methotrexate sodium, and acitretin in decreasing order.
• For concurrent skin and nail disease without joint involvement (defined as skin disease on at least 8% of the body surface and moderately to severely painful dystrophy of at least 5 of 10 nails), the board strongly recommended adalimumab, etanercept, and ustekinumab, and also recommended methotrexate, acitretin, infliximab, and apremilast.
• For concurrent nail, skin, and joint involvement (defined as skin disease on 8% of the body surface, a history of dactylitis and morning stiffness (psoriatic arthritis), and severe, painful involvement of at least 5 of 10 nails), the board most strongly recommended adalimumab, followed by etanercept, ustekinumab, infliximab, methotrexate, apremilast, and golimumab.
Because nails grow slowly, psoriatic joint and skin disease often improve before nail psoriasis does, the authors noted. “Few studies show any significant improvement before 12 weeks, and several studies with etanercept, infliximab, and ustekinumab demonstrate continued improvement beyond 6 months,” they wrote.
About half of patients with psoriasis have some amount of nail involvement, and about 70% of patients with psoriatic arthritis have nail disease, according to the literature review. Dermatophyte infections can further complicate treatment of nail psoriasis, and immunosuppressive therapies can lead to onychomycosis in patients whose psoriasis includes the toenails, the authors added.
Dr. Crowley reported speaker and consulting honoraria from AbbVie, Abbott, and Amgen, and research funding from Abbott, AbbVie, Amgen, AstraZeneca, Celgene, Eli Lilly, Janssen Pharmaceutica, Merck, Pfizer, and Regeneron Pharmaceuticals. Four coauthors reported advisory, consulting, or financial relationships with Amgen, Abbott, Janssen Biotech Inc., Celgene, Novartis International AG, Abbvie, Merck, Celgene, Leo Pharma, Eli Lilly, Pfizer, and the National Psoriasis Foundation.
FROM JAMA DERMATOLOGY
Early psoriatic arthritis treatment with etanercept gives better outcomes
Patients with psoriatic arthritis and psoriasis report having a better response to etanercept the earlier they are treated, according to a post hoc analysis of the PRESTA trial.
Patients with shorter psoriatic arthritis (PsA) duration had greater improvements in arthritis scores and several patient-reported outcomes at 24 weeks of treatment with etanercept 50 mg a week, compared with patients with longer disease duration.
The researchers, led by Dr. Bruce Kirkham from Guy’s and St. Thomas’ NHS Foundation Trust, London, said the results showed “clinicians should consider treating their PsA patients with therapies effective in PsA early rather than late.”
The industry-sponsored PRESTA (Psoriasis Randomized Etanercept Study in Patients with Psoriatic Arthritis) trial was a randomized, blinded, 24-week, multicenter study enrolling adults with active but stable plaque psoriasis involving at least 10% body surface area and active PsA defined as 2 or more swollen joints, 2 or more tender joints, joint pain for 3 months or longer, and a negative serum rheumatoid factor within 6 months prior to baseline.
Overall, 372 patients who received etanercept 50 mg once a week for 24 weeks were included in the current post hoc analysis (Clin. Exp. Rheumatol. 2014 Dec. 22).
Baseline and after treatment changes were compared between patients with PsA disease duration of 2 years or less (n = 103) and those with disease more than 2 years (n = 269).
Baseline efficacy measures were similar between the shorter duration and longer duration groups, with the exception of Physicians Global Assessment (PGA) arthritis score, which was significantly lower in the group with 2 years or less duration (44.9 vs. 51.8; P = .006), the authors reported.
At week 24, joint disease improved, based on the PGA arthritis score, by a significantly greater amount in the shorter duration group (–39.8 vs. –35.7; P = .03).
Clinically meaningful improvements in patient-reported outcomes with etanercept treatment occurred in both groups, the study authors said, but changes in scores from baseline to week 24 were significantly higher in the shorter duration group for visual analog scale reports of joint pain (P = .007) and arthritis activity (P = .01) as well as quality of life on EuroQol 5D utility (P = .046) and EuroQol 5D visual analog scale (P = .04) responses.
The mean number of swollen joints that had improved from baseline to week 24 was not significantly different between the groups, and no significant between-group differences were seen in the percentages of patients achieving the ACR20, ACR50, and ACR70 responses.
While all patients responded to treatment irrespective of disease duration, patients with shorter disease duration had greater improvements on some measures, the authors concluded.
However, the study was limited by the fact that it was a post hoc analysis and the original trial was not designed to explore the effect of early treatment versus later treatment in patients with PsA and moderate-to-severe psoriasis, they noted.
