User login
VIDEO: What’s new in AAP’s pediatric hypertension guidelines
SAN FRANCISCO – The American Academy of Pediatrics recently released new hypertension guidelines for children and adolescents.
Some of the advice is similar to the group’s last effort in 2004, but there are a few key changes that clinicians need to know, according to lead author Joseph Flynn, MD, professor of pediatrics and chief of nephrology at Seattle Children’s Hospital. He explained what they are, and the reasons behind them, in an interview at the joint hypertension scientific sessions sponsored by the American Heart Association and the American Society of Hypertension (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
The prevalence of pediatric hypertension, he said, now rivals asthma.
SAN FRANCISCO – The American Academy of Pediatrics recently released new hypertension guidelines for children and adolescents.
Some of the advice is similar to the group’s last effort in 2004, but there are a few key changes that clinicians need to know, according to lead author Joseph Flynn, MD, professor of pediatrics and chief of nephrology at Seattle Children’s Hospital. He explained what they are, and the reasons behind them, in an interview at the joint hypertension scientific sessions sponsored by the American Heart Association and the American Society of Hypertension (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
The prevalence of pediatric hypertension, he said, now rivals asthma.
SAN FRANCISCO – The American Academy of Pediatrics recently released new hypertension guidelines for children and adolescents.
Some of the advice is similar to the group’s last effort in 2004, but there are a few key changes that clinicians need to know, according to lead author Joseph Flynn, MD, professor of pediatrics and chief of nephrology at Seattle Children’s Hospital. He explained what they are, and the reasons behind them, in an interview at the joint hypertension scientific sessions sponsored by the American Heart Association and the American Society of Hypertension (Pediatrics. 2017 Aug 21. doi: 10.1542/peds.2017-1904).
The prevalence of pediatric hypertension, he said, now rivals asthma.
EXPERT ANALYSIS FROM THE AHA/ASH JOINT SCIENTIFIC SESSIONS
VIDEO: Alopecia areata patients seek emotional support
SILVER SPRING, MD. – The emotional challenges facing alopecia areata patients are as tough, or tougher, than the physical challenges, according to many patients participating in a public meeting on alopecia areata patient-focused drug development.
A panel of patients shared their experiences of living with alopecia areata, including Elizabeth DeCarlo of Wilmington, Delaware. In a video interview at the meeting, held at FDA headquarters on Sept. 11, Ms. DeCarlo elaborated on what she would like clinicians to understand about alopecia patients that might surprise them, and what matters to her as a patient.
“I would tell them to be more compassionate,” Ms. DeCarlo said. “It’s very emotional.” She also emphasized the value of giving alopecia patients information about local support groups, as well as national organizations such as the National Alopecia Areata Foundation.
Ms. DeCarlo had no financial conflicts to disclose.
SILVER SPRING, MD. – The emotional challenges facing alopecia areata patients are as tough, or tougher, than the physical challenges, according to many patients participating in a public meeting on alopecia areata patient-focused drug development.
A panel of patients shared their experiences of living with alopecia areata, including Elizabeth DeCarlo of Wilmington, Delaware. In a video interview at the meeting, held at FDA headquarters on Sept. 11, Ms. DeCarlo elaborated on what she would like clinicians to understand about alopecia patients that might surprise them, and what matters to her as a patient.
“I would tell them to be more compassionate,” Ms. DeCarlo said. “It’s very emotional.” She also emphasized the value of giving alopecia patients information about local support groups, as well as national organizations such as the National Alopecia Areata Foundation.
Ms. DeCarlo had no financial conflicts to disclose.
SILVER SPRING, MD. – The emotional challenges facing alopecia areata patients are as tough, or tougher, than the physical challenges, according to many patients participating in a public meeting on alopecia areata patient-focused drug development.
A panel of patients shared their experiences of living with alopecia areata, including Elizabeth DeCarlo of Wilmington, Delaware. In a video interview at the meeting, held at FDA headquarters on Sept. 11, Ms. DeCarlo elaborated on what she would like clinicians to understand about alopecia patients that might surprise them, and what matters to her as a patient.
“I would tell them to be more compassionate,” Ms. DeCarlo said. “It’s very emotional.” She also emphasized the value of giving alopecia patients information about local support groups, as well as national organizations such as the National Alopecia Areata Foundation.
Ms. DeCarlo had no financial conflicts to disclose.
AT AN FDA PUBLIC MEETING
Advances in ablative and non-ablative lasers in gynecology: A clinician’s guide

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
Anxiety disorders in children and adolescents
What FPs need to know about naloxone kits
VIDEO: Prescription-strength ibuprofen worsens blood pressure more than other NSAIDs
BARCELONA – Prescription-strength ibuprofen has a bigger adverse effect on blood pressure than celecoxib or naproxen, a finding that suggests a likely mechanism for the worse cardiovascular event rate documented in ibuprofen-treated arthritis patients in the PRECISION trial, Frank Ruschitzka, MD, said at the annual congress of the European Society of Cardiology.
“Prescription-strength ibuprofen is under pressure – it has a high incidence of new-onset hypertension, particularly when compared to the more selective COX-2 inhibitor celecoxib. Before we did this study, many would have said it’s the other way around,” observed Dr. Ruschitzka, professor of cardiology at the University of Zurich.
He presented the results of PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement).
“These results will have impact on your daily practice when you go home,” the cardiologist promised.
PRECISION-ABPM was a prespecified double-blind, randomized, 60-center substudy of the published PRECISION trial, which included 24,081 U.S. patients who needed daily NSAIDs for arthritis and were also at increased cardiovascular risk. They were randomized to the COX-2 inhibitor celecoxib at 100-200 mg b.i.d. or the nonselective NSAIDs ibuprofen at 600-800 mg three times a day or naproxen at 375-500 mg twice daily. Participants also received a proton pump inhibitor to protect against NSAID-related GI bleeding. In the on-treatment analysis, the ibuprofen group was significantly more likely to experience cardiovascular and all-cause mortality and renal events than were those on celecoxib (N Engl J Med. 2016 Dec 29;375[26]:2519-29).
The PRECISION-ABPM substudy included 444 arthritis patients, 92% of whom had osteoarthritis. During the 4-month study, investigators amassed roughly 60,000 automated blood pressure measurements across the three study arms.
