User login
VTE risk after bariatric surgery should be assessed
SEATTLE – Preop thromboelastometry can identify patients who need extra according to a prospective investigation of 40 patients at Conemaugh Memorial Medical Center in Johnstown, Pa.
Enoxaparin 40 mg twice daily just wasn’t enough for people who were hypercoagulable before surgery. The goal of the study was to find the best way to prevent venous thromboembolism (VTE) after weight loss surgery. At present, there’s no consensus on prophylaxis dosing, timing, duration, or even what agent to use for these patients. Conemaugh uses postop enoxaparin, a low-molecular-weight heparin. Among many other options, some hospitals opt for preop dosing with traditional heparin, which is less expensive.
The Conemaugh team turned to thromboelastometry (TEM) to look at the question of VTE risk in bariatric surgery patients. The test gauges coagulation status by measuring elasticity as a small blood sample clots over a few minutes. The investigators found that patients who were hypercoagulable before surgery were likely to be hypercoagulable afterwards. The finding argues for baseline TEM testing to guide postop anticoagulation.
The problem is that bariatric services don’t often have access to TEM equipment, and insurance doesn’t cover the $60 test. In this instance, the Lake Erie College of Osteopathic Medicine in Erie, Pa., had the equipment and covered the testing for the study.
The patients had TEM at baseline and then received 40 mg of enoxaparin about 4 hours after surgery – mostly laparoscopic gastric bypasses – and a second dose about 12 hours after the first. TEM was repeated about 2 hours after the second dose.
At baseline, 2 (5%) of the patients were hypocoagulable, 15 (37.5%) were normal, and 23 (57.5%) were hypercoagulable. On postop TEM, 17 patients (42.5%) were normal and 23 (57.5%) were hypercoagulable: “These 23 were inadequately anticoagulated,” said lead investigator Daniel Urias, MD, a general surgery resident at the medical center.
“There was an association between being normal at baseline and being normal postop, and being hypercoagulable at baseline and hypercoagulable postop. We didn’t anticipate finding such similarity between the numbers. Our suspicion that baseline status plays a major role is holding true,” Dr. Urias said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
When patients test hypercoagulable at baseline, “we are [now] leaning towards [enoxaparin] 60 mg twice daily,” he said.
Ultimately, anticoagulation TEM could be used to titrate patients into the normal range. For best outcomes, it’s likely that “obese patients require goal-directed therapy instead of weight-based or fixed dosing,” he said, but nothing is going to happen until insurance steps up.
The patients did not have underlying coagulopathies, and 33 (82.5%) were women; the average age was 44 years and average body mass index was 43.6 kg/m2. The mean preop Caprini score was 4, indicating moderate VTE risk. Surgery lasted about 200 minutes. Patients were out of bed and walking on postop day 0.
The investigators had no relevant disclosures.
SOURCE: Urias D et al. World Congress of Endoscopic Surgery hosted by SAGES & CAGS abstract S023.
SEATTLE – Preop thromboelastometry can identify patients who need extra according to a prospective investigation of 40 patients at Conemaugh Memorial Medical Center in Johnstown, Pa.
Enoxaparin 40 mg twice daily just wasn’t enough for people who were hypercoagulable before surgery. The goal of the study was to find the best way to prevent venous thromboembolism (VTE) after weight loss surgery. At present, there’s no consensus on prophylaxis dosing, timing, duration, or even what agent to use for these patients. Conemaugh uses postop enoxaparin, a low-molecular-weight heparin. Among many other options, some hospitals opt for preop dosing with traditional heparin, which is less expensive.
The Conemaugh team turned to thromboelastometry (TEM) to look at the question of VTE risk in bariatric surgery patients. The test gauges coagulation status by measuring elasticity as a small blood sample clots over a few minutes. The investigators found that patients who were hypercoagulable before surgery were likely to be hypercoagulable afterwards. The finding argues for baseline TEM testing to guide postop anticoagulation.
The problem is that bariatric services don’t often have access to TEM equipment, and insurance doesn’t cover the $60 test. In this instance, the Lake Erie College of Osteopathic Medicine in Erie, Pa., had the equipment and covered the testing for the study.
The patients had TEM at baseline and then received 40 mg of enoxaparin about 4 hours after surgery – mostly laparoscopic gastric bypasses – and a second dose about 12 hours after the first. TEM was repeated about 2 hours after the second dose.
At baseline, 2 (5%) of the patients were hypocoagulable, 15 (37.5%) were normal, and 23 (57.5%) were hypercoagulable. On postop TEM, 17 patients (42.5%) were normal and 23 (57.5%) were hypercoagulable: “These 23 were inadequately anticoagulated,” said lead investigator Daniel Urias, MD, a general surgery resident at the medical center.
“There was an association between being normal at baseline and being normal postop, and being hypercoagulable at baseline and hypercoagulable postop. We didn’t anticipate finding such similarity between the numbers. Our suspicion that baseline status plays a major role is holding true,” Dr. Urias said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
When patients test hypercoagulable at baseline, “we are [now] leaning towards [enoxaparin] 60 mg twice daily,” he said.
Ultimately, anticoagulation TEM could be used to titrate patients into the normal range. For best outcomes, it’s likely that “obese patients require goal-directed therapy instead of weight-based or fixed dosing,” he said, but nothing is going to happen until insurance steps up.
The patients did not have underlying coagulopathies, and 33 (82.5%) were women; the average age was 44 years and average body mass index was 43.6 kg/m2. The mean preop Caprini score was 4, indicating moderate VTE risk. Surgery lasted about 200 minutes. Patients were out of bed and walking on postop day 0.
The investigators had no relevant disclosures.
SOURCE: Urias D et al. World Congress of Endoscopic Surgery hosted by SAGES & CAGS abstract S023.
SEATTLE – Preop thromboelastometry can identify patients who need extra according to a prospective investigation of 40 patients at Conemaugh Memorial Medical Center in Johnstown, Pa.
Enoxaparin 40 mg twice daily just wasn’t enough for people who were hypercoagulable before surgery. The goal of the study was to find the best way to prevent venous thromboembolism (VTE) after weight loss surgery. At present, there’s no consensus on prophylaxis dosing, timing, duration, or even what agent to use for these patients. Conemaugh uses postop enoxaparin, a low-molecular-weight heparin. Among many other options, some hospitals opt for preop dosing with traditional heparin, which is less expensive.
