User login
Same-day discharge for hysterectomy
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
Think methotrexate for granulomatous mastitis
MAUI, HAWAII – Methotrexate is the most effective therapy for granulomatous mastitis, according to Anna Postolova, MD, a rheumatology fellow at Stanford (Calif.) University.
Granulomatous mastitis is a rare inflammatory disease of the breast of uncertain but possibly autoimmune etiology. The most common treatments – antibiotics, prednisone, and incision and drainage – are often ineffective and have a roughly 50% recurrence rate. That’s why Stanford rheumatologists began using methotrexate more than a decade ago with impressive results, she explained at the 2018 Rheumatology Winter Clinical Symposium.
Dr. Postolova presented a retrospective series of 19 women referred to Stanford for recurrent or refractory granulomatous mastitis. At diagnosis, they averaged 33.5 years of age with a 6-month history of symptoms prior to diagnosis. Of the 19 women, 11 were Hispanic, and only 2 were Caucasian. A total of 17 women were multiparous, with an average of two children, and 3 women were breastfeeding at symptom onset.
The women were placed on methotrexate at 15 mg/week. At 3 months, 17 of the 19 patients showed improvement, but none had disease resolution. At that point the dose was raised to 20 mg/week. After 3 months at the higher dose, 16 of 18 patients were improved and 4 had experienced resolution of their granulomatous mastitis. After 9 months on methotrexate – 6 at the higher dose – the granulomatous mastitis showed continued improvement in 13 of 15 women and resolution in 8. One woman experienced recurrent disease at 9 months of follow-up after her methotrexate was withheld because of liver test abnormalities and lack of birth control; however, she went into remission upon restarting therapy.
By 12 months, 12 of 15 women, or 80%, had experienced disease resolution. Their methotrexate was then slowly tapered over the course of 18-24 months without disease recurrence.
On the other hand, two women who had previously shown improvement were experiencing mild recurrences at the 12-month mark. They were switched to subcutaneous methotrexate. One responded favorably to the change, and the other had not yet returned for follow-up.
Dr. Postolova reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Methotrexate is the most effective therapy for granulomatous mastitis, according to Anna Postolova, MD, a rheumatology fellow at Stanford (Calif.) University.
Granulomatous mastitis is a rare inflammatory disease of the breast of uncertain but possibly autoimmune etiology. The most common treatments – antibiotics, prednisone, and incision and drainage – are often ineffective and have a roughly 50% recurrence rate. That’s why Stanford rheumatologists began using methotrexate more than a decade ago with impressive results, she explained at the 2018 Rheumatology Winter Clinical Symposium.
Dr. Postolova presented a retrospective series of 19 women referred to Stanford for recurrent or refractory granulomatous mastitis. At diagnosis, they averaged 33.5 years of age with a 6-month history of symptoms prior to diagnosis. Of the 19 women, 11 were Hispanic, and only 2 were Caucasian. A total of 17 women were multiparous, with an average of two children, and 3 women were breastfeeding at symptom onset.
The women were placed on methotrexate at 15 mg/week. At 3 months, 17 of the 19 patients showed improvement, but none had disease resolution. At that point the dose was raised to 20 mg/week. After 3 months at the higher dose, 16 of 18 patients were improved and 4 had experienced resolution of their granulomatous mastitis. After 9 months on methotrexate – 6 at the higher dose – the granulomatous mastitis showed continued improvement in 13 of 15 women and resolution in 8. One woman experienced recurrent disease at 9 months of follow-up after her methotrexate was withheld because of liver test abnormalities and lack of birth control; however, she went into remission upon restarting therapy.
By 12 months, 12 of 15 women, or 80%, had experienced disease resolution. Their methotrexate was then slowly tapered over the course of 18-24 months without disease recurrence.
On the other hand, two women who had previously shown improvement were experiencing mild recurrences at the 12-month mark. They were switched to subcutaneous methotrexate. One responded favorably to the change, and the other had not yet returned for follow-up.
Dr. Postolova reported having no financial conflicts of interest regarding her presentation.
MAUI, HAWAII – Methotrexate is the most effective therapy for granulomatous mastitis, according to Anna Postolova, MD, a rheumatology fellow at Stanford (Calif.) University.
Granulomatous mastitis is a rare inflammatory disease of the breast of uncertain but possibly autoimmune etiology. The most common treatments – antibiotics, prednisone, and incision and drainage – are often ineffective and have a roughly 50% recurrence rate. That’s why Stanford rheumatologists began using methotrexate more than a decade ago with impressive results, she explained at the 2018 Rheumatology Winter Clinical Symposium.
Dr. Postolova presented a retrospective series of 19 women referred to Stanford for recurrent or refractory granulomatous mastitis. At diagnosis, they averaged 33.5 years of age with a 6-month history of symptoms prior to diagnosis. Of the 19 women, 11 were Hispanic, and only 2 were Caucasian. A total of 17 women were multiparous, with an average of two children, and 3 women were breastfeeding at symptom onset.
The women were placed on methotrexate at 15 mg/week. At 3 months, 17 of the 19 patients showed improvement, but none had disease resolution. At that point the dose was raised to 20 mg/week. After 3 months at the higher dose, 16 of 18 patients were improved and 4 had experienced resolution of their granulomatous mastitis. After 9 months on methotrexate – 6 at the higher dose – the granulomatous mastitis showed continued improvement in 13 of 15 women and resolution in 8. One woman experienced recurrent disease at 9 months of follow-up after her methotrexate was withheld because of liver test abnormalities and lack of birth control; however, she went into remission upon restarting therapy.
By 12 months, 12 of 15 women, or 80%, had experienced disease resolution. Their methotrexate was then slowly tapered over the course of 18-24 months without disease recurrence.
On the other hand, two women who had previously shown improvement were experiencing mild recurrences at the 12-month mark. They were switched to subcutaneous methotrexate. One responded favorably to the change, and the other had not yet returned for follow-up.
Dr. Postolova reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM RWCS 2018
Key clinical point:
Major finding: At 3 months, 17 of 19 patients showed improvement on methotrexate at 15 mg/week, and at 12 months, 12 of 15 had experienced disease resolution on 20 mg/week.
Study details: A single-center retrospective review of 19 patients with granulomatous mastitis.
Disclosures: The presenter reported having no financial conflicts of interest regarding her presentation.
FDA updates breast implant–associated lymphoma cases, risk
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
VIDEO: Andexanet alfa effectively reverses factor Xa anticoagulant
ORLANDO – Andexanet alfa, a new agent that reverses the anticoagulant effect of direct factor Xa inhibitors, showed an acceptable level of efficacy and safety in 227 patients who received the drug in the agent’s pivotal trial.
These results, which placed andexanet in the same ballpark for efficacy and safety as idarucizumab (Praxbind), approved in 2015 for reversing the anticoagulant dabigatran (Pradaxa), suggest that andexanet is likely on track for its own Food and Drug Administration marketing approval, Stuart Connolly, MD, said at the annual meeting of the American College of Cardiology.
Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa) previously announced that it expected Food and Drug Administration action on its marketing application by May 2018.
Andexanet reversal “has similar efficacy and safety as seen with other reversal agents” for other types of anticoagulants, said Dr. Connolly, a professor of medicine and an electrophysiologist at McMaster University in Hamilton, Ont. In the trial results he reported, andexanet treatment of patients who were bleeding while on treatment with a direct factor Xa inhibitor had an 83% rate of hemostatic efficacy and an 11% rate of thrombotic events. By comparison, idarucizumab, the FDA-approved reversal agent for the anticoagulant dabigatran, produced a 68% hemostatic efficacy and a 6% rate of thrombotic events in the idarucizumab pivotal trial, RE-VERSE AD (N Engl J Med. 2015 Aug 6;373[6]:511-20).
The Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding (ANNEXA-4) enrolled 227 patients at any of 60 centers, with efficacy data available from 132 of the patients. About 60% of the patients had an intracranial bleed, and about 30% had a gastrointestinal bleed, and their average age was 77 years. Roughly three-quarters of patients were on an anticoagulant for atrial fibrillation, with the rest treated for venous thromboembolism, with 4% having both conditions. The most commonly used direct factor Xa inhbitors in these patients were apixaban (Eliquis) in 105 and rivaroxaban (Xarelto) in 75. The ANNEXA-4 study has not enrolled patients treated with a direct factor Xa inhibitor anticoagulant and undergoing surgery, a setting that will be the subject of a future study, Dr. Connolly said.
Clinicians administered andexanet alfa as a bolus followed by a 2-hour continuous infusion, with hemostatic efficacy assessed 12 hours after the start of treatment. The results showed that factor Xa inhibition fell by about 75%-90% within minutes of starting the bolus and remained depressed at that level during the infusion but then began recovering by 2 hours after the stop of infusion. Andexanet is a factor Xa “decoy” molecule that acts by latching onto the inhibitor molecules and thereby preventing them from interacting with actual factor Xa, but andexanet also has a short half life and hence the effect quickly reduces once treatment stops.
“There is no doubt that andexanet rapidly decreases anti–factor Xa activity,” he said.
Adjudicated efficacy results were available for 132 patients and showed good or excellent hemostasis achieved on andexanet in 109 patients (83%), Dr. Connolly reported. The effect on hemostasis was consistent regardless of patient age, sex, bleeding site, type of anticoagulant, and dosage tested.
Thrombotic events during the 30 days following treatment occurred in 24 of 227 patients (11%) who received andexanet and were evaluable for safety. Notably, no clustering of thrombotic events occurred early, even among the 129 patients who restarted on an anticoagulant during the 30 days after treatment. Among the 129 patients who restarted on an anticoagulant, 9 (7%) had a thrombotic event during the 30-day follow-up, compared with 15 events among 98 patients (15%) who did not restart on an anticoagulant.
Dr. Connolly acknowledged that a limitation of the ANNEXA-4 study is the absence of a control group, but he added that he and his associates believed randomizing patients with a serious bleed to placebo control would not have been “practical, feasible, or ethical.”
ANNEXA-4 is sponsored by Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa). Dr. Connolly has been a consultant to Portola, and also to Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Sanofi-Aventis. Dr. Kirtane has received research support from several device manufacturers.
SOURCE: Connolly S. ACC 2018.
Treatment with andexanet alfa produced good or excellent hemostasis in 83% of patients in the ANNEXA-4 study, which is what matters when patients are bleeding. Clinicians want to know that you can restore coagulation to a level where you can stop bleeding, and that’s what the results show.
The lack of a reversal agent until now for direct-acting factor Xa inhibitor drugs has probably been a modest but real obstacle to widespread adoption of these agents. We can look at the example of another new oral anticoagulant, dabigatran (Pradaxa), which works by a different mechanism, specifically by inhibiting thrombin. After a reversal agent for dabigatran, idarucizumab (Praxbind) received Food and Drug Administration approval and became available in late 2015, an uptick in dabigatran prescriptions occurred. That experience shows that patients and providers want the safety net of a reversal agent. They want to know that, if there is bleeding or need for urgent surgery, there is a way to facilitate restoration of hemostasis.
