User login
WCD: Ixekizumab beats etanercept for psoriatic itch
VANCOUVER, B.C. – Ixekizumab-treated patients with plaque psoriasis reported markedly faster and larger improvements in itch severity than those on high-dose etanercept or placebo in a large head-to-head phase III randomized trial.
Ixekizumab is an investigational humanized IgG4 monoclonal antibody directed against the proinflammatory cytokines interleukin-17A. In the 1,224-patient phase III UNCOVER-2 trial, itch severity was rapidly reduced in ixekizumab-treated patients as early as week 1, when the first assessment occurred, Dr. Gil Yosipovitch reported at the World Congress of Dermatology.
Moreover, a clinically meaningful itch response – defined by Dr. Yosipovitch and coinvestigators as at least a 4-point drop from baseline on a 0-10 Itch Numeric Rating Scale based upon 24-hour recall – occurred in the ixekizumab-treated patients by week 4. Patients randomized to etanercept (Enbrel) at 50 mg twice per week never reached that bar during the 12-week study. Neither did the placebo-treated controls, noted Dr. Yosipovitch, professor and chairman of the department of dermatology at Temple University in Philadelphia.
The average baseline itch severity score in UNCOVER-2 was 6.6 points. The mean 12-week reduction in itch score was 0.4 points, or 14%, with placebo; 3.6 points, or 51%, with etanercept; 4.9 points, or 75%, with ixekizumab at 80 mg every 4 weeks; and 5.2 points, or 76%, with ixekizumab 80 mg every 2 weeks.
“The most important part of my presentation today is the rapid response to ixekizumab. There’s a significant reduction in itch in the first week and a 75% reduction at 12 weeks. I have to tell you, as someone who’s been in the itch field for a very long time, those are very impressive results,” the dermatologist declared. “Fast improvement is the issue, really. It we want a drug that claims to have an antipruritic effect, it has to work fast, because that’s what really bothers the patient.”
Another impressive statistic: 41% of ixekizumab-treated patients reported “no itching” at 12 weeks, compared with 17% on high-dose etanercept and 2% with placebo, he continued.
A recurring theme at WCD 2015, bolstered by an impressively large survey of North American and European dermatologists, rheumatologists, and patients, is that psoriasis patients feel itch hasn’t gotten the attention it deserves from physicians. Itch is the most common symptom of psoriasis, and more psoriasis patients rate itch as the most bothersome aspect of their chronic inflammatory skin disease than they do any other symptom, Dr. Yosipovitch noted.
Aside from this UNCOVER-2 analysis, very few data exist regarding the antipruritic effects of various biologic agents, he observed.
“I am very excited about this type of approach. Hopefully other companies wil now put more focus on itch. I think this is what the patients want, and if we can achieve fast responses, we are doing our job well,” the dermatologist said.
One audience member asked why the itch score is based upon recall over the previous 24 hours. Why not a week?
Dr. Yosipovitch replied that from other studies he’s done, he has found itch recall beyond 24 hours to be highly problematic.
“I actually recommend doing it twice a day, because itch is not constant throughout the day. It fluctuates. It is worst at nighttime, and that affects quality of life. In trials, I try to have patients keep a diary capturing itch evening and morning,” Dr. Yosipovitch said.
Another audience member asked whether a rapid response to itch might be a biomarker predictive of a later favorable response in terms of lesion size. If so, he continued, perhaps failure to experience a reduction in itch within the first week or so on an agent such a ixekizumab might be a signal to switch to another drug, rather than waiting for 4-8 weeks.
Dr. Yosipovitch replied that it’s an excellent thought and worthy of study, but the answer simply isn’t known yet.
The primary outcomes of UNCOVER-2 have previously been presented (Lancet 2015 June 10 [doi: 10.1016/S0140-6736(15)60125-8]). Briefly, the Psoriasis Area Severity Index (PASI) 75 response rate was 2% with placebo, 42% with etanercept, 78% with ixekizumab every 4 weeks, and 90% with ixekizumab every 2 weeks. When the bar was set higher – a PASI 100 – the response rate was 1% with placebo, 5% with etanercept, and 31% and 41% with ixekizumab every 4 and 2 weeks.
The UNCOVER-2 trial was sponsored by Eli Lilly. Dr. Yosipovitch is a recipient of research grants from and/or a consultant to Eli Lilly and roughly a dozen other pharmaceutical companies.
VANCOUVER, B.C. – Ixekizumab-treated patients with plaque psoriasis reported markedly faster and larger improvements in itch severity than those on high-dose etanercept or placebo in a large head-to-head phase III randomized trial.
Ixekizumab is an investigational humanized IgG4 monoclonal antibody directed against the proinflammatory cytokines interleukin-17A. In the 1,224-patient phase III UNCOVER-2 trial, itch severity was rapidly reduced in ixekizumab-treated patients as early as week 1, when the first assessment occurred, Dr. Gil Yosipovitch reported at the World Congress of Dermatology.
Moreover, a clinically meaningful itch response – defined by Dr. Yosipovitch and coinvestigators as at least a 4-point drop from baseline on a 0-10 Itch Numeric Rating Scale based upon 24-hour recall – occurred in the ixekizumab-treated patients by week 4. Patients randomized to etanercept (Enbrel) at 50 mg twice per week never reached that bar during the 12-week study. Neither did the placebo-treated controls, noted Dr. Yosipovitch, professor and chairman of the department of dermatology at Temple University in Philadelphia.
The average baseline itch severity score in UNCOVER-2 was 6.6 points. The mean 12-week reduction in itch score was 0.4 points, or 14%, with placebo; 3.6 points, or 51%, with etanercept; 4.9 points, or 75%, with ixekizumab at 80 mg every 4 weeks; and 5.2 points, or 76%, with ixekizumab 80 mg every 2 weeks.
“The most important part of my presentation today is the rapid response to ixekizumab. There’s a significant reduction in itch in the first week and a 75% reduction at 12 weeks. I have to tell you, as someone who’s been in the itch field for a very long time, those are very impressive results,” the dermatologist declared. “Fast improvement is the issue, really. It we want a drug that claims to have an antipruritic effect, it has to work fast, because that’s what really bothers the patient.”
Another impressive statistic: 41% of ixekizumab-treated patients reported “no itching” at 12 weeks, compared with 17% on high-dose etanercept and 2% with placebo, he continued.
A recurring theme at WCD 2015, bolstered by an impressively large survey of North American and European dermatologists, rheumatologists, and patients, is that psoriasis patients feel itch hasn’t gotten the attention it deserves from physicians. Itch is the most common symptom of psoriasis, and more psoriasis patients rate itch as the most bothersome aspect of their chronic inflammatory skin disease than they do any other symptom, Dr. Yosipovitch noted.
Aside from this UNCOVER-2 analysis, very few data exist regarding the antipruritic effects of various biologic agents, he observed.
“I am very excited about this type of approach. Hopefully other companies wil now put more focus on itch. I think this is what the patients want, and if we can achieve fast responses, we are doing our job well,” the dermatologist said.
One audience member asked why the itch score is based upon recall over the previous 24 hours. Why not a week?
Dr. Yosipovitch replied that from other studies he’s done, he has found itch recall beyond 24 hours to be highly problematic.
“I actually recommend doing it twice a day, because itch is not constant throughout the day. It fluctuates. It is worst at nighttime, and that affects quality of life. In trials, I try to have patients keep a diary capturing itch evening and morning,” Dr. Yosipovitch said.
Another audience member asked whether a rapid response to itch might be a biomarker predictive of a later favorable response in terms of lesion size. If so, he continued, perhaps failure to experience a reduction in itch within the first week or so on an agent such a ixekizumab might be a signal to switch to another drug, rather than waiting for 4-8 weeks.
Dr. Yosipovitch replied that it’s an excellent thought and worthy of study, but the answer simply isn’t known yet.
The primary outcomes of UNCOVER-2 have previously been presented (Lancet 2015 June 10 [doi: 10.1016/S0140-6736(15)60125-8]). Briefly, the Psoriasis Area Severity Index (PASI) 75 response rate was 2% with placebo, 42% with etanercept, 78% with ixekizumab every 4 weeks, and 90% with ixekizumab every 2 weeks. When the bar was set higher – a PASI 100 – the response rate was 1% with placebo, 5% with etanercept, and 31% and 41% with ixekizumab every 4 and 2 weeks.
The UNCOVER-2 trial was sponsored by Eli Lilly. Dr. Yosipovitch is a recipient of research grants from and/or a consultant to Eli Lilly and roughly a dozen other pharmaceutical companies.
VANCOUVER, B.C. – Ixekizumab-treated patients with plaque psoriasis reported markedly faster and larger improvements in itch severity than those on high-dose etanercept or placebo in a large head-to-head phase III randomized trial.
Ixekizumab is an investigational humanized IgG4 monoclonal antibody directed against the proinflammatory cytokines interleukin-17A. In the 1,224-patient phase III UNCOVER-2 trial, itch severity was rapidly reduced in ixekizumab-treated patients as early as week 1, when the first assessment occurred, Dr. Gil Yosipovitch reported at the World Congress of Dermatology.
Moreover, a clinically meaningful itch response – defined by Dr. Yosipovitch and coinvestigators as at least a 4-point drop from baseline on a 0-10 Itch Numeric Rating Scale based upon 24-hour recall – occurred in the ixekizumab-treated patients by week 4. Patients randomized to etanercept (Enbrel) at 50 mg twice per week never reached that bar during the 12-week study. Neither did the placebo-treated controls, noted Dr. Yosipovitch, professor and chairman of the department of dermatology at Temple University in Philadelphia.
The average baseline itch severity score in UNCOVER-2 was 6.6 points. The mean 12-week reduction in itch score was 0.4 points, or 14%, with placebo; 3.6 points, or 51%, with etanercept; 4.9 points, or 75%, with ixekizumab at 80 mg every 4 weeks; and 5.2 points, or 76%, with ixekizumab 80 mg every 2 weeks.
“The most important part of my presentation today is the rapid response to ixekizumab. There’s a significant reduction in itch in the first week and a 75% reduction at 12 weeks. I have to tell you, as someone who’s been in the itch field for a very long time, those are very impressive results,” the dermatologist declared. “Fast improvement is the issue, really. It we want a drug that claims to have an antipruritic effect, it has to work fast, because that’s what really bothers the patient.”
Another impressive statistic: 41% of ixekizumab-treated patients reported “no itching” at 12 weeks, compared with 17% on high-dose etanercept and 2% with placebo, he continued.
A recurring theme at WCD 2015, bolstered by an impressively large survey of North American and European dermatologists, rheumatologists, and patients, is that psoriasis patients feel itch hasn’t gotten the attention it deserves from physicians. Itch is the most common symptom of psoriasis, and more psoriasis patients rate itch as the most bothersome aspect of their chronic inflammatory skin disease than they do any other symptom, Dr. Yosipovitch noted.
Aside from this UNCOVER-2 analysis, very few data exist regarding the antipruritic effects of various biologic agents, he observed.
“I am very excited about this type of approach. Hopefully other companies wil now put more focus on itch. I think this is what the patients want, and if we can achieve fast responses, we are doing our job well,” the dermatologist said.
One audience member asked why the itch score is based upon recall over the previous 24 hours. Why not a week?
Dr. Yosipovitch replied that from other studies he’s done, he has found itch recall beyond 24 hours to be highly problematic.
“I actually recommend doing it twice a day, because itch is not constant throughout the day. It fluctuates. It is worst at nighttime, and that affects quality of life. In trials, I try to have patients keep a diary capturing itch evening and morning,” Dr. Yosipovitch said.
Another audience member asked whether a rapid response to itch might be a biomarker predictive of a later favorable response in terms of lesion size. If so, he continued, perhaps failure to experience a reduction in itch within the first week or so on an agent such a ixekizumab might be a signal to switch to another drug, rather than waiting for 4-8 weeks.
Dr. Yosipovitch replied that it’s an excellent thought and worthy of study, but the answer simply isn’t known yet.
The primary outcomes of UNCOVER-2 have previously been presented (Lancet 2015 June 10 [doi: 10.1016/S0140-6736(15)60125-8]). Briefly, the Psoriasis Area Severity Index (PASI) 75 response rate was 2% with placebo, 42% with etanercept, 78% with ixekizumab every 4 weeks, and 90% with ixekizumab every 2 weeks. When the bar was set higher – a PASI 100 – the response rate was 1% with placebo, 5% with etanercept, and 31% and 41% with ixekizumab every 4 and 2 weeks.
The UNCOVER-2 trial was sponsored by Eli Lilly. Dr. Yosipovitch is a recipient of research grants from and/or a consultant to Eli Lilly and roughly a dozen other pharmaceutical companies.
AT WCD 2015
Key clinical point: Ixekizumab reduces psoriatic itch markedly faster and more profoundly than etanercept.
Major finding: Psoriasis patients randomized to ixekizumab showed a significant reduction in itch scores within the first week.
Data source: The UNCOVER-2 trial was a 12-week, phase III randomized trial involving 1,224 patients with plaque psoriasis.
Disclosures: UNCOVER-2 was sponsored by Eli Lilly. The presenter reported receiving research grants from and/or serving as a consultant to Eli Lilly and roughly a dozen other pharmaceutical companies.
Amgen’s termination of brodalumab stuns psoriasis world
VANCOUVER, B.C. – Amgen’s recent announcement that it is pulling the plug on further development of the investigational interleukin-17 receptor A inhibitor brodalumab was the red hot topic among psoriasis experts at the World Congress of Dermatology.
The biologic agent had seemingly been breezing through phase III clinical trials, racking up head-turning efficacy and reasonable safety numbers in a series of very large randomized studies conducted in patients with moderate-to-severe chronic plaque psoriasis. Then came the surprise announcement a scant couple of weeks before WCD 2015.
