User login
VIDEO: Public reporting of congenital heart disease outcomes should be easily understood
HOUSTON – Survival statistics, surgeon-specific experience, and complication rates are the types of information most sought by parents of children with congenital heart disease, results from a large survey suggest.
Future efforts in public reporting for congenital heart surgery outcomes should have better methods for presenting the data in a valid, easily interpreted format, explained study investigator Mallory L. Irons, MD, an integrated cardiac surgery resident at the Hospital of the University of Pennsylvania, Philadelphia.
“We’re doing a good job of public reporting currently, but what we’re doing is not meeting the needs of all of our stakeholders – in this case, the parents of children with congenital heart disease,” Dr. Irons said in an interview at the annual meeting of the Society of Thoracic Surgeons. “The optimal public reporting scheme still has yet to be determined.”
Dr. Irons reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON – Survival statistics, surgeon-specific experience, and complication rates are the types of information most sought by parents of children with congenital heart disease, results from a large survey suggest.
Future efforts in public reporting for congenital heart surgery outcomes should have better methods for presenting the data in a valid, easily interpreted format, explained study investigator Mallory L. Irons, MD, an integrated cardiac surgery resident at the Hospital of the University of Pennsylvania, Philadelphia.
“We’re doing a good job of public reporting currently, but what we’re doing is not meeting the needs of all of our stakeholders – in this case, the parents of children with congenital heart disease,” Dr. Irons said in an interview at the annual meeting of the Society of Thoracic Surgeons. “The optimal public reporting scheme still has yet to be determined.”
Dr. Irons reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON – Survival statistics, surgeon-specific experience, and complication rates are the types of information most sought by parents of children with congenital heart disease, results from a large survey suggest.
Future efforts in public reporting for congenital heart surgery outcomes should have better methods for presenting the data in a valid, easily interpreted format, explained study investigator Mallory L. Irons, MD, an integrated cardiac surgery resident at the Hospital of the University of Pennsylvania, Philadelphia.
“We’re doing a good job of public reporting currently, but what we’re doing is not meeting the needs of all of our stakeholders – in this case, the parents of children with congenital heart disease,” Dr. Irons said in an interview at the annual meeting of the Society of Thoracic Surgeons. “The optimal public reporting scheme still has yet to be determined.”
Dr. Irons reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FROM THE STS ANNUAL MEETING HOUSTON
After TAVR, 1 in 10 Medicare patients need permanent pacemaker
HOUSTON – About 1 in 10 Medicare patients require implantation of a permanent pacemaker following transcatheter aortic valve replacement, results from a large analysis showed.
“There is conflicting evidence and some debate over permanent pacemaker placement following transcatheter aortic valve replacement – whether it has a protective or adverse effect, and how often it takes place,” study investigator Fenton H. McCarthy, MD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
One recent study found that permanent pacemaker placement within 30 days post TAVR was found in 6.7% of patients undergoing balloon-expanding or self-expanding valve implantation, and is associated with increased mortality and hospitalizations (JACC Cardiovasc Interv. 2016 Nov 14;9[21]:2189-2199).
To evaluate the relationship between permanent pacemaker implantation and long-term patient outcomes among Medicare beneficiaries undergoing TAVR, Dr. McCarthy, a cardiothoracic surgery fellow at the University of Pennsylvania, Philadelphia, and his associates used Medicare carrier claims and Medicare Provider Analysis and Review files to identify 14,305 TAVR patients between January 2011 and December 2013.
The researchers used univariate Kaplan survival estimates and multivariable models to analyze survival, readmission and risk factors for pacemaker implantation.
The mean age of the 14,305 TAVR patients studied was 83 years, and 11% received a permanent pacemaker after TAVR. Of these, 9% received the pacemaker at index hospitalization, 1% at 30 days after implant, 0.5% at 90 days after implant, and 1% at 1 year after implant. Patient age of greater than 90 years was a significant predictor of pacemaker placement, with an odds ratio of 1.7 (P less than .01).
Dr. McCarthy and his associates observed that the readmission rates for pacemaker placement and no pacemaker placement at index hospitalization were similar at 30 days (21% vs. 19%, respectively), at 90 days (33% vs. 31%) and at 1 year (43% in both groups of patients).
In addition, Kaplan Meier estimates revealed no significant difference in long-term survival for patients with pacemaker placement within 30 days of TAVR, while multivariate Cox proportional hazard modeling revealed that pacemaker placement is not a predictor of long-term mortality (hazard ratio, 1.03; P = .65).
“This was the largest study to evaluate the question of incidence and effect of permanent pacemaker in the transcatheter aortic valve replacement population in the United States,” Dr. McCarthy said. “The size of our data set and the fact that the Medicare database includes all types of patients, regardless of trial participation, study or registry, is a strength of this study. Some other studies have used different inclusion and exclusion criteria. We used broad inclusion criteria and evaluated patients from as many different centers as possible.”
A key limitation of the study, he said, was that the researchers were unable to determine whether a patient received a balloon-expanding or self-expanding TAVR.
Dr. McCarthy reported having no financial disclosures.
HOUSTON – About 1 in 10 Medicare patients require implantation of a permanent pacemaker following transcatheter aortic valve replacement, results from a large analysis showed.
“There is conflicting evidence and some debate over permanent pacemaker placement following transcatheter aortic valve replacement – whether it has a protective or adverse effect, and how often it takes place,” study investigator Fenton H. McCarthy, MD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
One recent study found that permanent pacemaker placement within 30 days post TAVR was found in 6.7% of patients undergoing balloon-expanding or self-expanding valve implantation, and is associated with increased mortality and hospitalizations (JACC Cardiovasc Interv. 2016 Nov 14;9[21]:2189-2199).
To evaluate the relationship between permanent pacemaker implantation and long-term patient outcomes among Medicare beneficiaries undergoing TAVR, Dr. McCarthy, a cardiothoracic surgery fellow at the University of Pennsylvania, Philadelphia, and his associates used Medicare carrier claims and Medicare Provider Analysis and Review files to identify 14,305 TAVR patients between January 2011 and December 2013.
The researchers used univariate Kaplan survival estimates and multivariable models to analyze survival, readmission and risk factors for pacemaker implantation.
The mean age of the 14,305 TAVR patients studied was 83 years, and 11% received a permanent pacemaker after TAVR. Of these, 9% received the pacemaker at index hospitalization, 1% at 30 days after implant, 0.5% at 90 days after implant, and 1% at 1 year after implant. Patient age of greater than 90 years was a significant predictor of pacemaker placement, with an odds ratio of 1.7 (P less than .01).
Dr. McCarthy and his associates observed that the readmission rates for pacemaker placement and no pacemaker placement at index hospitalization were similar at 30 days (21% vs. 19%, respectively), at 90 days (33% vs. 31%) and at 1 year (43% in both groups of patients).
In addition, Kaplan Meier estimates revealed no significant difference in long-term survival for patients with pacemaker placement within 30 days of TAVR, while multivariate Cox proportional hazard modeling revealed that pacemaker placement is not a predictor of long-term mortality (hazard ratio, 1.03; P = .65).
“This was the largest study to evaluate the question of incidence and effect of permanent pacemaker in the transcatheter aortic valve replacement population in the United States,” Dr. McCarthy said. “The size of our data set and the fact that the Medicare database includes all types of patients, regardless of trial participation, study or registry, is a strength of this study. Some other studies have used different inclusion and exclusion criteria. We used broad inclusion criteria and evaluated patients from as many different centers as possible.”
A key limitation of the study, he said, was that the researchers were unable to determine whether a patient received a balloon-expanding or self-expanding TAVR.
Dr. McCarthy reported having no financial disclosures.
HOUSTON – About 1 in 10 Medicare patients require implantation of a permanent pacemaker following transcatheter aortic valve replacement, results from a large analysis showed.
“There is conflicting evidence and some debate over permanent pacemaker placement following transcatheter aortic valve replacement – whether it has a protective or adverse effect, and how often it takes place,” study investigator Fenton H. McCarthy, MD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
One recent study found that permanent pacemaker placement within 30 days post TAVR was found in 6.7% of patients undergoing balloon-expanding or self-expanding valve implantation, and is associated with increased mortality and hospitalizations (JACC Cardiovasc Interv. 2016 Nov 14;9[21]:2189-2199).
To evaluate the relationship between permanent pacemaker implantation and long-term patient outcomes among Medicare beneficiaries undergoing TAVR, Dr. McCarthy, a cardiothoracic surgery fellow at the University of Pennsylvania, Philadelphia, and his associates used Medicare carrier claims and Medicare Provider Analysis and Review files to identify 14,305 TAVR patients between January 2011 and December 2013.
The researchers used univariate Kaplan survival estimates and multivariable models to analyze survival, readmission and risk factors for pacemaker implantation.
The mean age of the 14,305 TAVR patients studied was 83 years, and 11% received a permanent pacemaker after TAVR. Of these, 9% received the pacemaker at index hospitalization, 1% at 30 days after implant, 0.5% at 90 days after implant, and 1% at 1 year after implant. Patient age of greater than 90 years was a significant predictor of pacemaker placement, with an odds ratio of 1.7 (P less than .01).
Dr. McCarthy and his associates observed that the readmission rates for pacemaker placement and no pacemaker placement at index hospitalization were similar at 30 days (21% vs. 19%, respectively), at 90 days (33% vs. 31%) and at 1 year (43% in both groups of patients).
In addition, Kaplan Meier estimates revealed no significant difference in long-term survival for patients with pacemaker placement within 30 days of TAVR, while multivariate Cox proportional hazard modeling revealed that pacemaker placement is not a predictor of long-term mortality (hazard ratio, 1.03; P = .65).
“This was the largest study to evaluate the question of incidence and effect of permanent pacemaker in the transcatheter aortic valve replacement population in the United States,” Dr. McCarthy said. “The size of our data set and the fact that the Medicare database includes all types of patients, regardless of trial participation, study or registry, is a strength of this study. Some other studies have used different inclusion and exclusion criteria. We used broad inclusion criteria and evaluated patients from as many different centers as possible.”
A key limitation of the study, he said, was that the researchers were unable to determine whether a patient received a balloon-expanding or self-expanding TAVR.
Dr. McCarthy reported having no financial disclosures.
Key clinical point:
Major finding: Pacemaker placement is not a predictor of long-term mortality (hazard ratio, 1.03; P = .65).
Data source: A study of 14,305 Medicare beneficiaries who underwent TAVR between January 2011 and December 2013.
Disclosures: Dr. McCarthy reported having no financial disclosures.
7 Myomectomy myths debunked
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
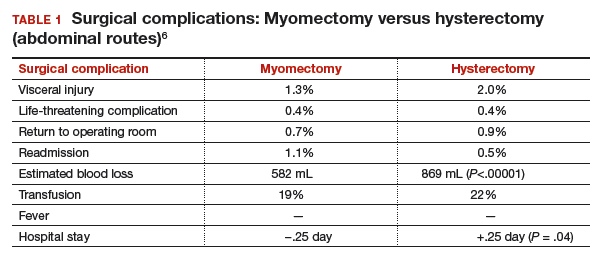
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6
MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
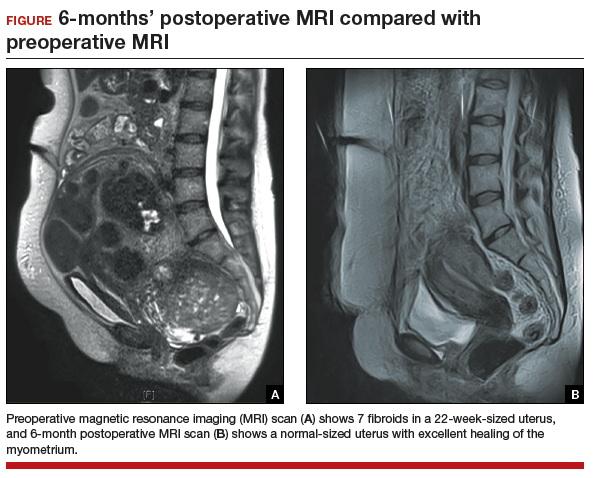
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.
MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15
Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
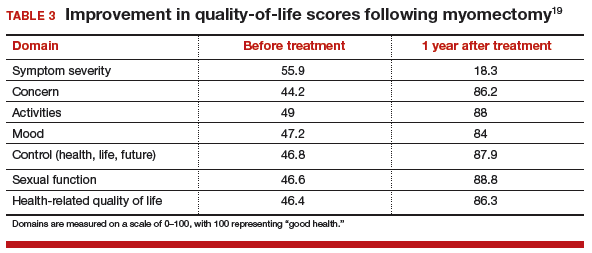
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20
For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6
MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.
MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15
Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20
For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6
MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.
MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15
Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20
For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
Should coffee consumption be added as an adjunct to the postoperative care of gynecologic oncology patients?
EXPERT COMMENTARY
Postoperative ileus is a common complication following abdominal surgery, particularly for patients undergoing laparotomy. Ileus is frustrating for patients and providers alike, and its occurrence may prolong the length of hospital stay, increase the cost of care, worsen patient satisfaction, and potentially delay postoperative treatments, such as chemotherapy for patients with gynecologic malignancies. The etiology of ileus is multifactorial, but it is thought to be caused primarily by a local inflammatory response from mechanical handling and irritation of the bowel. Although various interventions, such as laxatives, peripheral mu antagonists, and chewing gum, have been shown to reduce the occurrence of ileus, the effectiveness of these interventions varies, and ileus remains a major source of morbidity.1
Details of the study
To investigate whether coffee consumption accelerates recovery of bowel function following surgery, Güngördük and colleagues conducted a randomized controlled trial of coffee consumption after laparotomy with hysterectomy and staging for patients with gynecologic malignancies. This intervention avoids costs associated with drugs such as oral mu antagonists.
The trial included 114 women; after surgery, 58 were assigned to consume coffee 3 times daily and 56 received routine postoperative care without coffee consumption. The primary outcome measure was the time to the first passage of flatus after surgery. Time to first bowel movement and time to tolerance of a solid diet were secondary outcomes.
The results of this trial are consistent with prior study findings in colorectal surgery.2 Güngördük and associates found that patients in the coffee-consumption group, compared with controls, had reduced the time to flatus by 12 hours (mean [SD] time to flatus, 30.2 [8.0] vs 40.2 [12.1] hours; P<.001), shortened time to full diet by 1.3 days (mean [SD] time to tolerate food, 3.4 [1.2] vs 4.7 [1.6] days; P<.001), reduced time to first bowel movement by 12 hours (43.1 [9.4] vs 58.5 [17.0] hours; P<.001), and shortened length of hospital stay by 1 day. Symptoms of ileus were reduced from 52% to 14% with coffee consumption.
Study limitation. An important weakness of this study is that although the authors defined the severity of ileus by time to resolution, they did not define what constituted a diagnosis of ileus in the first place.