The study was sponsored by Wyeth, which was acquired by Pfizer, the manufacturer of etanercept, in October 2009. Several of the authors declared receiving honoraria from several pharmaceutical companies. Two authors were employees of Pfizer during the PRESTA study and development of the current manuscript, and two other authors are current employees of Pfizer.
Patients with psoriatic arthritis and psoriasis report having a better response to etanercept the earlier they are treated, according to a post hoc analysis of the PRESTA trial.
Patients with shorter psoriatic arthritis (PsA) duration had greater improvements in arthritis scores and several patient-reported outcomes at 24 weeks of treatment with etanercept 50 mg a week, compared with patients with longer disease duration.
The researchers, led by Dr. Bruce Kirkham from Guy’s and St. Thomas’ NHS Foundation Trust, London, said the results showed “clinicians should consider treating their PsA patients with therapies effective in PsA early rather than late.”
The industry-sponsored PRESTA (Psoriasis Randomized Etanercept Study in Patients with Psoriatic Arthritis) trial was a randomized, blinded, 24-week, multicenter study enrolling adults with active but stable plaque psoriasis involving at least 10% body surface area and active PsA defined as 2 or more swollen joints, 2 or more tender joints, joint pain for 3 months or longer, and a negative serum rheumatoid factor within 6 months prior to baseline.
Overall, 372 patients who received etanercept 50 mg once a week for 24 weeks were included in the current post hoc analysis (Clin. Exp. Rheumatol. 2014 Dec. 22).
Baseline and after treatment changes were compared between patients with PsA disease duration of 2 years or less (n = 103) and those with disease more than 2 years (n = 269).
Baseline efficacy measures were similar between the shorter duration and longer duration groups, with the exception of Physicians Global Assessment (PGA) arthritis score, which was significantly lower in the group with 2 years or less duration (44.9 vs. 51.8; P = .006), the authors reported.
At week 24, joint disease improved, based on the PGA arthritis score, by a significantly greater amount in the shorter duration group (–39.8 vs. –35.7; P = .03).
Clinically meaningful improvements in patient-reported outcomes with etanercept treatment occurred in both groups, the study authors said, but changes in scores from baseline to week 24 were significantly higher in the shorter duration group for visual analog scale reports of joint pain (P = .007) and arthritis activity (P = .01) as well as quality of life on EuroQol 5D utility (P = .046) and EuroQol 5D visual analog scale (P = .04) responses.
The mean number of swollen joints that had improved from baseline to week 24 was not significantly different between the groups, and no significant between-group differences were seen in the percentages of patients achieving the ACR20, ACR50, and ACR70 responses.
While all patients responded to treatment irrespective of disease duration, patients with shorter disease duration had greater improvements on some measures, the authors concluded.
However, the study was limited by the fact that it was a post hoc analysis and the original trial was not designed to explore the effect of early treatment versus later treatment in patients with PsA and moderate-to-severe psoriasis, they noted.
The study was sponsored by Wyeth, which was acquired by Pfizer, the manufacturer of etanercept, in October 2009. Several of the authors declared receiving honoraria from several pharmaceutical companies. Two authors were employees of Pfizer during the PRESTA study and development of the current manuscript, and two other authors are current employees of Pfizer.
Patients with psoriatic arthritis and psoriasis report having a better response to etanercept the earlier they are treated, according to a post hoc analysis of the PRESTA trial.
Patients with shorter psoriatic arthritis (PsA) duration had greater improvements in arthritis scores and several patient-reported outcomes at 24 weeks of treatment with etanercept 50 mg a week, compared with patients with longer disease duration.
The researchers, led by Dr. Bruce Kirkham from Guy’s and St. Thomas’ NHS Foundation Trust, London, said the results showed “clinicians should consider treating their PsA patients with therapies effective in PsA early rather than late.”
The industry-sponsored PRESTA (Psoriasis Randomized Etanercept Study in Patients with Psoriatic Arthritis) trial was a randomized, blinded, 24-week, multicenter study enrolling adults with active but stable plaque psoriasis involving at least 10% body surface area and active PsA defined as 2 or more swollen joints, 2 or more tender joints, joint pain for 3 months or longer, and a negative serum rheumatoid factor within 6 months prior to baseline.
Overall, 372 patients who received etanercept 50 mg once a week for 24 weeks were included in the current post hoc analysis (Clin. Exp. Rheumatol. 2014 Dec. 22).
Baseline and after treatment changes were compared between patients with PsA disease duration of 2 years or less (n = 103) and those with disease more than 2 years (n = 269).
