User login
Update on Sinusitis
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
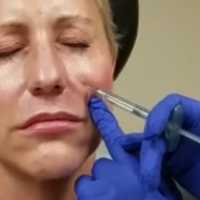
Juvéderm Voluma for Cheek Rejuvenation



Tatiana Falcone, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Andrew G. Herzog, MD
Thapanee Somboon, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Justin Gover
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MACRA Monday: Depression screening
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #134: Preventive Care and Screening: Screening for Depression and Follow-Up Plan
This measure aims to capture the percentage of patients aged 12 years and older who have been screened for depression and have a documented follow-up plan of care, if appropriate.
What you need to do: Use an age-appropriate, standardized tool to screen patients for depression during the visit. If they screen positive, develop a follow-up plan during the visit and document it.
Eligible cases include patients who were aged 12 years or older on the date of the encounter and have a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 92625, 96116, 96118, 96150, 96151, 97165, 97166, 97167, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, G0101, G0402, G0438, G0439, G0444.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8431 indicates that depression screening was positive and a follow-up plan was documented, while G8510 indicates that the depression screening was negative and a follow-up plan is not required. Use exclusion code G9717 if the patient has an active diagnosis of depression for bipolar disorder.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #134: Preventive Care and Screening: Screening for Depression and Follow-Up Plan
This measure aims to capture the percentage of patients aged 12 years and older who have been screened for depression and have a documented follow-up plan of care, if appropriate.
What you need to do: Use an age-appropriate, standardized tool to screen patients for depression during the visit. If they screen positive, develop a follow-up plan during the visit and document it.
Eligible cases include patients who were aged 12 years or older on the date of the encounter and have a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 92625, 96116, 96118, 96150, 96151, 97165, 97166, 97167, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, G0101, G0402, G0438, G0439, G0444.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8431 indicates that depression screening was positive and a follow-up plan was documented, while G8510 indicates that the depression screening was negative and a follow-up plan is not required. Use exclusion code G9717 if the patient has an active diagnosis of depression for bipolar disorder.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #134: Preventive Care and Screening: Screening for Depression and Follow-Up Plan
This measure aims to capture the percentage of patients aged 12 years and older who have been screened for depression and have a documented follow-up plan of care, if appropriate.
What you need to do: Use an age-appropriate, standardized tool to screen patients for depression during the visit. If they screen positive, develop a follow-up plan during the visit and document it.
Eligible cases include patients who were aged 12 years or older on the date of the encounter and have a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 92625, 96116, 96118, 96150, 96151, 97165, 97166, 97167, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, G0101, G0402, G0438, G0439, G0444.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8431 indicates that depression screening was positive and a follow-up plan was documented, while G8510 indicates that the depression screening was negative and a follow-up plan is not required. Use exclusion code G9717 if the patient has an active diagnosis of depression for bipolar disorder.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
Differentiate Acute Bronchitis and Community Acquired Pneumonia
Preview of Best of Acne: 2017
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO - New lymphoma drug approvals: Clinical use, future directions
ATLANTA – 2017 was a banner year for the approval of new drugs to treat hematologic disorders.
At a special interest session at the annual meeting of American Society of Hematology, representatives from the Food and Drug Administration joined forces with clinicians to discuss the use of the newly approved treatments in the real-world setting.
In this video interview,
“This is extremely exciting,” she said regarding the pace of new approvals for hematologic malignancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Axicabtagene ciloleucel, a CAR T-cell product approved in October for the treatment of relapsed/refractory large B-cell lymphoma in adults, is particularly interesting, she said.
“The data shows that if you look at a population of diffuse large B-cell lymphoma patients, that historically have a very poor outcome, there is definitely an impressive response rate and improved survival, compared to the natural history cohort,” said Dr. Heslop of Baylor College of Medicine, Houston.
However, while the findings are encouraging, only 30%-40% are having a durable response, she added.
“So I think there’ll be lots of efforts to try and improve the response rate by combination with other agents such as checkpoint inhibitors or other immunomodulators,” she said.