Unanswered questions. Coffee is a known diarrhetic, so it is not unexpected that its use shortened time to flatus and first bowel movement. What is not known, however, is whether coffee consumption improves recovery. The significance of a 1-day reduction in hospital stay is unclear given the relatively prolonged hospitalization (6 to 7 days) seen in this investigation of patients with mixed gynecologic malignancies who underwent staging only. In contrast, another study showed that, for patients managed within an enhanced recovery pathway (a multimodal perioperative care enhancement protocol), median length of stay was 4 days for patients who underwent staging alone and 5 days for patients with ovarian cancer (40% underwent enteric resections).3 Thus, the effects of coffee consumption are unclear for patients managed with an optimized perioperative pathway.
The improvement in oral intake is also of questionable significance since these patients tolerated a solid diet 3 to 4 days after surgery, compared with the evening of surgery for most patients managed with enhanced recovery.
Incisional injection of liposomal bupivacaine has been associated with a reduction in the rate of ileus from 22% to 11% after complex cytoreduction for ovarian cancer when added to an existing enhanced recovery pathway; rates were only 5% for patients undergoing staging alone.4 These findings may be due to the significant reduction in opioid use that accompanied the use of liposomal bupivacaine.
--SEAN C. DOWDY, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Nelson G, Altman AD, Nick A, et al. Guidelines for post- operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140(2):323-332.
- Müller SA, Rahbari NN, Schneider F, et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br J Surg. 2012;99(11):1530-1538.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122 (2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128(5):1009-1017.
EXPERT COMMENTARY
Postoperative ileus is a common complication following abdominal surgery, particularly for patients undergoing laparotomy. Ileus is frustrating for patients and providers alike, and its occurrence may prolong the length of hospital stay, increase the cost of care, worsen patient satisfaction, and potentially delay postoperative treatments, such as chemotherapy for patients with gynecologic malignancies. The etiology of ileus is multifactorial, but it is thought to be caused primarily by a local inflammatory response from mechanical handling and irritation of the bowel. Although various interventions, such as laxatives, peripheral mu antagonists, and chewing gum, have been shown to reduce the occurrence of ileus, the effectiveness of these interventions varies, and ileus remains a major source of morbidity.1
Details of the study
To investigate whether coffee consumption accelerates recovery of bowel function following surgery, Güngördük and colleagues conducted a randomized controlled trial of coffee consumption after laparotomy with hysterectomy and staging for patients with gynecologic malignancies. This intervention avoids costs associated with drugs such as oral mu antagonists.
The trial included 114 women; after surgery, 58 were assigned to consume coffee 3 times daily and 56 received routine postoperative care without coffee consumption. The primary outcome measure was the time to the first passage of flatus after surgery. Time to first bowel movement and time to tolerance of a solid diet were secondary outcomes.
The results of this trial are consistent with prior study findings in colorectal surgery.2 Güngördük and associates found that patients in the coffee-consumption group, compared with controls, had reduced the time to flatus by 12 hours (mean [SD] time to flatus, 30.2 [8.0] vs 40.2 [12.1] hours; P<.001), shortened time to full diet by 1.3 days (mean [SD] time to tolerate food, 3.4 [1.2] vs 4.7 [1.6] days; P<.001), reduced time to first bowel movement by 12 hours (43.1 [9.4] vs 58.5 [17.0] hours; P<.001), and shortened length of hospital stay by 1 day. Symptoms of ileus were reduced from 52% to 14% with coffee consumption.
Study limitation. An important weakness of this study is that although the authors defined the severity of ileus by time to resolution, they did not define what constituted a diagnosis of ileus in the first place.
Unanswered questions. Coffee is a known diarrhetic, so it is not unexpected that its use shortened time to flatus and first bowel movement. What is not known, however, is whether coffee consumption improves recovery. The significance of a 1-day reduction in hospital stay is unclear given the relatively prolonged hospitalization (6 to 7 days) seen in this investigation of patients with mixed gynecologic malignancies who underwent staging only. In contrast, another study showed that, for patients managed within an enhanced recovery pathway (a multimodal perioperative care enhancement protocol), median length of stay was 4 days for patients who underwent staging alone and 5 days for patients with ovarian cancer (40% underwent enteric resections).3 Thus, the effects of coffee consumption are unclear for patients managed with an optimized perioperative pathway.
The improvement in oral intake is also of questionable significance since these patients tolerated a solid diet 3 to 4 days after surgery, compared with the evening of surgery for most patients managed with enhanced recovery.
Incisional injection of liposomal bupivacaine has been associated with a reduction in the rate of ileus from 22% to 11% after complex cytoreduction for ovarian cancer when added to an existing enhanced recovery pathway; rates were only 5% for patients undergoing staging alone.4 These findings may be due to the significant reduction in opioid use that accompanied the use of liposomal bupivacaine.
--SEAN C. DOWDY, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Postoperative ileus is a common complication following abdominal surgery, particularly for patients undergoing laparotomy. Ileus is frustrating for patients and providers alike, and its occurrence may prolong the length of hospital stay, increase the cost of care, worsen patient satisfaction, and potentially delay postoperative treatments, such as chemotherapy for patients with gynecologic malignancies. The etiology of ileus is multifactorial, but it is thought to be caused primarily by a local inflammatory response from mechanical handling and irritation of the bowel. Although various interventions, such as laxatives, peripheral mu antagonists, and chewing gum, have been shown to reduce the occurrence of ileus, the effectiveness of these interventions varies, and ileus remains a major source of morbidity.1
Details of the study
To investigate whether coffee consumption accelerates recovery of bowel function following surgery, Güngördük and colleagues conducted a randomized controlled trial of coffee consumption after laparotomy with hysterectomy and staging for patients with gynecologic malignancies. This intervention avoids costs associated with drugs such as oral mu antagonists.
The trial included 114 women; after surgery, 58 were assigned to consume coffee 3 times daily and 56 received routine postoperative care without coffee consumption. The primary outcome measure was the time to the first passage of flatus after surgery. Time to first bowel movement and time to tolerance of a solid diet were secondary outcomes.
The results of this trial are consistent with prior study findings in colorectal surgery.2 Güngördük and associates found that patients in the coffee-consumption group, compared with controls, had reduced the time to flatus by 12 hours (mean [SD] time to flatus, 30.2 [8.0] vs 40.2 [12.1] hours; P<.001), shortened time to full diet by 1.3 days (mean [SD] time to tolerate food, 3.4 [1.2] vs 4.7 [1.6] days; P<.001), reduced time to first bowel movement by 12 hours (43.1 [9.4] vs 58.5 [17.0] hours; P<.001), and shortened length of hospital stay by 1 day. Symptoms of ileus were reduced from 52% to 14% with coffee consumption.
Study limitation. An important weakness of this study is that although the authors defined the severity of ileus by time to resolution, they did not define what constituted a diagnosis of ileus in the first place.
Unanswered questions. Coffee is a known diarrhetic, so it is not unexpected that its use shortened time to flatus and first bowel movement. What is not known, however, is whether coffee consumption improves recovery. The significance of a 1-day reduction in hospital stay is unclear given the relatively prolonged hospitalization (6 to 7 days) seen in this investigation of patients with mixed gynecologic malignancies who underwent staging only. In contrast, another study showed that, for patients managed within an enhanced recovery pathway (a multimodal perioperative care enhancement protocol), median length of stay was 4 days for patients who underwent staging alone and 5 days for patients with ovarian cancer (40% underwent enteric resections).3 Thus, the effects of coffee consumption are unclear for patients managed with an optimized perioperative pathway.
The improvement in oral intake is also of questionable significance since these patients tolerated a solid diet 3 to 4 days after surgery, compared with the evening of surgery for most patients managed with enhanced recovery.
Incisional injection of liposomal bupivacaine has been associated with a reduction in the rate of ileus from 22% to 11% after complex cytoreduction for ovarian cancer when added to an existing enhanced recovery pathway; rates were only 5% for patients undergoing staging alone.4 These findings may be due to the significant reduction in opioid use that accompanied the use of liposomal bupivacaine.
--SEAN C. DOWDY, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Nelson G, Altman AD, Nick A, et al. Guidelines for post- operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140(2):323-332.
- Müller SA, Rahbari NN, Schneider F, et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br J Surg. 2012;99(11):1530-1538.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122 (2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128(5):1009-1017.
- Nelson G, Altman AD, Nick A, et al. Guidelines for post- operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations--part II. Gynecol Oncol. 2016;140(2):323-332.
- Müller SA, Rahbari NN, Schneider F, et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br J Surg. 2012;99(11):1530-1538.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122 (2 pt 1):319-328.
- Kalogera E, Bakkum-Gamez JN, Weaver AL, et al. Abdominal incision injection of liposomal bupivacaine and opioid use after laparotomy for gynecologic malignancies. Obstet Gynecol. 2016;128(5):1009-1017.
Musculoskeletal Hand Pain Group Visits: An Adaptive Health Care Model
From Cooper Medical School of Rowan University (Dr. Patel, Dr. Fuller) and Cooper University Hospital (Dr. Kaufman), Camden, NJ.
Abstract
- Objective: To describe an adaptive musculoskeletal hand clinic that offers accessible and economically viable musculoskeletal care for an underserved, urban population.
- Methods: Descriptive report.
- Results: An enhanced access group visit model was developed offering both nonsurgical and surgical care pathways for patients with musculoskeletal disorders of the hand. Both patient education and care were provided in the group environment. Staffing included an orthopedic surgeon, nurse practitioner, medical student, orthopedic technician, and medical assistant. Over a 12-month period, group visit efficiency improved to accommodate an equivalent number of patients as compared to the traditional model. Access (time to appointment) was improved in the group visit. The model allows for the addition of non-physician clinical staff to improve access and limit costs in a manner not feasible with traditional office visits.
- Conclusion: A group visit model may offer a sustainable process to increase patient access to musculoskeletal subspecialty care and accommodate care of greater numbers of patients while maintaining quality. The group model offers flexible staffing, enhanced access, and educational benefit.
Key words: Group medical visit, team-based care, hand pain, access, underserved populations.
Group visits are a relatively new health care delivery model [1–3]. The term is applied to a wide variety of visits designed for groups of patients, rather than individual patient-provider appointments. The group visit format has been used for various disease- or condition-specific populations. Group visits can increase access to care and have been associated with improved clinical outcomes [4].
The Urban Health Institute, a dedicated business unit at Cooper University Health System in Camden, NJ, was established to focus on care of the underserved. The business unit is working to reduce cost of care delivery, increase access, and improve quality through the use of workflow redesign, task shifting, dashboards, and other methods. With a large startup grant from the Nicholson Foundation, the Urban Health Institute launched the Cooper Advanced Care Center to provide the local under-served population with access to a collaborative practice of 23 medical and surgical specialties under one roof. The center incorporates traditional one-on-one provider visits with innovative models of care, including group visits. Multiple partners were required in the group visit design and implementation starting with buy-in from Cooper Health System’s leadership for existing space to be redesigned for the group visit activities.
The Cooper Advanced Care Center, which had high no-show rates of 30% to 40%, and low reimbursement for a primarily Medicaid and self-pay population, initially operated at a financial loss. Meanwhile, most physicians had extended lag time until their next available appointment. In a traditional patient–physician office visit, additional physician time is required to increase access to care. This costly solution is often not financially viable. Group visits were considered as a means of using an interdisciplinary team to increase access while controlling or decreasing the per-visit cost.
Musculoskeletal medicine was identified as an area of need in part due to limited access to care. Patients were waiting more than 2 months to see a musculoskeletal specialist and were being scheduled beyond capacity in our existing traditional weekly hand and knee/sports medicine clinics. Within musculoskeletal medicine, 4 areas of musculoskeletal medicine were considered for group visits: back pain, knee pain, hand pain, and foot and ankle disorders. The decision was made to focus the subspecialty musculoskeletal clinic on disorders of the hand and wrist to provide nonsurgical and surgical care options for atraumatic and traumatic disorders of the hand in a teaching environment at an urban hospital. The purpose of this article is to discuss the design and implementation of a hand pain group visit model to increase access to care without increasing cost.
Setting and Patients
Camden City, New Jersey, is a medically underserved, resource-poor community. The population is 48% African American and 47% Hispanic and nearly 40% of individuals live below the poverty level [5]. The group visit was intentionally set up as a means to provide access to the un- or underinsured. Patients attending the group visits were 33% African American, 33% Hispanic, and 30% Caucasian. Most patients had Medicaid insurance (67%) with the remaining patients covered by commercial insurance (15%), dual Medicare/Medicaid (11%), Medicare (5%), or self pay (2%).
Group Visit Staffing and Structure
In a traditional office visit, used nearly ubiquitously in outpatient medical offices, patients arrive at individual appointment times for a prescribed time encounter with the physician, are registered and roomed by support staff, and are then seen by a clinician for diagnosis and treatment. While assistants and trainees participate in the patient’s care with attending physician supervision, the majority of direct care falls to the physician. Access is coupled to physician availability; increasing access to care requires crowding the schedule with additional patients. We used this model as the benchmark for volume and scheduling against which to compare the group visit.
The group visit staffing was the same as for the traditional visit: hand surgeon, nurse practitioner, orthopedic technician, medical student, and medical assistant. However, each clinical session consists of four 1-hour, consecutive group visits scheduled once a month on a Monday morning. Up to 10 people could be scheduled for each 1-hour group visit. We continued to offer our traditional office visit clinic on the other 3 Mondays in the month.
The hand surgeon begins the group visit with a 10-minute educational session and group discussion held in a meeting room. He reviews common disorders of the hand, including carpal tunnel syndrome, trigger fingers, hand arthritis, cysts, sprains and fractures, how they are treated, and risks and benefits of treatments. Patients sign a confidentiality agreement at check-in. Time is allowed for questions and experiential sharing is encouraged. Expectations are set at the start of the visit to honor each patient’s input to provide a safe environment for asking questions and expressing concerns about their shared health condition to enhance the learning experience [6]. A medical assistant enters the chief complaint using an electronic standardized questionnaire into the EMR along with basic vital signs for each patient either prior to, during, or after the group presentation.
After the group educational session with the surgeon, patients transition to a large, open clinical room with 6 separate workstations, each consisting of a small table with 4 chairs and a laptop computer. Small procedures can be performed on the table (suture removal, dressing changes, injections) and the table is appropriately sized to accommodate a care provider, the patient, and their support person(s). Tables are spaced comfortably such that conversations do not carry much from one to the other. The clinical space has white noise speakers for sound dampening while patients receive individual history, vital signs, physical examination, and review of relevant studies. Patients may see the clinicians in a private exam room if they wish or require.
In a traditional office setting, workflow through the major tasks (check-in, data gathering, diagnosis, treatment) is often linear, as dictated by the configuration of individual patient rooms and the patient’s expectations of a traditional doctor’s visit. In the group visit, major tasks are performed simultaneously by the advance practice providers (nurse practitioners, physician assistants) in conjunction with supervision of the attending physician. The workstations (tables, chairs, laptops) in the open clinical room allows for greater efficiency; providers can easily transition to other tasks from one workstation to another during time that may have been spent waiting for other team members in the more linear, traditional clinic. For example, while waiting for the attending physician’s approval of a diagnosis and treatment plan for one patient, a nurse practitioner may be able to begin assessing and gathering data with a second patient until the physician becomes available.
