User login
Accelerated aging in schizophrenia
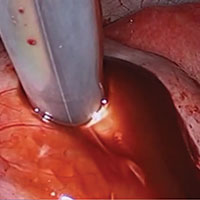
Laparoscopic excision of type I and type II endometriomas

Read the accompanying article: “Endometriomas: Classification and surgical management”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.

Read the accompanying article: “Endometriomas: Classification and surgical management”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.

Read the accompanying article: “Endometriomas: Classification and surgical management”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
VIDEO: Hip, knee replacements fall in Danish RA patients
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: RA patient hip replacements fell from nearly 9/1,000 person-years in 1996 to about 4.5/1,000 person-years in 2011.
Data source: Records from more than 300,000 people in the Danish National Patient Register.
Disclosures: Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
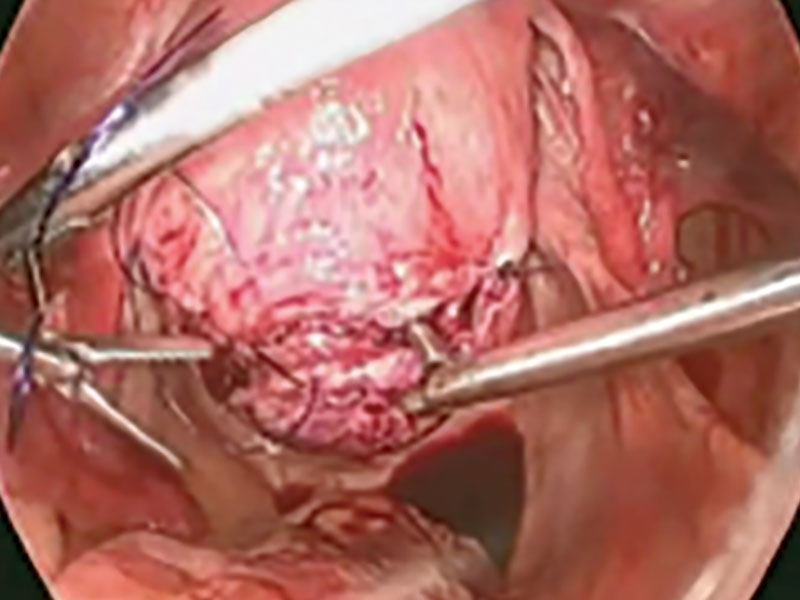
Laparoscopic myomectomy technique
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
VIDEO: Does biologic immunogenicity matter in daily practice?
MADRID – Measuring the formation of antibodies against biologic agents has no real value in daily practice as their presence or absence does not really change how patients are likely to be treated, Johannes W.J. Bijlsma, MD, observed at the European Congress of Rheumatology.
Consider a female patient who is 59 years old, diagnosed with rheumatoid arthritis (RA) in 2014, he said. She was being treated with methotrexate at a dose of 20 mg with additional glucocorticoids, initially given at a dose of 10 mg, reduced to 5 mg after 2 years, and then stopped. The patient experiences a disease flare, however, and for various other reasons is given a tumor necrosis factor inhibitor (TNFi). She does well initially but then has another flare, so would there be any point of measuring anti-drug antibodies (ADAbs) as this point? Not really, Dr. Bijlsma suggested, as the same decision to change the biologic agent would probably result if ADAbs were detected or not.
“If I do not measure them, I decide to change the biological. If I measure them and they are present, I change the biological, and if they are absent, I still change the biological,” said Dr. Bijlsma, professor and head of the department of rheumatology and clinical immunology at University Medical Center Utrecht (the Netherlands).
Following the European League Against Rheumatism (EULAR) recommendations for biologic disease-modifying antirheumatic drug (bDMARD) use (Ann Rheum Dis. 2017;76:960-77) would then mean that the first bDMARD, in this case adalimumab (Humira), would be replaced by another biologic with a different mechanism of action or a second TNFi.
“The immune response is always there,” Dr. Bijlsma said. It does not matter what or how it is administered, introducing any foreign protein, humanized or not, will instigate some kind of immune reaction, he said. The extent to which an immune reaction is raised might vary between biologic agents, but it will be there. He cited a review paper (Rheumatology [Oxford]. 2016;55:210-20) showing that the mean estimated occurrence of ADAbs in patients with RA ranges from 0.6% with the interleukin-6-targeting agent tocilizumab (Actemra) to 30% with infliximab (Remicade).
Measuring the level of ADAbs becomes problematic when considering that different biologics will induce different levels of immune response. The level of detection also will be dependent on which of three current types of assays are used. In addition, “humanization of biological agents is not the key point in preventing anti-drug antibodies,” Dr. Bijlsma said, pointing out that the prevalence of ADAbs against adalimumab did not appear to by any lower than ADAbs against infliximab.
Preventing ADAbs can be achieved by co-administering methotrexate or alternating the treatment schedule, Dr. Bijlsma said. Treatment with methotrexate, which is usually continued when patients start a biologic, “diminishes the immune response,” he noted. Indeed, while 50% of patients who are not treated with this conventional DMARD develop ADAbs, only 14%-35% develop them while taking methotrexate, depending on the dose used.
