User login
Olanzapine-samidorphan combination for schizophrenia or bipolar I disorder
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
Treating homeless patients: Book offers key insights
As a psychiatrist dedicated to working with people who are experiencing homelessness, I was very impressed with the new book edited by Col. (Ret.) Elspeth Cameron Ritchie, MD, MPH, and Maria D. Llorente, MD, about treating and providing services to this vulnerable population.
The book, “Clinical Management of the Homeless Patient: Social, Psychiatric, and Medical Issues” (Cham, Switzerland: Springer Nature Switzerland, 2021), offers an in-depth review and analysis of the biopsychosocial complexities that affect how medical and behavioral health conditions present in those who are unhoused. Notably, the book recommends with great sensitivity best practices to address these conditions with care, understanding, and love.
This text, invaluable in particular for those of us clinicians who work with people experiencing homelessness (PEH), provides a historical context of homelessness in the United States, an evaluation of the current state, and indispensable guidance for medical and behavioral health practitioners, case managers, housing navigators, and policy makers alike. It also serves as an inspiring source for those who are considering work in the public sector while reminding those of us in the field why we continue to do this challenging and rewarding work.
Tips can provide hope to clinicians
The volume is divided into four clear sections that are easy to navigate depending on your area of expertise and interest. Each chapter consolidates an extensive literature review into an intriguing and thought-provoking analysis. Part I, “The Big Picture – Social and Medical Issues,” focuses on conditions that disproportionately affect those who are unhoused. The authors offer a glimpse into the unique challenges of managing routine health conditions. They also detail the practical knowledge that’s needed to best care for our most vulnerable neighbors; for example, promoting a shared decision-making model; simplifying treatment plans; prescribing, when possible, medications that are dosed daily – instead of multiple times per day; allowing for walk-in appointments; and addressing cultural, linguistic, and educational barriers.
Most chapters highlight informative case examples that bring the text to life. It can be heartbreaking to recognize and witness the inhumane conditions in which PEH live, and these practical tips and suggestions for future policies based on best practices can help prevent burnout and provide hope for those who care for this community.
Part II, “Psychiatric Issues and Treatments,” presents a brief yet comprehensive history on homelessness, beginning with the deep shame that PEH experienced in Colonial times as the result of cultural and religious influences. Sadly, that negative judgment continues to this day.
The authors also explain how deinstitutionalization and transinstitutionalization have shaped the current state of homelessness, including why many PEH receive their care in emergency departments while incarcerated. This section highlights the barriers of care that are created not just by the patient, but also by the clinicians and systems of care – and what’s needed practically to overcome those challenges.
I appreciate the chapter on substance use disorders. It reminds us that the most commonly used substance among PEH is tobacco, which has serious health effects and for which we have treatment; nevertheless, . This section also provides examples of the trauma-informed language to use when addressing difficult and sometimes stigmatizing topics, such as survival sex and trauma history.
The evidence-based discussion continues in Part III with a focus on topics that everyone working with PEH should understand, including food insecurity, the criminal justice system, and sex trafficking. Part IV highlights best practices that should be replicated in every community, including Housing First approaches, medical respite care, and multiple Veterans Administration programs.
Throughout the text, major themes reverberate across the chapters, beginning with empathy. All who work with PEH must understand the conditions and challenges PEH face every day that affect their physical and mental health. The authors offer a stark and pointed reminder that being unhoused amounts to a full-time job just to meet basic needs. In addition, the devastating role of trauma and structural racism in creating and promoting the conditions that lead someone to be unhoused cannot be underestimated.
Fortunately, the primary aim of the book is to highlight solutions, and it’s here that the book shines. While some interventions are well-known, such as the importance of working in multidisciplinary teams, building trust and rapport with our patients, and urging clinicians and institutions to examine their own judgments and biases that might interfere with humane treatment, other suggestions will lead some readers into new territory. The authors, for example, maintain that we need more data and evidence-based research that include PEH. They also make a case for more preventive care and enhanced professional education for all health care workers that centers on trauma-informed care, social determinants of health, and the unique needs of especially vulnerable communities, such as the unhoused LBGTQ+ community and policies that promote best practices, such as Housing First. The book is a stirring read. It offers both inspiration and practical guidance for all who are currently working with or interested in caring for people experiencing homelessness.
Dr. Bird is a psychiatrist with Alameda County Health Care for the Homeless and the TRUST Clinic in Oakland, Calif. She also is a cofounder of StreetHealth, a backpack street medicine team that provides psychiatric and substance use disorder treatment to people experiencing homelessness in downtown Oakland.
Dr. Bird has no disclosures.
As a psychiatrist dedicated to working with people who are experiencing homelessness, I was very impressed with the new book edited by Col. (Ret.) Elspeth Cameron Ritchie, MD, MPH, and Maria D. Llorente, MD, about treating and providing services to this vulnerable population.
The book, “Clinical Management of the Homeless Patient: Social, Psychiatric, and Medical Issues” (Cham, Switzerland: Springer Nature Switzerland, 2021), offers an in-depth review and analysis of the biopsychosocial complexities that affect how medical and behavioral health conditions present in those who are unhoused. Notably, the book recommends with great sensitivity best practices to address these conditions with care, understanding, and love.
This text, invaluable in particular for those of us clinicians who work with people experiencing homelessness (PEH), provides a historical context of homelessness in the United States, an evaluation of the current state, and indispensable guidance for medical and behavioral health practitioners, case managers, housing navigators, and policy makers alike. It also serves as an inspiring source for those who are considering work in the public sector while reminding those of us in the field why we continue to do this challenging and rewarding work.
Tips can provide hope to clinicians
The volume is divided into four clear sections that are easy to navigate depending on your area of expertise and interest. Each chapter consolidates an extensive literature review into an intriguing and thought-provoking analysis. Part I, “The Big Picture – Social and Medical Issues,” focuses on conditions that disproportionately affect those who are unhoused. The authors offer a glimpse into the unique challenges of managing routine health conditions. They also detail the practical knowledge that’s needed to best care for our most vulnerable neighbors; for example, promoting a shared decision-making model; simplifying treatment plans; prescribing, when possible, medications that are dosed daily – instead of multiple times per day; allowing for walk-in appointments; and addressing cultural, linguistic, and educational barriers.
Most chapters highlight informative case examples that bring the text to life. It can be heartbreaking to recognize and witness the inhumane conditions in which PEH live, and these practical tips and suggestions for future policies based on best practices can help prevent burnout and provide hope for those who care for this community.
Part II, “Psychiatric Issues and Treatments,” presents a brief yet comprehensive history on homelessness, beginning with the deep shame that PEH experienced in Colonial times as the result of cultural and religious influences. Sadly, that negative judgment continues to this day.
The authors also explain how deinstitutionalization and transinstitutionalization have shaped the current state of homelessness, including why many PEH receive their care in emergency departments while incarcerated. This section highlights the barriers of care that are created not just by the patient, but also by the clinicians and systems of care – and what’s needed practically to overcome those challenges.
I appreciate the chapter on substance use disorders. It reminds us that the most commonly used substance among PEH is tobacco, which has serious health effects and for which we have treatment; nevertheless, . This section also provides examples of the trauma-informed language to use when addressing difficult and sometimes stigmatizing topics, such as survival sex and trauma history.
The evidence-based discussion continues in Part III with a focus on topics that everyone working with PEH should understand, including food insecurity, the criminal justice system, and sex trafficking. Part IV highlights best practices that should be replicated in every community, including Housing First approaches, medical respite care, and multiple Veterans Administration programs.
Throughout the text, major themes reverberate across the chapters, beginning with empathy. All who work with PEH must understand the conditions and challenges PEH face every day that affect their physical and mental health. The authors offer a stark and pointed reminder that being unhoused amounts to a full-time job just to meet basic needs. In addition, the devastating role of trauma and structural racism in creating and promoting the conditions that lead someone to be unhoused cannot be underestimated.
Fortunately, the primary aim of the book is to highlight solutions, and it’s here that the book shines. While some interventions are well-known, such as the importance of working in multidisciplinary teams, building trust and rapport with our patients, and urging clinicians and institutions to examine their own judgments and biases that might interfere with humane treatment, other suggestions will lead some readers into new territory. The authors, for example, maintain that we need more data and evidence-based research that include PEH. They also make a case for more preventive care and enhanced professional education for all health care workers that centers on trauma-informed care, social determinants of health, and the unique needs of especially vulnerable communities, such as the unhoused LBGTQ+ community and policies that promote best practices, such as Housing First. The book is a stirring read. It offers both inspiration and practical guidance for all who are currently working with or interested in caring for people experiencing homelessness.