Scheduling and Access
A primary aim of the group visit pilot was to develop a model of care delivery that allowed scheduling beyond capacity for the traditional office hand clinic. At the inception of the group visit, all patients were offered a visit in either the traditional office or group visit model by our scheduling secretaries based upon availability, with emphasis on scheduling a defined underserved population into the group visit.
In traditional 1:1 appointments, the number of people who can access care is dependent on physician availability. The team-based model uncouples the number of scheduled patients from the physician availability, allowing increased efficiency in the model and/or additional staff to increase the number of patients the group visit can accommodate. Thus, patients were essentially guaranteed an appointment in the next clinic because there was no cap on the number of patients that could be scheduled into the group visit. If the number of patients exceeded the limit of 10 per hour, a non-physician clinician was added to accommodate the patient demand. As our group visit matures, the ability to increase the staffing model enables patients to get care without adding more physician time.
Task Shifting
A central pillar of making the group visit a sustainable model for more accessible care is shifting tasks to non-physician health care workers. Adding specialist time enhances access but drives up the cost of care. Non-physician clinical staff members in subspecialty orthopedic offices with experience diagnosing and treating common conditions are capable of providing the standard of care for those conditions with variable physician oversight [7].
In the group visit, there is a deliberate assignment of patients to clinical staff by the physician based on anticipated level of care required. Given the attending physician’s experience with the most common disorders related to hand pain, it is possible to anticipate the approximate complexity of care required for each patient based on the nature and duration of the presenting complaint. With some degree of clinical supervision by the physician, members of the team operate maximally independently to assist patients. The nurse practitioner can operate largely autonomously in the group visit. The overall goal is to encourage all team members to function at the top of their licenses and abilities. Task shifting in this manner increases the productivity of all members of the team and minimizes redundancy. Despite more autonomy of mid-level providers and support staff in the group visit, there is still direct supervision of care by the attending physician.
The current body of literature in task shifting to non-physician health care workers has mostly concerned low-income countries with marked physician and provider shortages [8]. However, given the increased patient volume already seen with the Affordable Care Act and further expected increases, the health care system is likely to see provider shortages, especially in primary care [9–11]. This will necessitate the adoption of strategies to increase access, maintain quality care, and decrease systemic cost. Task shifting provides one such strategy.
Patient Satisfaction
One concern with shifting clinical duties to non-physician clinical staff is patient satisfaction. An abbreviated interaction with physicians can make patients dissatisfied with medical care independent of eventual clinical outcome [12]. However, it has also been demonstrated in an outpatient hand surgery clinic that quality of time spent with the physician may have a greater impact on satisfaction than quantity of time [13].
Our group visit is structured to allow high physician-patient visibility and interaction. The introductory remarks by the physician engage patients with the physician early in the visit and establish a group and individual rapport. The physician introduces the clinical team and the idea of patients being seen by other clinical staff up front, which establishes comfort for later patient–staff interaction. This is also an important time for patient education, which has been shown as a significant determinant of patient satisfaction in the outpatient setting [14]. The patient education at the beginning of the visit answers questions by one person that another person may not have considered, and generates patient questions to be addressed individually with a clinician. One common example is when a person considering carpal tunnel surgery hears from a person who has recently completed the procedure and can talk about their operative experience.
In the group room, the physician and staff can move between patients quickly and efficiently without waiting for turnover of rooms and resources. The structure of the visit allows staff to dedicate more time to patient care by bypassing the extra time required when patients are roomed individually. The group/communal structure also allows patients to see the staff at work, as compared to time spent waiting alone in an exam room in the traditional office. This enables patients to appreciate the efforts of the clinical staff and avoids giving the impression that the physician is inattentive or cursory in patient interaction.
Medical Education
An important consideration at any academic medical center is education. However, education often introduces redundancies and inefficiency into the medical care visit. The work a trainee does is either extensively overseen or the clinical questions are repeated by a supervising provider. However, it is possible to increase efficiency and utility of trainees in the group visit setting while maintaining educational value.
Given the relatively narrow scope and the nature of conditions encountered in the hand clinic, medical decision making for many patients is limited to a “straightforward” or “low complexity” level. These designations assume a limited number of diagnoses, management options, and amount and complexity of diagnostic workup. Most importantly, risk of complications or morbidity/mortality at these levels is minimal to low. For these conditions, a supervising physician can allow a trainee more independence to practice employing simple treatment and management guidelines and progress to working independently when addressing simpler conditions as the trainee’s experience grows. As independence grows, trainees can build confidence in medical management as well as focus on other core educational competencies once they are comfortable with the evaluation and management of a limited scope of conditions. Conditions such as trigger fingers, hand arthritis, ganglion cysts, and carpal tunnel are those the trainee is likely to encounter in a primary care practice. While there may be a decrease in direct physician teaching, the trainee gains clinical autonomy and experience in educational core competencies such as patient communication, patient education, systems-based practice, procedural skill, cultural competency, and interdisciplinary teamwork [15,16].
Lessons Learned
The success of the group visit required buy-in from hospital and physician leadership, the clinical team, and multiple partners in the hospital system. The hospital administration supported group visits as an integral component of the Urban Health Institute. Buy-in from key hospital leadership ensured resources and dedicated space for the group visit program. Grant support allowed additional programmatic support to acquire the necessary assistance from information services, EMR support, legal, and marketing. Physician buy-in was the most significant piece to the success of an individual group visit. Accepting the movement away from physician autonomy to team-based care is challenging for many providers. Physician willingness to start a high-demand group visit, recognition of the start-up inefficiencies, and working with the administrative and clinical team on program improvement strategies has succeeded in launching a sustaining group visit model.
Conclusion
There is a need for an adaptive and economically viable model of patient care to meet increasing demand, as well as provide care for indigent populations in a way that is more economically sustainable than providing care through the emergency department. The development and implementation of an urban hand group visit at our institution has demonstrated that such a model, based on group visit models more commonly seen in primary care, can be effectively implemented in a subspecialty care setting. This model is capable of increasing patient access to care and effectively handling increased patient volume with room for cost-effective growth in the future, all while maintaining quality of care. We anticipate further subspecialty clinics within hand pain to emerge, such as a group visit dedicated specifically to carpal tunnel syndrome or hand arthritis. This will allow each group to be more focused and will streamline education and mutual support among the patients.
Corresponding author: Steven Kaufman, MD, 3 Cooper Plaza, Suite 211, Camden, NJ 08103, kaufman-steven@cooperhealth.edu.
Funding/support: The Nicholson Foundation.
Financial disclosures: None reported.
1. Gardiner P, Dresner D, Barnett KG, et al. Medical group visits: a feasibility study to manage patients with chronic pain in an underserved urban clinic. Glob Adv Health Med 2014;3:20–6.
2. Remick RA, Remick AK. Do patients really prefer individual outpatient follow-up visits, compared with group medical visits?. Can J Psychiatry 2014;59:50–3.
3. Thompson C, Meeuwisse I, Dahlke R, Drummond N. Group medical visits in primary care for patients with diabetes and low socioeconomic status: users’ perspectives and lessons for practitioners. Can J Diabetes 2014;38:198–204.
4. Eisenstat S, Lipps SA, Carlson K, Ulman K. Putting group visits into practice: a practical overview to preparation, implementation, and maintenance of group visits at Massachusetts General Hospital. Women’s Health Associates, The John D. Stoeckle Center for Primary Care Innovation, Massachusetts General Hospital; January 2012.
5. U.S. Census Bureau. State and city Quickfacts: Camden (city), New Jersey. Accessed 29 Sep 2015 at http://quickfacts.census.gov/qfd/states/34/3410000.html.
6. Slocum YS. A survey of expectations about group therapy among clinical and nonclinical populations. Int J Group Psychother 1987;37:39–54.
7. Newhouse RP, Stanik-hutt J, White KM, et al. Advanced practice nurse outcomes 1990-2008: a systematic review. Nurs Econ 2011;29:230–50.
8. Joshi R, Alim M, Kengne AP, et al. Task shifting for non-communicable disease management in low and middle income countries--a systematic review. PLoS ONE 2014;9:e103754.
9. Hofer AN, Abraham JM, Moscovice I. Expansion of coverage under the Patient Protection and Affordable Care Act and primary care utilization. Milbank Q 2011;89:69–89.
10. Kushnir T, Greenberg D, Madjar N, et al. Is burnout associated with referral rates among primary care physicians in community clinics?. Fam Pract 2014;31:44–50.
11. Calfee RP, Shah CM, Canham CD, et al. The influence of insurance status on access to and utilization of a tertiary hand surgery referral center. J Bone Joint Surg Am 2012;94:2177–84.
12. Lin CT, Albertson GA, Schilling LM, et al. Is patients’ perception of time spent with the physician a determinant of ambulatory patient satisfaction?. Arch Intern Med 2001;161:1437–42.
13. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated with patient satisfaction. Clin Orthop Relat Res 2014.
14. Murdock A, Griffin B. How is patient education linked to patient satisfaction?. Nursing 2013;43:43–5.
15. Accreditation Council for Graduate Medical Education. Common program requirements. Approved 2014. Available at www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRs_07012016.pdf.
16. Liaison Committee on Medical Education. Functions and structure of a medical school: standards for accreditation of medical education programs leading to the M.D. degree. [updated June 2013]. Available at www.lcme.org/publications/functions.pdf.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf 2011;20:46–51.
From Cooper Medical School of Rowan University (Dr. Patel, Dr. Fuller) and Cooper University Hospital (Dr. Kaufman), Camden, NJ.
Abstract
- Objective: To describe an adaptive musculoskeletal hand clinic that offers accessible and economically viable musculoskeletal care for an underserved, urban population.
- Methods: Descriptive report.
- Results: An enhanced access group visit model was developed offering both nonsurgical and surgical care pathways for patients with musculoskeletal disorders of the hand. Both patient education and care were provided in the group environment. Staffing included an orthopedic surgeon, nurse practitioner, medical student, orthopedic technician, and medical assistant. Over a 12-month period, group visit efficiency improved to accommodate an equivalent number of patients as compared to the traditional model. Access (time to appointment) was improved in the group visit. The model allows for the addition of non-physician clinical staff to improve access and limit costs in a manner not feasible with traditional office visits.
- Conclusion: A group visit model may offer a sustainable process to increase patient access to musculoskeletal subspecialty care and accommodate care of greater numbers of patients while maintaining quality. The group model offers flexible staffing, enhanced access, and educational benefit.
Key words: Group medical visit, team-based care, hand pain, access, underserved populations.
Group visits are a relatively new health care delivery model [1–3]. The term is applied to a wide variety of visits designed for groups of patients, rather than individual patient-provider appointments. The group visit format has been used for various disease- or condition-specific populations. Group visits can increase access to care and have been associated with improved clinical outcomes [4].
The Urban Health Institute, a dedicated business unit at Cooper University Health System in Camden, NJ, was established to focus on care of the underserved. The business unit is working to reduce cost of care delivery, increase access, and improve quality through the use of workflow redesign, task shifting, dashboards, and other methods. With a large startup grant from the Nicholson Foundation, the Urban Health Institute launched the Cooper Advanced Care Center to provide the local under-served population with access to a collaborative practice of 23 medical and surgical specialties under one roof. The center incorporates traditional one-on-one provider visits with innovative models of care, including group visits. Multiple partners were required in the group visit design and implementation starting with buy-in from Cooper Health System’s leadership for existing space to be redesigned for the group visit activities.
The Cooper Advanced Care Center, which had high no-show rates of 30% to 40%, and low reimbursement for a primarily Medicaid and self-pay population, initially operated at a financial loss. Meanwhile, most physicians had extended lag time until their next available appointment. In a traditional patient–physician office visit, additional physician time is required to increase access to care. This costly solution is often not financially viable. Group visits were considered as a means of using an interdisciplinary team to increase access while controlling or decreasing the per-visit cost.
Musculoskeletal medicine was identified as an area of need in part due to limited access to care. Patients were waiting more than 2 months to see a musculoskeletal specialist and were being scheduled beyond capacity in our existing traditional weekly hand and knee/sports medicine clinics. Within musculoskeletal medicine, 4 areas of musculoskeletal medicine were considered for group visits: back pain, knee pain, hand pain, and foot and ankle disorders. The decision was made to focus the subspecialty musculoskeletal clinic on disorders of the hand and wrist to provide nonsurgical and surgical care options for atraumatic and traumatic disorders of the hand in a teaching environment at an urban hospital. The purpose of this article is to discuss the design and implementation of a hand pain group visit model to increase access to care without increasing cost.
Setting and Patients
Camden City, New Jersey, is a medically underserved, resource-poor community. The population is 48% African American and 47% Hispanic and nearly 40% of individuals live below the poverty level [5]. The group visit was intentionally set up as a means to provide access to the un- or underinsured. Patients attending the group visits were 33% African American, 33% Hispanic, and 30% Caucasian. Most patients had Medicaid insurance (67%) with the remaining patients covered by commercial insurance (15%), dual Medicare/Medicaid (11%), Medicare (5%), or self pay (2%).
Group Visit Staffing and Structure
In a traditional office visit, used nearly ubiquitously in outpatient medical offices, patients arrive at individual appointment times for a prescribed time encounter with the physician, are registered and roomed by support staff, and are then seen by a clinician for diagnosis and treatment. While assistants and trainees participate in the patient’s care with attending physician supervision, the majority of direct care falls to the physician. Access is coupled to physician availability; increasing access to care requires crowding the schedule with additional patients. We used this model as the benchmark for volume and scheduling against which to compare the group visit.
The group visit staffing was the same as for the traditional visit: hand surgeon, nurse practitioner, orthopedic technician, medical student, and medical assistant. However, each clinical session consists of four 1-hour, consecutive group visits scheduled once a month on a Monday morning. Up to 10 people could be scheduled for each 1-hour group visit. We continued to offer our traditional office visit clinic on the other 3 Mondays in the month.
The hand surgeon begins the group visit with a 10-minute educational session and group discussion held in a meeting room. He reviews common disorders of the hand, including carpal tunnel syndrome, trigger fingers, hand arthritis, cysts, sprains and fractures, how they are treated, and risks and benefits of treatments. Patients sign a confidentiality agreement at check-in. Time is allowed for questions and experiential sharing is encouraged. Expectations are set at the start of the visit to honor each patient’s input to provide a safe environment for asking questions and expressing concerns about their shared health condition to enhance the learning experience [6]. A medical assistant enters the chief complaint using an electronic standardized questionnaire into the EMR along with basic vital signs for each patient either prior to, during, or after the group presentation.
After the group educational session with the surgeon, patients transition to a large, open clinical room with 6 separate workstations, each consisting of a small table with 4 chairs and a laptop computer. Small procedures can be performed on the table (suture removal, dressing changes, injections) and the table is appropriately sized to accommodate a care provider, the patient, and their support person(s). Tables are spaced comfortably such that conversations do not carry much from one to the other. The clinical space has white noise speakers for sound dampening while patients receive individual history, vital signs, physical examination, and review of relevant studies. Patients may see the clinicians in a private exam room if they wish or require.