The Conemaugh team turned to thromboelastometry (TEM) to look at the question of VTE risk in bariatric surgery patients. The test gauges coagulation status by measuring elasticity as a small blood sample clots over a few minutes. The investigators found that patients who were hypercoagulable before surgery were likely to be hypercoagulable afterwards. The finding argues for baseline TEM testing to guide postop anticoagulation.
The problem is that bariatric services don’t often have access to TEM equipment, and insurance doesn’t cover the $60 test. In this instance, the Lake Erie College of Osteopathic Medicine in Erie, Pa., had the equipment and covered the testing for the study.
The patients had TEM at baseline and then received 40 mg of enoxaparin about 4 hours after surgery – mostly laparoscopic gastric bypasses – and a second dose about 12 hours after the first. TEM was repeated about 2 hours after the second dose.
At baseline, 2 (5%) of the patients were hypocoagulable, 15 (37.5%) were normal, and 23 (57.5%) were hypercoagulable. On postop TEM, 17 patients (42.5%) were normal and 23 (57.5%) were hypercoagulable: “These 23 were inadequately anticoagulated,” said lead investigator Daniel Urias, MD, a general surgery resident at the medical center.
“There was an association between being normal at baseline and being normal postop, and being hypercoagulable at baseline and hypercoagulable postop. We didn’t anticipate finding such similarity between the numbers. Our suspicion that baseline status plays a major role is holding true,” Dr. Urias said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
When patients test hypercoagulable at baseline, “we are [now] leaning towards [enoxaparin] 60 mg twice daily,” he said.
Ultimately, anticoagulation TEM could be used to titrate patients into the normal range. For best outcomes, it’s likely that “obese patients require goal-directed therapy instead of weight-based or fixed dosing,” he said, but nothing is going to happen until insurance steps up.
The patients did not have underlying coagulopathies, and 33 (82.5%) were women; the average age was 44 years and average body mass index was 43.6 kg/m2. The mean preop Caprini score was 4, indicating moderate VTE risk. Surgery lasted about 200 minutes. Patients were out of bed and walking on postop day 0.
The investigators had no relevant disclosures.
SOURCE: Urias D et al. World Congress of Endoscopic Surgery hosted by SAGES & CAGS abstract S023.
REPORTING FROM SAGES 2018
Key clinical point: Preoperative thromboelastometry identifies patients who need extra anticoagulation against venous thromboembolism following bariatric surgery.
Major finding: Baseline and postop coagulation were similar: 37.5% vs. 42.5% were normal and 57.5% vs 57.5% were hypercoagulable.
Study details: Prospective study of 40 bariatric surgery patients.
Disclosures: The investigators did not have any relevant disclosures. The Lake Erie College of Osteopathic Medicine paid for the testing.
Source: Urias D et al. World Congress of Endoscopic Surgery hosted by SAGES & CAGS abstract S023.
What is HIPEC?
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Ovarian cancer most commonly follows a pattern of intraperitoneal spread, and even in the setting of bulky extra-ovarian disease, it can be thought of as being largely localized to the peritoneal compartment. This forms some of the rationale for performing extensive cytoreductive surgery (CRS) on ovarian cancer metastatic within the peritoneal cavity, and also some of the rationale for delivery of cytotoxic therapy directly to this compartment (intraperitoneal or “IP” chemotherapy). To be most effective, IP chemotherapy should be able to contact all peritoneal surfaces and be exposed to very low volume tumors (ideally no thicker than 2-mm implants).
There is a large body of evidence demonstrating the benefits of conventional IP chemotherapy in women who have received complete or “optimal” CRS to disease measuring less than 1 cm3.1 However, IP chemotherapy is complicated by difficult administration and can be difficult for patients to tolerate. It is associated with significant toxicity, more so than what is seen from intravenous chemotherapy, and this toxicity is drawn out over the 18 (or more) weeks of therapy. It requires placement of an intraperitoneal port, and there are many problems associated with this foreign body including infection, malposition, and even erosions into underlying visceral structures. There are also concerns regarding the ability of the intraperitoneal infusions to reach all peritoneal surfaces when postoperative adhesions may have formed to pocket-off areas of the peritoneal cavity.
Hyperthermic intraperitoneal chemotherapy (HIPEC), at the time of CRS, is a strategy that has been explored to overcome some of these challenges.2 HIPEC has the most history as an adjunct to the surgical management of gastrointestinal cancers (particularly appendiceal and colorectal). The technique first described by Dr. Paul H. Sugarbaker for gastrointestinal tumors remains similar to that performed in ovarian cancer.3 Patients first undergo extensive CRS until there is no macroscopic residual disease. Immediately following cytoreduction, catheters are placed into the peritoneal cavity, the main incision is temporarily closed (to prevent spillage), and an infusion of cytotoxic agents (commonly cisplatin, often with a second agent such as mitomycin C or doxorubicin) is warmed and then distilled into the peritoneal cavity until it is “moderately distended.” The patient’s body is then rolled back and forth to “wash” down the entire peritoneal cavity. All peritoneal surfaces can be touched by the agent as this procedure is happening intraoperatively prior to adhesion formation.
The “H” in HIPEC stands for hyperthermic, which is a key differentiator from traditional intraperitoneal and intravenous chemotherapy administration. Some chemotherapy agents, such as cisplatin, have a synergistic effect with hyperthermia. Some of these effects include increased oxygen free radical formation, increased cellular uptake of drug, reversal of mechanisms of drug resistance, and increases in DNA damage. The ideal range of hyperthermia is between 41° C and 44° C. At higher temperatures, infusions rates can be faster; however, higher temperatures are associated with more toxicity, particularly of the small bowel.4
Toxicity is a concern with HIPEC.5 Cytoreductive surgery for ovarian cancer can be associated with substantial morbidity, and exposing patients to prolonged operative time, extended periods of hyperthermia, and high doses of cytotoxic agents is a concern. When performed by high volume, experienced practitioners, approximately 12% of patients experience serious (grade 3-5) toxicity after CRS with HIPEC, with a procedure-related mortality of 1.2%. The majority of major toxicities were bone marrow suppression and nephrotoxicity (which in some cases can result in patients requiring hemodialysis in the immediate postoperative period). It is for this reason that most HIPEC protocols include a routine ICU admission postoperatively to closely monitor patients for major renal toxicity and electrolyte disturbances. These complications are associated with higher doses of administered cisplatin. Febrile neutropenia and gastrointestinal complications (such as bowel perforation, fistula, or anastomotic leak) also comprise the most common major toxicities. Patient factors to consider as higher risk for morbidity and mortality include underlying cardiac or pulmonary disease, poor performance status, extreme obesity, and preexisting renal disease.
