User login
VIDEO: Large distal nongranular colorectal polyps were most likely to contain occult invasive cancers
Large sessile or flat colorectal polyps or laterally spreading lesions were most likely to contain covert malignancies when their location was rectosigmoid, their Paris classification was 0-Is or 0-IIa+Is, and they were nongranular, according to the results of a multicenter prospective cohort study of 2,106 consecutive patients reported in the September issue of Gastroenterology (doi: 10.1053/j.gastro.2017.05.047).
“Distal nongranular lesions have a high risk of occult SMIC [submucosal invasive cancer], whereas proximal, granular 0-IIa lesions, after a careful assessment for features associated with SMIC, have a very low risk,” wrote Nicholas G. Burgess, MD, of Westmead Hospital, Sydney, with his associates. “These findings can be used to inform decisions [about] which patients should undergo endoscopic submucosal dissection, endoscopic mucosal resection, or surgery.”
Source: American Gastroenterological Association
Many studies of colonic lesions have examined predictors of SMIC. Nonetheless, clinicians need more information on factors that improve clinical decision making, especially as colonic endoscopic submucosal dissection becomes more accessible, the researchers said. Large colonic lesions can contain submucosal invasive SMICs that are not visible on endoscopy, and characterizing predictors of this occurrence could help patients and clinicians decide between endoscopic submucosal dissection and endoscopic mucosal resection. To do so, the researchers analyzed histologic specimens from 2,277 colonic lesions above 20 mm (average size, 37 mm) that lacked overt endoscopic high-risk features. The study ran from 2008 through 2016, study participants averaged 68 years of age, and 53% were male. A total of 171 lesions (8%) had evidence of SMIC on pathologic review, and 138 lesions had covert SMIC. Predictors of overt and occult SMIC included Kudo pit pattern V, a depressed component (0-IIc), rectosigmoid location, 0-Is or 0-IIa+Is Paris classification, nongranular surface morphology, and larger size. After excluding lesions with obvious SMIC features – including serrated lesions and those with depressed components (Kudo pit pattern of V and Paris 0-IIc) – the strongest predictors of occult SMIC included Paris classification, surface morphology, size, and location.
“Proximal 0-IIa G or 0-Is granular lesions had the lowest risk of SMIC (0.7% and 2.3%), whereas distal 0-Is nongranular lesions had the highest risk (21.4%),” the investigators added. Lesion location, size, and combined Paris classification and surface topography showed the best fit in a multivariable model. Notably, rectosigmoid lesions had nearly twice the odds of containing covert SMIC, compared with proximal lesions (odds ratio, 1.9; 95% confidence interval, 1.2-3.0; P = .01). Other significant predictors of covert SMIC in the multivariable model included combined Paris classification, surface morphology (OR, 4.0; 95% CI, 1.2-12.7; P = .02), and increasing size (OR, 1.2 per 10-mm increase; 95% CI, 1.04-1.3; P = .01). Increased size showed an even greater effect in lesions exceeding 50 mm.
Clinicians can use these factors to help evaluate risk of invasive cancer in lesions without overt SMIC, the researchers said. “One lesion type that differs from the pattern is 0-IIa nongranular lesions,” they noted. “Once lesions with overt evidence of SMIC are excluded, these lesions have a low risk (4.2%) of harboring underlying cancer.” Although 42% of lesions with covert SMIC were SM1 (potentially curable by endoscopic resection), no predictor of covert SMIC also predicted SMI status.
Funders included Cancer Institute of New South Wales and Gallipoli Medical Research Foundation. The investigators had no conflicts of interest.
In recent years, substantial efforts have been made to improve both colonoscopy preparation and endoscopic image quality to achieve improved polyp detection. In addition, while large, complex colon polyps (typically greater than 20 mm in size) previously were often referred for surgical resection, improved polyp resection techniques and equipment have led to the ability to remove many such lesions in a piecemeal fashion or en bloc via endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD).
The authors are to be congratulated for their meticulous and sustained efforts in acquiring and analyzing this data. These results provide endoscopists with some important, practical, and entirely visual criteria to assess upon identification of large colon polyps that can aid in determining which type of endoscopy therapy, if any, to embark upon. Avoiding EMR when there is a reasonably high probability of invasive disease will allow for choosing a more appropriate technique such as ESD (which is becoming increasingly available in the West) or surgery. In addition, patients can avoid the unnecessary EMR-related risks of bleeding and perforation when this technique is likely to result in an inadequate resection. Future work should assess whether this information can be widely adopted and utilized to achieve similar predictive accuracy in nonexpert settings.
V. Raman Muthusamy, MD, is director, interventional and general endoscopy, clinical professor of medicine, digestive diseases/gastroenterology, University of California, Los Angeles School of Medicine. He is a consultant for Medtronic and Boston Scientific.
In recent years, substantial efforts have been made to improve both colonoscopy preparation and endoscopic image quality to achieve improved polyp detection. In addition, while large, complex colon polyps (typically greater than 20 mm in size) previously were often referred for surgical resection, improved polyp resection techniques and equipment have led to the ability to remove many such lesions in a piecemeal fashion or en bloc via endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD).
The authors are to be congratulated for their meticulous and sustained efforts in acquiring and analyzing this data. These results provide endoscopists with some important, practical, and entirely visual criteria to assess upon identification of large colon polyps that can aid in determining which type of endoscopy therapy, if any, to embark upon. Avoiding EMR when there is a reasonably high probability of invasive disease will allow for choosing a more appropriate technique such as ESD (which is becoming increasingly available in the West) or surgery. In addition, patients can avoid the unnecessary EMR-related risks of bleeding and perforation when this technique is likely to result in an inadequate resection. Future work should assess whether this information can be widely adopted and utilized to achieve similar predictive accuracy in nonexpert settings.
V. Raman Muthusamy, MD, is director, interventional and general endoscopy, clinical professor of medicine, digestive diseases/gastroenterology, University of California, Los Angeles School of Medicine. He is a consultant for Medtronic and Boston Scientific.
In recent years, substantial efforts have been made to improve both colonoscopy preparation and endoscopic image quality to achieve improved polyp detection. In addition, while large, complex colon polyps (typically greater than 20 mm in size) previously were often referred for surgical resection, improved polyp resection techniques and equipment have led to the ability to remove many such lesions in a piecemeal fashion or en bloc via endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD).
