User login
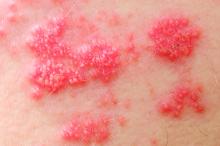
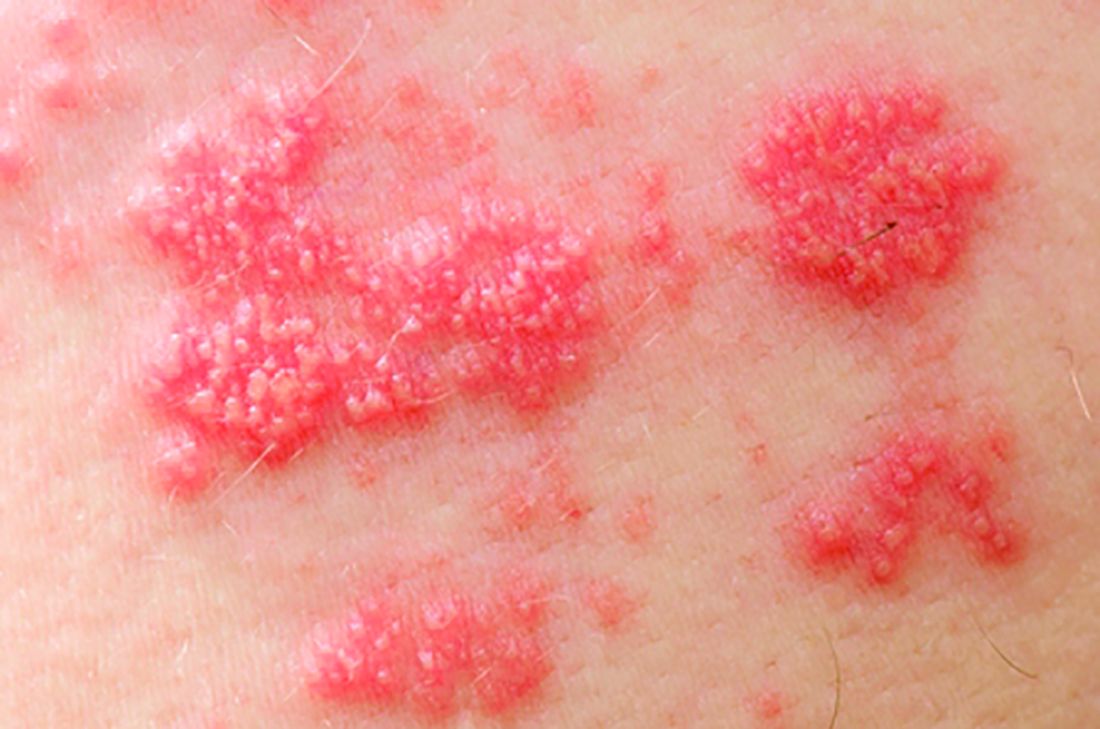
Secukinumab beat etanercept in 52-week psoriasis quality of life analysis
Compared with etanercept, secukinumab was associated with significantly faster and greater improvements in skin-specific quality of life among adults with moderate to severe psoriasis, according to a pooled analysis of data from the randomized, multicenter phase III ERASURE and FIXTURE trials.
The findings confirm the clinical superiority of secukinumab and extend prior head-to-head comparisons of this IL-17A inhibitor and etanercept, said Bruce E. Strober, MD, PhD, of the University of Connecticut in Farmington, Conn.
ERASURE and FIXTURE compared secukinumab (weekly for 4 weeks, then once-monthly) with etanercept (twice weekly for 12 weeks, then once-weekly) and placebo. Both secukinumab doses outperformed etanercept and placebo based on coprimary endpoints of PASI 75 and the proportion of patients who were clear or almost clear on a 5-point modified investigator’s global assessment (N Engl J Med. 2014 Jul 24;371[4]:326-38). A subsequent analysis showed that the clinical superiority of secukinumab held up at 52 weeks (J Dermatolog Treat. 2016;27[1]:1).
Because health-related quality of life is of increasing interest in psoriasis, Dr. Strober and his associates analyzed ERASURE and FIXTURE data on the Dermatology Life Quality Index (DLQI), a 10-item assessment of daily activities, leisure, personal relationships, symptoms and feelings, treatment, and work or school. Their analysis included 572 patients who received 300 mg secukinumab, 572 patients who received 150 mg secukinumab, and 326 patients who received 50 mg etanercept.
Baseline DLQI scores were similar among treatment groups. By week 4, significantly more secukinumab than etanercept recipients reported DLQI scores of 0 or 1 (DLQI 0/1), indicating that psoriasis was not impairing their quality of life (P < .05). This difference remained significant through week 52 at the 300-mg dose. The 150-mg dose significantly outperformed etanercept by week 8, and the difference remained significant through week 48.
In all, 81% of patients who received 300 mg secukinumab reported DLQI 0/1 at week 24, compared with 71% of those who received 150 mg secukinumab and 55% of those who received etanercept (P < .001 for each comparison with etanercept).
Among patients with complete data available, 86% of 300-mg secukinumab recipients achieved DLQI 0/1 at week 24, maintained this response through 52 weeks, and achieved a 90%-100% reduction in PASI total score at week 24. That was true of only 75% of etanercept recipients (P = .02, compared with 300 mg secukinumab) and 79% of 150-mg secukinumab recipients (P = .4, compared with etanercept).
In an adjusted model, patients who received 300 mg secukinumab were 1.9 times more likely to reach DLQI 0/1 than those who received etanercept (95% confidence interval, 1.6-2.3). Median time to response to both doses of secukinumab was 12 weeks, versus 24 weeks for etanercept (P < .0001).
This analysis offers “a robust, longer-term head-to-head comparison” of how secukinumab and etanercept affect quality of life in psoriasis, the researchers wrote. “Future research should consider replicating this evaluation using real-world long-term data and including other aspects of patient-centered outcomes, such as work productivity and psoriasis-related symptoms.”
Novartis funded ERASURE and FIXTURE. Dr. Strober reported financial ties to AbbVie, Amgen, Novartis, and several other pharmaceutical companies.
Compared with etanercept, secukinumab was associated with significantly faster and greater improvements in skin-specific quality of life among adults with moderate to severe psoriasis, according to a pooled analysis of data from the randomized, multicenter phase III ERASURE and FIXTURE trials.
The findings confirm the clinical superiority of secukinumab and extend prior head-to-head comparisons of this IL-17A inhibitor and etanercept, said Bruce E. Strober, MD, PhD, of the University of Connecticut in Farmington, Conn.
ERASURE and FIXTURE compared secukinumab (weekly for 4 weeks, then once-monthly) with etanercept (twice weekly for 12 weeks, then once-weekly) and placebo. Both secukinumab doses outperformed etanercept and placebo based on coprimary endpoints of PASI 75 and the proportion of patients who were clear or almost clear on a 5-point modified investigator’s global assessment (N Engl J Med. 2014 Jul 24;371[4]:326-38). A subsequent analysis showed that the clinical superiority of secukinumab held up at 52 weeks (J Dermatolog Treat. 2016;27[1]:1).
Because health-related quality of life is of increasing interest in psoriasis, Dr. Strober and his associates analyzed ERASURE and FIXTURE data on the Dermatology Life Quality Index (DLQI), a 10-item assessment of daily activities, leisure, personal relationships, symptoms and feelings, treatment, and work or school. Their analysis included 572 patients who received 300 mg secukinumab, 572 patients who received 150 mg secukinumab, and 326 patients who received 50 mg etanercept.
Baseline DLQI scores were similar among treatment groups. By week 4, significantly more secukinumab than etanercept recipients reported DLQI scores of 0 or 1 (DLQI 0/1), indicating that psoriasis was not impairing their quality of life (P < .05). This difference remained significant through week 52 at the 300-mg dose. The 150-mg dose significantly outperformed etanercept by week 8, and the difference remained significant through week 48.
In all, 81% of patients who received 300 mg secukinumab reported DLQI 0/1 at week 24, compared with 71% of those who received 150 mg secukinumab and 55% of those who received etanercept (P < .001 for each comparison with etanercept).
Among patients with complete data available, 86% of 300-mg secukinumab recipients achieved DLQI 0/1 at week 24, maintained this response through 52 weeks, and achieved a 90%-100% reduction in PASI total score at week 24. That was true of only 75% of etanercept recipients (P = .02, compared with 300 mg secukinumab) and 79% of 150-mg secukinumab recipients (P = .4, compared with etanercept).
