User login
AHA: COPD Doubles Sudden Cardiac Death Risk in Hypertensives
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
AT THE AHA SCIENTIFIC SESSIONS
AHA: COPD doubles sudden cardiac death risk in hypertensives
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
ORLANDO – A second, confirmatory major study has shown that chronic obstructive pulmonary disease independently increases the risk of sudden cardiac death severalfold.
COPD was associated with a roughly twofold increased risk of sudden cardiac death (SCD) in hypertensive patients with COPD, compared with those without the pulmonary disease, in the Scandinavian Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial, Dr. Peter M. Okin reported at the American Heart Association scientific sessions.
Moreover, aggressive blood pressure lowering in the hypertensive COPD patients didn’t negate this risk, added Dr. Okin of Cornell University in New York.
The impetus for his secondary analysis of LIFE data was an earlier report from the landmark, population-based Rotterdam Heart Study. Among 1,615 participants with COPD, the age- and sex-adjusted risk of SCD was 1.34-fold greater than in nearly 12,000 controls. This increased SCD risk climbed to 2.12-fold during the first 2,000 days following diagnosis of COPD and reached 3.58-fold among those with frequent COPD exacerbations during this period (Eur Heart J. 2015 Jul 14;36[27]:1754-61).
Dr. Okin’s secondary analysis of LIFE data included 9,193 hypertensive subjects with ECG evidence of left ventricular hypertrophy who were randomized to lisinopril- or atenolol-based blood pressure lowering to a target of 140/90 mm Hg or less. A history of COPD was present in 385 patients (4.2%) at enrollment.
During a mean 4.8 years of prospective follow-up, 178 patients experienced SCD, a prespecified secondary endpoint in the LIFE trial. The incidence rate was 9 cases per 1,000 patient-years in those with COPD and 3.8 per 1,000 person-years in those without the pulmonary disease.
In a univariate analysis, a history of COPD was associated with a 2.36-fold increased risk of SCD during follow-up. In a multivariate analysis extensively adjusted for potential confounders – treatment arm, age, race, gender, history of atrial fibrillation, baseline serum creatinine and serum glucose, stroke or TIA, as well as on-treatment blood pressure, heart rate, QRS duration, HDL cholesterol level, use of a statin or hydrochlorothiazide, and incident MI or heart failure – COPD remained associated with a 1.82-fold increased risk of SCD, the cardiologist reported.
“These results suggest the need for additional studies to assess whether there are targeted therapies that can reduce the risk of SCD in patients with COPD,” he concluded.
As previously reported, the main finding in LIFE was that losartan conferred benefits beyond blood pressure control (Lancet. 2002 Mar 23;359[9311]:995-1003).
Dr. Okin reported serving as a consultant to Novartis.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Two large studies link chronic obstructive pulmonary disease with increased risk of sudden cardiac death.
Major finding: Patients with COPD and hypertension had nearly a twofold increased risk of sudden cardiac death, compared with hypertensives without the pulmonary disease.
Data source: This was a secondary analysis comparing sudden cardiac death rates in 385 hypertensive patients with and nearly 12,000 without COPD, all participants in the LIFE trial.
Disclosures: The presenter reported serving as a consultant to Novartis.
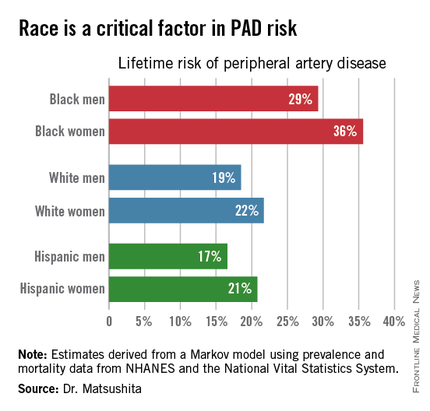
AHA: One in Three Black Americans Will Experience PAD
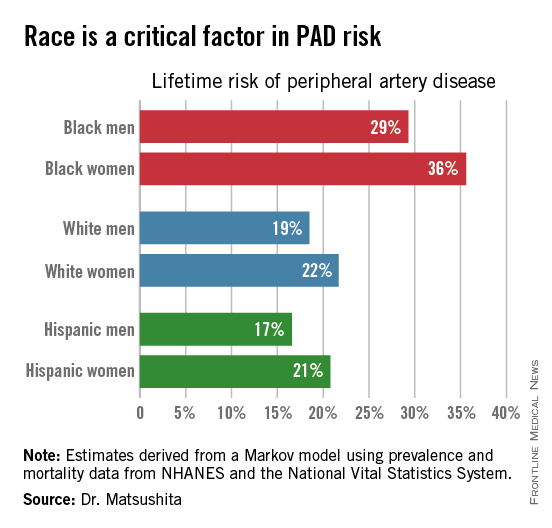
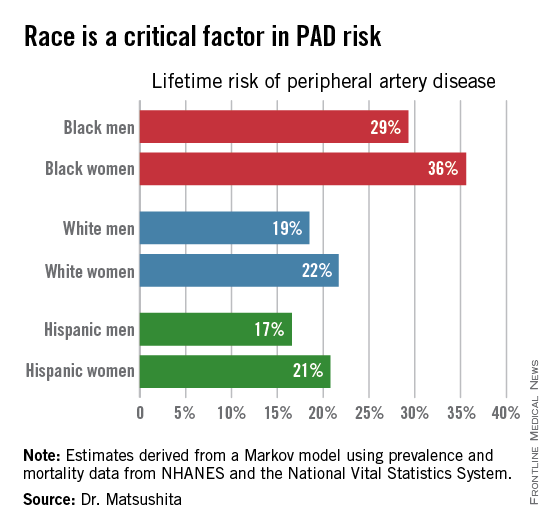
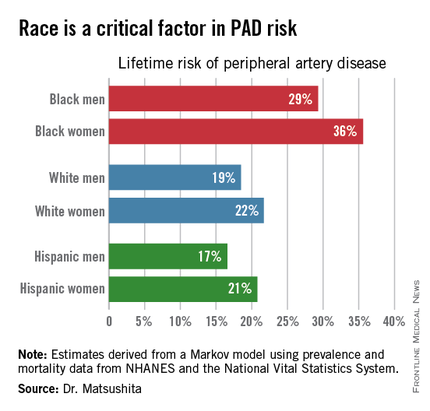
ORLANDO – One in three black Americans and one in five whites and Hispanics will develop lower extremity peripheral artery disease during their lifetime, according to the first-ever lifetime risk estimate calculated for this important manifestation of atherosclerotic vascular disease.
“Our results suggest that race is a critical factor in PAD [peripheral artery disease] risk. Current clinical guidelines recommend measuring ankle-brachial index according to age, traditional cardiovascular risk factors, and leg symptoms. Our results suggest race should also be taken into account,” Dr. Kunihiro Matsushita said at the American Heart Association scientific sessions.
This lifetime risk estimate for PAD was derived from national prevalence and mortality data from the National Health and Nutrition Examination Survey (NHANES) and the National Vital Statistics System. The analytic methods employed have previously been used to estimate lifetime risk of kidney disease and other major health issues with significant impact upon quality of life and longevity, noted Dr. Matsushita of Johns Hopkins University, Baltimore.
Over an 80-year time horizon, the projected risk of experiencing PAD was similar for men and women of the same race, but 1.5-fold higher for blacks, compared with whites or Hispanics (see chart).
An estimated 10% of black Americans will develop PAD by age 60. Among whites and Hispanics, a 10% prevalence is not reached until age 70. For individuals who don’t have PAD by age 65, their risk during the next 15 years is in the range of 28%-30% for black men and women, and 16%-18% in white or Hispanic men and women, according to Dr. Matsushita.
He declared having no financial conflicts related to this study. His work is supported by an AHA award.
ORLANDO – One in three black Americans and one in five whites and Hispanics will develop lower extremity peripheral artery disease during their lifetime, according to the first-ever lifetime risk estimate calculated for this important manifestation of atherosclerotic vascular disease.
“Our results suggest that race is a critical factor in PAD [peripheral artery disease] risk. Current clinical guidelines recommend measuring ankle-brachial index according to age, traditional cardiovascular risk factors, and leg symptoms. Our results suggest race should also be taken into account,” Dr. Kunihiro Matsushita said at the American Heart Association scientific sessions.