The authors are to be congratulated for their meticulous and sustained efforts in acquiring and analyzing this data. These results provide endoscopists with some important, practical, and entirely visual criteria to assess upon identification of large colon polyps that can aid in determining which type of endoscopy therapy, if any, to embark upon. Avoiding EMR when there is a reasonably high probability of invasive disease will allow for choosing a more appropriate technique such as ESD (which is becoming increasingly available in the West) or surgery. In addition, patients can avoid the unnecessary EMR-related risks of bleeding and perforation when this technique is likely to result in an inadequate resection. Future work should assess whether this information can be widely adopted and utilized to achieve similar predictive accuracy in nonexpert settings.
V. Raman Muthusamy, MD, is director, interventional and general endoscopy, clinical professor of medicine, digestive diseases/gastroenterology, University of California, Los Angeles School of Medicine. He is a consultant for Medtronic and Boston Scientific.
Large sessile or flat colorectal polyps or laterally spreading lesions were most likely to contain covert malignancies when their location was rectosigmoid, their Paris classification was 0-Is or 0-IIa+Is, and they were nongranular, according to the results of a multicenter prospective cohort study of 2,106 consecutive patients reported in the September issue of Gastroenterology (doi: 10.1053/j.gastro.2017.05.047).
“Distal nongranular lesions have a high risk of occult SMIC [submucosal invasive cancer], whereas proximal, granular 0-IIa lesions, after a careful assessment for features associated with SMIC, have a very low risk,” wrote Nicholas G. Burgess, MD, of Westmead Hospital, Sydney, with his associates. “These findings can be used to inform decisions [about] which patients should undergo endoscopic submucosal dissection, endoscopic mucosal resection, or surgery.”
Source: American Gastroenterological Association
Many studies of colonic lesions have examined predictors of SMIC. Nonetheless, clinicians need more information on factors that improve clinical decision making, especially as colonic endoscopic submucosal dissection becomes more accessible, the researchers said. Large colonic lesions can contain submucosal invasive SMICs that are not visible on endoscopy, and characterizing predictors of this occurrence could help patients and clinicians decide between endoscopic submucosal dissection and endoscopic mucosal resection. To do so, the researchers analyzed histologic specimens from 2,277 colonic lesions above 20 mm (average size, 37 mm) that lacked overt endoscopic high-risk features. The study ran from 2008 through 2016, study participants averaged 68 years of age, and 53% were male. A total of 171 lesions (8%) had evidence of SMIC on pathologic review, and 138 lesions had covert SMIC. Predictors of overt and occult SMIC included Kudo pit pattern V, a depressed component (0-IIc), rectosigmoid location, 0-Is or 0-IIa+Is Paris classification, nongranular surface morphology, and larger size. After excluding lesions with obvious SMIC features – including serrated lesions and those with depressed components (Kudo pit pattern of V and Paris 0-IIc) – the strongest predictors of occult SMIC included Paris classification, surface morphology, size, and location.
“Proximal 0-IIa G or 0-Is granular lesions had the lowest risk of SMIC (0.7% and 2.3%), whereas distal 0-Is nongranular lesions had the highest risk (21.4%),” the investigators added. Lesion location, size, and combined Paris classification and surface topography showed the best fit in a multivariable model. Notably, rectosigmoid lesions had nearly twice the odds of containing covert SMIC, compared with proximal lesions (odds ratio, 1.9; 95% confidence interval, 1.2-3.0; P = .01). Other significant predictors of covert SMIC in the multivariable model included combined Paris classification, surface morphology (OR, 4.0; 95% CI, 1.2-12.7; P = .02), and increasing size (OR, 1.2 per 10-mm increase; 95% CI, 1.04-1.3; P = .01). Increased size showed an even greater effect in lesions exceeding 50 mm.
Clinicians can use these factors to help evaluate risk of invasive cancer in lesions without overt SMIC, the researchers said. “One lesion type that differs from the pattern is 0-IIa nongranular lesions,” they noted. “Once lesions with overt evidence of SMIC are excluded, these lesions have a low risk (4.2%) of harboring underlying cancer.” Although 42% of lesions with covert SMIC were SM1 (potentially curable by endoscopic resection), no predictor of covert SMIC also predicted SMI status.
Funders included Cancer Institute of New South Wales and Gallipoli Medical Research Foundation. The investigators had no conflicts of interest.
Large sessile or flat colorectal polyps or laterally spreading lesions were most likely to contain covert malignancies when their location was rectosigmoid, their Paris classification was 0-Is or 0-IIa+Is, and they were nongranular, according to the results of a multicenter prospective cohort study of 2,106 consecutive patients reported in the September issue of Gastroenterology (doi: 10.1053/j.gastro.2017.05.047).
“Distal nongranular lesions have a high risk of occult SMIC [submucosal invasive cancer], whereas proximal, granular 0-IIa lesions, after a careful assessment for features associated with SMIC, have a very low risk,” wrote Nicholas G. Burgess, MD, of Westmead Hospital, Sydney, with his associates. “These findings can be used to inform decisions [about] which patients should undergo endoscopic submucosal dissection, endoscopic mucosal resection, or surgery.”
Source: American Gastroenterological Association
Many studies of colonic lesions have examined predictors of SMIC. Nonetheless, clinicians need more information on factors that improve clinical decision making, especially as colonic endoscopic submucosal dissection becomes more accessible, the researchers said. Large colonic lesions can contain submucosal invasive SMICs that are not visible on endoscopy, and characterizing predictors of this occurrence could help patients and clinicians decide between endoscopic submucosal dissection and endoscopic mucosal resection. To do so, the researchers analyzed histologic specimens from 2,277 colonic lesions above 20 mm (average size, 37 mm) that lacked overt endoscopic high-risk features. The study ran from 2008 through 2016, study participants averaged 68 years of age, and 53% were male. A total of 171 lesions (8%) had evidence of SMIC on pathologic review, and 138 lesions had covert SMIC. Predictors of overt and occult SMIC included Kudo pit pattern V, a depressed component (0-IIc), rectosigmoid location, 0-Is or 0-IIa+Is Paris classification, nongranular surface morphology, and larger size. After excluding lesions with obvious SMIC features – including serrated lesions and those with depressed components (Kudo pit pattern of V and Paris 0-IIc) – the strongest predictors of occult SMIC included Paris classification, surface morphology, size, and location.
“Proximal 0-IIa G or 0-Is granular lesions had the lowest risk of SMIC (0.7% and 2.3%), whereas distal 0-Is nongranular lesions had the highest risk (21.4%),” the investigators added. Lesion location, size, and combined Paris classification and surface topography showed the best fit in a multivariable model. Notably, rectosigmoid lesions had nearly twice the odds of containing covert SMIC, compared with proximal lesions (odds ratio, 1.9; 95% confidence interval, 1.2-3.0; P = .01). Other significant predictors of covert SMIC in the multivariable model included combined Paris classification, surface morphology (OR, 4.0; 95% CI, 1.2-12.7; P = .02), and increasing size (OR, 1.2 per 10-mm increase; 95% CI, 1.04-1.3; P = .01). Increased size showed an even greater effect in lesions exceeding 50 mm.