In an adjusted model, patients who received 300 mg secukinumab were 1.9 times more likely to reach DLQI 0/1 than those who received etanercept (95% confidence interval, 1.6-2.3). Median time to response to both doses of secukinumab was 12 weeks, versus 24 weeks for etanercept (P < .0001).
This analysis offers “a robust, longer-term head-to-head comparison” of how secukinumab and etanercept affect quality of life in psoriasis, the researchers wrote. “Future research should consider replicating this evaluation using real-world long-term data and including other aspects of patient-centered outcomes, such as work productivity and psoriasis-related symptoms.”
Novartis funded ERASURE and FIXTURE. Dr. Strober reported financial ties to AbbVie, Amgen, Novartis, and several other pharmaceutical companies.
Compared with etanercept, secukinumab was associated with significantly faster and greater improvements in skin-specific quality of life among adults with moderate to severe psoriasis, according to a pooled analysis of data from the randomized, multicenter phase III ERASURE and FIXTURE trials.
The findings confirm the clinical superiority of secukinumab and extend prior head-to-head comparisons of this IL-17A inhibitor and etanercept, said Bruce E. Strober, MD, PhD, of the University of Connecticut in Farmington, Conn.
ERASURE and FIXTURE compared secukinumab (weekly for 4 weeks, then once-monthly) with etanercept (twice weekly for 12 weeks, then once-weekly) and placebo. Both secukinumab doses outperformed etanercept and placebo based on coprimary endpoints of PASI 75 and the proportion of patients who were clear or almost clear on a 5-point modified investigator’s global assessment (N Engl J Med. 2014 Jul 24;371[4]:326-38). A subsequent analysis showed that the clinical superiority of secukinumab held up at 52 weeks (J Dermatolog Treat. 2016;27[1]:1).
Because health-related quality of life is of increasing interest in psoriasis, Dr. Strober and his associates analyzed ERASURE and FIXTURE data on the Dermatology Life Quality Index (DLQI), a 10-item assessment of daily activities, leisure, personal relationships, symptoms and feelings, treatment, and work or school. Their analysis included 572 patients who received 300 mg secukinumab, 572 patients who received 150 mg secukinumab, and 326 patients who received 50 mg etanercept.
Baseline DLQI scores were similar among treatment groups. By week 4, significantly more secukinumab than etanercept recipients reported DLQI scores of 0 or 1 (DLQI 0/1), indicating that psoriasis was not impairing their quality of life (P < .05). This difference remained significant through week 52 at the 300-mg dose. The 150-mg dose significantly outperformed etanercept by week 8, and the difference remained significant through week 48.
In all, 81% of patients who received 300 mg secukinumab reported DLQI 0/1 at week 24, compared with 71% of those who received 150 mg secukinumab and 55% of those who received etanercept (P < .001 for each comparison with etanercept).
Among patients with complete data available, 86% of 300-mg secukinumab recipients achieved DLQI 0/1 at week 24, maintained this response through 52 weeks, and achieved a 90%-100% reduction in PASI total score at week 24. That was true of only 75% of etanercept recipients (P = .02, compared with 300 mg secukinumab) and 79% of 150-mg secukinumab recipients (P = .4, compared with etanercept).
In an adjusted model, patients who received 300 mg secukinumab were 1.9 times more likely to reach DLQI 0/1 than those who received etanercept (95% confidence interval, 1.6-2.3). Median time to response to both doses of secukinumab was 12 weeks, versus 24 weeks for etanercept (P < .0001).
This analysis offers “a robust, longer-term head-to-head comparison” of how secukinumab and etanercept affect quality of life in psoriasis, the researchers wrote. “Future research should consider replicating this evaluation using real-world long-term data and including other aspects of patient-centered outcomes, such as work productivity and psoriasis-related symptoms.”
Novartis funded ERASURE and FIXTURE. Dr. Strober reported financial ties to AbbVie, Amgen, Novartis, and several other pharmaceutical companies.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Secukinumab was associated with faster and greater improvements in self-reported quality of life, compared with etanercept, in adults with moderate to severe plaque psoriasis.
Major finding: In all, 81% of patients who received 300 mg secukinumab reported DLQI 0/1 at week 24, compared with 71% of those who received 150 mg secukinumab and 55% of those who received etanercept (P < .001 for each comparison with etanercept).
Data source: A pooled analysis of the randomized, multicenter, phase III ERASURE and FIXTURE trials.
Disclosures: Novartis funded ERASURE and FIXTURE. Dr. Strober reported financial ties to AbbVie, Amgen, Novartis, and several other pharmaceutical companies.
Debunking Psoriasis Myths: Do Treatments for Psoriasis Cause Suicide?
Myth: Psoriasis Therapies Can Cause Suicidal Ideation in Psoriasis Patients
Psoriasis takes a toll on patients, both physically and emotionally. Depression is one of the comorbidities of psoriasis due to biological changes that cause psoriasis as well as the stigma of visible psoriasis. Severe depression and suicidal ideation have been perceived to be features of life-threatening medical disorders, but dermatologists need to be aware of the relationship between depressive symptoms, suicidal ideation, and psoriasis severity.
A 2010 United Kingdom study of 916,948 patients with mild psoriasis, severe psoriasis, or controls without psoriasis indicated that patients with psoriasis have an increased risk for depression, anxiety, and suicidality. The relative risk of these outcomes is elevated in younger patients with psoriasis, with the greatest relative risk being for depression in patients with severe psoriasis.
Kimball et al conducted a study in the United States of 7404 patients with psoriasis and 37,020 controls without psoriasis (age, <18 years). They reported that pediatric patients with psoriasis were significantly more at risk of developing psychiatric disorders versus controls (P=.0001), especially depression (P=.0036) and anxiety (P=.0048).
In February 2017, the US Food and Drug Administration (FDA) announced approval of brodalumab for use in adults with moderate to severe plaque psoriasis. It is intended for patients who are candidates for systemic therapy or phototherapy but have failed to respond or have stopped responding to other systemic therapies. Lebwohl et al published the results of the phase 3 clinical trials, which showed that brodalumab was highly effective in reducing plaque psoriasis, even compared to ustekinumab. In fact, psoriasis area and severity index scores of 100 were significantly higher in the brodalumab 210-mg group versus ustekinumab group by week 12 (P<.001).
However, the approval is accompanied with a strict warning from the FDA and tightly regulated access to the drug, as suicidal ideation and behavior, including 4 suicides, occurred in patients treated with brodalumab during clinical trials, particularly patients with a history of depression or suicidality. According to the FDA, "[a] causal association between treatment with [brodalumab] and increased risk of suicidal ideation and behavior has not been established." The label includes a black box warning and the drug will only be available through a restricted Risk Evaluation and Mitigation Strategy program, which has the following requirements from the FDA:
- Prescribers must be certified with the program and counsel patients about this risk. Patients with new or worsening symptoms of depression or suicidality should be referred to a mental health professional, as appropriate.
- Patients must sign a Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety, or other mood changes.
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive the drug.
A medication guide is available for patients to inform them of the risk for suicidal ideation and behavior. The benefit of treatment must be weighed carefully against the seriousness of the risks associated with use.
Regardless of the therapy prescribed, dermatologists should be aware of the symptoms of depression. The National Psoriasis Foundation suggests you ask patients how they dress: Do they always wear long-sleeved shirts when they leave the house? Do they wear black? These questions can help determine if patients feel socially isolated or stigmatized by the disease. The National Psoriasis Foundation offers a Patient Navigation Center to help patients find a psychologist who specializes in issues related to psoriatic disease. Antidepressants and seeing a mental health professional can help, but ultimately taking control of the disease is the best way to improve depression.
Expert Commentary
According to the prescribing information for brodalumab, "Eight of the 10 subjects who attempted or completed suicide had a history of depression and/or suicidal ideation or behavior." Thus, 80% of these cases were at risk even before receiving 1 injection of brodalumab. Long-term registries will determine if there is truly an increased risk for suicidal ideation or behavior when taking brodalumab.
Brodalumab will be commercially available around the fall 2017. Before prescribing brodalumab, I will counsel patients about this potential increased risk of suicidal ideation or behavior as noted in the prescribing information, but I will tell them that a true risk has not yet been determined in long-term registries. I will mention to patients that if they really do feel depressed or experience suicidal ideation or behavior after starting brodalumab, they should stop taking brodalumab and contact me or a mental health professional.
—Jashin J. Wu, MD (Los Angeles, California)
FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed April 5, 2017.
Gupta MA, Schork NJ, Gupta AK, et al. Suicidal ideation in psoriasis. Int J Dermatol. 1993;32:188-190.
