User login
Disease Burden and Treatment Adherence in Psoriasis Patients
Test your knowledge on QOL issues for patients with psoriasis with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.
Biologic therapy doesn’t preclude vaccinating psoriasis patients
LAS VEGAS – Dermatologists, like all physicians, play an important role not just in the treatment of disease, but also in the prevention of disease, and that means vaccinating patients when indicated, according to Dr. Stephen K. Tyring.
The varicella vaccines, including the chicken pox and shingles vaccines, and the human papillomavirus (HPV), hepatitis, and influenza virus vaccines are among those to consider offering to patients when appropriate, Dr. Tyring said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Concerns arise, however, when it comes to vaccinating psoriasis patients who are – or will be – treated with biologics.
Ideally, vaccination should be offered before treatment is initiated, he said, but for some patients – such as those who are under the age for which their insurance company will cover a vaccine (age 50 or 60 for the shingles vaccine, depending on the insurance company, for example) – this may not be possible. In those already taking a biologic drug, the question is how to vaccinate safely and without interfering with treatment efficacy, he said.
"What we usually do – and there’s no absolute golden rule – is ask the patient to stop the drug for one to two half-lives of the drug," said Dr. Tyring of the University of Texas, Houston. We suggest stopping one half-life for killed, subunit, or recombinant virus vaccines and stopping for two half-lives for live attenuated virus vaccines.
With the tumor necrosis factor (TNF) inhibitor etanercept (Enbrel), for example, that means skipping one to two weekly injections.
"That’s a little bit more than a half-life, but if a patient is using Enbrel once or twice a week, the next week, instead of injecting the Enbrel, they can just come in and get the shingles vaccine [Zostavax]," he said.
Adalimumab (Humira), another anti-TNF drug, has a longer half-life, so that has to be taken into account. Things get a little more complicated when it comes to patients treated with ustekinumab (Stelera), which has a particularly long half-life, he noted.
"It’s not quite as easy, because it’s not clear how long to wait after Stelera to give a vaccine. Most efficacy is seen in the first month, and when patients come back at 3 months, they often are starting to get a little psoriasis back. Therefore, 2 months following an injection of Stelera is about the right time to get the vaccine; that way you get the minimum immunosuppression and the maximum chance to respond," he said.
Similarly, with other vaccines like the Gardasil HPV vaccine and the hepatitis A and B vaccines that require a series of shots, the biologic should be stopped for a half-life. This will mean skipping biologic dosing multiple times, but in most cases this won’t be problematic, because it typically takes about 3 months for a patient who is clear to experience significant psoriasis recurrence.
"There’s really no danger in skipping," he said.
While some may advocate waiting two half-lives after vaccination, most experts agree that is unnecessary for killed, recombinant, or subunit (for example, injectable influenza) virus vaccines but is advisable for live attenuated virus vaccines like the intranasal influenza virus vaccine or the herpes zoster vaccine Zostavax, he said.
Dr. Tyring also noted during his presentation that in his experience, concerns about increased infection risk in patients taking TNF inhibitors have been unfounded. In fact, treatment appears to provide an unexpected benefit for those who do become infected with herpes zoster: a reduced risk of postherpetic neuralgia, even among older individuals who are generally at particularly high risk.
After noticing this benefit in his own patients, Dr. Tyring asked his colleagues and found that they, too, had noticed a similar pattern. In 2011, he and his colleagues published a retrospective study of 206 patients on TNF inhibitors who developed herpes zoster, and with only two exceptions involving patients on both a TNF inhibitor and methotrexate, patients universally experienced milder symptoms if they developed shingles while on a TNF inhibitor, he said, noting that this is much lower than rates reported in the literature in the general population (J. Med. Virol. 2011;83:2051-5).
SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Dermatologists, like all physicians, play an important role not just in the treatment of disease, but also in the prevention of disease, and that means vaccinating patients when indicated, according to Dr. Stephen K. Tyring.
The varicella vaccines, including the chicken pox and shingles vaccines, and the human papillomavirus (HPV), hepatitis, and influenza virus vaccines are among those to consider offering to patients when appropriate, Dr. Tyring said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Concerns arise, however, when it comes to vaccinating psoriasis patients who are – or will be – treated with biologics.
Ideally, vaccination should be offered before treatment is initiated, he said, but for some patients – such as those who are under the age for which their insurance company will cover a vaccine (age 50 or 60 for the shingles vaccine, depending on the insurance company, for example) – this may not be possible. In those already taking a biologic drug, the question is how to vaccinate safely and without interfering with treatment efficacy, he said.
"What we usually do – and there’s no absolute golden rule – is ask the patient to stop the drug for one to two half-lives of the drug," said Dr. Tyring of the University of Texas, Houston. We suggest stopping one half-life for killed, subunit, or recombinant virus vaccines and stopping for two half-lives for live attenuated virus vaccines.
With the tumor necrosis factor (TNF) inhibitor etanercept (Enbrel), for example, that means skipping one to two weekly injections.
"That’s a little bit more than a half-life, but if a patient is using Enbrel once or twice a week, the next week, instead of injecting the Enbrel, they can just come in and get the shingles vaccine [Zostavax]," he said.
Adalimumab (Humira), another anti-TNF drug, has a longer half-life, so that has to be taken into account. Things get a little more complicated when it comes to patients treated with ustekinumab (Stelera), which has a particularly long half-life, he noted.
"It’s not quite as easy, because it’s not clear how long to wait after Stelera to give a vaccine. Most efficacy is seen in the first month, and when patients come back at 3 months, they often are starting to get a little psoriasis back. Therefore, 2 months following an injection of Stelera is about the right time to get the vaccine; that way you get the minimum immunosuppression and the maximum chance to respond," he said.
Similarly, with other vaccines like the Gardasil HPV vaccine and the hepatitis A and B vaccines that require a series of shots, the biologic should be stopped for a half-life. This will mean skipping biologic dosing multiple times, but in most cases this won’t be problematic, because it typically takes about 3 months for a patient who is clear to experience significant psoriasis recurrence.
"There’s really no danger in skipping," he said.
While some may advocate waiting two half-lives after vaccination, most experts agree that is unnecessary for killed, recombinant, or subunit (for example, injectable influenza) virus vaccines but is advisable for live attenuated virus vaccines like the intranasal influenza virus vaccine or the herpes zoster vaccine Zostavax, he said.
Dr. Tyring also noted during his presentation that in his experience, concerns about increased infection risk in patients taking TNF inhibitors have been unfounded. In fact, treatment appears to provide an unexpected benefit for those who do become infected with herpes zoster: a reduced risk of postherpetic neuralgia, even among older individuals who are generally at particularly high risk.
After noticing this benefit in his own patients, Dr. Tyring asked his colleagues and found that they, too, had noticed a similar pattern. In 2011, he and his colleagues published a retrospective study of 206 patients on TNF inhibitors who developed herpes zoster, and with only two exceptions involving patients on both a TNF inhibitor and methotrexate, patients universally experienced milder symptoms if they developed shingles while on a TNF inhibitor, he said, noting that this is much lower than rates reported in the literature in the general population (J. Med. Virol. 2011;83:2051-5).
SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Dermatologists, like all physicians, play an important role not just in the treatment of disease, but also in the prevention of disease, and that means vaccinating patients when indicated, according to Dr. Stephen K. Tyring.
The varicella vaccines, including the chicken pox and shingles vaccines, and the human papillomavirus (HPV), hepatitis, and influenza virus vaccines are among those to consider offering to patients when appropriate, Dr. Tyring said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Concerns arise, however, when it comes to vaccinating psoriasis patients who are – or will be – treated with biologics.
Ideally, vaccination should be offered before treatment is initiated, he said, but for some patients – such as those who are under the age for which their insurance company will cover a vaccine (age 50 or 60 for the shingles vaccine, depending on the insurance company, for example) – this may not be possible. In those already taking a biologic drug, the question is how to vaccinate safely and without interfering with treatment efficacy, he said.
"What we usually do – and there’s no absolute golden rule – is ask the patient to stop the drug for one to two half-lives of the drug," said Dr. Tyring of the University of Texas, Houston. We suggest stopping one half-life for killed, subunit, or recombinant virus vaccines and stopping for two half-lives for live attenuated virus vaccines.
With the tumor necrosis factor (TNF) inhibitor etanercept (Enbrel), for example, that means skipping one to two weekly injections.
"That’s a little bit more than a half-life, but if a patient is using Enbrel once or twice a week, the next week, instead of injecting the Enbrel, they can just come in and get the shingles vaccine [Zostavax]," he said.
Adalimumab (Humira), another anti-TNF drug, has a longer half-life, so that has to be taken into account. Things get a little more complicated when it comes to patients treated with ustekinumab (Stelera), which has a particularly long half-life, he noted.
"It’s not quite as easy, because it’s not clear how long to wait after Stelera to give a vaccine. Most efficacy is seen in the first month, and when patients come back at 3 months, they often are starting to get a little psoriasis back. Therefore, 2 months following an injection of Stelera is about the right time to get the vaccine; that way you get the minimum immunosuppression and the maximum chance to respond," he said.
Similarly, with other vaccines like the Gardasil HPV vaccine and the hepatitis A and B vaccines that require a series of shots, the biologic should be stopped for a half-life. This will mean skipping biologic dosing multiple times, but in most cases this won’t be problematic, because it typically takes about 3 months for a patient who is clear to experience significant psoriasis recurrence.
"There’s really no danger in skipping," he said.
While some may advocate waiting two half-lives after vaccination, most experts agree that is unnecessary for killed, recombinant, or subunit (for example, injectable influenza) virus vaccines but is advisable for live attenuated virus vaccines like the intranasal influenza virus vaccine or the herpes zoster vaccine Zostavax, he said.
Dr. Tyring also noted during his presentation that in his experience, concerns about increased infection risk in patients taking TNF inhibitors have been unfounded. In fact, treatment appears to provide an unexpected benefit for those who do become infected with herpes zoster: a reduced risk of postherpetic neuralgia, even among older individuals who are generally at particularly high risk.
After noticing this benefit in his own patients, Dr. Tyring asked his colleagues and found that they, too, had noticed a similar pattern. In 2011, he and his colleagues published a retrospective study of 206 patients on TNF inhibitors who developed herpes zoster, and with only two exceptions involving patients on both a TNF inhibitor and methotrexate, patients universally experienced milder symptoms if they developed shingles while on a TNF inhibitor, he said, noting that this is much lower than rates reported in the literature in the general population (J. Med. Virol. 2011;83:2051-5).
SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Don’t brush off topical therapies for psoriasis
Biologics and systemic therapies command much of the spotlight for treating psoriasis, but topical therapy remains an effective option for many psoriasis patients, according to Dr. Linda Stein Gold.
In fact, up to 80% of psoriasis patients can be adequately treated with topical therapy (N. Engl. J. Med. 2005;352:1899-912), said Dr. Stein Gold at the Skin Disease Education Foundation’s (SDEF’s) annual Las Vegas dermatology seminar.
