User login
Pediatricians urged to check for vision problems after concussion
Pediatricians should consider screening children suspected of having a concussion for resulting vision problems that are often overlooked, according to the American Academy of Pediatrics.
Christina Master, MD, a pediatrician and sports medicine specialist at the Children’s Hospital of Philadelphia, said many doctors don’t think of vision problems when examining children who’ve experienced a head injury. But the issues are common and can significantly affect a child’s performance in school and sports, and disrupt daily life.
Dr. Master led a team of sports medicine and vision specialists who wrote an AAP policy statement on vision and concussion. She summarized the new recommendations during a plenary session Oct. 9 at the American Academy of Pediatrics National Conference.
Dr. Master told this news organization that the vast majority of the estimated 1.4 million U.S. children and adolescents who have concussions annually are treated in pediatricians’ offices.
Up to 40% of young patients experience symptoms such as blurred vision, light sensitivity, and double vision following a concussion, the panel said. In addition, children with vision problems are more likely to have prolonged recoveries and delays in returning to school than children who have concussions but don’t have similar eyesight issues.
Concussions affect neurologic pathways of the visual system and disturb basic functions such as the ability of the eyes to change focus from a distant object to a near one.
Dr. Master said most pediatricians do not routinely check for vision problems following a concussion, and children themselves may not recognize that they have vision deficits “unless you ask them very specifically.”
In addition to asking children about their vision, the policy statement recommends pediatricians conduct a thorough exam to assess ocular alignment, the ability to track a moving object, and the ability to maintain focus on an image while moving.
Dr. Master said that an assessment of vision and balance, which is described in an accompanying clinical report, lasts about 5 minutes and is easy for pediatricians to learn.
Managing vision problems
Pediatricians can guide parents in talking to their child’s school about accommodations such as extra time on classroom tasks, creating materials with enlarged fonts, and using preprinted or audio notes, the statement said.
At school, vision deficits can interfere with reading by causing children to skip words, lose their place, become fatigued, or lose interest, according to the statement.
Children can also take breaks from visual stressors such as bright lights and screens, and use prescription glasses temporarily to correct blurred vision, the panel noted.
Although most children will recover from a concussion on their own within 4 weeks, up to one-third will have persistent symptoms and may benefit from seeing a specialist who can provide treatment such as rehabilitative exercises. While evidence suggests that referring some children to specialty care within a week of a concussion improves outcomes, the signs of who would benefit are not always clear, according to the panel.
Specialties such as sports medicine, neurology, physiatry, otorhinolaryngology, and occupational therapy may provide care for prolonged symptoms, Dr. Master said.
The panel noted that more study is needed on treatment options such as rehabilitation exercises, which have been shown to help with balance and dizziness.
Dr. Master said the panel did not recommend that pediatricians provide a home exercise program to treat concussion, as she does in her practice, explaining that “it’s not clear that it’s necessary for all kids.”
One author of the policy statement, Ankoor Shah, MD, PhD, reported an intellectual property relationship with Rebion involving a patent application for a pediatric vision screener. Others, including Dr. Master, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians should consider screening children suspected of having a concussion for resulting vision problems that are often overlooked, according to the American Academy of Pediatrics.
Christina Master, MD, a pediatrician and sports medicine specialist at the Children’s Hospital of Philadelphia, said many doctors don’t think of vision problems when examining children who’ve experienced a head injury. But the issues are common and can significantly affect a child’s performance in school and sports, and disrupt daily life.
Dr. Master led a team of sports medicine and vision specialists who wrote an AAP policy statement on vision and concussion. She summarized the new recommendations during a plenary session Oct. 9 at the American Academy of Pediatrics National Conference.
Dr. Master told this news organization that the vast majority of the estimated 1.4 million U.S. children and adolescents who have concussions annually are treated in pediatricians’ offices.
Up to 40% of young patients experience symptoms such as blurred vision, light sensitivity, and double vision following a concussion, the panel said. In addition, children with vision problems are more likely to have prolonged recoveries and delays in returning to school than children who have concussions but don’t have similar eyesight issues.
Concussions affect neurologic pathways of the visual system and disturb basic functions such as the ability of the eyes to change focus from a distant object to a near one.
Dr. Master said most pediatricians do not routinely check for vision problems following a concussion, and children themselves may not recognize that they have vision deficits “unless you ask them very specifically.”
In addition to asking children about their vision, the policy statement recommends pediatricians conduct a thorough exam to assess ocular alignment, the ability to track a moving object, and the ability to maintain focus on an image while moving.
Dr. Master said that an assessment of vision and balance, which is described in an accompanying clinical report, lasts about 5 minutes and is easy for pediatricians to learn.
Managing vision problems
Pediatricians can guide parents in talking to their child’s school about accommodations such as extra time on classroom tasks, creating materials with enlarged fonts, and using preprinted or audio notes, the statement said.
At school, vision deficits can interfere with reading by causing children to skip words, lose their place, become fatigued, or lose interest, according to the statement.
Children can also take breaks from visual stressors such as bright lights and screens, and use prescription glasses temporarily to correct blurred vision, the panel noted.
Although most children will recover from a concussion on their own within 4 weeks, up to one-third will have persistent symptoms and may benefit from seeing a specialist who can provide treatment such as rehabilitative exercises. While evidence suggests that referring some children to specialty care within a week of a concussion improves outcomes, the signs of who would benefit are not always clear, according to the panel.
Specialties such as sports medicine, neurology, physiatry, otorhinolaryngology, and occupational therapy may provide care for prolonged symptoms, Dr. Master said.
The panel noted that more study is needed on treatment options such as rehabilitation exercises, which have been shown to help with balance and dizziness.
Dr. Master said the panel did not recommend that pediatricians provide a home exercise program to treat concussion, as she does in her practice, explaining that “it’s not clear that it’s necessary for all kids.”
One author of the policy statement, Ankoor Shah, MD, PhD, reported an intellectual property relationship with Rebion involving a patent application for a pediatric vision screener. Others, including Dr. Master, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians should consider screening children suspected of having a concussion for resulting vision problems that are often overlooked, according to the American Academy of Pediatrics.
Christina Master, MD, a pediatrician and sports medicine specialist at the Children’s Hospital of Philadelphia, said many doctors don’t think of vision problems when examining children who’ve experienced a head injury. But the issues are common and can significantly affect a child’s performance in school and sports, and disrupt daily life.
Dr. Master led a team of sports medicine and vision specialists who wrote an AAP policy statement on vision and concussion. She summarized the new recommendations during a plenary session Oct. 9 at the American Academy of Pediatrics National Conference.
Dr. Master told this news organization that the vast majority of the estimated 1.4 million U.S. children and adolescents who have concussions annually are treated in pediatricians’ offices.
Up to 40% of young patients experience symptoms such as blurred vision, light sensitivity, and double vision following a concussion, the panel said. In addition, children with vision problems are more likely to have prolonged recoveries and delays in returning to school than children who have concussions but don’t have similar eyesight issues.
Concussions affect neurologic pathways of the visual system and disturb basic functions such as the ability of the eyes to change focus from a distant object to a near one.
Dr. Master said most pediatricians do not routinely check for vision problems following a concussion, and children themselves may not recognize that they have vision deficits “unless you ask them very specifically.”
In addition to asking children about their vision, the policy statement recommends pediatricians conduct a thorough exam to assess ocular alignment, the ability to track a moving object, and the ability to maintain focus on an image while moving.
Dr. Master said that an assessment of vision and balance, which is described in an accompanying clinical report, lasts about 5 minutes and is easy for pediatricians to learn.
Managing vision problems
Pediatricians can guide parents in talking to their child’s school about accommodations such as extra time on classroom tasks, creating materials with enlarged fonts, and using preprinted or audio notes, the statement said.
At school, vision deficits can interfere with reading by causing children to skip words, lose their place, become fatigued, or lose interest, according to the statement.
Children can also take breaks from visual stressors such as bright lights and screens, and use prescription glasses temporarily to correct blurred vision, the panel noted.
Although most children will recover from a concussion on their own within 4 weeks, up to one-third will have persistent symptoms and may benefit from seeing a specialist who can provide treatment such as rehabilitative exercises. While evidence suggests that referring some children to specialty care within a week of a concussion improves outcomes, the signs of who would benefit are not always clear, according to the panel.
Specialties such as sports medicine, neurology, physiatry, otorhinolaryngology, and occupational therapy may provide care for prolonged symptoms, Dr. Master said.
The panel noted that more study is needed on treatment options such as rehabilitation exercises, which have been shown to help with balance and dizziness.
Dr. Master said the panel did not recommend that pediatricians provide a home exercise program to treat concussion, as she does in her practice, explaining that “it’s not clear that it’s necessary for all kids.”
One author of the policy statement, Ankoor Shah, MD, PhD, reported an intellectual property relationship with Rebion involving a patent application for a pediatric vision screener. Others, including Dr. Master, reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAP 2022
A history of head trauma may predict Parkinson’s disease progression
, new research suggests.
In a longitudinal online study, among patients with Parkinson’s disease who had a history of head injury, motor impairment developed 25% faster and cognitive impairment developed 45% faster than among those without such a history.
In addition, severe head injuries were associated with an even more rapid onset of impairment. The results give weight to the idea that “it’s head injuries themselves” prior to the development of Parkinson’s disease that might exacerbate motor and cognitive symptoms, said study investigator Ethan Brown, MD, assistant professor, Weill Institute of Neurosciences, department of neurology, University of California, San Francisco.
The findings emphasize the importance of “doing everything we can” to prevent falls and head injuries for patients with Parkinson’s disease, Dr. Brown said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reverse causality concerns
Head injury is a risk factor for Parkinson’s disease, but its relationship to Parkinson’s disease progression is not well established. “There has always been this concern in Parkinson’s disease that maybe it’s problems with motor impairment that lead to head injuries, so reverse causality is an issue,” said Dr. Brown. “We wanted to look at whether risk factors we know relate to the development of Parkinson’s disease can also have a bearing on its progression,” he added.
The analysis was part of the online Fox Insight study that is evaluating motor and nonmotor symptoms in individuals with and those without Parkinson’s disease. The study included participants who had completed questionnaires on such things as head trauma.
The study included 1,065 patients (47% women; mean age, 63 years) with Parkinson’s disease who reported having had a head injury at least 5 years prior to their diagnosis. Among the participants, the mean duration of Parkinson’s disease was 7.5 years.
The investigators employed a 5-year lag time in their study to exclude head injuries caused by early motor dysfunction, they noted. “We wanted to look at people who had these head injuries we think might be part of the cause of Parkinson’s disease as opposed to a result of them,” Dr. Brown said.
In this head injury group, 51% had received one head injury, 28% had received two injuries, and 22% had received more than two injuries.
The study also included 1,457 participants (56% women; mean age, 65 years) with Parkinson’s disease who had not had a head injury prior to their diagnosis. Of these patients, the mean time with a Parkinson’s disease diagnosis was 8 years.
Dr. Brown noted that the age and sex distribution of the study group was “probably representative” of the general Parkinson’s disease population. However, because the participants had to be able to go online and complete questionnaires, it is unlikely that, among these patients, Parkinson’s disease was far advanced, he said.
The investigators adjusted for age, sex, years of education, and Parkinson’s disease duration.
Two-hit hypothesis?
The researchers compared time from diagnosis to the development of significant motor impairment, such as the need for assistance with walking, and cognitive impairment, such as having a score of less than 43 on the Penn Daily Activities Questionnaire.
They also examined the role of more severe head injuries. In the head injury group, over half (54%) had had a severe head injury, including 543 who had lost consciousness and others who had suffered a fracture or had had a seizure.
Results showed that the adjusted hazard ratio for developing motor impairment among those with a head injury, compared with those who had not had a head injury was 1.24 (95% confidence interval, 1.01-1.53; P = .037). For severe injuries, the aHR for motor impairment was 1.44 (95% CI, 1.13-1.83; P = .003).
For cognitive impairment, the aHR for those with versus without head injuries was 1.45 (95% CI, 1.14-1.86; P = .003); and for severe injuries, the aHR was 1.49 (95% CI, 1.11-2.0; P = .008).
Aside from severity, the researchers did not examine subgroups. However, Dr. Brown reported that his team would like to stratify results by sex and other variables in the future.
He noted that various mechanisms may explain why Parkinson’s disease progression is faster for patients who have a history of head injury, compared with others. Chronic inflammation due to the injury and “co-pathology” might play some role, he said. He noted that head injuries are associated with cognitive impairment in other conditions, including Alzheimer’s disease.
There is also the “two hit” hypothesis, Dr. Brown said. “A head injury could cause such broad damage that once people develop Parkinson’s disease, it’s harder for them to compensate.”
Dr. Brown also noted there might have been a “higher magnitude” of a difference between groups had the study captured participants with more severe symptoms.
‘Provocative’ findings
Michael S. Okun, MD, medical advisor at the Parkinson’s Foundation and professor and director at the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the new data are “provocative.”
“The idea that a head injury may be important in predicting how quickly and how severely deficits will manifest could be important to the treating clinician,” said Dr. Okun, who was not involved with the research.
He noted that the results suggest clinicians should elicit more information from patients about head trauma. “They should be seeking more than a binary ‘yes or no’ answer to head injury when questioning patients,” he added.
Dr. Okun reiterated that head injury is a “known and important risk factor” not only for Parkinson’s disease but also for other neurodegenerative diseases. “It’s important to counsel patients about the association,” he said.
The study was supported by the Michael J. Fox Foundation. Dr. Brown reports having received grant support from the Michael J. Fox Foundation. Dr. Okun has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a longitudinal online study, among patients with Parkinson’s disease who had a history of head injury, motor impairment developed 25% faster and cognitive impairment developed 45% faster than among those without such a history.
In addition, severe head injuries were associated with an even more rapid onset of impairment. The results give weight to the idea that “it’s head injuries themselves” prior to the development of Parkinson’s disease that might exacerbate motor and cognitive symptoms, said study investigator Ethan Brown, MD, assistant professor, Weill Institute of Neurosciences, department of neurology, University of California, San Francisco.
The findings emphasize the importance of “doing everything we can” to prevent falls and head injuries for patients with Parkinson’s disease, Dr. Brown said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reverse causality concerns
Head injury is a risk factor for Parkinson’s disease, but its relationship to Parkinson’s disease progression is not well established. “There has always been this concern in Parkinson’s disease that maybe it’s problems with motor impairment that lead to head injuries, so reverse causality is an issue,” said Dr. Brown. “We wanted to look at whether risk factors we know relate to the development of Parkinson’s disease can also have a bearing on its progression,” he added.
The analysis was part of the online Fox Insight study that is evaluating motor and nonmotor symptoms in individuals with and those without Parkinson’s disease. The study included participants who had completed questionnaires on such things as head trauma.
The study included 1,065 patients (47% women; mean age, 63 years) with Parkinson’s disease who reported having had a head injury at least 5 years prior to their diagnosis. Among the participants, the mean duration of Parkinson’s disease was 7.5 years.
The investigators employed a 5-year lag time in their study to exclude head injuries caused by early motor dysfunction, they noted. “We wanted to look at people who had these head injuries we think might be part of the cause of Parkinson’s disease as opposed to a result of them,” Dr. Brown said.
In this head injury group, 51% had received one head injury, 28% had received two injuries, and 22% had received more than two injuries.
The study also included 1,457 participants (56% women; mean age, 65 years) with Parkinson’s disease who had not had a head injury prior to their diagnosis. Of these patients, the mean time with a Parkinson’s disease diagnosis was 8 years.
Dr. Brown noted that the age and sex distribution of the study group was “probably representative” of the general Parkinson’s disease population. However, because the participants had to be able to go online and complete questionnaires, it is unlikely that, among these patients, Parkinson’s disease was far advanced, he said.
The investigators adjusted for age, sex, years of education, and Parkinson’s disease duration.
Two-hit hypothesis?
The researchers compared time from diagnosis to the development of significant motor impairment, such as the need for assistance with walking, and cognitive impairment, such as having a score of less than 43 on the Penn Daily Activities Questionnaire.
They also examined the role of more severe head injuries. In the head injury group, over half (54%) had had a severe head injury, including 543 who had lost consciousness and others who had suffered a fracture or had had a seizure.
Results showed that the adjusted hazard ratio for developing motor impairment among those with a head injury, compared with those who had not had a head injury was 1.24 (95% confidence interval, 1.01-1.53; P = .037). For severe injuries, the aHR for motor impairment was 1.44 (95% CI, 1.13-1.83; P = .003).
For cognitive impairment, the aHR for those with versus without head injuries was 1.45 (95% CI, 1.14-1.86; P = .003); and for severe injuries, the aHR was 1.49 (95% CI, 1.11-2.0; P = .008).
Aside from severity, the researchers did not examine subgroups. However, Dr. Brown reported that his team would like to stratify results by sex and other variables in the future.
He noted that various mechanisms may explain why Parkinson’s disease progression is faster for patients who have a history of head injury, compared with others. Chronic inflammation due to the injury and “co-pathology” might play some role, he said. He noted that head injuries are associated with cognitive impairment in other conditions, including Alzheimer’s disease.
There is also the “two hit” hypothesis, Dr. Brown said. “A head injury could cause such broad damage that once people develop Parkinson’s disease, it’s harder for them to compensate.”
Dr. Brown also noted there might have been a “higher magnitude” of a difference between groups had the study captured participants with more severe symptoms.
‘Provocative’ findings
Michael S. Okun, MD, medical advisor at the Parkinson’s Foundation and professor and director at the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the new data are “provocative.”
“The idea that a head injury may be important in predicting how quickly and how severely deficits will manifest could be important to the treating clinician,” said Dr. Okun, who was not involved with the research.
