User login
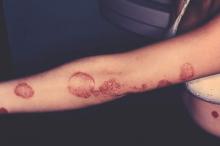
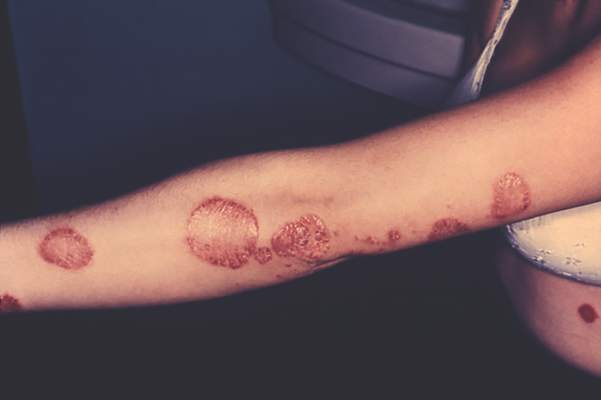
Don’t overlook topical tazarotene for psoriasis
WAIKOLOA, HAWAII – Tazarotene remains an important and effective albeit greatly underutilized topical therapy in psoriasis – but it’s on its way to becoming an even better drug, Dr. Linda Stein Gold said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The topical retinoid has been formulated together with the superpotent steroid halobetasol propionate 0.01% in an investigational fixed combination lotion known for now as IDP-118. This medication, under development by Valeant, is in an ongoing, phase III multicenter, double-blind randomized trial with the vehicle lotion serving as control in adults with moderate to severe plaque psoriasis. A year-long, open-label, phase III safety study is also in progress, according to Dr. Stein Gold of Henry Ford Hospital in Detroit.
Tazarotene (Tazorac) is approved by the Food and Drug Administration as a 0.05% and 0.1% cream or gel for psoriasis and in the 0.1% cream or gel for acne. But when Dr. Stein Gold asked her large Hawaii audience for a show of hands as to who is prescribing tazarotene for their psoriasis patients, not a hand went up.
“Tazarotene carries some baggage,” she observed. “It’s pregnancy category X, and it also is quite irritating. If you use tazarotene on psoriatic skin, you’ll get a lot of irritation. But if you do so in combination with a potent or superpotent topical steroid, you’re not only able to increase the efficacy, but you also minimize the tolerability issues.
These dual benefits are the result of the two treatments’ differing mechanisms of action. This has been known for a long time. Indeed, it was demonstrated in a randomized trial nearly 2 decades ago (J Am Acad Dermatol. 1998 Oct;39[4 Pt 2]:S139-43). But only with the recent appreciation that 80% of psoriasis patients treat their disease exclusively with topical therapies has a pharmaceutical company moved to take advantage of these synergistic effects.
Dr. Stein Gold, director of dermatology research at Detroit’s Henry Ford Health System, said that the pharmaceutical industry has finally noted the considerable unmet need for additional topical psoriasis therapies that will be more effective and/or cosmetically elegant or have a novel mechanism of action. A slew of novel topical agents are now in the developmental pipeline in phase II studies for psoriasis. Among these new molecules are a topical formulation of methotrexate in a proprietary vehicle; the Janus kinase (JAK) 1 and 2 inhibitor ruxolitinib (Jakafi) in a cream for treatment of both psoriasis and atopic dermatitis; a tyrosine kinase inhibitor cream and ointment; an integrin inhibitor cream, and a phosphodiesterase-4 inhibitor ointment.
In addition, in October 2015, the FDA approved an aerosol foam fixed combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% (Enstilar) for psoriasis. It’s more effective and cosmetically elegant than the fixed-combination ointment, she noted.
“Topical therapy is still going strong. I think there is always going to be a need for topical psoriasis therapies,” the dermatologist declared.
She reported serving as a consultant to and/or scientific advisory board member for numerous pharmaceutical companies.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Tazarotene remains an important and effective albeit greatly underutilized topical therapy in psoriasis – but it’s on its way to becoming an even better drug, Dr. Linda Stein Gold said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The topical retinoid has been formulated together with the superpotent steroid halobetasol propionate 0.01% in an investigational fixed combination lotion known for now as IDP-118. This medication, under development by Valeant, is in an ongoing, phase III multicenter, double-blind randomized trial with the vehicle lotion serving as control in adults with moderate to severe plaque psoriasis. A year-long, open-label, phase III safety study is also in progress, according to Dr. Stein Gold of Henry Ford Hospital in Detroit.
Tazarotene (Tazorac) is approved by the Food and Drug Administration as a 0.05% and 0.1% cream or gel for psoriasis and in the 0.1% cream or gel for acne. But when Dr. Stein Gold asked her large Hawaii audience for a show of hands as to who is prescribing tazarotene for their psoriasis patients, not a hand went up.
“Tazarotene carries some baggage,” she observed. “It’s pregnancy category X, and it also is quite irritating. If you use tazarotene on psoriatic skin, you’ll get a lot of irritation. But if you do so in combination with a potent or superpotent topical steroid, you’re not only able to increase the efficacy, but you also minimize the tolerability issues.
These dual benefits are the result of the two treatments’ differing mechanisms of action. This has been known for a long time. Indeed, it was demonstrated in a randomized trial nearly 2 decades ago (J Am Acad Dermatol. 1998 Oct;39[4 Pt 2]:S139-43). But only with the recent appreciation that 80% of psoriasis patients treat their disease exclusively with topical therapies has a pharmaceutical company moved to take advantage of these synergistic effects.
Dr. Stein Gold, director of dermatology research at Detroit’s Henry Ford Health System, said that the pharmaceutical industry has finally noted the considerable unmet need for additional topical psoriasis therapies that will be more effective and/or cosmetically elegant or have a novel mechanism of action. A slew of novel topical agents are now in the developmental pipeline in phase II studies for psoriasis. Among these new molecules are a topical formulation of methotrexate in a proprietary vehicle; the Janus kinase (JAK) 1 and 2 inhibitor ruxolitinib (Jakafi) in a cream for treatment of both psoriasis and atopic dermatitis; a tyrosine kinase inhibitor cream and ointment; an integrin inhibitor cream, and a phosphodiesterase-4 inhibitor ointment.
In addition, in October 2015, the FDA approved an aerosol foam fixed combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% (Enstilar) for psoriasis. It’s more effective and cosmetically elegant than the fixed-combination ointment, she noted.
“Topical therapy is still going strong. I think there is always going to be a need for topical psoriasis therapies,” the dermatologist declared.
She reported serving as a consultant to and/or scientific advisory board member for numerous pharmaceutical companies.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Tazarotene remains an important and effective albeit greatly underutilized topical therapy in psoriasis – but it’s on its way to becoming an even better drug, Dr. Linda Stein Gold said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The topical retinoid has been formulated together with the superpotent steroid halobetasol propionate 0.01% in an investigational fixed combination lotion known for now as IDP-118. This medication, under development by Valeant, is in an ongoing, phase III multicenter, double-blind randomized trial with the vehicle lotion serving as control in adults with moderate to severe plaque psoriasis. A year-long, open-label, phase III safety study is also in progress, according to Dr. Stein Gold of Henry Ford Hospital in Detroit.
Tazarotene (Tazorac) is approved by the Food and Drug Administration as a 0.05% and 0.1% cream or gel for psoriasis and in the 0.1% cream or gel for acne. But when Dr. Stein Gold asked her large Hawaii audience for a show of hands as to who is prescribing tazarotene for their psoriasis patients, not a hand went up.
“Tazarotene carries some baggage,” she observed. “It’s pregnancy category X, and it also is quite irritating. If you use tazarotene on psoriatic skin, you’ll get a lot of irritation. But if you do so in combination with a potent or superpotent topical steroid, you’re not only able to increase the efficacy, but you also minimize the tolerability issues.
These dual benefits are the result of the two treatments’ differing mechanisms of action. This has been known for a long time. Indeed, it was demonstrated in a randomized trial nearly 2 decades ago (J Am Acad Dermatol. 1998 Oct;39[4 Pt 2]:S139-43). But only with the recent appreciation that 80% of psoriasis patients treat their disease exclusively with topical therapies has a pharmaceutical company moved to take advantage of these synergistic effects.
Dr. Stein Gold, director of dermatology research at Detroit’s Henry Ford Health System, said that the pharmaceutical industry has finally noted the considerable unmet need for additional topical psoriasis therapies that will be more effective and/or cosmetically elegant or have a novel mechanism of action. A slew of novel topical agents are now in the developmental pipeline in phase II studies for psoriasis. Among these new molecules are a topical formulation of methotrexate in a proprietary vehicle; the Janus kinase (JAK) 1 and 2 inhibitor ruxolitinib (Jakafi) in a cream for treatment of both psoriasis and atopic dermatitis; a tyrosine kinase inhibitor cream and ointment; an integrin inhibitor cream, and a phosphodiesterase-4 inhibitor ointment.
In addition, in October 2015, the FDA approved an aerosol foam fixed combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% (Enstilar) for psoriasis. It’s more effective and cosmetically elegant than the fixed-combination ointment, she noted.
“Topical therapy is still going strong. I think there is always going to be a need for topical psoriasis therapies,” the dermatologist declared.
She reported serving as a consultant to and/or scientific advisory board member for numerous pharmaceutical companies.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
60 weeks of ixekizumab effective, well tolerated in moderate-to-severe plaque psoriasis
WASHINGTON – After 60 weeks of continuous therapy with the investigational drug ixekizumab, 722 patients who initially had moderate to severe plaque psoriasis had Psoriasis Area Severity Index (PASI) 75, 90, and 100 response rates of 87%, 78%, and 57%, respectively, based on study results presented by Dr. Andrew Blauvelt at the annual meeting of the American Academy of Dermatology.
Additionally, the rate of complete clearance or mild severity of plaque psoriasis based on the Physician Global Assessment measure was 79% in the study patients. Dr. Blauvelt said “these were tremendous results over time.”
The findings come from the open-label continuation study of ixekizumab in UNCOVER-3. At week 12 of that study, 722 ixekizumab-treated patients were continued on or converted to a regimen of 80 mg ixekizumab every 4 weeks.
In UNCOVER-3, all study participants initially received induction therapy with ixekizumab and then were randomized to placebo (193 patients), etanercept 50 mg twice weekly (382 patients), 80 mg ixekizumab every 4 weeks (386 patients) after a 160 mg starting dose, or 80 mg ixekizumab every 2 weeks (385 patients) after an initial 160 mg starting dose. After 12 weeks, patients entered open-label treatment with the study drug once every 4 weeks.
The long-term safety and tolerability profile was similar to the one seen during the induction period and cited in previously published data. Dr. Blauvelt, President of the Oregon Medical Research Center, said the finding was “good news, in that there were no unexpected safety signals [not already] seen with initial therapy.”