It is likely to be more useful in clinical practice to measure individual patients’ drug trough levels than to measure ADAb levels, he suggested, with dosing continued or adjusted accordingly for each patient. Using drug trough levels to personalize adalimumab treatment has been tested (Ann Rheum Dis. 2015;74:361-8) using a theoretical algorithm based on whether patients achieve a EULAR response at 6 months. If they do achieve a EULAR response and drug trough levels are between 5 and 12 mg/L or greater than 12 mg/L, then adalimumab treatment should continue. However, if the trough levels fall below 5 mg/L, there is probably no point in continuing treatment and this TNFi should be stopped. If patients do not respond and drug testing shows a trough level above 5 mg/L, then a switch to infliximab might be advantageous, while a trough level below this threshold could indicated that a TNFi with a lower immunogenic potential such as etanercept might be a better choice.
Using drug trough levels is still very much research based right now and is not ready for clinical practice just yet, but the theory is that it could help decide if patients should continue, stop, or perhaps switch their biologic, Dr. Bijlsma said.
Dr. Bijlsma spoke about these issues in a video interview at the congress.
Dr. Bijlsma has worked with many of the pharmaceutical companies that produce biologic agents for the management of rheumatic diseases but had no specific disclosures in relation to his comments.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Measuring the formation of antibodies against biologic agents has no real value in daily practice as their presence or absence does not really change how patients are likely to be treated, Johannes W.J. Bijlsma, MD, observed at the European Congress of Rheumatology.
Consider a female patient who is 59 years old, diagnosed with rheumatoid arthritis (RA) in 2014, he said. She was being treated with methotrexate at a dose of 20 mg with additional glucocorticoids, initially given at a dose of 10 mg, reduced to 5 mg after 2 years, and then stopped. The patient experiences a disease flare, however, and for various other reasons is given a tumor necrosis factor inhibitor (TNFi). She does well initially but then has another flare, so would there be any point of measuring anti-drug antibodies (ADAbs) as this point? Not really, Dr. Bijlsma suggested, as the same decision to change the biologic agent would probably result if ADAbs were detected or not.
“If I do not measure them, I decide to change the biological. If I measure them and they are present, I change the biological, and if they are absent, I still change the biological,” said Dr. Bijlsma, professor and head of the department of rheumatology and clinical immunology at University Medical Center Utrecht (the Netherlands).
Following the European League Against Rheumatism (EULAR) recommendations for biologic disease-modifying antirheumatic drug (bDMARD) use (Ann Rheum Dis. 2017;76:960-77) would then mean that the first bDMARD, in this case adalimumab (Humira), would be replaced by another biologic with a different mechanism of action or a second TNFi.
“The immune response is always there,” Dr. Bijlsma said. It does not matter what or how it is administered, introducing any foreign protein, humanized or not, will instigate some kind of immune reaction, he said. The extent to which an immune reaction is raised might vary between biologic agents, but it will be there. He cited a review paper (Rheumatology [Oxford]. 2016;55:210-20) showing that the mean estimated occurrence of ADAbs in patients with RA ranges from 0.6% with the interleukin-6-targeting agent tocilizumab (Actemra) to 30% with infliximab (Remicade).
Measuring the level of ADAbs becomes problematic when considering that different biologics will induce different levels of immune response. The level of detection also will be dependent on which of three current types of assays are used. In addition, “humanization of biological agents is not the key point in preventing anti-drug antibodies,” Dr. Bijlsma said, pointing out that the prevalence of ADAbs against adalimumab did not appear to by any lower than ADAbs against infliximab.
Preventing ADAbs can be achieved by co-administering methotrexate or alternating the treatment schedule, Dr. Bijlsma said. Treatment with methotrexate, which is usually continued when patients start a biologic, “diminishes the immune response,” he noted. Indeed, while 50% of patients who are not treated with this conventional DMARD develop ADAbs, only 14%-35% develop them while taking methotrexate, depending on the dose used.
It is likely to be more useful in clinical practice to measure individual patients’ drug trough levels than to measure ADAb levels, he suggested, with dosing continued or adjusted accordingly for each patient. Using drug trough levels to personalize adalimumab treatment has been tested (Ann Rheum Dis. 2015;74:361-8) using a theoretical algorithm based on whether patients achieve a EULAR response at 6 months. If they do achieve a EULAR response and drug trough levels are between 5 and 12 mg/L or greater than 12 mg/L, then adalimumab treatment should continue. However, if the trough levels fall below 5 mg/L, there is probably no point in continuing treatment and this TNFi should be stopped. If patients do not respond and drug testing shows a trough level above 5 mg/L, then a switch to infliximab might be advantageous, while a trough level below this threshold could indicated that a TNFi with a lower immunogenic potential such as etanercept might be a better choice.