Clinicians can use these factors to help evaluate risk of invasive cancer in lesions without overt SMIC, the researchers said. “One lesion type that differs from the pattern is 0-IIa nongranular lesions,” they noted. “Once lesions with overt evidence of SMIC are excluded, these lesions have a low risk (4.2%) of harboring underlying cancer.” Although 42% of lesions with covert SMIC were SM1 (potentially curable by endoscopic resection), no predictor of covert SMIC also predicted SMI status.
Funders included Cancer Institute of New South Wales and Gallipoli Medical Research Foundation. The investigators had no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Large sessile or flat colorectal polyps or laterally spreading lesions had the highest risk of occult malignancy when they were distal 0-Is or 0–IIa+Is nongranular lesions. Proximally located 0-Is or 0-IIa granular lesions had the lowest risk.
Major finding: Only 0.7% of proximal 0-IIa granular lesions and 2.3% of 0-Is granular lesions contained occult submucosal invasive malignancies, compared with 21% of distal 0-Is nongranular lesions.
Data source: A multicenter prospective cohort study of 2,277 large colonic lesions from 2,106 consecutive patients.
Disclosures: Funders included Cancer Institute of New South Wales and Gallipoli Medical Research Foundation. The investigators had no conflicts of interest.
VIDEO: High myristic acid intake linked to relapse in ulcerative colitis
High intake of myristic acid approximately tripled the odds of relapse in patients with ulcerative colitis (UC), compared with low intake, according to the results of a 12-month multicenter, prospective, observational study reported in the September 2017 issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.12.036).
Relapsers consumed an average of 2.2 g of this saturated fatty acid daily from sources such as palm and coconut oils, as well as dairy fats, reported Edward L. Barnes, MD, MPH, and his associates at Brigham and Women’s Hospital, Boston, on behalf of the DREAM (Diet’s Role in Exacerbations of Mesalamine Maintenance) investigators. Nonrelapsers averaged 1.4 g/day. “Our broader goal is to determine how alterations in diet can improve the care of people with IBD [inflammatory bowel disease],” the researchers wrote. “These findings can help design interventional dietary studies to determine if supplementation or avoidance of certain compounds might reduce the risk of a flare for patients with ulcerative colitis in remission.”
Dietary factors are thought to underlie relapse in ulcerative colitis, but specific culprits are poorly defined, the investigators said. Therefore, the DREAM study prospectively tracked dietary intake and flares among a homogeneous group of 412 patients with UC from 25 academic and community gastroenterology practices in the United States. Between 2007 and 2014, patients were interviewed by telephone every 3 months for 1 year or until they reported a flare, defined as a Simple Clinical Colitis Activity Index score of at least 5 or a change in disease activity that entailed a change in medication.
Source: American Gastroenterological Association
A total of 34 patients were lost to follow-up, and 45 (11% of those remaining) flared within a year of study enrollment. “When analyzed in tertiles, increasing intake of multiple fatty acids was associated with increasing odds of relapse,” the researchers wrote. Predictors of flare in the univariate analysis included high intake of myristic acid, oleic acid, eicosenoic acid, palmitelaidic acid, total translinoleic acid, saturated fat, monounsaturated fat, and omega-3 fatty acids. These predictors also included moderate or high intake of alpha-linolenic acid. Only high intake of myristic acid maintained a significant dose-response relationship in the multivariable analysis (odds ratio, 3.0; 95% confidence interval, 1.2-7.7; P = .02 for high vs. low intake). Moderate intake of alpha-linolenic acid predicted flare (OR, 5.5; CI, 95%, 1.6-19.3; P = .001) in the multivariable analysis, but high intake did not (OR, 1.3; CI, 95%, 0.3-7.0; P = .4). “Other foods previously implicated in flares of UC, such as processed meat, alcohol, and foods high in sulfur, were not associated with an increased risk of flare,” the researchers wrote.
Study participants were generally in their mid- to late 40s, white, and not current smokers. More than half were male. Most had proctitis or left-sided colitis, not pancolitis. Relapsers averaged 2.4 flares in the 18 months before enrollment (standard deviation, 1.9), compared with 1.8 flares for nonrelapsers (SD, 2.4; P = .003).
This observational study not only was subject to unmeasured confounding, but also excluded many types of patients. Among those excluded were anyone with a history of allergy to salicylates, aminosalicylates, or mesalamine tablets. Also excluded were those who had recent exposure to NSAIDs, oral or parenteral antibiotics, antidiarrheals, antispasmodics, immunosuppressives, biologics, or corticosteroids (except budesonide). Requiring monotherapy with an aminosalicylate might limit the generalizability of the findings, the investigators noted. Patients also were on variable doses of aminosalicylates, and higher doses might have helped inhibit flares.
Actavis and the National Institutes of Health provided funding. The investigators reported having no relevant financial conflicts.
Patients with inflammatory bowel disease commonly ask their physicians if dietary modifications can be made to control their disease. Despite the interest from patients, we have limited data to provide informed recommendations.
These results provide additional information to better guide our discussions with patients regarding diet and disease activity. However, the overall body of information remains sparse, and we should reinforce that dietary manipulation is an adjunct measure, at best, to our current medical therapies.
Rajesh Rasik Shah, MD, is an assistant professor of internal medicine and gastroenterology at Baylor College of Medicine, Houston. He has no conflicts of interest.
Patients with inflammatory bowel disease commonly ask their physicians if dietary modifications can be made to control their disease. Despite the interest from patients, we have limited data to provide informed recommendations.
These results provide additional information to better guide our discussions with patients regarding diet and disease activity. However, the overall body of information remains sparse, and we should reinforce that dietary manipulation is an adjunct measure, at best, to our current medical therapies.
Rajesh Rasik Shah, MD, is an assistant professor of internal medicine and gastroenterology at Baylor College of Medicine, Houston. He has no conflicts of interest.
Patients with inflammatory bowel disease commonly ask their physicians if dietary modifications can be made to control their disease. Despite the interest from patients, we have limited data to provide informed recommendations.
These results provide additional information to better guide our discussions with patients regarding diet and disease activity. However, the overall body of information remains sparse, and we should reinforce that dietary manipulation is an adjunct measure, at best, to our current medical therapies.
Rajesh Rasik Shah, MD, is an assistant professor of internal medicine and gastroenterology at Baylor College of Medicine, Houston. He has no conflicts of interest.
