User login
WCD: Topical tofacitinib hits marks in atopic dermatitis
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
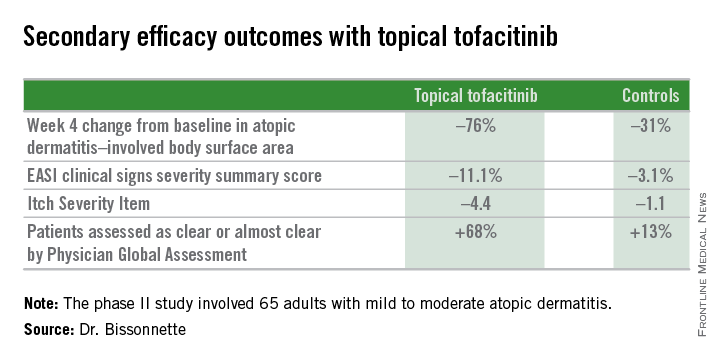
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.
VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
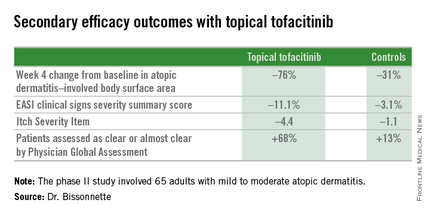
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

VANCOUVER, B.C. – Topical tofacitinib shows promise as a novel treatment for atopic dermatitis on the basis of a highly positive phase II randomized clinical trial.
The topical Janus kinase inhibitor hit all of its efficacy endpoints and was well tolerated, with infrequent side effects, none of them serious, Dr. Robert Bissonnette reported at the World Congress of Dermatology.
An unmet need exists for additional topical therapies for atopic dermatitis, a condition whose prevalence has been estimated at up to 20%. Existing topical agents, including corticosteroids and calcineurin inhibitors, have limitations involving long-term safety concerns and application site reactions, noted Dr. Bissonnette, president of Innovaderm Research in Montreal.
The Janus kinases have been implicated in the pathogenesis of atopic dermatitis due to their effects upon the interleukin-4, IL-5, and IL-31 signaling pathways and the resultant dysregulation of the immune response.
Dr. Bissonnette presented a phase II, 4-week, double-blind, vehicle-controlled, multicenter Canadian study involving 65 adults with mild to moderate atopic dermatitis. They applied tofacitinib ointment 2% or its vehicle twice daily for 4 weeks. Participants averaged 31 years of age, with a median 21 years since receiving the diagnosis of atopic dermatitis. Roughly three-quarters of subjects had moderate disease based upon Physician Global Assessment.
The primary study endpoint was change in Eczema Area and Severity Index (EASI) total score after 4 weeks. From a baseline EASI score of 5.4, the topical tofacitinib group experienced a mean 82% reduction, significantly outperforming the control group, which showed a 30% reduction. The difference between the two study arms reached significance at the first assessment, at week 1.
Patients on the topical Janus kinase (JAK) inhibitor also showed significantly better outcomes than controls on all secondary endpoints, with the differences reaching statistical significance at week 1 with the exception of improvement in self-assessed Itch Severity Item, where topical tofacitinib’s advantage became significant on treatment day 2.
Two patients on topical tofacitinib and five controls developed treatment area adverse events, consisting of skin irritation and/or pain, which was mild in all cases. There were no cases of herpes simplex or zoster, no opportunistic infections, no laboratory abnormalities, and no one required a dose reduction or temporary discontinuation of the topical JAK inhibitor, according to the dermatologist.
Oral tofacitinib is Food and Drug Administration–approved as Xeljanz for the treatment of rheumatoid arthritis and is currently under FDA review as a potential treatment for moderate to severe chronic plaque psoriasis, with a regulatory decision anticipated in October 2015.
The atopic dermatitis study was funded by Pfizer. Dr. Bissonnette is a consultant to and recipient of research grants from more than a dozen pharmaceutical companies, including Pfizer.

AT WCD 2015
Key clinical point: Topical tofacitinib, a Janus kinase inhibitor, may provide a novel safe and effective therapy for atopic dermatitis.
Major finding: After 4 weeks of topical tofacitinib, atopic dermatitis patients experienced a mean 82% reduction in their Eczema Area and Severity Index total score, compared with a 30% decrease in vehicle-treated controls.
Data source: A five-center, randomized, double-blind, prospective, vehicle-controlled phase II study involving 65 adults with atopic dermatitis.
Disclosures: Dr. Robert Bissonnette disclosed ties with Pfizer – the study sponsor – and more than a dozen other pharmaceutical companies.
Contrast injection approximates adenosine-derived FFR
PARIS – Measurement of fractional flow reserve using adenosine remains the gold standard for assessing the functional significance of coronary stenoses, but when that’s not an option then intracoronary infusion of contrast medium is a good alternative, according to the results of the CONTRAST trial.
“Contrast injection is an easy, inexpensive, and safe method of providing hyperemia. It displays excellent test/retest stability. And it does not depend on a specific software platform or ECG gating. So if you need to use it, all of you can start next week,” Dr. Nils P. Johnson said in presenting the CONTRAST study findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He was quick to emphasize that contrast measurement of Pd/Pa – that is, the pressure distal to a stenosis relative to the pressure proximal to a stenosis – had a diagnostic accuracy of 86% relative to adenosine-derived fractional flow reserve (FFR) as the reference standard in the CONTRAST trial. So the contrast-based measurement is imperfect, and interventionalists who use it run the risk of making the wrong clinical diagnosis. However, a key clinical finding in CONTRAST was that if the contrast-based Pd/Pa value was 0.8 or less, then the adenosine-derived FFR was, too, thus confirming the functional significance and actionability of the lesion under evaluation.
Adenosine maximizes hyperemia, and adenosine-derived FFR is the version of the test with a IA recommendation in European Society of Cardiology guidelines based upon the persuasive findings of the FAME and FAME 2 (N. Engl. J. Med. 2014;371:1208-17) studies. It is, Dr. Johnson stressed, the best technique, but inducing hyperemia via adenosine is expensive, time-consuming, and burdensome. And when it’s not an option, either because a patient has a contraindication to adenosine or a health care system deems adenosine prohibitively expensive as a first-line agent, then achieving hyperemia via an injection of contrast media is a reasonable strategy for measurement of Pd/Pa, according to Dr. Johnson, coprincipal investigator for the CONTRAST study and a cardiologist at the University of Texas, Houston.
He noted that in the study, contrast Pd/Pa proved to have a significantly better diagnostic accuracy than the 78%-79% achieved via measurement of Pd/Pa at physiologic rest or based upon the instantaneous wave-free rate (iFR), a proprietary Volcano Corp. technology that assesses a lesion’s significance over the course of five heartbeats without need for hypemic agents. Moreover, the contrast Pd/Pa’s test/retest stability was as good as with intracoronary or intravenous adenosine and superior to that of iFR or resting Pd/Pa.
CONTRAST was a 750-patient, nine-country, prospective diagnostic accuracy study. It was designed as a pragmatic, real-world study in which investigators utilized an average of 8.2 mL of any of eight contrast agents in assessing a variety of coronary lesions in consecutive patients. Thus, the results are broadly applicable to anyone who performs percutaneous coronary intervention (PCI), the cardiologist said.
Once the pressure wire was introduced into a participant in the CONTRAST study, measurement of Pd/Pa was obtained during two intracoronary contrast infusions, two intracoronary adenosine infusions, two intravenous adenosine infusions, twice at rest, and twice using iFR. The tracings were then sent to a core physiology lab for interpretation by blinded evaluators.
A study limitation was that investigators didn’t collect data on the incidence of contrast-induced nephropathy. However, with an average contrast dose of just 8.2 mL, it’s likely the clinical impact was negligible, according to Dr. Johnson.
The CONTRAST study was sponsored by the University of Texas Medical Center at Houston. Dr. Johnson reported receiving a research grant from St. Jude Medical to conduct the study.
PARIS – Measurement of fractional flow reserve using adenosine remains the gold standard for assessing the functional significance of coronary stenoses, but when that’s not an option then intracoronary infusion of contrast medium is a good alternative, according to the results of the CONTRAST trial.
“Contrast injection is an easy, inexpensive, and safe method of providing hyperemia. It displays excellent test/retest stability. And it does not depend on a specific software platform or ECG gating. So if you need to use it, all of you can start next week,” Dr. Nils P. Johnson said in presenting the CONTRAST study findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He was quick to emphasize that contrast measurement of Pd/Pa – that is, the pressure distal to a stenosis relative to the pressure proximal to a stenosis – had a diagnostic accuracy of 86% relative to adenosine-derived fractional flow reserve (FFR) as the reference standard in the CONTRAST trial. So the contrast-based measurement is imperfect, and interventionalists who use it run the risk of making the wrong clinical diagnosis. However, a key clinical finding in CONTRAST was that if the contrast-based Pd/Pa value was 0.8 or less, then the adenosine-derived FFR was, too, thus confirming the functional significance and actionability of the lesion under evaluation.
Adenosine maximizes hyperemia, and adenosine-derived FFR is the version of the test with a IA recommendation in European Society of Cardiology guidelines based upon the persuasive findings of the FAME and FAME 2 (N. Engl. J. Med. 2014;371:1208-17) studies. It is, Dr. Johnson stressed, the best technique, but inducing hyperemia via adenosine is expensive, time-consuming, and burdensome. And when it’s not an option, either because a patient has a contraindication to adenosine or a health care system deems adenosine prohibitively expensive as a first-line agent, then achieving hyperemia via an injection of contrast media is a reasonable strategy for measurement of Pd/Pa, according to Dr. Johnson, coprincipal investigator for the CONTRAST study and a cardiologist at the University of Texas, Houston.