In a traditional office setting, workflow through the major tasks (check-in, data gathering, diagnosis, treatment) is often linear, as dictated by the configuration of individual patient rooms and the patient’s expectations of a traditional doctor’s visit. In the group visit, major tasks are performed simultaneously by the advance practice providers (nurse practitioners, physician assistants) in conjunction with supervision of the attending physician. The workstations (tables, chairs, laptops) in the open clinical room allows for greater efficiency; providers can easily transition to other tasks from one workstation to another during time that may have been spent waiting for other team members in the more linear, traditional clinic. For example, while waiting for the attending physician’s approval of a diagnosis and treatment plan for one patient, a nurse practitioner may be able to begin assessing and gathering data with a second patient until the physician becomes available.
Scheduling and Access
A primary aim of the group visit pilot was to develop a model of care delivery that allowed scheduling beyond capacity for the traditional office hand clinic. At the inception of the group visit, all patients were offered a visit in either the traditional office or group visit model by our scheduling secretaries based upon availability, with emphasis on scheduling a defined underserved population into the group visit.
In traditional 1:1 appointments, the number of people who can access care is dependent on physician availability. The team-based model uncouples the number of scheduled patients from the physician availability, allowing increased efficiency in the model and/or additional staff to increase the number of patients the group visit can accommodate. Thus, patients were essentially guaranteed an appointment in the next clinic because there was no cap on the number of patients that could be scheduled into the group visit. If the number of patients exceeded the limit of 10 per hour, a non-physician clinician was added to accommodate the patient demand. As our group visit matures, the ability to increase the staffing model enables patients to get care without adding more physician time.
Task Shifting
A central pillar of making the group visit a sustainable model for more accessible care is shifting tasks to non-physician health care workers. Adding specialist time enhances access but drives up the cost of care. Non-physician clinical staff members in subspecialty orthopedic offices with experience diagnosing and treating common conditions are capable of providing the standard of care for those conditions with variable physician oversight [7].
In the group visit, there is a deliberate assignment of patients to clinical staff by the physician based on anticipated level of care required. Given the attending physician’s experience with the most common disorders related to hand pain, it is possible to anticipate the approximate complexity of care required for each patient based on the nature and duration of the presenting complaint. With some degree of clinical supervision by the physician, members of the team operate maximally independently to assist patients. The nurse practitioner can operate largely autonomously in the group visit. The overall goal is to encourage all team members to function at the top of their licenses and abilities. Task shifting in this manner increases the productivity of all members of the team and minimizes redundancy. Despite more autonomy of mid-level providers and support staff in the group visit, there is still direct supervision of care by the attending physician.
The current body of literature in task shifting to non-physician health care workers has mostly concerned low-income countries with marked physician and provider shortages [8]. However, given the increased patient volume already seen with the Affordable Care Act and further expected increases, the health care system is likely to see provider shortages, especially in primary care [9–11]. This will necessitate the adoption of strategies to increase access, maintain quality care, and decrease systemic cost. Task shifting provides one such strategy.
Patient Satisfaction
One concern with shifting clinical duties to non-physician clinical staff is patient satisfaction. An abbreviated interaction with physicians can make patients dissatisfied with medical care independent of eventual clinical outcome [12]. However, it has also been demonstrated in an outpatient hand surgery clinic that quality of time spent with the physician may have a greater impact on satisfaction than quantity of time [13].
Our group visit is structured to allow high physician-patient visibility and interaction. The introductory remarks by the physician engage patients with the physician early in the visit and establish a group and individual rapport. The physician introduces the clinical team and the idea of patients being seen by other clinical staff up front, which establishes comfort for later patient–staff interaction. This is also an important time for patient education, which has been shown as a significant determinant of patient satisfaction in the outpatient setting [14]. The patient education at the beginning of the visit answers questions by one person that another person may not have considered, and generates patient questions to be addressed individually with a clinician. One common example is when a person considering carpal tunnel surgery hears from a person who has recently completed the procedure and can talk about their operative experience.
In the group room, the physician and staff can move between patients quickly and efficiently without waiting for turnover of rooms and resources. The structure of the visit allows staff to dedicate more time to patient care by bypassing the extra time required when patients are roomed individually. The group/communal structure also allows patients to see the staff at work, as compared to time spent waiting alone in an exam room in the traditional office. This enables patients to appreciate the efforts of the clinical staff and avoids giving the impression that the physician is inattentive or cursory in patient interaction.
Medical Education
An important consideration at any academic medical center is education. However, education often introduces redundancies and inefficiency into the medical care visit. The work a trainee does is either extensively overseen or the clinical questions are repeated by a supervising provider. However, it is possible to increase efficiency and utility of trainees in the group visit setting while maintaining educational value.
Given the relatively narrow scope and the nature of conditions encountered in the hand clinic, medical decision making for many patients is limited to a “straightforward” or “low complexity” level. These designations assume a limited number of diagnoses, management options, and amount and complexity of diagnostic workup. Most importantly, risk of complications or morbidity/mortality at these levels is minimal to low. For these conditions, a supervising physician can allow a trainee more independence to practice employing simple treatment and management guidelines and progress to working independently when addressing simpler conditions as the trainee’s experience grows. As independence grows, trainees can build confidence in medical management as well as focus on other core educational competencies once they are comfortable with the evaluation and management of a limited scope of conditions. Conditions such as trigger fingers, hand arthritis, ganglion cysts, and carpal tunnel are those the trainee is likely to encounter in a primary care practice. While there may be a decrease in direct physician teaching, the trainee gains clinical autonomy and experience in educational core competencies such as patient communication, patient education, systems-based practice, procedural skill, cultural competency, and interdisciplinary teamwork [15,16].
Lessons Learned
The success of the group visit required buy-in from hospital and physician leadership, the clinical team, and multiple partners in the hospital system. The hospital administration supported group visits as an integral component of the Urban Health Institute. Buy-in from key hospital leadership ensured resources and dedicated space for the group visit program. Grant support allowed additional programmatic support to acquire the necessary assistance from information services, EMR support, legal, and marketing. Physician buy-in was the most significant piece to the success of an individual group visit. Accepting the movement away from physician autonomy to team-based care is challenging for many providers. Physician willingness to start a high-demand group visit, recognition of the start-up inefficiencies, and working with the administrative and clinical team on program improvement strategies has succeeded in launching a sustaining group visit model.
Conclusion
There is a need for an adaptive and economically viable model of patient care to meet increasing demand, as well as provide care for indigent populations in a way that is more economically sustainable than providing care through the emergency department. The development and implementation of an urban hand group visit at our institution has demonstrated that such a model, based on group visit models more commonly seen in primary care, can be effectively implemented in a subspecialty care setting. This model is capable of increasing patient access to care and effectively handling increased patient volume with room for cost-effective growth in the future, all while maintaining quality of care. We anticipate further subspecialty clinics within hand pain to emerge, such as a group visit dedicated specifically to carpal tunnel syndrome or hand arthritis. This will allow each group to be more focused and will streamline education and mutual support among the patients.
Corresponding author: Steven Kaufman, MD, 3 Cooper Plaza, Suite 211, Camden, NJ 08103, kaufman-steven@cooperhealth.edu.
Funding/support: The Nicholson Foundation.
Financial disclosures: None reported.
From Cooper Medical School of Rowan University (Dr. Patel, Dr. Fuller) and Cooper University Hospital (Dr. Kaufman), Camden, NJ.
Abstract
- Objective: To describe an adaptive musculoskeletal hand clinic that offers accessible and economically viable musculoskeletal care for an underserved, urban population.
- Methods: Descriptive report.
- Results: An enhanced access group visit model was developed offering both nonsurgical and surgical care pathways for patients with musculoskeletal disorders of the hand. Both patient education and care were provided in the group environment. Staffing included an orthopedic surgeon, nurse practitioner, medical student, orthopedic technician, and medical assistant. Over a 12-month period, group visit efficiency improved to accommodate an equivalent number of patients as compared to the traditional model. Access (time to appointment) was improved in the group visit. The model allows for the addition of non-physician clinical staff to improve access and limit costs in a manner not feasible with traditional office visits.
- Conclusion: A group visit model may offer a sustainable process to increase patient access to musculoskeletal subspecialty care and accommodate care of greater numbers of patients while maintaining quality. The group model offers flexible staffing, enhanced access, and educational benefit.
Key words: Group medical visit, team-based care, hand pain, access, underserved populations.
Group visits are a relatively new health care delivery model [1–3]. The term is applied to a wide variety of visits designed for groups of patients, rather than individual patient-provider appointments. The group visit format has been used for various disease- or condition-specific populations. Group visits can increase access to care and have been associated with improved clinical outcomes [4].
The Urban Health Institute, a dedicated business unit at Cooper University Health System in Camden, NJ, was established to focus on care of the underserved. The business unit is working to reduce cost of care delivery, increase access, and improve quality through the use of workflow redesign, task shifting, dashboards, and other methods. With a large startup grant from the Nicholson Foundation, the Urban Health Institute launched the Cooper Advanced Care Center to provide the local under-served population with access to a collaborative practice of 23 medical and surgical specialties under one roof. The center incorporates traditional one-on-one provider visits with innovative models of care, including group visits. Multiple partners were required in the group visit design and implementation starting with buy-in from Cooper Health System’s leadership for existing space to be redesigned for the group visit activities.
The Cooper Advanced Care Center, which had high no-show rates of 30% to 40%, and low reimbursement for a primarily Medicaid and self-pay population, initially operated at a financial loss. Meanwhile, most physicians had extended lag time until their next available appointment. In a traditional patient–physician office visit, additional physician time is required to increase access to care. This costly solution is often not financially viable. Group visits were considered as a means of using an interdisciplinary team to increase access while controlling or decreasing the per-visit cost.
Musculoskeletal medicine was identified as an area of need in part due to limited access to care. Patients were waiting more than 2 months to see a musculoskeletal specialist and were being scheduled beyond capacity in our existing traditional weekly hand and knee/sports medicine clinics. Within musculoskeletal medicine, 4 areas of musculoskeletal medicine were considered for group visits: back pain, knee pain, hand pain, and foot and ankle disorders. The decision was made to focus the subspecialty musculoskeletal clinic on disorders of the hand and wrist to provide nonsurgical and surgical care options for atraumatic and traumatic disorders of the hand in a teaching environment at an urban hospital. The purpose of this article is to discuss the design and implementation of a hand pain group visit model to increase access to care without increasing cost.
Setting and Patients
Camden City, New Jersey, is a medically underserved, resource-poor community. The population is 48% African American and 47% Hispanic and nearly 40% of individuals live below the poverty level [5]. The group visit was intentionally set up as a means to provide access to the un- or underinsured. Patients attending the group visits were 33% African American, 33% Hispanic, and 30% Caucasian. Most patients had Medicaid insurance (67%) with the remaining patients covered by commercial insurance (15%), dual Medicare/Medicaid (11%), Medicare (5%), or self pay (2%).
Group Visit Staffing and Structure
In a traditional office visit, used nearly ubiquitously in outpatient medical offices, patients arrive at individual appointment times for a prescribed time encounter with the physician, are registered and roomed by support staff, and are then seen by a clinician for diagnosis and treatment. While assistants and trainees participate in the patient’s care with attending physician supervision, the majority of direct care falls to the physician. Access is coupled to physician availability; increasing access to care requires crowding the schedule with additional patients. We used this model as the benchmark for volume and scheduling against which to compare the group visit.
The group visit staffing was the same as for the traditional visit: hand surgeon, nurse practitioner, orthopedic technician, medical student, and medical assistant. However, each clinical session consists of four 1-hour, consecutive group visits scheduled once a month on a Monday morning. Up to 10 people could be scheduled for each 1-hour group visit. We continued to offer our traditional office visit clinic on the other 3 Mondays in the month.
The hand surgeon begins the group visit with a 10-minute educational session and group discussion held in a meeting room. He reviews common disorders of the hand, including carpal tunnel syndrome, trigger fingers, hand arthritis, cysts, sprains and fractures, how they are treated, and risks and benefits of treatments. Patients sign a confidentiality agreement at check-in. Time is allowed for questions and experiential sharing is encouraged. Expectations are set at the start of the visit to honor each patient’s input to provide a safe environment for asking questions and expressing concerns about their shared health condition to enhance the learning experience [6]. A medical assistant enters the chief complaint using an electronic standardized questionnaire into the EMR along with basic vital signs for each patient either prior to, during, or after the group presentation.
After the group educational session with the surgeon, patients transition to a large, open clinical room with 6 separate workstations, each consisting of a small table with 4 chairs and a laptop computer. Small procedures can be performed on the table (suture removal, dressing changes, injections) and the table is appropriately sized to accommodate a care provider, the patient, and their support person(s). Tables are spaced comfortably such that conversations do not carry much from one to the other. The clinical space has white noise speakers for sound dampening while patients receive individual history, vital signs, physical examination, and review of relevant studies. Patients may see the clinicians in a private exam room if they wish or require.
In a traditional office setting, workflow through the major tasks (check-in, data gathering, diagnosis, treatment) is often linear, as dictated by the configuration of individual patient rooms and the patient’s expectations of a traditional doctor’s visit. In the group visit, major tasks are performed simultaneously by the advance practice providers (nurse practitioners, physician assistants) in conjunction with supervision of the attending physician. The workstations (tables, chairs, laptops) in the open clinical room allows for greater efficiency; providers can easily transition to other tasks from one workstation to another during time that may have been spent waiting for other team members in the more linear, traditional clinic. For example, while waiting for the attending physician’s approval of a diagnosis and treatment plan for one patient, a nurse practitioner may be able to begin assessing and gathering data with a second patient until the physician becomes available.
Scheduling and Access
A primary aim of the group visit pilot was to develop a model of care delivery that allowed scheduling beyond capacity for the traditional office hand clinic. At the inception of the group visit, all patients were offered a visit in either the traditional office or group visit model by our scheduling secretaries based upon availability, with emphasis on scheduling a defined underserved population into the group visit.
In traditional 1:1 appointments, the number of people who can access care is dependent on physician availability. The team-based model uncouples the number of scheduled patients from the physician availability, allowing increased efficiency in the model and/or additional staff to increase the number of patients the group visit can accommodate. Thus, patients were essentially guaranteed an appointment in the next clinic because there was no cap on the number of patients that could be scheduled into the group visit. If the number of patients exceeded the limit of 10 per hour, a non-physician clinician was added to accommodate the patient demand. As our group visit matures, the ability to increase the staffing model enables patients to get care without adding more physician time.
Task Shifting
A central pillar of making the group visit a sustainable model for more accessible care is shifting tasks to non-physician health care workers. Adding specialist time enhances access but drives up the cost of care. Non-physician clinical staff members in subspecialty orthopedic offices with experience diagnosing and treating common conditions are capable of providing the standard of care for those conditions with variable physician oversight [7].