Kimball AB, Wu EQ, Guérin A, et al. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J Am Acad Dermatol. 2012;67:651-7.e1-651-7.e2.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
Life with psoriasis: depression. National Psoriasis Foundation website. https://www.psoriasis.org/life-with-psoriasis/depression. Accessed April 5, 2017.
Özkaya Ö. Biologic psoriasis treatment, Siliq, approved by FDA with strong warning of possible suicide risk. https://psoriasisnewstoday.com/2017/02/16/psoriasis-drug-siliq-approved-by-fda-with-warning-of-possible-suicide-risk/. Published February 16, 2017. Accessed April 5, 2017.
Myth: Psoriasis Therapies Can Cause Suicidal Ideation in Psoriasis Patients
Psoriasis takes a toll on patients, both physically and emotionally. Depression is one of the comorbidities of psoriasis due to biological changes that cause psoriasis as well as the stigma of visible psoriasis. Severe depression and suicidal ideation have been perceived to be features of life-threatening medical disorders, but dermatologists need to be aware of the relationship between depressive symptoms, suicidal ideation, and psoriasis severity.
A 2010 United Kingdom study of 916,948 patients with mild psoriasis, severe psoriasis, or controls without psoriasis indicated that patients with psoriasis have an increased risk for depression, anxiety, and suicidality. The relative risk of these outcomes is elevated in younger patients with psoriasis, with the greatest relative risk being for depression in patients with severe psoriasis.
Kimball et al conducted a study in the United States of 7404 patients with psoriasis and 37,020 controls without psoriasis (age, <18 years). They reported that pediatric patients with psoriasis were significantly more at risk of developing psychiatric disorders versus controls (P=.0001), especially depression (P=.0036) and anxiety (P=.0048).
In February 2017, the US Food and Drug Administration (FDA) announced approval of brodalumab for use in adults with moderate to severe plaque psoriasis. It is intended for patients who are candidates for systemic therapy or phototherapy but have failed to respond or have stopped responding to other systemic therapies. Lebwohl et al published the results of the phase 3 clinical trials, which showed that brodalumab was highly effective in reducing plaque psoriasis, even compared to ustekinumab. In fact, psoriasis area and severity index scores of 100 were significantly higher in the brodalumab 210-mg group versus ustekinumab group by week 12 (P<.001).
However, the approval is accompanied with a strict warning from the FDA and tightly regulated access to the drug, as suicidal ideation and behavior, including 4 suicides, occurred in patients treated with brodalumab during clinical trials, particularly patients with a history of depression or suicidality. According to the FDA, "[a] causal association between treatment with [brodalumab] and increased risk of suicidal ideation and behavior has not been established." The label includes a black box warning and the drug will only be available through a restricted Risk Evaluation and Mitigation Strategy program, which has the following requirements from the FDA:
- Prescribers must be certified with the program and counsel patients about this risk. Patients with new or worsening symptoms of depression or suicidality should be referred to a mental health professional, as appropriate.
- Patients must sign a Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety, or other mood changes.
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive the drug.
A medication guide is available for patients to inform them of the risk for suicidal ideation and behavior. The benefit of treatment must be weighed carefully against the seriousness of the risks associated with use.
Regardless of the therapy prescribed, dermatologists should be aware of the symptoms of depression. The National Psoriasis Foundation suggests you ask patients how they dress: Do they always wear long-sleeved shirts when they leave the house? Do they wear black? These questions can help determine if patients feel socially isolated or stigmatized by the disease. The National Psoriasis Foundation offers a Patient Navigation Center to help patients find a psychologist who specializes in issues related to psoriatic disease. Antidepressants and seeing a mental health professional can help, but ultimately taking control of the disease is the best way to improve depression.
Expert Commentary
According to the prescribing information for brodalumab, "Eight of the 10 subjects who attempted or completed suicide had a history of depression and/or suicidal ideation or behavior." Thus, 80% of these cases were at risk even before receiving 1 injection of brodalumab. Long-term registries will determine if there is truly an increased risk for suicidal ideation or behavior when taking brodalumab.
Brodalumab will be commercially available around the fall 2017. Before prescribing brodalumab, I will counsel patients about this potential increased risk of suicidal ideation or behavior as noted in the prescribing information, but I will tell them that a true risk has not yet been determined in long-term registries. I will mention to patients that if they really do feel depressed or experience suicidal ideation or behavior after starting brodalumab, they should stop taking brodalumab and contact me or a mental health professional.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Psoriasis Therapies Can Cause Suicidal Ideation in Psoriasis Patients
Psoriasis takes a toll on patients, both physically and emotionally. Depression is one of the comorbidities of psoriasis due to biological changes that cause psoriasis as well as the stigma of visible psoriasis. Severe depression and suicidal ideation have been perceived to be features of life-threatening medical disorders, but dermatologists need to be aware of the relationship between depressive symptoms, suicidal ideation, and psoriasis severity.
A 2010 United Kingdom study of 916,948 patients with mild psoriasis, severe psoriasis, or controls without psoriasis indicated that patients with psoriasis have an increased risk for depression, anxiety, and suicidality. The relative risk of these outcomes is elevated in younger patients with psoriasis, with the greatest relative risk being for depression in patients with severe psoriasis.
Kimball et al conducted a study in the United States of 7404 patients with psoriasis and 37,020 controls without psoriasis (age, <18 years). They reported that pediatric patients with psoriasis were significantly more at risk of developing psychiatric disorders versus controls (P=.0001), especially depression (P=.0036) and anxiety (P=.0048).
In February 2017, the US Food and Drug Administration (FDA) announced approval of brodalumab for use in adults with moderate to severe plaque psoriasis. It is intended for patients who are candidates for systemic therapy or phototherapy but have failed to respond or have stopped responding to other systemic therapies. Lebwohl et al published the results of the phase 3 clinical trials, which showed that brodalumab was highly effective in reducing plaque psoriasis, even compared to ustekinumab. In fact, psoriasis area and severity index scores of 100 were significantly higher in the brodalumab 210-mg group versus ustekinumab group by week 12 (P<.001).
However, the approval is accompanied with a strict warning from the FDA and tightly regulated access to the drug, as suicidal ideation and behavior, including 4 suicides, occurred in patients treated with brodalumab during clinical trials, particularly patients with a history of depression or suicidality. According to the FDA, "[a] causal association between treatment with [brodalumab] and increased risk of suicidal ideation and behavior has not been established." The label includes a black box warning and the drug will only be available through a restricted Risk Evaluation and Mitigation Strategy program, which has the following requirements from the FDA:
- Prescribers must be certified with the program and counsel patients about this risk. Patients with new or worsening symptoms of depression or suicidality should be referred to a mental health professional, as appropriate.
- Patients must sign a Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety, or other mood changes.
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive the drug.
A medication guide is available for patients to inform them of the risk for suicidal ideation and behavior. The benefit of treatment must be weighed carefully against the seriousness of the risks associated with use.
Regardless of the therapy prescribed, dermatologists should be aware of the symptoms of depression. The National Psoriasis Foundation suggests you ask patients how they dress: Do they always wear long-sleeved shirts when they leave the house? Do they wear black? These questions can help determine if patients feel socially isolated or stigmatized by the disease. The National Psoriasis Foundation offers a Patient Navigation Center to help patients find a psychologist who specializes in issues related to psoriatic disease. Antidepressants and seeing a mental health professional can help, but ultimately taking control of the disease is the best way to improve depression.
Expert Commentary
According to the prescribing information for brodalumab, "Eight of the 10 subjects who attempted or completed suicide had a history of depression and/or suicidal ideation or behavior." Thus, 80% of these cases were at risk even before receiving 1 injection of brodalumab. Long-term registries will determine if there is truly an increased risk for suicidal ideation or behavior when taking brodalumab.
Brodalumab will be commercially available around the fall 2017. Before prescribing brodalumab, I will counsel patients about this potential increased risk of suicidal ideation or behavior as noted in the prescribing information, but I will tell them that a true risk has not yet been determined in long-term registries. I will mention to patients that if they really do feel depressed or experience suicidal ideation or behavior after starting brodalumab, they should stop taking brodalumab and contact me or a mental health professional.
—Jashin J. Wu, MD (Los Angeles, California)
FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed April 5, 2017.
Gupta MA, Schork NJ, Gupta AK, et al. Suicidal ideation in psoriasis. Int J Dermatol. 1993;32:188-190.