He noted that in the study, contrast Pd/Pa proved to have a significantly better diagnostic accuracy than the 78%-79% achieved via measurement of Pd/Pa at physiologic rest or based upon the instantaneous wave-free rate (iFR), a proprietary Volcano Corp. technology that assesses a lesion’s significance over the course of five heartbeats without need for hypemic agents. Moreover, the contrast Pd/Pa’s test/retest stability was as good as with intracoronary or intravenous adenosine and superior to that of iFR or resting Pd/Pa.
CONTRAST was a 750-patient, nine-country, prospective diagnostic accuracy study. It was designed as a pragmatic, real-world study in which investigators utilized an average of 8.2 mL of any of eight contrast agents in assessing a variety of coronary lesions in consecutive patients. Thus, the results are broadly applicable to anyone who performs percutaneous coronary intervention (PCI), the cardiologist said.
Once the pressure wire was introduced into a participant in the CONTRAST study, measurement of Pd/Pa was obtained during two intracoronary contrast infusions, two intracoronary adenosine infusions, two intravenous adenosine infusions, twice at rest, and twice using iFR. The tracings were then sent to a core physiology lab for interpretation by blinded evaluators.
A study limitation was that investigators didn’t collect data on the incidence of contrast-induced nephropathy. However, with an average contrast dose of just 8.2 mL, it’s likely the clinical impact was negligible, according to Dr. Johnson.
The CONTRAST study was sponsored by the University of Texas Medical Center at Houston. Dr. Johnson reported receiving a research grant from St. Jude Medical to conduct the study.
PARIS – Measurement of fractional flow reserve using adenosine remains the gold standard for assessing the functional significance of coronary stenoses, but when that’s not an option then intracoronary infusion of contrast medium is a good alternative, according to the results of the CONTRAST trial.
“Contrast injection is an easy, inexpensive, and safe method of providing hyperemia. It displays excellent test/retest stability. And it does not depend on a specific software platform or ECG gating. So if you need to use it, all of you can start next week,” Dr. Nils P. Johnson said in presenting the CONTRAST study findings at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
He was quick to emphasize that contrast measurement of Pd/Pa – that is, the pressure distal to a stenosis relative to the pressure proximal to a stenosis – had a diagnostic accuracy of 86% relative to adenosine-derived fractional flow reserve (FFR) as the reference standard in the CONTRAST trial. So the contrast-based measurement is imperfect, and interventionalists who use it run the risk of making the wrong clinical diagnosis. However, a key clinical finding in CONTRAST was that if the contrast-based Pd/Pa value was 0.8 or less, then the adenosine-derived FFR was, too, thus confirming the functional significance and actionability of the lesion under evaluation.
Adenosine maximizes hyperemia, and adenosine-derived FFR is the version of the test with a IA recommendation in European Society of Cardiology guidelines based upon the persuasive findings of the FAME and FAME 2 (N. Engl. J. Med. 2014;371:1208-17) studies. It is, Dr. Johnson stressed, the best technique, but inducing hyperemia via adenosine is expensive, time-consuming, and burdensome. And when it’s not an option, either because a patient has a contraindication to adenosine or a health care system deems adenosine prohibitively expensive as a first-line agent, then achieving hyperemia via an injection of contrast media is a reasonable strategy for measurement of Pd/Pa, according to Dr. Johnson, coprincipal investigator for the CONTRAST study and a cardiologist at the University of Texas, Houston.
He noted that in the study, contrast Pd/Pa proved to have a significantly better diagnostic accuracy than the 78%-79% achieved via measurement of Pd/Pa at physiologic rest or based upon the instantaneous wave-free rate (iFR), a proprietary Volcano Corp. technology that assesses a lesion’s significance over the course of five heartbeats without need for hypemic agents. Moreover, the contrast Pd/Pa’s test/retest stability was as good as with intracoronary or intravenous adenosine and superior to that of iFR or resting Pd/Pa.
CONTRAST was a 750-patient, nine-country, prospective diagnostic accuracy study. It was designed as a pragmatic, real-world study in which investigators utilized an average of 8.2 mL of any of eight contrast agents in assessing a variety of coronary lesions in consecutive patients. Thus, the results are broadly applicable to anyone who performs percutaneous coronary intervention (PCI), the cardiologist said.
Once the pressure wire was introduced into a participant in the CONTRAST study, measurement of Pd/Pa was obtained during two intracoronary contrast infusions, two intracoronary adenosine infusions, two intravenous adenosine infusions, twice at rest, and twice using iFR. The tracings were then sent to a core physiology lab for interpretation by blinded evaluators.
A study limitation was that investigators didn’t collect data on the incidence of contrast-induced nephropathy. However, with an average contrast dose of just 8.2 mL, it’s likely the clinical impact was negligible, according to Dr. Johnson.
The CONTRAST study was sponsored by the University of Texas Medical Center at Houston. Dr. Johnson reported receiving a research grant from St. Jude Medical to conduct the study.
AT EUROPCR 2015
Key clinical point: Intracoronary injection of contrast media to achieve hyperemia is a safe, simple, and inexpensive alternative to adenosine-derived fractional flow reserve in assessing the functional significance of coronary stenoses.
Major finding: When a stenotic lesion’s Pd/Pa is 0.8 or less as measured during hyperemia induced by an intracoronary injection of contrast media, it’s 0.8 or less according to adenosine-derived FFR as well.
Data source: CONTRAST, a prospective, 750-patient, nine-country diagnostic accuracy study in which the same coronary stenoses were evaluated multiple times using standard adenosine-based FFR as well as measurements obtained under hyperemia induced by an intracoronary contrast media injection, at physiologic rest, and using instantaneous wave-free rate technology.
Disclosures: The study was sponsored by the University of Texas Medical Center at Houston. The presenter reported receiving a research grant from St. Jude Medical to conduct the study.
Elderly-onset atopic dermatitis is on the rise
VANCOUVER – Atopic dermatitis arising de novo in patients in their 60s or older with no history of the disease poses a diagnostic challenge, and a low threshold for biopsy is warranted, Dr. Thomas Bieber said at the World Congress of Dermatology.
“The diagnosis is not very easy, and if you are not sure what you are facing, I urge you to take biopsies in order to exclude cutaneous T-cell lymphoma before treating the patient with any kind of active compound,” cautioned Dr. Bieber, professor and chair of the department of dermatology and allergy at the University of Bonn (Germany).
Very-late-onset atopic dermatitis and cutaneous T-cell lymphoma (CTCL) may look quite similar clinically. There is but a single exception: Primary CTCL usually doesn’t itch, while pruritus is a prominent feature of atopic dermatitis arising in seniors, he added.
New-onset atopic dermatitis at an advanced age is increasing in prevalence, as is true of atopic dermatitis across the rest of the age spectrum. Dr. Bieber said that statistic is certainly borne out in his own clinical practice, where with the graying of the population he is seeing more cases.
He credited Dr. Ryoji Tanei of Tokyo Metropolitan Geriatric Hospital with doing pioneering work in bringing this particular variant of atopic dermatitis to wider attention (J. Clin. Med. 2015;4:979-97). Roughly 30% of patients with atopic dermatitis in their 60s or older report they never had the disease before. Another 20% had atopic dermatitis in childhood, while it arose in early adulthood in the rest.
Atopic dermatitis arising de novo in seniors is a special form of the disease that characteristically involves the face, neck, and trunk while sparing the flexural areas which are so prominently involved in younger patients. The eczema is often erythrodermic. Older men are affected threefold more often than women.
The disorder is characterized by extraordinarily high serum IgE levels: a mean of 8,000 IU in one series reported by Dr. Tanei.
This very-late-onset form of atopic dermatitis tends not to fade away over time. Dr. Tanei has reported that many affected patients die with the inflammatory skin disease, never outgrowing it.
Very-late-onset atopic dermatitis is often resistant to topical therapies; repeated courses of oral corticosteroids may be required.
The differential diagnosis is quite different than in children, where genetic immunodeficiency syndromes are a real concern. While CTCL is the biggie in the differential diagnosis of very-late-onset atopic dermatitis, other conditions that need to be considered include psoriasis, contact dermatitis, pityriasis rubra pilaris, and pityriasis rosea.
Dr. Bieber is a consultant to and recipient of research grants from numerous pharmaceutical companies having an interest in dermatology.
VANCOUVER – Atopic dermatitis arising de novo in patients in their 60s or older with no history of the disease poses a diagnostic challenge, and a low threshold for biopsy is warranted, Dr. Thomas Bieber said at the World Congress of Dermatology.
“The diagnosis is not very easy, and if you are not sure what you are facing, I urge you to take biopsies in order to exclude cutaneous T-cell lymphoma before treating the patient with any kind of active compound,” cautioned Dr. Bieber, professor and chair of the department of dermatology and allergy at the University of Bonn (Germany).
Very-late-onset atopic dermatitis and cutaneous T-cell lymphoma (CTCL) may look quite similar clinically. There is but a single exception: Primary CTCL usually doesn’t itch, while pruritus is a prominent feature of atopic dermatitis arising in seniors, he added.
New-onset atopic dermatitis at an advanced age is increasing in prevalence, as is true of atopic dermatitis across the rest of the age spectrum. Dr. Bieber said that statistic is certainly borne out in his own clinical practice, where with the graying of the population he is seeing more cases.
He credited Dr. Ryoji Tanei of Tokyo Metropolitan Geriatric Hospital with doing pioneering work in bringing this particular variant of atopic dermatitis to wider attention (J. Clin. Med. 2015;4:979-97). Roughly 30% of patients with atopic dermatitis in their 60s or older report they never had the disease before. Another 20% had atopic dermatitis in childhood, while it arose in early adulthood in the rest.
Atopic dermatitis arising de novo in seniors is a special form of the disease that characteristically involves the face, neck, and trunk while sparing the flexural areas which are so prominently involved in younger patients. The eczema is often erythrodermic. Older men are affected threefold more often than women.