Using drug trough levels is still very much research based right now and is not ready for clinical practice just yet, but the theory is that it could help decide if patients should continue, stop, or perhaps switch their biologic, Dr. Bijlsma said.
Dr. Bijlsma spoke about these issues in a video interview at the congress.
Dr. Bijlsma has worked with many of the pharmaceutical companies that produce biologic agents for the management of rheumatic diseases but had no specific disclosures in relation to his comments.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Measuring the formation of antibodies against biologic agents has no real value in daily practice as their presence or absence does not really change how patients are likely to be treated, Johannes W.J. Bijlsma, MD, observed at the European Congress of Rheumatology.
Consider a female patient who is 59 years old, diagnosed with rheumatoid arthritis (RA) in 2014, he said. She was being treated with methotrexate at a dose of 20 mg with additional glucocorticoids, initially given at a dose of 10 mg, reduced to 5 mg after 2 years, and then stopped. The patient experiences a disease flare, however, and for various other reasons is given a tumor necrosis factor inhibitor (TNFi). She does well initially but then has another flare, so would there be any point of measuring anti-drug antibodies (ADAbs) as this point? Not really, Dr. Bijlsma suggested, as the same decision to change the biologic agent would probably result if ADAbs were detected or not.
“If I do not measure them, I decide to change the biological. If I measure them and they are present, I change the biological, and if they are absent, I still change the biological,” said Dr. Bijlsma, professor and head of the department of rheumatology and clinical immunology at University Medical Center Utrecht (the Netherlands).
Following the European League Against Rheumatism (EULAR) recommendations for biologic disease-modifying antirheumatic drug (bDMARD) use (Ann Rheum Dis. 2017;76:960-77) would then mean that the first bDMARD, in this case adalimumab (Humira), would be replaced by another biologic with a different mechanism of action or a second TNFi.
“The immune response is always there,” Dr. Bijlsma said. It does not matter what or how it is administered, introducing any foreign protein, humanized or not, will instigate some kind of immune reaction, he said. The extent to which an immune reaction is raised might vary between biologic agents, but it will be there. He cited a review paper (Rheumatology [Oxford]. 2016;55:210-20) showing that the mean estimated occurrence of ADAbs in patients with RA ranges from 0.6% with the interleukin-6-targeting agent tocilizumab (Actemra) to 30% with infliximab (Remicade).
Measuring the level of ADAbs becomes problematic when considering that different biologics will induce different levels of immune response. The level of detection also will be dependent on which of three current types of assays are used. In addition, “humanization of biological agents is not the key point in preventing anti-drug antibodies,” Dr. Bijlsma said, pointing out that the prevalence of ADAbs against adalimumab did not appear to by any lower than ADAbs against infliximab.
Preventing ADAbs can be achieved by co-administering methotrexate or alternating the treatment schedule, Dr. Bijlsma said. Treatment with methotrexate, which is usually continued when patients start a biologic, “diminishes the immune response,” he noted. Indeed, while 50% of patients who are not treated with this conventional DMARD develop ADAbs, only 14%-35% develop them while taking methotrexate, depending on the dose used.
It is likely to be more useful in clinical practice to measure individual patients’ drug trough levels than to measure ADAb levels, he suggested, with dosing continued or adjusted accordingly for each patient. Using drug trough levels to personalize adalimumab treatment has been tested (Ann Rheum Dis. 2015;74:361-8) using a theoretical algorithm based on whether patients achieve a EULAR response at 6 months. If they do achieve a EULAR response and drug trough levels are between 5 and 12 mg/L or greater than 12 mg/L, then adalimumab treatment should continue. However, if the trough levels fall below 5 mg/L, there is probably no point in continuing treatment and this TNFi should be stopped. If patients do not respond and drug testing shows a trough level above 5 mg/L, then a switch to infliximab might be advantageous, while a trough level below this threshold could indicated that a TNFi with a lower immunogenic potential such as etanercept might be a better choice.
Using drug trough levels is still very much research based right now and is not ready for clinical practice just yet, but the theory is that it could help decide if patients should continue, stop, or perhaps switch their biologic, Dr. Bijlsma said.
Dr. Bijlsma spoke about these issues in a video interview at the congress.
Dr. Bijlsma has worked with many of the pharmaceutical companies that produce biologic agents for the management of rheumatic diseases but had no specific disclosures in relation to his comments.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE EULAR 2017 CONGRESS
VIDEO: Cancer immunotherapies activate rheumatologic adverse effects
MADRID – The introduction of immune checkpoint inhibitor drugs has “been great for cancer but bad for rheumatology.”
That’s the gist of the immunologic adverse effect fallout from the immunomodulatory revolution that’s recently swept oncology, Leonard Calabrese, DO, said in a video interview during the European Congress of Rheumatology.
Results from a recent survey of U.S. rheumatologists run by Dr. Calabrese and his associates showed that “more than a quarter” now have seen at least one patient who experienced activation of a rheumatologic disease after starting treatment with an immune checkpoint inhibitor, said Dr. Calabrese, head of the section of clinical immunology at the Cleveland Clinic in Ohio.