Brodalumab was also in codevelopment by Amgen and AstraZeneca for psoriatic arthritis and axial spondyloarthritis. Amgen is halting all participation in those research projects as well.
The company’s decision was based upon preliminary indications from the Food and Drug Administration that, as a condition for marketing approval, there would likely need to be restrictive labeling calling for physician monitoring for suicidality, something few dermatologists or rheumatologists are comfortable with.
“During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” Dr. Sean E. Harper, executive vice president of research and development at Amgen, said in the company’s announcement.
AstraZeneca officials said in a separate statement that they will review all the brodalumab data in detail and make a decision as soon as possible regarding whether to pursue unilateral development of the biologic agent.
Dr. Richard G. Langley, who at WCD 2015 presented the results of the AMAGINE-3 trial – 1,881 psoriasis patients randomized to ustekinumab (Stelara) at the approved dosing, placebo, or brodalumab at either 140 mg or 210 mg subcutaneously – noted that rates of depression and suicidal ideation were similarly low in the ustekinumab and brodalumab groups during the 52-week study, and there were no suicide attempts.
The problem for Amgen was a single-digit numeric imbalance in these adverse events in some of the earlier, considerably smaller studies of brodalumab, according to Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
“We have to remember that we can sometimes make spurious assumptions about rare events and perhaps cast a shadow on a pathway or molecule before we have full information,” he said diplomatically. “The Amgen decision is particularly unfortunate for patients because with brodalumab the only patients I’ve had who were depressed were the ones I’ve told are now no longer going to get the medication.”
Session chair Dr. Yves Poulin of Laval University in Quebec City, Quebec, said his experience has been similar: Patients love the unprecedented effectiveness of brodalumab and are crestfallen at learning they have to go off it.
Indeed, in AMAGINE-3, the 12-week PASI 75 rate among patients on brodalumab at 210 mg every 2 weeks – the more effective dose of the IL-17A inhibitor – was 85.1%, compared with 69.2% for ustekinumab, which is one of the most widely prescribed biologics. The PASI 100 rate was 36.7% for brodalumab, twice the 18.5% rate with ustekinumab. And the Investigator’s Global Assessment rating of 0 or 1 – clear or almost clear – was 79.6% with brodalumab, compared with 59% for ustekinumab, Dr. Langley reported.
“A consistent finding with brodalumab and the other IL-17 inhibitors is the remarkably fast improvement: within the first 2-4 weeks there’s a robust response,” he added.
Elsewhere at WCD 2015, Dr. Alan Menter presented the findings of the AMAGINE-2 trial, an 1,831-patient study identical in design to AMAGINE-3. The efficacy and safety results were virtually the same as in AMAGINE-3 as well, both in the 12-week induction phase and out to 52 weeks.
“We have a major issue that I think people haven’t understood well over the years relating to depression and psoriasis. Suicidal ideation has always been an issue. If you’re a 25-year-old and you’re trying to make your way in life and you’ve got significant psoriasis, you’re going to get depressed. But the suicidal ideation is twice as high in young people with psoriasis at baseline than in all the other autoimmune diseases put together,” said Dr. Menter, chair of dermatology at Baylor University Medical Center, Dallas, and the founding president of the International Psoriasis Council.
There has been no suicidality signal with secukinumab (Cosentyx), which in January became the first and, to date, only IL-17 inhibitor approved for marketing, nor with ixekizumab, which is in phase III clinical trials. There are distinct differences between the IL-17 inhibitor molecules – for example, brodalumab is IgG2, secukinumab is IgG1, and ixekizumab is IgG4. And brodalumab is a receptor antibody that not only inhibits the activity of IL-17A, but of IL-17F and -E as well, whereas secukinumab and ixekizumab work by other mechanisms. Investigators are now taking a close look at these differences to see if they have effects in terms of clinical outcomes and side effect profiles, he said.
Dr. Langley and Dr. Menter reported having financial relationships with Amgen and numerous other pharmaceutical companies.
VANCOUVER, B.C. – Amgen’s recent announcement that it is pulling the plug on further development of the investigational interleukin-17 receptor A inhibitor brodalumab was the red hot topic among psoriasis experts at the World Congress of Dermatology.
The biologic agent had seemingly been breezing through phase III clinical trials, racking up head-turning efficacy and reasonable safety numbers in a series of very large randomized studies conducted in patients with moderate-to-severe chronic plaque psoriasis. Then came the surprise announcement a scant couple of weeks before WCD 2015.
Brodalumab was also in codevelopment by Amgen and AstraZeneca for psoriatic arthritis and axial spondyloarthritis. Amgen is halting all participation in those research projects as well.
The company’s decision was based upon preliminary indications from the Food and Drug Administration that, as a condition for marketing approval, there would likely need to be restrictive labeling calling for physician monitoring for suicidality, something few dermatologists or rheumatologists are comfortable with.
“During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” Dr. Sean E. Harper, executive vice president of research and development at Amgen, said in the company’s announcement.
AstraZeneca officials said in a separate statement that they will review all the brodalumab data in detail and make a decision as soon as possible regarding whether to pursue unilateral development of the biologic agent.
Dr. Richard G. Langley, who at WCD 2015 presented the results of the AMAGINE-3 trial – 1,881 psoriasis patients randomized to ustekinumab (Stelara) at the approved dosing, placebo, or brodalumab at either 140 mg or 210 mg subcutaneously – noted that rates of depression and suicidal ideation were similarly low in the ustekinumab and brodalumab groups during the 52-week study, and there were no suicide attempts.
The problem for Amgen was a single-digit numeric imbalance in these adverse events in some of the earlier, considerably smaller studies of brodalumab, according to Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
“We have to remember that we can sometimes make spurious assumptions about rare events and perhaps cast a shadow on a pathway or molecule before we have full information,” he said diplomatically. “The Amgen decision is particularly unfortunate for patients because with brodalumab the only patients I’ve had who were depressed were the ones I’ve told are now no longer going to get the medication.”
Session chair Dr. Yves Poulin of Laval University in Quebec City, Quebec, said his experience has been similar: Patients love the unprecedented effectiveness of brodalumab and are crestfallen at learning they have to go off it.
Indeed, in AMAGINE-3, the 12-week PASI 75 rate among patients on brodalumab at 210 mg every 2 weeks – the more effective dose of the IL-17A inhibitor – was 85.1%, compared with 69.2% for ustekinumab, which is one of the most widely prescribed biologics. The PASI 100 rate was 36.7% for brodalumab, twice the 18.5% rate with ustekinumab. And the Investigator’s Global Assessment rating of 0 or 1 – clear or almost clear – was 79.6% with brodalumab, compared with 59% for ustekinumab, Dr. Langley reported.
“A consistent finding with brodalumab and the other IL-17 inhibitors is the remarkably fast improvement: within the first 2-4 weeks there’s a robust response,” he added.
Elsewhere at WCD 2015, Dr. Alan Menter presented the findings of the AMAGINE-2 trial, an 1,831-patient study identical in design to AMAGINE-3. The efficacy and safety results were virtually the same as in AMAGINE-3 as well, both in the 12-week induction phase and out to 52 weeks.
“We have a major issue that I think people haven’t understood well over the years relating to depression and psoriasis. Suicidal ideation has always been an issue. If you’re a 25-year-old and you’re trying to make your way in life and you’ve got significant psoriasis, you’re going to get depressed. But the suicidal ideation is twice as high in young people with psoriasis at baseline than in all the other autoimmune diseases put together,” said Dr. Menter, chair of dermatology at Baylor University Medical Center, Dallas, and the founding president of the International Psoriasis Council.
There has been no suicidality signal with secukinumab (Cosentyx), which in January became the first and, to date, only IL-17 inhibitor approved for marketing, nor with ixekizumab, which is in phase III clinical trials. There are distinct differences between the IL-17 inhibitor molecules – for example, brodalumab is IgG2, secukinumab is IgG1, and ixekizumab is IgG4. And brodalumab is a receptor antibody that not only inhibits the activity of IL-17A, but of IL-17F and -E as well, whereas secukinumab and ixekizumab work by other mechanisms. Investigators are now taking a close look at these differences to see if they have effects in terms of clinical outcomes and side effect profiles, he said.
Dr. Langley and Dr. Menter reported having financial relationships with Amgen and numerous other pharmaceutical companies.
VANCOUVER, B.C. – Amgen’s recent announcement that it is pulling the plug on further development of the investigational interleukin-17 receptor A inhibitor brodalumab was the red hot topic among psoriasis experts at the World Congress of Dermatology.
The biologic agent had seemingly been breezing through phase III clinical trials, racking up head-turning efficacy and reasonable safety numbers in a series of very large randomized studies conducted in patients with moderate-to-severe chronic plaque psoriasis. Then came the surprise announcement a scant couple of weeks before WCD 2015.
Brodalumab was also in codevelopment by Amgen and AstraZeneca for psoriatic arthritis and axial spondyloarthritis. Amgen is halting all participation in those research projects as well.
The company’s decision was based upon preliminary indications from the Food and Drug Administration that, as a condition for marketing approval, there would likely need to be restrictive labeling calling for physician monitoring for suicidality, something few dermatologists or rheumatologists are comfortable with.
“During our preparation process for regulatory submissions, we came to believe that labeling requirements likely would limit the appropriate patient population for brodalumab,” Dr. Sean E. Harper, executive vice president of research and development at Amgen, said in the company’s announcement.
AstraZeneca officials said in a separate statement that they will review all the brodalumab data in detail and make a decision as soon as possible regarding whether to pursue unilateral development of the biologic agent.
Dr. Richard G. Langley, who at WCD 2015 presented the results of the AMAGINE-3 trial – 1,881 psoriasis patients randomized to ustekinumab (Stelara) at the approved dosing, placebo, or brodalumab at either 140 mg or 210 mg subcutaneously – noted that rates of depression and suicidal ideation were similarly low in the ustekinumab and brodalumab groups during the 52-week study, and there were no suicide attempts.
The problem for Amgen was a single-digit numeric imbalance in these adverse events in some of the earlier, considerably smaller studies of brodalumab, according to Dr. Langley, professor of dermatology at Dalhousie University in Halifax, Nova Scotia.
“We have to remember that we can sometimes make spurious assumptions about rare events and perhaps cast a shadow on a pathway or molecule before we have full information,” he said diplomatically. “The Amgen decision is particularly unfortunate for patients because with brodalumab the only patients I’ve had who were depressed were the ones I’ve told are now no longer going to get the medication.”
Session chair Dr. Yves Poulin of Laval University in Quebec City, Quebec, said his experience has been similar: Patients love the unprecedented effectiveness of brodalumab and are crestfallen at learning they have to go off it.
Indeed, in AMAGINE-3, the 12-week PASI 75 rate among patients on brodalumab at 210 mg every 2 weeks – the more effective dose of the IL-17A inhibitor – was 85.1%, compared with 69.2% for ustekinumab, which is one of the most widely prescribed biologics. The PASI 100 rate was 36.7% for brodalumab, twice the 18.5% rate with ustekinumab. And the Investigator’s Global Assessment rating of 0 or 1 – clear or almost clear – was 79.6% with brodalumab, compared with 59% for ustekinumab, Dr. Langley reported.
“A consistent finding with brodalumab and the other IL-17 inhibitors is the remarkably fast improvement: within the first 2-4 weeks there’s a robust response,” he added.
Elsewhere at WCD 2015, Dr. Alan Menter presented the findings of the AMAGINE-2 trial, an 1,831-patient study identical in design to AMAGINE-3. The efficacy and safety results were virtually the same as in AMAGINE-3 as well, both in the 12-week induction phase and out to 52 weeks.
“We have a major issue that I think people haven’t understood well over the years relating to depression and psoriasis. Suicidal ideation has always been an issue. If you’re a 25-year-old and you’re trying to make your way in life and you’ve got significant psoriasis, you’re going to get depressed. But the suicidal ideation is twice as high in young people with psoriasis at baseline than in all the other autoimmune diseases put together,” said Dr. Menter, chair of dermatology at Baylor University Medical Center, Dallas, and the founding president of the International Psoriasis Council.
There has been no suicidality signal with secukinumab (Cosentyx), which in January became the first and, to date, only IL-17 inhibitor approved for marketing, nor with ixekizumab, which is in phase III clinical trials. There are distinct differences between the IL-17 inhibitor molecules – for example, brodalumab is IgG2, secukinumab is IgG1, and ixekizumab is IgG4. And brodalumab is a receptor antibody that not only inhibits the activity of IL-17A, but of IL-17F and -E as well, whereas secukinumab and ixekizumab work by other mechanisms. Investigators are now taking a close look at these differences to see if they have effects in terms of clinical outcomes and side effect profiles, he said.
Dr. Langley and Dr. Menter reported having financial relationships with Amgen and numerous other pharmaceutical companies.
EXPERT ANALYSIS FROM WCD 2015
Expert touts ivermectin 1% cream as treatment of choice for rosacea
VANCOUVER, B.C. – A persuasive case can be made for ivermectin 1% cream as the new treatment of choice for papulopustular rosacea, Dr. Leon Kircik asserted at the World Congress of Dermatology.
He cited the results of a large recent head-to-head randomized trial in which ivermectin 1% cream (Soolantra), approved last December by the Food and Drug Administration, proved superior to metronidazole 0.75% cream (Metrocream), which is the market leader and until now the topical agent most physicians have considered their first-line treatment for papulopustular rosacea.
The study, known as ATTRACT (Assessment of a Topical Treatment in Rosacea: Activity, Compliance, Tolerability), was a 962-patient, phase III, single-blind, European randomized trial. Ivermectin demonstrated faster onset of action, a greater clinical success rate, delayed time to relapse following treatment discontinuation, a lower relapse rate, and better tolerability (Br. J. Dermatol. 2015;172:1103-10).
Dr. Kircik, a Louisville dermatologist in private practice, and a clinical trialist, was not involved in the ATTRACT study but was impressed that it was undertaken by Galderma, which markets both drugs.
