User login
Infliximab biosimilar noninferior to originator in IBD – NOR-SWITCH
CHICAGO – The biosimilar infliximab CT-P13 is not inferior to the originator infliximab in terms of efficacy, safety, and immunogenicity in the treatment of inflammatory bowel disease (IBD), a phase IV randomized trial showed.
Patient outcomes were not compromised with the use of the biosimilar, and the cost of treatment was much lower, said lead author Kristin K. Jørgensen, MD, PhD, at Digestive Disease Week®.
“Biologics represent a substantial source of IBD expenditure,” said Dr. Jørgensen of Akershus University Hospital, Lørenskog, Norway. “The medication is expensive, patients are treated on a long-term basis, and the incidence of IBD is increasing.”
Biosimilars are biotherapeutic products that are similar in terms of quality, safety, and efficacy to the already licensed reference biologic product. “Use of biosimilars can potentially dramatically decrease costs and may lead to better patient care,” said Dr. Jørgensen. “The patient gets increased access to biologic therapy, and it is easier to intensify dosing if indicated.”
Tumor necrosis factor–inhibitors are commonly used to treat Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, and chronic plaque psoriasis, and, while they have altered the treatment paradigm, they are expensive products.
The goal of the NOR-SWITCH was to evaluate switching from originator infliximab to CT-P13, in terms of efficacy, safety, and immunogenicity.
Dr. Jørgensen and her colleagues conducted a randomized phase IV trial that enrolled 482 patients who were randomly assigned to either infliximab originator (n = 241) or CT-P13 (n = 241). The primary endpoint was disease worsening during follow-up.
Of the group, 155 patients (32%) had Crohn’s disease, 93 (19%) had ulcerative colitis, 91 (19%) had spondyloarthritis, 77 (16%) had rheumatoid arthritis, 30 (6%) had psoriatic arthritis, and 35 (7%) had chronic plaque psoriasis.
Disease worsening occurred at a similar rate in both groups. In the infliximab originator group, 53 patients (26%) experienced a worsening of their symptoms, compared with 61 patients (30%) in the CT-P13 group. The 95% confidence interval of the adjusted risk difference (−4.4%) was −12.7% to 3.9%, which fell within the prespecified noninferiority margin of 15%.
Therefore, the findings demonstrated that CT-P13 is not inferior to infliximab originator, and the adjusted relative risk of disease worsening for CT-P13 patients was 1.17 (95% CI, 0.82-1.52), compared with the infliximab originator group.
An almost equal number of patients achieved disease remission, 123 patients (61%) in the infliximab originator group and 126 patients (61%) in the CT-P13 group, and the adjusted rate difference was 0.6% (95% CI, –7.5%-8.8%; per-protocol set).
An explorative subgroup analysis that looked at patients with Crohn’s disease and ulcerative colitis showed similar findings between patients treated with either agent.
“Our results support switching from the originator to a biosimilar for nonmedical reasons,” concluded Dr. Jørgensen.
However, she urged caution in generalizing these findings to other biologic agents.
The study was funded by the Norwegian Ministry of Health and Care Services. Dr. Jorgensen reported receiving personal fees from Tillotts, Intercept, and Celltrion. Several coauthors also reported relationships with industry.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – The biosimilar infliximab CT-P13 is not inferior to the originator infliximab in terms of efficacy, safety, and immunogenicity in the treatment of inflammatory bowel disease (IBD), a phase IV randomized trial showed.
Patient outcomes were not compromised with the use of the biosimilar, and the cost of treatment was much lower, said lead author Kristin K. Jørgensen, MD, PhD, at Digestive Disease Week®.
“Biologics represent a substantial source of IBD expenditure,” said Dr. Jørgensen of Akershus University Hospital, Lørenskog, Norway. “The medication is expensive, patients are treated on a long-term basis, and the incidence of IBD is increasing.”
Biosimilars are biotherapeutic products that are similar in terms of quality, safety, and efficacy to the already licensed reference biologic product. “Use of biosimilars can potentially dramatically decrease costs and may lead to better patient care,” said Dr. Jørgensen. “The patient gets increased access to biologic therapy, and it is easier to intensify dosing if indicated.”
Tumor necrosis factor–inhibitors are commonly used to treat Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, and chronic plaque psoriasis, and, while they have altered the treatment paradigm, they are expensive products.
The goal of the NOR-SWITCH was to evaluate switching from originator infliximab to CT-P13, in terms of efficacy, safety, and immunogenicity.
Dr. Jørgensen and her colleagues conducted a randomized phase IV trial that enrolled 482 patients who were randomly assigned to either infliximab originator (n = 241) or CT-P13 (n = 241). The primary endpoint was disease worsening during follow-up.
Of the group, 155 patients (32%) had Crohn’s disease, 93 (19%) had ulcerative colitis, 91 (19%) had spondyloarthritis, 77 (16%) had rheumatoid arthritis, 30 (6%) had psoriatic arthritis, and 35 (7%) had chronic plaque psoriasis.
Disease worsening occurred at a similar rate in both groups. In the infliximab originator group, 53 patients (26%) experienced a worsening of their symptoms, compared with 61 patients (30%) in the CT-P13 group. The 95% confidence interval of the adjusted risk difference (−4.4%) was −12.7% to 3.9%, which fell within the prespecified noninferiority margin of 15%.
Therefore, the findings demonstrated that CT-P13 is not inferior to infliximab originator, and the adjusted relative risk of disease worsening for CT-P13 patients was 1.17 (95% CI, 0.82-1.52), compared with the infliximab originator group.
An almost equal number of patients achieved disease remission, 123 patients (61%) in the infliximab originator group and 126 patients (61%) in the CT-P13 group, and the adjusted rate difference was 0.6% (95% CI, –7.5%-8.8%; per-protocol set).
An explorative subgroup analysis that looked at patients with Crohn’s disease and ulcerative colitis showed similar findings between patients treated with either agent.
“Our results support switching from the originator to a biosimilar for nonmedical reasons,” concluded Dr. Jørgensen.
However, she urged caution in generalizing these findings to other biologic agents.
The study was funded by the Norwegian Ministry of Health and Care Services. Dr. Jorgensen reported receiving personal fees from Tillotts, Intercept, and Celltrion. Several coauthors also reported relationships with industry.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – The biosimilar infliximab CT-P13 is not inferior to the originator infliximab in terms of efficacy, safety, and immunogenicity in the treatment of inflammatory bowel disease (IBD), a phase IV randomized trial showed.
Patient outcomes were not compromised with the use of the biosimilar, and the cost of treatment was much lower, said lead author Kristin K. Jørgensen, MD, PhD, at Digestive Disease Week®.
“Biologics represent a substantial source of IBD expenditure,” said Dr. Jørgensen of Akershus University Hospital, Lørenskog, Norway. “The medication is expensive, patients are treated on a long-term basis, and the incidence of IBD is increasing.”
Biosimilars are biotherapeutic products that are similar in terms of quality, safety, and efficacy to the already licensed reference biologic product. “Use of biosimilars can potentially dramatically decrease costs and may lead to better patient care,” said Dr. Jørgensen. “The patient gets increased access to biologic therapy, and it is easier to intensify dosing if indicated.”
Tumor necrosis factor–inhibitors are commonly used to treat Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, and chronic plaque psoriasis, and, while they have altered the treatment paradigm, they are expensive products.
The goal of the NOR-SWITCH was to evaluate switching from originator infliximab to CT-P13, in terms of efficacy, safety, and immunogenicity.
Dr. Jørgensen and her colleagues conducted a randomized phase IV trial that enrolled 482 patients who were randomly assigned to either infliximab originator (n = 241) or CT-P13 (n = 241). The primary endpoint was disease worsening during follow-up.
Of the group, 155 patients (32%) had Crohn’s disease, 93 (19%) had ulcerative colitis, 91 (19%) had spondyloarthritis, 77 (16%) had rheumatoid arthritis, 30 (6%) had psoriatic arthritis, and 35 (7%) had chronic plaque psoriasis.
Disease worsening occurred at a similar rate in both groups. In the infliximab originator group, 53 patients (26%) experienced a worsening of their symptoms, compared with 61 patients (30%) in the CT-P13 group. The 95% confidence interval of the adjusted risk difference (−4.4%) was −12.7% to 3.9%, which fell within the prespecified noninferiority margin of 15%.
Therefore, the findings demonstrated that CT-P13 is not inferior to infliximab originator, and the adjusted relative risk of disease worsening for CT-P13 patients was 1.17 (95% CI, 0.82-1.52), compared with the infliximab originator group.
An almost equal number of patients achieved disease remission, 123 patients (61%) in the infliximab originator group and 126 patients (61%) in the CT-P13 group, and the adjusted rate difference was 0.6% (95% CI, –7.5%-8.8%; per-protocol set).
An explorative subgroup analysis that looked at patients with Crohn’s disease and ulcerative colitis showed similar findings between patients treated with either agent.
“Our results support switching from the originator to a biosimilar for nonmedical reasons,” concluded Dr. Jørgensen.
However, she urged caution in generalizing these findings to other biologic agents.
The study was funded by the Norwegian Ministry of Health and Care Services. Dr. Jorgensen reported receiving personal fees from Tillotts, Intercept, and Celltrion. Several coauthors also reported relationships with industry.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
AT DDW
Key clinical point: An infliximab biosimilar was not inferior to the originator in terms of efficacy, safety, and immunogenicity in the treatment of inflammatory bowel disease (IBD).
Major finding: In the infliximab originator group, 53 patients (26%) experienced disease worsening, vs. 61 patients (30%) in the CT-P13 group, which fell within the prespecified noninferiority margin of 15%.
Data source: A phase IV randomized trial that included 482 patients with inflammatory bowel disease.
Disclosures: The study was funded by the Norwegian Ministry of Health and Care Services. Dr. Jorgensen reported receiving personal fees from Tillotts, Intercept, and Celltrion. Several coauthors also reported relationships with industry.
Adalimumab outperforms methotrexate in treating severe pediatric plaque psoriasis
Adalimumab appears to be a safe and effective treatment option for severe plaque psoriasis in children, outperforming methotrexate, based on the results of a phase III study, said Kim Papp, MD, PhD, of Probity Medical Research, Waterloo, Ont., and his associates.
“To our knowledge, this is the first randomized controlled study of either adalimumab or methotrexate in children and adolescents with psoriasis,” the researchers said, noting that the study did not include a placebo group because of ethical issues related to treating children with a severe chronic disorder.
At week 16 of the initial treatment period, an improvement of at least 75% in the Psoriasis Area and Severity Index (PASI75) score was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group – 22 (58%) – than in the methotrexate group – 12 (32%). In the 0.4-mg/kg adalimumab group, 17 (44%) of patients reached a PASI75. The PASI75 response was rapid in the 0.8 mg/kg adalimumab group, a significant difference, compared with the methotrexate group. It was reached by week 4 (P = .002).
“At week 16, the 26% difference between adalimumab 0.8 mg/kg and methotrexate in the proportion of patients who achieved PASI75 was significant and clinically relevant,” Dr. Papp and his associates concluded.
At week 16 of treatment, the proportion of patients who achieved a physician global assessment (PGA) score of 0 or 1 (clear or minimal) was higher in the adalimumab 0.8 mg/kg group (23 of 38 [61%]) than in the methotrexate group (15 of 37 [41%]) or in the adalimumab 0.4-mg/kg group (16 of 39 [41%]) (P = .083). At week 16, the difference between the adalimumab 0.8-mg/kg and methotrexate groups was not significant, the investigators said (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31189-3).
After the withdrawal period, PASI75 was achieved in 15 of 19 (79%) patients who were initial responders to adalimumab 0.8 mg/kg and 6 of 11 (55%) patients who were initial responders to adalimumab 0.4 mg/kg. PASI75 was achieved in six of eight (75%) patients who had responded to methotrexate treatment in the initial treatment period and who had loss of disease control in the withdrawal period.
