User login
Study: No increased risk of serious AEs with combined urogyn/gyn onc surgery
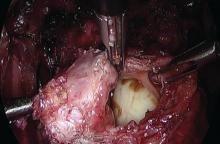
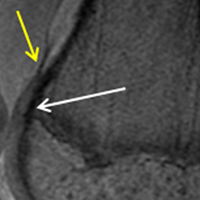
ORLANDO – Intraoperative and serious postoperative adverse events do not occur more frequently with concurrent urogynecologic and gynecologic oncology procedures versus the latter alone, but minor adverse events are more common, according to findings from a retrospective matched cohort study.
The study also showed that 10% of planned urogynecologic procedures are modified or abandoned at the time of gynecologic oncology surgery, Emily R. Davidson, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
“Concurrent cases were longer by 76 minutes, which is not surprising given that additional procedures were performed, and on univariate analysis there were differences in the frequency of multiple postoperative adverse events between the cohorts, including estimated blood loss, discharge with a Foley catheter, perioperative transfusion, postoperative pulmonary complications, ileus, renal failure, and urinary tract infection,” she said.
However, on multivariate analysis controlling for preoperative cardiovascular and pulmonary comorbidities, only urinary tract infection and discharge with a Foley catheter related to postoperative voiding dysfunction, which were significantly more common in the combined surgery group (26% vs. 7% and 35% vs. 1%, respectively), remained significantly different between the groups, she noted.
No differences were seen between the groups in length of hospital stay, reoperation, readmission within 1 month, surgical site infection, or death within 1 year of surgery, but patients undergoing concurrent procedures had more Clavien-Dindo grade 2 complications (44% vs. 19%), and this was primarily related to the prescription of antibiotics for urinary tract infections, she said.
As for the 11 cases (10%) with planned urogynecologic surgeries that were significantly changed or aborted at the time of gynecologic oncology surgery, 5 were because of intraoperative complications, 3 because of technical limitations, and 3 because of a change in oncologic care plan, including the need for postoperative radiation, she noted.
Case patients were women who underwent planned concurrent procedures at a large tertiary care center from January 2004 to June 2017. Of these women, 77% had stress urinary incontinence, 74% had pelvic organ prolapse (with 55% having stage 3 or 4 prolapse), 71% had prolapse repair – most commonly a native-tissue transvaginal colpopexy – as part of their procedure, and 74% had an anti-incontinence procedure – most commonly a transobturator sling.
The most common final histologic diagnosis was benign disease (in 51% of patients), and uterine cancer was the most common malignancy encountered (36% of patients), Dr. Davidson said, noting that other diagnoses included ovarian and vulvar cancer, in 12% and 1% of cases, respectively.
Most surgeries were minimally invasive abdominal hysterectomies (56%), followed by laparotomies in 32%, minor vaginal surgeries in 6%, laparoscopy without hysterectomy in 5%, and vaginal hysterectomy in 1%.
Controls were matched 2:1 based on surgeon, surgery date and invasiveness (surgical route), and final pathological diagnosis.
The median age of all patients was 59 years. Case patients undergoing concurrent procedures were more likely to be older (median of 64 vs. 57 years) and postmenopausal.
“Other statistically significant differences were that women undergoing combined surgery had higher vaginal parity, and were more likely to have undergone preoperative chemotherapy. They were also more likely to have a prior diagnosis of cardiovascular or pulmonary disease,” Dr. Davidson said.
“Women undergoing treatment for suspected gynecologic malignancy have the same or higher prevalence of pelvic floor disorders, compared with the general population, and they may choose to have combined surgery if both subspecialists are available,” she continued. “However, there are limited data regarding the incidence of adverse events in these concurrent procedures, or how often the planned urogynecology portion of the procedure is modified intraoperatively.”
Though limited by factors inherent in retrospective chart review, such as information bias at the time of data collection (which was mitigated by cross-checking data and having only two data collectors), the findings of the current study suggest that “concurrent urogynecologic and gynecologic oncology surgery should be offered to appropriate patients, as adding urogynecology surgery does not increase the risk of serious adverse events,” she concluded, adding that the study “highlights the importance of preoperative counseling, including discussion of the increased risk of minor postoperative adverse events, such as postoperative voiding symptoms and urinary tract infection, as well as discussion of the 10% risk of a change in intraoperative plan in the urogynecologic procedure.”
“This study certainly helps us know that we’re doing no harm [in offering concurrent surgery],” he said.
Dr. Davidson and Dr. Noone each reported having no relevant disclosures.
SOURCE: Davidson ER et al. SGS 2018, Oral Presentation 13.
ORLANDO – Intraoperative and serious postoperative adverse events do not occur more frequently with concurrent urogynecologic and gynecologic oncology procedures versus the latter alone, but minor adverse events are more common, according to findings from a retrospective matched cohort study.
The study also showed that 10% of planned urogynecologic procedures are modified or abandoned at the time of gynecologic oncology surgery, Emily R. Davidson, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
“Concurrent cases were longer by 76 minutes, which is not surprising given that additional procedures were performed, and on univariate analysis there were differences in the frequency of multiple postoperative adverse events between the cohorts, including estimated blood loss, discharge with a Foley catheter, perioperative transfusion, postoperative pulmonary complications, ileus, renal failure, and urinary tract infection,” she said.
However, on multivariate analysis controlling for preoperative cardiovascular and pulmonary comorbidities, only urinary tract infection and discharge with a Foley catheter related to postoperative voiding dysfunction, which were significantly more common in the combined surgery group (26% vs. 7% and 35% vs. 1%, respectively), remained significantly different between the groups, she noted.
No differences were seen between the groups in length of hospital stay, reoperation, readmission within 1 month, surgical site infection, or death within 1 year of surgery, but patients undergoing concurrent procedures had more Clavien-Dindo grade 2 complications (44% vs. 19%), and this was primarily related to the prescription of antibiotics for urinary tract infections, she said.
As for the 11 cases (10%) with planned urogynecologic surgeries that were significantly changed or aborted at the time of gynecologic oncology surgery, 5 were because of intraoperative complications, 3 because of technical limitations, and 3 because of a change in oncologic care plan, including the need for postoperative radiation, she noted.
Case patients were women who underwent planned concurrent procedures at a large tertiary care center from January 2004 to June 2017. Of these women, 77% had stress urinary incontinence, 74% had pelvic organ prolapse (with 55% having stage 3 or 4 prolapse), 71% had prolapse repair – most commonly a native-tissue transvaginal colpopexy – as part of their procedure, and 74% had an anti-incontinence procedure – most commonly a transobturator sling.
The most common final histologic diagnosis was benign disease (in 51% of patients), and uterine cancer was the most common malignancy encountered (36% of patients), Dr. Davidson said, noting that other diagnoses included ovarian and vulvar cancer, in 12% and 1% of cases, respectively.
Most surgeries were minimally invasive abdominal hysterectomies (56%), followed by laparotomies in 32%, minor vaginal surgeries in 6%, laparoscopy without hysterectomy in 5%, and vaginal hysterectomy in 1%.
Controls were matched 2:1 based on surgeon, surgery date and invasiveness (surgical route), and final pathological diagnosis.
The median age of all patients was 59 years. Case patients undergoing concurrent procedures were more likely to be older (median of 64 vs. 57 years) and postmenopausal.
“Other statistically significant differences were that women undergoing combined surgery had higher vaginal parity, and were more likely to have undergone preoperative chemotherapy. They were also more likely to have a prior diagnosis of cardiovascular or pulmonary disease,” Dr. Davidson said.
“Women undergoing treatment for suspected gynecologic malignancy have the same or higher prevalence of pelvic floor disorders, compared with the general population, and they may choose to have combined surgery if both subspecialists are available,” she continued. “However, there are limited data regarding the incidence of adverse events in these concurrent procedures, or how often the planned urogynecology portion of the procedure is modified intraoperatively.”
Though limited by factors inherent in retrospective chart review, such as information bias at the time of data collection (which was mitigated by cross-checking data and having only two data collectors), the findings of the current study suggest that “concurrent urogynecologic and gynecologic oncology surgery should be offered to appropriate patients, as adding urogynecology surgery does not increase the risk of serious adverse events,” she concluded, adding that the study “highlights the importance of preoperative counseling, including discussion of the increased risk of minor postoperative adverse events, such as postoperative voiding symptoms and urinary tract infection, as well as discussion of the 10% risk of a change in intraoperative plan in the urogynecologic procedure.”
“This study certainly helps us know that we’re doing no harm [in offering concurrent surgery],” he said.
Dr. Davidson and Dr. Noone each reported having no relevant disclosures.
SOURCE: Davidson ER et al. SGS 2018, Oral Presentation 13.
ORLANDO – Intraoperative and serious postoperative adverse events do not occur more frequently with concurrent urogynecologic and gynecologic oncology procedures versus the latter alone, but minor adverse events are more common, according to findings from a retrospective matched cohort study.
The study also showed that 10% of planned urogynecologic procedures are modified or abandoned at the time of gynecologic oncology surgery, Emily R. Davidson, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
“Concurrent cases were longer by 76 minutes, which is not surprising given that additional procedures were performed, and on univariate analysis there were differences in the frequency of multiple postoperative adverse events between the cohorts, including estimated blood loss, discharge with a Foley catheter, perioperative transfusion, postoperative pulmonary complications, ileus, renal failure, and urinary tract infection,” she said.
However, on multivariate analysis controlling for preoperative cardiovascular and pulmonary comorbidities, only urinary tract infection and discharge with a Foley catheter related to postoperative voiding dysfunction, which were significantly more common in the combined surgery group (26% vs. 7% and 35% vs. 1%, respectively), remained significantly different between the groups, she noted.
No differences were seen between the groups in length of hospital stay, reoperation, readmission within 1 month, surgical site infection, or death within 1 year of surgery, but patients undergoing concurrent procedures had more Clavien-Dindo grade 2 complications (44% vs. 19%), and this was primarily related to the prescription of antibiotics for urinary tract infections, she said.
As for the 11 cases (10%) with planned urogynecologic surgeries that were significantly changed or aborted at the time of gynecologic oncology surgery, 5 were because of intraoperative complications, 3 because of technical limitations, and 3 because of a change in oncologic care plan, including the need for postoperative radiation, she noted.
Case patients were women who underwent planned concurrent procedures at a large tertiary care center from January 2004 to June 2017. Of these women, 77% had stress urinary incontinence, 74% had pelvic organ prolapse (with 55% having stage 3 or 4 prolapse), 71% had prolapse repair – most commonly a native-tissue transvaginal colpopexy – as part of their procedure, and 74% had an anti-incontinence procedure – most commonly a transobturator sling.
The most common final histologic diagnosis was benign disease (in 51% of patients), and uterine cancer was the most common malignancy encountered (36% of patients), Dr. Davidson said, noting that other diagnoses included ovarian and vulvar cancer, in 12% and 1% of cases, respectively.
Most surgeries were minimally invasive abdominal hysterectomies (56%), followed by laparotomies in 32%, minor vaginal surgeries in 6%, laparoscopy without hysterectomy in 5%, and vaginal hysterectomy in 1%.
Controls were matched 2:1 based on surgeon, surgery date and invasiveness (surgical route), and final pathological diagnosis.
The median age of all patients was 59 years. Case patients undergoing concurrent procedures were more likely to be older (median of 64 vs. 57 years) and postmenopausal.
“Other statistically significant differences were that women undergoing combined surgery had higher vaginal parity, and were more likely to have undergone preoperative chemotherapy. They were also more likely to have a prior diagnosis of cardiovascular or pulmonary disease,” Dr. Davidson said.
“Women undergoing treatment for suspected gynecologic malignancy have the same or higher prevalence of pelvic floor disorders, compared with the general population, and they may choose to have combined surgery if both subspecialists are available,” she continued. “However, there are limited data regarding the incidence of adverse events in these concurrent procedures, or how often the planned urogynecology portion of the procedure is modified intraoperatively.”
Though limited by factors inherent in retrospective chart review, such as information bias at the time of data collection (which was mitigated by cross-checking data and having only two data collectors), the findings of the current study suggest that “concurrent urogynecologic and gynecologic oncology surgery should be offered to appropriate patients, as adding urogynecology surgery does not increase the risk of serious adverse events,” she concluded, adding that the study “highlights the importance of preoperative counseling, including discussion of the increased risk of minor postoperative adverse events, such as postoperative voiding symptoms and urinary tract infection, as well as discussion of the 10% risk of a change in intraoperative plan in the urogynecologic procedure.”
“This study certainly helps us know that we’re doing no harm [in offering concurrent surgery],” he said.
Dr. Davidson and Dr. Noone each reported having no relevant disclosures.
SOURCE: Davidson ER et al. SGS 2018, Oral Presentation 13.
REPORTING FROM SGS 2018
Key clinical point: Concurrent urogynecologic/gynecologic oncology surgery does not increase the risk of serious adverse events.
Major finding: Concurrent surgery patients had more grade 2 complications (44% vs. 19%).
Study details: A retrospective study of 108 cases and 216 matched controls.
Disclosures: Dr. Davidson and Dr. Noone each reported having no disclosures.
Source: Davidson ER et al. SGS 2018, Oral Presentation 13.
Failure to find cancer earlier; patient dies: $4.69M verdict
Failure to find cancer earlier; patient dies: $4.69M verdict
On July 19, a 26-year-old woman presented to the emergency department (ED) with abnormal vaginal bleeding 3 months after giving birth. She was found to have endometrial thickening and an elevated ß human chorionic gonadotropin level. An ObGyn (Dr. A) assumed that the patient was having a miscarriage and sent her home.
On July 30, when the patient returned to the ED with continued bleeding, lesions on her cervix and urethra were discovered. A second ObGyn, Dr. B, addressed the bleeding, removed the lesion, and ordered testing. On August 17, the patient saw a third ObGyn (Dr. C), who did not conduct an examination.
Days later, the patient suffered a brain hemorrhage that was suspicious for hemorrhagic metastasis. After that, stage IV choriocarcinoma was identified. Although she underwent chemotherapy, the patient died 18 months later.
ESTATE'S CLAIM: All 3 ObGyns failed to take a proper history, conduct adequate examinations, and order appropriate testing. Even at stage IV, 75% of patients with choriocarcinoma survive past 5 years. The stroke rendered chemotherapy less effective and substantially contributed to the patient's death. Failure to diagnose the cancer before the stroke allowed the disease to progress beyond the point at which the patient's life could be saved.
DEFENDANTS' DEFENSE: The ObGyns and hospital claimed that appropriate care was provided and that they were not negligent in failing to consider the diagnosis of a very rare form of cancer.
VERDICT: A $4.69 million New Jersey verdict was returned, with all 3 physicians held partially liable.
Hot speculum burns patient: $547,090 award
A 54-year-old woman underwent a hysterectomy performed at a government-operated hospital. After she was anesthetized and unconscious, a second-year resident took a speculum that had been placed in the sterile field by a nurse, and inserted it in the patient's vagina.
When the patient awoke from surgery, she discovered significant burns to her vaginal area, perineum, anus, and buttocks.
PATIENT'S CLAIM: The speculum had just been removed from the autoclave and was very hot. The patient incurred substantial medical bills to treat her injuries and was unable to work for several months. She sued the hospital and resident, alleging error by the nurse in placing the hot speculum in the sterile field without cooling it or advising the resident that it was still hot. The resident was blamed for using the speculum without confirming that it was hot.
DEFENDANTS' DEFENSE: The resident claimed that she reasonably relied on the nurse to not place a hot instrument in the surgical field without first cooling it. The hospital, representing the nurse, denied fault, blaming the resident for not checking the speculum.
VERDICT: A $547,090 Louisiana verdict was awarded by a judge against the resident and the hospital, but it was halved by comparative fault to $273,545.
Surgeon's breast exam insufficient: $375,000 verdict
After a woman in her early 40s found a lump in her left breast, she underwent a radiographic study, which a radiologist interpreted as showing a 3-mm cyst. Without performing additional tests, a general surgeon immediately scheduled her for surgery.
On May 17, the radiologist performed an ultrasound-guided needle-localized biopsy and found a nodule. The patient was immediately sent to the operating room where the surgeon performed a segmental resection of the nodule.
On May 24, the patient presented to the surgeon's office for a postoperative visit. She told the nurse that the palpable mass was still there. The nurse examined the mass, told the patient that the incision was healing nicely, and suggested follow-up in a month.
Four months later, the patient sought a second opinion. On September 15, she underwent a diagnostic mammogram, ultrasound, and biopsy. The biopsy was positive for invasive ductal carcinoma. On September 30, magnetic resonance imaging and a second biopsy further confirmed the diagnosis. On November 2, she underwent a segmental mastectomy with sentinel lymph node biopsy. The pathology report noted a 3-cm invasive ductal carcinoma with necrosis. The patient underwent chemotherapy and radiation treatment.
PATIENT'S CLAIM: She sued the general surgeon, radiologist, and surgical center, alleging that her breast cancer went undiagnosed. Prior to trial, the radiologist and surgical center were dismissed from the case.
The surgeon failed to perform a thorough physical examination and nodal evaluation of the left breast and axilla. His substandard methods to diagnose and treat the patient's breast cancer delayed proper treatment and significantly altered the outcome.
PHYSICIAN'S CLAIM: The surgeon's treatment met the standard of care. The outcome and treatment were not significantly changed by the delay.
VERDICT: A $375,000 Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Failure to find cancer earlier; patient dies: $4.69M verdict
On July 19, a 26-year-old woman presented to the emergency department (ED) with abnormal vaginal bleeding 3 months after giving birth. She was found to have endometrial thickening and an elevated ß human chorionic gonadotropin level. An ObGyn (Dr. A) assumed that the patient was having a miscarriage and sent her home.
On July 30, when the patient returned to the ED with continued bleeding, lesions on her cervix and urethra were discovered. A second ObGyn, Dr. B, addressed the bleeding, removed the lesion, and ordered testing. On August 17, the patient saw a third ObGyn (Dr. C), who did not conduct an examination.
Days later, the patient suffered a brain hemorrhage that was suspicious for hemorrhagic metastasis. After that, stage IV choriocarcinoma was identified. Although she underwent chemotherapy, the patient died 18 months later.
ESTATE'S CLAIM: All 3 ObGyns failed to take a proper history, conduct adequate examinations, and order appropriate testing. Even at stage IV, 75% of patients with choriocarcinoma survive past 5 years. The stroke rendered chemotherapy less effective and substantially contributed to the patient's death. Failure to diagnose the cancer before the stroke allowed the disease to progress beyond the point at which the patient's life could be saved.
DEFENDANTS' DEFENSE: The ObGyns and hospital claimed that appropriate care was provided and that they were not negligent in failing to consider the diagnosis of a very rare form of cancer.
VERDICT: A $4.69 million New Jersey verdict was returned, with all 3 physicians held partially liable.
Hot speculum burns patient: $547,090 award
A 54-year-old woman underwent a hysterectomy performed at a government-operated hospital. After she was anesthetized and unconscious, a second-year resident took a speculum that had been placed in the sterile field by a nurse, and inserted it in the patient's vagina.
When the patient awoke from surgery, she discovered significant burns to her vaginal area, perineum, anus, and buttocks.
PATIENT'S CLAIM: The speculum had just been removed from the autoclave and was very hot. The patient incurred substantial medical bills to treat her injuries and was unable to work for several months. She sued the hospital and resident, alleging error by the nurse in placing the hot speculum in the sterile field without cooling it or advising the resident that it was still hot. The resident was blamed for using the speculum without confirming that it was hot.
DEFENDANTS' DEFENSE: The resident claimed that she reasonably relied on the nurse to not place a hot instrument in the surgical field without first cooling it. The hospital, representing the nurse, denied fault, blaming the resident for not checking the speculum.
VERDICT: A $547,090 Louisiana verdict was awarded by a judge against the resident and the hospital, but it was halved by comparative fault to $273,545.
Surgeon's breast exam insufficient: $375,000 verdict
After a woman in her early 40s found a lump in her left breast, she underwent a radiographic study, which a radiologist interpreted as showing a 3-mm cyst. Without performing additional tests, a general surgeon immediately scheduled her for surgery.
On May 17, the radiologist performed an ultrasound-guided needle-localized biopsy and found a nodule. The patient was immediately sent to the operating room where the surgeon performed a segmental resection of the nodule.
On May 24, the patient presented to the surgeon's office for a postoperative visit. She told the nurse that the palpable mass was still there. The nurse examined the mass, told the patient that the incision was healing nicely, and suggested follow-up in a month.
Four months later, the patient sought a second opinion. On September 15, she underwent a diagnostic mammogram, ultrasound, and biopsy. The biopsy was positive for invasive ductal carcinoma. On September 30, magnetic resonance imaging and a second biopsy further confirmed the diagnosis. On November 2, she underwent a segmental mastectomy with sentinel lymph node biopsy. The pathology report noted a 3-cm invasive ductal carcinoma with necrosis. The patient underwent chemotherapy and radiation treatment.
PATIENT'S CLAIM: She sued the general surgeon, radiologist, and surgical center, alleging that her breast cancer went undiagnosed. Prior to trial, the radiologist and surgical center were dismissed from the case.
The surgeon failed to perform a thorough physical examination and nodal evaluation of the left breast and axilla. His substandard methods to diagnose and treat the patient's breast cancer delayed proper treatment and significantly altered the outcome.
PHYSICIAN'S CLAIM: The surgeon's treatment met the standard of care. The outcome and treatment were not significantly changed by the delay.
VERDICT: A $375,000 Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Failure to find cancer earlier; patient dies: $4.69M verdict
On July 19, a 26-year-old woman presented to the emergency department (ED) with abnormal vaginal bleeding 3 months after giving birth. She was found to have endometrial thickening and an elevated ß human chorionic gonadotropin level. An ObGyn (Dr. A) assumed that the patient was having a miscarriage and sent her home.
On July 30, when the patient returned to the ED with continued bleeding, lesions on her cervix and urethra were discovered. A second ObGyn, Dr. B, addressed the bleeding, removed the lesion, and ordered testing. On August 17, the patient saw a third ObGyn (Dr. C), who did not conduct an examination.
Days later, the patient suffered a brain hemorrhage that was suspicious for hemorrhagic metastasis. After that, stage IV choriocarcinoma was identified. Although she underwent chemotherapy, the patient died 18 months later.
ESTATE'S CLAIM: All 3 ObGyns failed to take a proper history, conduct adequate examinations, and order appropriate testing. Even at stage IV, 75% of patients with choriocarcinoma survive past 5 years. The stroke rendered chemotherapy less effective and substantially contributed to the patient's death. Failure to diagnose the cancer before the stroke allowed the disease to progress beyond the point at which the patient's life could be saved.
DEFENDANTS' DEFENSE: The ObGyns and hospital claimed that appropriate care was provided and that they were not negligent in failing to consider the diagnosis of a very rare form of cancer.
VERDICT: A $4.69 million New Jersey verdict was returned, with all 3 physicians held partially liable.
Hot speculum burns patient: $547,090 award
A 54-year-old woman underwent a hysterectomy performed at a government-operated hospital. After she was anesthetized and unconscious, a second-year resident took a speculum that had been placed in the sterile field by a nurse, and inserted it in the patient's vagina.
When the patient awoke from surgery, she discovered significant burns to her vaginal area, perineum, anus, and buttocks.
PATIENT'S CLAIM: The speculum had just been removed from the autoclave and was very hot. The patient incurred substantial medical bills to treat her injuries and was unable to work for several months. She sued the hospital and resident, alleging error by the nurse in placing the hot speculum in the sterile field without cooling it or advising the resident that it was still hot. The resident was blamed for using the speculum without confirming that it was hot.
DEFENDANTS' DEFENSE: The resident claimed that she reasonably relied on the nurse to not place a hot instrument in the surgical field without first cooling it. The hospital, representing the nurse, denied fault, blaming the resident for not checking the speculum.
VERDICT: A $547,090 Louisiana verdict was awarded by a judge against the resident and the hospital, but it was halved by comparative fault to $273,545.
Surgeon's breast exam insufficient: $375,000 verdict
After a woman in her early 40s found a lump in her left breast, she underwent a radiographic study, which a radiologist interpreted as showing a 3-mm cyst. Without performing additional tests, a general surgeon immediately scheduled her for surgery.
On May 17, the radiologist performed an ultrasound-guided needle-localized biopsy and found a nodule. The patient was immediately sent to the operating room where the surgeon performed a segmental resection of the nodule.
On May 24, the patient presented to the surgeon's office for a postoperative visit. She told the nurse that the palpable mass was still there. The nurse examined the mass, told the patient that the incision was healing nicely, and suggested follow-up in a month.
Four months later, the patient sought a second opinion. On September 15, she underwent a diagnostic mammogram, ultrasound, and biopsy. The biopsy was positive for invasive ductal carcinoma. On September 30, magnetic resonance imaging and a second biopsy further confirmed the diagnosis. On November 2, she underwent a segmental mastectomy with sentinel lymph node biopsy. The pathology report noted a 3-cm invasive ductal carcinoma with necrosis. The patient underwent chemotherapy and radiation treatment.
PATIENT'S CLAIM: She sued the general surgeon, radiologist, and surgical center, alleging that her breast cancer went undiagnosed. Prior to trial, the radiologist and surgical center were dismissed from the case.
The surgeon failed to perform a thorough physical examination and nodal evaluation of the left breast and axilla. His substandard methods to diagnose and treat the patient's breast cancer delayed proper treatment and significantly altered the outcome.
PHYSICIAN'S CLAIM: The surgeon's treatment met the standard of care. The outcome and treatment were not significantly changed by the delay.
VERDICT: A $375,000 Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
It costs what?! How we can educate residents and students on how much things cost
“Why are you ordering a CBC on the patient when her white blood cell count, hemoglobin, and platelets have been stable for the past 3 days?” sternly inquired the attending gynecologic oncologist. “Don’t order tests without any clinical indication. If she is infected or bleeding, there will be signs and thus an indication to order a CBC. The physical exam is your test.” There was an authoritative pause before he invoked the “value-based care” maxim.
For many residents who graduated in the past decade, education in value-based care and alternative payment models (APMs) was cobbled together from experience, demonstrated by attendings who labeled it as such, and from rare didactic education classroom sessions and inpatient environments.
In today’s health care environment, professional survival requires the ability to successfully deliver high-value care to patients. Attendings often illustrate and champion how to do this by using patient care to highlight the definition: Value = Quality ÷ Cost.
For residency education programs to create the ObGyns of the future, they must teach trainees what they will be evaluated on and held accountable for.1 Today’s clinicians will have to take responsibility for reigning in health care costs from the fee-for-service era, which in the United States have snowballed into one of the unhealthiest cost-to-outcomes ratios worldwide. Residents will be required to understand not only value but also areas in which they can influence the cost of care and how their outcome metrics are valued.
Modifiable factors in value-based care
As mentioned, value is defined by the equation, Value = Quality ÷ Cost. The granularity of these terms helps clarify the depth and the multitude of levels that clinicians can modify and influence to achieve the highest value.
Quality, as defined by the National Academy of Medicine, includes2:
- effectiveness: providing care processes and achieving outcomes as supported by scientific evidence
- efficiency: maximizing the quality of a comparable unit of health care delivered or unit of health benefit achieved for a given unit of health care resources used
- equity: providing health care of equal quality to those who may differ in personal characteristics other than their clinical condition or preferences for care
- patient-centeredness: meeting patient needs and preferences and providing education and support
- safety: actual or potential bodily harm
- timeliness: obtaining needed care while minimizing delays.
From electronic health records, which were mandated in the Patient Protection and Affordable Care Act of 2010, offices, hospitals, and medical systems have gained robust databases of mineable information. Even data abstraction from paper records has been made easier, allowing better reflection of practitioner-based delivery of care.
Understanding cost breakdown in the overall value equation
With regard to value-based care, cost is generally related to money. When broadly explored, however, cost can be broken down into cost to the patient, the health care system, and society this way:
- patient: time spent receiving evaluation and management from a clinician; money spent for family care needs while undergoing management; money spent for procedures and tests; wages lost due to appointments
- health system: preventive services versus costly emergency room visit; community-based interventions to improve population health
- society: cost to tax payers; equitable distribution of vital resources (for example, vaccines); prevention of iatrogenic antibiotic resistance.
To understand how physicians are paid, it is important to see how payers value our services. The Centers for Medicare and Medicaid Services states that it is “promoting value-based care as part of its larger quality strategy to reform how health care is delivered and paid for.” In 2018, the US Department of Health and Human Services is striving to have half of Medicare payments in APMs.3
It is the physician’s responsibility to recognize that costs to the patient, payer, health system, and society can compete with and directly influence the outcome of each other. For example, because the patient pays an insurance premium to participate in a risk pool where cost-sharing is the primary cost-containment strategy, poor-value interventions can directly translate into increased premiums, copayments, or deductibles for the entire pool.4
By clearly identifying the different variables involved in the value-based care equation, residents can better understand their responsibility in their day-to-day work in medicine to address value, not just quality or cost. Clarifying the tenets of value-based care will help guide educators in identifying “teaching moments” and organizing didactic sessions focused on practical implementation of value.
Less is more
In our opening anecdote, the attending shows how curbing overuse of resources can increase the value of care delivered. But that example illustrates only one of the many levels on which educators can help residents understand their impact on value. A multidisciplinary education that incorporates outpatient and inpatient pharmacists, social workers, occupational therapists, pelvic floor physiotherapists, office staff, billing specialists, operating room (OR) technologists, and others can be beneficial in learning how to deliver high-value care.
Read about selecting value-based interventions at work.
Value-based interventions at work
In the discussion that follows, we illustrate how residents can identify, evaluate, and put into practice value-based interventions that can occur at multiple levels.
Antibiotic selection. Resident choices for outpatient antibiotics can severely affect patient adherence. Subtle differences in the formulation of certain antibiotics affect the price and thus pose a significant potential obstacle. Judicious use of inexpensive drug formulations with fewer dosing frequencies can help patients engage in their own care.
Knowing the pharmacologic difference between doxycycline hyclate and doxycycline monohydrate, for example, is to know the difference between esoteric salts—undeniably worthless information with regard to successfully treating a patient’s infection. Knowing that one formula is on the bargain formulary at the patient’s local pharmacy, or that one drug requires twice-daily dosing versus 4-times-daily dosing, however, can mean the difference between the patient’s adherence or nonadherence to your expert recommendation.
Contraception options. Contraceptives pose a challenge with respect to value because of the myriad delivery systems, doses, and generic formulations available. There are dozens of oral contraceptive pills (OCPs) on the market that vary in their dosing, phasic nature (monophasic, multiphasic), iron content in the hormone-free week, and different progestogens for different conditions (such as drospirenone for androgen excess).
When weighing contraceptive options, the clinician must look at value not only from a cost perspective but also from an effectiveness perspective. The desired outcome in this scenario is preventing unwanted pregnancy with ideal or typical contraceptive use at the most inexpensive price point. When working within the value equation, the clinician must individualize the prescribed contraceptive to one that is most acceptable to the patient and that optimizes the various costs and quality measures. “Cost” can mean the cost of OCPs, menstrual control products, backup contraception, failed or unwanted pregnancy management, or suffering lost wages from missed days of work from, for example, dysmenorrhea. “Quality” can mean a low contraceptive failure rate, predictable cyclicality, the need for patient administration and the risk of forgetting, and the need for backup contraceptives.
In comparing the subdermal contraceptive implant (which can cost up to $1,300 every 3 years, equivalent to $36.11 per month) with OCPs (which can cost as low as $324 for 3 years for an ethinyl estradiol and norgestimate combination, or $9 per month), the OCPs significantly outweigh the implant in terms of cost. When comparing failure rates, the degree of patient intervention, and decreased use of menstrual control products due to amenorrhea, the subdermal contraceptive wins. As we know, long-acting reversible contraception (LARC), including the intrauterine device (IUD) and subdermal implant, is the most effective but often the most expensive contraceptive option.5 When cost is evaluated from a global perspective, as highlighted by the adage “an IUD is cheaper than a baby,” the LARC’s value is derived from its overall high effectiveness and low cost.
If the patient elects to choose OCPs, the clinician should direct the prescription to a pharmacy that has discounted generic pills on its formulary. Generic OCPs have a low- cost burden without loss of efficacy, thus providing maximal value.6 This requires an intimate knowledge of the local pharmacies and what their formularies provide. Sometimes the patient will need to drive out of her way to access cost-effective, quality medications, or the high-value option.
Surgery considerations. Judicious instrument selection in the OR can decrease overall operative costs. While most advanced sealing and cutting instrumentation is for single use, for example, it also can be reprocessed for reuse. Although the cost of reprocessed, single-use instruments is lower, studies evaluating the quality of these instruments “found a significant rate of physical defects, performance issues, or improper decontamination.”7
Marketing largely has driven physician choice in the use of certain vessel sealing and cutting devices, but there has yet to be evidence that using any one device actually improves performance or outcomes, such as length of surgery, blood loss, or postoperative complications. Technology companies that create these instruments likely will have to start designing studies to test performance and outcomes as they relate to their devices to persuade hospital systems that using their products improves outcomes and reduces costs.
While learning laparoscopic hysterectomy, residents may see that some attending surgeons can complete the entire procedure with monopolar scissors, bipolar forceps, and laparoscopic needle drivers, while other surgeons use those instruments plus others, such as a LigaSure instrument or a Harmonic scalpel. With outcomes being the same between these surgeons, it is reasonable for hospitals to audit each surgeon using the Value = Quality ÷ Cost equation and to seek data to describe why the latter surgeon requires additional instrumentation.
Residency training poses a unique opportunity for physicians to learn numerous ways to perform the same procedure so they can fill their armamentarium with various effective techniques. Residency also should be a time in which proficiency with basic surgical instrumentation is emphasized. Attending physicians can help residents improve their skills, for example, by having them use only one advanced sealing and cutting device, or no device at all. This practice will make the trainee better able to adapt to situations in which an advanced device may fail or be unavailable. Future performance metrics may evaluate the physician’s cost effectiveness with regard to single-use instruments during routine surgical procedures.
Standardized order sets. Evidence-based order sets help in the management of pneumonia, sepsis, deep vein thrombosis prophylaxis, and numerous other conditions. In the era of computerized physician order entry systems (CPOEs), a resident needs to enter just a few clicks to order all necessary tests, interventions, and imaging studies for a condition. In one fell swoop, orders are placed not only for admission but also for the patient’s entire hospitalization. The paradox of the order set is that it uses a template to deliver individualized patient-centered care.
In the age of enhanced recovery pathways after surgery, we see patients who undergo a hysterectomy being discharged home directly from the postoperative anesthesia care unit (PACU). Generally, follow-up laboratory testing is not ordered on an outpatient basis. If, however, the patient needs to remain in the hospital for social reasons (such as delayed PACU transfer, transportation, weather), she receives the standardized orders from the post hysterectomy order set: a morning complete blood count ($55) with a basic metabolic panel ($45). As an academic exercise, the order set may help residents learn which orders they must consider when admitting a postoperative hysterectomy patient, but overuse of order sets can be a setback for a value-based care system.
Read about evaluating competence and individualizing care.
Evaluating competence in value-based care
Research is an integral component of all residency programs accredited by the Accreditation Council for Graduate Medical Education (ACGME). The implementation of value-based care—with all its nuances, quality metrics, and cost parameters—creates a space for resident-led studies to contribute to peer education. The ACGME’s Obstetrics and Gynecology Milestones project was developed to assess the development of ObGyn residents’ competence as they progress through training. Despite national laws tying reimbursements to value-based care, there is no mention of value as it relates to the basic formula, Value = Quality ÷ Cost, in the project.
With the nuances that value-based care offers, it would behoove the Council on Resident Education in Obstetrics and Gynecology of the American College of Obstetricians and Gynecologists to incorporate a method of evaluation to determine competence in this evolving field.
Care also must be individualized
Academic ObGyns and instructors should focus their pedagogy not only on value-based care but also on individualized care that will maximize desired outcomes for each patient. Incorporating multidisciplinary didactics, focused research, and a 360-degree evaluation in the residency curriculum will create new ObGyns who are known for successfully delivering high-value care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Wieand E, Lagrew DC Jr. Value-based payment: what does it mean, and how can ObGyns get out ahead? OBG Manag. 2018;30(1):17–19, 25–26.
- Agency for Healthcare Research and Quality. The six domains of health care quality. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/sixdomains.html. Reviewed March 2016. Accessed March 22, 2018.
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Published January 26, 2015. Accessed March 22, 2018.
- Society for Human Resource Management. Managing health care costs. https://www.shrm.org/resourcesandtools/tools-and-samples/toolkits/pages/managinghealthcarecosts.aspx. Published January 11, 2017. Accessed March 18, 2018.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 642: Increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126(4):e44–e48.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 375: Brand versus generic oral contraceptives. Obstet Gynecol. 2007;110(2 pt 1):447–448.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 537: R
eprocessed single-use devices. Obstet Gynecol. 2012;120(4):974–976.
“Why are you ordering a CBC on the patient when her white blood cell count, hemoglobin, and platelets have been stable for the past 3 days?” sternly inquired the attending gynecologic oncologist. “Don’t order tests without any clinical indication. If she is infected or bleeding, there will be signs and thus an indication to order a CBC. The physical exam is your test.” There was an authoritative pause before he invoked the “value-based care” maxim.
For many residents who graduated in the past decade, education in value-based care and alternative payment models (APMs) was cobbled together from experience, demonstrated by attendings who labeled it as such, and from rare didactic education classroom sessions and inpatient environments.
In today’s health care environment, professional survival requires the ability to successfully deliver high-value care to patients. Attendings often illustrate and champion how to do this by using patient care to highlight the definition: Value = Quality ÷ Cost.
For residency education programs to create the ObGyns of the future, they must teach trainees what they will be evaluated on and held accountable for.1 Today’s clinicians will have to take responsibility for reigning in health care costs from the fee-for-service era, which in the United States have snowballed into one of the unhealthiest cost-to-outcomes ratios worldwide. Residents will be required to understand not only value but also areas in which they can influence the cost of care and how their outcome metrics are valued.
Modifiable factors in value-based care
As mentioned, value is defined by the equation, Value = Quality ÷ Cost. The granularity of these terms helps clarify the depth and the multitude of levels that clinicians can modify and influence to achieve the highest value.
Quality, as defined by the National Academy of Medicine, includes2:
- effectiveness: providing care processes and achieving outcomes as supported by scientific evidence
- efficiency: maximizing the quality of a comparable unit of health care delivered or unit of health benefit achieved for a given unit of health care resources used
- equity: providing health care of equal quality to those who may differ in personal characteristics other than their clinical condition or preferences for care
- patient-centeredness: meeting patient needs and preferences and providing education and support
- safety: actual or potential bodily harm
- timeliness: obtaining needed care while minimizing delays.
From electronic health records, which were mandated in the Patient Protection and Affordable Care Act of 2010, offices, hospitals, and medical systems have gained robust databases of mineable information. Even data abstraction from paper records has been made easier, allowing better reflection of practitioner-based delivery of care.
Understanding cost breakdown in the overall value equation
With regard to value-based care, cost is generally related to money. When broadly explored, however, cost can be broken down into cost to the patient, the health care system, and society this way:
- patient: time spent receiving evaluation and management from a clinician; money spent for family care needs while undergoing management; money spent for procedures and tests; wages lost due to appointments
- health system: preventive services versus costly emergency room visit; community-based interventions to improve population health
- society: cost to tax payers; equitable distribution of vital resources (for example, vaccines); prevention of iatrogenic antibiotic resistance.
To understand how physicians are paid, it is important to see how payers value our services. The Centers for Medicare and Medicaid Services states that it is “promoting value-based care as part of its larger quality strategy to reform how health care is delivered and paid for.” In 2018, the US Department of Health and Human Services is striving to have half of Medicare payments in APMs.3
It is the physician’s responsibility to recognize that costs to the patient, payer, health system, and society can compete with and directly influence the outcome of each other. For example, because the patient pays an insurance premium to participate in a risk pool where cost-sharing is the primary cost-containment strategy, poor-value interventions can directly translate into increased premiums, copayments, or deductibles for the entire pool.4
By clearly identifying the different variables involved in the value-based care equation, residents can better understand their responsibility in their day-to-day work in medicine to address value, not just quality or cost. Clarifying the tenets of value-based care will help guide educators in identifying “teaching moments” and organizing didactic sessions focused on practical implementation of value.
Less is more
In our opening anecdote, the attending shows how curbing overuse of resources can increase the value of care delivered. But that example illustrates only one of the many levels on which educators can help residents understand their impact on value. A multidisciplinary education that incorporates outpatient and inpatient pharmacists, social workers, occupational therapists, pelvic floor physiotherapists, office staff, billing specialists, operating room (OR) technologists, and others can be beneficial in learning how to deliver high-value care.
Read about selecting value-based interventions at work.
Value-based interventions at work
In the discussion that follows, we illustrate how residents can identify, evaluate, and put into practice value-based interventions that can occur at multiple levels.
Antibiotic selection. Resident choices for outpatient antibiotics can severely affect patient adherence. Subtle differences in the formulation of certain antibiotics affect the price and thus pose a significant potential obstacle. Judicious use of inexpensive drug formulations with fewer dosing frequencies can help patients engage in their own care.
Knowing the pharmacologic difference between doxycycline hyclate and doxycycline monohydrate, for example, is to know the difference between esoteric salts—undeniably worthless information with regard to successfully treating a patient’s infection. Knowing that one formula is on the bargain formulary at the patient’s local pharmacy, or that one drug requires twice-daily dosing versus 4-times-daily dosing, however, can mean the difference between the patient’s adherence or nonadherence to your expert recommendation.
Contraception options. Contraceptives pose a challenge with respect to value because of the myriad delivery systems, doses, and generic formulations available. There are dozens of oral contraceptive pills (OCPs) on the market that vary in their dosing, phasic nature (monophasic, multiphasic), iron content in the hormone-free week, and different progestogens for different conditions (such as drospirenone for androgen excess).
When weighing contraceptive options, the clinician must look at value not only from a cost perspective but also from an effectiveness perspective. The desired outcome in this scenario is preventing unwanted pregnancy with ideal or typical contraceptive use at the most inexpensive price point. When working within the value equation, the clinician must individualize the prescribed contraceptive to one that is most acceptable to the patient and that optimizes the various costs and quality measures. “Cost” can mean the cost of OCPs, menstrual control products, backup contraception, failed or unwanted pregnancy management, or suffering lost wages from missed days of work from, for example, dysmenorrhea. “Quality” can mean a low contraceptive failure rate, predictable cyclicality, the need for patient administration and the risk of forgetting, and the need for backup contraceptives.
In comparing the subdermal contraceptive implant (which can cost up to $1,300 every 3 years, equivalent to $36.11 per month) with OCPs (which can cost as low as $324 for 3 years for an ethinyl estradiol and norgestimate combination, or $9 per month), the OCPs significantly outweigh the implant in terms of cost. When comparing failure rates, the degree of patient intervention, and decreased use of menstrual control products due to amenorrhea, the subdermal contraceptive wins. As we know, long-acting reversible contraception (LARC), including the intrauterine device (IUD) and subdermal implant, is the most effective but often the most expensive contraceptive option.5 When cost is evaluated from a global perspective, as highlighted by the adage “an IUD is cheaper than a baby,” the LARC’s value is derived from its overall high effectiveness and low cost.
If the patient elects to choose OCPs, the clinician should direct the prescription to a pharmacy that has discounted generic pills on its formulary. Generic OCPs have a low- cost burden without loss of efficacy, thus providing maximal value.6 This requires an intimate knowledge of the local pharmacies and what their formularies provide. Sometimes the patient will need to drive out of her way to access cost-effective, quality medications, or the high-value option.
Surgery considerations. Judicious instrument selection in the OR can decrease overall operative costs. While most advanced sealing and cutting instrumentation is for single use, for example, it also can be reprocessed for reuse. Although the cost of reprocessed, single-use instruments is lower, studies evaluating the quality of these instruments “found a significant rate of physical defects, performance issues, or improper decontamination.”7
Marketing largely has driven physician choice in the use of certain vessel sealing and cutting devices, but there has yet to be evidence that using any one device actually improves performance or outcomes, such as length of surgery, blood loss, or postoperative complications. Technology companies that create these instruments likely will have to start designing studies to test performance and outcomes as they relate to their devices to persuade hospital systems that using their products improves outcomes and reduces costs.
While learning laparoscopic hysterectomy, residents may see that some attending surgeons can complete the entire procedure with monopolar scissors, bipolar forceps, and laparoscopic needle drivers, while other surgeons use those instruments plus others, such as a LigaSure instrument or a Harmonic scalpel. With outcomes being the same between these surgeons, it is reasonable for hospitals to audit each surgeon using the Value = Quality ÷ Cost equation and to seek data to describe why the latter surgeon requires additional instrumentation.
Residency training poses a unique opportunity for physicians to learn numerous ways to perform the same procedure so they can fill their armamentarium with various effective techniques. Residency also should be a time in which proficiency with basic surgical instrumentation is emphasized. Attending physicians can help residents improve their skills, for example, by having them use only one advanced sealing and cutting device, or no device at all. This practice will make the trainee better able to adapt to situations in which an advanced device may fail or be unavailable. Future performance metrics may evaluate the physician’s cost effectiveness with regard to single-use instruments during routine surgical procedures.
Standardized order sets. Evidence-based order sets help in the management of pneumonia, sepsis, deep vein thrombosis prophylaxis, and numerous other conditions. In the era of computerized physician order entry systems (CPOEs), a resident needs to enter just a few clicks to order all necessary tests, interventions, and imaging studies for a condition. In one fell swoop, orders are placed not only for admission but also for the patient’s entire hospitalization. The paradox of the order set is that it uses a template to deliver individualized patient-centered care.
In the age of enhanced recovery pathways after surgery, we see patients who undergo a hysterectomy being discharged home directly from the postoperative anesthesia care unit (PACU). Generally, follow-up laboratory testing is not ordered on an outpatient basis. If, however, the patient needs to remain in the hospital for social reasons (such as delayed PACU transfer, transportation, weather), she receives the standardized orders from the post hysterectomy order set: a morning complete blood count ($55) with a basic metabolic panel ($45). As an academic exercise, the order set may help residents learn which orders they must consider when admitting a postoperative hysterectomy patient, but overuse of order sets can be a setback for a value-based care system.
Read about evaluating competence and individualizing care.
Evaluating competence in value-based care
Research is an integral component of all residency programs accredited by the Accreditation Council for Graduate Medical Education (ACGME). The implementation of value-based care—with all its nuances, quality metrics, and cost parameters—creates a space for resident-led studies to contribute to peer education. The ACGME’s Obstetrics and Gynecology Milestones project was developed to assess the development of ObGyn residents’ competence as they progress through training. Despite national laws tying reimbursements to value-based care, there is no mention of value as it relates to the basic formula, Value = Quality ÷ Cost, in the project.
With the nuances that value-based care offers, it would behoove the Council on Resident Education in Obstetrics and Gynecology of the American College of Obstetricians and Gynecologists to incorporate a method of evaluation to determine competence in this evolving field.
Care also must be individualized
Academic ObGyns and instructors should focus their pedagogy not only on value-based care but also on individualized care that will maximize desired outcomes for each patient. Incorporating multidisciplinary didactics, focused research, and a 360-degree evaluation in the residency curriculum will create new ObGyns who are known for successfully delivering high-value care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
“Why are you ordering a CBC on the patient when her white blood cell count, hemoglobin, and platelets have been stable for the past 3 days?” sternly inquired the attending gynecologic oncologist. “Don’t order tests without any clinical indication. If she is infected or bleeding, there will be signs and thus an indication to order a CBC. The physical exam is your test.” There was an authoritative pause before he invoked the “value-based care” maxim.
For many residents who graduated in the past decade, education in value-based care and alternative payment models (APMs) was cobbled together from experience, demonstrated by attendings who labeled it as such, and from rare didactic education classroom sessions and inpatient environments.
In today’s health care environment, professional survival requires the ability to successfully deliver high-value care to patients. Attendings often illustrate and champion how to do this by using patient care to highlight the definition: Value = Quality ÷ Cost.
For residency education programs to create the ObGyns of the future, they must teach trainees what they will be evaluated on and held accountable for.1 Today’s clinicians will have to take responsibility for reigning in health care costs from the fee-for-service era, which in the United States have snowballed into one of the unhealthiest cost-to-outcomes ratios worldwide. Residents will be required to understand not only value but also areas in which they can influence the cost of care and how their outcome metrics are valued.
Modifiable factors in value-based care
As mentioned, value is defined by the equation, Value = Quality ÷ Cost. The granularity of these terms helps clarify the depth and the multitude of levels that clinicians can modify and influence to achieve the highest value.
Quality, as defined by the National Academy of Medicine, includes2:
- effectiveness: providing care processes and achieving outcomes as supported by scientific evidence
- efficiency: maximizing the quality of a comparable unit of health care delivered or unit of health benefit achieved for a given unit of health care resources used
- equity: providing health care of equal quality to those who may differ in personal characteristics other than their clinical condition or preferences for care
- patient-centeredness: meeting patient needs and preferences and providing education and support
- safety: actual or potential bodily harm
- timeliness: obtaining needed care while minimizing delays.
From electronic health records, which were mandated in the Patient Protection and Affordable Care Act of 2010, offices, hospitals, and medical systems have gained robust databases of mineable information. Even data abstraction from paper records has been made easier, allowing better reflection of practitioner-based delivery of care.
Understanding cost breakdown in the overall value equation
With regard to value-based care, cost is generally related to money. When broadly explored, however, cost can be broken down into cost to the patient, the health care system, and society this way:
- patient: time spent receiving evaluation and management from a clinician; money spent for family care needs while undergoing management; money spent for procedures and tests; wages lost due to appointments
- health system: preventive services versus costly emergency room visit; community-based interventions to improve population health
- society: cost to tax payers; equitable distribution of vital resources (for example, vaccines); prevention of iatrogenic antibiotic resistance.
To understand how physicians are paid, it is important to see how payers value our services. The Centers for Medicare and Medicaid Services states that it is “promoting value-based care as part of its larger quality strategy to reform how health care is delivered and paid for.” In 2018, the US Department of Health and Human Services is striving to have half of Medicare payments in APMs.3
It is the physician’s responsibility to recognize that costs to the patient, payer, health system, and society can compete with and directly influence the outcome of each other. For example, because the patient pays an insurance premium to participate in a risk pool where cost-sharing is the primary cost-containment strategy, poor-value interventions can directly translate into increased premiums, copayments, or deductibles for the entire pool.4
By clearly identifying the different variables involved in the value-based care equation, residents can better understand their responsibility in their day-to-day work in medicine to address value, not just quality or cost. Clarifying the tenets of value-based care will help guide educators in identifying “teaching moments” and organizing didactic sessions focused on practical implementation of value.
Less is more
In our opening anecdote, the attending shows how curbing overuse of resources can increase the value of care delivered. But that example illustrates only one of the many levels on which educators can help residents understand their impact on value. A multidisciplinary education that incorporates outpatient and inpatient pharmacists, social workers, occupational therapists, pelvic floor physiotherapists, office staff, billing specialists, operating room (OR) technologists, and others can be beneficial in learning how to deliver high-value care.
Read about selecting value-based interventions at work.
Value-based interventions at work
In the discussion that follows, we illustrate how residents can identify, evaluate, and put into practice value-based interventions that can occur at multiple levels.
Antibiotic selection. Resident choices for outpatient antibiotics can severely affect patient adherence. Subtle differences in the formulation of certain antibiotics affect the price and thus pose a significant potential obstacle. Judicious use of inexpensive drug formulations with fewer dosing frequencies can help patients engage in their own care.
Knowing the pharmacologic difference between doxycycline hyclate and doxycycline monohydrate, for example, is to know the difference between esoteric salts—undeniably worthless information with regard to successfully treating a patient’s infection. Knowing that one formula is on the bargain formulary at the patient’s local pharmacy, or that one drug requires twice-daily dosing versus 4-times-daily dosing, however, can mean the difference between the patient’s adherence or nonadherence to your expert recommendation.
Contraception options. Contraceptives pose a challenge with respect to value because of the myriad delivery systems, doses, and generic formulations available. There are dozens of oral contraceptive pills (OCPs) on the market that vary in their dosing, phasic nature (monophasic, multiphasic), iron content in the hormone-free week, and different progestogens for different conditions (such as drospirenone for androgen excess).
When weighing contraceptive options, the clinician must look at value not only from a cost perspective but also from an effectiveness perspective. The desired outcome in this scenario is preventing unwanted pregnancy with ideal or typical contraceptive use at the most inexpensive price point. When working within the value equation, the clinician must individualize the prescribed contraceptive to one that is most acceptable to the patient and that optimizes the various costs and quality measures. “Cost” can mean the cost of OCPs, menstrual control products, backup contraception, failed or unwanted pregnancy management, or suffering lost wages from missed days of work from, for example, dysmenorrhea. “Quality” can mean a low contraceptive failure rate, predictable cyclicality, the need for patient administration and the risk of forgetting, and the need for backup contraceptives.
In comparing the subdermal contraceptive implant (which can cost up to $1,300 every 3 years, equivalent to $36.11 per month) with OCPs (which can cost as low as $324 for 3 years for an ethinyl estradiol and norgestimate combination, or $9 per month), the OCPs significantly outweigh the implant in terms of cost. When comparing failure rates, the degree of patient intervention, and decreased use of menstrual control products due to amenorrhea, the subdermal contraceptive wins. As we know, long-acting reversible contraception (LARC), including the intrauterine device (IUD) and subdermal implant, is the most effective but often the most expensive contraceptive option.5 When cost is evaluated from a global perspective, as highlighted by the adage “an IUD is cheaper than a baby,” the LARC’s value is derived from its overall high effectiveness and low cost.
If the patient elects to choose OCPs, the clinician should direct the prescription to a pharmacy that has discounted generic pills on its formulary. Generic OCPs have a low- cost burden without loss of efficacy, thus providing maximal value.6 This requires an intimate knowledge of the local pharmacies and what their formularies provide. Sometimes the patient will need to drive out of her way to access cost-effective, quality medications, or the high-value option.
Surgery considerations. Judicious instrument selection in the OR can decrease overall operative costs. While most advanced sealing and cutting instrumentation is for single use, for example, it also can be reprocessed for reuse. Although the cost of reprocessed, single-use instruments is lower, studies evaluating the quality of these instruments “found a significant rate of physical defects, performance issues, or improper decontamination.”7
Marketing largely has driven physician choice in the use of certain vessel sealing and cutting devices, but there has yet to be evidence that using any one device actually improves performance or outcomes, such as length of surgery, blood loss, or postoperative complications. Technology companies that create these instruments likely will have to start designing studies to test performance and outcomes as they relate to their devices to persuade hospital systems that using their products improves outcomes and reduces costs.
While learning laparoscopic hysterectomy, residents may see that some attending surgeons can complete the entire procedure with monopolar scissors, bipolar forceps, and laparoscopic needle drivers, while other surgeons use those instruments plus others, such as a LigaSure instrument or a Harmonic scalpel. With outcomes being the same between these surgeons, it is reasonable for hospitals to audit each surgeon using the Value = Quality ÷ Cost equation and to seek data to describe why the latter surgeon requires additional instrumentation.
Residency training poses a unique opportunity for physicians to learn numerous ways to perform the same procedure so they can fill their armamentarium with various effective techniques. Residency also should be a time in which proficiency with basic surgical instrumentation is emphasized. Attending physicians can help residents improve their skills, for example, by having them use only one advanced sealing and cutting device, or no device at all. This practice will make the trainee better able to adapt to situations in which an advanced device may fail or be unavailable. Future performance metrics may evaluate the physician’s cost effectiveness with regard to single-use instruments during routine surgical procedures.
Standardized order sets. Evidence-based order sets help in the management of pneumonia, sepsis, deep vein thrombosis prophylaxis, and numerous other conditions. In the era of computerized physician order entry systems (CPOEs), a resident needs to enter just a few clicks to order all necessary tests, interventions, and imaging studies for a condition. In one fell swoop, orders are placed not only for admission but also for the patient’s entire hospitalization. The paradox of the order set is that it uses a template to deliver individualized patient-centered care.
In the age of enhanced recovery pathways after surgery, we see patients who undergo a hysterectomy being discharged home directly from the postoperative anesthesia care unit (PACU). Generally, follow-up laboratory testing is not ordered on an outpatient basis. If, however, the patient needs to remain in the hospital for social reasons (such as delayed PACU transfer, transportation, weather), she receives the standardized orders from the post hysterectomy order set: a morning complete blood count ($55) with a basic metabolic panel ($45). As an academic exercise, the order set may help residents learn which orders they must consider when admitting a postoperative hysterectomy patient, but overuse of order sets can be a setback for a value-based care system.
Read about evaluating competence and individualizing care.
Evaluating competence in value-based care
Research is an integral component of all residency programs accredited by the Accreditation Council for Graduate Medical Education (ACGME). The implementation of value-based care—with all its nuances, quality metrics, and cost parameters—creates a space for resident-led studies to contribute to peer education. The ACGME’s Obstetrics and Gynecology Milestones project was developed to assess the development of ObGyn residents’ competence as they progress through training. Despite national laws tying reimbursements to value-based care, there is no mention of value as it relates to the basic formula, Value = Quality ÷ Cost, in the project.
With the nuances that value-based care offers, it would behoove the Council on Resident Education in Obstetrics and Gynecology of the American College of Obstetricians and Gynecologists to incorporate a method of evaluation to determine competence in this evolving field.
Care also must be individualized
Academic ObGyns and instructors should focus their pedagogy not only on value-based care but also on individualized care that will maximize desired outcomes for each patient. Incorporating multidisciplinary didactics, focused research, and a 360-degree evaluation in the residency curriculum will create new ObGyns who are known for successfully delivering high-value care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Wieand E, Lagrew DC Jr. Value-based payment: what does it mean, and how can ObGyns get out ahead? OBG Manag. 2018;30(1):17–19, 25–26.
- Agency for Healthcare Research and Quality. The six domains of health care quality. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/sixdomains.html. Reviewed March 2016. Accessed March 22, 2018.
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Published January 26, 2015. Accessed March 22, 2018.
- Society for Human Resource Management. Managing health care costs. https://www.shrm.org/resourcesandtools/tools-and-samples/toolkits/pages/managinghealthcarecosts.aspx. Published January 11, 2017. Accessed March 18, 2018.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 642: Increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126(4):e44–e48.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 375: Brand versus generic oral contraceptives. Obstet Gynecol. 2007;110(2 pt 1):447–448.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 537: R
eprocessed single-use devices. Obstet Gynecol. 2012;120(4):974–976.
- Wieand E, Lagrew DC Jr. Value-based payment: what does it mean, and how can ObGyns get out ahead? OBG Manag. 2018;30(1):17–19, 25–26.
- Agency for Healthcare Research and Quality. The six domains of health care quality. https://www.ahrq.gov/professionals/quality-patient-safety/talkingquality/create/sixdomains.html. Reviewed March 2016. Accessed March 22, 2018.
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Published January 26, 2015. Accessed March 22, 2018.
- Society for Human Resource Management. Managing health care costs. https://www.shrm.org/resourcesandtools/tools-and-samples/toolkits/pages/managinghealthcarecosts.aspx. Published January 11, 2017. Accessed March 18, 2018.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 642: Increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126(4):e44–e48.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 375: Brand versus generic oral contraceptives. Obstet Gynecol. 2007;110(2 pt 1):447–448.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Committee Opinion No. 537: R
eprocessed single-use devices. Obstet Gynecol. 2012;120(4):974–976.
Read all parts of this series
PART 1 Value-based payment: What does it mean and how can ObGyns get out ahead
PART 2 What makes a “quality” quality measure?