The primary outcome was change from baseline in mean 24-hour systolic blood pressure (SBP). It increased by 3.7 mm Hg in the ibuprofen group and declined by 0.3 mm Hg in the celecoxib group, while the naproxen group occupied the middle ground with a 1.6-mm Hg increase.
The nearly 4-mm Hg increase in mean 24-hour SBP at 4 months in the ibuprofen group is of sufficient magnitude to be clinically important, Dr. Ruschitzka noted. He noted that fully 23.2% of ibuprofen-treated patients who had normal baseline blood pressure developed hypertension as defined by a mean 24-hour SBP of at least 130 and/or a diastolic blood pressure of at least 80 mm Hg. In contrast, incident hypertension occurred in only 10.3% of the celecoxib group and 19% of naproxen-treated patients. Thus, the likelihood of developing hypertension was 61% less with celecoxib than ibuprofen and 51% less with celecoxib than naproxen.
Not treating chronic arthritic pain to avoid the cardiovascular risk of NSAIDs is not a legitimate option.
“Pain is a cardiovascular risk factor,” Dr. Ruschitzka emphasized. “It’s unethical not to treat it. If you don’t treat pain, the patient’s blood pressure goes up, heart rate goes up, and you’re driving patients into inactivity.”
Although he’s convinced there’s no such thing as a safe NSAID from a cardiovascular risk standpoint, the PRECISION and PRECISION-ABPM data show celecoxib is less unsafe than ibuprofen. And as for the oft-heard statement that naproxen is the safest NSAID for the heart, Dr. Ruschitzka snorted, “What an urban legend.”
Discussant Scott Solomon, MD, opined that, while PRECISION-ABPM doesn’t support the notion that conventional NSAIDs such as naproxen or ibuprofen are any safer than celecoxib, it would be wrong to conclude from the study that celecoxib doesn’t affect blood pressure and is safer than the others from a cardiovascular standpoint. That’s because the three study drugs weren’t compared in an equipotent way. Because of safety concerns, the Food and Drug Administration required that the daily dose of celecoxib be capped at the low end of the therapeutic range, while no such constraints were placed on the two nonselective NSAIDS.
“Compared to placebo, all NSAIDs likely raise blood pressure, especially in patients prone to hypertension, those with chronic kidney disease, the elderly – and this is exactly the type of patients who require NSAIDs for arthritis. Whichever NSAID is chosen, clinicians should be aware of this effect and treat hypertension according to guidelines,” said Dr. Solomon, director of noninvasive cardiology at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
Dr. Solomon has been a key figure in the COX-2 inhibitor controversy of the last decade. He was lead author of a 2005 review of data from clinical trials of COX-2 inhibitors for colorectal adenoma prevention, which concluded that the drugs had a cardiovascular safety issue in that setting (N Engl J Med. 2005 Mar 17;352[11]:1071-80).
“Our analysis of celecoxib concluded that a dose-dependent increase in cardiovascular events was there, was real, but notably occurred at doses which were substantially higher than what we typically use for patients with arthritis,” he said.
That report triggered a fevered reaction.
“Amid an enormous amount of hype, hyperbole, and hysteria, the safety of these agents was thrown into question, leading to the withdrawal of all but one of them from the market and a black-box warning around the one remaining agent, celecoxib,” he recalled.
Dr. Ruschitzka discussed his findings in a video interview.
PRECISION-ABPM was sponsored by Pfizer. Dr. Ruschitzka and Dr. Solomon reported having no financial conflicts of interest regarding their presentations.
BARCELONA – Prescription-strength ibuprofen has a bigger adverse effect on blood pressure than celecoxib or naproxen, a finding that suggests a likely mechanism for the worse cardiovascular event rate documented in ibuprofen-treated arthritis patients in the PRECISION trial, Frank Ruschitzka, MD, said at the annual congress of the European Society of Cardiology.
“Prescription-strength ibuprofen is under pressure – it has a high incidence of new-onset hypertension, particularly when compared to the more selective COX-2 inhibitor celecoxib. Before we did this study, many would have said it’s the other way around,” observed Dr. Ruschitzka, professor of cardiology at the University of Zurich.
He presented the results of PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement).
“These results will have impact on your daily practice when you go home,” the cardiologist promised.
PRECISION-ABPM was a prespecified double-blind, randomized, 60-center substudy of the published PRECISION trial, which included 24,081 U.S. patients who needed daily NSAIDs for arthritis and were also at increased cardiovascular risk. They were randomized to the COX-2 inhibitor celecoxib at 100-200 mg b.i.d. or the nonselective NSAIDs ibuprofen at 600-800 mg three times a day or naproxen at 375-500 mg twice daily. Participants also received a proton pump inhibitor to protect against NSAID-related GI bleeding. In the on-treatment analysis, the ibuprofen group was significantly more likely to experience cardiovascular and all-cause mortality and renal events than were those on celecoxib (N Engl J Med. 2016 Dec 29;375[26]:2519-29).
The PRECISION-ABPM substudy included 444 arthritis patients, 92% of whom had osteoarthritis. During the 4-month study, investigators amassed roughly 60,000 automated blood pressure measurements across the three study arms.
The primary outcome was change from baseline in mean 24-hour systolic blood pressure (SBP). It increased by 3.7 mm Hg in the ibuprofen group and declined by 0.3 mm Hg in the celecoxib group, while the naproxen group occupied the middle ground with a 1.6-mm Hg increase.
The nearly 4-mm Hg increase in mean 24-hour SBP at 4 months in the ibuprofen group is of sufficient magnitude to be clinically important, Dr. Ruschitzka noted. He noted that fully 23.2% of ibuprofen-treated patients who had normal baseline blood pressure developed hypertension as defined by a mean 24-hour SBP of at least 130 and/or a diastolic blood pressure of at least 80 mm Hg. In contrast, incident hypertension occurred in only 10.3% of the celecoxib group and 19% of naproxen-treated patients. Thus, the likelihood of developing hypertension was 61% less with celecoxib than ibuprofen and 51% less with celecoxib than naproxen.
Not treating chronic arthritic pain to avoid the cardiovascular risk of NSAIDs is not a legitimate option.