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor.
According to a spokesperson from Eli Lilly, sponsor of the UNCOVER trial and maker of ixekizumab, a decision from the U.S. Food and Drug Administration on whether to approve ixekizumab for use in psoriasis is expected during the first quarter of 2016.
Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
On Twitter @whitneymcknight
WASHINGTON – After 60 weeks of continuous therapy with the investigational drug ixekizumab, 722 patients who initially had moderate to severe plaque psoriasis had Psoriasis Area Severity Index (PASI) 75, 90, and 100 response rates of 87%, 78%, and 57%, respectively, based on study results presented by Dr. Andrew Blauvelt at the annual meeting of the American Academy of Dermatology.
Additionally, the rate of complete clearance or mild severity of plaque psoriasis based on the Physician Global Assessment measure was 79% in the study patients. Dr. Blauvelt said “these were tremendous results over time.”
The findings come from the open-label continuation study of ixekizumab in UNCOVER-3. At week 12 of that study, 722 ixekizumab-treated patients were continued on or converted to a regimen of 80 mg ixekizumab every 4 weeks.
In UNCOVER-3, all study participants initially received induction therapy with ixekizumab and then were randomized to placebo (193 patients), etanercept 50 mg twice weekly (382 patients), 80 mg ixekizumab every 4 weeks (386 patients) after a 160 mg starting dose, or 80 mg ixekizumab every 2 weeks (385 patients) after an initial 160 mg starting dose. After 12 weeks, patients entered open-label treatment with the study drug once every 4 weeks.
The long-term safety and tolerability profile was similar to the one seen during the induction period and cited in previously published data. Dr. Blauvelt, President of the Oregon Medical Research Center, said the finding was “good news, in that there were no unexpected safety signals [not already] seen with initial therapy.”
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor.
According to a spokesperson from Eli Lilly, sponsor of the UNCOVER trial and maker of ixekizumab, a decision from the U.S. Food and Drug Administration on whether to approve ixekizumab for use in psoriasis is expected during the first quarter of 2016.
Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
On Twitter @whitneymcknight
WASHINGTON – After 60 weeks of continuous therapy with the investigational drug ixekizumab, 722 patients who initially had moderate to severe plaque psoriasis had Psoriasis Area Severity Index (PASI) 75, 90, and 100 response rates of 87%, 78%, and 57%, respectively, based on study results presented by Dr. Andrew Blauvelt at the annual meeting of the American Academy of Dermatology.
Additionally, the rate of complete clearance or mild severity of plaque psoriasis based on the Physician Global Assessment measure was 79% in the study patients. Dr. Blauvelt said “these were tremendous results over time.”
The findings come from the open-label continuation study of ixekizumab in UNCOVER-3. At week 12 of that study, 722 ixekizumab-treated patients were continued on or converted to a regimen of 80 mg ixekizumab every 4 weeks.
In UNCOVER-3, all study participants initially received induction therapy with ixekizumab and then were randomized to placebo (193 patients), etanercept 50 mg twice weekly (382 patients), 80 mg ixekizumab every 4 weeks (386 patients) after a 160 mg starting dose, or 80 mg ixekizumab every 2 weeks (385 patients) after an initial 160 mg starting dose. After 12 weeks, patients entered open-label treatment with the study drug once every 4 weeks.
The long-term safety and tolerability profile was similar to the one seen during the induction period and cited in previously published data. Dr. Blauvelt, President of the Oregon Medical Research Center, said the finding was “good news, in that there were no unexpected safety signals [not already] seen with initial therapy.”
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor.
According to a spokesperson from Eli Lilly, sponsor of the UNCOVER trial and maker of ixekizumab, a decision from the U.S. Food and Drug Administration on whether to approve ixekizumab for use in psoriasis is expected during the first quarter of 2016.
Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
On Twitter @whitneymcknight
AT AAD 2016
Key clinical point: Continuous therapy with the investigational drug ixekizumab appears to be safe and effective for long-term use.
Major finding: At Week 60, the PASI 75, 90, and 100 response rates were 87%, 78%, and 57%, respectively.
Data source: Phase 3 UNCOVER-3 trial of 1,346 patients randomized to placebo, active control, or ixekizumab at every 4 weeks or every 2 weeks after induction.
Disclosures: This trial was sponsored by Eli Lilly, maker of ixekizumab. Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
Minimal disease activity criteria in PsA fall short
The minimal disease activity criteria used to identify low disease activity in psoriatic arthritis (PsA) may not be useful as a treatment target in patients with extended skin involvement, according to the results of a small study.
Minimal disease activity (MDA) for PsA is a composite measure encompassing clinically important aspects of the disease: arthritis, psoriasis, enthesitis, pain, patient-assessed global disease activity, and physical function and provides an objective target for therapy in clinical trials, Dr. Josefina Marin of the Hospital Italiano de Buenos Aires and her associates wrote in the Journal of Rheumatology.
To evaluate components of the MDA criteria, the research team enrolled 83 consecutive patients with PsA aged over 18 years who were attending their clinic. Patients needed to fulfill five out of the seven criteria to be classified as having MDA.
An assessment by a single experienced rheumatologist revealed that 41 patients met MDA criteria, with only 7.4% of these patients not satisfying the tender/swollen joint–count criteria.
However, only 51% (n = 21) of patients fulfilling MDA fulfilled the skin criteria (Psoriasis Area and Severity Index [PASI] and body surface area [BSA]), a percentage not statistically different from the 36% of patients not in MDA (n = 15; P = .154). “This is important because MDA may therefore not be useful as a treatment target in patients with extended skin involvement,” the investigators wrote (J Rheum. 2016 Mar 1. doi: 10.3899/jrheum.151101).
The investigators noted that the selection of the target was of crucial importance when implementing a treat-to-target and tight control strategy. “In this sense our study provides reassurance that almost all patients at MDA will have no swollen/tender joints but also raises some concerns that the skin might not be well controlled if the MDA is used as the only target to treat patients with both joint and skin involvement,” they wrote. One explanation could be that the cutoff values selected in the MDA criteria for skin involvement are too stringent for therapies currently available, they suggested.
MDA seems to be a valuable tool to define low disease activity in patients with PsA, but the skin component requires further evaluation, they concluded.
The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
The minimal disease activity criteria used to identify low disease activity in psoriatic arthritis (PsA) may not be useful as a treatment target in patients with extended skin involvement, according to the results of a small study.
Minimal disease activity (MDA) for PsA is a composite measure encompassing clinically important aspects of the disease: arthritis, psoriasis, enthesitis, pain, patient-assessed global disease activity, and physical function and provides an objective target for therapy in clinical trials, Dr. Josefina Marin of the Hospital Italiano de Buenos Aires and her associates wrote in the Journal of Rheumatology.
To evaluate components of the MDA criteria, the research team enrolled 83 consecutive patients with PsA aged over 18 years who were attending their clinic. Patients needed to fulfill five out of the seven criteria to be classified as having MDA.
An assessment by a single experienced rheumatologist revealed that 41 patients met MDA criteria, with only 7.4% of these patients not satisfying the tender/swollen joint–count criteria.
However, only 51% (n = 21) of patients fulfilling MDA fulfilled the skin criteria (Psoriasis Area and Severity Index [PASI] and body surface area [BSA]), a percentage not statistically different from the 36% of patients not in MDA (n = 15; P = .154). “This is important because MDA may therefore not be useful as a treatment target in patients with extended skin involvement,” the investigators wrote (J Rheum. 2016 Mar 1. doi: 10.3899/jrheum.151101).
The investigators noted that the selection of the target was of crucial importance when implementing a treat-to-target and tight control strategy. “In this sense our study provides reassurance that almost all patients at MDA will have no swollen/tender joints but also raises some concerns that the skin might not be well controlled if the MDA is used as the only target to treat patients with both joint and skin involvement,” they wrote. One explanation could be that the cutoff values selected in the MDA criteria for skin involvement are too stringent for therapies currently available, they suggested.
MDA seems to be a valuable tool to define low disease activity in patients with PsA, but the skin component requires further evaluation, they concluded.
The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
The minimal disease activity criteria used to identify low disease activity in psoriatic arthritis (PsA) may not be useful as a treatment target in patients with extended skin involvement, according to the results of a small study.
Minimal disease activity (MDA) for PsA is a composite measure encompassing clinically important aspects of the disease: arthritis, psoriasis, enthesitis, pain, patient-assessed global disease activity, and physical function and provides an objective target for therapy in clinical trials, Dr. Josefina Marin of the Hospital Italiano de Buenos Aires and her associates wrote in the Journal of Rheumatology.
To evaluate components of the MDA criteria, the research team enrolled 83 consecutive patients with PsA aged over 18 years who were attending their clinic. Patients needed to fulfill five out of the seven criteria to be classified as having MDA.
An assessment by a single experienced rheumatologist revealed that 41 patients met MDA criteria, with only 7.4% of these patients not satisfying the tender/swollen joint–count criteria.
However, only 51% (n = 21) of patients fulfilling MDA fulfilled the skin criteria (Psoriasis Area and Severity Index [PASI] and body surface area [BSA]), a percentage not statistically different from the 36% of patients not in MDA (n = 15; P = .154). “This is important because MDA may therefore not be useful as a treatment target in patients with extended skin involvement,” the investigators wrote (J Rheum. 2016 Mar 1. doi: 10.3899/jrheum.151101).
The investigators noted that the selection of the target was of crucial importance when implementing a treat-to-target and tight control strategy. “In this sense our study provides reassurance that almost all patients at MDA will have no swollen/tender joints but also raises some concerns that the skin might not be well controlled if the MDA is used as the only target to treat patients with both joint and skin involvement,” they wrote. One explanation could be that the cutoff values selected in the MDA criteria for skin involvement are too stringent for therapies currently available, they suggested.
MDA seems to be a valuable tool to define low disease activity in patients with PsA, but the skin component requires further evaluation, they concluded.
The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point: Minimal disease activity criteria in PsA may not be stringent enough for people with extended skin involvement.
Major finding: Only half of the PsA patients classified as having minimal disease activity met PASI and body surface area criteria.
Data source: A single-center study of 83 consecutive PsA patients aged over 18 years.
Disclosures: The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
Brodalumab met primary endpoints, deaths called ‘unrelated’
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
Key clinical point: The interleukin-17 inhibitor brodalumab met its coprimary endpoints in a phase III trial of patients with moderate to severe plaque psoriasis.
Major finding: At week 12, 83% of patients on 210 mg brodalumab achieved PASI 75, as did 60% of 140-mg patients and 3% of the placebo group. The coprimary endpoint, a static Physician Global Assessment score of 0/1, was met for 76% of 210-mg patients, 54% of 140-mg patients, and 1% of placebo patients. All four deaths were considered unrelated to treatment.