PART 3 The role of patient-reported outcomes in women’s health
PART 4 It costs what?! How we can educate residents and students on how much things cost
Optimal surgical management of stage 3 and 4 pelvic organ prolapse
Effective surgical management of advanced pelvic organ prolapse (POP) depends on prolapse location and stage, presence of urinary incontinence, need for hysterectomy, the patient’s desire to maintain sexual function, type of surgery, and the surgeon’s skill and experience, among other factors. For these reasons, POP repair is not a one-size-fits all procedure.
In this article, experts in minimally invasive prolapse repair offer their perspectives on 3 surgical approaches: use of native tissue (Drs. White, Aguilar, and Rogers), abdominal sacrocolpopexy (Drs. Huber and Culligan), and transvaginal mesh (Drs. Lucente and Ton). They evaluate the evidence on these procedures and provide recommendations based on their experience of best practices for achieving surgical success and minimizing adverse events.
Using native tissue for vaginal anatomy repair
Amanda White, MD; Vivian Aguilar, MD; and Rebecca G. Rogers, MD
Dr. Rogers reports that she receives royalties from UpToDate. Drs. White and Aguilar report no financial relationships relevant to this article.
Surgical therapy is the mainstay of treatment for POP, and 20% of US women will undergo prolapse and/or stress incontinence surgery by age 80.1 Prolapse surgery either restores the vaginal anatomy (reconstructive surgery) or obliterates the vaginal canal (obliterative surgery). Vaginal reconstruction can be performed using the patient's native tissue or mesh. Because of concerns associated with mesh use, native tissue repairs continue to be commonly performed.
Unfortunately, not all prolapse surgeries result in prolapse cure, and recurrent prolapse that necessitates repeat operation is not rare, regardless of whether or not mesh is used.2,3 Native tissue repairs are most commonly performed through the vaginal route, the first minimally invasive approach to prolapse surgery. Restoration of the vaginal apex has been identified as critically important in these surgeries. Apical native tissue repairs include reconstructive procedures, such as sacrospinous ligament suspension (SSLS) or uterosacral ligament suspension (USLS), and obliterative procedures, such as colpocleisis.
In this discussion, we present 2 case vignettes that highlight surgical decision making for repair of stage 3 or 4 pelvic organ prolapse utilizing these techniques.
- Native tissue repair offers a minimally invasive approach to prolapse repair.
- Sacrospinous and uterosacral ligament suspensions have equivalent success rates.
- Prophylactic midurethral slings reduce postoperative incontinence at the time of transvaginal native tissue repair.
- Hysterectomy at the time of colpocleisis should not be performed routinely.
CASE 1 Active woman with prolapse
A 65-year-old woman (G2P2) presents with stage 3 prolapse, with the anterior compartment at +3 and the cervix at the hymen with straining. She is sexually active and desires to retain coital function. A trial of pessary has failed.
What surgical options can be considered for this patient?
Reconstruction procedures for prolapse
This patient presents with a typical configuration of prolapse; the anterior and apical compartments are the most likely to prolapse.4 Importantly, conservative management of her prolapse has failed. While it is not required that women have a trial with pessary prior to undergoing surgery, all women should be offered conservative management of prolapse, according to the American Urogynecologic Society (AUGS) and the American College of Obstetricians and Gynecologists (ACOG).4,5
Apical suspension
Since this patient desires to retain coital function, her gynecologist recommends a reconstructive procedure. The combination of apical and anterior vaginal wall prolapse will require an apical suspension procedure (FIGURES 1 and 2). If suspension of the apex does not correct the anterior wall prolapse, the patient also may require anterior compartment reconstruction.
The 2 most commonly performed native tissue apical suspension procedures, SSLS and USLS, have equivalent outcomes at 2 years, according to a multicenter randomized trial.6 Therefore, the choice of procedure is at the surgeon's discretion. USLS is most commonly performed at the time of hysterectomy via an intraperitoneal approach, while SSLS is often selected for posthysterectomy vault prolapse, given its extraperitoneal location.
Suture type. Whether to use permanent suture at the time of SSLS or USLS is controversial. Some data suggest that permanent suture provides greater long-term success compared with delayed absorbable suture.7 However, permanent suture has been reported to be associated with higher rates of suture complications--up to 44% in USLS and 36% in SSLS--compared with a 3.5% complication rate in a USLS cohort treated with absorbable suture.8-10
Hysterectomy versus hysteropexy. Considerable debate exists regarding whether a patient requires hysterectomy at the time of prolapse repair. In a randomized trial at 12 months' follow-up, uterine preservation by sacrospinous hysteropexy was noninferior to vaginal hysterectomy with suspension of the uterosacral ligaments for surgical failure of the apical compartment.11 A recent meta-analysis found that apical failure rates after sacrospinous hysteropexy versus vaginal hysterectomy were not different.12 Repeat surgery rates for prolapse also were not different between groups. The most significant disadvantage of uterine-preservation prolapse surgery, when compared with hysterectomy, is the lack of prevention and diagnosis of uterine malignancy.12 From 2002 to 2012, rates of hysteropexy significantly increased in the United States, although rates remain low.13
Sling procedure pros and cons. This case patient did not report urinary incontinence, but she may develop incontinence with reduction of the anterior wall prolapse. A large randomized controlled trial that included 337 women compared sling with no sling procedures among women with prolapse undergoing transvaginal prolapse repair.14 Management with a prophylactic sling resulted in less incontinence (27.3% and 43.0%, respectively, at 12 months postoperatively) but higher rates of urinary tract infection (31.0% vs 18.3%), major bleeding complications (3.1% vs 0%), and incomplete bladder emptying 6 weeks after surgery (3.7% vs 0%) (P≤.05 for all).14
CASE 1 Recommendations for this patient
For this case, we would offer the patient a transvaginal hysterectomy and USLS. At the time of repair, we would assess whether she needed an anterior repair as well. We would offer a prophylactic sling procedure and also would discuss the risks and benefits of concomitant versus interval incontinence procedures.
CASE 2 Elderly woman with severe prolapse
An 85-year-old woman (G3P3) presents with procidentia, or complete eversion of the vagina, with the cervix 10 cm outside of the hymen. She has difficulty voiding, and the prolapse is uncomfortable when walking. A trial of pessary has failed. The patient denies vaginal bleeding. She is not sexually active and does not desire to retain coital function.
What treatment options would be appropriate for this patient?
Obliterative surgery
This elderly patient presents with advanced pelvic organ prolapse, and conservative management has failed. She is not sexually active and does not desire coital function in the future, so an obliterative procedure is indicated. Colpocleisis is a minimally invasive procedure that has cure rates ranging from 91% to 100%.15 It is likely that this patient's voiding dysfunction will improve after surgery and that she will be highly satisfied with the surgery.16
The question of hysterectomy with colpocleisis
The role of hysterectomy at the time of colpocleisis is controversial. LeFort colpocleisis preserves the uterus, with the anterior and posterior vaginal walls sutured together (FIGURE 3). Hysterectomy at the time of vaginal closure increases the operative time and blood loss.15 On the other hand, closure without hysterectomy prohibits future endometrial or cervical cancer screening.
In a recent review using the American College of Surgeons National Surgical Quality Improvement Program database, investigators compared women who underwent colopocleisis alone with those who underwent colpocleisis with hysterectomy.17 They found that the incidence of major complications was greater among women who underwent concomitant hysterectomy, and they concluded that hysterectomy should not be performed routinely at the time of colpocleisis.17
Among 322 urogynecologists who responded to a web-based survey, only 18% routinely performed hysterectomy at the time of colpocleisis.18 Further, in a decision analysis model, the utility for colpocleisis without hysterectomy was higher in women older than age 40, suggesting that hysterectomy should be performed only in special circumstances.19
Evaluating the endometrium. If the uterus remains in situ, should endometrial evaluation be performed? If so, should ultrasonography or endometrial biopsy be used? Authors of a decision analysis model found that among women at low risk for cancer and without abnormal uterine bleeding, endometrial biopsy was not favored until the probability of cancer reached 64%.20 Specifically, no evaluation or evaluation by transvaginal ultrasonography is adequate in the majority of cases.20 When screened by transvaginal ultrasonography, the high, 99% negative predictive value for endometrial disease, using a cutoff value of 5 mm for endometrial stripe width, will allow most patients to avoid unnecessary tissue sampling.
Stress incontinence. It is likely that this patient's voiding dysfunction will resolve with reduction of the prolapse, and she may develop stress incontinence symptoms. In up to 68% of women, occult stress incontinence will be revealed with reduction of stage 3 or stage 4 prolapse.21 If the patient demonstrates stress incontinence, a midurethral sling is likely to treat her incontinence effectively, with little added risk from the procedure.22 Even among women who have an elevated postvoid residual urine volume, the incidence of sling revision is low.15
CASE 2 Procedure recommendation for this patient
For this case, we would perform a LeFort colpocleisis and discuss whether or not the patient would prefer a midurethral sling if stress incontinence was demonstrated on examination. We would not perform endometrial evaluation in this patient, as she has not been bleeding and her risk for endometrial cancer is low.
Weighing the benefits of native tissue repair
Native tissue repair when performed transvaginally is a minimally invasive approach to prolapse repair. In a multicenter randomized trial, anatomic success was reported to be 64.5% at 2 years.6 Long-term follow up of patients undergoing mesh sacrocolpopexy shows a similar anatomic failure rate, with up to one-third of patients meeting the definition of composite failure.3 Unlike mesh-augmented repairs, however, adverse events, including bowel obstruction, mesh exposure, and thromboembolism, are more likely to occur in the mesh sacrocolpopexy group.23
Obliterative procedures have the highest success rates of all prolapse repairs and carry with them low morbidity. However, women must forego the ability for coitus in the future. For all native tissue vaginal repairs, the surgeon and patient must weigh the risks and benefits of concomitant anti-incontinence procedures.
Read about using abdominal sacrocolpopexy for apical prolapse repair.
Abdominal sacrocolpopexy: A tried-and-true approach for apical prolapse repair
Sarah Huber, MD, and Patrick Culligan, MD
Dr. Culligan reports that he is a shareholder in Oragami Surgical LLC and a consultant and speaker for Coloplast and Intuitive Surgical Inc. Dr. Huber reports no financial relationships relevant to this article.
CASE Woman with advanced prolapse desires surgical repair
A 55-year-old woman (G2P2) presents to her gynecologist's office reporting a vaginal bulge and pressure that has been worsening for the past year. She describes a nontender ball of tissue the size of an orange protruding past the introitus that worsens with ambulating and lifting heavy objects. She reports some urinary urgency and increased frequency and at times feels as though her bladder does not empty completely with voiding. She denies any urinary incontinence. The patient has regular bowel movements but does report some difficulty with stool evacuation. She has a history of 2 vaginal deliveries and is sexually active. She is postmenopausal, with the last menses about 4 years ago. She is active and exercises regularly.
The patient's Pap smears, mammograms, and colonoscopy are up to date and test results have been normal. She has no significant medical or surgical history and no significant family history of cancer. On examination, her body mass index is normal, as is the cardiopulmonary exam. Her pelvic organ prolapse quantification system (POP-Q) score is Aa +3, Ba +3, C +4, GH 3, PB 3, TVL 10, Ap +2, Bp +2, and D +2. The patient is interested in surgical management.
What urodynamic tests would be appropriate for this patient, and what treatment options would you recommend?
- Robot-assisted laparoscopic sacrocolpopexy is a safe, effective, and durable treatment for advanced-stage pelvic organ prolapse.
- This procedure can completely correct stage 3 or 4 prolapse when the dissection of the anterior vaginal wall extends to the bladder neck and the dissection of the posterior vaginal wall extends to the perineal body.
- One can avoid the need for concomitant vaginal prolapse repair by gathering up stretched out vaginal epithelium while suturing to the mesh arms.
- Sacral attachment sutures should be placed in the anterior longitudinal ligament distal to the sacral promontory to avoid the L5-S1 disc.
- Unless contraindicated, lightweight macroporous polypropylene mesh is the current implant of choice.
Additional tests needed
Patients with advanced-stage pelvic organ prolapse are at an increased risk for stress urinary incontinence that may be masked by urethral "kinking" due to anatomic distortion of the periurethral support mechanism. Based on recommendations from the American Urological Association (AUA) and Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU), we routinely perform a postvoid residual urine volume measurement, urinalysis, urine culture, and a prolapse reduction stress test.24 If the urinalysis is positive for blood, then a preoperative cystoscopy would be indicated.
If stress incontinence is confirmed by reduction stress testing, the patient should be offered an anti-incontinence procedure, such as a mesh midurethral sling.
This patient's overactive bladder symptoms warrant investigation via complex urodynamic testing to allow for comprehensive counseling about her postoperative expectations.
Counseling the patient on the sacrocolpopexy option
Abdominal sacrocolpopexy initially was described in 1962 by Lane as a technique to affix the vaginal apex to the sacral promontory using a graft. Although the procedure has been modified over the years, the principles of using an implanted strengthening material to permanently attach the apex to the anterior longitudinal ligament at the sacrum has proven to be a highly effective and safe treatment, establishing it as the gold standard for apical prolapse repair.25,26
Compared with other methods of apical prolapse repair, sacrocolpopexy via any approach is superior to vaginal surgery in terms of subjective and objective outcomes. In a recent systematic review comparing apical prolapse repairs, patients who underwent a vaginal approach were more likely to report awareness of their prolapse after surgery, undergo repeat surgery, have objective recurrent prolapse, and were at increased risk for postoperative stress urinary incontinence and dyspareunia.26 Prospective studies within our practice have shown 1-year composite subjective and objective cure rates of 94% to 95%.27,28
Selecting a route for sacrocolpopexy
Although sacrocolpopexy can be approached via laparotomy or conventional laparoscopy, we routinely use a robot-assisted approach, as it has been shown to be especially beneficial for complex situations, such as in patients with prior pelvic surgery, a foreshortened vagina, or obesity.29,30
Potential complications
Sacrocolpopexy complications are rare, especially when a minimally invasive approach is used.31 Reported complications of minimally invasive sacrocolpopexy include gastrointestinal or genitourinary injury, bowel obstruction or ileus, incisional hernia, vascular injury, discitis or osteomyelitis, conversion to open procedure, and mesh exposure.
Vaginal mesh exposure is rare following sacrocolpopexy, but it can occur at any time following surgery.31 Some risk factors include mesh material selection (specifically polytetrafluoroethylene [PTFE] mesh), concurrent total hysterectomy, vaginal atrophy, and smoking.32,33 As a result, recent recommendations have advised the use of polypropylene mesh with uterine preservation or supracervical hysterectomy at the time of sacrocolpopexy.34 In fact, supracervical hysterectomy alone appears to cut down or eliminate the risk of mesh exposure in laparoscopic sacrocolpopexy.35
In our practice, avoiding split-thickness vaginal dissection, employing supracervical hysterectomy techniques, and using ultralightweight mesh has resulted in mesh exposure rates approaching zero.28
For atrophic vaginal tissue, one can consider prescribing preoperative vaginal estrogen for 4 to 6 weeks, but this is not essential and should not routinely delay pelvic reconstructive surgery.
What type of implant material is best?
While various materials have been used as the fixation media in sacrocolpopexy, loosely knitted synthetic type I macroporous polypropylene mesh is the best choice due to its efficacy, availability, and low adverse effect profile. We recommend a lightweight mesh with a maximum weight of 25 g/m2. Two such products currently available are the UPsylon Y-Mesh (Boston Scientific, Marlborough, Massachusetts) and Restorelle Y mesh (Coloplast, Minneapolis, Minnesota). Lightweight mesh has been proven to maintain integrity, guaranteeing a successful outcome, while reducing the "mesh load" on the attached tissue.27,28
Comparative studies with fascia lata or cross-linked porcine dermal grafts demonstrated inferior outcomes versus synthetic mesh, and currently the only biologic material on the market indicated for prolapse repair augmentation, ACell Pelvic Floor Matrix (ACell, Columbia, Maryland), has not been extensively tested in sacrocolpopexy.36-38
Vaginal anatomy restored by sacrocolpopexy
Abdominal sacrocolpopexy, specifically via a minimally invasive approach, is an effective and long-lasting treatment that should be offered to women with advanced-stage prolapse.
Using the surgical techniques described below, including attachment of the mesh along the lengths of the anterior and posterior vaginal walls and gathering up excess tissue with mesh attachment, can provide women with adequate support for the entire vagina with restoration of normal vaginal anatomy and caliber.
Step-by-step tips for surgical efficiency
Robotic port placement
- Place the trocars in a "W" layout for the da Vinci Si Surgical System (FIGURE 4, VIDEO 1) or in a linear layout for the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, California). Both Si and Xi port placement includes a 3- to 5-mm assistant port in the right upper quadrant of the abdomen.
Supracervical hysterectomy, if indicated
- Maneuver the uterus with the robotic tenaculum, which obviates the need for a uterine manipulator during the hysterectomy (VIDEO 2).
- Create the bladder flap just above the upper edge of the bladder to facilitate the upcoming anterior wall dissection. This helps to prevent the development of a split-thickness dissection plane.
- 1.5 to 2 cm of cervix should be left in place, and conization should be avoided.
Anterior vaginal wall dissection
- The key to a good full-thickness dissection is sustained tissue traction and countertraction. The bedside assistant pulls the anterior peritoneal cut edge anteriorly for "gross" traction, and further "fine" traction can be created by pulling the areolar tissue with robotic forceps. The cervix is grasped with the tenaculum, which applies a constant midline cephalad countertraction (VIDEO 3).
- Sharp dissection with cold scissors allows for creation of the dissection plane, while cautery is judiciously applied only for hemostasis. If bleeding is encountered, this usually indicates that a split thickness of the vaginal wall has been created, and the surgeon should correct to the proper dissection plane.
- Dissection is made easier by taking down the bladder pillars before advancing down toward the bladder neck.
- The anterior dissection is always carried down to level of the trigone, confirmed by visualization of the Foley bulb (FIGURE 5).
Posterior vaginal wall dissection
- Begin dissection just above the rectal reflection, leaving peritoneum on the posterior cervix (VIDEO 4).
- Extend the incision bilaterally to the uterosacral ligaments only after the correct dissection plane is confirmed by visualization of the areolar tissue.
- Apply cervical traction using the tenaculum in a cephalad midline direction, and place traction on the cut edge of the posterior peritoneum using the bipolar forceps. The tenaculum wrist must be turned away from the working instruments to avoid internal clashing.
- Completely transect the right uterosacral ligament to better facilitate the creation of a contiguous peritoneal opening for burying the mesh. The remainder of the opening will be created later.
- While it is important to avoid split-thickness dissection, the vaginal plane must be "clean" (that is, without fat or adventitia) to allow for robust suturing.
- Dissection at least halfway down the posterior vaginal wall is recommended but proceeding down to the perineal body provides the most optimal support (FIGURE 6).
Sacral dissection
- Use a noncrushing instrument to laterally sweep the bowel to the left side, effectively "plastering" the peritoneum over the sacral promontory (FIGURE 7; VIDEO 5).
- Extend the superficial peritoneal incision down the right paracolic gutter halfway between the ureter and colon until it communicates with the incised posterior peritoneal edge created during the posterior dissection.
- Identify the middle sacral artery to avoid vascular injury, but there is no need to prophylactically coagulate it.
Vaginal mesh attachment
- Cut a lightweight Y-mesh to a length of 6 to 8 cm anteriorly and 8 to 11 cm posteriorly and place it into the surgical field (FIGURE 8; VIDEO 6). The length is determined based on the preoperative office examination and examination under anesthesia prior to starting the procedure.
- Attach the mesh securely and evenly to the anterior and posterior vaginal walls using multiple interrupted monofilament sutures. We aim to place sutures that provide mesh stability without excess vaginal wall incorporation to avoid "through-and-through" suturing.
- The posterior wall suturing is performed first, starting at the perineal body and continuing cephalad (VIDEO 7). We find it easiest to tie the knots between the mesh and the vagina in this space.
- Suture the crotch of the Y-mesh to the cervix so that no gap exists between tissue and mesh.
- For advanced-stage prolapse with significant anterior prolapse, the stretched out vaginal epithelium can be systematically gathered up to reconfigure the tissue to conform to the desired mesh dimensions (VIDEO 8). This tissue remodeling is evident even at the 2- to 4-week postoperative visit.
Peritoneal closure: Step 1
- Reapproximate the cut edges of peritoneum surrounding the vagina and cervix using a continuous purse-string suture of 0 Monocryl (poliglecaprone 25) on an SH needle (Ethicon, Somerville, New Jersey) with a fisherman's knot tied at the end (VIDEO 9). The needle passes are placed close together and close to the incised edge of the cut peritoneum.
- We typically start our peritoneal suture at the 5 o'clock position of the posterior peritoneum, extending in a clockwise direction and ultimately jumping anteriorly around the sacral arm of the mesh.
- Place the mesh within the paracolic peritoneal canal, and secure the needle for later use.
Sacral mesh attachment
- The mesh is tensioned so that a vaginal examination confirms adequate support of all the walls without excess tension or tissue banding. Some laxity of the anterior vaginal wall consistent with a mild cystocele is appropriate.
- Place 2 permanent PTFE sutures along the slope of the sacral promontory into the anterior longitudinal ligament (VIDEO 10). This avoids injury to the disc space that sits at the edge of the promontory. We do not advise the use of bone anchors as they increase the risk for discitis and osteomyelitis.
- Secure the mesh to the anterior longitudinal ligament without any tension. This is facilitated by creating mesh slack via cephalad pressure from a vaginal probe.
Peritoneal closure: Step 2
- Close the remaining paracolic peritoneal incision, completely burying the mesh within the created canal (FIGURE 9).
- At the end of the procedure, perform a repeat vaginal exam, rectal exam, and cystoscopy.
Technique with prior total hysterectomy
- In patients with a prior total hysterectomy, place a 13 x 3.5 cm Breisky vaginal retractor and/or coated nonconductive stent (Marina Medical, Sunrise, Florida) into the vagina to delineate the anterior and posterior walls at the vaginal apex during dissection.
- Some surgeons may opt to retrograde fill the bladder to better identify its location.
- We routinely leave a segment of peritoneum attached to the dome of the vaginal apex for added tissue integrity to prevent erosion.
Read about using transvaginal mesh for POP repair.
Transvaginal mesh: An effective, durable option for POP repair
Vincent R. Lucente, MD, MBA, and Jessica B. Ton, MD
Dr. Lucente reports that he has received grant or research support from Advanced Tactile Imaging, Boston Scientific, Coloplast, and Valencia; is a consultant to Coloplast; is a speaker for Allergan, Boston Scientific, Coloplast, and Shionogi; and serves as an expert witness for American Medical Systems and C.R. Bard. Dr. Ton reports no financial relationships relevant to this article.
As baseline health in the elderly population continues to improve, the number of women in the United States with symptomatic POP will increase by approximately 50% by 2050.39 Unfortunately, after native tissue repair (NTR) the rate of prolapse recurrence is extremely high: approximately 40% regardless of approach, as demonstrated in the OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss) trial by Barber and colleagues.6 The authors of that clinical trial recently revealed that at the 5-year follow-up, these failure rates progressed to 70% for sacrospinous ligament fixation and 61% for uterosacral ligament suspension (data presented at the Society of Gynecologic Surgeons Annual Scientific Meeting 2018, Orlando, Florida). This establishes that NTR is not durable enough to meet the increasing physical demands of this age group and that mesh augmentation must be considered.
For patients at increased risk of prolapse recurrence, using transvaginal mesh (TVM) is the most minimally invasive approach and is an excellent option for mesh augmentation. Avoiding adverse events during placement of TVM depends largely on optimal surgical technique.40 (VIDEO: “Demonstration of an anterior vaginal wall dissection into the true vesicovaginal space”)
- Active advanced age requires a durable reconstructive pelvic surgery for pelvic organ prolapse, and native tissue repair does not meet that demand.
- Mesh augmentation reduces the risk of prolapse recurrence, and vaginal placement of mesh is the most minimally invasive approach.
- Rates of exposure with transvaginal mesh would be minimized with use of a full-thickness vaginal wall dissection.
- Optimal surgical technique could be highly reproducible with better surgical training.
The evidence on TVM versus NTR
Several studies have examined whether TVM has a measurable benefit over NTR.
A 2016 Cochrane review by Maher and colleagues included 37 randomized trials (4,023 women) that compared TVM and biologic grafts with NTR.41 Three primary outcomes were defined: awareness of prolapse, recurrence, and repeat surgery. Compared with women treated with NTR, those treated with synthetic nonabsorbable TVM exhibited a greater reduction in awareness of prolapse (risk ratio [RR], 0.66; 95% confidence interval [CI], 0.54-0.81), decreased recurrence in the anterior compartment (RR, 0.33; 95% CI, 0.26-0.40), and decreased reoperation for prolapse (RR, 0.53; 95% CI, 0.31-0.88). The overall calculated exposure rate was 12%, with a range of 3.2% to 20.8%.41 As we will discuss, this wide range most likely is attributed to a suboptimal, split-thickness dissection. There were no differences in other key secondary outcomes, including dyspareunia, operating time, and estimated blood loss.41
Longitudinal studies are emerging as almost 2 decades have passed since TVM was introduced. In a study of 5-year follow-up after TVM placement, Meyer and colleagues reported that patients had continued significant improvements in both subjective and objective outcomes.42 The mesh exposure rate was 6%, attributed to severe vaginal atrophy.42 A 10-year observational study by Weintraub and colleagues demonstrated a recurrence rate of only 2.6% in the anterior compartment, 7.6% in the posterior (nonaugmented) compartment, and no exposures or extrusions after anterior TVM placement.43
In contrast to the Cochrane review, in the 2017 multicenter PROSPECT (Prolapse surgery: Pragmatic evaluation and randomized controlled trials) trial, Glazener and colleagues found no difference in desired outcomes with TVM compared with NTR.44 There was an overall 6% to 7% exposure rate over 2 years.44 To reflect "real-world" practice, however, this study was intentionally designed without rigorous standardization of surgical technique. The authors reported that "appropriately experienced surgeons" performed the procedure, but it is unclear how experience was determined given that 20% of the cases were performed by "registrars," the equivalent of US residents or fellows.45
The PROSPECT study protocol described the TVM procedure as "a standard repair with a nonabsorbable mesh inlay to support the stitches," implying that there was no apical attachment of the mesh to the sacrospinous ligament.45 This is a suboptimal use of TVM because it does not address a detachment-type defect common in advanced prolapse. The PROSPECT study reinforces the need for better surgical training and standardization of the TVM procedure.44
How TVM compares with sacrocolpopexy
When comparing the use of TVM with sacrocolpopexy, our experience has been that TVM yields similar outcomes to sacrocolpopexy with additional benefits. We completed a 1-year retrospective cohort study comparing robot-assisted laparoscopic sacrocolpopexy (RALS) with TVM in a total of 86 patients, with both approaches performed by the same surgeon. Both treatment groups showed statistically significant improvements in nearly all functional and quality-of-life measures, including urinary symptoms, sexual function, and POP-Q scores.40 In particular, points Aa and Ba on the POP-Q score were significantly improved with TVM as compared to RALS. This suggests that TVM can achieve both lateral and apical support, where sacrocolpopexy addresses only the apex.40 This has clinical significance when considering DeLancey and colleagues' dynamic magnetic resonance imaging study, which demonstrated advanced prolapse results from both lateral and apical detachment.46 In addition, TVM placement also was considerably faster than RALS by approximately 96 minutes and could be performed using regional anesthesia. Only 1 mesh exposure in each study arm was reported.40
Finally, as with other vaginal procedures, patients who undergo TVM placement require minimal to no pain medication postoperatively and report faster return to daily activities. Almost none of our patients require narcotics, which is a significant benefit in the face of the ongoing national opioid crisis.
Gutman and colleagues compared laparoscopic mesh hysteropexy with TVM; they demonstrated comparable cure rates and, again, significantly longer operative times for the laparoscopic approach (174 vs 64 minutes; P<.0001).47 This multicenter study reported mesh exposure rates of 2.7% for laparoscopy and 6.6% for TVM,47 again likely due to a split-thickness dissection.
Safety of TVM depends on the surgeon factor
Because of the reported complications associated with TVM, in 2011 the US Food and Drug Administration (FDA) issued an update on the safety and efficacy of TVM augmentation and mandated postmarket studies.48 While we do not dispute that the mesh exposure rates were accurate at the time the FDA document was issued, we recognize that exposure has been erroneously attributed to inherent properties of the mesh.
Mesh exposure rates reported in the literature vary widely, ranging from 0% to 30%, even when surgeons used identical mesh products.49 This clearly establishes that the main contributing variable is surgical technique. It is critically important to recognize the "surgeon factor" as a confounder in trials that compare surgical procedures.50 Studies on TVM have shown that low-volume surgeons had significantly higher reoperation rates, while high-volume surgeons achieved a 41% reduction in reoperations.51,52 When TVM is performed by expert surgeons, the reported mesh exposure rates for TVM are noticeably lower.40,42,43,53,54
Decreasing mesh exposure rates would reduce the most common adverse event associated with TVM, thus improving its safety. The critical step to successful TVM placement is the initial dissection. Gynecologists traditionally have performed a split-thickness, colporrhaphy-style dissection to place the mesh within the layers of the vaginal wall.55 Placement within these planes, however, is too superficial and increases the risk of exposure. By contrast, by consistently performing a full-thickness vaginal wall dissection (FIGURE 10) and placing the mesh in the true vesicovaginal space,56 we have achieved a TVM exposure rate as low as 0% to 3%.40,54 If we can standardize the dissection component across our subspecialty, the rate of mesh exposure undoubtedly will decrease.
The PROSPECT investigators readily admitted what the study was not: a trial conducted "exclusively by the most experienced surgeons in the highest volume centres¬with a highly protocolised technique."44 In reality, that is the kind of rigorous study on TVM that our subspecialty demands. We must hold ourselves accountable and ensure that only the most qualified surgeons are placing TVM.
Keep the mesh option available
We support the position of the American Urogynecologic Society in opposing an outright ban of TVM because such a restriction would deny our patients access to an effective, durable, and minimally invasive approach for prolapse repair.57
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201-1206.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501-506.
- Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016-2024.
- American College of Obstetricians and Gynecologists, American Urogynecologic Society. Practice Bulletin No. 185 Summary: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):1170-1172.
- American Urogynecologic Society Best Practice Statement: Evaluation and counseling of patients with pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2017;23(5):281-287.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023-1034.
- Chung CP, Miskimins R, Kuehl TJ, Yandell PM, Shull BL. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23(2):223-227.
- Yazdany T, Yip S, Bhatia NN, Nguyen JN. Suture complications in a teaching institution among patients undergoing uterosacral ligament suspension with permanent braided suture. Int Urogynecol J. 2010;21(7):813-818.
- Toglia MR, Fagan MJ. Suture erosion rates and long-term surgical outcomes in patients undergoing sacrospinous ligament suspension with braided polyester suture. Am J Obstet Gynecol. 2008;198(5):600.e1-e4.
- Wong MJ, Rezvan A, Bhatia NN, Yazdany T. Uterosacral ligament vaginal vault suspension using delayed absorbable monofilament suture. Int Urogynecol J. 2011;22(11):1389-1394.
- Detollenaere RJ, den Boon J, Stekelenburg J, IntHout J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- Kapoor S, Sivanesan K, Robertson JA, Veerasingham M, Kapoor V. Sacrospinous hysteropexy: review and meta-analysis of outcomes. Int Urogynecol J. 2017;28(9):1285-1294.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23(6):365-371.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358-2367.
- Buchsbaum GM, Lee TG. Vaginal obliterative procedures for pelvic organ prolapse: a systematic review. Obstet Gynecol Surv. 2017;72(3):175-183.
- Zebede S, Smith AL, Plowright LN, Hegde A, Aguilar VC, Davila GW. Obliterative LeFort colpocleisis in a large group of elderly women. Obstet Gynecol. 2013;121(2 pt 1):279-284.
- Bochenska K, Leader-Cramer A, Mueller M, Dave B, Alverdy A, Kenton K. Perioperative complications following colpocleisis with and without concomitant vaginal hysterectomy. Int Urogynecol J. 2017;28(11):1671-1675.
- Jones K, Wang G, Romano R, St Marie P, Harmanli O. Colpocleisis: a survey of current practice patterns. Female Pelvic Med Reconstr Surg. 2017;23(4):276-280.
- Jones KA, Zhuo Y, Solak S, Harmanli O. Hysterectomy at the time of colpocleisis: a decision analysis. Int Urogynecol J. 2016;27(5):805-810.
- Kandadai P, Flynn M, Zweizig S, Patterson D. Cost-utility of routine endometrial evaluation before le fort colpocleisis. Female Pelvic Med Reconstr Surg. 2014;20(3):168-173.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97(1):31-34.
- Oliphant SS, Shepherd JP, Lowder JL. Midurethral sling for treatment of occult stress urinary incontinence at the time of colpocleisis: a decision analysis. Female Pelvic Med Reconstr Surg. 2012;18(4):216-220.
- Siddiqui NY, Grimes CL, Casiano ER, et al; Society of Gynecologic Surgeons Systematic Review Group. Mesh sacrocolpopexy compared with native tissue vaginal repair: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(1):44-55.
- Winters JC, Dmochowski RR, Goldman HB, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction. Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2464-2472.
- Barber MD, Maher C. Apical prolapse. Int Urogynecol J. 2013;24(11):1815-1833.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev. 2016;10:CD012376.
- Salamon CG, Lewis C, Priestley J, Gurshumov E, Culligan PJ. Prospective study of an ultra-lightweight polypropylene Y mesh for robotic sacrocolpopexy. Int Urogynecol J. 2013;24(8):1371-1375.
- Culligan PJ, Gurshumov E, Lewis C, et al. Subjective and objective results 1 year after robotic sacrocolpopexy using a lightweight Y-mesh. Int Urogynecol J. 2014;25(6):731-735.
- Eddib A, Danakas A, Hughes S, et al. Influence of morbid obesity on surgical outcomes in robotic-assisted gynecologic surgery. J Gynecol Surg. 2014;30(2):81-86.
- Gallo T, Kashani S, Patel DA, Elsahwi K, Silasi D-A, Azodi M. Robotic-assisted laparoscopic hysterectomy: outcomes in obese and morbidly obese patients. JSLS. 2012;16(3):421-427.
- Serati M, Bogani G, Sorice P, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol. 2014;66(2):303-318.
- Cundiff GW, Varner E, Visco AG, et al; Pelvic Floor Disorders Network. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol. 2008;199(6):688.e1-e5.
- Wu JM, Wells EC, Hundley AF, Connolly A, Williams KS, Visco AG. Mesh erosion in abdominal sacral colpopexy with and without concomitant hysterectomy. Am J Obstet Gynecol. 2006;194(5):1418-1422.
- Costantini E, Brubaker L, Cervigni M, et al. Sacrocolpopexy for pelvic organ prolapse: evidence-based review and recommendations. Eur J Obstet Gynecol Reprod Biol. 2016;205:60-65.
- Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J. 2011;22:205-212.
- Culligan PJ, Salamon C, Priestley JL, Shariati A. Porcine dermis compared with polypropylene mesh for laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol. 2013;121(1):143-151.
- Tate SB, Blackwell L, Lorenz DJ, Steptoe MM, Culligan PJ. Randomized trial of fascia lata and polypropylene mesh for abdominal sacrocolpopexy: 5-year follow-up. Int Urogynecol J. 2011;22(2):137-143.
- Culligan PJ, Blackwell L, Goldsmith LJ, Graham CA, Rogers A, Heit MH. A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol. 2005;106(1):29-37.
- ACOG Committee on Practice Bulletins-Gynecology, American Urogynecologic Society. ACOG Practice Bulletin No. 185: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):e234-e250.
- Jambusaria LH, Murphy M, Lucente VR. One-year functional and anatomic outcomes of robotic sacrocolpopexy versus vaginal extraperitoneal colpopexy with mesh. Female Pelvic Med Reconstr Surg. 2015;21(2):87-92.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database System Rev. 2016:CD012079.
- Meyer I, McGwin G, Swain T, Alvarez MD, Ellington DR, Richter HE. Synthetic graft augmentation in vaginal prolapse surgery: long-term objective and subjective outcomes. J Minim Invasive Gynecol. 2016;23(4):614-621.
- Weintraub AY, Friedman T, Baumfeld Y, Neymeyer J, Neuman M, Krissi H. Long‐term functional outcomes following mesh‐augmented posterior vaginal prolapse repair. Int J Gynecol Obstet. 2016;135(1):107-111.
- Glazener CM, Breeman S, Elders A, et al; PROSPECT Study Group. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT). Lancet. 2017;389(10067):381-392.
- Clinical and cost-effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomized controlled trials within Comprehensive Cohort Study. PROSPECT study protocol. The National Institute for Health Research. https://www.journalslibrary.nihr.ac.uk/programmes/hta/076018. Accessed January 17, 2018.
- Chen L, Lisse S, Larson K, Berger MB, Ashton-Miller JA, DeLancey JO. Structural failure sites in anterior vaginal wall prolapse: identification of a collinear triad. Obstet Gynecol. 2016;128(4):853-862.
- Gutman RE, Rardin CR, Sokol ER, et al. Vaginal and laparoscopic mesh hysteropexy for uterovaginal prolapse: a parallel cohort study. Am J Obstet Gynecol. 2017;216(1):38.e1-e11.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. https://www.fda.gov/downloads/medicaldevices/safety/alertsandnotices/ucm262760.pdf. Published July 2011. Accessed January 9, 2017.
- Murphy M, Holzberg A, van Raalte H, et al; Pelvic Surgeons Network. Time to rethink: an evidence-based response from pelvic surgeons to the FDA Safety Communication: "Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse." Int Urogynecol J. 2012;23(1):5-9.
- Roman H, Marpeau L, Hulsey TC. Surgeons' experience and interaction effect in randomized controlled trials regarding new surgical procedures. Am J Obstet Gynecol. 2008;199(2):108.e1-e6.
- Eilber KS, Alperin M, Khan A, et al. The role of the surgeon on outcomes of vaginal prolapse surgery with mesh. Female Pelvic Med Reconstr Surg. 2017;23 (5):293-296.
- Kelly EC, Winick-Ng J, Welk B. Surgeon experience and complications of transvaginal prolapse mesh. Obstet Gynecol. 2016;128(1):65-72.
- Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C; Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364(19):1826-1836.
- van Raalte HM, Lucente VR, Molden SM, Haff R, Murphy M. One-year anatomic and quality-of-life outcomes after the Prolift procedure for treatment of posthysterectomy prolapse. Am J Obstet Gynecol. 2008:199(6):694.e1-e6.
- Iyer S, Botros SM. Transvaginal mesh: a historical review and update of the current state of affairs in the United States. Int Urogynecol J. 2017;28(4):527-535.
- Ting M, Gonzalez A, Ephraim S, Murphy M, Lucente V. The importance of a full thickness vaginal wall dissection. Comment on "Transvaginal mesh: a historical review and update of the current state of affairs in the United States." Int Urogynecol J. 2017;28(10):1609-1610.
- American Urogynecologic Society. Position statement on restriction of surgical options for pelvic floor disorders. https://www.augs.org/assets/1/6/Position_Statement_Surgical_Options_for_PFDs.pdf. Published March 26, 2013. Accessed January 9, 2017.
Effective surgical management of advanced pelvic organ prolapse (POP) depends on prolapse location and stage, presence of urinary incontinence, need for hysterectomy, the patient’s desire to maintain sexual function, type of surgery, and the surgeon’s skill and experience, among other factors. For these reasons, POP repair is not a one-size-fits all procedure.
In this article, experts in minimally invasive prolapse repair offer their perspectives on 3 surgical approaches: use of native tissue (Drs. White, Aguilar, and Rogers), abdominal sacrocolpopexy (Drs. Huber and Culligan), and transvaginal mesh (Drs. Lucente and Ton). They evaluate the evidence on these procedures and provide recommendations based on their experience of best practices for achieving surgical success and minimizing adverse events.
Using native tissue for vaginal anatomy repair
Amanda White, MD; Vivian Aguilar, MD; and Rebecca G. Rogers, MD
Dr. Rogers reports that she receives royalties from UpToDate. Drs. White and Aguilar report no financial relationships relevant to this article.
Surgical therapy is the mainstay of treatment for POP, and 20% of US women will undergo prolapse and/or stress incontinence surgery by age 80.1 Prolapse surgery either restores the vaginal anatomy (reconstructive surgery) or obliterates the vaginal canal (obliterative surgery). Vaginal reconstruction can be performed using the patient's native tissue or mesh. Because of concerns associated with mesh use, native tissue repairs continue to be commonly performed.
Unfortunately, not all prolapse surgeries result in prolapse cure, and recurrent prolapse that necessitates repeat operation is not rare, regardless of whether or not mesh is used.2,3 Native tissue repairs are most commonly performed through the vaginal route, the first minimally invasive approach to prolapse surgery. Restoration of the vaginal apex has been identified as critically important in these surgeries. Apical native tissue repairs include reconstructive procedures, such as sacrospinous ligament suspension (SSLS) or uterosacral ligament suspension (USLS), and obliterative procedures, such as colpocleisis.
In this discussion, we present 2 case vignettes that highlight surgical decision making for repair of stage 3 or 4 pelvic organ prolapse utilizing these techniques.
- Native tissue repair offers a minimally invasive approach to prolapse repair.
- Sacrospinous and uterosacral ligament suspensions have equivalent success rates.
- Prophylactic midurethral slings reduce postoperative incontinence at the time of transvaginal native tissue repair.
- Hysterectomy at the time of colpocleisis should not be performed routinely.
CASE 1 Active woman with prolapse
A 65-year-old woman (G2P2) presents with stage 3 prolapse, with the anterior compartment at +3 and the cervix at the hymen with straining. She is sexually active and desires to retain coital function. A trial of pessary has failed.
What surgical options can be considered for this patient?
Reconstruction procedures for prolapse
This patient presents with a typical configuration of prolapse; the anterior and apical compartments are the most likely to prolapse.4 Importantly, conservative management of her prolapse has failed. While it is not required that women have a trial with pessary prior to undergoing surgery, all women should be offered conservative management of prolapse, according to the American Urogynecologic Society (AUGS) and the American College of Obstetricians and Gynecologists (ACOG).4,5
Apical suspension
Since this patient desires to retain coital function, her gynecologist recommends a reconstructive procedure. The combination of apical and anterior vaginal wall prolapse will require an apical suspension procedure (FIGURES 1 and 2). If suspension of the apex does not correct the anterior wall prolapse, the patient also may require anterior compartment reconstruction.
The 2 most commonly performed native tissue apical suspension procedures, SSLS and USLS, have equivalent outcomes at 2 years, according to a multicenter randomized trial.6 Therefore, the choice of procedure is at the surgeon's discretion. USLS is most commonly performed at the time of hysterectomy via an intraperitoneal approach, while SSLS is often selected for posthysterectomy vault prolapse, given its extraperitoneal location.
Suture type. Whether to use permanent suture at the time of SSLS or USLS is controversial. Some data suggest that permanent suture provides greater long-term success compared with delayed absorbable suture.7 However, permanent suture has been reported to be associated with higher rates of suture complications--up to 44% in USLS and 36% in SSLS--compared with a 3.5% complication rate in a USLS cohort treated with absorbable suture.8-10
Hysterectomy versus hysteropexy. Considerable debate exists regarding whether a patient requires hysterectomy at the time of prolapse repair. In a randomized trial at 12 months' follow-up, uterine preservation by sacrospinous hysteropexy was noninferior to vaginal hysterectomy with suspension of the uterosacral ligaments for surgical failure of the apical compartment.11 A recent meta-analysis found that apical failure rates after sacrospinous hysteropexy versus vaginal hysterectomy were not different.12 Repeat surgery rates for prolapse also were not different between groups. The most significant disadvantage of uterine-preservation prolapse surgery, when compared with hysterectomy, is the lack of prevention and diagnosis of uterine malignancy.12 From 2002 to 2012, rates of hysteropexy significantly increased in the United States, although rates remain low.13
Sling procedure pros and cons. This case patient did not report urinary incontinence, but she may develop incontinence with reduction of the anterior wall prolapse. A large randomized controlled trial that included 337 women compared sling with no sling procedures among women with prolapse undergoing transvaginal prolapse repair.14 Management with a prophylactic sling resulted in less incontinence (27.3% and 43.0%, respectively, at 12 months postoperatively) but higher rates of urinary tract infection (31.0% vs 18.3%), major bleeding complications (3.1% vs 0%), and incomplete bladder emptying 6 weeks after surgery (3.7% vs 0%) (P≤.05 for all).14
CASE 1 Recommendations for this patient
For this case, we would offer the patient a transvaginal hysterectomy and USLS. At the time of repair, we would assess whether she needed an anterior repair as well. We would offer a prophylactic sling procedure and also would discuss the risks and benefits of concomitant versus interval incontinence procedures.
CASE 2 Elderly woman with severe prolapse
An 85-year-old woman (G3P3) presents with procidentia, or complete eversion of the vagina, with the cervix 10 cm outside of the hymen. She has difficulty voiding, and the prolapse is uncomfortable when walking. A trial of pessary has failed. The patient denies vaginal bleeding. She is not sexually active and does not desire to retain coital function.
What treatment options would be appropriate for this patient?
Obliterative surgery
This elderly patient presents with advanced pelvic organ prolapse, and conservative management has failed. She is not sexually active and does not desire coital function in the future, so an obliterative procedure is indicated. Colpocleisis is a minimally invasive procedure that has cure rates ranging from 91% to 100%.15 It is likely that this patient's voiding dysfunction will improve after surgery and that she will be highly satisfied with the surgery.16
The question of hysterectomy with colpocleisis
The role of hysterectomy at the time of colpocleisis is controversial. LeFort colpocleisis preserves the uterus, with the anterior and posterior vaginal walls sutured together (FIGURE 3). Hysterectomy at the time of vaginal closure increases the operative time and blood loss.15 On the other hand, closure without hysterectomy prohibits future endometrial or cervical cancer screening.
In a recent review using the American College of Surgeons National Surgical Quality Improvement Program database, investigators compared women who underwent colopocleisis alone with those who underwent colpocleisis with hysterectomy.17 They found that the incidence of major complications was greater among women who underwent concomitant hysterectomy, and they concluded that hysterectomy should not be performed routinely at the time of colpocleisis.17
Among 322 urogynecologists who responded to a web-based survey, only 18% routinely performed hysterectomy at the time of colpocleisis.18 Further, in a decision analysis model, the utility for colpocleisis without hysterectomy was higher in women older than age 40, suggesting that hysterectomy should be performed only in special circumstances.19
Evaluating the endometrium. If the uterus remains in situ, should endometrial evaluation be performed? If so, should ultrasonography or endometrial biopsy be used? Authors of a decision analysis model found that among women at low risk for cancer and without abnormal uterine bleeding, endometrial biopsy was not favored until the probability of cancer reached 64%.20 Specifically, no evaluation or evaluation by transvaginal ultrasonography is adequate in the majority of cases.20 When screened by transvaginal ultrasonography, the high, 99% negative predictive value for endometrial disease, using a cutoff value of 5 mm for endometrial stripe width, will allow most patients to avoid unnecessary tissue sampling.
Stress incontinence. It is likely that this patient's voiding dysfunction will resolve with reduction of the prolapse, and she may develop stress incontinence symptoms. In up to 68% of women, occult stress incontinence will be revealed with reduction of stage 3 or stage 4 prolapse.21 If the patient demonstrates stress incontinence, a midurethral sling is likely to treat her incontinence effectively, with little added risk from the procedure.22 Even among women who have an elevated postvoid residual urine volume, the incidence of sling revision is low.15
CASE 2 Procedure recommendation for this patient
For this case, we would perform a LeFort colpocleisis and discuss whether or not the patient would prefer a midurethral sling if stress incontinence was demonstrated on examination. We would not perform endometrial evaluation in this patient, as she has not been bleeding and her risk for endometrial cancer is low.
Weighing the benefits of native tissue repair
Native tissue repair when performed transvaginally is a minimally invasive approach to prolapse repair. In a multicenter randomized trial, anatomic success was reported to be 64.5% at 2 years.6 Long-term follow up of patients undergoing mesh sacrocolpopexy shows a similar anatomic failure rate, with up to one-third of patients meeting the definition of composite failure.3 Unlike mesh-augmented repairs, however, adverse events, including bowel obstruction, mesh exposure, and thromboembolism, are more likely to occur in the mesh sacrocolpopexy group.23
Obliterative procedures have the highest success rates of all prolapse repairs and carry with them low morbidity. However, women must forego the ability for coitus in the future. For all native tissue vaginal repairs, the surgeon and patient must weigh the risks and benefits of concomitant anti-incontinence procedures.
Read about using abdominal sacrocolpopexy for apical prolapse repair.
Abdominal sacrocolpopexy: A tried-and-true approach for apical prolapse repair
Sarah Huber, MD, and Patrick Culligan, MD
Dr. Culligan reports that he is a shareholder in Oragami Surgical LLC and a consultant and speaker for Coloplast and Intuitive Surgical Inc. Dr. Huber reports no financial relationships relevant to this article.
CASE Woman with advanced prolapse desires surgical repair
A 55-year-old woman (G2P2) presents to her gynecologist's office reporting a vaginal bulge and pressure that has been worsening for the past year. She describes a nontender ball of tissue the size of an orange protruding past the introitus that worsens with ambulating and lifting heavy objects. She reports some urinary urgency and increased frequency and at times feels as though her bladder does not empty completely with voiding. She denies any urinary incontinence. The patient has regular bowel movements but does report some difficulty with stool evacuation. She has a history of 2 vaginal deliveries and is sexually active. She is postmenopausal, with the last menses about 4 years ago. She is active and exercises regularly.
The patient's Pap smears, mammograms, and colonoscopy are up to date and test results have been normal. She has no significant medical or surgical history and no significant family history of cancer. On examination, her body mass index is normal, as is the cardiopulmonary exam. Her pelvic organ prolapse quantification system (POP-Q) score is Aa +3, Ba +3, C +4, GH 3, PB 3, TVL 10, Ap +2, Bp +2, and D +2. The patient is interested in surgical management.
What urodynamic tests would be appropriate for this patient, and what treatment options would you recommend?
- Robot-assisted laparoscopic sacrocolpopexy is a safe, effective, and durable treatment for advanced-stage pelvic organ prolapse.
- This procedure can completely correct stage 3 or 4 prolapse when the dissection of the anterior vaginal wall extends to the bladder neck and the dissection of the posterior vaginal wall extends to the perineal body.
- One can avoid the need for concomitant vaginal prolapse repair by gathering up stretched out vaginal epithelium while suturing to the mesh arms.
- Sacral attachment sutures should be placed in the anterior longitudinal ligament distal to the sacral promontory to avoid the L5-S1 disc.
- Unless contraindicated, lightweight macroporous polypropylene mesh is the current implant of choice.
Additional tests needed
Patients with advanced-stage pelvic organ prolapse are at an increased risk for stress urinary incontinence that may be masked by urethral "kinking" due to anatomic distortion of the periurethral support mechanism. Based on recommendations from the American Urological Association (AUA) and Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU), we routinely perform a postvoid residual urine volume measurement, urinalysis, urine culture, and a prolapse reduction stress test.24 If the urinalysis is positive for blood, then a preoperative cystoscopy would be indicated.
If stress incontinence is confirmed by reduction stress testing, the patient should be offered an anti-incontinence procedure, such as a mesh midurethral sling.
This patient's overactive bladder symptoms warrant investigation via complex urodynamic testing to allow for comprehensive counseling about her postoperative expectations.
Counseling the patient on the sacrocolpopexy option
Abdominal sacrocolpopexy initially was described in 1962 by Lane as a technique to affix the vaginal apex to the sacral promontory using a graft. Although the procedure has been modified over the years, the principles of using an implanted strengthening material to permanently attach the apex to the anterior longitudinal ligament at the sacrum has proven to be a highly effective and safe treatment, establishing it as the gold standard for apical prolapse repair.25,26
Compared with other methods of apical prolapse repair, sacrocolpopexy via any approach is superior to vaginal surgery in terms of subjective and objective outcomes. In a recent systematic review comparing apical prolapse repairs, patients who underwent a vaginal approach were more likely to report awareness of their prolapse after surgery, undergo repeat surgery, have objective recurrent prolapse, and were at increased risk for postoperative stress urinary incontinence and dyspareunia.26 Prospective studies within our practice have shown 1-year composite subjective and objective cure rates of 94% to 95%.27,28
Selecting a route for sacrocolpopexy
Although sacrocolpopexy can be approached via laparotomy or conventional laparoscopy, we routinely use a robot-assisted approach, as it has been shown to be especially beneficial for complex situations, such as in patients with prior pelvic surgery, a foreshortened vagina, or obesity.29,30
Potential complications
Sacrocolpopexy complications are rare, especially when a minimally invasive approach is used.31 Reported complications of minimally invasive sacrocolpopexy include gastrointestinal or genitourinary injury, bowel obstruction or ileus, incisional hernia, vascular injury, discitis or osteomyelitis, conversion to open procedure, and mesh exposure.
Vaginal mesh exposure is rare following sacrocolpopexy, but it can occur at any time following surgery.31 Some risk factors include mesh material selection (specifically polytetrafluoroethylene [PTFE] mesh), concurrent total hysterectomy, vaginal atrophy, and smoking.32,33 As a result, recent recommendations have advised the use of polypropylene mesh with uterine preservation or supracervical hysterectomy at the time of sacrocolpopexy.34 In fact, supracervical hysterectomy alone appears to cut down or eliminate the risk of mesh exposure in laparoscopic sacrocolpopexy.35
In our practice, avoiding split-thickness vaginal dissection, employing supracervical hysterectomy techniques, and using ultralightweight mesh has resulted in mesh exposure rates approaching zero.28
For atrophic vaginal tissue, one can consider prescribing preoperative vaginal estrogen for 4 to 6 weeks, but this is not essential and should not routinely delay pelvic reconstructive surgery.
What type of implant material is best?
While various materials have been used as the fixation media in sacrocolpopexy, loosely knitted synthetic type I macroporous polypropylene mesh is the best choice due to its efficacy, availability, and low adverse effect profile. We recommend a lightweight mesh with a maximum weight of 25 g/m2. Two such products currently available are the UPsylon Y-Mesh (Boston Scientific, Marlborough, Massachusetts) and Restorelle Y mesh (Coloplast, Minneapolis, Minnesota). Lightweight mesh has been proven to maintain integrity, guaranteeing a successful outcome, while reducing the "mesh load" on the attached tissue.27,28
Comparative studies with fascia lata or cross-linked porcine dermal grafts demonstrated inferior outcomes versus synthetic mesh, and currently the only biologic material on the market indicated for prolapse repair augmentation, ACell Pelvic Floor Matrix (ACell, Columbia, Maryland), has not been extensively tested in sacrocolpopexy.36-38
Vaginal anatomy restored by sacrocolpopexy
Abdominal sacrocolpopexy, specifically via a minimally invasive approach, is an effective and long-lasting treatment that should be offered to women with advanced-stage prolapse.
Using the surgical techniques described below, including attachment of the mesh along the lengths of the anterior and posterior vaginal walls and gathering up excess tissue with mesh attachment, can provide women with adequate support for the entire vagina with restoration of normal vaginal anatomy and caliber.
Step-by-step tips for surgical efficiency
Robotic port placement
- Place the trocars in a "W" layout for the da Vinci Si Surgical System (FIGURE 4, VIDEO 1) or in a linear layout for the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, California). Both Si and Xi port placement includes a 3- to 5-mm assistant port in the right upper quadrant of the abdomen.
Supracervical hysterectomy, if indicated
- Maneuver the uterus with the robotic tenaculum, which obviates the need for a uterine manipulator during the hysterectomy (VIDEO 2).
- Create the bladder flap just above the upper edge of the bladder to facilitate the upcoming anterior wall dissection. This helps to prevent the development of a split-thickness dissection plane.
- 1.5 to 2 cm of cervix should be left in place, and conization should be avoided.
Anterior vaginal wall dissection
- The key to a good full-thickness dissection is sustained tissue traction and countertraction. The bedside assistant pulls the anterior peritoneal cut edge anteriorly for "gross" traction, and further "fine" traction can be created by pulling the areolar tissue with robotic forceps. The cervix is grasped with the tenaculum, which applies a constant midline cephalad countertraction (VIDEO 3).
- Sharp dissection with cold scissors allows for creation of the dissection plane, while cautery is judiciously applied only for hemostasis. If bleeding is encountered, this usually indicates that a split thickness of the vaginal wall has been created, and the surgeon should correct to the proper dissection plane.
- Dissection is made easier by taking down the bladder pillars before advancing down toward the bladder neck.
- The anterior dissection is always carried down to level of the trigone, confirmed by visualization of the Foley bulb (FIGURE 5).
Posterior vaginal wall dissection
- Begin dissection just above the rectal reflection, leaving peritoneum on the posterior cervix (VIDEO 4).
- Extend the incision bilaterally to the uterosacral ligaments only after the correct dissection plane is confirmed by visualization of the areolar tissue.
- Apply cervical traction using the tenaculum in a cephalad midline direction, and place traction on the cut edge of the posterior peritoneum using the bipolar forceps. The tenaculum wrist must be turned away from the working instruments to avoid internal clashing.
- Completely transect the right uterosacral ligament to better facilitate the creation of a contiguous peritoneal opening for burying the mesh. The remainder of the opening will be created later.
- While it is important to avoid split-thickness dissection, the vaginal plane must be "clean" (that is, without fat or adventitia) to allow for robust suturing.
- Dissection at least halfway down the posterior vaginal wall is recommended but proceeding down to the perineal body provides the most optimal support (FIGURE 6).
Sacral dissection
- Use a noncrushing instrument to laterally sweep the bowel to the left side, effectively "plastering" the peritoneum over the sacral promontory (FIGURE 7; VIDEO 5).
- Extend the superficial peritoneal incision down the right paracolic gutter halfway between the ureter and colon until it communicates with the incised posterior peritoneal edge created during the posterior dissection.
- Identify the middle sacral artery to avoid vascular injury, but there is no need to prophylactically coagulate it.
Vaginal mesh attachment
- Cut a lightweight Y-mesh to a length of 6 to 8 cm anteriorly and 8 to 11 cm posteriorly and place it into the surgical field (FIGURE 8; VIDEO 6). The length is determined based on the preoperative office examination and examination under anesthesia prior to starting the procedure.
- Attach the mesh securely and evenly to the anterior and posterior vaginal walls using multiple interrupted monofilament sutures. We aim to place sutures that provide mesh stability without excess vaginal wall incorporation to avoid "through-and-through" suturing.
- The posterior wall suturing is performed first, starting at the perineal body and continuing cephalad (VIDEO 7). We find it easiest to tie the knots between the mesh and the vagina in this space.
- Suture the crotch of the Y-mesh to the cervix so that no gap exists between tissue and mesh.
- For advanced-stage prolapse with significant anterior prolapse, the stretched out vaginal epithelium can be systematically gathered up to reconfigure the tissue to conform to the desired mesh dimensions (VIDEO 8). This tissue remodeling is evident even at the 2- to 4-week postoperative visit.
Peritoneal closure: Step 1
- Reapproximate the cut edges of peritoneum surrounding the vagina and cervix using a continuous purse-string suture of 0 Monocryl (poliglecaprone 25) on an SH needle (Ethicon, Somerville, New Jersey) with a fisherman's knot tied at the end (VIDEO 9). The needle passes are placed close together and close to the incised edge of the cut peritoneum.
- We typically start our peritoneal suture at the 5 o'clock position of the posterior peritoneum, extending in a clockwise direction and ultimately jumping anteriorly around the sacral arm of the mesh.
- Place the mesh within the paracolic peritoneal canal, and secure the needle for later use.
Sacral mesh attachment
- The mesh is tensioned so that a vaginal examination confirms adequate support of all the walls without excess tension or tissue banding. Some laxity of the anterior vaginal wall consistent with a mild cystocele is appropriate.
- Place 2 permanent PTFE sutures along the slope of the sacral promontory into the anterior longitudinal ligament (VIDEO 10). This avoids injury to the disc space that sits at the edge of the promontory. We do not advise the use of bone anchors as they increase the risk for discitis and osteomyelitis.
- Secure the mesh to the anterior longitudinal ligament without any tension. This is facilitated by creating mesh slack via cephalad pressure from a vaginal probe.
Peritoneal closure: Step 2
- Close the remaining paracolic peritoneal incision, completely burying the mesh within the created canal (FIGURE 9).
- At the end of the procedure, perform a repeat vaginal exam, rectal exam, and cystoscopy.
Technique with prior total hysterectomy
- In patients with a prior total hysterectomy, place a 13 x 3.5 cm Breisky vaginal retractor and/or coated nonconductive stent (Marina Medical, Sunrise, Florida) into the vagina to delineate the anterior and posterior walls at the vaginal apex during dissection.
- Some surgeons may opt to retrograde fill the bladder to better identify its location.
- We routinely leave a segment of peritoneum attached to the dome of the vaginal apex for added tissue integrity to prevent erosion.
Read about using transvaginal mesh for POP repair.
Transvaginal mesh: An effective, durable option for POP repair
Vincent R. Lucente, MD, MBA, and Jessica B. Ton, MD
Dr. Lucente reports that he has received grant or research support from Advanced Tactile Imaging, Boston Scientific, Coloplast, and Valencia; is a consultant to Coloplast; is a speaker for Allergan, Boston Scientific, Coloplast, and Shionogi; and serves as an expert witness for American Medical Systems and C.R. Bard. Dr. Ton reports no financial relationships relevant to this article.
As baseline health in the elderly population continues to improve, the number of women in the United States with symptomatic POP will increase by approximately 50% by 2050.39 Unfortunately, after native tissue repair (NTR) the rate of prolapse recurrence is extremely high: approximately 40% regardless of approach, as demonstrated in the OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss) trial by Barber and colleagues.6 The authors of that clinical trial recently revealed that at the 5-year follow-up, these failure rates progressed to 70% for sacrospinous ligament fixation and 61% for uterosacral ligament suspension (data presented at the Society of Gynecologic Surgeons Annual Scientific Meeting 2018, Orlando, Florida). This establishes that NTR is not durable enough to meet the increasing physical demands of this age group and that mesh augmentation must be considered.
For patients at increased risk of prolapse recurrence, using transvaginal mesh (TVM) is the most minimally invasive approach and is an excellent option for mesh augmentation. Avoiding adverse events during placement of TVM depends largely on optimal surgical technique.40 (VIDEO: “Demonstration of an anterior vaginal wall dissection into the true vesicovaginal space”)
- Active advanced age requires a durable reconstructive pelvic surgery for pelvic organ prolapse, and native tissue repair does not meet that demand.
- Mesh augmentation reduces the risk of prolapse recurrence, and vaginal placement of mesh is the most minimally invasive approach.
- Rates of exposure with transvaginal mesh would be minimized with use of a full-thickness vaginal wall dissection.
- Optimal surgical technique could be highly reproducible with better surgical training.
The evidence on TVM versus NTR
Several studies have examined whether TVM has a measurable benefit over NTR.
A 2016 Cochrane review by Maher and colleagues included 37 randomized trials (4,023 women) that compared TVM and biologic grafts with NTR.41 Three primary outcomes were defined: awareness of prolapse, recurrence, and repeat surgery. Compared with women treated with NTR, those treated with synthetic nonabsorbable TVM exhibited a greater reduction in awareness of prolapse (risk ratio [RR], 0.66; 95% confidence interval [CI], 0.54-0.81), decreased recurrence in the anterior compartment (RR, 0.33; 95% CI, 0.26-0.40), and decreased reoperation for prolapse (RR, 0.53; 95% CI, 0.31-0.88). The overall calculated exposure rate was 12%, with a range of 3.2% to 20.8%.41 As we will discuss, this wide range most likely is attributed to a suboptimal, split-thickness dissection. There were no differences in other key secondary outcomes, including dyspareunia, operating time, and estimated blood loss.41
Longitudinal studies are emerging as almost 2 decades have passed since TVM was introduced. In a study of 5-year follow-up after TVM placement, Meyer and colleagues reported that patients had continued significant improvements in both subjective and objective outcomes.42 The mesh exposure rate was 6%, attributed to severe vaginal atrophy.42 A 10-year observational study by Weintraub and colleagues demonstrated a recurrence rate of only 2.6% in the anterior compartment, 7.6% in the posterior (nonaugmented) compartment, and no exposures or extrusions after anterior TVM placement.43
In contrast to the Cochrane review, in the 2017 multicenter PROSPECT (Prolapse surgery: Pragmatic evaluation and randomized controlled trials) trial, Glazener and colleagues found no difference in desired outcomes with TVM compared with NTR.44 There was an overall 6% to 7% exposure rate over 2 years.44 To reflect "real-world" practice, however, this study was intentionally designed without rigorous standardization of surgical technique. The authors reported that "appropriately experienced surgeons" performed the procedure, but it is unclear how experience was determined given that 20% of the cases were performed by "registrars," the equivalent of US residents or fellows.45
The PROSPECT study protocol described the TVM procedure as "a standard repair with a nonabsorbable mesh inlay to support the stitches," implying that there was no apical attachment of the mesh to the sacrospinous ligament.45 This is a suboptimal use of TVM because it does not address a detachment-type defect common in advanced prolapse. The PROSPECT study reinforces the need for better surgical training and standardization of the TVM procedure.44
How TVM compares with sacrocolpopexy
When comparing the use of TVM with sacrocolpopexy, our experience has been that TVM yields similar outcomes to sacrocolpopexy with additional benefits. We completed a 1-year retrospective cohort study comparing robot-assisted laparoscopic sacrocolpopexy (RALS) with TVM in a total of 86 patients, with both approaches performed by the same surgeon. Both treatment groups showed statistically significant improvements in nearly all functional and quality-of-life measures, including urinary symptoms, sexual function, and POP-Q scores.40 In particular, points Aa and Ba on the POP-Q score were significantly improved with TVM as compared to RALS. This suggests that TVM can achieve both lateral and apical support, where sacrocolpopexy addresses only the apex.40 This has clinical significance when considering DeLancey and colleagues' dynamic magnetic resonance imaging study, which demonstrated advanced prolapse results from both lateral and apical detachment.46 In addition, TVM placement also was considerably faster than RALS by approximately 96 minutes and could be performed using regional anesthesia. Only 1 mesh exposure in each study arm was reported.40