“Pain is a cardiovascular risk factor,” Dr. Ruschitzka emphasized. “It’s unethical not to treat it. If you don’t treat pain, the patient’s blood pressure goes up, heart rate goes up, and you’re driving patients into inactivity.”
Although he’s convinced there’s no such thing as a safe NSAID from a cardiovascular risk standpoint, the PRECISION and PRECISION-ABPM data show celecoxib is less unsafe than ibuprofen. And as for the oft-heard statement that naproxen is the safest NSAID for the heart, Dr. Ruschitzka snorted, “What an urban legend.”
Discussant Scott Solomon, MD, opined that, while PRECISION-ABPM doesn’t support the notion that conventional NSAIDs such as naproxen or ibuprofen are any safer than celecoxib, it would be wrong to conclude from the study that celecoxib doesn’t affect blood pressure and is safer than the others from a cardiovascular standpoint. That’s because the three study drugs weren’t compared in an equipotent way. Because of safety concerns, the Food and Drug Administration required that the daily dose of celecoxib be capped at the low end of the therapeutic range, while no such constraints were placed on the two nonselective NSAIDS.
“Compared to placebo, all NSAIDs likely raise blood pressure, especially in patients prone to hypertension, those with chronic kidney disease, the elderly – and this is exactly the type of patients who require NSAIDs for arthritis. Whichever NSAID is chosen, clinicians should be aware of this effect and treat hypertension according to guidelines,” said Dr. Solomon, director of noninvasive cardiology at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
Dr. Solomon has been a key figure in the COX-2 inhibitor controversy of the last decade. He was lead author of a 2005 review of data from clinical trials of COX-2 inhibitors for colorectal adenoma prevention, which concluded that the drugs had a cardiovascular safety issue in that setting (N Engl J Med. 2005 Mar 17;352[11]:1071-80).
“Our analysis of celecoxib concluded that a dose-dependent increase in cardiovascular events was there, was real, but notably occurred at doses which were substantially higher than what we typically use for patients with arthritis,” he said.
That report triggered a fevered reaction.
“Amid an enormous amount of hype, hyperbole, and hysteria, the safety of these agents was thrown into question, leading to the withdrawal of all but one of them from the market and a black-box warning around the one remaining agent, celecoxib,” he recalled.
Dr. Ruschitzka discussed his findings in a video interview.
PRECISION-ABPM was sponsored by Pfizer. Dr. Ruschitzka and Dr. Solomon reported having no financial conflicts of interest regarding their presentations.
BARCELONA – Prescription-strength ibuprofen has a bigger adverse effect on blood pressure than celecoxib or naproxen, a finding that suggests a likely mechanism for the worse cardiovascular event rate documented in ibuprofen-treated arthritis patients in the PRECISION trial, Frank Ruschitzka, MD, said at the annual congress of the European Society of Cardiology.
“Prescription-strength ibuprofen is under pressure – it has a high incidence of new-onset hypertension, particularly when compared to the more selective COX-2 inhibitor celecoxib. Before we did this study, many would have said it’s the other way around,” observed Dr. Ruschitzka, professor of cardiology at the University of Zurich.
He presented the results of PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement).
“These results will have impact on your daily practice when you go home,” the cardiologist promised.
PRECISION-ABPM was a prespecified double-blind, randomized, 60-center substudy of the published PRECISION trial, which included 24,081 U.S. patients who needed daily NSAIDs for arthritis and were also at increased cardiovascular risk. They were randomized to the COX-2 inhibitor celecoxib at 100-200 mg b.i.d. or the nonselective NSAIDs ibuprofen at 600-800 mg three times a day or naproxen at 375-500 mg twice daily. Participants also received a proton pump inhibitor to protect against NSAID-related GI bleeding. In the on-treatment analysis, the ibuprofen group was significantly more likely to experience cardiovascular and all-cause mortality and renal events than were those on celecoxib (N Engl J Med. 2016 Dec 29;375[26]:2519-29).
The PRECISION-ABPM substudy included 444 arthritis patients, 92% of whom had osteoarthritis. During the 4-month study, investigators amassed roughly 60,000 automated blood pressure measurements across the three study arms.
The primary outcome was change from baseline in mean 24-hour systolic blood pressure (SBP). It increased by 3.7 mm Hg in the ibuprofen group and declined by 0.3 mm Hg in the celecoxib group, while the naproxen group occupied the middle ground with a 1.6-mm Hg increase.
The nearly 4-mm Hg increase in mean 24-hour SBP at 4 months in the ibuprofen group is of sufficient magnitude to be clinically important, Dr. Ruschitzka noted. He noted that fully 23.2% of ibuprofen-treated patients who had normal baseline blood pressure developed hypertension as defined by a mean 24-hour SBP of at least 130 and/or a diastolic blood pressure of at least 80 mm Hg. In contrast, incident hypertension occurred in only 10.3% of the celecoxib group and 19% of naproxen-treated patients. Thus, the likelihood of developing hypertension was 61% less with celecoxib than ibuprofen and 51% less with celecoxib than naproxen.
Not treating chronic arthritic pain to avoid the cardiovascular risk of NSAIDs is not a legitimate option.
“Pain is a cardiovascular risk factor,” Dr. Ruschitzka emphasized. “It’s unethical not to treat it. If you don’t treat pain, the patient’s blood pressure goes up, heart rate goes up, and you’re driving patients into inactivity.”
Although he’s convinced there’s no such thing as a safe NSAID from a cardiovascular risk standpoint, the PRECISION and PRECISION-ABPM data show celecoxib is less unsafe than ibuprofen. And as for the oft-heard statement that naproxen is the safest NSAID for the heart, Dr. Ruschitzka snorted, “What an urban legend.”
Discussant Scott Solomon, MD, opined that, while PRECISION-ABPM doesn’t support the notion that conventional NSAIDs such as naproxen or ibuprofen are any safer than celecoxib, it would be wrong to conclude from the study that celecoxib doesn’t affect blood pressure and is safer than the others from a cardiovascular standpoint. That’s because the three study drugs weren’t compared in an equipotent way. Because of safety concerns, the Food and Drug Administration required that the daily dose of celecoxib be capped at the low end of the therapeutic range, while no such constraints were placed on the two nonselective NSAIDS.
