User login
Patient Has No Complaints, But Family Is Concerned
ANSWER
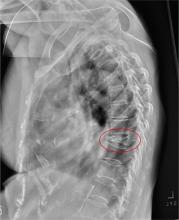
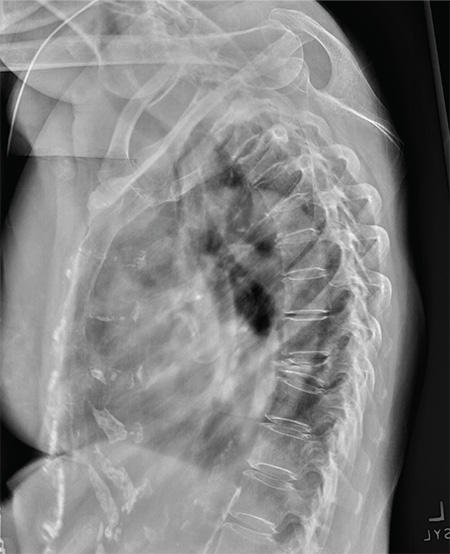
The radiograph shows diffuse osteopenia and spondylosis. Of note is a moderate to severe compression deformity of the T8 vertebral body. However, by plain radiograph, it is age indeterminate as to its acuity. For definitive diagnosis, MRI without contrast is required to assess for marrow edema, which then suggests an acute fracture.
This patient was admitted for further workup. MRI was ultimately obtained and revealed marrow edema within that vertebral body. She was treated with a rigid custom-made brace.
ANSWER
The radiograph shows diffuse osteopenia and spondylosis. Of note is a moderate to severe compression deformity of the T8 vertebral body. However, by plain radiograph, it is age indeterminate as to its acuity. For definitive diagnosis, MRI without contrast is required to assess for marrow edema, which then suggests an acute fracture.
This patient was admitted for further workup. MRI was ultimately obtained and revealed marrow edema within that vertebral body. She was treated with a rigid custom-made brace.
ANSWER
The radiograph shows diffuse osteopenia and spondylosis. Of note is a moderate to severe compression deformity of the T8 vertebral body. However, by plain radiograph, it is age indeterminate as to its acuity. For definitive diagnosis, MRI without contrast is required to assess for marrow edema, which then suggests an acute fracture.
This patient was admitted for further workup. MRI was ultimately obtained and revealed marrow edema within that vertebral body. She was treated with a rigid custom-made brace.
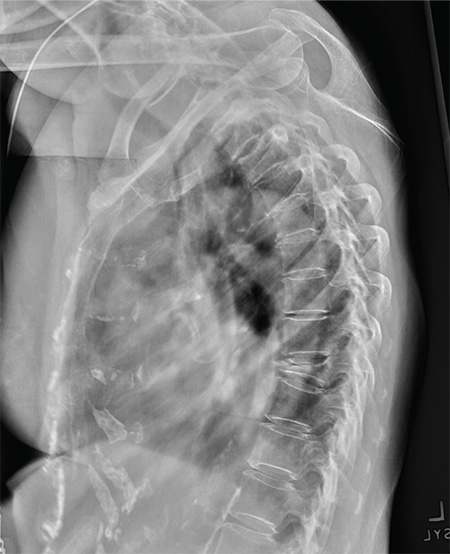
An 80-year-old woman presents to the emergency department for evaluation. Her family reports that she has baseline dementia and resides in an assisted living facility. Staff there report that recently the patient has fallen multiple times. The patient herself does not voice any specific complaints, but her family has noticed she is not as active or walking as much as usual. Medical history is significant for mild hypertension. On physical exam, you note that the patient is awake and alert but oriented only to person. Her vital signs are stable. Primary and secondary survey do not demonstrate any obvious injury or trauma. You order some basic blood work, as well as some imaging studies—including thoracic and lumbar radiographs. The lateral thoracic radiograph is shown. What is your impression?
The Ears Have It (and She Doesn’t Want It)
ANSWER
The correct answer is to obtain a biopsy (choice “d”), the results of which would likely dictate rational and effective treatment. The other choices are largely empirical and not based on available evidence.
DISCUSSION
In this case, the biopsy (with samples from the arm rash as well as from the ears) showed unequivocal evidence of connective tissue disease—almost certainly lupus.
Systemic lupus erythematosus (SLE) has protean manifestations because it can affect so many different organs in so many different ways. Reduced to its simplest elements, lupus is an autoimmune process that results in a form of vasculitis that can affect any perfused tissue.
In terms of the skin, the visible manifestations of lupus are numerous and not always obvious. Sun is a known exacerbating factor, as this case demonstrated quite well: The patient’s rash was pronounced on sun-exposed skin but spared covered skin. When this was brought to her attention, the patient recalled a baby-sitting job earlier in the year (summer) that had required her to spend time outdoors. She also acknowledged that her smoking habit often took her into the backyard, where she would stand in the sun.
That being said, neither the arm rash nor the ear changes are “typical” of lupus (although the latter did include patulous follicular orifices, enlarged pores often seen focally with lupus). The effect is simply the result of the patient’s normal dark skin color; on a white person, the discoloration would have been pink or red.
Although these changes were suspicious for lupus, it was necessary to establish the diagnosis by biopsy—especially since the patient was already being treated for the disease. With that accomplished, the patient was sent back to her rheumatologist, who indicated he would probably treat her with a biologic, plus or minus methotrexate.
ANSWER
The correct answer is to obtain a biopsy (choice “d”), the results of which would likely dictate rational and effective treatment. The other choices are largely empirical and not based on available evidence.
DISCUSSION
In this case, the biopsy (with samples from the arm rash as well as from the ears) showed unequivocal evidence of connective tissue disease—almost certainly lupus.
Systemic lupus erythematosus (SLE) has protean manifestations because it can affect so many different organs in so many different ways. Reduced to its simplest elements, lupus is an autoimmune process that results in a form of vasculitis that can affect any perfused tissue.
In terms of the skin, the visible manifestations of lupus are numerous and not always obvious. Sun is a known exacerbating factor, as this case demonstrated quite well: The patient’s rash was pronounced on sun-exposed skin but spared covered skin. When this was brought to her attention, the patient recalled a baby-sitting job earlier in the year (summer) that had required her to spend time outdoors. She also acknowledged that her smoking habit often took her into the backyard, where she would stand in the sun.
That being said, neither the arm rash nor the ear changes are “typical” of lupus (although the latter did include patulous follicular orifices, enlarged pores often seen focally with lupus). The effect is simply the result of the patient’s normal dark skin color; on a white person, the discoloration would have been pink or red.
Although these changes were suspicious for lupus, it was necessary to establish the diagnosis by biopsy—especially since the patient was already being treated for the disease. With that accomplished, the patient was sent back to her rheumatologist, who indicated he would probably treat her with a biologic, plus or minus methotrexate.
ANSWER
The correct answer is to obtain a biopsy (choice “d”), the results of which would likely dictate rational and effective treatment. The other choices are largely empirical and not based on available evidence.
DISCUSSION
In this case, the biopsy (with samples from the arm rash as well as from the ears) showed unequivocal evidence of connective tissue disease—almost certainly lupus.
Systemic lupus erythematosus (SLE) has protean manifestations because it can affect so many different organs in so many different ways. Reduced to its simplest elements, lupus is an autoimmune process that results in a form of vasculitis that can affect any perfused tissue.
In terms of the skin, the visible manifestations of lupus are numerous and not always obvious. Sun is a known exacerbating factor, as this case demonstrated quite well: The patient’s rash was pronounced on sun-exposed skin but spared covered skin. When this was brought to her attention, the patient recalled a baby-sitting job earlier in the year (summer) that had required her to spend time outdoors. She also acknowledged that her smoking habit often took her into the backyard, where she would stand in the sun.
That being said, neither the arm rash nor the ear changes are “typical” of lupus (although the latter did include patulous follicular orifices, enlarged pores often seen focally with lupus). The effect is simply the result of the patient’s normal dark skin color; on a white person, the discoloration would have been pink or red.
Although these changes were suspicious for lupus, it was necessary to establish the diagnosis by biopsy—especially since the patient was already being treated for the disease. With that accomplished, the patient was sent back to her rheumatologist, who indicated he would probably treat her with a biologic, plus or minus methotrexate.
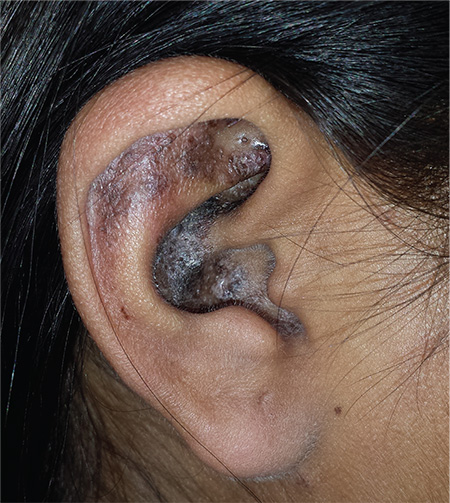
A 34-year-old black woman is sent to dermatology by her rheumatologist for evaluation of changes to her ears that began several months ago. The patient reports no symptoms, but she is quite distressed by the appearance of her ears. She has been under the care of the rheumatologist for several years for her systemic lupus erythematosus. She takes hydroxychloroquine (400 mg/d), which she says controls most of her systemic symptoms (ie, joint pain and malaise). Further history taking reveals that, within the time frame of the ear changes, the patient also developed an itchy rash on both arms. Application of triamcinolone 0.1% cream has not helped. On examination, the changes to the patient’s ears are immediately obvious: the dark brown to black discoloration contrasts sharply with her light brown skin. In addition to the color change, the surface of the ears is scaly and rough, with enlarged pores evident. There is no redness or swelling noted; palpation elicits neither increased warmth nor adenopathy around the ears or on the adjacent neck. The scaly rash on the patient’s arms is remarkably symmetrical. It affects the sun-exposed lateral portions of both arms, sparing the skin on the medial aspects and on the proximal portions normally covered by clothing.