High intake of myristic acid approximately tripled the odds of relapse in patients with ulcerative colitis (UC), compared with low intake, according to the results of a 12-month multicenter, prospective, observational study reported in the September 2017 issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.12.036).
Relapsers consumed an average of 2.2 g of this saturated fatty acid daily from sources such as palm and coconut oils, as well as dairy fats, reported Edward L. Barnes, MD, MPH, and his associates at Brigham and Women’s Hospital, Boston, on behalf of the DREAM (Diet’s Role in Exacerbations of Mesalamine Maintenance) investigators. Nonrelapsers averaged 1.4 g/day. “Our broader goal is to determine how alterations in diet can improve the care of people with IBD [inflammatory bowel disease],” the researchers wrote. “These findings can help design interventional dietary studies to determine if supplementation or avoidance of certain compounds might reduce the risk of a flare for patients with ulcerative colitis in remission.”
Dietary factors are thought to underlie relapse in ulcerative colitis, but specific culprits are poorly defined, the investigators said. Therefore, the DREAM study prospectively tracked dietary intake and flares among a homogeneous group of 412 patients with UC from 25 academic and community gastroenterology practices in the United States. Between 2007 and 2014, patients were interviewed by telephone every 3 months for 1 year or until they reported a flare, defined as a Simple Clinical Colitis Activity Index score of at least 5 or a change in disease activity that entailed a change in medication.
Source: American Gastroenterological Association
A total of 34 patients were lost to follow-up, and 45 (11% of those remaining) flared within a year of study enrollment. “When analyzed in tertiles, increasing intake of multiple fatty acids was associated with increasing odds of relapse,” the researchers wrote. Predictors of flare in the univariate analysis included high intake of myristic acid, oleic acid, eicosenoic acid, palmitelaidic acid, total translinoleic acid, saturated fat, monounsaturated fat, and omega-3 fatty acids. These predictors also included moderate or high intake of alpha-linolenic acid. Only high intake of myristic acid maintained a significant dose-response relationship in the multivariable analysis (odds ratio, 3.0; 95% confidence interval, 1.2-7.7; P = .02 for high vs. low intake). Moderate intake of alpha-linolenic acid predicted flare (OR, 5.5; CI, 95%, 1.6-19.3; P = .001) in the multivariable analysis, but high intake did not (OR, 1.3; CI, 95%, 0.3-7.0; P = .4). “Other foods previously implicated in flares of UC, such as processed meat, alcohol, and foods high in sulfur, were not associated with an increased risk of flare,” the researchers wrote.
Study participants were generally in their mid- to late 40s, white, and not current smokers. More than half were male. Most had proctitis or left-sided colitis, not pancolitis. Relapsers averaged 2.4 flares in the 18 months before enrollment (standard deviation, 1.9), compared with 1.8 flares for nonrelapsers (SD, 2.4; P = .003).
This observational study not only was subject to unmeasured confounding, but also excluded many types of patients. Among those excluded were anyone with a history of allergy to salicylates, aminosalicylates, or mesalamine tablets. Also excluded were those who had recent exposure to NSAIDs, oral or parenteral antibiotics, antidiarrheals, antispasmodics, immunosuppressives, biologics, or corticosteroids (except budesonide). Requiring monotherapy with an aminosalicylate might limit the generalizability of the findings, the investigators noted. Patients also were on variable doses of aminosalicylates, and higher doses might have helped inhibit flares.
Actavis and the National Institutes of Health provided funding. The investigators reported having no relevant financial conflicts.
High intake of myristic acid approximately tripled the odds of relapse in patients with ulcerative colitis (UC), compared with low intake, according to the results of a 12-month multicenter, prospective, observational study reported in the September 2017 issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.12.036).
Relapsers consumed an average of 2.2 g of this saturated fatty acid daily from sources such as palm and coconut oils, as well as dairy fats, reported Edward L. Barnes, MD, MPH, and his associates at Brigham and Women’s Hospital, Boston, on behalf of the DREAM (Diet’s Role in Exacerbations of Mesalamine Maintenance) investigators. Nonrelapsers averaged 1.4 g/day. “Our broader goal is to determine how alterations in diet can improve the care of people with IBD [inflammatory bowel disease],” the researchers wrote. “These findings can help design interventional dietary studies to determine if supplementation or avoidance of certain compounds might reduce the risk of a flare for patients with ulcerative colitis in remission.”
Dietary factors are thought to underlie relapse in ulcerative colitis, but specific culprits are poorly defined, the investigators said. Therefore, the DREAM study prospectively tracked dietary intake and flares among a homogeneous group of 412 patients with UC from 25 academic and community gastroenterology practices in the United States. Between 2007 and 2014, patients were interviewed by telephone every 3 months for 1 year or until they reported a flare, defined as a Simple Clinical Colitis Activity Index score of at least 5 or a change in disease activity that entailed a change in medication.
Source: American Gastroenterological Association
A total of 34 patients were lost to follow-up, and 45 (11% of those remaining) flared within a year of study enrollment. “When analyzed in tertiles, increasing intake of multiple fatty acids was associated with increasing odds of relapse,” the researchers wrote. Predictors of flare in the univariate analysis included high intake of myristic acid, oleic acid, eicosenoic acid, palmitelaidic acid, total translinoleic acid, saturated fat, monounsaturated fat, and omega-3 fatty acids. These predictors also included moderate or high intake of alpha-linolenic acid. Only high intake of myristic acid maintained a significant dose-response relationship in the multivariable analysis (odds ratio, 3.0; 95% confidence interval, 1.2-7.7; P = .02 for high vs. low intake). Moderate intake of alpha-linolenic acid predicted flare (OR, 5.5; CI, 95%, 1.6-19.3; P = .001) in the multivariable analysis, but high intake did not (OR, 1.3; CI, 95%, 0.3-7.0; P = .4). “Other foods previously implicated in flares of UC, such as processed meat, alcohol, and foods high in sulfur, were not associated with an increased risk of flare,” the researchers wrote.
Study participants were generally in their mid- to late 40s, white, and not current smokers. More than half were male. Most had proctitis or left-sided colitis, not pancolitis. Relapsers averaged 2.4 flares in the 18 months before enrollment (standard deviation, 1.9), compared with 1.8 flares for nonrelapsers (SD, 2.4; P = .003).
This observational study not only was subject to unmeasured confounding, but also excluded many types of patients. Among those excluded were anyone with a history of allergy to salicylates, aminosalicylates, or mesalamine tablets. Also excluded were those who had recent exposure to NSAIDs, oral or parenteral antibiotics, antidiarrheals, antispasmodics, immunosuppressives, biologics, or corticosteroids (except budesonide). Requiring monotherapy with an aminosalicylate might limit the generalizability of the findings, the investigators noted. Patients also were on variable doses of aminosalicylates, and higher doses might have helped inhibit flares.