Data source: AMAGINE-1, a phase III, multicenter, randomized, double-blind study of brodalumab (210 or 140 mg) or placebo in 661 patients.
Disclosures: Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
Expert advises how to use shingles vaccine in rheumatology patients
MAUI, HAWAII – The herpes zoster vaccine is particularly important in patients with rheumatic diseases because their risks of shingles and postherpetic neuralgia are substantially higher than in the general population, Dr. John J. Cush observed at the 2016 Rheumatology Winter Clinical Symposium.
This is a live attenuated virus vaccine, and the rules regarding its use in patients with rheumatic diseases are fairly complicated. Here’s what physicians need to know: the Centers for Disease Control and Prevention and the Advisory Committee on Immunization Practices say the shingles vaccine can safely be given to patients on prednisone at less than 20 mg/day, azathioprine at up to 3 mg/kg/day, or methotrexate at up to 0.4 mg/kg/week, which works out to about 25 mg/week in anyone weighing more than 136 pounds.
However, the shingles vaccine is contraindicated in patients on recombinant biologic agents, including tumor necrosis factor (TNF) inhibitors, abatacept (Orencia), rituximab (Rituxan), or Janus kinase inhibitors, explained Dr. Cush, professor of medicine and rheumatology at Baylor University, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
The lifetime risk of shingles in the general population is roughly one in three. The risk in patients with rheumatoid arthritis is roughly twice that of the age-matched general population, and the risks are substantially greater than that in individuals with other rheumatic diseases, including lupus and granulomatosis with polyangiitis.
Payers cover the vaccine in patients age 60 or older. The vaccine is approved for and has been shown to be effective in 50- to 59-year-olds as well, but that typically entails an out-of-pocket expense of around $200.
Given that close to 60% of all rheumatoid arthritis patients will eventually be placed on biologic therapy, Dr. Cush believes in seizing any opportunity to give the shingles vaccine in age-appropriate patients beforehand. However, he advises against temporarily stopping a biologic for the express purpose of administering the live virus vaccine.
“Find the opportunity: between changes in medication, after they have surgery, during a lapse in therapy,” he suggested.
It’s recommended that Zostavax be deferred until after a patient has been off biologic therapy or high-dose steroids for at least 4 weeks, and that a biologic agent shouldn’t be started for 2-4 weeks after vaccination.
The shingles vaccine can safely be given with multiple inactivated virus vaccines such as an influenza vaccine or pneumococcal vaccine on a single day.
Controversy surrounds the issue of whether TNF inhibitors increase the risk of shingles. Several retrospective studies have reported they do. But the largest retrospective study, involving more than 33,000 new users of anti-TNF agents, found that patients with rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis, and other inflammatory diseases who initiated anti-TNF therapy weren’t at any higher risk of herpes zoster than those who started on methotrexate or other nonbiologic disease-modifying antirheumatic drugs (JAMA. 2013 Mar 6;309[9]:887-95). The lead investigator in this study, Dr. Kevin L. Winthrop of Oregon Health and Science University, Portland, is currently conducting a prospective study in an effort to confirm these findings.
The recommendation against giving the zoster vaccine to patients while on biologics is based upon the theoretical risk that exposure to the live attenuated virus will trigger an acute shingles attack. However, when Dr. Cush conducted a survey of his fellow rheumatologists, they reported that among more than a collective 200 patients inadvertently given the vaccine while on biologic therapy, not one case of shingles subsequently occurred over the short term.
More persuasively, Dr. Jeffrey R. Curtis of the University of Alabama at Birmingham and coinvestigators conducted a formal retrospective study of close to a half-million Medicare patients and found there were no cases of herpes zoster or varicella within 42 days following inadvertent vaccination of 633 patients while on biologics. During a median 2-year follow-up of Medicare patients with rheumatoid arthritis and other immune-mediated diseases, herpes zoster vaccination was associated with a 39% reduction in the risk of shingles (JAMA. 2012 Jul 4;308[1]:43-9).
“You shouldn’t be vaccinating for herpes zoster while patients are on a biologic, but you know what? If it happens, don’t wig out. Move on and try to avoid it,” Dr. Cush advised.
In any event, this is an issue that is eventually likely to go away. An inactivated virus vaccine for the prevention of shingles is now in clinical trials. It appears to be more effective than the current vaccine, according to the rheumatologist.
He reported having no financial interests relevant to his presentation.
MAUI, HAWAII – The herpes zoster vaccine is particularly important in patients with rheumatic diseases because their risks of shingles and postherpetic neuralgia are substantially higher than in the general population, Dr. John J. Cush observed at the 2016 Rheumatology Winter Clinical Symposium.
This is a live attenuated virus vaccine, and the rules regarding its use in patients with rheumatic diseases are fairly complicated. Here’s what physicians need to know: the Centers for Disease Control and Prevention and the Advisory Committee on Immunization Practices say the shingles vaccine can safely be given to patients on prednisone at less than 20 mg/day, azathioprine at up to 3 mg/kg/day, or methotrexate at up to 0.4 mg/kg/week, which works out to about 25 mg/week in anyone weighing more than 136 pounds.
However, the shingles vaccine is contraindicated in patients on recombinant biologic agents, including tumor necrosis factor (TNF) inhibitors, abatacept (Orencia), rituximab (Rituxan), or Janus kinase inhibitors, explained Dr. Cush, professor of medicine and rheumatology at Baylor University, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
The lifetime risk of shingles in the general population is roughly one in three. The risk in patients with rheumatoid arthritis is roughly twice that of the age-matched general population, and the risks are substantially greater than that in individuals with other rheumatic diseases, including lupus and granulomatosis with polyangiitis.
Payers cover the vaccine in patients age 60 or older. The vaccine is approved for and has been shown to be effective in 50- to 59-year-olds as well, but that typically entails an out-of-pocket expense of around $200.
Given that close to 60% of all rheumatoid arthritis patients will eventually be placed on biologic therapy, Dr. Cush believes in seizing any opportunity to give the shingles vaccine in age-appropriate patients beforehand. However, he advises against temporarily stopping a biologic for the express purpose of administering the live virus vaccine.
“Find the opportunity: between changes in medication, after they have surgery, during a lapse in therapy,” he suggested.
It’s recommended that Zostavax be deferred until after a patient has been off biologic therapy or high-dose steroids for at least 4 weeks, and that a biologic agent shouldn’t be started for 2-4 weeks after vaccination.
The shingles vaccine can safely be given with multiple inactivated virus vaccines such as an influenza vaccine or pneumococcal vaccine on a single day.
Controversy surrounds the issue of whether TNF inhibitors increase the risk of shingles. Several retrospective studies have reported they do. But the largest retrospective study, involving more than 33,000 new users of anti-TNF agents, found that patients with rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis, and other inflammatory diseases who initiated anti-TNF therapy weren’t at any higher risk of herpes zoster than those who started on methotrexate or other nonbiologic disease-modifying antirheumatic drugs (JAMA. 2013 Mar 6;309[9]:887-95). The lead investigator in this study, Dr. Kevin L. Winthrop of Oregon Health and Science University, Portland, is currently conducting a prospective study in an effort to confirm these findings.
The recommendation against giving the zoster vaccine to patients while on biologics is based upon the theoretical risk that exposure to the live attenuated virus will trigger an acute shingles attack. However, when Dr. Cush conducted a survey of his fellow rheumatologists, they reported that among more than a collective 200 patients inadvertently given the vaccine while on biologic therapy, not one case of shingles subsequently occurred over the short term.
More persuasively, Dr. Jeffrey R. Curtis of the University of Alabama at Birmingham and coinvestigators conducted a formal retrospective study of close to a half-million Medicare patients and found there were no cases of herpes zoster or varicella within 42 days following inadvertent vaccination of 633 patients while on biologics. During a median 2-year follow-up of Medicare patients with rheumatoid arthritis and other immune-mediated diseases, herpes zoster vaccination was associated with a 39% reduction in the risk of shingles (JAMA. 2012 Jul 4;308[1]:43-9).
“You shouldn’t be vaccinating for herpes zoster while patients are on a biologic, but you know what? If it happens, don’t wig out. Move on and try to avoid it,” Dr. Cush advised.
In any event, this is an issue that is eventually likely to go away. An inactivated virus vaccine for the prevention of shingles is now in clinical trials. It appears to be more effective than the current vaccine, according to the rheumatologist.
He reported having no financial interests relevant to his presentation.
MAUI, HAWAII – The herpes zoster vaccine is particularly important in patients with rheumatic diseases because their risks of shingles and postherpetic neuralgia are substantially higher than in the general population, Dr. John J. Cush observed at the 2016 Rheumatology Winter Clinical Symposium.
This is a live attenuated virus vaccine, and the rules regarding its use in patients with rheumatic diseases are fairly complicated. Here’s what physicians need to know: the Centers for Disease Control and Prevention and the Advisory Committee on Immunization Practices say the shingles vaccine can safely be given to patients on prednisone at less than 20 mg/day, azathioprine at up to 3 mg/kg/day, or methotrexate at up to 0.4 mg/kg/week, which works out to about 25 mg/week in anyone weighing more than 136 pounds.
However, the shingles vaccine is contraindicated in patients on recombinant biologic agents, including tumor necrosis factor (TNF) inhibitors, abatacept (Orencia), rituximab (Rituxan), or Janus kinase inhibitors, explained Dr. Cush, professor of medicine and rheumatology at Baylor University, Dallas, and director of clinical rheumatology at the Baylor Research Institute.
The lifetime risk of shingles in the general population is roughly one in three. The risk in patients with rheumatoid arthritis is roughly twice that of the age-matched general population, and the risks are substantially greater than that in individuals with other rheumatic diseases, including lupus and granulomatosis with polyangiitis.
Payers cover the vaccine in patients age 60 or older. The vaccine is approved for and has been shown to be effective in 50- to 59-year-olds as well, but that typically entails an out-of-pocket expense of around $200.
Given that close to 60% of all rheumatoid arthritis patients will eventually be placed on biologic therapy, Dr. Cush believes in seizing any opportunity to give the shingles vaccine in age-appropriate patients beforehand. However, he advises against temporarily stopping a biologic for the express purpose of administering the live virus vaccine.
“Find the opportunity: between changes in medication, after they have surgery, during a lapse in therapy,” he suggested.
It’s recommended that Zostavax be deferred until after a patient has been off biologic therapy or high-dose steroids for at least 4 weeks, and that a biologic agent shouldn’t be started for 2-4 weeks after vaccination.
The shingles vaccine can safely be given with multiple inactivated virus vaccines such as an influenza vaccine or pneumococcal vaccine on a single day.
