User login
FDA approves abatacept for adults with psoriatic arthritis
The Food and Drug Administration has approved abatacept, a selective T-cell costimulation modulator, for treating adults with active psoriatic arthritis (PsA), the manufacturer, Bristol-Myers Squibb, has announced.
Approval of abatacept (Orencia) was based on two randomized, double-blind, placebo-controlled studies (PsA-I and PsA-II) in 594 adults with PsA for more than 7 years, according to the July 6 announcement. Patients had active PsA (at least three swollen joints and at least three tender joints), despite previous disease-modifying antirheumatic drug (DMARD) therapy and had one qualifying psoriatic skin lesion measuring at least 2 cm in diameter. The studies included patients treated with TNF inhibitors (TNFi) previously.
In the PsA-II trial, 424 patients received weekly doses of placebo or abatacept 25 mg administered subcutaneously (SC) without a loading dose for 24 weeks, followed by open-label abatacept at a dose of 125 mg SC weekly.
Compared with those on placebo, more patients treated with abatacept 10 mg/kg IV or 125 mg SC achieved an ACR 20 (American College of Rheumatology 20) response at 24 weeks: 47.5% vs. 19.0% and 39.4% vs. 22.3%, respectively (P less than .05).
Other results included a greater proportion of abatacept SC patients with at least a 0.35 decrease from baseline on the Health Assessment Questionnaire-Disability Index: 31% vs. 24% on placebo at 24 weeks. Responses were seen regardless of prior anti-TNFi treatment and regardless of concomitant non-biologic DMARD treatment. In addition, patients on abatacept IV and SC had improvements in enthesitis and dactylitis at 24 weeks.
The safety profile of abatacept in the two studies was “consistent with the safety profile” in rheumatoid arthritis, according to the company release.
Abatacept, initially approved in 2005, was previously approved for RA in adults and for juvenile idiopathic arthritis
Find the updated prescribing information for abatacept here.
The Food and Drug Administration has approved abatacept, a selective T-cell costimulation modulator, for treating adults with active psoriatic arthritis (PsA), the manufacturer, Bristol-Myers Squibb, has announced.
Approval of abatacept (Orencia) was based on two randomized, double-blind, placebo-controlled studies (PsA-I and PsA-II) in 594 adults with PsA for more than 7 years, according to the July 6 announcement. Patients had active PsA (at least three swollen joints and at least three tender joints), despite previous disease-modifying antirheumatic drug (DMARD) therapy and had one qualifying psoriatic skin lesion measuring at least 2 cm in diameter. The studies included patients treated with TNF inhibitors (TNFi) previously.
In the PsA-II trial, 424 patients received weekly doses of placebo or abatacept 25 mg administered subcutaneously (SC) without a loading dose for 24 weeks, followed by open-label abatacept at a dose of 125 mg SC weekly.
Compared with those on placebo, more patients treated with abatacept 10 mg/kg IV or 125 mg SC achieved an ACR 20 (American College of Rheumatology 20) response at 24 weeks: 47.5% vs. 19.0% and 39.4% vs. 22.3%, respectively (P less than .05).
Other results included a greater proportion of abatacept SC patients with at least a 0.35 decrease from baseline on the Health Assessment Questionnaire-Disability Index: 31% vs. 24% on placebo at 24 weeks. Responses were seen regardless of prior anti-TNFi treatment and regardless of concomitant non-biologic DMARD treatment. In addition, patients on abatacept IV and SC had improvements in enthesitis and dactylitis at 24 weeks.
The safety profile of abatacept in the two studies was “consistent with the safety profile” in rheumatoid arthritis, according to the company release.
Abatacept, initially approved in 2005, was previously approved for RA in adults and for juvenile idiopathic arthritis
Find the updated prescribing information for abatacept here.
The Food and Drug Administration has approved abatacept, a selective T-cell costimulation modulator, for treating adults with active psoriatic arthritis (PsA), the manufacturer, Bristol-Myers Squibb, has announced.
Approval of abatacept (Orencia) was based on two randomized, double-blind, placebo-controlled studies (PsA-I and PsA-II) in 594 adults with PsA for more than 7 years, according to the July 6 announcement. Patients had active PsA (at least three swollen joints and at least three tender joints), despite previous disease-modifying antirheumatic drug (DMARD) therapy and had one qualifying psoriatic skin lesion measuring at least 2 cm in diameter. The studies included patients treated with TNF inhibitors (TNFi) previously.
In the PsA-II trial, 424 patients received weekly doses of placebo or abatacept 25 mg administered subcutaneously (SC) without a loading dose for 24 weeks, followed by open-label abatacept at a dose of 125 mg SC weekly.
Compared with those on placebo, more patients treated with abatacept 10 mg/kg IV or 125 mg SC achieved an ACR 20 (American College of Rheumatology 20) response at 24 weeks: 47.5% vs. 19.0% and 39.4% vs. 22.3%, respectively (P less than .05).
Other results included a greater proportion of abatacept SC patients with at least a 0.35 decrease from baseline on the Health Assessment Questionnaire-Disability Index: 31% vs. 24% on placebo at 24 weeks. Responses were seen regardless of prior anti-TNFi treatment and regardless of concomitant non-biologic DMARD treatment. In addition, patients on abatacept IV and SC had improvements in enthesitis and dactylitis at 24 weeks.
The safety profile of abatacept in the two studies was “consistent with the safety profile” in rheumatoid arthritis, according to the company release.
Abatacept, initially approved in 2005, was previously approved for RA in adults and for juvenile idiopathic arthritis
Find the updated prescribing information for abatacept here.
Phototherapy Coding and Documentation in the Time of Biologics
In this era of biologics for psoriasis with ever-increasing effectiveness and safety as well as patients who have less and less time to visit the physician's office, it would seem that the days of in-office UV treatments would be numbered. However, rumors of the demise of phototherapy may be greatly exaggerated. Phototherapy is still one of the safest and most cost-effective treatments for psoriasis and other dermatoses.1 Its use often is a prerequisite for biologic therapy, and it may be the only therapeutic option for certain subsets of patients, such as children, pregnant women, and immunosuppressed patients. Moreover, narrowband UVB technology has breathed new life into phototherapy, with better efficacy and less long-term risk. Although the utilization of psoralen plus UVA (PUVA) light therapy has indeed decreased over the last 2 decades, the use of UVB therapies continues to increase dramatically.2
Phototherapy Codes
There are 4 chief Current Procedural Terminology (CPT) codes for reporting phototherapy services: (1) 96900: actinotherapy (UV light treatment); (2) 96910: photochemotherapy, tar, and UVB (Goeckerman treatment) or petrolatum and UVB; (3) 96912: photochemotherapy and PUVA; and (4) 96913: photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician.3
There is lack of specificity of the CPT code descriptions for phototherapy. Moreover, insurer guidance for documentation for phototherapy is vague to nonexistent, and of course whenever the use of any medical service increases, insurer scrutiny is sure to follow. Therefore, it is not surprising that dermatology practices have reported that private insurers as well as Medicare are auditing medical records for phototherapy treatments.4 In fact, recently we have seen a Midwest private insurer demand payment from dermatologists for hundreds of 96910 phototherapy services, which the insurer asserted should have been coded as 96900 because topical therapies were not applied by the dermatology staff. The insurer did not just evaluate medical records but also contacted patients directly and asked how services had been provided. Clearly, more detailed guidance for dermatologists and insurers on documentation and performance standards for each phototherapy service is needed.
Existing coding guidance for phototherapy indicates that actinotherapy (96900) defines the basic service of treating a patient with a UV light unit.5 Actinotherapy does not involve application of topical medications while the patient is in the office.
In contrast, photochemotherapy (96910) implies addition of a chemo agent to phototherapy. Despite the somewhat nonspecific nature of the code descriptor, it is apparent that application of photoenhancing agents such as tar, petrolatum, or distillates of petrolatum meet the requirements of 96910. The Coder's Desk Reference for Procedures 2017 describes 96910 as "the physician uses photosensitizing chemicals and light rays to treat skin ailments."6 Application of light-enhancing topical products should occur within the office by either staff or the patient. In fact, examination of practice expense data from the Centers for Medicare & Medicaid Services indicated that the 96910 code includes payment for clinical staff time to apply topical products as well as the cost of the topical agent(s).7
The PUVA code 96912 is defined by the use of photosensitizing psoralen medication, which can be administered topically or orally, followed by UVA treatment. In my experience, PUVA has similar performance standards with in-office application of psoralen, if applicable. If application of topical photoenhancing products occurs outside the office, the requirements of photochemotherapy are not met, and 96900 should be reported.
The 96913 code defines prolonged phototherapy service with intensive topical therapy requirements and multiple phototherapy sessions per day.3 This code is rarely reported (average of fewer than 100 times in the Medicare population per year), and most insurers do not reimburse this service.
Protecting Yourself From an Audit
In my experience, review of private insurer audits of phototherapy services has yielded important lessons. First, having a written standard operating procedure in place regarding the performance of phototherapy services and how application of topicals will be handled has been helpful in audit defense. The other key to beating audits for phototherapy services is to have detailed documentation or a flowchart in the medical record regarding the topical agent and the light administration. The medical record should include what topical agent was applied, if any; whether the topical agent was applied in the office; where the topical product was applied; and who applied the topical product. Sometimes topical product application by a physician or staff is not feasible because of patient preference or the site of application. If the patient applied the topical, document that assistance was offered and refused, along with what type of UV light was used and the dosage. Inclusion of these elements in the medical record provides a clear picture of the delivery of the phototherapy service and will aid in responding to medical record audit.
Final Thoughts
Phototherapy is a critical treatment modality that continues to be utilized frequently in the expanding armamentarium of treatments for dermatoses. Phototherapy is performed almost exclusively by dermatologists and allows dermatologists to offer a unique level of care and value in the treatment of skin disease. Careful documentation, a written standard operating procedure, and adherence to proper performance standards will allow dermatologists to be compensated fairly for this important treatment modality and pass audits that are likely to occur.
- Lapolla W, Yentzer BA, Bagel J, et al. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936-949.
- Simpson GL, Yelverton CB, Rittenberg S, et al. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359-361.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- American Academy of Dermatology Association. Insurers review billing for photochemotherapy (CPT 96910). Derm Coding Consult. Spring 2009;13:4.
- American Academy of Dermatology Association. Coding Q&A's. Derm Coding Consult. Spring 2007;11:5, 7, 8.
- Coders' Desk Reference for Procedures 2017. Chicago, IL: Optum360; 2017.
- Relative Value Scale Update Committee Database. Chicago, IL: American Medical Association; 2016.
In this era of biologics for psoriasis with ever-increasing effectiveness and safety as well as patients who have less and less time to visit the physician's office, it would seem that the days of in-office UV treatments would be numbered. However, rumors of the demise of phototherapy may be greatly exaggerated. Phototherapy is still one of the safest and most cost-effective treatments for psoriasis and other dermatoses.1 Its use often is a prerequisite for biologic therapy, and it may be the only therapeutic option for certain subsets of patients, such as children, pregnant women, and immunosuppressed patients. Moreover, narrowband UVB technology has breathed new life into phototherapy, with better efficacy and less long-term risk. Although the utilization of psoralen plus UVA (PUVA) light therapy has indeed decreased over the last 2 decades, the use of UVB therapies continues to increase dramatically.2
Phototherapy Codes
There are 4 chief Current Procedural Terminology (CPT) codes for reporting phototherapy services: (1) 96900: actinotherapy (UV light treatment); (2) 96910: photochemotherapy, tar, and UVB (Goeckerman treatment) or petrolatum and UVB; (3) 96912: photochemotherapy and PUVA; and (4) 96913: photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician.3
There is lack of specificity of the CPT code descriptions for phototherapy. Moreover, insurer guidance for documentation for phototherapy is vague to nonexistent, and of course whenever the use of any medical service increases, insurer scrutiny is sure to follow. Therefore, it is not surprising that dermatology practices have reported that private insurers as well as Medicare are auditing medical records for phototherapy treatments.4 In fact, recently we have seen a Midwest private insurer demand payment from dermatologists for hundreds of 96910 phototherapy services, which the insurer asserted should have been coded as 96900 because topical therapies were not applied by the dermatology staff. The insurer did not just evaluate medical records but also contacted patients directly and asked how services had been provided. Clearly, more detailed guidance for dermatologists and insurers on documentation and performance standards for each phototherapy service is needed.
Existing coding guidance for phototherapy indicates that actinotherapy (96900) defines the basic service of treating a patient with a UV light unit.5 Actinotherapy does not involve application of topical medications while the patient is in the office.
In contrast, photochemotherapy (96910) implies addition of a chemo agent to phototherapy. Despite the somewhat nonspecific nature of the code descriptor, it is apparent that application of photoenhancing agents such as tar, petrolatum, or distillates of petrolatum meet the requirements of 96910. The Coder's Desk Reference for Procedures 2017 describes 96910 as "the physician uses photosensitizing chemicals and light rays to treat skin ailments."6 Application of light-enhancing topical products should occur within the office by either staff or the patient. In fact, examination of practice expense data from the Centers for Medicare & Medicaid Services indicated that the 96910 code includes payment for clinical staff time to apply topical products as well as the cost of the topical agent(s).7
The PUVA code 96912 is defined by the use of photosensitizing psoralen medication, which can be administered topically or orally, followed by UVA treatment. In my experience, PUVA has similar performance standards with in-office application of psoralen, if applicable. If application of topical photoenhancing products occurs outside the office, the requirements of photochemotherapy are not met, and 96900 should be reported.
The 96913 code defines prolonged phototherapy service with intensive topical therapy requirements and multiple phototherapy sessions per day.3 This code is rarely reported (average of fewer than 100 times in the Medicare population per year), and most insurers do not reimburse this service.
Protecting Yourself From an Audit
In my experience, review of private insurer audits of phototherapy services has yielded important lessons. First, having a written standard operating procedure in place regarding the performance of phototherapy services and how application of topicals will be handled has been helpful in audit defense. The other key to beating audits for phototherapy services is to have detailed documentation or a flowchart in the medical record regarding the topical agent and the light administration. The medical record should include what topical agent was applied, if any; whether the topical agent was applied in the office; where the topical product was applied; and who applied the topical product. Sometimes topical product application by a physician or staff is not feasible because of patient preference or the site of application. If the patient applied the topical, document that assistance was offered and refused, along with what type of UV light was used and the dosage. Inclusion of these elements in the medical record provides a clear picture of the delivery of the phototherapy service and will aid in responding to medical record audit.
Final Thoughts
Phototherapy is a critical treatment modality that continues to be utilized frequently in the expanding armamentarium of treatments for dermatoses. Phototherapy is performed almost exclusively by dermatologists and allows dermatologists to offer a unique level of care and value in the treatment of skin disease. Careful documentation, a written standard operating procedure, and adherence to proper performance standards will allow dermatologists to be compensated fairly for this important treatment modality and pass audits that are likely to occur.