While the history of HIPEC was focused around the treatment of GI peritoneal malignancies, in more recent years, the technique has been applied and studied in women with ovarian cancer.
The indications for use can vary from the upfront setting (at the time of primary CRS), following neoadjuvant chemotherapy (at the time of interval CRS), or in the recurrent setting (at the time of secondary CRS).2 Possibly the most compelling study exploring HIPEC in ovarian cancer was published earlier this year in the New England Journal of Medicine.6 This study explored the use of HIPEC at the time of interval CRS following three cycles of neoadjuvant platinum and taxane intravenous chemotherapy. Two hundred and forty-five patients were randomly assigned at the time of optimal interval CRS to either CRS alone or CRS with HIPEC with cisplatin administered over 90 minutes. All patients received three additional cycles of intravenous platinum and taxane chemotherapy. Death was observed in a significantly higher proportion of patients in the non-HIPEC group (62% versus 50%). HIPEC was associated with a hazard ratio for death of 0.67 (P = .02). In this study, a similar number of severe adverse outcomes were observed in the two groups, though specific information was lacking, particularly with respect to renal toxicity.
These findings are intriguing and have energized interest in HIPEC by many gynecologic oncology providers; however, there are some concerns regarding the results. Patients in this study received not one intervention, but in fact three interventions (hyperthermia, an additional cycle of chemotherapy, and the peritoneal administration of chemotherapy). Any one of these three variables could explain the outcomes and it is difficult to know if all three (in the form of HIPEC) are necessary to see this observed benefit. Others have questioned the finding of no added toxicity when HIPEC is applied. This is inconsistent with what has been presented elsewhere. It is unclear from the data whether or not the nephrotoxicity was comparable between the two groups or more severe among those who received HIPEC.
An additional concern regarding HIPEC is the feasibility. Additional operative times (by up to 90 minutes), increased duration of hospitalization (including ICU admission), and additional equipment and specialized personnel are required for this technique. This may further hinder its uptake and routine practice. In the meantime, we continue to await further clinical trials that will better define the ovarian cancer patient population who might benefit from this technique and provide further data regarding its risk/benefit profile.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
2. Helm CW et al. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98(4):283-90.
3. Glehen O et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242-6.
4. Kusamura S et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98(4):247-52.
5. Kusamura S et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-8.
6. van Driel WJ et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan;378(3):230-240.
Surgeries account for almost half of hospital costs
according to the Agency for Healthcare Research and Quality (AHRQ).
Of the 35.4 million inpatient stays in 2014 – the last full year of ICD-9-CM coding – 10.1 million (28.6%) involved at least one any-listed surgical procedure, and 25.2 million (71.4%) did not. The total cost of all admissions was $386.2 billion, of which $187.1 billion (48.4%) went for stays with surgeries and $199.1 billion (51.6%) went for nonsurgical stays, the AHRQ reported in a statistical brief.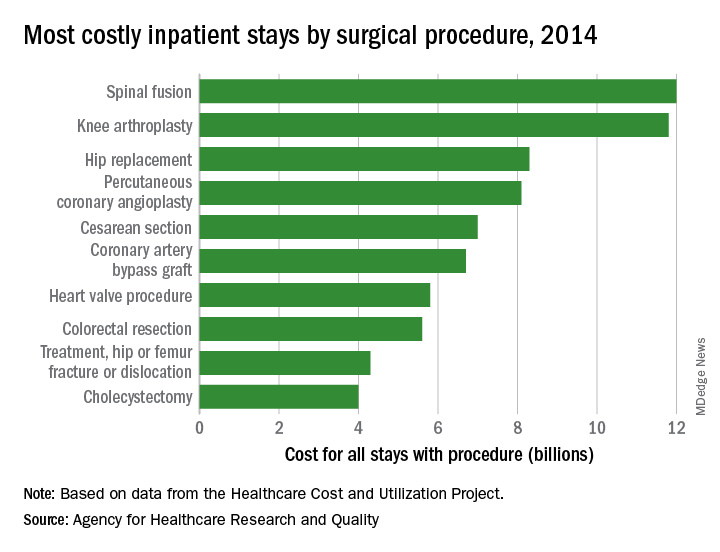
There were five other musculoskeletal procedures among the 20 most costly surgery-related admissions: knee arthroplasty was second at $11.8 billion, hip replacement was third at $8.3 billion, treatment of hip and femur fracture/dislocation was ninth at $4.3 billion, amputation of lower extremity was 13th at $2.5 billion, and treatment of lower extremity (other than hip or femur) fracture/dislocation was 14th at $2.4 billion. Those six procedures combined were $41.2 billion in hospital costs, which was a quarter of the total for all stays with a first-listed OR procedure, the AHRQ said.
The nonmusculoskeletal procedures in the top five were percutaneous coronary angioplasty in fourth, with an aggregate cost of $8.1 billion, and cesarean section in fifth at an even $7 billion. Coronary artery bypass graft, the most expensive procedure per stay ($52,000) among the top 20 procedures, was sixth in aggregate cost at $6.7 billion, according to the AHRQ researchers.
according to the Agency for Healthcare Research and Quality (AHRQ).
Of the 35.4 million inpatient stays in 2014 – the last full year of ICD-9-CM coding – 10.1 million (28.6%) involved at least one any-listed surgical procedure, and 25.2 million (71.4%) did not. The total cost of all admissions was $386.2 billion, of which $187.1 billion (48.4%) went for stays with surgeries and $199.1 billion (51.6%) went for nonsurgical stays, the AHRQ reported in a statistical brief.