This lifetime risk estimate for PAD was derived from national prevalence and mortality data from the National Health and Nutrition Examination Survey (NHANES) and the National Vital Statistics System. The analytic methods employed have previously been used to estimate lifetime risk of kidney disease and other major health issues with significant impact upon quality of life and longevity, noted Dr. Matsushita of Johns Hopkins University, Baltimore.
Over an 80-year time horizon, the projected risk of experiencing PAD was similar for men and women of the same race, but 1.5-fold higher for blacks, compared with whites or Hispanics (see chart).
An estimated 10% of black Americans will develop PAD by age 60. Among whites and Hispanics, a 10% prevalence is not reached until age 70. For individuals who don’t have PAD by age 65, their risk during the next 15 years is in the range of 28%-30% for black men and women, and 16%-18% in white or Hispanic men and women, according to Dr. Matsushita.
He declared having no financial conflicts related to this study. His work is supported by an AHA award.
ORLANDO – One in three black Americans and one in five whites and Hispanics will develop lower extremity peripheral artery disease during their lifetime, according to the first-ever lifetime risk estimate calculated for this important manifestation of atherosclerotic vascular disease.
“Our results suggest that race is a critical factor in PAD [peripheral artery disease] risk. Current clinical guidelines recommend measuring ankle-brachial index according to age, traditional cardiovascular risk factors, and leg symptoms. Our results suggest race should also be taken into account,” Dr. Kunihiro Matsushita said at the American Heart Association scientific sessions.

This lifetime risk estimate for PAD was derived from national prevalence and mortality data from the National Health and Nutrition Examination Survey (NHANES) and the National Vital Statistics System. The analytic methods employed have previously been used to estimate lifetime risk of kidney disease and other major health issues with significant impact upon quality of life and longevity, noted Dr. Matsushita of Johns Hopkins University, Baltimore.
Over an 80-year time horizon, the projected risk of experiencing PAD was similar for men and women of the same race, but 1.5-fold higher for blacks, compared with whites or Hispanics (see chart).
An estimated 10% of black Americans will develop PAD by age 60. Among whites and Hispanics, a 10% prevalence is not reached until age 70. For individuals who don’t have PAD by age 65, their risk during the next 15 years is in the range of 28%-30% for black men and women, and 16%-18% in white or Hispanic men and women, according to Dr. Matsushita.
He declared having no financial conflicts related to this study. His work is supported by an AHA award.
AT THE AHA SCIENTIFIC SESSIONS
AHA: One in three black Americans will experience PAD
ORLANDO – One in three black Americans and one in five whites and Hispanics will develop lower extremity peripheral artery disease during their lifetime, according to the first-ever lifetime risk estimate calculated for this important manifestation of atherosclerotic vascular disease.
“Our results suggest that race is a critical factor in PAD [peripheral artery disease] risk. Current clinical guidelines recommend measuring ankle-brachial index according to age, traditional cardiovascular risk factors, and leg symptoms. Our results suggest race should also be taken into account,” Dr. Kunihiro Matsushita said at the American Heart Association scientific sessions.
This lifetime risk estimate for PAD was derived from national prevalence and mortality data from the National Health and Nutrition Examination Survey (NHANES) and the National Vital Statistics System. The analytic methods employed have previously been used to estimate lifetime risk of kidney disease and other major health issues with significant impact upon quality of life and longevity, noted Dr. Matsushita of Johns Hopkins University, Baltimore.
Over an 80-year time horizon, the projected risk of experiencing PAD was similar for men and women of the same race, but 1.5-fold higher for blacks, compared with whites or Hispanics (see chart).
An estimated 10% of black Americans will develop PAD by age 60. Among whites and Hispanics, a 10% prevalence is not reached until age 70. For individuals who don’t have PAD by age 65, their risk during the next 15 years is in the range of 28%-30% for black men and women, and 16%-18% in white or Hispanic men and women, according to Dr. Matsushita.
He declared having no financial conflicts related to this study. His work is supported by an AHA award.
ORLANDO – One in three black Americans and one in five whites and Hispanics will develop lower extremity peripheral artery disease during their lifetime, according to the first-ever lifetime risk estimate calculated for this important manifestation of atherosclerotic vascular disease.
“Our results suggest that race is a critical factor in PAD [peripheral artery disease] risk. Current clinical guidelines recommend measuring ankle-brachial index according to age, traditional cardiovascular risk factors, and leg symptoms. Our results suggest race should also be taken into account,” Dr. Kunihiro Matsushita said at the American Heart Association scientific sessions.

This lifetime risk estimate for PAD was derived from national prevalence and mortality data from the National Health and Nutrition Examination Survey (NHANES) and the National Vital Statistics System. The analytic methods employed have previously been used to estimate lifetime risk of kidney disease and other major health issues with significant impact upon quality of life and longevity, noted Dr. Matsushita of Johns Hopkins University, Baltimore.
Over an 80-year time horizon, the projected risk of experiencing PAD was similar for men and women of the same race, but 1.5-fold higher for blacks, compared with whites or Hispanics (see chart).
An estimated 10% of black Americans will develop PAD by age 60. Among whites and Hispanics, a 10% prevalence is not reached until age 70. For individuals who don’t have PAD by age 65, their risk during the next 15 years is in the range of 28%-30% for black men and women, and 16%-18% in white or Hispanic men and women, according to Dr. Matsushita.
He declared having no financial conflicts related to this study. His work is supported by an AHA award.
ORLANDO – One in three black Americans and one in five whites and Hispanics will develop lower extremity peripheral artery disease during their lifetime, according to the first-ever lifetime risk estimate calculated for this important manifestation of atherosclerotic vascular disease.
“Our results suggest that race is a critical factor in PAD [peripheral artery disease] risk. Current clinical guidelines recommend measuring ankle-brachial index according to age, traditional cardiovascular risk factors, and leg symptoms. Our results suggest race should also be taken into account,” Dr. Kunihiro Matsushita said at the American Heart Association scientific sessions.

This lifetime risk estimate for PAD was derived from national prevalence and mortality data from the National Health and Nutrition Examination Survey (NHANES) and the National Vital Statistics System. The analytic methods employed have previously been used to estimate lifetime risk of kidney disease and other major health issues with significant impact upon quality of life and longevity, noted Dr. Matsushita of Johns Hopkins University, Baltimore.
Over an 80-year time horizon, the projected risk of experiencing PAD was similar for men and women of the same race, but 1.5-fold higher for blacks, compared with whites or Hispanics (see chart).
An estimated 10% of black Americans will develop PAD by age 60. Among whites and Hispanics, a 10% prevalence is not reached until age 70. For individuals who don’t have PAD by age 65, their risk during the next 15 years is in the range of 28%-30% for black men and women, and 16%-18% in white or Hispanic men and women, according to Dr. Matsushita.
He declared having no financial conflicts related to this study. His work is supported by an AHA award.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: The threshold for screening for peripheral artery disease should be lower in black patients.
Major finding: One in three black Americans will develop peripheral artery disease by age 80, as will one in five whites or Hispanics.
Data source: This lifetime risk estimate for peripheral artery disease was derived from a Markov model with data input on the prevalence of the disorder and its associated mortality obtained from NHANES and the National Vital Statistics System.
Disclosures: The presenter reported having no financial conflicts. His research is supported by an AHA award.
AHA: Should BP Targets Be Higher in Asymptomatic Aortic Stenosis?
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
AT THE AHA SCIENTIFIC SESSIONS
AHA: Should BP targets be higher in asymptomatic aortic stenosis?
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Optimal blood pressures in patients with asymptomatic aortic stenosis appear to be significantly higher than those identified for the general hypertensive population in the recent SPRINT trial.
Major finding: All-cause mortality in patients with asymptomatic mild to moderate aortic stenosis is lowest at a blood pressure of 145/82 mm Hg.
Data source: This was an analysis of all-cause mortality over a mean follow-up of 4.3 years in 1,873 patients with asymptomatic mild to moderate aortic stenosis.
Disclosures: This secondary analysis of a completed clinical trial was conducted without commercial support. The presenter reported having no financial conflicts.
AHA: New spotlight on peripheral artery disease
ORLANDO – Peripheral artery disease constitutes “a health crisis that is largely unnoticed” by the public and all too often by physicians as well – but that’s all about to change, Dr. Mark A. Creager said in his presidential address at the American Heart Association scientific sessions.
In the coming months, look for rollout of major AHA initiatives on peripheral vascular disease. These programs grew out of a summit meeting of thought leaders in the field of vascular disease convened recently by the AHA in order to find ways to boost public awareness and improve the quality of care for patients with peripheral artery disease (PAD), venous thromboembolism, and aortic aneurysm.