In this era of biologics for psoriasis with ever-increasing effectiveness and safety as well as patients who have less and less time to visit the physician's office, it would seem that the days of in-office UV treatments would be numbered. However, rumors of the demise of phototherapy may be greatly exaggerated. Phototherapy is still one of the safest and most cost-effective treatments for psoriasis and other dermatoses.1 Its use often is a prerequisite for biologic therapy, and it may be the only therapeutic option for certain subsets of patients, such as children, pregnant women, and immunosuppressed patients. Moreover, narrowband UVB technology has breathed new life into phototherapy, with better efficacy and less long-term risk. Although the utilization of psoralen plus UVA (PUVA) light therapy has indeed decreased over the last 2 decades, the use of UVB therapies continues to increase dramatically.2
Phototherapy Codes
There are 4 chief Current Procedural Terminology (CPT) codes for reporting phototherapy services: (1) 96900: actinotherapy (UV light treatment); (2) 96910: photochemotherapy, tar, and UVB (Goeckerman treatment) or petrolatum and UVB; (3) 96912: photochemotherapy and PUVA; and (4) 96913: photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician.3
There is lack of specificity of the CPT code descriptions for phototherapy. Moreover, insurer guidance for documentation for phototherapy is vague to nonexistent, and of course whenever the use of any medical service increases, insurer scrutiny is sure to follow. Therefore, it is not surprising that dermatology practices have reported that private insurers as well as Medicare are auditing medical records for phototherapy treatments.4 In fact, recently we have seen a Midwest private insurer demand payment from dermatologists for hundreds of 96910 phototherapy services, which the insurer asserted should have been coded as 96900 because topical therapies were not applied by the dermatology staff. The insurer did not just evaluate medical records but also contacted patients directly and asked how services had been provided. Clearly, more detailed guidance for dermatologists and insurers on documentation and performance standards for each phototherapy service is needed.
Existing coding guidance for phototherapy indicates that actinotherapy (96900) defines the basic service of treating a patient with a UV light unit.5 Actinotherapy does not involve application of topical medications while the patient is in the office.
In contrast, photochemotherapy (96910) implies addition of a chemo agent to phototherapy. Despite the somewhat nonspecific nature of the code descriptor, it is apparent that application of photoenhancing agents such as tar, petrolatum, or distillates of petrolatum meet the requirements of 96910. The Coder's Desk Reference for Procedures 2017 describes 96910 as "the physician uses photosensitizing chemicals and light rays to treat skin ailments."6 Application of light-enhancing topical products should occur within the office by either staff or the patient. In fact, examination of practice expense data from the Centers for Medicare & Medicaid Services indicated that the 96910 code includes payment for clinical staff time to apply topical products as well as the cost of the topical agent(s).7
The PUVA code 96912 is defined by the use of photosensitizing psoralen medication, which can be administered topically or orally, followed by UVA treatment. In my experience, PUVA has similar performance standards with in-office application of psoralen, if applicable. If application of topical photoenhancing products occurs outside the office, the requirements of photochemotherapy are not met, and 96900 should be reported.
The 96913 code defines prolonged phototherapy service with intensive topical therapy requirements and multiple phototherapy sessions per day.3 This code is rarely reported (average of fewer than 100 times in the Medicare population per year), and most insurers do not reimburse this service.
Protecting Yourself From an Audit
In my experience, review of private insurer audits of phototherapy services has yielded important lessons. First, having a written standard operating procedure in place regarding the performance of phototherapy services and how application of topicals will be handled has been helpful in audit defense. The other key to beating audits for phototherapy services is to have detailed documentation or a flowchart in the medical record regarding the topical agent and the light administration. The medical record should include what topical agent was applied, if any; whether the topical agent was applied in the office; where the topical product was applied; and who applied the topical product. Sometimes topical product application by a physician or staff is not feasible because of patient preference or the site of application. If the patient applied the topical, document that assistance was offered and refused, along with what type of UV light was used and the dosage. Inclusion of these elements in the medical record provides a clear picture of the delivery of the phototherapy service and will aid in responding to medical record audit.
Final Thoughts
Phototherapy is a critical treatment modality that continues to be utilized frequently in the expanding armamentarium of treatments for dermatoses. Phototherapy is performed almost exclusively by dermatologists and allows dermatologists to offer a unique level of care and value in the treatment of skin disease. Careful documentation, a written standard operating procedure, and adherence to proper performance standards will allow dermatologists to be compensated fairly for this important treatment modality and pass audits that are likely to occur.
- Lapolla W, Yentzer BA, Bagel J, et al. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936-949.
- Simpson GL, Yelverton CB, Rittenberg S, et al. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359-361.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- American Academy of Dermatology Association. Insurers review billing for photochemotherapy (CPT 96910). Derm Coding Consult. Spring 2009;13:4.
- American Academy of Dermatology Association. Coding Q&A's. Derm Coding Consult. Spring 2007;11:5, 7, 8.
- Coders' Desk Reference for Procedures 2017. Chicago, IL: Optum360; 2017.
- Relative Value Scale Update Committee Database. Chicago, IL: American Medical Association; 2016.
- Lapolla W, Yentzer BA, Bagel J, et al. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936-949.
- Simpson GL, Yelverton CB, Rittenberg S, et al. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359-361.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- American Academy of Dermatology Association. Insurers review billing for photochemotherapy (CPT 96910). Derm Coding Consult. Spring 2009;13:4.
- American Academy of Dermatology Association. Coding Q&A's. Derm Coding Consult. Spring 2007;11:5, 7, 8.
- Coders' Desk Reference for Procedures 2017. Chicago, IL: Optum360; 2017.
- Relative Value Scale Update Committee Database. Chicago, IL: American Medical Association; 2016.
Topical Cannabinoids in Dermatology
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
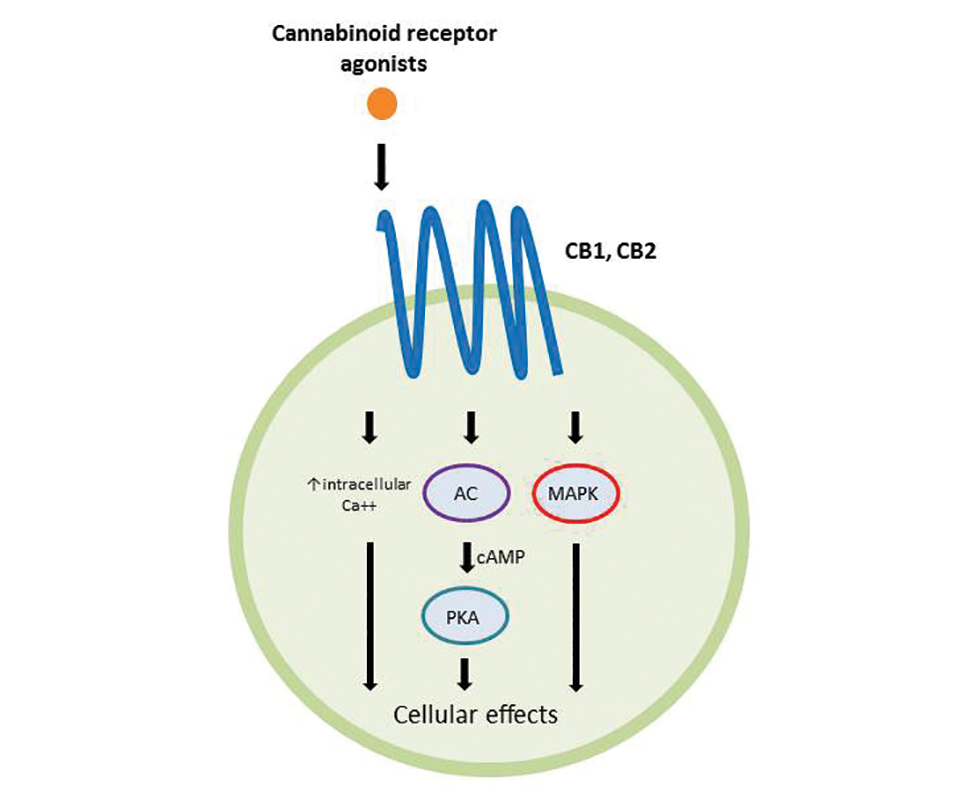
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.
Topical Cannabinoids in Inflammatory Disorders
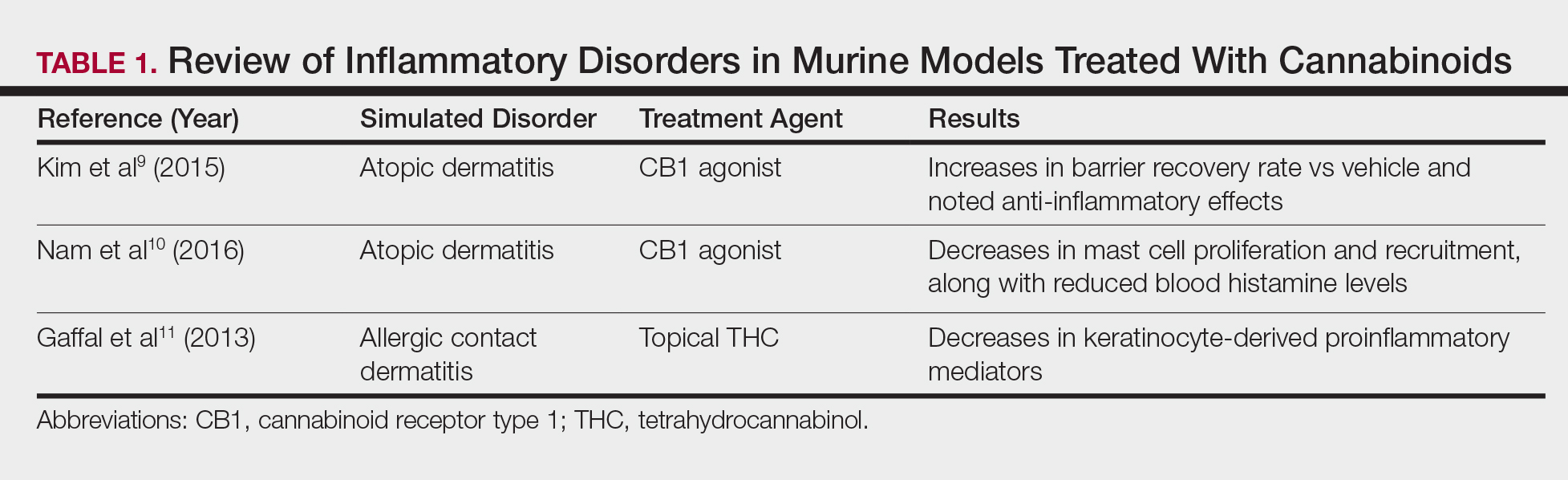
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
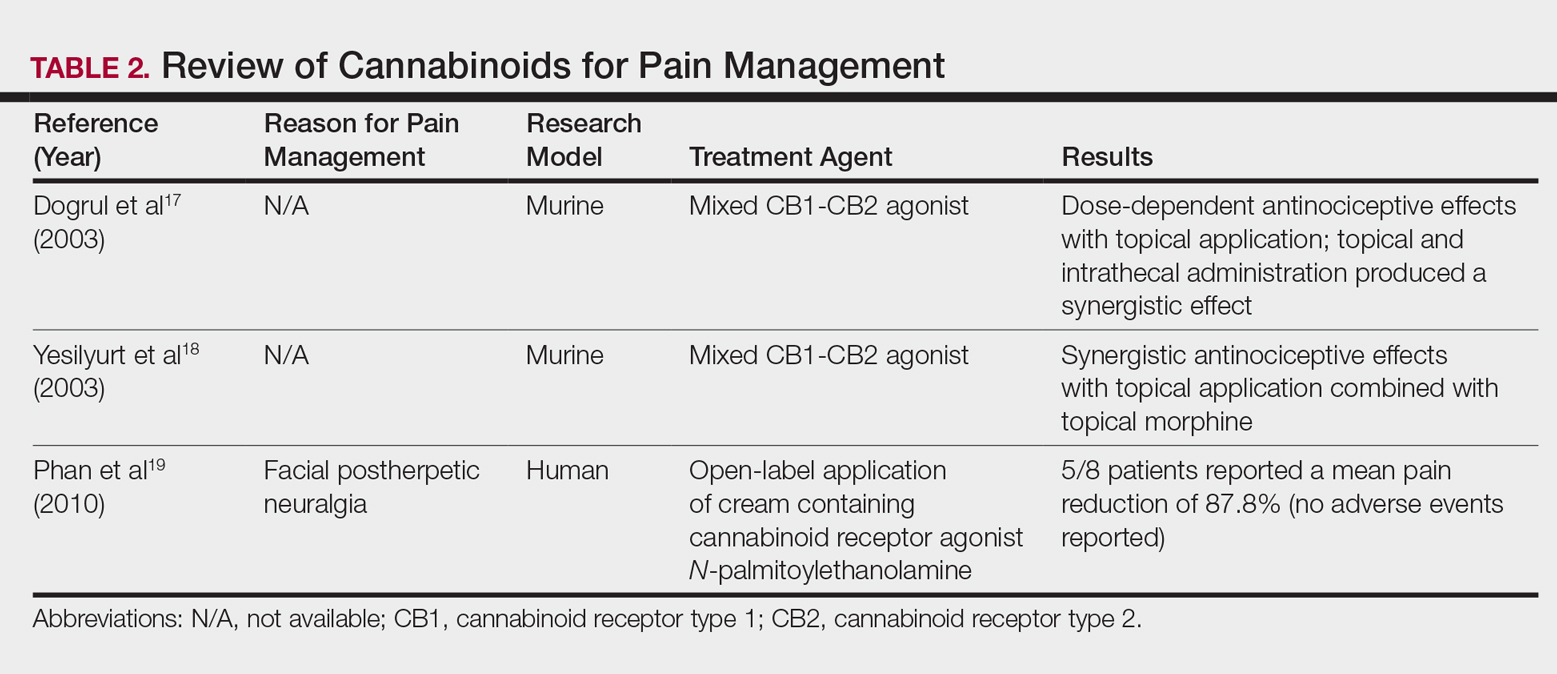
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
Practice Points
- Topical cannabinoids are advertised by companies as treatment options for numerous dermatologic conditions.
- Despite promising data in rodent models, there have been no rigorous studies to date confirming efficacy or safety in humans.
- Dermatologists should therefore inquire with patients about the use of any topical cannabinoid products, especially around the time of planned procedures, as they may affect treatment outcomes.
Early phase III data positive for adalimumab biosimilar, for both psoriasis and PsA
MADRID – To date, an adalimumab biosimilar has proven itself in a large, phase III trial of patients with psoriasis, including a subset with mild to moderate psoriatic arthritis (PsA).
The biosimilar, known as CHS-1420, cleared psoriatic plaques and improved health-related quality of life just as well as adalimumab after 12 weeks of treatment, Barbara Finck, MD, said at the European Congress of Rheumatology. It also suppressed high-sensitivity C-reactive protein (CRP) as well as the originator molecule, she said.