In the group visit, there is a deliberate assignment of patients to clinical staff by the physician based on anticipated level of care required. Given the attending physician’s experience with the most common disorders related to hand pain, it is possible to anticipate the approximate complexity of care required for each patient based on the nature and duration of the presenting complaint. With some degree of clinical supervision by the physician, members of the team operate maximally independently to assist patients. The nurse practitioner can operate largely autonomously in the group visit. The overall goal is to encourage all team members to function at the top of their licenses and abilities. Task shifting in this manner increases the productivity of all members of the team and minimizes redundancy. Despite more autonomy of mid-level providers and support staff in the group visit, there is still direct supervision of care by the attending physician.
The current body of literature in task shifting to non-physician health care workers has mostly concerned low-income countries with marked physician and provider shortages [8]. However, given the increased patient volume already seen with the Affordable Care Act and further expected increases, the health care system is likely to see provider shortages, especially in primary care [9–11]. This will necessitate the adoption of strategies to increase access, maintain quality care, and decrease systemic cost. Task shifting provides one such strategy.
Patient Satisfaction
One concern with shifting clinical duties to non-physician clinical staff is patient satisfaction. An abbreviated interaction with physicians can make patients dissatisfied with medical care independent of eventual clinical outcome [12]. However, it has also been demonstrated in an outpatient hand surgery clinic that quality of time spent with the physician may have a greater impact on satisfaction than quantity of time [13].
Our group visit is structured to allow high physician-patient visibility and interaction. The introductory remarks by the physician engage patients with the physician early in the visit and establish a group and individual rapport. The physician introduces the clinical team and the idea of patients being seen by other clinical staff up front, which establishes comfort for later patient–staff interaction. This is also an important time for patient education, which has been shown as a significant determinant of patient satisfaction in the outpatient setting [14]. The patient education at the beginning of the visit answers questions by one person that another person may not have considered, and generates patient questions to be addressed individually with a clinician. One common example is when a person considering carpal tunnel surgery hears from a person who has recently completed the procedure and can talk about their operative experience.
In the group room, the physician and staff can move between patients quickly and efficiently without waiting for turnover of rooms and resources. The structure of the visit allows staff to dedicate more time to patient care by bypassing the extra time required when patients are roomed individually. The group/communal structure also allows patients to see the staff at work, as compared to time spent waiting alone in an exam room in the traditional office. This enables patients to appreciate the efforts of the clinical staff and avoids giving the impression that the physician is inattentive or cursory in patient interaction.
Medical Education
An important consideration at any academic medical center is education. However, education often introduces redundancies and inefficiency into the medical care visit. The work a trainee does is either extensively overseen or the clinical questions are repeated by a supervising provider. However, it is possible to increase efficiency and utility of trainees in the group visit setting while maintaining educational value.
Given the relatively narrow scope and the nature of conditions encountered in the hand clinic, medical decision making for many patients is limited to a “straightforward” or “low complexity” level. These designations assume a limited number of diagnoses, management options, and amount and complexity of diagnostic workup. Most importantly, risk of complications or morbidity/mortality at these levels is minimal to low. For these conditions, a supervising physician can allow a trainee more independence to practice employing simple treatment and management guidelines and progress to working independently when addressing simpler conditions as the trainee’s experience grows. As independence grows, trainees can build confidence in medical management as well as focus on other core educational competencies once they are comfortable with the evaluation and management of a limited scope of conditions. Conditions such as trigger fingers, hand arthritis, ganglion cysts, and carpal tunnel are those the trainee is likely to encounter in a primary care practice. While there may be a decrease in direct physician teaching, the trainee gains clinical autonomy and experience in educational core competencies such as patient communication, patient education, systems-based practice, procedural skill, cultural competency, and interdisciplinary teamwork [15,16].
Lessons Learned
The success of the group visit required buy-in from hospital and physician leadership, the clinical team, and multiple partners in the hospital system. The hospital administration supported group visits as an integral component of the Urban Health Institute. Buy-in from key hospital leadership ensured resources and dedicated space for the group visit program. Grant support allowed additional programmatic support to acquire the necessary assistance from information services, EMR support, legal, and marketing. Physician buy-in was the most significant piece to the success of an individual group visit. Accepting the movement away from physician autonomy to team-based care is challenging for many providers. Physician willingness to start a high-demand group visit, recognition of the start-up inefficiencies, and working with the administrative and clinical team on program improvement strategies has succeeded in launching a sustaining group visit model.
Conclusion
There is a need for an adaptive and economically viable model of patient care to meet increasing demand, as well as provide care for indigent populations in a way that is more economically sustainable than providing care through the emergency department. The development and implementation of an urban hand group visit at our institution has demonstrated that such a model, based on group visit models more commonly seen in primary care, can be effectively implemented in a subspecialty care setting. This model is capable of increasing patient access to care and effectively handling increased patient volume with room for cost-effective growth in the future, all while maintaining quality of care. We anticipate further subspecialty clinics within hand pain to emerge, such as a group visit dedicated specifically to carpal tunnel syndrome or hand arthritis. This will allow each group to be more focused and will streamline education and mutual support among the patients.
Corresponding author: Steven Kaufman, MD, 3 Cooper Plaza, Suite 211, Camden, NJ 08103, kaufman-steven@cooperhealth.edu.
Funding/support: The Nicholson Foundation.
Financial disclosures: None reported.
1. Gardiner P, Dresner D, Barnett KG, et al. Medical group visits: a feasibility study to manage patients with chronic pain in an underserved urban clinic. Glob Adv Health Med 2014;3:20–6.
2. Remick RA, Remick AK. Do patients really prefer individual outpatient follow-up visits, compared with group medical visits?. Can J Psychiatry 2014;59:50–3.
3. Thompson C, Meeuwisse I, Dahlke R, Drummond N. Group medical visits in primary care for patients with diabetes and low socioeconomic status: users’ perspectives and lessons for practitioners. Can J Diabetes 2014;38:198–204.
4. Eisenstat S, Lipps SA, Carlson K, Ulman K. Putting group visits into practice: a practical overview to preparation, implementation, and maintenance of group visits at Massachusetts General Hospital. Women’s Health Associates, The John D. Stoeckle Center for Primary Care Innovation, Massachusetts General Hospital; January 2012.
5. U.S. Census Bureau. State and city Quickfacts: Camden (city), New Jersey. Accessed 29 Sep 2015 at http://quickfacts.census.gov/qfd/states/34/3410000.html.
6. Slocum YS. A survey of expectations about group therapy among clinical and nonclinical populations. Int J Group Psychother 1987;37:39–54.
7. Newhouse RP, Stanik-hutt J, White KM, et al. Advanced practice nurse outcomes 1990-2008: a systematic review. Nurs Econ 2011;29:230–50.
8. Joshi R, Alim M, Kengne AP, et al. Task shifting for non-communicable disease management in low and middle income countries--a systematic review. PLoS ONE 2014;9:e103754.
9. Hofer AN, Abraham JM, Moscovice I. Expansion of coverage under the Patient Protection and Affordable Care Act and primary care utilization. Milbank Q 2011;89:69–89.
10. Kushnir T, Greenberg D, Madjar N, et al. Is burnout associated with referral rates among primary care physicians in community clinics?. Fam Pract 2014;31:44–50.
11. Calfee RP, Shah CM, Canham CD, et al. The influence of insurance status on access to and utilization of a tertiary hand surgery referral center. J Bone Joint Surg Am 2012;94:2177–84.
12. Lin CT, Albertson GA, Schilling LM, et al. Is patients’ perception of time spent with the physician a determinant of ambulatory patient satisfaction?. Arch Intern Med 2001;161:1437–42.
13. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated with patient satisfaction. Clin Orthop Relat Res 2014.
14. Murdock A, Griffin B. How is patient education linked to patient satisfaction?. Nursing 2013;43:43–5.
15. Accreditation Council for Graduate Medical Education. Common program requirements. Approved 2014. Available at www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRs_07012016.pdf.
16. Liaison Committee on Medical Education. Functions and structure of a medical school: standards for accreditation of medical education programs leading to the M.D. degree. [updated June 2013]. Available at www.lcme.org/publications/functions.pdf.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf 2011;20:46–51.
1. Gardiner P, Dresner D, Barnett KG, et al. Medical group visits: a feasibility study to manage patients with chronic pain in an underserved urban clinic. Glob Adv Health Med 2014;3:20–6.
2. Remick RA, Remick AK. Do patients really prefer individual outpatient follow-up visits, compared with group medical visits?. Can J Psychiatry 2014;59:50–3.
3. Thompson C, Meeuwisse I, Dahlke R, Drummond N. Group medical visits in primary care for patients with diabetes and low socioeconomic status: users’ perspectives and lessons for practitioners. Can J Diabetes 2014;38:198–204.
4. Eisenstat S, Lipps SA, Carlson K, Ulman K. Putting group visits into practice: a practical overview to preparation, implementation, and maintenance of group visits at Massachusetts General Hospital. Women’s Health Associates, The John D. Stoeckle Center for Primary Care Innovation, Massachusetts General Hospital; January 2012.
5. U.S. Census Bureau. State and city Quickfacts: Camden (city), New Jersey. Accessed 29 Sep 2015 at http://quickfacts.census.gov/qfd/states/34/3410000.html.
6. Slocum YS. A survey of expectations about group therapy among clinical and nonclinical populations. Int J Group Psychother 1987;37:39–54.
7. Newhouse RP, Stanik-hutt J, White KM, et al. Advanced practice nurse outcomes 1990-2008: a systematic review. Nurs Econ 2011;29:230–50.
8. Joshi R, Alim M, Kengne AP, et al. Task shifting for non-communicable disease management in low and middle income countries--a systematic review. PLoS ONE 2014;9:e103754.
9. Hofer AN, Abraham JM, Moscovice I. Expansion of coverage under the Patient Protection and Affordable Care Act and primary care utilization. Milbank Q 2011;89:69–89.
10. Kushnir T, Greenberg D, Madjar N, et al. Is burnout associated with referral rates among primary care physicians in community clinics?. Fam Pract 2014;31:44–50.
11. Calfee RP, Shah CM, Canham CD, et al. The influence of insurance status on access to and utilization of a tertiary hand surgery referral center. J Bone Joint Surg Am 2012;94:2177–84.
12. Lin CT, Albertson GA, Schilling LM, et al. Is patients’ perception of time spent with the physician a determinant of ambulatory patient satisfaction?. Arch Intern Med 2001;161:1437–42.
13. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated with patient satisfaction. Clin Orthop Relat Res 2014.
14. Murdock A, Griffin B. How is patient education linked to patient satisfaction?. Nursing 2013;43:43–5.
15. Accreditation Council for Graduate Medical Education. Common program requirements. Approved 2014. Available at www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRs_07012016.pdf.
16. Liaison Committee on Medical Education. Functions and structure of a medical school: standards for accreditation of medical education programs leading to the M.D. degree. [updated June 2013]. Available at www.lcme.org/publications/functions.pdf.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf 2011;20:46–51.
Parents seek easily understood public reporting of cardiac outcome measures
HOUSTON – Parents of children with congenital heart disease cite survival statistics, surgeon-specific experience, and complication rates as the three most important congenital heart surgery outcome measures to report publicly, results from a large survey show.
“Recently, an increasing demand for the public reporting of pediatric heart surgery outcomes has led to the development of several different reporting schemes, including a hospital star rating system and procedure-specific mortality data tables for the Society of Thoracic Surgeons benchmark operations,” study investigator Mallory L. Irons, MD, said during a press briefing at the annual meeting of the Society of Thoracic Surgeons. “However, despite the availability of these reporting schemes, there remain unanswered questions about the optimal format and content of public reporting for pediatric heart surgery outcomes.”
“These procedures have been determined to represent more homogenous patient groups,” she said. “How data [are] presented may be just as important as the data itself. Noticeably absent from these frameworks is parent input regarding the information that may be necessary to make an informed choice about their child’s care.
“Failure to consider this perspective may contribute to suboptimal reporting and misunderstanding of the data presented. The goal of the current study was to identify parent preferences regarding the content of pediatric heart surgery outcomes reports, specifically with respect to the type of data that is included as well as the optimal format for presentation of this data.”
She and her associates developed a 43-question survey for 1,862 parents of children born with heart defects. The parents were invited to participate through member lists from patient advocacy groups Mended Little Hearts, the Pediatric Congenital Heart Association, and Sisters by Heart, and from a cohort of patients who underwent surgical correction for an STS benchmark procedure at the Children’s Hospital of Philadelphia after Jan. 1, 2007.
Of the 1,862 parents, 1,281 (69%) provided complete responses for inclusion in the final analysis. The mean age of respondents was 37 years, 92% were mothers of children with congenital heart disease, and 92% were white. “Most reported household incomes in excess of $65,000 per year, but lower income households were also significantly represented,” Dr. Irons added. More than half of the children (57%) were diagnosed with CHD prenatally, 63% underwent an initial repair in the neonatal period, and 60% of families were referred to a cardiac surgical center by a physician, while 23% were transferred from their birth hospital.
When the respondents were asked to rank categories of outcome measures or other types of information to include in an optimal public reporting scheme on a scale of 1 (most important) to 8 (least important), they identified survival statistics, surgeons’ experience with congenital heart surgery, and complication rates as the three most important. These preferences were stable across differences in education levels, household incomes, and race or ethnicity, Dr. Irons said.
Presented with three display formats for hospital-specific mortality rates, most parents (89%) identified a numerical procedure-based approach as the best format, and more than half (60%) identified the hospital star rating system as the worst potential format to display mortality data. These preferences also were stable across differences in education levels, household incomes, and race or ethnicity.
Dr. Irons acknowledged certain limitations of the study, including its retrospective design, and the fact that it lacked input from parents who do not have Internet access. “Similarly, the availability of the survey in English only prevents non-native English speakers from contributing,” she said. “Finally, while we have identified what types of information parents want, we must acknowledge that small case numbers limit the presentation of certain types of data, such as surgeon-specific outcomes, in a statistically meaningful way.”
In her opinion, an optimal reporting system requires a common database in which to collect and analyze data, a robust methodology for risk adjustment, and a way to compare performance across a field that is inherently heterogeneous.
“The ideal public reporting system should be valid as well as easily understood by all stakeholders,” Dr. Irons concluded. “We must recognize that just because parents want certain data, [it] does not mean that we can provide it in a meaningful, statistically valid way. Perhaps the most important takeaway from our study is the importance of involving parents and other stakeholders in the design and planning of methodologies for public reporting of congenital heart surgery outcomes. Ultimately, the optimal platform will represent a melding of what parents want, and what is statistically meaningful and valid.”
Jeffrey P. Jacobs, MD, a pediatric heart surgeon at Johns Hopkins University, Baltimore, who also chairs the STS Workforce on National Databases, characterized the study as “an extremely important paper that examines the format and structure of how it might be best to share information about cardiac surgical outcomes with patients and their families.” He said the STS “has studied multiple different strategies, and currently we use a method where we report outcomes using a categorical system of star ratings and augment that with detailed specific data with point estimates for confidence intervals. We continue to study and explore better ways to share this information with our patients and with their families.”
Dr. Irons reported having no financial disclosures.