Actavis and the National Institutes of Health provided funding. The investigators reported having no relevant financial conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: High intake of myristic acid tripled the odds of relapse in patients with ulcerative colitis.
Major finding: Protein, processed meat, alcohol, and sulfur intake were not linked to UC relapse.
Data source: A multicenter prospective study of 412 patients with UC.
Disclosures: Actavis and the National Institutes of Health provided funding. The investigators reported having no relevant financial conflicts.
Ex Vivo Confocal Microscopy in Clinical Practice: Report From the AAD Meeting
Evaluating suicidality
VIDEO: Less follow-up proposed for low-risk thyroid cancer
BOSTON – , Bryan R. Haugen, MD, suggested in a keynote lecture during the World Congress on Thyroid Cancer.
Traditionally, thyroid cancer specialists have monitored these patients for persistent or recurrent disease as often as every 6 or 12 months. “But what we’ve realized with recent assessments of response to treatment is that some patients do well without a recurrence over many years; so, the concept of doing less monitoring and less imaging, especially in patients with an excellent response [to their initial treatment], is being studied,” Dr. Haugen said in a video interview following his talk.
He estimated that perhaps two-thirds or as many as three-quarters of patients with differentiated thyroid cancer fall into the category of having low- or intermediate-risk disease with an excellent or good response to treatment, and hence they are potential candidates for eventually transitioning to less frequent follow-up.
During his talk, Dr. Haugen suggested that after several years with no sign of disease recurrence, lower-risk patients with an excellent treatment response may be able to stop undergoing regular monitoring, and those with a good treatment response may be able to safely have their monitoring intervals extended.
According to the most recent (2015) guidelines for differentiated thyroid cancer management from the American Thyroid Association, lower-risk patients with an excellent treatment response should have their serum thyroglobulin measured every 12-24 months and undergo an ultrasound examination every 3-5 years, while patients with a good response are targeted for serum thyroglobulin measurement annually with an ultrasound every 1-3 years (Thyroid. 2016 Jan;26[1]:1-133). Dr. Haugen chaired the expert panel that wrote these guidelines.
In another provocative suggestion, Dr. Haugen proposed that once well-responsive, lower-risk patients have remained disease free for several years, their less frequent follow-up monitoring could be continued by a primary care physician or another less specialized clinician.
At some time in the future, “a patient’s primary care physician could follow a simple tumor marker, thyroglobulin, maybe once every 5 years,” said Dr. Haugen, professor of medicine and head of the division of endocrinology, metabolism, and diabetes at the University of Colorado in Aurora. “At the University of Colorado, we use advanced-practice providers to do long-term follow-up” for lower-risk, treatment-responsive patients, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
BOSTON – , Bryan R. Haugen, MD, suggested in a keynote lecture during the World Congress on Thyroid Cancer.
Traditionally, thyroid cancer specialists have monitored these patients for persistent or recurrent disease as often as every 6 or 12 months. “But what we’ve realized with recent assessments of response to treatment is that some patients do well without a recurrence over many years; so, the concept of doing less monitoring and less imaging, especially in patients with an excellent response [to their initial treatment], is being studied,” Dr. Haugen said in a video interview following his talk.
He estimated that perhaps two-thirds or as many as three-quarters of patients with differentiated thyroid cancer fall into the category of having low- or intermediate-risk disease with an excellent or good response to treatment, and hence they are potential candidates for eventually transitioning to less frequent follow-up.
During his talk, Dr. Haugen suggested that after several years with no sign of disease recurrence, lower-risk patients with an excellent treatment response may be able to stop undergoing regular monitoring, and those with a good treatment response may be able to safely have their monitoring intervals extended.
According to the most recent (2015) guidelines for differentiated thyroid cancer management from the American Thyroid Association, lower-risk patients with an excellent treatment response should have their serum thyroglobulin measured every 12-24 months and undergo an ultrasound examination every 3-5 years, while patients with a good response are targeted for serum thyroglobulin measurement annually with an ultrasound every 1-3 years (Thyroid. 2016 Jan;26[1]:1-133). Dr. Haugen chaired the expert panel that wrote these guidelines.
In another provocative suggestion, Dr. Haugen proposed that once well-responsive, lower-risk patients have remained disease free for several years, their less frequent follow-up monitoring could be continued by a primary care physician or another less specialized clinician.
At some time in the future, “a patient’s primary care physician could follow a simple tumor marker, thyroglobulin, maybe once every 5 years,” said Dr. Haugen, professor of medicine and head of the division of endocrinology, metabolism, and diabetes at the University of Colorado in Aurora. “At the University of Colorado, we use advanced-practice providers to do long-term follow-up” for lower-risk, treatment-responsive patients, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
BOSTON – , Bryan R. Haugen, MD, suggested in a keynote lecture during the World Congress on Thyroid Cancer.
Traditionally, thyroid cancer specialists have monitored these patients for persistent or recurrent disease as often as every 6 or 12 months. “But what we’ve realized with recent assessments of response to treatment is that some patients do well without a recurrence over many years; so, the concept of doing less monitoring and less imaging, especially in patients with an excellent response [to their initial treatment], is being studied,” Dr. Haugen said in a video interview following his talk.
He estimated that perhaps two-thirds or as many as three-quarters of patients with differentiated thyroid cancer fall into the category of having low- or intermediate-risk disease with an excellent or good response to treatment, and hence they are potential candidates for eventually transitioning to less frequent follow-up.
During his talk, Dr. Haugen suggested that after several years with no sign of disease recurrence, lower-risk patients with an excellent treatment response may be able to stop undergoing regular monitoring, and those with a good treatment response may be able to safely have their monitoring intervals extended.
According to the most recent (2015) guidelines for differentiated thyroid cancer management from the American Thyroid Association, lower-risk patients with an excellent treatment response should have their serum thyroglobulin measured every 12-24 months and undergo an ultrasound examination every 3-5 years, while patients with a good response are targeted for serum thyroglobulin measurement annually with an ultrasound every 1-3 years (Thyroid. 2016 Jan;26[1]:1-133). Dr. Haugen chaired the expert panel that wrote these guidelines.
In another provocative suggestion, Dr. Haugen proposed that once well-responsive, lower-risk patients have remained disease free for several years, their less frequent follow-up monitoring could be continued by a primary care physician or another less specialized clinician.