Dr. Bird is a psychiatrist with Alameda County Health Care for the Homeless and the TRUST Clinic in Oakland, Calif. She also is a cofounder of StreetHealth, a backpack street medicine team that provides psychiatric and substance use disorder treatment to people experiencing homelessness in downtown Oakland.
Dr. Bird has no disclosures.
As a psychiatrist dedicated to working with people who are experiencing homelessness, I was very impressed with the new book edited by Col. (Ret.) Elspeth Cameron Ritchie, MD, MPH, and Maria D. Llorente, MD, about treating and providing services to this vulnerable population.
The book, “Clinical Management of the Homeless Patient: Social, Psychiatric, and Medical Issues” (Cham, Switzerland: Springer Nature Switzerland, 2021), offers an in-depth review and analysis of the biopsychosocial complexities that affect how medical and behavioral health conditions present in those who are unhoused. Notably, the book recommends with great sensitivity best practices to address these conditions with care, understanding, and love.
This text, invaluable in particular for those of us clinicians who work with people experiencing homelessness (PEH), provides a historical context of homelessness in the United States, an evaluation of the current state, and indispensable guidance for medical and behavioral health practitioners, case managers, housing navigators, and policy makers alike. It also serves as an inspiring source for those who are considering work in the public sector while reminding those of us in the field why we continue to do this challenging and rewarding work.
Tips can provide hope to clinicians
The volume is divided into four clear sections that are easy to navigate depending on your area of expertise and interest. Each chapter consolidates an extensive literature review into an intriguing and thought-provoking analysis. Part I, “The Big Picture – Social and Medical Issues,” focuses on conditions that disproportionately affect those who are unhoused. The authors offer a glimpse into the unique challenges of managing routine health conditions. They also detail the practical knowledge that’s needed to best care for our most vulnerable neighbors; for example, promoting a shared decision-making model; simplifying treatment plans; prescribing, when possible, medications that are dosed daily – instead of multiple times per day; allowing for walk-in appointments; and addressing cultural, linguistic, and educational barriers.
Most chapters highlight informative case examples that bring the text to life. It can be heartbreaking to recognize and witness the inhumane conditions in which PEH live, and these practical tips and suggestions for future policies based on best practices can help prevent burnout and provide hope for those who care for this community.
Part II, “Psychiatric Issues and Treatments,” presents a brief yet comprehensive history on homelessness, beginning with the deep shame that PEH experienced in Colonial times as the result of cultural and religious influences. Sadly, that negative judgment continues to this day.
The authors also explain how deinstitutionalization and transinstitutionalization have shaped the current state of homelessness, including why many PEH receive their care in emergency departments while incarcerated. This section highlights the barriers of care that are created not just by the patient, but also by the clinicians and systems of care – and what’s needed practically to overcome those challenges.
I appreciate the chapter on substance use disorders. It reminds us that the most commonly used substance among PEH is tobacco, which has serious health effects and for which we have treatment; nevertheless, . This section also provides examples of the trauma-informed language to use when addressing difficult and sometimes stigmatizing topics, such as survival sex and trauma history.
The evidence-based discussion continues in Part III with a focus on topics that everyone working with PEH should understand, including food insecurity, the criminal justice system, and sex trafficking. Part IV highlights best practices that should be replicated in every community, including Housing First approaches, medical respite care, and multiple Veterans Administration programs.
Throughout the text, major themes reverberate across the chapters, beginning with empathy. All who work with PEH must understand the conditions and challenges PEH face every day that affect their physical and mental health. The authors offer a stark and pointed reminder that being unhoused amounts to a full-time job just to meet basic needs. In addition, the devastating role of trauma and structural racism in creating and promoting the conditions that lead someone to be unhoused cannot be underestimated.
Fortunately, the primary aim of the book is to highlight solutions, and it’s here that the book shines. While some interventions are well-known, such as the importance of working in multidisciplinary teams, building trust and rapport with our patients, and urging clinicians and institutions to examine their own judgments and biases that might interfere with humane treatment, other suggestions will lead some readers into new territory. The authors, for example, maintain that we need more data and evidence-based research that include PEH. They also make a case for more preventive care and enhanced professional education for all health care workers that centers on trauma-informed care, social determinants of health, and the unique needs of especially vulnerable communities, such as the unhoused LBGTQ+ community and policies that promote best practices, such as Housing First. The book is a stirring read. It offers both inspiration and practical guidance for all who are currently working with or interested in caring for people experiencing homelessness.
Dr. Bird is a psychiatrist with Alameda County Health Care for the Homeless and the TRUST Clinic in Oakland, Calif. She also is a cofounder of StreetHealth, a backpack street medicine team that provides psychiatric and substance use disorder treatment to people experiencing homelessness in downtown Oakland.
Dr. Bird has no disclosures.
Is anosognosia a delusion, a negative symptom, or a cognitive deficit?
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.
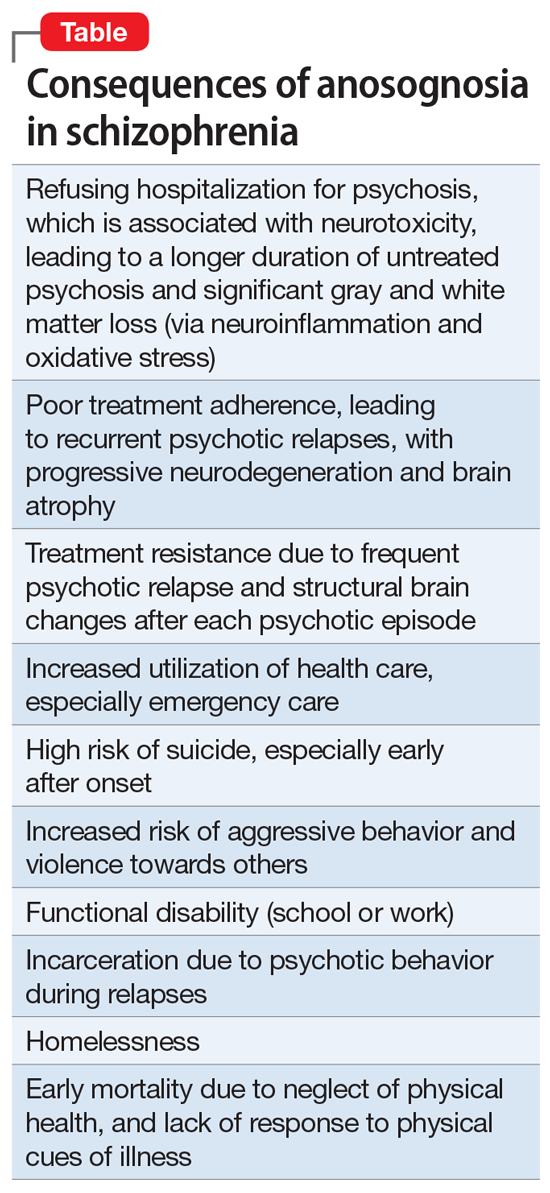
Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
More evidence ties some antipsychotics to increased breast cancer risk
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHOPHARMACOLOGY
Adolescents, THC, and the risk of psychosis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact letters@currentpsychiatry.com.
Since the recent legalization and decriminalization of cannabis (marijuana) use throughout the United States, adolescents’ access to, and use of, cannabis has increased.1 Cannabis products have been marketed in ways that attract adolescents, such as edible gummies, cookies, and hard candies, as well as by vaping.1 The adolescent years are a delicate period of development during which individuals are prone to psychiatric illness, including depression, anxiety, and psychosis.2,3 Here we discuss the relationship between adolescent cannabis use and the development of psychosis.