He noted that the results suggest clinicians should elicit more information from patients about head trauma. “They should be seeking more than a binary ‘yes or no’ answer to head injury when questioning patients,” he added.
Dr. Okun reiterated that head injury is a “known and important risk factor” not only for Parkinson’s disease but also for other neurodegenerative diseases. “It’s important to counsel patients about the association,” he said.
The study was supported by the Michael J. Fox Foundation. Dr. Brown reports having received grant support from the Michael J. Fox Foundation. Dr. Okun has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a longitudinal online study, among patients with Parkinson’s disease who had a history of head injury, motor impairment developed 25% faster and cognitive impairment developed 45% faster than among those without such a history.
In addition, severe head injuries were associated with an even more rapid onset of impairment. The results give weight to the idea that “it’s head injuries themselves” prior to the development of Parkinson’s disease that might exacerbate motor and cognitive symptoms, said study investigator Ethan Brown, MD, assistant professor, Weill Institute of Neurosciences, department of neurology, University of California, San Francisco.
The findings emphasize the importance of “doing everything we can” to prevent falls and head injuries for patients with Parkinson’s disease, Dr. Brown said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reverse causality concerns
Head injury is a risk factor for Parkinson’s disease, but its relationship to Parkinson’s disease progression is not well established. “There has always been this concern in Parkinson’s disease that maybe it’s problems with motor impairment that lead to head injuries, so reverse causality is an issue,” said Dr. Brown. “We wanted to look at whether risk factors we know relate to the development of Parkinson’s disease can also have a bearing on its progression,” he added.
The analysis was part of the online Fox Insight study that is evaluating motor and nonmotor symptoms in individuals with and those without Parkinson’s disease. The study included participants who had completed questionnaires on such things as head trauma.
The study included 1,065 patients (47% women; mean age, 63 years) with Parkinson’s disease who reported having had a head injury at least 5 years prior to their diagnosis. Among the participants, the mean duration of Parkinson’s disease was 7.5 years.
The investigators employed a 5-year lag time in their study to exclude head injuries caused by early motor dysfunction, they noted. “We wanted to look at people who had these head injuries we think might be part of the cause of Parkinson’s disease as opposed to a result of them,” Dr. Brown said.
In this head injury group, 51% had received one head injury, 28% had received two injuries, and 22% had received more than two injuries.
The study also included 1,457 participants (56% women; mean age, 65 years) with Parkinson’s disease who had not had a head injury prior to their diagnosis. Of these patients, the mean time with a Parkinson’s disease diagnosis was 8 years.
Dr. Brown noted that the age and sex distribution of the study group was “probably representative” of the general Parkinson’s disease population. However, because the participants had to be able to go online and complete questionnaires, it is unlikely that, among these patients, Parkinson’s disease was far advanced, he said.
The investigators adjusted for age, sex, years of education, and Parkinson’s disease duration.
Two-hit hypothesis?
The researchers compared time from diagnosis to the development of significant motor impairment, such as the need for assistance with walking, and cognitive impairment, such as having a score of less than 43 on the Penn Daily Activities Questionnaire.
They also examined the role of more severe head injuries. In the head injury group, over half (54%) had had a severe head injury, including 543 who had lost consciousness and others who had suffered a fracture or had had a seizure.
Results showed that the adjusted hazard ratio for developing motor impairment among those with a head injury, compared with those who had not had a head injury was 1.24 (95% confidence interval, 1.01-1.53; P = .037). For severe injuries, the aHR for motor impairment was 1.44 (95% CI, 1.13-1.83; P = .003).
For cognitive impairment, the aHR for those with versus without head injuries was 1.45 (95% CI, 1.14-1.86; P = .003); and for severe injuries, the aHR was 1.49 (95% CI, 1.11-2.0; P = .008).
Aside from severity, the researchers did not examine subgroups. However, Dr. Brown reported that his team would like to stratify results by sex and other variables in the future.
He noted that various mechanisms may explain why Parkinson’s disease progression is faster for patients who have a history of head injury, compared with others. Chronic inflammation due to the injury and “co-pathology” might play some role, he said. He noted that head injuries are associated with cognitive impairment in other conditions, including Alzheimer’s disease.
There is also the “two hit” hypothesis, Dr. Brown said. “A head injury could cause such broad damage that once people develop Parkinson’s disease, it’s harder for them to compensate.”
Dr. Brown also noted there might have been a “higher magnitude” of a difference between groups had the study captured participants with more severe symptoms.
‘Provocative’ findings
Michael S. Okun, MD, medical advisor at the Parkinson’s Foundation and professor and director at the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the new data are “provocative.”
“The idea that a head injury may be important in predicting how quickly and how severely deficits will manifest could be important to the treating clinician,” said Dr. Okun, who was not involved with the research.
He noted that the results suggest clinicians should elicit more information from patients about head trauma. “They should be seeking more than a binary ‘yes or no’ answer to head injury when questioning patients,” he added.
Dr. Okun reiterated that head injury is a “known and important risk factor” not only for Parkinson’s disease but also for other neurodegenerative diseases. “It’s important to counsel patients about the association,” he said.
The study was supported by the Michael J. Fox Foundation. Dr. Brown reports having received grant support from the Michael J. Fox Foundation. Dr. Okun has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From MDS 2022
TBI is an unrecognized risk factor for cardiovascular disease
(CVD). More severe TBI is associated with higher risk of CVD, new research shows.
Given the relatively young age of post-9/11–era veterans with TBI, there may be an increased burden of heart disease in the future as these veterans age and develop traditional risk factors for CVD, the investigators, led by Ian J. Stewart, MD, with Uniformed Services University, Bethesda, Md., wrote.
The study was published online in JAMA Neurology.
Novel data
Since Sept. 11, 2001, 4.5 million people have served in the U.S. military, with their time in service defined by the long-running wars in Iraq and Afghanistan. Estimates suggest that up to 20% of post-9/11 veterans sustained a TBI.
While some evidence suggests that TBI increases the risk of CVD, prior reports have focused mainly on cerebrovascular outcomes. Until now, the potential association of TBI with CVD has not been comprehensively examined in post-9/11–era veterans.
The retrospective cohort study included 1,559,928 predominantly male post-9/11 veterans, including 301,169 (19.3%) with a history of TBI and 1,258,759 (81%) with no TBI history.
In fully adjusted models, compared with veterans with no TBI history, a history of mild, moderate/severe, or penetrating TBI was associated with increased risk of developing the composite CVD endpoint (coronary artery disease, stroke, peripheral artery disease, and CVD death).
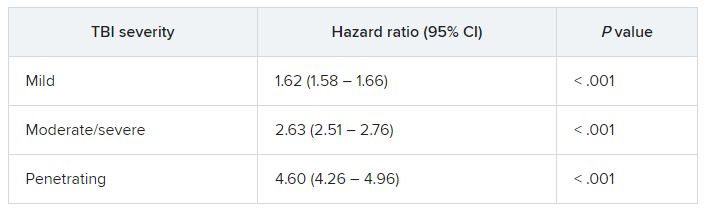
TBIs of all severities were associated with the individual components of the composite outcome, except penetrating TBI and CVD death.
“The association of TBI with subsequent CVD was not attenuated in multivariable models, suggesting that TBI may be accounting for risk that is independent from the other variables,” Dr. Stewart and colleagues wrote.
They noted that the risk was highest shortly after injury, but TBI remained significantly associated with CVD for years after the initial insult.
Why TBI may raise the risk of subsequent CVD remains unclear.
It’s possible that patients with TBI develop more traditional risk factors for CVD through time than do patients without TBI. A study in mice found that TBI led to increased rates of atherosclerosis, the researchers said.
An additional mechanism may be disruption of autonomic regulation, which has been known to occur after TBI.
Another potential pathway is through mental health diagnoses, such as posttraumatic stress disorder; a large body of work has identified associations between PTSD and CVD, including among post-9/11 veterans.
Further work is needed to determine how this risk can be modified to improve outcomes for post-9/11–era veterans, the researchers write.
Unrecognized CVD risk factor?
Reached for comment, Shaheen E. Lakhan, MD, PhD, a neurologist and researcher from Boston who wasn’t involved in the study, said the effects of TBI on heart health are “very underreported, and most clinicians would not make the link.”
“When the brain suffers a traumatic injury, it activates a cascade of neuro-inflammation that goes haywire in an attempt to protect further brain damage. Oftentimes, these inflammatory by-products leak into the body, especially in trauma, when the barriers are broken between brain and body, and can cause systemic body inflammation, which is well associated with heart disease,” Dr. Lakhan said.
In addition, Dr. Lakhan said, “TBI itself localized to just the brain can negatively affect good health habits, leading to worsening heart health, too.”
“Research like this brings light where not much exists and underscores the importance of protecting our brains from physical trauma,” he said.
The study was supported by the assistant secretary of defense for health affairs, endorsed by the Department of Defense through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium, and by the U.S. Department of Veterans Affairs. Dr. Stewart and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(CVD). More severe TBI is associated with higher risk of CVD, new research shows.
Given the relatively young age of post-9/11–era veterans with TBI, there may be an increased burden of heart disease in the future as these veterans age and develop traditional risk factors for CVD, the investigators, led by Ian J. Stewart, MD, with Uniformed Services University, Bethesda, Md., wrote.
The study was published online in JAMA Neurology.
Novel data
Since Sept. 11, 2001, 4.5 million people have served in the U.S. military, with their time in service defined by the long-running wars in Iraq and Afghanistan. Estimates suggest that up to 20% of post-9/11 veterans sustained a TBI.
While some evidence suggests that TBI increases the risk of CVD, prior reports have focused mainly on cerebrovascular outcomes. Until now, the potential association of TBI with CVD has not been comprehensively examined in post-9/11–era veterans.
The retrospective cohort study included 1,559,928 predominantly male post-9/11 veterans, including 301,169 (19.3%) with a history of TBI and 1,258,759 (81%) with no TBI history.
In fully adjusted models, compared with veterans with no TBI history, a history of mild, moderate/severe, or penetrating TBI was associated with increased risk of developing the composite CVD endpoint (coronary artery disease, stroke, peripheral artery disease, and CVD death).

TBIs of all severities were associated with the individual components of the composite outcome, except penetrating TBI and CVD death.
“The association of TBI with subsequent CVD was not attenuated in multivariable models, suggesting that TBI may be accounting for risk that is independent from the other variables,” Dr. Stewart and colleagues wrote.
They noted that the risk was highest shortly after injury, but TBI remained significantly associated with CVD for years after the initial insult.
Why TBI may raise the risk of subsequent CVD remains unclear.
It’s possible that patients with TBI develop more traditional risk factors for CVD through time than do patients without TBI. A study in mice found that TBI led to increased rates of atherosclerosis, the researchers said.
An additional mechanism may be disruption of autonomic regulation, which has been known to occur after TBI.
Another potential pathway is through mental health diagnoses, such as posttraumatic stress disorder; a large body of work has identified associations between PTSD and CVD, including among post-9/11 veterans.
Further work is needed to determine how this risk can be modified to improve outcomes for post-9/11–era veterans, the researchers write.
Unrecognized CVD risk factor?
Reached for comment, Shaheen E. Lakhan, MD, PhD, a neurologist and researcher from Boston who wasn’t involved in the study, said the effects of TBI on heart health are “very underreported, and most clinicians would not make the link.”
“When the brain suffers a traumatic injury, it activates a cascade of neuro-inflammation that goes haywire in an attempt to protect further brain damage. Oftentimes, these inflammatory by-products leak into the body, especially in trauma, when the barriers are broken between brain and body, and can cause systemic body inflammation, which is well associated with heart disease,” Dr. Lakhan said.
In addition, Dr. Lakhan said, “TBI itself localized to just the brain can negatively affect good health habits, leading to worsening heart health, too.”
“Research like this brings light where not much exists and underscores the importance of protecting our brains from physical trauma,” he said.
The study was supported by the assistant secretary of defense for health affairs, endorsed by the Department of Defense through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium, and by the U.S. Department of Veterans Affairs. Dr. Stewart and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(CVD). More severe TBI is associated with higher risk of CVD, new research shows.
Given the relatively young age of post-9/11–era veterans with TBI, there may be an increased burden of heart disease in the future as these veterans age and develop traditional risk factors for CVD, the investigators, led by Ian J. Stewart, MD, with Uniformed Services University, Bethesda, Md., wrote.
The study was published online in JAMA Neurology.
Novel data
Since Sept. 11, 2001, 4.5 million people have served in the U.S. military, with their time in service defined by the long-running wars in Iraq and Afghanistan. Estimates suggest that up to 20% of post-9/11 veterans sustained a TBI.
While some evidence suggests that TBI increases the risk of CVD, prior reports have focused mainly on cerebrovascular outcomes. Until now, the potential association of TBI with CVD has not been comprehensively examined in post-9/11–era veterans.
The retrospective cohort study included 1,559,928 predominantly male post-9/11 veterans, including 301,169 (19.3%) with a history of TBI and 1,258,759 (81%) with no TBI history.
In fully adjusted models, compared with veterans with no TBI history, a history of mild, moderate/severe, or penetrating TBI was associated with increased risk of developing the composite CVD endpoint (coronary artery disease, stroke, peripheral artery disease, and CVD death).

TBIs of all severities were associated with the individual components of the composite outcome, except penetrating TBI and CVD death.
“The association of TBI with subsequent CVD was not attenuated in multivariable models, suggesting that TBI may be accounting for risk that is independent from the other variables,” Dr. Stewart and colleagues wrote.
They noted that the risk was highest shortly after injury, but TBI remained significantly associated with CVD for years after the initial insult.
Why TBI may raise the risk of subsequent CVD remains unclear.
It’s possible that patients with TBI develop more traditional risk factors for CVD through time than do patients without TBI. A study in mice found that TBI led to increased rates of atherosclerosis, the researchers said.
An additional mechanism may be disruption of autonomic regulation, which has been known to occur after TBI.
Another potential pathway is through mental health diagnoses, such as posttraumatic stress disorder; a large body of work has identified associations between PTSD and CVD, including among post-9/11 veterans.
Further work is needed to determine how this risk can be modified to improve outcomes for post-9/11–era veterans, the researchers write.
Unrecognized CVD risk factor?
Reached for comment, Shaheen E. Lakhan, MD, PhD, a neurologist and researcher from Boston who wasn’t involved in the study, said the effects of TBI on heart health are “very underreported, and most clinicians would not make the link.”
“When the brain suffers a traumatic injury, it activates a cascade of neuro-inflammation that goes haywire in an attempt to protect further brain damage. Oftentimes, these inflammatory by-products leak into the body, especially in trauma, when the barriers are broken between brain and body, and can cause systemic body inflammation, which is well associated with heart disease,” Dr. Lakhan said.
In addition, Dr. Lakhan said, “TBI itself localized to just the brain can negatively affect good health habits, leading to worsening heart health, too.”
“Research like this brings light where not much exists and underscores the importance of protecting our brains from physical trauma,” he said.
The study was supported by the assistant secretary of defense for health affairs, endorsed by the Department of Defense through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium, and by the U.S. Department of Veterans Affairs. Dr. Stewart and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Blood biomarkers predict TBI disability and mortality
, new research suggests.
In new data from the TRACK-TBI study group, high levels of glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) proteins found in glial cells and neurons, respectively, correlated with death and severe injury. Investigators note that measuring these biomarkers may give a more accurate assessment of a patient’s prognosis following TBI.
This study is the “first report of the accuracy of a blood test that can be obtained rapidly on the day of injury to predict neurological recovery at 6 months after injury,” lead author Frederick Korley, MD, PhD, associate professor of emergency medicine at the University of Michigan, Ann Arbor, said in a news release.
The findings were published online in the Lancet Neurology.
Added value
The researchers measured GFAP and UCH-L1 in blood samples taken from 1,696 patients with TBI on the day of their injury, and they assessed patient recovery 6 months later.
The markers were measured using the i-STAT TBI Plasma test (Abbott Labs). The test was approved in 2021 by the U.S. Food and Drug Administration to determine which patients with mild TBI should undergo computed tomography scans.
About two-thirds of the study population were men, and the average age was 39 years. All patients were evaluated at Level I trauma centers for injuries caused primarily by traffic accidents or falls.
Six months following injury, 7% of the patients had died and 14% had an unfavorable outcome, ranging from vegetative state to severe disability requiring daily support. In addition, 67% had incomplete recovery, ranging from moderate disabilities requiring assistance outside of the home to minor disabling neurological or psychological deficits.
Day-of-injury GFAP and UCH-L1 levels had a high probability of predicting death (87% for GFAP and 89% for UCH-L1) and severe disability (86% for both GFAP and UCH-L1) at 6 months, the investigators reported.
The biomarkers were less accurate in predicting incomplete recovery (62% for GFAP and 61% for UCH-L1).
The researchers also assessed the added value of combining the blood biomarkers to current TBI prognostic models that take into account variables such as age, motor score, pupil reactivity, and CT characteristics.
In patients with a Glasgow Coma Scale (GCS) score of 3-12, adding GFAP and UCH-L1 alone or combined to each of the three International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) models significantly increased their accuracy for predicting death (range, 90%-94%) and unfavorable outcome (range, 83%-89%).
In patients with milder TBI (GCS score, 13-15), adding GFAP and UCH-L1 to the UPFRONT prognostic model modestly increased accuracy for predicting incomplete recovery (69%).
‘Important’ findings
Commenting on the study, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said this “critical” study shows that these biomarkers can “predict key outcomes,” including mortality and severe disability. “Thus, in conjunction with clinical evaluations and related data such as neuroimaging, these tests may warrant translation to broader clinical practice, particularly in acute settings,” said Dr. Raji, who was not involved in the research.
Also weighing in, Heidi Fusco, MD, assistant director of the traumatic brain injury program at NYU Langone Rusk Rehabilitation, said the findings are “important.”