Kimball AB, Wu EQ, Guérin A, et al. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J Am Acad Dermatol. 2012;67:651-7.e1-651-7.e2.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
Life with psoriasis: depression. National Psoriasis Foundation website. https://www.psoriasis.org/life-with-psoriasis/depression. Accessed April 5, 2017.
Özkaya Ö. Biologic psoriasis treatment, Siliq, approved by FDA with strong warning of possible suicide risk. https://psoriasisnewstoday.com/2017/02/16/psoriasis-drug-siliq-approved-by-fda-with-warning-of-possible-suicide-risk/. Published February 16, 2017. Accessed April 5, 2017.
FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed April 5, 2017.
Gupta MA, Schork NJ, Gupta AK, et al. Suicidal ideation in psoriasis. Int J Dermatol. 1993;32:188-190.
Kimball AB, Wu EQ, Guérin A, et al. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J Am Acad Dermatol. 2012;67:651-7.e1-651-7.e2.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
Life with psoriasis: depression. National Psoriasis Foundation website. https://www.psoriasis.org/life-with-psoriasis/depression. Accessed April 5, 2017.
Özkaya Ö. Biologic psoriasis treatment, Siliq, approved by FDA with strong warning of possible suicide risk. https://psoriasisnewstoday.com/2017/02/16/psoriasis-drug-siliq-approved-by-fda-with-warning-of-possible-suicide-risk/. Published February 16, 2017. Accessed April 5, 2017.
What’s old is new in topical psoriasis therapy
WAILEA, HAWAII – A fixed combination of halobetasol and tazarotene formulated as a once-daily lotion proved safe and effective for patients with moderate or severe plaque psoriasis in a phase II randomized clinical trial, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The rationale for developing this product is that it should provide the efficacy of a class 1 superpotent topical corticosteroid, albeit with the topical retinoid lessening concern about the possibility of steroid-induced skin atrophy and other long-term safety issues, explained Dr. Stein Gold, director of dermatology research at Henry Ford Health System in Detroit.
The double-blind, multicenter, 8-week, phase II study included 212 patients randomized 2:2:1 to once-daily application of fixed-combination halobetasol propionate 0.01% and tazarotene 0.045% lotion, either topical agent as monotherapy, or vehicle. Ninety percent of participants had moderate plaque psoriasis as defined by a baseline Investigator’s Global Assessment (IGA) score of 3 and a median 5% body surface area involvement. The remaining patients were classified as having severe psoriasis, with a baseline IGA of 4, but no one with psoriasis over more than 12% of their body surface area was eligible for the trial.
The fixed-combination lotion, known for now as IDP-118, established superior efficacy over placebo by 2 weeks. At week 8, treatment success, as defined by at least a two-grade improvement from baseline in IGA score plus a rating of clear or almost clear, was documented in 53% of the IDP-118 lotion group, compared with 33% on halobetasol propionate monotherapy, 19% with tazarotene alone, and 10% with vehicle, Dr. Stein Gold reported.
Each patient had a target lesion 25-32 cm2 in size, which was designated for careful assessment of changes in the domains of lesional erythema, plaque elevation, and scaling. At week 8, the IDP-118 had a target lesion treatment success rate of 54% for erythema, 68% for plaque elevation, and 64% for scaling, she said.
The treatment discontinuation rate was 3.4% in the IDP-118 group, 12.1% with tazarotene, zero for halobetasol propionate, and 3.2% for vehicle. The most frequently reported treatment-emergent adverse events were mild or moderate application-site reactions. Skin atrophy was rare. No treatment-related serious adverse events occurred.
A 555-patient, phase III, open-label, 12-month safety study of IDP-118 is due to be completed later in 2017.
The phase II study was funded by Valeant Pharmaceuticals. Dr. Stein Gold reported serving as an investigator, advisor, and speaker for the company. SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – A fixed combination of halobetasol and tazarotene formulated as a once-daily lotion proved safe and effective for patients with moderate or severe plaque psoriasis in a phase II randomized clinical trial, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The rationale for developing this product is that it should provide the efficacy of a class 1 superpotent topical corticosteroid, albeit with the topical retinoid lessening concern about the possibility of steroid-induced skin atrophy and other long-term safety issues, explained Dr. Stein Gold, director of dermatology research at Henry Ford Health System in Detroit.
The double-blind, multicenter, 8-week, phase II study included 212 patients randomized 2:2:1 to once-daily application of fixed-combination halobetasol propionate 0.01% and tazarotene 0.045% lotion, either topical agent as monotherapy, or vehicle. Ninety percent of participants had moderate plaque psoriasis as defined by a baseline Investigator’s Global Assessment (IGA) score of 3 and a median 5% body surface area involvement. The remaining patients were classified as having severe psoriasis, with a baseline IGA of 4, but no one with psoriasis over more than 12% of their body surface area was eligible for the trial.
The fixed-combination lotion, known for now as IDP-118, established superior efficacy over placebo by 2 weeks. At week 8, treatment success, as defined by at least a two-grade improvement from baseline in IGA score plus a rating of clear or almost clear, was documented in 53% of the IDP-118 lotion group, compared with 33% on halobetasol propionate monotherapy, 19% with tazarotene alone, and 10% with vehicle, Dr. Stein Gold reported.
Each patient had a target lesion 25-32 cm2 in size, which was designated for careful assessment of changes in the domains of lesional erythema, plaque elevation, and scaling. At week 8, the IDP-118 had a target lesion treatment success rate of 54% for erythema, 68% for plaque elevation, and 64% for scaling, she said.
The treatment discontinuation rate was 3.4% in the IDP-118 group, 12.1% with tazarotene, zero for halobetasol propionate, and 3.2% for vehicle. The most frequently reported treatment-emergent adverse events were mild or moderate application-site reactions. Skin atrophy was rare. No treatment-related serious adverse events occurred.
A 555-patient, phase III, open-label, 12-month safety study of IDP-118 is due to be completed later in 2017.
The phase II study was funded by Valeant Pharmaceuticals. Dr. Stein Gold reported serving as an investigator, advisor, and speaker for the company. SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – A fixed combination of halobetasol and tazarotene formulated as a once-daily lotion proved safe and effective for patients with moderate or severe plaque psoriasis in a phase II randomized clinical trial, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The rationale for developing this product is that it should provide the efficacy of a class 1 superpotent topical corticosteroid, albeit with the topical retinoid lessening concern about the possibility of steroid-induced skin atrophy and other long-term safety issues, explained Dr. Stein Gold, director of dermatology research at Henry Ford Health System in Detroit.
The double-blind, multicenter, 8-week, phase II study included 212 patients randomized 2:2:1 to once-daily application of fixed-combination halobetasol propionate 0.01% and tazarotene 0.045% lotion, either topical agent as monotherapy, or vehicle. Ninety percent of participants had moderate plaque psoriasis as defined by a baseline Investigator’s Global Assessment (IGA) score of 3 and a median 5% body surface area involvement. The remaining patients were classified as having severe psoriasis, with a baseline IGA of 4, but no one with psoriasis over more than 12% of their body surface area was eligible for the trial.
The fixed-combination lotion, known for now as IDP-118, established superior efficacy over placebo by 2 weeks. At week 8, treatment success, as defined by at least a two-grade improvement from baseline in IGA score plus a rating of clear or almost clear, was documented in 53% of the IDP-118 lotion group, compared with 33% on halobetasol propionate monotherapy, 19% with tazarotene alone, and 10% with vehicle, Dr. Stein Gold reported.
Each patient had a target lesion 25-32 cm2 in size, which was designated for careful assessment of changes in the domains of lesional erythema, plaque elevation, and scaling. At week 8, the IDP-118 had a target lesion treatment success rate of 54% for erythema, 68% for plaque elevation, and 64% for scaling, she said.
The treatment discontinuation rate was 3.4% in the IDP-118 group, 12.1% with tazarotene, zero for halobetasol propionate, and 3.2% for vehicle. The most frequently reported treatment-emergent adverse events were mild or moderate application-site reactions. Skin atrophy was rare. No treatment-related serious adverse events occurred.
A 555-patient, phase III, open-label, 12-month safety study of IDP-118 is due to be completed later in 2017.
The phase II study was funded by Valeant Pharmaceuticals. Dr. Stein Gold reported serving as an investigator, advisor, and speaker for the company. SDEF and this news organization are owned by the same parent company.
Key clinical point:
Major finding: An investigational fixed-combination lotion consisting of halobetasol propionate 0.01% and tazarotene 0.045% achieved scores of clear or almost clear in 53% of patients, significantly better than either component as monotherapy.
Data source: This phase II, double-blind, multicenter, vehicle-controlled, 8-week randomized clinical trial included 212 patients with moderate or severe plaque psoriasis.