Baseline efficacy measures were similar between the shorter duration and longer duration groups, with the exception of Physicians Global Assessment (PGA) arthritis score, which was significantly lower in the group with 2 years or less duration (44.9 vs. 51.8; P = .006), the authors reported.
At week 24, joint disease improved, based on the PGA arthritis score, by a significantly greater amount in the shorter duration group (–39.8 vs. –35.7; P = .03).
Clinically meaningful improvements in patient-reported outcomes with etanercept treatment occurred in both groups, the study authors said, but changes in scores from baseline to week 24 were significantly higher in the shorter duration group for visual analog scale reports of joint pain (P = .007) and arthritis activity (P = .01) as well as quality of life on EuroQol 5D utility (P = .046) and EuroQol 5D visual analog scale (P = .04) responses.
The mean number of swollen joints that had improved from baseline to week 24 was not significantly different between the groups, and no significant between-group differences were seen in the percentages of patients achieving the ACR20, ACR50, and ACR70 responses.
While all patients responded to treatment irrespective of disease duration, patients with shorter disease duration had greater improvements on some measures, the authors concluded.
However, the study was limited by the fact that it was a post hoc analysis and the original trial was not designed to explore the effect of early treatment versus later treatment in patients with PsA and moderate-to-severe psoriasis, they noted.
The study was sponsored by Wyeth, which was acquired by Pfizer, the manufacturer of etanercept, in October 2009. Several of the authors declared receiving honoraria from several pharmaceutical companies. Two authors were employees of Pfizer during the PRESTA study and development of the current manuscript, and two other authors are current employees of Pfizer.
FROM CLINICAL AND EXPERIMENTAL RHEUMATOLOGY
Key clinical point: PsA treatment with etanercept within 2 years of diagnosis may lead to better patient-reported quality of life outcomes than does treatment starting more than 2 years after diagnosis.
Major finding: After 24 weeks of etanercept 50 mg per week, joint disease improved, based on the PGA arthritis score, by a significantly greater amount in patients with PsA for 2 years or less vs. those with the disease for more than 2 years (–39.8 vs. –35.7, respectively; P = .03) .
Data source: A post hoc analysis of 372 patients with PsA and psoriasis who were enrolled in the PRESTA trial.
Disclosures: The study was sponsored by Wyeth, which was acquired by Pfizer, the manufacturer of etanercept, in October 2009. Several of the authors declared receiving honoraria from several pharmaceutical companies. Two authors were employees of Pfizer during the PRESTA study and development of the current manuscript, and two other authors are current employees of Pfizer.
Case series: Ustekinumab for psoriasis helps skin, hurts joints
Ustekinumab treatment was associated with new-onset or worsening psoriatic arthritis in a series of seven patients with psoriasis.
The findings, which support previous observations that patients treated with ustekinumab (Stelara) “sometimes have discordant responses of their skin and joint disease,” underscore the need for regularly asking patients about joint symptoms, and for referral to a rheumatologist for suspected psoriatic arthritis, Ben B. Jones of the University of Utah, Salt Lake City, and his colleagues reported (Br. J. Dermatol. 2014 Dec. 30 [doi:10.1111/bjd.13645]).
All seven patients in the case series had well-controlled psoriasis on ustekinumab. Five had new-onset psoriatic arthritis, and two had worsening psoriatic arthritis on treatment. The patients had phenotypic similarities; most were women over age 49 years, and all five of those with new-onset disease were women. Also, five of the seven patients had exposure to tumor necrosis factor inhibitors prior to switching to ustekinumab.
Three other case series have reported similar findings of marked improvement in cutaneous symptoms with worsening of joint symptoms among patients treated with ustekinumab, the investigators noted, concluding that the findings – which may reflect a lack of efficacy at the administered doses or a need for more frequent dosing – may support arguments that psoriatic arthritis and psoriasis involve distinct inflammatory pathways.
“It is also possible that ustekinumab may trigger or unmask inflammation in the joints of patients with psoriatic arthritis,” they wrote, concluding that “larger epidemiologic studies comparing patients with discordant and concordant cutaneous and articular responses to ustekinumab may better define patients at risk for psoriatic arthritis worsening with ustekinumab.”
Two of the five authors have reported serving as a consultant or advisory board member, receiving payment for lectures, and/or serving as an investigator for Janssen, which markets ustekinumab, as well as other manufacturers of biologics. The other authors reported having no conflicts of interest.
Ustekinumab treatment was associated with new-onset or worsening psoriatic arthritis in a series of seven patients with psoriasis.