Controversy surrounds the issue of whether TNF inhibitors increase the risk of shingles. Several retrospective studies have reported they do. But the largest retrospective study, involving more than 33,000 new users of anti-TNF agents, found that patients with rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis, and other inflammatory diseases who initiated anti-TNF therapy weren’t at any higher risk of herpes zoster than those who started on methotrexate or other nonbiologic disease-modifying antirheumatic drugs (JAMA. 2013 Mar 6;309[9]:887-95). The lead investigator in this study, Dr. Kevin L. Winthrop of Oregon Health and Science University, Portland, is currently conducting a prospective study in an effort to confirm these findings.
The recommendation against giving the zoster vaccine to patients while on biologics is based upon the theoretical risk that exposure to the live attenuated virus will trigger an acute shingles attack. However, when Dr. Cush conducted a survey of his fellow rheumatologists, they reported that among more than a collective 200 patients inadvertently given the vaccine while on biologic therapy, not one case of shingles subsequently occurred over the short term.
More persuasively, Dr. Jeffrey R. Curtis of the University of Alabama at Birmingham and coinvestigators conducted a formal retrospective study of close to a half-million Medicare patients and found there were no cases of herpes zoster or varicella within 42 days following inadvertent vaccination of 633 patients while on biologics. During a median 2-year follow-up of Medicare patients with rheumatoid arthritis and other immune-mediated diseases, herpes zoster vaccination was associated with a 39% reduction in the risk of shingles (JAMA. 2012 Jul 4;308[1]:43-9).
“You shouldn’t be vaccinating for herpes zoster while patients are on a biologic, but you know what? If it happens, don’t wig out. Move on and try to avoid it,” Dr. Cush advised.
In any event, this is an issue that is eventually likely to go away. An inactivated virus vaccine for the prevention of shingles is now in clinical trials. It appears to be more effective than the current vaccine, according to the rheumatologist.
He reported having no financial interests relevant to his presentation.
EXPERT ANALYSIS FROM RWCS 2016
Subtle radiographic progression in axial SpA cannot be reliably distinguished from error
Sacroiliitis observed in patients with axial spondyloarthritis more often regressed rather than progressed on radiography over nearly 5 years of follow-up of the Assessment of SpondyloArthritis international Society (ASAS) cohort, which lead author Dr. Alexandre Sepriano and his colleagues called “strange” and “sobering.”
The findings call into question the reliability of plain pelvic radiographs for detecting subtle change in sacroiliitis and should prompt the evaluation of alternative imaging modalities such as MRI and low-dose CT, according to Dr. Sepriano of Leiden (the Netherlands) University Medical Center and his associates.
Determining the presence of radiographic sacroiliitis is prognostically relevant and can pave the way for treatment with biologics, but ambiguity in making this decision and in tracking progression has been revealed in the large inter-and intrareader variability found in previous studies. Furthermore, previous studies tracking progression of nonradiographic axial spondyloarthritis (axSpA) to radiographic axSpA have addressed only disease progression and ignored regression. While regression is likely to be rare, it cannot be ignored from a methodologic standpoint, the investigators wrote.
The researchers therefore set out in the current study to assess positive and negative changes in sacroiliitis on plain pelvic radiographs over time in 975 patients from the ASAS cohort who had chronic back pain of unknown origin or undiagnosed peripheral symptoms (Ann Rheum Dis. 2016 Feb 22. doi: 10.1136/annrheumdis-2015-208964).
Of the 357 of the patients who had paired plain pelvic radiographs available at baseline and follow-up, 17.4% (62/357) fulfilled the criteria for radiographic axSpA at baseline, as defined by modified New York criteria (mNY). At a mean follow-up of 4.4 years, this figure had risen to 22.4% (80/357), suggesting a net progression of 5%.
However, when the authors cross-tabulated their figures, more than half (36/62) of the patients considered mNY positive at baseline were assessed as mNY negative at follow-up. This would mean that radiographic sacroiliitis would have regressed in 58% of the cases; conversely, only 54 of 295 patients (18.3%) became mNY positive at follow-up.
“If only positive change (progression) is valued and negative change is ignored, one would disregard measurement error and spuriously attribute part of the observed positive change to real progression,” the research team explained. “The most likely explanation of our strange and extreme observation is that subtle radiographic progression (the signal) – if truly present – cannot be reliably distinguished from measurement error (the noise). These sobering data clearly illustrate that more research is needed in visualising progression in axSpA.”
ASAS funded the study. The authors had no competing interests to declare.
Sacroiliitis observed in patients with axial spondyloarthritis more often regressed rather than progressed on radiography over nearly 5 years of follow-up of the Assessment of SpondyloArthritis international Society (ASAS) cohort, which lead author Dr. Alexandre Sepriano and his colleagues called “strange” and “sobering.”
The findings call into question the reliability of plain pelvic radiographs for detecting subtle change in sacroiliitis and should prompt the evaluation of alternative imaging modalities such as MRI and low-dose CT, according to Dr. Sepriano of Leiden (the Netherlands) University Medical Center and his associates.
Determining the presence of radiographic sacroiliitis is prognostically relevant and can pave the way for treatment with biologics, but ambiguity in making this decision and in tracking progression has been revealed in the large inter-and intrareader variability found in previous studies. Furthermore, previous studies tracking progression of nonradiographic axial spondyloarthritis (axSpA) to radiographic axSpA have addressed only disease progression and ignored regression. While regression is likely to be rare, it cannot be ignored from a methodologic standpoint, the investigators wrote.
The researchers therefore set out in the current study to assess positive and negative changes in sacroiliitis on plain pelvic radiographs over time in 975 patients from the ASAS cohort who had chronic back pain of unknown origin or undiagnosed peripheral symptoms (Ann Rheum Dis. 2016 Feb 22. doi: 10.1136/annrheumdis-2015-208964).
Of the 357 of the patients who had paired plain pelvic radiographs available at baseline and follow-up, 17.4% (62/357) fulfilled the criteria for radiographic axSpA at baseline, as defined by modified New York criteria (mNY). At a mean follow-up of 4.4 years, this figure had risen to 22.4% (80/357), suggesting a net progression of 5%.
However, when the authors cross-tabulated their figures, more than half (36/62) of the patients considered mNY positive at baseline were assessed as mNY negative at follow-up. This would mean that radiographic sacroiliitis would have regressed in 58% of the cases; conversely, only 54 of 295 patients (18.3%) became mNY positive at follow-up.
“If only positive change (progression) is valued and negative change is ignored, one would disregard measurement error and spuriously attribute part of the observed positive change to real progression,” the research team explained. “The most likely explanation of our strange and extreme observation is that subtle radiographic progression (the signal) – if truly present – cannot be reliably distinguished from measurement error (the noise). These sobering data clearly illustrate that more research is needed in visualising progression in axSpA.”
ASAS funded the study. The authors had no competing interests to declare.
Sacroiliitis observed in patients with axial spondyloarthritis more often regressed rather than progressed on radiography over nearly 5 years of follow-up of the Assessment of SpondyloArthritis international Society (ASAS) cohort, which lead author Dr. Alexandre Sepriano and his colleagues called “strange” and “sobering.”
The findings call into question the reliability of plain pelvic radiographs for detecting subtle change in sacroiliitis and should prompt the evaluation of alternative imaging modalities such as MRI and low-dose CT, according to Dr. Sepriano of Leiden (the Netherlands) University Medical Center and his associates.
Determining the presence of radiographic sacroiliitis is prognostically relevant and can pave the way for treatment with biologics, but ambiguity in making this decision and in tracking progression has been revealed in the large inter-and intrareader variability found in previous studies. Furthermore, previous studies tracking progression of nonradiographic axial spondyloarthritis (axSpA) to radiographic axSpA have addressed only disease progression and ignored regression. While regression is likely to be rare, it cannot be ignored from a methodologic standpoint, the investigators wrote.
The researchers therefore set out in the current study to assess positive and negative changes in sacroiliitis on plain pelvic radiographs over time in 975 patients from the ASAS cohort who had chronic back pain of unknown origin or undiagnosed peripheral symptoms (Ann Rheum Dis. 2016 Feb 22. doi: 10.1136/annrheumdis-2015-208964).
Of the 357 of the patients who had paired plain pelvic radiographs available at baseline and follow-up, 17.4% (62/357) fulfilled the criteria for radiographic axSpA at baseline, as defined by modified New York criteria (mNY). At a mean follow-up of 4.4 years, this figure had risen to 22.4% (80/357), suggesting a net progression of 5%.
However, when the authors cross-tabulated their figures, more than half (36/62) of the patients considered mNY positive at baseline were assessed as mNY negative at follow-up. This would mean that radiographic sacroiliitis would have regressed in 58% of the cases; conversely, only 54 of 295 patients (18.3%) became mNY positive at follow-up.
“If only positive change (progression) is valued and negative change is ignored, one would disregard measurement error and spuriously attribute part of the observed positive change to real progression,” the research team explained. “The most likely explanation of our strange and extreme observation is that subtle radiographic progression (the signal) – if truly present – cannot be reliably distinguished from measurement error (the noise). These sobering data clearly illustrate that more research is needed in visualising progression in axSpA.”
ASAS funded the study. The authors had no competing interests to declare.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Subtle radiographic progression in axSpA cannot be reliably distinguished from measurement error.
Major finding: Using plain radiographs, more than half of the patients identified as mNY positive for axSpA at baseline were assessed as mNY negative at a mean follow-up of 4.4 years.
Data source: 975 patients with chronic back pain of unknown origin or undiagnosed peripheral symptoms taking part in the Assessment of SpondyloArthritis international Society (ASAS) cohort.
Disclosures: ASAS funded the study. The authors had no competing interests to declare.
Subclinical inflammation predicts progression from psoriasis to PsA
People with cutaneous psoriasis who have arthralgia and signs of subclinical inflammation on MRI are at an increased risk of progressing to psoriatic arthritis, according to findings from a cross-sectional, longitudinal study.
First author Dr. Francesca Faustini of the University of Erlangen-Nuremberg, Erlangen, Germany, and her colleagues said the fact that psoriatic skin disease has a higher prevalence than arthritis raises the question whether patients with psoriasis without psoriatic arthritis (PsA) are spared from joint inflammation or whether mild changes, which escape physical examination, can be found in some patients (Ann Rheum Dis. 2016 Feb. 25. doi: 10.1136/annrheumdis-2015-208821).
Research published last year by the researchers shed some light on this by showing that psoriasis patients without PsA exhibit enthesiophytes as the result of pathological bone formation in the joint (Ann Rheum Dis. 2015 Feb 4. doi: 10.1136/annrheumdis-2014-206347).