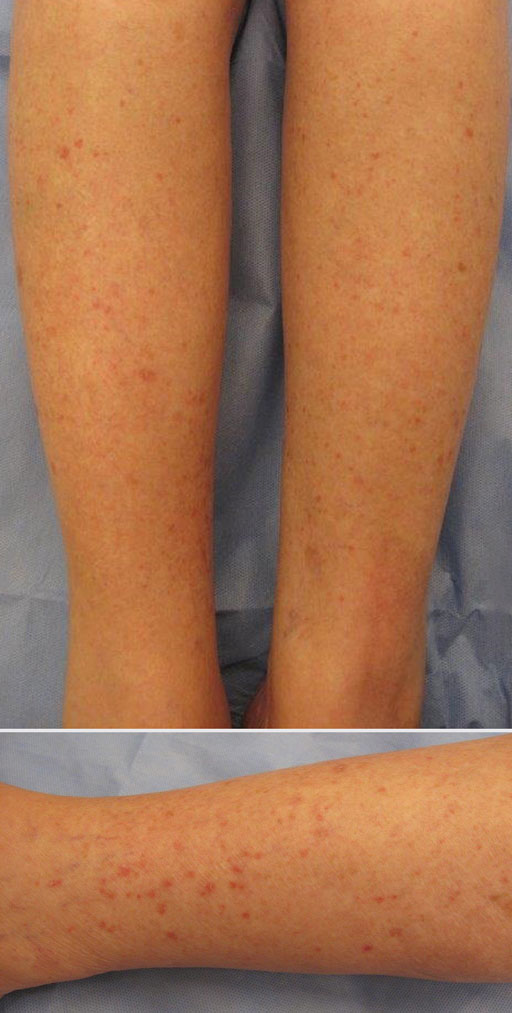
Erythematous Scaly Papules on the Shins and Calves
The Diagnosis: Hyperkeratosis Lenticularis Perstans
A shave biopsy of a lesion on the right leg was performed. Histopathology revealed a discrete papule with overlying compact hyperkeratosis. There was parakeratosis with an absent granular layer and a lichenoid lymphocytic infiltrate within the papillary dermis (Figure). Given the clinical context, these changes were consistent with a diagnosis of hyperkeratosis lenticularis perstans (HLP), also known as Flegel disease.
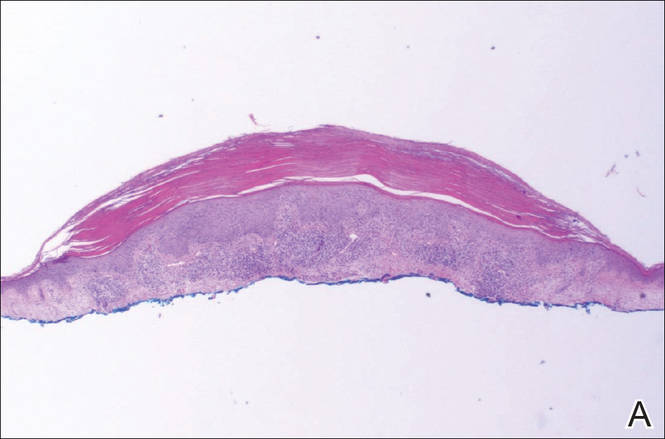
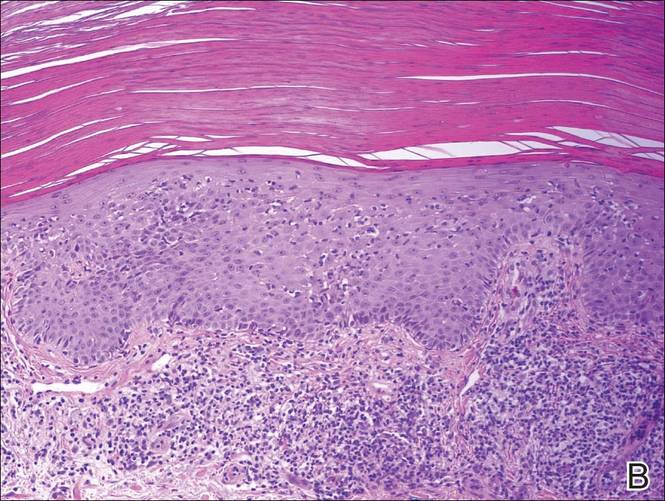
The patient was started on tretinoin cream 0.1% nightly for 3 months and triamcinolone ointment 0.1% as needed for pruritus but showed no clinical response. Given the benign nature of the condition and because the lesions were asymptomatic, additional treatment options were not pursued.
Originally described by Flegel1 in 1958, HLP is a rare skin disorder commonly seen in white individuals with onset in the fourth or fifth decades of life.1,2 While most cases are sporadic,3-6 HLP also has been associated with autosomal dominant inheritance.7-10
Patients with HLP typically present with multiple 1- to 5-mm reddish-brown, hyperkeratotic, scaly papules that reveal a moist, erythematous base with pinpoint bleeding upon removal of the scale. Lesions usually are distributed symmetrically and most commonly present on the extensor surfaces of the lower legs and dorsal feet.1,2,7 Lesions also may appear on the extensor surfaces of the arms, pinna, periocular region, antecubital and popliteal fossae, and oral mucosa and also may present as pits on the palms and soles.2,4,7,8 Furthermore, unilateral and localized variants of HLP have been described.11,12 Hyperkeratosis lenticularis perstans usually is asymptomatic but can present with mild pruritus or burning.3,5,13
The etiology and pathogenesis of HLP are unknown. Exposure to UV light has been implicated as an inciting factor14; however, reports of spontaneous resolution in the summer13 and upon treatment with psoralen plus UVA therapy15 make the role of UV light unclear. Furthermore, investigators disagree as to whether the primary pathogenic event in HLP is an inflammatory process or one of abnormal keratinization.1,3,7,10 Fernandez-Flores and Manjon16 suggested HLP is an inflammatory process with periods of exacerbations and remissions after finding mounds of parakeratosis with neutrophils arranged in different strata in the stratum corneum.
Histologically, compact hyperkeratosis usually is noted, often with associated parakeratosis, epidermal atrophy with thinning or absence of the granular layer, and a bandlike lymphohistiocytic infiltrate in the papillary dermis.1-3 Histopathologic differences between recent-onset versus longstanding lesions have been found, with old lesions lacking an inflammatory infiltrate.3 Furthermore, new lesions often show abnormalities in quantity and/or morphology of membrane-coating granules, also known as Odland bodies, in keratinocytes on electron microscopy,3,10,17 while old lesions do not.3 Odland bodies are involved in normal desquamation, leading some to speculate on their role in HLP.10 Currently, it is unclear whether abnormalities in these organelles cause the retention hyperkeratosis seen in HLP or if such abnormalities are a secondary phenomenon.3,17
There are questionable associations between HLP and diabetes mellitus type 2, hyperthyroidism, basal and squamous cell carcinomas of the skin, and gastrointestinal malignancy.4,9,18 Our patient had a history of basal cell carcinoma on the face, diet-controlled diabetes mellitus, and hypothyroidism. Given the high prevalence of these diseases in the general population, however, it is difficult to ascertain whether a true association with HLP exists.
While HLP can slowly progress to involve additional body sites, it is overall a benign condition that does not require treatment. Therapeutic options are based on case reports, with no single treatment showing a consistent response. From review of the literature, therapies that have been most effective include dermabrasion, excision,19 topical 5-fluorouracil,2,17,20 and oral retinoids.8 Hyperkeratosis lenticularis perstans generally is resistant to topical steroids, retinoids, and vitamin D3 analogs, although success with betamethasone dipropionate,5 isotretinoin
gel 0.05%,11 and calcipotriol have been reported.6 A case of HLP with clinical response to psoralen plus UVA therapy also has been described.15
- Flegel H. Hyperkeratosis lenticularis perstans. Hautarzt. 1958;9:363-364.
- Pearson LH, Smith JG, Chalker DK. Hyperkeratosis lenticularis perstans (Flegel’s disease). J Am Acad Dermatol. 1987;16:190-195.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel’s disease). Am J Dermatopathol. 2006;28:122-126.
- Fernández-Crehuet P, Rodríguez-Rey E, Ríos-Martín JJ, et al. Hyperkeratosis lenticularis perstans, or Flegel disease, with palmoplantar involvement. Actas Dermosifiliogr. 2009;100:157-159.
- Sterneberg-Vos H, van Marion AM, Frank J, et al. Hyperkeratosis lenticularis perstans (Flegel’s disease)—successful treatment with topical corticosteroids. Int J Dermatol. 2008;47:38-41.
- Bayramgürler D, Apaydin R, Dökmeci S, et al. Flegel’s disease: treatment with topical calcipotriol. Clin Exp Dermatol. 2002;27:161-162.