The disorder is characterized by extraordinarily high serum IgE levels: a mean of 8,000 IU in one series reported by Dr. Tanei.
This very-late-onset form of atopic dermatitis tends not to fade away over time. Dr. Tanei has reported that many affected patients die with the inflammatory skin disease, never outgrowing it.
Very-late-onset atopic dermatitis is often resistant to topical therapies; repeated courses of oral corticosteroids may be required.
The differential diagnosis is quite different than in children, where genetic immunodeficiency syndromes are a real concern. While CTCL is the biggie in the differential diagnosis of very-late-onset atopic dermatitis, other conditions that need to be considered include psoriasis, contact dermatitis, pityriasis rubra pilaris, and pityriasis rosea.
Dr. Bieber is a consultant to and recipient of research grants from numerous pharmaceutical companies having an interest in dermatology.
VANCOUVER – Atopic dermatitis arising de novo in patients in their 60s or older with no history of the disease poses a diagnostic challenge, and a low threshold for biopsy is warranted, Dr. Thomas Bieber said at the World Congress of Dermatology.
“The diagnosis is not very easy, and if you are not sure what you are facing, I urge you to take biopsies in order to exclude cutaneous T-cell lymphoma before treating the patient with any kind of active compound,” cautioned Dr. Bieber, professor and chair of the department of dermatology and allergy at the University of Bonn (Germany).
Very-late-onset atopic dermatitis and cutaneous T-cell lymphoma (CTCL) may look quite similar clinically. There is but a single exception: Primary CTCL usually doesn’t itch, while pruritus is a prominent feature of atopic dermatitis arising in seniors, he added.
New-onset atopic dermatitis at an advanced age is increasing in prevalence, as is true of atopic dermatitis across the rest of the age spectrum. Dr. Bieber said that statistic is certainly borne out in his own clinical practice, where with the graying of the population he is seeing more cases.
He credited Dr. Ryoji Tanei of Tokyo Metropolitan Geriatric Hospital with doing pioneering work in bringing this particular variant of atopic dermatitis to wider attention (J. Clin. Med. 2015;4:979-97). Roughly 30% of patients with atopic dermatitis in their 60s or older report they never had the disease before. Another 20% had atopic dermatitis in childhood, while it arose in early adulthood in the rest.
Atopic dermatitis arising de novo in seniors is a special form of the disease that characteristically involves the face, neck, and trunk while sparing the flexural areas which are so prominently involved in younger patients. The eczema is often erythrodermic. Older men are affected threefold more often than women.
The disorder is characterized by extraordinarily high serum IgE levels: a mean of 8,000 IU in one series reported by Dr. Tanei.
This very-late-onset form of atopic dermatitis tends not to fade away over time. Dr. Tanei has reported that many affected patients die with the inflammatory skin disease, never outgrowing it.
Very-late-onset atopic dermatitis is often resistant to topical therapies; repeated courses of oral corticosteroids may be required.
The differential diagnosis is quite different than in children, where genetic immunodeficiency syndromes are a real concern. While CTCL is the biggie in the differential diagnosis of very-late-onset atopic dermatitis, other conditions that need to be considered include psoriasis, contact dermatitis, pityriasis rubra pilaris, and pityriasis rosea.
Dr. Bieber is a consultant to and recipient of research grants from numerous pharmaceutical companies having an interest in dermatology.
EXPERT ANALYSIS FROM WCD 2015
WCD: As MRSA Situation Worsens, Don’t Overlook Strep
VANCOUVER – Here’s a key message to bear in mind in the current era of methicillin-resistant Staphylococcus aureus: “Don’t forget streptococci,” Dr. Dirk M. Elston urged at the World Congress of Dermatology.
“Cellulitis is usually not staphylococcal, it’s streptococcal. Erysipelas, of course, is also streptococcal. And almost all impetigo probably begins as a streptococcal infection, even though the staphylococci outnumber the streptococci over time,” said Dr. Elston, managing director of the Ackerman Academy of Dermatopathology in New York.
The reason this message is important is because sulfa drugs are unreliable for the treatment of streptococcal infections.
“There is no indication for the treatment of active streptococcal disease with sulfa drugs,” the dermatologist said.
He cited a retrospective case-control study of 2,096 children with undrained, noncultured skin and soft tissue infections treated empirically on an outpatient basis with beta-lactams, clindamycin, or trimethoprim-sulfamethoxazole as monotherapy. The treatment failure rate was significantly higher in patients receiving trimethoprim-sulfamethoxazole. Clindamycin and beta-lactams were equally effective as first-line empiric therapy (Pediatrics 2009;123:e959-66).
Clindamycin has the added advantage of providing a two for one benefit: It’s effective against both MRSA and strep, Dr. Elston noted.
Necrotizing fasciitis is increasing in prevalence, and in a recent case series from Belfast, the most commonly isolated causative organism obtained from debrided tissue cultures was group A Streptococcus. The investigators found the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scoring system lacked diagnostic sensitivity; however, an elevated serum lactate at presentation had 90% diagnostic sensitivity and provided added value as a prognostic indicator (J. Plast. Reconstr. Aesthet. Surg. 2015;68:304-11).
“Necrotizing fasciitis often begins as a minor skin infection. Any patient with pain out of proportion to the clinical appearance or just the opposite – they look horrible and yet it’s relatively nontender – those are both potentially true medical emergencies,” Dr. Elston said.
It’s important to understand that streptococcal virulence is not a static phenomenon, he added.
“We’ve seen changes in strep virulence periodically. You’ll have waves of much more toxic strep. There was a wave a number of years ago in Missouri where there was a very high death rate among 19- and 20-year-olds. Right now Australia is seeing an epidemic of severe streptococcal disease, with sepsis and toxic shock,” according to the dermatologist.
Many of these Northern Australian cases begin with pyoderma, and many of those pyodermas occurred secondary to scabies (Trop. Med. Int. Health 2015;20:40-7).
Dr. Elston reported receiving research support from more than a dozen pharmaceutical companies and serving as a consultant to half a dozen.
VANCOUVER – Here’s a key message to bear in mind in the current era of methicillin-resistant Staphylococcus aureus: “Don’t forget streptococci,” Dr. Dirk M. Elston urged at the World Congress of Dermatology.
“Cellulitis is usually not staphylococcal, it’s streptococcal. Erysipelas, of course, is also streptococcal. And almost all impetigo probably begins as a streptococcal infection, even though the staphylococci outnumber the streptococci over time,” said Dr. Elston, managing director of the Ackerman Academy of Dermatopathology in New York.
The reason this message is important is because sulfa drugs are unreliable for the treatment of streptococcal infections.
“There is no indication for the treatment of active streptococcal disease with sulfa drugs,” the dermatologist said.
He cited a retrospective case-control study of 2,096 children with undrained, noncultured skin and soft tissue infections treated empirically on an outpatient basis with beta-lactams, clindamycin, or trimethoprim-sulfamethoxazole as monotherapy. The treatment failure rate was significantly higher in patients receiving trimethoprim-sulfamethoxazole. Clindamycin and beta-lactams were equally effective as first-line empiric therapy (Pediatrics 2009;123:e959-66).
Clindamycin has the added advantage of providing a two for one benefit: It’s effective against both MRSA and strep, Dr. Elston noted.
Necrotizing fasciitis is increasing in prevalence, and in a recent case series from Belfast, the most commonly isolated causative organism obtained from debrided tissue cultures was group A Streptococcus. The investigators found the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scoring system lacked diagnostic sensitivity; however, an elevated serum lactate at presentation had 90% diagnostic sensitivity and provided added value as a prognostic indicator (J. Plast. Reconstr. Aesthet. Surg. 2015;68:304-11).
“Necrotizing fasciitis often begins as a minor skin infection. Any patient with pain out of proportion to the clinical appearance or just the opposite – they look horrible and yet it’s relatively nontender – those are both potentially true medical emergencies,” Dr. Elston said.
It’s important to understand that streptococcal virulence is not a static phenomenon, he added.
“We’ve seen changes in strep virulence periodically. You’ll have waves of much more toxic strep. There was a wave a number of years ago in Missouri where there was a very high death rate among 19- and 20-year-olds. Right now Australia is seeing an epidemic of severe streptococcal disease, with sepsis and toxic shock,” according to the dermatologist.
Many of these Northern Australian cases begin with pyoderma, and many of those pyodermas occurred secondary to scabies (Trop. Med. Int. Health 2015;20:40-7).
Dr. Elston reported receiving research support from more than a dozen pharmaceutical companies and serving as a consultant to half a dozen.
VANCOUVER – Here’s a key message to bear in mind in the current era of methicillin-resistant Staphylococcus aureus: “Don’t forget streptococci,” Dr. Dirk M. Elston urged at the World Congress of Dermatology.
“Cellulitis is usually not staphylococcal, it’s streptococcal. Erysipelas, of course, is also streptococcal. And almost all impetigo probably begins as a streptococcal infection, even though the staphylococci outnumber the streptococci over time,” said Dr. Elston, managing director of the Ackerman Academy of Dermatopathology in New York.
The reason this message is important is because sulfa drugs are unreliable for the treatment of streptococcal infections.
“There is no indication for the treatment of active streptococcal disease with sulfa drugs,” the dermatologist said.
He cited a retrospective case-control study of 2,096 children with undrained, noncultured skin and soft tissue infections treated empirically on an outpatient basis with beta-lactams, clindamycin, or trimethoprim-sulfamethoxazole as monotherapy. The treatment failure rate was significantly higher in patients receiving trimethoprim-sulfamethoxazole. Clindamycin and beta-lactams were equally effective as first-line empiric therapy (Pediatrics 2009;123:e959-66).
Clindamycin has the added advantage of providing a two for one benefit: It’s effective against both MRSA and strep, Dr. Elston noted.
