User login
Don’t shy away from vaginal salpingectomy
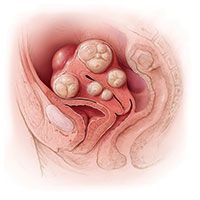
SAN ANTONIO – Surgeons at Houston Methodist Hospital reported a 75% success rate in removing both fallopian tubes during vaginal hysterectomy in a study presented at the annual scientific meeting of the Society of Gynecologic Surgeons.
Serous ovarian carcinoma is now thought to arise from the distal fallopian tube, and it’s estimated that salpingectomy prevents diagnosis of ovarian cancer in 1 in 225 women and death from ovarian cancer in 1 in 450 women. The American Congress of Obstetricians and Gynecologists recommends that surgeons and patients “discuss the potential benefits of the removal of the fallopian tubes” during hysterectomy in women not having an oophorectomy.
The findings from the Houston team show that “it’s feasible in most cases, with very little risk,” said Danielle Antosh, MD, lead investigator and director of the Center for Restorative Pelvic Medicine at Houston Methodist Urogynecology Associates.
“People are doing laparoscopic hysterectomies or robotic hysterectomies” to get at the fallopian tubes, “but they shouldn’t be deterred from trying to remove the fallopian tubes vaginally,” Dr. Antosh said at the SGS 2017 meeting. When women are having a vaginal hysterectomy, “why not try to remove the fallopian tubes? It’s something I would definitely consider counseling your patients about.”
Dr. Antosh said that residents should be taught how to perform salpingectomy during vaginal hysterectomy. “I think it is definitely feasible for residents to do.” Technically, “it’s a lot easier than removing the ovaries” vaginally, she said.
The 70 women in the study were undergoing vaginal hysterectomies by attending physicians for benign reasons, mostly uterine prolapse, followed by heavy menstrual flow and fibroids. In total, 52 (75%) had successful concomitant bilateral vaginal salpingectomies, and 7 additional women had one tube removed. Success was more likely with increasing parity and a history of prolapse. Most of the failures were because the tubes were too high in the pelvis or there were adhesions from prior adnexal surgery. Even with prior adnexal surgery, however, the success rate was 50%.
Vaginal salpingectomy added a mean of 11 minutes to surgery and a mean of 5 mL blood loss. There were no complications reported from including salpingectomy with vaginal hysterectomy. The study wasn’t powered to detect an impact on menopause symptoms, but there was a decrease in menopause symptoms at 16 week follow-up in the salpingectomy group, perhaps related to less sexual dysfunction and urinary incontinence.
The mean age in the study was 51 years, and mean body mass index was 27 kg/m2. There were no malignancies found on tubal pathology.
Five women were transferred to an abdominal approach because of a large uterus or discovery of ovarian pathology. None were transferred for the purpose of salpingectomy.
There was no external funding for the study, and the investigators reported no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
SAN ANTONIO – Surgeons at Houston Methodist Hospital reported a 75% success rate in removing both fallopian tubes during vaginal hysterectomy in a study presented at the annual scientific meeting of the Society of Gynecologic Surgeons.
Serous ovarian carcinoma is now thought to arise from the distal fallopian tube, and it’s estimated that salpingectomy prevents diagnosis of ovarian cancer in 1 in 225 women and death from ovarian cancer in 1 in 450 women. The American Congress of Obstetricians and Gynecologists recommends that surgeons and patients “discuss the potential benefits of the removal of the fallopian tubes” during hysterectomy in women not having an oophorectomy.
The findings from the Houston team show that “it’s feasible in most cases, with very little risk,” said Danielle Antosh, MD, lead investigator and director of the Center for Restorative Pelvic Medicine at Houston Methodist Urogynecology Associates.
“People are doing laparoscopic hysterectomies or robotic hysterectomies” to get at the fallopian tubes, “but they shouldn’t be deterred from trying to remove the fallopian tubes vaginally,” Dr. Antosh said at the SGS 2017 meeting. When women are having a vaginal hysterectomy, “why not try to remove the fallopian tubes? It’s something I would definitely consider counseling your patients about.”
Dr. Antosh said that residents should be taught how to perform salpingectomy during vaginal hysterectomy. “I think it is definitely feasible for residents to do.” Technically, “it’s a lot easier than removing the ovaries” vaginally, she said.
The 70 women in the study were undergoing vaginal hysterectomies by attending physicians for benign reasons, mostly uterine prolapse, followed by heavy menstrual flow and fibroids. In total, 52 (75%) had successful concomitant bilateral vaginal salpingectomies, and 7 additional women had one tube removed. Success was more likely with increasing parity and a history of prolapse. Most of the failures were because the tubes were too high in the pelvis or there were adhesions from prior adnexal surgery. Even with prior adnexal surgery, however, the success rate was 50%.
Vaginal salpingectomy added a mean of 11 minutes to surgery and a mean of 5 mL blood loss. There were no complications reported from including salpingectomy with vaginal hysterectomy. The study wasn’t powered to detect an impact on menopause symptoms, but there was a decrease in menopause symptoms at 16 week follow-up in the salpingectomy group, perhaps related to less sexual dysfunction and urinary incontinence.
The mean age in the study was 51 years, and mean body mass index was 27 kg/m2. There were no malignancies found on tubal pathology.
Five women were transferred to an abdominal approach because of a large uterus or discovery of ovarian pathology. None were transferred for the purpose of salpingectomy.
There was no external funding for the study, and the investigators reported no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
SAN ANTONIO – Surgeons at Houston Methodist Hospital reported a 75% success rate in removing both fallopian tubes during vaginal hysterectomy in a study presented at the annual scientific meeting of the Society of Gynecologic Surgeons.
Serous ovarian carcinoma is now thought to arise from the distal fallopian tube, and it’s estimated that salpingectomy prevents diagnosis of ovarian cancer in 1 in 225 women and death from ovarian cancer in 1 in 450 women. The American Congress of Obstetricians and Gynecologists recommends that surgeons and patients “discuss the potential benefits of the removal of the fallopian tubes” during hysterectomy in women not having an oophorectomy.
The findings from the Houston team show that “it’s feasible in most cases, with very little risk,” said Danielle Antosh, MD, lead investigator and director of the Center for Restorative Pelvic Medicine at Houston Methodist Urogynecology Associates.
“People are doing laparoscopic hysterectomies or robotic hysterectomies” to get at the fallopian tubes, “but they shouldn’t be deterred from trying to remove the fallopian tubes vaginally,” Dr. Antosh said at the SGS 2017 meeting. When women are having a vaginal hysterectomy, “why not try to remove the fallopian tubes? It’s something I would definitely consider counseling your patients about.”
Dr. Antosh said that residents should be taught how to perform salpingectomy during vaginal hysterectomy. “I think it is definitely feasible for residents to do.” Technically, “it’s a lot easier than removing the ovaries” vaginally, she said.
The 70 women in the study were undergoing vaginal hysterectomies by attending physicians for benign reasons, mostly uterine prolapse, followed by heavy menstrual flow and fibroids. In total, 52 (75%) had successful concomitant bilateral vaginal salpingectomies, and 7 additional women had one tube removed. Success was more likely with increasing parity and a history of prolapse. Most of the failures were because the tubes were too high in the pelvis or there were adhesions from prior adnexal surgery. Even with prior adnexal surgery, however, the success rate was 50%.
Vaginal salpingectomy added a mean of 11 minutes to surgery and a mean of 5 mL blood loss. There were no complications reported from including salpingectomy with vaginal hysterectomy. The study wasn’t powered to detect an impact on menopause symptoms, but there was a decrease in menopause symptoms at 16 week follow-up in the salpingectomy group, perhaps related to less sexual dysfunction and urinary incontinence.
The mean age in the study was 51 years, and mean body mass index was 27 kg/m2. There were no malignancies found on tubal pathology.
Five women were transferred to an abdominal approach because of a large uterus or discovery of ovarian pathology. None were transferred for the purpose of salpingectomy.
There was no external funding for the study, and the investigators reported no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
AT SGS 2017
Key clinical point:
Major finding: Three-quarters of women undergoing vaginal hysterectomy for benign reasons had successful concomitant bilateral vaginal salpingectomy.
Data source: A single-center, observational study among 70 women undergoing vaginal hysterectomy for benign reasons.
Disclosures: There was no external funding and the investigators reported no relevant financial disclosures.
Abdominal myomectomy: Patient and surgical technique considerations
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
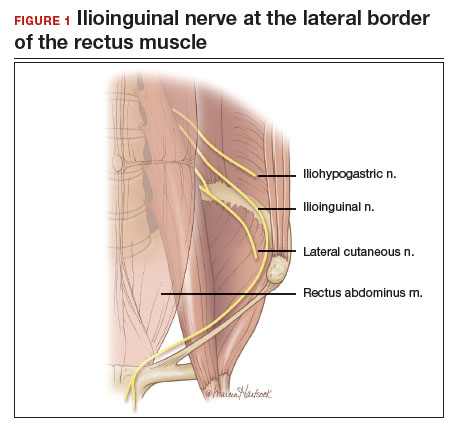
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.
Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
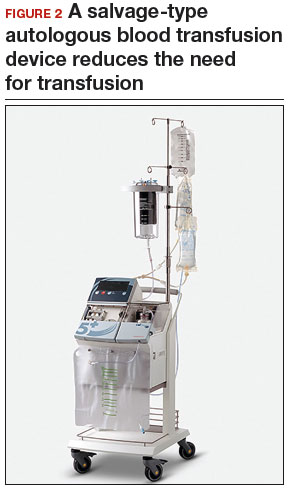
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.
Additional surgical considerations
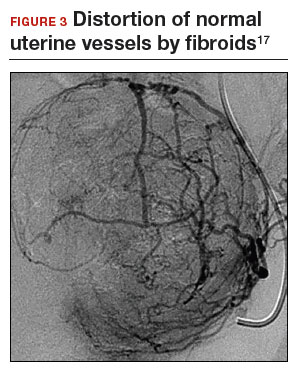
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17
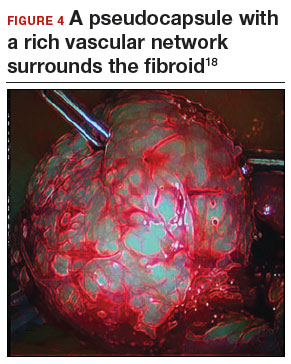
Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20
Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.
Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.
Additional surgical considerations
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17
Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20
Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.
Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.
Additional surgical considerations
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17
Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20
Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
The role of moisturizers and lubricants in genitourinary syndrome of menopause and beyond
Delivering clinician should be seated
“MANAGEMENT OF WOUND COMPLICATIONS FOLLOWING OBSTETRIC ANAL SPHINCTER INJURY (OASIS)”
ROBERT L. BARBIERI, MD, AND JEANNINE M. MIRANNE, MD, MS (EDITORIAL; DECEMBER 2016)
Delivering clinician should be seated
Indeed, obstetric anal sphincter injuries (OASIS),1 with their short- and long-term consequences, merit clinical attention, as spotlighted in Dr. Barbieri and Dr. Miranne’s article. An issue not discussed is the position of the obstetrician.