HOUSTON – Parents of children with congenital heart disease cite survival statistics, surgeon-specific experience, and complication rates as the three most important congenital heart surgery outcome measures to report publicly, results from a large survey show.
“Recently, an increasing demand for the public reporting of pediatric heart surgery outcomes has led to the development of several different reporting schemes, including a hospital star rating system and procedure-specific mortality data tables for the Society of Thoracic Surgeons benchmark operations,” study investigator Mallory L. Irons, MD, said during a press briefing at the annual meeting of the Society of Thoracic Surgeons. “However, despite the availability of these reporting schemes, there remain unanswered questions about the optimal format and content of public reporting for pediatric heart surgery outcomes.”
“These procedures have been determined to represent more homogenous patient groups,” she said. “How data [are] presented may be just as important as the data itself. Noticeably absent from these frameworks is parent input regarding the information that may be necessary to make an informed choice about their child’s care.
“Failure to consider this perspective may contribute to suboptimal reporting and misunderstanding of the data presented. The goal of the current study was to identify parent preferences regarding the content of pediatric heart surgery outcomes reports, specifically with respect to the type of data that is included as well as the optimal format for presentation of this data.”
She and her associates developed a 43-question survey for 1,862 parents of children born with heart defects. The parents were invited to participate through member lists from patient advocacy groups Mended Little Hearts, the Pediatric Congenital Heart Association, and Sisters by Heart, and from a cohort of patients who underwent surgical correction for an STS benchmark procedure at the Children’s Hospital of Philadelphia after Jan. 1, 2007.
Of the 1,862 parents, 1,281 (69%) provided complete responses for inclusion in the final analysis. The mean age of respondents was 37 years, 92% were mothers of children with congenital heart disease, and 92% were white. “Most reported household incomes in excess of $65,000 per year, but lower income households were also significantly represented,” Dr. Irons added. More than half of the children (57%) were diagnosed with CHD prenatally, 63% underwent an initial repair in the neonatal period, and 60% of families were referred to a cardiac surgical center by a physician, while 23% were transferred from their birth hospital.
When the respondents were asked to rank categories of outcome measures or other types of information to include in an optimal public reporting scheme on a scale of 1 (most important) to 8 (least important), they identified survival statistics, surgeons’ experience with congenital heart surgery, and complication rates as the three most important. These preferences were stable across differences in education levels, household incomes, and race or ethnicity, Dr. Irons said.
Presented with three display formats for hospital-specific mortality rates, most parents (89%) identified a numerical procedure-based approach as the best format, and more than half (60%) identified the hospital star rating system as the worst potential format to display mortality data. These preferences also were stable across differences in education levels, household incomes, and race or ethnicity.
Dr. Irons acknowledged certain limitations of the study, including its retrospective design, and the fact that it lacked input from parents who do not have Internet access. “Similarly, the availability of the survey in English only prevents non-native English speakers from contributing,” she said. “Finally, while we have identified what types of information parents want, we must acknowledge that small case numbers limit the presentation of certain types of data, such as surgeon-specific outcomes, in a statistically meaningful way.”
In her opinion, an optimal reporting system requires a common database in which to collect and analyze data, a robust methodology for risk adjustment, and a way to compare performance across a field that is inherently heterogeneous.
“The ideal public reporting system should be valid as well as easily understood by all stakeholders,” Dr. Irons concluded. “We must recognize that just because parents want certain data, [it] does not mean that we can provide it in a meaningful, statistically valid way. Perhaps the most important takeaway from our study is the importance of involving parents and other stakeholders in the design and planning of methodologies for public reporting of congenital heart surgery outcomes. Ultimately, the optimal platform will represent a melding of what parents want, and what is statistically meaningful and valid.”
Jeffrey P. Jacobs, MD, a pediatric heart surgeon at Johns Hopkins University, Baltimore, who also chairs the STS Workforce on National Databases, characterized the study as “an extremely important paper that examines the format and structure of how it might be best to share information about cardiac surgical outcomes with patients and their families.” He said the STS “has studied multiple different strategies, and currently we use a method where we report outcomes using a categorical system of star ratings and augment that with detailed specific data with point estimates for confidence intervals. We continue to study and explore better ways to share this information with our patients and with their families.”
Dr. Irons reported having no financial disclosures.
HOUSTON – Parents of children with congenital heart disease cite survival statistics, surgeon-specific experience, and complication rates as the three most important congenital heart surgery outcome measures to report publicly, results from a large survey show.
“Recently, an increasing demand for the public reporting of pediatric heart surgery outcomes has led to the development of several different reporting schemes, including a hospital star rating system and procedure-specific mortality data tables for the Society of Thoracic Surgeons benchmark operations,” study investigator Mallory L. Irons, MD, said during a press briefing at the annual meeting of the Society of Thoracic Surgeons. “However, despite the availability of these reporting schemes, there remain unanswered questions about the optimal format and content of public reporting for pediatric heart surgery outcomes.”
“These procedures have been determined to represent more homogenous patient groups,” she said. “How data [are] presented may be just as important as the data itself. Noticeably absent from these frameworks is parent input regarding the information that may be necessary to make an informed choice about their child’s care.
“Failure to consider this perspective may contribute to suboptimal reporting and misunderstanding of the data presented. The goal of the current study was to identify parent preferences regarding the content of pediatric heart surgery outcomes reports, specifically with respect to the type of data that is included as well as the optimal format for presentation of this data.”
She and her associates developed a 43-question survey for 1,862 parents of children born with heart defects. The parents were invited to participate through member lists from patient advocacy groups Mended Little Hearts, the Pediatric Congenital Heart Association, and Sisters by Heart, and from a cohort of patients who underwent surgical correction for an STS benchmark procedure at the Children’s Hospital of Philadelphia after Jan. 1, 2007.
Of the 1,862 parents, 1,281 (69%) provided complete responses for inclusion in the final analysis. The mean age of respondents was 37 years, 92% were mothers of children with congenital heart disease, and 92% were white. “Most reported household incomes in excess of $65,000 per year, but lower income households were also significantly represented,” Dr. Irons added. More than half of the children (57%) were diagnosed with CHD prenatally, 63% underwent an initial repair in the neonatal period, and 60% of families were referred to a cardiac surgical center by a physician, while 23% were transferred from their birth hospital.
When the respondents were asked to rank categories of outcome measures or other types of information to include in an optimal public reporting scheme on a scale of 1 (most important) to 8 (least important), they identified survival statistics, surgeons’ experience with congenital heart surgery, and complication rates as the three most important. These preferences were stable across differences in education levels, household incomes, and race or ethnicity, Dr. Irons said.
Presented with three display formats for hospital-specific mortality rates, most parents (89%) identified a numerical procedure-based approach as the best format, and more than half (60%) identified the hospital star rating system as the worst potential format to display mortality data. These preferences also were stable across differences in education levels, household incomes, and race or ethnicity.
Dr. Irons acknowledged certain limitations of the study, including its retrospective design, and the fact that it lacked input from parents who do not have Internet access. “Similarly, the availability of the survey in English only prevents non-native English speakers from contributing,” she said. “Finally, while we have identified what types of information parents want, we must acknowledge that small case numbers limit the presentation of certain types of data, such as surgeon-specific outcomes, in a statistically meaningful way.”
In her opinion, an optimal reporting system requires a common database in which to collect and analyze data, a robust methodology for risk adjustment, and a way to compare performance across a field that is inherently heterogeneous.
“The ideal public reporting system should be valid as well as easily understood by all stakeholders,” Dr. Irons concluded. “We must recognize that just because parents want certain data, [it] does not mean that we can provide it in a meaningful, statistically valid way. Perhaps the most important takeaway from our study is the importance of involving parents and other stakeholders in the design and planning of methodologies for public reporting of congenital heart surgery outcomes. Ultimately, the optimal platform will represent a melding of what parents want, and what is statistically meaningful and valid.”
Jeffrey P. Jacobs, MD, a pediatric heart surgeon at Johns Hopkins University, Baltimore, who also chairs the STS Workforce on National Databases, characterized the study as “an extremely important paper that examines the format and structure of how it might be best to share information about cardiac surgical outcomes with patients and their families.” He said the STS “has studied multiple different strategies, and currently we use a method where we report outcomes using a categorical system of star ratings and augment that with detailed specific data with point estimates for confidence intervals. We continue to study and explore better ways to share this information with our patients and with their families.”
Dr. Irons reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point:
Major finding: When parents of children with congenital heart disease were asked to rank categories of outcome measures or other types of information to include in an optimal public reporting scheme, they identified survival statistics, surgeons’ experience with congenital heart surgery, and complication rates as the three most important
Data source: A retrospective survey of 1,862 parents of children born with heart defects.
Disclosures: Dr. Irons reported having no financial disclosures.
Triclosan sutures halve surgical site infections in children
The use of triclosan-impregnated sutures reduced by half the incidence of surgical site infections in children, a large randomized study has determined.
Overall, the antibiotic-treated sutures cut the number of these infections by 52%, but they were particularly effective in reducing the risk of deep surgical site infections (SSIs), Marjo Renko, MD, wrote (Lancet Infect Dis. 2017;17[1]:50-7).
The study was conducted in clean wounds in healthy children and in a center that already had a very low rate of surgical site infections (just 5%) – showing that improvement is possible even in optimal care settings, wrote Dr. Renko, of the University of Oulu, Finland, and her colleagues.
“This randomized, controlled study shows that even in low-risk settings, where other prophylactic measures are available to use, triclosan-containing sutures effectively prevented the occurrence of SSIs in children,” the team wrote.
The study cohort comprised 1,633 children aged 7-17 who underwent surgery at a single Finnish hospital from 2010-2014. Most were there for planned surgery (87%); the remainder had emergency surgery. The most common surgical site was musculoskeletal (40%), followed by abdominal wall surgery (about 25%), and urogenital surgery (about 13%). The rest were intraabdominal or procedures on the nervous system, chest, and skin or subcutaneous tissue.
The children were randomized to either plain or triclosan-impregnated sutures. The primary outcome was the occurrence of a superficial or deep surgical site infection, based on Centers for Disease Control and Prevention criteria. The procedures were performed by 69 surgeons.
In a modified intent-to-treat analysis, a surgical site infection occurred in 3% of the triclosan-suture group (20 children) and in 5% of the control suture group (42 children). In the control group, these infections were most often of chest incisions (15%), followed by skin incisions (10%) and nervous system, intraabdominal, and musculoskeletal incisions (8% each). In the triclosan group, the most common site of infection was skin (10%), followed by musculoskeletal (4%), nervous system (2%), and urogenital and abdominal wall incisions (1% each).
Compared with control sutures, triclosan sutures reduced the overall risk of a surgical site infection by 52% (relative risk, 0.48; 95% confidence interval, 0.28-0.80). The number needed to treat to avoid one infection was 36.
The sutures were significantly more effective in reducing deep infections than superficial infections. Superficial infections occurred in 2% of the triclosan group (17) and 4% of the control group (28) – a risk reduction of 39% (RR, 0.61; 95% CI, 0.34-1.09) Deep infections occurred in less than 1% of the triclosan group (3) and 2% of the control group (14) – a risk reduction of 79% (RR, 0.21’ CI, 0.07-0.66).
Infections were associated with an increased incidence of wound dehiscence in the control group (6% vs. 4%), the need for additional antimicrobial agents (7% vs. 2%), and wound revisions (2% vs. less than 1%). Children in the control group also had more outpatient visits (8% vs. 4%) and were more often readmitted because of their infection (2% vs. 1%).
The authors noted that triclosan, in the setting of increased household use, “has raised concerns about the toxic effects of the drug on the human body. Observational studies have reported associations between triclosan exposures and altered thyroid hormone levels, body mass index, and waist circumference.”
Two Norwegian studies found that the drug was associated with inhalation allergies and seasonal allergies.
“Because of the agent’s suspected toxicity and to prevent further development of resistant bacteria, use of triclosan should be restricted and reserved only for medical procedures with adequate evidence,” they noted. However, “SSIs cause much morbidity and mortality after surgical procedures, and economic evaluations recommend the use of triclosan-containing material.”
Dr. Renko received grants from the Alma and K.A. Snellman Foundation, the Finnish Medical Foundation, and the Foundation for Pediatric Research.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
The study by Dr. Marjo Renko and her colleagues is impressive in its sheer numbers, if not so much in its findings, Felix J. Hüttner, MD, and Markus K. Diener, MD, wrote in an accompanying editorial (Lancet Infect Dis. 2017;17[1]:3-4).
“We congratulate the authors on successfully doing a pragmatic, large-scale trial in a difficult setting; randomized controlled trials in children are known to pose specific challenges to researchers. However, the monocenter design raises some concerns about the generalizability of the results.”
Single-center trials can overestimate treatment effects, the colleagues noted. Dr. Renko’s conclusions don’t line up with their own metaanalysis of triclosan-containing sutures for abdominal wall closure. In it, three single-center trials found in favor of the triclosan sutures, but two multicenter trials did not.
The variation in infection rates in each type of surgery is a clue to the difficulty of a one-size-fits-all intervention like the treated sutures. “The differences between the intervention group and the control group vary widely by surgery type – for example, 0% versus 15% for thoracic surgery, compared with 1% versus 1% for surgery of the urinary system and genitals. Thus, triclosan-containing sutures might only be beneficial for specific types of operations and in our opinion, it cannot be concluded that triclosan-containing sutures reduce surgical site infections in all of these indications. Future trials should focus at individual types of pediatric surgery to evaluate a potential beneficial effect.”
Dr. Hüttner and Dr. Diener are surgeons at the University of Heidelberg, Germany. Dr. Hüttner had no financial disclosures. Dr. Diener has received grants from Johnson & Johnson Medical Limited.
The study by Dr. Marjo Renko and her colleagues is impressive in its sheer numbers, if not so much in its findings, Felix J. Hüttner, MD, and Markus K. Diener, MD, wrote in an accompanying editorial (Lancet Infect Dis. 2017;17[1]:3-4).
“We congratulate the authors on successfully doing a pragmatic, large-scale trial in a difficult setting; randomized controlled trials in children are known to pose specific challenges to researchers. However, the monocenter design raises some concerns about the generalizability of the results.”
Single-center trials can overestimate treatment effects, the colleagues noted. Dr. Renko’s conclusions don’t line up with their own metaanalysis of triclosan-containing sutures for abdominal wall closure. In it, three single-center trials found in favor of the triclosan sutures, but two multicenter trials did not.
The variation in infection rates in each type of surgery is a clue to the difficulty of a one-size-fits-all intervention like the treated sutures. “The differences between the intervention group and the control group vary widely by surgery type – for example, 0% versus 15% for thoracic surgery, compared with 1% versus 1% for surgery of the urinary system and genitals. Thus, triclosan-containing sutures might only be beneficial for specific types of operations and in our opinion, it cannot be concluded that triclosan-containing sutures reduce surgical site infections in all of these indications. Future trials should focus at individual types of pediatric surgery to evaluate a potential beneficial effect.”
