User login
Palmar-plantar psoriasis? Anti-TNF therapy may be culprit
LAS VEGAS – Psoriasiform eruptions are increasingly recognized as a "real event" among patients treated with anti–tumor necrosis factor therapy, according to Dr. Kenneth B. Gordon.
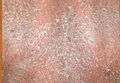
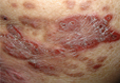
At Perspectives in Rheumatic Diseases 2013, Dr. Gordon of Northwestern University in Chicago presented the case of a 56-year-old woman with Crohn’s disease who developed a painful rash with fissures on both hands about 8 months after starting adalimumab therapy.
The patient first presented with Crohn’s disease at age 28 and was initially treated with mesalamine and systemic corticosteroids for flares. She became prednisone dependent and was started on azathioprine, but could not tolerate the drug, which caused pancreatitis. She was switched to adalimumab at a dose of 80 mg at week 1, 40 mg at week 2, and then 40 mg every other week thereafter.
The patient had an excellent response, including resolution of the gastrointestinal symptoms she experienced on azathioprine, but the rash, which involved well-demarcated plaques suggestive of a psoriasiform structure, was debilitating, Dr. Gordon said.
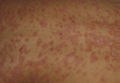
Another case, presented by Dr. Iain McInnes of Glasgow University, Scotland, involved a patient undergoing anti–tumor necrosis factor (anti-TNF) therapy for rheumatoid arthritis. That patient also developed palmar-plantar pustular psoriasis, which is the most common presentation of psoriasis induced by anti-TNF agents.
Data on the frequency of this condition are lacking, but Dr. Gordon said his "best guess" based on the available literature is that it occurs in about 1%-2% of patients on TNF blockers.
Dr. McInnes described the frequency as "sufficiently common to know it happens and reassure patients about it; sufficiently uncommon enough not to influence decision making" about prescribing anti-TNF therapy.
According to a 2010 systematic literature review by Dr. Angelique N. Collamer and Dr. Daniel F. Battafarano of the rheumatology service at Brooke Army Medical Center in Fort Sam Houston, Tex., pustular psoriasis occurs in 56% of cases, plaque psoriasis occurs in 50%, and guttate psoriasis occurs in 12% of cases. Most (85%) are new-onset cases, and 15% represent an exacerbation of existing psoriasis. Also, patients who are being treated for a variety of diseases – including rheumatoid arthritis, seronegative spondyloarthropathy, inflammatory bowel disease, and psoriasis – may be affected, and all anti-TNF therapies have been implicated (Semin. Arthritis Rheum. 2010;40:233-40).
However, treatment does not necessarily require stopping the TNF blockers, which is important because many patients don’t want to stop – they’re pleased with the effects of treatment on their underlying disease, Dr. Gordon noted.
He said that the first-line treatment is topical therapy, such as a potent topical corticosteroid, but retinoids may also be needed. Other options include phototherapy and switching to another anti-TNF therapy.
"My philosophy is to try to treat the patient while maintaining [anti-TNF] treatment," he said, noting that stopping anti-TNF therapy is a last resort.
Although the patient Dr. Gordon discussed was not a smoker, smoking is a risk factor for worsening of palm psoriasis. Patients with this condition should be advised to quit smoking, and many smokers will get better just by quitting smoking, Dr. Gordon noted.
Dr. McInnes also outlined his approach to patients presenting with possible anti-TNF therapy–induced psoriasis.
He stressed the importance of a good differential diagnosis, noting that infection may be the actual cause.
A biopsy can sometimes help with the diagnosis, he said.
In general, his treatment approach in cases of true anti-TNF–induced psoriasis is to:
• Continue the anti-TNF therapy when less than 5% of body surface area is affected.
• Change to a different anti-TNF drug if the psoriasis is tolerable but appears "a little more severe or is a palmar-pustular variant." The timing of the change will depend on how the patient responds to cutaneous management.
• Stop the drug if the psoriasis is intolerable, and treat the psoriasis aggressively.
In any of these circumstances, a change in the mode of action of treatment may be warranted, he said at the meeting.
In the review by Dr. Collamer and Dr. Battafarano, 24% of patients resolved off anti-TNF therapy, 5% had partial or no resolution off anti-TNF therapy, 25% had partial resolution on anti-TNF therapy, and 6% had no recurrence with change of anti-TNF. Only 1% had no resolution on anti-TNF therapy, and only 2% who resolved after anti-TNF discontinuation then had recurrence after reintroduction of anti-TNF therapy, while 6% resolved off anti-TNF therapy and then had recurrence with introduction of a different anti-TNF. The outcome was unknown in 5% of patients. A Mayo Clinic series showed a similar breakdown (J. Am. Acad. Dermatol. 2012;67:e179-85).
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Gordon reported receiving research support and/or honoraria from AbbVie, Amgen, and other companies. Dr. McInnes reported serving as a speaker, adviser, and/or researcher for Janssen, Roche, and other companies.
LAS VEGAS – Psoriasiform eruptions are increasingly recognized as a "real event" among patients treated with anti–tumor necrosis factor therapy, according to Dr. Kenneth B. Gordon.
At Perspectives in Rheumatic Diseases 2013, Dr. Gordon of Northwestern University in Chicago presented the case of a 56-year-old woman with Crohn’s disease who developed a painful rash with fissures on both hands about 8 months after starting adalimumab therapy.
The patient first presented with Crohn’s disease at age 28 and was initially treated with mesalamine and systemic corticosteroids for flares. She became prednisone dependent and was started on azathioprine, but could not tolerate the drug, which caused pancreatitis. She was switched to adalimumab at a dose of 80 mg at week 1, 40 mg at week 2, and then 40 mg every other week thereafter.
The patient had an excellent response, including resolution of the gastrointestinal symptoms she experienced on azathioprine, but the rash, which involved well-demarcated plaques suggestive of a psoriasiform structure, was debilitating, Dr. Gordon said.
Another case, presented by Dr. Iain McInnes of Glasgow University, Scotland, involved a patient undergoing anti–tumor necrosis factor (anti-TNF) therapy for rheumatoid arthritis. That patient also developed palmar-plantar pustular psoriasis, which is the most common presentation of psoriasis induced by anti-TNF agents.
Data on the frequency of this condition are lacking, but Dr. Gordon said his "best guess" based on the available literature is that it occurs in about 1%-2% of patients on TNF blockers.
Dr. McInnes described the frequency as "sufficiently common to know it happens and reassure patients about it; sufficiently uncommon enough not to influence decision making" about prescribing anti-TNF therapy.
According to a 2010 systematic literature review by Dr. Angelique N. Collamer and Dr. Daniel F. Battafarano of the rheumatology service at Brooke Army Medical Center in Fort Sam Houston, Tex., pustular psoriasis occurs in 56% of cases, plaque psoriasis occurs in 50%, and guttate psoriasis occurs in 12% of cases. Most (85%) are new-onset cases, and 15% represent an exacerbation of existing psoriasis. Also, patients who are being treated for a variety of diseases – including rheumatoid arthritis, seronegative spondyloarthropathy, inflammatory bowel disease, and psoriasis – may be affected, and all anti-TNF therapies have been implicated (Semin. Arthritis Rheum. 2010;40:233-40).