At some time in the future, “a patient’s primary care physician could follow a simple tumor marker, thyroglobulin, maybe once every 5 years,” said Dr. Haugen, professor of medicine and head of the division of endocrinology, metabolism, and diabetes at the University of Colorado in Aurora. “At the University of Colorado, we use advanced-practice providers to do long-term follow-up” for lower-risk, treatment-responsive patients, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT WCTC 2017
VIDEO: How to discharge new pediatric diabetes cases in 2 days
NASHVILLE, TENN. – Pediatric endocrinologist Cassandra Brady, MD, caught the attention of her audience at Pediatric Hospital Medicine when she mentioned that children presenting with new-onset diabetes rarely spend more than 2 days at Vanderbilt University’s children’s hospital, even if they present in diabetic ketoacidosis.
In many places, children with new-onset diabetes spend quite a bit longer in the hospital – even if they are medically stable and feeling fine – for diabetes education.
That’s not the case at Vanderbilt, where Dr. Brady is an assistant professor. Once kids are stabilized, they and their parents undergo a 3-hour crash course – sometimes even in the PICU – on diabetes survival skills, and then they’re sent home with insulin. They learn the finer points about carbohydrate counting and tight glucose control at subsequent outpatient visits.
More and more payers are probably going to push for that model, Dr. Brady noted at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
For those interested in making the transition to outpatient eduction, she explained in an interview exactly how Vanderbilt’s been doing it safely for years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN. – Pediatric endocrinologist Cassandra Brady, MD, caught the attention of her audience at Pediatric Hospital Medicine when she mentioned that children presenting with new-onset diabetes rarely spend more than 2 days at Vanderbilt University’s children’s hospital, even if they present in diabetic ketoacidosis.
In many places, children with new-onset diabetes spend quite a bit longer in the hospital – even if they are medically stable and feeling fine – for diabetes education.
That’s not the case at Vanderbilt, where Dr. Brady is an assistant professor. Once kids are stabilized, they and their parents undergo a 3-hour crash course – sometimes even in the PICU – on diabetes survival skills, and then they’re sent home with insulin. They learn the finer points about carbohydrate counting and tight glucose control at subsequent outpatient visits.
More and more payers are probably going to push for that model, Dr. Brady noted at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
For those interested in making the transition to outpatient eduction, she explained in an interview exactly how Vanderbilt’s been doing it safely for years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN. – Pediatric endocrinologist Cassandra Brady, MD, caught the attention of her audience at Pediatric Hospital Medicine when she mentioned that children presenting with new-onset diabetes rarely spend more than 2 days at Vanderbilt University’s children’s hospital, even if they present in diabetic ketoacidosis.
In many places, children with new-onset diabetes spend quite a bit longer in the hospital – even if they are medically stable and feeling fine – for diabetes education.
That’s not the case at Vanderbilt, where Dr. Brady is an assistant professor. Once kids are stabilized, they and their parents undergo a 3-hour crash course – sometimes even in the PICU – on diabetes survival skills, and then they’re sent home with insulin. They learn the finer points about carbohydrate counting and tight glucose control at subsequent outpatient visits.
More and more payers are probably going to push for that model, Dr. Brady noted at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
For those interested in making the transition to outpatient eduction, she explained in an interview exactly how Vanderbilt’s been doing it safely for years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT PHM 2017
Vaginal salpingo-oophorectomy: Tips and tricks

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
VIDEO: Software predicts septic shock in hospitalized patients
WASHINGTON – Researchers have devised a program that can predict severe sepsis or septic shock about 12-30 hours in advance of its onset in hospitalized patients, a feat they hope will allow them to apply early interventions to stave off impending sepsis.
“We’d love to see a change in sepsis mortality. Will earlier recognition and implementation of the sepsis bundle – fluids, antibiotics, etc. – improve outcomes?” wondered Heather M. Giannini, MD, in a video interview at an international conference of the American Thoracic Society.
The computer program works by monitoring all the data that enter a patient’s electronic health record during hospitalization. Researchers developed it and designed it specifically for inpatients who are not in the intensive care unit or emergency department.
Results from initial testing during October-December 2015 in 10,448 patients hospitalized at one of three participating Philadelphia hospitals showed the program predicted subsequent severe sepsis or septic shock with a sensitivity of 26% and a specificity of 98%, reported Dr. Giannini, a researcher in the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Analysis also showed a positive likelihood ratio of 13 for severe sepsis or septic shock actually occurring following an alert generated by the computer program, a level indicating a “very strong” ability to predict sepsis, she said.
Dr. Giannini and her associates developed the prediction program using a technique called “computational machine learning,” an alternative to standard logistic regression modeling that is better suited to analyzing large data sets and can better integrate outlier data points. They took EHR data for all non-ICU, non-ED inpatients at three Philadelphia hospitals during a 3-year period during 2011-2014 and had the program focus particularly on EHR data gleaned from the nearly 1,000 patients who developed severe sepsis or septic shock during the 12 hours preceding the start of these sepsis events. The analysis identified patients as having developed severe sepsis or shock if they had a blood draw positive for infection at the same time as having a blood lactate level above 2.2 mmol/L or a systolic blood pressure below 90 mm Hg.
To create the algorithm the machine-learning device compared the EHR entries for patients who developed severe sepsis or septic shock with EHR data from patients who did not, a process that involved hundred of thousands of data points, Dr. Giannini said. This identified 587 individual types of relevant EHR data entries and ranked them from most important to least important. Important, novel determinants of impending severe sepsis identified this way included anion gap, blood urea nitrogen, and platelet count. The development process also confirmed an important role for many classic markers of septic shock, such as respiration rate, heart rate, and temperature.
The researchers designed the algorithm to have a moderate level of sensitivity to avoid “alert fatigue” from generating too many alarms for impending severe sepsis. Their goal was for clinicians to receive no more than about 10 alerts per day for each hospital.
“We are satisfied with the sensitivity. We felt it was better to have too few alerts rather than overwhelm clinicians. About 10 alerts a day is reasonable,” Dr. Giannini explained. During initial 2015 testing, the system generated a daily average of 11 alerts.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – Researchers have devised a program that can predict severe sepsis or septic shock about 12-30 hours in advance of its onset in hospitalized patients, a feat they hope will allow them to apply early interventions to stave off impending sepsis.
“We’d love to see a change in sepsis mortality. Will earlier recognition and implementation of the sepsis bundle – fluids, antibiotics, etc. – improve outcomes?” wondered Heather M. Giannini, MD, in a video interview at an international conference of the American Thoracic Society.
The computer program works by monitoring all the data that enter a patient’s electronic health record during hospitalization. Researchers developed it and designed it specifically for inpatients who are not in the intensive care unit or emergency department.