- Price ML, Jones EW, MacDonald DM. A clinicopathological study of Flegel’s disease (hyperkeratosis lenticularis perstans). Br J Dermatol. 1987;116:681-691.
- Krishnan A, Kar S. Photoletter to the editor: hyperkeratosis lenticularis perstans (Flegel’s disease) with unusual clinical presentation. response to isotretinoin therapy. J Dermatol Case Rep. 2012;6:93-95.
- Beveridge GW, Langlands AO. Familial hyperkeratosis lenticularis perstans associated with tumours of the skin. Br J Dermatol. 1973;88:453-458.
- Frenk E, Tapernoux B. Hyperkeratosis lenticularis perstans (Flegel): a biological model for keratinization occurring in the absence of Odland bodies? Dermatologica. 1976;153:253-262.
- Miranda-Romero A, Sánchez Sambucety P, Bajo del Pozo C, et al. Unilateral hyperkeratosis lenticularis perstans (Flegel's disease). J Am Acad Dermatol. 1998;39:655-657.
- Gutiérrez MC, Hasson A, Arias MD, et al. Localized hyperkeratosis lenticularis perstans (Flegel's disease). Cutis. 1991;48:201-204.
- Fathy S, Azadeh B. Hyperkeratosis lenticularis perstans. Int J Dermatol. 1988;27:120-121.
- Rosdahl I, Rosen K. Hyperkeratosis lenticularis perstans: report on two cases. Acta Derm Venerol. 1985;65:562-564.
- Cooper SM, George S. Flegel's disease treated with psoralen ultraviolet A. Br J Dermatol. 2000;142:340-342.
- Fernandez-Flores A, Manjon JA. Morphological evidence of periodical exacerbation of hyperkeratosis lenticularis perstans. Acta Dermatovenerol Croat. 2009;17:16-19.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Ishibashi A, Tsuboi R, Fujita K. Familial hyperkeratosis lenticularis perstans. associated with cancers of the digestive organs. J Dermatol. 1984;11:407-409.
- Cunha Filho RR, Almeida Jr HL. Hyperkeratosis lenticularis perstans. An Bras Dermatol. 2011;86(4 suppl 1):S76-S77.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)—lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.
The Diagnosis: Hyperkeratosis Lenticularis Perstans
A shave biopsy of a lesion on the right leg was performed. Histopathology revealed a discrete papule with overlying compact hyperkeratosis. There was parakeratosis with an absent granular layer and a lichenoid lymphocytic infiltrate within the papillary dermis (Figure). Given the clinical context, these changes were consistent with a diagnosis of hyperkeratosis lenticularis perstans (HLP), also known as Flegel disease.


The patient was started on tretinoin cream 0.1% nightly for 3 months and triamcinolone ointment 0.1% as needed for pruritus but showed no clinical response. Given the benign nature of the condition and because the lesions were asymptomatic, additional treatment options were not pursued.
Originally described by Flegel1 in 1958, HLP is a rare skin disorder commonly seen in white individuals with onset in the fourth or fifth decades of life.1,2 While most cases are sporadic,3-6 HLP also has been associated with autosomal dominant inheritance.7-10
Patients with HLP typically present with multiple 1- to 5-mm reddish-brown, hyperkeratotic, scaly papules that reveal a moist, erythematous base with pinpoint bleeding upon removal of the scale. Lesions usually are distributed symmetrically and most commonly present on the extensor surfaces of the lower legs and dorsal feet.1,2,7 Lesions also may appear on the extensor surfaces of the arms, pinna, periocular region, antecubital and popliteal fossae, and oral mucosa and also may present as pits on the palms and soles.2,4,7,8 Furthermore, unilateral and localized variants of HLP have been described.11,12 Hyperkeratosis lenticularis perstans usually is asymptomatic but can present with mild pruritus or burning.3,5,13
The etiology and pathogenesis of HLP are unknown. Exposure to UV light has been implicated as an inciting factor14; however, reports of spontaneous resolution in the summer13 and upon treatment with psoralen plus UVA therapy15 make the role of UV light unclear. Furthermore, investigators disagree as to whether the primary pathogenic event in HLP is an inflammatory process or one of abnormal keratinization.1,3,7,10 Fernandez-Flores and Manjon16 suggested HLP is an inflammatory process with periods of exacerbations and remissions after finding mounds of parakeratosis with neutrophils arranged in different strata in the stratum corneum.
Histologically, compact hyperkeratosis usually is noted, often with associated parakeratosis, epidermal atrophy with thinning or absence of the granular layer, and a bandlike lymphohistiocytic infiltrate in the papillary dermis.1-3 Histopathologic differences between recent-onset versus longstanding lesions have been found, with old lesions lacking an inflammatory infiltrate.3 Furthermore, new lesions often show abnormalities in quantity and/or morphology of membrane-coating granules, also known as Odland bodies, in keratinocytes on electron microscopy,3,10,17 while old lesions do not.3 Odland bodies are involved in normal desquamation, leading some to speculate on their role in HLP.10 Currently, it is unclear whether abnormalities in these organelles cause the retention hyperkeratosis seen in HLP or if such abnormalities are a secondary phenomenon.3,17
There are questionable associations between HLP and diabetes mellitus type 2, hyperthyroidism, basal and squamous cell carcinomas of the skin, and gastrointestinal malignancy.4,9,18 Our patient had a history of basal cell carcinoma on the face, diet-controlled diabetes mellitus, and hypothyroidism. Given the high prevalence of these diseases in the general population, however, it is difficult to ascertain whether a true association with HLP exists.
While HLP can slowly progress to involve additional body sites, it is overall a benign condition that does not require treatment. Therapeutic options are based on case reports, with no single treatment showing a consistent response. From review of the literature, therapies that have been most effective include dermabrasion, excision,19 topical 5-fluorouracil,2,17,20 and oral retinoids.8 Hyperkeratosis lenticularis perstans generally is resistant to topical steroids, retinoids, and vitamin D3 analogs, although success with betamethasone dipropionate,5 isotretinoin
gel 0.05%,11 and calcipotriol have been reported.6 A case of HLP with clinical response to psoralen plus UVA therapy also has been described.15
The Diagnosis: Hyperkeratosis Lenticularis Perstans
A shave biopsy of a lesion on the right leg was performed. Histopathology revealed a discrete papule with overlying compact hyperkeratosis. There was parakeratosis with an absent granular layer and a lichenoid lymphocytic infiltrate within the papillary dermis (Figure). Given the clinical context, these changes were consistent with a diagnosis of hyperkeratosis lenticularis perstans (HLP), also known as Flegel disease.


The patient was started on tretinoin cream 0.1% nightly for 3 months and triamcinolone ointment 0.1% as needed for pruritus but showed no clinical response. Given the benign nature of the condition and because the lesions were asymptomatic, additional treatment options were not pursued.
Originally described by Flegel1 in 1958, HLP is a rare skin disorder commonly seen in white individuals with onset in the fourth or fifth decades of life.1,2 While most cases are sporadic,3-6 HLP also has been associated with autosomal dominant inheritance.7-10
Patients with HLP typically present with multiple 1- to 5-mm reddish-brown, hyperkeratotic, scaly papules that reveal a moist, erythematous base with pinpoint bleeding upon removal of the scale. Lesions usually are distributed symmetrically and most commonly present on the extensor surfaces of the lower legs and dorsal feet.1,2,7 Lesions also may appear on the extensor surfaces of the arms, pinna, periocular region, antecubital and popliteal fossae, and oral mucosa and also may present as pits on the palms and soles.2,4,7,8 Furthermore, unilateral and localized variants of HLP have been described.11,12 Hyperkeratosis lenticularis perstans usually is asymptomatic but can present with mild pruritus or burning.3,5,13
The etiology and pathogenesis of HLP are unknown. Exposure to UV light has been implicated as an inciting factor14; however, reports of spontaneous resolution in the summer13 and upon treatment with psoralen plus UVA therapy15 make the role of UV light unclear. Furthermore, investigators disagree as to whether the primary pathogenic event in HLP is an inflammatory process or one of abnormal keratinization.1,3,7,10 Fernandez-Flores and Manjon16 suggested HLP is an inflammatory process with periods of exacerbations and remissions after finding mounds of parakeratosis with neutrophils arranged in different strata in the stratum corneum.
Histologically, compact hyperkeratosis usually is noted, often with associated parakeratosis, epidermal atrophy with thinning or absence of the granular layer, and a bandlike lymphohistiocytic infiltrate in the papillary dermis.1-3 Histopathologic differences between recent-onset versus longstanding lesions have been found, with old lesions lacking an inflammatory infiltrate.3 Furthermore, new lesions often show abnormalities in quantity and/or morphology of membrane-coating granules, also known as Odland bodies, in keratinocytes on electron microscopy,3,10,17 while old lesions do not.3 Odland bodies are involved in normal desquamation, leading some to speculate on their role in HLP.10 Currently, it is unclear whether abnormalities in these organelles cause the retention hyperkeratosis seen in HLP or if such abnormalities are a secondary phenomenon.3,17
There are questionable associations between HLP and diabetes mellitus type 2, hyperthyroidism, basal and squamous cell carcinomas of the skin, and gastrointestinal malignancy.4,9,18 Our patient had a history of basal cell carcinoma on the face, diet-controlled diabetes mellitus, and hypothyroidism. Given the high prevalence of these diseases in the general population, however, it is difficult to ascertain whether a true association with HLP exists.