She offered several principles to help clinicians make the most of topical therapies by troubleshooting potential problems.
• Check the amount. One gram of most topical psoriasis products covers 4% of body surface area per application, so a 60-gram tube should treat 4% of body surface area for a month, said Dr. Stein Gold, citing guidelines developed by Dr. Alan Menter and his colleagues and approved by the American Academy of Dermatology in 2009.
• Don’t miss corticosteroid allergies. "We are missing this," said Dr. Stein Gold, director of dermatology research at Henry Ford Hospital in Detroit. Suspect a possible allergy if a patient returns with a worsening rash after using hydrocortisone, for example. Data have shown that between 0.2% and 5% of all dermatitis patients have a steroid allergy. "Allergy to the active molecule or to the vehicle should be suspected in all patients who don’t respond as expected to topical steroids," she noted.
• Visit (or revisit) vitamin D. Another plus for topical therapy is the usefulness of topical vitamin D for tricky areas, such as the forehead, armpit, groin, and the area behind the ears. Data from a randomized trial of 75 patients found calcitriol ointment to be significantly more effective against target lesions and better tolerated by patients than calcipotriene ointment (Br. J. Dermatol. 2003;148:326-33).
• Don’t forget coal tar. Simple, but effective, coal tar is a proven safe topical psoriasis treatment and is available in several vehicles, including solution and foam. Data from a study of more than 13,000 patients with psoriasis and eczema found that coal tar was safe, and that it did not increase the risk for skin cancer (J. Invest. Dermatol. 2010;130:953-61).
The future of topical psoriasis therapy is not static, said Dr. Stein Gold. New molecules – notably topical Janus kinase inhibitors and phosphodiesterase-4 inhibitors – are currently being explored in clinical trials.
Dr. Stein Gold disclosed relationships with Leo, Medicis, and other companies. SDEF and this news organization are owned by Frontline Medical Communications.
Biologics and systemic therapies command much of the spotlight for treating psoriasis, but topical therapy remains an effective option for many psoriasis patients, according to Dr. Linda Stein Gold.
In fact, up to 80% of psoriasis patients can be adequately treated with topical therapy (N. Engl. J. Med. 2005;352:1899-912), said Dr. Stein Gold at the Skin Disease Education Foundation’s (SDEF’s) annual Las Vegas dermatology seminar.
She offered several principles to help clinicians make the most of topical therapies by troubleshooting potential problems.
• Check the amount. One gram of most topical psoriasis products covers 4% of body surface area per application, so a 60-gram tube should treat 4% of body surface area for a month, said Dr. Stein Gold, citing guidelines developed by Dr. Alan Menter and his colleagues and approved by the American Academy of Dermatology in 2009.
• Don’t miss corticosteroid allergies. "We are missing this," said Dr. Stein Gold, director of dermatology research at Henry Ford Hospital in Detroit. Suspect a possible allergy if a patient returns with a worsening rash after using hydrocortisone, for example. Data have shown that between 0.2% and 5% of all dermatitis patients have a steroid allergy. "Allergy to the active molecule or to the vehicle should be suspected in all patients who don’t respond as expected to topical steroids," she noted.
• Visit (or revisit) vitamin D. Another plus for topical therapy is the usefulness of topical vitamin D for tricky areas, such as the forehead, armpit, groin, and the area behind the ears. Data from a randomized trial of 75 patients found calcitriol ointment to be significantly more effective against target lesions and better tolerated by patients than calcipotriene ointment (Br. J. Dermatol. 2003;148:326-33).
• Don’t forget coal tar. Simple, but effective, coal tar is a proven safe topical psoriasis treatment and is available in several vehicles, including solution and foam. Data from a study of more than 13,000 patients with psoriasis and eczema found that coal tar was safe, and that it did not increase the risk for skin cancer (J. Invest. Dermatol. 2010;130:953-61).
The future of topical psoriasis therapy is not static, said Dr. Stein Gold. New molecules – notably topical Janus kinase inhibitors and phosphodiesterase-4 inhibitors – are currently being explored in clinical trials.
Dr. Stein Gold disclosed relationships with Leo, Medicis, and other companies. SDEF and this news organization are owned by Frontline Medical Communications.
Biologics and systemic therapies command much of the spotlight for treating psoriasis, but topical therapy remains an effective option for many psoriasis patients, according to Dr. Linda Stein Gold.
In fact, up to 80% of psoriasis patients can be adequately treated with topical therapy (N. Engl. J. Med. 2005;352:1899-912), said Dr. Stein Gold at the Skin Disease Education Foundation’s (SDEF’s) annual Las Vegas dermatology seminar.
She offered several principles to help clinicians make the most of topical therapies by troubleshooting potential problems.
• Check the amount. One gram of most topical psoriasis products covers 4% of body surface area per application, so a 60-gram tube should treat 4% of body surface area for a month, said Dr. Stein Gold, citing guidelines developed by Dr. Alan Menter and his colleagues and approved by the American Academy of Dermatology in 2009.
• Don’t miss corticosteroid allergies. "We are missing this," said Dr. Stein Gold, director of dermatology research at Henry Ford Hospital in Detroit. Suspect a possible allergy if a patient returns with a worsening rash after using hydrocortisone, for example. Data have shown that between 0.2% and 5% of all dermatitis patients have a steroid allergy. "Allergy to the active molecule or to the vehicle should be suspected in all patients who don’t respond as expected to topical steroids," she noted.
• Visit (or revisit) vitamin D. Another plus for topical therapy is the usefulness of topical vitamin D for tricky areas, such as the forehead, armpit, groin, and the area behind the ears. Data from a randomized trial of 75 patients found calcitriol ointment to be significantly more effective against target lesions and better tolerated by patients than calcipotriene ointment (Br. J. Dermatol. 2003;148:326-33).
• Don’t forget coal tar. Simple, but effective, coal tar is a proven safe topical psoriasis treatment and is available in several vehicles, including solution and foam. Data from a study of more than 13,000 patients with psoriasis and eczema found that coal tar was safe, and that it did not increase the risk for skin cancer (J. Invest. Dermatol. 2010;130:953-61).
The future of topical psoriasis therapy is not static, said Dr. Stein Gold. New molecules – notably topical Janus kinase inhibitors and phosphodiesterase-4 inhibitors – are currently being explored in clinical trials.
Dr. Stein Gold disclosed relationships with Leo, Medicis, and other companies. SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Antirheumatic drugs don’t boost surgical infection risk
SAN DIEGO – Rheumatoid arthritis patients undergoing surgery who stayed on their antirheumatic medication perioperatively didn’t have a higher risk of early postoperative infection compared with those who temporarily stopped treatment before surgery, according to findings from a large national Veterans Affairs study.
Rheumatologists are frequently consulted about this issue. Evidence to guide practice has been scarce, however, and until now many rheumatologists and surgeons have taken a conservative approach, reasoning that the immunosuppressive drugs employed in controlling inflammation in rheumatoid arthritis might also increase the risk of surgical wound infection.
A common practice has been to have RA patients stop their medication a month ahead of elective surgery, or at least two drug half-lives beforehand, then start treatment again roughly a month after the operation, or when the wound has healed. The new Veterans Affairs (VA) study findings suggest this practice may be unnecessary, Dr. Zaki Abou Zahr said at the annual meeting of the American College of Rheumatology.
Dr. Bernard Ng, his senior coinvestigator in the study, added that temporarily stopping antirheumatic agents before surgery may actually be harmful in that it increases the risk of a flare of the RA, which in turn would impede postoperative rehabilitation.
But there is a major caveat regarding the VA study: Participation was restricted to RA patients on only a single conventional disease-modifying antirheumatic drug (DMARD) or biologic agent leading up to surgery. This restriction, imposed to make for a more clear-cut analysis, means that the study results can’t be extrapolated to patients on multidrug therapy. And multidrug therapy is quite common. Indeed, slightly more than half of RA patients in the VA health care system are on combination therapy, most often methotrexate plus a biologic agent, noted Dr. Ng, chief of rheumatology at the VA Puget Sound Health Care System, Seattle.
Dr. Abou Zahr presented the retrospective cohort study involving 6,548 RA patients in VA administrative databases, all of whom were on antirheumatic drug monotherapy prior to surgery. The surgery was of all types, including cardiothoracic, gastrointestinal, vascular, and orthopedic, as well as emergent and elective.
The primary endpoints were the rate of wound infections, both superficial and deep, within 30 days post surgery, and the general infection rate – including pneumonia, sepsis, and urinary tract infections – during the same time frame.
Sixty-two percent of the 1,480 RA patients on a single biologic agent did not stop taking it preoperatively. One key study finding was that neither their postoperative wound infection rate nor their general infection rate differed significantly from rates in patients who temporarily halted their biologic agent. The same held true among the 70% of patients on a single conventional DMARD who did not stop taking their medication preoperatively, according to Dr. Abou Zahr of Baylor College of Medicine, Houston.
Dr. Ng said the investigators plan to extend their work to include RA patients on multiple antirheumatic drugs that they do or don’t temporarily stop when undergoing surgery within the VA system. The researchers also plan to take a close look at patients undergoing specific types of surgery to see if the postoperative infection risk in patients who remain on treatment varies according to their operation.
Dr. Fehmida Zahabi, a rheumatologist from Plano, Tex., who chaired a press conference highlighting the VA study findings, said that while she’d like to see a confirmatory study, "I think we’re getting to the point where we’re saying we should cautiously keep these patients on their medications. That’s what the data suggest."
She noted that before the VA study, the very limited evidence available to guide practice in this area centered on a 12-year-old British randomized trial involving RA patients on methotrexate undergoing elective orthopedic surgery. Those assigned to stop the drug from 2 weeks before surgery to 2 weeks post surgery had significantly more infections, surgical complications, and RA flares within 6 weeks after surgery (Ann. Rheum. Dis. 2001;60:214-7).
As for patients on multidrug therapy who are scheduled for surgery, her inclination until evidence becomes available for guidance is to pare down the regimen preoperatively, while keeping the patient on one or two drugs.
The VA study was funded by the Department of Veterans Affairs. Dr. Abou Zahr and Dr. Ng reported having no conflicts of interest.
SAN DIEGO – Rheumatoid arthritis patients undergoing surgery who stayed on their antirheumatic medication perioperatively didn’t have a higher risk of early postoperative infection compared with those who temporarily stopped treatment before surgery, according to findings from a large national Veterans Affairs study.
Rheumatologists are frequently consulted about this issue. Evidence to guide practice has been scarce, however, and until now many rheumatologists and surgeons have taken a conservative approach, reasoning that the immunosuppressive drugs employed in controlling inflammation in rheumatoid arthritis might also increase the risk of surgical wound infection.
A common practice has been to have RA patients stop their medication a month ahead of elective surgery, or at least two drug half-lives beforehand, then start treatment again roughly a month after the operation, or when the wound has healed. The new Veterans Affairs (VA) study findings suggest this practice may be unnecessary, Dr. Zaki Abou Zahr said at the annual meeting of the American College of Rheumatology.