In our practice, we sit down to perform a vaginal delivery, as taught by Soranus of Ephesus.2 We strive to be at the bedside sooner than when the nurse calls “she is crowning.” This allows communication with the woman, attending nurse, and support person(s), as well as for a brief review of recent estimated fetal weight, length of the second stage, position of the presenting part, degree of flexion, presence of caput, and other last-minute details. Sitting down in front of the outlet permits uninterrupted visual evaluation of the distention of the soft perineal tissues. All traditional maneuvers are performed comfortably from the sitting position: the vertex is controlled by hands-on, and a quick reach with the nonpredominant hand searches for a loop of cord or a small part procidentia to resolve it. The patient is coached either for the next bearing-down effort or to not push to allow for gradual, controlled delivery of the fetal shoulder girdle. We avoid use of the fetal head for traction and move to facilitate “shrugging” with reduction of the bisacromial to facilitate delivery.
In our experience, the sitting position is ideal to observe uninterruptedly the tension of the perineal body during vertex and shoulders delivery, without having to flex and rotate our back and neck in repeatedly nonergonomic positions.
If an obstetrician of above-average height stands for the delivery, the obstetric bed should be elevated to fit her or his reach. Should shoulder dystocia occur, an assistant will stand on a chair and hover over the maternal abdomen to provide suprapubic pressure (indeed, an indelible memory for any parturient and her family). From the sitting position, exploration of the birth canal and repair of any injury, if necessary, can be conducted without technical impediments.
These simple steps have provided our patients and ourselves with clinical and professional satisfaction with minimal OASIS events as shown by others.3 Ironically, if we successfully avoid perineal injuries, our young trainees may require simulation training to learn this tedious repair procedure. In our geographic practice area, a new “collaborative” expects the frequency of episiotomy to be less than 4.6%. Third- and 4th-degree spontaneous or procedure-related perineal injuries still are used to measure quality of care despite demonstrated reasons for this parameter to be a noncredible metric.
Federico G. Mariona, MD
Dearborn, Michigan
Dr. Barbieri responds
I agree with Dr. Mariona that in some cases the fetal head delivers without causing a 3rd- or 4th-degree laceration, but then the delivery of the posterior shoulder causes a severe perineal injury. Dr. Mariona’s clinical pearl is that the delivering clinician should be seated, carefully observe the delivery of the shoulders, and facilitate fetal shrugging by gently reducing the bisacromial diameter as the posterior shoulder transitions over the perineal body.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Verghese TS, Champaneria R, Kapoor DS, Latthe PM. Obstetric anal sphincter injuries after episiotomy: systematic review and meta-analysis. Int Urogynecol J. 2016;27(10):1459–1467.
- Drife J. The start of life: a history of obstetrics. Postgrad Med J. 2002;78(919):311–315.
- Basu M, Smith D, Edwards R; STOMP Project Team. Can the incidence of obstetric anal sphincter injury be reduced? The STOMP experience. Eur J Obstet Gynecol Reprod Biol. 2016;202:55–59.
“MANAGEMENT OF WOUND COMPLICATIONS FOLLOWING OBSTETRIC ANAL SPHINCTER INJURY (OASIS)”
ROBERT L. BARBIERI, MD, AND JEANNINE M. MIRANNE, MD, MS (EDITORIAL; DECEMBER 2016)
Delivering clinician should be seated
Indeed, obstetric anal sphincter injuries (OASIS),1 with their short- and long-term consequences, merit clinical attention, as spotlighted in Dr. Barbieri and Dr. Miranne’s article. An issue not discussed is the position of the obstetrician.
In our practice, we sit down to perform a vaginal delivery, as taught by Soranus of Ephesus.2 We strive to be at the bedside sooner than when the nurse calls “she is crowning.” This allows communication with the woman, attending nurse, and support person(s), as well as for a brief review of recent estimated fetal weight, length of the second stage, position of the presenting part, degree of flexion, presence of caput, and other last-minute details. Sitting down in front of the outlet permits uninterrupted visual evaluation of the distention of the soft perineal tissues. All traditional maneuvers are performed comfortably from the sitting position: the vertex is controlled by hands-on, and a quick reach with the nonpredominant hand searches for a loop of cord or a small part procidentia to resolve it. The patient is coached either for the next bearing-down effort or to not push to allow for gradual, controlled delivery of the fetal shoulder girdle. We avoid use of the fetal head for traction and move to facilitate “shrugging” with reduction of the bisacromial to facilitate delivery.
In our experience, the sitting position is ideal to observe uninterruptedly the tension of the perineal body during vertex and shoulders delivery, without having to flex and rotate our back and neck in repeatedly nonergonomic positions.
If an obstetrician of above-average height stands for the delivery, the obstetric bed should be elevated to fit her or his reach. Should shoulder dystocia occur, an assistant will stand on a chair and hover over the maternal abdomen to provide suprapubic pressure (indeed, an indelible memory for any parturient and her family). From the sitting position, exploration of the birth canal and repair of any injury, if necessary, can be conducted without technical impediments.
These simple steps have provided our patients and ourselves with clinical and professional satisfaction with minimal OASIS events as shown by others.3 Ironically, if we successfully avoid perineal injuries, our young trainees may require simulation training to learn this tedious repair procedure. In our geographic practice area, a new “collaborative” expects the frequency of episiotomy to be less than 4.6%. Third- and 4th-degree spontaneous or procedure-related perineal injuries still are used to measure quality of care despite demonstrated reasons for this parameter to be a noncredible metric.
Federico G. Mariona, MD
Dearborn, Michigan
Dr. Barbieri responds
I agree with Dr. Mariona that in some cases the fetal head delivers without causing a 3rd- or 4th-degree laceration, but then the delivery of the posterior shoulder causes a severe perineal injury. Dr. Mariona’s clinical pearl is that the delivering clinician should be seated, carefully observe the delivery of the shoulders, and facilitate fetal shrugging by gently reducing the bisacromial diameter as the posterior shoulder transitions over the perineal body.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
“MANAGEMENT OF WOUND COMPLICATIONS FOLLOWING OBSTETRIC ANAL SPHINCTER INJURY (OASIS)”
ROBERT L. BARBIERI, MD, AND JEANNINE M. MIRANNE, MD, MS (EDITORIAL; DECEMBER 2016)
Delivering clinician should be seated
Indeed, obstetric anal sphincter injuries (OASIS),1 with their short- and long-term consequences, merit clinical attention, as spotlighted in Dr. Barbieri and Dr. Miranne’s article. An issue not discussed is the position of the obstetrician.
In our practice, we sit down to perform a vaginal delivery, as taught by Soranus of Ephesus.2 We strive to be at the bedside sooner than when the nurse calls “she is crowning.” This allows communication with the woman, attending nurse, and support person(s), as well as for a brief review of recent estimated fetal weight, length of the second stage, position of the presenting part, degree of flexion, presence of caput, and other last-minute details. Sitting down in front of the outlet permits uninterrupted visual evaluation of the distention of the soft perineal tissues. All traditional maneuvers are performed comfortably from the sitting position: the vertex is controlled by hands-on, and a quick reach with the nonpredominant hand searches for a loop of cord or a small part procidentia to resolve it. The patient is coached either for the next bearing-down effort or to not push to allow for gradual, controlled delivery of the fetal shoulder girdle. We avoid use of the fetal head for traction and move to facilitate “shrugging” with reduction of the bisacromial to facilitate delivery.
In our experience, the sitting position is ideal to observe uninterruptedly the tension of the perineal body during vertex and shoulders delivery, without having to flex and rotate our back and neck in repeatedly nonergonomic positions.
If an obstetrician of above-average height stands for the delivery, the obstetric bed should be elevated to fit her or his reach. Should shoulder dystocia occur, an assistant will stand on a chair and hover over the maternal abdomen to provide suprapubic pressure (indeed, an indelible memory for any parturient and her family). From the sitting position, exploration of the birth canal and repair of any injury, if necessary, can be conducted without technical impediments.
These simple steps have provided our patients and ourselves with clinical and professional satisfaction with minimal OASIS events as shown by others.3 Ironically, if we successfully avoid perineal injuries, our young trainees may require simulation training to learn this tedious repair procedure. In our geographic practice area, a new “collaborative” expects the frequency of episiotomy to be less than 4.6%. Third- and 4th-degree spontaneous or procedure-related perineal injuries still are used to measure quality of care despite demonstrated reasons for this parameter to be a noncredible metric.
Federico G. Mariona, MD
Dearborn, Michigan
Dr. Barbieri responds
I agree with Dr. Mariona that in some cases the fetal head delivers without causing a 3rd- or 4th-degree laceration, but then the delivery of the posterior shoulder causes a severe perineal injury. Dr. Mariona’s clinical pearl is that the delivering clinician should be seated, carefully observe the delivery of the shoulders, and facilitate fetal shrugging by gently reducing the bisacromial diameter as the posterior shoulder transitions over the perineal body.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Verghese TS, Champaneria R, Kapoor DS, Latthe PM. Obstetric anal sphincter injuries after episiotomy: systematic review and meta-analysis. Int Urogynecol J. 2016;27(10):1459–1467.
- Drife J. The start of life: a history of obstetrics. Postgrad Med J. 2002;78(919):311–315.
- Basu M, Smith D, Edwards R; STOMP Project Team. Can the incidence of obstetric anal sphincter injury be reduced? The STOMP experience. Eur J Obstet Gynecol Reprod Biol. 2016;202:55–59.
- Verghese TS, Champaneria R, Kapoor DS, Latthe PM. Obstetric anal sphincter injuries after episiotomy: systematic review and meta-analysis. Int Urogynecol J. 2016;27(10):1459–1467.
- Drife J. The start of life: a history of obstetrics. Postgrad Med J. 2002;78(919):311–315.
- Basu M, Smith D, Edwards R; STOMP Project Team. Can the incidence of obstetric anal sphincter injury be reduced? The STOMP experience. Eur J Obstet Gynecol Reprod Biol. 2016;202:55–59.
Direct from San Antonio: SGS Fellow Scholar reports from society’s 2017 annual meeting
3/28/17. DAY 3 AT SGS
Exciting presentations continue, society groups provide updates
The team from Mayo Clinic was still riding high this morning after winning last night’s armadillo race, which was part of the Texas hoedown fundraiser for SHARE.
The seventh scientific session started with a nice presentation by Cara Grimes, MD, entitled, “Evaluating ureteral patency in the post-indigo carmine era: a randomized controlled trial.” Several quality presentations followed before Ike Rahn, MD, updated the society on the work of the Fellows Pelvic Research Network, a group currently celebrating its 10th anniversary.
Drs. Star Hampton and Peter Jeppson then took the stage to share the progress of the SGS Pelvic Anatomy Group as they undertake the daunting task of a systematic review of anatomic terms used in the medical literature.
Leadership transition
Surely the moment that SGS President Vivian Sung, MD, had been waiting for all week was the passing of the gavel to incoming president John Gebhart, MD. On acceptance of his role as the incoming president, Dr. Gebhart’s remarks focused on the honor of serving in that role, and he stated that Dr. Sung, in her usual fashion, set the bar for performance very high.
After the midmorning break, where attendants usually say their temporary goodbyes to friends and mentors, old and new, the eighth and final scientific session began.
Come to next year’s meeting!
If you consider yourself a gynecologic surgeon (and if you’re reading this you probably do), please consider adding attendance at the next SGS meeting to your list of “things to do” in 2018. This family-friendly meeting is filled with opportunities for surgical teaching, learning, rest, relaxation, networking, and reconnecting. Most of all, it is a place where mentoring relationships begin and are nurtured, recognized, and appreciated.
See you in Orlando!