Disclosures: The study was funded by Valeant Pharmaceuticals. The presenter reported serving as an investigator, advisor, and speaker for the company.
Psoriasis Symptoms With the Greatest Impact on Patients
Flaking/scaling and itching, followed by dry cracked skin that may bleed, pain or soreness, and burning/stinging were noted by psoriasis patients as the symptoms with the most significant impact on daily life in a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than two-thirds of respondents identified flaking/scaling as one of their most significant symptoms of psoriasis, either localized to psoriasis-prone areas such as the elbows and knees or more widespread. Patients reported that this symptom is constant, leaving them to absentmindedly rub certain areas of the skin.
A similar number of respondents indicated that itching was their most significant symptom. One patient called it “an intense subcutaneous itch… deep down in the skin,” a description that resonated with other patients in the room.
Nearly 40% identified dry cracked skin that may bleed as a significant symptom, noting that areas where skin is thinner are affected more, such as the folds of the body. Patients described this symptom as interrelated with other symptoms such as itching. “The thicker the scales get on my skin, the more they itch, and the more they itch, the more I am likely to scratch them, and the more I scratch them, the more they start to crack, and then more come back and it keeps going and going,” one patient said.
More than one-quarter of respondents indicated that pain, soreness, or burning/stinging were the most significant symptoms. Patients indicated that the stinging/burning was more episodic, while the pain was more constant, with the pain being under the skin.
Triggers of these symptoms included stress (primary trigger), changes in weather, hormonal changes, diet, lotions, prolonged exposure to sunlight, sweat, aging, and other medical conditions.
Dermatologists may use these patient insights to prescribe therapies that target these symptoms.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Flaking/scaling and itching, followed by dry cracked skin that may bleed, pain or soreness, and burning/stinging were noted by psoriasis patients as the symptoms with the most significant impact on daily life in a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than two-thirds of respondents identified flaking/scaling as one of their most significant symptoms of psoriasis, either localized to psoriasis-prone areas such as the elbows and knees or more widespread. Patients reported that this symptom is constant, leaving them to absentmindedly rub certain areas of the skin.
A similar number of respondents indicated that itching was their most significant symptom. One patient called it “an intense subcutaneous itch… deep down in the skin,” a description that resonated with other patients in the room.
Nearly 40% identified dry cracked skin that may bleed as a significant symptom, noting that areas where skin is thinner are affected more, such as the folds of the body. Patients described this symptom as interrelated with other symptoms such as itching. “The thicker the scales get on my skin, the more they itch, and the more they itch, the more I am likely to scratch them, and the more I scratch them, the more they start to crack, and then more come back and it keeps going and going,” one patient said.
More than one-quarter of respondents indicated that pain, soreness, or burning/stinging were the most significant symptoms. Patients indicated that the stinging/burning was more episodic, while the pain was more constant, with the pain being under the skin.
Triggers of these symptoms included stress (primary trigger), changes in weather, hormonal changes, diet, lotions, prolonged exposure to sunlight, sweat, aging, and other medical conditions.
Dermatologists may use these patient insights to prescribe therapies that target these symptoms.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Flaking/scaling and itching, followed by dry cracked skin that may bleed, pain or soreness, and burning/stinging were noted by psoriasis patients as the symptoms with the most significant impact on daily life in a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than two-thirds of respondents identified flaking/scaling as one of their most significant symptoms of psoriasis, either localized to psoriasis-prone areas such as the elbows and knees or more widespread. Patients reported that this symptom is constant, leaving them to absentmindedly rub certain areas of the skin.
A similar number of respondents indicated that itching was their most significant symptom. One patient called it “an intense subcutaneous itch… deep down in the skin,” a description that resonated with other patients in the room.
Nearly 40% identified dry cracked skin that may bleed as a significant symptom, noting that areas where skin is thinner are affected more, such as the folds of the body. Patients described this symptom as interrelated with other symptoms such as itching. “The thicker the scales get on my skin, the more they itch, and the more they itch, the more I am likely to scratch them, and the more I scratch them, the more they start to crack, and then more come back and it keeps going and going,” one patient said.
More than one-quarter of respondents indicated that pain, soreness, or burning/stinging were the most significant symptoms. Patients indicated that the stinging/burning was more episodic, while the pain was more constant, with the pain being under the skin.
Triggers of these symptoms included stress (primary trigger), changes in weather, hormonal changes, diet, lotions, prolonged exposure to sunlight, sweat, aging, and other medical conditions.
Dermatologists may use these patient insights to prescribe therapies that target these symptoms.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Psoriasis on the Hands and Feet: How Patients Should Care for These Areas
What does your patient need to know at the first visit?
Patients with this condition need to avoid friction and excessive moisture. They should be counseled to use gloves for excessive wet work. I recommend they use cotton gloves on the hands, and then cover those with rubber gloves. Patients should use a hand emollient regularly, including after each time they wash their hands or have exposure to water. If the patient lifts weights, I recommend he/she use weight-lifting gloves to reduce friction.
What are your go to treatments? What are the side effects?
The first line of therapy for hand and foot psoriasis is a topical agent. I most often use a combination of topical steroids and a topical vitamin D analogue. If insurance is amenable, I may use a fixed combination of topical steroid and vitamin D analogue.
If topical therapies are not successful, I often consider using excimer laser therapy, which requires the patient to come to the office twice weekly, so it is important to determine if this therapy is compatible with the patient's schedule. Other options include oral and biological therapies. Apremilast is a reasonable first-line systemic therapy given that it is an oral therapy, requires no laboratory monitoring, and has a favorable safety profile. Alternatively, biologic agents can be utilized. There are several analyses available looking at the efficacy of different biologics in hand and foot psoriasis, but at this point there is no consensus first choice for a biologic in this condition. Many available biologics may have a notable impact though.
The side effects of therapies for psoriasis are well established. Topical therapies and excimer laser are relatively safe choices. Apremilast has been associated with early gastrointestinal tract side effects that tend to resolve over time. Each biologic has a unique safety profile, with a rare incidence of side effects that should be reviewed carefully with any prospective patients before starting therapy.
How do you keep patients compliant with treatment?
It is important to reinforce gentle hand care and foot care. Patients need to understand that lack of compliance with treatment will lead to recurrence of disease.
What do you do if patients refuse treatment?
I try to educate them as best as possible, and ask them to return and reconsider therapy if they find that this condition affects their quality of life.
What does your patient need to know at the first visit?
Patients with this condition need to avoid friction and excessive moisture. They should be counseled to use gloves for excessive wet work. I recommend they use cotton gloves on the hands, and then cover those with rubber gloves. Patients should use a hand emollient regularly, including after each time they wash their hands or have exposure to water. If the patient lifts weights, I recommend he/she use weight-lifting gloves to reduce friction.
What are your go to treatments? What are the side effects?
The first line of therapy for hand and foot psoriasis is a topical agent. I most often use a combination of topical steroids and a topical vitamin D analogue. If insurance is amenable, I may use a fixed combination of topical steroid and vitamin D analogue.
If topical therapies are not successful, I often consider using excimer laser therapy, which requires the patient to come to the office twice weekly, so it is important to determine if this therapy is compatible with the patient's schedule. Other options include oral and biological therapies. Apremilast is a reasonable first-line systemic therapy given that it is an oral therapy, requires no laboratory monitoring, and has a favorable safety profile. Alternatively, biologic agents can be utilized. There are several analyses available looking at the efficacy of different biologics in hand and foot psoriasis, but at this point there is no consensus first choice for a biologic in this condition. Many available biologics may have a notable impact though.
The side effects of therapies for psoriasis are well established. Topical therapies and excimer laser are relatively safe choices. Apremilast has been associated with early gastrointestinal tract side effects that tend to resolve over time. Each biologic has a unique safety profile, with a rare incidence of side effects that should be reviewed carefully with any prospective patients before starting therapy.
How do you keep patients compliant with treatment?
It is important to reinforce gentle hand care and foot care. Patients need to understand that lack of compliance with treatment will lead to recurrence of disease.
What do you do if patients refuse treatment?
I try to educate them as best as possible, and ask them to return and reconsider therapy if they find that this condition affects their quality of life.
What does your patient need to know at the first visit?
Patients with this condition need to avoid friction and excessive moisture. They should be counseled to use gloves for excessive wet work. I recommend they use cotton gloves on the hands, and then cover those with rubber gloves. Patients should use a hand emollient regularly, including after each time they wash their hands or have exposure to water. If the patient lifts weights, I recommend he/she use weight-lifting gloves to reduce friction.