“Prognosis after brain injury often is based on the initial presentation, ongoing clinical exams, and neuroimaging; and the addition of biomarkers would contribute to creating a more objective prognostic model,” Dr. Fusco said.
She noted “it’s unclear” whether clinical hospital laboratories would be able to accommodate this type of laboratory drawing.
“It is imperative that clinicians still use the patient history [and] clinical and radiological exam when making clinical decisions for a patient and not just lab values. It would be best to incorporate the GFAP and UCH-L1 into a preexisting prognostic model,” Dr. Fusco said.
The study was funded by the U.S. National Institutes of Health, the National Institute of Neurologic Disorders and Stroke, the U.S. Department of Defense, One Mind, and U.S. Army Medical Research and Development Command. Dr. Korley reported having previously consulted for Abbott Laboratories and has received research funding from Abbott Laboratories, which makes the assays used in the study. Dr. Raji is a consultant for Brainreader ApS and Neurevolution. Dr. Fusco has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In new data from the TRACK-TBI study group, high levels of glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) proteins found in glial cells and neurons, respectively, correlated with death and severe injury. Investigators note that measuring these biomarkers may give a more accurate assessment of a patient’s prognosis following TBI.
This study is the “first report of the accuracy of a blood test that can be obtained rapidly on the day of injury to predict neurological recovery at 6 months after injury,” lead author Frederick Korley, MD, PhD, associate professor of emergency medicine at the University of Michigan, Ann Arbor, said in a news release.
The findings were published online in the Lancet Neurology.
Added value
The researchers measured GFAP and UCH-L1 in blood samples taken from 1,696 patients with TBI on the day of their injury, and they assessed patient recovery 6 months later.
The markers were measured using the i-STAT TBI Plasma test (Abbott Labs). The test was approved in 2021 by the U.S. Food and Drug Administration to determine which patients with mild TBI should undergo computed tomography scans.
About two-thirds of the study population were men, and the average age was 39 years. All patients were evaluated at Level I trauma centers for injuries caused primarily by traffic accidents or falls.
Six months following injury, 7% of the patients had died and 14% had an unfavorable outcome, ranging from vegetative state to severe disability requiring daily support. In addition, 67% had incomplete recovery, ranging from moderate disabilities requiring assistance outside of the home to minor disabling neurological or psychological deficits.
Day-of-injury GFAP and UCH-L1 levels had a high probability of predicting death (87% for GFAP and 89% for UCH-L1) and severe disability (86% for both GFAP and UCH-L1) at 6 months, the investigators reported.
The biomarkers were less accurate in predicting incomplete recovery (62% for GFAP and 61% for UCH-L1).
The researchers also assessed the added value of combining the blood biomarkers to current TBI prognostic models that take into account variables such as age, motor score, pupil reactivity, and CT characteristics.
In patients with a Glasgow Coma Scale (GCS) score of 3-12, adding GFAP and UCH-L1 alone or combined to each of the three International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) models significantly increased their accuracy for predicting death (range, 90%-94%) and unfavorable outcome (range, 83%-89%).
In patients with milder TBI (GCS score, 13-15), adding GFAP and UCH-L1 to the UPFRONT prognostic model modestly increased accuracy for predicting incomplete recovery (69%).
‘Important’ findings
Commenting on the study, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said this “critical” study shows that these biomarkers can “predict key outcomes,” including mortality and severe disability. “Thus, in conjunction with clinical evaluations and related data such as neuroimaging, these tests may warrant translation to broader clinical practice, particularly in acute settings,” said Dr. Raji, who was not involved in the research.
Also weighing in, Heidi Fusco, MD, assistant director of the traumatic brain injury program at NYU Langone Rusk Rehabilitation, said the findings are “important.”
“Prognosis after brain injury often is based on the initial presentation, ongoing clinical exams, and neuroimaging; and the addition of biomarkers would contribute to creating a more objective prognostic model,” Dr. Fusco said.
She noted “it’s unclear” whether clinical hospital laboratories would be able to accommodate this type of laboratory drawing.
“It is imperative that clinicians still use the patient history [and] clinical and radiological exam when making clinical decisions for a patient and not just lab values. It would be best to incorporate the GFAP and UCH-L1 into a preexisting prognostic model,” Dr. Fusco said.
The study was funded by the U.S. National Institutes of Health, the National Institute of Neurologic Disorders and Stroke, the U.S. Department of Defense, One Mind, and U.S. Army Medical Research and Development Command. Dr. Korley reported having previously consulted for Abbott Laboratories and has received research funding from Abbott Laboratories, which makes the assays used in the study. Dr. Raji is a consultant for Brainreader ApS and Neurevolution. Dr. Fusco has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In new data from the TRACK-TBI study group, high levels of glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) proteins found in glial cells and neurons, respectively, correlated with death and severe injury. Investigators note that measuring these biomarkers may give a more accurate assessment of a patient’s prognosis following TBI.
This study is the “first report of the accuracy of a blood test that can be obtained rapidly on the day of injury to predict neurological recovery at 6 months after injury,” lead author Frederick Korley, MD, PhD, associate professor of emergency medicine at the University of Michigan, Ann Arbor, said in a news release.
The findings were published online in the Lancet Neurology.
Added value
The researchers measured GFAP and UCH-L1 in blood samples taken from 1,696 patients with TBI on the day of their injury, and they assessed patient recovery 6 months later.
The markers were measured using the i-STAT TBI Plasma test (Abbott Labs). The test was approved in 2021 by the U.S. Food and Drug Administration to determine which patients with mild TBI should undergo computed tomography scans.
About two-thirds of the study population were men, and the average age was 39 years. All patients were evaluated at Level I trauma centers for injuries caused primarily by traffic accidents or falls.
Six months following injury, 7% of the patients had died and 14% had an unfavorable outcome, ranging from vegetative state to severe disability requiring daily support. In addition, 67% had incomplete recovery, ranging from moderate disabilities requiring assistance outside of the home to minor disabling neurological or psychological deficits.
Day-of-injury GFAP and UCH-L1 levels had a high probability of predicting death (87% for GFAP and 89% for UCH-L1) and severe disability (86% for both GFAP and UCH-L1) at 6 months, the investigators reported.
The biomarkers were less accurate in predicting incomplete recovery (62% for GFAP and 61% for UCH-L1).
The researchers also assessed the added value of combining the blood biomarkers to current TBI prognostic models that take into account variables such as age, motor score, pupil reactivity, and CT characteristics.
In patients with a Glasgow Coma Scale (GCS) score of 3-12, adding GFAP and UCH-L1 alone or combined to each of the three International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) models significantly increased their accuracy for predicting death (range, 90%-94%) and unfavorable outcome (range, 83%-89%).
In patients with milder TBI (GCS score, 13-15), adding GFAP and UCH-L1 to the UPFRONT prognostic model modestly increased accuracy for predicting incomplete recovery (69%).
‘Important’ findings
Commenting on the study, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said this “critical” study shows that these biomarkers can “predict key outcomes,” including mortality and severe disability. “Thus, in conjunction with clinical evaluations and related data such as neuroimaging, these tests may warrant translation to broader clinical practice, particularly in acute settings,” said Dr. Raji, who was not involved in the research.
Also weighing in, Heidi Fusco, MD, assistant director of the traumatic brain injury program at NYU Langone Rusk Rehabilitation, said the findings are “important.”
“Prognosis after brain injury often is based on the initial presentation, ongoing clinical exams, and neuroimaging; and the addition of biomarkers would contribute to creating a more objective prognostic model,” Dr. Fusco said.
She noted “it’s unclear” whether clinical hospital laboratories would be able to accommodate this type of laboratory drawing.
“It is imperative that clinicians still use the patient history [and] clinical and radiological exam when making clinical decisions for a patient and not just lab values. It would be best to incorporate the GFAP and UCH-L1 into a preexisting prognostic model,” Dr. Fusco said.
The study was funded by the U.S. National Institutes of Health, the National Institute of Neurologic Disorders and Stroke, the U.S. Department of Defense, One Mind, and U.S. Army Medical Research and Development Command. Dr. Korley reported having previously consulted for Abbott Laboratories and has received research funding from Abbott Laboratories, which makes the assays used in the study. Dr. Raji is a consultant for Brainreader ApS and Neurevolution. Dr. Fusco has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET NEUROLOGY
Incomplete recovery common 6 months after mild TBI
, new data from the TRACK-TBI study shows.
“Seeing that more than half of the GCS [Glasgow Coma Score] 15, CT-negative TBI cohort in our study were not back to their preinjury baseline at 6 months was surprising and impacts the millions of Americans who suffer from concussions annually,” said lead author Debbie Madhok, MD, with department of emergency medicine, University of California, San Francisco.
“These results highlight the importance of improving care pathways for concussion, particularly from the emergency department,” Dr. Madhok said.
The findings were published online in JAMA Network Open.
The short- and long-term outcomes in the large group of patients who come into the ED with TBI, a GCS of 15, and without acute intracranial traumatic injury (defined as a negative head CT scan) remain poorly understood, the investigators noted. To investigate further, they evaluated outcomes at 2 weeks and 6 months in 991 of these patients (mean age, 38 years; 64% men) from the TRACK-TBI study.
Among the 751 (76%) participants followed up at 2 weeks after the injury, only 204 (27%) had functional recovery – with a Glasgow Outcome Scale-Extended (GOS-E) score of 8. The remaining 547 (73%) had incomplete recovery (GOS-E scores < 8).
Among the 659 patients (66%) followed up at 6 months after the injury, 287 (44%) had functional recovery and 372 (56%) had incomplete recovery.
Most patients who failed to recover completely reported they had not returned to their preinjury life (88%). They described trouble returning to social activities outside the home and disruptions in family relationships and friendships.
The researchers noted that the study population had a high rate of preinjury psychiatric comorbidities, and these patients were more likely to have incomplete recovery than those without psychiatric comorbidities. This aligns with results from previous studies, they added.
The investigators also noted that patients with mild TBI without acute intracranial trauma are typically managed by ED personnel.
“These findings highlight the importance of ED clinicians being aware of the risk of incomplete recovery for patients with a mild TBI (that is, GCS score of 15 and negative head CT scan) and providing accurate education and timely referral information before ED discharge,” they wrote.
The study was funded by grants from the National Foundation of Emergency Medicine, the National Institute of Neurological Disorders and Stroke, and the U.S. Department of Defense Traumatic Brain Injury Endpoints Development Initiative. Dr. Madhok has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new data from the TRACK-TBI study shows.
“Seeing that more than half of the GCS [Glasgow Coma Score] 15, CT-negative TBI cohort in our study were not back to their preinjury baseline at 6 months was surprising and impacts the millions of Americans who suffer from concussions annually,” said lead author Debbie Madhok, MD, with department of emergency medicine, University of California, San Francisco.
“These results highlight the importance of improving care pathways for concussion, particularly from the emergency department,” Dr. Madhok said.
The findings were published online in JAMA Network Open.
The short- and long-term outcomes in the large group of patients who come into the ED with TBI, a GCS of 15, and without acute intracranial traumatic injury (defined as a negative head CT scan) remain poorly understood, the investigators noted. To investigate further, they evaluated outcomes at 2 weeks and 6 months in 991 of these patients (mean age, 38 years; 64% men) from the TRACK-TBI study.
Among the 751 (76%) participants followed up at 2 weeks after the injury, only 204 (27%) had functional recovery – with a Glasgow Outcome Scale-Extended (GOS-E) score of 8. The remaining 547 (73%) had incomplete recovery (GOS-E scores < 8).
Among the 659 patients (66%) followed up at 6 months after the injury, 287 (44%) had functional recovery and 372 (56%) had incomplete recovery.
Most patients who failed to recover completely reported they had not returned to their preinjury life (88%). They described trouble returning to social activities outside the home and disruptions in family relationships and friendships.
The researchers noted that the study population had a high rate of preinjury psychiatric comorbidities, and these patients were more likely to have incomplete recovery than those without psychiatric comorbidities. This aligns with results from previous studies, they added.
The investigators also noted that patients with mild TBI without acute intracranial trauma are typically managed by ED personnel.
“These findings highlight the importance of ED clinicians being aware of the risk of incomplete recovery for patients with a mild TBI (that is, GCS score of 15 and negative head CT scan) and providing accurate education and timely referral information before ED discharge,” they wrote.
The study was funded by grants from the National Foundation of Emergency Medicine, the National Institute of Neurological Disorders and Stroke, and the U.S. Department of Defense Traumatic Brain Injury Endpoints Development Initiative. Dr. Madhok has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new data from the TRACK-TBI study shows.
“Seeing that more than half of the GCS [Glasgow Coma Score] 15, CT-negative TBI cohort in our study were not back to their preinjury baseline at 6 months was surprising and impacts the millions of Americans who suffer from concussions annually,” said lead author Debbie Madhok, MD, with department of emergency medicine, University of California, San Francisco.
“These results highlight the importance of improving care pathways for concussion, particularly from the emergency department,” Dr. Madhok said.
The findings were published online in JAMA Network Open.
The short- and long-term outcomes in the large group of patients who come into the ED with TBI, a GCS of 15, and without acute intracranial traumatic injury (defined as a negative head CT scan) remain poorly understood, the investigators noted. To investigate further, they evaluated outcomes at 2 weeks and 6 months in 991 of these patients (mean age, 38 years; 64% men) from the TRACK-TBI study.
Among the 751 (76%) participants followed up at 2 weeks after the injury, only 204 (27%) had functional recovery – with a Glasgow Outcome Scale-Extended (GOS-E) score of 8. The remaining 547 (73%) had incomplete recovery (GOS-E scores < 8).
Among the 659 patients (66%) followed up at 6 months after the injury, 287 (44%) had functional recovery and 372 (56%) had incomplete recovery.
Most patients who failed to recover completely reported they had not returned to their preinjury life (88%). They described trouble returning to social activities outside the home and disruptions in family relationships and friendships.
The researchers noted that the study population had a high rate of preinjury psychiatric comorbidities, and these patients were more likely to have incomplete recovery than those without psychiatric comorbidities. This aligns with results from previous studies, they added.
The investigators also noted that patients with mild TBI without acute intracranial trauma are typically managed by ED personnel.
“These findings highlight the importance of ED clinicians being aware of the risk of incomplete recovery for patients with a mild TBI (that is, GCS score of 15 and negative head CT scan) and providing accurate education and timely referral information before ED discharge,” they wrote.
The study was funded by grants from the National Foundation of Emergency Medicine, the National Institute of Neurological Disorders and Stroke, and the U.S. Department of Defense Traumatic Brain Injury Endpoints Development Initiative. Dr. Madhok has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Federal Health Care Data Trends 2022
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
Federal Health Care Data Trends (click to view the digital edition) is a special supplement to Federal Practitioner highlighting the latest research and study outcomes related to the health of veteran and active-duty populations.
In this issue:
- Vaccinations
- Mental Health and Related Disorders
- LGBTQ+ Veterans
- Military Sexual Trauma
- Sleep Disorders
- Respiratory Illnesses
- HIV Care in the VA
- Rheumatologic Diseases
- The Cancer-Obesity Connection
- Skin Health for Active-Duty Personnel
- Contraception
- Chronic Kidney Disease
- Cardiovascular Diseases
- Neurologic Disorders
- Hearing, Vision, and Balance
Federal Practitioner would like to thank the following experts for their review of content and helpful guidance in developing this issue:
Kelvin N.V. Bush, MD, FACC, CCDS; Sonya Borrero, MD, MS; Kenneth L. Cameron, PhD, MPH, ATC, FNATA; Jason DeViva, PhD; Ellen Lockard Edens, MD; Leonard E. Egede, MD, MS; Amy Justice, MD, PhD; Stephanie Knudson, MD; Willis H. Lyford, MD; Sarah O. Meadows, PhD; Tamara Schult, PhD, MPH; Eric L. Singman, MD, PhD; Art Wallace, MD, PhD; Elizabeth Waterhouse, MD, FAAN
CBT may improve comorbid posttraumatic headache, PTSD
Results from a randomized clinical trial of almost 200 military veterans showed that, compared with usual care, CBT for headache led to significant improvement in both headache disability and PTSD symptoms. Cognitive-processing therapy (CPT) also led to significant improvement in PTSD symptoms, but it did not improve headache disability.
Lead author Donald McGeary, PhD, department of rehabilitation medicine, the University of Texas Health Science Center,San Antonio, noted the improvements shown in headache disability after CBT were likely caused by its building of patients’ confidence that they could control or manage their headaches themselves.
That sense of control was key to helping patients “get their lives back. If you can improve a person’s belief that they can control their headache, they function better,” Dr. McGeary said in a news release.
The findings were published online in JAMA Neurology.
Signature wounds
Both mild traumatic brain injury (TBI) and PTSD are signature wounds of post-9/11 military conflicts. The two conditions commonly occur together and can harm quality of life and functioning, the investigators noted. Following mild TBI, many veterans experience persistent posttraumatic headache, which often co-occurs with PTSD.
To gauge the impact of CBTs for this patient population, researchers recruited 193 post-9/11 combat veterans (mean age, 39.7 years) with clinically significant PTSD symptoms and posttraumatic headache that had persisted more than 3 months after TBI. Of these, 167 were men.
All participants were receiving care at the Polytrauma Rehabilitation Center of the South Texas Veterans Health Care System in Houston.
They were randomly allocated to undergo 8 sessions of manualized CBT for headache, 12 sessions of manualized CPT for PTSD, or usual headache treatment.
CBT for headache uses CBT concepts to reduce headache disability and improve mood – and includes key components, such as relaxation, setting goals for activities patients want to resume, and planning for those situations.
CPT is a leading psychotherapy for PTSD. It teaches patients how to evaluate and change upsetting and maladaptive thoughts related to their trauma. The idea is that, by changing thoughts, patients can change the way they feel.
