User login
WCD: Psoriasis plus depression magnifies MI risk
VANCOUVER, B.C. – The combination of even mild psoriasis and a diagnosis of depression is associated with a greater risk of acute MI than is either condition alone, according to a Danish national study.
Moreover, when depression coincided with a severe case of psoriasis, not only was the risk of incident acute MI elevated, but the risk that the MI would be fatal was significantly increased as well, Dr. Alexander Egeberg reported at the World Congress of Dermatology.
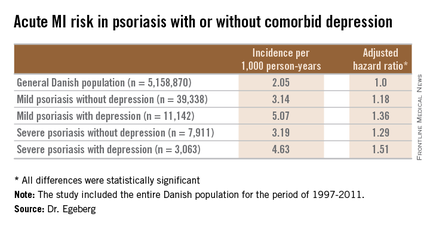
He presented a population-based cohort study of the entire Danish population, for the period of 1997-2011. The study population comprised 5,158,870 Danish citizens, including 50,480 Danes diagnosed with mild psoriasis and 10,974 others diagnosed with severe psoriasis during the study years. During that period, 11,142 members of the group with mild psoriasis and 3,063 with severe psoriasis were diagnosed with incident depression.
In a multivariate Cox regression analysis adjusted for age, gender, comorbid conditions, and concomitant medications, Danes with mild psoriasis but no diagnosis of depression were at a statistically significant 18% increased risk of having an acute MI compared to the general Danish population free of both diseases; the risk climbed upward from there (see chart).
A consistent trend for an increased risk of fatal MI was evident in patients with psoriasis, be it mild or severe, and with or without comorbid depression. However, this risk achieved statistical significance only in the group with severe psoriasis plus depression, a state associated with a 64% increased risk of fatal MI compared with the general population.
Although recent years have brought clear evidence that psoriasis is associated with increased risks of heart disease and depression, and that stand-alone depression is a risk factor for ischemic coronary events, the link between psoriasis, depression, and MI previously was unclear, explained Dr. Egeberg, who performed the research while at the University of Copenhagen but is now employed by Pfizer.
The retrospective study was conducted with the use of linked comprehensive national databases. The work was supported by a research grant from Pfizer.
VANCOUVER, B.C. – The combination of even mild psoriasis and a diagnosis of depression is associated with a greater risk of acute MI than is either condition alone, according to a Danish national study.
Moreover, when depression coincided with a severe case of psoriasis, not only was the risk of incident acute MI elevated, but the risk that the MI would be fatal was significantly increased as well, Dr. Alexander Egeberg reported at the World Congress of Dermatology.

He presented a population-based cohort study of the entire Danish population, for the period of 1997-2011. The study population comprised 5,158,870 Danish citizens, including 50,480 Danes diagnosed with mild psoriasis and 10,974 others diagnosed with severe psoriasis during the study years. During that period, 11,142 members of the group with mild psoriasis and 3,063 with severe psoriasis were diagnosed with incident depression.
In a multivariate Cox regression analysis adjusted for age, gender, comorbid conditions, and concomitant medications, Danes with mild psoriasis but no diagnosis of depression were at a statistically significant 18% increased risk of having an acute MI compared to the general Danish population free of both diseases; the risk climbed upward from there (see chart).
A consistent trend for an increased risk of fatal MI was evident in patients with psoriasis, be it mild or severe, and with or without comorbid depression. However, this risk achieved statistical significance only in the group with severe psoriasis plus depression, a state associated with a 64% increased risk of fatal MI compared with the general population.
Although recent years have brought clear evidence that psoriasis is associated with increased risks of heart disease and depression, and that stand-alone depression is a risk factor for ischemic coronary events, the link between psoriasis, depression, and MI previously was unclear, explained Dr. Egeberg, who performed the research while at the University of Copenhagen but is now employed by Pfizer.
The retrospective study was conducted with the use of linked comprehensive national databases. The work was supported by a research grant from Pfizer.
VANCOUVER, B.C. – The combination of even mild psoriasis and a diagnosis of depression is associated with a greater risk of acute MI than is either condition alone, according to a Danish national study.
Moreover, when depression coincided with a severe case of psoriasis, not only was the risk of incident acute MI elevated, but the risk that the MI would be fatal was significantly increased as well, Dr. Alexander Egeberg reported at the World Congress of Dermatology.

He presented a population-based cohort study of the entire Danish population, for the period of 1997-2011. The study population comprised 5,158,870 Danish citizens, including 50,480 Danes diagnosed with mild psoriasis and 10,974 others diagnosed with severe psoriasis during the study years. During that period, 11,142 members of the group with mild psoriasis and 3,063 with severe psoriasis were diagnosed with incident depression.
In a multivariate Cox regression analysis adjusted for age, gender, comorbid conditions, and concomitant medications, Danes with mild psoriasis but no diagnosis of depression were at a statistically significant 18% increased risk of having an acute MI compared to the general Danish population free of both diseases; the risk climbed upward from there (see chart).
A consistent trend for an increased risk of fatal MI was evident in patients with psoriasis, be it mild or severe, and with or without comorbid depression. However, this risk achieved statistical significance only in the group with severe psoriasis plus depression, a state associated with a 64% increased risk of fatal MI compared with the general population.
Although recent years have brought clear evidence that psoriasis is associated with increased risks of heart disease and depression, and that stand-alone depression is a risk factor for ischemic coronary events, the link between psoriasis, depression, and MI previously was unclear, explained Dr. Egeberg, who performed the research while at the University of Copenhagen but is now employed by Pfizer.
The retrospective study was conducted with the use of linked comprehensive national databases. The work was supported by a research grant from Pfizer.
AT WCD 2015
Key clinical point: In psoriasis patients, comorbid depression is associated with an increased risk of a first acute MI.
Major finding: The incidence rate of acute MI was 2.05 cases per 1,000 person-years in the general Danish population, climbing to 3.14/1,000 in those with mild psoriasis without diagnosed depression, and 5.07/1,000 for mild psoriasis with depression.
Data source: A retrospective, population-based, cohort study of the entire Danish adult population – more than 5.1 million individuals – during 1997-2011.
Disclosures: The presenter is an employee of Pfizer, which supported the study.
How Common Is Psoriasis in Patients With Skin of Color?
The prevalence of psoriasis in individuals with skin of color is lower than in white individuals, but a number of clinical differences can complicate diagnosis and treatment of psoriasis in patients with darker skin types. Dr. Andrew Alexis discusses some clinical features that may be unique to those with skin of color, including association with persistent pigmentary alterations, as well as special considerations and treatment approaches for psoriasis in darker-skinned patients.
The psoriasis audiocast series is created in collaboration with Cutis® and the National Psoriasis Foundation®.
Suggested Reading
Report on the psycho-social impacts of psoriasis. National Psoriasis Foundation Web site. Published in 2009. https://www.psoriasis.org/Document.DOC?id=619. Accessed June 15, 2015.
The prevalence of psoriasis in individuals with skin of color is lower than in white individuals, but a number of clinical differences can complicate diagnosis and treatment of psoriasis in patients with darker skin types. Dr. Andrew Alexis discusses some clinical features that may be unique to those with skin of color, including association with persistent pigmentary alterations, as well as special considerations and treatment approaches for psoriasis in darker-skinned patients.
The psoriasis audiocast series is created in collaboration with Cutis® and the National Psoriasis Foundation®.
The prevalence of psoriasis in individuals with skin of color is lower than in white individuals, but a number of clinical differences can complicate diagnosis and treatment of psoriasis in patients with darker skin types. Dr. Andrew Alexis discusses some clinical features that may be unique to those with skin of color, including association with persistent pigmentary alterations, as well as special considerations and treatment approaches for psoriasis in darker-skinned patients.
The psoriasis audiocast series is created in collaboration with Cutis® and the National Psoriasis Foundation®.
Suggested Reading
Report on the psycho-social impacts of psoriasis. National Psoriasis Foundation Web site. Published in 2009. https://www.psoriasis.org/Document.DOC?id=619. Accessed June 15, 2015.
Suggested Reading
Report on the psycho-social impacts of psoriasis. National Psoriasis Foundation Web site. Published in 2009. https://www.psoriasis.org/Document.DOC?id=619. Accessed June 15, 2015.
EULAR: Epigenetics points to anti-TNF efficacy in psoriatic arthritis
ROME – Tumor necrosis factor–alpha (TNF-alpha) inhibitors are well established as a treatment for psoriatic arthritis patients, but efficacy can vary greatly within that population. New work from Canadian researchers demonstrates that the global DNA methylation pattern differs between TNF-alpha–inhibitor responders and secondary failures, which could help explain the variability in medication success rates.
“Although TNF inhibitors [TNFi] work very well in certain individuals with psoriatic arthritis [PsA], up to 40% of individuals receiving this treatment fail to achieve a therapeutic response, and 20%-50% of individuals who have an initial response to treatment become refractory weeks or months after receiving therapy.” lead study author Darren O’Rielly, Ph.D., said at the European Congress of Rheumatology.
The preliminary study findings eventually could lead to the identification of biomarkers that would indicate patients who are not good candidates for TNFi medications, said Dr. O’Rielly of Memorial University, St. John’s, Nfld.
Given recent advances in epigenetics and that the epigenetic signature is affected by environmental factors, the investigators set out to determine if methylation alterations could help explain why PsA patients respond or fail with TNFi. The researchers performed genome-wide DNA methylation profiling on blood samples from 41 PsA patients, using machinery that measures about 480,000 CpG sites per sample and covers 96% of RefSeq genes. A total of 21 patients were considered TNFi responders, of whom 13 were treated with etanercept and 8 with adalimumab; median follow-up duration was 18 months. Twenty patients were considered secondary TNFi failures, of whom 15 were treated with etanercept and 5 with adalimumab; median follow-up duration was 36 months.
Dr. O’Rielly, a senior research scientist at Memorial and director of the Molecular Genetics Laboratory at Eastern Health in St. John’s, and his associates measured the methylation level at CpG sites using a genome-wide approach and selected regions of interest based on functional relevance to TNF-mediated signaling pathways with methylation level differences of 5% or greater and an adjusted P-value less than .05.
After quality-control filtering, investigators evaluated 384,599 CpG sites for TNFi responders and 368,863 CpG sites for TNFi failures. Researchers found 72 CpG sites of interest in the TNFi responder group and 91 CpG sites of interest in the TNFi failure group. Top candidate genes for TNFi responders included TRAPPC9 (which functions as an activator of NF-kB), CCR6 (which regulates the migration and recruitment of dendritic and T cells), and PSORS1C3 (psoriasis susceptibility 1 candidate 3), while top candidate genes for TNFi secondary failures included CD70 (an encoded protein that is a ligand for TNFRSF27/CD27) and TNFRSF1B (a member of the TNF receptor ‘superfamily’ that mediates most of the metabolic effects of TNF-alpha).
“We are very encouraged by these findings,” Dr. O’Rielly said. “We were a little surprised that several of our best candidate genes, such as TNFRSF1B and CD70, appear to play a role in TNF-alpha signaling, and that their methylation change occurs in a gene region that is consistent with a possible functional effect. We were expecting to find some methylation changes in genes but not necessarily in pathways with a direct connection with TNF-alpha–mediated signaling.”
The group plans to confirm these findings for the best candidate genes using other methylation-specific polymerase chain reaction technology, he said, and will investigate additional CpG sites adjacent to the region of interest, including promoters and enhancers, in the best candidate genes to better characterize the full extent of methylation changes in these regions. They also would like to replicate the findings in a prospective, independent cohort.
Dr. O’Rielly reported no relevant financial conflicts.
ROME – Tumor necrosis factor–alpha (TNF-alpha) inhibitors are well established as a treatment for psoriatic arthritis patients, but efficacy can vary greatly within that population. New work from Canadian researchers demonstrates that the global DNA methylation pattern differs between TNF-alpha–inhibitor responders and secondary failures, which could help explain the variability in medication success rates.
“Although TNF inhibitors [TNFi] work very well in certain individuals with psoriatic arthritis [PsA], up to 40% of individuals receiving this treatment fail to achieve a therapeutic response, and 20%-50% of individuals who have an initial response to treatment become refractory weeks or months after receiving therapy.” lead study author Darren O’Rielly, Ph.D., said at the European Congress of Rheumatology.