Dr. Finck, the chief medical officer of Coherus Biosciences, the developer of CHS-1420, reported results from the first 16-week phase of the 48-week study. Data are still to come on a 6-week period during which half those taking adalimumab switched to CHS-1420 in a blinded fashion, and 26 weeks of open-label CHS-1420 for all patients.
The study’s primary endpoint was a 75% reduction in the Psoriasis Area and Severity Index (PASI) score (PASI 75) Two additional endpoints were evaluated in patients with PsA: change in the Health Assessment Questionnaire-Disability Index (HAQ-DI) and changes in CRP.
Dr. Finck bemoaned the lack of the clinical rheumatologic endpoint, tender and swollen joint count. “I advocated for this but was unable to convince our dermatology colleagues” to conduct this exam, she said. “I think we have a ways to go to educate our colleagues in this regard.”
The study comprised 545 patients with mild to moderate psoriasis; of these, 127 had PsA. They received subcutaneous injections of either CHS-1420 or adalimumab at identical doses (80 mg at week 1, followed by 40 mg every other week). They were a mean of 44 years o
In the entire study population, treatment with CHS-1420 and adalimumab followed almost identical response curves. By week 4, 22% of the CHS-1420 group and 20% of the adalimumab group had reached a PASI75 response. By week 8, those numbers were 57% and 61%, respectively, and by week 12, they were 69% and 72% – not significantly different.
Response was similar in the subgroup of PsA patients: By week 12, 82% of the CHS-1420 group and 77% of the adalimumab group had reached a PASI 75. PsA patients also responded equally well to both medications on the HAQ-DI by week 12. At baseline, the mean HAQ-DI was about 1 in each group. At 12 weeks, it was reduced by about half a point in both groups. High-sensitivity CRP decreased similarly in the CHS-1420 and adalimumab groups as well (reductions of 8.9 mg/L and 6.3 mg/L, respectively).
Adalimumab, a tumor necrosis factor blocker, is a highly immunogenic molecule, and as such, many patients developed antibodies to both it and to CHS-1420. By week 12, 84% of both treatment groups had developed anti-drug antibodies and 32%, neutralizing antibodies. Among those with PsA, 82% taking CHS-1420 and 88% of those taking adalimumab developed antidrug antibodies. Neutralizing antibodies developed in 33% and 30%, respectively. Neither of these differences was statistically significant.
Other adverse events were similar, Dr. Finck noted. These included nasopharyngitis (9% of both groups), upper respiratory tract infection (6%), injection site reaction (4%), headache (3%), and worsening of psoriasis (1% for CHS-1420, and 3% for adalimumab).
If the switching study data are similarly positive, Coherus expects to file a Biologics License Application with the Food and Drug Administration in early 2018, Dr. Finck said.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
MADRID – To date, an adalimumab biosimilar has proven itself in a large, phase III trial of patients with psoriasis, including a subset with mild to moderate psoriatic arthritis (PsA).
The biosimilar, known as CHS-1420, cleared psoriatic plaques and improved health-related quality of life just as well as adalimumab after 12 weeks of treatment, Barbara Finck, MD, said at the European Congress of Rheumatology. It also suppressed high-sensitivity C-reactive protein (CRP) as well as the originator molecule, she said.
Dr. Finck, the chief medical officer of Coherus Biosciences, the developer of CHS-1420, reported results from the first 16-week phase of the 48-week study. Data are still to come on a 6-week period during which half those taking adalimumab switched to CHS-1420 in a blinded fashion, and 26 weeks of open-label CHS-1420 for all patients.
The study’s primary endpoint was a 75% reduction in the Psoriasis Area and Severity Index (PASI) score (PASI 75) Two additional endpoints were evaluated in patients with PsA: change in the Health Assessment Questionnaire-Disability Index (HAQ-DI) and changes in CRP.
Dr. Finck bemoaned the lack of the clinical rheumatologic endpoint, tender and swollen joint count. “I advocated for this but was unable to convince our dermatology colleagues” to conduct this exam, she said. “I think we have a ways to go to educate our colleagues in this regard.”
The study comprised 545 patients with mild to moderate psoriasis; of these, 127 had PsA. They received subcutaneous injections of either CHS-1420 or adalimumab at identical doses (80 mg at week 1, followed by 40 mg every other week). They were a mean of 44 years o
In the entire study population, treatment with CHS-1420 and adalimumab followed almost identical response curves. By week 4, 22% of the CHS-1420 group and 20% of the adalimumab group had reached a PASI75 response. By week 8, those numbers were 57% and 61%, respectively, and by week 12, they were 69% and 72% – not significantly different.
Response was similar in the subgroup of PsA patients: By week 12, 82% of the CHS-1420 group and 77% of the adalimumab group had reached a PASI 75. PsA patients also responded equally well to both medications on the HAQ-DI by week 12. At baseline, the mean HAQ-DI was about 1 in each group. At 12 weeks, it was reduced by about half a point in both groups. High-sensitivity CRP decreased similarly in the CHS-1420 and adalimumab groups as well (reductions of 8.9 mg/L and 6.3 mg/L, respectively).
Adalimumab, a tumor necrosis factor blocker, is a highly immunogenic molecule, and as such, many patients developed antibodies to both it and to CHS-1420. By week 12, 84% of both treatment groups had developed anti-drug antibodies and 32%, neutralizing antibodies. Among those with PsA, 82% taking CHS-1420 and 88% of those taking adalimumab developed antidrug antibodies. Neutralizing antibodies developed in 33% and 30%, respectively. Neither of these differences was statistically significant.
Other adverse events were similar, Dr. Finck noted. These included nasopharyngitis (9% of both groups), upper respiratory tract infection (6%), injection site reaction (4%), headache (3%), and worsening of psoriasis (1% for CHS-1420, and 3% for adalimumab).
If the switching study data are similarly positive, Coherus expects to file a Biologics License Application with the Food and Drug Administration in early 2018, Dr. Finck said.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
MADRID – To date, an adalimumab biosimilar has proven itself in a large, phase III trial of patients with psoriasis, including a subset with mild to moderate psoriatic arthritis (PsA).
The biosimilar, known as CHS-1420, cleared psoriatic plaques and improved health-related quality of life just as well as adalimumab after 12 weeks of treatment, Barbara Finck, MD, said at the European Congress of Rheumatology. It also suppressed high-sensitivity C-reactive protein (CRP) as well as the originator molecule, she said.
Dr. Finck, the chief medical officer of Coherus Biosciences, the developer of CHS-1420, reported results from the first 16-week phase of the 48-week study. Data are still to come on a 6-week period during which half those taking adalimumab switched to CHS-1420 in a blinded fashion, and 26 weeks of open-label CHS-1420 for all patients.
The study’s primary endpoint was a 75% reduction in the Psoriasis Area and Severity Index (PASI) score (PASI 75) Two additional endpoints were evaluated in patients with PsA: change in the Health Assessment Questionnaire-Disability Index (HAQ-DI) and changes in CRP.
Dr. Finck bemoaned the lack of the clinical rheumatologic endpoint, tender and swollen joint count. “I advocated for this but was unable to convince our dermatology colleagues” to conduct this exam, she said. “I think we have a ways to go to educate our colleagues in this regard.”
The study comprised 545 patients with mild to moderate psoriasis; of these, 127 had PsA. They received subcutaneous injections of either CHS-1420 or adalimumab at identical doses (80 mg at week 1, followed by 40 mg every other week). They were a mean of 44 years o
In the entire study population, treatment with CHS-1420 and adalimumab followed almost identical response curves. By week 4, 22% of the CHS-1420 group and 20% of the adalimumab group had reached a PASI75 response. By week 8, those numbers were 57% and 61%, respectively, and by week 12, they were 69% and 72% – not significantly different.
Response was similar in the subgroup of PsA patients: By week 12, 82% of the CHS-1420 group and 77% of the adalimumab group had reached a PASI 75. PsA patients also responded equally well to both medications on the HAQ-DI by week 12. At baseline, the mean HAQ-DI was about 1 in each group. At 12 weeks, it was reduced by about half a point in both groups. High-sensitivity CRP decreased similarly in the CHS-1420 and adalimumab groups as well (reductions of 8.9 mg/L and 6.3 mg/L, respectively).
Adalimumab, a tumor necrosis factor blocker, is a highly immunogenic molecule, and as such, many patients developed antibodies to both it and to CHS-1420. By week 12, 84% of both treatment groups had developed anti-drug antibodies and 32%, neutralizing antibodies. Among those with PsA, 82% taking CHS-1420 and 88% of those taking adalimumab developed antidrug antibodies. Neutralizing antibodies developed in 33% and 30%, respectively. Neither of these differences was statistically significant.
Other adverse events were similar, Dr. Finck noted. These included nasopharyngitis (9% of both groups), upper respiratory tract infection (6%), injection site reaction (4%), headache (3%), and worsening of psoriasis (1% for CHS-1420, and 3% for adalimumab).
If the switching study data are similarly positive, Coherus expects to file a Biologics License Application with the Food and Drug Administration in early 2018, Dr. Finck said.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: By week 12, 69% of those who received CHS-1420 and 72% of those who received adalimumab had reached a PASI75, response rates that were not significantly different.
Data source: The phase III trial randomized 545 patients with psoriasis, including 127 with PsA, to treatment with adalimumab or the biosimilar.
Disclosures: Dr. Finck is chief medical officer of Coherus Biosciences, which is developing CHS-1420.
TWEAKing inflammation: Studies reflect potential treatment target for psoriasis, atopic dermatitis
An immunomodulatory pathway that has been linked to cancer, kidney disease, and other disease processes is becoming a focus of dermatologic research.
New evidence suggests that TNF-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis family (TNF) superfamily, may be involved in both atopic dermatitis (AD) and psoriasis (Nat Commun. 2017 May 22;8:15395. doi: 10.1038/ncomms15395). The research showed that mice engineered to have low TWEAK levels had less severe disease when both AD and psoriasis were induced.
The TWEAK receptor, Fn14, was upregulated in keratinocytes and dermal fibroblasts in mouse disease models of AD and psoriasis, and TWEAK induced production of a range of cytokines associated with both AD and psoriasis. Subcutaneous injection of recombinant TWEAK led to cutaneous inflammation, as well as histological and molecular signals of the two diseases.
The pathophysiology of both AD and psoriasis is nebulously complex, sharing a similar theme of immune dysregulation, but historically polar opposites based on the different branches of the immune response implicated.
The study is not the only recent work tying TWEAK/Fn14 to dermatologic diseases. Other recent papers have shown evidence of their involvement in chronic cutaneous lupus (J Invest Dermatol. 2015;135[8]:1986-95), UVB irradiation-induced cutaneous lupus (Exp Dermatol. 2016 Dec;25[12]:969-76), and bullous pemphigoid (J Invest Dermatol. 2017 Jul;137[7]:1512-22).
The spate of findings hint that TWEAK/Fn14 could be a novel therapeutic pathway to attack inflammatory disease. Many therapies for autoimmune disease focus on immunosuppressive agents, which are associated with an increased risk of infection. But mice engineered to lack either TWEAK or Fn14 appear normal, and a phase I trial of an anti-TWEAK antibody in patients with rheumatoid arthritis did not reveal any worrisome safety concerns. “It doesn’t seem to have the broad immunosuppressive effects which characterize the therapies we currently use,” said Chaim Putterman, MD, chief of the division of rheumatology and professor of medicine and microbiology & immunology at the Albert Einstein College of Medicine, New York.
Instead, TWEAK seems to be regulating inflammation in target organs. It almost certainly plays a role in healthy functions like wound healing and cell survival, but Dr. Putterman believes there are redundant mechanisms that can pick up the slack, as the healthy knockout mice attest. The evidence suggests that the TWEAK pathway may become overactive in some diseases and, if so, a therapeutic antibody might be able to reset it to a more normal balance. “The utopian vision is that you would block this cytokine and bring its downstream effects back to normal levels, rather than totally abrogating its homeostatic functions,” Dr. Putterman noted.
Because blocking TWEAK has no apparent immunosuppressive effects, it might be a candidate for combination therapy with existing cytotoxic drugs. “If you have a disease like psoriasis where some standard of care medications are immunosuppressive, such as methotrexate, you might not get more risk by adding an antibody targeting TWEAK, as opposed to using immunosuppressives in combination. That, I think, has potential,” he said.
Work remains, however. A proof-of-concept study in lupus nephritis, sponsored by Biogen, failed to show a benefit when an anti-TWEAK antibody was combined with the standard of care.
But the potential impact of this approach holds much promise, and the fact that TWEAK has been linked to multiple diseases should make it a more attractive drug target for drug companies. “Now we have a target, that if you knock it out, or its receptor, you can potentially affect both diseases. This may the start of a whole new direction for biologics to treat inflammatory disease, and cancer as well,” Dr. Friedman said.
Dr. Putterman and Dr. Friedman were among the authors of the 2015 JID study on TWEAK/Fn14 signaling in spontaneous lupus and the Experimental Dermatology study. Dr. Putterman has research funding from Biogen Idec. Dr. Friedman had no related disclosures. The authors of the Nature Communications study were from the La Jolla Institute for Allergy and Immunology, and Biogen.
An immunomodulatory pathway that has been linked to cancer, kidney disease, and other disease processes is becoming a focus of dermatologic research.
New evidence suggests that TNF-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis family (TNF) superfamily, may be involved in both atopic dermatitis (AD) and psoriasis (Nat Commun. 2017 May 22;8:15395. doi: 10.1038/ncomms15395). The research showed that mice engineered to have low TWEAK levels had less severe disease when both AD and psoriasis were induced.
The TWEAK receptor, Fn14, was upregulated in keratinocytes and dermal fibroblasts in mouse disease models of AD and psoriasis, and TWEAK induced production of a range of cytokines associated with both AD and psoriasis. Subcutaneous injection of recombinant TWEAK led to cutaneous inflammation, as well as histological and molecular signals of the two diseases.
The pathophysiology of both AD and psoriasis is nebulously complex, sharing a similar theme of immune dysregulation, but historically polar opposites based on the different branches of the immune response implicated.
The study is not the only recent work tying TWEAK/Fn14 to dermatologic diseases. Other recent papers have shown evidence of their involvement in chronic cutaneous lupus (J Invest Dermatol. 2015;135[8]:1986-95), UVB irradiation-induced cutaneous lupus (Exp Dermatol. 2016 Dec;25[12]:969-76), and bullous pemphigoid (J Invest Dermatol. 2017 Jul;137[7]:1512-22).
The spate of findings hint that TWEAK/Fn14 could be a novel therapeutic pathway to attack inflammatory disease. Many therapies for autoimmune disease focus on immunosuppressive agents, which are associated with an increased risk of infection. But mice engineered to lack either TWEAK or Fn14 appear normal, and a phase I trial of an anti-TWEAK antibody in patients with rheumatoid arthritis did not reveal any worrisome safety concerns. “It doesn’t seem to have the broad immunosuppressive effects which characterize the therapies we currently use,” said Chaim Putterman, MD, chief of the division of rheumatology and professor of medicine and microbiology & immunology at the Albert Einstein College of Medicine, New York.