Unlike most other immunological adverse effects caused by immune checkpoint inhibitors, the rheumatologic complications usually don’t resolve when treatment stops, he added.
These adverse effects represent a new wrinkle for the practice of rheumatology and are now something that clinicians must familiarize themselves with, Dr. Calabrese advised.
Dr. Calabrese reported that he is a consultant to Bristol-Myers Squibb.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – The introduction of immune checkpoint inhibitor drugs has “been great for cancer but bad for rheumatology.”
That’s the gist of the immunologic adverse effect fallout from the immunomodulatory revolution that’s recently swept oncology, Leonard Calabrese, DO, said in a video interview during the European Congress of Rheumatology.
Results from a recent survey of U.S. rheumatologists run by Dr. Calabrese and his associates showed that “more than a quarter” now have seen at least one patient who experienced activation of a rheumatologic disease after starting treatment with an immune checkpoint inhibitor, said Dr. Calabrese, head of the section of clinical immunology at the Cleveland Clinic in Ohio.
Unlike most other immunological adverse effects caused by immune checkpoint inhibitors, the rheumatologic complications usually don’t resolve when treatment stops, he added.
These adverse effects represent a new wrinkle for the practice of rheumatology and are now something that clinicians must familiarize themselves with, Dr. Calabrese advised.
Dr. Calabrese reported that he is a consultant to Bristol-Myers Squibb.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – The introduction of immune checkpoint inhibitor drugs has “been great for cancer but bad for rheumatology.”
That’s the gist of the immunologic adverse effect fallout from the immunomodulatory revolution that’s recently swept oncology, Leonard Calabrese, DO, said in a video interview during the European Congress of Rheumatology.
Results from a recent survey of U.S. rheumatologists run by Dr. Calabrese and his associates showed that “more than a quarter” now have seen at least one patient who experienced activation of a rheumatologic disease after starting treatment with an immune checkpoint inhibitor, said Dr. Calabrese, head of the section of clinical immunology at the Cleveland Clinic in Ohio.
Unlike most other immunological adverse effects caused by immune checkpoint inhibitors, the rheumatologic complications usually don’t resolve when treatment stops, he added.
These adverse effects represent a new wrinkle for the practice of rheumatology and are now something that clinicians must familiarize themselves with, Dr. Calabrese advised.
Dr. Calabrese reported that he is a consultant to Bristol-Myers Squibb.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE EULAR 2017 CONGRESS
VIDEO: Dr. William J. Gradishar shares breast cancer take-aways from ASCO 2017
CHICAGO – William J. Gradishar, MD, outlines key research on breast cancer treatment presented at the annual meeting of the American Society of Clinical Oncology.
In a video interview, Dr. Gradishar, the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, discusses the take-home messages on pertuzumab for HER2+ breast cancer, PARP inhibitors for BRCA-mutated breast cancer, and CDK4/6 inhibitors for ER+ breast cancers.
In another video interview, Katherine O’Brien of the Metastatic Breast Cancer Network provides the patient advocate view on this years’ meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – William J. Gradishar, MD, outlines key research on breast cancer treatment presented at the annual meeting of the American Society of Clinical Oncology.
In a video interview, Dr. Gradishar, the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, discusses the take-home messages on pertuzumab for HER2+ breast cancer, PARP inhibitors for BRCA-mutated breast cancer, and CDK4/6 inhibitors for ER+ breast cancers.
In another video interview, Katherine O’Brien of the Metastatic Breast Cancer Network provides the patient advocate view on this years’ meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – William J. Gradishar, MD, outlines key research on breast cancer treatment presented at the annual meeting of the American Society of Clinical Oncology.
In a video interview, Dr. Gradishar, the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, discusses the take-home messages on pertuzumab for HER2+ breast cancer, PARP inhibitors for BRCA-mutated breast cancer, and CDK4/6 inhibitors for ER+ breast cancers.
In another video interview, Katherine O’Brien of the Metastatic Breast Cancer Network provides the patient advocate view on this years’ meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM ASCO 2017
VIDEO: Tocilizumab tested in children with sJIA under 2 years old
MADRID – The results of the first trial of a biologic agent in children less than 2 years of age with systemic juvenile idiopathic arthritis (sJIA) suggest that tocilizumab is likely to be effective in this age group.
“sJIA is the most severe form of childhood arthritis, and as you are aware, it’s the most difficult to treat as well,” said Navita L. Mallalieu, PhD, director of clinical pharmacology at Roche Innovation Center New York, the company that funded the study.
Tocilizumab (Actemra) has been available for the treatment of sJIA, both in the United States and the European Union since 2011, she observed at the European Congress of Rheumatology, but only for children aged 2 years or older at the current time.
Because of this prior history of use in sJIA, “we have confidence in the safety profile, and so we were able to go to the next step of testing children who were even younger than 2 years of age,” Dr. Mallalieu said in a video interview.