The preliminary study findings eventually could lead to the identification of biomarkers that would indicate patients who are not good candidates for TNFi medications, said Dr. O’Rielly of Memorial University, St. John’s, Nfld.
Given recent advances in epigenetics and that the epigenetic signature is affected by environmental factors, the investigators set out to determine if methylation alterations could help explain why PsA patients respond or fail with TNFi. The researchers performed genome-wide DNA methylation profiling on blood samples from 41 PsA patients, using machinery that measures about 480,000 CpG sites per sample and covers 96% of RefSeq genes. A total of 21 patients were considered TNFi responders, of whom 13 were treated with etanercept and 8 with adalimumab; median follow-up duration was 18 months. Twenty patients were considered secondary TNFi failures, of whom 15 were treated with etanercept and 5 with adalimumab; median follow-up duration was 36 months.
Dr. O’Rielly, a senior research scientist at Memorial and director of the Molecular Genetics Laboratory at Eastern Health in St. John’s, and his associates measured the methylation level at CpG sites using a genome-wide approach and selected regions of interest based on functional relevance to TNF-mediated signaling pathways with methylation level differences of 5% or greater and an adjusted P-value less than .05.
After quality-control filtering, investigators evaluated 384,599 CpG sites for TNFi responders and 368,863 CpG sites for TNFi failures. Researchers found 72 CpG sites of interest in the TNFi responder group and 91 CpG sites of interest in the TNFi failure group. Top candidate genes for TNFi responders included TRAPPC9 (which functions as an activator of NF-kB), CCR6 (which regulates the migration and recruitment of dendritic and T cells), and PSORS1C3 (psoriasis susceptibility 1 candidate 3), while top candidate genes for TNFi secondary failures included CD70 (an encoded protein that is a ligand for TNFRSF27/CD27) and TNFRSF1B (a member of the TNF receptor ‘superfamily’ that mediates most of the metabolic effects of TNF-alpha).
“We are very encouraged by these findings,” Dr. O’Rielly said. “We were a little surprised that several of our best candidate genes, such as TNFRSF1B and CD70, appear to play a role in TNF-alpha signaling, and that their methylation change occurs in a gene region that is consistent with a possible functional effect. We were expecting to find some methylation changes in genes but not necessarily in pathways with a direct connection with TNF-alpha–mediated signaling.”
The group plans to confirm these findings for the best candidate genes using other methylation-specific polymerase chain reaction technology, he said, and will investigate additional CpG sites adjacent to the region of interest, including promoters and enhancers, in the best candidate genes to better characterize the full extent of methylation changes in these regions. They also would like to replicate the findings in a prospective, independent cohort.
Dr. O’Rielly reported no relevant financial conflicts.
ROME – Tumor necrosis factor–alpha (TNF-alpha) inhibitors are well established as a treatment for psoriatic arthritis patients, but efficacy can vary greatly within that population. New work from Canadian researchers demonstrates that the global DNA methylation pattern differs between TNF-alpha–inhibitor responders and secondary failures, which could help explain the variability in medication success rates.
“Although TNF inhibitors [TNFi] work very well in certain individuals with psoriatic arthritis [PsA], up to 40% of individuals receiving this treatment fail to achieve a therapeutic response, and 20%-50% of individuals who have an initial response to treatment become refractory weeks or months after receiving therapy.” lead study author Darren O’Rielly, Ph.D., said at the European Congress of Rheumatology.
The preliminary study findings eventually could lead to the identification of biomarkers that would indicate patients who are not good candidates for TNFi medications, said Dr. O’Rielly of Memorial University, St. John’s, Nfld.
Given recent advances in epigenetics and that the epigenetic signature is affected by environmental factors, the investigators set out to determine if methylation alterations could help explain why PsA patients respond or fail with TNFi. The researchers performed genome-wide DNA methylation profiling on blood samples from 41 PsA patients, using machinery that measures about 480,000 CpG sites per sample and covers 96% of RefSeq genes. A total of 21 patients were considered TNFi responders, of whom 13 were treated with etanercept and 8 with adalimumab; median follow-up duration was 18 months. Twenty patients were considered secondary TNFi failures, of whom 15 were treated with etanercept and 5 with adalimumab; median follow-up duration was 36 months.
Dr. O’Rielly, a senior research scientist at Memorial and director of the Molecular Genetics Laboratory at Eastern Health in St. John’s, and his associates measured the methylation level at CpG sites using a genome-wide approach and selected regions of interest based on functional relevance to TNF-mediated signaling pathways with methylation level differences of 5% or greater and an adjusted P-value less than .05.
After quality-control filtering, investigators evaluated 384,599 CpG sites for TNFi responders and 368,863 CpG sites for TNFi failures. Researchers found 72 CpG sites of interest in the TNFi responder group and 91 CpG sites of interest in the TNFi failure group. Top candidate genes for TNFi responders included TRAPPC9 (which functions as an activator of NF-kB), CCR6 (which regulates the migration and recruitment of dendritic and T cells), and PSORS1C3 (psoriasis susceptibility 1 candidate 3), while top candidate genes for TNFi secondary failures included CD70 (an encoded protein that is a ligand for TNFRSF27/CD27) and TNFRSF1B (a member of the TNF receptor ‘superfamily’ that mediates most of the metabolic effects of TNF-alpha).
“We are very encouraged by these findings,” Dr. O’Rielly said. “We were a little surprised that several of our best candidate genes, such as TNFRSF1B and CD70, appear to play a role in TNF-alpha signaling, and that their methylation change occurs in a gene region that is consistent with a possible functional effect. We were expecting to find some methylation changes in genes but not necessarily in pathways with a direct connection with TNF-alpha–mediated signaling.”
The group plans to confirm these findings for the best candidate genes using other methylation-specific polymerase chain reaction technology, he said, and will investigate additional CpG sites adjacent to the region of interest, including promoters and enhancers, in the best candidate genes to better characterize the full extent of methylation changes in these regions. They also would like to replicate the findings in a prospective, independent cohort.
Dr. O’Rielly reported no relevant financial conflicts.
AT THE EULAR 2015 CONGRESS
Key clinical point:If preliminary findings are confirmed, methylation level profiling could lead to the identification of patients who are not good candidates for TNFi medications.
Major finding: Nonresponders to TNF inhibitors had significant methylation pattern differences in two key genes that appear to play a role in TNF-alpha signaling, TNFRSF1B and CD70.
Data source: A study of psoriatic arthritis patients comparing 21 TNFi responders and 20 nonresponders.
Disclosures: Dr. O’Rielly reported no relevant financial conflicts.
VIDEO: Predicting anti-TNF failure in psoriatic arthritis
ROME – Changes in the methylation status of particular genes in psoriatic arthritis patients might provide the ability to predict failure to respond to tumor necrosis factor-alpha inhibitors, according to preliminary research in 41 psoriatic arthritis patients.
Two genes stood out to the researchers from Memorial University of Newfoundland, St. John’s: TNFRSF1B and CD70. Patients who had methylation changes to those genes were more likely to have secondary failure of TNF inhibitors, said Dr. Proton Rahman, professor of internal medicine at the university and coinvestigator on the study.
It will be necessary to conduct validation studies of the results in larger numbers of patients, as well as functional studies of the effects of methylation changes on the expression of those genes and their proteins, he said in a video interview at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Changes in the methylation status of particular genes in psoriatic arthritis patients might provide the ability to predict failure to respond to tumor necrosis factor-alpha inhibitors, according to preliminary research in 41 psoriatic arthritis patients.
Two genes stood out to the researchers from Memorial University of Newfoundland, St. John’s: TNFRSF1B and CD70. Patients who had methylation changes to those genes were more likely to have secondary failure of TNF inhibitors, said Dr. Proton Rahman, professor of internal medicine at the university and coinvestigator on the study.
It will be necessary to conduct validation studies of the results in larger numbers of patients, as well as functional studies of the effects of methylation changes on the expression of those genes and their proteins, he said in a video interview at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Changes in the methylation status of particular genes in psoriatic arthritis patients might provide the ability to predict failure to respond to tumor necrosis factor-alpha inhibitors, according to preliminary research in 41 psoriatic arthritis patients.
Two genes stood out to the researchers from Memorial University of Newfoundland, St. John’s: TNFRSF1B and CD70. Patients who had methylation changes to those genes were more likely to have secondary failure of TNF inhibitors, said Dr. Proton Rahman, professor of internal medicine at the university and coinvestigator on the study.
It will be necessary to conduct validation studies of the results in larger numbers of patients, as well as functional studies of the effects of methylation changes on the expression of those genes and their proteins, he said in a video interview at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2015 CONGRESS
WCD: Ustekinumab succeeds as switch agent in psoriasis
VANCOUVER, B.C. – When adults with moderate to severe psoriasis fail one anti-TNF agent, most will still respond to another anti-TNF or ustekinumab, according to data from an investigation at two University of Toronto hospitals.
Researchers reviewed the outcomes of 155 patients who were switched to a second biologic after failing at least 12 weeks of treatment with their first, which was usually an anti–tumor necrosis factor (TNF) agent. Ustekinumab was often plan B. Of the 65 patients switched to it after failing initial anti-TNF therapy, 47 (72%) had a significant response, defined as at least a 75% improvement in the Psoriasis Area & Severity Index score (PASI 75) after 12 weeks of treatment. Ten more patients (15%) achieved PASI 50.
Meanwhile, of the 82 switched to a second anti-TNF, 48 (59%) reached PASI 75 and more achieved PASI 50 after 12 weeks.
“Clinicians switch biologics all the time, but there are no clear guidelines” on how to do it, “so it was worth looking into,” investigator Whan Kim, a medical student at McMaster University in Hamilton, Ont., said at the World Congress of Dermatology.
“This is one of the largest studies to date that determines the effectiveness of real-life switching of biologic therapies in patients with moderate to severe psoriasis. This study suggests switching to ustekinumab would induce the highest response rate – 72% was surprising,” he said. “Failing a biologic doesn’t preclude responding to another one. Don’t give up,” Mr. Kim added.
The team did find, however, that patients with two previously failed biologics were about half as likely to respond to a third biologic, compared with those who had failed just one (odds ratio, 0.474; 95% confidence interval, 0.23-0.96; P = 0.04).
The patients averaged 50 years of age and had psoriasis for approximately 17 years. Half had psoriatic arthritis, and the study population included slightly more women than men. Of the 155 patients, 93 failed initial treatment with etanercept. Seven of 12 (58%) who switched to infliximab, 24 of 43 (56%) who switched to adalimumab, and 27 of 38 (71%) who switched to ustekinumab achieved PASI 75.
Twenty-three failed initial infliximab. Three of four (75%) who switched to etanercept, four of seven (57%) who switched to adalimumab, and nine of 12 (75%) who switched to ustekinumab had a significant response.
Thirty-one patients failed initial adalimumab. Seven of 11 (64%) who switched to etanercept, 3 of 5 (60%) who switched to infliximab, and 11 of 15 (73%) who switched to ustekinumab had a significant response.
Of eight patients who failed initial ustekinumab, three (38%) switched to etanercept and had a significant response after 12 weeks.
There was no outside funding for the work, and Mr. Kim had no disclosures. The senior investigator is a speaker, consultant, and investigator for AbbVie, Amgen, and Janssen.
VANCOUVER, B.C. – When adults with moderate to severe psoriasis fail one anti-TNF agent, most will still respond to another anti-TNF or ustekinumab, according to data from an investigation at two University of Toronto hospitals.
Researchers reviewed the outcomes of 155 patients who were switched to a second biologic after failing at least 12 weeks of treatment with their first, which was usually an anti–tumor necrosis factor (TNF) agent. Ustekinumab was often plan B. Of the 65 patients switched to it after failing initial anti-TNF therapy, 47 (72%) had a significant response, defined as at least a 75% improvement in the Psoriasis Area & Severity Index score (PASI 75) after 12 weeks of treatment. Ten more patients (15%) achieved PASI 50.
Meanwhile, of the 82 switched to a second anti-TNF, 48 (59%) reached PASI 75 and more achieved PASI 50 after 12 weeks.
“Clinicians switch biologics all the time, but there are no clear guidelines” on how to do it, “so it was worth looking into,” investigator Whan Kim, a medical student at McMaster University in Hamilton, Ont., said at the World Congress of Dermatology.