Finally, as with other vaginal procedures, patients who undergo TVM placement require minimal to no pain medication postoperatively and report faster return to daily activities. Almost none of our patients require narcotics, which is a significant benefit in the face of the ongoing national opioid crisis.
Gutman and colleagues compared laparoscopic mesh hysteropexy with TVM; they demonstrated comparable cure rates and, again, significantly longer operative times for the laparoscopic approach (174 vs 64 minutes; P<.0001).47 This multicenter study reported mesh exposure rates of 2.7% for laparoscopy and 6.6% for TVM,47 again likely due to a split-thickness dissection.
Safety of TVM depends on the surgeon factor
Because of the reported complications associated with TVM, in 2011 the US Food and Drug Administration (FDA) issued an update on the safety and efficacy of TVM augmentation and mandated postmarket studies.48 While we do not dispute that the mesh exposure rates were accurate at the time the FDA document was issued, we recognize that exposure has been erroneously attributed to inherent properties of the mesh.
Mesh exposure rates reported in the literature vary widely, ranging from 0% to 30%, even when surgeons used identical mesh products.49 This clearly establishes that the main contributing variable is surgical technique. It is critically important to recognize the "surgeon factor" as a confounder in trials that compare surgical procedures.50 Studies on TVM have shown that low-volume surgeons had significantly higher reoperation rates, while high-volume surgeons achieved a 41% reduction in reoperations.51,52 When TVM is performed by expert surgeons, the reported mesh exposure rates for TVM are noticeably lower.40,42,43,53,54
Decreasing mesh exposure rates would reduce the most common adverse event associated with TVM, thus improving its safety. The critical step to successful TVM placement is the initial dissection. Gynecologists traditionally have performed a split-thickness, colporrhaphy-style dissection to place the mesh within the layers of the vaginal wall.55 Placement within these planes, however, is too superficial and increases the risk of exposure. By contrast, by consistently performing a full-thickness vaginal wall dissection (FIGURE 10) and placing the mesh in the true vesicovaginal space,56 we have achieved a TVM exposure rate as low as 0% to 3%.40,54 If we can standardize the dissection component across our subspecialty, the rate of mesh exposure undoubtedly will decrease.
The PROSPECT investigators readily admitted what the study was not: a trial conducted "exclusively by the most experienced surgeons in the highest volume centres¬with a highly protocolised technique."44 In reality, that is the kind of rigorous study on TVM that our subspecialty demands. We must hold ourselves accountable and ensure that only the most qualified surgeons are placing TVM.
Keep the mesh option available
We support the position of the American Urogynecologic Society in opposing an outright ban of TVM because such a restriction would deny our patients access to an effective, durable, and minimally invasive approach for prolapse repair.57
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Effective surgical management of advanced pelvic organ prolapse (POP) depends on prolapse location and stage, presence of urinary incontinence, need for hysterectomy, the patient’s desire to maintain sexual function, type of surgery, and the surgeon’s skill and experience, among other factors. For these reasons, POP repair is not a one-size-fits all procedure.
In this article, experts in minimally invasive prolapse repair offer their perspectives on 3 surgical approaches: use of native tissue (Drs. White, Aguilar, and Rogers), abdominal sacrocolpopexy (Drs. Huber and Culligan), and transvaginal mesh (Drs. Lucente and Ton). They evaluate the evidence on these procedures and provide recommendations based on their experience of best practices for achieving surgical success and minimizing adverse events.
Using native tissue for vaginal anatomy repair
Amanda White, MD; Vivian Aguilar, MD; and Rebecca G. Rogers, MD
Dr. Rogers reports that she receives royalties from UpToDate. Drs. White and Aguilar report no financial relationships relevant to this article.
Surgical therapy is the mainstay of treatment for POP, and 20% of US women will undergo prolapse and/or stress incontinence surgery by age 80.1 Prolapse surgery either restores the vaginal anatomy (reconstructive surgery) or obliterates the vaginal canal (obliterative surgery). Vaginal reconstruction can be performed using the patient's native tissue or mesh. Because of concerns associated with mesh use, native tissue repairs continue to be commonly performed.
Unfortunately, not all prolapse surgeries result in prolapse cure, and recurrent prolapse that necessitates repeat operation is not rare, regardless of whether or not mesh is used.2,3 Native tissue repairs are most commonly performed through the vaginal route, the first minimally invasive approach to prolapse surgery. Restoration of the vaginal apex has been identified as critically important in these surgeries. Apical native tissue repairs include reconstructive procedures, such as sacrospinous ligament suspension (SSLS) or uterosacral ligament suspension (USLS), and obliterative procedures, such as colpocleisis.
In this discussion, we present 2 case vignettes that highlight surgical decision making for repair of stage 3 or 4 pelvic organ prolapse utilizing these techniques.
- Native tissue repair offers a minimally invasive approach to prolapse repair.
- Sacrospinous and uterosacral ligament suspensions have equivalent success rates.
- Prophylactic midurethral slings reduce postoperative incontinence at the time of transvaginal native tissue repair.
- Hysterectomy at the time of colpocleisis should not be performed routinely.
CASE 1 Active woman with prolapse
A 65-year-old woman (G2P2) presents with stage 3 prolapse, with the anterior compartment at +3 and the cervix at the hymen with straining. She is sexually active and desires to retain coital function. A trial of pessary has failed.
What surgical options can be considered for this patient?
Reconstruction procedures for prolapse
This patient presents with a typical configuration of prolapse; the anterior and apical compartments are the most likely to prolapse.4 Importantly, conservative management of her prolapse has failed. While it is not required that women have a trial with pessary prior to undergoing surgery, all women should be offered conservative management of prolapse, according to the American Urogynecologic Society (AUGS) and the American College of Obstetricians and Gynecologists (ACOG).4,5
Apical suspension
Since this patient desires to retain coital function, her gynecologist recommends a reconstructive procedure. The combination of apical and anterior vaginal wall prolapse will require an apical suspension procedure (FIGURES 1 and 2). If suspension of the apex does not correct the anterior wall prolapse, the patient also may require anterior compartment reconstruction.
The 2 most commonly performed native tissue apical suspension procedures, SSLS and USLS, have equivalent outcomes at 2 years, according to a multicenter randomized trial.6 Therefore, the choice of procedure is at the surgeon's discretion. USLS is most commonly performed at the time of hysterectomy via an intraperitoneal approach, while SSLS is often selected for posthysterectomy vault prolapse, given its extraperitoneal location.
Suture type. Whether to use permanent suture at the time of SSLS or USLS is controversial. Some data suggest that permanent suture provides greater long-term success compared with delayed absorbable suture.7 However, permanent suture has been reported to be associated with higher rates of suture complications--up to 44% in USLS and 36% in SSLS--compared with a 3.5% complication rate in a USLS cohort treated with absorbable suture.8-10
Hysterectomy versus hysteropexy. Considerable debate exists regarding whether a patient requires hysterectomy at the time of prolapse repair. In a randomized trial at 12 months' follow-up, uterine preservation by sacrospinous hysteropexy was noninferior to vaginal hysterectomy with suspension of the uterosacral ligaments for surgical failure of the apical compartment.11 A recent meta-analysis found that apical failure rates after sacrospinous hysteropexy versus vaginal hysterectomy were not different.12 Repeat surgery rates for prolapse also were not different between groups. The most significant disadvantage of uterine-preservation prolapse surgery, when compared with hysterectomy, is the lack of prevention and diagnosis of uterine malignancy.12 From 2002 to 2012, rates of hysteropexy significantly increased in the United States, although rates remain low.13
Sling procedure pros and cons. This case patient did not report urinary incontinence, but she may develop incontinence with reduction of the anterior wall prolapse. A large randomized controlled trial that included 337 women compared sling with no sling procedures among women with prolapse undergoing transvaginal prolapse repair.14 Management with a prophylactic sling resulted in less incontinence (27.3% and 43.0%, respectively, at 12 months postoperatively) but higher rates of urinary tract infection (31.0% vs 18.3%), major bleeding complications (3.1% vs 0%), and incomplete bladder emptying 6 weeks after surgery (3.7% vs 0%) (P≤.05 for all).14
CASE 1 Recommendations for this patient
For this case, we would offer the patient a transvaginal hysterectomy and USLS. At the time of repair, we would assess whether she needed an anterior repair as well. We would offer a prophylactic sling procedure and also would discuss the risks and benefits of concomitant versus interval incontinence procedures.
CASE 2 Elderly woman with severe prolapse
An 85-year-old woman (G3P3) presents with procidentia, or complete eversion of the vagina, with the cervix 10 cm outside of the hymen. She has difficulty voiding, and the prolapse is uncomfortable when walking. A trial of pessary has failed. The patient denies vaginal bleeding. She is not sexually active and does not desire to retain coital function.
What treatment options would be appropriate for this patient?
Obliterative surgery
This elderly patient presents with advanced pelvic organ prolapse, and conservative management has failed. She is not sexually active and does not desire coital function in the future, so an obliterative procedure is indicated. Colpocleisis is a minimally invasive procedure that has cure rates ranging from 91% to 100%.15 It is likely that this patient's voiding dysfunction will improve after surgery and that she will be highly satisfied with the surgery.16
The question of hysterectomy with colpocleisis
The role of hysterectomy at the time of colpocleisis is controversial. LeFort colpocleisis preserves the uterus, with the anterior and posterior vaginal walls sutured together (FIGURE 3). Hysterectomy at the time of vaginal closure increases the operative time and blood loss.15 On the other hand, closure without hysterectomy prohibits future endometrial or cervical cancer screening.
In a recent review using the American College of Surgeons National Surgical Quality Improvement Program database, investigators compared women who underwent colopocleisis alone with those who underwent colpocleisis with hysterectomy.17 They found that the incidence of major complications was greater among women who underwent concomitant hysterectomy, and they concluded that hysterectomy should not be performed routinely at the time of colpocleisis.17
Among 322 urogynecologists who responded to a web-based survey, only 18% routinely performed hysterectomy at the time of colpocleisis.18 Further, in a decision analysis model, the utility for colpocleisis without hysterectomy was higher in women older than age 40, suggesting that hysterectomy should be performed only in special circumstances.19
Evaluating the endometrium. If the uterus remains in situ, should endometrial evaluation be performed? If so, should ultrasonography or endometrial biopsy be used? Authors of a decision analysis model found that among women at low risk for cancer and without abnormal uterine bleeding, endometrial biopsy was not favored until the probability of cancer reached 64%.20 Specifically, no evaluation or evaluation by transvaginal ultrasonography is adequate in the majority of cases.20 When screened by transvaginal ultrasonography, the high, 99% negative predictive value for endometrial disease, using a cutoff value of 5 mm for endometrial stripe width, will allow most patients to avoid unnecessary tissue sampling.
Stress incontinence. It is likely that this patient's voiding dysfunction will resolve with reduction of the prolapse, and she may develop stress incontinence symptoms. In up to 68% of women, occult stress incontinence will be revealed with reduction of stage 3 or stage 4 prolapse.21 If the patient demonstrates stress incontinence, a midurethral sling is likely to treat her incontinence effectively, with little added risk from the procedure.22 Even among women who have an elevated postvoid residual urine volume, the incidence of sling revision is low.15
CASE 2 Procedure recommendation for this patient
For this case, we would perform a LeFort colpocleisis and discuss whether or not the patient would prefer a midurethral sling if stress incontinence was demonstrated on examination. We would not perform endometrial evaluation in this patient, as she has not been bleeding and her risk for endometrial cancer is low.
Weighing the benefits of native tissue repair
Native tissue repair when performed transvaginally is a minimally invasive approach to prolapse repair. In a multicenter randomized trial, anatomic success was reported to be 64.5% at 2 years.6 Long-term follow up of patients undergoing mesh sacrocolpopexy shows a similar anatomic failure rate, with up to one-third of patients meeting the definition of composite failure.3 Unlike mesh-augmented repairs, however, adverse events, including bowel obstruction, mesh exposure, and thromboembolism, are more likely to occur in the mesh sacrocolpopexy group.23
Obliterative procedures have the highest success rates of all prolapse repairs and carry with them low morbidity. However, women must forego the ability for coitus in the future. For all native tissue vaginal repairs, the surgeon and patient must weigh the risks and benefits of concomitant anti-incontinence procedures.
Read about using abdominal sacrocolpopexy for apical prolapse repair.
Abdominal sacrocolpopexy: A tried-and-true approach for apical prolapse repair
Sarah Huber, MD, and Patrick Culligan, MD
Dr. Culligan reports that he is a shareholder in Oragami Surgical LLC and a consultant and speaker for Coloplast and Intuitive Surgical Inc. Dr. Huber reports no financial relationships relevant to this article.
CASE Woman with advanced prolapse desires surgical repair
A 55-year-old woman (G2P2) presents to her gynecologist's office reporting a vaginal bulge and pressure that has been worsening for the past year. She describes a nontender ball of tissue the size of an orange protruding past the introitus that worsens with ambulating and lifting heavy objects. She reports some urinary urgency and increased frequency and at times feels as though her bladder does not empty completely with voiding. She denies any urinary incontinence. The patient has regular bowel movements but does report some difficulty with stool evacuation. She has a history of 2 vaginal deliveries and is sexually active. She is postmenopausal, with the last menses about 4 years ago. She is active and exercises regularly.
The patient's Pap smears, mammograms, and colonoscopy are up to date and test results have been normal. She has no significant medical or surgical history and no significant family history of cancer. On examination, her body mass index is normal, as is the cardiopulmonary exam. Her pelvic organ prolapse quantification system (POP-Q) score is Aa +3, Ba +3, C +4, GH 3, PB 3, TVL 10, Ap +2, Bp +2, and D +2. The patient is interested in surgical management.
What urodynamic tests would be appropriate for this patient, and what treatment options would you recommend?
- Robot-assisted laparoscopic sacrocolpopexy is a safe, effective, and durable treatment for advanced-stage pelvic organ prolapse.
- This procedure can completely correct stage 3 or 4 prolapse when the dissection of the anterior vaginal wall extends to the bladder neck and the dissection of the posterior vaginal wall extends to the perineal body.
- One can avoid the need for concomitant vaginal prolapse repair by gathering up stretched out vaginal epithelium while suturing to the mesh arms.
- Sacral attachment sutures should be placed in the anterior longitudinal ligament distal to the sacral promontory to avoid the L5-S1 disc.
- Unless contraindicated, lightweight macroporous polypropylene mesh is the current implant of choice.
Additional tests needed
Patients with advanced-stage pelvic organ prolapse are at an increased risk for stress urinary incontinence that may be masked by urethral "kinking" due to anatomic distortion of the periurethral support mechanism. Based on recommendations from the American Urological Association (AUA) and Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU), we routinely perform a postvoid residual urine volume measurement, urinalysis, urine culture, and a prolapse reduction stress test.24 If the urinalysis is positive for blood, then a preoperative cystoscopy would be indicated.
If stress incontinence is confirmed by reduction stress testing, the patient should be offered an anti-incontinence procedure, such as a mesh midurethral sling.
This patient's overactive bladder symptoms warrant investigation via complex urodynamic testing to allow for comprehensive counseling about her postoperative expectations.
Counseling the patient on the sacrocolpopexy option
Abdominal sacrocolpopexy initially was described in 1962 by Lane as a technique to affix the vaginal apex to the sacral promontory using a graft. Although the procedure has been modified over the years, the principles of using an implanted strengthening material to permanently attach the apex to the anterior longitudinal ligament at the sacrum has proven to be a highly effective and safe treatment, establishing it as the gold standard for apical prolapse repair.25,26
Compared with other methods of apical prolapse repair, sacrocolpopexy via any approach is superior to vaginal surgery in terms of subjective and objective outcomes. In a recent systematic review comparing apical prolapse repairs, patients who underwent a vaginal approach were more likely to report awareness of their prolapse after surgery, undergo repeat surgery, have objective recurrent prolapse, and were at increased risk for postoperative stress urinary incontinence and dyspareunia.26 Prospective studies within our practice have shown 1-year composite subjective and objective cure rates of 94% to 95%.27,28
Selecting a route for sacrocolpopexy
Although sacrocolpopexy can be approached via laparotomy or conventional laparoscopy, we routinely use a robot-assisted approach, as it has been shown to be especially beneficial for complex situations, such as in patients with prior pelvic surgery, a foreshortened vagina, or obesity.29,30
Potential complications
Sacrocolpopexy complications are rare, especially when a minimally invasive approach is used.31 Reported complications of minimally invasive sacrocolpopexy include gastrointestinal or genitourinary injury, bowel obstruction or ileus, incisional hernia, vascular injury, discitis or osteomyelitis, conversion to open procedure, and mesh exposure.
Vaginal mesh exposure is rare following sacrocolpopexy, but it can occur at any time following surgery.31 Some risk factors include mesh material selection (specifically polytetrafluoroethylene [PTFE] mesh), concurrent total hysterectomy, vaginal atrophy, and smoking.32,33 As a result, recent recommendations have advised the use of polypropylene mesh with uterine preservation or supracervical hysterectomy at the time of sacrocolpopexy.34 In fact, supracervical hysterectomy alone appears to cut down or eliminate the risk of mesh exposure in laparoscopic sacrocolpopexy.35
In our practice, avoiding split-thickness vaginal dissection, employing supracervical hysterectomy techniques, and using ultralightweight mesh has resulted in mesh exposure rates approaching zero.28
For atrophic vaginal tissue, one can consider prescribing preoperative vaginal estrogen for 4 to 6 weeks, but this is not essential and should not routinely delay pelvic reconstructive surgery.
What type of implant material is best?
While various materials have been used as the fixation media in sacrocolpopexy, loosely knitted synthetic type I macroporous polypropylene mesh is the best choice due to its efficacy, availability, and low adverse effect profile. We recommend a lightweight mesh with a maximum weight of 25 g/m2. Two such products currently available are the UPsylon Y-Mesh (Boston Scientific, Marlborough, Massachusetts) and Restorelle Y mesh (Coloplast, Minneapolis, Minnesota). Lightweight mesh has been proven to maintain integrity, guaranteeing a successful outcome, while reducing the "mesh load" on the attached tissue.27,28
Comparative studies with fascia lata or cross-linked porcine dermal grafts demonstrated inferior outcomes versus synthetic mesh, and currently the only biologic material on the market indicated for prolapse repair augmentation, ACell Pelvic Floor Matrix (ACell, Columbia, Maryland), has not been extensively tested in sacrocolpopexy.36-38
Vaginal anatomy restored by sacrocolpopexy
Abdominal sacrocolpopexy, specifically via a minimally invasive approach, is an effective and long-lasting treatment that should be offered to women with advanced-stage prolapse.
Using the surgical techniques described below, including attachment of the mesh along the lengths of the anterior and posterior vaginal walls and gathering up excess tissue with mesh attachment, can provide women with adequate support for the entire vagina with restoration of normal vaginal anatomy and caliber.
Step-by-step tips for surgical efficiency
Robotic port placement
- Place the trocars in a "W" layout for the da Vinci Si Surgical System (FIGURE 4, VIDEO 1) or in a linear layout for the da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, California). Both Si and Xi port placement includes a 3- to 5-mm assistant port in the right upper quadrant of the abdomen.
Supracervical hysterectomy, if indicated
- Maneuver the uterus with the robotic tenaculum, which obviates the need for a uterine manipulator during the hysterectomy (VIDEO 2).
- Create the bladder flap just above the upper edge of the bladder to facilitate the upcoming anterior wall dissection. This helps to prevent the development of a split-thickness dissection plane.
- 1.5 to 2 cm of cervix should be left in place, and conization should be avoided.
Anterior vaginal wall dissection
- The key to a good full-thickness dissection is sustained tissue traction and countertraction. The bedside assistant pulls the anterior peritoneal cut edge anteriorly for "gross" traction, and further "fine" traction can be created by pulling the areolar tissue with robotic forceps. The cervix is grasped with the tenaculum, which applies a constant midline cephalad countertraction (VIDEO 3).
- Sharp dissection with cold scissors allows for creation of the dissection plane, while cautery is judiciously applied only for hemostasis. If bleeding is encountered, this usually indicates that a split thickness of the vaginal wall has been created, and the surgeon should correct to the proper dissection plane.
- Dissection is made easier by taking down the bladder pillars before advancing down toward the bladder neck.
- The anterior dissection is always carried down to level of the trigone, confirmed by visualization of the Foley bulb (FIGURE 5).
Posterior vaginal wall dissection
- Begin dissection just above the rectal reflection, leaving peritoneum on the posterior cervix (VIDEO 4).
- Extend the incision bilaterally to the uterosacral ligaments only after the correct dissection plane is confirmed by visualization of the areolar tissue.
- Apply cervical traction using the tenaculum in a cephalad midline direction, and place traction on the cut edge of the posterior peritoneum using the bipolar forceps. The tenaculum wrist must be turned away from the working instruments to avoid internal clashing.
- Completely transect the right uterosacral ligament to better facilitate the creation of a contiguous peritoneal opening for burying the mesh. The remainder of the opening will be created later.
- While it is important to avoid split-thickness dissection, the vaginal plane must be "clean" (that is, without fat or adventitia) to allow for robust suturing.
- Dissection at least halfway down the posterior vaginal wall is recommended but proceeding down to the perineal body provides the most optimal support (FIGURE 6).
Sacral dissection
- Use a noncrushing instrument to laterally sweep the bowel to the left side, effectively "plastering" the peritoneum over the sacral promontory (FIGURE 7; VIDEO 5).
- Extend the superficial peritoneal incision down the right paracolic gutter halfway between the ureter and colon until it communicates with the incised posterior peritoneal edge created during the posterior dissection.
- Identify the middle sacral artery to avoid vascular injury, but there is no need to prophylactically coagulate it.
Vaginal mesh attachment
- Cut a lightweight Y-mesh to a length of 6 to 8 cm anteriorly and 8 to 11 cm posteriorly and place it into the surgical field (FIGURE 8; VIDEO 6). The length is determined based on the preoperative office examination and examination under anesthesia prior to starting the procedure.
- Attach the mesh securely and evenly to the anterior and posterior vaginal walls using multiple interrupted monofilament sutures. We aim to place sutures that provide mesh stability without excess vaginal wall incorporation to avoid "through-and-through" suturing.
- The posterior wall suturing is performed first, starting at the perineal body and continuing cephalad (VIDEO 7). We find it easiest to tie the knots between the mesh and the vagina in this space.
- Suture the crotch of the Y-mesh to the cervix so that no gap exists between tissue and mesh.
- For advanced-stage prolapse with significant anterior prolapse, the stretched out vaginal epithelium can be systematically gathered up to reconfigure the tissue to conform to the desired mesh dimensions (VIDEO 8). This tissue remodeling is evident even at the 2- to 4-week postoperative visit.
Peritoneal closure: Step 1
- Reapproximate the cut edges of peritoneum surrounding the vagina and cervix using a continuous purse-string suture of 0 Monocryl (poliglecaprone 25) on an SH needle (Ethicon, Somerville, New Jersey) with a fisherman's knot tied at the end (VIDEO 9). The needle passes are placed close together and close to the incised edge of the cut peritoneum.
- We typically start our peritoneal suture at the 5 o'clock position of the posterior peritoneum, extending in a clockwise direction and ultimately jumping anteriorly around the sacral arm of the mesh.
- Place the mesh within the paracolic peritoneal canal, and secure the needle for later use.
Sacral mesh attachment
- The mesh is tensioned so that a vaginal examination confirms adequate support of all the walls without excess tension or tissue banding. Some laxity of the anterior vaginal wall consistent with a mild cystocele is appropriate.
- Place 2 permanent PTFE sutures along the slope of the sacral promontory into the anterior longitudinal ligament (VIDEO 10). This avoids injury to the disc space that sits at the edge of the promontory. We do not advise the use of bone anchors as they increase the risk for discitis and osteomyelitis.
- Secure the mesh to the anterior longitudinal ligament without any tension. This is facilitated by creating mesh slack via cephalad pressure from a vaginal probe.
Peritoneal closure: Step 2
- Close the remaining paracolic peritoneal incision, completely burying the mesh within the created canal (FIGURE 9).
- At the end of the procedure, perform a repeat vaginal exam, rectal exam, and cystoscopy.
Technique with prior total hysterectomy
- In patients with a prior total hysterectomy, place a 13 x 3.5 cm Breisky vaginal retractor and/or coated nonconductive stent (Marina Medical, Sunrise, Florida) into the vagina to delineate the anterior and posterior walls at the vaginal apex during dissection.
- Some surgeons may opt to retrograde fill the bladder to better identify its location.
- We routinely leave a segment of peritoneum attached to the dome of the vaginal apex for added tissue integrity to prevent erosion.
Read about using transvaginal mesh for POP repair.
Transvaginal mesh: An effective, durable option for POP repair
Vincent R. Lucente, MD, MBA, and Jessica B. Ton, MD
Dr. Lucente reports that he has received grant or research support from Advanced Tactile Imaging, Boston Scientific, Coloplast, and Valencia; is a consultant to Coloplast; is a speaker for Allergan, Boston Scientific, Coloplast, and Shionogi; and serves as an expert witness for American Medical Systems and C.R. Bard. Dr. Ton reports no financial relationships relevant to this article.
As baseline health in the elderly population continues to improve, the number of women in the United States with symptomatic POP will increase by approximately 50% by 2050.39 Unfortunately, after native tissue repair (NTR) the rate of prolapse recurrence is extremely high: approximately 40% regardless of approach, as demonstrated in the OPTIMAL (Operations and Pelvic Muscle Training in the Management of Apical Support Loss) trial by Barber and colleagues.6 The authors of that clinical trial recently revealed that at the 5-year follow-up, these failure rates progressed to 70% for sacrospinous ligament fixation and 61% for uterosacral ligament suspension (data presented at the Society of Gynecologic Surgeons Annual Scientific Meeting 2018, Orlando, Florida). This establishes that NTR is not durable enough to meet the increasing physical demands of this age group and that mesh augmentation must be considered.
For patients at increased risk of prolapse recurrence, using transvaginal mesh (TVM) is the most minimally invasive approach and is an excellent option for mesh augmentation. Avoiding adverse events during placement of TVM depends largely on optimal surgical technique.40 (VIDEO: “Demonstration of an anterior vaginal wall dissection into the true vesicovaginal space”)
- Active advanced age requires a durable reconstructive pelvic surgery for pelvic organ prolapse, and native tissue repair does not meet that demand.
- Mesh augmentation reduces the risk of prolapse recurrence, and vaginal placement of mesh is the most minimally invasive approach.
- Rates of exposure with transvaginal mesh would be minimized with use of a full-thickness vaginal wall dissection.
- Optimal surgical technique could be highly reproducible with better surgical training.
The evidence on TVM versus NTR
Several studies have examined whether TVM has a measurable benefit over NTR.
A 2016 Cochrane review by Maher and colleagues included 37 randomized trials (4,023 women) that compared TVM and biologic grafts with NTR.41 Three primary outcomes were defined: awareness of prolapse, recurrence, and repeat surgery. Compared with women treated with NTR, those treated with synthetic nonabsorbable TVM exhibited a greater reduction in awareness of prolapse (risk ratio [RR], 0.66; 95% confidence interval [CI], 0.54-0.81), decreased recurrence in the anterior compartment (RR, 0.33; 95% CI, 0.26-0.40), and decreased reoperation for prolapse (RR, 0.53; 95% CI, 0.31-0.88). The overall calculated exposure rate was 12%, with a range of 3.2% to 20.8%.41 As we will discuss, this wide range most likely is attributed to a suboptimal, split-thickness dissection. There were no differences in other key secondary outcomes, including dyspareunia, operating time, and estimated blood loss.41
Longitudinal studies are emerging as almost 2 decades have passed since TVM was introduced. In a study of 5-year follow-up after TVM placement, Meyer and colleagues reported that patients had continued significant improvements in both subjective and objective outcomes.42 The mesh exposure rate was 6%, attributed to severe vaginal atrophy.42 A 10-year observational study by Weintraub and colleagues demonstrated a recurrence rate of only 2.6% in the anterior compartment, 7.6% in the posterior (nonaugmented) compartment, and no exposures or extrusions after anterior TVM placement.43
In contrast to the Cochrane review, in the 2017 multicenter PROSPECT (Prolapse surgery: Pragmatic evaluation and randomized controlled trials) trial, Glazener and colleagues found no difference in desired outcomes with TVM compared with NTR.44 There was an overall 6% to 7% exposure rate over 2 years.44 To reflect "real-world" practice, however, this study was intentionally designed without rigorous standardization of surgical technique. The authors reported that "appropriately experienced surgeons" performed the procedure, but it is unclear how experience was determined given that 20% of the cases were performed by "registrars," the equivalent of US residents or fellows.45
The PROSPECT study protocol described the TVM procedure as "a standard repair with a nonabsorbable mesh inlay to support the stitches," implying that there was no apical attachment of the mesh to the sacrospinous ligament.45 This is a suboptimal use of TVM because it does not address a detachment-type defect common in advanced prolapse. The PROSPECT study reinforces the need for better surgical training and standardization of the TVM procedure.44
How TVM compares with sacrocolpopexy
When comparing the use of TVM with sacrocolpopexy, our experience has been that TVM yields similar outcomes to sacrocolpopexy with additional benefits. We completed a 1-year retrospective cohort study comparing robot-assisted laparoscopic sacrocolpopexy (RALS) with TVM in a total of 86 patients, with both approaches performed by the same surgeon. Both treatment groups showed statistically significant improvements in nearly all functional and quality-of-life measures, including urinary symptoms, sexual function, and POP-Q scores.40 In particular, points Aa and Ba on the POP-Q score were significantly improved with TVM as compared to RALS. This suggests that TVM can achieve both lateral and apical support, where sacrocolpopexy addresses only the apex.40 This has clinical significance when considering DeLancey and colleagues' dynamic magnetic resonance imaging study, which demonstrated advanced prolapse results from both lateral and apical detachment.46 In addition, TVM placement also was considerably faster than RALS by approximately 96 minutes and could be performed using regional anesthesia. Only 1 mesh exposure in each study arm was reported.40
Finally, as with other vaginal procedures, patients who undergo TVM placement require minimal to no pain medication postoperatively and report faster return to daily activities. Almost none of our patients require narcotics, which is a significant benefit in the face of the ongoing national opioid crisis.
Gutman and colleagues compared laparoscopic mesh hysteropexy with TVM; they demonstrated comparable cure rates and, again, significantly longer operative times for the laparoscopic approach (174 vs 64 minutes; P<.0001).47 This multicenter study reported mesh exposure rates of 2.7% for laparoscopy and 6.6% for TVM,47 again likely due to a split-thickness dissection.
Safety of TVM depends on the surgeon factor
Because of the reported complications associated with TVM, in 2011 the US Food and Drug Administration (FDA) issued an update on the safety and efficacy of TVM augmentation and mandated postmarket studies.48 While we do not dispute that the mesh exposure rates were accurate at the time the FDA document was issued, we recognize that exposure has been erroneously attributed to inherent properties of the mesh.
Mesh exposure rates reported in the literature vary widely, ranging from 0% to 30%, even when surgeons used identical mesh products.49 This clearly establishes that the main contributing variable is surgical technique. It is critically important to recognize the "surgeon factor" as a confounder in trials that compare surgical procedures.50 Studies on TVM have shown that low-volume surgeons had significantly higher reoperation rates, while high-volume surgeons achieved a 41% reduction in reoperations.51,52 When TVM is performed by expert surgeons, the reported mesh exposure rates for TVM are noticeably lower.40,42,43,53,54
Decreasing mesh exposure rates would reduce the most common adverse event associated with TVM, thus improving its safety. The critical step to successful TVM placement is the initial dissection. Gynecologists traditionally have performed a split-thickness, colporrhaphy-style dissection to place the mesh within the layers of the vaginal wall.55 Placement within these planes, however, is too superficial and increases the risk of exposure. By contrast, by consistently performing a full-thickness vaginal wall dissection (FIGURE 10) and placing the mesh in the true vesicovaginal space,56 we have achieved a TVM exposure rate as low as 0% to 3%.40,54 If we can standardize the dissection component across our subspecialty, the rate of mesh exposure undoubtedly will decrease.
The PROSPECT investigators readily admitted what the study was not: a trial conducted "exclusively by the most experienced surgeons in the highest volume centres¬with a highly protocolised technique."44 In reality, that is the kind of rigorous study on TVM that our subspecialty demands. We must hold ourselves accountable and ensure that only the most qualified surgeons are placing TVM.
Keep the mesh option available
We support the position of the American Urogynecologic Society in opposing an outright ban of TVM because such a restriction would deny our patients access to an effective, durable, and minimally invasive approach for prolapse repair.57
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201-1206.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501-506.
- Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016-2024.
- American College of Obstetricians and Gynecologists, American Urogynecologic Society. Practice Bulletin No. 185 Summary: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):1170-1172.
- American Urogynecologic Society Best Practice Statement: Evaluation and counseling of patients with pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2017;23(5):281-287.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023-1034.
- Chung CP, Miskimins R, Kuehl TJ, Yandell PM, Shull BL. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23(2):223-227.
- Yazdany T, Yip S, Bhatia NN, Nguyen JN. Suture complications in a teaching institution among patients undergoing uterosacral ligament suspension with permanent braided suture. Int Urogynecol J. 2010;21(7):813-818.
- Toglia MR, Fagan MJ. Suture erosion rates and long-term surgical outcomes in patients undergoing sacrospinous ligament suspension with braided polyester suture. Am J Obstet Gynecol. 2008;198(5):600.e1-e4.
- Wong MJ, Rezvan A, Bhatia NN, Yazdany T. Uterosacral ligament vaginal vault suspension using delayed absorbable monofilament suture. Int Urogynecol J. 2011;22(11):1389-1394.
- Detollenaere RJ, den Boon J, Stekelenburg J, IntHout J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- Kapoor S, Sivanesan K, Robertson JA, Veerasingham M, Kapoor V. Sacrospinous hysteropexy: review and meta-analysis of outcomes. Int Urogynecol J. 2017;28(9):1285-1294.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23(6):365-371.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358-2367.
- Buchsbaum GM, Lee TG. Vaginal obliterative procedures for pelvic organ prolapse: a systematic review. Obstet Gynecol Surv. 2017;72(3):175-183.
- Zebede S, Smith AL, Plowright LN, Hegde A, Aguilar VC, Davila GW. Obliterative LeFort colpocleisis in a large group of elderly women. Obstet Gynecol. 2013;121(2 pt 1):279-284.
- Bochenska K, Leader-Cramer A, Mueller M, Dave B, Alverdy A, Kenton K. Perioperative complications following colpocleisis with and without concomitant vaginal hysterectomy. Int Urogynecol J. 2017;28(11):1671-1675.
- Jones K, Wang G, Romano R, St Marie P, Harmanli O. Colpocleisis: a survey of current practice patterns. Female Pelvic Med Reconstr Surg. 2017;23(4):276-280.
- Jones KA, Zhuo Y, Solak S, Harmanli O. Hysterectomy at the time of colpocleisis: a decision analysis. Int Urogynecol J. 2016;27(5):805-810.
- Kandadai P, Flynn M, Zweizig S, Patterson D. Cost-utility of routine endometrial evaluation before le fort colpocleisis. Female Pelvic Med Reconstr Surg. 2014;20(3):168-173.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97(1):31-34.
- Oliphant SS, Shepherd JP, Lowder JL. Midurethral sling for treatment of occult stress urinary incontinence at the time of colpocleisis: a decision analysis. Female Pelvic Med Reconstr Surg. 2012;18(4):216-220.
- Siddiqui NY, Grimes CL, Casiano ER, et al; Society of Gynecologic Surgeons Systematic Review Group. Mesh sacrocolpopexy compared with native tissue vaginal repair: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(1):44-55.
- Winters JC, Dmochowski RR, Goldman HB, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction. Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2464-2472.
- Barber MD, Maher C. Apical prolapse. Int Urogynecol J. 2013;24(11):1815-1833.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev. 2016;10:CD012376.
- Salamon CG, Lewis C, Priestley J, Gurshumov E, Culligan PJ. Prospective study of an ultra-lightweight polypropylene Y mesh for robotic sacrocolpopexy. Int Urogynecol J. 2013;24(8):1371-1375.
- Culligan PJ, Gurshumov E, Lewis C, et al. Subjective and objective results 1 year after robotic sacrocolpopexy using a lightweight Y-mesh. Int Urogynecol J. 2014;25(6):731-735.
- Eddib A, Danakas A, Hughes S, et al. Influence of morbid obesity on surgical outcomes in robotic-assisted gynecologic surgery. J Gynecol Surg. 2014;30(2):81-86.
- Gallo T, Kashani S, Patel DA, Elsahwi K, Silasi D-A, Azodi M. Robotic-assisted laparoscopic hysterectomy: outcomes in obese and morbidly obese patients. JSLS. 2012;16(3):421-427.
- Serati M, Bogani G, Sorice P, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol. 2014;66(2):303-318.
- Cundiff GW, Varner E, Visco AG, et al; Pelvic Floor Disorders Network. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol. 2008;199(6):688.e1-e5.
- Wu JM, Wells EC, Hundley AF, Connolly A, Williams KS, Visco AG. Mesh erosion in abdominal sacral colpopexy with and without concomitant hysterectomy. Am J Obstet Gynecol. 2006;194(5):1418-1422.
- Costantini E, Brubaker L, Cervigni M, et al. Sacrocolpopexy for pelvic organ prolapse: evidence-based review and recommendations. Eur J Obstet Gynecol Reprod Biol. 2016;205:60-65.
- Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J. 2011;22:205-212.
- Culligan PJ, Salamon C, Priestley JL, Shariati A. Porcine dermis compared with polypropylene mesh for laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol. 2013;121(1):143-151.
- Tate SB, Blackwell L, Lorenz DJ, Steptoe MM, Culligan PJ. Randomized trial of fascia lata and polypropylene mesh for abdominal sacrocolpopexy: 5-year follow-up. Int Urogynecol J. 2011;22(2):137-143.
- Culligan PJ, Blackwell L, Goldsmith LJ, Graham CA, Rogers A, Heit MH. A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol. 2005;106(1):29-37.
- ACOG Committee on Practice Bulletins-Gynecology, American Urogynecologic Society. ACOG Practice Bulletin No. 185: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):e234-e250.
- Jambusaria LH, Murphy M, Lucente VR. One-year functional and anatomic outcomes of robotic sacrocolpopexy versus vaginal extraperitoneal colpopexy with mesh. Female Pelvic Med Reconstr Surg. 2015;21(2):87-92.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database System Rev. 2016:CD012079.
- Meyer I, McGwin G, Swain T, Alvarez MD, Ellington DR, Richter HE. Synthetic graft augmentation in vaginal prolapse surgery: long-term objective and subjective outcomes. J Minim Invasive Gynecol. 2016;23(4):614-621.
- Weintraub AY, Friedman T, Baumfeld Y, Neymeyer J, Neuman M, Krissi H. Long‐term functional outcomes following mesh‐augmented posterior vaginal prolapse repair. Int J Gynecol Obstet. 2016;135(1):107-111.
- Glazener CM, Breeman S, Elders A, et al; PROSPECT Study Group. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT). Lancet. 2017;389(10067):381-392.
- Clinical and cost-effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomized controlled trials within Comprehensive Cohort Study. PROSPECT study protocol. The National Institute for Health Research. https://www.journalslibrary.nihr.ac.uk/programmes/hta/076018. Accessed January 17, 2018.
- Chen L, Lisse S, Larson K, Berger MB, Ashton-Miller JA, DeLancey JO. Structural failure sites in anterior vaginal wall prolapse: identification of a collinear triad. Obstet Gynecol. 2016;128(4):853-862.
- Gutman RE, Rardin CR, Sokol ER, et al. Vaginal and laparoscopic mesh hysteropexy for uterovaginal prolapse: a parallel cohort study. Am J Obstet Gynecol. 2017;216(1):38.e1-e11.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. https://www.fda.gov/downloads/medicaldevices/safety/alertsandnotices/ucm262760.pdf. Published July 2011. Accessed January 9, 2017.
- Murphy M, Holzberg A, van Raalte H, et al; Pelvic Surgeons Network. Time to rethink: an evidence-based response from pelvic surgeons to the FDA Safety Communication: "Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse." Int Urogynecol J. 2012;23(1):5-9.
- Roman H, Marpeau L, Hulsey TC. Surgeons' experience and interaction effect in randomized controlled trials regarding new surgical procedures. Am J Obstet Gynecol. 2008;199(2):108.e1-e6.
- Eilber KS, Alperin M, Khan A, et al. The role of the surgeon on outcomes of vaginal prolapse surgery with mesh. Female Pelvic Med Reconstr Surg. 2017;23 (5):293-296.
- Kelly EC, Winick-Ng J, Welk B. Surgeon experience and complications of transvaginal prolapse mesh. Obstet Gynecol. 2016;128(1):65-72.
- Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C; Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364(19):1826-1836.
- van Raalte HM, Lucente VR, Molden SM, Haff R, Murphy M. One-year anatomic and quality-of-life outcomes after the Prolift procedure for treatment of posthysterectomy prolapse. Am J Obstet Gynecol. 2008:199(6):694.e1-e6.
- Iyer S, Botros SM. Transvaginal mesh: a historical review and update of the current state of affairs in the United States. Int Urogynecol J. 2017;28(4):527-535.
- Ting M, Gonzalez A, Ephraim S, Murphy M, Lucente V. The importance of a full thickness vaginal wall dissection. Comment on "Transvaginal mesh: a historical review and update of the current state of affairs in the United States." Int Urogynecol J. 2017;28(10):1609-1610.
- American Urogynecologic Society. Position statement on restriction of surgical options for pelvic floor disorders. https://www.augs.org/assets/1/6/Position_Statement_Surgical_Options_for_PFDs.pdf. Published March 26, 2013. Accessed January 9, 2017.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201-1206.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501-506.
- Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016-2024.
- American College of Obstetricians and Gynecologists, American Urogynecologic Society. Practice Bulletin No. 185 Summary: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):1170-1172.
- American Urogynecologic Society Best Practice Statement: Evaluation and counseling of patients with pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2017;23(5):281-287.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023-1034.
- Chung CP, Miskimins R, Kuehl TJ, Yandell PM, Shull BL. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23(2):223-227.
- Yazdany T, Yip S, Bhatia NN, Nguyen JN. Suture complications in a teaching institution among patients undergoing uterosacral ligament suspension with permanent braided suture. Int Urogynecol J. 2010;21(7):813-818.
- Toglia MR, Fagan MJ. Suture erosion rates and long-term surgical outcomes in patients undergoing sacrospinous ligament suspension with braided polyester suture. Am J Obstet Gynecol. 2008;198(5):600.e1-e4.
- Wong MJ, Rezvan A, Bhatia NN, Yazdany T. Uterosacral ligament vaginal vault suspension using delayed absorbable monofilament suture. Int Urogynecol J. 2011;22(11):1389-1394.
- Detollenaere RJ, den Boon J, Stekelenburg J, IntHout J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- Kapoor S, Sivanesan K, Robertson JA, Veerasingham M, Kapoor V. Sacrospinous hysteropexy: review and meta-analysis of outcomes. Int Urogynecol J. 2017;28(9):1285-1294.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23(6):365-371.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358-2367.
- Buchsbaum GM, Lee TG. Vaginal obliterative procedures for pelvic organ prolapse: a systematic review. Obstet Gynecol Surv. 2017;72(3):175-183.
- Zebede S, Smith AL, Plowright LN, Hegde A, Aguilar VC, Davila GW. Obliterative LeFort colpocleisis in a large group of elderly women. Obstet Gynecol. 2013;121(2 pt 1):279-284.
- Bochenska K, Leader-Cramer A, Mueller M, Dave B, Alverdy A, Kenton K. Perioperative complications following colpocleisis with and without concomitant vaginal hysterectomy. Int Urogynecol J. 2017;28(11):1671-1675.
- Jones K, Wang G, Romano R, St Marie P, Harmanli O. Colpocleisis: a survey of current practice patterns. Female Pelvic Med Reconstr Surg. 2017;23(4):276-280.
- Jones KA, Zhuo Y, Solak S, Harmanli O. Hysterectomy at the time of colpocleisis: a decision analysis. Int Urogynecol J. 2016;27(5):805-810.
- Kandadai P, Flynn M, Zweizig S, Patterson D. Cost-utility of routine endometrial evaluation before le fort colpocleisis. Female Pelvic Med Reconstr Surg. 2014;20(3):168-173.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97(1):31-34.
- Oliphant SS, Shepherd JP, Lowder JL. Midurethral sling for treatment of occult stress urinary incontinence at the time of colpocleisis: a decision analysis. Female Pelvic Med Reconstr Surg. 2012;18(4):216-220.
- Siddiqui NY, Grimes CL, Casiano ER, et al; Society of Gynecologic Surgeons Systematic Review Group. Mesh sacrocolpopexy compared with native tissue vaginal repair: a systematic review and meta-analysis. Obstet Gynecol. 2015;125(1):44-55.
- Winters JC, Dmochowski RR, Goldman HB, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction. Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2464-2472.
- Barber MD, Maher C. Apical prolapse. Int Urogynecol J. 2013;24(11):1815-1833.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev. 2016;10:CD012376.
- Salamon CG, Lewis C, Priestley J, Gurshumov E, Culligan PJ. Prospective study of an ultra-lightweight polypropylene Y mesh for robotic sacrocolpopexy. Int Urogynecol J. 2013;24(8):1371-1375.
- Culligan PJ, Gurshumov E, Lewis C, et al. Subjective and objective results 1 year after robotic sacrocolpopexy using a lightweight Y-mesh. Int Urogynecol J. 2014;25(6):731-735.
- Eddib A, Danakas A, Hughes S, et al. Influence of morbid obesity on surgical outcomes in robotic-assisted gynecologic surgery. J Gynecol Surg. 2014;30(2):81-86.
- Gallo T, Kashani S, Patel DA, Elsahwi K, Silasi D-A, Azodi M. Robotic-assisted laparoscopic hysterectomy: outcomes in obese and morbidly obese patients. JSLS. 2012;16(3):421-427.
- Serati M, Bogani G, Sorice P, et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol. 2014;66(2):303-318.
- Cundiff GW, Varner E, Visco AG, et al; Pelvic Floor Disorders Network. Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol. 2008;199(6):688.e1-e5.
- Wu JM, Wells EC, Hundley AF, Connolly A, Williams KS, Visco AG. Mesh erosion in abdominal sacral colpopexy with and without concomitant hysterectomy. Am J Obstet Gynecol. 2006;194(5):1418-1422.
- Costantini E, Brubaker L, Cervigni M, et al. Sacrocolpopexy for pelvic organ prolapse: evidence-based review and recommendations. Eur J Obstet Gynecol Reprod Biol. 2016;205:60-65.
- Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES. Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J. 2011;22:205-212.
- Culligan PJ, Salamon C, Priestley JL, Shariati A. Porcine dermis compared with polypropylene mesh for laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol. 2013;121(1):143-151.
- Tate SB, Blackwell L, Lorenz DJ, Steptoe MM, Culligan PJ. Randomized trial of fascia lata and polypropylene mesh for abdominal sacrocolpopexy: 5-year follow-up. Int Urogynecol J. 2011;22(2):137-143.
- Culligan PJ, Blackwell L, Goldsmith LJ, Graham CA, Rogers A, Heit MH. A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol. 2005;106(1):29-37.
- ACOG Committee on Practice Bulletins-Gynecology, American Urogynecologic Society. ACOG Practice Bulletin No. 185: Pelvic organ prolapse. Obstet Gynecol. 2017;130(5):e234-e250.
- Jambusaria LH, Murphy M, Lucente VR. One-year functional and anatomic outcomes of robotic sacrocolpopexy versus vaginal extraperitoneal colpopexy with mesh. Female Pelvic Med Reconstr Surg. 2015;21(2):87-92.
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database System Rev. 2016:CD012079.
- Meyer I, McGwin G, Swain T, Alvarez MD, Ellington DR, Richter HE. Synthetic graft augmentation in vaginal prolapse surgery: long-term objective and subjective outcomes. J Minim Invasive Gynecol. 2016;23(4):614-621.
- Weintraub AY, Friedman T, Baumfeld Y, Neymeyer J, Neuman M, Krissi H. Long‐term functional outcomes following mesh‐augmented posterior vaginal prolapse repair. Int J Gynecol Obstet. 2016;135(1):107-111.
- Glazener CM, Breeman S, Elders A, et al; PROSPECT Study Group. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT). Lancet. 2017;389(10067):381-392.
- Clinical and cost-effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomized controlled trials within Comprehensive Cohort Study. PROSPECT study protocol. The National Institute for Health Research. https://www.journalslibrary.nihr.ac.uk/programmes/hta/076018. Accessed January 17, 2018.
- Chen L, Lisse S, Larson K, Berger MB, Ashton-Miller JA, DeLancey JO. Structural failure sites in anterior vaginal wall prolapse: identification of a collinear triad. Obstet Gynecol. 2016;128(4):853-862.
- Gutman RE, Rardin CR, Sokol ER, et al. Vaginal and laparoscopic mesh hysteropexy for uterovaginal prolapse: a parallel cohort study. Am J Obstet Gynecol. 2017;216(1):38.e1-e11.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. https://www.fda.gov/downloads/medicaldevices/safety/alertsandnotices/ucm262760.pdf. Published July 2011. Accessed January 9, 2017.
- Murphy M, Holzberg A, van Raalte H, et al; Pelvic Surgeons Network. Time to rethink: an evidence-based response from pelvic surgeons to the FDA Safety Communication: "Update on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse." Int Urogynecol J. 2012;23(1):5-9.
- Roman H, Marpeau L, Hulsey TC. Surgeons' experience and interaction effect in randomized controlled trials regarding new surgical procedures. Am J Obstet Gynecol. 2008;199(2):108.e1-e6.
- Eilber KS, Alperin M, Khan A, et al. The role of the surgeon on outcomes of vaginal prolapse surgery with mesh. Female Pelvic Med Reconstr Surg. 2017;23 (5):293-296.
- Kelly EC, Winick-Ng J, Welk B. Surgeon experience and complications of transvaginal prolapse mesh. Obstet Gynecol. 2016;128(1):65-72.
- Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C; Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic-organ prolapse. N Engl J Med 2011;364(19):1826-1836.
- van Raalte HM, Lucente VR, Molden SM, Haff R, Murphy M. One-year anatomic and quality-of-life outcomes after the Prolift procedure for treatment of posthysterectomy prolapse. Am J Obstet Gynecol. 2008:199(6):694.e1-e6.
- Iyer S, Botros SM. Transvaginal mesh: a historical review and update of the current state of affairs in the United States. Int Urogynecol J. 2017;28(4):527-535.
- Ting M, Gonzalez A, Ephraim S, Murphy M, Lucente V. The importance of a full thickness vaginal wall dissection. Comment on "Transvaginal mesh: a historical review and update of the current state of affairs in the United States." Int Urogynecol J. 2017;28(10):1609-1610.
- American Urogynecologic Society. Position statement on restriction of surgical options for pelvic floor disorders. https://www.augs.org/assets/1/6/Position_Statement_Surgical_Options_for_PFDs.pdf. Published March 26, 2013. Accessed January 9, 2017.
Leading best gynecologic surgical care into the next decade
With today’s rapid health care transformation from fee for service to fee for value, it is imperative that gynecologic surgeons understand, engage in, and lead this transformation. The value equation is defined as patient experience times clinical outcome divided by cost. This 2-part special issue highlights some of the key content shared at the 2018 SGS annual meeting, held in Orlando, Florida, to help you engage and lead.
The keynote address was “Patient Experience: It is not about making people happy” and was presented by James Merlino, MD (author of Service Fanatics: How to Build Superior Patient Experience the Cleveland Clinic Way), who is former Chief Experience Officer and colorectal surgeon at the Cleveland Clinic and currently President and Chief Medical Officer, Strategic Consulting at Press Ganey. Dr. Merlino clearly defines that the patient experience is really about patient safety and quality. He shares practical tips to help physicians improve communication with patients, which not only increases patient satisfaction but also physician satisfaction. His wife Amy Merlino, MD, an ObGyn, coauthored the piece with him and shares their journey to implement programs that were impactful and designed to create greater personal appreciation and mindfulness of physicians’ clinical work.
Optimal surgical outcomes delivered at lowest cost are the other key components of value health care. Endometriosis and the management of stage 3 and 4 pelvic organ prolapse remain challenging clinical scenarios that we face often. Rosanne Kho, MD, and colleagues taught a postgraduate course on contemporary management of deep infiltrating endometriosis and, in part 2 of this special section, share key highlights and pearls from that course. A highpoint of the meeting was a debate on the optimal management of stage 3 and 4 pelvic organ prolapse. Peter Rosenblatt, MD, moderated a lively discussion involving Rebecca Rogers, MD, who advocated for native tissue repair; Patrick Culligan, MD, who promoted abdominal sacrocolpopexy; and Vincent Lucente, MD, backing transvaginal mesh. They summarize their arguments beginning on page SS4 for you to decide.
Lastly, with increasing demand for minimally invasive hysterectomy, many surgeons could benefit from simulation training to enhance their practice, hone up on skills, and provide warm-up to sharpen technical skills prior to the day in the operating room. Simulation training improves patient safety and outcomes and lowers cost. Simulation training is also key in training residents and fellows. Christine Vaccaro, MD, and colleagues taught a postgraduate course on what is new in simulation training for hysterectomy and summarize important technologies in part 2 of this special section.
I hope you enjoy the content of this special section and find it impactful to your practice and future.
With today’s rapid health care transformation from fee for service to fee for value, it is imperative that gynecologic surgeons understand, engage in, and lead this transformation. The value equation is defined as patient experience times clinical outcome divided by cost. This 2-part special issue highlights some of the key content shared at the 2018 SGS annual meeting, held in Orlando, Florida, to help you engage and lead.
The keynote address was “Patient Experience: It is not about making people happy” and was presented by James Merlino, MD (author of Service Fanatics: How to Build Superior Patient Experience the Cleveland Clinic Way), who is former Chief Experience Officer and colorectal surgeon at the Cleveland Clinic and currently President and Chief Medical Officer, Strategic Consulting at Press Ganey. Dr. Merlino clearly defines that the patient experience is really about patient safety and quality. He shares practical tips to help physicians improve communication with patients, which not only increases patient satisfaction but also physician satisfaction. His wife Amy Merlino, MD, an ObGyn, coauthored the piece with him and shares their journey to implement programs that were impactful and designed to create greater personal appreciation and mindfulness of physicians’ clinical work.
Optimal surgical outcomes delivered at lowest cost are the other key components of value health care. Endometriosis and the management of stage 3 and 4 pelvic organ prolapse remain challenging clinical scenarios that we face often. Rosanne Kho, MD, and colleagues taught a postgraduate course on contemporary management of deep infiltrating endometriosis and, in part 2 of this special section, share key highlights and pearls from that course. A highpoint of the meeting was a debate on the optimal management of stage 3 and 4 pelvic organ prolapse. Peter Rosenblatt, MD, moderated a lively discussion involving Rebecca Rogers, MD, who advocated for native tissue repair; Patrick Culligan, MD, who promoted abdominal sacrocolpopexy; and Vincent Lucente, MD, backing transvaginal mesh. They summarize their arguments beginning on page SS4 for you to decide.
Lastly, with increasing demand for minimally invasive hysterectomy, many surgeons could benefit from simulation training to enhance their practice, hone up on skills, and provide warm-up to sharpen technical skills prior to the day in the operating room. Simulation training improves patient safety and outcomes and lowers cost. Simulation training is also key in training residents and fellows. Christine Vaccaro, MD, and colleagues taught a postgraduate course on what is new in simulation training for hysterectomy and summarize important technologies in part 2 of this special section.
I hope you enjoy the content of this special section and find it impactful to your practice and future.
With today’s rapid health care transformation from fee for service to fee for value, it is imperative that gynecologic surgeons understand, engage in, and lead this transformation. The value equation is defined as patient experience times clinical outcome divided by cost. This 2-part special issue highlights some of the key content shared at the 2018 SGS annual meeting, held in Orlando, Florida, to help you engage and lead.
The keynote address was “Patient Experience: It is not about making people happy” and was presented by James Merlino, MD (author of Service Fanatics: How to Build Superior Patient Experience the Cleveland Clinic Way), who is former Chief Experience Officer and colorectal surgeon at the Cleveland Clinic and currently President and Chief Medical Officer, Strategic Consulting at Press Ganey. Dr. Merlino clearly defines that the patient experience is really about patient safety and quality. He shares practical tips to help physicians improve communication with patients, which not only increases patient satisfaction but also physician satisfaction. His wife Amy Merlino, MD, an ObGyn, coauthored the piece with him and shares their journey to implement programs that were impactful and designed to create greater personal appreciation and mindfulness of physicians’ clinical work.
Optimal surgical outcomes delivered at lowest cost are the other key components of value health care. Endometriosis and the management of stage 3 and 4 pelvic organ prolapse remain challenging clinical scenarios that we face often. Rosanne Kho, MD, and colleagues taught a postgraduate course on contemporary management of deep infiltrating endometriosis and, in part 2 of this special section, share key highlights and pearls from that course. A highpoint of the meeting was a debate on the optimal management of stage 3 and 4 pelvic organ prolapse. Peter Rosenblatt, MD, moderated a lively discussion involving Rebecca Rogers, MD, who advocated for native tissue repair; Patrick Culligan, MD, who promoted abdominal sacrocolpopexy; and Vincent Lucente, MD, backing transvaginal mesh. They summarize their arguments beginning on page SS4 for you to decide.
Lastly, with increasing demand for minimally invasive hysterectomy, many surgeons could benefit from simulation training to enhance their practice, hone up on skills, and provide warm-up to sharpen technical skills prior to the day in the operating room. Simulation training improves patient safety and outcomes and lowers cost. Simulation training is also key in training residents and fellows. Christine Vaccaro, MD, and colleagues taught a postgraduate course on what is new in simulation training for hysterectomy and summarize important technologies in part 2 of this special section.
I hope you enjoy the content of this special section and find it impactful to your practice and future.
Tactics for reducing the rate of surgical site infection following cesarean delivery
The 25-year-old patient (G1P0) is at 41 weeks’ gestation. She has been fully dilated and pushing for 3.5 hours, at station 0, with regular strong contractions, no descent and a Category II fetal heart-rate tracing. The estimated fetal weight is 8 lb. Membranes have been ruptured for 10 hours. Maternal temperature is 99° F and her prepregnancy body mass index (BMI) was 32 kg/m2. After examining the patient and reviewing the labor progress, you recommend a cesarean delivery. As you prepare for the delivery, you identify the patient as high risk for surgical site infection and begin to recall all the interventions that might reduce postoperative infection for a patient at high risk for infection.
Halsted’s surgical principles
Dr. William Steward Halsted, the first chief of surgery at Johns Hopkins Hospital, articulated a set of surgical principles that included strict aseptic technique, gentle tissue handling, meticulous hemostasis, minimum tension on tissue, accurate tissue apposition, preservation of blood supply, and obliteration of dead space where appropriate. These principles of “safe surgery” are believed to improve surgical outcomes and reduce the risk of surgical site infection.1
Preoperative antibiotics
All obstetricians who perform cesarean delivery know the importance of administering a narrow-spectrum antibiotic, such as cefazolin or ampicillin, prior to the skin incision, but not more than 60 minutes before the incision, to help reduce the risk of wound infection and endometritis. In a meta-analysis of 82 studies involving more than 13,000 women the administration of a preoperative antibiotic compared with placebo reduced the risk of wound infection (relative risk [RR], 0.40; 95% confidence interval [CI], 0.35–0.46) and endometritis (RR, 0.38; 95% CI, 0.34–0.42).2
Cefazolin 3 g versus 2 g for obese patients
There are no data from randomized trials of cesarean delivery that directly compare the efficacy of preoperative cefazolin at doses of 2 g and 3 g to reduce the risk of infection. However, based on the observation that, for any given dose of cefazolin, circulating levels are reduced in obese patients, many authorities recommend that if the patient weighs ≥120 kg that 3 g of cefazolin should be administered.3
Extended-spectrum preoperative antibiotics
Some experts recommend that, for women in labor and for women with more than 4 hours of ruptured membranes, IV azithromycin 500 mg be added to the standard narrow-spectrum cefazolin regimen to reduce the rate of postoperative infection. In one trial, 2,013 women who were in labor or had more than 4 hours of ruptured membranes were randomly assigned to IV cefazolin alone or IV cefazolin plus azithromycin 500 mg prior to cesarean delivery.4 The cefazolin dose was reported to be weight-based utilizing the BMI at the time of delivery. The rates of endometritis (3.8% vs 6.1%) and wound infection (2.4% vs 6.6%) were lower in the women receiving extended-spectrum antibiotics versus cefazolin monotherapy.
Concerns have been raised about the impact of extended-spectrum antibiotics on the newborn microbiome and risk of accelerating the emergence of bacteria resistant to available antibiotics. Limiting the use of azithromycin to those cesarean delivery cases in which the patient is immunosuppressed, diabetic, obese, in labor and/or with prolonged ruptured membranes would reduce the number of women and newborns exposed to the drug and achieve the immediate health goal of reducing surgical infection.
Preoperative vaginal preparation
Many authorities recommend the use of a preoperative povidone- iodine vaginal scrub for 30 seconds prior to cesarean delivery for women in labor and women with ruptured membranes. In a meta-analysis of 16 trials involving 4,837 women, the women who received vaginal cleansing before cesarean delivery had a significantly lower incidence of endometritis (4.5% vs 8.8%) and postoperative fever (9.4% vs 14.9%) compared with those who did not have vaginal cleansing.5 Most of the benefit in reducing the risk of endometritis was confined to women in labor before the cesarean delivery (8.1% vs 13.8%) and women with ruptured membranes (4.3% vs 20.1%).5
Metronidazole gel 5 g also has been reported to be effective in reducing the rate of endometritis associated with cesarean delivery. In one study, 224 women having a cesarean delivery for various indications were randomly assigned to preoperative treatment with vaginally administered metronidazole gel 5 g or placebo gel. All women also received one dose of preoperative intravenous antibiotics. The rates of endometritis were 7% and 17% in the metronidazole and placebo groups, respectively.6
Povidone-iodine is approved for vaginal surgical site cleansing. For women with allergies to iodine or povidone-iodine, the options for vaginal cleansing are limited. The American College of Obstetricians and Gynecologists has noted the chlorhexidine gluconate solutions with a high concentration of alcohol should not be used for vaginal cleansing because the alcohol can irritate the mucosal epithelium. However, although not US Food and Drug Administration–approved for vaginal cleansing, solutions of chlorhexidine with a low alcohol content (Hibiclens, chlorhexidine with 4% alcohol concentration) are thought to be safe and may be considered for off-label use in vaginal cleansing.7
Preoperative abdominal preparation with chlorhexidine
Some authorities recommend skin preparation with chlorhexidine rather than povidone-iodine prior to cesarean delivery. Two recent randomized trials in women undergoing cesarean delivery8,9 and one trial in patients undergoing general surgery operations10 reported a reduction in surgical site infection with chlorhexidine. However, other trials have reported no difference in the rate of surgical site infection with these two skin preparation methods.11,12
Changing gloves and equipment after delivery of the newborn
Currently there is no high-quality evidence that changing gloves after delivery of the newborn or using new surgical instruments for closure reduces the risk of postcesarean infection. Two small clinical trials reported that changing gloves after delivery of the newborn did not reduce the rate of postcesarean infection.13,14
Postoperative antibiotics (a heretical challenge to the central dogma of antibiotic prophylaxis in surgery)
The central dogma of antibiotic prevention of postoperative infection is that antibiotics administered just before skin incision are effective, and postoperative antibiotics to prevent surgical infection generally are not useful. For the case of cesarean delivery, where the rate of postcesarean infection is very high, that dogma is being questioned. In a recent clinical trial, 403 women with a prepregnancy BMI ≥30 kg/m2 were randomly assigned to postcesarean treatment with oral cephalexin plus metronidazole (500 mg of each medication every 8 hours for 6 doses) or placebo pills.15 All women received preoperative IV cefazolin 2 g, indicating that the dosing was probably not weight-based. The surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 6.4% and 15.4%, respectively (RR, 0.41; 95% CI, 0.22–0.77; P = .01). In a subgroup analysis based on the presence or absence of ruptured membranes, postoperative oral cephalexin plus metronidazole was most beneficial for the women with ruptured membranes. Among women with ruptured membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 9.5% and 30.2%, respectively. Among women with intact membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 5% and 8.7%, respectively.
Given that these findings are not consistent with current dogma, clinicians should be cautious about using postcesarean antibiotics and await confirmation in additional trials. Of relevance, a randomized study of women with chorioamnionitis who were treated precesarean delivery with ampicillin, gentamicin, and clindamycin did not benefit from the administration of additional postoperative antibiotics (one additional dose of gentamicin and clindamycin) compared with no postdelivery antibiotics.16
Does suture selection matter?
In one randomized trial comparing two suture types, 550 women undergoing nonemergent cesarean delivery were randomly assigned to subcuticular skin closure with polyglactin 910 (Vicryl) or poliglecaprone 25 (Monocryl) suture. The poliglecaprone 25 suture was associated with a lower rate of wound complications (8.8% vs 14.4%; 95% CI, 0.37–99; P = .04).17 However, a post-hoc analysis of a randomized trial of skin preparation did not observe a difference in wound complications between the use of polyglactinor poliglecaprone suture for skin closure.18
Prophylactic negative-pressure wound therapy: An evolving best practice?
A meta-analysis of 6 randomized trials and 3 cohort studies reported that in high-risk obese women the use of prophylactic negative-pressure wound therapy compared with standard wound dressing resulted in a decrease in surgical site infection (RR, 0.45; 95% CI, 0.31–0.66).19 The number needed to treat was 17. In one recent study, the wound outcomes following cesarean delivery among women with a BMI ≥40 kg/m2 were compared in 234 women who received and 233 women who did not receive negative-pressure wound therapy.20 Wound infection was observed in 5.6% and 9.9% of the treated and untreated women, respectively.20 However, another meta-analysis of prophylactic negative-pressure wound therapy for obese women undergoing cesarean delivery did not report any benefit.21
Let’s work on continuous improvement
Cesarean delivery is a common major operation and is associated with wound infections and endometritis at rates much greater than those observed after vaginal delivery or other major intra-abdominal operations. As obstetricians, we can do more to guide practice toward continuous improvement in surgical outcomes. Systematically using a bundle of evidence-based interventions, including proper antibiotic selection, timing, and dosing; use of hair removal with clippers; use of chlorhexidine abdominal prep; removal of the placenta with gentle traction; and closure of the subcutaneous layer if tissue depth is ≥2 cm, will reduce the rate of postcesarean infection.22 Although aspirational, we may, someday, achieve a post‑cesarean infection rate less than 1%!
CASE Conclusion
The patient was noted to be at high risk for postcesarean infection because she had both an elevated BMI and ruptured membranes. The surgeon astutely decided to administer cefazolin 3 g and azithromycin 500 mg, cleanse the vagina with povidone-iodine, use chlorhexidine for the abdominal prep, use poliglecaprone 25 subcuticular skin closure, and did not use postoperative antibiotics or prophylactic wound vacuum. Following an uneventful cesarean delivery, the patient was discharged without an infection on postoperative day 4.

Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Cameron JL. William Steward Halsted: our surgical heritage. Ann Surg. 1997;225(5):445–458.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;(10):CD007482.
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systemic review and meta-analysis. Obstet Gynecol. 2017;130(3):527–538.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgicalpreparation of the vagina. Obstet Gynecol. 2013;122(3):718–720.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2015;28(5):573–577.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. 2017;217(4):463.e1–e8.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4(1):16–19.
- Cernadas M, Smulian JC, Giannina G, Ananth CV. Effects of placental delivery method and intraoperative glove changing on postcesareanfebrile morbidity. J Matern Fetal Med. 1998;7(2):100–104.
- Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA. 2017;318(11):1026–1034.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol. 2016;33(8):732–737.
- Buresch AM, Van Arsdale A, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2017;130(3): 521–526.
- Tuuli MG, Stout MJ, Martin S, Rampersad RM, Cahill AG, Macones GA. Comparison of suture materials for subcuticular skin closure at cesarean delivery. Am J Obstet Gynecol. 2016;215(4): 490.e1–e5.
- Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200–210.e1.
- Looby MA, Vogel RI, Bangdiwala A, Hyer B, Das K. Prophylactic negative pressure wound therapy in obese patients following cesarean delivery. Surg Innov. 2018;25(1):43–49.
- Smid MD, Dotters-Katz SK, Grace M, et al. Prophylactic negative pressure wound therapy for obese women after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(5):969–978.
- Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–746.
The 25-year-old patient (G1P0) is at 41 weeks’ gestation. She has been fully dilated and pushing for 3.5 hours, at station 0, with regular strong contractions, no descent and a Category II fetal heart-rate tracing. The estimated fetal weight is 8 lb. Membranes have been ruptured for 10 hours. Maternal temperature is 99° F and her prepregnancy body mass index (BMI) was 32 kg/m2. After examining the patient and reviewing the labor progress, you recommend a cesarean delivery. As you prepare for the delivery, you identify the patient as high risk for surgical site infection and begin to recall all the interventions that might reduce postoperative infection for a patient at high risk for infection.
Halsted’s surgical principles
Dr. William Steward Halsted, the first chief of surgery at Johns Hopkins Hospital, articulated a set of surgical principles that included strict aseptic technique, gentle tissue handling, meticulous hemostasis, minimum tension on tissue, accurate tissue apposition, preservation of blood supply, and obliteration of dead space where appropriate. These principles of “safe surgery” are believed to improve surgical outcomes and reduce the risk of surgical site infection.1
Preoperative antibiotics
All obstetricians who perform cesarean delivery know the importance of administering a narrow-spectrum antibiotic, such as cefazolin or ampicillin, prior to the skin incision, but not more than 60 minutes before the incision, to help reduce the risk of wound infection and endometritis. In a meta-analysis of 82 studies involving more than 13,000 women the administration of a preoperative antibiotic compared with placebo reduced the risk of wound infection (relative risk [RR], 0.40; 95% confidence interval [CI], 0.35–0.46) and endometritis (RR, 0.38; 95% CI, 0.34–0.42).2
Cefazolin 3 g versus 2 g for obese patients
There are no data from randomized trials of cesarean delivery that directly compare the efficacy of preoperative cefazolin at doses of 2 g and 3 g to reduce the risk of infection. However, based on the observation that, for any given dose of cefazolin, circulating levels are reduced in obese patients, many authorities recommend that if the patient weighs ≥120 kg that 3 g of cefazolin should be administered.3
Extended-spectrum preoperative antibiotics
Some experts recommend that, for women in labor and for women with more than 4 hours of ruptured membranes, IV azithromycin 500 mg be added to the standard narrow-spectrum cefazolin regimen to reduce the rate of postoperative infection. In one trial, 2,013 women who were in labor or had more than 4 hours of ruptured membranes were randomly assigned to IV cefazolin alone or IV cefazolin plus azithromycin 500 mg prior to cesarean delivery.4 The cefazolin dose was reported to be weight-based utilizing the BMI at the time of delivery. The rates of endometritis (3.8% vs 6.1%) and wound infection (2.4% vs 6.6%) were lower in the women receiving extended-spectrum antibiotics versus cefazolin monotherapy.
Concerns have been raised about the impact of extended-spectrum antibiotics on the newborn microbiome and risk of accelerating the emergence of bacteria resistant to available antibiotics. Limiting the use of azithromycin to those cesarean delivery cases in which the patient is immunosuppressed, diabetic, obese, in labor and/or with prolonged ruptured membranes would reduce the number of women and newborns exposed to the drug and achieve the immediate health goal of reducing surgical infection.
Preoperative vaginal preparation
Many authorities recommend the use of a preoperative povidone- iodine vaginal scrub for 30 seconds prior to cesarean delivery for women in labor and women with ruptured membranes. In a meta-analysis of 16 trials involving 4,837 women, the women who received vaginal cleansing before cesarean delivery had a significantly lower incidence of endometritis (4.5% vs 8.8%) and postoperative fever (9.4% vs 14.9%) compared with those who did not have vaginal cleansing.5 Most of the benefit in reducing the risk of endometritis was confined to women in labor before the cesarean delivery (8.1% vs 13.8%) and women with ruptured membranes (4.3% vs 20.1%).5
Metronidazole gel 5 g also has been reported to be effective in reducing the rate of endometritis associated with cesarean delivery. In one study, 224 women having a cesarean delivery for various indications were randomly assigned to preoperative treatment with vaginally administered metronidazole gel 5 g or placebo gel. All women also received one dose of preoperative intravenous antibiotics. The rates of endometritis were 7% and 17% in the metronidazole and placebo groups, respectively.6
Povidone-iodine is approved for vaginal surgical site cleansing. For women with allergies to iodine or povidone-iodine, the options for vaginal cleansing are limited. The American College of Obstetricians and Gynecologists has noted the chlorhexidine gluconate solutions with a high concentration of alcohol should not be used for vaginal cleansing because the alcohol can irritate the mucosal epithelium. However, although not US Food and Drug Administration–approved for vaginal cleansing, solutions of chlorhexidine with a low alcohol content (Hibiclens, chlorhexidine with 4% alcohol concentration) are thought to be safe and may be considered for off-label use in vaginal cleansing.7
Preoperative abdominal preparation with chlorhexidine
Some authorities recommend skin preparation with chlorhexidine rather than povidone-iodine prior to cesarean delivery. Two recent randomized trials in women undergoing cesarean delivery8,9 and one trial in patients undergoing general surgery operations10 reported a reduction in surgical site infection with chlorhexidine. However, other trials have reported no difference in the rate of surgical site infection with these two skin preparation methods.11,12
Changing gloves and equipment after delivery of the newborn
Currently there is no high-quality evidence that changing gloves after delivery of the newborn or using new surgical instruments for closure reduces the risk of postcesarean infection. Two small clinical trials reported that changing gloves after delivery of the newborn did not reduce the rate of postcesarean infection.13,14
Postoperative antibiotics (a heretical challenge to the central dogma of antibiotic prophylaxis in surgery)
The central dogma of antibiotic prevention of postoperative infection is that antibiotics administered just before skin incision are effective, and postoperative antibiotics to prevent surgical infection generally are not useful. For the case of cesarean delivery, where the rate of postcesarean infection is very high, that dogma is being questioned. In a recent clinical trial, 403 women with a prepregnancy BMI ≥30 kg/m2 were randomly assigned to postcesarean treatment with oral cephalexin plus metronidazole (500 mg of each medication every 8 hours for 6 doses) or placebo pills.15 All women received preoperative IV cefazolin 2 g, indicating that the dosing was probably not weight-based. The surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 6.4% and 15.4%, respectively (RR, 0.41; 95% CI, 0.22–0.77; P = .01). In a subgroup analysis based on the presence or absence of ruptured membranes, postoperative oral cephalexin plus metronidazole was most beneficial for the women with ruptured membranes. Among women with ruptured membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 9.5% and 30.2%, respectively. Among women with intact membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 5% and 8.7%, respectively.
Given that these findings are not consistent with current dogma, clinicians should be cautious about using postcesarean antibiotics and await confirmation in additional trials. Of relevance, a randomized study of women with chorioamnionitis who were treated precesarean delivery with ampicillin, gentamicin, and clindamycin did not benefit from the administration of additional postoperative antibiotics (one additional dose of gentamicin and clindamycin) compared with no postdelivery antibiotics.16
Does suture selection matter?
In one randomized trial comparing two suture types, 550 women undergoing nonemergent cesarean delivery were randomly assigned to subcuticular skin closure with polyglactin 910 (Vicryl) or poliglecaprone 25 (Monocryl) suture. The poliglecaprone 25 suture was associated with a lower rate of wound complications (8.8% vs 14.4%; 95% CI, 0.37–99; P = .04).17 However, a post-hoc analysis of a randomized trial of skin preparation did not observe a difference in wound complications between the use of polyglactinor poliglecaprone suture for skin closure.18
Prophylactic negative-pressure wound therapy: An evolving best practice?
A meta-analysis of 6 randomized trials and 3 cohort studies reported that in high-risk obese women the use of prophylactic negative-pressure wound therapy compared with standard wound dressing resulted in a decrease in surgical site infection (RR, 0.45; 95% CI, 0.31–0.66).19 The number needed to treat was 17. In one recent study, the wound outcomes following cesarean delivery among women with a BMI ≥40 kg/m2 were compared in 234 women who received and 233 women who did not receive negative-pressure wound therapy.20 Wound infection was observed in 5.6% and 9.9% of the treated and untreated women, respectively.20 However, another meta-analysis of prophylactic negative-pressure wound therapy for obese women undergoing cesarean delivery did not report any benefit.21
Let’s work on continuous improvement
Cesarean delivery is a common major operation and is associated with wound infections and endometritis at rates much greater than those observed after vaginal delivery or other major intra-abdominal operations. As obstetricians, we can do more to guide practice toward continuous improvement in surgical outcomes. Systematically using a bundle of evidence-based interventions, including proper antibiotic selection, timing, and dosing; use of hair removal with clippers; use of chlorhexidine abdominal prep; removal of the placenta with gentle traction; and closure of the subcutaneous layer if tissue depth is ≥2 cm, will reduce the rate of postcesarean infection.22 Although aspirational, we may, someday, achieve a post‑cesarean infection rate less than 1%!
CASE Conclusion
The patient was noted to be at high risk for postcesarean infection because she had both an elevated BMI and ruptured membranes. The surgeon astutely decided to administer cefazolin 3 g and azithromycin 500 mg, cleanse the vagina with povidone-iodine, use chlorhexidine for the abdominal prep, use poliglecaprone 25 subcuticular skin closure, and did not use postoperative antibiotics or prophylactic wound vacuum. Following an uneventful cesarean delivery, the patient was discharged without an infection on postoperative day 4.

Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The 25-year-old patient (G1P0) is at 41 weeks’ gestation. She has been fully dilated and pushing for 3.5 hours, at station 0, with regular strong contractions, no descent and a Category II fetal heart-rate tracing. The estimated fetal weight is 8 lb. Membranes have been ruptured for 10 hours. Maternal temperature is 99° F and her prepregnancy body mass index (BMI) was 32 kg/m2. After examining the patient and reviewing the labor progress, you recommend a cesarean delivery. As you prepare for the delivery, you identify the patient as high risk for surgical site infection and begin to recall all the interventions that might reduce postoperative infection for a patient at high risk for infection.
Halsted’s surgical principles
Dr. William Steward Halsted, the first chief of surgery at Johns Hopkins Hospital, articulated a set of surgical principles that included strict aseptic technique, gentle tissue handling, meticulous hemostasis, minimum tension on tissue, accurate tissue apposition, preservation of blood supply, and obliteration of dead space where appropriate. These principles of “safe surgery” are believed to improve surgical outcomes and reduce the risk of surgical site infection.1
Preoperative antibiotics
All obstetricians who perform cesarean delivery know the importance of administering a narrow-spectrum antibiotic, such as cefazolin or ampicillin, prior to the skin incision, but not more than 60 minutes before the incision, to help reduce the risk of wound infection and endometritis. In a meta-analysis of 82 studies involving more than 13,000 women the administration of a preoperative antibiotic compared with placebo reduced the risk of wound infection (relative risk [RR], 0.40; 95% confidence interval [CI], 0.35–0.46) and endometritis (RR, 0.38; 95% CI, 0.34–0.42).2
Cefazolin 3 g versus 2 g for obese patients
There are no data from randomized trials of cesarean delivery that directly compare the efficacy of preoperative cefazolin at doses of 2 g and 3 g to reduce the risk of infection. However, based on the observation that, for any given dose of cefazolin, circulating levels are reduced in obese patients, many authorities recommend that if the patient weighs ≥120 kg that 3 g of cefazolin should be administered.3
Extended-spectrum preoperative antibiotics
Some experts recommend that, for women in labor and for women with more than 4 hours of ruptured membranes, IV azithromycin 500 mg be added to the standard narrow-spectrum cefazolin regimen to reduce the rate of postoperative infection. In one trial, 2,013 women who were in labor or had more than 4 hours of ruptured membranes were randomly assigned to IV cefazolin alone or IV cefazolin plus azithromycin 500 mg prior to cesarean delivery.4 The cefazolin dose was reported to be weight-based utilizing the BMI at the time of delivery. The rates of endometritis (3.8% vs 6.1%) and wound infection (2.4% vs 6.6%) were lower in the women receiving extended-spectrum antibiotics versus cefazolin monotherapy.
Concerns have been raised about the impact of extended-spectrum antibiotics on the newborn microbiome and risk of accelerating the emergence of bacteria resistant to available antibiotics. Limiting the use of azithromycin to those cesarean delivery cases in which the patient is immunosuppressed, diabetic, obese, in labor and/or with prolonged ruptured membranes would reduce the number of women and newborns exposed to the drug and achieve the immediate health goal of reducing surgical infection.
Preoperative vaginal preparation
Many authorities recommend the use of a preoperative povidone- iodine vaginal scrub for 30 seconds prior to cesarean delivery for women in labor and women with ruptured membranes. In a meta-analysis of 16 trials involving 4,837 women, the women who received vaginal cleansing before cesarean delivery had a significantly lower incidence of endometritis (4.5% vs 8.8%) and postoperative fever (9.4% vs 14.9%) compared with those who did not have vaginal cleansing.5 Most of the benefit in reducing the risk of endometritis was confined to women in labor before the cesarean delivery (8.1% vs 13.8%) and women with ruptured membranes (4.3% vs 20.1%).5
Metronidazole gel 5 g also has been reported to be effective in reducing the rate of endometritis associated with cesarean delivery. In one study, 224 women having a cesarean delivery for various indications were randomly assigned to preoperative treatment with vaginally administered metronidazole gel 5 g or placebo gel. All women also received one dose of preoperative intravenous antibiotics. The rates of endometritis were 7% and 17% in the metronidazole and placebo groups, respectively.6
Povidone-iodine is approved for vaginal surgical site cleansing. For women with allergies to iodine or povidone-iodine, the options for vaginal cleansing are limited. The American College of Obstetricians and Gynecologists has noted the chlorhexidine gluconate solutions with a high concentration of alcohol should not be used for vaginal cleansing because the alcohol can irritate the mucosal epithelium. However, although not US Food and Drug Administration–approved for vaginal cleansing, solutions of chlorhexidine with a low alcohol content (Hibiclens, chlorhexidine with 4% alcohol concentration) are thought to be safe and may be considered for off-label use in vaginal cleansing.7
Preoperative abdominal preparation with chlorhexidine
Some authorities recommend skin preparation with chlorhexidine rather than povidone-iodine prior to cesarean delivery. Two recent randomized trials in women undergoing cesarean delivery8,9 and one trial in patients undergoing general surgery operations10 reported a reduction in surgical site infection with chlorhexidine. However, other trials have reported no difference in the rate of surgical site infection with these two skin preparation methods.11,12
Changing gloves and equipment after delivery of the newborn
Currently there is no high-quality evidence that changing gloves after delivery of the newborn or using new surgical instruments for closure reduces the risk of postcesarean infection. Two small clinical trials reported that changing gloves after delivery of the newborn did not reduce the rate of postcesarean infection.13,14
Postoperative antibiotics (a heretical challenge to the central dogma of antibiotic prophylaxis in surgery)
The central dogma of antibiotic prevention of postoperative infection is that antibiotics administered just before skin incision are effective, and postoperative antibiotics to prevent surgical infection generally are not useful. For the case of cesarean delivery, where the rate of postcesarean infection is very high, that dogma is being questioned. In a recent clinical trial, 403 women with a prepregnancy BMI ≥30 kg/m2 were randomly assigned to postcesarean treatment with oral cephalexin plus metronidazole (500 mg of each medication every 8 hours for 6 doses) or placebo pills.15 All women received preoperative IV cefazolin 2 g, indicating that the dosing was probably not weight-based. The surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 6.4% and 15.4%, respectively (RR, 0.41; 95% CI, 0.22–0.77; P = .01). In a subgroup analysis based on the presence or absence of ruptured membranes, postoperative oral cephalexin plus metronidazole was most beneficial for the women with ruptured membranes. Among women with ruptured membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 9.5% and 30.2%, respectively. Among women with intact membranes the surgical site infection rates in the cephalexin plus metronidazole and placebo groups were 5% and 8.7%, respectively.
Given that these findings are not consistent with current dogma, clinicians should be cautious about using postcesarean antibiotics and await confirmation in additional trials. Of relevance, a randomized study of women with chorioamnionitis who were treated precesarean delivery with ampicillin, gentamicin, and clindamycin did not benefit from the administration of additional postoperative antibiotics (one additional dose of gentamicin and clindamycin) compared with no postdelivery antibiotics.16
Does suture selection matter?
In one randomized trial comparing two suture types, 550 women undergoing nonemergent cesarean delivery were randomly assigned to subcuticular skin closure with polyglactin 910 (Vicryl) or poliglecaprone 25 (Monocryl) suture. The poliglecaprone 25 suture was associated with a lower rate of wound complications (8.8% vs 14.4%; 95% CI, 0.37–99; P = .04).17 However, a post-hoc analysis of a randomized trial of skin preparation did not observe a difference in wound complications between the use of polyglactinor poliglecaprone suture for skin closure.18
Prophylactic negative-pressure wound therapy: An evolving best practice?
A meta-analysis of 6 randomized trials and 3 cohort studies reported that in high-risk obese women the use of prophylactic negative-pressure wound therapy compared with standard wound dressing resulted in a decrease in surgical site infection (RR, 0.45; 95% CI, 0.31–0.66).19 The number needed to treat was 17. In one recent study, the wound outcomes following cesarean delivery among women with a BMI ≥40 kg/m2 were compared in 234 women who received and 233 women who did not receive negative-pressure wound therapy.20 Wound infection was observed in 5.6% and 9.9% of the treated and untreated women, respectively.20 However, another meta-analysis of prophylactic negative-pressure wound therapy for obese women undergoing cesarean delivery did not report any benefit.21
Let’s work on continuous improvement
Cesarean delivery is a common major operation and is associated with wound infections and endometritis at rates much greater than those observed after vaginal delivery or other major intra-abdominal operations. As obstetricians, we can do more to guide practice toward continuous improvement in surgical outcomes. Systematically using a bundle of evidence-based interventions, including proper antibiotic selection, timing, and dosing; use of hair removal with clippers; use of chlorhexidine abdominal prep; removal of the placenta with gentle traction; and closure of the subcutaneous layer if tissue depth is ≥2 cm, will reduce the rate of postcesarean infection.22 Although aspirational, we may, someday, achieve a post‑cesarean infection rate less than 1%!
CASE Conclusion
The patient was noted to be at high risk for postcesarean infection because she had both an elevated BMI and ruptured membranes. The surgeon astutely decided to administer cefazolin 3 g and azithromycin 500 mg, cleanse the vagina with povidone-iodine, use chlorhexidine for the abdominal prep, use poliglecaprone 25 subcuticular skin closure, and did not use postoperative antibiotics or prophylactic wound vacuum. Following an uneventful cesarean delivery, the patient was discharged without an infection on postoperative day 4.

Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Cameron JL. William Steward Halsted: our surgical heritage. Ann Surg. 1997;225(5):445–458.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;(10):CD007482.
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systemic review and meta-analysis. Obstet Gynecol. 2017;130(3):527–538.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgicalpreparation of the vagina. Obstet Gynecol. 2013;122(3):718–720.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2015;28(5):573–577.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. 2017;217(4):463.e1–e8.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4(1):16–19.
- Cernadas M, Smulian JC, Giannina G, Ananth CV. Effects of placental delivery method and intraoperative glove changing on postcesareanfebrile morbidity. J Matern Fetal Med. 1998;7(2):100–104.
- Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA. 2017;318(11):1026–1034.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol. 2016;33(8):732–737.
- Buresch AM, Van Arsdale A, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2017;130(3): 521–526.
- Tuuli MG, Stout MJ, Martin S, Rampersad RM, Cahill AG, Macones GA. Comparison of suture materials for subcuticular skin closure at cesarean delivery. Am J Obstet Gynecol. 2016;215(4): 490.e1–e5.
- Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200–210.e1.
- Looby MA, Vogel RI, Bangdiwala A, Hyer B, Das K. Prophylactic negative pressure wound therapy in obese patients following cesarean delivery. Surg Innov. 2018;25(1):43–49.
- Smid MD, Dotters-Katz SK, Grace M, et al. Prophylactic negative pressure wound therapy for obese women after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(5):969–978.
- Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–746.
- Cameron JL. William Steward Halsted: our surgical heritage. Ann Surg. 1997;225(5):445–458.
- Smaill FM, Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014;(10):CD007482.
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283.
- Tita AT, Szychowski JM, Boggess K, et al; C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231–1241.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systemic review and meta-analysis. Obstet Gynecol. 2017;130(3):527–538.
- Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 pt 1):745–750.
- American College of Obstetricians and Gynecologists; Committee on Gynecologic Practice. Committee Opinion No. 571: solutions for surgicalpreparation of the vagina. Obstet Gynecol. 2013;122(3):718–720.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. J Matern Fetal Neonatal Med. 2015;28(5):573–577.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol vs povidone-iodine for cesarean antisepsis: the CAPICA trial. Am J Obstet Gynecol. 2017;217(4):463.e1–e8.
- Turrentine MA, Banks TA. Effect of changing gloves before placental extraction on incidence of postcesarean endometritis. Infect Dis Obstet Gynecol. 1996;4(1):16–19.
- Cernadas M, Smulian JC, Giannina G, Ananth CV. Effects of placental delivery method and intraoperative glove changing on postcesareanfebrile morbidity. J Matern Fetal Med. 1998;7(2):100–104.
- Valent AM, DeArmond C, Houston JM, et al. Effect of post-cesarean delivery oral cephalexin and metronidazole on surgical site infection among obese women: a randomized clinical trial. JAMA. 2017;318(11):1026–1034.
- Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol. 2016;33(8):732–737.
- Buresch AM, Van Arsdale A, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2017;130(3): 521–526.
- Tuuli MG, Stout MJ, Martin S, Rampersad RM, Cahill AG, Macones GA. Comparison of suture materials for subcuticular skin closure at cesarean delivery. Am J Obstet Gynecol. 2016;215(4): 490.e1–e5.
- Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200–210.e1.
- Looby MA, Vogel RI, Bangdiwala A, Hyer B, Das K. Prophylactic negative pressure wound therapy in obese patients following cesarean delivery. Surg Innov. 2018;25(1):43–49.
- Smid MD, Dotters-Katz SK, Grace M, et al. Prophylactic negative pressure wound therapy for obese women after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(5):969–978.
- Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(4):735–746.
Sling revisions: pain as indication linked with SUI recurrence
ORLANDO – Pain as an indication for midurethral sling revision is associated with an elevated risk of postoperative stress urinary incontinence recurrence, according to a review of 129 cases.
At a mean follow-up of 21 months, the overall rate of recurrent stress urinary incontinence (SUI) among study subjects was 39.5%, which is similar to what has been previously reported in the literature. However, women who underwent revision for the indication of pain, including dyspareunia, pelvic pain, or muscle spasms, had an SUI recurrence rate of 70%, Meagan S. Cramer, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Women older than 55 years at the time of revision were significantly less likely to recur (adjusted odds ratio, 0.34 vs. younger women), and those whose original slings were placed less than 1 year prior to revision had a significantly lower rate of SUI recurrence, compared with those whose slings were placed more than 1 year prior (23.1% vs. 46.7%), she noted.
“There was no difference in BMI [body mass index], race, or baseline comorbidities between the recurrence vs. no recurrence group. There was also no difference in estrogen use preoperatively, concurrent replacement with another sling, preoperative [pelvic organ prolapse quantification] exam, length of sling excised, or the original type of sling placed between the recurrence versus non recurrence groups,” she said. “On multivariable regression analysis controlling for age, BMI, race, tobacco use, time since original sling, erosion on exam, technique used for revision, and presenting indication, pain as an indication of revision was still associated with an increased risk for recurrent incontinence, with an adjusted odds ratio of 9.08.”
Study subjects were women aged 18 years and older who underwent mesh sling revision from January 2009 to July 2016 at a single center. Women were excluded if they underwent revision or excision of vaginal mesh, or if they underwent sling adjustment for persistent incontinence if the incontinence had never resolved after the original sling placement. Those without postoperative follow-up were also excluded.
“Approximately 2%-4% of women who undergo midurethral sling surgery for stress incontinence will later require revision or excision of the sling due to erosion, pain, and/or voiding dysfunction. Based on current literature, up to 56% of these women can experience postoperative recurrence of stress incontinence following their revision,” Dr. Cramer said, noting that there is no consensus on which women will require future surgeries for stress incontinence after revision.
In the current study, women with pain were significantly more likely to undergo a complete excision of their sling (45.0% vs. 17.9% with other indications).
“We also discovered that of all women who underwent complete excision of a prior sling, those with pain had a higher rate of recurrent SUI, compared with those without pain [66.7% vs. 31.6%], but the difference was not statistically significant,” Dr. Cramer said in an interview.
She noted, however, that partial sling excision has been shown in at least one prior study “to provide just as much benefit as complete sling excision for patients with pain without leading to an increased risk of SUI.”
Though limited by the retrospective study design, the current findings suggest that pain as an indication for sling revision is associated with a higher risk of postoperative recurrent SUI, regardless of the type of revision, she said.
“Future prospective studies investigating the relationship between the indication for revision and postoperative stress incontinence would help to further define the roles of pain in this process,” she said, adding that in the meantime, given the prior findings regarding the benefits of partial sling excision, it may be reasonable to advise patients with pain that undergoing a partial sling excision could reduce the risk of recurrent SUI.
Dr. Cramer reported having no disclosures.
SOURCE: Cramer MS et al. SGS 2018, Oral Presentation 04.
ORLANDO – Pain as an indication for midurethral sling revision is associated with an elevated risk of postoperative stress urinary incontinence recurrence, according to a review of 129 cases.
At a mean follow-up of 21 months, the overall rate of recurrent stress urinary incontinence (SUI) among study subjects was 39.5%, which is similar to what has been previously reported in the literature. However, women who underwent revision for the indication of pain, including dyspareunia, pelvic pain, or muscle spasms, had an SUI recurrence rate of 70%, Meagan S. Cramer, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Women older than 55 years at the time of revision were significantly less likely to recur (adjusted odds ratio, 0.34 vs. younger women), and those whose original slings were placed less than 1 year prior to revision had a significantly lower rate of SUI recurrence, compared with those whose slings were placed more than 1 year prior (23.1% vs. 46.7%), she noted.
“There was no difference in BMI [body mass index], race, or baseline comorbidities between the recurrence vs. no recurrence group. There was also no difference in estrogen use preoperatively, concurrent replacement with another sling, preoperative [pelvic organ prolapse quantification] exam, length of sling excised, or the original type of sling placed between the recurrence versus non recurrence groups,” she said. “On multivariable regression analysis controlling for age, BMI, race, tobacco use, time since original sling, erosion on exam, technique used for revision, and presenting indication, pain as an indication of revision was still associated with an increased risk for recurrent incontinence, with an adjusted odds ratio of 9.08.”
Study subjects were women aged 18 years and older who underwent mesh sling revision from January 2009 to July 2016 at a single center. Women were excluded if they underwent revision or excision of vaginal mesh, or if they underwent sling adjustment for persistent incontinence if the incontinence had never resolved after the original sling placement. Those without postoperative follow-up were also excluded.
“Approximately 2%-4% of women who undergo midurethral sling surgery for stress incontinence will later require revision or excision of the sling due to erosion, pain, and/or voiding dysfunction. Based on current literature, up to 56% of these women can experience postoperative recurrence of stress incontinence following their revision,” Dr. Cramer said, noting that there is no consensus on which women will require future surgeries for stress incontinence after revision.
In the current study, women with pain were significantly more likely to undergo a complete excision of their sling (45.0% vs. 17.9% with other indications).
“We also discovered that of all women who underwent complete excision of a prior sling, those with pain had a higher rate of recurrent SUI, compared with those without pain [66.7% vs. 31.6%], but the difference was not statistically significant,” Dr. Cramer said in an interview.
She noted, however, that partial sling excision has been shown in at least one prior study “to provide just as much benefit as complete sling excision for patients with pain without leading to an increased risk of SUI.”
Though limited by the retrospective study design, the current findings suggest that pain as an indication for sling revision is associated with a higher risk of postoperative recurrent SUI, regardless of the type of revision, she said.
“Future prospective studies investigating the relationship between the indication for revision and postoperative stress incontinence would help to further define the roles of pain in this process,” she said, adding that in the meantime, given the prior findings regarding the benefits of partial sling excision, it may be reasonable to advise patients with pain that undergoing a partial sling excision could reduce the risk of recurrent SUI.
Dr. Cramer reported having no disclosures.
SOURCE: Cramer MS et al. SGS 2018, Oral Presentation 04.
ORLANDO – Pain as an indication for midurethral sling revision is associated with an elevated risk of postoperative stress urinary incontinence recurrence, according to a review of 129 cases.
At a mean follow-up of 21 months, the overall rate of recurrent stress urinary incontinence (SUI) among study subjects was 39.5%, which is similar to what has been previously reported in the literature. However, women who underwent revision for the indication of pain, including dyspareunia, pelvic pain, or muscle spasms, had an SUI recurrence rate of 70%, Meagan S. Cramer, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Women older than 55 years at the time of revision were significantly less likely to recur (adjusted odds ratio, 0.34 vs. younger women), and those whose original slings were placed less than 1 year prior to revision had a significantly lower rate of SUI recurrence, compared with those whose slings were placed more than 1 year prior (23.1% vs. 46.7%), she noted.
“There was no difference in BMI [body mass index], race, or baseline comorbidities between the recurrence vs. no recurrence group. There was also no difference in estrogen use preoperatively, concurrent replacement with another sling, preoperative [pelvic organ prolapse quantification] exam, length of sling excised, or the original type of sling placed between the recurrence versus non recurrence groups,” she said. “On multivariable regression analysis controlling for age, BMI, race, tobacco use, time since original sling, erosion on exam, technique used for revision, and presenting indication, pain as an indication of revision was still associated with an increased risk for recurrent incontinence, with an adjusted odds ratio of 9.08.”
Study subjects were women aged 18 years and older who underwent mesh sling revision from January 2009 to July 2016 at a single center. Women were excluded if they underwent revision or excision of vaginal mesh, or if they underwent sling adjustment for persistent incontinence if the incontinence had never resolved after the original sling placement. Those without postoperative follow-up were also excluded.
“Approximately 2%-4% of women who undergo midurethral sling surgery for stress incontinence will later require revision or excision of the sling due to erosion, pain, and/or voiding dysfunction. Based on current literature, up to 56% of these women can experience postoperative recurrence of stress incontinence following their revision,” Dr. Cramer said, noting that there is no consensus on which women will require future surgeries for stress incontinence after revision.
In the current study, women with pain were significantly more likely to undergo a complete excision of their sling (45.0% vs. 17.9% with other indications).
“We also discovered that of all women who underwent complete excision of a prior sling, those with pain had a higher rate of recurrent SUI, compared with those without pain [66.7% vs. 31.6%], but the difference was not statistically significant,” Dr. Cramer said in an interview.
She noted, however, that partial sling excision has been shown in at least one prior study “to provide just as much benefit as complete sling excision for patients with pain without leading to an increased risk of SUI.”
Though limited by the retrospective study design, the current findings suggest that pain as an indication for sling revision is associated with a higher risk of postoperative recurrent SUI, regardless of the type of revision, she said.
“Future prospective studies investigating the relationship between the indication for revision and postoperative stress incontinence would help to further define the roles of pain in this process,” she said, adding that in the meantime, given the prior findings regarding the benefits of partial sling excision, it may be reasonable to advise patients with pain that undergoing a partial sling excision could reduce the risk of recurrent SUI.
Dr. Cramer reported having no disclosures.
SOURCE: Cramer MS et al. SGS 2018, Oral Presentation 04.
REPORTING FROM SGS 2018
Key clinical point: Sling revisions for pain may increase the risk of postoperative stress urinary incontinence recurrence.
Major finding: The recurrent SUI rate after revision was 39.5% overall vs. 70% among those with pain as an indication.
Study details: A retrospective cohort study of 129 women.
Disclosures: Dr. Cramer reported having no disclosures.
Source: Cramer MS et al. SGS 2018, Oral Presentation 04.
Anterior discoid resection using a ‘squeeze’ technique
Rectosigmoid endometriosis has been estimated to affect between 4% and 37% of patients with endometriosis and is one of the most advanced and complex forms of the disease. Bowel endometriosis can be asymptomatic but often involves severe dysmenorrhea, dyspareunia, and a spectrum of bowel symptoms such as dyschezia, diarrhea, constipation, bloating, and rectal bleeding. Deep infiltrating rectovaginal endometriosis causes persistent or recurrent pain and is best treated by surgical removal of nodular lesions.
I have found that laparoscopic full-thickness disc resection (anterior discoid resection) with primary two-layer closure is often feasible and avoids the need for a complete bowel reanastomosis. It may not be an option in cases of multifocal rectal involvement (which may affect between one-quarter and one-third of patients with bowel endometriosis) or in cases involving large rectal nodules or luminal stenosis secondary to advanced fibrosis. In these cases, segmental bowel resection (low anterior resection) is often necessary. When anterior discoid resection is feasible, however, patients face significantly less morbidity with comparable outcomes.
Less morbidity
Preoperative evaluation is far from straightforward, and practices vary. Transvaginal ultrasonography is used for diagnosing rectal endometriosis in select centers in certain regions of the world, but there are important limitations; not only is it highly operator dependent, but its limited range does not allow for the detection of endometriosis higher in the sigmoid colon. Endorectal ultrasonography can be an excellent tool for more fully evaluating rectal wall involvement, but it does not usually allow for the evaluation of disease elsewhere in the pelvis.
The preoperative tool we utilize most often along with clinical examination is MRI with vaginal and rectal contrast. MRI provides us with a superior anatomic perspective on the disease. Not only can we assess the depth of bowel wall infiltration and the distribution of the affected areas of the bowel, but we can see the bladder, the uterosacral ligaments, and how the uterus is situated relative to areas of disease. However, there are individualized limits to how high the contrast will travel, even with bowel preparation; disease that occurs significantly above the uterus often cannot be visualized as well as disease that occurs lower.
My general surgeon colleague and I have been working together for years, and we both are involved in counseling the patient suspected of having deep infiltrating disease. I typically talk with the patient about the probability of segmental resection based on my exam and preoperative MRI, and my colleague expands on this discussion with further explanation of the risks of bowel surgery.
Segmental resection has been associated with significant postoperative complications. In a single-center series of 436 laparoscopic colorectal resections for deep infiltrating endometriosis, rectovaginal and anastomotic fistula were among the most frequent postoperative complications (3.2% and 1.1%), along with transient urinary retention, which occurred in almost 20% (Surg Endosc. 2010 Jan;24:63-7).
Patients undergoing discoid resection for deep infiltrating endometriosis also had a significantly lower rate of temporary ileostomy (2.1% vs. 9.1%), a reduced rate of postoperative fever, and a reduced rate of gastrointestinal complications, mainly anastomotic leak or rectovaginal fistula (2.1% vs. 5.6%). There were no significant differences in the recurrence rate (13.8% vs. 11.5%).
A retrospective cohort study from our institution similarly showed decreased operative time, blood loss, hospital stay, and a lower rate of anastomotic strictures in patients who underwent laparoscopic anterior discoid resection between 2001 and 2009. The ADR group consistently had higher increments of improvement in bowel symptoms and dyspareunia, compared with patients who were selected to have segmental resection. Patients were followed for a mean of 41 months (JSLS. 2011;15[3]:331-8).
In general, there is agreement among surgeons that for consideration of discoid resection, nodule diameter should not exceed 3 cm, with a maximum of half of the bowel circumference and a maximum of 60% stenosis. I view these numbers as guiding principles, however, and not firm rules. Surgical decisions should be personalized based on the patient, the surgeon’s impression of the extent of the disease, and the ability to perform anterior discoid resection without compromising the rectal lumen with primary closure of the defect.
The technique
Rectosigmoid endometriotic nodules may present within the context of an obliterated posterior cul-de-sac, but the avascular pararectal space can be used to approach the nodules. Detailed knowledge of the avascular planes of this space, as well as the rectovaginal space, is crucial. Development of the rectovaginal space frees the bowel from its attachments to the posterior uterus and vagina. Judicious use of energized instruments in sharp dissection, and frequently sharp cold cutting, should be used near the bowel serosa to prevent thermal injury.
Presurgical imaging usually offers a good assessment of a nodule’s size and location, but intraoperatively, I typically use an atraumatic grasper to further assess size and contour and to determine if the nodule is suitable for discoid resection. If so, a suture is placed through the nodule to improve manipulation, and enucleation of the nodule itself is achieved through a “squeeze” technique in which an advanced bipolar device is used to circumscribe the lesion, dissecting the nodule as the device bounces off the thick endometriotic tissue.
The ENSEAL bipolar device (Ethicon, Somerville, N.J.) was designed as a vessel sealer, but because it will not cut through hard tissue as will other laparoscopic cutting devices, it serves as a useful tool for resecting endometriotic nodules while minimizing the removal of healthy rectal tissue. The device bounces off the nodule because it will avoid cutting through the thick tissue; in the process, it facilitates a fairly complete enucleation of the endometriotic nodule, starting with dissection until an intentional colotomy/enterotomy is made and followed by circumscription of the lesion once the rectum is entered.
Gentle traction and counter-traction increase the efficiency of dissection and minimize the amount of normal rectal tissue removed. Quick cutting with short bursts of energy allows for good hemostasis and minimizes thermal spread, which will maximize tissue healing from subsequent repair.
I then use a rectal probe as a template for repair. The probe is advanced underneath the defect between the distal and proximal portions, and the tissue is moved over the probe to ensure that the repair will be tension free. An ability to reapproximate the defect while keeping the probe in place indicates that the defect can be safely closed. (For a video presentation of the surgery, see www.surgeryu.com/leeobgyn.) If suturing is not feasible, the general surgeon is called to perform segmental resection.
The integrity of the repair is then thoroughly assessed with an air leak test. A bowel clamp is placed across the rectum and the pelvis is filled with sterile saline. Air is placed into the rectum with a rigid proctoscope while the operative field is inspected for evidence of an air leak.
Discoid resection may also be performed with a circular stapler. While this technique is faster than suturing, its use is limited by nodule size and has the potential to compromise complete excision of the nodule.
Dr. Lee is director of minimally invasive gynecologic surgery, Magee-Women’s Hospital of the University of Pittsburgh Medical Center.
Rectosigmoid endometriosis has been estimated to affect between 4% and 37% of patients with endometriosis and is one of the most advanced and complex forms of the disease. Bowel endometriosis can be asymptomatic but often involves severe dysmenorrhea, dyspareunia, and a spectrum of bowel symptoms such as dyschezia, diarrhea, constipation, bloating, and rectal bleeding. Deep infiltrating rectovaginal endometriosis causes persistent or recurrent pain and is best treated by surgical removal of nodular lesions.
I have found that laparoscopic full-thickness disc resection (anterior discoid resection) with primary two-layer closure is often feasible and avoids the need for a complete bowel reanastomosis. It may not be an option in cases of multifocal rectal involvement (which may affect between one-quarter and one-third of patients with bowel endometriosis) or in cases involving large rectal nodules or luminal stenosis secondary to advanced fibrosis. In these cases, segmental bowel resection (low anterior resection) is often necessary. When anterior discoid resection is feasible, however, patients face significantly less morbidity with comparable outcomes.
Less morbidity
Preoperative evaluation is far from straightforward, and practices vary. Transvaginal ultrasonography is used for diagnosing rectal endometriosis in select centers in certain regions of the world, but there are important limitations; not only is it highly operator dependent, but its limited range does not allow for the detection of endometriosis higher in the sigmoid colon. Endorectal ultrasonography can be an excellent tool for more fully evaluating rectal wall involvement, but it does not usually allow for the evaluation of disease elsewhere in the pelvis.
The preoperative tool we utilize most often along with clinical examination is MRI with vaginal and rectal contrast. MRI provides us with a superior anatomic perspective on the disease. Not only can we assess the depth of bowel wall infiltration and the distribution of the affected areas of the bowel, but we can see the bladder, the uterosacral ligaments, and how the uterus is situated relative to areas of disease. However, there are individualized limits to how high the contrast will travel, even with bowel preparation; disease that occurs significantly above the uterus often cannot be visualized as well as disease that occurs lower.
My general surgeon colleague and I have been working together for years, and we both are involved in counseling the patient suspected of having deep infiltrating disease. I typically talk with the patient about the probability of segmental resection based on my exam and preoperative MRI, and my colleague expands on this discussion with further explanation of the risks of bowel surgery.
Segmental resection has been associated with significant postoperative complications. In a single-center series of 436 laparoscopic colorectal resections for deep infiltrating endometriosis, rectovaginal and anastomotic fistula were among the most frequent postoperative complications (3.2% and 1.1%), along with transient urinary retention, which occurred in almost 20% (Surg Endosc. 2010 Jan;24:63-7).
Patients undergoing discoid resection for deep infiltrating endometriosis also had a significantly lower rate of temporary ileostomy (2.1% vs. 9.1%), a reduced rate of postoperative fever, and a reduced rate of gastrointestinal complications, mainly anastomotic leak or rectovaginal fistula (2.1% vs. 5.6%). There were no significant differences in the recurrence rate (13.8% vs. 11.5%).
A retrospective cohort study from our institution similarly showed decreased operative time, blood loss, hospital stay, and a lower rate of anastomotic strictures in patients who underwent laparoscopic anterior discoid resection between 2001 and 2009. The ADR group consistently had higher increments of improvement in bowel symptoms and dyspareunia, compared with patients who were selected to have segmental resection. Patients were followed for a mean of 41 months (JSLS. 2011;15[3]:331-8).
In general, there is agreement among surgeons that for consideration of discoid resection, nodule diameter should not exceed 3 cm, with a maximum of half of the bowel circumference and a maximum of 60% stenosis. I view these numbers as guiding principles, however, and not firm rules. Surgical decisions should be personalized based on the patient, the surgeon’s impression of the extent of the disease, and the ability to perform anterior discoid resection without compromising the rectal lumen with primary closure of the defect.
The technique
Rectosigmoid endometriotic nodules may present within the context of an obliterated posterior cul-de-sac, but the avascular pararectal space can be used to approach the nodules. Detailed knowledge of the avascular planes of this space, as well as the rectovaginal space, is crucial. Development of the rectovaginal space frees the bowel from its attachments to the posterior uterus and vagina. Judicious use of energized instruments in sharp dissection, and frequently sharp cold cutting, should be used near the bowel serosa to prevent thermal injury.
Presurgical imaging usually offers a good assessment of a nodule’s size and location, but intraoperatively, I typically use an atraumatic grasper to further assess size and contour and to determine if the nodule is suitable for discoid resection. If so, a suture is placed through the nodule to improve manipulation, and enucleation of the nodule itself is achieved through a “squeeze” technique in which an advanced bipolar device is used to circumscribe the lesion, dissecting the nodule as the device bounces off the thick endometriotic tissue.
The ENSEAL bipolar device (Ethicon, Somerville, N.J.) was designed as a vessel sealer, but because it will not cut through hard tissue as will other laparoscopic cutting devices, it serves as a useful tool for resecting endometriotic nodules while minimizing the removal of healthy rectal tissue. The device bounces off the nodule because it will avoid cutting through the thick tissue; in the process, it facilitates a fairly complete enucleation of the endometriotic nodule, starting with dissection until an intentional colotomy/enterotomy is made and followed by circumscription of the lesion once the rectum is entered.
Gentle traction and counter-traction increase the efficiency of dissection and minimize the amount of normal rectal tissue removed. Quick cutting with short bursts of energy allows for good hemostasis and minimizes thermal spread, which will maximize tissue healing from subsequent repair.
I then use a rectal probe as a template for repair. The probe is advanced underneath the defect between the distal and proximal portions, and the tissue is moved over the probe to ensure that the repair will be tension free. An ability to reapproximate the defect while keeping the probe in place indicates that the defect can be safely closed. (For a video presentation of the surgery, see www.surgeryu.com/leeobgyn.) If suturing is not feasible, the general surgeon is called to perform segmental resection.
The integrity of the repair is then thoroughly assessed with an air leak test. A bowel clamp is placed across the rectum and the pelvis is filled with sterile saline. Air is placed into the rectum with a rigid proctoscope while the operative field is inspected for evidence of an air leak.
Discoid resection may also be performed with a circular stapler. While this technique is faster than suturing, its use is limited by nodule size and has the potential to compromise complete excision of the nodule.
Dr. Lee is director of minimally invasive gynecologic surgery, Magee-Women’s Hospital of the University of Pittsburgh Medical Center.
Rectosigmoid endometriosis has been estimated to affect between 4% and 37% of patients with endometriosis and is one of the most advanced and complex forms of the disease. Bowel endometriosis can be asymptomatic but often involves severe dysmenorrhea, dyspareunia, and a spectrum of bowel symptoms such as dyschezia, diarrhea, constipation, bloating, and rectal bleeding. Deep infiltrating rectovaginal endometriosis causes persistent or recurrent pain and is best treated by surgical removal of nodular lesions.
I have found that laparoscopic full-thickness disc resection (anterior discoid resection) with primary two-layer closure is often feasible and avoids the need for a complete bowel reanastomosis. It may not be an option in cases of multifocal rectal involvement (which may affect between one-quarter and one-third of patients with bowel endometriosis) or in cases involving large rectal nodules or luminal stenosis secondary to advanced fibrosis. In these cases, segmental bowel resection (low anterior resection) is often necessary. When anterior discoid resection is feasible, however, patients face significantly less morbidity with comparable outcomes.
Less morbidity
Preoperative evaluation is far from straightforward, and practices vary. Transvaginal ultrasonography is used for diagnosing rectal endometriosis in select centers in certain regions of the world, but there are important limitations; not only is it highly operator dependent, but its limited range does not allow for the detection of endometriosis higher in the sigmoid colon. Endorectal ultrasonography can be an excellent tool for more fully evaluating rectal wall involvement, but it does not usually allow for the evaluation of disease elsewhere in the pelvis.
The preoperative tool we utilize most often along with clinical examination is MRI with vaginal and rectal contrast. MRI provides us with a superior anatomic perspective on the disease. Not only can we assess the depth of bowel wall infiltration and the distribution of the affected areas of the bowel, but we can see the bladder, the uterosacral ligaments, and how the uterus is situated relative to areas of disease. However, there are individualized limits to how high the contrast will travel, even with bowel preparation; disease that occurs significantly above the uterus often cannot be visualized as well as disease that occurs lower.
My general surgeon colleague and I have been working together for years, and we both are involved in counseling the patient suspected of having deep infiltrating disease. I typically talk with the patient about the probability of segmental resection based on my exam and preoperative MRI, and my colleague expands on this discussion with further explanation of the risks of bowel surgery.
Segmental resection has been associated with significant postoperative complications. In a single-center series of 436 laparoscopic colorectal resections for deep infiltrating endometriosis, rectovaginal and anastomotic fistula were among the most frequent postoperative complications (3.2% and 1.1%), along with transient urinary retention, which occurred in almost 20% (Surg Endosc. 2010 Jan;24:63-7).
Patients undergoing discoid resection for deep infiltrating endometriosis also had a significantly lower rate of temporary ileostomy (2.1% vs. 9.1%), a reduced rate of postoperative fever, and a reduced rate of gastrointestinal complications, mainly anastomotic leak or rectovaginal fistula (2.1% vs. 5.6%). There were no significant differences in the recurrence rate (13.8% vs. 11.5%).
A retrospective cohort study from our institution similarly showed decreased operative time, blood loss, hospital stay, and a lower rate of anastomotic strictures in patients who underwent laparoscopic anterior discoid resection between 2001 and 2009. The ADR group consistently had higher increments of improvement in bowel symptoms and dyspareunia, compared with patients who were selected to have segmental resection. Patients were followed for a mean of 41 months (JSLS. 2011;15[3]:331-8).
In general, there is agreement among surgeons that for consideration of discoid resection, nodule diameter should not exceed 3 cm, with a maximum of half of the bowel circumference and a maximum of 60% stenosis. I view these numbers as guiding principles, however, and not firm rules. Surgical decisions should be personalized based on the patient, the surgeon’s impression of the extent of the disease, and the ability to perform anterior discoid resection without compromising the rectal lumen with primary closure of the defect.
The technique
Rectosigmoid endometriotic nodules may present within the context of an obliterated posterior cul-de-sac, but the avascular pararectal space can be used to approach the nodules. Detailed knowledge of the avascular planes of this space, as well as the rectovaginal space, is crucial. Development of the rectovaginal space frees the bowel from its attachments to the posterior uterus and vagina. Judicious use of energized instruments in sharp dissection, and frequently sharp cold cutting, should be used near the bowel serosa to prevent thermal injury.
Presurgical imaging usually offers a good assessment of a nodule’s size and location, but intraoperatively, I typically use an atraumatic grasper to further assess size and contour and to determine if the nodule is suitable for discoid resection. If so, a suture is placed through the nodule to improve manipulation, and enucleation of the nodule itself is achieved through a “squeeze” technique in which an advanced bipolar device is used to circumscribe the lesion, dissecting the nodule as the device bounces off the thick endometriotic tissue.
The ENSEAL bipolar device (Ethicon, Somerville, N.J.) was designed as a vessel sealer, but because it will not cut through hard tissue as will other laparoscopic cutting devices, it serves as a useful tool for resecting endometriotic nodules while minimizing the removal of healthy rectal tissue. The device bounces off the nodule because it will avoid cutting through the thick tissue; in the process, it facilitates a fairly complete enucleation of the endometriotic nodule, starting with dissection until an intentional colotomy/enterotomy is made and followed by circumscription of the lesion once the rectum is entered.
Gentle traction and counter-traction increase the efficiency of dissection and minimize the amount of normal rectal tissue removed. Quick cutting with short bursts of energy allows for good hemostasis and minimizes thermal spread, which will maximize tissue healing from subsequent repair.
I then use a rectal probe as a template for repair. The probe is advanced underneath the defect between the distal and proximal portions, and the tissue is moved over the probe to ensure that the repair will be tension free. An ability to reapproximate the defect while keeping the probe in place indicates that the defect can be safely closed. (For a video presentation of the surgery, see www.surgeryu.com/leeobgyn.) If suturing is not feasible, the general surgeon is called to perform segmental resection.
The integrity of the repair is then thoroughly assessed with an air leak test. A bowel clamp is placed across the rectum and the pelvis is filled with sterile saline. Air is placed into the rectum with a rigid proctoscope while the operative field is inspected for evidence of an air leak.
Discoid resection may also be performed with a circular stapler. While this technique is faster than suturing, its use is limited by nodule size and has the potential to compromise complete excision of the nodule.
Dr. Lee is director of minimally invasive gynecologic surgery, Magee-Women’s Hospital of the University of Pittsburgh Medical Center.
Discoid resection of rectal endometriotic nodules
The treatment of the rectovaginal endometriotic nodule continues to be controversial. While proponents of “shaving” the nodule are quick to point out that compared with segmental bowel resection, pelvic pain, dyspareunia, dysmenorrhea, and postoperative pregnancy rates are similarly reduced, most comparative studies are retrospective and are not randomized. That is, patients with larger nodules or multifocal disease with deep infiltration into the muscularis layer of the bowel, or involving more than half of the bowel wall circumference, with surrounding severe fibrosis, invariably are more likely to undergo segmental bowel resection. Even with performance of segmental bowel resection to treat more extensive disease, there is a trend toward greater improvement of pain-related symptoms when compared with the “shaving” technique. Furthermore, the risk of rectal recurrence is acknowledged to be greater in patients undergoing endometriotic rectal nodule shaving.
Concern must be raised with segmental bowel resection. Not only is the risk of temporary ileostomy increased, but subsequent anastomotic leakage and rectovaginal fistula is noted in up to 10% of women. Although reduced with nerve sparing techniques, bladder denervation secondary to damage of the parasympathetic plexus causes urinary retention. In a study of 436 cases of laparoscopic colorectal resection, 9.5% presented after 30 days with persistent urinary retention and 4.2% with constipation (Surg Endosc. 2010 Jan;24:63-7).
For this edition of the Master Class in Gynecologic Surgery, I have invited Ted Lee, MD, director of minimally invasive gynecologic surgery, Magee-Womens Hospital of the University of Pittsburgh Medical Center, to discuss laparoscopic rectosigmoid resection for a deep endometriotic nodule. While many surgeons utilize a single-use curved circular stapler, I appreciate Dr. Lee’s innovative technique, for both its ease of use and its safety.
Dr. Lee has received multiple awards for his efforts, including best surgical video presentation by the AAGL. He is also the only five-time winner of the prestigious Golden Laparoscope Award for best surgical video from the AAGL.
A highly-regarded lecturer and surgeon, Dr. Lee has taught and performed live surgeries around the world.
Dr. Lee’s practice is entirely dedicated to minimally invasive surgical options for women. He is a firm believer that virtually all benign gynecologic surgical conditions should be treated using a minimally invasive approach. Dr. Lee’s clinical expertise includes minimally invasive surgery for treatments of endometriosis (including severe endometriosis involving bowel, bladder, and ureter); fibroids; abnormal uterine bleeding; urinary incontinence; and pelvic organ prolapse.
It is a great honor for the Master Class in Gynecologic Surgery to have Dr. Lee as guest author for this important area of our surgical arena.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
The treatment of the rectovaginal endometriotic nodule continues to be controversial. While proponents of “shaving” the nodule are quick to point out that compared with segmental bowel resection, pelvic pain, dyspareunia, dysmenorrhea, and postoperative pregnancy rates are similarly reduced, most comparative studies are retrospective and are not randomized. That is, patients with larger nodules or multifocal disease with deep infiltration into the muscularis layer of the bowel, or involving more than half of the bowel wall circumference, with surrounding severe fibrosis, invariably are more likely to undergo segmental bowel resection. Even with performance of segmental bowel resection to treat more extensive disease, there is a trend toward greater improvement of pain-related symptoms when compared with the “shaving” technique. Furthermore, the risk of rectal recurrence is acknowledged to be greater in patients undergoing endometriotic rectal nodule shaving.
Concern must be raised with segmental bowel resection. Not only is the risk of temporary ileostomy increased, but subsequent anastomotic leakage and rectovaginal fistula is noted in up to 10% of women. Although reduced with nerve sparing techniques, bladder denervation secondary to damage of the parasympathetic plexus causes urinary retention. In a study of 436 cases of laparoscopic colorectal resection, 9.5% presented after 30 days with persistent urinary retention and 4.2% with constipation (Surg Endosc. 2010 Jan;24:63-7).
For this edition of the Master Class in Gynecologic Surgery, I have invited Ted Lee, MD, director of minimally invasive gynecologic surgery, Magee-Womens Hospital of the University of Pittsburgh Medical Center, to discuss laparoscopic rectosigmoid resection for a deep endometriotic nodule. While many surgeons utilize a single-use curved circular stapler, I appreciate Dr. Lee’s innovative technique, for both its ease of use and its safety.
Dr. Lee has received multiple awards for his efforts, including best surgical video presentation by the AAGL. He is also the only five-time winner of the prestigious Golden Laparoscope Award for best surgical video from the AAGL.
A highly-regarded lecturer and surgeon, Dr. Lee has taught and performed live surgeries around the world.
Dr. Lee’s practice is entirely dedicated to minimally invasive surgical options for women. He is a firm believer that virtually all benign gynecologic surgical conditions should be treated using a minimally invasive approach. Dr. Lee’s clinical expertise includes minimally invasive surgery for treatments of endometriosis (including severe endometriosis involving bowel, bladder, and ureter); fibroids; abnormal uterine bleeding; urinary incontinence; and pelvic organ prolapse.
It is a great honor for the Master Class in Gynecologic Surgery to have Dr. Lee as guest author for this important area of our surgical arena.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
The treatment of the rectovaginal endometriotic nodule continues to be controversial. While proponents of “shaving” the nodule are quick to point out that compared with segmental bowel resection, pelvic pain, dyspareunia, dysmenorrhea, and postoperative pregnancy rates are similarly reduced, most comparative studies are retrospective and are not randomized. That is, patients with larger nodules or multifocal disease with deep infiltration into the muscularis layer of the bowel, or involving more than half of the bowel wall circumference, with surrounding severe fibrosis, invariably are more likely to undergo segmental bowel resection. Even with performance of segmental bowel resection to treat more extensive disease, there is a trend toward greater improvement of pain-related symptoms when compared with the “shaving” technique. Furthermore, the risk of rectal recurrence is acknowledged to be greater in patients undergoing endometriotic rectal nodule shaving.
Concern must be raised with segmental bowel resection. Not only is the risk of temporary ileostomy increased, but subsequent anastomotic leakage and rectovaginal fistula is noted in up to 10% of women. Although reduced with nerve sparing techniques, bladder denervation secondary to damage of the parasympathetic plexus causes urinary retention. In a study of 436 cases of laparoscopic colorectal resection, 9.5% presented after 30 days with persistent urinary retention and 4.2% with constipation (Surg Endosc. 2010 Jan;24:63-7).
For this edition of the Master Class in Gynecologic Surgery, I have invited Ted Lee, MD, director of minimally invasive gynecologic surgery, Magee-Womens Hospital of the University of Pittsburgh Medical Center, to discuss laparoscopic rectosigmoid resection for a deep endometriotic nodule. While many surgeons utilize a single-use curved circular stapler, I appreciate Dr. Lee’s innovative technique, for both its ease of use and its safety.
Dr. Lee has received multiple awards for his efforts, including best surgical video presentation by the AAGL. He is also the only five-time winner of the prestigious Golden Laparoscope Award for best surgical video from the AAGL.
A highly-regarded lecturer and surgeon, Dr. Lee has taught and performed live surgeries around the world.
Dr. Lee’s practice is entirely dedicated to minimally invasive surgical options for women. He is a firm believer that virtually all benign gynecologic surgical conditions should be treated using a minimally invasive approach. Dr. Lee’s clinical expertise includes minimally invasive surgery for treatments of endometriosis (including severe endometriosis involving bowel, bladder, and ureter); fibroids; abnormal uterine bleeding; urinary incontinence; and pelvic organ prolapse.
It is a great honor for the Master Class in Gynecologic Surgery to have Dr. Lee as guest author for this important area of our surgical arena.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
Special Considerations for Pediatric Patellar Instability
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.
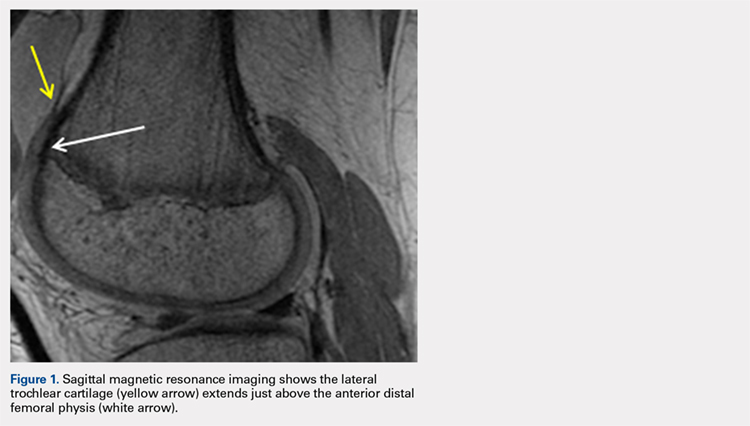
LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29
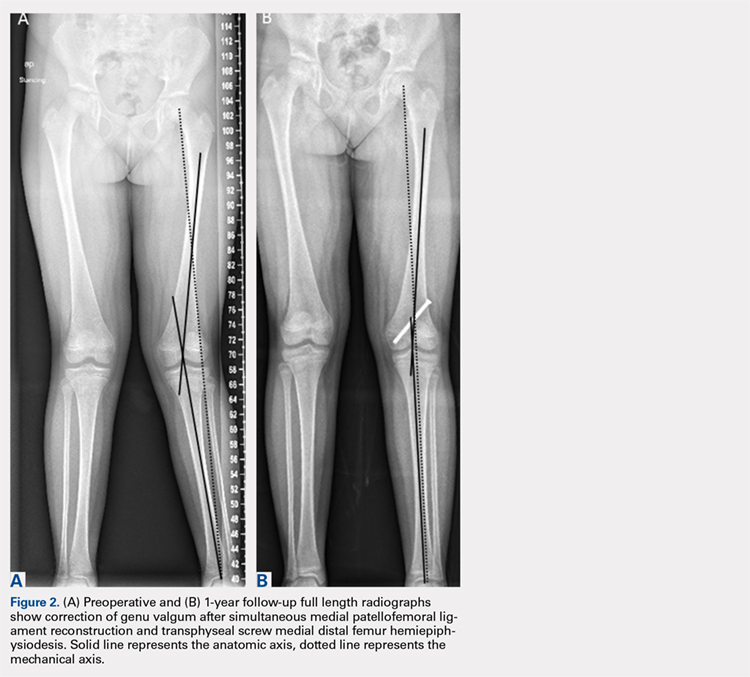
PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60
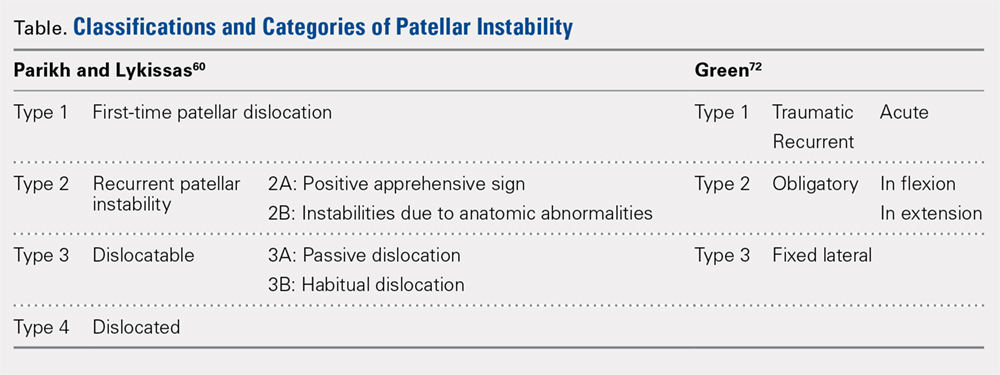
The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92
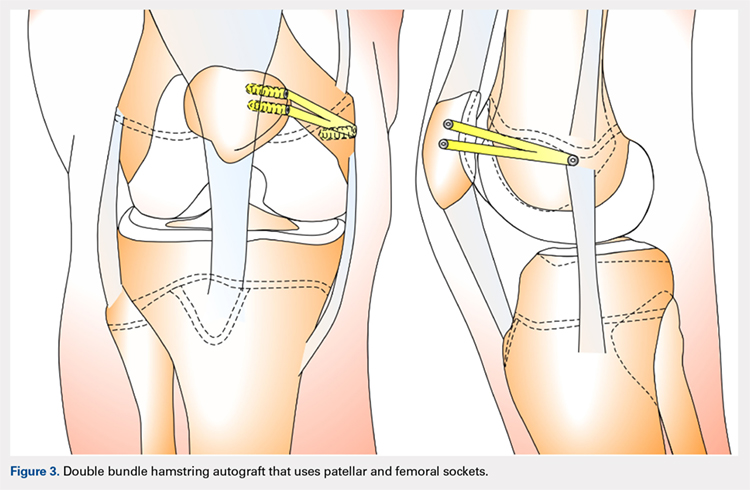
QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.
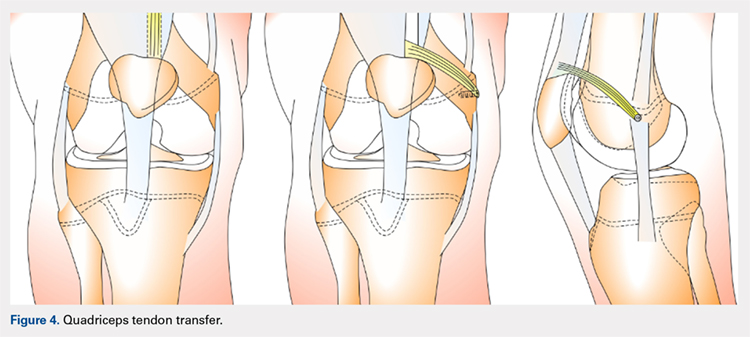
Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.
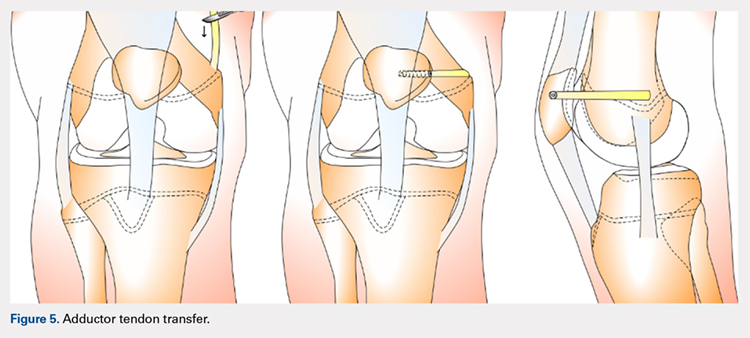
HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130
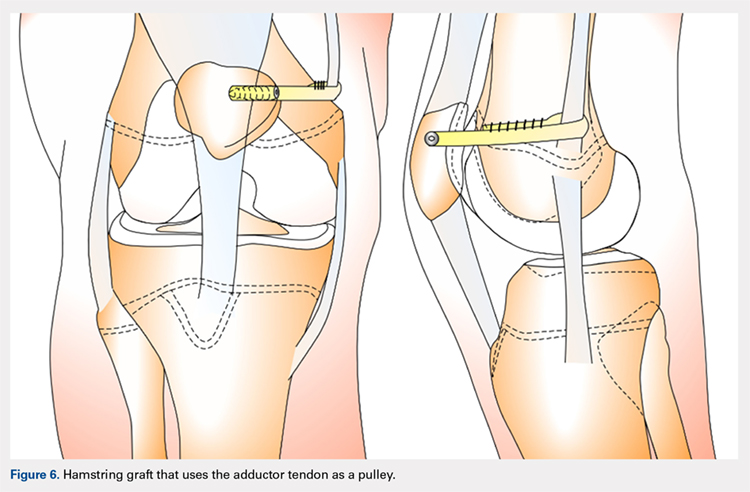
HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.
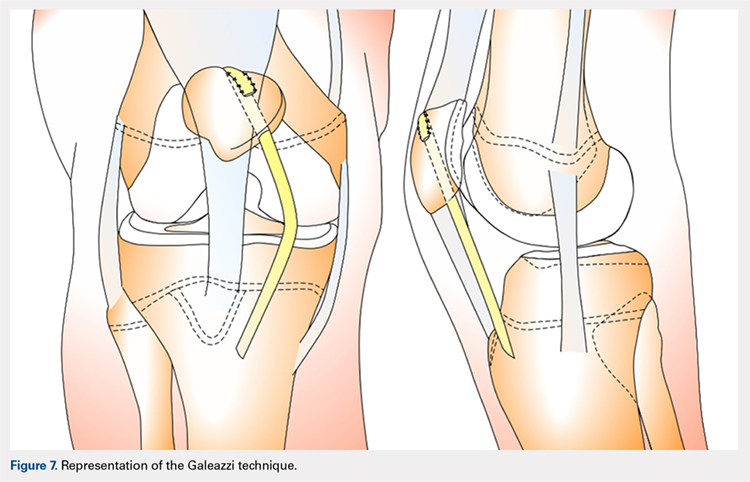
ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150
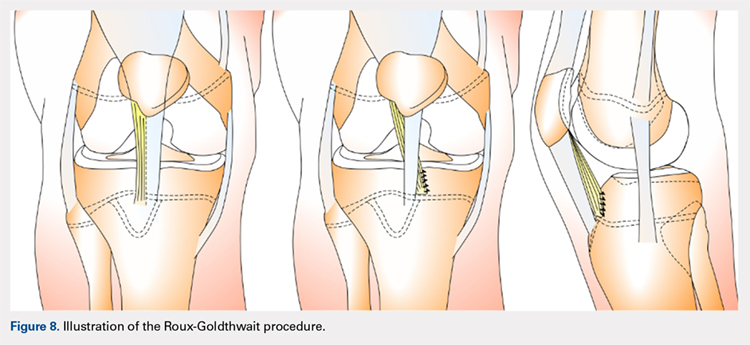
COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178
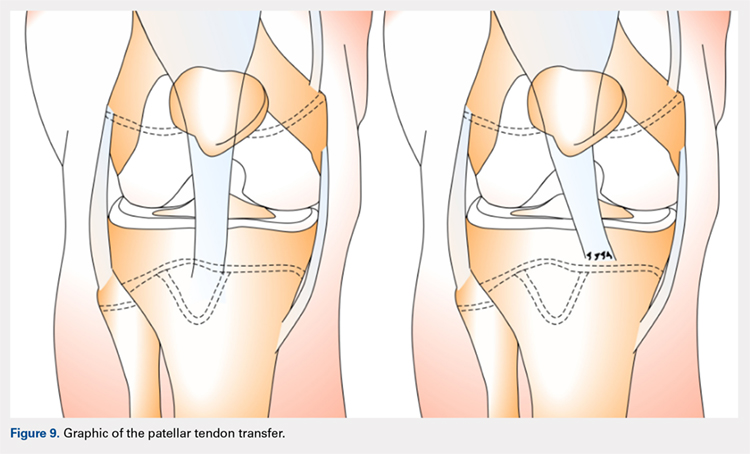
Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.
CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.

LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29

PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60

The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92

QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.

Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.

HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130

HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.

ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150

COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178

Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.

CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
ABSTRACT
Patellar instability in children and adolescents is a challenging subset to treat. Varied forms of instability, ranging from episodic dislocation to fixed dislocation, have been recognized. It is of utmost importance for the treating physician to recognize these different patterns of instability and their associated risk factors, as more complex patterns of instability would require more extensive surgical procedures. Medial patellofemoral ligament (MPFL) reconstruction, by itself, may not suffice or may not be appropriate for the more complex instability patterns. Appropriate and early treatment of such instability in children would allow for functional progression and possible remodeling of the trochlea. However, early treatment has the associated risk of growth disturbances when surgical procedures are performed around open physis or if adult-type bony procedures are performed in children. Recent knowledge about the relationship between trochlea, MPFL femoral attachment, and distal femoral physis could help to advance safe surgical care for these patients. This article reviews the pathophysiology, risk factors, and the existing classification systems for patellar instability in children and adolescents. It focuses on varied surgical techniques, which are unique to the pediatric population, and summarizes the outcomes of these surgical techniques.
Continue to: EPIDEMIOLOGY
EPIDEMIOLOGY
In a prospective 2-year study of Finnish children, the annual incidence rate of patellar instability was 43/100,000 pediatric population.1 In patients 9 to 15 years of age, the incidence was approximately 1/1000.1 In another study, patients at highest risk for a first-time patellar dislocation were females aged 10 to 17 years.2 In a study in patients with traumatic hemarthrosis, 36% in the younger age group (10-14 years) and 28% in the older age group (15-18 years) had sustained patellar dislocation. In contrast, 22% in the younger age group and 40% in the older age group had sustained an anterior cruciate ligament tear.3
Approximately one-half of patients who dislocate their patella suffer from long-term complications.4,5 These complications include recurrent instability, patellofemoral pain, osteochondral lesions, and eventual arthritis.1,4,5 Young, active individuals are more prone to these issues.6 Also, 39% or more of patellar dislocation patients have an associated osteochondral fracture that might influence the management.1 Thus, patellar instability in young patients is an area of concern.
DEVELOPMENTAL ANATOMY
At 4-week gestation, the patellofemoral joint is an ectodermal sac filled with mesenchyme of the somatic mesoderm.7 Mesenchymal condensations then appear at 4 to 5 weeks gestation, followed by chondrification of both the femur and patella.7 The joint space is present by 6 weeks, and the patellar and distal femoral condyles are present at 7 weeks gestation.7 By 8 weeks gestation, the basic knee anatomy resembles that of an adult with the chondroepiphysis forming the articular surfaces of the femur, tibia, and patella.7 By this time, the extensor mechanism is formed, and active joint motion has begun, facilitating the development of the trochlear sulcus.7 The secondary ossification center in the distal femoral epiphysis forms around 36 weeks gestation.8 Postnatally, both the patella and distal femur grow through endochondral ossification.9,10
The patella is the largest sesamoid bone in the human body.11 The patella begins as a dense consolidation of cells that differentiate as the quadriceps mechanisms develop.12,13 The patellar anlage becomes distinguishable within the quadriceps tendon around 7.5-week gestation.12 The morphology of the patella conforms to the distal femur.12 The patella molds or re-models as the knee begins to move in response to mechanical stresses.7 The patella increases in relative size during the first 6 months of gestation, then enlarges proportionately to the rest of the bones.7 Ossification begins around 3 years of age for females and 4 to 5 years of age for males.8,14 The ossification center may appear irregular as it rapidly expands.14 Ossification proceeds in a proximal to distal direction, thus giving a spurious estimation of patellar height on radiographs in children. The overall morphology of the cartilaginous patella during development is comparable to the final mature shape.14 Abnormal contact stresses on the articular surface of the patella during skeletal immaturity can lead to deformation.7
Ultrasonographic measurements in normal patients show that trochlear groove (TG) morphology is present early and becomes more radiographically apparent as distal femoral ossification is completed.15 Anatomic dissections of aborted fetuses have verified the morphology of the TG as it remains constant during growth and the groove morphology is the same for both fetuses and adults.16 An ultrasound study performed on patients aged 12 to 18 years showed the cartilaginous sulcus angle (CSA) remained constant throughout all age groups (146°).17 The CSA however, differed in patients who suffered a patellar dislocation (average, 164°; range, 154°-195°) compared with normal knees (average CSA, 145°; range, 131°-158°).15,17,18 The osseous sulcus angle, on the other hand, appears flat at birth and the TG deepens with age. This increase in depth is more of a reflection of progressive ossification of a well-formed cartilaginous trochlea, rather than a true deepening of the sulcus.17 Thus, the axial radiographic view of the patella provides misleading information about the sulcus angle in children and should not be used to define trochlear morphology.
Continue to: MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
MEDIAL PATELLOFEMORAL LIGAMENT ANATOMY
The medial patellofemoral ligament (MPFL) functions to limit the lateral translation of the patella.19 The attachment sites on the femur and patella for the MPFL have been studied in children.20-23 Cadaveric dissections in specimens aged 2 to 11 years have noted the patellar attachment to be an average of 12 mm in length with the midpoint approximately 5 mm superior to the mid-pole of the patella.22 The patellar footprint of the MPFL insertion was a mean 41% of the entire patellar length.22
It is important to be aware of the characteristic anatomy of the MPFL, as fixation points should mimic the anatomic insertion as best as possible while also avoiding violation of the nearby physis. The MPFL originates between the adductor tubercle and the medial femoral epicondyle just distal to the distal femoral physis and attaches to the superomedial aspect of the patella.20-25 In relation to the physis in pediatric patients, the midpoint of MPFL insertion has been measured to be 4 mm to 9 mm distal to the femoral physis.21,24,25 These measurements represent averages as cadaveric studies have reported that some part of MPFL femoral insertion extends proximal to the distal femoral physis.21 A recent report of physeal injury to the posterior distal femoral physis during MPFL reconstruction leading to femoral flexion deformity highlights the importance of physeal-respecting surgery.26
TROCHLEA AND ANTERIOR DISTAL FEMORAL PHYSIS
The relationship between the proximal aspect of the trochlea and the anterior distal femoral physis has been recently studied in 175 knees with dysplastic trochlea.27 Based on magnetic resonance imaging evaluation, the lateral aspect of the trochlea extended proximal to the anterior distal femoral physis in 13% of patients and was at the level of the anterior physis in another 13% of patients (Figure 1).27 Hence, a cautious approach is recommended for any surgery to address trochlear dysplasia or trochlear bump in younger patients to prevent iatrogenic injury to anterior distal femoral physis and resultant genu recurvatum. The distance between the trochlea and the physis increased with increasing age.

LIMB ALIGNMENT
Physiologically, the quadriceps angle (Q angle) changes through the course of growth. As children begin standing and walking, they stand with their feet wider apart and in genu varum.28 Physiologic genu varum can reach 15°.28 This degree lessens during the first 1.5 to 2 years of life, transitioning to physiologic valgus of nearly 12° by 3 years of age.28 Genu valgum, thereafter, gradually decreases to reach the adult value of around 7° to 8° by age 7 years.28 Increased genu valgum is a risk factor for patellar instability. In skeletally immature patients, correction of genu valgum through guided growth may be desirable in patients undergoing patellar stabilization surgery (Figures 2A, 2B).29

PATHOPHYSIOLOGY OF PEDIATRIC PATELLAR DISLOCATION
TROCHLEAR DYSPLASIA
Trochlear dysplasia is an abnormal shape and depth of the TG.30 Up to 96% of patients with patellar dislocation have trochlear dysplasia.30-33 In a study of patellar instability in children, at least 1 of the 3 signs of trochlear dysplasia (the crossing sign, supratrochlear bump, and double contour sign) was present on lateral radiographs.34 In another study on the growth of trochlear dysplasia in children and adolescents, all grades of trochlear dysplasia were present at all ages (ie, the dysplasia was most likely present at birth and did not necessarily worsen with age and growth).35 The linear dimensions of lateral and medial condylar height as well as trochlear bump increased with age but both the sulcus angle and shape of the trochlea did not change significantly.35 Remodeling of a dysplastic trochlea can happen if the patella is stabilized and appropriately located at a younger age, preferably before 10 years of age.36,37
Continue to: PATELLAR HEIGHT
PATELLAR HEIGHT
The role of patellar height in patellar instability has been well established.38 In patients with patella alta, the patella remains proximal to the TG during the greater arc of knee motion, which predisposes it to patellar instability. Calculation of patellar height in children could be challenging due to incomplete ossification, as well as asymmetric ossification of the patella and the tibial tubercle (TT). Since the patella ossifies from proximal to distal, most radiographic methods that measure the patellar height from the distal aspect of the patella provide a spurious elevation of the measurement.
The Caton-Deschamps (CD) method measures the length of the patellar articular surface and the distance from the inferior edge of the articular surface to the anterosuperior corner of the tibial plateau.39 A ratio >1.3 signifies patella alta. The CD ratio has been verified as a simple and reliable index for measuring patellar height in children.40 Two other methods have been described for determining patellar height in children.41,42 Based on anteroposterior (AP) radiographs of the knee in full extension, Micheli and colleagues41 calculated the difference between the distance from the superior pole of the patella to the tibial plateau and the length of the patella. A positive difference signified patella alta. The Koshino method involves the ratio between a reference line from the midpoint of the patella to the midpoint of the proximal tibial physis and a second distance from the midpoint of the distal femoral physis to the midpoint of the proximal tibial physis on lateral knee radiographs.42 Normal values range from 0.99 to 1.20 with the knee in >30° flexion, in children 3 to 18 years of age.
HYPERLAXITY
In contrast to adults, children have increased levels of collagen III compared with collagen I, which is responsible for tissue elasticity.43 Tissue elasticity leads to increased joint mobility, which is more common in children. Joint hypermobility or hyperlaxity has to be differentiated from symptomatic instability. The traditional Beighton score identifies individuals as having joint hypermobility with a score of 5/9 or higher in school-aged children.44-46 Smits-Engelsman and colleagues44 suggested using stricter criteria with scores of 7/9 or higher being indicative of hyperlaxity in school-aged children. A study of 1845 Swedish school children noted that females have a higher degree of joint laxity.45 Maximal laxity was noted in females at 15 years of age.45 Hyperlaxity has been demonstrated to be greater on the left side of the body44 and can be part of generalized syndromes including Down’s syndrome, Marfan’s syndrome, or Ehlers-Danlos syndrome.
LIMB TORSION
Staheli and colleagues47 described the normative values of a lower extremity rotational profile, including femoral anteversion and tibial torsion. Children normally have increased femoral anteversion, which decreases with growth. Miserable malalignment is a term used to denote increased femoral anteversion and increased external tibial torsion.48,49 These rotational abnormalities can increase the Q angle and the lateral forces on the patella. Femoral anteversion or internal rotation of the femur of 30° significantly increases strain in all areas of the MPFL.48 This increased strain may lead to MPFL failure and patellar instability.48 Increased internal rotation of the femur also increases contact pressure on the lateral aspect of the patellofemoral joint.48 Miserable malalignment frequently manifests following a pubertal growth spurt and may require femoral and tibial osteotomy.50
SYNDROMIC ASSOCIATIONS
Several syndromes have patellar instability as a part of their manifestation. The more common syndromes include nail-patella syndrome, Kabuki syndrome, Down’s syndrome, and Rubinstein-Taybi syndrome.51-54 Other syndromes less commonly associated with patellar instability include Turner syndrome, patella aplasia, or absent patella syndrome. Since many patients with syndromic patellar instability are functionally limited, they may not require an aggressive approach to treatment. When treating these patients, it is important to recognize the unique features of a specific syndrome, which may affect the anesthesia risk profile, management decisions, rehabilitation, and prognosis.
Continue to: MPFL TEAR PATTERN
MPFL TEAR PATTERN
The MPFL serves as an important constraint to the patella to prevent lateral dislocation, primarily during the first 20° to 30° of knee flexion.55,56 Injury to the MPFL is noted in over 90% of patients who suffer a patellar dislocation.57 The location of MPFL tears in pediatric patients is variably reported at the patellar attachment (10%-61%), femoral attachment (12%-73%), both (12%-35%) or mid-substance (2.5%-15%).25,57 The most common tear patterns in pediatric patients are tears at the patellar attachment.25,57 This tear pattern may be accompanied by an avulsion fracture of the medial rim of the patella, though this fracture, being extra-articular, seldom needs treatment.
CLASSIFICATION
While several authors have established extensive classification systems of patellar dislocation based on both clinical and radiographic presentation and reviews of the literature, a single classification system has not been recognized as the gold standard. In this section, in addition to presenting our preferred methods of classification, we will review some of the more recent and extensive classification systems for patellar dislocation and patellar instability.
Dejour and colleagues31 initially used both the presence of patellofemoral anatomic abnormalities and pain to define 3 types of patellar instability: major, objective, and potential patellar instability. Major patellar instability indicates that the patient has experienced more than 1 documented dislocation, objective instability involves one dislocation in addition to an associated anatomic abnormality, and potential patellar instability refers to cases in which the patient has radiographic abnormalities and patellar pain.31 Garin and colleagues58 more simplistically divided patellar dislocation patients into 2 groups: major (permanent or habitual) dislocation of the patella and recurrent dislocation. Sillanpaa59 stressed the distinction between first-time dislocation and recurrent dislocation specifically in the context of acute injuries. These classification systems were formulated with adults as the most relevant population; however, classifications targeted specifically to pediatric patients have recently been presented in the literature.
Historically, pediatric patella dislocations were simply categorized as traumatic or congenital.60 In 2014, Chotel and colleagues61 focused on classifying patellar dislocation by extensively reviewing anatomic, biomechanical, pathophysiological, and clinical patterns seen most commonly in children. They included 5 categories: congenital dislocation, permanent dislocation, habitual dislocation during knee flexion, habitual dislocation during knee extension, and recurrent dislocation; however, they did not address traumatic dislocations.61 Congenital dislocation is a rare condition, typically presenting at birth, which produces a pattern of functional genu valgum.62 Permanent dislocation typically presents after the child has started walking, but before the age of 5 years.61 The 2 variations of habitual dislocation typically present between ages 5 and 8 years.61 The final category is the most common and typically occurs during pre-adolescence or adolescence as a result of an atraumatic or trivial traumatic event or sports injury.1 Using more specific terminology, Hiemstra and colleagues63 modeled a classification system based on the traumatic, unilateral, bankart lesion, surgery (TUBS)/atraumatic, multidirectional, bilateral, rehabilitation, inferior shift (AMBRI) for shoulder dislocation classifications. The patellar dislocation system is used to identify 2 distinct subsets of patients in the patellofemoral instability population. One subset is defined by the acronym WARPS (weak, atraumatic, risky anatomy, pain, and subluxation), the other is STAID (strong, traumatic, anatomy normal, instability, and dislocation).64 Patients categorized by the WARPS acronym tend to experience atraumatic onsets of patellofemoral instability and demonstrate anatomic issues that increase this instability. These underlying anatomic issues include valgus alignment, ligamentous laxity, rotational abnormalities, shallow and short TG, and patella alta. On the other hand, STAID patients describe a traumatic dislocation event and do not have underlying anatomic abnormalities that predispose them to instability.64
Taking into account these previous classifications, Frosch and colleagues65 added specific pathologies including “instability,” “maltracking,” and “loss of patellar tracking,” in addition to both clinical and radiographic factors to define 5 types of patellar dislocation and their specific treatment recommendations.65 Type 1 involves simple dislocation with neither maltracking nor instability and a low risk of redislocation.65 Type 2 is defined as primary dislocation followed by subsequent high risk of dislocation and no maltracking.65 Type 3 is divided into 5 subcategories of instability and maltracking issues involving soft tissue contracture, patella alta, pathological tibial tuberosity, and TG distance.65 Type 4 is defined as the highly unstable “floating patella,” and type 5 involves patellar maltracking without instability 65. In terms of treatment, conservative rehabilitation is recommended for type 1 whereas MPFL reconstruction tends to show positive outcomes for both types 2 and 3.66-70
Continue to: Parikh and Lykissas recently published...
Parikh and Lykissas recently published a comprehensive classification system of 4 defined types of patellar dislocation in addition to voluntary patellar instability and syndromic patellar instability (Table).60 The 4 types are Type 1, first-time patellar dislocation; Type 2, recurrent patellar instability; Type 3, dislocatable; and Type 4, dislocated. Type 2 is further subdivided into Type 2A, which presents with positive apprehension signs, and Type 2B, which involves instabilities related to anatomic abnormalities.60 A distinction is also made between Type 3A or passive patellar dislocation and Type 3B habitual patellar dislocation.60

The classification system proposed by Green and colleagues is more simplified with 3 main categories (Table) of pediatric patellar dislocation: traumatic (acute or recurrent), obligatory (either in flexion or extension), and fixed laterally.71,72 The acute traumatic categorization refers to patients who experienced an initial dislocation event due to trauma whereas recurrent traumatic involves repeated patella dislocations following an initial incident. Studies report that between 60% to 70% of these acute traumatic dislocations occur as a result of a sports-related incident.2,33,73 Obligatory dislocations occur with every episode of either knee flexion or extension, depending on the subtype. Obligatory patella dislocation in flexion typically cannot be manipulated or relocated into the trochlea while the knee is fixed but does reduce into the trochlea in full extension. Fixed lateral dislocations are rare, irreducible dislocations in which the patella stays dislocated laterally in flexion and extension. These dislocations often present with other congenital abnormalities. Each of these categories can be further specified as syndromic if the dislocation is associated with genetic or congenital conditions including skeletal dysplasia, Ehlers-Danlos syndrome, cerebral palsy, Marfan disease, nail-patella syndrome, Down syndrome, Rubenstein-Taybi syndrome, and Kabuki syndrome.51-54,61,74-76
SURGICAL TECHNIQUES IN SKELETALLY IMMATURE PATIENTS
While nonsurgical, conservative treatment involving physical therapy and activity modification is recommended for most patients who experience first-time traumatic patellar dislocations, many patients experience complicating factors that indicate them for surgery. These factors include recurrent dislocation, risk factors for patellofemoral instability, underlying malalignment issues, and congenital deformities. When evaluating these factors, particularly patellofemoral instability, the authors recommend assessing osteochondral lesions, age, skeletal maturity, number of previous dislocations, family history, and anatomic risk factors.2,5,77-79 Extra care should be taken when considering surgical treatment for skeletally immature patients at elevated risk for recurrent instability as the risk of cartilage damage in these cases is high.80-82
Recently, there has been a reported increase in surgical treatment for patellar instability in the skeletally immature.83 This finding may be attributed to heightened awareness of factors that indicate patients for surgical treatment and increased familiarity of surgeons with newer techniques.83 Many surgical techniques have been described to address patellar instability involving both soft-tissue procedures and bony corrections.84 In this article, we discuss the various surgical techniques for MPFL reconstruction, quadricepsplasty, and distal realignment. These procedures can be paired with any number of additional procedures including, but not limited to, lateral retinacular release or lengthening, chondroplasty, TT osteotomy (in skeletally mature patients), and removal of loose bodies.83
There is a need for more comprehensive studies, particularly randomized controlled trials, to evaluate the outcomes for both surgical and nonsurgical treatments for first-time dislocations. In the current literature, only very recently have surgical treatments shown outcomes that are more positive. In 2009, Nietosvaara and colleagues85 conducted a randomized controlled trial of nonoperative and operative treatment of primary acute patellar dislocation in both children and adolescents. After a long-term mean follow-up of 14 years, there was not a significant difference between the groups in recurrent dislocation and instability, subjective outcome, or activity scores.85 In a subsequent review of 5 studies including 339 knees, Hing and colleagues86 also found similar results in both the operative and nonoperative cohorts at risk of recurrent dislocations, Kujala scores, and reoperations. However, a recent systematic review comparing redislocation rates and clinical outcomes between surgical and conservative management of acute patellar dislocation reported more positive outcomes for the surgical cohort.87 This review included 627 knees, 470 of which received conservative management, 157 of which received operative treatment. The conservative cohort was followed for an average of 3.9 years and had a 31% rate of recurrent dislocation while the surgical group was followed for a mean 4.7 years and experienced a 22% redislocation rate.87 This study indicates that operative management for acute first-time dislocations may be the preferred treatment option.
Continue to: A potential reason some of these studies...
A potential reason some of these studies did not show any significant difference between the operative and nonoperative cohort could be that the surgical cohorts included a wide range of procedures including lateral releases and MPFL repairs. Recent publications have indicated that these techniques do not produce overall positive outcomes. While each surgical treatment plan is unique depending on the patient; recently, MPFL reconstruction has been shown to have better outcomes than both nonoperative management and simple medial repair and/or lateral
release.67,88-90
MPFL RECONSTRUCTION
INDICATIONS/OVERVIEW
The MPFL is an important stabilizer for the knee that primarily resists lateral translation of the patella. Damage to the MPFL is very common in acute patellar dislocations with up to 90% of first-time dislocations resulting in injury to the MPFL.91,92 Historically, simple medial and/or lateral MPFL repairs have not been shown to improve patellofemoral kinematics significantly and often result in recurrence.90,93 To address this issue, during the past few decades, numerous MPFL reconstruction techniques have been developed to reconstruct a stronger ligament with the same kinematics as the anatomic MPFL.2,19,69,81,94-106 The ultimate goal of MPFL reconstruction is to reestablish the anatomic “checkrein” to guide the patella into the trochlea between 0° and 30° of knee flexion.107,108 An essential secondary surgical goal in skeletally immature patients is to avoid damaging the distal femoral physis.
There are many variations in both the grafts used to replace the MPFL and the means by which to secure them. The ones discussed below include free semitendinosus or gracilis autografts or grafts constructed from a pedicled adductor, patellar, or quadriceps tendon.69,105,109 While not used as frequently, allografts have also been used.110 Methods to secure these grafts in osseous tunnels include suture anchors or tenodesis screws. Incomplete osseous sockets or medial-sided bone tunnels have also been used as a method to decrease patellar fractures as they preserve the lateral patellar cortex.111-114
DOUBLE-BUNDLE HAMSTRING AUTOGRAFT
The technique most often used by the author is a double-bundle hamstring autograft harvested from either the semitendinosus or the gracilis secured by short patellar and femoral sockets (Figure 3). After harvesting the hamstring graft from a posteromedial incision, an approximately 90-mm graft is prepared with Krackow stitches to secure 15 mm of the tendon in each socket.115 Lateral radiographs are used intraoperatively to ensure the guidewire for the femoral drill hole falls along the posterior cortex of the diaphysis of the femur while AP radiographs confirm placement distal to the physis. It is important to take both AP and lateral radiographs intraoperatively due to the concave curvature of the distal femoral physis. This unique anatomy can make a point that is located distally to the physis on the AP view appear on or proximal to it on the lateral cross reference view.24,116 For the patellar socket, 2 short sockets are made in the superior half of the patella. Once the sockets have been drilled, the graft is adjusted so that the patella stays seated in the center of the trochlea between 20° and 30° of flexion. This anchoring is accomplished by securing the graft while the knee is kept at 30° of flexion. Proper tension is confirmed by ensuring that the graft does not allow lateral patella movement over one-fourth the width of the patella in extension while crepitation must not appear throughout the ROM.92

QUADRICPETS TENDON TRANSFER
A combination of techniques by Steensen and colleagues,105 Goyal,109 Noyes and Albright,117 and Pinkowsky and Hennrikus118 describe an MPFL reconstruction in which the proximal end of a small medial portion of the quadriceps tendon is released and then attached to the medial epicondyle through a subcutaneous tunnel (Figure 4). This technique is particularly useful for cases in which the extra strength provided by the bone-quadriceps tendon is necessary to correct more severe dysplasia. Leaving the distal end of the quadriceps tendon intact at its patellar insertion, a graft of about 8 mm x 70 mm thickness is harvested from the tendon. The free distal end of the tendon is then run anatomically through the synovium and retinaculum to be either sutured to the medial intermuscular septum at the medial femoral epicondyle or fixed in femoral tunnel using interference screw.105,109,118 The placement of the femoral fixation point is essential to ensure positive surgical outcomes. If the graft is secured too anteriorly, it may be too loose in extension and too tight in flexion, both of which can lead to postoperative pain, loss of normal kinematics, and overload of the medial patellofemoral cartilage.119-121 Once the ideal placement of the femoral fixation point has been confirmed by intraoperative radiographs, the graft is secured with a small absorbable suture.122,123 While this technique has good clinical results, the longitudinal scar that results from graft harvesting is cosmetically unappealing, and it is technically challenging to harvest a consistent strip of the quadriceps tendon. To address some of these concerns, Fink and colleagues124 described a new harvesting technique that produces more consistent grafts and requires a smaller incision.

Continue to: ADDUCTOR MAGNUS TENDON TRANSFER
ADDUCTOR MAGNUS TENDON TRANSFER
This technique is a double-bundle MPFL reconstruction that uses a pedicled graft of the distal adductor magnus tendon and suture anchors or incomplete osseous sockets to recreate the MPFL anatomically (Figure 5). Avikainen and colleagues96 and Sillanpää and colleagues125 described this procedure as a progression from the original single-strand adductor magnus transfer technique. First, maintaining the distal insertion, a graft of approximately 14 cm to 18 cm is harvested from the adductor tendon and then passed through a subcutaneous tunnel between the distal vastus medialis obliquus and the superficial joint capsule. The graft is then looped at the medial patella so that the distal bundle runs back to the adductor tubercle.125 With the knee at 30° of flexion to assure proper tension, the graft is secured at both the patella and near the adductor tubercle with suture anchors.125 Hambridge and colleagues126 compared a similar adductor magnus transfer with other pedicled techniques including bone-quadriceps tendon autograft and bone-patellar tendon allograft and found positive results for all 3 methods of reconstruction.

HEMI-PATELLA TENDON TRANSFER
In a similar technique to the adductor tendon transfer, the medial section of the patellar tendon is harvested from the TT and run from its proximal insertion at the medial patella to the medial femoral attachment via a subcutaneous tunnel. The free end of the graft is then secured with suture anchors or incomplete osseous sockets with the knee at 30° of flexion.127
HAMSTRING GRAFT WITH ADDUCTOR TENDON AS A PULLEY
Several techniques opt to use a more dynamic model of MPFL reconstruction in which the adductor tendon or medial collateral ligament (MCL) is used as a pulley for the hamstring graft (Figure 6).128,129 The site of the pulley approximates the normal attachment of the MPFL to the femur and so acts as an effective anatomic replica of the MPFL origin. A semitendinosus graft is harvested and is prepared with continuous sutures, and 2 tunnels to secure the graft are drilled into the patella. The graft is then run subcutaneously from the medial side of the patella to the adductor magnus tubercle into which an osteoperiosteal tunnel is drilled at its distal femoral insertion. The graft is looped through the adductor tunnel and secured with sutures. Proper knee kinematics was ensured by placing the knee at 30° of flexion as the ends of the tendon are secured to the patella.114,130

HAMSTRING GRAFT WITH MCL AS A PULLEY
The MCL can also be used as a pulley rather than the adductor tendon. The semitendinosus graft is harvested and prepared and the patella drilled as it is in the previous technique. The MCL was fashioned into a pulley by making a slit in its posterior one-third. The semitendinosus graft is looped through this slit, and both ends of the graft are held in place with suture anchors on the surface of the patella.129
ADDITIONAL PROCEDURAL COMBINATIONS
Depending on the needs of the individual patient, MPFL reconstruction, and other patellar stabilization techniques can also be combined with additional procedures. Arshi and colleagues83 conducted a review of 6190 adolescents surgically treated for patellar instability and reported the most common additional procedures performed at the time of the stabilization. They found 43.7% of the population underwent lateral retinacular release, which while not effective as an isolated technique to treat patellar instability, has often been used in combination with MPFL reconstruction.131-133 There is currently a lack of consensus regarding the success of adding a lateral release to the reconstruction. Some studies report no difference while others report a decrease in stability after lateral release.90,134-136 While lateral retinacular release has been shown to decrease the force required to displace the patella, it can be surgically indicated in certain patients undergoing MPFL reconstruction.131 The authors advocate that if the lateral retinaculum is tight such that centralized patellar tracking is inhibited following the reconstruction, or if the patella cannot be pushed passively from a laterally tilted position to the neutral horizontal position, lateral retinacular lengthening should be performed to improve kinematics.132
Continue to: Arshi and colleagues...
Arshi and colleagues83 also reported a high rate of cartilage procedures, with chondroplasty performed in 31.1% and chondral fragment/loose body removal in 10.2%. These statistics suggest that a significant level of cartilage damage has occurred by the time of surgery.83
COMPLICATIONS
As MPFL reconstruction techniques have only recently been popularized and developed, there are not many comprehensive studies evaluating the outcomes and complications associated with these procedures. However, in the current literature, there is a general consensus that patients usually experience positive short-term clinical outcomes and relatively low complication rates.68,77 In one of the largest retrospective cohort studies of pediatric patients undergoing MPFL reconstruction, Parikh and colleagues114 reported both the type and rate of complications. They found complications occurred in 16.2% of patients, and the most common complications were recurrent patellar instability, patellar fractures, patellofemoral arthrosis, motion deficits, and stiffness with over half classified as avoidable. Most of these complications were due to technical errors with episodes of recurrent instability only reported in 4.5% of patients.114 In a comprehensive meta-analysis of MPFL reconstruction studies, Shah and colleagues137 reported a complication rate of 26% in both pediatric and adult patients. The cohort was not stratified by age, yet complications were similar to those reported by Parikh and colleagues,114 including pain, loss of knee flexion, wound complications, and patellar fracture.137
As indicated by the frequency of technical complications reported by Parikh and colleagues,114 extra caution should be taken in the operating room to minimize potential errors. In techniques that require drilling of femoral sockets, proper length for and placement of the graft is essential to reestablish proper kinematics. Studies have reported that placing the femoral socket too proximally can result in loss of ROM during flexion and increased compressive forces across the patella.138 A graft that is too short can have similar negative outcomes, and a graft that is too long can result in recurrent instability. Positioning the graft while the knee is in 30° of flexion can help ensure the proper length and tension is achieved. Once the graft is in place, it is important to ensure the ROM and isometry before completing the fixation.72 It is also essential to be vigilant about potential violation of the physes and subsequent growth disturbances. To establish the safest angles for drilling the distal femoral epiphysis for graft placement, Nguyen and colleagues139 conducted a study using high-resolution 3-dimensional images of cadaveric distal femoral epiphyses. By recording which tunnels disrupted the physis before reaching 20 mm of depth, the authors concluded that it is safest to drill distally and anteriorly at an angle between 15° and 20°.139 This technique should minimize damage to the physis, notch, and distal femoral cartilage and decrease potential complications.139
OUTCOMES
In general, the literature reports positive outcomes for MPFL reconstruction—in both studies that address a specific technique and all-encompassing studies. Outcomes are typically reported as Kujala and Tegner scores, results from clinical examinations, and rates of subsequence recurrences. Several recent studies have also evaluated the ability of MPFL reconstruction to restore proper kinematics. Edmonds and colleagues140 evaluated the difference in patellofemoral joint reaction forces and load experienced by 3 groups of adolescents: a cohort treated with MPFL reconstruction, a cohort treated with soft-tissue realignment of the extensor mechanism (the Insall method), and controls. While both surgical techniques were able to restore medial constraints to the patella, the study showed that only the MPFL reconstruction cohort experienced joint reaction forces that were analogous to the control group. In comparison, the cohort that was treated with soft-tissue realignment alone experienced higher patellofemoral joint reaction forces and did not regain normal joint mechanics.140 These results can be used to advocate for the further use of MPFL reconstruction as an effective anatomic replacement of the native ligament. Radiographic studies have similarly reported MPFL reconstruction as an effective means to restore anatomic normality. Fabricant and colleagues141 conducted a radiographic study in which patella alta was corrected to normal childhood ranges in patients who underwent MPFL reconstruction technique using a hamstring autograft. Lykissas and colleagues142 corroborated these results with another radiographic study that reported small but significant decreases in the Blackburne-Peel index and CD index following MPFL reconstruction in 25 adolescents. As correction of patella alta allows the patella to rest in a deeper, more secure position in the TG, these results indicate that effective early MPFL reconstruction can correct for patellar anatomic abnormalities that could be future risk factors.143,144 Several studies have also reported outcomes addressing specific MPFL techniques; these are reported and discussed in this article.
OUTCOMES BY TECHNIQUE
HAMSTRING AUTOGRAFT
Reports on outcomes following MPFL reconstructions using hamstring autografts have been particularly promising. A cohort of 21 skeletally immature patients who underwent MPFL reconstruction was evaluated pre- and postoperatively with an average of a 2.8-year follow-up. The authors of the study reported no redislocation events and significant improvement in the Kujala scores, and patients were able to return to athletic activities safely.145 Previous studies report similar positive increases in Kujala scores, subjective patient reports, and lack of subsequent redislocation for patients who underwent either semitendinosus or gracilis autograft MPFL reconstructions. One such study further documented an average patellar inclination angle decrease from 34.3° to 18.6° following MPFL reconstruction.146 However, while the literature typically reports positive Kujala scores and subjective outcomes for the hamstring autograft procedure, a study arthroscopically evaluating patellar tracking immediately following surgery and then at 6 to 26 months follow-up found that patellar tracking correction was not maintained for all patients who underwent this type of MPFL reconstruction.147
Continue to: QUADRICEPS TENDON TRANSFER OUTCOMES
QUADRICEPS TENDON TRANSFER OUTCOMES
Studies specifically evaluating the quadriceps tendon transfer technique for MPFL reconstruction in children are sparse, but authors have reported positive clinical outcomes and low complication rates in adults. After following 32 young adults who underwent this MPFL reconstruction technique for 3 years, Goyal109 reported a significant increase in mean Kujala scores from 49.31 to 91.25 and no complications or redislocation. He argues this type of quadriceps graft has a high success rate because it is anatomically more similar to the MPFL than other grafts and does not require additional patellar fixation.101,109 Similar positive Kujala scores and minimal complications have been reported in adult patient populations.148 Abouelsoud and colleagues149 conducted one of the few studies in skeletally immature patients and reported similarly positive results with no redislocations and significantly improved Kujala scores at a mean follow-up of 29.25 months in their 16-patient cohorts.
ADDUCTOR MAGNUS TENDON TRANSFER
After initially describing this technique in 14 adult patients, Avikainen and colleagues96 followed this cohort and reported positive subjective results and only 1 redislocation. In a more recent study in which the adductor tendon transfer technique was compared with the quadriceps tendon transfer described above and the bone-patellar tendon allograft, Steiner and colleagues69 reported similarly significant improvement in all cohorts in Lysholm, Kujala, and Tegner scores with no redislocations. Additionally, Malecki and colleagues150 followed a cohort of 33 children with 39 knees diagnosed with recurrent patellar dislocation, who underwent MPFL reconstruction using the adductor magnus tendon. After evaluating this cohort functionally and radiographically, the authors reported improvements in Lysholm and Kujala scores, patellar tilt and congruence angles, and peak torque of the quadriceps muscle and flexor.150 However, this cohort did report postoperative redislocations in 36.4% of patients (4 of 11).150
HEMI-PATELLA TENDON TRANSFER
In 2012, in the first randomized controlled trial, Bitar and colleagues67 compared the outcomes of patients who underwent MPFL reconstruction via the hemi-patellar tendon technique with those who were managed nonoperatively with immobilization and physiotherapy after first-time patellar dislocation. At 2-year follow-up, the surgical cohort presented positive results with a significantly higher mean Kujala score (88.9 to 70.8) and no redislocations or subluxations. In contrast, 35% of nonoperative cases presented with recurrences and subluxations over the 2-year period.67
MCL OR ADDUCTOR TENDON AS A PULLEY
Studies have reported good postoperative results and low complication rates for these dynamic techniques.128,129 In terms of kinematics, while hypermobility and patellar height were not fully corrected, improvements in patellar tilt and lateral shift were reported in a cohort of 6 patients with a minimum 4-year follow-up.129 To further evaluate whether the more dynamic pulley reconstruction technique resulted in better outcomes, Gomes and colleagues128 compared the subjective reports, clinical evaluations, and complication rates of patients who underwent MPFL reconstruction with a rigid adductor magnus fixation vs a semitendinosus tendon dynamic femoral fixation. One case in the rigid cohort experienced a subsequent subluxation, while patients in the semitendinosus group had better subjective reports and a higher rate of return to sport.128 More recently, Kumahashi and colleagues151 specifically studied the outcomes of the MCL tendon as a pulley in 5 patients aged 14 to 15 years. They reported similar successful results as no patients experienced recurrence, and all patients exhibited improvement in radiographic measures of patellar tilt and congruence angle, lateral shift ratio, and both Kujala and Lysholm scores.151
While there has yet to be a randomized controlled trial comparing all of these different techniques, there is a general consensus in the literature that patients tend to perform better following MPFL reconstruction vs MPFL repair.
OTHER STABILIZATION PROCEDURES, INCLUDING DISTAL REALIGNMENT
Patients with additional underlying deficits and malalignment issues such as significant trochlear dysplasia, increased TT-TG distance, patella alta, increased Q angle, and/or positive J sign may require stabilization procedures beyond MPFL reconstruction.152,153 TT osteotomies are often used to correct alignment issues in the adult patient population; however, these procedures are typically contraindicated in skeletally immature patients. Alternative realignment procedures for the pediatric population include both proximal and distal realignment, with proximal realignment performed primarily in children under the age of 12 years.153 Many variations on these procedures exist, some of which are no longer regularly performed due to poor reported outcomes. In this article, we discuss several of the techniques, focusing primarily on those that have demonstrated higher success rates.
Continue to: GALEAZZI TECHNIQUE
GALEAZZI TECHNIQUE
One of the first and most famous soft-tissue techniques to address patellar instability was the semitendinosus tenodesis, published by Galeazzi154 in 1922 (Figure 7). This technique stabilizes the patella without altering the TT. In the original technique, a portion of the semitendinosus tendon is harvested with its tibial insertion left intact. The free end of the tendon is then secured with sutures at the periosteal groove of the medial patella.154,155 Fiume156 modified this technique by adding a lateral release and medial retinacular reefing. The most recent addition to this procedure was introduced by Baker and colleagues,157 in which a tunnel is drilled from the medial to the lateral border of the patella. Tension placed on the grafted tendon is used to reposition the patella medially and draw it downward. Preliminary literature on this modified procedure reported fair clinical results with success rates of approximately 75%.155,158-160 A recent study evaluating both the clinical and radiographic outcomes of this technique also indicated that while clinical results were excellent in 62.5% of patients, this technique alone was unsuccessful in fully addressing patellar instability in patients with underlying anatomic abnormalities such as patellar alta.161 In light of these less than ideal reports, the authors no longer recommend this technique for patellofemoral instability cases.