“We don’t get many head-to-head studies in dermatology. Those are typically studies no one wants to sponsor. And most of what few head-to-head studies are done are designed as noninferiority studies. In this case, ATTRACT was designed as a superiority study. That’s a higher bar to reach,” noted Dr. Kircik, a consultant to, and member of the speakers bureau for, Galderma and other pharmaceutical companies.
ATTRACT was a two-part study. In part A, 962 patients with moderate to severe rosacea were randomized to ivermectin 1% cream once daily or metronidazole 0.75% cream b.i.d. for 16 weeks. As early as the first assessment at 3 weeks, a significant difference in favor of ivermectin was evident in terms of reduction in inflammatory lesion count. At week 16, the ivermectin group showed an 83% reduction from baseline in inflammatory lesions, significantly better than the 73.7% reduction with metronidazole. Moreover, 84.9% of the ivermectin group was rated clear or almost clear by Investigator’s Global Assessment (IGA) compared with 75.4% of the metronidazole group.
In addition, 34.9% of ivermectin-treated patients achieved an IGA of 0 by 16 weeks, meaning they were totally lesion free, compared with 21.7% of the metronidazole group. That “completely clear” status is really important psychosocially to a significant proportion of patients, said Dr. Kircik.
“How many times have you had a patient come in asking for an intralesional steroid injection for their single remaining lesion after months of topical treatment?” he asked rhetorically.
Ivermectin was better tolerated than was metronidazole. The rate of treatment-related adverse events leading to discontinuation – typically for skin irritation, redness, or itching – was 0.6% in the ivermectin group compared with 2.1% with metronidazole.
Part B of the ATTRACT study was an unusually designed 36-week extension study.
“In my mind, part B is much more important and much more relevant because rosacea is a chronic disease. It doesn’t last for just 16 weeks. Part B asks what happens after we stop therapy. This is the rare clinical trial that actually mimics real life,” Dr. Kircik said.
Despite physicians’ standard advice to continue with maintenance therapy after clearing, the reality is that most patients stop treatment once they clear, figuring they’ll resume if they relapse, he asserted. Part B of ATTRACT reflected that approach. Seven hundred fifty-seven patients who were clear/almost clear as defined by an IGA of 0/1 at the end of 16 weeks enrolled in the 36-week extension study. They surrendered their medication and returned to their physician monthly. If they had relapsed during that month, meaning they showed up with an IGA of 2 or more, they got their medication back; if they were still IGA 0/1, they did not.
The median time to relapse off treatment in the ivermectin group was 115 days, a full month later than the 85 days for the metronidazole group. Also, the relapse rate in the ivermectin group was significantly lower: 62.7% compared with 68.4% in the metronidazole group.
“The impact on both clinical practice and pharmacoeconomic practice is huge here. It makes sense to switch from metronidazole to ivermectin as first-line therapy because you know that your patients will do better and will relapse later and less,” Dr. Kircik declared.
He was a coinvestigator in the twin pivotal phase III randomized Galderma-funded trials that led to FDA approval of ivermectin for papulopustular rosacea. Although those studies have been published (J. Drugs Dermatol. 2014;13:1380-6), Dr. Kircik highlighted a couple of findings he said haven’t drawn the attention they deserve. Both came from the long-term 40-week extension that followed the initial 12-week, double-blind stage.
One of these underappreciated findings concerned safety. The rate of treatment-related dermatologic adverse events during the double-blind first 3 months of the study was 2.5% in the ivermectin group compared with 6.3% in vehicle-treated controls.
“That’s pretty impressive. That the active treatment arm of the study had less treatment-related dermatologic adverse events than placebo has never been seen before in any other topical study. It tells you something: The assumption here is that the potent inherent anti-inflammatory activity of ivermectin is overwhelming,” the dermatologist said.
The other particularly noteworthy finding came in the long-term, 40-week extension study that followed the initial 12-week, double-blind stage. What was impressive here was the way the proportion of patients who were IGA clear/almost clear rose steadily throughout, he noted. At the end of the initial 3-month phase of the two studies, 38%-40% of ivermectin-treated patients were clear/almost clear. At 12 months, nearly 70% were.
“In most studies, efficacy sort of plateaus at some point. Here it keeps going up through 12 months. So if somebody comes to your office after 3 months using ivermectin and says, ‘Eh, I’m okay, but I’m not clear or almost clear,’ there’s no reason to switch to another medication, because if they continue you know there’s a high chance they will become clear/almost clear,” Dr. Kircik said.
On the other hand, study participants who were still IGA ‘severe’ after 3 months remained severe after 12 months on the drug. So the message here is if a patient still has severe rosacea after 3 months on ivermectin it’s time to change drugs, he added.
To say that ivermectin should be the treatment of choice because it outperformed metronidazole cream .75% seems a bit overzealous. The reality is, and I believe most of my colleagues agree, metronidazole .75% cream is not very effective. We give it because it is what insurances force us to give, or we gave it because we had nothing else to give. Fortunately this has changed over the years with the advent of azelaic gel and now ivermectin 1% cream.
I do agree, however, that it is rare to see a company structure a superiority head-to-head study, so I will give credit where it is due. However, my guess here is that it was anticipated that ivermectin would at the very least prove noninferior, if not superior, given the poor success rate of this long-standing workhorse. This should not distract from the fact that a) the studies were thorough and well structured and b) held for a good time frame. The data are certainly compelling, so I don’t want that to be overshadowed by the heavy focus on comparing to metronidazole twice a day. To me, that’s a red herring; had they only compared to placebo, we wouldn’t be having this discussion.
The data herein presented are more than supportive of its addition to our limited armamentarium, but to say first line is premature at this early stage. The once-daily dosing and limited adverse events are supportive features as patient compliance is always an issue. Probably more important, and only time will tell, is will insurance companies cover it? Or, will they reject our prescriptions and continue the current trend of recommending medications that bear no similarity to mechanism of action or efficacy. I am suddenly reminded of the all too frequent notice sent, stating that I should give an acne patient benzoyl peroxide, instead of the retinoid I initially selected.
Kudos to Galderma for keeping innovation alive and bringing a new topical drug forward – curious to see if I can actually prescribe it.
Dr. Adam Friedman is associate professor of dermatology and director of translational research in the department of dermatology at George Washington University, Washington, D.C.
To say that ivermectin should be the treatment of choice because it outperformed metronidazole cream .75% seems a bit overzealous. The reality is, and I believe most of my colleagues agree, metronidazole .75% cream is not very effective. We give it because it is what insurances force us to give, or we gave it because we had nothing else to give. Fortunately this has changed over the years with the advent of azelaic gel and now ivermectin 1% cream.
I do agree, however, that it is rare to see a company structure a superiority head-to-head study, so I will give credit where it is due. However, my guess here is that it was anticipated that ivermectin would at the very least prove noninferior, if not superior, given the poor success rate of this long-standing workhorse. This should not distract from the fact that a) the studies were thorough and well structured and b) held for a good time frame. The data are certainly compelling, so I don’t want that to be overshadowed by the heavy focus on comparing to metronidazole twice a day. To me, that’s a red herring; had they only compared to placebo, we wouldn’t be having this discussion.
The data herein presented are more than supportive of its addition to our limited armamentarium, but to say first line is premature at this early stage. The once-daily dosing and limited adverse events are supportive features as patient compliance is always an issue. Probably more important, and only time will tell, is will insurance companies cover it? Or, will they reject our prescriptions and continue the current trend of recommending medications that bear no similarity to mechanism of action or efficacy. I am suddenly reminded of the all too frequent notice sent, stating that I should give an acne patient benzoyl peroxide, instead of the retinoid I initially selected.
Kudos to Galderma for keeping innovation alive and bringing a new topical drug forward – curious to see if I can actually prescribe it.
Dr. Adam Friedman is associate professor of dermatology and director of translational research in the department of dermatology at George Washington University, Washington, D.C.
To say that ivermectin should be the treatment of choice because it outperformed metronidazole cream .75% seems a bit overzealous. The reality is, and I believe most of my colleagues agree, metronidazole .75% cream is not very effective. We give it because it is what insurances force us to give, or we gave it because we had nothing else to give. Fortunately this has changed over the years with the advent of azelaic gel and now ivermectin 1% cream.
I do agree, however, that it is rare to see a company structure a superiority head-to-head study, so I will give credit where it is due. However, my guess here is that it was anticipated that ivermectin would at the very least prove noninferior, if not superior, given the poor success rate of this long-standing workhorse. This should not distract from the fact that a) the studies were thorough and well structured and b) held for a good time frame. The data are certainly compelling, so I don’t want that to be overshadowed by the heavy focus on comparing to metronidazole twice a day. To me, that’s a red herring; had they only compared to placebo, we wouldn’t be having this discussion.
The data herein presented are more than supportive of its addition to our limited armamentarium, but to say first line is premature at this early stage. The once-daily dosing and limited adverse events are supportive features as patient compliance is always an issue. Probably more important, and only time will tell, is will insurance companies cover it? Or, will they reject our prescriptions and continue the current trend of recommending medications that bear no similarity to mechanism of action or efficacy. I am suddenly reminded of the all too frequent notice sent, stating that I should give an acne patient benzoyl peroxide, instead of the retinoid I initially selected.
Kudos to Galderma for keeping innovation alive and bringing a new topical drug forward – curious to see if I can actually prescribe it.
Dr. Adam Friedman is associate professor of dermatology and director of translational research in the department of dermatology at George Washington University, Washington, D.C.
VANCOUVER, B.C. – A persuasive case can be made for ivermectin 1% cream as the new treatment of choice for papulopustular rosacea, Dr. Leon Kircik asserted at the World Congress of Dermatology.
He cited the results of a large recent head-to-head randomized trial in which ivermectin 1% cream (Soolantra), approved last December by the Food and Drug Administration, proved superior to metronidazole 0.75% cream (Metrocream), which is the market leader and until now the topical agent most physicians have considered their first-line treatment for papulopustular rosacea.
The study, known as ATTRACT (Assessment of a Topical Treatment in Rosacea: Activity, Compliance, Tolerability), was a 962-patient, phase III, single-blind, European randomized trial. Ivermectin demonstrated faster onset of action, a greater clinical success rate, delayed time to relapse following treatment discontinuation, a lower relapse rate, and better tolerability (Br. J. Dermatol. 2015;172:1103-10).
Dr. Kircik, a Louisville dermatologist in private practice, and a clinical trialist, was not involved in the ATTRACT study but was impressed that it was undertaken by Galderma, which markets both drugs.
“We don’t get many head-to-head studies in dermatology. Those are typically studies no one wants to sponsor. And most of what few head-to-head studies are done are designed as noninferiority studies. In this case, ATTRACT was designed as a superiority study. That’s a higher bar to reach,” noted Dr. Kircik, a consultant to, and member of the speakers bureau for, Galderma and other pharmaceutical companies.
ATTRACT was a two-part study. In part A, 962 patients with moderate to severe rosacea were randomized to ivermectin 1% cream once daily or metronidazole 0.75% cream b.i.d. for 16 weeks. As early as the first assessment at 3 weeks, a significant difference in favor of ivermectin was evident in terms of reduction in inflammatory lesion count. At week 16, the ivermectin group showed an 83% reduction from baseline in inflammatory lesions, significantly better than the 73.7% reduction with metronidazole. Moreover, 84.9% of the ivermectin group was rated clear or almost clear by Investigator’s Global Assessment (IGA) compared with 75.4% of the metronidazole group.
In addition, 34.9% of ivermectin-treated patients achieved an IGA of 0 by 16 weeks, meaning they were totally lesion free, compared with 21.7% of the metronidazole group. That “completely clear” status is really important psychosocially to a significant proportion of patients, said Dr. Kircik.
“How many times have you had a patient come in asking for an intralesional steroid injection for their single remaining lesion after months of topical treatment?” he asked rhetorically.
Ivermectin was better tolerated than was metronidazole. The rate of treatment-related adverse events leading to discontinuation – typically for skin irritation, redness, or itching – was 0.6% in the ivermectin group compared with 2.1% with metronidazole.
Part B of the ATTRACT study was an unusually designed 36-week extension study.
“In my mind, part B is much more important and much more relevant because rosacea is a chronic disease. It doesn’t last for just 16 weeks. Part B asks what happens after we stop therapy. This is the rare clinical trial that actually mimics real life,” Dr. Kircik said.
Despite physicians’ standard advice to continue with maintenance therapy after clearing, the reality is that most patients stop treatment once they clear, figuring they’ll resume if they relapse, he asserted. Part B of ATTRACT reflected that approach. Seven hundred fifty-seven patients who were clear/almost clear as defined by an IGA of 0/1 at the end of 16 weeks enrolled in the 36-week extension study. They surrendered their medication and returned to their physician monthly. If they had relapsed during that month, meaning they showed up with an IGA of 2 or more, they got their medication back; if they were still IGA 0/1, they did not.
The median time to relapse off treatment in the ivermectin group was 115 days, a full month later than the 85 days for the metronidazole group. Also, the relapse rate in the ivermectin group was significantly lower: 62.7% compared with 68.4% in the metronidazole group.
“The impact on both clinical practice and pharmacoeconomic practice is huge here. It makes sense to switch from metronidazole to ivermectin as first-line therapy because you know that your patients will do better and will relapse later and less,” Dr. Kircik declared.
He was a coinvestigator in the twin pivotal phase III randomized Galderma-funded trials that led to FDA approval of ivermectin for papulopustular rosacea. Although those studies have been published (J. Drugs Dermatol. 2014;13:1380-6), Dr. Kircik highlighted a couple of findings he said haven’t drawn the attention they deserve. Both came from the long-term 40-week extension that followed the initial 12-week, double-blind stage.
One of these underappreciated findings concerned safety. The rate of treatment-related dermatologic adverse events during the double-blind first 3 months of the study was 2.5% in the ivermectin group compared with 6.3% in vehicle-treated controls.