Instead, TWEAK seems to be regulating inflammation in target organs. It almost certainly plays a role in healthy functions like wound healing and cell survival, but Dr. Putterman believes there are redundant mechanisms that can pick up the slack, as the healthy knockout mice attest. The evidence suggests that the TWEAK pathway may become overactive in some diseases and, if so, a therapeutic antibody might be able to reset it to a more normal balance. “The utopian vision is that you would block this cytokine and bring its downstream effects back to normal levels, rather than totally abrogating its homeostatic functions,” Dr. Putterman noted.
Because blocking TWEAK has no apparent immunosuppressive effects, it might be a candidate for combination therapy with existing cytotoxic drugs. “If you have a disease like psoriasis where some standard of care medications are immunosuppressive, such as methotrexate, you might not get more risk by adding an antibody targeting TWEAK, as opposed to using immunosuppressives in combination. That, I think, has potential,” he said.
Work remains, however. A proof-of-concept study in lupus nephritis, sponsored by Biogen, failed to show a benefit when an anti-TWEAK antibody was combined with the standard of care.
But the potential impact of this approach holds much promise, and the fact that TWEAK has been linked to multiple diseases should make it a more attractive drug target for drug companies. “Now we have a target, that if you knock it out, or its receptor, you can potentially affect both diseases. This may the start of a whole new direction for biologics to treat inflammatory disease, and cancer as well,” Dr. Friedman said.
Dr. Putterman and Dr. Friedman were among the authors of the 2015 JID study on TWEAK/Fn14 signaling in spontaneous lupus and the Experimental Dermatology study. Dr. Putterman has research funding from Biogen Idec. Dr. Friedman had no related disclosures. The authors of the Nature Communications study were from the La Jolla Institute for Allergy and Immunology, and Biogen.
An immunomodulatory pathway that has been linked to cancer, kidney disease, and other disease processes is becoming a focus of dermatologic research.
New evidence suggests that TNF-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis family (TNF) superfamily, may be involved in both atopic dermatitis (AD) and psoriasis (Nat Commun. 2017 May 22;8:15395. doi: 10.1038/ncomms15395). The research showed that mice engineered to have low TWEAK levels had less severe disease when both AD and psoriasis were induced.
The TWEAK receptor, Fn14, was upregulated in keratinocytes and dermal fibroblasts in mouse disease models of AD and psoriasis, and TWEAK induced production of a range of cytokines associated with both AD and psoriasis. Subcutaneous injection of recombinant TWEAK led to cutaneous inflammation, as well as histological and molecular signals of the two diseases.
The pathophysiology of both AD and psoriasis is nebulously complex, sharing a similar theme of immune dysregulation, but historically polar opposites based on the different branches of the immune response implicated.
The study is not the only recent work tying TWEAK/Fn14 to dermatologic diseases. Other recent papers have shown evidence of their involvement in chronic cutaneous lupus (J Invest Dermatol. 2015;135[8]:1986-95), UVB irradiation-induced cutaneous lupus (Exp Dermatol. 2016 Dec;25[12]:969-76), and bullous pemphigoid (J Invest Dermatol. 2017 Jul;137[7]:1512-22).
The spate of findings hint that TWEAK/Fn14 could be a novel therapeutic pathway to attack inflammatory disease. Many therapies for autoimmune disease focus on immunosuppressive agents, which are associated with an increased risk of infection. But mice engineered to lack either TWEAK or Fn14 appear normal, and a phase I trial of an anti-TWEAK antibody in patients with rheumatoid arthritis did not reveal any worrisome safety concerns. “It doesn’t seem to have the broad immunosuppressive effects which characterize the therapies we currently use,” said Chaim Putterman, MD, chief of the division of rheumatology and professor of medicine and microbiology & immunology at the Albert Einstein College of Medicine, New York.
Instead, TWEAK seems to be regulating inflammation in target organs. It almost certainly plays a role in healthy functions like wound healing and cell survival, but Dr. Putterman believes there are redundant mechanisms that can pick up the slack, as the healthy knockout mice attest. The evidence suggests that the TWEAK pathway may become overactive in some diseases and, if so, a therapeutic antibody might be able to reset it to a more normal balance. “The utopian vision is that you would block this cytokine and bring its downstream effects back to normal levels, rather than totally abrogating its homeostatic functions,” Dr. Putterman noted.
Because blocking TWEAK has no apparent immunosuppressive effects, it might be a candidate for combination therapy with existing cytotoxic drugs. “If you have a disease like psoriasis where some standard of care medications are immunosuppressive, such as methotrexate, you might not get more risk by adding an antibody targeting TWEAK, as opposed to using immunosuppressives in combination. That, I think, has potential,” he said.
Work remains, however. A proof-of-concept study in lupus nephritis, sponsored by Biogen, failed to show a benefit when an anti-TWEAK antibody was combined with the standard of care.
But the potential impact of this approach holds much promise, and the fact that TWEAK has been linked to multiple diseases should make it a more attractive drug target for drug companies. “Now we have a target, that if you knock it out, or its receptor, you can potentially affect both diseases. This may the start of a whole new direction for biologics to treat inflammatory disease, and cancer as well,” Dr. Friedman said.
Dr. Putterman and Dr. Friedman were among the authors of the 2015 JID study on TWEAK/Fn14 signaling in spontaneous lupus and the Experimental Dermatology study. Dr. Putterman has research funding from Biogen Idec. Dr. Friedman had no related disclosures. The authors of the Nature Communications study were from the La Jolla Institute for Allergy and Immunology, and Biogen.
Pain often persists despite biologic treatment in PsA
MADRID – Pain is common in patients with psoriatic arthritis (PsA) and can be disruptive to their lives and jobs, even among those whose inflammatory symptoms have been treated with biologic drugs for 3 months or longer, according to findings from a multinational survey.
At the European Congress of Rheumatology, Dr. Philip G. Conaghan of the University of Leeds (England) presented findings from the survey of 782 consecutive PsA patients from 13 countries in Europe, the Middle East, Asia, and the Americas, as well as Australia. All patients included in the analysis were on biologic agents – mainly tumor necrosis factor inhibitors – for at least 90 days.
In an interview. Dr. Conaghan said that it’s important for clinicians not to assume that pain in a PsA patient on a biologic means that the drug is not working.
“The main limitation of our study is that we haven’t worked out how well-controlled patients’ psoriatic arthritis is, so, although we know they’re on a biologic for more than 3 months, we don’t know if they were responding well to it.” But, even in the absence of systemic inflammation, he said, there are other potential causes for pain that should not be overlooked.
“There’s no reason why PsA patients wouldn’t have pain due to tendinitis, enthesitis, and osteoarthritis – the same mechanical-type joint pain that we see in the whole community of people over 40,” Dr. Conaghan said. “I am concerned that, once we give someone a label of inflammatory arthritis, we stop looking at all the other things that can happen to their musculoskeletal system.”
Moreover, he said, “people who’ve had arthritis severe enough to need a biologic treatment will have muscle deconditioning and weakness. It’s very common that PsA patients have trouble opening jars and getting out of chairs.”
Such weakness “can lead to mechanical joint pain, which fortunately can be improved – along with the pain – through muscle strengthening and rehabilitation.”
For their study, Dr. Conaghan and his colleagues collected information from clinicians on treatment and from patients. The questionnaires incorporated several measures of disability, pain, functional impairment, and health-related quality of life that have been validated for use in PsA patients.
Severe pain was significantly associated with increased use of prescription nonsteroidal anti-inflammatory drugs and opioids, as well as nonprescription pain medication. Patients 65 years and older had a significantly greater likelihood of being unemployed or retired because of PsA if they reported severe pain, compared with those reporting mild or moderate pain.
A number of quality of life and work-related measures were also associated with pain severity. Dr. Conaghan and his colleagues found that the risk of disability increased with bodily pain, and more severe pain was associated with greater activity impairment, worse social functioning, more work impairment, and work time missed, among other measures (P less than .0001 for all).
“What we saw is that, the more pain you have, the more your world shrinks in,” Dr. Conaghan said.
Dr. Conaghan reported financial relationships with AbbVie, Eli Lilly, Novartis, Pfizer, Bristol-Myers Squibb, and Roche. Some of his study coauthors have similar disclosures. Four coauthors are employees of Novartis.
MADRID – Pain is common in patients with psoriatic arthritis (PsA) and can be disruptive to their lives and jobs, even among those whose inflammatory symptoms have been treated with biologic drugs for 3 months or longer, according to findings from a multinational survey.
At the European Congress of Rheumatology, Dr. Philip G. Conaghan of the University of Leeds (England) presented findings from the survey of 782 consecutive PsA patients from 13 countries in Europe, the Middle East, Asia, and the Americas, as well as Australia. All patients included in the analysis were on biologic agents – mainly tumor necrosis factor inhibitors – for at least 90 days.
In an interview. Dr. Conaghan said that it’s important for clinicians not to assume that pain in a PsA patient on a biologic means that the drug is not working.
“The main limitation of our study is that we haven’t worked out how well-controlled patients’ psoriatic arthritis is, so, although we know they’re on a biologic for more than 3 months, we don’t know if they were responding well to it.” But, even in the absence of systemic inflammation, he said, there are other potential causes for pain that should not be overlooked.
“There’s no reason why PsA patients wouldn’t have pain due to tendinitis, enthesitis, and osteoarthritis – the same mechanical-type joint pain that we see in the whole community of people over 40,” Dr. Conaghan said. “I am concerned that, once we give someone a label of inflammatory arthritis, we stop looking at all the other things that can happen to their musculoskeletal system.”
Moreover, he said, “people who’ve had arthritis severe enough to need a biologic treatment will have muscle deconditioning and weakness. It’s very common that PsA patients have trouble opening jars and getting out of chairs.”
Such weakness “can lead to mechanical joint pain, which fortunately can be improved – along with the pain – through muscle strengthening and rehabilitation.”
For their study, Dr. Conaghan and his colleagues collected information from clinicians on treatment and from patients. The questionnaires incorporated several measures of disability, pain, functional impairment, and health-related quality of life that have been validated for use in PsA patients.
Severe pain was significantly associated with increased use of prescription nonsteroidal anti-inflammatory drugs and opioids, as well as nonprescription pain medication. Patients 65 years and older had a significantly greater likelihood of being unemployed or retired because of PsA if they reported severe pain, compared with those reporting mild or moderate pain.
A number of quality of life and work-related measures were also associated with pain severity. Dr. Conaghan and his colleagues found that the risk of disability increased with bodily pain, and more severe pain was associated with greater activity impairment, worse social functioning, more work impairment, and work time missed, among other measures (P less than .0001 for all).
“What we saw is that, the more pain you have, the more your world shrinks in,” Dr. Conaghan said.
Dr. Conaghan reported financial relationships with AbbVie, Eli Lilly, Novartis, Pfizer, Bristol-Myers Squibb, and Roche. Some of his study coauthors have similar disclosures. Four coauthors are employees of Novartis.
MADRID – Pain is common in patients with psoriatic arthritis (PsA) and can be disruptive to their lives and jobs, even among those whose inflammatory symptoms have been treated with biologic drugs for 3 months or longer, according to findings from a multinational survey.
At the European Congress of Rheumatology, Dr. Philip G. Conaghan of the University of Leeds (England) presented findings from the survey of 782 consecutive PsA patients from 13 countries in Europe, the Middle East, Asia, and the Americas, as well as Australia. All patients included in the analysis were on biologic agents – mainly tumor necrosis factor inhibitors – for at least 90 days.
In an interview. Dr. Conaghan said that it’s important for clinicians not to assume that pain in a PsA patient on a biologic means that the drug is not working.
“The main limitation of our study is that we haven’t worked out how well-controlled patients’ psoriatic arthritis is, so, although we know they’re on a biologic for more than 3 months, we don’t know if they were responding well to it.” But, even in the absence of systemic inflammation, he said, there are other potential causes for pain that should not be overlooked.
“There’s no reason why PsA patients wouldn’t have pain due to tendinitis, enthesitis, and osteoarthritis – the same mechanical-type joint pain that we see in the whole community of people over 40,” Dr. Conaghan said. “I am concerned that, once we give someone a label of inflammatory arthritis, we stop looking at all the other things that can happen to their musculoskeletal system.”
Moreover, he said, “people who’ve had arthritis severe enough to need a biologic treatment will have muscle deconditioning and weakness. It’s very common that PsA patients have trouble opening jars and getting out of chairs.”
Such weakness “can lead to mechanical joint pain, which fortunately can be improved – along with the pain – through muscle strengthening and rehabilitation.”
For their study, Dr. Conaghan and his colleagues collected information from clinicians on treatment and from patients. The questionnaires incorporated several measures of disability, pain, functional impairment, and health-related quality of life that have been validated for use in PsA patients.
Severe pain was significantly associated with increased use of prescription nonsteroidal anti-inflammatory drugs and opioids, as well as nonprescription pain medication. Patients 65 years and older had a significantly greater likelihood of being unemployed or retired because of PsA if they reported severe pain, compared with those reporting mild or moderate pain.
A number of quality of life and work-related measures were also associated with pain severity. Dr. Conaghan and his colleagues found that the risk of disability increased with bodily pain, and more severe pain was associated with greater activity impairment, worse social functioning, more work impairment, and work time missed, among other measures (P less than .0001 for all).
“What we saw is that, the more pain you have, the more your world shrinks in,” Dr. Conaghan said.
Dr. Conaghan reported financial relationships with AbbVie, Eli Lilly, Novartis, Pfizer, Bristol-Myers Squibb, and Roche. Some of his study coauthors have similar disclosures. Four coauthors are employees of Novartis.
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Overall, 37% of PsA patients reported severe pain despite treatment with biologic agents.
Data source: A multinational survey of 782 consecutive PsA patients on biologic agents.
Disclosures: Dr. Conaghan reported financial relationships with AbbVie, Eli Lilly, Novartis, Pfizer, Bristol-Myers Squibb, and Roche. Some of his study coauthors have similar disclosures. Four coauthors are employees of Novartis.
Phototherapy and Nondrug Therapies for Psoriasis Considered Beneficial by Patients
Oral or injected medications for psoriasis can be burdensome for patients, making them inclined to use alternative therapies such as phototherapy and other nondrug therapies, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than half of participants indicated that they have used phototherapy. Both positive and negative experiences were reported. One participant reported that a home UVB 3-panel light box "dramatically changed [his/her] life." Other participants indicated phototherapy was less successful for them. Participants also indicated fears about skin cancer.
RELATED ARTICLE: Does UVB phototherapy cause skin cancer?