Necrotizing fasciitis is increasing in prevalence, and in a recent case series from Belfast, the most commonly isolated causative organism obtained from debrided tissue cultures was group A Streptococcus. The investigators found the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scoring system lacked diagnostic sensitivity; however, an elevated serum lactate at presentation had 90% diagnostic sensitivity and provided added value as a prognostic indicator (J. Plast. Reconstr. Aesthet. Surg. 2015;68:304-11).
“Necrotizing fasciitis often begins as a minor skin infection. Any patient with pain out of proportion to the clinical appearance or just the opposite – they look horrible and yet it’s relatively nontender – those are both potentially true medical emergencies,” Dr. Elston said.
It’s important to understand that streptococcal virulence is not a static phenomenon, he added.
“We’ve seen changes in strep virulence periodically. You’ll have waves of much more toxic strep. There was a wave a number of years ago in Missouri where there was a very high death rate among 19- and 20-year-olds. Right now Australia is seeing an epidemic of severe streptococcal disease, with sepsis and toxic shock,” according to the dermatologist.
Many of these Northern Australian cases begin with pyoderma, and many of those pyodermas occurred secondary to scabies (Trop. Med. Int. Health 2015;20:40-7).
Dr. Elston reported receiving research support from more than a dozen pharmaceutical companies and serving as a consultant to half a dozen.
EXPERT ANALYSIS FROM WCD 2015
WCD: As MRSA situation worsens, don’t overlook strep
VANCOUVER – Here’s a key message to bear in mind in the current era of methicillin-resistant Staphylococcus aureus: “Don’t forget streptococci,” Dr. Dirk M. Elston urged at the World Congress of Dermatology.
“Cellulitis is usually not staphylococcal, it’s streptococcal. Erysipelas, of course, is also streptococcal. And almost all impetigo probably begins as a streptococcal infection, even though the staphylococci outnumber the streptococci over time,” said Dr. Elston, managing director of the Ackerman Academy of Dermatopathology in New York.
The reason this message is important is because sulfa drugs are unreliable for the treatment of streptococcal infections.
“There is no indication for the treatment of active streptococcal disease with sulfa drugs,” the dermatologist said.
He cited a retrospective case-control study of 2,096 children with undrained, noncultured skin and soft tissue infections treated empirically on an outpatient basis with beta-lactams, clindamycin, or trimethoprim-sulfamethoxazole as monotherapy. The treatment failure rate was significantly higher in patients receiving trimethoprim-sulfamethoxazole. Clindamycin and beta-lactams were equally effective as first-line empiric therapy (Pediatrics 2009;123:e959-66).
Clindamycin has the added advantage of providing a two for one benefit: It’s effective against both MRSA and strep, Dr. Elston noted.
Necrotizing fasciitis is increasing in prevalence, and in a recent case series from Belfast, the most commonly isolated causative organism obtained from debrided tissue cultures was group A Streptococcus. The investigators found the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scoring system lacked diagnostic sensitivity; however, an elevated serum lactate at presentation had 90% diagnostic sensitivity and provided added value as a prognostic indicator (J. Plast. Reconstr. Aesthet. Surg. 2015;68:304-11).
“Necrotizing fasciitis often begins as a minor skin infection. Any patient with pain out of proportion to the clinical appearance or just the opposite – they look horrible and yet it’s relatively nontender – those are both potentially true medical emergencies,” Dr. Elston said.
It’s important to understand that streptococcal virulence is not a static phenomenon, he added.
“We’ve seen changes in strep virulence periodically. You’ll have waves of much more toxic strep. There was a wave a number of years ago in Missouri where there was a very high death rate among 19- and 20-year-olds. Right now Australia is seeing an epidemic of severe streptococcal disease, with sepsis and toxic shock,” according to the dermatologist.
Many of these Northern Australian cases begin with pyoderma, and many of those pyodermas occurred secondary to scabies (Trop. Med. Int. Health 2015;20:40-7).
Dr. Elston reported receiving research support from more than a dozen pharmaceutical companies and serving as a consultant to half a dozen.
VANCOUVER – Here’s a key message to bear in mind in the current era of methicillin-resistant Staphylococcus aureus: “Don’t forget streptococci,” Dr. Dirk M. Elston urged at the World Congress of Dermatology.
“Cellulitis is usually not staphylococcal, it’s streptococcal. Erysipelas, of course, is also streptococcal. And almost all impetigo probably begins as a streptococcal infection, even though the staphylococci outnumber the streptococci over time,” said Dr. Elston, managing director of the Ackerman Academy of Dermatopathology in New York.
The reason this message is important is because sulfa drugs are unreliable for the treatment of streptococcal infections.
“There is no indication for the treatment of active streptococcal disease with sulfa drugs,” the dermatologist said.
He cited a retrospective case-control study of 2,096 children with undrained, noncultured skin and soft tissue infections treated empirically on an outpatient basis with beta-lactams, clindamycin, or trimethoprim-sulfamethoxazole as monotherapy. The treatment failure rate was significantly higher in patients receiving trimethoprim-sulfamethoxazole. Clindamycin and beta-lactams were equally effective as first-line empiric therapy (Pediatrics 2009;123:e959-66).
Clindamycin has the added advantage of providing a two for one benefit: It’s effective against both MRSA and strep, Dr. Elston noted.
Necrotizing fasciitis is increasing in prevalence, and in a recent case series from Belfast, the most commonly isolated causative organism obtained from debrided tissue cultures was group A Streptococcus. The investigators found the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scoring system lacked diagnostic sensitivity; however, an elevated serum lactate at presentation had 90% diagnostic sensitivity and provided added value as a prognostic indicator (J. Plast. Reconstr. Aesthet. Surg. 2015;68:304-11).
“Necrotizing fasciitis often begins as a minor skin infection. Any patient with pain out of proportion to the clinical appearance or just the opposite – they look horrible and yet it’s relatively nontender – those are both potentially true medical emergencies,” Dr. Elston said.
It’s important to understand that streptococcal virulence is not a static phenomenon, he added.
“We’ve seen changes in strep virulence periodically. You’ll have waves of much more toxic strep. There was a wave a number of years ago in Missouri where there was a very high death rate among 19- and 20-year-olds. Right now Australia is seeing an epidemic of severe streptococcal disease, with sepsis and toxic shock,” according to the dermatologist.
Many of these Northern Australian cases begin with pyoderma, and many of those pyodermas occurred secondary to scabies (Trop. Med. Int. Health 2015;20:40-7).
Dr. Elston reported receiving research support from more than a dozen pharmaceutical companies and serving as a consultant to half a dozen.
VANCOUVER – Here’s a key message to bear in mind in the current era of methicillin-resistant Staphylococcus aureus: “Don’t forget streptococci,” Dr. Dirk M. Elston urged at the World Congress of Dermatology.
“Cellulitis is usually not staphylococcal, it’s streptococcal. Erysipelas, of course, is also streptococcal. And almost all impetigo probably begins as a streptococcal infection, even though the staphylococci outnumber the streptococci over time,” said Dr. Elston, managing director of the Ackerman Academy of Dermatopathology in New York.
The reason this message is important is because sulfa drugs are unreliable for the treatment of streptococcal infections.
“There is no indication for the treatment of active streptococcal disease with sulfa drugs,” the dermatologist said.
He cited a retrospective case-control study of 2,096 children with undrained, noncultured skin and soft tissue infections treated empirically on an outpatient basis with beta-lactams, clindamycin, or trimethoprim-sulfamethoxazole as monotherapy. The treatment failure rate was significantly higher in patients receiving trimethoprim-sulfamethoxazole. Clindamycin and beta-lactams were equally effective as first-line empiric therapy (Pediatrics 2009;123:e959-66).
Clindamycin has the added advantage of providing a two for one benefit: It’s effective against both MRSA and strep, Dr. Elston noted.
Necrotizing fasciitis is increasing in prevalence, and in a recent case series from Belfast, the most commonly isolated causative organism obtained from debrided tissue cultures was group A Streptococcus. The investigators found the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) scoring system lacked diagnostic sensitivity; however, an elevated serum lactate at presentation had 90% diagnostic sensitivity and provided added value as a prognostic indicator (J. Plast. Reconstr. Aesthet. Surg. 2015;68:304-11).
“Necrotizing fasciitis often begins as a minor skin infection. Any patient with pain out of proportion to the clinical appearance or just the opposite – they look horrible and yet it’s relatively nontender – those are both potentially true medical emergencies,” Dr. Elston said.
It’s important to understand that streptococcal virulence is not a static phenomenon, he added.
“We’ve seen changes in strep virulence periodically. You’ll have waves of much more toxic strep. There was a wave a number of years ago in Missouri where there was a very high death rate among 19- and 20-year-olds. Right now Australia is seeing an epidemic of severe streptococcal disease, with sepsis and toxic shock,” according to the dermatologist.
Many of these Northern Australian cases begin with pyoderma, and many of those pyodermas occurred secondary to scabies (Trop. Med. Int. Health 2015;20:40-7).
Dr. Elston reported receiving research support from more than a dozen pharmaceutical companies and serving as a consultant to half a dozen.
EXPERT ANALYSIS FROM WCD 2015
Real-time detection of aortic regurgitation during TAVI without TEE
PARIS – Real-time monitoring of von Willebrand factor during transcatheter aortic valve implantation shows promise as a means of detecting clinically significant aortic regurgitation, enabling interventionalists to make an on-the-spot fix and end up with a better result.
“We believe this is a nice way to expand the use of TAVI, to simplify the procedure, and to decrease the cost,” Dr. Eric Van Belle said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Clinically significant aortic regurgitation post-TAVI remains a major problem. It occurs in 10%-30% of patients who undergo TAVI to treat severe aortic regurgitation. It’s tough to identify and grade the severity of aortic regurgitation in the catheterization lab using echocardiography, angiography, and hemodynamic measurements, so the current standard is transesophageal echocardiography (TEE), typically performed under general anesthesia.