There were five other musculoskeletal procedures among the 20 most costly surgery-related admissions: knee arthroplasty was second at $11.8 billion, hip replacement was third at $8.3 billion, treatment of hip and femur fracture/dislocation was ninth at $4.3 billion, amputation of lower extremity was 13th at $2.5 billion, and treatment of lower extremity (other than hip or femur) fracture/dislocation was 14th at $2.4 billion. Those six procedures combined were $41.2 billion in hospital costs, which was a quarter of the total for all stays with a first-listed OR procedure, the AHRQ said.
The nonmusculoskeletal procedures in the top five were percutaneous coronary angioplasty in fourth, with an aggregate cost of $8.1 billion, and cesarean section in fifth at an even $7 billion. Coronary artery bypass graft, the most expensive procedure per stay ($52,000) among the top 20 procedures, was sixth in aggregate cost at $6.7 billion, according to the AHRQ researchers.
according to the Agency for Healthcare Research and Quality (AHRQ).
Of the 35.4 million inpatient stays in 2014 – the last full year of ICD-9-CM coding – 10.1 million (28.6%) involved at least one any-listed surgical procedure, and 25.2 million (71.4%) did not. The total cost of all admissions was $386.2 billion, of which $187.1 billion (48.4%) went for stays with surgeries and $199.1 billion (51.6%) went for nonsurgical stays, the AHRQ reported in a statistical brief.
There were five other musculoskeletal procedures among the 20 most costly surgery-related admissions: knee arthroplasty was second at $11.8 billion, hip replacement was third at $8.3 billion, treatment of hip and femur fracture/dislocation was ninth at $4.3 billion, amputation of lower extremity was 13th at $2.5 billion, and treatment of lower extremity (other than hip or femur) fracture/dislocation was 14th at $2.4 billion. Those six procedures combined were $41.2 billion in hospital costs, which was a quarter of the total for all stays with a first-listed OR procedure, the AHRQ said.
The nonmusculoskeletal procedures in the top five were percutaneous coronary angioplasty in fourth, with an aggregate cost of $8.1 billion, and cesarean section in fifth at an even $7 billion. Coronary artery bypass graft, the most expensive procedure per stay ($52,000) among the top 20 procedures, was sixth in aggregate cost at $6.7 billion, according to the AHRQ researchers.
Tips for performing complex laparoscopic gyn surgery

Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.

Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.

Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
ERAS reduced opioid use, improved same-day discharge after gyn surgery
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
REPORTING FROM SGS 2018
Key clinical point: ERAS pathways improve same-day discharge rates and reduce opioid use in gynecologic surgery.
Major finding: Same-day discharge rates before and after ERAS were 25.9% and 91.7%, respectively.
Study details: A retrospective review of 258 patients; a study of 161 patients.
Disclosures: Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
Sources: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
Study: Preop EKGs have little utility for benign hysterectomy
ORLANDO – Preoperative electrocardiograms (EKGs) had no effect on management or perioperative complications in women undergoing benign hysterectomy over a 12-month period at a single medical center, according to a review of records.
Of 587 patients included in the review, 182 (31%) underwent EKG as part of their preoperative evaluation, and the majority of those were indicated according to institutional criteria (166; 28%) or National Institute for Health Care Excellence (NICE) guidelines (177; 30%), Nemi M. Shah, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
“EKG was indicated in 91% of these patients according to institutional criteria, and in 97% of patients per NICE criteria,” said Dr. Shah, a third-year resident at the University of Texas Southwestern Medical Center, Dallas.
By institutional criteria, hypertension was the most common indication (68% of cases), and by NICE criteria, American Society of Anesthesia class 2 physical status was the most common indication (80% of cases), she noted, adding that obesity, which was present in 70% of patients, was the most common comorbidity classifying patients with American Society of Anesthesia class 2 or above.
Of the 182 EKGs performed, findings were abnormal in 89, but further workup was pursued in only 16 patients, and included repeat EKG, echocardiogram, and/or stress testing and cardiology consultation. Surgical delays of 1 and 4 months occurred in 2 patients as a result of the additional workup, and ultimately, all planned hysterectomies were completed by the primary surgical team without changes in management, she said.
Perioperative complications occurred in two patients, and included nonspecific postinduction EKG changes that led to surgery being aborted in one patient who had left ventricular hypertrophy on the preoperative EKG, and failed extubation in a patient with airway edema whose preoperative EKG showed a nonacute inferior infarct.
For the first, cardiology was consulted and determined the findings to be benign; the patient underwent hysterectomy at a later date without complications. The second patient was taken to the surgical intensive care unit for management, Dr. Shah said.
“Preoperative testing for benign hysterectomy is variable as there is no single standard of care,” she explained. “Though tests such as EKG are commonly ordered, there are no data linking study results to surgical outcomes.”
The current study was conducted to evaluate the rate of preoperative EKG performed in concordance with institutional and NICE guidelines, and to assess implications for management and perioperative complications.
Patients included in the review were adult women who underwent scheduled benign hysterectomy during 2016. Women who underwent emergency surgery or whose surgery was performed by gynecologic oncologists were excluded.
Subjects were primarily Hispanic, and had a mean age of 45 years, Dr. Shah noted.
Though limited by the single-center design and retrospective nature of the study, the findings suggest that preoperative EKG has little clinical utility.
“We found that practice patterns were highly concordant with institutional and NICE guidelines. However, EKG resulted in minimal impact on perioperative management, and no association between abnormal EKG and perioperative complications was found,” she said. “EKG may not accurately stratify perioperative cardiopulmonary risk, and alternative methods for preoperative evaluation should be considered.”
Dr. Shah reported having no disclosures.
SOURCE: Shah N et al. SGS 2018 Oral Poster 3.
ORLANDO – Preoperative electrocardiograms (EKGs) had no effect on management or perioperative complications in women undergoing benign hysterectomy over a 12-month period at a single medical center, according to a review of records.
Of 587 patients included in the review, 182 (31%) underwent EKG as part of their preoperative evaluation, and the majority of those were indicated according to institutional criteria (166; 28%) or National Institute for Health Care Excellence (NICE) guidelines (177; 30%), Nemi M. Shah, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
“EKG was indicated in 91% of these patients according to institutional criteria, and in 97% of patients per NICE criteria,” said Dr. Shah, a third-year resident at the University of Texas Southwestern Medical Center, Dallas.