However, several participants reported that phototherapy was more effective when used in combination with other medical therapies. Similarly, most participants indicated using 1 or more nondrug therapies to manage their psoriatic symptoms. Approximately one-third used over-the-counter products, such as coal tar, salicylic acid, and Epsom salt. Slightly more than one-fourth indicated the importance of complementary or alternative therapy, including exercise and meditation, to manage their psoriasis symptoms. Diet modifications, such as eliminating alcohol, sugar, processed foods, drugs, gluten, and tobacco, also were reported as successful.
RELATED ARTICLE: Yoga for dermatologic conditions
RELATED VIDEO: Answering patient questions about diet
Psoriasis patients emphasized that an effective multimodal approach including drug, phototherapy, and nondrug therapies usually is done through trial and error based on each patient's individual needs. Dermatologists would benefit from knowing that nearly all participants in this public meeting indicated they value the benefits of nondrug therapies, and combination therapies using drug and nondrug therapies should be discussed with patients.
The psoriasis public meeting in March 2016 was the FDA's 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Oral or injected medications for psoriasis can be burdensome for patients, making them inclined to use alternative therapies such as phototherapy and other nondrug therapies, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than half of participants indicated that they have used phototherapy. Both positive and negative experiences were reported. One participant reported that a home UVB 3-panel light box "dramatically changed [his/her] life." Other participants indicated phototherapy was less successful for them. Participants also indicated fears about skin cancer.
RELATED ARTICLE: Does UVB phototherapy cause skin cancer?
However, several participants reported that phototherapy was more effective when used in combination with other medical therapies. Similarly, most participants indicated using 1 or more nondrug therapies to manage their psoriatic symptoms. Approximately one-third used over-the-counter products, such as coal tar, salicylic acid, and Epsom salt. Slightly more than one-fourth indicated the importance of complementary or alternative therapy, including exercise and meditation, to manage their psoriasis symptoms. Diet modifications, such as eliminating alcohol, sugar, processed foods, drugs, gluten, and tobacco, also were reported as successful.
RELATED ARTICLE: Yoga for dermatologic conditions
RELATED VIDEO: Answering patient questions about diet
Psoriasis patients emphasized that an effective multimodal approach including drug, phototherapy, and nondrug therapies usually is done through trial and error based on each patient's individual needs. Dermatologists would benefit from knowing that nearly all participants in this public meeting indicated they value the benefits of nondrug therapies, and combination therapies using drug and nondrug therapies should be discussed with patients.
The psoriasis public meeting in March 2016 was the FDA's 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Oral or injected medications for psoriasis can be burdensome for patients, making them inclined to use alternative therapies such as phototherapy and other nondrug therapies, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than half of participants indicated that they have used phototherapy. Both positive and negative experiences were reported. One participant reported that a home UVB 3-panel light box "dramatically changed [his/her] life." Other participants indicated phototherapy was less successful for them. Participants also indicated fears about skin cancer.
RELATED ARTICLE: Does UVB phototherapy cause skin cancer?
However, several participants reported that phototherapy was more effective when used in combination with other medical therapies. Similarly, most participants indicated using 1 or more nondrug therapies to manage their psoriatic symptoms. Approximately one-third used over-the-counter products, such as coal tar, salicylic acid, and Epsom salt. Slightly more than one-fourth indicated the importance of complementary or alternative therapy, including exercise and meditation, to manage their psoriasis symptoms. Diet modifications, such as eliminating alcohol, sugar, processed foods, drugs, gluten, and tobacco, also were reported as successful.
RELATED ARTICLE: Yoga for dermatologic conditions
RELATED VIDEO: Answering patient questions about diet
Psoriasis patients emphasized that an effective multimodal approach including drug, phototherapy, and nondrug therapies usually is done through trial and error based on each patient's individual needs. Dermatologists would benefit from knowing that nearly all participants in this public meeting indicated they value the benefits of nondrug therapies, and combination therapies using drug and nondrug therapies should be discussed with patients.
The psoriasis public meeting in March 2016 was the FDA's 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Narrowband UVB Treatment Increases Serum 25-Hydroxyvitamin D Levels in Patients With Chronic Plaque Psoriasis
Psoriasis is a chronic, inflammatory, T-cell–mediated skin disease. Phototherapy, which consists of light used at various wavelengths, is a well-established treatment method for psoriasis vulgaris. Although successful results have been obtained with phototherapy in psoriasis, its mechanism of action is not fully understood. UV light has been shown to have an effect on T-lymphocyte function as well as various components of the natural and acquired immune response. It also has a suppressive effect on the immune system caused by many independent effects.1 Phototherapy currently is available using broadband UVB (290–320 nm), narrowband UVB (NB-UVB)(311–313 nm), 308-nm excimer laser, UVA1 (340–400 nm), psoralen plus UVA, and photopheresis.2 Narrowband UVB treatment with light sources that peak at 311 to 313 nm have been used with high efficacy and a low side-effect profile, becoming the standard phototherapy method for chronic plaque-type psoriasis.3
More than 90% of vitamin D synthesis is formed in the skin following UV exposure, and the wavelengths and the solar spectrum that stimulate vitamin D synthesis have been a focus of research.4 7-Dehydrocholesterol (provitamin D3) is first converted to previtamin D3. Although the necessary UV wavelength for previtamin D3 synthesis is 295 to 300 nm, it is known that production stops below 260 nm and above 315 nm.4-6 Previtamin D3 is unstable and is quickly converted to vitamin D3 in the skinand then to the biologically active form of 1,25-dihydroxyvitamin D3 (calcitriol) following hydroxylation in the liver and kidneys. Calcitriol shows its effect by binding to the special nuclear receptor for vitamin D.7 Many tissues including the keratinocytes, dendritic cells, melanocytes, and sebocytes in the skin have been shown to possess the enzymatic mechanism necessary for 1,25-dihydroxyvitamin D3 production. Vitamin D also is known to have paracrine, autocrine, and intracrine effects on immunomodulation, cell proliferation, differentiation, and apoptosis, in addition to its role in calcium metabolism.5-9 Topical vitamin D and its analogues are used effectively and safely in psoriasis treatment with these effects.10 A correlation between low serum vitamin D levels and chronic inflammation severity has been shown in psoriasis patients in some studies.11,12
In this study, we sought to evaluate the effect of NB-UVB on vitamin D status and related metabolic markers in patients with psoriasis.
Methods
This prospective, single-center study included patients living in or around Eskisehir, Turkey, who were 18 years of age or older and had been diagnosed with chronic plaque psoriasis with a psoriasis area and severity index (PASI) score of 5 or higher. Permission was granted by the local ethics committee. Patients provided written informed consent prior to enrollment. Patients were excluded if they were younger than 18 years; were pregnant or breastfeeding; stayed in open environments for more than 2 hours per day during the summer months (May through September); used drugs affecting calcium metabolism in the last 8 weeks (eg, barbiturates, anticonvulsants, corticosteroids, vitamin D supplements, bisphosphonates); used systemic treatment for psoriasis in the last 8 weeks; used phototherapy or sunbathing in the last 8 weeks; used topical vitamin D analogues in the last 4 weeks; or had a history of psoriatic arthritis and other inflammatory disorders, renal disease, known calcium metabolism disorders, granulomatous disorders, thyroid disease, diabetes mellitus, skin cancer, or abnormal photosensitivity and known lack of response or hypersensitivity to phototherapy.
Clinical Evaluation and Laboratory Studies
The participants’ age, gender, Fitzpatrick skin type, disease duration, dairy intake and vitamin supplement levels, hours of sun exposure per week, detailed medical history, and medications were obtained and documented in the medical records.
Serum 25(OH)D levels were measured using high-performance liquid chromatography/mass spectrometry, serum calcium and phosphorus levels using colorimetric analysis, serum alkaline phosphatase (ALP) levels using the enzymatic colorimetric method, and serum parathyroid hormone (PTH) levels using electrochemiluminescence at baseline and after PASI 75 was achieved with treatment. Vitamin D levels were classified in 3 groups: (1) deficient (<20 ng/mL); (2) inadequate (20–30 ng/mL); and (3) adequate (>30 ng/mL). The PASI scores at baseline and posttreatment were calculated by the same dermatologist (S.S.).
Treatment Protocol and Patient Follow-up
Narrowband UVB treatment was started at 70% of the minimal erythema dose (MED). Phototherapy was administered 3 times weekly for 6 months or until PASI 75 response was achieved. An increase of 20% to 30% from the prior dose was made according to the participants’ clinical status at each treatment session, and the dose was stabilized once the maximum dose was achieved according to skin type—up to 2000 mJ/cm2 for Fitzpatrick skin types I and II, 3000 mJ/cm2 for skin types III and IV, and 5000 mJ/cm2 for skin types V and VI. Participants were allowed to use low- and moderate-potency topical corticosteroids and moisturizers containing urea during the course of treatment. The study physician (S.S.) clinically evaluated participants every 4 weeks for 6 months or until PASI 75 was achieved, and the clinical improvement was calculated as the percentage decrease in PASI score.
Statistical Analysis
The Shapiro-Wilk normalcy test was used for the continuous variables in the study. Variables with a normal distribution were analyzed with the paired t test and 1-way analysis of variance test and presented as mean (SD). Variables without a normal distribution were analyzed with the Wilcoxon t test and the Kruskal-Wallis test and presented as the median and 25th and 75th quartiles. The serum 25(OH)D levels were evaluated according to the seasons with the Kruskal-Wallis test. Categorical variables were expressed as frequency and percentages. The Pearson and Spearman correlation analysis and regression analysis were used to show the relationship between the variables (ie, age, Fitzpatrick skin type, PASI score, maximum NB-UVB dose, and number of sessions). The statistical significance level was set at P≤.05. Statistical analyses were performed using SPSS software version 21.
Results
A total of 49 participants (30 [61.22%] males; 19 [38.78%] females) were included in the study. The mean age (SD) was 40.27 (14.62) years (range, 19–74 years). Three (6.12%) participants were Fitzpatrick skin type I, 15 (30.61%) were skin type II, and 31 (63.27%) were skin type III.
The baseline median PASI score for the 49 participants was 10.20 (7.85–13.65). Baseline serum 25(OH)D levels were noted to be deficient in 40 participants (81.63%) and inadequate in 9 participants (18.37%). The distribution of the serum 25(OH)D levels of the participants according to the season was evaluated with the Kruskal-Wallis test and no association was found between serum 25(OH)D levels and seasonal changes (P=.685). Comparison of 25(OH)D basal values with Fitzpatrick skin type revealed a statistically significant relationship between skin type and vitamin D level (P=.024). The basal serum 25(OH)D levels were significantly lower in Fitzpatrick skin type II versus skin type I (P=.039).
Thirty-two (65.31%) participants achieved PASI 75 by the end of treatment. The baseline median PASI score (25th-75th quartiles) for the 32 patients was 10.45 (8.20-13.83) and the posttreatment PASI score was 1.95 (1.20-3.55), a statistically significant decrease following treatment (P<.001)(Table 1). Mean (SD) baseline serum 25(OH)D levels were 14.14 (6.70) ng/mL and posttreatment levels were 46.42 (15.51) ng/mL in these participants, which demonstrated a statistically significant increase during NB-UVB treatment (P<.001). None of the participants reached the toxicity levels (>80 ng/mL) for serum 25(OH)D. There were no significant changes in serum calcium or phosphorus levels posttreatment (Table 1), but statistically significant decreases in serum ALP and PTH levels were noted (P=.001 and P=.019, respectively)(Table 1).
Participants who completed the study (n=32) received an average (SD) of 30.09 (7.53) sessions of NB-UVB treatment and the mean (SD) MED was 611.88 (240.14) mJ/cm2. The mean (SD) maximum dose was 2090.09 (341.78) mJ/cm2 (Table 2).
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and the maximum dose values. We found that the posttreatment serum 25(OH)D levels correlated with the number of sessions (P=.031) but not with the maximum dose (P=.498).
Using regression analysis, we also evaluated the effect of the increase in vitamin D levels—posttreatment serum 25(OH)D level minus baseline serum 25(OH)D levels—on the decrease in PASI scores—baseline PASI score minus posttreatment PASI score—and found no effect of serum 25(OH)D level increase on PASI decrease (P=.530). There was no correlation between increased serum 25(OH)D levels and age, Fitzpatrick skin type, or baseline PASI score.
Comment
The most effective UV wavelength for vitamin D synthesis is 295 to 300 nm, and therefore broadband UVB is frequently studied when determining the relationship between phototherapy and serum vitamin D levels.4 The current study demonstrated a statistically significant increase in serum 25(OH)D levels following NB-UVB treatment in patients with moderate to severe chronic plaque psoriasis (P<.001). This result supports other studies reporting that NB-UVB treatment in psoriasis patients increases serum 25(OH)D levels.13-18
The main factor in the effective UVB level for vitamin D synthesis is the angle at which solar radiation reaches the earth, which is affected by the longitude, latitude, and time of day.19 For this reason, we planned to perform our study at a single center. Patients who stayed in open areas for more than 2 hours per day during the summer months (May through September) were excluded from the study to decrease the effect of seasonal changes on vitamin D levels. We evaluated the seasonal variation of vitamin D levels and found no relationship between seasonal changes and serum 25(OH)D levels. Therefore, the potential effect of seasonal changes on the vitamin D levels of study participants was excluded from the study.
The response to UV radiation changes according to age and Fitzpatrick skin type because 7-dehydrocholesterol levels decrease with age and melanin prevents the access of UVB photons to 7-dehydrocholesterol.20 The basal serum 25(OH)D levels were deficient in 81.63% of participants and inadequate in 18.37%. In this study, we also observed that the basal serum 25(OH)D levels were significantly lower in patients with Fitzpatrick skin type II than in Fitzpatrick skin type I (P=.039). The mean (SD) serum 25(OH)D level at baseline was 14.14 (6.70) ng/mL and posttreatment was 46.42 (15.51) ng/mL in the 32 patients who completed the study. Serum 25(OH)D levels showed a statistically significant increase after NB-UVB treatment (P<.001). The increased serum 25(OH)D levels after NB-UVB phototherapy were not associated with Fitzpatrick skin type, which was consistent with the results of Osmancevic et al.17 The adjusted NB-UVB doses according to the different skin types might be responsible for this result in our study.
Participant age did not have a significant effect on serum 25(OH)D levels, similar to other studies in the literature.13,17 We believe that artificial UVB radiation at high doses can compensate for the 7-dehydrocholesterol that decreases in the skin with aging.
We observed no significant change in the serum calcium and phosphorus levels with NB-UVB treatment in our study. None of the participants had a metabolic disorder related to increased 25(OH)D levels. The serum ALP and PTH levels decreased significantly following treatment (P=.001 and P=.019, respectively), which may have been secondary to increased serum 25(OH)D levels.
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and maximum dose values. The posttreatment serum 25(OH)D levels were found to be related to the number of sessions received, but this value was not correlated with the maximum dose received. The MED and maximum dose were determined according to the Fitzpatrick skin type of the participants. Therefore, increased serum 25(OH)D levels with an increased number of sessions was an expected result. Our observation is in accordance with the finding described by Ryan et al.14 On the other hand, an in vitro study conducted by Olds et al21 reported that the relationship between UV light and cholecalciferol synthesis was not linear.