3/28/17. DAY 2 AT SGS
Morning highlights: Prize-winning paper, presidential address
Scholarly activity continued this morning at the Society of Gynecologic Surgeons Annual Scientific meeting at the La Cantera Resort in San Antonio, Texas. After early morning reviews and coffee with good friends, the scientific program began with a comparison of barbed and nonbarbed sutures, after which Eric Jelovsek, MD, presented the prize-winning paper for the Pelvic Floor Disorders Network, “A Randomized Trial of Uterosacral Ligament Suspension or Sacrospinous Ligament Fixation for Apical Pelvic Organ Prolapse: Five-year Outcomes.”
The highlight of the morning was almost certainly the presidential address in which Dr. Vivian Sung shared with the audience the recipe for the “secret sauce” that makes SGS a special organization:
- Be everyday leaders and mentors. Do the little things to teach, coach, and encourage others in the field.
- Maintain a safe environment. Allow others to be brave, be creative, and make mistakes. It will make them more effective.
- Consider the “WHY.” Focus on why SGS exists and continue to strive for that mission.
Stimulating scientific sessions
After the fifth scientific session, where we learned that prophylactic salpingectomy at the time of vaginal hysterectomy not only is feasible but also cost-effective, participants were treated to the TeLinde Lecture. Dr. Richard Reznick, Dean of Health Sciences and Professor in the Department of Surgery at Queen’s University in Kingston, Ontario, shared exciting and intriguing data regarding competency-based learning in surgical training. The lecture, entitled “Great Expectations: The Promise of Competency-based Education,” sparked questions and conversation that could have gone on for hours. Alas, program director Rob Gutman, MD, kept the program on track and, after a brief break for lunch, the sixth scientific session was underway.
In the sixth session, Dr. Gutman moderated a lively panel discussion that set out to answer the question, “How can we increase the percentage and quality of minimally invasive hysterectomy for benign disease among low/intermediate volume gynecologic surgeons?” Panelists shared thoughts and information—from organizations and institutions around the country—outlining the data on current hysterectomy rates, trends in policymaking, learning through simulation, incremental quality improvement planning, and surgical pathways.
Afternoon fun and a Texas hoedown
The scientific meeting was then adjourned, making way for the SGS business meeting and then an afternoon of well-deserved fun in the Texas sun.
Evening events included an old-fashioned Texas hoedown—a time for two-stepping, armadillo racing, and camaraderie to raise money for SHARE. My money’s on the armadillo from the University of New Mexico!
3/27/17. DAY 1 AT SGS
Debate, postgrad courses, videos galore
The Annual Scientific Meeting of the Society of Gynecologic Surgeons, held in San Antonio, Texas, opened to an energetic crowd when SGS President, Vivan Sung, MD, welcomed participants from 10 countries before introducing the society’s 10 newest members. The first scientific session then quickly got underway with oral presentations and videos covering a variety of topics.
Janet Bickel, MS, a national leader in mentorship and faculty development, reinforced the meeting’s theme with her keynote lecture, “Hard Work and Talent Aren’t Enough: Mentoring and Finding Mentors across Career Stages.” She shared with attendees the keys to mentoring women and minorities before outlining the characteristics associated with both effective mentors and mentees. It turns out that many of her key points had been on display just a day earlier during the postgraduate courses in which physicians from around the country were coached by experts on surgical complications, pelvic anatomy and computer modeling, surgical teaching, and enhanced surgical recovery.
After a brief break for lunch at the beautiful La Cantera Resort, attendees returned for a lively debate between Kim Kenton, MD and Geoff Cundiff, MD entitled “Should we separate the O from the G in Obstetrics and Gynecology?” Dee Fenner, MD acted as moderator and referee as both sides passionately shared their convincing arguments. In the end, both parties agreed that this century-old debate would continue as we constantly evaluate the best approach to caring for the female patient.
Promises of popcorn brought attendees back to the meeting hall for the afternoon videofest where 13 videos were presented on a variety of surgical topics.
Specialists learn from each other
Meanwhile, the Fellows Pelvic Research Network had the pleasure a special lecture and Q&A session with Linda Brubaker, MD. Among the many pearls of wisdom she shared was an evergreen piece of advice, “Enthusiasm is good. Focus is better.” The fellows then turned their focus to the review of current projects and evaluation of proposals for new research.
Eight academic roundtables hosted by experts from across the country provided an opportunity for attendees to discuss best practices in various areas of pelvic surgery including bladder pain syndrome, chronic pelvic pain, transgender care, billing and coding, social media, and more.
The day ended with a delightful awards ceremony in which Dr. Sung recognized outstanding scholarly and service activity in the gynecologic surgery community. Notably, Dr. Peter Jeppson was presented with the 2017 Distinguished Service Award. Members then joined meeting sponsors and staff in the exhibit hall for an evening reception—a fitting ending to a phenomenal first day.
Click for more…
For more details about the scientific presentations and to read abstracts of presentations, videos, and posters, see the March 2017 supplemental issue of the American Journal of Obstetrics and Gynecology.
For an up-to-the minute report of the week’s events, follow the #SGS2017 hashtag on Twitter. Be sure to follow @obgmanagement, @gynsurg, and @stuboo as well.
3/28/17. DAY 3 AT SGS
Exciting presentations continue, society groups provide updates
The team from Mayo Clinic was still riding high this morning after winning last night’s armadillo race, which was part of the Texas hoedown fundraiser for SHARE.
The seventh scientific session started with a nice presentation by Cara Grimes, MD, entitled, “Evaluating ureteral patency in the post-indigo carmine era: a randomized controlled trial.” Several quality presentations followed before Ike Rahn, MD, updated the society on the work of the Fellows Pelvic Research Network, a group currently celebrating its 10th anniversary.
Drs. Star Hampton and Peter Jeppson then took the stage to share the progress of the SGS Pelvic Anatomy Group as they undertake the daunting task of a systematic review of anatomic terms used in the medical literature.
Leadership transition
Surely the moment that SGS President Vivian Sung, MD, had been waiting for all week was the passing of the gavel to incoming president John Gebhart, MD. On acceptance of his role as the incoming president, Dr. Gebhart’s remarks focused on the honor of serving in that role, and he stated that Dr. Sung, in her usual fashion, set the bar for performance very high.
After the midmorning break, where attendants usually say their temporary goodbyes to friends and mentors, old and new, the eighth and final scientific session began.
Come to next year’s meeting!
If you consider yourself a gynecologic surgeon (and if you’re reading this you probably do), please consider adding attendance at the next SGS meeting to your list of “things to do” in 2018. This family-friendly meeting is filled with opportunities for surgical teaching, learning, rest, relaxation, networking, and reconnecting. Most of all, it is a place where mentoring relationships begin and are nurtured, recognized, and appreciated.
See you in Orlando!
3/28/17. DAY 2 AT SGS
Morning highlights: Prize-winning paper, presidential address
Scholarly activity continued this morning at the Society of Gynecologic Surgeons Annual Scientific meeting at the La Cantera Resort in San Antonio, Texas. After early morning reviews and coffee with good friends, the scientific program began with a comparison of barbed and nonbarbed sutures, after which Eric Jelovsek, MD, presented the prize-winning paper for the Pelvic Floor Disorders Network, “A Randomized Trial of Uterosacral Ligament Suspension or Sacrospinous Ligament Fixation for Apical Pelvic Organ Prolapse: Five-year Outcomes.”
The highlight of the morning was almost certainly the presidential address in which Dr. Vivian Sung shared with the audience the recipe for the “secret sauce” that makes SGS a special organization:
- Be everyday leaders and mentors. Do the little things to teach, coach, and encourage others in the field.
- Maintain a safe environment. Allow others to be brave, be creative, and make mistakes. It will make them more effective.
- Consider the “WHY.” Focus on why SGS exists and continue to strive for that mission.
Stimulating scientific sessions
After the fifth scientific session, where we learned that prophylactic salpingectomy at the time of vaginal hysterectomy not only is feasible but also cost-effective, participants were treated to the TeLinde Lecture. Dr. Richard Reznick, Dean of Health Sciences and Professor in the Department of Surgery at Queen’s University in Kingston, Ontario, shared exciting and intriguing data regarding competency-based learning in surgical training. The lecture, entitled “Great Expectations: The Promise of Competency-based Education,” sparked questions and conversation that could have gone on for hours. Alas, program director Rob Gutman, MD, kept the program on track and, after a brief break for lunch, the sixth scientific session was underway.
In the sixth session, Dr. Gutman moderated a lively panel discussion that set out to answer the question, “How can we increase the percentage and quality of minimally invasive hysterectomy for benign disease among low/intermediate volume gynecologic surgeons?” Panelists shared thoughts and information—from organizations and institutions around the country—outlining the data on current hysterectomy rates, trends in policymaking, learning through simulation, incremental quality improvement planning, and surgical pathways.
Afternoon fun and a Texas hoedown
The scientific meeting was then adjourned, making way for the SGS business meeting and then an afternoon of well-deserved fun in the Texas sun.
Evening events included an old-fashioned Texas hoedown—a time for two-stepping, armadillo racing, and camaraderie to raise money for SHARE. My money’s on the armadillo from the University of New Mexico!
3/27/17. DAY 1 AT SGS
Debate, postgrad courses, videos galore
The Annual Scientific Meeting of the Society of Gynecologic Surgeons, held in San Antonio, Texas, opened to an energetic crowd when SGS President, Vivan Sung, MD, welcomed participants from 10 countries before introducing the society’s 10 newest members. The first scientific session then quickly got underway with oral presentations and videos covering a variety of topics.
Janet Bickel, MS, a national leader in mentorship and faculty development, reinforced the meeting’s theme with her keynote lecture, “Hard Work and Talent Aren’t Enough: Mentoring and Finding Mentors across Career Stages.” She shared with attendees the keys to mentoring women and minorities before outlining the characteristics associated with both effective mentors and mentees. It turns out that many of her key points had been on display just a day earlier during the postgraduate courses in which physicians from around the country were coached by experts on surgical complications, pelvic anatomy and computer modeling, surgical teaching, and enhanced surgical recovery.
After a brief break for lunch at the beautiful La Cantera Resort, attendees returned for a lively debate between Kim Kenton, MD and Geoff Cundiff, MD entitled “Should we separate the O from the G in Obstetrics and Gynecology?” Dee Fenner, MD acted as moderator and referee as both sides passionately shared their convincing arguments. In the end, both parties agreed that this century-old debate would continue as we constantly evaluate the best approach to caring for the female patient.
Promises of popcorn brought attendees back to the meeting hall for the afternoon videofest where 13 videos were presented on a variety of surgical topics.
Specialists learn from each other
Meanwhile, the Fellows Pelvic Research Network had the pleasure a special lecture and Q&A session with Linda Brubaker, MD. Among the many pearls of wisdom she shared was an evergreen piece of advice, “Enthusiasm is good. Focus is better.” The fellows then turned their focus to the review of current projects and evaluation of proposals for new research.
Eight academic roundtables hosted by experts from across the country provided an opportunity for attendees to discuss best practices in various areas of pelvic surgery including bladder pain syndrome, chronic pelvic pain, transgender care, billing and coding, social media, and more.
The day ended with a delightful awards ceremony in which Dr. Sung recognized outstanding scholarly and service activity in the gynecologic surgery community. Notably, Dr. Peter Jeppson was presented with the 2017 Distinguished Service Award. Members then joined meeting sponsors and staff in the exhibit hall for an evening reception—a fitting ending to a phenomenal first day.