During the initial treatment period, adverse events were reported by 26 of 38 (68%) in the adalimumab 0.8-mg/kg group, 30 of 39 (77%) in the adalimumab 0.4-mg/kg group, and 28 of 37 (76%) in the methotrexate group. Infections were the most frequently reported adverse events. Serious adverse events were infrequent, reported by three patients in the adalimumab 0.4-mg/kg group, and were not considered to be related to the study drug, the researchers said. Eleven severe adverse events were reported by 8 of the 114 (7%) children. Of these, headache was the most common. A case of urticaria during retreatment that led to discontinuation of adalimumab in the patient (who had received methotrexate in the first treatment period), was considered by the investigator as “probably related” to adalimumab.
“No new safety risks were identified in our study; however, longer-term data are needed to fully assess the safety profile of adalimumab in the pediatric population,” Dr. Papp and his associates commented.
“Our results showed better quality of life outcomes in children and adolescents treated with adalimumab compared with methotrexate. The mean 10.8-point change in PedsQL [pediatric quality of life inventory] from baseline to week 16 in the adalimumab 0.8-mg/kg group exceeded the minimal clinically important difference of 4.36, whereas the 1.9-point change in the methotrexate group did not,” they noted.
The study was funded by AbbVie, the manufacturer of adalimumab (Humira). Dr. Papp has served as a consultant for AbbVie and a number of other pharmaceutical companies, for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
The initial treatment response to adalimumab was quick with “significant clinical difference compared with methotrexate seen as early as 4 weeks.” Improvement in the pediatric quality of life inventory (PedsQL) score from baseline “was significantly greater” in children in the adalimumab 0.8-mg/kg group than in children in the methotrexate group. Adalimumab provided “several clinically and statistically significant improvements” in children with severe plaque psoriasis, compared with methotrexate.
However, further studies are needed to determine both the short-term and long-term effectiveness and the safety of systemic treatments in children and adolescents with psoriasis.
Dario Kivelevitch, MD, is a third year dermatology resident, and Alan Menter, MD, is chief of dermatology at Baylor University Medical Center, Dallas. These comments are from an editorial that accompanied the study (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31190-X). Dr. Kivelevitch said he had no relevant financial disclosures. Dr. Menter disclosed grants and personal fees from AbbVie and other pharmaceutical companies, all outside the submitted work.
The initial treatment response to adalimumab was quick with “significant clinical difference compared with methotrexate seen as early as 4 weeks.” Improvement in the pediatric quality of life inventory (PedsQL) score from baseline “was significantly greater” in children in the adalimumab 0.8-mg/kg group than in children in the methotrexate group. Adalimumab provided “several clinically and statistically significant improvements” in children with severe plaque psoriasis, compared with methotrexate.
However, further studies are needed to determine both the short-term and long-term effectiveness and the safety of systemic treatments in children and adolescents with psoriasis.
Dario Kivelevitch, MD, is a third year dermatology resident, and Alan Menter, MD, is chief of dermatology at Baylor University Medical Center, Dallas. These comments are from an editorial that accompanied the study (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31190-X). Dr. Kivelevitch said he had no relevant financial disclosures. Dr. Menter disclosed grants and personal fees from AbbVie and other pharmaceutical companies, all outside the submitted work.
The initial treatment response to adalimumab was quick with “significant clinical difference compared with methotrexate seen as early as 4 weeks.” Improvement in the pediatric quality of life inventory (PedsQL) score from baseline “was significantly greater” in children in the adalimumab 0.8-mg/kg group than in children in the methotrexate group. Adalimumab provided “several clinically and statistically significant improvements” in children with severe plaque psoriasis, compared with methotrexate.
However, further studies are needed to determine both the short-term and long-term effectiveness and the safety of systemic treatments in children and adolescents with psoriasis.
Dario Kivelevitch, MD, is a third year dermatology resident, and Alan Menter, MD, is chief of dermatology at Baylor University Medical Center, Dallas. These comments are from an editorial that accompanied the study (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31190-X). Dr. Kivelevitch said he had no relevant financial disclosures. Dr. Menter disclosed grants and personal fees from AbbVie and other pharmaceutical companies, all outside the submitted work.
Adalimumab appears to be a safe and effective treatment option for severe plaque psoriasis in children, outperforming methotrexate, based on the results of a phase III study, said Kim Papp, MD, PhD, of Probity Medical Research, Waterloo, Ont., and his associates.
“To our knowledge, this is the first randomized controlled study of either adalimumab or methotrexate in children and adolescents with psoriasis,” the researchers said, noting that the study did not include a placebo group because of ethical issues related to treating children with a severe chronic disorder.
At week 16 of the initial treatment period, an improvement of at least 75% in the Psoriasis Area and Severity Index (PASI75) score was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group – 22 (58%) – than in the methotrexate group – 12 (32%). In the 0.4-mg/kg adalimumab group, 17 (44%) of patients reached a PASI75. The PASI75 response was rapid in the 0.8 mg/kg adalimumab group, a significant difference, compared with the methotrexate group. It was reached by week 4 (P = .002).
“At week 16, the 26% difference between adalimumab 0.8 mg/kg and methotrexate in the proportion of patients who achieved PASI75 was significant and clinically relevant,” Dr. Papp and his associates concluded.
At week 16 of treatment, the proportion of patients who achieved a physician global assessment (PGA) score of 0 or 1 (clear or minimal) was higher in the adalimumab 0.8 mg/kg group (23 of 38 [61%]) than in the methotrexate group (15 of 37 [41%]) or in the adalimumab 0.4-mg/kg group (16 of 39 [41%]) (P = .083). At week 16, the difference between the adalimumab 0.8-mg/kg and methotrexate groups was not significant, the investigators said (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31189-3).
After the withdrawal period, PASI75 was achieved in 15 of 19 (79%) patients who were initial responders to adalimumab 0.8 mg/kg and 6 of 11 (55%) patients who were initial responders to adalimumab 0.4 mg/kg. PASI75 was achieved in six of eight (75%) patients who had responded to methotrexate treatment in the initial treatment period and who had loss of disease control in the withdrawal period.
During the initial treatment period, adverse events were reported by 26 of 38 (68%) in the adalimumab 0.8-mg/kg group, 30 of 39 (77%) in the adalimumab 0.4-mg/kg group, and 28 of 37 (76%) in the methotrexate group. Infections were the most frequently reported adverse events. Serious adverse events were infrequent, reported by three patients in the adalimumab 0.4-mg/kg group, and were not considered to be related to the study drug, the researchers said. Eleven severe adverse events were reported by 8 of the 114 (7%) children. Of these, headache was the most common. A case of urticaria during retreatment that led to discontinuation of adalimumab in the patient (who had received methotrexate in the first treatment period), was considered by the investigator as “probably related” to adalimumab.
“No new safety risks were identified in our study; however, longer-term data are needed to fully assess the safety profile of adalimumab in the pediatric population,” Dr. Papp and his associates commented.
“Our results showed better quality of life outcomes in children and adolescents treated with adalimumab compared with methotrexate. The mean 10.8-point change in PedsQL [pediatric quality of life inventory] from baseline to week 16 in the adalimumab 0.8-mg/kg group exceeded the minimal clinically important difference of 4.36, whereas the 1.9-point change in the methotrexate group did not,” they noted.
The study was funded by AbbVie, the manufacturer of adalimumab (Humira). Dr. Papp has served as a consultant for AbbVie and a number of other pharmaceutical companies, for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
Adalimumab appears to be a safe and effective treatment option for severe plaque psoriasis in children, outperforming methotrexate, based on the results of a phase III study, said Kim Papp, MD, PhD, of Probity Medical Research, Waterloo, Ont., and his associates.
“To our knowledge, this is the first randomized controlled study of either adalimumab or methotrexate in children and adolescents with psoriasis,” the researchers said, noting that the study did not include a placebo group because of ethical issues related to treating children with a severe chronic disorder.
At week 16 of the initial treatment period, an improvement of at least 75% in the Psoriasis Area and Severity Index (PASI75) score was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group – 22 (58%) – than in the methotrexate group – 12 (32%). In the 0.4-mg/kg adalimumab group, 17 (44%) of patients reached a PASI75. The PASI75 response was rapid in the 0.8 mg/kg adalimumab group, a significant difference, compared with the methotrexate group. It was reached by week 4 (P = .002).
“At week 16, the 26% difference between adalimumab 0.8 mg/kg and methotrexate in the proportion of patients who achieved PASI75 was significant and clinically relevant,” Dr. Papp and his associates concluded.
At week 16 of treatment, the proportion of patients who achieved a physician global assessment (PGA) score of 0 or 1 (clear or minimal) was higher in the adalimumab 0.8 mg/kg group (23 of 38 [61%]) than in the methotrexate group (15 of 37 [41%]) or in the adalimumab 0.4-mg/kg group (16 of 39 [41%]) (P = .083). At week 16, the difference between the adalimumab 0.8-mg/kg and methotrexate groups was not significant, the investigators said (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31189-3).
After the withdrawal period, PASI75 was achieved in 15 of 19 (79%) patients who were initial responders to adalimumab 0.8 mg/kg and 6 of 11 (55%) patients who were initial responders to adalimumab 0.4 mg/kg. PASI75 was achieved in six of eight (75%) patients who had responded to methotrexate treatment in the initial treatment period and who had loss of disease control in the withdrawal period.
During the initial treatment period, adverse events were reported by 26 of 38 (68%) in the adalimumab 0.8-mg/kg group, 30 of 39 (77%) in the adalimumab 0.4-mg/kg group, and 28 of 37 (76%) in the methotrexate group. Infections were the most frequently reported adverse events. Serious adverse events were infrequent, reported by three patients in the adalimumab 0.4-mg/kg group, and were not considered to be related to the study drug, the researchers said. Eleven severe adverse events were reported by 8 of the 114 (7%) children. Of these, headache was the most common. A case of urticaria during retreatment that led to discontinuation of adalimumab in the patient (who had received methotrexate in the first treatment period), was considered by the investigator as “probably related” to adalimumab.
“No new safety risks were identified in our study; however, longer-term data are needed to fully assess the safety profile of adalimumab in the pediatric population,” Dr. Papp and his associates commented.
“Our results showed better quality of life outcomes in children and adolescents treated with adalimumab compared with methotrexate. The mean 10.8-point change in PedsQL [pediatric quality of life inventory] from baseline to week 16 in the adalimumab 0.8-mg/kg group exceeded the minimal clinically important difference of 4.36, whereas the 1.9-point change in the methotrexate group did not,” they noted.
The study was funded by AbbVie, the manufacturer of adalimumab (Humira). Dr. Papp has served as a consultant for AbbVie and a number of other pharmaceutical companies, for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
FROM THE LANCET
Key clinical point:
Major finding: At week 16 of the initial treatment period, Psoriasis Area and Severity Index (PASI)175 was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group (22 of 38 [58%]) than in the methotrexate group (12 of 37 [32%]).
Data source: A double-blind, phase III trial was done at 38 clinics in 13 countries with 114 children aged 4-17 years, with severe plaque psoriasis that had not responded to topical therapy.
Disclosures: The study was funded by AbbVie. Dr. Papp has served as a consultant for adalimumab manufacturer AbbVie and a number of other pharmaceutical companies for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
Living With Psoriasis: How the Disease Impacts the Daily Activities of Patients
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Some data support botulinum toxin for psoriasis and rosacea
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
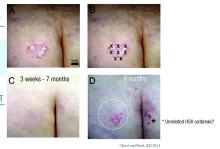
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
Pediatric psoriasis may have a distinct presentation
Children may have a distinctive presentation of psoriasis, compared with adults, Dr. Wynnis Tom said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Infants may present with diaper involvement, also known as “napkin psoriasis,” which may be confused with irritant dermatitis or perineal infections. Moreover, guttate psoriasis, which can be preceded by infectious triggers such as Streptococcus and appears as small, salmon-pink bumps on the skin, is more frequent in children than adults, said Dr. Tom, associate professor of dermatology and pediatrics at the university.