By institutional criteria, hypertension was the most common indication (68% of cases), and by NICE criteria, American Society of Anesthesia class 2 physical status was the most common indication (80% of cases), she noted, adding that obesity, which was present in 70% of patients, was the most common comorbidity classifying patients with American Society of Anesthesia class 2 or above.
Of the 182 EKGs performed, findings were abnormal in 89, but further workup was pursued in only 16 patients, and included repeat EKG, echocardiogram, and/or stress testing and cardiology consultation. Surgical delays of 1 and 4 months occurred in 2 patients as a result of the additional workup, and ultimately, all planned hysterectomies were completed by the primary surgical team without changes in management, she said.
Perioperative complications occurred in two patients, and included nonspecific postinduction EKG changes that led to surgery being aborted in one patient who had left ventricular hypertrophy on the preoperative EKG, and failed extubation in a patient with airway edema whose preoperative EKG showed a nonacute inferior infarct.
For the first, cardiology was consulted and determined the findings to be benign; the patient underwent hysterectomy at a later date without complications. The second patient was taken to the surgical intensive care unit for management, Dr. Shah said.
“Preoperative testing for benign hysterectomy is variable as there is no single standard of care,” she explained. “Though tests such as EKG are commonly ordered, there are no data linking study results to surgical outcomes.”
The current study was conducted to evaluate the rate of preoperative EKG performed in concordance with institutional and NICE guidelines, and to assess implications for management and perioperative complications.
Patients included in the review were adult women who underwent scheduled benign hysterectomy during 2016. Women who underwent emergency surgery or whose surgery was performed by gynecologic oncologists were excluded.
Subjects were primarily Hispanic, and had a mean age of 45 years, Dr. Shah noted.
Though limited by the single-center design and retrospective nature of the study, the findings suggest that preoperative EKG has little clinical utility.
“We found that practice patterns were highly concordant with institutional and NICE guidelines. However, EKG resulted in minimal impact on perioperative management, and no association between abnormal EKG and perioperative complications was found,” she said. “EKG may not accurately stratify perioperative cardiopulmonary risk, and alternative methods for preoperative evaluation should be considered.”
Dr. Shah reported having no disclosures.
SOURCE: Shah N et al. SGS 2018 Oral Poster 3.
ORLANDO – Preoperative electrocardiograms (EKGs) had no effect on management or perioperative complications in women undergoing benign hysterectomy over a 12-month period at a single medical center, according to a review of records.
Of 587 patients included in the review, 182 (31%) underwent EKG as part of their preoperative evaluation, and the majority of those were indicated according to institutional criteria (166; 28%) or National Institute for Health Care Excellence (NICE) guidelines (177; 30%), Nemi M. Shah, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
“EKG was indicated in 91% of these patients according to institutional criteria, and in 97% of patients per NICE criteria,” said Dr. Shah, a third-year resident at the University of Texas Southwestern Medical Center, Dallas.
By institutional criteria, hypertension was the most common indication (68% of cases), and by NICE criteria, American Society of Anesthesia class 2 physical status was the most common indication (80% of cases), she noted, adding that obesity, which was present in 70% of patients, was the most common comorbidity classifying patients with American Society of Anesthesia class 2 or above.
Of the 182 EKGs performed, findings were abnormal in 89, but further workup was pursued in only 16 patients, and included repeat EKG, echocardiogram, and/or stress testing and cardiology consultation. Surgical delays of 1 and 4 months occurred in 2 patients as a result of the additional workup, and ultimately, all planned hysterectomies were completed by the primary surgical team without changes in management, she said.
Perioperative complications occurred in two patients, and included nonspecific postinduction EKG changes that led to surgery being aborted in one patient who had left ventricular hypertrophy on the preoperative EKG, and failed extubation in a patient with airway edema whose preoperative EKG showed a nonacute inferior infarct.
For the first, cardiology was consulted and determined the findings to be benign; the patient underwent hysterectomy at a later date without complications. The second patient was taken to the surgical intensive care unit for management, Dr. Shah said.
“Preoperative testing for benign hysterectomy is variable as there is no single standard of care,” she explained. “Though tests such as EKG are commonly ordered, there are no data linking study results to surgical outcomes.”
The current study was conducted to evaluate the rate of preoperative EKG performed in concordance with institutional and NICE guidelines, and to assess implications for management and perioperative complications.
Patients included in the review were adult women who underwent scheduled benign hysterectomy during 2016. Women who underwent emergency surgery or whose surgery was performed by gynecologic oncologists were excluded.
Subjects were primarily Hispanic, and had a mean age of 45 years, Dr. Shah noted.
Though limited by the single-center design and retrospective nature of the study, the findings suggest that preoperative EKG has little clinical utility.
“We found that practice patterns were highly concordant with institutional and NICE guidelines. However, EKG resulted in minimal impact on perioperative management, and no association between abnormal EKG and perioperative complications was found,” she said. “EKG may not accurately stratify perioperative cardiopulmonary risk, and alternative methods for preoperative evaluation should be considered.”
Dr. Shah reported having no disclosures.
SOURCE: Shah N et al. SGS 2018 Oral Poster 3.
REPORTING FROM SGS 2018
Key clinical point: Preoperative EKG appears to have little utility in patients undergoing benign hysterectomy.
Major finding: Only 16 of 89 patients with abnormal preoperative EKG underwent further workup, and surgery was delayed in only two cases.
Study details: A retrospective review of 587 cases.
Disclosures: Dr. Shah reported having no disclosures.
Source: Shah N et al. SGS 2018 Oral Poster 3.
Complication rates rise after decline in uterine fibroid morcellation
The rate of major and minor 30-day complications from the treatment of uterine fibroids has increased significantly since the Food and Drug Administration’s black-box warning against the use of power morcellation, data suggest.
Researchers examined the incidence of 30-day posthysterectomy complications in 75,487 women who underwent treatment for benign gynecologic indications before and after November 2014, when the FDA’s edict was issued over concerns about the risk of disseminating benign or malignant disease. Of these women, 25,571 had uterine fibroids as the indication for hysterectomy.
The retrospective cohort study, published online April 11 in JAMA Surgery, showed that while the overall rate of complications in the cohort was relatively unchanged before and after the FDA’s warning, complication rates increased significantly in women undergoing treatment for uterine fibroids.