“That’s pretty impressive. That the active treatment arm of the study had less treatment-related dermatologic adverse events than placebo has never been seen before in any other topical study. It tells you something: The assumption here is that the potent inherent anti-inflammatory activity of ivermectin is overwhelming,” the dermatologist said.
The other particularly noteworthy finding came in the long-term, 40-week extension study that followed the initial 12-week, double-blind stage. What was impressive here was the way the proportion of patients who were IGA clear/almost clear rose steadily throughout, he noted. At the end of the initial 3-month phase of the two studies, 38%-40% of ivermectin-treated patients were clear/almost clear. At 12 months, nearly 70% were.
“In most studies, efficacy sort of plateaus at some point. Here it keeps going up through 12 months. So if somebody comes to your office after 3 months using ivermectin and says, ‘Eh, I’m okay, but I’m not clear or almost clear,’ there’s no reason to switch to another medication, because if they continue you know there’s a high chance they will become clear/almost clear,” Dr. Kircik said.
On the other hand, study participants who were still IGA ‘severe’ after 3 months remained severe after 12 months on the drug. So the message here is if a patient still has severe rosacea after 3 months on ivermectin it’s time to change drugs, he added.
VANCOUVER, B.C. – A persuasive case can be made for ivermectin 1% cream as the new treatment of choice for papulopustular rosacea, Dr. Leon Kircik asserted at the World Congress of Dermatology.
He cited the results of a large recent head-to-head randomized trial in which ivermectin 1% cream (Soolantra), approved last December by the Food and Drug Administration, proved superior to metronidazole 0.75% cream (Metrocream), which is the market leader and until now the topical agent most physicians have considered their first-line treatment for papulopustular rosacea.
The study, known as ATTRACT (Assessment of a Topical Treatment in Rosacea: Activity, Compliance, Tolerability), was a 962-patient, phase III, single-blind, European randomized trial. Ivermectin demonstrated faster onset of action, a greater clinical success rate, delayed time to relapse following treatment discontinuation, a lower relapse rate, and better tolerability (Br. J. Dermatol. 2015;172:1103-10).
Dr. Kircik, a Louisville dermatologist in private practice, and a clinical trialist, was not involved in the ATTRACT study but was impressed that it was undertaken by Galderma, which markets both drugs.
“We don’t get many head-to-head studies in dermatology. Those are typically studies no one wants to sponsor. And most of what few head-to-head studies are done are designed as noninferiority studies. In this case, ATTRACT was designed as a superiority study. That’s a higher bar to reach,” noted Dr. Kircik, a consultant to, and member of the speakers bureau for, Galderma and other pharmaceutical companies.
ATTRACT was a two-part study. In part A, 962 patients with moderate to severe rosacea were randomized to ivermectin 1% cream once daily or metronidazole 0.75% cream b.i.d. for 16 weeks. As early as the first assessment at 3 weeks, a significant difference in favor of ivermectin was evident in terms of reduction in inflammatory lesion count. At week 16, the ivermectin group showed an 83% reduction from baseline in inflammatory lesions, significantly better than the 73.7% reduction with metronidazole. Moreover, 84.9% of the ivermectin group was rated clear or almost clear by Investigator’s Global Assessment (IGA) compared with 75.4% of the metronidazole group.
In addition, 34.9% of ivermectin-treated patients achieved an IGA of 0 by 16 weeks, meaning they were totally lesion free, compared with 21.7% of the metronidazole group. That “completely clear” status is really important psychosocially to a significant proportion of patients, said Dr. Kircik.
“How many times have you had a patient come in asking for an intralesional steroid injection for their single remaining lesion after months of topical treatment?” he asked rhetorically.
Ivermectin was better tolerated than was metronidazole. The rate of treatment-related adverse events leading to discontinuation – typically for skin irritation, redness, or itching – was 0.6% in the ivermectin group compared with 2.1% with metronidazole.
Part B of the ATTRACT study was an unusually designed 36-week extension study.
“In my mind, part B is much more important and much more relevant because rosacea is a chronic disease. It doesn’t last for just 16 weeks. Part B asks what happens after we stop therapy. This is the rare clinical trial that actually mimics real life,” Dr. Kircik said.
Despite physicians’ standard advice to continue with maintenance therapy after clearing, the reality is that most patients stop treatment once they clear, figuring they’ll resume if they relapse, he asserted. Part B of ATTRACT reflected that approach. Seven hundred fifty-seven patients who were clear/almost clear as defined by an IGA of 0/1 at the end of 16 weeks enrolled in the 36-week extension study. They surrendered their medication and returned to their physician monthly. If they had relapsed during that month, meaning they showed up with an IGA of 2 or more, they got their medication back; if they were still IGA 0/1, they did not.
The median time to relapse off treatment in the ivermectin group was 115 days, a full month later than the 85 days for the metronidazole group. Also, the relapse rate in the ivermectin group was significantly lower: 62.7% compared with 68.4% in the metronidazole group.
“The impact on both clinical practice and pharmacoeconomic practice is huge here. It makes sense to switch from metronidazole to ivermectin as first-line therapy because you know that your patients will do better and will relapse later and less,” Dr. Kircik declared.
He was a coinvestigator in the twin pivotal phase III randomized Galderma-funded trials that led to FDA approval of ivermectin for papulopustular rosacea. Although those studies have been published (J. Drugs Dermatol. 2014;13:1380-6), Dr. Kircik highlighted a couple of findings he said haven’t drawn the attention they deserve. Both came from the long-term 40-week extension that followed the initial 12-week, double-blind stage.
One of these underappreciated findings concerned safety. The rate of treatment-related dermatologic adverse events during the double-blind first 3 months of the study was 2.5% in the ivermectin group compared with 6.3% in vehicle-treated controls.
“That’s pretty impressive. That the active treatment arm of the study had less treatment-related dermatologic adverse events than placebo has never been seen before in any other topical study. It tells you something: The assumption here is that the potent inherent anti-inflammatory activity of ivermectin is overwhelming,” the dermatologist said.
The other particularly noteworthy finding came in the long-term, 40-week extension study that followed the initial 12-week, double-blind stage. What was impressive here was the way the proportion of patients who were IGA clear/almost clear rose steadily throughout, he noted. At the end of the initial 3-month phase of the two studies, 38%-40% of ivermectin-treated patients were clear/almost clear. At 12 months, nearly 70% were.
“In most studies, efficacy sort of plateaus at some point. Here it keeps going up through 12 months. So if somebody comes to your office after 3 months using ivermectin and says, ‘Eh, I’m okay, but I’m not clear or almost clear,’ there’s no reason to switch to another medication, because if they continue you know there’s a high chance they will become clear/almost clear,” Dr. Kircik said.
On the other hand, study participants who were still IGA ‘severe’ after 3 months remained severe after 12 months on the drug. So the message here is if a patient still has severe rosacea after 3 months on ivermectin it’s time to change drugs, he added.
EXPERT ANALYSIS FROM WCD 2015
Erectile dysfunction: New frontier in interventional cardiology
PARIS – Balloon angioplasty for isolated penile artery stenoses improved erectile dysfunction scores, and the results endured at 12-month follow-ups for 55% of treated men, Dr. Tzung-Dau Wang reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A 41% restenosis rate was noted, however, on CT angiography of the penile arteries, which was performed routinely at 6-9 months post PCI as part of the PERFECT-2 study. Even among the patients with clinically meaningful improvements in their erectile dysfunction scores, one-third had angiographic restenosis, which doesn’t bode well for long-term outcomes.
“Our findings highlight an unmet need for a more enduring treatment modality for penile artery stenotic disease,” said Dr. Wang of National Taiwan University in Taipei. As most penile arteries are less than 2 mm in diameter, they are too small for interventionalists to take on with anything in their toolbox except for plain old balloon angioplasty (POBA).
Last year, Dr. Wang presented the results of the PERFECT-1 study (EuroIntervention 2014;10:147-56), his first-in-man study of POBA for isolated penile artery stenoses in 20 men with erectile dysfunction. At 6 months’ follow-up, 11 (55%) had at least a 4-point improvement from baseline in the International Index for Erectile Dysfunction-5 (IIED-5) score or normalization of erectile function, defined as an IIED-5 score of at least 22.
At EuroPCR 2015, Dr. Wang presented PERFECT-2, a confirmatory study with 12-month follow-up rather than the 6 months in PERFECT-1, and with mandatory CT angiography to learn what happened to the treated vessels.
The study began with 28 treated patients, but 2 of them were excluded because they declined follow-up CT angiography and 4 others had inadequate-quality imaging, leaving a final population of 22 patients with 34 treated penile artery lesions. Their baseline IIED-5 scores averaged 10.1. Fifteen patients had known CAD. Three-quarters of lesions were located in the common penile artery, the rest in the dorsal penile or cavernosal artery. The average target-vessel diameter was 1.7 mm, with a mean lesion length of 11.1 mm. None of the lesions were calcified.
Twenty of the 22 patients were treated with a 1.5-mm balloon. The degree of stenosis was 77% before treatment and 9.5% after treatment. Flow-limiting dissection occurred in two patients, with the complication being successfully managed by prolonged balloon dilation in both cases. No penile hematomas or any other complications occurred.
Binary restenosis, as defined by at least a 50% diameter stenosis upon CT angiography at 6-9 months, occurred in 14 of 34 treated lesions, or 41%. Many patients, however, had more than one treated lesion, and the per-patient restenosis rate was 59%, or 13 of 22 patients. The mean lesion length at follow-up was 4.3 mm.
Nine of the 10 patients without significant clinical improvement as reflected by their change in IIED-5 scores had angiographic binary restenosis. So did 4 of the 12 patients who did experience meaningful improvement in their erectile dysfunction.
Session cochair Dr. Flavio Ribichini, head of interventional cardiology at the University of Verona (Italy), noted that, back in the 1980s, there was considerable interest in balloon angioplasty as a treatment for erectile dysfunction, but the practice was abandoned mainly due to an unacceptably high restenosis rate. He asked why Dr. Wang thought he was getting better results now with POBA.
Much improved technology, Dr. Wang replied.
He reported having no financial conflicts with regard to this study, which was sponsored by National Taiwan University Hospital.
PARIS – Balloon angioplasty for isolated penile artery stenoses improved erectile dysfunction scores, and the results endured at 12-month follow-ups for 55% of treated men, Dr. Tzung-Dau Wang reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A 41% restenosis rate was noted, however, on CT angiography of the penile arteries, which was performed routinely at 6-9 months post PCI as part of the PERFECT-2 study. Even among the patients with clinically meaningful improvements in their erectile dysfunction scores, one-third had angiographic restenosis, which doesn’t bode well for long-term outcomes.
“Our findings highlight an unmet need for a more enduring treatment modality for penile artery stenotic disease,” said Dr. Wang of National Taiwan University in Taipei. As most penile arteries are less than 2 mm in diameter, they are too small for interventionalists to take on with anything in their toolbox except for plain old balloon angioplasty (POBA).
Last year, Dr. Wang presented the results of the PERFECT-1 study (EuroIntervention 2014;10:147-56), his first-in-man study of POBA for isolated penile artery stenoses in 20 men with erectile dysfunction. At 6 months’ follow-up, 11 (55%) had at least a 4-point improvement from baseline in the International Index for Erectile Dysfunction-5 (IIED-5) score or normalization of erectile function, defined as an IIED-5 score of at least 22.
At EuroPCR 2015, Dr. Wang presented PERFECT-2, a confirmatory study with 12-month follow-up rather than the 6 months in PERFECT-1, and with mandatory CT angiography to learn what happened to the treated vessels.
The study began with 28 treated patients, but 2 of them were excluded because they declined follow-up CT angiography and 4 others had inadequate-quality imaging, leaving a final population of 22 patients with 34 treated penile artery lesions. Their baseline IIED-5 scores averaged 10.1. Fifteen patients had known CAD. Three-quarters of lesions were located in the common penile artery, the rest in the dorsal penile or cavernosal artery. The average target-vessel diameter was 1.7 mm, with a mean lesion length of 11.1 mm. None of the lesions were calcified.
Twenty of the 22 patients were treated with a 1.5-mm balloon. The degree of stenosis was 77% before treatment and 9.5% after treatment. Flow-limiting dissection occurred in two patients, with the complication being successfully managed by prolonged balloon dilation in both cases. No penile hematomas or any other complications occurred.
Binary restenosis, as defined by at least a 50% diameter stenosis upon CT angiography at 6-9 months, occurred in 14 of 34 treated lesions, or 41%. Many patients, however, had more than one treated lesion, and the per-patient restenosis rate was 59%, or 13 of 22 patients. The mean lesion length at follow-up was 4.3 mm.
Nine of the 10 patients without significant clinical improvement as reflected by their change in IIED-5 scores had angiographic binary restenosis. So did 4 of the 12 patients who did experience meaningful improvement in their erectile dysfunction.
Session cochair Dr. Flavio Ribichini, head of interventional cardiology at the University of Verona (Italy), noted that, back in the 1980s, there was considerable interest in balloon angioplasty as a treatment for erectile dysfunction, but the practice was abandoned mainly due to an unacceptably high restenosis rate. He asked why Dr. Wang thought he was getting better results now with POBA.
Much improved technology, Dr. Wang replied.
He reported having no financial conflicts with regard to this study, which was sponsored by National Taiwan University Hospital.
PARIS – Balloon angioplasty for isolated penile artery stenoses improved erectile dysfunction scores, and the results endured at 12-month follow-ups for 55% of treated men, Dr. Tzung-Dau Wang reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A 41% restenosis rate was noted, however, on CT angiography of the penile arteries, which was performed routinely at 6-9 months post PCI as part of the PERFECT-2 study. Even among the patients with clinically meaningful improvements in their erectile dysfunction scores, one-third had angiographic restenosis, which doesn’t bode well for long-term outcomes.