Click for more…
For more details about the scientific presentations and to read abstracts of presentations, videos, and posters, see the March 2017 supplemental issue of the American Journal of Obstetrics and Gynecology.
For an up-to-the minute report of the week’s events, follow the #SGS2017 hashtag on Twitter. Be sure to follow @obgmanagement, @gynsurg, and @stuboo as well.
3/28/17. DAY 3 AT SGS
Exciting presentations continue, society groups provide updates
The team from Mayo Clinic was still riding high this morning after winning last night’s armadillo race, which was part of the Texas hoedown fundraiser for SHARE.
The seventh scientific session started with a nice presentation by Cara Grimes, MD, entitled, “Evaluating ureteral patency in the post-indigo carmine era: a randomized controlled trial.” Several quality presentations followed before Ike Rahn, MD, updated the society on the work of the Fellows Pelvic Research Network, a group currently celebrating its 10th anniversary.
Drs. Star Hampton and Peter Jeppson then took the stage to share the progress of the SGS Pelvic Anatomy Group as they undertake the daunting task of a systematic review of anatomic terms used in the medical literature.
Leadership transition
Surely the moment that SGS President Vivian Sung, MD, had been waiting for all week was the passing of the gavel to incoming president John Gebhart, MD. On acceptance of his role as the incoming president, Dr. Gebhart’s remarks focused on the honor of serving in that role, and he stated that Dr. Sung, in her usual fashion, set the bar for performance very high.
After the midmorning break, where attendants usually say their temporary goodbyes to friends and mentors, old and new, the eighth and final scientific session began.
Come to next year’s meeting!
If you consider yourself a gynecologic surgeon (and if you’re reading this you probably do), please consider adding attendance at the next SGS meeting to your list of “things to do” in 2018. This family-friendly meeting is filled with opportunities for surgical teaching, learning, rest, relaxation, networking, and reconnecting. Most of all, it is a place where mentoring relationships begin and are nurtured, recognized, and appreciated.
See you in Orlando!
3/28/17. DAY 2 AT SGS
Morning highlights: Prize-winning paper, presidential address
Scholarly activity continued this morning at the Society of Gynecologic Surgeons Annual Scientific meeting at the La Cantera Resort in San Antonio, Texas. After early morning reviews and coffee with good friends, the scientific program began with a comparison of barbed and nonbarbed sutures, after which Eric Jelovsek, MD, presented the prize-winning paper for the Pelvic Floor Disorders Network, “A Randomized Trial of Uterosacral Ligament Suspension or Sacrospinous Ligament Fixation for Apical Pelvic Organ Prolapse: Five-year Outcomes.”
The highlight of the morning was almost certainly the presidential address in which Dr. Vivian Sung shared with the audience the recipe for the “secret sauce” that makes SGS a special organization:
- Be everyday leaders and mentors. Do the little things to teach, coach, and encourage others in the field.
- Maintain a safe environment. Allow others to be brave, be creative, and make mistakes. It will make them more effective.
- Consider the “WHY.” Focus on why SGS exists and continue to strive for that mission.
Stimulating scientific sessions
After the fifth scientific session, where we learned that prophylactic salpingectomy at the time of vaginal hysterectomy not only is feasible but also cost-effective, participants were treated to the TeLinde Lecture. Dr. Richard Reznick, Dean of Health Sciences and Professor in the Department of Surgery at Queen’s University in Kingston, Ontario, shared exciting and intriguing data regarding competency-based learning in surgical training. The lecture, entitled “Great Expectations: The Promise of Competency-based Education,” sparked questions and conversation that could have gone on for hours. Alas, program director Rob Gutman, MD, kept the program on track and, after a brief break for lunch, the sixth scientific session was underway.
In the sixth session, Dr. Gutman moderated a lively panel discussion that set out to answer the question, “How can we increase the percentage and quality of minimally invasive hysterectomy for benign disease among low/intermediate volume gynecologic surgeons?” Panelists shared thoughts and information—from organizations and institutions around the country—outlining the data on current hysterectomy rates, trends in policymaking, learning through simulation, incremental quality improvement planning, and surgical pathways.
Afternoon fun and a Texas hoedown
The scientific meeting was then adjourned, making way for the SGS business meeting and then an afternoon of well-deserved fun in the Texas sun.
Evening events included an old-fashioned Texas hoedown—a time for two-stepping, armadillo racing, and camaraderie to raise money for SHARE. My money’s on the armadillo from the University of New Mexico!
3/27/17. DAY 1 AT SGS
Debate, postgrad courses, videos galore
The Annual Scientific Meeting of the Society of Gynecologic Surgeons, held in San Antonio, Texas, opened to an energetic crowd when SGS President, Vivan Sung, MD, welcomed participants from 10 countries before introducing the society’s 10 newest members. The first scientific session then quickly got underway with oral presentations and videos covering a variety of topics.
Janet Bickel, MS, a national leader in mentorship and faculty development, reinforced the meeting’s theme with her keynote lecture, “Hard Work and Talent Aren’t Enough: Mentoring and Finding Mentors across Career Stages.” She shared with attendees the keys to mentoring women and minorities before outlining the characteristics associated with both effective mentors and mentees. It turns out that many of her key points had been on display just a day earlier during the postgraduate courses in which physicians from around the country were coached by experts on surgical complications, pelvic anatomy and computer modeling, surgical teaching, and enhanced surgical recovery.
After a brief break for lunch at the beautiful La Cantera Resort, attendees returned for a lively debate between Kim Kenton, MD and Geoff Cundiff, MD entitled “Should we separate the O from the G in Obstetrics and Gynecology?” Dee Fenner, MD acted as moderator and referee as both sides passionately shared their convincing arguments. In the end, both parties agreed that this century-old debate would continue as we constantly evaluate the best approach to caring for the female patient.
Promises of popcorn brought attendees back to the meeting hall for the afternoon videofest where 13 videos were presented on a variety of surgical topics.
Specialists learn from each other
Meanwhile, the Fellows Pelvic Research Network had the pleasure a special lecture and Q&A session with Linda Brubaker, MD. Among the many pearls of wisdom she shared was an evergreen piece of advice, “Enthusiasm is good. Focus is better.” The fellows then turned their focus to the review of current projects and evaluation of proposals for new research.
Eight academic roundtables hosted by experts from across the country provided an opportunity for attendees to discuss best practices in various areas of pelvic surgery including bladder pain syndrome, chronic pelvic pain, transgender care, billing and coding, social media, and more.
The day ended with a delightful awards ceremony in which Dr. Sung recognized outstanding scholarly and service activity in the gynecologic surgery community. Notably, Dr. Peter Jeppson was presented with the 2017 Distinguished Service Award. Members then joined meeting sponsors and staff in the exhibit hall for an evening reception—a fitting ending to a phenomenal first day.
Click for more…
For more details about the scientific presentations and to read abstracts of presentations, videos, and posters, see the March 2017 supplemental issue of the American Journal of Obstetrics and Gynecology.
For an up-to-the minute report of the week’s events, follow the #SGS2017 hashtag on Twitter. Be sure to follow @obgmanagement, @gynsurg, and @stuboo as well.
Laparoscopic and abdominal hysterectomy yield equivalent survival
Laparoscopic hysterectomy yields equivalent disease-free and overall survival at 4.5 years, compared with abdominal hysterectomy in stage I endometrial cancer, according to a report published online March 28 in JAMA.
Several short-term advantages with the laparoscopic approach have been well documented, including less pain, less morbidity, better quality of life, decreased risk of surgery-related adverse events, and cost savings. But until now, no large international trial has demonstrated that longer-term survival outcomes are at least as good with laparoscopic as with open abdominal hysterectomy in this patient population, reported Monika Janda, PhD, of Queensland University of Technology, Brisbane (Australia) and her colleagues.
They conducted the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial, a randomized equivalence study involving 760 women treated at 20 medical centers in Australia, New Zealand, and Hong Kong during 2005-2010. The women were followed for a median of 4.5 years.
All of the women had histologically confirmed stage I adenocarcinoma of the endometrium. A total of 407 patients were randomly assigned to undergo total laparoscopic hysterectomy and 353 patients to undergo total abdominal hysterectomy. Medical comorbidities were equally distributed between the two study groups, and there were no significant between-group differences in tumor type, histologic grade, number of involved lymph nodes, or adjuvant treatments.
Disease-free survival at 4.5 years was 81.6% with laparoscopic hysterectomy and 81.3% with abdominal hysterectomy, meeting the criteria for equivalence. Overall survival at 4.5 years was 92.0% and 92.4%, respectively. Cancer recurred near the operative site in 3% of each group and at a regional or distant site in 2% or less of each group. Causes of death also were similar between the two study groups, with 56% of all deaths attributed to endometrial cancer (JAMA. 2017;317[12]:1224-33).
Of note, two patients who underwent laparoscopic surgery developed port-site metastases and two patients who underwent abdominal surgery developed metastases at the site of the abdominal wound.
The study was funded by Cancer Councils in Australia, the National Health and Medical Research Council, Cancer Australia, QLD Health, and numerous others. Dr. Janda reported having no relevant financial disclosures; one of her coauthors reported ties to the O.R. Company, SurgicalPerformance Pty, and Covidien.
This study adds to a growing body of literature that suggests laparoscopic hysterectomy is not only safe, but also the preferred modality of hysterectomy for women with endometrial cancer.
Despite the clear benefits of laparoscopic hysterectomy, the findings from the LACE trial should be interpreted in the context of the study design. Importantly, patients randomized to the study represent a highly select group of women with endometrial cancer. The study entry criteria involved a low-risk population of women with stage I tumors of endometrioid histology with a uterine size of less than 10 weeks’ gestation. In practice, laparoscopic hysterectomy is now routinely used for women with nonendometrioid histologies and in those with more advanced disease.
The LACE trial reported by Janda et al. provides confirmation that laparoscopic hysterectomy is a safe and effective treatment modality for women with early-stage endometrial cancer. The favorable short-term outcomes along with equivalent oncological outcomes make laparoscopic hysterectomy the preferred surgical modality in this setting. Even though the road to defining the benefits of laparoscopic hysterectomy has been long, efforts to promote the procedure for women with endometrial cancer should now be a priority.
Jason D. Wright, MD, is at the Herbert Irving Comprehensive Cancer Center and the department of ob.gyn. at Columbia University, New York. He reported having no relevant financial disclosures. These comments are excerpted from an accompanying editorial (JAMA 2017;317[12]:1215-6).
This study adds to a growing body of literature that suggests laparoscopic hysterectomy is not only safe, but also the preferred modality of hysterectomy for women with endometrial cancer.
Despite the clear benefits of laparoscopic hysterectomy, the findings from the LACE trial should be interpreted in the context of the study design. Importantly, patients randomized to the study represent a highly select group of women with endometrial cancer. The study entry criteria involved a low-risk population of women with stage I tumors of endometrioid histology with a uterine size of less than 10 weeks’ gestation. In practice, laparoscopic hysterectomy is now routinely used for women with nonendometrioid histologies and in those with more advanced disease.
The LACE trial reported by Janda et al. provides confirmation that laparoscopic hysterectomy is a safe and effective treatment modality for women with early-stage endometrial cancer. The favorable short-term outcomes along with equivalent oncological outcomes make laparoscopic hysterectomy the preferred surgical modality in this setting. Even though the road to defining the benefits of laparoscopic hysterectomy has been long, efforts to promote the procedure for women with endometrial cancer should now be a priority.