Treatment as usual was consistent with multidisciplinary treatment in a large Veterans Affairs multiple-trauma center and could include pharmacotherapies, physical and occupational therapies, pain medications, acupuncture, and massage.
The coprimary outcomes were headache-related disability on the six-item Headache Impact Test (HIT-6) and PTSD symptom severity on the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (PCL-5), assessed from end of treatment to 6 months post treatment.
At baseline, all participants reported severe headache-related disability (mean HIT-6 score, 65.8 points) and severe PTSD symptoms (mean PCL-5 score, 48.4 points).
Significant improvement
Compared with usual care, CBT for headache led to significant improvement in headache disability (posttreatment mean change in HIT-6 score, –3.4 points; P < .01) and PTSD symptoms (posttreatment change in PCL-5, –6.5 points; P = .04).
CPT also led to significant improvement in PTSD symptoms (8.9 points lower on the PCL-5 after treatment; P = .01), but it had only a modest effect on headache disability (1.4 points lower after treatment; P = .21).
“This was a surprise,” Dr. McGeary said. “If theories about PTSD driving posttraumatic headache are correct, you’d expect CPT to help both PTSD and headache. Our findings call that into question.”
Despite improvements in headache disability, CBT for headache did not significantly reduce headache frequency or intensity.
The researchers are now hoping to replicate their findings in a larger trial at multiple military and VA sites around the United States.
“We need more women, more racial and ethnic diversity, veterans as well as active military of different branches with varying comorbidities in different geographic regions attached to different hospitals and medical systems, because we’re comparing to usual care,” Dr. McGeary said.
A step forward
Commenting on the study, retired Col. Elspeth Cameron Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, said she was “pleased” to see that this study was conducted and that she was pleased with the results.
“It’s been 20 years since 9/11, and wars are pretty much forgotten, but people are still suffering from the effects of traumatic brain injury and posttraumatic stress disorder. These are not conditions that go away quickly or lightly. They do take work,” said Dr. Ritchie, who was not involved with the research.
Finding therapies besides medication that are helpful is “good and is a step forward. The more alternatives we have, the better,” she concluded.
The study was supported in part by the Department of Defense and the Department of Veterans Affairs. Dr. McGeary and Dr. Ritchie have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a randomized clinical trial of almost 200 military veterans showed that, compared with usual care, CBT for headache led to significant improvement in both headache disability and PTSD symptoms. Cognitive-processing therapy (CPT) also led to significant improvement in PTSD symptoms, but it did not improve headache disability.
Lead author Donald McGeary, PhD, department of rehabilitation medicine, the University of Texas Health Science Center,San Antonio, noted the improvements shown in headache disability after CBT were likely caused by its building of patients’ confidence that they could control or manage their headaches themselves.
That sense of control was key to helping patients “get their lives back. If you can improve a person’s belief that they can control their headache, they function better,” Dr. McGeary said in a news release.
The findings were published online in JAMA Neurology.
Signature wounds
Both mild traumatic brain injury (TBI) and PTSD are signature wounds of post-9/11 military conflicts. The two conditions commonly occur together and can harm quality of life and functioning, the investigators noted. Following mild TBI, many veterans experience persistent posttraumatic headache, which often co-occurs with PTSD.
To gauge the impact of CBTs for this patient population, researchers recruited 193 post-9/11 combat veterans (mean age, 39.7 years) with clinically significant PTSD symptoms and posttraumatic headache that had persisted more than 3 months after TBI. Of these, 167 were men.
All participants were receiving care at the Polytrauma Rehabilitation Center of the South Texas Veterans Health Care System in Houston.
They were randomly allocated to undergo 8 sessions of manualized CBT for headache, 12 sessions of manualized CPT for PTSD, or usual headache treatment.
CBT for headache uses CBT concepts to reduce headache disability and improve mood – and includes key components, such as relaxation, setting goals for activities patients want to resume, and planning for those situations.
CPT is a leading psychotherapy for PTSD. It teaches patients how to evaluate and change upsetting and maladaptive thoughts related to their trauma. The idea is that, by changing thoughts, patients can change the way they feel.
Treatment as usual was consistent with multidisciplinary treatment in a large Veterans Affairs multiple-trauma center and could include pharmacotherapies, physical and occupational therapies, pain medications, acupuncture, and massage.
The coprimary outcomes were headache-related disability on the six-item Headache Impact Test (HIT-6) and PTSD symptom severity on the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (PCL-5), assessed from end of treatment to 6 months post treatment.
At baseline, all participants reported severe headache-related disability (mean HIT-6 score, 65.8 points) and severe PTSD symptoms (mean PCL-5 score, 48.4 points).
Significant improvement
Compared with usual care, CBT for headache led to significant improvement in headache disability (posttreatment mean change in HIT-6 score, –3.4 points; P < .01) and PTSD symptoms (posttreatment change in PCL-5, –6.5 points; P = .04).
CPT also led to significant improvement in PTSD symptoms (8.9 points lower on the PCL-5 after treatment; P = .01), but it had only a modest effect on headache disability (1.4 points lower after treatment; P = .21).
“This was a surprise,” Dr. McGeary said. “If theories about PTSD driving posttraumatic headache are correct, you’d expect CPT to help both PTSD and headache. Our findings call that into question.”
Despite improvements in headache disability, CBT for headache did not significantly reduce headache frequency or intensity.
The researchers are now hoping to replicate their findings in a larger trial at multiple military and VA sites around the United States.
“We need more women, more racial and ethnic diversity, veterans as well as active military of different branches with varying comorbidities in different geographic regions attached to different hospitals and medical systems, because we’re comparing to usual care,” Dr. McGeary said.
A step forward
Commenting on the study, retired Col. Elspeth Cameron Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, said she was “pleased” to see that this study was conducted and that she was pleased with the results.
“It’s been 20 years since 9/11, and wars are pretty much forgotten, but people are still suffering from the effects of traumatic brain injury and posttraumatic stress disorder. These are not conditions that go away quickly or lightly. They do take work,” said Dr. Ritchie, who was not involved with the research.
Finding therapies besides medication that are helpful is “good and is a step forward. The more alternatives we have, the better,” she concluded.
The study was supported in part by the Department of Defense and the Department of Veterans Affairs. Dr. McGeary and Dr. Ritchie have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a randomized clinical trial of almost 200 military veterans showed that, compared with usual care, CBT for headache led to significant improvement in both headache disability and PTSD symptoms. Cognitive-processing therapy (CPT) also led to significant improvement in PTSD symptoms, but it did not improve headache disability.
Lead author Donald McGeary, PhD, department of rehabilitation medicine, the University of Texas Health Science Center,San Antonio, noted the improvements shown in headache disability after CBT were likely caused by its building of patients’ confidence that they could control or manage their headaches themselves.
That sense of control was key to helping patients “get their lives back. If you can improve a person’s belief that they can control their headache, they function better,” Dr. McGeary said in a news release.
The findings were published online in JAMA Neurology.
Signature wounds
Both mild traumatic brain injury (TBI) and PTSD are signature wounds of post-9/11 military conflicts. The two conditions commonly occur together and can harm quality of life and functioning, the investigators noted. Following mild TBI, many veterans experience persistent posttraumatic headache, which often co-occurs with PTSD.
To gauge the impact of CBTs for this patient population, researchers recruited 193 post-9/11 combat veterans (mean age, 39.7 years) with clinically significant PTSD symptoms and posttraumatic headache that had persisted more than 3 months after TBI. Of these, 167 were men.
All participants were receiving care at the Polytrauma Rehabilitation Center of the South Texas Veterans Health Care System in Houston.
They were randomly allocated to undergo 8 sessions of manualized CBT for headache, 12 sessions of manualized CPT for PTSD, or usual headache treatment.
CBT for headache uses CBT concepts to reduce headache disability and improve mood – and includes key components, such as relaxation, setting goals for activities patients want to resume, and planning for those situations.
CPT is a leading psychotherapy for PTSD. It teaches patients how to evaluate and change upsetting and maladaptive thoughts related to their trauma. The idea is that, by changing thoughts, patients can change the way they feel.
Treatment as usual was consistent with multidisciplinary treatment in a large Veterans Affairs multiple-trauma center and could include pharmacotherapies, physical and occupational therapies, pain medications, acupuncture, and massage.
The coprimary outcomes were headache-related disability on the six-item Headache Impact Test (HIT-6) and PTSD symptom severity on the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (PCL-5), assessed from end of treatment to 6 months post treatment.
At baseline, all participants reported severe headache-related disability (mean HIT-6 score, 65.8 points) and severe PTSD symptoms (mean PCL-5 score, 48.4 points).
Significant improvement
Compared with usual care, CBT for headache led to significant improvement in headache disability (posttreatment mean change in HIT-6 score, –3.4 points; P < .01) and PTSD symptoms (posttreatment change in PCL-5, –6.5 points; P = .04).
CPT also led to significant improvement in PTSD symptoms (8.9 points lower on the PCL-5 after treatment; P = .01), but it had only a modest effect on headache disability (1.4 points lower after treatment; P = .21).
“This was a surprise,” Dr. McGeary said. “If theories about PTSD driving posttraumatic headache are correct, you’d expect CPT to help both PTSD and headache. Our findings call that into question.”
Despite improvements in headache disability, CBT for headache did not significantly reduce headache frequency or intensity.
The researchers are now hoping to replicate their findings in a larger trial at multiple military and VA sites around the United States.
“We need more women, more racial and ethnic diversity, veterans as well as active military of different branches with varying comorbidities in different geographic regions attached to different hospitals and medical systems, because we’re comparing to usual care,” Dr. McGeary said.
A step forward
Commenting on the study, retired Col. Elspeth Cameron Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, said she was “pleased” to see that this study was conducted and that she was pleased with the results.
“It’s been 20 years since 9/11, and wars are pretty much forgotten, but people are still suffering from the effects of traumatic brain injury and posttraumatic stress disorder. These are not conditions that go away quickly or lightly. They do take work,” said Dr. Ritchie, who was not involved with the research.
Finding therapies besides medication that are helpful is “good and is a step forward. The more alternatives we have, the better,” she concluded.
The study was supported in part by the Department of Defense and the Department of Veterans Affairs. Dr. McGeary and Dr. Ritchie have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Benign Pneumatosis Intestinalis: A Case Report and Review of the Literature
Pneumatosis intestinalis (PI) is the finding of gas within the walls of the intestine on imaging. It is most commonly detected via radiograph or computed tomography (CT). The diseases leading to the accumulation of gas within the submucosal space of the gastrointestinal (GI) tract are heterogenous, and the finding of PI itself has a wide range of clinical implications from impending clinical deterioration to an incidental finding of minimal consequence.
We present the case of a veteran who had sustained a remote anoxic brain injury resulting in chronic dependence on a gastrostomy tube for enteral nutrition, found incidentally to have PI without signs of intra-abdominal catastrophe. An exclusion of other, more lifethreatening causes of PI led to a diagnosis of benign PI secondary to the presence of his gastrostomy tube. This case highlights the importance of interpreting the finding of PI in the clinical context of the specific patient and how conservative management may be appropriate in some cases.
Case Presentation
A 61-year-old male patient was admitted for fever. The patient had a remote history of cardiac arrest complicated by anoxic brain injury requiring tracheostomy, gastrostomy tube, and a suprapubic catheter with recurrent catheter-associated urinary tract infections (CAUTI), secondary seizure disorder, atrial fibrillation off anticoagulation due to recurrent GI bleeding, and treatment naive chronic hepatitis C virus. His ability to provide a clinical history was limited by his nonverbal status. He had no prior surgical history but had presented a month earlier for a high-grade small bowel obstruction (SBO) with pneumobilia that was managed conservatively as the surgical team deemed him a poor candidate for surgical intervention with his extensive comorbidities. A bioethics consultation at the time supported minimizing potential surgical risk in favor of conservative medical management; this was discussed with the patient’s surrogate decision maker, who also wished to avoid surgery. The SBO resolved with conservative management. He had been residing in a nursing home and doing well until 24 hours prior to admission when he developed fevers.
Vital signs on admission showed a temperature of 100.8 °F, heart rate 100 beats per minute, blood pressure 116/85, respiratory rate 22 per minute, and oxygen saturation of 100% on 6 L of oxygen via tracheostomy collar. His initial examination was notable for clear lung sounds, a nondistended nonrigid abdomen with an indwelling percutaneous gastrostomy tube, and absence of areas of skin breakdown or erythema. Notable laboratory studies showed a leukocytosis and urinalysis suggestive of CAUTI (Table). His urinary catheter was exchanged, he was fluid resuscitated and started on empiric vancomycin and piperacillin-tazobactam for management of sepsis due to CAUTI.
For the first 3 days of his hospitalization, he demonstrated clinical improvement on vancomycin and piperacillin-tazobactam while awaiting results from his urine bacterial culture. On hospital day 3, hedeveloped recurrent nonbloody, nonbilious emesis despite no change in the rate or formulation of his enteral nutrition. He also had 3 watery brown bowel movements. His vital signs remained within normal limits. His abdominal examination at this point showed mild distention and was hypertympanic to percussion, but there was no rigidity or involuntary guarding. On hospital day 4, he continued to have emesis with an unchanged abdominal examination. The differential diagnosis included recurrence of prior SBO, ileus, intestinal ischemia, enteral nutrition intolerance, Clostridioides difficile (C difficile) colitis, and GI dysmotility because of his anoxic brain injury.
Testing for C difficile was negative. An abdominal radiograph was obtained and revealed no bowel obstruction but, alarmingly, showed extensive intramural bowel gas, suggestive of PI (Figure 1). His leukocyte count, serum bicarbonate, and serum lactate levels remained within normal limits. A CT with contrast of the abdomen and pelvis demonstrated no vascular obstruction but confirmed the presence of diffuse intramural gas in his stomach and proximal small bowel, as well as the presence of mesenteric and portal venous gas (Figures 2 and 3). Although his abdominal examination had not changed and did not suggest peritonitis, general surgery was consulted to discuss the need for surgical intervention. Given his overall clinical stability and high surgical risk due to his many comorbidities, surgery recommended a conservative approach.
Through the following hospital days, his enteral nutrition was held and serial abdominal examinations were performed without change. Serial laboratory studies, including serum lactate and leukocyte count, remained reassuringly within normal limits. His urine culture eventually revealed multidrugresistant Pseudomonas aeruginosa. Antimicrobial therapy was narrowed to piperacillintazobactam for a complete course. Enteral nutrition was gradually reintroduced at a low rate, ultimately reaching goal rate with return of bowel function by hospital day 9. Despite extensive workup, the etiology of his transient enteral nutrition intolerance remained uncertain, though an adverse effect of antibiotic therapy was thought possible. Follow-up abdominal radiographs demonstrated interval improvement of PI. He was discharged back to his skilled nursing facility on hospital day 11 without incident.
Discussion
PI is an incompletely understood condition seen in multiple diseases. Patients may present with highly variable symptoms, often more attributable to the underlying disease causing the PI than the presence of PI, as patients may be entirely asymptomatic. When symptoms are attributed to PI, those most reported are abdominal pain, bloody stools, and diarrhea.1 It is often detected on abdominal plain films. Alternative methods of diagnosis include ultrasonography, barium enema, and endoscopy although the last method has been known to occasionally lead to bowel perforation.2-6 The most sensitive method of detection is CT, which also provides additional information about abdominal pathology and may identify the underlying process responsible for the PI.7
While not fully understood, much information about PI and its pathogenesis is known. Understanding the mechanisms of PI is vital to direct the clinician’s evaluation of the patient for reversible conditions that may cause PI. Early descriptions of PI in the literature documented an association with pyloric stenosis, leading to the theory that gas from the intestinal lumen is driven into the submucosal space during episodes of forceful vomiting with increased intraluminal pressure.8 As PI was subsequently described in multiple other disease states not typically associated with increased intraluminal pressure such as inflammatory bowel disease, GI malignancy, cryptosporidiosis and CMV infection, additional theories about the pathogenesis of PI have arisen.9-24 There is now experimental data to support multiple mechanisms of intramural gas accumulation. It has become accepted that PI represents a common pathway shared across various pathologic states and results from multifactorial mechanisms of gas entry into the intestinal wall.25-29
Factors leading to the development of PI include bacterial production of gas, intraluminal GI gas compositions, increased intraluminal pressure, pulmonary gas tracking through vessels communicating with the thorax, and mucosal disruption. PI has been linked to bacterial infections of the GI tract in humans including C difficile, Klebsiella, and Whipple disease.14-18 In animal models, C difficile within the walls of rat intestine results in the appearance of pneumocysts, or discrete collections of submucosal gas, which are the hallmark feature of PI.30 It is thought that direct invasion of bacteria into intramural spaces can cause PI in humans, although bacteria have yet to be directly isolated from the pneumocysts. Translocation of luminal gas into pneumocysts found in PI is theorized to be driven by differences in partial pressures.31 The concentration of hydrogen within the intestinal lumen is high due to bacterial production. Hydrogen, diffusing along its partial pressure gradient between the lumen and blood, accumulates within the intestinal wall and causes the formation of pneumocysts. This phenomenon has been hypothesized to explain the tendency for pneumocysts to form around the mesenteric vasculature.