“Our findings highlight an unmet need for a more enduring treatment modality for penile artery stenotic disease,” said Dr. Wang of National Taiwan University in Taipei. As most penile arteries are less than 2 mm in diameter, they are too small for interventionalists to take on with anything in their toolbox except for plain old balloon angioplasty (POBA).
Last year, Dr. Wang presented the results of the PERFECT-1 study (EuroIntervention 2014;10:147-56), his first-in-man study of POBA for isolated penile artery stenoses in 20 men with erectile dysfunction. At 6 months’ follow-up, 11 (55%) had at least a 4-point improvement from baseline in the International Index for Erectile Dysfunction-5 (IIED-5) score or normalization of erectile function, defined as an IIED-5 score of at least 22.
At EuroPCR 2015, Dr. Wang presented PERFECT-2, a confirmatory study with 12-month follow-up rather than the 6 months in PERFECT-1, and with mandatory CT angiography to learn what happened to the treated vessels.
The study began with 28 treated patients, but 2 of them were excluded because they declined follow-up CT angiography and 4 others had inadequate-quality imaging, leaving a final population of 22 patients with 34 treated penile artery lesions. Their baseline IIED-5 scores averaged 10.1. Fifteen patients had known CAD. Three-quarters of lesions were located in the common penile artery, the rest in the dorsal penile or cavernosal artery. The average target-vessel diameter was 1.7 mm, with a mean lesion length of 11.1 mm. None of the lesions were calcified.
Twenty of the 22 patients were treated with a 1.5-mm balloon. The degree of stenosis was 77% before treatment and 9.5% after treatment. Flow-limiting dissection occurred in two patients, with the complication being successfully managed by prolonged balloon dilation in both cases. No penile hematomas or any other complications occurred.
Binary restenosis, as defined by at least a 50% diameter stenosis upon CT angiography at 6-9 months, occurred in 14 of 34 treated lesions, or 41%. Many patients, however, had more than one treated lesion, and the per-patient restenosis rate was 59%, or 13 of 22 patients. The mean lesion length at follow-up was 4.3 mm.
Nine of the 10 patients without significant clinical improvement as reflected by their change in IIED-5 scores had angiographic binary restenosis. So did 4 of the 12 patients who did experience meaningful improvement in their erectile dysfunction.
Session cochair Dr. Flavio Ribichini, head of interventional cardiology at the University of Verona (Italy), noted that, back in the 1980s, there was considerable interest in balloon angioplasty as a treatment for erectile dysfunction, but the practice was abandoned mainly due to an unacceptably high restenosis rate. He asked why Dr. Wang thought he was getting better results now with POBA.
Much improved technology, Dr. Wang replied.
He reported having no financial conflicts with regard to this study, which was sponsored by National Taiwan University Hospital.
AT EUROPCR 2015
Key clinical point: Balloon angioplasty of penile stenoses was an effective treatment for erectile dysfunction in the majority of treated patients.
Major finding: Twelve of 22 men with erectile dysfunction who underwent balloon angioplasty for penile artery stenoses had clinically meaningful improvement in erectile function at 12 months of follow-up.
Data source: Prospective study of 22 patients with erectile dysfunction.
Disclosures: The study was conducted without commercial support and the presenter reported having no financial conflicts.
New tools aid decisions on length of dual-antiplatelet therapy
PARIS – A novel method of quantifying the risks of major bleeding and stent thrombosis may guide decisions about the duration of dual-antiplatelet therapy in stent recipients, according to Dr. Francesco Costa.
It’s a two-pronged approach that relies upon a CRUSADE bleeding risk score greater than 40 as a red flag cautioning against 24 months of dual-antiplatelet therapy (DAPT) in favor of 6 months, while also taking into consideration the anatomic location of an individual’s coronary artery disease as a guide to ischemic risk, such as stent thrombosis, Dr. Costa said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A patient with at least 30% luminal narrowing of the left main coronary artery and/or the proximal LAD (left anterior descending) artery is at markedly reduced risk of stent thrombosis with a DAPT regimen of 24 months rather than 6 months, according to Dr. Costa of Erasmus University in Rotterdam, the Netherlands. These findings were borne out in a retrospective analysis of data from the previously published PRODIGY trial, in which 2,013 patients undergoing percutaneous coronary intervention were randomized to receive a first- or second-generation drug-eluting stent or a bare metal stent, and then further randomized to 6 or 24 months of DAPT (Circulation 2012;125:2015-26).
As these findings about how to guide DAPT duration come from an exploratory retrospective analysis, Dr. Costa stressed, they must be considered hypothesis generating. A definitive prospective randomized trial is warranted to confirm the hypothesis. Such a trial is sorely needed, the cardiologist added.
“International guidelines suggest tailoring DAPT duration according to a patient’s ischemic and bleeding risks. However, currently a reproducible method of weighing these risks has not yet been proposed,” he said. “I think if we put 10 different [physicians] in front of a patient and asked them to define that patient’s bleeding risk, almost everyone would have a different idea.”
The PRODIGY-tested approach, while not ideal, is a definite step forward, according to Dr. Costa.
He and his coworkers evaluated three different bleeding risk scoring systems – HAS-BLED, ACUITY, and CRUSADE – before concluding that a CRUSADE score greater than 40 was superior as a predictor of major bleeding in the PRODIGY population.
Roughly 16% of participants in this all-comers study had a CRUSADE score above 40. A 24-month course of DAPT in this group was associated with a 2.7-fold increased risk of major bleeding events, compared with a 6-month course. The number-needed-to-harm with a 24-month course of DAPT was 17, compared with a number-needed-to-harm of 67 in an unselected population. In contrast, there was no significant increase in major bleeding risk with 24 months of DAPT in patients with a CRUSADE score of 40 or less.
Patients with a CRUSADE score greater than 40 also had a sharply increased need for RBC transfusion if they were on 24 months of DAPT.
The investigators chose 30% luminal narrowing of the left main or proximal LAD coronary arteries as their cutpoint for increased risk of ischemic events during follow-up because they consider it a good marker for more diffuse atherosclerotic disease.
PRODIGY participants with luminal narrowing at either location were 55% less likely to experience stent thrombosis with 24 months of DAPT than with 6.
Dr. Andreas Baumbach said the DAPT decision-making aid presented by Dr. Costa is just what interventional cardiologists have been looking for.
“We’re always talking about patients at high bleeding risk and high ischemic risk, but we haven’t really had a tool to identify those other than our clinical judgment, thinking that high bleeding risk comes with age and renal impairment. So to have a score that’s almost validated for this purpose is really important,” according to Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
This analysis was conducted without external funding. Dr. Costa reported having no relevant financial conflicts.
PARIS – A novel method of quantifying the risks of major bleeding and stent thrombosis may guide decisions about the duration of dual-antiplatelet therapy in stent recipients, according to Dr. Francesco Costa.
It’s a two-pronged approach that relies upon a CRUSADE bleeding risk score greater than 40 as a red flag cautioning against 24 months of dual-antiplatelet therapy (DAPT) in favor of 6 months, while also taking into consideration the anatomic location of an individual’s coronary artery disease as a guide to ischemic risk, such as stent thrombosis, Dr. Costa said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A patient with at least 30% luminal narrowing of the left main coronary artery and/or the proximal LAD (left anterior descending) artery is at markedly reduced risk of stent thrombosis with a DAPT regimen of 24 months rather than 6 months, according to Dr. Costa of Erasmus University in Rotterdam, the Netherlands. These findings were borne out in a retrospective analysis of data from the previously published PRODIGY trial, in which 2,013 patients undergoing percutaneous coronary intervention were randomized to receive a first- or second-generation drug-eluting stent or a bare metal stent, and then further randomized to 6 or 24 months of DAPT (Circulation 2012;125:2015-26).
As these findings about how to guide DAPT duration come from an exploratory retrospective analysis, Dr. Costa stressed, they must be considered hypothesis generating. A definitive prospective randomized trial is warranted to confirm the hypothesis. Such a trial is sorely needed, the cardiologist added.
“International guidelines suggest tailoring DAPT duration according to a patient’s ischemic and bleeding risks. However, currently a reproducible method of weighing these risks has not yet been proposed,” he said. “I think if we put 10 different [physicians] in front of a patient and asked them to define that patient’s bleeding risk, almost everyone would have a different idea.”
The PRODIGY-tested approach, while not ideal, is a definite step forward, according to Dr. Costa.
He and his coworkers evaluated three different bleeding risk scoring systems – HAS-BLED, ACUITY, and CRUSADE – before concluding that a CRUSADE score greater than 40 was superior as a predictor of major bleeding in the PRODIGY population.
Roughly 16% of participants in this all-comers study had a CRUSADE score above 40. A 24-month course of DAPT in this group was associated with a 2.7-fold increased risk of major bleeding events, compared with a 6-month course. The number-needed-to-harm with a 24-month course of DAPT was 17, compared with a number-needed-to-harm of 67 in an unselected population. In contrast, there was no significant increase in major bleeding risk with 24 months of DAPT in patients with a CRUSADE score of 40 or less.
Patients with a CRUSADE score greater than 40 also had a sharply increased need for RBC transfusion if they were on 24 months of DAPT.
The investigators chose 30% luminal narrowing of the left main or proximal LAD coronary arteries as their cutpoint for increased risk of ischemic events during follow-up because they consider it a good marker for more diffuse atherosclerotic disease.
PRODIGY participants with luminal narrowing at either location were 55% less likely to experience stent thrombosis with 24 months of DAPT than with 6.
Dr. Andreas Baumbach said the DAPT decision-making aid presented by Dr. Costa is just what interventional cardiologists have been looking for.
“We’re always talking about patients at high bleeding risk and high ischemic risk, but we haven’t really had a tool to identify those other than our clinical judgment, thinking that high bleeding risk comes with age and renal impairment. So to have a score that’s almost validated for this purpose is really important,” according to Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
This analysis was conducted without external funding. Dr. Costa reported having no relevant financial conflicts.
PARIS – A novel method of quantifying the risks of major bleeding and stent thrombosis may guide decisions about the duration of dual-antiplatelet therapy in stent recipients, according to Dr. Francesco Costa.
It’s a two-pronged approach that relies upon a CRUSADE bleeding risk score greater than 40 as a red flag cautioning against 24 months of dual-antiplatelet therapy (DAPT) in favor of 6 months, while also taking into consideration the anatomic location of an individual’s coronary artery disease as a guide to ischemic risk, such as stent thrombosis, Dr. Costa said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
A patient with at least 30% luminal narrowing of the left main coronary artery and/or the proximal LAD (left anterior descending) artery is at markedly reduced risk of stent thrombosis with a DAPT regimen of 24 months rather than 6 months, according to Dr. Costa of Erasmus University in Rotterdam, the Netherlands. These findings were borne out in a retrospective analysis of data from the previously published PRODIGY trial, in which 2,013 patients undergoing percutaneous coronary intervention were randomized to receive a first- or second-generation drug-eluting stent or a bare metal stent, and then further randomized to 6 or 24 months of DAPT (Circulation 2012;125:2015-26).
As these findings about how to guide DAPT duration come from an exploratory retrospective analysis, Dr. Costa stressed, they must be considered hypothesis generating. A definitive prospective randomized trial is warranted to confirm the hypothesis. Such a trial is sorely needed, the cardiologist added.
“International guidelines suggest tailoring DAPT duration according to a patient’s ischemic and bleeding risks. However, currently a reproducible method of weighing these risks has not yet been proposed,” he said. “I think if we put 10 different [physicians] in front of a patient and asked them to define that patient’s bleeding risk, almost everyone would have a different idea.”
The PRODIGY-tested approach, while not ideal, is a definite step forward, according to Dr. Costa.
He and his coworkers evaluated three different bleeding risk scoring systems – HAS-BLED, ACUITY, and CRUSADE – before concluding that a CRUSADE score greater than 40 was superior as a predictor of major bleeding in the PRODIGY population.
Roughly 16% of participants in this all-comers study had a CRUSADE score above 40. A 24-month course of DAPT in this group was associated with a 2.7-fold increased risk of major bleeding events, compared with a 6-month course. The number-needed-to-harm with a 24-month course of DAPT was 17, compared with a number-needed-to-harm of 67 in an unselected population. In contrast, there was no significant increase in major bleeding risk with 24 months of DAPT in patients with a CRUSADE score of 40 or less.
Patients with a CRUSADE score greater than 40 also had a sharply increased need for RBC transfusion if they were on 24 months of DAPT.
The investigators chose 30% luminal narrowing of the left main or proximal LAD coronary arteries as their cutpoint for increased risk of ischemic events during follow-up because they consider it a good marker for more diffuse atherosclerotic disease.
PRODIGY participants with luminal narrowing at either location were 55% less likely to experience stent thrombosis with 24 months of DAPT than with 6.
Dr. Andreas Baumbach said the DAPT decision-making aid presented by Dr. Costa is just what interventional cardiologists have been looking for.
“We’re always talking about patients at high bleeding risk and high ischemic risk, but we haven’t really had a tool to identify those other than our clinical judgment, thinking that high bleeding risk comes with age and renal impairment. So to have a score that’s almost validated for this purpose is really important,” according to Dr. Baumbach, professor of interventional cardiology at the University of Bristol (England).
This analysis was conducted without external funding. Dr. Costa reported having no relevant financial conflicts.
AT EuroPCR 2015
Key clinical point: Stent location and CRUSADE score can inform decisions about the duration of dual-antiplatelet therapy.
Major finding: Coronary stent recipients with a CRUSADE bleeding risk score above 40 had a 2.7-fold greater risk of a major bleeding event if randomized to 24 months rather than 6 months of dual-antiplatelet therapy.
Data source: A retrospective, hypothesis-generating secondary analysis of the 2,103-patient prospective randomized PRODIGY study.
Disclosures: This analysis was conducted without external funding. The presenter reported having no relevant financial conflicts.