[polldaddy:9771949]
Dr. Mallalieu presented findings from an open-label, single-arm, phase I trial that evaluated a 12 mg/kg dosing regimen of tocilizumab, which was given intravenously every 2 weeks for 12 weeks. Eleven children were studied who had a mean age of 1.3 years and active disease for at least 1 month despite treatment with glucocorticoids or nonsteroidal anti-inflammatory drugs.
The primary endpoint was the pharmacokinetics of tocilizumab in this younger patient population, and secondary endpoints were safety, pharmacodynamics, and exploring the efficacy over 12 weeks on top of stable background therapy, she explained.
Results showed that tocilizumab in children under 2 years of age could achieve pharmacokinetics similar to those seen in older children in the TENDER trial (N Engl J Med. 2012;367:2385-95), which is the trial that helped the biologic get licensed for use in the older sJIA population. Reductions in soluble interleukin-6 receptor, C-reactive protein, and the erythrocyte sedimentation rate were seen, again to a similar extent as seen in the TENDER trial. There was also an indication that similar reductions in the Juvenile Arthritis Disease Activity Score (JADAS)-71 score could be achieved, Dr. Mallalieu reported.
While the pattern and nature of adverse events were similar to those seen in the TENDER trial, there were more cases of hypersensitivity in this phase I study. Four cases of hypersensitivity were clinically confirmed, three of which were deemed serious. The three serious cases were observed at day 15, with two of the cases associated with multiple signs and symptoms that were confounded by either subclinical macrophage activation syndrome (MAS) or a faster infusion rate. One patient had urticaria and was hospitalized for observation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – The results of the first trial of a biologic agent in children less than 2 years of age with systemic juvenile idiopathic arthritis (sJIA) suggest that tocilizumab is likely to be effective in this age group.
“sJIA is the most severe form of childhood arthritis, and as you are aware, it’s the most difficult to treat as well,” said Navita L. Mallalieu, PhD, director of clinical pharmacology at Roche Innovation Center New York, the company that funded the study.
Tocilizumab (Actemra) has been available for the treatment of sJIA, both in the United States and the European Union since 2011, she observed at the European Congress of Rheumatology, but only for children aged 2 years or older at the current time.
Because of this prior history of use in sJIA, “we have confidence in the safety profile, and so we were able to go to the next step of testing children who were even younger than 2 years of age,” Dr. Mallalieu said in a video interview.
[polldaddy:9771949]
Dr. Mallalieu presented findings from an open-label, single-arm, phase I trial that evaluated a 12 mg/kg dosing regimen of tocilizumab, which was given intravenously every 2 weeks for 12 weeks. Eleven children were studied who had a mean age of 1.3 years and active disease for at least 1 month despite treatment with glucocorticoids or nonsteroidal anti-inflammatory drugs.
The primary endpoint was the pharmacokinetics of tocilizumab in this younger patient population, and secondary endpoints were safety, pharmacodynamics, and exploring the efficacy over 12 weeks on top of stable background therapy, she explained.
Results showed that tocilizumab in children under 2 years of age could achieve pharmacokinetics similar to those seen in older children in the TENDER trial (N Engl J Med. 2012;367:2385-95), which is the trial that helped the biologic get licensed for use in the older sJIA population. Reductions in soluble interleukin-6 receptor, C-reactive protein, and the erythrocyte sedimentation rate were seen, again to a similar extent as seen in the TENDER trial. There was also an indication that similar reductions in the Juvenile Arthritis Disease Activity Score (JADAS)-71 score could be achieved, Dr. Mallalieu reported.
While the pattern and nature of adverse events were similar to those seen in the TENDER trial, there were more cases of hypersensitivity in this phase I study. Four cases of hypersensitivity were clinically confirmed, three of which were deemed serious. The three serious cases were observed at day 15, with two of the cases associated with multiple signs and symptoms that were confounded by either subclinical macrophage activation syndrome (MAS) or a faster infusion rate. One patient had urticaria and was hospitalized for observation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – The results of the first trial of a biologic agent in children less than 2 years of age with systemic juvenile idiopathic arthritis (sJIA) suggest that tocilizumab is likely to be effective in this age group.
“sJIA is the most severe form of childhood arthritis, and as you are aware, it’s the most difficult to treat as well,” said Navita L. Mallalieu, PhD, director of clinical pharmacology at Roche Innovation Center New York, the company that funded the study.
Tocilizumab (Actemra) has been available for the treatment of sJIA, both in the United States and the European Union since 2011, she observed at the European Congress of Rheumatology, but only for children aged 2 years or older at the current time.
Because of this prior history of use in sJIA, “we have confidence in the safety profile, and so we were able to go to the next step of testing children who were even younger than 2 years of age,” Dr. Mallalieu said in a video interview.
[polldaddy:9771949]
Dr. Mallalieu presented findings from an open-label, single-arm, phase I trial that evaluated a 12 mg/kg dosing regimen of tocilizumab, which was given intravenously every 2 weeks for 12 weeks. Eleven children were studied who had a mean age of 1.3 years and active disease for at least 1 month despite treatment with glucocorticoids or nonsteroidal anti-inflammatory drugs.