With respect to the second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib, which was approved in October for adults with mantle cell lymphoma who have been treated with at least one prior therapy, she discussed the potential for improved outcomes and the importance of looking further into its use in patients who have failed ibrutinib therapy, as well as its use in combination with other agents, such as bendamustine and rituximab early in the course of disease.
Copanlisib, a PI3 kinase inhibitor approved in September, is an addition to the armamentarium for adult patients with relapsed follicular lymphoma after two lines of previous therapy.
“It still does have some side effects, as do other drugs in this class, so I think it’s place will still need to be defined,” Dr. Heslop said.
She reported having no relevant financial disclosures.
ATLANTA – 2017 was a banner year for the approval of new drugs to treat hematologic disorders.
At a special interest session at the annual meeting of American Society of Hematology, representatives from the Food and Drug Administration joined forces with clinicians to discuss the use of the newly approved treatments in the real-world setting.
In this video interview,
“This is extremely exciting,” she said regarding the pace of new approvals for hematologic malignancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Axicabtagene ciloleucel, a CAR T-cell product approved in October for the treatment of relapsed/refractory large B-cell lymphoma in adults, is particularly interesting, she said.
“The data shows that if you look at a population of diffuse large B-cell lymphoma patients, that historically have a very poor outcome, there is definitely an impressive response rate and improved survival, compared to the natural history cohort,” said Dr. Heslop of Baylor College of Medicine, Houston.
However, while the findings are encouraging, only 30%-40% are having a durable response, she added.
“So I think there’ll be lots of efforts to try and improve the response rate by combination with other agents such as checkpoint inhibitors or other immunomodulators,” she said.
With respect to the second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib, which was approved in October for adults with mantle cell lymphoma who have been treated with at least one prior therapy, she discussed the potential for improved outcomes and the importance of looking further into its use in patients who have failed ibrutinib therapy, as well as its use in combination with other agents, such as bendamustine and rituximab early in the course of disease.
Copanlisib, a PI3 kinase inhibitor approved in September, is an addition to the armamentarium for adult patients with relapsed follicular lymphoma after two lines of previous therapy.
“It still does have some side effects, as do other drugs in this class, so I think it’s place will still need to be defined,” Dr. Heslop said.
She reported having no relevant financial disclosures.
ATLANTA – 2017 was a banner year for the approval of new drugs to treat hematologic disorders.
At a special interest session at the annual meeting of American Society of Hematology, representatives from the Food and Drug Administration joined forces with clinicians to discuss the use of the newly approved treatments in the real-world setting.
In this video interview,
“This is extremely exciting,” she said regarding the pace of new approvals for hematologic malignancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Axicabtagene ciloleucel, a CAR T-cell product approved in October for the treatment of relapsed/refractory large B-cell lymphoma in adults, is particularly interesting, she said.
“The data shows that if you look at a population of diffuse large B-cell lymphoma patients, that historically have a very poor outcome, there is definitely an impressive response rate and improved survival, compared to the natural history cohort,” said Dr. Heslop of Baylor College of Medicine, Houston.
However, while the findings are encouraging, only 30%-40% are having a durable response, she added.
“So I think there’ll be lots of efforts to try and improve the response rate by combination with other agents such as checkpoint inhibitors or other immunomodulators,” she said.
With respect to the second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib, which was approved in October for adults with mantle cell lymphoma who have been treated with at least one prior therapy, she discussed the potential for improved outcomes and the importance of looking further into its use in patients who have failed ibrutinib therapy, as well as its use in combination with other agents, such as bendamustine and rituximab early in the course of disease.
Copanlisib, a PI3 kinase inhibitor approved in September, is an addition to the armamentarium for adult patients with relapsed follicular lymphoma after two lines of previous therapy.
“It still does have some side effects, as do other drugs in this class, so I think it’s place will still need to be defined,” Dr. Heslop said.
She reported having no relevant financial disclosures.
REPORTING FROM ASH 2017