IHC: Lifestyle regularity helps quell migraine
VALENCIA, SPAIN – Following a schedule of regular daily mealtimes, bedtimes, and aerobic exercise is an effective yet underutilized tool for the management of migraine, Dr. Yohannes W. Woldeamanuel said at the International Headache Congress.
“We call these three ‘the RLB [regular lifestyle behavior] triumvirate’: daily regular sleep times, mealtime hours, and aerobic exercise. This is something physicians should be advocating ... it provides the patient with a tool to help control his or her own migraine problems. Along with avoidance of daily headache medications, it has added value for clinical practice,” according to Dr. Woldeamanuel, an Ethiopian neurologist who is currently a fellow in the Stanford (Calif.) University Headache and Facial Pain Program.
He presented a retrospective study of 100 patients with chronic migraine aged 15 and older and 100 age- and gender-matched patients with episodic migraine treated at the headache center. Collectively, the chronic migraine patients averaged 2,497 migraine attacks per month, while the episodic migraine group averaged 578.
Although all migraine patients seen at the Stanford center are encouraged to pursue RLB, only 6% of the chronic migraine group did so regularly, compared with a 10-fold higher rate among those with episodic migraine. Slicing the data another way, only 9% of patients who were on the RLB bandwagon had chronic migraine, compared with 70% of those without regular lifestyle behavior.
An observational study such as this cannot establish causality. Yet Dr. Woldeamanuel saw evidence suggestive of cause and effect with regard to RLB.
“Interestingly, among the small cohort of chronic migraineurs who were following the RLB, they were progressively month after month converting into episodic migraineurs, while the episodic migraineurs who were not following the RLB were converting to chronic migraine, month after month,” the neurologist said in an interview at the meeting sponsored by the International Headache Society and the American Headache Society.
By definition, patients with chronic migraine take headache medications most days of the month, raising the possibility that medication use might be a study confounder. To check that out, Dr. Woldeamanuel stratified patients who adhered to the RLB triumvirate into those who regularly used rescue and/or preventive medications and those who didn’t. The two subgroups didn’t differ significantly in terms of the prevalence of chronic migraine.
“This supports the genuine impact of RLB as a migraine management modality. It’s therapeutic. That’s the message,” he declared.
What’s the mechanism of benefit?
“We have biological rhythms that maintain our health: ultradian, circadian, diurnal, infradian. RLB promotes release of endorphins and keeps levels stable, increasing the threshold for sensory change and peripheral pain,” he explained.
Dr. Woldeamanuel reported having no financial conflicts with regard to this study, conducted with institutional funds.
VALENCIA, SPAIN – Following a schedule of regular daily mealtimes, bedtimes, and aerobic exercise is an effective yet underutilized tool for the management of migraine, Dr. Yohannes W. Woldeamanuel said at the International Headache Congress.
“We call these three ‘the RLB [regular lifestyle behavior] triumvirate’: daily regular sleep times, mealtime hours, and aerobic exercise. This is something physicians should be advocating ... it provides the patient with a tool to help control his or her own migraine problems. Along with avoidance of daily headache medications, it has added value for clinical practice,” according to Dr. Woldeamanuel, an Ethiopian neurologist who is currently a fellow in the Stanford (Calif.) University Headache and Facial Pain Program.
He presented a retrospective study of 100 patients with chronic migraine aged 15 and older and 100 age- and gender-matched patients with episodic migraine treated at the headache center. Collectively, the chronic migraine patients averaged 2,497 migraine attacks per month, while the episodic migraine group averaged 578.
Although all migraine patients seen at the Stanford center are encouraged to pursue RLB, only 6% of the chronic migraine group did so regularly, compared with a 10-fold higher rate among those with episodic migraine. Slicing the data another way, only 9% of patients who were on the RLB bandwagon had chronic migraine, compared with 70% of those without regular lifestyle behavior.
An observational study such as this cannot establish causality. Yet Dr. Woldeamanuel saw evidence suggestive of cause and effect with regard to RLB.
“Interestingly, among the small cohort of chronic migraineurs who were following the RLB, they were progressively month after month converting into episodic migraineurs, while the episodic migraineurs who were not following the RLB were converting to chronic migraine, month after month,” the neurologist said in an interview at the meeting sponsored by the International Headache Society and the American Headache Society.
By definition, patients with chronic migraine take headache medications most days of the month, raising the possibility that medication use might be a study confounder. To check that out, Dr. Woldeamanuel stratified patients who adhered to the RLB triumvirate into those who regularly used rescue and/or preventive medications and those who didn’t. The two subgroups didn’t differ significantly in terms of the prevalence of chronic migraine.
“This supports the genuine impact of RLB as a migraine management modality. It’s therapeutic. That’s the message,” he declared.
What’s the mechanism of benefit?
“We have biological rhythms that maintain our health: ultradian, circadian, diurnal, infradian. RLB promotes release of endorphins and keeps levels stable, increasing the threshold for sensory change and peripheral pain,” he explained.
Dr. Woldeamanuel reported having no financial conflicts with regard to this study, conducted with institutional funds.
VALENCIA, SPAIN – Following a schedule of regular daily mealtimes, bedtimes, and aerobic exercise is an effective yet underutilized tool for the management of migraine, Dr. Yohannes W. Woldeamanuel said at the International Headache Congress.
“We call these three ‘the RLB [regular lifestyle behavior] triumvirate’: daily regular sleep times, mealtime hours, and aerobic exercise. This is something physicians should be advocating ... it provides the patient with a tool to help control his or her own migraine problems. Along with avoidance of daily headache medications, it has added value for clinical practice,” according to Dr. Woldeamanuel, an Ethiopian neurologist who is currently a fellow in the Stanford (Calif.) University Headache and Facial Pain Program.
He presented a retrospective study of 100 patients with chronic migraine aged 15 and older and 100 age- and gender-matched patients with episodic migraine treated at the headache center. Collectively, the chronic migraine patients averaged 2,497 migraine attacks per month, while the episodic migraine group averaged 578.
Although all migraine patients seen at the Stanford center are encouraged to pursue RLB, only 6% of the chronic migraine group did so regularly, compared with a 10-fold higher rate among those with episodic migraine. Slicing the data another way, only 9% of patients who were on the RLB bandwagon had chronic migraine, compared with 70% of those without regular lifestyle behavior.
An observational study such as this cannot establish causality. Yet Dr. Woldeamanuel saw evidence suggestive of cause and effect with regard to RLB.
“Interestingly, among the small cohort of chronic migraineurs who were following the RLB, they were progressively month after month converting into episodic migraineurs, while the episodic migraineurs who were not following the RLB were converting to chronic migraine, month after month,” the neurologist said in an interview at the meeting sponsored by the International Headache Society and the American Headache Society.
By definition, patients with chronic migraine take headache medications most days of the month, raising the possibility that medication use might be a study confounder. To check that out, Dr. Woldeamanuel stratified patients who adhered to the RLB triumvirate into those who regularly used rescue and/or preventive medications and those who didn’t. The two subgroups didn’t differ significantly in terms of the prevalence of chronic migraine.
“This supports the genuine impact of RLB as a migraine management modality. It’s therapeutic. That’s the message,” he declared.
What’s the mechanism of benefit?
“We have biological rhythms that maintain our health: ultradian, circadian, diurnal, infradian. RLB promotes release of endorphins and keeps levels stable, increasing the threshold for sensory change and peripheral pain,” he explained.
Dr. Woldeamanuel reported having no financial conflicts with regard to this study, conducted with institutional funds.
AT IHC 2015
Key clinical point: Regular lifestyle behavior in terms of mealtimes, bedtimes, and daily aerobic exercise is a self-management tool with a therapeutic effect in migraine patients.
Major finding: Migraine patients who follow regular lifestyle behavior with regard to mealtimes, bedtimes, and daily aerobic exercise are 10-fold more likely to have episodic than chronic migraine.
Data source: This was a retrospective study of 100 chronic migraineurs and 100 patients with episodic migraine.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted with institutional funds.
European cardiologists seek involvement in acute stroke
PARIS – The leaders of European interventional cardiology have thrown down the gauntlet to their colleagues, declaring during a special call-to-action session at EuroPCR that a revolution is underway in the treatment of acute stroke, and interventional cardiologists need to train up and become part of it.
“Something big is going on today. If we want to be transformative and impactful, I think stroke intervention is one of the main points where we can do so as interventional cardiologists,” said Dr. Alberto Cremonesi of Villa Maria Cecilia Hospital in Cotignola, Italy, a past president of the Italian Society of Interventional Cardiology.
Dr. Petr Widimsky highlighted the five prospective, randomized, controlled trials that have come out in the past few months and triggered the revolution in acute stroke therapy. All five studies – MR CLEAN, ESCAPE, EXTENT IA, SWIFT PRIME, and REVASCAT – were halted early because of the significant advantage mechanical endovascular therapy with stents or thrombus retrieval devices demonstrated over standard therapy featuring clot thrombolysis with tissue plasminogen activator.
Collectively, the five trials showed a 60% greater chance for good functional recovery from stroke with endovascular interventions. The rate of a favorable neurologic outcome as reflected in a modified Rankin score of 0-2 was 48% with the use of stent/retriever devices, compared with 30% with thrombolysis alone, noted Dr. Widimsky, professor and chair of the cardiology department at Charles University in Prague.
The Food and Drug Administration began approving these endovascular therapy devices in 2012. The major challenge is how to make this therapy available to the vast numbers of patients in need. After all, the successful clinical trials were carried out by highly skilled interventional neuroradiologists operating in centers of excellence – yet such centers are few and far between.
“There should be no fight between the specialties. In hospitals with high patient volume and good work flow and experienced neuroradiologists available 24/7, there is no need for cardiologists to jump in. But in hospitals where that’s not the case then cardiologists can be of help,” he asserted at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
There aren’t nearly enough interventional neuroradiologists or endovascularly trained neurosurgeons to fill the enormous need, and neurologists simply don’t have the mindset for this sort of work, Dr. Widimsky added.
“Neurologists, with few exceptions, don’t do interventions. In general, they are people who think conservatively. These procedures should be done by someone who is working with procedures every day, and that’s not what neurologists do,” he continued.
Because interventional neuroradiology services weren’t available at Dr. Widimsky’s hospital, he and his fellow interventional cardiologists took on the task several years ago, gaining specialized training and then forming a multidisciplinary acute stroke team. The results, he said, have been gratifying.
The new endovascular therapy for acute stroke has much in common with contemporary management of ST-elevation MI, Dr. Widimsky observed. Just as in an acute MI, where time is heart muscle, in acute stroke time is brain. In most patients, the endovascular procedures are most effective when done within 3 hours after acute stroke onset. By 6 hours, the rate of good functional recovery falls to about 20%. But some patients can derive benefit even with much later intervention provided they have sufficient collateral circulation, which can be determined by sophisticated perfusion imaging techniques.
Dr. Widimsky pointed out a couple of ways to streamline today’s standard acute stroke management flow in order to save substantial time. The typical pathway today is for EMS personnel to take a patient to the emergency department for evaluation for suspected stroke, which can take up to 30 minutes. That patient then goes to CT imaging to determine whether the stroke is ischemic or hemorrhagic, then to the neurology unit for thrombolytic therapy, which can take another 30-60 minutes. Only afterwards, if indicated, does the patient go to the catheterization laboratory for endovascular intervention.
A faster, better approach, he said, is to train EMS personnel to recognize suspected cases of acute stroke, have them bypass the ED and instead take those patients straight to a hospital with high-quality CT imaging available 24/7, and if imaging indicates the patient is a candidate for mechanical revascularization, to then bypass the thrombolysis suite and go directly to the catheterization laboratory. That can save an hour to an hour-and-a-half in total.
Who should be performing these endovascular interventions? Dr. Alain Bonafe presented highlights of a recent joint consensus statement by the European Stroke Organization, the European Society of Minimally Invasive Neurological Therapy, and the European Society of Neuroradiology that declared the decision to undertake these procedures should be made jointly by a multidisciplinary team in experienced centers providing comprehensive stroke care, and that the procedures should be carried out by accredited interventionalists with certified expertise, regardless of their specialty.
“We must offer this intervention to as many patients as possible,” stressed Dr. Bonafe, professor of neuroradiology at the University of Toulouse and president of the French Society of Neuroradiology. “In most places it’s not offered at all, or only part-time by a few experts. So I think cardiologists should join the force, and everybody who is expert in procedural interventions should be trained for this in order to cover the need for the whole population.”
Dr. Kenneth K. Snyder observed that as recently as 2013, the rumor was that endovascular stroke therapy was dead. Three randomized trials published in the New England Journal of Medicine – IMS III, SYNTHESIS, and MR RESCUE – had found no difference between endovascular therapy and standard medical therapy.
But only 5% of the participants in those trials were treated with modern clot retrievers, which are much more effective than earlier-generation devices. And the negative trials didn’t specifically target large-vessel occlusions, which is where device therapy clearly works best.
“Stroke is now a surgical disease. Many of us have believed this from the get go. In centers with advanced systems of stroke care, endovascular therapy can significantly improve functional outcomes without compromising safety as compared to standard therapy,” said Dr. Snyder, a neurosurgeon specializing in endovascular therapy at the State University of New York at Buffalo.
In the United States, he noted, stroke is the fourth leading cause of mortality, the No. 1 cause of long-term disability, the most common discharge diagnosis to nursing homes, and carries a cost of $70 billion annually. Worldwide, stroke is the second leading cause of mortality. And stroke rates will continue to grow.
He said conflict between specialties regarding provision of state-of-the-art acute stroke therapy is not inevitable, as can be seen at the acute stroke unit at SUNY Buffalo.
“Our center is collaborative and multidisciplinary. We have 20 interventional suites. We all work next to each other and with each other – the cardiologists next to the interventional radiologists next to the neurosurgeons. It forces a great deal of collaboration. And we have a track record of training cardiologists both in observerships and also in formal training programs,” Dr. Snyder said.