The findings, which support previous observations that patients treated with ustekinumab (Stelara) “sometimes have discordant responses of their skin and joint disease,” underscore the need for regularly asking patients about joint symptoms, and for referral to a rheumatologist for suspected psoriatic arthritis, Ben B. Jones of the University of Utah, Salt Lake City, and his colleagues reported (Br. J. Dermatol. 2014 Dec. 30 [doi:10.1111/bjd.13645]).
All seven patients in the case series had well-controlled psoriasis on ustekinumab. Five had new-onset psoriatic arthritis, and two had worsening psoriatic arthritis on treatment. The patients had phenotypic similarities; most were women over age 49 years, and all five of those with new-onset disease were women. Also, five of the seven patients had exposure to tumor necrosis factor inhibitors prior to switching to ustekinumab.
Three other case series have reported similar findings of marked improvement in cutaneous symptoms with worsening of joint symptoms among patients treated with ustekinumab, the investigators noted, concluding that the findings – which may reflect a lack of efficacy at the administered doses or a need for more frequent dosing – may support arguments that psoriatic arthritis and psoriasis involve distinct inflammatory pathways.
“It is also possible that ustekinumab may trigger or unmask inflammation in the joints of patients with psoriatic arthritis,” they wrote, concluding that “larger epidemiologic studies comparing patients with discordant and concordant cutaneous and articular responses to ustekinumab may better define patients at risk for psoriatic arthritis worsening with ustekinumab.”
Two of the five authors have reported serving as a consultant or advisory board member, receiving payment for lectures, and/or serving as an investigator for Janssen, which markets ustekinumab, as well as other manufacturers of biologics. The other authors reported having no conflicts of interest.
Ustekinumab treatment was associated with new-onset or worsening psoriatic arthritis in a series of seven patients with psoriasis.
The findings, which support previous observations that patients treated with ustekinumab (Stelara) “sometimes have discordant responses of their skin and joint disease,” underscore the need for regularly asking patients about joint symptoms, and for referral to a rheumatologist for suspected psoriatic arthritis, Ben B. Jones of the University of Utah, Salt Lake City, and his colleagues reported (Br. J. Dermatol. 2014 Dec. 30 [doi:10.1111/bjd.13645]).
All seven patients in the case series had well-controlled psoriasis on ustekinumab. Five had new-onset psoriatic arthritis, and two had worsening psoriatic arthritis on treatment. The patients had phenotypic similarities; most were women over age 49 years, and all five of those with new-onset disease were women. Also, five of the seven patients had exposure to tumor necrosis factor inhibitors prior to switching to ustekinumab.
Three other case series have reported similar findings of marked improvement in cutaneous symptoms with worsening of joint symptoms among patients treated with ustekinumab, the investigators noted, concluding that the findings – which may reflect a lack of efficacy at the administered doses or a need for more frequent dosing – may support arguments that psoriatic arthritis and psoriasis involve distinct inflammatory pathways.
“It is also possible that ustekinumab may trigger or unmask inflammation in the joints of patients with psoriatic arthritis,” they wrote, concluding that “larger epidemiologic studies comparing patients with discordant and concordant cutaneous and articular responses to ustekinumab may better define patients at risk for psoriatic arthritis worsening with ustekinumab.”
Two of the five authors have reported serving as a consultant or advisory board member, receiving payment for lectures, and/or serving as an investigator for Janssen, which markets ustekinumab, as well as other manufacturers of biologics. The other authors reported having no conflicts of interest.
Key clinical point: Increasing evidence suggests that ustekinumab is associated with discordant joint and skin responses.
Major finding: Five of seven psoriasis patients treated with ustekinumab experienced new-onset psoriatic arthritis, and two had worsening psoriatic arthritis.
Data source: A series of seven cases.
Disclosures: Two of the five authors have reported serving as a consultant or advisory board member, receiving payment for lectures, and/or serving as an investigator for Janssen, which markets ustekinumab, as well as other manufacturers of biologics. The other authors reported having no conflicts of interest.
Framingham score underestimates CVD risk in psoriatic arthritis patients
Most newly diagnosed psoriatic arthritis patients have an increased risk for cardiovascular disease that is markedly underestimated by the Framingham Risk Score, according to findings from a retrospective, population-based, cohort study.
The mean Framingham Risk Score in 126 patients with psoriatic arthritis who were aged 30 years or older and who had no prior cardiovascular disease (CVD) history, was 9.7% during the first 10 years of follow-up. However, the 10-year cumulative incidence of CVD events was nearly double that at 17% (standardized incidence ratio, 1.80), Dr. Floranne C. Ernste of the Mayo Clinic, Rochester, Minn., and her colleagues reported (Arthritis Care Res. 2015 Jan. 7 [doi:10.1002/acr.22536]).