Dr. Hüttner and Dr. Diener are surgeons at the University of Heidelberg, Germany. Dr. Hüttner had no financial disclosures. Dr. Diener has received grants from Johnson & Johnson Medical Limited.
The study by Dr. Marjo Renko and her colleagues is impressive in its sheer numbers, if not so much in its findings, Felix J. Hüttner, MD, and Markus K. Diener, MD, wrote in an accompanying editorial (Lancet Infect Dis. 2017;17[1]:3-4).
“We congratulate the authors on successfully doing a pragmatic, large-scale trial in a difficult setting; randomized controlled trials in children are known to pose specific challenges to researchers. However, the monocenter design raises some concerns about the generalizability of the results.”
Single-center trials can overestimate treatment effects, the colleagues noted. Dr. Renko’s conclusions don’t line up with their own metaanalysis of triclosan-containing sutures for abdominal wall closure. In it, three single-center trials found in favor of the triclosan sutures, but two multicenter trials did not.
The variation in infection rates in each type of surgery is a clue to the difficulty of a one-size-fits-all intervention like the treated sutures. “The differences between the intervention group and the control group vary widely by surgery type – for example, 0% versus 15% for thoracic surgery, compared with 1% versus 1% for surgery of the urinary system and genitals. Thus, triclosan-containing sutures might only be beneficial for specific types of operations and in our opinion, it cannot be concluded that triclosan-containing sutures reduce surgical site infections in all of these indications. Future trials should focus at individual types of pediatric surgery to evaluate a potential beneficial effect.”
Dr. Hüttner and Dr. Diener are surgeons at the University of Heidelberg, Germany. Dr. Hüttner had no financial disclosures. Dr. Diener has received grants from Johnson & Johnson Medical Limited.
The use of triclosan-impregnated sutures reduced by half the incidence of surgical site infections in children, a large randomized study has determined.
Overall, the antibiotic-treated sutures cut the number of these infections by 52%, but they were particularly effective in reducing the risk of deep surgical site infections (SSIs), Marjo Renko, MD, wrote (Lancet Infect Dis. 2017;17[1]:50-7).
The study was conducted in clean wounds in healthy children and in a center that already had a very low rate of surgical site infections (just 5%) – showing that improvement is possible even in optimal care settings, wrote Dr. Renko, of the University of Oulu, Finland, and her colleagues.
“This randomized, controlled study shows that even in low-risk settings, where other prophylactic measures are available to use, triclosan-containing sutures effectively prevented the occurrence of SSIs in children,” the team wrote.
The study cohort comprised 1,633 children aged 7-17 who underwent surgery at a single Finnish hospital from 2010-2014. Most were there for planned surgery (87%); the remainder had emergency surgery. The most common surgical site was musculoskeletal (40%), followed by abdominal wall surgery (about 25%), and urogenital surgery (about 13%). The rest were intraabdominal or procedures on the nervous system, chest, and skin or subcutaneous tissue.
The children were randomized to either plain or triclosan-impregnated sutures. The primary outcome was the occurrence of a superficial or deep surgical site infection, based on Centers for Disease Control and Prevention criteria. The procedures were performed by 69 surgeons.
In a modified intent-to-treat analysis, a surgical site infection occurred in 3% of the triclosan-suture group (20 children) and in 5% of the control suture group (42 children). In the control group, these infections were most often of chest incisions (15%), followed by skin incisions (10%) and nervous system, intraabdominal, and musculoskeletal incisions (8% each). In the triclosan group, the most common site of infection was skin (10%), followed by musculoskeletal (4%), nervous system (2%), and urogenital and abdominal wall incisions (1% each).
Compared with control sutures, triclosan sutures reduced the overall risk of a surgical site infection by 52% (relative risk, 0.48; 95% confidence interval, 0.28-0.80). The number needed to treat to avoid one infection was 36.
The sutures were significantly more effective in reducing deep infections than superficial infections. Superficial infections occurred in 2% of the triclosan group (17) and 4% of the control group (28) – a risk reduction of 39% (RR, 0.61; 95% CI, 0.34-1.09) Deep infections occurred in less than 1% of the triclosan group (3) and 2% of the control group (14) – a risk reduction of 79% (RR, 0.21’ CI, 0.07-0.66).
Infections were associated with an increased incidence of wound dehiscence in the control group (6% vs. 4%), the need for additional antimicrobial agents (7% vs. 2%), and wound revisions (2% vs. less than 1%). Children in the control group also had more outpatient visits (8% vs. 4%) and were more often readmitted because of their infection (2% vs. 1%).
The authors noted that triclosan, in the setting of increased household use, “has raised concerns about the toxic effects of the drug on the human body. Observational studies have reported associations between triclosan exposures and altered thyroid hormone levels, body mass index, and waist circumference.”
Two Norwegian studies found that the drug was associated with inhalation allergies and seasonal allergies.
“Because of the agent’s suspected toxicity and to prevent further development of resistant bacteria, use of triclosan should be restricted and reserved only for medical procedures with adequate evidence,” they noted. However, “SSIs cause much morbidity and mortality after surgical procedures, and economic evaluations recommend the use of triclosan-containing material.”
Dr. Renko received grants from the Alma and K.A. Snellman Foundation, the Finnish Medical Foundation, and the Foundation for Pediatric Research.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
The use of triclosan-impregnated sutures reduced by half the incidence of surgical site infections in children, a large randomized study has determined.
Overall, the antibiotic-treated sutures cut the number of these infections by 52%, but they were particularly effective in reducing the risk of deep surgical site infections (SSIs), Marjo Renko, MD, wrote (Lancet Infect Dis. 2017;17[1]:50-7).
The study was conducted in clean wounds in healthy children and in a center that already had a very low rate of surgical site infections (just 5%) – showing that improvement is possible even in optimal care settings, wrote Dr. Renko, of the University of Oulu, Finland, and her colleagues.
“This randomized, controlled study shows that even in low-risk settings, where other prophylactic measures are available to use, triclosan-containing sutures effectively prevented the occurrence of SSIs in children,” the team wrote.
The study cohort comprised 1,633 children aged 7-17 who underwent surgery at a single Finnish hospital from 2010-2014. Most were there for planned surgery (87%); the remainder had emergency surgery. The most common surgical site was musculoskeletal (40%), followed by abdominal wall surgery (about 25%), and urogenital surgery (about 13%). The rest were intraabdominal or procedures on the nervous system, chest, and skin or subcutaneous tissue.
The children were randomized to either plain or triclosan-impregnated sutures. The primary outcome was the occurrence of a superficial or deep surgical site infection, based on Centers for Disease Control and Prevention criteria. The procedures were performed by 69 surgeons.
In a modified intent-to-treat analysis, a surgical site infection occurred in 3% of the triclosan-suture group (20 children) and in 5% of the control suture group (42 children). In the control group, these infections were most often of chest incisions (15%), followed by skin incisions (10%) and nervous system, intraabdominal, and musculoskeletal incisions (8% each). In the triclosan group, the most common site of infection was skin (10%), followed by musculoskeletal (4%), nervous system (2%), and urogenital and abdominal wall incisions (1% each).
Compared with control sutures, triclosan sutures reduced the overall risk of a surgical site infection by 52% (relative risk, 0.48; 95% confidence interval, 0.28-0.80). The number needed to treat to avoid one infection was 36.
The sutures were significantly more effective in reducing deep infections than superficial infections. Superficial infections occurred in 2% of the triclosan group (17) and 4% of the control group (28) – a risk reduction of 39% (RR, 0.61; 95% CI, 0.34-1.09) Deep infections occurred in less than 1% of the triclosan group (3) and 2% of the control group (14) – a risk reduction of 79% (RR, 0.21’ CI, 0.07-0.66).
Infections were associated with an increased incidence of wound dehiscence in the control group (6% vs. 4%), the need for additional antimicrobial agents (7% vs. 2%), and wound revisions (2% vs. less than 1%). Children in the control group also had more outpatient visits (8% vs. 4%) and were more often readmitted because of their infection (2% vs. 1%).
The authors noted that triclosan, in the setting of increased household use, “has raised concerns about the toxic effects of the drug on the human body. Observational studies have reported associations between triclosan exposures and altered thyroid hormone levels, body mass index, and waist circumference.”
Two Norwegian studies found that the drug was associated with inhalation allergies and seasonal allergies.
“Because of the agent’s suspected toxicity and to prevent further development of resistant bacteria, use of triclosan should be restricted and reserved only for medical procedures with adequate evidence,” they noted. However, “SSIs cause much morbidity and mortality after surgical procedures, and economic evaluations recommend the use of triclosan-containing material.”
Dr. Renko received grants from the Alma and K.A. Snellman Foundation, the Finnish Medical Foundation, and the Foundation for Pediatric Research.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
FROM LANCET INFECTIOUS DISEASES
Key clinical point:
Major finding: Overall, the sutures were associated with a 52% decrease in SSIs.
Data source: The study randomized 1,633 children undergoing surgery to the triclosan sutures or to a control suture.
Disclosures: Dr. Renko received grants from the Alma and K.A. Snellman Foundation, the Finnish Medical Foundation, and the Foundation for Pediatric Research.
Strategies for prophylactic oophoropexy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by
Early, in-hospital shunt failure common among infants
HOUSTON – Among neonates and infants who underwent shunt construction as a source of pulmonary blood flow, early, in-hospital shunt failure occurred in 7.3% of cases, results from a large retrospective study showed.
“Approximately one in seven patients who experiences cardiac surgery in the first year of life undergoes construction of a systemic to pulmonary artery shunt of some type,” one of the study investigators, Marshall L. Jacobs, MD, said in an interview. The study was presented at the annual meeting of the Society of Thoracic Surgeons.
“Early failure of such shunts is an incompletely understood phenomenon which accounts for important morbidity and mortality among infants and neonates. Much of what is known about shunt failure is based on experiences reported from individual institutions. The few multicenter studies to date have been clinical trials that focused primarily on pharmacologic strategies intended to reduce the risk of shunt failure due to thrombosis. Their utility for guiding clinical decision making has been limited. Some have been underpowered; some have had limited risk adjustment of subjects.”
Dr. Do, who presented the findings at the meeting and is currently a Congenital Heart Surgery Fellow at the Children’s Hospital of Philadelphia, and a team of 11 other investigators utilized the STS Congenital Heart Surgery Database to identify 9,172 neonates and infants who underwent shunt construction as a source of pulmonary blood flow at 118 institutions from 2010 to 2015. Criteria for shunt failure included a documented diagnosis of in-hospital shunt failure, shunt revision, or catheter-based shunt intervention. The investigators used multivariable logistic regression to evaluate risk factors for in-hospital shunt failure.
Of the 9,172 at-risk neonates and infants, 674 (7.3%) experienced early, in-hospital shunt failure. “The observed rate of early shunt failure varied across the many specific types of shunts, and was lower with systemic ventricle to pulmonary artery shunts (as in the Sano modification of the Norwood procedure) than with the systemic artery to pulmonary artery shunts,” said Dr. Jacobs, who is a cardiothoracic surgeon at Johns Hopkins University, Baltimore.
In multivariable analysis, risk factors for in-hospital shunt failure included lower weight at operation for both neonates and infants, preoperative hypercoagulable state, and the collective presence of any other STS Congenital Heart Surgery Database preoperative risk factors. Neither cardiopulmonary bypass nor single ventricle diagnosis were risk factors for shunt failure. The investigators also observed that patients with in-hospital shunt failure had significantly higher rates of operative mortality (31.9% vs. 11.1%) and major morbidity (84.4% vs. 29.4%), and longer postoperative length of stay among survivors (a median of 45 vs. 22 days).
“Understanding the characteristics of the patient groups found to be at highest risk for early shunt failure is helpful in identifying individual patients that may warrant expectant surveillance, enhanced pharmacologic management, or other strategies to reduce the risk of shunt failure,” Dr. Jacobs concluded.
“But perhaps more importantly it provides key information that may be helpful in the design and development of future clinical trials and/or collaborative quality improvement initiatives designed to reduce the cost in lives and resources that is associated with early shunt dysfunction.”
He acknowledged certain limitations of the study, including its retrospective observational design and the voluntary nature of the STS Congenital Heart Surgery Database. “In addition, some potentially important variables, such as detailed data concerning preoperative test results of coagulation assays are not collected in the STS Congenital Heart Surgery Database,” he said.
The research was supported by the STS Access & Publications Research program. The investigators reported having no financial disclosures.
HOUSTON – Among neonates and infants who underwent shunt construction as a source of pulmonary blood flow, early, in-hospital shunt failure occurred in 7.3% of cases, results from a large retrospective study showed.
“Approximately one in seven patients who experiences cardiac surgery in the first year of life undergoes construction of a systemic to pulmonary artery shunt of some type,” one of the study investigators, Marshall L. Jacobs, MD, said in an interview. The study was presented at the annual meeting of the Society of Thoracic Surgeons.
“Early failure of such shunts is an incompletely understood phenomenon which accounts for important morbidity and mortality among infants and neonates. Much of what is known about shunt failure is based on experiences reported from individual institutions. The few multicenter studies to date have been clinical trials that focused primarily on pharmacologic strategies intended to reduce the risk of shunt failure due to thrombosis. Their utility for guiding clinical decision making has been limited. Some have been underpowered; some have had limited risk adjustment of subjects.”
Dr. Do, who presented the findings at the meeting and is currently a Congenital Heart Surgery Fellow at the Children’s Hospital of Philadelphia, and a team of 11 other investigators utilized the STS Congenital Heart Surgery Database to identify 9,172 neonates and infants who underwent shunt construction as a source of pulmonary blood flow at 118 institutions from 2010 to 2015. Criteria for shunt failure included a documented diagnosis of in-hospital shunt failure, shunt revision, or catheter-based shunt intervention. The investigators used multivariable logistic regression to evaluate risk factors for in-hospital shunt failure.
Of the 9,172 at-risk neonates and infants, 674 (7.3%) experienced early, in-hospital shunt failure. “The observed rate of early shunt failure varied across the many specific types of shunts, and was lower with systemic ventricle to pulmonary artery shunts (as in the Sano modification of the Norwood procedure) than with the systemic artery to pulmonary artery shunts,” said Dr. Jacobs, who is a cardiothoracic surgeon at Johns Hopkins University, Baltimore.
In multivariable analysis, risk factors for in-hospital shunt failure included lower weight at operation for both neonates and infants, preoperative hypercoagulable state, and the collective presence of any other STS Congenital Heart Surgery Database preoperative risk factors. Neither cardiopulmonary bypass nor single ventricle diagnosis were risk factors for shunt failure. The investigators also observed that patients with in-hospital shunt failure had significantly higher rates of operative mortality (31.9% vs. 11.1%) and major morbidity (84.4% vs. 29.4%), and longer postoperative length of stay among survivors (a median of 45 vs. 22 days).
“Understanding the characteristics of the patient groups found to be at highest risk for early shunt failure is helpful in identifying individual patients that may warrant expectant surveillance, enhanced pharmacologic management, or other strategies to reduce the risk of shunt failure,” Dr. Jacobs concluded.