We found that increased serum 25(OH)D levels after treatment were not correlated with the decrease in PASI score, similar to studies by Romaní et al18 and Ryan et al.14 These results suggest that the clinical improvement following NB-UVB treatment is independent of the increased serum 25(OH)D levels in psoriasis patients.
Conclusion
In conclusion, we found that the serum 25(OH)D levels that increase as a result of NB-UVB therapy for the treatment of chronic plaque psoriasis has no statistically significant relationship with the age, Fitzpatrick skin type, baseline PASI score, changes in PASI, or maximum dose, while a positive relationship is present between the serum 25(OH)D levels and the number of sessions of NB-UVB.
- Şavk E. Immunology of Photo(chemo)therapy. Turkderm. 2010;44(suppl 2):62-66.
- Ferahbaş A. Phototherapy modalities and protocols. Turkderm. 2010;44(suppl 2):67-72.
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol. 2004;151:283-297.
- Norval M, Björn LO, de Gruijl FR. Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9:11-17.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- McKenzie RL, Liley JB, Björn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377-393.
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618-625.
- Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931-938.
- Osmancevic A, Landin-Wilhelmsen K, Larkö O, et al. UVB therapy increases 25 (OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007;23:172-178.
- Ryan C, Moran B, McKenna MJ, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-842.
- Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321-328.
- Lesiak A, Narbutt J, Pawlaczyk M, et al. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol Photoimmunol Photomed. 2011;27:304-310.
- Osmancevic A, Landin-Wilhelmsen K, Larko O, et al.Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2009;25:119-123.
- Romaní J, Caixàs A, Carrascosa JM, et al. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. 2012;166:1237-1244.
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48-60.
- Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588-593.
- Olds WJ, McKinley AR, Moore MR, et al. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J Photochem Photobiol B. 2008;93:88-93.
Psoriasis is a chronic, inflammatory, T-cell–mediated skin disease. Phototherapy, which consists of light used at various wavelengths, is a well-established treatment method for psoriasis vulgaris. Although successful results have been obtained with phototherapy in psoriasis, its mechanism of action is not fully understood. UV light has been shown to have an effect on T-lymphocyte function as well as various components of the natural and acquired immune response. It also has a suppressive effect on the immune system caused by many independent effects.1 Phototherapy currently is available using broadband UVB (290–320 nm), narrowband UVB (NB-UVB)(311–313 nm), 308-nm excimer laser, UVA1 (340–400 nm), psoralen plus UVA, and photopheresis.2 Narrowband UVB treatment with light sources that peak at 311 to 313 nm have been used with high efficacy and a low side-effect profile, becoming the standard phototherapy method for chronic plaque-type psoriasis.3
More than 90% of vitamin D synthesis is formed in the skin following UV exposure, and the wavelengths and the solar spectrum that stimulate vitamin D synthesis have been a focus of research.4 7-Dehydrocholesterol (provitamin D3) is first converted to previtamin D3. Although the necessary UV wavelength for previtamin D3 synthesis is 295 to 300 nm, it is known that production stops below 260 nm and above 315 nm.4-6 Previtamin D3 is unstable and is quickly converted to vitamin D3 in the skinand then to the biologically active form of 1,25-dihydroxyvitamin D3 (calcitriol) following hydroxylation in the liver and kidneys. Calcitriol shows its effect by binding to the special nuclear receptor for vitamin D.7 Many tissues including the keratinocytes, dendritic cells, melanocytes, and sebocytes in the skin have been shown to possess the enzymatic mechanism necessary for 1,25-dihydroxyvitamin D3 production. Vitamin D also is known to have paracrine, autocrine, and intracrine effects on immunomodulation, cell proliferation, differentiation, and apoptosis, in addition to its role in calcium metabolism.5-9 Topical vitamin D and its analogues are used effectively and safely in psoriasis treatment with these effects.10 A correlation between low serum vitamin D levels and chronic inflammation severity has been shown in psoriasis patients in some studies.11,12
In this study, we sought to evaluate the effect of NB-UVB on vitamin D status and related metabolic markers in patients with psoriasis.
Methods
This prospective, single-center study included patients living in or around Eskisehir, Turkey, who were 18 years of age or older and had been diagnosed with chronic plaque psoriasis with a psoriasis area and severity index (PASI) score of 5 or higher. Permission was granted by the local ethics committee. Patients provided written informed consent prior to enrollment. Patients were excluded if they were younger than 18 years; were pregnant or breastfeeding; stayed in open environments for more than 2 hours per day during the summer months (May through September); used drugs affecting calcium metabolism in the last 8 weeks (eg, barbiturates, anticonvulsants, corticosteroids, vitamin D supplements, bisphosphonates); used systemic treatment for psoriasis in the last 8 weeks; used phototherapy or sunbathing in the last 8 weeks; used topical vitamin D analogues in the last 4 weeks; or had a history of psoriatic arthritis and other inflammatory disorders, renal disease, known calcium metabolism disorders, granulomatous disorders, thyroid disease, diabetes mellitus, skin cancer, or abnormal photosensitivity and known lack of response or hypersensitivity to phototherapy.
Clinical Evaluation and Laboratory Studies
The participants’ age, gender, Fitzpatrick skin type, disease duration, dairy intake and vitamin supplement levels, hours of sun exposure per week, detailed medical history, and medications were obtained and documented in the medical records.
Serum 25(OH)D levels were measured using high-performance liquid chromatography/mass spectrometry, serum calcium and phosphorus levels using colorimetric analysis, serum alkaline phosphatase (ALP) levels using the enzymatic colorimetric method, and serum parathyroid hormone (PTH) levels using electrochemiluminescence at baseline and after PASI 75 was achieved with treatment. Vitamin D levels were classified in 3 groups: (1) deficient (<20 ng/mL); (2) inadequate (20–30 ng/mL); and (3) adequate (>30 ng/mL). The PASI scores at baseline and posttreatment were calculated by the same dermatologist (S.S.).
Treatment Protocol and Patient Follow-up
Narrowband UVB treatment was started at 70% of the minimal erythema dose (MED). Phototherapy was administered 3 times weekly for 6 months or until PASI 75 response was achieved. An increase of 20% to 30% from the prior dose was made according to the participants’ clinical status at each treatment session, and the dose was stabilized once the maximum dose was achieved according to skin type—up to 2000 mJ/cm2 for Fitzpatrick skin types I and II, 3000 mJ/cm2 for skin types III and IV, and 5000 mJ/cm2 for skin types V and VI. Participants were allowed to use low- and moderate-potency topical corticosteroids and moisturizers containing urea during the course of treatment. The study physician (S.S.) clinically evaluated participants every 4 weeks for 6 months or until PASI 75 was achieved, and the clinical improvement was calculated as the percentage decrease in PASI score.
Statistical Analysis
The Shapiro-Wilk normalcy test was used for the continuous variables in the study. Variables with a normal distribution were analyzed with the paired t test and 1-way analysis of variance test and presented as mean (SD). Variables without a normal distribution were analyzed with the Wilcoxon t test and the Kruskal-Wallis test and presented as the median and 25th and 75th quartiles. The serum 25(OH)D levels were evaluated according to the seasons with the Kruskal-Wallis test. Categorical variables were expressed as frequency and percentages. The Pearson and Spearman correlation analysis and regression analysis were used to show the relationship between the variables (ie, age, Fitzpatrick skin type, PASI score, maximum NB-UVB dose, and number of sessions). The statistical significance level was set at P≤.05. Statistical analyses were performed using SPSS software version 21.
Results
A total of 49 participants (30 [61.22%] males; 19 [38.78%] females) were included in the study. The mean age (SD) was 40.27 (14.62) years (range, 19–74 years). Three (6.12%) participants were Fitzpatrick skin type I, 15 (30.61%) were skin type II, and 31 (63.27%) were skin type III.
The baseline median PASI score for the 49 participants was 10.20 (7.85–13.65). Baseline serum 25(OH)D levels were noted to be deficient in 40 participants (81.63%) and inadequate in 9 participants (18.37%). The distribution of the serum 25(OH)D levels of the participants according to the season was evaluated with the Kruskal-Wallis test and no association was found between serum 25(OH)D levels and seasonal changes (P=.685). Comparison of 25(OH)D basal values with Fitzpatrick skin type revealed a statistically significant relationship between skin type and vitamin D level (P=.024). The basal serum 25(OH)D levels were significantly lower in Fitzpatrick skin type II versus skin type I (P=.039).
Thirty-two (65.31%) participants achieved PASI 75 by the end of treatment. The baseline median PASI score (25th-75th quartiles) for the 32 patients was 10.45 (8.20-13.83) and the posttreatment PASI score was 1.95 (1.20-3.55), a statistically significant decrease following treatment (P<.001)(Table 1). Mean (SD) baseline serum 25(OH)D levels were 14.14 (6.70) ng/mL and posttreatment levels were 46.42 (15.51) ng/mL in these participants, which demonstrated a statistically significant increase during NB-UVB treatment (P<.001). None of the participants reached the toxicity levels (>80 ng/mL) for serum 25(OH)D. There were no significant changes in serum calcium or phosphorus levels posttreatment (Table 1), but statistically significant decreases in serum ALP and PTH levels were noted (P=.001 and P=.019, respectively)(Table 1).
Participants who completed the study (n=32) received an average (SD) of 30.09 (7.53) sessions of NB-UVB treatment and the mean (SD) MED was 611.88 (240.14) mJ/cm2. The mean (SD) maximum dose was 2090.09 (341.78) mJ/cm2 (Table 2).
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and the maximum dose values. We found that the posttreatment serum 25(OH)D levels correlated with the number of sessions (P=.031) but not with the maximum dose (P=.498).
Using regression analysis, we also evaluated the effect of the increase in vitamin D levels—posttreatment serum 25(OH)D level minus baseline serum 25(OH)D levels—on the decrease in PASI scores—baseline PASI score minus posttreatment PASI score—and found no effect of serum 25(OH)D level increase on PASI decrease (P=.530). There was no correlation between increased serum 25(OH)D levels and age, Fitzpatrick skin type, or baseline PASI score.
Comment
The most effective UV wavelength for vitamin D synthesis is 295 to 300 nm, and therefore broadband UVB is frequently studied when determining the relationship between phototherapy and serum vitamin D levels.4 The current study demonstrated a statistically significant increase in serum 25(OH)D levels following NB-UVB treatment in patients with moderate to severe chronic plaque psoriasis (P<.001). This result supports other studies reporting that NB-UVB treatment in psoriasis patients increases serum 25(OH)D levels.13-18
The main factor in the effective UVB level for vitamin D synthesis is the angle at which solar radiation reaches the earth, which is affected by the longitude, latitude, and time of day.19 For this reason, we planned to perform our study at a single center. Patients who stayed in open areas for more than 2 hours per day during the summer months (May through September) were excluded from the study to decrease the effect of seasonal changes on vitamin D levels. We evaluated the seasonal variation of vitamin D levels and found no relationship between seasonal changes and serum 25(OH)D levels. Therefore, the potential effect of seasonal changes on the vitamin D levels of study participants was excluded from the study.
The response to UV radiation changes according to age and Fitzpatrick skin type because 7-dehydrocholesterol levels decrease with age and melanin prevents the access of UVB photons to 7-dehydrocholesterol.20 The basal serum 25(OH)D levels were deficient in 81.63% of participants and inadequate in 18.37%. In this study, we also observed that the basal serum 25(OH)D levels were significantly lower in patients with Fitzpatrick skin type II than in Fitzpatrick skin type I (P=.039). The mean (SD) serum 25(OH)D level at baseline was 14.14 (6.70) ng/mL and posttreatment was 46.42 (15.51) ng/mL in the 32 patients who completed the study. Serum 25(OH)D levels showed a statistically significant increase after NB-UVB treatment (P<.001). The increased serum 25(OH)D levels after NB-UVB phototherapy were not associated with Fitzpatrick skin type, which was consistent with the results of Osmancevic et al.17 The adjusted NB-UVB doses according to the different skin types might be responsible for this result in our study.
Participant age did not have a significant effect on serum 25(OH)D levels, similar to other studies in the literature.13,17 We believe that artificial UVB radiation at high doses can compensate for the 7-dehydrocholesterol that decreases in the skin with aging.
We observed no significant change in the serum calcium and phosphorus levels with NB-UVB treatment in our study. None of the participants had a metabolic disorder related to increased 25(OH)D levels. The serum ALP and PTH levels decreased significantly following treatment (P=.001 and P=.019, respectively), which may have been secondary to increased serum 25(OH)D levels.
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and maximum dose values. The posttreatment serum 25(OH)D levels were found to be related to the number of sessions received, but this value was not correlated with the maximum dose received. The MED and maximum dose were determined according to the Fitzpatrick skin type of the participants. Therefore, increased serum 25(OH)D levels with an increased number of sessions was an expected result. Our observation is in accordance with the finding described by Ryan et al.14 On the other hand, an in vitro study conducted by Olds et al21 reported that the relationship between UV light and cholecalciferol synthesis was not linear.
We found that increased serum 25(OH)D levels after treatment were not correlated with the decrease in PASI score, similar to studies by Romaní et al18 and Ryan et al.14 These results suggest that the clinical improvement following NB-UVB treatment is independent of the increased serum 25(OH)D levels in psoriasis patients.
Conclusion
In conclusion, we found that the serum 25(OH)D levels that increase as a result of NB-UVB therapy for the treatment of chronic plaque psoriasis has no statistically significant relationship with the age, Fitzpatrick skin type, baseline PASI score, changes in PASI, or maximum dose, while a positive relationship is present between the serum 25(OH)D levels and the number of sessions of NB-UVB.
Psoriasis is a chronic, inflammatory, T-cell–mediated skin disease. Phototherapy, which consists of light used at various wavelengths, is a well-established treatment method for psoriasis vulgaris. Although successful results have been obtained with phototherapy in psoriasis, its mechanism of action is not fully understood. UV light has been shown to have an effect on T-lymphocyte function as well as various components of the natural and acquired immune response. It also has a suppressive effect on the immune system caused by many independent effects.1 Phototherapy currently is available using broadband UVB (290–320 nm), narrowband UVB (NB-UVB)(311–313 nm), 308-nm excimer laser, UVA1 (340–400 nm), psoralen plus UVA, and photopheresis.2 Narrowband UVB treatment with light sources that peak at 311 to 313 nm have been used with high efficacy and a low side-effect profile, becoming the standard phototherapy method for chronic plaque-type psoriasis.3
More than 90% of vitamin D synthesis is formed in the skin following UV exposure, and the wavelengths and the solar spectrum that stimulate vitamin D synthesis have been a focus of research.4 7-Dehydrocholesterol (provitamin D3) is first converted to previtamin D3. Although the necessary UV wavelength for previtamin D3 synthesis is 295 to 300 nm, it is known that production stops below 260 nm and above 315 nm.4-6 Previtamin D3 is unstable and is quickly converted to vitamin D3 in the skinand then to the biologically active form of 1,25-dihydroxyvitamin D3 (calcitriol) following hydroxylation in the liver and kidneys. Calcitriol shows its effect by binding to the special nuclear receptor for vitamin D.7 Many tissues including the keratinocytes, dendritic cells, melanocytes, and sebocytes in the skin have been shown to possess the enzymatic mechanism necessary for 1,25-dihydroxyvitamin D3 production. Vitamin D also is known to have paracrine, autocrine, and intracrine effects on immunomodulation, cell proliferation, differentiation, and apoptosis, in addition to its role in calcium metabolism.5-9 Topical vitamin D and its analogues are used effectively and safely in psoriasis treatment with these effects.10 A correlation between low serum vitamin D levels and chronic inflammation severity has been shown in psoriasis patients in some studies.11,12
In this study, we sought to evaluate the effect of NB-UVB on vitamin D status and related metabolic markers in patients with psoriasis.