Patients with psoriasis are at higher risk for psychiatric disorders, especially depression and anxiety. A study by Varni et al. discussed QOL ratings by 208 children aged 4-17 years with moderate to severe plaque disease. The study demonstrated a significant negative QOL impact in patients with plaque psoriasis, comparable to the impairment of QOL from arthritis or asthma (Eur J Pediatr. 2011 Sep 30;171[3]485-92).
Dr. Tom talked about other comorbidities associated with psoriasis, including psoriatic arthritis, and encouraged physicians to inquire about morning stiffness, joint pains, swelling, and gait abnormalities. “Psoriatic arthritis occurs in about 10% of children, and it is essential to detect early to prevent permanent joint damage,” she said. “Over the past decade, psoriasis has resurfaced as a systemic disorder as it may be associated with obesity, metabolic syndrome, and inflammatory bowel disease.” Psoriasis also entails an increased risk for cardiovascular disease, myocardial infarction, and stroke.
Dr. Tom emphasized, “because of these risks, we need to extend comorbidity screening to the pediatric population.”
Management of pediatric psoriasis has focused on topical and systemic therapies, in addition to phototherapies. Most systemic agents are used off-label on the basis of experience rather than evidence. Clinical trials are currently underway to extend indications for systemic therapy to the pediatric age group, she said.
Dr. Tom disclosed she is an investigator for Promius Pharma, Celgene, and Janssen.
Children may have a distinctive presentation of psoriasis, compared with adults, Dr. Wynnis Tom said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Infants may present with diaper involvement, also known as “napkin psoriasis,” which may be confused with irritant dermatitis or perineal infections. Moreover, guttate psoriasis, which can be preceded by infectious triggers such as Streptococcus and appears as small, salmon-pink bumps on the skin, is more frequent in children than adults, said Dr. Tom, associate professor of dermatology and pediatrics at the university.
Patients with psoriasis are at higher risk for psychiatric disorders, especially depression and anxiety. A study by Varni et al. discussed QOL ratings by 208 children aged 4-17 years with moderate to severe plaque disease. The study demonstrated a significant negative QOL impact in patients with plaque psoriasis, comparable to the impairment of QOL from arthritis or asthma (Eur J Pediatr. 2011 Sep 30;171[3]485-92).
Dr. Tom talked about other comorbidities associated with psoriasis, including psoriatic arthritis, and encouraged physicians to inquire about morning stiffness, joint pains, swelling, and gait abnormalities. “Psoriatic arthritis occurs in about 10% of children, and it is essential to detect early to prevent permanent joint damage,” she said. “Over the past decade, psoriasis has resurfaced as a systemic disorder as it may be associated with obesity, metabolic syndrome, and inflammatory bowel disease.” Psoriasis also entails an increased risk for cardiovascular disease, myocardial infarction, and stroke.
Dr. Tom emphasized, “because of these risks, we need to extend comorbidity screening to the pediatric population.”
Management of pediatric psoriasis has focused on topical and systemic therapies, in addition to phototherapies. Most systemic agents are used off-label on the basis of experience rather than evidence. Clinical trials are currently underway to extend indications for systemic therapy to the pediatric age group, she said.
Dr. Tom disclosed she is an investigator for Promius Pharma, Celgene, and Janssen.
Children may have a distinctive presentation of psoriasis, compared with adults, Dr. Wynnis Tom said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Infants may present with diaper involvement, also known as “napkin psoriasis,” which may be confused with irritant dermatitis or perineal infections. Moreover, guttate psoriasis, which can be preceded by infectious triggers such as Streptococcus and appears as small, salmon-pink bumps on the skin, is more frequent in children than adults, said Dr. Tom, associate professor of dermatology and pediatrics at the university.
Patients with psoriasis are at higher risk for psychiatric disorders, especially depression and anxiety. A study by Varni et al. discussed QOL ratings by 208 children aged 4-17 years with moderate to severe plaque disease. The study demonstrated a significant negative QOL impact in patients with plaque psoriasis, comparable to the impairment of QOL from arthritis or asthma (Eur J Pediatr. 2011 Sep 30;171[3]485-92).
Dr. Tom talked about other comorbidities associated with psoriasis, including psoriatic arthritis, and encouraged physicians to inquire about morning stiffness, joint pains, swelling, and gait abnormalities. “Psoriatic arthritis occurs in about 10% of children, and it is essential to detect early to prevent permanent joint damage,” she said. “Over the past decade, psoriasis has resurfaced as a systemic disorder as it may be associated with obesity, metabolic syndrome, and inflammatory bowel disease.” Psoriasis also entails an increased risk for cardiovascular disease, myocardial infarction, and stroke.
Dr. Tom emphasized, “because of these risks, we need to extend comorbidity screening to the pediatric population.”
Management of pediatric psoriasis has focused on topical and systemic therapies, in addition to phototherapies. Most systemic agents are used off-label on the basis of experience rather than evidence. Clinical trials are currently underway to extend indications for systemic therapy to the pediatric age group, she said.
Dr. Tom disclosed she is an investigator for Promius Pharma, Celgene, and Janssen.
Study underscores antipsoriatic effect of gastric bypass surgery
Gastric bypass surgery was associated with more than a 50% drop in baseline rates of psoriasis, and with about a 70% decrease in the incidence of psoriatic arthritis, investigators reported.
In contrast, gastric banding did not appear to affect baselines rates of either of these autoimmune conditions, Alexander Egeberg, MD, of Herlev and Gentofte Hospital, Hellerup, Denmark, and associates reported in JAMA Surgery. “Although speculative, these findings may be the result of post-operative differences in weight loss and nutrient uptake, as well as differences in the postsurgical secretion of a number of gut hormones, including [glucagon-like peptide-1],” they wrote.
Psoriasis strongly correlates with obesity, and weight loss appears to mitigate psoriatic symptoms, the investigators noted. Previously, small studies and case series indicated that bariatric surgery might induce remission of psoriasis. To further investigate this possibility, Dr. Egeberg and his associates conducted a longitudinal cohort study of all 12,364 patients who underwent gastric bypass surgery and all 1,071 patients who underwent gastric banding in Denmark between 1997 and 2012 (JAMA Surg. 2017;152:344-349). No patient had psoriasis symptoms at the start of the study. A total of 272 (2%) gastric bypass patients developed psoriasis before their surgery, while only 0.5% did so afterward. In contrast, gastric banding was not tied to a significant change in the incidence of psoriasis – the preoperative rate was 0.5%, and the postoperative rate was 0.4%. Similarly, respective rates of psoriatic arthritis were 0.5% and 0.1% before and after gastric bypass, but were 0.3% and 0.6% before and after gastric banding. Additionally, respective rates of severe psoriasis were 0.8% and 0% before and after gastric bypass, but were about 0.2% and 0.5% before and after gastric banding.
After adjusting for age, sex, alcohol abuse, socioeconomic status, smoking, and diabetes status, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis (P = .004), with about a 56% drop in the rate of severe psoriasis (P = .02), and with about a 71% drop in the rate of psoriatic arthritis (P = .01). In contrast, neither crude nor adjusted models linked gastric banding to a decrease in the incidence of psoriasis, severe psoriasis, or psoriatic arthritis, the researchers said.
Gastric banding is “a purely restrictive procedure,” while gastric bypass – especially Roux-en-Y bypass – diverts nutrients to the distal small intestine, where enteroendocrine cells secrete GLP-1, the researchers wrote.
“These postoperative hormonal changes may, in addition to the weight loss, be important for the antipsoriatic effect of gastric bypass,” they added. “Both gastric bypass and gastric banding have been shown to lead to sustained weight loss, suggesting that the observed differences in our study might be caused by factors other than weight loss.”
An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies.
Gastric bypass surgery was associated with more than a 50% drop in baseline rates of psoriasis, and with about a 70% decrease in the incidence of psoriatic arthritis, investigators reported.
In contrast, gastric banding did not appear to affect baselines rates of either of these autoimmune conditions, Alexander Egeberg, MD, of Herlev and Gentofte Hospital, Hellerup, Denmark, and associates reported in JAMA Surgery. “Although speculative, these findings may be the result of post-operative differences in weight loss and nutrient uptake, as well as differences in the postsurgical secretion of a number of gut hormones, including [glucagon-like peptide-1],” they wrote.
Psoriasis strongly correlates with obesity, and weight loss appears to mitigate psoriatic symptoms, the investigators noted. Previously, small studies and case series indicated that bariatric surgery might induce remission of psoriasis. To further investigate this possibility, Dr. Egeberg and his associates conducted a longitudinal cohort study of all 12,364 patients who underwent gastric bypass surgery and all 1,071 patients who underwent gastric banding in Denmark between 1997 and 2012 (JAMA Surg. 2017;152:344-349). No patient had psoriasis symptoms at the start of the study. A total of 272 (2%) gastric bypass patients developed psoriasis before their surgery, while only 0.5% did so afterward. In contrast, gastric banding was not tied to a significant change in the incidence of psoriasis – the preoperative rate was 0.5%, and the postoperative rate was 0.4%. Similarly, respective rates of psoriatic arthritis were 0.5% and 0.1% before and after gastric bypass, but were 0.3% and 0.6% before and after gastric banding. Additionally, respective rates of severe psoriasis were 0.8% and 0% before and after gastric bypass, but were about 0.2% and 0.5% before and after gastric banding.
After adjusting for age, sex, alcohol abuse, socioeconomic status, smoking, and diabetes status, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis (P = .004), with about a 56% drop in the rate of severe psoriasis (P = .02), and with about a 71% drop in the rate of psoriatic arthritis (P = .01). In contrast, neither crude nor adjusted models linked gastric banding to a decrease in the incidence of psoriasis, severe psoriasis, or psoriatic arthritis, the researchers said.
Gastric banding is “a purely restrictive procedure,” while gastric bypass – especially Roux-en-Y bypass – diverts nutrients to the distal small intestine, where enteroendocrine cells secrete GLP-1, the researchers wrote.
“These postoperative hormonal changes may, in addition to the weight loss, be important for the antipsoriatic effect of gastric bypass,” they added. “Both gastric bypass and gastric banding have been shown to lead to sustained weight loss, suggesting that the observed differences in our study might be caused by factors other than weight loss.”
An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies.
Gastric bypass surgery was associated with more than a 50% drop in baseline rates of psoriasis, and with about a 70% decrease in the incidence of psoriatic arthritis, investigators reported.
In contrast, gastric banding did not appear to affect baselines rates of either of these autoimmune conditions, Alexander Egeberg, MD, of Herlev and Gentofte Hospital, Hellerup, Denmark, and associates reported in JAMA Surgery. “Although speculative, these findings may be the result of post-operative differences in weight loss and nutrient uptake, as well as differences in the postsurgical secretion of a number of gut hormones, including [glucagon-like peptide-1],” they wrote.
Psoriasis strongly correlates with obesity, and weight loss appears to mitigate psoriatic symptoms, the investigators noted. Previously, small studies and case series indicated that bariatric surgery might induce remission of psoriasis. To further investigate this possibility, Dr. Egeberg and his associates conducted a longitudinal cohort study of all 12,364 patients who underwent gastric bypass surgery and all 1,071 patients who underwent gastric banding in Denmark between 1997 and 2012 (JAMA Surg. 2017;152:344-349). No patient had psoriasis symptoms at the start of the study. A total of 272 (2%) gastric bypass patients developed psoriasis before their surgery, while only 0.5% did so afterward. In contrast, gastric banding was not tied to a significant change in the incidence of psoriasis – the preoperative rate was 0.5%, and the postoperative rate was 0.4%. Similarly, respective rates of psoriatic arthritis were 0.5% and 0.1% before and after gastric bypass, but were 0.3% and 0.6% before and after gastric banding. Additionally, respective rates of severe psoriasis were 0.8% and 0% before and after gastric bypass, but were about 0.2% and 0.5% before and after gastric banding.
After adjusting for age, sex, alcohol abuse, socioeconomic status, smoking, and diabetes status, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis (P = .004), with about a 56% drop in the rate of severe psoriasis (P = .02), and with about a 71% drop in the rate of psoriatic arthritis (P = .01). In contrast, neither crude nor adjusted models linked gastric banding to a decrease in the incidence of psoriasis, severe psoriasis, or psoriatic arthritis, the researchers said.