“This is one of the largest studies to date that determines the effectiveness of real-life switching of biologic therapies in patients with moderate to severe psoriasis. This study suggests switching to ustekinumab would induce the highest response rate – 72% was surprising,” he said. “Failing a biologic doesn’t preclude responding to another one. Don’t give up,” Mr. Kim added.
The team did find, however, that patients with two previously failed biologics were about half as likely to respond to a third biologic, compared with those who had failed just one (odds ratio, 0.474; 95% confidence interval, 0.23-0.96; P = 0.04).
The patients averaged 50 years of age and had psoriasis for approximately 17 years. Half had psoriatic arthritis, and the study population included slightly more women than men. Of the 155 patients, 93 failed initial treatment with etanercept. Seven of 12 (58%) who switched to infliximab, 24 of 43 (56%) who switched to adalimumab, and 27 of 38 (71%) who switched to ustekinumab achieved PASI 75.
Twenty-three failed initial infliximab. Three of four (75%) who switched to etanercept, four of seven (57%) who switched to adalimumab, and nine of 12 (75%) who switched to ustekinumab had a significant response.
Thirty-one patients failed initial adalimumab. Seven of 11 (64%) who switched to etanercept, 3 of 5 (60%) who switched to infliximab, and 11 of 15 (73%) who switched to ustekinumab had a significant response.
Of eight patients who failed initial ustekinumab, three (38%) switched to etanercept and had a significant response after 12 weeks.
There was no outside funding for the work, and Mr. Kim had no disclosures. The senior investigator is a speaker, consultant, and investigator for AbbVie, Amgen, and Janssen.
VANCOUVER, B.C. – When adults with moderate to severe psoriasis fail one anti-TNF agent, most will still respond to another anti-TNF or ustekinumab, according to data from an investigation at two University of Toronto hospitals.
Researchers reviewed the outcomes of 155 patients who were switched to a second biologic after failing at least 12 weeks of treatment with their first, which was usually an anti–tumor necrosis factor (TNF) agent. Ustekinumab was often plan B. Of the 65 patients switched to it after failing initial anti-TNF therapy, 47 (72%) had a significant response, defined as at least a 75% improvement in the Psoriasis Area & Severity Index score (PASI 75) after 12 weeks of treatment. Ten more patients (15%) achieved PASI 50.
Meanwhile, of the 82 switched to a second anti-TNF, 48 (59%) reached PASI 75 and more achieved PASI 50 after 12 weeks.
“Clinicians switch biologics all the time, but there are no clear guidelines” on how to do it, “so it was worth looking into,” investigator Whan Kim, a medical student at McMaster University in Hamilton, Ont., said at the World Congress of Dermatology.
“This is one of the largest studies to date that determines the effectiveness of real-life switching of biologic therapies in patients with moderate to severe psoriasis. This study suggests switching to ustekinumab would induce the highest response rate – 72% was surprising,” he said. “Failing a biologic doesn’t preclude responding to another one. Don’t give up,” Mr. Kim added.
The team did find, however, that patients with two previously failed biologics were about half as likely to respond to a third biologic, compared with those who had failed just one (odds ratio, 0.474; 95% confidence interval, 0.23-0.96; P = 0.04).
The patients averaged 50 years of age and had psoriasis for approximately 17 years. Half had psoriatic arthritis, and the study population included slightly more women than men. Of the 155 patients, 93 failed initial treatment with etanercept. Seven of 12 (58%) who switched to infliximab, 24 of 43 (56%) who switched to adalimumab, and 27 of 38 (71%) who switched to ustekinumab achieved PASI 75.
Twenty-three failed initial infliximab. Three of four (75%) who switched to etanercept, four of seven (57%) who switched to adalimumab, and nine of 12 (75%) who switched to ustekinumab had a significant response.
Thirty-one patients failed initial adalimumab. Seven of 11 (64%) who switched to etanercept, 3 of 5 (60%) who switched to infliximab, and 11 of 15 (73%) who switched to ustekinumab had a significant response.
Of eight patients who failed initial ustekinumab, three (38%) switched to etanercept and had a significant response after 12 weeks.
There was no outside funding for the work, and Mr. Kim had no disclosures. The senior investigator is a speaker, consultant, and investigator for AbbVie, Amgen, and Janssen.
AT WCD 2015
Key clinical point: Consider ustekinumab if psoriasis patients fail their first anti-TNF agent.
Major finding: Of 65 patients switched to ustekinumab after failing initial anti-TNF therapy, 47 (72%) achieved PASI 75 within 12 weeks.
Data source: Chart review of 155 psoriasis patients.
Disclosures: There was no outside funding for the work. The senior investigator is a speaker, consultant, and investigator for Abbvie, Amgen, and Janssen.
Hidradenitis Suppurativa and Concomitant Pyoderma Gangrenosum Treated With Infliximab
Pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS) are rare chronic inflammatory dermatoses of unknown etiologies that often are refractory to conventional treatments. Multiple case reports have described the coexistence of these 2 diseases in the same patient.1-11 The diagnosis of PG commonly followed the diagnosis of HS by 5 months to 30 years in several of these cases, suggesting that HS may have triggered a possible underlying inflammatory process that resulted in subsequent development of PG. When presenting in the setting of preexisting HS, PG lesions have occurred both in the same sites of the body affected by HS as well as in distinct sites such as the legs.1
Although the treatment of either PG or HS alone can be difficult, the combination of these 2 diseases presents a further therapeutic challenge. Tumor necrosis factor a (TNF-α) has been implicated in the pathogenesis of both of these diseases, and several cases have demonstrated great potential for the use of TNF-α inhibitors, particularly infliximab, in the treatment of resistant cases of each of these conditions.2,12 We report a case of severe concurrent PG and HS in a 51-year-old man that was refractory to other treatments but clinically remitted in response to infliximab therapy.
Case Report
A 51-year-old man was transferred to our institution from an outside hospital where he had presented with fevers and a worsening rash that had prevented him from ambulating for several days secondary to severe pain. At the outside facility, he was noted to have extensive ulcerations with granulation tissue on the scalp, face, sacrum, buttocks, and bilateral legs. A skin biopsy performed at the outside facility revealed a neutrophilic dermal infiltrate with abscesses and granulation tissue consistent with PG. He was transferred to our institution for continued local wound care, corticosteroid administration, and hyperbaric oxygen therapy.
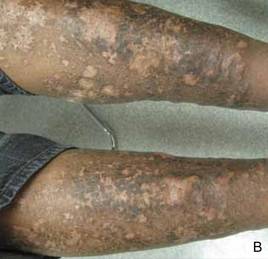
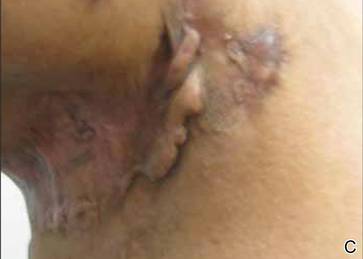
On presentation at our institution, continued ulcerative skin lesions were noted in the previously mentioned areas (Figures 1A and 1B), and deeper purulent sinus tracts were noted in the axillae and extending onto the chest (Figure 1C). A biopsy from the chest showed folliculitis with a prominent plasmacytic infiltrate consistent with HS. Cultures from the sinus tracts showed abundant growth of Enterobacter aerogenes, Klebsiella pneumoniae, Enterobacter cloacae, and Proteus mirabilis. The patient was subsequently treated with intravenous ampicillin-sulbactam and vancomycin as well as oral prednisone 80 mg daily for 2 weeks with only mild improvement of the lesions. The prednisone was gradually tapered to a dose of 10 mg daily in preparation for a trial of infliximab. During the early course of his therapy, subsequent cultures from draining sinus tracts grew Pseudomonas aeruginosa and Acinetobacter baumannii, requiring concomitant treatment with oral antimicrobials including doxycycline, sulfamethoxazole-trimethoprim, ciprofloxacin, and amoxicillin–clavulanic acid for control of the superinfection.
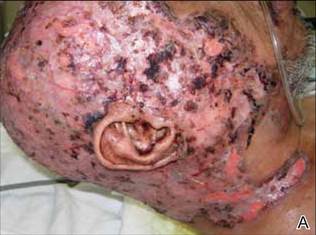 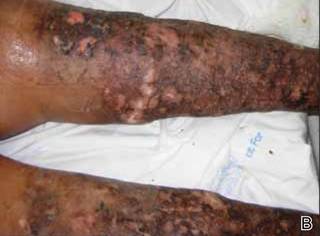 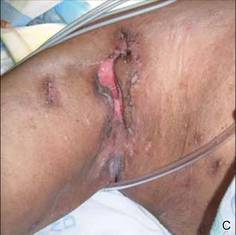 |
The patient received 3 induction infusions of infliximab at a dosage of 5 mg/kg per treatment at weeks 0, 2, and 6. At the time of the first treatment, he was noted to have numerous crusted draining erosions on the head, neck, and chest. The patient reported no adverse effects during or after each treatment. Following completion of the 3 infliximab infusions, the skin lesions showed considerable improvement, with only 1 draining lesion remaining on the left temple and only erythematous plaques remaining on the scalp, upper back, and axillae. Maintenance infusions were continued every 8 weeks with an increased infliximab dose of 7.5 mg/kg.
After 1 year of treatment, the lesions had healed to form cicatrices with no evidence of erythema, drainage, or infection (Figure 2). Doxycycline and prednisone were discontinued, and the infliximab dose was decreased to 5 mg/kg per infusion every 8 weeks. Following sustained improvement after 2 infusions at this lower dose, infliximab was successfully tapered to 2.5 mg/kg every 8 weeks for 2 doses and then was subsequently discontinued; however, the patient’s disease relapsed approximately 7 months after discontinuation of the infliximab and was immediately resumed at a dose of 5 mg/kg per infusion every 8 weeks. He has remained disease free on this dose to date.
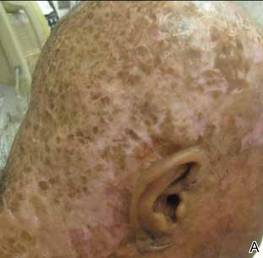   |
Comment
Pyoderma gangrenosum is a chronic inflammatory ulcerative skin condition that most commonly occurs on the lower legs.3 In its most common form, PG lesions typically begin as tender erythematous nodules or pustules that evolve into enlarging painful ulcers with raised, undermined, violaceous borders.13 Biopsy specimens taken from the edges of the lesions typically show a diffuse neutrophilic infiltrate, and pseudoepitheliomatous hyperplasia also may be seen. Lesions tend to persist for months to years, ultimately healing as cribriform scars. The etiology of PG is unknown and its pathogenesis is poorly understood.13 Pyoderma gangrenosum is associated with an underlying systemic disease in approximately 50% of cases, most commonly inflammatory bowel disease, hematologic malignancies, and inflammatory arthritis.3 An underlying immunologic abnormality is therefore postulated to contribute to the pathogenesis of PG, as it is frequently associated with these immune-mediated systemic diseases.
There is no specific and uniformly effective treatment of PG, but the main therapeutic goals include the reduction of inflammation to promote wound healing, pain reduction, and the treatment of any comorbid diseases that may contribute to the severity of PG, all while minimizing adverse side effects. Systemic corticosteroids and cyclosporine have been reported to produce the most effective results,13 but due to the side effects and toxicities of these drugs, they are often reserved for severe cases in which the benefits of their use outweigh the risks for other comorbidities. Other reported treatments include antimicrobial agents, topical and intralesional corticosteroids, steroid-sparing immunosuppressive agents, colchicine, hyperbaric oxygen therapy, and more recently drugs that function via immune modulation such as TNF-α inhibitors.