“It costs money for TEE under general anesthesia, and it lengthens the hospital stay,” observed Dr. Van Belle, professor of cardiology and head of the cardiac catheterization laboratory at Lille (France) University Hospital.
If this novel real-time monitoring of von Willebrand factor defects works out as well as it has in a new proof-of-concept study, it will essentially serve as a screening test for clinically significant aortic regurgitation. The need for TEE would then be limited to the minority of patients with evidence of moderate or severe aortic regurgitation on the screening test, he explained.
Dr. Van Belle and his coinvestigators have previously shown that patients with clinically significant aortic stenosis or valvular regurgitation exhibit high-molecular-weight multimers of von Willebrand factor defects and that these defects are corrected upon surgical valve replacement (Circ. Res. 2015;27:116:1193-201). Something that’s not widely known except among hematologists is that these defects can be monitored in real time by commercially available, bedside point-of-care platelet function analyzer closure time adenosine diphosphate(PFA-CADP) assays. Indeed, the cardiologist continued, hematologists use the PFA-CADP assay to screen for von Willebrand disease; a normal value is 114 seconds.
He presented a proof-of-concept study involving 88 consecutive patients who underwent TAVI with transfemoral delivery of the Sapien XT valve. All were monitored using the PFA-CADP assay, had their high-molecular-weight multimers of von Willebrand factor measured, and also underwent TEE to see how those definitive imaging results correlated with the assay findings.
The assay results were uniformly abnormal at baseline, prior to TAVI. The results normalized in most patients when PFA-CADP was measured 5 minutes after valve implantation, and TEE confirmed that patients with a normal assay result had only mild or no aortic regurgitation. Those with more than mild aortic regurgitation by TEE then underwent dilation with a larger balloon in an effort to correct the problem. Repeat PFA-CADP testing performed 5 minutes after the corrective procedure as well as TEE showed that the aortic regurgitation had been corrected.
One commentator said he’d really like to see evidence that the level of PFA-CADP reproducibly correlates with the degree of aortic regurgitation.
“If you could come up with a way of quantifying the degree of aortic regurgitation, that to me would be very, very appealing,” the cardiologist said.
Dr. Van Belle replied that “we’re relatively confident” that this is indeed the case, but an 88-patient study isn’t big enough to prove it. However, the study is ongoing, and with a planned 200 TAVI patients he says he believes it will be possible to demonstrate that the real-time assay discriminates between severe, moderate, and mild aortic regurgitation. Also, the investigators plan to evaluate the impact of PFA-CADP–guided interventions upon long-term clinical outcomes.
The study was funded by Lille University. Dr. Van Belle reported having no financial conflicts.
PARIS – Real-time monitoring of von Willebrand factor during transcatheter aortic valve implantation shows promise as a means of detecting clinically significant aortic regurgitation, enabling interventionalists to make an on-the-spot fix and end up with a better result.
“We believe this is a nice way to expand the use of TAVI, to simplify the procedure, and to decrease the cost,” Dr. Eric Van Belle said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Clinically significant aortic regurgitation post-TAVI remains a major problem. It occurs in 10%-30% of patients who undergo TAVI to treat severe aortic regurgitation. It’s tough to identify and grade the severity of aortic regurgitation in the catheterization lab using echocardiography, angiography, and hemodynamic measurements, so the current standard is transesophageal echocardiography (TEE), typically performed under general anesthesia.
“It costs money for TEE under general anesthesia, and it lengthens the hospital stay,” observed Dr. Van Belle, professor of cardiology and head of the cardiac catheterization laboratory at Lille (France) University Hospital.
If this novel real-time monitoring of von Willebrand factor defects works out as well as it has in a new proof-of-concept study, it will essentially serve as a screening test for clinically significant aortic regurgitation. The need for TEE would then be limited to the minority of patients with evidence of moderate or severe aortic regurgitation on the screening test, he explained.
Dr. Van Belle and his coinvestigators have previously shown that patients with clinically significant aortic stenosis or valvular regurgitation exhibit high-molecular-weight multimers of von Willebrand factor defects and that these defects are corrected upon surgical valve replacement (Circ. Res. 2015;27:116:1193-201). Something that’s not widely known except among hematologists is that these defects can be monitored in real time by commercially available, bedside point-of-care platelet function analyzer closure time adenosine diphosphate(PFA-CADP) assays. Indeed, the cardiologist continued, hematologists use the PFA-CADP assay to screen for von Willebrand disease; a normal value is 114 seconds.
He presented a proof-of-concept study involving 88 consecutive patients who underwent TAVI with transfemoral delivery of the Sapien XT valve. All were monitored using the PFA-CADP assay, had their high-molecular-weight multimers of von Willebrand factor measured, and also underwent TEE to see how those definitive imaging results correlated with the assay findings.
The assay results were uniformly abnormal at baseline, prior to TAVI. The results normalized in most patients when PFA-CADP was measured 5 minutes after valve implantation, and TEE confirmed that patients with a normal assay result had only mild or no aortic regurgitation. Those with more than mild aortic regurgitation by TEE then underwent dilation with a larger balloon in an effort to correct the problem. Repeat PFA-CADP testing performed 5 minutes after the corrective procedure as well as TEE showed that the aortic regurgitation had been corrected.
One commentator said he’d really like to see evidence that the level of PFA-CADP reproducibly correlates with the degree of aortic regurgitation.
“If you could come up with a way of quantifying the degree of aortic regurgitation, that to me would be very, very appealing,” the cardiologist said.
Dr. Van Belle replied that “we’re relatively confident” that this is indeed the case, but an 88-patient study isn’t big enough to prove it. However, the study is ongoing, and with a planned 200 TAVI patients he says he believes it will be possible to demonstrate that the real-time assay discriminates between severe, moderate, and mild aortic regurgitation. Also, the investigators plan to evaluate the impact of PFA-CADP–guided interventions upon long-term clinical outcomes.
The study was funded by Lille University. Dr. Van Belle reported having no financial conflicts.
PARIS – Real-time monitoring of von Willebrand factor during transcatheter aortic valve implantation shows promise as a means of detecting clinically significant aortic regurgitation, enabling interventionalists to make an on-the-spot fix and end up with a better result.
“We believe this is a nice way to expand the use of TAVI, to simplify the procedure, and to decrease the cost,” Dr. Eric Van Belle said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Clinically significant aortic regurgitation post-TAVI remains a major problem. It occurs in 10%-30% of patients who undergo TAVI to treat severe aortic regurgitation. It’s tough to identify and grade the severity of aortic regurgitation in the catheterization lab using echocardiography, angiography, and hemodynamic measurements, so the current standard is transesophageal echocardiography (TEE), typically performed under general anesthesia.
“It costs money for TEE under general anesthesia, and it lengthens the hospital stay,” observed Dr. Van Belle, professor of cardiology and head of the cardiac catheterization laboratory at Lille (France) University Hospital.
If this novel real-time monitoring of von Willebrand factor defects works out as well as it has in a new proof-of-concept study, it will essentially serve as a screening test for clinically significant aortic regurgitation. The need for TEE would then be limited to the minority of patients with evidence of moderate or severe aortic regurgitation on the screening test, he explained.
Dr. Van Belle and his coinvestigators have previously shown that patients with clinically significant aortic stenosis or valvular regurgitation exhibit high-molecular-weight multimers of von Willebrand factor defects and that these defects are corrected upon surgical valve replacement (Circ. Res. 2015;27:116:1193-201). Something that’s not widely known except among hematologists is that these defects can be monitored in real time by commercially available, bedside point-of-care platelet function analyzer closure time adenosine diphosphate(PFA-CADP) assays. Indeed, the cardiologist continued, hematologists use the PFA-CADP assay to screen for von Willebrand disease; a normal value is 114 seconds.
He presented a proof-of-concept study involving 88 consecutive patients who underwent TAVI with transfemoral delivery of the Sapien XT valve. All were monitored using the PFA-CADP assay, had their high-molecular-weight multimers of von Willebrand factor measured, and also underwent TEE to see how those definitive imaging results correlated with the assay findings.
The assay results were uniformly abnormal at baseline, prior to TAVI. The results normalized in most patients when PFA-CADP was measured 5 minutes after valve implantation, and TEE confirmed that patients with a normal assay result had only mild or no aortic regurgitation. Those with more than mild aortic regurgitation by TEE then underwent dilation with a larger balloon in an effort to correct the problem. Repeat PFA-CADP testing performed 5 minutes after the corrective procedure as well as TEE showed that the aortic regurgitation had been corrected.
One commentator said he’d really like to see evidence that the level of PFA-CADP reproducibly correlates with the degree of aortic regurgitation.
“If you could come up with a way of quantifying the degree of aortic regurgitation, that to me would be very, very appealing,” the cardiologist said.
Dr. Van Belle replied that “we’re relatively confident” that this is indeed the case, but an 88-patient study isn’t big enough to prove it. However, the study is ongoing, and with a planned 200 TAVI patients he says he believes it will be possible to demonstrate that the real-time assay discriminates between severe, moderate, and mild aortic regurgitation. Also, the investigators plan to evaluate the impact of PFA-CADP–guided interventions upon long-term clinical outcomes.
The study was funded by Lille University. Dr. Van Belle reported having no financial conflicts.
AT EUROPCR
Key clinical point: A real-time assay for defects in von Willebrand factor may enable most patients undergoing transcatheter aortic valve replacement to avoid transesophageal echocardiography under general anesthesia in order to assess for clinically significant aortic regurgitation following valve replacement.
Major finding: An assay for PFA-CADP identifies patients who have more than mild aortic regurgitation immediately after valve implantation, while they are still in the cath lab and can readily undergo dilation with a larger balloon in order to fix the problem.
Data source: This ongoing study includes to date 88 consecutive patients undergoing transcatheter aortic valve implantation via the transfemoral route.
Disclosures: This study is sponsored by Lille (France) University. The presenter reported having no financial conflicts.