Gastric banding is “a purely restrictive procedure,” while gastric bypass – especially Roux-en-Y bypass – diverts nutrients to the distal small intestine, where enteroendocrine cells secrete GLP-1, the researchers wrote.
“These postoperative hormonal changes may, in addition to the weight loss, be important for the antipsoriatic effect of gastric bypass,” they added. “Both gastric bypass and gastric banding have been shown to lead to sustained weight loss, suggesting that the observed differences in our study might be caused by factors other than weight loss.”
An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies.
Key clinical point: Gastric bypass, but not gastric banding, was associated with significant drops in rates of psoriasis and psoriatic arthritis.
Major finding: In an adjusted model, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis, with a 56% drop in the rate of severe psoriasis, and with a 71% drop in the rate of psoriatic arthritis.
Data source: A population-based cohort study of 12,364 gastric bypass patients and 1,071 gastric banding patients.
Disclosures: An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies. The other coinvestigators reported having no ties to industry.
Renflexis approved as second infliximab biosimilar
Infliximab-abda is the second infliximab biosimilar approved by the Food and Drug Administration, the agency announced April 21.
Infliximab-abda, to be marketed as Renflexis, is approved for all indications as the reference product, including Crohn’s diseases in adults and children, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, according to the product label.
Like Remicade, Renflexis will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections, lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Renflexis will be marketed by Merck Sharp & Dohme and is manufactured by Samsung Bioepis.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
Infliximab-abda is the second infliximab biosimilar approved by the Food and Drug Administration, the agency announced April 21.
Infliximab-abda, to be marketed as Renflexis, is approved for all indications as the reference product, including Crohn’s diseases in adults and children, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, according to the product label.
Like Remicade, Renflexis will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections, lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Renflexis will be marketed by Merck Sharp & Dohme and is manufactured by Samsung Bioepis.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
Infliximab-abda is the second infliximab biosimilar approved by the Food and Drug Administration, the agency announced April 21.
Infliximab-abda, to be marketed as Renflexis, is approved for all indications as the reference product, including Crohn’s diseases in adults and children, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, according to the product label.
Like Remicade, Renflexis will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections, lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Renflexis will be marketed by Merck Sharp & Dohme and is manufactured by Samsung Bioepis.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
Recovery of Hair in the Psoriatic Plaques of a Patient With Coexistent Alopecia Universalis
To the Editor:
Both alopecia areata (AA) and psoriasis vulgaris are chronic relapsing autoimmune diseases, with AA causing nonscarring hair loss in approximately 0.1% to 0.2%1 of the population with a lifetime risk of 1.7%,2 and psoriasis more broadly impacting 1.5% to 2% of the population.3 The helper T cell (TH1) cytokine milieu is pathogenic in both conditions.4-6 IFN-γ knockout mice, unlike their wild-type counterparts, do not exhibit AA.7 Psoriasis is notably improved by IL-10 injections, which dampen the TH1 response.8 Distinct from AA, TH17 and TH22 cells have been implicated as key players in psoriasis pathogenesis, along with the associated IL-17 and IL-22 cytokines.9-12
Few cases of patients with concurrent AA and psoriasis have been described. Interestingly, these cases document normal hair regrowth in the areas of psoriasis.13-16 These cases may offer unique insight into the immune factors driving each disease. We describe a case of a man with both alopecia universalis (AU) and psoriasis who developed hair regrowth in some of the psoriatic plaques.
A 34-year-old man with concurrent AU and psoriasis who had not used any systemic or topical medication for either condition in the last year presented to our clinic seeking treatment. The patient had a history of alopecia totalis as a toddler that completely resolved by 4 years of age with the use of squaric acid dibutylester (SADBE). At 31 years of age, the alopecia recurred and was localized to the scalp. It was partially responsive to intralesional triamcinolone acetonide. The patient’s alopecia worsened over the 2 years following recurrence, ultimately progressing to AU. Two months after the alopecia recurrence, he developed the first psoriatic plaques. As the plaque psoriasis progressed, systemic therapy was initiated, first methotrexate and then etanercept. Shortly after developing AU, he lost his health insurance and discontinued all therapy. The patient’s psoriasis began to recur approximately 3 months after stopping etanercept. He was not using any other psoriasis medications. At that time, he noted terminal hair regrowth within some of the psoriatic plaques. No terminal hairs grew outside of the psoriatic plaques, and all regions with growth had previously been without hair for an extended period of time. The patient presented to our clinic approximately 1 year later. He had no other medical conditions and no relevant family history.
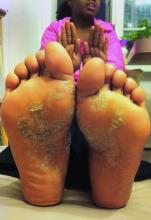
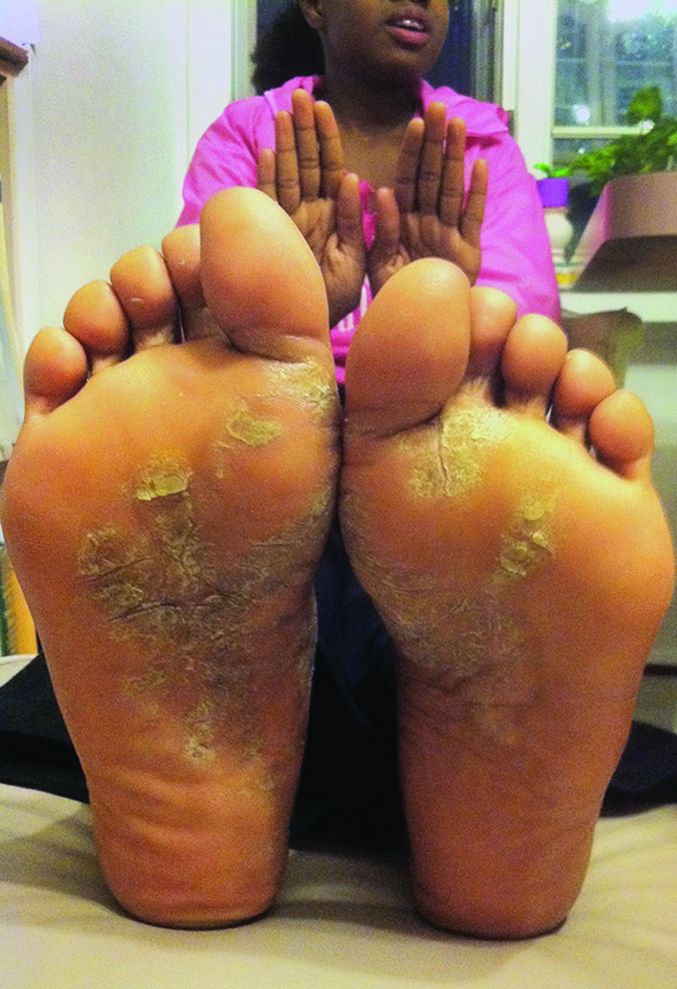
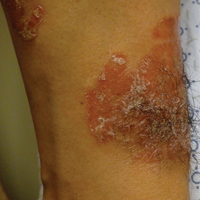
On initial physical examination, he had nonscarring hair loss involving nearly 100% of the body with psoriatic plaques on approximately 30% of the body surface area. Regions of terminal hair growth were confined to some but not all of the psoriatic plaques (Figure). Interestingly, the terminal hairs were primarily localized to the thickest central regions of the plaques. The patient’s psoriasis was treated with a combination of topical clobetasol and calcipotriene. In addition, he was started on tacrolimus ointment to the face and eyebrows for the AA. Maintenance of terminal hair within a region of topically treated psoriasis on the forearm persisted at the 2-month follow-up despite complete clearance of the corresponding psoriatic plaque. A small psoriatic plaque on the scalp cleared early with topical therapy without noticeable hair regrowth. The patient subsequently was started on contact immunotherapy with SADBE and intralesional triamcinolone acetonide for the scalp alopecia without satisfactory response. He decided to discontinue further attempts at treating the alopecia and requested to be restarted on etanercept therapy for recalcitrant psoriatic plaques. His psoriasis responded well to this therapy and he continues to be followed in our psoriasis clinic. One year after clearance of the treated psoriatic plaques, the corresponding terminal hairs persist.
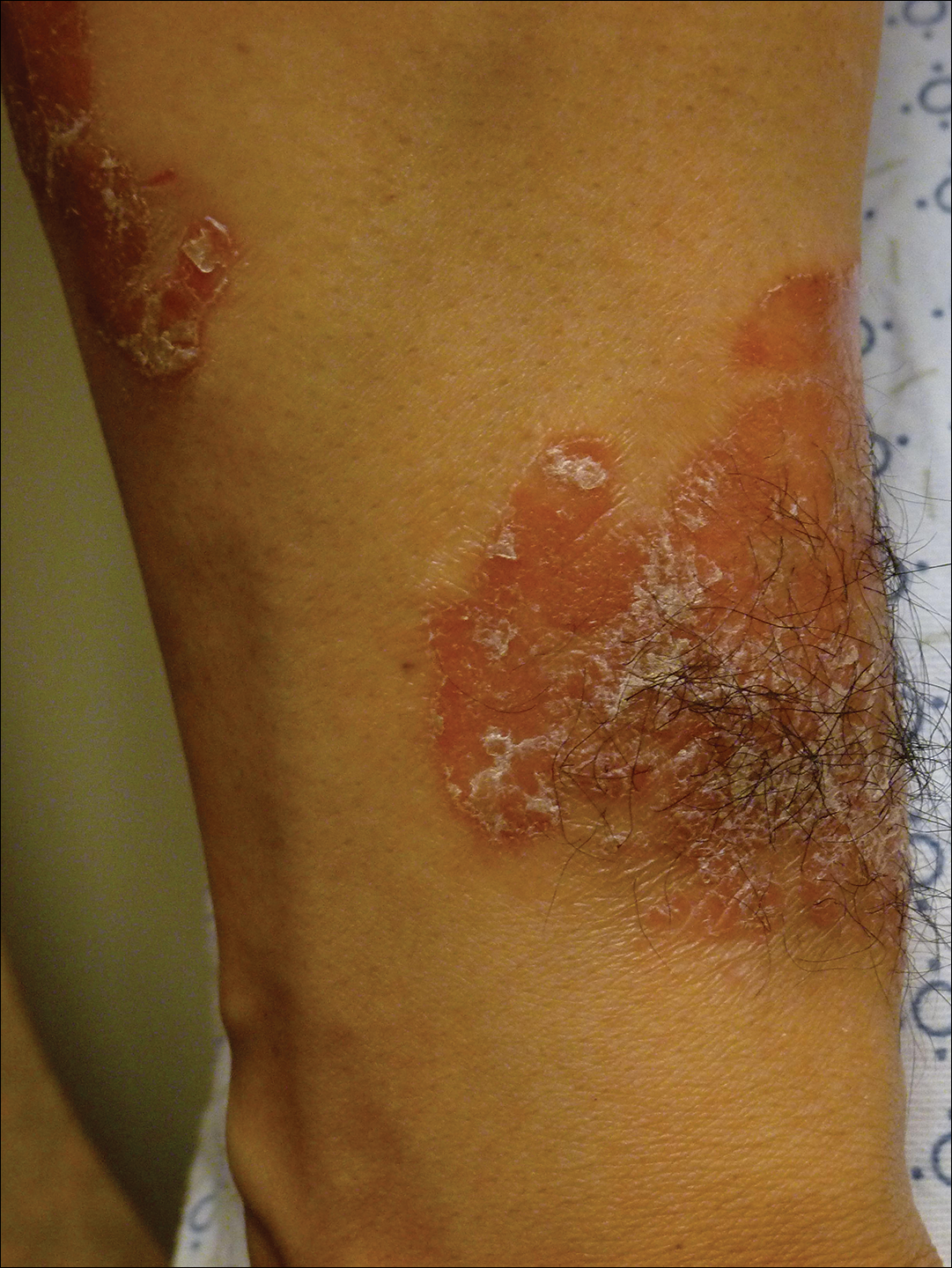
Contact immunotherapy, most commonly with diphenylcyclopropenone or SADBE, is reported to have a 50% to 60% success rate in extensive AA, with a broad range of 9% to 87%17; however, randomized controlled trials testing the efficacy of contact immunotherapy are lacking. Although the mechanism of action of these topical sensitizers is not clearly delineated, it has been postulated that by inducing a new type of inflammatory response in the region, the immunologic milieu is changed, allowing the hair to grow. Some proposed mechanisms include promoting perifollicular lymphocyte apoptosis, preventing new recruitment of autoreactive lymphocytes, and allowing for the correction of aberrant major histocompatibility complex expression on the hair matrix epithelium to regain follicle immune privilege.18-20
Iatrogenic immunotherapy may work analogously to the natural immune system deviation demonstrated in our patient. Psoriasis and AA are believed to form competing immune cells and cytokine milieus, thus explaining how an individual with AA could regain normal hair growth in areas of psoriasis.15,16 The Renbök phenomenon, or reverse Köbner phenomenon, coined by Happle et al13 can be used to describe both the iatrogenic and natural cases of dermatologic disease improvement in response to secondary insults.14
A complex cascade of immune cells and cytokines coordinate AA pathogenesis. In the acute stage of AA, an inflammatory infiltrate of CD4+ T cells, CD8+ T cells, and antigen-presenting cells target anagen phase follicles, with a higher CD4+:CD8+ ratio in clinically active disease.21-23 Subcutaneous injections of either CD4+ or CD8+ lymphocyte subsets from mice with AA into normal-haired mice induces disease. However, CD8+ T cell injections rapidly produce apparent hair loss, whereas CD4+ T cells cause hair loss after several weeks, suggesting that CD8+ T cells directly modulate AA hair loss and CD4+ T cells act as an aide.24 The growth, differentiation, and survival of CD8+ T cells are stimulated by IL-2 and IFN-γ. Alopecia areata biopsies demonstrate a prevalence of TH1 cytokines, and patients with localized AA, alopecia totalis, and AU have notably higher serum IFN-γ levels compared to controls.25 In murine models, IL-1α and IL-1β increase during the catagen phase of the hair cycle and peak during the telogen phase.26 Excessive IL-1β expression is detected in the early stages of human disease, and certain IL-1β polymorphisms are associated with severe forms of AA.26 The role of tumor necrosis factor (TNF) α in AA is not well understood. In vitro studies show it inhibits hair growth, suggesting the cytokine may play a role in AA.27 However, anti–TNF-α therapy is not effective in AA, and case reports propose these therapies rarely induce AA.28-31
The TH1 response is likewise critical to psoriatic plaque development. IFN-γ and TNF-α are overexpressed in psoriatic plaques.32 IFN-γ has an antiproliferative and differentiation-inducing effect on normal keratinocytes, but psoriatic epithelial cells in vitro respond differently to the cytokine with a notably diminished growth inhibition.33,34 One explanation for the role of IFN-γ is that it stimulates dendritic cells to produce IL-1 and IL-23.35 IL-23 activates TH17 cells36; TH1 and TH17 conditions produce IL-22 whose serum level correlates with disease severity.37-39 IL-22 induces keratinocyte proliferation and migration and inhibits keratinocyte differentiation, helping account for hallmarks of the disease.40 Patients with psoriasis have increased levels of TH1, TH17, and TH22 cells, as well as their associated cytokines, in the skin and blood compared to controls.4,11,32,39,41