What are your go to treatments? What are the side effects?
The first line of therapy for hand and foot psoriasis is a topical agent. I most often use a combination of topical steroids and a topical vitamin D analogue. If insurance is amenable, I may use a fixed combination of topical steroid and vitamin D analogue.
If topical therapies are not successful, I often consider using excimer laser therapy, which requires the patient to come to the office twice weekly, so it is important to determine if this therapy is compatible with the patient's schedule. Other options include oral and biological therapies. Apremilast is a reasonable first-line systemic therapy given that it is an oral therapy, requires no laboratory monitoring, and has a favorable safety profile. Alternatively, biologic agents can be utilized. There are several analyses available looking at the efficacy of different biologics in hand and foot psoriasis, but at this point there is no consensus first choice for a biologic in this condition. Many available biologics may have a notable impact though.
The side effects of therapies for psoriasis are well established. Topical therapies and excimer laser are relatively safe choices. Apremilast has been associated with early gastrointestinal tract side effects that tend to resolve over time. Each biologic has a unique safety profile, with a rare incidence of side effects that should be reviewed carefully with any prospective patients before starting therapy.
How do you keep patients compliant with treatment?
It is important to reinforce gentle hand care and foot care. Patients need to understand that lack of compliance with treatment will lead to recurrence of disease.
What do you do if patients refuse treatment?
I try to educate them as best as possible, and ask them to return and reconsider therapy if they find that this condition affects their quality of life.
Ixekizumab found superior to ustekinumab in psoriasis at 24 weeks
ORLANDO – The interleukin-17A inhibitor ixekizumab was associated with superior efficacy and safety when compared with ustekinumab at 24 weeks, a head-to-head trial of the two monoclonal antibodies in plaque psoriasis has shown.
The 24-week data from the IXORA-S trial were presented during a late-breaking clinical trial session at the annual meeting of the American Academy of Dermatology by Kristian Reich, MD, PhD, a founder of SCIderm in Hamburg, Germany.
At 24 weeks, 49% in the ixekizumab arm achieved a Psoriasis Area and Severity Index (PASI) 100 level of skin clearance, compared with 24% in the ustekinumab arm (P = .001). Ixekizumab treatment also reached significantly higher skin clearances than ustekinumab at 24 weeks at the level of PASI 90 (83% vs. 59%; P less than .001) and PASI 75 (91% vs. 82%; P = .015).
Treatment with ixekizumab produced a Static Physician’s Global Assessment (sPGA) score of 0 in 54% at 24 weeks, compared with 24% with ustekinumab (P less than .001). A sPGA score of 0 or 1 occurred in 87% of patients who took ixekizumab and in 59% of the ustekinumab group.
An improvement of 4 or more points in the pruritus Numeric Rating Scale was reported by 86% of ixekizumab patients at 24 weeks, compared with 72% of those who took ustekinumab (P = .018).
Dr. Reich reported that there were no deaths or any significant differences in overall treatment-related adverse events across both arms, although he cautioned against putting too much stock in 24-week safety data. “I hesitate to show safety data for only 300 patients at 24 weeks, but it’s good to see here that there doesn’t seem to be a difference. I still would want to see larger patient numbers and more long-term data,” he said.
Dr. Reich had numerous disclosures, including honoraria for serving as a speaker for Eli Lilly, the sponsor of this trial.
ORLANDO – The interleukin-17A inhibitor ixekizumab was associated with superior efficacy and safety when compared with ustekinumab at 24 weeks, a head-to-head trial of the two monoclonal antibodies in plaque psoriasis has shown.
The 24-week data from the IXORA-S trial were presented during a late-breaking clinical trial session at the annual meeting of the American Academy of Dermatology by Kristian Reich, MD, PhD, a founder of SCIderm in Hamburg, Germany.
At 24 weeks, 49% in the ixekizumab arm achieved a Psoriasis Area and Severity Index (PASI) 100 level of skin clearance, compared with 24% in the ustekinumab arm (P = .001). Ixekizumab treatment also reached significantly higher skin clearances than ustekinumab at 24 weeks at the level of PASI 90 (83% vs. 59%; P less than .001) and PASI 75 (91% vs. 82%; P = .015).
Treatment with ixekizumab produced a Static Physician’s Global Assessment (sPGA) score of 0 in 54% at 24 weeks, compared with 24% with ustekinumab (P less than .001). A sPGA score of 0 or 1 occurred in 87% of patients who took ixekizumab and in 59% of the ustekinumab group.
An improvement of 4 or more points in the pruritus Numeric Rating Scale was reported by 86% of ixekizumab patients at 24 weeks, compared with 72% of those who took ustekinumab (P = .018).
Dr. Reich reported that there were no deaths or any significant differences in overall treatment-related adverse events across both arms, although he cautioned against putting too much stock in 24-week safety data. “I hesitate to show safety data for only 300 patients at 24 weeks, but it’s good to see here that there doesn’t seem to be a difference. I still would want to see larger patient numbers and more long-term data,” he said.
Dr. Reich had numerous disclosures, including honoraria for serving as a speaker for Eli Lilly, the sponsor of this trial.
ORLANDO – The interleukin-17A inhibitor ixekizumab was associated with superior efficacy and safety when compared with ustekinumab at 24 weeks, a head-to-head trial of the two monoclonal antibodies in plaque psoriasis has shown.
The 24-week data from the IXORA-S trial were presented during a late-breaking clinical trial session at the annual meeting of the American Academy of Dermatology by Kristian Reich, MD, PhD, a founder of SCIderm in Hamburg, Germany.
At 24 weeks, 49% in the ixekizumab arm achieved a Psoriasis Area and Severity Index (PASI) 100 level of skin clearance, compared with 24% in the ustekinumab arm (P = .001). Ixekizumab treatment also reached significantly higher skin clearances than ustekinumab at 24 weeks at the level of PASI 90 (83% vs. 59%; P less than .001) and PASI 75 (91% vs. 82%; P = .015).
Treatment with ixekizumab produced a Static Physician’s Global Assessment (sPGA) score of 0 in 54% at 24 weeks, compared with 24% with ustekinumab (P less than .001). A sPGA score of 0 or 1 occurred in 87% of patients who took ixekizumab and in 59% of the ustekinumab group.
An improvement of 4 or more points in the pruritus Numeric Rating Scale was reported by 86% of ixekizumab patients at 24 weeks, compared with 72% of those who took ustekinumab (P = .018).
Dr. Reich reported that there were no deaths or any significant differences in overall treatment-related adverse events across both arms, although he cautioned against putting too much stock in 24-week safety data. “I hesitate to show safety data for only 300 patients at 24 weeks, but it’s good to see here that there doesn’t seem to be a difference. I still would want to see larger patient numbers and more long-term data,” he said.
Dr. Reich had numerous disclosures, including honoraria for serving as a speaker for Eli Lilly, the sponsor of this trial.
AT AAD 2017
Key clinical point:
Major finding: At 24 weeks, ixekizumab was associated with significantly better PASI response rates and reduction in itch than ustekinumab in patients with moderate to severe plaque psoriasis.
Data source: Head-to-head trial of 302 adults with moderate to severe plaque psoriasis treated with either ixekizumab or ustekinumab.
Disclosures: Dr. Reich had numerous disclosures, including honoraria for serving as a speaker for Eli Lilly, the sponsor of this trial.
Shingles vaccine deemed effective in people with autoimmune disease
The herpes zoster vaccine reduces the risk of shingles in older adults with autoimmune disease, even if they are taking immunosuppressants for their condition, but the protection begins to wane after about 5 years, a recent retrospective study found.
“There has been some concern that patients with autoimmune conditions might have a lower immunogenic response to herpes zoster vaccination, especially when treated with immunosuppressive medications such as glucocorticoids,” wrote Huifeng Yun, PhD, of the University of Alabama at Birmingham, and her colleagues.
The researchers used 2006-2013 Medicare data to calculate the risk of shingles among Medicare recipients who had an autoimmune disease and either did or did not receive the herpes zoster vaccine. All the patients had been enrolled in Medicare for at least 12 continuous months and had a diagnosis of ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis.
The researchers matched 59,627 patients who received the herpes zoster vaccine with 119,254 unvaccinated patients, based on age, sex, race, calendar year, autoimmune disease type, and use of autoimmune drugs (biologics, disease-modifying antirheumatic drugs, and glucocorticoids). During a follow-up of up to 7 years, the researchers additionally accounted for comorbid medical conditions and concurrent medications each year.