While HLP can slowly progress to involve additional body sites, it is overall a benign condition that does not require treatment. Therapeutic options are based on case reports, with no single treatment showing a consistent response. From review of the literature, therapies that have been most effective include dermabrasion, excision,19 topical 5-fluorouracil,2,17,20 and oral retinoids.8 Hyperkeratosis lenticularis perstans generally is resistant to topical steroids, retinoids, and vitamin D3 analogs, although success with betamethasone dipropionate,5 isotretinoin
gel 0.05%,11 and calcipotriol have been reported.6 A case of HLP with clinical response to psoralen plus UVA therapy also has been described.15
- Flegel H. Hyperkeratosis lenticularis perstans. Hautarzt. 1958;9:363-364.
- Pearson LH, Smith JG, Chalker DK. Hyperkeratosis lenticularis perstans (Flegel’s disease). J Am Acad Dermatol. 1987;16:190-195.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel’s disease). Am J Dermatopathol. 2006;28:122-126.
- Fernández-Crehuet P, Rodríguez-Rey E, Ríos-Martín JJ, et al. Hyperkeratosis lenticularis perstans, or Flegel disease, with palmoplantar involvement. Actas Dermosifiliogr. 2009;100:157-159.
- Sterneberg-Vos H, van Marion AM, Frank J, et al. Hyperkeratosis lenticularis perstans (Flegel’s disease)—successful treatment with topical corticosteroids. Int J Dermatol. 2008;47:38-41.
- Bayramgürler D, Apaydin R, Dökmeci S, et al. Flegel’s disease: treatment with topical calcipotriol. Clin Exp Dermatol. 2002;27:161-162.
- Price ML, Jones EW, MacDonald DM. A clinicopathological study of Flegel’s disease (hyperkeratosis lenticularis perstans). Br J Dermatol. 1987;116:681-691.
- Krishnan A, Kar S. Photoletter to the editor: hyperkeratosis lenticularis perstans (Flegel’s disease) with unusual clinical presentation. response to isotretinoin therapy. J Dermatol Case Rep. 2012;6:93-95.
- Beveridge GW, Langlands AO. Familial hyperkeratosis lenticularis perstans associated with tumours of the skin. Br J Dermatol. 1973;88:453-458.
- Frenk E, Tapernoux B. Hyperkeratosis lenticularis perstans (Flegel): a biological model for keratinization occurring in the absence of Odland bodies? Dermatologica. 1976;153:253-262.
- Miranda-Romero A, Sánchez Sambucety P, Bajo del Pozo C, et al. Unilateral hyperkeratosis lenticularis perstans (Flegel's disease). J Am Acad Dermatol. 1998;39:655-657.
- Gutiérrez MC, Hasson A, Arias MD, et al. Localized hyperkeratosis lenticularis perstans (Flegel's disease). Cutis. 1991;48:201-204.
- Fathy S, Azadeh B. Hyperkeratosis lenticularis perstans. Int J Dermatol. 1988;27:120-121.
- Rosdahl I, Rosen K. Hyperkeratosis lenticularis perstans: report on two cases. Acta Derm Venerol. 1985;65:562-564.
- Cooper SM, George S. Flegel's disease treated with psoralen ultraviolet A. Br J Dermatol. 2000;142:340-342.
- Fernandez-Flores A, Manjon JA. Morphological evidence of periodical exacerbation of hyperkeratosis lenticularis perstans. Acta Dermatovenerol Croat. 2009;17:16-19.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Ishibashi A, Tsuboi R, Fujita K. Familial hyperkeratosis lenticularis perstans. associated with cancers of the digestive organs. J Dermatol. 1984;11:407-409.
- Cunha Filho RR, Almeida Jr HL. Hyperkeratosis lenticularis perstans. An Bras Dermatol. 2011;86(4 suppl 1):S76-S77.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)—lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.
- Flegel H. Hyperkeratosis lenticularis perstans. Hautarzt. 1958;9:363-364.
- Pearson LH, Smith JG, Chalker DK. Hyperkeratosis lenticularis perstans (Flegel’s disease). J Am Acad Dermatol. 1987;16:190-195.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel’s disease). Am J Dermatopathol. 2006;28:122-126.
- Fernández-Crehuet P, Rodríguez-Rey E, Ríos-Martín JJ, et al. Hyperkeratosis lenticularis perstans, or Flegel disease, with palmoplantar involvement. Actas Dermosifiliogr. 2009;100:157-159.
- Sterneberg-Vos H, van Marion AM, Frank J, et al. Hyperkeratosis lenticularis perstans (Flegel’s disease)—successful treatment with topical corticosteroids. Int J Dermatol. 2008;47:38-41.
- Bayramgürler D, Apaydin R, Dökmeci S, et al. Flegel’s disease: treatment with topical calcipotriol. Clin Exp Dermatol. 2002;27:161-162.
- Price ML, Jones EW, MacDonald DM. A clinicopathological study of Flegel’s disease (hyperkeratosis lenticularis perstans). Br J Dermatol. 1987;116:681-691.
- Krishnan A, Kar S. Photoletter to the editor: hyperkeratosis lenticularis perstans (Flegel’s disease) with unusual clinical presentation. response to isotretinoin therapy. J Dermatol Case Rep. 2012;6:93-95.
- Beveridge GW, Langlands AO. Familial hyperkeratosis lenticularis perstans associated with tumours of the skin. Br J Dermatol. 1973;88:453-458.
- Frenk E, Tapernoux B. Hyperkeratosis lenticularis perstans (Flegel): a biological model for keratinization occurring in the absence of Odland bodies? Dermatologica. 1976;153:253-262.
- Miranda-Romero A, Sánchez Sambucety P, Bajo del Pozo C, et al. Unilateral hyperkeratosis lenticularis perstans (Flegel's disease). J Am Acad Dermatol. 1998;39:655-657.
- Gutiérrez MC, Hasson A, Arias MD, et al. Localized hyperkeratosis lenticularis perstans (Flegel's disease). Cutis. 1991;48:201-204.
- Fathy S, Azadeh B. Hyperkeratosis lenticularis perstans. Int J Dermatol. 1988;27:120-121.
- Rosdahl I, Rosen K. Hyperkeratosis lenticularis perstans: report on two cases. Acta Derm Venerol. 1985;65:562-564.
- Cooper SM, George S. Flegel's disease treated with psoralen ultraviolet A. Br J Dermatol. 2000;142:340-342.
- Fernandez-Flores A, Manjon JA. Morphological evidence of periodical exacerbation of hyperkeratosis lenticularis perstans. Acta Dermatovenerol Croat. 2009;17:16-19.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Ishibashi A, Tsuboi R, Fujita K. Familial hyperkeratosis lenticularis perstans. associated with cancers of the digestive organs. J Dermatol. 1984;11:407-409.
- Cunha Filho RR, Almeida Jr HL. Hyperkeratosis lenticularis perstans. An Bras Dermatol. 2011;86(4 suppl 1):S76-S77.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)—lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.

Holiday Trip Marred by Illness
ANSWER
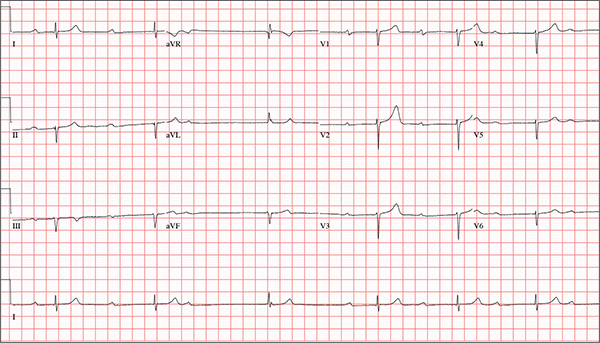
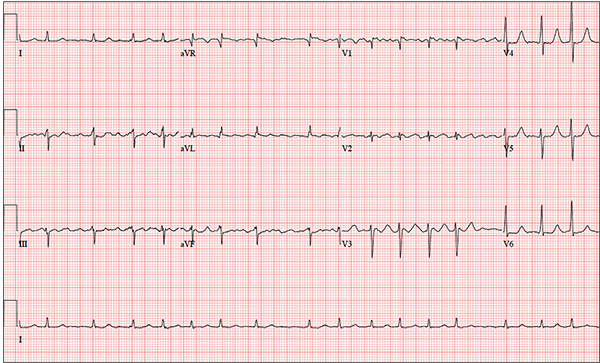
This ECG is representative of marked sinus bradycardia with second-degree atrioventricular block (Mobitz I) with an occasional junctional escape, left-axis deviation, and poor R-wave progression in the precordial leads.
Marked sinus bradycardia is evidenced by P waves that are regular except where expected, prior to the third QRS complex on the rhythm strip (lead 1).
Second-degree Mobitz I (Wenckebach) block is indicated by a gradual prolonging of the PR interval until there is loss of conduction from the atria to the ventricle (following the third P wave). Careful inspection of the third QRS complex shows a slight difference in the normally conducted sinus beat, indicative of a junctional escape beat. Left-axis deviation entails an R-wave axis of –78°.
Finally, there is poor R-wave progression in the precordial leads, including the lateral leads.