Before the FDA’s announcement, the 30-day major complication rate in women undergoing hysterectomy for uterine fibroids was 1.9%, which increased to 2.4% after the FDA’s warning (odds ratio, 1.23; 95% confidence interval, 1.04-1.47; P = .02). Similarly, the rate of minor 30-day complications increased from 2.7% before the warning to 3.3% after the warning (OR, 1.21; 95% CI, 1.04-1.40; P = .01), after adjustment for factors such as age, body mass index, comorbidities, and other associated procedures.
“This 20% increase in the odds of major and minor complications could translate into a large number of additional complications among the 200,000 hysterectomies performed annually for uterine fibroids in the United States,” wrote Francesco Multinu, MD, of the department of obstetrics and gynecology at the Mayo Clinic, Rochester, Minn., and his coauthors.
Overall, the researchers saw a much higher rate of major complications in women undergoing open abdominal surgery, compared with women who underwent minimally invasive surgery or vaginal hysterectomy (3.5% vs. 1.7% vs. 1.7%). A similar pattern was seen in the subgroup of women who underwent hysterectomy for uterine fibroids (2.8% vs. 1.8% vs. 1.8%).
However, minor 30-day complication rates were higher in women who underwent vaginal hysterectomy (4.5%), compared with open abdominal surgery (4.1%) and minimally invasive surgery (3.2%). In women with uterine fibroids, the minor complication rates were slightly higher in those who underwent open hysterectomy or vaginal hysterectomy than in those who had minimally invasive surgery, but this was not statistically significant.
The type of surgery in women with uterine fibroids changed significantly after the FDA announcement. Before the black-box warning against power morcellation was issued, 37.2% of hysterectomy procedures for uterine fibroids were open, 56.1% were minimally invasive, and 6.7% were vaginal. After the announcement, the percentage that were open procedures increased to 43%, minimally invasive procedures decreased to 49.7%, and vaginal hysterectomies increased to 7.3% (P less than .001).
A similar but less pronounced trend was seen across all hysterectomies for benign gynecological indications.
The authors noted that the study analyzed data on 30-day complications, so they weren’t able to draw conclusions about longer-term outcomes.
“Although caution is required to avoid morcellation of unexpected uterine malignant neoplasms, our results should be considered by women and clinicians during the process of shared decision making and by medical societies and regulatory bodies when issuing safety communications,” the authors wrote.
The study was supported by a grant from the National Center for Advancing Translational Sciences, and one author was supported by the University of Insubria, and by Fondo Miglierina, Varese, Italy. No conflicts of interest were declared.
SOURCE: Multinu F et al. JAMA Surg. 2018 Apr 11. doi: 10.1001/jamasurg.2018.0141.
The rate of major and minor 30-day complications from the treatment of uterine fibroids has increased significantly since the Food and Drug Administration’s black-box warning against the use of power morcellation, data suggest.
Researchers examined the incidence of 30-day posthysterectomy complications in 75,487 women who underwent treatment for benign gynecologic indications before and after November 2014, when the FDA’s edict was issued over concerns about the risk of disseminating benign or malignant disease. Of these women, 25,571 had uterine fibroids as the indication for hysterectomy.
The retrospective cohort study, published online April 11 in JAMA Surgery, showed that while the overall rate of complications in the cohort was relatively unchanged before and after the FDA’s warning, complication rates increased significantly in women undergoing treatment for uterine fibroids.
Before the FDA’s announcement, the 30-day major complication rate in women undergoing hysterectomy for uterine fibroids was 1.9%, which increased to 2.4% after the FDA’s warning (odds ratio, 1.23; 95% confidence interval, 1.04-1.47; P = .02). Similarly, the rate of minor 30-day complications increased from 2.7% before the warning to 3.3% after the warning (OR, 1.21; 95% CI, 1.04-1.40; P = .01), after adjustment for factors such as age, body mass index, comorbidities, and other associated procedures.
“This 20% increase in the odds of major and minor complications could translate into a large number of additional complications among the 200,000 hysterectomies performed annually for uterine fibroids in the United States,” wrote Francesco Multinu, MD, of the department of obstetrics and gynecology at the Mayo Clinic, Rochester, Minn., and his coauthors.
Overall, the researchers saw a much higher rate of major complications in women undergoing open abdominal surgery, compared with women who underwent minimally invasive surgery or vaginal hysterectomy (3.5% vs. 1.7% vs. 1.7%). A similar pattern was seen in the subgroup of women who underwent hysterectomy for uterine fibroids (2.8% vs. 1.8% vs. 1.8%).
However, minor 30-day complication rates were higher in women who underwent vaginal hysterectomy (4.5%), compared with open abdominal surgery (4.1%) and minimally invasive surgery (3.2%). In women with uterine fibroids, the minor complication rates were slightly higher in those who underwent open hysterectomy or vaginal hysterectomy than in those who had minimally invasive surgery, but this was not statistically significant.
The type of surgery in women with uterine fibroids changed significantly after the FDA announcement. Before the black-box warning against power morcellation was issued, 37.2% of hysterectomy procedures for uterine fibroids were open, 56.1% were minimally invasive, and 6.7% were vaginal. After the announcement, the percentage that were open procedures increased to 43%, minimally invasive procedures decreased to 49.7%, and vaginal hysterectomies increased to 7.3% (P less than .001).
A similar but less pronounced trend was seen across all hysterectomies for benign gynecological indications.
The authors noted that the study analyzed data on 30-day complications, so they weren’t able to draw conclusions about longer-term outcomes.
“Although caution is required to avoid morcellation of unexpected uterine malignant neoplasms, our results should be considered by women and clinicians during the process of shared decision making and by medical societies and regulatory bodies when issuing safety communications,” the authors wrote.
The study was supported by a grant from the National Center for Advancing Translational Sciences, and one author was supported by the University of Insubria, and by Fondo Miglierina, Varese, Italy. No conflicts of interest were declared.
SOURCE: Multinu F et al. JAMA Surg. 2018 Apr 11. doi: 10.1001/jamasurg.2018.0141.
The rate of major and minor 30-day complications from the treatment of uterine fibroids has increased significantly since the Food and Drug Administration’s black-box warning against the use of power morcellation, data suggest.
Researchers examined the incidence of 30-day posthysterectomy complications in 75,487 women who underwent treatment for benign gynecologic indications before and after November 2014, when the FDA’s edict was issued over concerns about the risk of disseminating benign or malignant disease. Of these women, 25,571 had uterine fibroids as the indication for hysterectomy.
The retrospective cohort study, published online April 11 in JAMA Surgery, showed that while the overall rate of complications in the cohort was relatively unchanged before and after the FDA’s warning, complication rates increased significantly in women undergoing treatment for uterine fibroids.
Before the FDA’s announcement, the 30-day major complication rate in women undergoing hysterectomy for uterine fibroids was 1.9%, which increased to 2.4% after the FDA’s warning (odds ratio, 1.23; 95% confidence interval, 1.04-1.47; P = .02). Similarly, the rate of minor 30-day complications increased from 2.7% before the warning to 3.3% after the warning (OR, 1.21; 95% CI, 1.04-1.40; P = .01), after adjustment for factors such as age, body mass index, comorbidities, and other associated procedures.
“This 20% increase in the odds of major and minor complications could translate into a large number of additional complications among the 200,000 hysterectomies performed annually for uterine fibroids in the United States,” wrote Francesco Multinu, MD, of the department of obstetrics and gynecology at the Mayo Clinic, Rochester, Minn., and his coauthors.
Overall, the researchers saw a much higher rate of major complications in women undergoing open abdominal surgery, compared with women who underwent minimally invasive surgery or vaginal hysterectomy (3.5% vs. 1.7% vs. 1.7%). A similar pattern was seen in the subgroup of women who underwent hysterectomy for uterine fibroids (2.8% vs. 1.8% vs. 1.8%).
However, minor 30-day complication rates were higher in women who underwent vaginal hysterectomy (4.5%), compared with open abdominal surgery (4.1%) and minimally invasive surgery (3.2%). In women with uterine fibroids, the minor complication rates were slightly higher in those who underwent open hysterectomy or vaginal hysterectomy than in those who had minimally invasive surgery, but this was not statistically significant.
The type of surgery in women with uterine fibroids changed significantly after the FDA announcement. Before the black-box warning against power morcellation was issued, 37.2% of hysterectomy procedures for uterine fibroids were open, 56.1% were minimally invasive, and 6.7% were vaginal. After the announcement, the percentage that were open procedures increased to 43%, minimally invasive procedures decreased to 49.7%, and vaginal hysterectomies increased to 7.3% (P less than .001).
A similar but less pronounced trend was seen across all hysterectomies for benign gynecological indications.
The authors noted that the study analyzed data on 30-day complications, so they weren’t able to draw conclusions about longer-term outcomes.
“Although caution is required to avoid morcellation of unexpected uterine malignant neoplasms, our results should be considered by women and clinicians during the process of shared decision making and by medical societies and regulatory bodies when issuing safety communications,” the authors wrote.
The study was supported by a grant from the National Center for Advancing Translational Sciences, and one author was supported by the University of Insubria, and by Fondo Miglierina, Varese, Italy. No conflicts of interest were declared.
SOURCE: Multinu F et al. JAMA Surg. 2018 Apr 11. doi: 10.1001/jamasurg.2018.0141.
FROM JAMA SURGERY
Key clinical point: Major complications from uterine fibroid hysterectomies have increased with more open surgeries.
Major finding: The rate of 30-day complications after uterine fibroid hysterectomy has increased by 20%.
Study details: A retrospective cohort study of 75,487 women.
Disclosures: The study was supported by a grant from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, and one author was supported by the University of Insubria, and by Fondo Miglierina, Varese, Italy. No conflicts of interest were declared.
Source: Multinu F et al. JAMA Surg. 2018 Apr 11. doi: 10.1001/jamasurg.2018.0141.
Life and health are not even across the U.S.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
FROM JAMA
Key clinical point: While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors.
Major finding: Life expectancy ranged from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, and previously decreasing death rates for adults have reversed in 19 states.
Study details: A U.S. state-level analysis of results from the Global Burden of Disease (GBD) study illustrating trends in diseases, injuries, risk factors, and deaths from 1990 to 2016.
Disclosures: The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Study authors reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
Source: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-1472.
VIDEO: Cervical cancer laparotomy outperforms minimally invasive surgery
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
REPORTING FROM SGO 2018
Key clinical point: Laparotomy produced better survival than did minimally invasive surgery for cervical cancer.
Major finding: Disease-free survival after 4.5 years was 96.5% with laparotomy and 86.0% with minimally invasive surgery.
Study details: LACC was a multicenter, randomized trial with 631 patients. The observational study included 2,221 patients from the National Cancer Database during 2010-2012.
Disclosures: Dr. Ramirez and Dr. Rauh-Hain had no disclosures.
Source: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
Fast-track catheter management offers little benefit after benign hysterectomy
ORLANDO – A fast-track approach to urinary catheter management after benign gynecologic surgery reduced catheter dwell time, but did not significantly improve outcomes or patient satisfaction in a prospective, randomized trial.
Catheter dwell times in 200 women randomized 1:1 to receive either fast-track catheter management with planned catheter removal at 4 hours after surgery, or conventional catheter management with planned catheter removal 1 day after surgery, were 650 minutes versus 1,196 minutes in the groups, respectively, Patrick Lang, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Overall, 93% of patients had a successful voiding trial for catheter removal, but failures occurred more often in the fast-track management group than in the conventional management group (12% vs. 2%), said Dr. Lang, a fellow at the Christ Hospital Health Network, Cincinnati.
Furthermore, the reduction in Urogenital Distress Inventory short form scores after surgery was significant overall but did not differ significantly in the fast-track and conventional management groups, and there was a trend toward less of a reduction in Urogenital Distress Inventory scores among those who failed the voiding trial versus those who passed, he noted.
Follow-up patient surveys at 2-3 weeks after surgery showed no significant difference between the groups in the rates of reported urinary tract infections (13% and 19% with fast-track and conventional management, respectively) and antibiotic exposure, or in lower urinary tract symptoms (P = .24 and .92), he said.