How cannabis can affect the adolescent brain
The 2 main psychotropic substances found within the cannabis plant are tetrahydrocannabinol (THC) and cannabidiol (CBD).1,4 Endocannabinoids are fatty acid derivatives produced in the brain that bind to cannabinoid (CB) receptors found in the brain and the peripheral nervous system.1,4
During adolescence, neurodevelopment and neurochemical balances are evolving, and it’s during this period that the bulk of prefrontal pruning occurs, especially in the glutamatergic and gamma aminobutyric acidergic (GABAergic) neural pathways.5 THC affects the CB1 receptors by downregulating the neuron receptors, which then alters the maturation of the prefrontal cortical GABAergic neurons. Also, THC affects the upregulation of the microglia located on the CB2 receptors, thereby altering synaptic pruning even further.2,5
All of these changes can cause brain insults that can contribute to the precipitation of psychotic decompensation in adolescents who ingest products that contain THC. In addition, consuming THC might hasten the progression of disorder in adolescents who are genetically predisposed to psychotic disorders. However, existing studies must be interpreted with caution because there are other contributing risk factors for psychosis, such as social isolation, that can alter dopamine signaling as well as oligodendrocyte maturation, which can affect myelination in the prefrontal area of the evolving brain. Factors such as increased academic demand can alter the release of cortisol, which in turn affects the dopamine response as well as the structure of the hippocampus as it responds to cortisol. With all of these contributing factors, it is difficult to attribute psychosis in adolescents solely to the use of THC.5
How to discuss cannabis usewith adolescents
Clinicians should engage in open-ended therapeutic conversations about cannabis use with their adolescent patients, including the various types of cannabis and methods of use (ingestion vs inhalation, etc). Educate patients about the acute and long-term effects of THC use, including an increased risk of depression, schizophrenia, and substance abuse in adulthood.
For a patient who has experienced a psychotic episode, early intervention has proven to result in greater treatment response and functional improvement because it reduces brain exposure to neurotoxic effects in adolescents.3 Access to community resources such as school counselors can help to create coping strategies and enhance family support, which can optimize treatment outcomes and medication adherence, all of which will minimize the likelihood of another psychotic episode. Kelleher et al6 found an increased risk of suicidal behavior after a psychotic experience from any cause in adolescents and young adults, and thereby recommended that clinicians conduct continuous assessment of suicidal ideation in such patients.
1. US Food & Drug Administration. 5 Things to know about delta-8 tetrahydrocannabinol – delta-8 THC. Updated September 14, 2021. Accessed November 3, 2021. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahy drocannabinol-delta-8-thc
2. Patel PK, Leathem LD, Currin DL, et al. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89(2):184-193. doi: 10.1016/j.biopsych.2020.06.028
3. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372. doi: 10.1176/appi.ajp.2015.15050632
4. Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta Neurol Belg. 2019;119(4):511-521. doi: 10.1007/s13760-019-01160-0
5. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21(2):152-162. doi: 10.1080/09540260902782802
6. Kelleher I, Cederlöf M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184-188. doi: 10.1002/wps.20131
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact letters@currentpsychiatry.com.
Since the recent legalization and decriminalization of cannabis (marijuana) use throughout the United States, adolescents’ access to, and use of, cannabis has increased.1 Cannabis products have been marketed in ways that attract adolescents, such as edible gummies, cookies, and hard candies, as well as by vaping.1 The adolescent years are a delicate period of development during which individuals are prone to psychiatric illness, including depression, anxiety, and psychosis.2,3 Here we discuss the relationship between adolescent cannabis use and the development of psychosis.
How cannabis can affect the adolescent brain
The 2 main psychotropic substances found within the cannabis plant are tetrahydrocannabinol (THC) and cannabidiol (CBD).1,4 Endocannabinoids are fatty acid derivatives produced in the brain that bind to cannabinoid (CB) receptors found in the brain and the peripheral nervous system.1,4
During adolescence, neurodevelopment and neurochemical balances are evolving, and it’s during this period that the bulk of prefrontal pruning occurs, especially in the glutamatergic and gamma aminobutyric acidergic (GABAergic) neural pathways.5 THC affects the CB1 receptors by downregulating the neuron receptors, which then alters the maturation of the prefrontal cortical GABAergic neurons. Also, THC affects the upregulation of the microglia located on the CB2 receptors, thereby altering synaptic pruning even further.2,5
All of these changes can cause brain insults that can contribute to the precipitation of psychotic decompensation in adolescents who ingest products that contain THC. In addition, consuming THC might hasten the progression of disorder in adolescents who are genetically predisposed to psychotic disorders. However, existing studies must be interpreted with caution because there are other contributing risk factors for psychosis, such as social isolation, that can alter dopamine signaling as well as oligodendrocyte maturation, which can affect myelination in the prefrontal area of the evolving brain. Factors such as increased academic demand can alter the release of cortisol, which in turn affects the dopamine response as well as the structure of the hippocampus as it responds to cortisol. With all of these contributing factors, it is difficult to attribute psychosis in adolescents solely to the use of THC.5
How to discuss cannabis usewith adolescents
Clinicians should engage in open-ended therapeutic conversations about cannabis use with their adolescent patients, including the various types of cannabis and methods of use (ingestion vs inhalation, etc). Educate patients about the acute and long-term effects of THC use, including an increased risk of depression, schizophrenia, and substance abuse in adulthood.
For a patient who has experienced a psychotic episode, early intervention has proven to result in greater treatment response and functional improvement because it reduces brain exposure to neurotoxic effects in adolescents.3 Access to community resources such as school counselors can help to create coping strategies and enhance family support, which can optimize treatment outcomes and medication adherence, all of which will minimize the likelihood of another psychotic episode. Kelleher et al6 found an increased risk of suicidal behavior after a psychotic experience from any cause in adolescents and young adults, and thereby recommended that clinicians conduct continuous assessment of suicidal ideation in such patients.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in Current Psychiatry. All submissions to Readers’ Forum undergo peer review and are subject to editing for length and style. For more information, contact letters@currentpsychiatry.com.
Since the recent legalization and decriminalization of cannabis (marijuana) use throughout the United States, adolescents’ access to, and use of, cannabis has increased.1 Cannabis products have been marketed in ways that attract adolescents, such as edible gummies, cookies, and hard candies, as well as by vaping.1 The adolescent years are a delicate period of development during which individuals are prone to psychiatric illness, including depression, anxiety, and psychosis.2,3 Here we discuss the relationship between adolescent cannabis use and the development of psychosis.
How cannabis can affect the adolescent brain
The 2 main psychotropic substances found within the cannabis plant are tetrahydrocannabinol (THC) and cannabidiol (CBD).1,4 Endocannabinoids are fatty acid derivatives produced in the brain that bind to cannabinoid (CB) receptors found in the brain and the peripheral nervous system.1,4
During adolescence, neurodevelopment and neurochemical balances are evolving, and it’s during this period that the bulk of prefrontal pruning occurs, especially in the glutamatergic and gamma aminobutyric acidergic (GABAergic) neural pathways.5 THC affects the CB1 receptors by downregulating the neuron receptors, which then alters the maturation of the prefrontal cortical GABAergic neurons. Also, THC affects the upregulation of the microglia located on the CB2 receptors, thereby altering synaptic pruning even further.2,5
All of these changes can cause brain insults that can contribute to the precipitation of psychotic decompensation in adolescents who ingest products that contain THC. In addition, consuming THC might hasten the progression of disorder in adolescents who are genetically predisposed to psychotic disorders. However, existing studies must be interpreted with caution because there are other contributing risk factors for psychosis, such as social isolation, that can alter dopamine signaling as well as oligodendrocyte maturation, which can affect myelination in the prefrontal area of the evolving brain. Factors such as increased academic demand can alter the release of cortisol, which in turn affects the dopamine response as well as the structure of the hippocampus as it responds to cortisol. With all of these contributing factors, it is difficult to attribute psychosis in adolescents solely to the use of THC.5
How to discuss cannabis usewith adolescents
Clinicians should engage in open-ended therapeutic conversations about cannabis use with their adolescent patients, including the various types of cannabis and methods of use (ingestion vs inhalation, etc). Educate patients about the acute and long-term effects of THC use, including an increased risk of depression, schizophrenia, and substance abuse in adulthood.
For a patient who has experienced a psychotic episode, early intervention has proven to result in greater treatment response and functional improvement because it reduces brain exposure to neurotoxic effects in adolescents.3 Access to community resources such as school counselors can help to create coping strategies and enhance family support, which can optimize treatment outcomes and medication adherence, all of which will minimize the likelihood of another psychotic episode. Kelleher et al6 found an increased risk of suicidal behavior after a psychotic experience from any cause in adolescents and young adults, and thereby recommended that clinicians conduct continuous assessment of suicidal ideation in such patients.