However, treatment does not necessarily require stopping the TNF blockers, which is important because many patients don’t want to stop – they’re pleased with the effects of treatment on their underlying disease, Dr. Gordon noted.
He said that the first-line treatment is topical therapy, such as a potent topical corticosteroid, but retinoids may also be needed. Other options include phototherapy and switching to another anti-TNF therapy.
"My philosophy is to try to treat the patient while maintaining [anti-TNF] treatment," he said, noting that stopping anti-TNF therapy is a last resort.
Although the patient Dr. Gordon discussed was not a smoker, smoking is a risk factor for worsening of palm psoriasis. Patients with this condition should be advised to quit smoking, and many smokers will get better just by quitting smoking, Dr. Gordon noted.
Dr. McInnes also outlined his approach to patients presenting with possible anti-TNF therapy–induced psoriasis.
He stressed the importance of a good differential diagnosis, noting that infection may be the actual cause.
A biopsy can sometimes help with the diagnosis, he said.
In general, his treatment approach in cases of true anti-TNF–induced psoriasis is to:
• Continue the anti-TNF therapy when less than 5% of body surface area is affected.
• Change to a different anti-TNF drug if the psoriasis is tolerable but appears "a little more severe or is a palmar-pustular variant." The timing of the change will depend on how the patient responds to cutaneous management.
• Stop the drug if the psoriasis is intolerable, and treat the psoriasis aggressively.
In any of these circumstances, a change in the mode of action of treatment may be warranted, he said at the meeting.
In the review by Dr. Collamer and Dr. Battafarano, 24% of patients resolved off anti-TNF therapy, 5% had partial or no resolution off anti-TNF therapy, 25% had partial resolution on anti-TNF therapy, and 6% had no recurrence with change of anti-TNF. Only 1% had no resolution on anti-TNF therapy, and only 2% who resolved after anti-TNF discontinuation then had recurrence after reintroduction of anti-TNF therapy, while 6% resolved off anti-TNF therapy and then had recurrence with introduction of a different anti-TNF. The outcome was unknown in 5% of patients. A Mayo Clinic series showed a similar breakdown (J. Am. Acad. Dermatol. 2012;67:e179-85).
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Gordon reported receiving research support and/or honoraria from AbbVie, Amgen, and other companies. Dr. McInnes reported serving as a speaker, adviser, and/or researcher for Janssen, Roche, and other companies.
LAS VEGAS – Psoriasiform eruptions are increasingly recognized as a "real event" among patients treated with anti–tumor necrosis factor therapy, according to Dr. Kenneth B. Gordon.
At Perspectives in Rheumatic Diseases 2013, Dr. Gordon of Northwestern University in Chicago presented the case of a 56-year-old woman with Crohn’s disease who developed a painful rash with fissures on both hands about 8 months after starting adalimumab therapy.
The patient first presented with Crohn’s disease at age 28 and was initially treated with mesalamine and systemic corticosteroids for flares. She became prednisone dependent and was started on azathioprine, but could not tolerate the drug, which caused pancreatitis. She was switched to adalimumab at a dose of 80 mg at week 1, 40 mg at week 2, and then 40 mg every other week thereafter.
The patient had an excellent response, including resolution of the gastrointestinal symptoms she experienced on azathioprine, but the rash, which involved well-demarcated plaques suggestive of a psoriasiform structure, was debilitating, Dr. Gordon said.
Another case, presented by Dr. Iain McInnes of Glasgow University, Scotland, involved a patient undergoing anti–tumor necrosis factor (anti-TNF) therapy for rheumatoid arthritis. That patient also developed palmar-plantar pustular psoriasis, which is the most common presentation of psoriasis induced by anti-TNF agents.
Data on the frequency of this condition are lacking, but Dr. Gordon said his "best guess" based on the available literature is that it occurs in about 1%-2% of patients on TNF blockers.
Dr. McInnes described the frequency as "sufficiently common to know it happens and reassure patients about it; sufficiently uncommon enough not to influence decision making" about prescribing anti-TNF therapy.
According to a 2010 systematic literature review by Dr. Angelique N. Collamer and Dr. Daniel F. Battafarano of the rheumatology service at Brooke Army Medical Center in Fort Sam Houston, Tex., pustular psoriasis occurs in 56% of cases, plaque psoriasis occurs in 50%, and guttate psoriasis occurs in 12% of cases. Most (85%) are new-onset cases, and 15% represent an exacerbation of existing psoriasis. Also, patients who are being treated for a variety of diseases – including rheumatoid arthritis, seronegative spondyloarthropathy, inflammatory bowel disease, and psoriasis – may be affected, and all anti-TNF therapies have been implicated (Semin. Arthritis Rheum. 2010;40:233-40).
However, treatment does not necessarily require stopping the TNF blockers, which is important because many patients don’t want to stop – they’re pleased with the effects of treatment on their underlying disease, Dr. Gordon noted.
He said that the first-line treatment is topical therapy, such as a potent topical corticosteroid, but retinoids may also be needed. Other options include phototherapy and switching to another anti-TNF therapy.
"My philosophy is to try to treat the patient while maintaining [anti-TNF] treatment," he said, noting that stopping anti-TNF therapy is a last resort.
Although the patient Dr. Gordon discussed was not a smoker, smoking is a risk factor for worsening of palm psoriasis. Patients with this condition should be advised to quit smoking, and many smokers will get better just by quitting smoking, Dr. Gordon noted.
Dr. McInnes also outlined his approach to patients presenting with possible anti-TNF therapy–induced psoriasis.
He stressed the importance of a good differential diagnosis, noting that infection may be the actual cause.
A biopsy can sometimes help with the diagnosis, he said.
In general, his treatment approach in cases of true anti-TNF–induced psoriasis is to:
• Continue the anti-TNF therapy when less than 5% of body surface area is affected.
• Change to a different anti-TNF drug if the psoriasis is tolerable but appears "a little more severe or is a palmar-pustular variant." The timing of the change will depend on how the patient responds to cutaneous management.
• Stop the drug if the psoriasis is intolerable, and treat the psoriasis aggressively.
In any of these circumstances, a change in the mode of action of treatment may be warranted, he said at the meeting.
In the review by Dr. Collamer and Dr. Battafarano, 24% of patients resolved off anti-TNF therapy, 5% had partial or no resolution off anti-TNF therapy, 25% had partial resolution on anti-TNF therapy, and 6% had no recurrence with change of anti-TNF. Only 1% had no resolution on anti-TNF therapy, and only 2% who resolved after anti-TNF discontinuation then had recurrence after reintroduction of anti-TNF therapy, while 6% resolved off anti-TNF therapy and then had recurrence with introduction of a different anti-TNF. The outcome was unknown in 5% of patients. A Mayo Clinic series showed a similar breakdown (J. Am. Acad. Dermatol. 2012;67:e179-85).
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
Dr. Gordon reported receiving research support and/or honoraria from AbbVie, Amgen, and other companies. Dr. McInnes reported serving as a speaker, adviser, and/or researcher for Janssen, Roche, and other companies.