Methods
This prospective, single-center study included patients living in or around Eskisehir, Turkey, who were 18 years of age or older and had been diagnosed with chronic plaque psoriasis with a psoriasis area and severity index (PASI) score of 5 or higher. Permission was granted by the local ethics committee. Patients provided written informed consent prior to enrollment. Patients were excluded if they were younger than 18 years; were pregnant or breastfeeding; stayed in open environments for more than 2 hours per day during the summer months (May through September); used drugs affecting calcium metabolism in the last 8 weeks (eg, barbiturates, anticonvulsants, corticosteroids, vitamin D supplements, bisphosphonates); used systemic treatment for psoriasis in the last 8 weeks; used phototherapy or sunbathing in the last 8 weeks; used topical vitamin D analogues in the last 4 weeks; or had a history of psoriatic arthritis and other inflammatory disorders, renal disease, known calcium metabolism disorders, granulomatous disorders, thyroid disease, diabetes mellitus, skin cancer, or abnormal photosensitivity and known lack of response or hypersensitivity to phototherapy.
Clinical Evaluation and Laboratory Studies
The participants’ age, gender, Fitzpatrick skin type, disease duration, dairy intake and vitamin supplement levels, hours of sun exposure per week, detailed medical history, and medications were obtained and documented in the medical records.
Serum 25(OH)D levels were measured using high-performance liquid chromatography/mass spectrometry, serum calcium and phosphorus levels using colorimetric analysis, serum alkaline phosphatase (ALP) levels using the enzymatic colorimetric method, and serum parathyroid hormone (PTH) levels using electrochemiluminescence at baseline and after PASI 75 was achieved with treatment. Vitamin D levels were classified in 3 groups: (1) deficient (<20 ng/mL); (2) inadequate (20–30 ng/mL); and (3) adequate (>30 ng/mL). The PASI scores at baseline and posttreatment were calculated by the same dermatologist (S.S.).
Treatment Protocol and Patient Follow-up
Narrowband UVB treatment was started at 70% of the minimal erythema dose (MED). Phototherapy was administered 3 times weekly for 6 months or until PASI 75 response was achieved. An increase of 20% to 30% from the prior dose was made according to the participants’ clinical status at each treatment session, and the dose was stabilized once the maximum dose was achieved according to skin type—up to 2000 mJ/cm2 for Fitzpatrick skin types I and II, 3000 mJ/cm2 for skin types III and IV, and 5000 mJ/cm2 for skin types V and VI. Participants were allowed to use low- and moderate-potency topical corticosteroids and moisturizers containing urea during the course of treatment. The study physician (S.S.) clinically evaluated participants every 4 weeks for 6 months or until PASI 75 was achieved, and the clinical improvement was calculated as the percentage decrease in PASI score.
Statistical Analysis
The Shapiro-Wilk normalcy test was used for the continuous variables in the study. Variables with a normal distribution were analyzed with the paired t test and 1-way analysis of variance test and presented as mean (SD). Variables without a normal distribution were analyzed with the Wilcoxon t test and the Kruskal-Wallis test and presented as the median and 25th and 75th quartiles. The serum 25(OH)D levels were evaluated according to the seasons with the Kruskal-Wallis test. Categorical variables were expressed as frequency and percentages. The Pearson and Spearman correlation analysis and regression analysis were used to show the relationship between the variables (ie, age, Fitzpatrick skin type, PASI score, maximum NB-UVB dose, and number of sessions). The statistical significance level was set at P≤.05. Statistical analyses were performed using SPSS software version 21.
Results
A total of 49 participants (30 [61.22%] males; 19 [38.78%] females) were included in the study. The mean age (SD) was 40.27 (14.62) years (range, 19–74 years). Three (6.12%) participants were Fitzpatrick skin type I, 15 (30.61%) were skin type II, and 31 (63.27%) were skin type III.
The baseline median PASI score for the 49 participants was 10.20 (7.85–13.65). Baseline serum 25(OH)D levels were noted to be deficient in 40 participants (81.63%) and inadequate in 9 participants (18.37%). The distribution of the serum 25(OH)D levels of the participants according to the season was evaluated with the Kruskal-Wallis test and no association was found between serum 25(OH)D levels and seasonal changes (P=.685). Comparison of 25(OH)D basal values with Fitzpatrick skin type revealed a statistically significant relationship between skin type and vitamin D level (P=.024). The basal serum 25(OH)D levels were significantly lower in Fitzpatrick skin type II versus skin type I (P=.039).
Thirty-two (65.31%) participants achieved PASI 75 by the end of treatment. The baseline median PASI score (25th-75th quartiles) for the 32 patients was 10.45 (8.20-13.83) and the posttreatment PASI score was 1.95 (1.20-3.55), a statistically significant decrease following treatment (P<.001)(Table 1). Mean (SD) baseline serum 25(OH)D levels were 14.14 (6.70) ng/mL and posttreatment levels were 46.42 (15.51) ng/mL in these participants, which demonstrated a statistically significant increase during NB-UVB treatment (P<.001). None of the participants reached the toxicity levels (>80 ng/mL) for serum 25(OH)D. There were no significant changes in serum calcium or phosphorus levels posttreatment (Table 1), but statistically significant decreases in serum ALP and PTH levels were noted (P=.001 and P=.019, respectively)(Table 1).
Participants who completed the study (n=32) received an average (SD) of 30.09 (7.53) sessions of NB-UVB treatment and the mean (SD) MED was 611.88 (240.14) mJ/cm2. The mean (SD) maximum dose was 2090.09 (341.78) mJ/cm2 (Table 2).
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and the maximum dose values. We found that the posttreatment serum 25(OH)D levels correlated with the number of sessions (P=.031) but not with the maximum dose (P=.498).
Using regression analysis, we also evaluated the effect of the increase in vitamin D levels—posttreatment serum 25(OH)D level minus baseline serum 25(OH)D levels—on the decrease in PASI scores—baseline PASI score minus posttreatment PASI score—and found no effect of serum 25(OH)D level increase on PASI decrease (P=.530). There was no correlation between increased serum 25(OH)D levels and age, Fitzpatrick skin type, or baseline PASI score.
Comment
The most effective UV wavelength for vitamin D synthesis is 295 to 300 nm, and therefore broadband UVB is frequently studied when determining the relationship between phototherapy and serum vitamin D levels.4 The current study demonstrated a statistically significant increase in serum 25(OH)D levels following NB-UVB treatment in patients with moderate to severe chronic plaque psoriasis (P<.001). This result supports other studies reporting that NB-UVB treatment in psoriasis patients increases serum 25(OH)D levels.13-18
The main factor in the effective UVB level for vitamin D synthesis is the angle at which solar radiation reaches the earth, which is affected by the longitude, latitude, and time of day.19 For this reason, we planned to perform our study at a single center. Patients who stayed in open areas for more than 2 hours per day during the summer months (May through September) were excluded from the study to decrease the effect of seasonal changes on vitamin D levels. We evaluated the seasonal variation of vitamin D levels and found no relationship between seasonal changes and serum 25(OH)D levels. Therefore, the potential effect of seasonal changes on the vitamin D levels of study participants was excluded from the study.
The response to UV radiation changes according to age and Fitzpatrick skin type because 7-dehydrocholesterol levels decrease with age and melanin prevents the access of UVB photons to 7-dehydrocholesterol.20 The basal serum 25(OH)D levels were deficient in 81.63% of participants and inadequate in 18.37%. In this study, we also observed that the basal serum 25(OH)D levels were significantly lower in patients with Fitzpatrick skin type II than in Fitzpatrick skin type I (P=.039). The mean (SD) serum 25(OH)D level at baseline was 14.14 (6.70) ng/mL and posttreatment was 46.42 (15.51) ng/mL in the 32 patients who completed the study. Serum 25(OH)D levels showed a statistically significant increase after NB-UVB treatment (P<.001). The increased serum 25(OH)D levels after NB-UVB phototherapy were not associated with Fitzpatrick skin type, which was consistent with the results of Osmancevic et al.17 The adjusted NB-UVB doses according to the different skin types might be responsible for this result in our study.
Participant age did not have a significant effect on serum 25(OH)D levels, similar to other studies in the literature.13,17 We believe that artificial UVB radiation at high doses can compensate for the 7-dehydrocholesterol that decreases in the skin with aging.
We observed no significant change in the serum calcium and phosphorus levels with NB-UVB treatment in our study. None of the participants had a metabolic disorder related to increased 25(OH)D levels. The serum ALP and PTH levels decreased significantly following treatment (P=.001 and P=.019, respectively), which may have been secondary to increased serum 25(OH)D levels.
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and maximum dose values. The posttreatment serum 25(OH)D levels were found to be related to the number of sessions received, but this value was not correlated with the maximum dose received. The MED and maximum dose were determined according to the Fitzpatrick skin type of the participants. Therefore, increased serum 25(OH)D levels with an increased number of sessions was an expected result. Our observation is in accordance with the finding described by Ryan et al.14 On the other hand, an in vitro study conducted by Olds et al21 reported that the relationship between UV light and cholecalciferol synthesis was not linear.
We found that increased serum 25(OH)D levels after treatment were not correlated with the decrease in PASI score, similar to studies by Romaní et al18 and Ryan et al.14 These results suggest that the clinical improvement following NB-UVB treatment is independent of the increased serum 25(OH)D levels in psoriasis patients.
Conclusion
In conclusion, we found that the serum 25(OH)D levels that increase as a result of NB-UVB therapy for the treatment of chronic plaque psoriasis has no statistically significant relationship with the age, Fitzpatrick skin type, baseline PASI score, changes in PASI, or maximum dose, while a positive relationship is present between the serum 25(OH)D levels and the number of sessions of NB-UVB.
- Şavk E. Immunology of Photo(chemo)therapy. Turkderm. 2010;44(suppl 2):62-66.
- Ferahbaş A. Phototherapy modalities and protocols. Turkderm. 2010;44(suppl 2):67-72.
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol. 2004;151:283-297.
- Norval M, Björn LO, de Gruijl FR. Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9:11-17.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- McKenzie RL, Liley JB, Björn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377-393.
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618-625.
- Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931-938.
- Osmancevic A, Landin-Wilhelmsen K, Larkö O, et al. UVB therapy increases 25 (OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007;23:172-178.
- Ryan C, Moran B, McKenna MJ, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-842.
- Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321-328.
- Lesiak A, Narbutt J, Pawlaczyk M, et al. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol Photoimmunol Photomed. 2011;27:304-310.
- Osmancevic A, Landin-Wilhelmsen K, Larko O, et al.Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2009;25:119-123.
- Romaní J, Caixàs A, Carrascosa JM, et al. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. 2012;166:1237-1244.
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48-60.
- Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588-593.
- Olds WJ, McKinley AR, Moore MR, et al. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J Photochem Photobiol B. 2008;93:88-93.
- Şavk E. Immunology of Photo(chemo)therapy. Turkderm. 2010;44(suppl 2):62-66.
- Ferahbaş A. Phototherapy modalities and protocols. Turkderm. 2010;44(suppl 2):67-72.
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol. 2004;151:283-297.
- Norval M, Björn LO, de Gruijl FR. Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9:11-17.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- McKenzie RL, Liley JB, Björn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377-393.
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618-625.
- Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931-938.
- Osmancevic A, Landin-Wilhelmsen K, Larkö O, et al. UVB therapy increases 25 (OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007;23:172-178.
- Ryan C, Moran B, McKenna MJ, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-842.
- Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321-328.
- Lesiak A, Narbutt J, Pawlaczyk M, et al. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol Photoimmunol Photomed. 2011;27:304-310.
- Osmancevic A, Landin-Wilhelmsen K, Larko O, et al.Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2009;25:119-123.
- Romaní J, Caixàs A, Carrascosa JM, et al. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. 2012;166:1237-1244.
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48-60.
- Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588-593.
- Olds WJ, McKinley AR, Moore MR, et al. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J Photochem Photobiol B. 2008;93:88-93.
Practice Points
- The 25-hydroxyvitamin D (25[OH]D) levels are increased by narrowband UVB (NB-UVB) treatment in psoriasis patients.
- The number of sessions of NB-UVB is associated with increased 25(OH)D levels.
BSA75, BSA90, and BSA100: New Clinical Tools for Measuring Improvement in Psoriasis
Currently, there is no widely accepted tool for assessing the severity of psoriasis in the clinical setting.1-5 Moreover, there is still a need for a simple assessment tool to assist in evaluating a patient’s response to therapy in clinical practice.6
The body surface area (BSA) is a familiar and widely used measurement by clinicians. It is easily calculated by the rule of nines or with the patient’s open palm and thumb approximating 1% of the BSA.7 Body surface area is an uncomplicated concept for patients to understand and interpret. It also promotes patient empowerment and self-care by allowing patients to monitor short-term and long-term response to therapy.
The National Psoriasis Foundation Medical Board published treatment targets for plaque psoriasis. One of the conclusions states, “The acceptable response at 3 months postinitiation was either BSA 3% or less or BSA improvement 75% or more from baseline.”8
We propose a new nomenclature that a 75% improvement in BSA be recognized as BSA75, a 90% improvement in BSA as BSA90, and a 100% improvement in BSA as BSA100. These classifications would be analogous to corresponding improvements in the following psoriasis area and severity index (PASI) scores: PASI 75, PASI 90, PASI 100.9 A loss of BSA goals/milestones (ie, BSA75) could encourage and facilitate physician-patient conversations and further direct modifications to disease management and treatment therapy.
A potential drawback to the implementation of this novel categorization system is that other notable aspects of psoriasis would not be assessed, such as erythema, induration, or scale; subjective measurements; patient quality of life; patient symptoms; areas of involvement (eg, palms, soles of feet); and disease course. Nevertheless, the BSA75, BSA90, and BSA100 classifications can serve as practical, objective, and straightforward tools to monitor disease progression and treatment response in psoriasis patients, which may potentially promote improved patient outcomes in clinical practice.