Results from initial testing during October-December 2015 in 10,448 patients hospitalized at one of three participating Philadelphia hospitals showed the program predicted subsequent severe sepsis or septic shock with a sensitivity of 26% and a specificity of 98%, reported Dr. Giannini, a researcher in the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Analysis also showed a positive likelihood ratio of 13 for severe sepsis or septic shock actually occurring following an alert generated by the computer program, a level indicating a “very strong” ability to predict sepsis, she said.
Dr. Giannini and her associates developed the prediction program using a technique called “computational machine learning,” an alternative to standard logistic regression modeling that is better suited to analyzing large data sets and can better integrate outlier data points. They took EHR data for all non-ICU, non-ED inpatients at three Philadelphia hospitals during a 3-year period during 2011-2014 and had the program focus particularly on EHR data gleaned from the nearly 1,000 patients who developed severe sepsis or septic shock during the 12 hours preceding the start of these sepsis events. The analysis identified patients as having developed severe sepsis or shock if they had a blood draw positive for infection at the same time as having a blood lactate level above 2.2 mmol/L or a systolic blood pressure below 90 mm Hg.
To create the algorithm the machine-learning device compared the EHR entries for patients who developed severe sepsis or septic shock with EHR data from patients who did not, a process that involved hundred of thousands of data points, Dr. Giannini said. This identified 587 individual types of relevant EHR data entries and ranked them from most important to least important. Important, novel determinants of impending severe sepsis identified this way included anion gap, blood urea nitrogen, and platelet count. The development process also confirmed an important role for many classic markers of septic shock, such as respiration rate, heart rate, and temperature.
The researchers designed the algorithm to have a moderate level of sensitivity to avoid “alert fatigue” from generating too many alarms for impending severe sepsis. Their goal was for clinicians to receive no more than about 10 alerts per day for each hospital.
“We are satisfied with the sensitivity. We felt it was better to have too few alerts rather than overwhelm clinicians. About 10 alerts a day is reasonable,” Dr. Giannini explained. During initial 2015 testing, the system generated a daily average of 11 alerts.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – Researchers have devised a program that can predict severe sepsis or septic shock about 12-30 hours in advance of its onset in hospitalized patients, a feat they hope will allow them to apply early interventions to stave off impending sepsis.
“We’d love to see a change in sepsis mortality. Will earlier recognition and implementation of the sepsis bundle – fluids, antibiotics, etc. – improve outcomes?” wondered Heather M. Giannini, MD, in a video interview at an international conference of the American Thoracic Society.
The computer program works by monitoring all the data that enter a patient’s electronic health record during hospitalization. Researchers developed it and designed it specifically for inpatients who are not in the intensive care unit or emergency department.
Results from initial testing during October-December 2015 in 10,448 patients hospitalized at one of three participating Philadelphia hospitals showed the program predicted subsequent severe sepsis or septic shock with a sensitivity of 26% and a specificity of 98%, reported Dr. Giannini, a researcher in the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Analysis also showed a positive likelihood ratio of 13 for severe sepsis or septic shock actually occurring following an alert generated by the computer program, a level indicating a “very strong” ability to predict sepsis, she said.
Dr. Giannini and her associates developed the prediction program using a technique called “computational machine learning,” an alternative to standard logistic regression modeling that is better suited to analyzing large data sets and can better integrate outlier data points. They took EHR data for all non-ICU, non-ED inpatients at three Philadelphia hospitals during a 3-year period during 2011-2014 and had the program focus particularly on EHR data gleaned from the nearly 1,000 patients who developed severe sepsis or septic shock during the 12 hours preceding the start of these sepsis events. The analysis identified patients as having developed severe sepsis or shock if they had a blood draw positive for infection at the same time as having a blood lactate level above 2.2 mmol/L or a systolic blood pressure below 90 mm Hg.
To create the algorithm the machine-learning device compared the EHR entries for patients who developed severe sepsis or septic shock with EHR data from patients who did not, a process that involved hundred of thousands of data points, Dr. Giannini said. This identified 587 individual types of relevant EHR data entries and ranked them from most important to least important. Important, novel determinants of impending severe sepsis identified this way included anion gap, blood urea nitrogen, and platelet count. The development process also confirmed an important role for many classic markers of septic shock, such as respiration rate, heart rate, and temperature.
The researchers designed the algorithm to have a moderate level of sensitivity to avoid “alert fatigue” from generating too many alarms for impending severe sepsis. Their goal was for clinicians to receive no more than about 10 alerts per day for each hospital.
“We are satisfied with the sensitivity. We felt it was better to have too few alerts rather than overwhelm clinicians. About 10 alerts a day is reasonable,” Dr. Giannini explained. During initial 2015 testing, the system generated a daily average of 11 alerts.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: The program predicted severe sepsis with a sensitivity of 26% and specificity of 98%.
Data source: A total of 10,448 inpatients at three Philadelphia hospitals during October-December 2015.
Disclosures: Dr. Giannini had no disclosures.
VIDEO: Cardiovascular events in rheumatoid arthritis have decreased over decades
MADRID – Recent improvements in the management of rheumatoid arthritis may have had a positive impact on common cardiovascular comorbidities, according to the results of a systematic review and meta-analysis.
Risk ratios (RR) for several CV events in rheumatoid arthritis (RA) patients were found to be lower for data published after 2000 and up to March 2016 when compared with data published up until 2000. Indeed, comparing these two time periods, French researchers found that the RR for myocardial infarction (MI) were a respective 1.32 and 1.18, for heart failure a respective 1.25 and 1.17, and for CV mortality a respective 1.21 and 1.07.
“Systemic inflammation is the cornerstone of both rheumatoid arthritis and atherosclerosis,” Cécile Gaujoux-Viala, MD, PhD, professor of rheumatology at Montpellier University, Nîmes, France, and chief of the rheumatology service at Nîmes University Hospital, said during a press briefing at the European Congress of Rheumatology.
“Over the past 15 years, new treatment strategies such as ‘tight control,’ ‘treat-to-target,’ methotrexate optimization, and use of biologic DMARDs [disease-modifying antirheumatic drugs] have led to better control of this inflammation,” Dr. Gaujoux-Viala added.
The aim of the meta-analysis was to look at the overall risk for CV events in RA patients versus the general population, she said, as well as to see if there had been any temporal shift by analyzing data obtained within two time periods – before 2000 and after 2000.
A systematic literature review was performed using the PubMed and Cochrane Library databases to search for observational studies that provided data about the occurrence of CV events in RA patients and controls. Of 5,714 papers that included reports of stroke, MI, heart failure, or CV death, 28 had the necessary data that could be used for the meta-analysis. Overall, the 28 studies included 227,871 RA patients, with a mean age of 55 years.