Although Mobitz I block is not an indication for pacemaker placement, symptomatic bradycardia is. The patient underwent implantation of a dual-chamber permanent pacemaker, with complete resolution of symptoms.
ANSWER
This ECG is representative of marked sinus bradycardia with second-degree atrioventricular block (Mobitz I) with an occasional junctional escape, left-axis deviation, and poor R-wave progression in the precordial leads.
Marked sinus bradycardia is evidenced by P waves that are regular except where expected, prior to the third QRS complex on the rhythm strip (lead 1).
Second-degree Mobitz I (Wenckebach) block is indicated by a gradual prolonging of the PR interval until there is loss of conduction from the atria to the ventricle (following the third P wave). Careful inspection of the third QRS complex shows a slight difference in the normally conducted sinus beat, indicative of a junctional escape beat. Left-axis deviation entails an R-wave axis of –78°.
Finally, there is poor R-wave progression in the precordial leads, including the lateral leads.
Although Mobitz I block is not an indication for pacemaker placement, symptomatic bradycardia is. The patient underwent implantation of a dual-chamber permanent pacemaker, with complete resolution of symptoms.
ANSWER
This ECG is representative of marked sinus bradycardia with second-degree atrioventricular block (Mobitz I) with an occasional junctional escape, left-axis deviation, and poor R-wave progression in the precordial leads.
Marked sinus bradycardia is evidenced by P waves that are regular except where expected, prior to the third QRS complex on the rhythm strip (lead 1).
Second-degree Mobitz I (Wenckebach) block is indicated by a gradual prolonging of the PR interval until there is loss of conduction from the atria to the ventricle (following the third P wave). Careful inspection of the third QRS complex shows a slight difference in the normally conducted sinus beat, indicative of a junctional escape beat. Left-axis deviation entails an R-wave axis of –78°.
Finally, there is poor R-wave progression in the precordial leads, including the lateral leads.
Although Mobitz I block is not an indication for pacemaker placement, symptomatic bradycardia is. The patient underwent implantation of a dual-chamber permanent pacemaker, with complete resolution of symptoms.
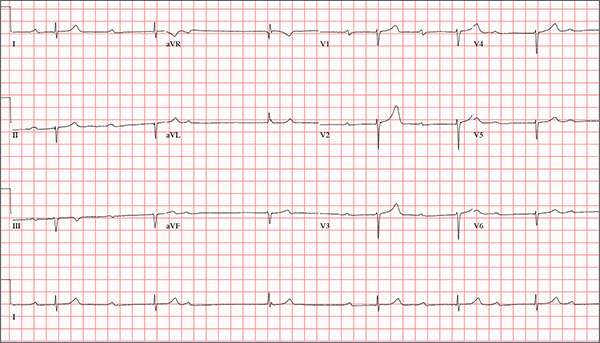
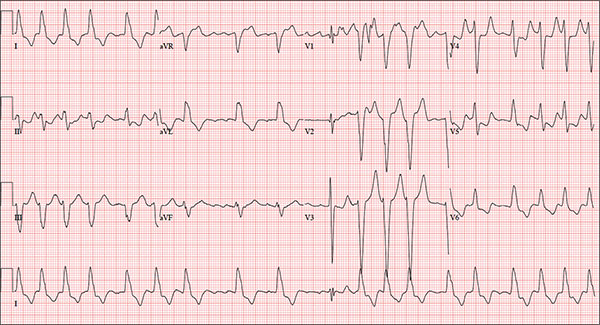
While visiting family over the holidays, an 84-year-old man is (plaintively) informed by his wife that he appears unwell; she suspects he has the flu. The patient’s son, whom they are visiting, learns through conversation that his father has been feeling very tired and lethargic and becomes dizzy if he stands too quickly. The son, concerned for his father’s well-being, brings him to your clinic in the hope of obtaining a prescription for antibiotics. According to the patient, his symptoms, which have waxed and waned for several weeks, have become constant in the past week. He denies fever, cough, nausea, and vomiting, as well as chest pain, shortness of breath, palpitations, and lower extremity swelling. He reports that he has not recently changed his medication regimen and, aside from this current visit, has not traveled anywhere; he reiterates that his symptoms started prior to this trip. Medical history is remarkable for hypertension, peripheral atherosclerosis, osteoarthritis, gout, and pneumonia. Surgical history is remarkable for appendectomy, cholecystectomy, and removal of multiple lipomas from the patient’s upper extremities. Via the family history, you learn that the man’s father had a myocardial infarction and died of complications from a stroke at age 92 and his mother died of complications of diabetes at age 87. The patient is married, with three sons and one daughter, all of whom are in good health. He is a retired owner of a hardware store. He has never smoked or used recreational drugs and says he rarely drinks alcohol. The patient’s medication list includes metoprolol, atorvastatin, furosemide, and a daily baby aspirin. He is allergic to sulfa, which causes shortness of breath and wheezing. Review of systems reveals that he wears corrective lenses and hearing aids. He walks with a cane due to pain in both knees but is not dependent on it. He denies constitutional symptoms. A review of cardiovascular, respiratory, gastrointestinal, urologic, neurologic, and integumentary systems is noncontributory. Chest x-ray and laboratory testing, including complete blood count and chemistry panel, yield normal results. Vital signs include a blood pressure of 162/92 mm Hg; pulse, 40 beats/min; respiratory rate, 14 breaths/min; temperature, 99.2°F; and O2 saturation, 97% on room air. Pertinent physical findings include clear lung fields bilaterally, no evidence of jugular venous distention, and a heart rate of 40 beats/min that is regular and without evidence of a murmur or rub. There are well-healed scars on the abdomen and no evidence of organomegaly. Peripheral pulses are diminished but present bilaterally in both lower extremities. The neurologic exam is grossly intact, and the patient is alert, cooperative, and cognizant. Your concern about the patient’s heart rate prompts you to order an ECG. It reveals a ventricular rate of 38 beats/min; no measurable PR interval; QRS duration, 78 ms; QT/QTc interval, 434/345 ms; P axis, 25°; R axis, –78°; and T axis, 13°. What is your interpretation of this ECG?
Friendly Advice Goes Awry
ANSWER
The correct interpretation is coarse atrial fibrillation with a rapid ventricular response and left-axis deviation.
Coarse atrial fibrillation is evidenced by the irregularly irregular rhythm with a normal QRS duration and flutter/fibrillation waves arising from the atria. Rapid ventricular response is defined as a ventricular response > 100 beats/min (seen in this case). Finally, an R-wave axis between –30° and –90° is indicative of left-axis deviation.
Correcting the patient’s hypokalemia and hypomagnesemia resulted in a return to normal sinus rhythm. At one-year follow-up, he had had no further episodes of atrial fibrillation.
ANSWER
The correct interpretation is coarse atrial fibrillation with a rapid ventricular response and left-axis deviation.
Coarse atrial fibrillation is evidenced by the irregularly irregular rhythm with a normal QRS duration and flutter/fibrillation waves arising from the atria. Rapid ventricular response is defined as a ventricular response > 100 beats/min (seen in this case). Finally, an R-wave axis between –30° and –90° is indicative of left-axis deviation.
Correcting the patient’s hypokalemia and hypomagnesemia resulted in a return to normal sinus rhythm. At one-year follow-up, he had had no further episodes of atrial fibrillation.
ANSWER
The correct interpretation is coarse atrial fibrillation with a rapid ventricular response and left-axis deviation.
Coarse atrial fibrillation is evidenced by the irregularly irregular rhythm with a normal QRS duration and flutter/fibrillation waves arising from the atria. Rapid ventricular response is defined as a ventricular response > 100 beats/min (seen in this case). Finally, an R-wave axis between –30° and –90° is indicative of left-axis deviation.
Correcting the patient’s hypokalemia and hypomagnesemia resulted in a return to normal sinus rhythm. At one-year follow-up, he had had no further episodes of atrial fibrillation.