Dr. Bernard Ng, his senior coinvestigator in the study, added that temporarily stopping antirheumatic agents before surgery may actually be harmful in that it increases the risk of a flare of the RA, which in turn would impede postoperative rehabilitation.
But there is a major caveat regarding the VA study: Participation was restricted to RA patients on only a single conventional disease-modifying antirheumatic drug (DMARD) or biologic agent leading up to surgery. This restriction, imposed to make for a more clear-cut analysis, means that the study results can’t be extrapolated to patients on multidrug therapy. And multidrug therapy is quite common. Indeed, slightly more than half of RA patients in the VA health care system are on combination therapy, most often methotrexate plus a biologic agent, noted Dr. Ng, chief of rheumatology at the VA Puget Sound Health Care System, Seattle.
Dr. Abou Zahr presented the retrospective cohort study involving 6,548 RA patients in VA administrative databases, all of whom were on antirheumatic drug monotherapy prior to surgery. The surgery was of all types, including cardiothoracic, gastrointestinal, vascular, and orthopedic, as well as emergent and elective.
The primary endpoints were the rate of wound infections, both superficial and deep, within 30 days post surgery, and the general infection rate – including pneumonia, sepsis, and urinary tract infections – during the same time frame.
Sixty-two percent of the 1,480 RA patients on a single biologic agent did not stop taking it preoperatively. One key study finding was that neither their postoperative wound infection rate nor their general infection rate differed significantly from rates in patients who temporarily halted their biologic agent. The same held true among the 70% of patients on a single conventional DMARD who did not stop taking their medication preoperatively, according to Dr. Abou Zahr of Baylor College of Medicine, Houston.
Dr. Ng said the investigators plan to extend their work to include RA patients on multiple antirheumatic drugs that they do or don’t temporarily stop when undergoing surgery within the VA system. The researchers also plan to take a close look at patients undergoing specific types of surgery to see if the postoperative infection risk in patients who remain on treatment varies according to their operation.
Dr. Fehmida Zahabi, a rheumatologist from Plano, Tex., who chaired a press conference highlighting the VA study findings, said that while she’d like to see a confirmatory study, "I think we’re getting to the point where we’re saying we should cautiously keep these patients on their medications. That’s what the data suggest."
She noted that before the VA study, the very limited evidence available to guide practice in this area centered on a 12-year-old British randomized trial involving RA patients on methotrexate undergoing elective orthopedic surgery. Those assigned to stop the drug from 2 weeks before surgery to 2 weeks post surgery had significantly more infections, surgical complications, and RA flares within 6 weeks after surgery (Ann. Rheum. Dis. 2001;60:214-7).
As for patients on multidrug therapy who are scheduled for surgery, her inclination until evidence becomes available for guidance is to pare down the regimen preoperatively, while keeping the patient on one or two drugs.
The VA study was funded by the Department of Veterans Affairs. Dr. Abou Zahr and Dr. Ng reported having no conflicts of interest.
SAN DIEGO – Rheumatoid arthritis patients undergoing surgery who stayed on their antirheumatic medication perioperatively didn’t have a higher risk of early postoperative infection compared with those who temporarily stopped treatment before surgery, according to findings from a large national Veterans Affairs study.
Rheumatologists are frequently consulted about this issue. Evidence to guide practice has been scarce, however, and until now many rheumatologists and surgeons have taken a conservative approach, reasoning that the immunosuppressive drugs employed in controlling inflammation in rheumatoid arthritis might also increase the risk of surgical wound infection.
A common practice has been to have RA patients stop their medication a month ahead of elective surgery, or at least two drug half-lives beforehand, then start treatment again roughly a month after the operation, or when the wound has healed. The new Veterans Affairs (VA) study findings suggest this practice may be unnecessary, Dr. Zaki Abou Zahr said at the annual meeting of the American College of Rheumatology.
Dr. Bernard Ng, his senior coinvestigator in the study, added that temporarily stopping antirheumatic agents before surgery may actually be harmful in that it increases the risk of a flare of the RA, which in turn would impede postoperative rehabilitation.
But there is a major caveat regarding the VA study: Participation was restricted to RA patients on only a single conventional disease-modifying antirheumatic drug (DMARD) or biologic agent leading up to surgery. This restriction, imposed to make for a more clear-cut analysis, means that the study results can’t be extrapolated to patients on multidrug therapy. And multidrug therapy is quite common. Indeed, slightly more than half of RA patients in the VA health care system are on combination therapy, most often methotrexate plus a biologic agent, noted Dr. Ng, chief of rheumatology at the VA Puget Sound Health Care System, Seattle.
Dr. Abou Zahr presented the retrospective cohort study involving 6,548 RA patients in VA administrative databases, all of whom were on antirheumatic drug monotherapy prior to surgery. The surgery was of all types, including cardiothoracic, gastrointestinal, vascular, and orthopedic, as well as emergent and elective.
The primary endpoints were the rate of wound infections, both superficial and deep, within 30 days post surgery, and the general infection rate – including pneumonia, sepsis, and urinary tract infections – during the same time frame.
Sixty-two percent of the 1,480 RA patients on a single biologic agent did not stop taking it preoperatively. One key study finding was that neither their postoperative wound infection rate nor their general infection rate differed significantly from rates in patients who temporarily halted their biologic agent. The same held true among the 70% of patients on a single conventional DMARD who did not stop taking their medication preoperatively, according to Dr. Abou Zahr of Baylor College of Medicine, Houston.
Dr. Ng said the investigators plan to extend their work to include RA patients on multiple antirheumatic drugs that they do or don’t temporarily stop when undergoing surgery within the VA system. The researchers also plan to take a close look at patients undergoing specific types of surgery to see if the postoperative infection risk in patients who remain on treatment varies according to their operation.
Dr. Fehmida Zahabi, a rheumatologist from Plano, Tex., who chaired a press conference highlighting the VA study findings, said that while she’d like to see a confirmatory study, "I think we’re getting to the point where we’re saying we should cautiously keep these patients on their medications. That’s what the data suggest."
She noted that before the VA study, the very limited evidence available to guide practice in this area centered on a 12-year-old British randomized trial involving RA patients on methotrexate undergoing elective orthopedic surgery. Those assigned to stop the drug from 2 weeks before surgery to 2 weeks post surgery had significantly more infections, surgical complications, and RA flares within 6 weeks after surgery (Ann. Rheum. Dis. 2001;60:214-7).
As for patients on multidrug therapy who are scheduled for surgery, her inclination until evidence becomes available for guidance is to pare down the regimen preoperatively, while keeping the patient on one or two drugs.
The VA study was funded by the Department of Veterans Affairs. Dr. Abou Zahr and Dr. Ng reported having no conflicts of interest.
AT THE ACR ANNUAL MEETING
Major finding: Rheumatoid arthritis patients who remained on their antirheumatic medication while they underwent various types of surgery did not have a significantly different 30-day wound infection rate than those who stopped treatment temporarily prior to surgery.
Data source: This was a retrospective observational cohort study involving 6,548 rheumatoid arthritis patients undergoing various types of surgery.
Disclosures: The study was funded by the Department of Veterans Affairs. The presenters reported having no financial conflicts.
Systemic sclerosis brings sharply increased MI risk
SAN DIEGO – Patients with systemic sclerosis are at greater than eightfold increased risk of having an acute myocardial infarction during the first year after diagnosis, compared with matched controls, according to the first large population-based cohort study to look at the issue.
After that first year, the MI risk drops off over time. Still, during years 1-5 post diagnosis the MI risk remains more than triple that of the matched general population, Dr. J. Antonio Avina-Zubieta reported at the annual meeting of the American College of Rheumatology.
"Our findings support increased vigilance in cardiovascular disease prevention, surveillance, and risk modification in patients with systemic sclerosis," declared Dr. Avina-Zubieta, a rheumatologist at the University of British Columbia, Vancouver.
He and his coinvestigators harnessed comprehensive provincial medical databases to identify all 1,245 adults diagnosed with rheumatologist-confirmed systemic sclerosis (SSc) in British Columbia during 1996-2010. The SSc patients averaged 53 years of age at the time of their rheumatologic diagnosis. Eighty-three percent were women.
During a mean follow-up period of 3.5 years following diagnosis of SSc, 89 patients experienced an acute MI, as did 289 controls. This translated to an incidence rate of 20.2 cases per 1,000 person-years among patients with SSc, compared with 5.3 per 1,000 among controls.
The risk was 8.2-fold greater in SSc patients than controls during the first year after diagnosis and 3.5 times greater during years 1-5 after diagnosis, but once patients got more than 5 years out from diagnosis there was no longer any significant increase in MI risk.
Patients with SSc who were in the 45- to 59-year-old age group were at greatest risk: They were 7.4 times more likely than controls to have an MI. SSc patients aged 60-74 years had a 4.4-fold greater risk of MI than did controls, and those aged 75 years and older were at 5.6-fold increased risk.
The SSc patients remained at a 4.3-fold increased risk of MI, compared with controls, in a multivariate analysis extensively adjusted for baseline chronic obstructive pulmonary disease, angina, obesity, hormone replacement therapy, Charlson Comorbidity Index, number of hospitalizations during the 12 months prior to diagnosis, and the use of NSAIDs, COX-2 inhibitors, glucocorticoids, lipid-lowering drugs, and antidiabetic medications.
It is well established that patients with rheumatoid arthritis or systemic lupus erythematosus have an increased risk of premature atherosclerotic disease. Up until now, however, information about the risk of major adverse cardiovascular events in patients with SSc has been scarce and has come from nondefinitive studies in selected populations or cross-sectional studies lacking adequate adjustment for potential confounding factors, according to Dr. Avina-Zubieta, who also is a research scientist at the Arthritis Research Centre of Canada in Richmond, B.C.
Why the sharp increase in MI risk during the first year after diagnosis of SSc? He offered two hypotheses. One is that this is a time of particularly great inflammatory load as physicians work to get the disease under control. Also, there is a phenomenon Dr. Avina-Zubieta called "the depletion of susceptibles," whereby, once the patients at higher risk have had their MI early on, those who remain aren’t at sufficient risk to stand out from the general population.
The study was sponsored by the Arthritis Centre of Canada. Dr. Avina-Zubieta reported having no financial conflicts of interest.
SAN DIEGO – Patients with systemic sclerosis are at greater than eightfold increased risk of having an acute myocardial infarction during the first year after diagnosis, compared with matched controls, according to the first large population-based cohort study to look at the issue.
After that first year, the MI risk drops off over time. Still, during years 1-5 post diagnosis the MI risk remains more than triple that of the matched general population, Dr. J. Antonio Avina-Zubieta reported at the annual meeting of the American College of Rheumatology.
"Our findings support increased vigilance in cardiovascular disease prevention, surveillance, and risk modification in patients with systemic sclerosis," declared Dr. Avina-Zubieta, a rheumatologist at the University of British Columbia, Vancouver.
He and his coinvestigators harnessed comprehensive provincial medical databases to identify all 1,245 adults diagnosed with rheumatologist-confirmed systemic sclerosis (SSc) in British Columbia during 1996-2010. The SSc patients averaged 53 years of age at the time of their rheumatologic diagnosis. Eighty-three percent were women.