“The presence of similar changes in patients with psoriasis strongly supports the hypothesis of subclinical joint pathology that antedates the clinical onset of PsA,” they said. The current study help to build on that finding by examining the extent to which inflammatory changes in the joints precede the onset of PsA, how such changes are related to structural pathology, and whether they influence the progression of psoriasis to PsA.
Dr. Faustini and her colleagues studied 55 patients with psoriasis who were attending a dermatology clinic and 30 healthy controls who underwent MRI of the hand and were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Patients with psoriasis also received a clinical investigation, high-resolution CT for detecting erosions and enthesiophytes, and were followed up for at least 1 year for the development of PsA.
Results showed that almost half of the patients with psoriasis (47%) had a least one inflammatory lesion shown on MRI.
Synovitis was the most prevalent inflammatory lesion (38%), while osteitis (11%), tenosynovitis (4%) and periarticular inflammation (4%) were less frequent.
Based on the PsA MRI scoring (PsAMRIS) system in patients with MRI lesions, the extent of inflammation was scored 3.0 units for synovitis, 1.8 for osteitis, 10.5 for tenosynovitis, and 3.0 for periarticular inflammation. Synovitis was deemed moderate (PsAMRIS of 2) in five joints and mild (score of 1) in all others where it was present; none of the joints scored 3 (severe).
Enthesiophytes and bone erosions were not different between patients with psoriasis with or without inflammatory MRI changes.
Among 41 patients who completed a follow-up examination a mean of about 14 months after the first, the risk for developing PsA at 1 year was as high as 56% if patients had subclinical synovitis and symptoms related to arthralgia (at least one tender joint), but was as low as 15% if patients had normal MRIs and did not report arthralgia. A total of 12 (30%) developed PsA according to CASPAR criteria.
“Subclinical inflammation appears to substantially influence the risk of patients with psoriasis to progress to PsA ... these findings indicate the possibility to define patients with psoriasis, in which preventive treatment for the development of PsA may be feasible,” they concluded.
However, they noted that it was important to consider that the presence of inflammatory lesions does not necessarily indicate that cutaneous psoriasis is causally linked to such lesions, particularly because neither disease activity and duration nor scalp and nail involvement was associated with MRI lesions.
“These findings suggest that skin and joint inflammation occur uncoupled and that skin disease may not represent the key pacemaker for joint inflammation,” they said.
The study received funding from the German Research Foundation, the Marie Curie project OSTEOIMMUNE, the German Ministry of Science and Education, the Innovative Medicines Initiative, and the Pfizer Competitive Grant Award Germany. The authors declared no conflicts of interest.
People with cutaneous psoriasis who have arthralgia and signs of subclinical inflammation on MRI are at an increased risk of progressing to psoriatic arthritis, according to findings from a cross-sectional, longitudinal study.
First author Dr. Francesca Faustini of the University of Erlangen-Nuremberg, Erlangen, Germany, and her colleagues said the fact that psoriatic skin disease has a higher prevalence than arthritis raises the question whether patients with psoriasis without psoriatic arthritis (PsA) are spared from joint inflammation or whether mild changes, which escape physical examination, can be found in some patients (Ann Rheum Dis. 2016 Feb. 25. doi: 10.1136/annrheumdis-2015-208821).
Research published last year by the researchers shed some light on this by showing that psoriasis patients without PsA exhibit enthesiophytes as the result of pathological bone formation in the joint (Ann Rheum Dis. 2015 Feb 4. doi: 10.1136/annrheumdis-2014-206347).
“The presence of similar changes in patients with psoriasis strongly supports the hypothesis of subclinical joint pathology that antedates the clinical onset of PsA,” they said. The current study help to build on that finding by examining the extent to which inflammatory changes in the joints precede the onset of PsA, how such changes are related to structural pathology, and whether they influence the progression of psoriasis to PsA.
Dr. Faustini and her colleagues studied 55 patients with psoriasis who were attending a dermatology clinic and 30 healthy controls who underwent MRI of the hand and were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Patients with psoriasis also received a clinical investigation, high-resolution CT for detecting erosions and enthesiophytes, and were followed up for at least 1 year for the development of PsA.
Results showed that almost half of the patients with psoriasis (47%) had a least one inflammatory lesion shown on MRI.
Synovitis was the most prevalent inflammatory lesion (38%), while osteitis (11%), tenosynovitis (4%) and periarticular inflammation (4%) were less frequent.
Based on the PsA MRI scoring (PsAMRIS) system in patients with MRI lesions, the extent of inflammation was scored 3.0 units for synovitis, 1.8 for osteitis, 10.5 for tenosynovitis, and 3.0 for periarticular inflammation. Synovitis was deemed moderate (PsAMRIS of 2) in five joints and mild (score of 1) in all others where it was present; none of the joints scored 3 (severe).
Enthesiophytes and bone erosions were not different between patients with psoriasis with or without inflammatory MRI changes.
Among 41 patients who completed a follow-up examination a mean of about 14 months after the first, the risk for developing PsA at 1 year was as high as 56% if patients had subclinical synovitis and symptoms related to arthralgia (at least one tender joint), but was as low as 15% if patients had normal MRIs and did not report arthralgia. A total of 12 (30%) developed PsA according to CASPAR criteria.
“Subclinical inflammation appears to substantially influence the risk of patients with psoriasis to progress to PsA ... these findings indicate the possibility to define patients with psoriasis, in which preventive treatment for the development of PsA may be feasible,” they concluded.
However, they noted that it was important to consider that the presence of inflammatory lesions does not necessarily indicate that cutaneous psoriasis is causally linked to such lesions, particularly because neither disease activity and duration nor scalp and nail involvement was associated with MRI lesions.
“These findings suggest that skin and joint inflammation occur uncoupled and that skin disease may not represent the key pacemaker for joint inflammation,” they said.
The study received funding from the German Research Foundation, the Marie Curie project OSTEOIMMUNE, the German Ministry of Science and Education, the Innovative Medicines Initiative, and the Pfizer Competitive Grant Award Germany. The authors declared no conflicts of interest.
People with cutaneous psoriasis who have arthralgia and signs of subclinical inflammation on MRI are at an increased risk of progressing to psoriatic arthritis, according to findings from a cross-sectional, longitudinal study.
First author Dr. Francesca Faustini of the University of Erlangen-Nuremberg, Erlangen, Germany, and her colleagues said the fact that psoriatic skin disease has a higher prevalence than arthritis raises the question whether patients with psoriasis without psoriatic arthritis (PsA) are spared from joint inflammation or whether mild changes, which escape physical examination, can be found in some patients (Ann Rheum Dis. 2016 Feb. 25. doi: 10.1136/annrheumdis-2015-208821).
Research published last year by the researchers shed some light on this by showing that psoriasis patients without PsA exhibit enthesiophytes as the result of pathological bone formation in the joint (Ann Rheum Dis. 2015 Feb 4. doi: 10.1136/annrheumdis-2014-206347).
“The presence of similar changes in patients with psoriasis strongly supports the hypothesis of subclinical joint pathology that antedates the clinical onset of PsA,” they said. The current study help to build on that finding by examining the extent to which inflammatory changes in the joints precede the onset of PsA, how such changes are related to structural pathology, and whether they influence the progression of psoriasis to PsA.
Dr. Faustini and her colleagues studied 55 patients with psoriasis who were attending a dermatology clinic and 30 healthy controls who underwent MRI of the hand and were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Patients with psoriasis also received a clinical investigation, high-resolution CT for detecting erosions and enthesiophytes, and were followed up for at least 1 year for the development of PsA.
Results showed that almost half of the patients with psoriasis (47%) had a least one inflammatory lesion shown on MRI.
Synovitis was the most prevalent inflammatory lesion (38%), while osteitis (11%), tenosynovitis (4%) and periarticular inflammation (4%) were less frequent.
Based on the PsA MRI scoring (PsAMRIS) system in patients with MRI lesions, the extent of inflammation was scored 3.0 units for synovitis, 1.8 for osteitis, 10.5 for tenosynovitis, and 3.0 for periarticular inflammation. Synovitis was deemed moderate (PsAMRIS of 2) in five joints and mild (score of 1) in all others where it was present; none of the joints scored 3 (severe).
Enthesiophytes and bone erosions were not different between patients with psoriasis with or without inflammatory MRI changes.
Among 41 patients who completed a follow-up examination a mean of about 14 months after the first, the risk for developing PsA at 1 year was as high as 56% if patients had subclinical synovitis and symptoms related to arthralgia (at least one tender joint), but was as low as 15% if patients had normal MRIs and did not report arthralgia. A total of 12 (30%) developed PsA according to CASPAR criteria.
“Subclinical inflammation appears to substantially influence the risk of patients with psoriasis to progress to PsA ... these findings indicate the possibility to define patients with psoriasis, in which preventive treatment for the development of PsA may be feasible,” they concluded.
However, they noted that it was important to consider that the presence of inflammatory lesions does not necessarily indicate that cutaneous psoriasis is causally linked to such lesions, particularly because neither disease activity and duration nor scalp and nail involvement was associated with MRI lesions.
“These findings suggest that skin and joint inflammation occur uncoupled and that skin disease may not represent the key pacemaker for joint inflammation,” they said.
The study received funding from the German Research Foundation, the Marie Curie project OSTEOIMMUNE, the German Ministry of Science and Education, the Innovative Medicines Initiative, and the Pfizer Competitive Grant Award Germany. The authors declared no conflicts of interest.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Subclinical inflammation appears to substantially influence the risk of patients with psoriasis progressing to PsA.
Major finding: The risk for developing PsA was as high as 56% if patients had subclinical synovitis and symptoms related to arthralgia.
Data source: A cross-sectional longitudinal analysis of 55 patients with cutaneous psoriasis and 30 healthy controls.
Disclosures: The study received funding from the German Research Foundation, the Marie Curie project OSTEOIMMUNE, the German Ministry of Science and Education, the Innovative Medicines Initiative, and the Pfizer Competitive Grant Award Germany. The authors declared no conflicts of interest.
NNTs show once-unimaginable psoriasis outcomes now readily attainable
WAIKOLOA, HAWAII – Scrutiny of the number needed to treat with various systemic drugs to achieve a Psoriasis Area and Severity Index–90 (PASI-90) response in psoriasis highlights the folly of current stepwise treatment strategies imposed upon dermatologists by many payers, Dr. Craig L. Leonardi asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“It’s time to rethink our goals,” declared Dr. Leonardi, a dermatologist at Saint Louis University and a noted clinical trialist.