“Compared to placebo, all NSAIDs likely raise blood pressure, especially in patients prone to hypertension, those with chronic kidney disease, the elderly – and this is exactly the type of patients who require NSAIDs for arthritis. Whichever NSAID is chosen, clinicians should be aware of this effect and treat hypertension according to guidelines,” said Dr. Solomon, director of noninvasive cardiology at Brigham and Women’s Hospital, Boston, and professor of medicine at Harvard Medical School.
Dr. Solomon has been a key figure in the COX-2 inhibitor controversy of the last decade. He was lead author of a 2005 review of data from clinical trials of COX-2 inhibitors for colorectal adenoma prevention, which concluded that the drugs had a cardiovascular safety issue in that setting (N Engl J Med. 2005 Mar 17;352[11]:1071-80).
“Our analysis of celecoxib concluded that a dose-dependent increase in cardiovascular events was there, was real, but notably occurred at doses which were substantially higher than what we typically use for patients with arthritis,” he said.
That report triggered a fevered reaction.
“Amid an enormous amount of hype, hyperbole, and hysteria, the safety of these agents was thrown into question, leading to the withdrawal of all but one of them from the market and a black-box warning around the one remaining agent, celecoxib,” he recalled.
Dr. Ruschitzka discussed his findings in a video interview.
PRECISION-ABPM was sponsored by Pfizer. Dr. Ruschitzka and Dr. Solomon reported having no financial conflicts of interest regarding their presentations.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Incident hypertension occurred within 4 months in 23.2% of arthritis patients on ibuprofen, compared with 10.3% taking celecoxib and 19% on naproxen.
Data source: This was a randomized, double-blind, multicenter, prospective trial including 444 arthritis patients at increased cardiovascular risk who underwent 4 months of ambulatory blood pressure monitoring after being assigned to prescription-strength ibuprofen, naproxen, or celecoxib.
Disclosures: The PRECISION-ABPM trial was sponsored by Pfizer. The presenter reported having no financial conflicts of interest.
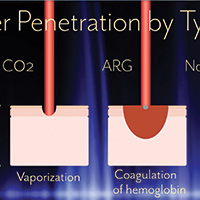
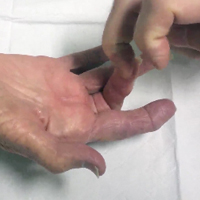
Percutaneous Trigger Finger Release
VIDEO: Rivaroxaban plus aspirin cut cardiovascular events in stable patients
BARCELONA – Combined treatment with a very low dosage of the anticoagulant rivaroxaban plus low-dose aspirin produced significant cuts in major adverse coronary, cerebral, and peripheral artery disease events with just a modest rise in major bleeding events in patients with stable disease in the COMPASS pivotal, randomized trial with more than 27,000 patients.
The benefits from the rivaroxaban plus aspirin regimen included a statistically significant 24% relative risk reduction in the study’s primary, combined endpoint, and a significant 18% relative risk reduction in all-cause death compared with a standard regimen of aspirin only, John W. Eikelboom, MD, said at the annual congress of the European Society of Cardiology. In addition, analysis of the net clinical benefit from treatment that took into account both the major adverse cardiovascular events prevented and major bleeding events induced, showed that the rivaroxaban-plus-aspirin regimen cut these by a statistically significant 20%, compared with aspirin alone.
Other notable benefits documented by the findings included a statistically significant 42% relative risk reduction for stroke and a statistically significant 46% relative risk reduction in the incidence of major adverse limb events among the roughly one-quarter of enrolled patients who entered the study with evidence of peripheral artery disease.
These risk reductions are similar in magnitude to the secondary-prevention benefits produced by controlling hypertension or dyslipidemia, noted Dr. Eikelboom, a researcher at McMaster University in Hamilton, Ont. “In the future, rivaroxaban will take its place among the other foundational treatments for long-term, secondary prevention,” he predicted in a video interview.
The COMPASS trial produced “unambiguous results that should change guidelines and the management of stable coronary artery disease,” commented Eugene Braunwald, MD, designated discussant for Dr. Eikelboom’s report. The results are “an important step for thrombocardiology,” said Dr. Braunwald, professor of medicine at Harvard Medical School in Boston.
Concurrently with Dr. Eikelboom’s report the results appeared in an article published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1709118). This publication also include an editorial by Dr. Braunwald (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMe1710241).
The Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS) trial enrolled 27,395 patients with stable coronary, carotid, or peripheral artery disease, or a combination of two or more of these, at 602 centers in 33 countries. About 90% of enrolled patients had coronary artery disease and 27% had peripheral artery disease. The enrolled patients averaged 68 year old and were an average of 7 years removed from their index arterial event. Randomization assigned patients to receive 2.5 mg rivaroxaban (Xarelto) twice daily plus 100 mg aspirin daily, 5 mg rivaroxaban twice daily, or 100 mg aspirin once daily. The trial stopped early, after an average follow-up of 23 months, because of the overwhelming benefit seen for the rivaroxaban plus aspirin combination. The rivaroxaban-monotherapy arm failed to show any statistically significant benefits, compared with the aspirin-monotherapy control group.
The study’s primary endpoint – the combined rate of cardiovascular disease death, nonfatal stroke, and nonfatal MI – occurred in 4.1% of patients in the rivaroxaban-plus-aspirin group and in 5.4% of patients on aspirin alone. The rate of major bleeding events was 3.1% among patients on rivaroxaban plus aspirin and 1.9% in those who received aspirin only, a 51% relative increase among patients on the dual regimen, but the results showed no statistically significant increase in the rates of fatal bleeds, intracerebral bleeds, or bleeding in other critical organs.
Sonia Anand, MD, a colleague of Dr. Eikelboom’s at McMaster, presented a separate set of analyses that focused on the 7,470 enrolled patients who had been diagnosed at enrollment with peripheral artery disease. In this subgroup, the rivaroxaban-plus-aspirin regimen produced a statistically significant 28% relative risk reduction in the rate of the primary endpoint, compared with the aspirin control group. The dual regimen also produced a statistically significant 46% relative risk reduction in major adverse limb events and a significant 70% relative reduction in the incidence of major lower-extremity amputations, reported Dr. Anand, professor of medicine and director of the vascular medicine clinic at McMaster.