1. US Food & Drug Administration. 5 Things to know about delta-8 tetrahydrocannabinol – delta-8 THC. Updated September 14, 2021. Accessed November 3, 2021. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahy drocannabinol-delta-8-thc
2. Patel PK, Leathem LD, Currin DL, et al. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89(2):184-193. doi: 10.1016/j.biopsych.2020.06.028
3. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372. doi: 10.1176/appi.ajp.2015.15050632
4. Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta Neurol Belg. 2019;119(4):511-521. doi: 10.1007/s13760-019-01160-0
5. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21(2):152-162. doi: 10.1080/09540260902782802
6. Kelleher I, Cederlöf M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184-188. doi: 10.1002/wps.20131
1. US Food & Drug Administration. 5 Things to know about delta-8 tetrahydrocannabinol – delta-8 THC. Updated September 14, 2021. Accessed November 3, 2021. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahy drocannabinol-delta-8-thc
2. Patel PK, Leathem LD, Currin DL, et al. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. 2021;89(2):184-193. doi: 10.1016/j.biopsych.2020.06.028
3. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372. doi: 10.1176/appi.ajp.2015.15050632
4. Mastrangelo M. Clinical approach to neurodegenerative disorders in childhood: an updated overview. Acta Neurol Belg. 2019;119(4):511-521. doi: 10.1007/s13760-019-01160-0
5. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21(2):152-162. doi: 10.1080/09540260902782802
6. Kelleher I, Cederlöf M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184-188. doi: 10.1002/wps.20131
New tool guides nutrition counseling in schizophrenia patients
A new tool designed by psychiatrists to help guide nutritional counseling in patients with schizophrenia spectrum disorders (SSD) has been released.
The worksheet and clinician guide were developed using results from a recent scoping review on the relationship between diet and mental health in patients with SSD, and a feedback process involving a focus group with psychiatrists and individuals who had lived experience with psychosis.
“Mental health clinicians already have the training to help our patients make behavioral changes,” lead author Laura LaChance, MD, lecturer, department of psychiatry, and a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said in an interview.
“We work every day with patients to help them to reduce their substance use, improve their sleep, take medications, etc., and nutrition should be added to the radar [since] eating well for mental health is part of self-care and can be included in mental health treatment plans,” she said.
The paper was published online Nov. 10 in BMC Psychiatry.
Nutrition frequently ignored
Dr. LaChance noted that “nutrition is largely absent from mental health training programs and often ignored in clinical practice.”
The investigators “wanted to create a tool to help incorporate basic nutritional counseling into the care of individuals with severe mental illness.” They wanted the tool “to be simple enough to understand for patients and simple enough to use for mental health care professionals who don’t have any official nutrition training.”
The team developed a worksheet that includes dietary recommendations, the majority of which are supported by the scoping review and consistent with Canada’s Food Guide. The review “identified all of the published literature related to the relationship between diet and psychiatric symptoms of SSD,” synthesizing the results of 822 prior articles.
and does not contradict generally accepted recommendations for weight management. It is suitable for all patients including those with low or normal body mass index and provides psychoeducation about the importance of quality nutrition as a determinant of mental health.
Positive tone
The worksheet was informed by social cognitive theory, which “highlights the important role of goal setting and behavior contracting, reinforcement, self-control, social norms, attitudes, and self-efficacy.”
It provides “basic education about important nutrition principles” as well as “very simple recommendations to increase knowledge about healthy eating” and “actionable tips for individuals to incorporate.” The researchers used a “positive” tone and included motivational interviewing questions.
“Delivery of the intervention by the patient’s mental health care provider is by design, in an attempt to address the widely held misbelief that nutrition intervention is of limited importance to mental health care and begin to change norms,” Dr. LaChance said.
The worksheet addresses monetary barriers to healthy eating; offers practical tips to “increase perceived control and self-efficacy”; is written in simple, accessible, nontechnical language; and includes foods from a range of cultural backgrounds.
To ensure that the worksheet and clinical guide met the needs of the target population, the researchers conducted a focus group with five psychiatrists and individual phone interviews with people who live with psychosis (n = 6).
Participants with psychosis were evenly divided between male and female and six age groups were represented: younger than 20 years; 21-30 years; 31-40 years; 41-50 years; 51-50 years; and older than 60 years. Of these participants, half scored in the “limited literacy” range, based on a nutritional literacy assessment tool (the Newest Vital Sign [NVS]).
A revised version of the worksheet, taking participants’ feedback into account, was mailed to all participants, who then provided further feedback on the revised version.
‘Unspoken area’
The clinician guide contains not only an overview and a suggested agenda to steer discussion, but also a sample visual representation of the recommended relative proportions of different food categories in an ideal meal as well as sample meals, a budgeting discussion, and a list of goals.
A closing statement encourages the clinician to “keep the messaging positive, celebrate small victories, and provide encouragement.”
Specific dietary recommendations include choosing complex carbohydrates and healthy fats, reducing highly processed foods and sugar, adding vegetables and fruits to meals and snacks, and eating protein-rich foods throughout the day.
A “noteworthy theme” that emerged in discussions with psychiatrists as well as participants with SSD was “the lack of nutrition training in medical education and psychiatric residency and the general absence of nutritional counseling in this field of medicine.”
One participant described nutrition as “definitely an unspoken area” in schizophrenia – especially in institutional settings, where “you are overloaded with sugars, not healthy grain, not complex grain. You get white bread sandwiches, shitty juice.”
Powerful tool
Commenting on the paper for this news organization, Uma Naidoo, MD, director of nutritional and lifestyle psychiatry, Massachusetts General Hospital, and a nutrition educator at Harvard Medical School, both in Boston, said she appreciates that this paper “is seeking methods to expand treatment options for those with SSD and improve provider understanding/knowledge of therapeutic foods.”
She called the pilot evaluation “notably small,” but added that it “provides results to suggest that scaling this worksheet/guide may hold promise to better provide nutritional counseling to those with psychiatric illness.”
Dr. Naidoo, also a chef and the author of “This Is Your Brain on Food,” who was not involved in the study said, “I’ve seen the power of food as medicine in my own hospital practice and do believe that food is one of the most powerful tools we have in supporting mental fitness and emotional well-being.”
The project was funded by the Canadian CAM Research Fund. Dr. LaChance and Dr. Naidoo have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new tool designed by psychiatrists to help guide nutritional counseling in patients with schizophrenia spectrum disorders (SSD) has been released.
The worksheet and clinician guide were developed using results from a recent scoping review on the relationship between diet and mental health in patients with SSD, and a feedback process involving a focus group with psychiatrists and individuals who had lived experience with psychosis.
“Mental health clinicians already have the training to help our patients make behavioral changes,” lead author Laura LaChance, MD, lecturer, department of psychiatry, and a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said in an interview.
“We work every day with patients to help them to reduce their substance use, improve their sleep, take medications, etc., and nutrition should be added to the radar [since] eating well for mental health is part of self-care and can be included in mental health treatment plans,” she said.
The paper was published online Nov. 10 in BMC Psychiatry.
Nutrition frequently ignored
Dr. LaChance noted that “nutrition is largely absent from mental health training programs and often ignored in clinical practice.”
The investigators “wanted to create a tool to help incorporate basic nutritional counseling into the care of individuals with severe mental illness.” They wanted the tool “to be simple enough to understand for patients and simple enough to use for mental health care professionals who don’t have any official nutrition training.”
The team developed a worksheet that includes dietary recommendations, the majority of which are supported by the scoping review and consistent with Canada’s Food Guide. The review “identified all of the published literature related to the relationship between diet and psychiatric symptoms of SSD,” synthesizing the results of 822 prior articles.
and does not contradict generally accepted recommendations for weight management. It is suitable for all patients including those with low or normal body mass index and provides psychoeducation about the importance of quality nutrition as a determinant of mental health.
Positive tone
The worksheet was informed by social cognitive theory, which “highlights the important role of goal setting and behavior contracting, reinforcement, self-control, social norms, attitudes, and self-efficacy.”