During a mean follow-up period of 3.5 years following diagnosis of SSc, 89 patients experienced an acute MI, as did 289 controls. This translated to an incidence rate of 20.2 cases per 1,000 person-years among patients with SSc, compared with 5.3 per 1,000 among controls.
The risk was 8.2-fold greater in SSc patients than controls during the first year after diagnosis and 3.5 times greater during years 1-5 after diagnosis, but once patients got more than 5 years out from diagnosis there was no longer any significant increase in MI risk.
Patients with SSc who were in the 45- to 59-year-old age group were at greatest risk: They were 7.4 times more likely than controls to have an MI. SSc patients aged 60-74 years had a 4.4-fold greater risk of MI than did controls, and those aged 75 years and older were at 5.6-fold increased risk.
The SSc patients remained at a 4.3-fold increased risk of MI, compared with controls, in a multivariate analysis extensively adjusted for baseline chronic obstructive pulmonary disease, angina, obesity, hormone replacement therapy, Charlson Comorbidity Index, number of hospitalizations during the 12 months prior to diagnosis, and the use of NSAIDs, COX-2 inhibitors, glucocorticoids, lipid-lowering drugs, and antidiabetic medications.
It is well established that patients with rheumatoid arthritis or systemic lupus erythematosus have an increased risk of premature atherosclerotic disease. Up until now, however, information about the risk of major adverse cardiovascular events in patients with SSc has been scarce and has come from nondefinitive studies in selected populations or cross-sectional studies lacking adequate adjustment for potential confounding factors, according to Dr. Avina-Zubieta, who also is a research scientist at the Arthritis Research Centre of Canada in Richmond, B.C.
Why the sharp increase in MI risk during the first year after diagnosis of SSc? He offered two hypotheses. One is that this is a time of particularly great inflammatory load as physicians work to get the disease under control. Also, there is a phenomenon Dr. Avina-Zubieta called "the depletion of susceptibles," whereby, once the patients at higher risk have had their MI early on, those who remain aren’t at sufficient risk to stand out from the general population.
The study was sponsored by the Arthritis Centre of Canada. Dr. Avina-Zubieta reported having no financial conflicts of interest.
SAN DIEGO – Patients with systemic sclerosis are at greater than eightfold increased risk of having an acute myocardial infarction during the first year after diagnosis, compared with matched controls, according to the first large population-based cohort study to look at the issue.
After that first year, the MI risk drops off over time. Still, during years 1-5 post diagnosis the MI risk remains more than triple that of the matched general population, Dr. J. Antonio Avina-Zubieta reported at the annual meeting of the American College of Rheumatology.
"Our findings support increased vigilance in cardiovascular disease prevention, surveillance, and risk modification in patients with systemic sclerosis," declared Dr. Avina-Zubieta, a rheumatologist at the University of British Columbia, Vancouver.
He and his coinvestigators harnessed comprehensive provincial medical databases to identify all 1,245 adults diagnosed with rheumatologist-confirmed systemic sclerosis (SSc) in British Columbia during 1996-2010. The SSc patients averaged 53 years of age at the time of their rheumatologic diagnosis. Eighty-three percent were women.
During a mean follow-up period of 3.5 years following diagnosis of SSc, 89 patients experienced an acute MI, as did 289 controls. This translated to an incidence rate of 20.2 cases per 1,000 person-years among patients with SSc, compared with 5.3 per 1,000 among controls.
The risk was 8.2-fold greater in SSc patients than controls during the first year after diagnosis and 3.5 times greater during years 1-5 after diagnosis, but once patients got more than 5 years out from diagnosis there was no longer any significant increase in MI risk.
Patients with SSc who were in the 45- to 59-year-old age group were at greatest risk: They were 7.4 times more likely than controls to have an MI. SSc patients aged 60-74 years had a 4.4-fold greater risk of MI than did controls, and those aged 75 years and older were at 5.6-fold increased risk.
The SSc patients remained at a 4.3-fold increased risk of MI, compared with controls, in a multivariate analysis extensively adjusted for baseline chronic obstructive pulmonary disease, angina, obesity, hormone replacement therapy, Charlson Comorbidity Index, number of hospitalizations during the 12 months prior to diagnosis, and the use of NSAIDs, COX-2 inhibitors, glucocorticoids, lipid-lowering drugs, and antidiabetic medications.
It is well established that patients with rheumatoid arthritis or systemic lupus erythematosus have an increased risk of premature atherosclerotic disease. Up until now, however, information about the risk of major adverse cardiovascular events in patients with SSc has been scarce and has come from nondefinitive studies in selected populations or cross-sectional studies lacking adequate adjustment for potential confounding factors, according to Dr. Avina-Zubieta, who also is a research scientist at the Arthritis Research Centre of Canada in Richmond, B.C.
Why the sharp increase in MI risk during the first year after diagnosis of SSc? He offered two hypotheses. One is that this is a time of particularly great inflammatory load as physicians work to get the disease under control. Also, there is a phenomenon Dr. Avina-Zubieta called "the depletion of susceptibles," whereby, once the patients at higher risk have had their MI early on, those who remain aren’t at sufficient risk to stand out from the general population.
The study was sponsored by the Arthritis Centre of Canada. Dr. Avina-Zubieta reported having no financial conflicts of interest.
AT THE ACR ANNUAL MEETING
Major finding: The incidence rate of acute MI following diagnosis of systemic sclerosis was 20.2 events per 1,000 person-years, compared with 5.3 per 1,000 in matched controls.
Data source: Population-based cohort study included all 1,245 adults diagnosed with systemic sclerosis in British Columbia in 1990-2010 and 12,678 matched controls drawn from the general population.
Disclosures: The study was sponsored by the Arthritis Centre of Canada. Dr. Avina-Zubieta reported having no financial conflicts of interest.
Treatment adherence poor among Medicaid beneficiaries with lupus
SAN DIEGO – Fewer than one in three Medicaid beneficiaries with systemic lupus erythematosus are adhering to their recommended treatment regimens at least 80% of the time, results from a large national analysis demonstrated.
"As a physician who spends my professional time taking care of people with lupus, these data are highly concerning," Dr. Jinoos Yazdany said in an interview prior to the annual meeting of the American College of Rheumatology, where the study was presented. "For many individuals with lupus, treatment is very effective and has a proven track record of preventing life-threatening complications and long-term organ damage such as kidney failure. The fact that less than one in three patients are adhering with treatment is alarming and should serve as a call to action for those of providing care to these patients."
Dr. Yazdany, associate director of the lupus clinic at the University of California-San Francisco Medical Center, and her colleagues used MAX (Medicaid Analytic eXtract) data from 2000 to 2006 to identify 23,187 patients with systemic lupus erythematosus (SLE) who were taking at least one immunosuppressive or antimalarial drug. They used pharmacy claims to assess adherence to drugs over a period of 180 days by calculating a medication possession ratio (MPR), defined as the proportion of days covered by the total days’ supply dispensed after the first claim for each drug. The researchers also evaluated the proportion of patients who had an MPR of 80% or greater. The oral drugs studied were hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, cyclosporine, tacrolimus, cyclophosphamide, and leflunomide.
The mean age of the 23,187 patients was 38 years, 94% were female, and the racial and ethnic makeup was diverse (40% black, 34% white, 16% Hispanic, 5% Asian, and 5% other). The highest proportion of SLE patients lived in the southern United States (36%).
Dr. Yazdany reported that the average MPR ranged from 31.1% for tacrolimus to 56% for hydroxychloroquine. In addition, for most drugs, fewer than one in three patients had an MPR of at least 80%. Overall adherence was poorest among those taking tacrolimus (14%) and highest among those taking mycophenolate mofetil (40%).
Across all medications, blacks had lower adherence, compared with whites, and adherence was highest for those residing in the Northeast.
"Our findings underscore the need to understand the reasons behind low adherence in this high-risk and vulnerable group of patients," Dr. Yazdany said. "And we urgently need to develop interventions to improve adherence. Physicians may be unaware of their patient’s adherence with medication, and patients may not be forthcoming about this issue. Concerns about side effects, inadequate understanding of the benefit, and the medication’s cost may be barriers for patients. Treatment-associated side effects may be another important barrier. We need better patient-physician communication around the issue of adherence."
The Medicaid administrative data allowed for an otherwise unobtainable nationwide view of adherence in SLE, but Dr. Yazdany acknowledged certain limitations of the study, including the fact that treatment "may be interrupted for clinically appropriate reasons, so the medication possession ratios in our study may underestimate actual adherence. Also, pharmacy claims are imperfect proxies for whether patients actually take medications that are dispensed, which might lead to an overestimation of adherence."
Dr. Yazdany disclosed that her research is funded by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases. She had no other relevant financial conflicts to disclose.
SAN DIEGO – Fewer than one in three Medicaid beneficiaries with systemic lupus erythematosus are adhering to their recommended treatment regimens at least 80% of the time, results from a large national analysis demonstrated.
"As a physician who spends my professional time taking care of people with lupus, these data are highly concerning," Dr. Jinoos Yazdany said in an interview prior to the annual meeting of the American College of Rheumatology, where the study was presented. "For many individuals with lupus, treatment is very effective and has a proven track record of preventing life-threatening complications and long-term organ damage such as kidney failure. The fact that less than one in three patients are adhering with treatment is alarming and should serve as a call to action for those of providing care to these patients."
Dr. Yazdany, associate director of the lupus clinic at the University of California-San Francisco Medical Center, and her colleagues used MAX (Medicaid Analytic eXtract) data from 2000 to 2006 to identify 23,187 patients with systemic lupus erythematosus (SLE) who were taking at least one immunosuppressive or antimalarial drug. They used pharmacy claims to assess adherence to drugs over a period of 180 days by calculating a medication possession ratio (MPR), defined as the proportion of days covered by the total days’ supply dispensed after the first claim for each drug. The researchers also evaluated the proportion of patients who had an MPR of 80% or greater. The oral drugs studied were hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, cyclosporine, tacrolimus, cyclophosphamide, and leflunomide.
The mean age of the 23,187 patients was 38 years, 94% were female, and the racial and ethnic makeup was diverse (40% black, 34% white, 16% Hispanic, 5% Asian, and 5% other). The highest proportion of SLE patients lived in the southern United States (36%).
Dr. Yazdany reported that the average MPR ranged from 31.1% for tacrolimus to 56% for hydroxychloroquine. In addition, for most drugs, fewer than one in three patients had an MPR of at least 80%. Overall adherence was poorest among those taking tacrolimus (14%) and highest among those taking mycophenolate mofetil (40%).
Across all medications, blacks had lower adherence, compared with whites, and adherence was highest for those residing in the Northeast.
"Our findings underscore the need to understand the reasons behind low adherence in this high-risk and vulnerable group of patients," Dr. Yazdany said. "And we urgently need to develop interventions to improve adherence. Physicians may be unaware of their patient’s adherence with medication, and patients may not be forthcoming about this issue. Concerns about side effects, inadequate understanding of the benefit, and the medication’s cost may be barriers for patients. Treatment-associated side effects may be another important barrier. We need better patient-physician communication around the issue of adherence."