PAD is all too often thought of as a disease of the legs, when in fact it is a clinical manifestation of systemic atherosclerosis, Dr. Creager noted. PAD affects on estimated 200 million people worldwide. In the United States alone, it accounts for $20 billion per year in health care costs. The mortality risk in affected patients is two- to fourfold greater than in individuals without PAD. Moreover, the risk of acute MI, stroke, or cardiovascular death among patients with PAD exceeds that of patients with established cerebrovascular disease.
“Clearly the unrecognized epidemic of vascular disease requires our attention,” observed Dr. Creager, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
Indeed, he made it clear that improved diagnosis and treatment of PAD will be a major AHA priority during his term as president. Noting that the annual rate of deaths due to cardiovascular disease and stroke has already dropped by 13.7% in the 5 years since the AHA set the ambitious goal of a 20% reduction by the year 2020, he emphasized that a critical element in getting the rest of the way there involves addressing peripheral vascular diseases more effectively.
Improved public awareness about PAD has to be a priority. One survey found that 75% of the public is unaware of PAD. It’s a condition which Dr. Creager and others have shown has its highest prevalence in Americans in the lowest income and educational strata, largely independent of traditional cardiovascular risk factors.
“Even physicians often fail to consider PAD, chalking up leg pain to age or arthritis. In one study, physicians missed the diagnosis of PAD half the time. And even when physicians diagnose PAD, they often don’t treat it adequately,” according to Dr. Creager.
The use of statins and antiplatelet therapy in patients with PAD each reduces the risk of MI, stroke, or cardiovascular death by about 25%. Yet when Dr. Creager and coworkers analyzed National Health and Nutrition Examination Survey data, they found only 19% of patients with PAD who didn’t have previously established coronary or cerebrovascular disease were on a statin, just 21% were on an ACE inhibitor or angiotensin receptor blocker, and 27% were on antiplatelet therapy (Circulation. 2011 Jul 5;124[1]:17-23).
Among patients with symptomatic PAD, studies have shown that supervised exercise training can double walking distance. That’s a major quality of life benefit, yet one that goes unconsidered if the diagnosis is missed. “It’s rarely instituted even with the diagnosis, largely because of the lack of reimbursement,” according to Dr. Creager.
He painted a picture of PAD as a field ripe with opportunities for improved outcomes.
“Our ability to diagnose and treat vascular diseases has never been greater. The field of vascular biology has virtually exploded in recent years,” he said. “I’d like to focus not only on the extent of this crisis, but also on the importance of using what we know to treat it and prevent it, and on the urgency of intensifying our research efforts to better understand it.”
In the diagnostic arena, optical coherence tomography, intravascular ultrasound, PET-CT, and contrast-enhanced MRI provide an unprecedented ability to image plaques and assess their vulnerability to rupture.
On the interventional front, innovative bioengineering has led to the development of drug-coated balloons and bioabsorbable vascular scaffolds with the potential to curb restenosis and preserve patency following endovascular treatment of critical lesions.
In terms of medical management, the novel, super-potent LDL cholesterol–lowering inhibitors of PCSK9 (proprotein convertase subtilisin-kexin type 9) will need to be studied in order to see if they have a special role in the treatment and/or prevention of PAD.
Roughly 2 decades ago, Dr. Creager and coinvestigators showed that endothelial function typically drops by 30% in our twenties and then in our thirties, and by 50% once we’re in our forties. A high research priority in PAD will be to learn how to more effectively prolong endothelial health and prevent vascular stiffness, he said.
He reported having no financial conflicts regarding his presentation.
ORLANDO – Peripheral artery disease constitutes “a health crisis that is largely unnoticed” by the public and all too often by physicians as well – but that’s all about to change, Dr. Mark A. Creager said in his presidential address at the American Heart Association scientific sessions.
In the coming months, look for rollout of major AHA initiatives on peripheral vascular disease. These programs grew out of a summit meeting of thought leaders in the field of vascular disease convened recently by the AHA in order to find ways to boost public awareness and improve the quality of care for patients with peripheral artery disease (PAD), venous thromboembolism, and aortic aneurysm.
PAD is all too often thought of as a disease of the legs, when in fact it is a clinical manifestation of systemic atherosclerosis, Dr. Creager noted. PAD affects on estimated 200 million people worldwide. In the United States alone, it accounts for $20 billion per year in health care costs. The mortality risk in affected patients is two- to fourfold greater than in individuals without PAD. Moreover, the risk of acute MI, stroke, or cardiovascular death among patients with PAD exceeds that of patients with established cerebrovascular disease.
“Clearly the unrecognized epidemic of vascular disease requires our attention,” observed Dr. Creager, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
Indeed, he made it clear that improved diagnosis and treatment of PAD will be a major AHA priority during his term as president. Noting that the annual rate of deaths due to cardiovascular disease and stroke has already dropped by 13.7% in the 5 years since the AHA set the ambitious goal of a 20% reduction by the year 2020, he emphasized that a critical element in getting the rest of the way there involves addressing peripheral vascular diseases more effectively.
Improved public awareness about PAD has to be a priority. One survey found that 75% of the public is unaware of PAD. It’s a condition which Dr. Creager and others have shown has its highest prevalence in Americans in the lowest income and educational strata, largely independent of traditional cardiovascular risk factors.
“Even physicians often fail to consider PAD, chalking up leg pain to age or arthritis. In one study, physicians missed the diagnosis of PAD half the time. And even when physicians diagnose PAD, they often don’t treat it adequately,” according to Dr. Creager.
The use of statins and antiplatelet therapy in patients with PAD each reduces the risk of MI, stroke, or cardiovascular death by about 25%. Yet when Dr. Creager and coworkers analyzed National Health and Nutrition Examination Survey data, they found only 19% of patients with PAD who didn’t have previously established coronary or cerebrovascular disease were on a statin, just 21% were on an ACE inhibitor or angiotensin receptor blocker, and 27% were on antiplatelet therapy (Circulation. 2011 Jul 5;124[1]:17-23).
Among patients with symptomatic PAD, studies have shown that supervised exercise training can double walking distance. That’s a major quality of life benefit, yet one that goes unconsidered if the diagnosis is missed. “It’s rarely instituted even with the diagnosis, largely because of the lack of reimbursement,” according to Dr. Creager.
He painted a picture of PAD as a field ripe with opportunities for improved outcomes.
“Our ability to diagnose and treat vascular diseases has never been greater. The field of vascular biology has virtually exploded in recent years,” he said. “I’d like to focus not only on the extent of this crisis, but also on the importance of using what we know to treat it and prevent it, and on the urgency of intensifying our research efforts to better understand it.”
In the diagnostic arena, optical coherence tomography, intravascular ultrasound, PET-CT, and contrast-enhanced MRI provide an unprecedented ability to image plaques and assess their vulnerability to rupture.
On the interventional front, innovative bioengineering has led to the development of drug-coated balloons and bioabsorbable vascular scaffolds with the potential to curb restenosis and preserve patency following endovascular treatment of critical lesions.
In terms of medical management, the novel, super-potent LDL cholesterol–lowering inhibitors of PCSK9 (proprotein convertase subtilisin-kexin type 9) will need to be studied in order to see if they have a special role in the treatment and/or prevention of PAD.
Roughly 2 decades ago, Dr. Creager and coinvestigators showed that endothelial function typically drops by 30% in our twenties and then in our thirties, and by 50% once we’re in our forties. A high research priority in PAD will be to learn how to more effectively prolong endothelial health and prevent vascular stiffness, he said.
He reported having no financial conflicts regarding his presentation.
ORLANDO – Peripheral artery disease constitutes “a health crisis that is largely unnoticed” by the public and all too often by physicians as well – but that’s all about to change, Dr. Mark A. Creager said in his presidential address at the American Heart Association scientific sessions.
In the coming months, look for rollout of major AHA initiatives on peripheral vascular disease. These programs grew out of a summit meeting of thought leaders in the field of vascular disease convened recently by the AHA in order to find ways to boost public awareness and improve the quality of care for patients with peripheral artery disease (PAD), venous thromboembolism, and aortic aneurysm.
PAD is all too often thought of as a disease of the legs, when in fact it is a clinical manifestation of systemic atherosclerosis, Dr. Creager noted. PAD affects on estimated 200 million people worldwide. In the United States alone, it accounts for $20 billion per year in health care costs. The mortality risk in affected patients is two- to fourfold greater than in individuals without PAD. Moreover, the risk of acute MI, stroke, or cardiovascular death among patients with PAD exceeds that of patients with established cerebrovascular disease.