The speakers declared having no financial conflicts.
The convergence of technological advancements for intracranial mechanical thrombectomy (stent retrievers) and the use of noninvasive imaging (CTA/MRA) to improve patient selection for revascularization have revolutionized the treatment of acute stroke as demonstrated by the recent publication of five randomized clinical trials supporting revascularization for acute ischemic stroke. Similar to our national goal for minimizing door to balloon time (DTB) for acute heart attacks, there will now be a similar effort directed at expediting stroke treatment.
 |
Dr. Christopher J. White |
However, we have not solved the manpower issue of offering this specialized therapy in the local hospitals where the stroke patients are. Unfortunately, the demand for endovascular stroke treatment has outstripped the ability of traditional radiology specialists to provide this care, in many hospitals. The good news is that many other specialists, including interventional neurologists, vascular surgeons, neurosurgeons, and interventional cardiologists have endovascular skills readily adaptable to treating patients with acute stroke.
At Ochsner Medical Center in New Orleans, we have demonstrated the feasibility of interventional cardiologists working 24-7–365 with neurologists as a team, to perform endovascular revascularization for acute stroke patients. Reassuringly, we found no difference in outcomes among those acute stroke patients treated by radiology specialists and those treated by the interventional cardiology team (Catheter. Cardiovasc. Interven. 2015;85:1043-50). Because there is an uneven distribution of radiology specialists in our communities where patients with strokes need time-sensitive treatment, we need to develop teams composed of a variety of physician specialties, including interventional cardiologists, who can deliver rapid and safe intracranial mechanical thrombectomy to selected patients with acute stroke in their local communities.
Dr. Christopher J. White is medical director of the John Ochsner Heart & Vascular Institute in New Orleans. He is an adviser to and consultant for Neovasc, and consults for Surmodics.
The convergence of technological advancements for intracranial mechanical thrombectomy (stent retrievers) and the use of noninvasive imaging (CTA/MRA) to improve patient selection for revascularization have revolutionized the treatment of acute stroke as demonstrated by the recent publication of five randomized clinical trials supporting revascularization for acute ischemic stroke. Similar to our national goal for minimizing door to balloon time (DTB) for acute heart attacks, there will now be a similar effort directed at expediting stroke treatment.
 |
Dr. Christopher J. White |
However, we have not solved the manpower issue of offering this specialized therapy in the local hospitals where the stroke patients are. Unfortunately, the demand for endovascular stroke treatment has outstripped the ability of traditional radiology specialists to provide this care, in many hospitals. The good news is that many other specialists, including interventional neurologists, vascular surgeons, neurosurgeons, and interventional cardiologists have endovascular skills readily adaptable to treating patients with acute stroke.
At Ochsner Medical Center in New Orleans, we have demonstrated the feasibility of interventional cardiologists working 24-7–365 with neurologists as a team, to perform endovascular revascularization for acute stroke patients. Reassuringly, we found no difference in outcomes among those acute stroke patients treated by radiology specialists and those treated by the interventional cardiology team (Catheter. Cardiovasc. Interven. 2015;85:1043-50). Because there is an uneven distribution of radiology specialists in our communities where patients with strokes need time-sensitive treatment, we need to develop teams composed of a variety of physician specialties, including interventional cardiologists, who can deliver rapid and safe intracranial mechanical thrombectomy to selected patients with acute stroke in their local communities.
Dr. Christopher J. White is medical director of the John Ochsner Heart & Vascular Institute in New Orleans. He is an adviser to and consultant for Neovasc, and consults for Surmodics.
The convergence of technological advancements for intracranial mechanical thrombectomy (stent retrievers) and the use of noninvasive imaging (CTA/MRA) to improve patient selection for revascularization have revolutionized the treatment of acute stroke as demonstrated by the recent publication of five randomized clinical trials supporting revascularization for acute ischemic stroke. Similar to our national goal for minimizing door to balloon time (DTB) for acute heart attacks, there will now be a similar effort directed at expediting stroke treatment.
 |
Dr. Christopher J. White |
However, we have not solved the manpower issue of offering this specialized therapy in the local hospitals where the stroke patients are. Unfortunately, the demand for endovascular stroke treatment has outstripped the ability of traditional radiology specialists to provide this care, in many hospitals. The good news is that many other specialists, including interventional neurologists, vascular surgeons, neurosurgeons, and interventional cardiologists have endovascular skills readily adaptable to treating patients with acute stroke.
At Ochsner Medical Center in New Orleans, we have demonstrated the feasibility of interventional cardiologists working 24-7–365 with neurologists as a team, to perform endovascular revascularization for acute stroke patients. Reassuringly, we found no difference in outcomes among those acute stroke patients treated by radiology specialists and those treated by the interventional cardiology team (Catheter. Cardiovasc. Interven. 2015;85:1043-50). Because there is an uneven distribution of radiology specialists in our communities where patients with strokes need time-sensitive treatment, we need to develop teams composed of a variety of physician specialties, including interventional cardiologists, who can deliver rapid and safe intracranial mechanical thrombectomy to selected patients with acute stroke in their local communities.
Dr. Christopher J. White is medical director of the John Ochsner Heart & Vascular Institute in New Orleans. He is an adviser to and consultant for Neovasc, and consults for Surmodics.
PARIS – The leaders of European interventional cardiology have thrown down the gauntlet to their colleagues, declaring during a special call-to-action session at EuroPCR that a revolution is underway in the treatment of acute stroke, and interventional cardiologists need to train up and become part of it.
“Something big is going on today. If we want to be transformative and impactful, I think stroke intervention is one of the main points where we can do so as interventional cardiologists,” said Dr. Alberto Cremonesi of Villa Maria Cecilia Hospital in Cotignola, Italy, a past president of the Italian Society of Interventional Cardiology.
Dr. Petr Widimsky highlighted the five prospective, randomized, controlled trials that have come out in the past few months and triggered the revolution in acute stroke therapy. All five studies – MR CLEAN, ESCAPE, EXTENT IA, SWIFT PRIME, and REVASCAT – were halted early because of the significant advantage mechanical endovascular therapy with stents or thrombus retrieval devices demonstrated over standard therapy featuring clot thrombolysis with tissue plasminogen activator.
Collectively, the five trials showed a 60% greater chance for good functional recovery from stroke with endovascular interventions. The rate of a favorable neurologic outcome as reflected in a modified Rankin score of 0-2 was 48% with the use of stent/retriever devices, compared with 30% with thrombolysis alone, noted Dr. Widimsky, professor and chair of the cardiology department at Charles University in Prague.
The Food and Drug Administration began approving these endovascular therapy devices in 2012. The major challenge is how to make this therapy available to the vast numbers of patients in need. After all, the successful clinical trials were carried out by highly skilled interventional neuroradiologists operating in centers of excellence – yet such centers are few and far between.
“There should be no fight between the specialties. In hospitals with high patient volume and good work flow and experienced neuroradiologists available 24/7, there is no need for cardiologists to jump in. But in hospitals where that’s not the case then cardiologists can be of help,” he asserted at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
There aren’t nearly enough interventional neuroradiologists or endovascularly trained neurosurgeons to fill the enormous need, and neurologists simply don’t have the mindset for this sort of work, Dr. Widimsky added.
“Neurologists, with few exceptions, don’t do interventions. In general, they are people who think conservatively. These procedures should be done by someone who is working with procedures every day, and that’s not what neurologists do,” he continued.
Because interventional neuroradiology services weren’t available at Dr. Widimsky’s hospital, he and his fellow interventional cardiologists took on the task several years ago, gaining specialized training and then forming a multidisciplinary acute stroke team. The results, he said, have been gratifying.
The new endovascular therapy for acute stroke has much in common with contemporary management of ST-elevation MI, Dr. Widimsky observed. Just as in an acute MI, where time is heart muscle, in acute stroke time is brain. In most patients, the endovascular procedures are most effective when done within 3 hours after acute stroke onset. By 6 hours, the rate of good functional recovery falls to about 20%. But some patients can derive benefit even with much later intervention provided they have sufficient collateral circulation, which can be determined by sophisticated perfusion imaging techniques.
Dr. Widimsky pointed out a couple of ways to streamline today’s standard acute stroke management flow in order to save substantial time. The typical pathway today is for EMS personnel to take a patient to the emergency department for evaluation for suspected stroke, which can take up to 30 minutes. That patient then goes to CT imaging to determine whether the stroke is ischemic or hemorrhagic, then to the neurology unit for thrombolytic therapy, which can take another 30-60 minutes. Only afterwards, if indicated, does the patient go to the catheterization laboratory for endovascular intervention.
A faster, better approach, he said, is to train EMS personnel to recognize suspected cases of acute stroke, have them bypass the ED and instead take those patients straight to a hospital with high-quality CT imaging available 24/7, and if imaging indicates the patient is a candidate for mechanical revascularization, to then bypass the thrombolysis suite and go directly to the catheterization laboratory. That can save an hour to an hour-and-a-half in total.
Who should be performing these endovascular interventions? Dr. Alain Bonafe presented highlights of a recent joint consensus statement by the European Stroke Organization, the European Society of Minimally Invasive Neurological Therapy, and the European Society of Neuroradiology that declared the decision to undertake these procedures should be made jointly by a multidisciplinary team in experienced centers providing comprehensive stroke care, and that the procedures should be carried out by accredited interventionalists with certified expertise, regardless of their specialty.
“We must offer this intervention to as many patients as possible,” stressed Dr. Bonafe, professor of neuroradiology at the University of Toulouse and president of the French Society of Neuroradiology. “In most places it’s not offered at all, or only part-time by a few experts. So I think cardiologists should join the force, and everybody who is expert in procedural interventions should be trained for this in order to cover the need for the whole population.”
Dr. Kenneth K. Snyder observed that as recently as 2013, the rumor was that endovascular stroke therapy was dead. Three randomized trials published in the New England Journal of Medicine – IMS III, SYNTHESIS, and MR RESCUE – had found no difference between endovascular therapy and standard medical therapy.
But only 5% of the participants in those trials were treated with modern clot retrievers, which are much more effective than earlier-generation devices. And the negative trials didn’t specifically target large-vessel occlusions, which is where device therapy clearly works best.
“Stroke is now a surgical disease. Many of us have believed this from the get go. In centers with advanced systems of stroke care, endovascular therapy can significantly improve functional outcomes without compromising safety as compared to standard therapy,” said Dr. Snyder, a neurosurgeon specializing in endovascular therapy at the State University of New York at Buffalo.
In the United States, he noted, stroke is the fourth leading cause of mortality, the No. 1 cause of long-term disability, the most common discharge diagnosis to nursing homes, and carries a cost of $70 billion annually. Worldwide, stroke is the second leading cause of mortality. And stroke rates will continue to grow.
He said conflict between specialties regarding provision of state-of-the-art acute stroke therapy is not inevitable, as can be seen at the acute stroke unit at SUNY Buffalo.
“Our center is collaborative and multidisciplinary. We have 20 interventional suites. We all work next to each other and with each other – the cardiologists next to the interventional radiologists next to the neurosurgeons. It forces a great deal of collaboration. And we have a track record of training cardiologists both in observerships and also in formal training programs,” Dr. Snyder said.
The speakers declared having no financial conflicts.
PARIS – The leaders of European interventional cardiology have thrown down the gauntlet to their colleagues, declaring during a special call-to-action session at EuroPCR that a revolution is underway in the treatment of acute stroke, and interventional cardiologists need to train up and become part of it.
“Something big is going on today. If we want to be transformative and impactful, I think stroke intervention is one of the main points where we can do so as interventional cardiologists,” said Dr. Alberto Cremonesi of Villa Maria Cecilia Hospital in Cotignola, Italy, a past president of the Italian Society of Interventional Cardiology.
Dr. Petr Widimsky highlighted the five prospective, randomized, controlled trials that have come out in the past few months and triggered the revolution in acute stroke therapy. All five studies – MR CLEAN, ESCAPE, EXTENT IA, SWIFT PRIME, and REVASCAT – were halted early because of the significant advantage mechanical endovascular therapy with stents or thrombus retrieval devices demonstrated over standard therapy featuring clot thrombolysis with tissue plasminogen activator.
Collectively, the five trials showed a 60% greater chance for good functional recovery from stroke with endovascular interventions. The rate of a favorable neurologic outcome as reflected in a modified Rankin score of 0-2 was 48% with the use of stent/retriever devices, compared with 30% with thrombolysis alone, noted Dr. Widimsky, professor and chair of the cardiology department at Charles University in Prague.
The Food and Drug Administration began approving these endovascular therapy devices in 2012. The major challenge is how to make this therapy available to the vast numbers of patients in need. After all, the successful clinical trials were carried out by highly skilled interventional neuroradiologists operating in centers of excellence – yet such centers are few and far between.
“There should be no fight between the specialties. In hospitals with high patient volume and good work flow and experienced neuroradiologists available 24/7, there is no need for cardiologists to jump in. But in hospitals where that’s not the case then cardiologists can be of help,” he asserted at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
There aren’t nearly enough interventional neuroradiologists or endovascularly trained neurosurgeons to fill the enormous need, and neurologists simply don’t have the mindset for this sort of work, Dr. Widimsky added.
“Neurologists, with few exceptions, don’t do interventions. In general, they are people who think conservatively. These procedures should be done by someone who is working with procedures every day, and that’s not what neurologists do,” he continued.
Because interventional neuroradiology services weren’t available at Dr. Widimsky’s hospital, he and his fellow interventional cardiologists took on the task several years ago, gaining specialized training and then forming a multidisciplinary acute stroke team. The results, he said, have been gratifying.