EXPERT ANALYSIS FROM PERSPECTIVES IN RHEUMATIC DISEASES 2013
Psoriasis Treatment With Tumor Necrosis Factor Inhibitors
Autoantibodies play role in myositis classification, treatment
LAS VEGAS – Autoantibodies and autoantibody subsets can be particularly helpful for classifying and treating patients with myositis, including those with myositis-associated interstitial lung disease, according to Dr. Chester V. Oddis.
Many autoantibody subsets are phenotypically and clinically well defined, and have clinical relevance, said Dr. Oddis, professor of medicine and associate director of the rheumatology fellowship training program at the University of Pittsburgh.
Serological classification isn’t always accurate or routinely available for all clinicians, but this may change in the near future as improved techniques for autoantibody detection become available, he said at Perspectives in Rheumatic Diseases 2013.
Anti-MDA-5 and interstitial lung disease
One autoantibody that has gained attention in recent years is anti-MDA-5, also known as anti-CADM-140, which is often seen in patients with amyopathic dermatomyositis (ADM).
Patients with ADM represent a subset of dermatomyositis patients who have cutaneous manifestations of dermatomyositis for 6 months or longer and have no clinical evidence of proximal muscle weakness but may have mild serum muscle enzyme abnormalities. More extensive muscle testing in these patients generally demonstrates no or minimal abnormalities. However, these patients should not be considered to have simply a benign cutaneous form of disease; in fact, they have a frequency of malignancy similar to that of patients with classic dermatomyositis (14% in one series of nearly 300 patients, compared with 15% in classic dermatomyositis).
In addition, ADM patients also have a relatively high frequency of lung disease, Dr. Oddis said. In a published review of the literature of nearly 200 patients with ADM, 10% had interstitial lung disease (ILD) – an important point given that the rash of dermatomyositis may be subtle and missed, he noted.
The Asian population seems to be particularly at risk for this complication. Two studies in recent years have demonstrated that Japanese ADM patients with anti-MDA-5 present with rapidly progressive ILD. A 2011 study showed an increased incidence of acute or subacute interstitial pneumonitis in Chinese patients. Other studies have shown similar findings in Korean and other Asian populations, Dr. Oddis noted.
The presence of anti-MDA-5 represents a novel cutaneous phenotype involving palmar papules and cutaneous ulcerations, severe vasculopathy, and rapidly progressive ILD. The target autoantigen in these cases is MDA-5, which is involved in innate immune defense against viruses, he explained, noting that this supports the possibility that a viral trigger plays a role in the disease.
"I think this complication is filtering into the U.S. population, as we have seen it in our myositis cohort," Dr. Oddis said of the anti-MDA-5 association with ADM and severe ILD. He noted that he recently cared for a 70-year-old white male with "double pneumonia" (a finding that "should always raise suspicion of autoimmune ILD") in June of 2012, a rash of dermatomyositis in September of 2012, and vasculitic skin changes in January of 2013. He presented without muscle weakness.
Anti-synthetase syndrome and ILD
Another autoantibody myositis subset involves the anti-synthetases, including PL-7, PL-12, EJ, and Jo-1.
Patients with anti-synthetase syndrome are generally a clinically homogeneous patient population characterized by fever, myositis, arthritis, Raynaud’s phenomenon, mechanic’s hands, and ILD. About 30%-40% of myositis patients have ILD, which is a significant contributor to morbidity and mortality.
Anti-Jo-1 is found in 50%-75% of these patients, and the coexistence of Ro52 may portend worse prognosis, Dr. Oddis said.
There are certain clinical features of ILD in polymyositis and dermatomyositis, including progressive dyspnea with or without nonproductive cough, lack of digital clubbing (unlike in idiopathic pulmonary fibrosis), and lack of pleuritis and pleural effusion in most cases (unlike in systemic lupus erythematosus). However, presentation can be variable, with about one-third of patients developing ILD before muscle or skin manifestations are apparent. Some patients present with acute disease (acute respiratory distress syndrome), and others present with subacute disease that is chronic and slowly progressing or asymptomatic.
It is important to understand when making a diagnosis of autoimmune ILD that not all patients will present with the classic anti-synthetase syndrome, Dr. Oddis said.
In some cases, patients will have an "incomplete" clinical syndrome with ILD alone or ILD with only subtle connective tissue disease findings, myositis-specific autoantibodies in the absence of myositis, and/or a negative antinuclear antibody (ANA) test, he explained.
The initial symptoms in patients may vary depending on the anti-synthetase autoantibody that is present. In a University of Pittsburgh study, for example, Jo-1 was found in 60% of cases; non-Jo-1 synthetase positive cases more often experienced Raynaud’s as their initial symptom, less often experienced muscle and joint problems as their initial symptom, and had a longer delay in diagnosis, compared with Jo-1 patients. Survival was also decreased, compared with Jo-1 patients.
As for ANA, about half of patients tested positive, whereas 72% demonstrated positive anti-cytoplasmic staining on indirect immunofluorescence.
The diagnosis of autoimmune ILD can be missed when there is a failure to recognize "incomplete" clinical syndromes, when there is a failure to order or detect myositis-specific autoantibodies – even in patients without myositis – and when a negative ANA is considered to be reassuring, Dr. Oddis said.
Dr. Oddis has served on an advisory board for Questcor.
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Autoantibodies and autoantibody subsets can be particularly helpful for classifying and treating patients with myositis, including those with myositis-associated interstitial lung disease, according to Dr. Chester V. Oddis.
Many autoantibody subsets are phenotypically and clinically well defined, and have clinical relevance, said Dr. Oddis, professor of medicine and associate director of the rheumatology fellowship training program at the University of Pittsburgh.
Serological classification isn’t always accurate or routinely available for all clinicians, but this may change in the near future as improved techniques for autoantibody detection become available, he said at Perspectives in Rheumatic Diseases 2013.
Anti-MDA-5 and interstitial lung disease
One autoantibody that has gained attention in recent years is anti-MDA-5, also known as anti-CADM-140, which is often seen in patients with amyopathic dermatomyositis (ADM).
Patients with ADM represent a subset of dermatomyositis patients who have cutaneous manifestations of dermatomyositis for 6 months or longer and have no clinical evidence of proximal muscle weakness but may have mild serum muscle enzyme abnormalities. More extensive muscle testing in these patients generally demonstrates no or minimal abnormalities. However, these patients should not be considered to have simply a benign cutaneous form of disease; in fact, they have a frequency of malignancy similar to that of patients with classic dermatomyositis (14% in one series of nearly 300 patients, compared with 15% in classic dermatomyositis).
In addition, ADM patients also have a relatively high frequency of lung disease, Dr. Oddis said. In a published review of the literature of nearly 200 patients with ADM, 10% had interstitial lung disease (ILD) – an important point given that the rash of dermatomyositis may be subtle and missed, he noted.
The Asian population seems to be particularly at risk for this complication. Two studies in recent years have demonstrated that Japanese ADM patients with anti-MDA-5 present with rapidly progressive ILD. A 2011 study showed an increased incidence of acute or subacute interstitial pneumonitis in Chinese patients. Other studies have shown similar findings in Korean and other Asian populations, Dr. Oddis noted.