Reports of TAVI leaflet thickening downplayed – for now
PARIS – Thought leaders in interventional cardiology have been quick to throw cold water on recent reports of valve leaflet thickening and abnormal leaflet motion being detected in roughly 10% of patients after transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
“The take home message for the interventional community is there is no need for clinicians to modify their practice in relation to patient selection, TAVI implantation, or follow-up protocols. Specifically, there is no role for systematic CT or transesophageal echocardiographic follow-up of asymptomatic TAVI patients because they’re not at clinical risk, and these additional procedures carry risk in themselves,” Dr. Bernard Prendergast said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Whilst there is room here for speculation and conjecture, I think most of us are confident that some of these findings may represent imaging artifact or reflect the natural history of biological valve leaflets, which has never been examined in such detail in the past,” added Dr. Prendergast, director of the cardiac structural intervention program at Guys and St. Thomas’ Hospital in London and cochair of the special EuroPCR session devoted to the emerging data on valve leaflet abnormalities.
The session featured three separate studies totaling 345 patients who underwent sophisticated, high-resolution 4D CT imaging or transesophageal echocardiography 5 days or more following TAVI or, less frequently, after surgical aortic valve replacement. Roughly 10% of patients showed a spectrum of leaflet abnormalities: thickening, mildly impaired motion, and/or thin films believed to be thrombi.
The leaflet abnormalities weren’t associated with any particular valve. And, as was emphasized by Dr. Prendergast and other speakers, to date these abnormalities haven’t been associated with a single case of stroke, systemic embolism, or valve failure.
Indeed, more than 100,000 TAVI procedures have been performed worldwide, and stroke rates in contemporary randomized trials and large registries are in the 1%-2% range. That’s better than with surgical valve replacement, Dr. Prendergast observed.
“Nowadays we can see much more than we could in the past, when we worked with 2D echocardiography,” observed discussant Dr. Jeroen J. Bax, professor of cardiology and director of noninvasive cardiology imaging at Leiden (the Netherlands) University Medical Center. “We see things that we do not completely understand. We could say that technology has outpaced our clinical understanding. But although we see things, at the moment there is no consequence in terms of hemodynamic performance or clinical outcomes. And this phenomenon of leaflet thickening has been occurring with surgical aortic valve replacement for many, many years and we simply didn’t realize it.”
Dr. Franz-Josef Neumann, who led one of the three studies, reported that 4D CT on day 5 post TAVI revealed leaflet abnormalities, all completely asymptomatic, in 16 of 154 patients. Two-thirds of the study population were on dual antiplatelet therapy at the time, the rest on a single antiplatelet agent. Dual antiplatelet therapy didn’t protect against leaflet thickening or other abnormalities.
All 16 affected patients were placed on an oral vitamin K antagonist with a target international normalized ratio (INR) of 2.5-3.5. To date, 11 of the 16 have undergone follow-up high-resolution CT after a median of 77 days. The leaflet thickening was resolved in all instances, according to Dr. Neumann, medical director of the department of cardiology and angiology at the University of Freiburg (Germany).
Dr. Raj R. Makkar presented a study of 125 patients who underwent high-resolution imaging after TAVI. Importantly, none of those who were on warfarin as part of their post-TAVI regimen developed leaflet abnormalities.
But he cautioned his colleagues against overreacting to the studies he and Dr. Neumann presented by placing all of their TAVI patients on an oral anticoagulant. He noted that the current TAVI population is elderly and laden with many comorbid conditions, placing them at high risk for bleeding complications.
There is at present no standard, guideline-recommended antiplatelet/antithrombotic regimen for before, during, or after TAVI. Working out the optimal protective drug regimen in this population is now a priority in light of the leaflet abnormality findings, but it will take time and require careful study, said Dr. Makkar, associate director of the Cedars Sinai Heart Institute in Los Angeles.
“Everyone is talking about anticoagulation as the imminent solution. But I want to emphasize that it comes with a price in terms of bleeding. These images are beautiful in terms of spatial resolution, but we must resist our temptation while the industry works on designing less thrombogenic valves,” the cardiologist added.
Discussant Dr. Christoph K. Naber confessed he was “stunned” by the images of leaflet abnormalities.
“Although we haven’t seen any clinical consequences, we have to keep in mind that the group of patients is still small. We have very good experience with TAVI; it has saved the lives of many patients. We know it’s a very good therapy. But if we believe we can go further and offer it to younger, lower-risk patients who will have their device for a longer time, then we should take the time and money to understand what is going on here and what consequences it could have. It’s something we should closely watch, especially if we want to extend the indication,” said Dr. Naber, director of the department of cardiology and angiology at the Contilia Cardiovascular Center in Essen, Germany.
He disclosed that he serves as a consultant to Abbott Vascular, Biotronik, Medtronic, and The Medicines Company. Dr. Makkar has received research grants from Edwards Lifesciences, St. Jude Medical, and Boston Scientific. Dr. Prendergast is on the speakers’ bureau for Edwards Lifesciences.
PARIS – Thought leaders in interventional cardiology have been quick to throw cold water on recent reports of valve leaflet thickening and abnormal leaflet motion being detected in roughly 10% of patients after transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
“The take home message for the interventional community is there is no need for clinicians to modify their practice in relation to patient selection, TAVI implantation, or follow-up protocols. Specifically, there is no role for systematic CT or transesophageal echocardiographic follow-up of asymptomatic TAVI patients because they’re not at clinical risk, and these additional procedures carry risk in themselves,” Dr. Bernard Prendergast said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Whilst there is room here for speculation and conjecture, I think most of us are confident that some of these findings may represent imaging artifact or reflect the natural history of biological valve leaflets, which has never been examined in such detail in the past,” added Dr. Prendergast, director of the cardiac structural intervention program at Guys and St. Thomas’ Hospital in London and cochair of the special EuroPCR session devoted to the emerging data on valve leaflet abnormalities.
The session featured three separate studies totaling 345 patients who underwent sophisticated, high-resolution 4D CT imaging or transesophageal echocardiography 5 days or more following TAVI or, less frequently, after surgical aortic valve replacement. Roughly 10% of patients showed a spectrum of leaflet abnormalities: thickening, mildly impaired motion, and/or thin films believed to be thrombi.
The leaflet abnormalities weren’t associated with any particular valve. And, as was emphasized by Dr. Prendergast and other speakers, to date these abnormalities haven’t been associated with a single case of stroke, systemic embolism, or valve failure.
Indeed, more than 100,000 TAVI procedures have been performed worldwide, and stroke rates in contemporary randomized trials and large registries are in the 1%-2% range. That’s better than with surgical valve replacement, Dr. Prendergast observed.
“Nowadays we can see much more than we could in the past, when we worked with 2D echocardiography,” observed discussant Dr. Jeroen J. Bax, professor of cardiology and director of noninvasive cardiology imaging at Leiden (the Netherlands) University Medical Center. “We see things that we do not completely understand. We could say that technology has outpaced our clinical understanding. But although we see things, at the moment there is no consequence in terms of hemodynamic performance or clinical outcomes. And this phenomenon of leaflet thickening has been occurring with surgical aortic valve replacement for many, many years and we simply didn’t realize it.”
Dr. Franz-Josef Neumann, who led one of the three studies, reported that 4D CT on day 5 post TAVI revealed leaflet abnormalities, all completely asymptomatic, in 16 of 154 patients. Two-thirds of the study population were on dual antiplatelet therapy at the time, the rest on a single antiplatelet agent. Dual antiplatelet therapy didn’t protect against leaflet thickening or other abnormalities.
All 16 affected patients were placed on an oral vitamin K antagonist with a target international normalized ratio (INR) of 2.5-3.5. To date, 11 of the 16 have undergone follow-up high-resolution CT after a median of 77 days. The leaflet thickening was resolved in all instances, according to Dr. Neumann, medical director of the department of cardiology and angiology at the University of Freiburg (Germany).
Dr. Raj R. Makkar presented a study of 125 patients who underwent high-resolution imaging after TAVI. Importantly, none of those who were on warfarin as part of their post-TAVI regimen developed leaflet abnormalities.
But he cautioned his colleagues against overreacting to the studies he and Dr. Neumann presented by placing all of their TAVI patients on an oral anticoagulant. He noted that the current TAVI population is elderly and laden with many comorbid conditions, placing them at high risk for bleeding complications.
There is at present no standard, guideline-recommended antiplatelet/antithrombotic regimen for before, during, or after TAVI. Working out the optimal protective drug regimen in this population is now a priority in light of the leaflet abnormality findings, but it will take time and require careful study, said Dr. Makkar, associate director of the Cedars Sinai Heart Institute in Los Angeles.
“Everyone is talking about anticoagulation as the imminent solution. But I want to emphasize that it comes with a price in terms of bleeding. These images are beautiful in terms of spatial resolution, but we must resist our temptation while the industry works on designing less thrombogenic valves,” the cardiologist added.
Discussant Dr. Christoph K. Naber confessed he was “stunned” by the images of leaflet abnormalities.
“Although we haven’t seen any clinical consequences, we have to keep in mind that the group of patients is still small. We have very good experience with TAVI; it has saved the lives of many patients. We know it’s a very good therapy. But if we believe we can go further and offer it to younger, lower-risk patients who will have their device for a longer time, then we should take the time and money to understand what is going on here and what consequences it could have. It’s something we should closely watch, especially if we want to extend the indication,” said Dr. Naber, director of the department of cardiology and angiology at the Contilia Cardiovascular Center in Essen, Germany.
He disclosed that he serves as a consultant to Abbott Vascular, Biotronik, Medtronic, and The Medicines Company. Dr. Makkar has received research grants from Edwards Lifesciences, St. Jude Medical, and Boston Scientific. Dr. Prendergast is on the speakers’ bureau for Edwards Lifesciences.