“Clearly the unrecognized epidemic of vascular disease requires our attention,” observed Dr. Creager, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
Indeed, he made it clear that improved diagnosis and treatment of PAD will be a major AHA priority during his term as president. Noting that the annual rate of deaths due to cardiovascular disease and stroke has already dropped by 13.7% in the 5 years since the AHA set the ambitious goal of a 20% reduction by the year 2020, he emphasized that a critical element in getting the rest of the way there involves addressing peripheral vascular diseases more effectively.
Improved public awareness about PAD has to be a priority. One survey found that 75% of the public is unaware of PAD. It’s a condition which Dr. Creager and others have shown has its highest prevalence in Americans in the lowest income and educational strata, largely independent of traditional cardiovascular risk factors.
“Even physicians often fail to consider PAD, chalking up leg pain to age or arthritis. In one study, physicians missed the diagnosis of PAD half the time. And even when physicians diagnose PAD, they often don’t treat it adequately,” according to Dr. Creager.
The use of statins and antiplatelet therapy in patients with PAD each reduces the risk of MI, stroke, or cardiovascular death by about 25%. Yet when Dr. Creager and coworkers analyzed National Health and Nutrition Examination Survey data, they found only 19% of patients with PAD who didn’t have previously established coronary or cerebrovascular disease were on a statin, just 21% were on an ACE inhibitor or angiotensin receptor blocker, and 27% were on antiplatelet therapy (Circulation. 2011 Jul 5;124[1]:17-23).
Among patients with symptomatic PAD, studies have shown that supervised exercise training can double walking distance. That’s a major quality of life benefit, yet one that goes unconsidered if the diagnosis is missed. “It’s rarely instituted even with the diagnosis, largely because of the lack of reimbursement,” according to Dr. Creager.
He painted a picture of PAD as a field ripe with opportunities for improved outcomes.
“Our ability to diagnose and treat vascular diseases has never been greater. The field of vascular biology has virtually exploded in recent years,” he said. “I’d like to focus not only on the extent of this crisis, but also on the importance of using what we know to treat it and prevent it, and on the urgency of intensifying our research efforts to better understand it.”
In the diagnostic arena, optical coherence tomography, intravascular ultrasound, PET-CT, and contrast-enhanced MRI provide an unprecedented ability to image plaques and assess their vulnerability to rupture.
On the interventional front, innovative bioengineering has led to the development of drug-coated balloons and bioabsorbable vascular scaffolds with the potential to curb restenosis and preserve patency following endovascular treatment of critical lesions.
In terms of medical management, the novel, super-potent LDL cholesterol–lowering inhibitors of PCSK9 (proprotein convertase subtilisin-kexin type 9) will need to be studied in order to see if they have a special role in the treatment and/or prevention of PAD.
Roughly 2 decades ago, Dr. Creager and coinvestigators showed that endothelial function typically drops by 30% in our twenties and then in our thirties, and by 50% once we’re in our forties. A high research priority in PAD will be to learn how to more effectively prolong endothelial health and prevent vascular stiffness, he said.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
EADV: New long-term data on biologics for pediatric psoriasis ‘encouraging’
COPENHAGEN – The longest-ever clinical trials of etanercept and adalimumab for pediatric psoriasis show reassuring maintenance of efficacy, coupled with safety and tolerability profiles similar to what has been seen in long-term trials in adults, investigators reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Richard G. Langley presented outcomes from a 5-year open-label extension of an initial 12-week, multicenter, double-blind, randomized trial of etanercept or placebo in children and adolescents with moderate to severe chronic plaque psoriasis. The primary results were published more than 7 years ago (N Engl J Med. 2008 Jan 17;358[3]:241-51).
“This is the largest and longest follow-up of any biologic in children and adolescents to date. I think it’s encouraging data and should be reassuring to those of us who are managing this important population of pediatric patients in our conversations with parents and families,”said Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
In the original 211-patient study, 57% of patients receiving etanercept (Enbrel) once weekly at 0.8 mg/kg to a maximum of 50 mg achieved a PASI 75 response at week 12, and 27% had a PASI 90. In the 69 patients who completed the full 264 weeks of follow-up, those response rates remained essentially unchanged.
Dr. Langley was quick to point out that this was an as-observed analysis, meaning results were counted only in those patients still participating at week 264. While conceding that the two-thirds dropout rate is an important study limitation, he added: “Notwithstanding that, what matters to us in the clinic are the patients we continue to treat and how they’re responding.”
Of note, most study discontinuations didn’t result from loss of response or adverse events, they were due to withdrawal of consent by families in which the patient began the study as a cooperative child and who as time went by turned into an independent and often willful teenager, he said.
No new safety signals arose over the course of 5 years. There were no opportunistic infections, no malignancies. The most common adverse events were the same ones seen in the original short-term study: upper respiratory infections, nasopharyngitis, and headaches. There was only one serious adverse event deemed by investigators as ‘possibly related’ to etanercept therapy: a case of cellulitis.
Separately, Dr. Diamant Thaci and Dr. Kim A. Papp presented 52-week outcomes for different aspects of the pivotal phase III randomized trial which earlier in 2015 earned adalimumab (Humira) European Commission marketing approval as the first biologic agent indicated for treatment of children as young as age 4 years, as well as for adolescents. The multi-arm trial included 114 patients aged 4-17 years with moderate to severe plaque psoriasis.
Dr. Thaci reported on the 37 subjects initially randomized double-blind to 16 weeks of oral methotrexate at 0.1-0.4 mg/kg weekly. The 19 patients (51%) who were deemed nonresponders to methotrexate because of inadequate PASI response at week 16 were then switched to open-label adalimumab at 0.8 mg/kg every other week for the remainder of the 52 weeks.
After 16 weeks on adalimumab, the methotrexate nonresponders had a PASI 75 of 90% and a PASI 90 of 74%, and 79% of the subjects were rated clear or almost clear by Physician’s Global Assessment. At week 52, the PASI 75 rate was 79%, the PASI 90 rate was 58%, and 68% of patients were rated clear or almost clear, according to Dr. Thaci of University Hospital Schleswig-Holstein, in L<scaps>ü</scaps>beck, Germany.
The side effects of methotrexate and adalimumab were similar to those seen in adults. There were no serious adverse events. The infections that occurred during adalimumab therapy were “very banal things,” mostly nasopharyngitis and upper respiratory tract infections, the dermatologist said. There were no opportunistic infections, malignancies, or cases of tuberculosis during the phase III study.
This was an important analysis because it recapitulates daily clinical practice, he explained. In most of the world, when dermatologists deem it time for systemic therapy, they generally start out with methotrexate, reserving biologics for second-line therapy because of the cost.
Dr. Papp reported on 39 patients on adalimumab at 0.4 mg/kg every other week, and 38 on 0.8 mg/kg every other week throughout the 52-week study. The response rate was essentially a flat line from week 16 to week 52, with PASI 75s of 44% at week 16 and 50% at week 52 in the low-dose group, and 58% and 56% in the high-dose group. Half of patients on 0.4 mg/kg were clear or almost clear at week 52, as were 56% on 0.8 mg/kg.
There was a nonsignificant trend for an increasing infection rate with greater exposure to adalimumab, which will require evaluation during the ongoing follow-up beyond 52 weeks, said Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“I think what’s gratifying about this is we see that these children actually have a robust response and that response is maintained over a full year of treatment, which is reassuring because that reflects what we’ve seen in the adult population as well,” he said.
Dr. Papp and Dr. Thaci receive research funding and serve as scientific advisers to AbbVie, which sponsored the adalimumab trial. They also have ties to other pharmaceutical companies.
Dr. Langley has served as principal investigator for and is on the scientific advisory boards of Amgen, which sponsored the etanercept pediatric psoriasis study. He also has ties to other pharmaceutical companies.
COPENHAGEN – The longest-ever clinical trials of etanercept and adalimumab for pediatric psoriasis show reassuring maintenance of efficacy, coupled with safety and tolerability profiles similar to what has been seen in long-term trials in adults, investigators reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Richard G. Langley presented outcomes from a 5-year open-label extension of an initial 12-week, multicenter, double-blind, randomized trial of etanercept or placebo in children and adolescents with moderate to severe chronic plaque psoriasis. The primary results were published more than 7 years ago (N Engl J Med. 2008 Jan 17;358[3]:241-51).