Hidradenitis suppurativa is a chronic suppurative inflammatory disease of follicular occlusion in apocrine gland–bearing areas of the skin such as the groin, axillae, and anogenital region. Hidradenitis suppurativa is characterized by recurrent skin abscesses, sinus tract and fistula formation, and subsequent fibrosis with bacterial overgrowth as a common secondary process.14 The pathogenesis of HS remains poorly understood, but histology typically shows a nonspecific inflammatory process with or without concomitant infection.15 Treatment of HS is complex and usually is transiently effective at best.14 In 2012, Rambhatla et al16 discussed the efficacy of various treatments of HS, including systemic and topical antimicrobial agents (eg, clindamycin-rifampicin combination treatment, tetracycline, topical clindamycin phosphate, isotretinoin, dapsone), antiandrogenic agents, biologic agents (eg, infliximab, etanercept, adalimumab, efalizumab), laser surgery (CO2 laser, Nd:YAG laser), and excisional surgery of sinus tracts. Other therapies include cryotherapy, photodynamic therapy, finasteride, zinc gluconate, topical resorcinol, and acitretin.16
Both PG and HS are categorized as chronic inflammatory disorders with nonspecific histopathologic findings. Although the etiologies of these 2 disorders remain poorly understood, several case reports have suggested an association between PG and HS.1,4 In many of the reported cases of concurrent HS and PG, HS preceded the diagnosis of PG by several years and no correlation in disease activity was observed between the 2 conditions.1-4,6-8 Additionally, the clinical triad of PG, acne, and suppurative hidradenitis, known as PASH syndrome, has been described, which may represent a new entity within the spectrum of autoinflammatory syndromes.5 It is not uncommon for either PG or HS to be refractory to conventional therapies, and therefore the management of concomitant PG and HS presents an even further therapeutic challenge.
Recently, biologic agents such as TNF-α inhibitors have been used with increased frequency as novel treatments for severe dermatologic diseases including psoriasis and pemphigus vulgaris, among others.17 Furthermore, several reports have presented convincing results in the off-label use of TNF-α inhibitors in the treatment of isolated PG as well as in the treatment of HS.12,14,17-22 Infliximab, a chimeric anti–TNF-α monoclonal antibody, has been used with particular success in recalcitrant cases of these conditions.12,17,19-21
In a double-blind, placebo-controlled, crossover trial analyzing 38 patients with moderate to severe refractory HS, Grant et al19 found that treatment with infliximab was associated with a significantly greater improvement in pain intensity (P<.001), disease severity (P<.001), and quality of life (P=.003), with concomitant reduction in clinical markers of inflammation compared to placebo. Similarly, a randomized, double-blind, placebo-controlled trial by Brooklyn et al12 evaluated 30 patients with refractory PG. In this study, infliximab was shown to be superior to placebo for rapid clinical response in patients with PG irrespective of the existence of concomitant inflammatory bowel disease.12
On the contrary, lack of efficacy and/or adverse reactions associated with infliximab and other TNF-α inhibitors in the treatment of PG and HS also have been reported in the literature. Fardet et al20 reported that 2 of 7 (28.6%) HS patients treated with at least 3 infusions of infliximab (5 mg/kg) at weeks 0, 2, and 6 had minimal or nonexistent improvement by week 6. Additionally, adverse events occurred in 3 of 7 (42.9%) HS patients, including abdominal pain caused by colon cancer, multifocal motor neuropathy with conduction block, and a severe allergic reaction.20 Usmani et al21 described 1 HS patient who developed an infliximab-induced lupus reaction, 1 who experienced a hypersensitivity reaction to infliximab, and 2 who had poor response to treatment despite 3 infusions. Kleinpenning et al22 reported a case of PG that failed consecutive trials of etanercept and adalimumab. Wolbing et al23 reported a case of septic shock after treatment of PG with infliximab. Shareef et al24 reported progression of IgA gammopathy to myeloma following infliximab treatment for PG in 1 patient.
Of the reported cases of concurrent HS and PG, 3 were treated with TNF-αinhibitors, both alone and in conjunction with other treatment modalities, with varying results. One patient demonstrated a partial response to treatment with etanercept followed by infliximab, 1 was resistant to treatment with infliximab as well as adalimumab, and 1 exhibited clinical improvement following treatment with infliximab.1,2
Conclusion
In our patient with concurrent PG and HS, both conditions showed dramatic improvement with in-fliximab therapy, and this response has been sustained on 5-mg/kg infliximab maintenance therapy every 8 weeks for the last 3 years. Our case suggests that infliximab may represent an effective therapeutic option for the treatment of concurrent PG and HS that is refractory to conventional therapies. It is yet to be seen whether our patient will continue to experience sustained remission in both his PG and HS permitting the discontinuation of infliximab. Continued study of infliximab and other TNF-α inhibitors is necessary to establish their long-term safety and efficacy for use in patients affected by both HS and PG.
1. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurative and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270.
2. Moschella S. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? a report of three treated cases. Int J Dermatol. 2007;46:1287-1291.
3. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
4. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671.
5. Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
6. Buckley DA, Rogers S. Cyclosporin-responsive hidradenitis suppurativa. J R Soc Med. 1995;88:289-290.
7. Shenefelt PD. Pyoderma gangrenosum associated with cystic acne and hidradenitis suppurativa controlled by adding minocycline and sulfasalazine to the treatment regimen. Cutis. 1996;57:315-319.
8. Raynor A, Askari AD. Behçet’s disease and treatment with colchicine. J Am Acad Dermatol. 1980;2:396-400.
9. Steinhoff JP, Cilursu A, Falasca GF, et al. A study of musculoskeletal manifestations in 12 patients with SAPHO syndrome. J Clin Rheumatol. 2002;8:13-22.
10. Rosner IA, Richter DE, Huettner TL, et al. Spondyloarthropathy associated with hidradenitis suppurative and acne conglobata. Ann Intern Med. 1982;97:520-525.
11. von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000-1005.
12. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomized, double-blind, placebo-controlled trial. Gut. 2006;55:505-509.
13. Ruocco E, Sangiulliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008-1017.
14. Mortimer P. Management of hidradenitis suppurativa. Clin Exp Dermatol. 2002;27:328.
15. Blanco R, Martinez-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580-584.
16. Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatment for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
17. Alecsandru D, Padilla B, Izquierdo JA, et al. Severe refractory hidradenitis suppurativa in an HIV-positive patient successfully treated with infliximab. Arch Dermatol. 2010;146:1343-1345.
18. Alexis A, Strober B. Off-label dermatologic uses of anti-TNF-a therapies. J Cutan Med Surg. 2006;9:296-301.
19. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
20. Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
21. Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
22. Kleinpenning MM, Langewouters AM, Van De Kerkhof PC, et al. Severe pyoderma gangrenosum unresponsive to etanercept and adalimumab. J Dermatolog Treat. 2011;22:261-265.
23. Wolbing F, Fierlbeck G, Hotzenecker W, et al. Septic shock after treatment of pyoderma gangrenosum with infliximab. Acta Dermato-Venereologica. 2009;89:93-94.
24. Shareef MS, Munro LR, Owen RG, et al. Progression of IgA gammopathy to myeloma following infliximab treatment for pyoderma gangrenosum. Clin Exp Dermatol. 2012;37:146-148.
Pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS) are rare chronic inflammatory dermatoses of unknown etiologies that often are refractory to conventional treatments. Multiple case reports have described the coexistence of these 2 diseases in the same patient.1-11 The diagnosis of PG commonly followed the diagnosis of HS by 5 months to 30 years in several of these cases, suggesting that HS may have triggered a possible underlying inflammatory process that resulted in subsequent development of PG. When presenting in the setting of preexisting HS, PG lesions have occurred both in the same sites of the body affected by HS as well as in distinct sites such as the legs.1
Although the treatment of either PG or HS alone can be difficult, the combination of these 2 diseases presents a further therapeutic challenge. Tumor necrosis factor a (TNF-α) has been implicated in the pathogenesis of both of these diseases, and several cases have demonstrated great potential for the use of TNF-α inhibitors, particularly infliximab, in the treatment of resistant cases of each of these conditions.2,12 We report a case of severe concurrent PG and HS in a 51-year-old man that was refractory to other treatments but clinically remitted in response to infliximab therapy.
Case Report
A 51-year-old man was transferred to our institution from an outside hospital where he had presented with fevers and a worsening rash that had prevented him from ambulating for several days secondary to severe pain. At the outside facility, he was noted to have extensive ulcerations with granulation tissue on the scalp, face, sacrum, buttocks, and bilateral legs. A skin biopsy performed at the outside facility revealed a neutrophilic dermal infiltrate with abscesses and granulation tissue consistent with PG. He was transferred to our institution for continued local wound care, corticosteroid administration, and hyperbaric oxygen therapy.
On presentation at our institution, continued ulcerative skin lesions were noted in the previously mentioned areas (Figures 1A and 1B), and deeper purulent sinus tracts were noted in the axillae and extending onto the chest (Figure 1C). A biopsy from the chest showed folliculitis with a prominent plasmacytic infiltrate consistent with HS. Cultures from the sinus tracts showed abundant growth of Enterobacter aerogenes, Klebsiella pneumoniae, Enterobacter cloacae, and Proteus mirabilis. The patient was subsequently treated with intravenous ampicillin-sulbactam and vancomycin as well as oral prednisone 80 mg daily for 2 weeks with only mild improvement of the lesions. The prednisone was gradually tapered to a dose of 10 mg daily in preparation for a trial of infliximab. During the early course of his therapy, subsequent cultures from draining sinus tracts grew Pseudomonas aeruginosa and Acinetobacter baumannii, requiring concomitant treatment with oral antimicrobials including doxycycline, sulfamethoxazole-trimethoprim, ciprofloxacin, and amoxicillin–clavulanic acid for control of the superinfection.
   |
The patient received 3 induction infusions of infliximab at a dosage of 5 mg/kg per treatment at weeks 0, 2, and 6. At the time of the first treatment, he was noted to have numerous crusted draining erosions on the head, neck, and chest. The patient reported no adverse effects during or after each treatment. Following completion of the 3 infliximab infusions, the skin lesions showed considerable improvement, with only 1 draining lesion remaining on the left temple and only erythematous plaques remaining on the scalp, upper back, and axillae. Maintenance infusions were continued every 8 weeks with an increased infliximab dose of 7.5 mg/kg.
After 1 year of treatment, the lesions had healed to form cicatrices with no evidence of erythema, drainage, or infection (Figure 2). Doxycycline and prednisone were discontinued, and the infliximab dose was decreased to 5 mg/kg per infusion every 8 weeks. Following sustained improvement after 2 infusions at this lower dose, infliximab was successfully tapered to 2.5 mg/kg every 8 weeks for 2 doses and then was subsequently discontinued; however, the patient’s disease relapsed approximately 7 months after discontinuation of the infliximab and was immediately resumed at a dose of 5 mg/kg per infusion every 8 weeks. He has remained disease free on this dose to date.
   |
Comment
Pyoderma gangrenosum is a chronic inflammatory ulcerative skin condition that most commonly occurs on the lower legs.3 In its most common form, PG lesions typically begin as tender erythematous nodules or pustules that evolve into enlarging painful ulcers with raised, undermined, violaceous borders.13 Biopsy specimens taken from the edges of the lesions typically show a diffuse neutrophilic infiltrate, and pseudoepitheliomatous hyperplasia also may be seen. Lesions tend to persist for months to years, ultimately healing as cribriform scars. The etiology of PG is unknown and its pathogenesis is poorly understood.13 Pyoderma gangrenosum is associated with an underlying systemic disease in approximately 50% of cases, most commonly inflammatory bowel disease, hematologic malignancies, and inflammatory arthritis.3 An underlying immunologic abnormality is therefore postulated to contribute to the pathogenesis of PG, as it is frequently associated with these immune-mediated systemic diseases.
There is no specific and uniformly effective treatment of PG, but the main therapeutic goals include the reduction of inflammation to promote wound healing, pain reduction, and the treatment of any comorbid diseases that may contribute to the severity of PG, all while minimizing adverse side effects. Systemic corticosteroids and cyclosporine have been reported to produce the most effective results,13 but due to the side effects and toxicities of these drugs, they are often reserved for severe cases in which the benefits of their use outweigh the risks for other comorbidities. Other reported treatments include antimicrobial agents, topical and intralesional corticosteroids, steroid-sparing immunosuppressive agents, colchicine, hyperbaric oxygen therapy, and more recently drugs that function via immune modulation such as TNF-α inhibitors.