“But perhaps more importantly it provides key information that may be helpful in the design and development of future clinical trials and/or collaborative quality improvement initiatives designed to reduce the cost in lives and resources that is associated with early shunt dysfunction.”
He acknowledged certain limitations of the study, including its retrospective observational design and the voluntary nature of the STS Congenital Heart Surgery Database. “In addition, some potentially important variables, such as detailed data concerning preoperative test results of coagulation assays are not collected in the STS Congenital Heart Surgery Database,” he said.
The research was supported by the STS Access & Publications Research program. The investigators reported having no financial disclosures.
HOUSTON – Among neonates and infants who underwent shunt construction as a source of pulmonary blood flow, early, in-hospital shunt failure occurred in 7.3% of cases, results from a large retrospective study showed.
“Approximately one in seven patients who experiences cardiac surgery in the first year of life undergoes construction of a systemic to pulmonary artery shunt of some type,” one of the study investigators, Marshall L. Jacobs, MD, said in an interview. The study was presented at the annual meeting of the Society of Thoracic Surgeons.
“Early failure of such shunts is an incompletely understood phenomenon which accounts for important morbidity and mortality among infants and neonates. Much of what is known about shunt failure is based on experiences reported from individual institutions. The few multicenter studies to date have been clinical trials that focused primarily on pharmacologic strategies intended to reduce the risk of shunt failure due to thrombosis. Their utility for guiding clinical decision making has been limited. Some have been underpowered; some have had limited risk adjustment of subjects.”
Dr. Do, who presented the findings at the meeting and is currently a Congenital Heart Surgery Fellow at the Children’s Hospital of Philadelphia, and a team of 11 other investigators utilized the STS Congenital Heart Surgery Database to identify 9,172 neonates and infants who underwent shunt construction as a source of pulmonary blood flow at 118 institutions from 2010 to 2015. Criteria for shunt failure included a documented diagnosis of in-hospital shunt failure, shunt revision, or catheter-based shunt intervention. The investigators used multivariable logistic regression to evaluate risk factors for in-hospital shunt failure.
Of the 9,172 at-risk neonates and infants, 674 (7.3%) experienced early, in-hospital shunt failure. “The observed rate of early shunt failure varied across the many specific types of shunts, and was lower with systemic ventricle to pulmonary artery shunts (as in the Sano modification of the Norwood procedure) than with the systemic artery to pulmonary artery shunts,” said Dr. Jacobs, who is a cardiothoracic surgeon at Johns Hopkins University, Baltimore.
In multivariable analysis, risk factors for in-hospital shunt failure included lower weight at operation for both neonates and infants, preoperative hypercoagulable state, and the collective presence of any other STS Congenital Heart Surgery Database preoperative risk factors. Neither cardiopulmonary bypass nor single ventricle diagnosis were risk factors for shunt failure. The investigators also observed that patients with in-hospital shunt failure had significantly higher rates of operative mortality (31.9% vs. 11.1%) and major morbidity (84.4% vs. 29.4%), and longer postoperative length of stay among survivors (a median of 45 vs. 22 days).
“Understanding the characteristics of the patient groups found to be at highest risk for early shunt failure is helpful in identifying individual patients that may warrant expectant surveillance, enhanced pharmacologic management, or other strategies to reduce the risk of shunt failure,” Dr. Jacobs concluded.
“But perhaps more importantly it provides key information that may be helpful in the design and development of future clinical trials and/or collaborative quality improvement initiatives designed to reduce the cost in lives and resources that is associated with early shunt dysfunction.”
He acknowledged certain limitations of the study, including its retrospective observational design and the voluntary nature of the STS Congenital Heart Surgery Database. “In addition, some potentially important variables, such as detailed data concerning preoperative test results of coagulation assays are not collected in the STS Congenital Heart Surgery Database,” he said.
The research was supported by the STS Access & Publications Research program. The investigators reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point:
Major finding: Among neonates and infants who underwent shunt operations, 7.3% experienced early, in-hospital shunt failure.
Data source: A retrospective analysis of 9,172 neonates and infants who underwent shunt construction as a source of pulmonary blood flow at 118 institutions from 2010 to 2015.
Disclosures: The research was supported by the STS Access & Publications Research program. The investigators reported having no financial disclosures.
Study IDs risk factors for ideal timing of stage 2 palliation following Norwood
HOUSTON – The optimal timing of stage 2 palliation after the Norwood operation depends on certain patient-specific risk factors, but in most cases should be done around 3-4 months of age, results from a multi-center study show.
While previous studies have investigated whether early stage-2 palliation (S2P) can be performed without increased post-S2P mortality, the effect of the timing of S2P on post-Norwood mortality remains unknown, Robert “Jake” Jaquiss, MD, said in an interview in advance of the annual meeting of the Society of Thoracic Surgeons.
“There has been a lot of dispute about how early is too early for S2P,” said Dr. Jaquiss, the study’s senior author, who is professor and division chief of pediatric cardiothoracic surgery at the University of Texas Southwestern Medical Center. “That is one of the few things that is in the control of the doctor. Most of the rest of the decisions are based entirely on the condition of the patient and the patient’s specific anatomy. So the timing of S2P is something that we can truly define most always. What we want to find out is, what is the ideal timing? How early is too early? Is there such a thing as too late?”
In an effort to determine the optimal timing of S2P that both minimizes pre-S2P attrition and maximizes long-term post-S2P survival, Dr. Jaquiss and his associates at 19 other institutions evaluated data from 534 neonates diagnosed with left ventricular outflow tract obstruction that precluded adequate systemic cardiac output through the aortic valve who initially underwent a Norwood operation from 2005 to 2016.
S2P was performed in 377 patients (71%) at a mean age of 5.4 months, while 115 (22%) died after Norwood, and the rest underwent biventricular repair or heart transplantation. After S2P, 38 (10%) died, 248 (66%) underwent Fontan, and the rest were alive awaiting Fontan or underwent heart transplantation.
Risk factors for death after Norwood included requiring pre-Norwood extracorporeal membrane oxygenation (P less than .0001), birth weight of less than 2.5 kg (P less than .0001), modified Blalock-Taussig shunt vs. a right ventricle to pulmonary artery conduit (P = .0003), larger baseline right pulmonary artery diameter (P = .0002), smaller baseline mitral valve diameter (P = .0002), smaller baseline tricuspid valve diameter (P = .0001), and nonwhite race (P = .03).
Risk factors for death after S2P included lower oxygen saturation at pre-S2P clinic visit (P = .02), having moderate or severe pre-S2P right ventricular dysfunction (P = .007), younger age at S2P (P = .03), and longer post-Norwood hospital length of stay (P = .03).
The risk-adjusted, 4-year, post-Norwood survival was 72%, with a confidence interval of 67%-75%. When plotted vs. the age at S2P, risk-adjusted, 4-year, post-Norwood survival for the 534 patients was maximized by S2P at 3-6 months of age. At the same time, risk-adjusted, 4-year survival in low-risk infants was compromised only by undergoing S2P earlier than 3 months of age. In high-risk infants, survival was severely compromised, especially when undergoing S2P earlier than 6 months of age.
“The results reinforced intuitions or expectations that most of the investigators already had,” Dr. Jaquiss said. “But we are in an era where evidence-based medicine is much preferable to intuition-based medicine. I’m very confident in the findings we have. I feel more confident in suggesting that we should be planning these surgeries around 3-4 months of age in usual-risk children and also more confident in suggesting that we need to consider transplantation earlier in children who are perceived to be at high risk. There is some hope [by clinicians in] some centers that you can convert a high-risk prognosis to a lower or intermediate risk prognosis by doing the S2P earlier or at some alternative time. Our data suggests that would not be helpful.”
Dr. Jaquiss and Dr. Meza reported having no financial disclosures.
HOUSTON – The optimal timing of stage 2 palliation after the Norwood operation depends on certain patient-specific risk factors, but in most cases should be done around 3-4 months of age, results from a multi-center study show.
While previous studies have investigated whether early stage-2 palliation (S2P) can be performed without increased post-S2P mortality, the effect of the timing of S2P on post-Norwood mortality remains unknown, Robert “Jake” Jaquiss, MD, said in an interview in advance of the annual meeting of the Society of Thoracic Surgeons.
“There has been a lot of dispute about how early is too early for S2P,” said Dr. Jaquiss, the study’s senior author, who is professor and division chief of pediatric cardiothoracic surgery at the University of Texas Southwestern Medical Center. “That is one of the few things that is in the control of the doctor. Most of the rest of the decisions are based entirely on the condition of the patient and the patient’s specific anatomy. So the timing of S2P is something that we can truly define most always. What we want to find out is, what is the ideal timing? How early is too early? Is there such a thing as too late?”
In an effort to determine the optimal timing of S2P that both minimizes pre-S2P attrition and maximizes long-term post-S2P survival, Dr. Jaquiss and his associates at 19 other institutions evaluated data from 534 neonates diagnosed with left ventricular outflow tract obstruction that precluded adequate systemic cardiac output through the aortic valve who initially underwent a Norwood operation from 2005 to 2016.
S2P was performed in 377 patients (71%) at a mean age of 5.4 months, while 115 (22%) died after Norwood, and the rest underwent biventricular repair or heart transplantation. After S2P, 38 (10%) died, 248 (66%) underwent Fontan, and the rest were alive awaiting Fontan or underwent heart transplantation.
Risk factors for death after Norwood included requiring pre-Norwood extracorporeal membrane oxygenation (P less than .0001), birth weight of less than 2.5 kg (P less than .0001), modified Blalock-Taussig shunt vs. a right ventricle to pulmonary artery conduit (P = .0003), larger baseline right pulmonary artery diameter (P = .0002), smaller baseline mitral valve diameter (P = .0002), smaller baseline tricuspid valve diameter (P = .0001), and nonwhite race (P = .03).
Risk factors for death after S2P included lower oxygen saturation at pre-S2P clinic visit (P = .02), having moderate or severe pre-S2P right ventricular dysfunction (P = .007), younger age at S2P (P = .03), and longer post-Norwood hospital length of stay (P = .03).
The risk-adjusted, 4-year, post-Norwood survival was 72%, with a confidence interval of 67%-75%. When plotted vs. the age at S2P, risk-adjusted, 4-year, post-Norwood survival for the 534 patients was maximized by S2P at 3-6 months of age. At the same time, risk-adjusted, 4-year survival in low-risk infants was compromised only by undergoing S2P earlier than 3 months of age. In high-risk infants, survival was severely compromised, especially when undergoing S2P earlier than 6 months of age.
“The results reinforced intuitions or expectations that most of the investigators already had,” Dr. Jaquiss said. “But we are in an era where evidence-based medicine is much preferable to intuition-based medicine. I’m very confident in the findings we have. I feel more confident in suggesting that we should be planning these surgeries around 3-4 months of age in usual-risk children and also more confident in suggesting that we need to consider transplantation earlier in children who are perceived to be at high risk. There is some hope [by clinicians in] some centers that you can convert a high-risk prognosis to a lower or intermediate risk prognosis by doing the S2P earlier or at some alternative time. Our data suggests that would not be helpful.”
Dr. Jaquiss and Dr. Meza reported having no financial disclosures.
HOUSTON – The optimal timing of stage 2 palliation after the Norwood operation depends on certain patient-specific risk factors, but in most cases should be done around 3-4 months of age, results from a multi-center study show.
While previous studies have investigated whether early stage-2 palliation (S2P) can be performed without increased post-S2P mortality, the effect of the timing of S2P on post-Norwood mortality remains unknown, Robert “Jake” Jaquiss, MD, said in an interview in advance of the annual meeting of the Society of Thoracic Surgeons.
“There has been a lot of dispute about how early is too early for S2P,” said Dr. Jaquiss, the study’s senior author, who is professor and division chief of pediatric cardiothoracic surgery at the University of Texas Southwestern Medical Center. “That is one of the few things that is in the control of the doctor. Most of the rest of the decisions are based entirely on the condition of the patient and the patient’s specific anatomy. So the timing of S2P is something that we can truly define most always. What we want to find out is, what is the ideal timing? How early is too early? Is there such a thing as too late?”
In an effort to determine the optimal timing of S2P that both minimizes pre-S2P attrition and maximizes long-term post-S2P survival, Dr. Jaquiss and his associates at 19 other institutions evaluated data from 534 neonates diagnosed with left ventricular outflow tract obstruction that precluded adequate systemic cardiac output through the aortic valve who initially underwent a Norwood operation from 2005 to 2016.
S2P was performed in 377 patients (71%) at a mean age of 5.4 months, while 115 (22%) died after Norwood, and the rest underwent biventricular repair or heart transplantation. After S2P, 38 (10%) died, 248 (66%) underwent Fontan, and the rest were alive awaiting Fontan or underwent heart transplantation.
Risk factors for death after Norwood included requiring pre-Norwood extracorporeal membrane oxygenation (P less than .0001), birth weight of less than 2.5 kg (P less than .0001), modified Blalock-Taussig shunt vs. a right ventricle to pulmonary artery conduit (P = .0003), larger baseline right pulmonary artery diameter (P = .0002), smaller baseline mitral valve diameter (P = .0002), smaller baseline tricuspid valve diameter (P = .0001), and nonwhite race (P = .03).
Risk factors for death after S2P included lower oxygen saturation at pre-S2P clinic visit (P = .02), having moderate or severe pre-S2P right ventricular dysfunction (P = .007), younger age at S2P (P = .03), and longer post-Norwood hospital length of stay (P = .03).
The risk-adjusted, 4-year, post-Norwood survival was 72%, with a confidence interval of 67%-75%. When plotted vs. the age at S2P, risk-adjusted, 4-year, post-Norwood survival for the 534 patients was maximized by S2P at 3-6 months of age. At the same time, risk-adjusted, 4-year survival in low-risk infants was compromised only by undergoing S2P earlier than 3 months of age. In high-risk infants, survival was severely compromised, especially when undergoing S2P earlier than 6 months of age.
“The results reinforced intuitions or expectations that most of the investigators already had,” Dr. Jaquiss said. “But we are in an era where evidence-based medicine is much preferable to intuition-based medicine. I’m very confident in the findings we have. I feel more confident in suggesting that we should be planning these surgeries around 3-4 months of age in usual-risk children and also more confident in suggesting that we need to consider transplantation earlier in children who are perceived to be at high risk. There is some hope [by clinicians in] some centers that you can convert a high-risk prognosis to a lower or intermediate risk prognosis by doing the S2P earlier or at some alternative time. Our data suggests that would not be helpful.”
Dr. Jaquiss and Dr. Meza reported having no financial disclosures.
Key clinical point:
Major finding: When plotted vs. the age at stage 2 palliation, risk-adjusted, 4-year, post-Norwood survival for the 534 patients was maximized by S2P at 3-6 months of age.
Data source: A multi-institutional analysis of 534 neonates diagnosed with left ventricular outflow tract obstruction that precluded adequate systemic cardiac output through the aortic valve who initially underwent a Norwood operation from 2005 to 2016.
Disclosures: Dr. Jaquiss and Dr. Meza reported having no financial disclosures.