Alopecia areata and psoriasis are regulated by complex and still not entirely understood immune interactions. The fact that many of the same therapies are used to treat both diseases emphasizes both their overlapping characteristics and the lack of targeted therapy. It is unclear if and how the topical or systemic therapies used in our patient to treat one disease affected the natural history of the other condition. It is important to highlight, however, that the patient had not been treated for months when he developed the psoriatic plaques with hair regrowth. Other case reports also document hair regrowth in untreated plaques,13,16 making it unlikely to be a side effect of the medication regimen. For both psoriasis and AA, the immune cell composition and cytokine levels in the skin or serum vary throughout a patient’s disease course depending on severity of disease or response to treatment.6,39,42,43 Therefore, we hypothesize that the 2 conditions interact in a similarly distinct manner based on each disease’s stage and intensity in the patient. Both our patient’s course thus far and the various presentations described by other groups support this hypothesis. Our patient had a small region of psoriasis on the scalp that cleared without any terminal hair growth. He also had larger plaques on the forearms that developed hair growth most predominantly within the thicker regions of the plaques. His unique presentation highlights the fluidity of the immune factors driving psoriasis vulgaris and AA.
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Wolff K, Johnson RA. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York, NY: McGraw-Hill; 2009.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Ghoreishi M, Martinka M, Dutz JP. Type 1 interferon signature in the scalp lesions of alopecia areata. Br J Dermatol. 2010;163:57-62.
- Rossi A, Cantisani C, Carlesimo M, et al. Serum concentrations of IL-2, IL-6, IL-12 and TNF-α in patients with alopecia areata. Int J Immunopathol Pharmacol. 2012;25:781-788.
- Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515-521.
- Reich K, Garbe C, Blaschke V, et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation. J Invest Dermatol. 2001;116:319-329.
- Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645-649.
- Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648-651.
- Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407-415.
- Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022-1030.e395.
- Happle R, van der Steen PHM, Perret CM. The Renbök phenomenon: an inverse Köebner reaction observed in alopecia areata. Eur J Dermatol. 1991;2:39-40.
- Ito T, Hashizume H, Takigawa M. Contact immunotherapy-induced Renbök phenomenon in a patient with alopecia areata and psoriasis vulgaris. Eur J Dermatol. 2010;20:126-127.
- Criado PR, Valente NY, Michalany NS, et al. An unusual association between scalp psoriasis and ophiasic alopecia areata: the Renbök phenomenon. Clin Exp Dermatol. 2007;32:320-321.
- Harris JE, Seykora JT, Lee RA. Renbök phenomenon and contact sensitization in a patient with alopecia universalis. Arch Dermatol. 2010;146:422-425.
- Alkhalifah A. Topical and intralesional therapies for alopecia areata. Dermatol Ther. 2011;24:355-363.
- Herbst V, Zöller M, Kissling S, et al. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16:537-542.
- Zöller M, Freyschmidt-Paul P, Vitacolonna M, et al. Chronic delayed-type hypersensitivity reaction as a means to treat alopecia areata. Clin Exp Immunol. 2004;135:398-408.
- Bröcker EB, Echternacht-Happle K, Hamm H, et al. Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest Dermatol. 1987;88:564-568.
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11:216-223.
- Perret C, Wiesner-Menzel L, Happle R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venereol. 1984;64:26-30.
- Wiesner-Menzel L, Happle R. Intrabulbar and peribulbar accumulation of dendritic OKT 6-positive cells in alopecia areata. Arch Dermatol Res. 1984;276:333-334.
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8+ cells induces localized hair loss whereas CD4+/CD25– cells promote systemic alopecia areata and CD4+/CD25+ cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947-957.
- Arca E, Muşabak U, Akar A, et al. Interferon-gamma in alopecia areata. Eur J Dermatol. 2004;14:33-36.
- Hoffmann R. The potential role of cytokines and T cells in alopecia areata. J Investig Dermatol Symp Proc. 1999;4:235-238.
- Philpott MP, Sanders DA, Bowen J, et al. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135:942-948.
- Le Bidre E, Chaby G, Martin L, et al. Alopecia areata during anti-TNF alpha therapy: nine cases. Ann Dermatol Venereol. 2011;138:285-293.
- Ferran M, Calvet J, Almirall M, et al. Alopecia areata as another immune-mediated disease developed in patients treated with tumour necrosis factor-α blocker agents: report of five cases and review of the literature. J Eur Acad Dermatol Venereol. 2011;25:479-484.
- Pan Y, Rao NA. Alopecia areata during etanercept therapy. Ocul Immunol Inflamm. 2009;17:127-129.
- Pelivani N, Hassan AS, Braathen LR, et al. Alopecia areata universalis elicited during treatment with adalimumab. Dermatology. 2008;216:320-323.
- Uyemura K, Yamamura M, Fivenson DF, et al. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701-705.
- Baker BS, Powles AV, Valdimarsson H, et al. An altered response by psoriatic keratinocytes to gamma interferon. Scan J Immunol. 1988;28:735-740.
- Jackson M, Howie SE, Weller R, et al. Psoriatic keratinocytes show reduced IRF-1 and STAT-1alpha activation in response to gamma-IFN. FASEB J. 1999;13:495-502.
- Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385-422.
- McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314-324.
- Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650-657.
- Boniface K, Blumenschein WM, Brovont-Porth K, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679-687.
- Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
- Boniface K, Bernard FX, Garcia M, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695-3702.
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183.
- Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699-711.
- Hoffmann R, Wenzel E, Huth A, et al. Cytokine mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994;103:530-533.
To the Editor:
Both alopecia areata (AA) and psoriasis vulgaris are chronic relapsing autoimmune diseases, with AA causing nonscarring hair loss in approximately 0.1% to 0.2%1 of the population with a lifetime risk of 1.7%,2 and psoriasis more broadly impacting 1.5% to 2% of the population.3 The helper T cell (TH1) cytokine milieu is pathogenic in both conditions.4-6 IFN-γ knockout mice, unlike their wild-type counterparts, do not exhibit AA.7 Psoriasis is notably improved by IL-10 injections, which dampen the TH1 response.8 Distinct from AA, TH17 and TH22 cells have been implicated as key players in psoriasis pathogenesis, along with the associated IL-17 and IL-22 cytokines.9-12
Few cases of patients with concurrent AA and psoriasis have been described. Interestingly, these cases document normal hair regrowth in the areas of psoriasis.13-16 These cases may offer unique insight into the immune factors driving each disease. We describe a case of a man with both alopecia universalis (AU) and psoriasis who developed hair regrowth in some of the psoriatic plaques.
A 34-year-old man with concurrent AU and psoriasis who had not used any systemic or topical medication for either condition in the last year presented to our clinic seeking treatment. The patient had a history of alopecia totalis as a toddler that completely resolved by 4 years of age with the use of squaric acid dibutylester (SADBE). At 31 years of age, the alopecia recurred and was localized to the scalp. It was partially responsive to intralesional triamcinolone acetonide. The patient’s alopecia worsened over the 2 years following recurrence, ultimately progressing to AU. Two months after the alopecia recurrence, he developed the first psoriatic plaques. As the plaque psoriasis progressed, systemic therapy was initiated, first methotrexate and then etanercept. Shortly after developing AU, he lost his health insurance and discontinued all therapy. The patient’s psoriasis began to recur approximately 3 months after stopping etanercept. He was not using any other psoriasis medications. At that time, he noted terminal hair regrowth within some of the psoriatic plaques. No terminal hairs grew outside of the psoriatic plaques, and all regions with growth had previously been without hair for an extended period of time. The patient presented to our clinic approximately 1 year later. He had no other medical conditions and no relevant family history.
On initial physical examination, he had nonscarring hair loss involving nearly 100% of the body with psoriatic plaques on approximately 30% of the body surface area. Regions of terminal hair growth were confined to some but not all of the psoriatic plaques (Figure). Interestingly, the terminal hairs were primarily localized to the thickest central regions of the plaques. The patient’s psoriasis was treated with a combination of topical clobetasol and calcipotriene. In addition, he was started on tacrolimus ointment to the face and eyebrows for the AA. Maintenance of terminal hair within a region of topically treated psoriasis on the forearm persisted at the 2-month follow-up despite complete clearance of the corresponding psoriatic plaque. A small psoriatic plaque on the scalp cleared early with topical therapy without noticeable hair regrowth. The patient subsequently was started on contact immunotherapy with SADBE and intralesional triamcinolone acetonide for the scalp alopecia without satisfactory response. He decided to discontinue further attempts at treating the alopecia and requested to be restarted on etanercept therapy for recalcitrant psoriatic plaques. His psoriasis responded well to this therapy and he continues to be followed in our psoriasis clinic. One year after clearance of the treated psoriatic plaques, the corresponding terminal hairs persist.