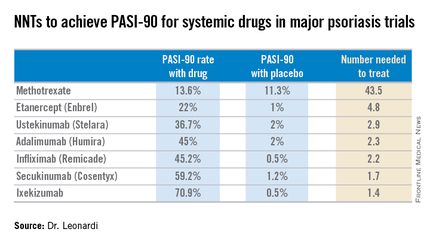
Most health plans insist that patients with moderate to severe psoriasis must have tried methotrexate and failed to achieve an adequate response before moving on to costlier biologic agents. But a PASI-90 response rate, indicative of 90% improvement in psoriasis area and severity, was achieved in only 13.6% of patients on methotrexate in the CHAMPION trial, compared with 59.2% and 70.9% of patients in phase III randomized trials of the interleukin-17 antagonists secukinumab (Cosentyx) and ixekizumab, respectively.
In other words, it is now routinely possible using highly effective medications to achieve a PASI-90 response in the majority of psoriasis patients, he noted.
Although statisticians say it isn’t appropriate to compare outcomes across clinical trials because study populations may differ, Dr. Leonardi decided it nevertheless would be illuminating to compare NNTs (the number of patients who needed to be treated with a medication instead of placebo to achieve one additional responder in a defined time period). In this analysis, he used data from phase III randomized, placebo-controlled clinical trials and Food and Drug Administration–regulated package inserts to calculate NNTs for systemic medications for psoriasis for the 10- to 16-week duration of phase III trials. The NNTs for a PASI-90 response ranged from a whopping 43.5 for methotrexate to 1.7 with secukinumab and 1.4 for ixekizumab.
“You can see immediately that methotrexate is outed as a weak and ineffective drug. And yet which drug are we usually asked to use first? Methotrexate. This is a structural problem that we have to solve in our specialty. We have to get with the insurance industry, we have to get with our guidelines of care and rewrite them. There is no reason that this drug should be placed in front of any of the other drugs, which are much more effective,” Dr. Leonardi said.
Viewing the data another way, the proportion of patients who achieved a PASI-75 response at the time of a major placebo-controlled clinical trial’s primary endpoint ranged from a low of 35.5% with methotrexate to 81.6% with secukinumab and 89.1% with ixekizumab. The NNTs to get a PASI-75 improvement with secukinumab and ixekizumab were, respectively, 1.3 and 1.2, compared to 6.0 for methotrexate.
“With an NNT of 1.2, if you treat 12 patients with ixekizumab, 10 of them are going to achieve PASI-75. This is a very different world than we had at the beginning of this adventure back in the early 2000s,” he observed.
In the modern era of highly effective biologic therapies for psoriasis, it makes sense to push as hard as possible in an effort to try to clear patients, according to Dr. Leonardi. That’s in part because the closer patients come to that once-nearly-unattainable goal, the better they actually feel, as underscored in the phase III results for ixekizumab.
In that study, the proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1 at week 12 rose stepwise with the size of their PASI response. Among patients with a week-12 PASI response of 50 to less than 75, 18.8% had a DLQI of 0 or 1. For patients with a PASI-75 to less than PASI-90, it jumped to 52.3%. Among subjects with a PASI-90 to less than 100, 66.9% had a DLQI of 0 or 1. And among the 35.3% of ixekizumab-treated patients who had a PASI-100 response, the likelihood of a DLQI of 0 or 1 rose to 71.1%.
Noting that etanercept (Enbrel) is the only biologic on the market that achieves a PASI-75 response in less than half of treated patients, Dr. Leonardi said, “I’m over using etanercept as a first-line biologic. It’s the weakest biologic we can pick right now. I really like the highly efficacious drugs. I like adalimumab. I like the efficacy I see with ustekinumab. I like secukinumab. They are all preferred first-line drugs if I can get them, but it’s an insurance company world. I can’t tell you how many times I’ve heard, ‘Well, we’re not saying you can’t prescribe it, Dr. Leonardi, we’re just not going to pay for it.’ ”
Ixekizumab is under FDA review for psoriasis and a decision is expected within the first quarter of 2016, according to a spokesperson for Eli Lilly.
Dr. Leonardi is a recipient of research grants from well over a dozen pharmaceutical companies and is a consultant to and/or member of the speakers bureaus for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Eli Lilly, Leo, Novartis, Pfizer, Sandoz, UCB, and Vitae.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Scrutiny of the number needed to treat with various systemic drugs to achieve a Psoriasis Area and Severity Index–90 (PASI-90) response in psoriasis highlights the folly of current stepwise treatment strategies imposed upon dermatologists by many payers, Dr. Craig L. Leonardi asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“It’s time to rethink our goals,” declared Dr. Leonardi, a dermatologist at Saint Louis University and a noted clinical trialist.
Most health plans insist that patients with moderate to severe psoriasis must have tried methotrexate and failed to achieve an adequate response before moving on to costlier biologic agents. But a PASI-90 response rate, indicative of 90% improvement in psoriasis area and severity, was achieved in only 13.6% of patients on methotrexate in the CHAMPION trial, compared with 59.2% and 70.9% of patients in phase III randomized trials of the interleukin-17 antagonists secukinumab (Cosentyx) and ixekizumab, respectively.
In other words, it is now routinely possible using highly effective medications to achieve a PASI-90 response in the majority of psoriasis patients, he noted.
Although statisticians say it isn’t appropriate to compare outcomes across clinical trials because study populations may differ, Dr. Leonardi decided it nevertheless would be illuminating to compare NNTs (the number of patients who needed to be treated with a medication instead of placebo to achieve one additional responder in a defined time period). In this analysis, he used data from phase III randomized, placebo-controlled clinical trials and Food and Drug Administration–regulated package inserts to calculate NNTs for systemic medications for psoriasis for the 10- to 16-week duration of phase III trials. The NNTs for a PASI-90 response ranged from a whopping 43.5 for methotrexate to 1.7 with secukinumab and 1.4 for ixekizumab.
“You can see immediately that methotrexate is outed as a weak and ineffective drug. And yet which drug are we usually asked to use first? Methotrexate. This is a structural problem that we have to solve in our specialty. We have to get with the insurance industry, we have to get with our guidelines of care and rewrite them. There is no reason that this drug should be placed in front of any of the other drugs, which are much more effective,” Dr. Leonardi said.

Viewing the data another way, the proportion of patients who achieved a PASI-75 response at the time of a major placebo-controlled clinical trial’s primary endpoint ranged from a low of 35.5% with methotrexate to 81.6% with secukinumab and 89.1% with ixekizumab. The NNTs to get a PASI-75 improvement with secukinumab and ixekizumab were, respectively, 1.3 and 1.2, compared to 6.0 for methotrexate.
“With an NNT of 1.2, if you treat 12 patients with ixekizumab, 10 of them are going to achieve PASI-75. This is a very different world than we had at the beginning of this adventure back in the early 2000s,” he observed.
In the modern era of highly effective biologic therapies for psoriasis, it makes sense to push as hard as possible in an effort to try to clear patients, according to Dr. Leonardi. That’s in part because the closer patients come to that once-nearly-unattainable goal, the better they actually feel, as underscored in the phase III results for ixekizumab.
In that study, the proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1 at week 12 rose stepwise with the size of their PASI response. Among patients with a week-12 PASI response of 50 to less than 75, 18.8% had a DLQI of 0 or 1. For patients with a PASI-75 to less than PASI-90, it jumped to 52.3%. Among subjects with a PASI-90 to less than 100, 66.9% had a DLQI of 0 or 1. And among the 35.3% of ixekizumab-treated patients who had a PASI-100 response, the likelihood of a DLQI of 0 or 1 rose to 71.1%.
Noting that etanercept (Enbrel) is the only biologic on the market that achieves a PASI-75 response in less than half of treated patients, Dr. Leonardi said, “I’m over using etanercept as a first-line biologic. It’s the weakest biologic we can pick right now. I really like the highly efficacious drugs. I like adalimumab. I like the efficacy I see with ustekinumab. I like secukinumab. They are all preferred first-line drugs if I can get them, but it’s an insurance company world. I can’t tell you how many times I’ve heard, ‘Well, we’re not saying you can’t prescribe it, Dr. Leonardi, we’re just not going to pay for it.’ ”
Ixekizumab is under FDA review for psoriasis and a decision is expected within the first quarter of 2016, according to a spokesperson for Eli Lilly.
Dr. Leonardi is a recipient of research grants from well over a dozen pharmaceutical companies and is a consultant to and/or member of the speakers bureaus for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Eli Lilly, Leo, Novartis, Pfizer, Sandoz, UCB, and Vitae.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Scrutiny of the number needed to treat with various systemic drugs to achieve a Psoriasis Area and Severity Index–90 (PASI-90) response in psoriasis highlights the folly of current stepwise treatment strategies imposed upon dermatologists by many payers, Dr. Craig L. Leonardi asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“It’s time to rethink our goals,” declared Dr. Leonardi, a dermatologist at Saint Louis University and a noted clinical trialist.
Most health plans insist that patients with moderate to severe psoriasis must have tried methotrexate and failed to achieve an adequate response before moving on to costlier biologic agents. But a PASI-90 response rate, indicative of 90% improvement in psoriasis area and severity, was achieved in only 13.6% of patients on methotrexate in the CHAMPION trial, compared with 59.2% and 70.9% of patients in phase III randomized trials of the interleukin-17 antagonists secukinumab (Cosentyx) and ixekizumab, respectively.
In other words, it is now routinely possible using highly effective medications to achieve a PASI-90 response in the majority of psoriasis patients, he noted.
Although statisticians say it isn’t appropriate to compare outcomes across clinical trials because study populations may differ, Dr. Leonardi decided it nevertheless would be illuminating to compare NNTs (the number of patients who needed to be treated with a medication instead of placebo to achieve one additional responder in a defined time period). In this analysis, he used data from phase III randomized, placebo-controlled clinical trials and Food and Drug Administration–regulated package inserts to calculate NNTs for systemic medications for psoriasis for the 10- to 16-week duration of phase III trials. The NNTs for a PASI-90 response ranged from a whopping 43.5 for methotrexate to 1.7 with secukinumab and 1.4 for ixekizumab.
“You can see immediately that methotrexate is outed as a weak and ineffective drug. And yet which drug are we usually asked to use first? Methotrexate. This is a structural problem that we have to solve in our specialty. We have to get with the insurance industry, we have to get with our guidelines of care and rewrite them. There is no reason that this drug should be placed in front of any of the other drugs, which are much more effective,” Dr. Leonardi said.

Viewing the data another way, the proportion of patients who achieved a PASI-75 response at the time of a major placebo-controlled clinical trial’s primary endpoint ranged from a low of 35.5% with methotrexate to 81.6% with secukinumab and 89.1% with ixekizumab. The NNTs to get a PASI-75 improvement with secukinumab and ixekizumab were, respectively, 1.3 and 1.2, compared to 6.0 for methotrexate.
“With an NNT of 1.2, if you treat 12 patients with ixekizumab, 10 of them are going to achieve PASI-75. This is a very different world than we had at the beginning of this adventure back in the early 2000s,” he observed.