Gas from the lumen can also be forced into the intestinal wall during an abrupt increase in intra-abdominal pressure, such as that seen with forceful vomiting. The final possible origin of the gas is the lungs, as PI has been associated with lung disease. It was previously thought that gas from ruptured alveoli tracks along mediastinal vessels, below the diaphragm, and into the mesentery.30 Newer theories argue that increased intra-abdominal pressure, typical of patients with obstructive lung disease and frequent coughing, is the driver of PI by the mechanism previously described.32-34 Additionally, mucosal disruption leads to increased permeability and allows accumulation of gas within the intestinal walls. Mucosal abnormalities have been described in histopathologic studies of patients with PI and associated with conditions known to compromise mucosal integrity, such as immunodeficiencies, inflammatory bowel disease, and the receipt of cytotoxic chemotherapy.10,12,19-23
Our patient likely had mucosal disruption due to his gastrostomy tube as well as increased intraluminal pressure from recurrent vomiting, contributing to translocation of otherwise normal intraluminal gas. The presence of portal venous gas, as seen in this case, has historically portended a worse prognosis, with 37% mortality in one series.7,35,36 However, portal venous gas as well as pneumoperitoneum occur in benign etiologies of PI as well. It is thought that this occurs due to rupture of the submucosal pneumocysts through the wall opposite the intestinal lumen and thus does not result in a direct communication between the intestinal lumen and the peritoneal cavity.12
PI is not a diagnosis but a manifestation of an underlying disease. As such, the treatment of PI is targeted toward the underlying condition. Of note, the pattern and extent of PI seen on imaging has not been shown to correlate with the severity of the underlying pathologic process.35,37 Instead, assessment of the patient and their clinical trajectory should determine the appropriate treatment. The decision facing the clinician when PI is discovered is whether urgent surgery is indicated, as is the case in mesenteric ischemia, bowel necrosis, or intestinal perforation, conditions known to be associated with PI. Otherwise, there is no definitive treatment for PI. Bowel rest is almost universally pursued. There are reports of treating with supranormal levels of supplemental oxygen, maintaining arterial partial pressure of oxygen above 300 mm Hg, with a face mask and 8 L/min flow rate.38,39 The proposed mechanisms of benefit include establishing a favorable diffusion gradient for intramural gas to exit the pneumocysts as well as creating an inhospitable, aerobic environment for hydrogenproducing anaerobic enteric bacteria. A prudent approach for most cases of PI is conservative management with bowel rest and supplemental oxygen unless there is a definitive indication for urgent surgical intervention, such as peritonitis, abdominal sepsis, or perforation.40,41 Management recommendations suggest that up to 50% of cases can be successfully managed nonoperatively.42
Conclusions
PI is the radiographic finding of gas within the walls of the intestinal tract and has variable clinical significance. It can represent a benign incidental finding or a sequela of intraabdominal emergencies such as mesenteric ischemia or bowel necrosis. Because PI is seen in a variety of disorders, several proposed mechanisms are supported in the medical literature. These include bacterial production of gas, gas pressure gradients between the intestinal lumen and the blood, increased intraluminal pressure, pulmonary gas tracking from intrathoracic vessels, and mucosal disruption. The evaluation of a patient with PI must begin with an assessment for the need for urgent surgical intervention. Additional management measures include bowel rest, IV hydration, and supplemental oxygen administration. Because of its wide variety of etiologies of varying clinical urgency, placing the finding of PI in the context of the patient is paramount to selecting an appropriate management strategy.
1. Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg). 1979;26(5):419-422.
2. Lafortune M, Trinh BC, Burns PN, et al. Air in the portal vein: sonographic and Doppler manifestations. Radiology. 1991;180(3):667-670. doi:10.1148/radiology.180.3.1871276
3. Kriegshauser JS, Reading CC, King BF, Welch TJ. Combined systemic and portal venous gas: sonographic and CT detection in two cases. AJR Am J Roentgenol. 1990;154(6):1219-1221. doi:10.2214/ajr.154.6.2110731
4. Goske MJ, Goldblum JR, Applegate KE, Mitchell CS, Bardo D. The “circle sign”: a new sonographic sign of pneumatosis intestinalis - clinical, pathologic and experimental findings. Pediatr Radiol. 1999;29(7):530-535. doi:10.1007/s002470050638
5. Marshak RH, Lindner AE, Maklansky D. Pneumatosis cystoides coli. Gastrointest Radiol. 1977;2(2):85-89. doi:10.1007/BF02256475
6. Jensen R, Gutnik SH. Pneumatosis cystoides intestinalis: a complication of colonoscopic polypectomy. S D J Med. 1991;44(7):177-179.
7. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160-165. doi:10.1097/00000658-199008000-00008
8. Koss LG. Abdominal gas cysts (Pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
9. Jona JZ. Benign pneumatosis intestinalis coli after blunt trauma to the abdomen in a child. J Pediatr Surg. 2000;35(7):1109-1111. doi:10.1053/jpsu.2000.7837
10. Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111-118. doi:10.1007/s003840050031
11. Seto T, Koide N, Taniuchi N, Yamada T, Hamaguchi M, Goto S. Pneumatosis cystoides intestinalis complicating carcinoma of the small intestine. Am J Surg. 2001;182(3):287-288. doi:10.1016/S0002-9610(01)00710-3
12. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohn’s disease. Report of two cases. Dis Colon Rectum. 1985;28(12):951-956. doi:10.1007/BF02554315
13. Parra JA, Acinas O, Bueno J, Madrazo C, Fariñas C. An unusual form of pneumatosis intestinalis associated with appendicitis. Br J Radiol. 1998;71(843):326-328. doi:10.1259/bjr.71.843.9616245
14. Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci. 1998;43(11):2455-2458. doi:10.1023/a:1026682131847
15. Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol. 1999;94(9):2560-2561. doi:10.1111/j.1572-0241.1999.01397.x
16. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988;151(1):85-87. doi:10.2214/ajr.151.1.85
17. Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: A case report. Medicine (Baltimore). 2019;98(2):e14079. doi:10.1097/MD.0000000000014079
18. Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of whipple’s disease in a patient mistakenly on anti-tnf therapy. ACG Case Rep J. 2013;1(1):25- 28. Published 2013 Oct 8. doi:10.14309/crj.2013.11
19. Burton EM, Mercado-Deane MG, Patel K. Pneumatosis intestinalis in a child with AIDS and pseudomembranous colitis. Pediatr Radiol. 1994;24(8):609-610. doi:10.1007/BF02012750
20. Berk RN, Wall SD, McArdle CB, et al. Cryptosporidiosis of the stomach and small intestine in patients with AIDS. AJR Am J Roentgenol. 1984;143(3):549-554. doi:10.2214/ajr.143.3.549
21. Samson VE, Brown WR. Pneumatosis cystoides intestinalis in AIDS-associated cryptosporidiosis. More than an incidental finding? J Clin Gastroenterol. 1996;22(4):311-312.doi:10.1097/00004836-199606000-00015
22. Tjon A Tham RT, Vlasveld LT, Willemze R. Gastrointestinal complications of cytosine-arabinoside chemotherapy: findings on plain abdominal radiographs. AJR Am J Roentgenol. 1990;154(1):95-98. doi:10.2214/ajr.154.1.2104733
23. Hashimoto S, Saitoh H, Wada K, et al. Pneumatosis cystoides intestinalis after chemotherapy for hematological malignancies: report of 4 cases. Intern Med. 1995;34(3):212-215. doi:10.2169/internalmedicine.34.212
24. Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646-650. doi:10.1111/j.1572-0241.1998.183_b.x
25. Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20(11):1008-1011. doi:10.1136/gut.20.11.1008
26. Hughes DT, Gordon KC, Swann JC, Bolt GL. Pneumatosis cystoides intestinalis. Gut. 1966;7(5):553-557. doi:10.1136/gut.7.5.553
27. Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839-845. doi:10.1136/gut.25.8.839
28. van der Linden W, Marsell R. Pneumatosis cystoides coli associated with high H2 excretion. Treatment with an elemental diet. Scand J Gastroenterol. 1979;14(2):173-174. doi:10.3109/00365527909179864
29. Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104(2):392-397. doi:10.1016/0016-5085(93)90406-3
30. Keyting WS, Mccarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733-741. doi:10.1148/76.5.733
31. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345(8959):1220-1222. doi:10.1016/S0140-6736(95)91996-1
32. Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61(6):423-426.
33. Micklefield GH, Kuntz HD, May B. Pneumatosis cystoides intestinalis: case reports and review of the literature. Mater Med Pol. 1990;22(2):70-72.
34. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974;109(1):89- 94. doi:10.1001/archsurg.1974.01360010067017
35. Fenton LZ, Buonomo C. Benign pneumatosis in children. Pediatr Radiol. 2000;30(11):786-793. doi:10.1007/s002470000303
36. Tobias R, Coleman S, Helman CA. Pneumatosis coli simulating hepatomegaly. Am J Gastroenterol. 1985;80(2):146-149.
37. Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel w a l l . Radiographics. 1992;12(6):1069-1078. doi:10.1148/radiographics.12.6.1439012
38. Masterson JS, Fratkin LB, Osler TR, Trapp WG. Treatment of pneumatosis cystoides intestinalis with hyperbaric oxygen. Ann Surg. 1978;187(3):245-247. doi:10.1097/00000658-197803000-00005
39. Höflin F, Linden W van der. Pneumatosis cystoides intestinalis treated by oxygen breathing. Scandinavian J Gastroenterol . 1974;9(5) :427-430. doi:10.1080/00365521.1974.12096852
40. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75. doi:10.1001/archsurg.138.1.68
41. Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176. Published 2019 Nov 6. doi:10.1186/s12876-019-1087-9
42. Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-682. doi:10.1016/j.amjsurg.2008.01.011
Pneumatosis intestinalis (PI) is the finding of gas within the walls of the intestine on imaging. It is most commonly detected via radiograph or computed tomography (CT). The diseases leading to the accumulation of gas within the submucosal space of the gastrointestinal (GI) tract are heterogenous, and the finding of PI itself has a wide range of clinical implications from impending clinical deterioration to an incidental finding of minimal consequence.
We present the case of a veteran who had sustained a remote anoxic brain injury resulting in chronic dependence on a gastrostomy tube for enteral nutrition, found incidentally to have PI without signs of intra-abdominal catastrophe. An exclusion of other, more lifethreatening causes of PI led to a diagnosis of benign PI secondary to the presence of his gastrostomy tube. This case highlights the importance of interpreting the finding of PI in the clinical context of the specific patient and how conservative management may be appropriate in some cases.
Case Presentation
A 61-year-old male patient was admitted for fever. The patient had a remote history of cardiac arrest complicated by anoxic brain injury requiring tracheostomy, gastrostomy tube, and a suprapubic catheter with recurrent catheter-associated urinary tract infections (CAUTI), secondary seizure disorder, atrial fibrillation off anticoagulation due to recurrent GI bleeding, and treatment naive chronic hepatitis C virus. His ability to provide a clinical history was limited by his nonverbal status. He had no prior surgical history but had presented a month earlier for a high-grade small bowel obstruction (SBO) with pneumobilia that was managed conservatively as the surgical team deemed him a poor candidate for surgical intervention with his extensive comorbidities. A bioethics consultation at the time supported minimizing potential surgical risk in favor of conservative medical management; this was discussed with the patient’s surrogate decision maker, who also wished to avoid surgery. The SBO resolved with conservative management. He had been residing in a nursing home and doing well until 24 hours prior to admission when he developed fevers.
Vital signs on admission showed a temperature of 100.8 °F, heart rate 100 beats per minute, blood pressure 116/85, respiratory rate 22 per minute, and oxygen saturation of 100% on 6 L of oxygen via tracheostomy collar. His initial examination was notable for clear lung sounds, a nondistended nonrigid abdomen with an indwelling percutaneous gastrostomy tube, and absence of areas of skin breakdown or erythema. Notable laboratory studies showed a leukocytosis and urinalysis suggestive of CAUTI (Table). His urinary catheter was exchanged, he was fluid resuscitated and started on empiric vancomycin and piperacillin-tazobactam for management of sepsis due to CAUTI.
For the first 3 days of his hospitalization, he demonstrated clinical improvement on vancomycin and piperacillin-tazobactam while awaiting results from his urine bacterial culture. On hospital day 3, hedeveloped recurrent nonbloody, nonbilious emesis despite no change in the rate or formulation of his enteral nutrition. He also had 3 watery brown bowel movements. His vital signs remained within normal limits. His abdominal examination at this point showed mild distention and was hypertympanic to percussion, but there was no rigidity or involuntary guarding. On hospital day 4, he continued to have emesis with an unchanged abdominal examination. The differential diagnosis included recurrence of prior SBO, ileus, intestinal ischemia, enteral nutrition intolerance, Clostridioides difficile (C difficile) colitis, and GI dysmotility because of his anoxic brain injury.
Testing for C difficile was negative. An abdominal radiograph was obtained and revealed no bowel obstruction but, alarmingly, showed extensive intramural bowel gas, suggestive of PI (Figure 1). His leukocyte count, serum bicarbonate, and serum lactate levels remained within normal limits. A CT with contrast of the abdomen and pelvis demonstrated no vascular obstruction but confirmed the presence of diffuse intramural gas in his stomach and proximal small bowel, as well as the presence of mesenteric and portal venous gas (Figures 2 and 3). Although his abdominal examination had not changed and did not suggest peritonitis, general surgery was consulted to discuss the need for surgical intervention. Given his overall clinical stability and high surgical risk due to his many comorbidities, surgery recommended a conservative approach.
Through the following hospital days, his enteral nutrition was held and serial abdominal examinations were performed without change. Serial laboratory studies, including serum lactate and leukocyte count, remained reassuringly within normal limits. His urine culture eventually revealed multidrugresistant Pseudomonas aeruginosa. Antimicrobial therapy was narrowed to piperacillintazobactam for a complete course. Enteral nutrition was gradually reintroduced at a low rate, ultimately reaching goal rate with return of bowel function by hospital day 9. Despite extensive workup, the etiology of his transient enteral nutrition intolerance remained uncertain, though an adverse effect of antibiotic therapy was thought possible. Follow-up abdominal radiographs demonstrated interval improvement of PI. He was discharged back to his skilled nursing facility on hospital day 11 without incident.
Discussion
PI is an incompletely understood condition seen in multiple diseases. Patients may present with highly variable symptoms, often more attributable to the underlying disease causing the PI than the presence of PI, as patients may be entirely asymptomatic. When symptoms are attributed to PI, those most reported are abdominal pain, bloody stools, and diarrhea.1 It is often detected on abdominal plain films. Alternative methods of diagnosis include ultrasonography, barium enema, and endoscopy although the last method has been known to occasionally lead to bowel perforation.2-6 The most sensitive method of detection is CT, which also provides additional information about abdominal pathology and may identify the underlying process responsible for the PI.7
While not fully understood, much information about PI and its pathogenesis is known. Understanding the mechanisms of PI is vital to direct the clinician’s evaluation of the patient for reversible conditions that may cause PI. Early descriptions of PI in the literature documented an association with pyloric stenosis, leading to the theory that gas from the intestinal lumen is driven into the submucosal space during episodes of forceful vomiting with increased intraluminal pressure.8 As PI was subsequently described in multiple other disease states not typically associated with increased intraluminal pressure such as inflammatory bowel disease, GI malignancy, cryptosporidiosis and CMV infection, additional theories about the pathogenesis of PI have arisen.9-24 There is now experimental data to support multiple mechanisms of intramural gas accumulation. It has become accepted that PI represents a common pathway shared across various pathologic states and results from multifactorial mechanisms of gas entry into the intestinal wall.25-29
Factors leading to the development of PI include bacterial production of gas, intraluminal GI gas compositions, increased intraluminal pressure, pulmonary gas tracking through vessels communicating with the thorax, and mucosal disruption. PI has been linked to bacterial infections of the GI tract in humans including C difficile, Klebsiella, and Whipple disease.14-18 In animal models, C difficile within the walls of rat intestine results in the appearance of pneumocysts, or discrete collections of submucosal gas, which are the hallmark feature of PI.30 It is thought that direct invasion of bacteria into intramural spaces can cause PI in humans, although bacteria have yet to be directly isolated from the pneumocysts. Translocation of luminal gas into pneumocysts found in PI is theorized to be driven by differences in partial pressures.31 The concentration of hydrogen within the intestinal lumen is high due to bacterial production. Hydrogen, diffusing along its partial pressure gradient between the lumen and blood, accumulates within the intestinal wall and causes the formation of pneumocysts. This phenomenon has been hypothesized to explain the tendency for pneumocysts to form around the mesenteric vasculature.