Contact immunotherapy, most commonly with diphenylcyclopropenone or SADBE, is reported to have a 50% to 60% success rate in extensive AA, with a broad range of 9% to 87%17; however, randomized controlled trials testing the efficacy of contact immunotherapy are lacking. Although the mechanism of action of these topical sensitizers is not clearly delineated, it has been postulated that by inducing a new type of inflammatory response in the region, the immunologic milieu is changed, allowing the hair to grow. Some proposed mechanisms include promoting perifollicular lymphocyte apoptosis, preventing new recruitment of autoreactive lymphocytes, and allowing for the correction of aberrant major histocompatibility complex expression on the hair matrix epithelium to regain follicle immune privilege.18-20
Iatrogenic immunotherapy may work analogously to the natural immune system deviation demonstrated in our patient. Psoriasis and AA are believed to form competing immune cells and cytokine milieus, thus explaining how an individual with AA could regain normal hair growth in areas of psoriasis.15,16 The Renbök phenomenon, or reverse Köbner phenomenon, coined by Happle et al13 can be used to describe both the iatrogenic and natural cases of dermatologic disease improvement in response to secondary insults.14
A complex cascade of immune cells and cytokines coordinate AA pathogenesis. In the acute stage of AA, an inflammatory infiltrate of CD4+ T cells, CD8+ T cells, and antigen-presenting cells target anagen phase follicles, with a higher CD4+:CD8+ ratio in clinically active disease.21-23 Subcutaneous injections of either CD4+ or CD8+ lymphocyte subsets from mice with AA into normal-haired mice induces disease. However, CD8+ T cell injections rapidly produce apparent hair loss, whereas CD4+ T cells cause hair loss after several weeks, suggesting that CD8+ T cells directly modulate AA hair loss and CD4+ T cells act as an aide.24 The growth, differentiation, and survival of CD8+ T cells are stimulated by IL-2 and IFN-γ. Alopecia areata biopsies demonstrate a prevalence of TH1 cytokines, and patients with localized AA, alopecia totalis, and AU have notably higher serum IFN-γ levels compared to controls.25 In murine models, IL-1α and IL-1β increase during the catagen phase of the hair cycle and peak during the telogen phase.26 Excessive IL-1β expression is detected in the early stages of human disease, and certain IL-1β polymorphisms are associated with severe forms of AA.26 The role of tumor necrosis factor (TNF) α in AA is not well understood. In vitro studies show it inhibits hair growth, suggesting the cytokine may play a role in AA.27 However, anti–TNF-α therapy is not effective in AA, and case reports propose these therapies rarely induce AA.28-31
The TH1 response is likewise critical to psoriatic plaque development. IFN-γ and TNF-α are overexpressed in psoriatic plaques.32 IFN-γ has an antiproliferative and differentiation-inducing effect on normal keratinocytes, but psoriatic epithelial cells in vitro respond differently to the cytokine with a notably diminished growth inhibition.33,34 One explanation for the role of IFN-γ is that it stimulates dendritic cells to produce IL-1 and IL-23.35 IL-23 activates TH17 cells36; TH1 and TH17 conditions produce IL-22 whose serum level correlates with disease severity.37-39 IL-22 induces keratinocyte proliferation and migration and inhibits keratinocyte differentiation, helping account for hallmarks of the disease.40 Patients with psoriasis have increased levels of TH1, TH17, and TH22 cells, as well as their associated cytokines, in the skin and blood compared to controls.4,11,32,39,41
Alopecia areata and psoriasis are regulated by complex and still not entirely understood immune interactions. The fact that many of the same therapies are used to treat both diseases emphasizes both their overlapping characteristics and the lack of targeted therapy. It is unclear if and how the topical or systemic therapies used in our patient to treat one disease affected the natural history of the other condition. It is important to highlight, however, that the patient had not been treated for months when he developed the psoriatic plaques with hair regrowth. Other case reports also document hair regrowth in untreated plaques,13,16 making it unlikely to be a side effect of the medication regimen. For both psoriasis and AA, the immune cell composition and cytokine levels in the skin or serum vary throughout a patient’s disease course depending on severity of disease or response to treatment.6,39,42,43 Therefore, we hypothesize that the 2 conditions interact in a similarly distinct manner based on each disease’s stage and intensity in the patient. Both our patient’s course thus far and the various presentations described by other groups support this hypothesis. Our patient had a small region of psoriasis on the scalp that cleared without any terminal hair growth. He also had larger plaques on the forearms that developed hair growth most predominantly within the thicker regions of the plaques. His unique presentation highlights the fluidity of the immune factors driving psoriasis vulgaris and AA.
To the Editor:
Both alopecia areata (AA) and psoriasis vulgaris are chronic relapsing autoimmune diseases, with AA causing nonscarring hair loss in approximately 0.1% to 0.2%1 of the population with a lifetime risk of 1.7%,2 and psoriasis more broadly impacting 1.5% to 2% of the population.3 The helper T cell (TH1) cytokine milieu is pathogenic in both conditions.4-6 IFN-γ knockout mice, unlike their wild-type counterparts, do not exhibit AA.7 Psoriasis is notably improved by IL-10 injections, which dampen the TH1 response.8 Distinct from AA, TH17 and TH22 cells have been implicated as key players in psoriasis pathogenesis, along with the associated IL-17 and IL-22 cytokines.9-12
Few cases of patients with concurrent AA and psoriasis have been described. Interestingly, these cases document normal hair regrowth in the areas of psoriasis.13-16 These cases may offer unique insight into the immune factors driving each disease. We describe a case of a man with both alopecia universalis (AU) and psoriasis who developed hair regrowth in some of the psoriatic plaques.
A 34-year-old man with concurrent AU and psoriasis who had not used any systemic or topical medication for either condition in the last year presented to our clinic seeking treatment. The patient had a history of alopecia totalis as a toddler that completely resolved by 4 years of age with the use of squaric acid dibutylester (SADBE). At 31 years of age, the alopecia recurred and was localized to the scalp. It was partially responsive to intralesional triamcinolone acetonide. The patient’s alopecia worsened over the 2 years following recurrence, ultimately progressing to AU. Two months after the alopecia recurrence, he developed the first psoriatic plaques. As the plaque psoriasis progressed, systemic therapy was initiated, first methotrexate and then etanercept. Shortly after developing AU, he lost his health insurance and discontinued all therapy. The patient’s psoriasis began to recur approximately 3 months after stopping etanercept. He was not using any other psoriasis medications. At that time, he noted terminal hair regrowth within some of the psoriatic plaques. No terminal hairs grew outside of the psoriatic plaques, and all regions with growth had previously been without hair for an extended period of time. The patient presented to our clinic approximately 1 year later. He had no other medical conditions and no relevant family history.
On initial physical examination, he had nonscarring hair loss involving nearly 100% of the body with psoriatic plaques on approximately 30% of the body surface area. Regions of terminal hair growth were confined to some but not all of the psoriatic plaques (Figure). Interestingly, the terminal hairs were primarily localized to the thickest central regions of the plaques. The patient’s psoriasis was treated with a combination of topical clobetasol and calcipotriene. In addition, he was started on tacrolimus ointment to the face and eyebrows for the AA. Maintenance of terminal hair within a region of topically treated psoriasis on the forearm persisted at the 2-month follow-up despite complete clearance of the corresponding psoriatic plaque. A small psoriatic plaque on the scalp cleared early with topical therapy without noticeable hair regrowth. The patient subsequently was started on contact immunotherapy with SADBE and intralesional triamcinolone acetonide for the scalp alopecia without satisfactory response. He decided to discontinue further attempts at treating the alopecia and requested to be restarted on etanercept therapy for recalcitrant psoriatic plaques. His psoriasis responded well to this therapy and he continues to be followed in our psoriasis clinic. One year after clearance of the treated psoriatic plaques, the corresponding terminal hairs persist.

Contact immunotherapy, most commonly with diphenylcyclopropenone or SADBE, is reported to have a 50% to 60% success rate in extensive AA, with a broad range of 9% to 87%17; however, randomized controlled trials testing the efficacy of contact immunotherapy are lacking. Although the mechanism of action of these topical sensitizers is not clearly delineated, it has been postulated that by inducing a new type of inflammatory response in the region, the immunologic milieu is changed, allowing the hair to grow. Some proposed mechanisms include promoting perifollicular lymphocyte apoptosis, preventing new recruitment of autoreactive lymphocytes, and allowing for the correction of aberrant major histocompatibility complex expression on the hair matrix epithelium to regain follicle immune privilege.18-20
Iatrogenic immunotherapy may work analogously to the natural immune system deviation demonstrated in our patient. Psoriasis and AA are believed to form competing immune cells and cytokine milieus, thus explaining how an individual with AA could regain normal hair growth in areas of psoriasis.15,16 The Renbök phenomenon, or reverse Köbner phenomenon, coined by Happle et al13 can be used to describe both the iatrogenic and natural cases of dermatologic disease improvement in response to secondary insults.14
A complex cascade of immune cells and cytokines coordinate AA pathogenesis. In the acute stage of AA, an inflammatory infiltrate of CD4+ T cells, CD8+ T cells, and antigen-presenting cells target anagen phase follicles, with a higher CD4+:CD8+ ratio in clinically active disease.21-23 Subcutaneous injections of either CD4+ or CD8+ lymphocyte subsets from mice with AA into normal-haired mice induces disease. However, CD8+ T cell injections rapidly produce apparent hair loss, whereas CD4+ T cells cause hair loss after several weeks, suggesting that CD8+ T cells directly modulate AA hair loss and CD4+ T cells act as an aide.24 The growth, differentiation, and survival of CD8+ T cells are stimulated by IL-2 and IFN-γ. Alopecia areata biopsies demonstrate a prevalence of TH1 cytokines, and patients with localized AA, alopecia totalis, and AU have notably higher serum IFN-γ levels compared to controls.25 In murine models, IL-1α and IL-1β increase during the catagen phase of the hair cycle and peak during the telogen phase.26 Excessive IL-1β expression is detected in the early stages of human disease, and certain IL-1β polymorphisms are associated with severe forms of AA.26 The role of tumor necrosis factor (TNF) α in AA is not well understood. In vitro studies show it inhibits hair growth, suggesting the cytokine may play a role in AA.27 However, anti–TNF-α therapy is not effective in AA, and case reports propose these therapies rarely induce AA.28-31
The TH1 response is likewise critical to psoriatic plaque development. IFN-γ and TNF-α are overexpressed in psoriatic plaques.32 IFN-γ has an antiproliferative and differentiation-inducing effect on normal keratinocytes, but psoriatic epithelial cells in vitro respond differently to the cytokine with a notably diminished growth inhibition.33,34 One explanation for the role of IFN-γ is that it stimulates dendritic cells to produce IL-1 and IL-23.35 IL-23 activates TH17 cells36; TH1 and TH17 conditions produce IL-22 whose serum level correlates with disease severity.37-39 IL-22 induces keratinocyte proliferation and migration and inhibits keratinocyte differentiation, helping account for hallmarks of the disease.40 Patients with psoriasis have increased levels of TH1, TH17, and TH22 cells, as well as their associated cytokines, in the skin and blood compared to controls.4,11,32,39,41
Alopecia areata and psoriasis are regulated by complex and still not entirely understood immune interactions. The fact that many of the same therapies are used to treat both diseases emphasizes both their overlapping characteristics and the lack of targeted therapy. It is unclear if and how the topical or systemic therapies used in our patient to treat one disease affected the natural history of the other condition. It is important to highlight, however, that the patient had not been treated for months when he developed the psoriatic plaques with hair regrowth. Other case reports also document hair regrowth in untreated plaques,13,16 making it unlikely to be a side effect of the medication regimen. For both psoriasis and AA, the immune cell composition and cytokine levels in the skin or serum vary throughout a patient’s disease course depending on severity of disease or response to treatment.6,39,42,43 Therefore, we hypothesize that the 2 conditions interact in a similarly distinct manner based on each disease’s stage and intensity in the patient. Both our patient’s course thus far and the various presentations described by other groups support this hypothesis. Our patient had a small region of psoriasis on the scalp that cleared without any terminal hair growth. He also had larger plaques on the forearms that developed hair growth most predominantly within the thicker regions of the plaques. His unique presentation highlights the fluidity of the immune factors driving psoriasis vulgaris and AA.