The Medicaid administrative data allowed for an otherwise unobtainable nationwide view of adherence in SLE, but Dr. Yazdany acknowledged certain limitations of the study, including the fact that treatment "may be interrupted for clinically appropriate reasons, so the medication possession ratios in our study may underestimate actual adherence. Also, pharmacy claims are imperfect proxies for whether patients actually take medications that are dispensed, which might lead to an overestimation of adherence."
Dr. Yazdany disclosed that her research is funded by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases. She had no other relevant financial conflicts to disclose.
SAN DIEGO – Fewer than one in three Medicaid beneficiaries with systemic lupus erythematosus are adhering to their recommended treatment regimens at least 80% of the time, results from a large national analysis demonstrated.
"As a physician who spends my professional time taking care of people with lupus, these data are highly concerning," Dr. Jinoos Yazdany said in an interview prior to the annual meeting of the American College of Rheumatology, where the study was presented. "For many individuals with lupus, treatment is very effective and has a proven track record of preventing life-threatening complications and long-term organ damage such as kidney failure. The fact that less than one in three patients are adhering with treatment is alarming and should serve as a call to action for those of providing care to these patients."
Dr. Yazdany, associate director of the lupus clinic at the University of California-San Francisco Medical Center, and her colleagues used MAX (Medicaid Analytic eXtract) data from 2000 to 2006 to identify 23,187 patients with systemic lupus erythematosus (SLE) who were taking at least one immunosuppressive or antimalarial drug. They used pharmacy claims to assess adherence to drugs over a period of 180 days by calculating a medication possession ratio (MPR), defined as the proportion of days covered by the total days’ supply dispensed after the first claim for each drug. The researchers also evaluated the proportion of patients who had an MPR of 80% or greater. The oral drugs studied were hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, cyclosporine, tacrolimus, cyclophosphamide, and leflunomide.
The mean age of the 23,187 patients was 38 years, 94% were female, and the racial and ethnic makeup was diverse (40% black, 34% white, 16% Hispanic, 5% Asian, and 5% other). The highest proportion of SLE patients lived in the southern United States (36%).
Dr. Yazdany reported that the average MPR ranged from 31.1% for tacrolimus to 56% for hydroxychloroquine. In addition, for most drugs, fewer than one in three patients had an MPR of at least 80%. Overall adherence was poorest among those taking tacrolimus (14%) and highest among those taking mycophenolate mofetil (40%).
Across all medications, blacks had lower adherence, compared with whites, and adherence was highest for those residing in the Northeast.
"Our findings underscore the need to understand the reasons behind low adherence in this high-risk and vulnerable group of patients," Dr. Yazdany said. "And we urgently need to develop interventions to improve adherence. Physicians may be unaware of their patient’s adherence with medication, and patients may not be forthcoming about this issue. Concerns about side effects, inadequate understanding of the benefit, and the medication’s cost may be barriers for patients. Treatment-associated side effects may be another important barrier. We need better patient-physician communication around the issue of adherence."
The Medicaid administrative data allowed for an otherwise unobtainable nationwide view of adherence in SLE, but Dr. Yazdany acknowledged certain limitations of the study, including the fact that treatment "may be interrupted for clinically appropriate reasons, so the medication possession ratios in our study may underestimate actual adherence. Also, pharmacy claims are imperfect proxies for whether patients actually take medications that are dispensed, which might lead to an overestimation of adherence."
Dr. Yazdany disclosed that her research is funded by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases. She had no other relevant financial conflicts to disclose.
AT THE ACR ANNUAL MEETING
Major finding: Among Medicaid beneficiaries with SLE, the average medication possession ratio ranged from 31.1% for tacrolimus to 56% for hydroxychloroquine.
Data source: A study of 23,187 Medicaid patients with SLE who were taking at least one immunosuppressive or antimalarial drug between 2000 and 2006.
Disclosures: Dr. Yazdany disclosed that her research is funded by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases. She had no other relevant financial conflicts to disclose.
Apremilast promising for Behçet’s syndrome
ISTANBUL, TURKEY – The investigational oral phosphodiesterase-4 inhibitor apremilast showed dramatic efficacy in the treatment of oral and genital ulcers of Behçet’s syndrome in a randomized, double-blind clinical trial.
The multicenter phase II study included 111 Behçet’s syndrome patients with active oral ulcers. They were randomized to 12 weeks of double-blind apremilast at 30 mg twice daily or placebo followed by an additional 12 weeks of open-label apremilast for all, then 4 weeks of post-treatment observation.
Participants had a mean of 2.8 oral ulcers at baseline. The primary study endpoint was the number present at week 12: a mean of 0.5 in the apremilast group, compared with 2.1 in controls, Dr. Gülen Hatemi reported at the annual congress of the European Academy of Dermatology and Venereology.
A total of 71% of patients in the apremilast group were oral ulcer–free at week 12, as were 29% of controls, added Dr. Hatemi, a rheumatologist at Istanbul (Turkey) University.
The benefit of apremilast occurred rapidly. The full response was seen at week 2 and was then maintained throughout the remainder of the 12 weeks of double-blind therapy and the following 12 weeks of open-label apremilast. However, oral ulcers returned in full force quickly after treatment cessation.
Pain from oral ulcers on a 0-100 visual analog scale plummeted in the apremilast group by a mean 44.7 points from a baseline of score of 54. In contrast, pain scores in the control group improved by 16 points, which is less than the 20-point threshold accepted as being clinically meaningful.
Behçet’s syndrome is an immune-mediated small-vessel vasculitis. Its mucocutaneous manifestations can be disabling and resistant to conventional therapies, Dr. Hatemi noted.
Only 16 patients had genital ulcers. All 10 randomized to apremilast were free of the ulcers at week 12, as were three of six affected patients in the control group.
Average scores on the Behçet’s Disease Activity Index dropped from 3.4 at baseline to 2.0 at 12 weeks in the apremilast arm while remaining unchanged in controls. Mean scores on the Behçet’s Disease Quality of Life instrument decreased by 4.5 points from a baseline of 12.6 in apremilast-treated patients, compared with a 1.6-point reduction in controls.
The investigators said no serious adverse events were related to apremilast.
Dr. Hatemi received a research grant from Celgene, the study sponsor, which is also developing the oral drug as a possible treatment for psoriasis, psoriatic arthritis, and ankylosing spondylitis.
ISTANBUL, TURKEY – The investigational oral phosphodiesterase-4 inhibitor apremilast showed dramatic efficacy in the treatment of oral and genital ulcers of Behçet’s syndrome in a randomized, double-blind clinical trial.
The multicenter phase II study included 111 Behçet’s syndrome patients with active oral ulcers. They were randomized to 12 weeks of double-blind apremilast at 30 mg twice daily or placebo followed by an additional 12 weeks of open-label apremilast for all, then 4 weeks of post-treatment observation.
Participants had a mean of 2.8 oral ulcers at baseline. The primary study endpoint was the number present at week 12: a mean of 0.5 in the apremilast group, compared with 2.1 in controls, Dr. Gülen Hatemi reported at the annual congress of the European Academy of Dermatology and Venereology.
A total of 71% of patients in the apremilast group were oral ulcer–free at week 12, as were 29% of controls, added Dr. Hatemi, a rheumatologist at Istanbul (Turkey) University.
The benefit of apremilast occurred rapidly. The full response was seen at week 2 and was then maintained throughout the remainder of the 12 weeks of double-blind therapy and the following 12 weeks of open-label apremilast. However, oral ulcers returned in full force quickly after treatment cessation.
Pain from oral ulcers on a 0-100 visual analog scale plummeted in the apremilast group by a mean 44.7 points from a baseline of score of 54. In contrast, pain scores in the control group improved by 16 points, which is less than the 20-point threshold accepted as being clinically meaningful.
Behçet’s syndrome is an immune-mediated small-vessel vasculitis. Its mucocutaneous manifestations can be disabling and resistant to conventional therapies, Dr. Hatemi noted.
Only 16 patients had genital ulcers. All 10 randomized to apremilast were free of the ulcers at week 12, as were three of six affected patients in the control group.
Average scores on the Behçet’s Disease Activity Index dropped from 3.4 at baseline to 2.0 at 12 weeks in the apremilast arm while remaining unchanged in controls. Mean scores on the Behçet’s Disease Quality of Life instrument decreased by 4.5 points from a baseline of 12.6 in apremilast-treated patients, compared with a 1.6-point reduction in controls.
The investigators said no serious adverse events were related to apremilast.
Dr. Hatemi received a research grant from Celgene, the study sponsor, which is also developing the oral drug as a possible treatment for psoriasis, psoriatic arthritis, and ankylosing spondylitis.
ISTANBUL, TURKEY – The investigational oral phosphodiesterase-4 inhibitor apremilast showed dramatic efficacy in the treatment of oral and genital ulcers of Behçet’s syndrome in a randomized, double-blind clinical trial.
The multicenter phase II study included 111 Behçet’s syndrome patients with active oral ulcers. They were randomized to 12 weeks of double-blind apremilast at 30 mg twice daily or placebo followed by an additional 12 weeks of open-label apremilast for all, then 4 weeks of post-treatment observation.
Participants had a mean of 2.8 oral ulcers at baseline. The primary study endpoint was the number present at week 12: a mean of 0.5 in the apremilast group, compared with 2.1 in controls, Dr. Gülen Hatemi reported at the annual congress of the European Academy of Dermatology and Venereology.
A total of 71% of patients in the apremilast group were oral ulcer–free at week 12, as were 29% of controls, added Dr. Hatemi, a rheumatologist at Istanbul (Turkey) University.
The benefit of apremilast occurred rapidly. The full response was seen at week 2 and was then maintained throughout the remainder of the 12 weeks of double-blind therapy and the following 12 weeks of open-label apremilast. However, oral ulcers returned in full force quickly after treatment cessation.
Pain from oral ulcers on a 0-100 visual analog scale plummeted in the apremilast group by a mean 44.7 points from a baseline of score of 54. In contrast, pain scores in the control group improved by 16 points, which is less than the 20-point threshold accepted as being clinically meaningful.
Behçet’s syndrome is an immune-mediated small-vessel vasculitis. Its mucocutaneous manifestations can be disabling and resistant to conventional therapies, Dr. Hatemi noted.
Only 16 patients had genital ulcers. All 10 randomized to apremilast were free of the ulcers at week 12, as were three of six affected patients in the control group.
Average scores on the Behçet’s Disease Activity Index dropped from 3.4 at baseline to 2.0 at 12 weeks in the apremilast arm while remaining unchanged in controls. Mean scores on the Behçet’s Disease Quality of Life instrument decreased by 4.5 points from a baseline of 12.6 in apremilast-treated patients, compared with a 1.6-point reduction in controls.
The investigators said no serious adverse events were related to apremilast.