The COMPASS findings follow a 2012 published report from the ATLAS ACS 2-TIMI 51 trial showing that treatment with the same low-dose rivaroxaban regimen plus aspirin and a thienopyridine (clopidogrel or ticlopidine) reduced the same combined triple endpoint by a statistically significant 16%, compared with aspirin and a thienopyridine alone, in patients with a recent acute coronary syndrome event (N Engl J Med. 2012 Jan 5;366[1]:9-19). Despite this evidence, the Food and Drug Administration never approved the 2.5-mg formulation of rivaroxaban, nor did it approve marketing of rivaroxaban for this acute coronary syndrome population. This decision may have been driven in part by a problem with incomplete follow-up of several of the enrolled patients.
The COMPASS results were “very consistent” with the ATLAS ACS 2-TIMI 51 results. noted Dr. Eikelboom. “I think it’s time to look at these two trials in combination,” he suggested. Availability of the 2.5-mg rivaroxaban formulation used in both trials would allow “a treatment strategy that could start early after an acute coronary syndrome event and then extend long term,” he said.
COMPASS was sponsored by Bayer, which markets rivaroxaban (Xarelto). Dr. Eikelboom has received research support from Bayer and also from Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi, Janssen, Pfizer, Portola, and Sanofi. Dr. Anand has received speaking honoraria from several drug compnies. Dr. Braunwald had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
The key message from COMPASS was that, although adding a very low dosage of rivaroxaban to aspirin in patients with stable coronary or peripheral artery disease resulted in a clear increase in major bleeding events, patients received an overall net beneficial effect from the combined regimen. The finding that clinches the net benefit from the rivaroxaban plus aspirin combination, compared with aspirin alone, was that the combined regimen produced a statistically significant relative risk reduction of 18% for all-cause mortality. This finding reinforces the idea that the primary outcome was beneficial despite an increase in major bleeding events.
The finding that rivaroxaban plus aspirin produced benefit with a modest increase in bleeding risk in patients with peripheral artery disease (PAD) is especially important because PAD is really difficult to treat. Very few interventions have previously been proven to have a beneficial effect for patients with PAD. It’s very important to find an intervention that can reduce critical limb ischemia events in addition to reducing coronary events, stroke, and overall mortality.
The very low dosage of rivaroxaban used in COMPASS, 2.5 mg twice daily, seems to be a very important part of the study’s design. This dosage appeared to hit the sweet spot of being large enough to reduce events but with a gentle enough anticoagulation effect to avoid a significant increase in fatal, intracerebral, or critical organ bleeds. However, the patients enrolled in COMPASS, like most patients who enter trials, were generally at a lower risk for bleeding complications than we usually see in routine practice in patients with stable coronary or peripheral artery disease. Presuming that the Food and Drug Administration will soon approve the 2.5-mg formulation of rivaroxaban used in COMPASS, clinicians will need to be careful using this regimen on patients at increased risk for bleeding, such as frail or elderly patients with a history of bleeding events or taking other treatments that could increase bleeding risk, such as nonsteroidal anti-inflammatory drugs. In general, clinicians are wary of using treatments that increase bleeding risk, and so they may hesitate to use this combination of rivaroxaban plus aspirin in patients with a high bleeding risk.
The success of the approach used in COMPASS became possible with the introduction of the new oral anticoagulant drugs. Now that this class of agents has been available for a few years, clinicians have grown increasingly comfortable with them, compared with warfarin. When the new oral anticoagulants first came out, many considered them similar to warfarin. Today, there is a better appreciation that these drugs are distinct from warfarin by really causing fewer bleeding complications.
Christopher B. Granger, MD , is a cardiologist and professor of medicine at Duke University in Durham, N.C. He has been a consultant to and has received research support from Bayer and from other drugs companies that market new oral anticoagulants. He made these comments in an interview.
The key message from COMPASS was that, although adding a very low dosage of rivaroxaban to aspirin in patients with stable coronary or peripheral artery disease resulted in a clear increase in major bleeding events, patients received an overall net beneficial effect from the combined regimen. The finding that clinches the net benefit from the rivaroxaban plus aspirin combination, compared with aspirin alone, was that the combined regimen produced a statistically significant relative risk reduction of 18% for all-cause mortality. This finding reinforces the idea that the primary outcome was beneficial despite an increase in major bleeding events.
The finding that rivaroxaban plus aspirin produced benefit with a modest increase in bleeding risk in patients with peripheral artery disease (PAD) is especially important because PAD is really difficult to treat. Very few interventions have previously been proven to have a beneficial effect for patients with PAD. It’s very important to find an intervention that can reduce critical limb ischemia events in addition to reducing coronary events, stroke, and overall mortality.
The very low dosage of rivaroxaban used in COMPASS, 2.5 mg twice daily, seems to be a very important part of the study’s design. This dosage appeared to hit the sweet spot of being large enough to reduce events but with a gentle enough anticoagulation effect to avoid a significant increase in fatal, intracerebral, or critical organ bleeds. However, the patients enrolled in COMPASS, like most patients who enter trials, were generally at a lower risk for bleeding complications than we usually see in routine practice in patients with stable coronary or peripheral artery disease. Presuming that the Food and Drug Administration will soon approve the 2.5-mg formulation of rivaroxaban used in COMPASS, clinicians will need to be careful using this regimen on patients at increased risk for bleeding, such as frail or elderly patients with a history of bleeding events or taking other treatments that could increase bleeding risk, such as nonsteroidal anti-inflammatory drugs. In general, clinicians are wary of using treatments that increase bleeding risk, and so they may hesitate to use this combination of rivaroxaban plus aspirin in patients with a high bleeding risk.
The success of the approach used in COMPASS became possible with the introduction of the new oral anticoagulant drugs. Now that this class of agents has been available for a few years, clinicians have grown increasingly comfortable with them, compared with warfarin. When the new oral anticoagulants first came out, many considered them similar to warfarin. Today, there is a better appreciation that these drugs are distinct from warfarin by really causing fewer bleeding complications.
Christopher B. Granger, MD , is a cardiologist and professor of medicine at Duke University in Durham, N.C. He has been a consultant to and has received research support from Bayer and from other drugs companies that market new oral anticoagulants. He made these comments in an interview.