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Wolff K, Johnson RA. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York, NY: McGraw-Hill; 2009.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Ghoreishi M, Martinka M, Dutz JP. Type 1 interferon signature in the scalp lesions of alopecia areata. Br J Dermatol. 2010;163:57-62.
- Rossi A, Cantisani C, Carlesimo M, et al. Serum concentrations of IL-2, IL-6, IL-12 and TNF-α in patients with alopecia areata. Int J Immunopathol Pharmacol. 2012;25:781-788.
- Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515-521.
- Reich K, Garbe C, Blaschke V, et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation. J Invest Dermatol. 2001;116:319-329.
- Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645-649.
- Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648-651.
- Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407-415.
- Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022-1030.e395.
- Happle R, van der Steen PHM, Perret CM. The Renbök phenomenon: an inverse Köebner reaction observed in alopecia areata. Eur J Dermatol. 1991;2:39-40.
- Ito T, Hashizume H, Takigawa M. Contact immunotherapy-induced Renbök phenomenon in a patient with alopecia areata and psoriasis vulgaris. Eur J Dermatol. 2010;20:126-127.
- Criado PR, Valente NY, Michalany NS, et al. An unusual association between scalp psoriasis and ophiasic alopecia areata: the Renbök phenomenon. Clin Exp Dermatol. 2007;32:320-321.
- Harris JE, Seykora JT, Lee RA. Renbök phenomenon and contact sensitization in a patient with alopecia universalis. Arch Dermatol. 2010;146:422-425.
- Alkhalifah A. Topical and intralesional therapies for alopecia areata. Dermatol Ther. 2011;24:355-363.
- Herbst V, Zöller M, Kissling S, et al. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16:537-542.
- Zöller M, Freyschmidt-Paul P, Vitacolonna M, et al. Chronic delayed-type hypersensitivity reaction as a means to treat alopecia areata. Clin Exp Immunol. 2004;135:398-408.
- Bröcker EB, Echternacht-Happle K, Hamm H, et al. Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest Dermatol. 1987;88:564-568.
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11:216-223.
- Perret C, Wiesner-Menzel L, Happle R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venereol. 1984;64:26-30.
- Wiesner-Menzel L, Happle R. Intrabulbar and peribulbar accumulation of dendritic OKT 6-positive cells in alopecia areata. Arch Dermatol Res. 1984;276:333-334.
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8+ cells induces localized hair loss whereas CD4+/CD25– cells promote systemic alopecia areata and CD4+/CD25+ cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947-957.
- Arca E, Muşabak U, Akar A, et al. Interferon-gamma in alopecia areata. Eur J Dermatol. 2004;14:33-36.
- Hoffmann R. The potential role of cytokines and T cells in alopecia areata. J Investig Dermatol Symp Proc. 1999;4:235-238.
- Philpott MP, Sanders DA, Bowen J, et al. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135:942-948.
- Le Bidre E, Chaby G, Martin L, et al. Alopecia areata during anti-TNF alpha therapy: nine cases. Ann Dermatol Venereol. 2011;138:285-293.
- Ferran M, Calvet J, Almirall M, et al. Alopecia areata as another immune-mediated disease developed in patients treated with tumour necrosis factor-α blocker agents: report of five cases and review of the literature. J Eur Acad Dermatol Venereol. 2011;25:479-484.
- Pan Y, Rao NA. Alopecia areata during etanercept therapy. Ocul Immunol Inflamm. 2009;17:127-129.
- Pelivani N, Hassan AS, Braathen LR, et al. Alopecia areata universalis elicited during treatment with adalimumab. Dermatology. 2008;216:320-323.
- Uyemura K, Yamamura M, Fivenson DF, et al. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701-705.
- Baker BS, Powles AV, Valdimarsson H, et al. An altered response by psoriatic keratinocytes to gamma interferon. Scan J Immunol. 1988;28:735-740.
- Jackson M, Howie SE, Weller R, et al. Psoriatic keratinocytes show reduced IRF-1 and STAT-1alpha activation in response to gamma-IFN. FASEB J. 1999;13:495-502.
- Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385-422.
- McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314-324.
- Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650-657.
- Boniface K, Blumenschein WM, Brovont-Porth K, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679-687.
- Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
- Boniface K, Bernard FX, Garcia M, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695-3702.
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183.
- Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699-711.
- Hoffmann R, Wenzel E, Huth A, et al. Cytokine mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994;103:530-533.
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Wolff K, Johnson RA. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. New York, NY: McGraw-Hill; 2009.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Ghoreishi M, Martinka M, Dutz JP. Type 1 interferon signature in the scalp lesions of alopecia areata. Br J Dermatol. 2010;163:57-62.
- Rossi A, Cantisani C, Carlesimo M, et al. Serum concentrations of IL-2, IL-6, IL-12 and TNF-α in patients with alopecia areata. Int J Immunopathol Pharmacol. 2012;25:781-788.
- Freyschmidt-Paul P, McElwee KJ, Hoffmann R, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515-521.
- Reich K, Garbe C, Blaschke V, et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation. J Invest Dermatol. 2001;116:319-329.
- Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645-649.
- Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648-651.
- Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407-415.
- Zaba LC, Suárez-Fariñas M, Fuentes-Duculan J, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022-1030.e395.
- Happle R, van der Steen PHM, Perret CM. The Renbök phenomenon: an inverse Köebner reaction observed in alopecia areata. Eur J Dermatol. 1991;2:39-40.
- Ito T, Hashizume H, Takigawa M. Contact immunotherapy-induced Renbök phenomenon in a patient with alopecia areata and psoriasis vulgaris. Eur J Dermatol. 2010;20:126-127.
- Criado PR, Valente NY, Michalany NS, et al. An unusual association between scalp psoriasis and ophiasic alopecia areata: the Renbök phenomenon. Clin Exp Dermatol. 2007;32:320-321.
- Harris JE, Seykora JT, Lee RA. Renbök phenomenon and contact sensitization in a patient with alopecia universalis. Arch Dermatol. 2010;146:422-425.
- Alkhalifah A. Topical and intralesional therapies for alopecia areata. Dermatol Ther. 2011;24:355-363.
- Herbst V, Zöller M, Kissling S, et al. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol. 2006;16:537-542.
- Zöller M, Freyschmidt-Paul P, Vitacolonna M, et al. Chronic delayed-type hypersensitivity reaction as a means to treat alopecia areata. Clin Exp Immunol. 2004;135:398-408.
- Bröcker EB, Echternacht-Happle K, Hamm H, et al. Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest Dermatol. 1987;88:564-568.
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11:216-223.
- Perret C, Wiesner-Menzel L, Happle R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venereol. 1984;64:26-30.
- Wiesner-Menzel L, Happle R. Intrabulbar and peribulbar accumulation of dendritic OKT 6-positive cells in alopecia areata. Arch Dermatol Res. 1984;276:333-334.
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, et al. Transfer of CD8+ cells induces localized hair loss whereas CD4+/CD25– cells promote systemic alopecia areata and CD4+/CD25+ cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947-957.
- Arca E, Muşabak U, Akar A, et al. Interferon-gamma in alopecia areata. Eur J Dermatol. 2004;14:33-36.
- Hoffmann R. The potential role of cytokines and T cells in alopecia areata. J Investig Dermatol Symp Proc. 1999;4:235-238.
- Philpott MP, Sanders DA, Bowen J, et al. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135:942-948.
- Le Bidre E, Chaby G, Martin L, et al. Alopecia areata during anti-TNF alpha therapy: nine cases. Ann Dermatol Venereol. 2011;138:285-293.
- Ferran M, Calvet J, Almirall M, et al. Alopecia areata as another immune-mediated disease developed in patients treated with tumour necrosis factor-α blocker agents: report of five cases and review of the literature. J Eur Acad Dermatol Venereol. 2011;25:479-484.
- Pan Y, Rao NA. Alopecia areata during etanercept therapy. Ocul Immunol Inflamm. 2009;17:127-129.
- Pelivani N, Hassan AS, Braathen LR, et al. Alopecia areata universalis elicited during treatment with adalimumab. Dermatology. 2008;216:320-323.
- Uyemura K, Yamamura M, Fivenson DF, et al. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993;101:701-705.
- Baker BS, Powles AV, Valdimarsson H, et al. An altered response by psoriatic keratinocytes to gamma interferon. Scan J Immunol. 1988;28:735-740.
- Jackson M, Howie SE, Weller R, et al. Psoriatic keratinocytes show reduced IRF-1 and STAT-1alpha activation in response to gamma-IFN. FASEB J. 1999;13:495-502.
- Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385-422.
- McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314-324.
- Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650-657.
- Boniface K, Blumenschein WM, Brovont-Porth K, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679-687.
- Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373-1383.
- Boniface K, Bernard FX, Garcia M, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695-3702.
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183.
- Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699-711.
- Hoffmann R, Wenzel E, Huth A, et al. Cytokine mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol. 1994;103:530-533.
Practice Points
- The Renbök phenomenon, or reverse Köbner phenomenon, describes cases where secondary insults improve dermatologic disease.
- Current evidence suggests that alopecia areata (AA) is driven by a helper T cell (TH1) response whereas psoriasis vulgaris is driven by TH1, TH17, and TH22.
- Patients with concurrent AA and psoriasis can develop normal hair regrowth confined to the psoriatic plaques. Developing methods to artificially alter the cytokine milieu in affected skin may lead to new therapeutic options for each condition.
Study finds psoriasis link to melanoma and hematologic cancers
Patients with psoriasis may be at a greater risk of melanoma and hematologic cancers, compared with the general population, but treatments do not appear to increase risk, according to Shivani P. Reddy of the University of Illinois at Chicago, and associates.
In a retrospective cohort study, they identified 815,765 patients at Kaiser Permanente Southern California who had at least one medical encounter from January 2004 through December 2013. Of these patients, 8,161 (1%) met the diagnostic and inclusion criteria for psoriasis, and there were 7,167 (0.89%) cases of melanoma and 5,399 (0.66%) cases of lymphoma or leukemia.
Among the patients with psoriasis, there were 62 (0.87%) melanoma cases and 47 (0.87%) cases of lymphoma or leukemia.
Of the 109 patients with psoriasis who went on to develop melanoma or lymphoma, the time to diagnosis of melanoma or hematologic cancers was significantly less for patients with psoriasis than for patients without psoriasis (P = .01). The patients with psoriasis had a 1.53 times higher risk of developing a malignancy compared with patients without psoriasis (P less than .01) in the multivariable Cox proportional hazards model.
“There were no differences between patients with melanoma and hematologic cancer by treatment type,” which were phototherapy, tumor necrosis factor inhibitor therapy, and topical medications, they wrote.
“Our study demonstrates that the risk of hematologic cancer and melanoma is increased in patients with psoriasis over time, although this risk is not impacted by psoriasis therapies,” the researchers concluded. “Defining the risk of malignancy in these patients is important for proper workup and management.”
Read the study in the Journal of the American Academy of Dermatology (doi: 10.1016/j.jaad.2016.09.047).
Patients with psoriasis may be at a greater risk of melanoma and hematologic cancers, compared with the general population, but treatments do not appear to increase risk, according to Shivani P. Reddy of the University of Illinois at Chicago, and associates.
In a retrospective cohort study, they identified 815,765 patients at Kaiser Permanente Southern California who had at least one medical encounter from January 2004 through December 2013. Of these patients, 8,161 (1%) met the diagnostic and inclusion criteria for psoriasis, and there were 7,167 (0.89%) cases of melanoma and 5,399 (0.66%) cases of lymphoma or leukemia.
Among the patients with psoriasis, there were 62 (0.87%) melanoma cases and 47 (0.87%) cases of lymphoma or leukemia.