In the modern era of highly effective biologic therapies for psoriasis, it makes sense to push as hard as possible in an effort to try to clear patients, according to Dr. Leonardi. That’s in part because the closer patients come to that once-nearly-unattainable goal, the better they actually feel, as underscored in the phase III results for ixekizumab.
In that study, the proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1 at week 12 rose stepwise with the size of their PASI response. Among patients with a week-12 PASI response of 50 to less than 75, 18.8% had a DLQI of 0 or 1. For patients with a PASI-75 to less than PASI-90, it jumped to 52.3%. Among subjects with a PASI-90 to less than 100, 66.9% had a DLQI of 0 or 1. And among the 35.3% of ixekizumab-treated patients who had a PASI-100 response, the likelihood of a DLQI of 0 or 1 rose to 71.1%.
Noting that etanercept (Enbrel) is the only biologic on the market that achieves a PASI-75 response in less than half of treated patients, Dr. Leonardi said, “I’m over using etanercept as a first-line biologic. It’s the weakest biologic we can pick right now. I really like the highly efficacious drugs. I like adalimumab. I like the efficacy I see with ustekinumab. I like secukinumab. They are all preferred first-line drugs if I can get them, but it’s an insurance company world. I can’t tell you how many times I’ve heard, ‘Well, we’re not saying you can’t prescribe it, Dr. Leonardi, we’re just not going to pay for it.’ ”
Ixekizumab is under FDA review for psoriasis and a decision is expected within the first quarter of 2016, according to a spokesperson for Eli Lilly.
Dr. Leonardi is a recipient of research grants from well over a dozen pharmaceutical companies and is a consultant to and/or member of the speakers bureaus for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Eli Lilly, Leo, Novartis, Pfizer, Sandoz, UCB, and Vitae.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Registry shows no increased cancer risk with biologics for psoriasis
WAIKOLOA, HAWAII – The latest update from the ongoing PSOLAR registry provides “very reassuring” evidence that the use of biologic agents to treat moderate to severe psoriasis doesn’t significantly increase malignancy risk other than for skin cancer, according to Dr. Kristina Callis Duffin.
Dr. Duffin of the department of dermatology at the University of Utah, Salt Lake City, cited a report presented by Dr. David Fiorentino, professor of dermatology at Stanford (Calif.) University, at the annual meeting of the European Academy of Dermatology and Venereology last October in Copenhagen. This update from the prospective international Psoriasis Longitudinal Assessment and Registry (PSOLAR) included 12,093 psoriasis patients deemed candidates for biologics, including 2,084 who did not go on a biologic agent while the rest did.
During 40,388 patient-years of prospective follow-up, or an average of 3.3 years, 455 patients were diagnosed with a malignancy other than skin cancer. The cumulative malignancy rate was 0.75 cases per 100 patient-years in patients on nonbiologic therapies, which was not significantly different from the rates of 0.51 per 100 patient-years in participants who started on ustekinumab (Stelara) at enrollment, 0.81 in patients on infliximab (Remicade), or 0.73 per 100 patient-years in those on other biologics, Dr. Duffin said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“These are all very low rates,” commented Dr. Duffin.
The PSOLAR data are particularly valuable because the registry was set up specifically to prospectively examine the long-term safety and efficacy of biologic agents in psoriasis patients. In contrast, the landmark epidemiologic study led by Dr. Joel M. Gelfand of the University of Pennsylvania, Philadelphia, which concluded that mild psoriasis was associated with a 34% increased risk of lymphoma and that severe psoriasis carried a 59% greater risk than in nonpsoriatic controls (J Invest Dermatol. 2006 Oct;126[10]:2194-201), involved a retrospective analysis of the U.K. General Practice Research Database. And while that study had strength in numbers – it included more than 153,000 British psoriasis patients and nearly 800,000 controls – it wasn’t designed to look specifically at psoriasis patients.
It’s reassuring that the lymphoma rate of 0.47 cases per 100 patient-years in patients with severe psoriasis in the U.K. registry during the prebiologics era is virtually identical to the rates associated with biologic agents in PSOLAR to date, which ranged from 0.3 to 0.5 cases per 100 patient-years, Dr. Duffin said.
The PSOLAR findings are worth sharing with patients. As a result of direct-to-consumer advertising by pharmaceutical companies, psoriasis patients are typically quite concerned about the risk of cancer associated with biologic agents, she added.
“They hear the comment in the ad that serious infections and malignancies have been reported in patients on these drugs as ‘these drugs increase the risk of malignancy.’ So where I start this conversation is, ‘Actually, patients with psoriasis do have some increased risk of malignancy, but those malignancies are mostly nonmelanoma skin cancers and lymphoproliferative diseases,’” the dermatologist explained.
Much of this risk is probably related to the fact that patients with moderate to severe psoriasis often have an extensive history of exposure to immunosuppressive agents such as cyclosporine as well as UV light therapies, which increase the risk of skin cancer.
“You also have to consider the fact that psoriasis patients tend to have a lot of smoking behaviors and alcohol behaviors that increase cancer risk,” Dr. Duffin continued.
In shared decision making regarding the option of biologic therapy in psoriasis patients having a history of cancer or who develop cancer while on a biologic, she likes to pose a question: What scares you more: the risk of your cancer coming back or not being able to have a good quality of life?
“That gets them thinking,” she said.
She stressed that as part of discussions regarding the risk/benefit profile of biologic therapy in an individual with a history of cancer, or of continuing a biologic in someone diagnosed with a malignancy while on treatment, it’s important for the dermatologist to talk with the patient’s oncologist, who is best positioned to provide insight into the risk of cancer recurrence.
Dr. Duffin is a recipient of research grants from and a consultant to Janssen, which sponsors the PSOLAR registry, as well as to more than half a dozen other pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The latest update from the ongoing PSOLAR registry provides “very reassuring” evidence that the use of biologic agents to treat moderate to severe psoriasis doesn’t significantly increase malignancy risk other than for skin cancer, according to Dr. Kristina Callis Duffin.
Dr. Duffin of the department of dermatology at the University of Utah, Salt Lake City, cited a report presented by Dr. David Fiorentino, professor of dermatology at Stanford (Calif.) University, at the annual meeting of the European Academy of Dermatology and Venereology last October in Copenhagen. This update from the prospective international Psoriasis Longitudinal Assessment and Registry (PSOLAR) included 12,093 psoriasis patients deemed candidates for biologics, including 2,084 who did not go on a biologic agent while the rest did.
During 40,388 patient-years of prospective follow-up, or an average of 3.3 years, 455 patients were diagnosed with a malignancy other than skin cancer. The cumulative malignancy rate was 0.75 cases per 100 patient-years in patients on nonbiologic therapies, which was not significantly different from the rates of 0.51 per 100 patient-years in participants who started on ustekinumab (Stelara) at enrollment, 0.81 in patients on infliximab (Remicade), or 0.73 per 100 patient-years in those on other biologics, Dr. Duffin said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“These are all very low rates,” commented Dr. Duffin.
The PSOLAR data are particularly valuable because the registry was set up specifically to prospectively examine the long-term safety and efficacy of biologic agents in psoriasis patients. In contrast, the landmark epidemiologic study led by Dr. Joel M. Gelfand of the University of Pennsylvania, Philadelphia, which concluded that mild psoriasis was associated with a 34% increased risk of lymphoma and that severe psoriasis carried a 59% greater risk than in nonpsoriatic controls (J Invest Dermatol. 2006 Oct;126[10]:2194-201), involved a retrospective analysis of the U.K. General Practice Research Database. And while that study had strength in numbers – it included more than 153,000 British psoriasis patients and nearly 800,000 controls – it wasn’t designed to look specifically at psoriasis patients.
It’s reassuring that the lymphoma rate of 0.47 cases per 100 patient-years in patients with severe psoriasis in the U.K. registry during the prebiologics era is virtually identical to the rates associated with biologic agents in PSOLAR to date, which ranged from 0.3 to 0.5 cases per 100 patient-years, Dr. Duffin said.
The PSOLAR findings are worth sharing with patients. As a result of direct-to-consumer advertising by pharmaceutical companies, psoriasis patients are typically quite concerned about the risk of cancer associated with biologic agents, she added.
“They hear the comment in the ad that serious infections and malignancies have been reported in patients on these drugs as ‘these drugs increase the risk of malignancy.’ So where I start this conversation is, ‘Actually, patients with psoriasis do have some increased risk of malignancy, but those malignancies are mostly nonmelanoma skin cancers and lymphoproliferative diseases,’” the dermatologist explained.
Much of this risk is probably related to the fact that patients with moderate to severe psoriasis often have an extensive history of exposure to immunosuppressive agents such as cyclosporine as well as UV light therapies, which increase the risk of skin cancer.
“You also have to consider the fact that psoriasis patients tend to have a lot of smoking behaviors and alcohol behaviors that increase cancer risk,” Dr. Duffin continued.
In shared decision making regarding the option of biologic therapy in psoriasis patients having a history of cancer or who develop cancer while on a biologic, she likes to pose a question: What scares you more: the risk of your cancer coming back or not being able to have a good quality of life?
“That gets them thinking,” she said.
She stressed that as part of discussions regarding the risk/benefit profile of biologic therapy in an individual with a history of cancer, or of continuing a biologic in someone diagnosed with a malignancy while on treatment, it’s important for the dermatologist to talk with the patient’s oncologist, who is best positioned to provide insight into the risk of cancer recurrence.
Dr. Duffin is a recipient of research grants from and a consultant to Janssen, which sponsors the PSOLAR registry, as well as to more than half a dozen other pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The latest update from the ongoing PSOLAR registry provides “very reassuring” evidence that the use of biologic agents to treat moderate to severe psoriasis doesn’t significantly increase malignancy risk other than for skin cancer, according to Dr. Kristina Callis Duffin.
Dr. Duffin of the department of dermatology at the University of Utah, Salt Lake City, cited a report presented by Dr. David Fiorentino, professor of dermatology at Stanford (Calif.) University, at the annual meeting of the European Academy of Dermatology and Venereology last October in Copenhagen. This update from the prospective international Psoriasis Longitudinal Assessment and Registry (PSOLAR) included 12,093 psoriasis patients deemed candidates for biologics, including 2,084 who did not go on a biologic agent while the rest did.
During 40,388 patient-years of prospective follow-up, or an average of 3.3 years, 455 patients were diagnosed with a malignancy other than skin cancer. The cumulative malignancy rate was 0.75 cases per 100 patient-years in patients on nonbiologic therapies, which was not significantly different from the rates of 0.51 per 100 patient-years in participants who started on ustekinumab (Stelara) at enrollment, 0.81 in patients on infliximab (Remicade), or 0.73 per 100 patient-years in those on other biologics, Dr. Duffin said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“These are all very low rates,” commented Dr. Duffin.