Hidradenitis suppurativa is a chronic suppurative inflammatory disease of follicular occlusion in apocrine gland–bearing areas of the skin such as the groin, axillae, and anogenital region. Hidradenitis suppurativa is characterized by recurrent skin abscesses, sinus tract and fistula formation, and subsequent fibrosis with bacterial overgrowth as a common secondary process.14 The pathogenesis of HS remains poorly understood, but histology typically shows a nonspecific inflammatory process with or without concomitant infection.15 Treatment of HS is complex and usually is transiently effective at best.14 In 2012, Rambhatla et al16 discussed the efficacy of various treatments of HS, including systemic and topical antimicrobial agents (eg, clindamycin-rifampicin combination treatment, tetracycline, topical clindamycin phosphate, isotretinoin, dapsone), antiandrogenic agents, biologic agents (eg, infliximab, etanercept, adalimumab, efalizumab), laser surgery (CO2 laser, Nd:YAG laser), and excisional surgery of sinus tracts. Other therapies include cryotherapy, photodynamic therapy, finasteride, zinc gluconate, topical resorcinol, and acitretin.16
Both PG and HS are categorized as chronic inflammatory disorders with nonspecific histopathologic findings. Although the etiologies of these 2 disorders remain poorly understood, several case reports have suggested an association between PG and HS.1,4 In many of the reported cases of concurrent HS and PG, HS preceded the diagnosis of PG by several years and no correlation in disease activity was observed between the 2 conditions.1-4,6-8 Additionally, the clinical triad of PG, acne, and suppurative hidradenitis, known as PASH syndrome, has been described, which may represent a new entity within the spectrum of autoinflammatory syndromes.5 It is not uncommon for either PG or HS to be refractory to conventional therapies, and therefore the management of concomitant PG and HS presents an even further therapeutic challenge.
Recently, biologic agents such as TNF-α inhibitors have been used with increased frequency as novel treatments for severe dermatologic diseases including psoriasis and pemphigus vulgaris, among others.17 Furthermore, several reports have presented convincing results in the off-label use of TNF-α inhibitors in the treatment of isolated PG as well as in the treatment of HS.12,14,17-22 Infliximab, a chimeric anti–TNF-α monoclonal antibody, has been used with particular success in recalcitrant cases of these conditions.12,17,19-21
In a double-blind, placebo-controlled, crossover trial analyzing 38 patients with moderate to severe refractory HS, Grant et al19 found that treatment with infliximab was associated with a significantly greater improvement in pain intensity (P<.001), disease severity (P<.001), and quality of life (P=.003), with concomitant reduction in clinical markers of inflammation compared to placebo. Similarly, a randomized, double-blind, placebo-controlled trial by Brooklyn et al12 evaluated 30 patients with refractory PG. In this study, infliximab was shown to be superior to placebo for rapid clinical response in patients with PG irrespective of the existence of concomitant inflammatory bowel disease.12
On the contrary, lack of efficacy and/or adverse reactions associated with infliximab and other TNF-α inhibitors in the treatment of PG and HS also have been reported in the literature. Fardet et al20 reported that 2 of 7 (28.6%) HS patients treated with at least 3 infusions of infliximab (5 mg/kg) at weeks 0, 2, and 6 had minimal or nonexistent improvement by week 6. Additionally, adverse events occurred in 3 of 7 (42.9%) HS patients, including abdominal pain caused by colon cancer, multifocal motor neuropathy with conduction block, and a severe allergic reaction.20 Usmani et al21 described 1 HS patient who developed an infliximab-induced lupus reaction, 1 who experienced a hypersensitivity reaction to infliximab, and 2 who had poor response to treatment despite 3 infusions. Kleinpenning et al22 reported a case of PG that failed consecutive trials of etanercept and adalimumab. Wolbing et al23 reported a case of septic shock after treatment of PG with infliximab. Shareef et al24 reported progression of IgA gammopathy to myeloma following infliximab treatment for PG in 1 patient.
Of the reported cases of concurrent HS and PG, 3 were treated with TNF-αinhibitors, both alone and in conjunction with other treatment modalities, with varying results. One patient demonstrated a partial response to treatment with etanercept followed by infliximab, 1 was resistant to treatment with infliximab as well as adalimumab, and 1 exhibited clinical improvement following treatment with infliximab.1,2
Conclusion
In our patient with concurrent PG and HS, both conditions showed dramatic improvement with in-fliximab therapy, and this response has been sustained on 5-mg/kg infliximab maintenance therapy every 8 weeks for the last 3 years. Our case suggests that infliximab may represent an effective therapeutic option for the treatment of concurrent PG and HS that is refractory to conventional therapies. It is yet to be seen whether our patient will continue to experience sustained remission in both his PG and HS permitting the discontinuation of infliximab. Continued study of infliximab and other TNF-α inhibitors is necessary to establish their long-term safety and efficacy for use in patients affected by both HS and PG.
Pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS) are rare chronic inflammatory dermatoses of unknown etiologies that often are refractory to conventional treatments. Multiple case reports have described the coexistence of these 2 diseases in the same patient.1-11 The diagnosis of PG commonly followed the diagnosis of HS by 5 months to 30 years in several of these cases, suggesting that HS may have triggered a possible underlying inflammatory process that resulted in subsequent development of PG. When presenting in the setting of preexisting HS, PG lesions have occurred both in the same sites of the body affected by HS as well as in distinct sites such as the legs.1
Although the treatment of either PG or HS alone can be difficult, the combination of these 2 diseases presents a further therapeutic challenge. Tumor necrosis factor a (TNF-α) has been implicated in the pathogenesis of both of these diseases, and several cases have demonstrated great potential for the use of TNF-α inhibitors, particularly infliximab, in the treatment of resistant cases of each of these conditions.2,12 We report a case of severe concurrent PG and HS in a 51-year-old man that was refractory to other treatments but clinically remitted in response to infliximab therapy.
Case Report
A 51-year-old man was transferred to our institution from an outside hospital where he had presented with fevers and a worsening rash that had prevented him from ambulating for several days secondary to severe pain. At the outside facility, he was noted to have extensive ulcerations with granulation tissue on the scalp, face, sacrum, buttocks, and bilateral legs. A skin biopsy performed at the outside facility revealed a neutrophilic dermal infiltrate with abscesses and granulation tissue consistent with PG. He was transferred to our institution for continued local wound care, corticosteroid administration, and hyperbaric oxygen therapy.
On presentation at our institution, continued ulcerative skin lesions were noted in the previously mentioned areas (Figures 1A and 1B), and deeper purulent sinus tracts were noted in the axillae and extending onto the chest (Figure 1C). A biopsy from the chest showed folliculitis with a prominent plasmacytic infiltrate consistent with HS. Cultures from the sinus tracts showed abundant growth of Enterobacter aerogenes, Klebsiella pneumoniae, Enterobacter cloacae, and Proteus mirabilis. The patient was subsequently treated with intravenous ampicillin-sulbactam and vancomycin as well as oral prednisone 80 mg daily for 2 weeks with only mild improvement of the lesions. The prednisone was gradually tapered to a dose of 10 mg daily in preparation for a trial of infliximab. During the early course of his therapy, subsequent cultures from draining sinus tracts grew Pseudomonas aeruginosa and Acinetobacter baumannii, requiring concomitant treatment with oral antimicrobials including doxycycline, sulfamethoxazole-trimethoprim, ciprofloxacin, and amoxicillin–clavulanic acid for control of the superinfection.
   |
The patient received 3 induction infusions of infliximab at a dosage of 5 mg/kg per treatment at weeks 0, 2, and 6. At the time of the first treatment, he was noted to have numerous crusted draining erosions on the head, neck, and chest. The patient reported no adverse effects during or after each treatment. Following completion of the 3 infliximab infusions, the skin lesions showed considerable improvement, with only 1 draining lesion remaining on the left temple and only erythematous plaques remaining on the scalp, upper back, and axillae. Maintenance infusions were continued every 8 weeks with an increased infliximab dose of 7.5 mg/kg.
After 1 year of treatment, the lesions had healed to form cicatrices with no evidence of erythema, drainage, or infection (Figure 2). Doxycycline and prednisone were discontinued, and the infliximab dose was decreased to 5 mg/kg per infusion every 8 weeks. Following sustained improvement after 2 infusions at this lower dose, infliximab was successfully tapered to 2.5 mg/kg every 8 weeks for 2 doses and then was subsequently discontinued; however, the patient’s disease relapsed approximately 7 months after discontinuation of the infliximab and was immediately resumed at a dose of 5 mg/kg per infusion every 8 weeks. He has remained disease free on this dose to date.
   |
Comment
Pyoderma gangrenosum is a chronic inflammatory ulcerative skin condition that most commonly occurs on the lower legs.3 In its most common form, PG lesions typically begin as tender erythematous nodules or pustules that evolve into enlarging painful ulcers with raised, undermined, violaceous borders.13 Biopsy specimens taken from the edges of the lesions typically show a diffuse neutrophilic infiltrate, and pseudoepitheliomatous hyperplasia also may be seen. Lesions tend to persist for months to years, ultimately healing as cribriform scars. The etiology of PG is unknown and its pathogenesis is poorly understood.13 Pyoderma gangrenosum is associated with an underlying systemic disease in approximately 50% of cases, most commonly inflammatory bowel disease, hematologic malignancies, and inflammatory arthritis.3 An underlying immunologic abnormality is therefore postulated to contribute to the pathogenesis of PG, as it is frequently associated with these immune-mediated systemic diseases.
There is no specific and uniformly effective treatment of PG, but the main therapeutic goals include the reduction of inflammation to promote wound healing, pain reduction, and the treatment of any comorbid diseases that may contribute to the severity of PG, all while minimizing adverse side effects. Systemic corticosteroids and cyclosporine have been reported to produce the most effective results,13 but due to the side effects and toxicities of these drugs, they are often reserved for severe cases in which the benefits of their use outweigh the risks for other comorbidities. Other reported treatments include antimicrobial agents, topical and intralesional corticosteroids, steroid-sparing immunosuppressive agents, colchicine, hyperbaric oxygen therapy, and more recently drugs that function via immune modulation such as TNF-α inhibitors.
Hidradenitis suppurativa is a chronic suppurative inflammatory disease of follicular occlusion in apocrine gland–bearing areas of the skin such as the groin, axillae, and anogenital region. Hidradenitis suppurativa is characterized by recurrent skin abscesses, sinus tract and fistula formation, and subsequent fibrosis with bacterial overgrowth as a common secondary process.14 The pathogenesis of HS remains poorly understood, but histology typically shows a nonspecific inflammatory process with or without concomitant infection.15 Treatment of HS is complex and usually is transiently effective at best.14 In 2012, Rambhatla et al16 discussed the efficacy of various treatments of HS, including systemic and topical antimicrobial agents (eg, clindamycin-rifampicin combination treatment, tetracycline, topical clindamycin phosphate, isotretinoin, dapsone), antiandrogenic agents, biologic agents (eg, infliximab, etanercept, adalimumab, efalizumab), laser surgery (CO2 laser, Nd:YAG laser), and excisional surgery of sinus tracts. Other therapies include cryotherapy, photodynamic therapy, finasteride, zinc gluconate, topical resorcinol, and acitretin.16
Both PG and HS are categorized as chronic inflammatory disorders with nonspecific histopathologic findings. Although the etiologies of these 2 disorders remain poorly understood, several case reports have suggested an association between PG and HS.1,4 In many of the reported cases of concurrent HS and PG, HS preceded the diagnosis of PG by several years and no correlation in disease activity was observed between the 2 conditions.1-4,6-8 Additionally, the clinical triad of PG, acne, and suppurative hidradenitis, known as PASH syndrome, has been described, which may represent a new entity within the spectrum of autoinflammatory syndromes.5 It is not uncommon for either PG or HS to be refractory to conventional therapies, and therefore the management of concomitant PG and HS presents an even further therapeutic challenge.