The presence of anti-MDA-5 represents a novel cutaneous phenotype involving palmar papules and cutaneous ulcerations, severe vasculopathy, and rapidly progressive ILD. The target autoantigen in these cases is MDA-5, which is involved in innate immune defense against viruses, he explained, noting that this supports the possibility that a viral trigger plays a role in the disease.
"I think this complication is filtering into the U.S. population, as we have seen it in our myositis cohort," Dr. Oddis said of the anti-MDA-5 association with ADM and severe ILD. He noted that he recently cared for a 70-year-old white male with "double pneumonia" (a finding that "should always raise suspicion of autoimmune ILD") in June of 2012, a rash of dermatomyositis in September of 2012, and vasculitic skin changes in January of 2013. He presented without muscle weakness.
Anti-synthetase syndrome and ILD
Another autoantibody myositis subset involves the anti-synthetases, including PL-7, PL-12, EJ, and Jo-1.
Patients with anti-synthetase syndrome are generally a clinically homogeneous patient population characterized by fever, myositis, arthritis, Raynaud’s phenomenon, mechanic’s hands, and ILD. About 30%-40% of myositis patients have ILD, which is a significant contributor to morbidity and mortality.
Anti-Jo-1 is found in 50%-75% of these patients, and the coexistence of Ro52 may portend worse prognosis, Dr. Oddis said.
There are certain clinical features of ILD in polymyositis and dermatomyositis, including progressive dyspnea with or without nonproductive cough, lack of digital clubbing (unlike in idiopathic pulmonary fibrosis), and lack of pleuritis and pleural effusion in most cases (unlike in systemic lupus erythematosus). However, presentation can be variable, with about one-third of patients developing ILD before muscle or skin manifestations are apparent. Some patients present with acute disease (acute respiratory distress syndrome), and others present with subacute disease that is chronic and slowly progressing or asymptomatic.
It is important to understand when making a diagnosis of autoimmune ILD that not all patients will present with the classic anti-synthetase syndrome, Dr. Oddis said.
In some cases, patients will have an "incomplete" clinical syndrome with ILD alone or ILD with only subtle connective tissue disease findings, myositis-specific autoantibodies in the absence of myositis, and/or a negative antinuclear antibody (ANA) test, he explained.
The initial symptoms in patients may vary depending on the anti-synthetase autoantibody that is present. In a University of Pittsburgh study, for example, Jo-1 was found in 60% of cases; non-Jo-1 synthetase positive cases more often experienced Raynaud’s as their initial symptom, less often experienced muscle and joint problems as their initial symptom, and had a longer delay in diagnosis, compared with Jo-1 patients. Survival was also decreased, compared with Jo-1 patients.
As for ANA, about half of patients tested positive, whereas 72% demonstrated positive anti-cytoplasmic staining on indirect immunofluorescence.
The diagnosis of autoimmune ILD can be missed when there is a failure to recognize "incomplete" clinical syndromes, when there is a failure to order or detect myositis-specific autoantibodies – even in patients without myositis – and when a negative ANA is considered to be reassuring, Dr. Oddis said.
Dr. Oddis has served on an advisory board for Questcor.
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Autoantibodies and autoantibody subsets can be particularly helpful for classifying and treating patients with myositis, including those with myositis-associated interstitial lung disease, according to Dr. Chester V. Oddis.
Many autoantibody subsets are phenotypically and clinically well defined, and have clinical relevance, said Dr. Oddis, professor of medicine and associate director of the rheumatology fellowship training program at the University of Pittsburgh.
Serological classification isn’t always accurate or routinely available for all clinicians, but this may change in the near future as improved techniques for autoantibody detection become available, he said at Perspectives in Rheumatic Diseases 2013.
Anti-MDA-5 and interstitial lung disease
One autoantibody that has gained attention in recent years is anti-MDA-5, also known as anti-CADM-140, which is often seen in patients with amyopathic dermatomyositis (ADM).
Patients with ADM represent a subset of dermatomyositis patients who have cutaneous manifestations of dermatomyositis for 6 months or longer and have no clinical evidence of proximal muscle weakness but may have mild serum muscle enzyme abnormalities. More extensive muscle testing in these patients generally demonstrates no or minimal abnormalities. However, these patients should not be considered to have simply a benign cutaneous form of disease; in fact, they have a frequency of malignancy similar to that of patients with classic dermatomyositis (14% in one series of nearly 300 patients, compared with 15% in classic dermatomyositis).
In addition, ADM patients also have a relatively high frequency of lung disease, Dr. Oddis said. In a published review of the literature of nearly 200 patients with ADM, 10% had interstitial lung disease (ILD) – an important point given that the rash of dermatomyositis may be subtle and missed, he noted.
The Asian population seems to be particularly at risk for this complication. Two studies in recent years have demonstrated that Japanese ADM patients with anti-MDA-5 present with rapidly progressive ILD. A 2011 study showed an increased incidence of acute or subacute interstitial pneumonitis in Chinese patients. Other studies have shown similar findings in Korean and other Asian populations, Dr. Oddis noted.
The presence of anti-MDA-5 represents a novel cutaneous phenotype involving palmar papules and cutaneous ulcerations, severe vasculopathy, and rapidly progressive ILD. The target autoantigen in these cases is MDA-5, which is involved in innate immune defense against viruses, he explained, noting that this supports the possibility that a viral trigger plays a role in the disease.
"I think this complication is filtering into the U.S. population, as we have seen it in our myositis cohort," Dr. Oddis said of the anti-MDA-5 association with ADM and severe ILD. He noted that he recently cared for a 70-year-old white male with "double pneumonia" (a finding that "should always raise suspicion of autoimmune ILD") in June of 2012, a rash of dermatomyositis in September of 2012, and vasculitic skin changes in January of 2013. He presented without muscle weakness.
Anti-synthetase syndrome and ILD
Another autoantibody myositis subset involves the anti-synthetases, including PL-7, PL-12, EJ, and Jo-1.
Patients with anti-synthetase syndrome are generally a clinically homogeneous patient population characterized by fever, myositis, arthritis, Raynaud’s phenomenon, mechanic’s hands, and ILD. About 30%-40% of myositis patients have ILD, which is a significant contributor to morbidity and mortality.
Anti-Jo-1 is found in 50%-75% of these patients, and the coexistence of Ro52 may portend worse prognosis, Dr. Oddis said.
There are certain clinical features of ILD in polymyositis and dermatomyositis, including progressive dyspnea with or without nonproductive cough, lack of digital clubbing (unlike in idiopathic pulmonary fibrosis), and lack of pleuritis and pleural effusion in most cases (unlike in systemic lupus erythematosus). However, presentation can be variable, with about one-third of patients developing ILD before muscle or skin manifestations are apparent. Some patients present with acute disease (acute respiratory distress syndrome), and others present with subacute disease that is chronic and slowly progressing or asymptomatic.
It is important to understand when making a diagnosis of autoimmune ILD that not all patients will present with the classic anti-synthetase syndrome, Dr. Oddis said.
In some cases, patients will have an "incomplete" clinical syndrome with ILD alone or ILD with only subtle connective tissue disease findings, myositis-specific autoantibodies in the absence of myositis, and/or a negative antinuclear antibody (ANA) test, he explained.