PARIS – Thought leaders in interventional cardiology have been quick to throw cold water on recent reports of valve leaflet thickening and abnormal leaflet motion being detected in roughly 10% of patients after transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
“The take home message for the interventional community is there is no need for clinicians to modify their practice in relation to patient selection, TAVI implantation, or follow-up protocols. Specifically, there is no role for systematic CT or transesophageal echocardiographic follow-up of asymptomatic TAVI patients because they’re not at clinical risk, and these additional procedures carry risk in themselves,” Dr. Bernard Prendergast said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Whilst there is room here for speculation and conjecture, I think most of us are confident that some of these findings may represent imaging artifact or reflect the natural history of biological valve leaflets, which has never been examined in such detail in the past,” added Dr. Prendergast, director of the cardiac structural intervention program at Guys and St. Thomas’ Hospital in London and cochair of the special EuroPCR session devoted to the emerging data on valve leaflet abnormalities.
The session featured three separate studies totaling 345 patients who underwent sophisticated, high-resolution 4D CT imaging or transesophageal echocardiography 5 days or more following TAVI or, less frequently, after surgical aortic valve replacement. Roughly 10% of patients showed a spectrum of leaflet abnormalities: thickening, mildly impaired motion, and/or thin films believed to be thrombi.
The leaflet abnormalities weren’t associated with any particular valve. And, as was emphasized by Dr. Prendergast and other speakers, to date these abnormalities haven’t been associated with a single case of stroke, systemic embolism, or valve failure.
Indeed, more than 100,000 TAVI procedures have been performed worldwide, and stroke rates in contemporary randomized trials and large registries are in the 1%-2% range. That’s better than with surgical valve replacement, Dr. Prendergast observed.
“Nowadays we can see much more than we could in the past, when we worked with 2D echocardiography,” observed discussant Dr. Jeroen J. Bax, professor of cardiology and director of noninvasive cardiology imaging at Leiden (the Netherlands) University Medical Center. “We see things that we do not completely understand. We could say that technology has outpaced our clinical understanding. But although we see things, at the moment there is no consequence in terms of hemodynamic performance or clinical outcomes. And this phenomenon of leaflet thickening has been occurring with surgical aortic valve replacement for many, many years and we simply didn’t realize it.”
Dr. Franz-Josef Neumann, who led one of the three studies, reported that 4D CT on day 5 post TAVI revealed leaflet abnormalities, all completely asymptomatic, in 16 of 154 patients. Two-thirds of the study population were on dual antiplatelet therapy at the time, the rest on a single antiplatelet agent. Dual antiplatelet therapy didn’t protect against leaflet thickening or other abnormalities.
All 16 affected patients were placed on an oral vitamin K antagonist with a target international normalized ratio (INR) of 2.5-3.5. To date, 11 of the 16 have undergone follow-up high-resolution CT after a median of 77 days. The leaflet thickening was resolved in all instances, according to Dr. Neumann, medical director of the department of cardiology and angiology at the University of Freiburg (Germany).
Dr. Raj R. Makkar presented a study of 125 patients who underwent high-resolution imaging after TAVI. Importantly, none of those who were on warfarin as part of their post-TAVI regimen developed leaflet abnormalities.
But he cautioned his colleagues against overreacting to the studies he and Dr. Neumann presented by placing all of their TAVI patients on an oral anticoagulant. He noted that the current TAVI population is elderly and laden with many comorbid conditions, placing them at high risk for bleeding complications.
There is at present no standard, guideline-recommended antiplatelet/antithrombotic regimen for before, during, or after TAVI. Working out the optimal protective drug regimen in this population is now a priority in light of the leaflet abnormality findings, but it will take time and require careful study, said Dr. Makkar, associate director of the Cedars Sinai Heart Institute in Los Angeles.
“Everyone is talking about anticoagulation as the imminent solution. But I want to emphasize that it comes with a price in terms of bleeding. These images are beautiful in terms of spatial resolution, but we must resist our temptation while the industry works on designing less thrombogenic valves,” the cardiologist added.
Discussant Dr. Christoph K. Naber confessed he was “stunned” by the images of leaflet abnormalities.
“Although we haven’t seen any clinical consequences, we have to keep in mind that the group of patients is still small. We have very good experience with TAVI; it has saved the lives of many patients. We know it’s a very good therapy. But if we believe we can go further and offer it to younger, lower-risk patients who will have their device for a longer time, then we should take the time and money to understand what is going on here and what consequences it could have. It’s something we should closely watch, especially if we want to extend the indication,” said Dr. Naber, director of the department of cardiology and angiology at the Contilia Cardiovascular Center in Essen, Germany.
He disclosed that he serves as a consultant to Abbott Vascular, Biotronik, Medtronic, and The Medicines Company. Dr. Makkar has received research grants from Edwards Lifesciences, St. Jude Medical, and Boston Scientific. Dr. Prendergast is on the speakers’ bureau for Edwards Lifesciences.
EXPERT ANALYSIS FROM EUROPCR
Naloxone lotion improves disabling itch in CTCL
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
AT WCD 2015
Key clinical point: Naloxone lotion shows promise for the severe pruritis that accompanies cutaneous T-cell lymphoma.
Major finding: Patients with cutaneous T-cell lymphoma reported an absolute 21% greater reduction in pruritis with naloxone lotion than with its vehicle.
Data source: This was a 15-patient, multicenter, double-blind, crossover study.
Disclosures: The study was funded by Elorac. The presenter reported having no financial conflicts.
July 2015: Click for Credit
Here are 7 articles in the July issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. BSR: Multiple Benefits Seen With Intensive Psoriatic Arthritis Therapy
Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis (PsA) until they achieve a set of minimal disease activity (MDA) criteria (see Table), an expert said at the British Society for Rheumatology annual conference.
To take the posttest, go to: http://bit.ly/1KaikxW
2. Subclinical Hyperthyroidism Linked to Higher Fracture Risk
Individuals with subclinical hyperthyroidism are at increased risk for hip and other fractures, according to the authors of a meta-analysis. The researchers examined data from 70,298 individuals—4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism—enrolled in 13 prospective cohort studies.
To take the posttest, go to: http://bit.ly/1H13j0t
3. Newer Oral Contraceptives Pose Higher VTE Risk
The risk for venous thromboembolism (VTE) is generally greater for women using oral contraceptives with newer types of progestogen hormones than for those taking older, second-generation birth control pills, study results showed.
To take the posttest, go to: http://bit.ly/1AKQert
4. Statins, Fibrates Lower Stroke Risk in Elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased risk for stroke by 30% in a community-dwelling population of elderly people, according to a prospective European study published online in the British Medical Journal.
To take the posttest, go to: http://bit.ly/1FuyYCb
5. Cystic Fibrosis–related Diabetes Requires Different Approach
Cystic fibrosis–related diabetes (CFRD) is a unique disease that requires a different mindset on the part of the treating clinician.
To take the posttest, go to: http://bit.ly/1BKGZCm
6. CVD Risk Persists for 40 Years in Hodgkin Survivors
People who survive Hodgkin lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease (CVD) for at least 40 years—the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
To take the posttest, go to: http://bit.ly/1M5ymYG
7. Asymptomatic Carotid Stenosis and Central Sleep Apnea Linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea, according to an observational study. The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea: 42% had obstructive sleep apnea (OSA) and 27%, central sleep apnea (CSA).
To take the posttest, go to: http://bit.ly/1SWGPmb
Here are 7 articles in the July issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. BSR: Multiple Benefits Seen With Intensive Psoriatic Arthritis Therapy
Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis (PsA) until they achieve a set of minimal disease activity (MDA) criteria (see Table), an expert said at the British Society for Rheumatology annual conference.
To take the posttest, go to: http://bit.ly/1KaikxW
2. Subclinical Hyperthyroidism Linked to Higher Fracture Risk
Individuals with subclinical hyperthyroidism are at increased risk for hip and other fractures, according to the authors of a meta-analysis. The researchers examined data from 70,298 individuals—4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism—enrolled in 13 prospective cohort studies.
To take the posttest, go to: http://bit.ly/1H13j0t
3. Newer Oral Contraceptives Pose Higher VTE Risk
The risk for venous thromboembolism (VTE) is generally greater for women using oral contraceptives with newer types of progestogen hormones than for those taking older, second-generation birth control pills, study results showed.
To take the posttest, go to: http://bit.ly/1AKQert
4. Statins, Fibrates Lower Stroke Risk in Elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased risk for stroke by 30% in a community-dwelling population of elderly people, according to a prospective European study published online in the British Medical Journal.
To take the posttest, go to: http://bit.ly/1FuyYCb
5. Cystic Fibrosis–related Diabetes Requires Different Approach
Cystic fibrosis–related diabetes (CFRD) is a unique disease that requires a different mindset on the part of the treating clinician.
To take the posttest, go to: http://bit.ly/1BKGZCm
6. CVD Risk Persists for 40 Years in Hodgkin Survivors
People who survive Hodgkin lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease (CVD) for at least 40 years—the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
To take the posttest, go to: http://bit.ly/1M5ymYG
7. Asymptomatic Carotid Stenosis and Central Sleep Apnea Linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea, according to an observational study. The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea: 42% had obstructive sleep apnea (OSA) and 27%, central sleep apnea (CSA).
To take the posttest, go to: http://bit.ly/1SWGPmb
Here are 7 articles in the July issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. BSR: Multiple Benefits Seen With Intensive Psoriatic Arthritis Therapy
Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis (PsA) until they achieve a set of minimal disease activity (MDA) criteria (see Table), an expert said at the British Society for Rheumatology annual conference.
To take the posttest, go to: http://bit.ly/1KaikxW
2. Subclinical Hyperthyroidism Linked to Higher Fracture Risk
Individuals with subclinical hyperthyroidism are at increased risk for hip and other fractures, according to the authors of a meta-analysis. The researchers examined data from 70,298 individuals—4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism—enrolled in 13 prospective cohort studies.