Recently, biologic agents such as TNF-α inhibitors have been used with increased frequency as novel treatments for severe dermatologic diseases including psoriasis and pemphigus vulgaris, among others.17 Furthermore, several reports have presented convincing results in the off-label use of TNF-α inhibitors in the treatment of isolated PG as well as in the treatment of HS.12,14,17-22 Infliximab, a chimeric anti–TNF-α monoclonal antibody, has been used with particular success in recalcitrant cases of these conditions.12,17,19-21
In a double-blind, placebo-controlled, crossover trial analyzing 38 patients with moderate to severe refractory HS, Grant et al19 found that treatment with infliximab was associated with a significantly greater improvement in pain intensity (P<.001), disease severity (P<.001), and quality of life (P=.003), with concomitant reduction in clinical markers of inflammation compared to placebo. Similarly, a randomized, double-blind, placebo-controlled trial by Brooklyn et al12 evaluated 30 patients with refractory PG. In this study, infliximab was shown to be superior to placebo for rapid clinical response in patients with PG irrespective of the existence of concomitant inflammatory bowel disease.12
On the contrary, lack of efficacy and/or adverse reactions associated with infliximab and other TNF-α inhibitors in the treatment of PG and HS also have been reported in the literature. Fardet et al20 reported that 2 of 7 (28.6%) HS patients treated with at least 3 infusions of infliximab (5 mg/kg) at weeks 0, 2, and 6 had minimal or nonexistent improvement by week 6. Additionally, adverse events occurred in 3 of 7 (42.9%) HS patients, including abdominal pain caused by colon cancer, multifocal motor neuropathy with conduction block, and a severe allergic reaction.20 Usmani et al21 described 1 HS patient who developed an infliximab-induced lupus reaction, 1 who experienced a hypersensitivity reaction to infliximab, and 2 who had poor response to treatment despite 3 infusions. Kleinpenning et al22 reported a case of PG that failed consecutive trials of etanercept and adalimumab. Wolbing et al23 reported a case of septic shock after treatment of PG with infliximab. Shareef et al24 reported progression of IgA gammopathy to myeloma following infliximab treatment for PG in 1 patient.
Of the reported cases of concurrent HS and PG, 3 were treated with TNF-αinhibitors, both alone and in conjunction with other treatment modalities, with varying results. One patient demonstrated a partial response to treatment with etanercept followed by infliximab, 1 was resistant to treatment with infliximab as well as adalimumab, and 1 exhibited clinical improvement following treatment with infliximab.1,2
Conclusion
In our patient with concurrent PG and HS, both conditions showed dramatic improvement with in-fliximab therapy, and this response has been sustained on 5-mg/kg infliximab maintenance therapy every 8 weeks for the last 3 years. Our case suggests that infliximab may represent an effective therapeutic option for the treatment of concurrent PG and HS that is refractory to conventional therapies. It is yet to be seen whether our patient will continue to experience sustained remission in both his PG and HS permitting the discontinuation of infliximab. Continued study of infliximab and other TNF-α inhibitors is necessary to establish their long-term safety and efficacy for use in patients affected by both HS and PG.
1. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurative and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270.
2. Moschella S. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? a report of three treated cases. Int J Dermatol. 2007;46:1287-1291.
3. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
4. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671.
5. Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
6. Buckley DA, Rogers S. Cyclosporin-responsive hidradenitis suppurativa. J R Soc Med. 1995;88:289-290.
7. Shenefelt PD. Pyoderma gangrenosum associated with cystic acne and hidradenitis suppurativa controlled by adding minocycline and sulfasalazine to the treatment regimen. Cutis. 1996;57:315-319.
8. Raynor A, Askari AD. Behçet’s disease and treatment with colchicine. J Am Acad Dermatol. 1980;2:396-400.
9. Steinhoff JP, Cilursu A, Falasca GF, et al. A study of musculoskeletal manifestations in 12 patients with SAPHO syndrome. J Clin Rheumatol. 2002;8:13-22.
10. Rosner IA, Richter DE, Huettner TL, et al. Spondyloarthropathy associated with hidradenitis suppurative and acne conglobata. Ann Intern Med. 1982;97:520-525.
11. von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000-1005.
12. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomized, double-blind, placebo-controlled trial. Gut. 2006;55:505-509.
13. Ruocco E, Sangiulliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008-1017.
14. Mortimer P. Management of hidradenitis suppurativa. Clin Exp Dermatol. 2002;27:328.
15. Blanco R, Martinez-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580-584.
16. Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatment for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
17. Alecsandru D, Padilla B, Izquierdo JA, et al. Severe refractory hidradenitis suppurativa in an HIV-positive patient successfully treated with infliximab. Arch Dermatol. 2010;146:1343-1345.
18. Alexis A, Strober B. Off-label dermatologic uses of anti-TNF-a therapies. J Cutan Med Surg. 2006;9:296-301.
19. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
20. Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
21. Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
22. Kleinpenning MM, Langewouters AM, Van De Kerkhof PC, et al. Severe pyoderma gangrenosum unresponsive to etanercept and adalimumab. J Dermatolog Treat. 2011;22:261-265.
23. Wolbing F, Fierlbeck G, Hotzenecker W, et al. Septic shock after treatment of pyoderma gangrenosum with infliximab. Acta Dermato-Venereologica. 2009;89:93-94.
24. Shareef MS, Munro LR, Owen RG, et al. Progression of IgA gammopathy to myeloma following infliximab treatment for pyoderma gangrenosum. Clin Exp Dermatol. 2012;37:146-148.
1. Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurative and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146:1265-1270.
2. Moschella S. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? a report of three treated cases. Int J Dermatol. 2007;46:1287-1291.
3. Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
4. Ah-Weng A, Langtry JAA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol. 2005;30:669-671.
5. Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
6. Buckley DA, Rogers S. Cyclosporin-responsive hidradenitis suppurativa. J R Soc Med. 1995;88:289-290.
7. Shenefelt PD. Pyoderma gangrenosum associated with cystic acne and hidradenitis suppurativa controlled by adding minocycline and sulfasalazine to the treatment regimen. Cutis. 1996;57:315-319.
8. Raynor A, Askari AD. Behçet’s disease and treatment with colchicine. J Am Acad Dermatol. 1980;2:396-400.
9. Steinhoff JP, Cilursu A, Falasca GF, et al. A study of musculoskeletal manifestations in 12 patients with SAPHO syndrome. J Clin Rheumatol. 2002;8:13-22.
10. Rosner IA, Richter DE, Huettner TL, et al. Spondyloarthropathy associated with hidradenitis suppurative and acne conglobata. Ann Intern Med. 1982;97:520-525.
11. von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137:1000-1005.
12. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomized, double-blind, placebo-controlled trial. Gut. 2006;55:505-509.
13. Ruocco E, Sangiulliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008-1017.
14. Mortimer P. Management of hidradenitis suppurativa. Clin Exp Dermatol. 2002;27:328.
15. Blanco R, Martinez-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580-584.
16. Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatment for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
17. Alecsandru D, Padilla B, Izquierdo JA, et al. Severe refractory hidradenitis suppurativa in an HIV-positive patient successfully treated with infliximab. Arch Dermatol. 2010;146:1343-1345.
18. Alexis A, Strober B. Off-label dermatologic uses of anti-TNF-a therapies. J Cutan Med Surg. 2006;9:296-301.
19. Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217.
20. Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624-628.
21. Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204-205.
22. Kleinpenning MM, Langewouters AM, Van De Kerkhof PC, et al. Severe pyoderma gangrenosum unresponsive to etanercept and adalimumab. J Dermatolog Treat. 2011;22:261-265.
23. Wolbing F, Fierlbeck G, Hotzenecker W, et al. Septic shock after treatment of pyoderma gangrenosum with infliximab. Acta Dermato-Venereologica. 2009;89:93-94.
24. Shareef MS, Munro LR, Owen RG, et al. Progression of IgA gammopathy to myeloma following infliximab treatment for pyoderma gangrenosum. Clin Exp Dermatol. 2012;37:146-148.
Practice Points
- Pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS) are rare chronic inflammatory dermatoses that may coexist in the same patient.
- Infliximab may represent an effective therapeutic option for the treatment of concurrent PG and HS that is refractory to conventional therapies.
WCD: Tofacitinib’s benefits for psoriasis persist for 2 years
VANCOUVER – Oral tofacitinib for adults with moderate to severe plaque psoriasis demonstrated sustained efficacy and a “generally manageable” safety profile throughout 2 years of follow-up in a large, multicenter ongoing long-term extension study, Dr. Matthias Augustin reported at the World Congress of Dermatology.
One month after enrolling in the phase III, open-label trial (known as OPT Extend), and going on the investigational oral Janus kinase inhibitor at 10 mg b.i.d., 56.2% of the 2,847 participants showed a PASI 75 response – that is, at least a 75% reduction from their baseline Psoriasis Area and Severity Index score. After 2 years in the OPT Extend study, 64.5% of the 1,912 patients remaining in the study were PASI 75 responders.
Similarly, 56.3% of patients went from moderate or severe disease at baseline to “clear” or “almost clear” by Physician Global Assessment (PGA) at 1 month, and 53.9% of participants had a PGA score of 0 or 1 at 24 months, added Dr. Augustin, professor of dermatology at the University of Hamburg-Eppendorf and director of the German Center for Health Services Research in Dermatology and Nursing.
Psoriasis patients who had participated in any of four earlier phase III or two phase II randomized, controlled trials of tofacitinib were eligible to participate in OPT Extend. Participants were placed on tofacitinib at 10 mg b.i.d. for the first 2 months of the open-label study; then investigators adjusted their dose individually – either 5 or 10 mg b.i.d. – at each follow-up visit based on efficacy and side effects. For purposes of OPT Extend, patients’ baseline PASI and PGA scores were defined as the values recorded on the day they were randomized in the earlier round of trials.
Through 2 years of follow-up in this interim analysis of OPT Extend, less than 10% of subjects discontinued tofacitinib because of adverse events. The adverse events were the same as the ones that arose in the earlier, briefer randomized trials, the longest of which lasted 1 year. No signs of cumulative organ toxicity were evident during the additional follow-up.
Slightly more than 4% of all adverse events were categorized as severe, while 62% were mild. The most frequent adverse events were nasopharyngitis in 16% of patients, increased creatinine phosphokinase in 10%, and upper respiratory tract infections in 7%. Serious infections requiring systemic antibiotics or hospitalization occurred in 1.8% of patients on tofacitinib for 2 years, the most common of which were pneumonia, herpes zoster, and urinary tract infection. Herpes zoster occurred in 3.5% of participants, with most cases being mild or moderate. Malignancies other than nonmelanoma skin cancers occurred in 1.2% of subjects.
In terms of laboratory findings of interest, patients’ LDL/HDL ratio remained stable throughout 24 months on tofacitinib. Nor were there any clinically meaningful changes in average levels of other laboratory parameters, including hemoglobin, creatinine phosphokinase, lymphocytes, and neutrophils. A lymphocyte count below 500/mm3 occurred in 0.4% of patients at some point; however, it was unrelated to duration of exposure to tofacitinib and wasn’t linked to an increased infection rate.
Dr. Augustin said an unmet need exists for effective and nontoxic oral medications for moderate to severe psoriasis, because many patients would prefer not to take an injectable biologic. Tofacitinib is aimed at filling that need, and serving as an easier, less toxic initial therapy. However, it is essential that an optimal oral agent have a favorable safety profile because psoriasis is a lifelong disease with an average lifetime duration in excess of 40 years in adults and more than 60 years in affected children, he said.
Pfizer, which is developing the drug, has filed for marketing approval for tofacitinib for treatment of adults with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy. The Food and Drug Administration has announced its intent to issue a ruling on the application in October 2015.
The OPT Expect study is funded by Pfizer. Dr. Augustin is an adviser to and/or a recipient of research grants from Pfizer and more than a dozen other medical companies.
VANCOUVER – Oral tofacitinib for adults with moderate to severe plaque psoriasis demonstrated sustained efficacy and a “generally manageable” safety profile throughout 2 years of follow-up in a large, multicenter ongoing long-term extension study, Dr. Matthias Augustin reported at the World Congress of Dermatology.
One month after enrolling in the phase III, open-label trial (known as OPT Extend), and going on the investigational oral Janus kinase inhibitor at 10 mg b.i.d., 56.2% of the 2,847 participants showed a PASI 75 response – that is, at least a 75% reduction from their baseline Psoriasis Area and Severity Index score. After 2 years in the OPT Extend study, 64.5% of the 1,912 patients remaining in the study were PASI 75 responders.
Similarly, 56.3% of patients went from moderate or severe disease at baseline to “clear” or “almost clear” by Physician Global Assessment (PGA) at 1 month, and 53.9% of participants had a PGA score of 0 or 1 at 24 months, added Dr. Augustin, professor of dermatology at the University of Hamburg-Eppendorf and director of the German Center for Health Services Research in Dermatology and Nursing.