The cohort, with an average age of 73.5 years in both groups, included 53.1% of adults with rheumatoid arthritis, 31.6% with psoriasis, 20.9% with inflammatory bowel disease, 4.7% with psoriatic arthritis, and 1.4% with ankylosing spondylitis.
Those who received the vaccine had a rate of 0.75 herpes zoster cases per 100 people during the first year, which rose to 1.25 cases per 100 people per year at the seventh year after vaccination. The rate among unvaccinated individuals stayed steady at approximately 1.3-1.7 cases per 100 people per year throughout the study period. These rates, as expected, were approximately 50% higher than in the general population over age 70 without autoimmune disease.
Compared with unvaccinated individuals, vaccinated individuals had a reduced relative risk for shingles of 0.74-0.77 after adjustment for confounders, but the risk reduction only remained statistically significant for the first 5 years after vaccination.
The waning seen with the vaccine’s effectiveness “raises the possibility that patients might benefit from a booster vaccine at some point after initial vaccination, although no recommendation currently exists that would support such a practice,” the authors wrote.
Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
The herpes zoster vaccine reduces the risk of shingles in older adults with autoimmune disease, even if they are taking immunosuppressants for their condition, but the protection begins to wane after about 5 years, a recent retrospective study found.
“There has been some concern that patients with autoimmune conditions might have a lower immunogenic response to herpes zoster vaccination, especially when treated with immunosuppressive medications such as glucocorticoids,” wrote Huifeng Yun, PhD, of the University of Alabama at Birmingham, and her colleagues.
The researchers used 2006-2013 Medicare data to calculate the risk of shingles among Medicare recipients who had an autoimmune disease and either did or did not receive the herpes zoster vaccine. All the patients had been enrolled in Medicare for at least 12 continuous months and had a diagnosis of ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis.
The researchers matched 59,627 patients who received the herpes zoster vaccine with 119,254 unvaccinated patients, based on age, sex, race, calendar year, autoimmune disease type, and use of autoimmune drugs (biologics, disease-modifying antirheumatic drugs, and glucocorticoids). During a follow-up of up to 7 years, the researchers additionally accounted for comorbid medical conditions and concurrent medications each year.
The cohort, with an average age of 73.5 years in both groups, included 53.1% of adults with rheumatoid arthritis, 31.6% with psoriasis, 20.9% with inflammatory bowel disease, 4.7% with psoriatic arthritis, and 1.4% with ankylosing spondylitis.
Those who received the vaccine had a rate of 0.75 herpes zoster cases per 100 people during the first year, which rose to 1.25 cases per 100 people per year at the seventh year after vaccination. The rate among unvaccinated individuals stayed steady at approximately 1.3-1.7 cases per 100 people per year throughout the study period. These rates, as expected, were approximately 50% higher than in the general population over age 70 without autoimmune disease.
Compared with unvaccinated individuals, vaccinated individuals had a reduced relative risk for shingles of 0.74-0.77 after adjustment for confounders, but the risk reduction only remained statistically significant for the first 5 years after vaccination.
The waning seen with the vaccine’s effectiveness “raises the possibility that patients might benefit from a booster vaccine at some point after initial vaccination, although no recommendation currently exists that would support such a practice,” the authors wrote.
Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
The herpes zoster vaccine reduces the risk of shingles in older adults with autoimmune disease, even if they are taking immunosuppressants for their condition, but the protection begins to wane after about 5 years, a recent retrospective study found.
“There has been some concern that patients with autoimmune conditions might have a lower immunogenic response to herpes zoster vaccination, especially when treated with immunosuppressive medications such as glucocorticoids,” wrote Huifeng Yun, PhD, of the University of Alabama at Birmingham, and her colleagues.
The researchers used 2006-2013 Medicare data to calculate the risk of shingles among Medicare recipients who had an autoimmune disease and either did or did not receive the herpes zoster vaccine. All the patients had been enrolled in Medicare for at least 12 continuous months and had a diagnosis of ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis.
The researchers matched 59,627 patients who received the herpes zoster vaccine with 119,254 unvaccinated patients, based on age, sex, race, calendar year, autoimmune disease type, and use of autoimmune drugs (biologics, disease-modifying antirheumatic drugs, and glucocorticoids). During a follow-up of up to 7 years, the researchers additionally accounted for comorbid medical conditions and concurrent medications each year.
The cohort, with an average age of 73.5 years in both groups, included 53.1% of adults with rheumatoid arthritis, 31.6% with psoriasis, 20.9% with inflammatory bowel disease, 4.7% with psoriatic arthritis, and 1.4% with ankylosing spondylitis.
Those who received the vaccine had a rate of 0.75 herpes zoster cases per 100 people during the first year, which rose to 1.25 cases per 100 people per year at the seventh year after vaccination. The rate among unvaccinated individuals stayed steady at approximately 1.3-1.7 cases per 100 people per year throughout the study period. These rates, as expected, were approximately 50% higher than in the general population over age 70 without autoimmune disease.
Compared with unvaccinated individuals, vaccinated individuals had a reduced relative risk for shingles of 0.74-0.77 after adjustment for confounders, but the risk reduction only remained statistically significant for the first 5 years after vaccination.
The waning seen with the vaccine’s effectiveness “raises the possibility that patients might benefit from a booster vaccine at some point after initial vaccination, although no recommendation currently exists that would support such a practice,” the authors wrote.
Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
Key clinical point:
Major finding: Medicare patients with autoimmune disease had a 23%-26% reduced risk of shingles for 5 years after receiving the herpes zoster vaccine.
Data source: The findings are based on analysis of 2006-2013 Medicare data on 59,627 patients who received the herpes zoster vaccine and 119,254 patients who didn’t.
Disclosures: Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
Clinicians still seek the best uses for apremilast
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
Adalimumab for psoriasis: Blocking TNF-alpha had no effect on vascular inflammation
ORLANDO – Vascular inflammation was no different in patients with moderate to severe psoriasis after 16 weeks of treatment with adalimumab than in untreated controls, according to a study that evaluated the impact of blocking tumor necrosis factor–alpha on vascular inflammation.
Further, there was a modest increase in vascular inflammation in carotid arteries after 52 weeks of treatment with the tumor necrosis factor (TNF) alpha-antagonist adalimumab, Robert Bissonnette, MD, president of Innovaderm Research, Montreal, reported in a late-breaking session at the annual meeting of the American Academy of Dermatology.
At 16 weeks, there were no significant differences in vascular inflammation between the treatment and control arms, based on the change from baseline in the vessel wall target to background ratio from the ascending aorta (the primary endpoint). In the carotid arteries at 16 weeks, differences in vascular inflammation between the adalimumab group and control group were also not significant.
At 52 weeks, there was no significant change in target to background ratio from the ascending aorta between baseline and the start of adalimumab, although in the carotid arteries, there was a modest increase in vascular inflammation.
Several previous studies have suggested that reducing inflammation in psoriasis can also reduce the risk of some cardiovascular events. As to why this study did not demonstrate a correlation between vascular inflammation and treatment, Dr. Bissonnette said during the question and answer portion of the presentation that “either the dose of adalimumab that is used for psoriasis has no impact on vascular inflammation or it may be possible that levels of interleukin-6, a key cytokine correlated with vascular inflammation, were very low in our study.”
Interleukin-6 typically is increased in patients with psoriatic arthritis, he explained, noting that in this study, only 7.5% of patients in the treatment group and about 10% of those in the control group had a history of psoriatic arthritis.
Additionally, at baseline, high-sensitivity C-reactive protein levels were significantly higher in controls: 5.32, compared with 2.72 in the treatment arm (P = .003).
Another possible reason for the results could be the molecule size of adalimumab, session comoderator Joel Gelfand, MD, noted in an interview. “The drug may not penetrate the aorta. These are large molecules. It also might be that [TNF–alpha] is not the main driver of aortic inflammation in people with psoriasis. Increasingly, we think of people with psoriasis as an IL-23 and IL-17 disease, so maybe that is part of it,” said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. He also directs the psoriasis and phototherapy treatment center at the university.
In addition to being presented at the meeting, the results were published in the Journal of Investigative Dermatology (2017 Feb 7. doi: 10.1016/j.jid.2017.02.977).
Adalimumab is marketed as Humira by Abbvie; a biosimilar version, adalimumab-atto (Amjevita), was approved in the United States in 2016.
Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
ORLANDO – Vascular inflammation was no different in patients with moderate to severe psoriasis after 16 weeks of treatment with adalimumab than in untreated controls, according to a study that evaluated the impact of blocking tumor necrosis factor–alpha on vascular inflammation.