The primary endpoint was the pharmacokinetics of tocilizumab in this younger patient population, and secondary endpoints were safety, pharmacodynamics, and exploring the efficacy over 12 weeks on top of stable background therapy, she explained.
Results showed that tocilizumab in children under 2 years of age could achieve pharmacokinetics similar to those seen in older children in the TENDER trial (N Engl J Med. 2012;367:2385-95), which is the trial that helped the biologic get licensed for use in the older sJIA population. Reductions in soluble interleukin-6 receptor, C-reactive protein, and the erythrocyte sedimentation rate were seen, again to a similar extent as seen in the TENDER trial. There was also an indication that similar reductions in the Juvenile Arthritis Disease Activity Score (JADAS)-71 score could be achieved, Dr. Mallalieu reported.
While the pattern and nature of adverse events were similar to those seen in the TENDER trial, there were more cases of hypersensitivity in this phase I study. Four cases of hypersensitivity were clinically confirmed, three of which were deemed serious. The three serious cases were observed at day 15, with two of the cases associated with multiple signs and symptoms that were confounded by either subclinical macrophage activation syndrome (MAS) or a faster infusion rate. One patient had urticaria and was hospitalized for observation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Similar pharmacokinetics were observed in children under 2 years of age as those seen in a prior study of older children.
Data source: Open-label, single-arm, phase I trial that evaluated a 12-mg/kg dosing regimen of tocilizumab given intravenously every 2 weeks for 12 weeks.
Disclosures: Roche funded the study. The presenter is an employee of Roche.
VIDEO: Childhood second-hand smoke boosts RA incidence
MADRID – Second-hand smoke exposure to children was about as potent a trigger for future rheumatoid arthritis as active smoking by an adult, based on an analysis of data collected from more than 70,000 French women followed for an average of more than 20 years
“This is the first demonstration of a rheumatoid arthritis risk associated with passive smoking,” Raphaèle Seror, MD, said at the European Congress of Rheumatology.
“This is an important finding because we can avoid passive smoke exposure,” Dr. Seror added in a video interview . The imperative to eliminate second-hand smoke exposure to children is particularly acute for those with a genetic risk for developing rheumatoid arthritis (RA), specifically children with a parent diagnosed with RA, suggested Dr. Seror, a professor of rheumatology at the University of Paris–South.
She and her associates used data collected in the E3N, a longitudinal French epidemiological study that enrolled nearly 100,000 women in 1990 when they were 40-65 years old and collected health data by questionnaire every 2-3 years for an average of 21 years. They identified from this cohort women with “confirmed” RA based on a self report of having incident RA during follow-up plus a coincident record of reimbursement for a prescription for an RA-specific treatment, such as methotrexate or a biological disease-modifying drug.
This identified 389 women with confirmed incident RA, including 350 with a complete smoking history that made the current analysis possible. The study also included 70,248 women who did not develop RA and who had provided a complete smoking history.
The analysis showed that women who reported a history of second-hand smoke exposure estimated at more than an hour daily as children but without a history of active smoking had a 43% higher rate of incident RA compared with never smoker women without a history of passive smoke exposure, Dr. Seror reported. This association just missed reaching statistical significance, a limitation that Dr. Seror attributed to a power issue as the analysis included only 30 women who had incident RA and a history of second-hand smoke exposure without adult smoke exposure. By comparison, women in the study with a history of active smoking without childhood exposure linked had a 37% increased incidence of RA, a finding that confirmed the well-known link between smoking and RA incidence.
The study also found that women with both second-hand smoke exposure as children and adult smoking linked with a 73% higher RA incidence, an indication that the contributions from second-hand smoke in children and active smoking by adults were not only similar in magnitude but also worked additively to promote RA development.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Second-hand smoke exposure to children was about as potent a trigger for future rheumatoid arthritis as active smoking by an adult, based on an analysis of data collected from more than 70,000 French women followed for an average of more than 20 years
“This is the first demonstration of a rheumatoid arthritis risk associated with passive smoking,” Raphaèle Seror, MD, said at the European Congress of Rheumatology.
“This is an important finding because we can avoid passive smoke exposure,” Dr. Seror added in a video interview . The imperative to eliminate second-hand smoke exposure to children is particularly acute for those with a genetic risk for developing rheumatoid arthritis (RA), specifically children with a parent diagnosed with RA, suggested Dr. Seror, a professor of rheumatology at the University of Paris–South.
She and her associates used data collected in the E3N, a longitudinal French epidemiological study that enrolled nearly 100,000 women in 1990 when they were 40-65 years old and collected health data by questionnaire every 2-3 years for an average of 21 years. They identified from this cohort women with “confirmed” RA based on a self report of having incident RA during follow-up plus a coincident record of reimbursement for a prescription for an RA-specific treatment, such as methotrexate or a biological disease-modifying drug.