Jason D. Wright, MD, is at the Herbert Irving Comprehensive Cancer Center and the department of ob.gyn. at Columbia University, New York. He reported having no relevant financial disclosures. These comments are excerpted from an accompanying editorial (JAMA 2017;317[12]:1215-6).
This study adds to a growing body of literature that suggests laparoscopic hysterectomy is not only safe, but also the preferred modality of hysterectomy for women with endometrial cancer.
Despite the clear benefits of laparoscopic hysterectomy, the findings from the LACE trial should be interpreted in the context of the study design. Importantly, patients randomized to the study represent a highly select group of women with endometrial cancer. The study entry criteria involved a low-risk population of women with stage I tumors of endometrioid histology with a uterine size of less than 10 weeks’ gestation. In practice, laparoscopic hysterectomy is now routinely used for women with nonendometrioid histologies and in those with more advanced disease.
The LACE trial reported by Janda et al. provides confirmation that laparoscopic hysterectomy is a safe and effective treatment modality for women with early-stage endometrial cancer. The favorable short-term outcomes along with equivalent oncological outcomes make laparoscopic hysterectomy the preferred surgical modality in this setting. Even though the road to defining the benefits of laparoscopic hysterectomy has been long, efforts to promote the procedure for women with endometrial cancer should now be a priority.
Jason D. Wright, MD, is at the Herbert Irving Comprehensive Cancer Center and the department of ob.gyn. at Columbia University, New York. He reported having no relevant financial disclosures. These comments are excerpted from an accompanying editorial (JAMA 2017;317[12]:1215-6).
Laparoscopic hysterectomy yields equivalent disease-free and overall survival at 4.5 years, compared with abdominal hysterectomy in stage I endometrial cancer, according to a report published online March 28 in JAMA.
Several short-term advantages with the laparoscopic approach have been well documented, including less pain, less morbidity, better quality of life, decreased risk of surgery-related adverse events, and cost savings. But until now, no large international trial has demonstrated that longer-term survival outcomes are at least as good with laparoscopic as with open abdominal hysterectomy in this patient population, reported Monika Janda, PhD, of Queensland University of Technology, Brisbane (Australia) and her colleagues.
They conducted the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial, a randomized equivalence study involving 760 women treated at 20 medical centers in Australia, New Zealand, and Hong Kong during 2005-2010. The women were followed for a median of 4.5 years.
All of the women had histologically confirmed stage I adenocarcinoma of the endometrium. A total of 407 patients were randomly assigned to undergo total laparoscopic hysterectomy and 353 patients to undergo total abdominal hysterectomy. Medical comorbidities were equally distributed between the two study groups, and there were no significant between-group differences in tumor type, histologic grade, number of involved lymph nodes, or adjuvant treatments.
Disease-free survival at 4.5 years was 81.6% with laparoscopic hysterectomy and 81.3% with abdominal hysterectomy, meeting the criteria for equivalence. Overall survival at 4.5 years was 92.0% and 92.4%, respectively. Cancer recurred near the operative site in 3% of each group and at a regional or distant site in 2% or less of each group. Causes of death also were similar between the two study groups, with 56% of all deaths attributed to endometrial cancer (JAMA. 2017;317[12]:1224-33).
Of note, two patients who underwent laparoscopic surgery developed port-site metastases and two patients who underwent abdominal surgery developed metastases at the site of the abdominal wound.
The study was funded by Cancer Councils in Australia, the National Health and Medical Research Council, Cancer Australia, QLD Health, and numerous others. Dr. Janda reported having no relevant financial disclosures; one of her coauthors reported ties to the O.R. Company, SurgicalPerformance Pty, and Covidien.
Laparoscopic hysterectomy yields equivalent disease-free and overall survival at 4.5 years, compared with abdominal hysterectomy in stage I endometrial cancer, according to a report published online March 28 in JAMA.
Several short-term advantages with the laparoscopic approach have been well documented, including less pain, less morbidity, better quality of life, decreased risk of surgery-related adverse events, and cost savings. But until now, no large international trial has demonstrated that longer-term survival outcomes are at least as good with laparoscopic as with open abdominal hysterectomy in this patient population, reported Monika Janda, PhD, of Queensland University of Technology, Brisbane (Australia) and her colleagues.
They conducted the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial, a randomized equivalence study involving 760 women treated at 20 medical centers in Australia, New Zealand, and Hong Kong during 2005-2010. The women were followed for a median of 4.5 years.
All of the women had histologically confirmed stage I adenocarcinoma of the endometrium. A total of 407 patients were randomly assigned to undergo total laparoscopic hysterectomy and 353 patients to undergo total abdominal hysterectomy. Medical comorbidities were equally distributed between the two study groups, and there were no significant between-group differences in tumor type, histologic grade, number of involved lymph nodes, or adjuvant treatments.
Disease-free survival at 4.5 years was 81.6% with laparoscopic hysterectomy and 81.3% with abdominal hysterectomy, meeting the criteria for equivalence. Overall survival at 4.5 years was 92.0% and 92.4%, respectively. Cancer recurred near the operative site in 3% of each group and at a regional or distant site in 2% or less of each group. Causes of death also were similar between the two study groups, with 56% of all deaths attributed to endometrial cancer (JAMA. 2017;317[12]:1224-33).
Of note, two patients who underwent laparoscopic surgery developed port-site metastases and two patients who underwent abdominal surgery developed metastases at the site of the abdominal wound.
The study was funded by Cancer Councils in Australia, the National Health and Medical Research Council, Cancer Australia, QLD Health, and numerous others. Dr. Janda reported having no relevant financial disclosures; one of her coauthors reported ties to the O.R. Company, SurgicalPerformance Pty, and Covidien.
FROM JAMA
Key clinical point:
Major finding: Disease-free survival at 4.5 years was 81.6% with laparoscopic hysterectomy and 81.3% with abdominal hysterectomy.
Data source: An international, randomized, phase III equivalence trial involving 760 women treated with total abdominal or total laparoscopic hysterectomy.
Disclosures: The study was funded by Cancer Councils in Australia, the National Health and Medical Research Council, Cancer Australia, QLD Health, and others. Dr. Janda reported having no relevant financial disclosures; one of her coauthors reported ties to the O.R. Company, SurgicalPerformance Pty, and Covidien.
Nonoperative management of pediatric appendicitis appears feasible
Nonoperative management of uncomplicated acute appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach, investigators reported March 27 in JAMA Pediatrics.
Nonoperative management, based on antibiotic treatment and close monitoring of the patient, is accepted as safe and effective in adults but has not been well studied in children and adolescents. “Owing to specific anatomical and pathophysiologic features of children, the clinical scenario of acute appendicitis in pediatric patients is different from that in adults, and treatment decisions for children are more difficult,” said Libin Huang, MD, of West China Hospital and Sichuan University, Chengdu, and his associates.
The few clinical trials that have been performed in children have had small sample sizes, so the investigators performed a meta-analysis to pool the results for 404 patients aged 5-18 years. They analyzed data from four single-center prospective but nonrandomized controlled trials and one single-center randomized controlled trial to compare outcomes between 168 patients initially treated with antibiotics and 236 who underwent immediate appendectomy.
Sixteen patients in the nonoperative group (9.5%) had treatment failure, defined as appendectomy within 48 hours (11 patients) or within 1 month of follow-up (5 patients). Three of these patients developed a complication (perforated appendicitis). In comparison, none of the surgery group had treatment failure, and one developed a complication requiring reoperation. Thus, the rate of success in the nonoperative group was 152 of 168 patients, or 90.5%, and the rate of complications was not significantly different between the two study groups, Dr. Huang and his associates said (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0057).
During the following year, 27 patients in the nonoperative group had a histopathologically confirmed recurrence of appendicitis and underwent appendectomy; another 8 had the surgery because of parents’ requests. Nonoperative management was significantly more likely to fail in patients who had an appendicolith, so this approach should be considered inappropriate for this subgroup of patients, the investigators said.
Larger clinical trials with a randomized design, standardized criteria for antibiotic therapy, and longer follow-up are needed to confirm these preliminary findings, they added.
No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
This is the first data synthesis on the effectiveness of nonoperative management compared with appendectomy in children, and it shows that the evidence at this time is simply insufficient to warrant a change in clinical practice. Appendectomy remains the standard of care for this disease.
Despite the high early “success rate” for nonoperative treatment, patients in this group were nearly nine times more likely to have “treatment failure” than those who underwent immediate appendectomy.
The nonoperative approach remains an experimental proposition and should be offered only under protocol in a clinical trial setting. It clearly merits ongoing consideration, but much more data from high-quality clinical trials are needed.
Monica E. Lopez, MD, and David E. Wesson, MD, are both with the division of pediatric surgery at Baylor College of Medicine and the department of surgery at Texas Children’s Hospital, both in Houston. They reported having no relevant financial disclosures. Dr. Lopez and Dr. Wesson made these remarks in an editorial accompanying Dr. Huang’s report (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0056).
This is the first data synthesis on the effectiveness of nonoperative management compared with appendectomy in children, and it shows that the evidence at this time is simply insufficient to warrant a change in clinical practice. Appendectomy remains the standard of care for this disease.
Despite the high early “success rate” for nonoperative treatment, patients in this group were nearly nine times more likely to have “treatment failure” than those who underwent immediate appendectomy.
The nonoperative approach remains an experimental proposition and should be offered only under protocol in a clinical trial setting. It clearly merits ongoing consideration, but much more data from high-quality clinical trials are needed.
Monica E. Lopez, MD, and David E. Wesson, MD, are both with the division of pediatric surgery at Baylor College of Medicine and the department of surgery at Texas Children’s Hospital, both in Houston. They reported having no relevant financial disclosures. Dr. Lopez and Dr. Wesson made these remarks in an editorial accompanying Dr. Huang’s report (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0056).
This is the first data synthesis on the effectiveness of nonoperative management compared with appendectomy in children, and it shows that the evidence at this time is simply insufficient to warrant a change in clinical practice. Appendectomy remains the standard of care for this disease.
Despite the high early “success rate” for nonoperative treatment, patients in this group were nearly nine times more likely to have “treatment failure” than those who underwent immediate appendectomy.
The nonoperative approach remains an experimental proposition and should be offered only under protocol in a clinical trial setting. It clearly merits ongoing consideration, but much more data from high-quality clinical trials are needed.
Monica E. Lopez, MD, and David E. Wesson, MD, are both with the division of pediatric surgery at Baylor College of Medicine and the department of surgery at Texas Children’s Hospital, both in Houston. They reported having no relevant financial disclosures. Dr. Lopez and Dr. Wesson made these remarks in an editorial accompanying Dr. Huang’s report (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0056).
Nonoperative management of uncomplicated acute appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach, investigators reported March 27 in JAMA Pediatrics.
Nonoperative management, based on antibiotic treatment and close monitoring of the patient, is accepted as safe and effective in adults but has not been well studied in children and adolescents. “Owing to specific anatomical and pathophysiologic features of children, the clinical scenario of acute appendicitis in pediatric patients is different from that in adults, and treatment decisions for children are more difficult,” said Libin Huang, MD, of West China Hospital and Sichuan University, Chengdu, and his associates.