Of the 109 patients with psoriasis who went on to develop melanoma or lymphoma, the time to diagnosis of melanoma or hematologic cancers was significantly less for patients with psoriasis than for patients without psoriasis (P = .01). The patients with psoriasis had a 1.53 times higher risk of developing a malignancy compared with patients without psoriasis (P less than .01) in the multivariable Cox proportional hazards model.
“There were no differences between patients with melanoma and hematologic cancer by treatment type,” which were phototherapy, tumor necrosis factor inhibitor therapy, and topical medications, they wrote.
“Our study demonstrates that the risk of hematologic cancer and melanoma is increased in patients with psoriasis over time, although this risk is not impacted by psoriasis therapies,” the researchers concluded. “Defining the risk of malignancy in these patients is important for proper workup and management.”
Read the study in the Journal of the American Academy of Dermatology (doi: 10.1016/j.jaad.2016.09.047).
Patients with psoriasis may be at a greater risk of melanoma and hematologic cancers, compared with the general population, but treatments do not appear to increase risk, according to Shivani P. Reddy of the University of Illinois at Chicago, and associates.
In a retrospective cohort study, they identified 815,765 patients at Kaiser Permanente Southern California who had at least one medical encounter from January 2004 through December 2013. Of these patients, 8,161 (1%) met the diagnostic and inclusion criteria for psoriasis, and there were 7,167 (0.89%) cases of melanoma and 5,399 (0.66%) cases of lymphoma or leukemia.
Among the patients with psoriasis, there were 62 (0.87%) melanoma cases and 47 (0.87%) cases of lymphoma or leukemia.
Of the 109 patients with psoriasis who went on to develop melanoma or lymphoma, the time to diagnosis of melanoma or hematologic cancers was significantly less for patients with psoriasis than for patients without psoriasis (P = .01). The patients with psoriasis had a 1.53 times higher risk of developing a malignancy compared with patients without psoriasis (P less than .01) in the multivariable Cox proportional hazards model.
“There were no differences between patients with melanoma and hematologic cancer by treatment type,” which were phototherapy, tumor necrosis factor inhibitor therapy, and topical medications, they wrote.
“Our study demonstrates that the risk of hematologic cancer and melanoma is increased in patients with psoriasis over time, although this risk is not impacted by psoriasis therapies,” the researchers concluded. “Defining the risk of malignancy in these patients is important for proper workup and management.”
Read the study in the Journal of the American Academy of Dermatology (doi: 10.1016/j.jaad.2016.09.047).
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Risankizumab tops ustekinumab in phase II psoriasis trial
At 12 weeks, 77% of psoriasis patients treated with risankizumab showed a 90% or greater reduction in Psoriasis Area and Severity Index (PASI) score, compared with 40% of ustekinumab patients, in a phase II randomized trial.
The results were published April 19 in the New England Journal of Medicine.
Ustekinumab (Stelara), approved by the Food and Drug Administration in 2009, blocks interleukin-12 and interleukin-23 and has demonstrated effectiveness in psoriasis patients. However, the humanized IgG1 monoclonal antibody risankizumab goes farther and “selectively inhibits interleukin-23 by specifically targeting p19,” wrote Kim A. Papp, MD, PhD, of K. Papp Clinical Research and Probity Medical Research, Waterloo, Ont., and associates (N. Engl. J. Med. 2017;376:1551-60. doi: 10.1056/NEJMoa1607017).
To compare clinical response and safety, the researchers enrolled 166 adults aged 18-75 years with moderate to severe plaque psoriasis, in the phase II study. Patients were randomized to subcutaneous injections of risankizumab at one of three doses, or ustekinumab at one of two doses. Risankizumab patients received a single 18-mg dose at week 0, or 90-mg or 180-mg doses at weeks 0, 4, and 16. Ustekinumab patients weighing 100 kg or less received 45 mg at weeks 0, 4, and 16; those weighing more than 100 kg received 90 mg at weeks 0, 4, and 16. Demographics were similar among the treatment groups.
The primary end point was a 90% or greater reduction in the PASI score at week 12, compared with baseline.
In pooled results of the risankizumab 90-mg and 180-mg groups, 77% of patients achieved a PASI 90 at 12 weeks (73% of the 90-mg group and 81% of the 180-mg group), vs. 40% of ustekinumab patients (P less than .001). Complete clearance of lesions (PASI 100) occurred among risankizumab patients in 14% of the 18-mg group, 41% of the 90-mg group, and 48% of the 180-mg group, compared with 18% of the ustekinumab group.
Among risankizumab patients, the rates of adverse events through 48 weeks were 81% in the 180-mg group, 80% in the 90-mg group, and 69% in the 180-mg group, compared with 72% in those on ustekinumab, with nasopharyngitis the most commonly reported adverse event in all the treatment groups. The rates of serious adverse events were 12% and 15% among those in the 18-mg and 90-mg risankizumab groups, respectively; 0% among those on the 180-mg dose, and 8% among those on ustekinumab.
The study was not large or long enough to provide conclusive safety data on risankizumab, and additional studies are needed to review psoriasis lesions over a longer time period and include both placebo and active comparators, researchers noted. However, the results suggest that “the selective blockade of interleukin-23 through the inhibition of the p19 subunit rather than p40 provides a more complete inhibition of interleukin-23 activity, potentially resulting in a greater efficacy in the treatment of plaque psoriasis at the doses used,” they said.
The study was supported by Boehringer Ingelheim. Several study coauthors, including lead author Dr. Papp, disclosed relationships with Boehringer Ingelheim and other companies. Several authors are Boehringer Ingelheim employees.
At 12 weeks, 77% of psoriasis patients treated with risankizumab showed a 90% or greater reduction in Psoriasis Area and Severity Index (PASI) score, compared with 40% of ustekinumab patients, in a phase II randomized trial.
The results were published April 19 in the New England Journal of Medicine.
Ustekinumab (Stelara), approved by the Food and Drug Administration in 2009, blocks interleukin-12 and interleukin-23 and has demonstrated effectiveness in psoriasis patients. However, the humanized IgG1 monoclonal antibody risankizumab goes farther and “selectively inhibits interleukin-23 by specifically targeting p19,” wrote Kim A. Papp, MD, PhD, of K. Papp Clinical Research and Probity Medical Research, Waterloo, Ont., and associates (N. Engl. J. Med. 2017;376:1551-60. doi: 10.1056/NEJMoa1607017).
To compare clinical response and safety, the researchers enrolled 166 adults aged 18-75 years with moderate to severe plaque psoriasis, in the phase II study. Patients were randomized to subcutaneous injections of risankizumab at one of three doses, or ustekinumab at one of two doses. Risankizumab patients received a single 18-mg dose at week 0, or 90-mg or 180-mg doses at weeks 0, 4, and 16. Ustekinumab patients weighing 100 kg or less received 45 mg at weeks 0, 4, and 16; those weighing more than 100 kg received 90 mg at weeks 0, 4, and 16. Demographics were similar among the treatment groups.
The primary end point was a 90% or greater reduction in the PASI score at week 12, compared with baseline.
In pooled results of the risankizumab 90-mg and 180-mg groups, 77% of patients achieved a PASI 90 at 12 weeks (73% of the 90-mg group and 81% of the 180-mg group), vs. 40% of ustekinumab patients (P less than .001). Complete clearance of lesions (PASI 100) occurred among risankizumab patients in 14% of the 18-mg group, 41% of the 90-mg group, and 48% of the 180-mg group, compared with 18% of the ustekinumab group.
Among risankizumab patients, the rates of adverse events through 48 weeks were 81% in the 180-mg group, 80% in the 90-mg group, and 69% in the 180-mg group, compared with 72% in those on ustekinumab, with nasopharyngitis the most commonly reported adverse event in all the treatment groups. The rates of serious adverse events were 12% and 15% among those in the 18-mg and 90-mg risankizumab groups, respectively; 0% among those on the 180-mg dose, and 8% among those on ustekinumab.
The study was not large or long enough to provide conclusive safety data on risankizumab, and additional studies are needed to review psoriasis lesions over a longer time period and include both placebo and active comparators, researchers noted. However, the results suggest that “the selective blockade of interleukin-23 through the inhibition of the p19 subunit rather than p40 provides a more complete inhibition of interleukin-23 activity, potentially resulting in a greater efficacy in the treatment of plaque psoriasis at the doses used,” they said.
The study was supported by Boehringer Ingelheim. Several study coauthors, including lead author Dr. Papp, disclosed relationships with Boehringer Ingelheim and other companies. Several authors are Boehringer Ingelheim employees.
At 12 weeks, 77% of psoriasis patients treated with risankizumab showed a 90% or greater reduction in Psoriasis Area and Severity Index (PASI) score, compared with 40% of ustekinumab patients, in a phase II randomized trial.
The results were published April 19 in the New England Journal of Medicine.
Ustekinumab (Stelara), approved by the Food and Drug Administration in 2009, blocks interleukin-12 and interleukin-23 and has demonstrated effectiveness in psoriasis patients. However, the humanized IgG1 monoclonal antibody risankizumab goes farther and “selectively inhibits interleukin-23 by specifically targeting p19,” wrote Kim A. Papp, MD, PhD, of K. Papp Clinical Research and Probity Medical Research, Waterloo, Ont., and associates (N. Engl. J. Med. 2017;376:1551-60. doi: 10.1056/NEJMoa1607017).
To compare clinical response and safety, the researchers enrolled 166 adults aged 18-75 years with moderate to severe plaque psoriasis, in the phase II study. Patients were randomized to subcutaneous injections of risankizumab at one of three doses, or ustekinumab at one of two doses. Risankizumab patients received a single 18-mg dose at week 0, or 90-mg or 180-mg doses at weeks 0, 4, and 16. Ustekinumab patients weighing 100 kg or less received 45 mg at weeks 0, 4, and 16; those weighing more than 100 kg received 90 mg at weeks 0, 4, and 16. Demographics were similar among the treatment groups.
The primary end point was a 90% or greater reduction in the PASI score at week 12, compared with baseline.
In pooled results of the risankizumab 90-mg and 180-mg groups, 77% of patients achieved a PASI 90 at 12 weeks (73% of the 90-mg group and 81% of the 180-mg group), vs. 40% of ustekinumab patients (P less than .001). Complete clearance of lesions (PASI 100) occurred among risankizumab patients in 14% of the 18-mg group, 41% of the 90-mg group, and 48% of the 180-mg group, compared with 18% of the ustekinumab group.
Among risankizumab patients, the rates of adverse events through 48 weeks were 81% in the 180-mg group, 80% in the 90-mg group, and 69% in the 180-mg group, compared with 72% in those on ustekinumab, with nasopharyngitis the most commonly reported adverse event in all the treatment groups. The rates of serious adverse events were 12% and 15% among those in the 18-mg and 90-mg risankizumab groups, respectively; 0% among those on the 180-mg dose, and 8% among those on ustekinumab.
The study was not large or long enough to provide conclusive safety data on risankizumab, and additional studies are needed to review psoriasis lesions over a longer time period and include both placebo and active comparators, researchers noted. However, the results suggest that “the selective blockade of interleukin-23 through the inhibition of the p19 subunit rather than p40 provides a more complete inhibition of interleukin-23 activity, potentially resulting in a greater efficacy in the treatment of plaque psoriasis at the doses used,” they said.
The study was supported by Boehringer Ingelheim. Several study coauthors, including lead author Dr. Papp, disclosed relationships with Boehringer Ingelheim and other companies. Several authors are Boehringer Ingelheim employees.
Key clinical point: Clinical responses in psoriasis patients treated with risankizumab were superior to responses in patients treated with ustekinumab.
Major finding: At 12 weeks, 77% of risankizumab patients showed a 90% or greater reduction in PASI score, compared with 40% of ustekinumab patients.
Data source: A phase II randomized trial of 166 adults with moderate to severe plaque psoriasis.
Disclosures: The study was supported by Boehringer Ingelheim. Several study coauthors, including lead author Dr. Papp, disclosed relationships with Boehringer Ingelheim and other companies; several were employees of Boehringer.