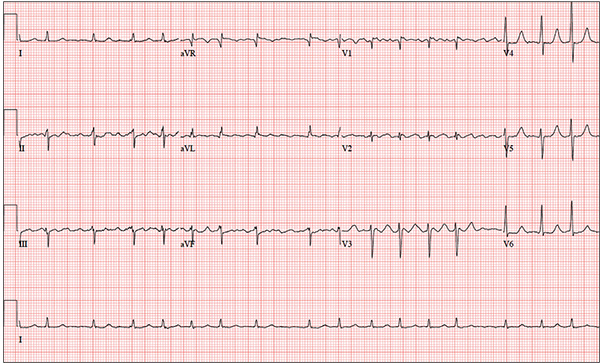
You have been following a 57-year-old man for gastroesophageal reflux disease (GERD). He presents for routine follow-up stating that his reflux has subsided; you presume this is a result of the 14-day course of a proton pump inhibitor that you prescribed. However, the patient confesses that, for about three months, he’s taken his omeprazole at twice the dose—because a friend told him that OTC medications are half the dose of the prescription versions. His primary concern today is that his heart has started flip-flopping in his chest for brief periods at bedtime. The symptoms typically last for 30 to 60 minutes and recur when he wakes in the morning—particularly if he is startled by his alarm clock. They began approximately a week ago, and he reports that they start and stop abruptly. The patient denies chest pain, dyspnea, and syncope or near-syncope, but he does note that it feels like something is “sticking in his throat.” His active medical problems include GERD, hypertension, and obesity. Surgical history is remarkable for repair of bilateral ankle fractures and a left femur fracture sustained in a motorcycle accident six years ago. Current medications include omeprazole, metoprolol, furosemide, and potassium chloride. He says he ran out of his potassium about a month ago and hasn’t refilled it yet. He also reports that he hasn’t taken his metoprolol in more than six months, because it makes him lethargic. He has no known drug allergies. The patient, who works as a welder, is married and has one son. He drinks approximately one six-pack of beer per week and smokes half a pack of cigarettes per day. He uses marijuana recreationally once or twice a month but denies use of any other illicit or naturopathic drugs. Review of systems is remarkable for a smoker’s cough, which clears with coughing. He also states his right eye twitches uncontrollably, and he feels weak and washed out. He denies nausea, vomiting, diarrhea, and constipation. While you are conducting the review, he states, “It just started again.” You immediately check the patient’s pulse; it is 110 beats/min and irregular. Additional vital statistics include a blood pressure of 124/74 mm Hg; respiratory rate, 14 breaths/min; O2 saturation, 96% on room air; and temperature, 98.4°F. His weight is 245 lb and his height, 72 in. Pertinent physical findings include inspiratory and expiratory crackles that change with coughing, an irregularly irregular rhythm without evidence of a murmur or rub, a soft abdomen, and no evidence of jugular venous distention or peripheral edema. Laboratory values are within normal limits, with the exception of the potassium (2.8 mmol/L; normal range, 3.6-5.2 mmol/L) and magnesium (0.9 mg/dL; normal range, 1.8-2.6 mg/dL). An ECG reveals a ventricular rate of 108 beats/min; PR interval, not measured; QRS duration, 78 ms; QT/QTc interval, 352/471 ms; no P axis; R axis, –64°; and T axis, –58°. What is your interpretation of this ECG?
Secondary Survey of Trauma Patient
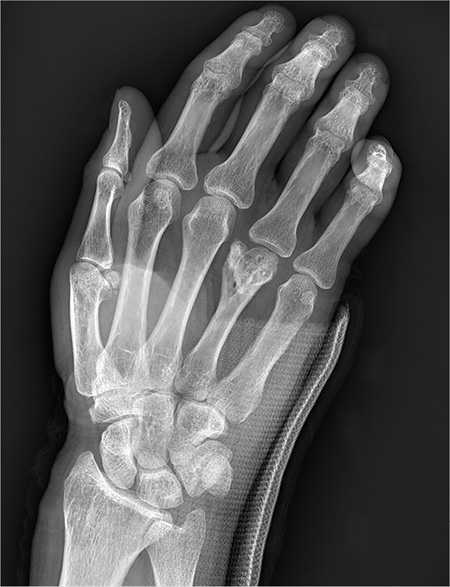
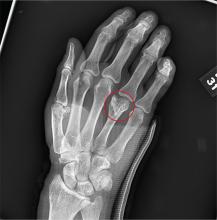
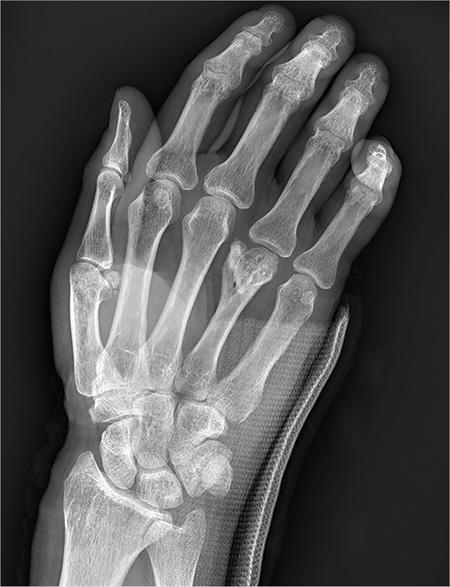
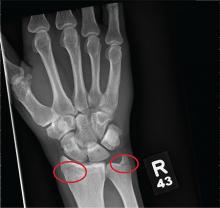
You are assisting in the evaluation and management of a trauma patient who was brought to your facility earlier today, following a motor vehicle collision. He is estimated to be in his 30s and is presumed to have been ejected, as he was found outside the vehicle. He was intubated in the field, and primary survey and resuscitation have been completed. History is otherwise unknown. The patient’s vital signs are currently stable. As you perform your secondary survey, you note that his right hand and wrist appear to be moderately swollen. He has been placed in a splint. You order a radiograph of the hand. What is your impression?
Boy Wrestles With Scalp Problem
ANSWER
The correct answer is all of the above (choice “e”). This particular form of tinea capitis is called black dot tinea capitis (BDTC), a somewhat unusual dermatophytosis (superficial fungal infection) that mostly affects children. The causative organisms are anthropophilic—that is, acquired from human sources, such as other children, during activities that involve skin-to-skin contact (eg, sports).
The vast majority of these organisms are from the Trichophyton family, such as T tonsurans or T violaceum. They invade the hair shaft itself, leaving the hard covering (the cuticle) intact. The black dots represent the tips of broken-off hairs, themselves full of fungal elements, seen in the photomicrograph. The term endothrix is given to this kind of fungal infection, in which the organisms are contained within the hair shaft, which, as a result, becomes brittle and breaks off. This is a relatively common type of infection.
A more unusual form of tinea capitis is caused by zoophilic organisms, such as Microsporum canis (from dogs and cats), Microsporum gypseum (pigs or cows), or T equinum (horses). These infect the external surface of the hair shaft, breaking down the cuticle. This allows for identification of the infection by Wood’s lamp, which causes the affected area to turn a yellowish color. These infections also tend to provoke a more brisk inflammatory response in the victim and are more difficult to treat.
Diagnosis can be made from a combination of clinical findings, KOH prep (as in this patient), and/or fungal culture.
Treatment can entail griseofulvin or terbinafine; the case patient was treated with a two-month course of the latter (125 mg/d). Topical treatment is of limited usefulness.
ANSWER
The correct answer is all of the above (choice “e”). This particular form of tinea capitis is called black dot tinea capitis (BDTC), a somewhat unusual dermatophytosis (superficial fungal infection) that mostly affects children. The causative organisms are anthropophilic—that is, acquired from human sources, such as other children, during activities that involve skin-to-skin contact (eg, sports).
The vast majority of these organisms are from the Trichophyton family, such as T tonsurans or T violaceum. They invade the hair shaft itself, leaving the hard covering (the cuticle) intact. The black dots represent the tips of broken-off hairs, themselves full of fungal elements, seen in the photomicrograph. The term endothrix is given to this kind of fungal infection, in which the organisms are contained within the hair shaft, which, as a result, becomes brittle and breaks off. This is a relatively common type of infection.
A more unusual form of tinea capitis is caused by zoophilic organisms, such as Microsporum canis (from dogs and cats), Microsporum gypseum (pigs or cows), or T equinum (horses). These infect the external surface of the hair shaft, breaking down the cuticle. This allows for identification of the infection by Wood’s lamp, which causes the affected area to turn a yellowish color. These infections also tend to provoke a more brisk inflammatory response in the victim and are more difficult to treat.
Diagnosis can be made from a combination of clinical findings, KOH prep (as in this patient), and/or fungal culture.
Treatment can entail griseofulvin or terbinafine; the case patient was treated with a two-month course of the latter (125 mg/d). Topical treatment is of limited usefulness.
ANSWER
The correct answer is all of the above (choice “e”). This particular form of tinea capitis is called black dot tinea capitis (BDTC), a somewhat unusual dermatophytosis (superficial fungal infection) that mostly affects children. The causative organisms are anthropophilic—that is, acquired from human sources, such as other children, during activities that involve skin-to-skin contact (eg, sports).
The vast majority of these organisms are from the Trichophyton family, such as T tonsurans or T violaceum. They invade the hair shaft itself, leaving the hard covering (the cuticle) intact. The black dots represent the tips of broken-off hairs, themselves full of fungal elements, seen in the photomicrograph. The term endothrix is given to this kind of fungal infection, in which the organisms are contained within the hair shaft, which, as a result, becomes brittle and breaks off. This is a relatively common type of infection.
A more unusual form of tinea capitis is caused by zoophilic organisms, such as Microsporum canis (from dogs and cats), Microsporum gypseum (pigs or cows), or T equinum (horses). These infect the external surface of the hair shaft, breaking down the cuticle. This allows for identification of the infection by Wood’s lamp, which causes the affected area to turn a yellowish color. These infections also tend to provoke a more brisk inflammatory response in the victim and are more difficult to treat.
Diagnosis can be made from a combination of clinical findings, KOH prep (as in this patient), and/or fungal culture.
Treatment can entail griseofulvin or terbinafine; the case patient was treated with a two-month course of the latter (125 mg/d). Topical treatment is of limited usefulness.