Dr. Hatemi received a research grant from Celgene, the study sponsor, which is also developing the oral drug as a possible treatment for psoriasis, psoriatic arthritis, and ankylosing spondylitis.
AT THE EADV CONGRESS
Major finding: The mean number of oral ulcers in Behçet’s syndrome patients treated with apremilast quickly dropped from 2.8 at baseline to 0.5, an improvement maintained during 24 weeks of therapy.
Data source: A randomized, double-blind, multicenter, 28-week phase II study involving 111 Behçet’s syndrome patients with active oral ulcers.
Disclosures: The presenter received a research grant from the study sponsor, Celgene.
Systemic sclerosis overlap syndromes called distinct entity
ISTANBUL – Systemic sclerosis overlap syndromes follow a path of disease progression distinctly different from that of limited or diffuse cutaneous systemic sclerosis, according to a new analysis from the German Network for Systemic Scleroderma.
"This study shows for the first time that patients suffering from systemic sclerosis overlap syndromes should be viewed as a distinct systemic sclerosis subset," Dr. Pia Moinzadeh noted at the annual congress of the European Academy of Dermatology and Venereology.
Those with systemic sclerosis overlap syndromes (SSc-OS) are a heterogeneous subgroup of patients who have the clinical features of SSc according to American College of Rheumatology criteria simultaneously with the characteristic findings of at least one other connective tissue disease, such as dermatomyositis, Sjögren’s syndrome, rheumatoid arthritis, or lupus erythematosus.
The registry of the German Network for Systemic Scleroderma is a uniquely large and inclusive ongoing prospective data base directed by a joint committee of dermatologists and rheumatologists. More than 6 years ago, when the investigators realized that a sizable fraction of patients enrolled in the national registry didn’t fit within the classic bimodal SSc categorization scheme composed of two subtypes – limited cutaneous and diffuse cutaneous SSc – they proposed three additional SSc subtypes. These are SSc-OS, undifferentiated scleroderma, and sclerosis sine scleroderma.
The focus of Dr. Moinzadeh’s EADV presentation was on SSc-OS. Of 3,240 SSc patients in the German registry, patients with OS comprise 11%, while those with limited cutaneous SSc make up 43%, those with diffuse cutaneous disease account for 31%, and the remainder of patients have undifferentiated scleroderma or sclerosis sine scleroderma.
The validity of the SSc-OS diagnostic construct has been controversial. Some experts have contended that patients with SSc-OS ought to be lumped under the headings of limited cutaneous SSc or diffuse cutaneous SSc, depending on the extent of skin involvement. However, the unparalleled size, inclusiveness, and long-term prospective follow-up provided by the German registry have resulted in evidence that argues to the contrary, according to Dr. Moinzadeh, a rheumatologist at the University of Cologne (Germany).
She reported on 326 patients with SSc-OS, 1,598 with limited cutaneous SSc, and 996 with diffuse cutaneous SSc prospectively followed through the German registry for a mean of 10.1 years. The follow-up data indicate SSc-OS evolves differently from either limited cutaneous SSc or diffuse cutaneous SSc. It also displays a distinctive pattern of organ involvement and autoantibody positivity, she noted.
The group with SSc-OS had a mean modified Rodnan skin score of 6.7, similar to the 7.2 score in the limited cutaneous SSc group and markedly less than the 15.8 in the diffuse cutaneous SSc patients. While this might suggest that patients with SSc-OS have a milder course of disease comparable to limited cutaneous SSc, in fact they progressed more rapidly, with earlier and more widespread significant organ involvement and a higher disease burden than the limited cutaneous SSc group, according to Dr. Moinzadeh.
Patients with SSc-OS developed musculoskeletal involvement far more frequently than others. Sixty-seven percent were affected, compared with 38% of patients with limited cutaneous SSc and 48% with diffuse cutaneous SSc. The patients in the SSc-OS group also developed musculoskeletal involvement earlier.
Lung fibrosis occurred in 38% of patients with SSc-OS, 27% of patients with limited cutaneous SSc, and 63% with diffuse cutaneous SSc. Cardiac involvement occurred in 15% with SSc-OS, 10% with limited cutaneous SSc, and 19% with diffuse cutaneous SSc. The onset of these manifestations of SSc in the SSc-OS subgroup was significantly earlier than in the limited cutaneous SSc patients but later than with diffuse cutaneous SSc.
Progression to esophageal and renal involvement, as well as pulmonary arterial hypertension, in the SSc-OS group occurred significantly earlier than in the limited cutaneous SSC patients, but again was later than with diffuse cutaneous SSC.
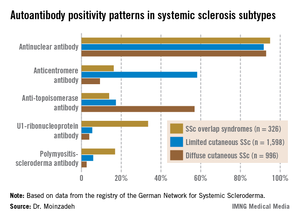
The autoantibody status of the three subgroups showed distinct differences (see graphic).
The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
ISTANBUL – Systemic sclerosis overlap syndromes follow a path of disease progression distinctly different from that of limited or diffuse cutaneous systemic sclerosis, according to a new analysis from the German Network for Systemic Scleroderma.
"This study shows for the first time that patients suffering from systemic sclerosis overlap syndromes should be viewed as a distinct systemic sclerosis subset," Dr. Pia Moinzadeh noted at the annual congress of the European Academy of Dermatology and Venereology.

Those with systemic sclerosis overlap syndromes (SSc-OS) are a heterogeneous subgroup of patients who have the clinical features of SSc according to American College of Rheumatology criteria simultaneously with the characteristic findings of at least one other connective tissue disease, such as dermatomyositis, Sjögren’s syndrome, rheumatoid arthritis, or lupus erythematosus.
The registry of the German Network for Systemic Scleroderma is a uniquely large and inclusive ongoing prospective data base directed by a joint committee of dermatologists and rheumatologists. More than 6 years ago, when the investigators realized that a sizable fraction of patients enrolled in the national registry didn’t fit within the classic bimodal SSc categorization scheme composed of two subtypes – limited cutaneous and diffuse cutaneous SSc – they proposed three additional SSc subtypes. These are SSc-OS, undifferentiated scleroderma, and sclerosis sine scleroderma.
The focus of Dr. Moinzadeh’s EADV presentation was on SSc-OS. Of 3,240 SSc patients in the German registry, patients with OS comprise 11%, while those with limited cutaneous SSc make up 43%, those with diffuse cutaneous disease account for 31%, and the remainder of patients have undifferentiated scleroderma or sclerosis sine scleroderma.
The validity of the SSc-OS diagnostic construct has been controversial. Some experts have contended that patients with SSc-OS ought to be lumped under the headings of limited cutaneous SSc or diffuse cutaneous SSc, depending on the extent of skin involvement. However, the unparalleled size, inclusiveness, and long-term prospective follow-up provided by the German registry have resulted in evidence that argues to the contrary, according to Dr. Moinzadeh, a rheumatologist at the University of Cologne (Germany).
She reported on 326 patients with SSc-OS, 1,598 with limited cutaneous SSc, and 996 with diffuse cutaneous SSc prospectively followed through the German registry for a mean of 10.1 years. The follow-up data indicate SSc-OS evolves differently from either limited cutaneous SSc or diffuse cutaneous SSc. It also displays a distinctive pattern of organ involvement and autoantibody positivity, she noted.
The group with SSc-OS had a mean modified Rodnan skin score of 6.7, similar to the 7.2 score in the limited cutaneous SSc group and markedly less than the 15.8 in the diffuse cutaneous SSc patients. While this might suggest that patients with SSc-OS have a milder course of disease comparable to limited cutaneous SSc, in fact they progressed more rapidly, with earlier and more widespread significant organ involvement and a higher disease burden than the limited cutaneous SSc group, according to Dr. Moinzadeh.
Patients with SSc-OS developed musculoskeletal involvement far more frequently than others. Sixty-seven percent were affected, compared with 38% of patients with limited cutaneous SSc and 48% with diffuse cutaneous SSc. The patients in the SSc-OS group also developed musculoskeletal involvement earlier.
Lung fibrosis occurred in 38% of patients with SSc-OS, 27% of patients with limited cutaneous SSc, and 63% with diffuse cutaneous SSc. Cardiac involvement occurred in 15% with SSc-OS, 10% with limited cutaneous SSc, and 19% with diffuse cutaneous SSc. The onset of these manifestations of SSc in the SSc-OS subgroup was significantly earlier than in the limited cutaneous SSc patients but later than with diffuse cutaneous SSc.
Progression to esophageal and renal involvement, as well as pulmonary arterial hypertension, in the SSc-OS group occurred significantly earlier than in the limited cutaneous SSC patients, but again was later than with diffuse cutaneous SSC.
The autoantibody status of the three subgroups showed distinct differences (see graphic).
The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
ISTANBUL – Systemic sclerosis overlap syndromes follow a path of disease progression distinctly different from that of limited or diffuse cutaneous systemic sclerosis, according to a new analysis from the German Network for Systemic Scleroderma.
"This study shows for the first time that patients suffering from systemic sclerosis overlap syndromes should be viewed as a distinct systemic sclerosis subset," Dr. Pia Moinzadeh noted at the annual congress of the European Academy of Dermatology and Venereology.

Those with systemic sclerosis overlap syndromes (SSc-OS) are a heterogeneous subgroup of patients who have the clinical features of SSc according to American College of Rheumatology criteria simultaneously with the characteristic findings of at least one other connective tissue disease, such as dermatomyositis, Sjögren’s syndrome, rheumatoid arthritis, or lupus erythematosus.
The registry of the German Network for Systemic Scleroderma is a uniquely large and inclusive ongoing prospective data base directed by a joint committee of dermatologists and rheumatologists. More than 6 years ago, when the investigators realized that a sizable fraction of patients enrolled in the national registry didn’t fit within the classic bimodal SSc categorization scheme composed of two subtypes – limited cutaneous and diffuse cutaneous SSc – they proposed three additional SSc subtypes. These are SSc-OS, undifferentiated scleroderma, and sclerosis sine scleroderma.
The focus of Dr. Moinzadeh’s EADV presentation was on SSc-OS. Of 3,240 SSc patients in the German registry, patients with OS comprise 11%, while those with limited cutaneous SSc make up 43%, those with diffuse cutaneous disease account for 31%, and the remainder of patients have undifferentiated scleroderma or sclerosis sine scleroderma.
The validity of the SSc-OS diagnostic construct has been controversial. Some experts have contended that patients with SSc-OS ought to be lumped under the headings of limited cutaneous SSc or diffuse cutaneous SSc, depending on the extent of skin involvement. However, the unparalleled size, inclusiveness, and long-term prospective follow-up provided by the German registry have resulted in evidence that argues to the contrary, according to Dr. Moinzadeh, a rheumatologist at the University of Cologne (Germany).
She reported on 326 patients with SSc-OS, 1,598 with limited cutaneous SSc, and 996 with diffuse cutaneous SSc prospectively followed through the German registry for a mean of 10.1 years. The follow-up data indicate SSc-OS evolves differently from either limited cutaneous SSc or diffuse cutaneous SSc. It also displays a distinctive pattern of organ involvement and autoantibody positivity, she noted.