“This is the largest and longest follow-up of any biologic in children and adolescents to date. I think it’s encouraging data and should be reassuring to those of us who are managing this important population of pediatric patients in our conversations with parents and families,”said Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
In the original 211-patient study, 57% of patients receiving etanercept (Enbrel) once weekly at 0.8 mg/kg to a maximum of 50 mg achieved a PASI 75 response at week 12, and 27% had a PASI 90. In the 69 patients who completed the full 264 weeks of follow-up, those response rates remained essentially unchanged.
Dr. Langley was quick to point out that this was an as-observed analysis, meaning results were counted only in those patients still participating at week 264. While conceding that the two-thirds dropout rate is an important study limitation, he added: “Notwithstanding that, what matters to us in the clinic are the patients we continue to treat and how they’re responding.”
Of note, most study discontinuations didn’t result from loss of response or adverse events, they were due to withdrawal of consent by families in which the patient began the study as a cooperative child and who as time went by turned into an independent and often willful teenager, he said.
No new safety signals arose over the course of 5 years. There were no opportunistic infections, no malignancies. The most common adverse events were the same ones seen in the original short-term study: upper respiratory infections, nasopharyngitis, and headaches. There was only one serious adverse event deemed by investigators as ‘possibly related’ to etanercept therapy: a case of cellulitis.
Separately, Dr. Diamant Thaci and Dr. Kim A. Papp presented 52-week outcomes for different aspects of the pivotal phase III randomized trial which earlier in 2015 earned adalimumab (Humira) European Commission marketing approval as the first biologic agent indicated for treatment of children as young as age 4 years, as well as for adolescents. The multi-arm trial included 114 patients aged 4-17 years with moderate to severe plaque psoriasis.
Dr. Thaci reported on the 37 subjects initially randomized double-blind to 16 weeks of oral methotrexate at 0.1-0.4 mg/kg weekly. The 19 patients (51%) who were deemed nonresponders to methotrexate because of inadequate PASI response at week 16 were then switched to open-label adalimumab at 0.8 mg/kg every other week for the remainder of the 52 weeks.
After 16 weeks on adalimumab, the methotrexate nonresponders had a PASI 75 of 90% and a PASI 90 of 74%, and 79% of the subjects were rated clear or almost clear by Physician’s Global Assessment. At week 52, the PASI 75 rate was 79%, the PASI 90 rate was 58%, and 68% of patients were rated clear or almost clear, according to Dr. Thaci of University Hospital Schleswig-Holstein, in L<scaps>ü</scaps>beck, Germany.
The side effects of methotrexate and adalimumab were similar to those seen in adults. There were no serious adverse events. The infections that occurred during adalimumab therapy were “very banal things,” mostly nasopharyngitis and upper respiratory tract infections, the dermatologist said. There were no opportunistic infections, malignancies, or cases of tuberculosis during the phase III study.
This was an important analysis because it recapitulates daily clinical practice, he explained. In most of the world, when dermatologists deem it time for systemic therapy, they generally start out with methotrexate, reserving biologics for second-line therapy because of the cost.
Dr. Papp reported on 39 patients on adalimumab at 0.4 mg/kg every other week, and 38 on 0.8 mg/kg every other week throughout the 52-week study. The response rate was essentially a flat line from week 16 to week 52, with PASI 75s of 44% at week 16 and 50% at week 52 in the low-dose group, and 58% and 56% in the high-dose group. Half of patients on 0.4 mg/kg were clear or almost clear at week 52, as were 56% on 0.8 mg/kg.
There was a nonsignificant trend for an increasing infection rate with greater exposure to adalimumab, which will require evaluation during the ongoing follow-up beyond 52 weeks, said Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“I think what’s gratifying about this is we see that these children actually have a robust response and that response is maintained over a full year of treatment, which is reassuring because that reflects what we’ve seen in the adult population as well,” he said.
Dr. Papp and Dr. Thaci receive research funding and serve as scientific advisers to AbbVie, which sponsored the adalimumab trial. They also have ties to other pharmaceutical companies.
Dr. Langley has served as principal investigator for and is on the scientific advisory boards of Amgen, which sponsored the etanercept pediatric psoriasis study. He also has ties to other pharmaceutical companies.
COPENHAGEN – The longest-ever clinical trials of etanercept and adalimumab for pediatric psoriasis show reassuring maintenance of efficacy, coupled with safety and tolerability profiles similar to what has been seen in long-term trials in adults, investigators reported at the annual congress of the European Academy of Dermatology and Venereology.
Dr. Richard G. Langley presented outcomes from a 5-year open-label extension of an initial 12-week, multicenter, double-blind, randomized trial of etanercept or placebo in children and adolescents with moderate to severe chronic plaque psoriasis. The primary results were published more than 7 years ago (N Engl J Med. 2008 Jan 17;358[3]:241-51).
“This is the largest and longest follow-up of any biologic in children and adolescents to date. I think it’s encouraging data and should be reassuring to those of us who are managing this important population of pediatric patients in our conversations with parents and families,”said Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
In the original 211-patient study, 57% of patients receiving etanercept (Enbrel) once weekly at 0.8 mg/kg to a maximum of 50 mg achieved a PASI 75 response at week 12, and 27% had a PASI 90. In the 69 patients who completed the full 264 weeks of follow-up, those response rates remained essentially unchanged.
Dr. Langley was quick to point out that this was an as-observed analysis, meaning results were counted only in those patients still participating at week 264. While conceding that the two-thirds dropout rate is an important study limitation, he added: “Notwithstanding that, what matters to us in the clinic are the patients we continue to treat and how they’re responding.”
Of note, most study discontinuations didn’t result from loss of response or adverse events, they were due to withdrawal of consent by families in which the patient began the study as a cooperative child and who as time went by turned into an independent and often willful teenager, he said.
No new safety signals arose over the course of 5 years. There were no opportunistic infections, no malignancies. The most common adverse events were the same ones seen in the original short-term study: upper respiratory infections, nasopharyngitis, and headaches. There was only one serious adverse event deemed by investigators as ‘possibly related’ to etanercept therapy: a case of cellulitis.
Separately, Dr. Diamant Thaci and Dr. Kim A. Papp presented 52-week outcomes for different aspects of the pivotal phase III randomized trial which earlier in 2015 earned adalimumab (Humira) European Commission marketing approval as the first biologic agent indicated for treatment of children as young as age 4 years, as well as for adolescents. The multi-arm trial included 114 patients aged 4-17 years with moderate to severe plaque psoriasis.
Dr. Thaci reported on the 37 subjects initially randomized double-blind to 16 weeks of oral methotrexate at 0.1-0.4 mg/kg weekly. The 19 patients (51%) who were deemed nonresponders to methotrexate because of inadequate PASI response at week 16 were then switched to open-label adalimumab at 0.8 mg/kg every other week for the remainder of the 52 weeks.
After 16 weeks on adalimumab, the methotrexate nonresponders had a PASI 75 of 90% and a PASI 90 of 74%, and 79% of the subjects were rated clear or almost clear by Physician’s Global Assessment. At week 52, the PASI 75 rate was 79%, the PASI 90 rate was 58%, and 68% of patients were rated clear or almost clear, according to Dr. Thaci of University Hospital Schleswig-Holstein, in L<scaps>ü</scaps>beck, Germany.
The side effects of methotrexate and adalimumab were similar to those seen in adults. There were no serious adverse events. The infections that occurred during adalimumab therapy were “very banal things,” mostly nasopharyngitis and upper respiratory tract infections, the dermatologist said. There were no opportunistic infections, malignancies, or cases of tuberculosis during the phase III study.
This was an important analysis because it recapitulates daily clinical practice, he explained. In most of the world, when dermatologists deem it time for systemic therapy, they generally start out with methotrexate, reserving biologics for second-line therapy because of the cost.
Dr. Papp reported on 39 patients on adalimumab at 0.4 mg/kg every other week, and 38 on 0.8 mg/kg every other week throughout the 52-week study. The response rate was essentially a flat line from week 16 to week 52, with PASI 75s of 44% at week 16 and 50% at week 52 in the low-dose group, and 58% and 56% in the high-dose group. Half of patients on 0.4 mg/kg were clear or almost clear at week 52, as were 56% on 0.8 mg/kg.
There was a nonsignificant trend for an increasing infection rate with greater exposure to adalimumab, which will require evaluation during the ongoing follow-up beyond 52 weeks, said Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
“I think what’s gratifying about this is we see that these children actually have a robust response and that response is maintained over a full year of treatment, which is reassuring because that reflects what we’ve seen in the adult population as well,” he said.