The initial symptoms in patients may vary depending on the anti-synthetase autoantibody that is present. In a University of Pittsburgh study, for example, Jo-1 was found in 60% of cases; non-Jo-1 synthetase positive cases more often experienced Raynaud’s as their initial symptom, less often experienced muscle and joint problems as their initial symptom, and had a longer delay in diagnosis, compared with Jo-1 patients. Survival was also decreased, compared with Jo-1 patients.
As for ANA, about half of patients tested positive, whereas 72% demonstrated positive anti-cytoplasmic staining on indirect immunofluorescence.
The diagnosis of autoimmune ILD can be missed when there is a failure to recognize "incomplete" clinical syndromes, when there is a failure to order or detect myositis-specific autoantibodies – even in patients without myositis – and when a negative ANA is considered to be reassuring, Dr. Oddis said.
Dr. Oddis has served on an advisory board for Questcor.
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
AT PERSPECTIVES IN RHEUMATIC DISEASES 2013
Limited data support multiple myositis treatment options
LAS VEGAS – Corticosteroids remain the initial treatment of choice for myositis and myositis-associated interstitial lung disease, but immunosuppressive agents, intravenous immunoglobulin, and biologics can also play a role in the treatment of one or both of these conditions, according to Dr. Chester V. Oddis.
For myositis in general, Dr. Oddis, professor of medicine and associate director of the rheumatology fellowship training program at the University of Pittsburgh, recommends an initial divided dose of 30 mg of prednisone twice daily, continued until serum creatine kinase (CK) levels fall to normal. At that time, the total daily prednisone dose can be consolidated into a single morning dose, he said at Perspectives in Rheumatic Diseases 2013.
The prednisone can then be tapered by 25% every 3-4 weeks down to a 5- to 10-mg daily maintenance dose that is continued until active disease is suppressed for 12 months. This is a general guideline that helps prevent disease flares, he noted.
Keep in mind that improvement in strength generally lags behind improvement in CK levels, he added.
Nonsteroidal immunosuppressives
Not all patients will need an additional immunosuppressive agent, but for those who do, methotrexate is a good option, Dr. Oddis said, noting that methotrexate is the drug he is most comfortable using in those cases.
The literature also supports the combined use of methotrexate and azathioprine, which when given together have been shown to be effective in treatment-resistant myositis and in those who failed either of the drugs alone.
"So that’s a regimen you might want to think about," he said.
Another immunosuppressive option is mycophenolate mofetil (MMF), which has been shown in several small studies and case series to be of benefit. In one study, 6 of 10 patients with dermatomyositis successfully tapered corticosteroids with MMF, and 10 of 12 in another study experienced improvement in cutaneous features of the disease.
The use of intravenous immunoglobulin (IVIg) as add-on therapy with MMF was effective in severe refractory patients, including four with polymyositis and three with dermatomyositis. In a retrospective review of 50 patients with juvenile dermatomyositis, MMF for 12 months was well tolerated, improved skin and muscle, and proved to be steroid-sparing, Dr. Oddis said.
Cyclosporine, tacrolimus, and cyclophosphamide are other immunosuppressive options.
While cyclophosphamide is more often used for myositis-associated interstitial lung disease (ILD), it can be of benefit for refractory skin disease, and can be useful in non-ILD myositis cases that involve severe skin disease.
The only available controlled data for IVIg alone are from a study published more than 20 years ago, but that randomized, double-blind, placebo-controlled study showed that treatment was safe, effective, and steroid sparing in 15 patients with dermatomyositis, he said.
Biologics
As for biologics, anti–tumor necrosis factor–alpha (anti-TNF-alpha) therapy and B-cell therapy have both been considered. Anti-TNF-alpha therapy makes sense because TNF-alpha and other proinflammatory cytokines are increased in muscle tissue of myositis patients; TNF-alpha is toxic to myofibers and prevents their regeneration; and TNF-alpha enhances other inflammatory cytokines in both dermatomyositis and polymyositis, but data are lacking on whether targeting TNF-alpha is worthwhile.
B cell therapy, on the other hand, is showing promise. In one open-label pilot study, rituximab was effective in seven patients with refractory dermatomyositis, and in others it was effective in anti-synthetase syndrome. Rituximab also was effective in two studies for refractory myositis and dermatomyositis rash, and it induced longstanding remission in some of the patients. In another study, however, rituximab was not effective for dermatomyositis rash.
The multicenter Rituximab in Myositis (RIM) study, the largest ever done in myositis, evaluated rituximab for the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis patients.
Although the primary and secondary endpoints of the RIM study were not achieved, 83% of refractory adult and juvenile myositis patients met the definition of improvement, there was a significant corticosteroid sparing effect between the baseline dose and the dose at study conclusion, and treatment was generally well tolerated, he said.
Other targets that are being explored include interleukin-6 and type 1 IFN genes. Findings suggest that coordinated dysregulation of type 1 IFN signaling and IL-6 production are contributors to dermatomyositis pathogenesis, he explained.
Treating myositis patients with ILD
The treatment approach to these patients is somewhat similar to those without ILD, with corticosteroids as initial treatment, Dr. Oddis said.
Cyclophosphamide and azathioprine have been used early on, and also in corticosteroid resistant cases, but with variable results. Cyclophosphamide can be given orally or by IV for 3-6 months.
MMF has been used with success in connective tissue disease–associated ILD, and based on small studies it appears to be effective in myositis-associated ILD as well.
Cyclosporine and tacrolimus have been used in both adult and pediatric patients with promising, steroid-sparing results, he said, noting that the use of anti-T-cell therapy in myositis-associated ILD makes sense, because findings from multiple studies have implicated activated CD8-positive T-cells in myositis-associated ILD.
"It’s an exciting time for therapeutic interventions in myositis, but even though we have all these therapeutic options, we have to temper our enthusiasm with what they do long term," he said.
Dr. Oddis has served on an advisory board for Questcor.
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Corticosteroids remain the initial treatment of choice for myositis and myositis-associated interstitial lung disease, but immunosuppressive agents, intravenous immunoglobulin, and biologics can also play a role in the treatment of one or both of these conditions, according to Dr. Chester V. Oddis.
For myositis in general, Dr. Oddis, professor of medicine and associate director of the rheumatology fellowship training program at the University of Pittsburgh, recommends an initial divided dose of 30 mg of prednisone twice daily, continued until serum creatine kinase (CK) levels fall to normal. At that time, the total daily prednisone dose can be consolidated into a single morning dose, he said at Perspectives in Rheumatic Diseases 2013.
The prednisone can then be tapered by 25% every 3-4 weeks down to a 5- to 10-mg daily maintenance dose that is continued until active disease is suppressed for 12 months. This is a general guideline that helps prevent disease flares, he noted.
Keep in mind that improvement in strength generally lags behind improvement in CK levels, he added.
Nonsteroidal immunosuppressives
Not all patients will need an additional immunosuppressive agent, but for those who do, methotrexate is a good option, Dr. Oddis said, noting that methotrexate is the drug he is most comfortable using in those cases.
The literature also supports the combined use of methotrexate and azathioprine, which when given together have been shown to be effective in treatment-resistant myositis and in those who failed either of the drugs alone.
"So that’s a regimen you might want to think about," he said.