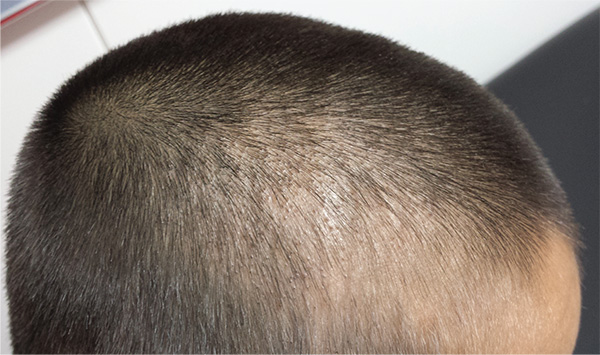
An 8-year-old boy is brought in by his mother for evaluation of a scalp condition that manifested several months ago. The first sign was hair loss in several locations, mostly on the sides, followed in a few weeks by faint scaling. As more hair came out, the scaling in the affected locations reduced, but uniformly spaced black dots began to appear. There has never been any redness. The boy was taken to a local urgent care center, where he was diagnosed with probable “ringworm” and given a prescription for topical antifungal cream (clotrimazole, bid application). This failed to help, so the family sought an appointment with dermatology. Additional history-taking reveals that the boy noticed the problem within a few weeks of starting wrestling at school. Examination of the scalp reveals several round areas of partial and uniform hair loss, averaging 3 cm in diameter. No redness or edema is seen, and only very faint scaling is observed on the surface of the skin. Distinct black dots are uniformly distributed within the lesions. A vigorous scrape of one of the areas is processed with potassium hydroxide 10% and examined under 10x magnification. The black dots are found to be broken-off hairs filled with hundreds of tiny round spheres. Several hyphae are seen adjacent to the hairs. Palpation reveals adenopathy in the adjacent nuchal scalp and neck. Wood’s lamp examination fails to highlight these areas.
Revenge of the Deer Stand
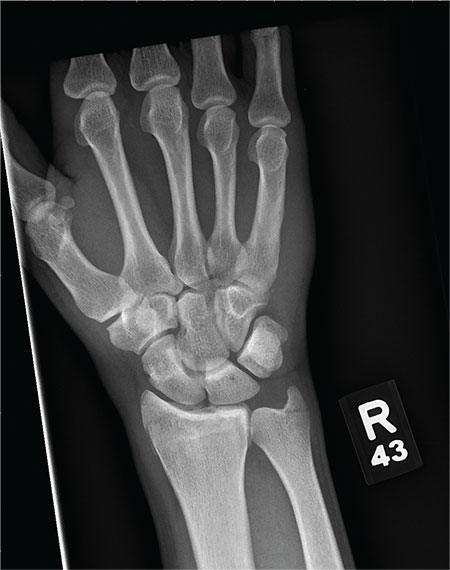
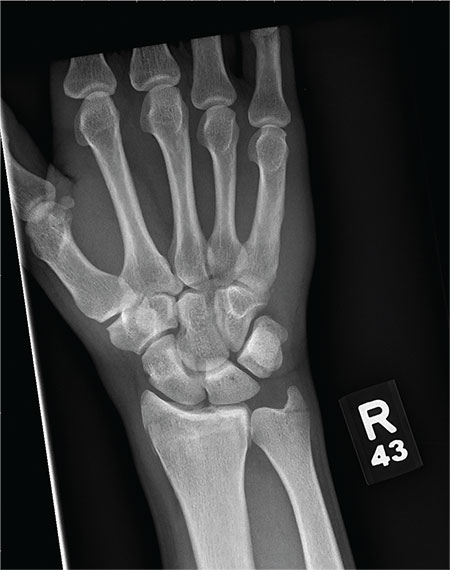
As you begin your shift, knowing deer season has started in your area, you wonder how long it will be before you see your first deer stand casualty of the day. With your first patient, you wonder no more: A 30-year-old man presents for evaluation of right wrist pain after falling from his deer stand. He says one of the straps holding the stand broke, causing him to fall forward and land on his outstretched hands. His medical history is unremarkable. Inspection of the right wrist shows no obvious deformity. No significant swelling is present. There is decreased range of motion and localized tenderness over the radius and ulna. Good pulses and capillary refill are noted. You obtain a radiograph of the wrist. What is your impression?
“Spry” Woman Reports Rapid Heart Rate
ANSWER
The correct interpretation includes atrial fibrillation with a rapid ventricular response and aberrantly conducted complexes, left axis deviation, and a left bundle branch block.
Atrial fibrillation is evidenced by the irregularly irregular heart rhythm without a measurable PR interval, and the rapid ventricular response is indicated by a ventricular rate > 100 beats/min.
Aberrant conduction, caused by conduction delay down the His-Purkinje system, is evidenced by the wide QRS complexes with a normally conducted beat (see first beat in leads V1-V3). Criteria for left axis deviation include an R axis between –30° and –90°, and left bundle branch block criteria include a QRS duration > 120 ms, a dominant S wave in V1, and broad monophasic R waves in leads I, aVL, and V5-V6.
ANSWER
The correct interpretation includes atrial fibrillation with a rapid ventricular response and aberrantly conducted complexes, left axis deviation, and a left bundle branch block.
Atrial fibrillation is evidenced by the irregularly irregular heart rhythm without a measurable PR interval, and the rapid ventricular response is indicated by a ventricular rate > 100 beats/min.
Aberrant conduction, caused by conduction delay down the His-Purkinje system, is evidenced by the wide QRS complexes with a normally conducted beat (see first beat in leads V1-V3). Criteria for left axis deviation include an R axis between –30° and –90°, and left bundle branch block criteria include a QRS duration > 120 ms, a dominant S wave in V1, and broad monophasic R waves in leads I, aVL, and V5-V6.
ANSWER
The correct interpretation includes atrial fibrillation with a rapid ventricular response and aberrantly conducted complexes, left axis deviation, and a left bundle branch block.
Atrial fibrillation is evidenced by the irregularly irregular heart rhythm without a measurable PR interval, and the rapid ventricular response is indicated by a ventricular rate > 100 beats/min.
Aberrant conduction, caused by conduction delay down the His-Purkinje system, is evidenced by the wide QRS complexes with a normally conducted beat (see first beat in leads V1-V3). Criteria for left axis deviation include an R axis between –30° and –90°, and left bundle branch block criteria include a QRS duration > 120 ms, a dominant S wave in V1, and broad monophasic R waves in leads I, aVL, and V5-V6.
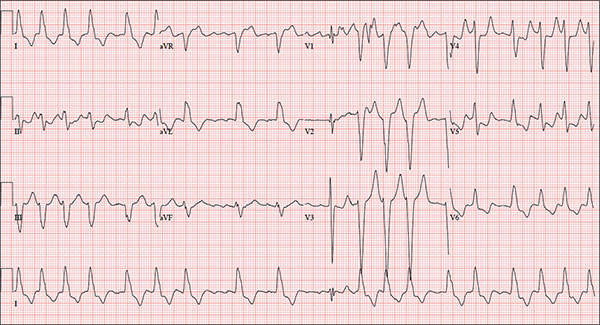
An 84-year-old woman who recently relocated to be closer to her children presents to your practice as a new patient. She is a resident of an assisted living facility near your office, and although she has no specific complaints, she does report that her home health nurse observed a rapid heart rate and recommended she get it checked. A comprehensive medical history—provided by the patient, her daughter, and the aforementioned nurse—includes hypertension, paroxysmal atrial fibrillation, hypothyroidism, and type 2 diabetes. She has taken medication for these diagnoses for more than 30 years. Surgical history is remarkable for cholecystectomy, appendectomy, and abdominal hysterectomy and oophorectomy, all of which were performed in the 1970s. Her current medication list—confirmed by the assisted living facility—includes furosemide, glyburide, metoprolol, potassium, and levothyroxine. She has not missed any doses. She is allergic to sulfa. The patient, a retired teacher, has never smoked, but she does “enjoy” one martini at dinner on a regular basis. She is widowed; her two daughters and four sons are all alive and well. The review of systems is remarkable for corrective lenses, bilateral hearing aids, and chronic joint pain. The patient does not routinely weigh herself but thinks, based on the fit of her clothes, that she may have gained some weight. She denies constitutional symptoms and shortness of breath. She thinks she may have a urinary tract infection, as she’s had burning with urination for several days, but says this is beginning to improve. Physical exam reveals a blood pressure of 168/90 mm Hg; pulse, 106 beats/min; temperature, 98.4° F; and O2 saturation, 94% on room air. Her weight is 132 lb and her height, 60 in. She is alert and quite spry, with a lot of energy. She wears glasses and bilateral hearing aids. Jugular distention is present to the angle of the jaw. There is no thyromegaly. The pulmonary exam is remarkable for crackles in both lung bases. The heart rhythm is irregularly irregular at a rate of 110 beats/min, and a grade II/VI murmur of mitral regurgitation is heard at the left lower sternal border. The abdomen is soft and nontender, with multiple surgical scars. The lower extremities are remarkable for 2+ pitting edema bilaterally to the level of the mid-calf. Osteoarthritic changes are present in both hands. The neurologic exam is grossly intact. An ECG reveals a ventricular rate of 110 beats/min; PR interval, not measured; QRS duration, 144 ms; QT/QTc interval, 298/403 ms; no P axis; R axis, –36°; and T axis, 169°. What is your interpretation of this ECG?
Painful Lesion Hasn’t Responded to Antibiotics
ANSWER
The correct answer is erythema nodosum (EN; choice “c”), a reactive form of septal panniculitis with many potential triggers.
Erythema induratum (choice “a”) is a manifestation of lobular panniculitis, which affects the fat lobules but not the septae. It too has numerous triggers, but it tends to manifest with more discrete nodules, which eventually open and drain. It is far less common than EN.
Urticaria (choice “b”), also known as hives, presents as itchy, stingy wheals that are typically evanescent (eg, they appear suddenly, within seconds, and disappear within hours). Despite the itching and stinging, urticaria rarely hurts when it presents on the skin.