The PSOLAR data are particularly valuable because the registry was set up specifically to prospectively examine the long-term safety and efficacy of biologic agents in psoriasis patients. In contrast, the landmark epidemiologic study led by Dr. Joel M. Gelfand of the University of Pennsylvania, Philadelphia, which concluded that mild psoriasis was associated with a 34% increased risk of lymphoma and that severe psoriasis carried a 59% greater risk than in nonpsoriatic controls (J Invest Dermatol. 2006 Oct;126[10]:2194-201), involved a retrospective analysis of the U.K. General Practice Research Database. And while that study had strength in numbers – it included more than 153,000 British psoriasis patients and nearly 800,000 controls – it wasn’t designed to look specifically at psoriasis patients.
It’s reassuring that the lymphoma rate of 0.47 cases per 100 patient-years in patients with severe psoriasis in the U.K. registry during the prebiologics era is virtually identical to the rates associated with biologic agents in PSOLAR to date, which ranged from 0.3 to 0.5 cases per 100 patient-years, Dr. Duffin said.
The PSOLAR findings are worth sharing with patients. As a result of direct-to-consumer advertising by pharmaceutical companies, psoriasis patients are typically quite concerned about the risk of cancer associated with biologic agents, she added.
“They hear the comment in the ad that serious infections and malignancies have been reported in patients on these drugs as ‘these drugs increase the risk of malignancy.’ So where I start this conversation is, ‘Actually, patients with psoriasis do have some increased risk of malignancy, but those malignancies are mostly nonmelanoma skin cancers and lymphoproliferative diseases,’” the dermatologist explained.
Much of this risk is probably related to the fact that patients with moderate to severe psoriasis often have an extensive history of exposure to immunosuppressive agents such as cyclosporine as well as UV light therapies, which increase the risk of skin cancer.
“You also have to consider the fact that psoriasis patients tend to have a lot of smoking behaviors and alcohol behaviors that increase cancer risk,” Dr. Duffin continued.
In shared decision making regarding the option of biologic therapy in psoriasis patients having a history of cancer or who develop cancer while on a biologic, she likes to pose a question: What scares you more: the risk of your cancer coming back or not being able to have a good quality of life?
“That gets them thinking,” she said.
She stressed that as part of discussions regarding the risk/benefit profile of biologic therapy in an individual with a history of cancer, or of continuing a biologic in someone diagnosed with a malignancy while on treatment, it’s important for the dermatologist to talk with the patient’s oncologist, who is best positioned to provide insight into the risk of cancer recurrence.
Dr. Duffin is a recipient of research grants from and a consultant to Janssen, which sponsors the PSOLAR registry, as well as to more than half a dozen other pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
NSAIDs effective and safe for axSpA in the short term
The findings of a recent Cochrane review back up guidelines that recommend NSAIDs as an appropriate first-line treatment for people with axial spondyloarthritis (axSpA).
NSAIDs have been associated with a variety of gastrointestinal effects and an increased risk of cardiovascular events, heart failure, and renal toxicity, an international team of researchers led by Dr. Féline P.B. Kroon of the Leiden University Medical Center in the Netherlands noted in the review, published in the Journal of Rheumatology.
“It is therefore crucial to know whether the benefits offset the risks, especially because the therapy is often given for extended periods of time,” the researchers wrote in background information to the article (J Rheumatol. 2016. doi: 10.3899/jrheum.150721).
The investigators analyzed the evidence to assess the benefits and harms of NSAIDs in controlling symptoms, disease activity, and radiographic progression in patients with axSpA.
Reviewing 29 randomized, controlled trials and two “quasi” RCT studies in a pooled analyses, the research team found that, compared with placebo, both traditional and cyclooxygenase-2 (COX-2) NSAIDs were consistently more efficacious at 6 weeks and equally safe after 12 weeks.
The researchers also observed no significant differences in benefits or harms between the two NSAID classes. An increased number of neurologic events were initially observed for indomethacin, but the finding was not statistically significant when studies with high or unclear risk of bias were excluded.
Two single studies included in the review also suggested NSAIDs might retard radiographic progression in the spine in axSpA, especially in certain subgroups of patients such as those with high C-reactive protein. Although this would most likely be best achieved by continuous rather than on-demand use, the researchers said.
“The results of the review are in keeping with current recommendations that NSAIDs are appropriate first-line treatments of patients with axSpA with active disease before tumor necrosis factor inhibitor biologicals are applied,” they concluded.
However, they said it was surprising that they were unable to confirm the safety concerns associated with traditional NSAIDs and COX-2 NSAIDs.
This finding could mean that short-term use of either class of NSAID in this population of patients is not associated with an increased risk of GI or other adverse events.
But it could also be because most patients with ankylosing spondylitis are younger and have fewer comorbidities than patients with other rheumatic diseases.
The finding that people with ankylosing spondylitis have fewer adverse events with biologics, compared with patients with other rheumatic diseases, supported this theory, they said.
“It is technically still possible that lack of statistical power is at the basis of this, but we feel it is more likely that in the studied population and within the studied time frame (i.e., short-term), the risks of GI or cardiovascular toxicity are really not increased,” they wrote.
The review had several limitations, including that many trials were older (61% of the included studies were published before 1990) and there were not sufficient data to draw conclusions on long-term safety.
The findings of a recent Cochrane review back up guidelines that recommend NSAIDs as an appropriate first-line treatment for people with axial spondyloarthritis (axSpA).
NSAIDs have been associated with a variety of gastrointestinal effects and an increased risk of cardiovascular events, heart failure, and renal toxicity, an international team of researchers led by Dr. Féline P.B. Kroon of the Leiden University Medical Center in the Netherlands noted in the review, published in the Journal of Rheumatology.
“It is therefore crucial to know whether the benefits offset the risks, especially because the therapy is often given for extended periods of time,” the researchers wrote in background information to the article (J Rheumatol. 2016. doi: 10.3899/jrheum.150721).
The investigators analyzed the evidence to assess the benefits and harms of NSAIDs in controlling symptoms, disease activity, and radiographic progression in patients with axSpA.
Reviewing 29 randomized, controlled trials and two “quasi” RCT studies in a pooled analyses, the research team found that, compared with placebo, both traditional and cyclooxygenase-2 (COX-2) NSAIDs were consistently more efficacious at 6 weeks and equally safe after 12 weeks.
The researchers also observed no significant differences in benefits or harms between the two NSAID classes. An increased number of neurologic events were initially observed for indomethacin, but the finding was not statistically significant when studies with high or unclear risk of bias were excluded.
Two single studies included in the review also suggested NSAIDs might retard radiographic progression in the spine in axSpA, especially in certain subgroups of patients such as those with high C-reactive protein. Although this would most likely be best achieved by continuous rather than on-demand use, the researchers said.
“The results of the review are in keeping with current recommendations that NSAIDs are appropriate first-line treatments of patients with axSpA with active disease before tumor necrosis factor inhibitor biologicals are applied,” they concluded.
However, they said it was surprising that they were unable to confirm the safety concerns associated with traditional NSAIDs and COX-2 NSAIDs.
This finding could mean that short-term use of either class of NSAID in this population of patients is not associated with an increased risk of GI or other adverse events.
But it could also be because most patients with ankylosing spondylitis are younger and have fewer comorbidities than patients with other rheumatic diseases.
The finding that people with ankylosing spondylitis have fewer adverse events with biologics, compared with patients with other rheumatic diseases, supported this theory, they said.
“It is technically still possible that lack of statistical power is at the basis of this, but we feel it is more likely that in the studied population and within the studied time frame (i.e., short-term), the risks of GI or cardiovascular toxicity are really not increased,” they wrote.
The review had several limitations, including that many trials were older (61% of the included studies were published before 1990) and there were not sufficient data to draw conclusions on long-term safety.
The findings of a recent Cochrane review back up guidelines that recommend NSAIDs as an appropriate first-line treatment for people with axial spondyloarthritis (axSpA).
NSAIDs have been associated with a variety of gastrointestinal effects and an increased risk of cardiovascular events, heart failure, and renal toxicity, an international team of researchers led by Dr. Féline P.B. Kroon of the Leiden University Medical Center in the Netherlands noted in the review, published in the Journal of Rheumatology.
“It is therefore crucial to know whether the benefits offset the risks, especially because the therapy is often given for extended periods of time,” the researchers wrote in background information to the article (J Rheumatol. 2016. doi: 10.3899/jrheum.150721).
The investigators analyzed the evidence to assess the benefits and harms of NSAIDs in controlling symptoms, disease activity, and radiographic progression in patients with axSpA.
Reviewing 29 randomized, controlled trials and two “quasi” RCT studies in a pooled analyses, the research team found that, compared with placebo, both traditional and cyclooxygenase-2 (COX-2) NSAIDs were consistently more efficacious at 6 weeks and equally safe after 12 weeks.
The researchers also observed no significant differences in benefits or harms between the two NSAID classes. An increased number of neurologic events were initially observed for indomethacin, but the finding was not statistically significant when studies with high or unclear risk of bias were excluded.
Two single studies included in the review also suggested NSAIDs might retard radiographic progression in the spine in axSpA, especially in certain subgroups of patients such as those with high C-reactive protein. Although this would most likely be best achieved by continuous rather than on-demand use, the researchers said.
“The results of the review are in keeping with current recommendations that NSAIDs are appropriate first-line treatments of patients with axSpA with active disease before tumor necrosis factor inhibitor biologicals are applied,” they concluded.
However, they said it was surprising that they were unable to confirm the safety concerns associated with traditional NSAIDs and COX-2 NSAIDs.
This finding could mean that short-term use of either class of NSAID in this population of patients is not associated with an increased risk of GI or other adverse events.
But it could also be because most patients with ankylosing spondylitis are younger and have fewer comorbidities than patients with other rheumatic diseases.
The finding that people with ankylosing spondylitis have fewer adverse events with biologics, compared with patients with other rheumatic diseases, supported this theory, they said.
“It is technically still possible that lack of statistical power is at the basis of this, but we feel it is more likely that in the studied population and within the studied time frame (i.e., short-term), the risks of GI or cardiovascular toxicity are really not increased,” they wrote.
The review had several limitations, including that many trials were older (61% of the included studies were published before 1990) and there were not sufficient data to draw conclusions on long-term safety.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: Traditional and COX-2 NSAIDs are appropriate first-line treatments of patients with axSpA with active disease.
Major finding: Compared with placebo, both traditional and COX-2 NSAIDs were consistently more efficacious at 6 weeks and equally safe after 12 weeks.
Data source: A Cochrane review of 29 randomized, controlled trials and 2 “quasi” RCTs were included in the meta-analysis.
Disclosures: No conflicts of interest were declared.