It’s the same with direct factor Xa inhibitors: Some patients are concerned about the lack of a reversal agent, and having such an agent may help increase access to these agents for such patients. I think that, once andexanet becomes available for routine U.S. practice, we’ll see an uptick in prescribing of direct factor Xa inhibitors. Also, some patients who have opted for treatment with warfarin will switch to a safer class of drugs, the direct factor X a inhibitors. A myth exists that reversal agents can easily negate the anticoagulant effect of warfarin. The reality is that, despite having treatments that reverse warfarin’s effect, this is often not an easy process in actual practice.
On the safety side, there was no indication in the ANNEXA-4 results of rebound thrombosis with andexanet alfa treatment. Patients receiving a direct factor Xa inhibitor are prothrombotic – that’s why they are on an anticoagulant – so their risk for a thrombotic event is always there, especially when they are not fully anticoagulated, such as when a reversal agent is administered. We need to look to restarting treatment with an anticoagulant because these patients have a high thrombotic risk.
Gregory Piazza, MD , is a cardiologist at Brigham and Women’s Hospital in Boston. He has been an advisor to Portola Pharmaceuticals, the company developing andexanet alfa, as well as to Bayer and Pfizer, and he has received research funding from Bristol-Myers Squibb, Janssen, and Daiichi Sankyo. He made these comments in an interview .
Treatment with andexanet alfa produced good or excellent hemostasis in 83% of patients in the ANNEXA-4 study, which is what matters when patients are bleeding. Clinicians want to know that you can restore coagulation to a level where you can stop bleeding, and that’s what the results show.
The lack of a reversal agent until now for direct-acting factor Xa inhibitor drugs has probably been a modest but real obstacle to widespread adoption of these agents. We can look at the example of another new oral anticoagulant, dabigatran (Pradaxa), which works by a different mechanism, specifically by inhibiting thrombin. After a reversal agent for dabigatran, idarucizumab (Praxbind) received Food and Drug Administration approval and became available in late 2015, an uptick in dabigatran prescriptions occurred. That experience shows that patients and providers want the safety net of a reversal agent. They want to know that, if there is bleeding or need for urgent surgery, there is a way to facilitate restoration of hemostasis.
It’s the same with direct factor Xa inhibitors: Some patients are concerned about the lack of a reversal agent, and having such an agent may help increase access to these agents for such patients. I think that, once andexanet becomes available for routine U.S. practice, we’ll see an uptick in prescribing of direct factor Xa inhibitors. Also, some patients who have opted for treatment with warfarin will switch to a safer class of drugs, the direct factor X a inhibitors. A myth exists that reversal agents can easily negate the anticoagulant effect of warfarin. The reality is that, despite having treatments that reverse warfarin’s effect, this is often not an easy process in actual practice.
On the safety side, there was no indication in the ANNEXA-4 results of rebound thrombosis with andexanet alfa treatment. Patients receiving a direct factor Xa inhibitor are prothrombotic – that’s why they are on an anticoagulant – so their risk for a thrombotic event is always there, especially when they are not fully anticoagulated, such as when a reversal agent is administered. We need to look to restarting treatment with an anticoagulant because these patients have a high thrombotic risk.
Gregory Piazza, MD , is a cardiologist at Brigham and Women’s Hospital in Boston. He has been an advisor to Portola Pharmaceuticals, the company developing andexanet alfa, as well as to Bayer and Pfizer, and he has received research funding from Bristol-Myers Squibb, Janssen, and Daiichi Sankyo. He made these comments in an interview .
Treatment with andexanet alfa produced good or excellent hemostasis in 83% of patients in the ANNEXA-4 study, which is what matters when patients are bleeding. Clinicians want to know that you can restore coagulation to a level where you can stop bleeding, and that’s what the results show.
The lack of a reversal agent until now for direct-acting factor Xa inhibitor drugs has probably been a modest but real obstacle to widespread adoption of these agents. We can look at the example of another new oral anticoagulant, dabigatran (Pradaxa), which works by a different mechanism, specifically by inhibiting thrombin. After a reversal agent for dabigatran, idarucizumab (Praxbind) received Food and Drug Administration approval and became available in late 2015, an uptick in dabigatran prescriptions occurred. That experience shows that patients and providers want the safety net of a reversal agent. They want to know that, if there is bleeding or need for urgent surgery, there is a way to facilitate restoration of hemostasis.
It’s the same with direct factor Xa inhibitors: Some patients are concerned about the lack of a reversal agent, and having such an agent may help increase access to these agents for such patients. I think that, once andexanet becomes available for routine U.S. practice, we’ll see an uptick in prescribing of direct factor Xa inhibitors. Also, some patients who have opted for treatment with warfarin will switch to a safer class of drugs, the direct factor X a inhibitors. A myth exists that reversal agents can easily negate the anticoagulant effect of warfarin. The reality is that, despite having treatments that reverse warfarin’s effect, this is often not an easy process in actual practice.
On the safety side, there was no indication in the ANNEXA-4 results of rebound thrombosis with andexanet alfa treatment. Patients receiving a direct factor Xa inhibitor are prothrombotic – that’s why they are on an anticoagulant – so their risk for a thrombotic event is always there, especially when they are not fully anticoagulated, such as when a reversal agent is administered. We need to look to restarting treatment with an anticoagulant because these patients have a high thrombotic risk.
Gregory Piazza, MD , is a cardiologist at Brigham and Women’s Hospital in Boston. He has been an advisor to Portola Pharmaceuticals, the company developing andexanet alfa, as well as to Bayer and Pfizer, and he has received research funding from Bristol-Myers Squibb, Janssen, and Daiichi Sankyo. He made these comments in an interview .
ORLANDO – Andexanet alfa, a new agent that reverses the anticoagulant effect of direct factor Xa inhibitors, showed an acceptable level of efficacy and safety in 227 patients who received the drug in the agent’s pivotal trial.
These results, which placed andexanet in the same ballpark for efficacy and safety as idarucizumab (Praxbind), approved in 2015 for reversing the anticoagulant dabigatran (Pradaxa), suggest that andexanet is likely on track for its own Food and Drug Administration marketing approval, Stuart Connolly, MD, said at the annual meeting of the American College of Cardiology.
Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa) previously announced that it expected Food and Drug Administration action on its marketing application by May 2018.
Andexanet reversal “has similar efficacy and safety as seen with other reversal agents” for other types of anticoagulants, said Dr. Connolly, a professor of medicine and an electrophysiologist at McMaster University in Hamilton, Ont. In the trial results he reported, andexanet treatment of patients who were bleeding while on treatment with a direct factor Xa inhibitor had an 83% rate of hemostatic efficacy and an 11% rate of thrombotic events. By comparison, idarucizumab, the FDA-approved reversal agent for the anticoagulant dabigatran, produced a 68% hemostatic efficacy and a 6% rate of thrombotic events in the idarucizumab pivotal trial, RE-VERSE AD (N Engl J Med. 2015 Aug 6;373[6]:511-20).
The Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding (ANNEXA-4) enrolled 227 patients at any of 60 centers, with efficacy data available from 132 of the patients. About 60% of the patients had an intracranial bleed, and about 30% had a gastrointestinal bleed, and their average age was 77 years. Roughly three-quarters of patients were on an anticoagulant for atrial fibrillation, with the rest treated for venous thromboembolism, with 4% having both conditions. The most commonly used direct factor Xa inhbitors in these patients were apixaban (Eliquis) in 105 and rivaroxaban (Xarelto) in 75. The ANNEXA-4 study has not enrolled patients treated with a direct factor Xa inhibitor anticoagulant and undergoing surgery, a setting that will be the subject of a future study, Dr. Connolly said.
Clinicians administered andexanet alfa as a bolus followed by a 2-hour continuous infusion, with hemostatic efficacy assessed 12 hours after the start of treatment. The results showed that factor Xa inhibition fell by about 75%-90% within minutes of starting the bolus and remained depressed at that level during the infusion but then began recovering by 2 hours after the stop of infusion. Andexanet is a factor Xa “decoy” molecule that acts by latching onto the inhibitor molecules and thereby preventing them from interacting with actual factor Xa, but andexanet also has a short half life and hence the effect quickly reduces once treatment stops.
“There is no doubt that andexanet rapidly decreases anti–factor Xa activity,” he said.
Adjudicated efficacy results were available for 132 patients and showed good or excellent hemostasis achieved on andexanet in 109 patients (83%), Dr. Connolly reported. The effect on hemostasis was consistent regardless of patient age, sex, bleeding site, type of anticoagulant, and dosage tested.
Thrombotic events during the 30 days following treatment occurred in 24 of 227 patients (11%) who received andexanet and were evaluable for safety. Notably, no clustering of thrombotic events occurred early, even among the 129 patients who restarted on an anticoagulant during the 30 days after treatment. Among the 129 patients who restarted on an anticoagulant, 9 (7%) had a thrombotic event during the 30-day follow-up, compared with 15 events among 98 patients (15%) who did not restart on an anticoagulant.
Dr. Connolly acknowledged that a limitation of the ANNEXA-4 study is the absence of a control group, but he added that he and his associates believed randomizing patients with a serious bleed to placebo control would not have been “practical, feasible, or ethical.”
ANNEXA-4 is sponsored by Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa). Dr. Connolly has been a consultant to Portola, and also to Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Sanofi-Aventis. Dr. Kirtane has received research support from several device manufacturers.
SOURCE: Connolly S. ACC 2018.
ORLANDO – Andexanet alfa, a new agent that reverses the anticoagulant effect of direct factor Xa inhibitors, showed an acceptable level of efficacy and safety in 227 patients who received the drug in the agent’s pivotal trial.
These results, which placed andexanet in the same ballpark for efficacy and safety as idarucizumab (Praxbind), approved in 2015 for reversing the anticoagulant dabigatran (Pradaxa), suggest that andexanet is likely on track for its own Food and Drug Administration marketing approval, Stuart Connolly, MD, said at the annual meeting of the American College of Cardiology.
Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa) previously announced that it expected Food and Drug Administration action on its marketing application by May 2018.
Andexanet reversal “has similar efficacy and safety as seen with other reversal agents” for other types of anticoagulants, said Dr. Connolly, a professor of medicine and an electrophysiologist at McMaster University in Hamilton, Ont. In the trial results he reported, andexanet treatment of patients who were bleeding while on treatment with a direct factor Xa inhibitor had an 83% rate of hemostatic efficacy and an 11% rate of thrombotic events. By comparison, idarucizumab, the FDA-approved reversal agent for the anticoagulant dabigatran, produced a 68% hemostatic efficacy and a 6% rate of thrombotic events in the idarucizumab pivotal trial, RE-VERSE AD (N Engl J Med. 2015 Aug 6;373[6]:511-20).
The Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding (ANNEXA-4) enrolled 227 patients at any of 60 centers, with efficacy data available from 132 of the patients. About 60% of the patients had an intracranial bleed, and about 30% had a gastrointestinal bleed, and their average age was 77 years. Roughly three-quarters of patients were on an anticoagulant for atrial fibrillation, with the rest treated for venous thromboembolism, with 4% having both conditions. The most commonly used direct factor Xa inhbitors in these patients were apixaban (Eliquis) in 105 and rivaroxaban (Xarelto) in 75. The ANNEXA-4 study has not enrolled patients treated with a direct factor Xa inhibitor anticoagulant and undergoing surgery, a setting that will be the subject of a future study, Dr. Connolly said.
Clinicians administered andexanet alfa as a bolus followed by a 2-hour continuous infusion, with hemostatic efficacy assessed 12 hours after the start of treatment. The results showed that factor Xa inhibition fell by about 75%-90% within minutes of starting the bolus and remained depressed at that level during the infusion but then began recovering by 2 hours after the stop of infusion. Andexanet is a factor Xa “decoy” molecule that acts by latching onto the inhibitor molecules and thereby preventing them from interacting with actual factor Xa, but andexanet also has a short half life and hence the effect quickly reduces once treatment stops.
“There is no doubt that andexanet rapidly decreases anti–factor Xa activity,” he said.
Adjudicated efficacy results were available for 132 patients and showed good or excellent hemostasis achieved on andexanet in 109 patients (83%), Dr. Connolly reported. The effect on hemostasis was consistent regardless of patient age, sex, bleeding site, type of anticoagulant, and dosage tested.
Thrombotic events during the 30 days following treatment occurred in 24 of 227 patients (11%) who received andexanet and were evaluable for safety. Notably, no clustering of thrombotic events occurred early, even among the 129 patients who restarted on an anticoagulant during the 30 days after treatment. Among the 129 patients who restarted on an anticoagulant, 9 (7%) had a thrombotic event during the 30-day follow-up, compared with 15 events among 98 patients (15%) who did not restart on an anticoagulant.
Dr. Connolly acknowledged that a limitation of the ANNEXA-4 study is the absence of a control group, but he added that he and his associates believed randomizing patients with a serious bleed to placebo control would not have been “practical, feasible, or ethical.”
ANNEXA-4 is sponsored by Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa). Dr. Connolly has been a consultant to Portola, and also to Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Sanofi-Aventis. Dr. Kirtane has received research support from several device manufacturers.
SOURCE: Connolly S. ACC 2018.
REPORTING FROM ACC 18
Key clinical point:
Major finding: Hemostatic efficacy of andexanet alfa was 83%, and thrombotic events occurred in 11%.
Study details: ANNEXA-4, a single arm cohort study with 227 patients.
Disclosures: ANNEXA-4 is sponsored by Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa). Dr. Connolly has been a consultant to Portola and also to Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Sanofi-Aventis.
Source: Connolly S. ACC 2018.
Survey: Litigation fears drive response to FDA power morcellator warnings
ORLANDO – A 2014 Food and Drug Administration Safety Communication warning about the risk of disseminating occult cancerous tissue when using power morcellation led to a sharp decline in its use among physicians in a large health care system, a survey and records review showed.
Of 126 ob.gyns. in the Charlotte, N.C., metro area who received the 24-question e-mail survey, 58% responded, and while 75% reported that they always or sometimes used power morcellation prior to receiving the FDA communication, more than 80% reported rarely or never using it after receiving the communication, Gerald Bernard Taylor, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Fear of litigation was cited as the reason for the change in practice by 45.7% of ob.gyns., followed by lack of access to power morcellators (34.7%), and concerns about undiagnosed malignancy (11.8%) and patient safety (5.7%), said Dr. Taylor of the Carolinas HealthCare System in Charlotte.
“We also heard from physicians about the guidelines,” he added, referring to informed consent protocols developed by a group of “key opinion leaders in gynecologic oncology and minimally invasive gynecology.” Many institutions developed moratoriums against the use of power morcellation in the wake of the FDA communication, but the Carolinas HealthCare System chose to commission these opinion leaders to address the issue, he explained.
“Interestingly enough, some [survey respondents] were not aware that the guidelines were available. Most (74.6%) thought that they were helpful when they actually reviewed them, and a few said that they were not helpful,” he said, noting that most of those who found the guidelines helpful said they felt that way because the guidelines “provide a standard of care and a possible defense against litigation.”
Analyses with respect to changes in practice after the FDA communication are ongoing but suggest that changes occurred mainly in the approach to myomectomies rather than in hysterectomy routes, Dr. Taylor said.
“We’re looking to see if there was increased use of minilaparotomy, and we are looking at the use of concealed morcellation bags right now,” he said.
The FDA Safety Communication was issued April 17, 2014 and stated that power morcellation used for hysterectomy or myomectomy in women with uterine fibroids “poses a risk of spreading unsuspected cancerous tissue, notably uterine sarcomas, beyond the uterus.”
“Health care providers and patients should carefully consider available alternative treatment options for symptomatic uterine fibroids. Based on currently available information, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids,” the communication stated.
A Nov. 24, 2014 update stated that “laparoscopic power morcellators are contraindicated for removal of uterine tissue containing suspected fibroids in patients who are peri- or postmenopausal or are candidates for en bloc tissue removal, for example, through the vagina or minilaparotomy incision,” and “in gynecologic surgery in which the tissue to be morcellated is known or suspected to contain malignancy.”
The update also urged manufacturers of new and existing laparoscopic power morcellators to include the contraindications and a boxed warning in their product labeling and recommended that doctors share the information with their patients.
On Dec. 24, 2017, the FDA issued an updated assessment reinforcing their earlier communications. This latest update was based on a review of recent medical studies that, according to the FDA, suggested that “approximately 1 in 225 to 1 in 580 women who undergo surgery (hysterectomy or myomectomy) for presumed benign uterine growths (‘fibroids’) may have ‘occult’ or hidden uterine cancers known as sarcomas.” The FDA also said that “this is generally consistent with the 1 in 350 incidence estimated by the FDA in a 2014 review. The FDA also estimates the rate of occult leiomyosarcoma, a particularly aggressive type of sarcoma, to be approximately 1 in 495 to 1 in 1,100, again in general agreement with our previous assessment of 1 in 498.”
The FDA acknowledged that some health organizations have reported a lower estimate of risk but maintained its findings and recommendations, noting that it will continue to review new and relevant data.
Dr. Taylor reported having no disclosures.
SOURCE: Taylor GB et al. SGS 2018, Oral Poster 19.
ORLANDO – A 2014 Food and Drug Administration Safety Communication warning about the risk of disseminating occult cancerous tissue when using power morcellation led to a sharp decline in its use among physicians in a large health care system, a survey and records review showed.
Of 126 ob.gyns. in the Charlotte, N.C., metro area who received the 24-question e-mail survey, 58% responded, and while 75% reported that they always or sometimes used power morcellation prior to receiving the FDA communication, more than 80% reported rarely or never using it after receiving the communication, Gerald Bernard Taylor, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Fear of litigation was cited as the reason for the change in practice by 45.7% of ob.gyns., followed by lack of access to power morcellators (34.7%), and concerns about undiagnosed malignancy (11.8%) and patient safety (5.7%), said Dr. Taylor of the Carolinas HealthCare System in Charlotte.
“We also heard from physicians about the guidelines,” he added, referring to informed consent protocols developed by a group of “key opinion leaders in gynecologic oncology and minimally invasive gynecology.” Many institutions developed moratoriums against the use of power morcellation in the wake of the FDA communication, but the Carolinas HealthCare System chose to commission these opinion leaders to address the issue, he explained.
“Interestingly enough, some [survey respondents] were not aware that the guidelines were available. Most (74.6%) thought that they were helpful when they actually reviewed them, and a few said that they were not helpful,” he said, noting that most of those who found the guidelines helpful said they felt that way because the guidelines “provide a standard of care and a possible defense against litigation.”
Analyses with respect to changes in practice after the FDA communication are ongoing but suggest that changes occurred mainly in the approach to myomectomies rather than in hysterectomy routes, Dr. Taylor said.
“We’re looking to see if there was increased use of minilaparotomy, and we are looking at the use of concealed morcellation bags right now,” he said.
The FDA Safety Communication was issued April 17, 2014 and stated that power morcellation used for hysterectomy or myomectomy in women with uterine fibroids “poses a risk of spreading unsuspected cancerous tissue, notably uterine sarcomas, beyond the uterus.”
“Health care providers and patients should carefully consider available alternative treatment options for symptomatic uterine fibroids. Based on currently available information, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids,” the communication stated.
A Nov. 24, 2014 update stated that “laparoscopic power morcellators are contraindicated for removal of uterine tissue containing suspected fibroids in patients who are peri- or postmenopausal or are candidates for en bloc tissue removal, for example, through the vagina or minilaparotomy incision,” and “in gynecologic surgery in which the tissue to be morcellated is known or suspected to contain malignancy.”
The update also urged manufacturers of new and existing laparoscopic power morcellators to include the contraindications and a boxed warning in their product labeling and recommended that doctors share the information with their patients.
On Dec. 24, 2017, the FDA issued an updated assessment reinforcing their earlier communications. This latest update was based on a review of recent medical studies that, according to the FDA, suggested that “approximately 1 in 225 to 1 in 580 women who undergo surgery (hysterectomy or myomectomy) for presumed benign uterine growths (‘fibroids’) may have ‘occult’ or hidden uterine cancers known as sarcomas.” The FDA also said that “this is generally consistent with the 1 in 350 incidence estimated by the FDA in a 2014 review. The FDA also estimates the rate of occult leiomyosarcoma, a particularly aggressive type of sarcoma, to be approximately 1 in 495 to 1 in 1,100, again in general agreement with our previous assessment of 1 in 498.”
The FDA acknowledged that some health organizations have reported a lower estimate of risk but maintained its findings and recommendations, noting that it will continue to review new and relevant data.
Dr. Taylor reported having no disclosures.
SOURCE: Taylor GB et al. SGS 2018, Oral Poster 19.
ORLANDO – A 2014 Food and Drug Administration Safety Communication warning about the risk of disseminating occult cancerous tissue when using power morcellation led to a sharp decline in its use among physicians in a large health care system, a survey and records review showed.
Of 126 ob.gyns. in the Charlotte, N.C., metro area who received the 24-question e-mail survey, 58% responded, and while 75% reported that they always or sometimes used power morcellation prior to receiving the FDA communication, more than 80% reported rarely or never using it after receiving the communication, Gerald Bernard Taylor, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
Fear of litigation was cited as the reason for the change in practice by 45.7% of ob.gyns., followed by lack of access to power morcellators (34.7%), and concerns about undiagnosed malignancy (11.8%) and patient safety (5.7%), said Dr. Taylor of the Carolinas HealthCare System in Charlotte.
“We also heard from physicians about the guidelines,” he added, referring to informed consent protocols developed by a group of “key opinion leaders in gynecologic oncology and minimally invasive gynecology.” Many institutions developed moratoriums against the use of power morcellation in the wake of the FDA communication, but the Carolinas HealthCare System chose to commission these opinion leaders to address the issue, he explained.