The group with SSc-OS had a mean modified Rodnan skin score of 6.7, similar to the 7.2 score in the limited cutaneous SSc group and markedly less than the 15.8 in the diffuse cutaneous SSc patients. While this might suggest that patients with SSc-OS have a milder course of disease comparable to limited cutaneous SSc, in fact they progressed more rapidly, with earlier and more widespread significant organ involvement and a higher disease burden than the limited cutaneous SSc group, according to Dr. Moinzadeh.
Patients with SSc-OS developed musculoskeletal involvement far more frequently than others. Sixty-seven percent were affected, compared with 38% of patients with limited cutaneous SSc and 48% with diffuse cutaneous SSc. The patients in the SSc-OS group also developed musculoskeletal involvement earlier.
Lung fibrosis occurred in 38% of patients with SSc-OS, 27% of patients with limited cutaneous SSc, and 63% with diffuse cutaneous SSc. Cardiac involvement occurred in 15% with SSc-OS, 10% with limited cutaneous SSc, and 19% with diffuse cutaneous SSc. The onset of these manifestations of SSc in the SSc-OS subgroup was significantly earlier than in the limited cutaneous SSc patients but later than with diffuse cutaneous SSc.
Progression to esophageal and renal involvement, as well as pulmonary arterial hypertension, in the SSc-OS group occurred significantly earlier than in the limited cutaneous SSC patients, but again was later than with diffuse cutaneous SSC.
The autoantibody status of the three subgroups showed distinct differences (see graphic).
The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
AT THE EADV CONGRESS
Major finding: Musculoskeletal involvement occurred in 67% of patients with systemic sclerosis overlap syndromes, compared with 38% of those with limited cutaneous systemic sclerosis and 48% with diffuse cutaneous systemic sclerosis.
Data source: This analysis from the ongoing prospective national registry maintained by the German Network for Systemic Scleroderma included nearly 3,000 patients with systemic sclerosis followed for a mean of 10.1 years.
Disclosures: The German Network for Systemic Scleroderma is funded by the German Federal Ministry of Education and Research. Dr. Moinzadeh reported having no financial conflicts.
Is PASI 90 becoming the new PASI 75?
ISTANBUL – With the majority of psoriasis patients now achieving PASI 90 responses in randomized trials of the latest-generation biologic agents, a push is on to replace PASI 75 with PASI 90 as the new goal defining treatment success. But some dermatologists have misgivings about raising the bar.
"More and more, editorialists are promoting the idea of silencing psoriasis in all patients. This is a tricky and challenging goal," Dr. Hervé Bachelez said at the annual congress of the European Academy of Dermatology and Venereology.
"You’ve probably noticed that PASI 90 and even PASI 100 are becoming important as secondary endpoints in virtually all clinical trials. It’s good, it’s legitimate, and the PASI 90 probably better reflects the wishes of the patient and the physician than the PASI 75. But we have to wait and see what the caveats of this are. You can say, ‘Let’s push the response rate up to PASI 100 in all patients,’ but the danger is that if you cross a line, you may be unable to precisely regulate the level of immunosuppression in some patients. Basically you can expect some safety issues in real life that you would not see in clinical trials," cautioned Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris.
Not so many years ago the notion of PASI 90 responses in high double figures seemed a pipedream, he observed. For example, the week-12 PASI 90 rate in published randomized trials of methotrexate was only 9%, while for the first-generation tumor necrosis factor inhibitor etanercept, the rates were 19%-23%. In contrast, among the highlights of this year’s EADV congress were the presentation of results from clinical trials of two investigational interleukin-17 inhibitors: In the pivotal Phase III FIXTURE trial, secukinumab-treated patients had a PASI 90 response rate of 72% at week 16, while the week-16 PASI 90 rate in the Phase II OLE trial was 87% in patients on brodalimab, and even out to week 96, it was 78%.
Underscoring Dr. Bachelez’ concern that the randomized trial experience likely underestimates the true extent of safety hazards posed by potent therapies in daily clinical practice was a report by a consortium of 13 Spanish dermatology departments responsible for the BIOBADADERM registry. The Spanish registry is focused on safety and includes only psoriasis patients on systemic therapy, whether biologics or classic drugs. Among the first 1,042 enrollees receiving systemic therapy, fully 30% would not have been eligible for participation in randomized controlled trials for various reasons, including age greater than 70 years, having chronic kidney or liver disease, a history of hepatitis B or C, HIV infection, or cancer, or having psoriasis of a type other than chronic plaque disease.
The disturbing finding was that during 2,179 person-years of prospective follow-up, the large group of patients ineligible for randomized trials had a 2.7-fold increased risk of serious adverse events compared with patients on systemic therapy who were eligible for study participation.
The number needed to harm was calculated as follows: For every 40 patients treated with systemic therapy for 2.1 years despite not being eligible for randomized trials, one additional serious adverse event can be expected compared with similar treatment in randomized trial-eligible patients, according to the investigators (Arch. Dermatol. 2012;148:463-70). And that’s without pushing the envelope by trying to aim for a PASI 90 response, Dr. Bachelez noted.
A contrary view regarding PASI 90 as an emerging standard of treatment excellence was put forth elsewhere at the meeting by Dr. Peter van de Kerkhof, professor and head of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
He cited multiple studies demonstrating that substantial PASI reductions may not translate into tangible improvements in patients’ quality of life. For example, among psoriasis patients who achieved a PASI 75 response in one European study, 65% still had a Dermatology Life Quality Index (DLQI) score of 2 or more (Eur. J. Dermatol. 2010;20:62-7).
"This implies that there is something more to be wished for by patients, even when PASI 75 is reached," according to Dr. van de Kerkhof.
Moreover, in another trial, even among patients with a PASI score of 0 at week 24, only 70% had an optimal DLQI of 0, not 100% as most dermatologists might expect (Br. J. Dermatol. 2006;154:1161-8).
A recent survey of 2,151 European psoriasis patients and their dermatologists highlighted a substantial degree of dissatisfaction with current therapies. Patients on biologics had higher rates of improvement from severe to moderate or mild disease than did those on any other forms of psoriasis therapy, yet 41% of patients on biologics were dissatisfied with their treatment (J. Dermatolog. Treat. 2013;24:193-8).
"I think it’s extremely important that we continue to innovate treatment possibilities for psoriasis in order to improve outcomes for our patients," Dr. van de Kerkhof said.
What patients really want, surveys suggest, are treatments that render them clear or nearly clear, and do so quickly. In one survey, patients rated as the most important characteristic about a therapy the rapidity with which it could achieve a moderate 50% improvement in symptoms. They rated that as higher in importance to them than the therapy’s long-term risks (Arch. Dermatol. 2007;143:1175-9), Dr. van de Kerkhof noted.
Patients also place a high priority on improvement of a broad array of symptoms that aren’t captured by either the PASI or the DLQI, Dr. Bruce E. Strober noted in a separate presentation. These include itching, plaque-related pain, altered skin appearance, flaking, and bleeding.
For this reason, he and a group of his coworkers have created and are now validating a new tool for the assessment of patient-related outcomes in psoriasis called the Psoriasis Symptom Diary (Value Health 2013;16:1014-22). The 16-item tool takes less than 5 minutes for a patient to fill out and is designed to replace the DLQI both in clinical trials and everyday practice. The goal is to be able to walk into the examination room, take a quick look at the Psoriasis Symptom Diary, and know from that how a patient is currently doing even before asking for the patient to disrobe.
"Let’s face it: Outside of skin cancer, when we’re doing dermatology, we’re in the quality of life business. That means we’re asking patients how they’re doing at every visit for psoriasis, atopic dermatitis, or severe acne. The DLQI does that, but it’s not psoriasis specific," said Dr. Strober, vice chair and director of the clinical trials unit in the department of dermatology at the University of Connecticut, Farmington.
As for the PASI, he doesn’t use it except in structured clinical trials. It’s too time consuming and has a high rate of inter- and intrarater variability.
"In the United States, dermatologists never do PASI scores in their clinics. The PASI score has numerous drawbacks that make it impractical in a regular practice setting," Dr. Strober said. "In my own practice, I routinely do a 5-point Physician’s Global Assessment along with an estimate of involved body surface area. I think that gives you a good picture of the objective level of psoriasis severity."
Dr. Bachelez, Dr. van de Kerkhof, and Dr. Strober each reported receiving research grants from and serving on advisory boards for 9-14 pharmaceutical companies engaged in developing new treatments for psoriasis.
ISTANBUL – With the majority of psoriasis patients now achieving PASI 90 responses in randomized trials of the latest-generation biologic agents, a push is on to replace PASI 75 with PASI 90 as the new goal defining treatment success. But some dermatologists have misgivings about raising the bar.
"More and more, editorialists are promoting the idea of silencing psoriasis in all patients. This is a tricky and challenging goal," Dr. Hervé Bachelez said at the annual congress of the European Academy of Dermatology and Venereology.
"You’ve probably noticed that PASI 90 and even PASI 100 are becoming important as secondary endpoints in virtually all clinical trials. It’s good, it’s legitimate, and the PASI 90 probably better reflects the wishes of the patient and the physician than the PASI 75. But we have to wait and see what the caveats of this are. You can say, ‘Let’s push the response rate up to PASI 100 in all patients,’ but the danger is that if you cross a line, you may be unable to precisely regulate the level of immunosuppression in some patients. Basically you can expect some safety issues in real life that you would not see in clinical trials," cautioned Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris.
Not so many years ago the notion of PASI 90 responses in high double figures seemed a pipedream, he observed. For example, the week-12 PASI 90 rate in published randomized trials of methotrexate was only 9%, while for the first-generation tumor necrosis factor inhibitor etanercept, the rates were 19%-23%. In contrast, among the highlights of this year’s EADV congress were the presentation of results from clinical trials of two investigational interleukin-17 inhibitors: In the pivotal Phase III FIXTURE trial, secukinumab-treated patients had a PASI 90 response rate of 72% at week 16, while the week-16 PASI 90 rate in the Phase II OLE trial was 87% in patients on brodalimab, and even out to week 96, it was 78%.
Underscoring Dr. Bachelez’ concern that the randomized trial experience likely underestimates the true extent of safety hazards posed by potent therapies in daily clinical practice was a report by a consortium of 13 Spanish dermatology departments responsible for the BIOBADADERM registry. The Spanish registry is focused on safety and includes only psoriasis patients on systemic therapy, whether biologics or classic drugs. Among the first 1,042 enrollees receiving systemic therapy, fully 30% would not have been eligible for participation in randomized controlled trials for various reasons, including age greater than 70 years, having chronic kidney or liver disease, a history of hepatitis B or C, HIV infection, or cancer, or having psoriasis of a type other than chronic plaque disease.
The disturbing finding was that during 2,179 person-years of prospective follow-up, the large group of patients ineligible for randomized trials had a 2.7-fold increased risk of serious adverse events compared with patients on systemic therapy who were eligible for study participation.