It provides “basic education about important nutrition principles” as well as “very simple recommendations to increase knowledge about healthy eating” and “actionable tips for individuals to incorporate.” The researchers used a “positive” tone and included motivational interviewing questions.
“Delivery of the intervention by the patient’s mental health care provider is by design, in an attempt to address the widely held misbelief that nutrition intervention is of limited importance to mental health care and begin to change norms,” Dr. LaChance said.
The worksheet addresses monetary barriers to healthy eating; offers practical tips to “increase perceived control and self-efficacy”; is written in simple, accessible, nontechnical language; and includes foods from a range of cultural backgrounds.
To ensure that the worksheet and clinical guide met the needs of the target population, the researchers conducted a focus group with five psychiatrists and individual phone interviews with people who live with psychosis (n = 6).
Participants with psychosis were evenly divided between male and female and six age groups were represented: younger than 20 years; 21-30 years; 31-40 years; 41-50 years; 51-50 years; and older than 60 years. Of these participants, half scored in the “limited literacy” range, based on a nutritional literacy assessment tool (the Newest Vital Sign [NVS]).
A revised version of the worksheet, taking participants’ feedback into account, was mailed to all participants, who then provided further feedback on the revised version.
‘Unspoken area’
The clinician guide contains not only an overview and a suggested agenda to steer discussion, but also a sample visual representation of the recommended relative proportions of different food categories in an ideal meal as well as sample meals, a budgeting discussion, and a list of goals.
A closing statement encourages the clinician to “keep the messaging positive, celebrate small victories, and provide encouragement.”
Specific dietary recommendations include choosing complex carbohydrates and healthy fats, reducing highly processed foods and sugar, adding vegetables and fruits to meals and snacks, and eating protein-rich foods throughout the day.
A “noteworthy theme” that emerged in discussions with psychiatrists as well as participants with SSD was “the lack of nutrition training in medical education and psychiatric residency and the general absence of nutritional counseling in this field of medicine.”
One participant described nutrition as “definitely an unspoken area” in schizophrenia – especially in institutional settings, where “you are overloaded with sugars, not healthy grain, not complex grain. You get white bread sandwiches, shitty juice.”
Powerful tool
Commenting on the paper for this news organization, Uma Naidoo, MD, director of nutritional and lifestyle psychiatry, Massachusetts General Hospital, and a nutrition educator at Harvard Medical School, both in Boston, said she appreciates that this paper “is seeking methods to expand treatment options for those with SSD and improve provider understanding/knowledge of therapeutic foods.”
She called the pilot evaluation “notably small,” but added that it “provides results to suggest that scaling this worksheet/guide may hold promise to better provide nutritional counseling to those with psychiatric illness.”
Dr. Naidoo, also a chef and the author of “This Is Your Brain on Food,” who was not involved in the study said, “I’ve seen the power of food as medicine in my own hospital practice and do believe that food is one of the most powerful tools we have in supporting mental fitness and emotional well-being.”
The project was funded by the Canadian CAM Research Fund. Dr. LaChance and Dr. Naidoo have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new tool designed by psychiatrists to help guide nutritional counseling in patients with schizophrenia spectrum disorders (SSD) has been released.
The worksheet and clinician guide were developed using results from a recent scoping review on the relationship between diet and mental health in patients with SSD, and a feedback process involving a focus group with psychiatrists and individuals who had lived experience with psychosis.
“Mental health clinicians already have the training to help our patients make behavioral changes,” lead author Laura LaChance, MD, lecturer, department of psychiatry, and a psychiatrist at St. Mary’s Hospital Centre, McGill University, Montreal, said in an interview.
“We work every day with patients to help them to reduce their substance use, improve their sleep, take medications, etc., and nutrition should be added to the radar [since] eating well for mental health is part of self-care and can be included in mental health treatment plans,” she said.
The paper was published online Nov. 10 in BMC Psychiatry.
Nutrition frequently ignored
Dr. LaChance noted that “nutrition is largely absent from mental health training programs and often ignored in clinical practice.”
The investigators “wanted to create a tool to help incorporate basic nutritional counseling into the care of individuals with severe mental illness.” They wanted the tool “to be simple enough to understand for patients and simple enough to use for mental health care professionals who don’t have any official nutrition training.”
The team developed a worksheet that includes dietary recommendations, the majority of which are supported by the scoping review and consistent with Canada’s Food Guide. The review “identified all of the published literature related to the relationship between diet and psychiatric symptoms of SSD,” synthesizing the results of 822 prior articles.
and does not contradict generally accepted recommendations for weight management. It is suitable for all patients including those with low or normal body mass index and provides psychoeducation about the importance of quality nutrition as a determinant of mental health.
Positive tone
The worksheet was informed by social cognitive theory, which “highlights the important role of goal setting and behavior contracting, reinforcement, self-control, social norms, attitudes, and self-efficacy.”
It provides “basic education about important nutrition principles” as well as “very simple recommendations to increase knowledge about healthy eating” and “actionable tips for individuals to incorporate.” The researchers used a “positive” tone and included motivational interviewing questions.
“Delivery of the intervention by the patient’s mental health care provider is by design, in an attempt to address the widely held misbelief that nutrition intervention is of limited importance to mental health care and begin to change norms,” Dr. LaChance said.
The worksheet addresses monetary barriers to healthy eating; offers practical tips to “increase perceived control and self-efficacy”; is written in simple, accessible, nontechnical language; and includes foods from a range of cultural backgrounds.
To ensure that the worksheet and clinical guide met the needs of the target population, the researchers conducted a focus group with five psychiatrists and individual phone interviews with people who live with psychosis (n = 6).
Participants with psychosis were evenly divided between male and female and six age groups were represented: younger than 20 years; 21-30 years; 31-40 years; 41-50 years; 51-50 years; and older than 60 years. Of these participants, half scored in the “limited literacy” range, based on a nutritional literacy assessment tool (the Newest Vital Sign [NVS]).
A revised version of the worksheet, taking participants’ feedback into account, was mailed to all participants, who then provided further feedback on the revised version.
‘Unspoken area’
The clinician guide contains not only an overview and a suggested agenda to steer discussion, but also a sample visual representation of the recommended relative proportions of different food categories in an ideal meal as well as sample meals, a budgeting discussion, and a list of goals.
A closing statement encourages the clinician to “keep the messaging positive, celebrate small victories, and provide encouragement.”
Specific dietary recommendations include choosing complex carbohydrates and healthy fats, reducing highly processed foods and sugar, adding vegetables and fruits to meals and snacks, and eating protein-rich foods throughout the day.
A “noteworthy theme” that emerged in discussions with psychiatrists as well as participants with SSD was “the lack of nutrition training in medical education and psychiatric residency and the general absence of nutritional counseling in this field of medicine.”
One participant described nutrition as “definitely an unspoken area” in schizophrenia – especially in institutional settings, where “you are overloaded with sugars, not healthy grain, not complex grain. You get white bread sandwiches, shitty juice.”
Powerful tool
Commenting on the paper for this news organization, Uma Naidoo, MD, director of nutritional and lifestyle psychiatry, Massachusetts General Hospital, and a nutrition educator at Harvard Medical School, both in Boston, said she appreciates that this paper “is seeking methods to expand treatment options for those with SSD and improve provider understanding/knowledge of therapeutic foods.”
She called the pilot evaluation “notably small,” but added that it “provides results to suggest that scaling this worksheet/guide may hold promise to better provide nutritional counseling to those with psychiatric illness.”
Dr. Naidoo, also a chef and the author of “This Is Your Brain on Food,” who was not involved in the study said, “I’ve seen the power of food as medicine in my own hospital practice and do believe that food is one of the most powerful tools we have in supporting mental fitness and emotional well-being.”
The project was funded by the Canadian CAM Research Fund. Dr. LaChance and Dr. Naidoo have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMC PSYCHIATRY
COVID-19 mortality risk factors: An unexpected finding
Schizophrenia and severe mood and anxiety disorders are associated with a significantly lower risk of COVID-19 but are tied to a two- to fourfold increased risk of death from the virus, new research shows.
The study results held after the researchers controlled for other risk factors, and they contradict an earlier study that showed no increased mortality risk associated with mood or anxiety disorders. The findings come as the overall number of deaths in the United States approaches 800,000.