Age-based analysis showed that the CVD risk in these patients was consistently twice as high as predicted by the FRS beginning after age 40 years, the authors noted.
The findings underscore the importance of CVD risk assessment in patients with psoriatic arthritis but suggest that the Framingham Risk Score may not be applicable in such patients, the investigators said, adding that “This study serves to illustrate the important need for further research to focus on the development of CVD risk assessment tools specific to psoriatic arthritis patients.”
The findings also suggest that aggressive therapy may be warranted early in the course of psoriatic arthritis to “attenuate the long-term burden of CVD,” they said.
This study was supported by the Rochester Epidemiology Project and by Amgen. Dr. Ernste reported having no disclosures.
Most newly diagnosed psoriatic arthritis patients have an increased risk for cardiovascular disease that is markedly underestimated by the Framingham Risk Score, according to findings from a retrospective, population-based, cohort study.
The mean Framingham Risk Score in 126 patients with psoriatic arthritis who were aged 30 years or older and who had no prior cardiovascular disease (CVD) history, was 9.7% during the first 10 years of follow-up. However, the 10-year cumulative incidence of CVD events was nearly double that at 17% (standardized incidence ratio, 1.80), Dr. Floranne C. Ernste of the Mayo Clinic, Rochester, Minn., and her colleagues reported (Arthritis Care Res. 2015 Jan. 7 [doi:10.1002/acr.22536]).
Age-based analysis showed that the CVD risk in these patients was consistently twice as high as predicted by the FRS beginning after age 40 years, the authors noted.
The findings underscore the importance of CVD risk assessment in patients with psoriatic arthritis but suggest that the Framingham Risk Score may not be applicable in such patients, the investigators said, adding that “This study serves to illustrate the important need for further research to focus on the development of CVD risk assessment tools specific to psoriatic arthritis patients.”
The findings also suggest that aggressive therapy may be warranted early in the course of psoriatic arthritis to “attenuate the long-term burden of CVD,” they said.
This study was supported by the Rochester Epidemiology Project and by Amgen. Dr. Ernste reported having no disclosures.
Most newly diagnosed psoriatic arthritis patients have an increased risk for cardiovascular disease that is markedly underestimated by the Framingham Risk Score, according to findings from a retrospective, population-based, cohort study.
The mean Framingham Risk Score in 126 patients with psoriatic arthritis who were aged 30 years or older and who had no prior cardiovascular disease (CVD) history, was 9.7% during the first 10 years of follow-up. However, the 10-year cumulative incidence of CVD events was nearly double that at 17% (standardized incidence ratio, 1.80), Dr. Floranne C. Ernste of the Mayo Clinic, Rochester, Minn., and her colleagues reported (Arthritis Care Res. 2015 Jan. 7 [doi:10.1002/acr.22536]).
Age-based analysis showed that the CVD risk in these patients was consistently twice as high as predicted by the FRS beginning after age 40 years, the authors noted.
The findings underscore the importance of CVD risk assessment in patients with psoriatic arthritis but suggest that the Framingham Risk Score may not be applicable in such patients, the investigators said, adding that “This study serves to illustrate the important need for further research to focus on the development of CVD risk assessment tools specific to psoriatic arthritis patients.”
The findings also suggest that aggressive therapy may be warranted early in the course of psoriatic arthritis to “attenuate the long-term burden of CVD,” they said.
This study was supported by the Rochester Epidemiology Project and by Amgen. Dr. Ernste reported having no disclosures.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: The Framingham Risk Score may not be applicable for estimating CVD risk in psoriatic arthritis patients.
Major finding: The 10-year cumulative CVD incidence rate was 17%, compared with 9.7% predicted by Framingham Risk Score.
Data source: A retrospective, population-based, cohort study of 126 patients.
Disclosures: This study was supported by the Rochester Epidemiology Project and by Amgen. Dr. Ernste reported having no disclosures.