The number needed to harm was calculated as follows: For every 40 patients treated with systemic therapy for 2.1 years despite not being eligible for randomized trials, one additional serious adverse event can be expected compared with similar treatment in randomized trial-eligible patients, according to the investigators (Arch. Dermatol. 2012;148:463-70). And that’s without pushing the envelope by trying to aim for a PASI 90 response, Dr. Bachelez noted.
A contrary view regarding PASI 90 as an emerging standard of treatment excellence was put forth elsewhere at the meeting by Dr. Peter van de Kerkhof, professor and head of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
He cited multiple studies demonstrating that substantial PASI reductions may not translate into tangible improvements in patients’ quality of life. For example, among psoriasis patients who achieved a PASI 75 response in one European study, 65% still had a Dermatology Life Quality Index (DLQI) score of 2 or more (Eur. J. Dermatol. 2010;20:62-7).
"This implies that there is something more to be wished for by patients, even when PASI 75 is reached," according to Dr. van de Kerkhof.
Moreover, in another trial, even among patients with a PASI score of 0 at week 24, only 70% had an optimal DLQI of 0, not 100% as most dermatologists might expect (Br. J. Dermatol. 2006;154:1161-8).
A recent survey of 2,151 European psoriasis patients and their dermatologists highlighted a substantial degree of dissatisfaction with current therapies. Patients on biologics had higher rates of improvement from severe to moderate or mild disease than did those on any other forms of psoriasis therapy, yet 41% of patients on biologics were dissatisfied with their treatment (J. Dermatolog. Treat. 2013;24:193-8).
"I think it’s extremely important that we continue to innovate treatment possibilities for psoriasis in order to improve outcomes for our patients," Dr. van de Kerkhof said.
What patients really want, surveys suggest, are treatments that render them clear or nearly clear, and do so quickly. In one survey, patients rated as the most important characteristic about a therapy the rapidity with which it could achieve a moderate 50% improvement in symptoms. They rated that as higher in importance to them than the therapy’s long-term risks (Arch. Dermatol. 2007;143:1175-9), Dr. van de Kerkhof noted.
Patients also place a high priority on improvement of a broad array of symptoms that aren’t captured by either the PASI or the DLQI, Dr. Bruce E. Strober noted in a separate presentation. These include itching, plaque-related pain, altered skin appearance, flaking, and bleeding.
For this reason, he and a group of his coworkers have created and are now validating a new tool for the assessment of patient-related outcomes in psoriasis called the Psoriasis Symptom Diary (Value Health 2013;16:1014-22). The 16-item tool takes less than 5 minutes for a patient to fill out and is designed to replace the DLQI both in clinical trials and everyday practice. The goal is to be able to walk into the examination room, take a quick look at the Psoriasis Symptom Diary, and know from that how a patient is currently doing even before asking for the patient to disrobe.
"Let’s face it: Outside of skin cancer, when we’re doing dermatology, we’re in the quality of life business. That means we’re asking patients how they’re doing at every visit for psoriasis, atopic dermatitis, or severe acne. The DLQI does that, but it’s not psoriasis specific," said Dr. Strober, vice chair and director of the clinical trials unit in the department of dermatology at the University of Connecticut, Farmington.
As for the PASI, he doesn’t use it except in structured clinical trials. It’s too time consuming and has a high rate of inter- and intrarater variability.
"In the United States, dermatologists never do PASI scores in their clinics. The PASI score has numerous drawbacks that make it impractical in a regular practice setting," Dr. Strober said. "In my own practice, I routinely do a 5-point Physician’s Global Assessment along with an estimate of involved body surface area. I think that gives you a good picture of the objective level of psoriasis severity."
Dr. Bachelez, Dr. van de Kerkhof, and Dr. Strober each reported receiving research grants from and serving on advisory boards for 9-14 pharmaceutical companies engaged in developing new treatments for psoriasis.
ISTANBUL – With the majority of psoriasis patients now achieving PASI 90 responses in randomized trials of the latest-generation biologic agents, a push is on to replace PASI 75 with PASI 90 as the new goal defining treatment success. But some dermatologists have misgivings about raising the bar.
"More and more, editorialists are promoting the idea of silencing psoriasis in all patients. This is a tricky and challenging goal," Dr. Hervé Bachelez said at the annual congress of the European Academy of Dermatology and Venereology.
"You’ve probably noticed that PASI 90 and even PASI 100 are becoming important as secondary endpoints in virtually all clinical trials. It’s good, it’s legitimate, and the PASI 90 probably better reflects the wishes of the patient and the physician than the PASI 75. But we have to wait and see what the caveats of this are. You can say, ‘Let’s push the response rate up to PASI 100 in all patients,’ but the danger is that if you cross a line, you may be unable to precisely regulate the level of immunosuppression in some patients. Basically you can expect some safety issues in real life that you would not see in clinical trials," cautioned Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris.
Not so many years ago the notion of PASI 90 responses in high double figures seemed a pipedream, he observed. For example, the week-12 PASI 90 rate in published randomized trials of methotrexate was only 9%, while for the first-generation tumor necrosis factor inhibitor etanercept, the rates were 19%-23%. In contrast, among the highlights of this year’s EADV congress were the presentation of results from clinical trials of two investigational interleukin-17 inhibitors: In the pivotal Phase III FIXTURE trial, secukinumab-treated patients had a PASI 90 response rate of 72% at week 16, while the week-16 PASI 90 rate in the Phase II OLE trial was 87% in patients on brodalimab, and even out to week 96, it was 78%.
Underscoring Dr. Bachelez’ concern that the randomized trial experience likely underestimates the true extent of safety hazards posed by potent therapies in daily clinical practice was a report by a consortium of 13 Spanish dermatology departments responsible for the BIOBADADERM registry. The Spanish registry is focused on safety and includes only psoriasis patients on systemic therapy, whether biologics or classic drugs. Among the first 1,042 enrollees receiving systemic therapy, fully 30% would not have been eligible for participation in randomized controlled trials for various reasons, including age greater than 70 years, having chronic kidney or liver disease, a history of hepatitis B or C, HIV infection, or cancer, or having psoriasis of a type other than chronic plaque disease.
The disturbing finding was that during 2,179 person-years of prospective follow-up, the large group of patients ineligible for randomized trials had a 2.7-fold increased risk of serious adverse events compared with patients on systemic therapy who were eligible for study participation.
The number needed to harm was calculated as follows: For every 40 patients treated with systemic therapy for 2.1 years despite not being eligible for randomized trials, one additional serious adverse event can be expected compared with similar treatment in randomized trial-eligible patients, according to the investigators (Arch. Dermatol. 2012;148:463-70). And that’s without pushing the envelope by trying to aim for a PASI 90 response, Dr. Bachelez noted.
A contrary view regarding PASI 90 as an emerging standard of treatment excellence was put forth elsewhere at the meeting by Dr. Peter van de Kerkhof, professor and head of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
He cited multiple studies demonstrating that substantial PASI reductions may not translate into tangible improvements in patients’ quality of life. For example, among psoriasis patients who achieved a PASI 75 response in one European study, 65% still had a Dermatology Life Quality Index (DLQI) score of 2 or more (Eur. J. Dermatol. 2010;20:62-7).
"This implies that there is something more to be wished for by patients, even when PASI 75 is reached," according to Dr. van de Kerkhof.
Moreover, in another trial, even among patients with a PASI score of 0 at week 24, only 70% had an optimal DLQI of 0, not 100% as most dermatologists might expect (Br. J. Dermatol. 2006;154:1161-8).
A recent survey of 2,151 European psoriasis patients and their dermatologists highlighted a substantial degree of dissatisfaction with current therapies. Patients on biologics had higher rates of improvement from severe to moderate or mild disease than did those on any other forms of psoriasis therapy, yet 41% of patients on biologics were dissatisfied with their treatment (J. Dermatolog. Treat. 2013;24:193-8).
"I think it’s extremely important that we continue to innovate treatment possibilities for psoriasis in order to improve outcomes for our patients," Dr. van de Kerkhof said.
What patients really want, surveys suggest, are treatments that render them clear or nearly clear, and do so quickly. In one survey, patients rated as the most important characteristic about a therapy the rapidity with which it could achieve a moderate 50% improvement in symptoms. They rated that as higher in importance to them than the therapy’s long-term risks (Arch. Dermatol. 2007;143:1175-9), Dr. van de Kerkhof noted.
Patients also place a high priority on improvement of a broad array of symptoms that aren’t captured by either the PASI or the DLQI, Dr. Bruce E. Strober noted in a separate presentation. These include itching, plaque-related pain, altered skin appearance, flaking, and bleeding.
For this reason, he and a group of his coworkers have created and are now validating a new tool for the assessment of patient-related outcomes in psoriasis called the Psoriasis Symptom Diary (Value Health 2013;16:1014-22). The 16-item tool takes less than 5 minutes for a patient to fill out and is designed to replace the DLQI both in clinical trials and everyday practice. The goal is to be able to walk into the examination room, take a quick look at the Psoriasis Symptom Diary, and know from that how a patient is currently doing even before asking for the patient to disrobe.
"Let’s face it: Outside of skin cancer, when we’re doing dermatology, we’re in the quality of life business. That means we’re asking patients how they’re doing at every visit for psoriasis, atopic dermatitis, or severe acne. The DLQI does that, but it’s not psoriasis specific," said Dr. Strober, vice chair and director of the clinical trials unit in the department of dermatology at the University of Connecticut, Farmington.
As for the PASI, he doesn’t use it except in structured clinical trials. It’s too time consuming and has a high rate of inter- and intrarater variability.
"In the United States, dermatologists never do PASI scores in their clinics. The PASI score has numerous drawbacks that make it impractical in a regular practice setting," Dr. Strober said. "In my own practice, I routinely do a 5-point Physician’s Global Assessment along with an estimate of involved body surface area. I think that gives you a good picture of the objective level of psoriasis severity."
Dr. Bachelez, Dr. van de Kerkhof, and Dr. Strober each reported receiving research grants from and serving on advisory boards for 9-14 pharmaceutical companies engaged in developing new treatments for psoriasis.
EXPERT ANALYSIS FROM THE eadv CONGRESS
New drugs, strategies advance rheumatoid arthritis treatment
New biologics and more aggressive intervention strategies have advanced rheumatoid arthritis treatment over the last decade, Dr. Iain McInnes explains, but remission remains too infrequent. New drugs under development in the coming years offer promise for the future. For more on rheumatoid arthritis advances, visit http://www.rheumatologynews.com.
New biologics and more aggressive intervention strategies have advanced rheumatoid arthritis treatment over the last decade, Dr. Iain McInnes explains, but remission remains too infrequent. New drugs under development in the coming years offer promise for the future. For more on rheumatoid arthritis advances, visit http://www.rheumatologynews.com.
New biologics and more aggressive intervention strategies have advanced rheumatoid arthritis treatment over the last decade, Dr. Iain McInnes explains, but remission remains too infrequent. New drugs under development in the coming years offer promise for the future. For more on rheumatoid arthritis advances, visit http://www.rheumatologynews.com.