“These patients were less likely to be infected because they were probably less exposed, but once they have the infection, they are more prone to worse outcomes,” lead author Antonio L. Teixeira, MD, PhD, professor of psychiatry with McGovern Medical School at the University of Texas Health Science Center at Houston, said in an interview.
The study was published online Nov. 23 in JAMA Network Open.
Unexpected finding
Researchers analyzed electronic health records for 2.5 million adults with private health insurance who were tested for COVID-19 in 2020.
The overall positivity rate for the entire cohort was 11.91%, and patients with severe psychiatric illness fell below that rate. Positivity rates were 9.86% for people with schizophrenia or mood disorders and 11.17% among those with anxiety disorder.
Despite their lower positivity rate, patients with schizophrenia had the highest odds of death from COVID-19 after adjustment for age, race, body mass index, and comorbidities (aOR, 3.74; 95% confidence interval, 2.66-5.24).
Those results were not very surprising, Dr. Teixeira said, as earlier studies have reported similar findings. However,
Patients with mood disorders were nearly three times as likely to die (aOR, 2.76; 95% CI, 2.00-3.81), and those with anxiety disorders had more than double the mortality risk (aOR, 2.34; 95% CI, 1.68-3.27).
“We were expecting some increase, but there was strong evidence in those populations as well,” he said. “We were especially surprised at the data on patients with anxiety disorders.”
An outstanding question
These findings contradict a study published Jan. 27, 2021, in JAMA Psychiatry, that showed no significant increase in mortality risk among those with mood or anxiety disorders.
Study methodology and timing might explain some of the differences, Katlyn Nemani, MD, a research assistant professor of psychiatry at New York University, who led that earlier study, said in an interview.
Dr. Nemani’s study had a smaller study sample, examined mortality over a 30-day period after a positive COVID-19 test, and was limited to the peak of the pandemic in New York, between March and May 2020. Dr. Teixeira’s team examined a full year of data and assessed mortality for 7 days following a positive test.
“It is possible patients with some psychiatric disorders were less likely to receive or successfully respond to treatment for severe COVD-19 which evolved during the course of the pandemic,” Dr. Nemani said, adding that it’s also possible that differences in mortality in the days following infection became attenuated over time.
While a meta-analysis published in July and reported by this news organization at that time did show higher COVID-19 mortality among patients with mood disorders, the risk was far lower than that reported in this new study. That report, which included 33 studies in 22 countries, also found no increase in risk among those with anxiety disorder.
In October, the Centers for Disease Control and Prevention added mood disorders to the list of medical conditions that increase the risk for more severe COVID-19. Schizophrenia was already on that list.
“The outstanding question is what underlies this increased risk,” Dr. Nemani said. “Future studies focused on immune-mediated mechanisms and other potential explanations will help guide targeted interventions to reduce morbidity and mortality in this vulnerable population.”
Funding for the study was not disclosed. Dr. Teixeira and Dr. Nemani report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Schizophrenia and severe mood and anxiety disorders are associated with a significantly lower risk of COVID-19 but are tied to a two- to fourfold increased risk of death from the virus, new research shows.
The study results held after the researchers controlled for other risk factors, and they contradict an earlier study that showed no increased mortality risk associated with mood or anxiety disorders. The findings come as the overall number of deaths in the United States approaches 800,000.
“These patients were less likely to be infected because they were probably less exposed, but once they have the infection, they are more prone to worse outcomes,” lead author Antonio L. Teixeira, MD, PhD, professor of psychiatry with McGovern Medical School at the University of Texas Health Science Center at Houston, said in an interview.
The study was published online Nov. 23 in JAMA Network Open.
Unexpected finding
Researchers analyzed electronic health records for 2.5 million adults with private health insurance who were tested for COVID-19 in 2020.
The overall positivity rate for the entire cohort was 11.91%, and patients with severe psychiatric illness fell below that rate. Positivity rates were 9.86% for people with schizophrenia or mood disorders and 11.17% among those with anxiety disorder.
Despite their lower positivity rate, patients with schizophrenia had the highest odds of death from COVID-19 after adjustment for age, race, body mass index, and comorbidities (aOR, 3.74; 95% confidence interval, 2.66-5.24).
Those results were not very surprising, Dr. Teixeira said, as earlier studies have reported similar findings. However,
Patients with mood disorders were nearly three times as likely to die (aOR, 2.76; 95% CI, 2.00-3.81), and those with anxiety disorders had more than double the mortality risk (aOR, 2.34; 95% CI, 1.68-3.27).
“We were expecting some increase, but there was strong evidence in those populations as well,” he said. “We were especially surprised at the data on patients with anxiety disorders.”
An outstanding question
These findings contradict a study published Jan. 27, 2021, in JAMA Psychiatry, that showed no significant increase in mortality risk among those with mood or anxiety disorders.
Study methodology and timing might explain some of the differences, Katlyn Nemani, MD, a research assistant professor of psychiatry at New York University, who led that earlier study, said in an interview.
Dr. Nemani’s study had a smaller study sample, examined mortality over a 30-day period after a positive COVID-19 test, and was limited to the peak of the pandemic in New York, between March and May 2020. Dr. Teixeira’s team examined a full year of data and assessed mortality for 7 days following a positive test.
“It is possible patients with some psychiatric disorders were less likely to receive or successfully respond to treatment for severe COVD-19 which evolved during the course of the pandemic,” Dr. Nemani said, adding that it’s also possible that differences in mortality in the days following infection became attenuated over time.
While a meta-analysis published in July and reported by this news organization at that time did show higher COVID-19 mortality among patients with mood disorders, the risk was far lower than that reported in this new study. That report, which included 33 studies in 22 countries, also found no increase in risk among those with anxiety disorder.
In October, the Centers for Disease Control and Prevention added mood disorders to the list of medical conditions that increase the risk for more severe COVID-19. Schizophrenia was already on that list.
“The outstanding question is what underlies this increased risk,” Dr. Nemani said. “Future studies focused on immune-mediated mechanisms and other potential explanations will help guide targeted interventions to reduce morbidity and mortality in this vulnerable population.”
Funding for the study was not disclosed. Dr. Teixeira and Dr. Nemani report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Schizophrenia and severe mood and anxiety disorders are associated with a significantly lower risk of COVID-19 but are tied to a two- to fourfold increased risk of death from the virus, new research shows.
The study results held after the researchers controlled for other risk factors, and they contradict an earlier study that showed no increased mortality risk associated with mood or anxiety disorders. The findings come as the overall number of deaths in the United States approaches 800,000.
“These patients were less likely to be infected because they were probably less exposed, but once they have the infection, they are more prone to worse outcomes,” lead author Antonio L. Teixeira, MD, PhD, professor of psychiatry with McGovern Medical School at the University of Texas Health Science Center at Houston, said in an interview.
The study was published online Nov. 23 in JAMA Network Open.
Unexpected finding
Researchers analyzed electronic health records for 2.5 million adults with private health insurance who were tested for COVID-19 in 2020.
The overall positivity rate for the entire cohort was 11.91%, and patients with severe psychiatric illness fell below that rate. Positivity rates were 9.86% for people with schizophrenia or mood disorders and 11.17% among those with anxiety disorder.
Despite their lower positivity rate, patients with schizophrenia had the highest odds of death from COVID-19 after adjustment for age, race, body mass index, and comorbidities (aOR, 3.74; 95% confidence interval, 2.66-5.24).
Those results were not very surprising, Dr. Teixeira said, as earlier studies have reported similar findings. However,
Patients with mood disorders were nearly three times as likely to die (aOR, 2.76; 95% CI, 2.00-3.81), and those with anxiety disorders had more than double the mortality risk (aOR, 2.34; 95% CI, 1.68-3.27).
“We were expecting some increase, but there was strong evidence in those populations as well,” he said. “We were especially surprised at the data on patients with anxiety disorders.”
An outstanding question
These findings contradict a study published Jan. 27, 2021, in JAMA Psychiatry, that showed no significant increase in mortality risk among those with mood or anxiety disorders.
Study methodology and timing might explain some of the differences, Katlyn Nemani, MD, a research assistant professor of psychiatry at New York University, who led that earlier study, said in an interview.
Dr. Nemani’s study had a smaller study sample, examined mortality over a 30-day period after a positive COVID-19 test, and was limited to the peak of the pandemic in New York, between March and May 2020. Dr. Teixeira’s team examined a full year of data and assessed mortality for 7 days following a positive test.