The key message from COMPASS was that, although adding a very low dosage of rivaroxaban to aspirin in patients with stable coronary or peripheral artery disease resulted in a clear increase in major bleeding events, patients received an overall net beneficial effect from the combined regimen. The finding that clinches the net benefit from the rivaroxaban plus aspirin combination, compared with aspirin alone, was that the combined regimen produced a statistically significant relative risk reduction of 18% for all-cause mortality. This finding reinforces the idea that the primary outcome was beneficial despite an increase in major bleeding events.
The finding that rivaroxaban plus aspirin produced benefit with a modest increase in bleeding risk in patients with peripheral artery disease (PAD) is especially important because PAD is really difficult to treat. Very few interventions have previously been proven to have a beneficial effect for patients with PAD. It’s very important to find an intervention that can reduce critical limb ischemia events in addition to reducing coronary events, stroke, and overall mortality.
The very low dosage of rivaroxaban used in COMPASS, 2.5 mg twice daily, seems to be a very important part of the study’s design. This dosage appeared to hit the sweet spot of being large enough to reduce events but with a gentle enough anticoagulation effect to avoid a significant increase in fatal, intracerebral, or critical organ bleeds. However, the patients enrolled in COMPASS, like most patients who enter trials, were generally at a lower risk for bleeding complications than we usually see in routine practice in patients with stable coronary or peripheral artery disease. Presuming that the Food and Drug Administration will soon approve the 2.5-mg formulation of rivaroxaban used in COMPASS, clinicians will need to be careful using this regimen on patients at increased risk for bleeding, such as frail or elderly patients with a history of bleeding events or taking other treatments that could increase bleeding risk, such as nonsteroidal anti-inflammatory drugs. In general, clinicians are wary of using treatments that increase bleeding risk, and so they may hesitate to use this combination of rivaroxaban plus aspirin in patients with a high bleeding risk.
The success of the approach used in COMPASS became possible with the introduction of the new oral anticoagulant drugs. Now that this class of agents has been available for a few years, clinicians have grown increasingly comfortable with them, compared with warfarin. When the new oral anticoagulants first came out, many considered them similar to warfarin. Today, there is a better appreciation that these drugs are distinct from warfarin by really causing fewer bleeding complications.
Christopher B. Granger, MD , is a cardiologist and professor of medicine at Duke University in Durham, N.C. He has been a consultant to and has received research support from Bayer and from other drugs companies that market new oral anticoagulants. He made these comments in an interview.
BARCELONA – Combined treatment with a very low dosage of the anticoagulant rivaroxaban plus low-dose aspirin produced significant cuts in major adverse coronary, cerebral, and peripheral artery disease events with just a modest rise in major bleeding events in patients with stable disease in the COMPASS pivotal, randomized trial with more than 27,000 patients.
The benefits from the rivaroxaban plus aspirin regimen included a statistically significant 24% relative risk reduction in the study’s primary, combined endpoint, and a significant 18% relative risk reduction in all-cause death compared with a standard regimen of aspirin only, John W. Eikelboom, MD, said at the annual congress of the European Society of Cardiology. In addition, analysis of the net clinical benefit from treatment that took into account both the major adverse cardiovascular events prevented and major bleeding events induced, showed that the rivaroxaban-plus-aspirin regimen cut these by a statistically significant 20%, compared with aspirin alone.
Other notable benefits documented by the findings included a statistically significant 42% relative risk reduction for stroke and a statistically significant 46% relative risk reduction in the incidence of major adverse limb events among the roughly one-quarter of enrolled patients who entered the study with evidence of peripheral artery disease.
These risk reductions are similar in magnitude to the secondary-prevention benefits produced by controlling hypertension or dyslipidemia, noted Dr. Eikelboom, a researcher at McMaster University in Hamilton, Ont. “In the future, rivaroxaban will take its place among the other foundational treatments for long-term, secondary prevention,” he predicted in a video interview.
The COMPASS trial produced “unambiguous results that should change guidelines and the management of stable coronary artery disease,” commented Eugene Braunwald, MD, designated discussant for Dr. Eikelboom’s report. The results are “an important step for thrombocardiology,” said Dr. Braunwald, professor of medicine at Harvard Medical School in Boston.
Concurrently with Dr. Eikelboom’s report the results appeared in an article published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1709118). This publication also include an editorial by Dr. Braunwald (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMe1710241).
The Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS) trial enrolled 27,395 patients with stable coronary, carotid, or peripheral artery disease, or a combination of two or more of these, at 602 centers in 33 countries. About 90% of enrolled patients had coronary artery disease and 27% had peripheral artery disease. The enrolled patients averaged 68 year old and were an average of 7 years removed from their index arterial event. Randomization assigned patients to receive 2.5 mg rivaroxaban (Xarelto) twice daily plus 100 mg aspirin daily, 5 mg rivaroxaban twice daily, or 100 mg aspirin once daily. The trial stopped early, after an average follow-up of 23 months, because of the overwhelming benefit seen for the rivaroxaban plus aspirin combination. The rivaroxaban-monotherapy arm failed to show any statistically significant benefits, compared with the aspirin-monotherapy control group.
The study’s primary endpoint – the combined rate of cardiovascular disease death, nonfatal stroke, and nonfatal MI – occurred in 4.1% of patients in the rivaroxaban-plus-aspirin group and in 5.4% of patients on aspirin alone. The rate of major bleeding events was 3.1% among patients on rivaroxaban plus aspirin and 1.9% in those who received aspirin only, a 51% relative increase among patients on the dual regimen, but the results showed no statistically significant increase in the rates of fatal bleeds, intracerebral bleeds, or bleeding in other critical organs.
Sonia Anand, MD, a colleague of Dr. Eikelboom’s at McMaster, presented a separate set of analyses that focused on the 7,470 enrolled patients who had been diagnosed at enrollment with peripheral artery disease. In this subgroup, the rivaroxaban-plus-aspirin regimen produced a statistically significant 28% relative risk reduction in the rate of the primary endpoint, compared with the aspirin control group. The dual regimen also produced a statistically significant 46% relative risk reduction in major adverse limb events and a significant 70% relative reduction in the incidence of major lower-extremity amputations, reported Dr. Anand, professor of medicine and director of the vascular medicine clinic at McMaster.