Erysipelas (choice “d”) is a superficial form of skin infection. More superficial than cellulitis, it usually is caused by a member of the Streptococcus family. It has a bright red appearance and sharply demarcated margins, with a peau d’orange (dimpled, like an orange peel) effect on its surface. It is acute in origin and responds readily to most common antibiotics.
DISCUSSION
Erythema nodosum, a reactive process involving fibrous septae that support and separate subcutaneous adipocytes, is notable for the complete lack of epidermal change (eg, scaling, broken skin, pointing, draining). The incidence is about 2 in 10,000 population, with women outnumbering men at a rate of 4:1 and the 18-to-34 age-group most affected. The anterior leg is involved in the vast majority of cases.
EN often starts with flulike symptoms, followed by the appearance of discrete, bright red nodules, measuring 2 to 4 cm, on the anterior legs; these darken and coalesce over a period of seven to 10 weeks. New lesions can continue to appear for up to six weeks. As they progress, the lesions often become ecchymotic. Idiopathic cases (at least 20%) can last months.
Notable triggers include Crohn disease flares and use of drugs such as sulfa, gold salts, and oral contraceptives. Several infections have been identified as triggers, including strep, mycoplasma, and campylobacter, as well as deep fungal infections (histoplasmosis, blastomycosis, coccidioidomycosis, and sporotrichosis). More unusual causes include pregnancy and diseases such as sarcoidosis, tuberculosis, Behçet disease, and leukemia/lymphoma.
The diagnostic workup for EN includes punch biopsy of deep adipose tissue and throat culture, and when indicated, ASO titer (if strep is suspected) and chest films (to rule out tuberculosis and sarcoid). In most cases, the diagnosis can be made on clinical grounds alone, although the triggering entity may be difficult to identify. The identification and elimination of the underlying trigger is nonetheless crucial for diagnosis and treatment.
That issue aside, most cases of EN resolve with minimal treatment; this can include the use of NSAIDs and elevation of the limbs when possible. In this particular case, the intense pain the patient was experiencing called for stronger therapy (ibuprofen 800 mg tid, plus a two-week taper of prednisone 40 mg). She responded quite well, and the problem was almost totally resolved at a follow-up visit several weeks later.
In the absence of findings to the contrary, it is likely that the trigger for this patient’s EN was strep, given the timing of its manifestation after a sore throat.
ANSWER
The correct answer is erythema nodosum (EN; choice “c”), a reactive form of septal panniculitis with many potential triggers.
Erythema induratum (choice “a”) is a manifestation of lobular panniculitis, which affects the fat lobules but not the septae. It too has numerous triggers, but it tends to manifest with more discrete nodules, which eventually open and drain. It is far less common than EN.
Urticaria (choice “b”), also known as hives, presents as itchy, stingy wheals that are typically evanescent (eg, they appear suddenly, within seconds, and disappear within hours). Despite the itching and stinging, urticaria rarely hurts when it presents on the skin.
Erysipelas (choice “d”) is a superficial form of skin infection. More superficial than cellulitis, it usually is caused by a member of the Streptococcus family. It has a bright red appearance and sharply demarcated margins, with a peau d’orange (dimpled, like an orange peel) effect on its surface. It is acute in origin and responds readily to most common antibiotics.
DISCUSSION
Erythema nodosum, a reactive process involving fibrous septae that support and separate subcutaneous adipocytes, is notable for the complete lack of epidermal change (eg, scaling, broken skin, pointing, draining). The incidence is about 2 in 10,000 population, with women outnumbering men at a rate of 4:1 and the 18-to-34 age-group most affected. The anterior leg is involved in the vast majority of cases.
EN often starts with flulike symptoms, followed by the appearance of discrete, bright red nodules, measuring 2 to 4 cm, on the anterior legs; these darken and coalesce over a period of seven to 10 weeks. New lesions can continue to appear for up to six weeks. As they progress, the lesions often become ecchymotic. Idiopathic cases (at least 20%) can last months.
Notable triggers include Crohn disease flares and use of drugs such as sulfa, gold salts, and oral contraceptives. Several infections have been identified as triggers, including strep, mycoplasma, and campylobacter, as well as deep fungal infections (histoplasmosis, blastomycosis, coccidioidomycosis, and sporotrichosis). More unusual causes include pregnancy and diseases such as sarcoidosis, tuberculosis, Behçet disease, and leukemia/lymphoma.
The diagnostic workup for EN includes punch biopsy of deep adipose tissue and throat culture, and when indicated, ASO titer (if strep is suspected) and chest films (to rule out tuberculosis and sarcoid). In most cases, the diagnosis can be made on clinical grounds alone, although the triggering entity may be difficult to identify. The identification and elimination of the underlying trigger is nonetheless crucial for diagnosis and treatment.
That issue aside, most cases of EN resolve with minimal treatment; this can include the use of NSAIDs and elevation of the limbs when possible. In this particular case, the intense pain the patient was experiencing called for stronger therapy (ibuprofen 800 mg tid, plus a two-week taper of prednisone 40 mg). She responded quite well, and the problem was almost totally resolved at a follow-up visit several weeks later.
In the absence of findings to the contrary, it is likely that the trigger for this patient’s EN was strep, given the timing of its manifestation after a sore throat.
ANSWER
The correct answer is erythema nodosum (EN; choice “c”), a reactive form of septal panniculitis with many potential triggers.
Erythema induratum (choice “a”) is a manifestation of lobular panniculitis, which affects the fat lobules but not the septae. It too has numerous triggers, but it tends to manifest with more discrete nodules, which eventually open and drain. It is far less common than EN.
Urticaria (choice “b”), also known as hives, presents as itchy, stingy wheals that are typically evanescent (eg, they appear suddenly, within seconds, and disappear within hours). Despite the itching and stinging, urticaria rarely hurts when it presents on the skin.
Erysipelas (choice “d”) is a superficial form of skin infection. More superficial than cellulitis, it usually is caused by a member of the Streptococcus family. It has a bright red appearance and sharply demarcated margins, with a peau d’orange (dimpled, like an orange peel) effect on its surface. It is acute in origin and responds readily to most common antibiotics.
DISCUSSION
Erythema nodosum, a reactive process involving fibrous septae that support and separate subcutaneous adipocytes, is notable for the complete lack of epidermal change (eg, scaling, broken skin, pointing, draining). The incidence is about 2 in 10,000 population, with women outnumbering men at a rate of 4:1 and the 18-to-34 age-group most affected. The anterior leg is involved in the vast majority of cases.
EN often starts with flulike symptoms, followed by the appearance of discrete, bright red nodules, measuring 2 to 4 cm, on the anterior legs; these darken and coalesce over a period of seven to 10 weeks. New lesions can continue to appear for up to six weeks. As they progress, the lesions often become ecchymotic. Idiopathic cases (at least 20%) can last months.
Notable triggers include Crohn disease flares and use of drugs such as sulfa, gold salts, and oral contraceptives. Several infections have been identified as triggers, including strep, mycoplasma, and campylobacter, as well as deep fungal infections (histoplasmosis, blastomycosis, coccidioidomycosis, and sporotrichosis). More unusual causes include pregnancy and diseases such as sarcoidosis, tuberculosis, Behçet disease, and leukemia/lymphoma.
The diagnostic workup for EN includes punch biopsy of deep adipose tissue and throat culture, and when indicated, ASO titer (if strep is suspected) and chest films (to rule out tuberculosis and sarcoid). In most cases, the diagnosis can be made on clinical grounds alone, although the triggering entity may be difficult to identify. The identification and elimination of the underlying trigger is nonetheless crucial for diagnosis and treatment.
That issue aside, most cases of EN resolve with minimal treatment; this can include the use of NSAIDs and elevation of the limbs when possible. In this particular case, the intense pain the patient was experiencing called for stronger therapy (ibuprofen 800 mg tid, plus a two-week taper of prednisone 40 mg). She responded quite well, and the problem was almost totally resolved at a follow-up visit several weeks later.
In the absence of findings to the contrary, it is likely that the trigger for this patient’s EN was strep, given the timing of its manifestation after a sore throat.
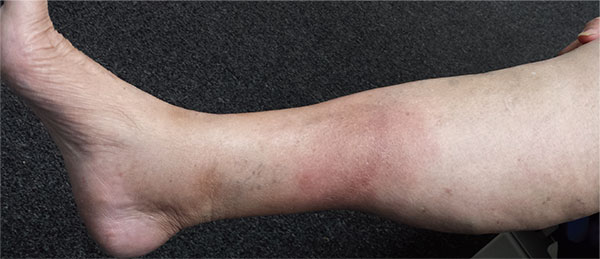
A 57-year-old woman is referred to dermatology for “cellulitis” that has persisted despite several courses of oral antibiotics (including cephalexin and trimethoprim/sulfamethoxazole). She denies taking any other medications and has no significant medical history. She states that the problem manifested as discrete red nodules, which eventually coalesced into a single large patch. At the time, she had just recovered from a sore throat and still felt a bit ill, although she denies cough, fever, and shortness of breath. Examination reveals a large (12 x 14 cm) red edematous plaque in the skin over her right anterior tibia. The deep intradermal and subdermal edema is exquisitely tender to touch, considerably warmer than the surrounding skin, and highly blanchable. No other changes are noted on the epidermal surface. A deep 5-mm punch biopsy is performed. Results show a dense lymphohistiocytic infiltrate in the pannicular septae.