“It is possible patients with some psychiatric disorders were less likely to receive or successfully respond to treatment for severe COVD-19 which evolved during the course of the pandemic,” Dr. Nemani said, adding that it’s also possible that differences in mortality in the days following infection became attenuated over time.
While a meta-analysis published in July and reported by this news organization at that time did show higher COVID-19 mortality among patients with mood disorders, the risk was far lower than that reported in this new study. That report, which included 33 studies in 22 countries, also found no increase in risk among those with anxiety disorder.
In October, the Centers for Disease Control and Prevention added mood disorders to the list of medical conditions that increase the risk for more severe COVID-19. Schizophrenia was already on that list.
“The outstanding question is what underlies this increased risk,” Dr. Nemani said. “Future studies focused on immune-mediated mechanisms and other potential explanations will help guide targeted interventions to reduce morbidity and mortality in this vulnerable population.”
Funding for the study was not disclosed. Dr. Teixeira and Dr. Nemani report no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
FDA puts clozapine REMS requirements on temporary hold
U.S. regulators have put some of the new clozapine risk evaluation and mitigation strategy (REMS) program on temporary hold because of start-up difficulties, including long telephone wait times.
In a Nov. 19 statement, the Food and Drug Administration announced it is temporarily suspending certain aspects of the program because of challenges reported by medical professionals who were trying to meet the original Nov. 15 deadline.
In response, the FDA has conceded that pharmacists can dispense clozapine without a REMS dispense authorization (RDA). Wholesalers can continue to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS, the FDA also said.
“We encourage pharmacists and prescribers to continue working with the clozapine REMS to complete certification and patient enrollment,” the FDA said in a statement.
In July, the FDA approved modifications to the clozapine REMS strategy. Clozapine is used to treat schizophrenia that is not well controlled with standard antipsychotics. It is also prescribed to patients with recurrent suicidal behavior associated with schizophrenia or schizoaffective disorder.
Although it is highly effective in some patients, it also carries serious risks. Specifically, it can decrease the neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count (ANC) monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
HCP frustration
, including a high call volume and long call wait times for stakeholders.
“We understand that this has caused frustration and has led to patient access issues for clozapine,” the FDA said in a statement.
“Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA added. “We are working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.”
Abrupt discontinuation of clozapine can result in significant complications, the FDA said. The agency urged use of “clinical judgment” with respect to prescribing and dispensing clozapine to patients with an absolute neutrophil count within the acceptable range.
As previously reported by this news organization, the American Psychiatric Association and other national groups in a September letter asked the FDA to delay the implementation of a new REMS program until after Jan. 1, 2022.
A version of this article first appeared on Medscape.com.
U.S. regulators have put some of the new clozapine risk evaluation and mitigation strategy (REMS) program on temporary hold because of start-up difficulties, including long telephone wait times.
In a Nov. 19 statement, the Food and Drug Administration announced it is temporarily suspending certain aspects of the program because of challenges reported by medical professionals who were trying to meet the original Nov. 15 deadline.
In response, the FDA has conceded that pharmacists can dispense clozapine without a REMS dispense authorization (RDA). Wholesalers can continue to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS, the FDA also said.
“We encourage pharmacists and prescribers to continue working with the clozapine REMS to complete certification and patient enrollment,” the FDA said in a statement.
In July, the FDA approved modifications to the clozapine REMS strategy. Clozapine is used to treat schizophrenia that is not well controlled with standard antipsychotics. It is also prescribed to patients with recurrent suicidal behavior associated with schizophrenia or schizoaffective disorder.
Although it is highly effective in some patients, it also carries serious risks. Specifically, it can decrease the neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count (ANC) monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
HCP frustration
, including a high call volume and long call wait times for stakeholders.
“We understand that this has caused frustration and has led to patient access issues for clozapine,” the FDA said in a statement.
“Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA added. “We are working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.”
Abrupt discontinuation of clozapine can result in significant complications, the FDA said. The agency urged use of “clinical judgment” with respect to prescribing and dispensing clozapine to patients with an absolute neutrophil count within the acceptable range.
As previously reported by this news organization, the American Psychiatric Association and other national groups in a September letter asked the FDA to delay the implementation of a new REMS program until after Jan. 1, 2022.
A version of this article first appeared on Medscape.com.
U.S. regulators have put some of the new clozapine risk evaluation and mitigation strategy (REMS) program on temporary hold because of start-up difficulties, including long telephone wait times.
In a Nov. 19 statement, the Food and Drug Administration announced it is temporarily suspending certain aspects of the program because of challenges reported by medical professionals who were trying to meet the original Nov. 15 deadline.
In response, the FDA has conceded that pharmacists can dispense clozapine without a REMS dispense authorization (RDA). Wholesalers can continue to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS, the FDA also said.
“We encourage pharmacists and prescribers to continue working with the clozapine REMS to complete certification and patient enrollment,” the FDA said in a statement.
In July, the FDA approved modifications to the clozapine REMS strategy. Clozapine is used to treat schizophrenia that is not well controlled with standard antipsychotics. It is also prescribed to patients with recurrent suicidal behavior associated with schizophrenia or schizoaffective disorder.
Although it is highly effective in some patients, it also carries serious risks. Specifically, it can decrease the neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count (ANC) monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
HCP frustration
, including a high call volume and long call wait times for stakeholders.
“We understand that this has caused frustration and has led to patient access issues for clozapine,” the FDA said in a statement.
“Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA added. “We are working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.”
Abrupt discontinuation of clozapine can result in significant complications, the FDA said. The agency urged use of “clinical judgment” with respect to prescribing and dispensing clozapine to patients with an absolute neutrophil count within the acceptable range.
As previously reported by this news organization, the American Psychiatric Association and other national groups in a September letter asked the FDA to delay the implementation of a new REMS program until after Jan. 1, 2022.
A version of this article first appeared on Medscape.com.
Transdermal patches ease extrapyramidal symptoms in schizophrenia
Use of a transdermal blonanserin patch significantly improved extrapyramidal symptoms (EPS), compared with oral blonanserin tablets in patients with schizophrenia, according to results of an open-label study of 155 adults.
Blonanserin, a second-generation antipsychotic, has been shown to reduce extrapyramidal symptoms when used to treat schizophrenia, but the impact of switching to a patch on extrapyramidal symptoms and on the use of antiparkinson drugs has not been well studied, Kazutaka Ohi, MD, of Gifu University Graduate School of Medicine, Seki, Japan, and colleagues wrote. Advantages of the patch include the ability to provide stable blood concentrations and the ability to be concealed under clothing to avoid patients’ embarrassment at taking oral medications.
In a study published in Progress in Neuropsychopharmacology & Biological Psychiatry, the researchers identified 155 adults aged 18 years and older diagnosed with schizophrenia who were treated at 37 medical institutions in Japan between February 2015 and May 2017.
The first cohort of 97 patients received blonanserin tablets (8-16 mg/day) for 6 weeks, followed by blonanserin transdermal patches (40-80 mg/day) once daily for 1 year. The second cohort of 58 patients received continuous blonanserin patch therapy. Extrapyramidal symptoms were assessed using the Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS); individual scores ranged from a 0 for normal to a 4 for severe.
Overall, DIEPSS scores decreased significantly in both cohorts after switching from blonanserin tablets or powders to transdermal patches. The average DIEPSS change from baseline at 3, 6, and 12 months was –0.44, –0.07, and –0.14, respectively, in cohort 1, and –0.16, –0.74, and –0.81, respectively, in cohort 2.
The researchers also assessed the impact of transition to transdermal patches on the use of antiparkinsonism drugs using the biperiden equivalents of total antiparkinsonian drugs (BPD-eq) measure. At baseline, about 22% of patients used concomitant antiparkinsonism drugs, compared with 25.8% at 1 year after starting patch treatment. The dose of antiparkinson drugs was not significantly decreased after switching to transdermal patches, in part because of psychiatrists’ prescribing behaviors, Dr. Ohi and colleagues noted.