Annual costs of psoriasis costs top $112 billion
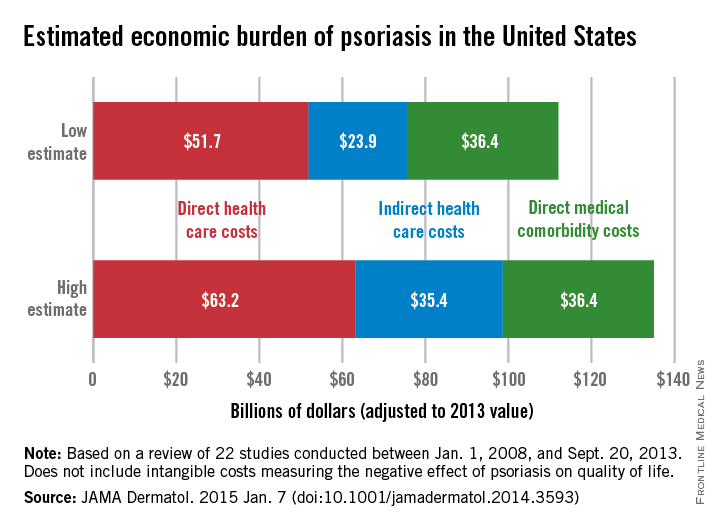
The total economic burden of psoriasis in the United States is at least $112 billion per year, and possibly as high as $135 billion, investigators estimated in a study published Jan. 7 in JAMA Dermatology.
Dr. Elizabeth A. Brezinski of the University of California, Davis, in Sacramento, and her associates, reviewed 22 studies conducted between Jan. 1, 2008, and Sept. 20, 2013, adjusting the results to 2013 dollars.
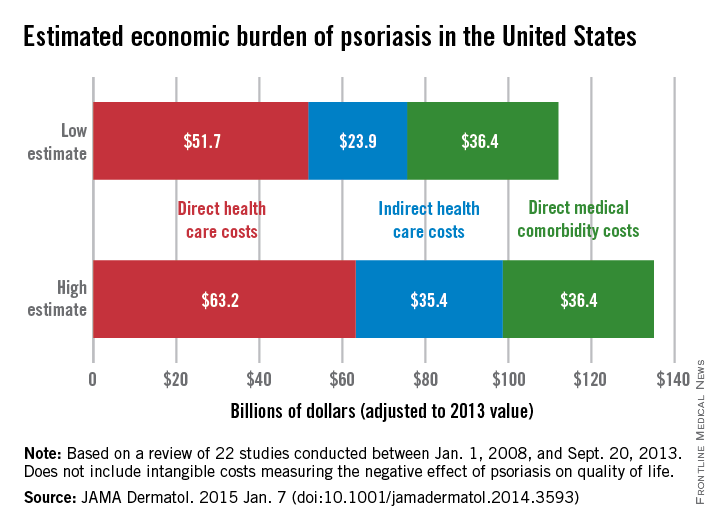
Estimates for the direct medical cost of psoriasis care ranged from $51.7 billion to $63.2 billion per year. Indirect costs from absenteeism or working while sick contributed another $23.9-$35.4 billion, with comorbidity costs estimated at $36.4 billion annually (JAMA Dermatol. 2014 Jan. 7 [doi:10.1001/jamadermatol.2014.3593]).
Intangible costs on quality of life, which were not included in the annual figures, were estimated to be $85.1 billion over the lifetimes of the psoriasis patient population (7.4 million as of 2013), they said.
“Defining the economic burden of psoriasis from a societal perspective is the foundation for innovating and providing access to cost-effective therapies that will result in improved patient outcomes,” Dr. Brezinski and her coauthors wrote.
One of the researchers reported serving as an investigator for, or consultant to, AbbVie, Amgen, Celgene, Janssen, Lilly, Merck, Pfizer, and UCB. No other disclosures were reported.
The total economic burden of psoriasis in the United States is at least $112 billion per year, and possibly as high as $135 billion, investigators estimated in a study published Jan. 7 in JAMA Dermatology.
Dr. Elizabeth A. Brezinski of the University of California, Davis, in Sacramento, and her associates, reviewed 22 studies conducted between Jan. 1, 2008, and Sept. 20, 2013, adjusting the results to 2013 dollars.

Estimates for the direct medical cost of psoriasis care ranged from $51.7 billion to $63.2 billion per year. Indirect costs from absenteeism or working while sick contributed another $23.9-$35.4 billion, with comorbidity costs estimated at $36.4 billion annually (JAMA Dermatol. 2014 Jan. 7 [doi:10.1001/jamadermatol.2014.3593]).
Intangible costs on quality of life, which were not included in the annual figures, were estimated to be $85.1 billion over the lifetimes of the psoriasis patient population (7.4 million as of 2013), they said.
“Defining the economic burden of psoriasis from a societal perspective is the foundation for innovating and providing access to cost-effective therapies that will result in improved patient outcomes,” Dr. Brezinski and her coauthors wrote.