Another immunosuppressive option is mycophenolate mofetil (MMF), which has been shown in several small studies and case series to be of benefit. In one study, 6 of 10 patients with dermatomyositis successfully tapered corticosteroids with MMF, and 10 of 12 in another study experienced improvement in cutaneous features of the disease.
The use of intravenous immunoglobulin (IVIg) as add-on therapy with MMF was effective in severe refractory patients, including four with polymyositis and three with dermatomyositis. In a retrospective review of 50 patients with juvenile dermatomyositis, MMF for 12 months was well tolerated, improved skin and muscle, and proved to be steroid-sparing, Dr. Oddis said.
Cyclosporine, tacrolimus, and cyclophosphamide are other immunosuppressive options.
While cyclophosphamide is more often used for myositis-associated interstitial lung disease (ILD), it can be of benefit for refractory skin disease, and can be useful in non-ILD myositis cases that involve severe skin disease.
The only available controlled data for IVIg alone are from a study published more than 20 years ago, but that randomized, double-blind, placebo-controlled study showed that treatment was safe, effective, and steroid sparing in 15 patients with dermatomyositis, he said.
Biologics
As for biologics, anti–tumor necrosis factor–alpha (anti-TNF-alpha) therapy and B-cell therapy have both been considered. Anti-TNF-alpha therapy makes sense because TNF-alpha and other proinflammatory cytokines are increased in muscle tissue of myositis patients; TNF-alpha is toxic to myofibers and prevents their regeneration; and TNF-alpha enhances other inflammatory cytokines in both dermatomyositis and polymyositis, but data are lacking on whether targeting TNF-alpha is worthwhile.
B cell therapy, on the other hand, is showing promise. In one open-label pilot study, rituximab was effective in seven patients with refractory dermatomyositis, and in others it was effective in anti-synthetase syndrome. Rituximab also was effective in two studies for refractory myositis and dermatomyositis rash, and it induced longstanding remission in some of the patients. In another study, however, rituximab was not effective for dermatomyositis rash.
The multicenter Rituximab in Myositis (RIM) study, the largest ever done in myositis, evaluated rituximab for the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis patients.
Although the primary and secondary endpoints of the RIM study were not achieved, 83% of refractory adult and juvenile myositis patients met the definition of improvement, there was a significant corticosteroid sparing effect between the baseline dose and the dose at study conclusion, and treatment was generally well tolerated, he said.
Other targets that are being explored include interleukin-6 and type 1 IFN genes. Findings suggest that coordinated dysregulation of type 1 IFN signaling and IL-6 production are contributors to dermatomyositis pathogenesis, he explained.
Treating myositis patients with ILD
The treatment approach to these patients is somewhat similar to those without ILD, with corticosteroids as initial treatment, Dr. Oddis said.
Cyclophosphamide and azathioprine have been used early on, and also in corticosteroid resistant cases, but with variable results. Cyclophosphamide can be given orally or by IV for 3-6 months.
MMF has been used with success in connective tissue disease–associated ILD, and based on small studies it appears to be effective in myositis-associated ILD as well.
Cyclosporine and tacrolimus have been used in both adult and pediatric patients with promising, steroid-sparing results, he said, noting that the use of anti-T-cell therapy in myositis-associated ILD makes sense, because findings from multiple studies have implicated activated CD8-positive T-cells in myositis-associated ILD.
"It’s an exciting time for therapeutic interventions in myositis, but even though we have all these therapeutic options, we have to temper our enthusiasm with what they do long term," he said.
Dr. Oddis has served on an advisory board for Questcor.
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Corticosteroids remain the initial treatment of choice for myositis and myositis-associated interstitial lung disease, but immunosuppressive agents, intravenous immunoglobulin, and biologics can also play a role in the treatment of one or both of these conditions, according to Dr. Chester V. Oddis.
For myositis in general, Dr. Oddis, professor of medicine and associate director of the rheumatology fellowship training program at the University of Pittsburgh, recommends an initial divided dose of 30 mg of prednisone twice daily, continued until serum creatine kinase (CK) levels fall to normal. At that time, the total daily prednisone dose can be consolidated into a single morning dose, he said at Perspectives in Rheumatic Diseases 2013.
The prednisone can then be tapered by 25% every 3-4 weeks down to a 5- to 10-mg daily maintenance dose that is continued until active disease is suppressed for 12 months. This is a general guideline that helps prevent disease flares, he noted.
Keep in mind that improvement in strength generally lags behind improvement in CK levels, he added.
Nonsteroidal immunosuppressives
Not all patients will need an additional immunosuppressive agent, but for those who do, methotrexate is a good option, Dr. Oddis said, noting that methotrexate is the drug he is most comfortable using in those cases.
The literature also supports the combined use of methotrexate and azathioprine, which when given together have been shown to be effective in treatment-resistant myositis and in those who failed either of the drugs alone.
"So that’s a regimen you might want to think about," he said.
Another immunosuppressive option is mycophenolate mofetil (MMF), which has been shown in several small studies and case series to be of benefit. In one study, 6 of 10 patients with dermatomyositis successfully tapered corticosteroids with MMF, and 10 of 12 in another study experienced improvement in cutaneous features of the disease.
The use of intravenous immunoglobulin (IVIg) as add-on therapy with MMF was effective in severe refractory patients, including four with polymyositis and three with dermatomyositis. In a retrospective review of 50 patients with juvenile dermatomyositis, MMF for 12 months was well tolerated, improved skin and muscle, and proved to be steroid-sparing, Dr. Oddis said.
Cyclosporine, tacrolimus, and cyclophosphamide are other immunosuppressive options.
While cyclophosphamide is more often used for myositis-associated interstitial lung disease (ILD), it can be of benefit for refractory skin disease, and can be useful in non-ILD myositis cases that involve severe skin disease.
The only available controlled data for IVIg alone are from a study published more than 20 years ago, but that randomized, double-blind, placebo-controlled study showed that treatment was safe, effective, and steroid sparing in 15 patients with dermatomyositis, he said.
Biologics
As for biologics, anti–tumor necrosis factor–alpha (anti-TNF-alpha) therapy and B-cell therapy have both been considered. Anti-TNF-alpha therapy makes sense because TNF-alpha and other proinflammatory cytokines are increased in muscle tissue of myositis patients; TNF-alpha is toxic to myofibers and prevents their regeneration; and TNF-alpha enhances other inflammatory cytokines in both dermatomyositis and polymyositis, but data are lacking on whether targeting TNF-alpha is worthwhile.
B cell therapy, on the other hand, is showing promise. In one open-label pilot study, rituximab was effective in seven patients with refractory dermatomyositis, and in others it was effective in anti-synthetase syndrome. Rituximab also was effective in two studies for refractory myositis and dermatomyositis rash, and it induced longstanding remission in some of the patients. In another study, however, rituximab was not effective for dermatomyositis rash.
The multicenter Rituximab in Myositis (RIM) study, the largest ever done in myositis, evaluated rituximab for the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis patients.
Although the primary and secondary endpoints of the RIM study were not achieved, 83% of refractory adult and juvenile myositis patients met the definition of improvement, there was a significant corticosteroid sparing effect between the baseline dose and the dose at study conclusion, and treatment was generally well tolerated, he said.