This identified 389 women with confirmed incident RA, including 350 with a complete smoking history that made the current analysis possible. The study also included 70,248 women who did not develop RA and who had provided a complete smoking history.
The analysis showed that women who reported a history of second-hand smoke exposure estimated at more than an hour daily as children but without a history of active smoking had a 43% higher rate of incident RA compared with never smoker women without a history of passive smoke exposure, Dr. Seror reported. This association just missed reaching statistical significance, a limitation that Dr. Seror attributed to a power issue as the analysis included only 30 women who had incident RA and a history of second-hand smoke exposure without adult smoke exposure. By comparison, women in the study with a history of active smoking without childhood exposure linked had a 37% increased incidence of RA, a finding that confirmed the well-known link between smoking and RA incidence.
The study also found that women with both second-hand smoke exposure as children and adult smoking linked with a 73% higher RA incidence, an indication that the contributions from second-hand smoke in children and active smoking by adults were not only similar in magnitude but also worked additively to promote RA development.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Second-hand smoke exposure to children was about as potent a trigger for future rheumatoid arthritis as active smoking by an adult, based on an analysis of data collected from more than 70,000 French women followed for an average of more than 20 years
“This is the first demonstration of a rheumatoid arthritis risk associated with passive smoking,” Raphaèle Seror, MD, said at the European Congress of Rheumatology.
“This is an important finding because we can avoid passive smoke exposure,” Dr. Seror added in a video interview . The imperative to eliminate second-hand smoke exposure to children is particularly acute for those with a genetic risk for developing rheumatoid arthritis (RA), specifically children with a parent diagnosed with RA, suggested Dr. Seror, a professor of rheumatology at the University of Paris–South.
She and her associates used data collected in the E3N, a longitudinal French epidemiological study that enrolled nearly 100,000 women in 1990 when they were 40-65 years old and collected health data by questionnaire every 2-3 years for an average of 21 years. They identified from this cohort women with “confirmed” RA based on a self report of having incident RA during follow-up plus a coincident record of reimbursement for a prescription for an RA-specific treatment, such as methotrexate or a biological disease-modifying drug.
This identified 389 women with confirmed incident RA, including 350 with a complete smoking history that made the current analysis possible. The study also included 70,248 women who did not develop RA and who had provided a complete smoking history.
The analysis showed that women who reported a history of second-hand smoke exposure estimated at more than an hour daily as children but without a history of active smoking had a 43% higher rate of incident RA compared with never smoker women without a history of passive smoke exposure, Dr. Seror reported. This association just missed reaching statistical significance, a limitation that Dr. Seror attributed to a power issue as the analysis included only 30 women who had incident RA and a history of second-hand smoke exposure without adult smoke exposure. By comparison, women in the study with a history of active smoking without childhood exposure linked had a 37% increased incidence of RA, a finding that confirmed the well-known link between smoking and RA incidence.
The study also found that women with both second-hand smoke exposure as children and adult smoking linked with a 73% higher RA incidence, an indication that the contributions from second-hand smoke in children and active smoking by adults were not only similar in magnitude but also worked additively to promote RA development.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Women with significant second-hand smoke exposure as children had a 43% increased rate of incident rheumatoid arthritis.
Data source: E3N, a prospective, longitudinal, observational study of nearly 100,000 French women begun in 1990.
Disclosures: Dr. Seror had no relevant disclosures.
VIDEO: Guselkumab bests placebo in psoriatic arthritis
MADRID – Guselkumab, an IL-23 blocker that has proved its mettle in psoriasis, also posted excellent results in an early-phase psoriatic arthritis trial.
The fully human monoclonal antibody, which is being developed by Janssen, targets the p19 subunit of interleukin-23, Atul Deodhar, MD, said at the European Congress of Rheumatology. The 52-week phase 2a study randomized 149 patients to 100 mg guselkumab or placebo given subcutaneously at baseline and week 4, then every 8 weeks, for 24 weeks. Patients who didn’t respond adequately in either arm could begin using ustekinumab. After 24 weeks, everyone remaining in the placebo group switched to guselkumab, and patients in both arms continued open-label treatment until 52 weeks.
Dr. Deodhar of the Oregon Health and Science University, Portland, reported 24-week outcomes. A full year of data will be presented at the American College of Rheumatology meeting in San Diego in November.
Guselkumab aced the study’s primary endpoint – ACR 20 response by week 24 (58% vs. 18% with the placebo, P less than .001). Improvement developed rapidly, with a significant separation between the groups by week 4 (21% vs. 0%; P less than .001). It also succeeded on the secondary endpoints of ACR 50 response (34% vs. 10%) and ACR 70 (14% vs. 2%). The antibody also significantly outperformed placebo on the Health Assessment Questionnaire Disability Index (HAQ-DI), 36-Item Short Form Health Survey (SF-36), and Minimal Disease Activity score. Skin clearance was strikingly good, Dr. Deodhar said: 39% achieved complete clearance, with a Psoriasis Area and Severity Index (PASI) score of 100; 66% achieved a PASI of 90; and 79% acheived a PASI of 75.