The COMPASS findings follow a 2012 published report from the ATLAS ACS 2-TIMI 51 trial showing that treatment with the same low-dose rivaroxaban regimen plus aspirin and a thienopyridine (clopidogrel or ticlopidine) reduced the same combined triple endpoint by a statistically significant 16%, compared with aspirin and a thienopyridine alone, in patients with a recent acute coronary syndrome event (N Engl J Med. 2012 Jan 5;366[1]:9-19). Despite this evidence, the Food and Drug Administration never approved the 2.5-mg formulation of rivaroxaban, nor did it approve marketing of rivaroxaban for this acute coronary syndrome population. This decision may have been driven in part by a problem with incomplete follow-up of several of the enrolled patients.
The COMPASS results were “very consistent” with the ATLAS ACS 2-TIMI 51 results. noted Dr. Eikelboom. “I think it’s time to look at these two trials in combination,” he suggested. Availability of the 2.5-mg rivaroxaban formulation used in both trials would allow “a treatment strategy that could start early after an acute coronary syndrome event and then extend long term,” he said.
COMPASS was sponsored by Bayer, which markets rivaroxaban (Xarelto). Dr. Eikelboom has received research support from Bayer and also from Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi, Janssen, Pfizer, Portola, and Sanofi. Dr. Anand has received speaking honoraria from several drug compnies. Dr. Braunwald had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
BARCELONA – Combined treatment with a very low dosage of the anticoagulant rivaroxaban plus low-dose aspirin produced significant cuts in major adverse coronary, cerebral, and peripheral artery disease events with just a modest rise in major bleeding events in patients with stable disease in the COMPASS pivotal, randomized trial with more than 27,000 patients.
The benefits from the rivaroxaban plus aspirin regimen included a statistically significant 24% relative risk reduction in the study’s primary, combined endpoint, and a significant 18% relative risk reduction in all-cause death compared with a standard regimen of aspirin only, John W. Eikelboom, MD, said at the annual congress of the European Society of Cardiology. In addition, analysis of the net clinical benefit from treatment that took into account both the major adverse cardiovascular events prevented and major bleeding events induced, showed that the rivaroxaban-plus-aspirin regimen cut these by a statistically significant 20%, compared with aspirin alone.
Other notable benefits documented by the findings included a statistically significant 42% relative risk reduction for stroke and a statistically significant 46% relative risk reduction in the incidence of major adverse limb events among the roughly one-quarter of enrolled patients who entered the study with evidence of peripheral artery disease.
These risk reductions are similar in magnitude to the secondary-prevention benefits produced by controlling hypertension or dyslipidemia, noted Dr. Eikelboom, a researcher at McMaster University in Hamilton, Ont. “In the future, rivaroxaban will take its place among the other foundational treatments for long-term, secondary prevention,” he predicted in a video interview.
The COMPASS trial produced “unambiguous results that should change guidelines and the management of stable coronary artery disease,” commented Eugene Braunwald, MD, designated discussant for Dr. Eikelboom’s report. The results are “an important step for thrombocardiology,” said Dr. Braunwald, professor of medicine at Harvard Medical School in Boston.
Concurrently with Dr. Eikelboom’s report the results appeared in an article published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1709118). This publication also include an editorial by Dr. Braunwald (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMe1710241).
The Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS) trial enrolled 27,395 patients with stable coronary, carotid, or peripheral artery disease, or a combination of two or more of these, at 602 centers in 33 countries. About 90% of enrolled patients had coronary artery disease and 27% had peripheral artery disease. The enrolled patients averaged 68 year old and were an average of 7 years removed from their index arterial event. Randomization assigned patients to receive 2.5 mg rivaroxaban (Xarelto) twice daily plus 100 mg aspirin daily, 5 mg rivaroxaban twice daily, or 100 mg aspirin once daily. The trial stopped early, after an average follow-up of 23 months, because of the overwhelming benefit seen for the rivaroxaban plus aspirin combination. The rivaroxaban-monotherapy arm failed to show any statistically significant benefits, compared with the aspirin-monotherapy control group.
The study’s primary endpoint – the combined rate of cardiovascular disease death, nonfatal stroke, and nonfatal MI – occurred in 4.1% of patients in the rivaroxaban-plus-aspirin group and in 5.4% of patients on aspirin alone. The rate of major bleeding events was 3.1% among patients on rivaroxaban plus aspirin and 1.9% in those who received aspirin only, a 51% relative increase among patients on the dual regimen, but the results showed no statistically significant increase in the rates of fatal bleeds, intracerebral bleeds, or bleeding in other critical organs.
Sonia Anand, MD, a colleague of Dr. Eikelboom’s at McMaster, presented a separate set of analyses that focused on the 7,470 enrolled patients who had been diagnosed at enrollment with peripheral artery disease. In this subgroup, the rivaroxaban-plus-aspirin regimen produced a statistically significant 28% relative risk reduction in the rate of the primary endpoint, compared with the aspirin control group. The dual regimen also produced a statistically significant 46% relative risk reduction in major adverse limb events and a significant 70% relative reduction in the incidence of major lower-extremity amputations, reported Dr. Anand, professor of medicine and director of the vascular medicine clinic at McMaster.
The COMPASS findings follow a 2012 published report from the ATLAS ACS 2-TIMI 51 trial showing that treatment with the same low-dose rivaroxaban regimen plus aspirin and a thienopyridine (clopidogrel or ticlopidine) reduced the same combined triple endpoint by a statistically significant 16%, compared with aspirin and a thienopyridine alone, in patients with a recent acute coronary syndrome event (N Engl J Med. 2012 Jan 5;366[1]:9-19). Despite this evidence, the Food and Drug Administration never approved the 2.5-mg formulation of rivaroxaban, nor did it approve marketing of rivaroxaban for this acute coronary syndrome population. This decision may have been driven in part by a problem with incomplete follow-up of several of the enrolled patients.
The COMPASS results were “very consistent” with the ATLAS ACS 2-TIMI 51 results. noted Dr. Eikelboom. “I think it’s time to look at these two trials in combination,” he suggested. Availability of the 2.5-mg rivaroxaban formulation used in both trials would allow “a treatment strategy that could start early after an acute coronary syndrome event and then extend long term,” he said.