The few clinical trials that have been performed in children have had small sample sizes, so the investigators performed a meta-analysis to pool the results for 404 patients aged 5-18 years. They analyzed data from four single-center prospective but nonrandomized controlled trials and one single-center randomized controlled trial to compare outcomes between 168 patients initially treated with antibiotics and 236 who underwent immediate appendectomy.
Sixteen patients in the nonoperative group (9.5%) had treatment failure, defined as appendectomy within 48 hours (11 patients) or within 1 month of follow-up (5 patients). Three of these patients developed a complication (perforated appendicitis). In comparison, none of the surgery group had treatment failure, and one developed a complication requiring reoperation. Thus, the rate of success in the nonoperative group was 152 of 168 patients, or 90.5%, and the rate of complications was not significantly different between the two study groups, Dr. Huang and his associates said (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0057).
During the following year, 27 patients in the nonoperative group had a histopathologically confirmed recurrence of appendicitis and underwent appendectomy; another 8 had the surgery because of parents’ requests. Nonoperative management was significantly more likely to fail in patients who had an appendicolith, so this approach should be considered inappropriate for this subgroup of patients, the investigators said.
Larger clinical trials with a randomized design, standardized criteria for antibiotic therapy, and longer follow-up are needed to confirm these preliminary findings, they added.
No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
Nonoperative management of uncomplicated acute appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach, investigators reported March 27 in JAMA Pediatrics.
Nonoperative management, based on antibiotic treatment and close monitoring of the patient, is accepted as safe and effective in adults but has not been well studied in children and adolescents. “Owing to specific anatomical and pathophysiologic features of children, the clinical scenario of acute appendicitis in pediatric patients is different from that in adults, and treatment decisions for children are more difficult,” said Libin Huang, MD, of West China Hospital and Sichuan University, Chengdu, and his associates.
The few clinical trials that have been performed in children have had small sample sizes, so the investigators performed a meta-analysis to pool the results for 404 patients aged 5-18 years. They analyzed data from four single-center prospective but nonrandomized controlled trials and one single-center randomized controlled trial to compare outcomes between 168 patients initially treated with antibiotics and 236 who underwent immediate appendectomy.
Sixteen patients in the nonoperative group (9.5%) had treatment failure, defined as appendectomy within 48 hours (11 patients) or within 1 month of follow-up (5 patients). Three of these patients developed a complication (perforated appendicitis). In comparison, none of the surgery group had treatment failure, and one developed a complication requiring reoperation. Thus, the rate of success in the nonoperative group was 152 of 168 patients, or 90.5%, and the rate of complications was not significantly different between the two study groups, Dr. Huang and his associates said (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0057).
During the following year, 27 patients in the nonoperative group had a histopathologically confirmed recurrence of appendicitis and underwent appendectomy; another 8 had the surgery because of parents’ requests. Nonoperative management was significantly more likely to fail in patients who had an appendicolith, so this approach should be considered inappropriate for this subgroup of patients, the investigators said.
Larger clinical trials with a randomized design, standardized criteria for antibiotic therapy, and longer follow-up are needed to confirm these preliminary findings, they added.
No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
FROM JAMA PEDIATRICS
Key clinical point: Nonoperative management of uncomplicated appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach.
Major finding: The rate of treatment success in the nonoperative group was 90.5% (152 of 168 patients).
Data source: A metaanalysis of five single-center clinical trials involving 404 patients aged 5-18 years.
Disclosures: No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
VIDEO: Looking at keloids from a different perspective
ORLANDO – It may be time to start considering new options for treating keloids, according to Amy McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
At the annual meeting of the American Academy of Dermatology, Dr. McMichael discussed one of the highlights of the annual symposium of the Skin of Color Society, held right before the annual meeting, a presentation by Michael Tirgan, MD, who has treated patients with keloids for about 10 years.
Dr. Tirgan, an oncologist based in New York, has a large database and registry of patients and shared some interesting data at the symposium. “What he’s found is that those who come with the supermassive and massive keloids are those who have had the most surgery on their keloid,” Dr. McMichael said in a video interview at the meeting.
His findings shed light on what she described as “a new perspective in the way that we think about keloids” and a new approach to treatment – considering keloids as tumors – not just a scar.
Dr. McMichael had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – It may be time to start considering new options for treating keloids, according to Amy McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
At the annual meeting of the American Academy of Dermatology, Dr. McMichael discussed one of the highlights of the annual symposium of the Skin of Color Society, held right before the annual meeting, a presentation by Michael Tirgan, MD, who has treated patients with keloids for about 10 years.
Dr. Tirgan, an oncologist based in New York, has a large database and registry of patients and shared some interesting data at the symposium. “What he’s found is that those who come with the supermassive and massive keloids are those who have had the most surgery on their keloid,” Dr. McMichael said in a video interview at the meeting.
His findings shed light on what she described as “a new perspective in the way that we think about keloids” and a new approach to treatment – considering keloids as tumors – not just a scar.
Dr. McMichael had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – It may be time to start considering new options for treating keloids, according to Amy McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
At the annual meeting of the American Academy of Dermatology, Dr. McMichael discussed one of the highlights of the annual symposium of the Skin of Color Society, held right before the annual meeting, a presentation by Michael Tirgan, MD, who has treated patients with keloids for about 10 years.
Dr. Tirgan, an oncologist based in New York, has a large database and registry of patients and shared some interesting data at the symposium. “What he’s found is that those who come with the supermassive and massive keloids are those who have had the most surgery on their keloid,” Dr. McMichael said in a video interview at the meeting.
His findings shed light on what she described as “a new perspective in the way that we think about keloids” and a new approach to treatment – considering keloids as tumors – not just a scar.
Dr. McMichael had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AAD 17
LVADs achieve cardiac palliation in muscular dystrophies
At one time, respiratory failure was the primary cause of death in young men and boys with muscular dystrophies, but since improvements in ventilator support have addressed this problem, cardiac complications such as cardiomyopathy have become the main cause of death in this group, with the highest risk of death in people with Duchenne muscular dystrophy (DMD). Researchers from Rome have reported that the novel use of ventricular assist devices in this population can prolong life.
Gianluigi Perri, MD, PhD, of University Hospital and Bambino Gesù Children Hospital in Rome, and his coauthors, shared their experience treating seven patients with dystrophinopathies and dilated cardiomyopathy (DCM) with left ventricular assist devices (LVADs) from February 2011 to February 2016 (J Thorac Cardiovasc Surg. 2017 March;153:669-74). “Our experience indicates that the use of an LVAD as destination therapy in patients with dystrophinopathies with end-stage DCM is feasible, suggesting that it may be suitable as a palliative therapy for the treatment of these patients with no other therapeutic options,” Dr. Perri and his coauthors said.
Heart transplantation is considered the procedure of choice for children with severe advanced heart failure, but transplantation is contraindicated for children with dystrophinopathies because of the risk of respiratory failure and progression of skeletal myopathy leads to limited functional capacity. Hence, Dr. Perri and his coauthors developed their alternative treatment for end-stage heart failure in these children. They used the Jarvik 2000 LVAD (Jarvik Heart Inc., New York) as destination therapy.
Six of the seven patients they operated on had DMD and one had beta-2 sarcoglycan deficit. Their ages ranged from 14.2 to 23.4 years. Two patients had early complications: retropharyngeal bleeding and cholecystectomy; and abdominal bleeding and splenectomy. Two different patients had late complications: gastrostomy; and osteolysis and infection at the pedestal site. Three patients died after the operation: one of stroke at 15 months; one of severe bleeding about 28 months later; and one of lung infection 45 months afterward. Follow-up for the surviving patients ranged from about 2 months to 40 months. Median hospital stay was 77 days.
Dr. Perri and his coauthors noted that the DMD Care Considerations Working Group expanded acceptable therapies for DMD cardiomyopathy to include novel treatments such as mechanical circulatory support and implantable cardioverter-defibrillators.
“Although the best approach remains unclear, it does seem clear that treatment should be more aggressive,” the researchers said. The limited life expectancy of these patients makes transplantation a complicated choice when a shortage of donors is a concern. “Therefore, the alternative therapeutic option is the use of LVAD,” Dr. Perri and his coauthors said.
These patients need care at centers “with a high level of experience of patients with DMD,” the researchers stated. Common comorbidities such as severe kyphoscoliosis and respiratory muscle weakness in this population increase surgical risks.
Dr. Perri and his coauthors used a surgical technique that involved avoiding the left thoracotomy approach common in adults who undergo VAD implantation, because of respiratory insufficiency in these younger patients. They also used cardiopulmonary bypass in all but one patient who had a minimally invasive off-pump procedure through a left anterior minithoracotomy.
The researchers “strongly suggest” noninvasive ventilation after surgery to assist in pulmonary function often compromised by scoliosis and muscle weakness. “Our experience shows that postoperative care can be extremely challenging and is often burdened by unexpected complications,” they noted.
Kyphoscoliosis poses challenges when placing drains, and complications of these patients should be treated only in a specialized center. “Indeed, one of our patients died in a peripheral hospital because they underwent bronchoscopic examination with an endoscope that caused severe and intractable retropharyngeal bleeding,” they said.
The researchers no relevant financial relationships to disclose.
Almost all young men living with Duchenne muscular dystrophy will develop heart failure, but for many of these patients, continuous-flow left ventricular assist devices can provide “reliable support” for up to a decade, David L. S. Morales, MD, of the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:675-6)
“The current series demonstrates, as has been shown at our institute as well as others, that one can provide an effective therapy for certain patients with DMD and heart failure,” Dr. Morales said of the work of Dr. Perri and coauthors. Dr. Morales added that maximizing outcomes in this population hinges on finding the appropriate time point for intervention in the disease process.
While “there is still much to be learned,” Dr. Morales said, Dr. Perri and his coauthors have shown that LVAD therapy is an option in patients with DMD and heart failure who have failed other treatments. “These young men may, therefore, have the option to extend their lives and possibly have the opportunity to benefit from the impressive medical advances being made,” he said. “Perhaps they and their families have been provided hope.”
Dr. Morales disclosed relationships with Berlin Heart, HeartWare and Oregon Total Artificial Heart.
Almost all young men living with Duchenne muscular dystrophy will develop heart failure, but for many of these patients, continuous-flow left ventricular assist devices can provide “reliable support” for up to a decade, David L. S. Morales, MD, of the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:675-6)
“The current series demonstrates, as has been shown at our institute as well as others, that one can provide an effective therapy for certain patients with DMD and heart failure,” Dr. Morales said of the work of Dr. Perri and coauthors. Dr. Morales added that maximizing outcomes in this population hinges on finding the appropriate time point for intervention in the disease process.
While “there is still much to be learned,” Dr. Morales said, Dr. Perri and his coauthors have shown that LVAD therapy is an option in patients with DMD and heart failure who have failed other treatments. “These young men may, therefore, have the option to extend their lives and possibly have the opportunity to benefit from the impressive medical advances being made,” he said. “Perhaps they and their families have been provided hope.”
Dr. Morales disclosed relationships with Berlin Heart, HeartWare and Oregon Total Artificial Heart.
Almost all young men living with Duchenne muscular dystrophy will develop heart failure, but for many of these patients, continuous-flow left ventricular assist devices can provide “reliable support” for up to a decade, David L. S. Morales, MD, of the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:675-6)
“The current series demonstrates, as has been shown at our institute as well as others, that one can provide an effective therapy for certain patients with DMD and heart failure,” Dr. Morales said of the work of Dr. Perri and coauthors. Dr. Morales added that maximizing outcomes in this population hinges on finding the appropriate time point for intervention in the disease process.