Dr. Papp and Dr. Thaci receive research funding and serve as scientific advisers to AbbVie, which sponsored the adalimumab trial. They also have ties to other pharmaceutical companies.
Dr. Langley has served as principal investigator for and is on the scientific advisory boards of Amgen, which sponsored the etanercept pediatric psoriasis study. He also has ties to other pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
EADV: Pediatric psoriasis called ‘grossly undertreated’
COPENHAGEN – Children and adolescents with moderate to severe psoriasis are generally undertreated, with resultant potential psychological impairment, social stigmatization, and isolation, psoriasis experts agreed at the annual congress of the European Academy of Dermatology and Venereology.
In a session featuring new long-term safety and efficacy data from extended pediatric clinical trials of etanercept and adalimumab, prominent clinical trial investigators who presented the findings asserted that many physicians and parents are overly timid about the use of systemic treatment options in children and adolescents when such therapy is clearly warranted.
“The burden of psoriasis is particularly acute in the pediatric population. I always ask patients, ‘How is psoriasis affecting you?’ And with children, it’s affecting them and it’s also affecting their parents. If it’s affecting their self-esteem – if it’s ruining your life and you can’t control it with topical agents – I think it’s time to think of other things,” declared Dr. Richard G. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
Yet all too often that doesn’t happen, as documented in the recently reported physician portion of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which included 391 dermatologists and 390 rheumatologists in North America and Western Europe (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
Dr. Langley was a coinvestigator in MAPP, which found that 39% of dermatologists reported treating their moderate to severe psoriasis patients with conventional oral and/or biologic agents (19.5% and 19.6%, respectively). A total of 75% indicated they prescribe topical medications as monotherapy for their patients with moderate to severe psoriasis. In children, that rate was even higher, he said, despite the fact that topical monotherapy is clearly inadequate for treatment of more severe disease.
“I think there’s a fear about using systemics among parents and among some practitioners, which was one of the top reasons in the MAPP survey that people didn’t get appropriate treatment,” he said.
That’s a shortsighted attitude, the dermatologist continued: “The risk is not just the risk of the drug, but the risk of untreated disease, because untreated disease devastates patients.”
MAPP was the largest-ever survey of physician and patient perspectives regarding psoriasis and psoriatic arthritis. The patient perspective, derived from interviews with nearly 3,500 patients, was published earlier (J Am Acad Dermatol. 2014 May;70[5]:871-81).
One impediment to more widespread use of systemic therapies for pediatric psoriasis is that it’s almost entirely off-label prescribing. In the United States, no conventional oral agents or biologics are Food and Drug Administration approved for use in psoriasis patients under age 18 years. That was the case in Europe as well until earlier this year, when adalimumab (Humira) received European marketing approval for children ages 4 years and up with severe chronic plaque psoriasis with an inadequate response to topical agents and phototherapy or in whom such therapies are contraindicated.
Dr. Kim A. Papp, who presented new 52-week safety and efficacy data from a phase III study of adalimumab in pediatric psoriasis patients, said that even when dermatologists utilize biologics in pediatric patients, the medications are typically underdosed. In the phase III pediatric adalimumab trial, for example, most participants were overweight or obese, and efficacy dropped off with increasing body mass index, as has been the case in trials of most of the biologics.
“When I look at the doses of the biologic agents that are used in treating the pediatric population and what we know about how these molecules are distributed in the body, we can safely say we are grossly underdosing children. We’re doing that because we believe somehow they’re at greater risk from exposures that are comparable to those in the adult population. And it’s not true. What we’ve seen over 20 years of using the biologics is that these agents are very safe and very effective when used appropriately in adequate doses,” according to Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
Asked which biologics he’s most comfortable with in prescribing for pediatric patients, Dr. Papp replied, “I think the best biologic therapies for pediatric patients are the ones that have been studied in the pediatric population: etanercept, ustekinumab, and adalimumab. Those three are the ones we want to choose because we at least have some guidance and some assurance in terms of expectations of response and adverse events.”
Dr. Papp and Dr. Langley have served as principal investigators of numerous clinical trials for and served as advisors to pharmaceutical companies developing dermatologic medications.
COPENHAGEN – Children and adolescents with moderate to severe psoriasis are generally undertreated, with resultant potential psychological impairment, social stigmatization, and isolation, psoriasis experts agreed at the annual congress of the European Academy of Dermatology and Venereology.
In a session featuring new long-term safety and efficacy data from extended pediatric clinical trials of etanercept and adalimumab, prominent clinical trial investigators who presented the findings asserted that many physicians and parents are overly timid about the use of systemic treatment options in children and adolescents when such therapy is clearly warranted.
“The burden of psoriasis is particularly acute in the pediatric population. I always ask patients, ‘How is psoriasis affecting you?’ And with children, it’s affecting them and it’s also affecting their parents. If it’s affecting their self-esteem – if it’s ruining your life and you can’t control it with topical agents – I think it’s time to think of other things,” declared Dr. Richard G. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
Yet all too often that doesn’t happen, as documented in the recently reported physician portion of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which included 391 dermatologists and 390 rheumatologists in North America and Western Europe (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
Dr. Langley was a coinvestigator in MAPP, which found that 39% of dermatologists reported treating their moderate to severe psoriasis patients with conventional oral and/or biologic agents (19.5% and 19.6%, respectively). A total of 75% indicated they prescribe topical medications as monotherapy for their patients with moderate to severe psoriasis. In children, that rate was even higher, he said, despite the fact that topical monotherapy is clearly inadequate for treatment of more severe disease.
“I think there’s a fear about using systemics among parents and among some practitioners, which was one of the top reasons in the MAPP survey that people didn’t get appropriate treatment,” he said.
That’s a shortsighted attitude, the dermatologist continued: “The risk is not just the risk of the drug, but the risk of untreated disease, because untreated disease devastates patients.”
MAPP was the largest-ever survey of physician and patient perspectives regarding psoriasis and psoriatic arthritis. The patient perspective, derived from interviews with nearly 3,500 patients, was published earlier (J Am Acad Dermatol. 2014 May;70[5]:871-81).
One impediment to more widespread use of systemic therapies for pediatric psoriasis is that it’s almost entirely off-label prescribing. In the United States, no conventional oral agents or biologics are Food and Drug Administration approved for use in psoriasis patients under age 18 years. That was the case in Europe as well until earlier this year, when adalimumab (Humira) received European marketing approval for children ages 4 years and up with severe chronic plaque psoriasis with an inadequate response to topical agents and phototherapy or in whom such therapies are contraindicated.
Dr. Kim A. Papp, who presented new 52-week safety and efficacy data from a phase III study of adalimumab in pediatric psoriasis patients, said that even when dermatologists utilize biologics in pediatric patients, the medications are typically underdosed. In the phase III pediatric adalimumab trial, for example, most participants were overweight or obese, and efficacy dropped off with increasing body mass index, as has been the case in trials of most of the biologics.
“When I look at the doses of the biologic agents that are used in treating the pediatric population and what we know about how these molecules are distributed in the body, we can safely say we are grossly underdosing children. We’re doing that because we believe somehow they’re at greater risk from exposures that are comparable to those in the adult population. And it’s not true. What we’ve seen over 20 years of using the biologics is that these agents are very safe and very effective when used appropriately in adequate doses,” according to Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
Asked which biologics he’s most comfortable with in prescribing for pediatric patients, Dr. Papp replied, “I think the best biologic therapies for pediatric patients are the ones that have been studied in the pediatric population: etanercept, ustekinumab, and adalimumab. Those three are the ones we want to choose because we at least have some guidance and some assurance in terms of expectations of response and adverse events.”
Dr. Papp and Dr. Langley have served as principal investigators of numerous clinical trials for and served as advisors to pharmaceutical companies developing dermatologic medications.
COPENHAGEN – Children and adolescents with moderate to severe psoriasis are generally undertreated, with resultant potential psychological impairment, social stigmatization, and isolation, psoriasis experts agreed at the annual congress of the European Academy of Dermatology and Venereology.
In a session featuring new long-term safety and efficacy data from extended pediatric clinical trials of etanercept and adalimumab, prominent clinical trial investigators who presented the findings asserted that many physicians and parents are overly timid about the use of systemic treatment options in children and adolescents when such therapy is clearly warranted.