COMPASS was sponsored by Bayer, which markets rivaroxaban (Xarelto). Dr. Eikelboom has received research support from Bayer and also from Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi, Janssen, Pfizer, Portola, and Sanofi. Dr. Anand has received speaking honoraria from several drug compnies. Dr. Braunwald had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: The dual regimen reduced the combined rate of cardiovascular disease events by 24%, compared with aspirin alone.
Data source: COMPASS is a multicenter, randomized controlled trial with 27,395 patients.
Disclosures: COMPASS was sponsored by Bayer, which markets rivaroxaban (Xarelto). Dr. Eikelboom has received research support from Bayer and also from Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi, Janssen, Pfizer, Portola, and Sanofi. Dr. Anand has received speaking honoraria from several drug compnies. Dr. Braunwald had no disclosures.
VIDEO: Inflammation’s role in atherosclerosis confirmed in CANTOS
BARCELONA – The results of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) mark the validation of many years of research on inflammation for Peter Libby, MD, Mallinckrodt Professor of Medicine, Harvard Medical School, Boston.
The CANTOS investigator said that, although some trials, most notably JUPITER, have linked reduced markers of inflammation with reduced cardiovascular events, none have been able to separate the effects of lowering LDL cholesterol from those of lowering the inflammatory marker interleukin-1B.
But using the monoclonal antibody canakinumab to target only interleukin-1B in CANTOS reduced the composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death by 15% at the highest dosage tested, compared with placebo, while lowering high-sensitivity C-reactive protein by 39 percentage points.
Dr. Libby has been studying interleukin-1B since the 1980s. “Now, today, for the first time, in a rigorous trial, we can show that an anti-inflammatory agent that is neutral for lipids (that doesn’t lower LDL) can provide a benefit for our patients, and that’s a real step forward,” Dr. Libby said in a video interview at the annual congress of the European Society of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Importantly, a dividend of the investigation was that “we found a decrease in fatal cancers, particularly lung cancer. So this again opens the door toward a whole new therapeutic window in patients not just in the cardiovascular space, but also in oncology. So it’s a doubly exciting day for us.”
CANTOS was presented at the meeting by Paul Ridker, MD, also of Harvard Medical School; the results were also published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
BARCELONA – The results of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) mark the validation of many years of research on inflammation for Peter Libby, MD, Mallinckrodt Professor of Medicine, Harvard Medical School, Boston.
The CANTOS investigator said that, although some trials, most notably JUPITER, have linked reduced markers of inflammation with reduced cardiovascular events, none have been able to separate the effects of lowering LDL cholesterol from those of lowering the inflammatory marker interleukin-1B.
But using the monoclonal antibody canakinumab to target only interleukin-1B in CANTOS reduced the composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death by 15% at the highest dosage tested, compared with placebo, while lowering high-sensitivity C-reactive protein by 39 percentage points.
Dr. Libby has been studying interleukin-1B since the 1980s. “Now, today, for the first time, in a rigorous trial, we can show that an anti-inflammatory agent that is neutral for lipids (that doesn’t lower LDL) can provide a benefit for our patients, and that’s a real step forward,” Dr. Libby said in a video interview at the annual congress of the European Society of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Importantly, a dividend of the investigation was that “we found a decrease in fatal cancers, particularly lung cancer. So this again opens the door toward a whole new therapeutic window in patients not just in the cardiovascular space, but also in oncology. So it’s a doubly exciting day for us.”
CANTOS was presented at the meeting by Paul Ridker, MD, also of Harvard Medical School; the results were also published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
BARCELONA – The results of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) mark the validation of many years of research on inflammation for Peter Libby, MD, Mallinckrodt Professor of Medicine, Harvard Medical School, Boston.
The CANTOS investigator said that, although some trials, most notably JUPITER, have linked reduced markers of inflammation with reduced cardiovascular events, none have been able to separate the effects of lowering LDL cholesterol from those of lowering the inflammatory marker interleukin-1B.
But using the monoclonal antibody canakinumab to target only interleukin-1B in CANTOS reduced the composite endpoint of nonfatal MI, nonfatal stroke, or cardiovascular death by 15% at the highest dosage tested, compared with placebo, while lowering high-sensitivity C-reactive protein by 39 percentage points.
Dr. Libby has been studying interleukin-1B since the 1980s. “Now, today, for the first time, in a rigorous trial, we can show that an anti-inflammatory agent that is neutral for lipids (that doesn’t lower LDL) can provide a benefit for our patients, and that’s a real step forward,” Dr. Libby said in a video interview at the annual congress of the European Society of Cardiology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Importantly, a dividend of the investigation was that “we found a decrease in fatal cancers, particularly lung cancer. So this again opens the door toward a whole new therapeutic window in patients not just in the cardiovascular space, but also in oncology. So it’s a doubly exciting day for us.”
CANTOS was presented at the meeting by Paul Ridker, MD, also of Harvard Medical School; the results were also published online (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
AT THE ESC CONGRESS 2017
Big risk of serious falls after first episode of syncope
BARCELONA – Patients have an exorbitant 80% increased risk of hospitalization for falls resulting in fracture or head injury in the first year after discharge following a first-ever episode of syncope, according to a Danish national cohort study. One in five patients who sustained a fall resulting in hospitalization experienced a hip fracture, according to Anna-Karin Nume, MD, of the University of Copenhagen.
In this interview at the annual congress of the European Society of Cardiology, Dr. Nume highlights findings from her study, which included 125,763 Danish adults with first-time syncope.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – Patients have an exorbitant 80% increased risk of hospitalization for falls resulting in fracture or head injury in the first year after discharge following a first-ever episode of syncope, according to a Danish national cohort study. One in five patients who sustained a fall resulting in hospitalization experienced a hip fracture, according to Anna-Karin Nume, MD, of the University of Copenhagen.
In this interview at the annual congress of the European Society of Cardiology, Dr. Nume highlights findings from her study, which included 125,763 Danish adults with first-time syncope.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – Patients have an exorbitant 80% increased risk of hospitalization for falls resulting in fracture or head injury in the first year after discharge following a first-ever episode of syncope, according to a Danish national cohort study. One in five patients who sustained a fall resulting in hospitalization experienced a hip fracture, according to Anna-Karin Nume, MD, of the University of Copenhagen.
In this interview at the annual congress of the European Society of Cardiology, Dr. Nume highlights findings from her study, which included 125,763 Danish adults with first-time syncope.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2017