“Interestingly enough, some [survey respondents] were not aware that the guidelines were available. Most (74.6%) thought that they were helpful when they actually reviewed them, and a few said that they were not helpful,” he said, noting that most of those who found the guidelines helpful said they felt that way because the guidelines “provide a standard of care and a possible defense against litigation.”
Analyses with respect to changes in practice after the FDA communication are ongoing but suggest that changes occurred mainly in the approach to myomectomies rather than in hysterectomy routes, Dr. Taylor said.
“We’re looking to see if there was increased use of minilaparotomy, and we are looking at the use of concealed morcellation bags right now,” he said.
The FDA Safety Communication was issued April 17, 2014 and stated that power morcellation used for hysterectomy or myomectomy in women with uterine fibroids “poses a risk of spreading unsuspected cancerous tissue, notably uterine sarcomas, beyond the uterus.”
“Health care providers and patients should carefully consider available alternative treatment options for symptomatic uterine fibroids. Based on currently available information, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids,” the communication stated.
A Nov. 24, 2014 update stated that “laparoscopic power morcellators are contraindicated for removal of uterine tissue containing suspected fibroids in patients who are peri- or postmenopausal or are candidates for en bloc tissue removal, for example, through the vagina or minilaparotomy incision,” and “in gynecologic surgery in which the tissue to be morcellated is known or suspected to contain malignancy.”
The update also urged manufacturers of new and existing laparoscopic power morcellators to include the contraindications and a boxed warning in their product labeling and recommended that doctors share the information with their patients.
On Dec. 24, 2017, the FDA issued an updated assessment reinforcing their earlier communications. This latest update was based on a review of recent medical studies that, according to the FDA, suggested that “approximately 1 in 225 to 1 in 580 women who undergo surgery (hysterectomy or myomectomy) for presumed benign uterine growths (‘fibroids’) may have ‘occult’ or hidden uterine cancers known as sarcomas.” The FDA also said that “this is generally consistent with the 1 in 350 incidence estimated by the FDA in a 2014 review. The FDA also estimates the rate of occult leiomyosarcoma, a particularly aggressive type of sarcoma, to be approximately 1 in 495 to 1 in 1,100, again in general agreement with our previous assessment of 1 in 498.”
The FDA acknowledged that some health organizations have reported a lower estimate of risk but maintained its findings and recommendations, noting that it will continue to review new and relevant data.
Dr. Taylor reported having no disclosures.
SOURCE: Taylor GB et al. SGS 2018, Oral Poster 19.
REPORTING FROM SGS 2018
Key clinical point: Litigation fears drove decline in power morcellation after FDA warning.
Study details: A survey of 126 physicians.
Disclosures: Dr. Taylor reported having no disclosures.
Source: Taylor GB et al. SGS 2018, Oral Poster 19.
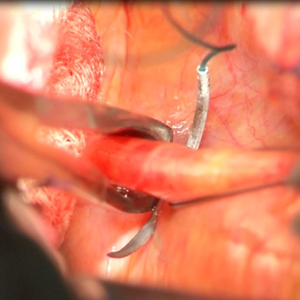
Uterosacral ligament colpopexy: The way we do it
The way we do it

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
The way we do it
The way we do it
This video is brought to you by
High MIH case volume may up risk for adverse events in women with large uteri
ORLANDO – High case volume for surgeons performing minimally invasive hysterectomies in women with large uteri is associated with an increased rate of perioperative adverse events – but also with a decreased rate of conversion to laparotomy – according to a review of 763 procedures.
The minimally invasive hysterectomy (MIH) procedures in the study were performed by 66 surgeons and included 416 total laparoscopic hysterectomies, 196 robotic-assisted laparoscopic hysterectomies, 90 total vaginal hysterectomies, and 61 laparoscopic-assisted vaginal hysterectomies, Carol E. Bretschneider, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
The mean monthly case volume was 16.4 and mean MIH volume was 23, said Dr. Bretschneider, a fellow at the Cleveland Clinic.
“The rate of postoperative adverse events was 17.8%, the rate of intraoperative adverse events was 4.2%, and the rate of conversion from a minimally invasive approach to a laparotomy was 5.5%,” she said, explaining that adverse events were defined as those greater than grade 2 on the Clavien-Dindo classification scale. “No differences were appreciated across routes [of MIH] in terms of perioperative adverse events or conversion to laparotomy,” she noted.
Even after investigators controlled for age, body mass index, uterine weight, history of laparotomy, and parity, as well as surgeon volume and operative time, they found that higher monthly MIH volume, estimated blood loss, and operative time remained significantly associated with both intraoperative adverse events (adjusted odds ratios, 1.9, 2.0, and 22.1, respectively) and postoperative adverse events (aOR, 1.3, 1.4, and 1.9, respectively), she said.
Higher BMI was associated with a lower incidence of intraoperative complications (aOR, 0.1).
“As for conversion to laparotomy, increasing surgeon volume was associated with a lower incidence of conversion (aOR, 0.4), but higher estimated blood loss and uterine weight were associated with a higher incidence of conversion (aOR, 2.6 and 7.1, respectively).”
Study subjects were women with a mean age of 47 years and mean BMI of 31 kg/m2 who underwent MIH during January 2014–June 2016 at a tertiary care referral center. Median uterine weight was 409 g, and indications for hysterectomy included fibroids, pelvic pain, abnormal uterine bleeding, and prolapse. Patients with malignancy were excluded.
“In the context of high-complexity cases, such as those for large uteri, many factors beyond surgical volume influence perioperative adverse events,” Dr. Bretschneider said, concluding that, to improve patient safety and outcomes, additional studies should be performed to explore the relationship between surgeon experience and patient factors on perioperative outcomes in the setting of common gynecologic procedures.
Dr. Bretschneider reported having no relevant disclosures.
sworcester@frontlinemedcom.com
SOURCE: Bretschneider C et al. SGS 2018, Oral Poster 12.
ORLANDO – High case volume for surgeons performing minimally invasive hysterectomies in women with large uteri is associated with an increased rate of perioperative adverse events – but also with a decreased rate of conversion to laparotomy – according to a review of 763 procedures.
The minimally invasive hysterectomy (MIH) procedures in the study were performed by 66 surgeons and included 416 total laparoscopic hysterectomies, 196 robotic-assisted laparoscopic hysterectomies, 90 total vaginal hysterectomies, and 61 laparoscopic-assisted vaginal hysterectomies, Carol E. Bretschneider, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
The mean monthly case volume was 16.4 and mean MIH volume was 23, said Dr. Bretschneider, a fellow at the Cleveland Clinic.
“The rate of postoperative adverse events was 17.8%, the rate of intraoperative adverse events was 4.2%, and the rate of conversion from a minimally invasive approach to a laparotomy was 5.5%,” she said, explaining that adverse events were defined as those greater than grade 2 on the Clavien-Dindo classification scale. “No differences were appreciated across routes [of MIH] in terms of perioperative adverse events or conversion to laparotomy,” she noted.
Even after investigators controlled for age, body mass index, uterine weight, history of laparotomy, and parity, as well as surgeon volume and operative time, they found that higher monthly MIH volume, estimated blood loss, and operative time remained significantly associated with both intraoperative adverse events (adjusted odds ratios, 1.9, 2.0, and 22.1, respectively) and postoperative adverse events (aOR, 1.3, 1.4, and 1.9, respectively), she said.
Higher BMI was associated with a lower incidence of intraoperative complications (aOR, 0.1).
“As for conversion to laparotomy, increasing surgeon volume was associated with a lower incidence of conversion (aOR, 0.4), but higher estimated blood loss and uterine weight were associated with a higher incidence of conversion (aOR, 2.6 and 7.1, respectively).”
Study subjects were women with a mean age of 47 years and mean BMI of 31 kg/m2 who underwent MIH during January 2014–June 2016 at a tertiary care referral center. Median uterine weight was 409 g, and indications for hysterectomy included fibroids, pelvic pain, abnormal uterine bleeding, and prolapse. Patients with malignancy were excluded.
“In the context of high-complexity cases, such as those for large uteri, many factors beyond surgical volume influence perioperative adverse events,” Dr. Bretschneider said, concluding that, to improve patient safety and outcomes, additional studies should be performed to explore the relationship between surgeon experience and patient factors on perioperative outcomes in the setting of common gynecologic procedures.
Dr. Bretschneider reported having no relevant disclosures.
sworcester@frontlinemedcom.com
SOURCE: Bretschneider C et al. SGS 2018, Oral Poster 12.
ORLANDO – High case volume for surgeons performing minimally invasive hysterectomies in women with large uteri is associated with an increased rate of perioperative adverse events – but also with a decreased rate of conversion to laparotomy – according to a review of 763 procedures.
The minimally invasive hysterectomy (MIH) procedures in the study were performed by 66 surgeons and included 416 total laparoscopic hysterectomies, 196 robotic-assisted laparoscopic hysterectomies, 90 total vaginal hysterectomies, and 61 laparoscopic-assisted vaginal hysterectomies, Carol E. Bretschneider, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
The mean monthly case volume was 16.4 and mean MIH volume was 23, said Dr. Bretschneider, a fellow at the Cleveland Clinic.
“The rate of postoperative adverse events was 17.8%, the rate of intraoperative adverse events was 4.2%, and the rate of conversion from a minimally invasive approach to a laparotomy was 5.5%,” she said, explaining that adverse events were defined as those greater than grade 2 on the Clavien-Dindo classification scale. “No differences were appreciated across routes [of MIH] in terms of perioperative adverse events or conversion to laparotomy,” she noted.
Even after investigators controlled for age, body mass index, uterine weight, history of laparotomy, and parity, as well as surgeon volume and operative time, they found that higher monthly MIH volume, estimated blood loss, and operative time remained significantly associated with both intraoperative adverse events (adjusted odds ratios, 1.9, 2.0, and 22.1, respectively) and postoperative adverse events (aOR, 1.3, 1.4, and 1.9, respectively), she said.
Higher BMI was associated with a lower incidence of intraoperative complications (aOR, 0.1).
“As for conversion to laparotomy, increasing surgeon volume was associated with a lower incidence of conversion (aOR, 0.4), but higher estimated blood loss and uterine weight were associated with a higher incidence of conversion (aOR, 2.6 and 7.1, respectively).”
Study subjects were women with a mean age of 47 years and mean BMI of 31 kg/m2 who underwent MIH during January 2014–June 2016 at a tertiary care referral center. Median uterine weight was 409 g, and indications for hysterectomy included fibroids, pelvic pain, abnormal uterine bleeding, and prolapse. Patients with malignancy were excluded.
“In the context of high-complexity cases, such as those for large uteri, many factors beyond surgical volume influence perioperative adverse events,” Dr. Bretschneider said, concluding that, to improve patient safety and outcomes, additional studies should be performed to explore the relationship between surgeon experience and patient factors on perioperative outcomes in the setting of common gynecologic procedures.
Dr. Bretschneider reported having no relevant disclosures.
sworcester@frontlinemedcom.com
SOURCE: Bretschneider C et al. SGS 2018, Oral Poster 12.