“The burden of psoriasis is particularly acute in the pediatric population. I always ask patients, ‘How is psoriasis affecting you?’ And with children, it’s affecting them and it’s also affecting their parents. If it’s affecting their self-esteem – if it’s ruining your life and you can’t control it with topical agents – I think it’s time to think of other things,” declared Dr. Richard G. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
Yet all too often that doesn’t happen, as documented in the recently reported physician portion of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey, which included 391 dermatologists and 390 rheumatologists in North America and Western Europe (J Eur Acad Dermatol Venereol. 2015 Oct;29[10]:2002-10).
Dr. Langley was a coinvestigator in MAPP, which found that 39% of dermatologists reported treating their moderate to severe psoriasis patients with conventional oral and/or biologic agents (19.5% and 19.6%, respectively). A total of 75% indicated they prescribe topical medications as monotherapy for their patients with moderate to severe psoriasis. In children, that rate was even higher, he said, despite the fact that topical monotherapy is clearly inadequate for treatment of more severe disease.
“I think there’s a fear about using systemics among parents and among some practitioners, which was one of the top reasons in the MAPP survey that people didn’t get appropriate treatment,” he said.
That’s a shortsighted attitude, the dermatologist continued: “The risk is not just the risk of the drug, but the risk of untreated disease, because untreated disease devastates patients.”
MAPP was the largest-ever survey of physician and patient perspectives regarding psoriasis and psoriatic arthritis. The patient perspective, derived from interviews with nearly 3,500 patients, was published earlier (J Am Acad Dermatol. 2014 May;70[5]:871-81).
One impediment to more widespread use of systemic therapies for pediatric psoriasis is that it’s almost entirely off-label prescribing. In the United States, no conventional oral agents or biologics are Food and Drug Administration approved for use in psoriasis patients under age 18 years. That was the case in Europe as well until earlier this year, when adalimumab (Humira) received European marketing approval for children ages 4 years and up with severe chronic plaque psoriasis with an inadequate response to topical agents and phototherapy or in whom such therapies are contraindicated.
Dr. Kim A. Papp, who presented new 52-week safety and efficacy data from a phase III study of adalimumab in pediatric psoriasis patients, said that even when dermatologists utilize biologics in pediatric patients, the medications are typically underdosed. In the phase III pediatric adalimumab trial, for example, most participants were overweight or obese, and efficacy dropped off with increasing body mass index, as has been the case in trials of most of the biologics.
“When I look at the doses of the biologic agents that are used in treating the pediatric population and what we know about how these molecules are distributed in the body, we can safely say we are grossly underdosing children. We’re doing that because we believe somehow they’re at greater risk from exposures that are comparable to those in the adult population. And it’s not true. What we’ve seen over 20 years of using the biologics is that these agents are very safe and very effective when used appropriately in adequate doses,” according to Dr. Papp, president of Probity Medical Research in Waterloo, Ont.
Asked which biologics he’s most comfortable with in prescribing for pediatric patients, Dr. Papp replied, “I think the best biologic therapies for pediatric patients are the ones that have been studied in the pediatric population: etanercept, ustekinumab, and adalimumab. Those three are the ones we want to choose because we at least have some guidance and some assurance in terms of expectations of response and adverse events.”
Dr. Papp and Dr. Langley have served as principal investigators of numerous clinical trials for and served as advisors to pharmaceutical companies developing dermatologic medications.
EXPERT ANALYSIS FROM THE EADV CONGRESS
AHA: Empagliflozin for T2D reduces heart failure endpoints
ORLANDO – The SGLT2 inhibitor empagliflozin reduced the risk of the composite endpoint of hospitalization for heart failure or death due to cardiovascular disease by 34% in patients with type 2 diabetes and prior cardiovascular events in the landmark EMPA-REG OUTCOME trial, Dr. Silvio E. Inzucchi reported at the American Heart Association scientific sessions.
The benefit was consistent regardless of whether or not patients had heart failure at enrollment, added Dr. Inzucchi, professor of medicine and clinical director of the endocrinology section at Yale University in New Haven, Conn.
He presented a prespecified secondary analysis from EMPA-REG OUTCOME that focused on heart failure–related endpoints. He had previously presented the primary outcome in Stockholm at the annual meeting of the European Association for the Study of Diabetes: a 14% relative risk reduction for empagliflozin in the composite of cardiovascular death, MI, or stroke, compared with placebo, driven largely by a 38% relative risk reduction in cardiovascular death.
The phase III, randomized, double-blind study has taken the worlds of cardiology and endocrinology by storm because it has convincingly demonstrated that empagliflozin (Jardiance) is the first glucose-lowering agent that also prevents cardiovascular complications.
“There are 12 different types of medications for lowering blood sugar levels in patients with type 2 diabetes – more than there are for lowering high blood pressure,” he noted. “We have been searching for decades for a diabetes medicine that will not only lower blood sugar but also reduce cardiovascular complications.”
The EMPA-REG OUTCOME trial included 7,020 patients with type 2 diabetes and a history of cardiovascular disease who were followed for a median of 3.1 years. Participants had a high rate of background optimal medical therapy for secondary cardiovascular prevention. They were randomized to empagliflozin at 10 or 25 mg/day or placebo. Results with the two doses of empagliflozin were pooled because the outcomes were so similar.
In addition to the 34% relative risk reduction in the combined endpoint of heart failure hospitalization or heart failure death, the empagliflozin group also experienced a 39% reduction in the composite of heart failure hospitalization or death due to heart failure.
These advantages for empagliflozin held true for all prespecified patient subgroups, including those based upon age, renal function, use of insulin, and background cardioprotective medications, Dr. Inzucchi noted.
Of study participants, 10% already had heart failure at baseline. Their rate of heart failure hospitalization or cardiovascular death was 20.1% on placebo and 16.2% with empagliflozin, a 28% relative risk reduction. In patients without heart failure at enrollment, the rates of this composite endpoint were 7.1% with placebo and 4.5% with the SGLT2 (sodium-glucose transporter 2) inhibitor, for a 37% relative risk reduction.
The only adverse event that occurred more frequently in empagliflozin-treated patients with or without heart failure than in controls was a threefold increase in genital infections. These easily treatable infections are a consequence of empagliflozin’s mechanism of action in reducing blood glucose, which entails increasing urinary excretion, the endocrinologist explained.
Discussant Dr. Allison B. Goldfine called Dr. Inzucchi’s update “really exciting.”
“Up to this time there has been little within the diabetes therapeutic pharmacologic options that has established clear safety, specifically with regard to heart failure. While we may not fully understand the mechanism behind the observed cardiovascular risk reduction, the uniformity of the findings is really remarkably consistent,” said Dr. Goldfine, head of the section of endocrinology at the Joslin Diabetes Center and an endocrinologist at Harvard Medical School, Boston.
“This is very promising,” Dr. David Goff, dean of the Colorado School of Public Health, declared in an interview. “We’ve had a lot of disappointments in the field of cardiovascular disease prevention for patients with diabetes mellitus. I think this medication should change the way we take care of people with diabetes.”
Metformin is widely accepted within endocrinology as the first-line agent for the treatment of type 2 diabetes, Dr. Inzucchi said. But he added that cardiovascular disease is a major problem in patients with diabetes. Heart failure, for example, is present in more than one in five type 2 diabetic patients over age 65.
Asked if he believes the cardiovascular benefits seen with empagliflozin in EMPA-REG OUTCOME are likely to be due to a class effect for SGLT2 inhibitors, Dr. Inzucchi replied that it’s impossible to say, since empagliflozin’s mechanism of cardiovascular benefit is unknown.
“Large randomized trials with hard cardiovascular endpoints are ongoing for the other two SGLT2 inhibitors, canagliflozin and dapagliflozin. Results should be available in 2-3 years. Then we’ll know,” he said.
Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
ORLANDO – The SGLT2 inhibitor empagliflozin reduced the risk of the composite endpoint of hospitalization for heart failure or death due to cardiovascular disease by 34% in patients with type 2 diabetes and prior cardiovascular events in the landmark EMPA-REG OUTCOME trial, Dr. Silvio E. Inzucchi reported at the American Heart Association scientific sessions.
The benefit was consistent regardless of whether or not patients had heart failure at enrollment, added Dr. Inzucchi, professor of medicine and clinical director of the endocrinology section at Yale University in New Haven, Conn.