Gas from the lumen can also be forced into the intestinal wall during an abrupt increase in intra-abdominal pressure, such as that seen with forceful vomiting. The final possible origin of the gas is the lungs, as PI has been associated with lung disease. It was previously thought that gas from ruptured alveoli tracks along mediastinal vessels, below the diaphragm, and into the mesentery.30 Newer theories argue that increased intra-abdominal pressure, typical of patients with obstructive lung disease and frequent coughing, is the driver of PI by the mechanism previously described.32-34 Additionally, mucosal disruption leads to increased permeability and allows accumulation of gas within the intestinal walls. Mucosal abnormalities have been described in histopathologic studies of patients with PI and associated with conditions known to compromise mucosal integrity, such as immunodeficiencies, inflammatory bowel disease, and the receipt of cytotoxic chemotherapy.10,12,19-23
Our patient likely had mucosal disruption due to his gastrostomy tube as well as increased intraluminal pressure from recurrent vomiting, contributing to translocation of otherwise normal intraluminal gas. The presence of portal venous gas, as seen in this case, has historically portended a worse prognosis, with 37% mortality in one series.7,35,36 However, portal venous gas as well as pneumoperitoneum occur in benign etiologies of PI as well. It is thought that this occurs due to rupture of the submucosal pneumocysts through the wall opposite the intestinal lumen and thus does not result in a direct communication between the intestinal lumen and the peritoneal cavity.12
PI is not a diagnosis but a manifestation of an underlying disease. As such, the treatment of PI is targeted toward the underlying condition. Of note, the pattern and extent of PI seen on imaging has not been shown to correlate with the severity of the underlying pathologic process.35,37 Instead, assessment of the patient and their clinical trajectory should determine the appropriate treatment. The decision facing the clinician when PI is discovered is whether urgent surgery is indicated, as is the case in mesenteric ischemia, bowel necrosis, or intestinal perforation, conditions known to be associated with PI. Otherwise, there is no definitive treatment for PI. Bowel rest is almost universally pursued. There are reports of treating with supranormal levels of supplemental oxygen, maintaining arterial partial pressure of oxygen above 300 mm Hg, with a face mask and 8 L/min flow rate.38,39 The proposed mechanisms of benefit include establishing a favorable diffusion gradient for intramural gas to exit the pneumocysts as well as creating an inhospitable, aerobic environment for hydrogenproducing anaerobic enteric bacteria. A prudent approach for most cases of PI is conservative management with bowel rest and supplemental oxygen unless there is a definitive indication for urgent surgical intervention, such as peritonitis, abdominal sepsis, or perforation.40,41 Management recommendations suggest that up to 50% of cases can be successfully managed nonoperatively.42
Conclusions
PI is the radiographic finding of gas within the walls of the intestinal tract and has variable clinical significance. It can represent a benign incidental finding or a sequela of intraabdominal emergencies such as mesenteric ischemia or bowel necrosis. Because PI is seen in a variety of disorders, several proposed mechanisms are supported in the medical literature. These include bacterial production of gas, gas pressure gradients between the intestinal lumen and the blood, increased intraluminal pressure, pulmonary gas tracking from intrathoracic vessels, and mucosal disruption. The evaluation of a patient with PI must begin with an assessment for the need for urgent surgical intervention. Additional management measures include bowel rest, IV hydration, and supplemental oxygen administration. Because of its wide variety of etiologies of varying clinical urgency, placing the finding of PI in the context of the patient is paramount to selecting an appropriate management strategy.
Pneumatosis intestinalis (PI) is the finding of gas within the walls of the intestine on imaging. It is most commonly detected via radiograph or computed tomography (CT). The diseases leading to the accumulation of gas within the submucosal space of the gastrointestinal (GI) tract are heterogenous, and the finding of PI itself has a wide range of clinical implications from impending clinical deterioration to an incidental finding of minimal consequence.
We present the case of a veteran who had sustained a remote anoxic brain injury resulting in chronic dependence on a gastrostomy tube for enteral nutrition, found incidentally to have PI without signs of intra-abdominal catastrophe. An exclusion of other, more lifethreatening causes of PI led to a diagnosis of benign PI secondary to the presence of his gastrostomy tube. This case highlights the importance of interpreting the finding of PI in the clinical context of the specific patient and how conservative management may be appropriate in some cases.
Case Presentation
A 61-year-old male patient was admitted for fever. The patient had a remote history of cardiac arrest complicated by anoxic brain injury requiring tracheostomy, gastrostomy tube, and a suprapubic catheter with recurrent catheter-associated urinary tract infections (CAUTI), secondary seizure disorder, atrial fibrillation off anticoagulation due to recurrent GI bleeding, and treatment naive chronic hepatitis C virus. His ability to provide a clinical history was limited by his nonverbal status. He had no prior surgical history but had presented a month earlier for a high-grade small bowel obstruction (SBO) with pneumobilia that was managed conservatively as the surgical team deemed him a poor candidate for surgical intervention with his extensive comorbidities. A bioethics consultation at the time supported minimizing potential surgical risk in favor of conservative medical management; this was discussed with the patient’s surrogate decision maker, who also wished to avoid surgery. The SBO resolved with conservative management. He had been residing in a nursing home and doing well until 24 hours prior to admission when he developed fevers.
Vital signs on admission showed a temperature of 100.8 °F, heart rate 100 beats per minute, blood pressure 116/85, respiratory rate 22 per minute, and oxygen saturation of 100% on 6 L of oxygen via tracheostomy collar. His initial examination was notable for clear lung sounds, a nondistended nonrigid abdomen with an indwelling percutaneous gastrostomy tube, and absence of areas of skin breakdown or erythema. Notable laboratory studies showed a leukocytosis and urinalysis suggestive of CAUTI (Table). His urinary catheter was exchanged, he was fluid resuscitated and started on empiric vancomycin and piperacillin-tazobactam for management of sepsis due to CAUTI.
For the first 3 days of his hospitalization, he demonstrated clinical improvement on vancomycin and piperacillin-tazobactam while awaiting results from his urine bacterial culture. On hospital day 3, hedeveloped recurrent nonbloody, nonbilious emesis despite no change in the rate or formulation of his enteral nutrition. He also had 3 watery brown bowel movements. His vital signs remained within normal limits. His abdominal examination at this point showed mild distention and was hypertympanic to percussion, but there was no rigidity or involuntary guarding. On hospital day 4, he continued to have emesis with an unchanged abdominal examination. The differential diagnosis included recurrence of prior SBO, ileus, intestinal ischemia, enteral nutrition intolerance, Clostridioides difficile (C difficile) colitis, and GI dysmotility because of his anoxic brain injury.
Testing for C difficile was negative. An abdominal radiograph was obtained and revealed no bowel obstruction but, alarmingly, showed extensive intramural bowel gas, suggestive of PI (Figure 1). His leukocyte count, serum bicarbonate, and serum lactate levels remained within normal limits. A CT with contrast of the abdomen and pelvis demonstrated no vascular obstruction but confirmed the presence of diffuse intramural gas in his stomach and proximal small bowel, as well as the presence of mesenteric and portal venous gas (Figures 2 and 3). Although his abdominal examination had not changed and did not suggest peritonitis, general surgery was consulted to discuss the need for surgical intervention. Given his overall clinical stability and high surgical risk due to his many comorbidities, surgery recommended a conservative approach.
Through the following hospital days, his enteral nutrition was held and serial abdominal examinations were performed without change. Serial laboratory studies, including serum lactate and leukocyte count, remained reassuringly within normal limits. His urine culture eventually revealed multidrugresistant Pseudomonas aeruginosa. Antimicrobial therapy was narrowed to piperacillintazobactam for a complete course. Enteral nutrition was gradually reintroduced at a low rate, ultimately reaching goal rate with return of bowel function by hospital day 9. Despite extensive workup, the etiology of his transient enteral nutrition intolerance remained uncertain, though an adverse effect of antibiotic therapy was thought possible. Follow-up abdominal radiographs demonstrated interval improvement of PI. He was discharged back to his skilled nursing facility on hospital day 11 without incident.
Discussion
PI is an incompletely understood condition seen in multiple diseases. Patients may present with highly variable symptoms, often more attributable to the underlying disease causing the PI than the presence of PI, as patients may be entirely asymptomatic. When symptoms are attributed to PI, those most reported are abdominal pain, bloody stools, and diarrhea.1 It is often detected on abdominal plain films. Alternative methods of diagnosis include ultrasonography, barium enema, and endoscopy although the last method has been known to occasionally lead to bowel perforation.2-6 The most sensitive method of detection is CT, which also provides additional information about abdominal pathology and may identify the underlying process responsible for the PI.7
While not fully understood, much information about PI and its pathogenesis is known. Understanding the mechanisms of PI is vital to direct the clinician’s evaluation of the patient for reversible conditions that may cause PI. Early descriptions of PI in the literature documented an association with pyloric stenosis, leading to the theory that gas from the intestinal lumen is driven into the submucosal space during episodes of forceful vomiting with increased intraluminal pressure.8 As PI was subsequently described in multiple other disease states not typically associated with increased intraluminal pressure such as inflammatory bowel disease, GI malignancy, cryptosporidiosis and CMV infection, additional theories about the pathogenesis of PI have arisen.9-24 There is now experimental data to support multiple mechanisms of intramural gas accumulation. It has become accepted that PI represents a common pathway shared across various pathologic states and results from multifactorial mechanisms of gas entry into the intestinal wall.25-29
Factors leading to the development of PI include bacterial production of gas, intraluminal GI gas compositions, increased intraluminal pressure, pulmonary gas tracking through vessels communicating with the thorax, and mucosal disruption. PI has been linked to bacterial infections of the GI tract in humans including C difficile, Klebsiella, and Whipple disease.14-18 In animal models, C difficile within the walls of rat intestine results in the appearance of pneumocysts, or discrete collections of submucosal gas, which are the hallmark feature of PI.30 It is thought that direct invasion of bacteria into intramural spaces can cause PI in humans, although bacteria have yet to be directly isolated from the pneumocysts. Translocation of luminal gas into pneumocysts found in PI is theorized to be driven by differences in partial pressures.31 The concentration of hydrogen within the intestinal lumen is high due to bacterial production. Hydrogen, diffusing along its partial pressure gradient between the lumen and blood, accumulates within the intestinal wall and causes the formation of pneumocysts. This phenomenon has been hypothesized to explain the tendency for pneumocysts to form around the mesenteric vasculature.
Gas from the lumen can also be forced into the intestinal wall during an abrupt increase in intra-abdominal pressure, such as that seen with forceful vomiting. The final possible origin of the gas is the lungs, as PI has been associated with lung disease. It was previously thought that gas from ruptured alveoli tracks along mediastinal vessels, below the diaphragm, and into the mesentery.30 Newer theories argue that increased intra-abdominal pressure, typical of patients with obstructive lung disease and frequent coughing, is the driver of PI by the mechanism previously described.32-34 Additionally, mucosal disruption leads to increased permeability and allows accumulation of gas within the intestinal walls. Mucosal abnormalities have been described in histopathologic studies of patients with PI and associated with conditions known to compromise mucosal integrity, such as immunodeficiencies, inflammatory bowel disease, and the receipt of cytotoxic chemotherapy.10,12,19-23
Our patient likely had mucosal disruption due to his gastrostomy tube as well as increased intraluminal pressure from recurrent vomiting, contributing to translocation of otherwise normal intraluminal gas. The presence of portal venous gas, as seen in this case, has historically portended a worse prognosis, with 37% mortality in one series.7,35,36 However, portal venous gas as well as pneumoperitoneum occur in benign etiologies of PI as well. It is thought that this occurs due to rupture of the submucosal pneumocysts through the wall opposite the intestinal lumen and thus does not result in a direct communication between the intestinal lumen and the peritoneal cavity.12
PI is not a diagnosis but a manifestation of an underlying disease. As such, the treatment of PI is targeted toward the underlying condition. Of note, the pattern and extent of PI seen on imaging has not been shown to correlate with the severity of the underlying pathologic process.35,37 Instead, assessment of the patient and their clinical trajectory should determine the appropriate treatment. The decision facing the clinician when PI is discovered is whether urgent surgery is indicated, as is the case in mesenteric ischemia, bowel necrosis, or intestinal perforation, conditions known to be associated with PI. Otherwise, there is no definitive treatment for PI. Bowel rest is almost universally pursued. There are reports of treating with supranormal levels of supplemental oxygen, maintaining arterial partial pressure of oxygen above 300 mm Hg, with a face mask and 8 L/min flow rate.38,39 The proposed mechanisms of benefit include establishing a favorable diffusion gradient for intramural gas to exit the pneumocysts as well as creating an inhospitable, aerobic environment for hydrogenproducing anaerobic enteric bacteria. A prudent approach for most cases of PI is conservative management with bowel rest and supplemental oxygen unless there is a definitive indication for urgent surgical intervention, such as peritonitis, abdominal sepsis, or perforation.40,41 Management recommendations suggest that up to 50% of cases can be successfully managed nonoperatively.42
Conclusions
PI is the radiographic finding of gas within the walls of the intestinal tract and has variable clinical significance. It can represent a benign incidental finding or a sequela of intraabdominal emergencies such as mesenteric ischemia or bowel necrosis. Because PI is seen in a variety of disorders, several proposed mechanisms are supported in the medical literature. These include bacterial production of gas, gas pressure gradients between the intestinal lumen and the blood, increased intraluminal pressure, pulmonary gas tracking from intrathoracic vessels, and mucosal disruption. The evaluation of a patient with PI must begin with an assessment for the need for urgent surgical intervention. Additional management measures include bowel rest, IV hydration, and supplemental oxygen administration. Because of its wide variety of etiologies of varying clinical urgency, placing the finding of PI in the context of the patient is paramount to selecting an appropriate management strategy.
1. Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg). 1979;26(5):419-422.
2. Lafortune M, Trinh BC, Burns PN, et al. Air in the portal vein: sonographic and Doppler manifestations. Radiology. 1991;180(3):667-670. doi:10.1148/radiology.180.3.1871276
3. Kriegshauser JS, Reading CC, King BF, Welch TJ. Combined systemic and portal venous gas: sonographic and CT detection in two cases. AJR Am J Roentgenol. 1990;154(6):1219-1221. doi:10.2214/ajr.154.6.2110731
4. Goske MJ, Goldblum JR, Applegate KE, Mitchell CS, Bardo D. The “circle sign”: a new sonographic sign of pneumatosis intestinalis - clinical, pathologic and experimental findings. Pediatr Radiol. 1999;29(7):530-535. doi:10.1007/s002470050638
5. Marshak RH, Lindner AE, Maklansky D. Pneumatosis cystoides coli. Gastrointest Radiol. 1977;2(2):85-89. doi:10.1007/BF02256475
6. Jensen R, Gutnik SH. Pneumatosis cystoides intestinalis: a complication of colonoscopic polypectomy. S D J Med. 1991;44(7):177-179.
7. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160-165. doi:10.1097/00000658-199008000-00008
8. Koss LG. Abdominal gas cysts (Pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
9. Jona JZ. Benign pneumatosis intestinalis coli after blunt trauma to the abdomen in a child. J Pediatr Surg. 2000;35(7):1109-1111. doi:10.1053/jpsu.2000.7837
10. Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111-118. doi:10.1007/s003840050031
11. Seto T, Koide N, Taniuchi N, Yamada T, Hamaguchi M, Goto S. Pneumatosis cystoides intestinalis complicating carcinoma of the small intestine. Am J Surg. 2001;182(3):287-288. doi:10.1016/S0002-9610(01)00710-3
12. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohn’s disease. Report of two cases. Dis Colon Rectum. 1985;28(12):951-956. doi:10.1007/BF02554315
13. Parra JA, Acinas O, Bueno J, Madrazo C, Fariñas C. An unusual form of pneumatosis intestinalis associated with appendicitis. Br J Radiol. 1998;71(843):326-328. doi:10.1259/bjr.71.843.9616245
14. Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci. 1998;43(11):2455-2458. doi:10.1023/a:1026682131847
15. Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol. 1999;94(9):2560-2561. doi:10.1111/j.1572-0241.1999.01397.x
16. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988;151(1):85-87. doi:10.2214/ajr.151.1.85
17. Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: A case report. Medicine (Baltimore). 2019;98(2):e14079. doi:10.1097/MD.0000000000014079
18. Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of whipple’s disease in a patient mistakenly on anti-tnf therapy. ACG Case Rep J. 2013;1(1):25- 28. Published 2013 Oct 8. doi:10.14309/crj.2013.11
19. Burton EM, Mercado-Deane MG, Patel K. Pneumatosis intestinalis in a child with AIDS and pseudomembranous colitis. Pediatr Radiol. 1994;24(8):609-610. doi:10.1007/BF02012750
20. Berk RN, Wall SD, McArdle CB, et al. Cryptosporidiosis of the stomach and small intestine in patients with AIDS. AJR Am J Roentgenol. 1984;143(3):549-554. doi:10.2214/ajr.143.3.549
21. Samson VE, Brown WR. Pneumatosis cystoides intestinalis in AIDS-associated cryptosporidiosis. More than an incidental finding? J Clin Gastroenterol. 1996;22(4):311-312.doi:10.1097/00004836-199606000-00015
22. Tjon A Tham RT, Vlasveld LT, Willemze R. Gastrointestinal complications of cytosine-arabinoside chemotherapy: findings on plain abdominal radiographs. AJR Am J Roentgenol. 1990;154(1):95-98. doi:10.2214/ajr.154.1.2104733
23. Hashimoto S, Saitoh H, Wada K, et al. Pneumatosis cystoides intestinalis after chemotherapy for hematological malignancies: report of 4 cases. Intern Med. 1995;34(3):212-215. doi:10.2169/internalmedicine.34.212
24. Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646-650. doi:10.1111/j.1572-0241.1998.183_b.x
25. Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20(11):1008-1011. doi:10.1136/gut.20.11.1008
26. Hughes DT, Gordon KC, Swann JC, Bolt GL. Pneumatosis cystoides intestinalis. Gut. 1966;7(5):553-557. doi:10.1136/gut.7.5.553
27. Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839-845. doi:10.1136/gut.25.8.839
28. van der Linden W, Marsell R. Pneumatosis cystoides coli associated with high H2 excretion. Treatment with an elemental diet. Scand J Gastroenterol. 1979;14(2):173-174. doi:10.3109/00365527909179864
29. Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104(2):392-397. doi:10.1016/0016-5085(93)90406-3
30. Keyting WS, Mccarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733-741. doi:10.1148/76.5.733
31. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345(8959):1220-1222. doi:10.1016/S0140-6736(95)91996-1
32. Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61(6):423-426.
33. Micklefield GH, Kuntz HD, May B. Pneumatosis cystoides intestinalis: case reports and review of the literature. Mater Med Pol. 1990;22(2):70-72.
34. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974;109(1):89- 94. doi:10.1001/archsurg.1974.01360010067017
35. Fenton LZ, Buonomo C. Benign pneumatosis in children. Pediatr Radiol. 2000;30(11):786-793. doi:10.1007/s002470000303
36. Tobias R, Coleman S, Helman CA. Pneumatosis coli simulating hepatomegaly. Am J Gastroenterol. 1985;80(2):146-149.
37. Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel w a l l . Radiographics. 1992;12(6):1069-1078. doi:10.1148/radiographics.12.6.1439012
38. Masterson JS, Fratkin LB, Osler TR, Trapp WG. Treatment of pneumatosis cystoides intestinalis with hyperbaric oxygen. Ann Surg. 1978;187(3):245-247. doi:10.1097/00000658-197803000-00005
39. Höflin F, Linden W van der. Pneumatosis cystoides intestinalis treated by oxygen breathing. Scandinavian J Gastroenterol . 1974;9(5) :427-430. doi:10.1080/00365521.1974.12096852
40. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75. doi:10.1001/archsurg.138.1.68
41. Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176. Published 2019 Nov 6. doi:10.1186/s12876-019-1087-9
42. Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-682. doi:10.1016/j.amjsurg.2008.01.011
1. Jamart J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg). 1979;26(5):419-422.
2. Lafortune M, Trinh BC, Burns PN, et al. Air in the portal vein: sonographic and Doppler manifestations. Radiology. 1991;180(3):667-670. doi:10.1148/radiology.180.3.1871276
3. Kriegshauser JS, Reading CC, King BF, Welch TJ. Combined systemic and portal venous gas: sonographic and CT detection in two cases. AJR Am J Roentgenol. 1990;154(6):1219-1221. doi:10.2214/ajr.154.6.2110731
4. Goske MJ, Goldblum JR, Applegate KE, Mitchell CS, Bardo D. The “circle sign”: a new sonographic sign of pneumatosis intestinalis - clinical, pathologic and experimental findings. Pediatr Radiol. 1999;29(7):530-535. doi:10.1007/s002470050638
5. Marshak RH, Lindner AE, Maklansky D. Pneumatosis cystoides coli. Gastrointest Radiol. 1977;2(2):85-89. doi:10.1007/BF02256475
6. Jensen R, Gutnik SH. Pneumatosis cystoides intestinalis: a complication of colonoscopic polypectomy. S D J Med. 1991;44(7):177-179.
7. Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212(2):160-165. doi:10.1097/00000658-199008000-00008
8. Koss LG. Abdominal gas cysts (Pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53(6):523-549.
9. Jona JZ. Benign pneumatosis intestinalis coli after blunt trauma to the abdomen in a child. J Pediatr Surg. 2000;35(7):1109-1111. doi:10.1053/jpsu.2000.7837
10. Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11(3):111-118. doi:10.1007/s003840050031
11. Seto T, Koide N, Taniuchi N, Yamada T, Hamaguchi M, Goto S. Pneumatosis cystoides intestinalis complicating carcinoma of the small intestine. Am J Surg. 2001;182(3):287-288. doi:10.1016/S0002-9610(01)00710-3
12. Galandiuk S, Fazio VW, Petras RE. Pneumatosis cystoides intestinalis in Crohn’s disease. Report of two cases. Dis Colon Rectum. 1985;28(12):951-956. doi:10.1007/BF02554315
13. Parra JA, Acinas O, Bueno J, Madrazo C, Fariñas C. An unusual form of pneumatosis intestinalis associated with appendicitis. Br J Radiol. 1998;71(843):326-328. doi:10.1259/bjr.71.843.9616245
14. Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci. 1998;43(11):2455-2458. doi:10.1023/a:1026682131847
15. Kreiss C, Forohar F, Smithline AE, Brandt LJ. Pneumatosis intestinalis complicating C. difficile pseudomembranous colitis. Am J Gastroenterol. 1999;94(9):2560-2561. doi:10.1111/j.1572-0241.1999.01397.x
16. Day DL, Ramsay NK, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol. 1988;151(1):85-87. doi:10.2214/ajr.151.1.85
17. Tahara S, Sakai Y, Katsuno H, Urano M, Kuroda M, Tsukamoto T. Pneumatosis intestinalis and hepatic portal venous gas associated with gas-forming bacterial translocation due to postoperative paralytic ileus: A case report. Medicine (Baltimore). 2019;98(2):e14079. doi:10.1097/MD.0000000000014079
18. Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of whipple’s disease in a patient mistakenly on anti-tnf therapy. ACG Case Rep J. 2013;1(1):25- 28. Published 2013 Oct 8. doi:10.14309/crj.2013.11
19. Burton EM, Mercado-Deane MG, Patel K. Pneumatosis intestinalis in a child with AIDS and pseudomembranous colitis. Pediatr Radiol. 1994;24(8):609-610. doi:10.1007/BF02012750
20. Berk RN, Wall SD, McArdle CB, et al. Cryptosporidiosis of the stomach and small intestine in patients with AIDS. AJR Am J Roentgenol. 1984;143(3):549-554. doi:10.2214/ajr.143.3.549
21. Samson VE, Brown WR. Pneumatosis cystoides intestinalis in AIDS-associated cryptosporidiosis. More than an incidental finding? J Clin Gastroenterol. 1996;22(4):311-312.doi:10.1097/00004836-199606000-00015
22. Tjon A Tham RT, Vlasveld LT, Willemze R. Gastrointestinal complications of cytosine-arabinoside chemotherapy: findings on plain abdominal radiographs. AJR Am J Roentgenol. 1990;154(1):95-98. doi:10.2214/ajr.154.1.2104733
23. Hashimoto S, Saitoh H, Wada K, et al. Pneumatosis cystoides intestinalis after chemotherapy for hematological malignancies: report of 4 cases. Intern Med. 1995;34(3):212-215. doi:10.2169/internalmedicine.34.212
24. Gelman SF, Brandt LJ. Pneumatosis intestinalis and AIDS: a case report and review of the literature. Am J Gastroenterol. 1998;93(4):646-650. doi:10.1111/j.1572-0241.1998.183_b.x
25. Gillon J, Tadesse K, Logan RF, Holt S, Sircus W. Breath hydrogen in pneumatosis cystoides intestinalis. Gut. 1979;20(11):1008-1011. doi:10.1136/gut.20.11.1008
26. Hughes DT, Gordon KC, Swann JC, Bolt GL. Pneumatosis cystoides intestinalis. Gut. 1966;7(5):553-557. doi:10.1136/gut.7.5.553
27. Read NW, Al-Janabi MN, Cann PA. Is raised breath hydrogen related to the pathogenesis of pneumatosis coli? Gut. 1984;25(8):839-845. doi:10.1136/gut.25.8.839
28. van der Linden W, Marsell R. Pneumatosis cystoides coli associated with high H2 excretion. Treatment with an elemental diet. Scand J Gastroenterol. 1979;14(2):173-174. doi:10.3109/00365527909179864
29. Christl SU, Gibson GR, Murgatroyd PR, Scheppach W, Cummings JH. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104(2):392-397. doi:10.1016/0016-5085(93)90406-3
30. Keyting WS, Mccarver RR, Kovarik JL, Daywitt AL. Pneumatosis intestinalis: a new concept. Radiology. 1961;76:733-741. doi:10.1148/76.5.733
31. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345(8959):1220-1222. doi:10.1016/S0140-6736(95)91996-1
32. Grieve DA, Unsworth IP. Pneumatosis cystoides intestinalis: an experience with hyperbaric oxygen treatment. Aust N Z J Surg. 1991;61(6):423-426.
33. Micklefield GH, Kuntz HD, May B. Pneumatosis cystoides intestinalis: case reports and review of the literature. Mater Med Pol. 1990;22(2):70-72.
34. Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg. 1974;109(1):89- 94. doi:10.1001/archsurg.1974.01360010067017
35. Fenton LZ, Buonomo C. Benign pneumatosis in children. Pediatr Radiol. 2000;30(11):786-793. doi:10.1007/s002470000303
36. Tobias R, Coleman S, Helman CA. Pneumatosis coli simulating hepatomegaly. Am J Gastroenterol. 1985;80(2):146-149.
37. Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel w a l l . Radiographics. 1992;12(6):1069-1078. doi:10.1148/radiographics.12.6.1439012
38. Masterson JS, Fratkin LB, Osler TR, Trapp WG. Treatment of pneumatosis cystoides intestinalis with hyperbaric oxygen. Ann Surg. 1978;187(3):245-247. doi:10.1097/00000658-197803000-00005
39. Höflin F, Linden W van der. Pneumatosis cystoides intestinalis treated by oxygen breathing. Scandinavian J Gastroenterol . 1974;9(5) :427-430. doi:10.1080/00365521.1974.12096852
40. St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138(1):68-75. doi:10.1001/archsurg.138.1.68
41. Ling F, Guo D, Zhu L. Pneumatosis cystoides intestinalis: a case report and literature review. BMC Gastroenterol. 2019;19(1):176. Published 2019 Nov 6. doi:10.1186/s12876-019-1087-9
42. Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679-682. doi:10.1016/j.amjsurg.2008.01.011
‘Double-edged’ impact of sparring on the brains of MMA fighters
, early research suggests.
Investigators found sparring, defined as strategically hitting opponents with kicks, punches, and other strikes during practice sessions, is linked to increased white matter hyperintensities in the brain, pointing to possible vascular damage from repeated head trauma. However, the study results also show sparring was associated with a larger bilateral caudate which, in theory, is neuroprotective.
“From our preliminary study, sparring practice in MMA fighters may have a ‘double-edged sword’ effect on the brain,” study investigator Aaron Esagoff, a second-year medical student at Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“The combination of complex movements along with constant strategy and anticipation of your opponent’s next move may provide a neuroprotective effect on the caudate,” Mr. Esagoff said. However, he added, more research is needed into understanding this particular finding.
The study results were presented at the American Psychiatric Association (APA) 2022 Annual Meeting.
Growing popularity
MMA is a full-contact combat sport that has become increasingly popular over the past 15 years. It combines techniques from boxing, wrestling, karate, judo, and jujitsu.
To prepare for fights, MMA practitioners incorporate sparring and grappling, which use techniques such as chokes and locks to submit an opponent. Head protection is sometimes incorporated during practice, but is not the norm during a fight, said Mr. Esagoff.
The study investigated sparring during practice rather than fights because, he said, MMA competitors only fight a few times a year but spend hundreds of hours training. “So the health effects of training are going to be really important,” he said.
As with other combat sports, MMA involves hits to the head. Previous research has shown repetitive head trauma can lead to neurodegenerative diseases, including chronic traumatic encephalopathy (CTE) and Alzheimer’s disease, Mr. Esagoff noted.
Previous studies have also linked more professional fights and years of fighting to a decrease in brain volume among MMA fighters, he added.
The new analysis was conducted as part of the Professional Fighters Brain Health Study, a longitudinal cohort study of MMA professional fighters. It included 92 fighters with data available on MRI and habits regarding practicing. The mean age of the participants was 30 years, 62% were White, and 85% were men.
The study examined sparring but did not include grappling because of “several challenges” with the current data analysis, Mr. Esagoff said. Researchers adjusted for age, sex, education, race, number of fights, total intracranial volume, and type of MRI scanner used.
A ‘highly strategic’ sport
Results showed a strong association between the number of sparring rounds per week and increased white matter hyperintensity volume (mcL) on MRI (P = .039).
This suggests white matter damage, possibly a result of direct neuronal injury, vascular damage, or immune modulation, said Mr. Esagoff. However, another mechanism may be involved, he added.
There was also a significant association between sparring and increased size of the caudate nucleus, an area of the brain involved in movement, learning, and memory (P = .014 for right caudate volume, P = .012 for left caudate volume).
There are some theories that might explain this finding, said Mr. Esagoff. For example, individuals who spar more may get better at avoiding impacts and injuries during a fight, which might in turn affect the size of the caudate.
The controlled movements and techniques used during sparring could also affect the caudate. “Some research has shown that behavior, learning, and/or exercise may increase the size of certain brain regions,” Mr. Esagoff said.
He noted the “highly strategic” nature of combat sports – and used the example of Brazilian jiu-jitsu. That sport “is known as human chess because it takes a thoughtful approach to defeat a larger opponent with base, leverage, and technique,” he said.
However, Mr. Esagoff stressed that while it is possible movements involved in MMA increase caudate size, this is just a theory at this point.
A study limitation was that fighters volunteered to participate and may not represent all fighters. As well, the study was cross-sectional and looked at only one point in time, so it cannot infer causation.
Overall, the new findings should help inform fighters, governing bodies, and the public about the potential risks and benefits of different styles of MMA fighting and practice, although more research is needed, said Mr. Esagoff.
He and his team now plan to conduct a longer-term study and investigate effects of grappling on brain structure and function in addition to sparring.
Jury still out
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry and incoming chair of the APA’s Council on Communications, said the jury “is clearly still out” when it comes to the investigation of brain impacts.
“We don’t know quite what these changes fully correlate to,” said Dr. Liu, who moderated a press briefing highlighting the study.
He underlined the importance of protecting athletes vulnerable to head trauma, be they professionals or those involved at the youth sports level.
Dr. Liu also noted the “extreme popularity” and rapid growth of MMA around the world, which he said provides an opportunity for researchers to study these professional fighters.
“This is a unique population that signed up in the midst of hundreds of hours of sparring to advance neuroscience, and that’s quite amazing,” he said.
A version of this article first appeared on Medscape.com.
, early research suggests.
Investigators found sparring, defined as strategically hitting opponents with kicks, punches, and other strikes during practice sessions, is linked to increased white matter hyperintensities in the brain, pointing to possible vascular damage from repeated head trauma. However, the study results also show sparring was associated with a larger bilateral caudate which, in theory, is neuroprotective.
“From our preliminary study, sparring practice in MMA fighters may have a ‘double-edged sword’ effect on the brain,” study investigator Aaron Esagoff, a second-year medical student at Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“The combination of complex movements along with constant strategy and anticipation of your opponent’s next move may provide a neuroprotective effect on the caudate,” Mr. Esagoff said. However, he added, more research is needed into understanding this particular finding.
The study results were presented at the American Psychiatric Association (APA) 2022 Annual Meeting.
Growing popularity
MMA is a full-contact combat sport that has become increasingly popular over the past 15 years. It combines techniques from boxing, wrestling, karate, judo, and jujitsu.
To prepare for fights, MMA practitioners incorporate sparring and grappling, which use techniques such as chokes and locks to submit an opponent. Head protection is sometimes incorporated during practice, but is not the norm during a fight, said Mr. Esagoff.
The study investigated sparring during practice rather than fights because, he said, MMA competitors only fight a few times a year but spend hundreds of hours training. “So the health effects of training are going to be really important,” he said.
As with other combat sports, MMA involves hits to the head. Previous research has shown repetitive head trauma can lead to neurodegenerative diseases, including chronic traumatic encephalopathy (CTE) and Alzheimer’s disease, Mr. Esagoff noted.
Previous studies have also linked more professional fights and years of fighting to a decrease in brain volume among MMA fighters, he added.
The new analysis was conducted as part of the Professional Fighters Brain Health Study, a longitudinal cohort study of MMA professional fighters. It included 92 fighters with data available on MRI and habits regarding practicing. The mean age of the participants was 30 years, 62% were White, and 85% were men.
The study examined sparring but did not include grappling because of “several challenges” with the current data analysis, Mr. Esagoff said. Researchers adjusted for age, sex, education, race, number of fights, total intracranial volume, and type of MRI scanner used.
A ‘highly strategic’ sport
Results showed a strong association between the number of sparring rounds per week and increased white matter hyperintensity volume (mcL) on MRI (P = .039).
This suggests white matter damage, possibly a result of direct neuronal injury, vascular damage, or immune modulation, said Mr. Esagoff. However, another mechanism may be involved, he added.
There was also a significant association between sparring and increased size of the caudate nucleus, an area of the brain involved in movement, learning, and memory (P = .014 for right caudate volume, P = .012 for left caudate volume).
There are some theories that might explain this finding, said Mr. Esagoff. For example, individuals who spar more may get better at avoiding impacts and injuries during a fight, which might in turn affect the size of the caudate.
The controlled movements and techniques used during sparring could also affect the caudate. “Some research has shown that behavior, learning, and/or exercise may increase the size of certain brain regions,” Mr. Esagoff said.
He noted the “highly strategic” nature of combat sports – and used the example of Brazilian jiu-jitsu. That sport “is known as human chess because it takes a thoughtful approach to defeat a larger opponent with base, leverage, and technique,” he said.
However, Mr. Esagoff stressed that while it is possible movements involved in MMA increase caudate size, this is just a theory at this point.
A study limitation was that fighters volunteered to participate and may not represent all fighters. As well, the study was cross-sectional and looked at only one point in time, so it cannot infer causation.
Overall, the new findings should help inform fighters, governing bodies, and the public about the potential risks and benefits of different styles of MMA fighting and practice, although more research is needed, said Mr. Esagoff.
He and his team now plan to conduct a longer-term study and investigate effects of grappling on brain structure and function in addition to sparring.
Jury still out
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry and incoming chair of the APA’s Council on Communications, said the jury “is clearly still out” when it comes to the investigation of brain impacts.
“We don’t know quite what these changes fully correlate to,” said Dr. Liu, who moderated a press briefing highlighting the study.
He underlined the importance of protecting athletes vulnerable to head trauma, be they professionals or those involved at the youth sports level.
Dr. Liu also noted the “extreme popularity” and rapid growth of MMA around the world, which he said provides an opportunity for researchers to study these professional fighters.
“This is a unique population that signed up in the midst of hundreds of hours of sparring to advance neuroscience, and that’s quite amazing,” he said.
A version of this article first appeared on Medscape.com.
, early research suggests.
Investigators found sparring, defined as strategically hitting opponents with kicks, punches, and other strikes during practice sessions, is linked to increased white matter hyperintensities in the brain, pointing to possible vascular damage from repeated head trauma. However, the study results also show sparring was associated with a larger bilateral caudate which, in theory, is neuroprotective.
“From our preliminary study, sparring practice in MMA fighters may have a ‘double-edged sword’ effect on the brain,” study investigator Aaron Esagoff, a second-year medical student at Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“The combination of complex movements along with constant strategy and anticipation of your opponent’s next move may provide a neuroprotective effect on the caudate,” Mr. Esagoff said. However, he added, more research is needed into understanding this particular finding.