Further, there was a modest increase in vascular inflammation in carotid arteries after 52 weeks of treatment with the tumor necrosis factor (TNF) alpha-antagonist adalimumab, Robert Bissonnette, MD, president of Innovaderm Research, Montreal, reported in a late-breaking session at the annual meeting of the American Academy of Dermatology.
At 16 weeks, there were no significant differences in vascular inflammation between the treatment and control arms, based on the change from baseline in the vessel wall target to background ratio from the ascending aorta (the primary endpoint). In the carotid arteries at 16 weeks, differences in vascular inflammation between the adalimumab group and control group were also not significant.
At 52 weeks, there was no significant change in target to background ratio from the ascending aorta between baseline and the start of adalimumab, although in the carotid arteries, there was a modest increase in vascular inflammation.
Several previous studies have suggested that reducing inflammation in psoriasis can also reduce the risk of some cardiovascular events. As to why this study did not demonstrate a correlation between vascular inflammation and treatment, Dr. Bissonnette said during the question and answer portion of the presentation that “either the dose of adalimumab that is used for psoriasis has no impact on vascular inflammation or it may be possible that levels of interleukin-6, a key cytokine correlated with vascular inflammation, were very low in our study.”
Interleukin-6 typically is increased in patients with psoriatic arthritis, he explained, noting that in this study, only 7.5% of patients in the treatment group and about 10% of those in the control group had a history of psoriatic arthritis.
Additionally, at baseline, high-sensitivity C-reactive protein levels were significantly higher in controls: 5.32, compared with 2.72 in the treatment arm (P = .003).
Another possible reason for the results could be the molecule size of adalimumab, session comoderator Joel Gelfand, MD, noted in an interview. “The drug may not penetrate the aorta. These are large molecules. It also might be that [TNF–alpha] is not the main driver of aortic inflammation in people with psoriasis. Increasingly, we think of people with psoriasis as an IL-23 and IL-17 disease, so maybe that is part of it,” said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. He also directs the psoriasis and phototherapy treatment center at the university.
In addition to being presented at the meeting, the results were published in the Journal of Investigative Dermatology (2017 Feb 7. doi: 10.1016/j.jid.2017.02.977).
Adalimumab is marketed as Humira by Abbvie; a biosimilar version, adalimumab-atto (Amjevita), was approved in the United States in 2016.
Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
ORLANDO – Vascular inflammation was no different in patients with moderate to severe psoriasis after 16 weeks of treatment with adalimumab than in untreated controls, according to a study that evaluated the impact of blocking tumor necrosis factor–alpha on vascular inflammation.
Further, there was a modest increase in vascular inflammation in carotid arteries after 52 weeks of treatment with the tumor necrosis factor (TNF) alpha-antagonist adalimumab, Robert Bissonnette, MD, president of Innovaderm Research, Montreal, reported in a late-breaking session at the annual meeting of the American Academy of Dermatology.
At 16 weeks, there were no significant differences in vascular inflammation between the treatment and control arms, based on the change from baseline in the vessel wall target to background ratio from the ascending aorta (the primary endpoint). In the carotid arteries at 16 weeks, differences in vascular inflammation between the adalimumab group and control group were also not significant.
At 52 weeks, there was no significant change in target to background ratio from the ascending aorta between baseline and the start of adalimumab, although in the carotid arteries, there was a modest increase in vascular inflammation.
Several previous studies have suggested that reducing inflammation in psoriasis can also reduce the risk of some cardiovascular events. As to why this study did not demonstrate a correlation between vascular inflammation and treatment, Dr. Bissonnette said during the question and answer portion of the presentation that “either the dose of adalimumab that is used for psoriasis has no impact on vascular inflammation or it may be possible that levels of interleukin-6, a key cytokine correlated with vascular inflammation, were very low in our study.”
Interleukin-6 typically is increased in patients with psoriatic arthritis, he explained, noting that in this study, only 7.5% of patients in the treatment group and about 10% of those in the control group had a history of psoriatic arthritis.
Additionally, at baseline, high-sensitivity C-reactive protein levels were significantly higher in controls: 5.32, compared with 2.72 in the treatment arm (P = .003).
Another possible reason for the results could be the molecule size of adalimumab, session comoderator Joel Gelfand, MD, noted in an interview. “The drug may not penetrate the aorta. These are large molecules. It also might be that [TNF–alpha] is not the main driver of aortic inflammation in people with psoriasis. Increasingly, we think of people with psoriasis as an IL-23 and IL-17 disease, so maybe that is part of it,” said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. He also directs the psoriasis and phototherapy treatment center at the university.
In addition to being presented at the meeting, the results were published in the Journal of Investigative Dermatology (2017 Feb 7. doi: 10.1016/j.jid.2017.02.977).
Adalimumab is marketed as Humira by Abbvie; a biosimilar version, adalimumab-atto (Amjevita), was approved in the United States in 2016.
Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
Key clinical point:
Major finding: At 16 weeks, there were no significant differences in aortic and carotid inflammation in the change from baseline in moderate psoriasis patients given adalimumab and controls.
Data source: A randomized, double-blind multicenter study that used PET-CT scans to evaluate the impact of treatment on vascular inflammation in 107 adults with moderate to severe psoriasis.
Disclosures: Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
VIDEO: Stress alleviation strategies boost inflammatory skin treatment regimens
ORLANDO – “Consider the effects of the mind on the skin when treating patients with some skin diseases.” That was the message of several speakers during a session titled “The Skin and the Mind” at the annual meeting of the American Academy of Dermatology.
“I certainly believe that the time has come to use stress alleviation techniques, at least for selected patients, and this should certainly be part of our armamentarium in the clinic,” said Richard D. Granstein, MD, George W. Hambrick Jr. professor and chairman of the department of dermatology, Cornell University, New York. During the session, he spoke about the emerging science of stress in dermatology.
While it has long been believed that stress exacerbates different skin diseases, the connection has been difficult to prove scientifically, he pointed out. However, “the overwhelming number of reports and studies indicate that stress probably does exacerbate a number of inflammatory skin diseases,” he added.
In a video interview at the meeting, Dr. Granstein notes that a number of pathways that help explain this link have now been identified and discusses one of those pathways, which involves neuropeptides and future directions in understanding the relationship between stress and inflammatory skin diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Granstein disclosed serving as an advisor to Castle Biosciences, Elysium Health, Galderma Laboratories, and Velius.
ORLANDO – “Consider the effects of the mind on the skin when treating patients with some skin diseases.” That was the message of several speakers during a session titled “The Skin and the Mind” at the annual meeting of the American Academy of Dermatology.
“I certainly believe that the time has come to use stress alleviation techniques, at least for selected patients, and this should certainly be part of our armamentarium in the clinic,” said Richard D. Granstein, MD, George W. Hambrick Jr. professor and chairman of the department of dermatology, Cornell University, New York. During the session, he spoke about the emerging science of stress in dermatology.
While it has long been believed that stress exacerbates different skin diseases, the connection has been difficult to prove scientifically, he pointed out. However, “the overwhelming number of reports and studies indicate that stress probably does exacerbate a number of inflammatory skin diseases,” he added.
In a video interview at the meeting, Dr. Granstein notes that a number of pathways that help explain this link have now been identified and discusses one of those pathways, which involves neuropeptides and future directions in understanding the relationship between stress and inflammatory skin diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Granstein disclosed serving as an advisor to Castle Biosciences, Elysium Health, Galderma Laboratories, and Velius.
ORLANDO – “Consider the effects of the mind on the skin when treating patients with some skin diseases.” That was the message of several speakers during a session titled “The Skin and the Mind” at the annual meeting of the American Academy of Dermatology.
“I certainly believe that the time has come to use stress alleviation techniques, at least for selected patients, and this should certainly be part of our armamentarium in the clinic,” said Richard D. Granstein, MD, George W. Hambrick Jr. professor and chairman of the department of dermatology, Cornell University, New York. During the session, he spoke about the emerging science of stress in dermatology.
While it has long been believed that stress exacerbates different skin diseases, the connection has been difficult to prove scientifically, he pointed out. However, “the overwhelming number of reports and studies indicate that stress probably does exacerbate a number of inflammatory skin diseases,” he added.
In a video interview at the meeting, Dr. Granstein notes that a number of pathways that help explain this link have now been identified and discusses one of those pathways, which involves neuropeptides and future directions in understanding the relationship between stress and inflammatory skin diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Granstein disclosed serving as an advisor to Castle Biosciences, Elysium Health, Galderma Laboratories, and Velius.
AT AAD 17