While “there is still much to be learned,” Dr. Morales said, Dr. Perri and his coauthors have shown that LVAD therapy is an option in patients with DMD and heart failure who have failed other treatments. “These young men may, therefore, have the option to extend their lives and possibly have the opportunity to benefit from the impressive medical advances being made,” he said. “Perhaps they and their families have been provided hope.”
Dr. Morales disclosed relationships with Berlin Heart, HeartWare and Oregon Total Artificial Heart.
At one time, respiratory failure was the primary cause of death in young men and boys with muscular dystrophies, but since improvements in ventilator support have addressed this problem, cardiac complications such as cardiomyopathy have become the main cause of death in this group, with the highest risk of death in people with Duchenne muscular dystrophy (DMD). Researchers from Rome have reported that the novel use of ventricular assist devices in this population can prolong life.
Gianluigi Perri, MD, PhD, of University Hospital and Bambino Gesù Children Hospital in Rome, and his coauthors, shared their experience treating seven patients with dystrophinopathies and dilated cardiomyopathy (DCM) with left ventricular assist devices (LVADs) from February 2011 to February 2016 (J Thorac Cardiovasc Surg. 2017 March;153:669-74). “Our experience indicates that the use of an LVAD as destination therapy in patients with dystrophinopathies with end-stage DCM is feasible, suggesting that it may be suitable as a palliative therapy for the treatment of these patients with no other therapeutic options,” Dr. Perri and his coauthors said.
Heart transplantation is considered the procedure of choice for children with severe advanced heart failure, but transplantation is contraindicated for children with dystrophinopathies because of the risk of respiratory failure and progression of skeletal myopathy leads to limited functional capacity. Hence, Dr. Perri and his coauthors developed their alternative treatment for end-stage heart failure in these children. They used the Jarvik 2000 LVAD (Jarvik Heart Inc., New York) as destination therapy.
Six of the seven patients they operated on had DMD and one had beta-2 sarcoglycan deficit. Their ages ranged from 14.2 to 23.4 years. Two patients had early complications: retropharyngeal bleeding and cholecystectomy; and abdominal bleeding and splenectomy. Two different patients had late complications: gastrostomy; and osteolysis and infection at the pedestal site. Three patients died after the operation: one of stroke at 15 months; one of severe bleeding about 28 months later; and one of lung infection 45 months afterward. Follow-up for the surviving patients ranged from about 2 months to 40 months. Median hospital stay was 77 days.
Dr. Perri and his coauthors noted that the DMD Care Considerations Working Group expanded acceptable therapies for DMD cardiomyopathy to include novel treatments such as mechanical circulatory support and implantable cardioverter-defibrillators.
“Although the best approach remains unclear, it does seem clear that treatment should be more aggressive,” the researchers said. The limited life expectancy of these patients makes transplantation a complicated choice when a shortage of donors is a concern. “Therefore, the alternative therapeutic option is the use of LVAD,” Dr. Perri and his coauthors said.
These patients need care at centers “with a high level of experience of patients with DMD,” the researchers stated. Common comorbidities such as severe kyphoscoliosis and respiratory muscle weakness in this population increase surgical risks.
Dr. Perri and his coauthors used a surgical technique that involved avoiding the left thoracotomy approach common in adults who undergo VAD implantation, because of respiratory insufficiency in these younger patients. They also used cardiopulmonary bypass in all but one patient who had a minimally invasive off-pump procedure through a left anterior minithoracotomy.
The researchers “strongly suggest” noninvasive ventilation after surgery to assist in pulmonary function often compromised by scoliosis and muscle weakness. “Our experience shows that postoperative care can be extremely challenging and is often burdened by unexpected complications,” they noted.
Kyphoscoliosis poses challenges when placing drains, and complications of these patients should be treated only in a specialized center. “Indeed, one of our patients died in a peripheral hospital because they underwent bronchoscopic examination with an endoscope that caused severe and intractable retropharyngeal bleeding,” they said.
The researchers no relevant financial relationships to disclose.
At one time, respiratory failure was the primary cause of death in young men and boys with muscular dystrophies, but since improvements in ventilator support have addressed this problem, cardiac complications such as cardiomyopathy have become the main cause of death in this group, with the highest risk of death in people with Duchenne muscular dystrophy (DMD). Researchers from Rome have reported that the novel use of ventricular assist devices in this population can prolong life.
Gianluigi Perri, MD, PhD, of University Hospital and Bambino Gesù Children Hospital in Rome, and his coauthors, shared their experience treating seven patients with dystrophinopathies and dilated cardiomyopathy (DCM) with left ventricular assist devices (LVADs) from February 2011 to February 2016 (J Thorac Cardiovasc Surg. 2017 March;153:669-74). “Our experience indicates that the use of an LVAD as destination therapy in patients with dystrophinopathies with end-stage DCM is feasible, suggesting that it may be suitable as a palliative therapy for the treatment of these patients with no other therapeutic options,” Dr. Perri and his coauthors said.
Heart transplantation is considered the procedure of choice for children with severe advanced heart failure, but transplantation is contraindicated for children with dystrophinopathies because of the risk of respiratory failure and progression of skeletal myopathy leads to limited functional capacity. Hence, Dr. Perri and his coauthors developed their alternative treatment for end-stage heart failure in these children. They used the Jarvik 2000 LVAD (Jarvik Heart Inc., New York) as destination therapy.
Six of the seven patients they operated on had DMD and one had beta-2 sarcoglycan deficit. Their ages ranged from 14.2 to 23.4 years. Two patients had early complications: retropharyngeal bleeding and cholecystectomy; and abdominal bleeding and splenectomy. Two different patients had late complications: gastrostomy; and osteolysis and infection at the pedestal site. Three patients died after the operation: one of stroke at 15 months; one of severe bleeding about 28 months later; and one of lung infection 45 months afterward. Follow-up for the surviving patients ranged from about 2 months to 40 months. Median hospital stay was 77 days.
Dr. Perri and his coauthors noted that the DMD Care Considerations Working Group expanded acceptable therapies for DMD cardiomyopathy to include novel treatments such as mechanical circulatory support and implantable cardioverter-defibrillators.
“Although the best approach remains unclear, it does seem clear that treatment should be more aggressive,” the researchers said. The limited life expectancy of these patients makes transplantation a complicated choice when a shortage of donors is a concern. “Therefore, the alternative therapeutic option is the use of LVAD,” Dr. Perri and his coauthors said.
These patients need care at centers “with a high level of experience of patients with DMD,” the researchers stated. Common comorbidities such as severe kyphoscoliosis and respiratory muscle weakness in this population increase surgical risks.
Dr. Perri and his coauthors used a surgical technique that involved avoiding the left thoracotomy approach common in adults who undergo VAD implantation, because of respiratory insufficiency in these younger patients. They also used cardiopulmonary bypass in all but one patient who had a minimally invasive off-pump procedure through a left anterior minithoracotomy.
The researchers “strongly suggest” noninvasive ventilation after surgery to assist in pulmonary function often compromised by scoliosis and muscle weakness. “Our experience shows that postoperative care can be extremely challenging and is often burdened by unexpected complications,” they noted.
Kyphoscoliosis poses challenges when placing drains, and complications of these patients should be treated only in a specialized center. “Indeed, one of our patients died in a peripheral hospital because they underwent bronchoscopic examination with an endoscope that caused severe and intractable retropharyngeal bleeding,” they said.
The researchers no relevant financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: A left ventricular assist device can be used as destination therapy in patients with Duchenne muscular dystrophy dystrophinopathies and end-stage dilated cardiomyopathy.
Major finding: Four of seven patients who had LVAD survived long term, and survival for the three who died ranged from 15 to 44 months.
Data source: Single-center, retrospective review of seven patients with DMD who had LVAD for DCM from February 2011 to February 2016.
Disclosure: Dr. Perri and his coauthors reported having no relevant financial disclosures.
Can better operations improve CABG in women?
Worse outcomes after cardiovascular arterial bypass grafting (CABG) in women have been attributed to a number of clinical and nonclinical factors: older age, delayed diagnosis and treatment, more comorbidities, smaller body size, underuse of arterial grafts, and referral bias. However, a team of Cleveland Clinic researchers reported that women were less likely than men to have bilateral–internal thoracic artery (ITA) grafting and complete revascularization, both of which are linked to better long-term survival.
“Women had less favorable preoperative characteristics than men but received fewer bilateral-ITA grafts and less all-arterial grafting as part of their revascularization strategy,” Tamer Attia, MD, MSc, and his coauthors said in the study published in the Journal of Thoracic and Cardiovascular Surgery. They also found that women were more likely to die in the hospital after CABG and had lower risk-adjusted long-term survival than men (2017 March;153:571-9).
Survival was lowest in women who were not completely revascularized and had no ITA grafting, while survival was highest in men who were completely revascularized and had bilateral grafting, Dr. Attia and coauthors said.
The researchers’ goal was to use extensive risk adjustment to evaluate how differences in revascularization strategies influenced survival of men and women after CABG. They analyzed 57,943 primary, isolated CABG procedures performed at Cleveland Clinic from 1972 to 2011, including 11,009 procedures in women.
The researchers identified differences between sexes in three key areas: revascularization strategies, in-hospital outcomes, and time-related survival.
With regard to revascularization strategies, while men were significantly more likely to have incomplete revascularization than were women, women received significantly fewer arterial grafts (8.4% vs. 9.3% for men). This included fewer bilateral-ITA grafts and radial artery grafts, less use of total arterial revascularization, and greater use of saphenous vein grafts (SVGs) to the left anterior descending artery. Women were also significantly more likely to receive only SVGs for revascularization (32% vs. 30% for men; P less than .0001). Most operations for both women and men were done with cardiopulmonary bypass, but significantly more women had off-pump procedures (5.4% vs. 2.9%).
In the hospital after operations, women were significantly more prone to postoperative deep sternal–wound infections and septicemias, as well as strokes and renal failure, including dialysis-dependent renal failure. Women also had higher rates of new-onset atrial fibrillation (15% vs. 13% in men), and higher rates of mechanical ventilation for more than 24 hours (13% vs. 9.2%). Women spent more time in the ICU and hospital overall, and their in-hospital death rate was more than double that of men (2.7% vs. 1.1%).
Women also had shorter long-term survival, “and this has persisted since the beginning of CABG at Cleveland Clinic,” Dr. Attia and coauthors said. What’s more, the survival gap between women in the study and women in the general population post CABG was wider than that in men. “After we adjusted for patient and revascularization strategy differences, female sex remained an independent risk factor for death overall and both early and late after CABG.”
Incomplete revascularization had greater consequences for women than for men. At 10 years, 58% of women with incomplete revascularization survived, vs. 70% of men. At 20 years, the rates were 25% for women and 35% for men. “Use of ITA grafts was associated with better survival than use of SVGs alone and was best when bilateral-ITA grafting was performed,” Dr. Attia and coauthors said. However, the study determined that bilateral grafting seems less effective in women long-term. “Hence, a patient’s sex deserves special consideration in operative planning.”
Many questions about CABG in women remain: Identifying which female patients would benefit from more meticulous conduit harvesting, from better coronary artery selection, and from bilateral-ITA grafting and which are less susceptible to sternal would infections could increase appropriate use of bilateral-ITA grafting, the researchers noted. “The difference in effectiveness of bilateral-ITA grafting needs to be considered in women at elevated risk for bilateral-ITA harvesting complications.”