He presented a prespecified secondary analysis from EMPA-REG OUTCOME that focused on heart failure–related endpoints. He had previously presented the primary outcome in Stockholm at the annual meeting of the European Association for the Study of Diabetes: a 14% relative risk reduction for empagliflozin in the composite of cardiovascular death, MI, or stroke, compared with placebo, driven largely by a 38% relative risk reduction in cardiovascular death.
The phase III, randomized, double-blind study has taken the worlds of cardiology and endocrinology by storm because it has convincingly demonstrated that empagliflozin (Jardiance) is the first glucose-lowering agent that also prevents cardiovascular complications.
“There are 12 different types of medications for lowering blood sugar levels in patients with type 2 diabetes – more than there are for lowering high blood pressure,” he noted. “We have been searching for decades for a diabetes medicine that will not only lower blood sugar but also reduce cardiovascular complications.”
The EMPA-REG OUTCOME trial included 7,020 patients with type 2 diabetes and a history of cardiovascular disease who were followed for a median of 3.1 years. Participants had a high rate of background optimal medical therapy for secondary cardiovascular prevention. They were randomized to empagliflozin at 10 or 25 mg/day or placebo. Results with the two doses of empagliflozin were pooled because the outcomes were so similar.
In addition to the 34% relative risk reduction in the combined endpoint of heart failure hospitalization or heart failure death, the empagliflozin group also experienced a 39% reduction in the composite of heart failure hospitalization or death due to heart failure.
These advantages for empagliflozin held true for all prespecified patient subgroups, including those based upon age, renal function, use of insulin, and background cardioprotective medications, Dr. Inzucchi noted.
Of study participants, 10% already had heart failure at baseline. Their rate of heart failure hospitalization or cardiovascular death was 20.1% on placebo and 16.2% with empagliflozin, a 28% relative risk reduction. In patients without heart failure at enrollment, the rates of this composite endpoint were 7.1% with placebo and 4.5% with the SGLT2 (sodium-glucose transporter 2) inhibitor, for a 37% relative risk reduction.
The only adverse event that occurred more frequently in empagliflozin-treated patients with or without heart failure than in controls was a threefold increase in genital infections. These easily treatable infections are a consequence of empagliflozin’s mechanism of action in reducing blood glucose, which entails increasing urinary excretion, the endocrinologist explained.
Discussant Dr. Allison B. Goldfine called Dr. Inzucchi’s update “really exciting.”
“Up to this time there has been little within the diabetes therapeutic pharmacologic options that has established clear safety, specifically with regard to heart failure. While we may not fully understand the mechanism behind the observed cardiovascular risk reduction, the uniformity of the findings is really remarkably consistent,” said Dr. Goldfine, head of the section of endocrinology at the Joslin Diabetes Center and an endocrinologist at Harvard Medical School, Boston.
“This is very promising,” Dr. David Goff, dean of the Colorado School of Public Health, declared in an interview. “We’ve had a lot of disappointments in the field of cardiovascular disease prevention for patients with diabetes mellitus. I think this medication should change the way we take care of people with diabetes.”
Metformin is widely accepted within endocrinology as the first-line agent for the treatment of type 2 diabetes, Dr. Inzucchi said. But he added that cardiovascular disease is a major problem in patients with diabetes. Heart failure, for example, is present in more than one in five type 2 diabetic patients over age 65.
Asked if he believes the cardiovascular benefits seen with empagliflozin in EMPA-REG OUTCOME are likely to be due to a class effect for SGLT2 inhibitors, Dr. Inzucchi replied that it’s impossible to say, since empagliflozin’s mechanism of cardiovascular benefit is unknown.
“Large randomized trials with hard cardiovascular endpoints are ongoing for the other two SGLT2 inhibitors, canagliflozin and dapagliflozin. Results should be available in 2-3 years. Then we’ll know,” he said.
Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
ORLANDO – The SGLT2 inhibitor empagliflozin reduced the risk of the composite endpoint of hospitalization for heart failure or death due to cardiovascular disease by 34% in patients with type 2 diabetes and prior cardiovascular events in the landmark EMPA-REG OUTCOME trial, Dr. Silvio E. Inzucchi reported at the American Heart Association scientific sessions.
The benefit was consistent regardless of whether or not patients had heart failure at enrollment, added Dr. Inzucchi, professor of medicine and clinical director of the endocrinology section at Yale University in New Haven, Conn.
He presented a prespecified secondary analysis from EMPA-REG OUTCOME that focused on heart failure–related endpoints. He had previously presented the primary outcome in Stockholm at the annual meeting of the European Association for the Study of Diabetes: a 14% relative risk reduction for empagliflozin in the composite of cardiovascular death, MI, or stroke, compared with placebo, driven largely by a 38% relative risk reduction in cardiovascular death.
The phase III, randomized, double-blind study has taken the worlds of cardiology and endocrinology by storm because it has convincingly demonstrated that empagliflozin (Jardiance) is the first glucose-lowering agent that also prevents cardiovascular complications.
“There are 12 different types of medications for lowering blood sugar levels in patients with type 2 diabetes – more than there are for lowering high blood pressure,” he noted. “We have been searching for decades for a diabetes medicine that will not only lower blood sugar but also reduce cardiovascular complications.”
The EMPA-REG OUTCOME trial included 7,020 patients with type 2 diabetes and a history of cardiovascular disease who were followed for a median of 3.1 years. Participants had a high rate of background optimal medical therapy for secondary cardiovascular prevention. They were randomized to empagliflozin at 10 or 25 mg/day or placebo. Results with the two doses of empagliflozin were pooled because the outcomes were so similar.
In addition to the 34% relative risk reduction in the combined endpoint of heart failure hospitalization or heart failure death, the empagliflozin group also experienced a 39% reduction in the composite of heart failure hospitalization or death due to heart failure.
These advantages for empagliflozin held true for all prespecified patient subgroups, including those based upon age, renal function, use of insulin, and background cardioprotective medications, Dr. Inzucchi noted.
Of study participants, 10% already had heart failure at baseline. Their rate of heart failure hospitalization or cardiovascular death was 20.1% on placebo and 16.2% with empagliflozin, a 28% relative risk reduction. In patients without heart failure at enrollment, the rates of this composite endpoint were 7.1% with placebo and 4.5% with the SGLT2 (sodium-glucose transporter 2) inhibitor, for a 37% relative risk reduction.
The only adverse event that occurred more frequently in empagliflozin-treated patients with or without heart failure than in controls was a threefold increase in genital infections. These easily treatable infections are a consequence of empagliflozin’s mechanism of action in reducing blood glucose, which entails increasing urinary excretion, the endocrinologist explained.
Discussant Dr. Allison B. Goldfine called Dr. Inzucchi’s update “really exciting.”
“Up to this time there has been little within the diabetes therapeutic pharmacologic options that has established clear safety, specifically with regard to heart failure. While we may not fully understand the mechanism behind the observed cardiovascular risk reduction, the uniformity of the findings is really remarkably consistent,” said Dr. Goldfine, head of the section of endocrinology at the Joslin Diabetes Center and an endocrinologist at Harvard Medical School, Boston.
“This is very promising,” Dr. David Goff, dean of the Colorado School of Public Health, declared in an interview. “We’ve had a lot of disappointments in the field of cardiovascular disease prevention for patients with diabetes mellitus. I think this medication should change the way we take care of people with diabetes.”
Metformin is widely accepted within endocrinology as the first-line agent for the treatment of type 2 diabetes, Dr. Inzucchi said. But he added that cardiovascular disease is a major problem in patients with diabetes. Heart failure, for example, is present in more than one in five type 2 diabetic patients over age 65.
Asked if he believes the cardiovascular benefits seen with empagliflozin in EMPA-REG OUTCOME are likely to be due to a class effect for SGLT2 inhibitors, Dr. Inzucchi replied that it’s impossible to say, since empagliflozin’s mechanism of cardiovascular benefit is unknown.
“Large randomized trials with hard cardiovascular endpoints are ongoing for the other two SGLT2 inhibitors, canagliflozin and dapagliflozin. Results should be available in 2-3 years. Then we’ll know,” he said.
Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Empagliflozin is the first glucose-lowering medication ever shown to reduce heart failure hospitalizations or cardiovascular death in patients with type 2 diabetes.
Major finding: The composite rate of heart failure hospitalization or death due to heart failure was reduced by 39% in type 2 diabetic patients on empagliflozin compared with placebo.
Data source: This was a secondary analysis of the EMPA-REG OUTCOME trial, a phase-III, double-blind, randomized trial of 7,020 patients with type 2 diabetes and prior cardiovascular disease.
Disclosures: The presenter disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.