Other targets that are being explored include interleukin-6 and type 1 IFN genes. Findings suggest that coordinated dysregulation of type 1 IFN signaling and IL-6 production are contributors to dermatomyositis pathogenesis, he explained.
Treating myositis patients with ILD
The treatment approach to these patients is somewhat similar to those without ILD, with corticosteroids as initial treatment, Dr. Oddis said.
Cyclophosphamide and azathioprine have been used early on, and also in corticosteroid resistant cases, but with variable results. Cyclophosphamide can be given orally or by IV for 3-6 months.
MMF has been used with success in connective tissue disease–associated ILD, and based on small studies it appears to be effective in myositis-associated ILD as well.
Cyclosporine and tacrolimus have been used in both adult and pediatric patients with promising, steroid-sparing results, he said, noting that the use of anti-T-cell therapy in myositis-associated ILD makes sense, because findings from multiple studies have implicated activated CD8-positive T-cells in myositis-associated ILD.
"It’s an exciting time for therapeutic interventions in myositis, but even though we have all these therapeutic options, we have to temper our enthusiasm with what they do long term," he said.
Dr. Oddis has served on an advisory board for Questcor.
The meeting was held by Global Academy for Medical Education. GAME and this news organization are owned by Frontline Medical Communications.
AT PERSPECTIVES IN RHEUMATIC DISEASES 2013
Ustekinumab approved for psoriatic arthritis
Ustekinumab, a human interleukin-12 and -23 antagonist, has been approved for the treatment of adults with active psoriatic arthritis, the manufacturer has announced.
The approved indication is for use alone or in combination with methotrexate, in the form of a 45-mg subcutaneous injection at weeks 0 and 4, and then every 12 weeks. For patients with coexistent moderate-to-severe plaque psoriasis who weigh more than 220 pounds, the recommended dose is a 90-mg subcutaneous injection at weeks 0 and 4, and then every 12 weeks.
Ustekinumab, approved for treating psoriasis in 2009, is marketed as Stelara by Janssen Biotech, which announced the approval in a press release on Sept. 23. Ustekinumab is the first treatment targeting the cytokines interleukin-12 and interleukin-23, according to the statement.
Approval was based on the results of two phase III multicenter, randomized double-blind studies comparing ustekinumab to placebo in 927 patients with active psoriatic arthritis, with at least five tender and five swollen joints and a C-reactive protein (CRP) level of at least 0.3 mg/dL, despite previous treatment with conventional therapy. The primary endpoint was the ACR 20 response at 24 weeks.
In the first trial, PSUMMIT 1, 42% of those on the 45-mg dose and 50% of those on the 90-mg dose achieved an ACR 20 at 24 weeks, vs. 23% of those on placebo. In addition, 57% of those on the 45-mg dose and 62% of those on the 90-mg dose achieved a PASI (Psoriasis Area and Severity Index) 75 response, vs. 11% of those on placebo.
In the second study, PSUMMIT II, 44% of those on a 45-mg dose and 44% of those on a 90-mg dose met the primary endpoint, vs 20% of those on placebo. Also, 51% of those on the 45-mg dose and 56% of those on the 90-mg dose achieved a PASI 75 response, vs. 5% of those on placebo.
The results of the PSUMMIT 1 study were published online in the Lancet on June 13 (doi:10.1016/S0140-6736[13]60594-2).
The warnings and precautions section of the ustekinumab label includes information about the risk of serious infections and other risks associated with treatment.
The updated label is available here.
Ustekinumab, a human interleukin-12 and -23 antagonist, has been approved for the treatment of adults with active psoriatic arthritis, the manufacturer has announced.
The approved indication is for use alone or in combination with methotrexate, in the form of a 45-mg subcutaneous injection at weeks 0 and 4, and then every 12 weeks. For patients with coexistent moderate-to-severe plaque psoriasis who weigh more than 220 pounds, the recommended dose is a 90-mg subcutaneous injection at weeks 0 and 4, and then every 12 weeks.
Ustekinumab, approved for treating psoriasis in 2009, is marketed as Stelara by Janssen Biotech, which announced the approval in a press release on Sept. 23. Ustekinumab is the first treatment targeting the cytokines interleukin-12 and interleukin-23, according to the statement.
Approval was based on the results of two phase III multicenter, randomized double-blind studies comparing ustekinumab to placebo in 927 patients with active psoriatic arthritis, with at least five tender and five swollen joints and a C-reactive protein (CRP) level of at least 0.3 mg/dL, despite previous treatment with conventional therapy. The primary endpoint was the ACR 20 response at 24 weeks.
In the first trial, PSUMMIT 1, 42% of those on the 45-mg dose and 50% of those on the 90-mg dose achieved an ACR 20 at 24 weeks, vs. 23% of those on placebo. In addition, 57% of those on the 45-mg dose and 62% of those on the 90-mg dose achieved a PASI (Psoriasis Area and Severity Index) 75 response, vs. 11% of those on placebo.
In the second study, PSUMMIT II, 44% of those on a 45-mg dose and 44% of those on a 90-mg dose met the primary endpoint, vs 20% of those on placebo. Also, 51% of those on the 45-mg dose and 56% of those on the 90-mg dose achieved a PASI 75 response, vs. 5% of those on placebo.
The results of the PSUMMIT 1 study were published online in the Lancet on June 13 (doi:10.1016/S0140-6736[13]60594-2).
The warnings and precautions section of the ustekinumab label includes information about the risk of serious infections and other risks associated with treatment.
The updated label is available here.
Ustekinumab, a human interleukin-12 and -23 antagonist, has been approved for the treatment of adults with active psoriatic arthritis, the manufacturer has announced.
The approved indication is for use alone or in combination with methotrexate, in the form of a 45-mg subcutaneous injection at weeks 0 and 4, and then every 12 weeks. For patients with coexistent moderate-to-severe plaque psoriasis who weigh more than 220 pounds, the recommended dose is a 90-mg subcutaneous injection at weeks 0 and 4, and then every 12 weeks.
Ustekinumab, approved for treating psoriasis in 2009, is marketed as Stelara by Janssen Biotech, which announced the approval in a press release on Sept. 23. Ustekinumab is the first treatment targeting the cytokines interleukin-12 and interleukin-23, according to the statement.
Approval was based on the results of two phase III multicenter, randomized double-blind studies comparing ustekinumab to placebo in 927 patients with active psoriatic arthritis, with at least five tender and five swollen joints and a C-reactive protein (CRP) level of at least 0.3 mg/dL, despite previous treatment with conventional therapy. The primary endpoint was the ACR 20 response at 24 weeks.
In the first trial, PSUMMIT 1, 42% of those on the 45-mg dose and 50% of those on the 90-mg dose achieved an ACR 20 at 24 weeks, vs. 23% of those on placebo. In addition, 57% of those on the 45-mg dose and 62% of those on the 90-mg dose achieved a PASI (Psoriasis Area and Severity Index) 75 response, vs. 11% of those on placebo.
In the second study, PSUMMIT II, 44% of those on a 45-mg dose and 44% of those on a 90-mg dose met the primary endpoint, vs 20% of those on placebo. Also, 51% of those on the 45-mg dose and 56% of those on the 90-mg dose achieved a PASI 75 response, vs. 5% of those on placebo.