Psoriasis patients who had participated in any of four earlier phase III or two phase II randomized, controlled trials of tofacitinib were eligible to participate in OPT Extend. Participants were placed on tofacitinib at 10 mg b.i.d. for the first 2 months of the open-label study; then investigators adjusted their dose individually – either 5 or 10 mg b.i.d. – at each follow-up visit based on efficacy and side effects. For purposes of OPT Extend, patients’ baseline PASI and PGA scores were defined as the values recorded on the day they were randomized in the earlier round of trials.
Through 2 years of follow-up in this interim analysis of OPT Extend, less than 10% of subjects discontinued tofacitinib because of adverse events. The adverse events were the same as the ones that arose in the earlier, briefer randomized trials, the longest of which lasted 1 year. No signs of cumulative organ toxicity were evident during the additional follow-up.
Slightly more than 4% of all adverse events were categorized as severe, while 62% were mild. The most frequent adverse events were nasopharyngitis in 16% of patients, increased creatinine phosphokinase in 10%, and upper respiratory tract infections in 7%. Serious infections requiring systemic antibiotics or hospitalization occurred in 1.8% of patients on tofacitinib for 2 years, the most common of which were pneumonia, herpes zoster, and urinary tract infection. Herpes zoster occurred in 3.5% of participants, with most cases being mild or moderate. Malignancies other than nonmelanoma skin cancers occurred in 1.2% of subjects.
In terms of laboratory findings of interest, patients’ LDL/HDL ratio remained stable throughout 24 months on tofacitinib. Nor were there any clinically meaningful changes in average levels of other laboratory parameters, including hemoglobin, creatinine phosphokinase, lymphocytes, and neutrophils. A lymphocyte count below 500/mm3 occurred in 0.4% of patients at some point; however, it was unrelated to duration of exposure to tofacitinib and wasn’t linked to an increased infection rate.
Dr. Augustin said an unmet need exists for effective and nontoxic oral medications for moderate to severe psoriasis, because many patients would prefer not to take an injectable biologic. Tofacitinib is aimed at filling that need, and serving as an easier, less toxic initial therapy. However, it is essential that an optimal oral agent have a favorable safety profile because psoriasis is a lifelong disease with an average lifetime duration in excess of 40 years in adults and more than 60 years in affected children, he said.
Pfizer, which is developing the drug, has filed for marketing approval for tofacitinib for treatment of adults with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy. The Food and Drug Administration has announced its intent to issue a ruling on the application in October 2015.
The OPT Expect study is funded by Pfizer. Dr. Augustin is an adviser to and/or a recipient of research grants from Pfizer and more than a dozen other medical companies.
VANCOUVER – Oral tofacitinib for adults with moderate to severe plaque psoriasis demonstrated sustained efficacy and a “generally manageable” safety profile throughout 2 years of follow-up in a large, multicenter ongoing long-term extension study, Dr. Matthias Augustin reported at the World Congress of Dermatology.
One month after enrolling in the phase III, open-label trial (known as OPT Extend), and going on the investigational oral Janus kinase inhibitor at 10 mg b.i.d., 56.2% of the 2,847 participants showed a PASI 75 response – that is, at least a 75% reduction from their baseline Psoriasis Area and Severity Index score. After 2 years in the OPT Extend study, 64.5% of the 1,912 patients remaining in the study were PASI 75 responders.
Similarly, 56.3% of patients went from moderate or severe disease at baseline to “clear” or “almost clear” by Physician Global Assessment (PGA) at 1 month, and 53.9% of participants had a PGA score of 0 or 1 at 24 months, added Dr. Augustin, professor of dermatology at the University of Hamburg-Eppendorf and director of the German Center for Health Services Research in Dermatology and Nursing.
Psoriasis patients who had participated in any of four earlier phase III or two phase II randomized, controlled trials of tofacitinib were eligible to participate in OPT Extend. Participants were placed on tofacitinib at 10 mg b.i.d. for the first 2 months of the open-label study; then investigators adjusted their dose individually – either 5 or 10 mg b.i.d. – at each follow-up visit based on efficacy and side effects. For purposes of OPT Extend, patients’ baseline PASI and PGA scores were defined as the values recorded on the day they were randomized in the earlier round of trials.
Through 2 years of follow-up in this interim analysis of OPT Extend, less than 10% of subjects discontinued tofacitinib because of adverse events. The adverse events were the same as the ones that arose in the earlier, briefer randomized trials, the longest of which lasted 1 year. No signs of cumulative organ toxicity were evident during the additional follow-up.
Slightly more than 4% of all adverse events were categorized as severe, while 62% were mild. The most frequent adverse events were nasopharyngitis in 16% of patients, increased creatinine phosphokinase in 10%, and upper respiratory tract infections in 7%. Serious infections requiring systemic antibiotics or hospitalization occurred in 1.8% of patients on tofacitinib for 2 years, the most common of which were pneumonia, herpes zoster, and urinary tract infection. Herpes zoster occurred in 3.5% of participants, with most cases being mild or moderate. Malignancies other than nonmelanoma skin cancers occurred in 1.2% of subjects.
In terms of laboratory findings of interest, patients’ LDL/HDL ratio remained stable throughout 24 months on tofacitinib. Nor were there any clinically meaningful changes in average levels of other laboratory parameters, including hemoglobin, creatinine phosphokinase, lymphocytes, and neutrophils. A lymphocyte count below 500/mm3 occurred in 0.4% of patients at some point; however, it was unrelated to duration of exposure to tofacitinib and wasn’t linked to an increased infection rate.
Dr. Augustin said an unmet need exists for effective and nontoxic oral medications for moderate to severe psoriasis, because many patients would prefer not to take an injectable biologic. Tofacitinib is aimed at filling that need, and serving as an easier, less toxic initial therapy. However, it is essential that an optimal oral agent have a favorable safety profile because psoriasis is a lifelong disease with an average lifetime duration in excess of 40 years in adults and more than 60 years in affected children, he said.
Pfizer, which is developing the drug, has filed for marketing approval for tofacitinib for treatment of adults with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy. The Food and Drug Administration has announced its intent to issue a ruling on the application in October 2015.
The OPT Expect study is funded by Pfizer. Dr. Augustin is an adviser to and/or a recipient of research grants from Pfizer and more than a dozen other medical companies.
AT WCD 2015
Key clinical point: Oral tofacitinib showed sustained efficacy for moderate to severe plaque psoriasis at 24 months in a large ongoing phase III study.
Major finding: At month 1 of the OPT Extend study, 56.2% of patients on tofacitinib had a PASI 75 response; at month 24, the PASI 75 rate was 64.5%.
Data source: The OPT Extend study is an ongoing, phase III multicenter open-label study of long-term therapy with oral tofacitinib at 5 or 10 mg b.i.d. in 2,847 patients with moderate to severe psoriasis.
Disclosures: The study is funded by Pfizer. The presenter is an adviser to and recipient of research grants from that company as well as others.
Anti-TNFs help psoriatic arthritis patients get back to work
ROME – Anti–tumor necrosis factor agents have a slight edge over conventional disease-modifying antirheumatic drugs when it comes to helping psoriatic arthritis patients who are having work issues, according to a large British observational study presented at the European Congress of Rheumatology.
Among 236 of 400 subjects working at baseline, presenteeism improved from 30% to 10% and productivity loss improved from 45% to 10% among patients who started taking anti-TNF (anti-tumor necrosis factor) agents. Gains were more modest when patients were started on DMARDs, with presenteeism improving from 30% to 20% and productivity loss from 40% to 25%. The difference in change of presenteeism between the two treatment groups became statistically significant at 2 weeks and remained so at 24 weeks.
“Work disability is a continuum,” said the presenting author, Dr. William Tillett of the Royal National Hospital for Rheumatic Diseases in Bath (England). It starts with the normal situation then graduates from presenteeism, where the individual is sick but still attends the workplace, to absenteeism, where the individual is sick and no longer attends the place of work, and eventual unemployment, he explained. “This study suggests that work disability is reversible in the real-world setting,” he added.
The study is from the Long-term Outcomes in Psoriatic ArthritiS (LOPAS II) working group, a 2-year, multicenter, prospective, observational cohort study of work disability in psoriatic arthritis. The group has previously reported that unemployment in psoriatic arthritis is associated with older age, disease duration of 2-5 years, and worse physical function, but that employer awareness and helpfulness enabled patients to stay on the job. Higher levels of global and joint-specific disease activity and worse physical function were associated with greater levels of reporting to work sick (presenteeism) and productivity loss (Rheumatology 2015;54:157-62).
The latest study by Dr. Tillett and his team is a follow-up to see how treatment affects work performance. At baseline, before treatment with anti-TNF or DMARDs, the LOPAS II team of investigators found that 164 (41%) of their 400 subjects were unemployed. Unemployed patients tended to be older (median of 59 years vs. 49 years) and to have worse physical function (a median score of 1.4 on the Health Assessment Questionnaire vs. 1.0). Subsequent treatment with anti-TNFs or DMARDs didn’t change overall employment levels.
Patients who started on anti-TNFs tended to have longer disease duration (median of 11 vs. 5 years) and a greater median number of tender (16 vs. 11) and swollen (7 vs. 5) joints, but otherwise there were no significant differences in demographic or clinical measures between the two treatment groups.
Median scores on the Disease Activity Index for Psoriatic Arthritis (DAPSA) improved over 24 weeks from 53 to 14 among anti-TNF patients, which is considered a good response, but only improved from 39 to 30 in the DMARD group, which is considered a poor response. All of the findings were statistically significant.
The results revealed a “surprisingly poor clinical response to synthetic DMARDs on clinical outcomes … as opposed to good response amongst patients commenced on TNF inhibitors,” Dr. Tillett said in an interview. The improvement in work disability and disease activity seen also was greater and more rapid among those who started on anti-TNF rather than synthetic DMARD.
Dr. Tillett reported receiving grant/research support from AbbVie and speaker or advisory board fees from UCB, Pfizer, and AbbVie. The other authors said they have no disclosures.
ROME – Anti–tumor necrosis factor agents have a slight edge over conventional disease-modifying antirheumatic drugs when it comes to helping psoriatic arthritis patients who are having work issues, according to a large British observational study presented at the European Congress of Rheumatology.
Among 236 of 400 subjects working at baseline, presenteeism improved from 30% to 10% and productivity loss improved from 45% to 10% among patients who started taking anti-TNF (anti-tumor necrosis factor) agents. Gains were more modest when patients were started on DMARDs, with presenteeism improving from 30% to 20% and productivity loss from 40% to 25%. The difference in change of presenteeism between the two treatment groups became statistically significant at 2 weeks and remained so at 24 weeks.
“Work disability is a continuum,” said the presenting author, Dr. William Tillett of the Royal National Hospital for Rheumatic Diseases in Bath (England). It starts with the normal situation then graduates from presenteeism, where the individual is sick but still attends the workplace, to absenteeism, where the individual is sick and no longer attends the place of work, and eventual unemployment, he explained. “This study suggests that work disability is reversible in the real-world setting,” he added.
The study is from the Long-term Outcomes in Psoriatic ArthritiS (LOPAS II) working group, a 2-year, multicenter, prospective, observational cohort study of work disability in psoriatic arthritis. The group has previously reported that unemployment in psoriatic arthritis is associated with older age, disease duration of 2-5 years, and worse physical function, but that employer awareness and helpfulness enabled patients to stay on the job. Higher levels of global and joint-specific disease activity and worse physical function were associated with greater levels of reporting to work sick (presenteeism) and productivity loss (Rheumatology 2015;54:157-62).
The latest study by Dr. Tillett and his team is a follow-up to see how treatment affects work performance. At baseline, before treatment with anti-TNF or DMARDs, the LOPAS II team of investigators found that 164 (41%) of their 400 subjects were unemployed. Unemployed patients tended to be older (median of 59 years vs. 49 years) and to have worse physical function (a median score of 1.4 on the Health Assessment Questionnaire vs. 1.0). Subsequent treatment with anti-TNFs or DMARDs didn’t change overall employment levels.