REPORTING FROM SGS 2018
Key clinical point: High case volume may increase risk for adverse events during minimally invasive hysterectomy with large uteri.
Major finding: MIH volume, estimated blood loss, and operative time were associated with intraoperative adverse events (odds ratios, 1.9, 2.0, and 22.1, respectively) and postoperative adverse events (ORs, 1.3, 1.4, and 1.9, respectively).
Study details: A retrospective cohort study of 763 women.
Disclosures: Dr. Bretschneider reported having no relevant disclosures.
Source: Bretschneider C et al. SGS 2018, Oral Poster 12.
Laparoscopic hysterectomy safest even for markedly enlarged uteri
ORLANDO – according to findings from a nationwide cohort of more than 27,000 women.
After adjusting for numerous potential confounding factors, including medical risk factors, procedure-related variables, and patient demographics, increasing uterine weight was significantly associated with increasing odds of complications – particularly after hysterectomy for uteri over 500 g, Michelle Louie, MD, reported during an oral poster session at the annual scientific meeting of the Society of Gynecologic Surgeons.
The same was true for uteri of 250-500 g (adjusted OR, 0.99, 1.73, and 1.06, respectively), she noted, adding that “abdominal hysterectomy always has the highest rate of a complication, except at above 850 g, when a vaginal hysterectomy is associated with a greater odds of complications.”
This secondary analysis was performed using prospectively collected quality improvement data abstracted from the American College of Surgeons National Surgical Quality Improvement Program database, which includes patient information and 30-day outcomes from more than 500 U.S. hospitals. Patients included in the analysis were 27,167 women who underwent a hysterectomy for benign conditions during 2014-2015 for whom uterine size was reported. Complications assessed included infection, vascular complications, reoperation, and readmission.
“Our study suggests that uterine weight is not an appropriate indication for abdominal hysterectomy – that we can, and should, offer a laparoscopic approach even for a markedly enlarged uterus,” she said. “We believe, therefore, that patients may benefit from referral to specialty surgeons who are able to offer a laparoscopic approach, even for a very large uterus.”
In response to a question from the audience about the role of physician experience in the findings, Dr. Louie said that it was not a covariate for which information was available, thus it was not included in the analysis.
“However, I think all of us realize that surgeon volume and surgeon experience is an important factor for patient safety,” she said.
Dr. Louie has received consulting fees from Teleflex.
sworcester@frontlinemedcom.com
SOURCE: Louie M et al. SGS 2018, Oral Poster 06.
ORLANDO – according to findings from a nationwide cohort of more than 27,000 women.
After adjusting for numerous potential confounding factors, including medical risk factors, procedure-related variables, and patient demographics, increasing uterine weight was significantly associated with increasing odds of complications – particularly after hysterectomy for uteri over 500 g, Michelle Louie, MD, reported during an oral poster session at the annual scientific meeting of the Society of Gynecologic Surgeons.
The same was true for uteri of 250-500 g (adjusted OR, 0.99, 1.73, and 1.06, respectively), she noted, adding that “abdominal hysterectomy always has the highest rate of a complication, except at above 850 g, when a vaginal hysterectomy is associated with a greater odds of complications.”
This secondary analysis was performed using prospectively collected quality improvement data abstracted from the American College of Surgeons National Surgical Quality Improvement Program database, which includes patient information and 30-day outcomes from more than 500 U.S. hospitals. Patients included in the analysis were 27,167 women who underwent a hysterectomy for benign conditions during 2014-2015 for whom uterine size was reported. Complications assessed included infection, vascular complications, reoperation, and readmission.
“Our study suggests that uterine weight is not an appropriate indication for abdominal hysterectomy – that we can, and should, offer a laparoscopic approach even for a markedly enlarged uterus,” she said. “We believe, therefore, that patients may benefit from referral to specialty surgeons who are able to offer a laparoscopic approach, even for a very large uterus.”
In response to a question from the audience about the role of physician experience in the findings, Dr. Louie said that it was not a covariate for which information was available, thus it was not included in the analysis.
“However, I think all of us realize that surgeon volume and surgeon experience is an important factor for patient safety,” she said.
Dr. Louie has received consulting fees from Teleflex.
sworcester@frontlinemedcom.com
SOURCE: Louie M et al. SGS 2018, Oral Poster 06.
ORLANDO – according to findings from a nationwide cohort of more than 27,000 women.
After adjusting for numerous potential confounding factors, including medical risk factors, procedure-related variables, and patient demographics, increasing uterine weight was significantly associated with increasing odds of complications – particularly after hysterectomy for uteri over 500 g, Michelle Louie, MD, reported during an oral poster session at the annual scientific meeting of the Society of Gynecologic Surgeons.
The same was true for uteri of 250-500 g (adjusted OR, 0.99, 1.73, and 1.06, respectively), she noted, adding that “abdominal hysterectomy always has the highest rate of a complication, except at above 850 g, when a vaginal hysterectomy is associated with a greater odds of complications.”
This secondary analysis was performed using prospectively collected quality improvement data abstracted from the American College of Surgeons National Surgical Quality Improvement Program database, which includes patient information and 30-day outcomes from more than 500 U.S. hospitals. Patients included in the analysis were 27,167 women who underwent a hysterectomy for benign conditions during 2014-2015 for whom uterine size was reported. Complications assessed included infection, vascular complications, reoperation, and readmission.
“Our study suggests that uterine weight is not an appropriate indication for abdominal hysterectomy – that we can, and should, offer a laparoscopic approach even for a markedly enlarged uterus,” she said. “We believe, therefore, that patients may benefit from referral to specialty surgeons who are able to offer a laparoscopic approach, even for a very large uterus.”
In response to a question from the audience about the role of physician experience in the findings, Dr. Louie said that it was not a covariate for which information was available, thus it was not included in the analysis.
“However, I think all of us realize that surgeon volume and surgeon experience is an important factor for patient safety,” she said.
Dr. Louie has received consulting fees from Teleflex.
sworcester@frontlinemedcom.com
SOURCE: Louie M et al. SGS 2018, Oral Poster 06.
REPORTING FROM SGS 2018
Key clinical point: Laparoscopic hysterectomy can and should be offered to women with uteri over 500 g.
Major finding: The odds ratios for complications after laparoscopic, abdominal, and vaginal hysterectomy for uteri over 500 g were 1.61, 2.16, and 2.57, respectively.
Study details: A secondary analysis of a nationwide cohort of 27,167 women.
Disclosures: Dr. Louie has received consulting fees from Teleflex.
Source: Louie M et al. SGS 2018, Oral Poster 06.
SGS 2018: SGS Fellow Scholar provides daily coverage of annual meeting
3/13/18. DAY 3 AT SGS
A busy day gets underway
During a busy morning scientific session, Dr. Amit Sood (@amitsoodMD) delivered the TeLinde lecture, titled "Building resilience," which he defined as doing well when you should not be doing well. He walked meeting attendees through a few exercises, including practicing morning gratitude and finding curious moments. He received a well-deserved standing ovation.
After a quick lunch, the group reconvened for a riveting panel discussion regarding the future of ObGyn in the session, "What is the future of gynecologic surgery and the subspecialties?" Dr. Eric Sokol moderated the discussion, and the panelists included Dr. Carl Zimmerman representing general gynecology, Dr. Dee Fenner from urogynecology, Dr. Arnold Advincula from minimally invasive gynecologic surgery (MIGS), Dr. Javier Magrina from gynecologic oncology, and Dr. Wilma Larsen representing the American Board of Obstetrics and Gynecology (ABOG). Many opinions were shared, including the possibility of allowing residents to choose a track in obstetrics or gynecology. Clearly there is a lot to explore in the future of our field. As a MIGS fellow, it was great to hear Dr. Advincula define our specialty as "non-malignant, advanced pelvic surgery."
The FMIGS Young Alumni Network then met to hear Dr. Samar Nahas give a talk on "Life as you see it, from all the struggles to all the success." She gave an inspiring overview of her life and encouraged those in attendance to keep searching for their next goal in life, whether it be academic, career-related, family, or personal.
Taking in Orlando sun and fun
The afternoon was free for leisure, and many meeting attendees took advantage of the activities offered, including a golf tournament as well as ziplining at nearby Gatorland. Others, like myself, simply took the opportunity to enjoy the sun and warm weather in Orlando, especially while there was a nor'easter occurring back home.
SGS' Got Talent was the festive evening activity on the agenda, during which a competitive talent show occurred. Attendees were able to vote for their favorites while giving donations to the organization Surgeons Helping Advance Research & Education (SHARE). Highlights included a Disney sing-along and a rousing medley of Michael Jackson songs sung on the kazoo.
See you next year!
Tomorrow is the last day of #SGS2018. I look forward to returning to my MIGS fellowship at Bridgeport Hospital in Bridgeport, Connecticut, and sharing all that I have learned. Thank you to Dr. Nancy Frankel, SGS, and OBG Management and all the sponsors supporting the Fellow Scholar program for allowing us trainees to take part in this excellent conference! I am looking forward to #SGS2019 in Tucson, Arizona.
3/12/18. DAY 2 AT SGS
Something for everyone at Day 2 SGS sessions
This morning’s Scientific Sessions began with the Keynote address by Dr. James Merlino, President and Chief Medical Officer, Strategic Consulting, at Press Ganey, who spoke on “Patient Experience: It Is Not About Making People Happy.” Dr. Merlino opened with a personal anecdote about an experience he had with a poor hospital outcome. He reminded us that showing empathy can make a huge difference in patients’ perspectives on their experience and that “an engaged culture is essential to high performance.” He also emphasized that all physicians are leaders and that our involvement in patient safety and decreasing errors is essential.
Surgeons debate best approach for POP
Another highlight of the Scientific Sessions was the debate moderated by Dr. Peter Rosenblatt on “Optimal Surgical Management of Stage 3 and 4 Pelvic Organ Prolapse.” Drs. Rebecca Rogers, Patrick Culligan, and Vincent Lucente participated. The consensus was that Dr. Rogers won over the crowd, convincing us that native tissue repair is the answer. Excellent job by all the panelists for an informative—and entertaining—debate!
Focus on fellows
Immediately following the third Scientific Session, the fellows convened at the Fellows’ Pelvic Research Network (FPRN)® meeting. After a quick joint session during which Dr. Donna Mazloomdoost gave an informative talk on the grant process at the National Institute of Child Health and Human Development (NICHD), the MIGS fellows broke out to review projects that are currently recruiting new sites and to hear a new proposal for a collaborative multicenter study.
President’s events
Evening activities included the President’s Awards Ceremony and the President’s Reception. Awards Ceremony notable moments included the “Honor your Mentor” session, during which the honored individuals were each recognized. Dr. Ralph Chesson then received the inaugural “Mentor of the Year” award, to much applause. Fellow/Resident awards were up next. Dr. Christina Jones received the President’s Award–Prize Paper for her oral presentation, “Liposomal bupivacaine efficacy for postoperative pain following posterior vaginal surgery: a randomized, double-blind, placebo-controlled trial.” Congratulations! The day concluded with the President’s Reception, where the food, drink, and conversation were plentiful.
Get appy
Finally, conference attendees were able to plan their meeting on their smartphones using the Event Pilot App, which also had a running Twitter thread under the hashtag #SGS2018. A Twitter scavenger hunt was also underway, with #speculumsunday, #maskmembermonday, and #trocartuesday. During this event attendees are encouraged to find the “object of the day,” Tweet a response to a prompt, and then bring the object to the social media booth to receive a prize.
Stay tuned to @ChrisTierneyMD for more live updates from #SGS2018.

3/11/18. DAY 1 AT SGS
Good morning from sunny Orlando!
It was a very early morning, due to the conveniently timed daylight savings to start the Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). First on the agenda was the postgraduate (PG) course, “Endometriosis: Contemporary Approaches to Diagnosis and Treatment” led by Dr. Rosanne Kho. Excellent talks were given and reminded us that, as a specialty, we have made great strides in advancing the diagnosis and treatment of endometriosis. Nevertheless, there is much work to be done in developing a better classification system, including identifying markers that can help stratify the extent of disease.
A highlight was a question from the audience regarding the important facets of a physical exam in diagnosing deep endometriosis. Dr. Mauricio Abrao reviewed his strategy, which included palpating for adnexal masses, pain, or nodules in the cul-de-sac and evaluating trigger points along the psoas muscle. Dr. Scott Young, a radiologist, then provided some key signs to look for in pelvic magnetic resonance imaging and endovaginal ultrasound in diagnosing deep endometriosis. He reminded all of us that a partnership with an excellent radiology department is key in diagnosing and treating deep endometriosis effectively. Dr. Tommaso Falcone then gave an excellent overview of anatomy in relation to deep endometriosis, teaching us all about the different layers of the retroperitoneum.
An afternoon of simulation
After a quick break, it was on to the next course: “Train the Trainer: Comprehensive Hysterectomy Simulation Course for Educators.” Dr. Christine Vaccaro headed this interactive session, and started us off with a reminder that “simulation is the future, and we need to jump on board!” Unfortunately for trainees, the overall number of hysterectomies performed during residency are decreasing secondary to medical management available for abnormal uterine bleeding and the fact that the 4 years of ObGyn residency have a heavy focus on obstetrics. Therefore, it was great to hear Dr. Vaccaro say that the American Board of Obstetrics and Gynecology (ABOG) is requiring Fundamentals of Laparoscopic Surgery (FLSTM) training for those seeking board certification. Attendees of this session included a combination of trainers and trainees, so to speak, and we took turns rotating around models for abdominal, vaginal, laparoscopic, and robotic hysterectomies. I took the opportunity to learn some tips and tricks from experts like Dr. Ernie Lockrow during a simulated laparoscopic myomectomy, in which the struggle felt very real.
Mix and mingle
Other PG courses offered and attended throughout the day included: “Surgical Coding: Working through the Entire Procedure,” “Variations in the Management of the Vaginal Apex during Reconstructive Pelvic Surgery,” Gynecologic Care of Girls and Young Women Born with Urogenital Anomalies,” and “Social Media, Medicine and your Digital Footprint.” SGS Fellow Scholars were then invited to the “Senior Members Engaging Young Learners” event, designed to connect senior SGS members with residents and fellows. Here, residents and fellows mingled with distinguished faculty, many of them with red badges on their nametags, indicating 20 years of membership with SGS. I learned firsthand that connections are very easy to make in this friendly group, where everyone is willing to introduce you to someone who will help advance your career. After this, we moved straight onto the Welcome Reception, where conversations and connections continued.
I look forward to a full day tomorrow! Stay tuned to @ChrisTierneyMD for live updates from #SGS2018.

3/13/18. DAY 3 AT SGS
A busy day gets underway
During a busy morning scientific session, Dr. Amit Sood (@amitsoodMD) delivered the TeLinde lecture, titled "Building resilience," which he defined as doing well when you should not be doing well. He walked meeting attendees through a few exercises, including practicing morning gratitude and finding curious moments. He received a well-deserved standing ovation.
After a quick lunch, the group reconvened for a riveting panel discussion regarding the future of ObGyn in the session, "What is the future of gynecologic surgery and the subspecialties?" Dr. Eric Sokol moderated the discussion, and the panelists included Dr. Carl Zimmerman representing general gynecology, Dr. Dee Fenner from urogynecology, Dr. Arnold Advincula from minimally invasive gynecologic surgery (MIGS), Dr. Javier Magrina from gynecologic oncology, and Dr. Wilma Larsen representing the American Board of Obstetrics and Gynecology (ABOG). Many opinions were shared, including the possibility of allowing residents to choose a track in obstetrics or gynecology. Clearly there is a lot to explore in the future of our field. As a MIGS fellow, it was great to hear Dr. Advincula define our specialty as "non-malignant, advanced pelvic surgery."
The FMIGS Young Alumni Network then met to hear Dr. Samar Nahas give a talk on "Life as you see it, from all the struggles to all the success." She gave an inspiring overview of her life and encouraged those in attendance to keep searching for their next goal in life, whether it be academic, career-related, family, or personal.
Taking in Orlando sun and fun
The afternoon was free for leisure, and many meeting attendees took advantage of the activities offered, including a golf tournament as well as ziplining at nearby Gatorland. Others, like myself, simply took the opportunity to enjoy the sun and warm weather in Orlando, especially while there was a nor'easter occurring back home.
SGS' Got Talent was the festive evening activity on the agenda, during which a competitive talent show occurred. Attendees were able to vote for their favorites while giving donations to the organization Surgeons Helping Advance Research & Education (SHARE). Highlights included a Disney sing-along and a rousing medley of Michael Jackson songs sung on the kazoo.
See you next year!
Tomorrow is the last day of #SGS2018. I look forward to returning to my MIGS fellowship at Bridgeport Hospital in Bridgeport, Connecticut, and sharing all that I have learned. Thank you to Dr. Nancy Frankel, SGS, and OBG Management and all the sponsors supporting the Fellow Scholar program for allowing us trainees to take part in this excellent conference! I am looking forward to #SGS2019 in Tucson, Arizona.
3/12/18. DAY 2 AT SGS
Something for everyone at Day 2 SGS sessions
This morning’s Scientific Sessions began with the Keynote address by Dr. James Merlino, President and Chief Medical Officer, Strategic Consulting, at Press Ganey, who spoke on “Patient Experience: It Is Not About Making People Happy.” Dr. Merlino opened with a personal anecdote about an experience he had with a poor hospital outcome. He reminded us that showing empathy can make a huge difference in patients’ perspectives on their experience and that “an engaged culture is essential to high performance.” He also emphasized that all physicians are leaders and that our involvement in patient safety and decreasing errors is essential.
Surgeons debate best approach for POP
Another highlight of the Scientific Sessions was the debate moderated by Dr. Peter Rosenblatt on “Optimal Surgical Management of Stage 3 and 4 Pelvic Organ Prolapse.” Drs. Rebecca Rogers, Patrick Culligan, and Vincent Lucente participated. The consensus was that Dr. Rogers won over the crowd, convincing us that native tissue repair is the answer. Excellent job by all the panelists for an informative—and entertaining—debate!
Focus on fellows
Immediately following the third Scientific Session, the fellows convened at the Fellows’ Pelvic Research Network (FPRN)® meeting. After a quick joint session during which Dr. Donna Mazloomdoost gave an informative talk on the grant process at the National Institute of Child Health and Human Development (NICHD), the MIGS fellows broke out to review projects that are currently recruiting new sites and to hear a new proposal for a collaborative multicenter study.
President’s events
Evening activities included the President’s Awards Ceremony and the President’s Reception. Awards Ceremony notable moments included the “Honor your Mentor” session, during which the honored individuals were each recognized. Dr. Ralph Chesson then received the inaugural “Mentor of the Year” award, to much applause. Fellow/Resident awards were up next. Dr. Christina Jones received the President’s Award–Prize Paper for her oral presentation, “Liposomal bupivacaine efficacy for postoperative pain following posterior vaginal surgery: a randomized, double-blind, placebo-controlled trial.” Congratulations! The day concluded with the President’s Reception, where the food, drink, and conversation were plentiful.
Get appy
Finally, conference attendees were able to plan their meeting on their smartphones using the Event Pilot App, which also had a running Twitter thread under the hashtag #SGS2018. A Twitter scavenger hunt was also underway, with #speculumsunday, #maskmembermonday, and #trocartuesday. During this event attendees are encouraged to find the “object of the day,” Tweet a response to a prompt, and then bring the object to the social media booth to receive a prize.
Stay tuned to @ChrisTierneyMD for more live updates from #SGS2018.

3/11/18. DAY 1 AT SGS
Good morning from sunny Orlando!
It was a very early morning, due to the conveniently timed daylight savings to start the Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). First on the agenda was the postgraduate (PG) course, “Endometriosis: Contemporary Approaches to Diagnosis and Treatment” led by Dr. Rosanne Kho. Excellent talks were given and reminded us that, as a specialty, we have made great strides in advancing the diagnosis and treatment of endometriosis. Nevertheless, there is much work to be done in developing a better classification system, including identifying markers that can help stratify the extent of disease.
A highlight was a question from the audience regarding the important facets of a physical exam in diagnosing deep endometriosis. Dr. Mauricio Abrao reviewed his strategy, which included palpating for adnexal masses, pain, or nodules in the cul-de-sac and evaluating trigger points along the psoas muscle. Dr. Scott Young, a radiologist, then provided some key signs to look for in pelvic magnetic resonance imaging and endovaginal ultrasound in diagnosing deep endometriosis. He reminded all of us that a partnership with an excellent radiology department is key in diagnosing and treating deep endometriosis effectively. Dr. Tommaso Falcone then gave an excellent overview of anatomy in relation to deep endometriosis, teaching us all about the different layers of the retroperitoneum.
An afternoon of simulation
After a quick break, it was on to the next course: “Train the Trainer: Comprehensive Hysterectomy Simulation Course for Educators.” Dr. Christine Vaccaro headed this interactive session, and started us off with a reminder that “simulation is the future, and we need to jump on board!” Unfortunately for trainees, the overall number of hysterectomies performed during residency are decreasing secondary to medical management available for abnormal uterine bleeding and the fact that the 4 years of ObGyn residency have a heavy focus on obstetrics. Therefore, it was great to hear Dr. Vaccaro say that the American Board of Obstetrics and Gynecology (ABOG) is requiring Fundamentals of Laparoscopic Surgery (FLSTM) training for those seeking board certification. Attendees of this session included a combination of trainers and trainees, so to speak, and we took turns rotating around models for abdominal, vaginal, laparoscopic, and robotic hysterectomies. I took the opportunity to learn some tips and tricks from experts like Dr. Ernie Lockrow during a simulated laparoscopic myomectomy, in which the struggle felt very real.
Mix and mingle
Other PG courses offered and attended throughout the day included: “Surgical Coding: Working through the Entire Procedure,” “Variations in the Management of the Vaginal Apex during Reconstructive Pelvic Surgery,” Gynecologic Care of Girls and Young Women Born with Urogenital Anomalies,” and “Social Media, Medicine and your Digital Footprint.” SGS Fellow Scholars were then invited to the “Senior Members Engaging Young Learners” event, designed to connect senior SGS members with residents and fellows. Here, residents and fellows mingled with distinguished faculty, many of them with red badges on their nametags, indicating 20 years of membership with SGS. I learned firsthand that connections are very easy to make in this friendly group, where everyone is willing to introduce you to someone who will help advance your career. After this, we moved straight onto the Welcome Reception, where conversations and connections continued.
I look forward to a full day tomorrow! Stay tuned to @ChrisTierneyMD for live updates from #SGS2018.

3/13/18. DAY 3 AT SGS
A busy day gets underway
During a busy morning scientific session, Dr. Amit Sood (@amitsoodMD) delivered the TeLinde lecture, titled "Building resilience," which he defined as doing well when you should not be doing well. He walked meeting attendees through a few exercises, including practicing morning gratitude and finding curious moments. He received a well-deserved standing ovation.
After a quick lunch, the group reconvened for a riveting panel discussion regarding the future of ObGyn in the session, "What is the future of gynecologic surgery and the subspecialties?" Dr. Eric Sokol moderated the discussion, and the panelists included Dr. Carl Zimmerman representing general gynecology, Dr. Dee Fenner from urogynecology, Dr. Arnold Advincula from minimally invasive gynecologic surgery (MIGS), Dr. Javier Magrina from gynecologic oncology, and Dr. Wilma Larsen representing the American Board of Obstetrics and Gynecology (ABOG). Many opinions were shared, including the possibility of allowing residents to choose a track in obstetrics or gynecology. Clearly there is a lot to explore in the future of our field. As a MIGS fellow, it was great to hear Dr. Advincula define our specialty as "non-malignant, advanced pelvic surgery."
The FMIGS Young Alumni Network then met to hear Dr. Samar Nahas give a talk on "Life as you see it, from all the struggles to all the success." She gave an inspiring overview of her life and encouraged those in attendance to keep searching for their next goal in life, whether it be academic, career-related, family, or personal.
Taking in Orlando sun and fun
The afternoon was free for leisure, and many meeting attendees took advantage of the activities offered, including a golf tournament as well as ziplining at nearby Gatorland. Others, like myself, simply took the opportunity to enjoy the sun and warm weather in Orlando, especially while there was a nor'easter occurring back home.
SGS' Got Talent was the festive evening activity on the agenda, during which a competitive talent show occurred. Attendees were able to vote for their favorites while giving donations to the organization Surgeons Helping Advance Research & Education (SHARE). Highlights included a Disney sing-along and a rousing medley of Michael Jackson songs sung on the kazoo.
See you next year!
Tomorrow is the last day of #SGS2018. I look forward to returning to my MIGS fellowship at Bridgeport Hospital in Bridgeport, Connecticut, and sharing all that I have learned. Thank you to Dr. Nancy Frankel, SGS, and OBG Management and all the sponsors supporting the Fellow Scholar program for allowing us trainees to take part in this excellent conference! I am looking forward to #SGS2019 in Tucson, Arizona.
3/12/18. DAY 2 AT SGS
Something for everyone at Day 2 SGS sessions
This morning’s Scientific Sessions began with the Keynote address by Dr. James Merlino, President and Chief Medical Officer, Strategic Consulting, at Press Ganey, who spoke on “Patient Experience: It Is Not About Making People Happy.” Dr. Merlino opened with a personal anecdote about an experience he had with a poor hospital outcome. He reminded us that showing empathy can make a huge difference in patients’ perspectives on their experience and that “an engaged culture is essential to high performance.” He also emphasized that all physicians are leaders and that our involvement in patient safety and decreasing errors is essential.
Surgeons debate best approach for POP
Another highlight of the Scientific Sessions was the debate moderated by Dr. Peter Rosenblatt on “Optimal Surgical Management of Stage 3 and 4 Pelvic Organ Prolapse.” Drs. Rebecca Rogers, Patrick Culligan, and Vincent Lucente participated. The consensus was that Dr. Rogers won over the crowd, convincing us that native tissue repair is the answer. Excellent job by all the panelists for an informative—and entertaining—debate!
Focus on fellows
Immediately following the third Scientific Session, the fellows convened at the Fellows’ Pelvic Research Network (FPRN)® meeting. After a quick joint session during which Dr. Donna Mazloomdoost gave an informative talk on the grant process at the National Institute of Child Health and Human Development (NICHD), the MIGS fellows broke out to review projects that are currently recruiting new sites and to hear a new proposal for a collaborative multicenter study.
President’s events
Evening activities included the President’s Awards Ceremony and the President’s Reception. Awards Ceremony notable moments included the “Honor your Mentor” session, during which the honored individuals were each recognized. Dr. Ralph Chesson then received the inaugural “Mentor of the Year” award, to much applause. Fellow/Resident awards were up next. Dr. Christina Jones received the President’s Award–Prize Paper for her oral presentation, “Liposomal bupivacaine efficacy for postoperative pain following posterior vaginal surgery: a randomized, double-blind, placebo-controlled trial.” Congratulations! The day concluded with the President’s Reception, where the food, drink, and conversation were plentiful.
Get appy
Finally, conference attendees were able to plan their meeting on their smartphones using the Event Pilot App, which also had a running Twitter thread under the hashtag #SGS2018. A Twitter scavenger hunt was also underway, with #speculumsunday, #maskmembermonday, and #trocartuesday. During this event attendees are encouraged to find the “object of the day,” Tweet a response to a prompt, and then bring the object to the social media booth to receive a prize.
Stay tuned to @ChrisTierneyMD for more live updates from #SGS2018.

3/11/18. DAY 1 AT SGS
Good morning from sunny Orlando!
It was a very early morning, due to the conveniently timed daylight savings to start the Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). First on the agenda was the postgraduate (PG) course, “Endometriosis: Contemporary Approaches to Diagnosis and Treatment” led by Dr. Rosanne Kho. Excellent talks were given and reminded us that, as a specialty, we have made great strides in advancing the diagnosis and treatment of endometriosis. Nevertheless, there is much work to be done in developing a better classification system, including identifying markers that can help stratify the extent of disease.
A highlight was a question from the audience regarding the important facets of a physical exam in diagnosing deep endometriosis. Dr. Mauricio Abrao reviewed his strategy, which included palpating for adnexal masses, pain, or nodules in the cul-de-sac and evaluating trigger points along the psoas muscle. Dr. Scott Young, a radiologist, then provided some key signs to look for in pelvic magnetic resonance imaging and endovaginal ultrasound in diagnosing deep endometriosis. He reminded all of us that a partnership with an excellent radiology department is key in diagnosing and treating deep endometriosis effectively. Dr. Tommaso Falcone then gave an excellent overview of anatomy in relation to deep endometriosis, teaching us all about the different layers of the retroperitoneum.
An afternoon of simulation
After a quick break, it was on to the next course: “Train the Trainer: Comprehensive Hysterectomy Simulation Course for Educators.” Dr. Christine Vaccaro headed this interactive session, and started us off with a reminder that “simulation is the future, and we need to jump on board!” Unfortunately for trainees, the overall number of hysterectomies performed during residency are decreasing secondary to medical management available for abnormal uterine bleeding and the fact that the 4 years of ObGyn residency have a heavy focus on obstetrics. Therefore, it was great to hear Dr. Vaccaro say that the American Board of Obstetrics and Gynecology (ABOG) is requiring Fundamentals of Laparoscopic Surgery (FLSTM) training for those seeking board certification. Attendees of this session included a combination of trainers and trainees, so to speak, and we took turns rotating around models for abdominal, vaginal, laparoscopic, and robotic hysterectomies. I took the opportunity to learn some tips and tricks from experts like Dr. Ernie Lockrow during a simulated laparoscopic myomectomy, in which the struggle felt very real.
Mix and mingle
Other PG courses offered and attended throughout the day included: “Surgical Coding: Working through the Entire Procedure,” “Variations in the Management of the Vaginal Apex during Reconstructive Pelvic Surgery,” Gynecologic Care of Girls and Young Women Born with Urogenital Anomalies,” and “Social Media, Medicine and your Digital Footprint.” SGS Fellow Scholars were then invited to the “Senior Members Engaging Young Learners” event, designed to connect senior SGS members with residents and fellows. Here, residents and fellows mingled with distinguished faculty, many of them with red badges on their nametags, indicating 20 years of membership with SGS. I learned firsthand that connections are very easy to make in this friendly group, where everyone is willing to introduce you to someone who will help advance your career. After this, we moved straight onto the Welcome Reception, where conversations and connections continued.
I look forward to a full day tomorrow! Stay tuned to @ChrisTierneyMD for live updates from #SGS2018.

VIDEO: Dabigatran effective for myocardial injury after noncardiac surgery
ORLANDO – Treating patients who developed myocardial injury after noncardiac surgery with the anticoagulant dabigatran significantly cut the rate of subsequent major vascular complications in a randomized, multicenter trial with 1,754 patients, a result that gives surgeons and physicians the first evidence-based intervention for treating a common postsurgical condition.
“Because we have not systematically followed noncardiac surgery patients, it’s easy to presume that everyone is okay, but all the epidemiology data show that these patients [who develop myocardial injury after noncardiac surgery] don’t do okay. We need to be aggressive with secondary prophylaxis,” P.J. Devereaux, MD, said at the annual meeting of the American College of Cardiology. “The unfortunate thing is that right now, we don’t do much for these patients,” said Dr. Devereaux, professor of medicine and director of cardiology at McMaster University in Hamilton, Ont.
Results from prior epidemiology studies have shown that, among the roughly 200 million patients who undergo noncardiac surgery worldwide each year, 8% will develop MINS (myocardial injury after noncardiac surgery) (Anesthesiology. 2014 March;120[3]:564-78). The myocardial injury that defines MINS is identified by either an overt MI that meets the universal definition, or an otherwise unexplained rise in serum troponin levels from baseline in the first couple of days after surgery. In the new study, Dr. Devereaux and his associates identified 80% of MINS by a troponin rise and 20% by a diagnosed MI.
The challenge in diagnosing MINS and then administering dabigatran will be implementation of this strategy into routine practice, commented Erin A. Bohula May, MD, a cardiologist at Brigham and Women’s Hospital in Boston. “The problem is, troponin is not routinely measured in postoperative patients. It will be hard to change practice,” she noted.
Dr. Devereaux agreed that a significant barrier is convincing clinicians, especially surgeons, to routinely measure a patient’s troponin levels just before and immediately after surgery. “People are lulled into a false sense of security because patients [who develop MINS] usually don’t have chest pain,” he said in a video interview. “When we first showed that patients with MINS have bad outcomes, that convinced some [surgeons] to measure troponin after surgery. “Showing we can do something about it” is another important step toward fostering more awareness of and interest in diagnosing and treating MINS.
The Management of Myocardial Injury After Noncardiac Surgery Trial (MANAGE) enrolled 1,754 patients at 82 centers in 19 countries. Researchers randomized patients to treatment with either 110 mg dabigatran b.i.d. or placebo. A majority of patients in both arms also received aspirin and a statin, treatments that Dr. Devereaux should be used along with dabigatran in routine practice, based on observational findings, although the efficacy of these drugs for MINS patients has not been tested in randomized studies. The study’s primary endpoint was the incidence of major vascular complications, a composite that included vascular mortality, nonfatal MI, nonfatal and nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, or symptomatic venous thromboembolism.
After an average follow-up of 16 months, the primary endpoint occurred in 11% of the dabigatran-treated patients and in 15% of controls, which represented a 28% risk reduction that was statistically significant. The study’s primary safety endpoint was a composite of life-threatening, major, and critical organ bleeds, which occurred in 3% of the dabigatran-treated patients and in 4% of controls, a nonsignificant difference. The dabigatran-treated patients showed a significant excess of both minor bleeds – 15% compared with 10% in controls – and “nonsignificant” lower gastrointestinal bleeds, 4% with dabigatran and 1% in the controls. The dabigatran-treated patients also had a significantly higher incidence of dyspepsia.
MANAGE was funded by the Population Health Research Institute and had no commercial funding. Dr. Devereaux has received research support from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, and Roche Diagnostics. Dr. May has been a consultant to Daiichi Sankyo, Merck, and Servier and has received research funding from Eisai.
SOURCE: Devereaux P et al. ACC 18.
Dr. Devereaux and his associates are to be congratulated on identifying a new disease entity, MINS (myocardial injury after noncardiac surgery), and now giving us a way to treat it. MINS is extremely common and quite morbid, and there had never before been a trial that studied its treatment. Identifying patients with MINS is extremely important. These are very-high-risk patients, and they are very hard to find. The results from MANAGE give us a way to do something about MINS and an opportunity to improve patient outcomes.
Pamela S. Douglas, MD , is a cardiologist and professor of medicine at Duke University in Durham, N.C. She had no disclosures. She made these comments as a discussant for MANAGE and in an interview.
Dr. Devereaux and his associates are to be congratulated on identifying a new disease entity, MINS (myocardial injury after noncardiac surgery), and now giving us a way to treat it. MINS is extremely common and quite morbid, and there had never before been a trial that studied its treatment. Identifying patients with MINS is extremely important. These are very-high-risk patients, and they are very hard to find. The results from MANAGE give us a way to do something about MINS and an opportunity to improve patient outcomes.
Pamela S. Douglas, MD , is a cardiologist and professor of medicine at Duke University in Durham, N.C. She had no disclosures. She made these comments as a discussant for MANAGE and in an interview.
Dr. Devereaux and his associates are to be congratulated on identifying a new disease entity, MINS (myocardial injury after noncardiac surgery), and now giving us a way to treat it. MINS is extremely common and quite morbid, and there had never before been a trial that studied its treatment. Identifying patients with MINS is extremely important. These are very-high-risk patients, and they are very hard to find. The results from MANAGE give us a way to do something about MINS and an opportunity to improve patient outcomes.
Pamela S. Douglas, MD , is a cardiologist and professor of medicine at Duke University in Durham, N.C. She had no disclosures. She made these comments as a discussant for MANAGE and in an interview.
ORLANDO – Treating patients who developed myocardial injury after noncardiac surgery with the anticoagulant dabigatran significantly cut the rate of subsequent major vascular complications in a randomized, multicenter trial with 1,754 patients, a result that gives surgeons and physicians the first evidence-based intervention for treating a common postsurgical condition.
“Because we have not systematically followed noncardiac surgery patients, it’s easy to presume that everyone is okay, but all the epidemiology data show that these patients [who develop myocardial injury after noncardiac surgery] don’t do okay. We need to be aggressive with secondary prophylaxis,” P.J. Devereaux, MD, said at the annual meeting of the American College of Cardiology. “The unfortunate thing is that right now, we don’t do much for these patients,” said Dr. Devereaux, professor of medicine and director of cardiology at McMaster University in Hamilton, Ont.
Results from prior epidemiology studies have shown that, among the roughly 200 million patients who undergo noncardiac surgery worldwide each year, 8% will develop MINS (myocardial injury after noncardiac surgery) (Anesthesiology. 2014 March;120[3]:564-78). The myocardial injury that defines MINS is identified by either an overt MI that meets the universal definition, or an otherwise unexplained rise in serum troponin levels from baseline in the first couple of days after surgery. In the new study, Dr. Devereaux and his associates identified 80% of MINS by a troponin rise and 20% by a diagnosed MI.
The challenge in diagnosing MINS and then administering dabigatran will be implementation of this strategy into routine practice, commented Erin A. Bohula May, MD, a cardiologist at Brigham and Women’s Hospital in Boston. “The problem is, troponin is not routinely measured in postoperative patients. It will be hard to change practice,” she noted.
Dr. Devereaux agreed that a significant barrier is convincing clinicians, especially surgeons, to routinely measure a patient’s troponin levels just before and immediately after surgery. “People are lulled into a false sense of security because patients [who develop MINS] usually don’t have chest pain,” he said in a video interview. “When we first showed that patients with MINS have bad outcomes, that convinced some [surgeons] to measure troponin after surgery. “Showing we can do something about it” is another important step toward fostering more awareness of and interest in diagnosing and treating MINS.
The Management of Myocardial Injury After Noncardiac Surgery Trial (MANAGE) enrolled 1,754 patients at 82 centers in 19 countries. Researchers randomized patients to treatment with either 110 mg dabigatran b.i.d. or placebo. A majority of patients in both arms also received aspirin and a statin, treatments that Dr. Devereaux should be used along with dabigatran in routine practice, based on observational findings, although the efficacy of these drugs for MINS patients has not been tested in randomized studies. The study’s primary endpoint was the incidence of major vascular complications, a composite that included vascular mortality, nonfatal MI, nonfatal and nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, or symptomatic venous thromboembolism.
After an average follow-up of 16 months, the primary endpoint occurred in 11% of the dabigatran-treated patients and in 15% of controls, which represented a 28% risk reduction that was statistically significant. The study’s primary safety endpoint was a composite of life-threatening, major, and critical organ bleeds, which occurred in 3% of the dabigatran-treated patients and in 4% of controls, a nonsignificant difference. The dabigatran-treated patients showed a significant excess of both minor bleeds – 15% compared with 10% in controls – and “nonsignificant” lower gastrointestinal bleeds, 4% with dabigatran and 1% in the controls. The dabigatran-treated patients also had a significantly higher incidence of dyspepsia.
MANAGE was funded by the Population Health Research Institute and had no commercial funding. Dr. Devereaux has received research support from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, and Roche Diagnostics. Dr. May has been a consultant to Daiichi Sankyo, Merck, and Servier and has received research funding from Eisai.
SOURCE: Devereaux P et al. ACC 18.
ORLANDO – Treating patients who developed myocardial injury after noncardiac surgery with the anticoagulant dabigatran significantly cut the rate of subsequent major vascular complications in a randomized, multicenter trial with 1,754 patients, a result that gives surgeons and physicians the first evidence-based intervention for treating a common postsurgical condition.
“Because we have not systematically followed noncardiac surgery patients, it’s easy to presume that everyone is okay, but all the epidemiology data show that these patients [who develop myocardial injury after noncardiac surgery] don’t do okay. We need to be aggressive with secondary prophylaxis,” P.J. Devereaux, MD, said at the annual meeting of the American College of Cardiology. “The unfortunate thing is that right now, we don’t do much for these patients,” said Dr. Devereaux, professor of medicine and director of cardiology at McMaster University in Hamilton, Ont.
Results from prior epidemiology studies have shown that, among the roughly 200 million patients who undergo noncardiac surgery worldwide each year, 8% will develop MINS (myocardial injury after noncardiac surgery) (Anesthesiology. 2014 March;120[3]:564-78). The myocardial injury that defines MINS is identified by either an overt MI that meets the universal definition, or an otherwise unexplained rise in serum troponin levels from baseline in the first couple of days after surgery. In the new study, Dr. Devereaux and his associates identified 80% of MINS by a troponin rise and 20% by a diagnosed MI.
The challenge in diagnosing MINS and then administering dabigatran will be implementation of this strategy into routine practice, commented Erin A. Bohula May, MD, a cardiologist at Brigham and Women’s Hospital in Boston. “The problem is, troponin is not routinely measured in postoperative patients. It will be hard to change practice,” she noted.
Dr. Devereaux agreed that a significant barrier is convincing clinicians, especially surgeons, to routinely measure a patient’s troponin levels just before and immediately after surgery. “People are lulled into a false sense of security because patients [who develop MINS] usually don’t have chest pain,” he said in a video interview. “When we first showed that patients with MINS have bad outcomes, that convinced some [surgeons] to measure troponin after surgery. “Showing we can do something about it” is another important step toward fostering more awareness of and interest in diagnosing and treating MINS.
The Management of Myocardial Injury After Noncardiac Surgery Trial (MANAGE) enrolled 1,754 patients at 82 centers in 19 countries. Researchers randomized patients to treatment with either 110 mg dabigatran b.i.d. or placebo. A majority of patients in both arms also received aspirin and a statin, treatments that Dr. Devereaux should be used along with dabigatran in routine practice, based on observational findings, although the efficacy of these drugs for MINS patients has not been tested in randomized studies. The study’s primary endpoint was the incidence of major vascular complications, a composite that included vascular mortality, nonfatal MI, nonfatal and nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, or symptomatic venous thromboembolism.
After an average follow-up of 16 months, the primary endpoint occurred in 11% of the dabigatran-treated patients and in 15% of controls, which represented a 28% risk reduction that was statistically significant. The study’s primary safety endpoint was a composite of life-threatening, major, and critical organ bleeds, which occurred in 3% of the dabigatran-treated patients and in 4% of controls, a nonsignificant difference. The dabigatran-treated patients showed a significant excess of both minor bleeds – 15% compared with 10% in controls – and “nonsignificant” lower gastrointestinal bleeds, 4% with dabigatran and 1% in the controls. The dabigatran-treated patients also had a significantly higher incidence of dyspepsia.
MANAGE was funded by the Population Health Research Institute and had no commercial funding. Dr. Devereaux has received research support from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, and Roche Diagnostics. Dr. May has been a consultant to Daiichi Sankyo, Merck, and Servier and has received research funding from Eisai.
SOURCE: Devereaux P et al. ACC 18.
REPORTING FROM ACC 18
Key clinical point: Dabigatran is the first intervention proven to benefit patients with MINS.
Major finding: Major vascular complications occurred in 11% of patients on dabigatran and 15% on placebo.
Study details: MANAGE, a multicenter, randomized trial with 1,754 patients.
Disclosures: MANAGE was funded by the Population Health Research Institute and had no commercial funding. Dr. Devereaux has received research support from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, and Roche Diagnostics. Dr. May has been a consultant to Daiichi Sankyo, Merck, and Servier and has received research funding from Eisai.
Source: Devereaux P et al. ACC 18.