The results of the PSUMMIT 1 study were published online in the Lancet on June 13 (doi:10.1016/S0140-6736[13]60594-2).
The warnings and precautions section of the ustekinumab label includes information about the risk of serious infections and other risks associated with treatment.
The updated label is available here.
Psoriasis linked to increased heart failure risk
AMSTERDAM – Psoriasis proved to be independently associated with an increased risk for new-onset heart failure in the first nationwide study to look at a possible relationship.
As word of the newly identified psoriasis/heart failure link spreads, it’s likely cardiologists will receive a growing number of referrals from dermatologists and primary care physicians for evaluation of possible heart failure in psoriasis patients who develop shortness of breath or other symptoms suggestive of ventricular dysfunction, Dr. Usman Khalid said at the annual congress of the European Society of Cardiology.
"Our results underlie the importance of considering the psoriatic population as a high-risk patient group in terms of cardiovascular risk. We encourage early screening for cardiovascular risk factors in psoriasis patients," he said.
Psoriasis is known to be associated with an increased cardiovascular event rate, presumably due to shared systemic inflammatory pathways, but the association between the dermatologic disease and heart failure, specifically, hadn’t been looked at in depth prior to Dr. Khalid’s presentation of a Danish study involving all 5.85 million Danish adults, who were followed from 1997 through 2009.
Among the Danish population without known heart failure at baseline were 57,049 individuals with mild psoriasis – identified by their use of prescription topical agents – and 11,638 others with severe psoriasis. The incidence of new-onset heart failure during follow-up was 2.27 cases per 1,000 person-years in subjects without psoriasis and a significantly greater 4.02 per 1,000 person-years in those with mild psoriasis and 4.50 per 1,000 person-years in individuals with severe psoriasis, reported Dr. Khalid of the University of Copenhagen.
In a multivariate regression analysis adjusted for demographic and socioeconomic factors, comorbid conditions, and use of cardiovascular medications, the likelihood of developing new-onset heart failure during follow-up was 64% greater among individuals with mild psoriasis and 85% greater in those with severe psoriasis than in psoriasis-free subjects.
Because of limitations in the registry database, it is not possible to determine the proportion of new-onset heart failure among psoriasis patients that involved systolic as opposed to diastolic dysfunction, or ischemic versus nonischemic etiology, Dr. Khalid said in response to audience questions.
This study was supported by a research grant from Leo Pharma. Dr. Khalid reported having no financial conflicts of interest.
AMSTERDAM – Psoriasis proved to be independently associated with an increased risk for new-onset heart failure in the first nationwide study to look at a possible relationship.
As word of the newly identified psoriasis/heart failure link spreads, it’s likely cardiologists will receive a growing number of referrals from dermatologists and primary care physicians for evaluation of possible heart failure in psoriasis patients who develop shortness of breath or other symptoms suggestive of ventricular dysfunction, Dr. Usman Khalid said at the annual congress of the European Society of Cardiology.
"Our results underlie the importance of considering the psoriatic population as a high-risk patient group in terms of cardiovascular risk. We encourage early screening for cardiovascular risk factors in psoriasis patients," he said.
Psoriasis is known to be associated with an increased cardiovascular event rate, presumably due to shared systemic inflammatory pathways, but the association between the dermatologic disease and heart failure, specifically, hadn’t been looked at in depth prior to Dr. Khalid’s presentation of a Danish study involving all 5.85 million Danish adults, who were followed from 1997 through 2009.
Among the Danish population without known heart failure at baseline were 57,049 individuals with mild psoriasis – identified by their use of prescription topical agents – and 11,638 others with severe psoriasis. The incidence of new-onset heart failure during follow-up was 2.27 cases per 1,000 person-years in subjects without psoriasis and a significantly greater 4.02 per 1,000 person-years in those with mild psoriasis and 4.50 per 1,000 person-years in individuals with severe psoriasis, reported Dr. Khalid of the University of Copenhagen.
In a multivariate regression analysis adjusted for demographic and socioeconomic factors, comorbid conditions, and use of cardiovascular medications, the likelihood of developing new-onset heart failure during follow-up was 64% greater among individuals with mild psoriasis and 85% greater in those with severe psoriasis than in psoriasis-free subjects.
Because of limitations in the registry database, it is not possible to determine the proportion of new-onset heart failure among psoriasis patients that involved systolic as opposed to diastolic dysfunction, or ischemic versus nonischemic etiology, Dr. Khalid said in response to audience questions.
This study was supported by a research grant from Leo Pharma. Dr. Khalid reported having no financial conflicts of interest.
AMSTERDAM – Psoriasis proved to be independently associated with an increased risk for new-onset heart failure in the first nationwide study to look at a possible relationship.
As word of the newly identified psoriasis/heart failure link spreads, it’s likely cardiologists will receive a growing number of referrals from dermatologists and primary care physicians for evaluation of possible heart failure in psoriasis patients who develop shortness of breath or other symptoms suggestive of ventricular dysfunction, Dr. Usman Khalid said at the annual congress of the European Society of Cardiology.
"Our results underlie the importance of considering the psoriatic population as a high-risk patient group in terms of cardiovascular risk. We encourage early screening for cardiovascular risk factors in psoriasis patients," he said.
Psoriasis is known to be associated with an increased cardiovascular event rate, presumably due to shared systemic inflammatory pathways, but the association between the dermatologic disease and heart failure, specifically, hadn’t been looked at in depth prior to Dr. Khalid’s presentation of a Danish study involving all 5.85 million Danish adults, who were followed from 1997 through 2009.
Among the Danish population without known heart failure at baseline were 57,049 individuals with mild psoriasis – identified by their use of prescription topical agents – and 11,638 others with severe psoriasis. The incidence of new-onset heart failure during follow-up was 2.27 cases per 1,000 person-years in subjects without psoriasis and a significantly greater 4.02 per 1,000 person-years in those with mild psoriasis and 4.50 per 1,000 person-years in individuals with severe psoriasis, reported Dr. Khalid of the University of Copenhagen.
In a multivariate regression analysis adjusted for demographic and socioeconomic factors, comorbid conditions, and use of cardiovascular medications, the likelihood of developing new-onset heart failure during follow-up was 64% greater among individuals with mild psoriasis and 85% greater in those with severe psoriasis than in psoriasis-free subjects.
Because of limitations in the registry database, it is not possible to determine the proportion of new-onset heart failure among psoriasis patients that involved systolic as opposed to diastolic dysfunction, or ischemic versus nonischemic etiology, Dr. Khalid said in response to audience questions.
This study was supported by a research grant from Leo Pharma. Dr. Khalid reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2013
Major finding: Danish patients with mild psoriasis developed new-onset heart failure at the rate of 4.02 cases per 1,000 person-years of follow-up, while those with severe psoriasis did so at a pace of 4.50 cases per 1,000 person-years, both significantly higher rates than the 2.27 per 1,000 person-years in the general psoriasis-free population.
Data source: This was a Danish nationwide registry study that included all 5.85 million Danish adults. They were followed during 1997-2009 for development of heart failure.
Disclosures: The study was supported by a research grant from Leo Pharma. The presenter reported having no financial conflicts of interest.