Guselkumab also significantly improved enthesitis – a symptom that can be terribly troubling for patients with psoriatic arthritis. Enthesitis was present in 106 at baseline. By 24 weeks, it had resolved in 56.6% of those taking the antibody and 29% of those taking the placebo (P = .012.).
A phase III trial in psoriatic arthritis will be forthcoming, Dr. Deodhar said.
Dr. Deodhar has received research funding and is a consultant for Janssen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
MADRID – Guselkumab, an IL-23 blocker that has proved its mettle in psoriasis, also posted excellent results in an early-phase psoriatic arthritis trial.
The fully human monoclonal antibody, which is being developed by Janssen, targets the p19 subunit of interleukin-23, Atul Deodhar, MD, said at the European Congress of Rheumatology. The 52-week phase 2a study randomized 149 patients to 100 mg guselkumab or placebo given subcutaneously at baseline and week 4, then every 8 weeks, for 24 weeks. Patients who didn’t respond adequately in either arm could begin using ustekinumab. After 24 weeks, everyone remaining in the placebo group switched to guselkumab, and patients in both arms continued open-label treatment until 52 weeks.
Dr. Deodhar of the Oregon Health and Science University, Portland, reported 24-week outcomes. A full year of data will be presented at the American College of Rheumatology meeting in San Diego in November.
Guselkumab aced the study’s primary endpoint – ACR 20 response by week 24 (58% vs. 18% with the placebo, P less than .001). Improvement developed rapidly, with a significant separation between the groups by week 4 (21% vs. 0%; P less than .001). It also succeeded on the secondary endpoints of ACR 50 response (34% vs. 10%) and ACR 70 (14% vs. 2%). The antibody also significantly outperformed placebo on the Health Assessment Questionnaire Disability Index (HAQ-DI), 36-Item Short Form Health Survey (SF-36), and Minimal Disease Activity score. Skin clearance was strikingly good, Dr. Deodhar said: 39% achieved complete clearance, with a Psoriasis Area and Severity Index (PASI) score of 100; 66% achieved a PASI of 90; and 79% acheived a PASI of 75.
Guselkumab also significantly improved enthesitis – a symptom that can be terribly troubling for patients with psoriatic arthritis. Enthesitis was present in 106 at baseline. By 24 weeks, it had resolved in 56.6% of those taking the antibody and 29% of those taking the placebo (P = .012.).
A phase III trial in psoriatic arthritis will be forthcoming, Dr. Deodhar said.
Dr. Deodhar has received research funding and is a consultant for Janssen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
MADRID – Guselkumab, an IL-23 blocker that has proved its mettle in psoriasis, also posted excellent results in an early-phase psoriatic arthritis trial.
The fully human monoclonal antibody, which is being developed by Janssen, targets the p19 subunit of interleukin-23, Atul Deodhar, MD, said at the European Congress of Rheumatology. The 52-week phase 2a study randomized 149 patients to 100 mg guselkumab or placebo given subcutaneously at baseline and week 4, then every 8 weeks, for 24 weeks. Patients who didn’t respond adequately in either arm could begin using ustekinumab. After 24 weeks, everyone remaining in the placebo group switched to guselkumab, and patients in both arms continued open-label treatment until 52 weeks.
Dr. Deodhar of the Oregon Health and Science University, Portland, reported 24-week outcomes. A full year of data will be presented at the American College of Rheumatology meeting in San Diego in November.
Guselkumab aced the study’s primary endpoint – ACR 20 response by week 24 (58% vs. 18% with the placebo, P less than .001). Improvement developed rapidly, with a significant separation between the groups by week 4 (21% vs. 0%; P less than .001). It also succeeded on the secondary endpoints of ACR 50 response (34% vs. 10%) and ACR 70 (14% vs. 2%). The antibody also significantly outperformed placebo on the Health Assessment Questionnaire Disability Index (HAQ-DI), 36-Item Short Form Health Survey (SF-36), and Minimal Disease Activity score. Skin clearance was strikingly good, Dr. Deodhar said: 39% achieved complete clearance, with a Psoriasis Area and Severity Index (PASI) score of 100; 66% achieved a PASI of 90; and 79% acheived a PASI of 75.
Guselkumab also significantly improved enthesitis – a symptom that can be terribly troubling for patients with psoriatic arthritis. Enthesitis was present in 106 at baseline. By 24 weeks, it had resolved in 56.6% of those taking the antibody and 29% of those taking the placebo (P = .012.).
A phase III trial in psoriatic arthritis will be forthcoming, Dr. Deodhar said.
Dr. Deodhar has received research funding and is a consultant for Janssen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: ACR 20 response at 24 weeks was 58% in the treated group, vs. 18% in the placebo group.
Data source: The 52-week 2a study randomized 149 patients to guselkumab or placebo.
Disclosures: Dr. Deodhar has received research monies and is a consultant for Janssen, which is developing guselkumab.