As a secondary outcome, the researchers examined psychotic symptoms and found that Positive and Negative Syndrome Scale (PANSS) negative symptom scores decreased significantly in patients in cohort 1 who switched from tablets or powders to patches. Changes in scores from baseline to 3, 6, and 12 months were –0.7, –1.0, and –1.3, respectively. Positive PANSS scores did not change significantly in cohort 1. In cohort 2, both positive and negative PANSS scores decreased significantly over 12 months after switching from blonanserin tablets/powders to patches. The mean changes in scores from baseline to 3, 6, and 12 months were –1.6, –2.3, and –2.4, respectively, for PANSS positive symptom scores, and –1.4, –2.7, and –2.8, respectively, for negative symptom scores.
A total of 41.2% of cohort 1 patients and 44.8% of cohort 2 patients discontinued patch treatments by 1 year. Four patients discontinued the patch because of EPS during the treatment period in cohort 1; no patients in cohort 2 discontinued because of EPS.
The study findings were limited by several factors, including the open-label design and lack of controls; also, the study did not examine crossover changes in patients who switched from tablets or powders to patches, the researchers noted.
However, the results indicate that direct switching from blonanserin tablets or powders to transdermal patches reduced EPS and psychotic symptoms in schizophrenia and may be more acceptable to patients, compared with oral medications, as well as more effective, they concluded.
The study received no outside funding, and Dr. Ohi and colleagues had no disclosures.
Use of a transdermal blonanserin patch significantly improved extrapyramidal symptoms (EPS), compared with oral blonanserin tablets in patients with schizophrenia, according to results of an open-label study of 155 adults.
Blonanserin, a second-generation antipsychotic, has been shown to reduce extrapyramidal symptoms when used to treat schizophrenia, but the impact of switching to a patch on extrapyramidal symptoms and on the use of antiparkinson drugs has not been well studied, Kazutaka Ohi, MD, of Gifu University Graduate School of Medicine, Seki, Japan, and colleagues wrote. Advantages of the patch include the ability to provide stable blood concentrations and the ability to be concealed under clothing to avoid patients’ embarrassment at taking oral medications.
In a study published in Progress in Neuropsychopharmacology & Biological Psychiatry, the researchers identified 155 adults aged 18 years and older diagnosed with schizophrenia who were treated at 37 medical institutions in Japan between February 2015 and May 2017.
The first cohort of 97 patients received blonanserin tablets (8-16 mg/day) for 6 weeks, followed by blonanserin transdermal patches (40-80 mg/day) once daily for 1 year. The second cohort of 58 patients received continuous blonanserin patch therapy. Extrapyramidal symptoms were assessed using the Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS); individual scores ranged from a 0 for normal to a 4 for severe.
Overall, DIEPSS scores decreased significantly in both cohorts after switching from blonanserin tablets or powders to transdermal patches. The average DIEPSS change from baseline at 3, 6, and 12 months was –0.44, –0.07, and –0.14, respectively, in cohort 1, and –0.16, –0.74, and –0.81, respectively, in cohort 2.
The researchers also assessed the impact of transition to transdermal patches on the use of antiparkinsonism drugs using the biperiden equivalents of total antiparkinsonian drugs (BPD-eq) measure. At baseline, about 22% of patients used concomitant antiparkinsonism drugs, compared with 25.8% at 1 year after starting patch treatment. The dose of antiparkinson drugs was not significantly decreased after switching to transdermal patches, in part because of psychiatrists’ prescribing behaviors, Dr. Ohi and colleagues noted.
As a secondary outcome, the researchers examined psychotic symptoms and found that Positive and Negative Syndrome Scale (PANSS) negative symptom scores decreased significantly in patients in cohort 1 who switched from tablets or powders to patches. Changes in scores from baseline to 3, 6, and 12 months were –0.7, –1.0, and –1.3, respectively. Positive PANSS scores did not change significantly in cohort 1. In cohort 2, both positive and negative PANSS scores decreased significantly over 12 months after switching from blonanserin tablets/powders to patches. The mean changes in scores from baseline to 3, 6, and 12 months were –1.6, –2.3, and –2.4, respectively, for PANSS positive symptom scores, and –1.4, –2.7, and –2.8, respectively, for negative symptom scores.
A total of 41.2% of cohort 1 patients and 44.8% of cohort 2 patients discontinued patch treatments by 1 year. Four patients discontinued the patch because of EPS during the treatment period in cohort 1; no patients in cohort 2 discontinued because of EPS.
The study findings were limited by several factors, including the open-label design and lack of controls; also, the study did not examine crossover changes in patients who switched from tablets or powders to patches, the researchers noted.
However, the results indicate that direct switching from blonanserin tablets or powders to transdermal patches reduced EPS and psychotic symptoms in schizophrenia and may be more acceptable to patients, compared with oral medications, as well as more effective, they concluded.
The study received no outside funding, and Dr. Ohi and colleagues had no disclosures.
Use of a transdermal blonanserin patch significantly improved extrapyramidal symptoms (EPS), compared with oral blonanserin tablets in patients with schizophrenia, according to results of an open-label study of 155 adults.
Blonanserin, a second-generation antipsychotic, has been shown to reduce extrapyramidal symptoms when used to treat schizophrenia, but the impact of switching to a patch on extrapyramidal symptoms and on the use of antiparkinson drugs has not been well studied, Kazutaka Ohi, MD, of Gifu University Graduate School of Medicine, Seki, Japan, and colleagues wrote. Advantages of the patch include the ability to provide stable blood concentrations and the ability to be concealed under clothing to avoid patients’ embarrassment at taking oral medications.
In a study published in Progress in Neuropsychopharmacology & Biological Psychiatry, the researchers identified 155 adults aged 18 years and older diagnosed with schizophrenia who were treated at 37 medical institutions in Japan between February 2015 and May 2017.
The first cohort of 97 patients received blonanserin tablets (8-16 mg/day) for 6 weeks, followed by blonanserin transdermal patches (40-80 mg/day) once daily for 1 year. The second cohort of 58 patients received continuous blonanserin patch therapy. Extrapyramidal symptoms were assessed using the Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS); individual scores ranged from a 0 for normal to a 4 for severe.
Overall, DIEPSS scores decreased significantly in both cohorts after switching from blonanserin tablets or powders to transdermal patches. The average DIEPSS change from baseline at 3, 6, and 12 months was –0.44, –0.07, and –0.14, respectively, in cohort 1, and –0.16, –0.74, and –0.81, respectively, in cohort 2.
The researchers also assessed the impact of transition to transdermal patches on the use of antiparkinsonism drugs using the biperiden equivalents of total antiparkinsonian drugs (BPD-eq) measure. At baseline, about 22% of patients used concomitant antiparkinsonism drugs, compared with 25.8% at 1 year after starting patch treatment. The dose of antiparkinson drugs was not significantly decreased after switching to transdermal patches, in part because of psychiatrists’ prescribing behaviors, Dr. Ohi and colleagues noted.
As a secondary outcome, the researchers examined psychotic symptoms and found that Positive and Negative Syndrome Scale (PANSS) negative symptom scores decreased significantly in patients in cohort 1 who switched from tablets or powders to patches. Changes in scores from baseline to 3, 6, and 12 months were –0.7, –1.0, and –1.3, respectively. Positive PANSS scores did not change significantly in cohort 1. In cohort 2, both positive and negative PANSS scores decreased significantly over 12 months after switching from blonanserin tablets/powders to patches. The mean changes in scores from baseline to 3, 6, and 12 months were –1.6, –2.3, and –2.4, respectively, for PANSS positive symptom scores, and –1.4, –2.7, and –2.8, respectively, for negative symptom scores.
A total of 41.2% of cohort 1 patients and 44.8% of cohort 2 patients discontinued patch treatments by 1 year. Four patients discontinued the patch because of EPS during the treatment period in cohort 1; no patients in cohort 2 discontinued because of EPS.
The study findings were limited by several factors, including the open-label design and lack of controls; also, the study did not examine crossover changes in patients who switched from tablets or powders to patches, the researchers noted.
However, the results indicate that direct switching from blonanserin tablets or powders to transdermal patches reduced EPS and psychotic symptoms in schizophrenia and may be more acceptable to patients, compared with oral medications, as well as more effective, they concluded.
The study received no outside funding, and Dr. Ohi and colleagues had no disclosures.
FROM PROGRESS IN NEUROPSYCHOPHARMACOLOGY & BIOLOGICAL PSYCHIATRY
Britney Spears – Reflections on conservatorship
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.