The new endovascular therapy for acute stroke has much in common with contemporary management of ST-elevation MI, Dr. Widimsky observed. Just as in an acute MI, where time is heart muscle, in acute stroke time is brain. In most patients, the endovascular procedures are most effective when done within 3 hours after acute stroke onset. By 6 hours, the rate of good functional recovery falls to about 20%. But some patients can derive benefit even with much later intervention provided they have sufficient collateral circulation, which can be determined by sophisticated perfusion imaging techniques.
Dr. Widimsky pointed out a couple of ways to streamline today’s standard acute stroke management flow in order to save substantial time. The typical pathway today is for EMS personnel to take a patient to the emergency department for evaluation for suspected stroke, which can take up to 30 minutes. That patient then goes to CT imaging to determine whether the stroke is ischemic or hemorrhagic, then to the neurology unit for thrombolytic therapy, which can take another 30-60 minutes. Only afterwards, if indicated, does the patient go to the catheterization laboratory for endovascular intervention.
A faster, better approach, he said, is to train EMS personnel to recognize suspected cases of acute stroke, have them bypass the ED and instead take those patients straight to a hospital with high-quality CT imaging available 24/7, and if imaging indicates the patient is a candidate for mechanical revascularization, to then bypass the thrombolysis suite and go directly to the catheterization laboratory. That can save an hour to an hour-and-a-half in total.
Who should be performing these endovascular interventions? Dr. Alain Bonafe presented highlights of a recent joint consensus statement by the European Stroke Organization, the European Society of Minimally Invasive Neurological Therapy, and the European Society of Neuroradiology that declared the decision to undertake these procedures should be made jointly by a multidisciplinary team in experienced centers providing comprehensive stroke care, and that the procedures should be carried out by accredited interventionalists with certified expertise, regardless of their specialty.
“We must offer this intervention to as many patients as possible,” stressed Dr. Bonafe, professor of neuroradiology at the University of Toulouse and president of the French Society of Neuroradiology. “In most places it’s not offered at all, or only part-time by a few experts. So I think cardiologists should join the force, and everybody who is expert in procedural interventions should be trained for this in order to cover the need for the whole population.”
Dr. Kenneth K. Snyder observed that as recently as 2013, the rumor was that endovascular stroke therapy was dead. Three randomized trials published in the New England Journal of Medicine – IMS III, SYNTHESIS, and MR RESCUE – had found no difference between endovascular therapy and standard medical therapy.
But only 5% of the participants in those trials were treated with modern clot retrievers, which are much more effective than earlier-generation devices. And the negative trials didn’t specifically target large-vessel occlusions, which is where device therapy clearly works best.
“Stroke is now a surgical disease. Many of us have believed this from the get go. In centers with advanced systems of stroke care, endovascular therapy can significantly improve functional outcomes without compromising safety as compared to standard therapy,” said Dr. Snyder, a neurosurgeon specializing in endovascular therapy at the State University of New York at Buffalo.
In the United States, he noted, stroke is the fourth leading cause of mortality, the No. 1 cause of long-term disability, the most common discharge diagnosis to nursing homes, and carries a cost of $70 billion annually. Worldwide, stroke is the second leading cause of mortality. And stroke rates will continue to grow.
He said conflict between specialties regarding provision of state-of-the-art acute stroke therapy is not inevitable, as can be seen at the acute stroke unit at SUNY Buffalo.
“Our center is collaborative and multidisciplinary. We have 20 interventional suites. We all work next to each other and with each other – the cardiologists next to the interventional radiologists next to the neurosurgeons. It forces a great deal of collaboration. And we have a track record of training cardiologists both in observerships and also in formal training programs,” Dr. Snyder said.
The speakers declared having no financial conflicts.
EXPERT ANALYSIS FROM EUROPCR 2015
WCD: Dermatologists Underappreciate Psoriatic Itch, Patients Say
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
AT WCD 2015
WCD: Dermatologists underappreciate psoriatic itch, patients say
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
VANCOUVER, B.C. – The largest-ever survey of North American and European physician and patient perspectives on psoriasis and psoriatic arthritis reveals a major disconnect between dermatologists and their psoriasis patients regarding the importance of pruritus.
Dermatologists characterized one-fifth of their psoriasis patients as having severe disease. The majority of dermatologists – fully 53% – regarded lesion size and location to be the most important factors contributing to disease severity. Only 7.4% of dermatologists considered itch to be the most important factor.
In contrast, 38% of patients considered itching to be the most important aspect of their skin disease, and 17% – less than one-third of the proportion of dermatologists – rated lesion size and location to be the top contributor to disease severity, Dr. Peter van de Kerkhof reported at the World Congress of Dermatology.
He presented new findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP), a survey of North American dermatologists, rheumatologists, psoriasis patients, and psoriatic arthritis patients unrivaled in its scope. The results of the first part of the survey, which entailed contacting 139,000 households and focused on unmet needs as characterized by 3,426 patients, have already been published (J. Am. Acad. Dermatol. 2014;70:871-81).
At the WCD, Dr. van de Kerkhof presented highlights of part 2 of the MAPP survey, which sought to obtain the real-world perspectives of dermatologists and rheumatologists on the management of psoriasis and psoriatic arthritis. Of the 6,530 dermatologists and 5,445 rheumatologists who were contacted, 391 and 390, respectively, completed the detailed interview, according to Dr. van de Kerkhof, professor and chairman of the department of dermatology at Radboud University in Nijmegen, the Netherlands.
Although 92% of dermatologists agreed that the disease burden of psoriasis is frequently underestimated, the marked physician/patient divergence regarding the importance of pruritus was particularly striking, he observed.
Nineteen percent of dermatologists reported that their patients with moderate to severe psoriasis were receiving conventional oral agents, and 19.6% indicated their patients were on biologic therapy. However, 54% of dermatologists indicated they prescribed topical agents as monotherapy for their patients with moderate to severe disease, which is out of step with current treatment guidelines and represents clear undertreatment, Dr. van de Kerkhof noted.
Thirty-six percent of dermatologists cited affordability and 22% cited uncertainty about long-term safety of current medications as the greatest challenge associated with management of psoriasis. Sixteen percent cited lack of effectiveness as the biggest challenge.
The top reasons dermatologists gave for not initiating treatment with conventional oral agents were concerns about long-term safety and tolerability. Those were also the main reasons dermatologists gave for not continuing conventional oral medications.
The story was slightly different when it came to reasons for not initiating or continuing biologic agents. Here cost joined tolerability and long-term safety concerns as the main reasons for not starting patients on biologic therapies. Those factors, along with lack or loss of response, which was cited by 18% of dermatologists, were also the chief reasons given for not continuing patients on biologic agents.
Dr. Gil Yosipovitch, who has devoted much of his research career to the study of itch, predicted that the sharp discrepancy between psoriasis patients and dermatologists regarding the importance of itch as documented in the new MAPP survey findings will improve soon. Dermatologists’ attitudes towards itch are beginning to change: “If you look at the textbooks of a decade ago, itch was not even mentioned as part of the symptoms,” he said.
Psoriasis patients clearly feel that effective treatment for their pruritus is an unmet need. Fortunately, studies on the itch impact of current and next-generation biologics are underway and in some cases completed, noted Dr. Yosipovitch, professor and chair of the department of dermatology at Temple University in Philadelphia.
The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof reported serving as a consultant to Celgene and a dozen other pharmaceutical companies, most of which also provide him with grants to conduct clinical trials.
AT WCD 2015
Key clinical point: Dermatologists and psoriasis patients don’t see eye to eye when it comes to the importance of itch as the defining symptom of disease severity.
Major finding: Thirty-eight percent of psoriasis patients consider itch to be the most troublesome aspect of their disease and the most important determinant of disease severity, as do 7.4% of dermatologists.
Data source: A random survey of 391 North American and European dermatologists, 390 rheumatologists, and more than 3,000 psoriasis or psoriatic arthritis patients.
Disclosures: The MAPP survey was sponsored by Celgene Corp. Dr. van de Kerkhof is a consultant to and recipient of research grants from Celgene and numerous other pharmaceutical companies.
WCD: Psoriasis plus depression magnifies MI risk
VANCOUVER, B.C. – The combination of even mild psoriasis and a diagnosis of depression is associated with a greater risk of acute MI than is either condition alone, according to a Danish national study.
Moreover, when depression coincided with a severe case of psoriasis, not only was the risk of incident acute MI elevated, but the risk that the MI would be fatal was significantly increased as well, Dr. Alexander Egeberg reported at the World Congress of Dermatology.
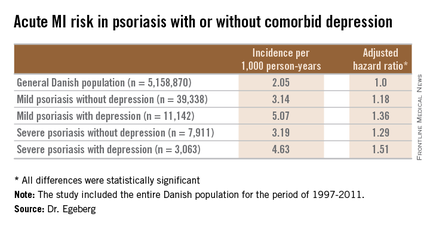
He presented a population-based cohort study of the entire Danish population, for the period of 1997-2011. The study population comprised 5,158,870 Danish citizens, including 50,480 Danes diagnosed with mild psoriasis and 10,974 others diagnosed with severe psoriasis during the study years. During that period, 11,142 members of the group with mild psoriasis and 3,063 with severe psoriasis were diagnosed with incident depression.
In a multivariate Cox regression analysis adjusted for age, gender, comorbid conditions, and concomitant medications, Danes with mild psoriasis but no diagnosis of depression were at a statistically significant 18% increased risk of having an acute MI compared to the general Danish population free of both diseases; the risk climbed upward from there (see chart).
A consistent trend for an increased risk of fatal MI was evident in patients with psoriasis, be it mild or severe, and with or without comorbid depression. However, this risk achieved statistical significance only in the group with severe psoriasis plus depression, a state associated with a 64% increased risk of fatal MI compared with the general population.
Although recent years have brought clear evidence that psoriasis is associated with increased risks of heart disease and depression, and that stand-alone depression is a risk factor for ischemic coronary events, the link between psoriasis, depression, and MI previously was unclear, explained Dr. Egeberg, who performed the research while at the University of Copenhagen but is now employed by Pfizer.
The retrospective study was conducted with the use of linked comprehensive national databases. The work was supported by a research grant from Pfizer.
VANCOUVER, B.C. – The combination of even mild psoriasis and a diagnosis of depression is associated with a greater risk of acute MI than is either condition alone, according to a Danish national study.
Moreover, when depression coincided with a severe case of psoriasis, not only was the risk of incident acute MI elevated, but the risk that the MI would be fatal was significantly increased as well, Dr. Alexander Egeberg reported at the World Congress of Dermatology.

He presented a population-based cohort study of the entire Danish population, for the period of 1997-2011. The study population comprised 5,158,870 Danish citizens, including 50,480 Danes diagnosed with mild psoriasis and 10,974 others diagnosed with severe psoriasis during the study years. During that period, 11,142 members of the group with mild psoriasis and 3,063 with severe psoriasis were diagnosed with incident depression.
In a multivariate Cox regression analysis adjusted for age, gender, comorbid conditions, and concomitant medications, Danes with mild psoriasis but no diagnosis of depression were at a statistically significant 18% increased risk of having an acute MI compared to the general Danish population free of both diseases; the risk climbed upward from there (see chart).
A consistent trend for an increased risk of fatal MI was evident in patients with psoriasis, be it mild or severe, and with or without comorbid depression. However, this risk achieved statistical significance only in the group with severe psoriasis plus depression, a state associated with a 64% increased risk of fatal MI compared with the general population.
Although recent years have brought clear evidence that psoriasis is associated with increased risks of heart disease and depression, and that stand-alone depression is a risk factor for ischemic coronary events, the link between psoriasis, depression, and MI previously was unclear, explained Dr. Egeberg, who performed the research while at the University of Copenhagen but is now employed by Pfizer.
The retrospective study was conducted with the use of linked comprehensive national databases. The work was supported by a research grant from Pfizer.
VANCOUVER, B.C. – The combination of even mild psoriasis and a diagnosis of depression is associated with a greater risk of acute MI than is either condition alone, according to a Danish national study.
Moreover, when depression coincided with a severe case of psoriasis, not only was the risk of incident acute MI elevated, but the risk that the MI would be fatal was significantly increased as well, Dr. Alexander Egeberg reported at the World Congress of Dermatology.

He presented a population-based cohort study of the entire Danish population, for the period of 1997-2011. The study population comprised 5,158,870 Danish citizens, including 50,480 Danes diagnosed with mild psoriasis and 10,974 others diagnosed with severe psoriasis during the study years. During that period, 11,142 members of the group with mild psoriasis and 3,063 with severe psoriasis were diagnosed with incident depression.
In a multivariate Cox regression analysis adjusted for age, gender, comorbid conditions, and concomitant medications, Danes with mild psoriasis but no diagnosis of depression were at a statistically significant 18% increased risk of having an acute MI compared to the general Danish population free of both diseases; the risk climbed upward from there (see chart).
A consistent trend for an increased risk of fatal MI was evident in patients with psoriasis, be it mild or severe, and with or without comorbid depression. However, this risk achieved statistical significance only in the group with severe psoriasis plus depression, a state associated with a 64% increased risk of fatal MI compared with the general population.
Although recent years have brought clear evidence that psoriasis is associated with increased risks of heart disease and depression, and that stand-alone depression is a risk factor for ischemic coronary events, the link between psoriasis, depression, and MI previously was unclear, explained Dr. Egeberg, who performed the research while at the University of Copenhagen but is now employed by Pfizer.
The retrospective study was conducted with the use of linked comprehensive national databases. The work was supported by a research grant from Pfizer.
AT WCD 2015
Key clinical point: In psoriasis patients, comorbid depression is associated with an increased risk of a first acute MI.
Major finding: The incidence rate of acute MI was 2.05 cases per 1,000 person-years in the general Danish population, climbing to 3.14/1,000 in those with mild psoriasis without diagnosed depression, and 5.07/1,000 for mild psoriasis with depression.
Data source: A retrospective, population-based, cohort study of the entire Danish adult population – more than 5.1 million individuals – during 1997-2011.
Disclosures: The presenter is an employee of Pfizer, which supported the study.