ROUX-GOLDTHWAIT PROCEDURE
The Roux-Goldthwait procedure, first described by both Roux162 and Goldthwait163 in 1888 and 1895 respectively, was later modified in 1985 to involve a lateral release, plication of the medial retinaculum, medial transfer of the lateral patellar tendon without advancement, and advancement of the vastus medialis (Figure 8).164 More recently, Marsh and colleagues152 introduced an addition to aligning the extensor mechanism with the femoral shaft better. In this technique modification, the patellar tendon is split longitudinally, and its lateral half is detached and transferred distally beneath its medial half. The free end is then sutured to the periosteum on the medial side of the tibia.152 With a mean long-term follow-up of 6.2 years, Marsh and colleagues152 reported excellent results in 65%, good in 11%, and fair in 3% of the knees operated on with this modified technique. Of the patients in this cohort whose strength was evaluated, 80% had their strength returned to 90% of preoperative levels in the operated leg.152 While this study and others report improved outcomes, an increasing body of literature has found high rates of recurrence, patella infera, and other complications following the modified Roux-Goldthwait procedure.36,165-171 Also, a study comparing MPFL reconstruction using adductus magnus transfer with the Roux-Goldthwait procedure reported that patients in the MPFL cohort reported less pain postoperatively.150 In addition, whereas the Kujala and Lysholm scores, recurrence rates, patellofemoral angles, and apprehension test results did not demonstrate significant differences between these 2 groups, the MPFL group had significantly fewer abnormal congruence angles, better patellar medialization, and higher peak torque of the hamstring.150

COMBINED MPFL AND MEDIAL PATELLOTIBIAL LIGAMENT RECONSTRUCTION
While the medial patellotibial ligament (MPTL) has not received much attention with regard to patellar stability, recent studies have indicated its role during higher degrees of both flexion and extension.172 The MPTL acts as a secondary restrictor ligament which helps release stress on the MPFL by decreasing the Q angle and further normalizing patellar kinematics.173 Patients who present with hyperlaxity or knee hyperextension combined with extension subluxation and flexion instability could be indicated for this additional stabilizing procedure. Both Nietosvaara and colleagues85 and Brown and Ahmad174 have described a dual MPTL and MPFL reconstruction technique using a semitendinosus hamstring graft. More recently Hinckel and colleagues172 described a combined MPFL and MPTL reconstruction, using a graft from the quadriceps tendon to reconstruct the MPFL and one from the patellar tendon to reconstruct the MPTL. In this technique, once the respective grafts have been harvested, a femoral insertion for the graft recreating the MPFL is fluoroscopically established so that an anchor can be inserted distal to the femoral physeal growth plate. For the MPTL insertion, attachment to the tibia below the joint line and 2 cm medial to the patellar tendon is established fluoroscopically just above the physeal growth plate on the proximal epiphysis.19,175 The MPTL graft is sutured first with the knee at 90° of flexion to establish tension similar to that of the patellar tendon.176 Then, the knee is placed in 30° of flexion to fix the MPFL graft to the medial patella to prevent excessive lateral translation of the patella.
PATELLAR TENDON TRANSFER
Patellar tendon transfer with proximal realignment is a technique used in particularly young patients to address cases of patellofemoral instability involving concomitant bony or anatomic abnormalities. This procedure is effective for young children with substantial amounts of remaining growth as it better mimics native anatomy than other realignment procedures and does not require bony remodeling.152-154 It is important to familiarize with surgical techniques to address malalignment issues in young patients as neglected alignment issues can lead to worsening of trochlear dysplasia and instability, which are very difficult to treat later on when patients are older.153
The patellar tendon transfer technique (Figure 9), as described by Gordon and Schoenecker,177 starts with an extensive lateral retinacular release. The patellar tendon is then released from its distal insertion at the TT so that it can be moved medially without moving it inferiorly. After confirming patellar tracking and alignment by flexing the knee from 0° to 90° with the graft in place, the patellar tendon graft is secured with multiple nonabsorbable horizontal sutures.177 Of note, in skeletally mature patients, a TT osteotomy is used to accomplish the same goal. This osteotomy has been shown to improve both patellar height and TT-TG distance in skeletally mature patients, but is contraindicated in skeletally immature patients.92,178

Continue to: Initial studies conducted on patellar tendon...
Initial studies conducted on patellar tendon transfer have positive outcomes.179 At a mean follow-up of 5.1 years, patients reported a decrease in pain and increased the ROM and activity, and only 1 reported a postoperative redislocation.179 In more recent studies, both Benoit and colleagues36 and Garin and colleagues58 reviewed cases of patellar instability treated with patellar tendon transfer to address concomitant patellar alignment and anatomic abnormalities. They reported good functional, clinical, and radiographic outcomes with 12.5% and 16% recurrence rates, respectively.36,58 They also noted radiographic improvements in femoral sulcus angle, particularly in younger patients, which indicate this procedure is effective in addressing bony abnormalities that can result from neglected malalignment issues.36,58,154
QUADRICEPSPLASTY
Quadricepsplasty is a lengthening and remodeling technique not frequently used in the pediatric population. The goal of this procedure in patients with significant amounts of growth remaining is to reposition the patella to ameliorate trochlear remodeling and prevent worsening symptoms and anatomic abnormalities.36 A quadricepsplasty accomplishes this by de-rotating and/or lengthening the extensor mechanism and may or may not involve a concomitant MPFL reconstruction. This procedure is particularly effective in young patients who experience obligatory dislocation.60,72 Several quadricepsplasty techniques have been described including Thompson, Curtis and Fisher, Judet, Stanisavljevic, and V-Y technique.180-186 Most techniques initially involve sharp dissection of the vastus medialis and lateralis from the rectus femoral tendon. A tongue is then fashioned out of the rectus femoral tendon. Once the vastus medialis and lateralis are detached from the margins of the patella, the knee is extended, and the distal ends of the vasti are sutured to the tongue of the rectus tendon. Effective extension facilitates flexion to 90°.184 The authors recommend a modification of this technique in which a Z lengthening of the quadriceps tendon is performed after the vastus lateralis is removed distally from the patella and the quadriceps tendon.
Several series and case reports evaluating quadricepsplasty in adult patients report positive outcomes with most patients achieving good or excellent flexion with minimal complications.183,185,187-189 Reports on quadricepsplasty used to treat conditions other than patellofemoral instability in children have reported similar positive outcomes.190-192 As quadricepsplasty for patellar instability is relatively rare in pediatric patients, there is not much relevant literature. However, Kocon and colleagues193 reported results of quadricepsplasty and quadricepsplasty combined with the modified Galeazzi procedure in 8 children (10 knees) with a mean follow-up of 3.25 years. Seventy percent of cases resulted in stabilization and correction of patellar position, and only 2 postoperative redislocations were noted.193 Additionally, in a study evaluating 6 patients suffering from patellar instability, 2 of whom were obligate dislocators, quadricepsplasty resulted in patellar stability, satisfaction, and near normal gait patterns.194
Figure 10 shows the surgical algorithm used for patellar instability characteristics.

CONCLUSION
Patellofemoral joint stability relies on a complex interplay of musculotendinous units, ligaments and the osteocartilaginous morphology of the patellofemoral joint. Patellar instability in pediatric patients is different from adults. Having an in-depth understanding of the remodeling potential, the insertion sites for the MPFL and its relationship to the physis are of utmost importance when planning surgery. Reducing and maintaining the patella within the patellofemoral joint early enough can allow for remodeling of the patella and/or the trochlea to provide for lasting stability. Appropriate surgical principles, such as tensioning, can help both prevent continued pain and minimize future complications.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
1. Nietosvaara Y, Aalto K, Kallio PE. Acute patellar dislocation in children: incidence and associated osteochondral fractures. J Pediatr Orthop. 1994;14(4):513-515.
2. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121. doi:10.1177/0363546503260788.
3. Abbasi D, May MM, Wall EJ, Chan G, Parikh SN. MRI findings in adolescent patients with acute traumatic knee hemarthrosis. J Pediatr Orthop. 2012;32(8):760-764. doi:10.1097/BPO.0b013e3182648d45.
4. Mäenpää H, Lehto MU. Patellar dislocation. The long-term results of nonoperative management in 100 patients. Am J Sports Med. 1997;25(2):213-217. doi:10.1177/036354659702500213.
5. Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations. The natural history. Am J Sports Med. 1986;14(2):117-120. doi:10.1177/036354658601400204.
6. Cofield RH, Bryan RS. Acute dislocation of the patella: results of conservative treatment. J Trauma. 1977;17(7):526-531.
7. Wasserlauf BL, Paletta GA. Developmental anatomy of the pediatric and adolescent knee. In: Micheli LJ, Kocher MS, eds. The Pediatric and Adolescent Knee. 1st ed. Elsevier; 2006:27-32.
8. Birch JG. Growth and development. In: Herring J, ed. Tachdjian’s Pediatric Orthopedics. 4th ed. Saunders/Elsevier; 2007:3-22.
9. Sarin VK, Carter DR. Mechanobiology and joint conformity regulate endochondral ossification of sesamoids. J Orthop Res. 2000;18(5):706-712. doi:10.1002/jor.1100180505.
10. Maes C, Kronenberg HM. Postnatal bone growth: growth plate biology, bone formation, and remodeling. In: Pediatric Bone. Elsevier; 2012:55-82.
11. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235-240. doi:10.1007/s00167-005-0683-0.
12. Walmsley R. The development of the patella. J Anat. 1940;74(Pt 3):360-368.3.
13. Fulkerson J, Hungerford D. Disorders of the patellofemoral Joint. In: Normal Anatomy. 2nd ed. Baltimore: Williams & Wilkins; 1990:1-24.
14. Ogden JA. Radiology of postnatal skeletal development. X. Patella and tibial tuberosity. Skeletal Radiol. 1984;11(4):246-257.
15. Nietosvaara Y, Aalto K. The cartilaginous femoral sulcus in children with patellar dislocation: an ultrasonographic study. J Pediatr Orthop. 1997;17(1):50-53.
16. Glard Y, Jouve JL, Garron E, Adalian P, Tardieu C, Bollini G. Anatomic study of femoral patellar groove in fetus. J Pediatr Orthop. 2005;25(3):305-308.
17. Nietosvaara Y. The femoral sulcus in children. An ultrasonographic study. J Bone Joint Surg Br. 1994;76(5):807-809.
18. Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;(297):62-64.
19. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 26(1):59-65. doi:10.1177/03635465980260012701.
20. Shea KG, Styhl AC, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: a cadaveric study. Am J Sports Med. 2016;44(11):2833-2837. doi:10.1177/0363546516656366.
21. Shea KG, Polousky JD, Jacobs JC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children. J Pediatr Orthop. 2014;34(8):808-813. doi:10.1097/BPO.0000000000000165.
22. Shea KG, Polousky JD, Jacobs JC, et al. The patellar insertion of the medial patellofemoral ligament in children. J Pediatr Orthop. 2015;35(4):e31-e35. doi:10.1097/BPO.0000000000000399.
23. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi:10.1016/j.arthro.2009.12.020.
24. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sport Traumatol Arthrosc. 2011;19(12):2067-2071. doi:10.1007/s00167-011-1548-3.
25. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi:10.1177/0363546510397174.
26. Seitlinger G, Moroder P, Fink C, Wierer G. Acquired femoral flexion deformity due to physeal injury during medial patellofemoral ligament reconstruction. Knee. 2017;24(3):680-685. doi:10.1016/j.knee.2017.02.003.
27. Parikh SN, Rajdev N. Relationship between trochlear dysplasia and the anterior distal femoral physis in pediatric and adolescent patients with patellar instability. In: 36th Annual Meething AANA. Denver; 2017.
28. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57(2):259-261.
29. Parikh SN. Medial patellofemoral ligament reconstruction and simultaneous guided correction of genu valgum for patellar instability in skeletally immature patients. In: EPOSNA. 2017.
30. Brattstroem H. Shape of the intercondylar groove normally and in recurrent dislocation of patella. A clinical and X-ray-Anatomical investigation. Acta Orthop Scand Suppl. 1964;68(suppl 68):1-148.
31. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
32. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi:10.1177/0363546512472873.
33. Jaquith BP, Parikh SN. Predictors of recurrent patellar instability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi:10.1097/BPO.0000000000000674.
34. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B. 2011;20(5):341-344. doi:10.1097/BPB.0b013e3283474c8b.
35. Parikh SN, Rajdev N, Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018. doi:10.1097/BPO.0000000000001168. [Epub ahead of print]
36. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177. doi:10.1302/0301-620X.89B9.19065.
37. Sugimoto D, Christino MA, Micheli LJ. Effects of surgical intervention on trochlear remodeling in pediatric patients with recurrent patella dislocation cases. J Pediatr Orthop B. 2016;25(4):349-353. doi:10.1097/BPB.0000000000000341.
38. Insall J, Goldberg V, Salvati E. Recurrent dislocation and the high-riding patella. Clin Orthop Relat Res. 1972;88:67-69.
39. Caton J. [Method of measuring the height of the patella]. Acta Orthop Belg. 1989;55(3):385-386.
40. Thévenin-Lemoine C, Ferrand M, Courvoisier A, Damsin JP, Ducou le Pointe H, Vialle R. Is the Caton-Deschamps index a valuable ratio to investigate patellar height in children? J Bone Joint Surg Am. 2011;93(8):e35. doi:10.2106/JBJS.J.00759.
41. Micheli LJ, Slater JA, Woods E, Gerbino PG. Patella alta and the adolescent growth spurt. Clin Orthop Relat Res. 1986;213:159-162.
42. Koshino T, Sugimoto K. New measurement of patellar height in the knees of children using the epiphyseal line midpoint. J Pediatr Orthop. 1989;9(2):216-218.
43. Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212.
44. Smits-Engelsman B, Klerks M, Kirby A. Beighton score: a valid measure for generalized hypermobility in children. J Pediatr. 2011;158(1):119-123. doi:10.1016/j.jpeds.2010.07.021.
45. Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202-1206.
46. Beighton P, Solomon L, Soskolnet CL. Articular mobility in an African population. Ann rheum Dis. 1973;32(5):413-418.
47. Staheli LT, Corbett M, Wyss C, King H. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67(1):39-47.
48. Kijowski R, Plagens D, Shaeh S, Teitge R. The effects of rotational deformities of the femur on contact pressure and contact area in the patellofemoral joint and on strain in the medial patellofemoral ligament. Annu Meet Int Patellofemoral Study Group, Napa Val San Fr CA. 1999.
49. Post W, Teitge R, Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3):521-546.
50. Bruce WD, Stevens PM. Surgical correction of miserable malalignment syndrome. J Pediatr Orthop. 2004;24(4):392-396.
51. Kurosawa K, Kawame H, Ochiai Y, Nakashima M, Tohma T, Ohashi H. Patellar dislocation in Kabuki syndrome. Am J Med Genet. 2002;108(2):160-163.
52. Mik G, Gholve PA, Scher DM, Widmann RF, Green DW. Down syndrome: orthopedic issues. Curr Opin Pediatr. 2008;20(1):30-36. doi:10.1097/MOP.0b013e3282f35f19.
53. Sweeney E, Fryer A, Mountford R, Green A, McIntosh I. Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet. 2003;40(3):153-162.
54. Stevens CA. Patellar dislocation in Rubenstein-Taybi syndrome. Am J Med Genet. 1997;72(2):188-190.
55. Andrish J. The biomechanics of patellofemoral stability. J Knee Surg. 2004;17(1):35-39.
56. Conlan T, Garth WP, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682-693.
57. Balcarek P, Ammon J, Frosch S, et al. Magnetic resonance imaging characteristics of the medial patellofemoral ligament lesion in acute lateral patellar dislocations considering trochlear dysplasia, patella alta, and tibial tuberosity-TG distance. Arthroscopy. 2010;26(7):926-935. doi:10.1016/j.arthro.2009.11.004.
58. Garin C, Chaker M, Dohin B, Kohler R. Permanent, habitual dislocation and recurrent dislocation of the patella in children: surgical management by patellar ligamentous transfer in 50 knees. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(7):690-700.
59. Sillanpaa P. Terminology of patellar dislocation. In: Sillanpaa P, ed. Trauma. Saarbrucken, Germany: Lambert Academic Publishing; 2010:16-18.
60. Parikh SN, Lykissas MG. Classification of lateral patellar instability in children and adolescents. Orthop Clin North Am. 2016;47(1):145-152. doi:10.1016/j.ocl.2015.08.016.
61. Chotel F, Bérard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(suppl 1):125-137. doi:10.1016/j.otsr.2013.06.014.
62. Wada A, Fujii T, Takamura K, Yanagida H, Surijamorn P. Congenital dislocation of the patella. J Child Orthop. 2008;2(2):119-123. doi:10.1007/s11832-008-0090-4.
63. Südkamp NP, Granrath M, Hoffmann R, Haas NP. Instability of the shoulder joint in the athlete. Chirurg. 1994;65(11):901-909.
64. Hiemstra LA, Kerslake S, Lafave M, Heard SM, Buchko GML. Introduction of a classification system for patients with patellofemoral instability (WARPS and STAID). Knee Surg Sport Traumatol Arthrosc. 2014;22(11):2776-2782. doi:10.1007/s00167-013-2477-0.
65. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497. doi:10.1007/s00402-015-2381-9.
66. Balcarek P, Oberthür S, Hopfensitz S, et al. Which patellae are likely to redislocate? Knee Surg Sport Traumatol Arthrosc. 2014;22(10):2308-2314. doi:10.1007/s00167-013-2650-5.
67. Bitar AC, Demange MK, D’Elia CO, Camanho GL. Traumatic patellar dislocation: nonoperative treatment compared with mpfl reconstruction using patellar tendon. Am J Sports Med. 2012;40(1):114-122. doi:10.1177/0363546511423742.
68. Smith TO, Walker J, Russell N. Outcomes of medial patellofemoral ligament reconstruction for patellar instability: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2007;15(11):1301-1314. doi:10.1007/s00167-007-0390-0.
69. Steiner TM, Torga-Spak R, Teitge RA. Medial patellofemoral ligament reconstruction in patients with lateral patellar instability and trochlear dysplasia. Am J Sports Med. 2006;34(8):1254-1261. doi:10.1177/0363546505285584.
70. Ma LF, Wang F, Chen BC, Wang CH, Zhou JW, Wang HY. Medial retinaculum plasty versus medial patellofemoral ligament reconstruction for recurrent patellar instability in adults: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2013;29(5):891-897. doi:10.1016/j.arthro.2013.01.030.
71. Weeks KD, Fabricant PD, Ladenhauf HN, Green DW. Surgical options for patellar stabilization in the skeletally immature patient. Sports Med Arthrosc Rev. 2012;20(3):194-202.
72. Green D. Surgical treatment of pediatric patella instability. Die Ther der Instabilen patella. 2016:80-89.
73. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309. doi:10.1055/s-0034-1398373.
74. Ghanem I, Wattincourt L, Seringe R. Congenital dislocation of the patella. Part I: pathologic anatomy. J Pediatr Orthop. 2000;20(6):812-816.
75. Bongers E, Van Kampen A, Van Bokhoven H, Knoers N. Human syndromes with congenital patellar anomalies and the underlying gene defects. Clin Genet. 2005;68(4):302-319. doi:10.1111/j.1399-0004.2005.00508.x.
76. Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51(3):444-453.
77. Gausden EB, Fabricant PD, Taylor SA, et al. Medial patellofemoral reconstruction in children and adolescents. JBJS Rev. 2015;3(10):1-11. doi:10.2106/JBJS.RVW.N.00091.
78. Palmu S, Kallio PE, Donell ST, Helenius I, Nietosvaara Y. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90(3):463-470. doi:10.2106/JBJS.G.00072.
79. Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics. 2013;36(11):e1444-e1449. doi:10.3928/01477447-20131021-30.
80. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
81. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):e47. doi:10.1053/jars.2003.50167.
82. Nomura E, Inoue M, Kobayashi S. Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2007;35(11):1851-1858. doi:10.1177/0363546507306161.
83. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States. Orthop J Sport Med. 2016;4(8):2325967116662873. doi:10.1177/2325967116662873.
84. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc. 2007;15(2):61-67. doi:10.1097/JSA.0b013e3180479464.
85. Nietosvaara Y, Paukku R, Palmu S, Donell ST. Acute patellar dislocation in children and adolescents. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2):139-145. doi:10.2106/JBJS.H.01289.
86. Hing CBC, Smith TO, Donell S, Song F. Surgical versus non-surgical interventions for treating patellar dislocation. In Hing CB, ed. Cochrane Database Syst Rev. 2011;(11):CD008106. doi:10.1002/14651858.CD008106.pub2.
87. Nwachukwu BU, So C, Schairer WW, Green DW, Dodwell ER. Surgical versus conservative management of acute patellar dislocation in children and adolescents: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):760-767. doi:10.1007/s00167-015-3948-2.
88. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi:10.1177/0363546510376230.
89. Nikku R, Nietosvaara Y, Aalto K, Kallio PE. Operative treatment of primary patellar dislocation does not improve medium-term outcome: a 7-year follow-up report and risk analysis of 127 randomized patients. Acta Orthop. 2005;76(5):699-704. doi:10.1080/17453670510041790.
90. Ostermeier S, Holst M, Hurschler C, Windhagen H, Stukenborg-Colsman C. Dynamic measurement of patellofemoral kinematics and contact pressure after lateral retinacular release: an in vitro study. Knee Surg Sport Traumatol Arthrosc. 2007;15(5):547-554. doi:10.1007/s00167-006-0261-0.
91. Sallay PI, Poggi J, Speer KP, Garrett WE. Acute dislocation of the patella. A correlative pathoanatomic study. Am J Sports Med. 1996;24(1):52-60. doi: 10.1177/036354659602400110.
92. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
93. Arendt EA, Moeller A, Agel J. Clinical outcomes of medial patellofemoral ligament repair in recurrent (chronic) lateral patella dislocations. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1909-1914. doi:10.1007/s00167-011-1516-y.
94. Ellera Gomes JL. Medial patellofemoral ligament reconstruction for recurrent dislocation of the patella: a preliminary report. Arthroscopy. 1992;8(3):335-340.
95. Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10(3):215-220.
96. Avikainen VJ, Nikku RK, Seppänen-Lehmonen TK. Adductor magnus tenodesis for patellar dislocation. Technique and preliminary results. Clin Orthop Relat Res. 1993;(297):12-16.
97. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop Relat Res. 2002;(402):38-52.
98. Dhillon MS, Mohan P, Nagi ON. Does harvesting the medial third of the patellar tendon cause lateral shift of the patella after ACL reconstruction? Acta Orthop Belg. 2003;69(4):334-340.
99. Drez D, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthrosc J Arthrosc Relat Surg. 2001;17(3):298-306. doi:10.1053/jars.2001.21490.
100. Ellera Gomes JL, Stigler Marczyk LR, César de César P, Jungblut CF. Medial patellofemoral ligament reconstruction with semitendinosus autograft for chronic patellar instability: a follow-up study. Arthrosc J Arthrosc Relat Surg. 2004;20(2):147-151. doi:10.1016/j.arthro.2003.11.006.
101. Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthrosc J Arthrosc Relat Surg. 2007;23(5):542-553. doi:10.1016/j.arthro.2007.03.006.
102. Nomura E, Horiuchi Y, Kihara M. Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee. 2000;7(2):121-127.
103. Ostermeier S, Stukenborg-Colsman C, Hurschler C, Wirth CJ. In vitro investigation of the effect of medial patellofemoral ligament reconstruction and medial tibial tuberosity transfer on lateral patellar stability. Arthrosc J Arthrosc Relat Surg. 2006;22(3):308-319. doi:10.1016/j.arthro.2005.09.024.
104. Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10(3):221-227.
105. Steensen RN, Dopirak RM, Maurus PB. A simple technique for reconstruction of the medial patellofemoral ligament using a quadriceps tendon graft. Arthroscopy. 2005;21(3):365-370. doi:10.1016/j.arthro.2004.10.007.
106. Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32(6):1509-1513. doi:10.1177/0363546503261505.
107. Farahmand F, Tahmasbi MN, Amis AA. Lateral force–displacement behaviour of the human patella and its variation with knee flexion—a biomechanical study in vitro. J Biomech. 1998;31(12):1147-1152.
108. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar 3-dimensional tracking. Clin Orthop Relat Res. 1994;(299):235-243.
109. Goyal D. Medial patellofemoral ligament reconstruction: the superficial quad technique. Am J Sports Med. 2013;41(5):1022-1029. doi:10.1177/0363546513477828.
110. Hohn E, Pandya NK. Does the utilization of allograft tissue in medial patellofemoral ligament reconstruction in pediatric and adolescent patients restore patellar stability? Clin Orthop Relat Res. 2017;475(6):1563-1569. doi:10.1007/s11999-016-5060-4.
111. Csintalan R, Latt L, Fornalski S, Raiszadeh K, Inacio M, Fithian D. Medial patellofemoral ligament (MPFL) reconstruction for the treatment of patellofemoral instability. J Knee Surg. 2013;27(2):139-146. doi:10.1055/s-0033-1360652.
112. Ahmad CS, Brown GD, Stein BS. The docking technique for medial patellofemoral ligament reconstruction: surgical technique and clinical outcome. Am J Sports Med. 2009;37(10):2021-2027. doi:10.1177/0363546509336261.
113. Fernandez E, Sala D, Castejon M. Reconstruction of the medial patellofemoral ligament for patellar instability using a semitendinosus autograft. Acta Orthop Belg. 2005;71(3):303-308.
114. Parikh SN, Nathan ST, Wall EJ, Eismann EA. Complications of medial patellofemoral ligament reconstruction in young patients. Am J Sports Med. 2013;41(5):1030-1038. doi:10.1177/0363546513482085.
115. Green D, Gausden E. Medial patellofemoral ligament reconstruction: hamstring technique. In: Green D, Cordasco F, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:150-157.
116. Craig JG, Cody DD, van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, 3-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33(6):337-344.
117. Noyes FR, Albright JC. Reconstruction of the medial patellofemoral ligament with autologous quadriceps tendon. Arthroscopy. 2006;22(8):904.e1-e7. doi:10.1016/j.arthro.2005.12.058.
118. Pinkowsky G, Hennrikus. Technique: quad tendon medial patellofemoral ligament reconstruction. In: Green DW, Cordasco FA, eds. Pediatr. Adolesc. Knee Surg. New York, NY: Wolters Kluwer; 2015:133-139.
119. Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27(8):1153-1159. doi:10.1016/j.arthro.2011.02.014.
120. Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34(9):1478-1485. doi:10.1177/0363546506287486.
121. Thaunat M, Erasmus PJ. Management of overtight medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):480-483. doi:10.1007/s00167-008-0702-z.
122. Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
123. Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293-297. doi:10.1177/0363546509347602.
124. Fink C, Veselko M, Herbort M, Hoser C. Minimally invasive reconstruction of the medial patellofemoral ligament using quadriceps tendon. Arthrosc Tech. 2014;3(3):e325-e329. doi:10.1016/j.eats.2014.01.012.
125. Sillanpää PJ, Mäenpää HM, Mattila VM, Visuri T, Pihlajamäki H. A mini-invasive adductor magnus tendon transfer technique for medial patellofemoral ligament reconstruction: a technical note. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):508-512. doi:10.1007/s00167-008-0713-9.
126. Hambidge SJ, Davidson AJ, Gonzales R, Steiner JF. Epidemiology of pediatric injury-related primary care office visits in the United States. Pediatrics. 2002;109(4):559-565.
127. Camanho GL, Bitar AC, Hernandez AJ, Olivi R. Medial patellofemoral ligament reconstruction: a novel technique using the patellar ligament. Arthroscopy. 2007;23(1):108.e1-e4. doi:10.1016/j.arthro.2006.07.008.
128. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435. doi:10.1016/j.arthro.2007.11.005.
129. Deie M, Ochi M, Sumen Y, Yasumoto M, Kobayashi K, Kimura H. Reconstruction of the medial patellofemoral ligament for the treatment of habitual or recurrent dislocation of the patella in children. J Bone Joint Surg Br. 2003;85(6):887-890.
130. Ladenhauf HN, Berkes MB, Green DW. Medial patellofemoral ligament reconstruction using hamstring autograft in children and adolescents. Arthrosc Tech. 2013;2(2):e151-e154. doi:10.1016/j.eats.2013.01.006.
131. Bedi H, Marzo J. The biomechanics of medial patellofemoral ligament repair followed by lateral retinacular release. Am J Sports Med. 2010;38(7):1462-1467. doi:10.1177/0363546510373581.
132. Lattermann C, Toth J, Bach BR. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc Rev. 2007;15(2):57-60. doi:10.1097/JSA.0b013e318042af30
133. Ricchetti ET, Mehta S, Sennett BJ, Huffman GR. Comparison of lateral release versus lateral release with medial soft-tissue realignment for the treatment of recurrent patellar instability: a systematic review. Arthroscopy. 2007;23(5):463-468. doi:10.1016/j.arthro.2007.01.007.
134. Dainer RD, Barrack RL, Buckley SL, Alexander AH. Arthroscopic treatment of acute patellar dislocations. Arthroscopy. 1988;4(4):267-271.
135. Miller JR, Adamson GJ, Pink MM, Fraipont MJ, Durand P. Arthroscopically assisted medial reefing without routine lateral release for patellar instability. Am J Sports Med. 2007;35(4):622-629. doi:10.1177/0363546506296041.
136. Vainionpää S, Laasonen E, Silvennoinen T, Vasenius J, Rokkanen P. Acute dislocation of the patella. A prospective review of operative treatment. J Bone Joint Surg Br. 1990;72(3):366-369.
137. Shah JN, Howard JS, Flanigan DC, Brophy RH, Carey JL, Lattermann C. A systematic review of complications and failures associated with medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med. 2012;40(8):1916-1923. doi:10.1177/0363546512442330.
138. Tanaka MJ, Bollier MJ, Andrish JT, Fulkerson JP, Cosgarea AJ. Complications of medial patellofemoral ligament reconstruction: common technical errors and factors for success: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(12):e87. doi:10.2106/JBJS.K.01449.
139. Nguyen CV, Farrow LD, Liu RW, Gilmore A. Safe drilling paths in the distal femoral epiphysis for pediatric medial patellofemoral ligament reconstruction. Am J Sports Med. 2017;45(5):1085-1089. doi:10.1177/0363546516677795.
140. Edmonds EW, Glaser DA. Adolescent patella instability extensor mechanics. J Pediatr Orthop. 2016;36(3):262-267. doi:10.1097/BPO.0000000000000430.
141. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184. doi:10.1016/j.knee.2014.07.023.
142. Lykissas MG, Li T, Eismann EA, Parikh SN. Does medial patellofemoral ligament reconstruction decrease patellar height? A preliminary report. J Pediatr Orthop. 2014;34(1):78-85. doi:10.1097/BPO.0b013e3182a12102.
143. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surgery, Sport Traumatol Arthrosc. 2014;22(10):2545-2550. doi:10.1007/s00167-013-2445-8.
144. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521. doi:10.1007/s00167-005-0659-0.
145. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi:10.1177/0363546512463683.
146. Raghuveer RK, Mishra CB. Reconstruction of medial patellofemoral ligament for chronic patellar instability. Indian J Orthop. 2012;46(4):447-454. doi:10.4103/0019-5413.97259.
147. Kita K, Horibe S, Toritsuka Y, et al. Effects of medial patellofemoral ligament reconstruction on patellar tracking. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):829-837. doi:10.1007/s00167-011-1609-7.
148. Lenschow S, Herbort M, Fink C. Medial patellofemoral ligament reconstruction using quadriceps tendon. Oper Orthop Traumatol. 2015;27(6):474-483. doi:10.1007/s00064-015-0416-6.
149. Abouelsoud MM, Abdelhady A, Elshazly O. Anatomic physeal-sparing technique for medial patellofemoral ligament reconstruction in skeletally immature patients with ligamentous laxity. Eur J Orthop Surg Traumatol. 2015;25(5):921-926. doi:10.1007/s00590-015-1618-1.
150. Malecki K, Fabis J, Flont P, Niedzielski KR. The results of adductor magnus tenodesis in adolescents with recurrent patellar dislocation. Biomed Res Int. 2015;2015:1-7. doi:10.1155/2015/456858.
151. Kumahashi N, Kuwata S, Tadenuma T, Kadowaki M, Uchio Y. A “sandwich” method of reconstruction of the medial patellofemoral ligament using a titanium interference screw for patellar instability in skeletally immature patients. Arch Orthop Trauma Surg. 2012;132(8):1077-1083. doi:10.1007/s00402-012-1516-5.
152. Marsh JS, Daigneault JP, Sethi P, Polzhofer GK. Treatment of recurrent patellar instability with a modification of the Roux-Goldthwait technique. J Pediatr Orthop. 2006;26(4):461-465. doi:10.1097/01.bpo.0000217711.34492.48.
153. Nepple J, Luhmann S. Medial patellar tendon transfer with proximal realignment. In: Green D, Cordasco F, eds. Pediatric and Adolescent Knee Surgery. New York, NY: Wolters Kluwer; 2015:148-153.
154. Galeazzi R. Nuove applicazioni del trapianto musculare e tendineo. Ard Di Orthop Milano. 1922;38:315-323.
155. Grannatt K, Heyworth BE, Ogunwole O, Micheli LJ, Kocher MS. Galeazzi semitendinosus tenodesis for patellofemoral instability in skeletally immature patients. J Pediatr Orthop. 2012;32(6):621-625. doi:10.1097/BPO.0b013e318263a230.
156. Fiume M. La rotulopessi secondi Galeazzi nella lussazione recidivante di rotula [in Italian]. Minerva Ortop. 1954;5:171-174.
157. Baker RH, Carroll N, Dewar FP, Hall JE. The semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Br. 1972;54(1):103-109.
158. Hall JE, Micheli LJ, McManama GB. Semitendinosus tenodesis for recurrent subluxation or dislocation of the patella. Clin Orthop Relat Res. 1979;(144):31-35.
159. Moyad TF, Blakemore L. Modified Galeazzi technique for recurrent patellar dislocation in children. Orthopedics. 2006;29(4):302-304.
160. Letts RM, Davidson D, Beaule P. Semitendinosus tenodesis for repair of recurrent dislocation of the patella in children. J Pediatr Orthop. 1999;19(6):742-747.
161. Aulisa AG, Falciglia F, Giordano M, Savignoni P, Guzzanti V. Galeazzi’s modified technique for recurrent patella dislocation in skeletally immature patients. J Orthop Sci. 2012;17(2):148-155. doi:10.1007/s00776-011-0189-1.
162. Roux C. Recurrent dislocation of the patella: operative treatment. 1888. Clin Orthop Relat Res. 2006;452:17-20.
163. Goldthwait J. Dislocation of the Patella. Trans Am Orthop Assn. 1895.
164. Fondren FB, Goldner JL, Bassett FH. Recurrent dislocation of the patella treated by the modified Roux-Goldthwait procedure. A prospective study of forty-seven knees. J Bone Joint Surg Am. 1985;67(7):993-1005.
165. Aärimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446. doi:10.1111/j.1600-0838.2007.00730.x.
166. Nelitz M, Reichel H, Dornacher D, Lippacher S. Anatomical reconstruction of the medial patellofemoral ligament in children with open growth-plates. Arch Orthop Trauma Surg. 2012;132(11):1647-1651. doi:10.1007/s00402-012-1593-5.
167. Vähäsarja V, Kinnunen P, Lanning P, Serlo W. Operative realignment of patellar malalignment in children. J Pediatr Orthop. 1995;15(3):281-285.
168. Abraham E, Washington E, Huang TL. Insall proximal realignment for disorders of the patella. Clin Orthop Relat Res. 1989;248:61-65.
169. Insall JN, Aglietti P, Tria AJ. Patellar pain and incongruence. II: Clinical application. Clin Orthop Relat Res. 1983;176:225-232.
170. Chrisman OD, Snook GA, Wilson TC. A long-term prospective study of the Hauser and Roux-Goldthwait procedures for recurrent patellar dislocation. Clin Orthop Relat Res. 1979;144:27-30.
171. Niedzielski KR, Malecki K, Flont P, Fabis J. The results of an extensive soft-tissue procedure in the treatment of obligatory patellar dislocation in children with ligamentous laxity: a post-operative isokinetic study. Bone Joint J. 2015;97-B(1):129-133. doi:10.1302/0301-620X.97B1.33941.
172. Hinckel BB, Gobbi RG, Demange MK, Bonadio MB, Pécora JR, Camanho GL. Combined reconstruction of the medial patellofemoral ligament with quadricipital tendon and the medial patellotibial ligament with patellar tendon. Arthrosc Tech. 2016;5(1):e79-e84. doi:10.1016/j.eats.2015.10.004.
173. Mani S, Kirkpatrick MS, Saranathan A, Smith LG, Cosgarea AJ, Elias JJ. Tibial tuberosity osteotomy for patellofemoral realignment alters tibiofemoral kinematics. Am J Sports Med. 2011;39(5):1024-1031. doi:10.1177/0363546510390188.
174. Brown GD, Ahmad CS. Combined medial patellofemoral ligament and medial patellotibial ligament reconstruction in skeletally immature patients. J Knee Surg. 2008;21(4):328-332.
175. Panagiotopoulos E, Strzelczyk P, Herrmann M, Scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7-12. doi:10.1007/s00167-005-0631-z.
176. Philippot R, Boyer B, Testa R, Farizon F, Moyen B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):331-336. doi:10.1007/s00167-011-1598-6.
177. Gordon JE, Schoenecker PL. Surgical treatment of congenital dislocation of the patella. J Pediatr Orthop. 1999;19(2):260-264.
178. Koh JL, Stewart C. Patellar instability. Orthop Clin North Am. 2015;46(1):147-157. doi:10.1016/j.ocl.2014.09.011.
179. Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927-933. doi:10.1002/jor.22008.
180. Thompson TC. Quadricepsplasty. Ann Surg. 1945;121(5):751-754.
181. Daoud H, O’Farrell T, Cruess RL. Quadricepsplasty. The Judet technique and results of six cases. J Bone Joint Surg Br. 1982;64(2):194-197.
182. Stanisavljevic S, Zemenick G, Miller D. Congenital, irreducible, permanent lateral dislocation of the patella. Clin Orthop Relat Res. 1976;(116):190-199.
183. Kundu Z, Sangwan S, Guliani G, Siwach R, Kamboj P, Singh R. Thompson’s quadricepsplasty for stiff knee. Indian J Orthop. 2007;41(4):390-394. doi:10.4103/0019-5413.37004.
184. Tercier S, Shah H, Joseph B. Quadricepsplasty for congenital dislocation of the knee and congenital quadriceps contracture. J Child Orthop. 2012;6(5):397-410. doi:10.1007/s11832-012-0437-8.
185. Rose RE. Judet quadricepsplasty for extension contracture of the knee. West Indian Med J. 2005;54(4):238-241.
186. Tsukamoto N, Miura H, Matsuda S, Mawatari T, Kato H, Iwamoto Y. Functional evaluation of four patients treated with V-Y quadricepsplasty in total knee arthroplasty. J Orthop Sci. 2006;11(4):394-400. doi:10.1007/s00776-006-1023-z.
187. Dao Q, Chen DB, Scott RD. Proximal patellar quadricepsplasty realignment during total knee arthroplasty for irreducible congenital dislocation of the patella. J Bone Joint Surg Am. 2010;92(14):2457-2461. doi:10.2106/JBJS.H.00812.
188. Judet R, Judet J, Lord G. Results of treatment of stiffness of the knee caused by arthrolysis and disinsertion of the quadriceps femoris. Mem Acad Chir. 1959;85:645-654.
189. Oliveira VG, D’Elia LF, Tirico LEP, et al. Judet quadricepsplasty in the treatment of posttraumatic knee rigidity: long-term outcomes of 45 cases. J Trauma Acute Care Surg. 2012;72(2):e77-e80.
190. Hahn SB, Choi YR, Kang HJ, Lee SH. Prognostic factors and long-term outcomes following a modified Thompson’s quadricepsplasty for severely stiff knees. J Bone Joint Surg Br. 2010;92(2):217-221. doi:10.1302/0301-620X.92B2.22936.
191. Hosalkar HS, Jones S, Chowdhury M, Hartley J, Hill RA. Quadricepsplasty for knee stiffness after femoral lengthening in congenital short femur. J Bone Joint Surg Br. 2003;85(2):261-264.
192. Massè A, Biasibetti A, Demangos J, Dutto E, Pazzano S, Gallinaro P. The judet quadricepsplasty: long-term outcome of 21 cases. J Trauma. 2006;61(2):358-362. doi:10.1097/01.ta.0000230281.31144.1d.
193. Kocon H, Kabacyj M, Zgoda M. The results of the operative treatment of patellar instability in children with Down’s syndrome. J Pediatr Orthop B. 2012;21(5):407-410. doi:10.1097/BPB.0b013e328354f684.
194. Martin BD, Cherkashin AM, Tulchin K, Samchukov M, Birch JG. Treatment of femoral lengthening-related knee stiffness with a novel quadricepsplasty. J Pediatr Orthop. 2013;33(4):446-452. doi:10.1097/BPO.0b013e3182784e5d.
TAKE-HOME POINTS
- Patellofemoral joint stability is dependent on a complex interplay of musculotendinous units, ligaments, and the osteocartilaginous morphology of the patellofemoral joint.
- Varied patterns of patellar instability in the pediatric population should be recognized. Habitual dislocation in flexion and permanent dislocation are the more severe types.
- Assessment of major risk factors and, if required, their correction would influence management decisions and would have prognostic value related to outcomes.
- Physeal-sparing MPFL reconstruction can suffice for most children and adolescents with recurrent patellar dislocation.
- Distal stabilization techniques and quadricepsplasty are an important part of surgical armamentarium, especially for the more complex patellar instability patterns.