To take the posttest, go to: http://bit.ly/1H13j0t
3. Newer Oral Contraceptives Pose Higher VTE Risk
The risk for venous thromboembolism (VTE) is generally greater for women using oral contraceptives with newer types of progestogen hormones than for those taking older, second-generation birth control pills, study results showed.
To take the posttest, go to: http://bit.ly/1AKQert
4. Statins, Fibrates Lower Stroke Risk in Elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased risk for stroke by 30% in a community-dwelling population of elderly people, according to a prospective European study published online in the British Medical Journal.
To take the posttest, go to: http://bit.ly/1FuyYCb
5. Cystic Fibrosis–related Diabetes Requires Different Approach
Cystic fibrosis–related diabetes (CFRD) is a unique disease that requires a different mindset on the part of the treating clinician.
To take the posttest, go to: http://bit.ly/1BKGZCm
6. CVD Risk Persists for 40 Years in Hodgkin Survivors
People who survive Hodgkin lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease (CVD) for at least 40 years—the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
To take the posttest, go to: http://bit.ly/1M5ymYG
7. Asymptomatic Carotid Stenosis and Central Sleep Apnea Linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea, according to an observational study. The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea: 42% had obstructive sleep apnea (OSA) and 27%, central sleep apnea (CSA).
To take the posttest, go to: http://bit.ly/1SWGPmb
WCD: Secukinumab shows effectiveness for nail, palmoplantar psoriasis
VANCOUVER, B.C. – The interleukin-17A inhibitor secukinumab demonstrated the greatest improvement in nail psoriasis ever reported from a randomized, placebo-controlled trial in the phase IIIb TRANSFIGURE study, Dr. Kristian Reich reported at the World Congress of Dermatology.
At 198 patients, TRANSFIGURE is the largest-ever prospective study in patients with moderate to severe chronic plaque psoriasis and significant nail involvement. And while only the 16-week results are available thus far, when TRANSFIGURE is completed after a planned 132 weeks of treatment, it will also be the longest-ever study in the treatment of nail psoriasis, noted Dr. Reich, a dermatologist in group practice in Hamburg, Germany.
Elsewhere at WCD 2015, Dr. Alice B. Gottlieb presented the week 16 results of the phase IIIb GESTURE study, in which 205 psoriasis patients with moderate to severe psoriasis of the palms and soles were randomized to subcutaneous secukinumab (Cosentyx) at 150 or 300 mg or placebo. Dosing was weekly for the first 5 weeks and monthly thereafter.
The primary endpoint, a palmoplantar Investigator’s Global Assessment scale score of 0 or 1 – clear or almost clear – at week 16 was 33.3% with secukinumab at 300 mg, 22.1% at 150 mg, and 1.5% with placebo. The average reduction in palmoplantar PASI (Psoriasis Area Severity Index) score from baseline was 54.6% with high-dose and 35.3% with low-dose secukinumab, compared with 4.1% in placebo-treated controls, reported Dr. Gottlieb, professor and chair of dermatology at Tufts University, Boston.
Like the TRANSFIGURE trial, GESTURE will continue for 132 weeks, with the initial placebo-treated controls being randomized to secukinumab at 150 or 300 mg after week 16.
Dr. Reich reported that by 16 weeks in TRANSFIGURE, mean scores on the Nail Psoriasis Severity Index had improved by 45.3%, compared with baseline, in patients on secukinumab 300 mg, 37.9% in those on secukinumab 150 mg, and 10.8% with placebo.
The results on the skin were dramatic: a PASI 75 rate of 87.1% with secukinumab 300 mg, 77% with secukinumab 150 mg, and 5.1% with placebo. The PASI 100 response rate – meaning totally clear skin – was 31.9% with high-dose and 25.2% with lower-dose secukinumab, while there was a zero PASI 100 rate in controls.
The only adverse events more common than with placebo were nasopharyngitis and upper respiratory infections.
Dr. Reich predicted that as the ongoing TRANSFIGURE study continues well beyond 16 weeks, the nail psoriasis response rates will climb, since nails are so slow growing.
TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
VANCOUVER, B.C. – The interleukin-17A inhibitor secukinumab demonstrated the greatest improvement in nail psoriasis ever reported from a randomized, placebo-controlled trial in the phase IIIb TRANSFIGURE study, Dr. Kristian Reich reported at the World Congress of Dermatology.
At 198 patients, TRANSFIGURE is the largest-ever prospective study in patients with moderate to severe chronic plaque psoriasis and significant nail involvement. And while only the 16-week results are available thus far, when TRANSFIGURE is completed after a planned 132 weeks of treatment, it will also be the longest-ever study in the treatment of nail psoriasis, noted Dr. Reich, a dermatologist in group practice in Hamburg, Germany.
Elsewhere at WCD 2015, Dr. Alice B. Gottlieb presented the week 16 results of the phase IIIb GESTURE study, in which 205 psoriasis patients with moderate to severe psoriasis of the palms and soles were randomized to subcutaneous secukinumab (Cosentyx) at 150 or 300 mg or placebo. Dosing was weekly for the first 5 weeks and monthly thereafter.
The primary endpoint, a palmoplantar Investigator’s Global Assessment scale score of 0 or 1 – clear or almost clear – at week 16 was 33.3% with secukinumab at 300 mg, 22.1% at 150 mg, and 1.5% with placebo. The average reduction in palmoplantar PASI (Psoriasis Area Severity Index) score from baseline was 54.6% with high-dose and 35.3% with low-dose secukinumab, compared with 4.1% in placebo-treated controls, reported Dr. Gottlieb, professor and chair of dermatology at Tufts University, Boston.
Like the TRANSFIGURE trial, GESTURE will continue for 132 weeks, with the initial placebo-treated controls being randomized to secukinumab at 150 or 300 mg after week 16.
Dr. Reich reported that by 16 weeks in TRANSFIGURE, mean scores on the Nail Psoriasis Severity Index had improved by 45.3%, compared with baseline, in patients on secukinumab 300 mg, 37.9% in those on secukinumab 150 mg, and 10.8% with placebo.
The results on the skin were dramatic: a PASI 75 rate of 87.1% with secukinumab 300 mg, 77% with secukinumab 150 mg, and 5.1% with placebo. The PASI 100 response rate – meaning totally clear skin – was 31.9% with high-dose and 25.2% with lower-dose secukinumab, while there was a zero PASI 100 rate in controls.
The only adverse events more common than with placebo were nasopharyngitis and upper respiratory infections.
Dr. Reich predicted that as the ongoing TRANSFIGURE study continues well beyond 16 weeks, the nail psoriasis response rates will climb, since nails are so slow growing.
TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
VANCOUVER, B.C. – The interleukin-17A inhibitor secukinumab demonstrated the greatest improvement in nail psoriasis ever reported from a randomized, placebo-controlled trial in the phase IIIb TRANSFIGURE study, Dr. Kristian Reich reported at the World Congress of Dermatology.
At 198 patients, TRANSFIGURE is the largest-ever prospective study in patients with moderate to severe chronic plaque psoriasis and significant nail involvement. And while only the 16-week results are available thus far, when TRANSFIGURE is completed after a planned 132 weeks of treatment, it will also be the longest-ever study in the treatment of nail psoriasis, noted Dr. Reich, a dermatologist in group practice in Hamburg, Germany.
Elsewhere at WCD 2015, Dr. Alice B. Gottlieb presented the week 16 results of the phase IIIb GESTURE study, in which 205 psoriasis patients with moderate to severe psoriasis of the palms and soles were randomized to subcutaneous secukinumab (Cosentyx) at 150 or 300 mg or placebo. Dosing was weekly for the first 5 weeks and monthly thereafter.
The primary endpoint, a palmoplantar Investigator’s Global Assessment scale score of 0 or 1 – clear or almost clear – at week 16 was 33.3% with secukinumab at 300 mg, 22.1% at 150 mg, and 1.5% with placebo. The average reduction in palmoplantar PASI (Psoriasis Area Severity Index) score from baseline was 54.6% with high-dose and 35.3% with low-dose secukinumab, compared with 4.1% in placebo-treated controls, reported Dr. Gottlieb, professor and chair of dermatology at Tufts University, Boston.
Like the TRANSFIGURE trial, GESTURE will continue for 132 weeks, with the initial placebo-treated controls being randomized to secukinumab at 150 or 300 mg after week 16.
Dr. Reich reported that by 16 weeks in TRANSFIGURE, mean scores on the Nail Psoriasis Severity Index had improved by 45.3%, compared with baseline, in patients on secukinumab 300 mg, 37.9% in those on secukinumab 150 mg, and 10.8% with placebo.
The results on the skin were dramatic: a PASI 75 rate of 87.1% with secukinumab 300 mg, 77% with secukinumab 150 mg, and 5.1% with placebo. The PASI 100 response rate – meaning totally clear skin – was 31.9% with high-dose and 25.2% with lower-dose secukinumab, while there was a zero PASI 100 rate in controls.
The only adverse events more common than with placebo were nasopharyngitis and upper respiratory infections.
Dr. Reich predicted that as the ongoing TRANSFIGURE study continues well beyond 16 weeks, the nail psoriasis response rates will climb, since nails are so slow growing.
TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
AT WCD 2015
Key clinical point: Two phase IIIb trials show secukinumab at 300 mg is the most effective drug ever formally studied for nail or palmoplantar psoriasis.
Major finding: At 16 weeks, secukinumab at 300 mg improved nail psoriasis by 45.3% and palmoplantar psoriasis by 33.3%.
Data source: The phase IIIb TRANSFIGURE and GESTURE studies, ongoing randomized, prospective, initially double-blind studies in which 198 patients with significant nail psoriasis and 205 with palmoplantar psoriasis received secukinumab at 150 or 300 mg or placebo. Both studies will continue out to 132 weeks.
Disclosures: TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.