One of the researchers reported serving as an investigator for, or consultant to, AbbVie, Amgen, Celgene, Janssen, Lilly, Merck, Pfizer, and UCB. No other disclosures were reported.
The total economic burden of psoriasis in the United States is at least $112 billion per year, and possibly as high as $135 billion, investigators estimated in a study published Jan. 7 in JAMA Dermatology.
Dr. Elizabeth A. Brezinski of the University of California, Davis, in Sacramento, and her associates, reviewed 22 studies conducted between Jan. 1, 2008, and Sept. 20, 2013, adjusting the results to 2013 dollars.

Estimates for the direct medical cost of psoriasis care ranged from $51.7 billion to $63.2 billion per year. Indirect costs from absenteeism or working while sick contributed another $23.9-$35.4 billion, with comorbidity costs estimated at $36.4 billion annually (JAMA Dermatol. 2014 Jan. 7 [doi:10.1001/jamadermatol.2014.3593]).
Intangible costs on quality of life, which were not included in the annual figures, were estimated to be $85.1 billion over the lifetimes of the psoriasis patient population (7.4 million as of 2013), they said.
“Defining the economic burden of psoriasis from a societal perspective is the foundation for innovating and providing access to cost-effective therapies that will result in improved patient outcomes,” Dr. Brezinski and her coauthors wrote.
One of the researchers reported serving as an investigator for, or consultant to, AbbVie, Amgen, Celgene, Janssen, Lilly, Merck, Pfizer, and UCB. No other disclosures were reported.
FROM JAMA DERMATOLOGY
Immunogenicity to TNF-alpha blockers varies in psoriatic arthritis
Immunogenicity to three TNF-alpha blocking agents seems to vary substantially among patients with psoriatic arthritis, and the use of methotrexate appears to attenuate the presence of antidrug antibodies, according to findings from a cross-sectional study.
Although researchers have looked at the immunogenicity of TNF-alpha blockers in patients with diseases such as rheumatoid arthritis (RA), ankylosing spondylitis, psoriasis, and inflammatory bowel disease, their immunogenicity in psoriatic arthritis (PsA) patients has not been fully investigated, noted the current study’s investigators, led by Dr. Michael Zisapel of the department of rheumatology at Tel Aviv University (J. Rheumatol. 2014 Nov. 15 [doi:10.3899/jrheum.140685]).
The prevalence of antidrug antibodies (ADAb) to TNF-alpha blockers has been reported to be 20%-40% in RA, 25%-64% in ankylosing spondylitis, and about 33% in psoriasis, and evidence has shown that this is significantly reduced by the use of methotrexate, they said.
The study involved 93 patients with PsA who were taking adalimumab (n = 48), infliximab (n = 24), and etanercept (n = 21). A quarter of the patients were taking methotrexate at an average dose of 13.3 mg/week.
Overall, 77% of the patients had therapeutic drug levels. The prevalence of immunogenicity in the entire group was 33.3%, and one-fifth of patients had high concentrations of antibodies.
High levels of ADAb were found in 29% of patients taking adalimumab and 21% of the patients taking infliximab. No ADAb levels were found in patients taking etanercept.
Interestingly, the patients taking adalimumab demonstrated an immunogenicity of 54%, but only half of them (29%) showed high concentrations of antibodies.
“This finding suggests that a variety of levels might be found that might affect the significance of immunogenicity differently among patients producing ADAb,” the investigators wrote.
Fewer methotrexate-treated patients had high ADAb concentrations, compared with patients not taking methotrexate (16.7% vs. 21.7%).
A clear correlation was found between the presence of immunogenicity, lower drug levels, and decreased clinical response in PsA patients, just as other studies have found in RA and ankylosing spondylitis patients, the authors noted.
“Our results suggest that the use of methotrexate should be strongly considered in addition to monoclonal antibodies,” they said.
Limitations of their study included the small number of patients and the use of a bridging ELISA test which is reliable but considered to be less accurate than radioimmunoassay.
No disclosure information was available.
Immunogenicity to three TNF-alpha blocking agents seems to vary substantially among patients with psoriatic arthritis, and the use of methotrexate appears to attenuate the presence of antidrug antibodies, according to findings from a cross-sectional study.
Although researchers have looked at the immunogenicity of TNF-alpha blockers in patients with diseases such as rheumatoid arthritis (RA), ankylosing spondylitis, psoriasis, and inflammatory bowel disease, their immunogenicity in psoriatic arthritis (PsA) patients has not been fully investigated, noted the current study’s investigators, led by Dr. Michael Zisapel of the department of rheumatology at Tel Aviv University (J. Rheumatol. 2014 Nov. 15 [doi:10.3899/jrheum.140685]).