Results showed that RA patients had a 17% increased risk for stroke versus controls overall (P = .002), with a RR of 1.17. The RRs were 1.12 before 2000 and 1.23 after 2000, making stroke the only CV event that did not appear to show a downward trend.
Compared with the general population, RA patients had a 24% excess risk of MI, a 22% excess risk of heart failure, and a 18% excess risk of dying from a CV event (all P less than .00001).
These data provide “confirmation of an increased CV risk in RA patients compared to the general population,” said Dr. Gaujoux-Viala, who also discussed the study and its implications in a video interview.
Commenting on the study, Philip J. Mease, MD, of the University of Washington, Seattle, wondered where the studies used in the meta-analysis had been performed because of the potential impact that reduced access to CV medications or prevention strategies in certain countries could have on the results. However, the investigators did not determine where each of the studies used in the review took place.
Dr. Gaujoux-Viala had no relevant conflicts of interest to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Recent improvements in the management of rheumatoid arthritis may have had a positive impact on common cardiovascular comorbidities, according to the results of a systematic review and meta-analysis.
Risk ratios (RR) for several CV events in rheumatoid arthritis (RA) patients were found to be lower for data published after 2000 and up to March 2016 when compared with data published up until 2000. Indeed, comparing these two time periods, French researchers found that the RR for myocardial infarction (MI) were a respective 1.32 and 1.18, for heart failure a respective 1.25 and 1.17, and for CV mortality a respective 1.21 and 1.07.
“Systemic inflammation is the cornerstone of both rheumatoid arthritis and atherosclerosis,” Cécile Gaujoux-Viala, MD, PhD, professor of rheumatology at Montpellier University, Nîmes, France, and chief of the rheumatology service at Nîmes University Hospital, said during a press briefing at the European Congress of Rheumatology.
“Over the past 15 years, new treatment strategies such as ‘tight control,’ ‘treat-to-target,’ methotrexate optimization, and use of biologic DMARDs [disease-modifying antirheumatic drugs] have led to better control of this inflammation,” Dr. Gaujoux-Viala added.
The aim of the meta-analysis was to look at the overall risk for CV events in RA patients versus the general population, she said, as well as to see if there had been any temporal shift by analyzing data obtained within two time periods – before 2000 and after 2000.
A systematic literature review was performed using the PubMed and Cochrane Library databases to search for observational studies that provided data about the occurrence of CV events in RA patients and controls. Of 5,714 papers that included reports of stroke, MI, heart failure, or CV death, 28 had the necessary data that could be used for the meta-analysis. Overall, the 28 studies included 227,871 RA patients, with a mean age of 55 years.
Results showed that RA patients had a 17% increased risk for stroke versus controls overall (P = .002), with a RR of 1.17. The RRs were 1.12 before 2000 and 1.23 after 2000, making stroke the only CV event that did not appear to show a downward trend.
Compared with the general population, RA patients had a 24% excess risk of MI, a 22% excess risk of heart failure, and a 18% excess risk of dying from a CV event (all P less than .00001).
These data provide “confirmation of an increased CV risk in RA patients compared to the general population,” said Dr. Gaujoux-Viala, who also discussed the study and its implications in a video interview.
Commenting on the study, Philip J. Mease, MD, of the University of Washington, Seattle, wondered where the studies used in the meta-analysis had been performed because of the potential impact that reduced access to CV medications or prevention strategies in certain countries could have on the results. However, the investigators did not determine where each of the studies used in the review took place.
Dr. Gaujoux-Viala had no relevant conflicts of interest to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Recent improvements in the management of rheumatoid arthritis may have had a positive impact on common cardiovascular comorbidities, according to the results of a systematic review and meta-analysis.
Risk ratios (RR) for several CV events in rheumatoid arthritis (RA) patients were found to be lower for data published after 2000 and up to March 2016 when compared with data published up until 2000. Indeed, comparing these two time periods, French researchers found that the RR for myocardial infarction (MI) were a respective 1.32 and 1.18, for heart failure a respective 1.25 and 1.17, and for CV mortality a respective 1.21 and 1.07.
“Systemic inflammation is the cornerstone of both rheumatoid arthritis and atherosclerosis,” Cécile Gaujoux-Viala, MD, PhD, professor of rheumatology at Montpellier University, Nîmes, France, and chief of the rheumatology service at Nîmes University Hospital, said during a press briefing at the European Congress of Rheumatology.
“Over the past 15 years, new treatment strategies such as ‘tight control,’ ‘treat-to-target,’ methotrexate optimization, and use of biologic DMARDs [disease-modifying antirheumatic drugs] have led to better control of this inflammation,” Dr. Gaujoux-Viala added.
The aim of the meta-analysis was to look at the overall risk for CV events in RA patients versus the general population, she said, as well as to see if there had been any temporal shift by analyzing data obtained within two time periods – before 2000 and after 2000.
A systematic literature review was performed using the PubMed and Cochrane Library databases to search for observational studies that provided data about the occurrence of CV events in RA patients and controls. Of 5,714 papers that included reports of stroke, MI, heart failure, or CV death, 28 had the necessary data that could be used for the meta-analysis. Overall, the 28 studies included 227,871 RA patients, with a mean age of 55 years.
Results showed that RA patients had a 17% increased risk for stroke versus controls overall (P = .002), with a RR of 1.17. The RRs were 1.12 before 2000 and 1.23 after 2000, making stroke the only CV event that did not appear to show a downward trend.
Compared with the general population, RA patients had a 24% excess risk of MI, a 22% excess risk of heart failure, and a 18% excess risk of dying from a CV event (all P less than .00001).
These data provide “confirmation of an increased CV risk in RA patients compared to the general population,” said Dr. Gaujoux-Viala, who also discussed the study and its implications in a video interview.
Commenting on the study, Philip J. Mease, MD, of the University of Washington, Seattle, wondered where the studies used in the meta-analysis had been performed because of the potential impact that reduced access to CV medications or prevention strategies in certain countries could have on the results. However, the investigators did not determine where each of the studies used in the review took place.
Dr. Gaujoux-Viala had no relevant conflicts of interest to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Risk ratios for myocardial infarction, heart failure, and CV mortality were lower between the period of 2000-2016 than for the period up to 2000.
Data source: Meta-analysis of 28 studies published up to March 2016 that provided data on CV event rates in RA patients and the general population.
Disclosures: Dr. Gaujoux-Viala had no relevant conflicts of interest to disclose.
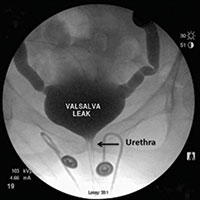
Video urodynamics for the evaluation of complex urinary symptoms
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
This video is brought to you by