Coauthor Ellen Mayer Sabik, MD, is a principal investigator for Abbott Laboratories and is on the scientific advisory board of Medtronic. Dr. Attia and all other coauthors reported having no relevant financial relationships to disclose.
As with other long-term analyses, the answer in this study “is in the shadows as opposed to the spotlight,” George L. Hicks Jr., MD, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153[3]:580-1).
Dr. Hicks of the University of Rochester (N.Y.) noted key limitations of the study: use of all-cause mortality, substandard use of bilateral– or single–internal thoracic artery grafting, little data about postdischarge cholesterol levels, diabetes incidence, or blood pressure, among others. However, “the authors raise the banner for the continued need for increased use of arterial revascularization with the eventual hope that the Arterial Revascularization Trial will reinforce the survival benefits manifested by that strategy,” he said.
Reducing risks and not changing the type of operation will even out the differences in postoperative survival between genders, he indicated. “Furthermore, the extension of similar therapies – for example, [bilateral–internal thoracic artery] or all-arterial grafting and improved long-term risk modification in both men and women – may improve the inequality but not eliminate the differences until we know that both men and women come from the same planet,” he said.
Dr. Hicks reported having no relevant financial relationships to disclose.
As with other long-term analyses, the answer in this study “is in the shadows as opposed to the spotlight,” George L. Hicks Jr., MD, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153[3]:580-1).
Dr. Hicks of the University of Rochester (N.Y.) noted key limitations of the study: use of all-cause mortality, substandard use of bilateral– or single–internal thoracic artery grafting, little data about postdischarge cholesterol levels, diabetes incidence, or blood pressure, among others. However, “the authors raise the banner for the continued need for increased use of arterial revascularization with the eventual hope that the Arterial Revascularization Trial will reinforce the survival benefits manifested by that strategy,” he said.
Reducing risks and not changing the type of operation will even out the differences in postoperative survival between genders, he indicated. “Furthermore, the extension of similar therapies – for example, [bilateral–internal thoracic artery] or all-arterial grafting and improved long-term risk modification in both men and women – may improve the inequality but not eliminate the differences until we know that both men and women come from the same planet,” he said.
Dr. Hicks reported having no relevant financial relationships to disclose.
As with other long-term analyses, the answer in this study “is in the shadows as opposed to the spotlight,” George L. Hicks Jr., MD, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153[3]:580-1).
Dr. Hicks of the University of Rochester (N.Y.) noted key limitations of the study: use of all-cause mortality, substandard use of bilateral– or single–internal thoracic artery grafting, little data about postdischarge cholesterol levels, diabetes incidence, or blood pressure, among others. However, “the authors raise the banner for the continued need for increased use of arterial revascularization with the eventual hope that the Arterial Revascularization Trial will reinforce the survival benefits manifested by that strategy,” he said.
Reducing risks and not changing the type of operation will even out the differences in postoperative survival between genders, he indicated. “Furthermore, the extension of similar therapies – for example, [bilateral–internal thoracic artery] or all-arterial grafting and improved long-term risk modification in both men and women – may improve the inequality but not eliminate the differences until we know that both men and women come from the same planet,” he said.
Dr. Hicks reported having no relevant financial relationships to disclose.
Worse outcomes after cardiovascular arterial bypass grafting (CABG) in women have been attributed to a number of clinical and nonclinical factors: older age, delayed diagnosis and treatment, more comorbidities, smaller body size, underuse of arterial grafts, and referral bias. However, a team of Cleveland Clinic researchers reported that women were less likely than men to have bilateral–internal thoracic artery (ITA) grafting and complete revascularization, both of which are linked to better long-term survival.
“Women had less favorable preoperative characteristics than men but received fewer bilateral-ITA grafts and less all-arterial grafting as part of their revascularization strategy,” Tamer Attia, MD, MSc, and his coauthors said in the study published in the Journal of Thoracic and Cardiovascular Surgery. They also found that women were more likely to die in the hospital after CABG and had lower risk-adjusted long-term survival than men (2017 March;153:571-9).
Survival was lowest in women who were not completely revascularized and had no ITA grafting, while survival was highest in men who were completely revascularized and had bilateral grafting, Dr. Attia and coauthors said.
The researchers’ goal was to use extensive risk adjustment to evaluate how differences in revascularization strategies influenced survival of men and women after CABG. They analyzed 57,943 primary, isolated CABG procedures performed at Cleveland Clinic from 1972 to 2011, including 11,009 procedures in women.
The researchers identified differences between sexes in three key areas: revascularization strategies, in-hospital outcomes, and time-related survival.
With regard to revascularization strategies, while men were significantly more likely to have incomplete revascularization than were women, women received significantly fewer arterial grafts (8.4% vs. 9.3% for men). This included fewer bilateral-ITA grafts and radial artery grafts, less use of total arterial revascularization, and greater use of saphenous vein grafts (SVGs) to the left anterior descending artery. Women were also significantly more likely to receive only SVGs for revascularization (32% vs. 30% for men; P less than .0001). Most operations for both women and men were done with cardiopulmonary bypass, but significantly more women had off-pump procedures (5.4% vs. 2.9%).
In the hospital after operations, women were significantly more prone to postoperative deep sternal–wound infections and septicemias, as well as strokes and renal failure, including dialysis-dependent renal failure. Women also had higher rates of new-onset atrial fibrillation (15% vs. 13% in men), and higher rates of mechanical ventilation for more than 24 hours (13% vs. 9.2%). Women spent more time in the ICU and hospital overall, and their in-hospital death rate was more than double that of men (2.7% vs. 1.1%).
Women also had shorter long-term survival, “and this has persisted since the beginning of CABG at Cleveland Clinic,” Dr. Attia and coauthors said. What’s more, the survival gap between women in the study and women in the general population post CABG was wider than that in men. “After we adjusted for patient and revascularization strategy differences, female sex remained an independent risk factor for death overall and both early and late after CABG.”
Incomplete revascularization had greater consequences for women than for men. At 10 years, 58% of women with incomplete revascularization survived, vs. 70% of men. At 20 years, the rates were 25% for women and 35% for men. “Use of ITA grafts was associated with better survival than use of SVGs alone and was best when bilateral-ITA grafting was performed,” Dr. Attia and coauthors said. However, the study determined that bilateral grafting seems less effective in women long-term. “Hence, a patient’s sex deserves special consideration in operative planning.”
Many questions about CABG in women remain: Identifying which female patients would benefit from more meticulous conduit harvesting, from better coronary artery selection, and from bilateral-ITA grafting and which are less susceptible to sternal would infections could increase appropriate use of bilateral-ITA grafting, the researchers noted. “The difference in effectiveness of bilateral-ITA grafting needs to be considered in women at elevated risk for bilateral-ITA harvesting complications.”
Coauthor Ellen Mayer Sabik, MD, is a principal investigator for Abbott Laboratories and is on the scientific advisory board of Medtronic. Dr. Attia and all other coauthors reported having no relevant financial relationships to disclose.
Worse outcomes after cardiovascular arterial bypass grafting (CABG) in women have been attributed to a number of clinical and nonclinical factors: older age, delayed diagnosis and treatment, more comorbidities, smaller body size, underuse of arterial grafts, and referral bias. However, a team of Cleveland Clinic researchers reported that women were less likely than men to have bilateral–internal thoracic artery (ITA) grafting and complete revascularization, both of which are linked to better long-term survival.
“Women had less favorable preoperative characteristics than men but received fewer bilateral-ITA grafts and less all-arterial grafting as part of their revascularization strategy,” Tamer Attia, MD, MSc, and his coauthors said in the study published in the Journal of Thoracic and Cardiovascular Surgery. They also found that women were more likely to die in the hospital after CABG and had lower risk-adjusted long-term survival than men (2017 March;153:571-9).
Survival was lowest in women who were not completely revascularized and had no ITA grafting, while survival was highest in men who were completely revascularized and had bilateral grafting, Dr. Attia and coauthors said.
The researchers’ goal was to use extensive risk adjustment to evaluate how differences in revascularization strategies influenced survival of men and women after CABG. They analyzed 57,943 primary, isolated CABG procedures performed at Cleveland Clinic from 1972 to 2011, including 11,009 procedures in women.
The researchers identified differences between sexes in three key areas: revascularization strategies, in-hospital outcomes, and time-related survival.
With regard to revascularization strategies, while men were significantly more likely to have incomplete revascularization than were women, women received significantly fewer arterial grafts (8.4% vs. 9.3% for men). This included fewer bilateral-ITA grafts and radial artery grafts, less use of total arterial revascularization, and greater use of saphenous vein grafts (SVGs) to the left anterior descending artery. Women were also significantly more likely to receive only SVGs for revascularization (32% vs. 30% for men; P less than .0001). Most operations for both women and men were done with cardiopulmonary bypass, but significantly more women had off-pump procedures (5.4% vs. 2.9%).
In the hospital after operations, women were significantly more prone to postoperative deep sternal–wound infections and septicemias, as well as strokes and renal failure, including dialysis-dependent renal failure. Women also had higher rates of new-onset atrial fibrillation (15% vs. 13% in men), and higher rates of mechanical ventilation for more than 24 hours (13% vs. 9.2%). Women spent more time in the ICU and hospital overall, and their in-hospital death rate was more than double that of men (2.7% vs. 1.1%).
Women also had shorter long-term survival, “and this has persisted since the beginning of CABG at Cleveland Clinic,” Dr. Attia and coauthors said. What’s more, the survival gap between women in the study and women in the general population post CABG was wider than that in men. “After we adjusted for patient and revascularization strategy differences, female sex remained an independent risk factor for death overall and both early and late after CABG.”
Incomplete revascularization had greater consequences for women than for men. At 10 years, 58% of women with incomplete revascularization survived, vs. 70% of men. At 20 years, the rates were 25% for women and 35% for men. “Use of ITA grafts was associated with better survival than use of SVGs alone and was best when bilateral-ITA grafting was performed,” Dr. Attia and coauthors said. However, the study determined that bilateral grafting seems less effective in women long-term. “Hence, a patient’s sex deserves special consideration in operative planning.”
Many questions about CABG in women remain: Identifying which female patients would benefit from more meticulous conduit harvesting, from better coronary artery selection, and from bilateral-ITA grafting and which are less susceptible to sternal would infections could increase appropriate use of bilateral-ITA grafting, the researchers noted. “The difference in effectiveness of bilateral-ITA grafting needs to be considered in women at elevated risk for bilateral-ITA harvesting complications.”
Coauthor Ellen Mayer Sabik, MD, is a principal investigator for Abbott Laboratories and is on the scientific advisory board of Medtronic. Dr. Attia and all other coauthors reported having no relevant financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Survival rates after CABG are worse for women than for men.
Major finding: In both men and women, complete revascularization and use of bilateral-ITA grafting achieve better long-term survival than incomplete revascularization and single-ITA grafting.
Data source: Analysis of 57,943 adults who had primary isolated CABG from 1972 to 2011 at Cleveland Clinic.
Disclosure: Coauthor Ellen Mayer Sabik, MD, is a principal investigator for Abbott Laboratories and is on the scientific advisory board of Medtronic. Dr. Attia and all other coauthors reported having no relevant financial disclosures.