The prevalence of antidrug antibodies (ADAb) to TNF-alpha blockers has been reported to be 20%-40% in RA, 25%-64% in ankylosing spondylitis, and about 33% in psoriasis, and evidence has shown that this is significantly reduced by the use of methotrexate, they said.
The study involved 93 patients with PsA who were taking adalimumab (n = 48), infliximab (n = 24), and etanercept (n = 21). A quarter of the patients were taking methotrexate at an average dose of 13.3 mg/week.
Overall, 77% of the patients had therapeutic drug levels. The prevalence of immunogenicity in the entire group was 33.3%, and one-fifth of patients had high concentrations of antibodies.
High levels of ADAb were found in 29% of patients taking adalimumab and 21% of the patients taking infliximab. No ADAb levels were found in patients taking etanercept.
Interestingly, the patients taking adalimumab demonstrated an immunogenicity of 54%, but only half of them (29%) showed high concentrations of antibodies.
“This finding suggests that a variety of levels might be found that might affect the significance of immunogenicity differently among patients producing ADAb,” the investigators wrote.
Fewer methotrexate-treated patients had high ADAb concentrations, compared with patients not taking methotrexate (16.7% vs. 21.7%).
A clear correlation was found between the presence of immunogenicity, lower drug levels, and decreased clinical response in PsA patients, just as other studies have found in RA and ankylosing spondylitis patients, the authors noted.
“Our results suggest that the use of methotrexate should be strongly considered in addition to monoclonal antibodies,” they said.
Limitations of their study included the small number of patients and the use of a bridging ELISA test which is reliable but considered to be less accurate than radioimmunoassay.
No disclosure information was available.
Immunogenicity to three TNF-alpha blocking agents seems to vary substantially among patients with psoriatic arthritis, and the use of methotrexate appears to attenuate the presence of antidrug antibodies, according to findings from a cross-sectional study.
Although researchers have looked at the immunogenicity of TNF-alpha blockers in patients with diseases such as rheumatoid arthritis (RA), ankylosing spondylitis, psoriasis, and inflammatory bowel disease, their immunogenicity in psoriatic arthritis (PsA) patients has not been fully investigated, noted the current study’s investigators, led by Dr. Michael Zisapel of the department of rheumatology at Tel Aviv University (J. Rheumatol. 2014 Nov. 15 [doi:10.3899/jrheum.140685]).
The prevalence of antidrug antibodies (ADAb) to TNF-alpha blockers has been reported to be 20%-40% in RA, 25%-64% in ankylosing spondylitis, and about 33% in psoriasis, and evidence has shown that this is significantly reduced by the use of methotrexate, they said.
The study involved 93 patients with PsA who were taking adalimumab (n = 48), infliximab (n = 24), and etanercept (n = 21). A quarter of the patients were taking methotrexate at an average dose of 13.3 mg/week.
Overall, 77% of the patients had therapeutic drug levels. The prevalence of immunogenicity in the entire group was 33.3%, and one-fifth of patients had high concentrations of antibodies.
High levels of ADAb were found in 29% of patients taking adalimumab and 21% of the patients taking infliximab. No ADAb levels were found in patients taking etanercept.
Interestingly, the patients taking adalimumab demonstrated an immunogenicity of 54%, but only half of them (29%) showed high concentrations of antibodies.
“This finding suggests that a variety of levels might be found that might affect the significance of immunogenicity differently among patients producing ADAb,” the investigators wrote.
Fewer methotrexate-treated patients had high ADAb concentrations, compared with patients not taking methotrexate (16.7% vs. 21.7%).
A clear correlation was found between the presence of immunogenicity, lower drug levels, and decreased clinical response in PsA patients, just as other studies have found in RA and ankylosing spondylitis patients, the authors noted.
“Our results suggest that the use of methotrexate should be strongly considered in addition to monoclonal antibodies,” they said.
Limitations of their study included the small number of patients and the use of a bridging ELISA test which is reliable but considered to be less accurate than radioimmunoassay.
No disclosure information was available.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: The use of methotrexate should be strongly considered in addition to TNF-alpha blockers in patients with psoriatic arthritis to reduce the presence and influence of antidrug antibodies.
Major finding: High levels of antidrug antibodies were found in 29% of patients taking adalimumab, 21% of the patients taking infliximab, and no patients taking etanercept.
Data source: A cross-sectional study of 93 consecutive psoriatic arthritis patients.
Disclosures: No disclosure information was available.