- van de Kerkhof PC. The Psoriasis Area and Severity Index and alternative approaches for the assessment of severity: persisting areas of confusion. Br J Dermatol. 1997;137:661-662.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563-569.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Ashcroft DM, Wan Po AL, Williams HC, et al. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999;141:185-191.
- Gottlieb AB, Chaudhari U, Baker DG, et al. The National Psoriasis Foundation Psoriasis Score (NPF-PS) system versus the Psoriasis Area Severity Index (PASI) and Physician’s Global Assessment (PGA): a comparison. J Drugs Dermatol. 2003;2:260-266.
- Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157:238-244.
- Sheridan RL, Petras L, Basha G, et al. Planimetry study of the percent of body surface represented by the hand and palm: sizing irregular burns is more accurately done with the palm. J Burn Care Rehabil. 1995;16:605-606.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to Psoriasis Area Severity Index 90 and 100. J Drugs Dermatol. 2015;14:1086-1088.
Currently, there is no widely accepted tool for assessing the severity of psoriasis in the clinical setting.1-5 Moreover, there is still a need for a simple assessment tool to assist in evaluating a patient’s response to therapy in clinical practice.6
The body surface area (BSA) is a familiar and widely used measurement by clinicians. It is easily calculated by the rule of nines or with the patient’s open palm and thumb approximating 1% of the BSA.7 Body surface area is an uncomplicated concept for patients to understand and interpret. It also promotes patient empowerment and self-care by allowing patients to monitor short-term and long-term response to therapy.
The National Psoriasis Foundation Medical Board published treatment targets for plaque psoriasis. One of the conclusions states, “The acceptable response at 3 months postinitiation was either BSA 3% or less or BSA improvement 75% or more from baseline.”8
We propose a new nomenclature that a 75% improvement in BSA be recognized as BSA75, a 90% improvement in BSA as BSA90, and a 100% improvement in BSA as BSA100. These classifications would be analogous to corresponding improvements in the following psoriasis area and severity index (PASI) scores: PASI 75, PASI 90, PASI 100.9 A loss of BSA goals/milestones (ie, BSA75) could encourage and facilitate physician-patient conversations and further direct modifications to disease management and treatment therapy.
A potential drawback to the implementation of this novel categorization system is that other notable aspects of psoriasis would not be assessed, such as erythema, induration, or scale; subjective measurements; patient quality of life; patient symptoms; areas of involvement (eg, palms, soles of feet); and disease course. Nevertheless, the BSA75, BSA90, and BSA100 classifications can serve as practical, objective, and straightforward tools to monitor disease progression and treatment response in psoriasis patients, which may potentially promote improved patient outcomes in clinical practice.
Currently, there is no widely accepted tool for assessing the severity of psoriasis in the clinical setting.1-5 Moreover, there is still a need for a simple assessment tool to assist in evaluating a patient’s response to therapy in clinical practice.6
The body surface area (BSA) is a familiar and widely used measurement by clinicians. It is easily calculated by the rule of nines or with the patient’s open palm and thumb approximating 1% of the BSA.7 Body surface area is an uncomplicated concept for patients to understand and interpret. It also promotes patient empowerment and self-care by allowing patients to monitor short-term and long-term response to therapy.
The National Psoriasis Foundation Medical Board published treatment targets for plaque psoriasis. One of the conclusions states, “The acceptable response at 3 months postinitiation was either BSA 3% or less or BSA improvement 75% or more from baseline.”8
We propose a new nomenclature that a 75% improvement in BSA be recognized as BSA75, a 90% improvement in BSA as BSA90, and a 100% improvement in BSA as BSA100. These classifications would be analogous to corresponding improvements in the following psoriasis area and severity index (PASI) scores: PASI 75, PASI 90, PASI 100.9 A loss of BSA goals/milestones (ie, BSA75) could encourage and facilitate physician-patient conversations and further direct modifications to disease management and treatment therapy.
A potential drawback to the implementation of this novel categorization system is that other notable aspects of psoriasis would not be assessed, such as erythema, induration, or scale; subjective measurements; patient quality of life; patient symptoms; areas of involvement (eg, palms, soles of feet); and disease course. Nevertheless, the BSA75, BSA90, and BSA100 classifications can serve as practical, objective, and straightforward tools to monitor disease progression and treatment response in psoriasis patients, which may potentially promote improved patient outcomes in clinical practice.
- van de Kerkhof PC. The Psoriasis Area and Severity Index and alternative approaches for the assessment of severity: persisting areas of confusion. Br J Dermatol. 1997;137:661-662.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563-569.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Ashcroft DM, Wan Po AL, Williams HC, et al. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999;141:185-191.
- Gottlieb AB, Chaudhari U, Baker DG, et al. The National Psoriasis Foundation Psoriasis Score (NPF-PS) system versus the Psoriasis Area Severity Index (PASI) and Physician’s Global Assessment (PGA): a comparison. J Drugs Dermatol. 2003;2:260-266.
- Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157:238-244.
- Sheridan RL, Petras L, Basha G, et al. Planimetry study of the percent of body surface represented by the hand and palm: sizing irregular burns is more accurately done with the palm. J Burn Care Rehabil. 1995;16:605-606.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to Psoriasis Area Severity Index 90 and 100. J Drugs Dermatol. 2015;14:1086-1088.
- van de Kerkhof PC. The Psoriasis Area and Severity Index and alternative approaches for the assessment of severity: persisting areas of confusion. Br J Dermatol. 1997;137:661-662.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563-569.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Ashcroft DM, Wan Po AL, Williams HC, et al. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999;141:185-191.
- Gottlieb AB, Chaudhari U, Baker DG, et al. The National Psoriasis Foundation Psoriasis Score (NPF-PS) system versus the Psoriasis Area Severity Index (PASI) and Physician’s Global Assessment (PGA): a comparison. J Drugs Dermatol. 2003;2:260-266.
- Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157:238-244.
- Sheridan RL, Petras L, Basha G, et al. Planimetry study of the percent of body surface represented by the hand and palm: sizing irregular burns is more accurately done with the palm. J Burn Care Rehabil. 1995;16:605-606.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to Psoriasis Area Severity Index 90 and 100. J Drugs Dermatol. 2015;14:1086-1088.
Experts endorse routine screening for pediatric psoriasis comorbidities
Pediatric psoriasis patients should be screened regularly to identify risk factors for comorbidities including depression, gastrointestinal problems, diabetes, and dyslipidemia, according to the debut guidelines issued by an expert panel.
The National Psoriasis Foundation and the Pediatric Dermatology Research Alliance joined forces to assess the literature and develop recommendations for screening comorbidities for children with psoriasis. The National Psoriasis Foundation has guidelines for comorbidity screening in adults with psoriasis, but no guidelines previously existed for children, wrote Emily Osier, MD, of Eastern Virginia Medical School, Norfolk, and her colleagues (JAMA Dermatol 2017 May 17. doi: 10.1001/jamadermatol.2017.0499).
The panelists reviewed the literature on psoriasis and comorbidities published between 1999 and 2015 and identified 153 studies, 26 of which involved children.
“The screening recommendations derived are largely consistent with those endorsed by the AAP for the general pediatric patient,” the researchers noted.
Although many young children are screened for a range of comorbid conditions at annual checkups, preteens and teenagers may be less likely to receive preventive services in primary care, they said. “Thus, all health care providers caring for patients with pediatric psoriasis should help assess and ensure that appropriate screening has been performed,” they emphasized.
Some notable recommendations include the following:
• Screen children with psoriasis for overweight and obesity annually using body mass index percentiles.
• Screen for diabetes every 3 years starting at age 10 years.
• Perform universal lipid screening at ages 9-11 years and 17-21 years.
• Screen for nonalcoholic fatty liver disease every 2-3 years starting at age 9-11 years.
• Screen for hypertension annually starting at age 3 years.
• Screen for arthritis at the time of psoriasis diagnosis and periodically.
• Screen yearly for depression and anxiety at all ages, with yearly screening for substance abuse starting at age 11 years.
Uveitis screening is recommended only for children with psoriatic arthritis, the researchers said.
In addition, clinicians “should recognize the profound psychosocial ramifications of psoriasis and the potential significant impact on quality of life of patients and caregivers,” the researchers wrote. Clinicians may consider a formal quality of life measurement, such as the Children’s Dermatology Life Quality Index, or at least asking questions about the impact of psoriasis on the child’s life at home, at school, and during other activities.
Awareness of comorbidities also impacts potential psoriasis treatment, the researchers said. “Direct baseline screening and monitoring tests should be performed as indicated by each individual’s therapeutic plan,” they said.
The consensus statement is a starting point for screening that will be refined over time, and may include stratifying patients by age, disease subtype, or disease severity, the researchers noted.
“Communication and collaboration between dermatologists, primary care providers, and other pediatric specialists will be critical to accomplish the recommended screenings and to limit the sequelae of this disorder,” they wrote.
The National Psoriasis Foundation and the University of California, San Diego, Eczema and Inflammatory Skin Disease Center supported the study. Dr. Osier was supported in part by a Medical Dermatology Research Fellowship grant from the National Psoriasis Foundation in 2014-2016, but she had no financial conflicts to disclose.
Pediatric psoriasis patients should be screened regularly to identify risk factors for comorbidities including depression, gastrointestinal problems, diabetes, and dyslipidemia, according to the debut guidelines issued by an expert panel.
The National Psoriasis Foundation and the Pediatric Dermatology Research Alliance joined forces to assess the literature and develop recommendations for screening comorbidities for children with psoriasis. The National Psoriasis Foundation has guidelines for comorbidity screening in adults with psoriasis, but no guidelines previously existed for children, wrote Emily Osier, MD, of Eastern Virginia Medical School, Norfolk, and her colleagues (JAMA Dermatol 2017 May 17. doi: 10.1001/jamadermatol.2017.0499).
The panelists reviewed the literature on psoriasis and comorbidities published between 1999 and 2015 and identified 153 studies, 26 of which involved children.
“The screening recommendations derived are largely consistent with those endorsed by the AAP for the general pediatric patient,” the researchers noted.
Although many young children are screened for a range of comorbid conditions at annual checkups, preteens and teenagers may be less likely to receive preventive services in primary care, they said. “Thus, all health care providers caring for patients with pediatric psoriasis should help assess and ensure that appropriate screening has been performed,” they emphasized.
Some notable recommendations include the following:
• Screen children with psoriasis for overweight and obesity annually using body mass index percentiles.
• Screen for diabetes every 3 years starting at age 10 years.
• Perform universal lipid screening at ages 9-11 years and 17-21 years.
• Screen for nonalcoholic fatty liver disease every 2-3 years starting at age 9-11 years.
• Screen for hypertension annually starting at age 3 years.
• Screen for arthritis at the time of psoriasis diagnosis and periodically.
• Screen yearly for depression and anxiety at all ages, with yearly screening for substance abuse starting at age 11 years.
Uveitis screening is recommended only for children with psoriatic arthritis, the researchers said.
In addition, clinicians “should recognize the profound psychosocial ramifications of psoriasis and the potential significant impact on quality of life of patients and caregivers,” the researchers wrote. Clinicians may consider a formal quality of life measurement, such as the Children’s Dermatology Life Quality Index, or at least asking questions about the impact of psoriasis on the child’s life at home, at school, and during other activities.
Awareness of comorbidities also impacts potential psoriasis treatment, the researchers said. “Direct baseline screening and monitoring tests should be performed as indicated by each individual’s therapeutic plan,” they said.
The consensus statement is a starting point for screening that will be refined over time, and may include stratifying patients by age, disease subtype, or disease severity, the researchers noted.
“Communication and collaboration between dermatologists, primary care providers, and other pediatric specialists will be critical to accomplish the recommended screenings and to limit the sequelae of this disorder,” they wrote.
The National Psoriasis Foundation and the University of California, San Diego, Eczema and Inflammatory Skin Disease Center supported the study. Dr. Osier was supported in part by a Medical Dermatology Research Fellowship grant from the National Psoriasis Foundation in 2014-2016, but she had no financial conflicts to disclose.
Pediatric psoriasis patients should be screened regularly to identify risk factors for comorbidities including depression, gastrointestinal problems, diabetes, and dyslipidemia, according to the debut guidelines issued by an expert panel.
The National Psoriasis Foundation and the Pediatric Dermatology Research Alliance joined forces to assess the literature and develop recommendations for screening comorbidities for children with psoriasis. The National Psoriasis Foundation has guidelines for comorbidity screening in adults with psoriasis, but no guidelines previously existed for children, wrote Emily Osier, MD, of Eastern Virginia Medical School, Norfolk, and her colleagues (JAMA Dermatol 2017 May 17. doi: 10.1001/jamadermatol.2017.0499).
The panelists reviewed the literature on psoriasis and comorbidities published between 1999 and 2015 and identified 153 studies, 26 of which involved children.
“The screening recommendations derived are largely consistent with those endorsed by the AAP for the general pediatric patient,” the researchers noted.
Although many young children are screened for a range of comorbid conditions at annual checkups, preteens and teenagers may be less likely to receive preventive services in primary care, they said. “Thus, all health care providers caring for patients with pediatric psoriasis should help assess and ensure that appropriate screening has been performed,” they emphasized.
Some notable recommendations include the following:
• Screen children with psoriasis for overweight and obesity annually using body mass index percentiles.
• Screen for diabetes every 3 years starting at age 10 years.
• Perform universal lipid screening at ages 9-11 years and 17-21 years.
• Screen for nonalcoholic fatty liver disease every 2-3 years starting at age 9-11 years.
• Screen for hypertension annually starting at age 3 years.
• Screen for arthritis at the time of psoriasis diagnosis and periodically.
• Screen yearly for depression and anxiety at all ages, with yearly screening for substance abuse starting at age 11 years.
Uveitis screening is recommended only for children with psoriatic arthritis, the researchers said.
In addition, clinicians “should recognize the profound psychosocial ramifications of psoriasis and the potential significant impact on quality of life of patients and caregivers,” the researchers wrote. Clinicians may consider a formal quality of life measurement, such as the Children’s Dermatology Life Quality Index, or at least asking questions about the impact of psoriasis on the child’s life at home, at school, and during other activities.
Awareness of comorbidities also impacts potential psoriasis treatment, the researchers said. “Direct baseline screening and monitoring tests should be performed as indicated by each individual’s therapeutic plan,” they said.
The consensus statement is a starting point for screening that will be refined over time, and may include stratifying patients by age, disease subtype, or disease severity, the researchers noted.
“Communication and collaboration between dermatologists, primary care providers, and other pediatric specialists will be critical to accomplish the recommended screenings and to limit the sequelae of this disorder,” they wrote.
The National Psoriasis Foundation and the University of California, San Diego, Eczema and Inflammatory Skin Disease Center supported the study. Dr. Osier was supported in part by a Medical Dermatology Research Fellowship grant from the National Psoriasis Foundation in 2014-2016, but she had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY