User login
Moving toward safer morcellation techniques
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
For minimally invasive surgeons throughout the world, particularly in the United States, as well as the patients we treat, April 17, 2014, is our day of infamy. It was on this day that the Food and Drug Administration recommended against the use of the electronic power morcellator. The basis of the agency’s decision was the concern about inadvertent spread of sarcomatous tissue. Many hospitals, medical centers, and hospital systems subsequently banned the use of power morcellation. With such bans, a subsequent study by Wright et al. noted a decrease in the percentage of both laparoscopic and vaginal hysterectomy (JAMA. 2016 Aug 23-30;316[8]:877-8). This is concerning when you consider that the complication rate for abdominal hysterectomy is around 17%, compared with about 4% for the minimally invasive procedure.
For this edition of the Master Class in Gynecologic Surgery, I have asked Tony Shibley, MD, to describe the PneumoLiner, the first FDA-approved bag for the purpose of contained laparoscopic morcellation. Dr. Shibley, who is in private practice in the Minneapolis area, first came to national attention because of his expertise in single-port surgery. He has been performing power morcellation in a contained system for 5 years and is the thought leader behind the design and creation of the PneumoLiner.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported receiving research funds from Espiner Medical Inc., and being a consultant to Olympus, which manufacturers the PneumoLiner.
VIDEO: Tips for performing contained power morcellation
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
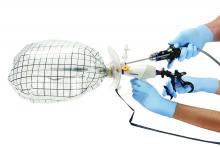
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
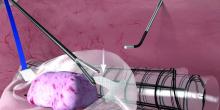
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
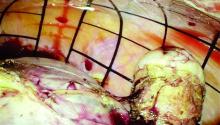
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Experience with electromechanical power morcellation in a bag has advanced in the last several years in an effort to achieve safe tissue removal for minimally invasive procedures such as myomectomy, laparoscopic supracervical hysterectomy, or total hysterectomy of a large uterus.
Tissue extraction using contained power morcellation has become favored over contained morcellation using a scalpel – not only because the latter approach is cumbersome but because of the risk of bag puncture and subsequent organ injury. Surgeons have experimented with various sizes and types of retrieval bags and with various techniques for contained power morcellation.
The PneumoLiner carries the same restrictions as do other laparoscopic power morcellation systems – namely that it should not be used in surgery in which the tissue to be morcellated is known or suspected to contain malignancy, and that it should not be used in women who are peri- or postmenopausal. Moreover, to further enhance safety, physicians must have successfully completed the FDA-required validated training program run by Advanced Surgical Concepts and Olympus in order to use the device.
The FDA reviewed the PneumoLiner through a regulatory process known as the de novo classification process. This regulatory process is for first of its kind, low- to moderate-risk medical devices. The PneumoLiner was tested in laboratory conditions to ensure that it could withstand stress force in excess of the normal forces of surgery, and was found to be impervious to substances similar in molecular size to tissues, cells, and body fluids. There could be no cellular migration or leakage.
As surgeons were advancing the idea of inflated bag morcellation, one promising adaptation was to puncture the inflated bag to place accessory ports. However, recent research has shown that contained morcellation involving intentional bag puncture with a trocar may result in tissue or fluid leakage.
Spillage was noted in 7 of 76 cases (9.2%) in a multicenter prospective cohort of women who underwent hysterectomy or myomectomy using a contained power morcellation technique that involved perforation of the containment bag with a balloon-tipped lateral trocar. Investigators had injected blue dye into the bag prior to morcellation and examined the abdomen and pelvis after removing the bag for signs of spillage of dye, fluid, or tissue. In all cases, the containment bags were intact (Am J Obstet Gynecol. 2016 Feb;214[2]:257.e1-6).
The authors prematurely closed this study and recommended against this puncture technique. For complete containment, it appears to be important that we morcellate using a bag that has a single opening and is not punctured with accessory trocars.
The technique
The PneumoLiner comes loaded in an insertion tube for placement. It has a plunger to deploy the device and a retrieval lanyard that closes the bag around the specimen, enabling retrieval of the neck of the bag outside the abdomen.
Included with the PneumoLiner is a multi-instrument port that can be used during the laparoscopic procedure and then converted to the active port for morcellation. The port has an opening for the laparoscope (either a 5-mm 30-degree straight or a 5-mm articulating laparoscope) and an opening for the morcellator, as well as two small openings for insufflation and for smoke exhaustion.
Surgery may be performed using this single-port or a multiport laparoscopic or robotic approach. For morcellation, the approach converts to a single-site technique that involves only one entry point for all instruments and no perforation of the bag.
At the beginning of the procedure (or at the end of the case if preferred), a 25-mm incision is made in the umbilicus and the system’s port is inserted and trimmed. The port cap is placed, the abdomen is insufflated, and the laparoscope is inserted. If placed at the beginning of the case, this port can be used as a camera or accessory port.
Before deployment of the PneumoLiner, the uterus or target tissue is placed out of the way; I recommend the upper right quadrant. The PneumoLiner is then inserted with its directional tab pointing upward, and the system’s plunger is depressed while the sleeve is pulled back. In essence, the PneumoLiner is advanced while the sleeve is simultaneously withdrawn, laying it flat in the pelvis.
With an atraumatic grasper, the uterus is placed within the opening of the bag, and the bag is grasped at the collar and elevated up and around the specimen. When full containment of the specimen is visualized, the retrieval lanyard is withdrawn until an opening ring partially protrudes outside the port. All lateral trocars must have been withdrawn prior to inflation of the bag to prevent it from being damaged.
At this point, the port cap is removed and the PneumoLiner neck is withdrawn until a black grid pattern on the bag is visible. The surgeon should then ensure there are no twists in the bag before replacing the port cap and insufflating the bag to a pressure of 15 mm Hg.*
The bag must be correctly in place and fully insufflated before the laparoscope is inserted. The laparoscope must be inserted prior to the morcellator. When the morcellator is inserted, care must be taken to ensure that the morcellator probe is in place.
Once the morcellator is placed, the probe is withdrawn and a closed tenaculum is placed. With the closed tenaculum, the surgeon can manipulate tissue and gauge depth and bearings without inadvertently grabbing the bag. The black grid pattern on the bag assists with estimation of tissue fragment size; morcellation proceeds under direct vision until the tissue fragments are smaller than four printed grids.
Instrumentation is removed in a set order, with the morcellator first and the laparoscope last. The port cap is detached and the PneumoLiner is removed while allowing fumes to escape. The morcellator, camera, tenaculum, and port cap are considered contaminated at this point and should not re-enter the field.
Pearls for morcellation
- The single-site nature of the procedure can sometimes be challenging. If you’ve placed your laparoscope and are having difficulty locating the morcellator, bring your laparoscope and morcellator shaft in parallel to each other, and you’ll be able to better orient yourself.
- To enlarge your field of view after you’ve inflated the PneumoLiner and captured the tissue within the bag, level the patient a bit and move the tissue further away from the laparoscope.
- If the morcellator tube is limiting visualization of the tenaculum tip, slide the morcellator back while leaving the tenaculum in a fixed position.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Courtesy Dr. Tony Shibley and Olympus
Dr. Shibley is an ob.gyn. in private practice in the Minneapolis area. He receives royalties from Advanced Surgical Concepts and serves as a consultant for Olympus.
*Correction 3/8/17: An earlier version of this article misstated the name of the Pneumoliner device in a photo caption. The pressure of the morcellation bag also was misstated.
Postoperative pain in women with preexisting chronic pain
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.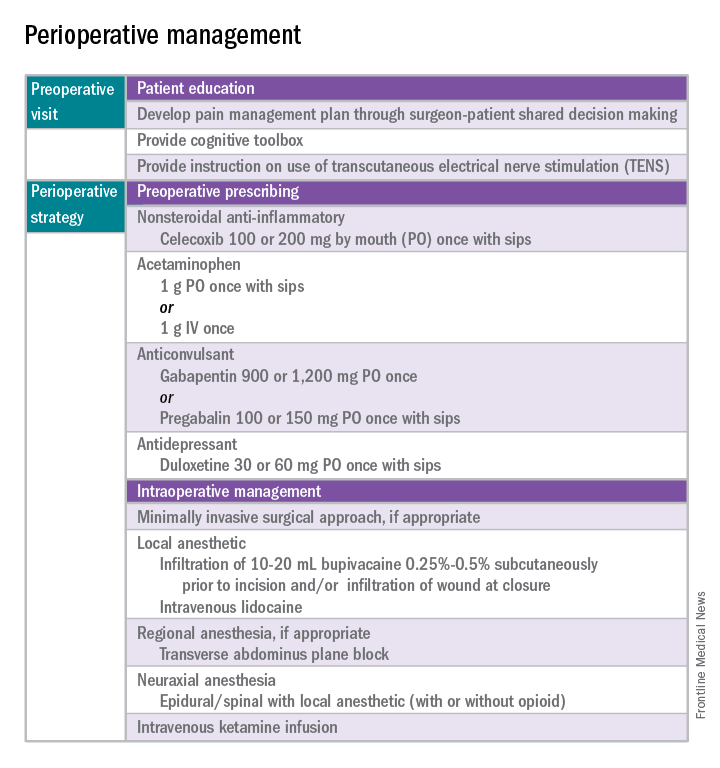
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 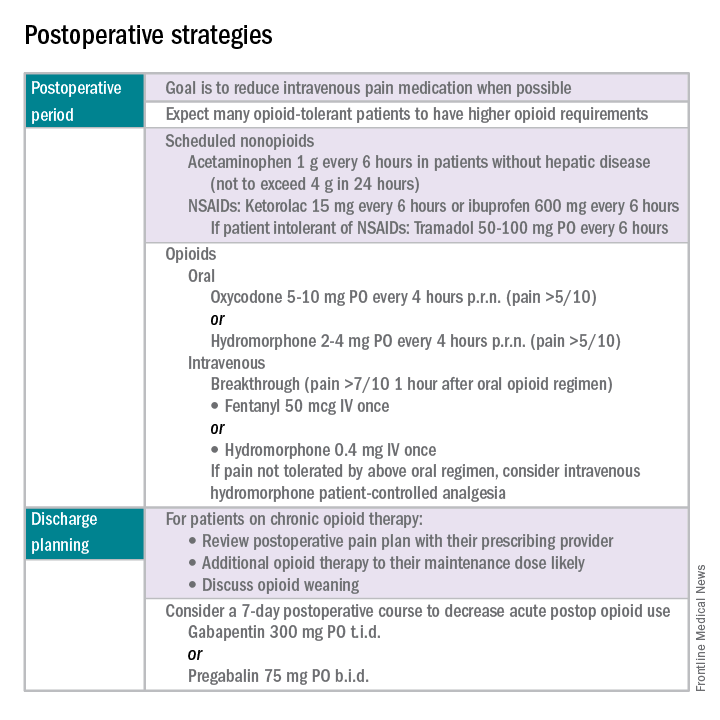
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Point/Counterpoint: Is endograft PAA repair durable?
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Primary prophylaxis of bleeding in portal hypertension safe in cirrhosis with high-risk varices
Primary prophylaxis of bleeding in children with portal hypertension is safe for treatment of cirrhosis associated with high-risk varices, according to Mathieu Duché, MD, of Hôpital Bicêtre in France, and his associates.
In a study from July 1989 to June 2014, researchers examined 1,300 children with various causes of liver disease, based on the presence of palpable splenomegaly and/or ultrasonographic signs of portal hypertension. During the study, a high-risk pattern – including grade 3 esophageal varices, grade 2 varices with red markings and/or gastric varices along the cardia, or gastric varices with or without esophageal varices – was present in 96% of the 246 children who bled spontaneously and in 11% of the 872 children who did not bleed. Of the 246 children who bled spontaneously, 170 children with high-risk varices underwent primary prophylaxis of bleeding with portal surgery, endoscopic banding/sclerotherapy of varices, or interventional radiology.
In 50 children with noncirrhotic causes of portal hypertension and the high risk varices, those with portal vein obstruction underwent portal surgery (Rex bypass or portosystemic shunt) as primary prophylaxis. Endoscopic banding or sclerotherapy was performed instead in younger children or when angiograms did not favor the surgery. One child who had a severe stenosis of the portal trunk underwent successful interventional radiology. After a mean follow-up of 5.5 years after primary prophylaxis, all these patients are alive.
Of the 120 children with cirrhosis with high risk varices, 10 children with well compensated cirrhosis received a portosystemic shunt; thrombosis of the shunt occurred in 1 child who later underwent liver transplantation, and 1 child with a patent shunt developed portosystemic encephalopathy and underwent liver transplantation. No gastrointestinal bleeding was recorded in these 10 children who were alive at the last follow-up.
Of 110 children who underwent endoscopic banding or sclerotherapy, in 76 children there was eradication of varices; there was a relapse of high-risk varices in 20 children, so further endoscopic was needed. In two children who initially underwent endoscopic treatment and achieved eradication of varices, a protein-losing enteropathy and bleeding from ectopic varices, respectively, required subsequent treatment by a surgical portosystemic shunt. Of the 34 children in whom no eradication was obtained with this primary prophylaxis, 3 died and 29 underwent liver transplantation before eradication could be obtained; 1 was lost to follow-up and 1 received a transjugular intrahepatic portosystemic shunt. After a mean follow-up of 5 years, 8 of the 120 children with cirrhosis who underwent primary prophylaxis have died: 4 children died before transplantation, 2 of septic shock unrelated to endoscopic treatment, and 1 of atrioventricular block during variceal injection of aethoxysklerol. Four other children died after transplantation.
It was noted that no esophageal or gastric perforation was observed as a consequence of endoscopic primary prophylaxis in the pre- or posttransplantation period.
“Although no statistical analysis was performed because this was not a controlled study, the results suggest that, compared with liver transplantation performed directly or with care after a spontaneous bleed, primary prophylaxis of bleeding has a fairly good safety record for the management of children with cirrhosis and high-risk varices,” researchers concluded.
Read the full study in the Journal of Hepatology (doi: 10.1016/j.jhep.2016.09.006).
Primary prophylaxis of bleeding in children with portal hypertension is safe for treatment of cirrhosis associated with high-risk varices, according to Mathieu Duché, MD, of Hôpital Bicêtre in France, and his associates.
In a study from July 1989 to June 2014, researchers examined 1,300 children with various causes of liver disease, based on the presence of palpable splenomegaly and/or ultrasonographic signs of portal hypertension. During the study, a high-risk pattern – including grade 3 esophageal varices, grade 2 varices with red markings and/or gastric varices along the cardia, or gastric varices with or without esophageal varices – was present in 96% of the 246 children who bled spontaneously and in 11% of the 872 children who did not bleed. Of the 246 children who bled spontaneously, 170 children with high-risk varices underwent primary prophylaxis of bleeding with portal surgery, endoscopic banding/sclerotherapy of varices, or interventional radiology.
In 50 children with noncirrhotic causes of portal hypertension and the high risk varices, those with portal vein obstruction underwent portal surgery (Rex bypass or portosystemic shunt) as primary prophylaxis. Endoscopic banding or sclerotherapy was performed instead in younger children or when angiograms did not favor the surgery. One child who had a severe stenosis of the portal trunk underwent successful interventional radiology. After a mean follow-up of 5.5 years after primary prophylaxis, all these patients are alive.
Of the 120 children with cirrhosis with high risk varices, 10 children with well compensated cirrhosis received a portosystemic shunt; thrombosis of the shunt occurred in 1 child who later underwent liver transplantation, and 1 child with a patent shunt developed portosystemic encephalopathy and underwent liver transplantation. No gastrointestinal bleeding was recorded in these 10 children who were alive at the last follow-up.
Of 110 children who underwent endoscopic banding or sclerotherapy, in 76 children there was eradication of varices; there was a relapse of high-risk varices in 20 children, so further endoscopic was needed. In two children who initially underwent endoscopic treatment and achieved eradication of varices, a protein-losing enteropathy and bleeding from ectopic varices, respectively, required subsequent treatment by a surgical portosystemic shunt. Of the 34 children in whom no eradication was obtained with this primary prophylaxis, 3 died and 29 underwent liver transplantation before eradication could be obtained; 1 was lost to follow-up and 1 received a transjugular intrahepatic portosystemic shunt. After a mean follow-up of 5 years, 8 of the 120 children with cirrhosis who underwent primary prophylaxis have died: 4 children died before transplantation, 2 of septic shock unrelated to endoscopic treatment, and 1 of atrioventricular block during variceal injection of aethoxysklerol. Four other children died after transplantation.
It was noted that no esophageal or gastric perforation was observed as a consequence of endoscopic primary prophylaxis in the pre- or posttransplantation period.
“Although no statistical analysis was performed because this was not a controlled study, the results suggest that, compared with liver transplantation performed directly or with care after a spontaneous bleed, primary prophylaxis of bleeding has a fairly good safety record for the management of children with cirrhosis and high-risk varices,” researchers concluded.
Read the full study in the Journal of Hepatology (doi: 10.1016/j.jhep.2016.09.006).
Primary prophylaxis of bleeding in children with portal hypertension is safe for treatment of cirrhosis associated with high-risk varices, according to Mathieu Duché, MD, of Hôpital Bicêtre in France, and his associates.
In a study from July 1989 to June 2014, researchers examined 1,300 children with various causes of liver disease, based on the presence of palpable splenomegaly and/or ultrasonographic signs of portal hypertension. During the study, a high-risk pattern – including grade 3 esophageal varices, grade 2 varices with red markings and/or gastric varices along the cardia, or gastric varices with or without esophageal varices – was present in 96% of the 246 children who bled spontaneously and in 11% of the 872 children who did not bleed. Of the 246 children who bled spontaneously, 170 children with high-risk varices underwent primary prophylaxis of bleeding with portal surgery, endoscopic banding/sclerotherapy of varices, or interventional radiology.
In 50 children with noncirrhotic causes of portal hypertension and the high risk varices, those with portal vein obstruction underwent portal surgery (Rex bypass or portosystemic shunt) as primary prophylaxis. Endoscopic banding or sclerotherapy was performed instead in younger children or when angiograms did not favor the surgery. One child who had a severe stenosis of the portal trunk underwent successful interventional radiology. After a mean follow-up of 5.5 years after primary prophylaxis, all these patients are alive.
Of the 120 children with cirrhosis with high risk varices, 10 children with well compensated cirrhosis received a portosystemic shunt; thrombosis of the shunt occurred in 1 child who later underwent liver transplantation, and 1 child with a patent shunt developed portosystemic encephalopathy and underwent liver transplantation. No gastrointestinal bleeding was recorded in these 10 children who were alive at the last follow-up.
Of 110 children who underwent endoscopic banding or sclerotherapy, in 76 children there was eradication of varices; there was a relapse of high-risk varices in 20 children, so further endoscopic was needed. In two children who initially underwent endoscopic treatment and achieved eradication of varices, a protein-losing enteropathy and bleeding from ectopic varices, respectively, required subsequent treatment by a surgical portosystemic shunt. Of the 34 children in whom no eradication was obtained with this primary prophylaxis, 3 died and 29 underwent liver transplantation before eradication could be obtained; 1 was lost to follow-up and 1 received a transjugular intrahepatic portosystemic shunt. After a mean follow-up of 5 years, 8 of the 120 children with cirrhosis who underwent primary prophylaxis have died: 4 children died before transplantation, 2 of septic shock unrelated to endoscopic treatment, and 1 of atrioventricular block during variceal injection of aethoxysklerol. Four other children died after transplantation.
It was noted that no esophageal or gastric perforation was observed as a consequence of endoscopic primary prophylaxis in the pre- or posttransplantation period.
“Although no statistical analysis was performed because this was not a controlled study, the results suggest that, compared with liver transplantation performed directly or with care after a spontaneous bleed, primary prophylaxis of bleeding has a fairly good safety record for the management of children with cirrhosis and high-risk varices,” researchers concluded.
Read the full study in the Journal of Hepatology (doi: 10.1016/j.jhep.2016.09.006).
FROM JOURNAL OF HEPATOLOGY
Aortic repair in Loeys-Dietz syndrome requires close follow-up
The knowledge about Loeys-Dietz syndrome has evolved quickly since Hal Dietz, MD, and Bart Loeys, MD, at Johns Hopkins University, Baltimore, first reported on it in 2005. Now, another team of Johns Hopkins investigators have reported that an aggressive approach with aortic root replacement coupled with valve-sparing whenever possible produces favorable results, but that clinicians must follow these patients closely with cardiovascular imaging.
“Growing experience with Loeys-Dietz syndrome has confirmed early impressions of its aggressive nature and proclivity toward aortic catastrophe,” Nishant D. Patel, MD, and his coauthors said in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:406-12). They reported on results of all 79 patients with Loeys-Dietz syndrome (LDS) who had cardiovascular surgery at Johns Hopkins. There were two (3%) deaths during surgery and eight (10%) late deaths.
Patients with LDS are at risk for dissection early when the aortic root reaches 4 cm. Despite what they termed “favorable” outcomes of surgery, Dr. Patel and his coauthors acknowledged that reintervention rates for this population are high – 19 patients (24%) had subsequent operations. That suggests cardiac surgeons must closely monitor these patients. “Meticulous follow-up with cardiovascular surveillance imaging remains important for management, particularly as clinical LDS subtypes are characterized and more tailored treatment is developed,” Dr. Patel and his coauthors reported.
They advise echocardiography every 3 to 6 months for the first year after surgery and then every 6 to 12 months afterward. Full-body imaging should occur at least every 2 years.
“In particular, patients with type B dissections should be monitored aggressively for aneurysm growth,” Dr. Patel and his coauthors said. They recommend imaging at seven to 14 days after dissection, then repeat imaging at 1, 3, 6, and 12 months, and then yearly thereafter.
They noted that four LDS subtypes have been identified. Although those with LDS1 and 2 subtypes are prone to aortic rupture at an earlier age and at smaller aortic diameters than other connective tissue disorders, the medical and surgical management for all subtypes are similar, Dr. Patel and his coauthors indicated.
“Certain congenital heart defects are more common among patients with LDS, compared with the normal population, including patent ductus arteriosus and mitral valve prolapse/insufficiency,” they said. Genotype is one factor that determines the need for surgery in LDS patients, Dr. Patel and his coauthors said. Others are growth rate, aortic valve function, family history, and severity of noncardiac phenotype.
The 79 patients in the study were divided almost evenly between gender, and the average age at first operation was 24.9 years; 38 were children younger than 18 years and 20 had a previous sternotomy.
Aortic root replacement represented the predominant operation in the group, accounting for 65 operations (82.3%), of which 52 (80%) were valve-sparing procedures and the remainder were composite valve-graft procedures. The other procedures the researchers performed were nine aortic arch replacements (11.4%), three open thoracoabdominal repairs (3.8%) and two ascending aorta replacements (2.5%).
“Valve-sparing root replacement has become a safe and reliable option for appropriately selected younger patients with LDS,” Dr. Patel and his coauthors wrote. Five patients needed a second operation on the aortic valve or root; three of them had a Florida sleeve procedure. “Based on these initial outcomes with the Florida sleeve at our institution, we have abandoned this procedure in favor of conventional valve-sparing root replacement,” Dr. Patel and his coauthors stated.
Dr. Patel and his coauthors had no financial relationships to disclose.
This report by Dr. Patel and his coauthors confirms the need for close surveillance of individuals with Loeys-Dietz syndrome who have had aortic operations, John S. Ikonomidis, MD, PhD, of the Medical University of South Carolina, Charleston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:413-4).
Dr. Ikonomidis noted this study is important because of its population size. “This is probably the largest single-center surgical report of this kind in the world,” he said.
The study highlighted a number of issues germane to LDS patients who have cardiovascular surgery, among them a critical need for genetic testing to help cardiac surgeons determine the disease genotype and what operation to perform, Dr. Ikonomidis said.
But Dr. Ikonomidis also pointed out the variation in aortic root size in the study patients. The smallest root in the series was 2 cm and 21 of 65 patients with a maximum root diameter smaller than 4 cm had root surgery. “This is a testament to the fact that surgical decision making in this population is dependent not just on the known genotype and aortic dimensions, but also on the rate of growth, aortic valve function, severity of noncardiac phenotype, and family history,” Dr. Ikonomidis said.
Dr. Ikonomidis had no financial relationships to disclose.
This report by Dr. Patel and his coauthors confirms the need for close surveillance of individuals with Loeys-Dietz syndrome who have had aortic operations, John S. Ikonomidis, MD, PhD, of the Medical University of South Carolina, Charleston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:413-4).
Dr. Ikonomidis noted this study is important because of its population size. “This is probably the largest single-center surgical report of this kind in the world,” he said.
The study highlighted a number of issues germane to LDS patients who have cardiovascular surgery, among them a critical need for genetic testing to help cardiac surgeons determine the disease genotype and what operation to perform, Dr. Ikonomidis said.
But Dr. Ikonomidis also pointed out the variation in aortic root size in the study patients. The smallest root in the series was 2 cm and 21 of 65 patients with a maximum root diameter smaller than 4 cm had root surgery. “This is a testament to the fact that surgical decision making in this population is dependent not just on the known genotype and aortic dimensions, but also on the rate of growth, aortic valve function, severity of noncardiac phenotype, and family history,” Dr. Ikonomidis said.
Dr. Ikonomidis had no financial relationships to disclose.
This report by Dr. Patel and his coauthors confirms the need for close surveillance of individuals with Loeys-Dietz syndrome who have had aortic operations, John S. Ikonomidis, MD, PhD, of the Medical University of South Carolina, Charleston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:413-4).
Dr. Ikonomidis noted this study is important because of its population size. “This is probably the largest single-center surgical report of this kind in the world,” he said.
The study highlighted a number of issues germane to LDS patients who have cardiovascular surgery, among them a critical need for genetic testing to help cardiac surgeons determine the disease genotype and what operation to perform, Dr. Ikonomidis said.
But Dr. Ikonomidis also pointed out the variation in aortic root size in the study patients. The smallest root in the series was 2 cm and 21 of 65 patients with a maximum root diameter smaller than 4 cm had root surgery. “This is a testament to the fact that surgical decision making in this population is dependent not just on the known genotype and aortic dimensions, but also on the rate of growth, aortic valve function, severity of noncardiac phenotype, and family history,” Dr. Ikonomidis said.
Dr. Ikonomidis had no financial relationships to disclose.
The knowledge about Loeys-Dietz syndrome has evolved quickly since Hal Dietz, MD, and Bart Loeys, MD, at Johns Hopkins University, Baltimore, first reported on it in 2005. Now, another team of Johns Hopkins investigators have reported that an aggressive approach with aortic root replacement coupled with valve-sparing whenever possible produces favorable results, but that clinicians must follow these patients closely with cardiovascular imaging.
“Growing experience with Loeys-Dietz syndrome has confirmed early impressions of its aggressive nature and proclivity toward aortic catastrophe,” Nishant D. Patel, MD, and his coauthors said in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:406-12). They reported on results of all 79 patients with Loeys-Dietz syndrome (LDS) who had cardiovascular surgery at Johns Hopkins. There were two (3%) deaths during surgery and eight (10%) late deaths.
Patients with LDS are at risk for dissection early when the aortic root reaches 4 cm. Despite what they termed “favorable” outcomes of surgery, Dr. Patel and his coauthors acknowledged that reintervention rates for this population are high – 19 patients (24%) had subsequent operations. That suggests cardiac surgeons must closely monitor these patients. “Meticulous follow-up with cardiovascular surveillance imaging remains important for management, particularly as clinical LDS subtypes are characterized and more tailored treatment is developed,” Dr. Patel and his coauthors reported.
They advise echocardiography every 3 to 6 months for the first year after surgery and then every 6 to 12 months afterward. Full-body imaging should occur at least every 2 years.
“In particular, patients with type B dissections should be monitored aggressively for aneurysm growth,” Dr. Patel and his coauthors said. They recommend imaging at seven to 14 days after dissection, then repeat imaging at 1, 3, 6, and 12 months, and then yearly thereafter.
They noted that four LDS subtypes have been identified. Although those with LDS1 and 2 subtypes are prone to aortic rupture at an earlier age and at smaller aortic diameters than other connective tissue disorders, the medical and surgical management for all subtypes are similar, Dr. Patel and his coauthors indicated.
“Certain congenital heart defects are more common among patients with LDS, compared with the normal population, including patent ductus arteriosus and mitral valve prolapse/insufficiency,” they said. Genotype is one factor that determines the need for surgery in LDS patients, Dr. Patel and his coauthors said. Others are growth rate, aortic valve function, family history, and severity of noncardiac phenotype.
The 79 patients in the study were divided almost evenly between gender, and the average age at first operation was 24.9 years; 38 were children younger than 18 years and 20 had a previous sternotomy.
Aortic root replacement represented the predominant operation in the group, accounting for 65 operations (82.3%), of which 52 (80%) were valve-sparing procedures and the remainder were composite valve-graft procedures. The other procedures the researchers performed were nine aortic arch replacements (11.4%), three open thoracoabdominal repairs (3.8%) and two ascending aorta replacements (2.5%).
“Valve-sparing root replacement has become a safe and reliable option for appropriately selected younger patients with LDS,” Dr. Patel and his coauthors wrote. Five patients needed a second operation on the aortic valve or root; three of them had a Florida sleeve procedure. “Based on these initial outcomes with the Florida sleeve at our institution, we have abandoned this procedure in favor of conventional valve-sparing root replacement,” Dr. Patel and his coauthors stated.
Dr. Patel and his coauthors had no financial relationships to disclose.
The knowledge about Loeys-Dietz syndrome has evolved quickly since Hal Dietz, MD, and Bart Loeys, MD, at Johns Hopkins University, Baltimore, first reported on it in 2005. Now, another team of Johns Hopkins investigators have reported that an aggressive approach with aortic root replacement coupled with valve-sparing whenever possible produces favorable results, but that clinicians must follow these patients closely with cardiovascular imaging.
“Growing experience with Loeys-Dietz syndrome has confirmed early impressions of its aggressive nature and proclivity toward aortic catastrophe,” Nishant D. Patel, MD, and his coauthors said in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:406-12). They reported on results of all 79 patients with Loeys-Dietz syndrome (LDS) who had cardiovascular surgery at Johns Hopkins. There were two (3%) deaths during surgery and eight (10%) late deaths.
Patients with LDS are at risk for dissection early when the aortic root reaches 4 cm. Despite what they termed “favorable” outcomes of surgery, Dr. Patel and his coauthors acknowledged that reintervention rates for this population are high – 19 patients (24%) had subsequent operations. That suggests cardiac surgeons must closely monitor these patients. “Meticulous follow-up with cardiovascular surveillance imaging remains important for management, particularly as clinical LDS subtypes are characterized and more tailored treatment is developed,” Dr. Patel and his coauthors reported.
They advise echocardiography every 3 to 6 months for the first year after surgery and then every 6 to 12 months afterward. Full-body imaging should occur at least every 2 years.
“In particular, patients with type B dissections should be monitored aggressively for aneurysm growth,” Dr. Patel and his coauthors said. They recommend imaging at seven to 14 days after dissection, then repeat imaging at 1, 3, 6, and 12 months, and then yearly thereafter.
They noted that four LDS subtypes have been identified. Although those with LDS1 and 2 subtypes are prone to aortic rupture at an earlier age and at smaller aortic diameters than other connective tissue disorders, the medical and surgical management for all subtypes are similar, Dr. Patel and his coauthors indicated.
“Certain congenital heart defects are more common among patients with LDS, compared with the normal population, including patent ductus arteriosus and mitral valve prolapse/insufficiency,” they said. Genotype is one factor that determines the need for surgery in LDS patients, Dr. Patel and his coauthors said. Others are growth rate, aortic valve function, family history, and severity of noncardiac phenotype.
The 79 patients in the study were divided almost evenly between gender, and the average age at first operation was 24.9 years; 38 were children younger than 18 years and 20 had a previous sternotomy.
Aortic root replacement represented the predominant operation in the group, accounting for 65 operations (82.3%), of which 52 (80%) were valve-sparing procedures and the remainder were composite valve-graft procedures. The other procedures the researchers performed were nine aortic arch replacements (11.4%), three open thoracoabdominal repairs (3.8%) and two ascending aorta replacements (2.5%).
“Valve-sparing root replacement has become a safe and reliable option for appropriately selected younger patients with LDS,” Dr. Patel and his coauthors wrote. Five patients needed a second operation on the aortic valve or root; three of them had a Florida sleeve procedure. “Based on these initial outcomes with the Florida sleeve at our institution, we have abandoned this procedure in favor of conventional valve-sparing root replacement,” Dr. Patel and his coauthors stated.
Dr. Patel and his coauthors had no financial relationships to disclose.
Key clinical point: Outcomes for aortic surgery in Loeys-Dietz syndrome are favorable, but reintervention rates are high.
Major finding: Patients require close postoperative follow-up with cardiovascular imaging.
Data source: Retrospective review of 79 patients who had cardiovascular surgery for LDS over 26 years at Johns Hopkins University.
Disclosure: Dr. Patel and his coauthors reported having no relevant financial disclosures.
Novel classification of labial anatomy and evaluation in the treatment of labial agglutination

References
Pardo J, Sola V, Ricci P, Guillof E. Laser labioplasty of labia minora. Int J Gynaecol Obstet. 2006;93(1):38–43.
Chang P, Salisbury MA, Narsete T, Buckspan R, Derrick D, Ersek RA. Vaginal labiaplasty: defense of the simple "clip and snip" and a new classification system. Aesthetic Plast Surg. 2013;37(5): p. 887–891.
Malone DG, Clark TB, Wei N. Ultrasound-guided percutaneous injection, hydrodissection, and fenestration for carpel tunnel syndrome description of a new technique. J Appl Res. 2010;10(3):116–123.
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

References
Pardo J, Sola V, Ricci P, Guillof E. Laser labioplasty of labia minora. Int J Gynaecol Obstet. 2006;93(1):38–43.
Chang P, Salisbury MA, Narsete T, Buckspan R, Derrick D, Ersek RA. Vaginal labiaplasty: defense of the simple "clip and snip" and a new classification system. Aesthetic Plast Surg. 2013;37(5): p. 887–891.
Malone DG, Clark TB, Wei N. Ultrasound-guided percutaneous injection, hydrodissection, and fenestration for carpel tunnel syndrome description of a new technique. J Appl Res. 2010;10(3):116–123.
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

References
Pardo J, Sola V, Ricci P, Guillof E. Laser labioplasty of labia minora. Int J Gynaecol Obstet. 2006;93(1):38–43.
Chang P, Salisbury MA, Narsete T, Buckspan R, Derrick D, Ersek RA. Vaginal labiaplasty: defense of the simple "clip and snip" and a new classification system. Aesthetic Plast Surg. 2013;37(5): p. 887–891.
Malone DG, Clark TB, Wei N. Ultrasound-guided percutaneous injection, hydrodissection, and fenestration for carpel tunnel syndrome description of a new technique. J Appl Res. 2010;10(3):116–123.
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by
Nicardipine okay to use after pediatric cardiac surgery
HOUSTON – The use of nicardipine following cardiac surgery in children appears to be safe and effective, results from a single-center study suggest.
“There has been a traditional hesitation to use calcium channel blockers, particularly in infants, due to underdevelopment of their calcium channels,” study investigator Matthew L. Stone, MD, PhD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
“Further, these agents have commonly lacked selectivity to the vascular smooth muscles affecting both the blood vessels and the heart. Nicardipine offers a unique advantage over other calcium channel blockers in that it has more direct effects on vascular smooth muscles than it does on the actual myocardium.”
In their study, Dr. Stone, a first-year fellow in the division of cardiothoracic surgery at the University of Virginia Health System, Charlottesville, and his associates noted that nicardipine offers a favorable pharmacokinetic profile with both rapid onset and short half-life. The purpose of the study was to evaluate the use of nicardipine as a first-line agent for treatment of postoperative hypertension and to compare outcomes between children younger than 6 months of age and those older than 6 months. The researchers retrospectively reviewed the medical records of 68 children who received nicardipine for postoperative hypertension after undergoing cardiac surgery at the University of Virginia during 2010-2015. They compared the incidence of adverse postoperative events between 33 children who were younger than 6 months (group 1) and 35 who were older than 6 months (group 2). Major events including stroke or cardiogenic shock were considered failure of therapy.
Dr. Stone and his associates found that all children received nicardipine within a median of 90 minutes following cardiac surgery; 22 (33%) were started on the drug prior to leaving the operating room and most required dosing for less than 24 hours. Clinically significant hypertension that required dose titration or cessation of therapy occurred in 13% of patients, but there were no significant differences between age groups (17% in group 1 vs. 9% in group 2; P = 0.47). “While the incidence of hypotension following nicardipine administration did not reach statistical significance, it’s important to note that going forward, a lower starting dose in infants less than 6 months of age may be most appropriate. This would certainly be an important focus for future prospective study in the development of postoperative blood pressure control protocols,” said Dr. Stone.
No significant adverse events including stroke or cardiogenic shock occurred in either group. In addition, no operative or postoperative factors reviewed were associated with the development of complications during administration of nicardipine. This included cardiopulmonary bypass time, cross-clamp time, ventilator time, nicardipine duration, ICU length of stay, and hospital length of stay.
“Our traditional hesitation to use this class of agents in infants should be reevaluated,” Dr. Stone concluded. “As we move toward standardization and optimization of perioperative care, our study supports the use and prospective clinical study of nicardipine. Additionally, further pharmacologic study of dose-specific responses within myocardial and vascular smooth muscle cells may further optimize this treatment strategy and provide a more reliable standard with which to control blood pressure.
“Our traditional agents such as beta-blockers and nitroprusside have side effects that need to be considered, the most significant of which being myocardial depression and cyanide toxicity. In a limited number of very-high-risk children, we’ve shown that nicardipine may provide an option with less deleterious side effects. It’s a foundation for future study.”
Dr. Stone reported having no financial disclosures.
HOUSTON – The use of nicardipine following cardiac surgery in children appears to be safe and effective, results from a single-center study suggest.
“There has been a traditional hesitation to use calcium channel blockers, particularly in infants, due to underdevelopment of their calcium channels,” study investigator Matthew L. Stone, MD, PhD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
“Further, these agents have commonly lacked selectivity to the vascular smooth muscles affecting both the blood vessels and the heart. Nicardipine offers a unique advantage over other calcium channel blockers in that it has more direct effects on vascular smooth muscles than it does on the actual myocardium.”
In their study, Dr. Stone, a first-year fellow in the division of cardiothoracic surgery at the University of Virginia Health System, Charlottesville, and his associates noted that nicardipine offers a favorable pharmacokinetic profile with both rapid onset and short half-life. The purpose of the study was to evaluate the use of nicardipine as a first-line agent for treatment of postoperative hypertension and to compare outcomes between children younger than 6 months of age and those older than 6 months. The researchers retrospectively reviewed the medical records of 68 children who received nicardipine for postoperative hypertension after undergoing cardiac surgery at the University of Virginia during 2010-2015. They compared the incidence of adverse postoperative events between 33 children who were younger than 6 months (group 1) and 35 who were older than 6 months (group 2). Major events including stroke or cardiogenic shock were considered failure of therapy.
Dr. Stone and his associates found that all children received nicardipine within a median of 90 minutes following cardiac surgery; 22 (33%) were started on the drug prior to leaving the operating room and most required dosing for less than 24 hours. Clinically significant hypertension that required dose titration or cessation of therapy occurred in 13% of patients, but there were no significant differences between age groups (17% in group 1 vs. 9% in group 2; P = 0.47). “While the incidence of hypotension following nicardipine administration did not reach statistical significance, it’s important to note that going forward, a lower starting dose in infants less than 6 months of age may be most appropriate. This would certainly be an important focus for future prospective study in the development of postoperative blood pressure control protocols,” said Dr. Stone.
No significant adverse events including stroke or cardiogenic shock occurred in either group. In addition, no operative or postoperative factors reviewed were associated with the development of complications during administration of nicardipine. This included cardiopulmonary bypass time, cross-clamp time, ventilator time, nicardipine duration, ICU length of stay, and hospital length of stay.
“Our traditional hesitation to use this class of agents in infants should be reevaluated,” Dr. Stone concluded. “As we move toward standardization and optimization of perioperative care, our study supports the use and prospective clinical study of nicardipine. Additionally, further pharmacologic study of dose-specific responses within myocardial and vascular smooth muscle cells may further optimize this treatment strategy and provide a more reliable standard with which to control blood pressure.
“Our traditional agents such as beta-blockers and nitroprusside have side effects that need to be considered, the most significant of which being myocardial depression and cyanide toxicity. In a limited number of very-high-risk children, we’ve shown that nicardipine may provide an option with less deleterious side effects. It’s a foundation for future study.”
Dr. Stone reported having no financial disclosures.
HOUSTON – The use of nicardipine following cardiac surgery in children appears to be safe and effective, results from a single-center study suggest.
“There has been a traditional hesitation to use calcium channel blockers, particularly in infants, due to underdevelopment of their calcium channels,” study investigator Matthew L. Stone, MD, PhD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
“Further, these agents have commonly lacked selectivity to the vascular smooth muscles affecting both the blood vessels and the heart. Nicardipine offers a unique advantage over other calcium channel blockers in that it has more direct effects on vascular smooth muscles than it does on the actual myocardium.”
In their study, Dr. Stone, a first-year fellow in the division of cardiothoracic surgery at the University of Virginia Health System, Charlottesville, and his associates noted that nicardipine offers a favorable pharmacokinetic profile with both rapid onset and short half-life. The purpose of the study was to evaluate the use of nicardipine as a first-line agent for treatment of postoperative hypertension and to compare outcomes between children younger than 6 months of age and those older than 6 months. The researchers retrospectively reviewed the medical records of 68 children who received nicardipine for postoperative hypertension after undergoing cardiac surgery at the University of Virginia during 2010-2015. They compared the incidence of adverse postoperative events between 33 children who were younger than 6 months (group 1) and 35 who were older than 6 months (group 2). Major events including stroke or cardiogenic shock were considered failure of therapy.
Dr. Stone and his associates found that all children received nicardipine within a median of 90 minutes following cardiac surgery; 22 (33%) were started on the drug prior to leaving the operating room and most required dosing for less than 24 hours. Clinically significant hypertension that required dose titration or cessation of therapy occurred in 13% of patients, but there were no significant differences between age groups (17% in group 1 vs. 9% in group 2; P = 0.47). “While the incidence of hypotension following nicardipine administration did not reach statistical significance, it’s important to note that going forward, a lower starting dose in infants less than 6 months of age may be most appropriate. This would certainly be an important focus for future prospective study in the development of postoperative blood pressure control protocols,” said Dr. Stone.
No significant adverse events including stroke or cardiogenic shock occurred in either group. In addition, no operative or postoperative factors reviewed were associated with the development of complications during administration of nicardipine. This included cardiopulmonary bypass time, cross-clamp time, ventilator time, nicardipine duration, ICU length of stay, and hospital length of stay.
“Our traditional hesitation to use this class of agents in infants should be reevaluated,” Dr. Stone concluded. “As we move toward standardization and optimization of perioperative care, our study supports the use and prospective clinical study of nicardipine. Additionally, further pharmacologic study of dose-specific responses within myocardial and vascular smooth muscle cells may further optimize this treatment strategy and provide a more reliable standard with which to control blood pressure.
“Our traditional agents such as beta-blockers and nitroprusside have side effects that need to be considered, the most significant of which being myocardial depression and cyanide toxicity. In a limited number of very-high-risk children, we’ve shown that nicardipine may provide an option with less deleterious side effects. It’s a foundation for future study.”
Dr. Stone reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point:
Major finding: The incidence of adverse postoperative events was similar between children who were younger than 6 months and those who were older than 6 months (17% vs. 9%, respectively), but no significant adverse events, including stroke and cardiogenic shock, occurred in either group.
Data source: A retrospective review of 68 children who received nicardipine for postoperative hypertension after undergoing cardiac surgery during 2010-2015.
Disclosures: Dr. Stone reported having no financial disclosures.
Liver transplantation largely effective in critically ill children
The use of advanced critical care in children and infants with liver failure is justified because orthotopic liver transplantation can be performed on the sickest children and achieve acceptable outcomes, results from a large analysis demonstrated.
“Hand in hand with improved care for critically ill children with liver failure, posttransplant critical care has made tremendous strides,” Abbas Rana, MD, wrote in a study published online in the Journal of the American College of Surgeons. “Our recipients have gotten sicker while our postoperative outcomes have improved. The question then becomes, have our operative skills and postoperative critical care management kept up with the abilities to keep sick children with liver failure alive? Just because transplantation is now possible in our sickest children, is it justified?”
To find out, Dr. Rana of the division of abdominal transplantation and hepatobiliary surgery at Baylor College of Medicine, Houston, and colleagues retrospectively analyzed United Network for Organ Sharing data from all orthotopic liver transplantation (OLT) recipients between Sept. 1, 1987, and June 30, 2015. The analysis paired the liver registry data with data collected by the Organ Procurement and Transplantation Network, and was limited to transplant recipients younger than age 18. The researchers followed a total of 13,723 recipients from date of transplant until either death or the date of last known follow-up (J Am Coll Surg. 2016 Dec 25. doi: 10.1016/j.jamcollsurg.2016.12.025).
In another part of the study, the researchers retrospectively reviewed the charts of 354 patients under 18 years of age who underwent OLT between March 1, 2002, and June 30, 2015, at Texas Children’s Hospital, including 65 who were admitted to the ICU at the time of transplantation.
In the analysis of national data, the researchers found that the rates of 1-year survival following OLT in children in the ICU improved from 60% in 1987 to 92% in 2013 (P less than .001). The rates of 1-year survival also improved for children on dialysis at the time of transplant (from 50% in 1995 to 95% in 2013; P less than .001) and for those dependent on a mechanical ventilator at the time of transplant (from 49% in 1994 to 94% in 2013; P less than .001). The significant risk factors were two previous transplants (hazard ratio, 4.2), one previous transplant (HR, 2.5), serum sodium greater than 150 mEq/L (HR, 2.0), dialysis or glomerular filtration rate less than 30 mL/min per 1.73 m2 (HR, 2.0), mechanical ventilator dependence (HR, 1.8), body weight under 6 kg (HR, 1.8), encephalopathy (HR, 1.8), and annual center volume of fewer than five cases (HR, 1.7).
In the experience at Texas Children’s Hospital, the researchers observed “preserved and successful patient survival outcomes” in many markers of acuity. For example, the 10-year survival rates for patients dependent on mechanical ventilation and dialysis were 85% and 96%, respectively, and reached 100% for those requiring therapeutic plasma exchange, molescular adsorbent recirculating system (MARS) liver dialysis, and vasopressors.
“Our collective ability to keep sick children alive with liver failure has improved considerably over the years,” the researchers wrote. “Keeping pace, this analysis demonstrates that the posttransplant outcomes have also improved dramatically. The survival outcomes are comparable to the general population, justifying the use of scarce donors. Although we cannot declare in absolute that no child should be left behind, we can demonstrate acceptable outcomes to date and urge the continual revisiting of our concepts of futility.
“We have learned throughout our experience that almost every child with end-stage liver disease and acute liver failure should be offered liver replacement, as long as the vasoactive medication and mechanical support are not maximized prior to the initiation of the OLT procedure. Every effort should be made to transplant our sickest children.”
This study was supported by the Cade R. Alpard Foundation. The researchers reported having no relevant financial disclosures.
The use of advanced critical care in children and infants with liver failure is justified because orthotopic liver transplantation can be performed on the sickest children and achieve acceptable outcomes, results from a large analysis demonstrated.
“Hand in hand with improved care for critically ill children with liver failure, posttransplant critical care has made tremendous strides,” Abbas Rana, MD, wrote in a study published online in the Journal of the American College of Surgeons. “Our recipients have gotten sicker while our postoperative outcomes have improved. The question then becomes, have our operative skills and postoperative critical care management kept up with the abilities to keep sick children with liver failure alive? Just because transplantation is now possible in our sickest children, is it justified?”
To find out, Dr. Rana of the division of abdominal transplantation and hepatobiliary surgery at Baylor College of Medicine, Houston, and colleagues retrospectively analyzed United Network for Organ Sharing data from all orthotopic liver transplantation (OLT) recipients between Sept. 1, 1987, and June 30, 2015. The analysis paired the liver registry data with data collected by the Organ Procurement and Transplantation Network, and was limited to transplant recipients younger than age 18. The researchers followed a total of 13,723 recipients from date of transplant until either death or the date of last known follow-up (J Am Coll Surg. 2016 Dec 25. doi: 10.1016/j.jamcollsurg.2016.12.025).
In another part of the study, the researchers retrospectively reviewed the charts of 354 patients under 18 years of age who underwent OLT between March 1, 2002, and June 30, 2015, at Texas Children’s Hospital, including 65 who were admitted to the ICU at the time of transplantation.
In the analysis of national data, the researchers found that the rates of 1-year survival following OLT in children in the ICU improved from 60% in 1987 to 92% in 2013 (P less than .001). The rates of 1-year survival also improved for children on dialysis at the time of transplant (from 50% in 1995 to 95% in 2013; P less than .001) and for those dependent on a mechanical ventilator at the time of transplant (from 49% in 1994 to 94% in 2013; P less than .001). The significant risk factors were two previous transplants (hazard ratio, 4.2), one previous transplant (HR, 2.5), serum sodium greater than 150 mEq/L (HR, 2.0), dialysis or glomerular filtration rate less than 30 mL/min per 1.73 m2 (HR, 2.0), mechanical ventilator dependence (HR, 1.8), body weight under 6 kg (HR, 1.8), encephalopathy (HR, 1.8), and annual center volume of fewer than five cases (HR, 1.7).
In the experience at Texas Children’s Hospital, the researchers observed “preserved and successful patient survival outcomes” in many markers of acuity. For example, the 10-year survival rates for patients dependent on mechanical ventilation and dialysis were 85% and 96%, respectively, and reached 100% for those requiring therapeutic plasma exchange, molescular adsorbent recirculating system (MARS) liver dialysis, and vasopressors.
“Our collective ability to keep sick children alive with liver failure has improved considerably over the years,” the researchers wrote. “Keeping pace, this analysis demonstrates that the posttransplant outcomes have also improved dramatically. The survival outcomes are comparable to the general population, justifying the use of scarce donors. Although we cannot declare in absolute that no child should be left behind, we can demonstrate acceptable outcomes to date and urge the continual revisiting of our concepts of futility.
“We have learned throughout our experience that almost every child with end-stage liver disease and acute liver failure should be offered liver replacement, as long as the vasoactive medication and mechanical support are not maximized prior to the initiation of the OLT procedure. Every effort should be made to transplant our sickest children.”
This study was supported by the Cade R. Alpard Foundation. The researchers reported having no relevant financial disclosures.
The use of advanced critical care in children and infants with liver failure is justified because orthotopic liver transplantation can be performed on the sickest children and achieve acceptable outcomes, results from a large analysis demonstrated.
“Hand in hand with improved care for critically ill children with liver failure, posttransplant critical care has made tremendous strides,” Abbas Rana, MD, wrote in a study published online in the Journal of the American College of Surgeons. “Our recipients have gotten sicker while our postoperative outcomes have improved. The question then becomes, have our operative skills and postoperative critical care management kept up with the abilities to keep sick children with liver failure alive? Just because transplantation is now possible in our sickest children, is it justified?”
To find out, Dr. Rana of the division of abdominal transplantation and hepatobiliary surgery at Baylor College of Medicine, Houston, and colleagues retrospectively analyzed United Network for Organ Sharing data from all orthotopic liver transplantation (OLT) recipients between Sept. 1, 1987, and June 30, 2015. The analysis paired the liver registry data with data collected by the Organ Procurement and Transplantation Network, and was limited to transplant recipients younger than age 18. The researchers followed a total of 13,723 recipients from date of transplant until either death or the date of last known follow-up (J Am Coll Surg. 2016 Dec 25. doi: 10.1016/j.jamcollsurg.2016.12.025).
In another part of the study, the researchers retrospectively reviewed the charts of 354 patients under 18 years of age who underwent OLT between March 1, 2002, and June 30, 2015, at Texas Children’s Hospital, including 65 who were admitted to the ICU at the time of transplantation.
In the analysis of national data, the researchers found that the rates of 1-year survival following OLT in children in the ICU improved from 60% in 1987 to 92% in 2013 (P less than .001). The rates of 1-year survival also improved for children on dialysis at the time of transplant (from 50% in 1995 to 95% in 2013; P less than .001) and for those dependent on a mechanical ventilator at the time of transplant (from 49% in 1994 to 94% in 2013; P less than .001). The significant risk factors were two previous transplants (hazard ratio, 4.2), one previous transplant (HR, 2.5), serum sodium greater than 150 mEq/L (HR, 2.0), dialysis or glomerular filtration rate less than 30 mL/min per 1.73 m2 (HR, 2.0), mechanical ventilator dependence (HR, 1.8), body weight under 6 kg (HR, 1.8), encephalopathy (HR, 1.8), and annual center volume of fewer than five cases (HR, 1.7).
In the experience at Texas Children’s Hospital, the researchers observed “preserved and successful patient survival outcomes” in many markers of acuity. For example, the 10-year survival rates for patients dependent on mechanical ventilation and dialysis were 85% and 96%, respectively, and reached 100% for those requiring therapeutic plasma exchange, molescular adsorbent recirculating system (MARS) liver dialysis, and vasopressors.
“Our collective ability to keep sick children alive with liver failure has improved considerably over the years,” the researchers wrote. “Keeping pace, this analysis demonstrates that the posttransplant outcomes have also improved dramatically. The survival outcomes are comparable to the general population, justifying the use of scarce donors. Although we cannot declare in absolute that no child should be left behind, we can demonstrate acceptable outcomes to date and urge the continual revisiting of our concepts of futility.
“We have learned throughout our experience that almost every child with end-stage liver disease and acute liver failure should be offered liver replacement, as long as the vasoactive medication and mechanical support are not maximized prior to the initiation of the OLT procedure. Every effort should be made to transplant our sickest children.”
This study was supported by the Cade R. Alpard Foundation. The researchers reported having no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point:
Major finding: The rates of 1-year survival following orthotopic liver transplantation in ICU children improved from 60% in 1987 to 92% in 2013 (P less than .001).
Data source: An analysis of 13,723 patients under the age of 18 years who underwent OLT between Sept. 1, 1987, and June 30, 2015.
Disclosures: The study was supported by the Cade R. Alpard Foundation. The researchers reported having no relevant financial disclosures.
Updated guidelines offer insight into pediatric obesity
Among other things, the guidelines offer new insight into the role of genetics in childhood obesity, provide more extensive guidance regarding bariatric surgery in adolescents, and suggest that measurements of insulin concentrations aren’t useful barometers.
The guidelines also point to research gaps in several areas and call for more studies.
Why issue new guidelines now? “Eight years have passed since the last publication. We did a very thorough job, but there’s been an incredible amount of interest in child obesity, and more information is available,” lead author Dennis M. Styne, MD, professor of pediatrics and the Yocha Dehe endowed chair in pediatric endocrinology at the University of California at Davis, said in an interview. Indeed, recent years have produced hundreds of studies into pediatric obesity, he noted.
The 49-page guidelines are cosponsored by the European Society of Endocrinology and the Pediatric Endocrine Society (J Clin Endocrinol Metab. 2017 March;102[3]:1-49).
Pediatric obesity is of special interest to endocrinologists, Dr. Styne said. “While there’s interest from many specialists now, we are at the forefront of evaluation and treatment of complications like polycystic ovary syndrome, metabolic syndrome, dyslipidemia, and type 2 diabetes.”
The guidelines provide recommendations about a wide variety of obesity-related topics including screening, diagnosis, and treatment. The Endocrine Society commissioned two systematic reviews to support the guidelines: One examined longer randomized controlled trials into medication, surgery, lifestyle, and community-based intervention treatments. The other examined how changing body mass index levels corresponded to cardiometabolic changes.
Several updated areas in the guidelines should be of special interest to endocrinologists: guidance regarding the genetic causes of pediatric obesity, the use of weight-loss medication and surgery, and the roles of insulin tests and breast-feeding, according to Dr. Styne.
In regard to genetics, the guidelines note that research suggests 7% of patients with extreme pediatric obesity “may have rare chromosomal abnormalities and/or highly penetrant genetic mutations that drive their obesity. This percentage is likely to increase with newer methods for genetic testing.”
Dr. Styne calls this finding “remarkable.” As he put it, “we didn’t appreciate that so much in the past.”
The guidelines suggest genetic testing for patients who become obese before the age of 5 years, have a family history of extreme obesity, or show clinical signs of genetic obesity syndromes, especially extreme hyperphagia.
As for the most extreme treatments for the most obese children, the guidelines recommend against weight-loss medication outside of clinical trials and note that “increasing evidence” supports bariatric surgery in teens who haven’t been able to lose enough pounds through lifestyle modification. However, “the use of surgery requires experienced teams with resources for long-term follow-up.”
The guidelines also recommend against measuring serum insulin concentrations as part of pediatric screening for obesity. “A lot of people like to get insulin levels and think it tells them about the future of the child,” Dr. Styne said. “But it doesn’t work very well.”
In another area that reflects new information, the guidelines note that breast-feeding hasn’t been definitively shown to be effective in reducing childhood obesity. “The literature is contradictory,” Dr. Styne said, although he noted that breast-feeding still has many other benefits.
The guidelines point to research gaps in several areas, including the prevention and treatment of pediatric obesity. There’s also “uncharted territory” regarding how to “effectively transition to adult care, with continued necessary monitoring, support, and intervention.”
In regard to the best treatment programs, “we didn’t come up with an answer regarding overall effectiveness,” Dr. Styne said. “School- and community-based programs have promise, but I can’t give you the percentage of success.”
As for the overall picture of pediatric obesity in the United States, “we’re in a better situation than we were 8 years ago,” he said. “Everyone is talking about the problem, and when you talk to families, they’re more aware of it.”
Still, he said, the prevalence of obesity in kids is high, estimated at 17% of those aged 2-19 years in 2014. “That’s not a good place to be,” he said. “We still have to work harder.”
The Endocrine Society funded the guidelines. Dr. Styne reports ownership interests in Teva, Bristol Myers and Organovo. Other authors report various disclosures.
Among other things, the guidelines offer new insight into the role of genetics in childhood obesity, provide more extensive guidance regarding bariatric surgery in adolescents, and suggest that measurements of insulin concentrations aren’t useful barometers.
The guidelines also point to research gaps in several areas and call for more studies.
Why issue new guidelines now? “Eight years have passed since the last publication. We did a very thorough job, but there’s been an incredible amount of interest in child obesity, and more information is available,” lead author Dennis M. Styne, MD, professor of pediatrics and the Yocha Dehe endowed chair in pediatric endocrinology at the University of California at Davis, said in an interview. Indeed, recent years have produced hundreds of studies into pediatric obesity, he noted.
The 49-page guidelines are cosponsored by the European Society of Endocrinology and the Pediatric Endocrine Society (J Clin Endocrinol Metab. 2017 March;102[3]:1-49).
Pediatric obesity is of special interest to endocrinologists, Dr. Styne said. “While there’s interest from many specialists now, we are at the forefront of evaluation and treatment of complications like polycystic ovary syndrome, metabolic syndrome, dyslipidemia, and type 2 diabetes.”
The guidelines provide recommendations about a wide variety of obesity-related topics including screening, diagnosis, and treatment. The Endocrine Society commissioned two systematic reviews to support the guidelines: One examined longer randomized controlled trials into medication, surgery, lifestyle, and community-based intervention treatments. The other examined how changing body mass index levels corresponded to cardiometabolic changes.
Several updated areas in the guidelines should be of special interest to endocrinologists: guidance regarding the genetic causes of pediatric obesity, the use of weight-loss medication and surgery, and the roles of insulin tests and breast-feeding, according to Dr. Styne.
In regard to genetics, the guidelines note that research suggests 7% of patients with extreme pediatric obesity “may have rare chromosomal abnormalities and/or highly penetrant genetic mutations that drive their obesity. This percentage is likely to increase with newer methods for genetic testing.”
Dr. Styne calls this finding “remarkable.” As he put it, “we didn’t appreciate that so much in the past.”
The guidelines suggest genetic testing for patients who become obese before the age of 5 years, have a family history of extreme obesity, or show clinical signs of genetic obesity syndromes, especially extreme hyperphagia.
As for the most extreme treatments for the most obese children, the guidelines recommend against weight-loss medication outside of clinical trials and note that “increasing evidence” supports bariatric surgery in teens who haven’t been able to lose enough pounds through lifestyle modification. However, “the use of surgery requires experienced teams with resources for long-term follow-up.”
The guidelines also recommend against measuring serum insulin concentrations as part of pediatric screening for obesity. “A lot of people like to get insulin levels and think it tells them about the future of the child,” Dr. Styne said. “But it doesn’t work very well.”
In another area that reflects new information, the guidelines note that breast-feeding hasn’t been definitively shown to be effective in reducing childhood obesity. “The literature is contradictory,” Dr. Styne said, although he noted that breast-feeding still has many other benefits.
The guidelines point to research gaps in several areas, including the prevention and treatment of pediatric obesity. There’s also “uncharted territory” regarding how to “effectively transition to adult care, with continued necessary monitoring, support, and intervention.”
In regard to the best treatment programs, “we didn’t come up with an answer regarding overall effectiveness,” Dr. Styne said. “School- and community-based programs have promise, but I can’t give you the percentage of success.”
As for the overall picture of pediatric obesity in the United States, “we’re in a better situation than we were 8 years ago,” he said. “Everyone is talking about the problem, and when you talk to families, they’re more aware of it.”
Still, he said, the prevalence of obesity in kids is high, estimated at 17% of those aged 2-19 years in 2014. “That’s not a good place to be,” he said. “We still have to work harder.”
The Endocrine Society funded the guidelines. Dr. Styne reports ownership interests in Teva, Bristol Myers and Organovo. Other authors report various disclosures.
Among other things, the guidelines offer new insight into the role of genetics in childhood obesity, provide more extensive guidance regarding bariatric surgery in adolescents, and suggest that measurements of insulin concentrations aren’t useful barometers.
The guidelines also point to research gaps in several areas and call for more studies.
Why issue new guidelines now? “Eight years have passed since the last publication. We did a very thorough job, but there’s been an incredible amount of interest in child obesity, and more information is available,” lead author Dennis M. Styne, MD, professor of pediatrics and the Yocha Dehe endowed chair in pediatric endocrinology at the University of California at Davis, said in an interview. Indeed, recent years have produced hundreds of studies into pediatric obesity, he noted.
The 49-page guidelines are cosponsored by the European Society of Endocrinology and the Pediatric Endocrine Society (J Clin Endocrinol Metab. 2017 March;102[3]:1-49).
Pediatric obesity is of special interest to endocrinologists, Dr. Styne said. “While there’s interest from many specialists now, we are at the forefront of evaluation and treatment of complications like polycystic ovary syndrome, metabolic syndrome, dyslipidemia, and type 2 diabetes.”
The guidelines provide recommendations about a wide variety of obesity-related topics including screening, diagnosis, and treatment. The Endocrine Society commissioned two systematic reviews to support the guidelines: One examined longer randomized controlled trials into medication, surgery, lifestyle, and community-based intervention treatments. The other examined how changing body mass index levels corresponded to cardiometabolic changes.
Several updated areas in the guidelines should be of special interest to endocrinologists: guidance regarding the genetic causes of pediatric obesity, the use of weight-loss medication and surgery, and the roles of insulin tests and breast-feeding, according to Dr. Styne.
In regard to genetics, the guidelines note that research suggests 7% of patients with extreme pediatric obesity “may have rare chromosomal abnormalities and/or highly penetrant genetic mutations that drive their obesity. This percentage is likely to increase with newer methods for genetic testing.”
Dr. Styne calls this finding “remarkable.” As he put it, “we didn’t appreciate that so much in the past.”
The guidelines suggest genetic testing for patients who become obese before the age of 5 years, have a family history of extreme obesity, or show clinical signs of genetic obesity syndromes, especially extreme hyperphagia.
As for the most extreme treatments for the most obese children, the guidelines recommend against weight-loss medication outside of clinical trials and note that “increasing evidence” supports bariatric surgery in teens who haven’t been able to lose enough pounds through lifestyle modification. However, “the use of surgery requires experienced teams with resources for long-term follow-up.”
The guidelines also recommend against measuring serum insulin concentrations as part of pediatric screening for obesity. “A lot of people like to get insulin levels and think it tells them about the future of the child,” Dr. Styne said. “But it doesn’t work very well.”
In another area that reflects new information, the guidelines note that breast-feeding hasn’t been definitively shown to be effective in reducing childhood obesity. “The literature is contradictory,” Dr. Styne said, although he noted that breast-feeding still has many other benefits.
The guidelines point to research gaps in several areas, including the prevention and treatment of pediatric obesity. There’s also “uncharted territory” regarding how to “effectively transition to adult care, with continued necessary monitoring, support, and intervention.”
In regard to the best treatment programs, “we didn’t come up with an answer regarding overall effectiveness,” Dr. Styne said. “School- and community-based programs have promise, but I can’t give you the percentage of success.”
As for the overall picture of pediatric obesity in the United States, “we’re in a better situation than we were 8 years ago,” he said. “Everyone is talking about the problem, and when you talk to families, they’re more aware of it.”
Still, he said, the prevalence of obesity in kids is high, estimated at 17% of those aged 2-19 years in 2014. “That’s not a good place to be,” he said. “We still have to work harder.”
The Endocrine Society funded the guidelines. Dr. Styne reports ownership interests in Teva, Bristol Myers and Organovo. Other authors report various disclosures.