Patients who started on anti-TNFs tended to have longer disease duration (median of 11 vs. 5 years) and a greater median number of tender (16 vs. 11) and swollen (7 vs. 5) joints, but otherwise there were no significant differences in demographic or clinical measures between the two treatment groups.
Median scores on the Disease Activity Index for Psoriatic Arthritis (DAPSA) improved over 24 weeks from 53 to 14 among anti-TNF patients, which is considered a good response, but only improved from 39 to 30 in the DMARD group, which is considered a poor response. All of the findings were statistically significant.
The results revealed a “surprisingly poor clinical response to synthetic DMARDs on clinical outcomes … as opposed to good response amongst patients commenced on TNF inhibitors,” Dr. Tillett said in an interview. The improvement in work disability and disease activity seen also was greater and more rapid among those who started on anti-TNF rather than synthetic DMARD.
Dr. Tillett reported receiving grant/research support from AbbVie and speaker or advisory board fees from UCB, Pfizer, and AbbVie. The other authors said they have no disclosures.
ROME – Anti–tumor necrosis factor agents have a slight edge over conventional disease-modifying antirheumatic drugs when it comes to helping psoriatic arthritis patients who are having work issues, according to a large British observational study presented at the European Congress of Rheumatology.
Among 236 of 400 subjects working at baseline, presenteeism improved from 30% to 10% and productivity loss improved from 45% to 10% among patients who started taking anti-TNF (anti-tumor necrosis factor) agents. Gains were more modest when patients were started on DMARDs, with presenteeism improving from 30% to 20% and productivity loss from 40% to 25%. The difference in change of presenteeism between the two treatment groups became statistically significant at 2 weeks and remained so at 24 weeks.
“Work disability is a continuum,” said the presenting author, Dr. William Tillett of the Royal National Hospital for Rheumatic Diseases in Bath (England). It starts with the normal situation then graduates from presenteeism, where the individual is sick but still attends the workplace, to absenteeism, where the individual is sick and no longer attends the place of work, and eventual unemployment, he explained. “This study suggests that work disability is reversible in the real-world setting,” he added.
The study is from the Long-term Outcomes in Psoriatic ArthritiS (LOPAS II) working group, a 2-year, multicenter, prospective, observational cohort study of work disability in psoriatic arthritis. The group has previously reported that unemployment in psoriatic arthritis is associated with older age, disease duration of 2-5 years, and worse physical function, but that employer awareness and helpfulness enabled patients to stay on the job. Higher levels of global and joint-specific disease activity and worse physical function were associated with greater levels of reporting to work sick (presenteeism) and productivity loss (Rheumatology 2015;54:157-62).
The latest study by Dr. Tillett and his team is a follow-up to see how treatment affects work performance. At baseline, before treatment with anti-TNF or DMARDs, the LOPAS II team of investigators found that 164 (41%) of their 400 subjects were unemployed. Unemployed patients tended to be older (median of 59 years vs. 49 years) and to have worse physical function (a median score of 1.4 on the Health Assessment Questionnaire vs. 1.0). Subsequent treatment with anti-TNFs or DMARDs didn’t change overall employment levels.
Patients who started on anti-TNFs tended to have longer disease duration (median of 11 vs. 5 years) and a greater median number of tender (16 vs. 11) and swollen (7 vs. 5) joints, but otherwise there were no significant differences in demographic or clinical measures between the two treatment groups.
Median scores on the Disease Activity Index for Psoriatic Arthritis (DAPSA) improved over 24 weeks from 53 to 14 among anti-TNF patients, which is considered a good response, but only improved from 39 to 30 in the DMARD group, which is considered a poor response. All of the findings were statistically significant.
The results revealed a “surprisingly poor clinical response to synthetic DMARDs on clinical outcomes … as opposed to good response amongst patients commenced on TNF inhibitors,” Dr. Tillett said in an interview. The improvement in work disability and disease activity seen also was greater and more rapid among those who started on anti-TNF rather than synthetic DMARD.
Dr. Tillett reported receiving grant/research support from AbbVie and speaker or advisory board fees from UCB, Pfizer, and AbbVie. The other authors said they have no disclosures.
AT THE EULAR 2015 CONGRESS
Key clinical point: Work disability is a reversible outcome in psoriatic arthritis and improves significantly more with use of anti-TNF drugs than with synthetic DMARDs.
Major finding: Presenteeism improved from 30% to 10% and productivity loss improved from 45% to 10% among patients who started taking anti-TNF agents.
Data source: A 2-year, multicenter, prospective, observational cohort study of work disability in 400 patients with psoriatic arthritis.
Disclosures: Dr. Tillett reported receiving research support from AbbVie and speaker or advisory board fees from UCB, Pfizer, and Abbvie.
Fragile Drug Development Process
We are currently in the midst of a new wave of drug developments and approvals for psoriasis; however, we recently have been reminded of the tenuous nature of bringing a new drug to market. Last month, Amgen Inc announced it was pulling out of the long-running collaboration on the high-profile IL-17 program after evaluating the likely commercial impact it would face in light of the suicidal thoughts some patients reported during the studies.
Brodalumab had successfully completed 3 phase 3 studies in patients with moderate to severe plaque psoriasis known as the AMAGINE program. Top-line results from AMAGINE-1 comparing brodalumab with placebo were released in May 2014. Top-line results from AMAGINE-2 and AMAGINE-3 comparing brodalumab with ustekinumab and placebo were announced in November 2014. AMAGINE-2 and AMAGINE-3 are identical in design. “During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” said Amgen Executive Vice President of Research and Development Sean Harper in a statement. AstraZeneca must now decide whether to pursue brodalumab independently.
Once the exact data are publicly released, we will be able to better evaluate the issues of suicidal ideation involved.
What’s the issue?
Brodalumab was eagerly anticipated in the dermatology community. In an instant, the drug’s future is in doubt, which once again demonstrates the fragility of the drug development process. How will this recent announcement affect your use of new biologics?
We are currently in the midst of a new wave of drug developments and approvals for psoriasis; however, we recently have been reminded of the tenuous nature of bringing a new drug to market. Last month, Amgen Inc announced it was pulling out of the long-running collaboration on the high-profile IL-17 program after evaluating the likely commercial impact it would face in light of the suicidal thoughts some patients reported during the studies.
Brodalumab had successfully completed 3 phase 3 studies in patients with moderate to severe plaque psoriasis known as the AMAGINE program. Top-line results from AMAGINE-1 comparing brodalumab with placebo were released in May 2014. Top-line results from AMAGINE-2 and AMAGINE-3 comparing brodalumab with ustekinumab and placebo were announced in November 2014. AMAGINE-2 and AMAGINE-3 are identical in design. “During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” said Amgen Executive Vice President of Research and Development Sean Harper in a statement. AstraZeneca must now decide whether to pursue brodalumab independently.
Once the exact data are publicly released, we will be able to better evaluate the issues of suicidal ideation involved.
What’s the issue?
Brodalumab was eagerly anticipated in the dermatology community. In an instant, the drug’s future is in doubt, which once again demonstrates the fragility of the drug development process. How will this recent announcement affect your use of new biologics?
We are currently in the midst of a new wave of drug developments and approvals for psoriasis; however, we recently have been reminded of the tenuous nature of bringing a new drug to market. Last month, Amgen Inc announced it was pulling out of the long-running collaboration on the high-profile IL-17 program after evaluating the likely commercial impact it would face in light of the suicidal thoughts some patients reported during the studies.
Brodalumab had successfully completed 3 phase 3 studies in patients with moderate to severe plaque psoriasis known as the AMAGINE program. Top-line results from AMAGINE-1 comparing brodalumab with placebo were released in May 2014. Top-line results from AMAGINE-2 and AMAGINE-3 comparing brodalumab with ustekinumab and placebo were announced in November 2014. AMAGINE-2 and AMAGINE-3 are identical in design. “During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” said Amgen Executive Vice President of Research and Development Sean Harper in a statement. AstraZeneca must now decide whether to pursue brodalumab independently.
Once the exact data are publicly released, we will be able to better evaluate the issues of suicidal ideation involved.
What’s the issue?
Brodalumab was eagerly anticipated in the dermatology community. In an instant, the drug’s future is in doubt, which once again demonstrates the fragility of the drug development process. How will this recent announcement affect your use of new biologics?
Fathers factor in psoriatic disease
Researchers have discovered further evidence of paternal transmission bias in psoriatic disease, according to a study published in Arthritis Care & Research.
Significantly more individuals with a first-degree relative affected with psoriatic disease reported an affected father (57%), compared with an affected mother (43%). Self-reported family history was obtained from 849 Canadian adults who reported a first-degree relative affected with either cutaneous psoriasis without arthritis (PsC) or psoriatic arthritis (PsA). A total of 532 (63%) reported an affected parent, and 23 probands reported that both parents were affected. The researchers found significantly more fathers with psoriatic disease than mothers: 289 (57%) of 509 discordant pairs vs. 220 (43%) of 509 discordant pairs, respectively (P = .003).
The paternal transmission bias did not reach statistical significance in PsC probands (P = .20), but the proportion of paternal PsC–proband PsA pairs (161 [75%] of 214 paternal transmissions) was significantly larger (P = .02) than maternal PsC–proband PsA pairs (103 [64%] of 161 maternal transmissions).
“If PsA is considered a more severe form of psoriatic disease than PsC alone, this finding suggests that there is a greater chance of an increase in disease severity when psoriatic disease is transmitted by an affected male compared to an affected female,” wrote the investigators, led by Remy A. Pollock of Toronto Western Hospital.
Read the full article here: (Arthritis Care Res. 2015 [doi:10.1002/acr.22625]).
Researchers have discovered further evidence of paternal transmission bias in psoriatic disease, according to a study published in Arthritis Care & Research.
Significantly more individuals with a first-degree relative affected with psoriatic disease reported an affected father (57%), compared with an affected mother (43%). Self-reported family history was obtained from 849 Canadian adults who reported a first-degree relative affected with either cutaneous psoriasis without arthritis (PsC) or psoriatic arthritis (PsA). A total of 532 (63%) reported an affected parent, and 23 probands reported that both parents were affected. The researchers found significantly more fathers with psoriatic disease than mothers: 289 (57%) of 509 discordant pairs vs. 220 (43%) of 509 discordant pairs, respectively (P = .003).
The paternal transmission bias did not reach statistical significance in PsC probands (P = .20), but the proportion of paternal PsC–proband PsA pairs (161 [75%] of 214 paternal transmissions) was significantly larger (P = .02) than maternal PsC–proband PsA pairs (103 [64%] of 161 maternal transmissions).
“If PsA is considered a more severe form of psoriatic disease than PsC alone, this finding suggests that there is a greater chance of an increase in disease severity when psoriatic disease is transmitted by an affected male compared to an affected female,” wrote the investigators, led by Remy A. Pollock of Toronto Western Hospital.
Read the full article here: (Arthritis Care Res. 2015 [doi:10.1002/acr.22625]).
Researchers have discovered further evidence of paternal transmission bias in psoriatic disease, according to a study published in Arthritis Care & Research.
Significantly more individuals with a first-degree relative affected with psoriatic disease reported an affected father (57%), compared with an affected mother (43%). Self-reported family history was obtained from 849 Canadian adults who reported a first-degree relative affected with either cutaneous psoriasis without arthritis (PsC) or psoriatic arthritis (PsA). A total of 532 (63%) reported an affected parent, and 23 probands reported that both parents were affected. The researchers found significantly more fathers with psoriatic disease than mothers: 289 (57%) of 509 discordant pairs vs. 220 (43%) of 509 discordant pairs, respectively (P = .003).
The paternal transmission bias did not reach statistical significance in PsC probands (P = .20), but the proportion of paternal PsC–proband PsA pairs (161 [75%] of 214 paternal transmissions) was significantly larger (P = .02) than maternal PsC–proband PsA pairs (103 [64%] of 161 maternal transmissions).
“If PsA is considered a more severe form of psoriatic disease than PsC alone, this finding suggests that there is a greater chance of an increase in disease severity when psoriatic disease is transmitted by an affected male compared to an affected female,” wrote the investigators, led by Remy A. Pollock of Toronto Western Hospital.
Read the full article here: (Arthritis Care Res. 2015 [doi:10.1002/acr.22625]).
FROM ARTHRITIS CARE & RESEARCH