Patients also had a positive overall impression of their catheter management, with no significant difference between the groups in the percentage of patients who strongly agreed that their catheter was well managed (80% and 87%, respectively).
In women undergoing gynecologic surgery, indwelling urinary catheters often are used for an extended period as postoperative voiding dysfunction is presumed, Dr. Lang noted.
For the current single-center study, women undergoing any benign gynecologic surgery with an anticipated hospital stay of at least 1 night were enrolled and randomized to fast-track or conventional catheter management. All underwent hysterectomy, and the approaches, including robotic in 42%, traditional laparoscopic in 33%, vaginal in 14.3%, and abdominal in 10.3%, did not differ among the groups. Neither the rates of voiding trial success nor catheter dwell time differed, based on hysterectomy approach, he said.
“Catheter dwell time is reduced with a fast-track approach, but dwell time does not appear to influence patient satisfaction or urinary tract symptomatology, urinary tract infection, or antibiotic exposure in the 2-3 weeks following benign gynecologic surgery,” he concluded.
Dr. Lang reported having no disclosures.
SOURCE: Lang P et al. SGS 2018, Oral Poster 20.
ORLANDO – A fast-track approach to urinary catheter management after benign gynecologic surgery reduced catheter dwell time, but did not significantly improve outcomes or patient satisfaction in a prospective, randomized trial.
Catheter dwell times in 200 women randomized 1:1 to receive either fast-track catheter management with planned catheter removal at 4 hours after surgery, or conventional catheter management with planned catheter removal 1 day after surgery, were 650 minutes versus 1,196 minutes in the groups, respectively, Patrick Lang, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Overall, 93% of patients had a successful voiding trial for catheter removal, but failures occurred more often in the fast-track management group than in the conventional management group (12% vs. 2%), said Dr. Lang, a fellow at the Christ Hospital Health Network, Cincinnati.
Furthermore, the reduction in Urogenital Distress Inventory short form scores after surgery was significant overall but did not differ significantly in the fast-track and conventional management groups, and there was a trend toward less of a reduction in Urogenital Distress Inventory scores among those who failed the voiding trial versus those who passed, he noted.
Follow-up patient surveys at 2-3 weeks after surgery showed no significant difference between the groups in the rates of reported urinary tract infections (13% and 19% with fast-track and conventional management, respectively) and antibiotic exposure, or in lower urinary tract symptoms (P = .24 and .92), he said.
Patients also had a positive overall impression of their catheter management, with no significant difference between the groups in the percentage of patients who strongly agreed that their catheter was well managed (80% and 87%, respectively).
In women undergoing gynecologic surgery, indwelling urinary catheters often are used for an extended period as postoperative voiding dysfunction is presumed, Dr. Lang noted.
For the current single-center study, women undergoing any benign gynecologic surgery with an anticipated hospital stay of at least 1 night were enrolled and randomized to fast-track or conventional catheter management. All underwent hysterectomy, and the approaches, including robotic in 42%, traditional laparoscopic in 33%, vaginal in 14.3%, and abdominal in 10.3%, did not differ among the groups. Neither the rates of voiding trial success nor catheter dwell time differed, based on hysterectomy approach, he said.
“Catheter dwell time is reduced with a fast-track approach, but dwell time does not appear to influence patient satisfaction or urinary tract symptomatology, urinary tract infection, or antibiotic exposure in the 2-3 weeks following benign gynecologic surgery,” he concluded.
Dr. Lang reported having no disclosures.
SOURCE: Lang P et al. SGS 2018, Oral Poster 20.
ORLANDO – A fast-track approach to urinary catheter management after benign gynecologic surgery reduced catheter dwell time, but did not significantly improve outcomes or patient satisfaction in a prospective, randomized trial.
Catheter dwell times in 200 women randomized 1:1 to receive either fast-track catheter management with planned catheter removal at 4 hours after surgery, or conventional catheter management with planned catheter removal 1 day after surgery, were 650 minutes versus 1,196 minutes in the groups, respectively, Patrick Lang, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Overall, 93% of patients had a successful voiding trial for catheter removal, but failures occurred more often in the fast-track management group than in the conventional management group (12% vs. 2%), said Dr. Lang, a fellow at the Christ Hospital Health Network, Cincinnati.
Furthermore, the reduction in Urogenital Distress Inventory short form scores after surgery was significant overall but did not differ significantly in the fast-track and conventional management groups, and there was a trend toward less of a reduction in Urogenital Distress Inventory scores among those who failed the voiding trial versus those who passed, he noted.
Follow-up patient surveys at 2-3 weeks after surgery showed no significant difference between the groups in the rates of reported urinary tract infections (13% and 19% with fast-track and conventional management, respectively) and antibiotic exposure, or in lower urinary tract symptoms (P = .24 and .92), he said.
Patients also had a positive overall impression of their catheter management, with no significant difference between the groups in the percentage of patients who strongly agreed that their catheter was well managed (80% and 87%, respectively).
In women undergoing gynecologic surgery, indwelling urinary catheters often are used for an extended period as postoperative voiding dysfunction is presumed, Dr. Lang noted.
For the current single-center study, women undergoing any benign gynecologic surgery with an anticipated hospital stay of at least 1 night were enrolled and randomized to fast-track or conventional catheter management. All underwent hysterectomy, and the approaches, including robotic in 42%, traditional laparoscopic in 33%, vaginal in 14.3%, and abdominal in 10.3%, did not differ among the groups. Neither the rates of voiding trial success nor catheter dwell time differed, based on hysterectomy approach, he said.
“Catheter dwell time is reduced with a fast-track approach, but dwell time does not appear to influence patient satisfaction or urinary tract symptomatology, urinary tract infection, or antibiotic exposure in the 2-3 weeks following benign gynecologic surgery,” he concluded.
Dr. Lang reported having no disclosures.
SOURCE: Lang P et al. SGS 2018, Oral Poster 20.
REPORTING FROM SGS 2018
Key clinical point: Fast-track catheter management after surgery improves dwell time, but not other outcomes.
Major finding: Catheter dwell times were 650 versus 1,196 minutes with fast-track and conventional management, respectively.
Study details: A prospective, randomized study of 200 women.
Disclosures: Dr. Lang reported having no disclosures.
Source: Lang P et al. SGS 2018, Oral Poster 20.