The study results were presented at the American Psychiatric Association (APA) 2022 Annual Meeting.
Growing popularity
MMA is a full-contact combat sport that has become increasingly popular over the past 15 years. It combines techniques from boxing, wrestling, karate, judo, and jujitsu.
To prepare for fights, MMA practitioners incorporate sparring and grappling, which use techniques such as chokes and locks to submit an opponent. Head protection is sometimes incorporated during practice, but is not the norm during a fight, said Mr. Esagoff.
The study investigated sparring during practice rather than fights because, he said, MMA competitors only fight a few times a year but spend hundreds of hours training. “So the health effects of training are going to be really important,” he said.
As with other combat sports, MMA involves hits to the head. Previous research has shown repetitive head trauma can lead to neurodegenerative diseases, including chronic traumatic encephalopathy (CTE) and Alzheimer’s disease, Mr. Esagoff noted.
Previous studies have also linked more professional fights and years of fighting to a decrease in brain volume among MMA fighters, he added.
The new analysis was conducted as part of the Professional Fighters Brain Health Study, a longitudinal cohort study of MMA professional fighters. It included 92 fighters with data available on MRI and habits regarding practicing. The mean age of the participants was 30 years, 62% were White, and 85% were men.
The study examined sparring but did not include grappling because of “several challenges” with the current data analysis, Mr. Esagoff said. Researchers adjusted for age, sex, education, race, number of fights, total intracranial volume, and type of MRI scanner used.
A ‘highly strategic’ sport
Results showed a strong association between the number of sparring rounds per week and increased white matter hyperintensity volume (mcL) on MRI (P = .039).
This suggests white matter damage, possibly a result of direct neuronal injury, vascular damage, or immune modulation, said Mr. Esagoff. However, another mechanism may be involved, he added.
There was also a significant association between sparring and increased size of the caudate nucleus, an area of the brain involved in movement, learning, and memory (P = .014 for right caudate volume, P = .012 for left caudate volume).
There are some theories that might explain this finding, said Mr. Esagoff. For example, individuals who spar more may get better at avoiding impacts and injuries during a fight, which might in turn affect the size of the caudate.
The controlled movements and techniques used during sparring could also affect the caudate. “Some research has shown that behavior, learning, and/or exercise may increase the size of certain brain regions,” Mr. Esagoff said.
He noted the “highly strategic” nature of combat sports – and used the example of Brazilian jiu-jitsu. That sport “is known as human chess because it takes a thoughtful approach to defeat a larger opponent with base, leverage, and technique,” he said.
However, Mr. Esagoff stressed that while it is possible movements involved in MMA increase caudate size, this is just a theory at this point.
A study limitation was that fighters volunteered to participate and may not represent all fighters. As well, the study was cross-sectional and looked at only one point in time, so it cannot infer causation.
Overall, the new findings should help inform fighters, governing bodies, and the public about the potential risks and benefits of different styles of MMA fighting and practice, although more research is needed, said Mr. Esagoff.
He and his team now plan to conduct a longer-term study and investigate effects of grappling on brain structure and function in addition to sparring.
Jury still out
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry and incoming chair of the APA’s Council on Communications, said the jury “is clearly still out” when it comes to the investigation of brain impacts.
“We don’t know quite what these changes fully correlate to,” said Dr. Liu, who moderated a press briefing highlighting the study.
He underlined the importance of protecting athletes vulnerable to head trauma, be they professionals or those involved at the youth sports level.
Dr. Liu also noted the “extreme popularity” and rapid growth of MMA around the world, which he said provides an opportunity for researchers to study these professional fighters.
“This is a unique population that signed up in the midst of hundreds of hours of sparring to advance neuroscience, and that’s quite amazing,” he said.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Premature return to play after concussion has decreased
, according to a recent chart review. Rates of premature return to learn (RTL) are essentially unchanged, however.
“Delay in recovery is the major reason why it’s important not to RTL or RTP prematurely,” said James Carson, MD, associate professor of family and community medicine, University of Toronto.
“That delay in recovery only sets students further back in terms of the stress they get from being delayed with their schoolwork – they could lose their year in school, lose all their social contacts. So, there are a number of psychosocial issues that come into play if recovery is delayed, and that is what premature RTL and premature RTP will do – they delay the student’s recovery,” he emphasized.
The study was published in Canadian Family Physician.
Differences by sex
The study involved 241 students who had 258 distinct cases of SRC. The researchers defined premature RTP and RTL as chart records documenting the relapse, recurrence, or worsening of concussion symptoms that accompanied the patient’s RTP or RTL. Between 2011 and 2016, 26.7% of students had evidence of premature RTP, while 42.6% of them had evidence of premature RTL, the authors noted.
Compared with findings from an earlier survey of data from 2006 to 2011, the incidence of premature RTP dropped by 38.6% (P = .0003). In contrast, symptoms associated with premature RTL dropped by only 4.7% from the previous survey. This change was not statistically significant.
There was also a significant difference between males and females in the proportion of SRC cases with relapse of symptoms. Relapse occurred in 43.4% of female athletes with SRC versus 29.7% of male athletes with SRC (P = .023).
Female athletes also had significantly longer times before being cleared for RTP. The mean time was 74.5 days for females, compared with a mean of 42.3 days for male athletes (P < .001). “The median time to RTP clearance was nearly double [for female athletes] at 49 days versus 25 days [for male athletes],” wrote the authors.
The rate of premature RTL was also higher among secondary school students (48.8%), compared with 28% among elementary students and 42% among postsecondary students.
More concussions coming?
Before the first consensus conference, organized by the Concussion in Sport Group in 2001, management of concussion was based on rating and grading scales that had no medical evidence to support them, said Dr. Carson. After the consensus conference, it was recommended that physicians manage each concussion individually and, when it came to RTP, recommendations were based upon symptom resolution.
In contrast, there was nothing in the literature regarding how student athletes who sustain a concussion should RTL. Some schools made generous accommodations, and others none. This situation changed around 2011, when experts started publishing data about how better to accommodate student athletes who have a temporary disability for which schools need to introduce temporary accommodations to help them recover.
“Recommendations for RTP essentially had a 12-year head-start,” Dr. Carson emphasized, “and RTL had a much slower start.” Unfortunately, Dr. Carson foresees more athletes sustaining concussions as pandemic restrictions ease over the next few months. “As athletes RTP after the pandemic, they just will not be in game shape,” he said.
“In other words, athletes may not have the neuromuscular control to avoid these injuries as easily,” he added. Worse, athletes may not realize they are not quite ready to return to the expected level of participation so quickly. “I believe this scenario will lead to more concussions that will be difficult to manage in the context of an already strained health care system,” said Dr. Carson.
A limitation of the study was that it was difficult to assess whether all patients followed medical advice consistently.
“Very positive shifts”
Commenting on the findings, Nick Reed, PhD, Canada research chair in pediatric concussion and associate professor of occupational science and occupational therapy, University of Toronto, said that sports medicine physicians are seeing “very positive shifts” in concussion awareness and related behaviors such as providing education, support, and accommodations to students within the school environment. “More and more teachers are seeking education to learn what a concussion is and what to do to best support their students with concussion,” he said. Dr. Reed was not involved in the current study.
Indeed, this increasing awareness led to the development of a concussion education tool for teachers – SCHOOLFirst – although Dr. Reed did acknowledge that not all teachers have either the knowledge or the resources they need to optimally support their students with concussion. In the meantime, to reduce the risk of injury, Dr. Reed stressed that it is important for students to wear equipment appropriate for the game being played and to play by the rules.
“It is key to play sports in a way that is fair and respectful and not [engage] in behaviors with the intent of injuring an opponent,” he stressed. It is also important for athletes themselves to know the signs and symptoms of concussion and, if they think they have a concussion, to immediately stop playing, report how they are feeling to a coach, teacher, or parent, and to seek medical assessment to determine if they have a concussion or not.
“The key here is to focus on what the athlete can do after a concussion rather than what they can’t do,” Dr. Reed said. After even a few days of complete rest, students with a concussion can gradually introduce low levels of physical and cognitive activity that won’t make their symptoms worse. This activity can include going back to school with temporary accommodations in place, such as shorter school days and increased rest breaks. “When returning to school and to sport after a concussion, it is important to follow a stepwise and gradual return to activities so that you aren’t doing too much too fast,” Dr. Reed emphasized.
The study was conducted without external funding. Dr. Carson and Dr. Reed reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, according to a recent chart review. Rates of premature return to learn (RTL) are essentially unchanged, however.
“Delay in recovery is the major reason why it’s important not to RTL or RTP prematurely,” said James Carson, MD, associate professor of family and community medicine, University of Toronto.
“That delay in recovery only sets students further back in terms of the stress they get from being delayed with their schoolwork – they could lose their year in school, lose all their social contacts. So, there are a number of psychosocial issues that come into play if recovery is delayed, and that is what premature RTL and premature RTP will do – they delay the student’s recovery,” he emphasized.
The study was published in Canadian Family Physician.
Differences by sex
The study involved 241 students who had 258 distinct cases of SRC. The researchers defined premature RTP and RTL as chart records documenting the relapse, recurrence, or worsening of concussion symptoms that accompanied the patient’s RTP or RTL. Between 2011 and 2016, 26.7% of students had evidence of premature RTP, while 42.6% of them had evidence of premature RTL, the authors noted.
Compared with findings from an earlier survey of data from 2006 to 2011, the incidence of premature RTP dropped by 38.6% (P = .0003). In contrast, symptoms associated with premature RTL dropped by only 4.7% from the previous survey. This change was not statistically significant.
There was also a significant difference between males and females in the proportion of SRC cases with relapse of symptoms. Relapse occurred in 43.4% of female athletes with SRC versus 29.7% of male athletes with SRC (P = .023).
Female athletes also had significantly longer times before being cleared for RTP. The mean time was 74.5 days for females, compared with a mean of 42.3 days for male athletes (P < .001). “The median time to RTP clearance was nearly double [for female athletes] at 49 days versus 25 days [for male athletes],” wrote the authors.
The rate of premature RTL was also higher among secondary school students (48.8%), compared with 28% among elementary students and 42% among postsecondary students.
More concussions coming?
Before the first consensus conference, organized by the Concussion in Sport Group in 2001, management of concussion was based on rating and grading scales that had no medical evidence to support them, said Dr. Carson. After the consensus conference, it was recommended that physicians manage each concussion individually and, when it came to RTP, recommendations were based upon symptom resolution.
In contrast, there was nothing in the literature regarding how student athletes who sustain a concussion should RTL. Some schools made generous accommodations, and others none. This situation changed around 2011, when experts started publishing data about how better to accommodate student athletes who have a temporary disability for which schools need to introduce temporary accommodations to help them recover.
“Recommendations for RTP essentially had a 12-year head-start,” Dr. Carson emphasized, “and RTL had a much slower start.” Unfortunately, Dr. Carson foresees more athletes sustaining concussions as pandemic restrictions ease over the next few months. “As athletes RTP after the pandemic, they just will not be in game shape,” he said.
“In other words, athletes may not have the neuromuscular control to avoid these injuries as easily,” he added. Worse, athletes may not realize they are not quite ready to return to the expected level of participation so quickly. “I believe this scenario will lead to more concussions that will be difficult to manage in the context of an already strained health care system,” said Dr. Carson.
A limitation of the study was that it was difficult to assess whether all patients followed medical advice consistently.
“Very positive shifts”
Commenting on the findings, Nick Reed, PhD, Canada research chair in pediatric concussion and associate professor of occupational science and occupational therapy, University of Toronto, said that sports medicine physicians are seeing “very positive shifts” in concussion awareness and related behaviors such as providing education, support, and accommodations to students within the school environment. “More and more teachers are seeking education to learn what a concussion is and what to do to best support their students with concussion,” he said. Dr. Reed was not involved in the current study.
Indeed, this increasing awareness led to the development of a concussion education tool for teachers – SCHOOLFirst – although Dr. Reed did acknowledge that not all teachers have either the knowledge or the resources they need to optimally support their students with concussion. In the meantime, to reduce the risk of injury, Dr. Reed stressed that it is important for students to wear equipment appropriate for the game being played and to play by the rules.
“It is key to play sports in a way that is fair and respectful and not [engage] in behaviors with the intent of injuring an opponent,” he stressed. It is also important for athletes themselves to know the signs and symptoms of concussion and, if they think they have a concussion, to immediately stop playing, report how they are feeling to a coach, teacher, or parent, and to seek medical assessment to determine if they have a concussion or not.
“The key here is to focus on what the athlete can do after a concussion rather than what they can’t do,” Dr. Reed said. After even a few days of complete rest, students with a concussion can gradually introduce low levels of physical and cognitive activity that won’t make their symptoms worse. This activity can include going back to school with temporary accommodations in place, such as shorter school days and increased rest breaks. “When returning to school and to sport after a concussion, it is important to follow a stepwise and gradual return to activities so that you aren’t doing too much too fast,” Dr. Reed emphasized.
The study was conducted without external funding. Dr. Carson and Dr. Reed reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, according to a recent chart review. Rates of premature return to learn (RTL) are essentially unchanged, however.
“Delay in recovery is the major reason why it’s important not to RTL or RTP prematurely,” said James Carson, MD, associate professor of family and community medicine, University of Toronto.
“That delay in recovery only sets students further back in terms of the stress they get from being delayed with their schoolwork – they could lose their year in school, lose all their social contacts. So, there are a number of psychosocial issues that come into play if recovery is delayed, and that is what premature RTL and premature RTP will do – they delay the student’s recovery,” he emphasized.
The study was published in Canadian Family Physician.
Differences by sex
The study involved 241 students who had 258 distinct cases of SRC. The researchers defined premature RTP and RTL as chart records documenting the relapse, recurrence, or worsening of concussion symptoms that accompanied the patient’s RTP or RTL. Between 2011 and 2016, 26.7% of students had evidence of premature RTP, while 42.6% of them had evidence of premature RTL, the authors noted.
Compared with findings from an earlier survey of data from 2006 to 2011, the incidence of premature RTP dropped by 38.6% (P = .0003). In contrast, symptoms associated with premature RTL dropped by only 4.7% from the previous survey. This change was not statistically significant.
There was also a significant difference between males and females in the proportion of SRC cases with relapse of symptoms. Relapse occurred in 43.4% of female athletes with SRC versus 29.7% of male athletes with SRC (P = .023).
Female athletes also had significantly longer times before being cleared for RTP. The mean time was 74.5 days for females, compared with a mean of 42.3 days for male athletes (P < .001). “The median time to RTP clearance was nearly double [for female athletes] at 49 days versus 25 days [for male athletes],” wrote the authors.
The rate of premature RTL was also higher among secondary school students (48.8%), compared with 28% among elementary students and 42% among postsecondary students.
More concussions coming?
Before the first consensus conference, organized by the Concussion in Sport Group in 2001, management of concussion was based on rating and grading scales that had no medical evidence to support them, said Dr. Carson. After the consensus conference, it was recommended that physicians manage each concussion individually and, when it came to RTP, recommendations were based upon symptom resolution.
In contrast, there was nothing in the literature regarding how student athletes who sustain a concussion should RTL. Some schools made generous accommodations, and others none. This situation changed around 2011, when experts started publishing data about how better to accommodate student athletes who have a temporary disability for which schools need to introduce temporary accommodations to help them recover.
“Recommendations for RTP essentially had a 12-year head-start,” Dr. Carson emphasized, “and RTL had a much slower start.” Unfortunately, Dr. Carson foresees more athletes sustaining concussions as pandemic restrictions ease over the next few months. “As athletes RTP after the pandemic, they just will not be in game shape,” he said.
“In other words, athletes may not have the neuromuscular control to avoid these injuries as easily,” he added. Worse, athletes may not realize they are not quite ready to return to the expected level of participation so quickly. “I believe this scenario will lead to more concussions that will be difficult to manage in the context of an already strained health care system,” said Dr. Carson.
A limitation of the study was that it was difficult to assess whether all patients followed medical advice consistently.
“Very positive shifts”
Commenting on the findings, Nick Reed, PhD, Canada research chair in pediatric concussion and associate professor of occupational science and occupational therapy, University of Toronto, said that sports medicine physicians are seeing “very positive shifts” in concussion awareness and related behaviors such as providing education, support, and accommodations to students within the school environment. “More and more teachers are seeking education to learn what a concussion is and what to do to best support their students with concussion,” he said. Dr. Reed was not involved in the current study.
Indeed, this increasing awareness led to the development of a concussion education tool for teachers – SCHOOLFirst – although Dr. Reed did acknowledge that not all teachers have either the knowledge or the resources they need to optimally support their students with concussion. In the meantime, to reduce the risk of injury, Dr. Reed stressed that it is important for students to wear equipment appropriate for the game being played and to play by the rules.
“It is key to play sports in a way that is fair and respectful and not [engage] in behaviors with the intent of injuring an opponent,” he stressed. It is also important for athletes themselves to know the signs and symptoms of concussion and, if they think they have a concussion, to immediately stop playing, report how they are feeling to a coach, teacher, or parent, and to seek medical assessment to determine if they have a concussion or not.
“The key here is to focus on what the athlete can do after a concussion rather than what they can’t do,” Dr. Reed said. After even a few days of complete rest, students with a concussion can gradually introduce low levels of physical and cognitive activity that won’t make their symptoms worse. This activity can include going back to school with temporary accommodations in place, such as shorter school days and increased rest breaks. “When returning to school and to sport after a concussion, it is important to follow a stepwise and gradual return to activities so that you aren’t doing too much too fast,” Dr. Reed emphasized.
The study was conducted without external funding. Dr. Carson and Dr. Reed reported no conflicts of interest.
A version of this article first appeared on Medscape.com.